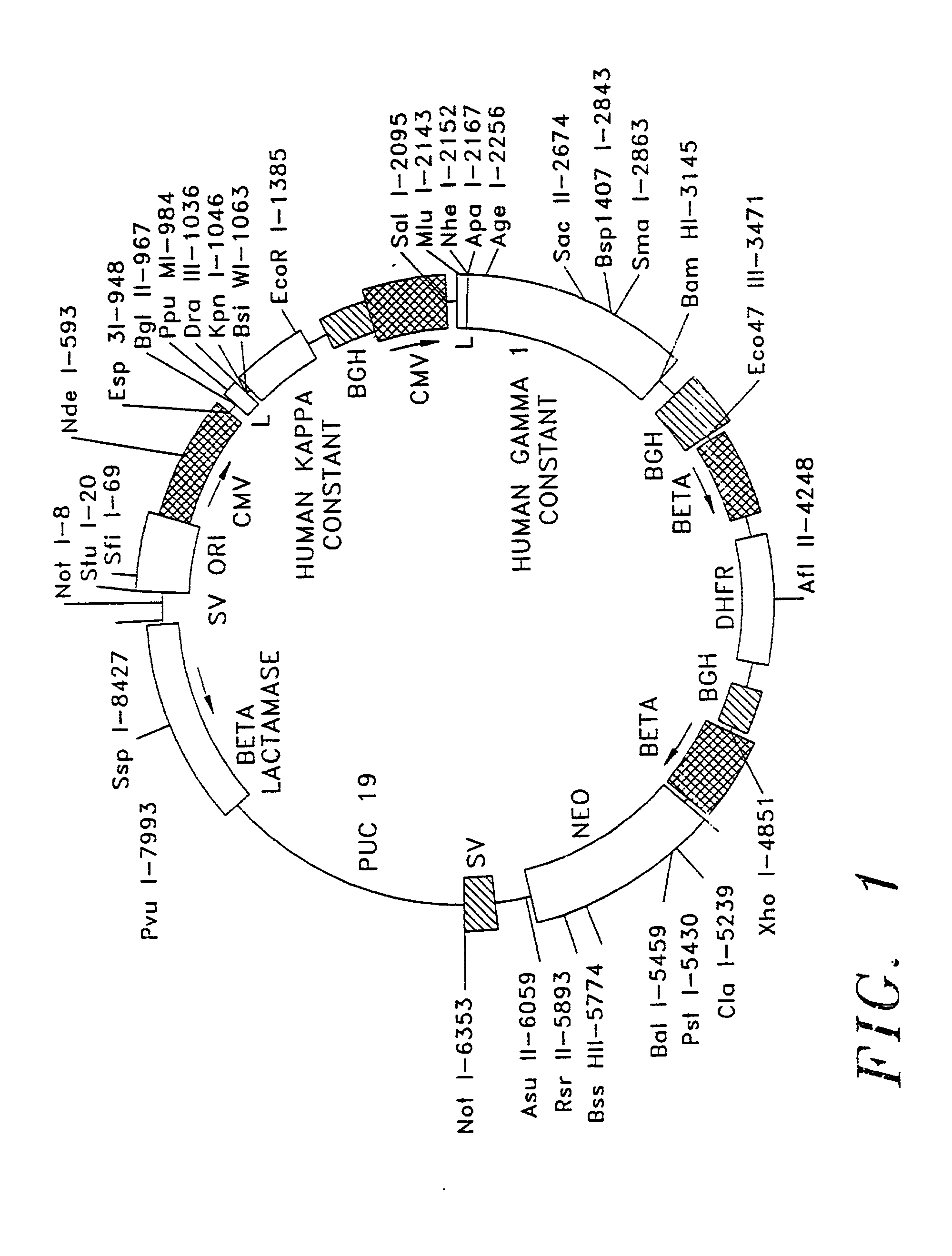
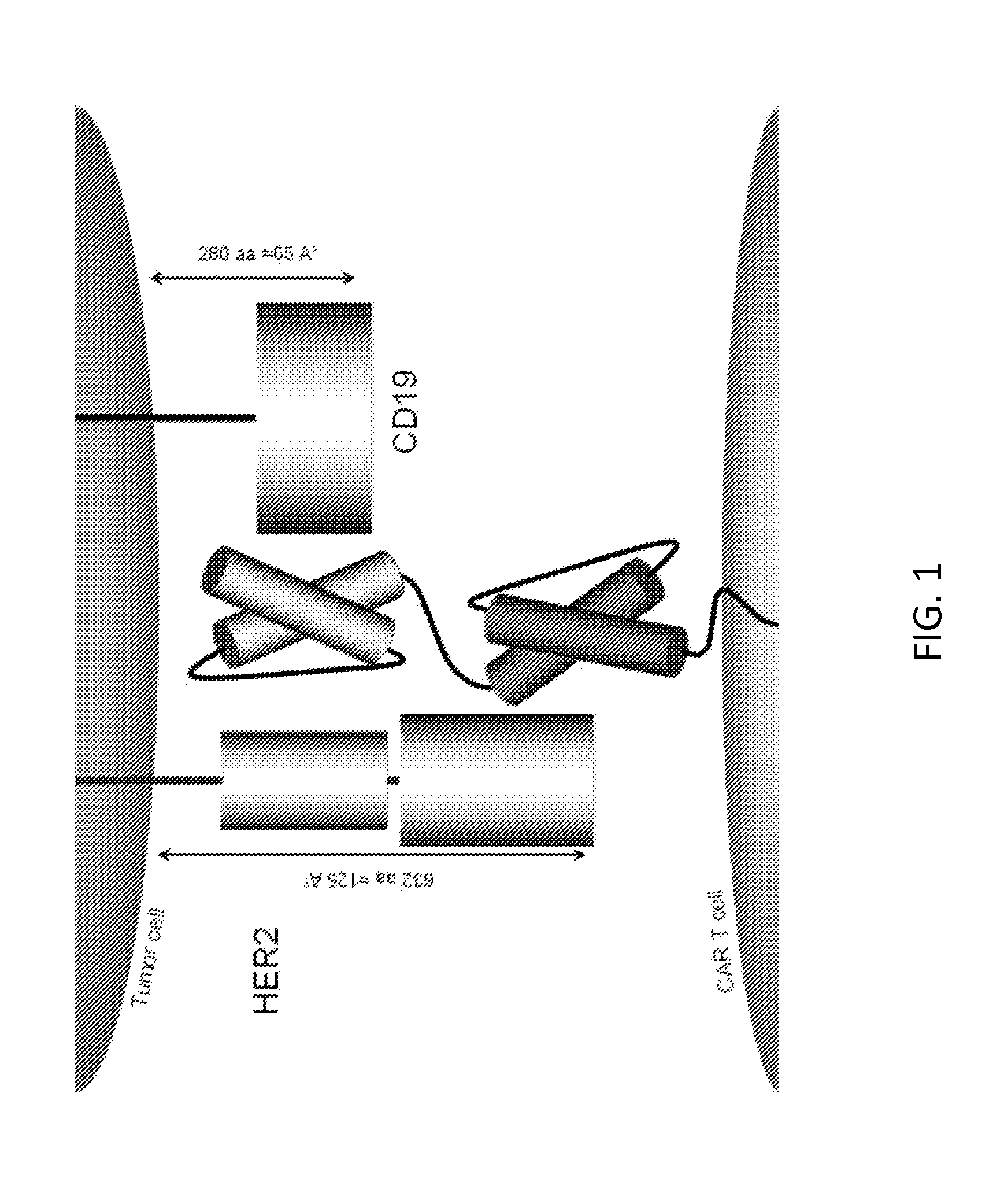
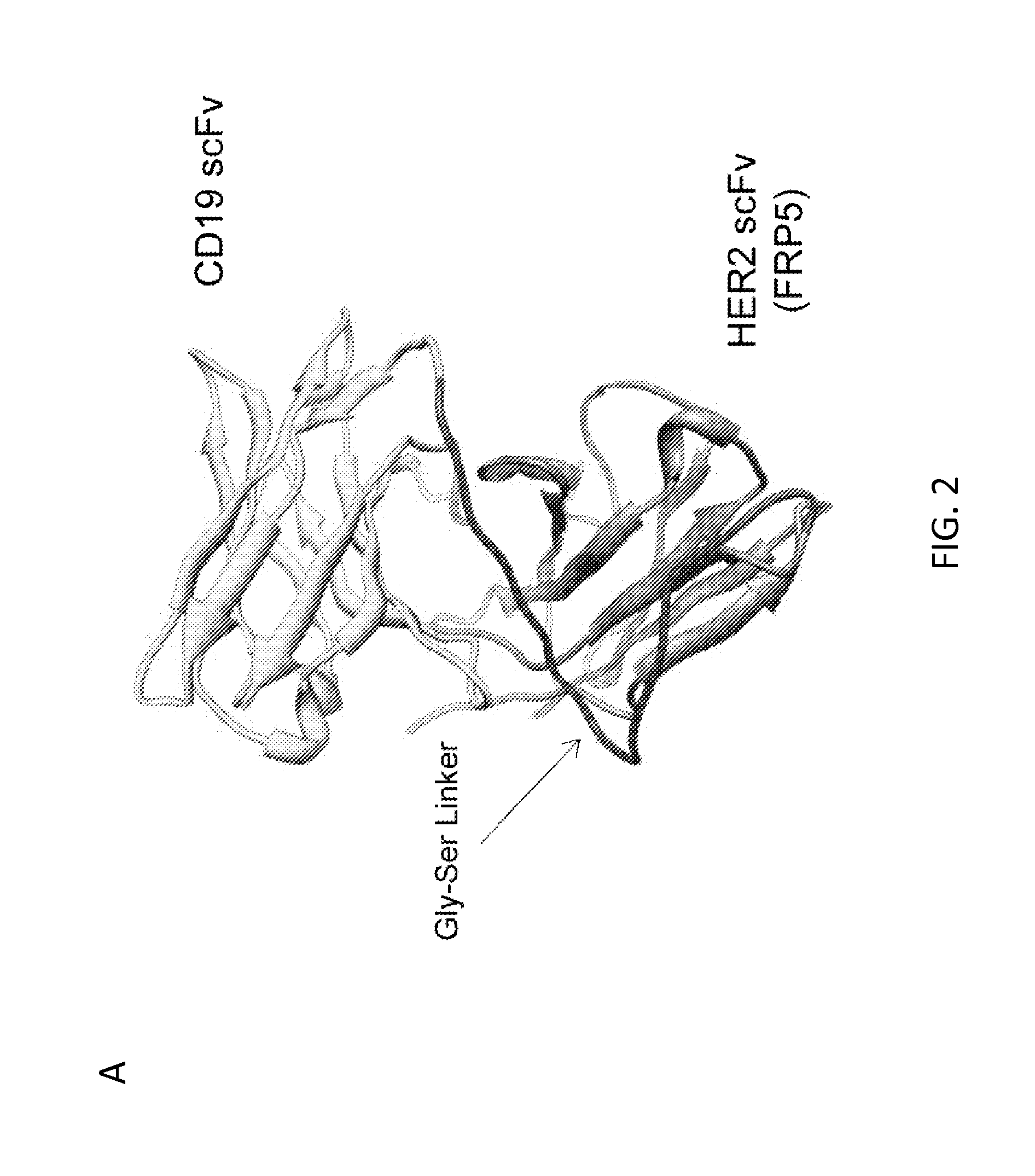
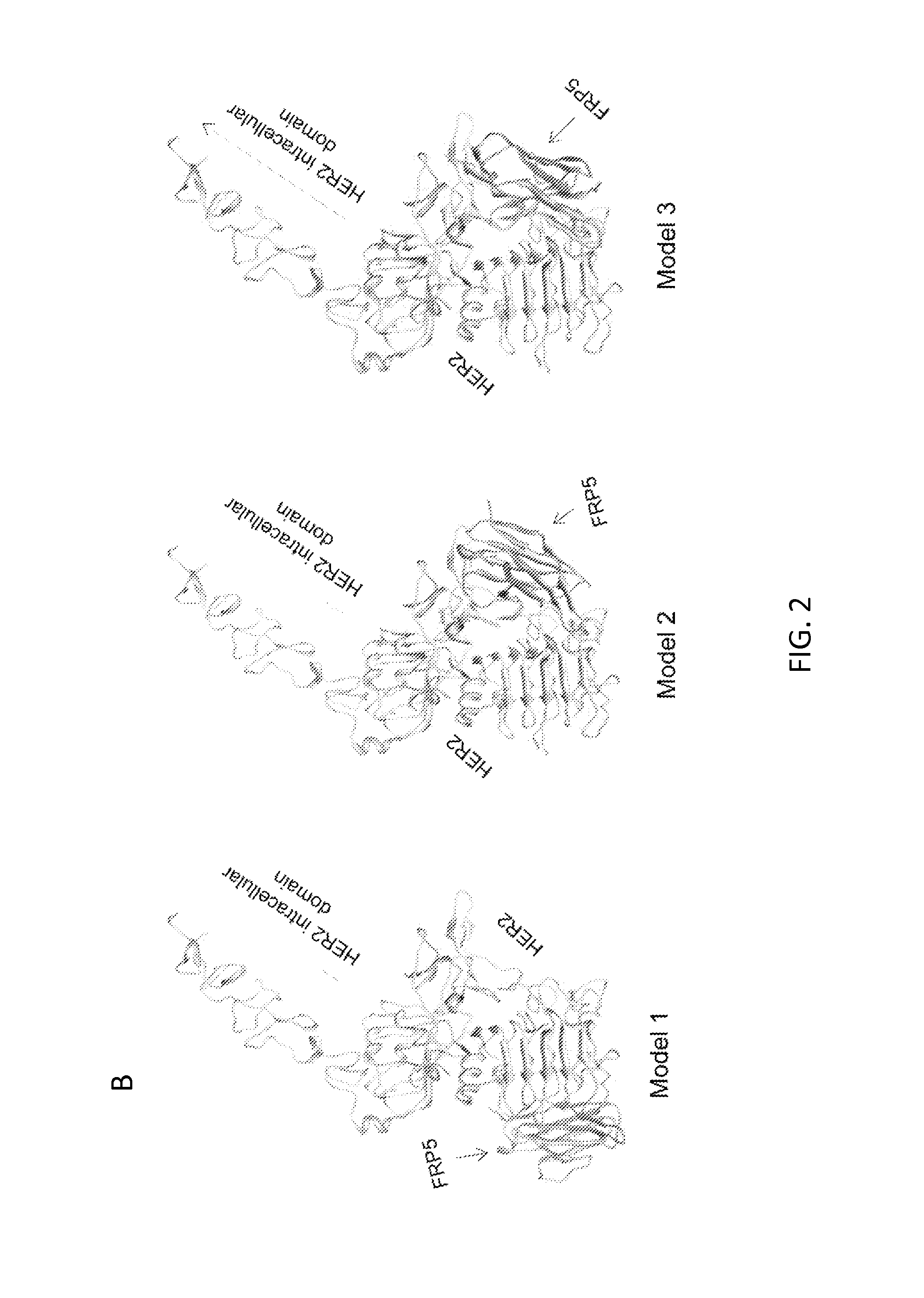
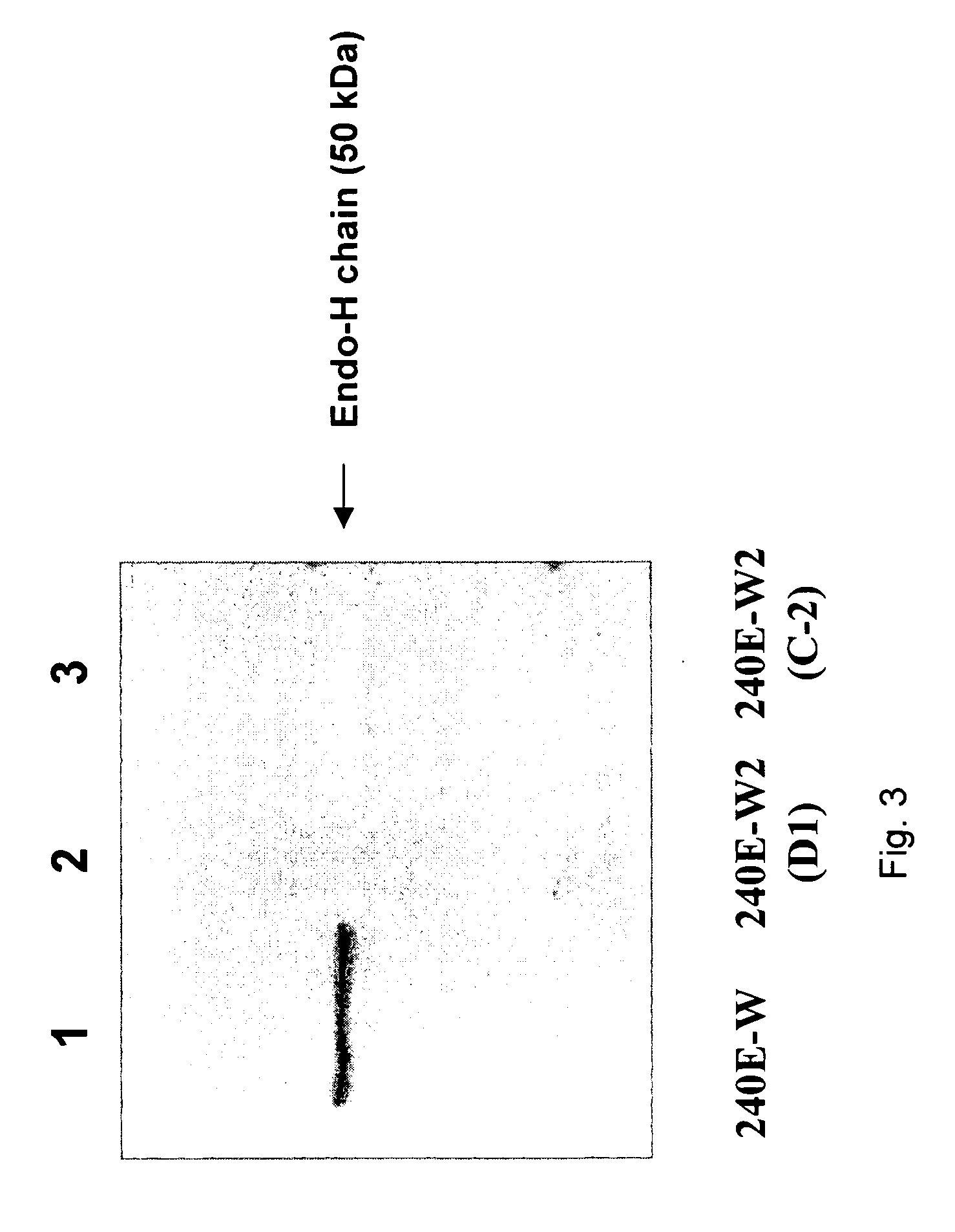
Patents
Literature
2853 results about "Lymphocyte" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
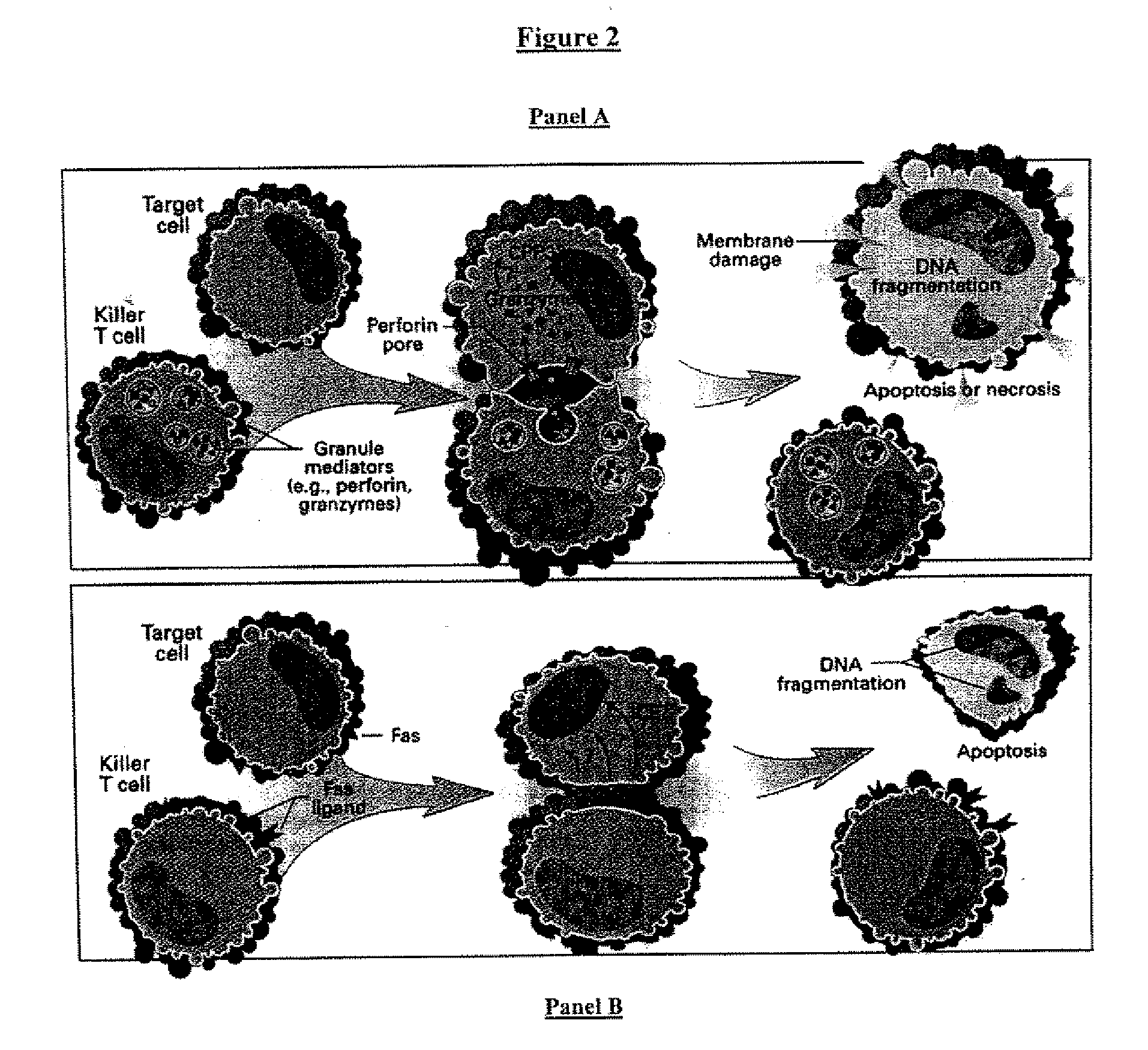
A lymphocyte is one of the subtypes of a white blood cell in a vertebrate's immune system. Lymphocytes include natural killer cells (which function in cell-mediated, cytotoxic innate immunity), T cells (for cell-mediated, cytotoxic adaptive immunity), and B cells (for humoral, antibody-driven adaptive immunity). They are the main type of cell found in lymph, which prompted the name "lymphocyte".
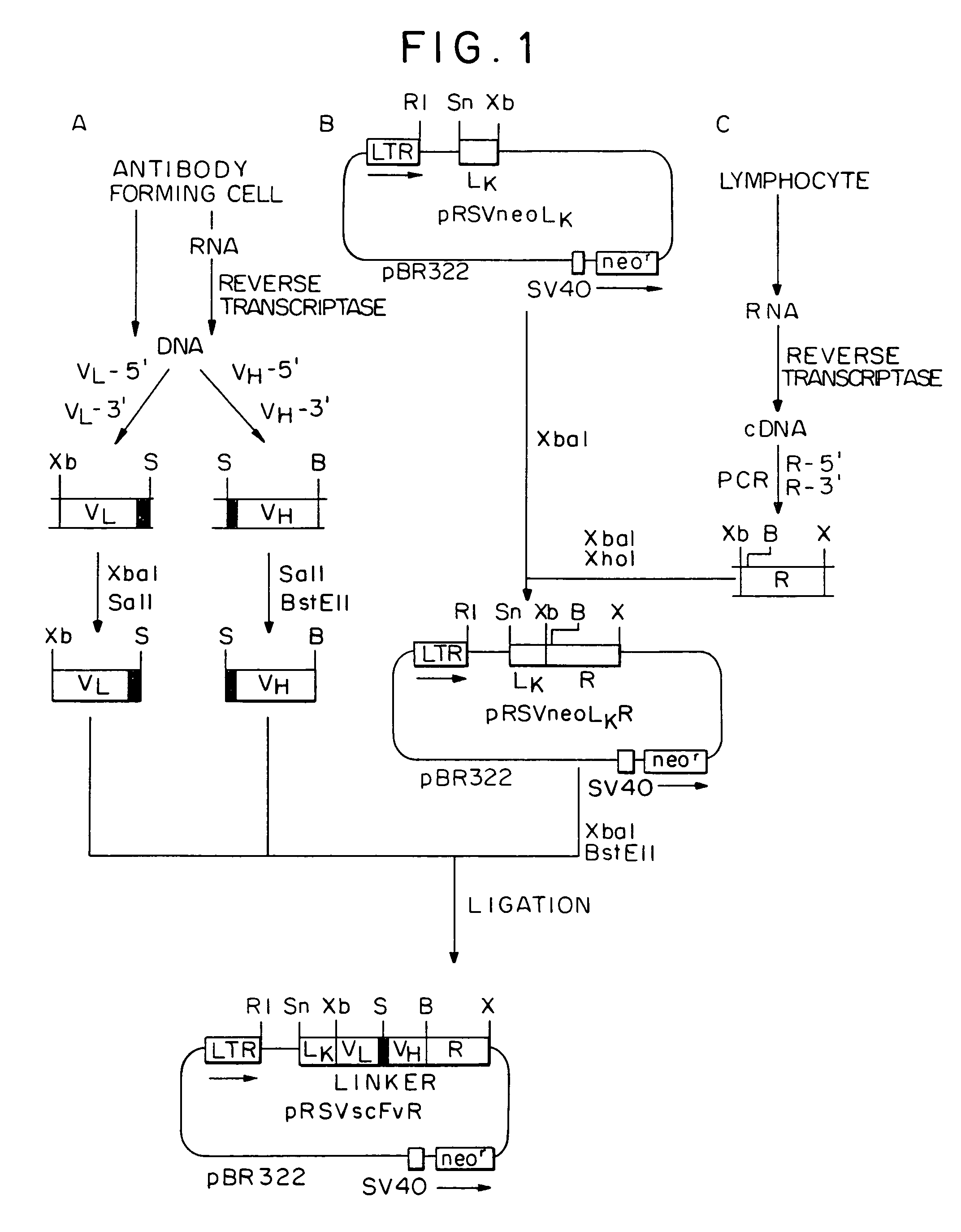
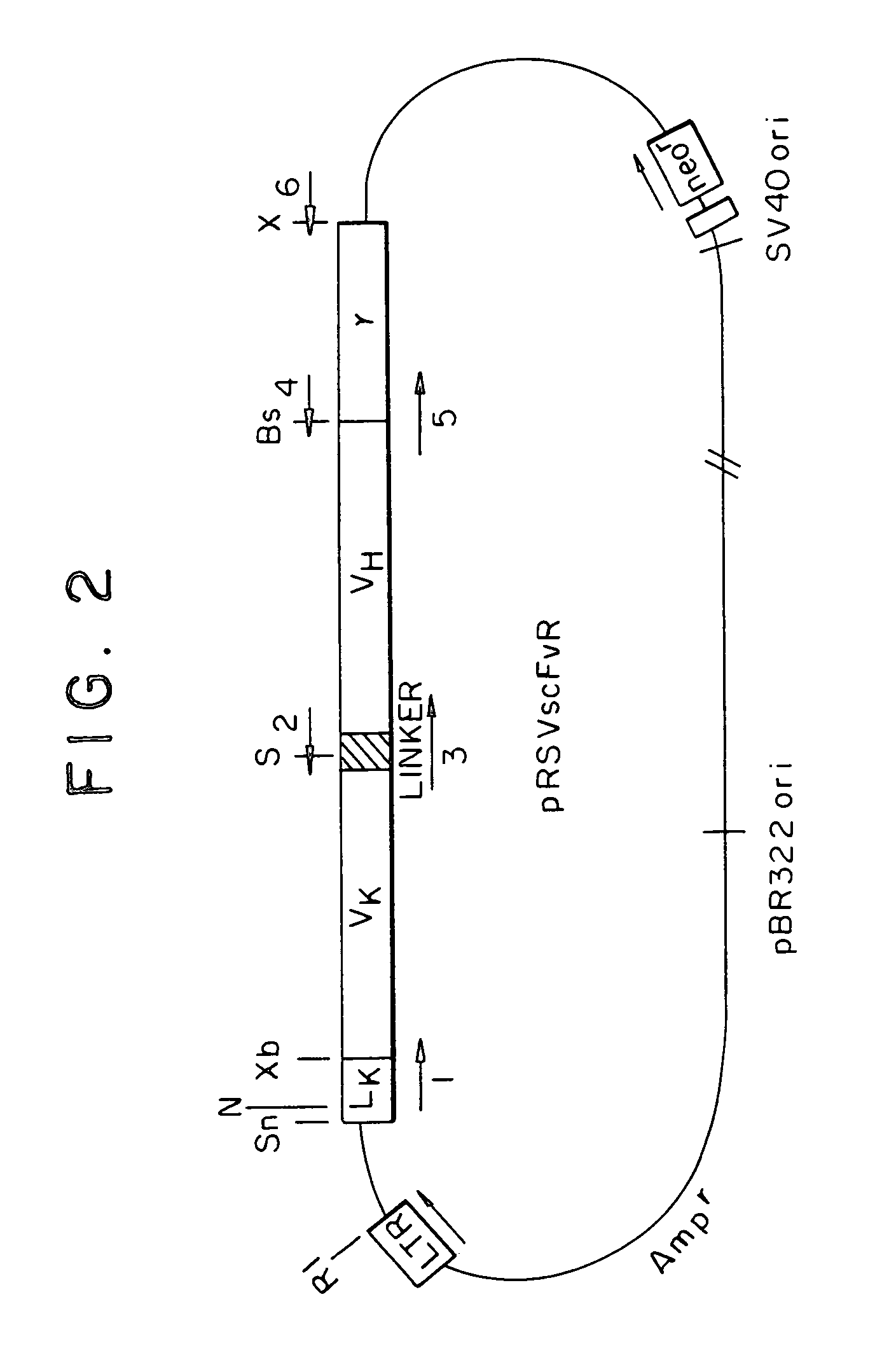
Chimeric receptor genes and cells transformed therewith
ActiveUS7741465B1Limit acquisitionMicroorganismsGenetic material ingredientsAntibody typesLymphocyte
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:HEALTH & HUMAN SERVICES GOVERNMENT OF THE UNITED STATES OF AMERICA AS REPRESENTED BY THE DEPT OF +1
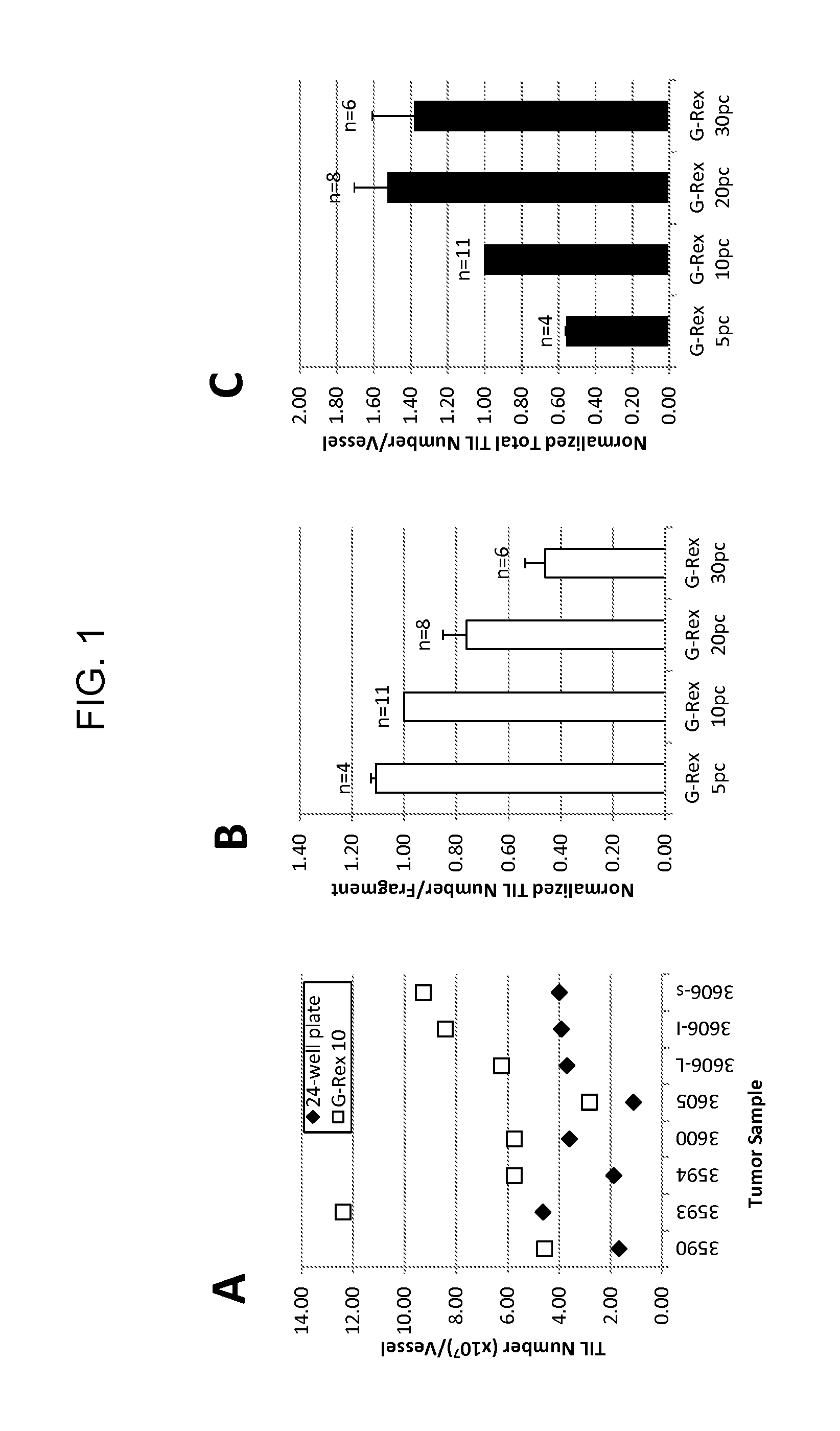
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20120244133A1Increase the number ofBiocideArtificial cell constructsTumour tissueCell culture media
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
Human B7.1-specific primatized antibodies and transfectomas expressing said antibodies
InactiveUS6113898AShrink tumorInhibit tumor growthPeptide/protein ingredientsAntipyreticDiseaseOrgan transplant rejection
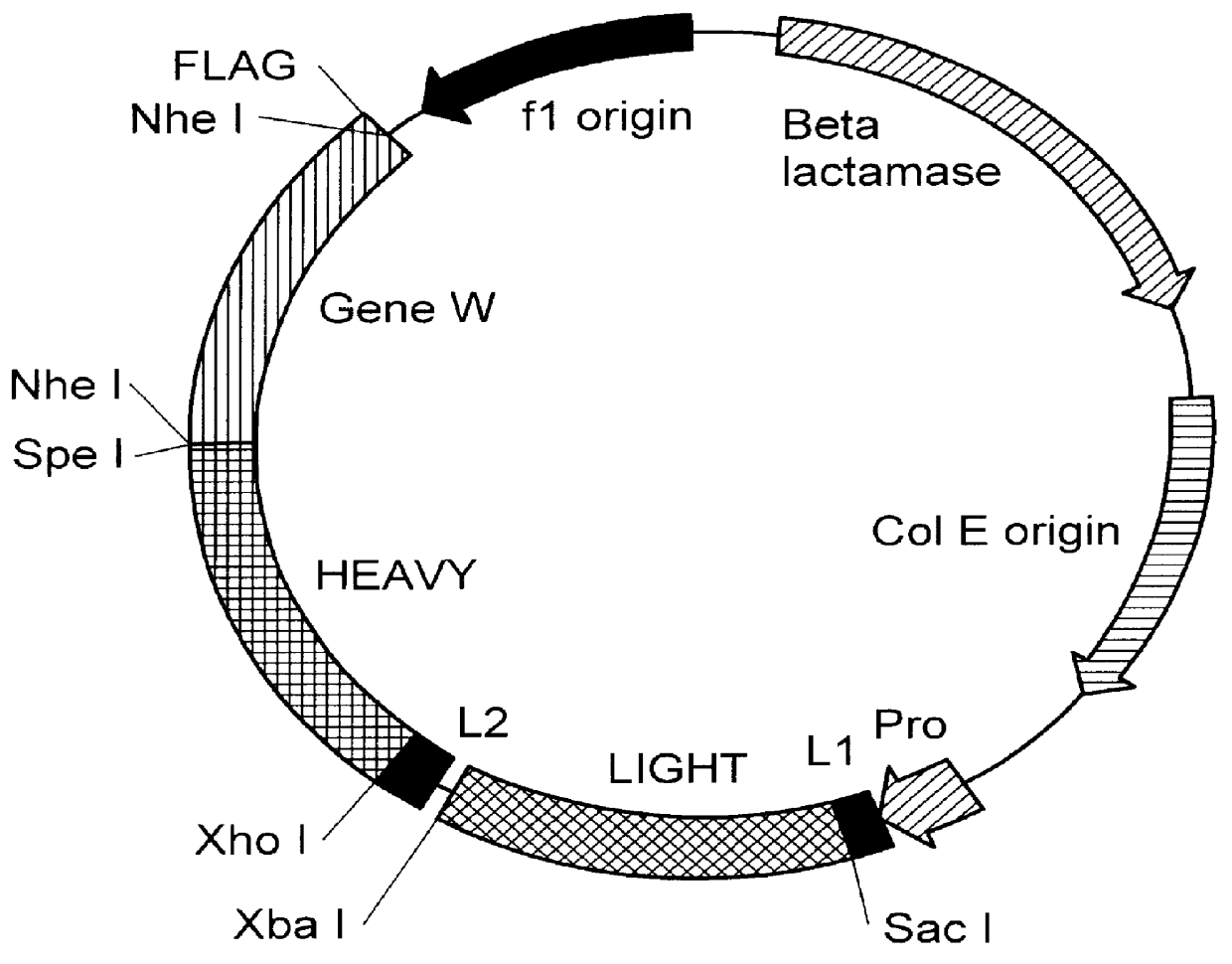
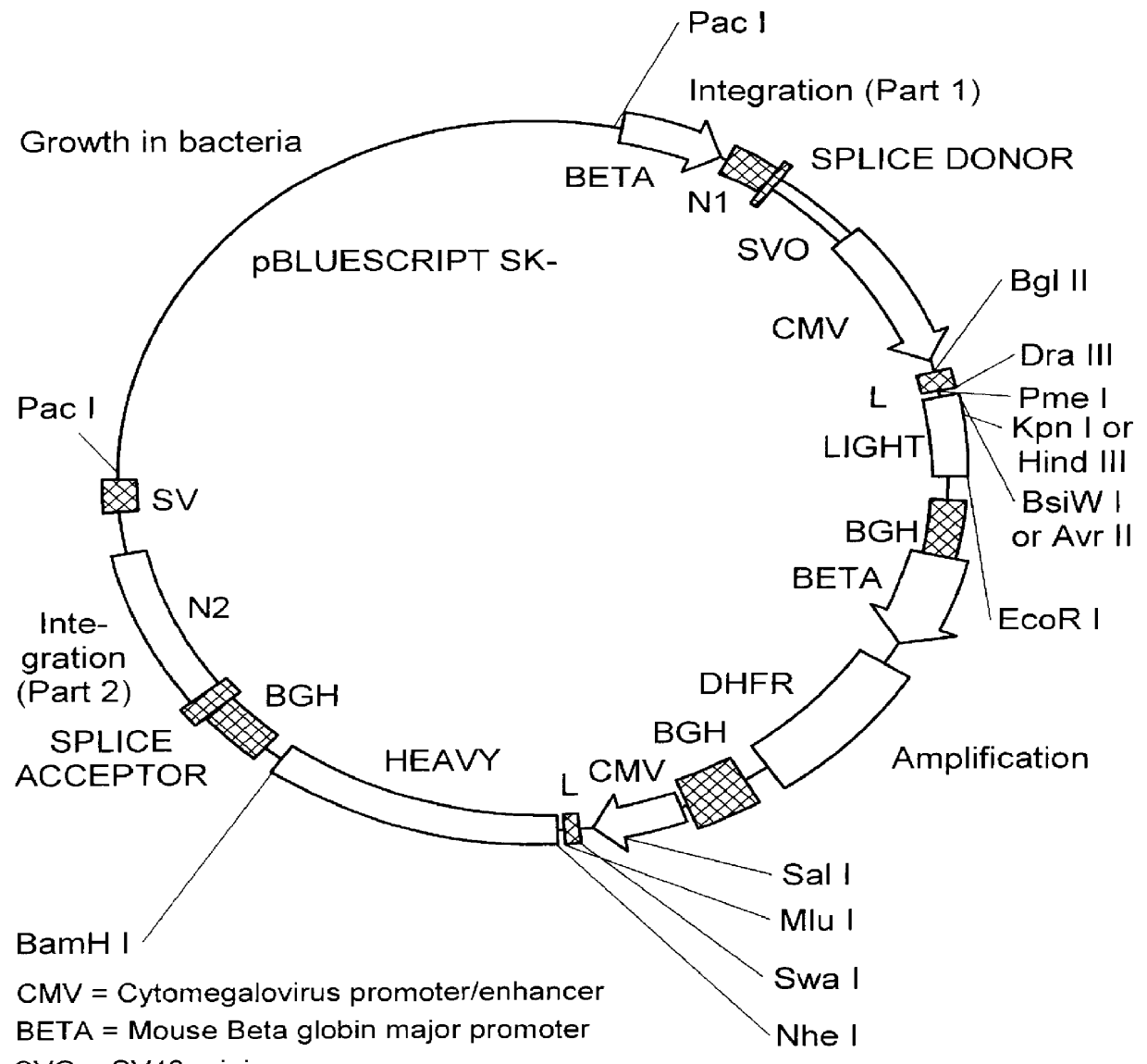
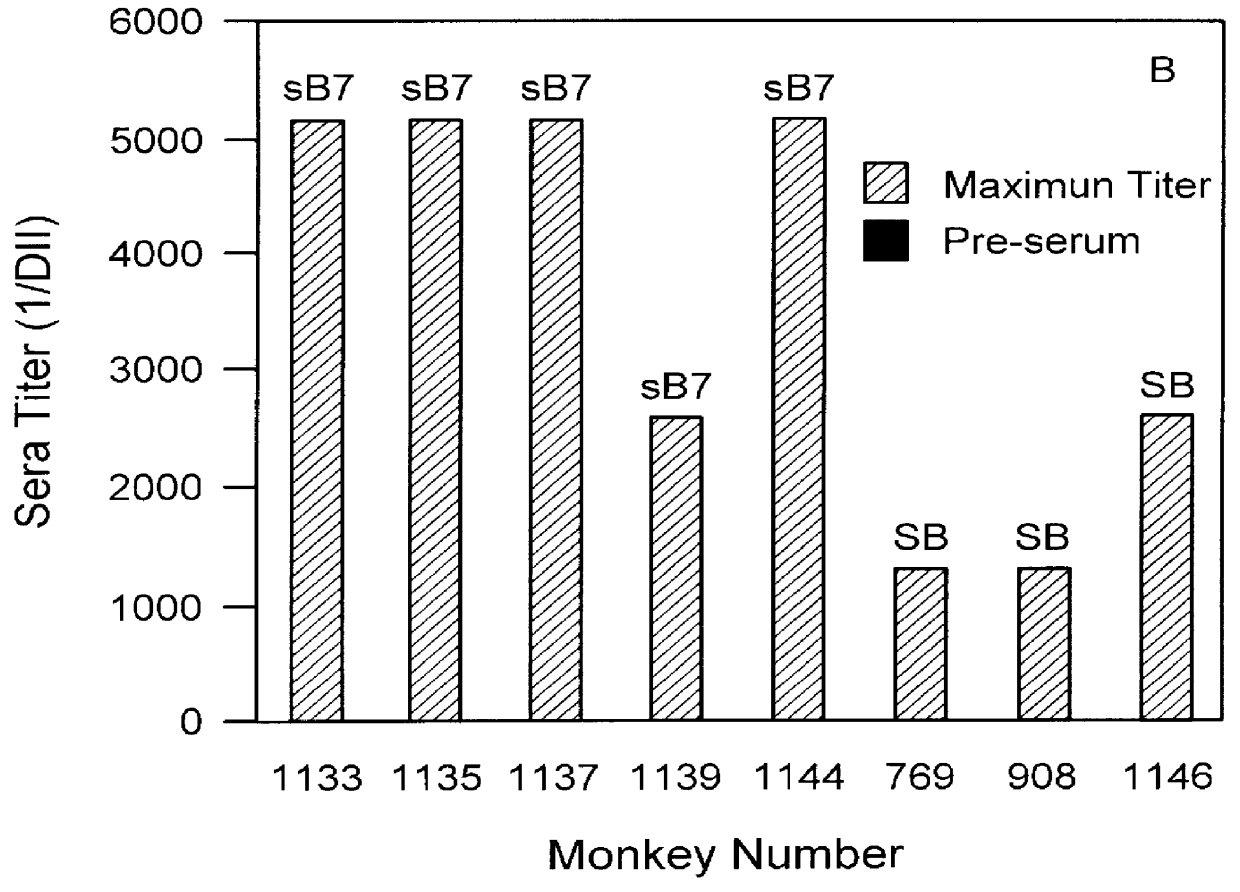
The present invention relates to the identification of macaque antibodies to human B7.1 and B7.2 by screening of phage display libraries or monkey heterohybridomas obtained using B lymphocytes from B7.1 and / or B7.2 immunized monkeys. More specifically, the invention provides four monkey monoclonal antibodies 7B6, 16C10, 7C10 and 20C9 which inhibit the B7:CD28 pathway and thereby function as effective immunosuppressants. The invention further provides the complete DNA and amino acid sequences of the light and heavy chain of three primatized antibodies derived from those monkey monoclonal antibodies which bind B7.1 and possibly B7.2, primatized 7C10, primatized 7B6 and primatized 16C10. These primatized and monkey antibodies may be used as specific immunosuppressants, e.g., for the treatment of autoimmune diseases and to prevent organ transplant rejection.
Owner:BIOGEN INC
Constitutive expression of costimulatory ligands on adoptively transferred T lymphocytes
ActiveUS8252592B2Increase self-toleranceIncrease and reduces immune responseFused cellsFermentationRegulatory T cellLymphocyte
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Adoptive cell therapy with young T cells
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:UNITED STATES OF AMERICA
Novel Anti-cd38 antibodies for the treatment of cancer
ActiveUS20090304710A1Improve propertiesLess immunogenicSenses disorderAntipyreticComplement-dependent cytotoxicityAntibody fragments
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to CD38, are capable of killing CD38+ cells by apoptosis, antibody-dependent cell-mediated cytotoxicity (ADCC), and / or complement-dependent cytotoxicity (CDC). Said antibodies and fragments thereof may be used in the treatment of tumors that express CD38 protein, such as multiple myeloma, chronic lymphocytic leukemia, chronic myelogenous leukemia, acute myelogenous leukemia, or acute lymphocytic leukemia, or the treatment of autoimmune and inflammatory diseases such as systemic lupus, rheumatoid arthritis, multiple sclerosis, erythematosus, and asthma. Said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of CD38. Also provided are cytotoxic conjugates comprising a cell binding agent and a cytotoxic agent, therapeutic compositions comprising the conjugate, methods for using the conjugates in the inhibition of cell growth and the treatment of disease, and a kit comprising the cytotoxic conjugate. In particular, the cell binding agent is a monoclonal antibody, and epitope-binding fragments thereof, that recognizes and binds the CD38 protein.
Owner:SANOFI AVENTIS US LLC
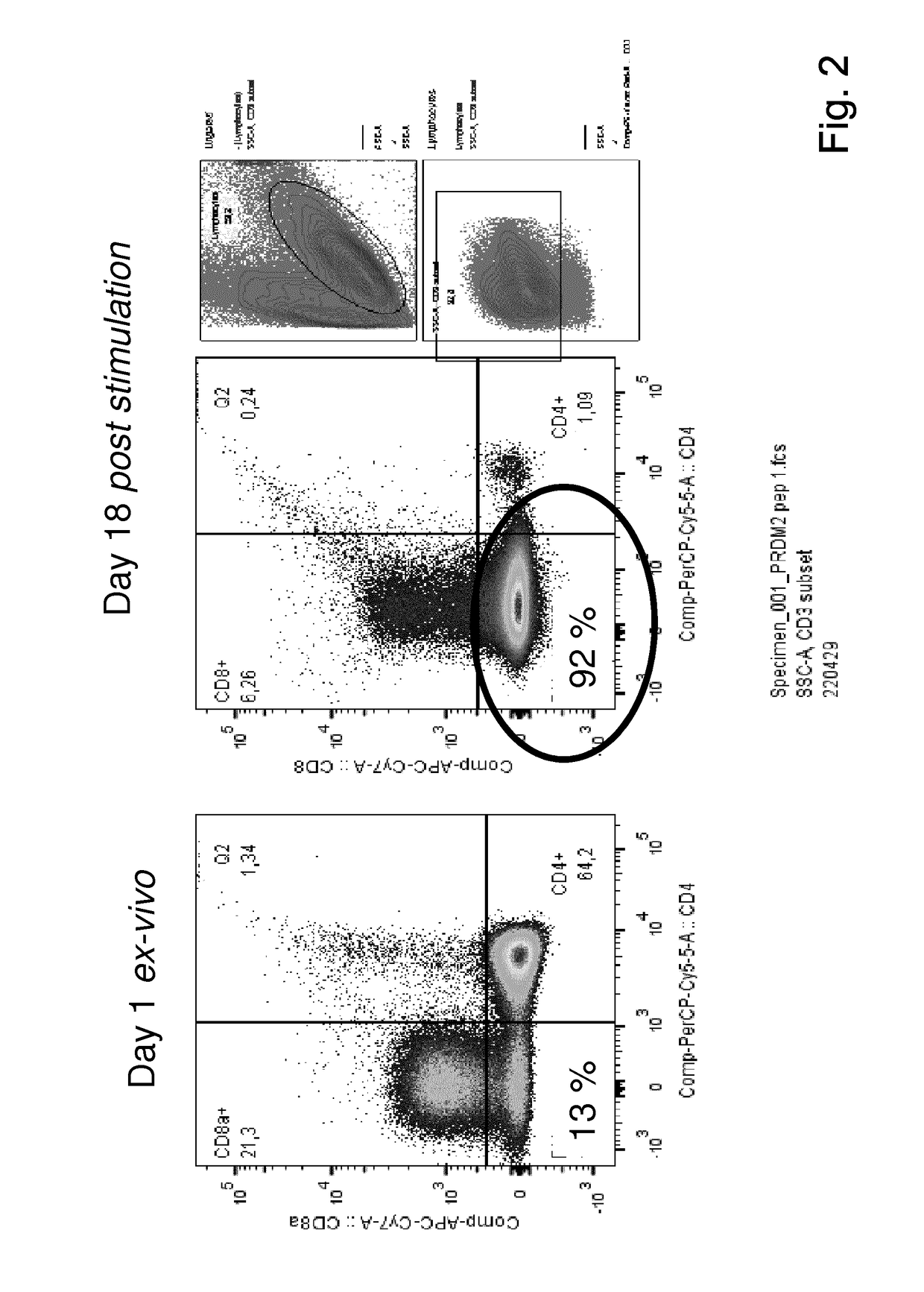
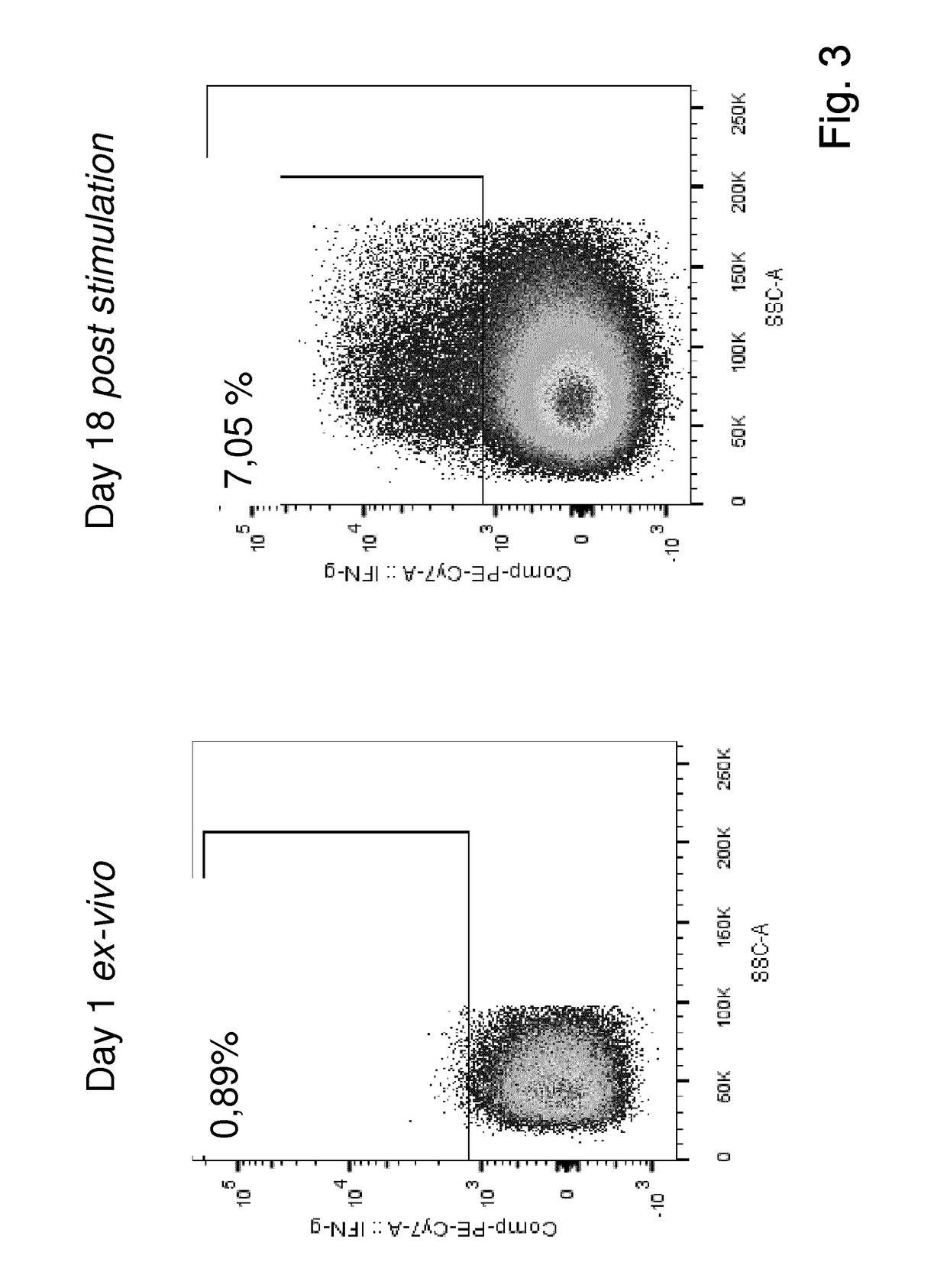
Method for the generation of antigen-specific lymphocytes
InactiveUS20070116690A1Function increaseEnhancing function of T cellBiocideVirusesAutoimmune conditionAutoimmune disease
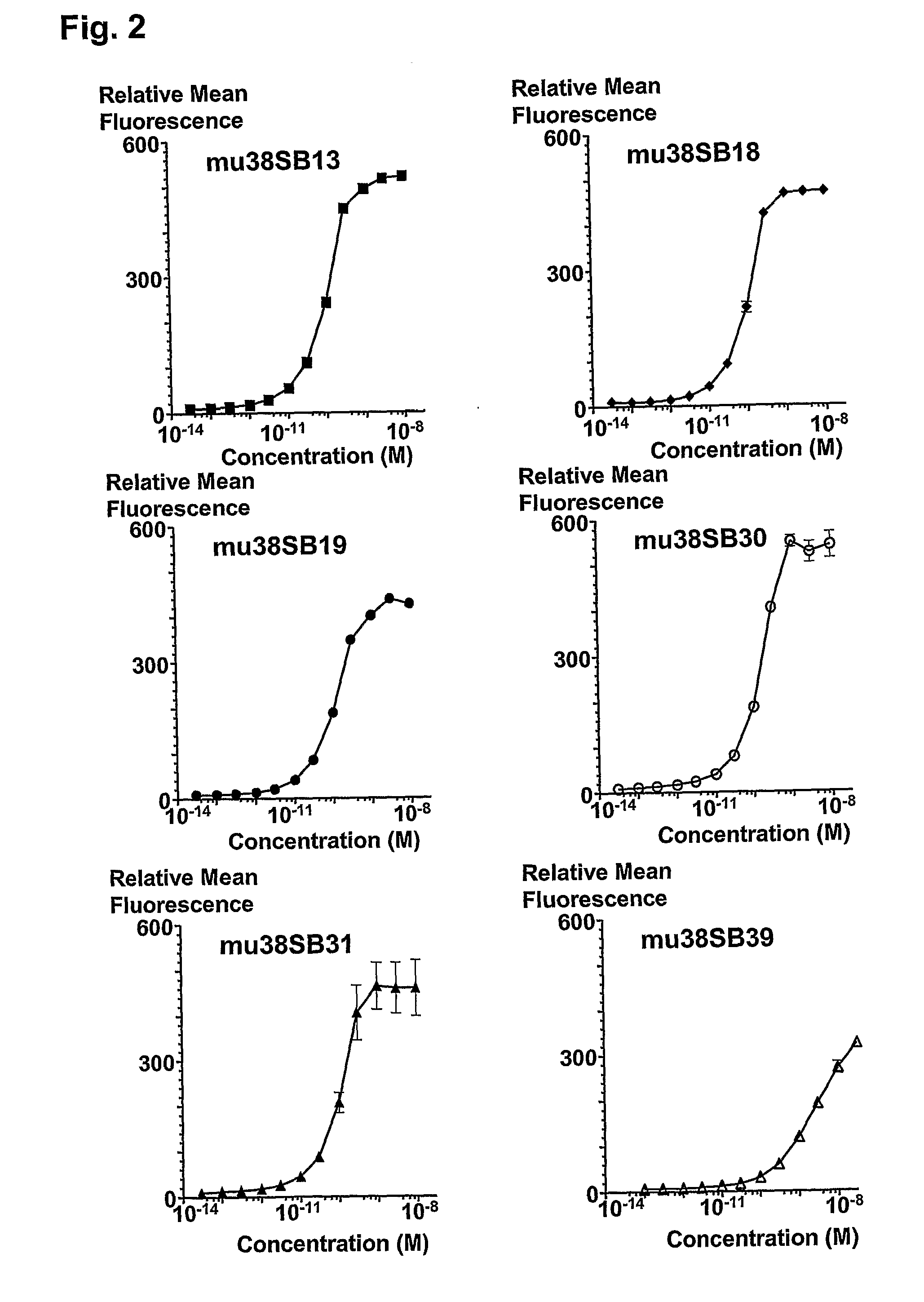
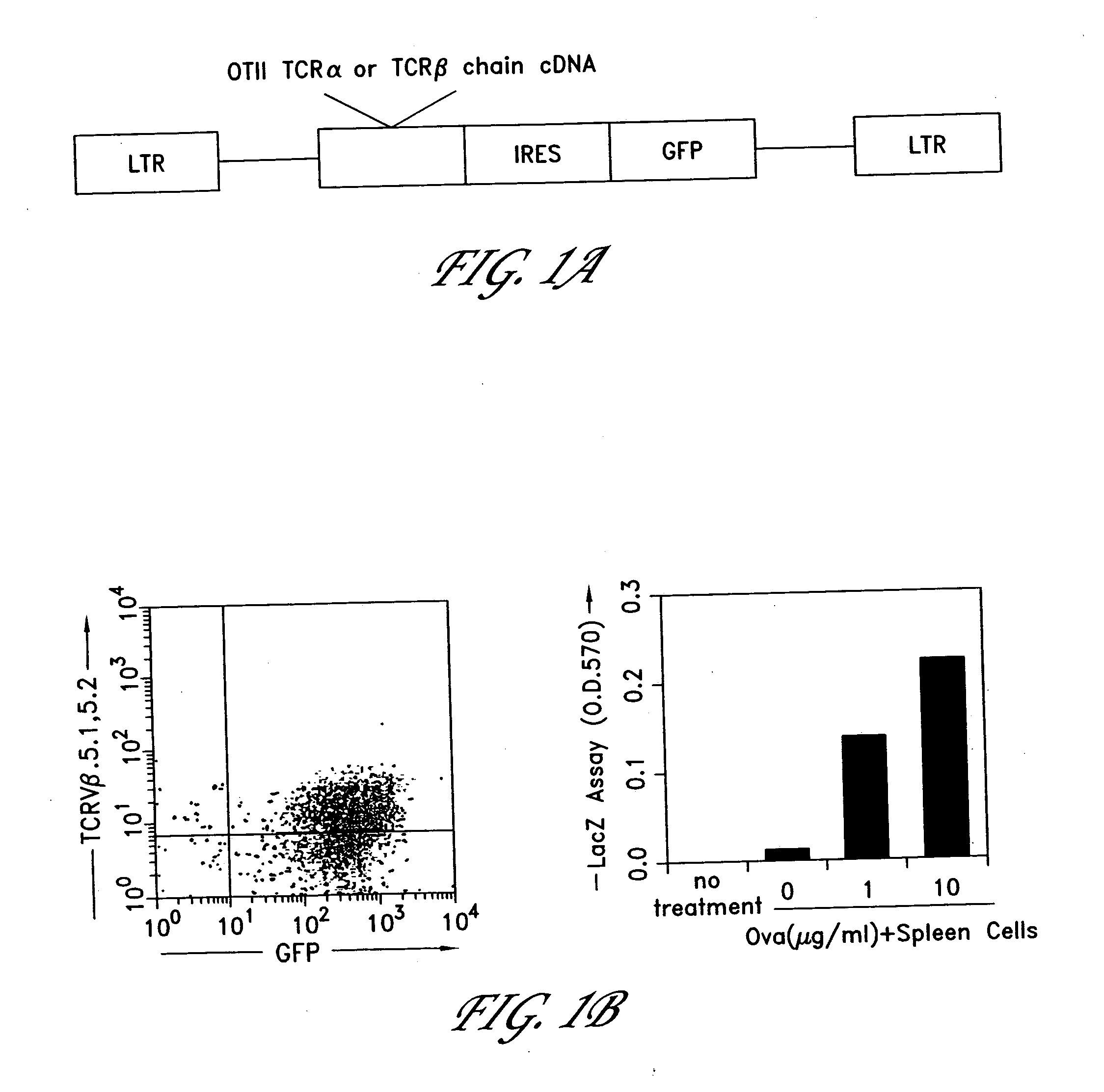
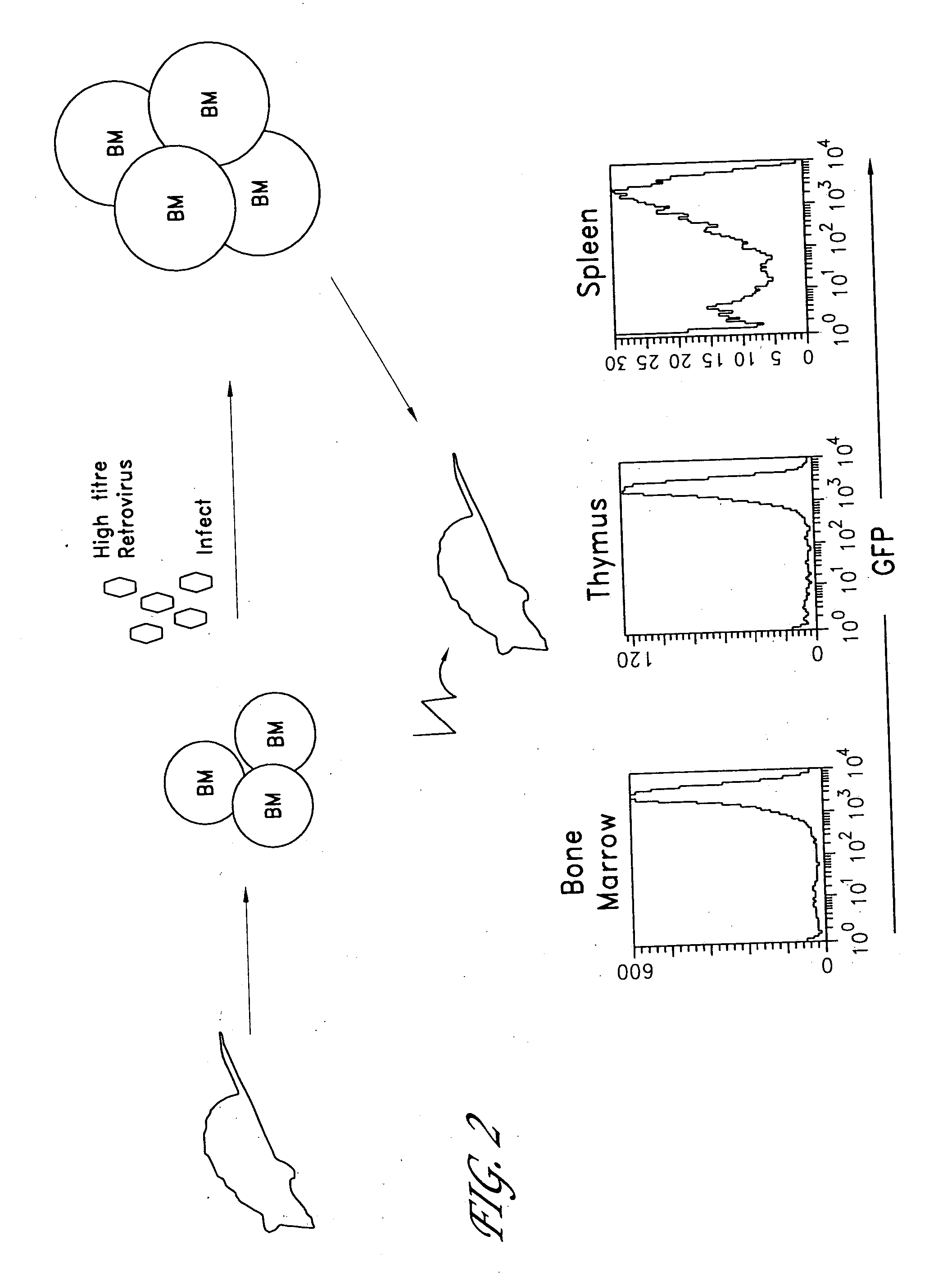
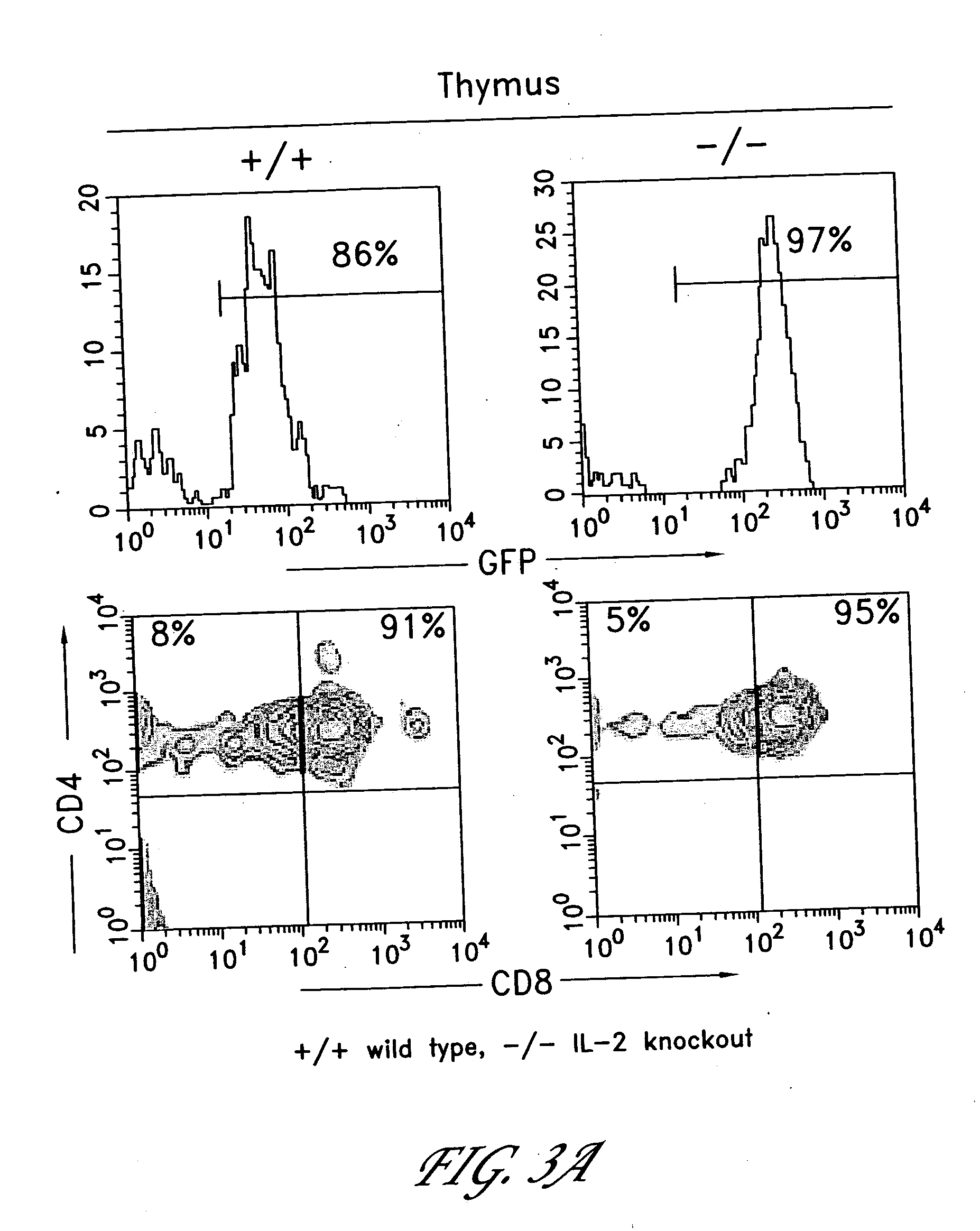
The invention provides systems and methods for the generation of lymphocytes having a unique antigen specificity. In a preferred embodiment, the invention provides methods of virally infecting cells from bone marrow with one or more viral vectors that encode antigen-specific antibodies for the production of, for example B cells and T cells. In some embodiments, the viral vectors include an IRES or 2A element to promote separation of, for example, the α subunit and β subunit of a T cell receptor (TCR) or heavy and light chains of a B-cell antibody. The resulting lymphocytes, express the particular antibody that was introduced in the case of B cells and TCR in the case of T cells. The lymphocytes generated can be used for a variety of therapeutic purposes including the treatment of various cancers and the generation of a desired immune response to viruses and other pathogens. The resulting cells develop normally and respond to antigen both in vitro and in vivo. We also show that it is possible to modify the function of lymphocytes by using stem cells from different genetic backgrounds. Thus our system constitutes a powerful tool to generate desired lymphocyte populations both for research and therapy. Future applications of this technology may include treatments for infectious diseases, such as HIV / AIDS, cancer therapy, allergy, and autoimmune disease.
Owner:CALIFORNIA INST OF TECH
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:IDEC PHARM CORP
Chimeric receptor genes and cells transformed therewith
Chimeric receptor genes suitable for endowing lymphocytes with antibody-type specificity include a first gene segment encoding a single-chain Fv domain of a specific antibody and a second gene segment encoding all or part of the transmembrane and cytoplasmic domains, and optionally the extracellular domain, of an immune cell-triggering molecule. The chimeric receptor gene, when transfected to immune cells, expresses the antibody-recognition site and the immune cell-triggering moiety into one continuous chain. The transformed lymphocytes are useful in therapeutic treatment methods.
Owner:UNITED STATES OF AMERICA +1
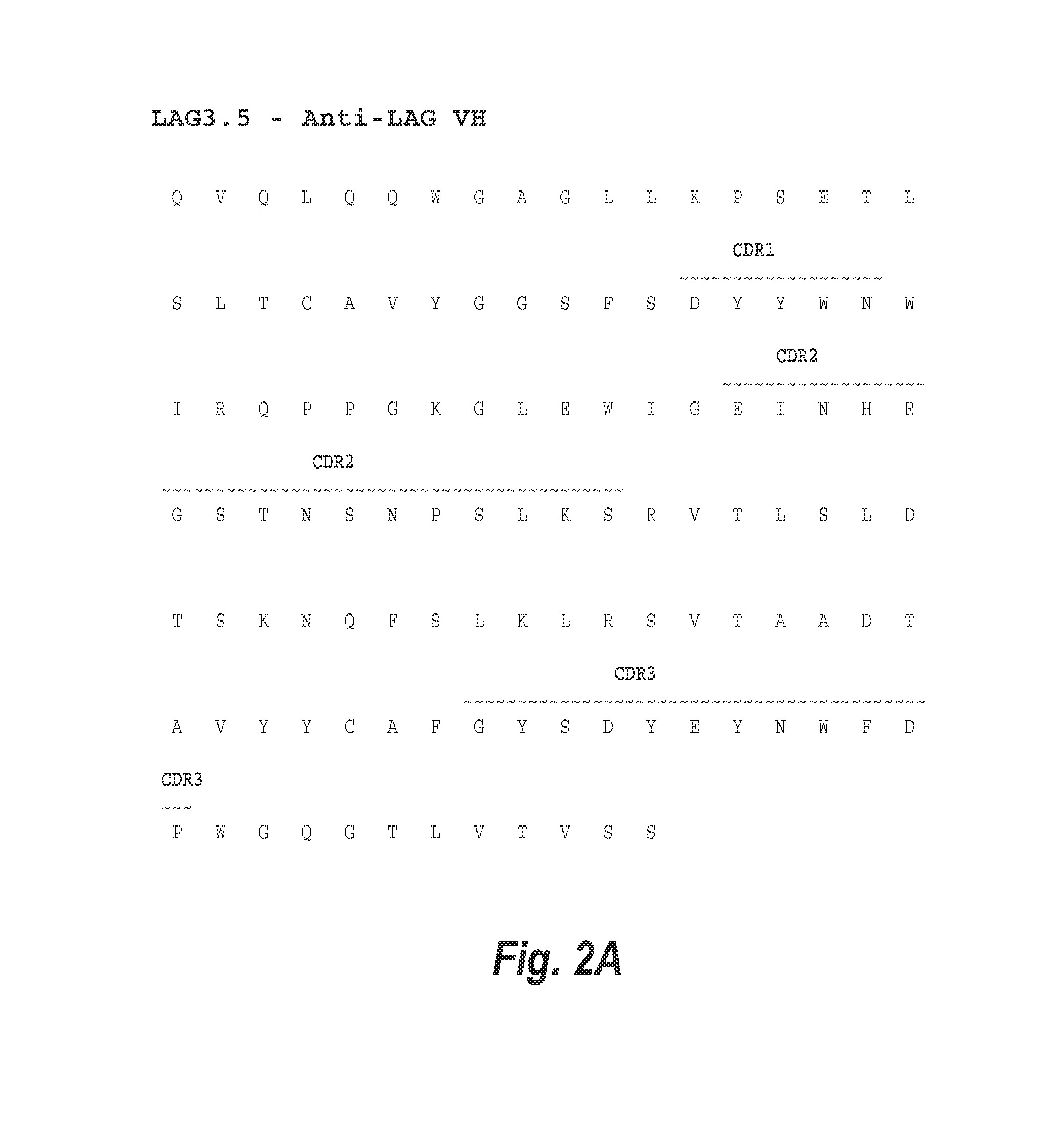
Optimization of antibodies that bind lymphocyte activation gene-3 (lag-3), and uses thereof
ActiveUS20140093511A1Improve physical stabilityGood chemical stabilityImmunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsLymphocyteLymphocyte activation
The present invention provides isolated monoclonal antibodies that specifically bind LAG-3, and have optimized functional properties compared to previously described anti-LAG-3 antibodies, such as antibody 25F7 (US 2011 / 0150892 A1). These properties include reduced deamidation sites, while still retaining high affinity binding to human LAG-3, and physical (i.e., thermal and chemical) stability. Nucleic acid molecules encoding the antibodies of the invention, expression vectors, host cells and methods for expressing the antibodies of the invention are also provided, as well as immunoconjugates, bispecific molecules and pharmaceutical compositions comprising the antibodies. The present invention also provides methods for detecting LAG-3, as well as methods for treating stimulating immune responses using an anti-LAG-3 antibody of the invention. Combination therapy, in which the antibodies are co-administered with at least one additional immunostimulatory antibody, is also provided.
Owner:BRISTOL MYERS SQUIBB CO
Chimeric immunoreceptor useful in treating human gliomas
InactiveUS7514537B2Negligible toxicityPotent and selectiveBiocideAntibody mimetics/scaffoldsIntracellular signallingHuman glioma
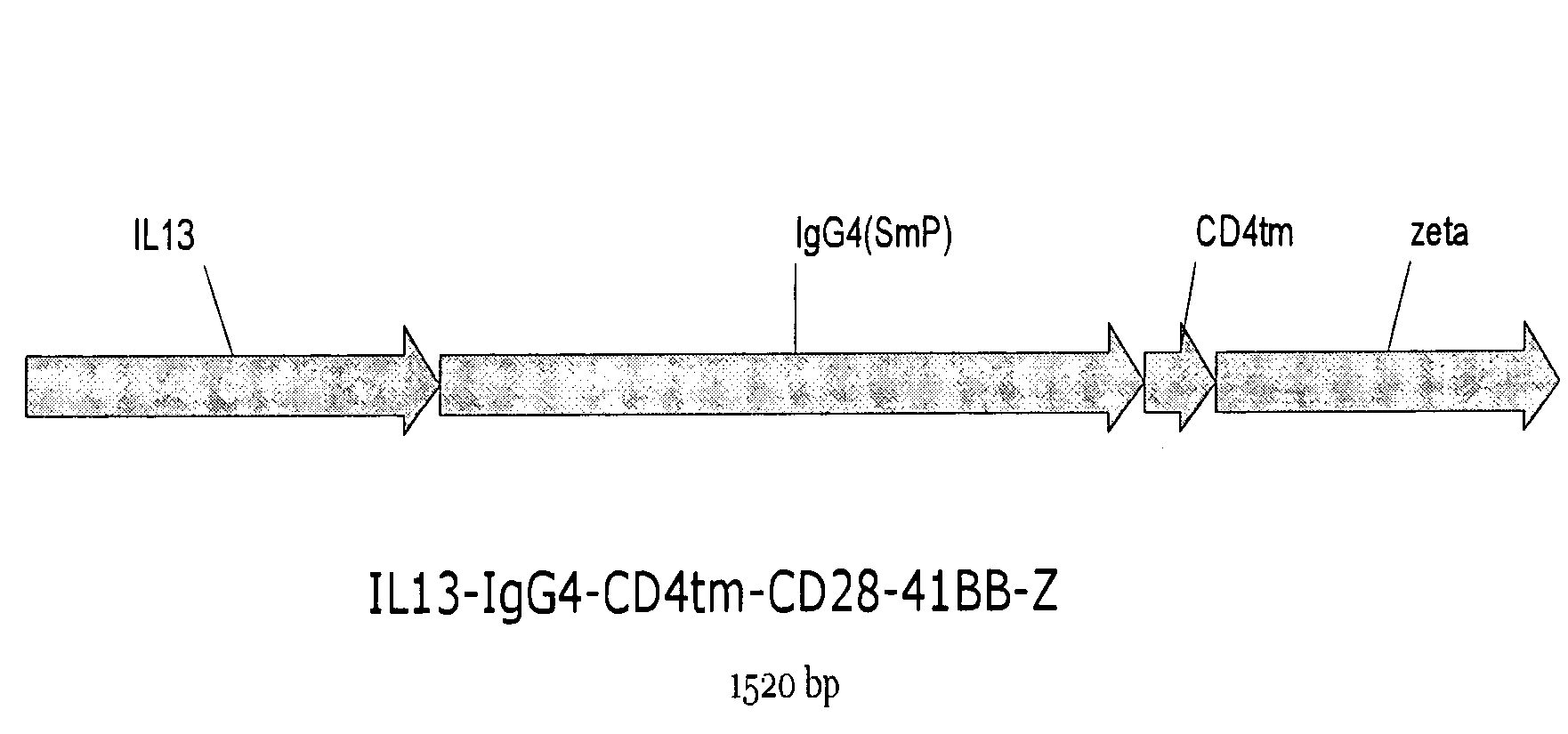
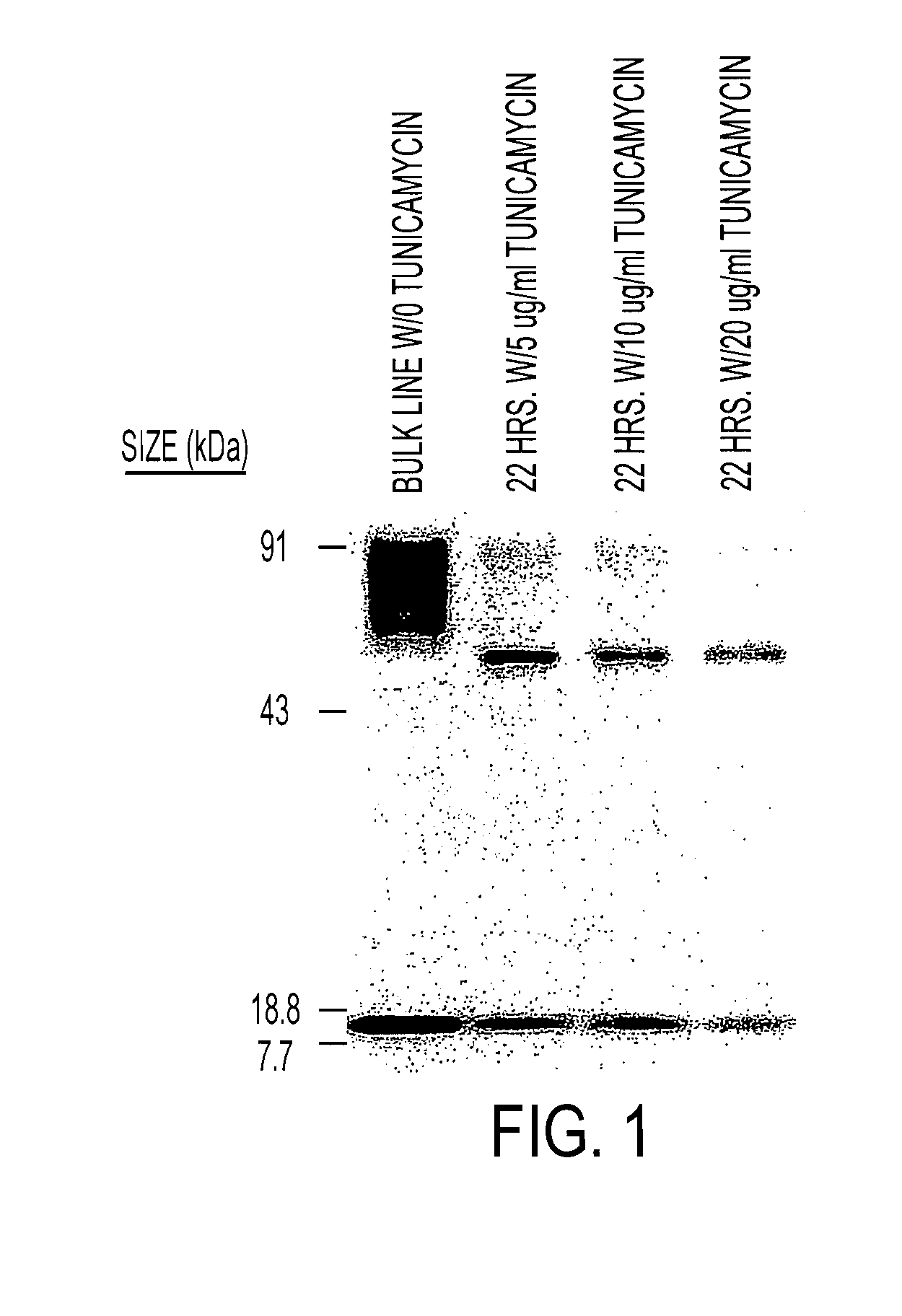
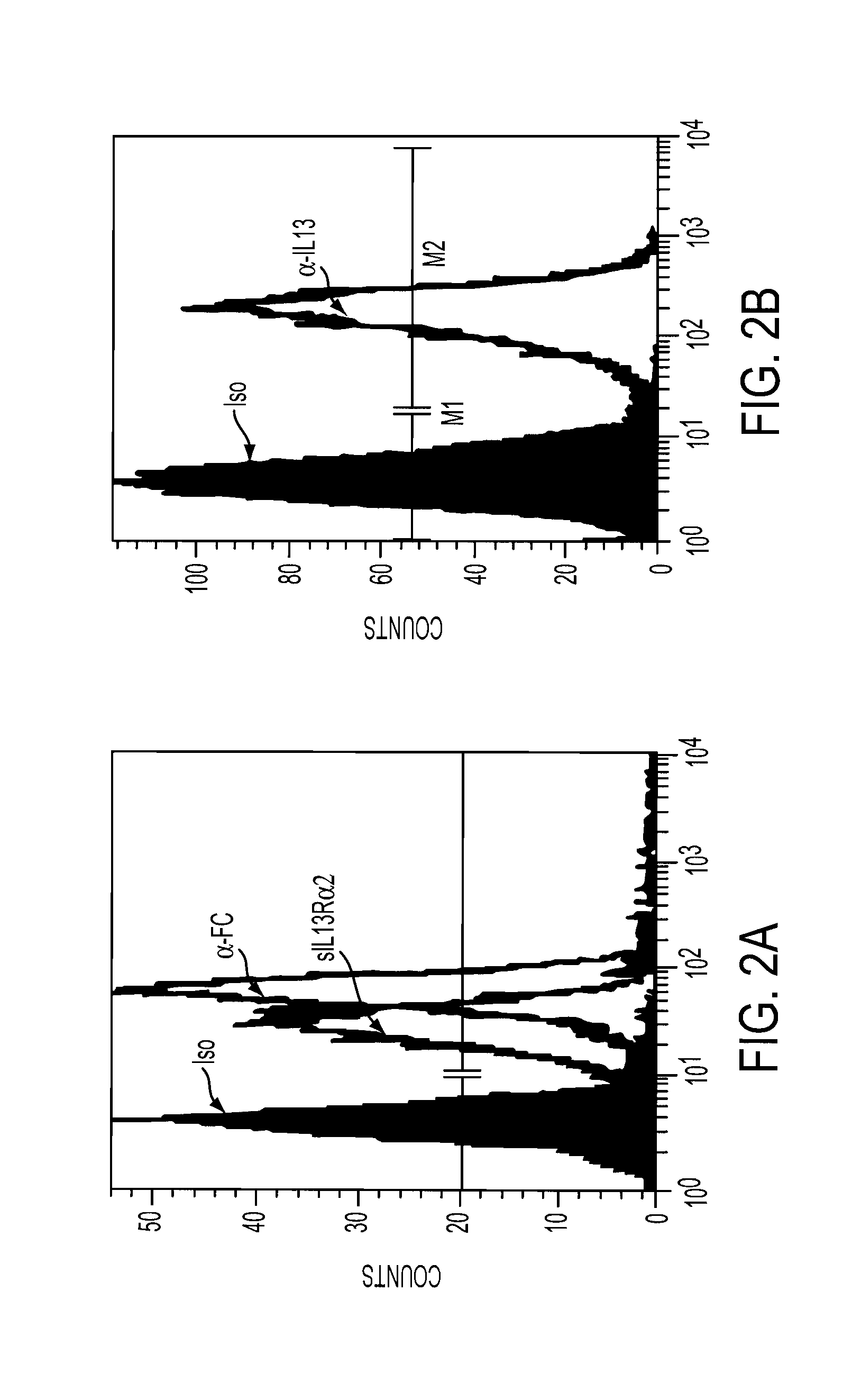
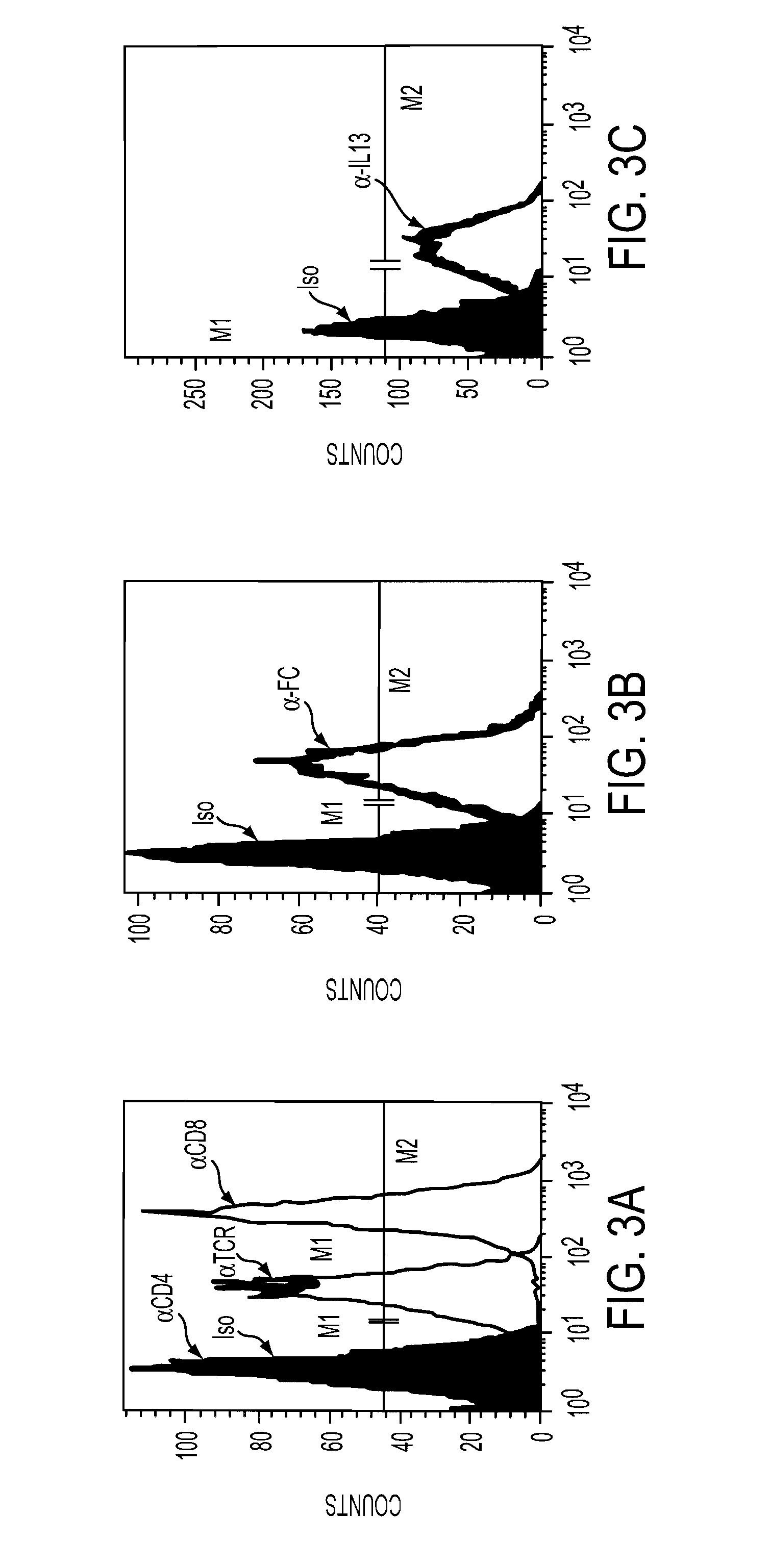
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signaling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human maligancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targeting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Therapeutic application of chimeric and radiolabelled antibodies to human B lymphocyte restricted differentiation antigen for treatment of B cell lymphoma
Disclosed herein are therapeutic treatment protocols designed for the treatment of B cell lymphoma. These protocols are based upon therapeutic strategies which include the use of administration of immunologically active mouse / human chimeric anti-CD20 antibodies, radiolabeled anti-CD20 antibodies, and cooperative strategies comprising the use of chimeric anti-CD20 antibodies and radiolabeled anti-CD20 antibodies.
Owner:BIOGEN INC
Chimeric antigen receptor for bispecific activation and targeting of t lymphocytes
InactiveUS20130280220A1Increase T cell activationEffectively offsetting tumor escapeBiocideGenetic material ingredientsLymphocyteT lymphocyte
Embodiments of the invention include methods and compositions related to improved cells encoding a chimeric antigen receptor that is specific for two or more antigens. In certain aspects the receptor encompasses two or more non-identical antigen recognition domains. The antigens are tumor antigens, in particular embodiments.
Owner:BAYLOR COLLEGE OF MEDICINE
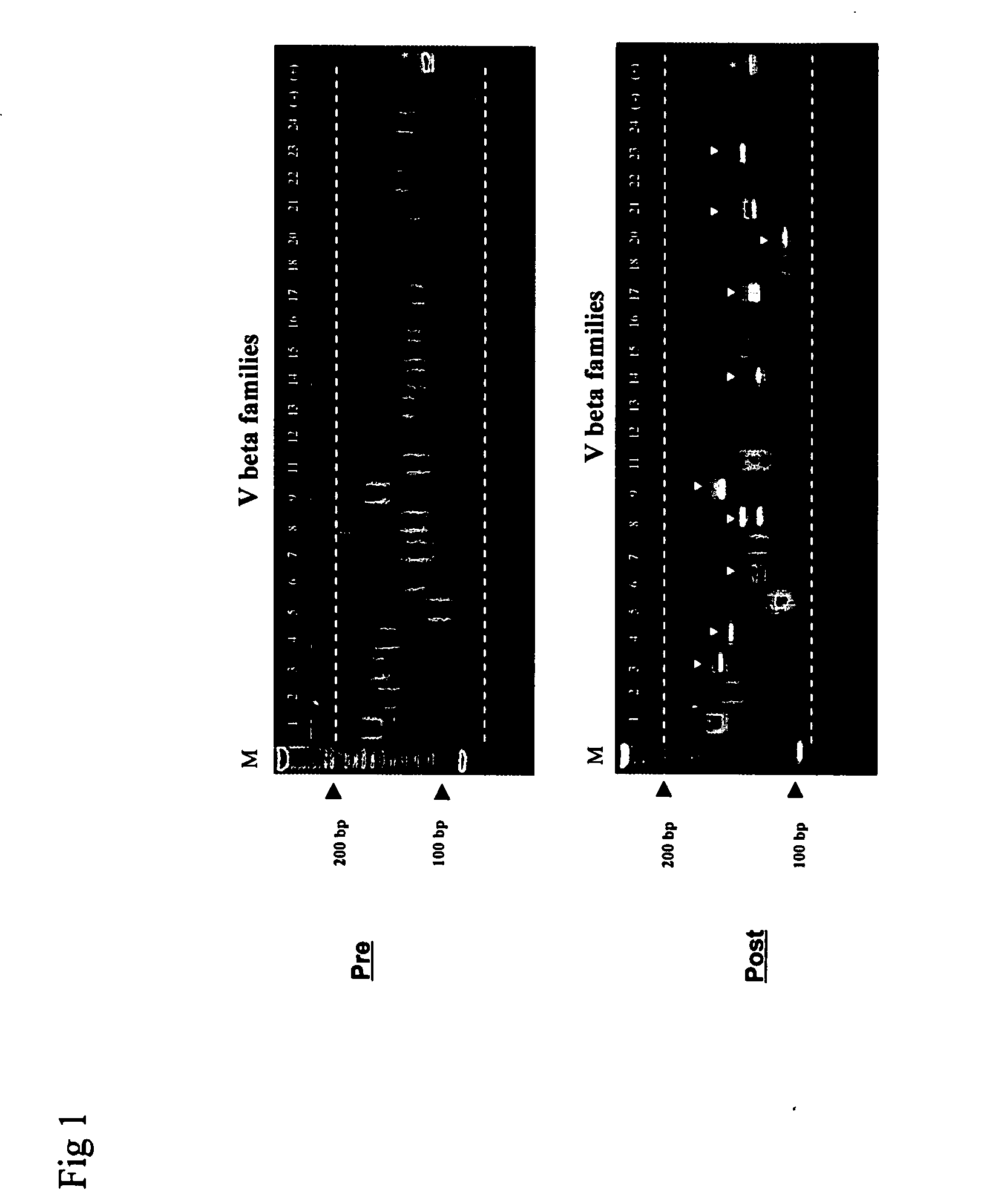
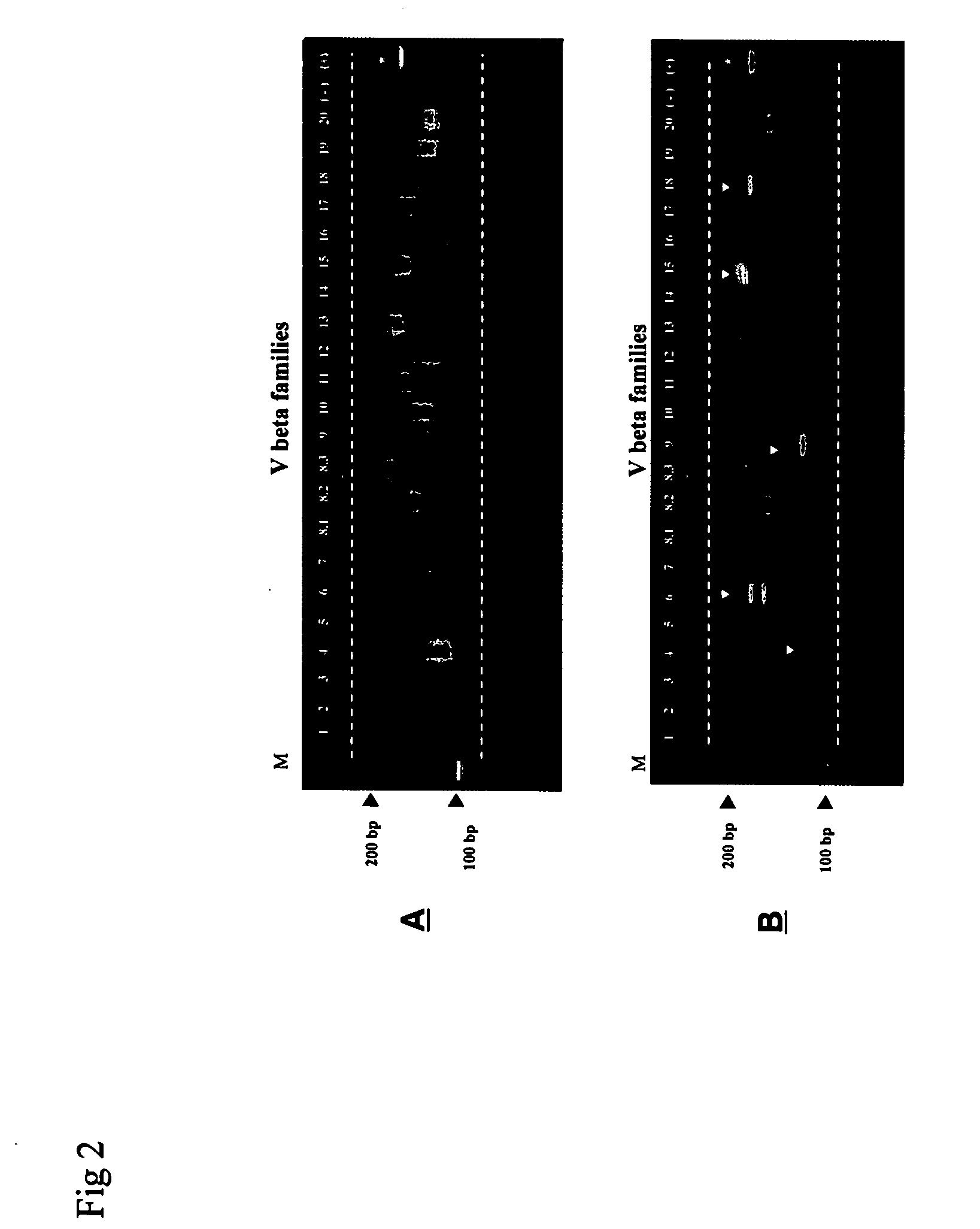
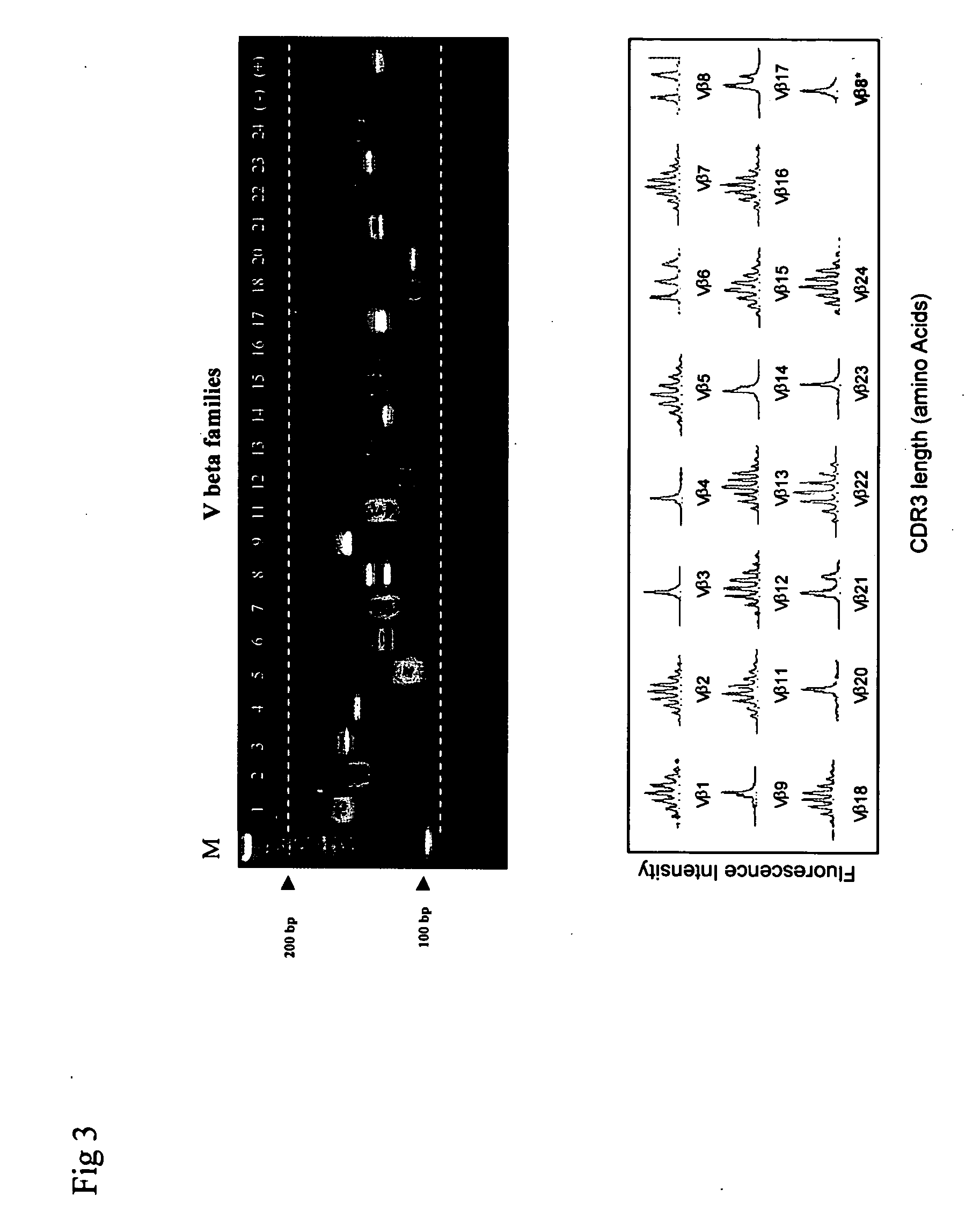
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Adoptive cell therapy with young t cells
The invention provides a method of promoting regression of a cancer in a mammal comprising (i) culturing autologous T cells; (ii) expanding the cultured T cells; (iii) administering to the mammal nonmyeloablative lymphodepleting chemotherapy; and (iv) after administering nonmyeloablative lymphodepleting chemotherapy, administering to the mammal the expanded T cells, wherein the T cells administered to the mammal are about 19 to about 35 days old and have not been screened for specific tumor reactivity, whereupon the regression of the cancer in the mammal is promoted.
Owner:US DEPT OF HEALTH & HUMAN SERVICES
Fusion partner for production of monoclonal rabbit antibodies
The invention provides a rabbit-derived immortal B-lymphocyte capable of fusion with a rabbit splenocyte to produce a hybrid cell that produces an antibody. The immortal B-lymphocyte does not detectably express endogenous immunoglobulin heavy chain and may contain, in certain embodiments, an altered immunoglobulin heavy chain-encoding gene. A hybridoma resulting from fusion between the subject immortal B-lymphocyte and a rabbit antibody-producing cell is provided, as is a method of using that hybridoma to produce an antibody. The subject invention finds use in a variety of different diagnostic, therapeutic and research applications.
Owner:EPITOMICS INC
Methods and compositions for the treatment of myeloproliferative diseases and other proliferative diseases
Compounds of the present invention, alone and in combination with other active agents, find utility in the treatment of hyperproliferative diseases, mammalian cancers and especially human cancers including but not limited to for example malignant melanomas, myeloproliferative diseases, chronic myelogenous leukemia, acute lymphocytic leukemia, a disease caused by c-ABL kinase, oncogenic forms thereof, aberrant fusion proteins thereof and polymorphs thereof.
Owner:DECIPHERA PHARMA LLC
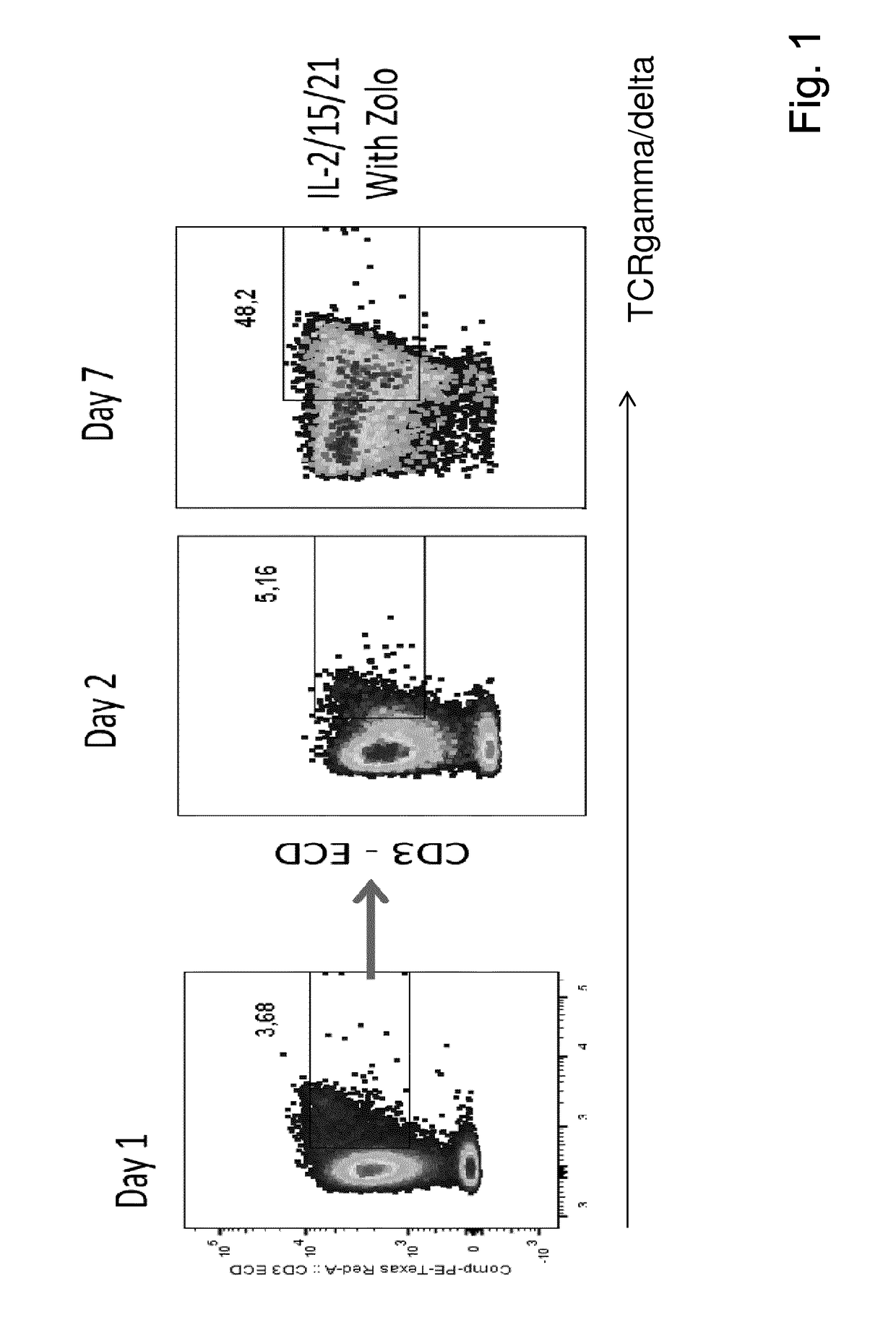
Expansion of lymphocytes with a cytokine composition for active cellular immunotherapy
PendingUS20170107490A1Increase stimulationImprove scalabilityPeptide/protein ingredientsBiological material analysisTissue sampleLymphocyte
The present invention relates to a composition for expanding lymphocytes comprising at least two types of cytokines selected from interleukin 2 (IL-2), interleukin 15 (IL-15) and interleukin 21 (IL-21). It further relates to a Method of preparing a population of clinically relevant lymphocytes, comprising the steps of: obtaining a body sample from a mammal in particular a tissue sample or body liquid sample, comprising at least one lymphocyte and optionally separating the cells in the body sample, culturing the body sample in-vitro to expand and / or stimulate lymphocytes in the sample wherein the culturing comprises using IL-2, IL-15 and / or IL-21, and optionally determining the presence of clinically relevant lymphocyte in the cultured sample. The present invention also relates to an immunotherapy and the population of clinically relevant lymphocytes.
Owner:POLYBIOCEPT GMBH
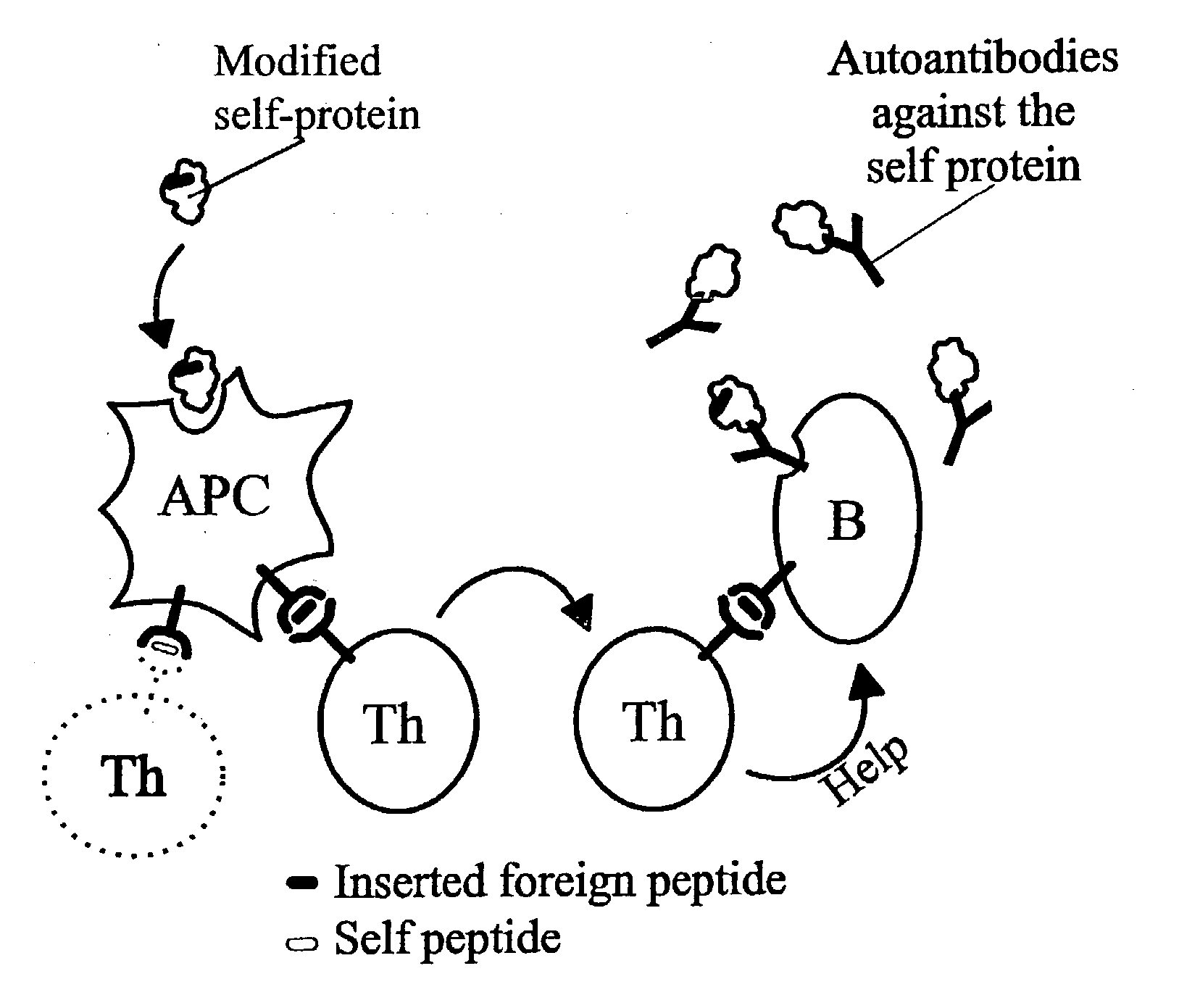
Novel methods for therapeutic vaccination
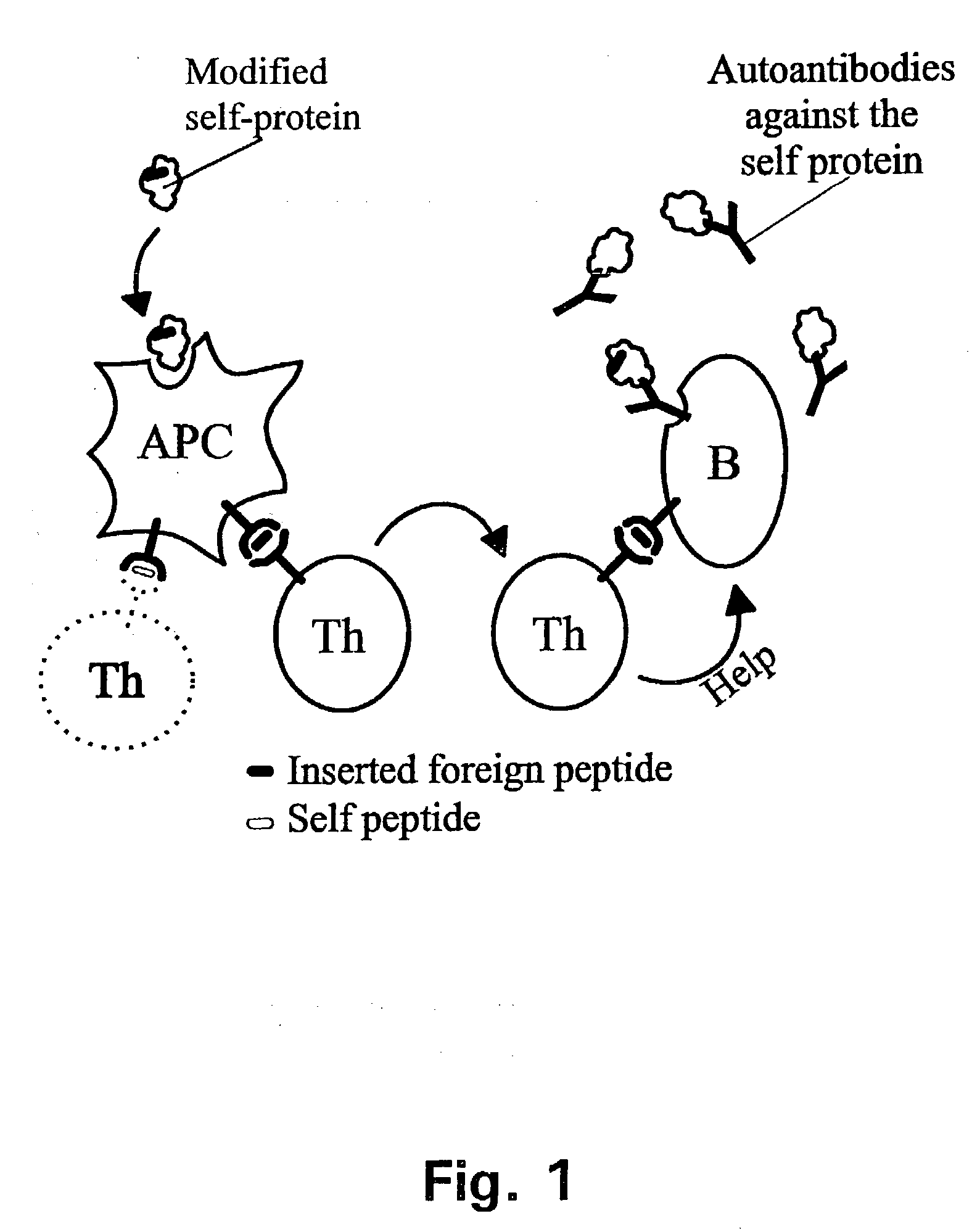
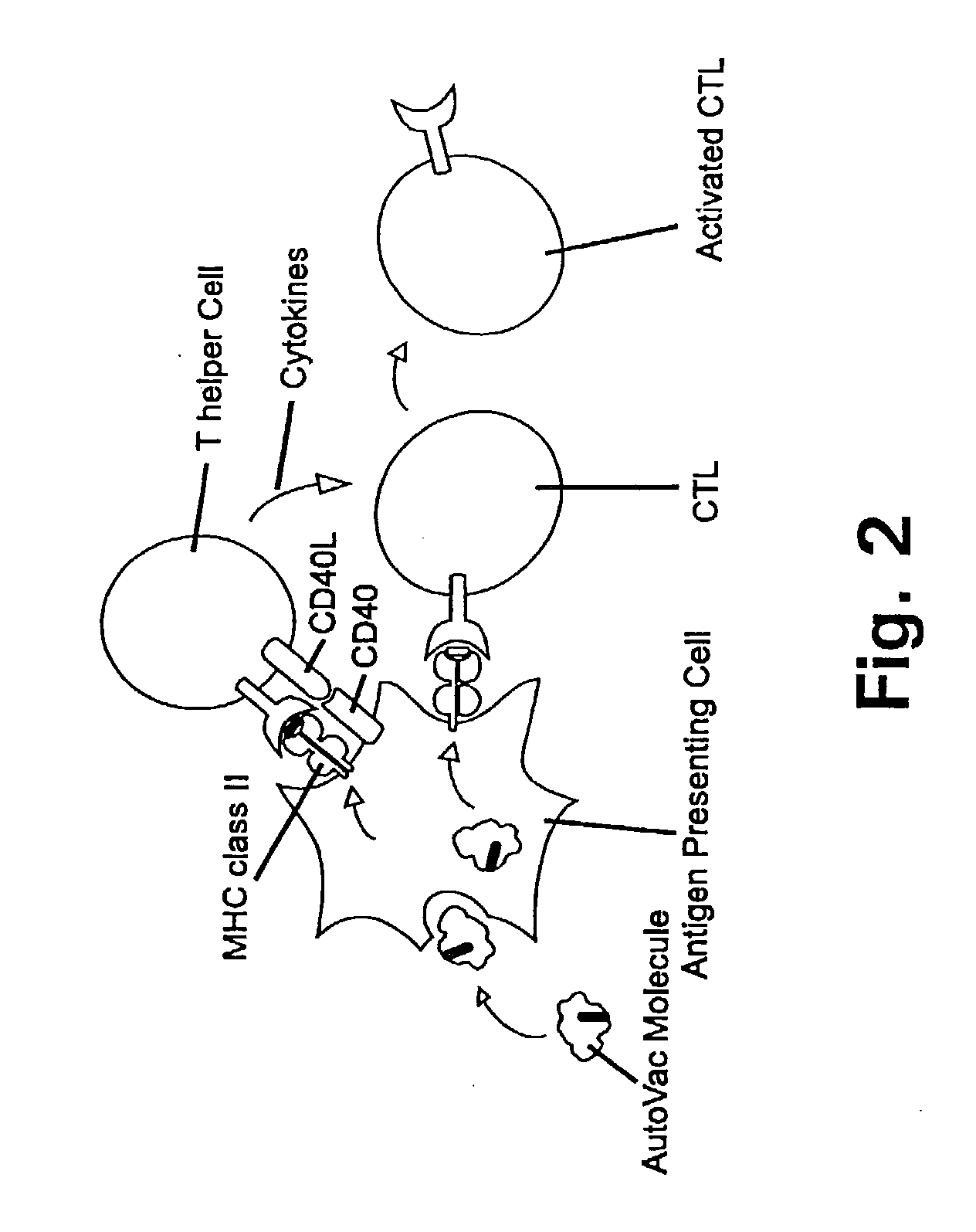
A method is disclosed for inducing cell-mediated immunity against cellular antigens. More specifically, the invention provides for a method for inducing cytotoxic T-lymphocyte immunity against weak antigens, notably self-proteins. The method entails that antigen presenting cells are induced to present at least one CTL epitope of the weak antigen and at the same time presenting at least one foreign T-helper lymphocyte epitope. In a preferred embodiment, the antigen is a cancer specific antigen, e.g. PSM, Her2, or FGF8b. The method can be exercised by using traditional polypeptide vaccination, but also by using live attenuated vaccines or nucleic acid vaccination. The invention furthermore provides immunogenic analogues of PSM, Her2 and FGF8b, as well as nucleic acid molecules encoding these analogues. Also vectors and transformed cells are disclosed. The invention also provides for a method for identification of immunogenic analogues of weak or non-immunogenic antigens.
Owner:BAVARIAN NORDIC AS
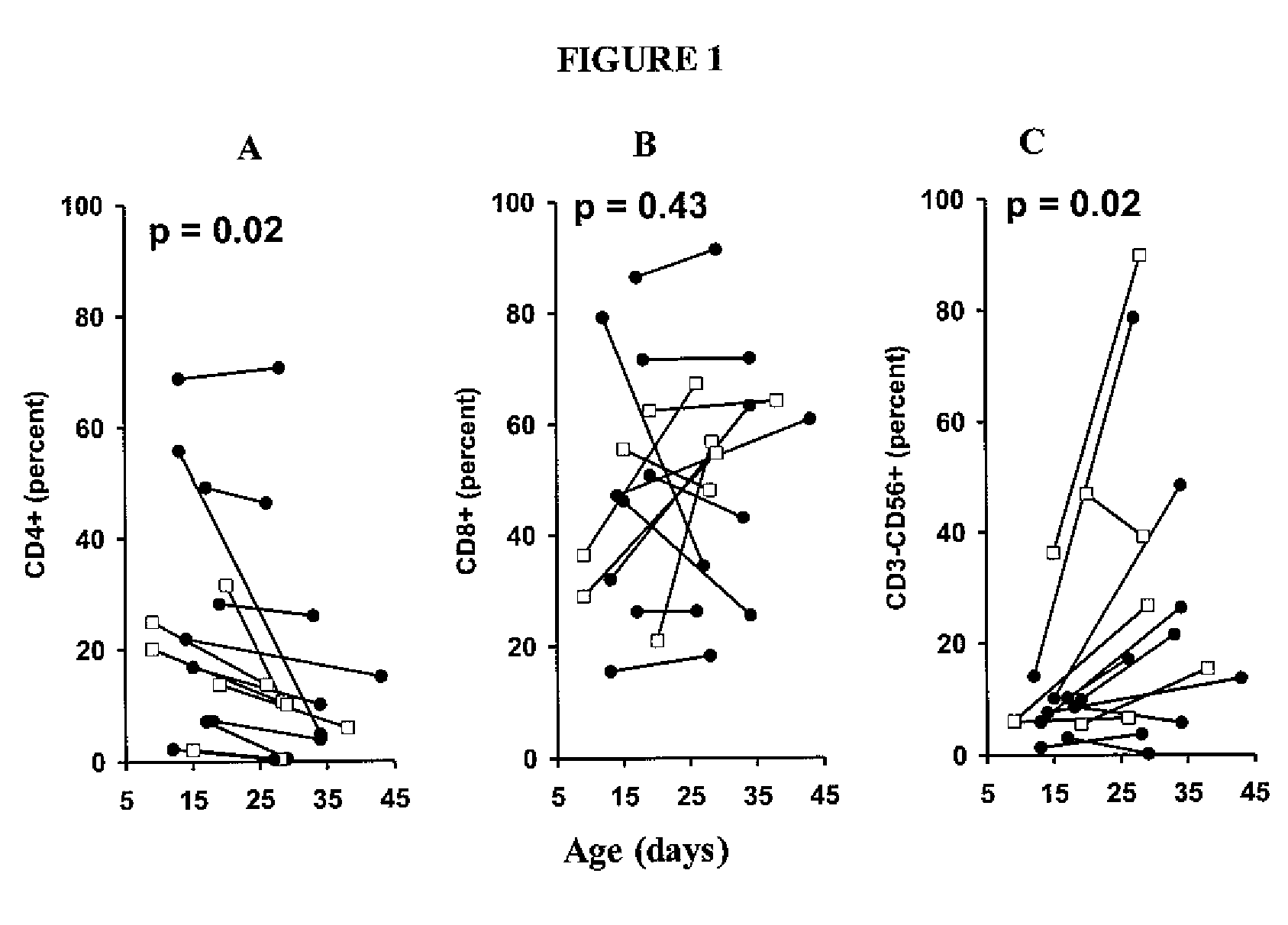
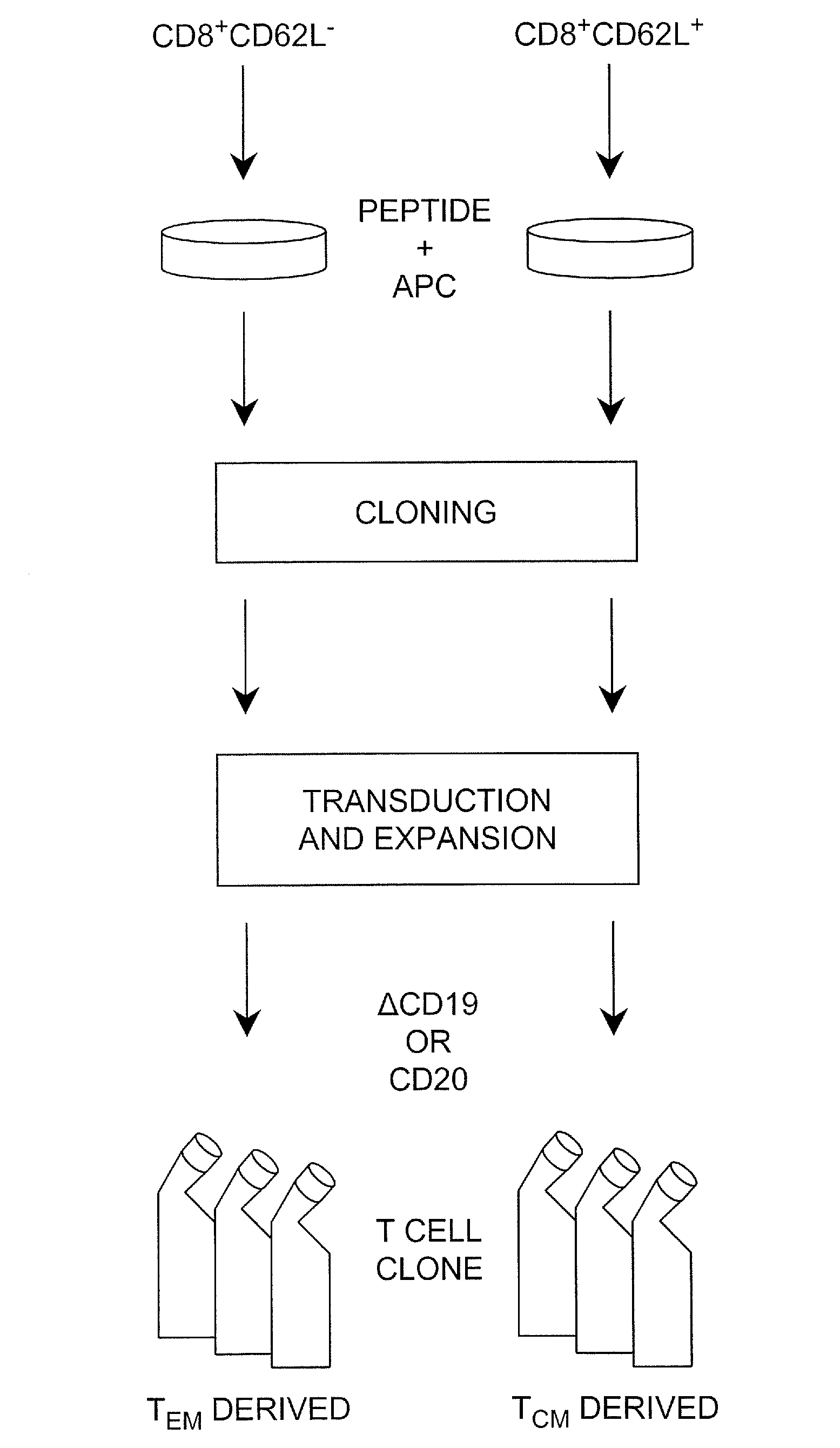
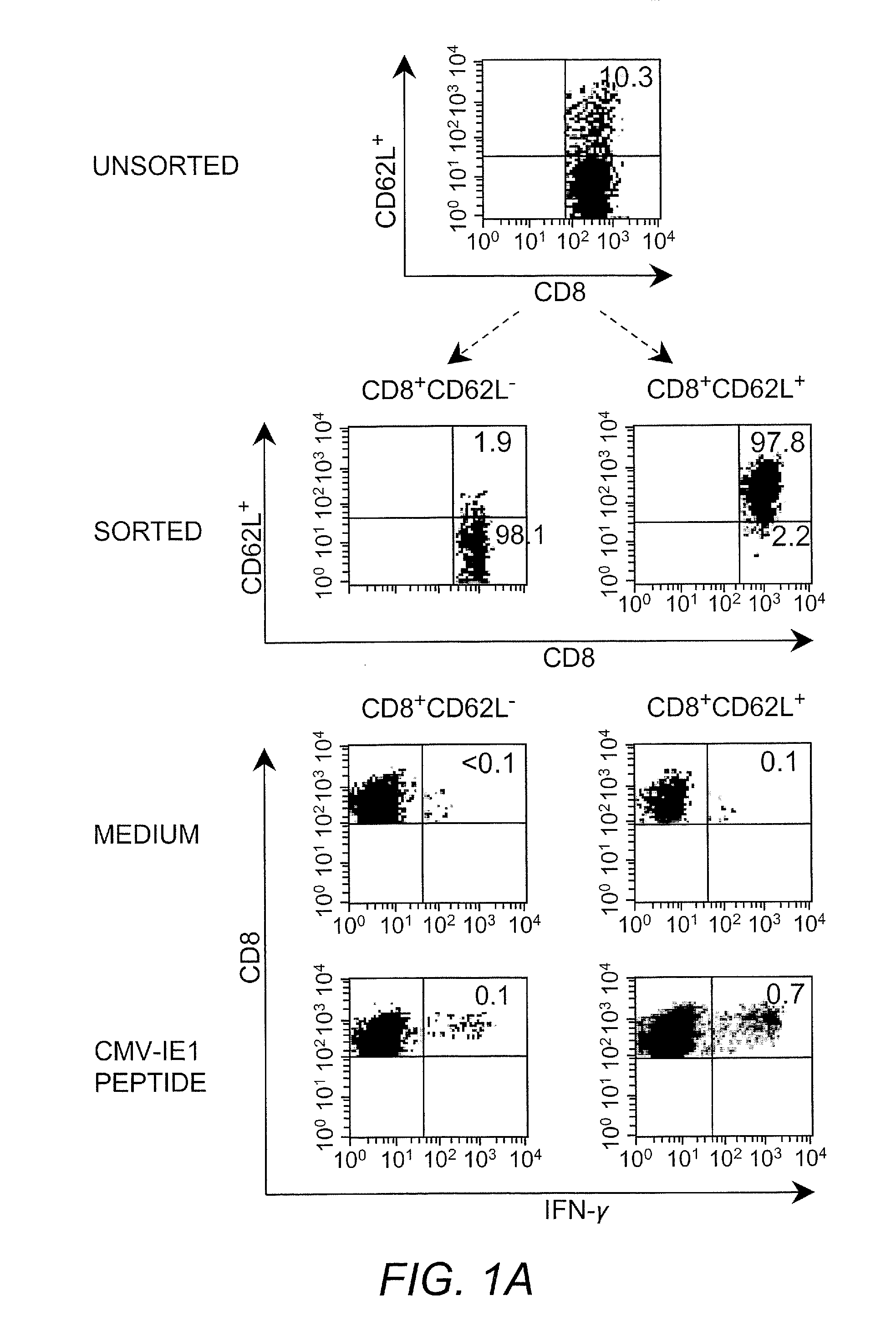
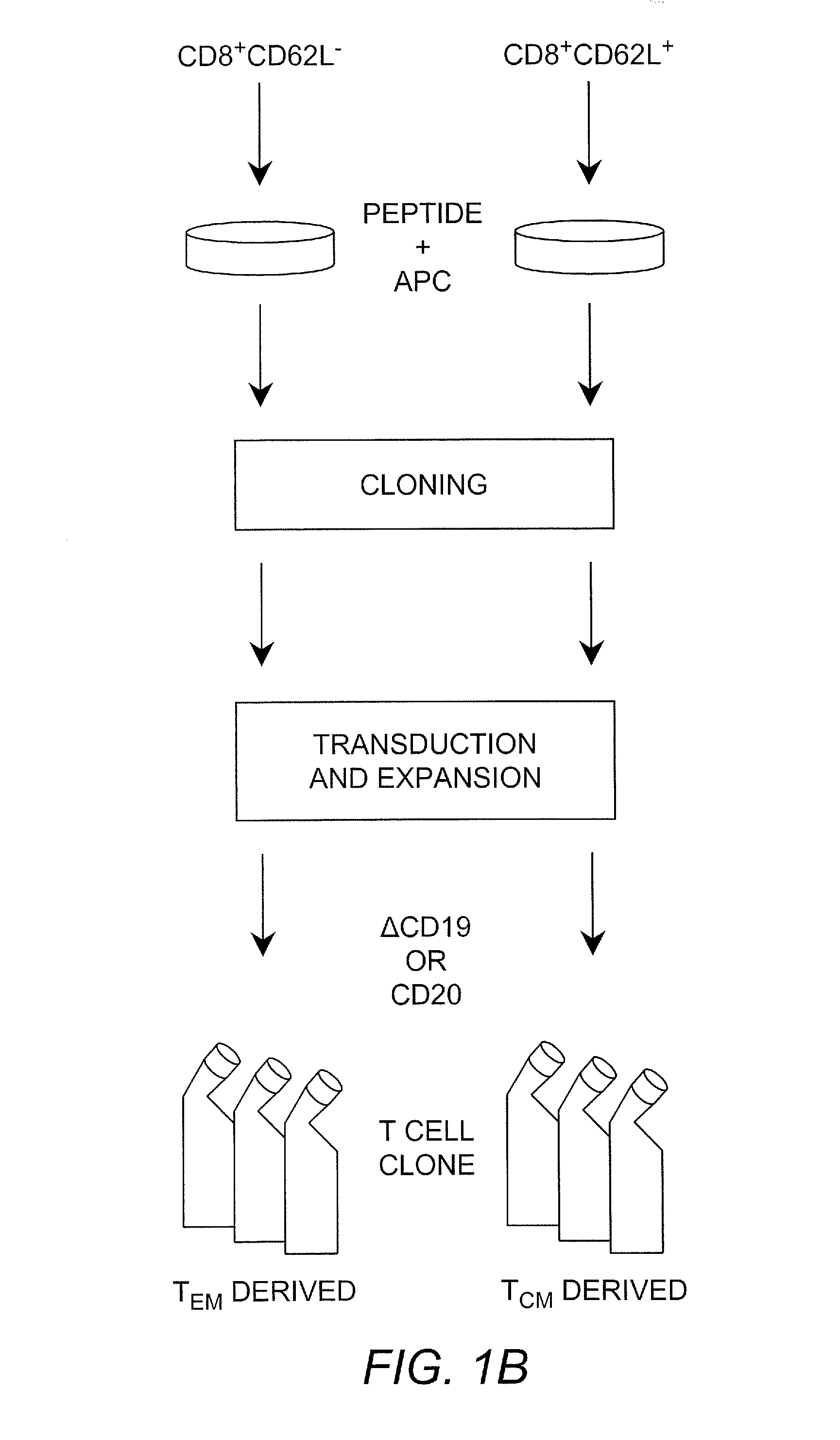
Adoptive transfer of cd8 + t cell clones derived from central memory cells
InactiveUS20080131415A1Increased proliferationBiocideArtificial cell constructsWhite blood cellPharmaceutical formulation
The present invention provides a method of carrying out adoptive immunotherapy in a primate subject in need thereof by administering the subject a cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount. The method comprises administering as the CTL preparation a preparation consisting essentially of an in vitro expanded primate CTL population, the CTL population enriched prior to expansion for central memory T lymphocytes, and depleted prior to expansion of effector memory T lymphocytes. In some embodiments, the method may further comprise concurrently administering Interleukin-15 to the subject in an amount effective to increase the proliferation of the central memory T cells in the subject. Pharmaceutical formulations produced by the method, and methods of using the same, are also described.
Owner:CITY OF HOPE +1
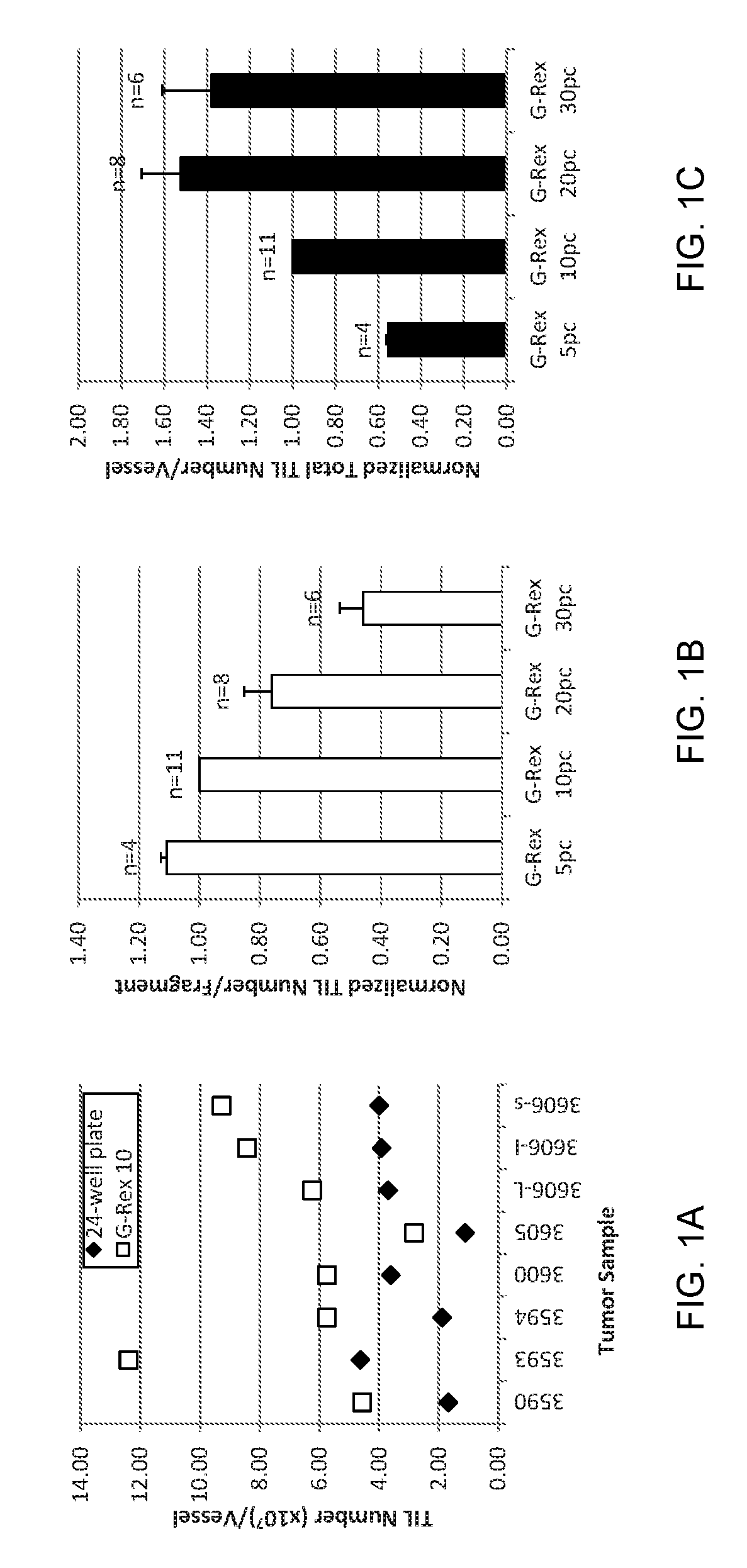
Methods of growing tumor infiltrating lymphocytes in gas-permeable containers
InactiveUS20170152478A1Mammal material medical ingredientsCancer antigen ingredientsAbnormal tissue growthTumour tissue
An embodiment of the invention provides a method of promoting regression of cancer in a mammal comprising obtaining a tumor tissue sample from the mammal; culturing the tumor tissue sample in a first gas permeable container containing cell medium therein; obtaining tumor infiltrating lymphocytes (TIL) from the tumor tissue sample; expanding the number of TIL in a second gas permeable container containing cell medium therein using irradiated allogeneic feeder cells and / or irradiated autologous feeder cells; and administering the expanded number of TIL to the mammal. Methods of obtaining an expanded number of TIL from a mammal for adoptive cell immunotherapy are also provided.
Owner:UNITED STATES OF AMERICA +1
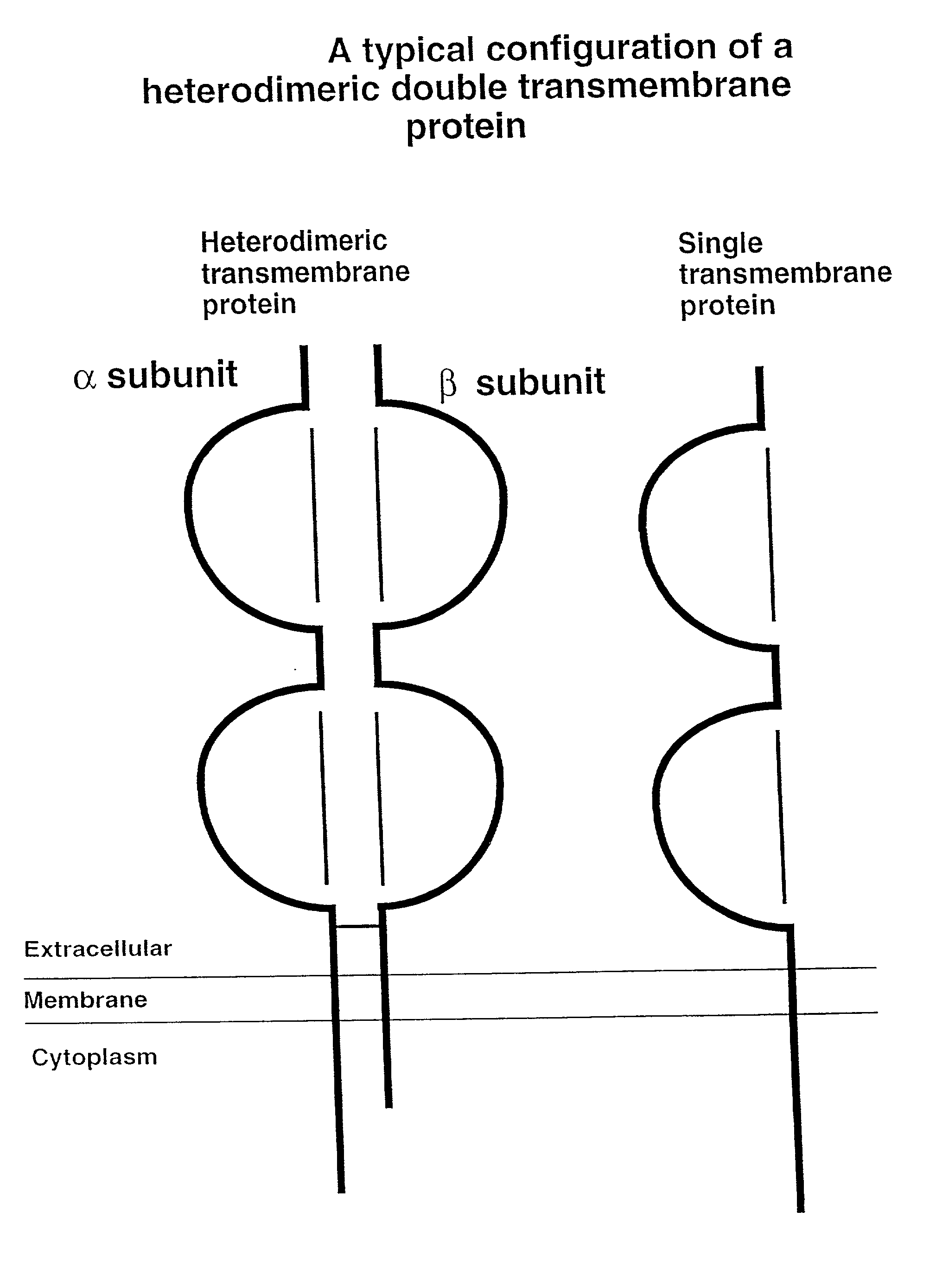
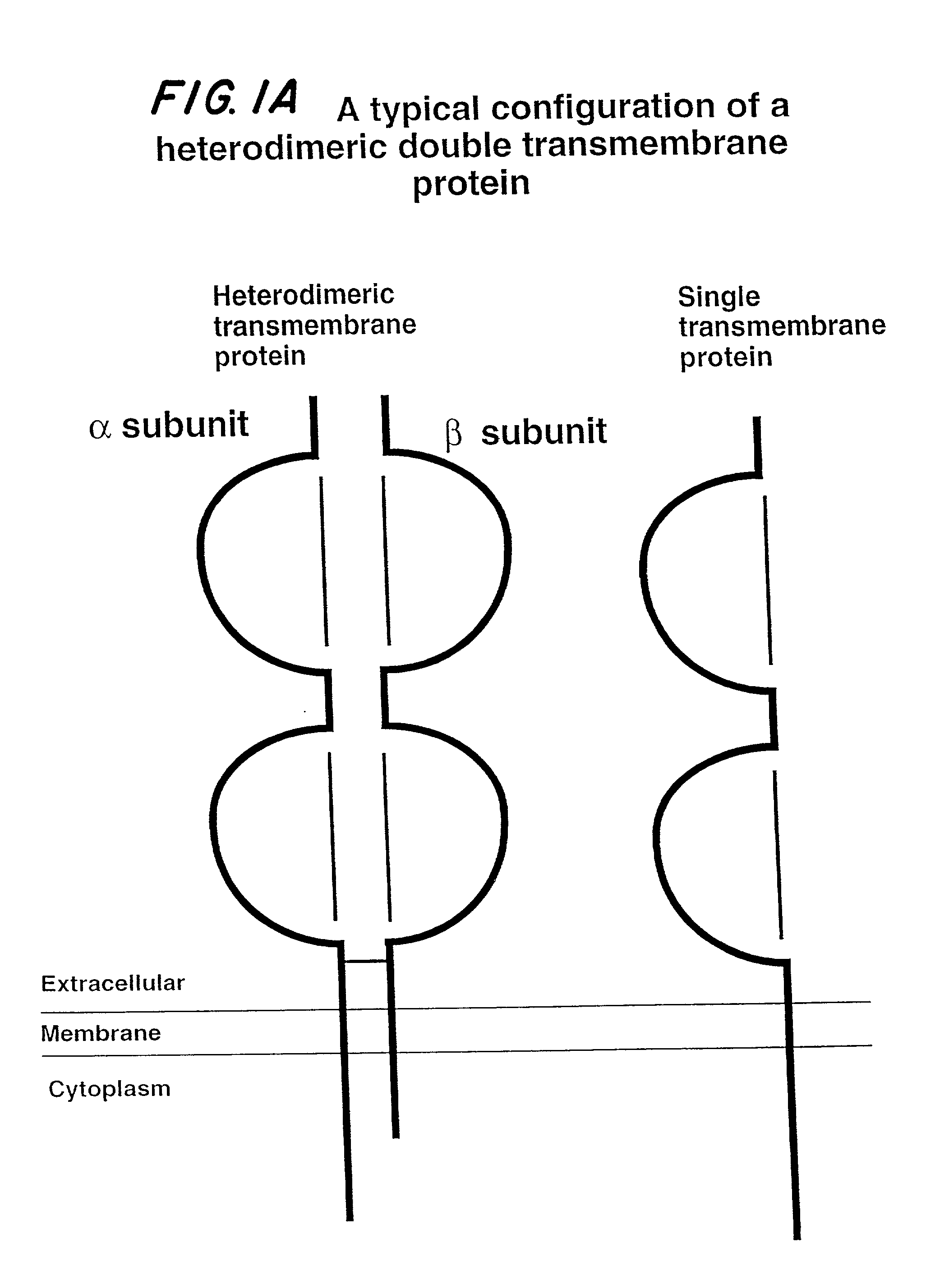
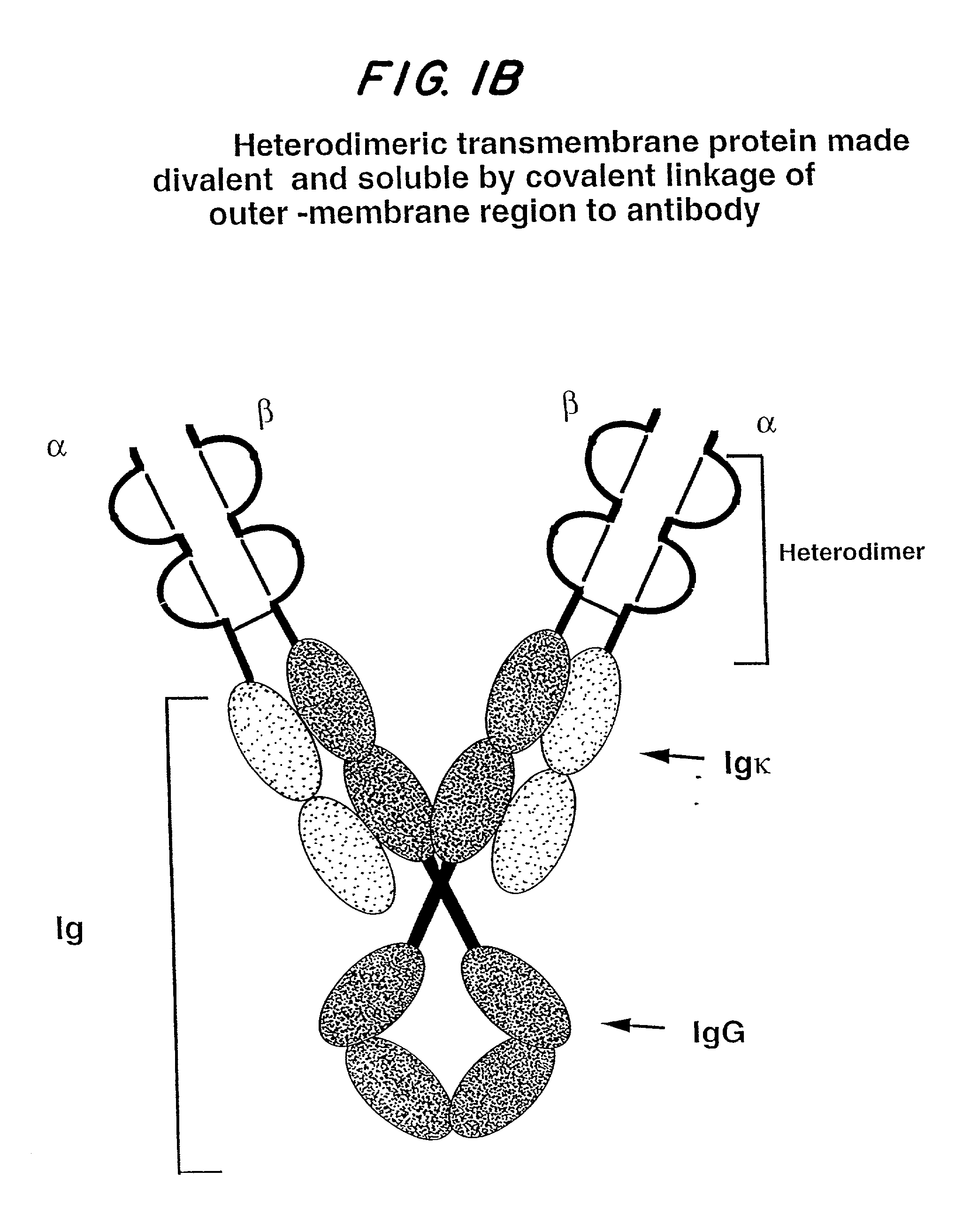
Soluble divalent and multivalent heterodimeric analogs of proteins
InactiveUS20020127231A1Well representedHigh affinityVirusesPeptide/protein ingredientsADAMTS ProteinsSpecific immunity
Specificity in immune responses is in part controlled by the selective interaction of T cell receptors with their cognate ligands, peptide / MHC molecules. The discriminating nature of this interaction makes these molecules, in soluble form, good candidates for selectively regulating immune responses. Attempts to exploit soluble analogs of these proteins has been hampered by the intrinsic low avidity of these molecules for their ligands. To increase the avidity of soluble analogs for their cognates to biologically relevant levels, divalent peptide / MHC complexes or T cell receptors (superdimers) were constructed. Using a recombinant DNA strategy, DNA encoding either the MHC class II / peptide or TCR heterodimers was ligated to DNA coding for murine Ig heavy and light chains. These constructs were subsequently expressed in a baculovirus expression system. Enzyme-linked immunosorbant assays (ELISA) specific for the Ig and polymorphic determinants of either the TCR or MHC fraction of the molecule indicated that infected insect cells secreted approximately 1 .mu.g / ml of soluble, conformnationally intact chimeric superdimers. SDS PAGE gel analysis of purified protein showed that expected molecular weight species. The results of flow cytometry demonstrated that the TCR and class II chimeras bound specifically with high avidity to cells bearing their cognate receptors. These superdimers will be useful for studying TCR / MHC interactions, lymphocyte tracking, identifying new antigens, and have possible uses as specific regulators of immune responses.
Owner:SCHNECK JONATHAN +1
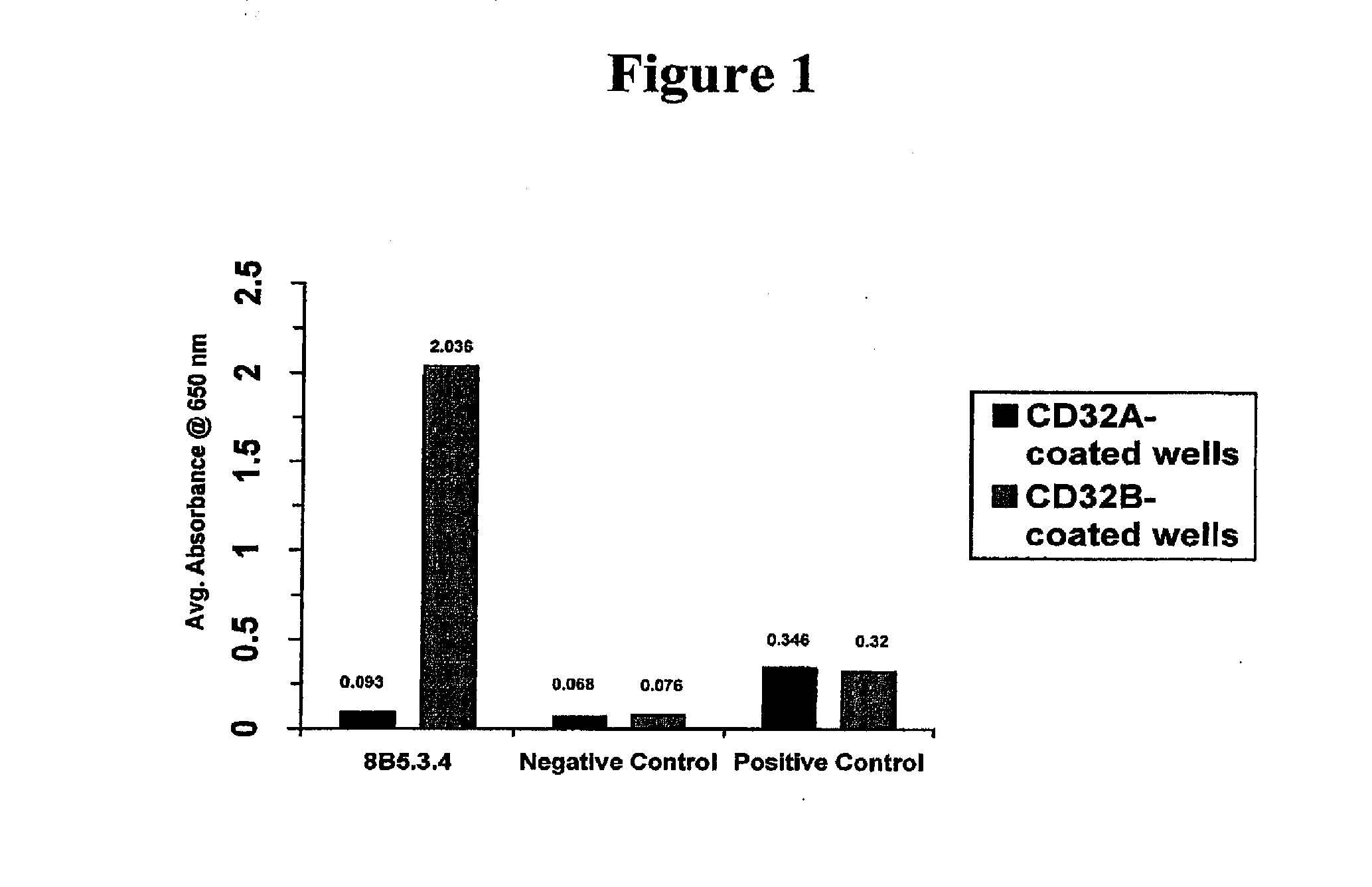
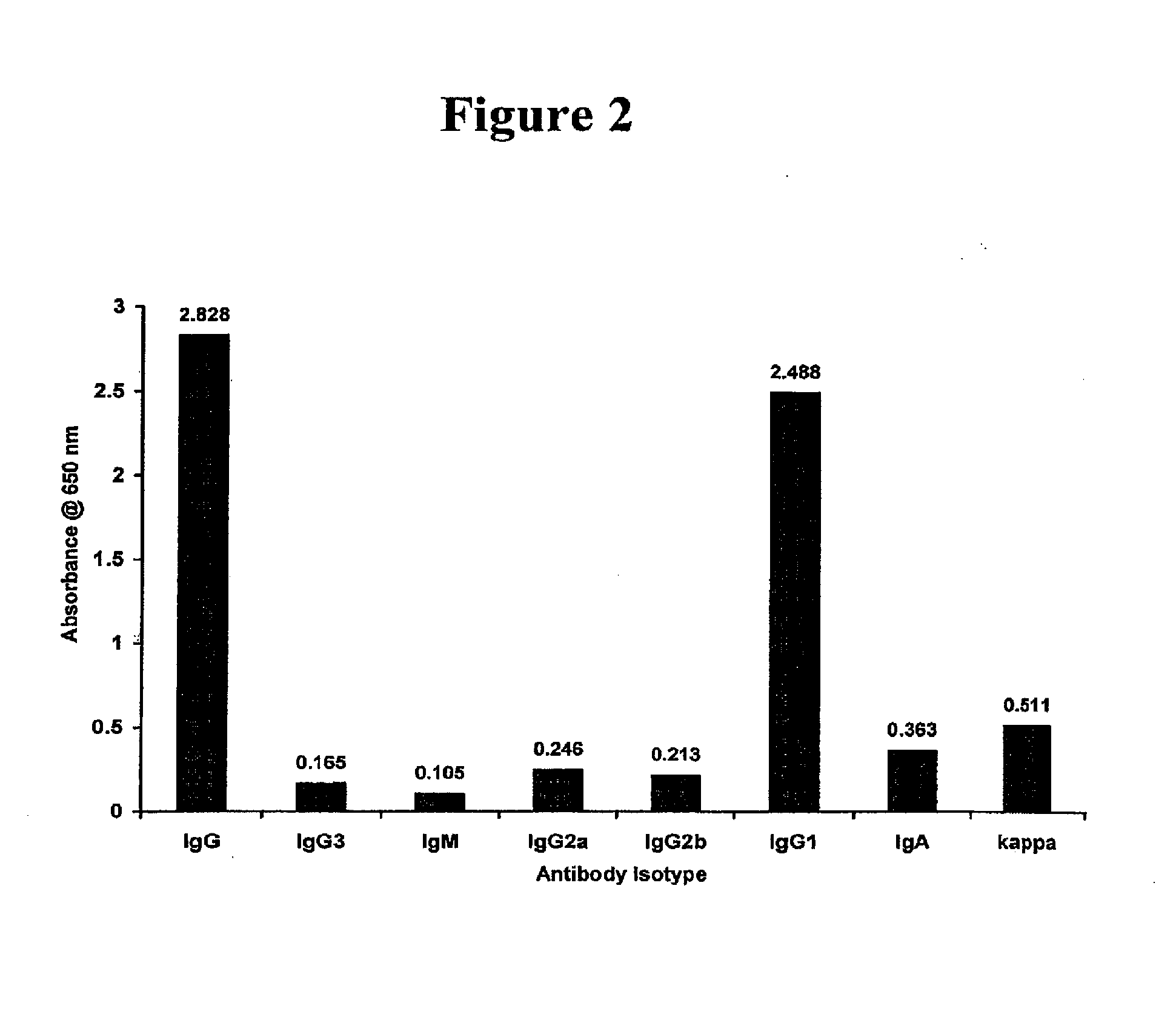
Fc.gamma.RIIB-Specific Antibodies and Methods of Use Thereof
ActiveUS20080044429A1Good curative effectEnhanced effector functionSugar derivativesPeptide/protein ingredientsTreatment effectAntigen Binding Fragment
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The present invention also provides the use of an anti-FcγRIIB antibody or an antigen-binding fragment thereof, as a single agent therapy for the treatment, prevention, management, or amelioration of a cancer, preferably a B-cell malignancy, particularly, B-cell chronic lymphocytic leukemia or non-Hodgkin's lymphoma, an autoimmune disorder, an inflammatory disorder, an IgE-mediated allergic disorder, or one or more symptoms thereof. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
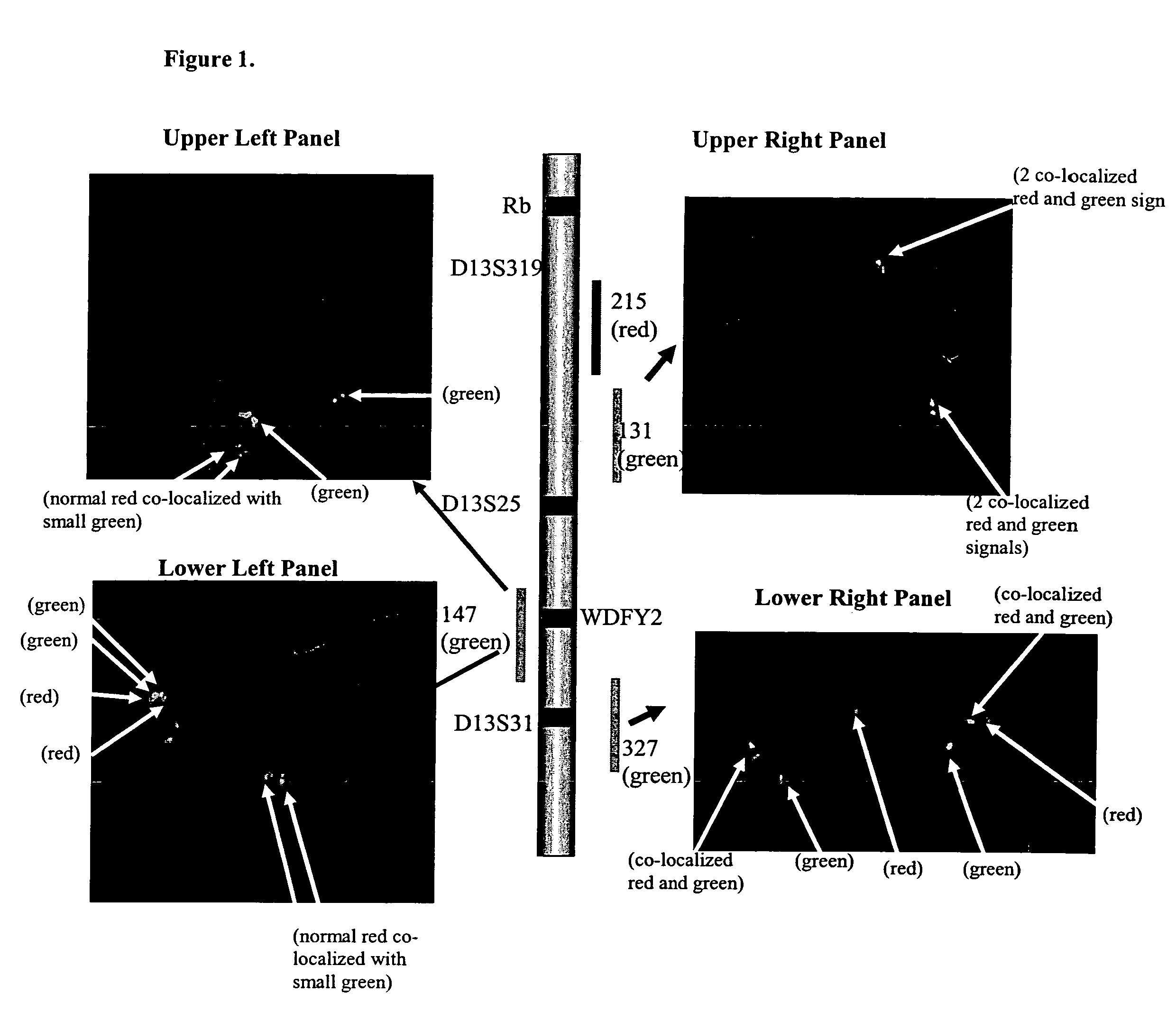
Detection of 13q14 chromosomal alterations
InactiveUS7479370B2Sugar derivativesMicrobiological testing/measurementLymphocyteChromosomal Alterations
The present invention relates to methods for detection of chromosomal alterations which are associated with the presence of various leukemias and lymphomas. The method comprises the steps of obtaining a biological sample comprising lymphocytes from an individual and assaying the sample to detect chromosomal deletions in the regions of chromosome 13 that corresponds to the region of chromosome 13 present in the RP11-147H23 or RP11-327P2.
Owner:HEALTH RES INC
Method for the early detection of breast cancer, lung cancer, pancreatic cancer and colon polyps, growths and cancers as well as other gastrointestinal disease conditions and the preoperative and postoperative monitoring of transplanted organs from the donor and in the recipient and their associated conditions related and unrelated to the organ transplantation
ActiveUS20060088876A1Early diagnosisMicrobiological testing/measurementDisease diagnosisLung cancerDisease cause
A method for the early diagnosis of breast, lung, pancreatic and colon growths and cancers as well as conditions associated with donor and recipient organ transplants, both before and after transplantation to identify and allow treatment of possible transplanted organ rejection and other disease conditions related and unrelated to the transplantation, compares the gene expression patterns from a patient's peripheral blood monocytes-lymphocyte's gene system with either the similar gene expression patterns of a normal person, or with the similar gene expression patterns of a person known to have the condition being screened for. Differences between the patient's gene expression patterns for particular genes and the normal patterns indicates the presence of the condition with the number of differences indicating the probability of the condition. Similarities between the patient's gene expression patterns for those particular genes and the patterns of a person known to have the condition indicates the presence of the condition with the number of similarities indicating the probability of the condition. For example, particular genes for use in identifying pancreatic cancer are disclosed.
Owner:BAUER A ROBERT
Modulating agonistic tnfr antibodies
InactiveUS20140010812A1High binding affinityExcellent agonistic activityOrganic active ingredientsPeptide/protein ingredientsCell specificLymphocyte
Owner:THE ROCKEFELLER UNIV
A cell therapy method for the treatment of tumors
T cell responses are often diminished in humans with a compromised immune system. We have developed a method to isolate, stimulate and expand naïve cytotoxic T lymphocyte precursors (CTLp) to antigen-specific effectors, capable of lysing tumor cells in vivo. This ex vivo protocol produces fully functional effectors. Artificial antigen presenting cells (AAPCs; Drosophila melanogaster) transfected with human HLA class I and defined accessory molecules, are used to stimulate CD8+ T cells from both normal donors and cancer patients. The class I molecules expressed to a high density on the surface of the Drosophila cells are empty, allowing for efficient loading of multiple peptides that results in the generation of polyclonal responses recognizing tumor cells endogenously expressing the specific peptides. The responses generated are robust, antigen-specific and reproducible if the peptide epitope is a defined immunogen. This artificial antigen expression system can be adapted to treat most cancers in a significant majority of the population.
Owner:JANSSEN PHARMA INC
Chimeric immunoreceptor useful in treating human cancers
InactiveUS20090257994A1Negligible toxicityPotent and selectiveBiocidePeptide/protein ingredientsIntracellular signallingMalignancy
The present invention relates to chimeric transmembrane immunoreceptors, named “zetakines,” comprised of an extracellular domain comprising a soluble receptor ligand linked to a support region capable of tethering the extracellular domain to a cell surface, a transmembrane region and an intracellular signalling domain. Zetakines, when expressed on the surface of T lymphocytes, direct T cell activity to those specific cells expressing a receptor for which the soluble receptor ligand is specific. Zetakine chimeric immunoreceptors represent a novel extension of antibody-based immunoreceptors for redirecting the antigen specificity of T cells, with application to treatment of a variety of cancers, particularly via the autocrin / paracrine cytokine systems utilized by human malignancy. In a preferred embodiment is a glioma-specific immunoreceptor comprising the extracellular targetting domain of the IL-13Rα2-specific IL-13 mutant IL-13(E13Y) linked to the Fc region of IgG, the transmembrane domain of human CD4, and the human CD3 zeta chain.
Owner:CITY OF HOPE
Compositions and methods for regulating lymphocyte activation
InactiveUS20020155604A1Increased proliferationHigh affinityPeptide/protein ingredientsAntibody mimetics/scaffoldsCell activationCell Surface Antigens
The present invention relates to regulation of lymphocyte activation. In particular, it relates to compositions and methods for regulating lymphocyte activation by selectively binding multiple cell surface antigens expressed by the same lymphocyte.
Owner:CYCLACEL PHARMA
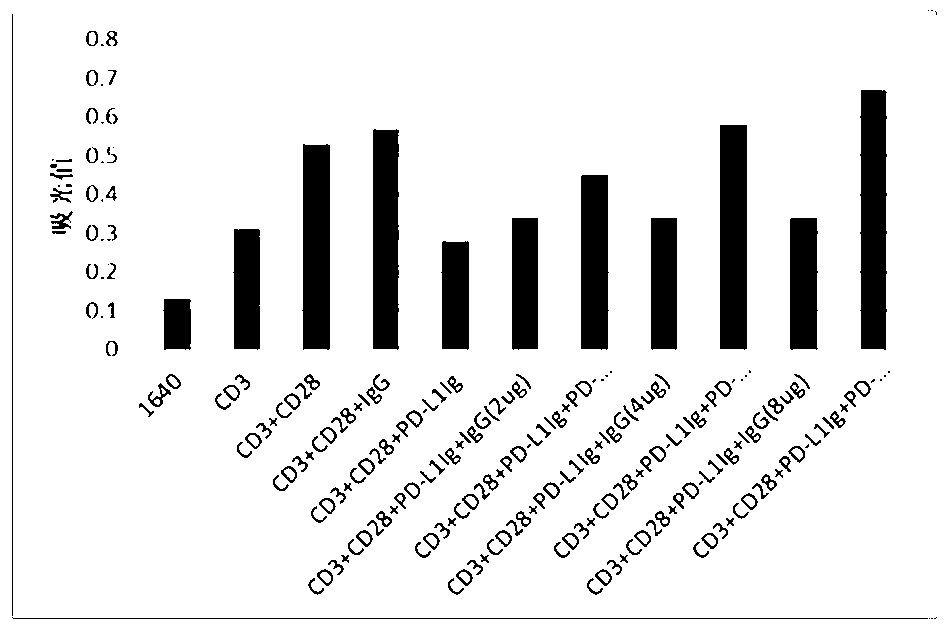
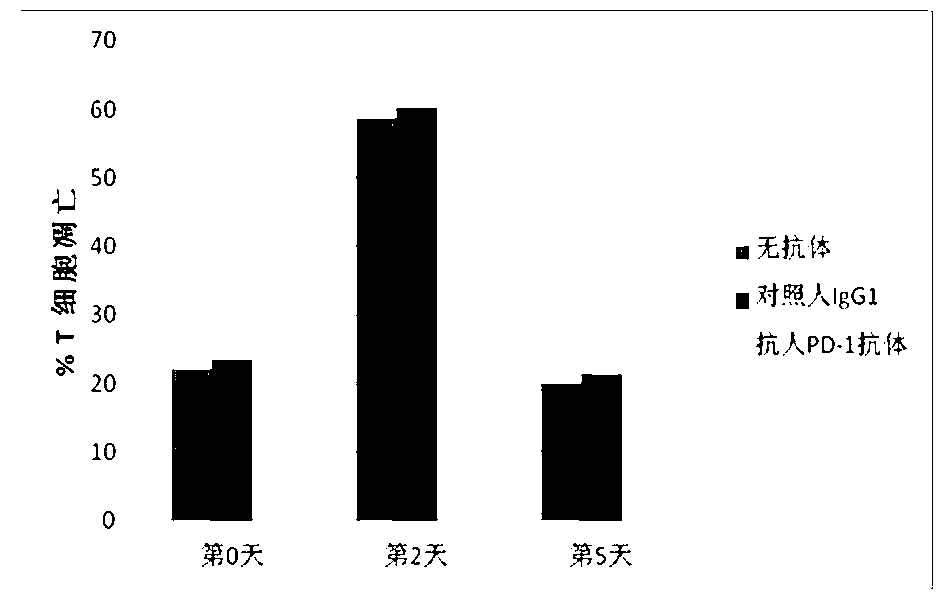
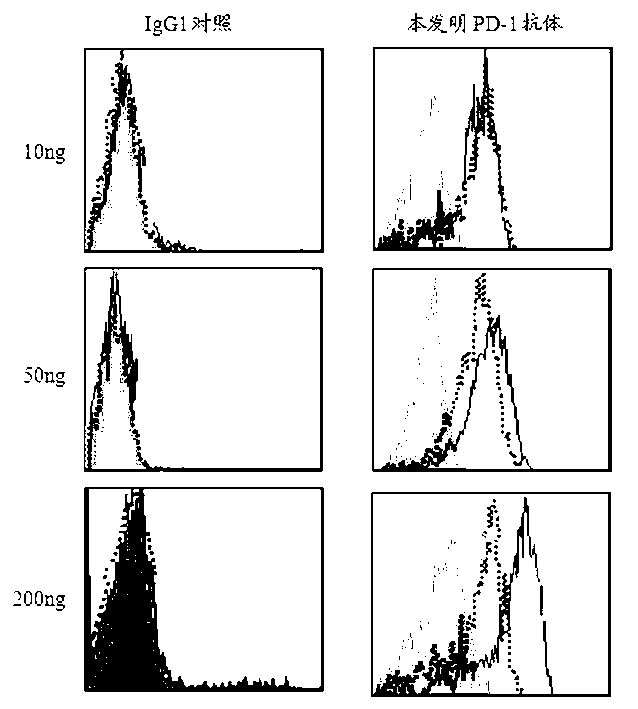
Full-humanized anti-PD-1 monoclonal antibody and preparation method and application thereof
InactiveCN103242448AHigh affinityImproving immunogenicityAntiviralsImmunoglobulins against cell receptors/antigens/surface-determinantsAutoimmune diseaseFactor ii
The invention discloses a full-humanized anti-PD-1 monoclonal antibody having a heavy-chain amino acid sequence shown in a sequence 7 in a sequence table and a light-chain amino acid sequence shown in a sequence 8 in the sequence table. The full-humanized anti-PD-1 monoclonal antibody has the advantages that the antibody has high appetency and low immunogenicity on PD-1, is efficiently expressed in animal cells, and can be used for industrial production. An experiment proves that the full-humanized anti-PD-1 monoclonal antibody specifically blocks a PD-1 / PD-L inhibiting signal, so that a disabled effector cell in an organism restores a biological function; activation proliferation of a tumor and a virus specificity CD8+T cell and secretion of a cell factor are facilitated; the killability of lymphocyte on tumor antigen, an exotic invasive virus and the like is enhanced; the immunity of the organism is improved; and the tumor cell and the virus are timely cleared. Therefore, the antibody disclosed by the invention has a wide application prospect on treatment of tumors, infectious diseases and autoimmune diseases.
Owner:ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com