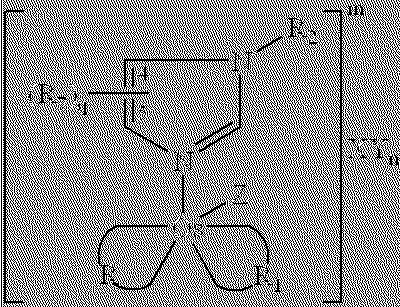Patents
Literature
671 results about "Osmium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Osmium (from Greek ὀσμή osme, "smell") is a chemical element with the symbol Os and atomic number 76. It is a hard, brittle, bluish-white transition metal in the platinum group that is found as a trace element in alloys, mostly in platinum ores. Osmium is the densest naturally occurring element, with an experimentally measured (using x-ray crystallography) density of 22.59 g/cm³. Manufacturers use its alloys with platinum, iridium, and other platinum-group metals to make fountain pen nib tipping, electrical contacts, and in other applications that require extreme durability and hardness. The element's abundance in the Earth's crust is among the rarest.
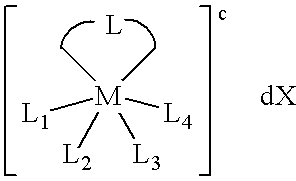
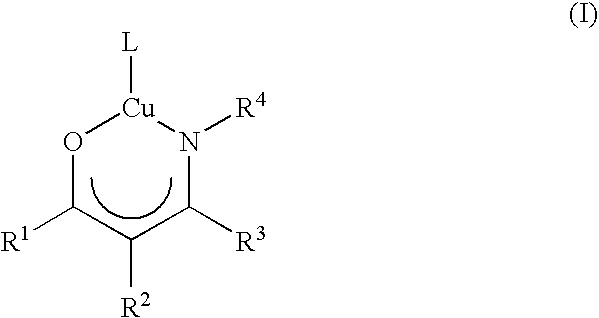
Volatile noble metal organometallic complexes
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
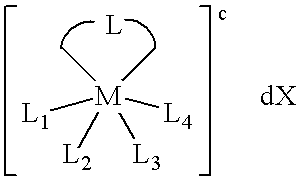
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
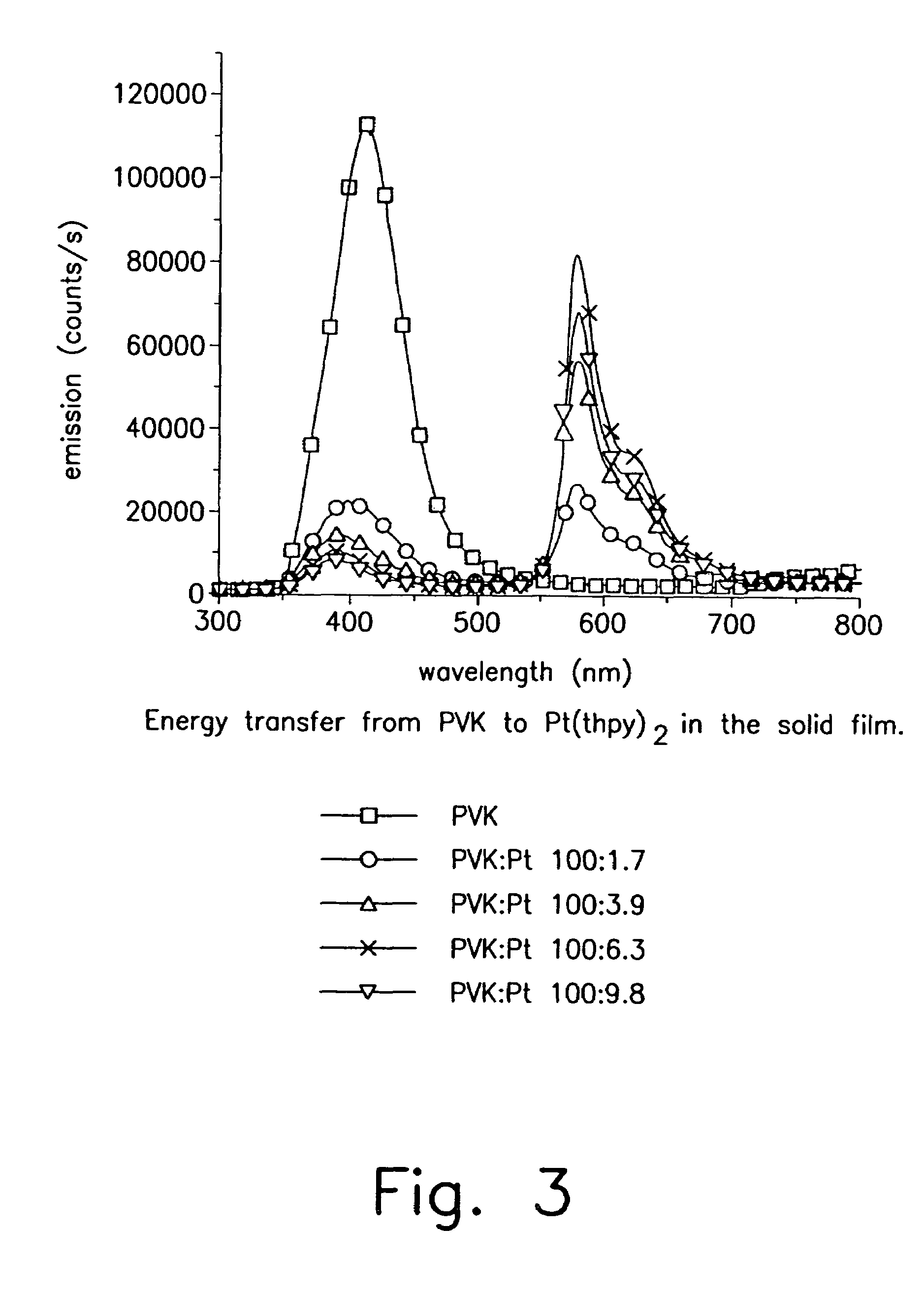
Organometallic complexes as phosphorescent emitters in organic LEDs
InactiveUS7001536B2Easy to combineDeterioration in emission qualityGroup 8/9/10/18 element organic compoundsElectroluminescent light sourcesIridiumPt element
Organic light emitting devices are described wherein the emissive layer comprises a host material containing an emissive molecule, which molecule is adapted to luminesce when a voltage is applied across the heterostructure, and the emissive molecule is selected from the group of phosphorescent organometallic complexes, including cyclometallated platinum, iridium and osmium complexes. The organic light emitting devices optionally contain an exciton blocking layer. Furthermore, improved electroluminescent efficiency in organic light emitting devices is obtained with an emitter layer comprising organometallic complexes of transition metals of formula L2MX, wherein L and X are distinct bidentate ligands. Compounds of this formula can be synthesized more facilely than in previous approaches and synthetic options allow insertion of fluorescent molecules into a phosphorescent complex, ligands to fine tune the color of emission, and ligands to trap carriers.
Owner:THE TRUSTEES FOR PRINCETON UNIV +1
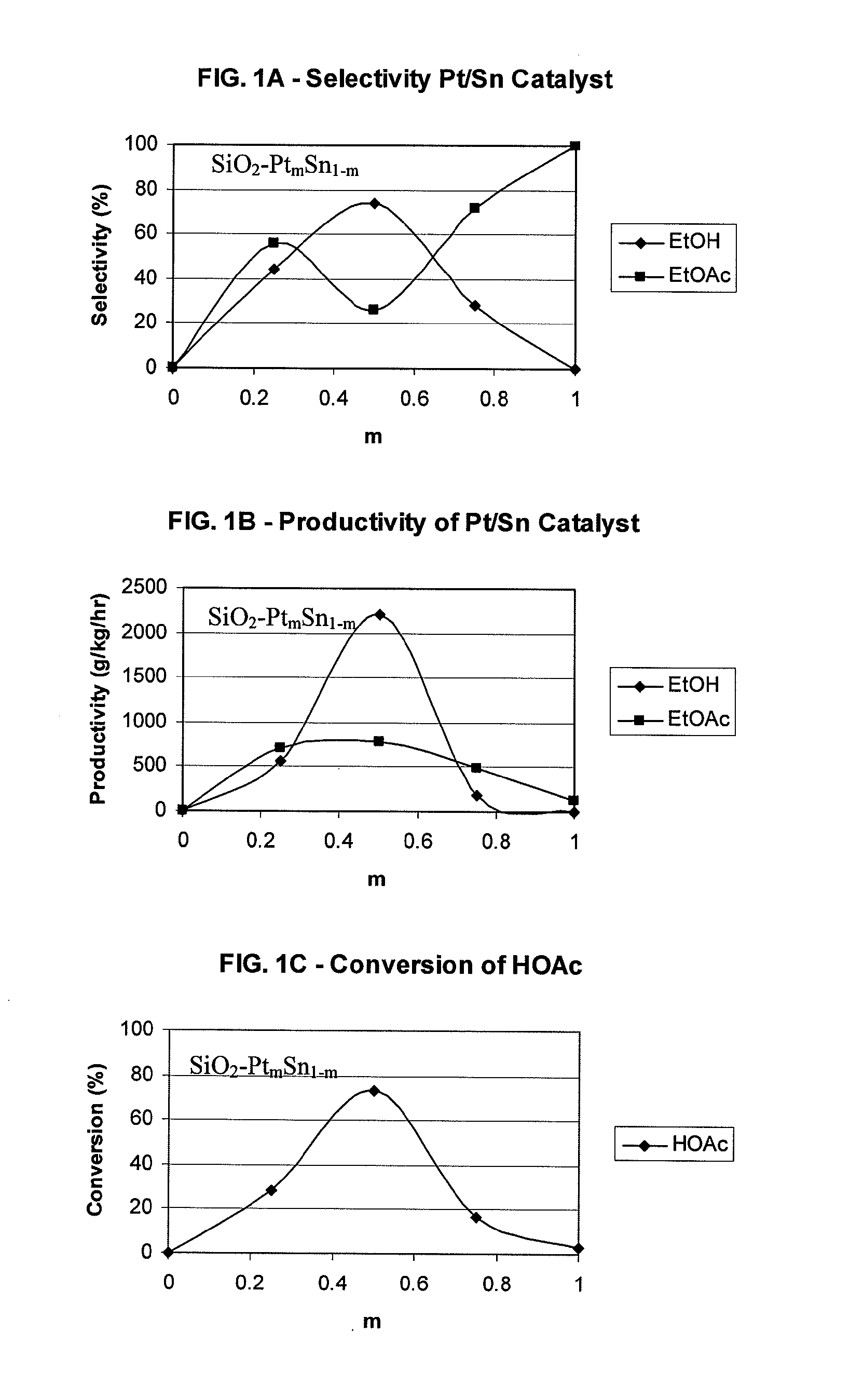
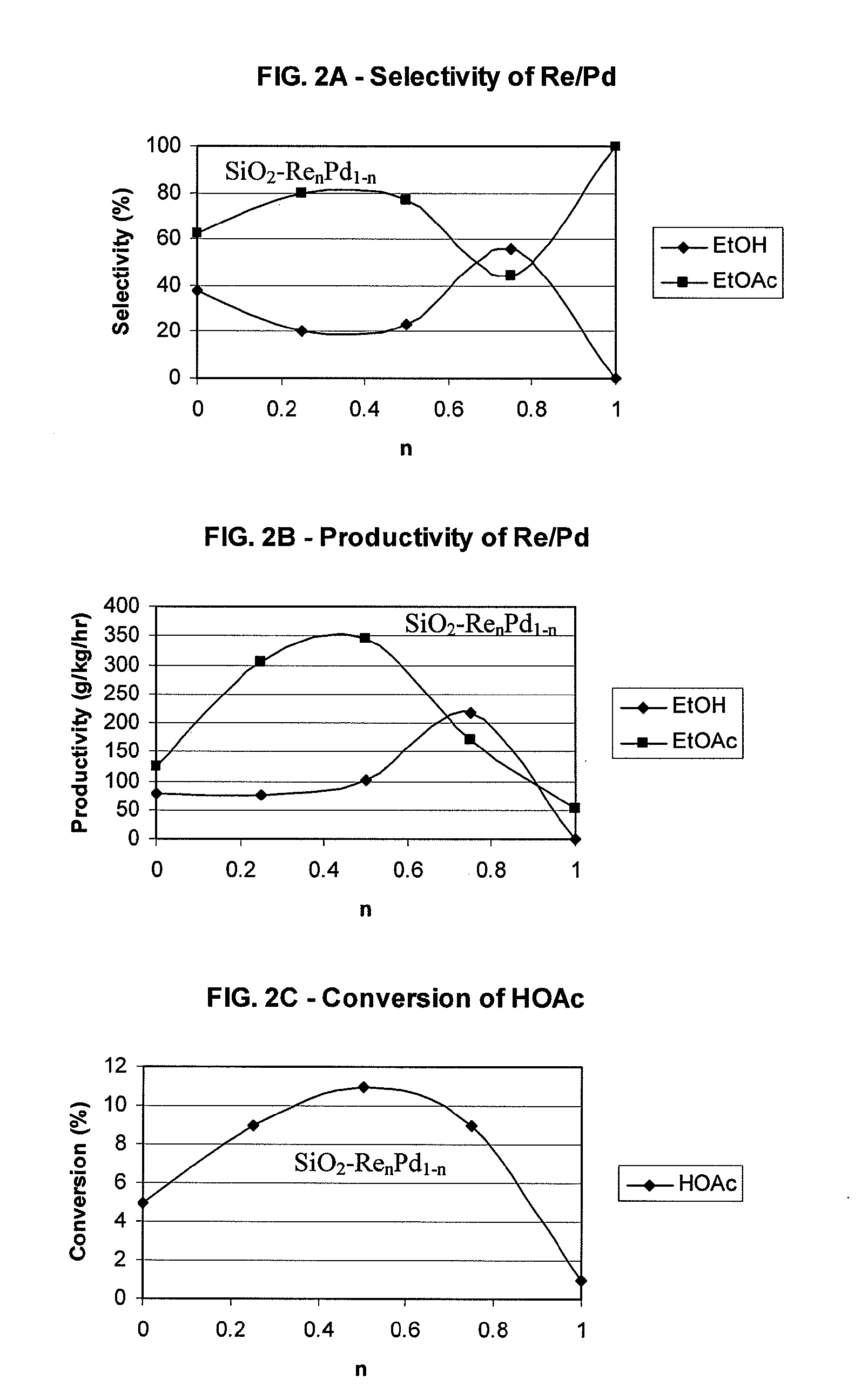
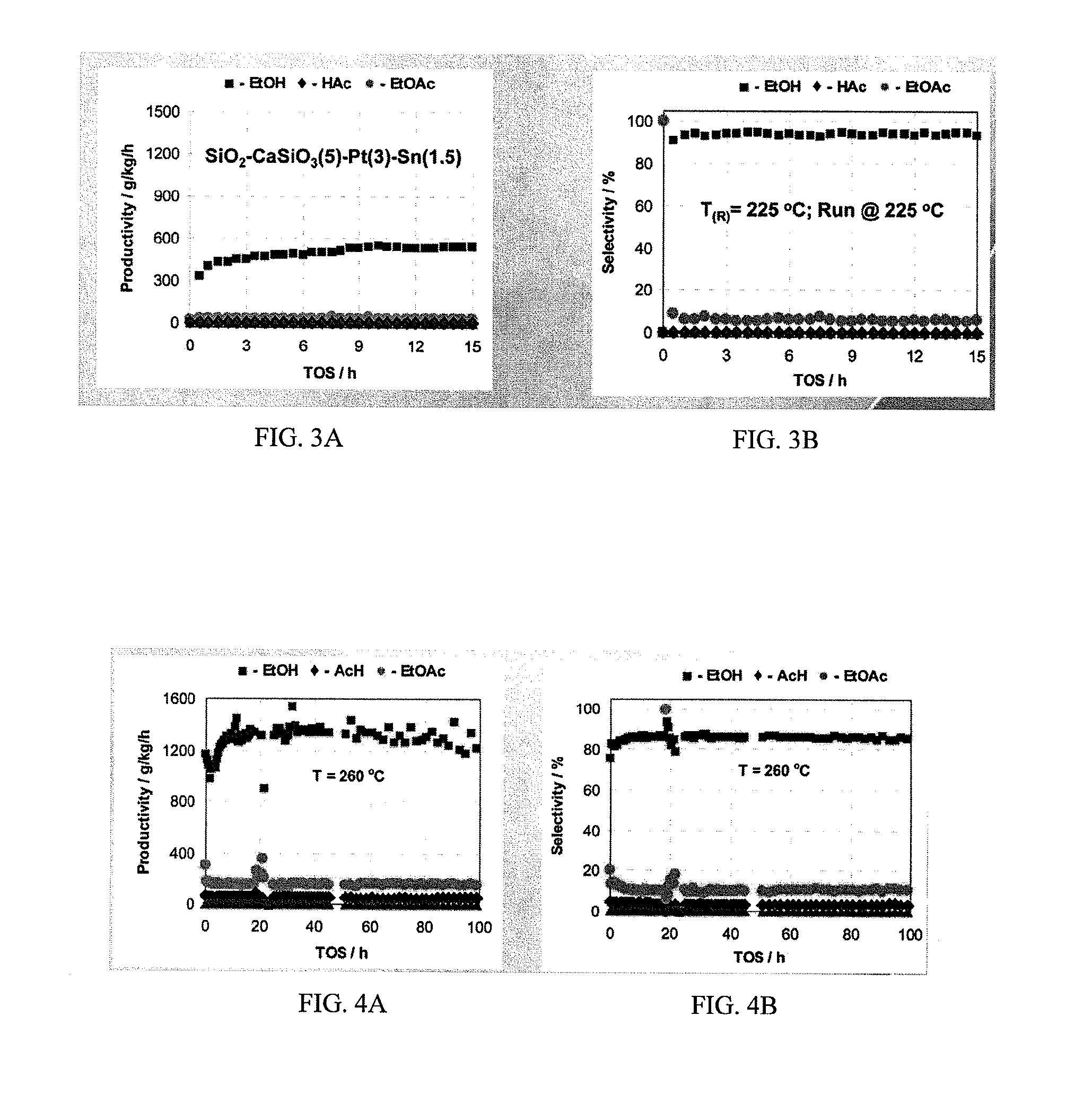
Processes for making ethanol from acetic acid
InactiveUS20100197985A1High selectivityPreparation by oxo-reaction and reductionEthylene productionCeriumCobalt
A process for selective formation of ethanol from acetic acid by hydrogenating acetic acid in the presence of first metal, a silicaceous support, and at least one support modifier. Preferably, the first metal is selected from the group consisting of copper, iron, cobalt, nickel, ruthenium, rhodium, palladium, osmium, iridium, platinum, titanium, zinc, chromium, rhenium, molybdenum, and tungsten. In addition the catalyst may comprise a second metal preferably selected from the group consisting of copper, molybdenum, tin, chromium, iron, cobalt, vanadium, tungsten, palladium, platinum, lanthanum, cerium, manganese, ruthenium, rhenium, gold, and nickel.
Owner:CELANESE INT CORP
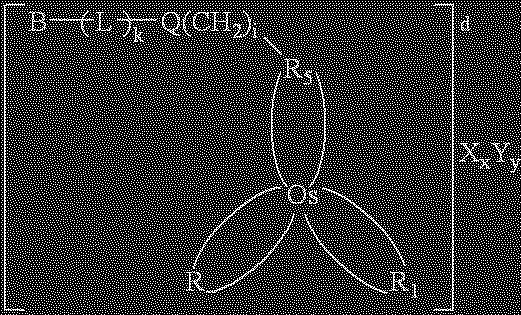
Redox reversible bipyridyl-osmium complex conjugates
InactiveUS7045310B2High measurement accuracyImprove accuracyBioreactor/fermenter combinationsBiological substance pretreatmentsRedoxElectrochemistry
Novel bipyridyl-osmium complex conjugates and their use in electrochemical assays are described. The redox reversible-osmium complexes can be prepared to exhibit unique reversible redox potentials and can thus be used in combination with other electroactive redox reversible species having redox potentials differing by at least 50 millivolts in electrochemical assays designed for use of multiple electroactive species in the same cell and in the same sample without interference between the two or more redox coupled conjugate systems.
Owner:ROCHE DIABETES CARE INC
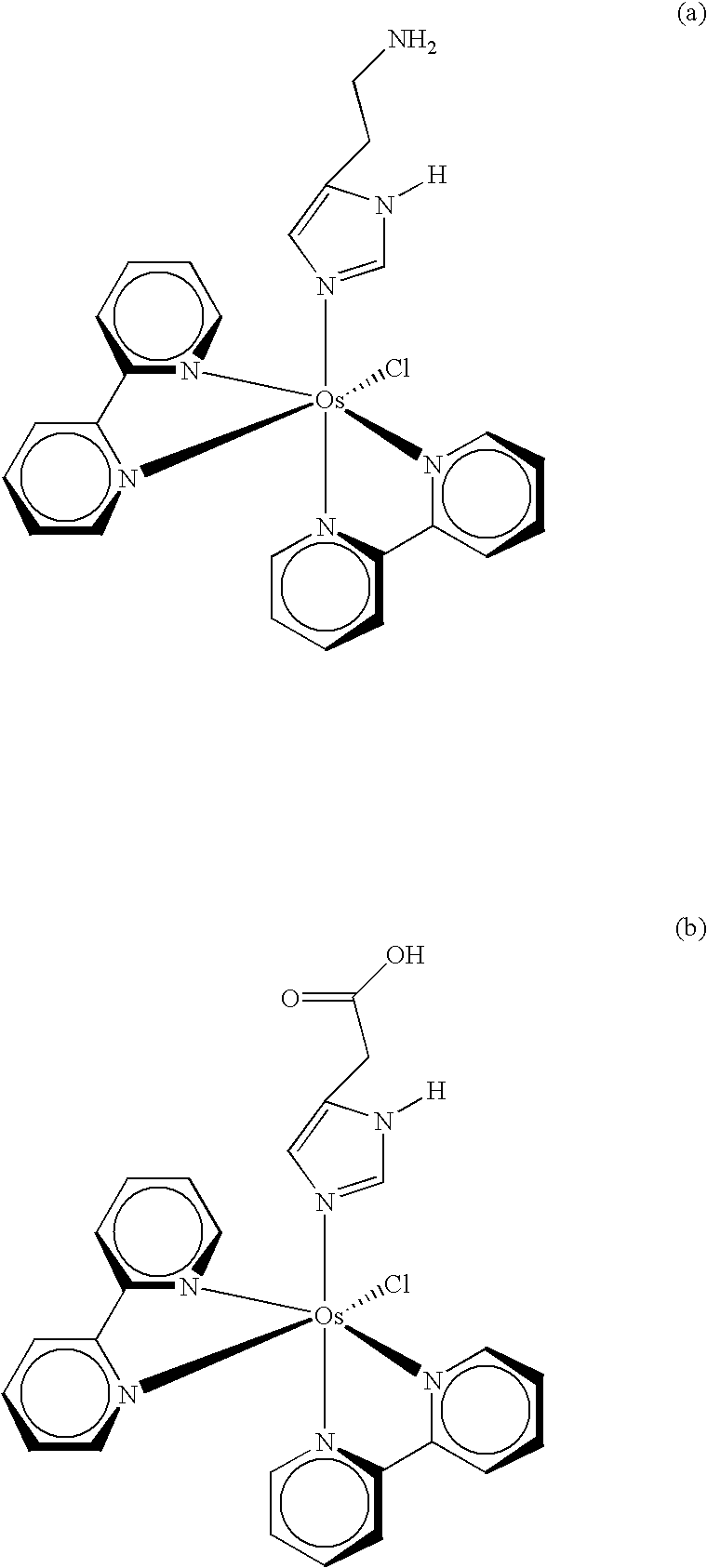
Transition metal complexes with (pyridyl)imidazole ligands
InactiveUS7074308B2Rapid electron exchangeFast dynamicsImmobilised enzymesBioreactor/fermenter combinationsOxidation-Reduction AgentRedox
Novel transition metal complexes of iron, cobalt, ruthenium, osmium, and vanadium are described. The transition metal complexes can be used as redox mediators in enzyme-based electrochemical sensors. The transition metal complexes include substituted or unsubstituted (pyridyl)imidazole ligands. Transition metal complexes attached to polymeric backbones are also described.
Owner:ABBOTT DIABETES CARE INC
Organometallic complexes as phosphorescent emitters in organic LEDS
InactiveUS7291406B2Easy to combineDeterioration in emission qualityOrganic chemistryDischarge tube luminescnet screensIridiumPt element
Organic light emitting devices are described wherein the emissive layer comprises a host material containing an emissive molecule, which molecule is adapted to luminesce when a voltage is applied across the heterostructure, and the emissive molecule is selected from the group of phosphorescent organometallic complexes, including cyclometallated platinum, iridium and osmium complexes. The organic light emitting devices optionally contain an exciton blocking layer. Furthermore, improved electroluminescent efficiency in organic light emitting devices is obtained with an emitter layer comprising organometallic complexes of transition metals of formula L2MX, wherein L and X are distinct bidentate ligands. Compounds of this formula can be synthesized more facilely than in previous approaches and synthetic options allow insertion of fluorescent molecules into a phosphorescent complex, ligands to fine tune the color of emission, and ligands to trap carriers.
Owner:THE TRUSTEES OF PRINCETON UNIV +1
Metathesis polymerizered olefin composites including sized reinforcement material
A reinforced polyolefin article is provided which includes a sized reinforcement material incorporated in the article. The article may be prepared by polymerizing a cyclic olefin monomer in the presence of the sizing agent and a metathesis polymerization catalyst which includes ruthenium or osmium.
Owner:ABELL III NELSON D +1
Metathesis polymerized olefin composites including sized reinforcement material
A reinforced polyolefin article is provided which includes a sized reinforcement material incorporated in the article. The article may be prepared by polymerizing a cyclic olefin monomer in the presence of the sizing agent and a metathesis polymerization catalyst which includes ruthenium or osmium.
Owner:WARNER MARK +2
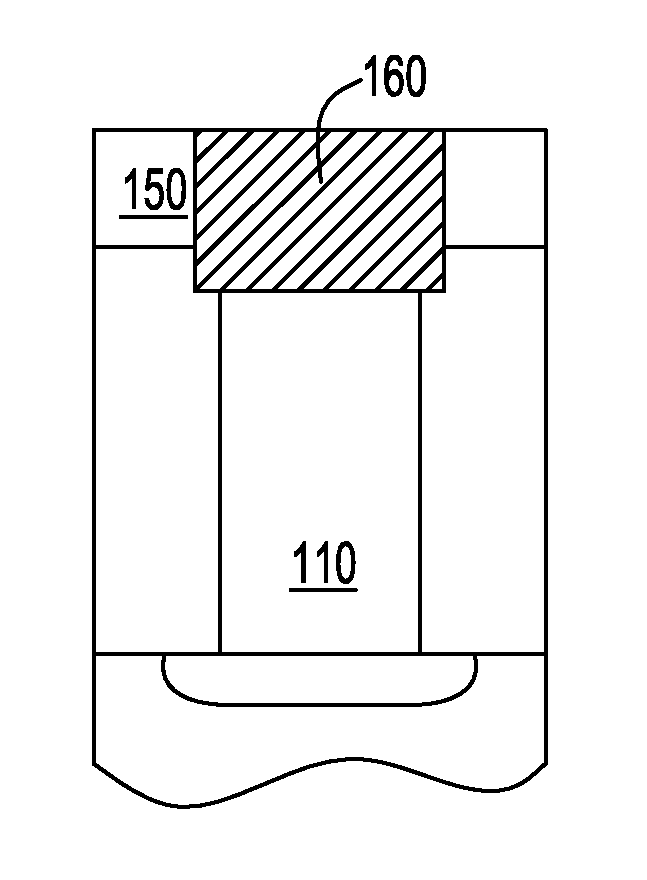
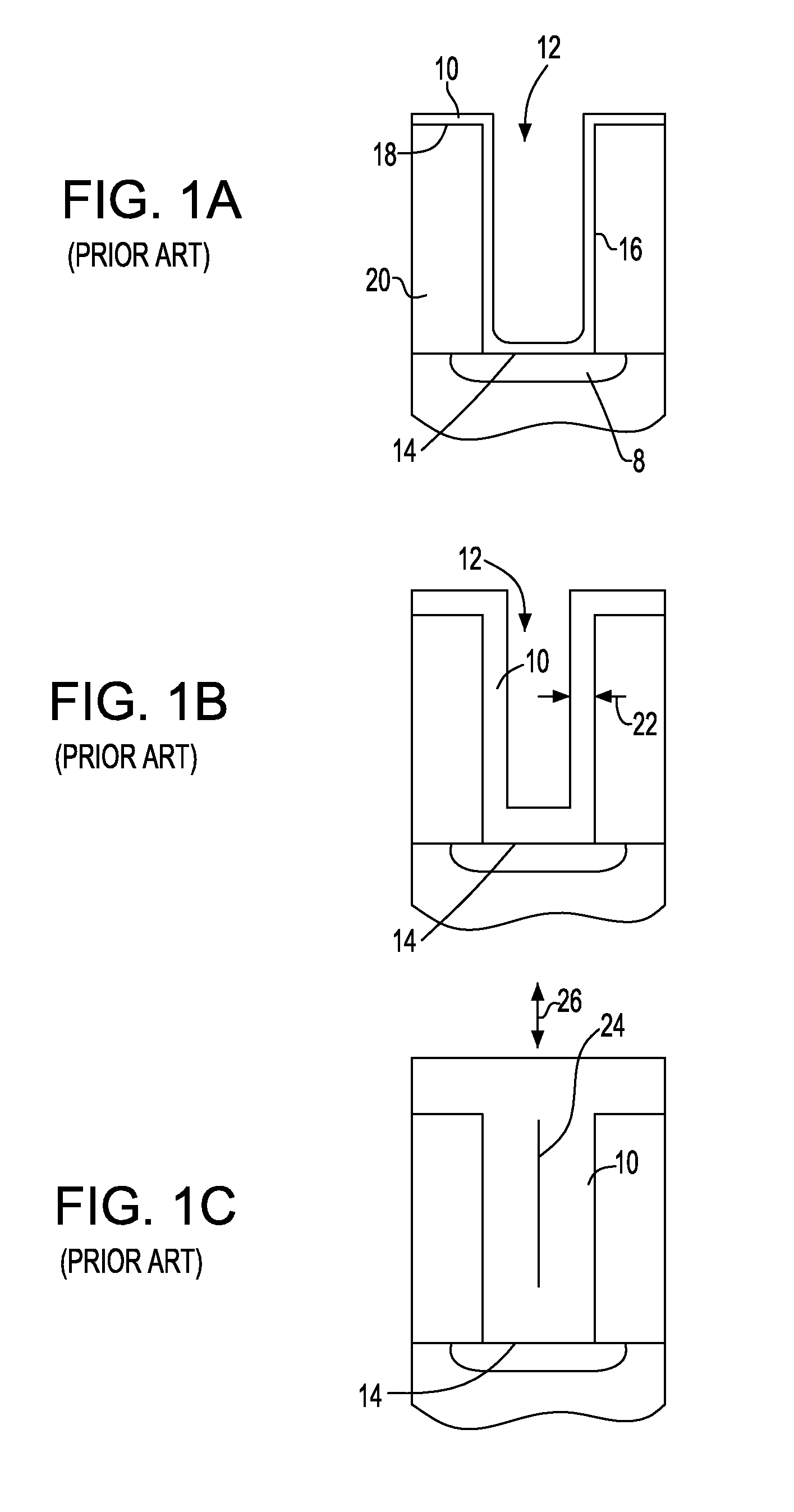
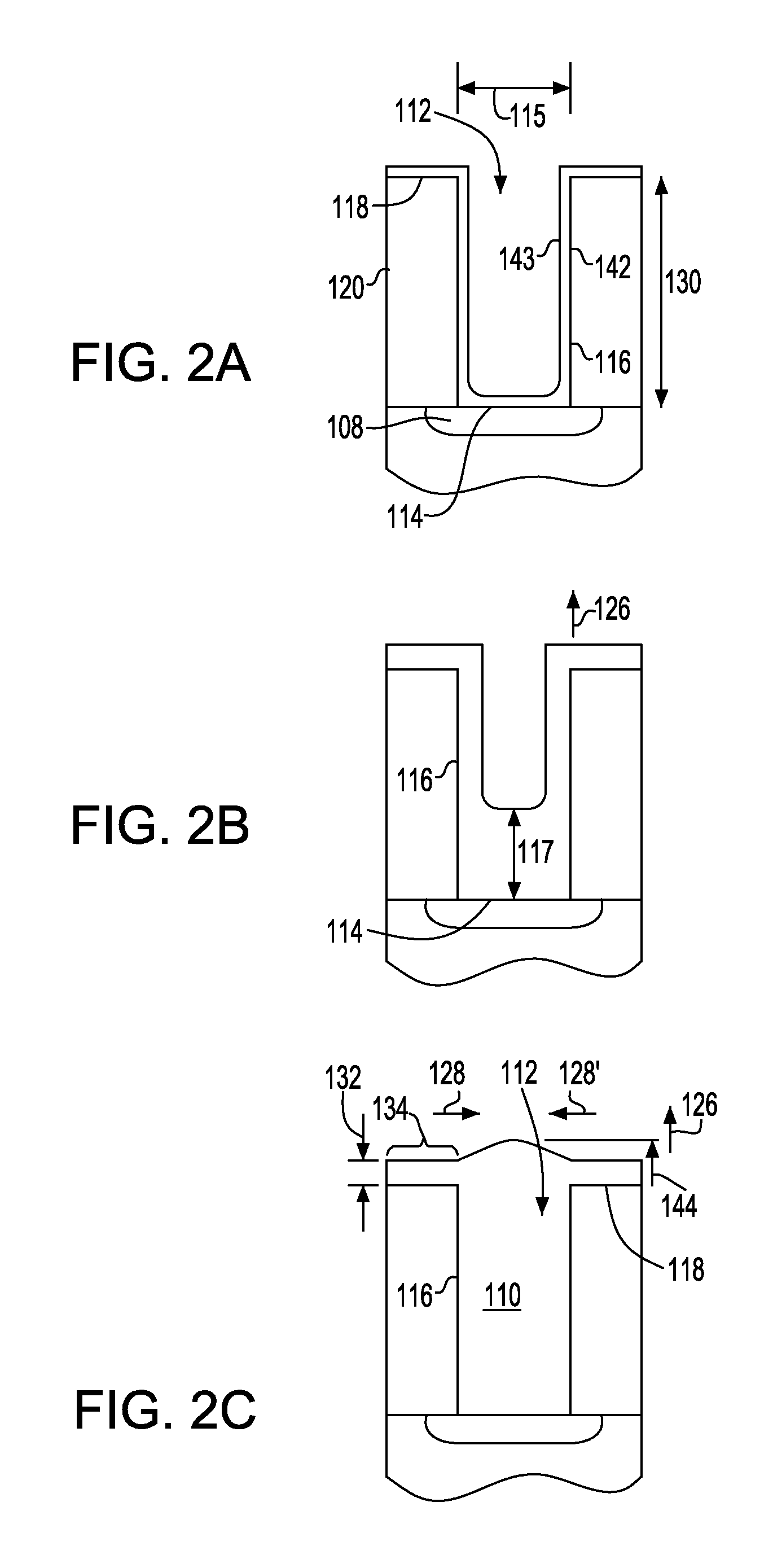
Superfilled metal contact vias for semiconductor devices
ActiveUS20110163449A1Semiconductor/solid-state device detailsSolid-state devicesIridiumConductive materials
In accordance with one aspect of the invention, a method is provided for fabricating a semiconductor element having a contact via. In such method, a hole can be formed in a dielectric layer to at least partially expose a region including at least one of semiconductor or conductive material. A seed layer can be deposited over a major surface of the dielectric layer and over a surface within the hole. In one embodiment, the seed layer can include a metal selected from the group consisting of iridium, osmium, palladium, platinum, rhodium, and ruthenium. A layer consisting essentially of cobalt can be electroplated over the seed layer within the hole to form a contact via in electrically conductive communication with the region.
Owner:GLOBALFOUNDRIES US INC
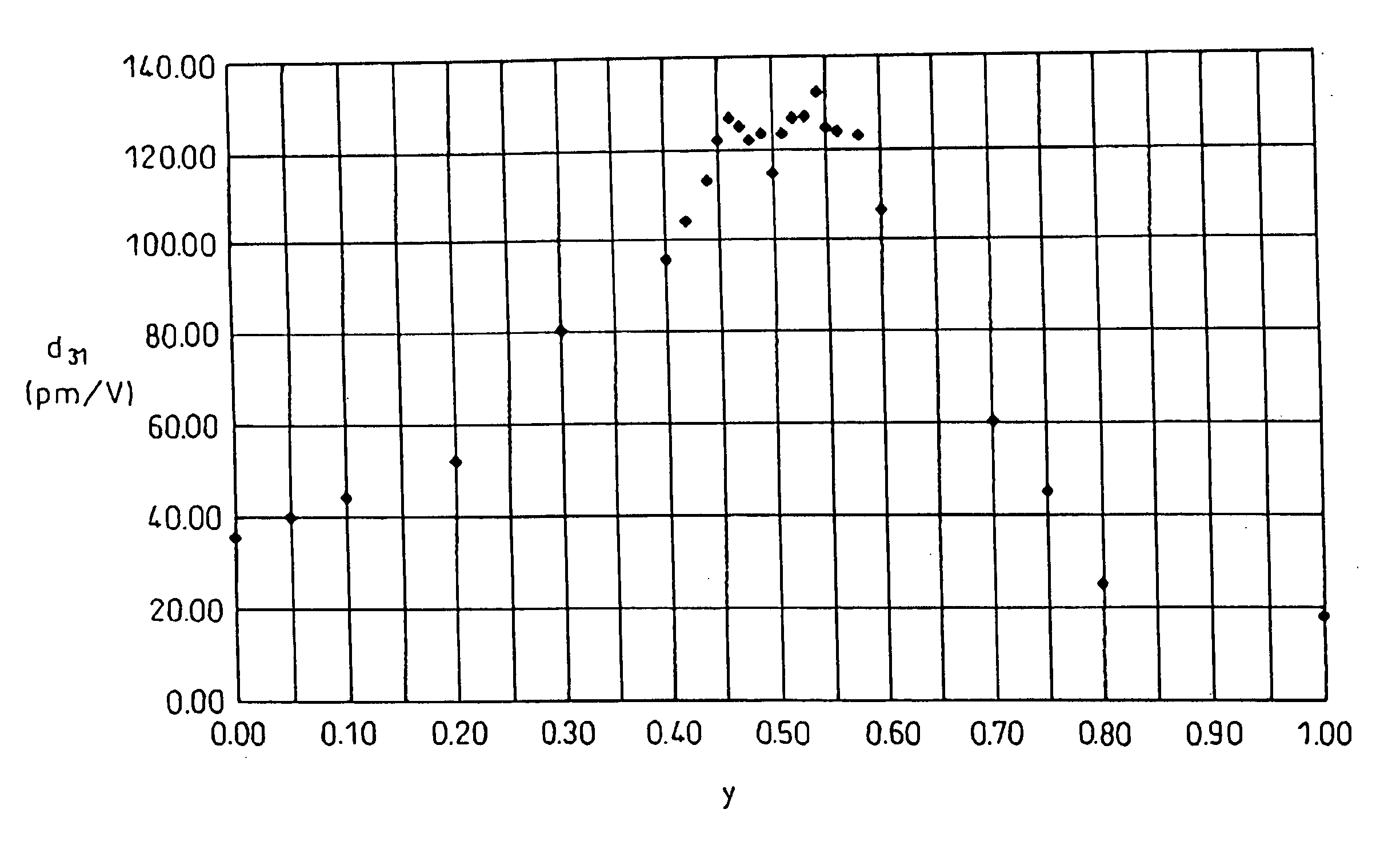
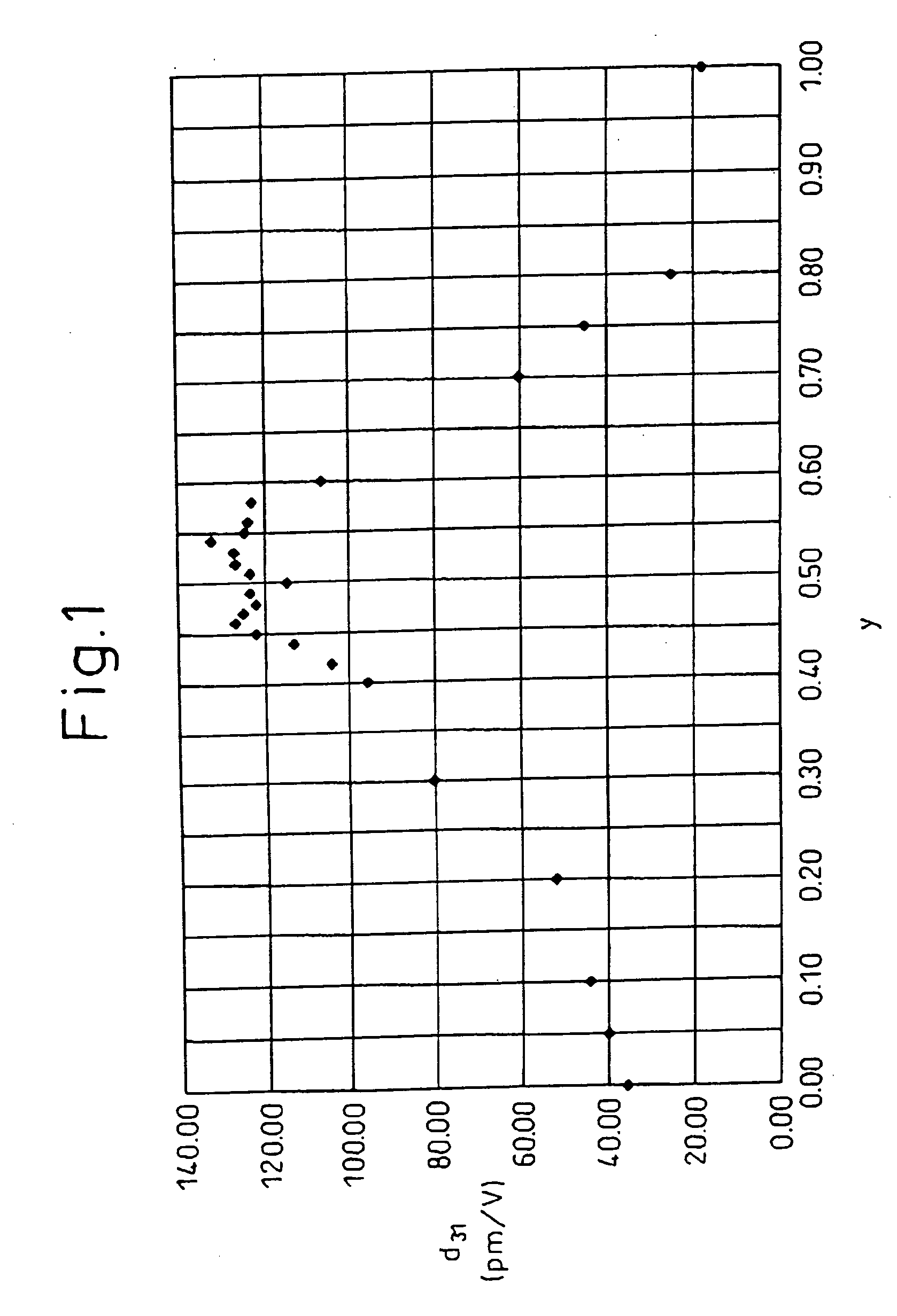
Piezoelectric ceramic composition and method of production of same, piezoelectric element, and dielectric element
InactiveUS20040058797A1Excellent piezoelectric propertiesIncrease temperaturePiezoelectric/electrostrictive device manufacture/assemblyPiezoelectric/electrostrictive device material selectionRheniumIridium
A piezoelectric ceramic composition not containing lead, able to be sintered at ordinary pressure, and superior to the past in at least one of the properties unique to piezoelectric ceramic compositions such as the piezoelectric d31 constant, that is, a piezoelectric ceramic composition having a compound of a general formula {Lix(K1-yNay)1-x}(Nb1-z-wTazSbw)O3 where x, y, z, and w are in the ranges of 0<=x<=0.2, 0<=y<=1, 0<z<=0.4, and 0<w<=0.2 as a main ingredient, where the piezoelectric ceramic composition contains at least one metal element selected from (1) palladium, silver, gold, ruthenium, rhodium, rhenium, osmium, iridium, and platinum, (2) nickel, iron, manganese, copper, and zinc, or (3) magnesium, calcium, strontium, and barium as an added element, and a method of production of the same and a piezoelectric element and dielectric element utilizing that piezoelectric ceramic composition.
Owner:DENSO CORP +1
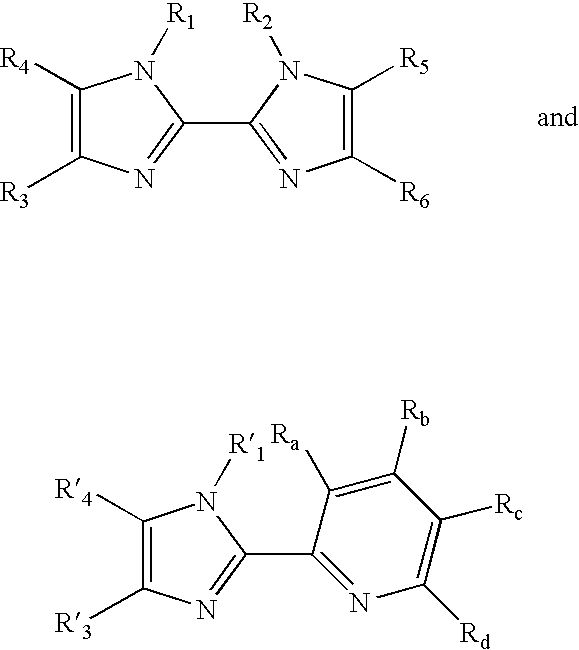
Transition metal complexes with bidentate ligand having an imidazole ring
InactiveUS7090756B2Quick exchangeImmobilised enzymesBioreactor/fermenter combinationsCoordination complexOsmium
Novel transition metal complexes of iron, cobalt, ruthenium, osmium, and vanadium are described. The transition metal complexes can be used as redox mediators in enzyme based electrochemical sensors. In such instances, transition metal complexes accept electrons from, or transfer electrons to, enzymes at a high rate and also exchange electrons rapidly with the sensor. The transition metal complexes include at least one substituted or unsubstituted biimidazole ligand and may further include a second substituted or unsubstituted biimidazole ligand or a substituted or unsubstituted bipyridine or pyridylimidazole ligand. Transition metal complexes attached to polymeric backbones are also described.
Owner:ABBOTT DIABETES CARE INC
Metal alloys for the reflective or the semi-reflective layer of an optical storage medium
InactiveUS6764735B2Improve reflectivitySimilar sputtering characteristicPhotomechanical apparatusRecord information storageIridiumHigh reflectivity
Owner:TARGET TECH
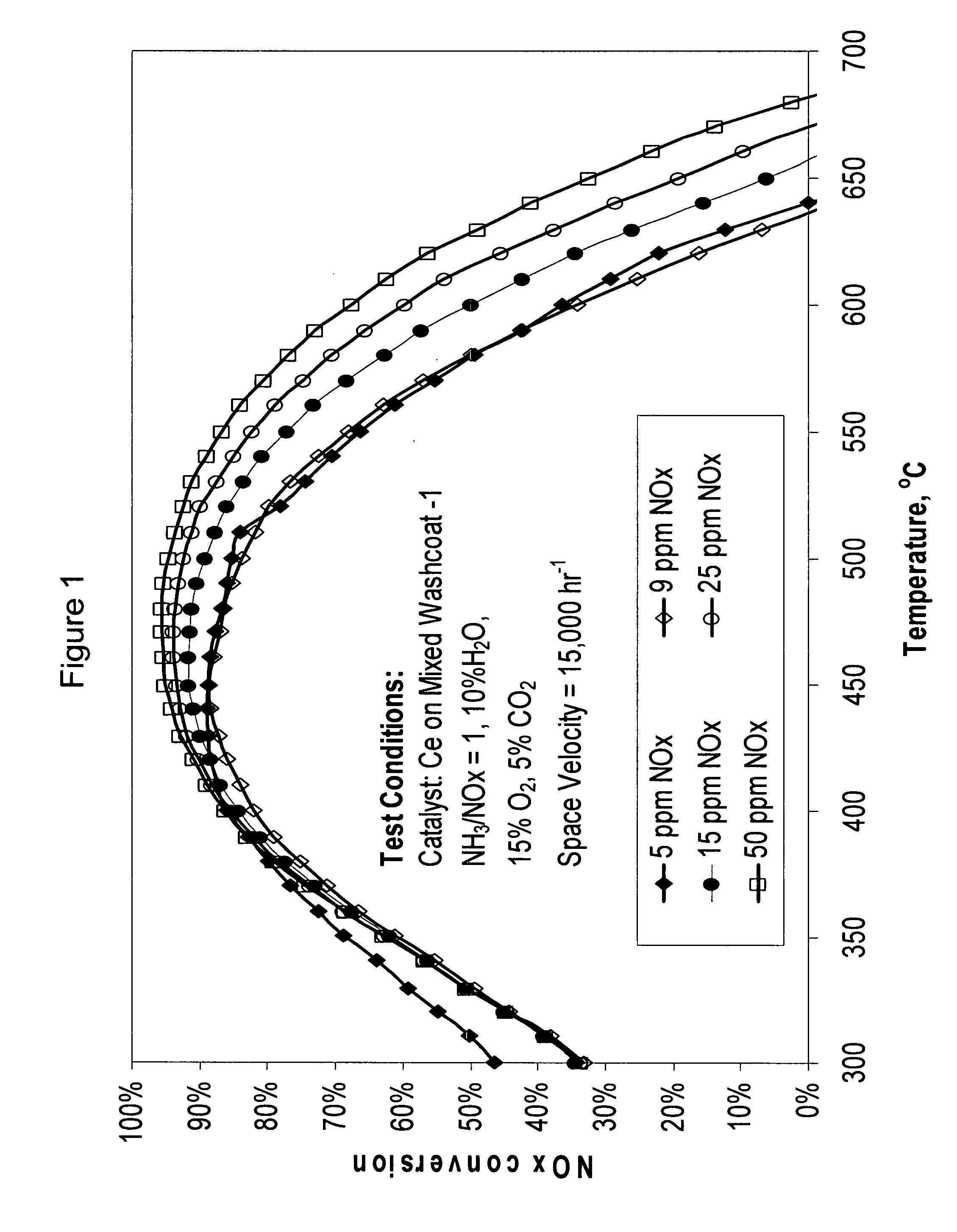
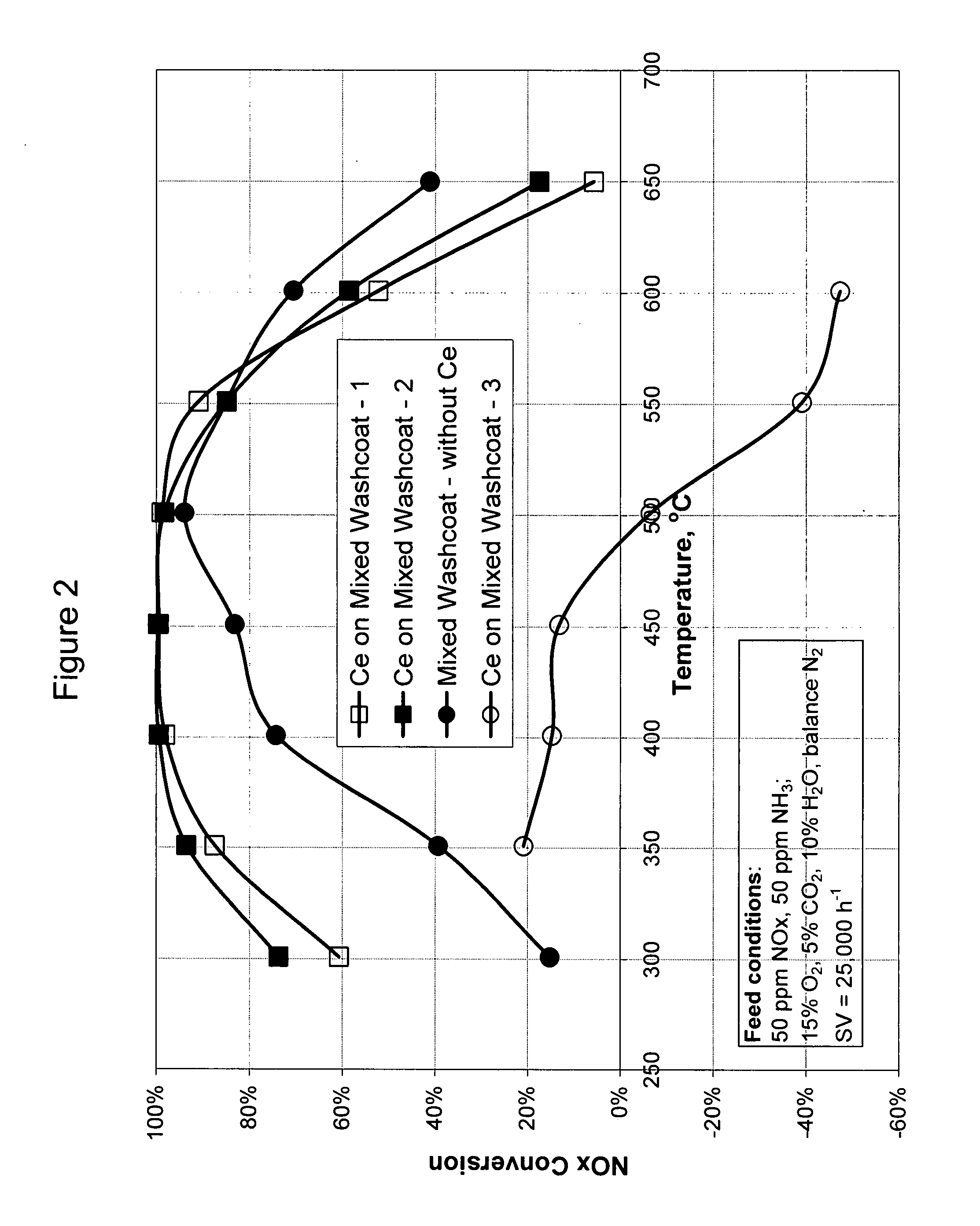
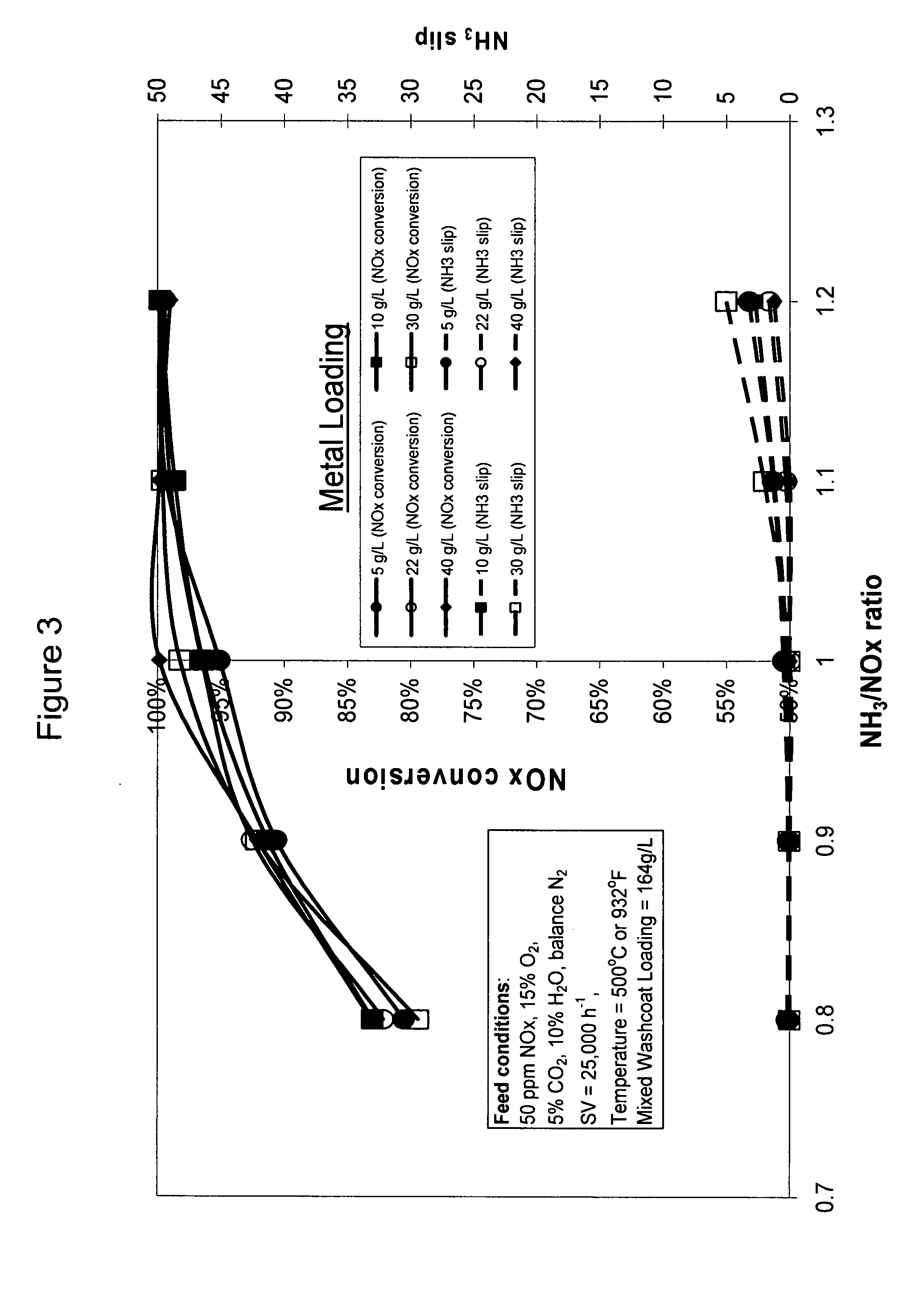
High temperature ammonia SCR catalyst and method of using the catalyst
ActiveUS20080167178A1Reduce selection requirementsMolecular sieve catalystsInternal combustion piston enginesCeriumMordenite
A catalyst and a method for selectively reducing nitrogen oxides (“NOx”) with ammonia are provided. The catalyst includes a first component comprising a zeolite or mixture of zeolites selected from the group consisting of ZSM-5, ZSM-11, ZSM-12, ZSM-18, ZSM-23, MCM-zeolites, mordenite, faujasite, ferrierite, zeolite beta, and mixtures thereof; a second component comprising at least one member selected from the group consisting of cerium, iron, copper, gallium, manganese, chromium, cobalt, molybdenum, tin, rhenium, tantalum, osmium, barium, boron, calcium, strontium, potassium, vanadium, nickel, tungsten, an actinide, mixtures of actinides, a lanthanide, mixtures of lanthanides, and mixtures thereof; optionally an oxygen storage material and optionally an inorganic oxide. The catalyst selectively reduces nitrogen oxides to nitrogen with ammonia at high temperatures. The catalyst has high hydrothermal stability. The catalyst has high activity for conversion of low levels of nitrogen oxides in exhaust streams. The catalyst and the method may have special application to selective reduction of nitrogen oxides in exhaust gas from gas turbines and gas engines, although the catalyst and the method have broad application to a wide range of gas streams that have excess oxygen and high temperatures. The temperature of exhaust gas from gas turbines and gas engines is high. Both the high temperature and the low levels of inlet NOx are challenging for selective catalytic reduction (SCR) catalysts.
Owner:CATALYTIC SOLUTIONS INC
Semiconductor device and method for manufacturing the same
InactiveUS20050020006A1Small amount of resistanceImprove mobilityStatic indicating devicesSolid-state devicesIridiumManganese
A method for manufacturing a semiconductor device such as a thin film transistor using a crystal silicon film is provided. The crystal silicon film is obtained by selectively forming films, particles or clusters containing nickel, iron, cobalt, ruthenium, rhodium, paradium, osmium, iridium, platinum, scandium, titanium, vanadium, chrome, manganese, copper, zinc, gold, silver or silicide thereof in a form of island, line, stripe, dot or film on or under an amorphous silicon film and using them as a starting point, by advancing its crystallization by annealing at a temperature lower than a normal crystallization temperature of an amorphous silicon. A transistor whose leak current is low and a transistor in which a mobility is high are obtained in the same time in structuring a dynamic circuit having a thin film transistor by selectively forming a cover film on a semiconductor layer which is to become an active layer of the transistor and by thermally crystallizing it thereafter.
Owner:SEMICON ENERGY LAB CO LTD
Tungsten carbide catalysts, their preparation and application in synthesis of ethylene glycol from cellulose
ActiveUS20100255983A1Improve efficiencyHigh selectivityOxygen-containing compound preparationOrganic compound preparationHydrogen pressureCobalt
Tungsten carbide catalysts are used in preparation of ethylene glycol by hydrogenating degradation of cellulose. The catalyst includes tungsten carbide as main catalytic active component, added with small amount of one or more transition metals such as nickel, cobalt, iron, ruthenium, rhodium, palladium, osmium, iridium, platinum, and copper as the second metal, supported on one or more porous complex supports such as active carbon, alumina, silica, titanium dioxide, silicon carbide, zirconium oxide, for conversion of cellulose to ethylene glycol. The catalyst realizes high efficiency, high selectivity, and high yield in the conversion of cellulose to ethylene glycol at the temperature of 120-300° C., hydrogen pressure of 1-10 MPa, and hydrothermal conditions. Compared to the existing industrial synthetic method of ethylene glycol using ethylene as feedstock, the invention has the advantages of using renewable raw material resources, environment friendly process, and excellent atom economy.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Nano molecular sieve catalyst for synthesizing paraxylene and preparation method thereof
ActiveCN101485994AMolecular sieve catalystsHydrocarbon by hydrocarbon and non-hydrocarbon condensationIridiumRare earth
The invention provides a catalyst for synthesizing a nanometer molecular sieve of para-xylene and a method for preparing the same, and mainly overcomes the defects of poor stability and low selectivity on para-xylene in the prior catalyst. In the method, the nanometer molecular sieve as a mother substance, silicon, phosphorus, magnesium and mixed rare earth are modified so as to adjust acid performance and porous channel structure of the catalyst and improve the selectivity of the catalyst, and the metals with hydrogenation function such as noble metals of platinum, palladium, ruthenium, rhodium, iridium, osmium, and the like or transition metals of iron, cobalt, nickel, and the like are modified so as to inhibit the deactivation of carbon accumulation of the catalyst and greatly improve the stability of the catalyst.
Owner:DALIAN UNIV OF TECH
Ag-based alloy wire for semiconductor package
InactiveUS20080240975A1Reduce manufacturing costImprove reliabilitySolid-state devicesSemiconductor/solid-state device manufacturingPlatinumSemiconductor package
An Ag-based alloy wire for a semiconductor package is highly reliable and can be fabricated with low costs. The Ag-based alloy wire includes 0.05˜5 wt % of at least one kind of a first additive ingredient selected from the group consisting of platinum (Pt), palladium (Pd), rhodium (Rh), osmium (Os), gold (Au), and nickel (Ni), and Ag as a remainder.
Owner:MK ELECTRON
Dehydrogenation catalysts
InactiveUS6191065B1High activityHigh selectivityHydrocarbon by dehydrogenationCatalystsIridiumRhenium
A catalyst for the production of alkenylaromatics from alkylaromatics, wherein the catalyst is predominantly iron oxide, an alkali metal compound and less than about 100 ppm of a source for a noble metal, such as palladium, platinum, ruthenium, rhenium, osmium, rhodium or iridium. Additional components of the catalyst may include compounds based on cerium, molybdenum, tungsten and other such promoters. Also a process for the production of alkenylaromatics from alkylaromatics using this catalyst.
Owner:SUD CHEM INC
Method for the Decomposition of N2O, Catalyst Therefor and Preparation of This Catalyst
ActiveUS20080044334A1Promote conversionImprove stabilityNitrous oxide captureNitrogen compoundsRheniumIridium
The invention relates to a method for the catalytic decomposition of N2O in a gas containing N2O in the presence of a catalyst, wherein the catalyst comprises a zeolite that has been loaded with a first metal selected from the group of noble metals consisting of ruthenium, rhodium, silver, rhenium, osmium, iridium, platinum and gold, and with a second metal selected from the group of transition metals consisting of chromium, manganese, iron cobalt, nickel and copper, and wherein the loading of the zeolite with metals has been obtained by first loading the zeolite with the noble metal and then with the transition metal, as well as a catalyst for this method and a method for the preparation of this catalyst.
Owner:NEDERLANDSE ORG VOOR TOEGEPAST-NATUURWETENSCHAPPELIJK ONDERZOEK (TNO)
Pipe made from metathesis polymerized olefins
InactiveUS6410110B1Extended validity periodImprove responseLayered productsBottlesPolymer sciencePipe fitting
Thermosetting resin pipes and pipe fittings are provided which are prepared by polymerizing a cyclic olefin monomer in the presence of a ruthenium or osmium metathesis polymerization catalyst. These articles may be prepared by various methods, such as centrifugal casting. Reinforced articles may also be prepared by filament winding.
Owner:A O SMITH +1
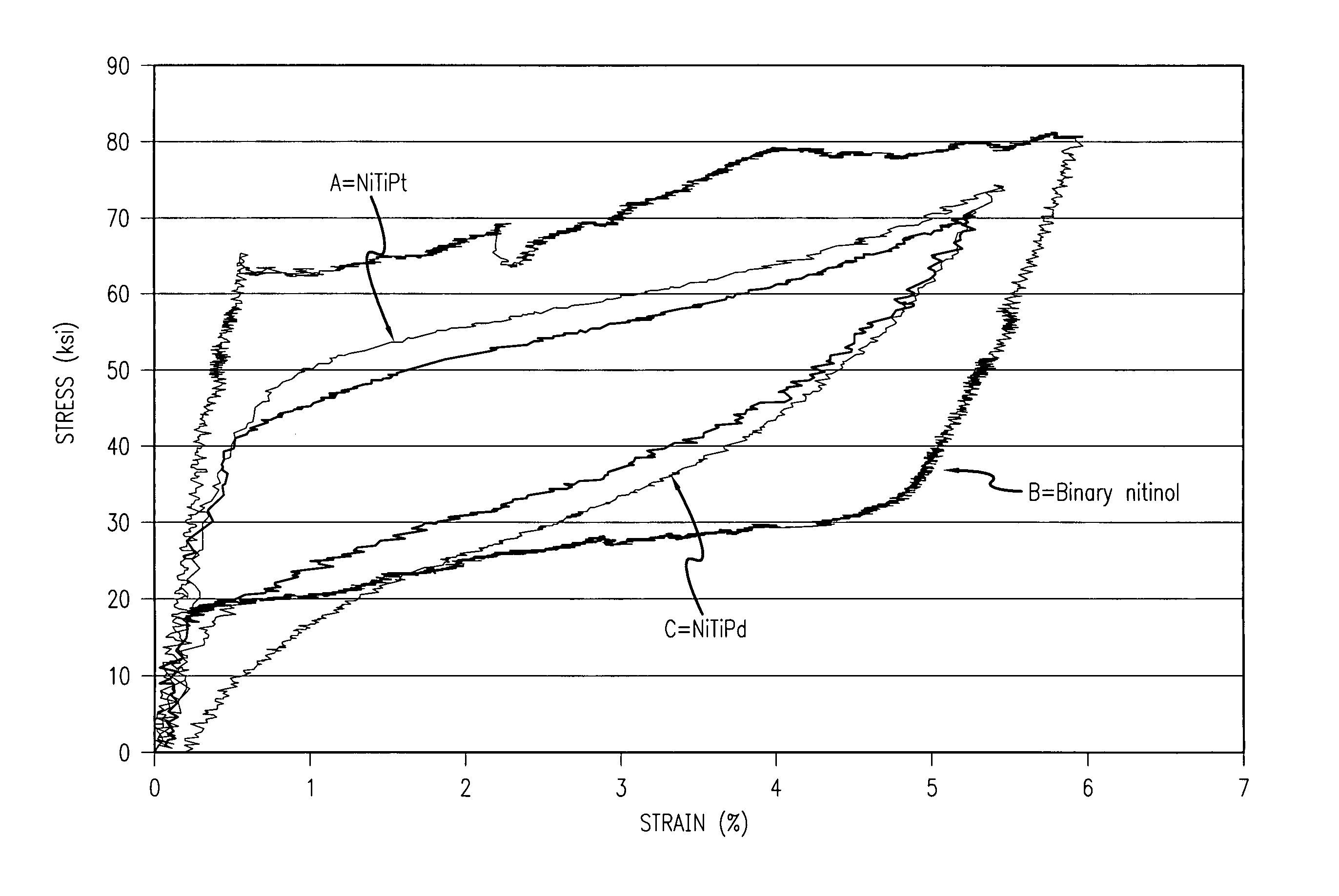
Radiopaque and MRI compatible nitinol alloys for medical devices
A radiopaque nitinol medical device such as a stent for use with or implantation in a body lumen is disclosed. The stent is made from a superelastic alloy such as nickel-titanium or nitinol, and includes a ternary element selected from the group of chemical elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, or hafnium. The nitinol stent has improved radiopacity yet retains its superelastic and shape memory behavior and further maintains a thin strut / wall thickness for high flexibility. Another embodiment includes a balloon expandable stent made from a radiopaque and MRI compatible alloy such as nitinol and includes a ternary element selected from the group of chemical elements consisting of iridium, platinum, gold, rhenium, tungsten, palladium, rhodium, tantalum, silver, ruthenium, hafnium, osmium, zirconium, niobium, or molybdenum.
Owner:ABBOTT CARDIOVASCULAR
Metal alloys for the reflective or the semi-reflective layer of an optical storage medium
InactiveUS6852384B2Improve reflectivitySimilar sputtering characteristicPhotomechanical apparatusRecord information storageIndiumCadmium Cation
A silver-based alloy thin film is provided for the highly reflective or semi-reflective layer of optical discs. Alloy additions to silver include gold, rhodium, ruthenium, osmium, platinum, palladium, copper, silicon, cadmium, tin, lithium, nickel, cobalt, indium, chromium, antimony, gallium, boron, molybdenum, zirconium, beryllium, titanium, and zinc. These alloys have moderate to high reflectivity and reasonable corrosion resistance in the ambient environment.
Owner:TARGET TECH
Method for the degradation of nitrile rubber by metathesis in the presence of ruthenium- or osmium-based catalysts
A novel process for the degradation of nitrile rubber by metathesis using ruthenium- or osmium-based catalysts which have both a substituted or unsubstituted imidazolidine ligand and a carbene ligand bearing a phosphonium radical is provided.
Owner:LANXESS DEUTDCHLAND GMBH
Organic EL element and method of manufacturing the same, organic EL display device using the element, organic EL material, and surface emission device and liquid crystal display device using the material
InactiveUS20060204788A1Avoiding degradation in luminescence characteristicSimplify manufacturing stepsOrganic chemistrySolid-state devicesRheniumIridium
In an organic EL element, an organic EL layer is interposed between anodes and cathodes formed on a substrate. Each of the cathodes is made of a first conductive film that comes into contact with the organic EL layer and a second conductive film that constitutes a laminated structure together with the first conductive film. The first conductive film contains any one of an alkaline metal and an alkaline earth metal. The second conductive film contains any one of at least one type metal selected from a group consisting of Ru (ruthenium), Rh (rhodium), Ir (iridium), Os (osmium) and Re (rhenium) and its oxide.
Owner:FUJITSU LTD
Metal alloys for the reflective or the semi-reflective layer of an optical storage medium
InactiveUS6841219B2Improve reflectivitySimilar sputtering characteristicLayered productsPhotomechanical apparatusIndiumCobalt
A silver-based alloy thin film is provided for the highly reflective or semi-reflective layer of optical discs. Alloy additions to silver include gold, rhodium, ruthenium, osmium, platinum, palladium, copper, silicon, cadmium, tin, lithium, nickel, cobalt, indium, chromium, antimony, gallium, boron, molybdenum, zirconium, beryllium, titanium, magnesium, and zinc. These alloys have moderate to high reflective and reasonable corrosion resistance in the ambient environment.
Owner:TARGET TECH
Volatile metal beta-ketoiminate and metal beta-diiminate complexes
ActiveUS7205422B2Group 1/11 organic compounds without C-metal linkagesCopper organic compoundsIndiumGas phase
Metal ketoiminate or diiminate complexes, containing copper, silver, gold, cobalt, ruthenium, rhodium, platinum, palladium, nickel, osmium, or indium, and methods for making and using same are described herein. In certain embodiments, the metal complexes described herein may be used as precursors to deposit metal and metal-containing films on a substrate through, for example, atomic layer deposition or chemical vapor deposition conditions.
Owner:VERSUM MATERIALS US LLC
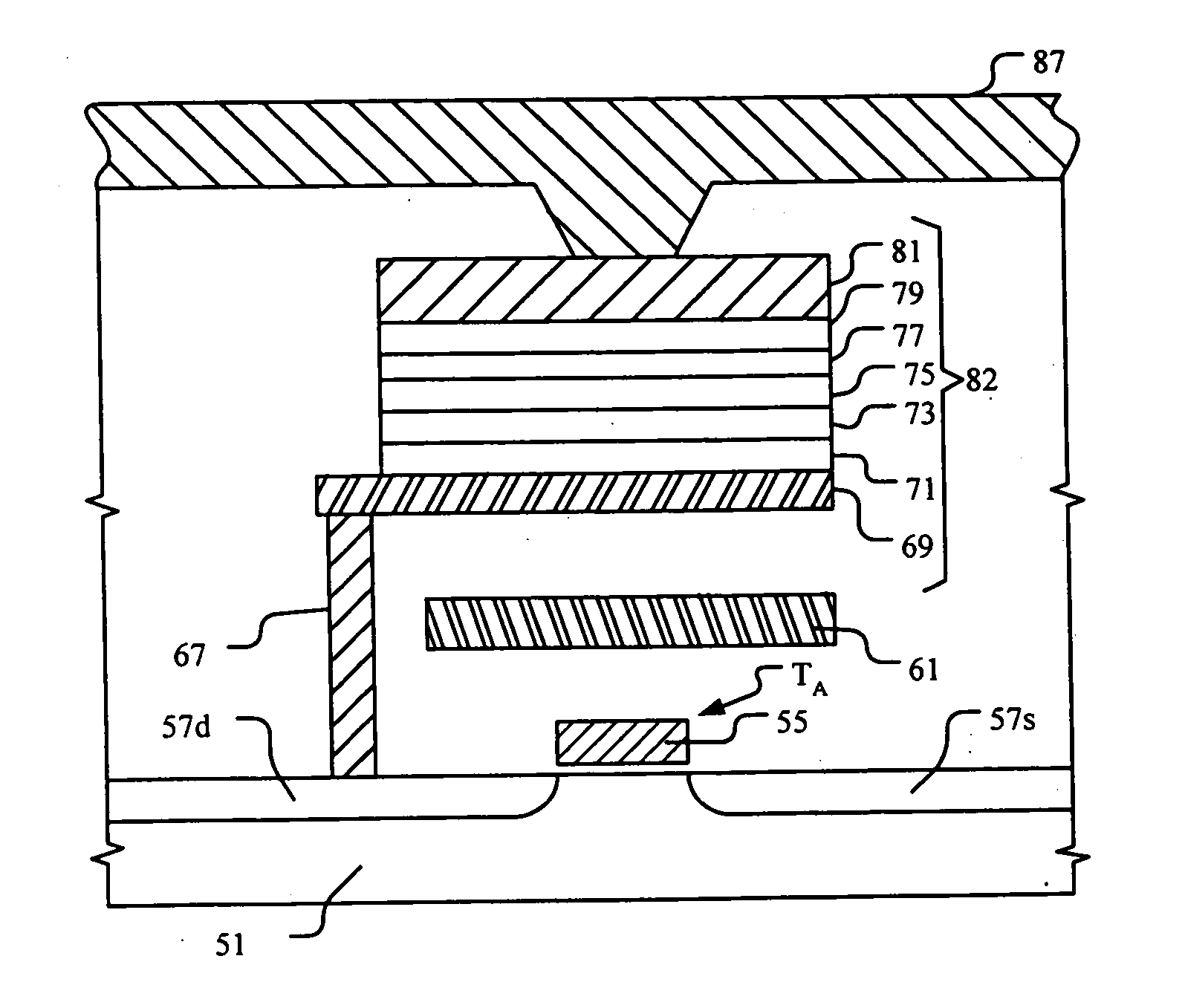
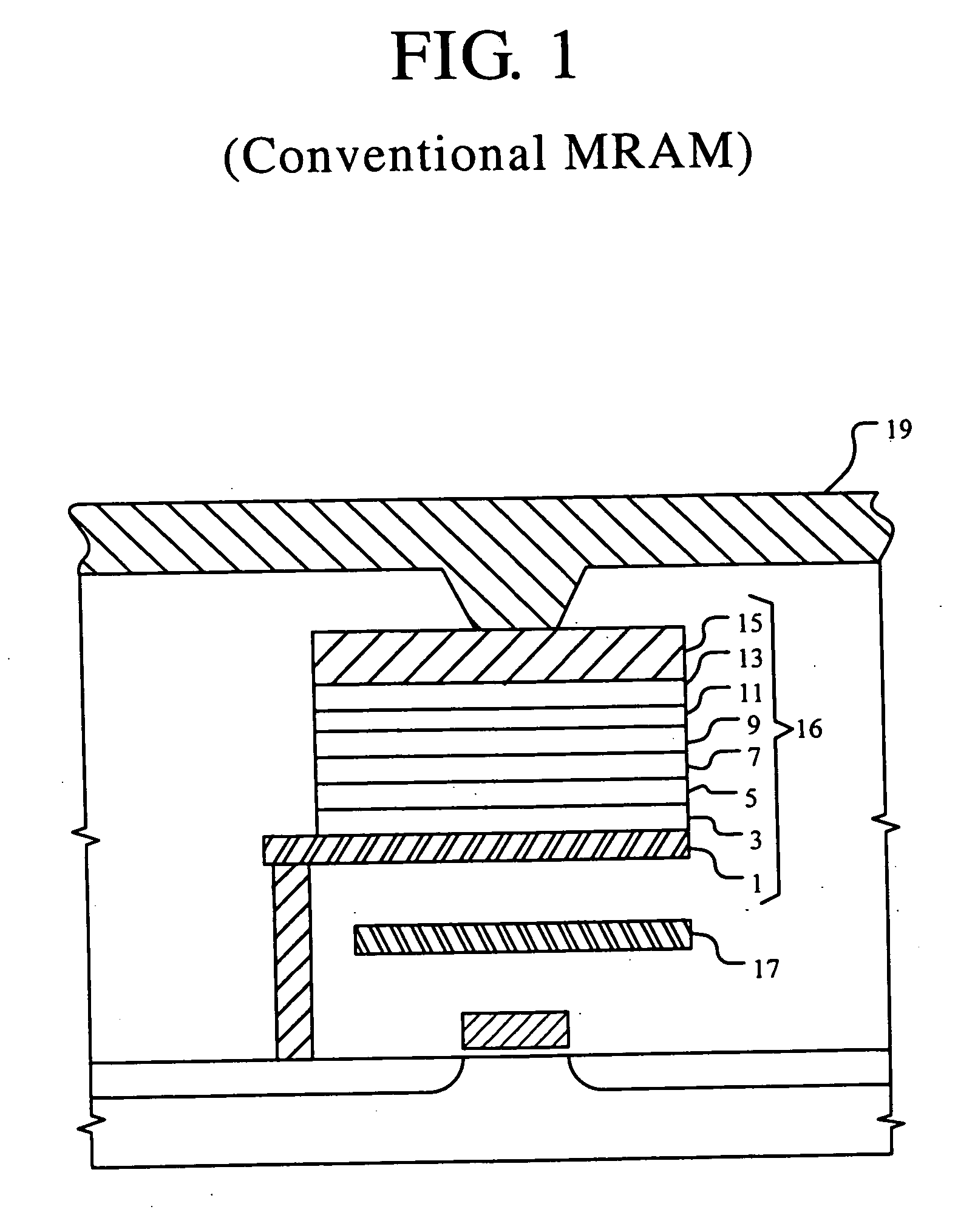
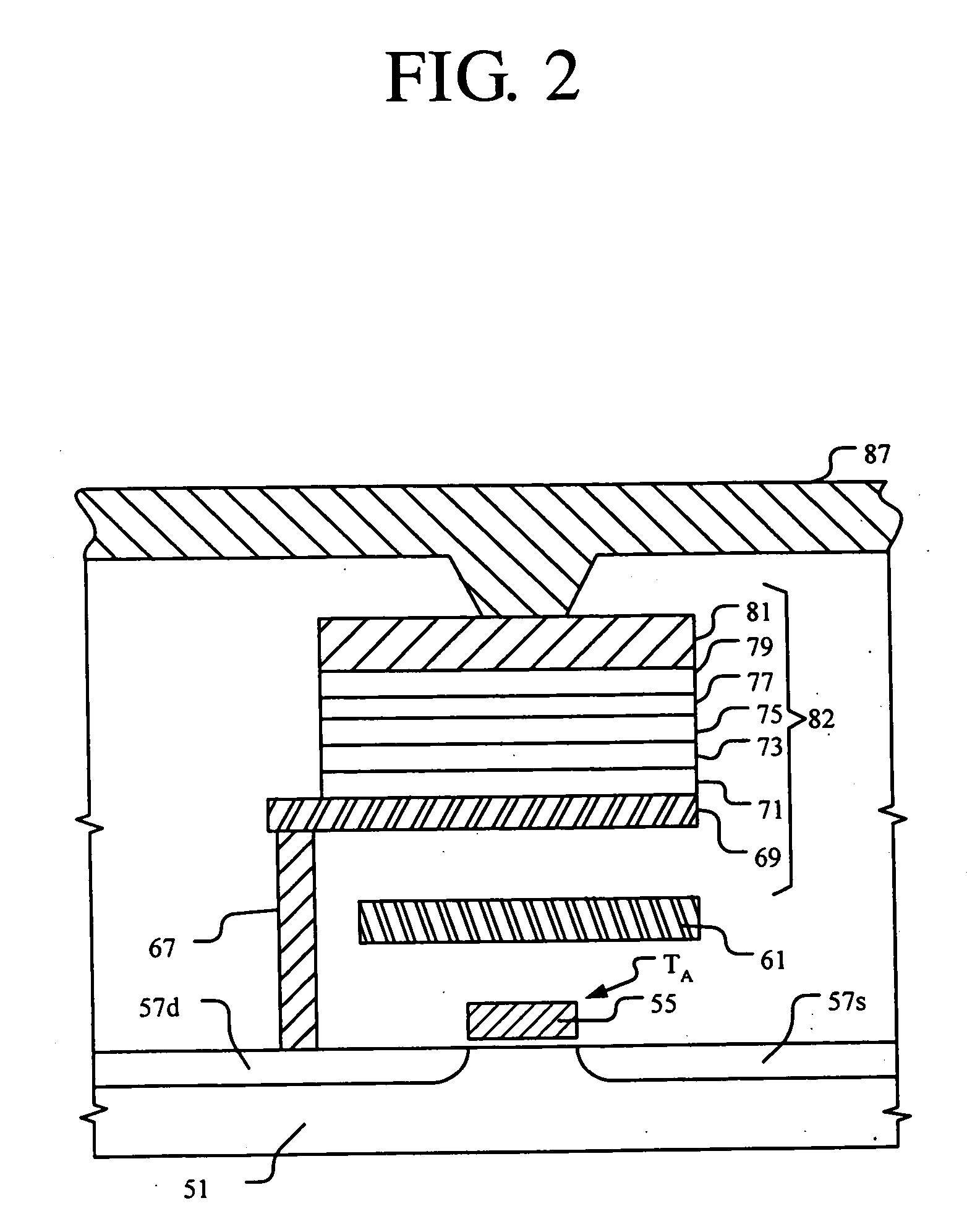
Magnetic tunnel junction structures and methods of fabrication
InactiveUS20050207219A1Reduce surface roughnessNanoinformaticsMagnetic-field-controlled resistorsIridiumHysteresis
A method for forming an MTJ structure suitable for use in a MRAM device having a bottom electrode including a layer of platinum, ruthenium, iridium, rhodium, osmium, palladium or their oxides and having reduced surface roughness to improve the hysteresis loop characteristics of the resulting MTJ structure. The bottom electrode layer may also combine the functions of both the seeding layer and bottom electrode of the conventional two-layer structure, thereby simplifying the manufacturing process.
Owner:SAMSUNG ELECTRONICS CO LTD
MRI compatible, radiopaque alloys for use in medical devices
A metallic alloy whose primary constituent elements are a relatively higher volume fraction of titanium and a relatively lower volume fraction of an added element or elements generally selected from the transition elements (excluding mercury, cadmium, osmium and copper). Medical devices formed from the metallic alloy are visible under MRI imaging and are sufficiently radiopaque for viewing by x-ray fluoroscopy. The alloy may be embodied in a multitude of medical devices.
Owner:ABBOTT CARDIOVASCULAR
Abrasive particles with metallurgically bonded metal coatings
InactiveUS20020069592A1Improve solubilityGood metallurgical bondPigmenting treatmentOther chemical processesBorideRhenium
An abrasive composite particle comprising a cubic abrasive core particle encapsulated within a deposit of hexagonal metallurgical bond forming material comprising at least about 50 volume percent rhenium, ruthenium, osmium or mixtures thereof. The metallurgical bonds serve to retain the core particle in a matrix / binder very strongly. Metallurgical bonds are formed by the encapsulating material taking into solution, at the interface with the cubic abrasive core particle, some element or compound from the abrasive particle such as, for example, carbon or cubic boron nitride, from the core particle. Chemical bonds are not formed between the abrasive particle and the deposit. Suitable abrasive core particles include diamond, cubic carbides, cubic borides, cubic nitrides, cubic oxides, and the like. Conventional fabrication procedures such as chemical vapor deposition are employed to form the metallurgical bond forming deposit on the core particle. The composite abrasive particles are useful in forming articles according to conventional powdered metal processing operations. The articles so formed are useful for their hardness as well as their abrasiveness.
Owner:POWDERMET
Organic EL element and method of manufacturing the same, organic EL display device using the element, organic EL material, and surface emission device and liquid crystal display device using the material
InactiveUS20050208206A1Simplify manufacturing stepsLow production costOrganic chemistryDischarge tube luminescnet screensRheniumIridium
In an organic EL element, an organic EL layer is interposed between anodes and cathodes formed on a substrate. Each of the cathodes is made of a first conductive film that comes into contact with the organic EL layer and a second conductive film that constitutes a laminated structure together with the first conductive film. The first conductive film contains any one of an alkaline metal and an alkaline earth metal. The second conductive film contains any one of at least one type metal selected from a group consisting of Ru (ruthenium), Rh (rhodium), Ir (iridium), Os (osmium) and Re (rhenium) and its oxide.
Owner:FUJITSU LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com