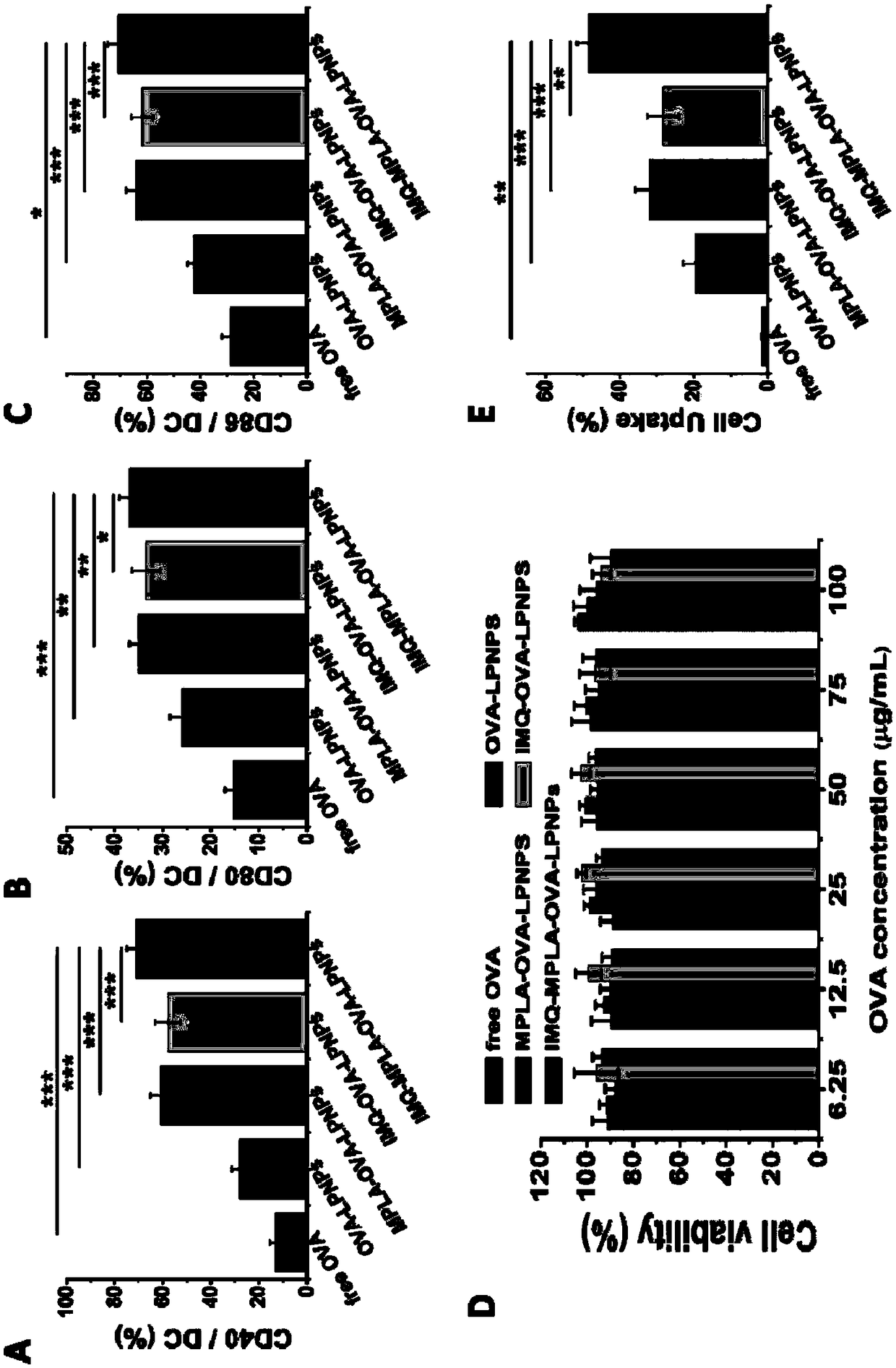
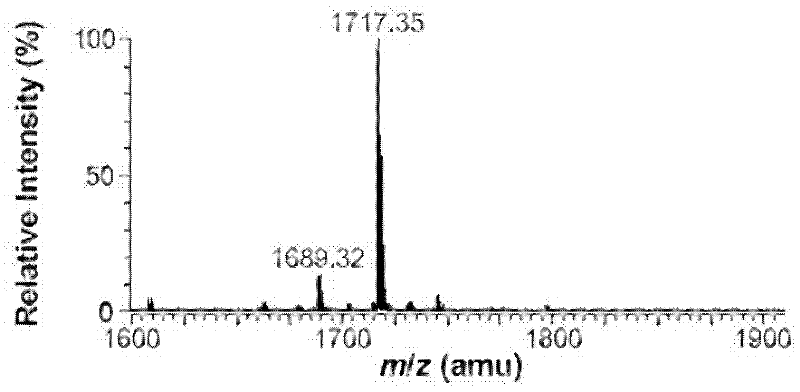
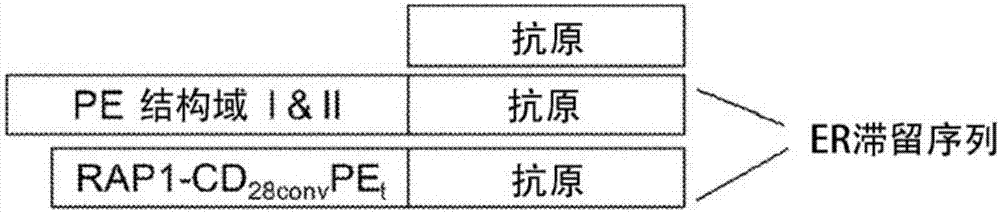
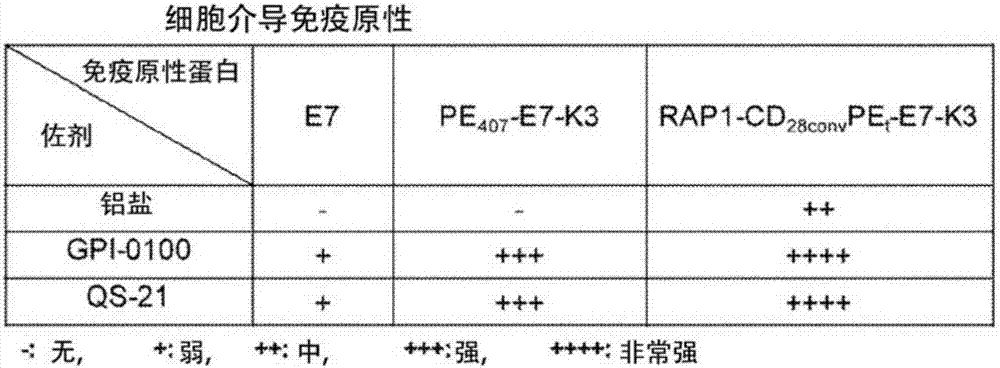
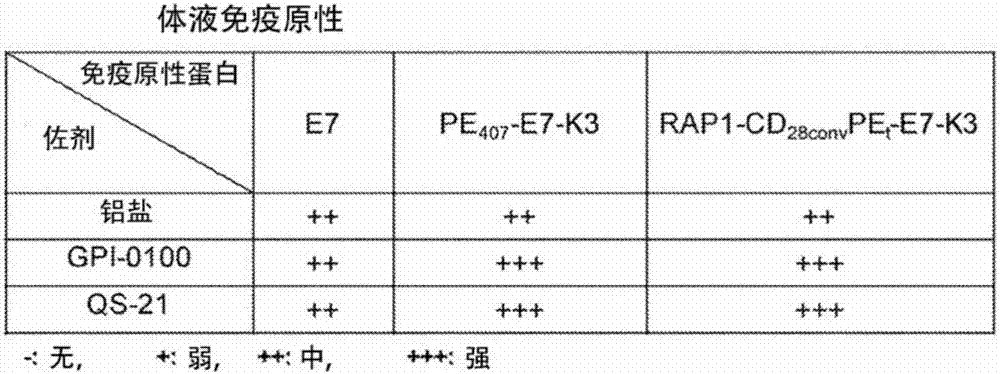
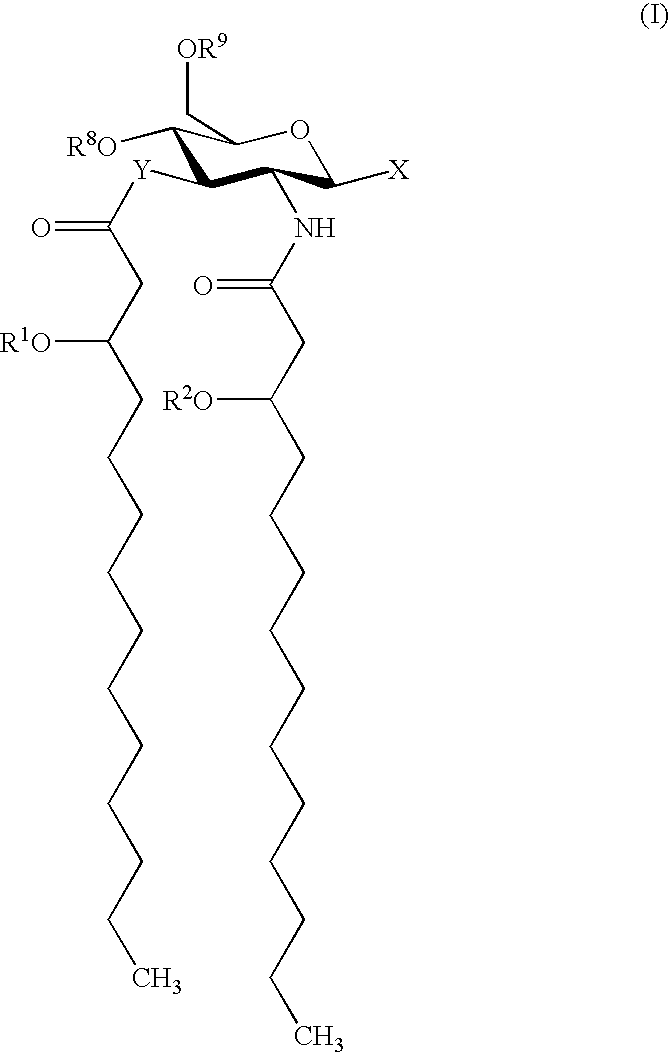
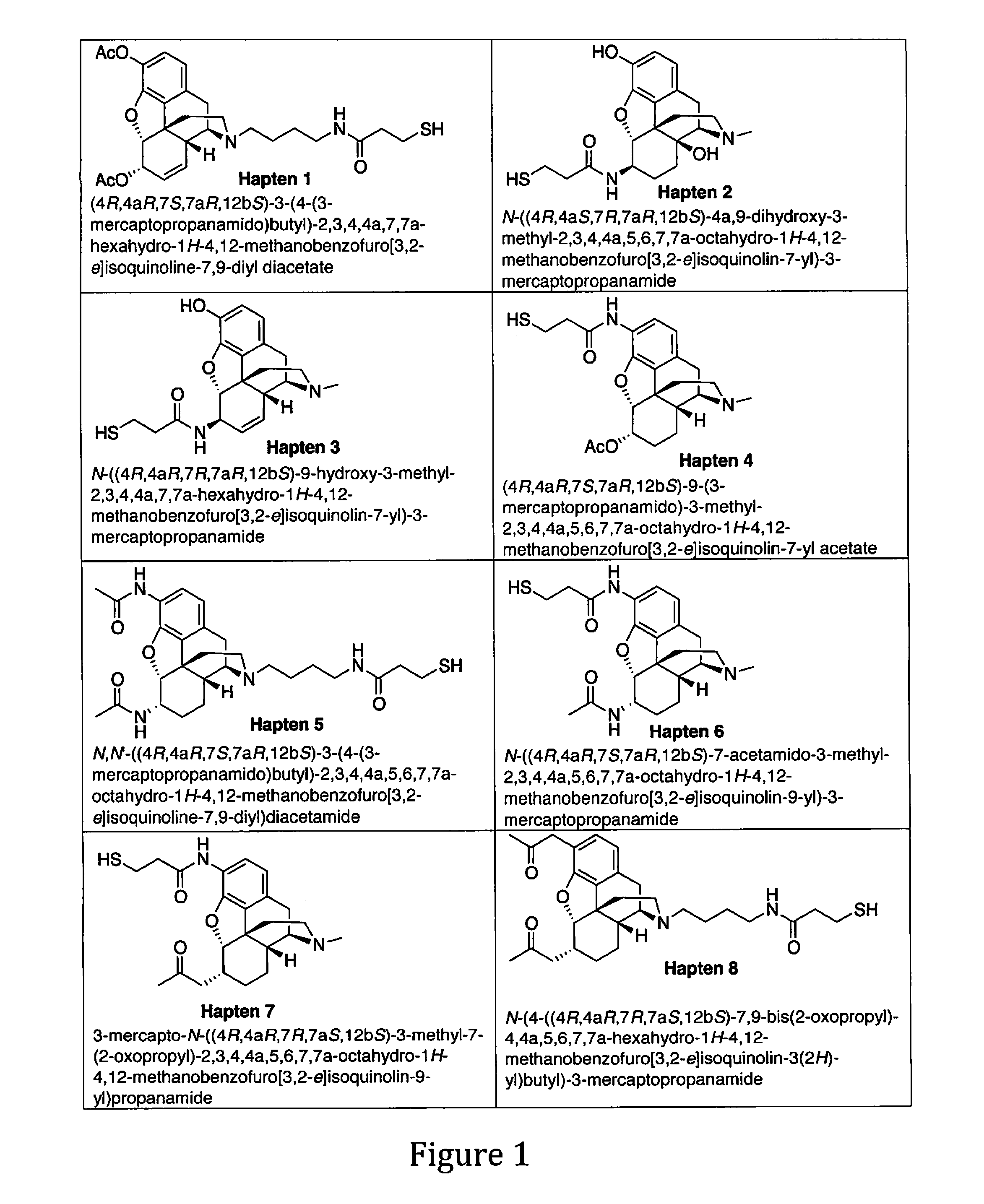
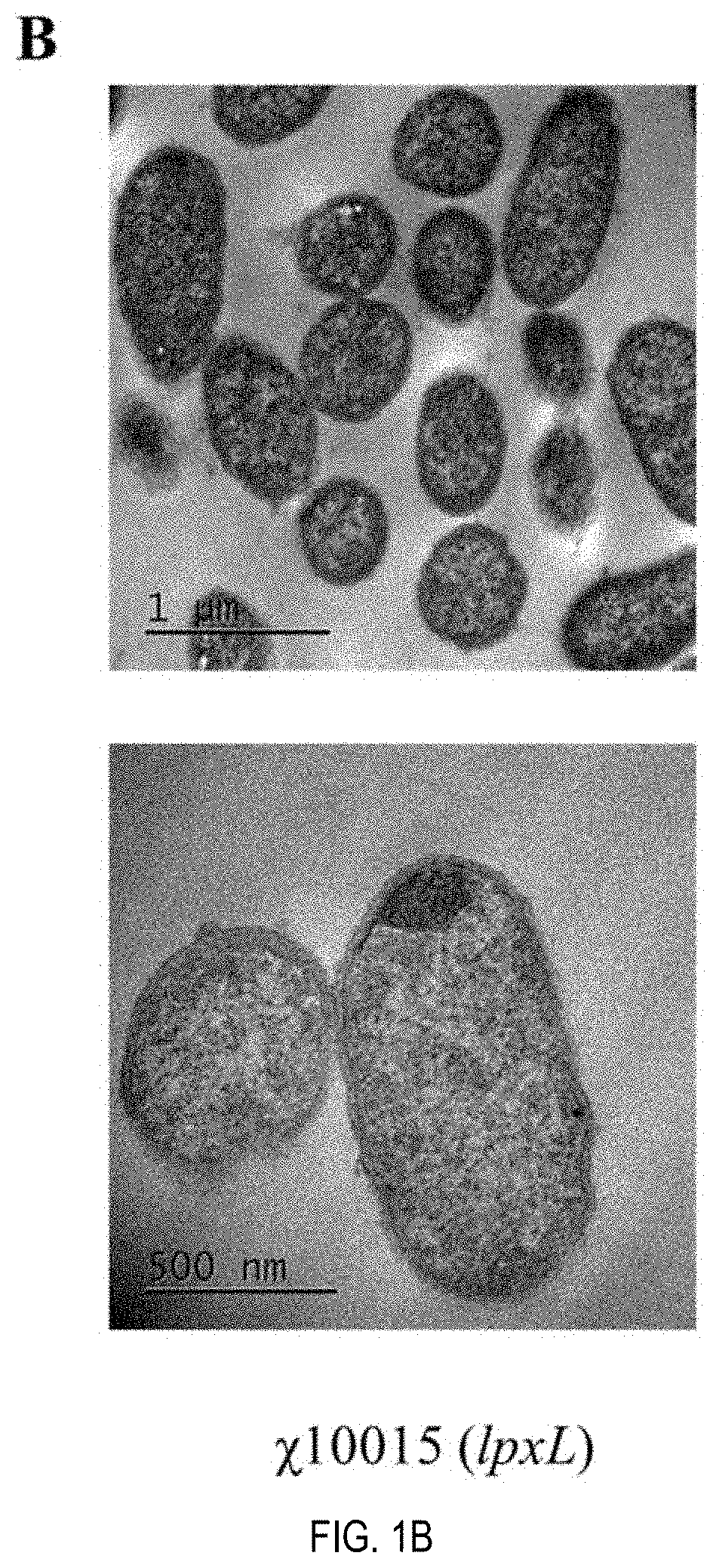
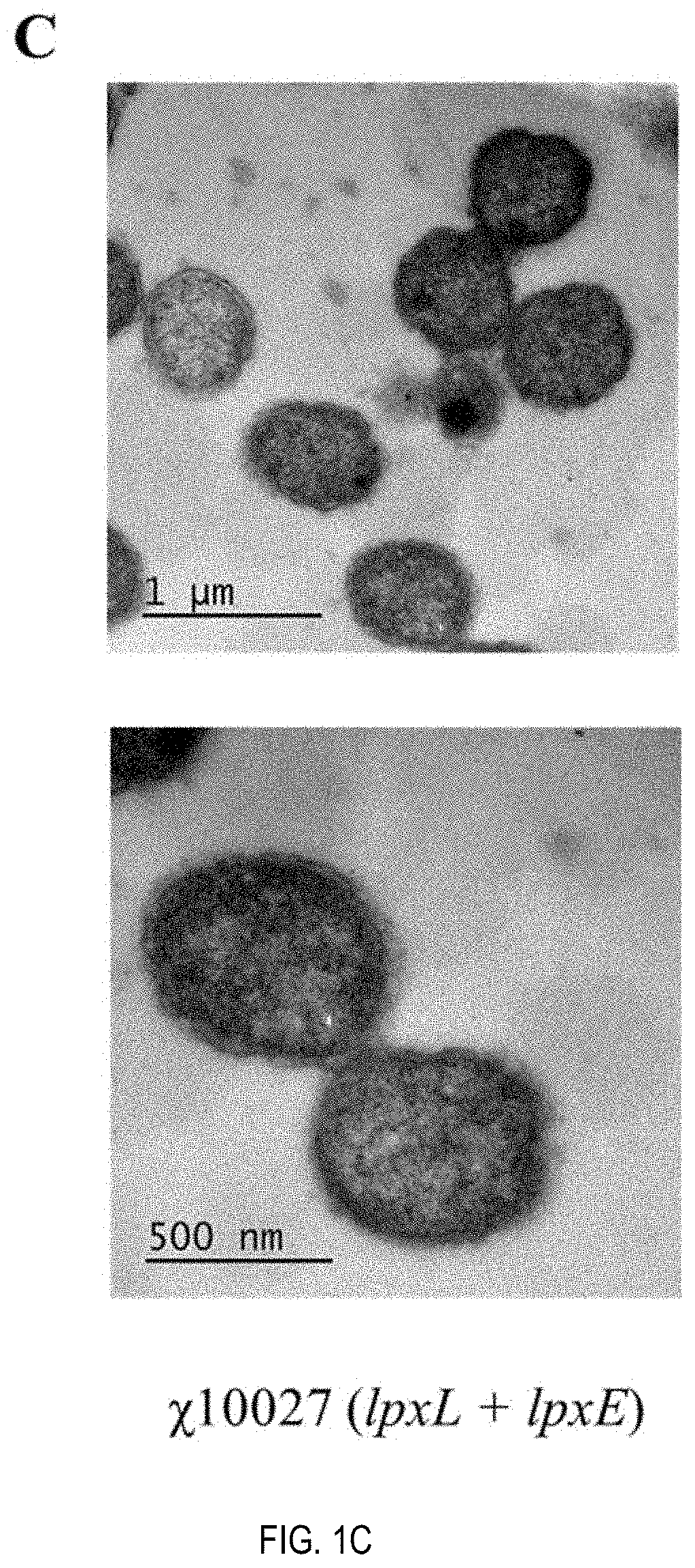
Patents
Literature
43 results about "Monophosphoryl Lipid A" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
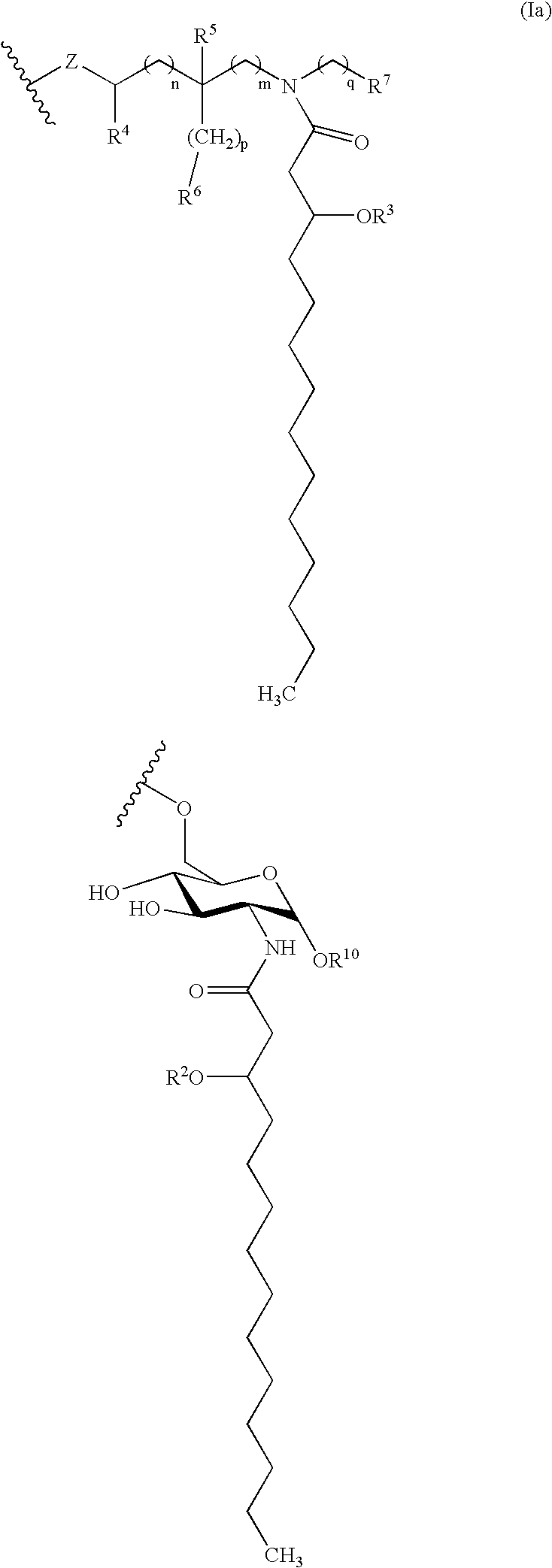
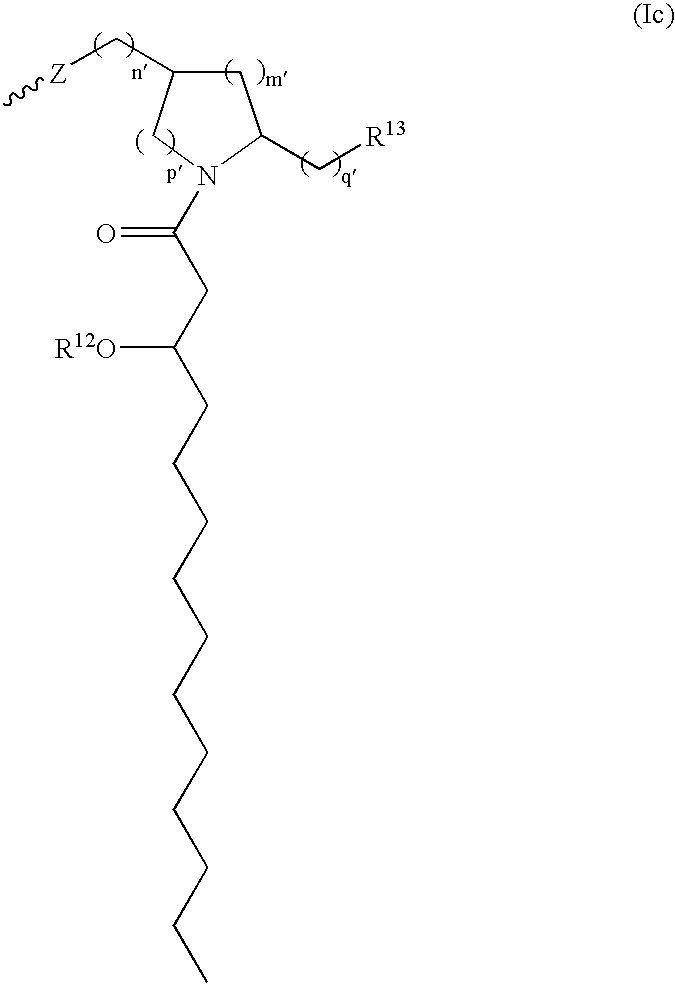
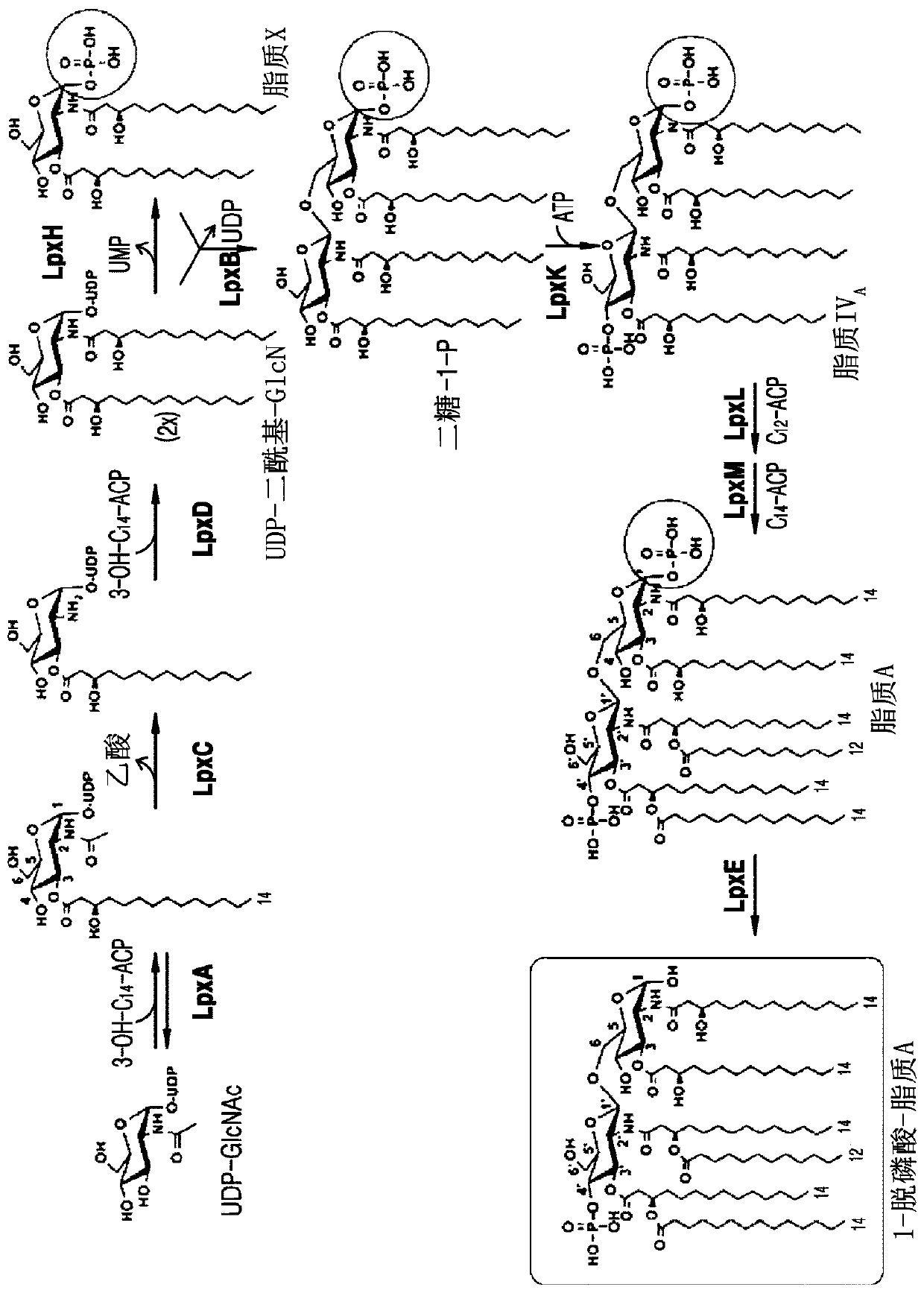
A modified form of lipid A, the biologically active part of Gram-negative bacterial lipopolysaccharide (LPS) endotoxin, and a Toll-like receptor 4 (TLR4) agonist, with potential immunostimulatory activity. As a vaccine adjuvant, monophosphoryl lipid A (MPLA) stimulates both cellular and humoral responses to the vaccine antigen. Compared to LPS, MPLA exerts a similar immunostimulatory activity but with reduced toxicity.
Quil a fraction with low toxicity and use thereof
ActiveUS20060239963A1Improve the level ofBacterial antigen ingredientsPeptide/protein ingredientsImmunomodulationsCell Wall Skeleton
Owner:NOVAVAX
Immunity enhancing agent, inactivated vaccine, and preparation method thereof
InactiveCN103083663AImprove immunityEnhance immune responseViral antigen ingredientsAntiviralsDipeptideOil phase
The invention provides an immunity enhancing agent, an inactivated vaccine, and a preparation method thereof. The invention relates to the field of biopharmaceutical. The immunity enhancing agent comprises 0.1-21mg / mL of monophosphoryl lipid A, 1.5-125mg / mL of muramyl dipeptide, and 0.7-4.5mg / mL of beta-glucan. The invention also provides the inactivated vaccine comprising the immunity enhancing agent, and a preparation method of the inactivated vaccine. According to the invention, the immunity enhancing agent is mixed with an inactivated antigen solution, such that a water-phase solution is obtained; and the water-phase solution is mixed with an oil-phase solution, such that the inactivated vaccine is obtained. According to the immunity enhancing agent provided by the invention, with a synergetic effect of the components, body immunity level can be improved, and immune response to antigen can be improved, such that antibody level after immunization can be increased, immune window period can be shortened, and vaccine immunization effect can be enhanced. According to the inactivated vaccine comprising the immunity enhancing agent, antibody level after immunization is high, a protection period is long, and immunization window period is short.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES +1
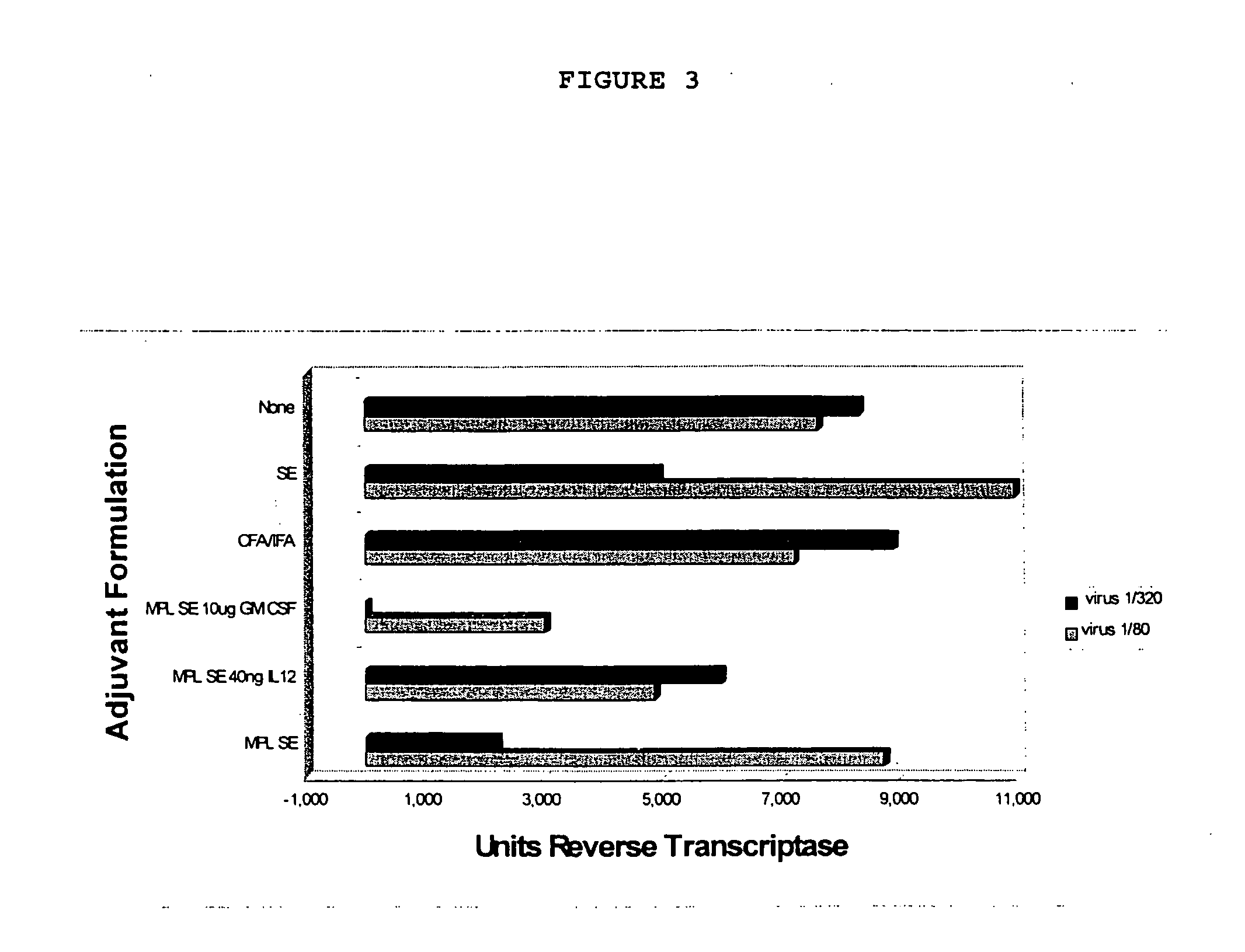
Adjuvant combination formulations
InactiveUS7611721B1Improve abilitiesSsRNA viruses negative-senseAntibacterial agentsWhite blood cellVertebrate Animals
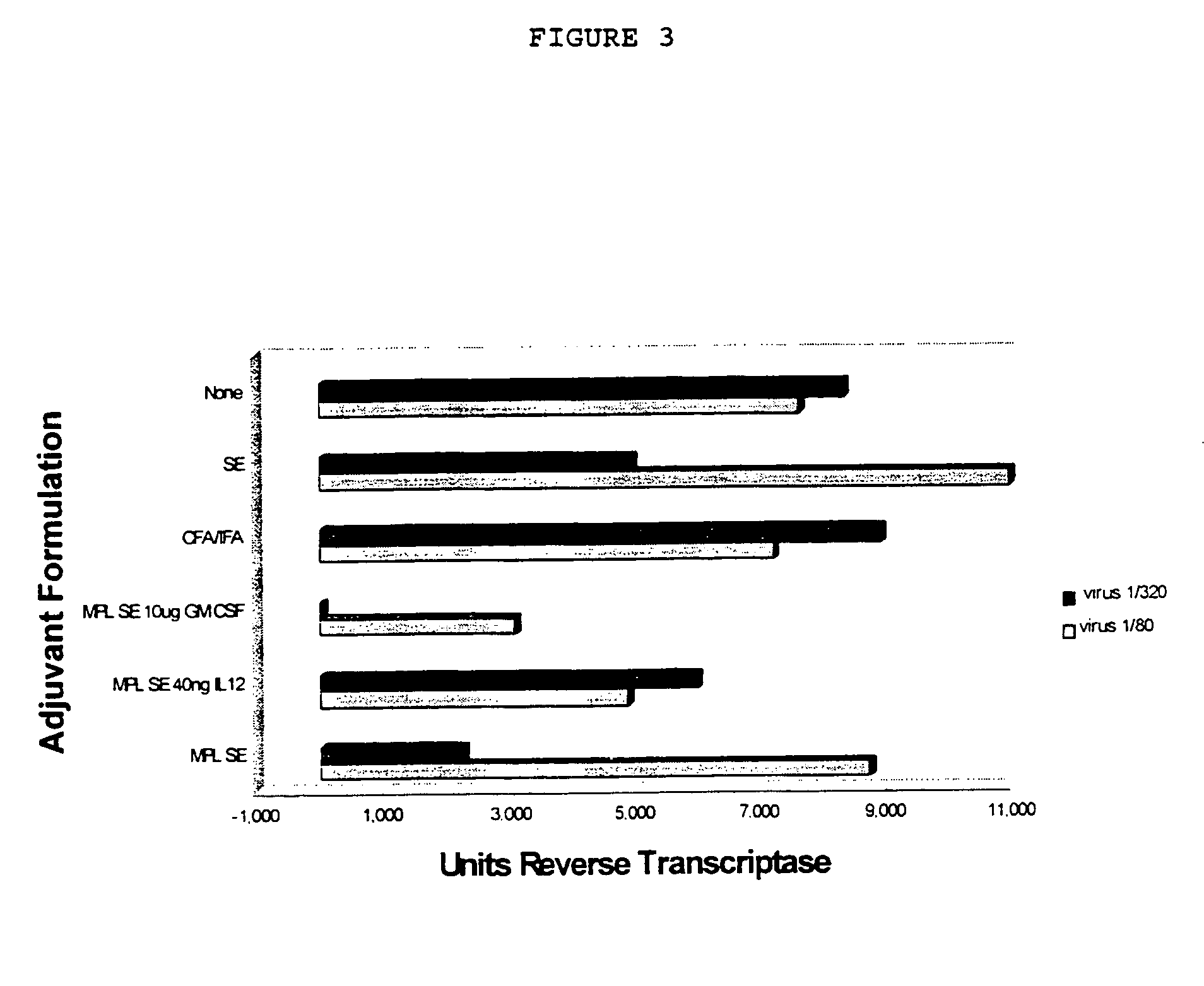
The use of 3-O-deacylated monophosphoryl lipid A or monophosphoryl lipid A and derivatives and analogs thereof, in combination with a cytokine or lymphokine such as granulocyte macrophage colony stimulating factor or interleukin-12 is useful as an adjuvant combination in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen.
Owner:ZOETIS SERVICE LLC
PED (Porcine Epedemic Diarrhea) inactivated vaccine and preparation method thereof
ActiveCN104383528AEnhance immune responseImprove the level ofOrganic active ingredientsDipeptide ingredientsAntiendomysial antibodiesDipeptide
The invention provides a PED (Porcine Epedemic Diarrhea) inactivated vaccine and a preparation method thereof and relates to the field of biopharmacy. The PED inactivated vaccine comprises inactivated PEDV (Porcine Epedemic Diarrhea Virus), and is characterized in that the PED inactivated vaccine comprises 0.05-10 mg / mL Beta-glucosylceramide, 0.1-21 mg / mL monophosphoryl lipid A, 1.5-125 mg / mL muramyl dipeptide and 0.7-4.5 mg / mL Beta-glucan. According to the ingredients of the PED inactivated vaccine, Beta-glucosylceramide, monophosphoryl phosphoryl lipid A, muramyl dipeptide and Beta-glucan have the synergistic effect, the immune response of animals to antigens in the vaccine is significantly improved, the immune window phase is shortened, the antibody production duration of the animal body is obviously prolonged, the serum antibody level is improved, and the level of total intestinal mucosa secretory antibodies (the total SIgA) is improved.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Genetic engineering bacterium for producing monophosphoryl lipid A as well as construction method and application thereof
InactiveCN102399736AReduce manufacturing costConducive to large-scale industrial productionBacteriaMicroorganism based processesMicrobiologyBacterial strain
The invention discloses a genetic engineering bacterium for producing a monophosphoryl lipid A as well as a construction method and application thereof, belonging to the field of a genetic engineering. The genetic engineering bacterium is E.coli W3110 delta lacI lacZ::FnlpxE, wherein the lacI takes place a deletion mutation to lose activity and an expression FnlpxE gene is knocked into the lacZ gene. According to the bacterial strain constructed in the invention, on the basis of keeping a lipid structure single, the production cost is also reduced; no exogenous resistance genes are introduced into the bacterial strain so that the industrial production of the bacterial strain is easier to realize in a large scale.
Owner:JIANGNAN UNIV
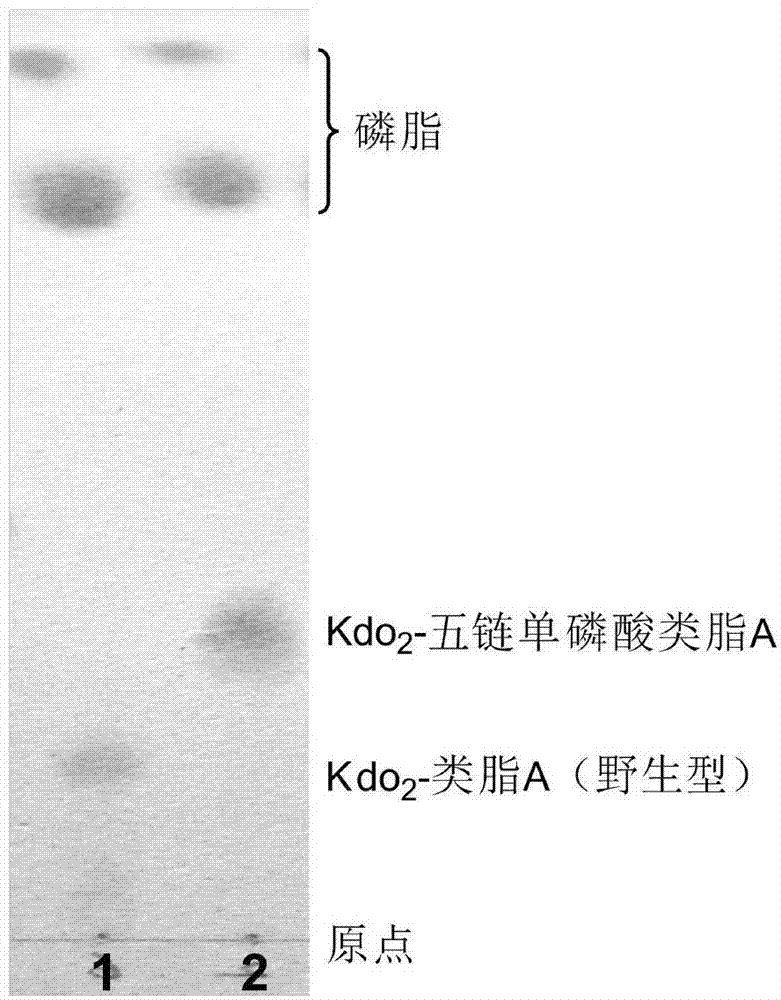
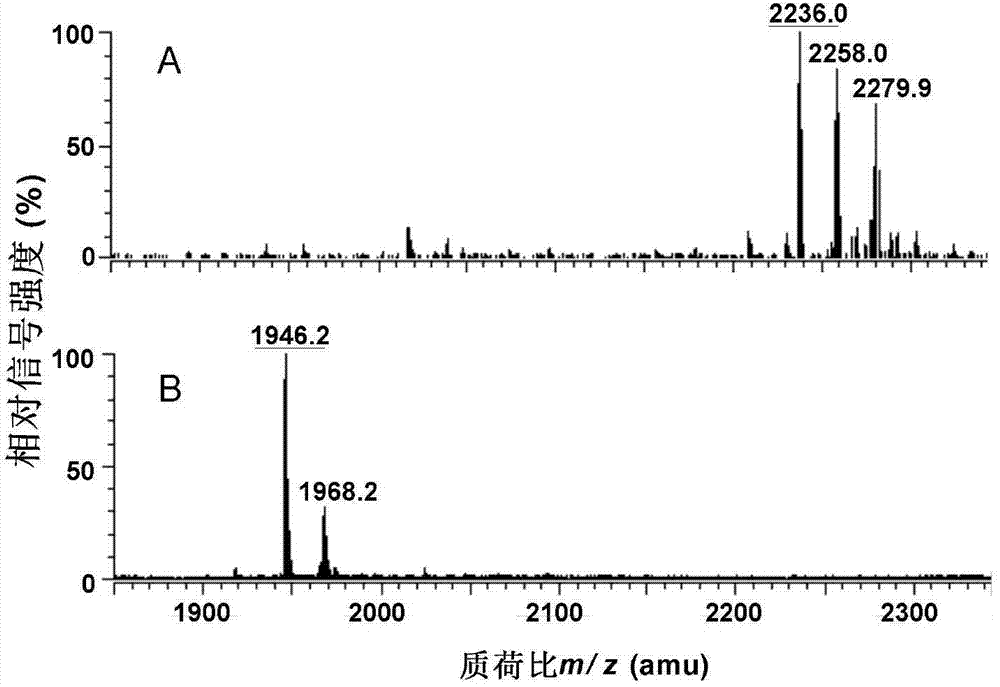
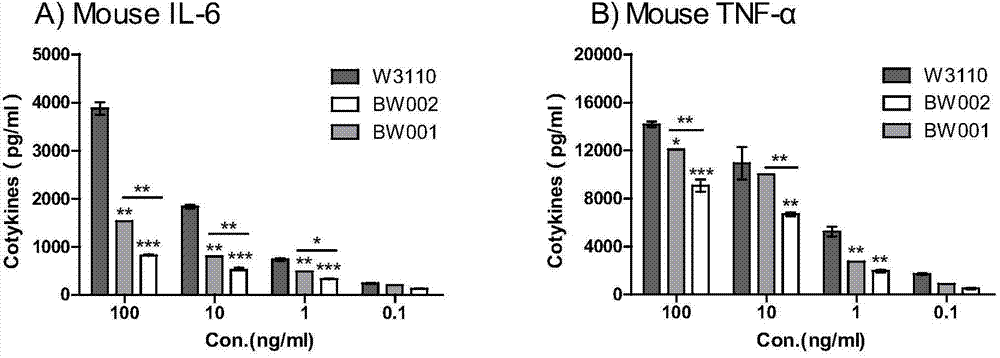
Preparation and application of low-toxicity Kdo2-monophosphoryl lipid A containing five fatty acid chains
ActiveCN104844665ALow cytotoxicityImprove solubilityEsterified saccharide compoundsBacteriaEscherichia coliImmunocompetence
The invention discloses preparation and application of a low-toxicity Kdo2-monophosphoryl lipid A containing five fatty acid chains, belonging to the field of bioengineering. By modifying Escherichia coli, a special-structure low-toxicity five-chain Kdo2-monophosphoryl lipid A is synthesized, and the Kdo2-lipid A molecule is not connected with the core polysaccharide long chain, and only composed of two 2-one-3-desoxyoctoic acids, five fatty acid chains and a C4' site single phosphoric acid. The Kdo2-lipid A is amphiphilic, is convenient for separation, extraction and purification, can be detected and directly identified, maintains the biological immunocompetence of the lipid A part, has obvious toxicity attenuation effect as compared with wild type W3110 LPS, and is a vaccine adjuvant with great development potential. Meanwhile, the method for extracting and purifying the Kdo2-lipid A is simple in steps and easy to operate.
Owner:JIANGNAN UNIV
Quil A fraction with low toxicity and use thereof
ActiveUS8821881B2Improve the level ofSnake antigen ingredientsCarrier-bound antigen/hapten ingredientsMedicineCell Wall Skeleton
Fraction A of Quil A can be used together with at least one other adjuvant for the preparation of an adjuvant composition, where the included adjuvant components act synergistically to enhance level of immune response and have synergistic immunomodulating activity on the co-administered antigens or immunogens.Other adjuvants can comprise saponins, naturally occurring, synthetic or semisynthetic saponin molecules; e.g. saponins and saponin fractions from Quil A, cell wall skeleton, blockpolymers, TDM, lipopeptides, LPS and LPS-derivatives, Lipid A from different bacterial species and derivatives thereof, e.g., monophosphoryl lipid A. CpG variants, CT and LT or fractions thereof.
Owner:NOVAVAX
EV71 subunit vaccine of mixed adjuvant and preparation method thereof
InactiveCN105535963AHigh School and ValenceGood immunogenic responseSsRNA viruses positive-senseViral antigen ingredientsEscherichia coliImmunogenicity
The invention discloses an EV71 subunit vaccine of a mixed adjuvant and a preparation method thereof. According to the vaccine, C4 sub-type EV71 virus VP1 protein is used as an antigen, and aluminum hydroxide and monophosphoryl lipid A are used as a mixed adjuvant. VP1 protein which is expressed and purified by escherichia coli and does not have any label is used as an antigen; the prepared protein is combined with an aluminum adjuvant under a denaturing condition, is subjected to detergence determination, and is mixed with an MPLA adjuvant to immune mice; and the prepared polyvalent antibody can be used for specifically neutralizing the EV71 virus to generate a neutral protective antibody having a titer nearly 1:128. Compared with an EV71 inactivated vaccine in current clinical tests, the human enterovirus 71 type subunit vaccine has the characteristics of low cost, simple operation, high safety, easy large-scale production and the like in the production process. The vaccine can generate relatively good immunogenicity in a human body, has a relatively high titer of the neutralizing antibody, and is an alternative vaccine with potential clinical application value.
Owner:BEIJING KYNING BIOSCI
Vaccine against streptococcus pneumoniae
InactiveUS20140072622A1Maintain good propertiesRaise immunogenic responseAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeQS21
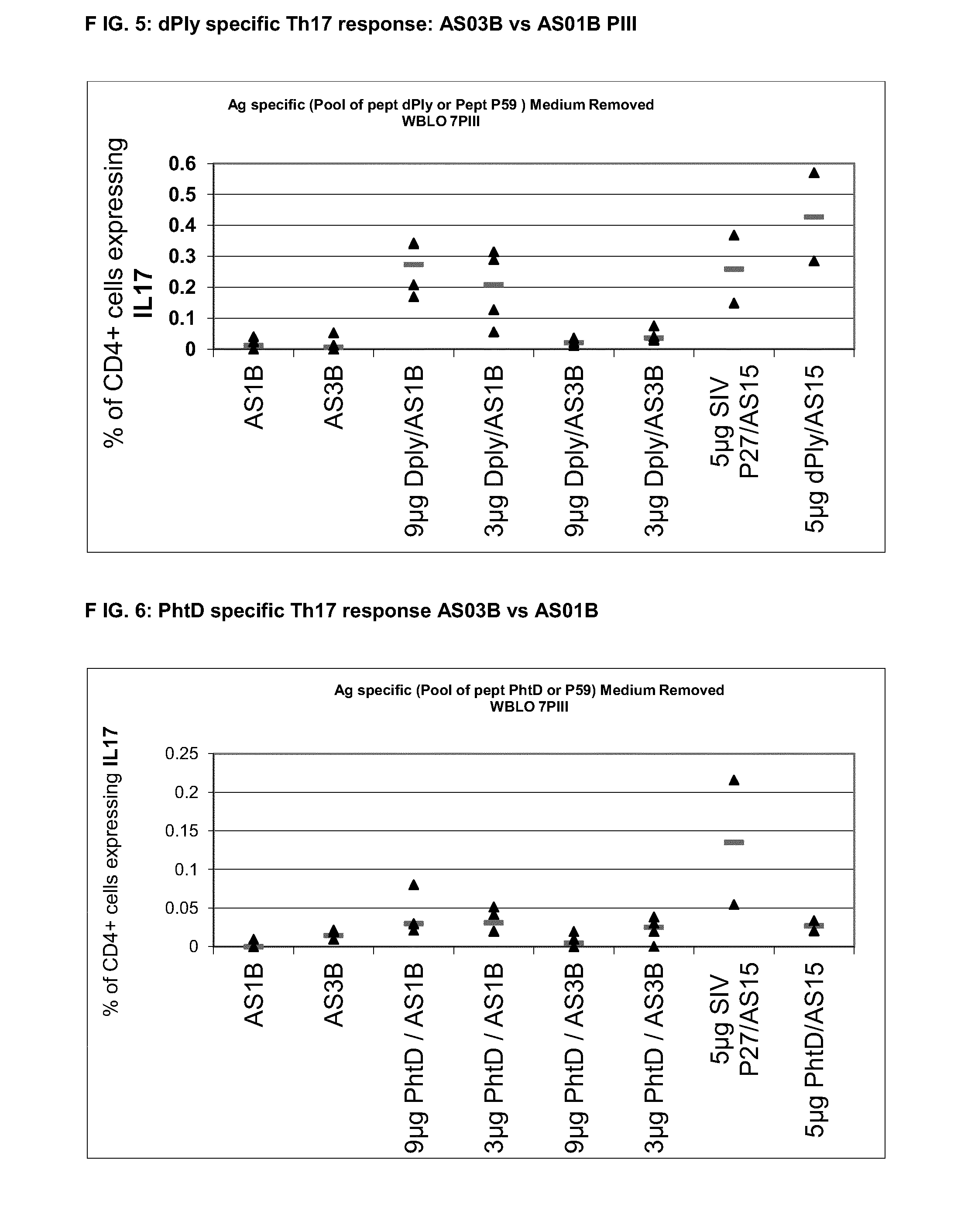
The present invention relates to improved immunogenic compositions and vaccines, methods for making them and their use in medicine. In particular the invention relates to immunogenic compositions of unconjugated Streptococcus pneumoniae proteins selected from: pneumolysin and member(s) of the Polyhistidine Triad family (e.g. PhtD), which comprise adjuvants comprising QS21 and monophosphoryl lipid A (MPL), and are presented in the form of a liposome.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
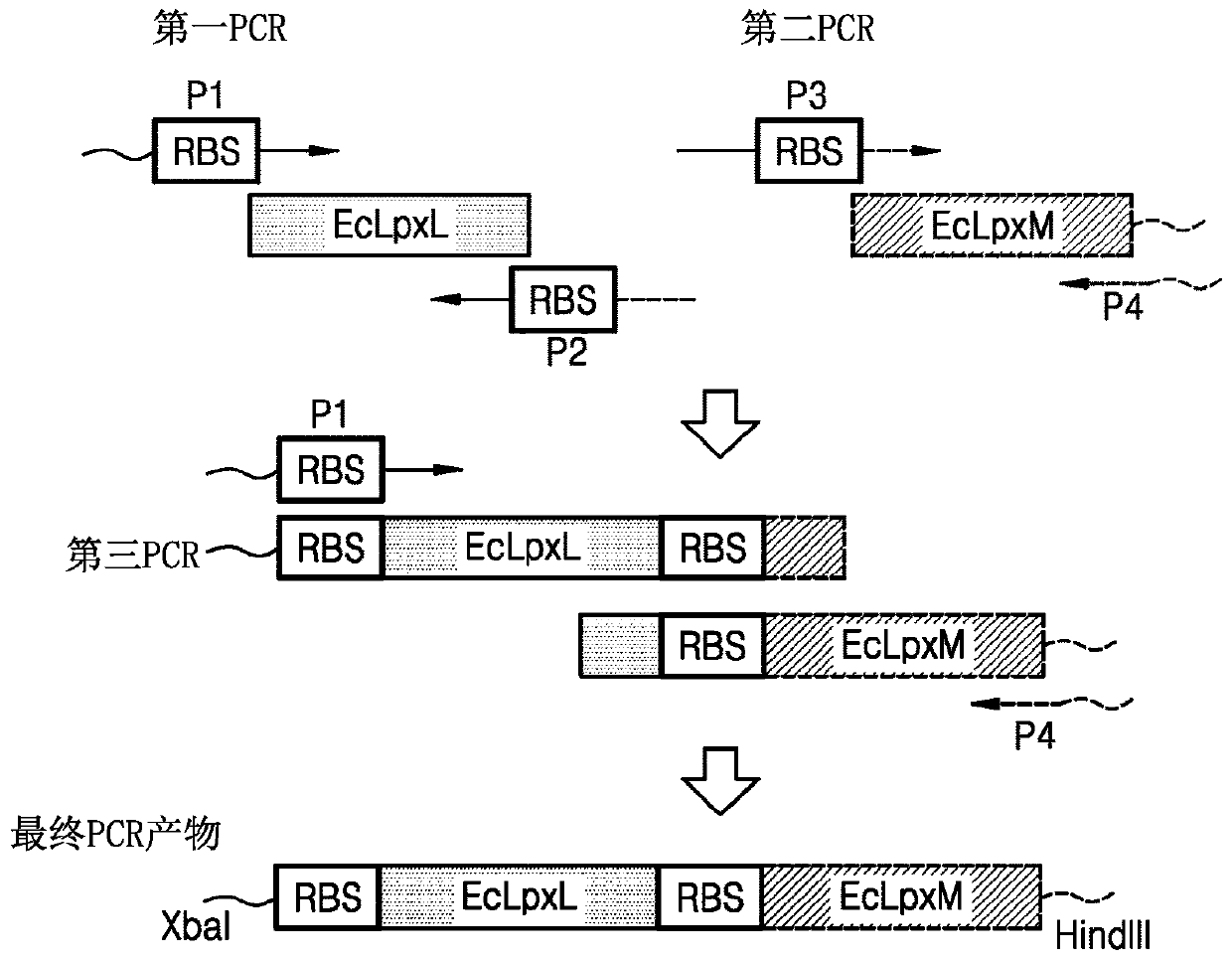
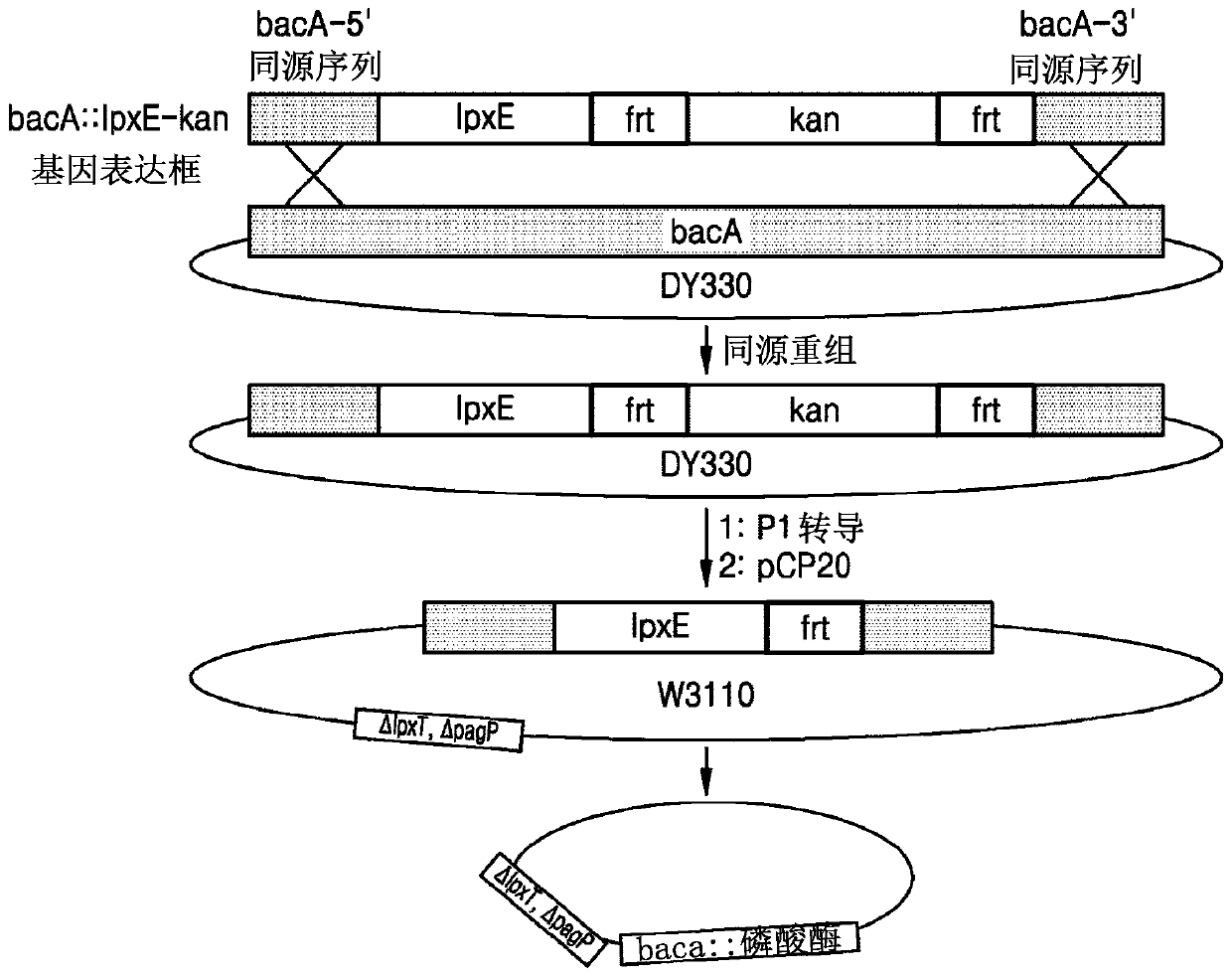
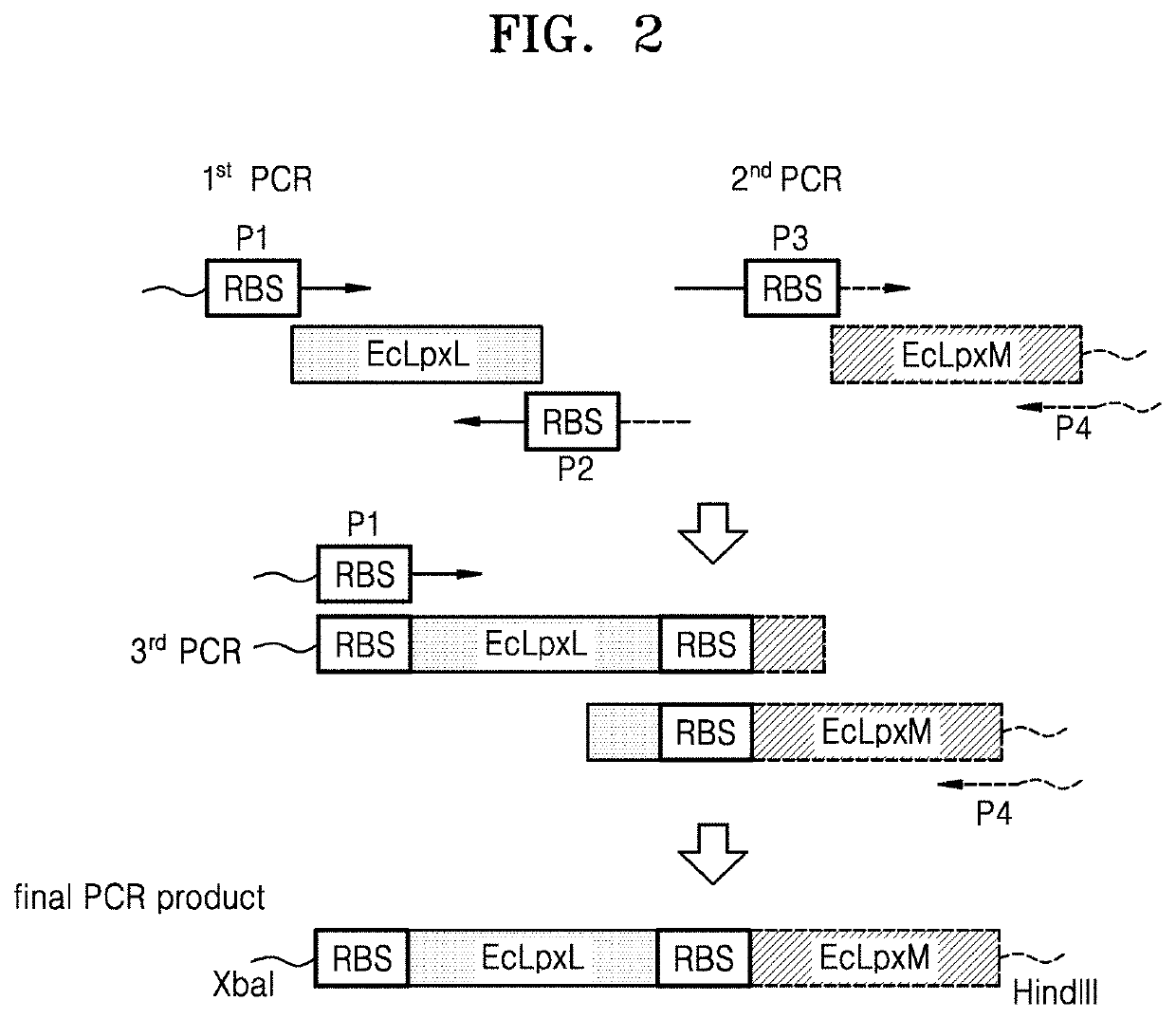
Bacterium producing monophosphoryl lipid A and method of producing monophosphoryl lipid A by using bacterium
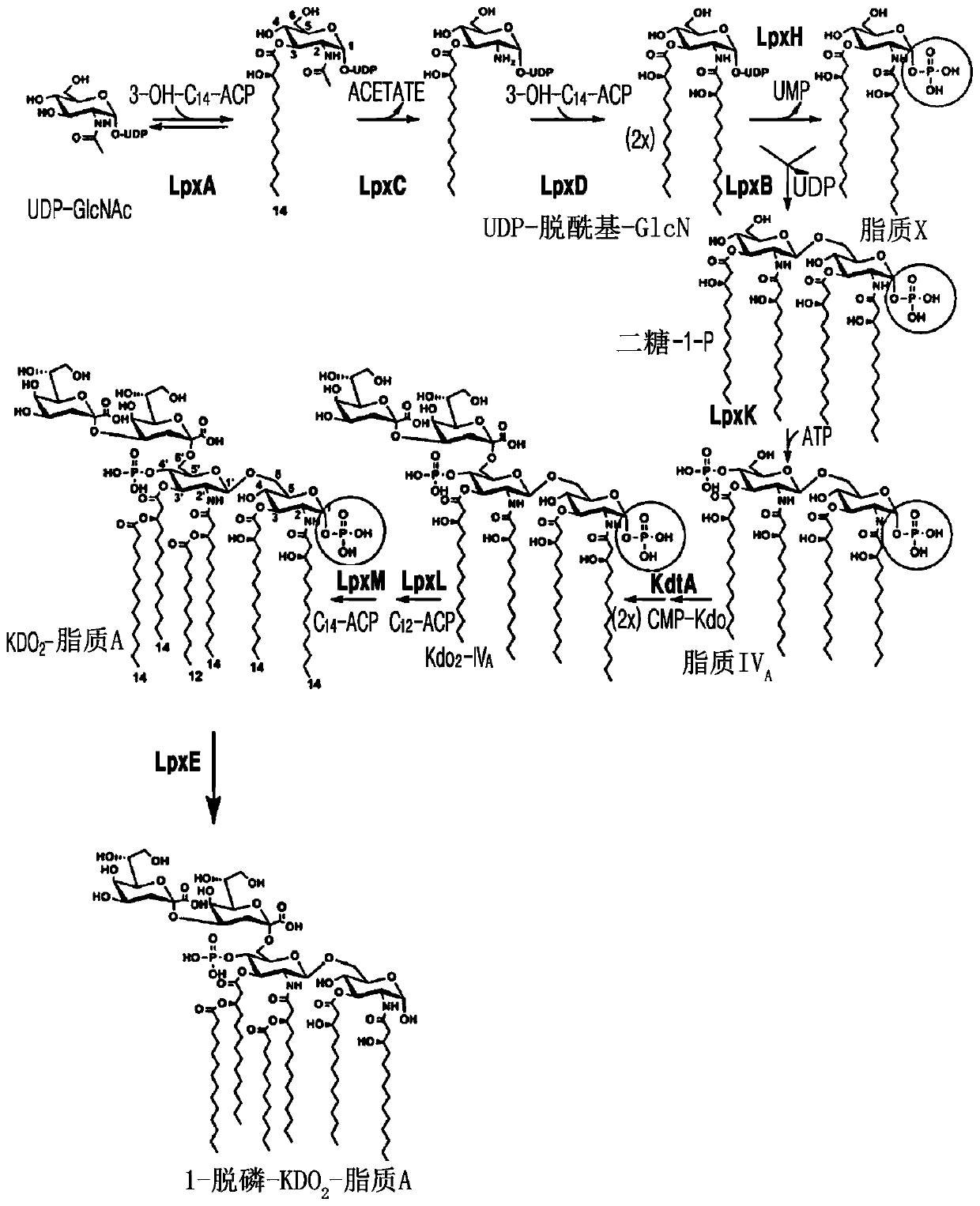
A bacterium producing monophosphoryl lipid A (MLA) comprising a genetic modification that increases expression of a gene encoding LpxE polypeptide and a method of producing MLA are provided. Accordingto the present invention, MLA may be produced in a simple manner without acid hydrolysis and / or base hydrolysis.
Owner:KOREA INST OF SCI & TECH
A vaccine composition comprising an immunogenic protein and combination adjuvants for use in eliciting antigen-specific t-cell responses
Owner:THEVAX GENETICS VACCINE
Compositions and methods for viral delivery
InactiveUS20030228279A1Good curative effectReduce doseOrganic active ingredientsBiocideDiseasePhosphate
Compositions and methods comprising a recombinant virus and an immunostimulant are provided for enhancing the immune response to a polypeptide expressed from the recombinant virus. Preferably this is done without also enhancing the neutralizing antibody response to the recombinant virus. Illustrative compositions comprise an adenovirus and an adjuvant such as, for example, monophosphoryl lipid A, an alkyl glucosaminide phosphate, a saponin, or a combination thereof. The disclosed compositions and methods are useful, for example, in the treatment of diseases such as cancer or infectious disease.
Owner:CORIXA CORP
Methods for the production of 3-o-deactivated-4'-monophosphoryl lipid a (3d-mla)
InactiveUS20070212758A1Low in phospholipidsSimple and inexpensive stepEsterified saccharide compoundsOrganic active ingredientsBiotechnologyAcyl group
Herein is disclosed a method for producing lipopolysaccharide (LPS), comprising: (a) growing a culture of deep rough mutant bacterial strain in a medium; (b) maintaining the culture in stationary phase for at least about 5 hr; (c) harvesting cells from the culture; and (d) extracting LPS from the cells. The method allows for the production of an LPS which can be used to produce a 3-O-deacylated monophosphoryl lipid A (3D-MLA) having at least about 20 mol % of the hexaacyl congener group. Also herein is disclosed a method of extracting lipopolysaccharide (LPS) from a culture of deep rough mutant bacterial strain cells, comprising: (a) extracting the cells with a solution consisting essentially of at least about 75 wt % of an aliphatic alcohol having from 1 to 4 carbon atoms and the balance water, thereby producing cells with reduced phospholipid content; and (b) extracting the cells with reduced phospholipid content with a solution comprising chloroform and methanol, thereby yielding a solution of LPS in chloroform and methanol (CM). This method provides LPS solutions in CM that have reduced phospholipid content and are produced by relatively simple and inexpensive process steps.
Owner:CORIXA CORP
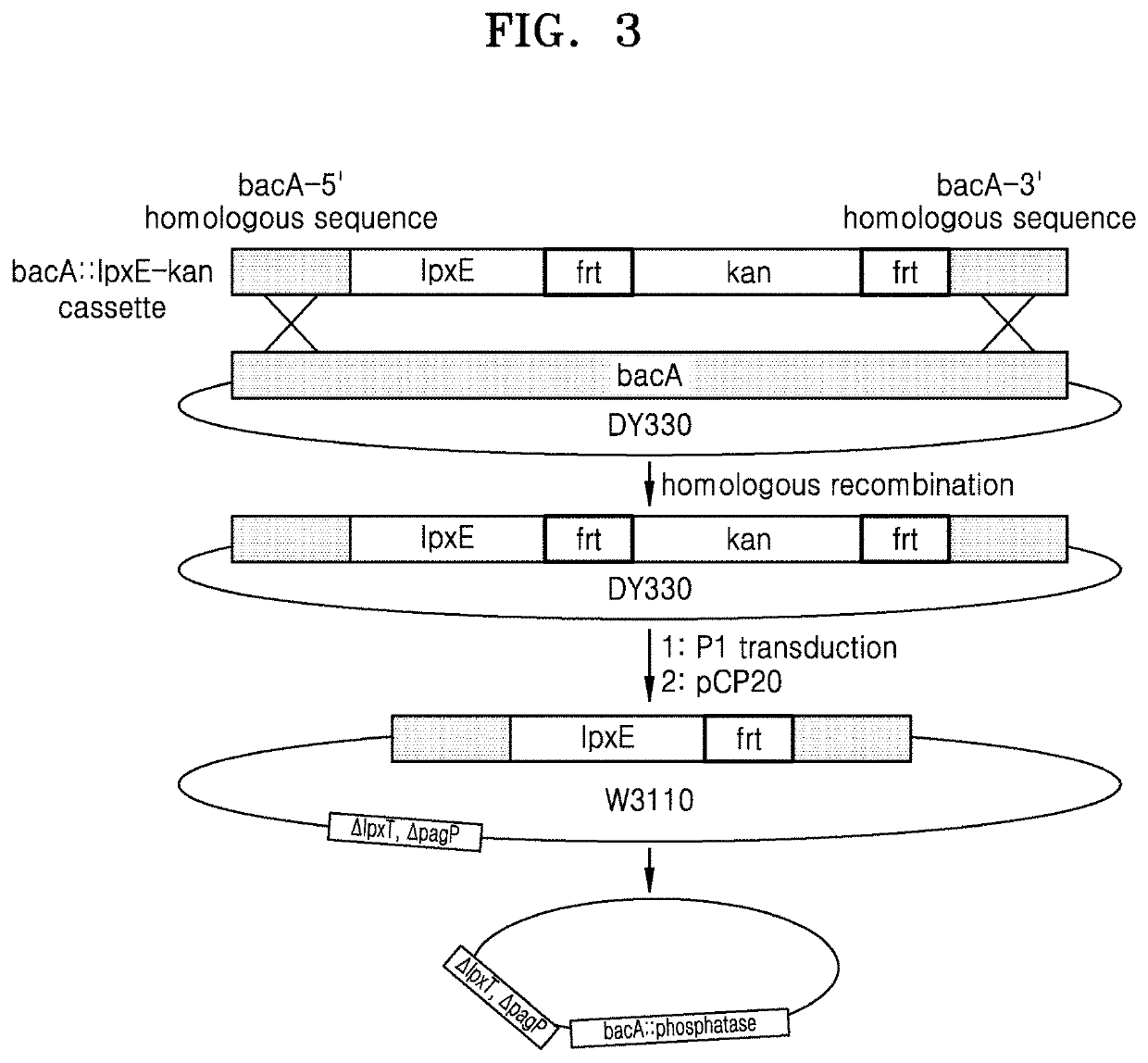
Bacterium constitutively producing monophosphoryl lipid a and method of producing monophosphoryl lipid a by using bacterium
A bacterium that constitutively produces monophosphoryl lipid A (MLA) and a method of producing MLA by using the bacterium may simply produce MLA and a derivative thereof without acid hydrolysis, reduce a probability of natural mutation, and increase yields of MLA and a derivative thereof by constitutive expression of the MLA and derivative thereof.
Owner:KOREA INST OF SCI & TECH
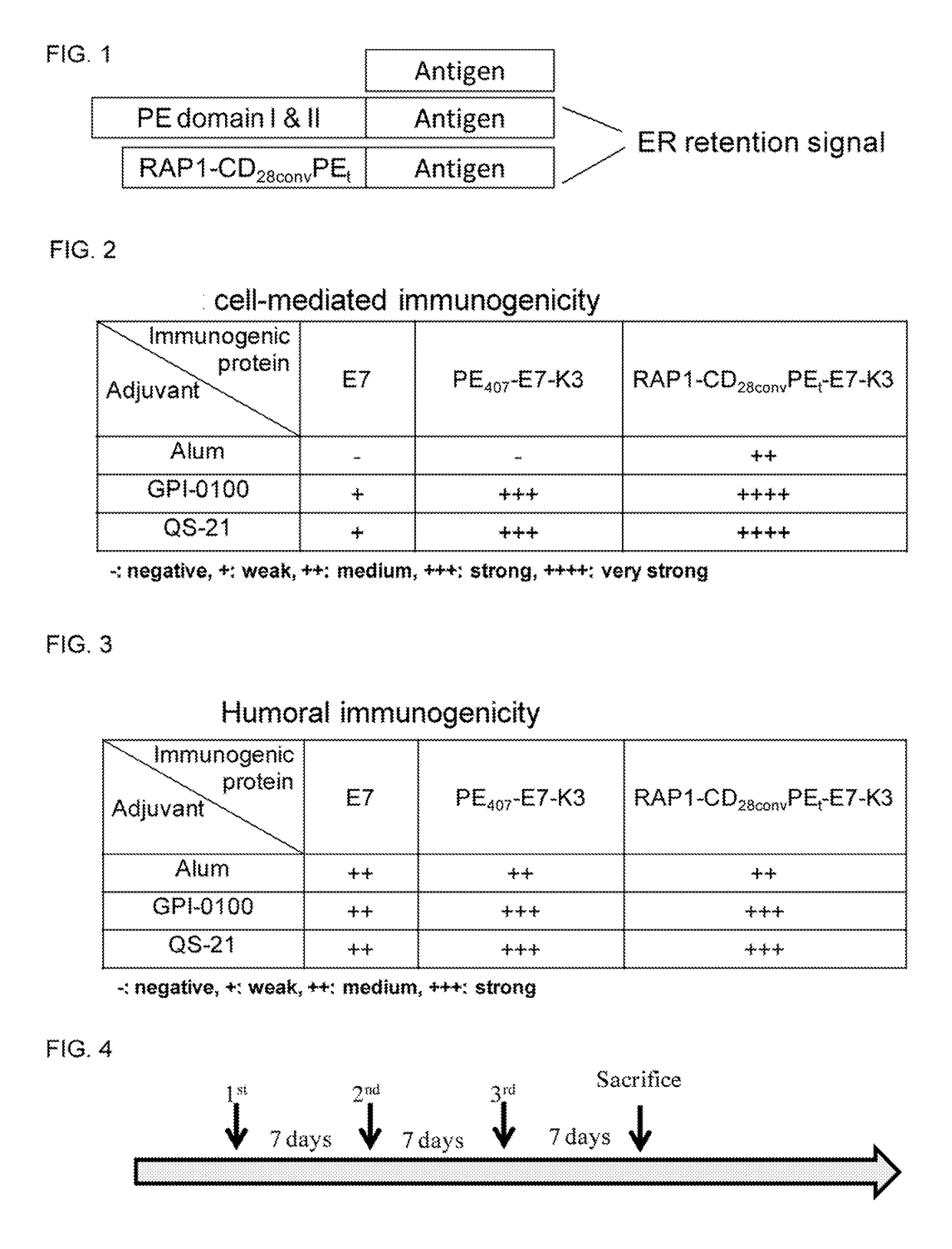
Vaccine composition comprising an immunogenic protein and combination adjuvants for use in eliciting antigen-specific T-cell responses
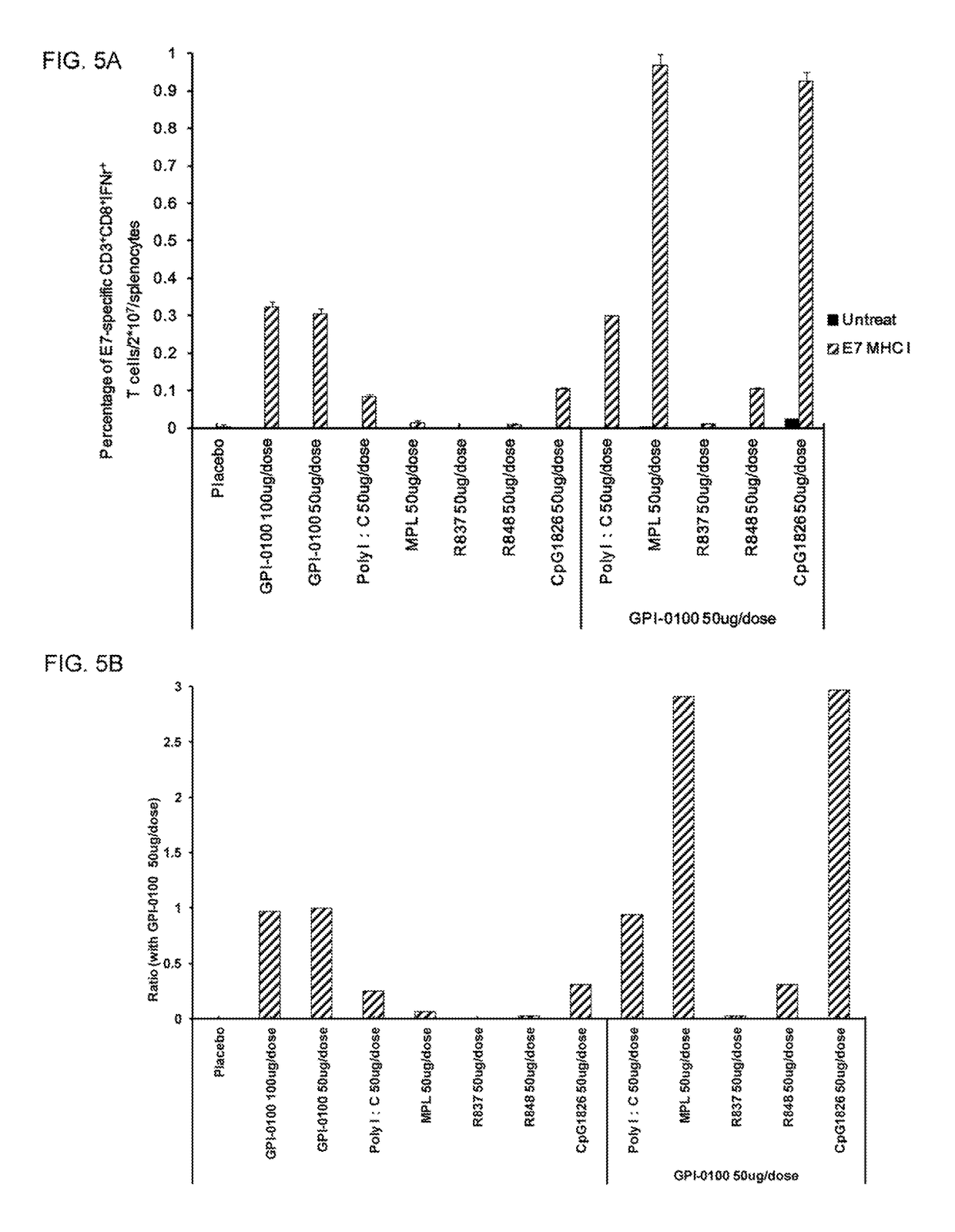
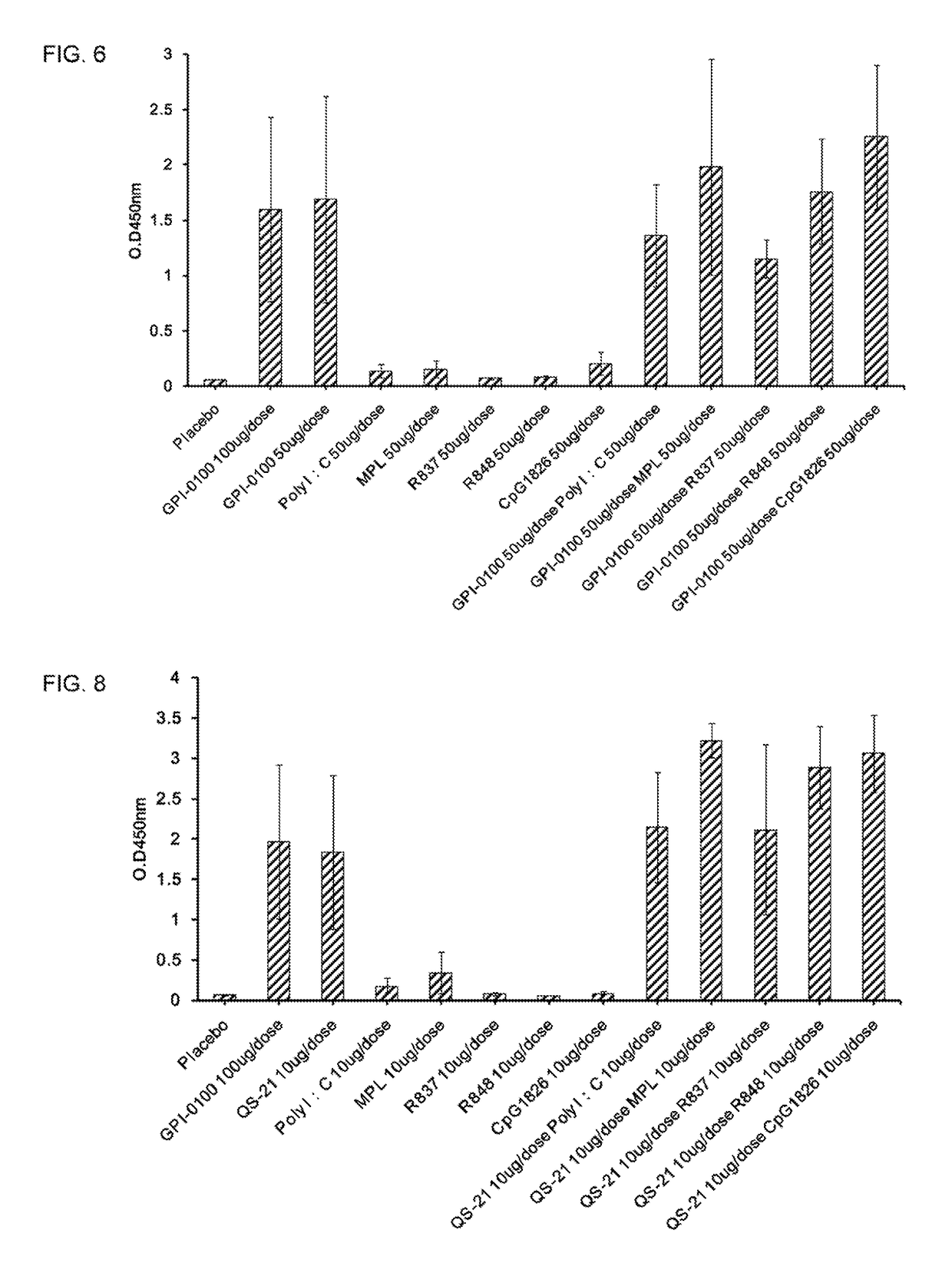
Vaccine compositions for use in inducing enhanced antigen-specific T cell-mediated immune responses in a subject in need thereof are disclosed. The composition comprises (a) a therapeutically effective amount of an immunogenic protein comprising at least an antigen of a pathogen; (b) a saponin-base adjuvant selected from the group consisting of GPI-0100, Quil A, QS-21; and (c) a Toll-like receptor (TLR) agonist adjuvant selected from the group consisting of monophosphoryl lipid A (MPL), and CpG1826.
Owner:THEVAX GENETICS VACCINE
Self-adjuvanting yersinia outer membrane vesicle as a vaccine against plague, anthrax and pseudomonas infection
ActiveUS20210177956A1Maximum protectionRemove virulence factorAntibacterial agentsBacterial antigen ingredientsVaccinationImmunogenicity
A vaccine platform using a Yersinia pestis mutant synthesizing an adjuvant form lipid A (monophosphoryl lipid A, MPLA) for the increased biogenesis of bacterial outer membrane vesicles (OMVs). To enhance the immunogenicity of the OMVs, an Asd-based balanced-lethal host-vector system was constructed to oversynthesize the LcrV antigen of Y. pestis, raise the amounts of LcrV enclosed in OMVs by Type II secretion system, and eliminate harmful factors like plasminogen activator (Pla) and murine toxin from the OMVs. Vaccination with OMVs containing MPLA and increased amounts of LcrV with diminished toxicity afforded complete protection in mice against subcutaneous challenge and intranasal challenge and was significantly superior to that resulting from vaccination with LcrV / alhydrogel. Additionally, the Yersinia OMV can be used as a platform to deliver the heterologous antigens of Bacillus anthraces. Vaccination with multiantigenic self-adjuvanting bionanoparticles from Pseudomonas was also successfully tested in connection with Pseudomonas aeruginosa.
Owner:ALBANY MEDICAL COLLEGE
Induction of highly specific antibodies to a hapten but not to a carrier peptide by immunization
ActiveUS20150098935A1Facilitating vaccination protocolAvoid poisoningBiocideImmunoglobulins against animals/humansCarrier proteinLiposome
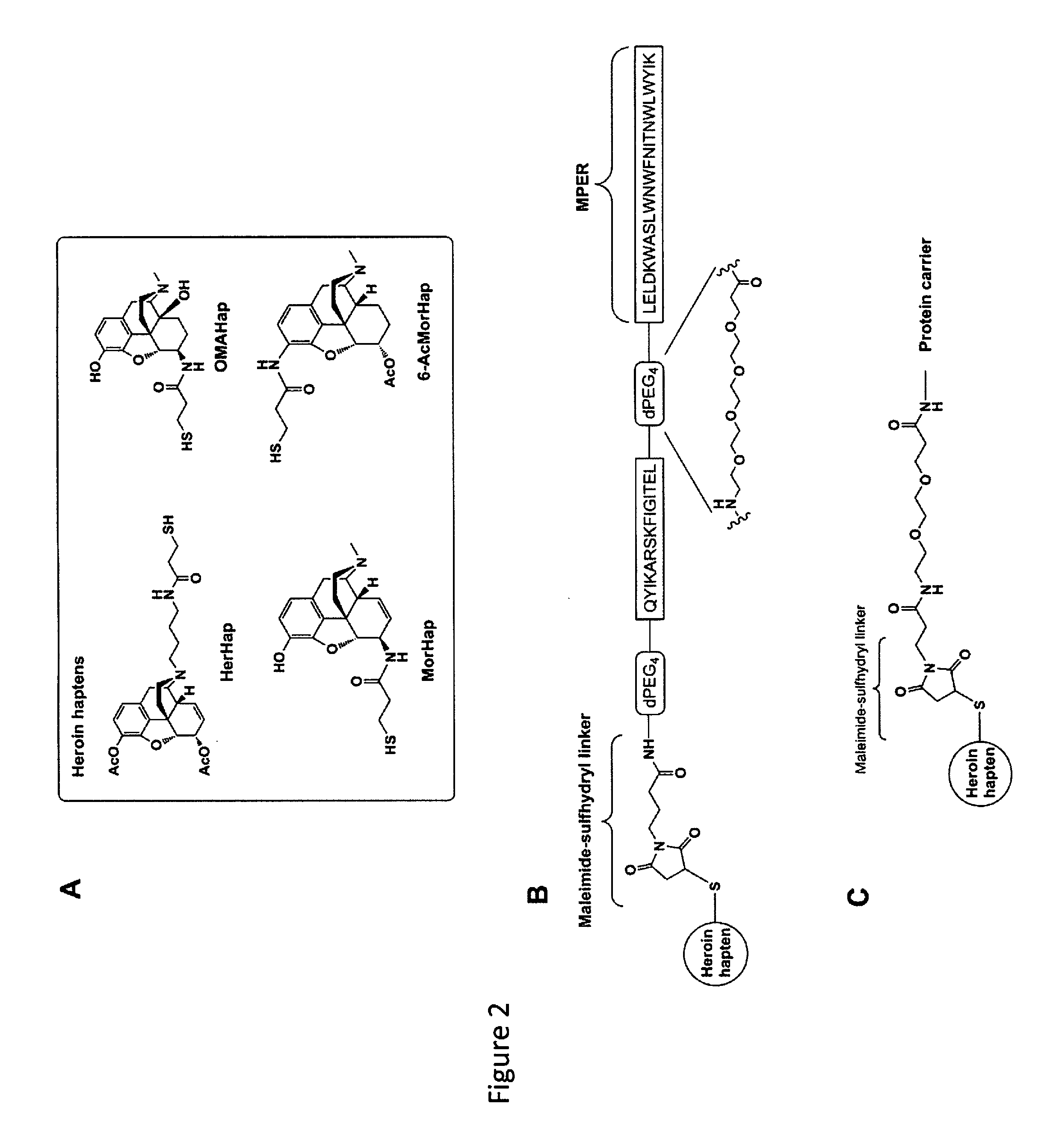
In this application Is described a composition and method for Inducing In a subject anti-hapten antibodies without Inducing antibodies to the carrier protein. Kits for designing and making compositions with desired haptens are also described. In this application Is disclosed a synthetic liposome composition comprising liposomes (L) containing monophosphoryl lipid A (MPLA) [L(MPLA)] and an immunoconjugate comprising a carrier and a hapten. In one embodiment, the carrier is a 23 amino acid hydrophobic membrane proximal external region peptide (MPER) derived from the gp41 transmembrane protein of HIV-1 that spontaneously associates with the outer surface of bilayers of liposomes containing MPLA during liposome formation.
Owner:UNITED STATES OF AMERICA THE AS REPRESENTED BY THE SEC OF THE ARMY
Adjuvant and antigen particle formulation
InactiveUS20140105970A1Reduce drippingNot to damageSsRNA viruses negative-sensePowder deliveryVirusCopolymer
A composition as disclosed is comprised of a plurality of particles and a pharmaceutically acceptable carrier. The particles are comprised of (1) an adjuvant; (2) a biocompatible polymer which maybe a co-polymer such as PLGA, and (3) a peptide of a sequence of interest, e.g. a sequence which corresponds to a sequence presented on a surface of a cell infected with a virus. The carrier includes an adjuvant such as a monophosphoryl lipid A (MPL) different from the adjuvant in the particles. The particles may be sized such that they are sufficiently large so as to prevent more than the contents of a single particle from being presented to a single immune system cell.
Owner:FLOW PHARMA
Site-directed mutagenesis carrier protein and purpose thereof to vaccine preparation
ActiveCN111533792AEfficient modification purposePurpose of specific modificationAntibacterial agentsBacterial antigen ingredientsProtein s antigenCarrier protein
The invention relates to a site-directed mutagenesis and site-directed modification protein antigen, and also relates to a site-directed mutagenesis and site-directed modification method of the protein antigen. The method comprises the following steps of introducing unnatural amino acids into specific sites of the protein antigen in a site-directed way by using a gene codon extension technology; and performing site-directed modification with the protein antigen by using the unnatural amino acids and a modifier, wherein the modifier is a receptor agonist such as tripalmitoyl-S-glyceryl cysteineand monophosphoryl lipid A. The invention further relates to application of the site-directed mutagenesis and site-directed modification protein antigen, such as a purpose as a vaccine and the like.
Owner:CANSINO BIOLOGICS INC
Compositions and Methods for Vaccine Delivery
The invention relates to pharmaceutical compositions comprising at least one antigen and an adjuvant composition, where the adjuvant composition comprises a saponin and a liposome. The liposome of the composition comprises monophosphoryl lipid A (MPLA), cholesterol and a phospholipid that is in a liquid crystalline state at greater than or equal to 23° C., and the concentration of cholesterol to lipid in the liposome is greater than 50% (mol / mol). The antigen in the composition is a soluble Plasmodium falciparum recombinant circumsporozoite protein (rCSP) comprising the amino acid sequence of SEQ ID NO:1, or a P. falciparum rCSP peptide that is at least 95% identical to the amino acid sequence of SEQ ID NO:1.
Owner:THE HENRY M JACKSON FOUND FOR THE ADVANCEMENT OF MILITARY MEDICINE INC +1
Self-adjuvanting yersinia outer membrane vesicle as a vaccine against plague, anthrax and pseudomonas infection
ActiveUS11167019B2Remove virulence factorIncrease productionAntibacterial agentsBacterial antigen ingredientsVaccinationImmunogenicity
A vaccine platform using a Yersinia pestis mutant synthesizing an adjuvant form lipid A (monophosphoryl lipid A, MPLA) for the increased biogenesis of bacterial outer membrane vesicles (OMVs). To enhance the immunogenicity of the OMVs, an Asd-based balanced-lethal host-vector system was constructed to oversynthesize the LcrV antigen of Y. pestis, raise the amounts of LcrV enclosed in OMVs by Type II secretion system, and eliminate harmful factors like plasminogen activator (Pla) and murine toxin from the OMVs. Vaccination with OMVs containing MPLA and increased amounts of LcrV with diminished toxicity afforded complete protection in mice against subcutaneous challenge and intranasal challenge and was significantly superior to that resulting from vaccination with LcrV / alhydrogel. Additionally, the Yersinia OMV can be used as a platform to deliver the heterologous antigens of Bacillus anthraces. Vaccination with multiantigenic self-adjuvanting bionanoparticles from Pseudomonas was also successfully tested in connection with Pseudomonas aeruginosa.
Owner:ALBANY MEDICAL COLLEGE
Acquisition method and application of individualized tumor neoantigen specific CD8 cells
PendingCN113151166AEasy to controlCulture processCancer antigen ingredientsPeripheral blood mononuclear cellCD8
The invention relates to an acquisition method and application of individualized tumor neoantigen specific CD8 cells. The acquisition method comprises the following steps: 1) injecting tumor neoantigen vaccines into a patient, and then sampling blood; 2) obtaining peripheral blood mononuclear cells by a density gradient centrifugation method, and cryopreserving the obtained peripheral blood mononuclear cells; (3) performing mononuclear cell culture; (4) adding monophosphoryl lipid A and gamma interferon for culture; (5) collecting mature DC cells for continuous culture; (6) resuscitating the cryopreserved resuscitated lymphocytes, mixing the resuscitated lymphocytes with the DC cells in proportion, continuing to culture, and adding human recombinant interleukin 2 on the third day and the tenth day; and (7) on the seventh day of co-culture of the DC cells and the lymphocytes, resuscitating the cryopreserved antigen-loaded DC cells, performing adding for co-culture on the fourteenth day, and harvesting the antigen-specific CD8 CELLs. According to the method, the specific antigen is obtained through individualization, and the CD8 cells are activated and tumor cells are killed through the antigen presented by the DC cells, so that tumor growth is more effectively controlled.
Owner:广州润生细胞医药科技有限责任公司
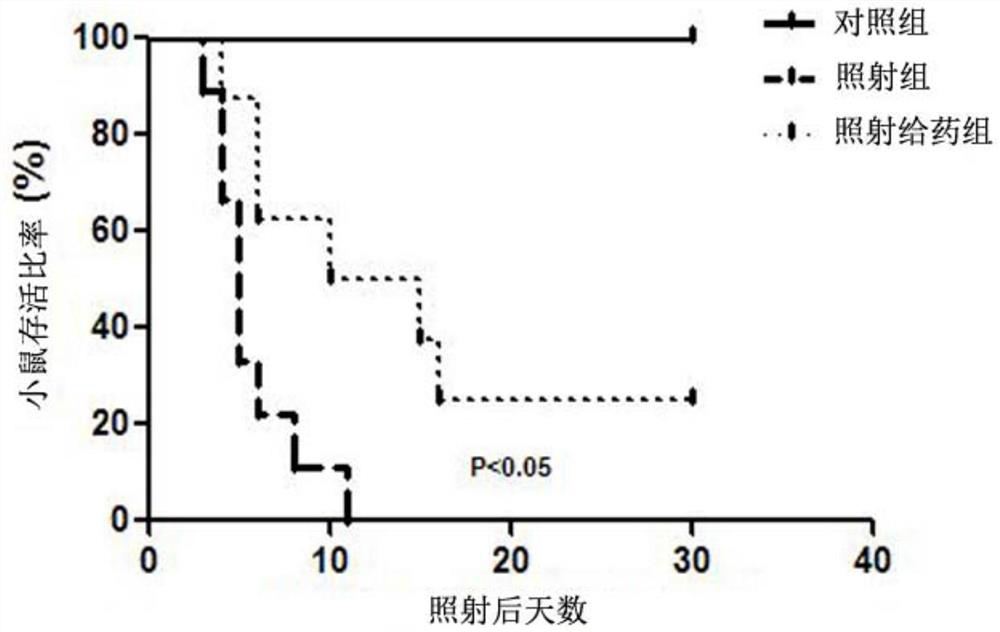
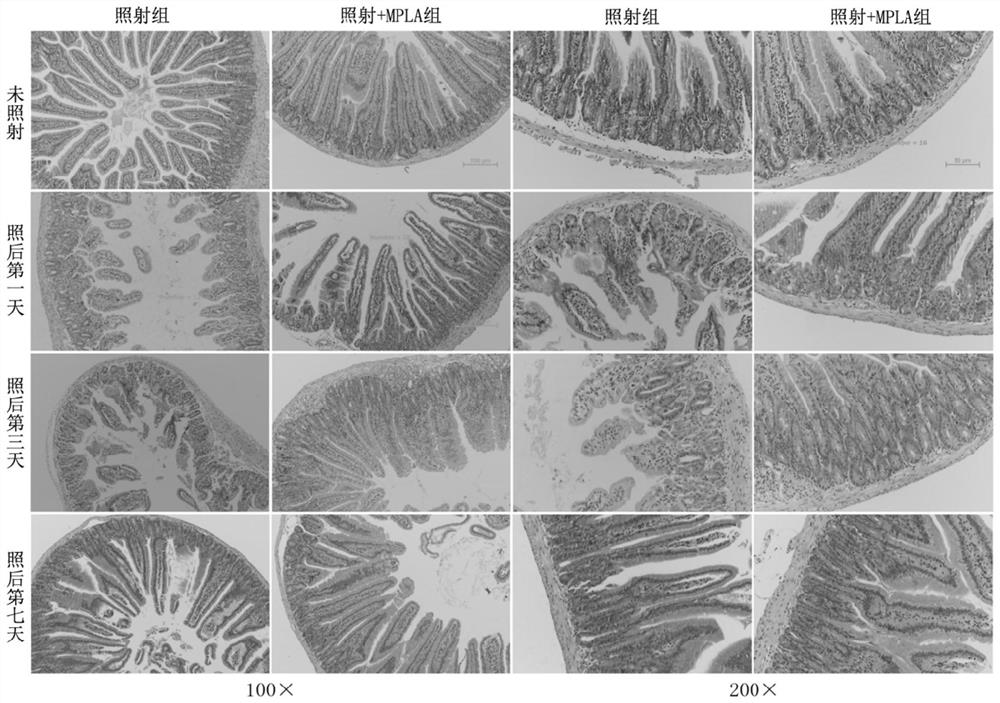
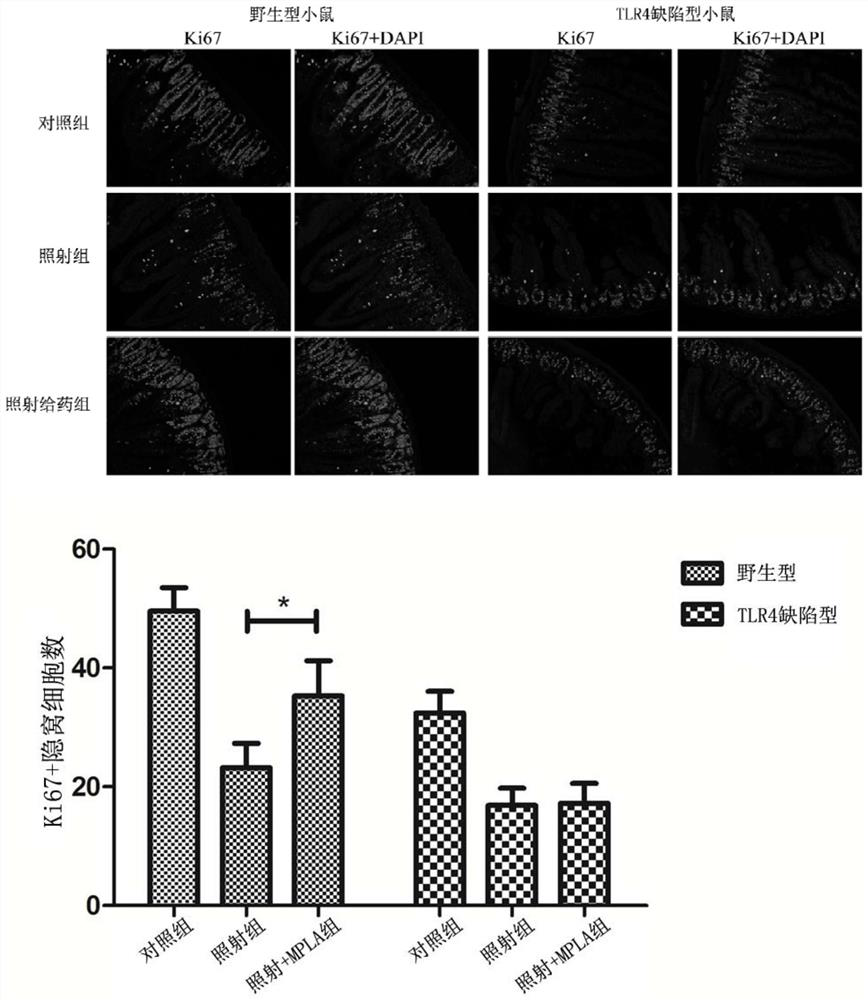
Application of mpla in the preparation of drugs for the prevention and treatment of intestinal damage caused by ionizing radiation
ActiveCN107669692BSmall side effectsObvious side effectsOrganic active ingredientsDigestive systemDiseaseSide effect
The invention belongs to the technical field of medicine, and more specially discloses applications of MPLA (Monophosphoryl lipid A) in preparation of drugs used for preventing and treating ionizationradiation induced intestinal injuries. Toxic and side effect of MPLA as a drug used for preventing and treating ionization radiation induced intestinal injuries is low, curative effect on ionizationradiation induced intestinal injuries is excellent, and drug using is safe and convenient. MPLA possesses unique advantages in preventing and treating ionization radiation induced intestinal injuries,and a novel method is provided for preventing and treating ionization radiation induced intestinal injuries. Effective preventing and treating methods on intestinal radiation injuries and intestinalradiation disease are few at home and abroad, development of the methods is a key problem in the field of radioactive medicine, so that application prospect of MPLA as a drug used for preventing and treating ionization radiation induced intestinal injuries is promising.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Adjuvant combination formulations
InactiveUS20100233117A1Improve abilitiesSsRNA viruses negative-senseAntibacterial agentsWhite blood cellVertebrate Animals
The use of 3-O-deacylated monophosphoryl lipid A or monophosphoryl lipid A and derivatives and analogs thereof, in combination with a cytokine or lymphokine such as granulocyte macrophage colony stimulating factor or interleukin-12 is useful as an adjuvant combination in an antigenic composition to enhance the immune response in a vertebrate host to a selected antigen.
Owner:ZOETIS WHC 2
Methods for the production of 3-O-deactivated-4′-monophosphoryl lipid A (3D-MLA)
InactiveUS9512159B2High proportionLow in phospholipidsEsterified saccharide compoundsOrganic active ingredientsPhospholipidChloroform
Owner:CORIXA CORP
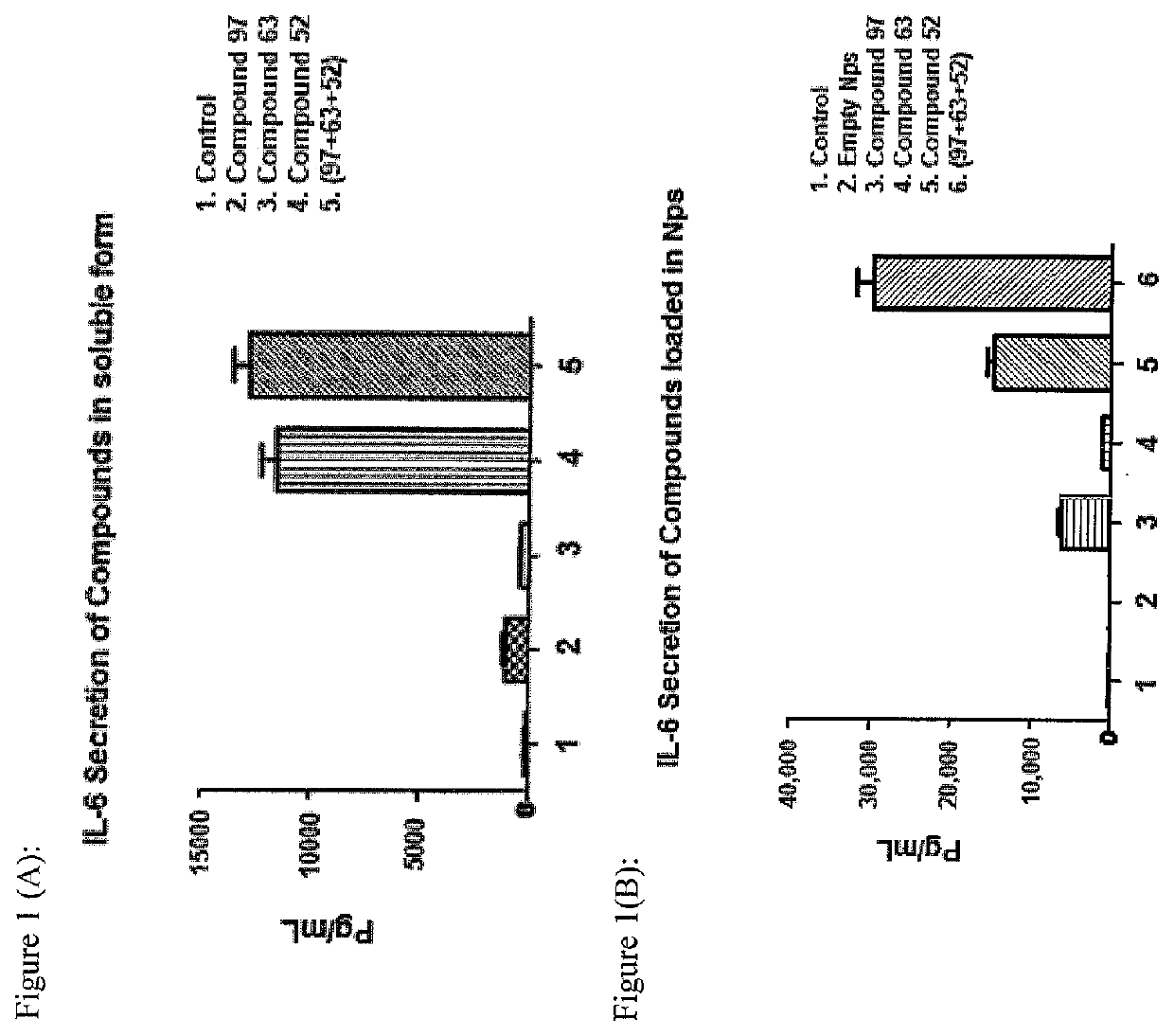
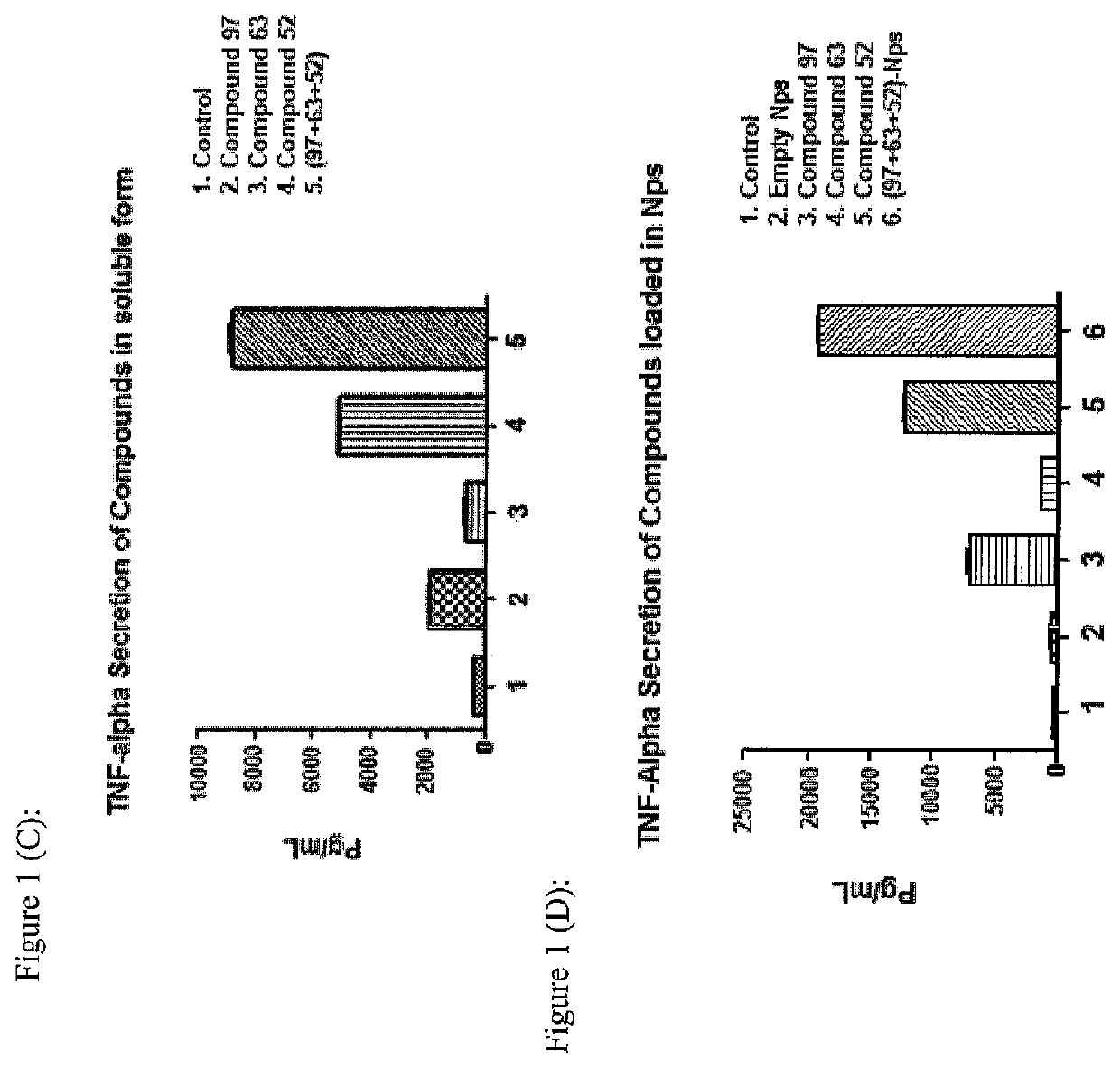
Synthetic innate immune receptor ligands and uses thereof
PendingUS20220047698A1Determine encapsulation efficiencyStrong immune responseEsterified saccharide compoundsSugar derivativesDipeptideGlycolic acid
An adjuvant formulation includes a monophosphoryl Lipid A (MPLA) analogue, a Pam3CSK4 analogue, or a muramyldipeptide (MDP) analogue, or combinations thereof. The adjuvant may be formulated in soluble form or in a nanoparticle, such as polylactic glycolic acid nanoparticles. A vaccine formulation comprises the adjuvant formulation and an immunogen. Methods of vaccinating an animal include delivering the vaccine formulation to the animal.
Owner:ALBERTA RES CHEM INC
Cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant co-loading antigen, mpla and imq, preparation method and application
ActiveCN108743939BGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The present invention relates to a cationic phospholipid-polymer hybrid nanoparticle vaccine adjuvant co-carrying antigen, MPLA and IMQ and its preparation method and application. The vaccine adjuvant carries the TLR7 agonist imiquimod in the hydrophobic core, The phospholipid layer encapsulates the TLR4 agonist monophosphoryl lipid A, absorbs the antigen through the cationic phospholipid DOTAP in the phospholipid layer, protects the antigen through the hybrid nanoparticle and improves the uptake of the antigen by dendritic cells, and significantly enhances the antigen through the TLR agonist Stimulated immune response and significantly improved antigen cross-presentation; as a vaccine adjuvant, the hybrid nanoparticles of the present invention can co-load antigens and different types of TLR agonists at the same time, and can deliver antigens in various immune pathways to promote DC activation and maturation , increase the level of cross-presentation, produce a strong T cell killing effect, induce cytokine secretion, produce long-lasting memory T cell response, and have a good preventive ability against tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Natural microorganism and plant source composite dual adjuvant capable of efficiently inducing body fluid and cellular immunity as well as preparation method and application of natural microorganism and plant source composite dual adjuvant
PendingCN114366810ARaw materials are readily availableSimple preparation processImmunological disordersAntibody medical ingredientsBiotechnologyNatural source
The invention belongs to the technical field of immunology, and discloses a natural microorganism and plant source composite dual adjuvant capable of efficiently inducing body fluid and cellular immunity, the natural microorganism and plant source composite dual adjuvant comprises monophosphoryl lipid A and ophiopogonin, and the preparation method comprises the following steps: S1, dissolving the monophosphoryl lipid A with Tween 80, castor oil, ethanol and the like; s2, dissolving ophiopogonin with Tween 85, hydrogenated castor oil, glycerol and the like; and S3, mixing the monophosphoryl lipid A solution and ophiopogonin, sub-packaging and sealing. The raw materials of the dual adjuvant are easy to obtain, the preparation process is simple, the cost is low, and the dual adjuvant is stable; two natural-source adjuvants with single immune response types are utilized to exert the synergistic advantages by utilizing the complementary principle of pathways, response types and target spots, so that the effects of slowly releasing a vaccination site and promoting antigen presentation are achieved, and efficient immune response reaction of Th2 type body fluid and Th1 type cells can be generated; the organism can be rejuvenated to generate efficient natural immune response, acquired immune response can be stimulated, and the safety and the applicability are high.
Owner:ARMY MEDICAL UNIV
Bacterium constitutively producing monophosphoryl lipid A and method of producing monophosphoryl lipid A by using bacterium
A bacterium that constitutively produces monophosphoryl lipid A (MLA) and a method of producing MLA by using the bacterium may simply produce MLA and a derivative thereof without acid hydrolysis, reduce a probability of natural mutation, and increase yields of MLA and a derivative thereof by constitutive expression of the MLA and derivative thereof.
Owner:KOREA INST OF SCI & TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com