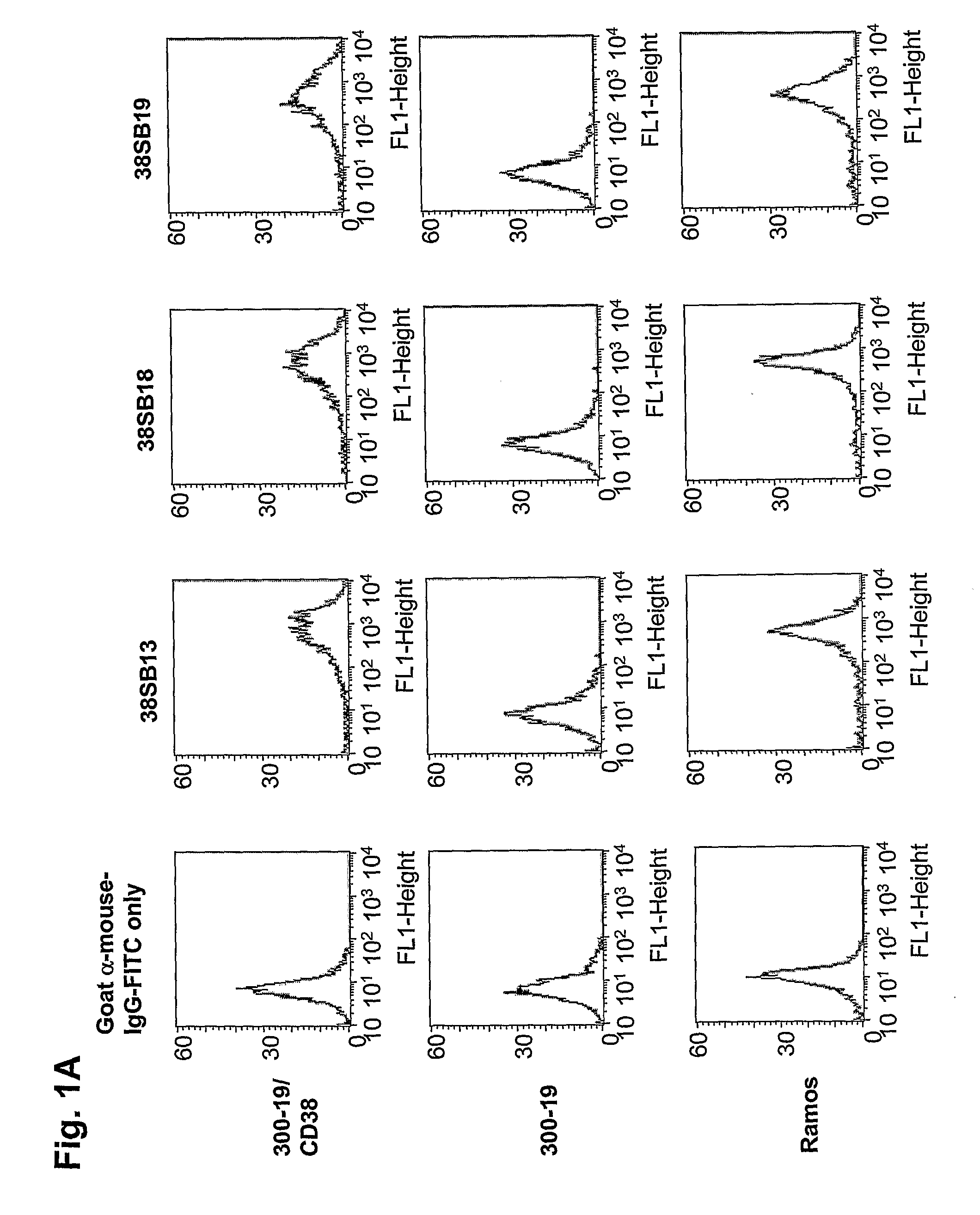
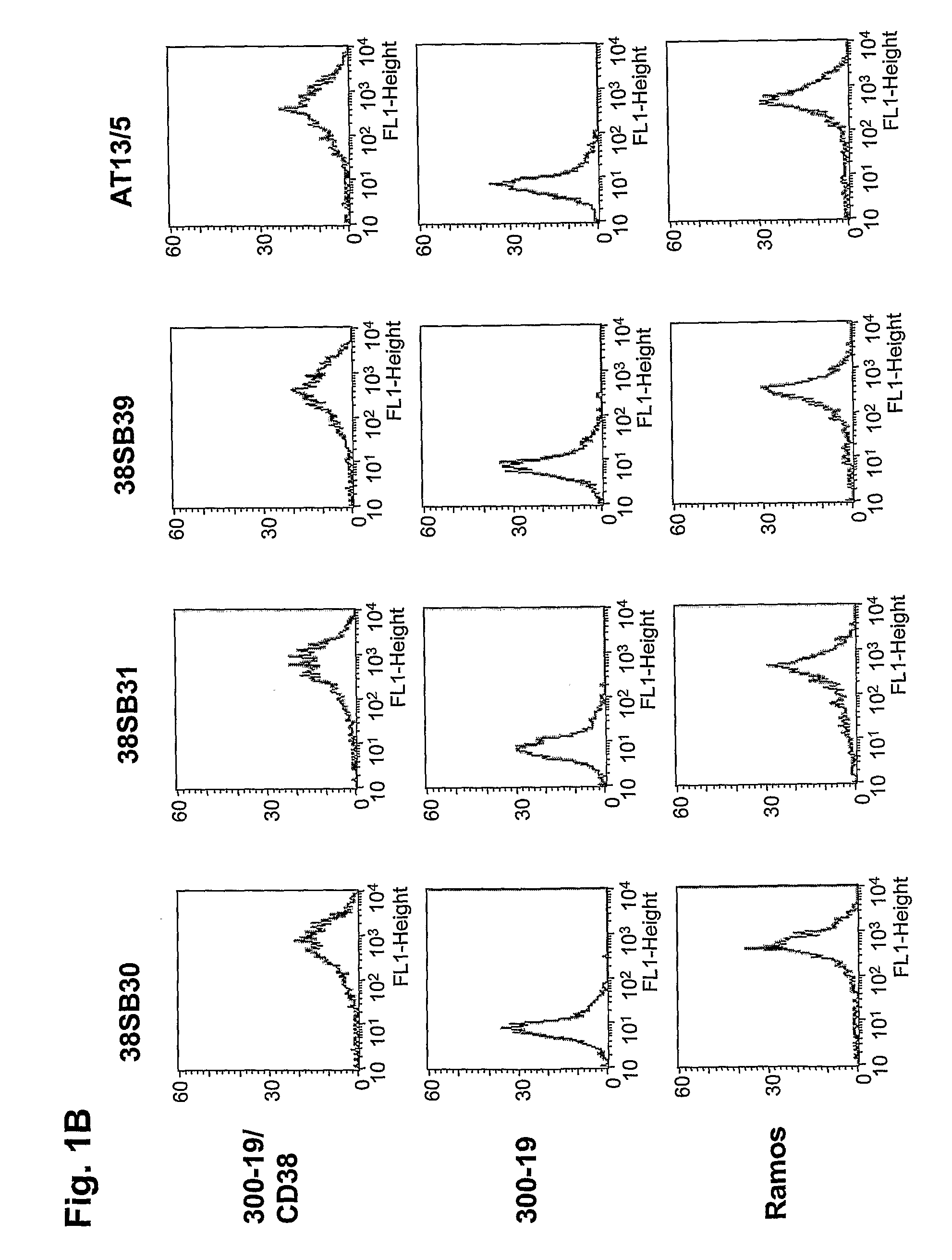
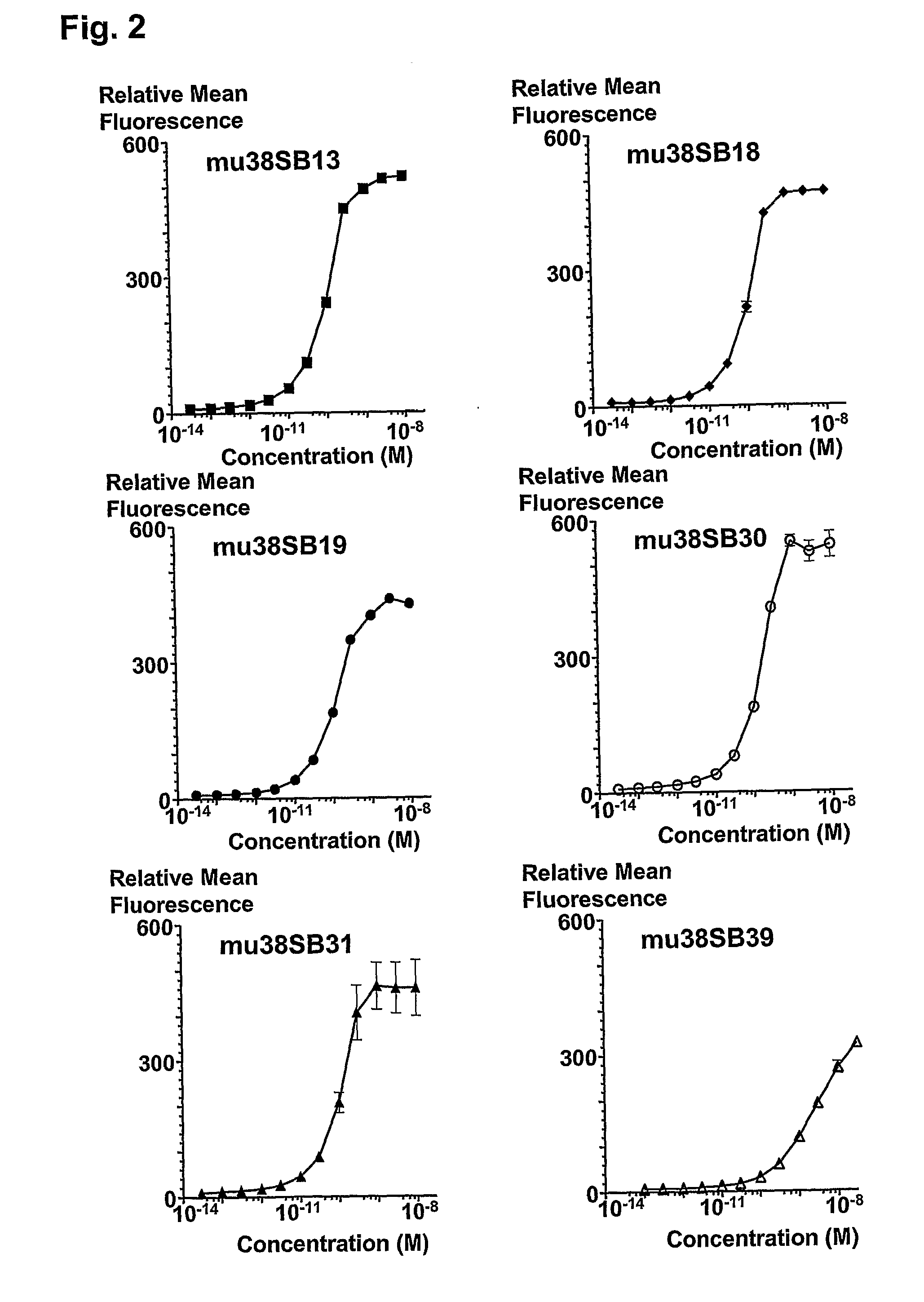
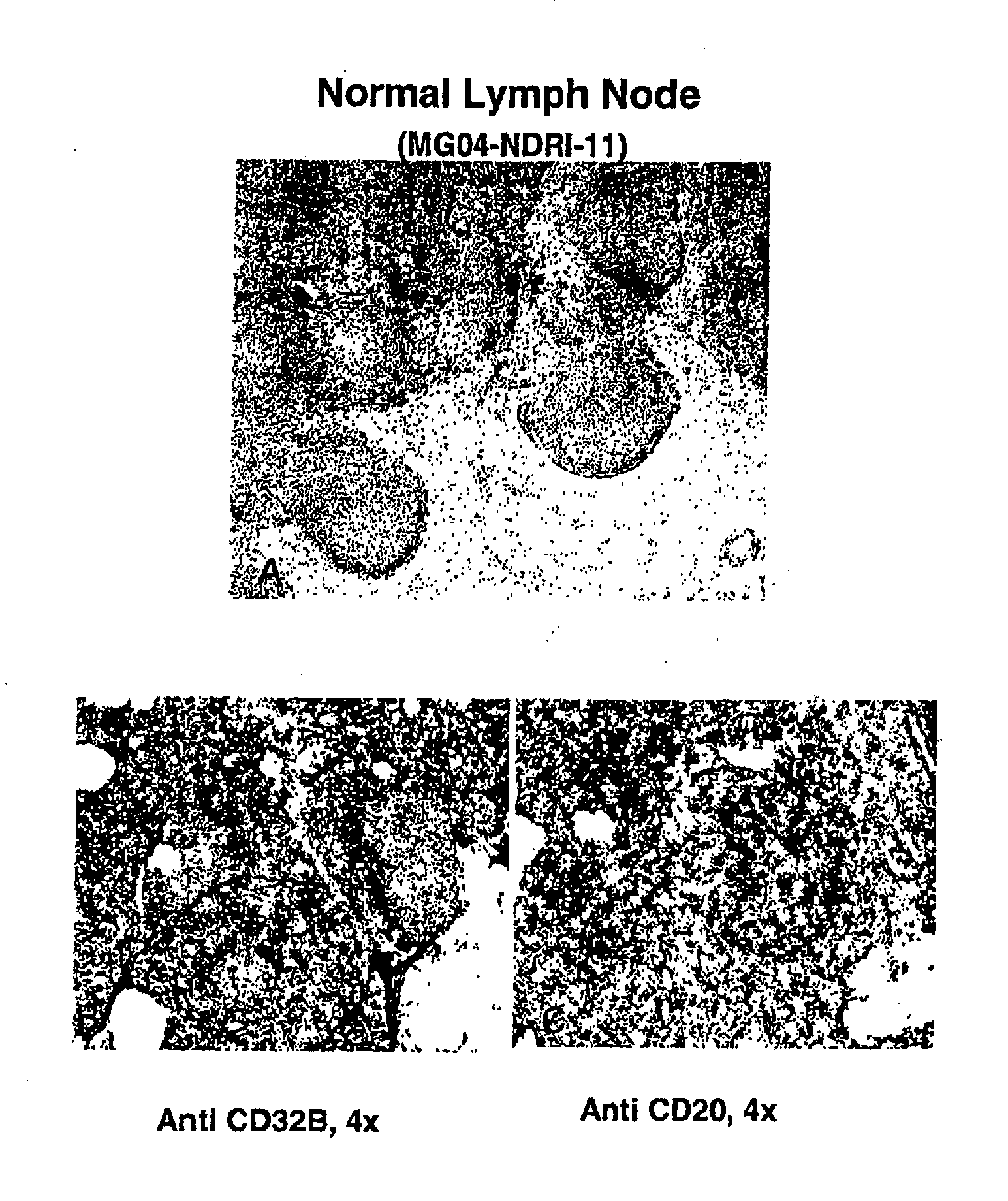
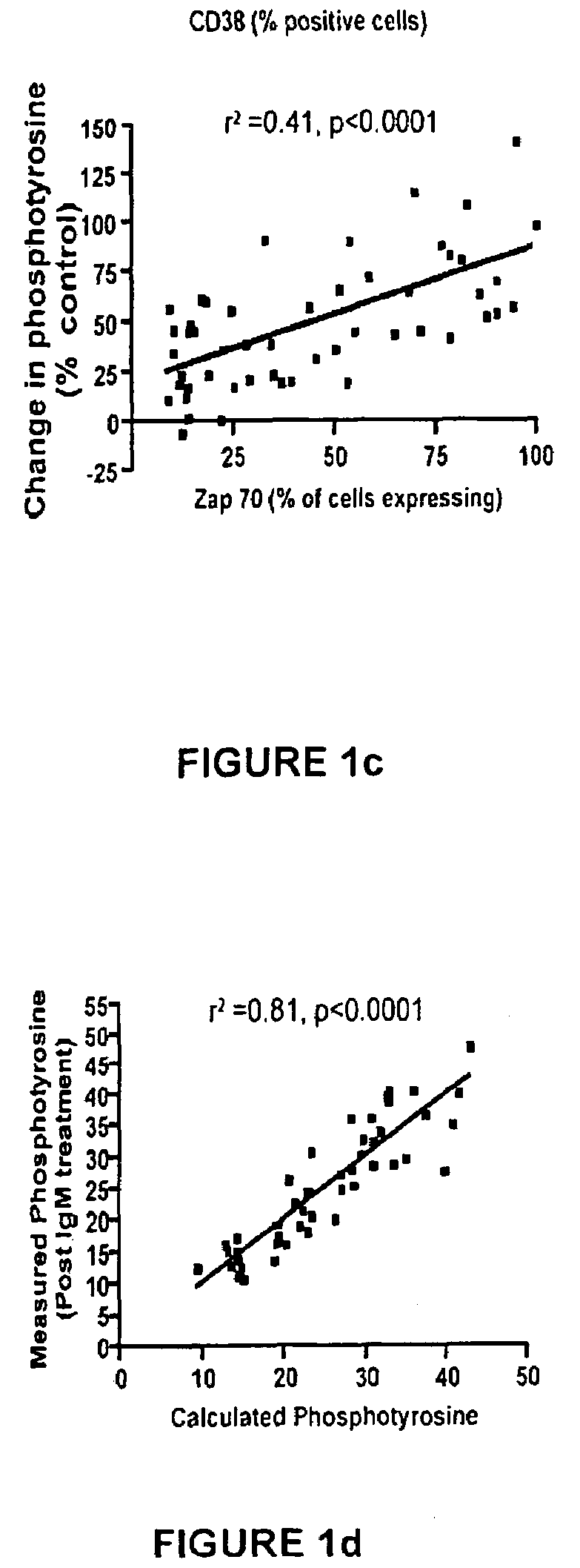
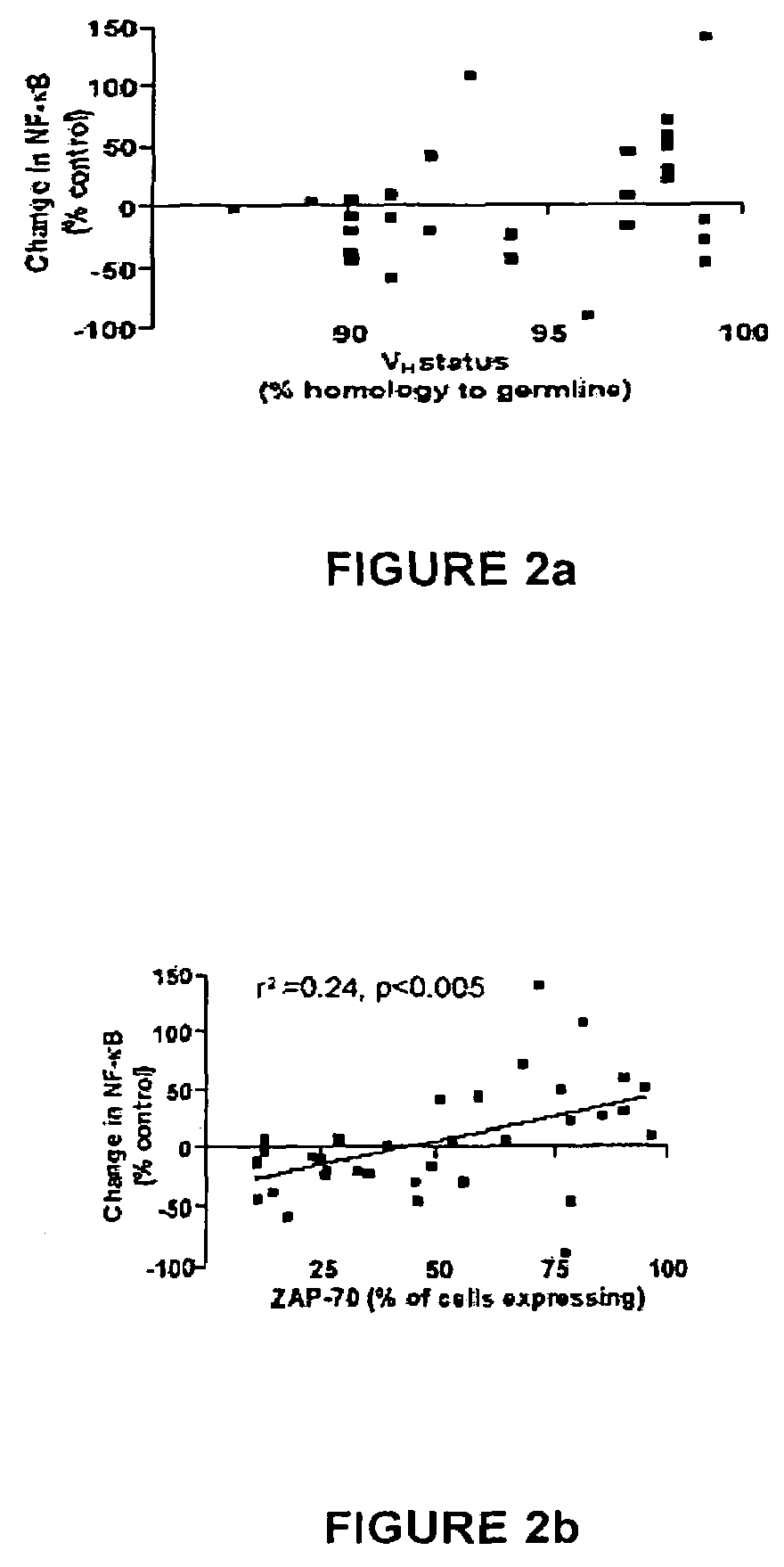
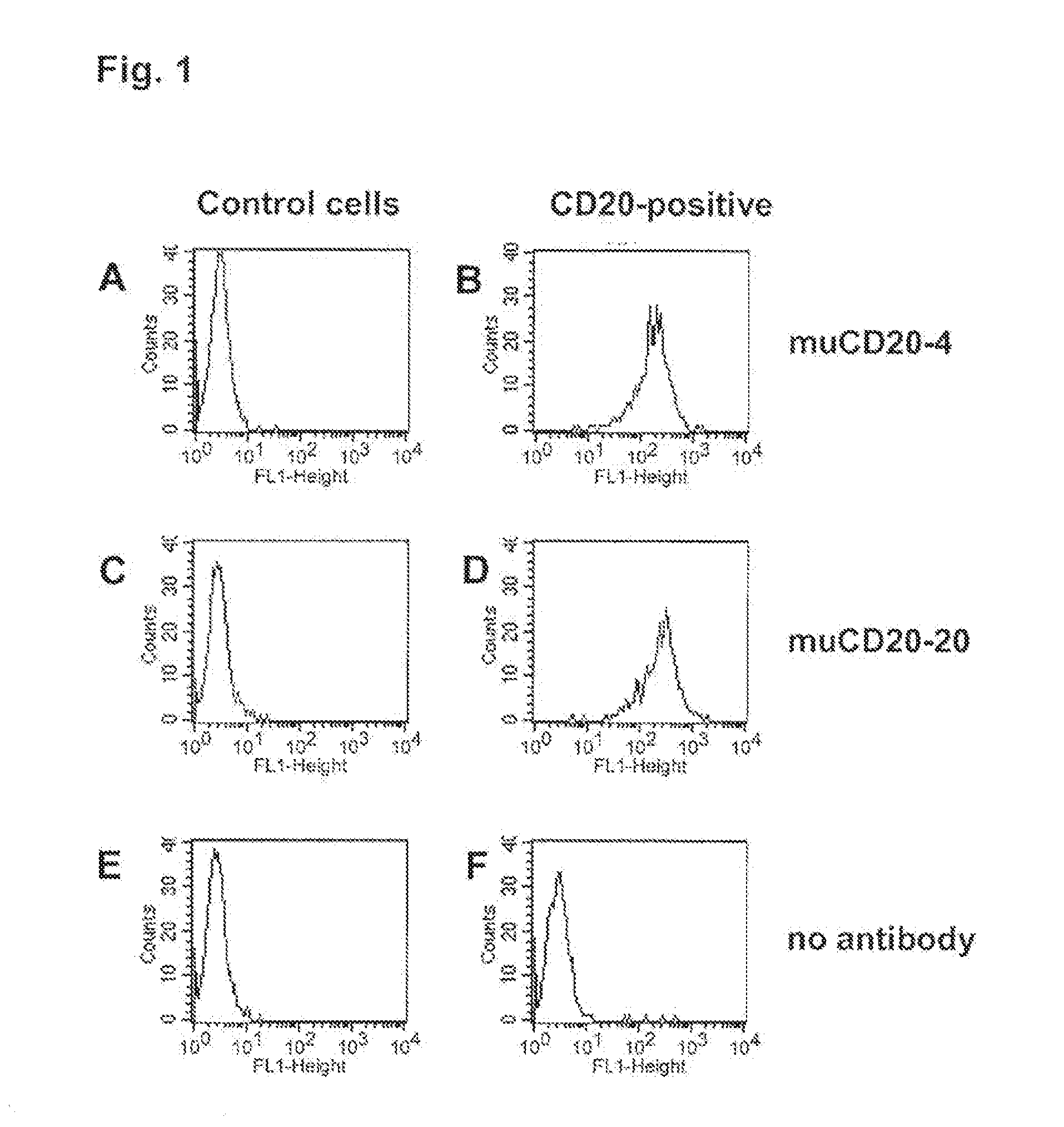
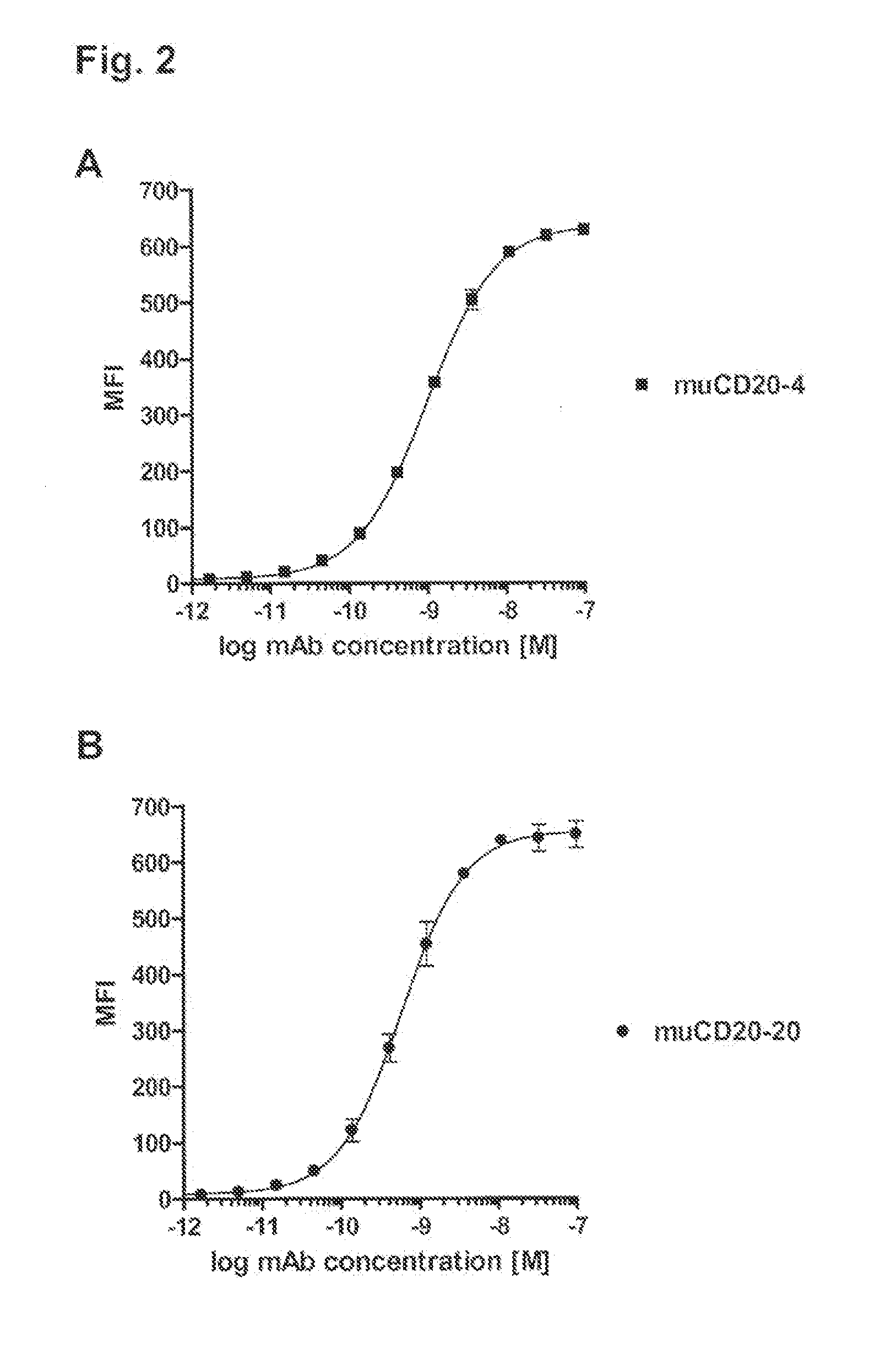
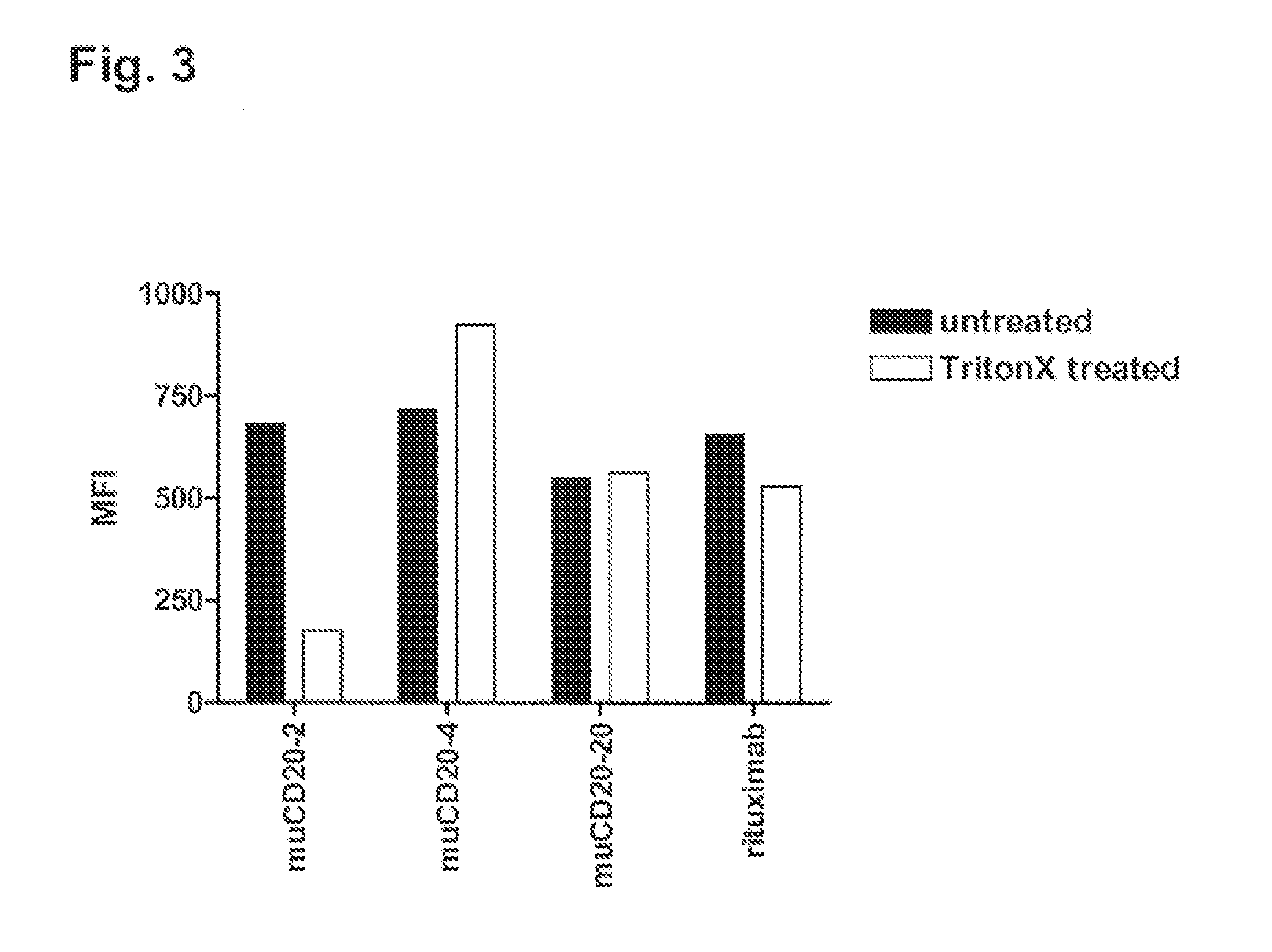
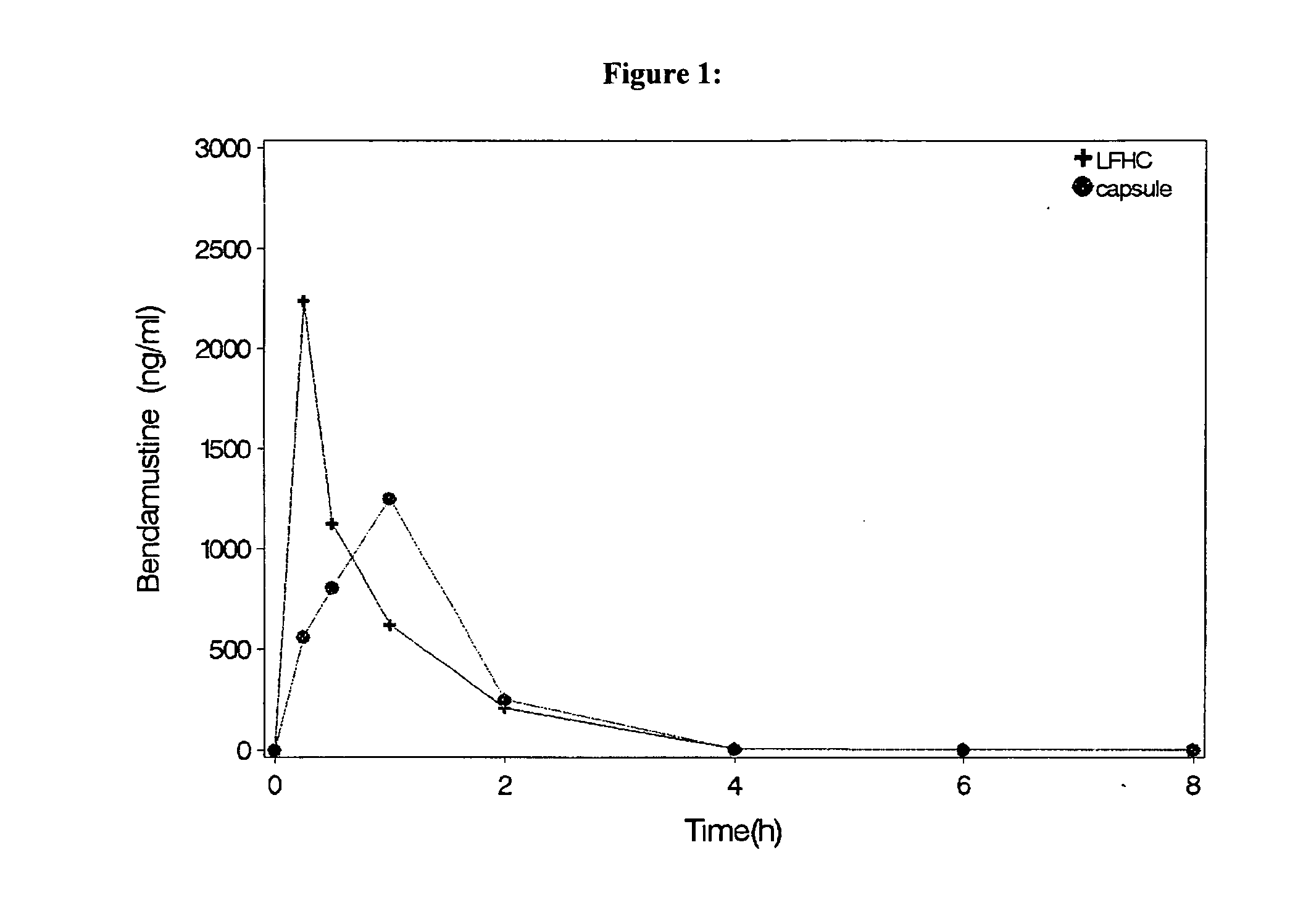
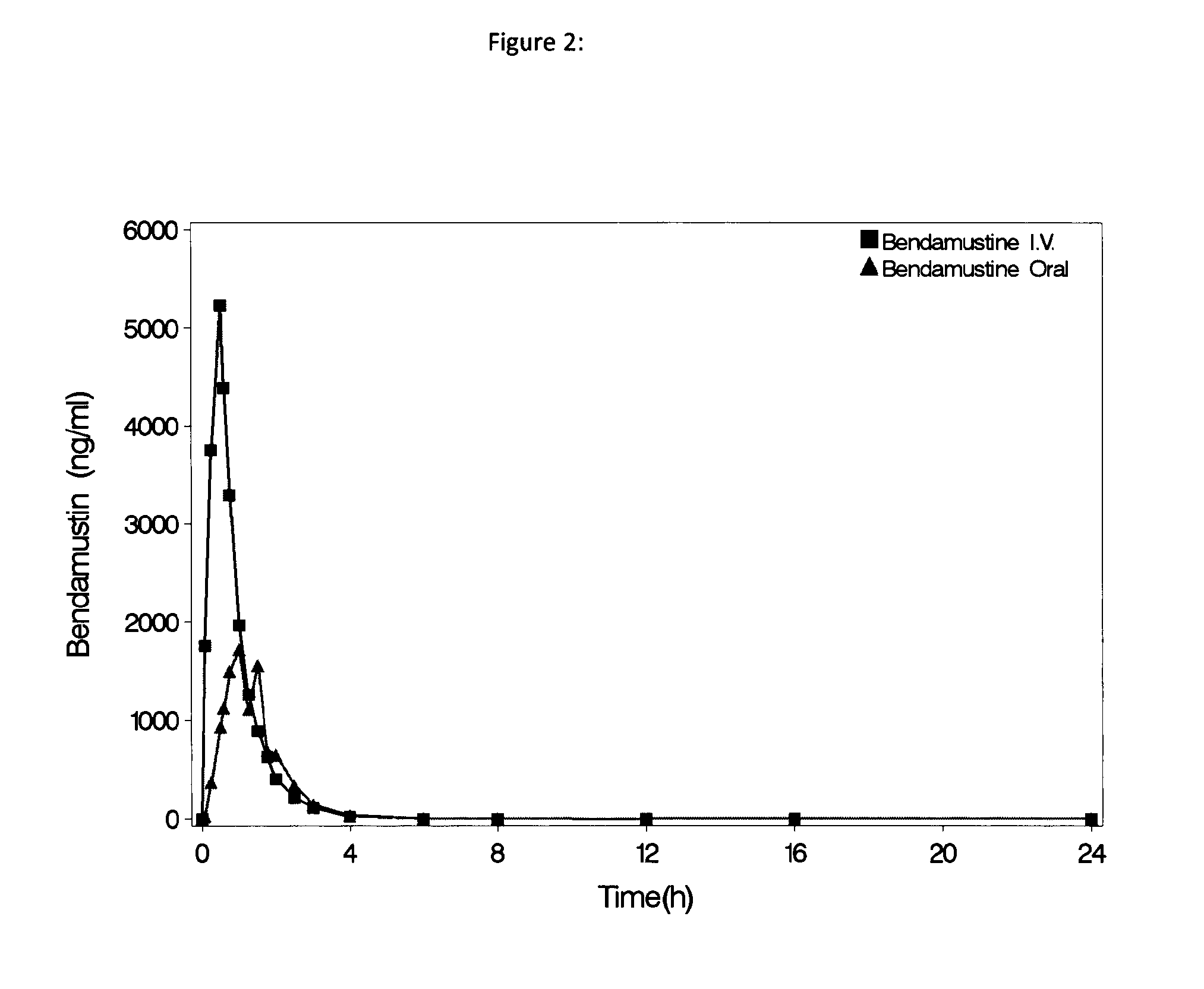
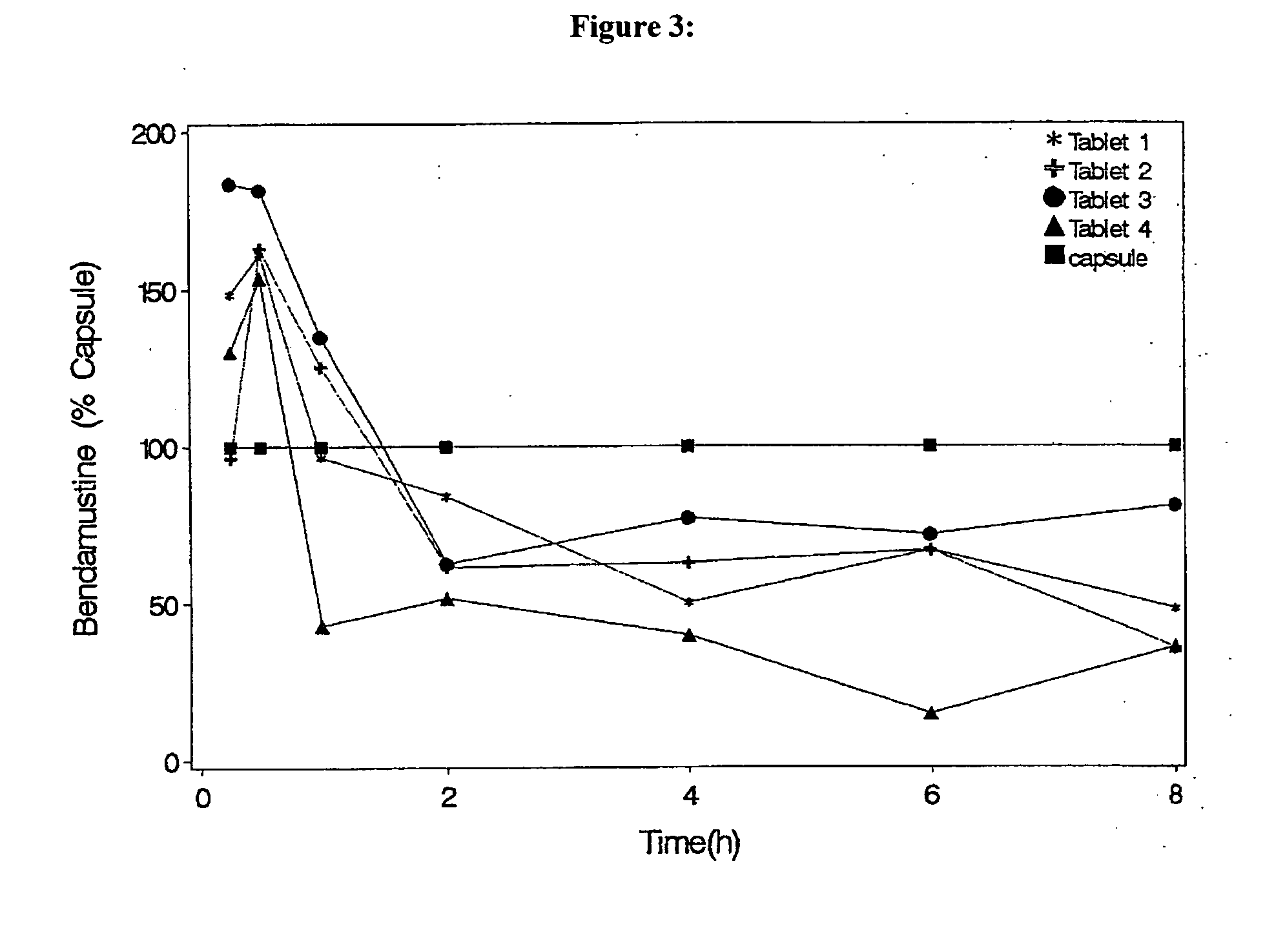
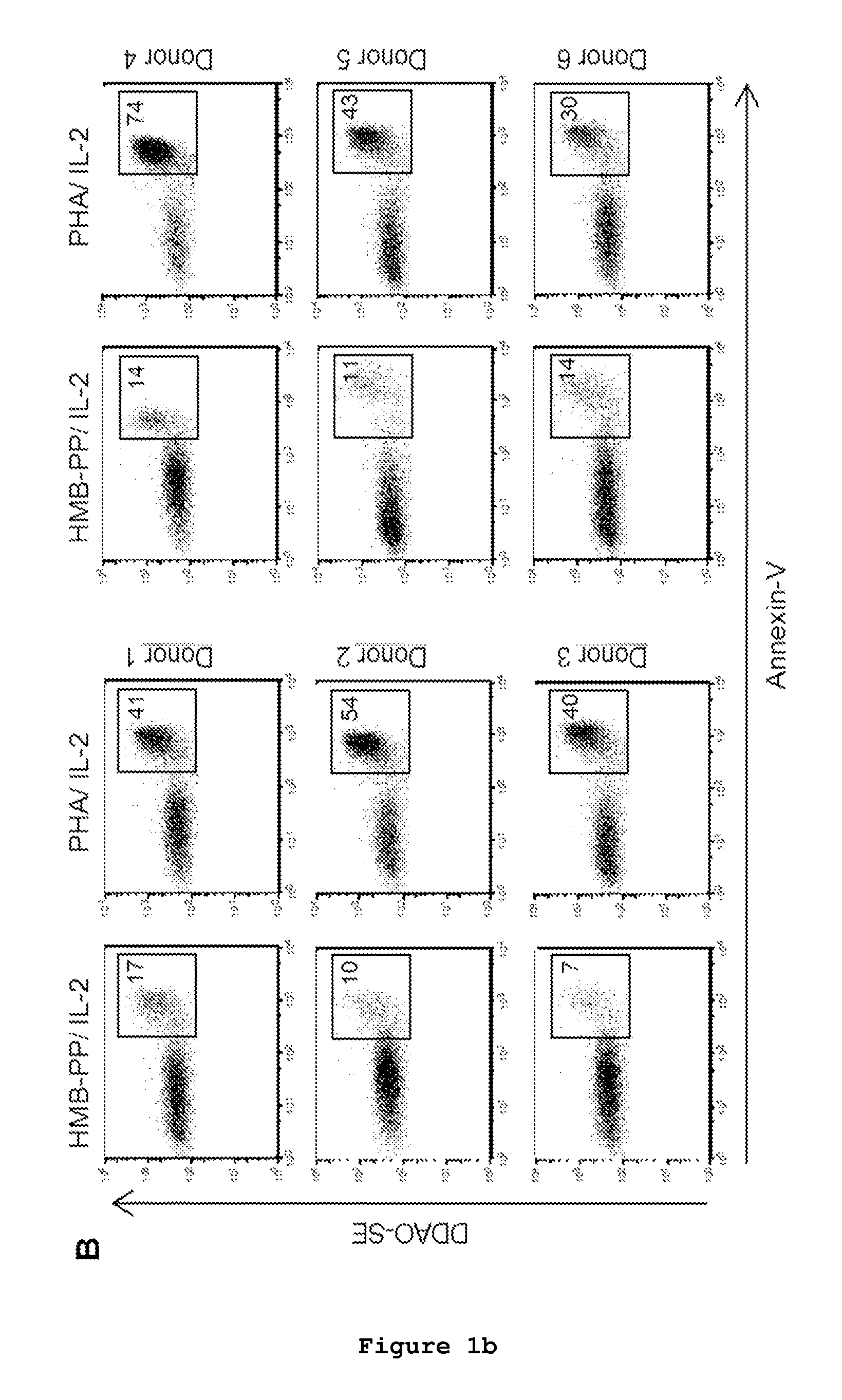
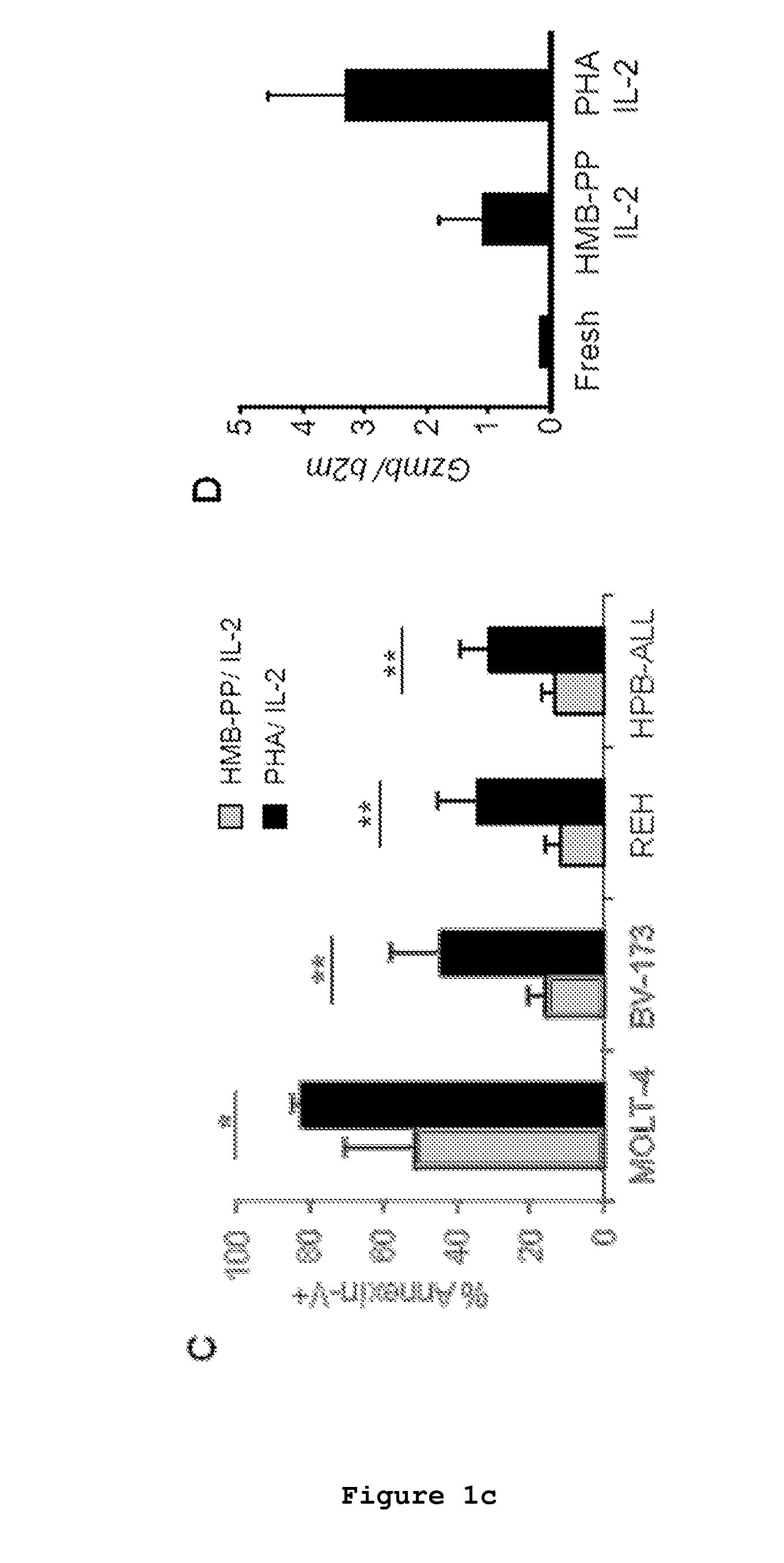
Patents
Literature
157 results about "Chronic lymphocytic leukemia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A type of cancer that begins in the lymphocytes of bone marrow and extends into the blood.
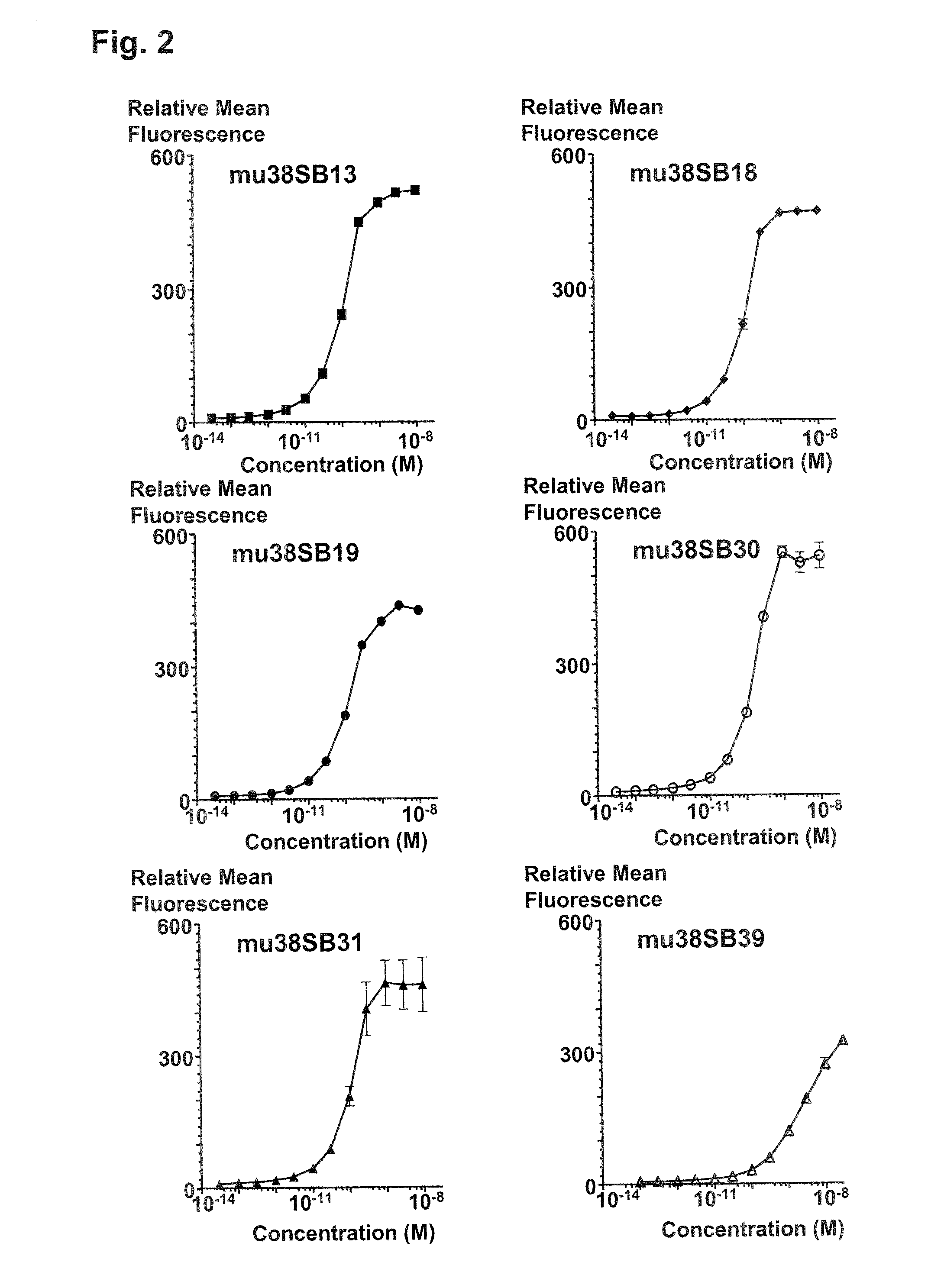
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Compositions and methods for cancer diagnosis and therapy
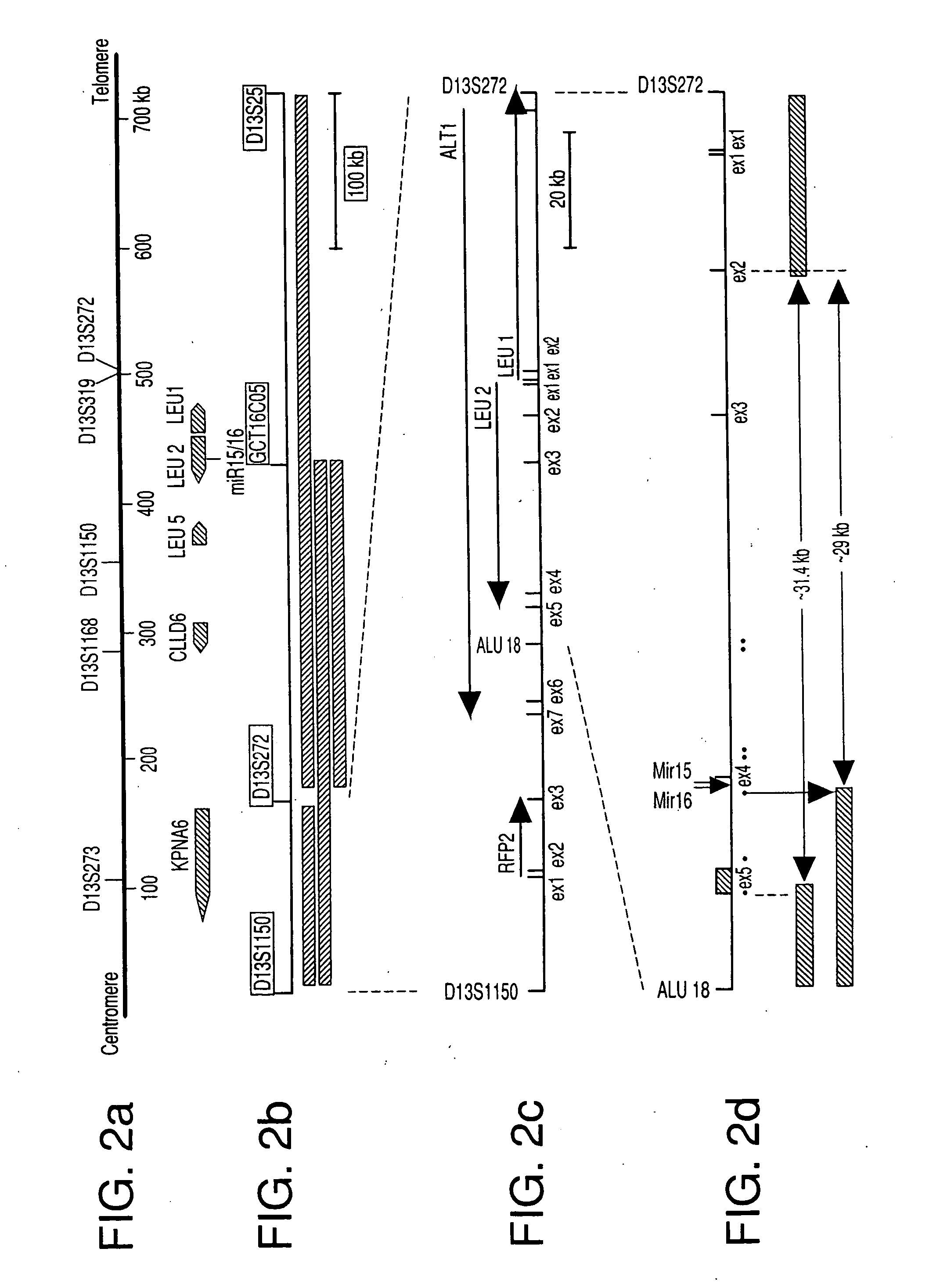
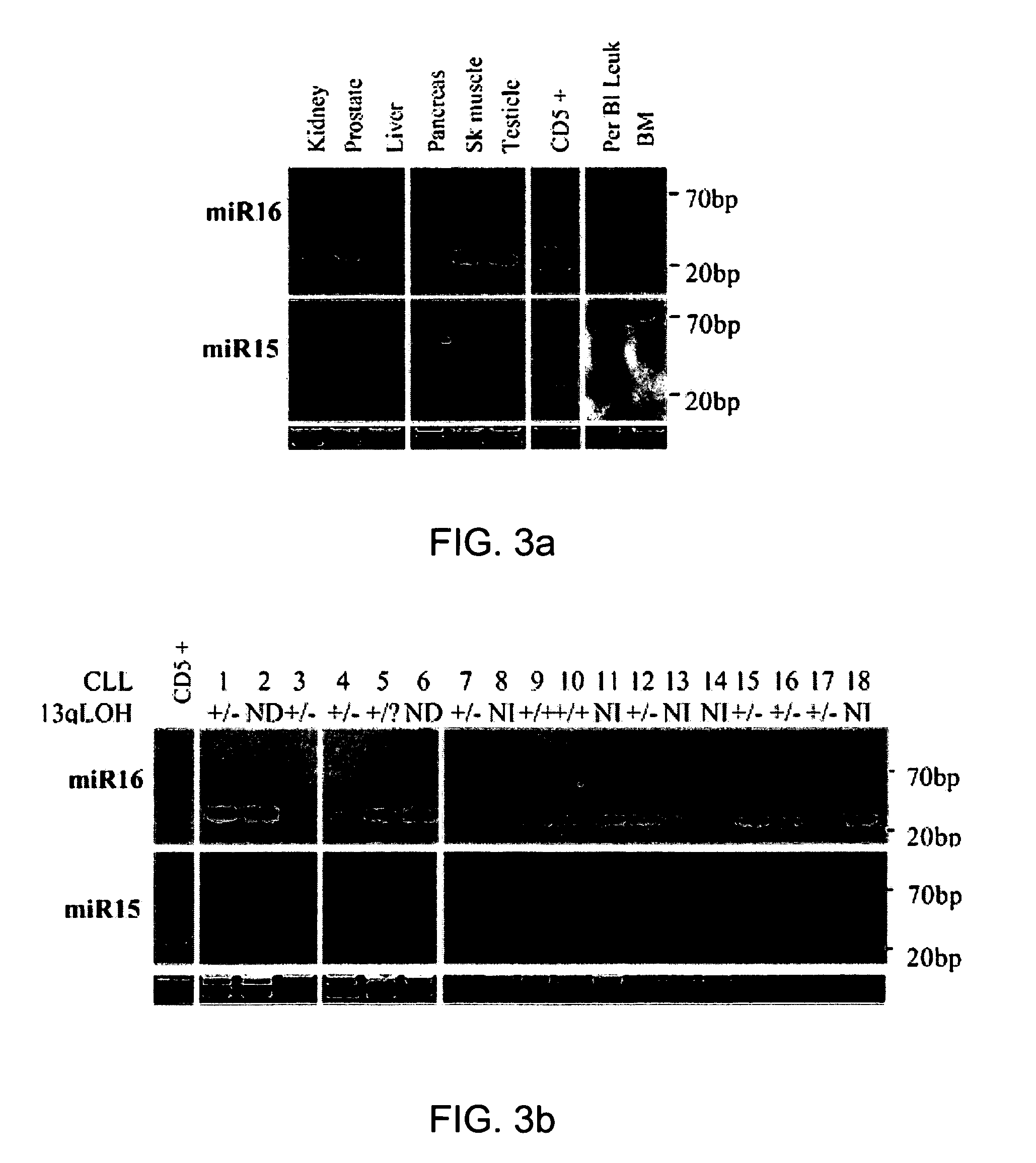
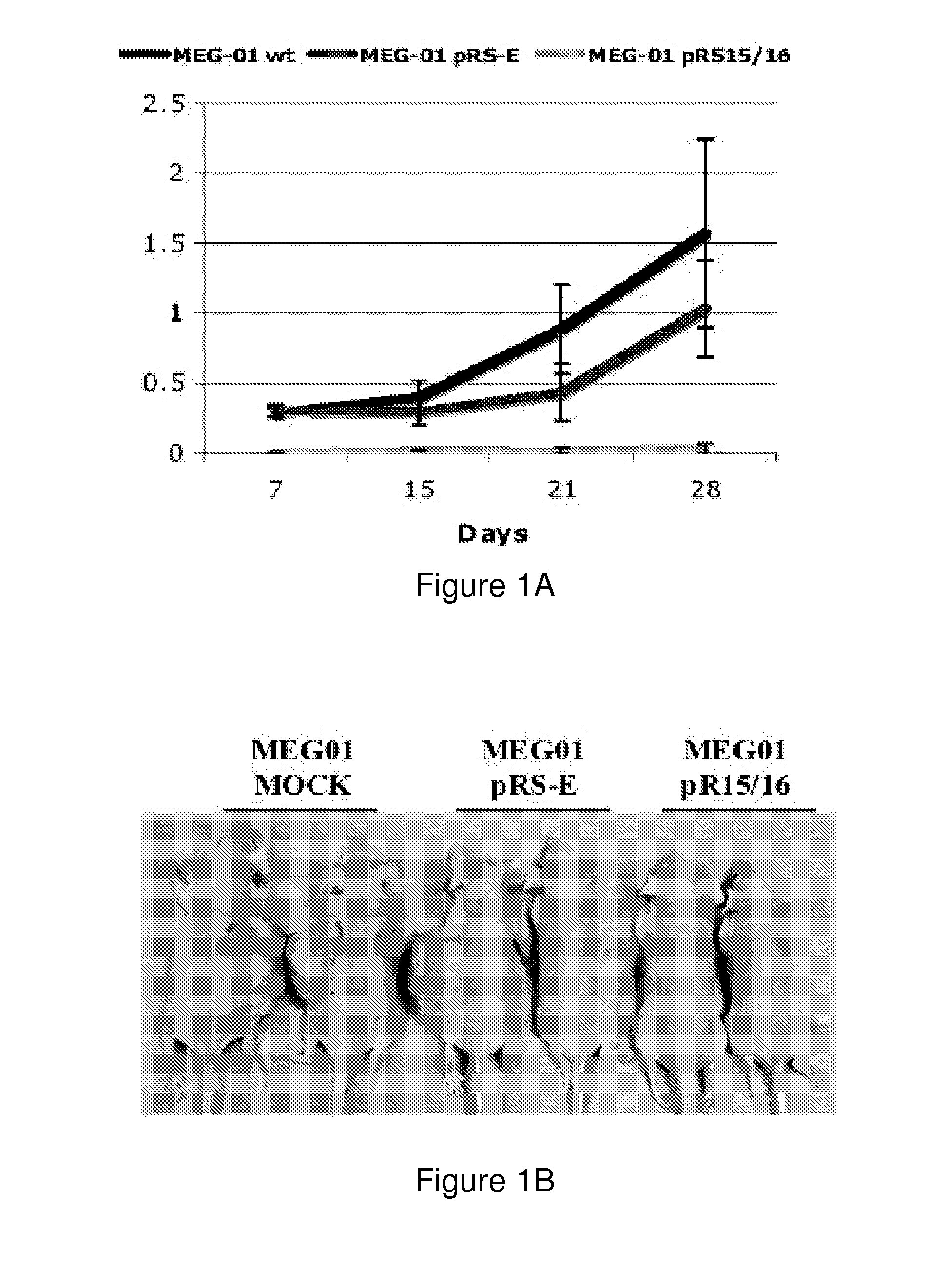
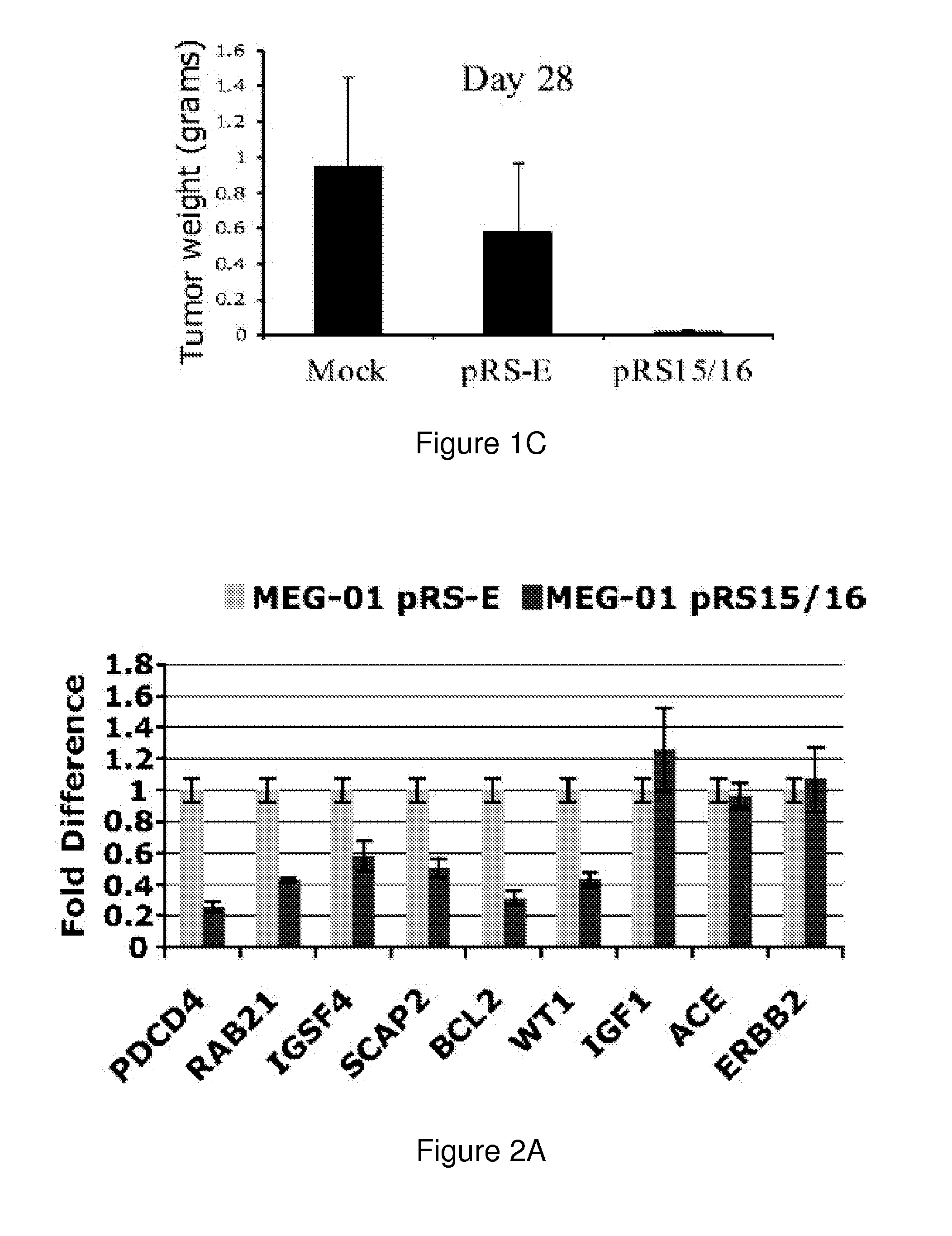
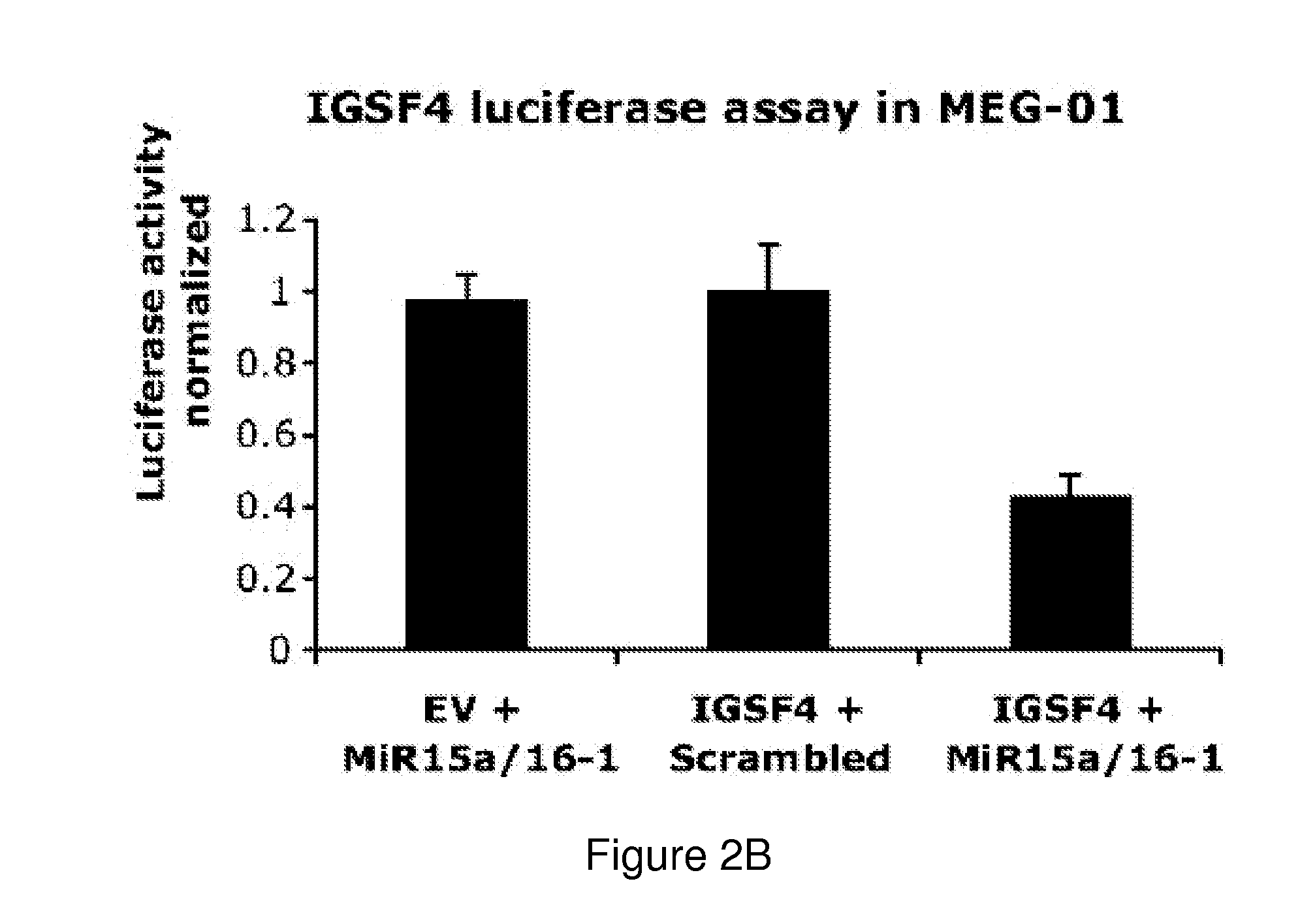
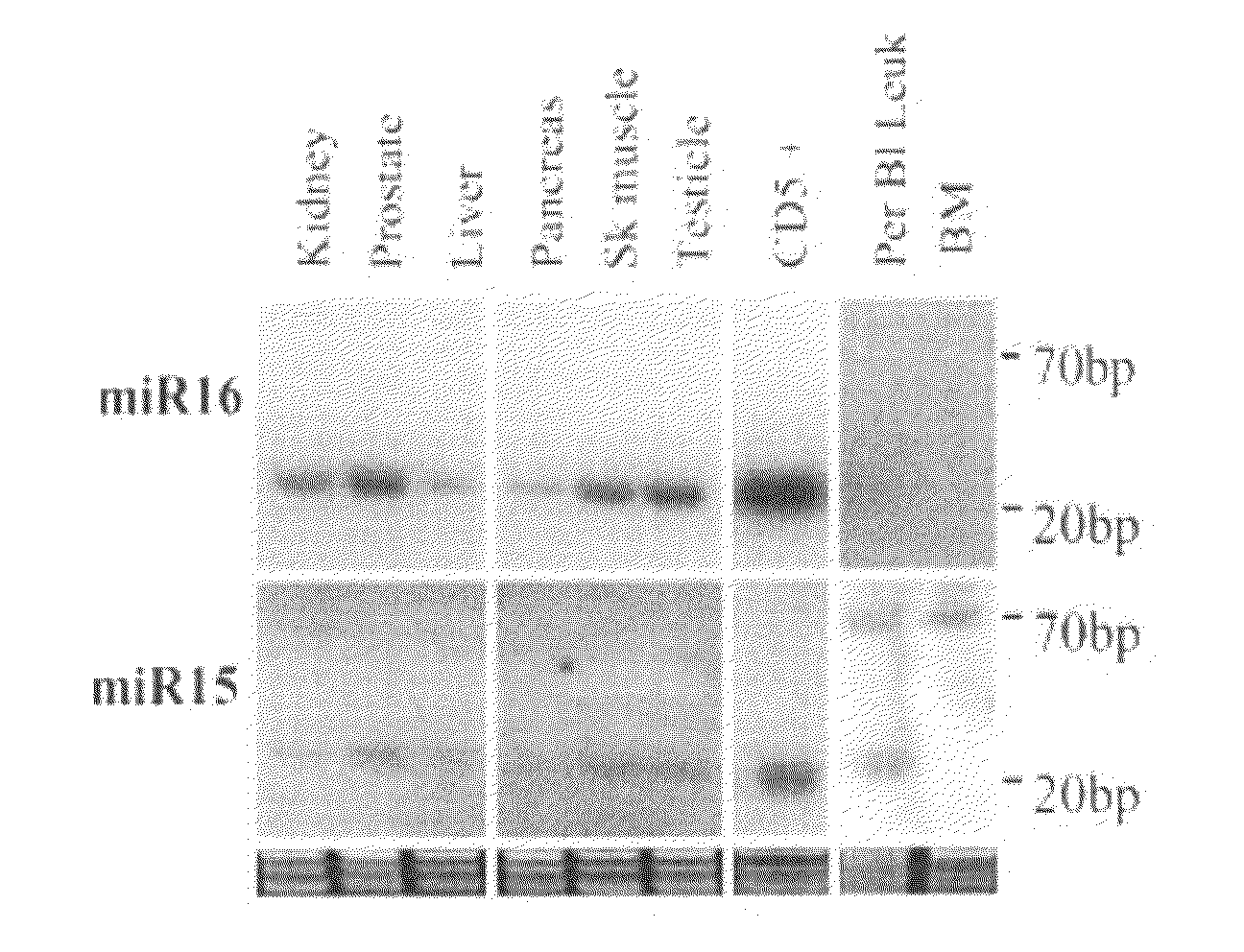
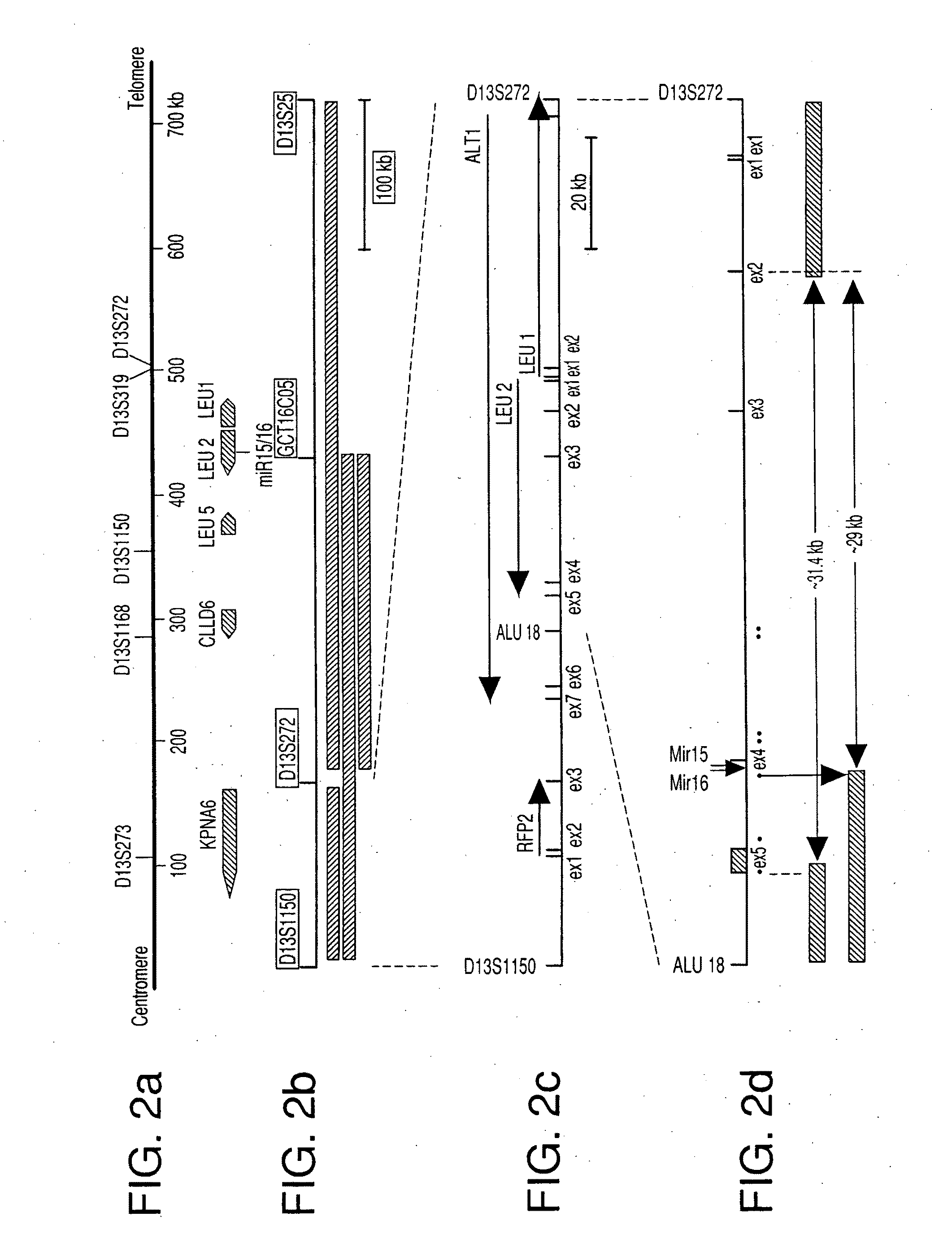
The miR15 and miR16 micro RNA genes are located at 13q14 within a 30 kb region of loss characteristic of cells from certain cancers, such as cells from chronic lymphocytic leukemia or prostate cancer. Chronic lymphocytic leukemia or prostate cancer can be diagnosed by detecting a reduction in miR15 or miR16 gene copy number, by determining miR15 or miR16 gene mutational status, or by detecting a reduction in the RNA transcribed from these genes. The miR15 or miR16 gene products can inhibit the neoplastic or tumorigenic growth of cancers such as chronic lymphocytic leukemia or prostate cancer cells when administered to subjects suffering from these diseases.
Owner:THOMAS JEFFERSON UNIV
Methods for diagnosis prognosis and methods of treatment
InactiveUS20090098594A1Minimal resistanceMicrobiological testing/measurementBiological material analysisCancer cellSignalling molecules
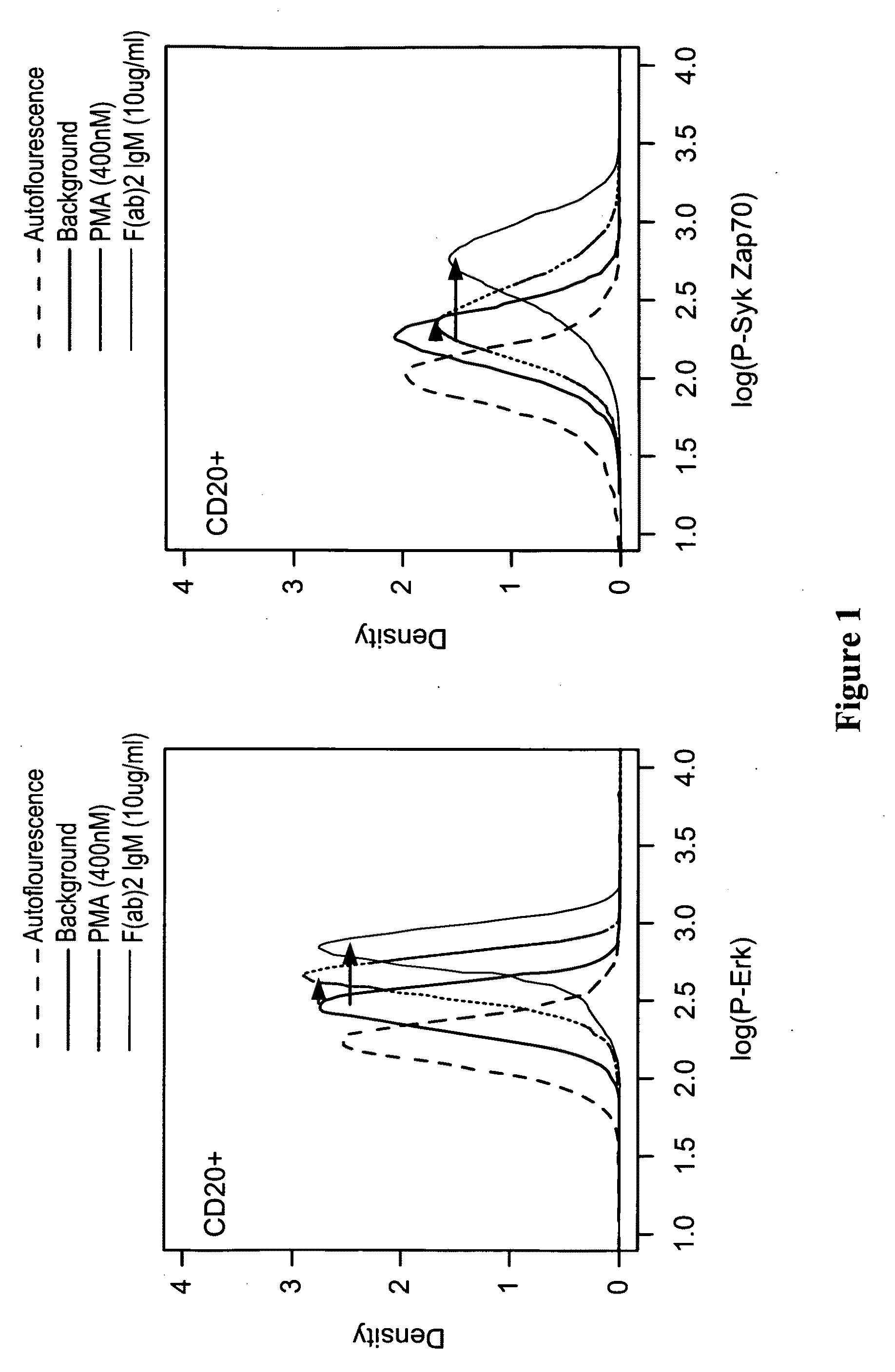
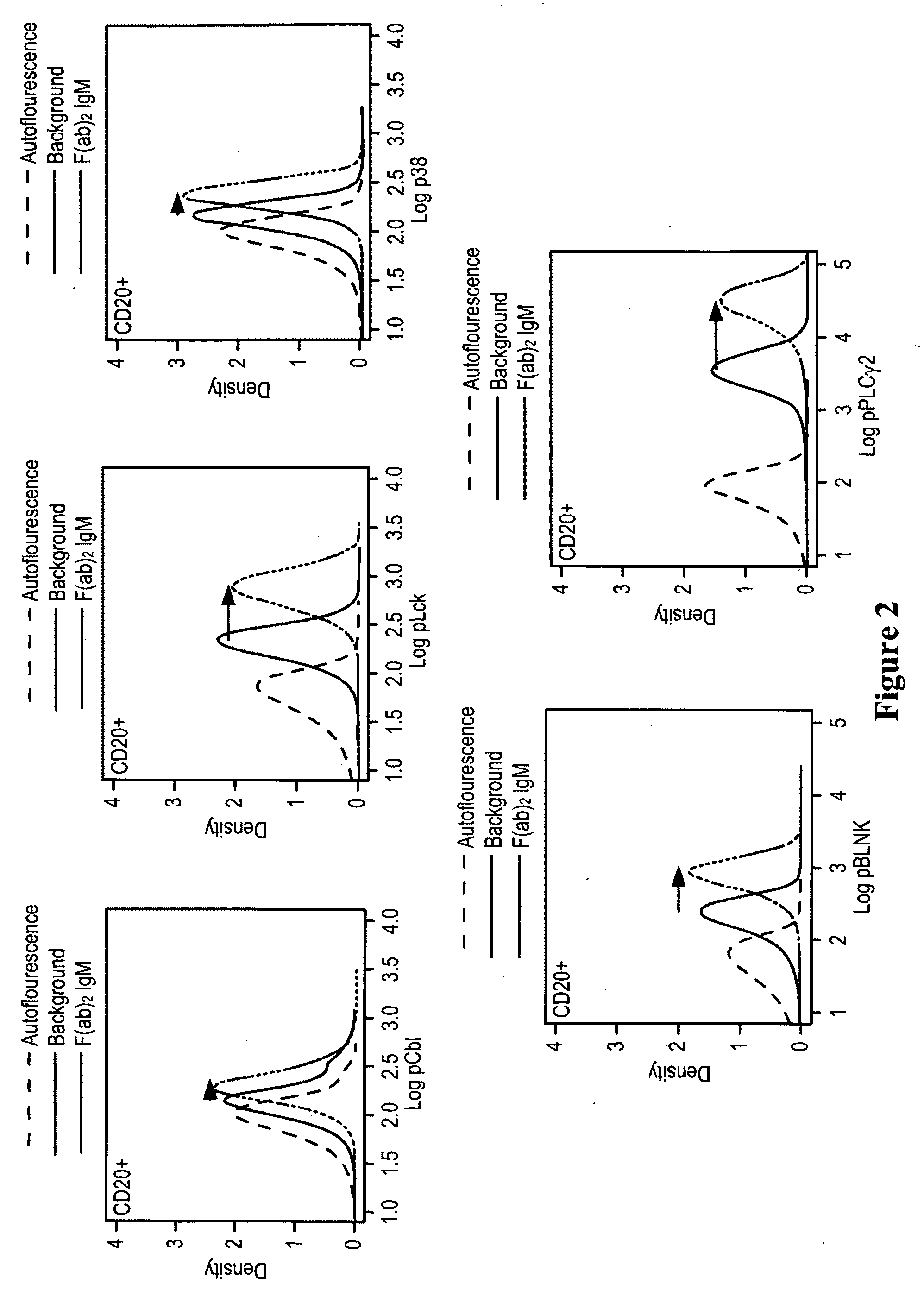
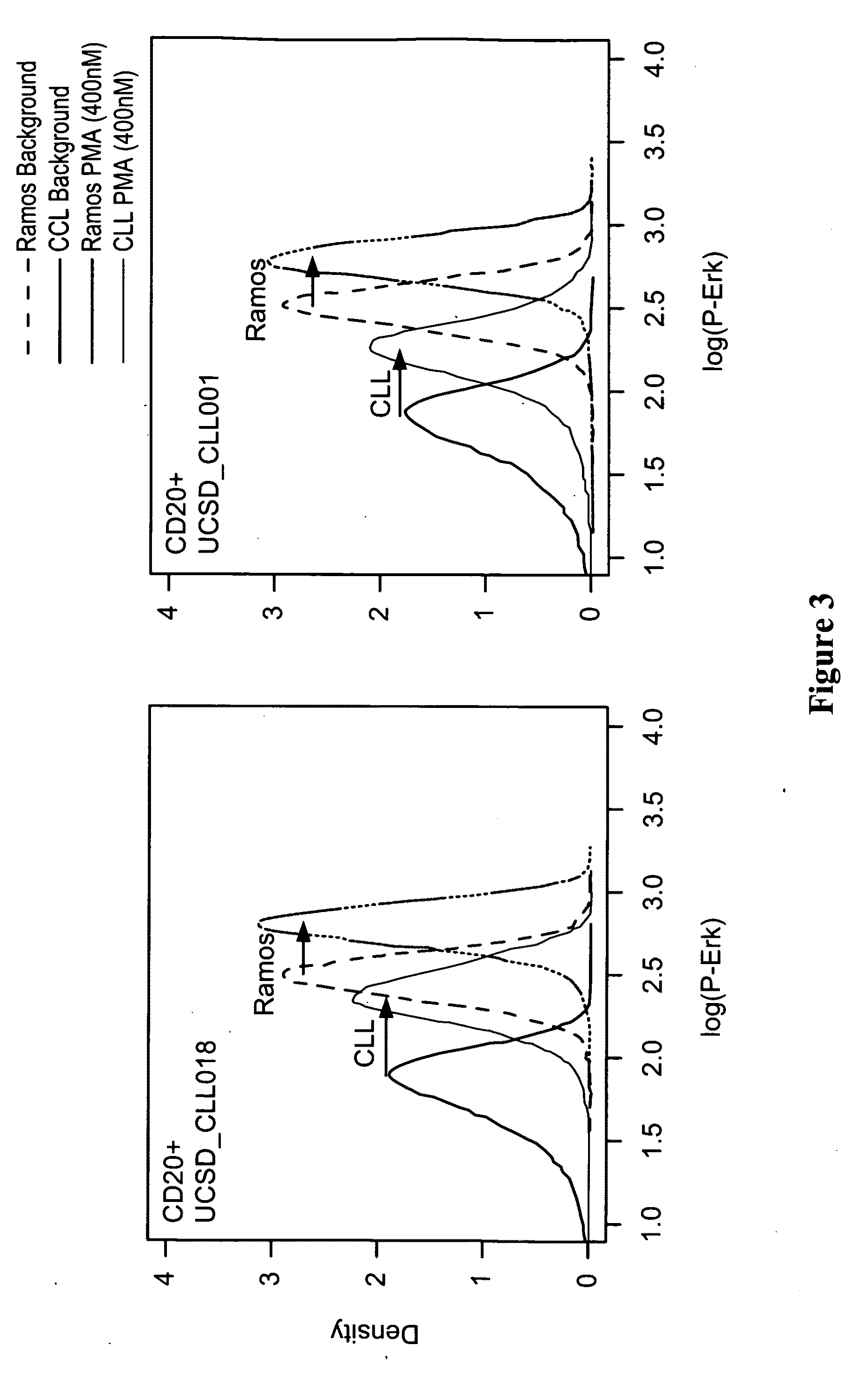
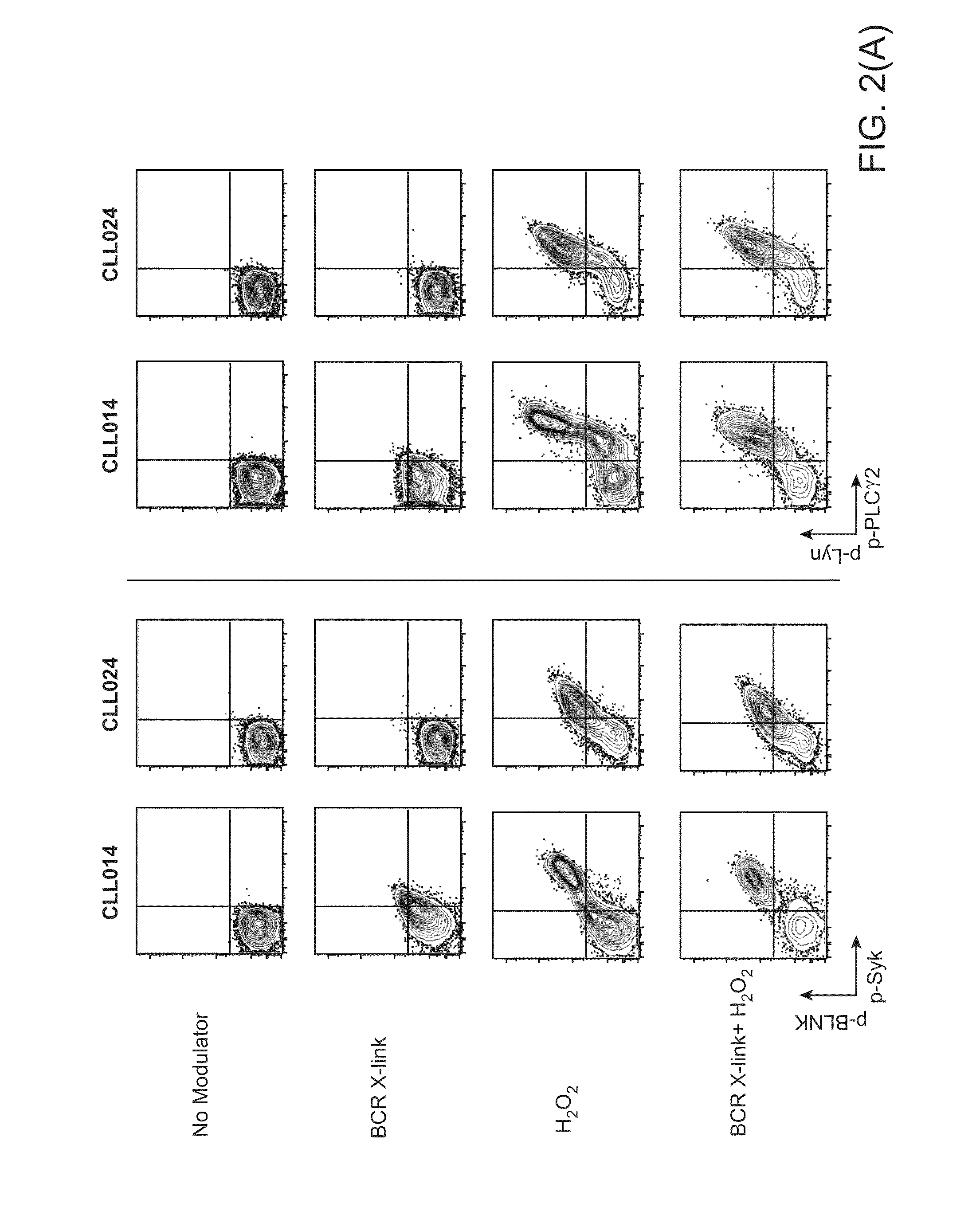
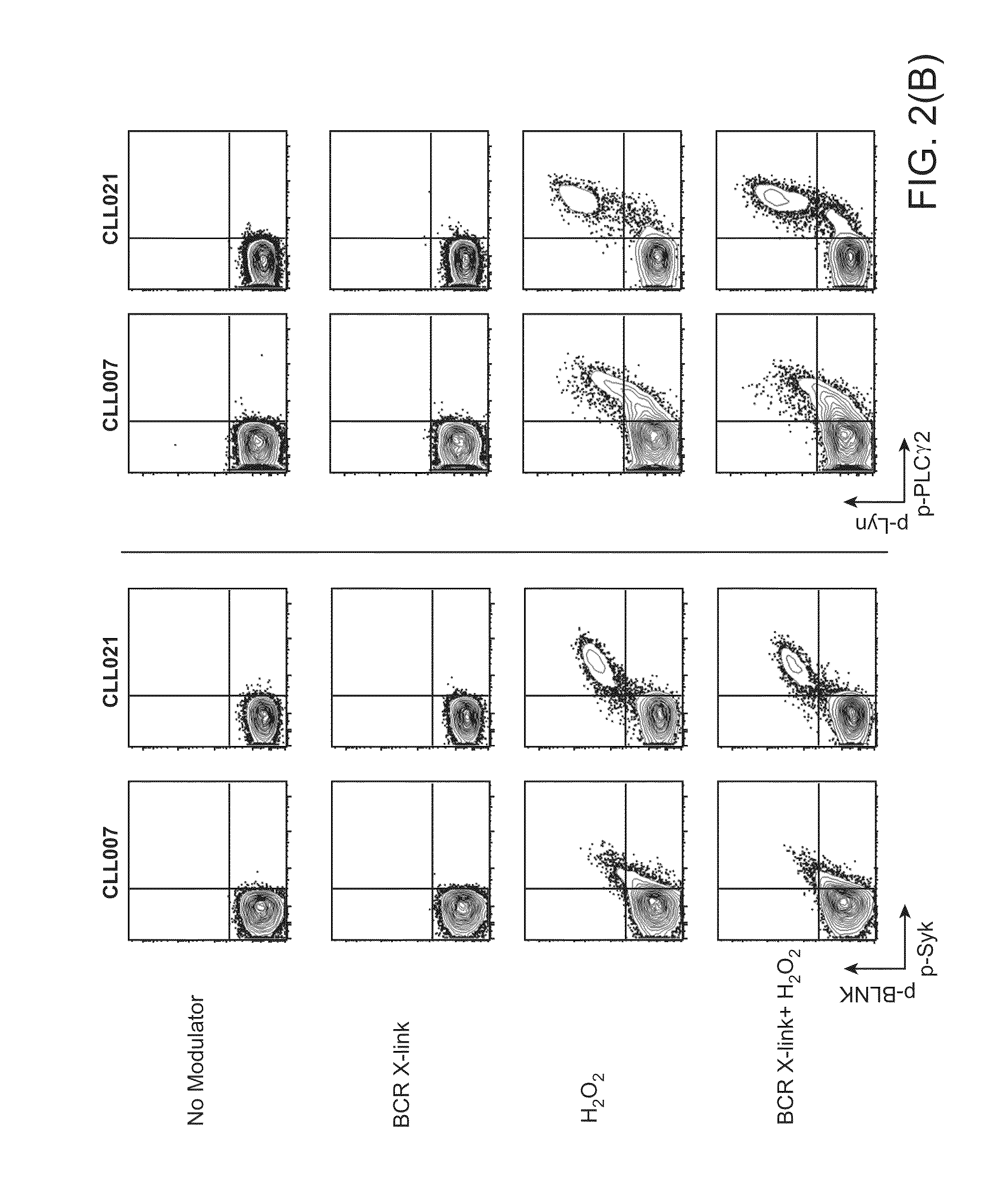
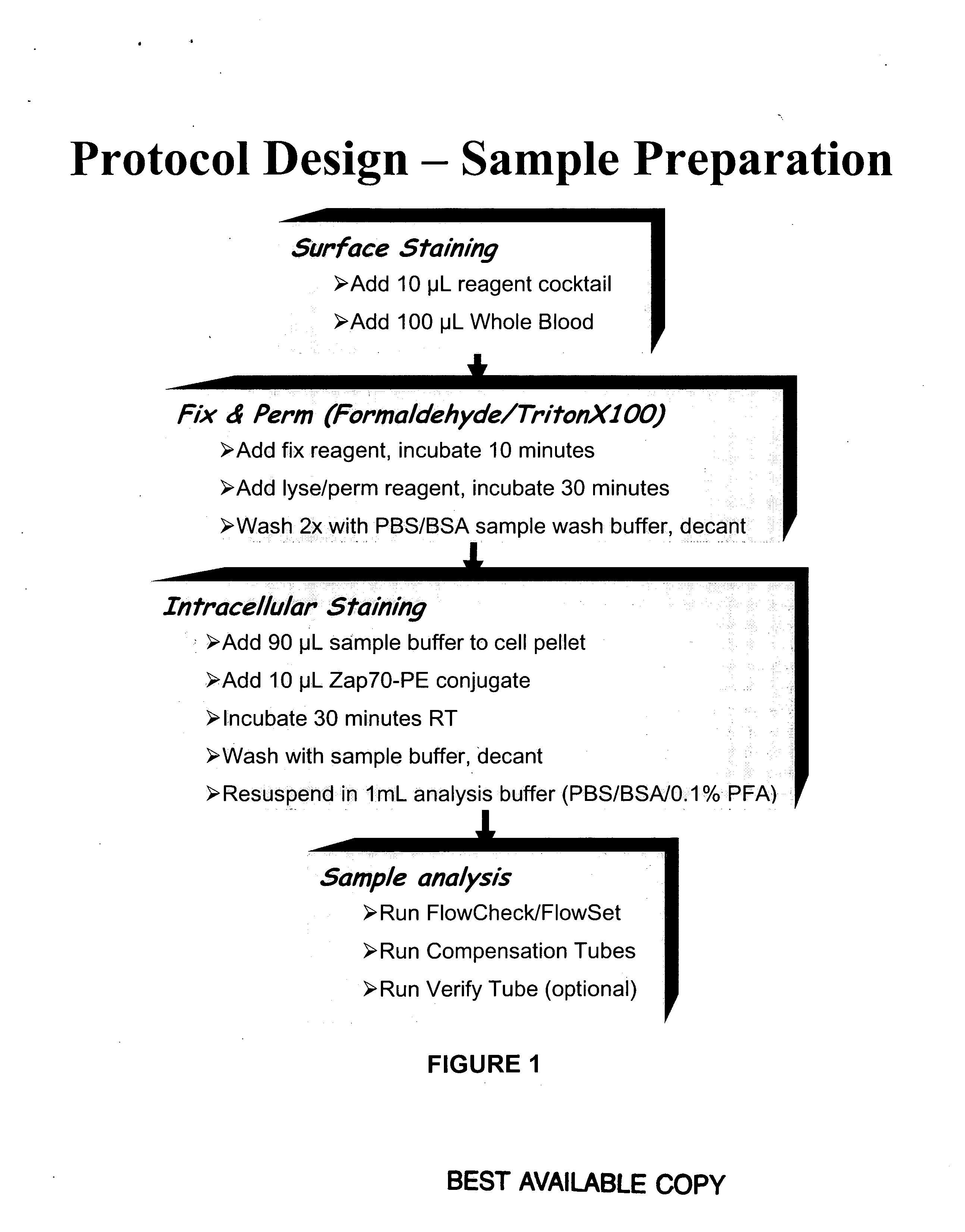
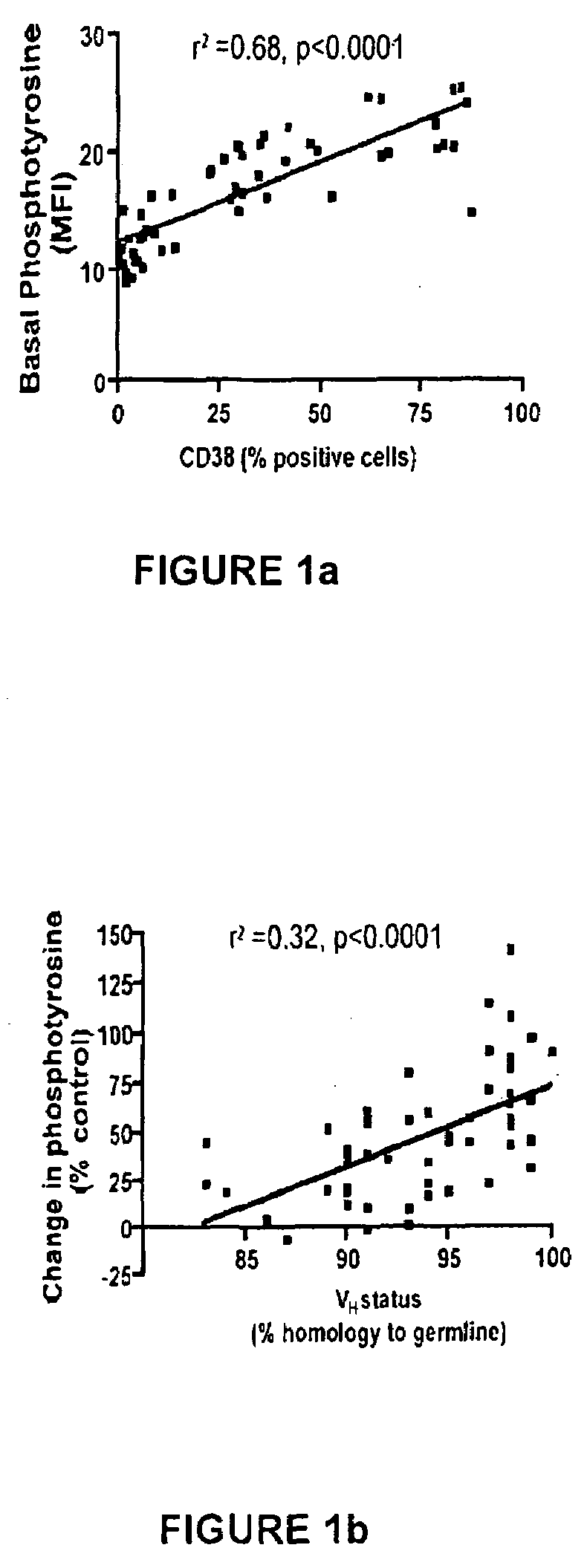
This invention is directed to methods and compositions for diagnosis, prognosis and for determining methods of treatment. The physiological status of cells present in a sample (e.g. clinical sample) can be used in diagnosis or prognosis of a condition (e.g. Chronic Lymphocytic Leukemia), in patient selection for therapy, to monitor treatment and to modify or optimize therapeutic regimens. The physiological status of a cell can be determined by comparing the intracellular status of one or more activation elements (e.g. the phosphorylation status of a signaling molecule) in a cell (e.g. a cancer cell) to that of another cell (e.g. a normal cell). The physiological status of a cell can be further classified by adding one or more modulators (e.g. an inhibitor or activator) to the cell in question. In some embodiments, the invention is directed to methods of determining a phenotypic profile of a population of cells.
Owner:NODALITY
MicroRNA Signatures Associated with Human Chronic Lymphocytic Leukemia (CLL) and Uses Thereof
InactiveUS20120283310A1Improve impactReduce expressionOrganic active ingredientsGenetic material ingredientsDiseaseChronic lymphocytic leukemia
Methods and compositions for the diagnosis, prognosis and / or treatment of leukemia associated diseases are disclosed.
Owner:THE OHIO STATE UNIV RES FOUND
Diagnosis and Treatment of Chronic Lymphocytic Leukemia (CLL)
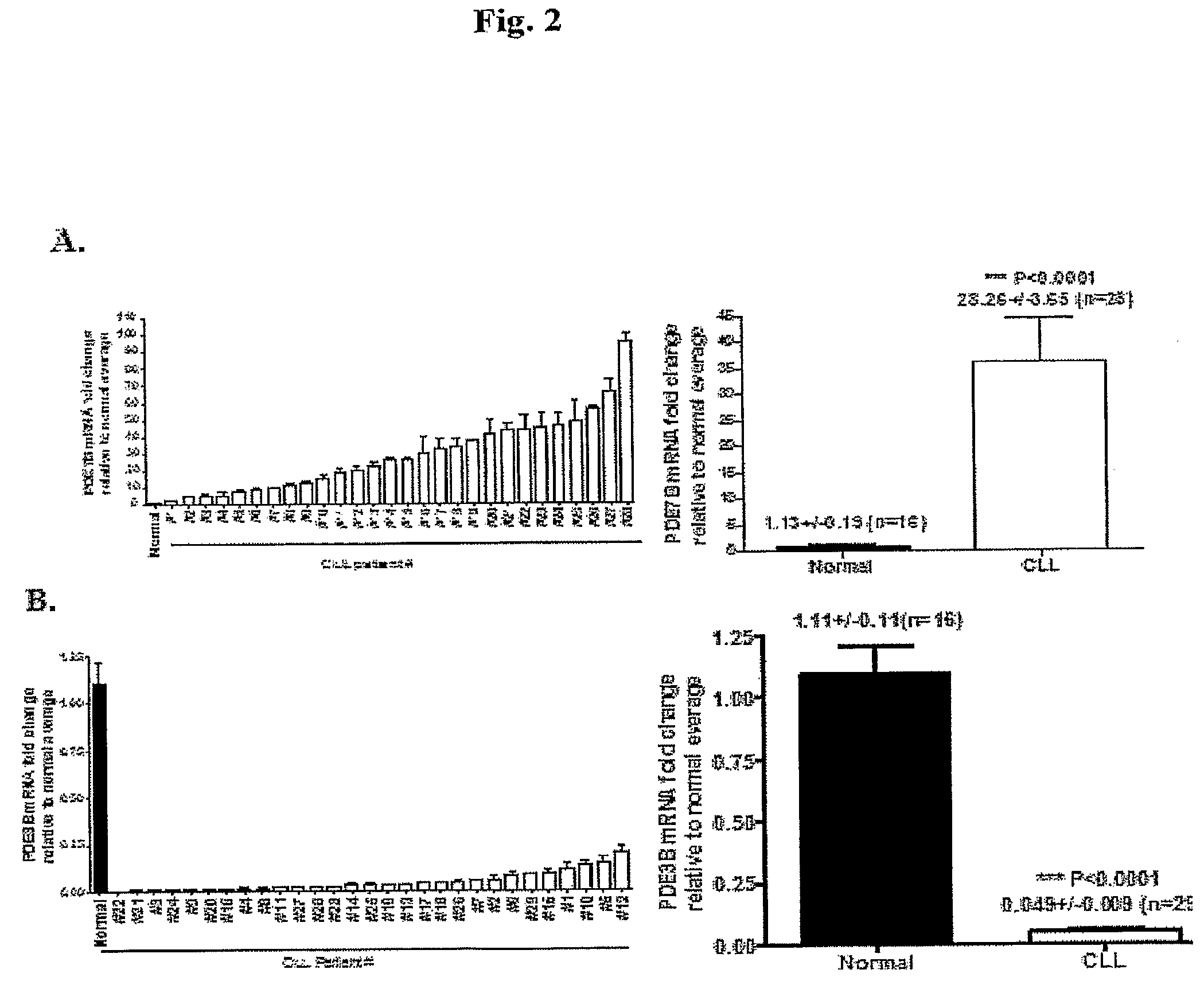
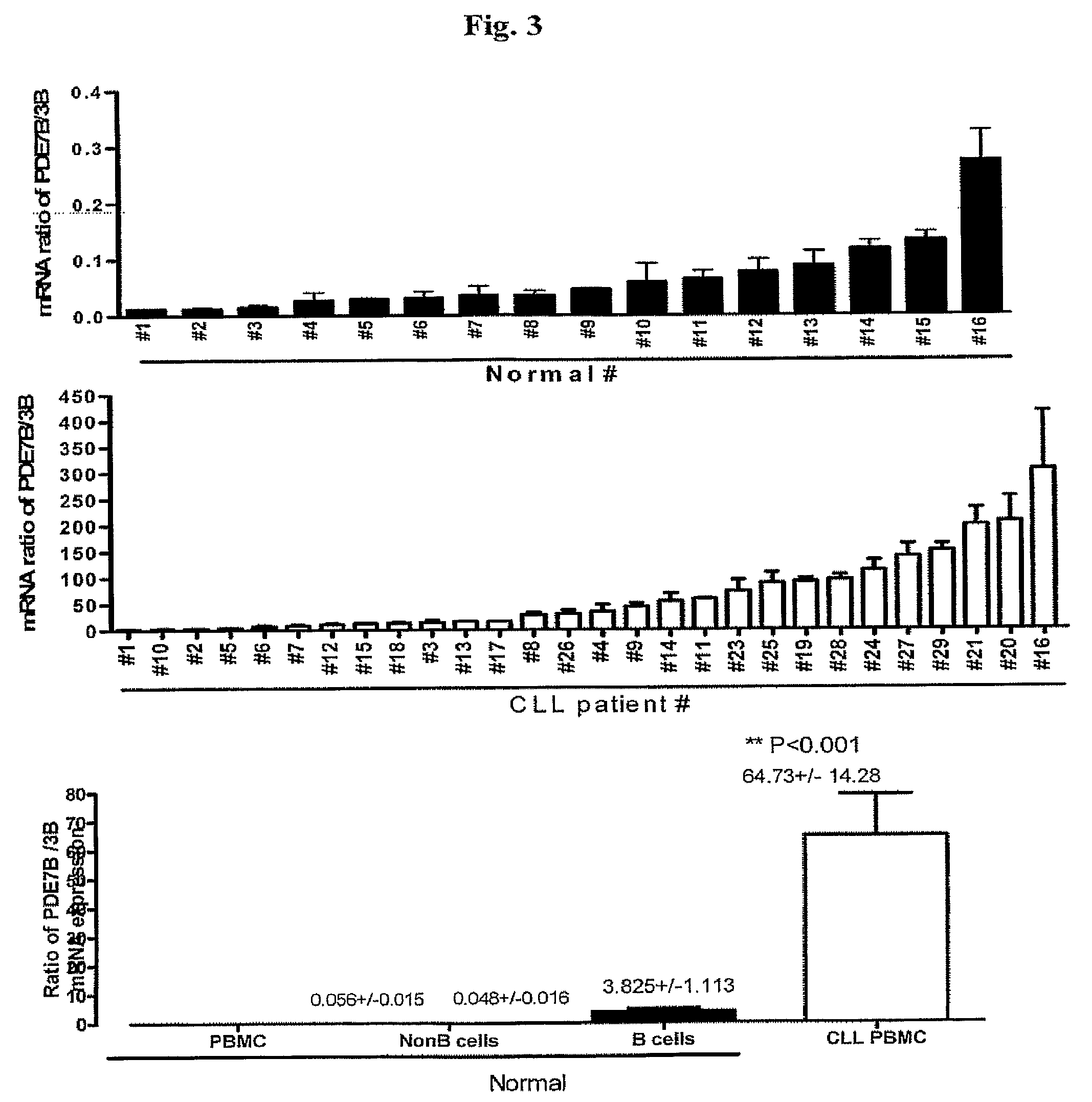
InactiveUS20090131353A1Improve expression levelIncrease protein expression levelOrganic active ingredientsSugar derivativesMedicineCyclic nucleotide phosphodiesterase
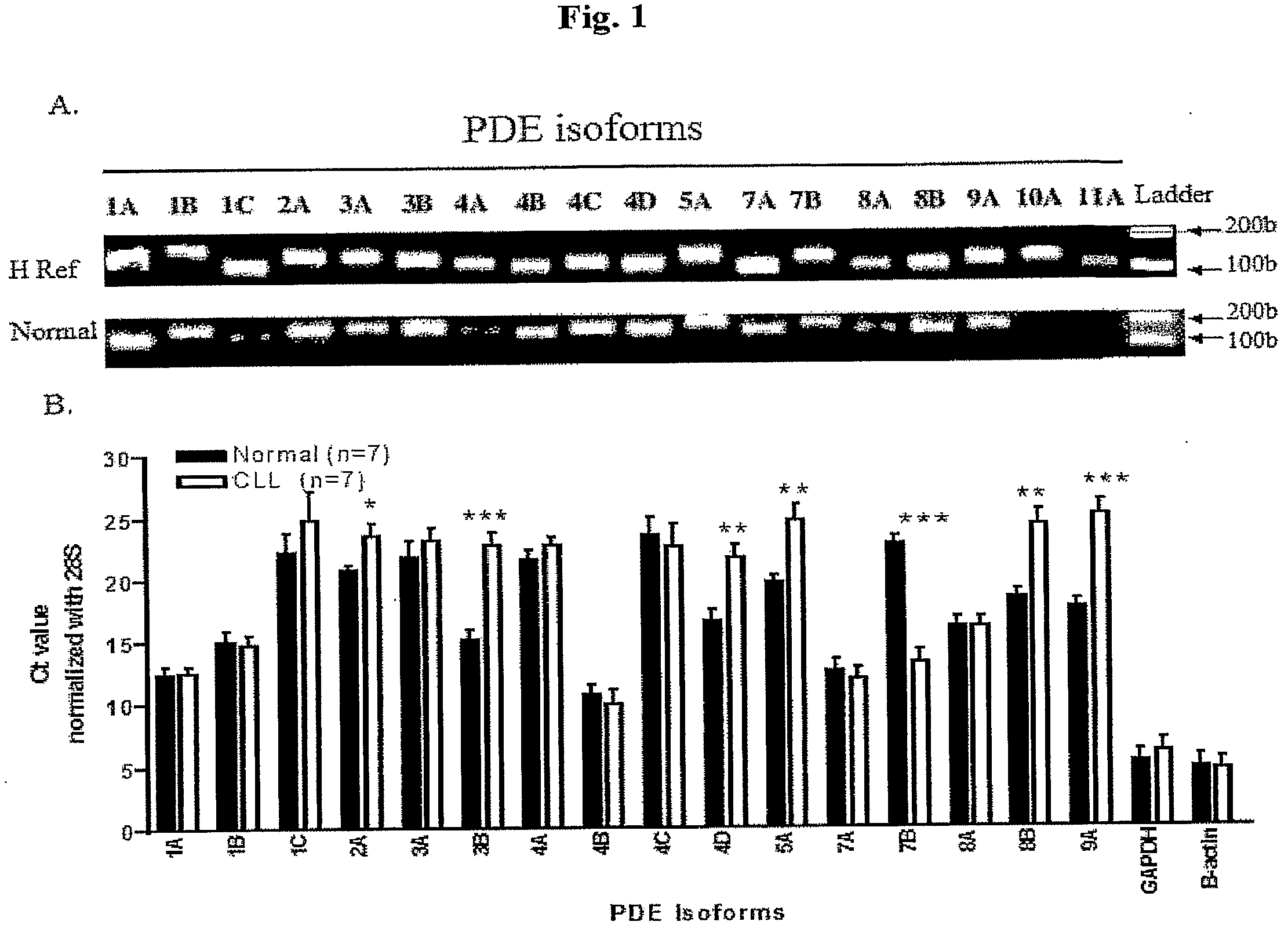
The present invention provides diagnostic methods and kits for diagnosis of chronic lymphocytic leukemia (CLL) by determining expression levels of isoforms of cyclic nucleotide phosphodiesterases (PDEs) associated with CLL particularly, PDE7B and / or PDE3B, and a ratio of mRNA expression of PDE7B to PDE3B. The present invention provides that CLL lymphocytes uniformly expressed high levels of PDE7B and low levels of PDE3B relative to those of normal lymphocytes. A method of treatment and a pharmaceutical composition for CLL comprising one or more therapeutic agents capable of modulating expression or activity levels of isoforms of PDEs associated with CLL, and / or reversing the ratio of PDE7B / PDE3B mRNA expression levels are also provided.
Owner:RGT UNIV OF CALIFORNIA
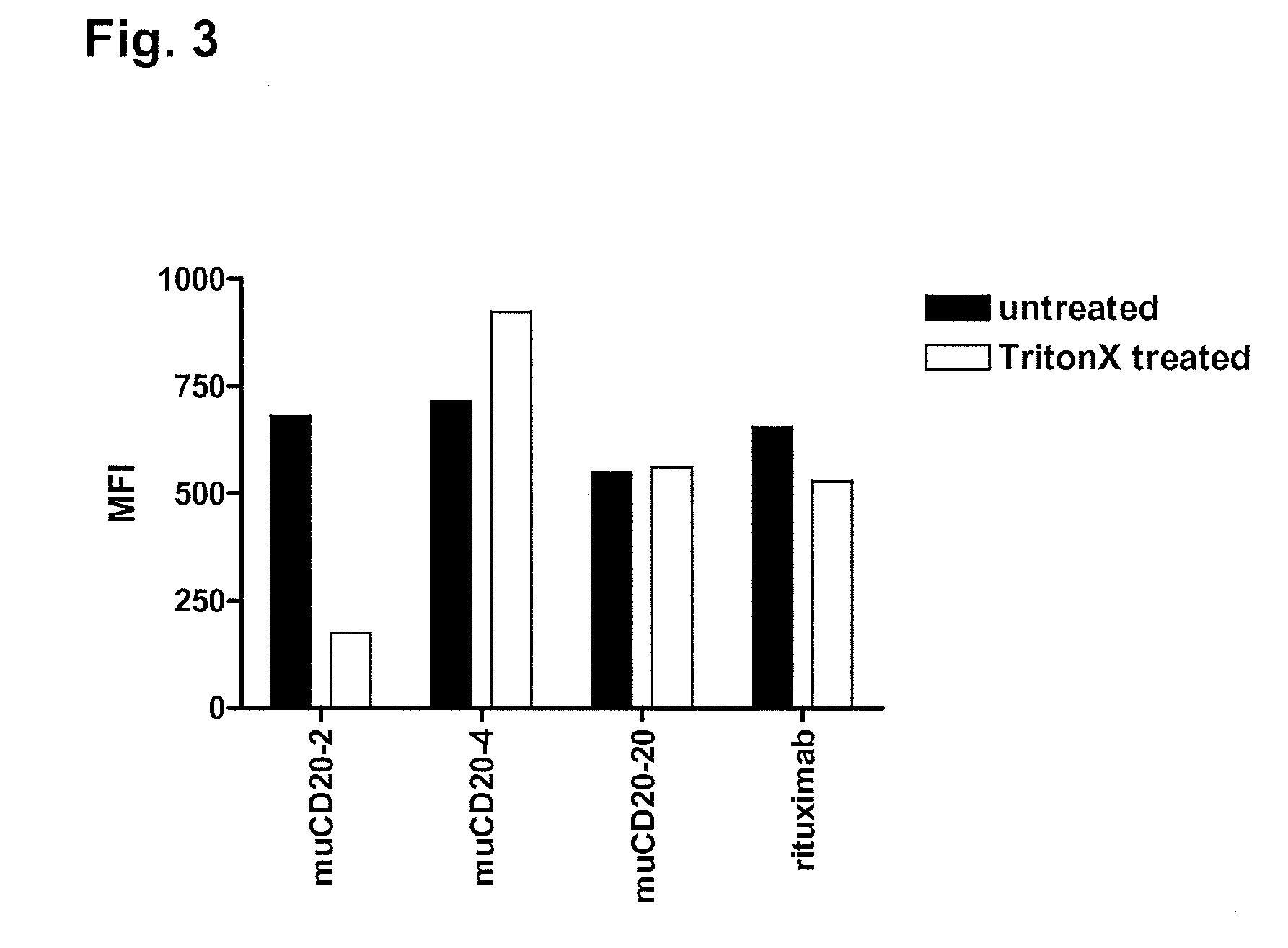
Cd20 antibodies and uses thereof
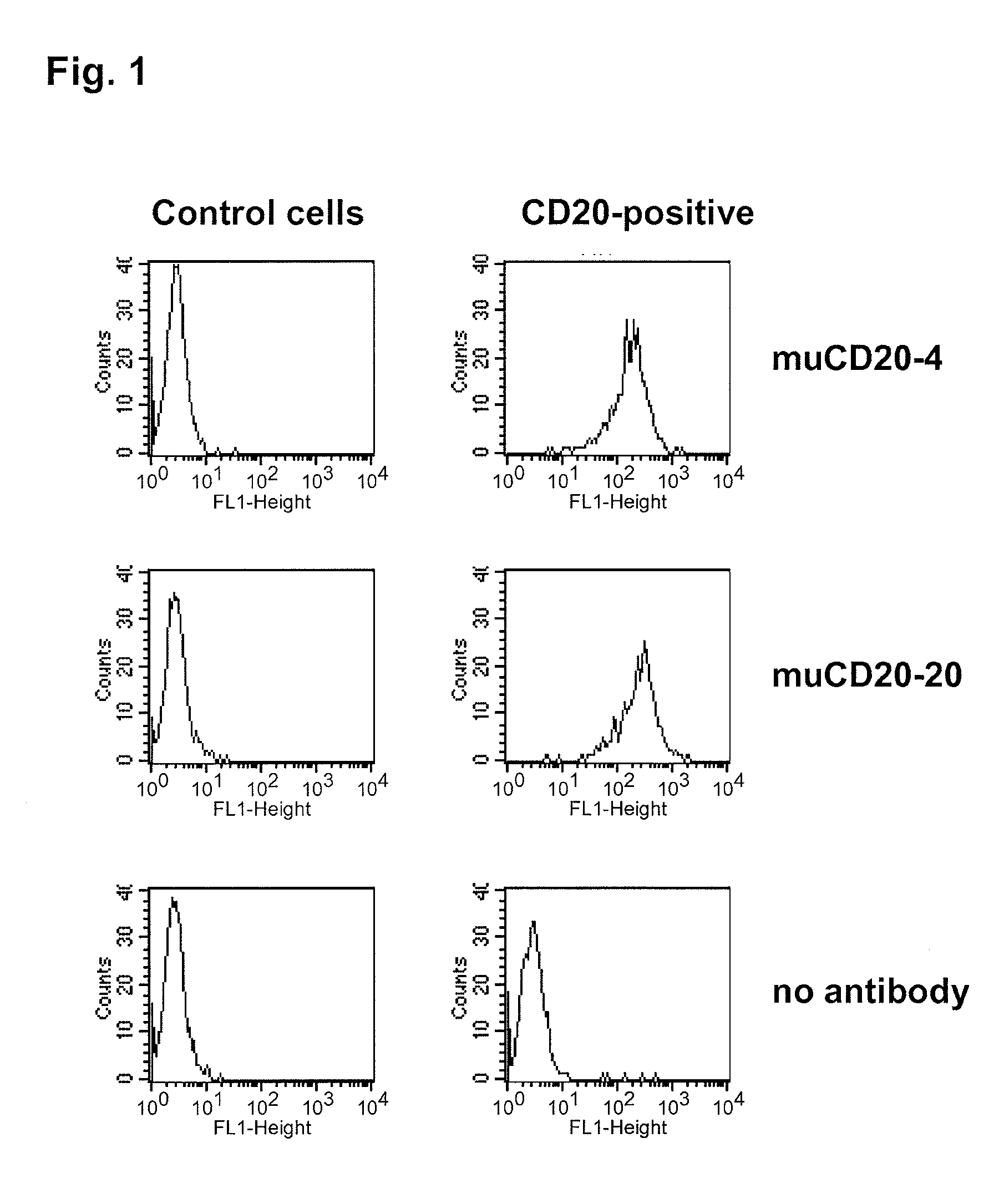
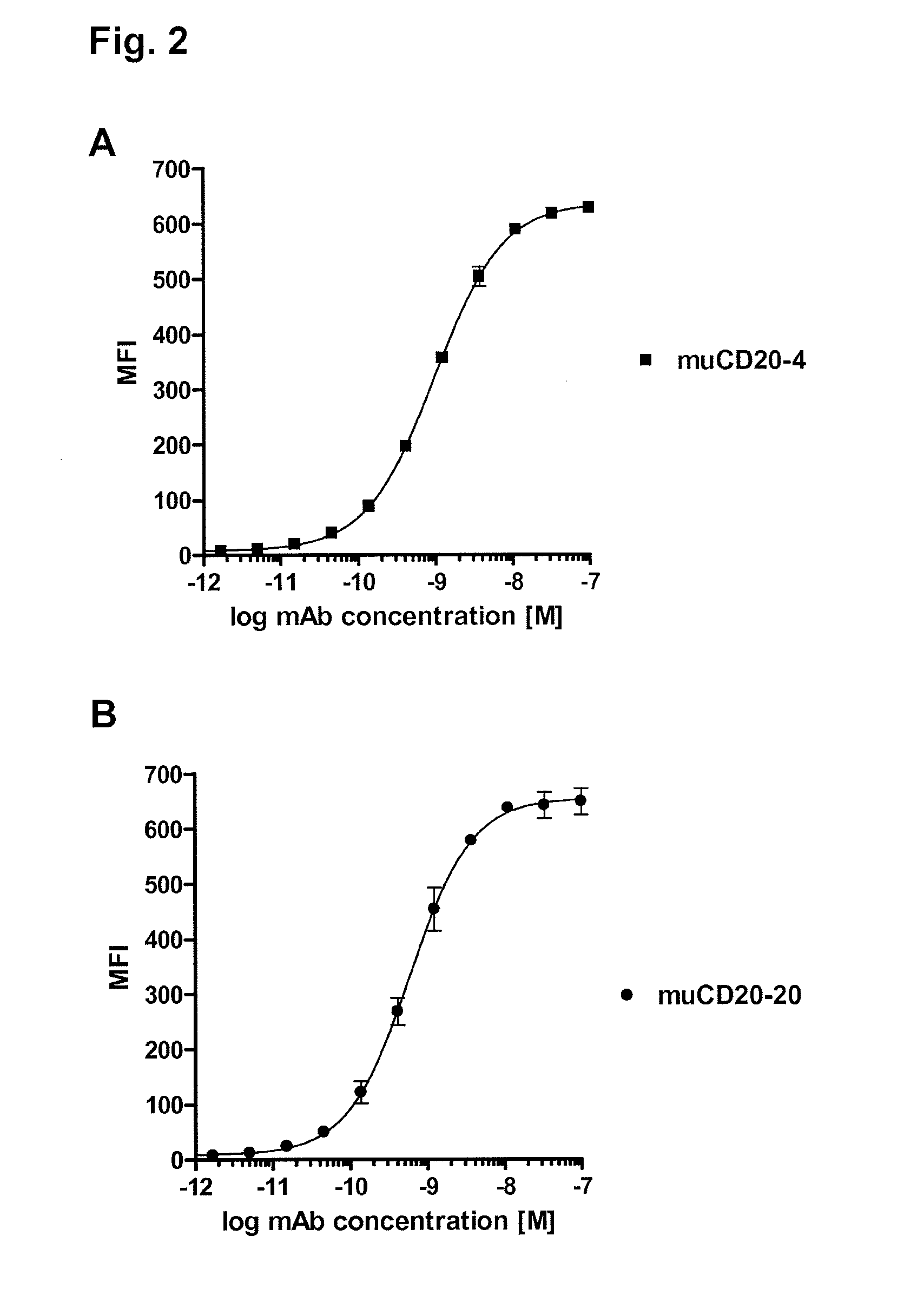
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells and has been found on B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. Conversely, it is not found on hematopoietic stem cells, pro-B cells, differentiated plasma cells or non-lymphoid tissues. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). CD20 expressing cells are known to play a role in other diseases and disorders, including inflammation. The present invention includes anti-CD20 antibodies, forms and fragments, having superior physical and functional properties; immunoconjugates, compositions, diagnostic reagents, methods for inhibiting growth, therapeutic methods, improved antibodies and cell lines; and polynucleotides, vectors and genetic constructs encoding same.
Owner:IMMUNOGEN INC
Compositions and methods for cancer diagnosis and therapy
The miR15 and miR16 micro RNA genes are located at 13q14 within a 30 kb region of loss characteristic of cells from certain cancers, such as cells from chronic lymphocytic leukemia or prostate cancer. Chronic lymphocytic leukemia or prostate cancer can be diagnosed by detecting a reduction in miR15 or miR16 gene copy number, by determining miR15 or miR16 gene mutational status, or by detecting a reduction in the RNA transcribed from these genes. The miR15 or miR16 gene products can inhibit the neoplastic or tumorigenic growth of cancers such as chronic lymphocytic leukemia or prostate cancer cells when administered to subjects suffering from these diseases.
Owner:THOMAS JEFFERSON UNIV
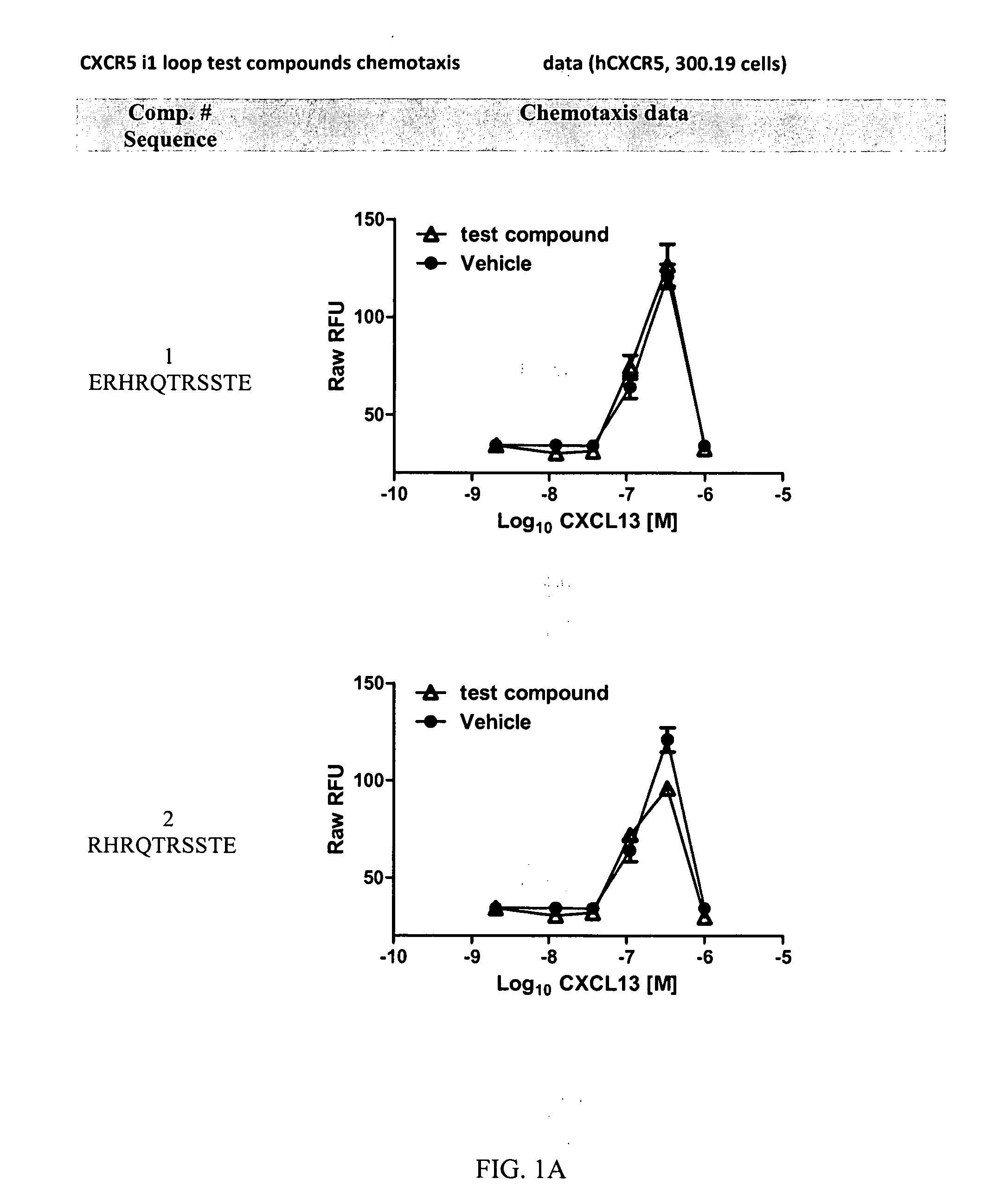
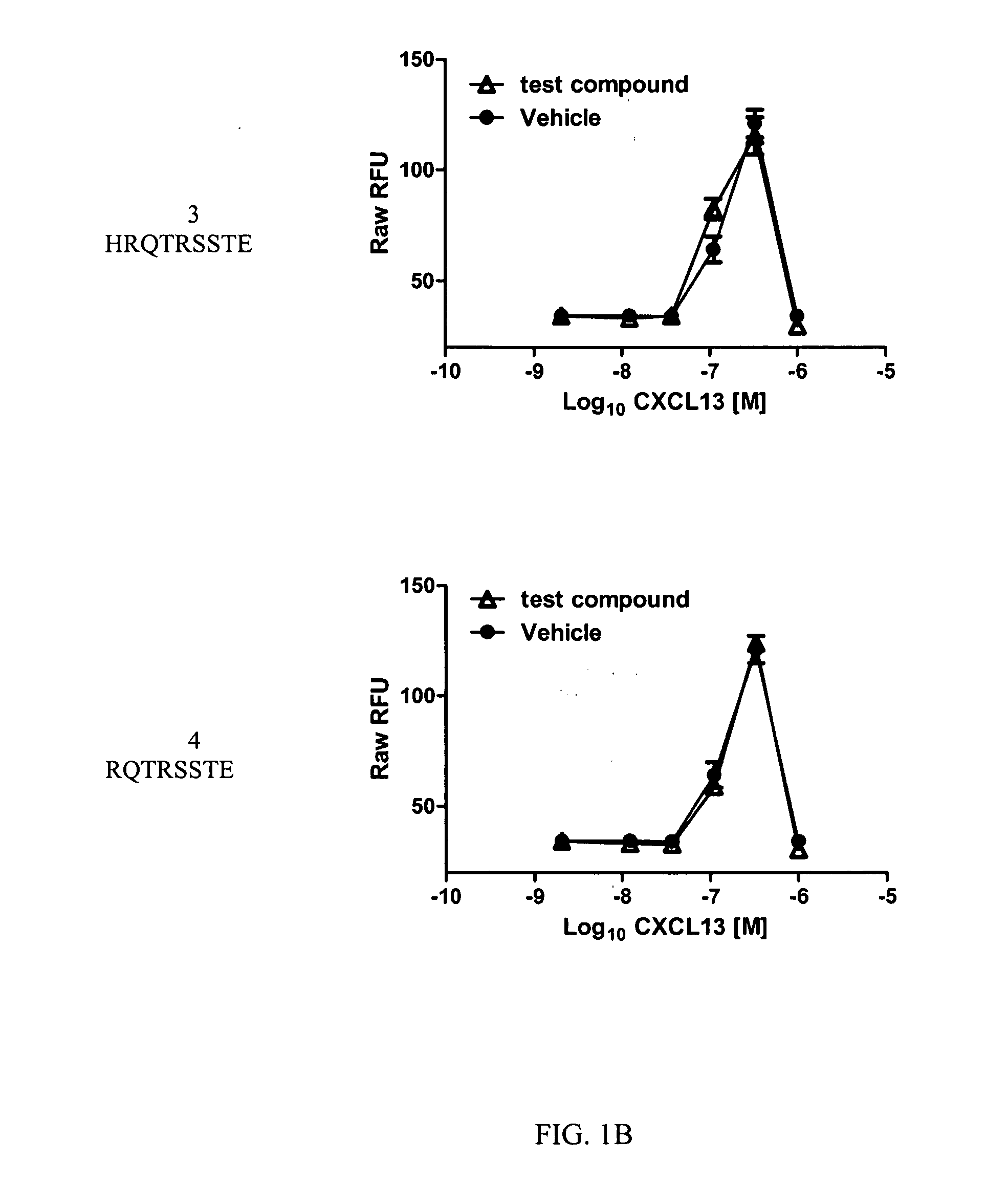
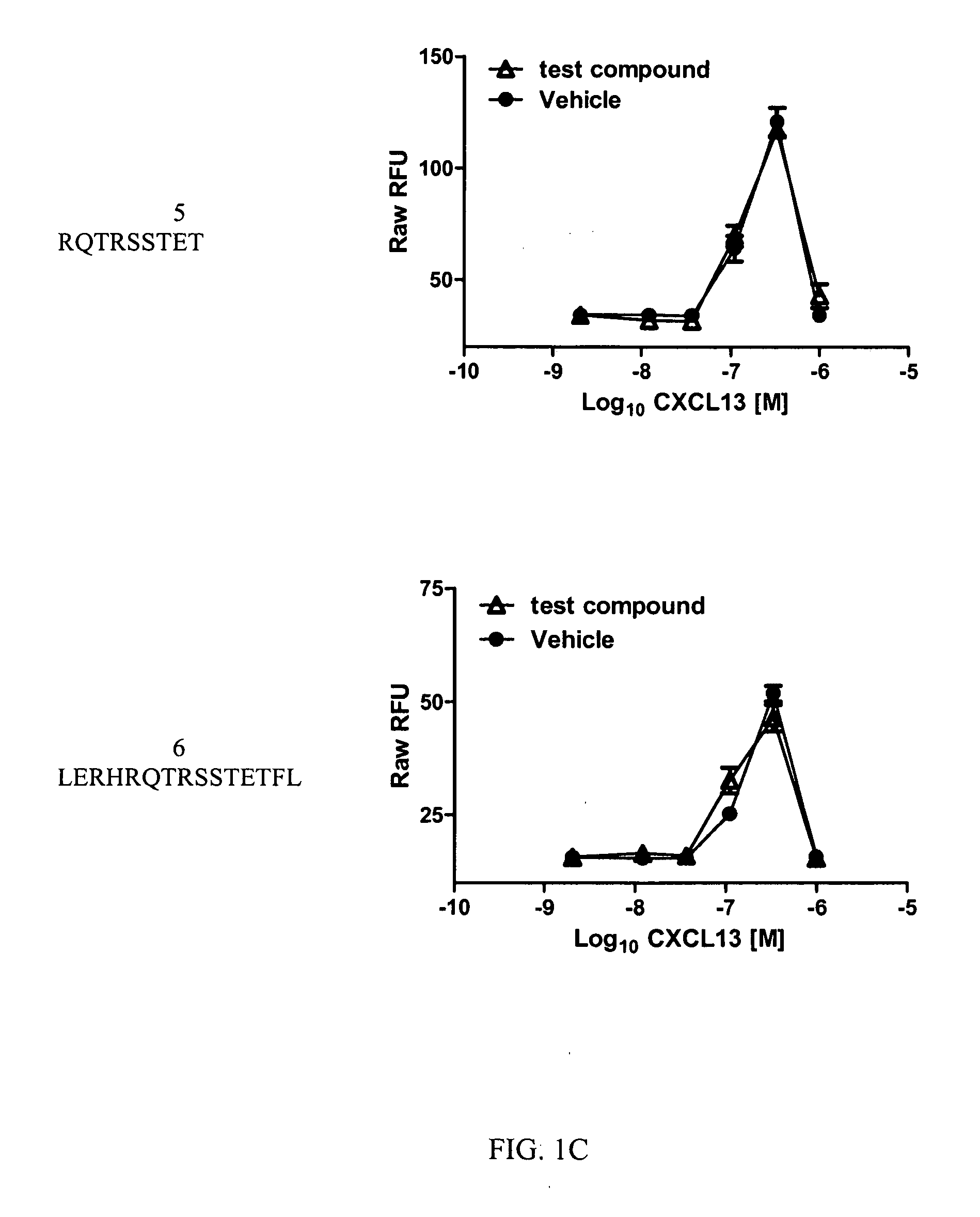
Cxcr5 receptor compounds
The invention relates generally to compounds which are allosteric modulators (e.g., negative and positive allosteric modulators, allosteric agonists, and ago-allosteric modulators) of the G protein coupled receptor CXCR5. The CXCR5 receptor compounds are derived from the intracellular loops and domains of the CXCR5 receptor. The invention also relates to the use of these CXCR5 receptor compounds and pharmaceutical compositions comprising the CXCR5 receptor compounds in the treatment of diseases and conditions associated with CXCR5 receptor modulation such as autoimmune diseases including lupus, HIV and rheumatoid arthritis, Primary Sjogren's Syndrome, chronic lymphocytic leukemia, Burkitt Lymphoma, colon and breast cancer tumor metastasis, Multiple Sclerosis and compromised immune function.
Owner:ANCHOR THERAPEUTICS
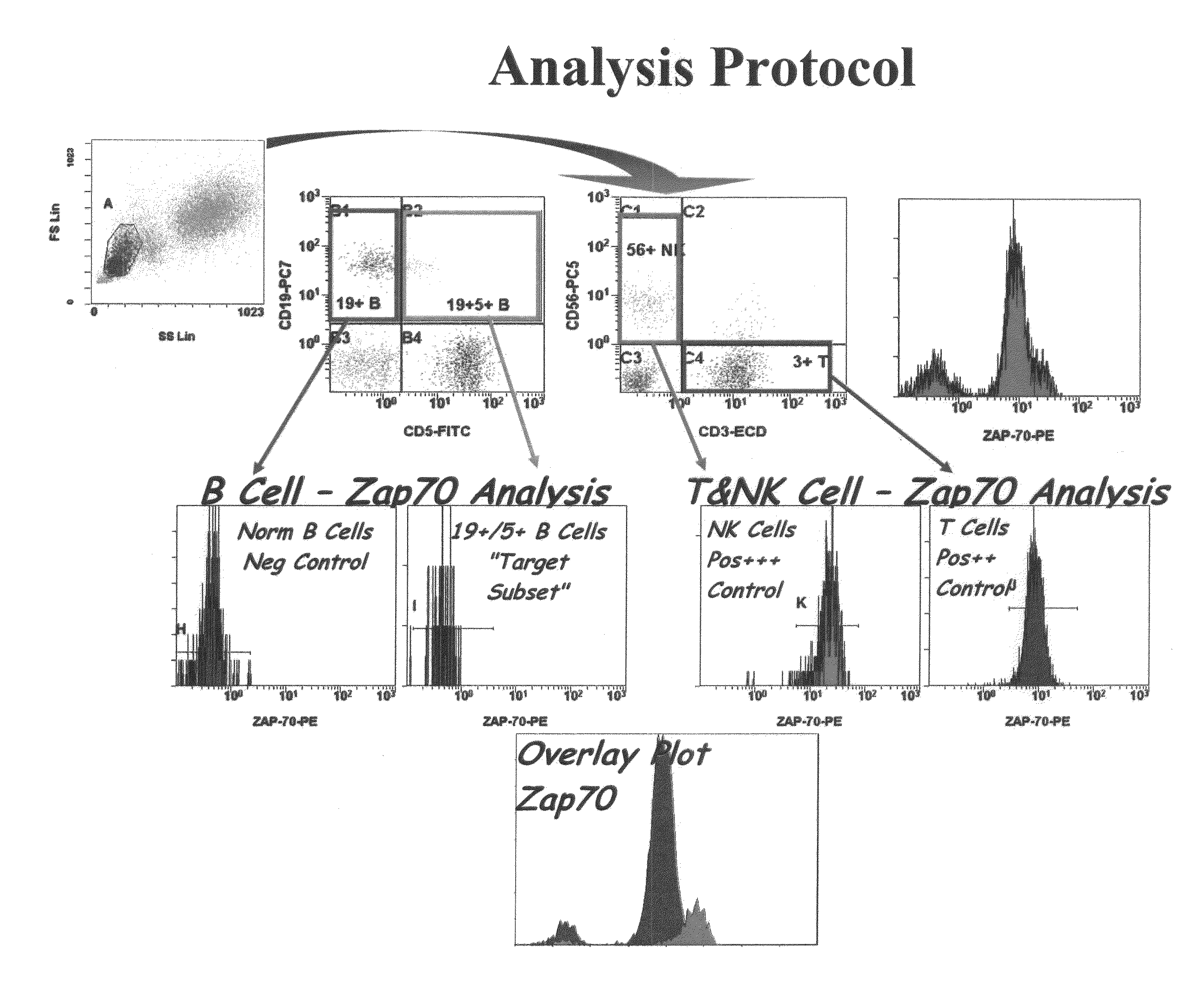
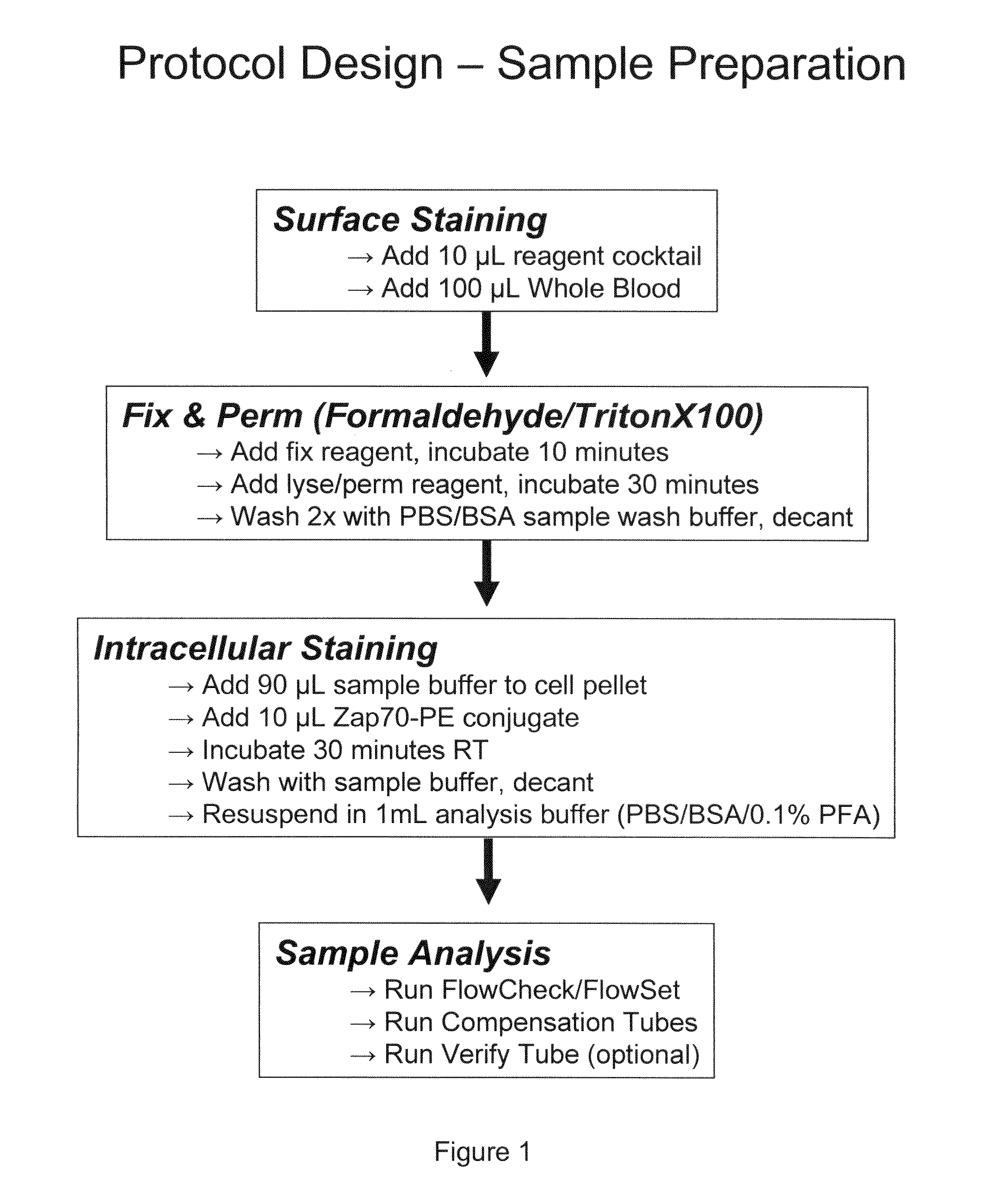
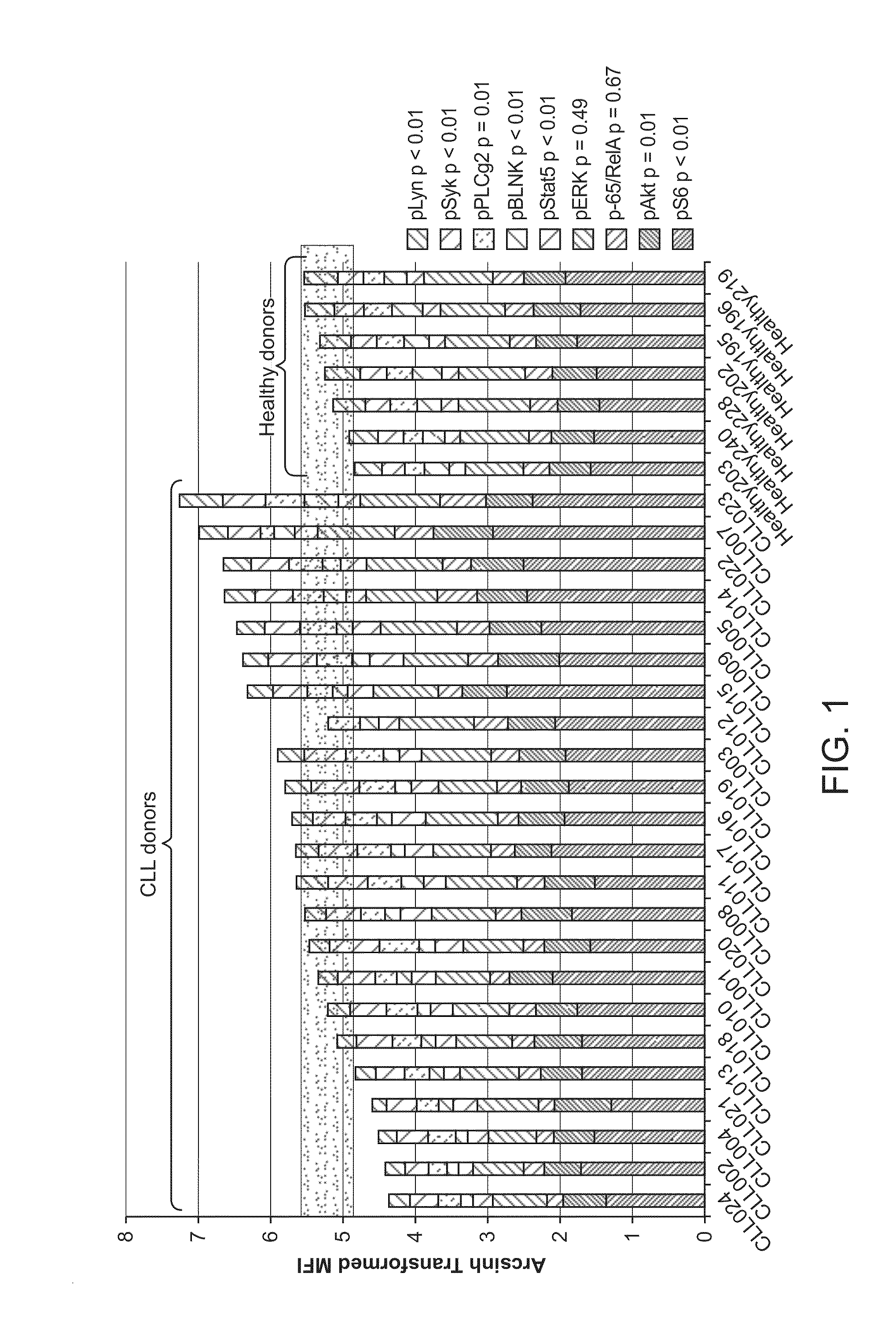
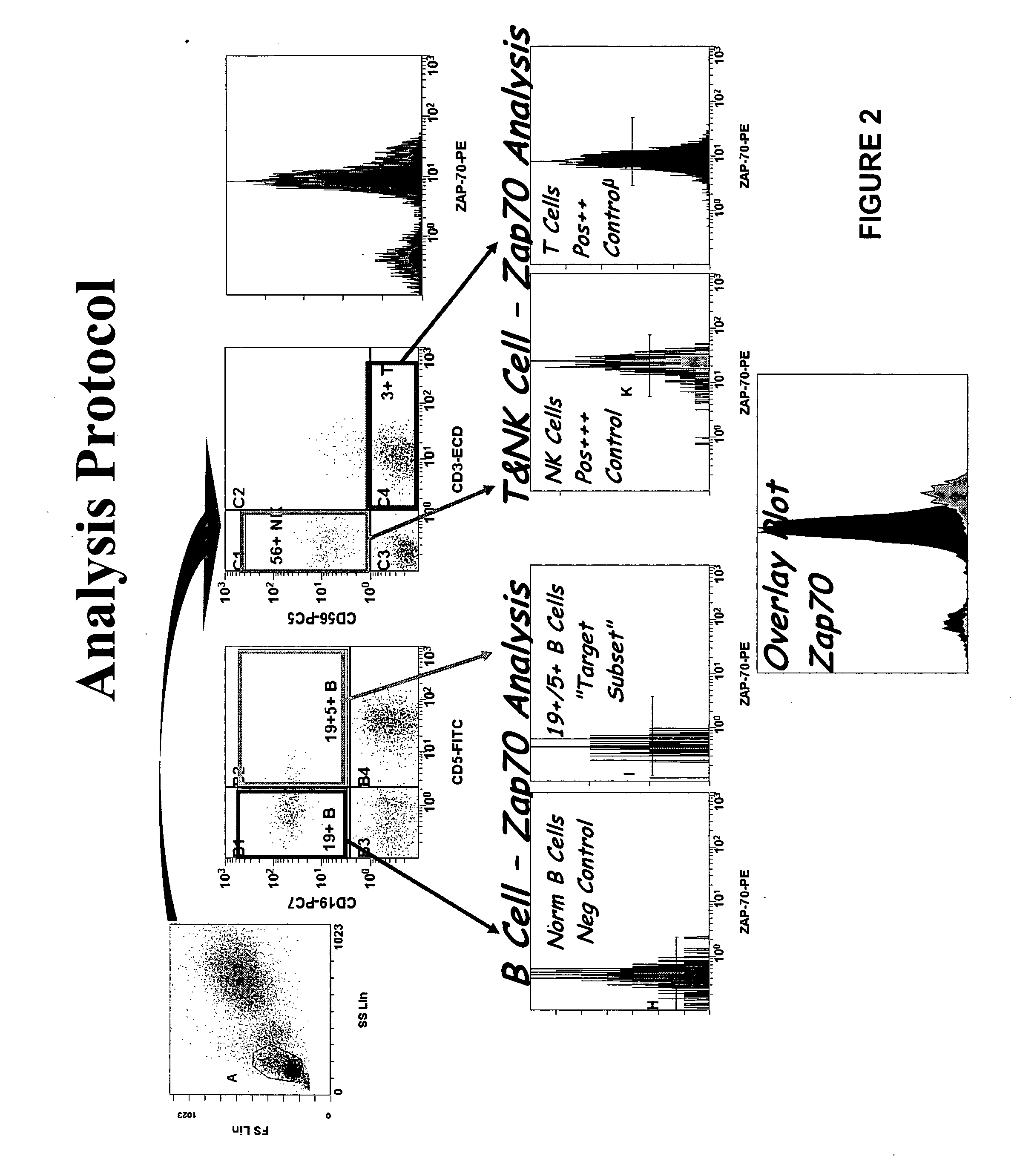
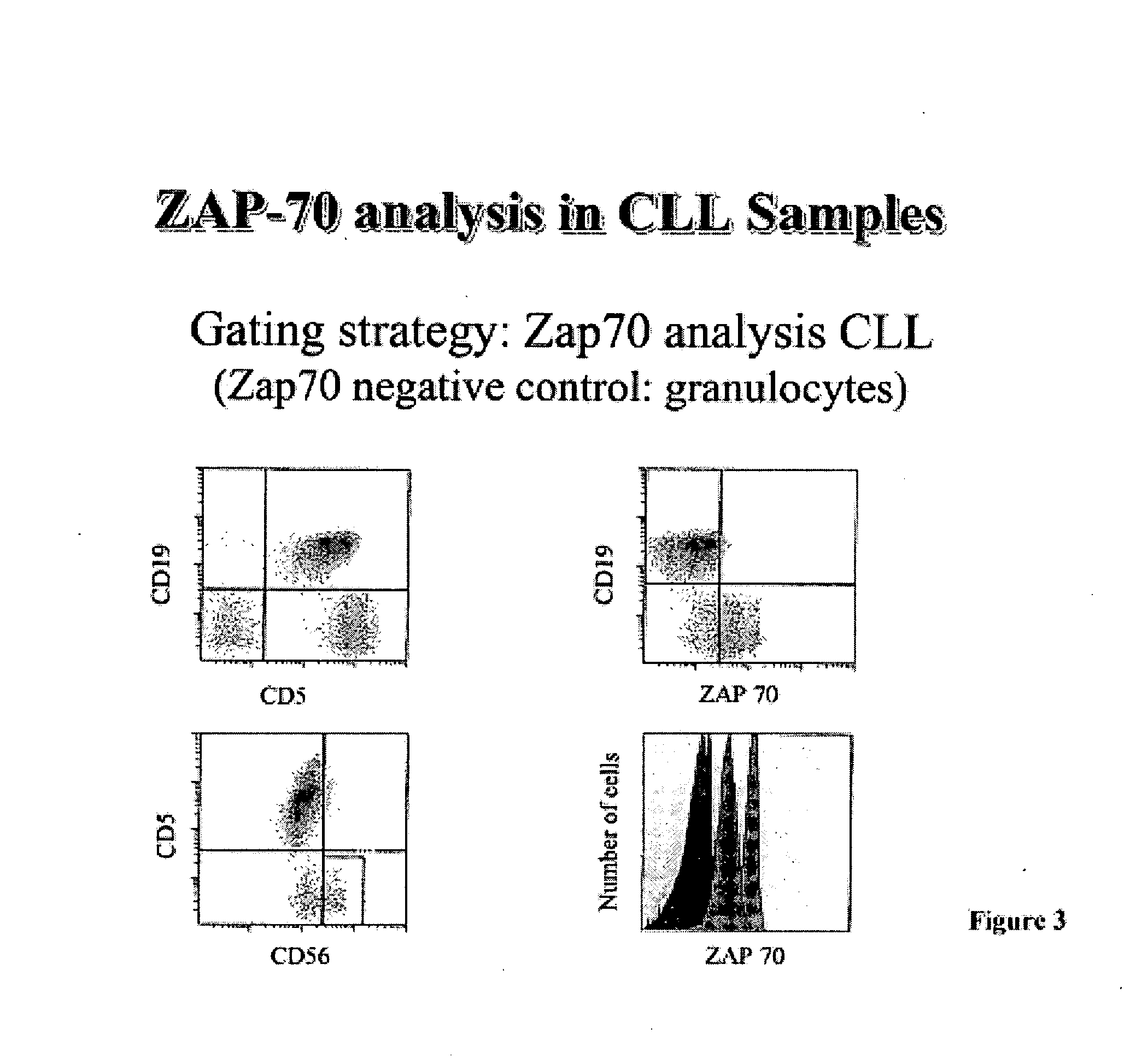
Composite Profiles of Cell Antigens and Target Signal Transduction Proteins for Analysis and Clinical Management of Hematologic Cancers
InactiveUS20100261204A1Increased riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
Owner:BECKMAN COULTER INC
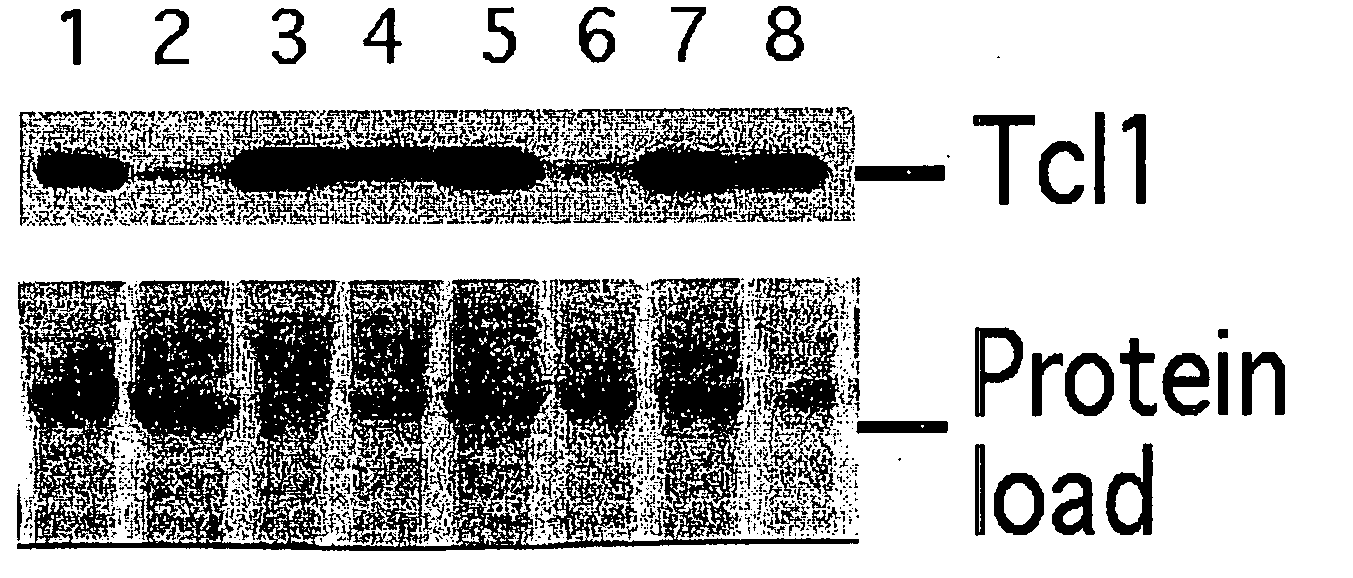
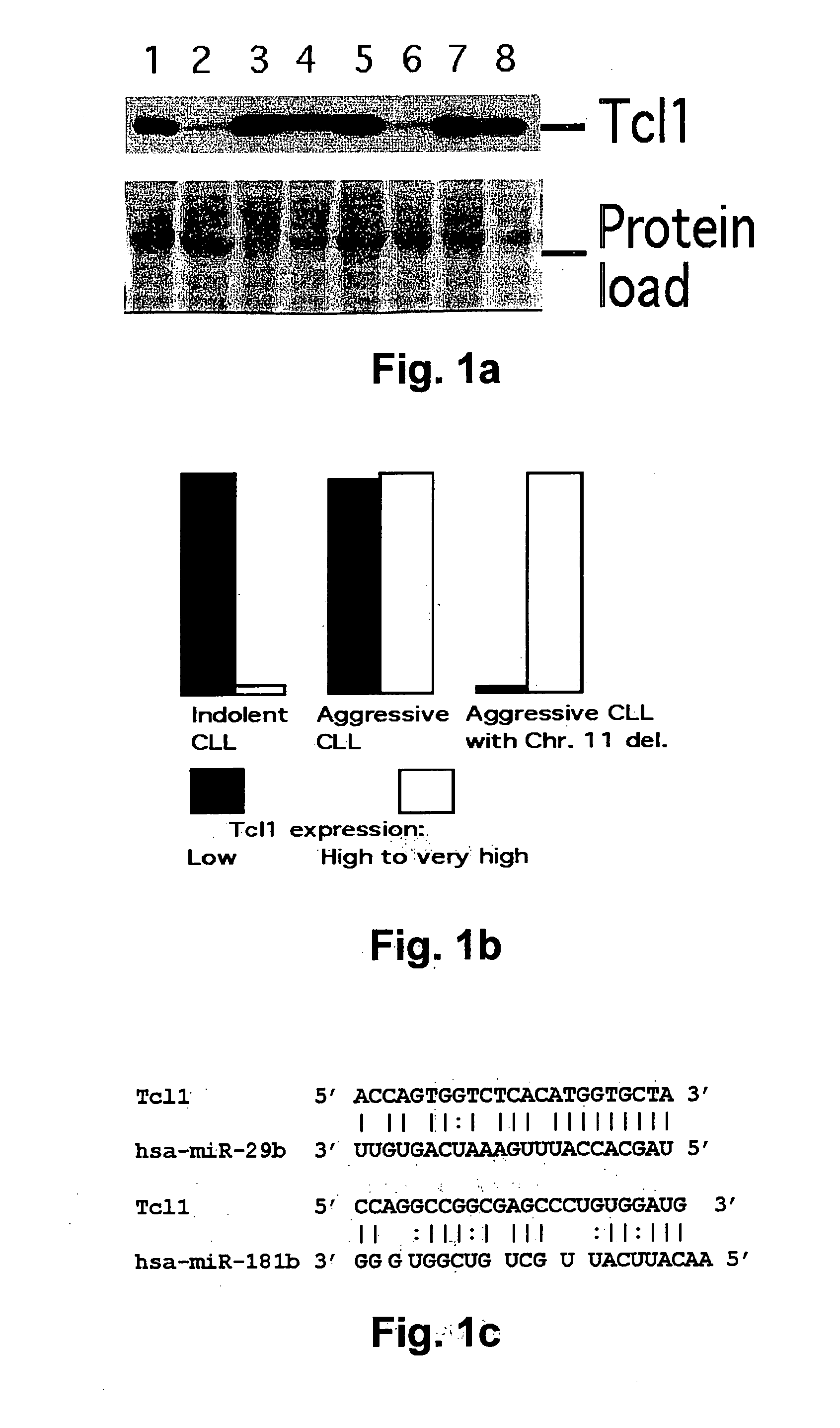
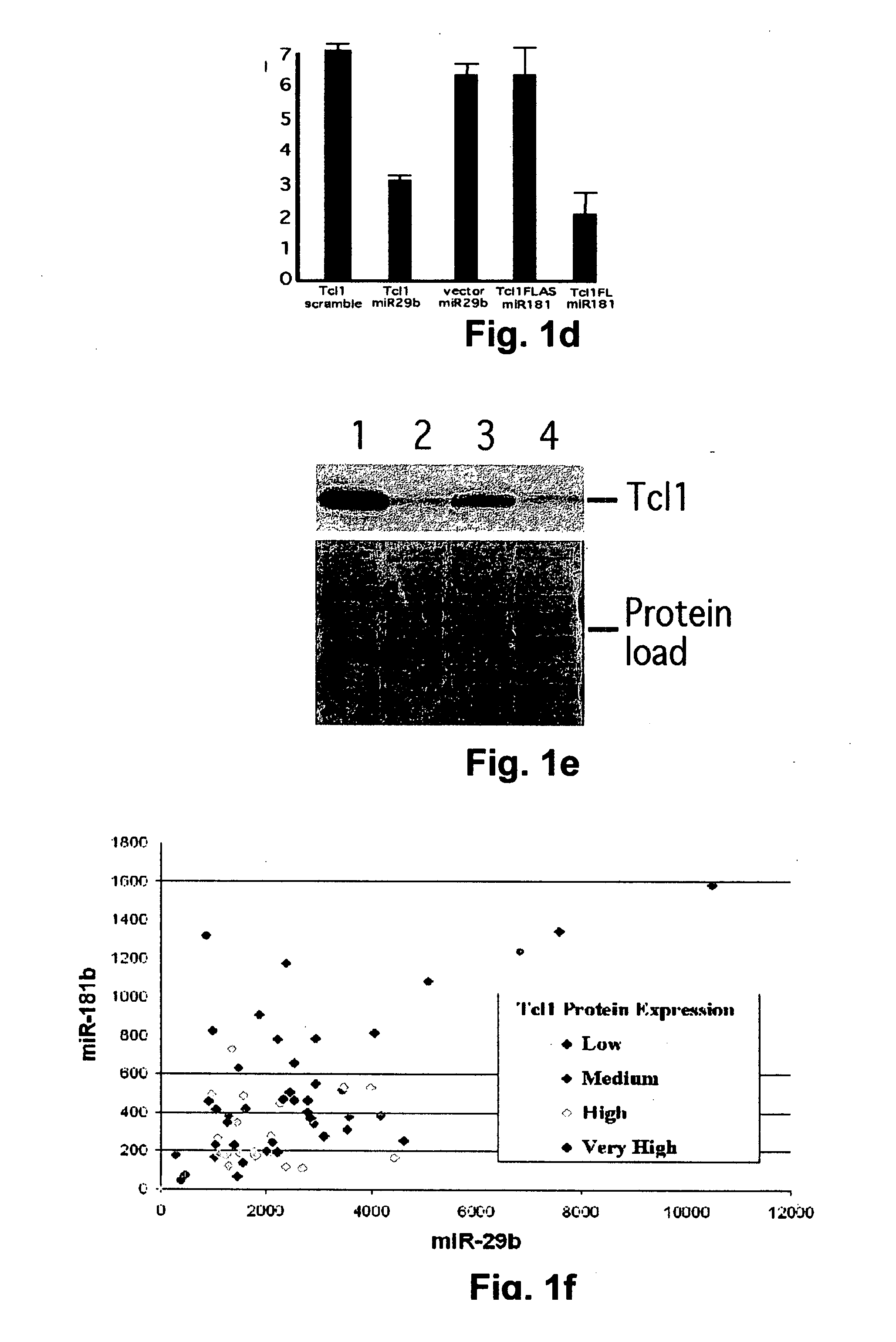
TCL1 Expression in Chronic Lymphocytic Leukemia (CLL) Regulated by MIR-29 and MIR-181
InactiveUS20100004322A1Prevent proliferationInhibit expressionBioreactor/fermenter combinationsOrganic active ingredientsChronic lymphocytic leukemia
The present invention provides novel methods and compositions for the diagnosis, prognosis and treatment of chronic lymphocytic leukemia (CLL). The invention also provides methods of identifying anti-CLL agents.
Owner:THE OHIO STATE UNIV RES FOUND
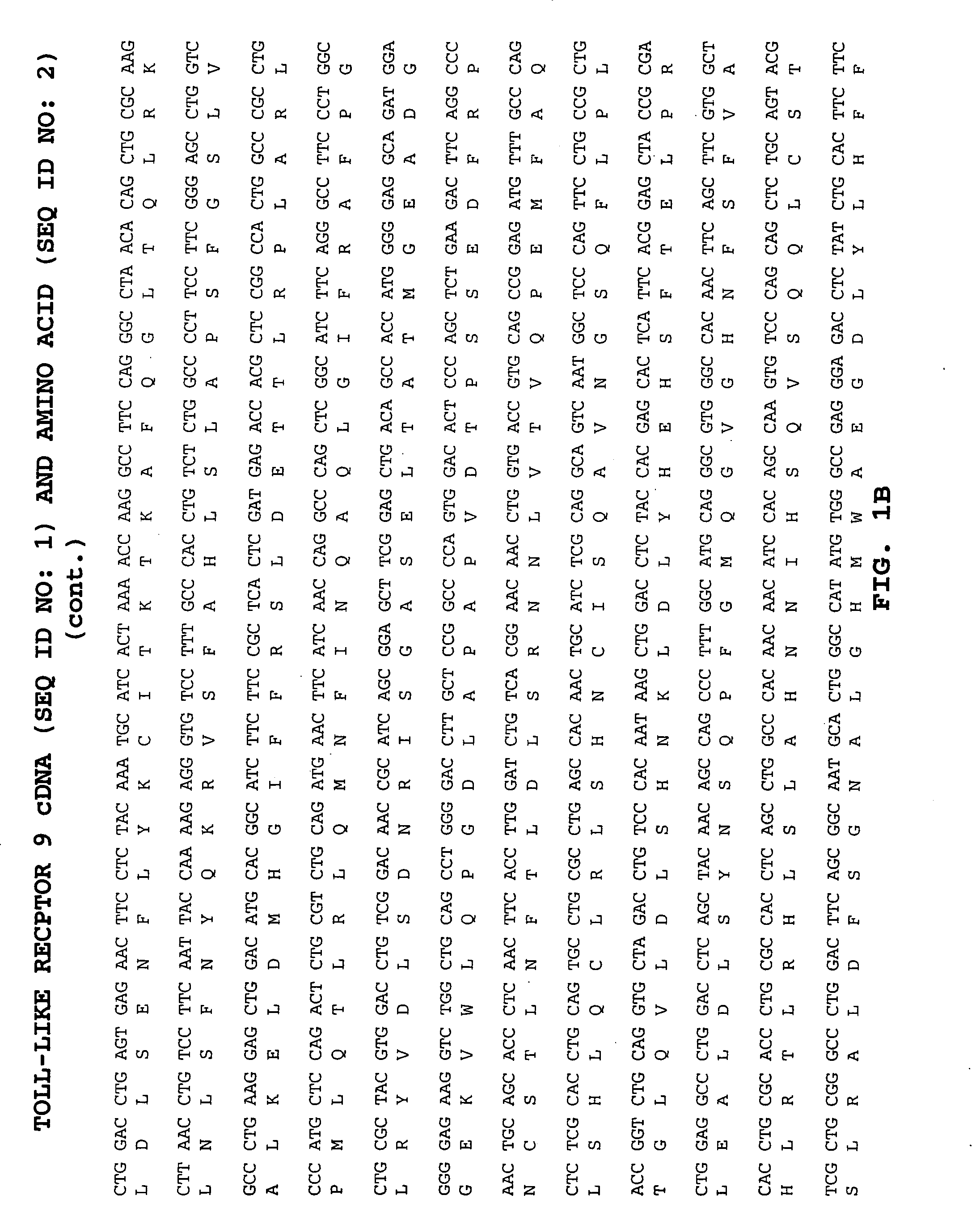
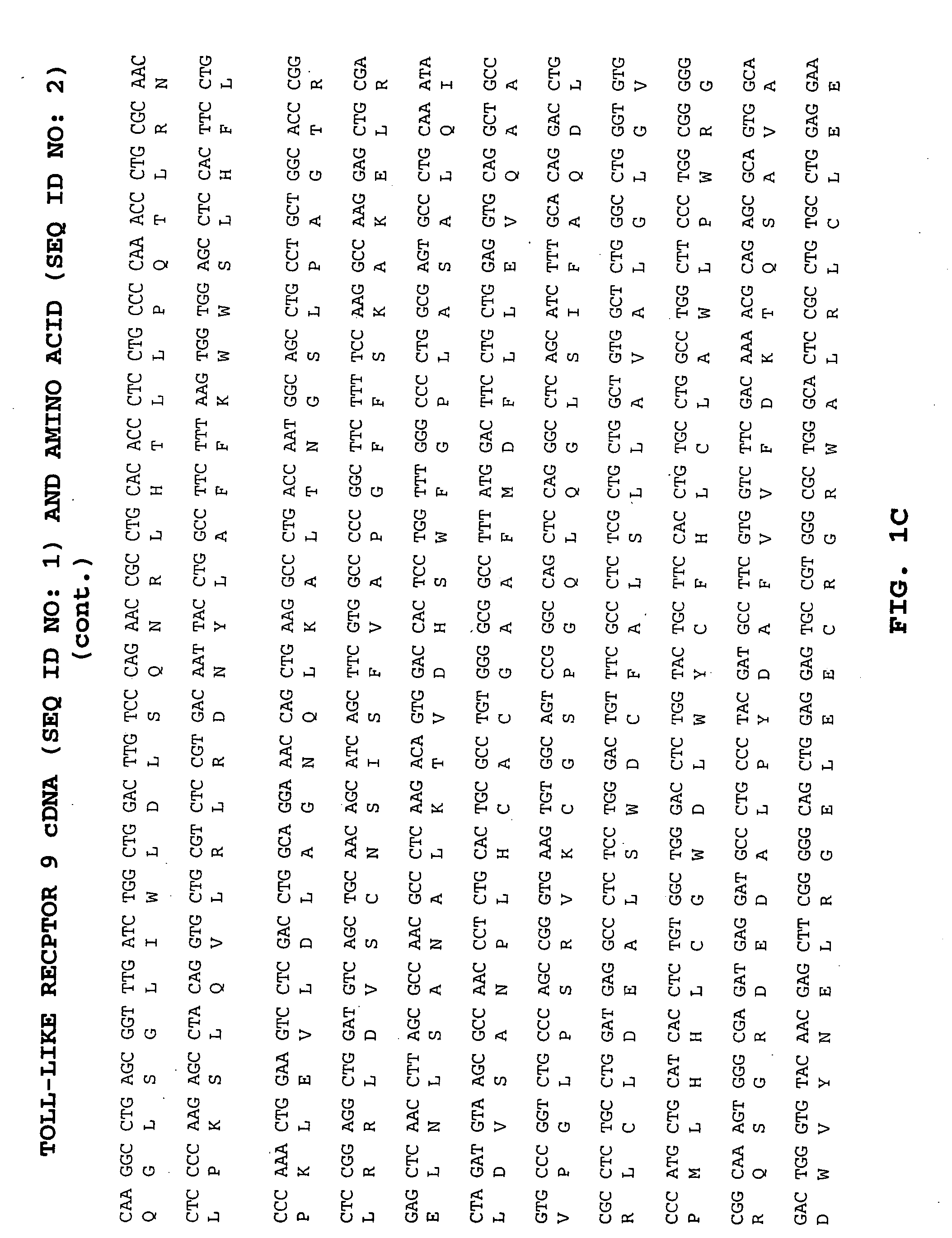
Methods of therapy and diagnosis using targeting of cells that express toll-like receptor proteins
InactiveUS20050281813A1Good effectPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsCancer cellT cell
Certain cells, including types of cancer cells such as B-cell lymphomas, T cell lymphomas, Hodgkin's disease and myeloid leukemias, are capable of expressing Toll-like Receptor 9 (TLR9) or Toll-like Receptor 10 (TLR10) mRNA. Immunotargeting using TLR9 or TLR10 polypeptides, nucleic acids encoding for TLR9 or TLR10 polypeptides and anti-TLR9 or anti-TLR10 antibodies provides a method of killing or inhibiting that growth of cancer cells that express the TLR9 or TLR10 protein. Methods of immunotherapy and diagnosis of disorders associated with TLR9 or TLR10 protein-expressing cells, such as B-cell lymphoma, T cell lymphoma, acute myeloid leukemia, Hodgkin's disease, B cell leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia and myelodysplastic syndromes, are described.
Owner:NUVELO INC
Methods for diagnosis, prognosis and methods of treatment
InactiveUS20100297676A1Increase level of activationMicrobiological testing/measurementDisease diagnosisPhysiologic StatesChronic lymphocytic leukemia
This invention is directed to methods and compositions for diagnosis, prognosis and for determining methods of treatment. The physiological status of a cell present in a sample (e.g. clinical sample) can be used in diagnosis or prognosis of a condition (e.g. Chronic Lymphocytic Leukemia), in patient selection for therapy, to monitor treatment and to modify or optimize therapeutic regimens.
Owner:NODALITY
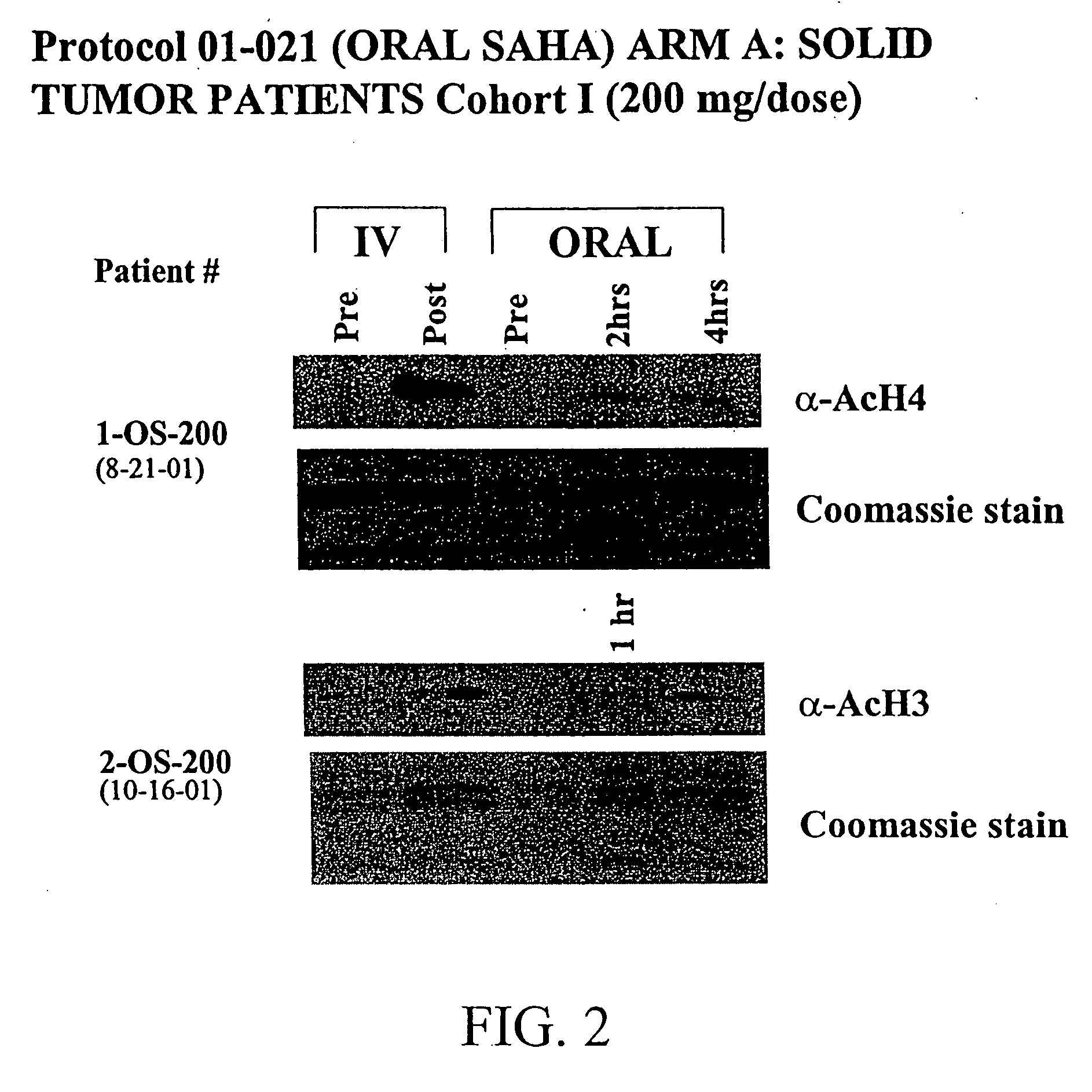
Methods of treating cancer with HDAC inhibitors
InactiveUS20080249179A1Better pharmacokinetic profileImprove bioavailabilityBiocideOrganic chemistryDosing regimenIn vivo
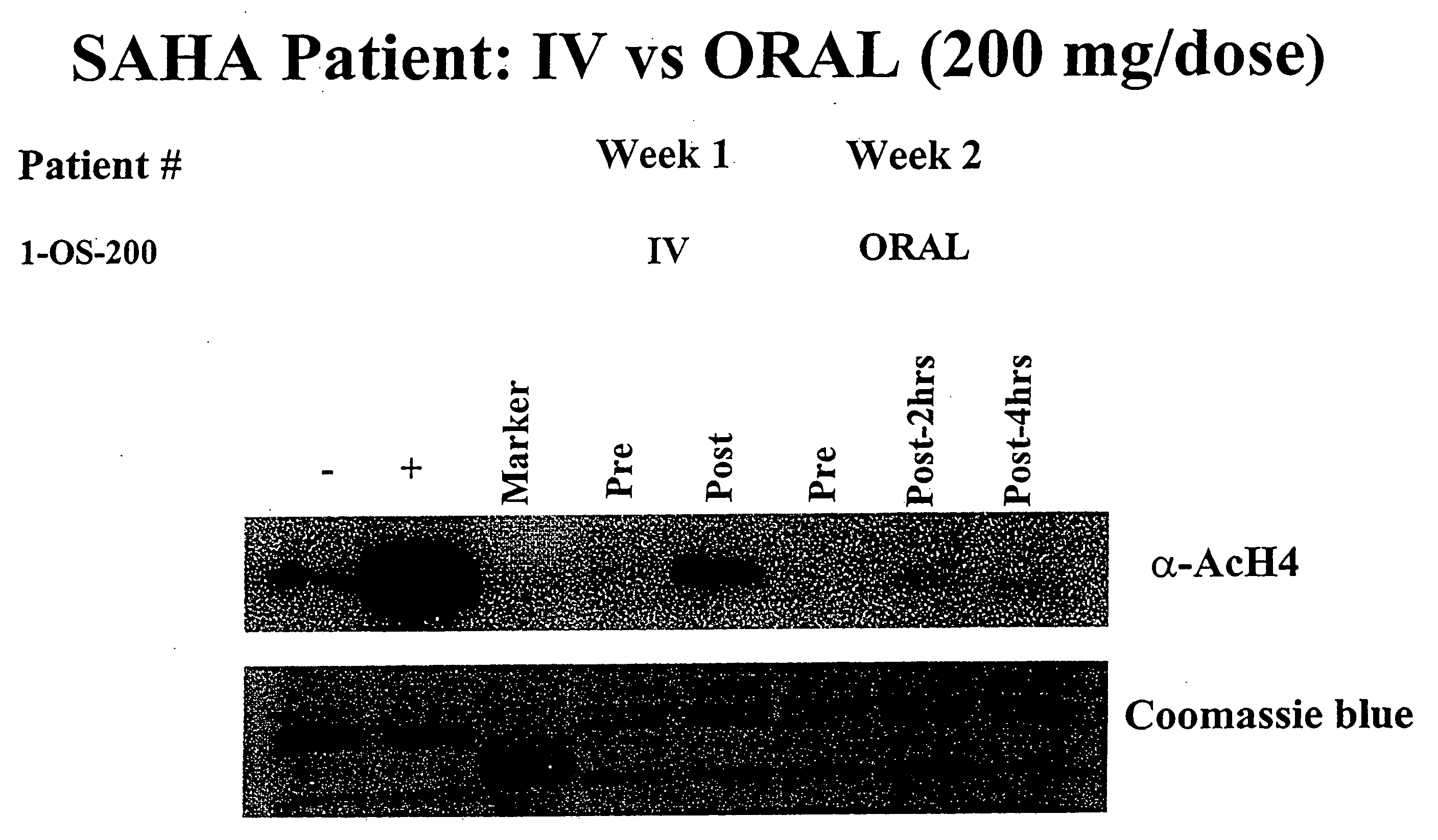
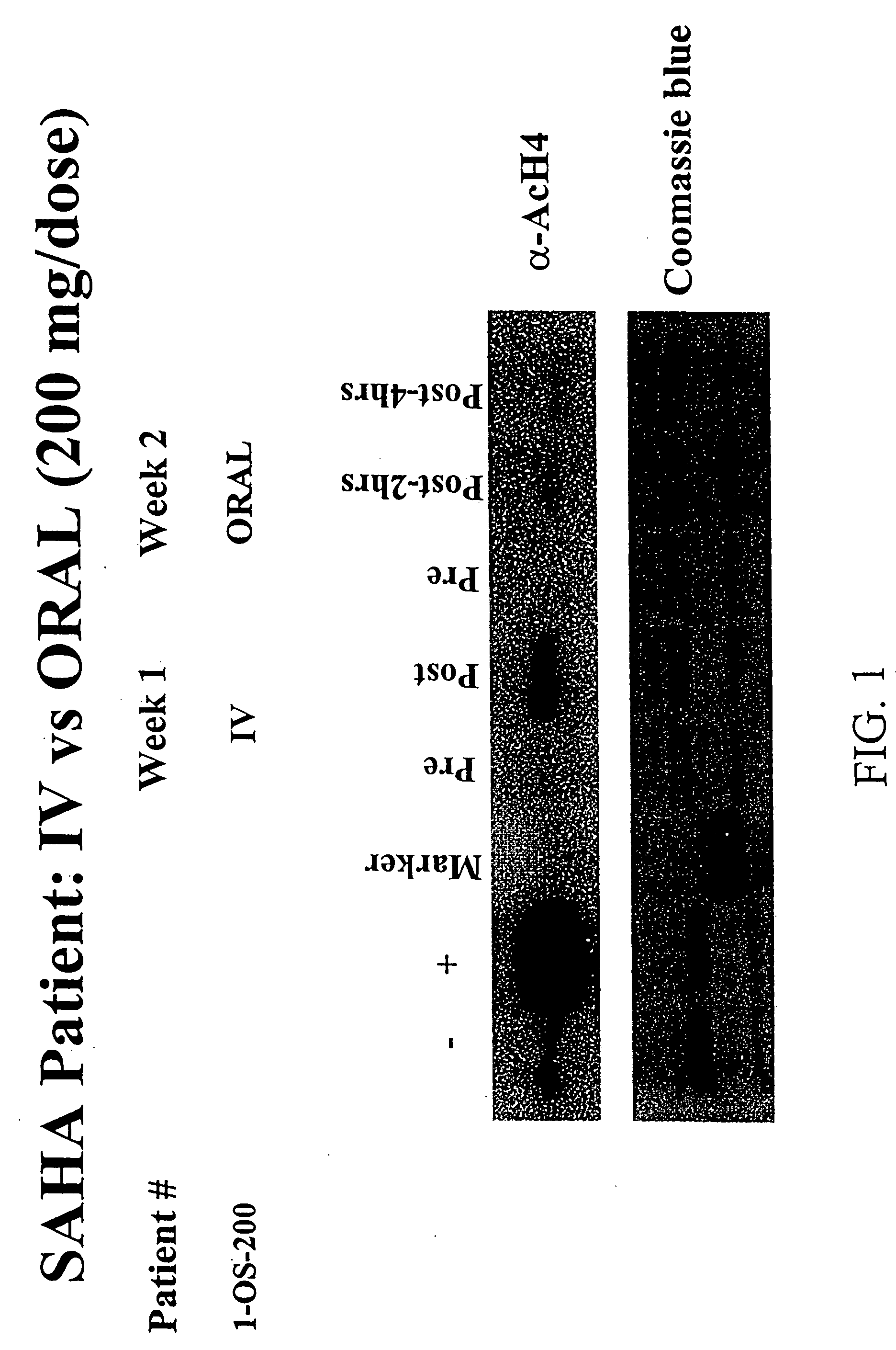
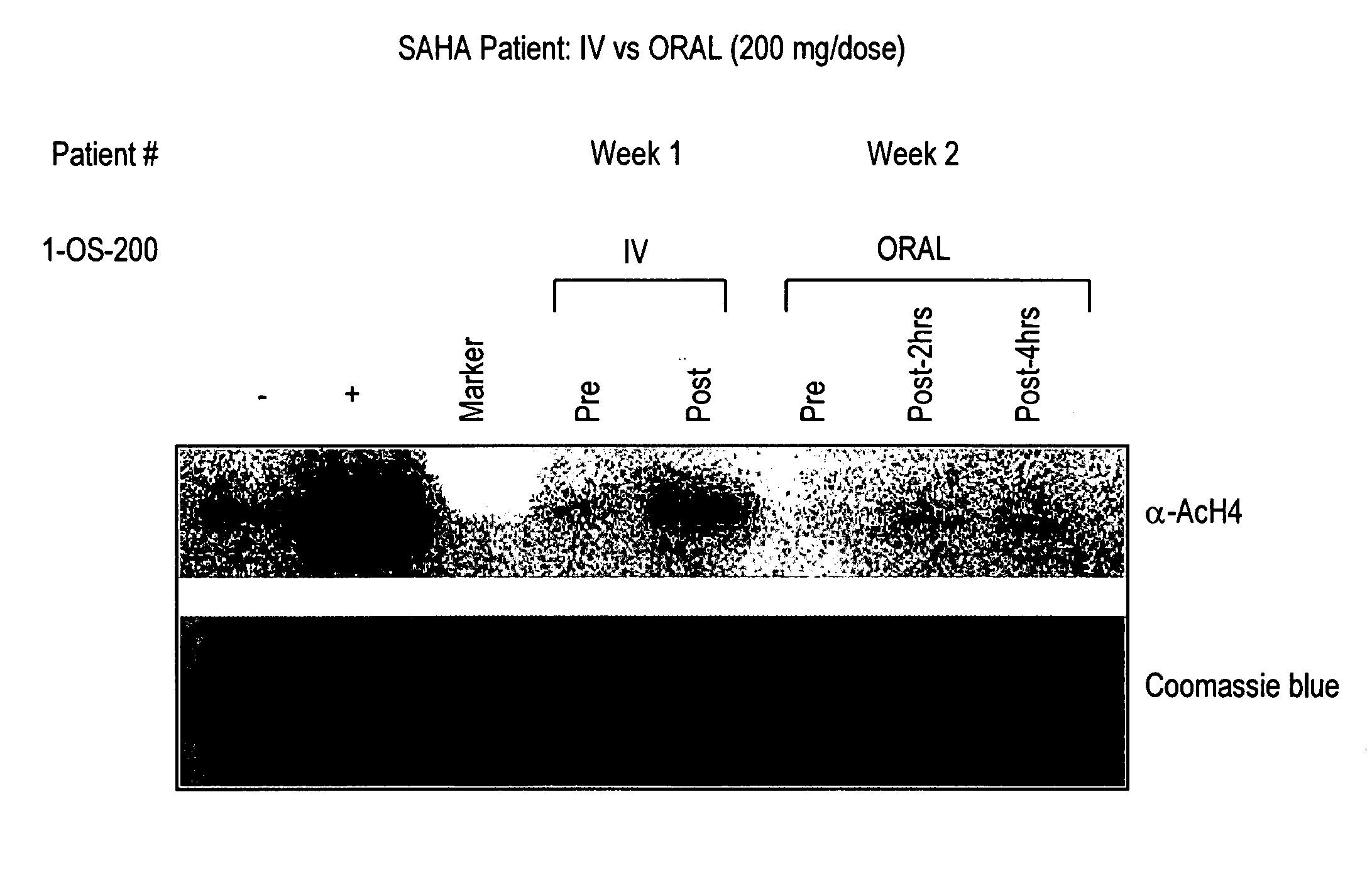
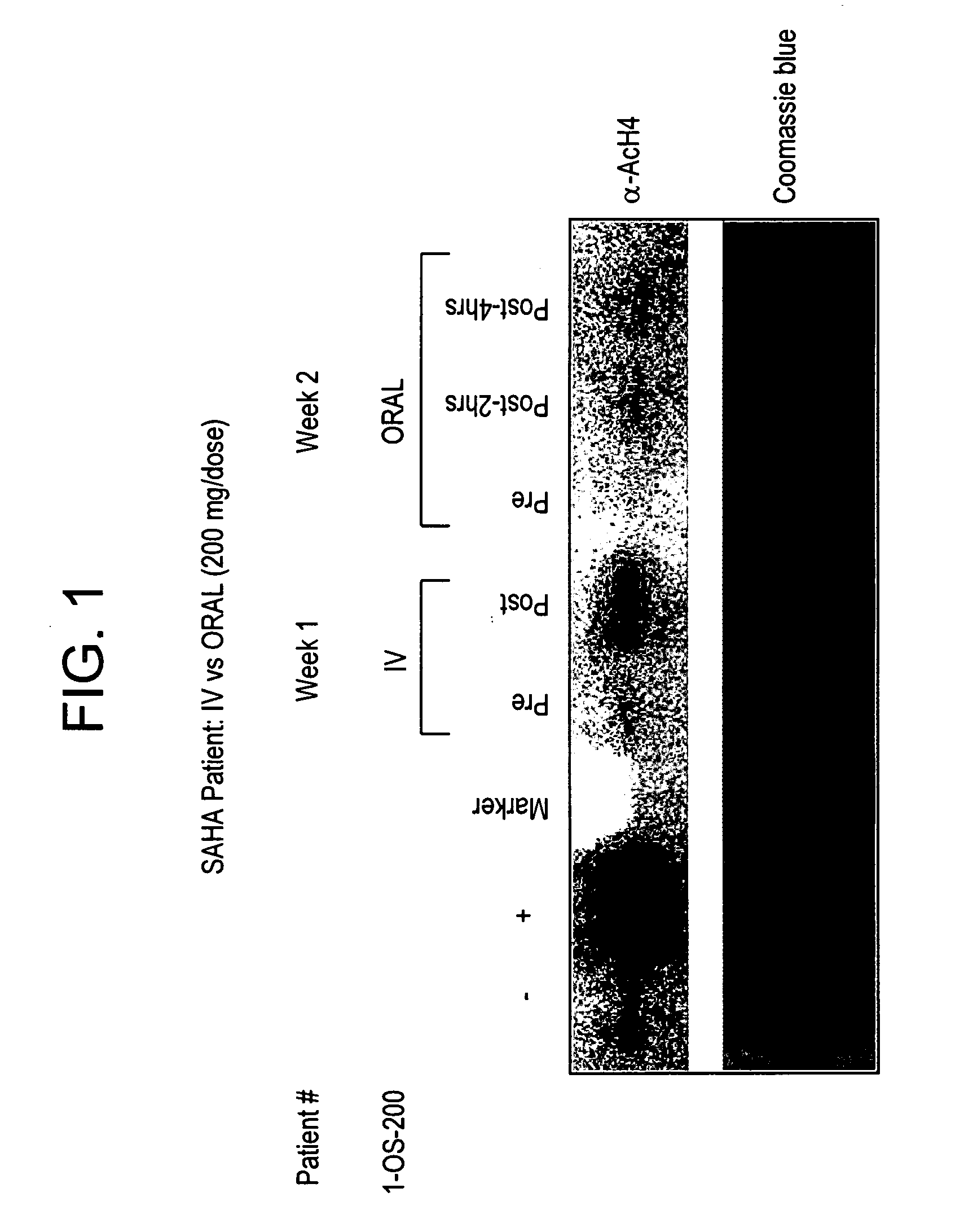
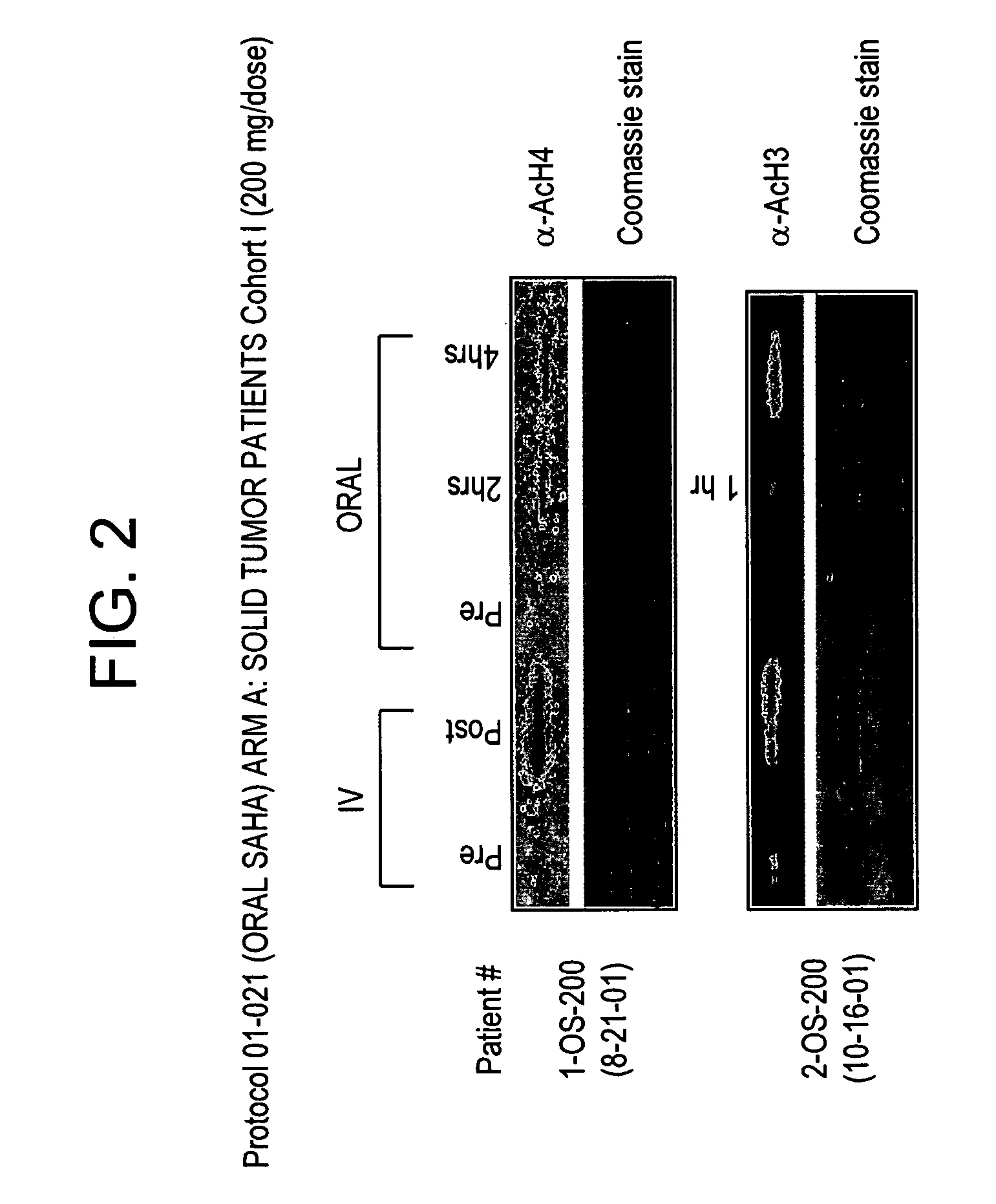
The present invention relates to methods of treating cancers, e.g., leukemia. More specifically, the present invention relates to methods of treating acute and chronic leukemias including Acute Lymphocytic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic leukemia (CLL), Chronic myeloid leukemia (CML) and Hairy Cell Leukemia, by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC +1
Novel Anti-cd38 antibodies for the treatment of cancer
InactiveUS20110262454A1Improved propertyLess immunogenicSenses disorderAntipyreticDiseaseComplement-dependent cytotoxicity
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI SA
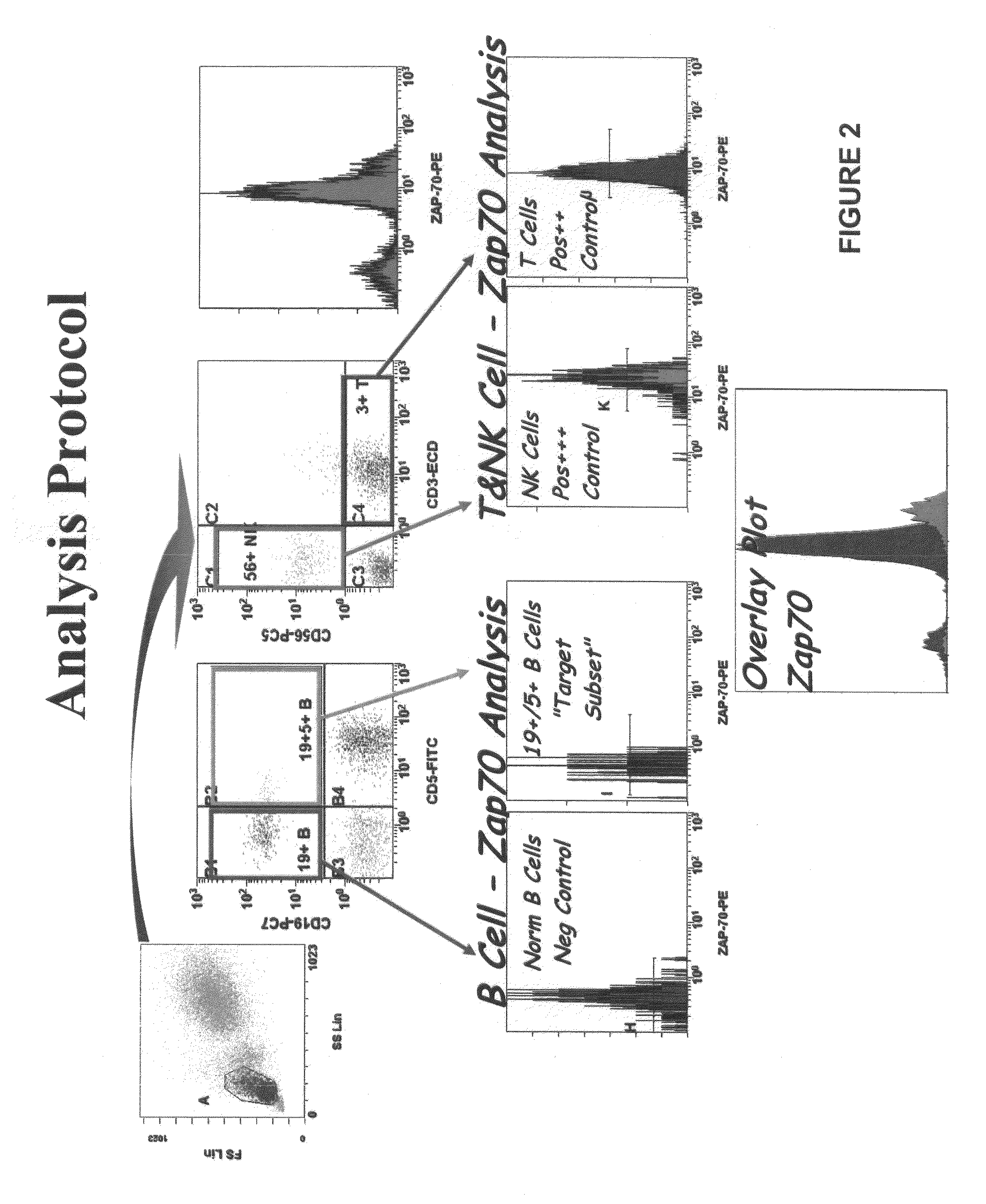
Composite profiles of cell antigens and target signal transduction proteins for analysis and clinical management of hematologic cancers
InactiveUS20070105165A1Increased relapse riskDetermining prognosisDisease diagnosisBlood/immune system cellsCellular antigensTarget signal
The present invention is directed to methods for establishing a composite marker profile for a sample derived from an individual suspected having a neoplastic condition. A composite marker profile of the invention allows for identification of prognostically and therapeutically relevant subgroups of neoplastic conditions and prediction of the clinical course of an individual. The methods of the invention provide tools useful in choosing a therapy for an individual afflicted with a neoplastic condition, including methods for assigning a risk group, methods of predicting an increased risk of relapse, methods of predicting an increased risk of developing secondary complications, methods of choosing a therapy for an individual, methods of determining the efficacy of a therapy in an individual, and methods of determining the prognosis for an individual. In particular, the method of the present invention discloses a method for establishing a composite marker profile that can serve as a prognostic indicator to predict whether the course of a neoplastic condition in a individual will be aggressive or indolent, thereby aiding the clinician in managing the patient and evaluating the modality of treatment to be used. In particular embodiments disclosed herein, the methods of the invention are directed to establishing a composite marker profile for a leukemia selected from the group consisting of Chronic Lymphocytic Leukemia (CLL), Acute Myelogenous Leukemia (AML), Chronic Myelogenous Leukemia (CML), and Acute Lymphocytic Leukemia (ALL).
Owner:BECKMAN COULTER INC +4
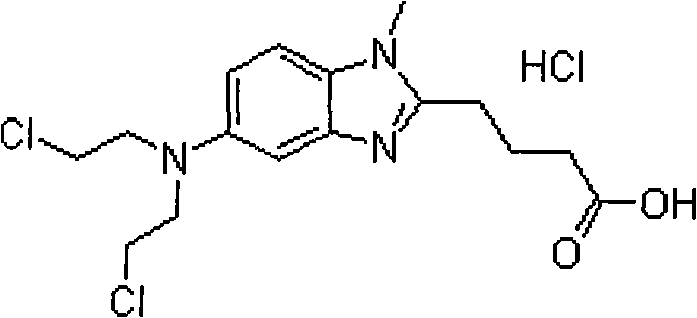
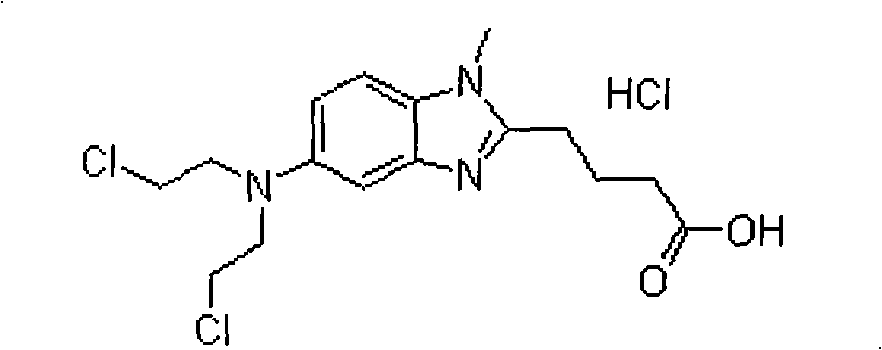
Bendamustine hydrochloride freeze-dried powder injection
InactiveCN101584668AImprove stabilityReduced stabilityPowder deliveryOrganic active ingredientsBendamustine hydrochlorideFreeze-drying
The invention relates to a bendamustine hydrochloride freeze-dried powder injection and its preparing method. The prepared bendamustine hydrochloride freeze-dried powder injection is used for chronic lymphocytic leukemia (CLL) and in the treating process of rituximab / MabThera or containing the same, or for inert B cell non hodgkin lymphoma (B-NHL) patients whose illness state still deteriorates during the treatment period in six months. The bendamustine hydrochloride freeze-dried powder injection contains bendamustine hydrochloride and uses the mixed solvent composed of the tertiary butanol and the injection water in the preparation process. The concentration of the bendamustine hydrochloride in the mixed solvents is 5-10 mg / ml. The volume ratio of the solvents is: 5-50% of tertiary butanol and the balance of injection water. The preparation process is that: measuring the tertiary butanol, adding injection water, mixing evenly, cooling to 2-15 DEG C, heat preserving, then adding the bendamustine hydrochloride, stirring to dissolve, filtering, filling, plugging, disking, freeze-drying, pressing the plug, out box, tying and packing.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Anti-lag-3 antibodies to treat hematological malignancies
Provided are methods for clinical treatment of hematological malignancies, such as relapsed or refractory chronic lymphocytic leukemia or lymphoma using an anti-LAG-3 antibody. Particular malignancies include, e.g., chronic lymphocytic leukemia (CLL), Hodgkin lymphoma (HL), or non-Hodgkin lymphoma (NHL).
Owner:BRISTOL MYERS SQUIBB CO
Methods for treatment of acute lymphocytic leukemia
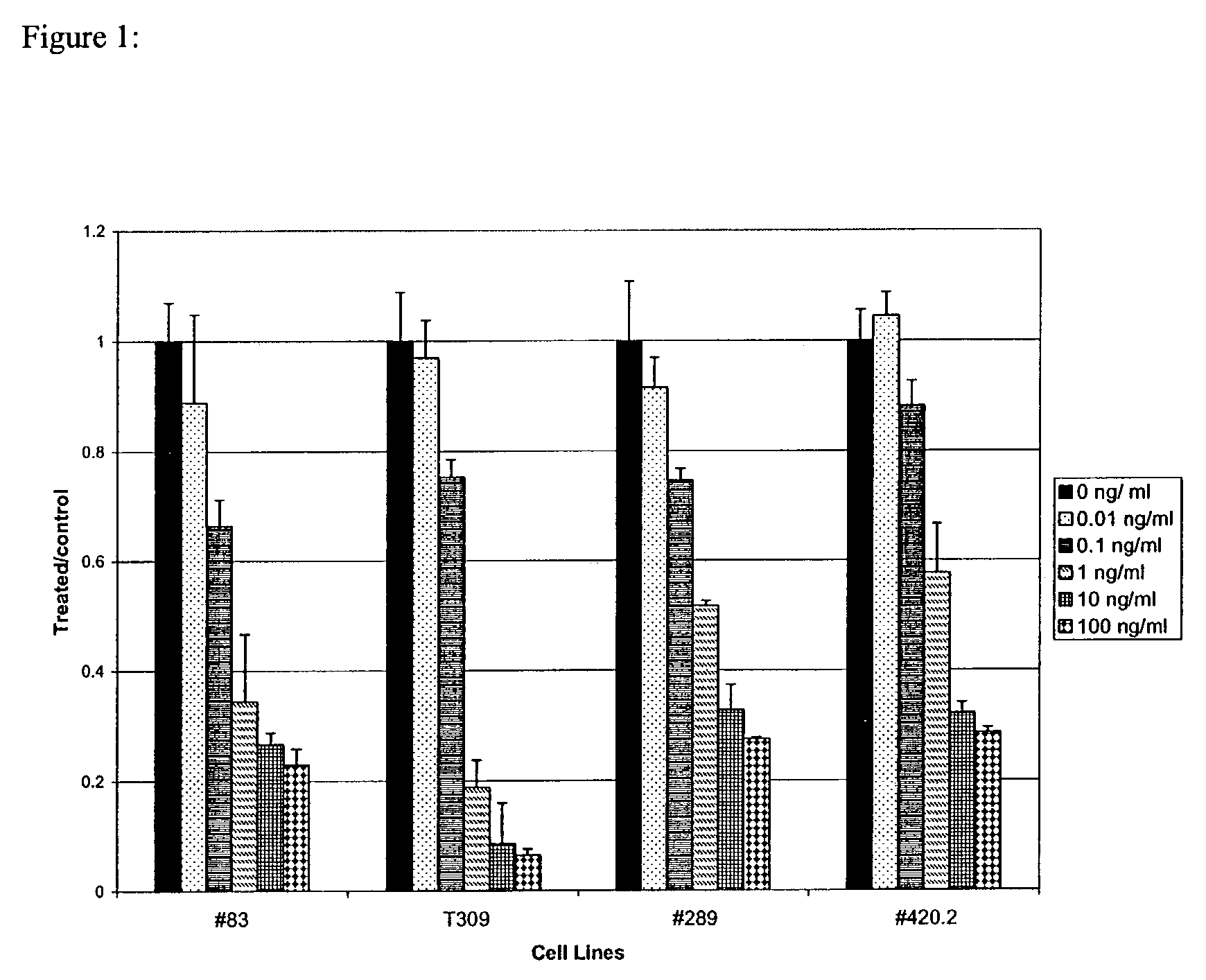
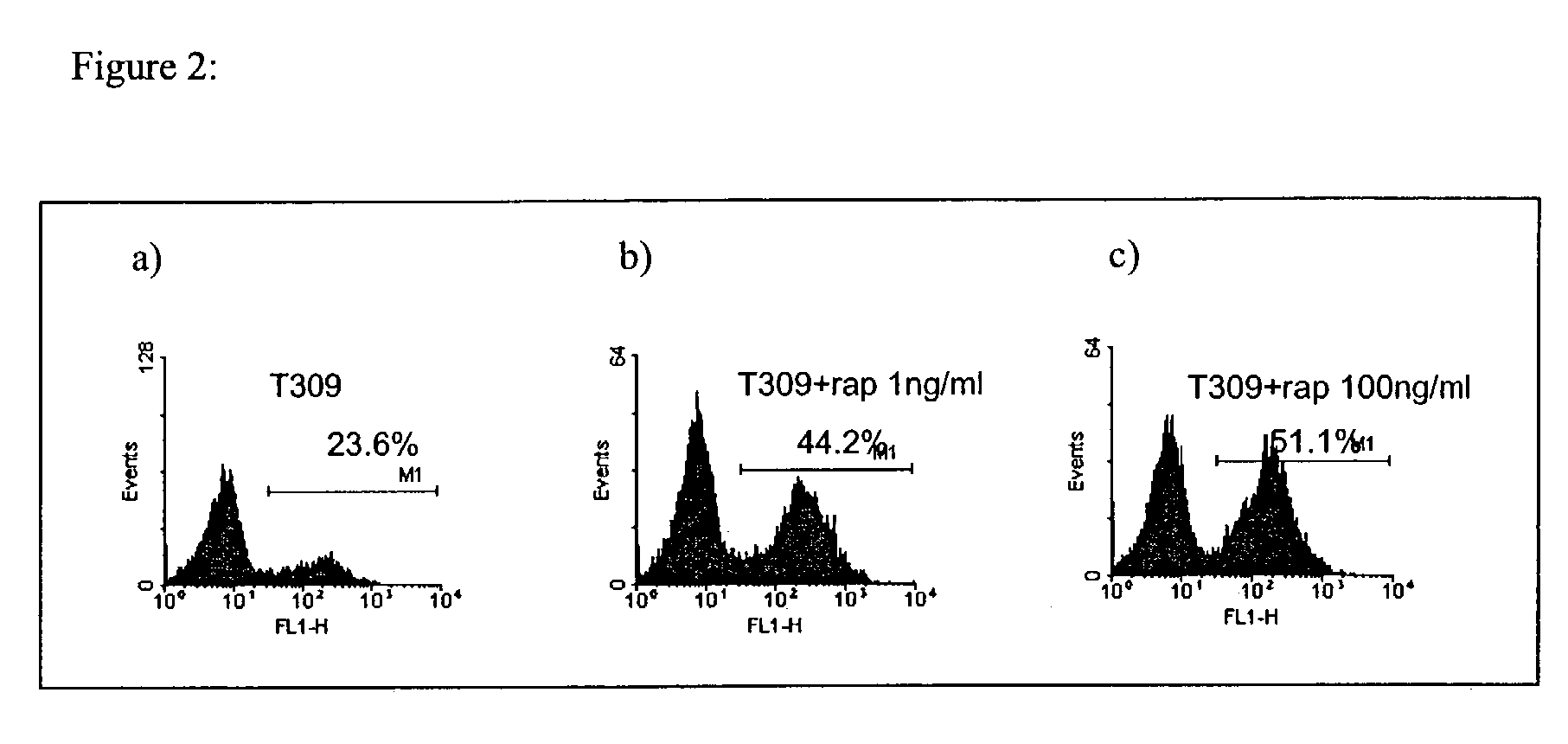
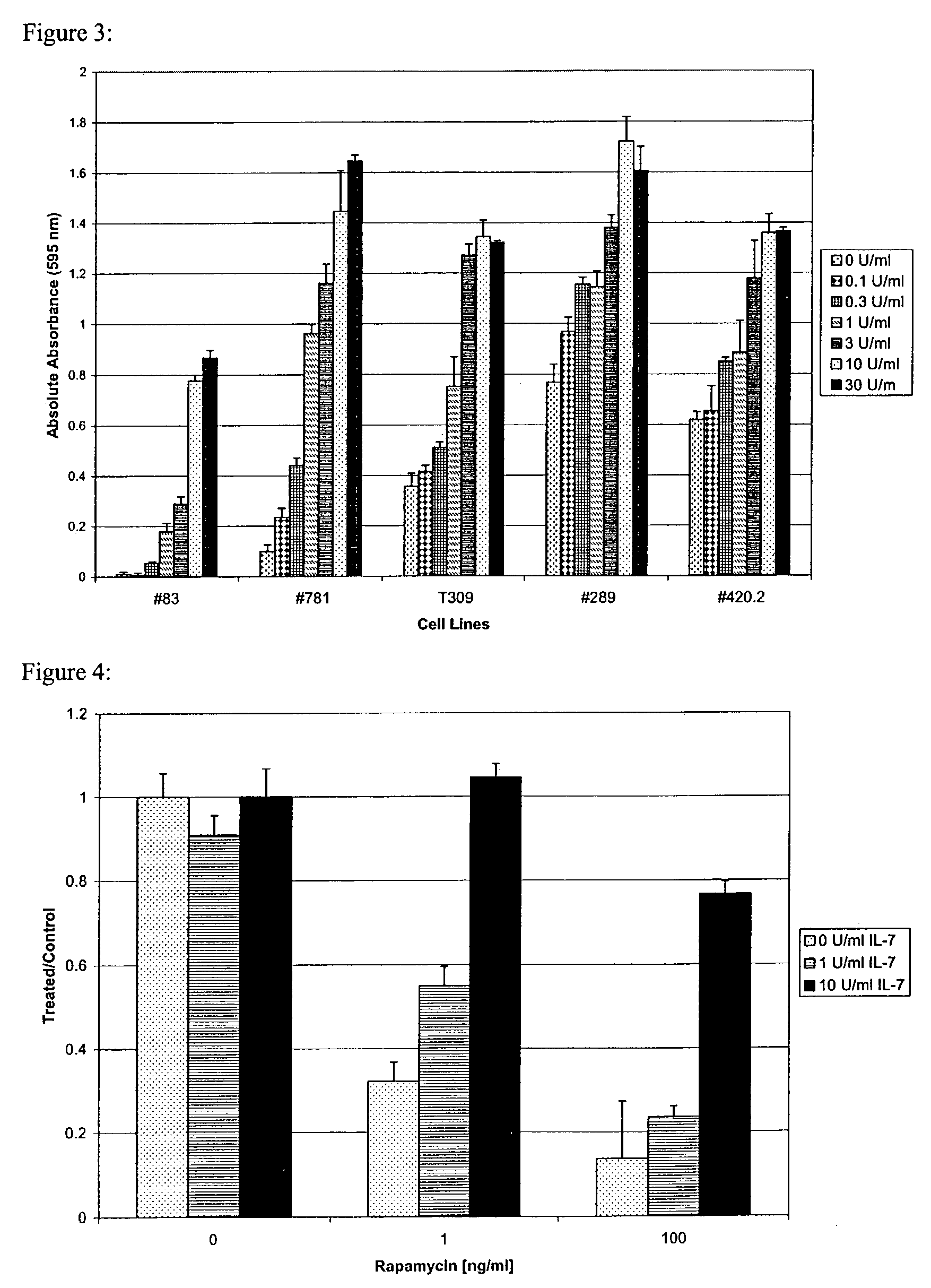
InactiveUS7026330B2Improved prognosisAvoid seizuresBiocideAntibody ingredientsChronic lymphocytic leukemiaAcute lymphocytic leukemia
Methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof are provided. Also provided are methods for treating patients having an early B cell derived acute lymphoblastic leukemia with rapamycin or a derivative thereof in combination with an IL-7 inhibitor. Finally methods for preventing GVHD in ALL patients following a bone marrow transplant are disclosed.
Owner:THE CHILDRENS HOSPITAL OF PHILADELPHIA
Method for determining the prognosis and therapeutic response in chronic lymphocytic leukemia (CLL) patients
InactiveUS20130210014A1Microbiological testing/measurementChronic lymphocytic leukemiaTreatment response
The present invention is based on the seminal discovery that using next generation sequencing, PCR or the like, one of skill in the art can determine the prognosis for or predict responsiveness to a particular therapeutic regimen in a subject diagnosed with CLL.
Owner:SHARMAN JEFF
Bendamustine hydrochloride compound
InactiveCN101606934AStable and uniform qualityImprove stabilityOrganic active ingredientsPharmaceutical product form changeBendamustine hydrochlorideFreeze-drying
The invention relates to a bendmustine hydrochloride compound and a method for preparing the same. The bendmustine hydrochloride compound prepared by the method can be used for treating chronic lymphocytic leukemia (CLL) and inertia B-cell non-Hodgkin's lymphoma (B-cell NHL). The bendmustine hydrochloride compound contains bendmustine hydrochloride and hydroxyproyl beta-cyclodextrin, wherein the mass ratio of bendmustine hydrochloride to hydroxyproyl beta-cyclodextrin is 1:1-20. During the preparation process, mixed solvent of tertiary butanol and water for injection can be used, wherein the concentration of bendmustine hydrochloride in the mixed solvent is 5-10mg / mL; the ratio in the solvent (100%) by volume is as follows: 5-50% of tertiary butanol and the balance of water. The preparation process comprises the following steps of: weighing the tertiary butanol, sequentially adding the solvent and the hydroxyproyl beta-cyclodextrin, dissolving, evenly mixing and cooling the mixture to 2-15 DEG C, maintaining the temperature, then adding the bendmustine hydrochloride, stirring until bendmustine hydrochloride dissolves, filtering, filling, stoppering, dishing-up, freeze-drying, corking, out-box, covering and packaging, and finally, the bendmustine hydrochloride compound is obtained.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
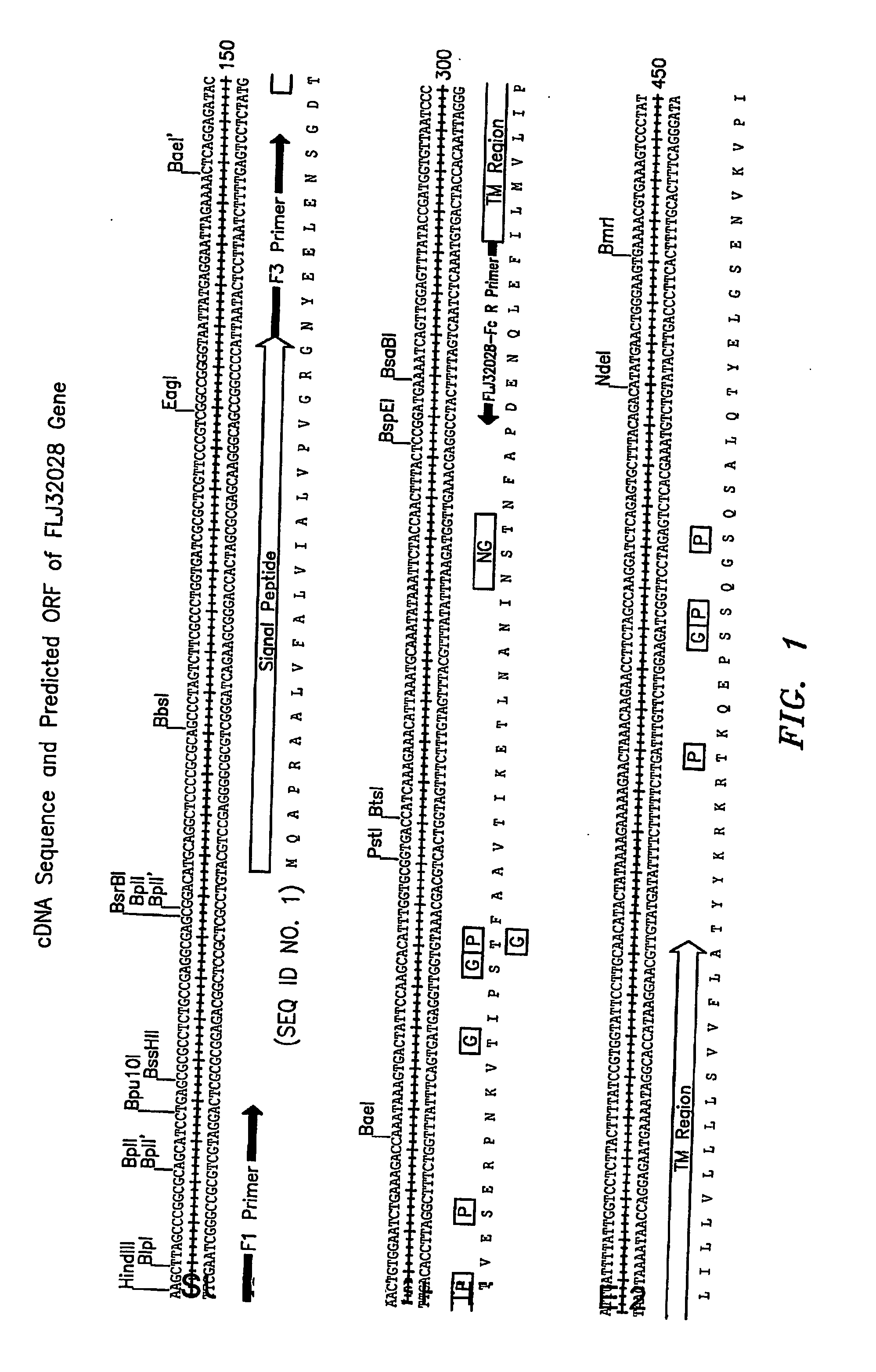
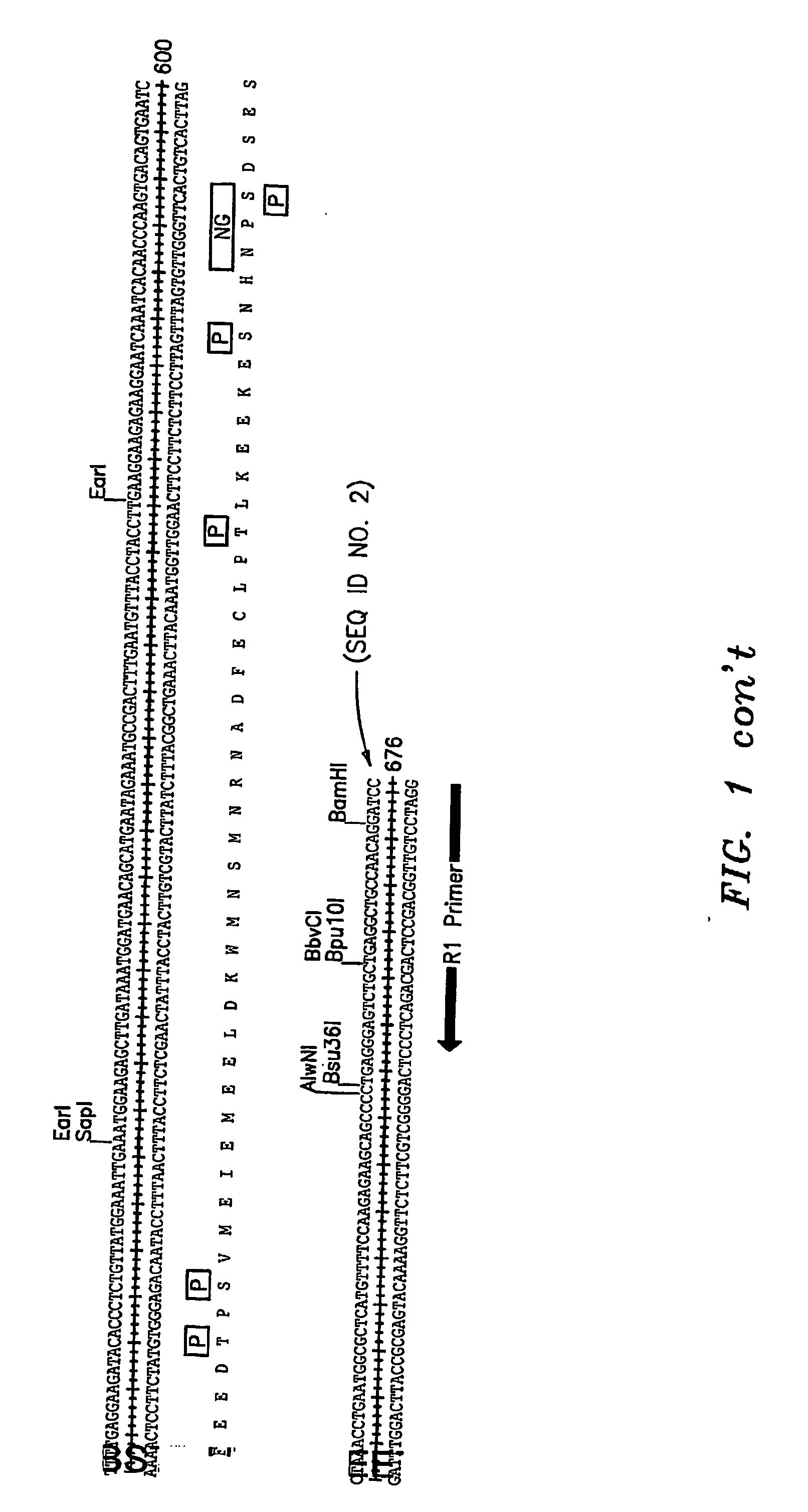
Cell Surface Protein Associated with Human Chronic Lymphocytic Leukemia
InactiveUS20080057519A1Sugar derivativesAntibody mimetics/scaffoldsChronic lymphoblastic leukemiaDiagnostic marker
Protein (referred to herein as “FLJ32028”) that is associated with B-cell chronic lymphocytic leukemia is isolated. Isolated nucleic acid encoding the protein, the generation of monoclonal antibodies recognizing at least a portion of this protein, and the use of this protein or antibodies thereto as a diagnostic marker or therapeutic target for B-CLL are also disclosed.
Owner:ALEXION PHARM INC
Methods of treating cancer with HDAC inhibitors
InactiveUS20080227862A1Better pharmacokinetic profileImprove bioavailabilityBiocideOrganic chemistryDosing regimenIn vivo
The present invention relates to methods of treating cancers, e.g., leukemia. More specifically, the present invention relates to methods of treating acute and chronic leukemias including Acute Lymphocytic Leukemia (ALL), Acute Myeloid Leukemia (AML), Chronic Lymphocytic leukemia (CLL), Chronic myeloid leukemia (CML) and Hairy Cell Leukemia, by administration of pharmaceutical compositions comprising HDAC inhibitors, e.g., suberoylanilide hydroxamic acid (SAHA). The oral formulations of the pharmaceutical compositions have favorable pharmacokinetic profiles such as high bioavailability and surprisingly give rise to high blood levels of the active compounds over an extended period of time. The present invention further provides a safe, daily dosing regimen of these pharmaceutical compositions, which is easy to follow, and which results in a therapeutically effective amount of the HDAC inhibitors in vivo.
Owner:MERCK HDAC RESEARCH LLC
Chronic lymphocytic leukaemia
InactiveUS20080026383A1Short time to first treatmentPrognosis is unfavourableMicrobiological testing/measurementBiological testingLymphocytic leukaemiaChronic lymphocytic leukemia
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
CD20 Antibodies and Uses Thereof
CD20 is a transmembrane protein of the tetra-spanin family expressed on the surface of B-cells from peripheral blood as well as lymphoid tissues. CD20 expression persists from the early pre-B cell stage until the plasma cell differentiation stage. In addition to expression in normal B-cells, CD20 is expressed in B-cell derived malignancies such as non-Hodgkin's lymphoma (NHL) and B-cell chronic lymphocytic leukemia (CLL). The present invention includes anti-CD20 antibodies and antigen-binding fragments thereof comprising a light chain variable region and a heavy chain variable region, wherein the CDR-L1, CDR-L2, and CDR-L3 of said light chain variable region comprise the amino acid sequences of SEQ ID NOs: 23-25, respectively, and wherein the CDR-H1, CDR-H2, and CDR-H3 of said heavy chain variable region comprise the amino acid sequences of SEQ ID NOs: 26-28, respectively.
Owner:IMMUNOGEN INC
Oral Dosage Forms of Bendamustine and Therapeutic Use Thereof
InactiveUS20130209558A1Improve stabilitySuperior dissolution profileBiocideHeavy metal active ingredientsDiseaseOral treatment
In the present invention there is provided a pharmaceutical composition for oral administration which comprises bendamustine or a pharmaceutically acceptable, ester, salt or solvate thereof as an active ingredient, and a pharmaceutically acceptable excipient and which shows a dissolution of the bendamustine of at least 60% in 20 minutes, 70% in 40 minutes and 80% in 60 minutes, as measured with a paddle apparatus at 50 rpm according to the European Pharmacopoeia in 500 ml of a dissolution medium at a pH of 1.5, and wherein the pharmaceutically acceptable excipient is either a pharmaceutically acceptable non-ionic surfactant, selected from the group consisting of a polyethoxylated castor oil or derivative thereof and a block copolymer of ethylene oxide and propylene oxide or a pharmaceutically acceptable saccharide selected from the group consisting of one or more of a monosaccharide, a disaccharide, an oligosaccharide, a cyclic oligosaccharide, a polysaccharide and a saccharide alcohol, wherein the ratio by weight of the active ingredient to the saccharide excipient(s) is in the range of 1:1-5. The invention further relates to the above pharmaceutical composition for use for the oral treatment of a medical condition which is selected from chronic lymphocytic leukemia, acute lymphocytic leukaemia, chronic myelocytic leukaemia, acute myelocytic leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, multiple myeloma, breast cancer, ovarian cancer, small cell lung cancer and non-small cell lung cancer. The invention moreover relates to the above pharmaceutical composition for the above use wherein the dosage regimen comprises at least the administration of a dose of 100 to 600 mg / m2 / per person of bendamustine on day 1 and day 2, optionally a dose of 50 to 150 mg / m2 i.v. or orally of a corticosteroid on days 1 to 5, and optionally a suitable dose of a further active agent selected from the group consisting of an antibody specific for CD20, an anthracyclin derivative, a vinca alkaloid or a platin derivative; and the repetition of said dosage regimen 4 to 15 times after intervals of two to four weeks.
Owner:ASTELLAS DEUTLAND
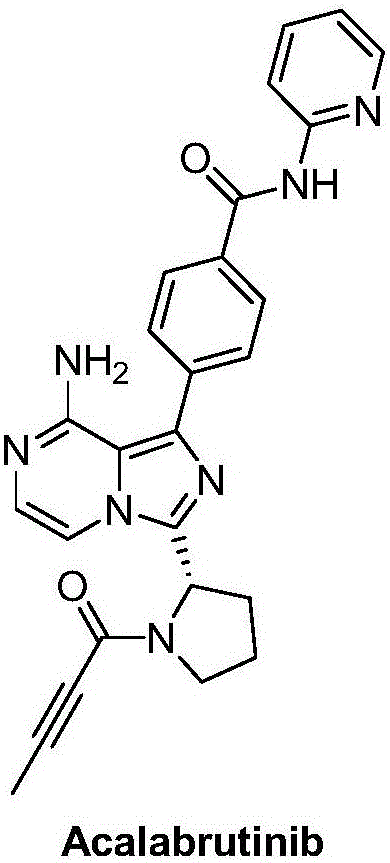
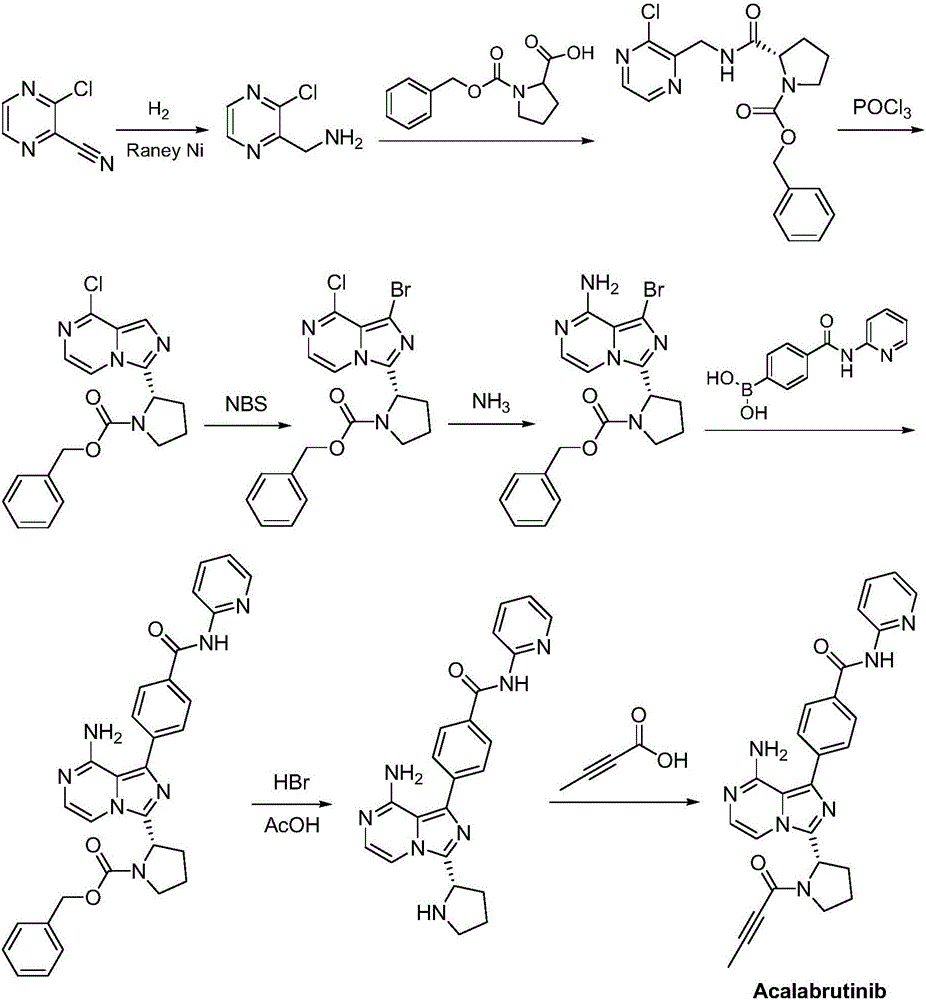
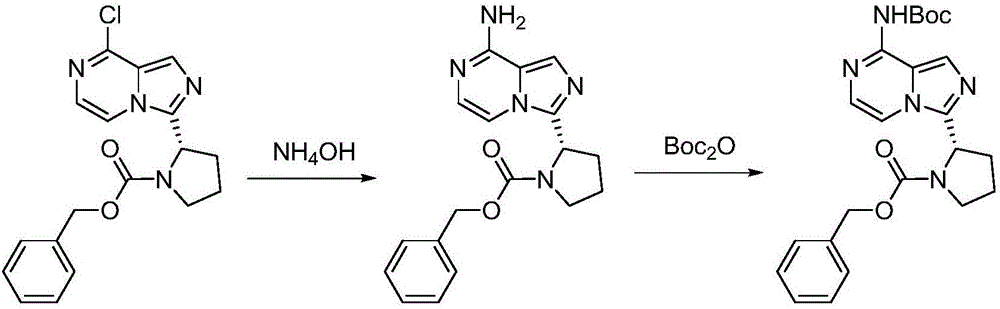
Synthesis method of BTK inhibitor Acalabrutinib for treating chronic lymphocytic leukemia
ActiveCN107522701AMeet the needs of useUse requirements applyOrganic chemistryTert-Butyloxycarbonyl protecting groupPyrazine
A synthesis method of a BTK inhibitor Acalabrutinib for treating chronic lymphocytic leukemia comprises the steps as follows: preparing (S)-2-(8-aminoimidazo[1,5-a]pyrazine-3-yl)-1-pyrrolidinecarboxylic aci benzyl ester; preparing (S)-2-(8-tert-butoxycarbonylaminoimidazo[1,5-a]pyrazine-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester; preparing (S)-2-(8-tert-butoxycarbonylamino-1-bromoimidazo[1,5-a]pyrazine-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester; preparing (S)-2-(8-tert-butoxycarbonylamino-1-[4-(2-pyridylcarbamoyl)phenyl]imidazo[1,5-a]pyrazine-3-yl)-1-pyrrolidinecarboxylic acid benzyl ester; preparing (S)-2-(8-tert-butoxycarbonylamino-1-[4-(2-pyridylcarbamoyl)phenyl]imidazo[1,5-a]pyrazine-3-yl)pyrrolidine; preparing (S)-2-(8-tert-butoxycarbonylamino-1-[4-(2-pyridylcarbamoyl)phenyl]imidazo[1,5-a]pyrazine-3-yl)-1-(2-butynoyl)pyrrolidine; preparing Acalabrutinib. The yield is high, the process is simplified, and the synthesis method is green and environmentally friendly.
Owner:SUZHOU FUSHILAI PHARMA CO LTD +1
Cell line of lymphocytes comprising gamma-delta T cells, composition and production method thereof
The present invention relates to a cell line of lymphocytes comprising γδ T cells, composition and production method thereof, and medical use namely for the use in medicine, namely in cancer immunotherapy.The cell line comprise a sample of human peripheral blood Vδ1+γδ T cells expressing functional natural cytotoxicity receptors (NCRs). These Vδ1+ NCR+ T lymphocytes can directly mediate killing of leukemia cell lines and chronic lymphocytic leukemia patient neoplastic cells.The present invention shows that human Vδ1+ NCR+ T cells can be differentiated and expanded from total γδ peripheral blood lymphocytes (PBLs), upon regular in vitro or ex vivo stimulation with γδTCR agonists and γc-family cytokines. This subset surprisingly expresses NKp30, NKp44 and NKp46, and high levels of Granzyme B that associate with highly enhanced cytotoxicity against lymphoid leukemias.
Owner:INST DE MEDICINA MOLECULAR JOAO LOBO ANTUNES
Chimeric antigen receptor of targeted CD19 as well as method and use for jointly expressing IL-15
ActiveCN108728459AMammal material medical ingredientsNucleic acid vectorAntiendomysial antibodiesTumor cells
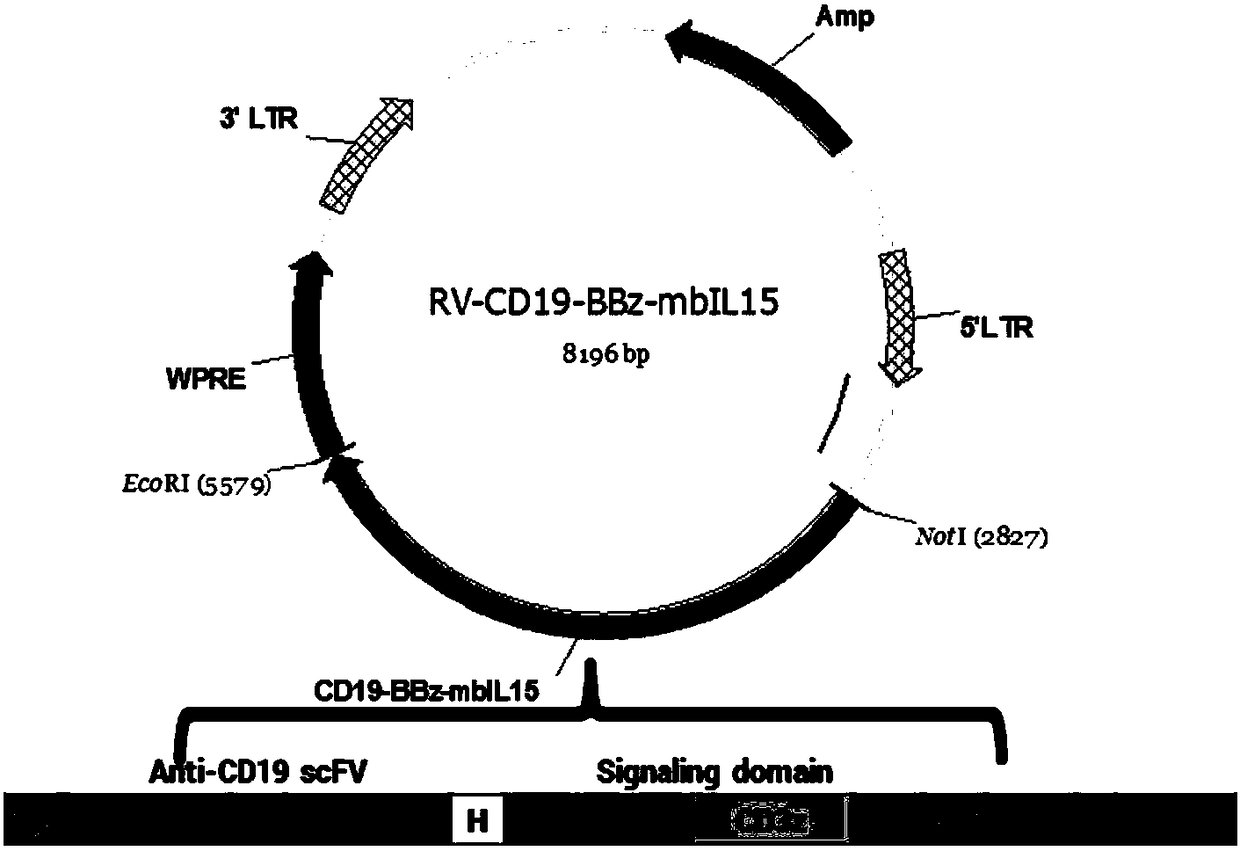
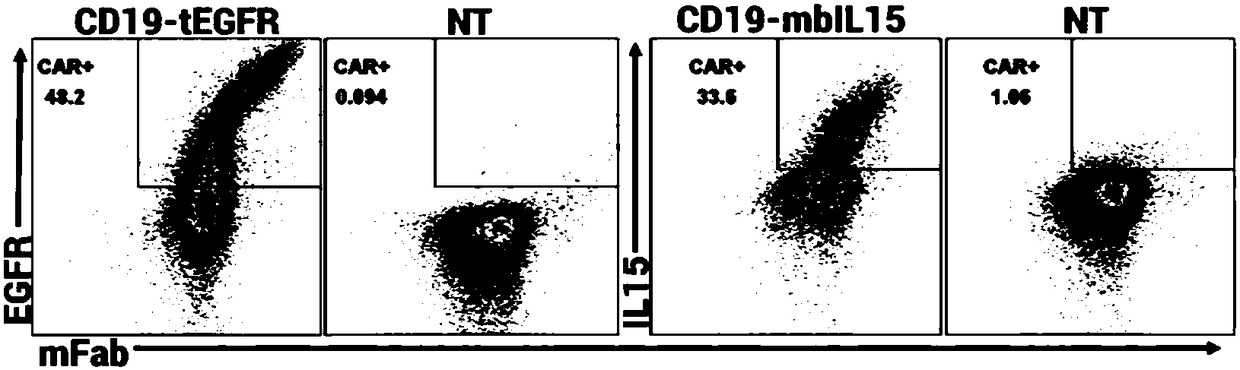
The invention discloses a chimeric antigen receptor CD19-CD8H&TM-41BB-CD3zeta-mbIL15 and use thereof. The chimeric antigen receptor is formed by series connection of a heavy chain and light chain variable region (CD19scFV) of a mouse anti-human CD19 monoclonal antibody (with a cloning number being FMC63), a human CD8alpha hinge region, a transmembrane region, a human 41BB intracellular region, a human CD3zeta intracellular region and an IL15+IL15Ralphla structure. The chimeric antigen receptor is used for modifying a human T-lymphocyte, and the modified T-cell (CAR-T cell) can be used for expressing CD19 positive acute / chronic lymphocytic leukemia and treating lymphoma. The prepared CD19 mbIL15 CAR-T cell has a strong killing function on specific tumor cells.
Owner:HRAIN BIOTECHNOLOGY CO LTD
Bispecific antibody for CD20 and CD3
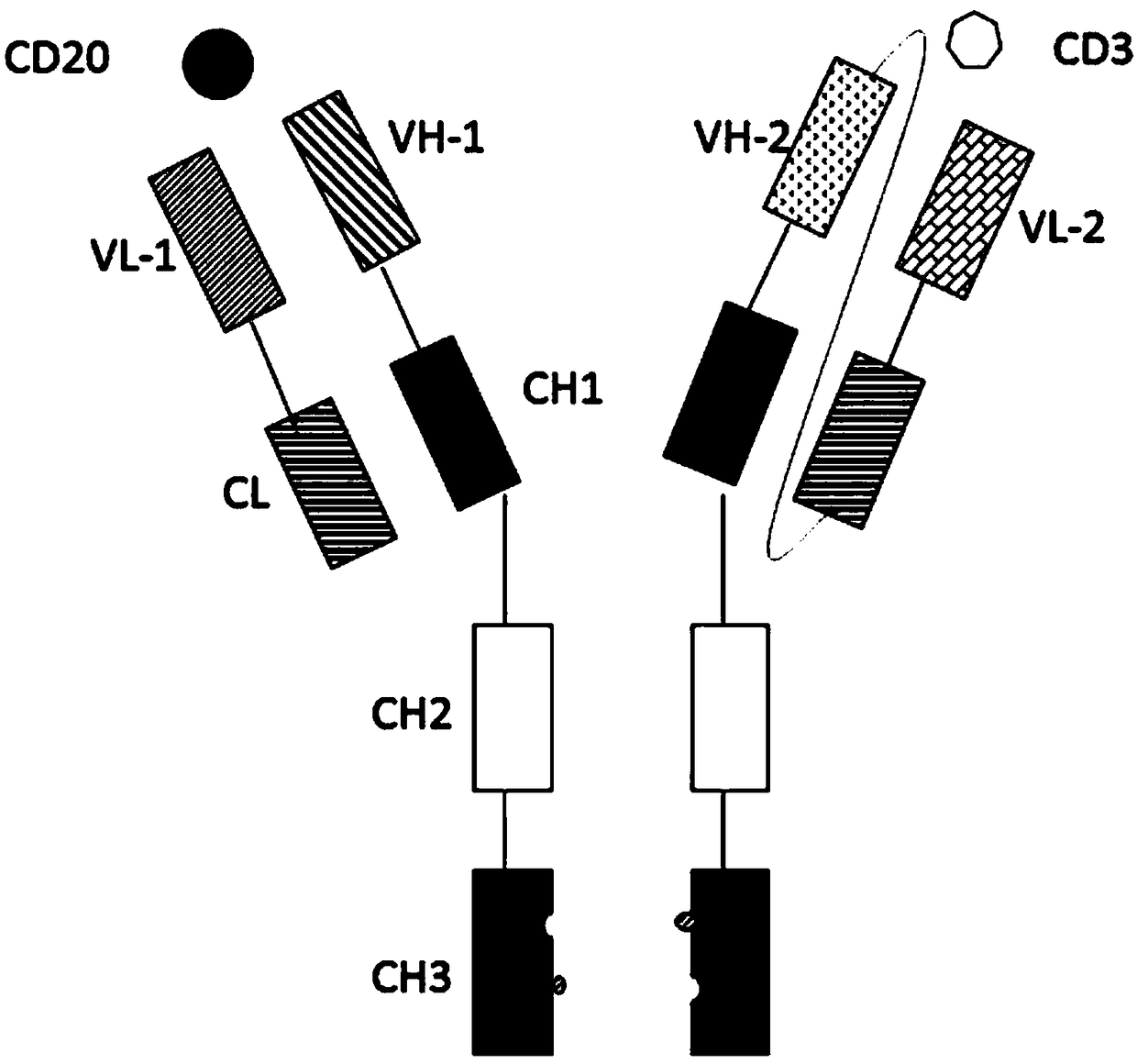
ActiveCN108059680AImprove stabilityOvercome stabilityHybrid immunoglobulinsAntibody ingredientsAntigenCD20
The invention relates to the technical field of biological pharmacy and particularly discloses a bispecific antibody for CD20 and CD3. The bispecific antibody comprises univalent unit, a single-chainunit and a linker, wherein the univalent unit is a light chain-heavy chain pair with a specific binding capability for an antigen CD20 on the surface of a tumor cell; the single-chain unit can be usedfor specifically binding a CD3 antigen on the surface of an immune cell; the end N of a heavy chain and the end C of a light chain of the single-chain unit are connected by the linker to form a Fab-Fc form; the univalent unit is connected with the single-chain unit through a disulfide bond of a hinge region and a pestle mortar structure of a CH3 structural domain. According to the bispecific antibody, CD20 and CD3 can be specifically bound and the immune effector cell can be targeted to the tumor cell, so that the killing effect of the immune effector cell on the tumor cell is improved; the bispecific antibody can be used for treating CD20 antigen positive B-cell neoplasms, comprising non-Hodgkin lymphoma, chronic lymphocytic leukemia and the like, and has a broad application prospect inthe field of immunotherapy of tumors.
Owner:BEIJING DONGFANG BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com