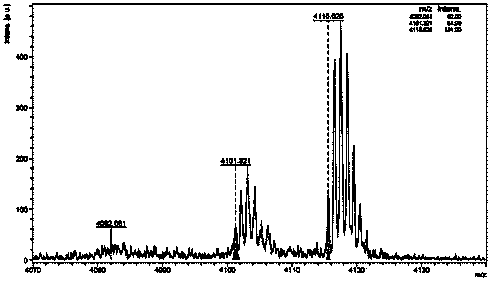
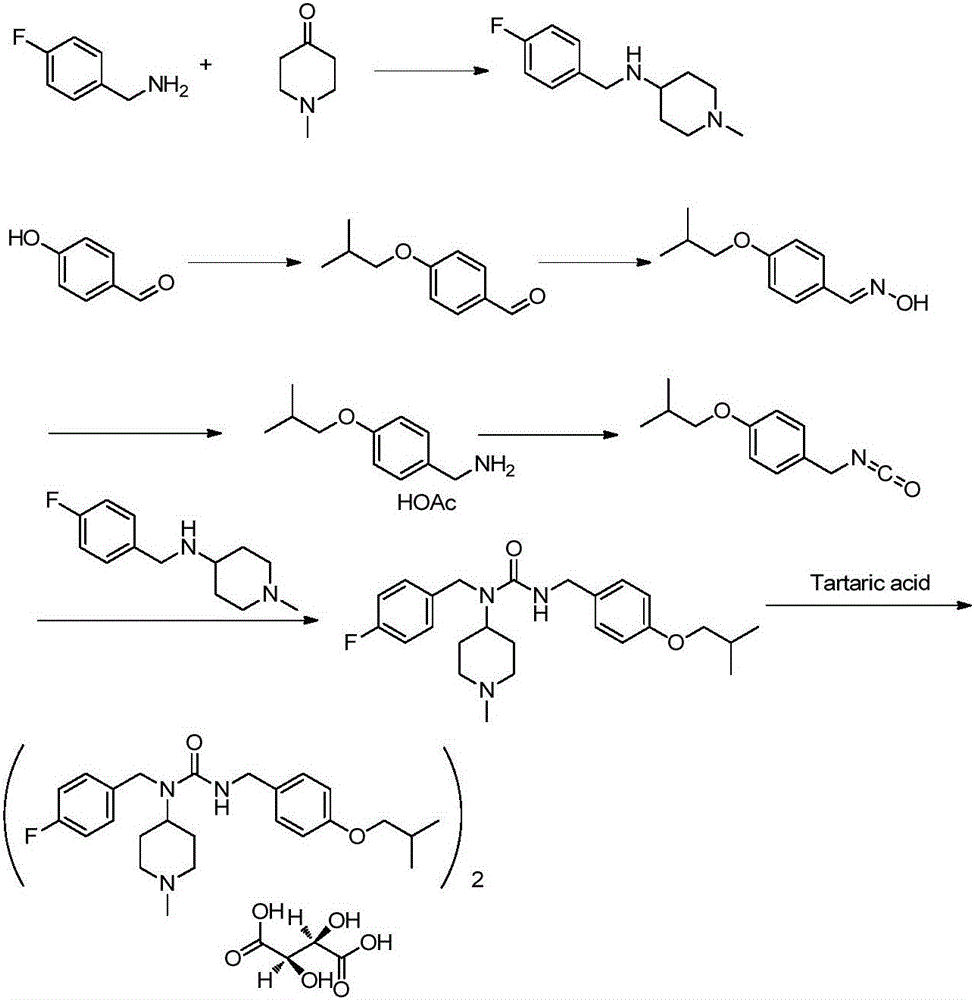
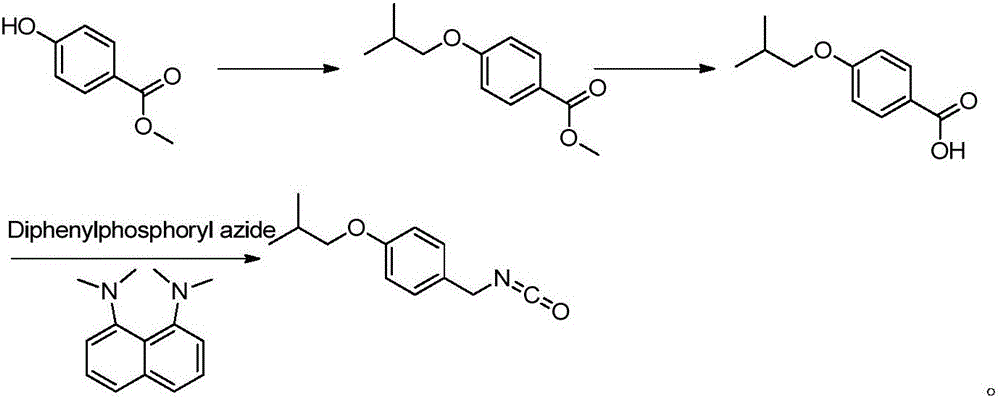
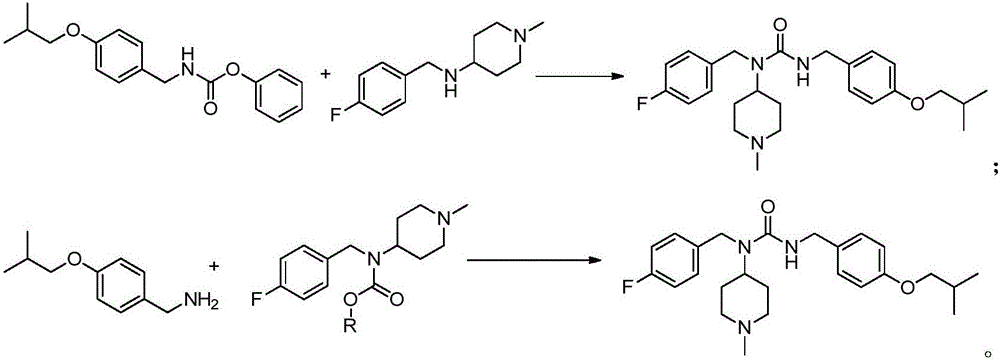
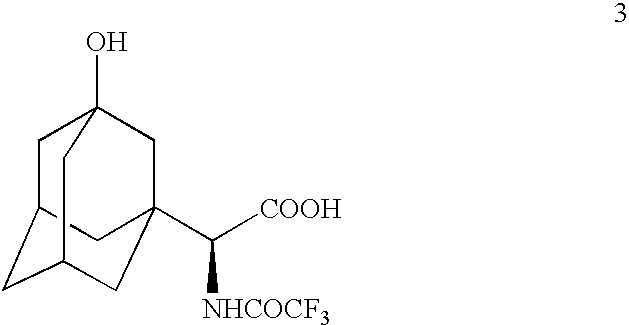
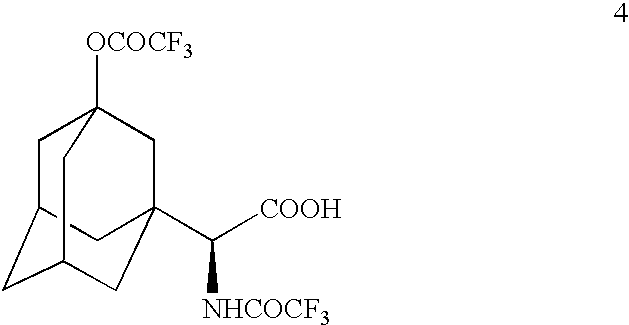
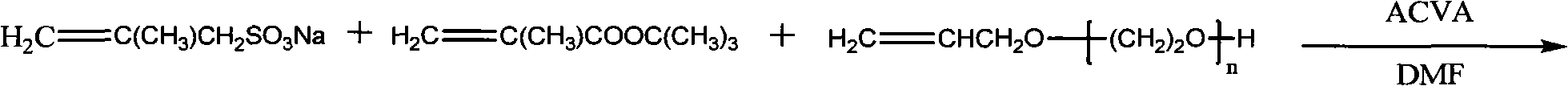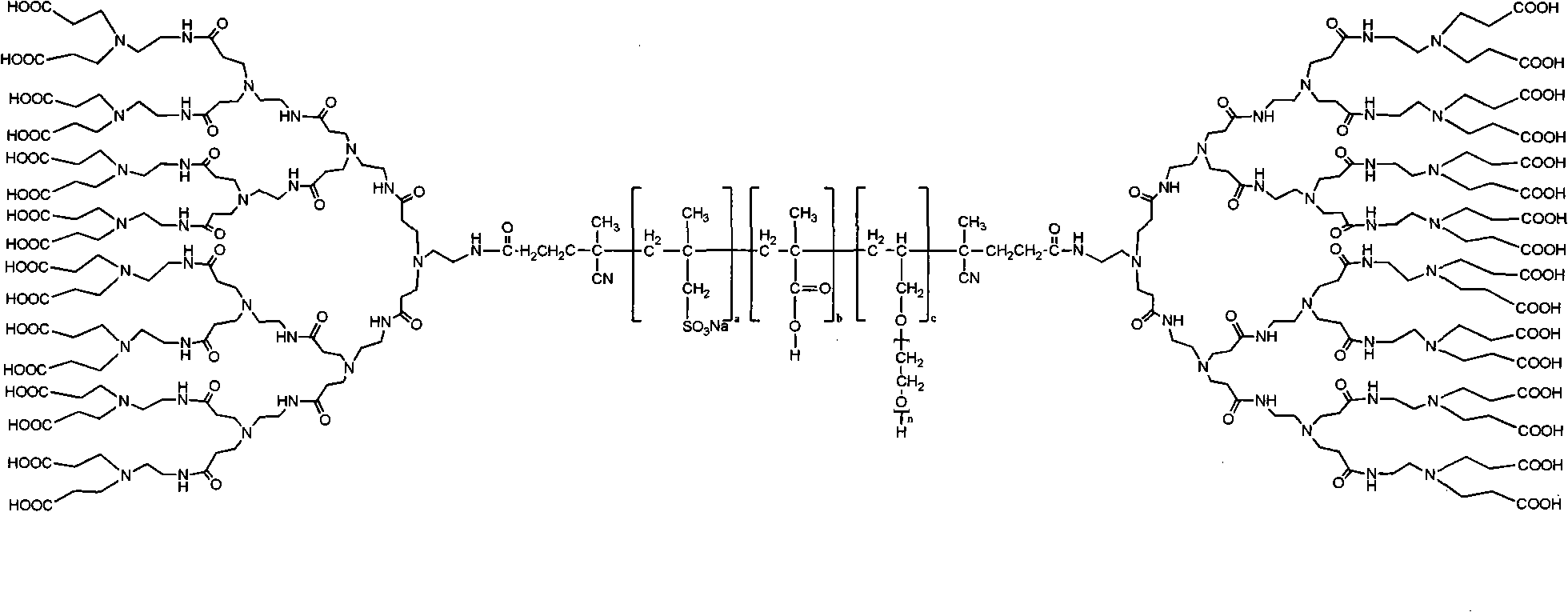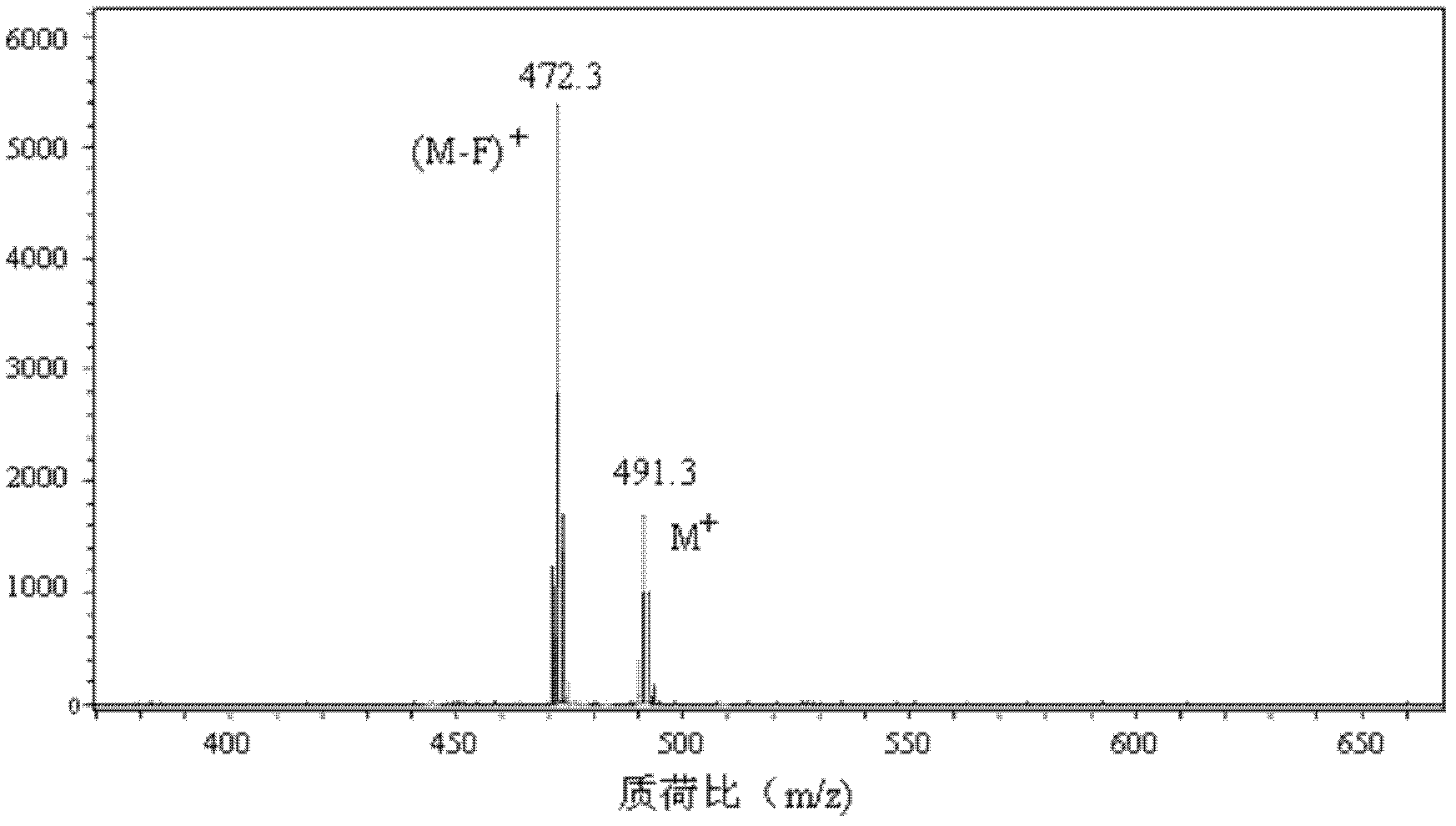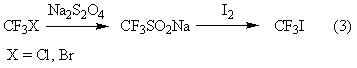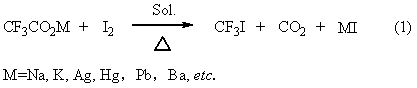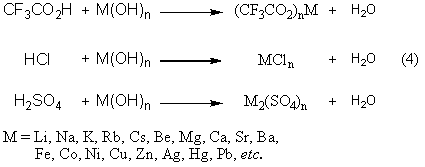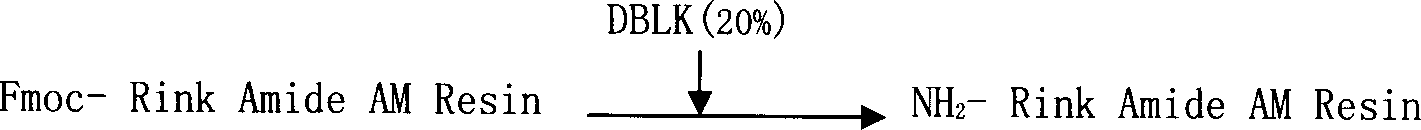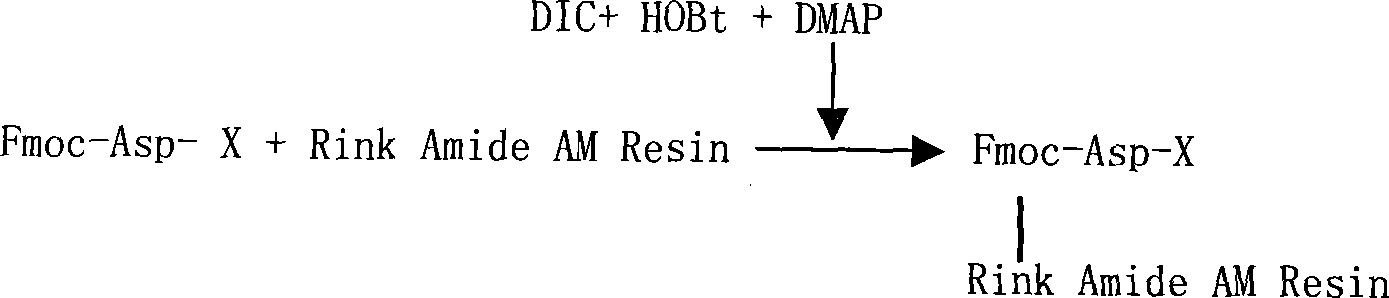Patents
Literature
1993 results about "Trifluoroacetic acid" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Trifluoroacetic acid (TFA) is an organofluorine compound with the chemical formula CF₃CO₂H. It is a structural analogue of acetic acid with all three of the acetyl group’s hydrogen atoms replaced by fluorine atoms and is a colorless liquid with a vinegar like odor. TFA is a stronger acid than acetic acid, having an acid ionisation constant that is approximately 34,000 times higher, as the highly electronegative fluorine atoms and consequent electron-withdrawing nature of the trifluoromethyl group weakens the oxygen-hydrogen bond (allowing for greater acidity) and stabilises the anionic conjugate base. TFA is widely used in organic chemistry for various purposes.
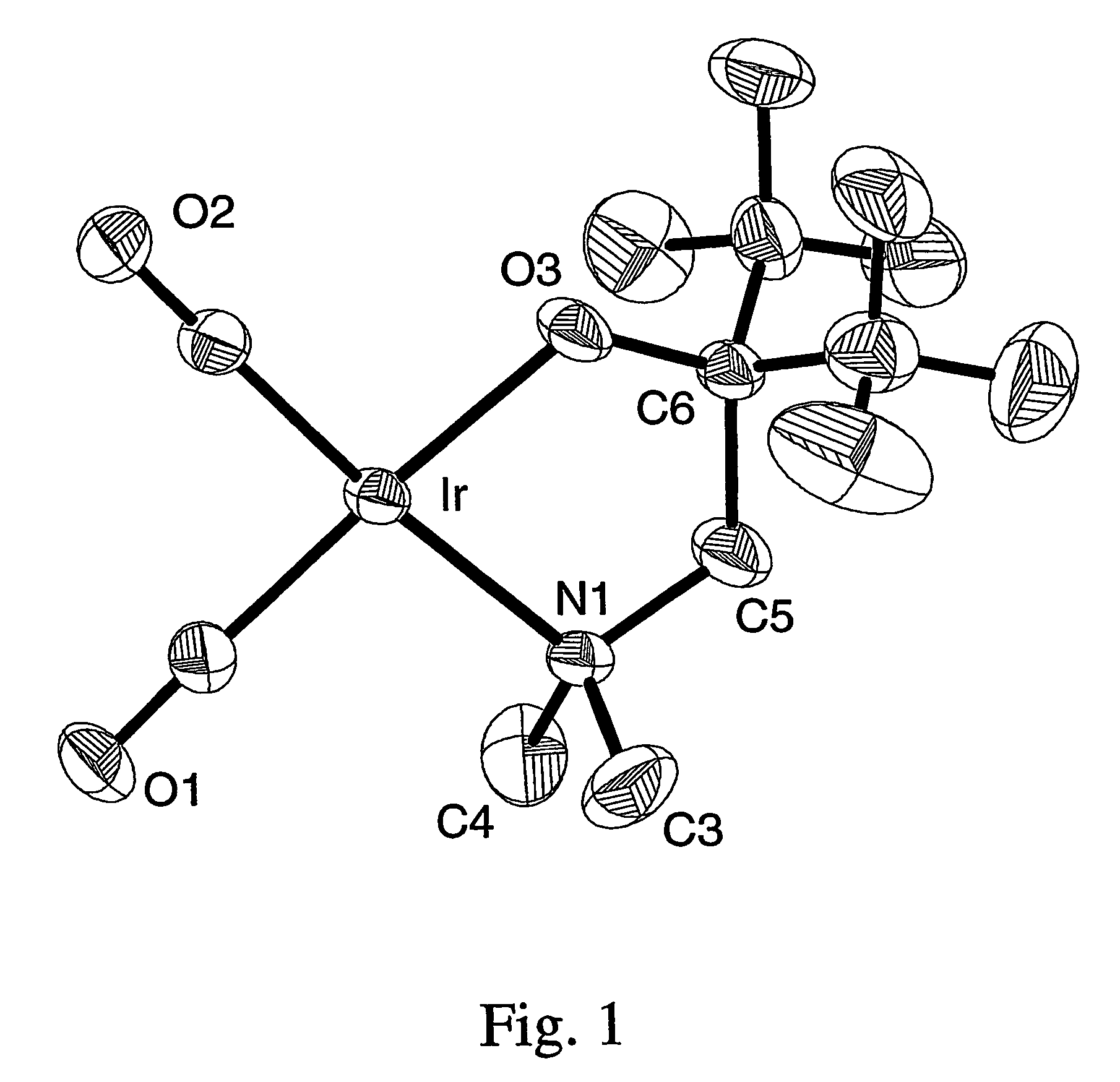
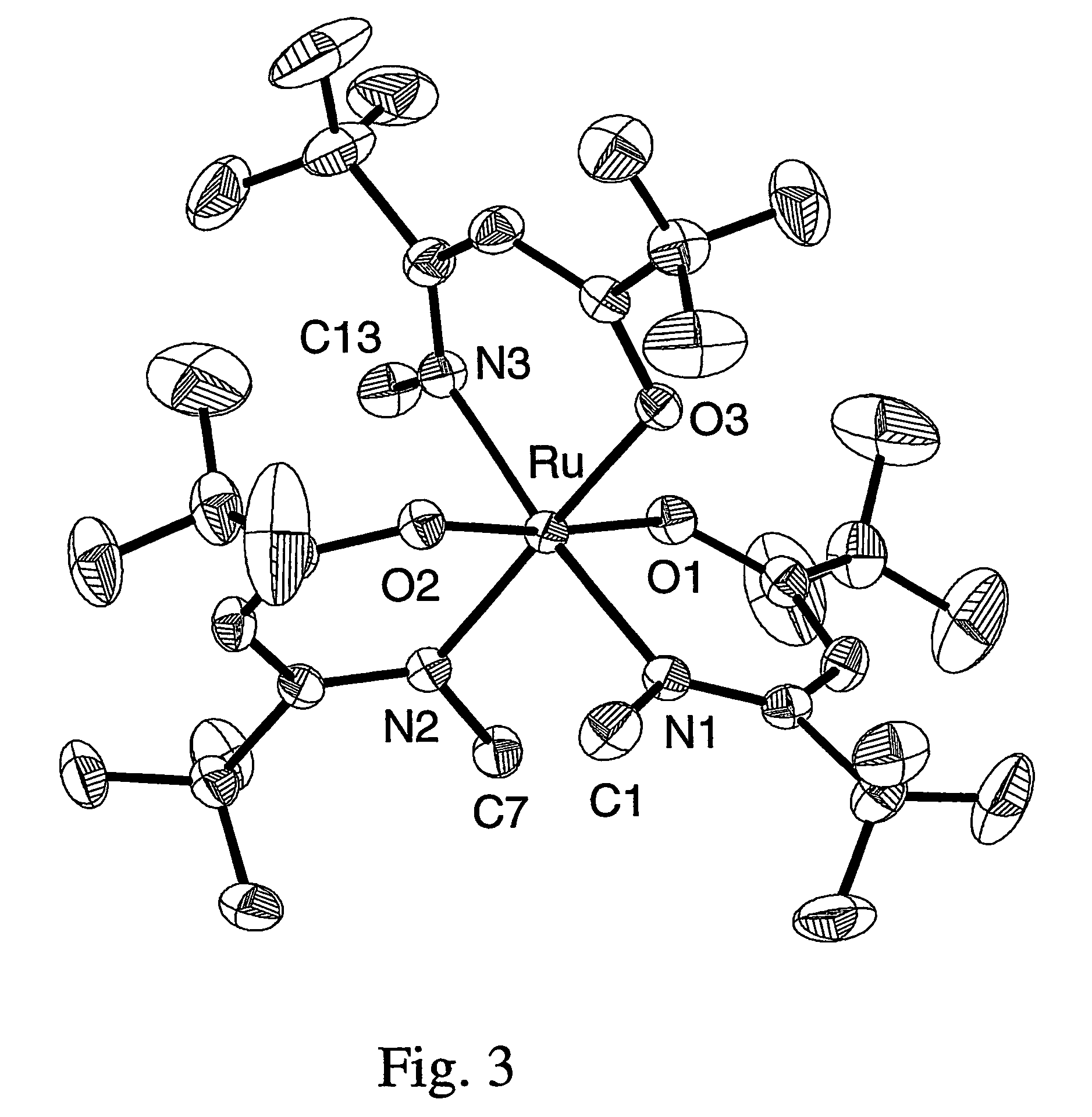
Volatile noble metal organometallic complexes
InactiveUS20050033075A1Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIridiumIodide
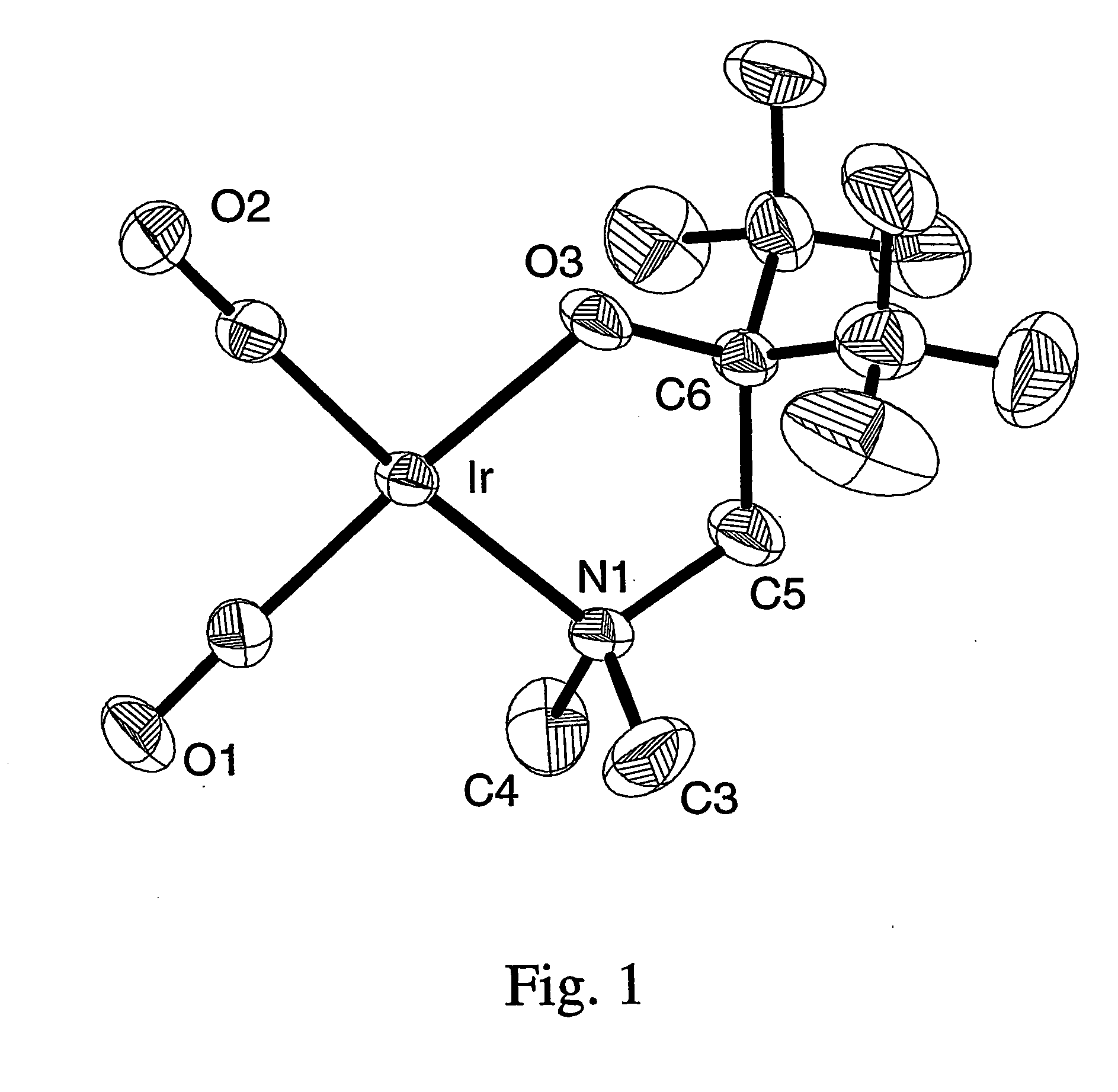
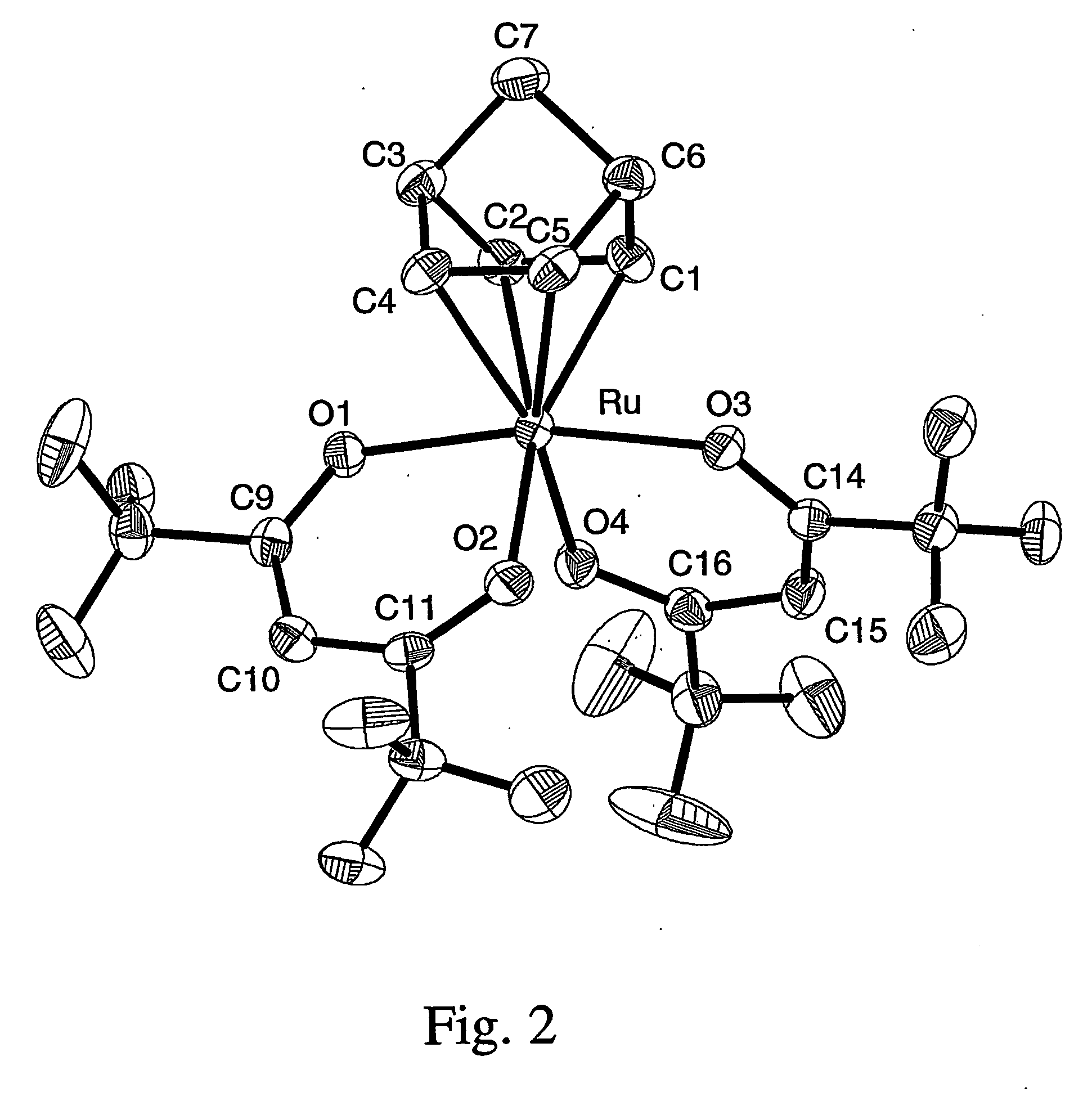
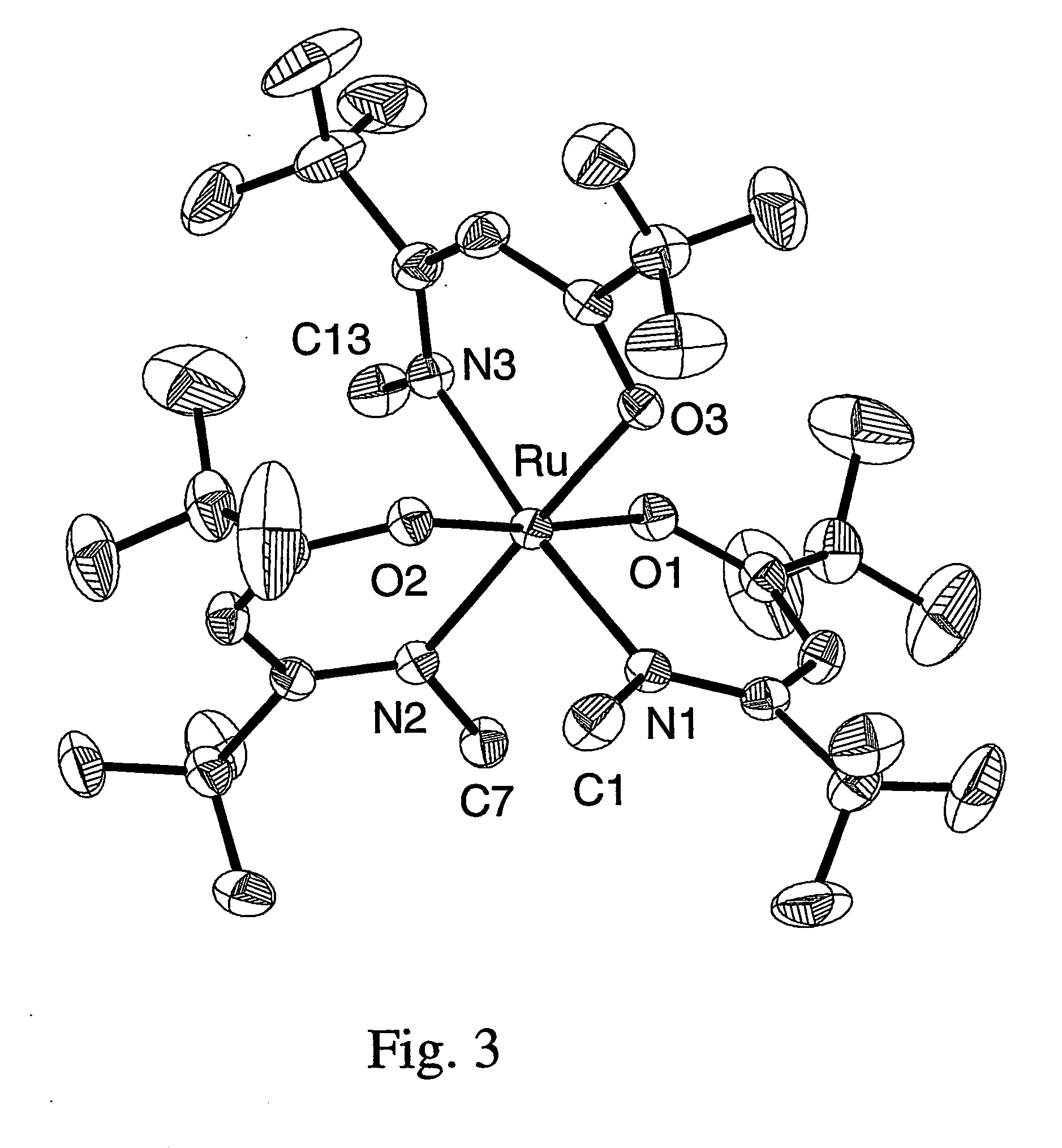
A series of noble metal organometallic complexes of the general formula (I): MLaXb(FBC)c, wherein M is a noble metal such as iridium, ruthenium or osmium, and L is a neutral ligand such as carbonyl, alkene or diene; X is an anionic ligand such as chloride, bromide, iodide and trifluoroacetate group; and FBC is a fluorinated bidentate chelate ligand such as beta diketonate, beta-ketoiminate, amino-alcoholate and amino-alcoholate ligand, wherein a is an integer of from zero (0) to three (3), b is an integer of from zero (0) to one (1) and c is an 10 integer of from one (1) to three (3). The resulting noble metal complexes possess enhanced volatility and thermal stability characteristics, and are suitable for chemical vapor deposition(CVD) applications. The corresponding noble metal complex is formed by treatment of the FBC ligand with a less volatile metal halide. Also disclosed are CVD methods for using the noble metal complexes as source reagents for deposition of noble metal-containing films such as Ir, Ru and Os, or even metal oxide film materials IrO2, OsO2 and RuO2.
Owner:NATIONAL TSING HUA UNIVERSITY +1
Prion-free collagen and collagen-derived products and implants for multiple biomedical applications; methods of making thereof
InactiveUS6197935B1Preserve integrityPeptide/protein ingredientsImmunoglobulinsIn vivo biocompatibilityCollagen VI
The use of collagen as a biomedical implant raises safety issues towards viruses and prions. The physicochemical changes and the in vitro and in vivo biocompatibility of collagen treated with heat, and by formic acid (FA), trifluoroacetic acid (TFA), tetrafluoroethanol (TFE) and hexafluoroiso-propanol (HFIP) were investigated. FA and TFA resulted in extensive depurination of nucleic acids while HFIP and TFE did so to a lesser degree. The molecules of FA, and most importantly of TFA, remained within collagen. Although these two acids induced modification in the secondary structure of collagen, resistance to collagenase was not affected and, in vitro, cell growth was not impaired. Severe dehydrothermal treatment, for example 110° C. for 1-3 days under high vacuum, also succeeded in removing completely nucleic acids. Since this treatment also leads to slight cross-linking, it could be advantageously used to eliminate prion and to stabilize gelatin products. Finally, prolonged treatment with TFA provides a transparent collagen, which transparency is further enhanced by adding glycosaminoglycans or proteoglycans, particularly hyaluronic acid. All the above treatments could offer a safe and biocompatible collagen-derived material for diverse biomedical uses, by providing a virus or prion-free product.
Owner:UNIV LAVAL
Process for Producing Self-Assembling Peptide Derivatives
ActiveUS20150175663A1Economical and efficientMass productionPeptide/protein ingredientsImmunoglobulinsTrifluoroacetic acidDisulfuric acid
An object of the present invention is to provide a process capable of producing a self-assembling peptide derivative that is useful in the fields of regenerative medicine and surgery in large quantities and in an economical and efficient manner. In particular, provided is a production process employing a combination of (i) a step of convergently constructing a sequence with use of a common repeating unit consisting of a specific amino acid sequence and (ii) a step of first isolating the peptide derivative as a disulfuric acid salt, a tetramethanesulfonic acid salt or a tetra(trifluoroacetic acid (TFA) salt), and then subjecting the peptide salt to a salt exchange reaction to yield a tetrahydrochloric acid salt.
Owner:MENICON CO LTD
Cosmetic compositions
ActiveUS20120288478A1Reducing activity of hyaluronidaseSkin stimulationBiocideCosmetic preparationsTrifluoroacetic acidHyaluronidase
Disclosed are compositions and methods for their use that can be used in cosmetic applications. The composition can include an effective amount of a Centella asiatica stem cells to reduce the activity of hyaluronidase in skin, an effective amount of tetradecyl aminobutyroylvalylamino butyric urea trifluoroacetate or Alpinia galanga leaf extract to promote the production of hyaluronic acid in skin, an effective amount of tripeptide-1 to promote the production of fibronectin and laminin in skin, and a dermatologically acceptable vehicle.
Owner:MARY KAY INC
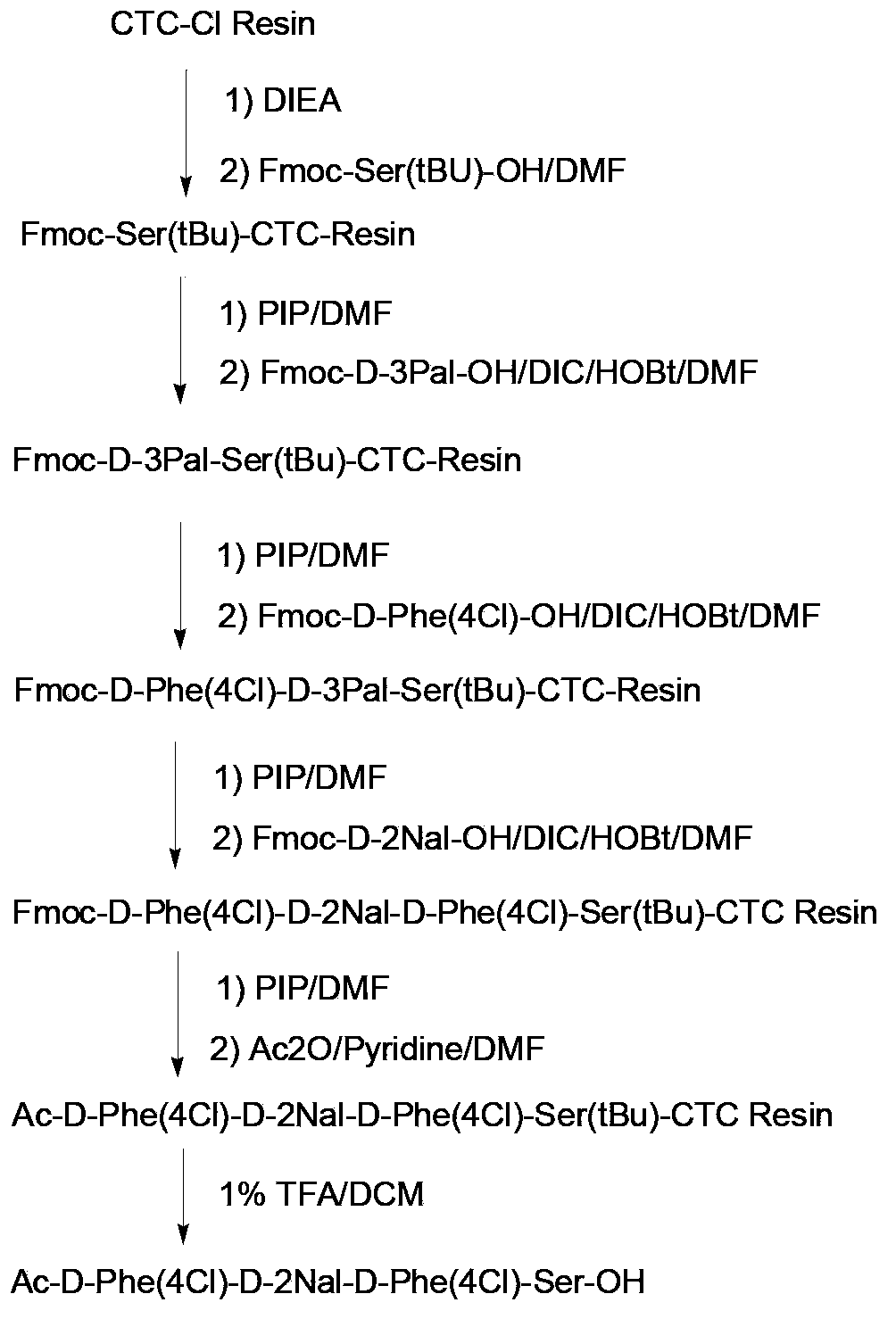
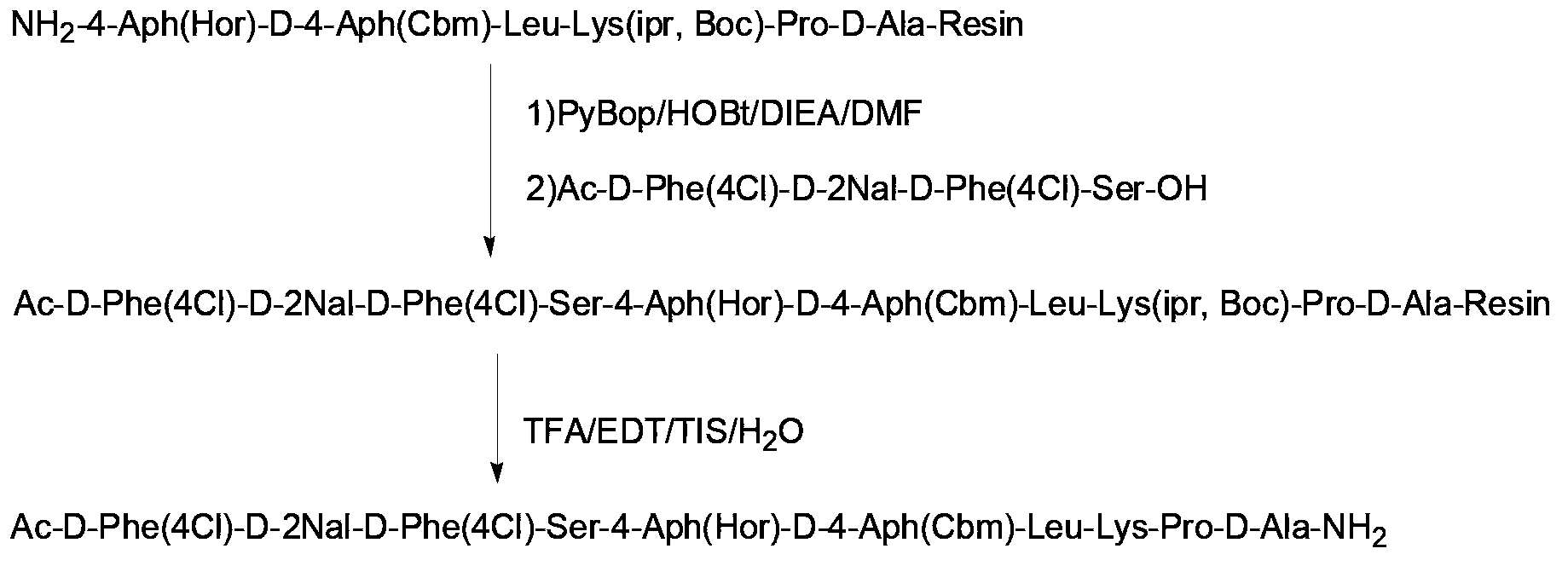
Solid-phase synthesis method for liraglutide
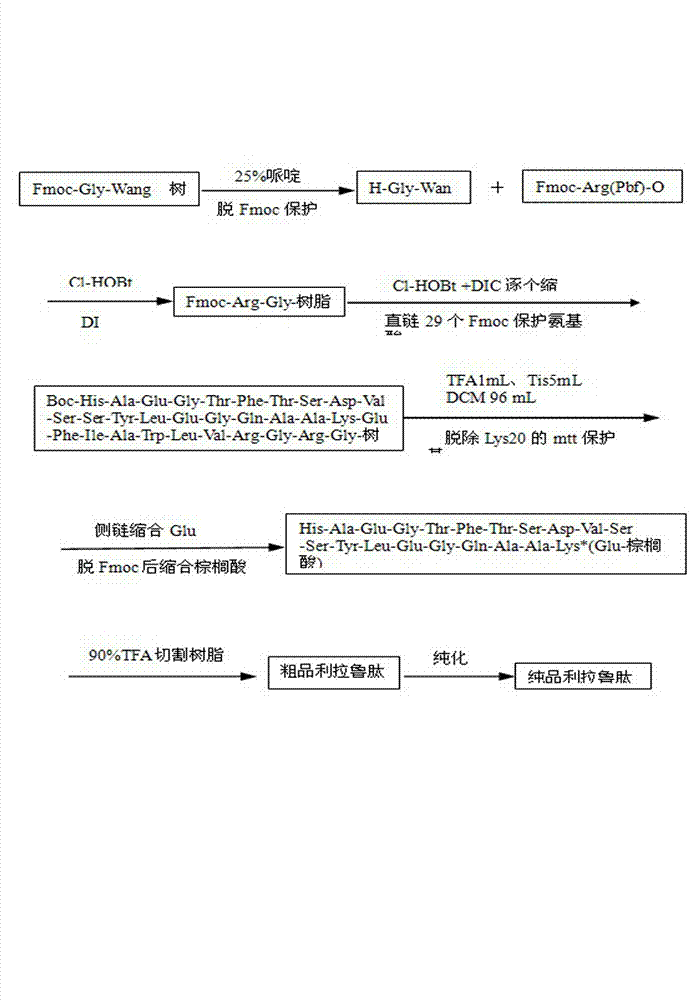
ActiveCN103864918AShort synthesis cycleImprove efficiencyPeptide preparation methodsBulk chemical productionSynthesis methodsSide chain
The invention discloses a solid-phase synthesis method for liraglutide. The solid-phase synthesis method for liraglutide comprises the following steps: 1) firstly synthesizing first to tenth amino acid segments 3, eleventh to nineteenth amino acid segments 2, and twentieth to thirty-first amino acid segments 1, wherein the twentieth lysine adopts Fmoc-Lys(Mtt)-OH, and the first histidine adopts Boc-His(Trt)-OH; 2) synthesizing pal-Glu(OH)-Otbu by adopting a liquid phase synthesis method; 3) selectively removing the Mtt protecting group on the twentieth lysine by use of 5% TFA (Trifluoroacetic Acid), connecting pal-Glu(OH)-Otbu with the side chain of the twentieth lysine to obtain a segment 4; 4) sequentially connecting the segments 2, the segments 3 and the segments 4 to obtain peptide resin completely protected by liraglutide; 5) cracking, purifying, and lyophilizing to obtain the liraglutide product.
Owner:哈尔滨吉象隆生物技术有限公司
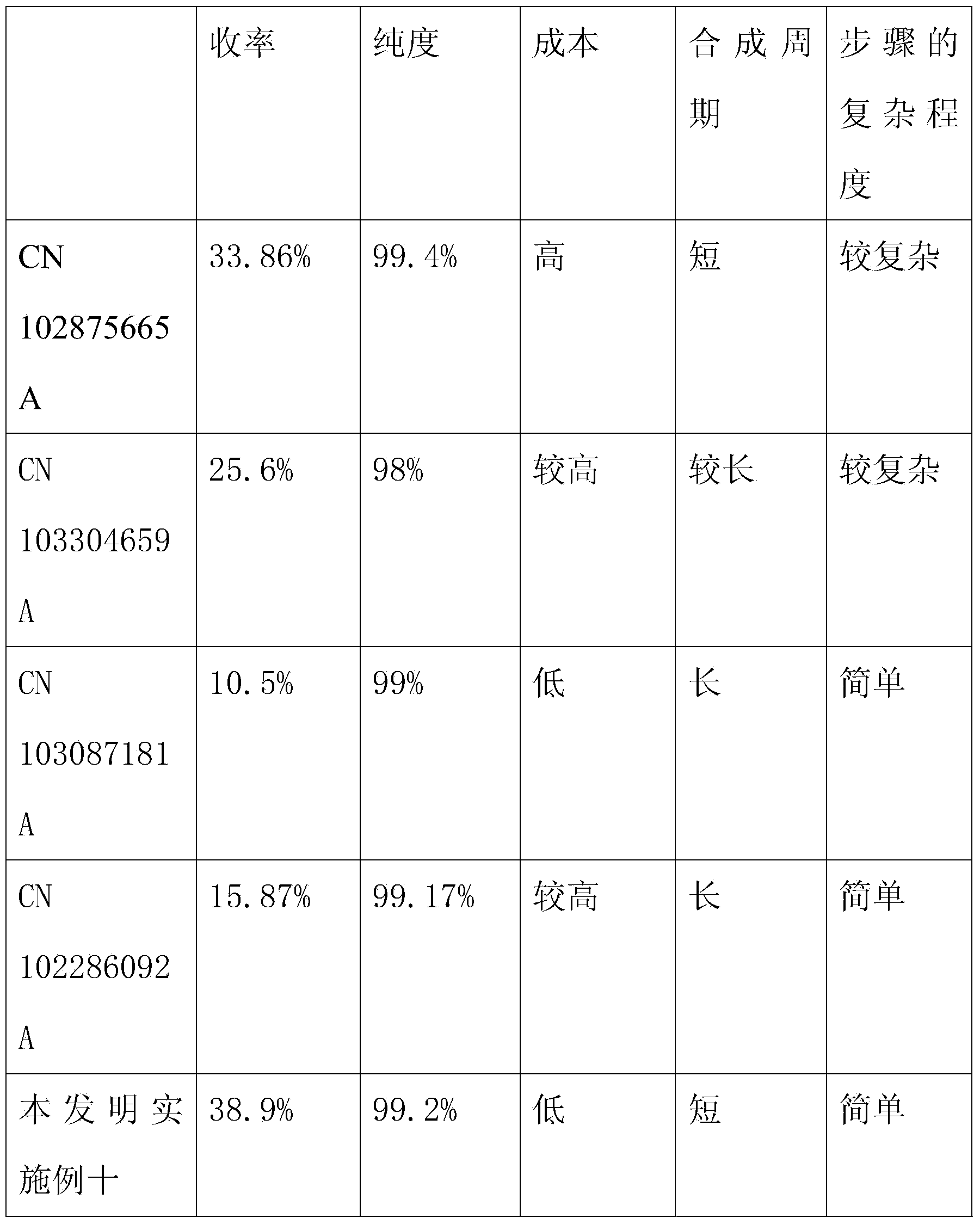
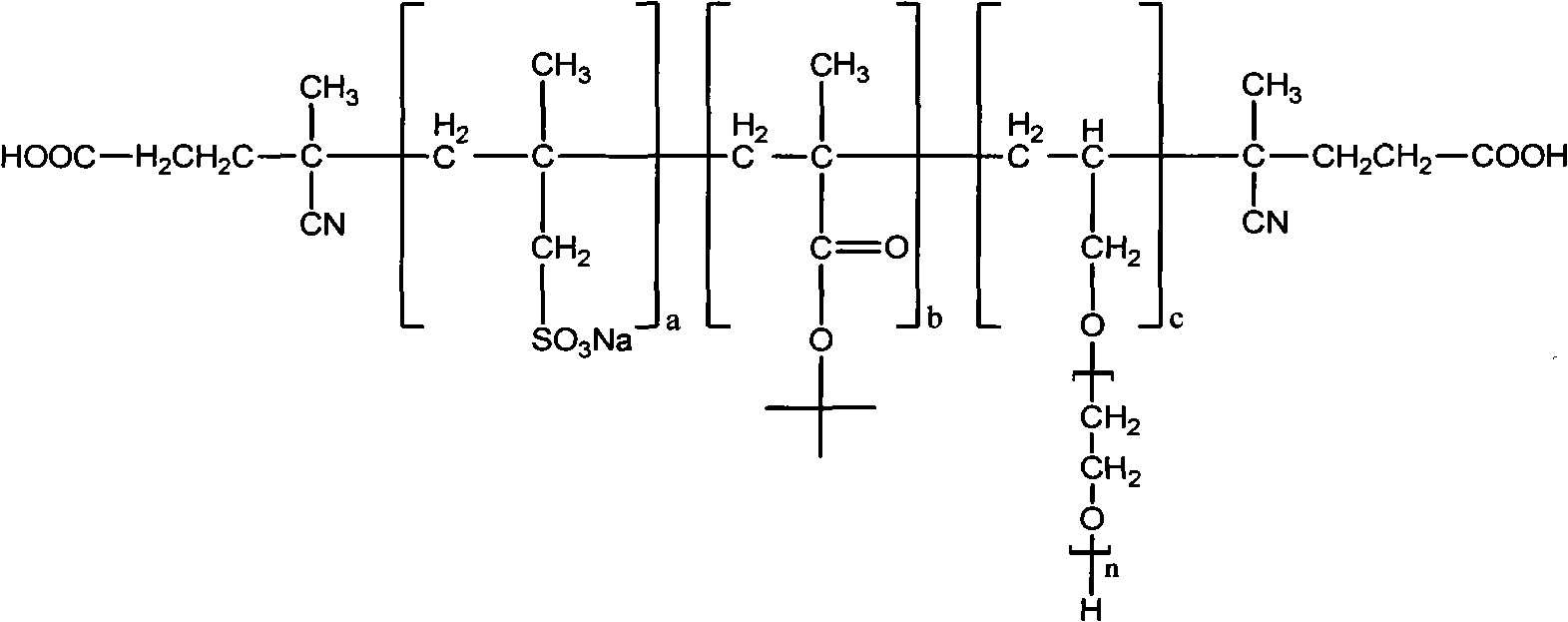
Hyper-branched polycarboxylate high-efficiency water reducing agent and preparation method thereof
The invention relates to a hyper-branched polycarboxylate high-efficiency water reducing agent and a preparation method thereof. The water reducing agent is prepared by polymerizing one of tert-butyl acrylate and methyl tert-butyl acrylate with sodium methyl-acryl sulfonate and allyl polyethenoxy ether to form a copolymer main chain, and then performing condensation polymerization on one of acrylic acid and methacrylic acid with ethylene diamine to form a hyper-branched polyamide structure which is grafted to two ends of the main chain. The preparation method comprises the following steps: (1) preparing the sodium methyl-acryl sulfonate into solution with DMF, and heating the solution in a nitrogen atmosphere; (2) dissolving the other two monomers and an initiating agent into the DMF to prepare mixed solution, and dripping the mixed solution into the step (1) to react for 1 to 20 hours; (3) adding a condensating agent CDI after the reaction and performing condensation reaction by using N-methyl morpholine as an organic base, the ethylene diamine and the acrylic acid as the monomers and the DMF as a solvent; and (4) performing vacuum distillation to remove the residual monomers and the solvent, and refluxing for 2 to 5 hours by using methylene chloride solution of trifluoroacetic acid to obtain the water reducing agent. The hyper-branched polycarboxylate water reducing agent has the advantages of low admixture, high water reducing rate, small slump loss, good compatibility with cement, no corrosivity to steel bars, strong frost resistance and the like.
Owner:厦门路桥翔通建材科技有限公司 +1
Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
ActiveCN102786569AReduce manufacturing costMild conditionsSugar derivativesSugar derivatives preparationEpoxyTert-Butyloxycarbonyl protecting group
The invention provides a tulathromycin intermediate, a preparation method of the tulathromycin intermediate, and a preparation method of the tulathromycin. The preparation method of the tulathromycin has the advantages of mild condition, convenience for operation, and low cost. The preparation method of the tulathromycin comprises the following steps of: using azithromycin A as a raw material; protecting 2'-hydroxy and 6'-amino in the azithromycin A through di-tert-butyl dicarbonate so as to obtain double-protective azithromycin A; carrying out Swern oxidation to 4''-hydroxy to the double-protective azithromycin A; salifying along with trifluoroacetic acid; and synchronously removing boc t-butyloxycarbonyl to obtain the azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl; and then reacting with trimethylsulfonium bromide to obtain 4''-epoxy compound; and finally carrying out nucleophilic addition on the 4''-epoxy compound by n-propylamine so as to obtain the phosphate of tulathromycin; and further neutralizing via alkaline to obtain the target compound tulathromycin; and synchronously obtaining the tulathromycin intermediate of azithromycin A bitrifluoroacetic acid salt of 4''-carbonyl.
Owner:TIANJIN ZHONGSHENG TIAOZHAN BIOTECH
Lanthanide-doped nayf4 nanocrystals, method of preparing and uses thereof
InactiveUS20090042314A1Improve commercial useCost-effectiveMaterial nanotechnologyInksAnalyteTrifluoroacetic acid
The present invention relates to a method of preparing lanthanide-doped NaYF4 nanocrystals, the method comprising: (A) providing a first solution comprising a non-coordinating solvent, a fatty acid coordinating ligand, sodium trifluoroacetate, yttrium trifluoroacetate, a first doping lanthanide trifluoroacetate and a second doping lanthanide trifluoroacetate, and a second solution comprising the non-coordinating solvent and the fatty acid coordinating ligand, the first and second solutions being substantially free of water and oxygen; (B) in an inert atmosphere, slowly adding the first solution heated at a temperature between about 100° C. and about 150° C. to the second solution heated at temperature between about 290° C. and about 330° C., thereby producing a reaction mixture containing the nanocrystals; and (C) recovering the nanocrystals from the reaction mixture. The invention also relates to lanthanide-doped uniformly shaped cubic NaYF4 nanocrystals having an average particle size of at most about 50 nm with a standard deviation of at most about 15%. Finally, the invention also relates to methods of (A) identifying or authenticating a product, (B) labelling an analyte, (C) detecting an analyte, and (D) producing a light source for the telecommunication industry using the above nanocrystals.
Owner:VALORBEC PARTNERSHIP
Process for preparing cefixime
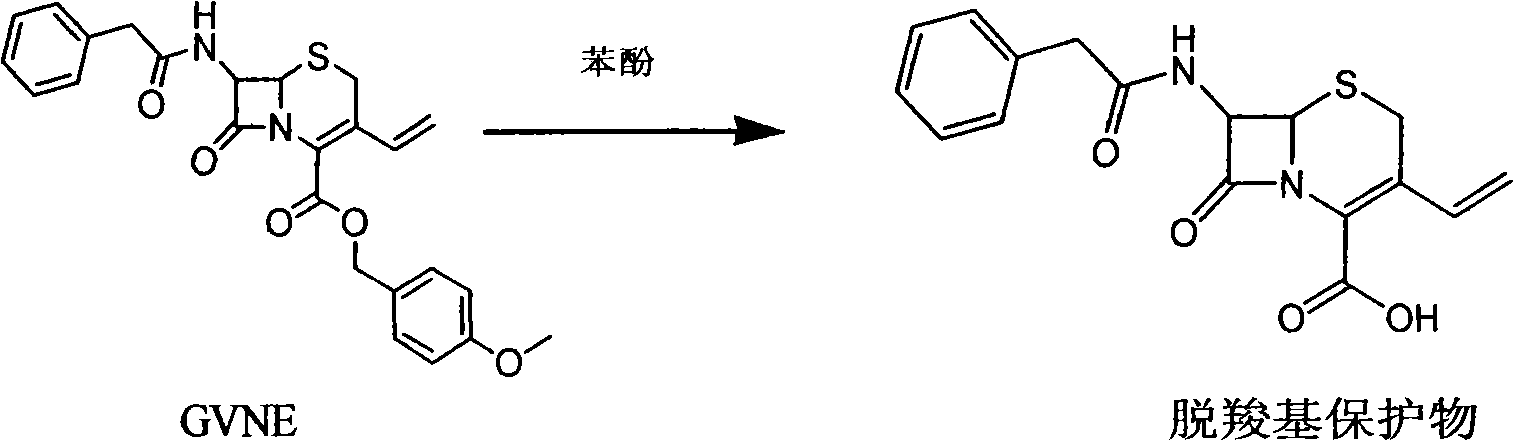
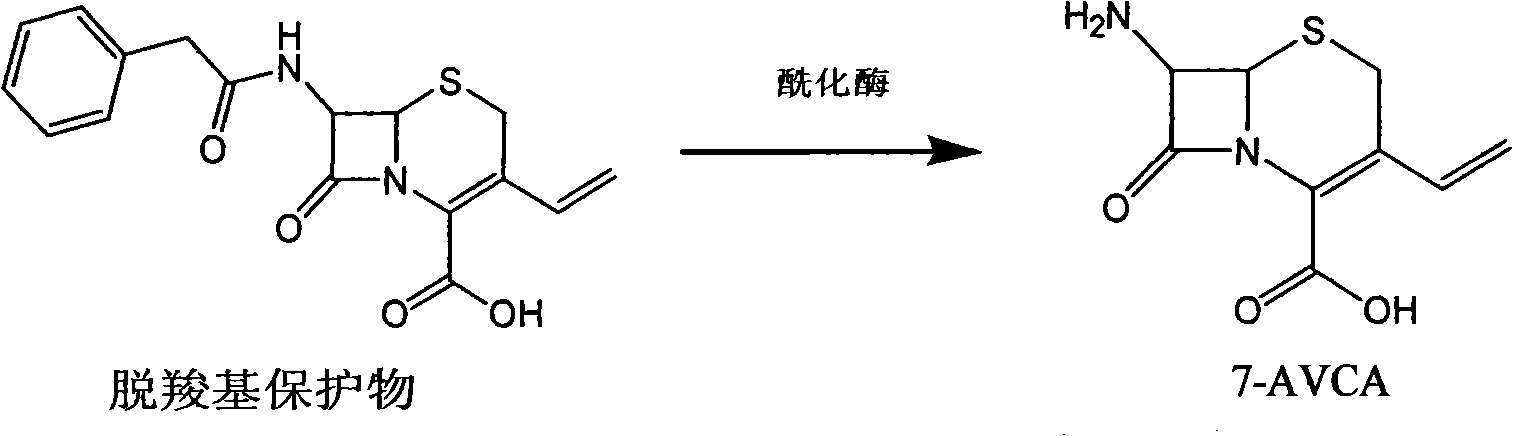
The invention provides a method for preparing cefixime, which belongs to the medicine synthesis technical field and comprises the steps of preparation of 7-AVCA, preparation of cefixime methyl ester, preparation of the cefixime and so on. The method aims at the prior amino de-protection to make an improvement, and uses penicillin acylase to substitute trifluoroacetic acid and so on in the prior process to ensure that an amino de-protection reaction is greatly improved, the use of organic solvents can be completely avoided, and acylase can also be reused at the same time. Methylene dichloride and tetrahydrofuran solvents in the prior process are substituted by adopting alcohol, ketone or ester solvents, so that an amidation reaction is greatly improved, which ensures that reaction solvents are easy to recover and reuse, can reduce production cost, reduce the discharge of waste liquid, reduce the pollution to the environment, can completely recover a byproduct, namely mercaptobenzothiazole produced by the reaction, improve the atom utilization rate of reaction raw-materials, and greatly improve the hydrolyzation and crystallization of subsequent products and the quality of a finished product.
Owner:ZHEJIANG ANGLIKANG PHARMA
Complete solid-phase synthesis method for liraglutide
InactiveCN103145828AImprove reaction efficiencyHigh purityPeptide preparation methodsBulk chemical productionFreeze-dryingSide chain
The invention discloses a complete solid-phase synthesis method for liraglutide. 2-Cl-TrtResin is enabled to serve as a solid-phase carrier. DIC / HOBt is enabled to serve as a condensation agent. After processing of microwave reaction technology, reaction time is shortened, and condensation efficiency is improved. According to side chain modification, novel ivDde side chain protected lysine is adopted and side chain modification synthesis is carried out. During the process, 20% piperidine is adopted to get rid of Fmoc protection until linear chain polypeptide synthesis is finished. Then, after hydrazine hydrate is adopted to get rid of ivDde protection, a side chain modification reaction is carried out. Obtained liraglutide with complete protection on the solid phase carrier is processed by trifluoroacetic acid, and crude liraglutide is obtained. After purification and freeze-drying by a C18 column, pure liraglutide is obtained. After strong negative ion salt conversion and free-drying, acetic acid liraglutide acetate is obtained. The complete solid-phase synthesis method for the liraglutide is simple in operation, short in synthesis cycle, low in production cost, few in accessory substance, high in product yield and beneficial for mass production.
Owner:宁波瑞达医药科技有限公司
Method for preparing teriparatide
InactiveCN104017064APromote resultsLow purityPeptide preparation methodsParathyroid hormonesDipeptideSide chain
The invention relates to a method for preparing teriparatide, which comprises the following steps: A) in the presence of an activator system, coupling a resin solid-phase carrier and Fmoc-Phe-OH to obtain a Fmoc-Phe-resin; B) sequentially coupling amino acids with N terminal Fmoc protection and side chain protection according to the teriparatide main chain peptide sequence by solid-phase synthesis, wherein the 16th-17th amino acids in the peptide sequence are coupled by using pseudoproline dipeptide Fmoc-Asn(Trt)-Ser(PsiMe,MePro)-OH; and C) carrying out peptide resin cracking by using a TFA (trifluoroacetic acid) cracking solution containing NH4I / Me2S, purifying the crude product by HPLC (high performance liquid chromatography), and freeze-drying to obtain the teriparatide. The content of the technical impurity Met8(O)-teriparatide is lower than 0.1%. The technique for preparing teriparatide has the advantages of high purity and low cost, is suitable for large-scale production, and can effective control the Met8(O)-teriparatide content without influencing the total yield of the teriparatide.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Novel synthesis method for pimavanserin
ActiveCN105820110AEasy to crystallize and purifyHigh yieldOrganic chemistrySynthesis methodsPhosphoric acid
The invention provides a novel synthesis method for pimavanserin. The method comprises the steps that a compound formula (1a) is adopted as a raw material for preparing an active urea compound formula (1) and then subjected to a reaction with a compound formula (2) under an alkaline system to obtain a pimavanserin free alkali formula (3), and then pimavanserin free alkali forms a salt with tartaric acid to obtain a pimavanserin half tartrate formula (4). The method is simple in technological path, low in cost and suitable for industrial production. The formula is shown in the description, wherein HmA is the general formula of m-basic acid, HnA is the general formula of n-basic acid, m and n are 1 or 2 or 3, and the m-basic acid and the n-basic acid are selected from sulfuric acid, hydrochloric acid, phosphoric acid, p-toluenesulfonic acid, benzenesulfonic acid, methanesulfonic acid, methanoic acid, acetic acid, trifluoroacetic acid, oxalic acid, citric acid or tartaric acid.
Owner:HANGZHOU CHEMINSPIRE TECH CO LTD
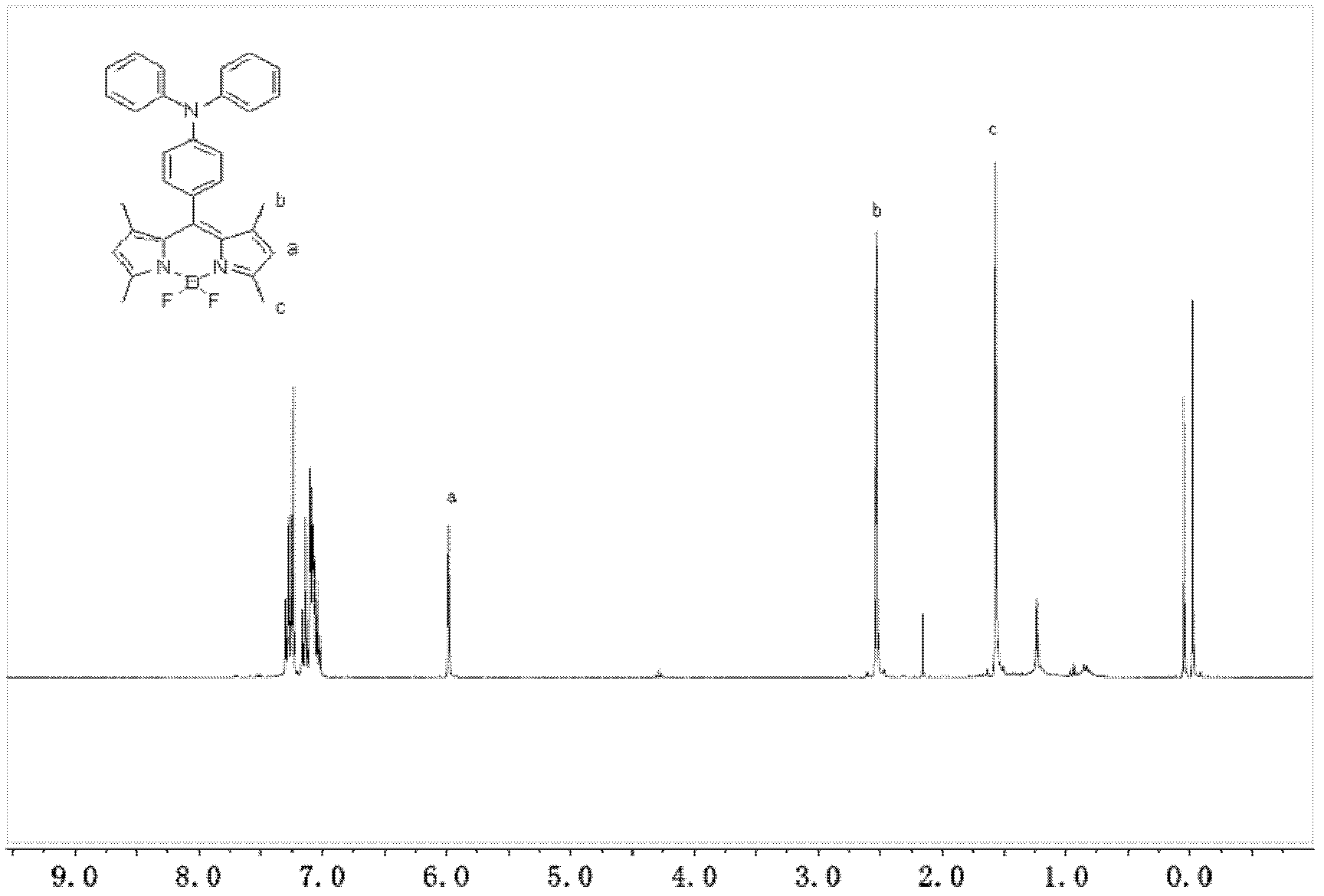
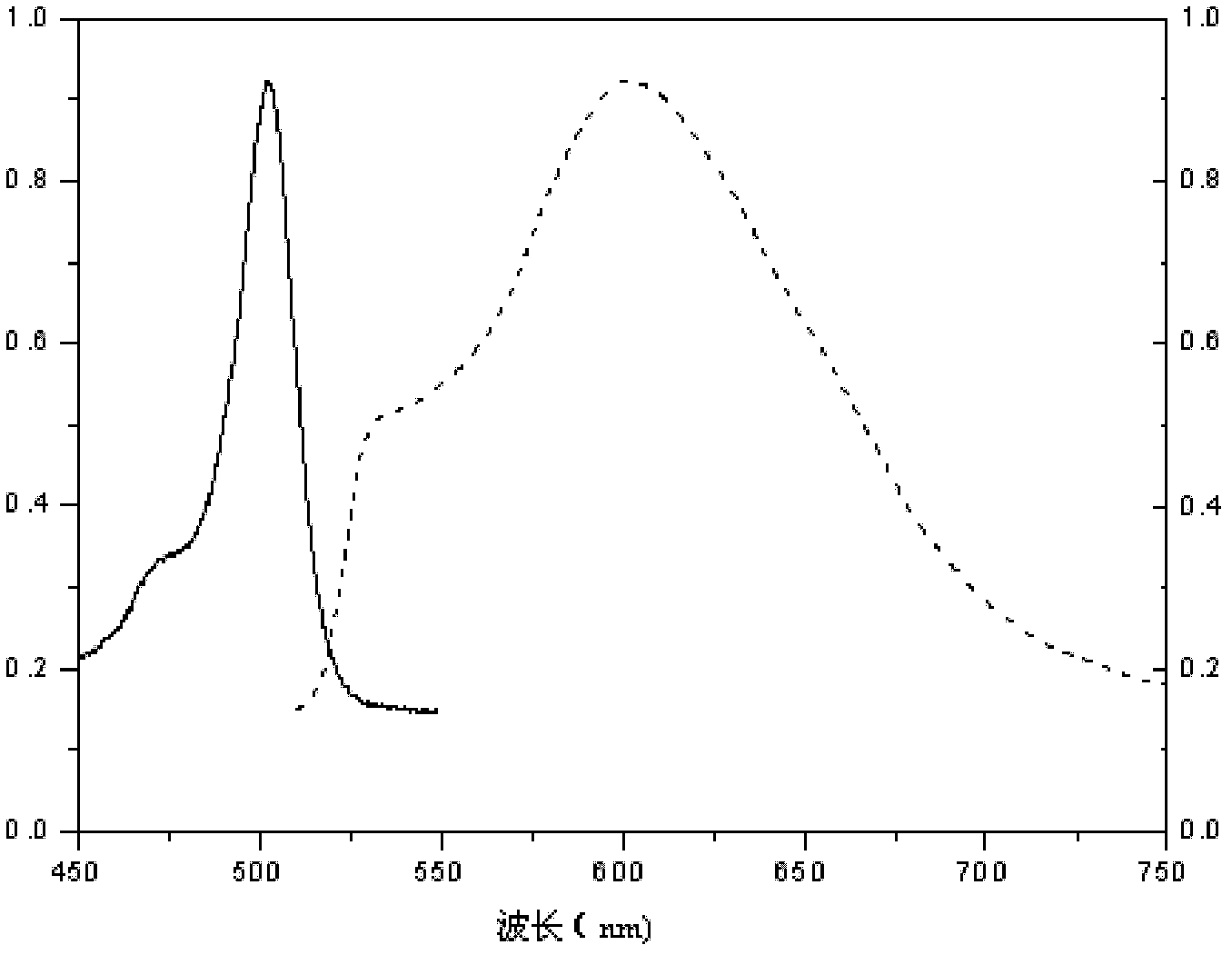
1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and preparation method thereof
InactiveCN102321109AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsMethylene DichlorideOrganic synthesis
The invention relates to a 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound and a preparation method thereof, which belong to the technical field of organic synthesis. The existing photocatalysts have few varieties and low conversion rate, and the existing bodipy dye has low infrared fluorescent efficiency and small Stokes displacement. The invention provides the 1,3,5,7-tetramethyl-8-triphenylamine base pyrrole methane-boron difluoride complex compound. The preparation method comprises the steps that: 4-formoxyl tetramethyl and 2,4-dimethyl pyrrole are dissolved in organic solvents, trifluoroacetic acid or monoprop is used as catalysts, and a reaction system is formed; 2,3-dichloro-5,6-dicyan-1,4-para-benzoquinone with the same mol ratio as the 4-formoxyl tetramethyl is taken, is dissolved in the methylene dichloride and is added into the reaction system; and one of triethylamine, triisopropyl amine and N,N-diisopropylethylamine is added into the reaction system, boron trifluoride etherate is added under the ice bath, and final products are generated.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
Liquid Chemical for Forming Protecting Film
ActiveUS20120017934A1Improve waterproof performanceReduce capillary forceNon-ionic surface-active compoundsOther chemical processesTrimethylsilyl trifluoromethanesulfonateCompound a
Disclosed is a liquid chemical for forming a water-repellent protecting film at least on a surface of a recessed portion of an uneven pattern at the time of cleaning a wafer having a finely uneven pattern at its surface and containing silicon at least a part of the uneven pattern. This liquid chemical contains a silicon compound A represented by the general formula: R1aSi(H)bX4-a-b and an acid A, the acid A being at least one selected from the group consisting of trimethylsilyl trifluoroactate, trimethylsilyl trifluoromethanesulfonate, dimethylsilyl trifluoroactate, dimethylsilyl trifluoromethanesulfonate, butyldimethylsilyl trifluoroactate, butyldimethylsilyl trifluoromethanesulfonate, hexyldimethylsilyl trifluoroacetate, hexyldimethylsilyl trifluoromethanesulfonate, octyldimethylsilyl trifluoroactate, octyldimethylsilyl trifluoromethanesulfonate, decyldimethylsilyl trifluoroacetate and decyldimethylsilyl trifluoromethanesulfonate.
Owner:CENT GLASS CO LTD
Solid-phase synthesis method of liraglutide
InactiveCN103087181ALess usableSmall quantityPeptide preparation methodsBulk chemical productionSide chainWang resin
The invention discloses a method for synthesizing liraglutide. The method comprises the following steps of: 1, selecting Fmoc-Gly-Wang resin and N terminal Fmoc protected and side chain protected amino acid as raw materials, wherein lysine on a 26th site adopts Fmoc-Lys(Mtt)-OH or Fmoc-Lys(Mmt)-OH, histidine on an N terminal adopts Boc-His(Trt)-OH or Boc-His(Boc)-OH; 2, selectively removing a Mtt or Mmt protecting group on the lysine by adopting 1 percent TFA (Trifluoroacetic Acid); 3, sequentially coupling one g-glutamic acid and palmitic acid on a side chain; and 4, cutting resin by the TFA to obtain a crude product of the liraglutide, and carrying out preparative liquid chromatography purification and lyophilization to obtain a pure product of the liraglutide. The method for synthesizing the liraglutide is simple in steps, saves time and labor, is less in difficult sequences in a coupling process, simple and easy to operate during side chain de-protection, less in byproducts and high in yield, and is suitable for industrialized production.
Owner:刘卫 +1
Method for synthesizing Arbekacin and intermediate dibekacin thereof
InactiveCN101575354AHigh yieldReduce generationSugar derivativesSugar derivatives preparationFluoroacetic acidSynthesis methods
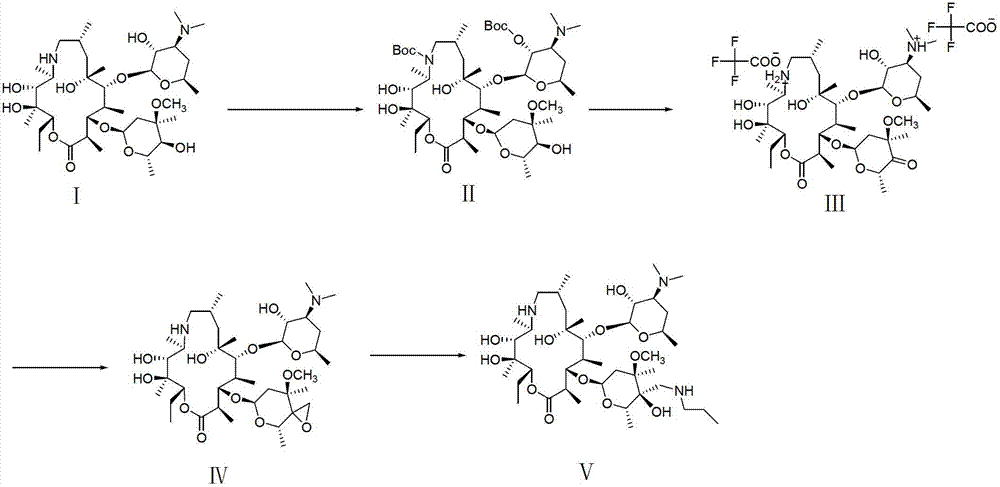
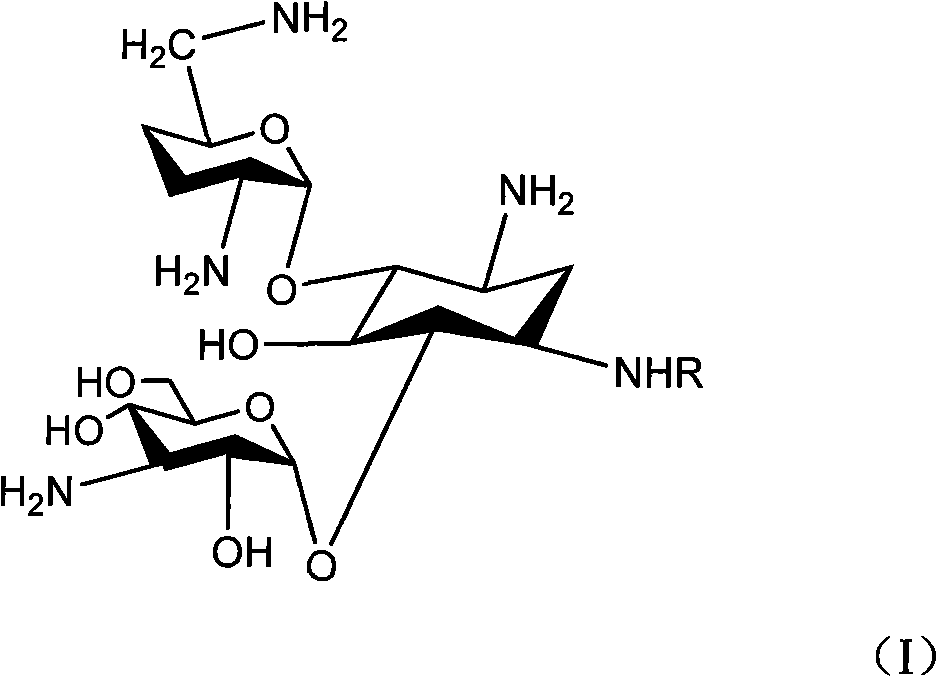
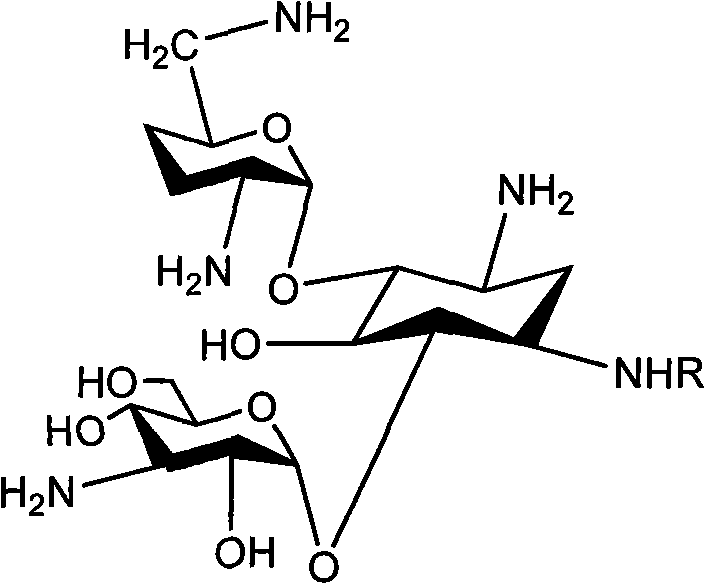
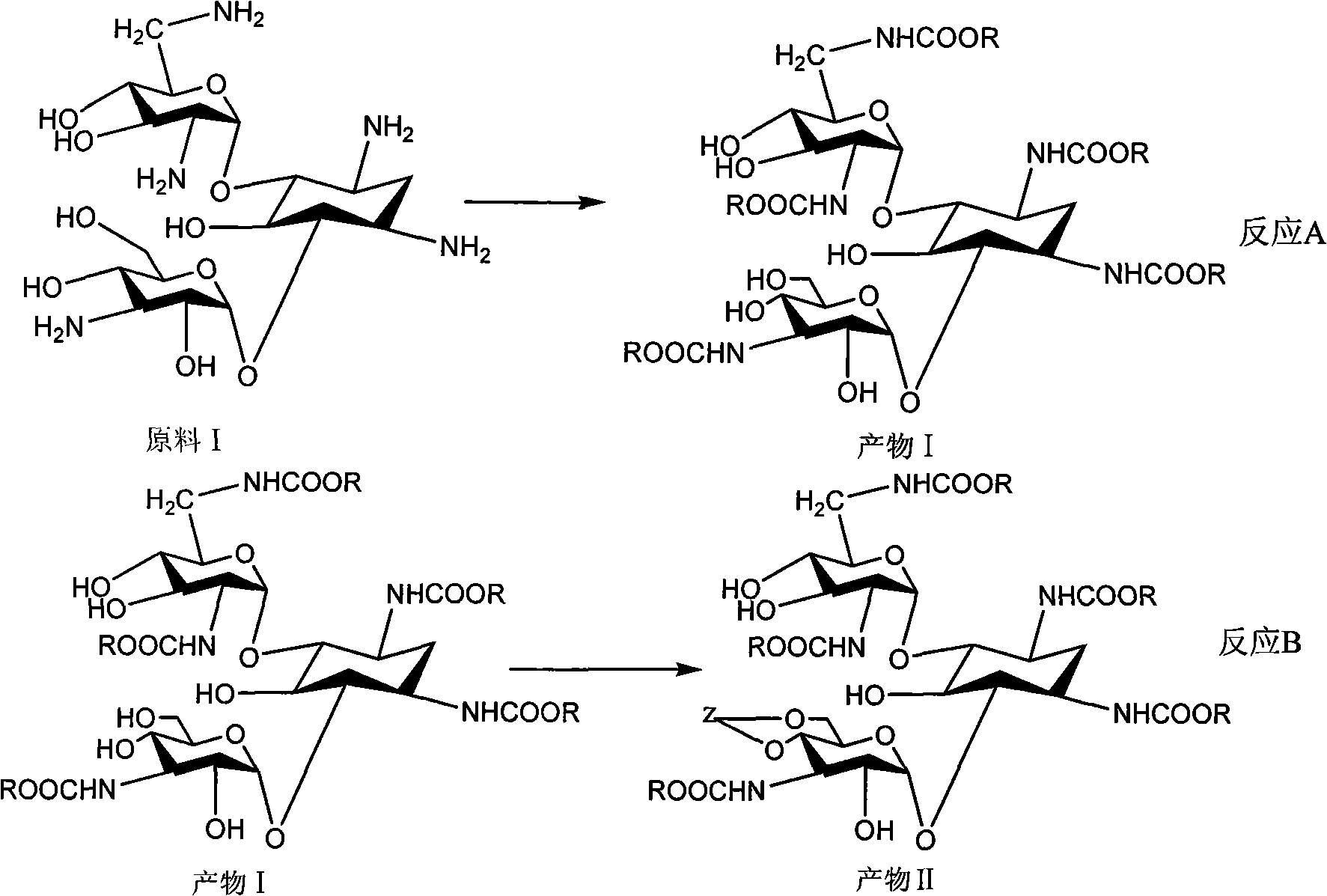
The invention relates to an organic synthesis method, in particular to a method for synthesizing an Arbekacin and an intermediate dibekacin thereof. The method comprises the following steps of: takinga kanamycin B as initial raw material, carrying out the following processes of aldol condensation, sulfonylation, sodium iodide replacement and elimination to form double bond, de-protection under acidic condition, amino-electron reduction and final hydrogenation, thus obtaining the dibekacin; taking 3',4'-dideoxy -3',4'-didehydro-kanamycin B as raw material, using a di-tert-butyl dicarbonate toselectively protect the amidogen of 3, 2', 6', 3'' sites; subsequently using the synthesized active ester to protect the 1-site amidogen; subsequently using tri-fluoroacetic acid to remove BOC; and carrying out hydrazinolysis and catalysis and hydrogenation of platinum oxide, thus obtaining the Arbekacin. The synthesis method has the advantages of simple operation, high outcome yield, reducing thecost of raw material, optimizing the reaction route, lowering the requirements to the reaction conditions and being beneficial to industrial production.
Owner:BEIJING UNIV OF CHEM TECH +1
Synthesis of degarelix by solid phase segment method
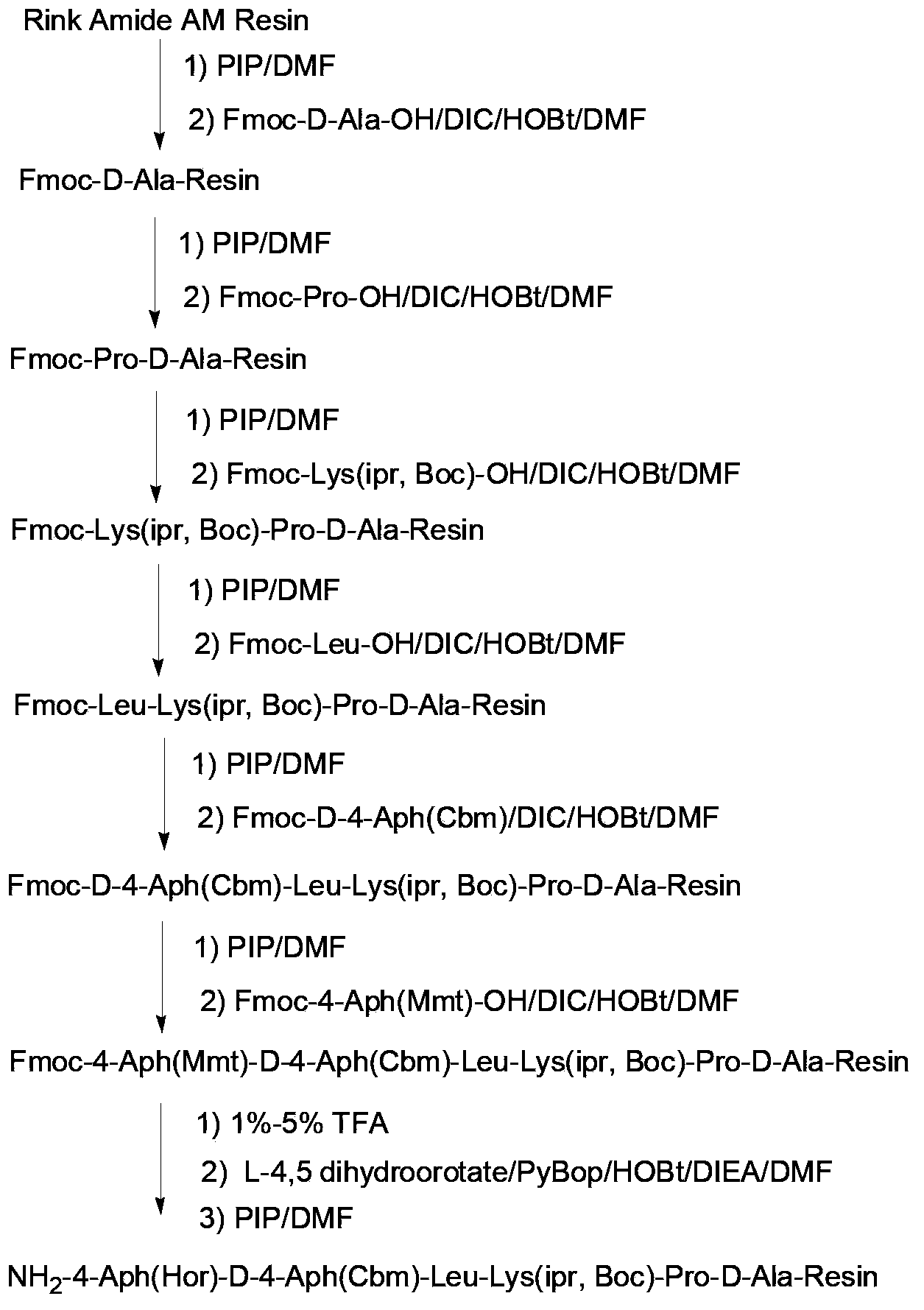
ActiveCN103351428AReduce usageAvoid repeated contactPeptide preparation methodsBulk chemical productionSide chainTrifluoroacetic acid
The invention discloses synthesis of degarelix by a solid phase segment method, wherein Fmoc-amino acid is connected with resin so that Fmoc-amino acid-resin can be obtained; a solid phase synthesis method is adopted, and the steps of orderly connecting amino acid from the end C to the end N and removing the Fmoc group are carried out, so that Fmoc protected polypeptide resin can be obtained; the method comprises the following steps of: (1) forming polypeptide resin as shown in formula III and polypeptide resin as shown in formula IV, respectively; (2) mixing the polypeptide resin as shown in formula III with 1-5% of trifluoroacetic acid (TFA), connecting L-4,5-dihydroorotate to the side chain amino of the polypeptide resin after the protecting group of the sixth amino acid residue at the end C is removed, and then removing the Fmoc group to obtain polypeptide resin as shown in formula V; removing the Fmoc group of the polypeptide resin as shown in formula IV and acetylating the polypeptide resin, thereby obtaining polypeptide resin as shown in formula VI, and then cutting to obtain segments as shown in formula VII; (3) coupling the polypeptide resin as shown in the formula V with the segments as shown in the formula VII, thereby obtaining the polypeptide resin as shown in formula II; (4) separating polypeptide on the polypeptide resin as shown in the formula II from the resin, thereby obtaining degarelix as shown in formula I.
Owner:HAINAN SHUANGCHENG PHARMA
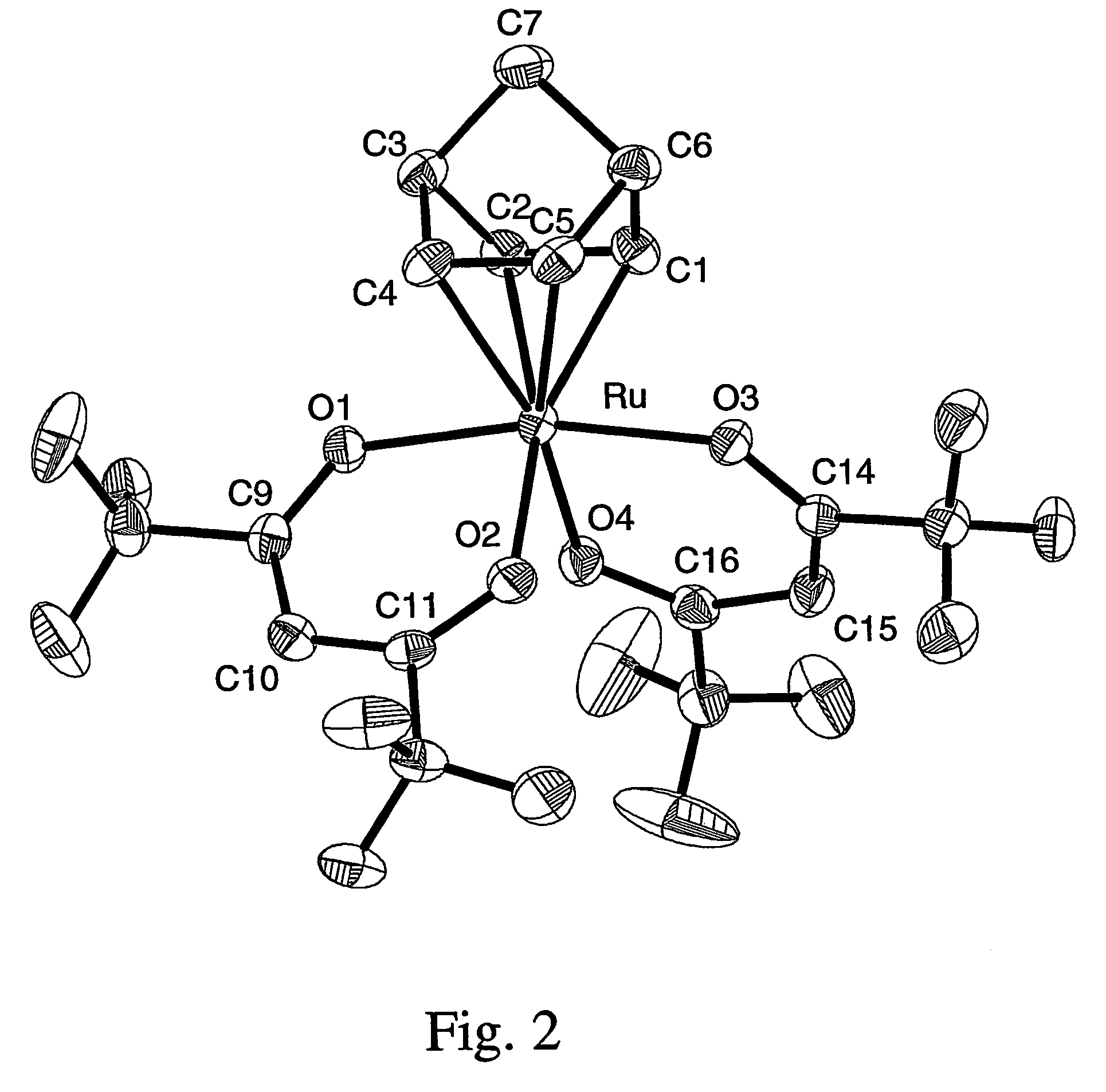
Volatile noble metal organometallic complexes
InactiveUS7112690B2Reduce Van der Waals interactionBoiling and sublimation temperatureFurnaces without endless coreRuthenium organic compoundsIodideChelating ligands
Owner:NATIONAL TSING HUA UNIVERSITY +1
Sea pumpkin and sea pumpkin product sea pumpkin polysaccharide content determination method
InactiveCN101285826AStrong absorption capacitySimple and fast operationComponent separationBiological testingHydrolysisPeak area
The invention discloses a method for determining sea cucumber polysaccharide content in a sea cucumber or a sea cucumber product. The method is characterized in that the sea cucumber or the sea cucumber product is enzymolyzed by protease; the sea cucumber polysaccharide produced by the enzymolysis is separated out by adopting alcohol precipitation; then tested solution is prepared by hydrolysis of trifluoroacetate; the tested solution is prepared into fucose standard water solution which is derived by 1-phenyl-3-methyl-5-pyrazolone; the peak area of fucose derivative in the obtained derivatives is measured by high performance liquid chromatography; and the sea cucumber polysaccharide content in the sea cucumber product is obtained by calculating with the fucose as a reference. The invention provides a simple, accurate, reliable and standardized quantitative analysis method for determining the sea cucumber polysaccharide in various sea cucumbers and sea cucumber products.
Owner:OCEAN UNIV OF CHINA
Post plasma ashing wafer cleaning formulation
InactiveUS7534752B2Increase productionImprove adhesionDetergent mixture composition preparationSemiconductor/solid-state device manufacturingSulfolaneInorganic compound
A semiconductor wafer cleaning formulation for use in post plasma ashing semiconductor fabrication comprising at least one organic chelating agent and at least one polar solvent, wherein the chelating agent and polar solvent are in sufficient amounts to effectively remove inorganic compound residue from a semiconductor wafer. Preferably, the chelating agent is selected from the group consisting of 2,4-Pentanedione, Malonic acid, Oxalic acid, p-Toluenesulfonic acid, and Trifluoroacetic acid; and the polar solvent is selected from the group consisting of Water, Ethylene glycol, N-Methylpyrrolidone (NMP), Gamma butyrolactone (BLO), Cyclohexylpyrrolidone (CHP), Sulfolane, 1,4-Butanediol, and Butyl carbitol.
Owner:ENTEGRIS INC
Trifluoroiodomethane preparation method
ActiveCN102992943AObvious cost advantageWorkmanship is feasibleChemical recyclingHalogenated hydrocarbon preparationActive agentEthylic acid
The invention provides a method for preparing trifluoroiodomethane through utilizing industrial waste trifluoroacetic acid and industrially recovered iodine. The method has the characteristics of low cost, simple operation, mild reaction, fast reaction speed, high yield, high selectivity, environmental protection and the like. The synthesized trifluoroiodomethane is an important fluorine-containing fine chemical engineering raw material, and can be used for producing fluorine-containing finishing agents, fluorine-containing surfactants and other fluorine-containing fine chemicals.
Owner:SINOCHEM LANTIAN +1
Process for preparing a dipeptidyl peptidase IV inhibitor and intermediates employed therein
A process is provided for preparing a dipeptidyl peptidase IV inhibitor of the structurewhereinis treated with TFAA in isopropyl acetate to protect the tertiary hydroxyl group as a trifluoroacetate group to form 4(which is a novel intermediate)which is converted to acid chloride compound 5(which is a novel compound)using Vilsmeier reagent or other chloro reagent and coupled with compound 6 in a heterogeneous mixture of ethyl acetate and aqueous bicarbonate to give compound 7The N,O-bis(trifluoroacetyl) groups of compound 7 are deprotected to give free base compound 10.
Owner:ASTRAZENECA AB
Collosol of yttrium barium cupper oxygen superconducting film and process for preparing high temp. superconducting film thereof
InactiveCN1792806AShorten heat treatment timeEasy to makeSuperconductors/hyperconductorsSuperconductor devicesPolymer scienceOxygen
A sol of the Y-Ba-Cu-O (YBCO) superconductor film is proportionally prepared from yttrium acetate, barium acetate, copper acetate, trifluoroacetic acid, water, alpha-methylacrylic acid, divinyltriamine and methanol. A process for preparing the high-temp superconductor film from said sol is also disclosed.
Owner:XIAN UNIV OF TECH
Phellinus igniarius mycelium liquid fermentation method by utilizing fungal elicitor for improving yield of flavone of phellinus igniarius
The invention discloses a phellinus igniarius mycelium liquid fermentation method by utilizing a fungal elicitor for improving the yield of flavone of phellinus igniarius, which comprises the steps of: inoculating a fungal elicitor strain for activated culture, and then inoculating and culturing a fungal block to obtain a fungal elicitor mycelium through filtration; then drying, grinding and crushing the fungal elicitor mycelium, adding the crushed fungal elicitor mycelium into ethanol for soaking, filtering the obtained solution, collecting filter residue, orderly adding a mixed solution of chloroform and normal butanol, acetone and water into the filter residue for washing, adding trifluoroacetic acid into a dry substance after the air-drying, performing boiling water bath for 0.5 to 2 hours, then filtering the obtained solution, taking a filtrate for neutralization, standing the filtrate, using a microporous filtering film with the pore diameter between 0.4 and 5mu m to filter the filtrate for sterilization to obtain the fungal elicitor, and cryopreserving the fungal elicitor at the temperature of between 10 and 20 DEG C below zero; inoculating a phellinus igniarius strain for activated culture, and then inoculating a fungal block for fermentation culture; and adding the fungal elicitor into the phellinus igniarius to continue culturing to obtain a phellinus igniarius mycelium within 1 to 5 days of liquid fermentation culture of the phellinus igniarius. The method has simple process, low cost and remarkable effect, can greatly improve the content of the flavone of phellinus igniarius cells, and is a production method with industrialization prospect.
Owner:LUDONG UNIVERSITY
Preparation method of trifluoroacetic acid
InactiveCN103524325ALow costPreparation from carboxylic acid halideHalogenated hydrocarbon preparationCatalytic oxidationTetrachloroethane
The invention discloses a preparation method of trifluoroacetic acid low in synthesis cost. The preparation method comprises the steps of (1) preparing 1,1-difluoro tetrachloroethane from 1,1-difluoroethane or chloride thereof through ultraviolet catalysis and a chlorine reaction; (2) implementing catalytic oxidation on the 1,1-difluoro tetrachloroethane to obtain 1,1-difluoro-1-chloroacetyl chloride, wherein in the catalytic oxidation, mol ratio of the 1,1-difluoro tetrachloroethane to an oxidizing agent is 1: (2-4), dosage of the catalyst is 0.5-5% of the total weight of an reactant, and reaction temperature is 50-70 DEG C; (3) carrying out hydrogen fluoride (HF) fluoridation on the 1,1-difluoro-1-chloroacetyl chloride under the catalyst to obtain trifluoroacetyl fluoride, wherein mol ratio of the 1,1-difluoro-1-chloroacetyl chloride to the HF is 1: (2-3), reaction temperature is 40-60 DEG C, and the catalyst is either antimony or antimony pentachloride; and (4) hydrolyzing the prepared trifluoroacetyl fluoride to obtain trifluoroacetic acid.
Owner:CHANGSHU 3F ZHENFU NEW MATERIALS CO LTD
Novel red BODIPY fluorescent dye and preparation method and application thereof
InactiveCN102702768ANarrow absorbencyNarrow fluorescence emission spectrumMethine/polymethine dyesMicrobiological testing/measurementChemical reaction1,4-Benzoquinone
The invention relates to a novel red BODIPY fluorescent dye with the chemical formula of C10+mH7+nBF2N2+xOy, wherein m, n, x and y are integers from 0 to 100. The preparation method comprises the following steps: dissolving pyrrole with substituent groups R1, R2 and R3 in an organic solution; adding ethyl glyoxylate together with nitrogen to the organic solution for a chemical reaction by using trifluoroacetic acid or toluenesulfonic acid as a catalyst; adding 2,3-dichloro-5,6-dicyano-1,4-benzoquinone oxidative dehydrogenation; and adding organic amine and a boron trifluoride diethyl ether solution for another reaction. After the reaction solution is concentrated, chromatography is performed with a silicagel column to obtain the fluorescent dye. The fluorescent dye can be used for cell imaging, fluorescent probe or laser dye. The fluorescent dye has the advantages that the ultraviolet-visible absorption spectrum and the fluorescence emission spectrum of the fluorescent dye are narrow; fluorescent quanta has high efficiency and good light stability; and the fluorescent dye has simple molecular structure and can be synthesized easily, so as to facilitate popularization and application.
Owner:TIANJIN UNIVERSITY OF TECHNOLOGY
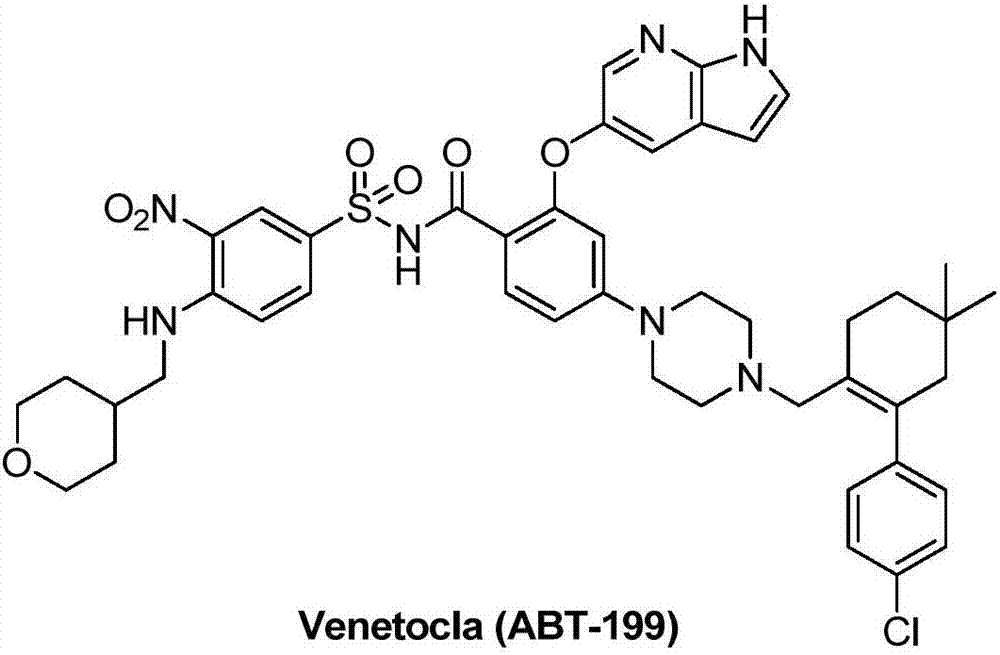
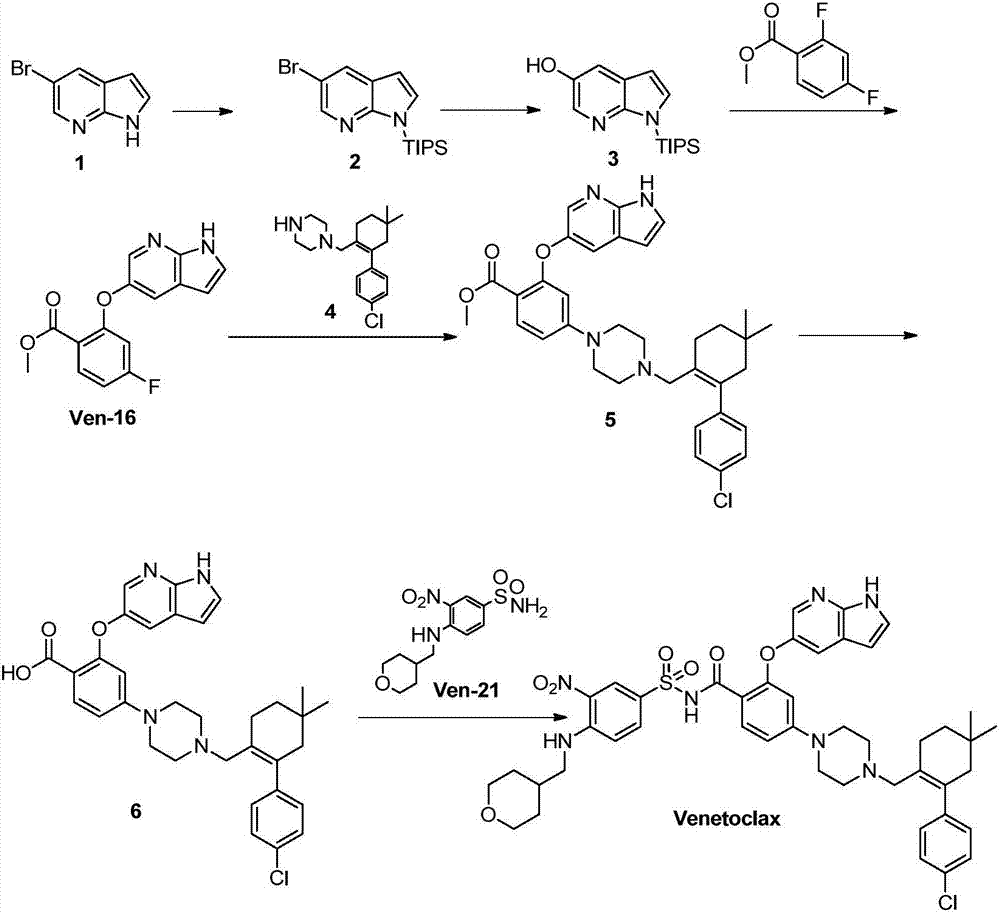
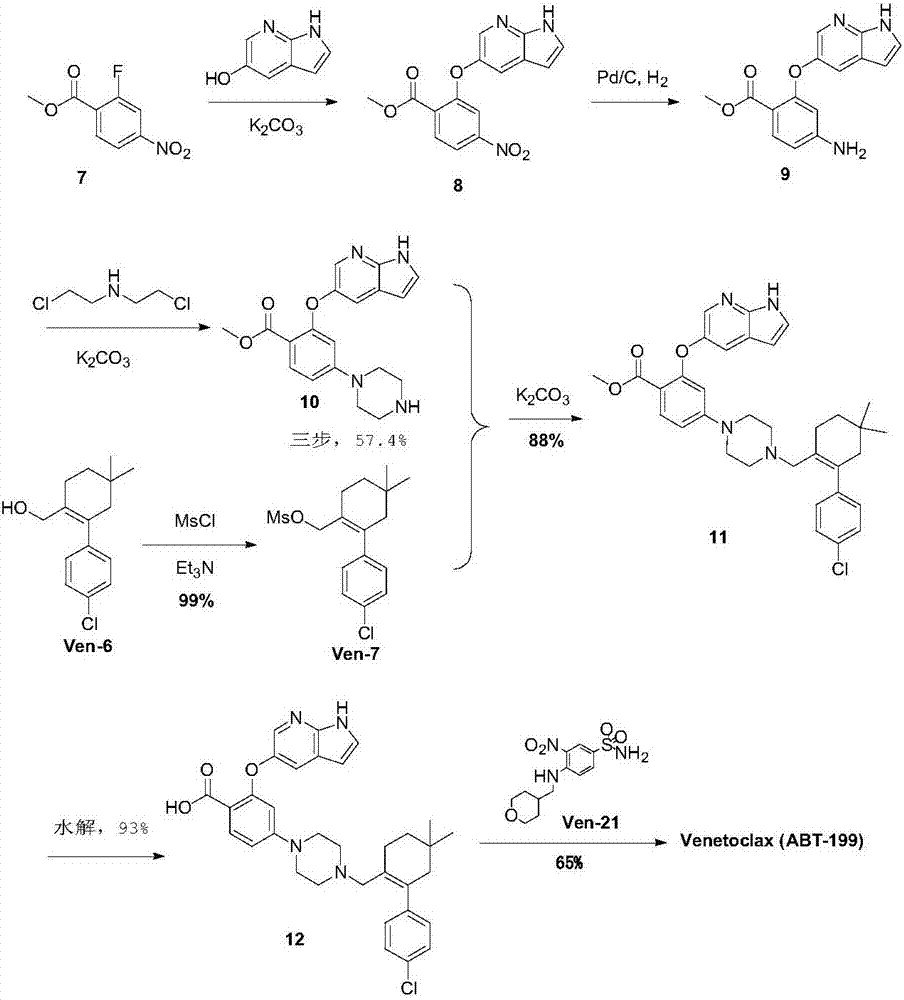
Synthesis method for BCL-2 inhibitor Venetoclax
InactiveCN107089981AFollow-up response is simpleHas industrial application valueOrganic chemistrySynthesis methodsReaction intermediate
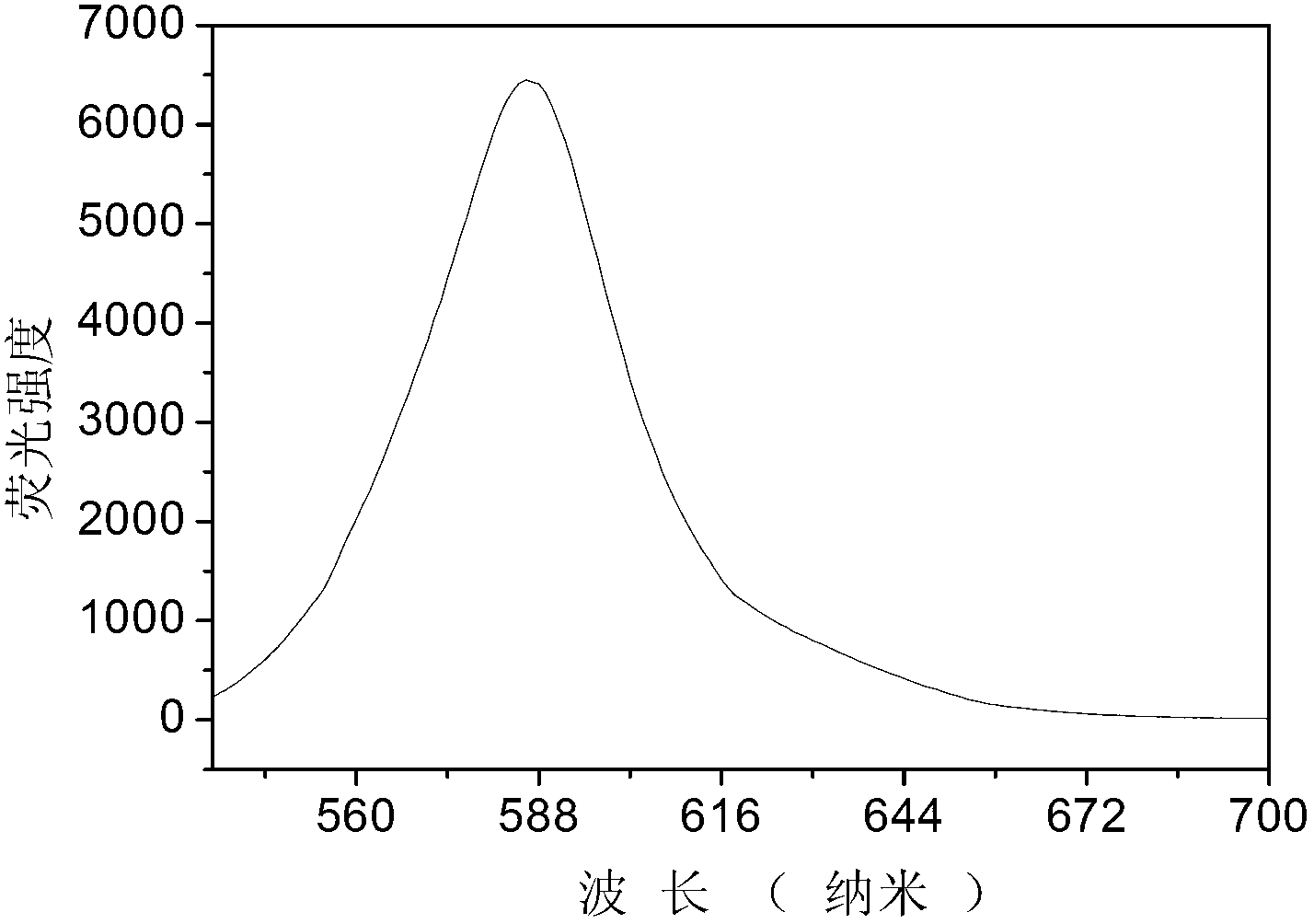
The invention discloses a new synthesis method for a BCL-2 inhibitor Venetoclax. The synthesis method includes the steps that 2,4-difluoro methyl benzoate serves as a raw material, the 2,4-difluoro methyl benzoate and 5-hydroxy-7-azaindole are subjected to a condensation reaction, Ven-16 is obtained, and 4-substituted isomer and other impurities are removed through recrystallization; the product and N-Boc-piperazine are reacted and hydrolyzed, and Ven-18 is obtained; Ven-18 and Ven-21 are condensed, Boc of trifluoroacetic acid is removed, and Ven-20 is obtained; the product and Ven-7 are reacted, reaction intermediates in all the steps have the good crystal forms, purification can be carried out through crystal to achieve the center control requirements of the technology, and a final product Venetoclax can also be subjected to recrystallization to obtain the product Venetoclax, wherein the HPLC purity of the Venetoclax is larger than 99.5%, and the individual impurity of the Venetoclax is smaller than 0.1%; the Venetoclax has the industrialized application value.
Owner:杭州科耀医药科技有限公司
Method for preparing carperitide acetate
ActiveCN102382188AReduce investmentEasy to operatePeptide preparation methodsDepsipeptidesTrifluoroacetic acidSolid phases
The invention discloses a method for preparing carperitide acetate. The method comprises the following steps of: 1) reacting Fmoc-Tyr(tBu)-OH with WangResin with the substitution value of 0.5-1.0mmol / g to obtain Fmoc-Tyr(tBu)-WangResin; 2) synthesizing the Fmoc-Tyr(tBu)-WangResin by adopting a mode of coupling one by one to obtain linear carperitide-WangResin; 3) oxidizing the carperitide-WangResin by using a solid-phase oxidation method; 4) cracking by using trifluoroacetic acid to obtain crude carperitide; and 5) purifying the crude carperitide by using reversed phase high performance liquid chromatography to obtain high-purity refined carperitide. The process has the advantages of simple reaction and operation, simple posttreatment, high yield, low cost and the like.
Owner:HYBIO PHARMA
Solid phase synthetic technique for thymosin alpha1
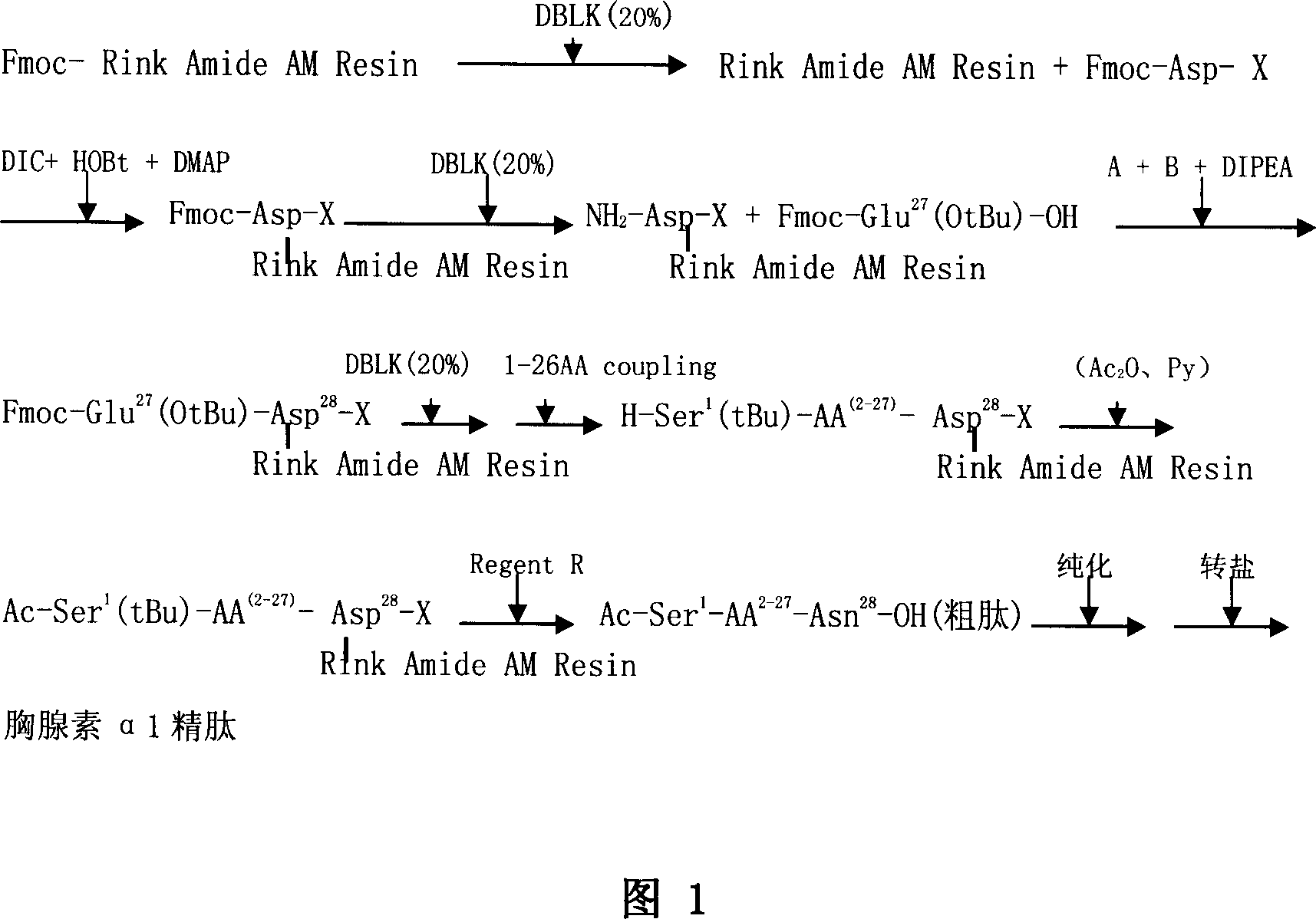
ActiveCN101104638AAdvantages of solid phase synthesis processEasy to purifyThymopoietinsPeptide preparation methodsFluoroacetic acidAcetic anhydride
The invention relates to a solid-phase synthesis process of a thymosin alpha 1, belonging to the polypeptide solid-phase synthesis technical field. The invention comprises the following steps: a. a Fmoc-Rink Amide AM resin or a Fmoc-Rink Amide MBHA resin is used as carrier, an H2N-Rink Amide AM resin or an H2N-Rink Amide MBHA resin is obtained after deprotection of the Fmoc; b. side chain carboxyl group of Fmoc-Asp-X is connected with resin amino by the method of solid-phase synthesis to obtain the Fmoc-Asp (resin)-X; c. the left amino acid in the sequence is synthesized in solid-phase with the Fmoc strategy; d. after the amino protection group Fmoc of N terminal amino acid is removed, the N terminal amino acid is acetylated by acetic anhydride and pyridine; e. then the acetylated N terminal amino acid is cut by a cracking agent (tri fluoroacetic acid / benzoylate sulfide / 1, 2- dithioglycol / Anisole) to obtain the thymosin alpha 1; f. crude product of the thymosin alpha 1 is prepared and separated by HPLC to obtain the pure thymosin alpha 1. The invention can increase significantly the yield of the thymosin alpha 1 and decrease the production cost, which is helpful for scale production and has better industrialization prospect.
Owner:苏州天马医药集团天吉生物制药有限公司
Dimension regulation method of porphyrin metal organic framework material
The invention provides a dimension regulation method of a porphyrin metal organic framework material. The method comprises the following steps of (1) adopting 1 to 5mL of DMF solution of ZrOCl2.8H2O with the concentration being 2 to 6mg / mL, adding benzoic acid, and uniformly dissolving; (2) adopting 1 to 5mL of DMF solution of tetracarboxyl-phenyl porphyrin with the concentration being 1 to 3mg / mL, wherein the mass ratio of the ZrOCl2.8H2O to the tetracarboxyl-phenyl porphyrin is 2:1; (3) after mixing the solution of the step (1) and the solution of the step (2), adding trifluoroacetic acid, mixing, adding CTAB or SDS, carrying out heating reaction at 100 to 150 DEG C for 0.5 to 1.5h, and postprocessing to obtain the porphyrin metal organic framework material. According to the dimension regulation method of the porphyrin metal organic framework material provided by the invention, the dimension can be controlled within an appropriate range, and a better hydrogen production performance can be achieved.
Owner:HENAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com