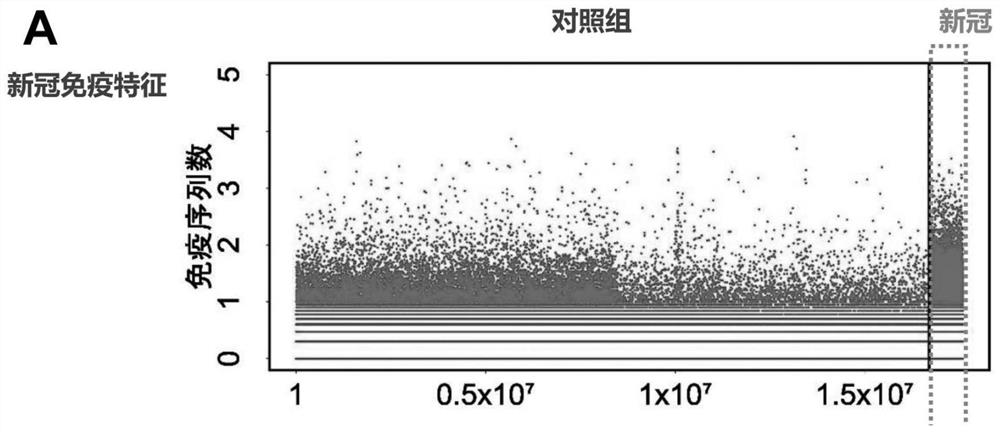
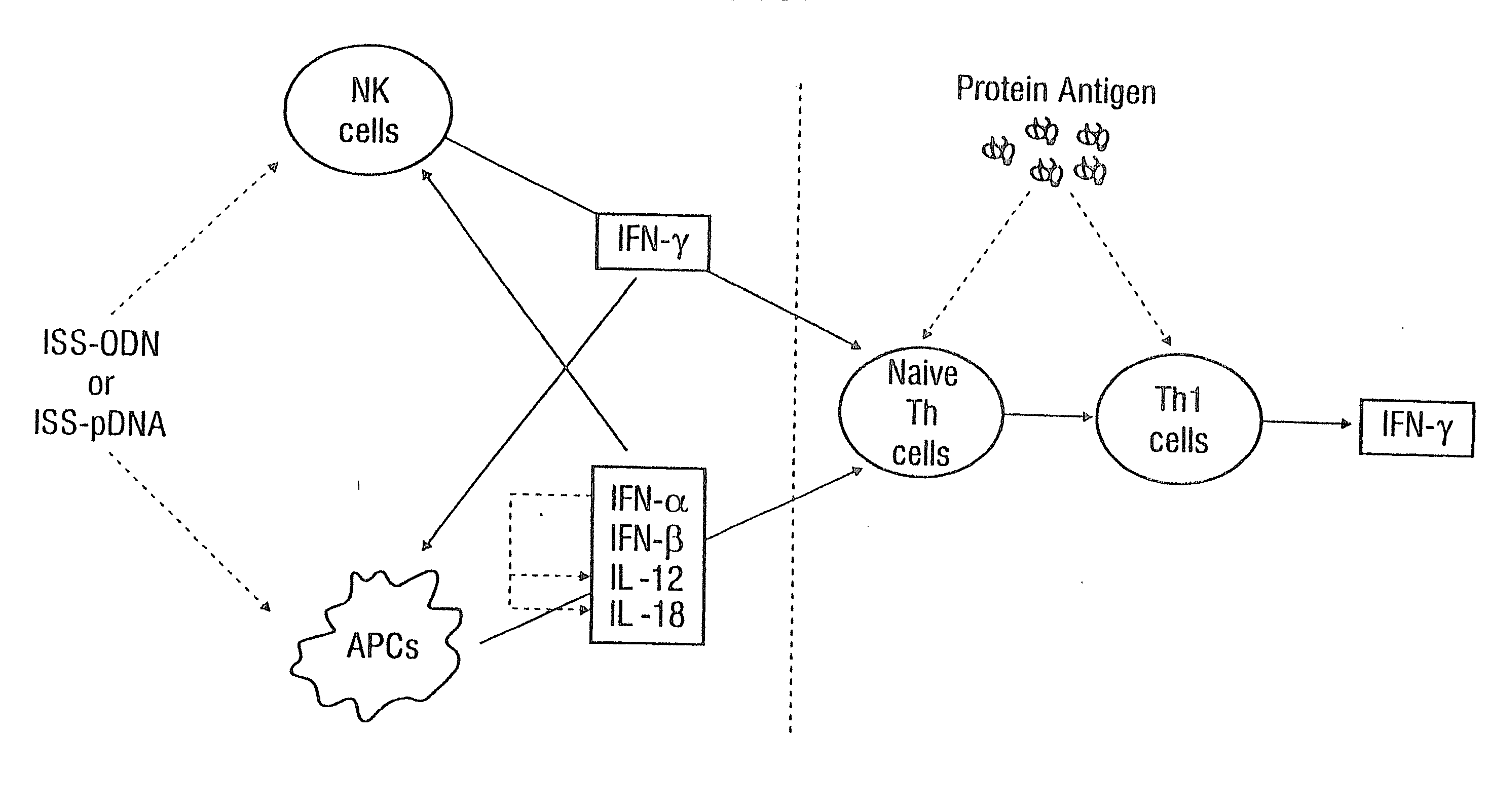
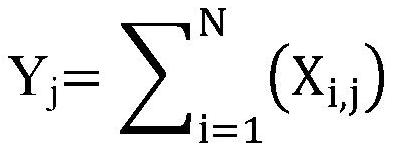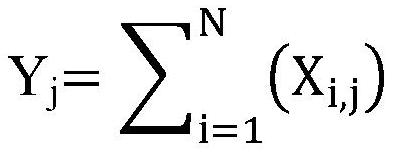Patents
Literature
80 results about "Antigen stimulation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Description. The Antigen Stimulation Study is used in the evaluation of immunodeficiency to determine the functional capabilities of peripheral blood mononuclear cells to respond to specific stimuli (Tetanus and/or Candida).
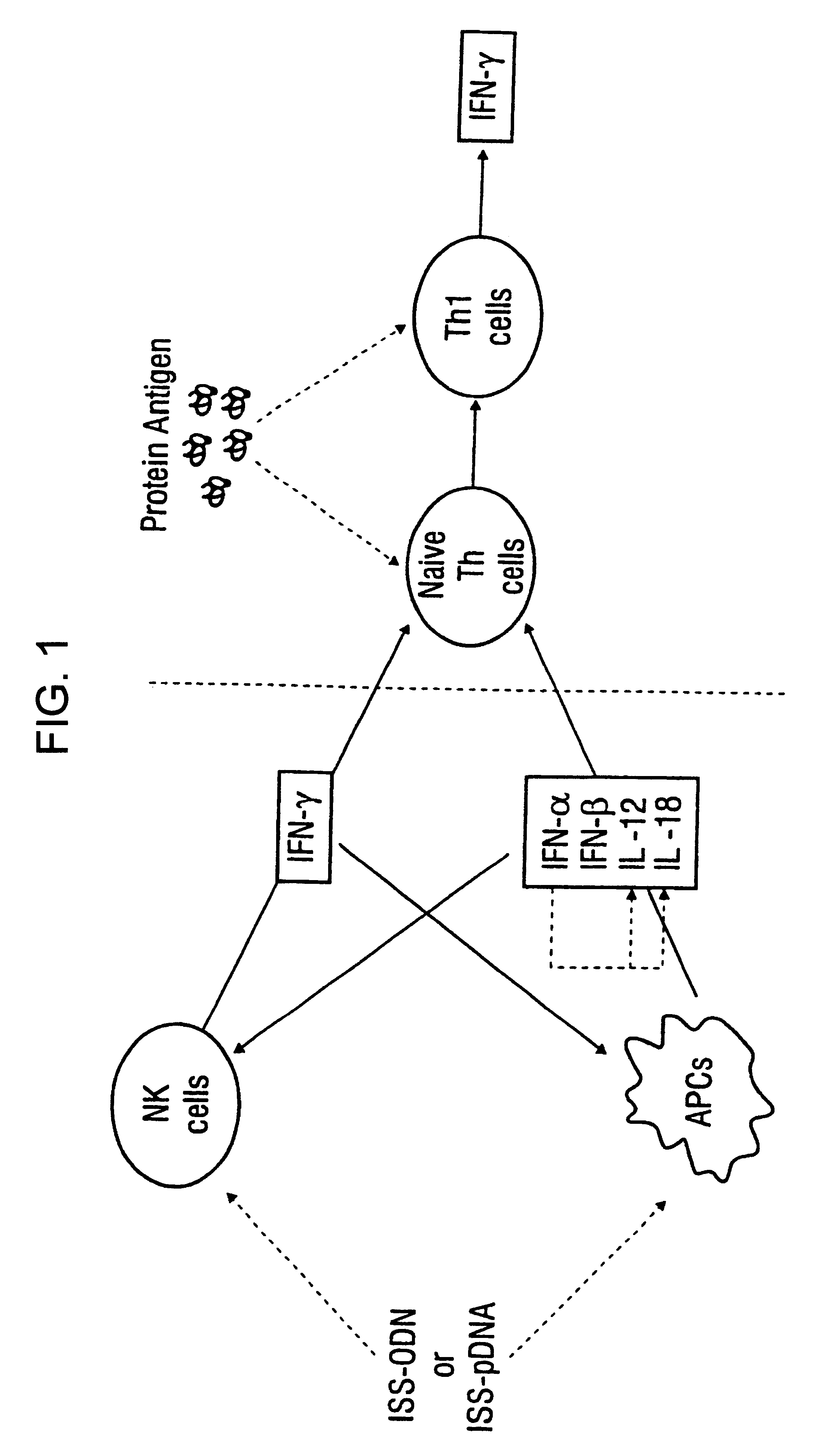
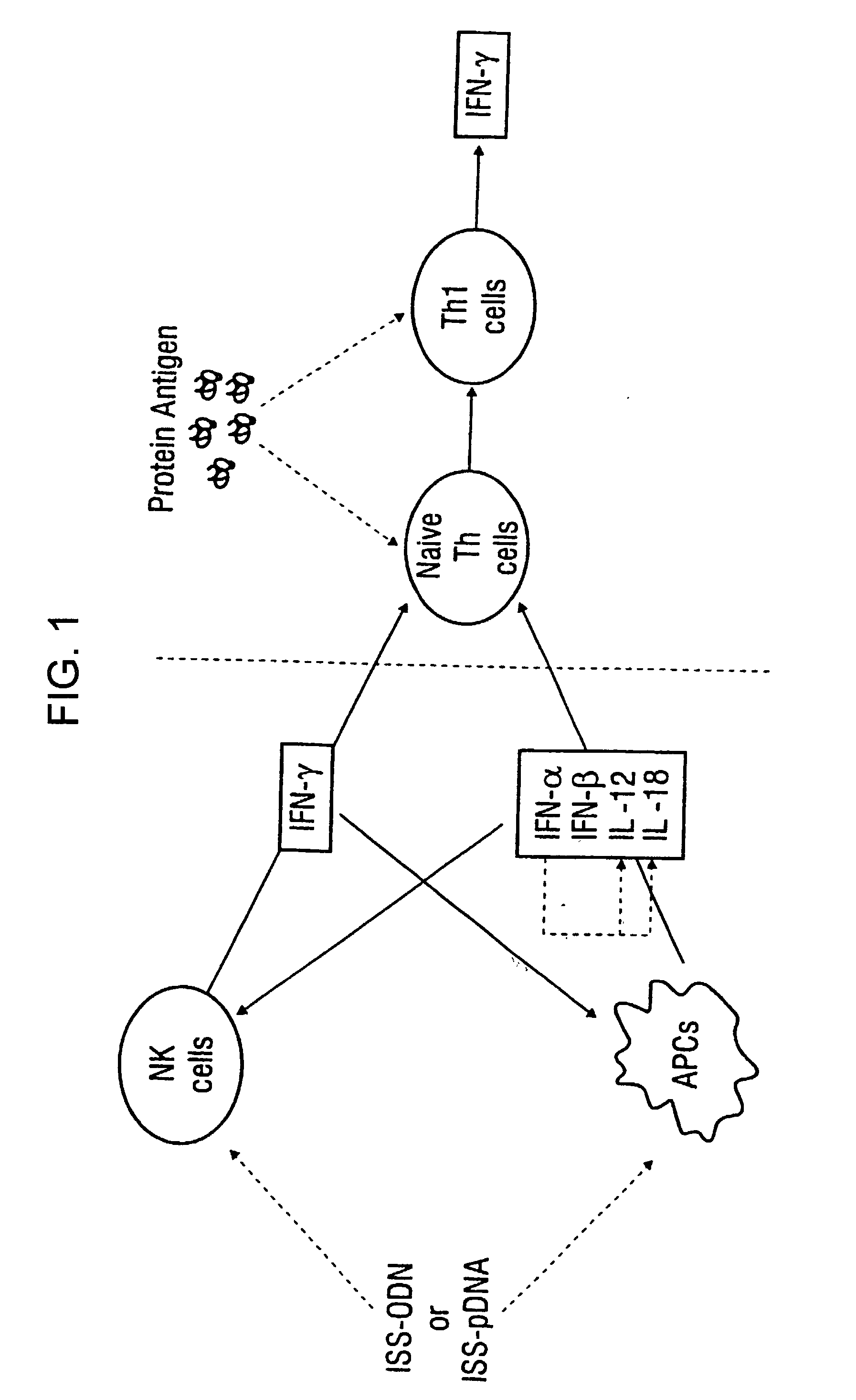
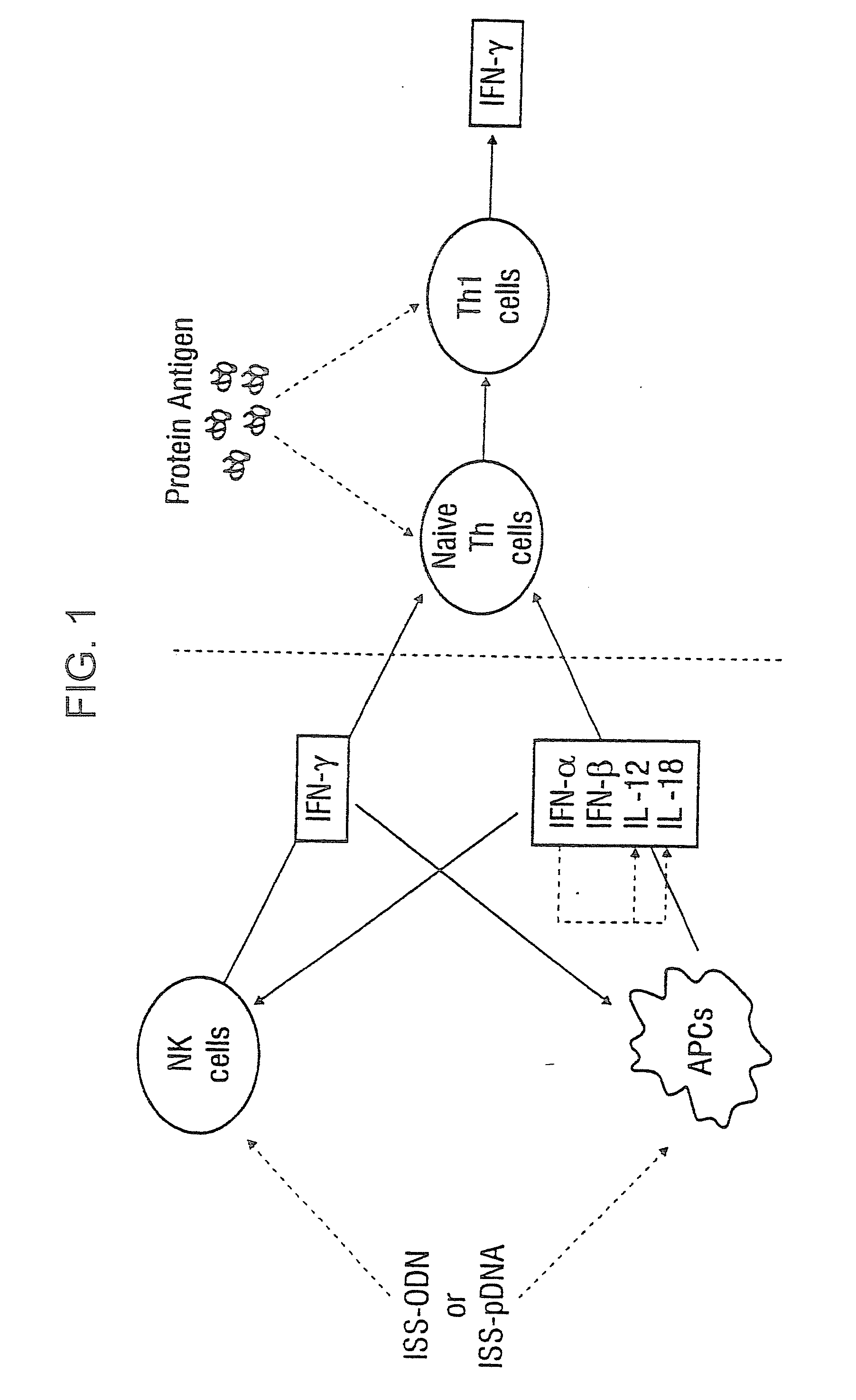
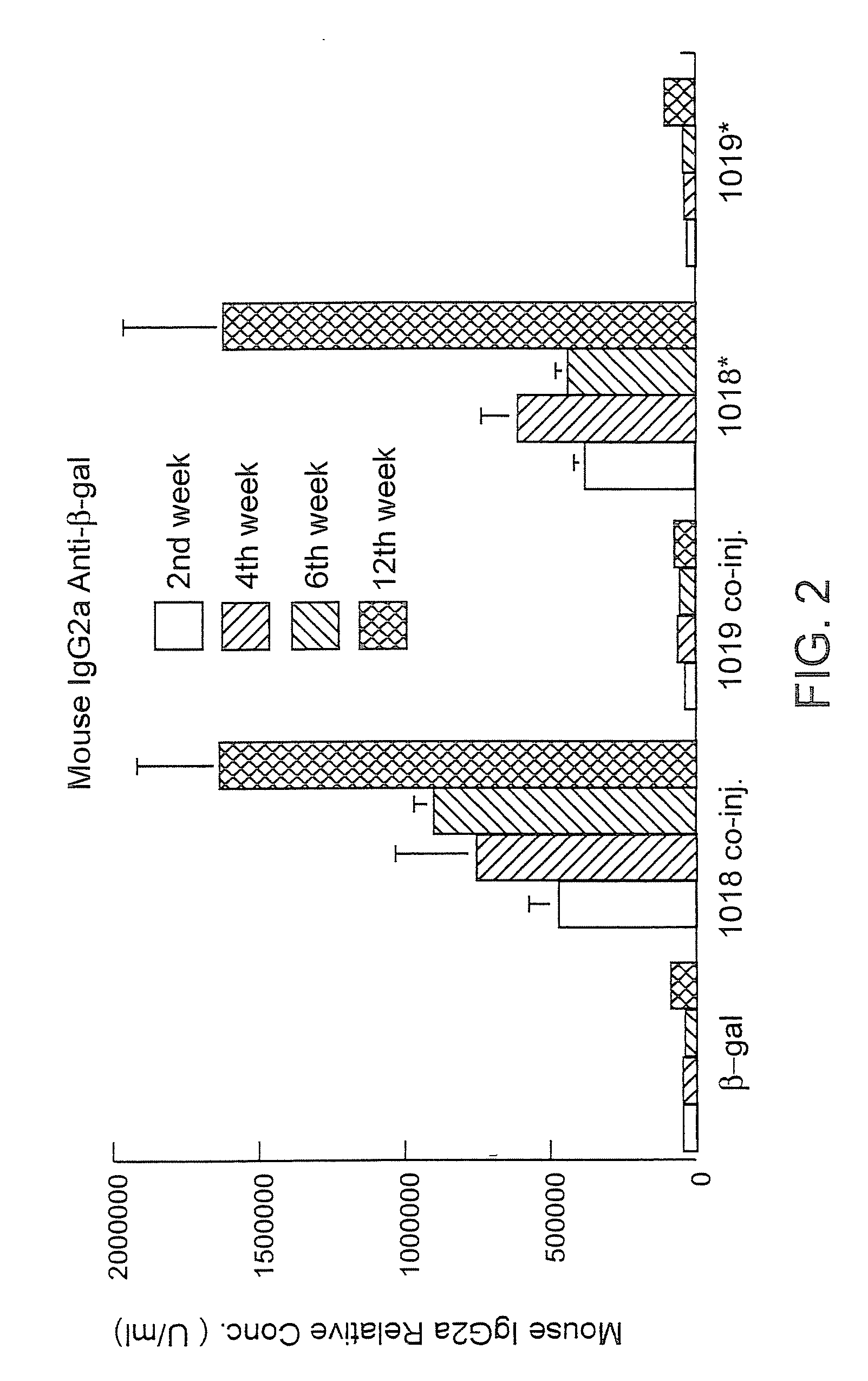
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a Th1 phenotype
InactiveUS6498148B1Treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsSenses disorderAntigen stimulationTherapeutic intent
The invention relates to methods for preventing or reducing antigen-stimulated, granulocyte-mediated inflammation in tissue of an antigen-sensitized mammal host by delivering an immunostimulatory oligonucleotide to the host. In addition, methods for using the immunostimulatory oligonucleotides to boost a mammal host's immune responsiveness to a sensitizing antigen (without immunization of the host by the antigen) and shifting the host's immune responsiveness to a Th1 phenotype to achieve various therapeutic ends are provided. Kits for practicing the methods of the invention are also provided.
Owner:RGT UNIV OF CALIFORNIA
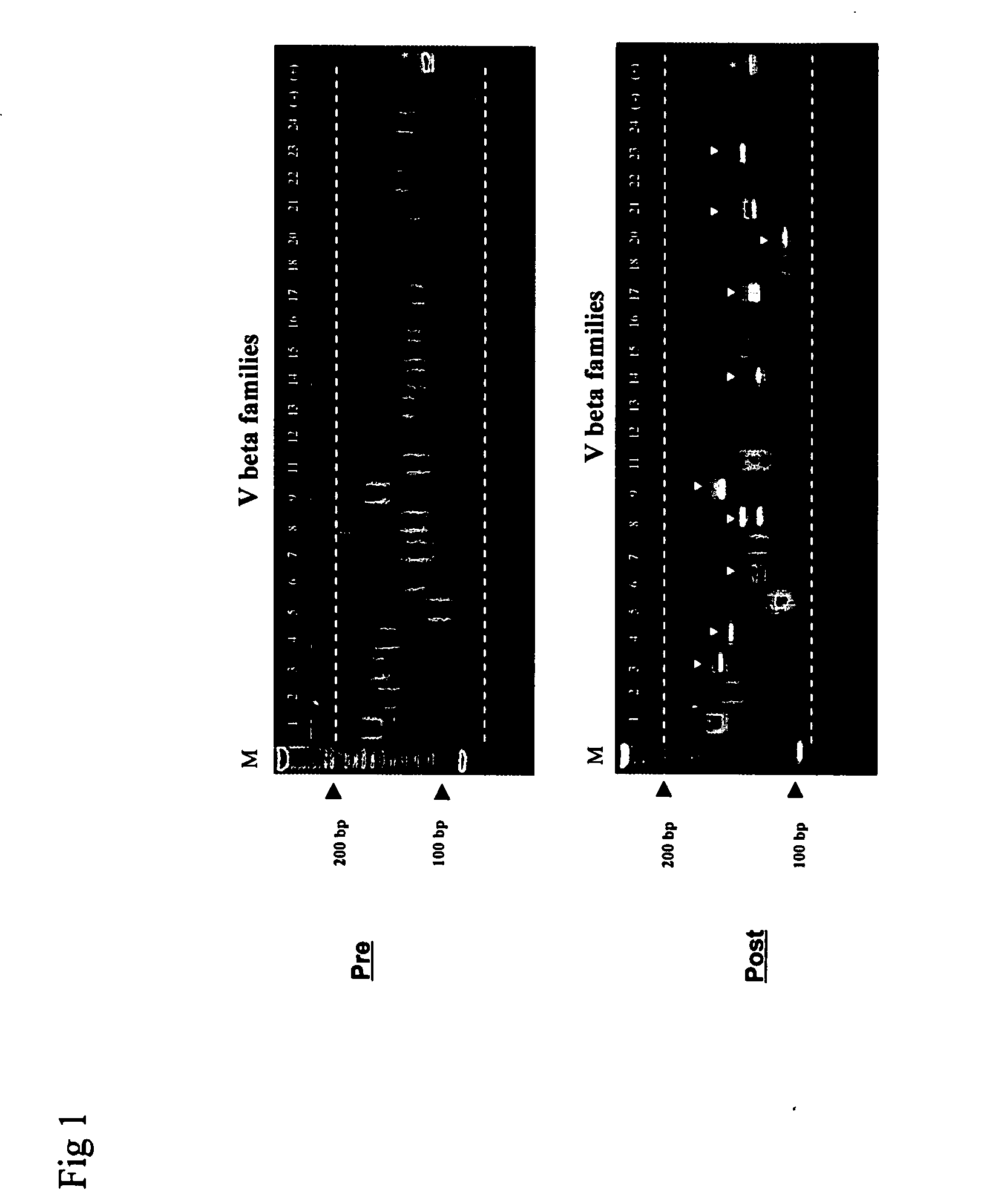
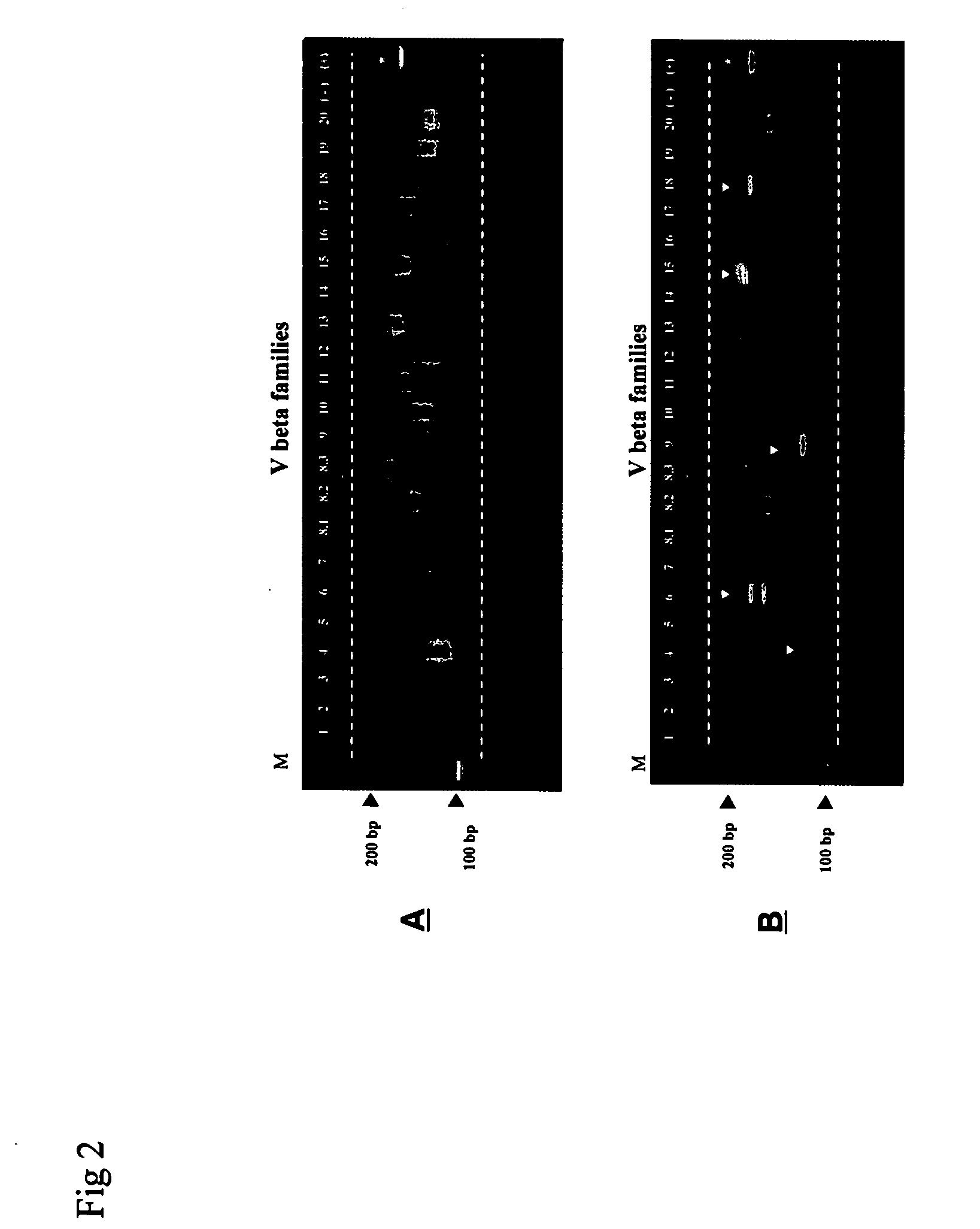
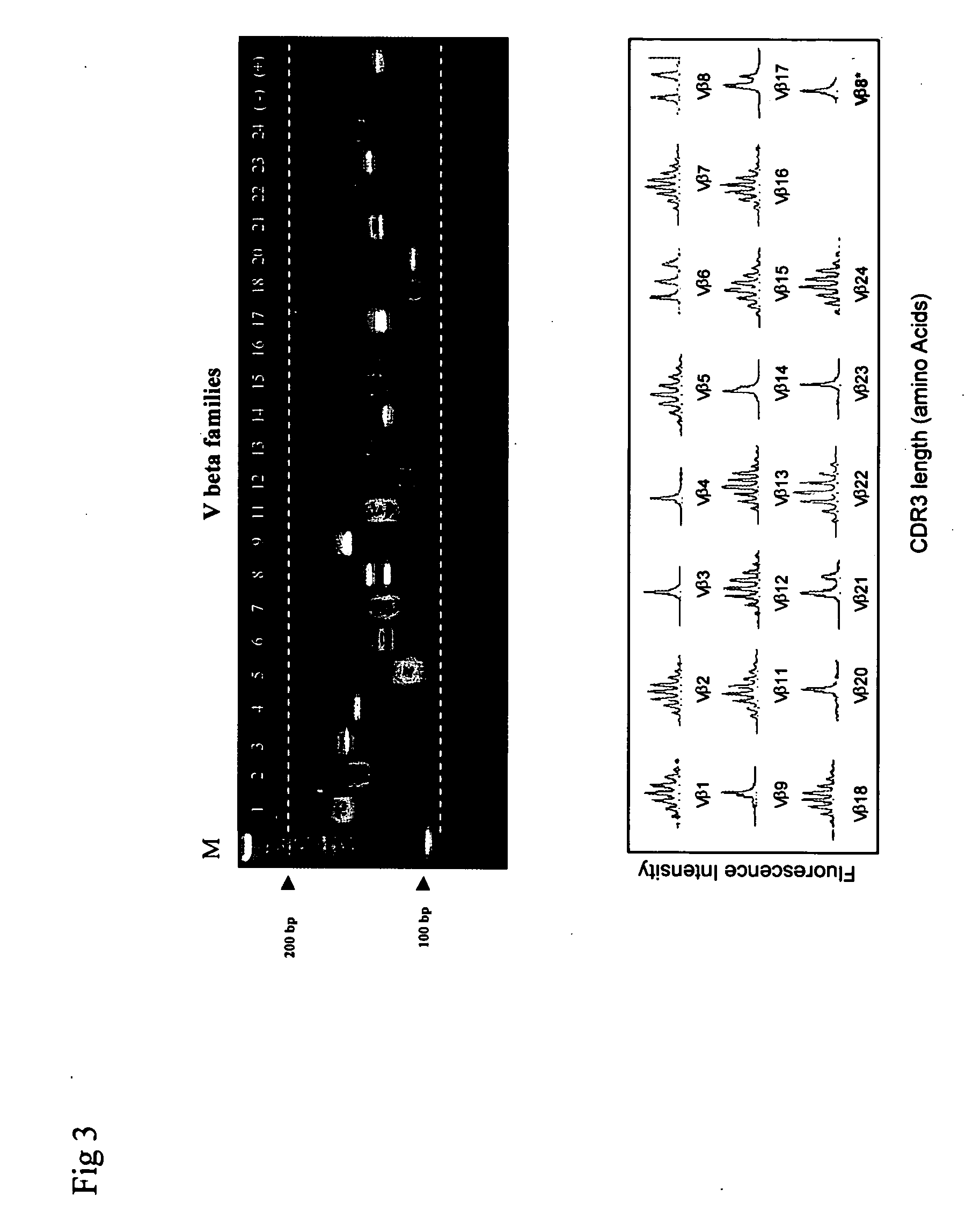
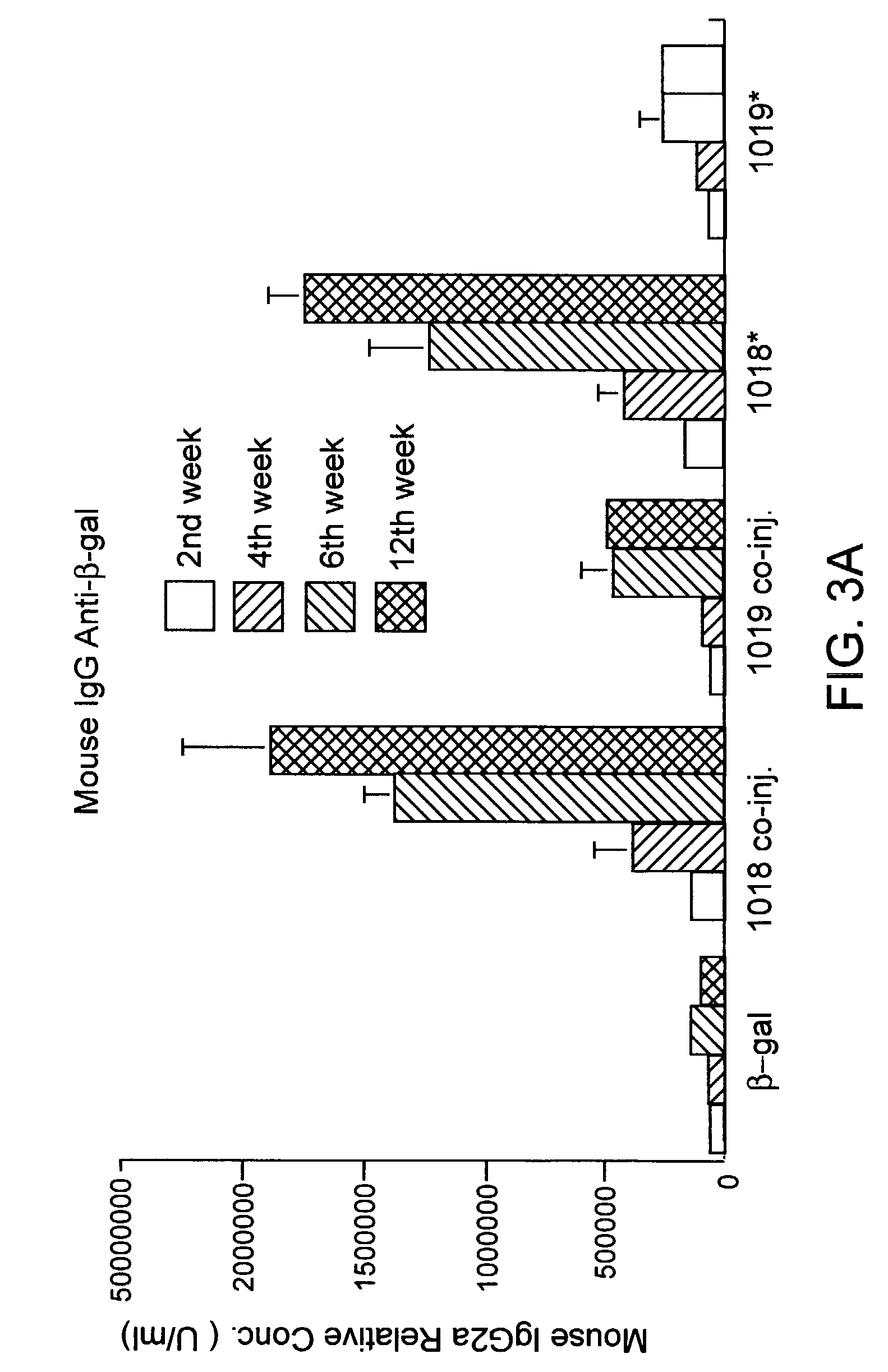
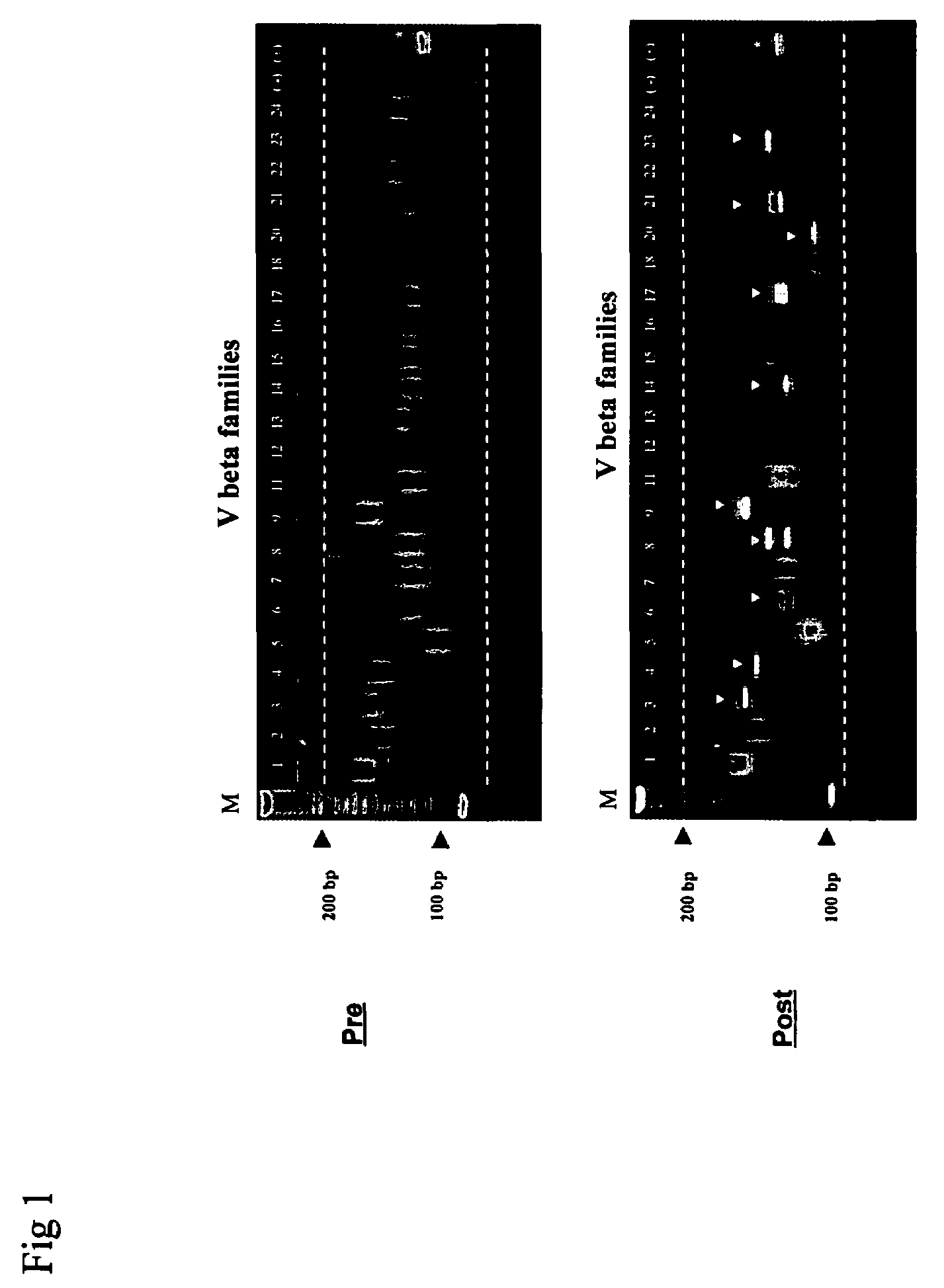
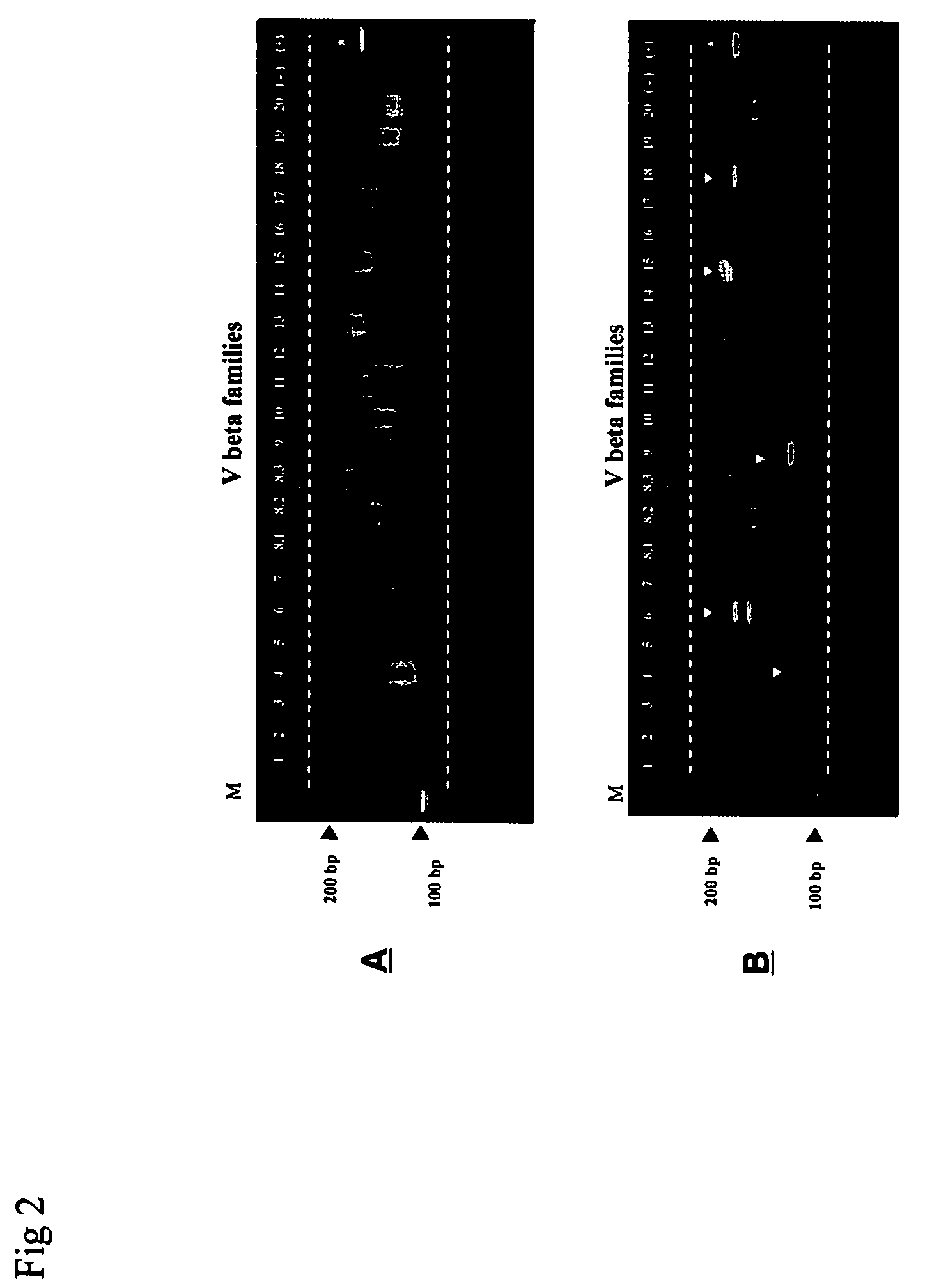
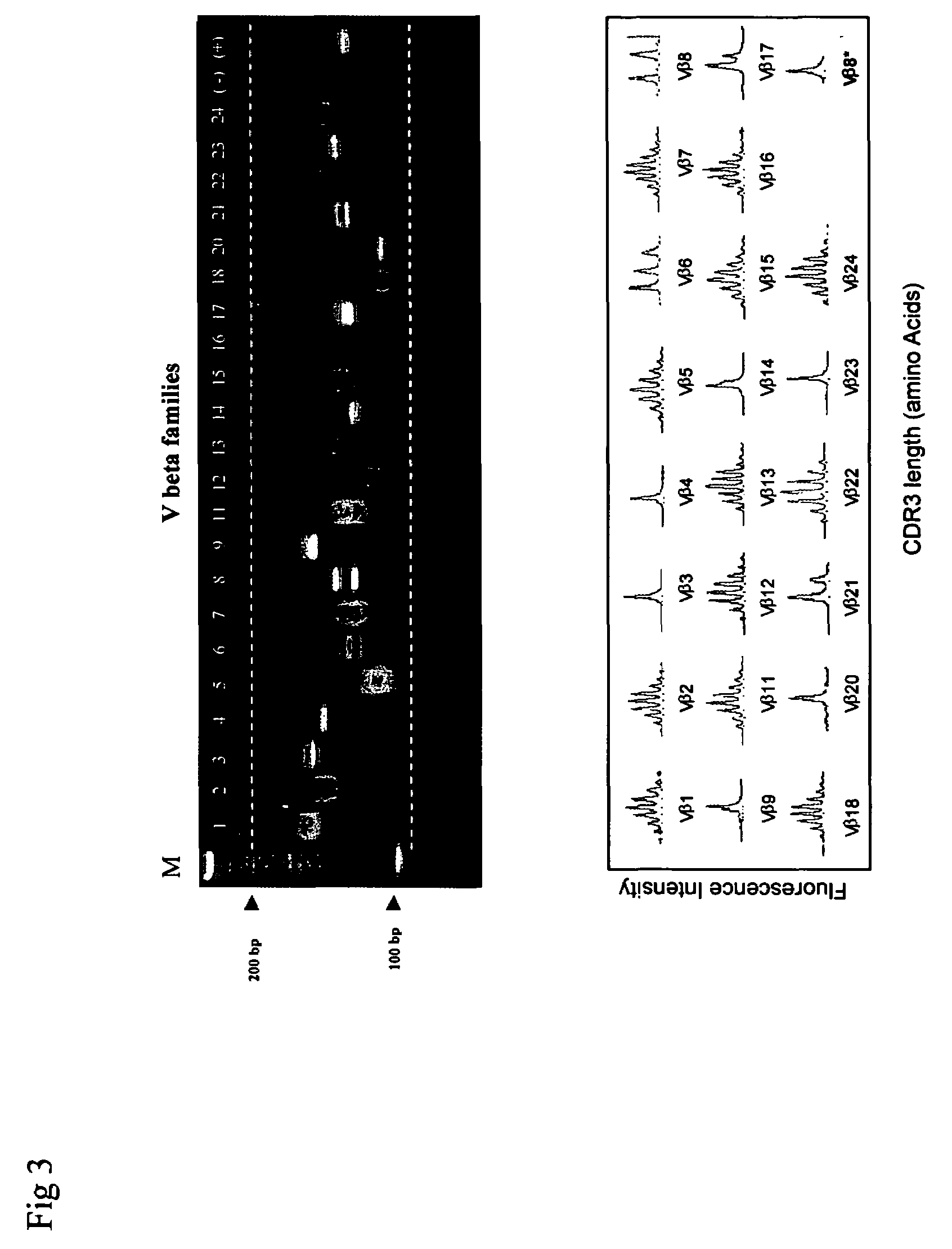
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS20070117134A1Enhances PCR reaction sensitivityRapid determinationAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a Th1 phenotype
InactiveUS20030092663A1Useful in treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsBiocideAntigen stimulationTherapeutic intent
Owner:RGT UNIV OF CALIFORNIA
Method for detection and quantification of T-cell receptor Vbeta repertoire
ActiveUS7375211B2Rapid determinationEasily detecting clonalityAnalysis using chemical indicatorsSugar derivativesVaccinationAntigen stimulation
The invention is a method for detecting and measuring T-cell receptor (TCR) repertoires from mammalian lymphocytes. The method is based on the use of the multiple sets of unique primers to amplify 22 regions of the TCR Vβ region and thereby detect clonal expansions related to antigen stimulation of the immune system. Kits containing sets of primers and specialized analytical statistical software for use in determining clonal expansion in humans and mice are disclosed. The reliability, efficiency and short assay time in using the method is well suited to monitoring immune response to vaccination and therapeutic treatments for immune disorders.
Owner:SHANGHAI CELLULAR BIOPHARMACEUTICAL GROUP LTD
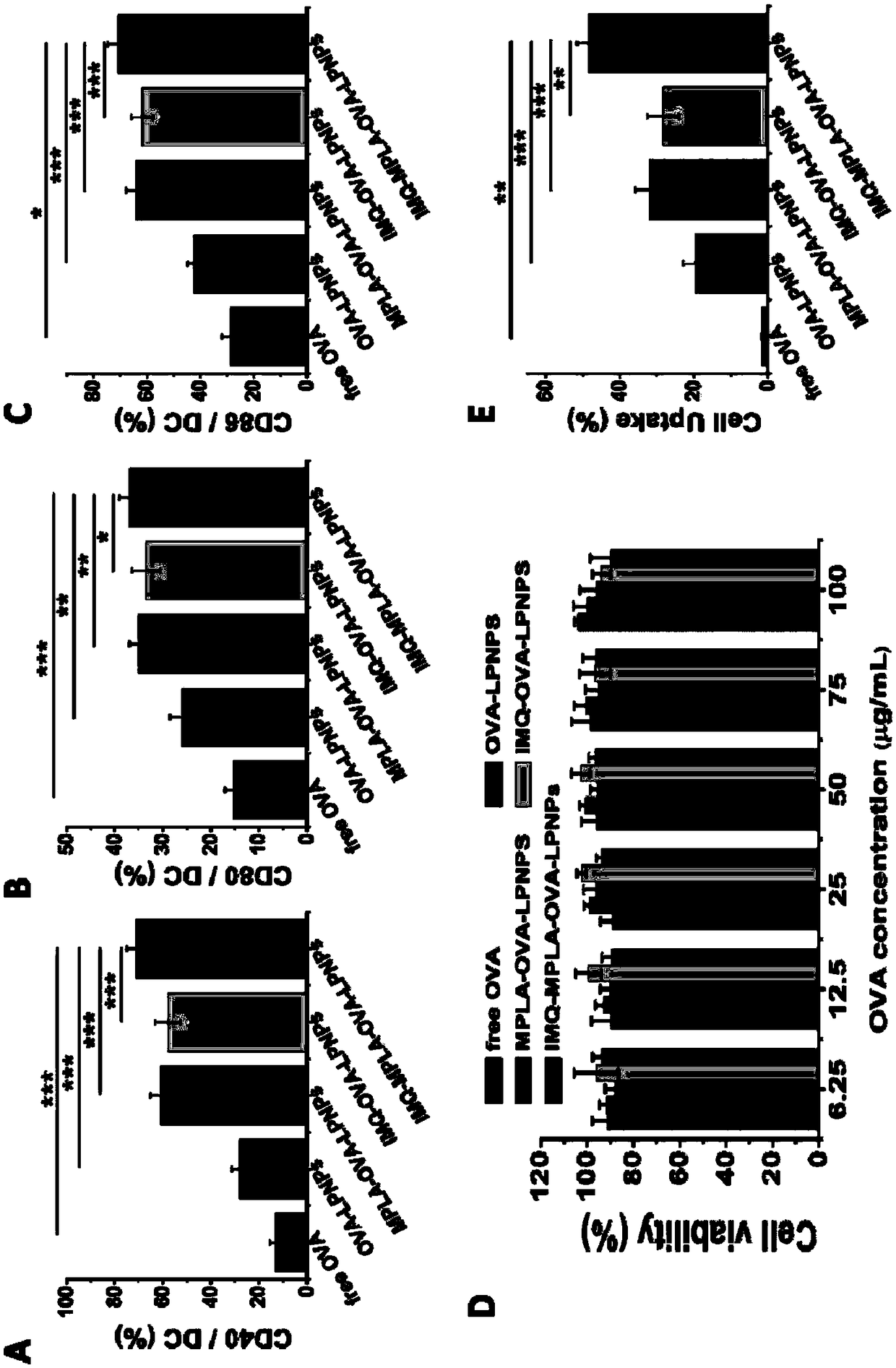
Antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and preparation method and application thereof
ActiveCN108992666AStrong activationRealize the loadCancer antigen ingredientsPharmaceutical non-active ingredientsDendritic cellPhospholipid
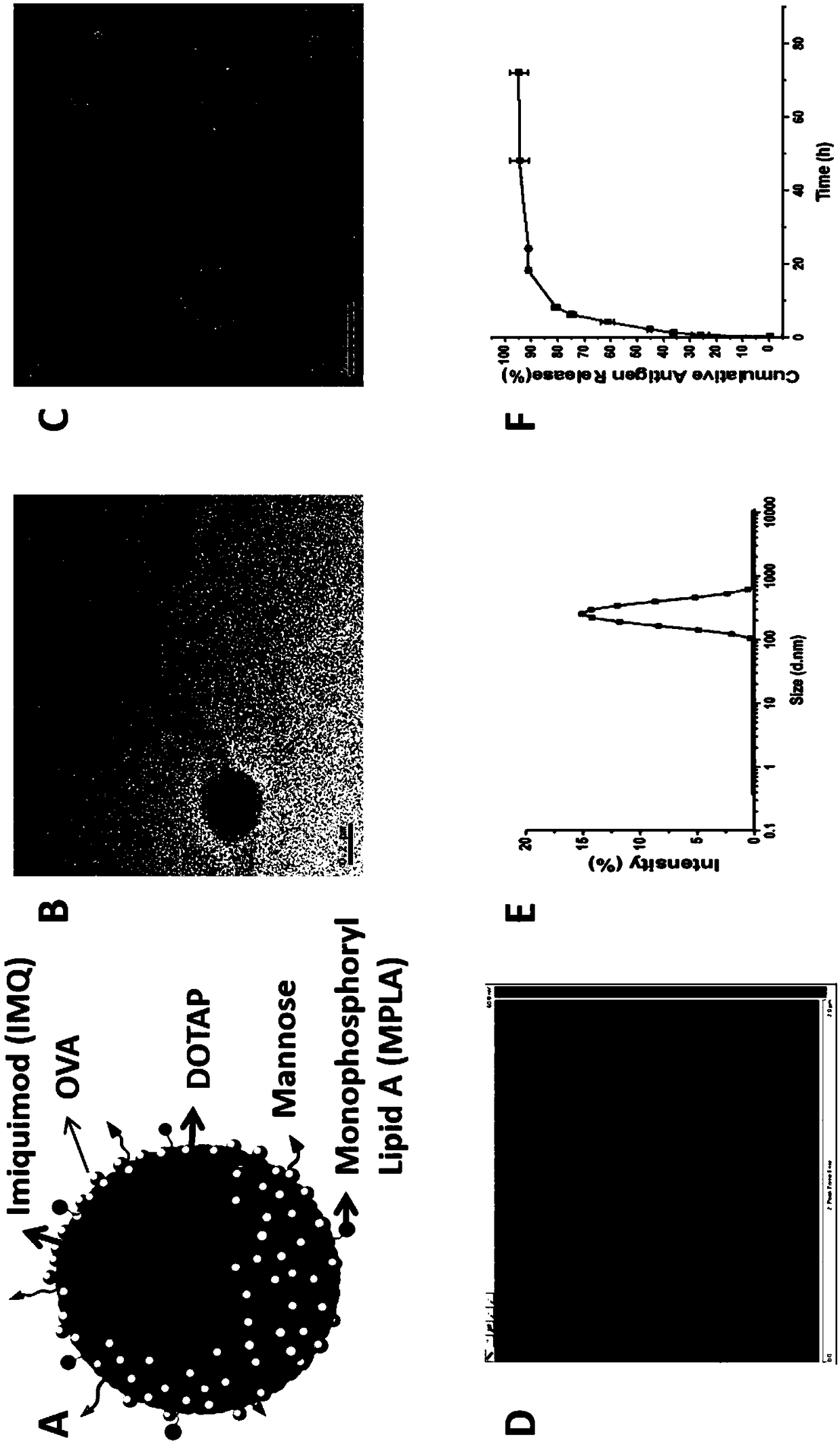
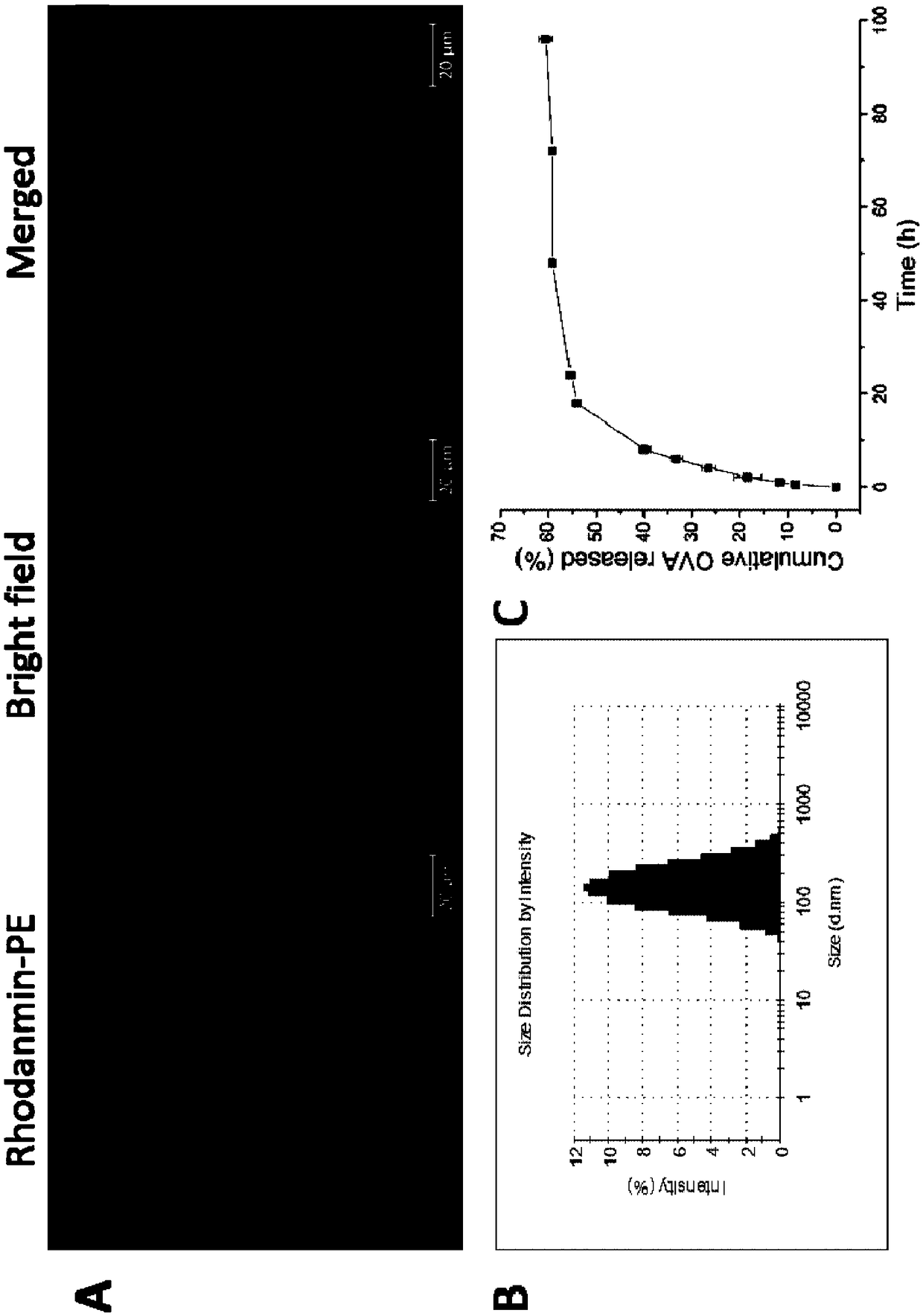
The invention relates to an antigen and TLR agonist targeting co-loaded cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant, and a preparation method and an application thereof. A hydrophobic inner core is encapsulated with a TLR7 agonist, a phospholipid layer is encapsulated with a TLR4 agonist, an antigen is adsorbed by cationic phospholipids in the phospholipid layer, and ligation of a mannose ligand makes the vaccine adjuvant like a specific targeting antigen to have the dendritic cell presenting ability, so the DC cell intake and the maturation promoting effect are enhanced; and the antigen is protected by hybridized nanoparticles, the antigen uptake by DC cells is improved, immune response after antigen stimulation is significantly enhanced by the TLR agonist, and thecross-presentation of the antigen is significantly improved, so vaccine adjuvant has a strong potent T cell killing effect, can induce cytokine secretion, has a long-acting memory T cell response, andhas an excellent prevention effect on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
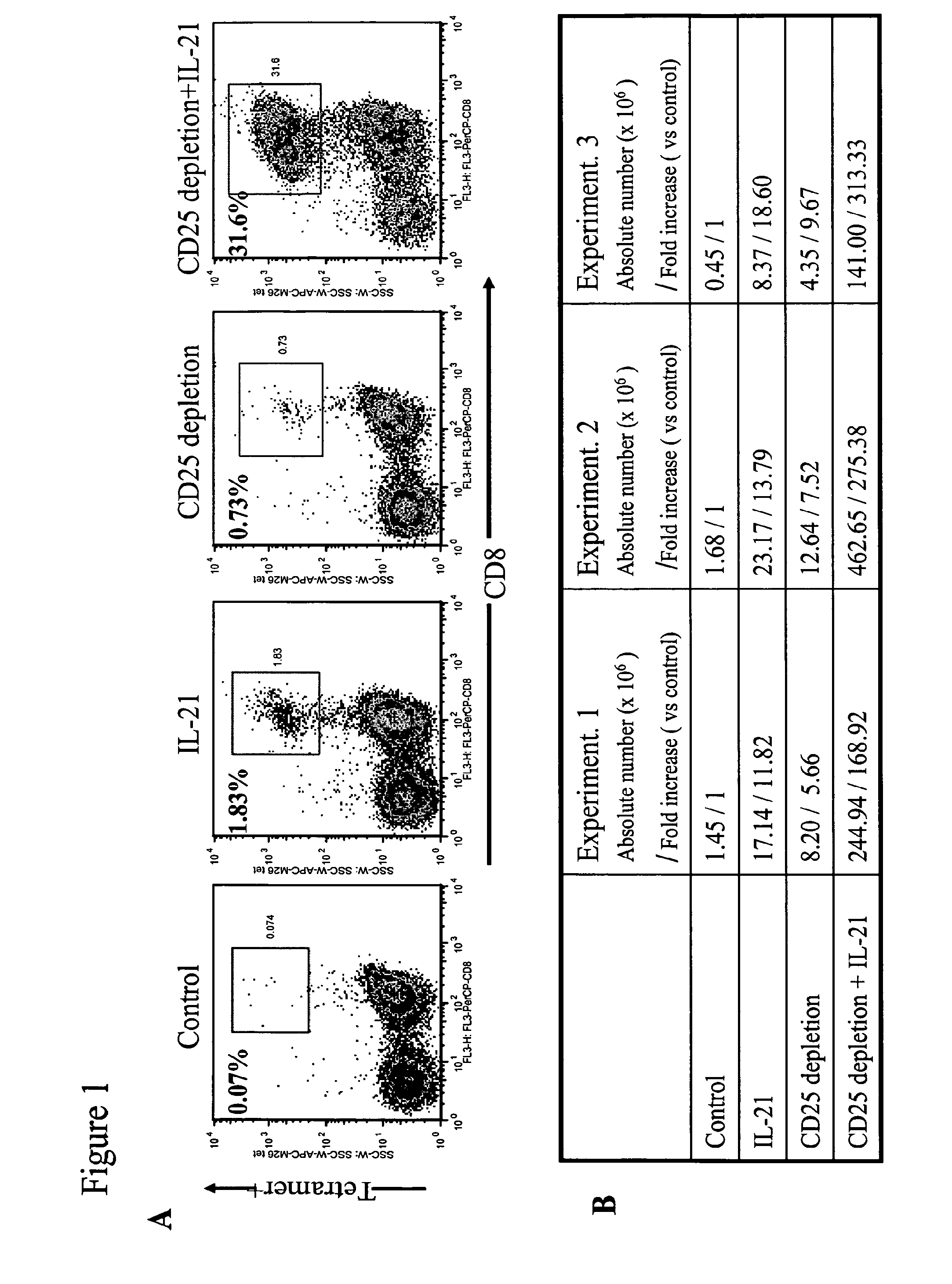
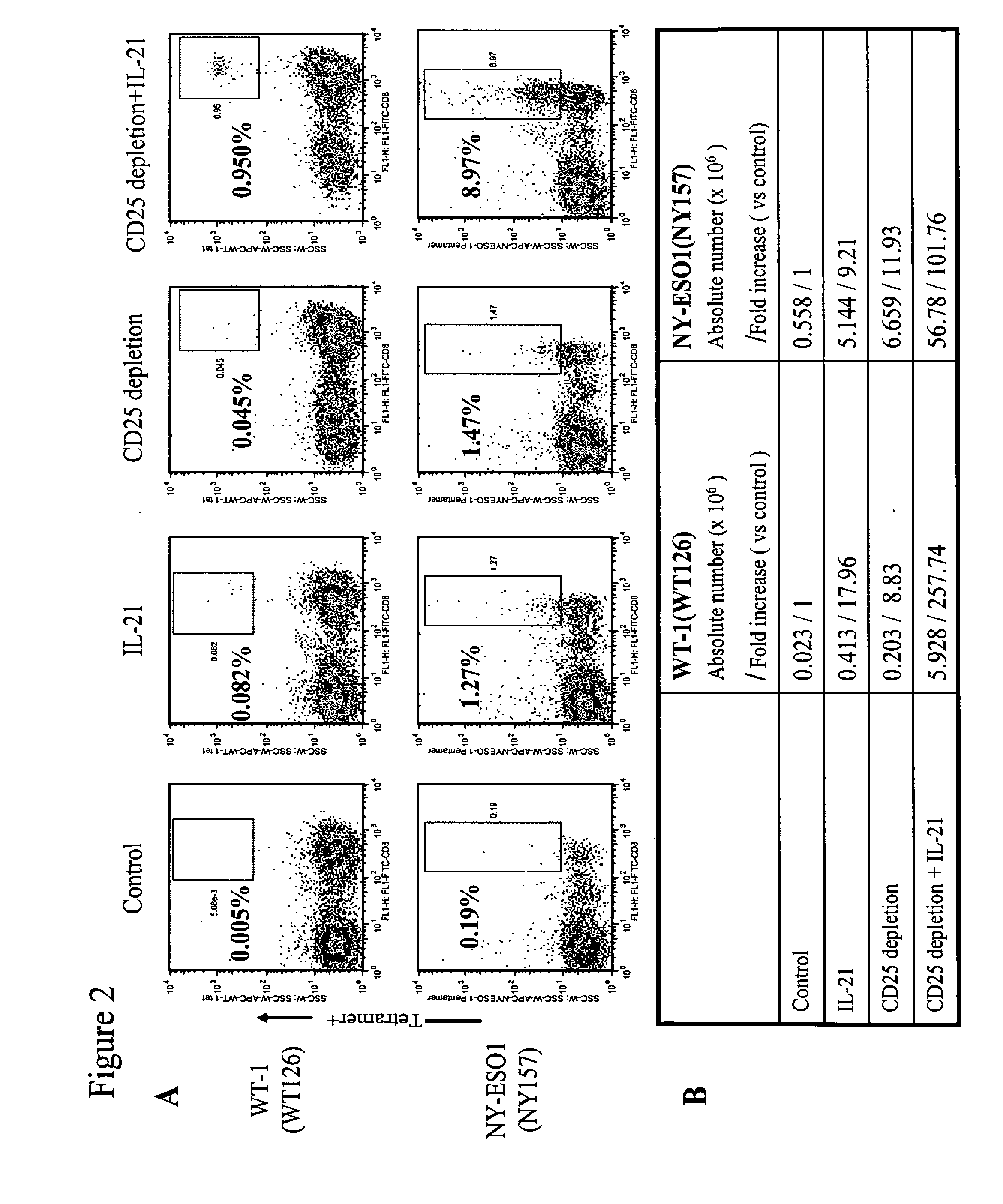
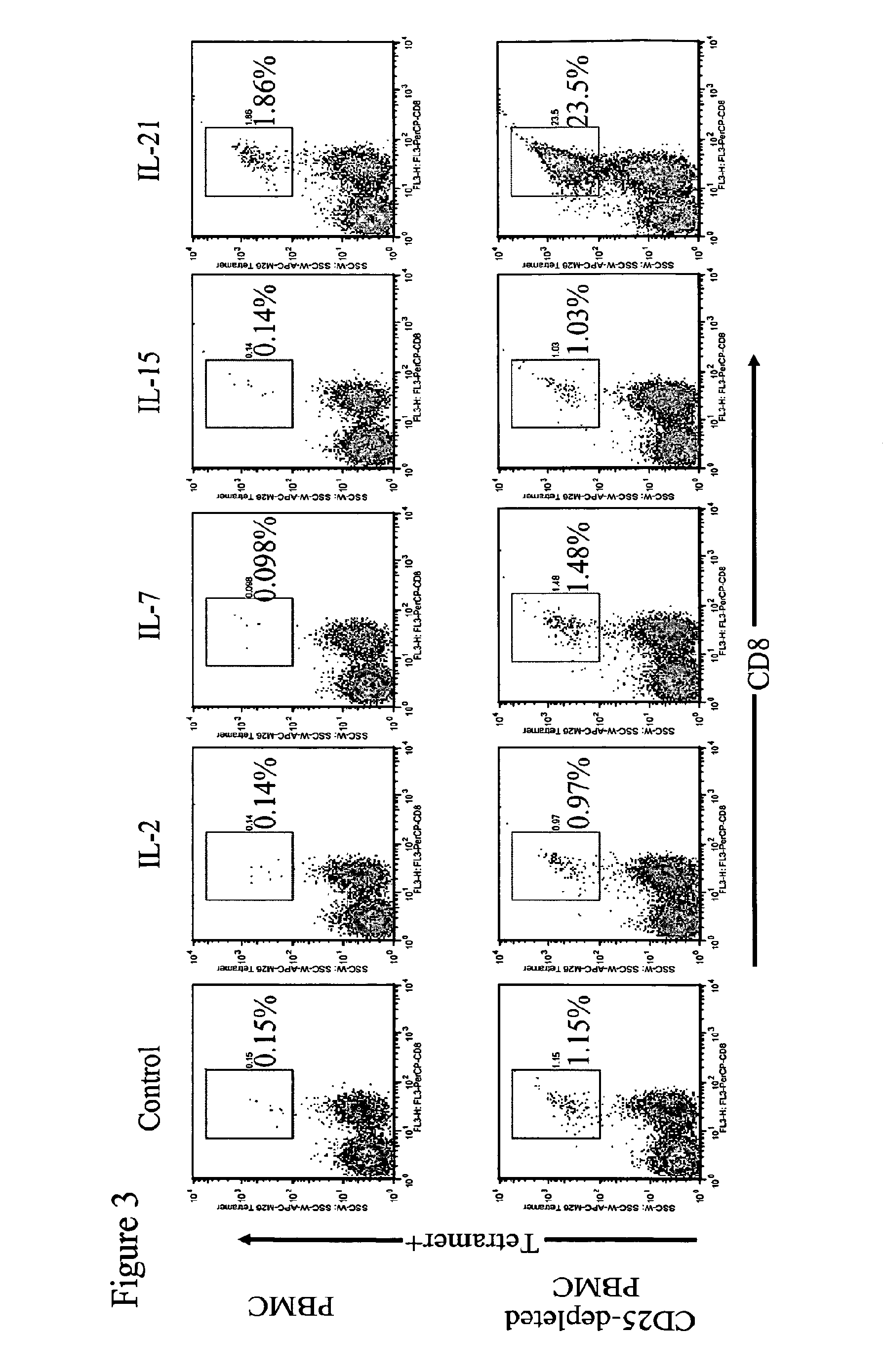
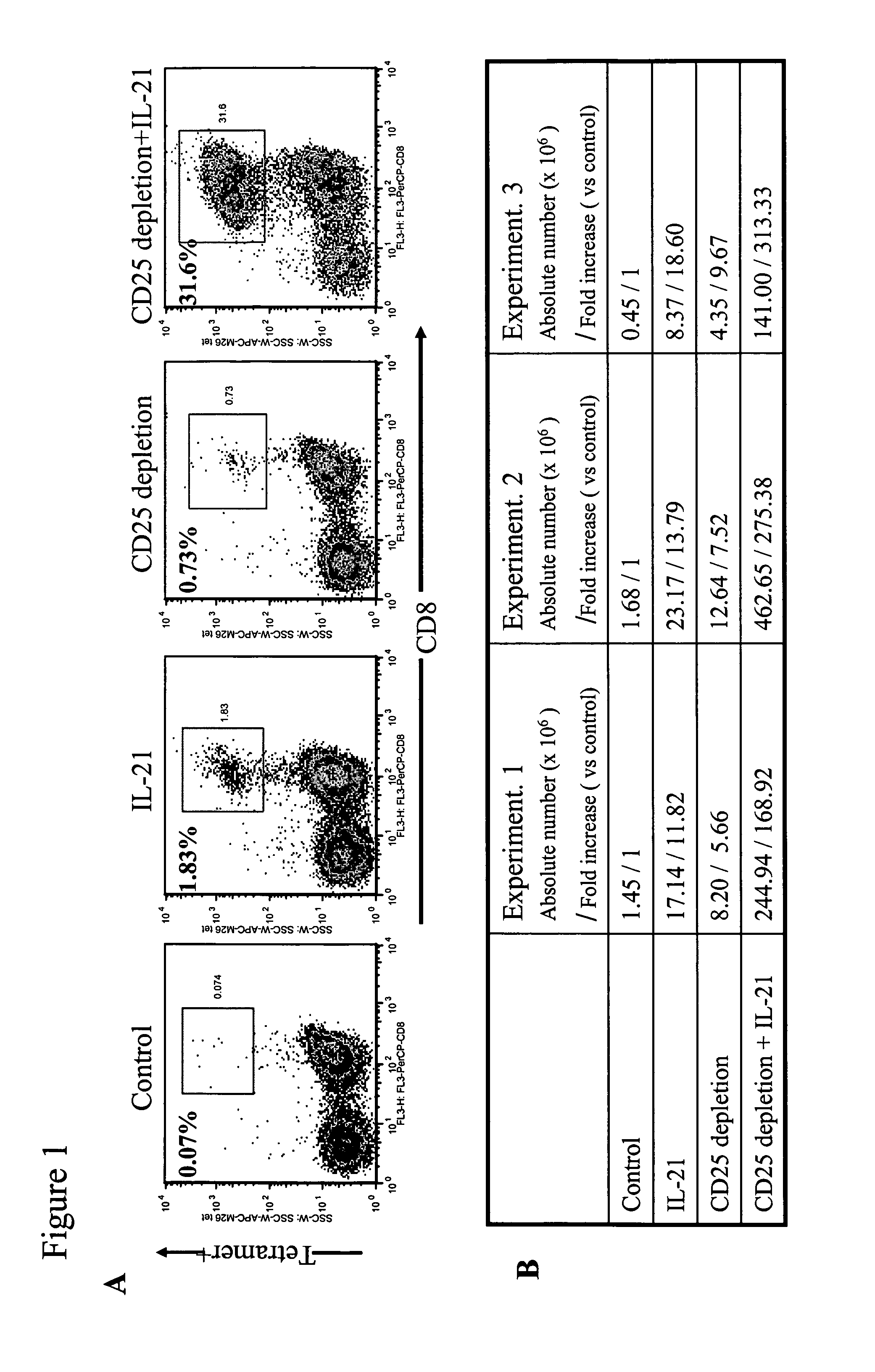
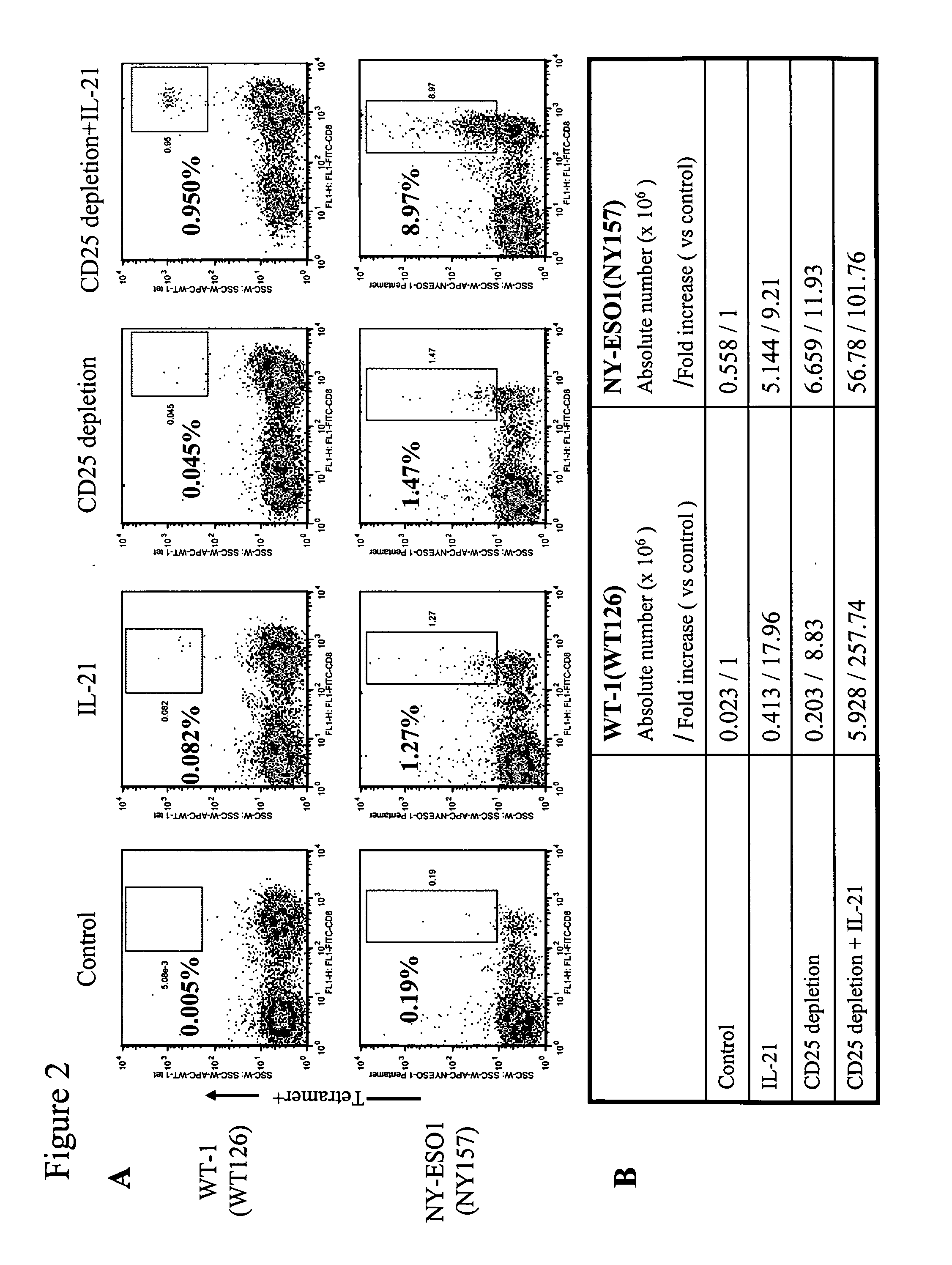
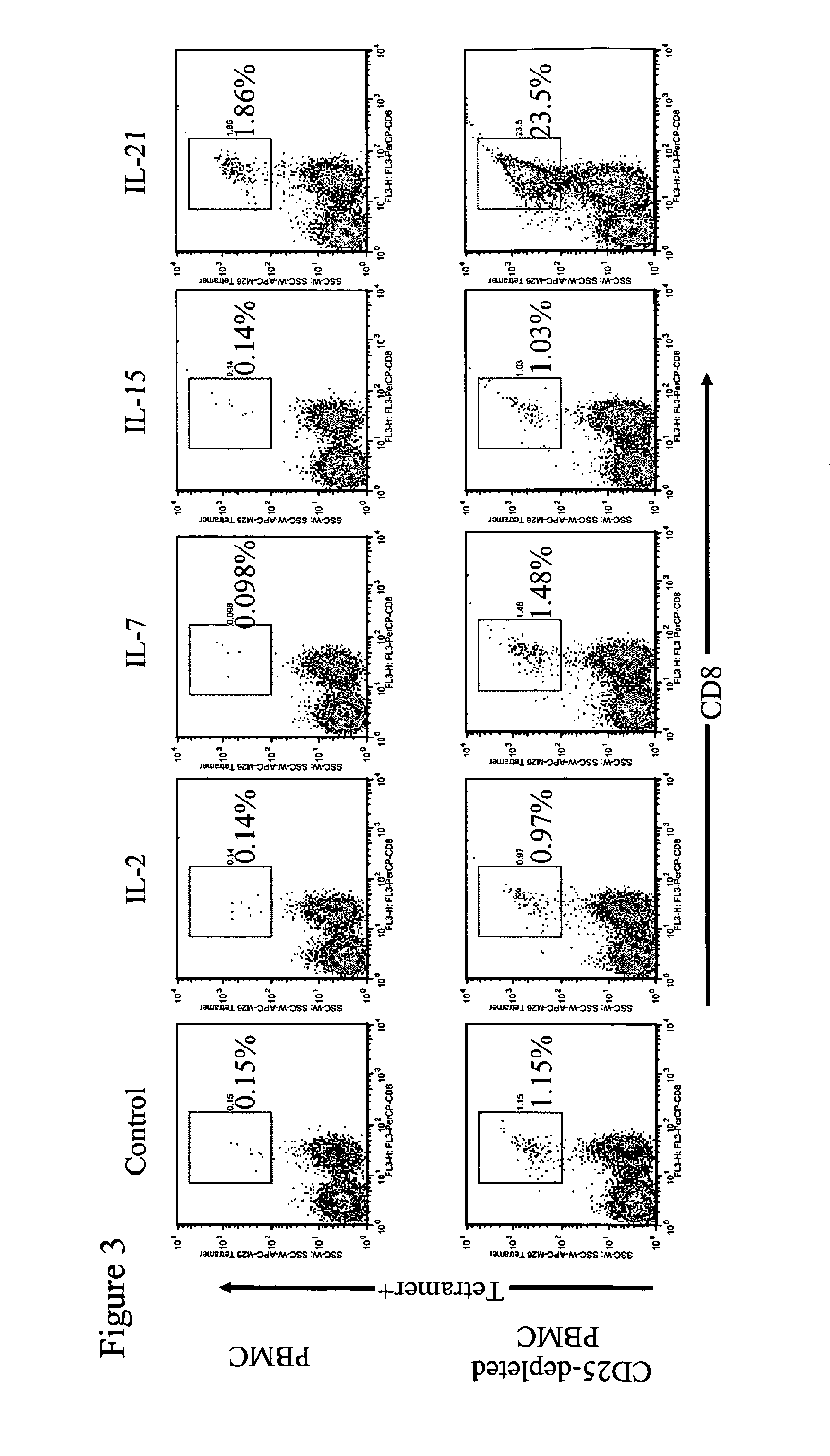
Enhanced generation of cytotoxic t lymphocytes by il-21 mediated foxp3 suppression
ActiveUS20160298081A1Mammal material medical ingredientsBlood/immune system cellsWhite blood cellAntigen stimulation
A method of carrying out adoptive immunotherapy by administering a subject an antigen-specific cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount is described. In the method, the CTL preparation is preferably administered as a preparation of an in vitro antigen-stimulated and expanded primate CTL population, the CTL population: (i) depleted of FoxP3+ T lymphocytes prior to antigen stimulation; (ii) antigen-stimulated in vitro in the presence of interleukin-21; or (iii) both depleted of FoxP3+ T lymphocytes prior to antigen stimulation and then antigen-stimulated in vitro in the presence of interleukin-21. Methods of preparing such compositions, and compositions useful for carrying out the adoptive immunotherapy, are also described.
Owner:FRED HUTCHINSON CANCER CENT
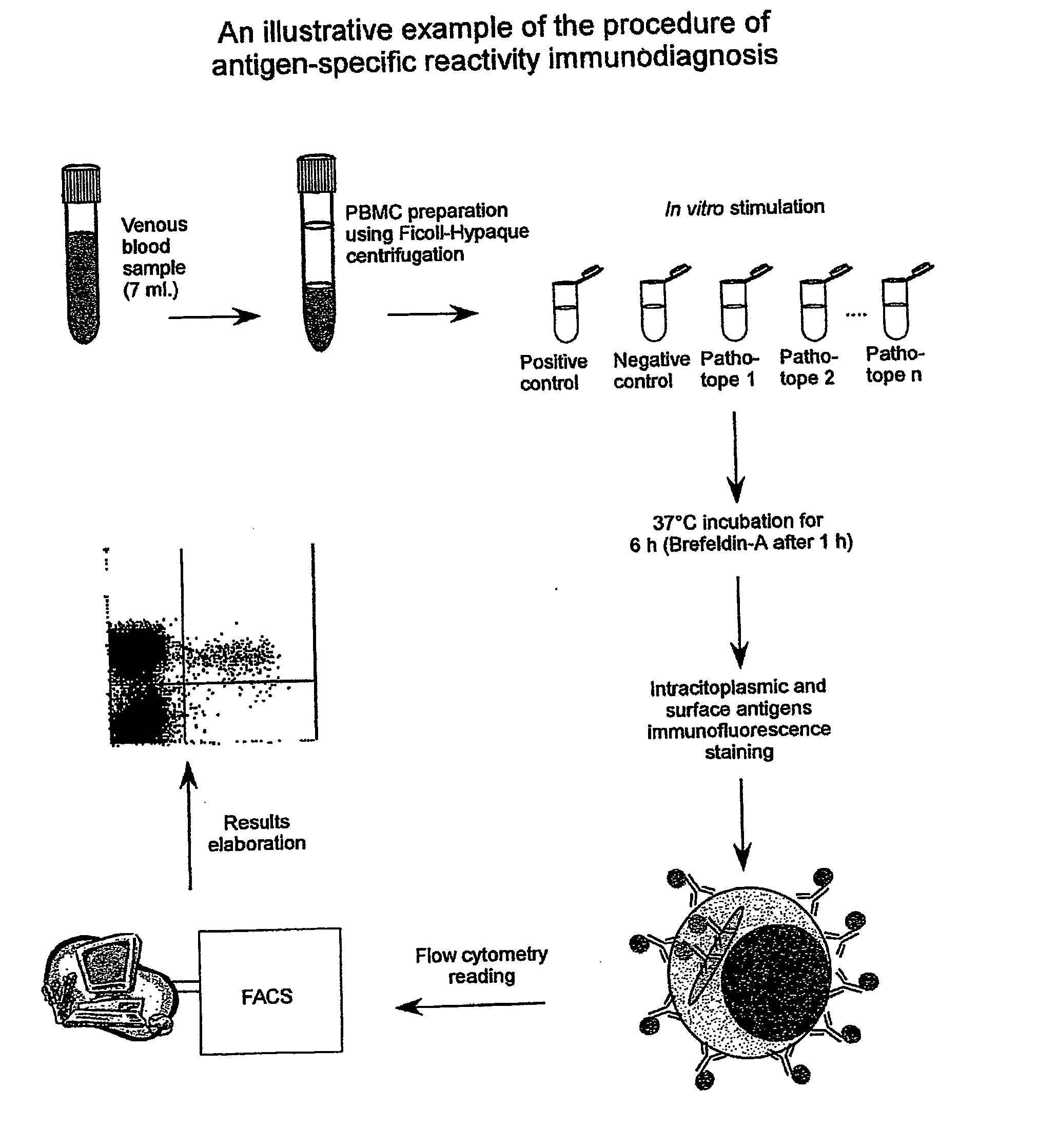
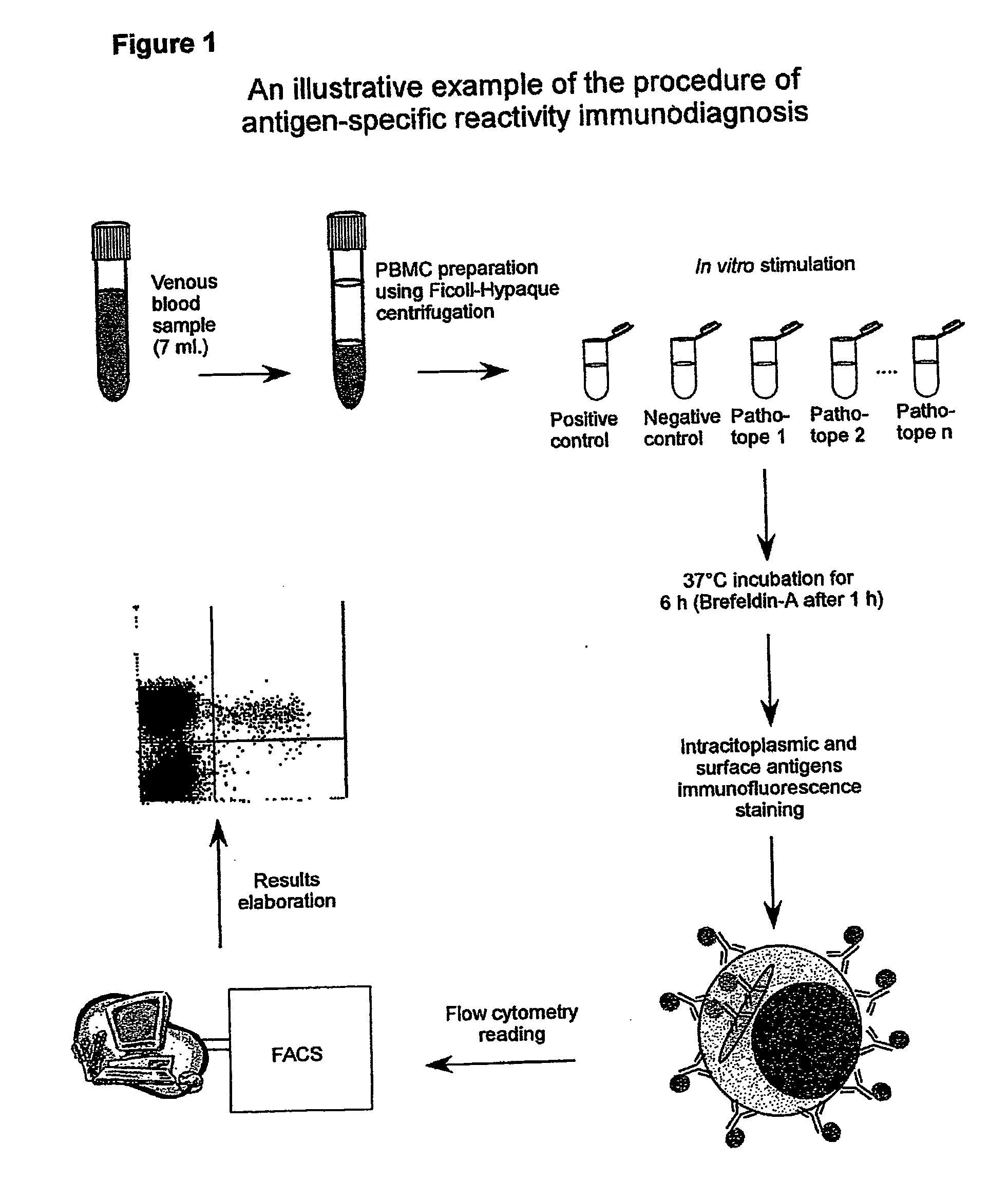
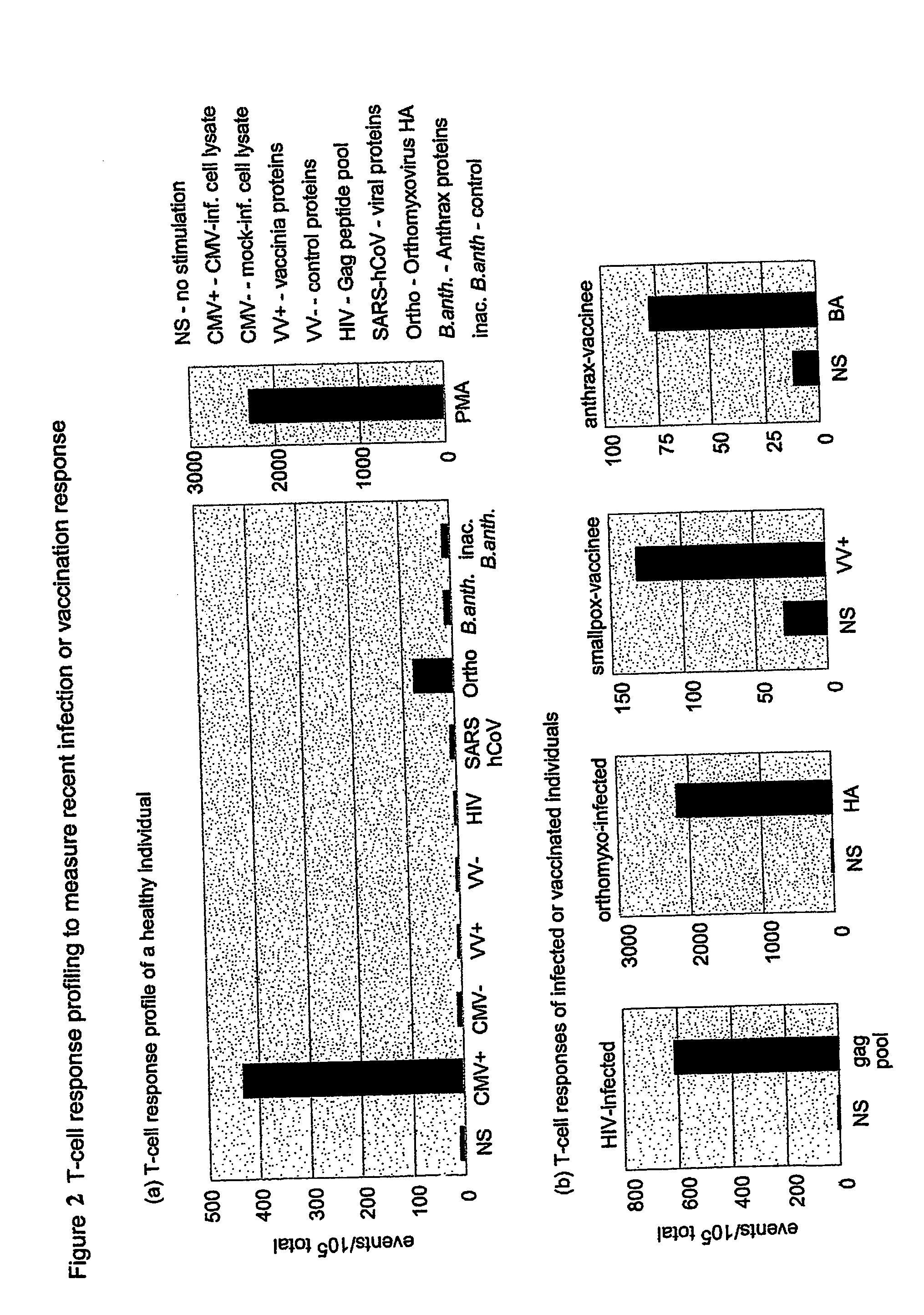
Method and diagnostic tests based on flow cytometric analysis of antigen-specific t lymphocytes
The present invention provides a method for the immuno-diagnosis of diseases with different aetiology (infectious diseases, tumors etc) by measurement of the T cell response J, B and NK lymphocytes) induced by a set of diseasespecific antigens. The method is based on the quantitative determination of antigenspecific T lymphocytes (referred as Ag-Sp), stimulated by using a newly devised pathology-specific antigen or epitope compositions which represent further embodiments of the invention. After stimulation, the selective measurement of the Ag-Sp T lymphocytes is performed by: A) monoclonal antibodies recognizing membrane structures of T lymphocytes and of their sub-populations; B) monoclonal antibodies binding to cytokines accumulating at intracellular level after the stimulation with the antigen; or C) mixtures of A) and B). The flow cytometric detection of the presence of markers of differentiation on T lymphocytes and of intracytoplasmic cytokines allows the acquisition of both qualitative and quantitative results. The invention also provides diagnostic kits for performing the method of the invention.
Owner:INST NAT PER LE MALATTIE INFETTIVE LAZZARO SPALLANZANI IRCCS
Preparation method for dendritic cell of umbilical cord blood source and dendritic cell vaccine
ActiveCN102676455ABlood/immune system cellsAntibody medical ingredientsHematopoietic cellCell culture media
The invention discloses a preparation method for the dendritic cell (DC) of an umbilical cord blood source and a dendritic cell (DC) vaccine, which relates to a preparation method for the dendritic cell. According to the method, various cell factors are adopted to induce DC obtained by umbilical cord blood separation, and then the DC is stimulated by a tumor specific antigen so as to improve the specific antigen presentation capability of the DC; and a stem cell growth factor and Flt3-L are added into a cell culture medium so as to effectively accelerate a hematopoietic cell in the umbilical cord blood to induce and proliferate to an immune cell. The DC vaccine prepared with the method has the specific antigen presentation capability, can be combined with a CIK (cytokine induced killer) cell to mutually treat the malignant tumor when being used as a tumor immunotherapy product, and is used as an important adjuvant therapy after operations and chemoradiotherapy. Recurrence and metastasis after the operations can be effectively prevented, and toxic and side effects caused by the chemoradiotherapy on patients are lowered so as to improve the treatment effect.
Owner:北京和泽普瑞生物科技有限公司 +1
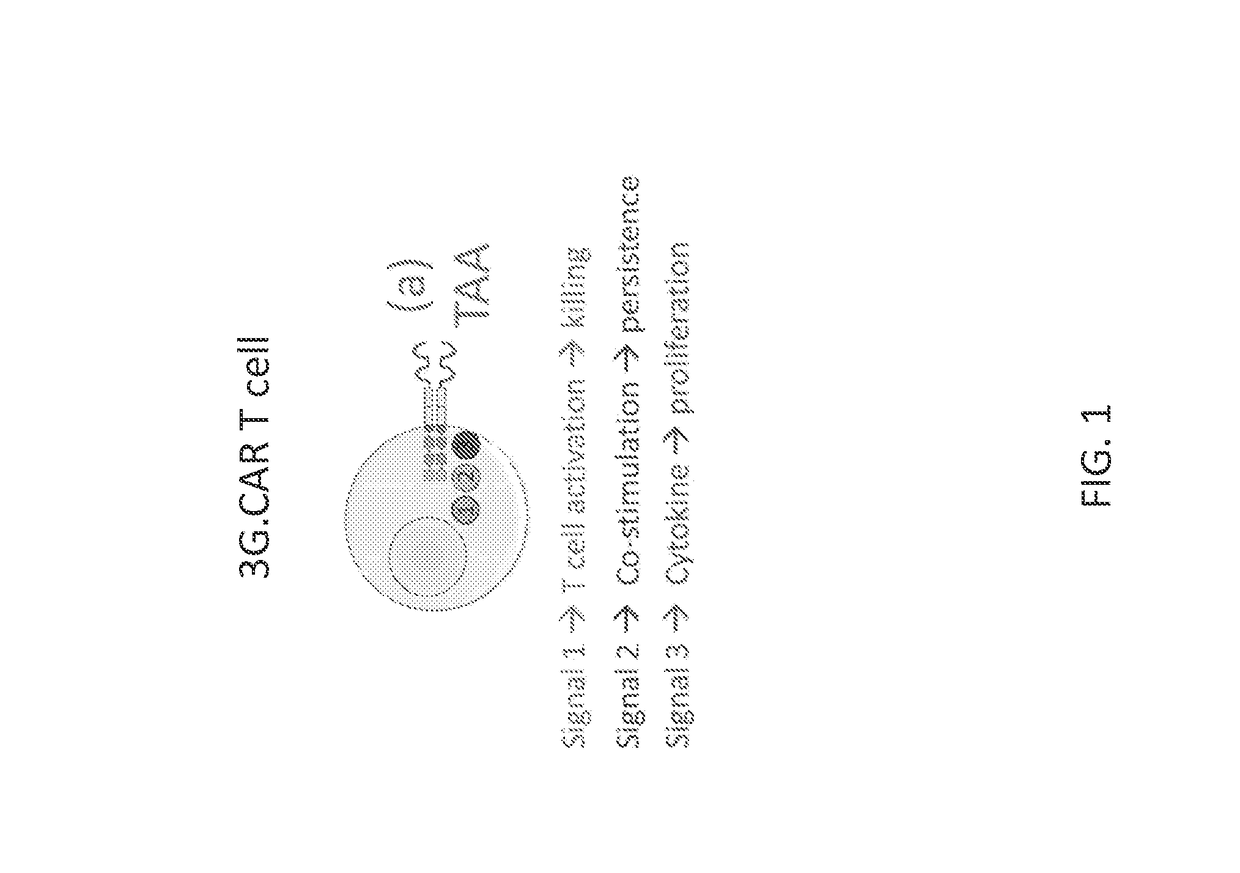
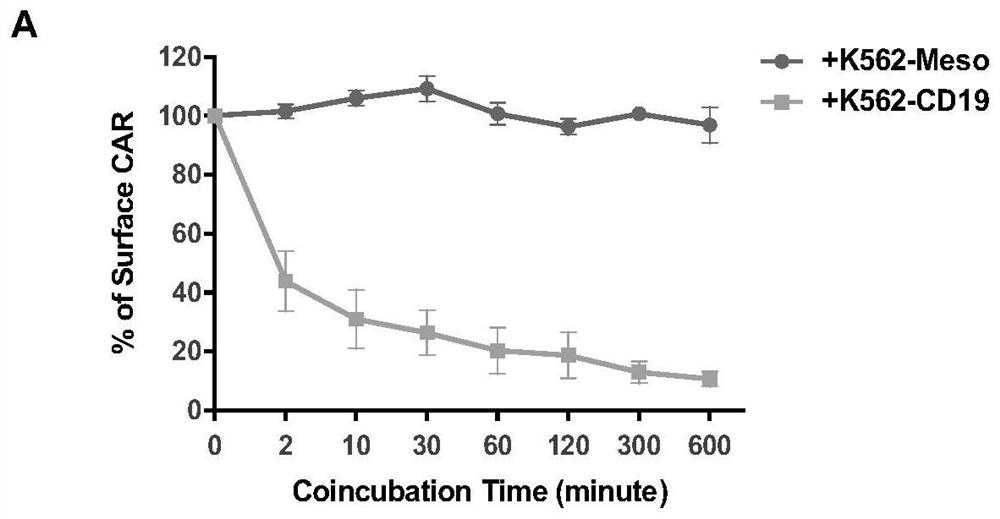
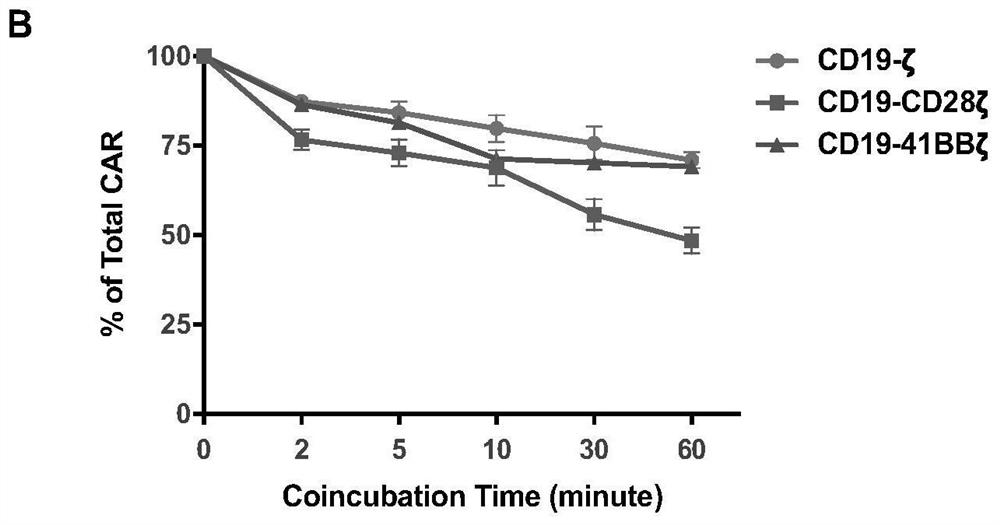
Bipartite and tripartite signaling immune cells
InactiveUS20170246278A1Polypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen stimulationTumor microenvironment
Embodiments of the disclosure include compositions and methods effective for immunotherapy, such as for cancer. The embodiments include cells that recognize a combination of two signals or three signals present at the tumor microenvironment. In certain embodiments, the signals for antigen stimulation, co-stimulation, and cytokine signaling act through separate molecules, although in certain embodiments the signals for antigen stimulation and co-stimulation are transmitted through the same molecule.
Owner:BAYLOR COLLEGE OF MEDICINE
DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating antigen and dual immunoagonists and preparation method and application thereof
ActiveCN108938568AMaximize Targeting EffectAchieving ImmunotherapyCancer antigen ingredientsPharmaceutical non-active ingredientsT lymphocyteBiological activation
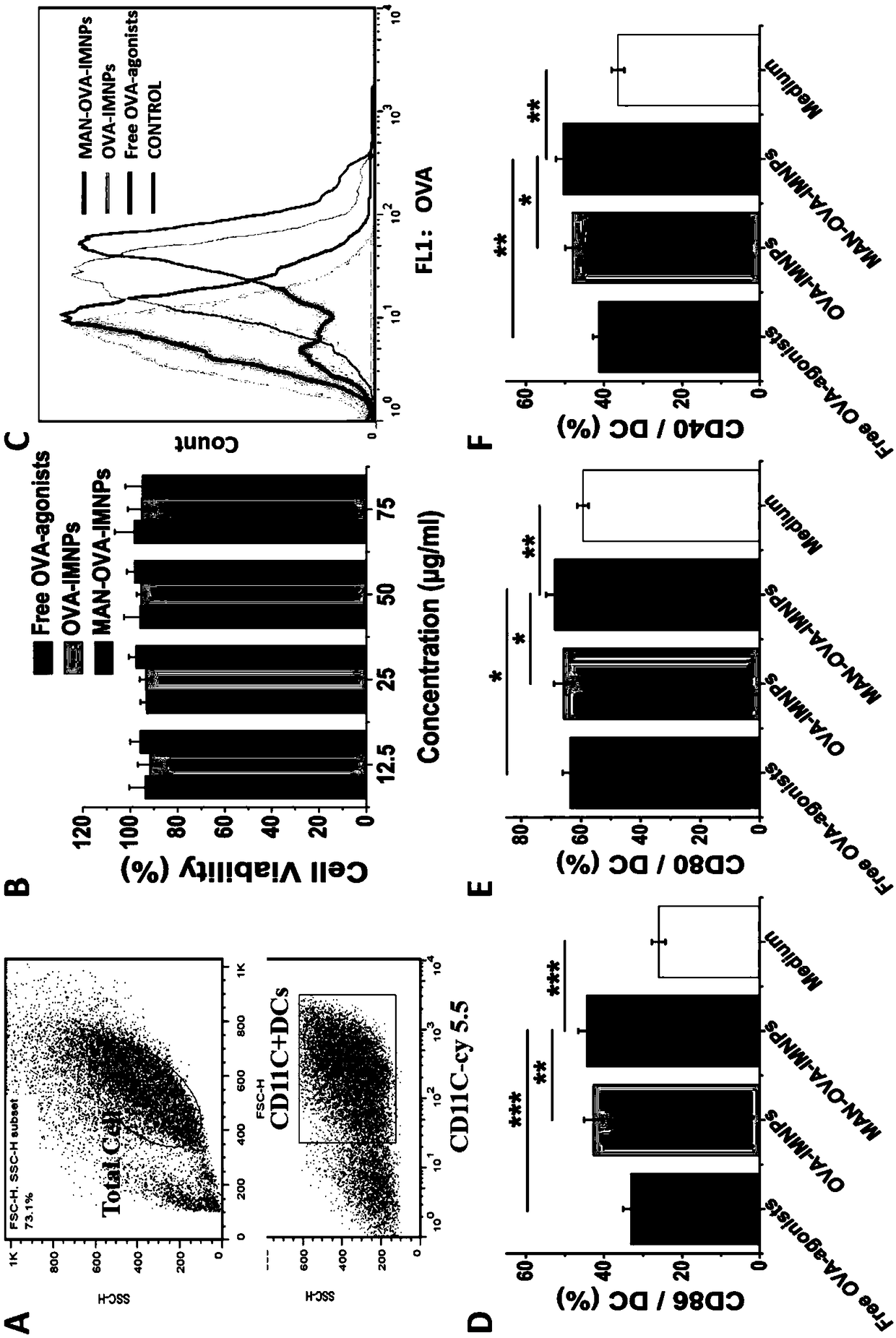
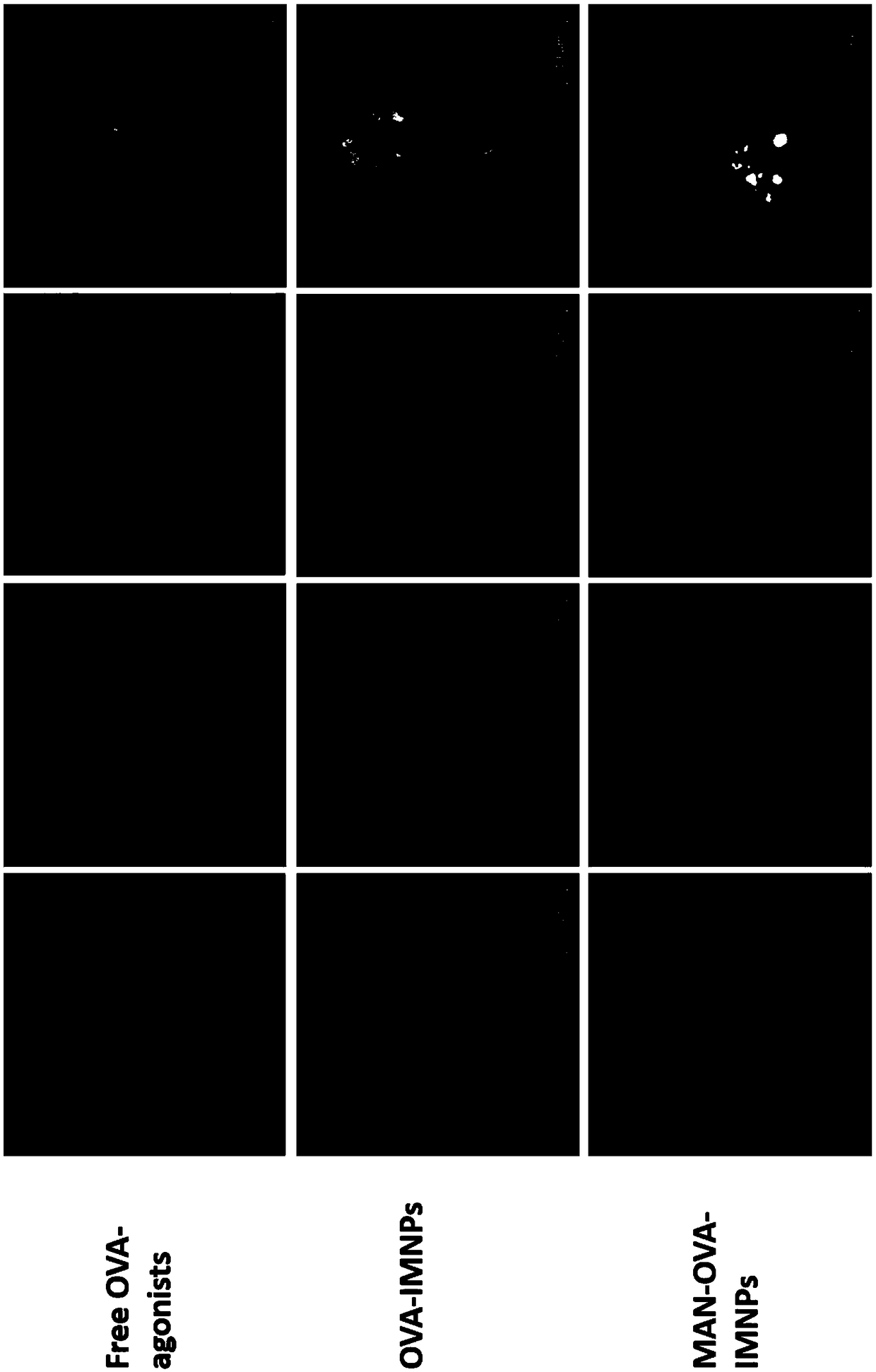
The invention relates to a DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating an antigen and dual immunoagonists, a preparation method and application thereof. The phospholipidhybrid polymersome which can jointly load a model antigen OVA and two types of TLR agonists (TLR7 / 8 and TLR4) is used for stimulation in vitro of the DCs so as to realize the effective phagocytosis of DCs cells. The rapid and long-term immunostimulatory effect on the DCs is achieved by the internal and external co-loading of the OVA antigen. The synergistic effect of the two types of TLR agonistssignificantly enhances the immune response after antigen stimulation; the phospholipid hybrid polymersome which jointly encapsulates the antigen and the dual immunoagonists can effectively promote the activation and maturation of the DCs, increases the level of cross-presentation, promotes the migration of the DC vaccine to secondary lymphoid organs, and produces a strong specific cytotoxic T lymphocytes (CTLs) killing effect, thereby effectively killing tumor cells and realizing the immunotherapy of the DCs vaccine on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
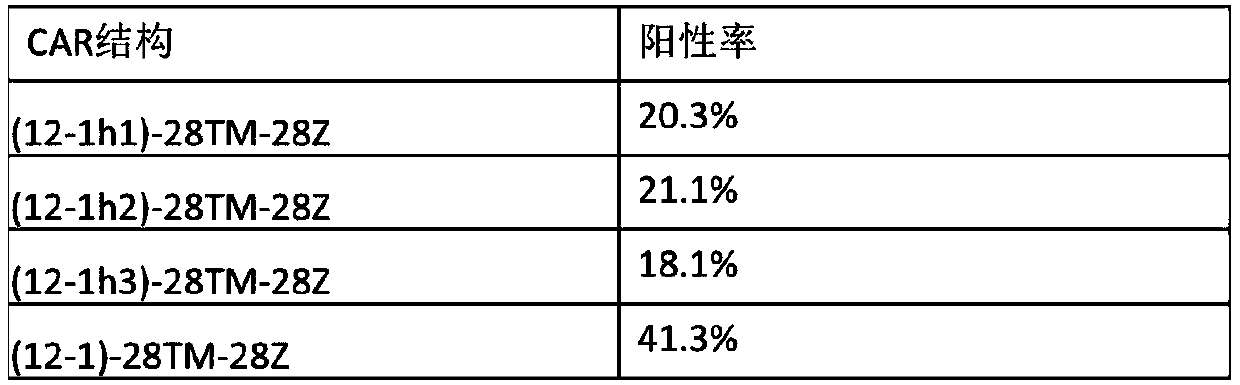
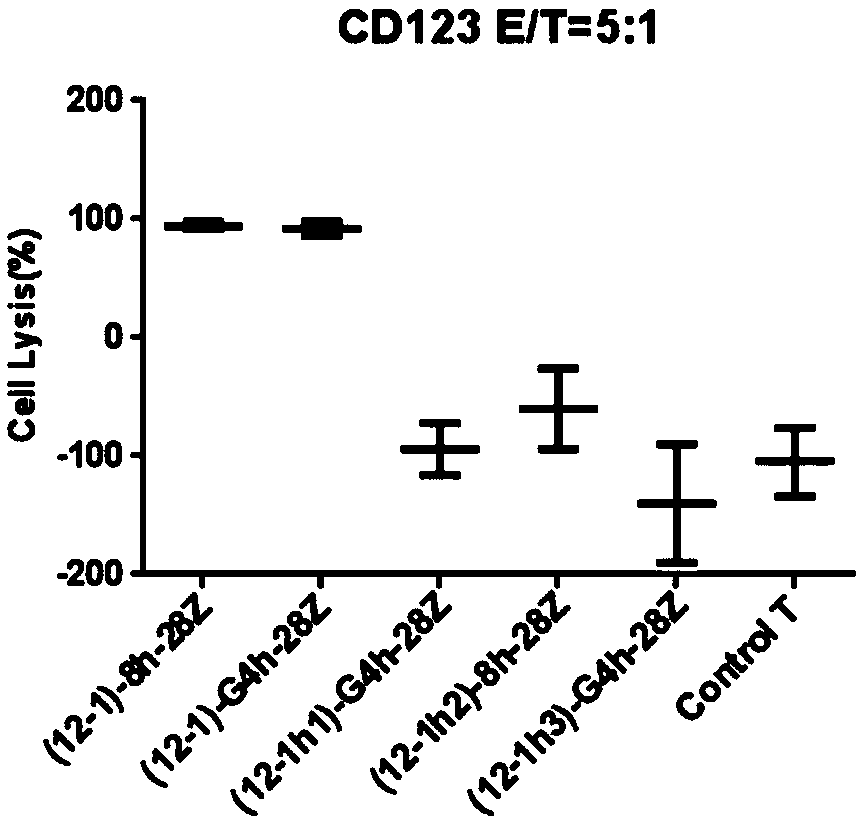
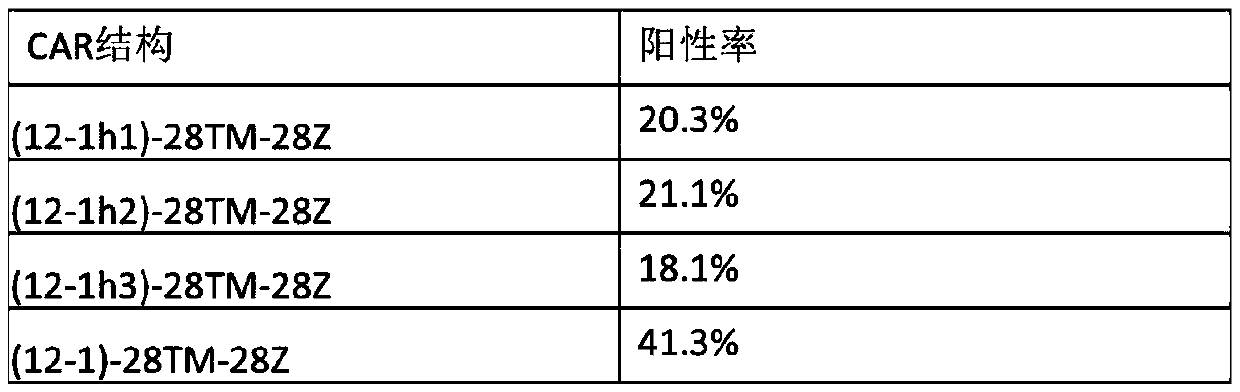
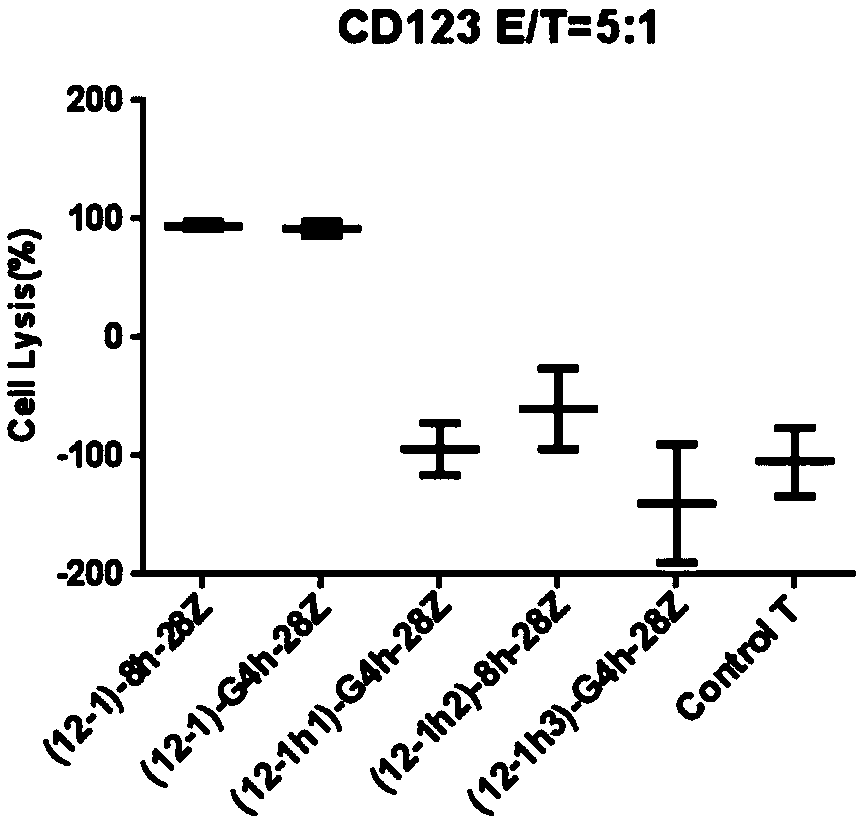
Chimeric antigen receptor of anti-human CD123 and application of chimeric antigen receptor
ActiveCN110950965AStrong specificityHigh degree of humanizationAntibody mimetics/scaffoldsMammal material medical ingredientsTumor targetAntigen receptors
The invention belongs to the field of gene engineering, and particularly relates to an anti-human CD123 chimeric antigen receptor and an application thereof. The polypeptide for recognizing a CD123 antigen comprises amino acid sequences as shown in SEQ ID NO: 4 to 7, and the polypeptide serving as an antigen recognition region in a (chimeric antigen receptor, CAR) structure can be used for inducing activation of CAR-T cells. After the chimeric antigen receptor of the anti-CD123 antigen is expressed in immune cells, tumor target cells expressing the CD123 antigen can be effectively removed, andthe chimeric antigen receptor has no toxic effect on tumor cells of a negative antigen (not expressing CD123). Moreover, the positive rate of the CD123-targeting CAR in the cell culture process of apatient can be maintained, and the CD123-targeting CAR can be proliferated for a long time after being stimulated by a target antigen and can be used for targeting treatment of tumors.
Owner:CHONGQING PRECISION BIOTECH CO LTD
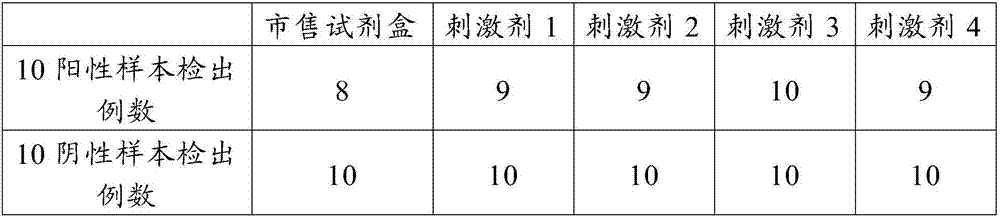
MTB (Mycobacterium Tuberculosis) infection diagnosis kit
ActiveCN105954521AInfection diagnosisStrong specificityBiological material analysisBiological testingBiotin-streptavidin complexInsulin-like growth factor
The invention belongs to the field of biomedicine examination, and relates to an MTB (Mycobacterium Tuberculosis) infection diagnosis kit. The MTB infection diagnosis kit provided by the invention is prepared from the following components: an antigen stimulant, a pre-coated ELISPOT (Enzyme-Linked Immunospot Assay) plate of a capture antibody, an insulin-like growth factor, a detection antibody, HRP (Horse Radish Peroxidase)-labeled streptavidin, a 3-amino-9-ethyl carbazole developing solution, antibody diluent and a positive control stimulant; the antigen stimulant is selected from one or multiple polypeptides in sequences as shown in SEQ ID NO.1 to 12. The MTB infection diagnosis kit provided by the invention is high in sensitivity and specificity and stable in property; meanwhile, when the MTB infection diagnosis kit provided by the invention is applied to in-vitro detection on MTB, the detection time can be saved, the detection steps can be simplified, and the detection efficiency can be increased.
Owner:WUHAN DANGKANG XING ZHONG BIOTECHNOLOGY CO LTD
Antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection, and application thereof
ActiveCN107011418AQuick checkSpecific detectionBiological material analysisDepsipeptidesPeripheral blood mononuclear cellMycobacterium Infections
The invention discloses an antigenic polypeptide pool capable of detecting mycobacterium tuberculosis infection. The antigenic polypeptide pool specifically stimulates mycobacterium tuberculosis infected fresh whole blood to specifically secrete IFN-gama and increase the sensitivity. The invention provides a new detection reagent for detecting mycobacterium tuberculosis infection, experiments prove that peripheral blood can be directly utilized to conduct antigenic simulation without separation of peripheral blood mononuclear cell, the experimental data shows that the antigenic polypeptide pool has high sensitivity and specificity for detecting mycobacterium tuberculosis infection, and is simple and convenient to operate and low in cost, thus having high clinical application values.
Owner:武汉海吉力生物科技有限公司
Methods for inducing systemic immune responses to cancer
InactiveUS20140205609A1Enhance all ongoing immune responseLow in DOCancer antigen ingredientsWhole bodyAntigen stimulation
Pharmaceutical compositions comprising molecules produced by antigen-stimulated peripheral blood mononuclear cells (PBMCs) that induce systemic immune responses to cancers are encompassed herein, as are methods for making and administering such molecules and pharmaceutical compositions comprising same. Also encompassed herein are mixtures of molecules produced by antigen-stimulated PBMCs and compositions thereof for use in treating cancer. Methods for stimulating a systemic immune response in a subject afflicted with a cancer are also envisioned, whereby molecules produced by antigen-stimulated PBMCs or supernatants comprising same are administered to the subject, wherein the molecules stimulate an immune response to the cancer in the subject.
Owner:NEW YORK UNIV
Chimeric antigen receptor carrying truncated or non-truncated myeloid cell triggered receptor signal structure
ActiveCN108752482AEnsure the safety of clinical applicationHigh clinical safetyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyAntigen receptorAntigen stimulation
Owner:NANJING CART MEDICAL TECH LTD
Method for simultaneously detecting neoantigen immunogenicity and neoantigen specificity TCR (T cell receptor)
PendingCN111909992AImprove efficiencyWide applicabilityMicrobiological testing/measurementBiological testingPeripheral blood mononuclear cellAntigen stimulation
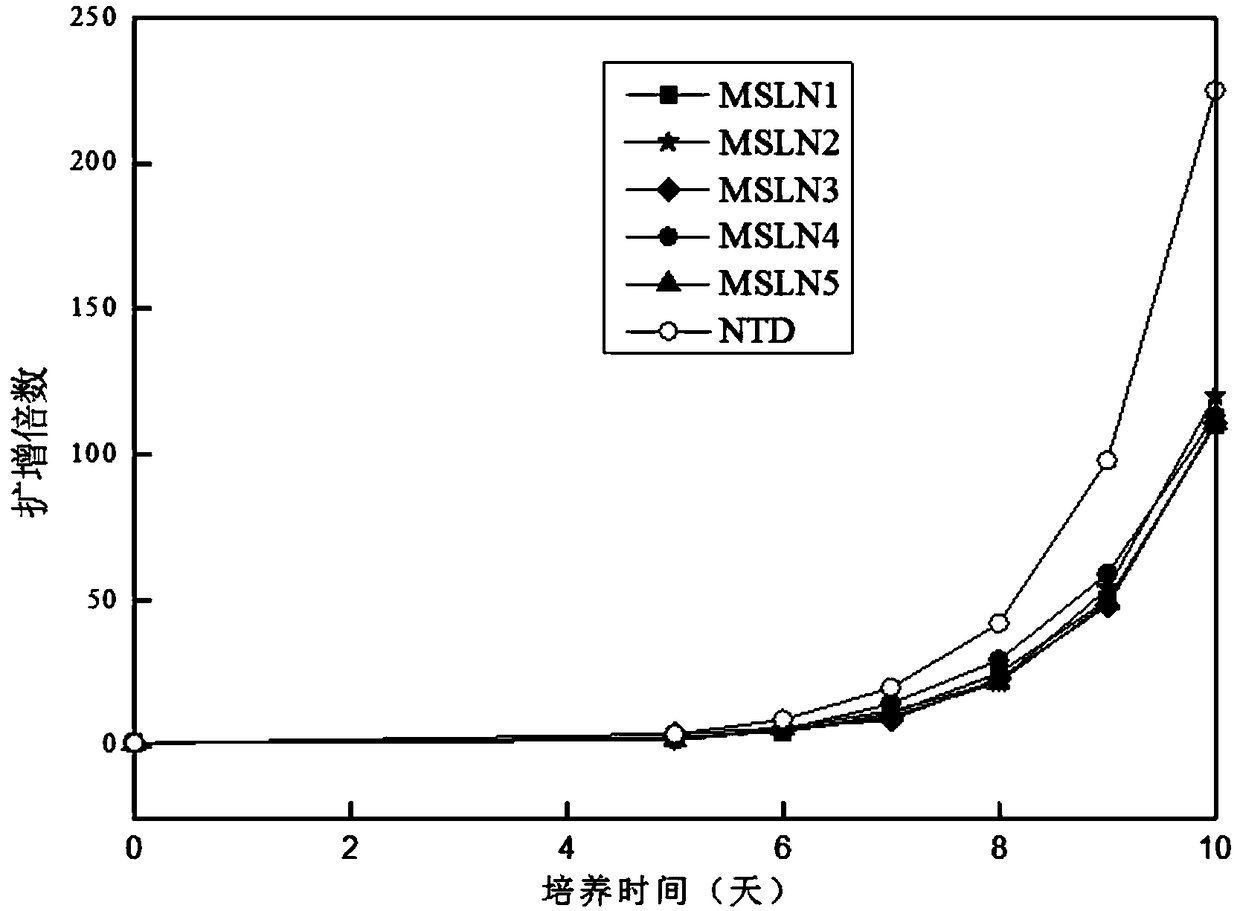
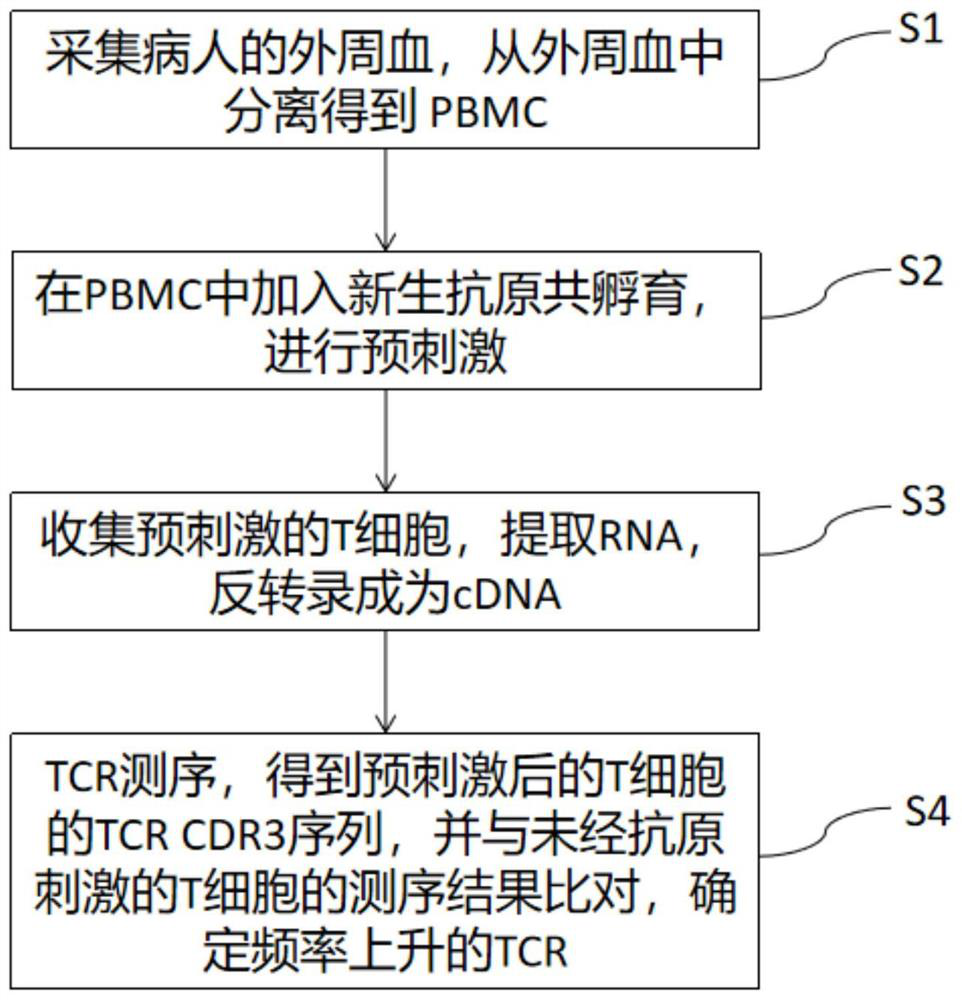
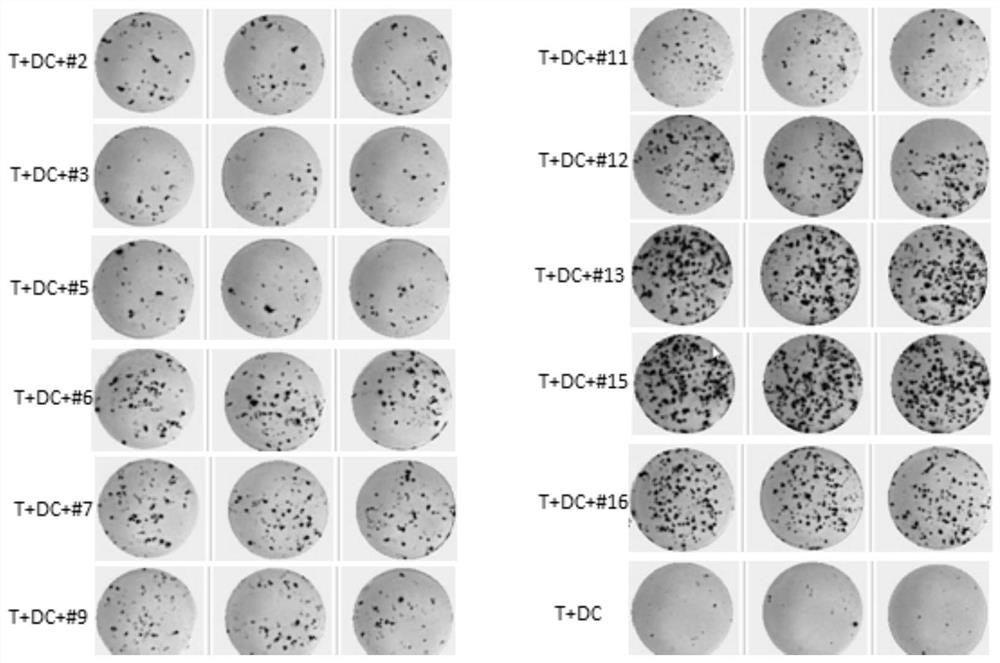
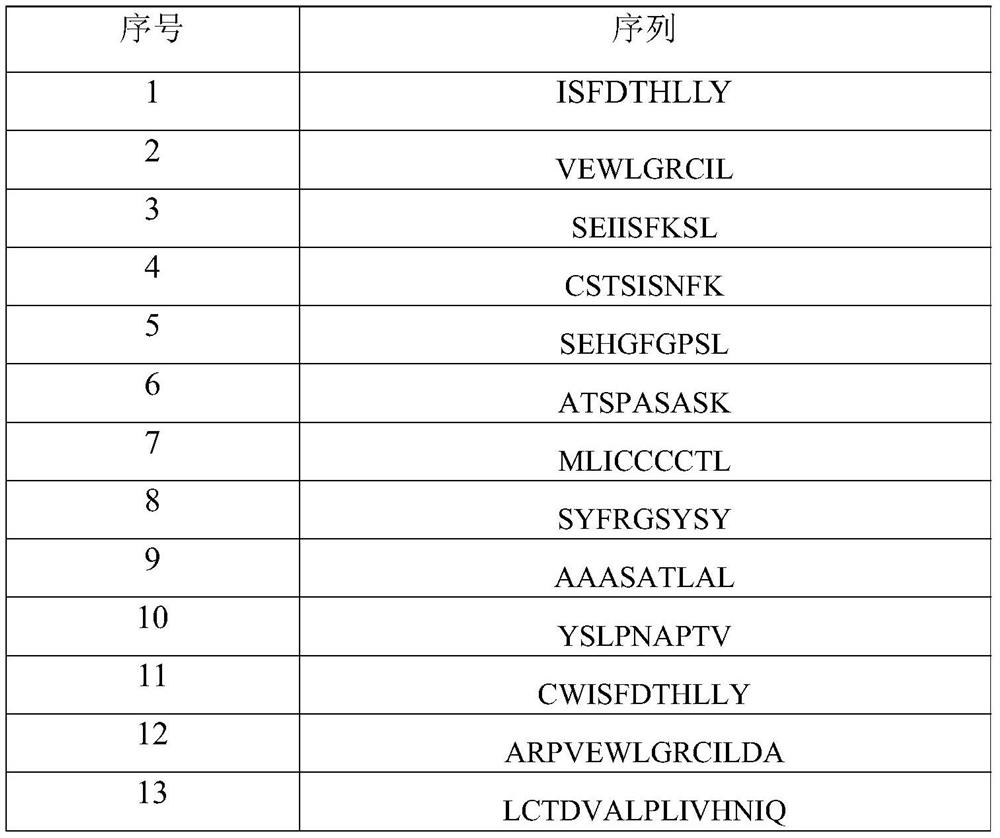
The invention discloses a method for simultaneously detecting neoantigen immunogenicity and a neoantigen specificity TCR (T cell receptor). A neoantigen polypeptide is added to peripheral blood mononuclear cells (PBMCs) to obtain antigen-stimulated cells, and compared with a traditional method, the method has the advantages that the time required for obtaining antigen-specific cells is shortened,the whole process of verifying the neoantigen immunogenicity and obtaining a TCR CDR3 sequence only needs 13 days, and compared with the 40-day treatment process of the traditional method, the methodsaves the time cost greatly. Compared with a traditional MHC-peptide tetramer technology, the method is low in cost, a special tetramer needs to be prepared for one antigen polypeptide in the MHC-peptide tetramer technology, the cost of the sequencing method is remarkably reduced, and compared with a traditional ELISPOT method with the test flux only reaching 10 pieces per batch, the sequencing method is not limited in test flux, higher in efficiency, high in test result accuracy and wide in applicability.
Owner:深圳裕泰抗原科技有限公司
Antigen for detecting tuberculosis infection T cells, kit and application
ActiveCN107219362AStop transmissionStop the epidemicMaterial analysisTrue positive rateAntigen stimulation
The invention discloses an antigen for detecting tuberculosis infection T cells. The amino acid sequence of the antigen is shown as SEQ ID NO.1 to SEQ ID NO.29; any eight polypeptides in SEQ ID NO.1 to SEQ ID NO.29 are coordinated with ESAT-6 and CFP-10 polypeptides and derivatives to have a common effect to specifically stimulate mycobacterium tuberculosis infected T cells to specifically secrete IFN-gamma, and the detection sensitivity is increased. The invention provides a novel detection kit for the tuberculosis infection T cells; when the kit is used, peripheral blood is directly subjected to antigen stimulation and mononuclear cells of the peripheral blood do not need to be separated; the detection sensitivity and the detection specificity are relatively high; the novel detection kit is simple and convenient to operate, has relatively low detection cost and has relatively high clinical application value.
Owner:武汉海吉力生物科技有限公司
Method for detecting B type hepatitis virus specificity T cell by flow cytometer cell factor detecting method
InactiveCN1811452AHigh sensitivityLow costMaterial analysis by optical meansImmune profilingStaining
The present invention relates to a flow cytometry cell factor detection method for detecting hepatitis B virus specific T cell. Said invention utilizes flow cytometry cell factor detection method to detect whole blood, and adopts the following steps: under the environment of having cell factor blocking agent brefeldin adding stirmulus antigen and synergic stimulant in whole blood to make antigen stimulation, after 6 hr of stimulation, washing, identifying lymphocyte and schizolyzing red blood cell, washing and breaking membrane, finally, using fluorescently-labelled antibody to fluorescently stain the cell factor in the cell, after the staining process is completed, utilizing flow cytometer to make analysis.
Owner:无锡市传染病医院
CAR (chimeric antigen receptor) carrying truncated or non-truncated natural cytotoxic receptor signal structure and application of CAR
ActiveCN108822216AEnsure the safety of clinical applicationHigh clinical safetyPolypeptide with localisation/targeting motifImmunoglobulin superfamilyReceptorAntigen receptors
The invention discloses a CAR (chimeric antigen receptor). The CAR comprises an antigen binding structural domain (scfv) and a signal conducting domain, wherein the signal conducting domain comprisesa first conducting domain and a second conducting domain, and the antigen binding structural domain is connected in series between the first conducting domain and the second conducting domain. When the CAR structure is stimulated by antigen, the level of secreted cytokines is extremely low, and safety of clinical application can be well ensured and is higher; killing capacity of antigen positive tumor cells in vitro is higher, and anti-tumor activity is better.
Owner:NANJING CART MEDICAL TECH LTD
Enhanced generation of cytotoxic t-lymphocytes by il-21 mediated foxp3 suppression
A method of carrying out adoptive immunotherapy by administering a subject an antigen-specific cytotoxic T lymphocytes (CTL) preparation in a treatment-effective amount is described. In the method, the CTL preparation is preferably administered as a preparation of an in vitro antigen-stimulated and expanded primate CTL population, the CTL population: (i) depleted of FoxP3+ T lymphocytes prior to antigen stimulation; (ii) antigen-stimulated in vitro in the presence of interleukin-21; or (iii) both depleted of FoxP3+ T lymphocytes prior to antigen stimulation and then antigen-stimulated in vitro in the presence of interleukin-21. Methods of preparing such compositions, and compositions useful for carrying out the adoptive immunotherapy, are also described.
Owner:FRED HUTCHINSON CANCER CENT
Immune cell capable of simultaneously expressing fusion protein and chimeric antigen receptor and application of immune cell
PendingCN114525260ASpecific recognitionEnhance tumor killing activityVirusesAntibody mimetics/scaffoldsReceptorAntigen receptors
The invention discloses an immune cell capable of simultaneously expressing a fusion protein of an intracellular truncated TGF-beta receptor and a cytokine intracellular stimulation domain and a chimeric antigen receptor on a cell membrane. The immune cell can enhance the proliferation capacity of the cell, reduce the influence of a tumor microenvironment, enhance the tumor killing activity of the CAR-T cell and reduce the depletion under the condition of receiving antigen stimulation, so that the negative regulation of the tumor microenvironment on the immune cell is weakened, the depletion of the immune cell is reduced, the tumor killing function of the immune cell is enhanced, and the inhibition of the tumor microenvironment on the immune cell is reduced; by combining with cell factor receptors, corresponding JAK and STAT signal channels are activated, the cells are induced to secrete various active substances such as IFN-gamma, perforin and granzyme, the growth of tumor cells is inhibited, the anti-tumor capability is enhanced, the killing function of immune cells is optimized, and the inhibition of a tumor microenvironment on the immune cells is reduced.
Owner:SHENZHEN FIRST CONDOR BIOSCIENCE CO LTD
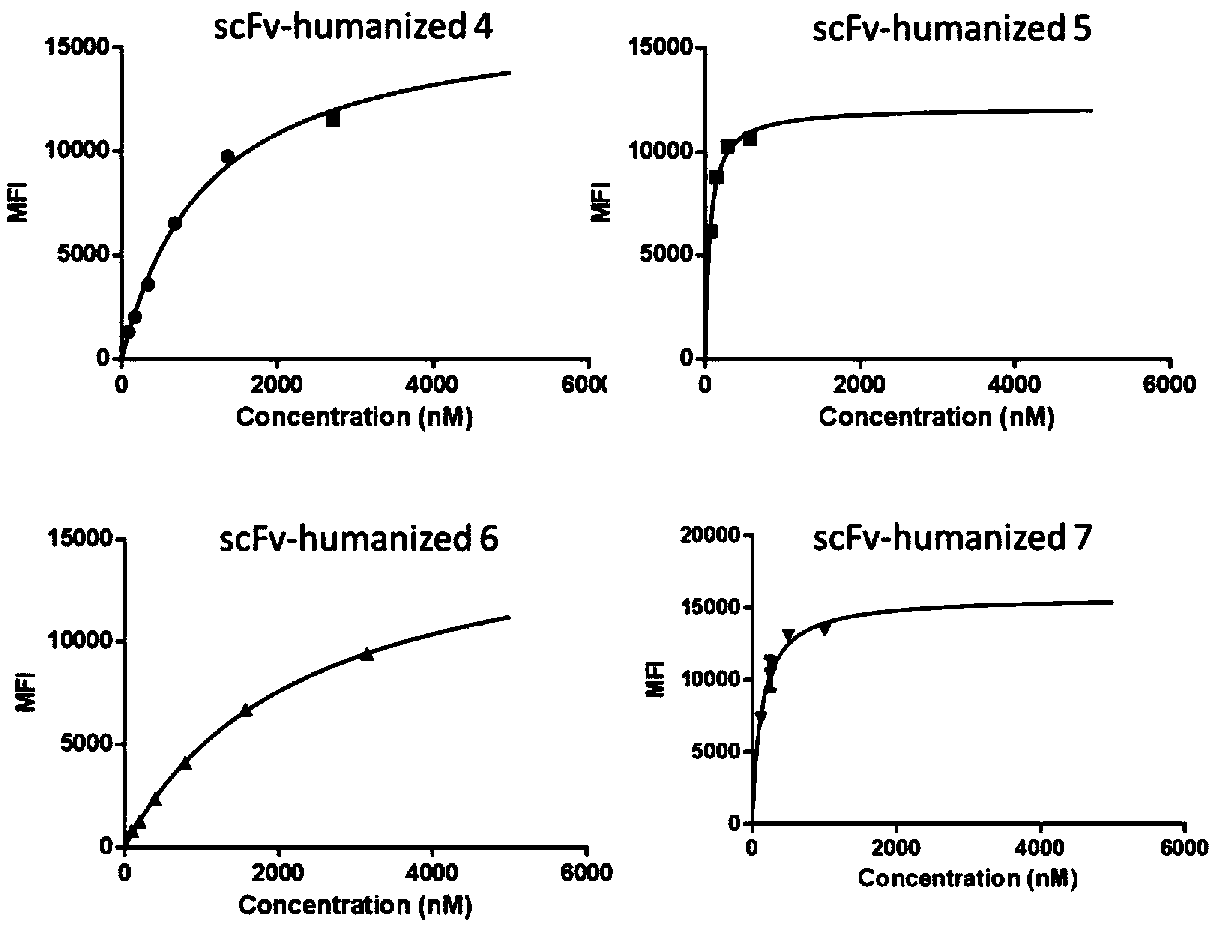
Anti-CD123 humanized single-chain antibody and application thereof
ActiveCN110950954AHigh degree of humanizationImprove stabilityMammal material medical ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsTumor targetAntiendomysial antibodies
The invention belongs to the field of gene engineering, and particularly relates to an anti-CD123 humanized single-chain antibody and an application thereof. The light-chain amino acid sequences of the single-chain antibody for identifying a CD123 antigen are shown as SEQ ID NO: 1, SEQ ID NO: 3, SEQ ID NO: 5 and SEQ ID NO: 7. The heavy chain amino acid sequences of the single-chain antibody are shown as SEQ ID NO: 2, SEQ ID NO: 4, SEQ ID NO: 6 and SEQ ID NO: 8. The invention provides a stable and suitable anti-CD123 humanized single-chain antibody (scFv) which is used for preparing a CAR structure. The binding specificity and affinity of the scFv are reserved, and the stability and safety of CAR-T in vivo are guaranteed. After expression in immune cells, not only can tumor target cells expressing the CD123 antigen be effectively removed, but also no toxic effect is caused to tumor cells expressing a negative antigen (not expressing CD123). Moreover, the positive rate of the CD123-targeting CAR in the cell culture process of a patient can be maintained, and the CD123-targeting CAR can be proliferated for a long time after being stimulated by a target antigen and can be used for targeting treatment of tumors.
Owner:CHONGQING PRECISION BIOTECH CO LTD
Methods of preparing lymphocytes that express interleukin-2 and their use in the treatment of cancer
InactiveUS7381405B2BiocideGenetic material ingredientsPeripheral blood mononuclear cellWhite blood cell
Owner:GOVERNMENT OF THE US REPRESENTED BY THE SEC
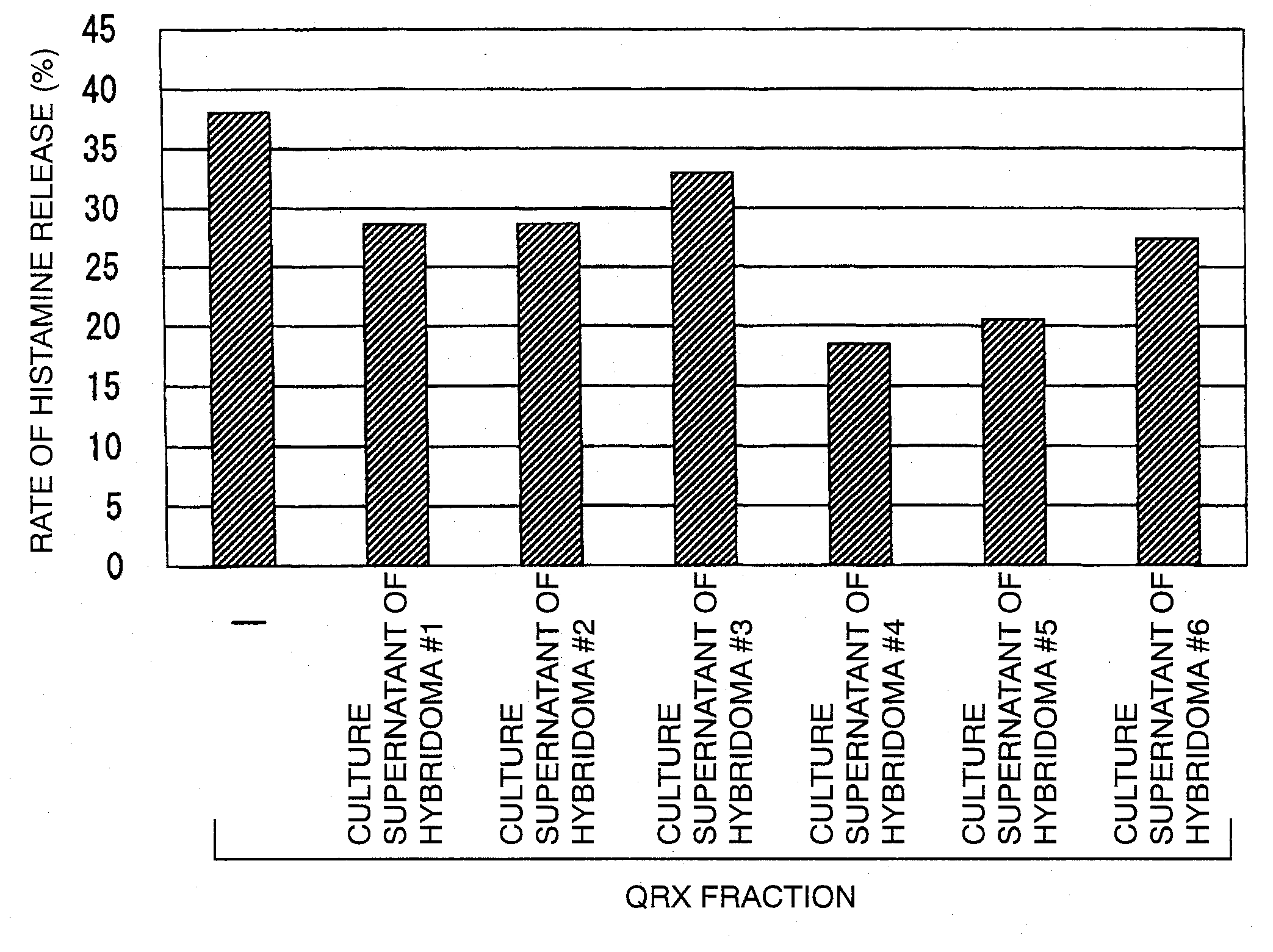
Anti-sweat antigen monoclonal antibody
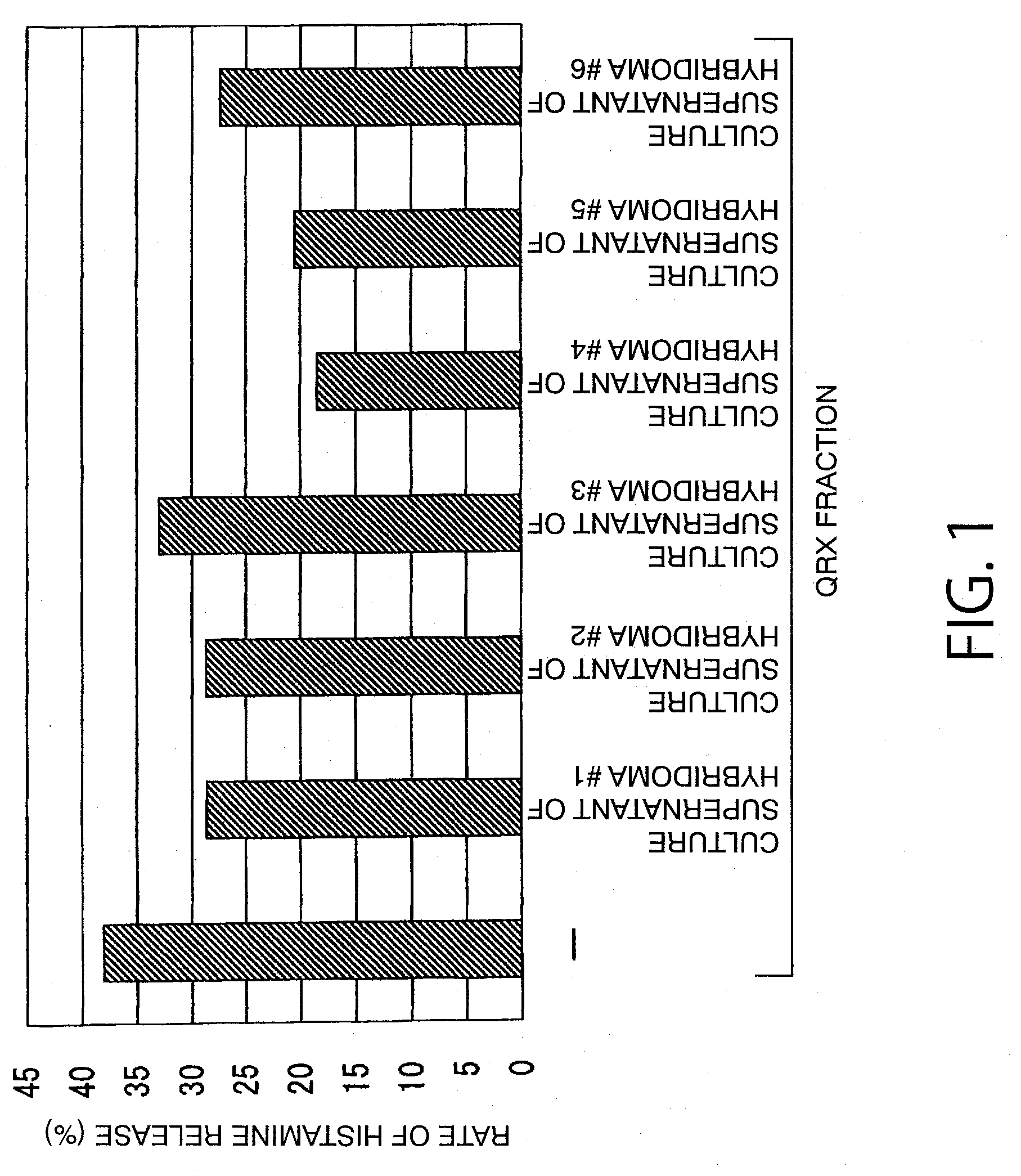
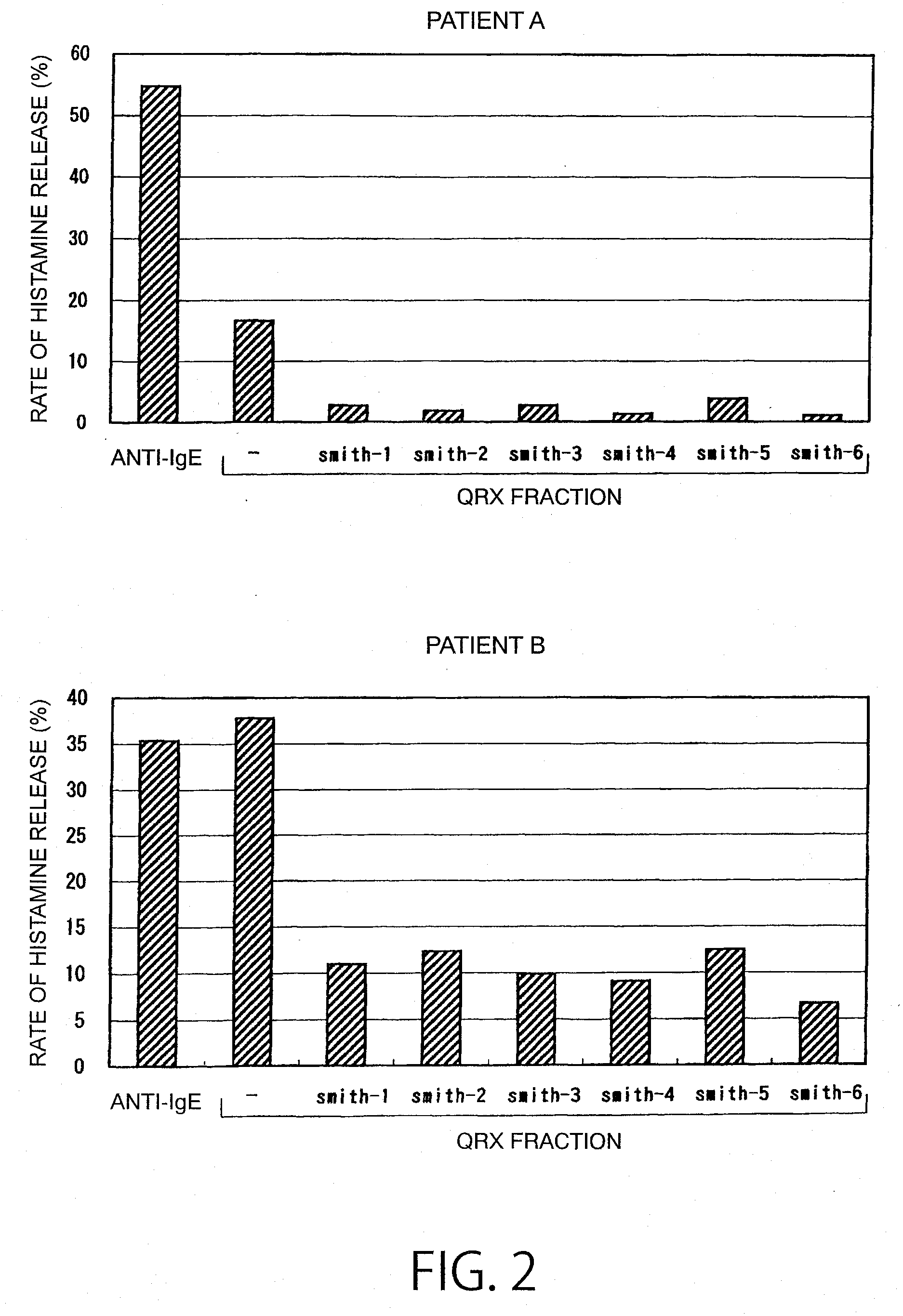
ActiveUS20110117103A1High sensitivity detectionEasy to optimizeCompound screeningSenses disorderAntigen stimulationMonoclonal antibody
[Object of the Invention] To provide an antibody that inhibits histamine releasing activity induced by an antigenic substance contained in sweat.[Means for Attaining the Object] An antibody which can react with a sweat antigen composition and inhibit the histamine releasing activity of the composition on a sweat antigen stimulation-responsive cell.
Owner:HIROSHIMA UNIVERSITY
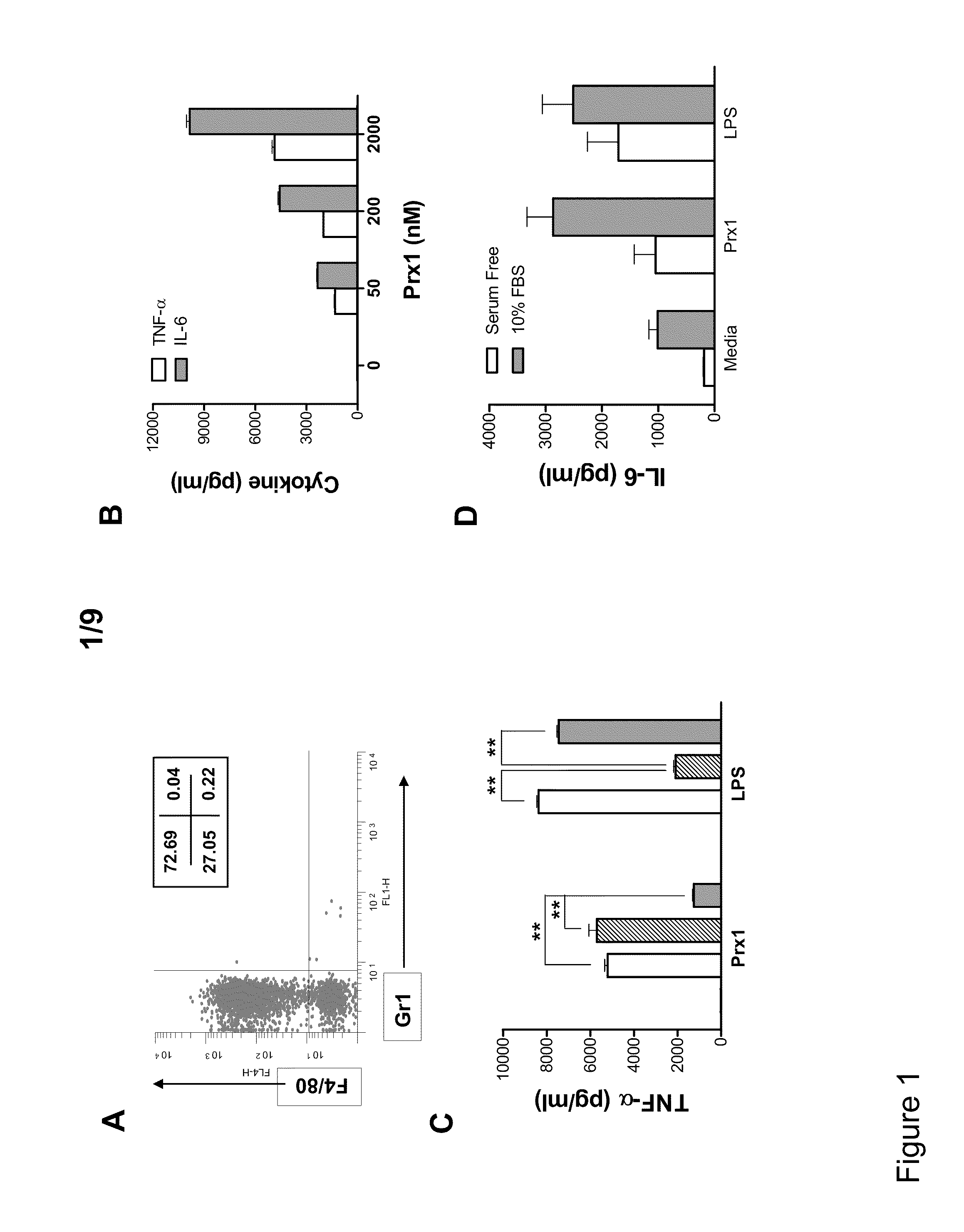
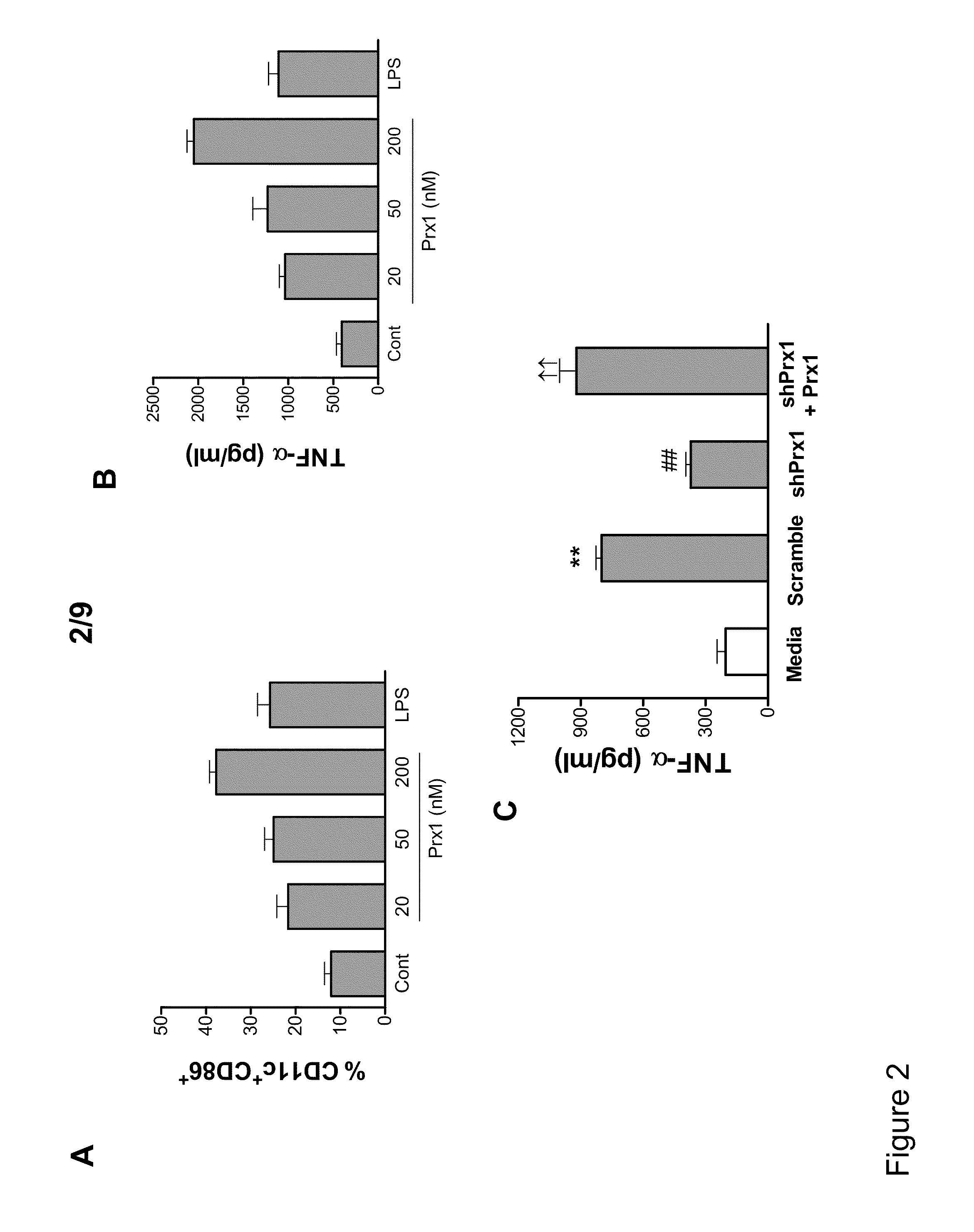
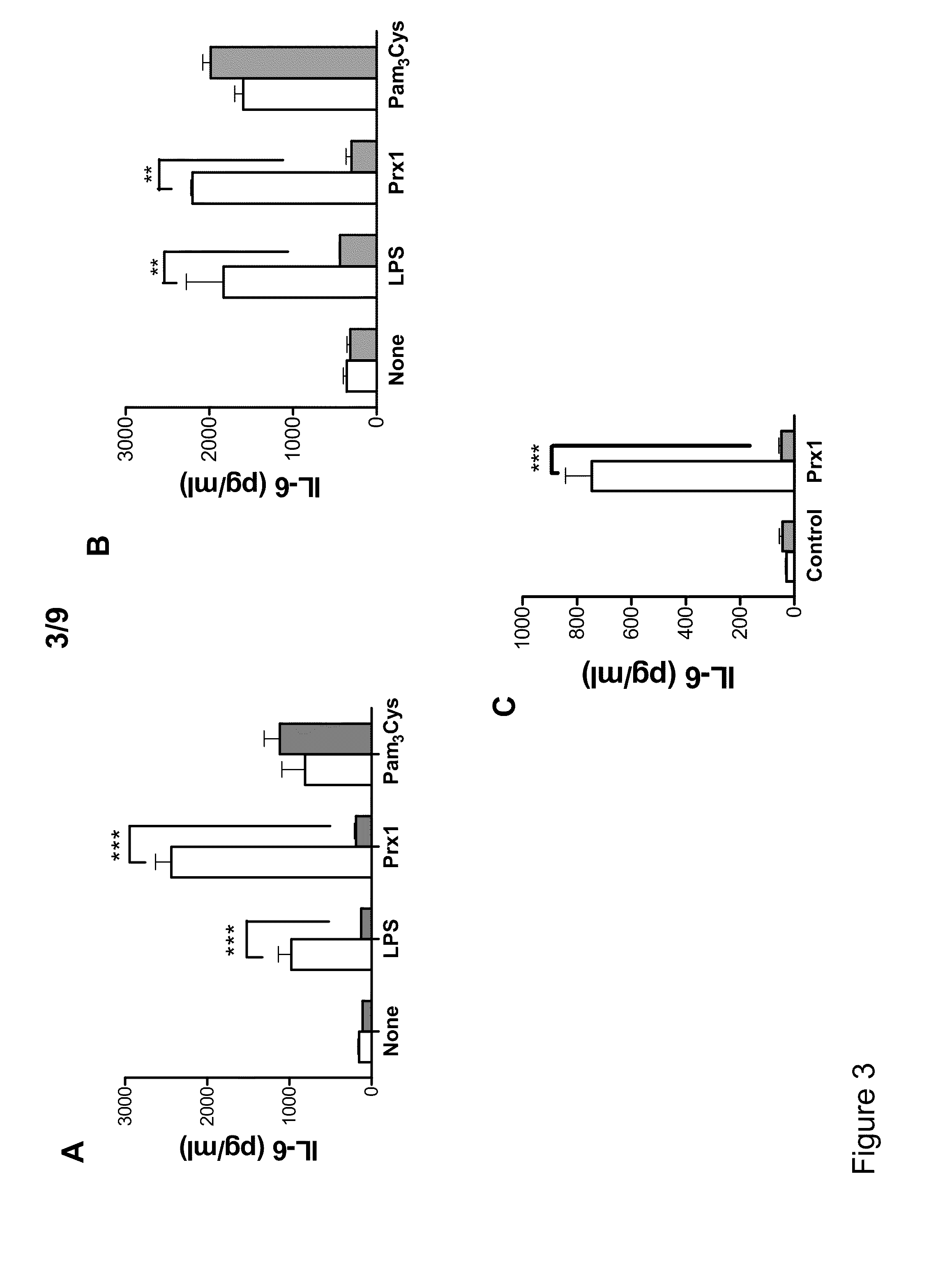
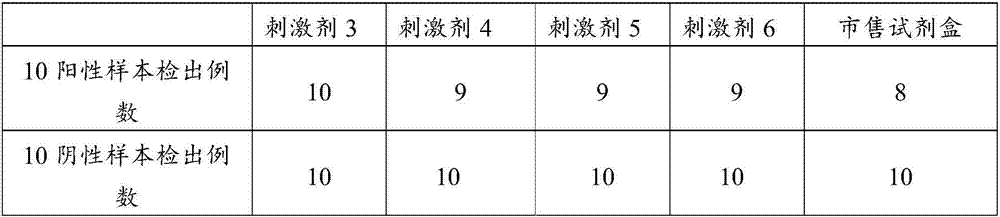
Methods and Compositions Using Peroxiredoxin 1 (PRX1) as an Adjuvant
InactiveUS20110177129A1Peptide/protein ingredientsSnake antigen ingredientsAdjuvantAntigen stimulation
Provided are compositions and methods for stimulating immune responses against antigens. The compositions contain an antigen to which a stimulated immune response is desired and an isolated Prx1 protein. The Prx1 protein functions as an adjuvant so that the immune response to the antigen stimulated by the composition comprising the antigen and Prx1 is greater than the immune response stimulated by the antigen alone.
Owner:HEALTH RES INC
Antigen for detecting tuberculosis infection T cells, kit and application
ActiveCN107219363AStop transmissionStop the epidemicChemiluminescene/bioluminescenceDisease diagnosisTrue positive rateAntigen stimulation
The invention discloses an antigen for detecting tuberculosis infection T cells. The amino acid sequence of the antigen is shown as SEQ ID NO.1 to SEQ ID NO.28; any eight polypeptides in SEQ ID NO.1 to SEQ ID NO.28 are coordinated with ESAT-6 and CFP-10 polypeptides and derivatives to have a common effect to specifically stimulate mycobacterium tuberculosis infected T cells to specifically secrete IFN-gamma, and the detection sensitivity is increased. The invention provides a novel detection kit for the tuberculosis infection T cells; when the kit is used, peripheral blood is directly subjected to antigen stimulation and mononuclear cells of the peripheral blood do not need to be separated; the detection sensitivity and the detection specificity are relatively high; the novel detection kit is simple and convenient to operate, has relatively low detection cost and has relatively high clinical application value.
Owner:武汉海吉力生物科技有限公司
Method and system for screening specific BCR/TCR
ActiveCN113122617AImprove the efficiency of screening and identificationImprove featuresMicrobiological testing/measurementBiostatisticsSpecific immunityAmino acid
The invention discloses a method and a system for screening specific BCR / TCR. The method for screening the specific BCR / TCR comprises the following steps of (1) extracting mRNA in lymphocytes of an immunized sample to be detected; (2) carrying out reverse transcription treatment on the mRNA obtained in the step (1), then carrying out multiple PCR amplification on the BCR / TCR variable region gene, then carrying out high-throughput sequencing, and determining nucleic acid and amino acid sequences of the BCR / TCR variable region; and (3) comparing the sequence obtained by sequencing with a lymphocyte BCR / TCR sequencing result of a sample which is not stimulated by the antigen to obtain a corresponding specific immune sequence. The method disclosed by the invention has the advantages that by comparing big data of the immune spectrum and comparing a huge number of BCR / TCR sequences, the specificity and the accuracy are higher, the efficiency of screening and identifying the antibody and the T cell receptor can be greatly improved, and the screening cost is low.
Owner:CHENGDU EXAB BIOTECH CO LTD
Immunization-free methods for treating antigen-stimulated inflammation in a mammalian host and shifting the host's antigen immune responsiveness to a th1 phenotype
InactiveUS20090131347A1Useful in treatment and prevention of inflammationSuppresses antigen-stimulated granulocyte infiltrationOrganic active ingredientsSenses disorderAntigen stimulationTherapeutic intent
The invention relates to methods for preventing or reducing antigen-stimulated, granulocytemediated inflammation in tissue of an antigen-sensitized mammal host by delivering an immunostimulatory oligonucleotide to the host. In addition, methods for using the immunostimulatory oligonucleotides to boost a mammal host's immune responsiveness to a sensitizing antigen (without immunization of the host by the antigen) and shifting the host's immune responsiveness to a Th1 phenotype to achieve various therapeutic ends are provided. Kits for practicing the methods of the invention are also provided.
Owner:RGT UNIV OF CALIFORNIA
Chimeric antigen receptor lack of ubiquitination and application of chimeric antigen receptor
ActiveCN111825769ABlock ubiquitinationHigh activityPeptide/protein ingredientsAntibody mimetics/scaffoldsWild typeTransmembrane domain
The invention provides a chimeric antigen receptor. The chimeric antigen receptor comprises an extracellular structural domain, a transmembrane structural domain and an intracellular structural domainwhich are sequentially connected, the extracellular domain comprises an antigen recognition region; the intracellular structural domain comprises a costimulatory signal conduction region and a CD3[zeta] intracellular region which are sequentially connected to form a costimulatory signal conduction region-CD3[zeta] intracellular region; the costimulatory signal conduction region-CD3[zeta] intracellular region is a polypeptide formed by mutating lysine in a wild type costimulatory signal conduction region-CD3[zeta] intracellular region into arginine. A method is provided for optimization and transformation of CAR-T, all lysine sites of an intracellular segment of the CAR are mutated into arginine, and ubiquitination modification generated after the CAR is stimulated by an antigen is blocked. The strategy is suitable for different CARs and transformation of different intracellular costimulatory domains, and a solution is provided for the problem that the proliferation ability of CAR-T insolid tumors is poor.
Owner:SHANGHAI TECH UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com