Tulathromycin intermediate and preparation method thereof, as well as preparation method of tulathromycin
A technology of teramycin and intermediates, which is applied in the field of teramycin drug synthesis, can solve the problems of easy ring opening, many by-products, low yield and the like, and achieves low production cost, convenient operation and improved product yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
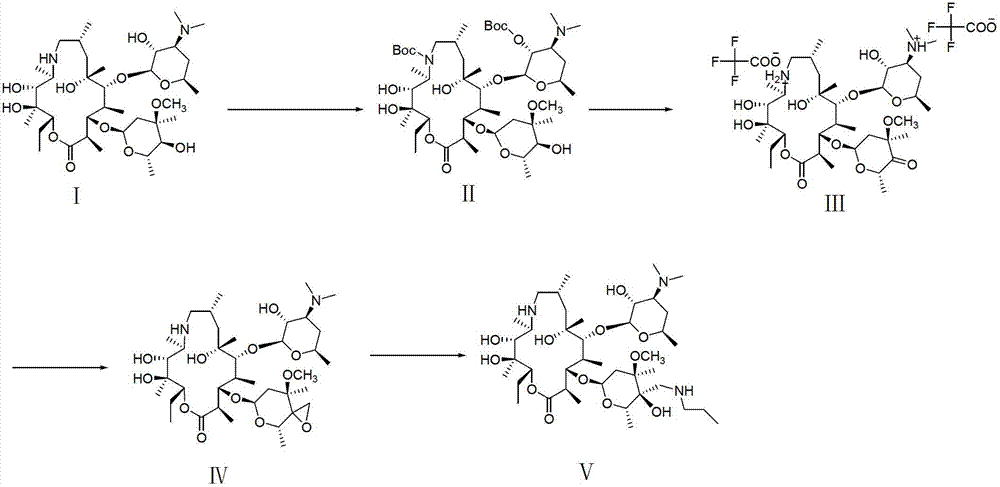
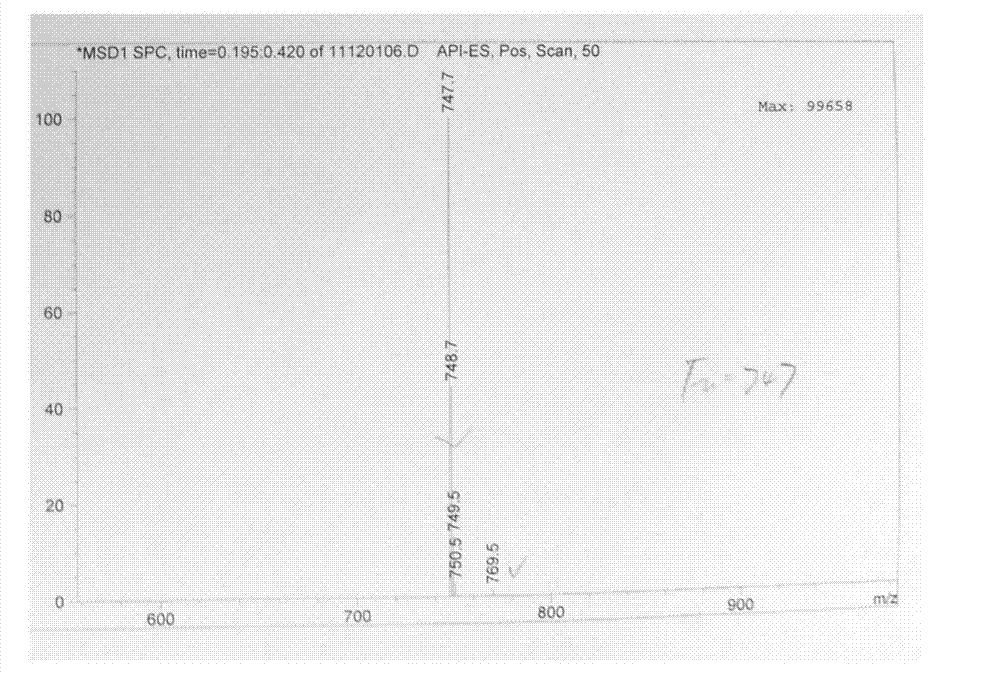
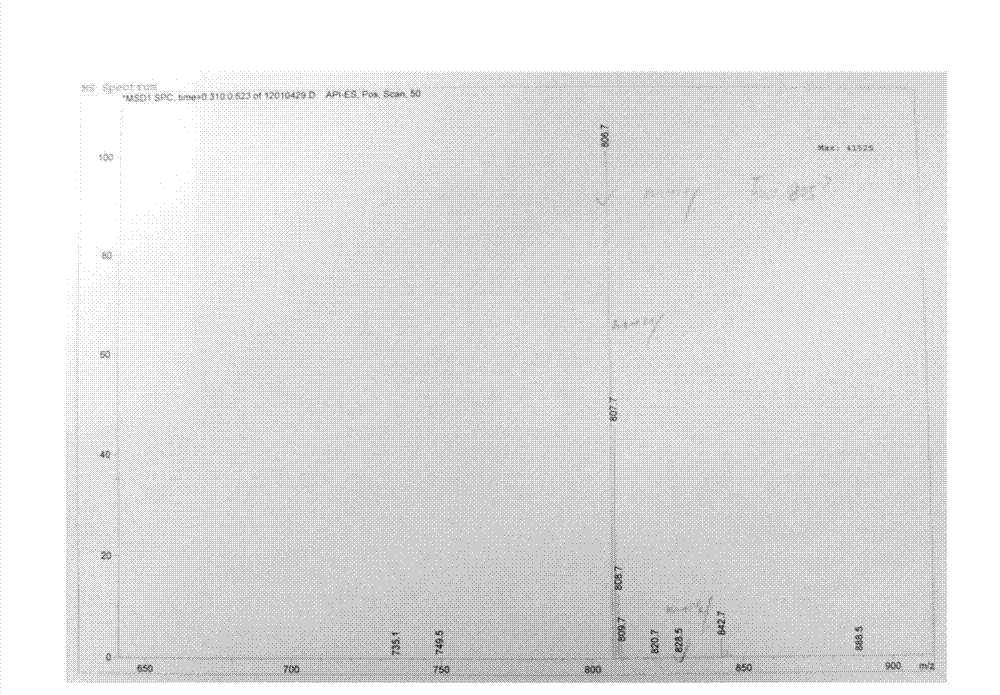
[0053] Such as Figure 1-3 Shown, a kind of synthetic method of telamycin, its steps are as follows:
[0054] 1. Double protected azithromycin A (Ⅱ), namely (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl- 3-O-methyl-α-L-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexamethyl Base-11-[[3,4,6-tridehydro-3-(dimethylamino)-2-O-(tert-butoxycarbonyl)-β-D-hexylopyranosyl]oxy] - Preparation of 1-oxa-6-(tert-butoxycarbonyl)azacyclopentadecan-15-one (Ⅱ)
[0055] Add 15g (20.4mml) (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl) into a 250ml three-neck flask -3-O-methyl-α-L-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexa Methyl-11-[[3,4,6-tridehydro-3-(dimethylamino)-β-D-hexylopyranosyl]oxy]-1-oxa-6-aza Cyclopentadecan-15-one (I), 90ml tetrahydrofuran, 1.2g 4-dimethylaminopyridine, temperature control -5-5 degrees, slowly drop 11.1g (51.0mml) di-tert-butyl dicarbonate into it , After dropping, after s...
Embodiment 2
[0066] Such as figure 1 Shown, a kind of synthetic method of telamycin, its steps are as follows:
[0067] 1. (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L -hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexamethyl-11-[[3,4, 6-Tridehydro-3-(dimethylamino)-2-O-(tert-butoxycarbonyl)-β-D-hexylopyranosyl]oxy]-1-oxa-6-(tert Preparation of butoxycarbonyl)azacyclopentadecan-15-one (Ⅱ)
[0068] Add 15g (20.4mml) (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl) into a 250ml three-neck flask -3-O-methyl-α-L-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexa Methyl-11-[[3,4,6-tridehydro-3-(dimethylamino)-β-D-hexylopyranosyl]oxy]-1-oxa-6-aza Cyclopentadecan-15-one (I), 90ml tetrahydrofuran, 8.4g potassium carbonate, temperature control -5-5 degrees, slowly drop 11.1g (51.0mml) di-tert-butyl dicarbonate into it, dropwise, stir Half an hour later, the temperature was raised to reflux, and afte...
Embodiment 3
[0079] Such as figure 1 Shown, a kind of synthetic method of telamycin, its steps are as follows:
[0080] 1. (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl-3-O-methyl-α-L -hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexamethyl-11-[[3,4, 6-Tridehydro-3-(dimethylamino)-2-O-(tert-butoxycarbonyl)-β-D-hexylopyranosyl]oxy]-1-oxa-6-(tert Preparation of butoxycarbonyl)azacyclopentadecan-15-one (Ⅱ)
[0081] Add 15g (20.4mml) (2R, 3S, 4R, 5R, 8R, 10R, 11R, 12S, 13S, 14R)-13-[(2,6-dideoxy-3-C-methyl) into a 250ml three-neck flask -3-O-methyl-α-L-hexapyranosyl)oxy]-2-ethyl-3,4,10-trihydro-3,5,8,10,12,14-hexa Methyl-11-[[3,4,6-tridehydro-3-(dimethylamino)-β-D-hexylopyranosyl]oxy]-1-oxa-6-aza Cyclopentadecan-15-one (I), 90ml dichloromethane, 1.2g 4-dimethylaminopyridine, temperature control -5-5 degrees, slowly dropwise add 22.2g (102.0mml) di-tert-dicarbonate Butyl ester, after dripping, stirred for half an hour, raised the temperature to re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com