Patents
Literature
58353 results about "Therapeutic effect" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Therapeutic effect refers to the response(s) after a treatment of any kind, the results of which are judged to be useful or favorable. This is true whether the result was expected, unexpected, or even an unintended consequence. An adverse effect (including nocebo) is the converse and refers to harmful or undesired response(s). What constitutes a therapeutic effect versus a side effect is a matter of both the nature of the situation and the goals of treatment. No inherent difference separates therapeutic and undesired side effects; both responses are behavioral/physiologic changes that occur as a response to the treatment strategy or agent.
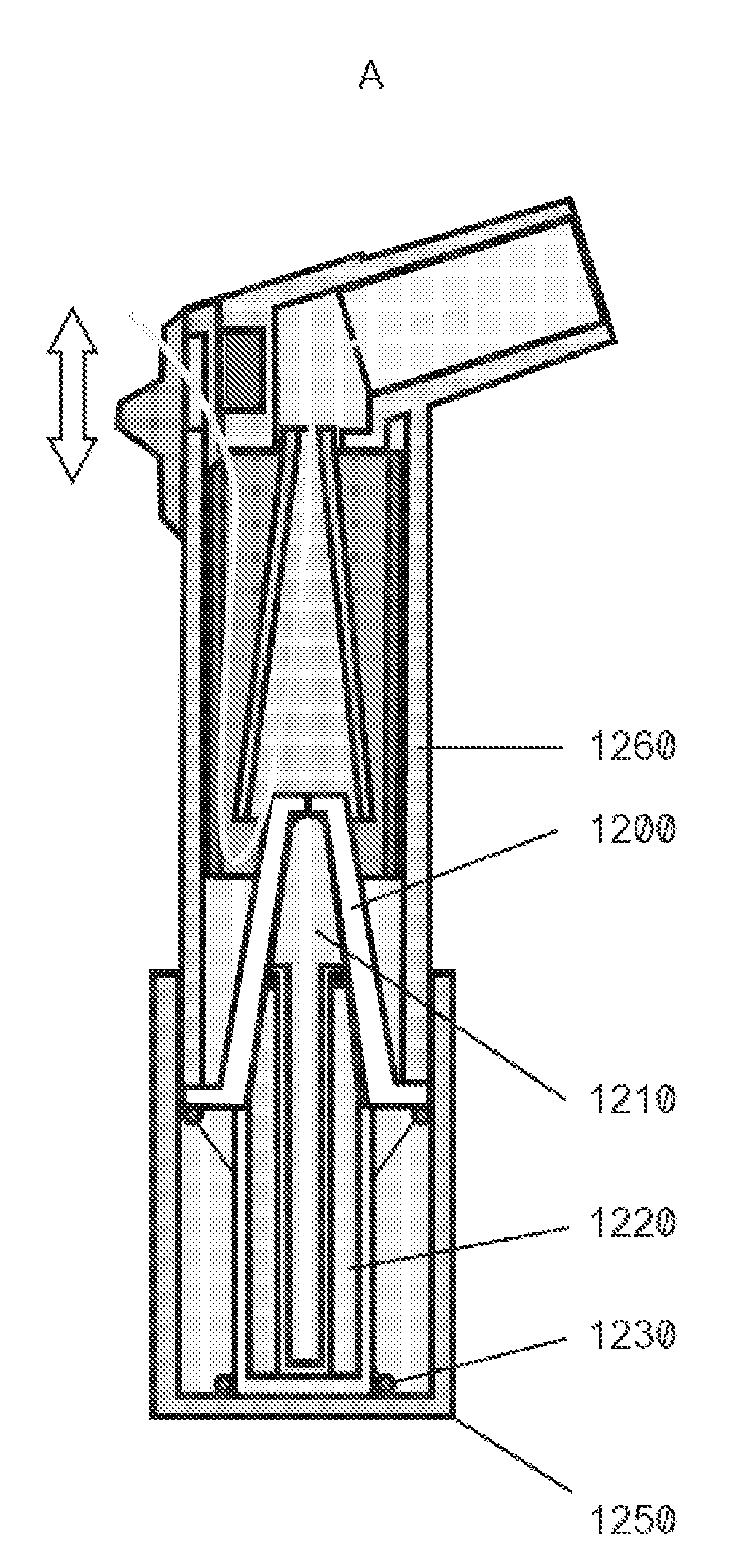
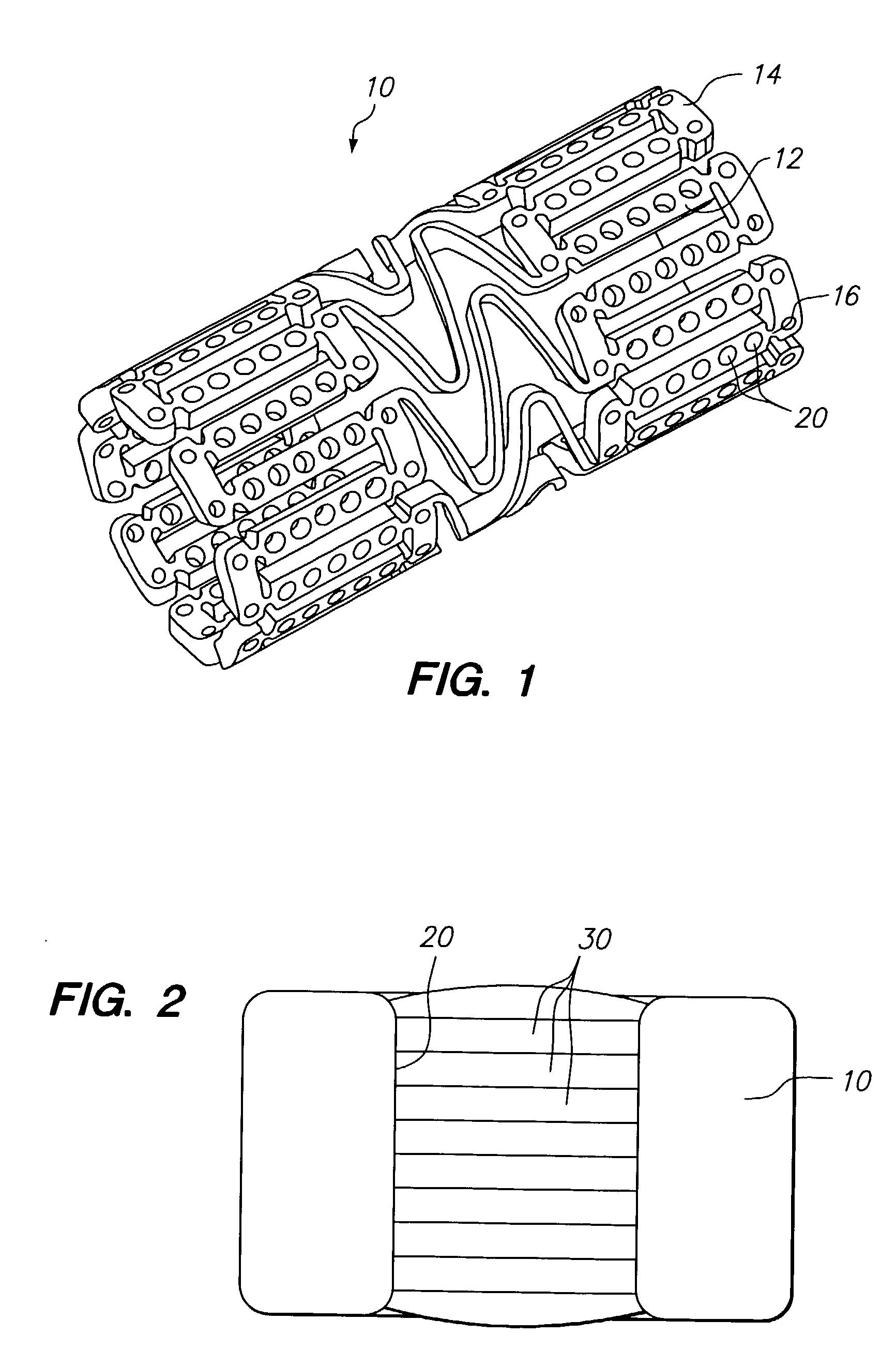
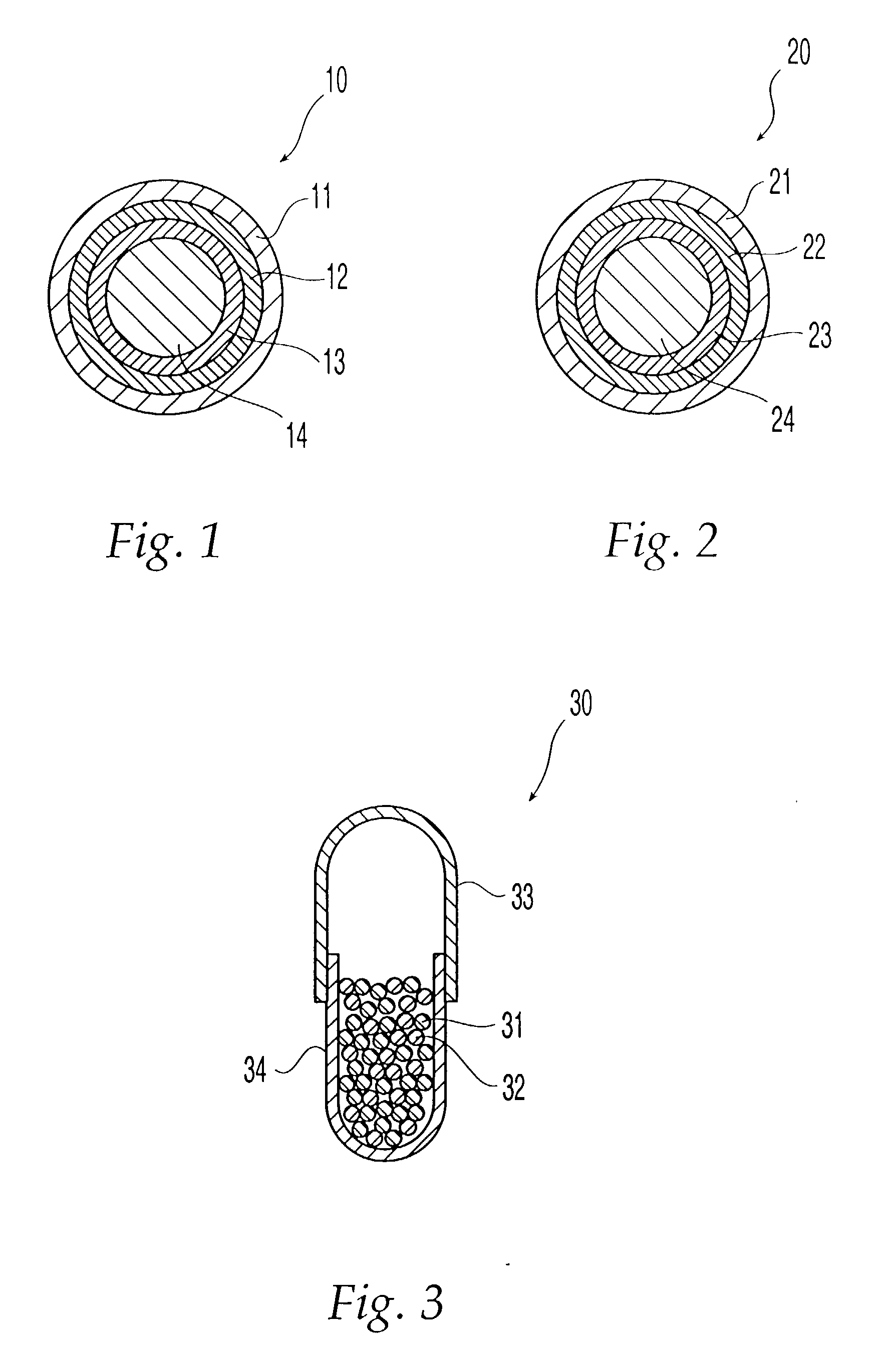
Drug depot implant designs
ActiveUS7727954B2Uniform drug distributionMinimal disruptionPowder deliveryPeptide/protein ingredientsSkeletal injuryChronic pain
Owner:WARSAW ORTHOPEDIC INC
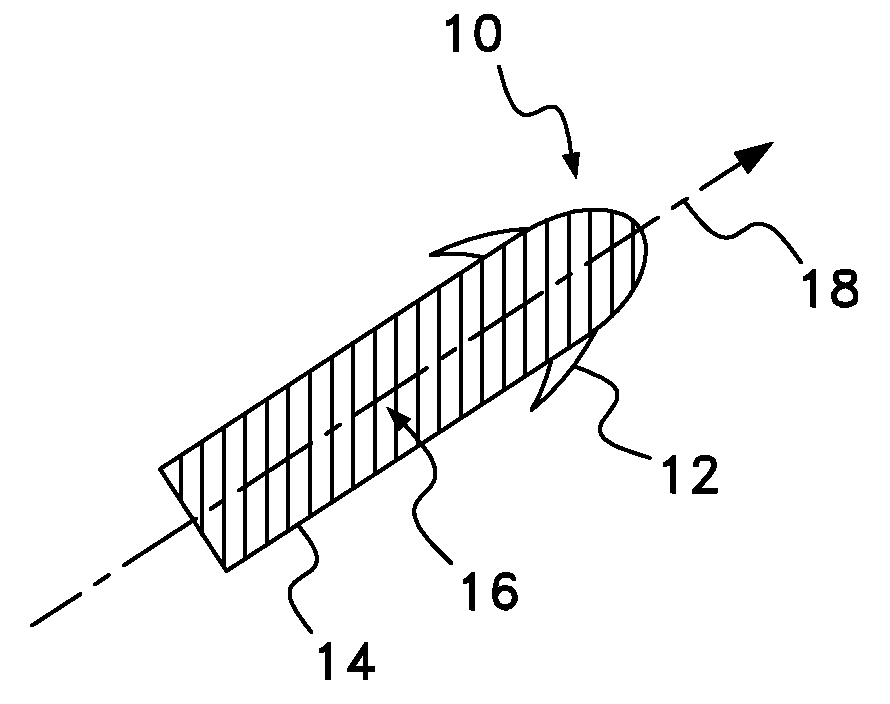
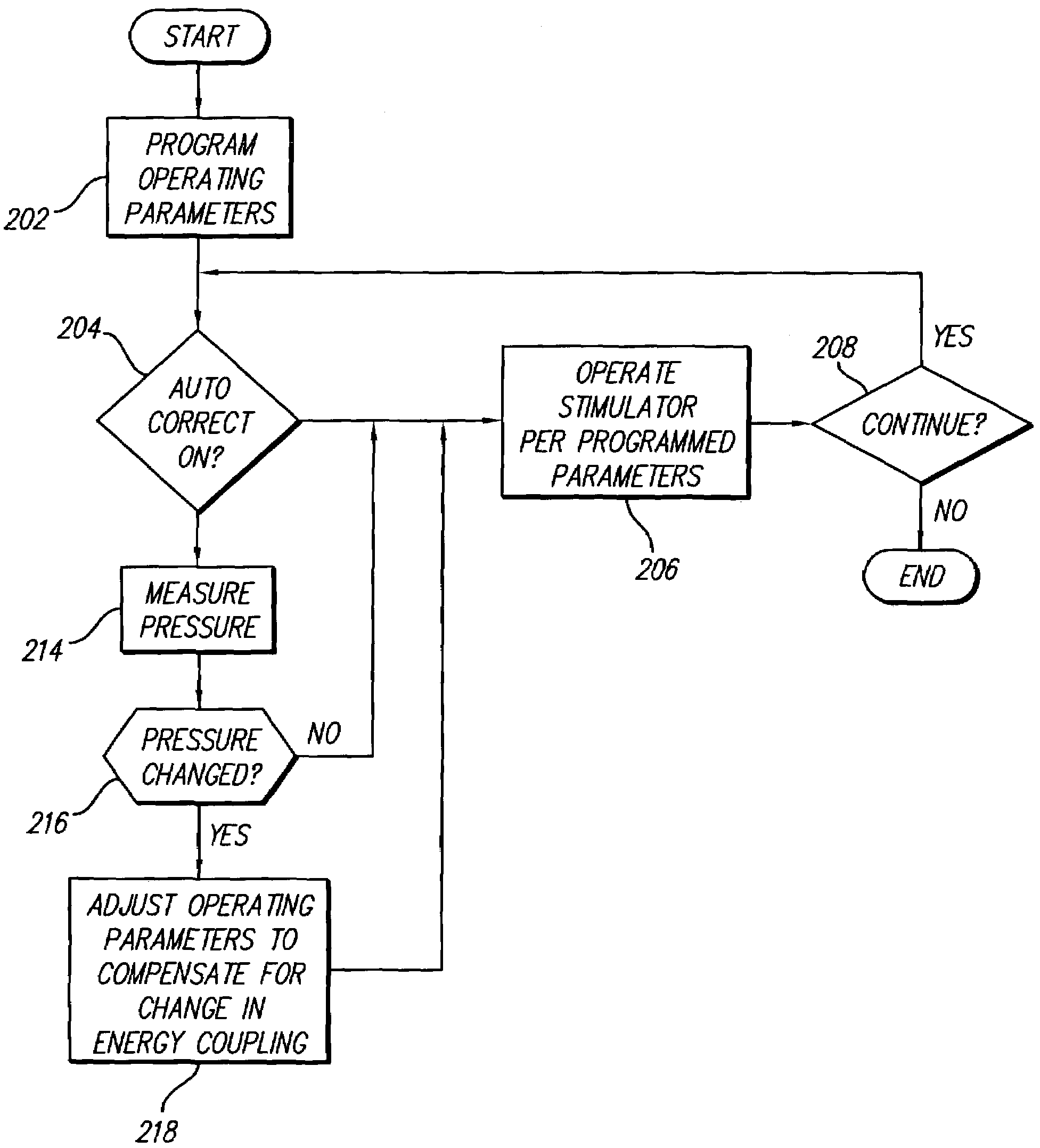
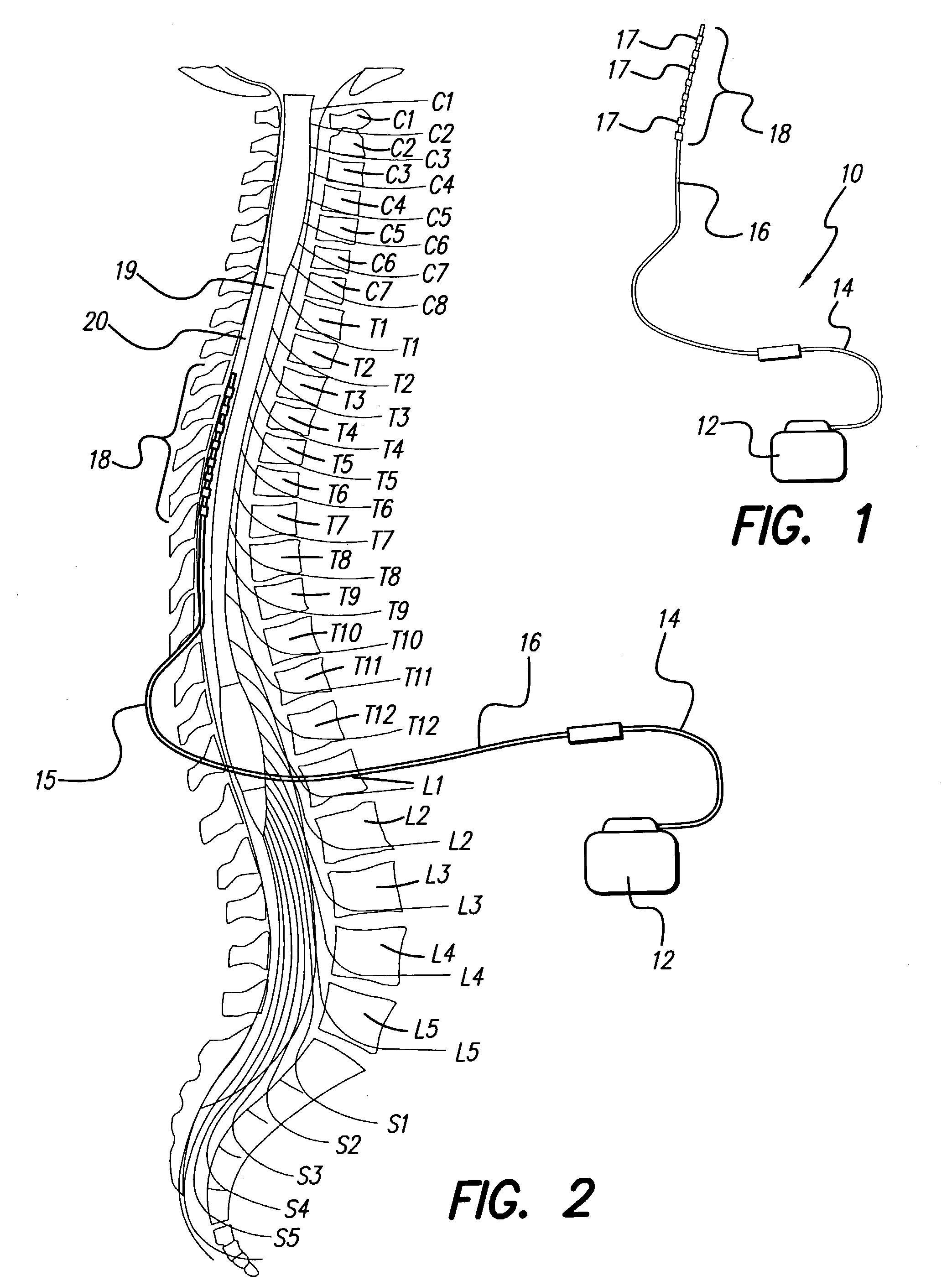
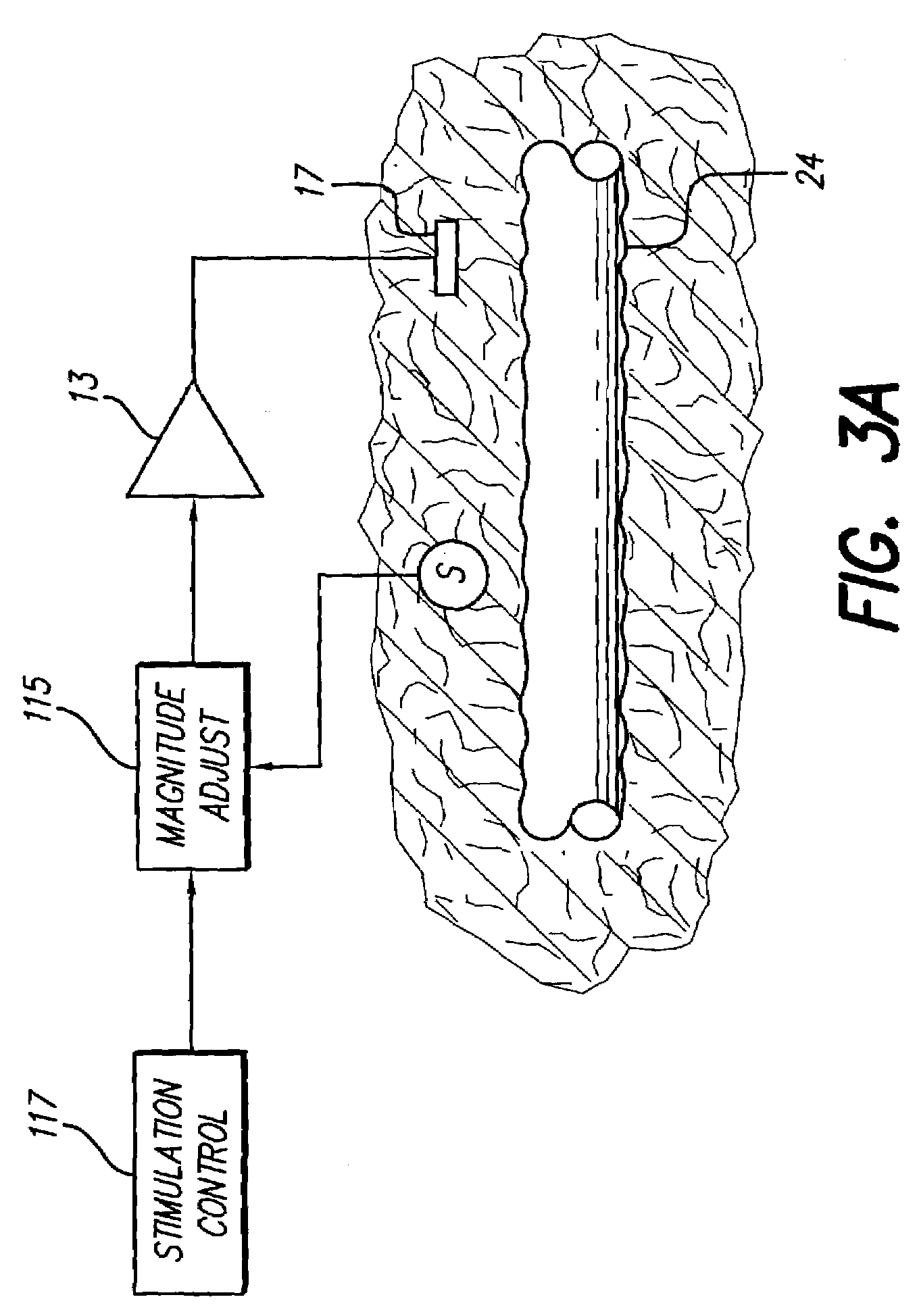
Neural stimulation system providing auto adjustment of stimulus output as a function of sensed impedance
InactiveUS7317948B1Effectively auto correct output amplitudeMinimize occurrenceElectrotherapyDiagnostic recording/measuringLead impedanceElectricity
A neural stimulation system automatically corrects or adjusts the stimulus magnitude (stimulation energy) in order to maintain a comfortable and effective stimulation therapy. Because the changes in impedance associated with the electrode-tissue interface can indicate obstruction of current flow and positional lead displacement, lead impedance can indicate the quantity of electrical stimulation energy that should be delivered to the target neural tissue to provide corrective adjustment. Hence, a change in impedance or morphology of an impedance curve may be used in a feedback loop to indicate that the stimulation energy needs to be adjusted and the system can effectively auto correct the magnitude of stimulation energy to maintain a desired therapeutic effect.
Owner:BOSTON SCI NEUROMODULATION CORP
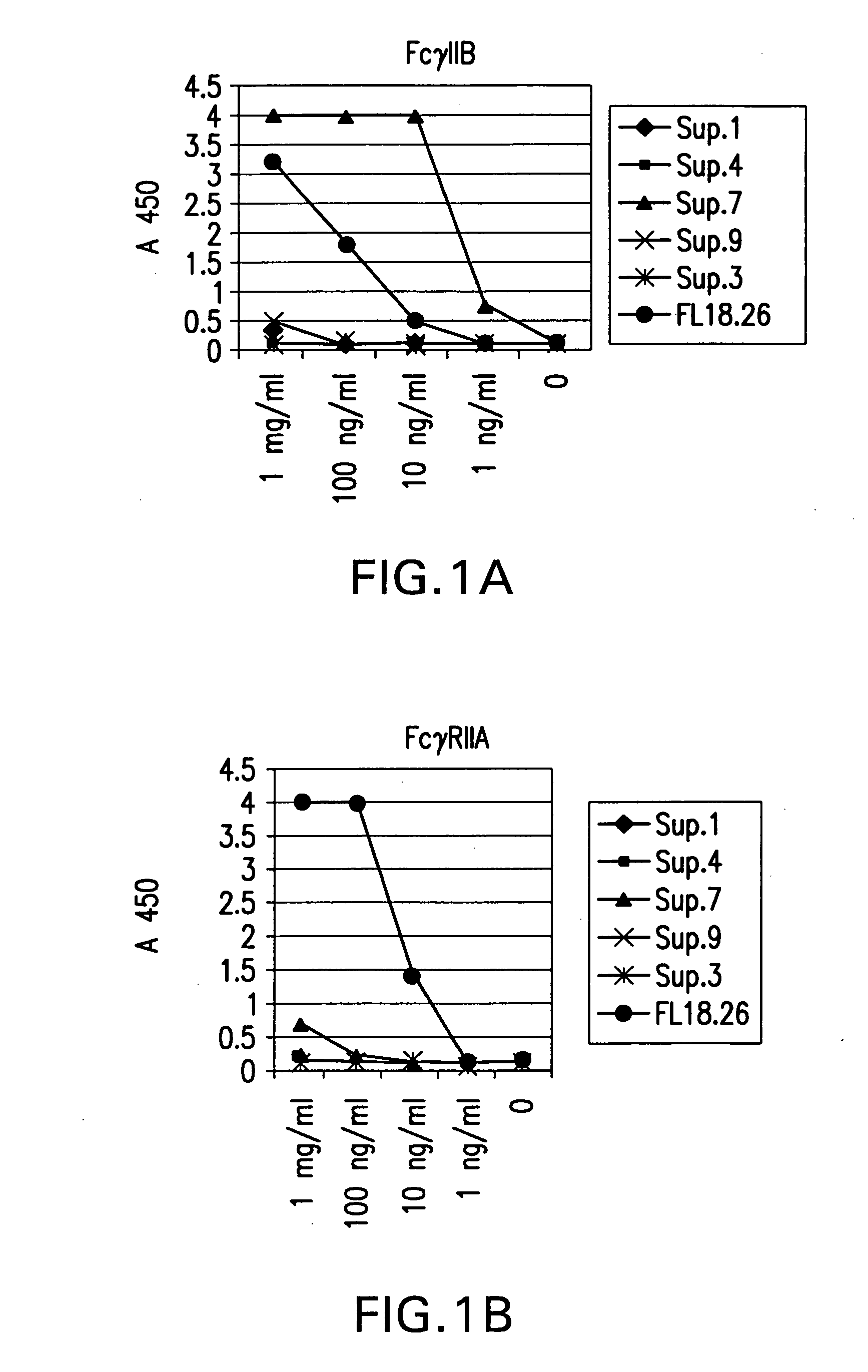
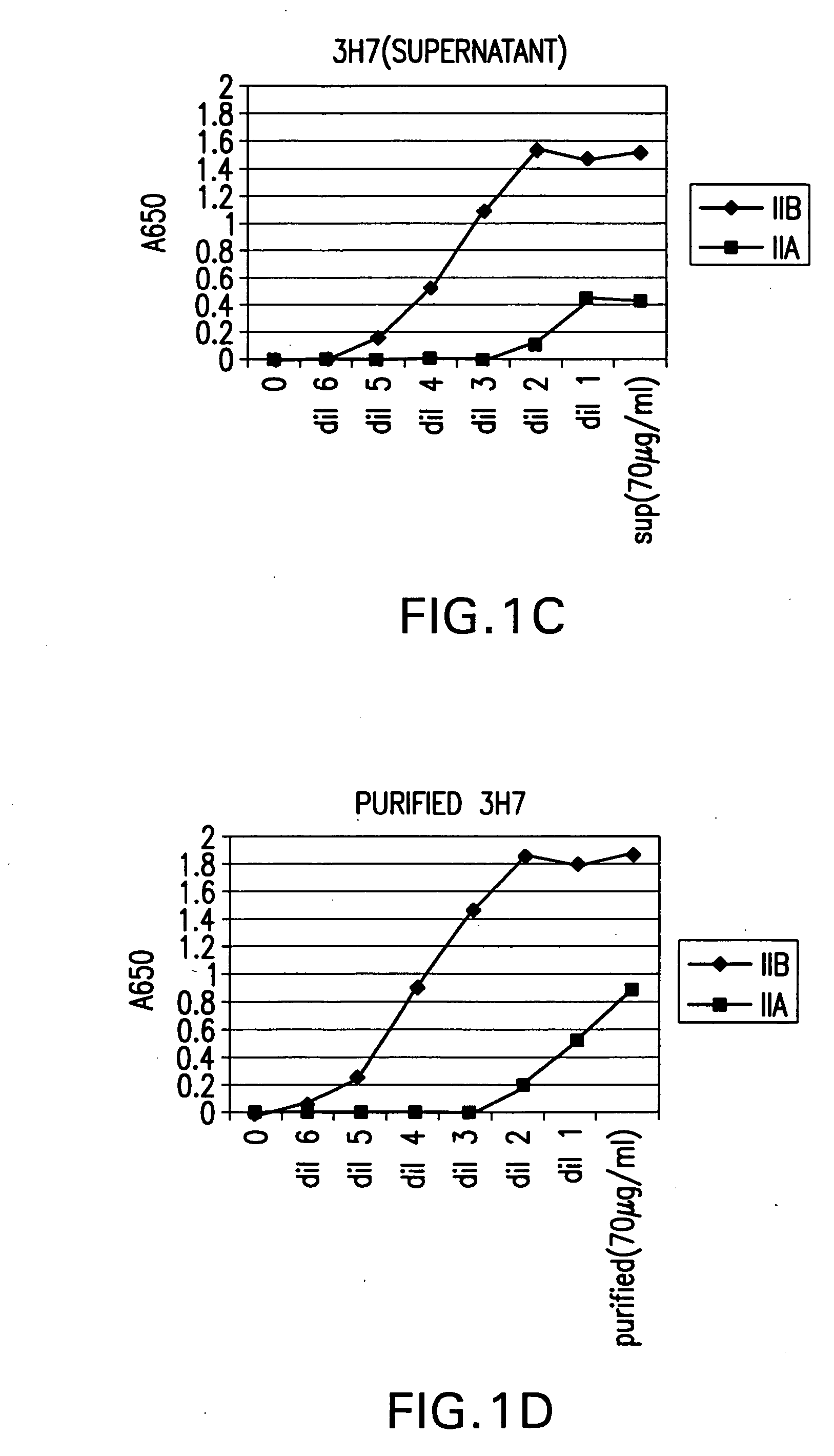
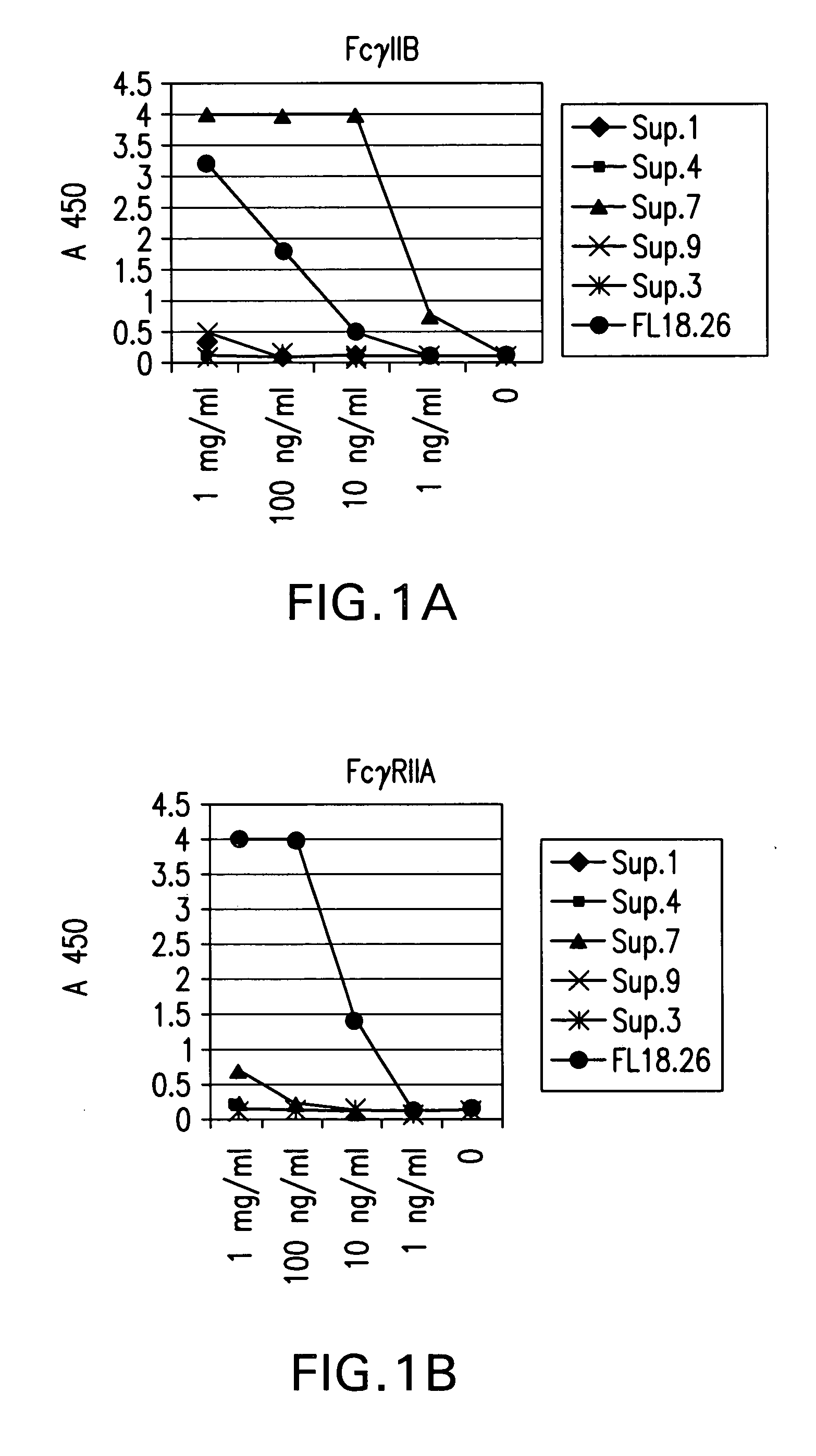
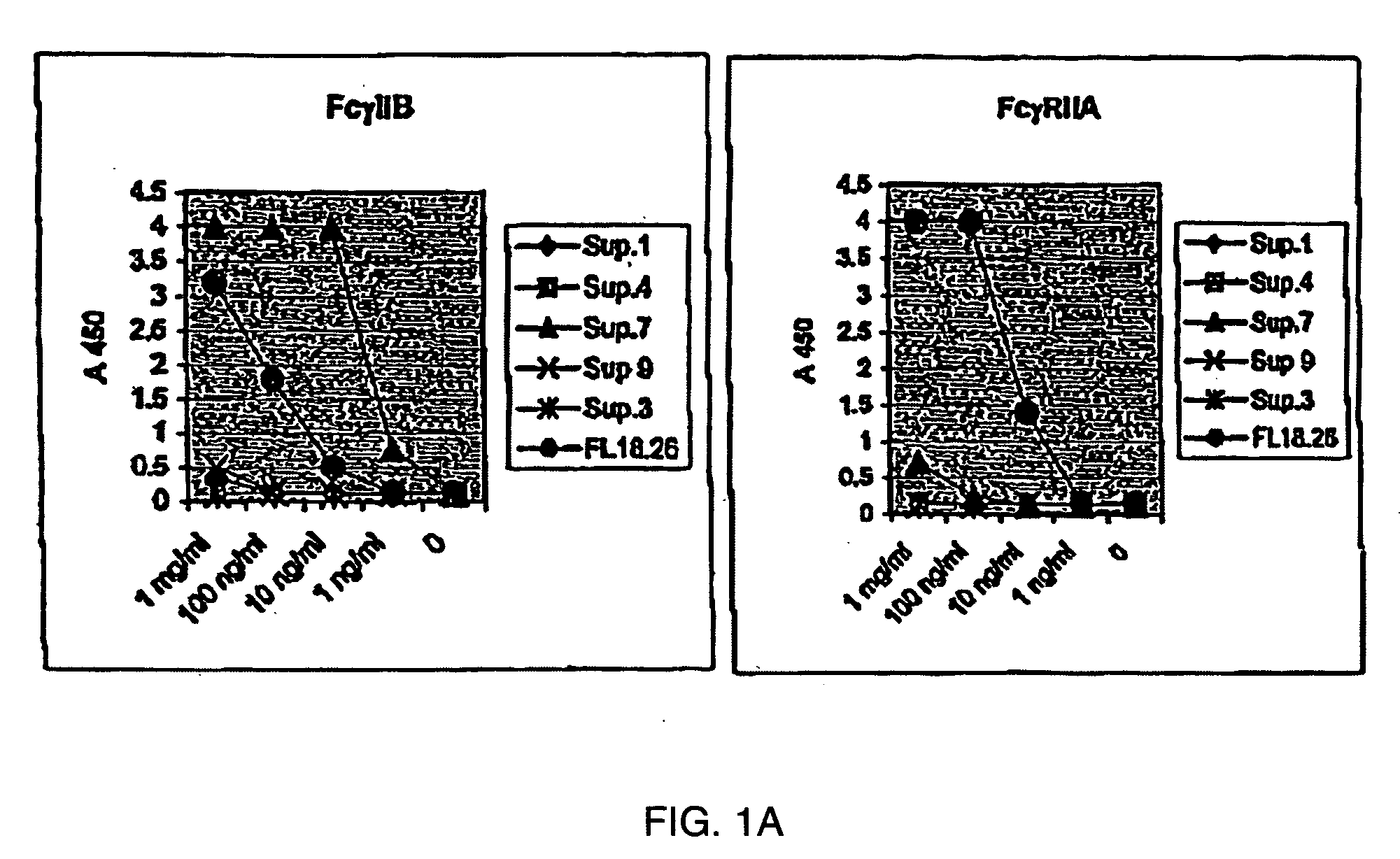
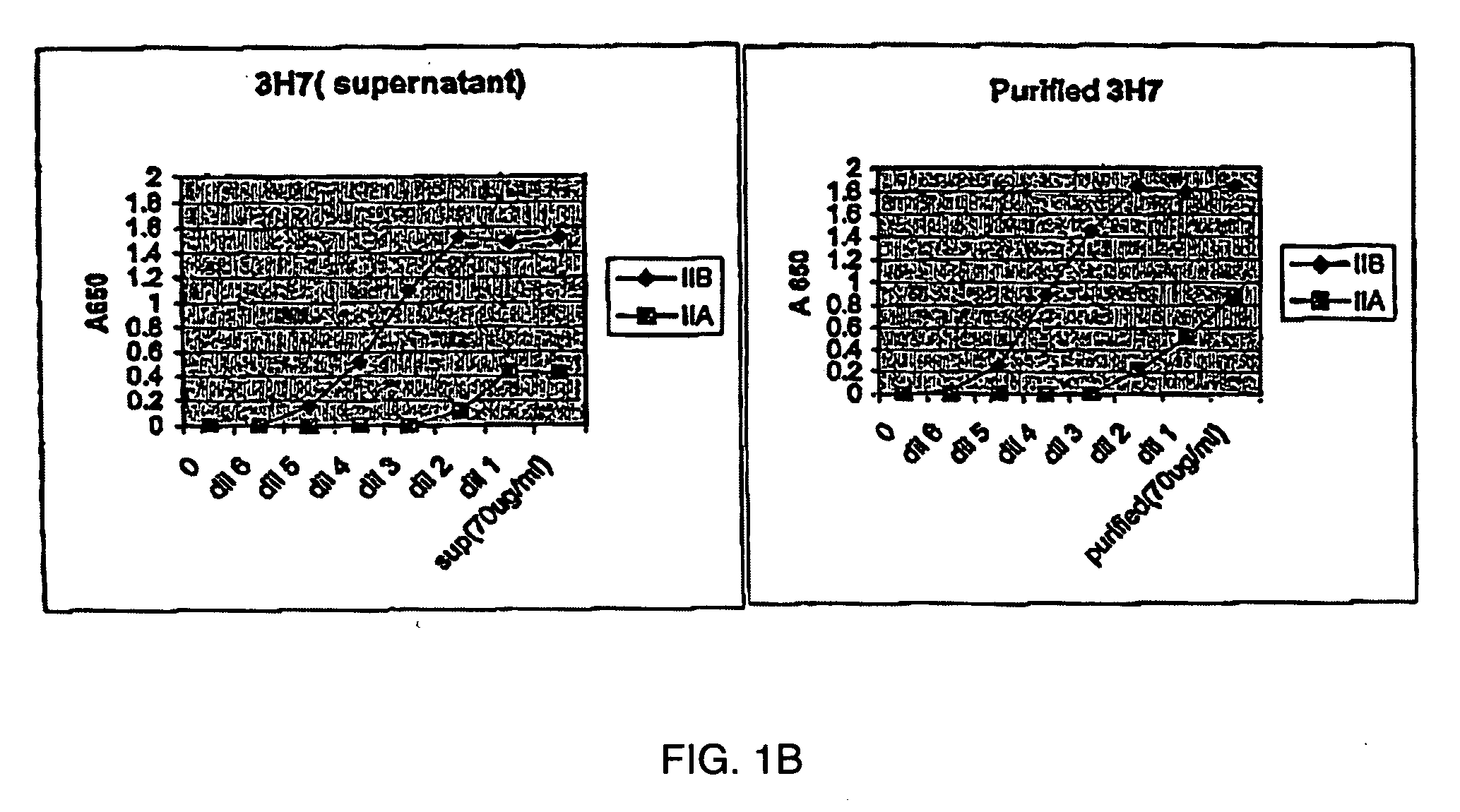
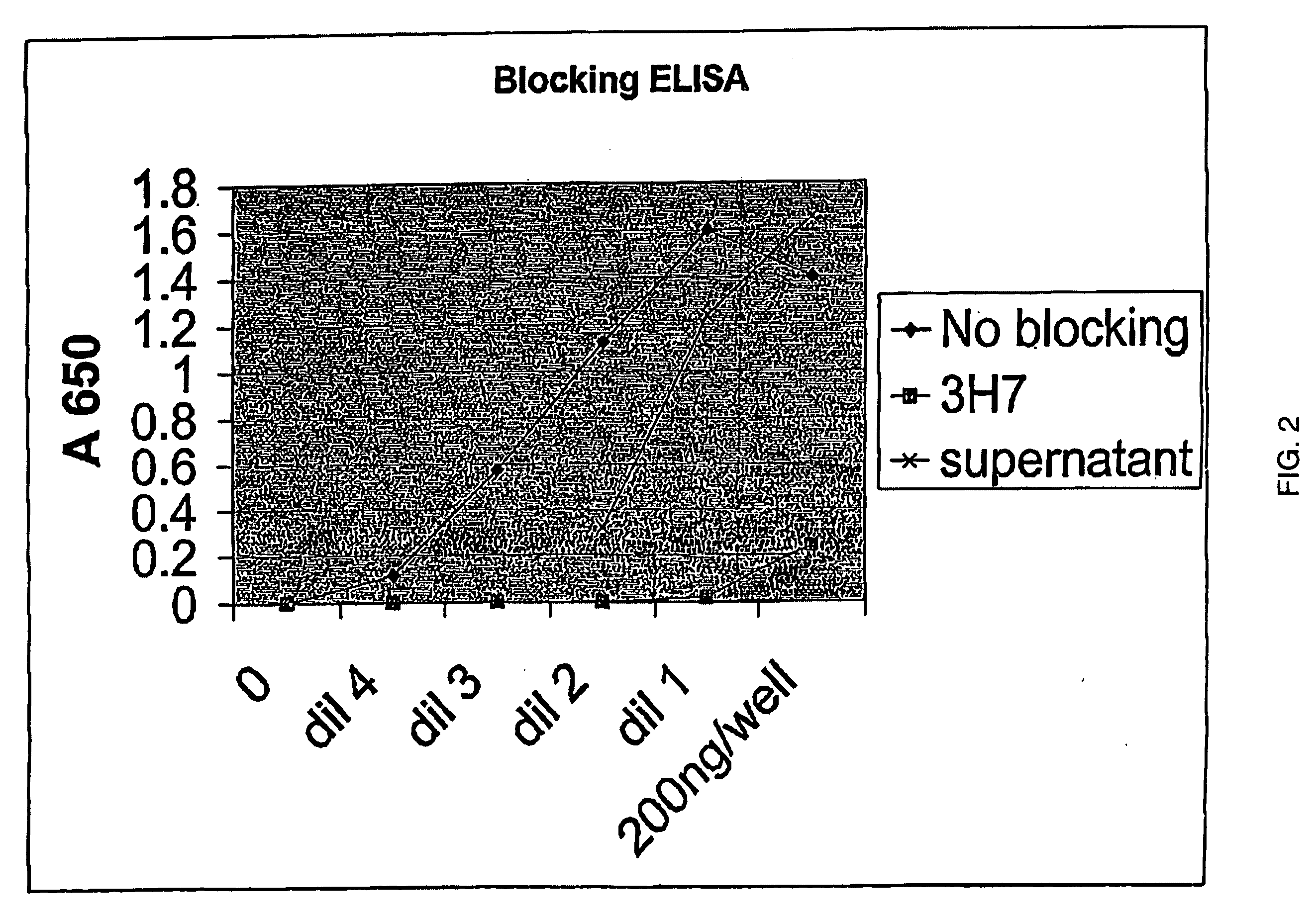
FcgammaRIIB-specific antibodies and methods of use thereof
ActiveUS20040185045A1Strong therapeutic activityEnhancing antibody-mediated effector functionSenses disorderAntipyreticTherapeutic antibodyTreatment effect
The present invention relates to antibodies or fragments thereof that specifically bind FcgammaRIIB, particularly human FcgammaRIIB, with greater affinity than said antibodies or fragments thereof bind FcgammaRIIA, particularly human FcgammaRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
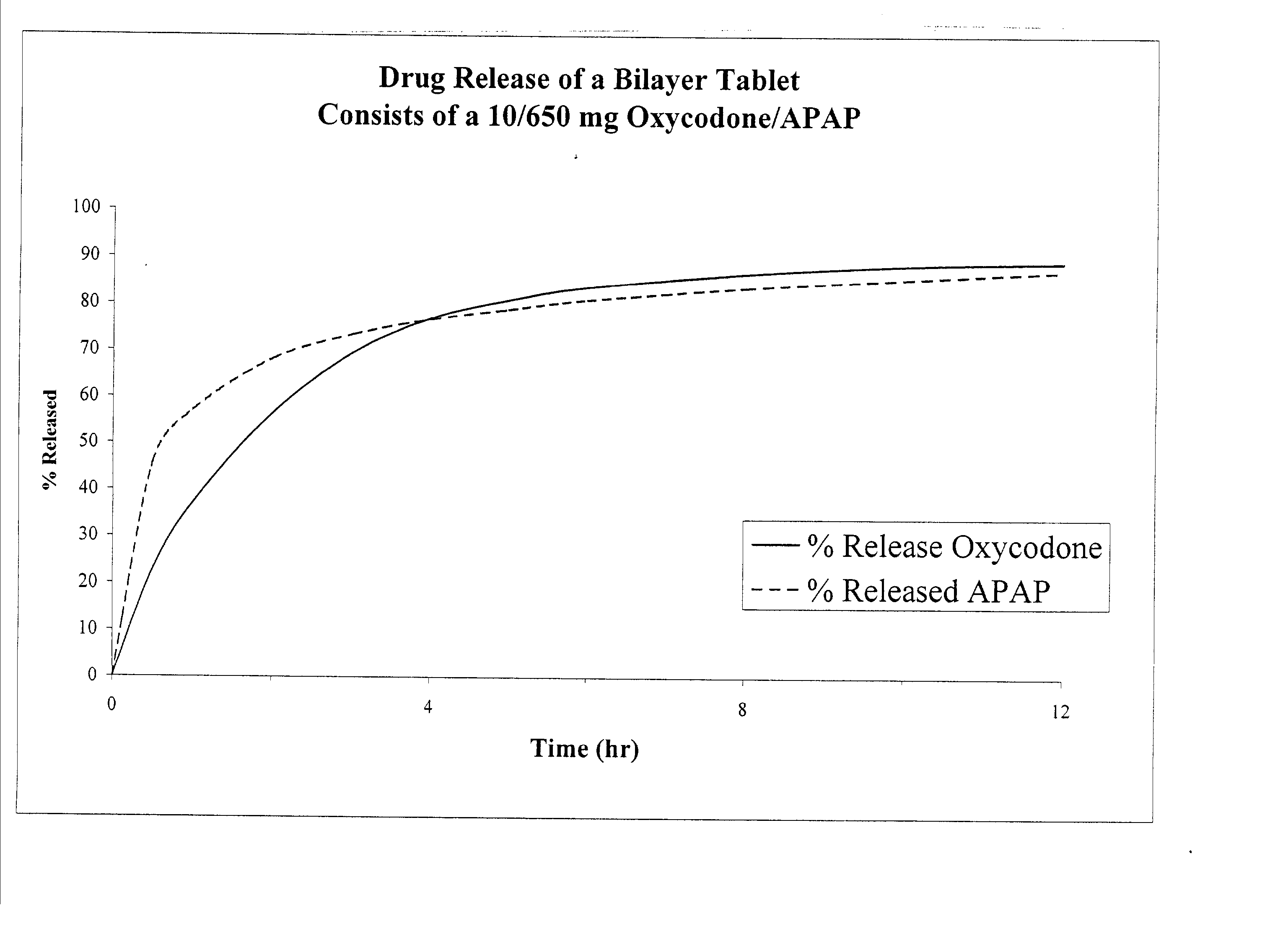
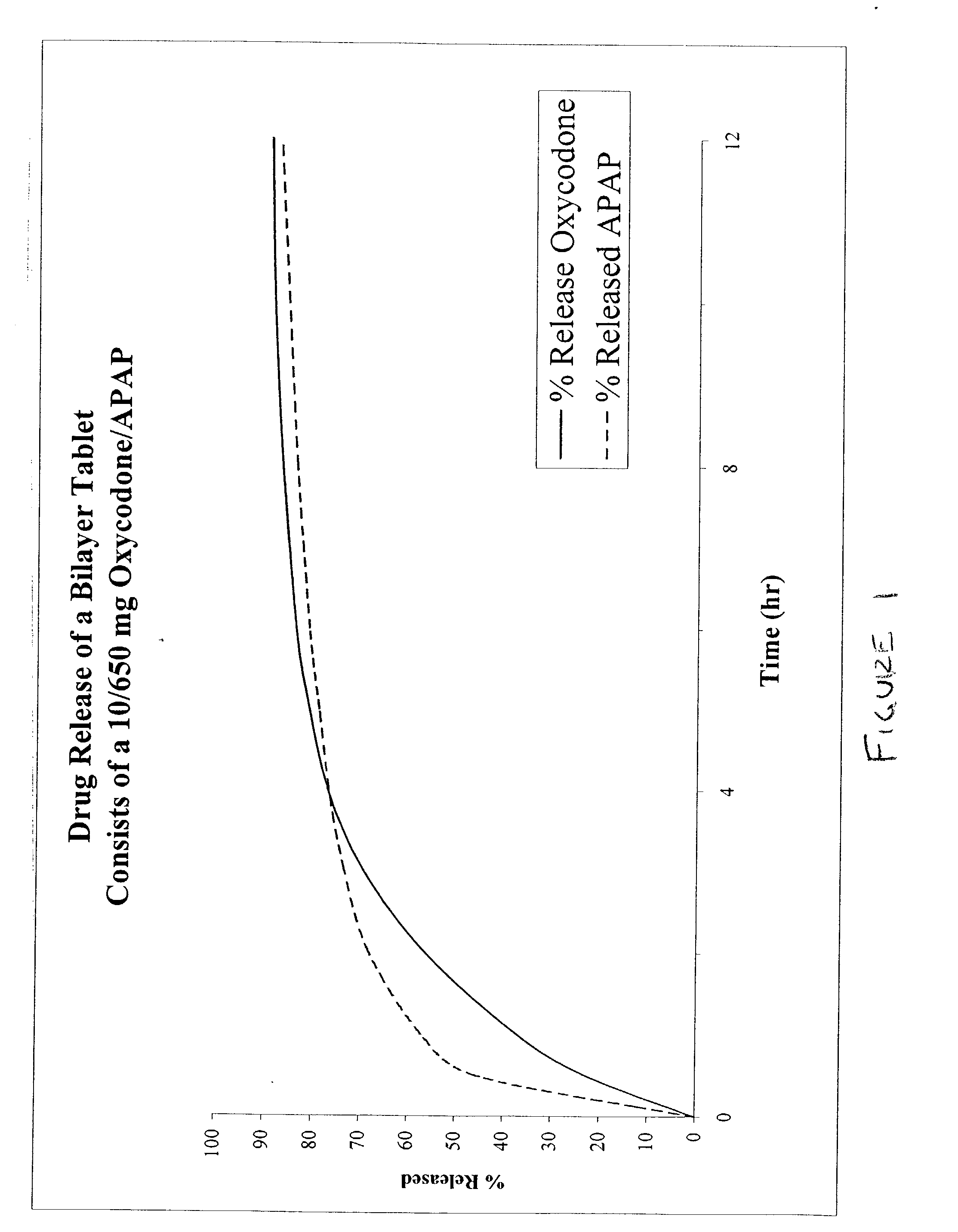
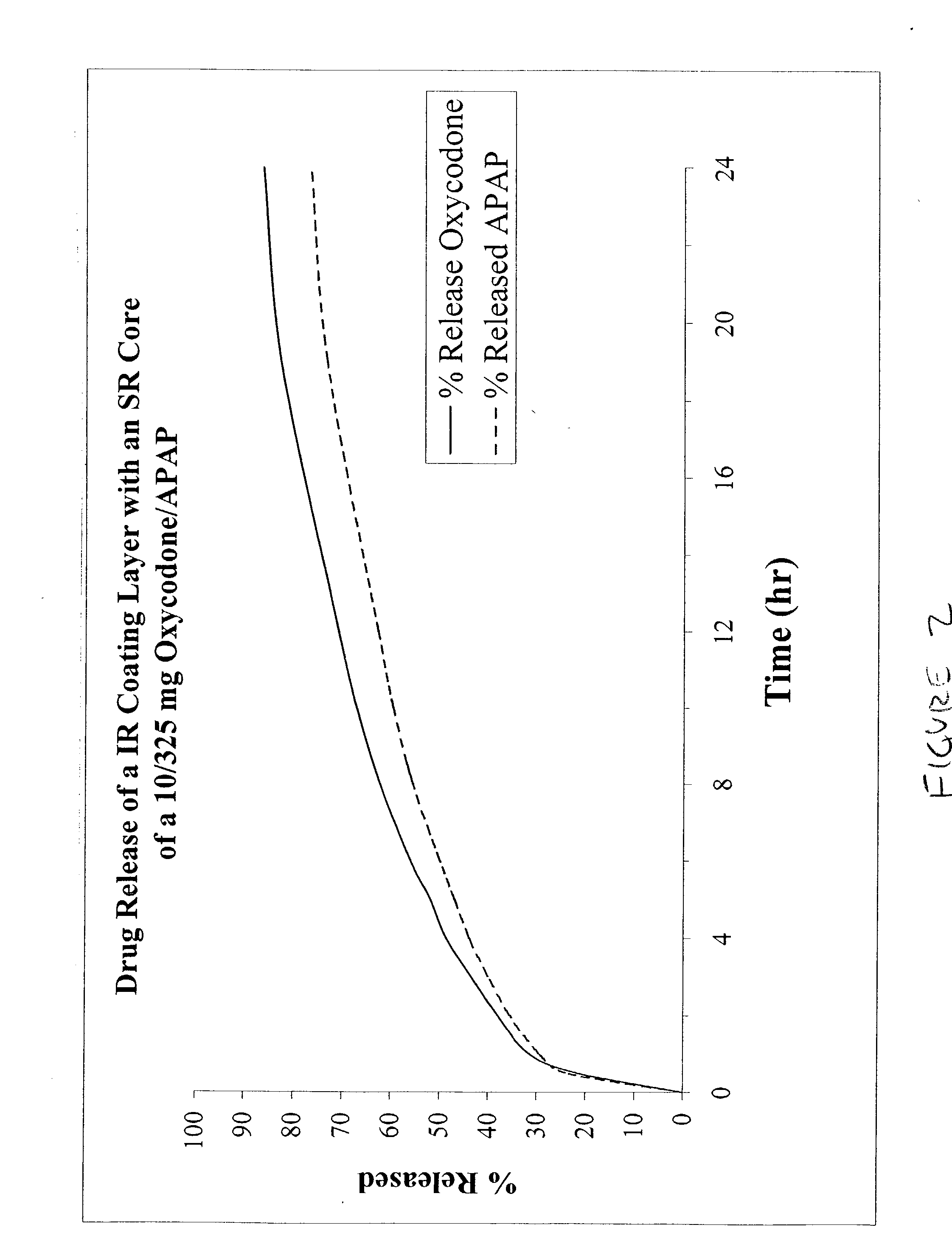
Combination sustained release-immediate release oral dosage forms with an opioid analgesic and a non-opioid analgesic
InactiveUS20030092724A1Long durationConstant plasma levels of opioid and non-opioid analgesicsBiocidePill deliveryImmediate releaseTherapeutic effect
The present invention relates to new and useful oral tablet compositions which include an immediate release portion having an opioid analgesic and a non-opioid analgesic, providing for a rapid onset of therapeutic effect, and a sustained release portion of an opioid analgesic and a non-opioid analgesic, providing for a relatively longer duration of therapeutic effect. A multilayer oral dosage form containing a sustained release layer, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release layer containing the same active ingredients as the sustained release layer, is also disclosed. Also disclosed are oral tablet compositions, containing a sustained release core, which includes oxycodone and APAP, hydrocodone and APAP, or oxymorphone and APAP, and an immediate release coating containing the same active ingredients as the sustained release core, are also disclosed. In addition, methods of making and using such oral tablet compositions are disclosed.
Owner:ENDO PHARMA INC
Apparatus and methods for the treatment of avian influenza with ultrasound
InactiveUS20070219481A1Increase blood flowBoosts immune systemUltrasound therapySurgeryConventional ultrasoundIncreased blood flow
The method and device of the present invention for the treatment of avian influenza and other viruses, bacteria, and infectious agents with ultrasonic waves includes a generator and a transducer to produce ultrasonic waves. The ultrasonic transducer has a specially designed ultrasound tip that is able to radiate ultrasound energy toward the body of the target animal or human at a level higher than a traditional ultrasound tip. The apparatus delivers ultrasonic energy without contacting the target through an air / gas medium or through a spray, or by contacting the target with or without a coupling medium. The ultrasonic waves provide a therapeutic effect such as destroying viruses, bacteria, or other infectious agents, increasing blood flow, or stimulating the immune system, etc; additionally, breathing ability is enhanced through the ultrasonic delivery of a medicament to the target's lungs.
Owner:BABAEV EILAZ
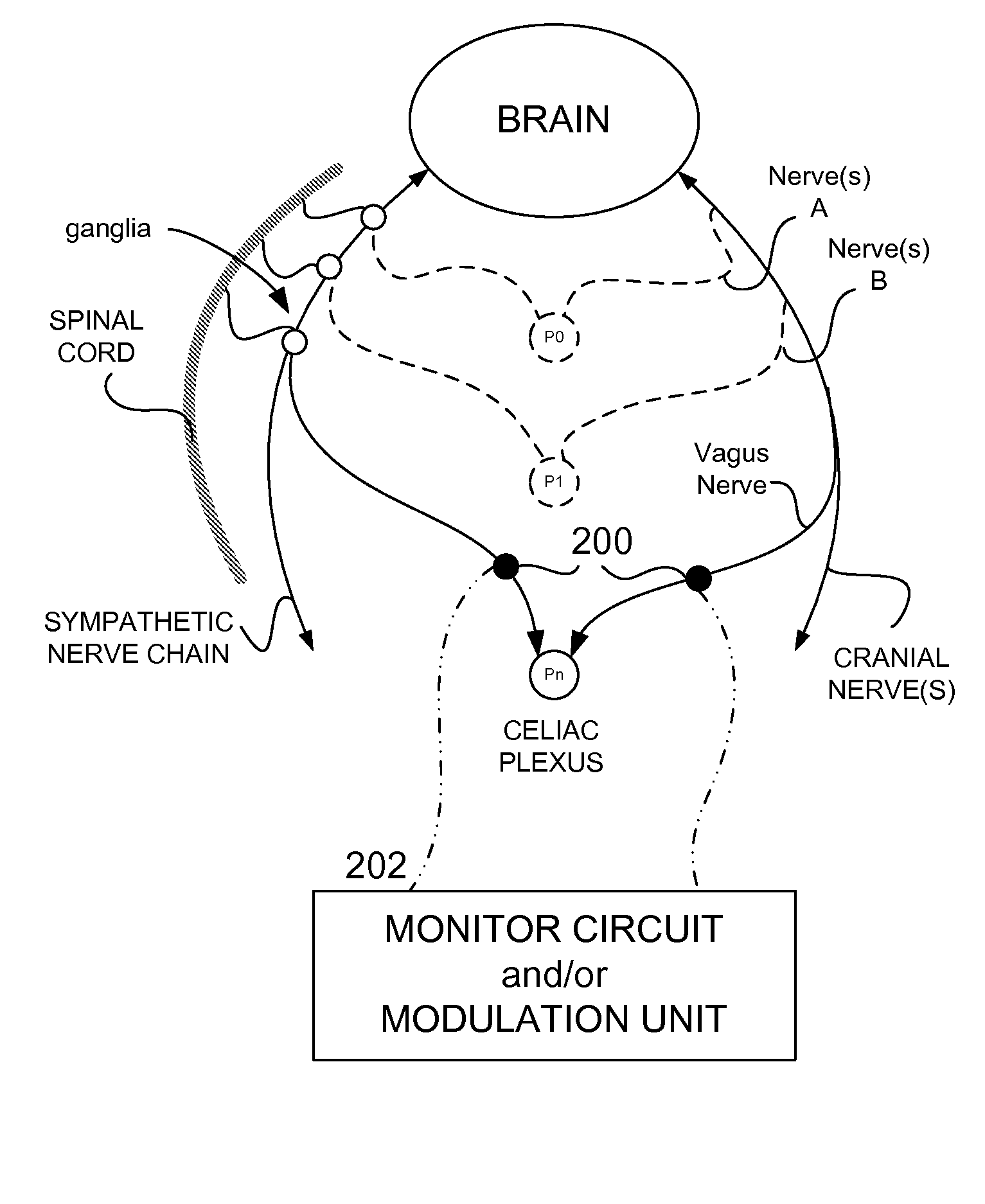
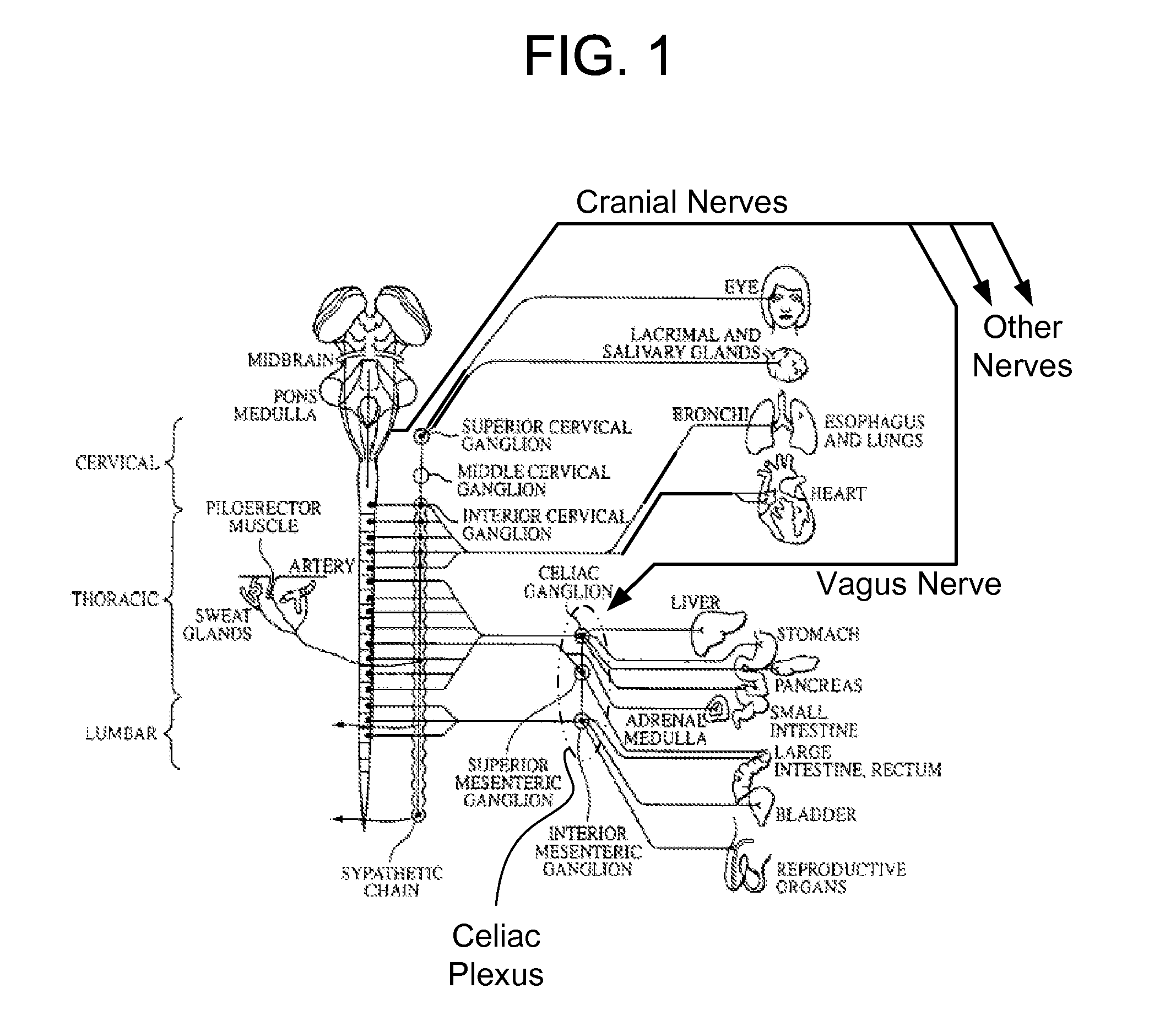
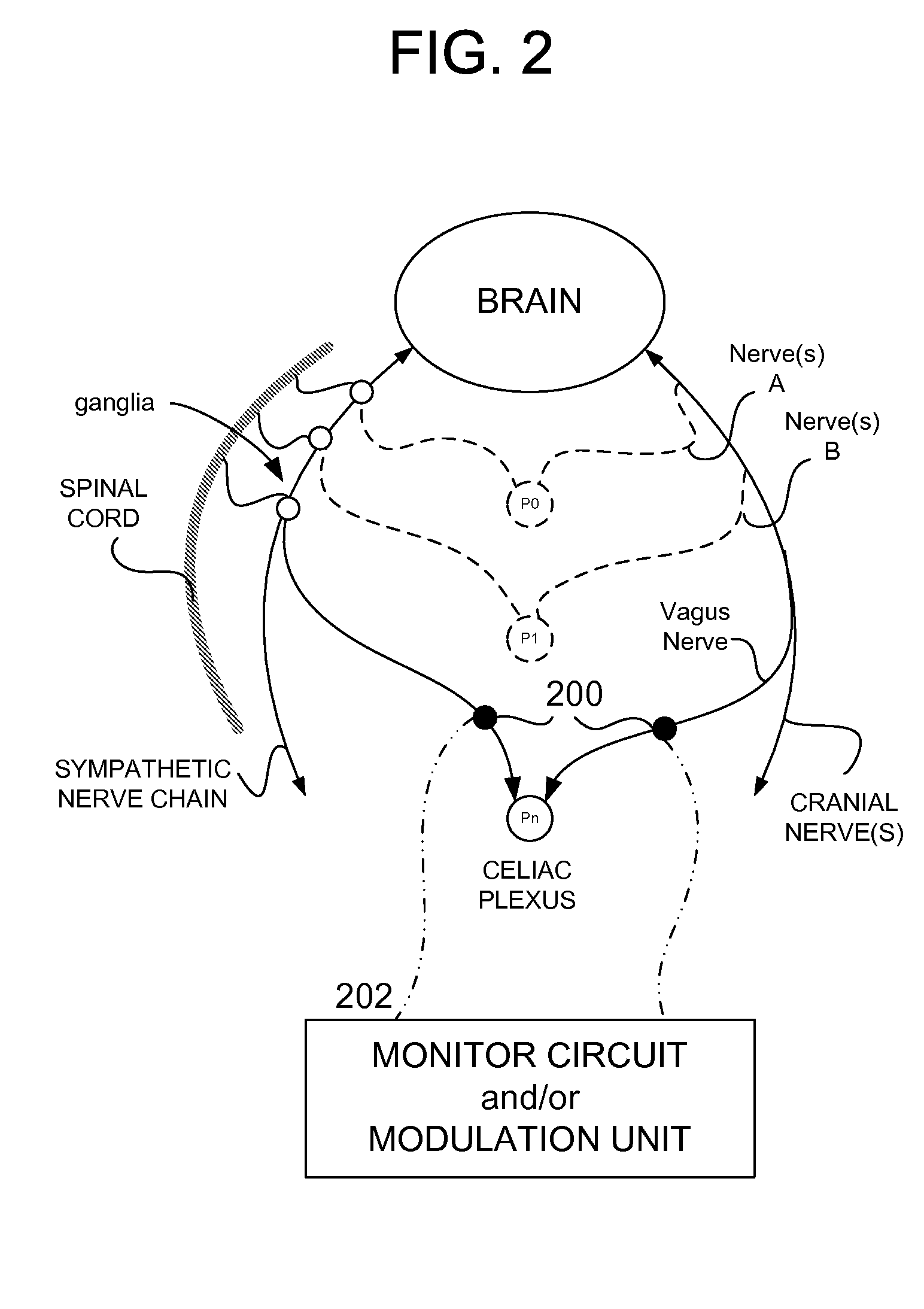
Methods And Apparatus For Treating Disorders Through Neurological And/Or Muscular Intervention
Methods and apparatus proved for: simultaneously monitoring at least one of nerve and muscle electrical activity on respective sides of a target plexus of a patient; identifying desired electrical activity of at least one of nerves and muscles on both sides of the target plexus based on the monitored activity; and modulating the electrical activity of the at least one of nerves and muscles on both sides of the target plexus to achieve a therapeutic result.
Owner:ELECTROCORE
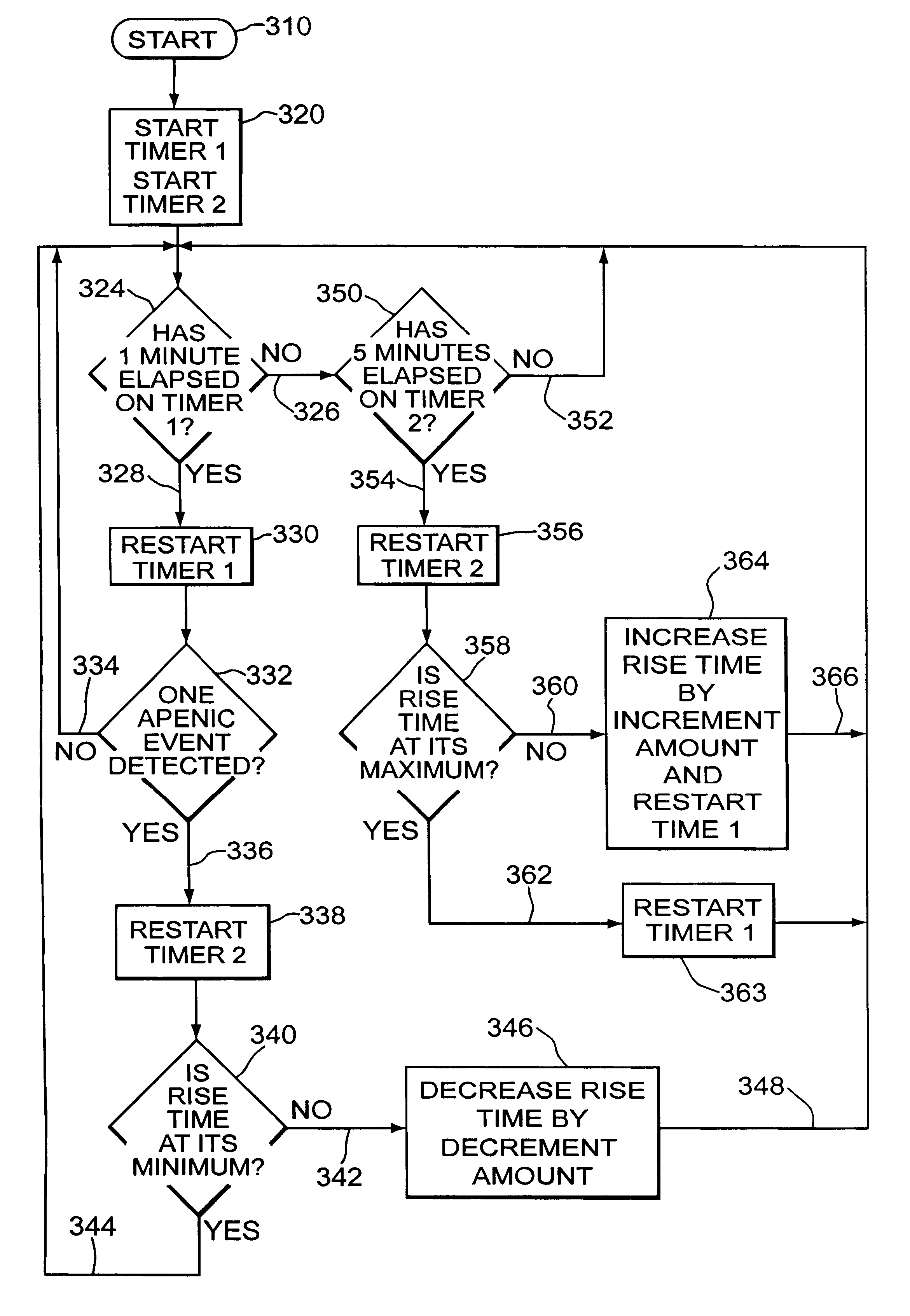
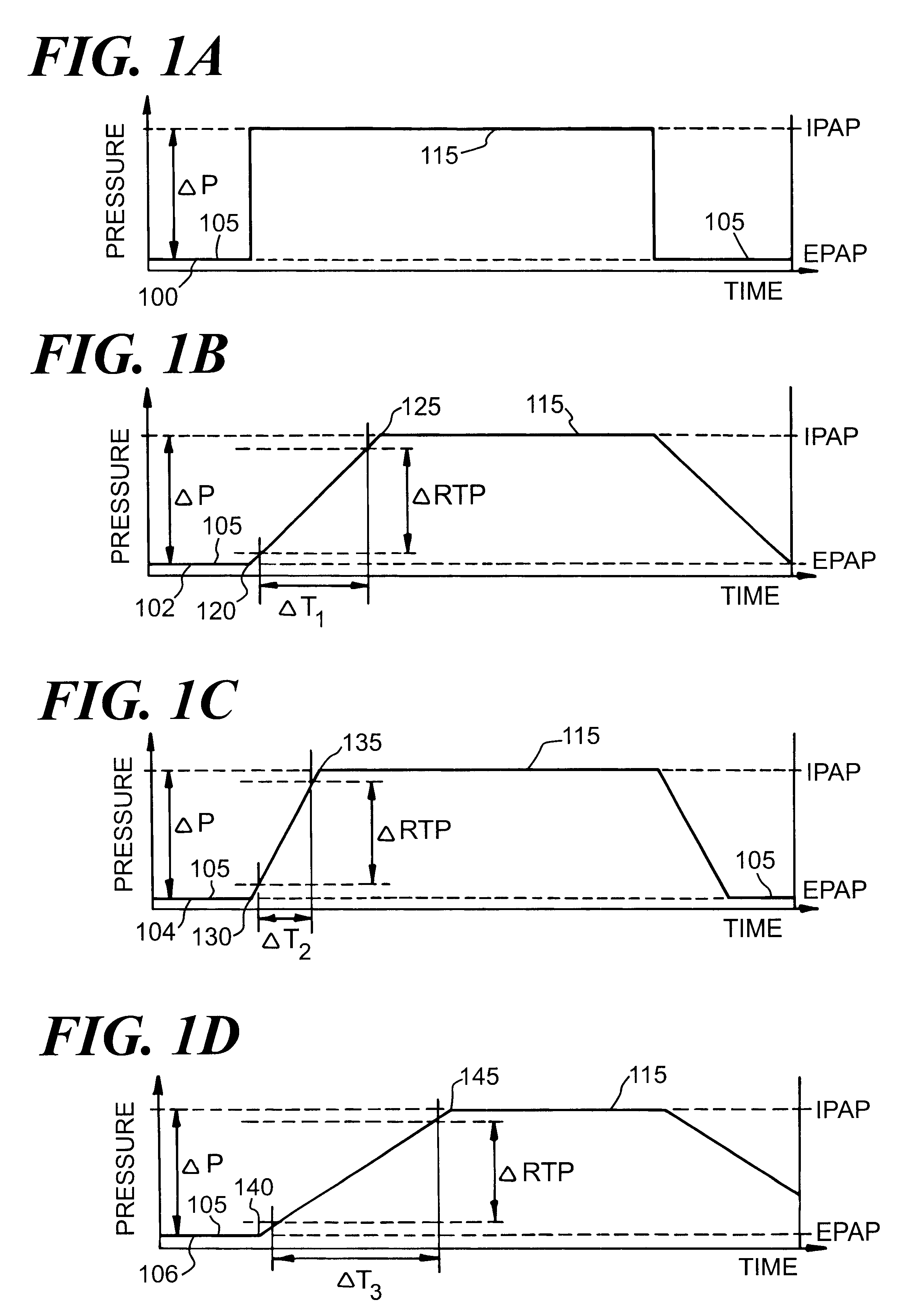
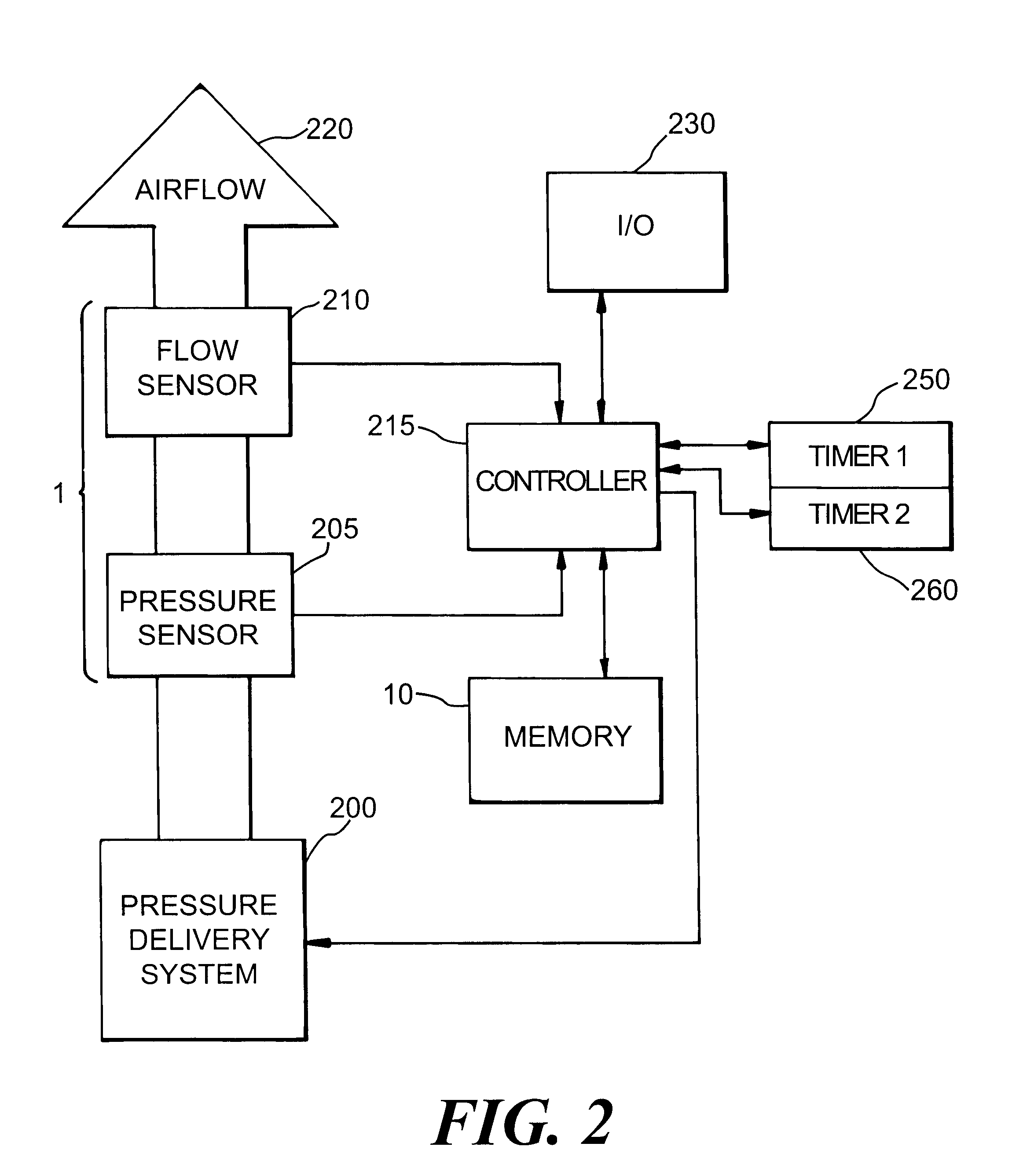
Automatic rise time adjustment for bi-level pressure support system
InactiveUS6532960B1Operating means/releasing devices for valvesRespiratory masksNon-invasive ventilationTreatment effect
An apparatus and method for a bi-level positive airway pressure support in which the rise time from the expiratory positive airway pressure to the inspiratory positive airway pressure is automatically controlled by the pressure support system. The pressure support system includes a sensor, a control system, and a pressure generating system. The sensor monitors the patient's respiration to detect respiratory events, such as an apnea, hyponea or other disturbance, and the control system responds to the sensor information to adjust the rise time from the expiratory positive airway pressure to the inspiratory positive airway pressure gas pressure to maximize patient comfort and pressure support treatment effectiveness.
Owner:RIC INVESTMENTS LLC
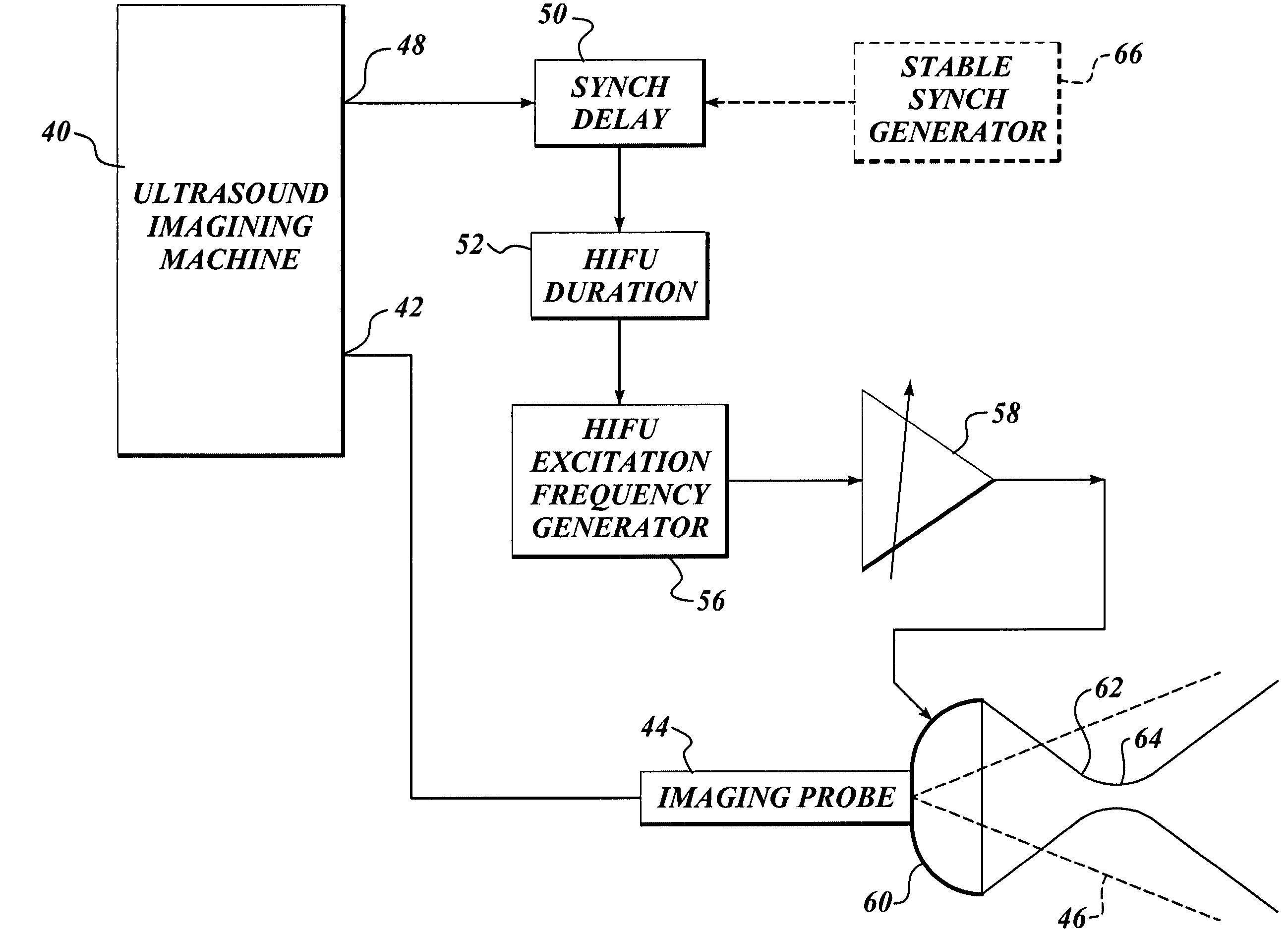
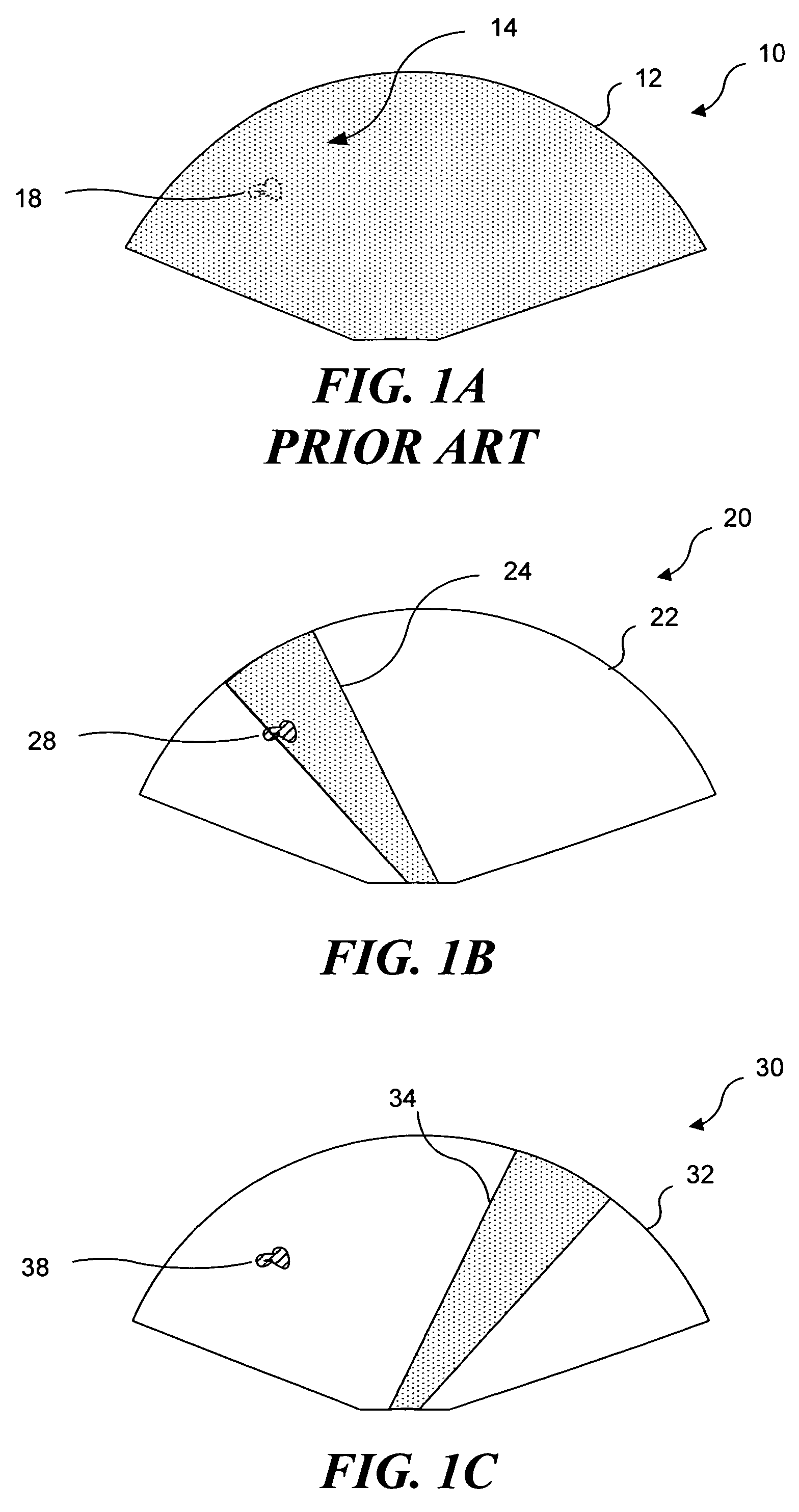
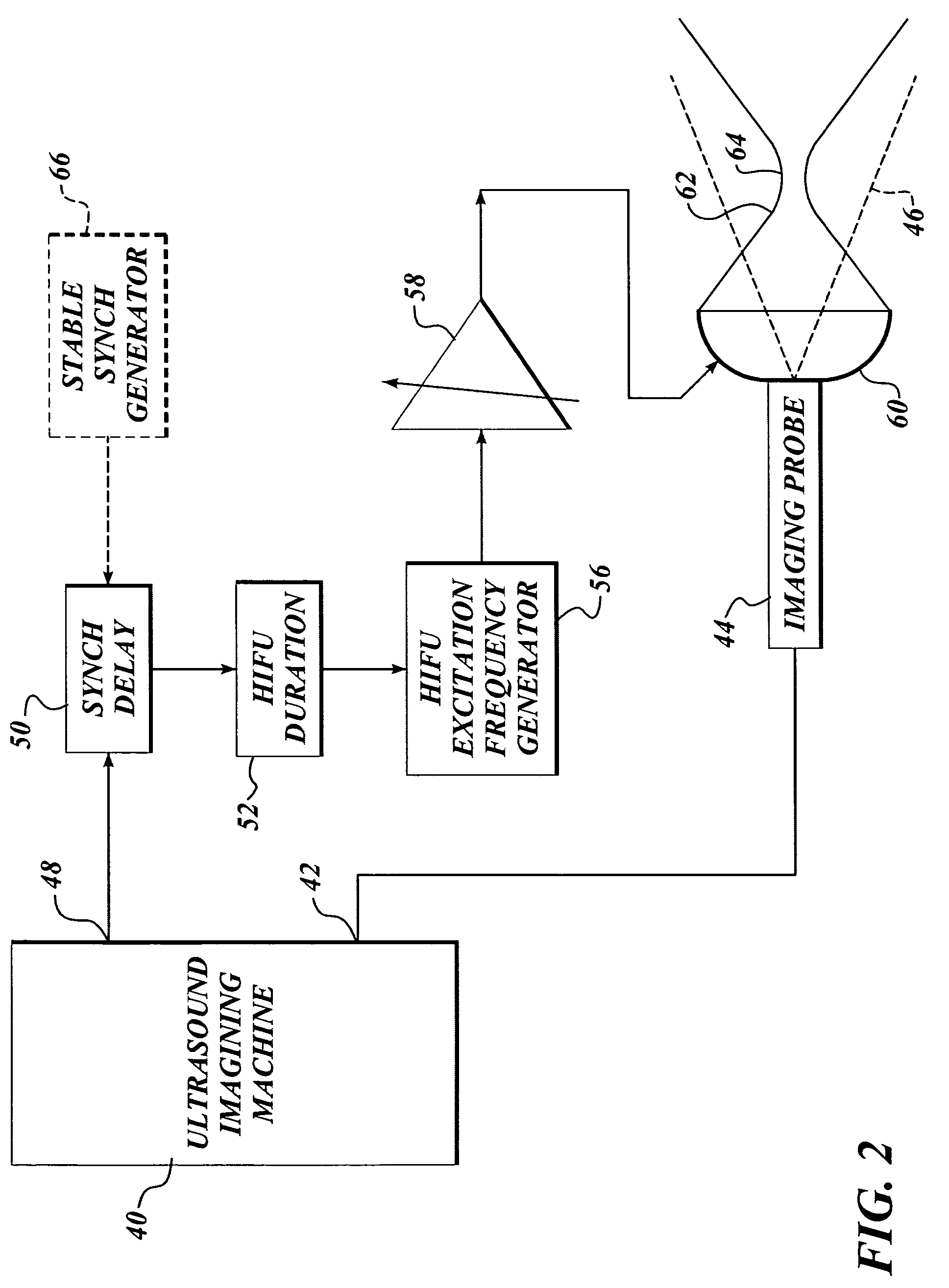
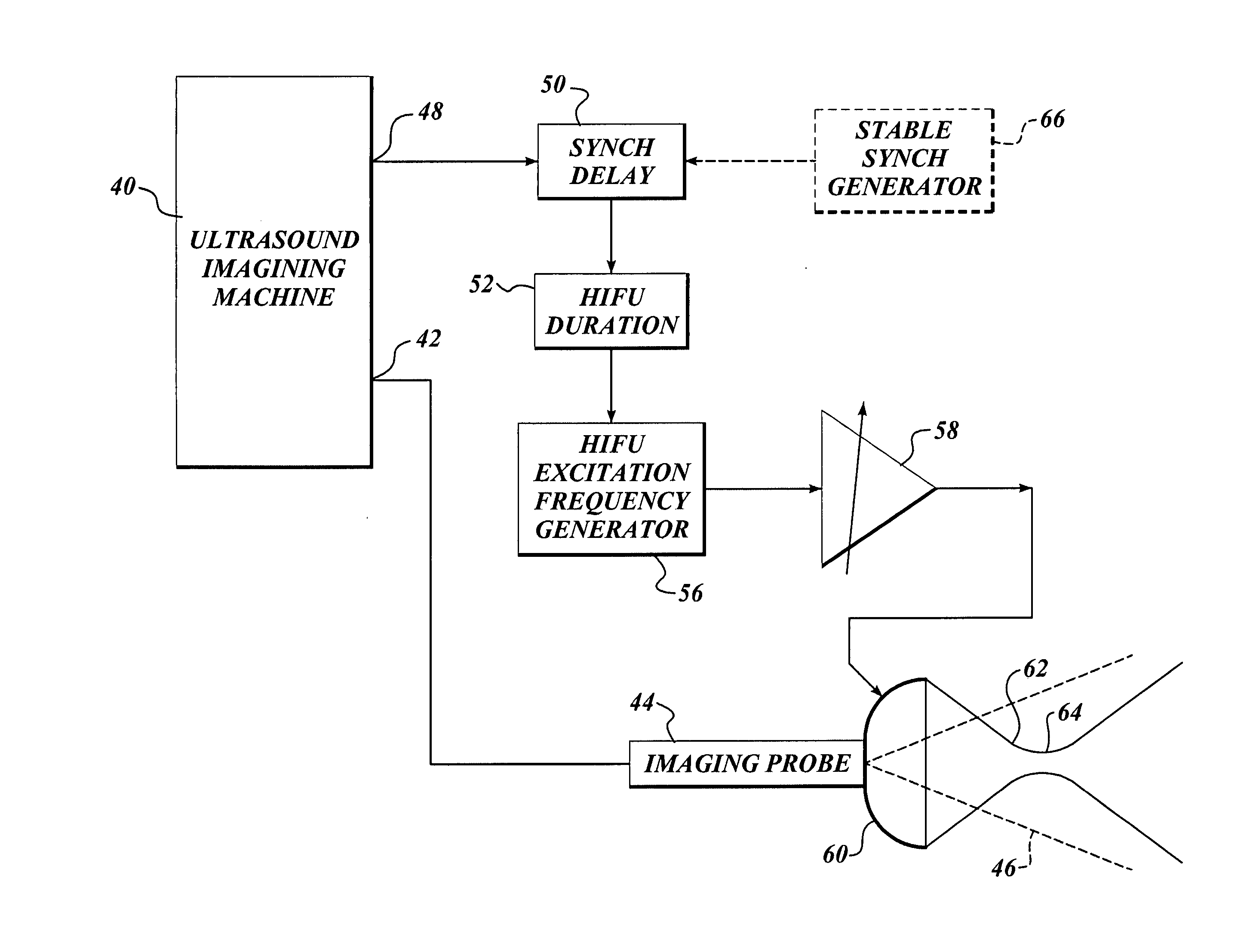
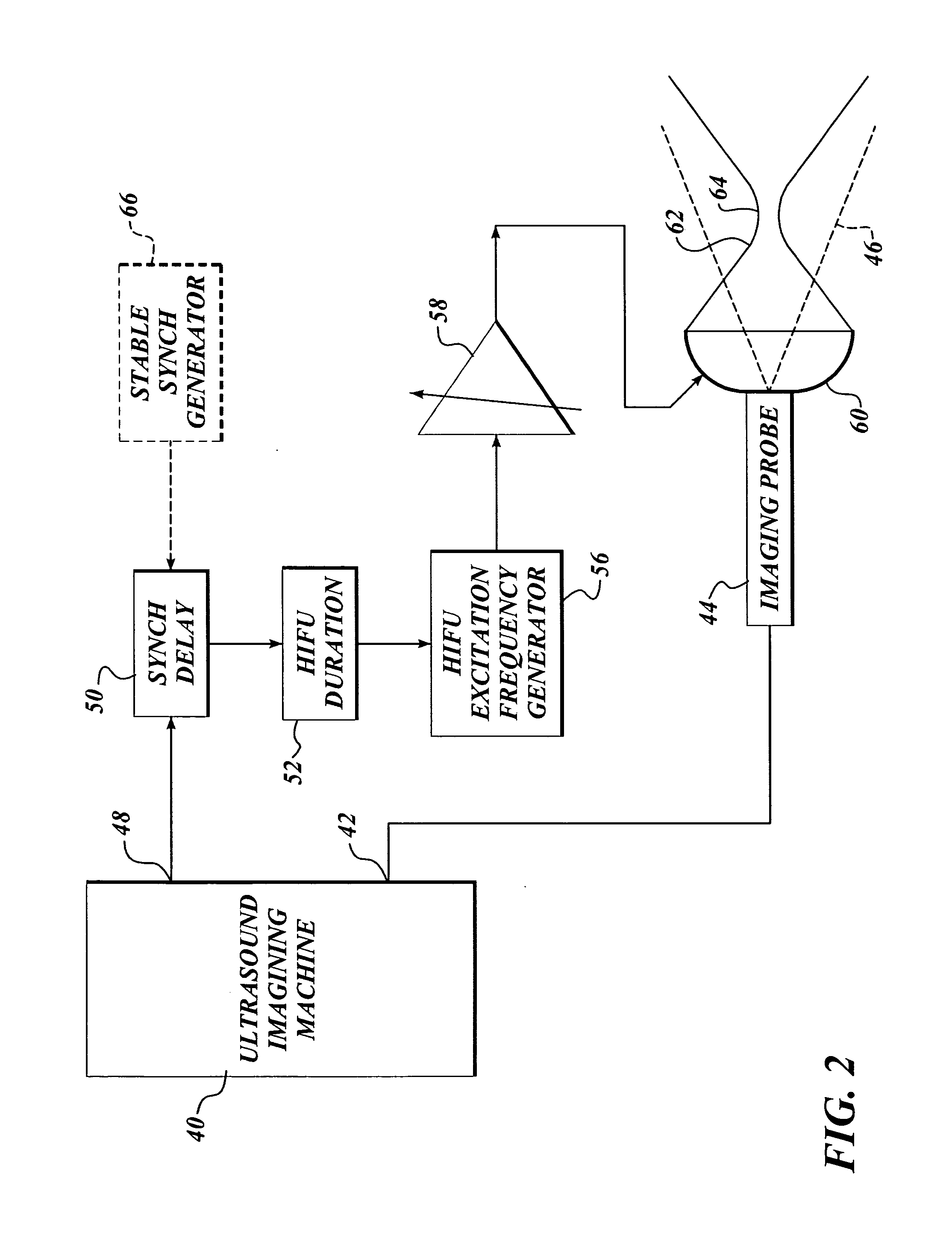
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS7510536B2Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesSonificationHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
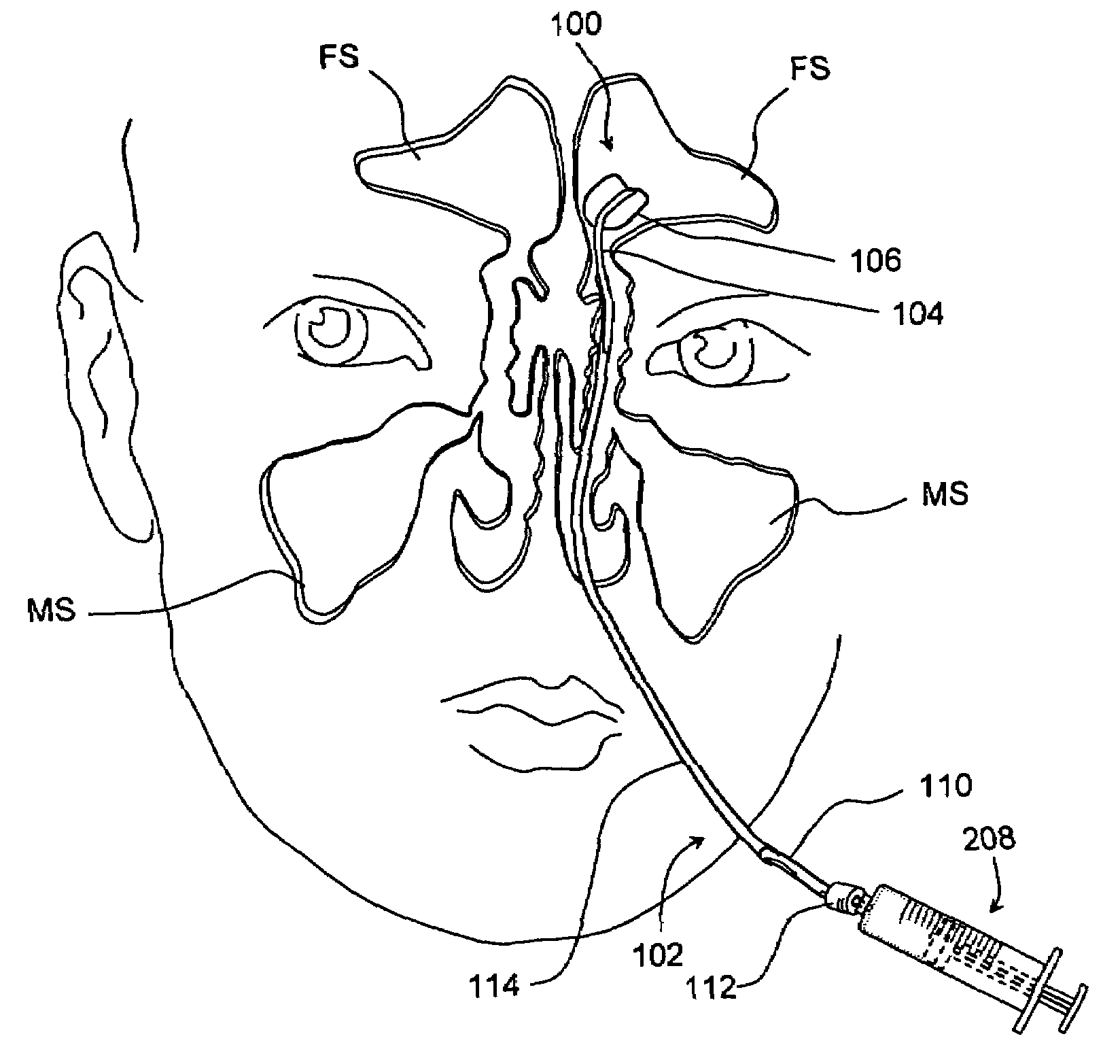
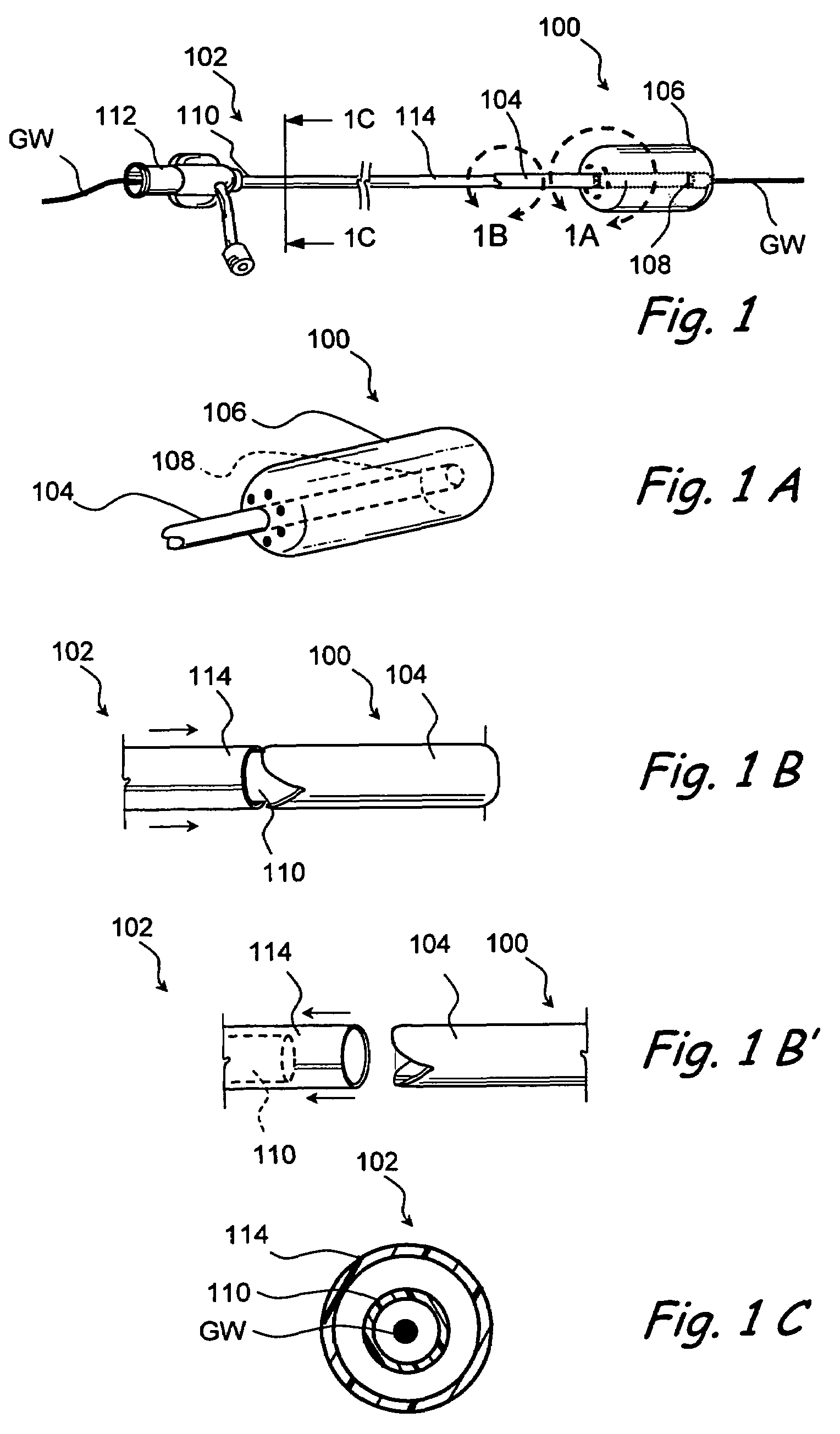
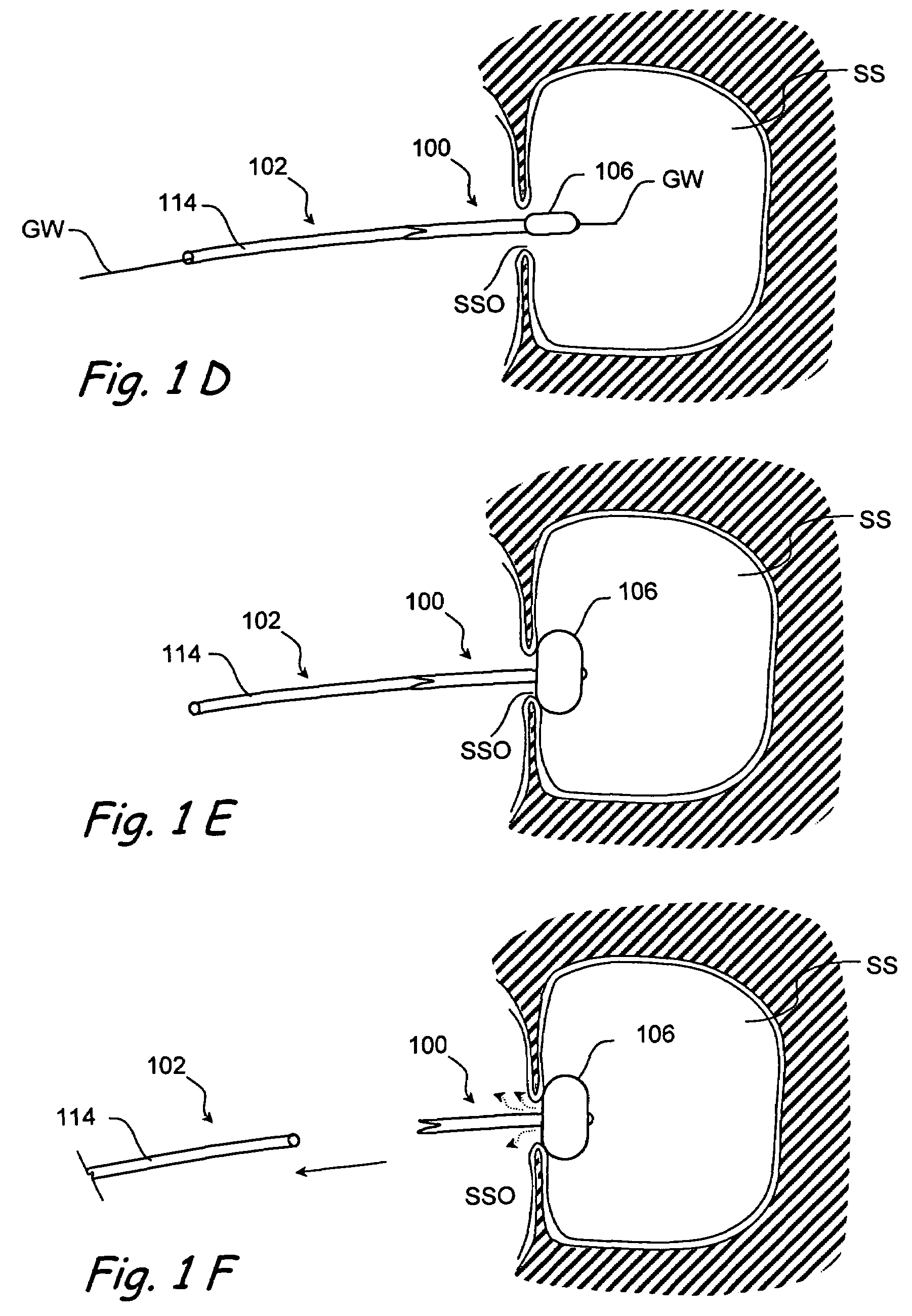
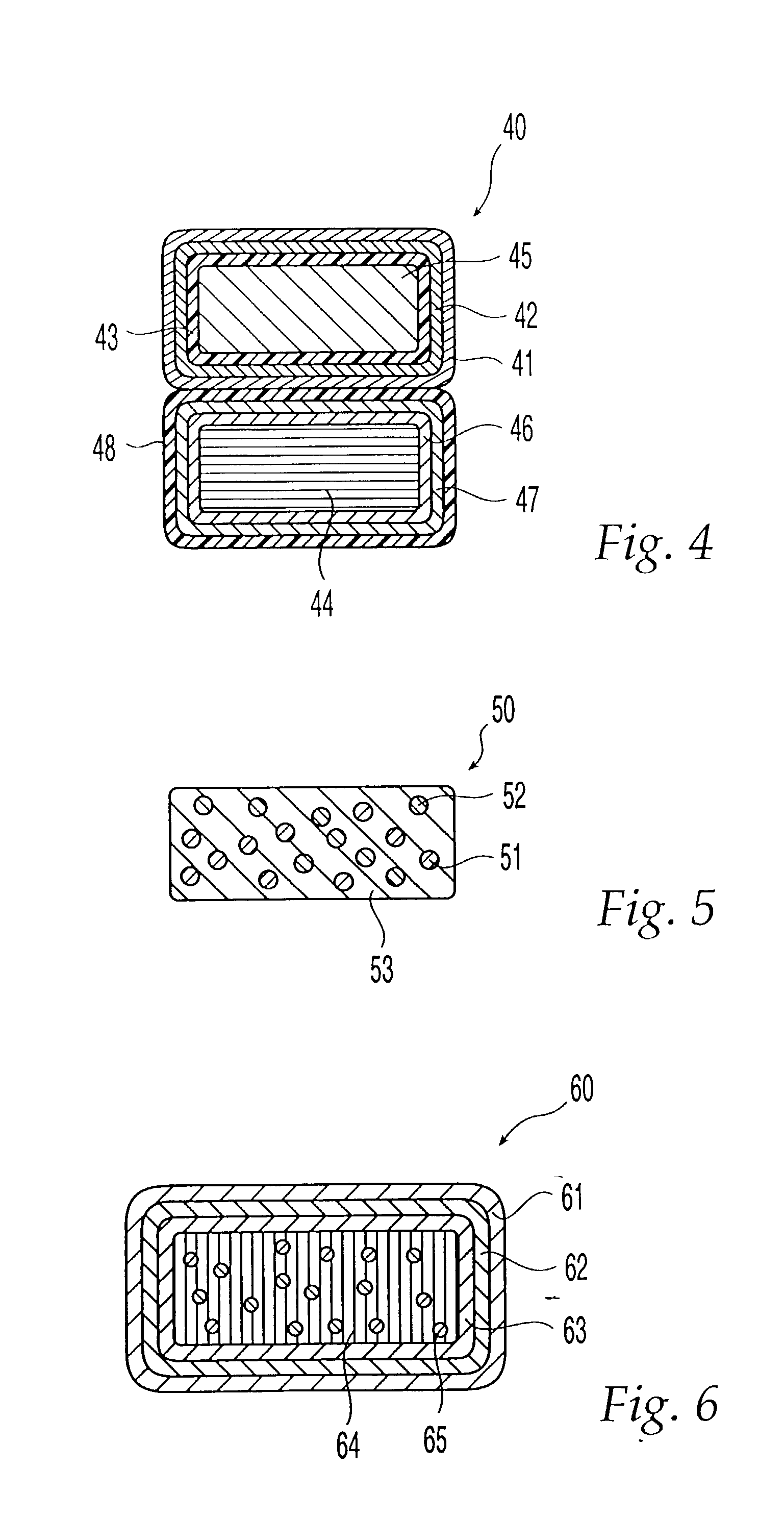
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
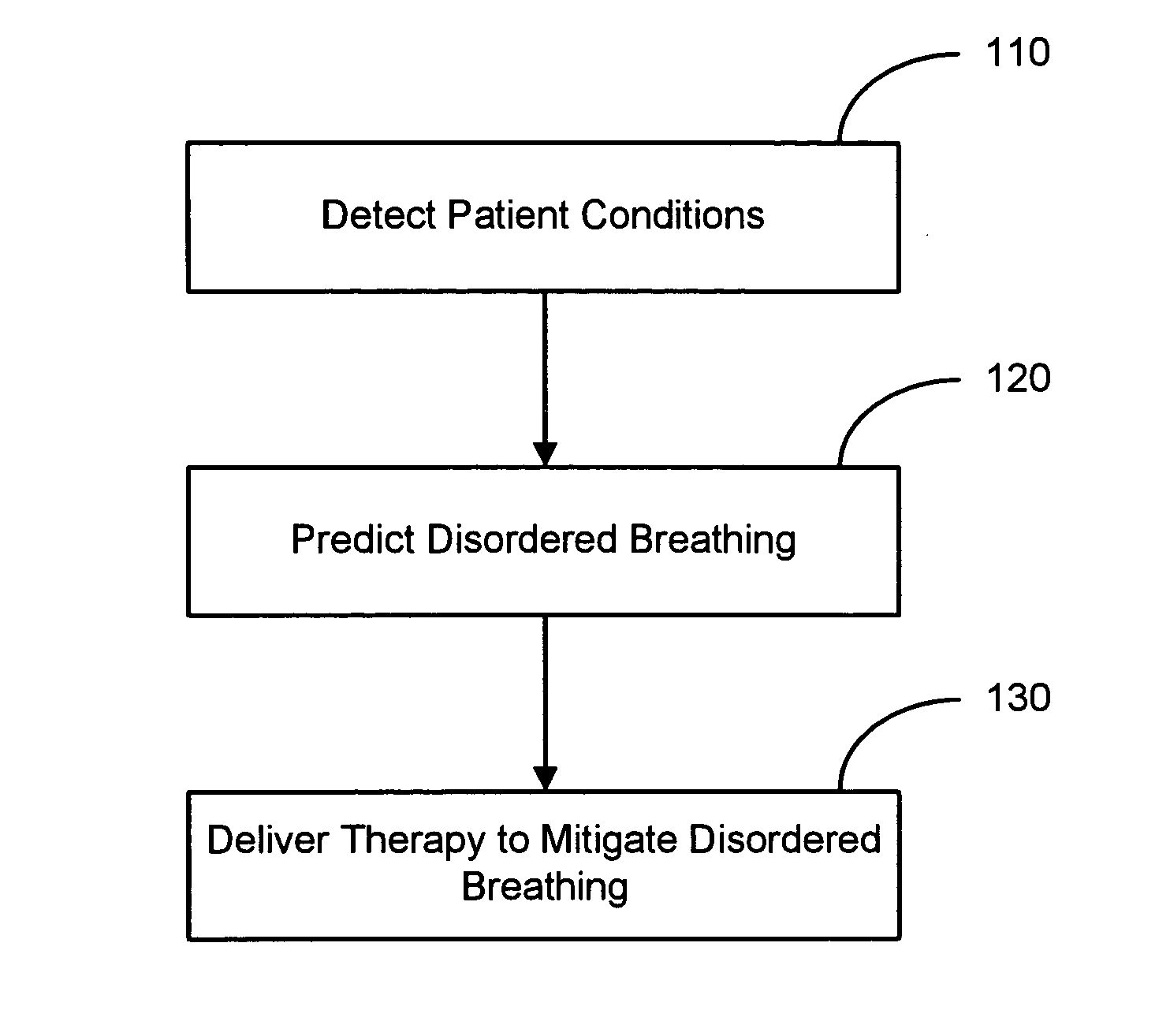
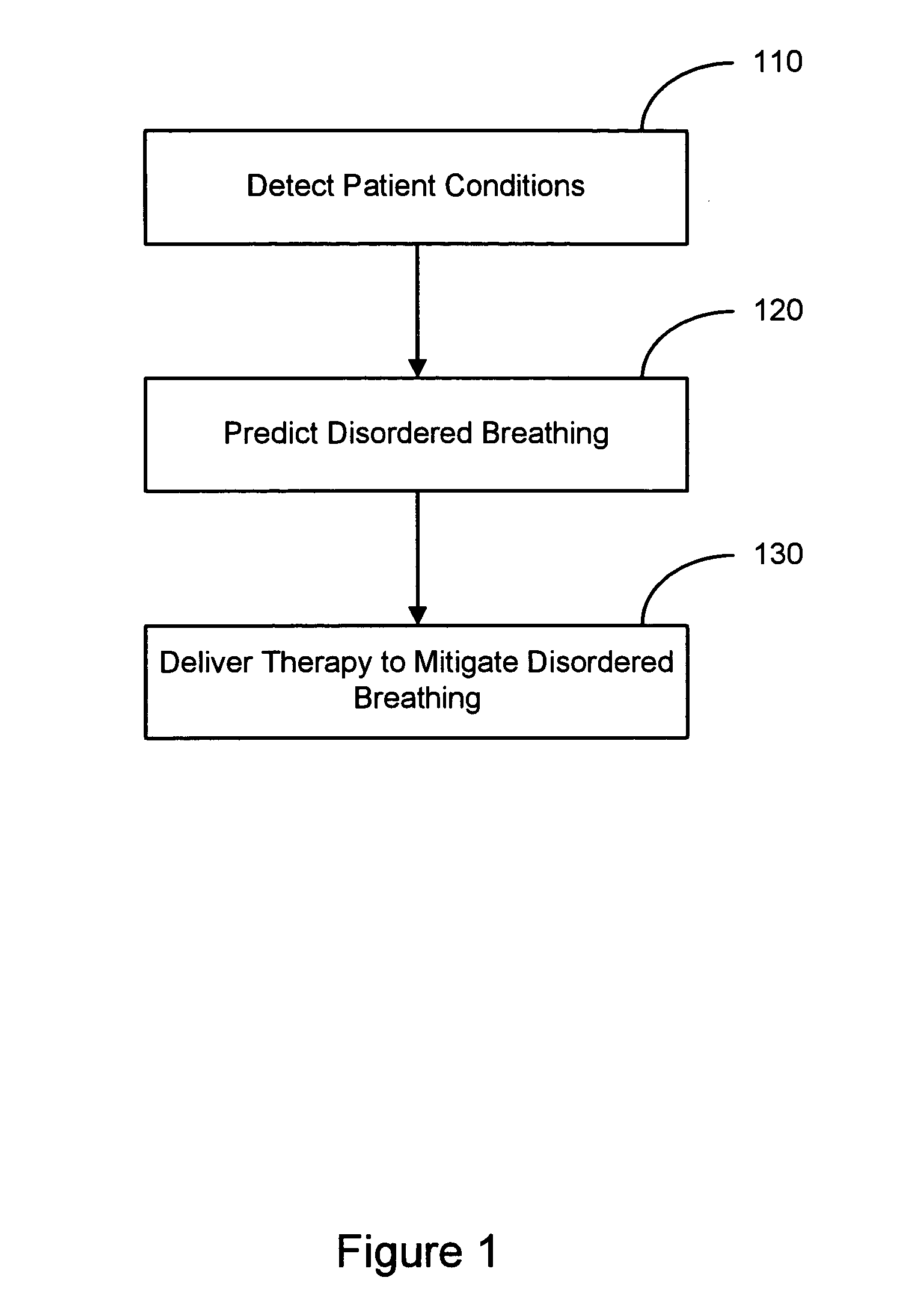
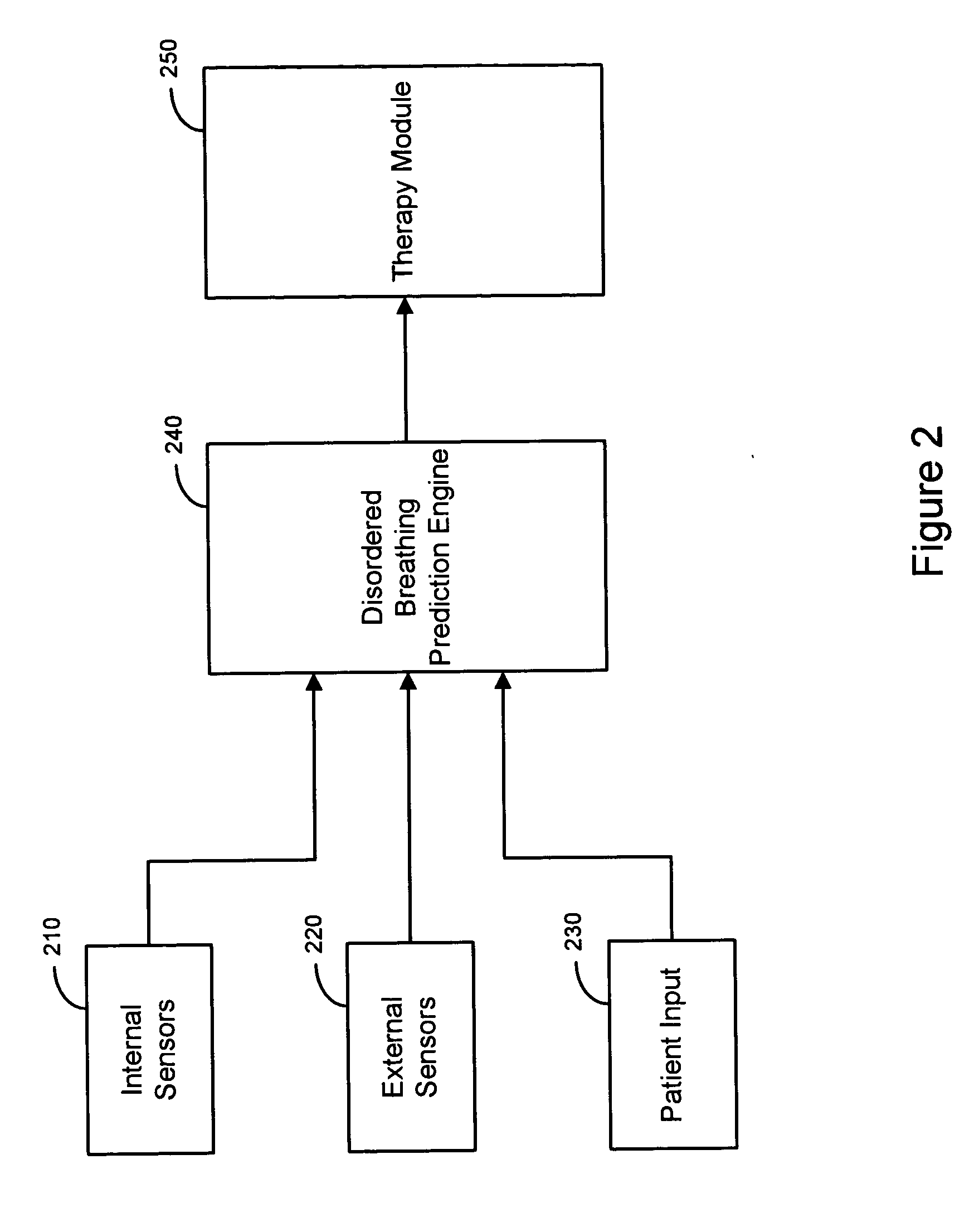
Therapy triggered by prediction of disordered breathing
ActiveUS20050043772A1Mitigate predicted disorderedHeart stimulatorsBreathing disturbancesTherapeutic effect
An approach to providing disordered breathing therapy includes providing therapy based on a prediction of disordered breathing. One or more patient conditions are detected and used to predict disordered breathing. Therapy is delivered to mitigate the predicted disordered breathing. The disordered breathing therapy may be adapted to enhance therapy efficacy and / or to reduce the impact of the therapy to the patient.
Owner:CARDIAC PACEMAKERS INC
Ultrasound guided high intensity focused ultrasound treatment of nerves
InactiveUS20050240126A1Relieve painEasy procedureUltrasound therapyBlood flow measurement devicesAbnormal tissue growthHigh doses
A method for using high intensity focused ultrasound (HIFU) to treat neurological structures to achieve a desired therapeutic affect. Depending on the dosage of HIFU applied, it can have a reversible or irreversible effect on neural structures. For example, a relatively high dose of HIFU can be used to permanently block nerve function, to provide a non-invasive alternative to severing a nerve to treat severe spasticity. Relatively lower doses of HIFU can be used to reversible a block nerve function, to alleviate pain, to achieve an anesthetic effect, or to achieve a cosmetic effect. Where sensory nerves are not necessary for voluntary function, but are involved in pain associated with tumors or bone cancer, HIFU can be used to non-invasively destroy such sensory nerves to alleviate pain without drugs. Preferably, ultrasound imaging synchronized to the HIFU therapy is used to provide real-time ultrasound image guided HIFU therapy of neural structures.
Owner:UNIV OF WASHINGTON
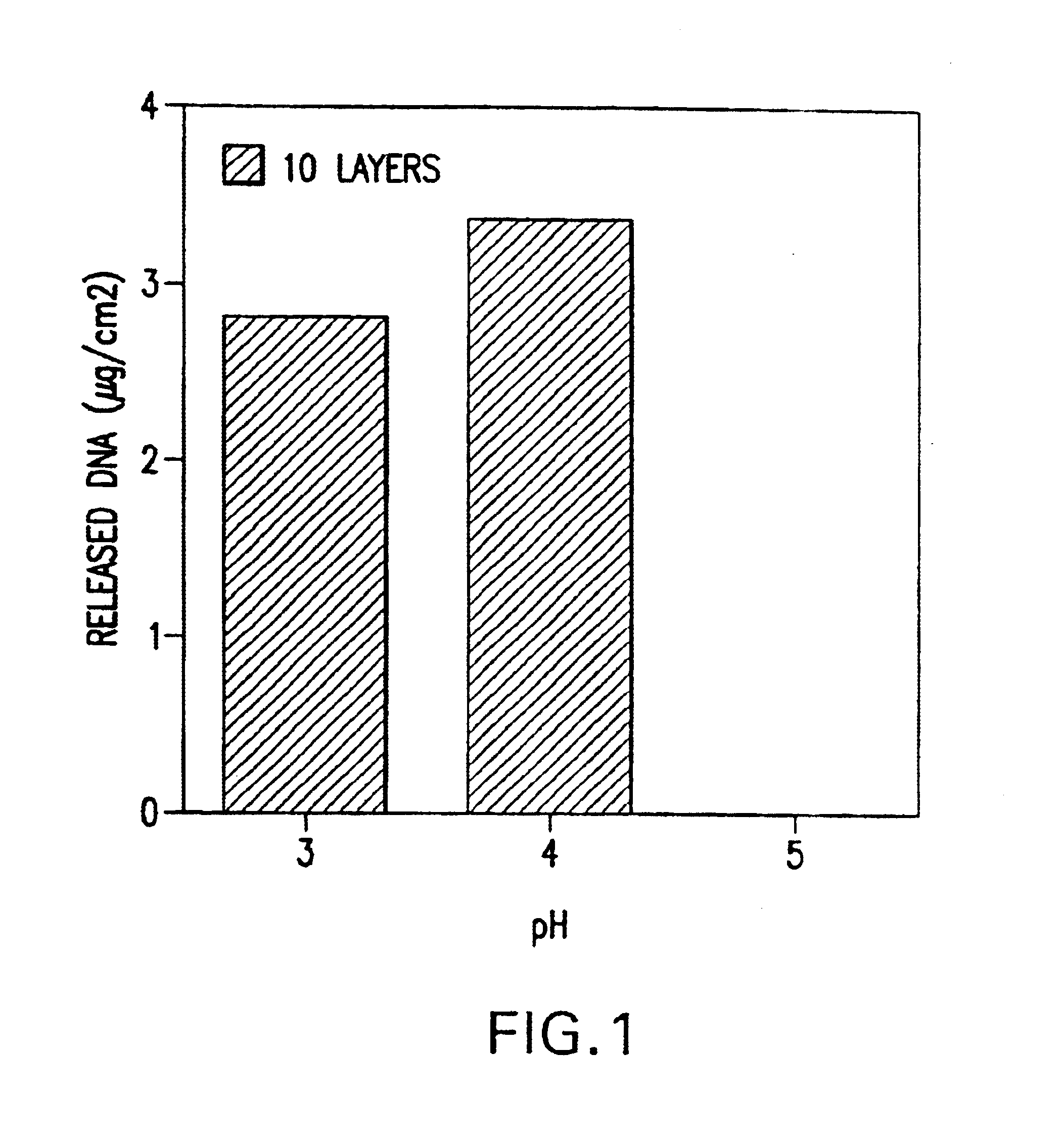
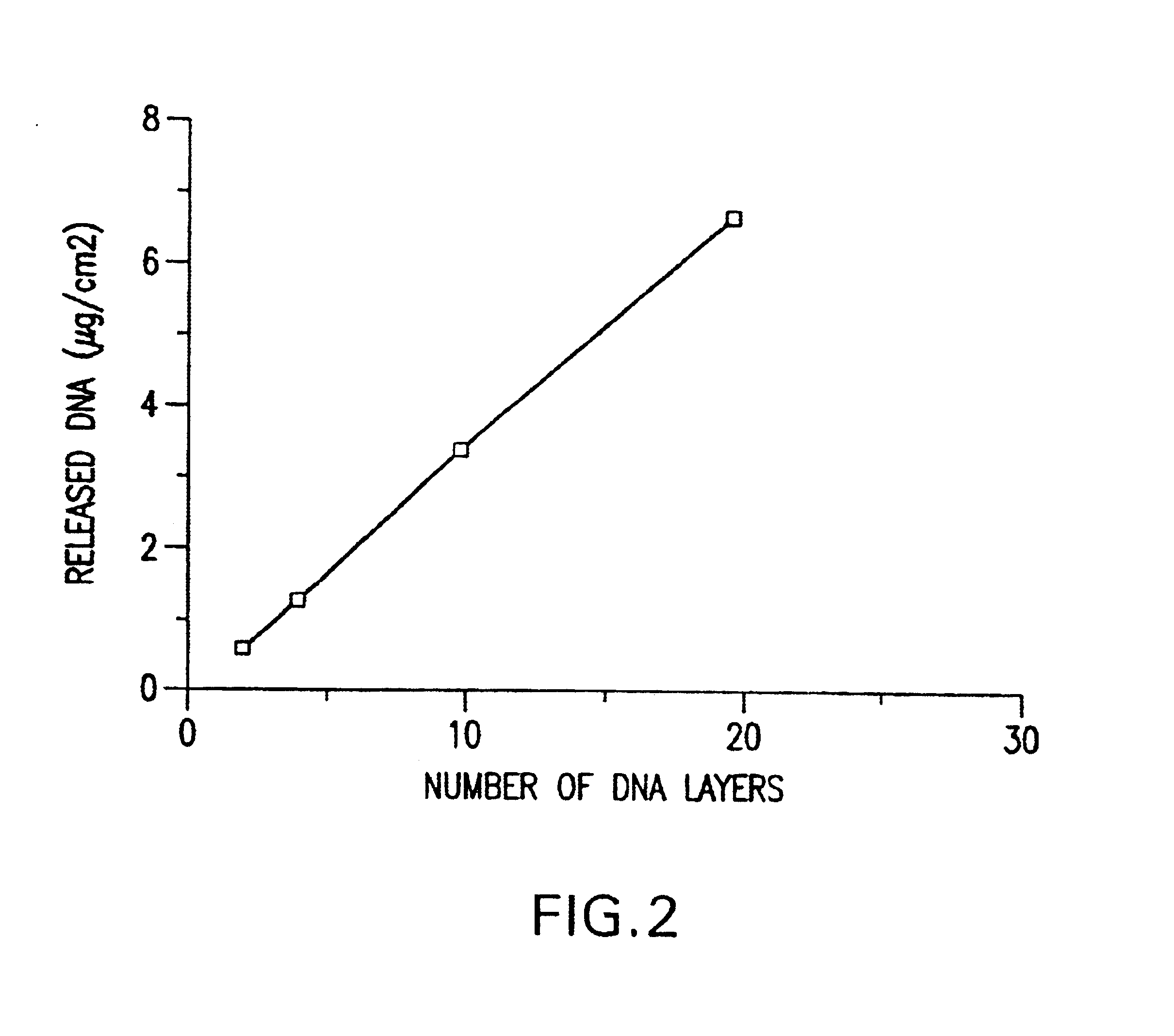
Controlled delivery of therapeutic agents by insertable medical devices
A medical device and method for transportation and release of a therapeutic agent into a mammalian body are disclosed. The medical device is coated with alternating layers of a negatively charged therapeutic agent and a cationic polyelectrolyte, following a controlled adsorption technique. The method is simple, with minimal perturbation to the therapeutic agent and uses clinically acceptable biopolymers such as human serum albumin. The amount of the therapeutic agent that can be delivered by this technique is optimized by the number of the layers of the therapeutic agent adsorbed on the surface of medical device. There is a washing step between alternate layers of the therapeutic agent and cationic polyelectrolyte carrier, so that the amount of the therapeutic agent on the insertable medical device represents the portion that is stably entrapped and adsorbed on to the medical device. The insertable medical device and method according to this invention are capable of reproducibly delivering therapeutic agent to a site in a mammalian body, and allow for a highly reproducible and controllable release kinetics of the therapeutic agent.
Owner:SCI MED LIFE SYST
Device and method for delivery of a medicament
The disclosure relates to a method of enhancing nicotine or other medicament concentrations in a gaseous carrier. The methods are adaptable to the delivery of nicotine or other medicaments for therapeutic effect in various diseases, in particular nicotine for tobacco product use cessation, substitution and / or harm reduction. The disclosure further relates various devices and device design principles for practicing these methods.
Owner:PHILIP MORRIS PROD SA
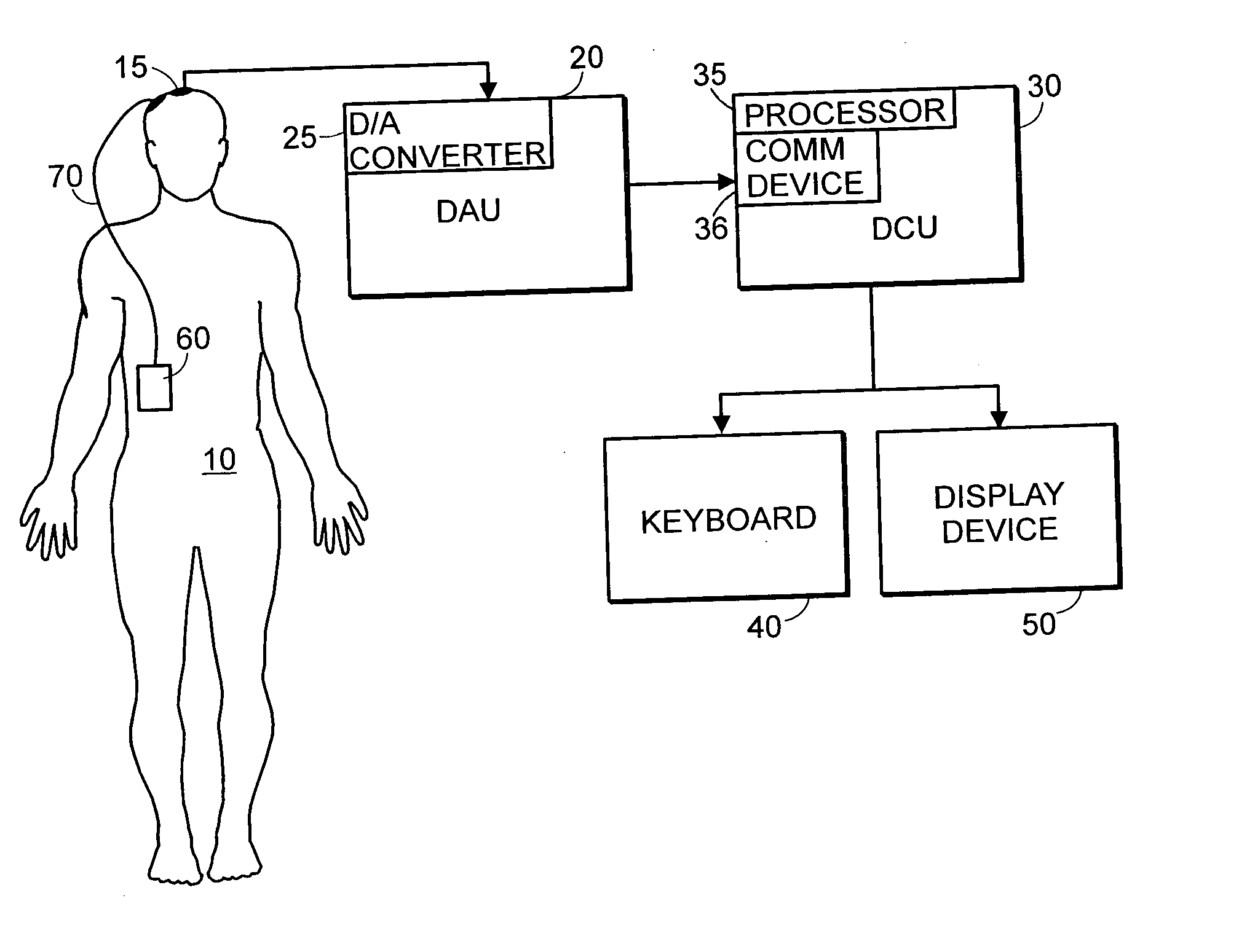
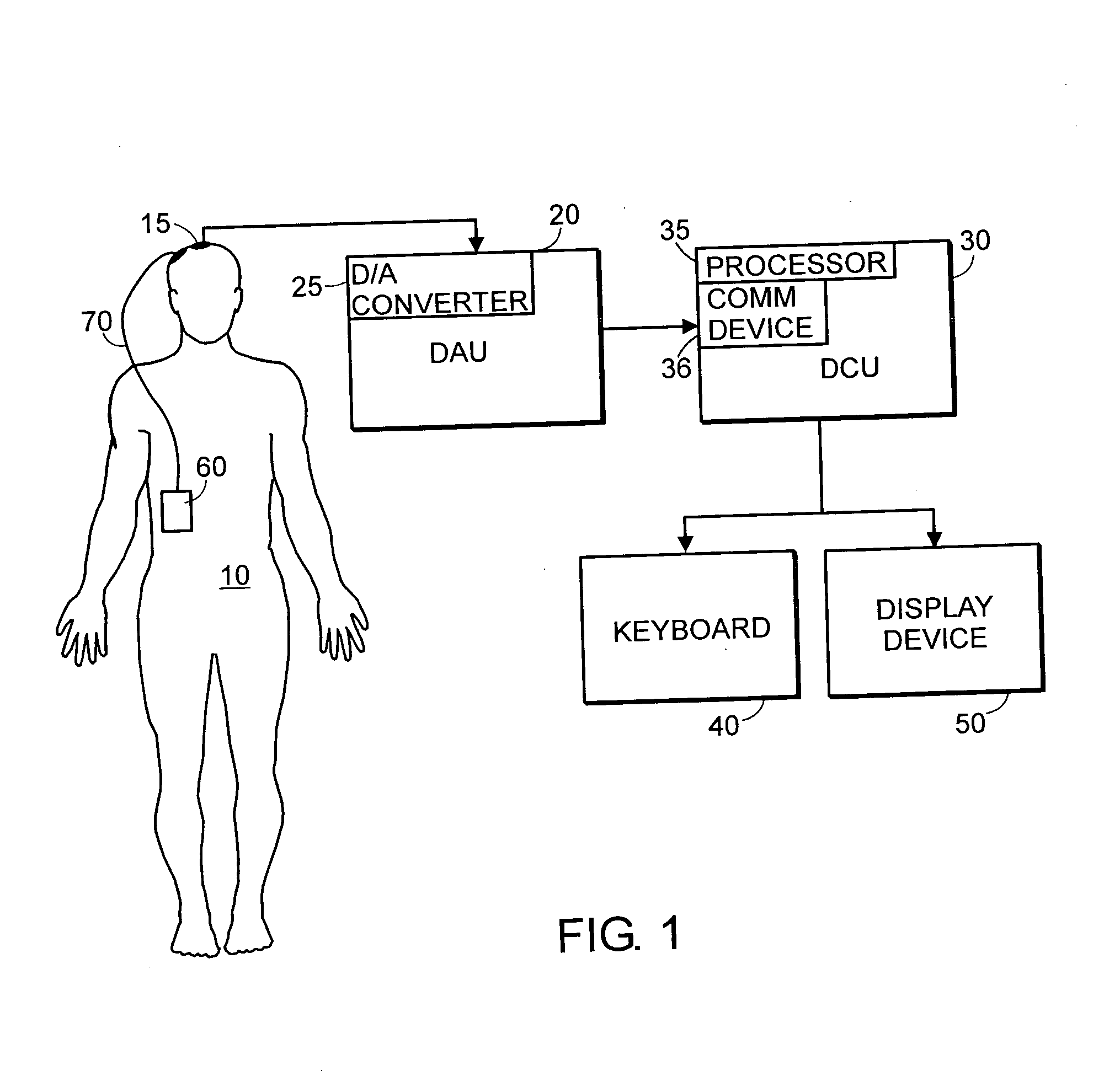
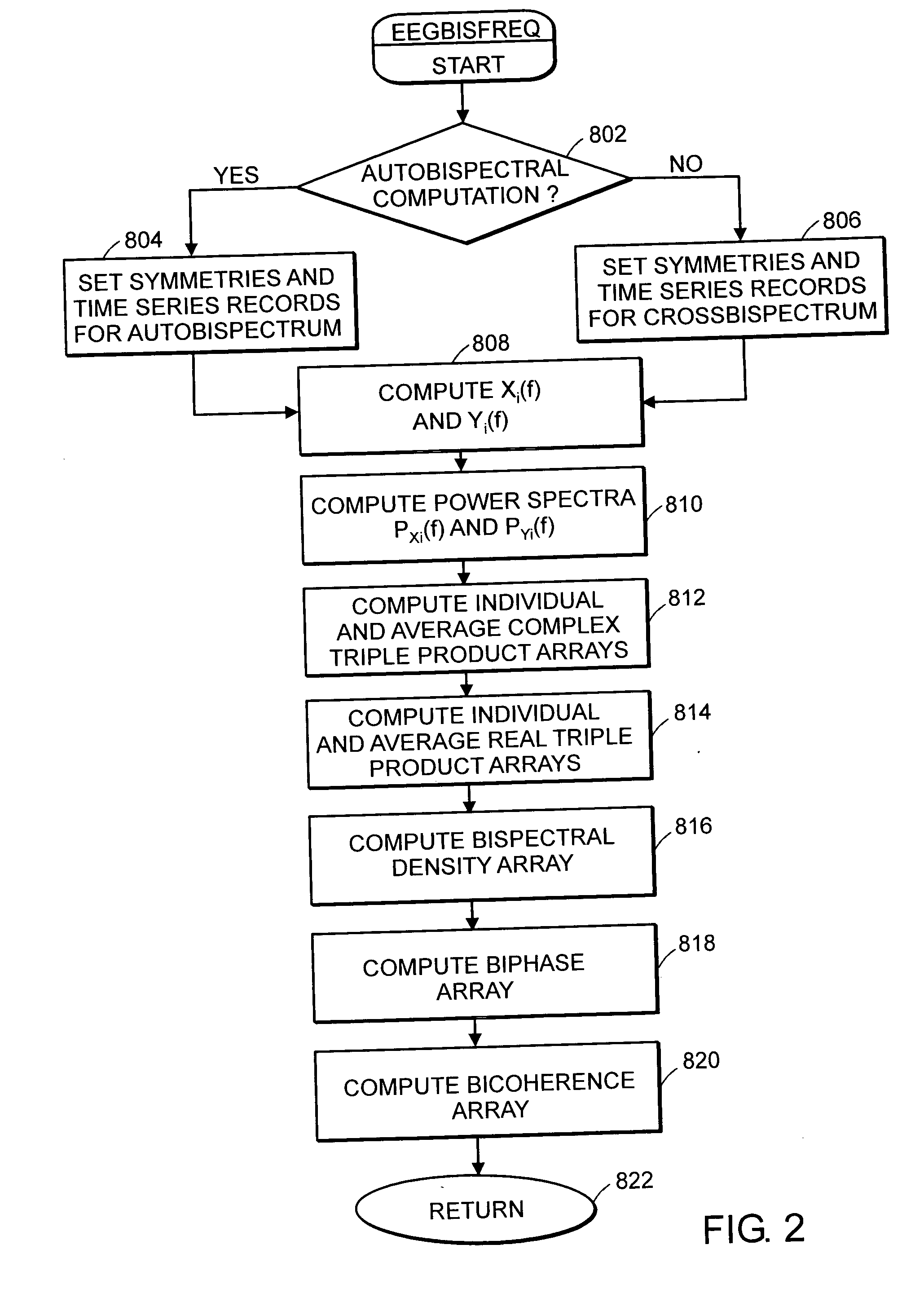
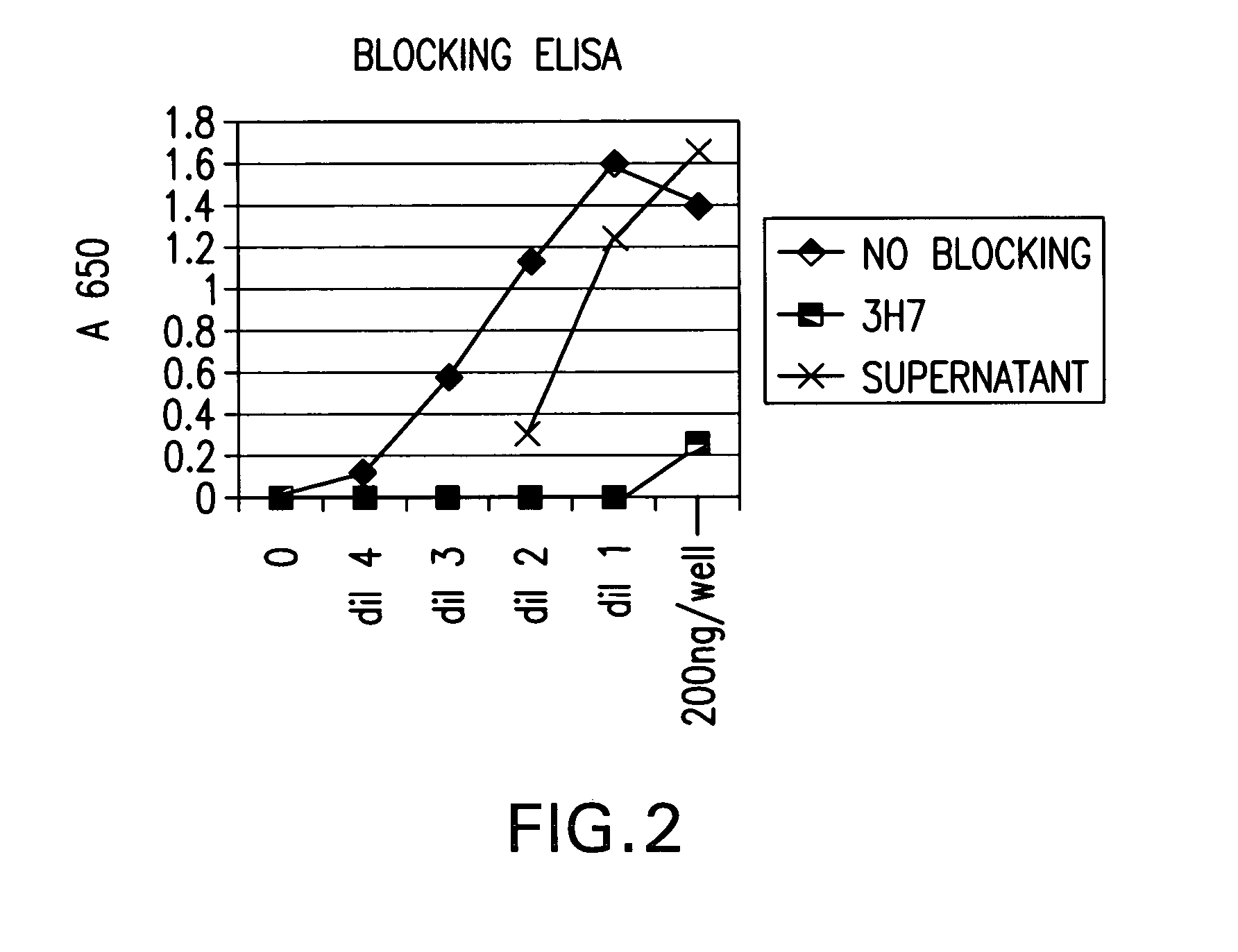
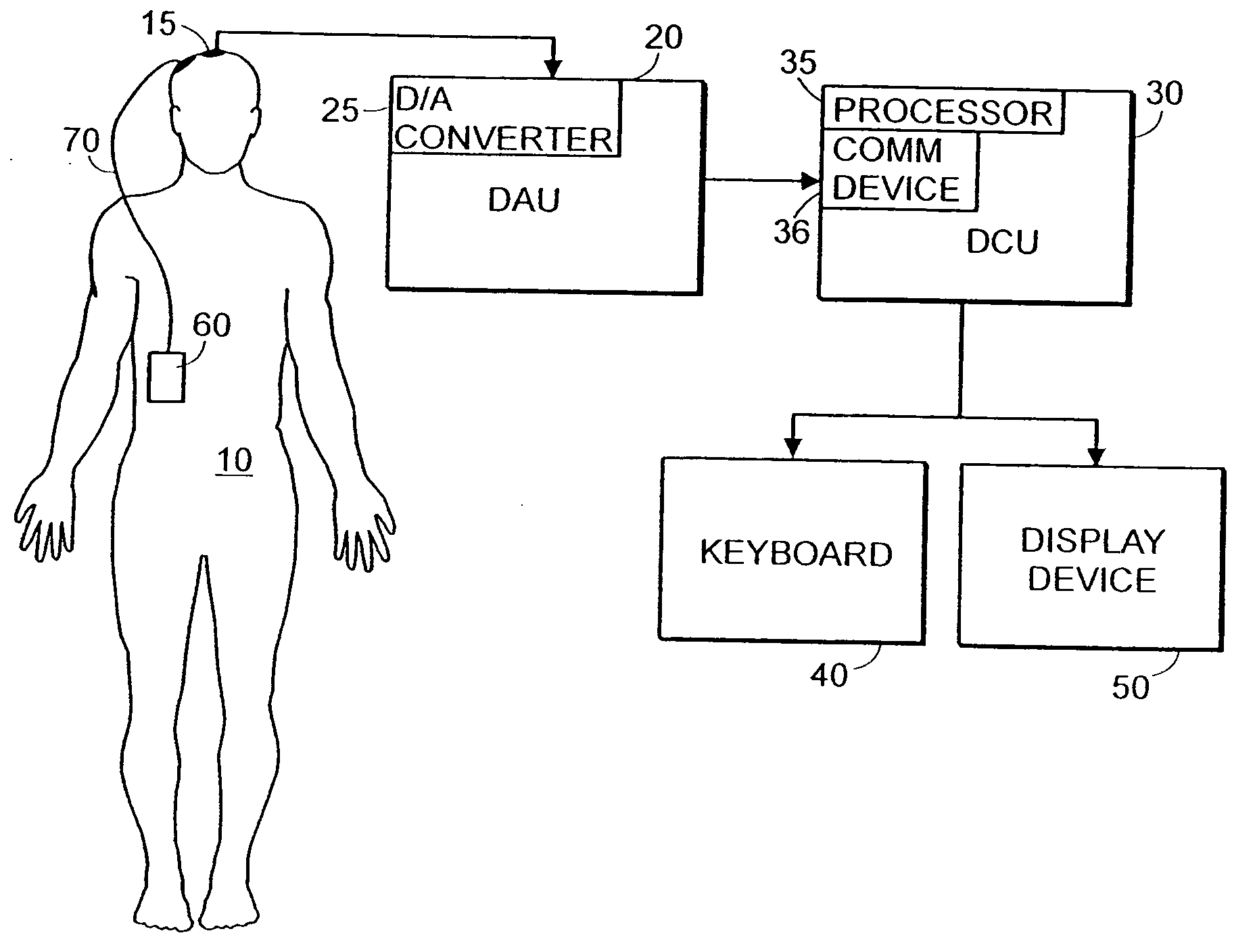
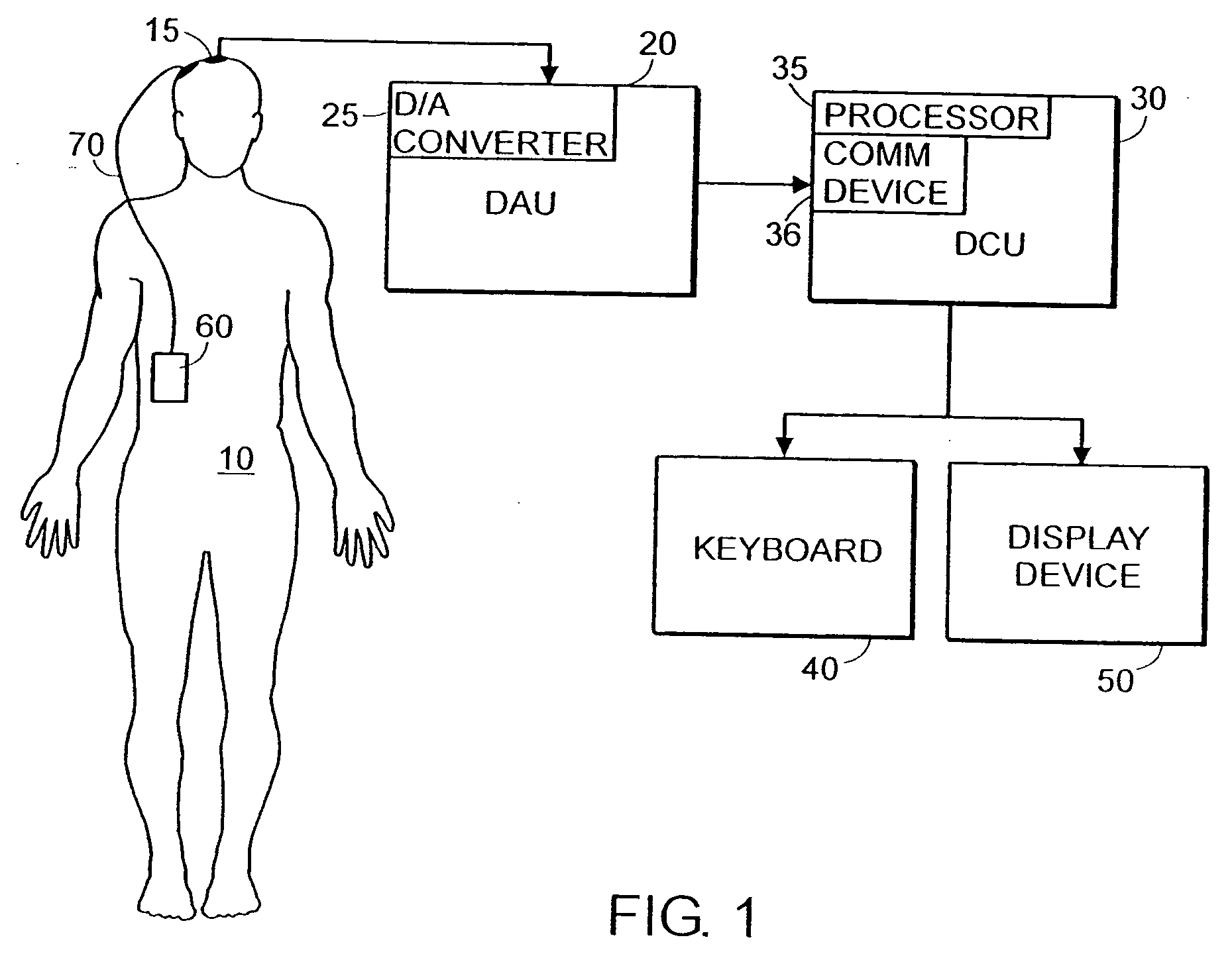
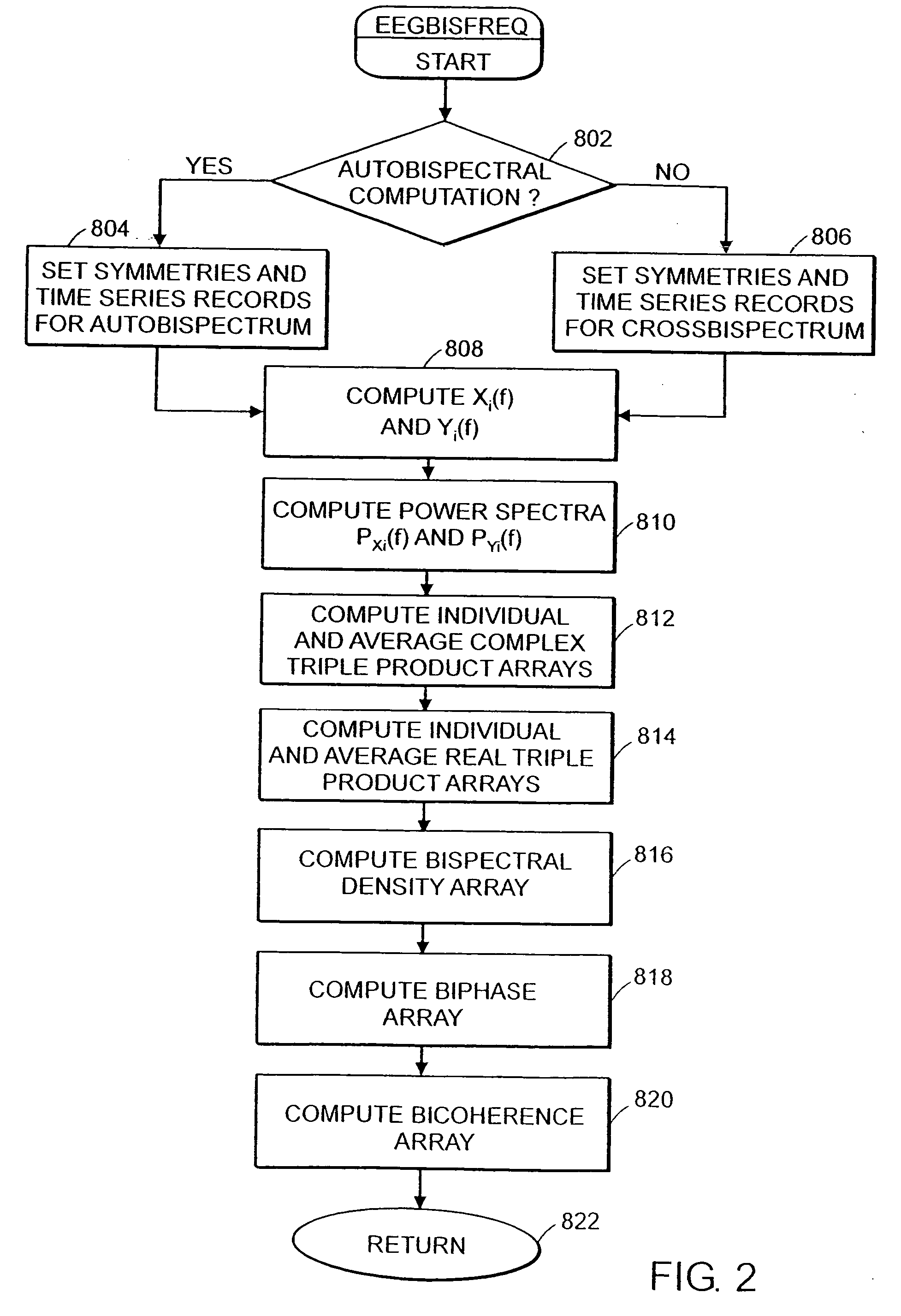
System and method of assessment of the efficacy of treatment of neurological disorders using the electroencephalogram
Disclosed is a system and method of assessing the efficacy of treatment of neurological or psychological disorders. The preferred embodiment uses at least two surface electrodes to acquire EEG signals from the surface of a patient's body, a processor for computing from the EEG signals various features and indices that are representative of the patient's neurological or psychological state. Changes in these parameters may be used to assess the efficacy of treatment and to modify the treatment to optimize the resultant patient state.
Owner:TYCO HEALTHCARE GRP LP
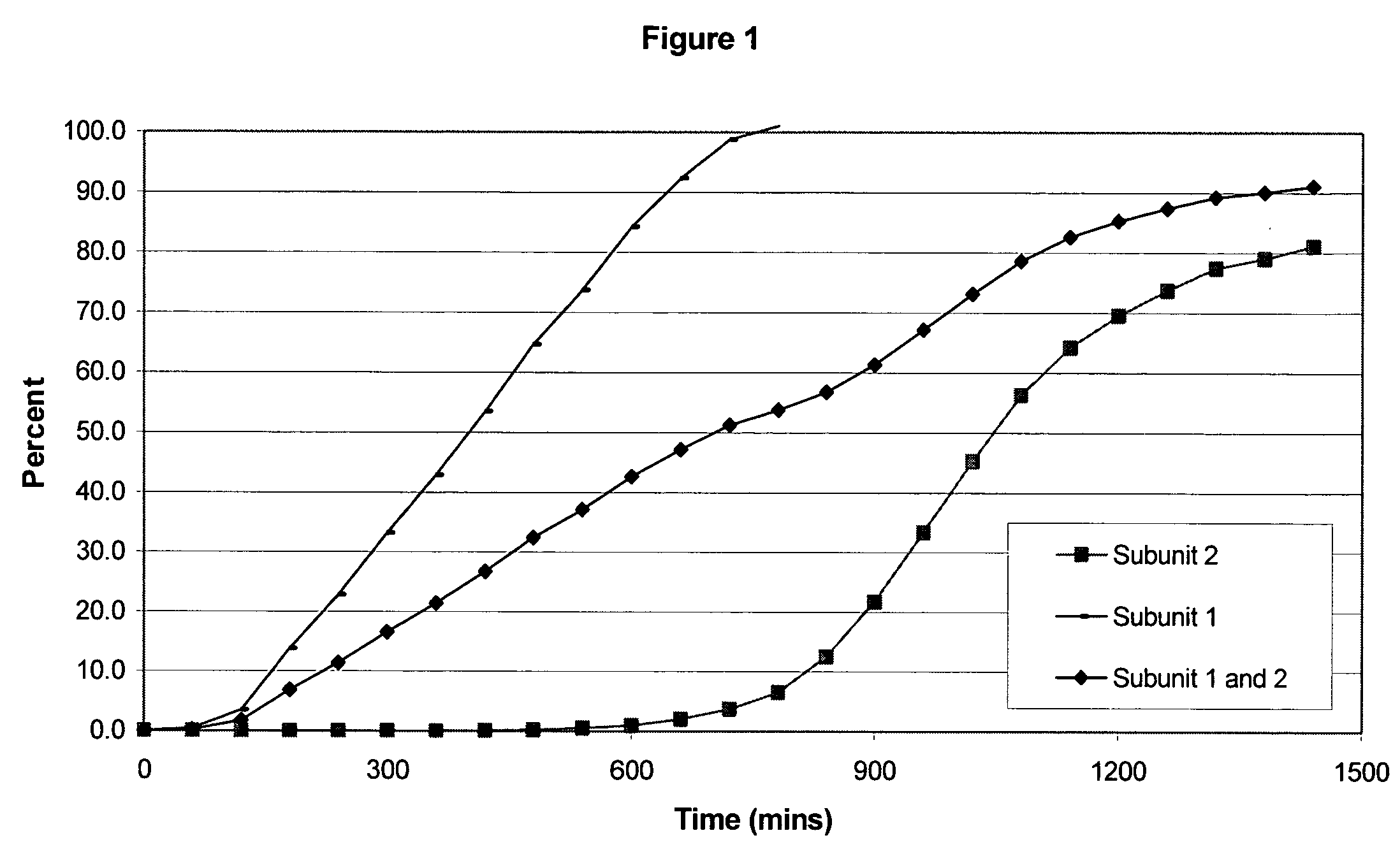
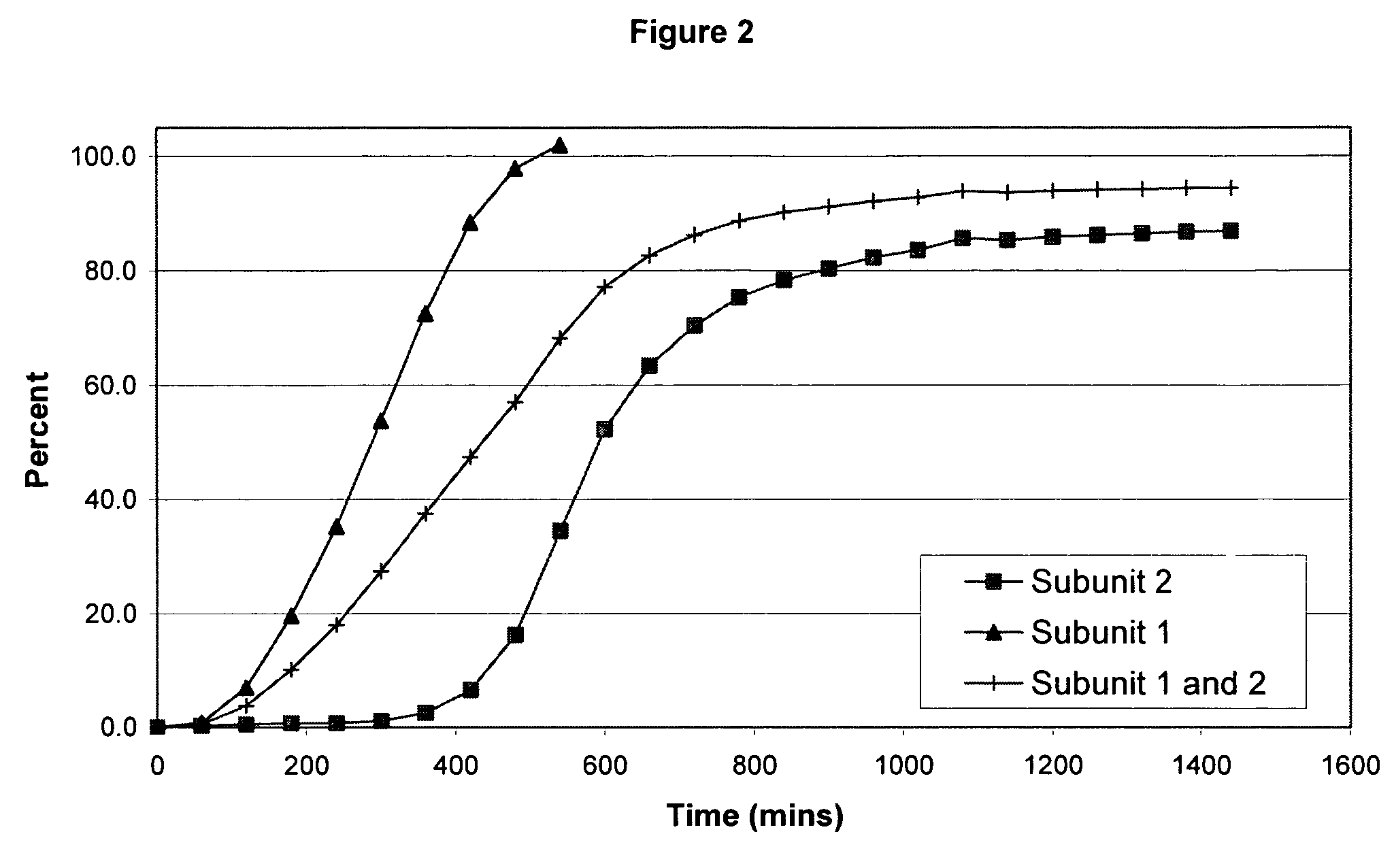
Sustained release opioid formulations and method of use
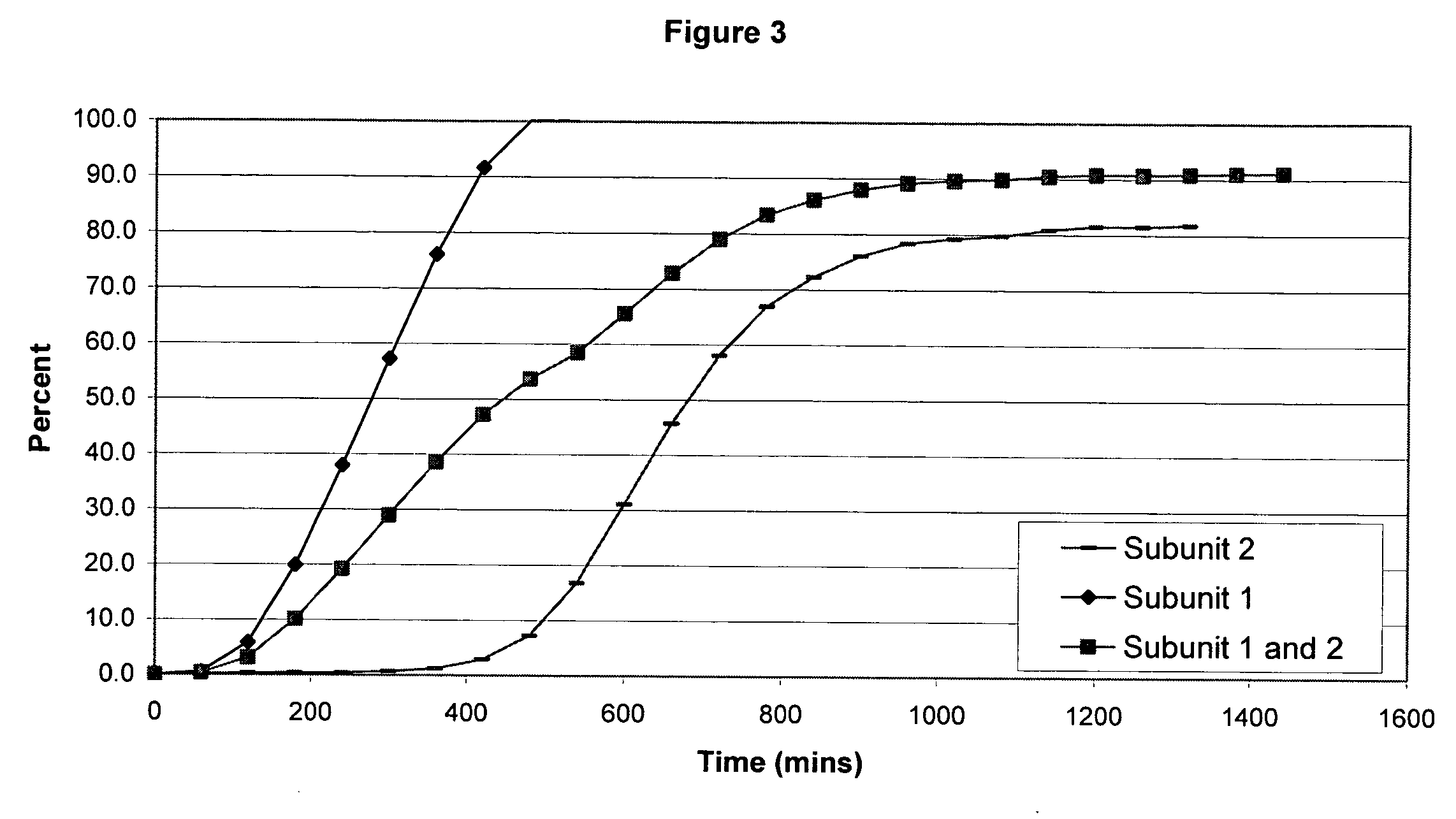
The invention combines two different subunits with different release profiles in novel sustained-release oral dosage forms. In particular, the oral dosage forms include a subunit that comprises an opioid analgesic and a sustained-release material, wherein the dissolution rate in-vitro of the subunit, when measured by the standard USP Drug Release test of U.S. Pharmacopeia XXVI (2003) <724>, is less than about 10% within about 6 hours and at least about 60% within about 24 hours; less than about 10% within about 8 hours and at least about 60% within about 24 hours; less than about 10% within about 10 hours and at least about 60% within about 24 hours; or less than about 10% within about 12 hours and at least about 60% within about 24 hours; the dosage form providing a duration of therapeutic effect of about 24 hours.
Owner:ALPHARMA PHARMA
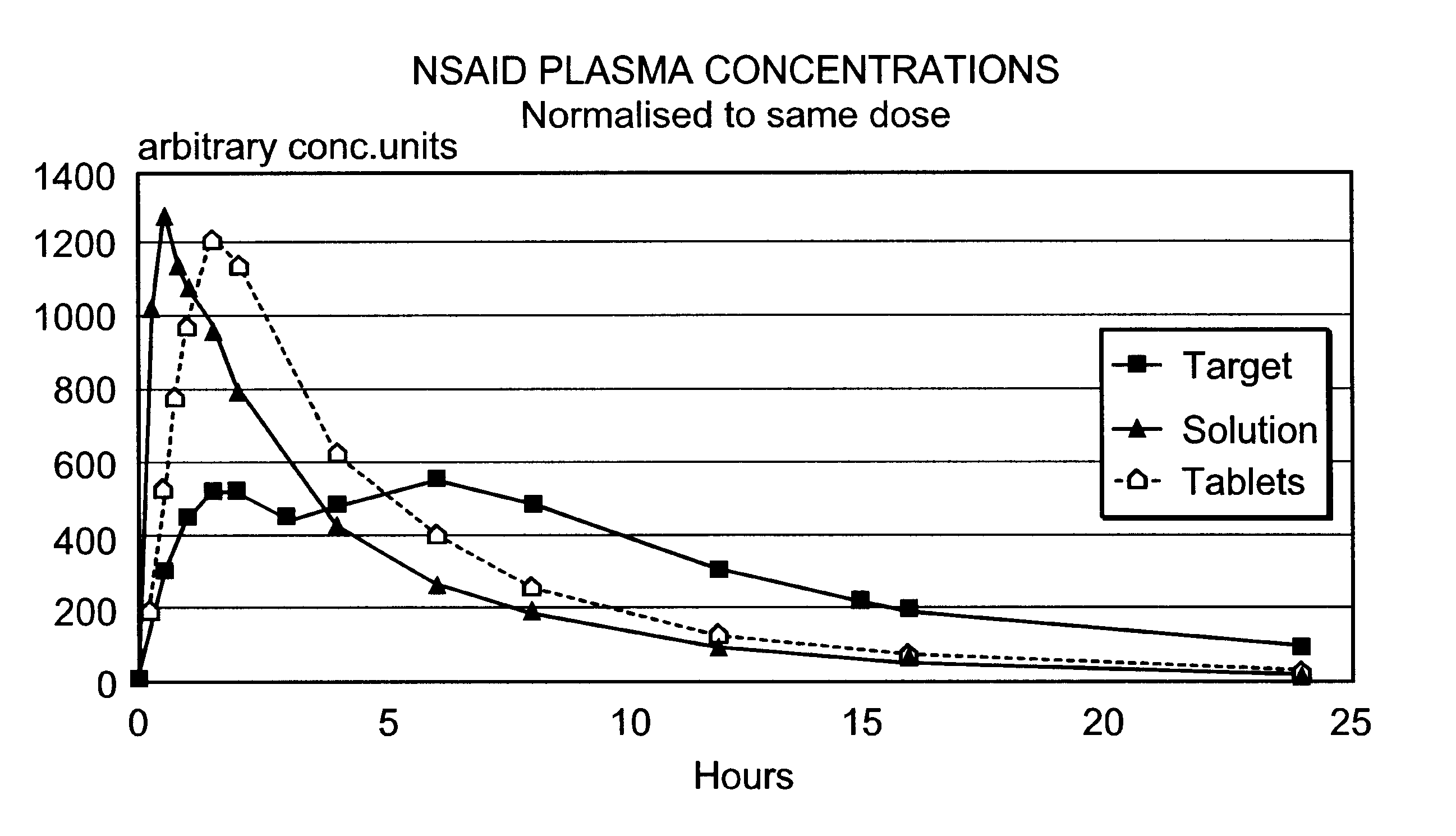
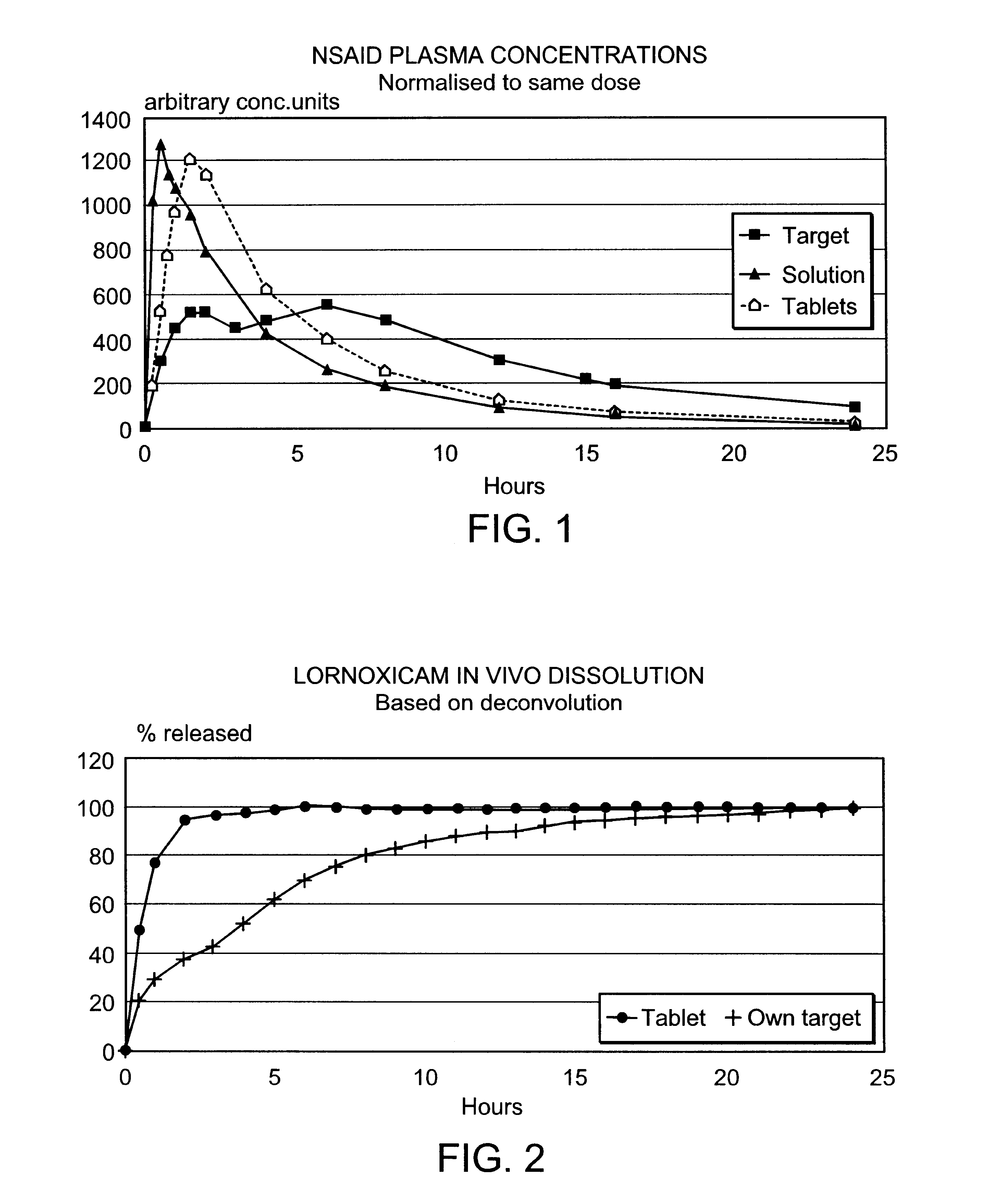
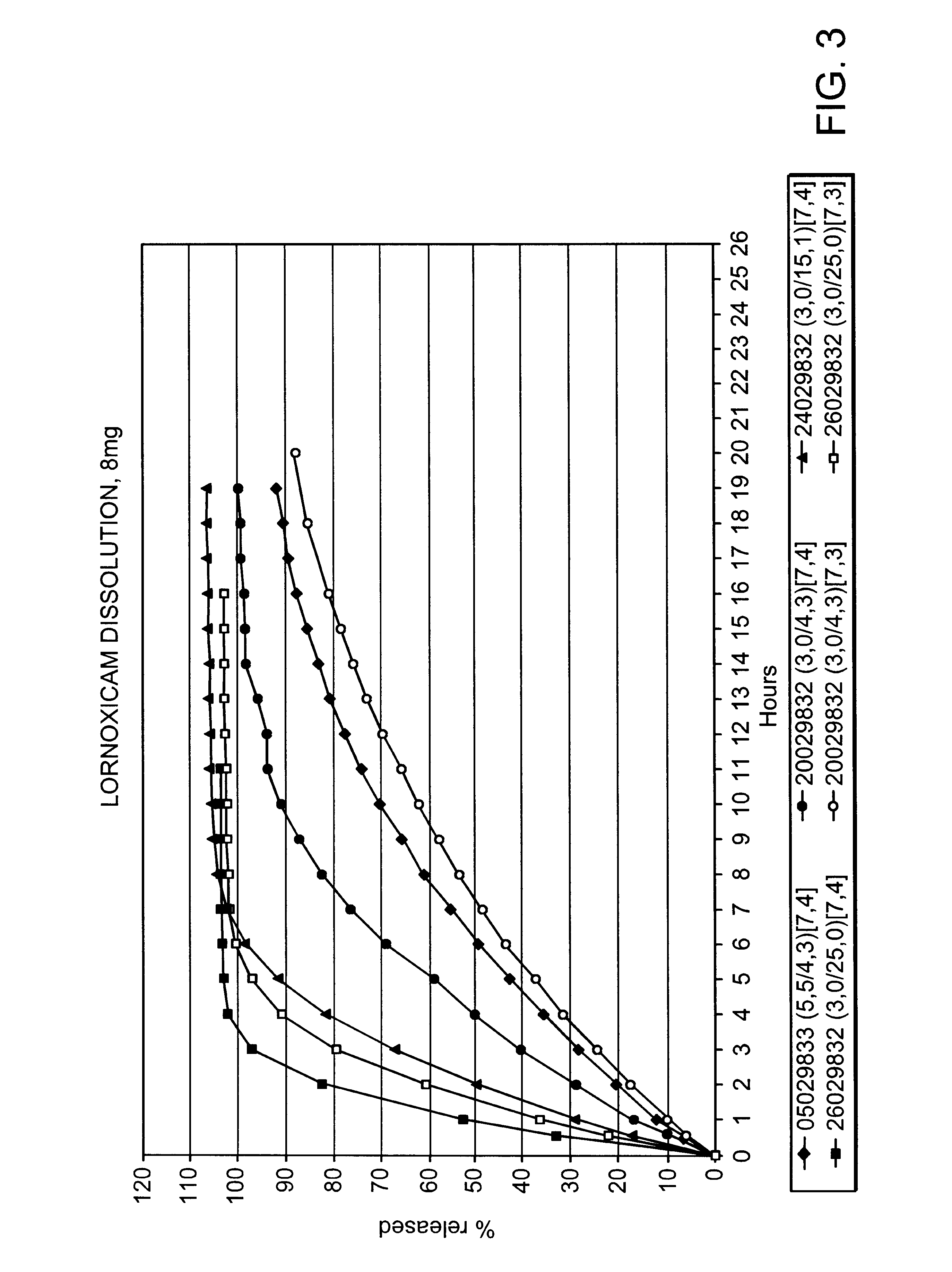
Modified release multiple-units compositions of non-steroid anti-inflammatory drug substances (NSAIDs)
InactiveUS6599529B1Keep low levelQuick releasePowder deliveryNervous disorderNon steroid anti inflammatory drugTherapeutic effect
An oral pharmaceutical modified release multiple-units composition for the administration of a therapeutically and / or prophylactically effective amount of a non-steroid anti-inflammatory drug substance to obtain both a relatively fast onset of the therapeutic effect and the maintenance of a therapeutically active plasma concentration for a relatively long period of time is disclosed.
Owner:TAKEDA PHARMA AS +1
Topically applied clotting material
A composition, system, articles and method for the enhancement of clotting in wounds with extravascular blood flow, especially where the surface of the tissue has been broken is described. The system consists of biotolerable, porous particulates applied to the surface of a wound with liquid blood thereon. The porous nature of the particulate material, either free-flowing or packaged or restrained on or in a surface, enhances clotting. Chemical or biochemical agents, such as additional clotting agents, therapeutic agents, antibiotics, clot strengthening agents (such as fibrous structural materials), and the like may optionally be included on, with or within the porous particles. The particles may comprise such diverse materials as organics, metallics, inorganics, ceramics, and the like, both natural and artificial. It is generally preferred that the pore size distribution lies within a general range, and this range may vary from animal to animal and condition to condition, but generally falls within about 0.5 to 1000 nanometers or 3,000 to 200,000 Daltons.
Owner:MEDAFOR
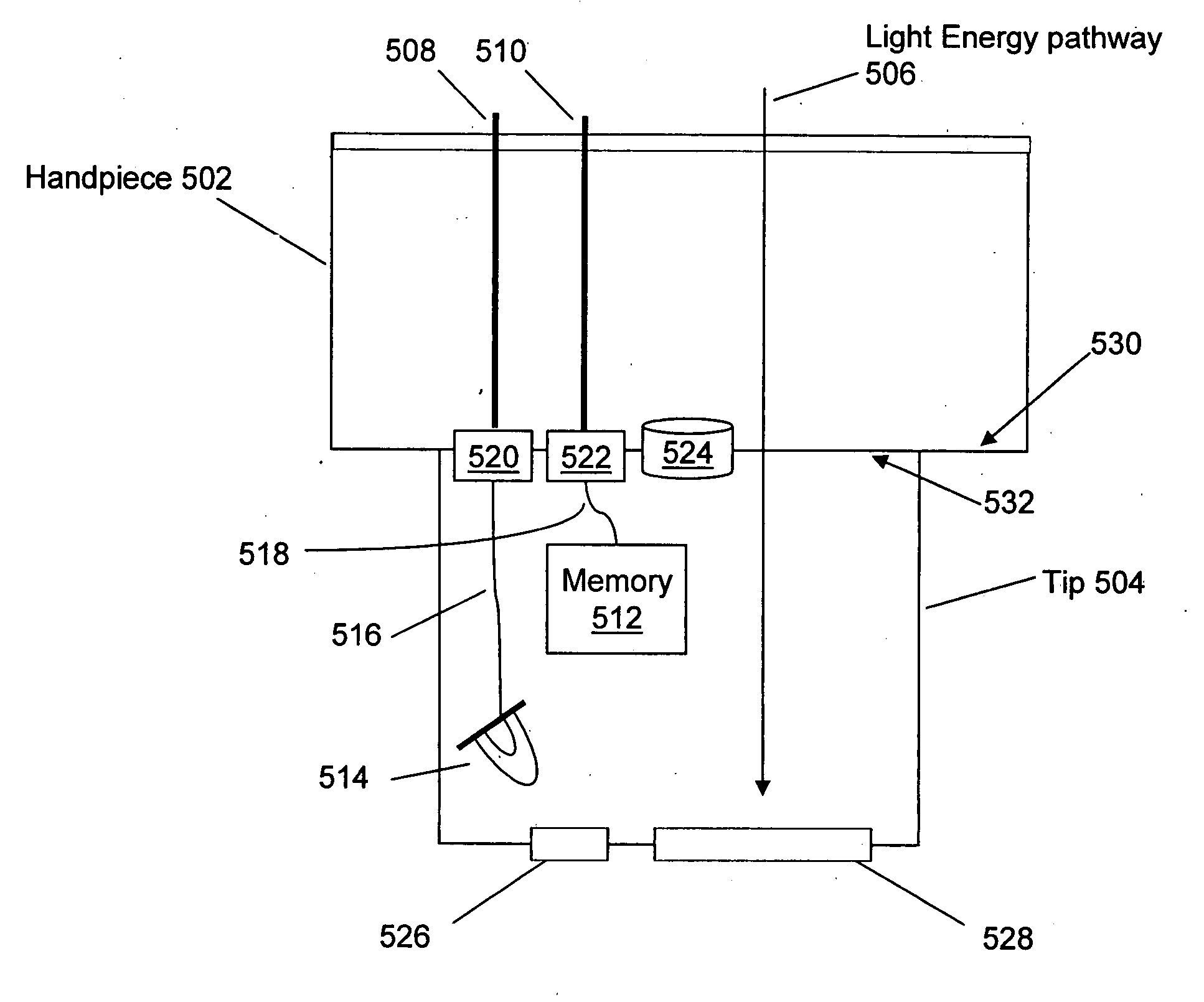
Interchangeable tips for medical laser treatments and methods for using same
InactiveUS20060095096A1Convenient treatmentDiagnosticsSurgical instrument detailsTreatment effectOptical energy
A typical treatment system for use with tip embodiments includes an optical energy source, such as, for example, a laser. A set of tips may be interchangeably attached to the treatment system, for example to alter the system parameters and the treatment provided through the individual tips. Embodiments of the present invention include tips with a security chip and / or a memory. A security chip protects the treatment system from use with unauthorized tips, and the memory stores information about the tips and / or the treatment system to enhance the treatment
Owner:RELIANT TECH INC
Therapeutic agent delivery device with protective separating layer
Owner:CONOR MEDSYST
Transdermal drug delivery devices having coated microprotrusions
A device (12) and method are provided for percutaneous transdermal delivery of a potent pharmacologically active agent. The agent is dissolved in water to form an aqueous coating solution having an appropriate viscosity for coating extremely tiny skin piercing elements (10). The coating solution is applied to the skin piercing elements (10) using known coating techniques and then dried. The device (12) is applied to the skin of a living animal (e.g., a human), causing the microprotrusions (10) to pierce the stratum corneum and deliver a therapeutically effect dose of the agent to the animal.
Owner:ALZA CORP
Composition comprising an agent providing a signal, an implant material and a drug
InactiveUS20060177379A1Facilitated releaseAvoid impairment of material compositionMaterial nanotechnologySurgeryTreatment effectBULK ACTIVE INGREDIENT
The present invention relates to compositions or combinations of materials for non-degradable and degradable implantable medical devices with regard to the setup of their signal generating properties and control of their therapeutic effectiveness, as well as to a method for the control of degradation of degradable or partially degradable medical devices composed like this, based on their signal generation, and to a method for supervision of their therapeutic effectiveness and / or the release of therapeutically active ingredients from such devices.
Owner:CINVENTION AG
Medical device rapid drug releasing coatings comprising a therapeutic agent and a contrast agent
ActiveUS20080255510A1Improve the immunityReduction tendencyBiocideMedical devicesTherapeutic effectDrug release
The invention relates to a coated medical device for rapid delivery of a therapeutic agent to a tissue in seconds to minutes. The medical device has a layer overlying the exterior surface of the medical device. The layer contains a therapeutic agent, a contrast agent, and an additive.
Owner:LUTONIX INC
Interactive transcutaneous electrical nerve stimulation device
InactiveUS20070276449A1Quickly and easily changedEffectively treats area greatExternal electrodesNervous tissueTherapeutic Area
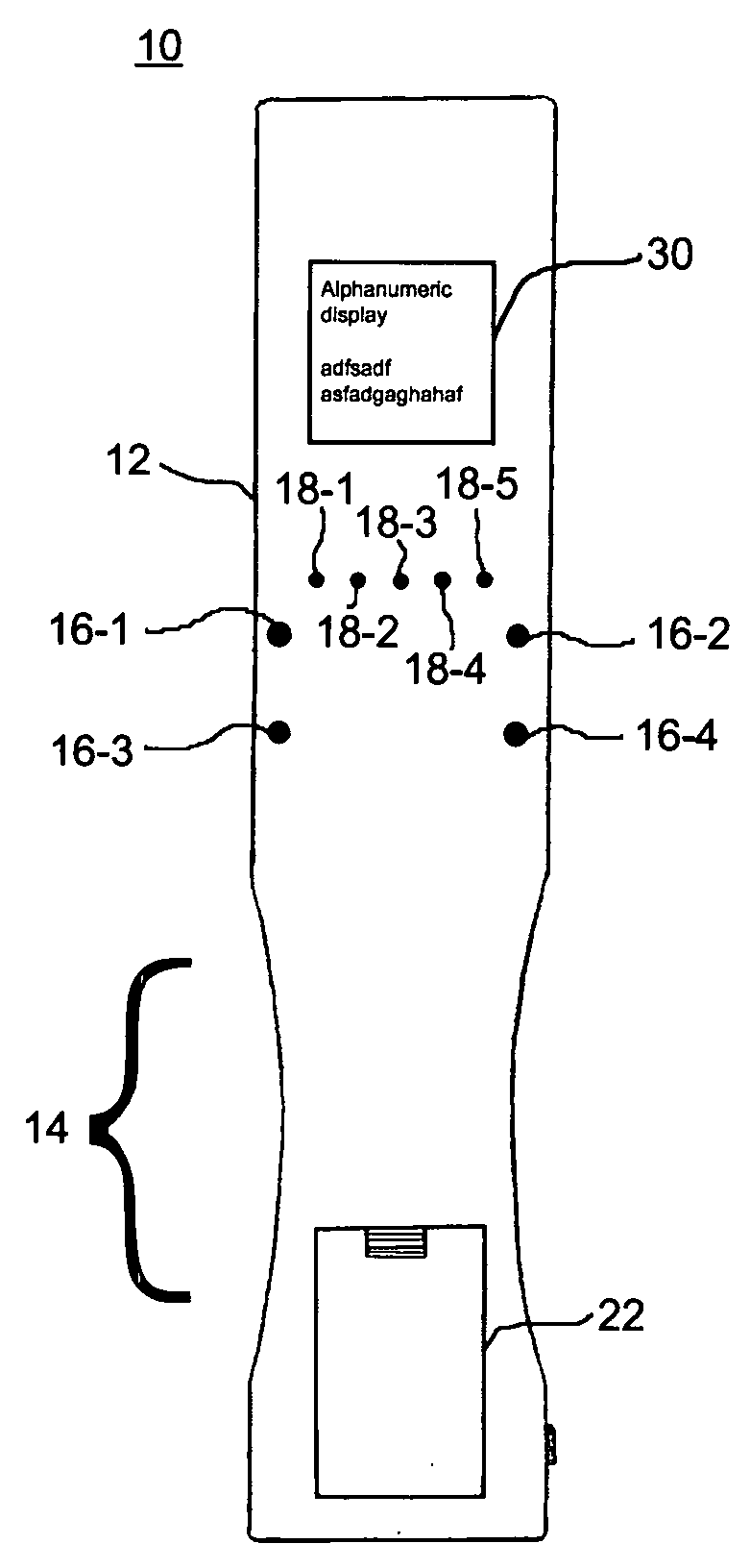
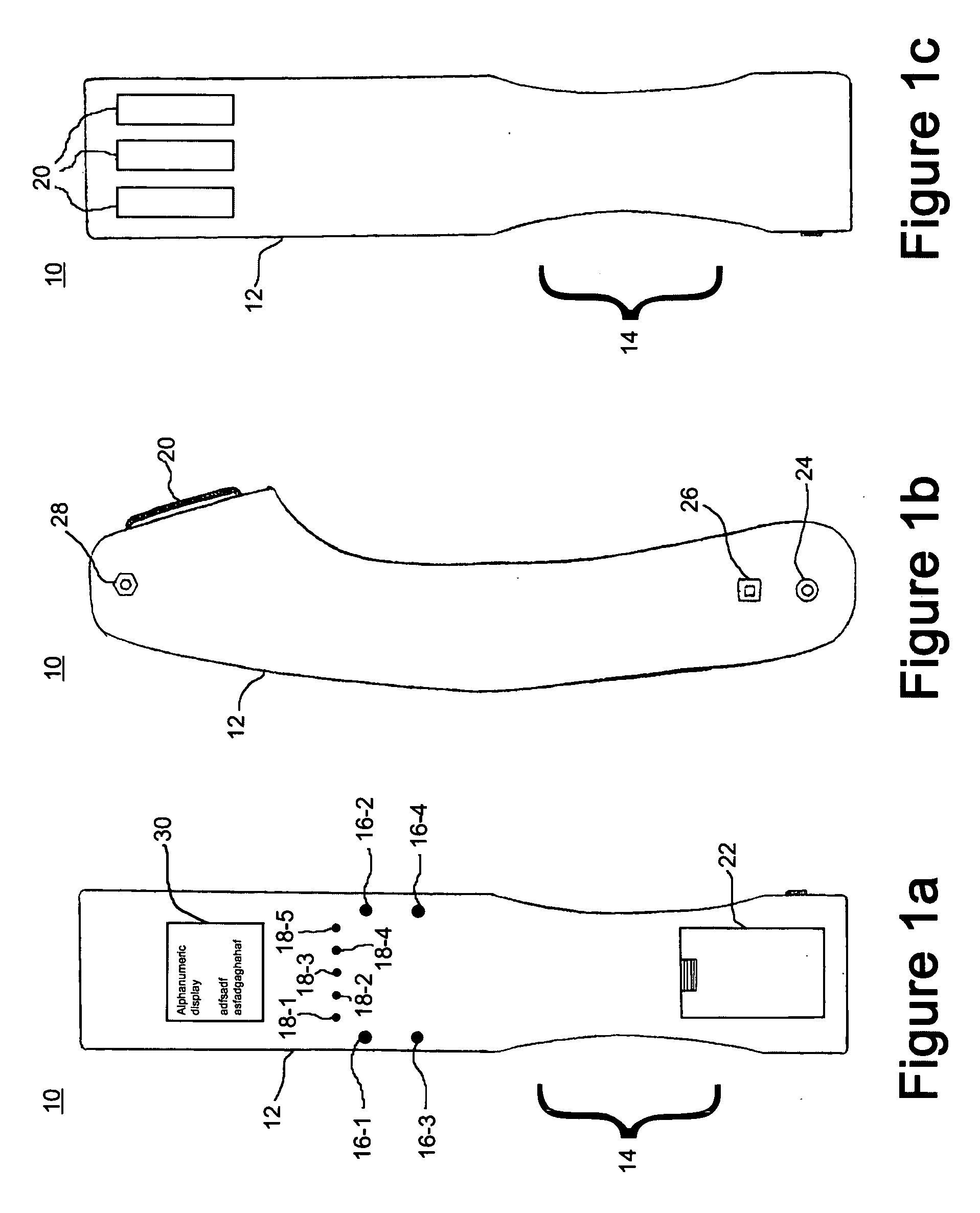
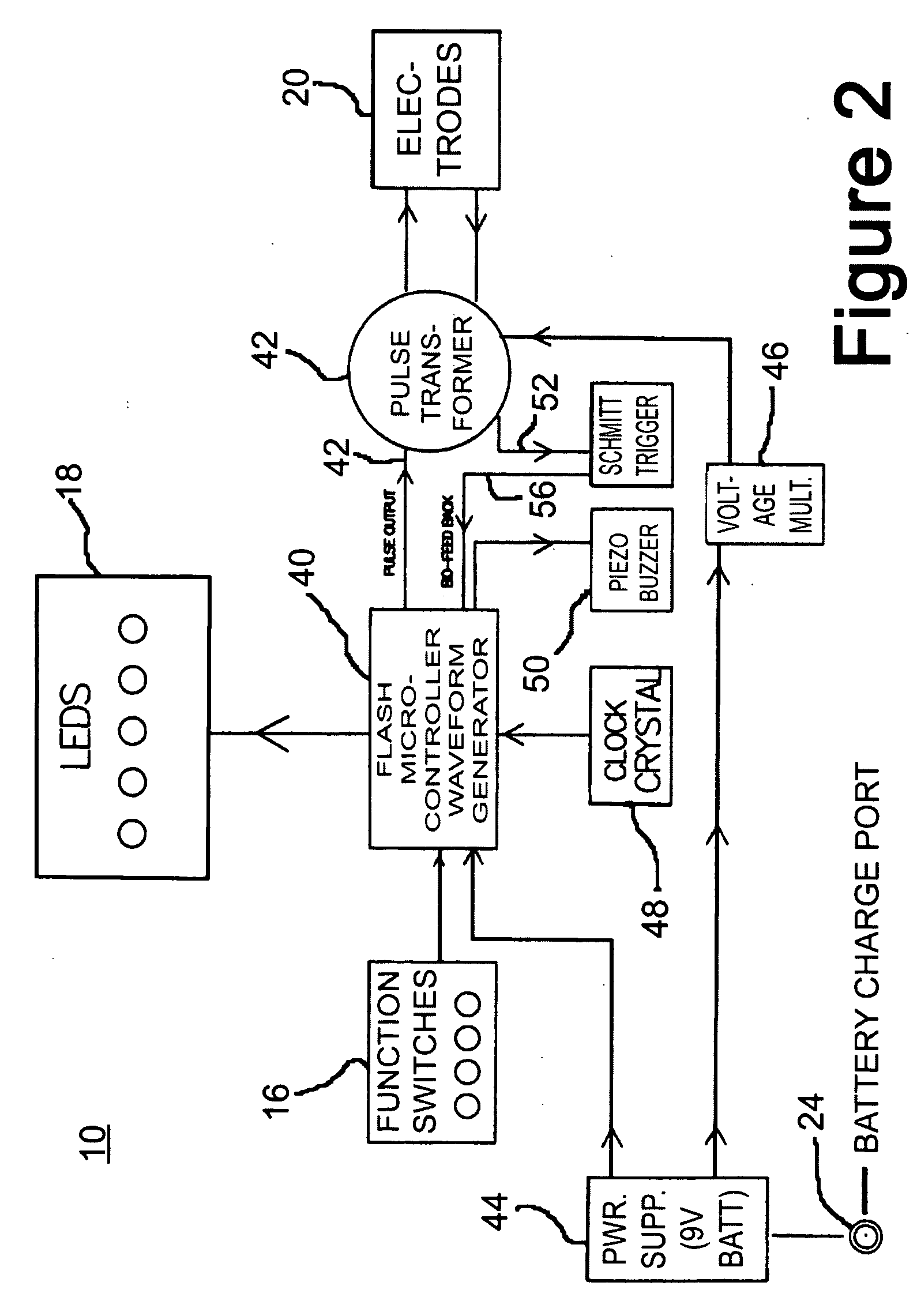
A wireless, handheld electrical therapy device delivers electrical pulses to a treatment area of a patient. In one embodiment, the device comprises a microcontroller-based pulse generator circuit selectively operable in a plurality of therapeutic modes. The device comprises an ergonomic housing adapted to be comfortably grasped by a user. A plurality of electrodes are disposed on a surface of the housing. In operation, a user brings the electrodes into contact with a patient's skin at a location on the patient to be treated. Electrical pulses are delivered between the electrodes, thereby electrically stimulating neural tissue at the treatment location. In one embodiment, the device is operable in a manual mode wherein the user selects from among a plurality of therapeutic regimens each corresponding to a set of predetermined operational parameters. Among the variable operational parameters are pulse amplitude, frequency, duration, damping, and shape. In another embodiment of the invention, the device is operable in an automatic mode wherein electrical conditions at the skin surface are periodically sensed and the operational parameters automatically adjusted to achieve optimal therapeutic effectiveness.
Owner:MED LECTRIC CORP
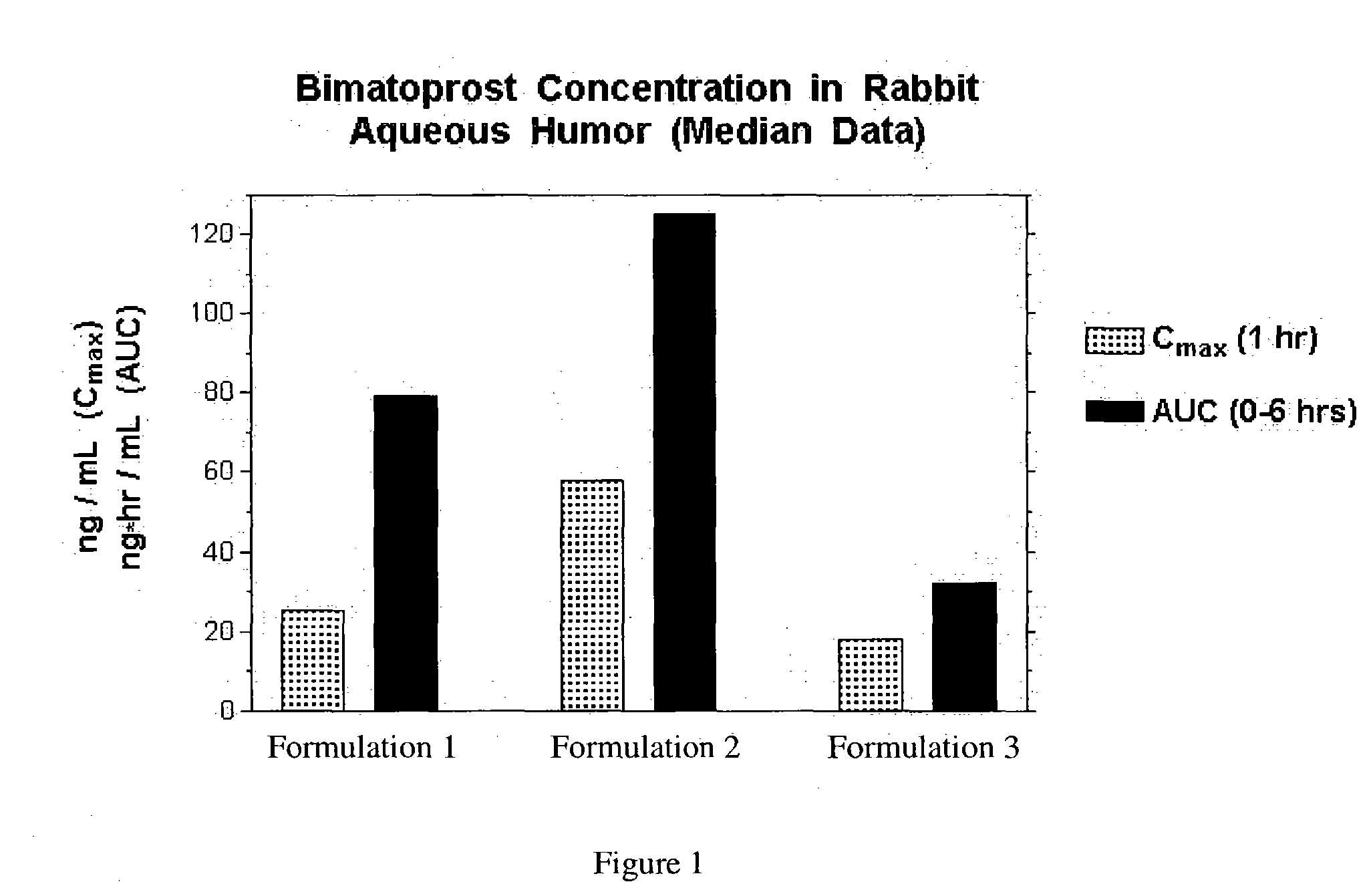
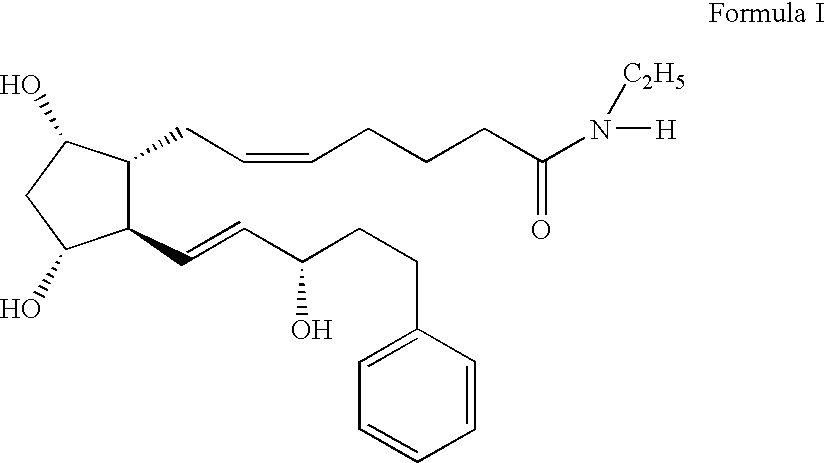
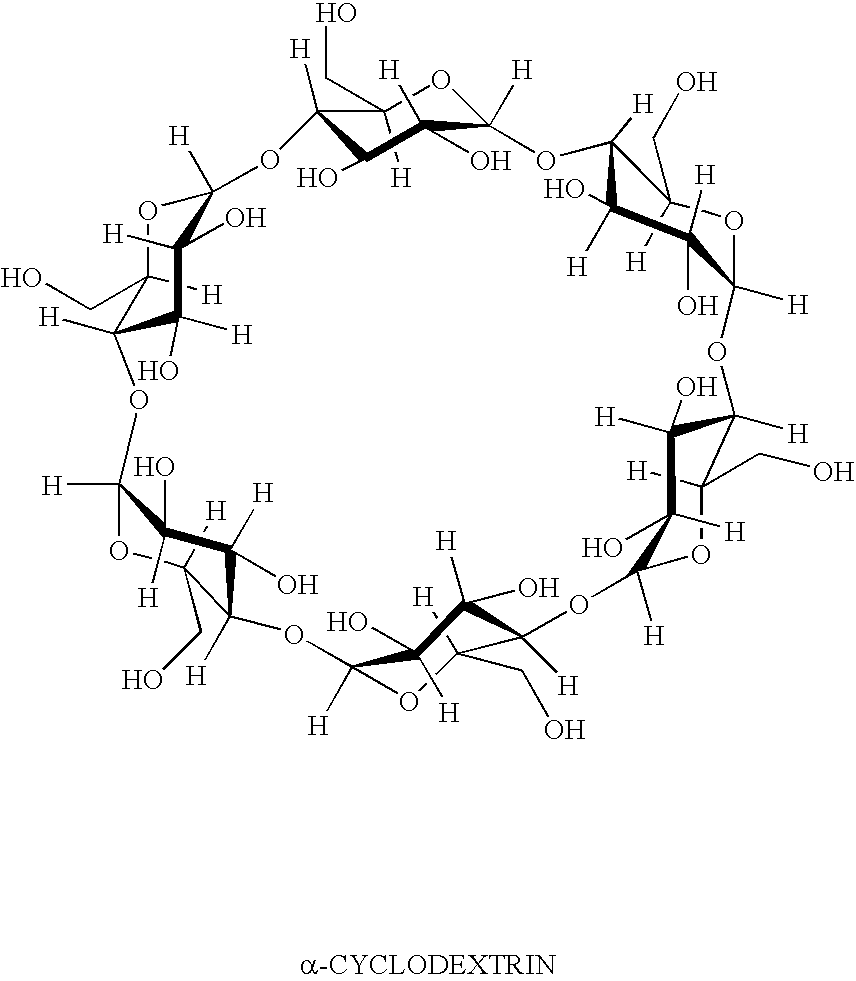
Inhibition of irritating side effects associated with use of a topical ophthalmic medication
InactiveUS20050004074A1Reduce severityEffective deliveryPowder deliveryBiocideCyclodextrin derivativeCyclodextrin Derivatives
This invention relates to a method of reducing an irritating or adverse side effect associated with the topical use of an active ophthalmic drug comprising incorporating an effective amount of a cyclodextrin or cyclodextrin derivative into a formulation to complex the active drug such that the concentration of the free active drug is reduced below a tolerable threshold, and incorporating an effective amount of a viscosity increasing agent in said formulation such that the bioavailability of said drug is high enough to be therapeutically effective, wherein the cyclodextrin or cyclodextrin derivative is not required to solubilize the active drug. Another aspect of this invention relates to topical ophthalmic formulations comprising an active drug, a cyclodextrin or cyclodextrin derivative, and a viscosity-enhancing agent, in effective amounts as stated above.
Owner:ALLERGAN INC
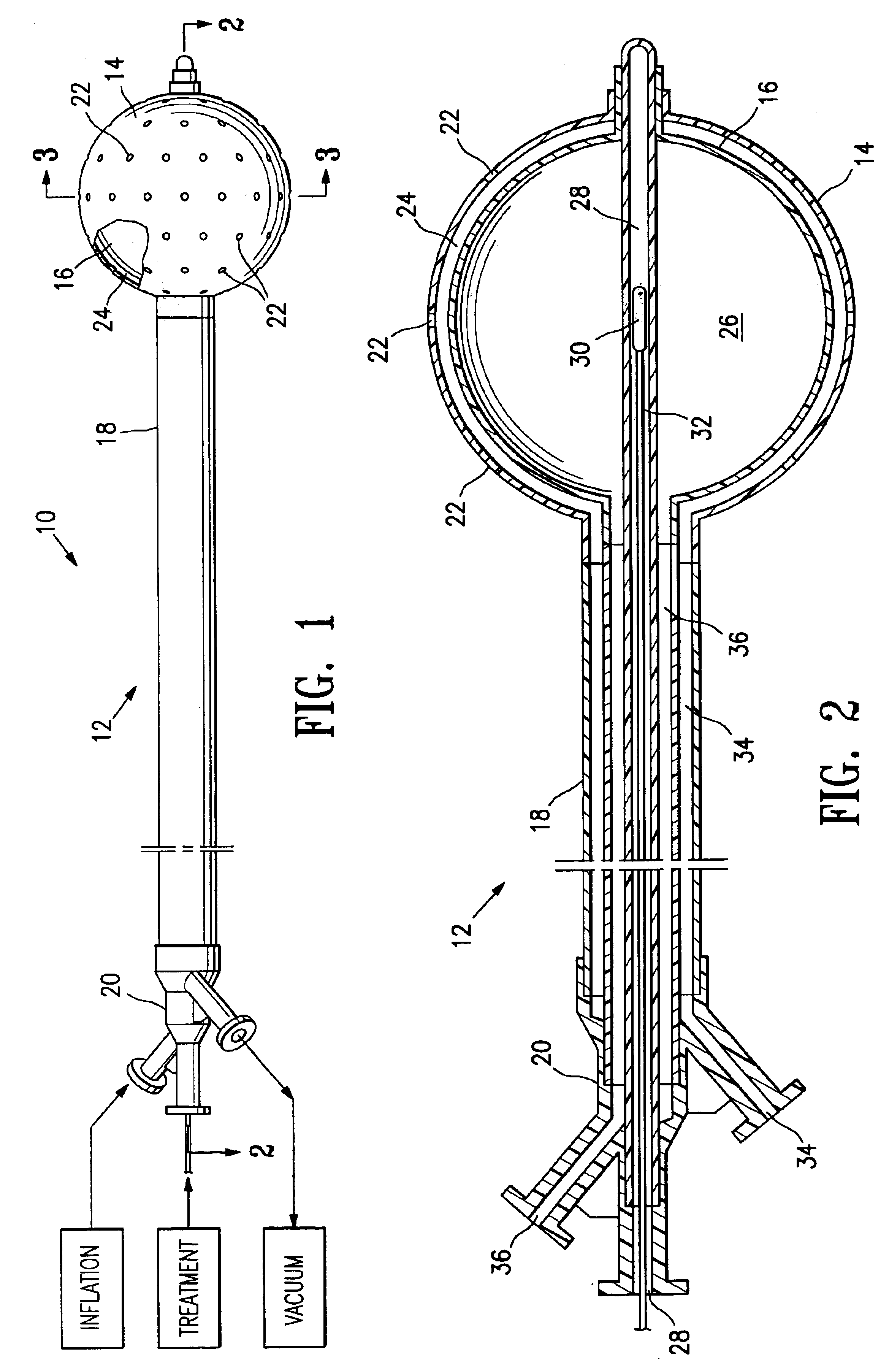
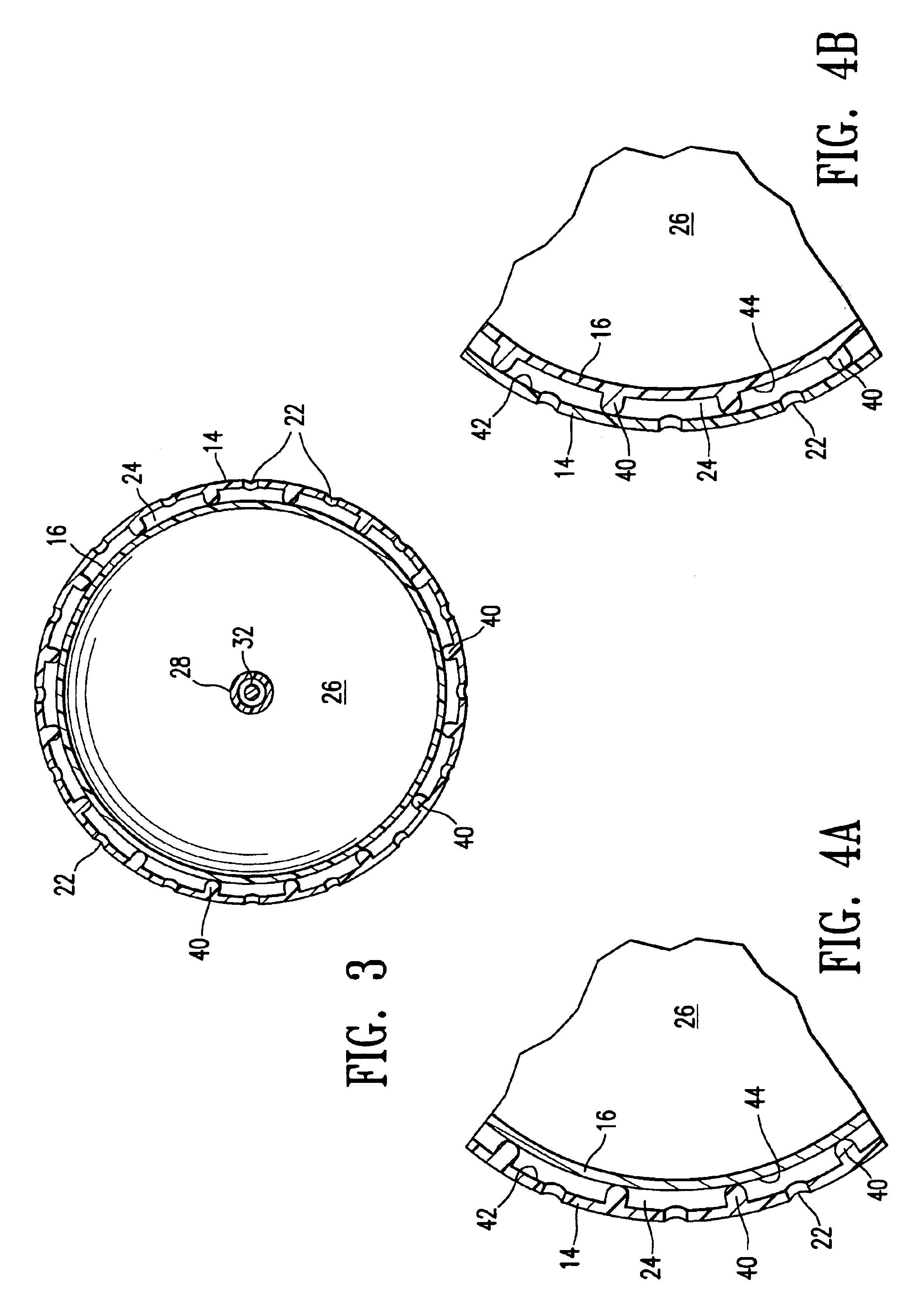
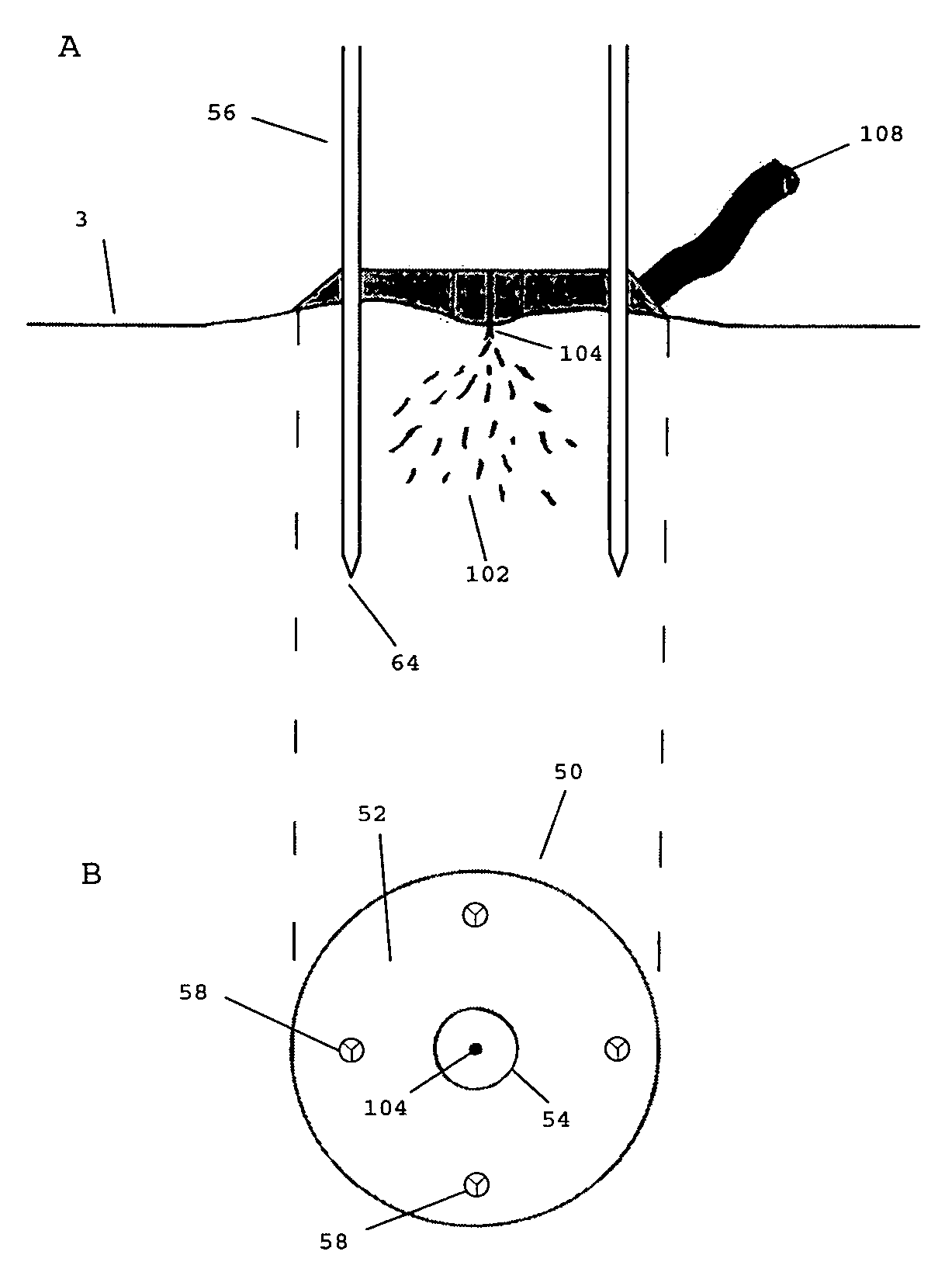
Vacuum device and method for treating tissue adjacent a body cavity
Devices and methods are provided for applying vacuum near to devices for delivering treatments to tissue adjacent a body cavity, effective to draw adjacent tissue near to such devices and to enhance treatment of the tissue. Body cavities include natural body cavities and cavities remaining after removal of tissue such as cancerous tissue. A device may include an inner balloon assembly with an inflation conduit. A sheath assembly having a fluid-permeable sheath wall may enclose the inner balloon assembly. Vacuum applied to the space between the sheath and the inner balloon is useful to draw tissue into contact with the device, improving treatment effectiveness. Methods for treating tissue with such devices and systems are also provided. Treatments may include providing radioactive material for radiation treatment, providing chemotherapeutic material for chemotherapy, providing thermal treatment, and combinations thereof. Systems may include devices of the invention and a vacuum source.
Owner:HOLOGIC INC
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
System and method of prediction of response to neurological treatment using the electroencephalogram
Disclosed is a system and method of assessing the efficacy of and predicting response to treatment of neurological or psychological disorders. The preferred embodiment uses at least two surface electrodes to acquire EEG signals from the surface of a patient's body, a processor for computing from the EEG signals various features and indices that are representative of the patient's neurological or psychological state. Pretreatment indices represent a patient's neurological or psychological state and therefore may be used to predict the response to treatment. Changes in these parameters may be used to assess the efficacy of treatment and to modify the treatment to optimize the resultant patient state.
Owner:TYCO HEALTHCARE GRP LP
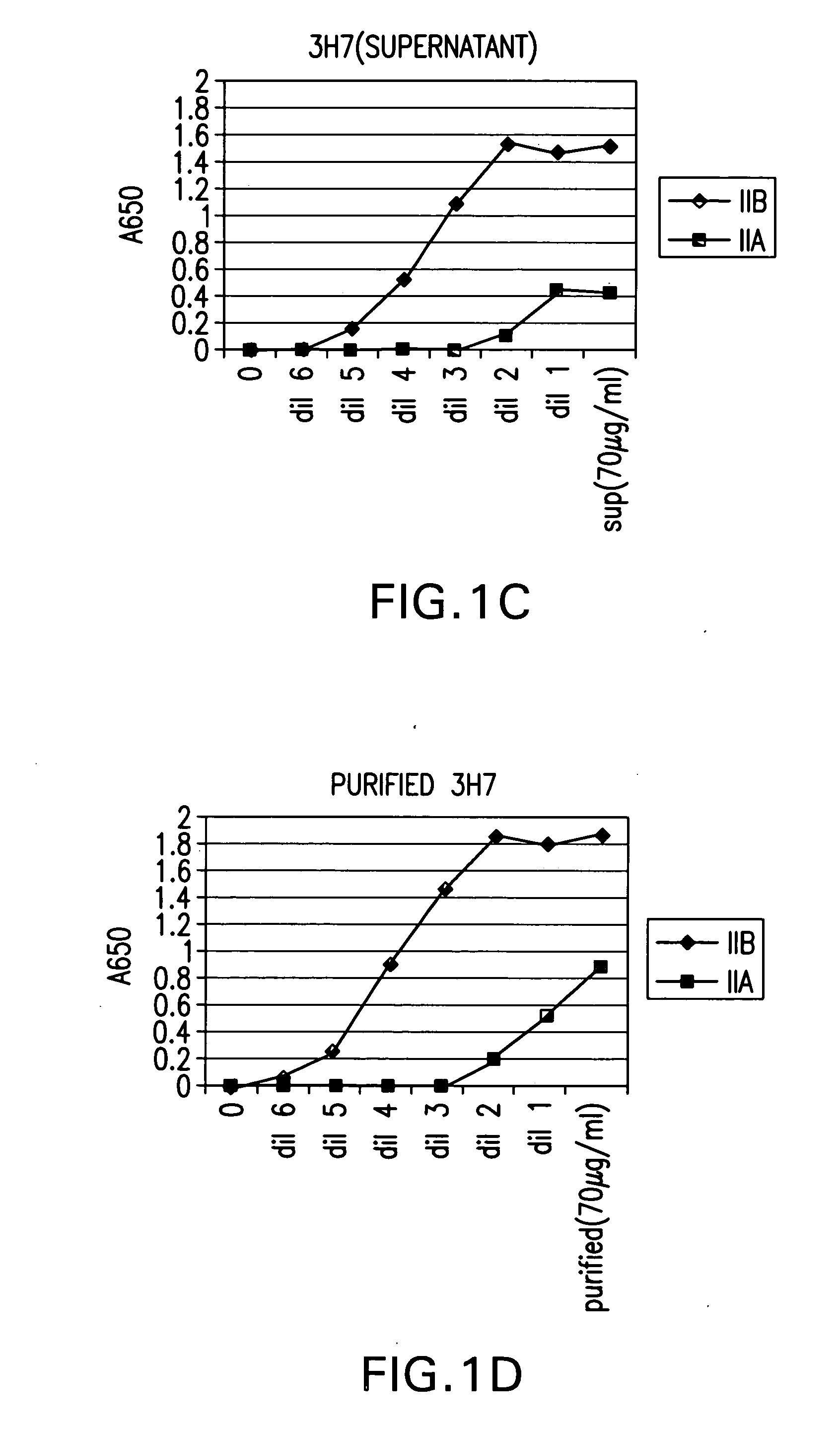
Fcgamma-RIIB-specific antibodies and methods of use thereof
InactiveUS20050260213A1Good curative effectAvoid managementHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigen Binding FragmentTherapeutic effect
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Oral dosage form comprising a therapeutic agent and an adverse-effect agent
InactiveUS20030044458A1Reduce eliminateReduce and eliminate pharmacological effectOrganic active ingredientsNervous disorderOral medicationTherapeutic effect
The present invention provides an oral dosage form comprising a first composition and a second composition. The first composition comprises an effective amount of a therapeutic agent and the second composition comprises an effective amount of an adverse-effect agent. The adverse-effect agent is covered with a coating that is substantially insoluble in the gastrointestinal tract. In one embodiment, the adverse-effect agent is coated with an outer base-soluble layer and an inner acid-soluble layer. The therapeutic agent can be uncoated or can be coated with a coating having an outer acid-soluble layer and an inner base-soluble layer. The dosage form discourages administration of the therapeutic agent by other than oral administration.
Owner:PURDUE PHARMA LP
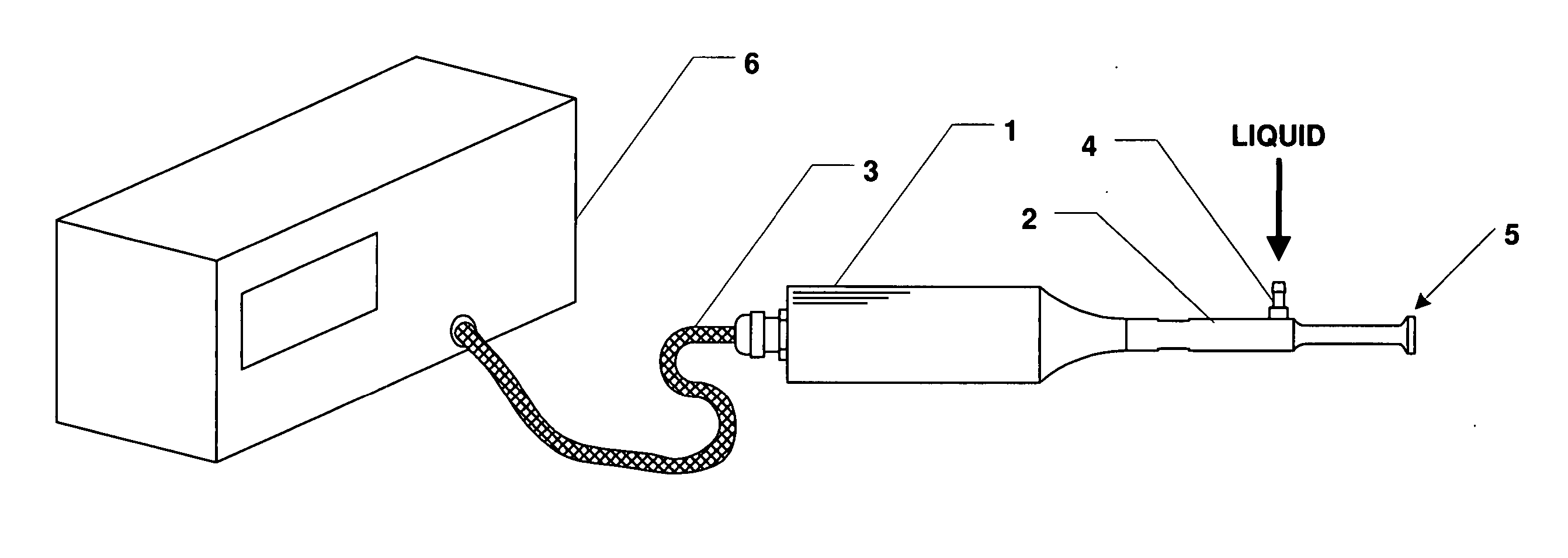
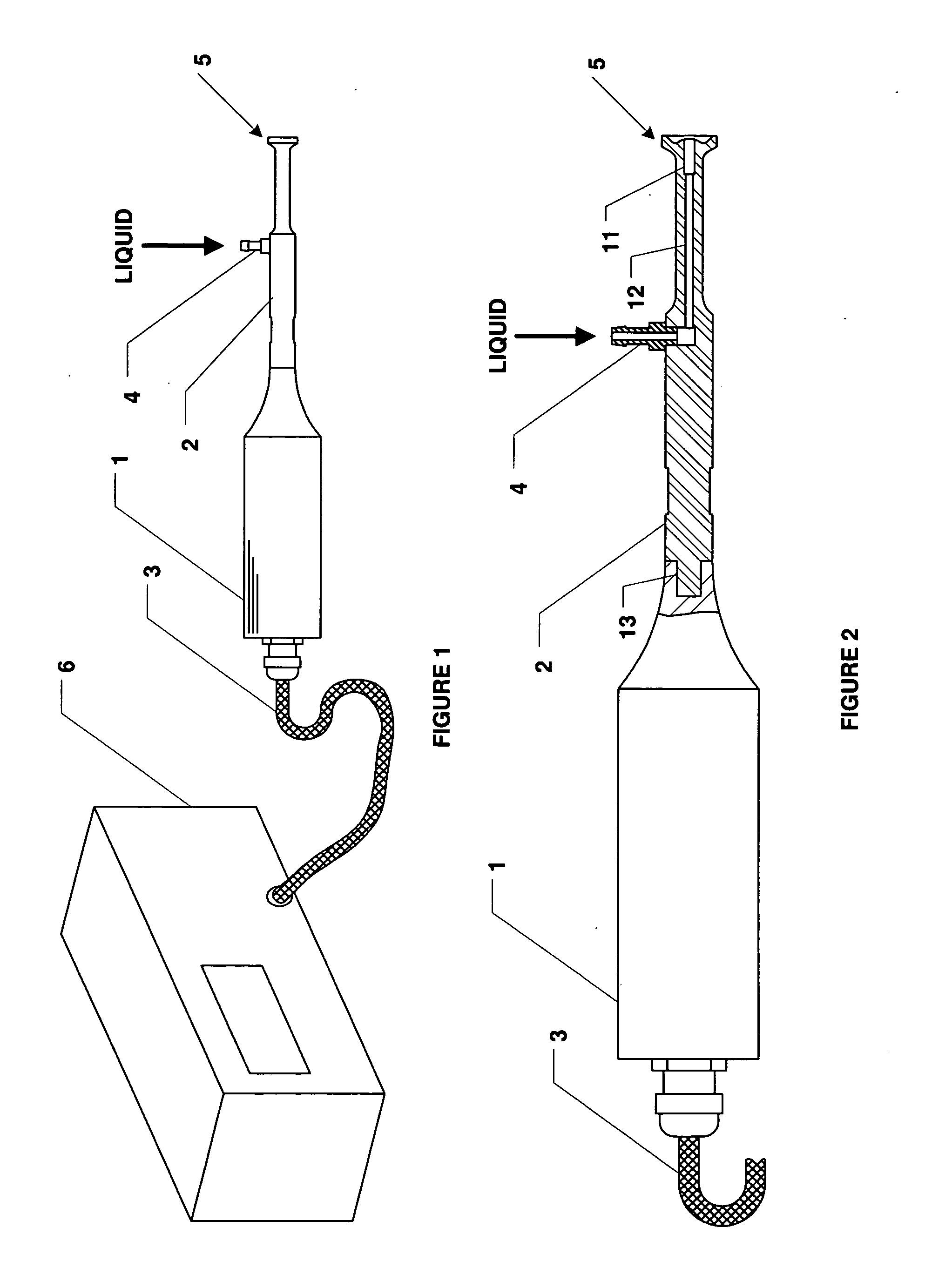
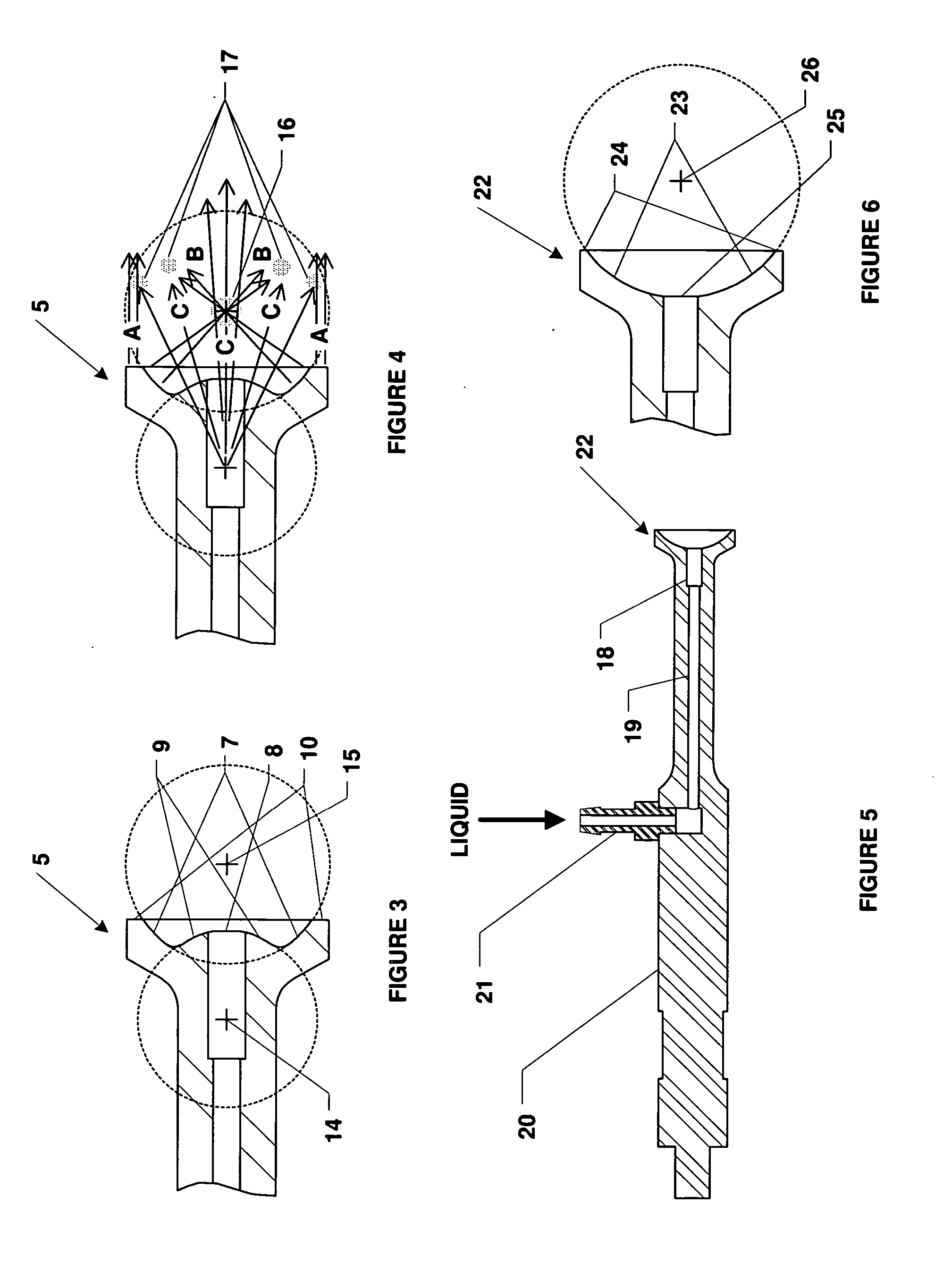
Method and apparatus for delivery of therapeutic agents
Methods and apparatus for the reproducible, consistent and efficacious delivery of a therapeutic agent to a patient. The invention comprises means for the controlled administration of the therapeutic agent through an orifice to the patient, a plurality of penetrating electrodes arranged with a predetermined spatial relationship relative to the orifice, and means for generating an electrical signal operatively connected to the electrodes.
Owner:ICHOR MEDICAL SYST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com