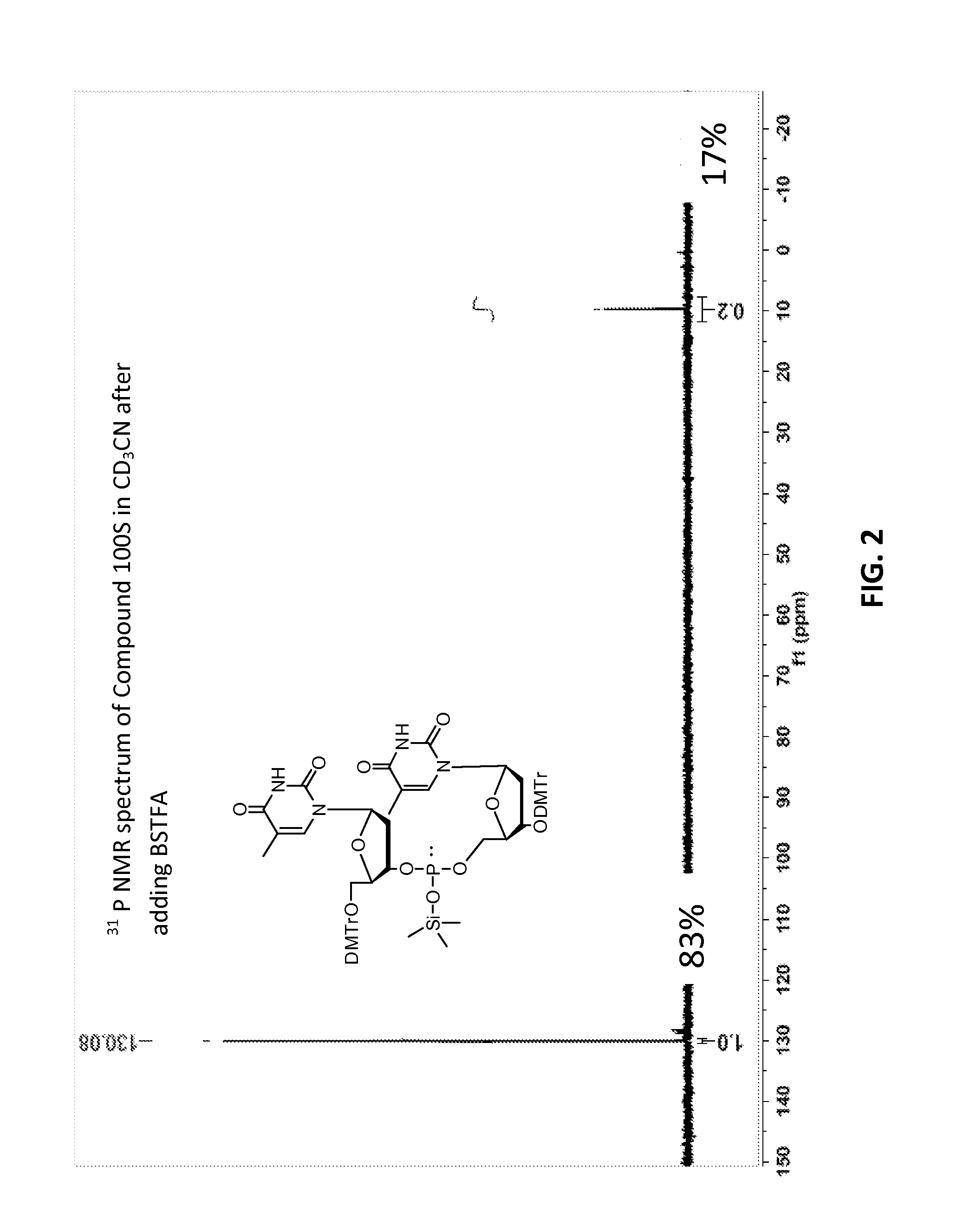
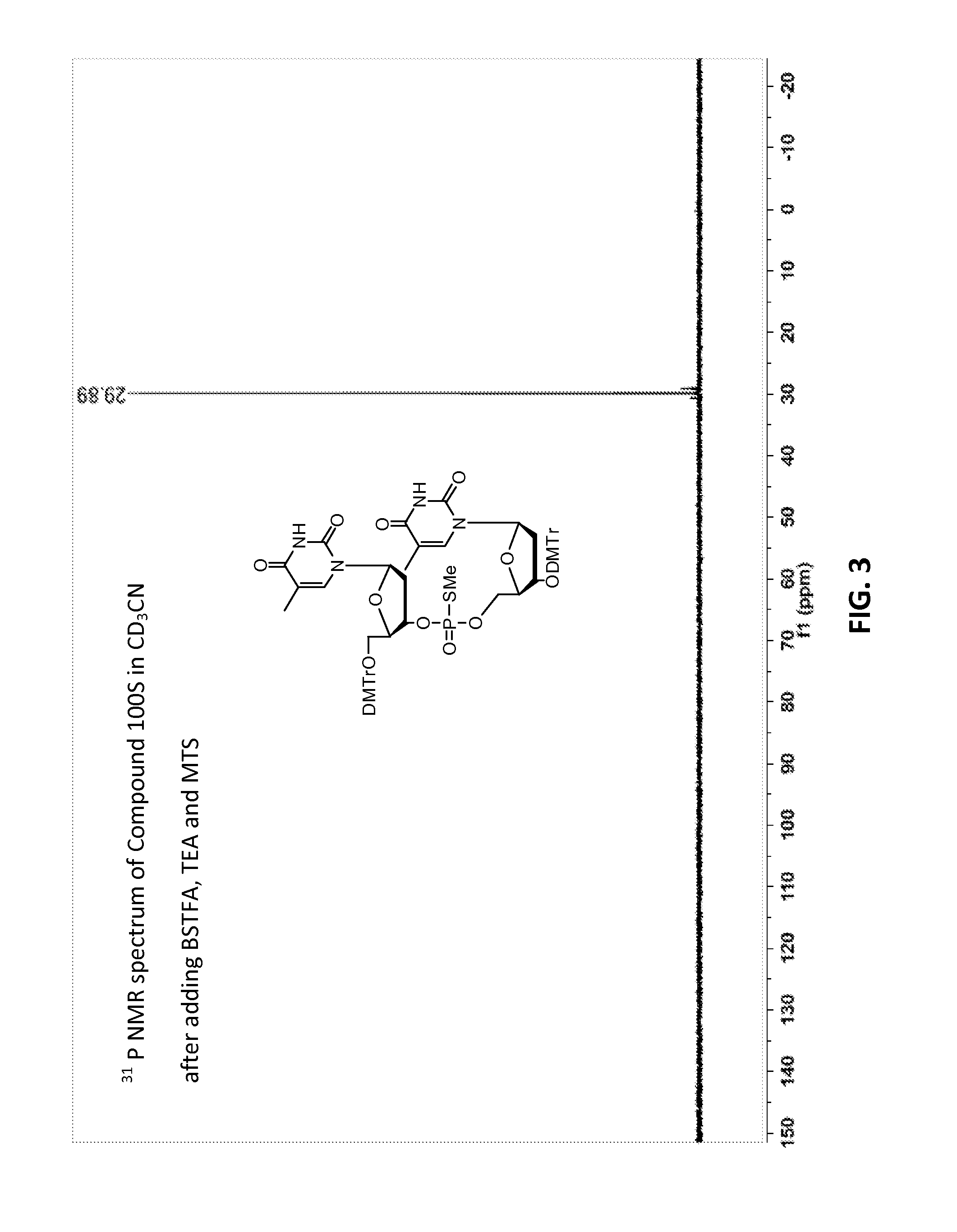
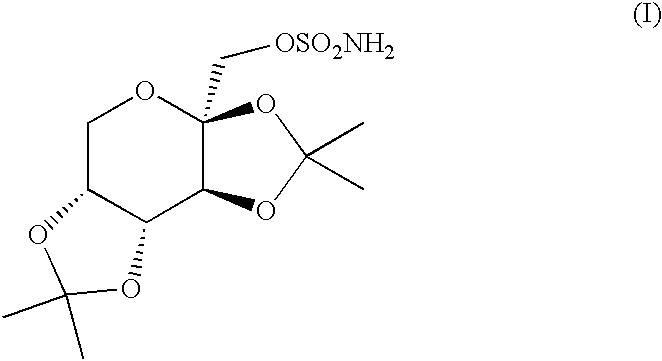
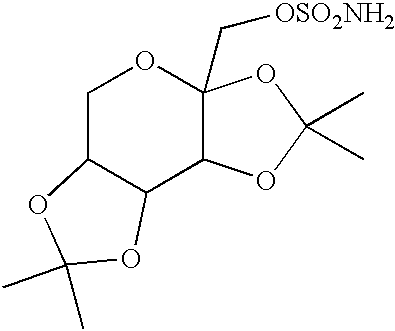
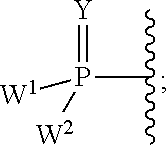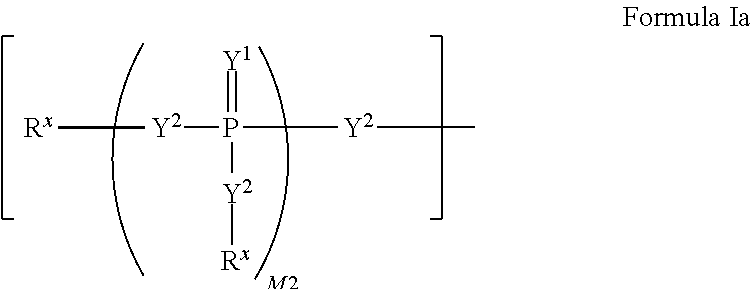Patents
Literature
2486results about "Esterified saccharide compounds" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
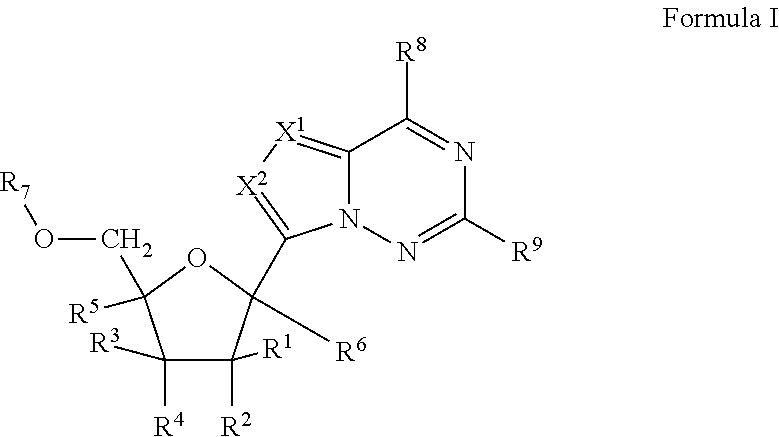
Bis-modified bicyclic nucleic acid analogs
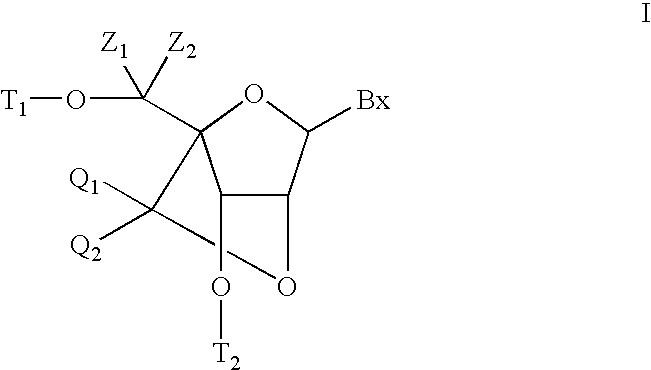
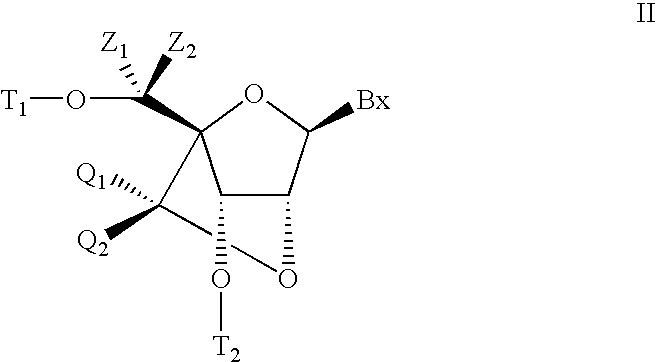
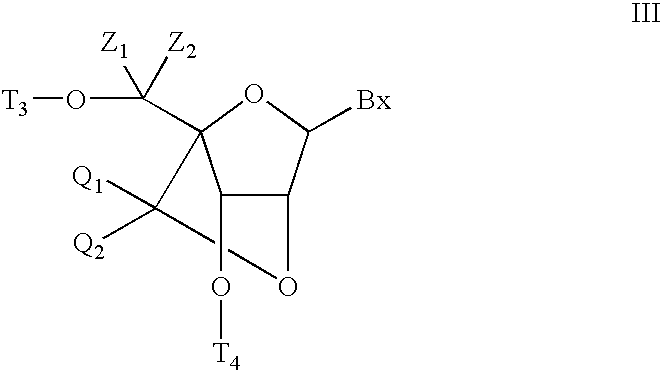
The present disclosure describes bis-modified bicyclic nucleosides and oligomeric compounds that can be prepared comprising at least one of these bis-modified bicyclic nucleosides. More particularly, the bis-modified bicyclic nucleosides have at least one substituent group at the 5′-methylene and on the bridge methylene and can be chiral. These bis-modified bicyclic nucleosides are expected to be useful for enhancing one or more property of oligomeric compounds including for example enhancing nuclease resistance.
Owner:IONIS PHARMA INC
Synthetic glucopyranosyl lipid adjuvants
Compounds, particularly, glucopyranosyl lipid adjuvant (GLA) compounds, having the following structure (I) are provided:or a pharmaceutically acceptable salt thereof, wherein L1, L2, L3, L4, L5, L6, L7, L8, L9, L10, Y1, Y2, Y3, Y4, R1, R2, R3, R4, R5, R6, are as defined herein. Pharmaceutical compositions, vaccine compositions, and related methods for inducing or enhancing immune responses, are also provided.
Owner:ACCESS TO ADVANCED HEALTH INST
Methods and compositions for making antibodies and antibody derivatives with reduced core fucosylation
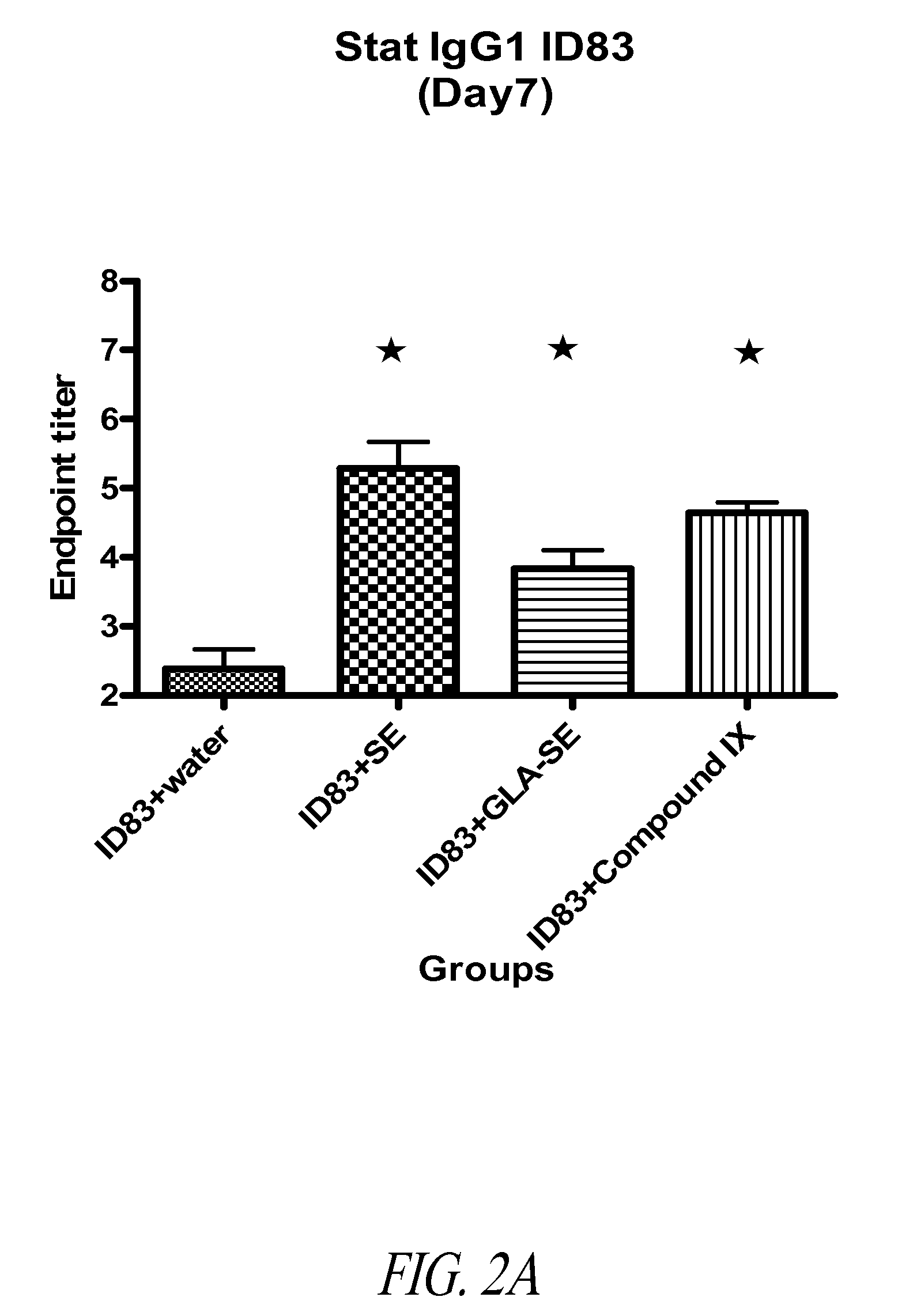
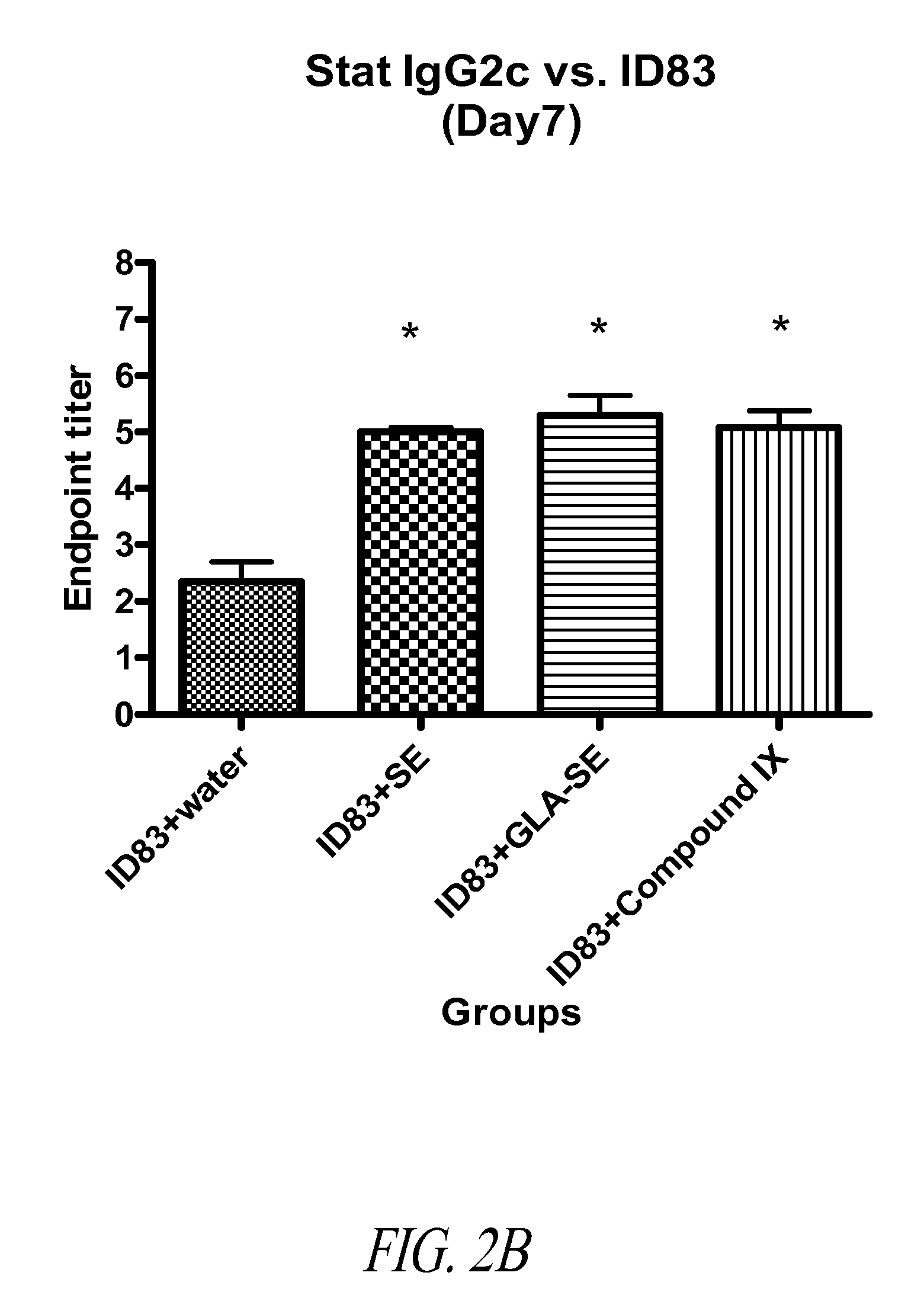
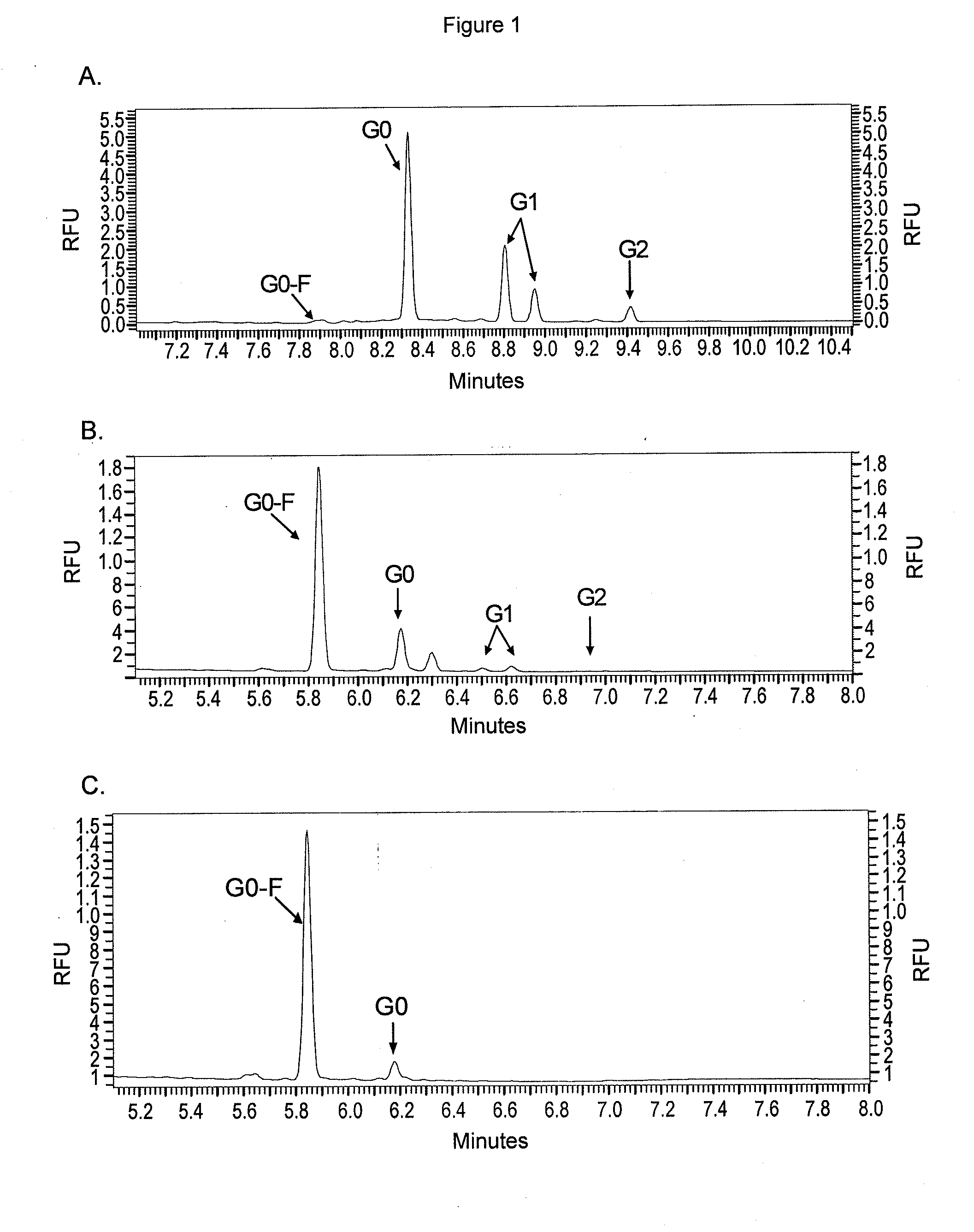
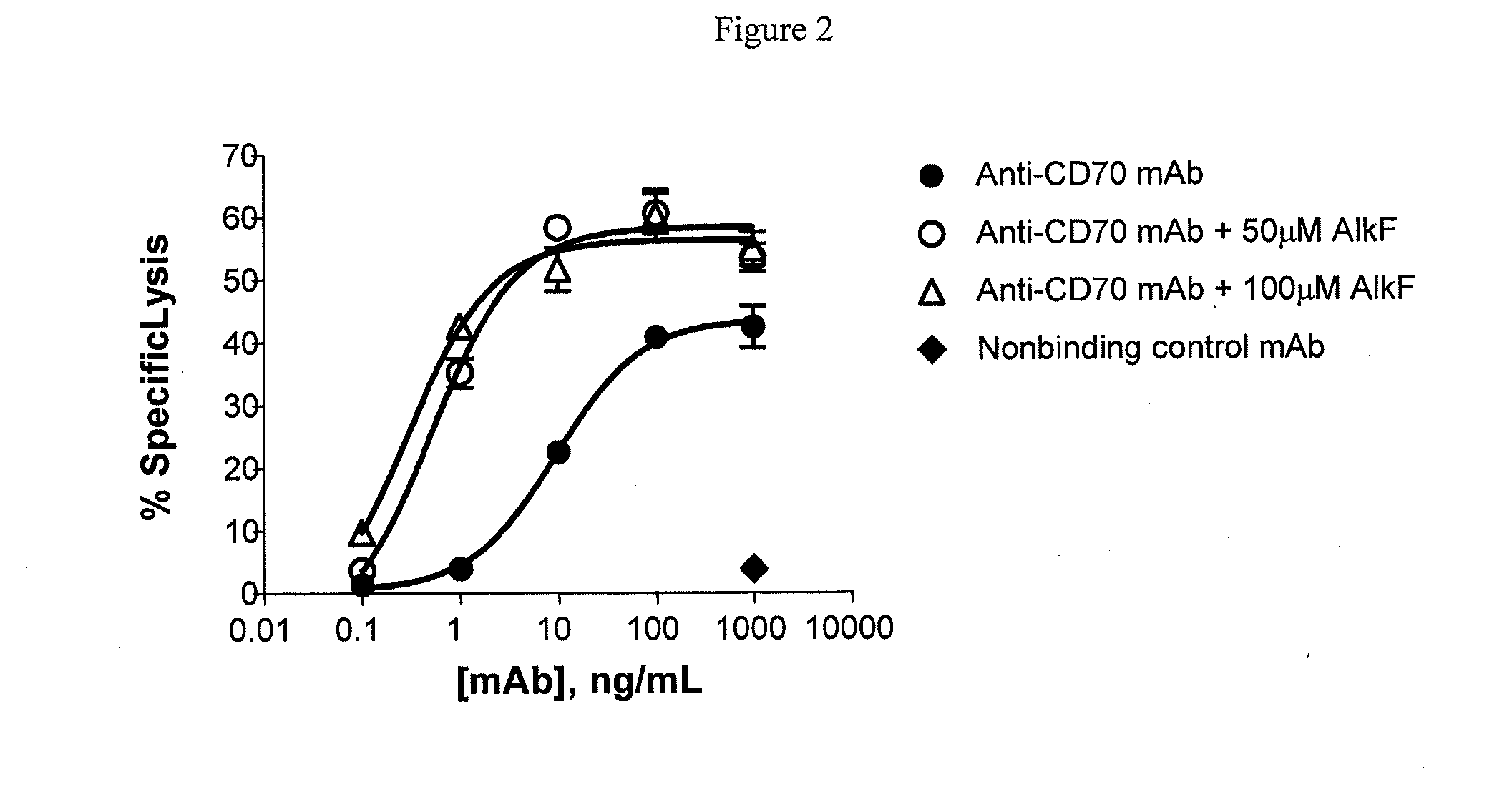
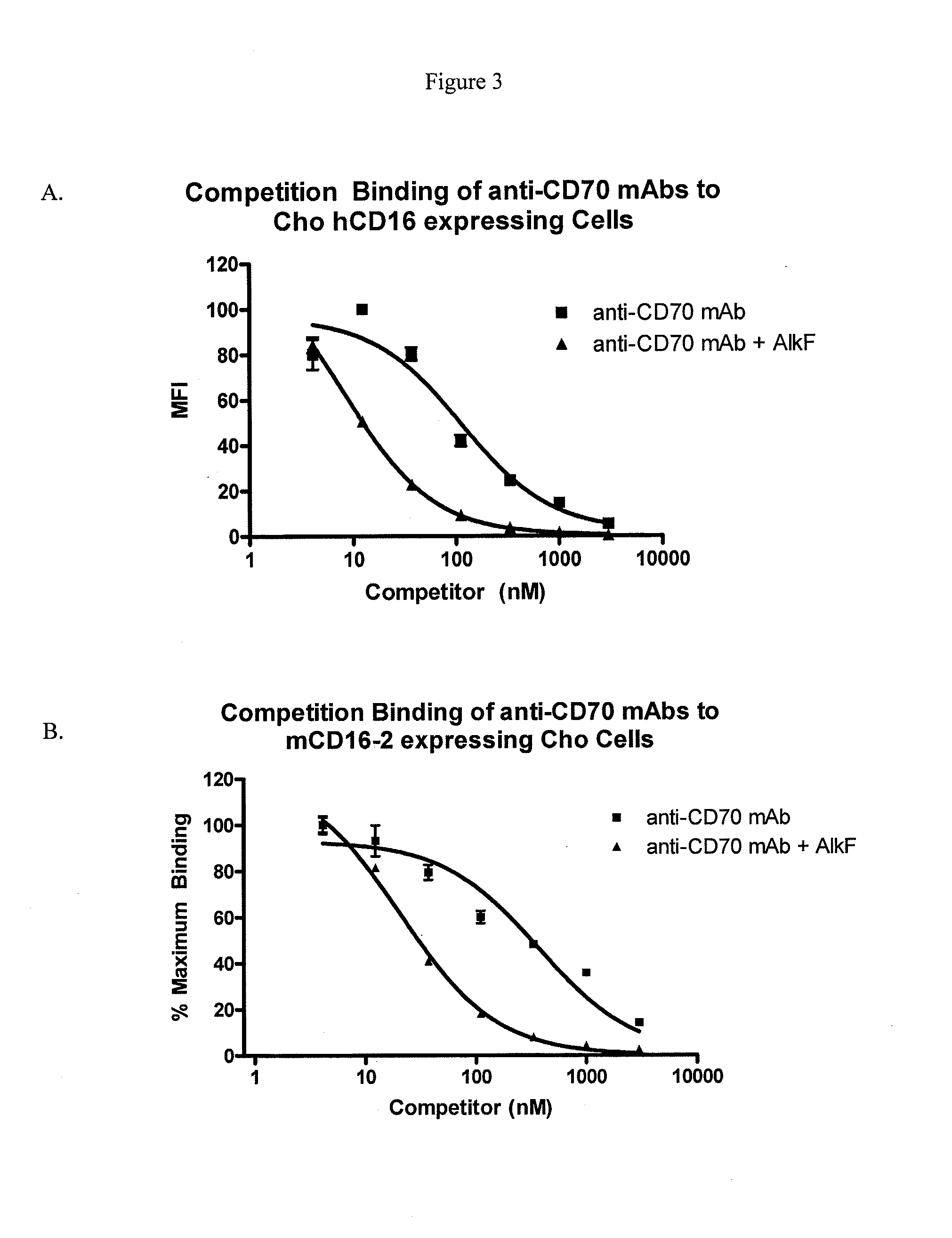
ActiveUS20090317869A1Inhibit and reduce core fucosylationEsterified saccharide compoundsSugar derivativesFucosylationAntibody
The invention provides methods and compositions for preparing antibodies and antibody derivatives with reduced core fucosylation.
Owner:SEAGEN INC
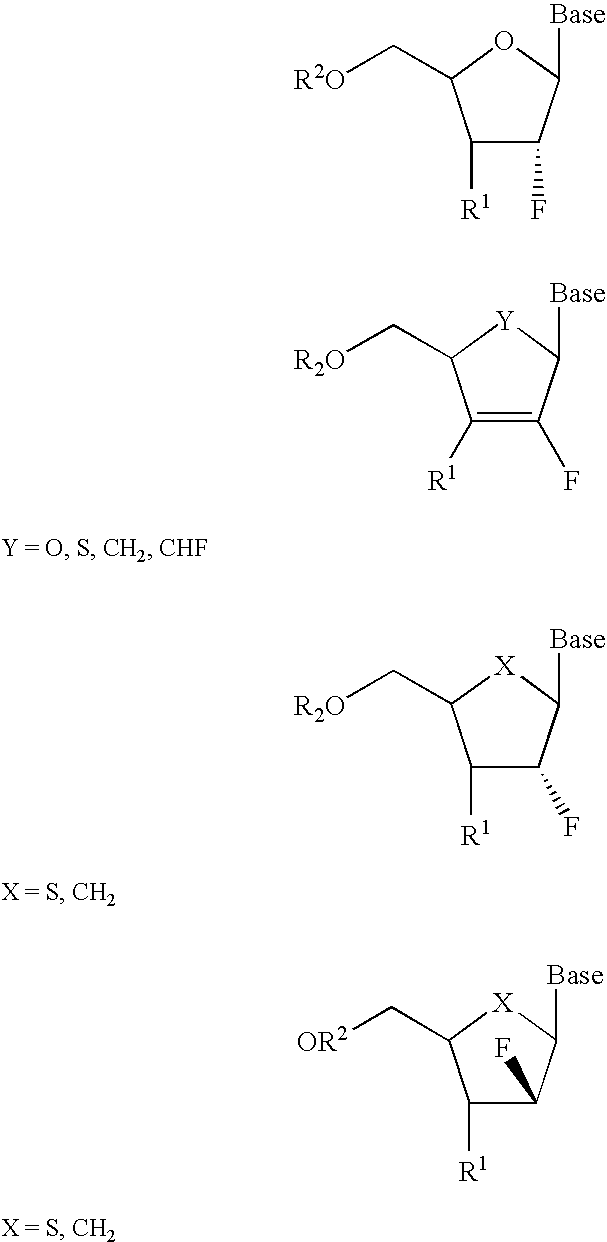
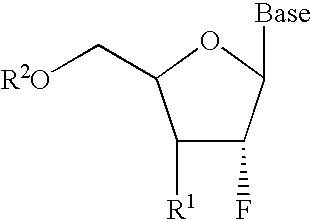
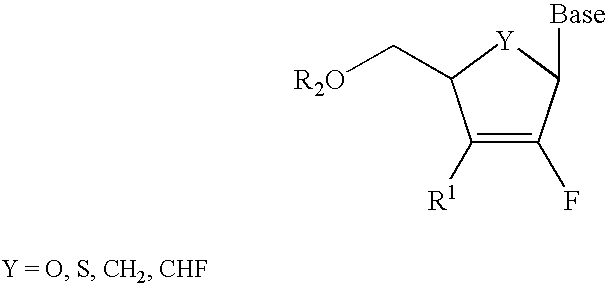
2′-fluoronucleosides
InactiveUS6911424B2Sure easyUseful in treatmentBiocideGroup 5/15 element organic compoundsPhosphoric Acid EstersPurine
A class of 2′-fluoro-nucleoside compounds are disclosed which are useful in the treatment of hepatitis B infection, hepatitis C infection, HIV and abnormal cellular proliferation, including tumors and cancer. The compounds have the general formulae: wherein[0001]Base is a purine or pyrimidine base;[0002]R1 is OH, H, OR3, N3, CN, halogen, including F, or CF3, lower alkyl, amino, loweralkylamino, di(lower)alkylamino, or alkoxy, and base refers to a purine or pyrimidine base;[0003]R2 is H, phosphate, including monophosphate, diphosphate, triphosphate, or a stabilized phosphate prodrug; acyl, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of providing a compound wherein R2 is H or phosphate; sulfonate ester including alkyl or arylalkyl sulfonyl including methanesulfonyl, benzyl, wherein the phenyl group is optionally substituted with one or more substituents as described in the definition of aryl given above, a lipid, an amino acid, peptide, or cholesterol; and[0004]R3 is acyl, alkyl, phosphate, or other pharmaceutically acceptable leaving group which when administered in vivo, is capable of being cleaved to the parent compound, or a pharmaceutically acceptable salt thereof.
Owner:EMORY UNIVERSITY
Inhibitors and methods of use thereof
New triterpenoid derivatives with various substituents at the C-17 position of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) were synthesized. Among them, 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-onitrile (CNDDO), 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl) imidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-2-methylimidazole, 1-(2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oyl)-4-methylimidazole show extremely high inhibitory activity (IC50=0.01-1 pM level) against production of nitric oxide induced by interferon-γ in mouse macrophages. These compounds can be used in the prevention or treatment of diseases such as cancer, Alzheimer's disease, Parkinson's disease, multiple sclerosis, rheumatoid arthritis, and other inflammatory diseases. All the new triterpenoid derivatives are more potent than previously known CDDO.
Owner:TRUSTEES OF DARTMOUTH COLLEGE THE
Reactive oil compositions and uses thereof
InactiveUS20020103091A1Low viscosityImprove abilitiesEsterified saccharide compoundsSugar derivativesPolyolOxygen
A process for modifying an unsaturated polyol fatty acid ester stock, such as an unsaturated triacylglycerol oil, to enhance its reactivity provided. The method includes reacting the unsaturated polyol fatty acid ester stock with an oxygenating agent, such as an oxygen-containing gas. Tempering oils containing reactive polyol fatty acid esters and methods for their production and use are also provided.
Owner:CARGILL INC
Method for reducing adenosine levels with a dehydroepiandrosterone and optionally a ubiquinone
A method and composition for reducing adenosine levels comprises administering a dehydroepiandrosterone, and optionally a ubiquinone.
Owner:EAST CAROLINA UNIVERISTY
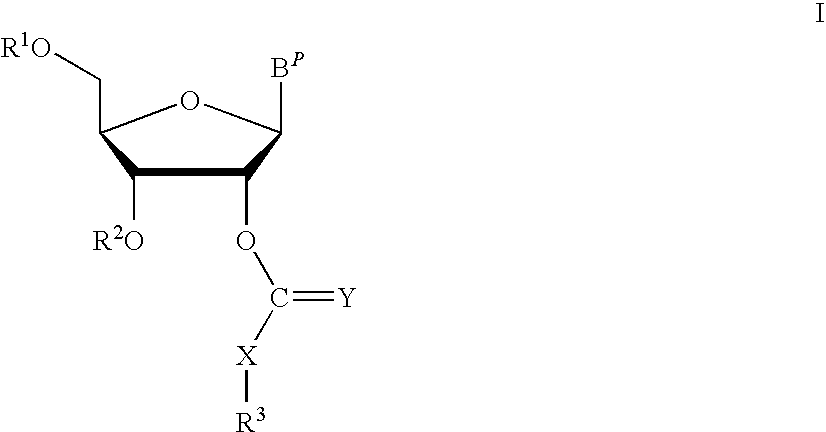
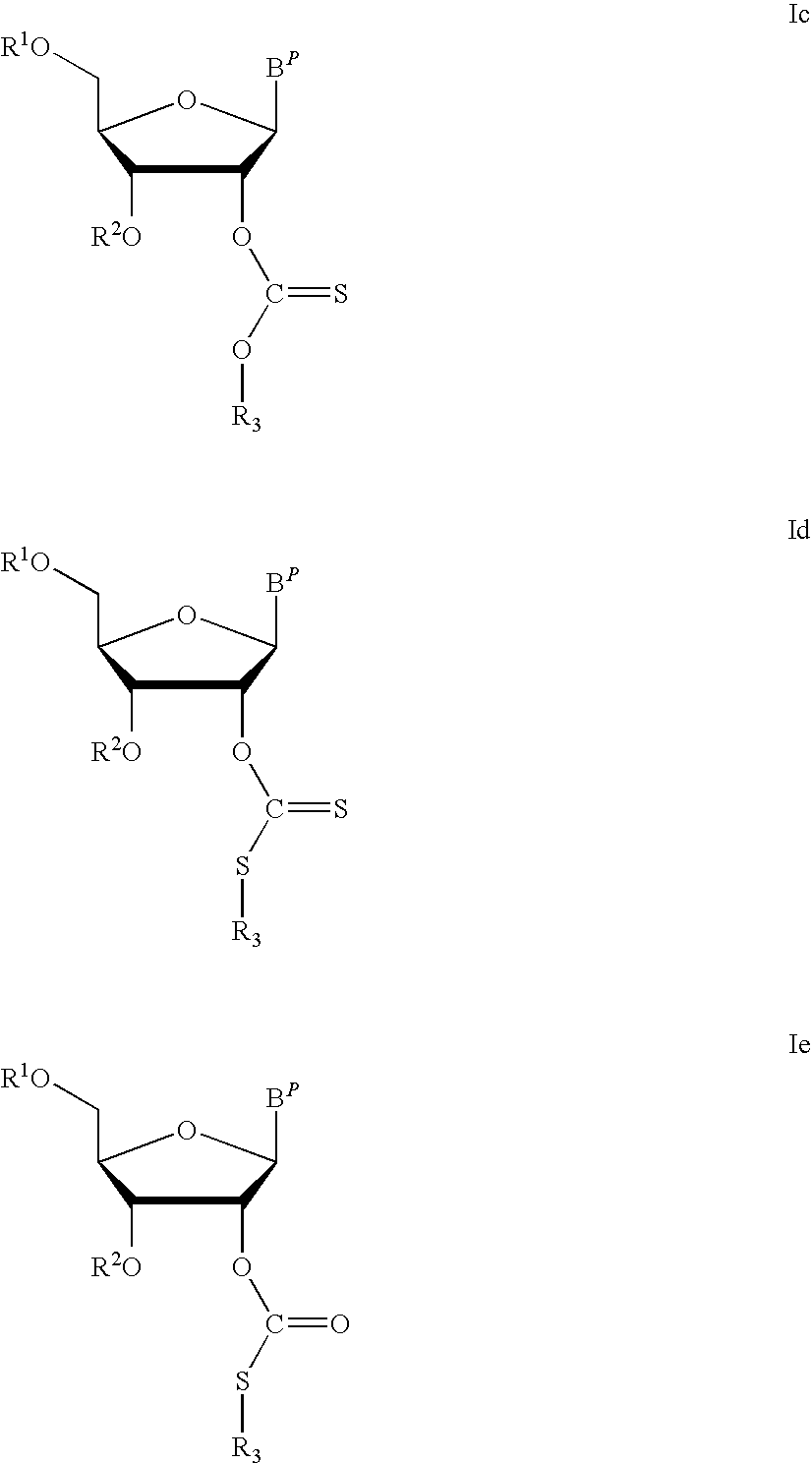
Thiocarbon-protecting groups for RNA synthesis
ActiveUS8202983B2Esterified saccharide compoundsOrganic active ingredientsProtecting groupMedicinal chemistry
Aspects of the invention include 2′ protected nucleoside monomers that are protected at the 2′ site with thiocarbon protecting groups. Thiocarbon protecting groups of interest include thiocarbonate, thionocarbonate, dithiocarbonate groups, as well as thionocarbamate protecting groups. Aspects of the invention further include nucleic acids that include the protecting groups of the invention, as well as methods of synthesizing nucleic acids using the protecting groups of the invention.
Owner:AGILENT TECH INC +1
2'-fluoro substituted carba-nucleoside analogs for antiviral treatment
Provided are select imidazo[1,2-f][1,2,4]triazinyl nucleosides, nucleoside phosphates and prodrugs thereof, wherein the 2′ position of the nucleoside sugar is substituted with halogen and carbon substituents. The compounds, compositions, and methods provided are useful for the treatment of Flaviviridae virus infections, particularly hepatitis C infections caused by both wild type and mutant strains of HCV.
Owner:GILEAD SCI INC
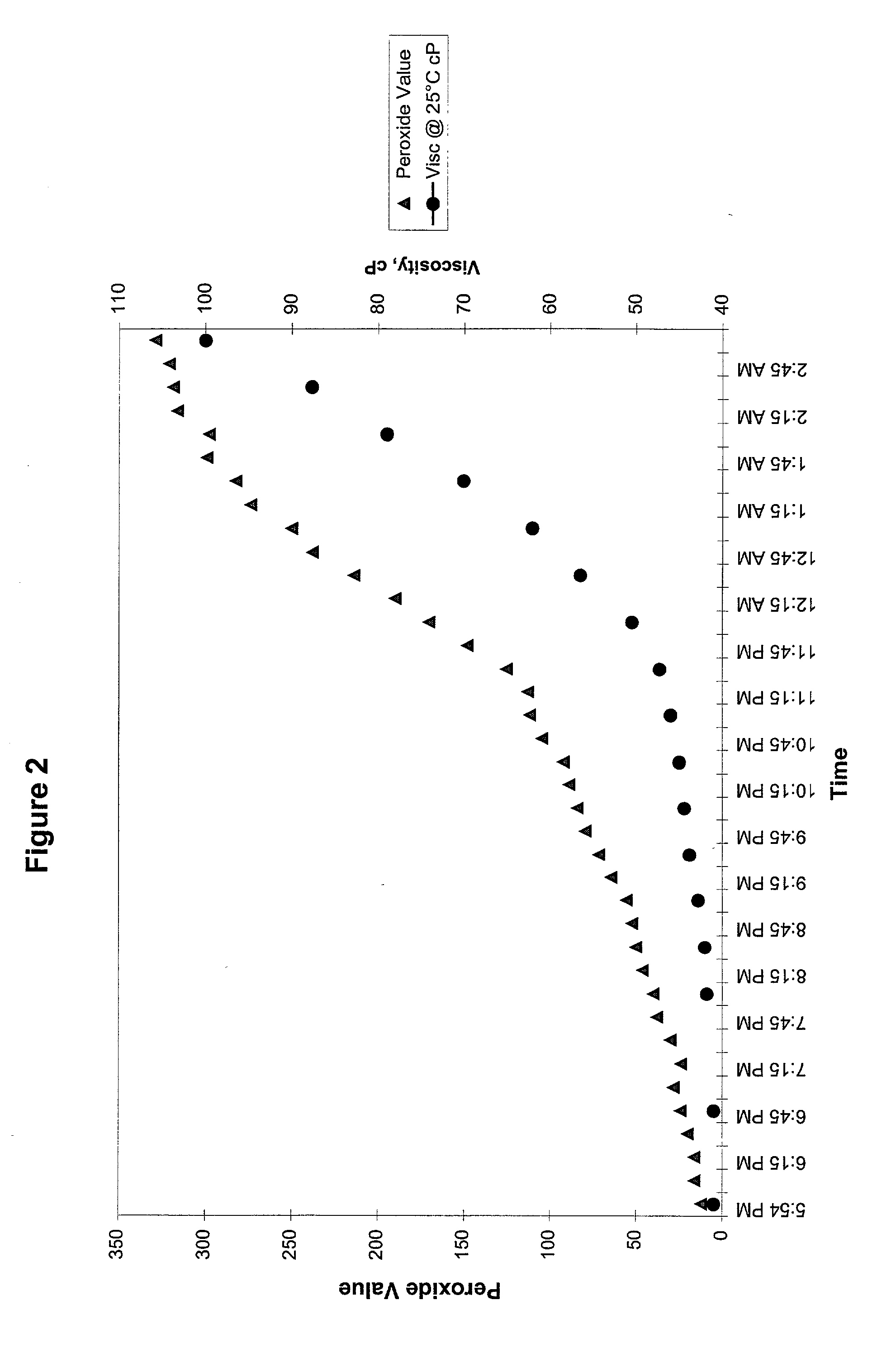
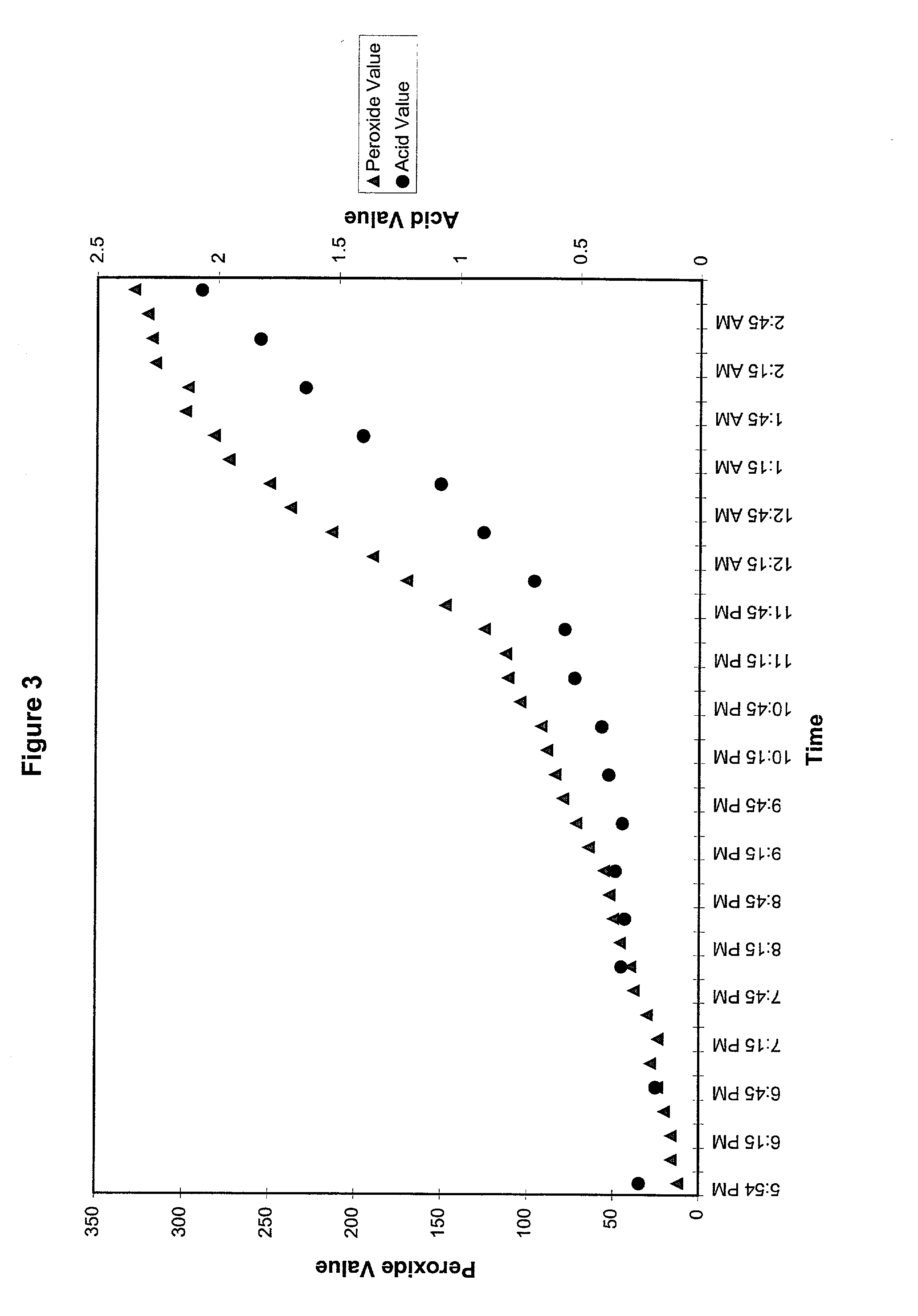
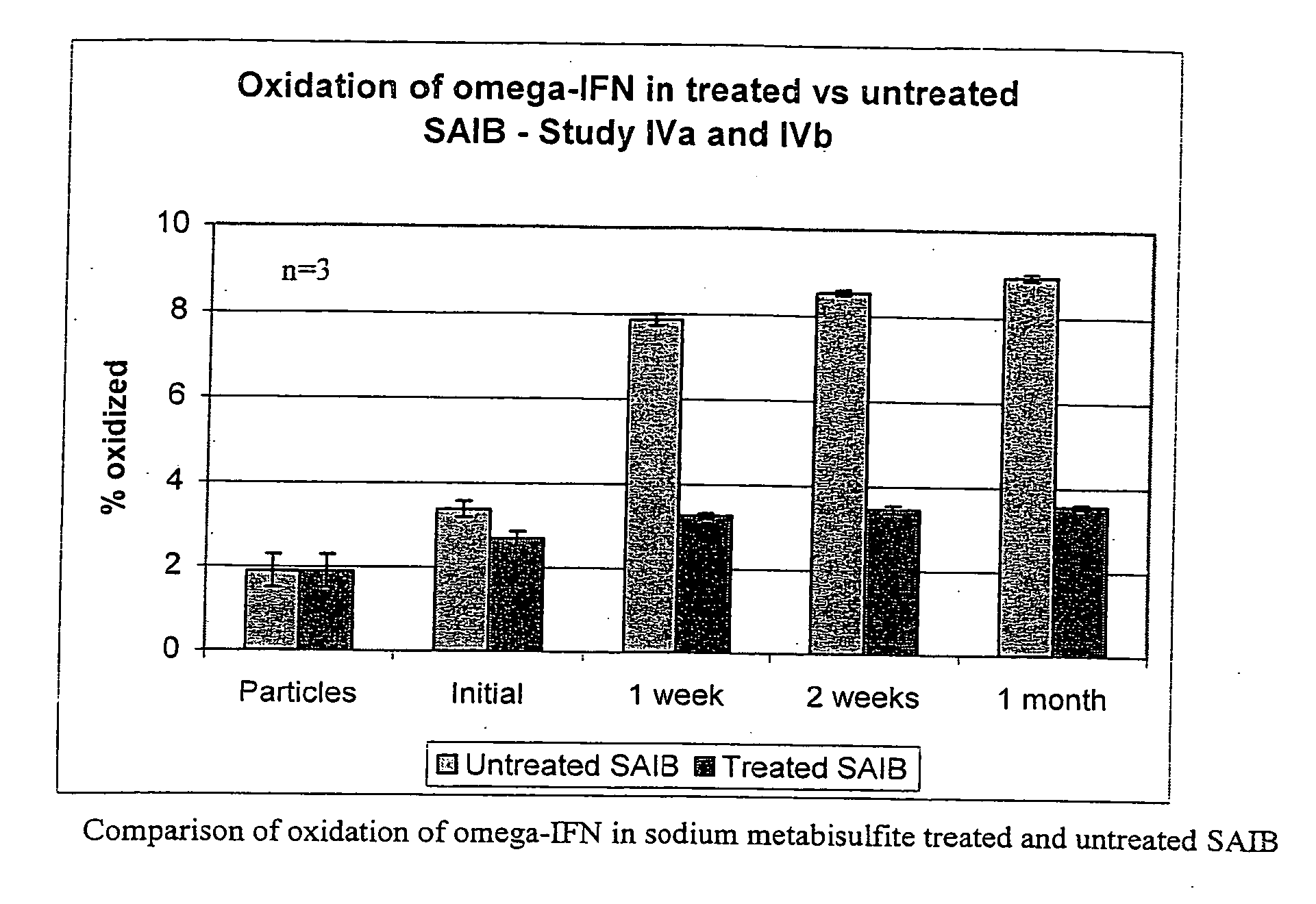
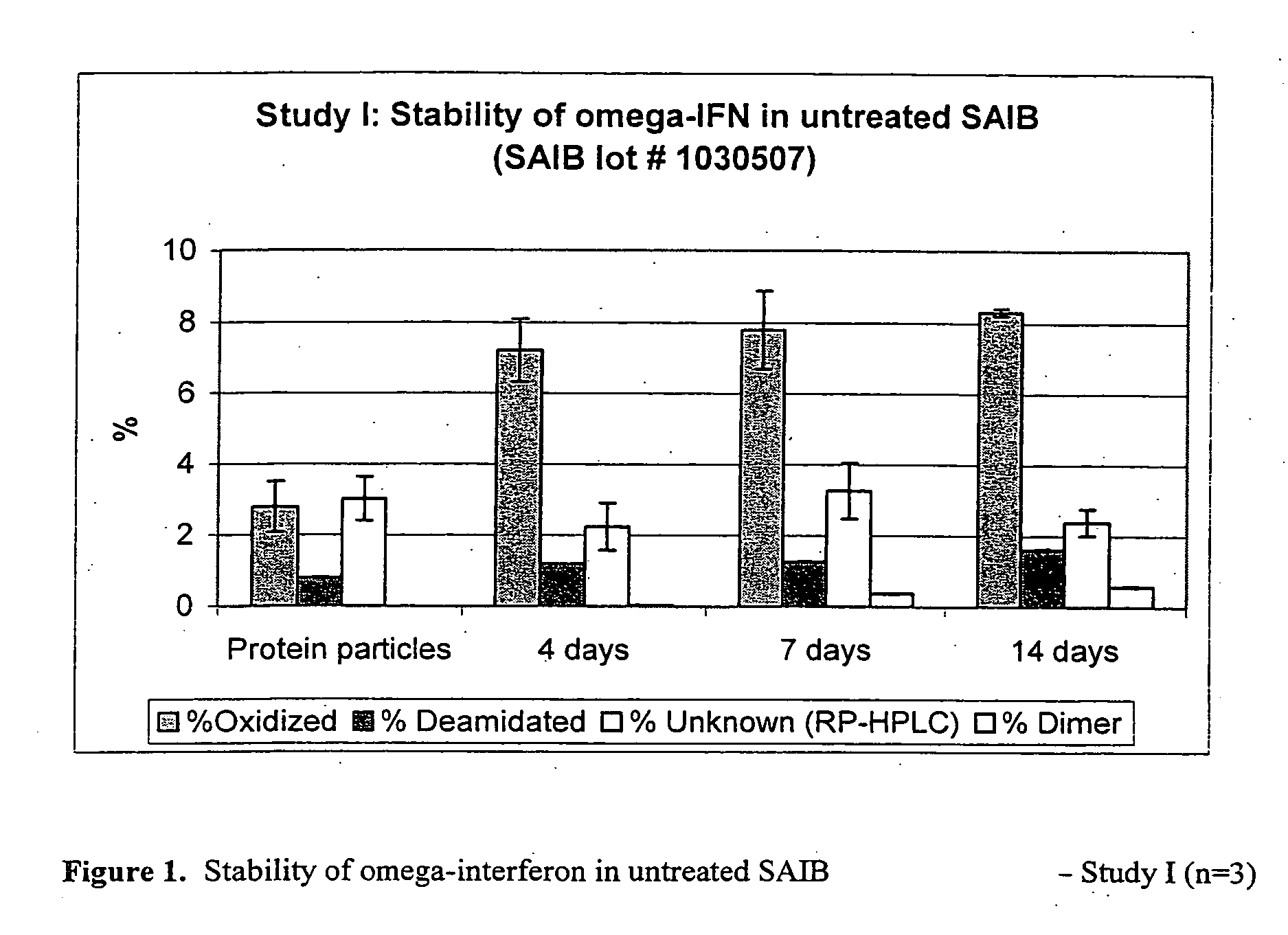
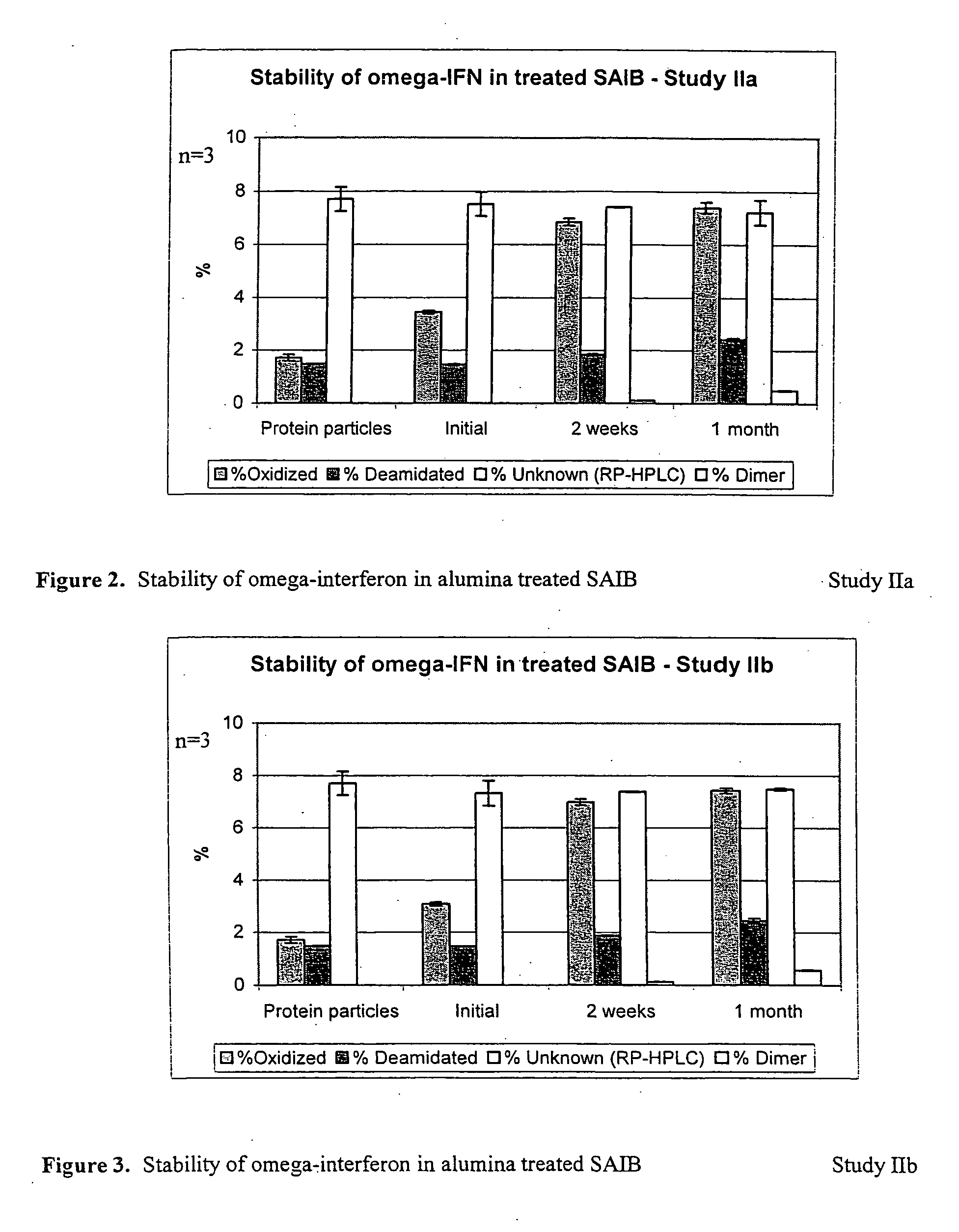
Peroxide removal from drug delivery vehicle
InactiveUS20070027105A1Improve drug stabilityReduced level of peroxideOrganic active ingredientsBiocideMedicineSucrose acetate isobutyrate
Owner:DURECT CORP
Methods for the synthesis of functionalized nucleic acids
ActiveUS20140194610A1Esterified saccharide compoundsSugar derivativesMedicinal chemistryNucleic acid
Owner:WAVE LIFE SCI LTD
Topiramate sodium trihydrate
The invention encompasses novel salts of topiramate, and pharmaceutically acceptable polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, or amorphous forms thereof, as well as pharmaceutical compositions and pharmaceutical unit dosage forms containing the same. In particular, the invention encompasses pharmaceutically acceptable salts of topiramate, including without limitation topiramate sodium, topiramate lithium, topiramate potassium, or polymorphs, solvates, hydrates, dehydrates, co-crystals, anhydrous, and amorphous forms thereof. The invention further encompasses novel co-crystals or complexes of topiramate, as well as pharmaceutical compositions comprising them. The invention also encompasses methods of treating or preventing a variety of diseases and conditions including, but not limited to, seizures, epileptic conditions, tremors, cerebral function disorders, obesity, neuropathic pain, affective disorders, tobacco cessation, migraines, and cluster headache.
Owner:ORTHO MCNEIL PHARM INC
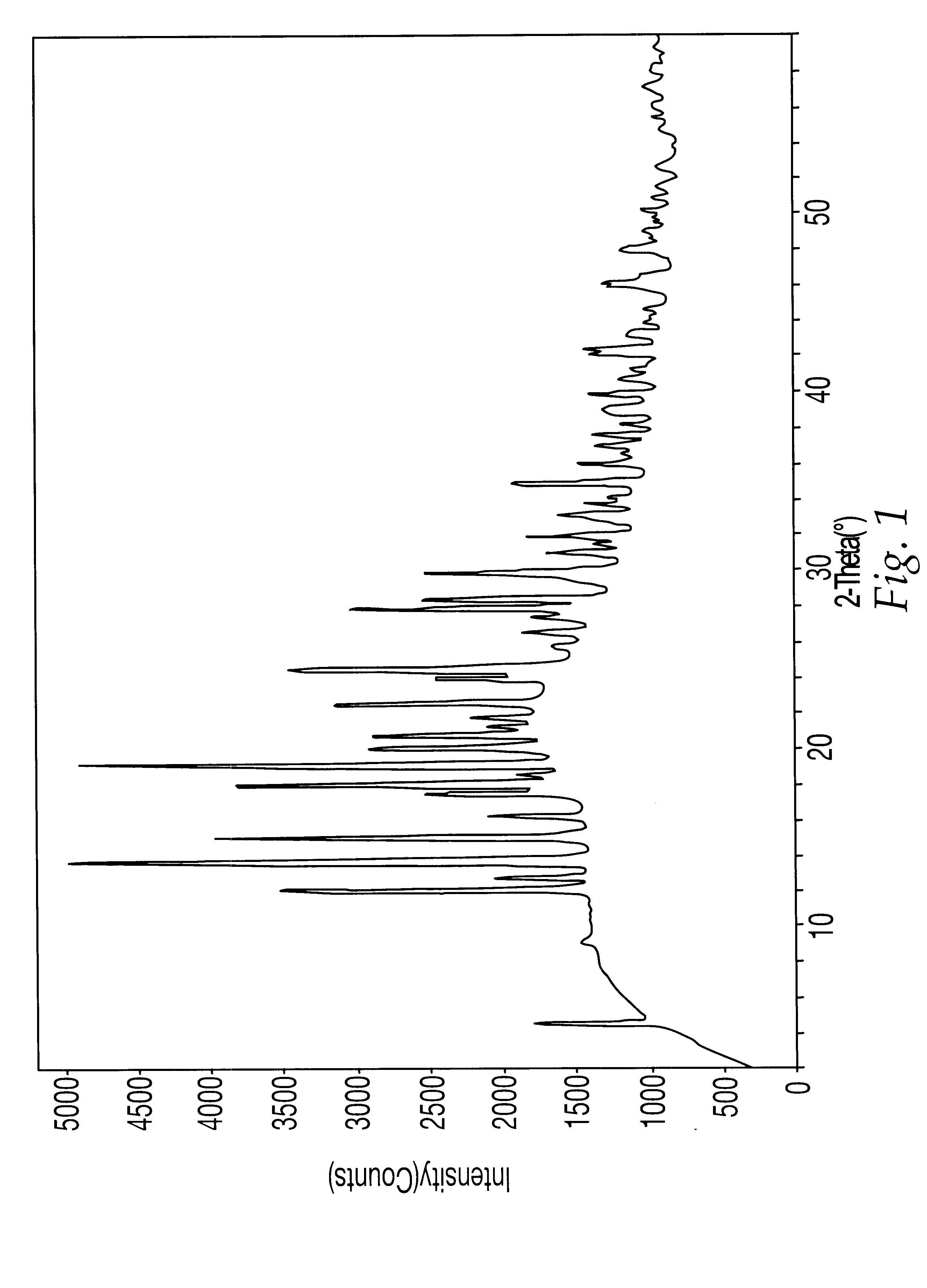
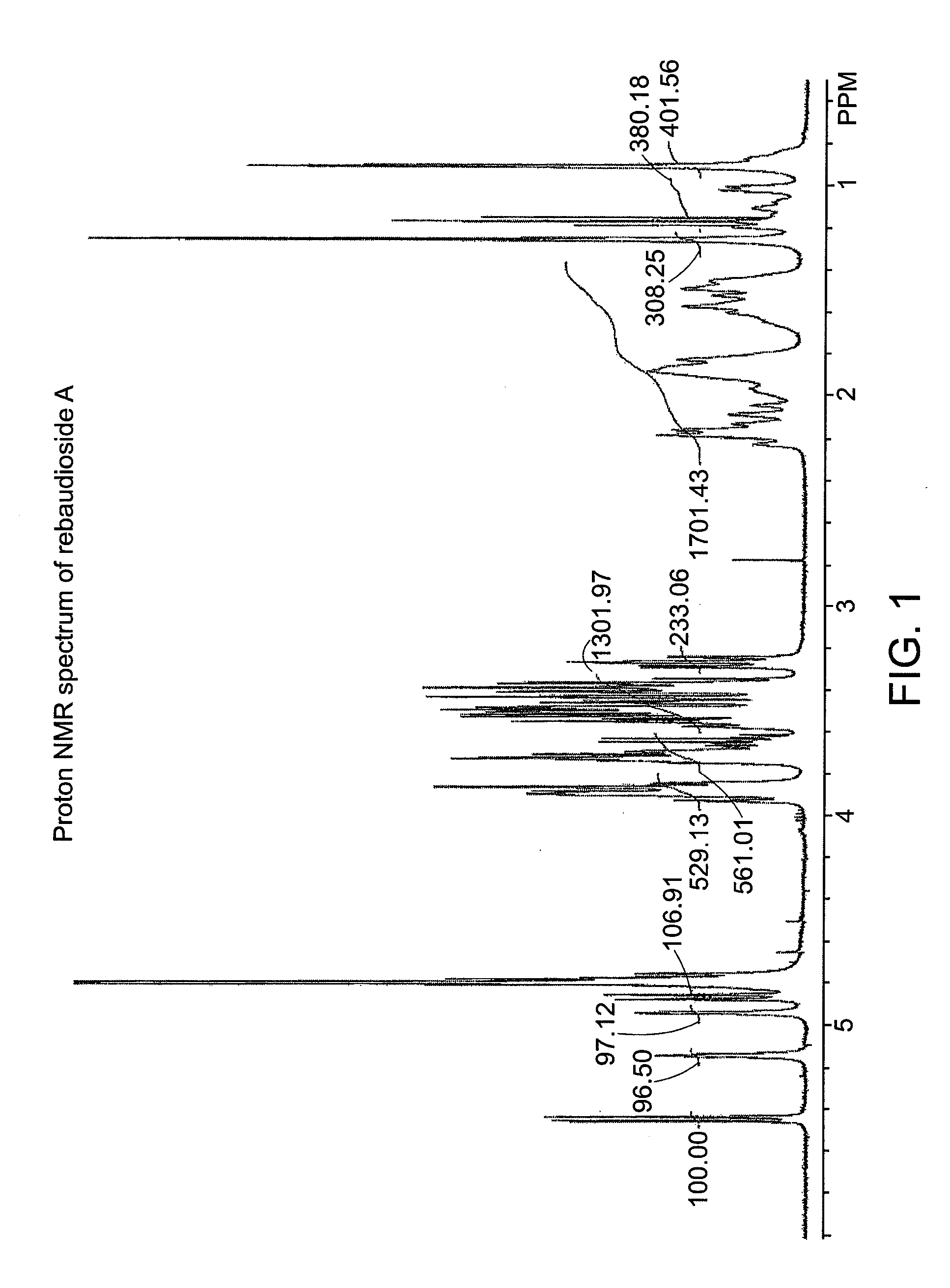
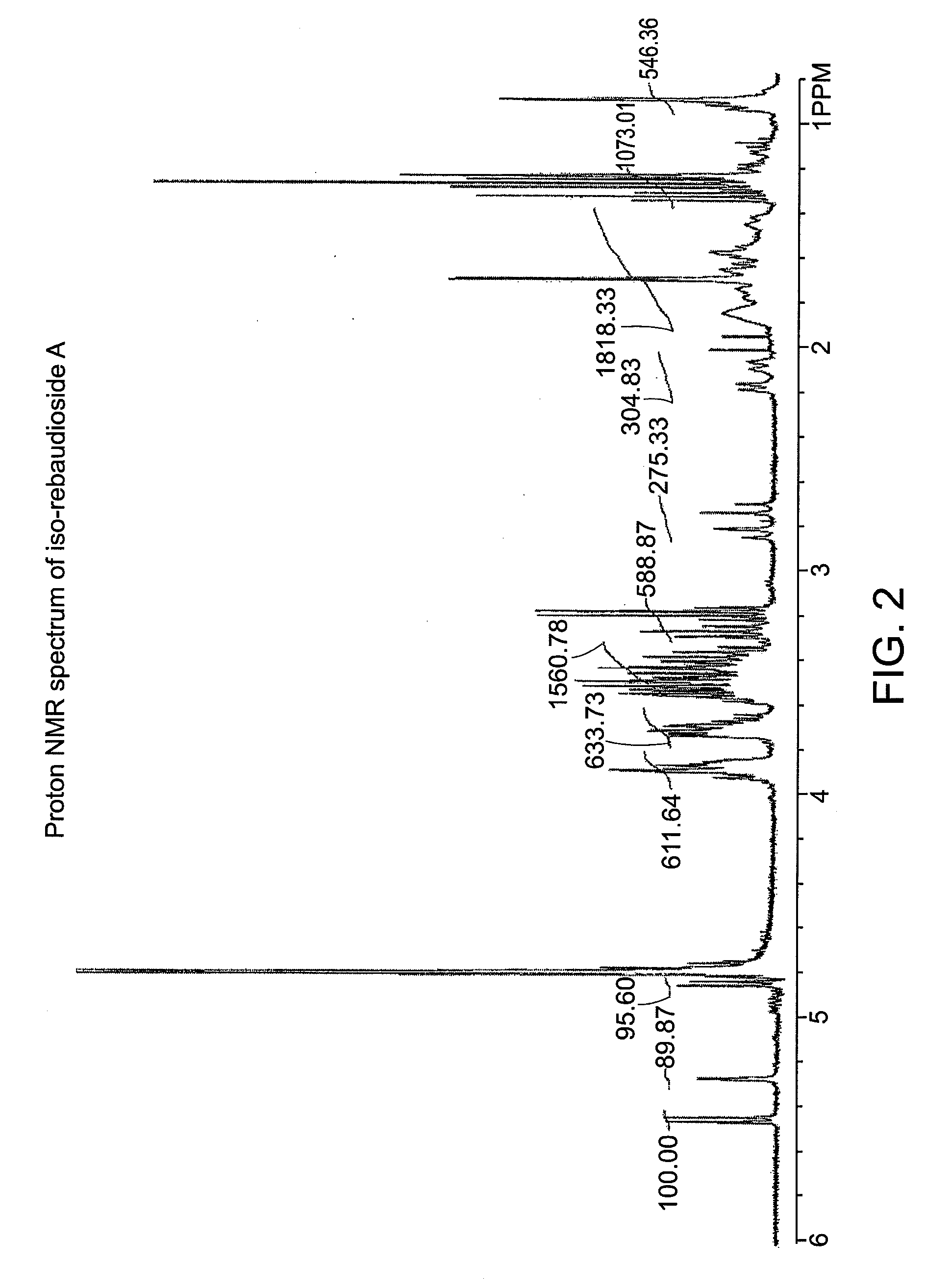
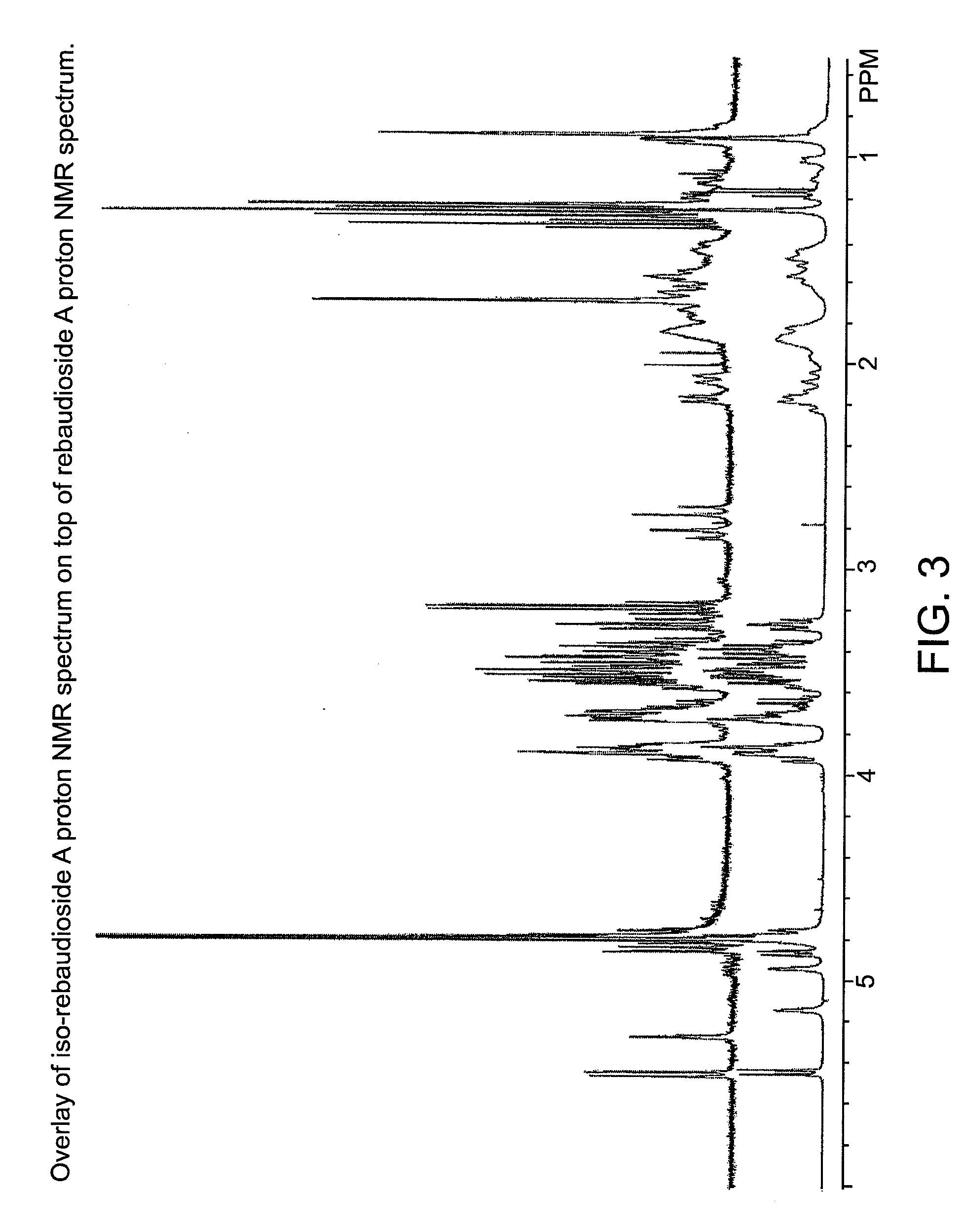
Steviol glycoside isomers
ActiveUS20090074935A1Esterified saccharide compoundsDough treatmentD-GlucopyranoseNutritive Sweeteners
Steviol glycoside isomers are provided having the formula:wherein R1 may be hydrogen, 1-β-D-glucopyranosyl, or 2-(1-β-D-glucopyranosyl)-1-β-D-glucopyranosyl, and R2 may be hydrogen, 1-β-D-glucopyranosyl, 2-(1-β-D-glucopyranosyl)-1-β-D-glucopyranosyl, 2,3-bis(1-β-D-glucopyranosyl)-1-β-D-glucopyranosyl, 2-(1-α-L-rhamnopyranosyl)-1-β-D-glucopyranosyl, 2-(1-α-L-rhamnopyranosyl)-3-(1-β-D-glucopyranosyl)-1-β-D-glucopyranosyl, or 2-(1-β-D-xylopyranosyl)-3-(1-β-D-glucopyranosyl)-1-β-D-glucopyranosyl. Methods for making steviol glycoside isomers are also disclosed. These compounds may be present in food and beverage products as non-nutritive sweeteners.
Owner:PEPSICO INC
Treatment of cystitis-like symptoms with chondroitin sulfate following administration of a challenge solution
Cystitis of the bladder and urinary tract, particularly interstitial cystitis, are treated using effective unit doses of chondroitin sulfate. Further, cystitis patients are screened for their response to a given cystitis treatment using a method in which patients are first challenged with an irritant and then treated with a selected cystitis therapeutic. Candidates for further treatment are identified as those patients who on receiving the selected therapeutic, report relief from at least one symptom elicited with the irritant. Also provided are kits comprising solutions for carrying out this screening method.
Owner:STELLAR INT
2'-fluoro substituted carba-nucleoside analogs for antiviral treatment
ActiveUS7973013B2Esterified saccharide compoundsSaccharide with heterocyclic radicalsHalogenPhosphate
Owner:GILEAD SCI INC
Preparation and use of sulfated oligosaccharides
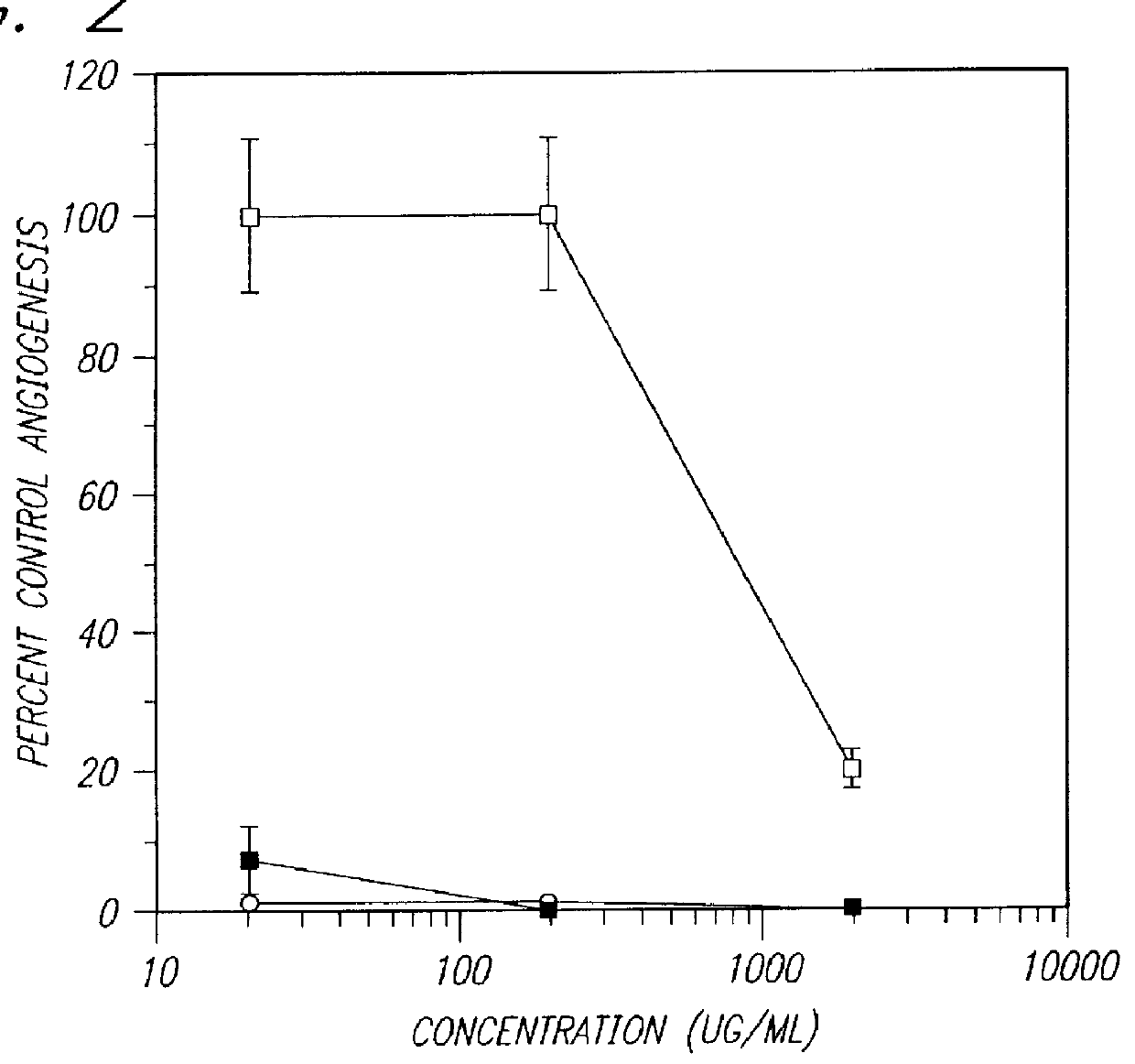
PCT No. PCT / AU96 / 00238 Sec. 371 Date Oct. 28, 1997 Sec. 102(e) Date Oct. 28, 1997 PCT Filed Apr. 24, 1996 PCT Pub. No. WO96 / 33726 PCT Pub. Date Oct. 31, 1996Sulfated oligosaccharides, wherein the oligosaccharide has the general formula I:R1-(Rx)n-R2(I)wherein R1 and R2 and each Rx represents a monosaccharide unit, all of which may be the same or different, adjacent monosaccharide units being linked by 1->2, 1->3, 1->4 and / or 1->6 glycosidic bonds and n is an integer of from 1 to 6, and use thereof as anti-angiogenic, anti-metastatic and / or anti-inflammatory agents.
Owner:AUSTRALIEN NAT UNIV
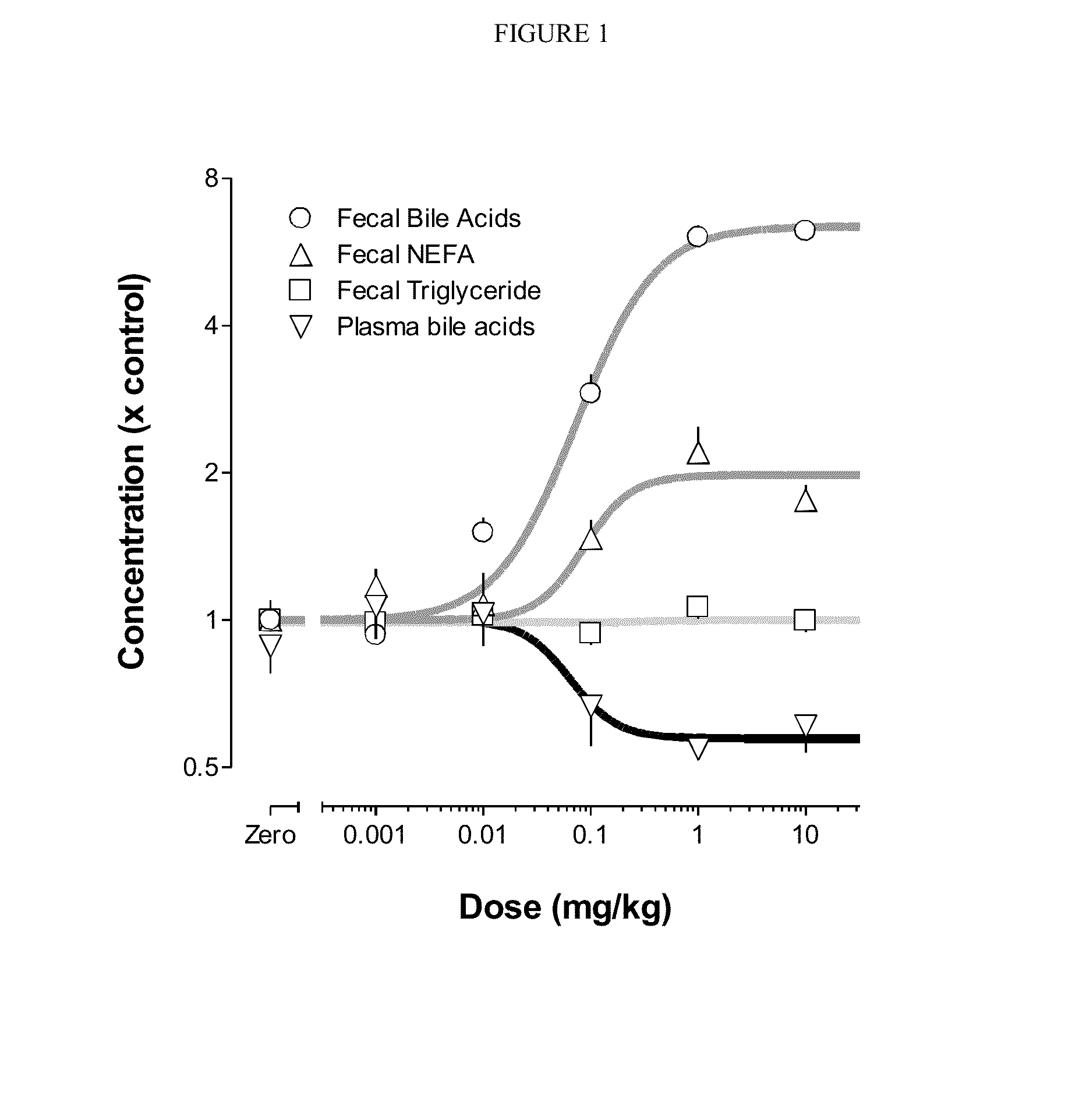
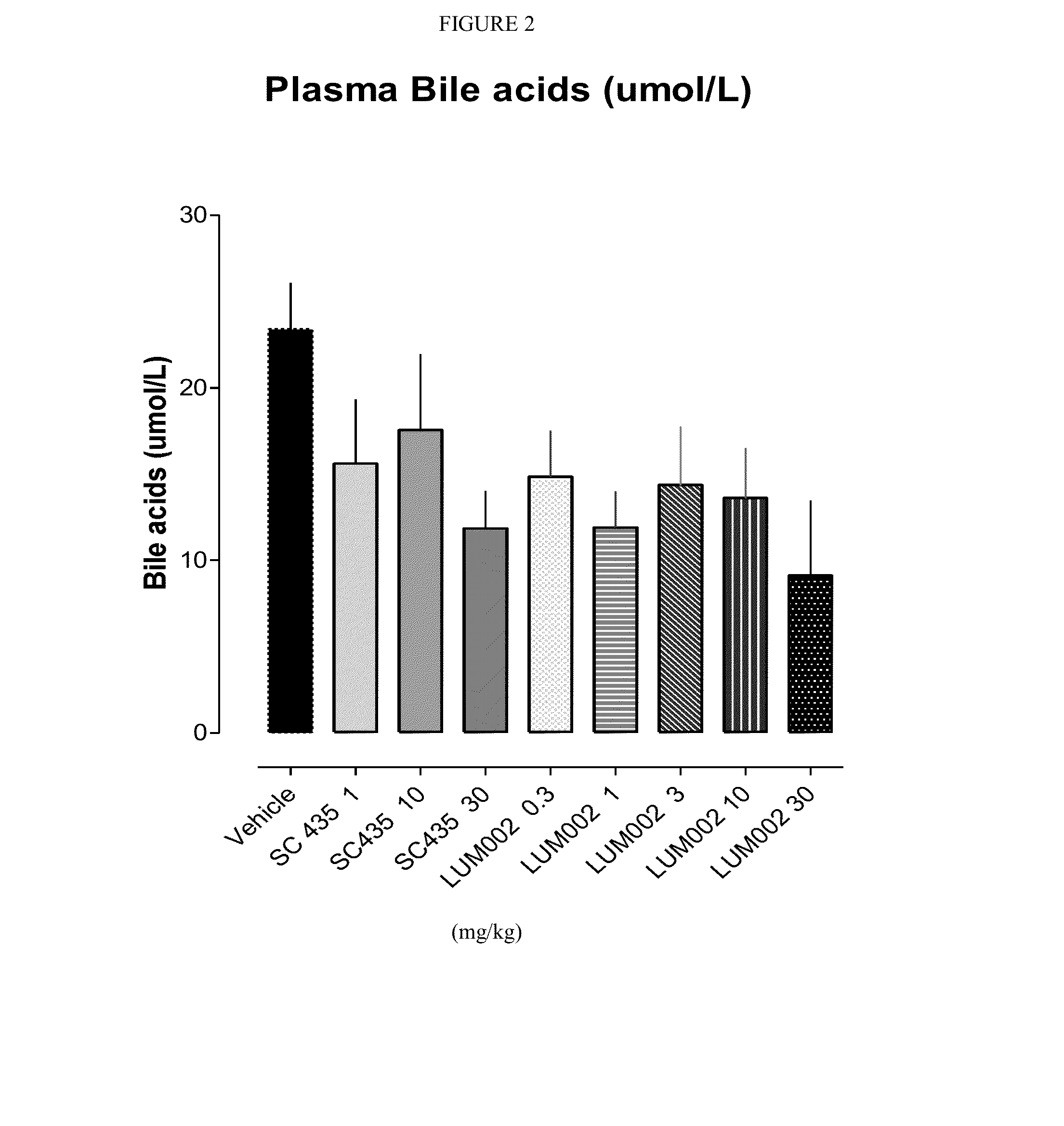
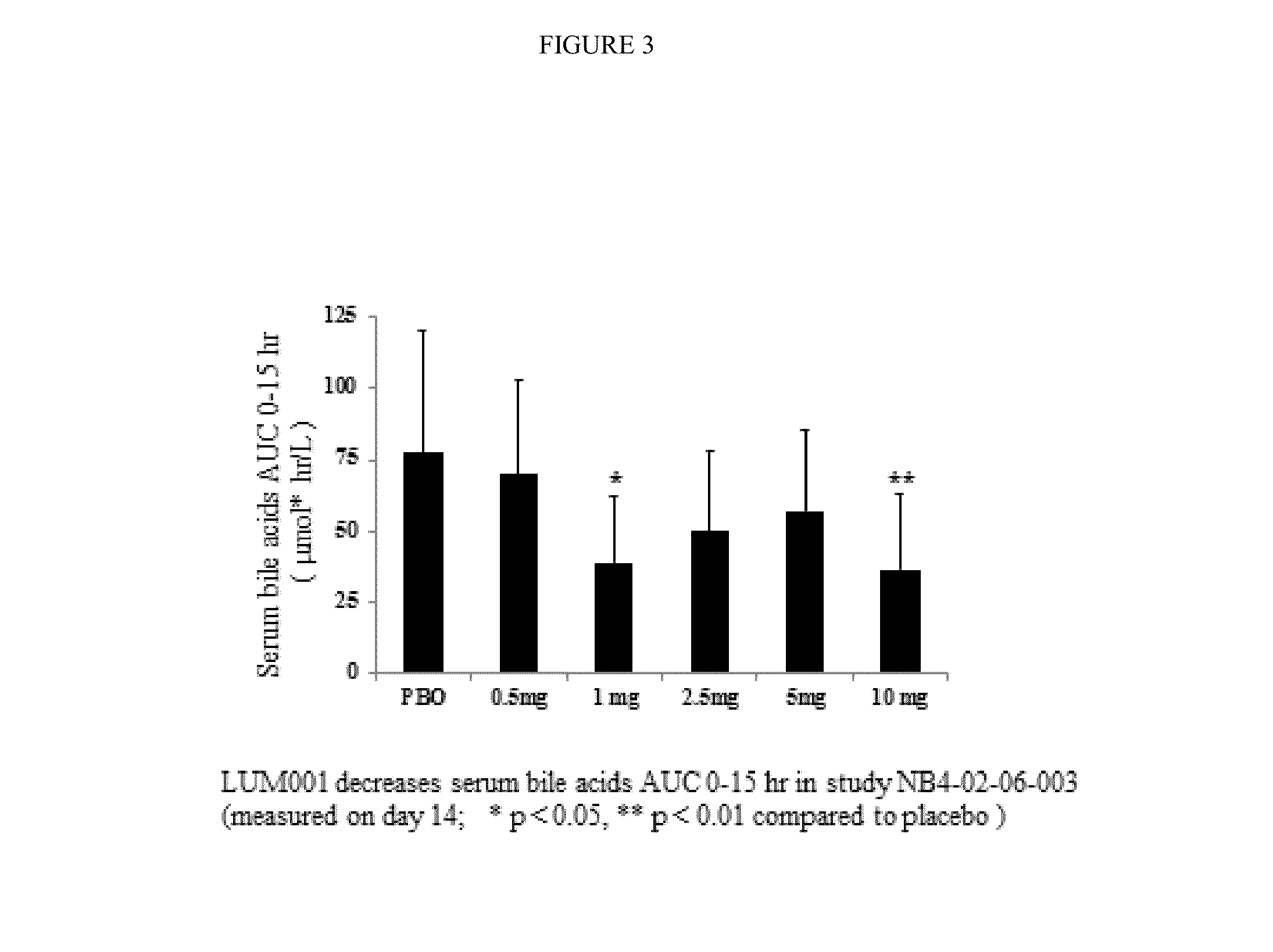
Bile Acid Recycling Inhibitors for Treatment of Hypercholemia and Cholestatic Liver Disease
InactiveUS20130108573A1Relieve symptomsReduce recurrenceBiocideCyclic peptide ingredientsDiseaseHepatic bile
Provided herein are methods of treating or ameliorating hypercholemia or a cholestatic liver disease by administering to an individual in need thereof a therapeutically effective amount of an Apical Sodium-dependent Bile Acid Transporter Inhibitor (ASBTI) or a pharmaceutically acceptable salt thereof. Also provided are methods for treating or ameliorating a liver disease, decreasing the levels of serum bile acids or hepatic bile acids, treating or ameliorating pruritis, reducing liver enzymes, or reducing bilirubin comprising administering to an individual in need thereof a therapeutically effective amount of ASBTI or a pharmaceutically acceptable salt thereof.
Owner:LUMENA PHARMA INC
Method for Preventing Cancer Metastasis
The present invention relates to the use of a specific family of glycerolipid compounds of formula (I) described in the detailed description or the manufacture of a medicament for the prevention or for the treatment of cancer metastasis.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +2
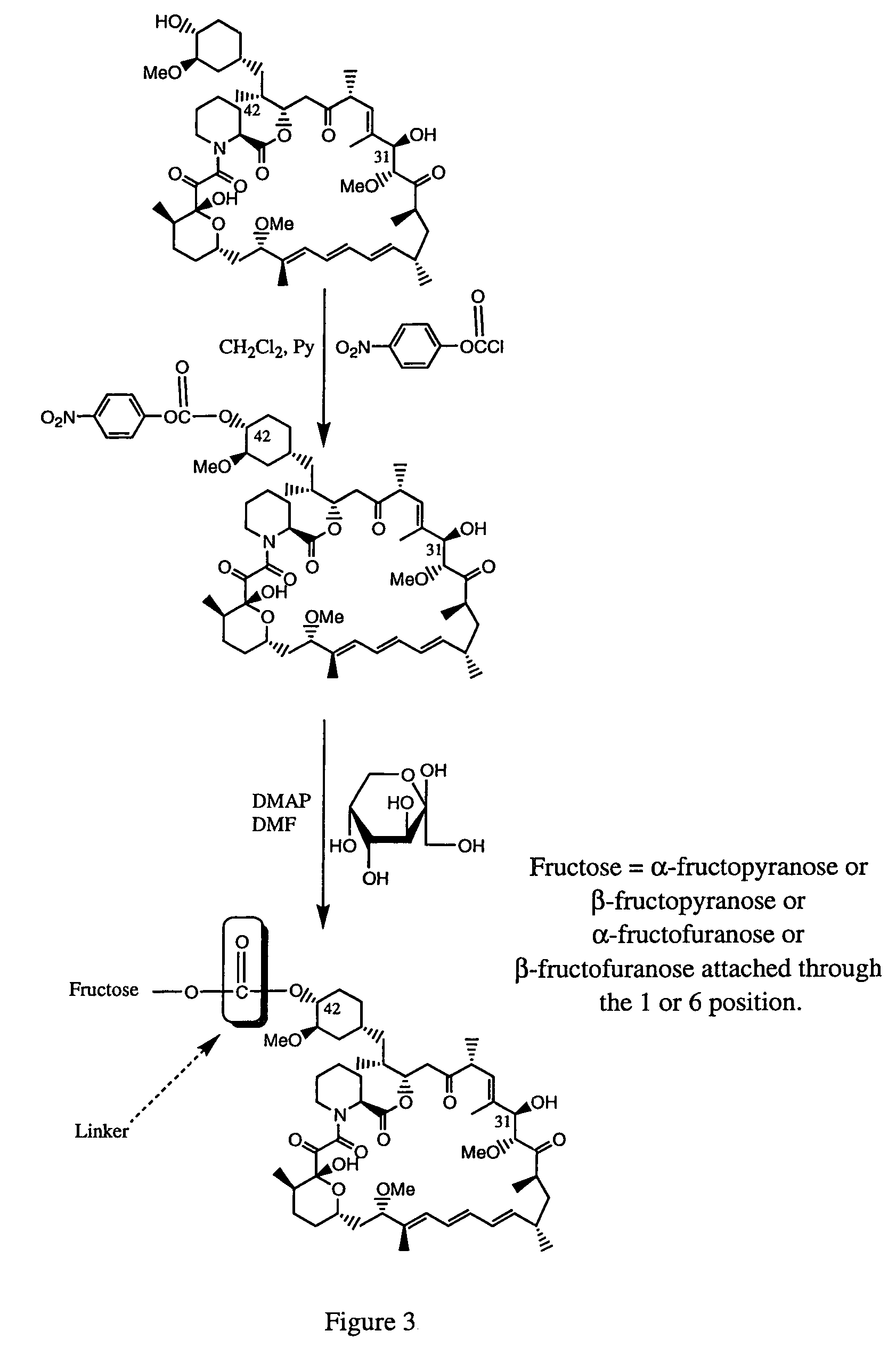
Rapamycin carbohydrate derivatives
InactiveUS7160867B2Low toxicityMore water solubleAntibacterial agentsBiocidePseudosugarsCarbohydrate derivative
This invention provides modified rapamycins that have specific monosaccharide(s), oligosaccharide(s), pseudosugar(s) or derivatives thereof attached through a linker to create rapamycin carbohydrate derivatives having enhanced pharmacokinetic and / or pharmacodynamic profiles. For example, administration of the rapamycin carbohydrate derivative results in altered pharmacokinetic profiles and reduced toxicities. Thus, the present invention provides compounds with characteristics that are distinct from other drugs in its class such as rapamycin.
Owner:ISOTECHNIKA INC
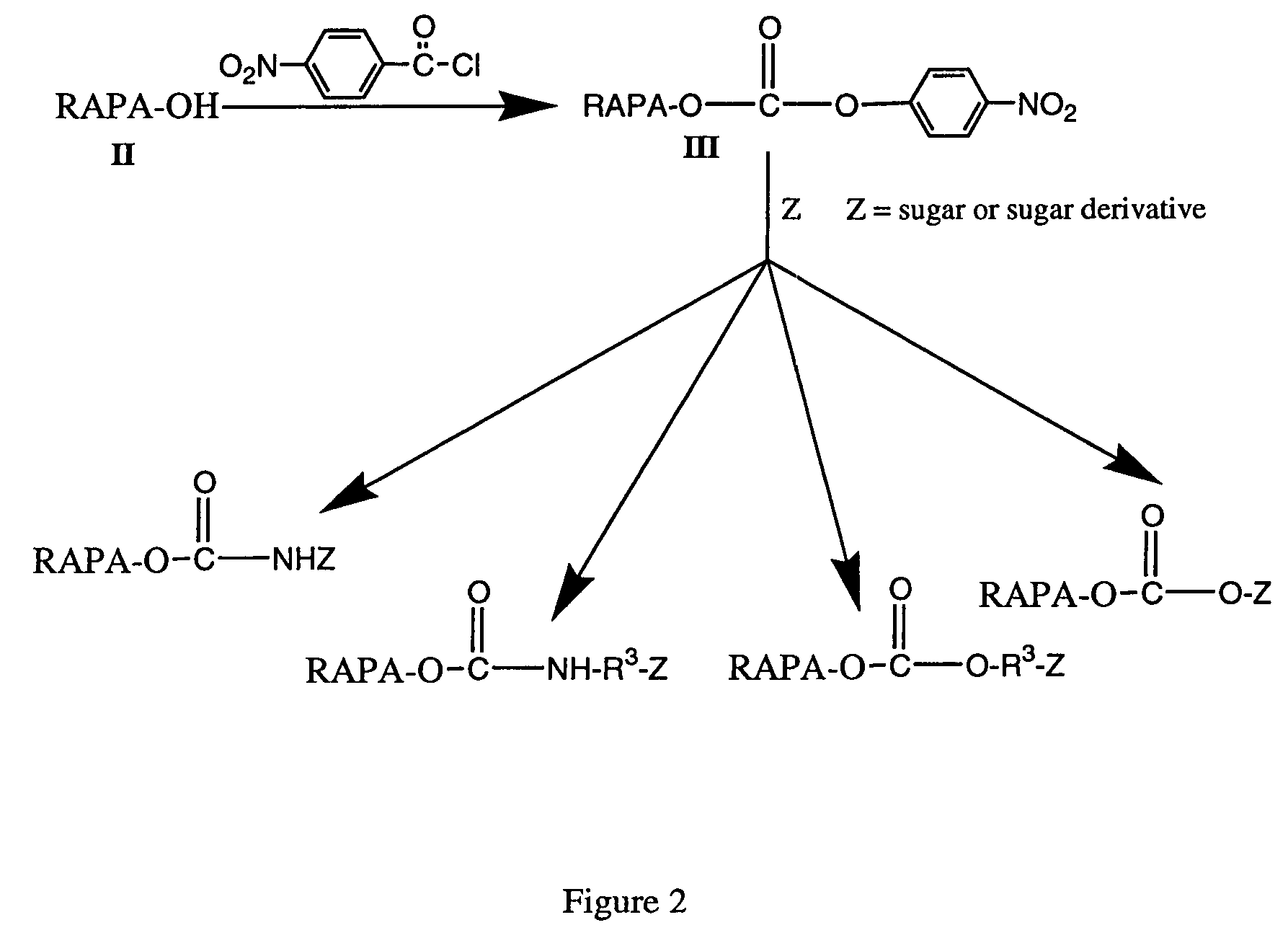
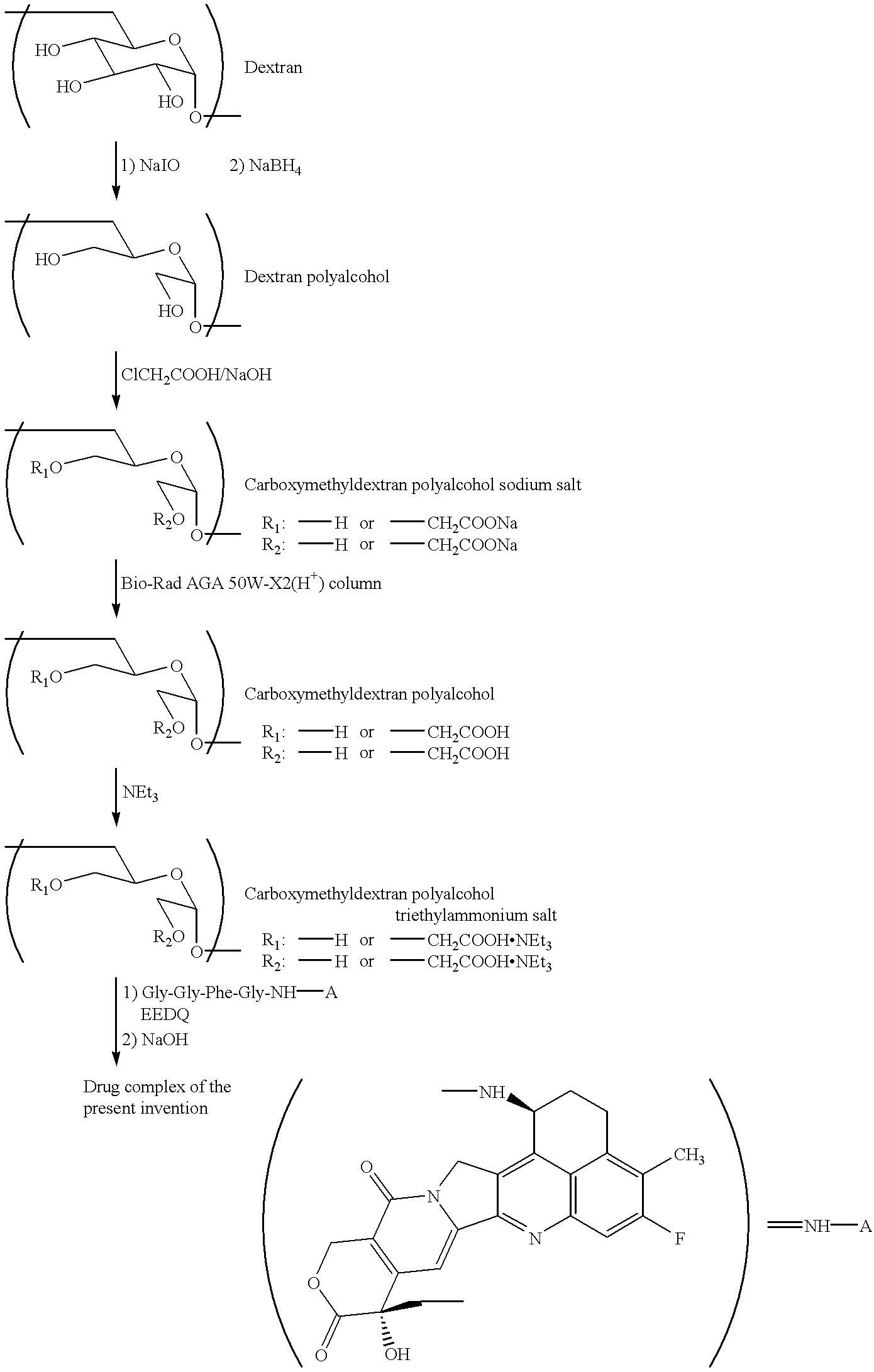
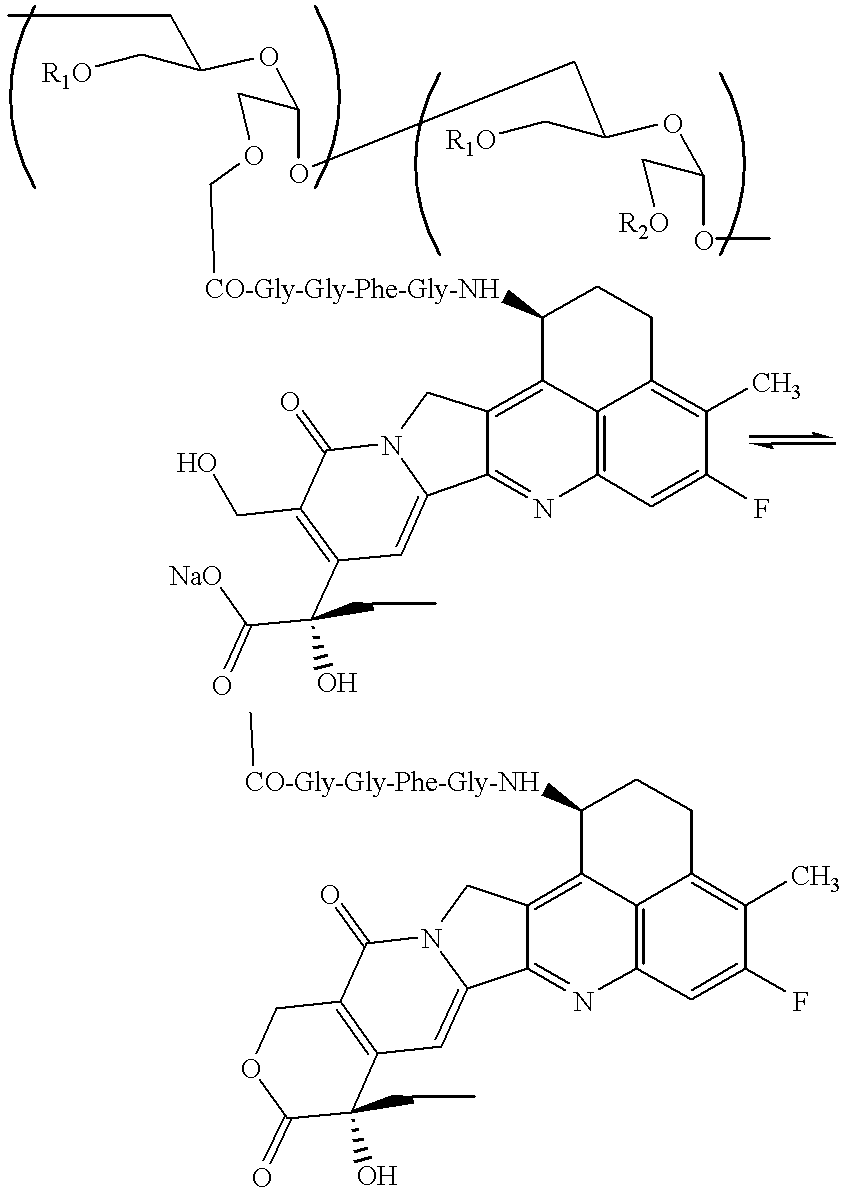
Process for producing drug complexes
InactiveUS6291671B1Efficient productionProduce significantEsterified saccharide compoundsSugar derivativesDrug compoundSide reaction
A method for preparing a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other by means of a spacer comprising an amino acid or a spacer comprising peptide-bonded 2 to 8 amino acids, or a drug complex in which a polysaccharide derivative having carboxyl groups and a residue of a drug compound are bound to each other without the spacer, characterized in that an organic amine salt of the polysaccharide derivative having carboxyl groups is reacted with the drug compound or the spacer bound to the drug compound in a non-aqueous system. The reaction between the polysaccharide derivative having carboxyl groups and the drug compound bound with the spacer or the like can be carried out in high yields, and when a drug compound having a lactone ring is subjected to the reaction, side reactions can be reduced.
Owner:DAIICHI PHARMA CO LTD
Galactoside inhibitors of galectins
InactiveUS7638623B2Process economyHigh affinityEsterified saccharide compoundsOrganic active ingredientsDiseaseMedicine
Owner:GALECTO BIOTECH
Pesticidal compositions
Owner:CORTEVA AGRISCIENCE LLC
2'-fluoro substituted carba-nucleoside analogs for antiviral treatment
Owner:GILEAD SCI INC
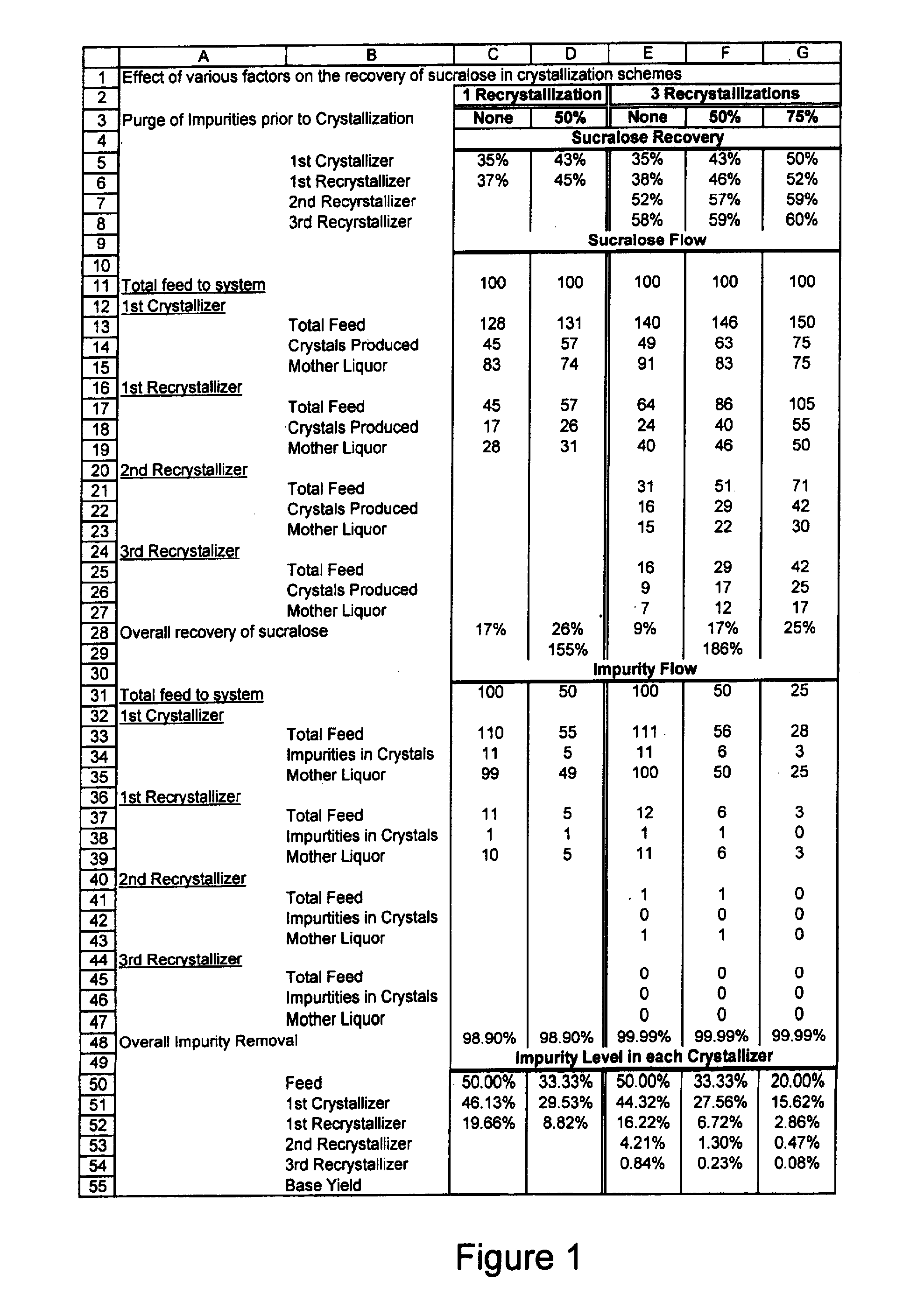
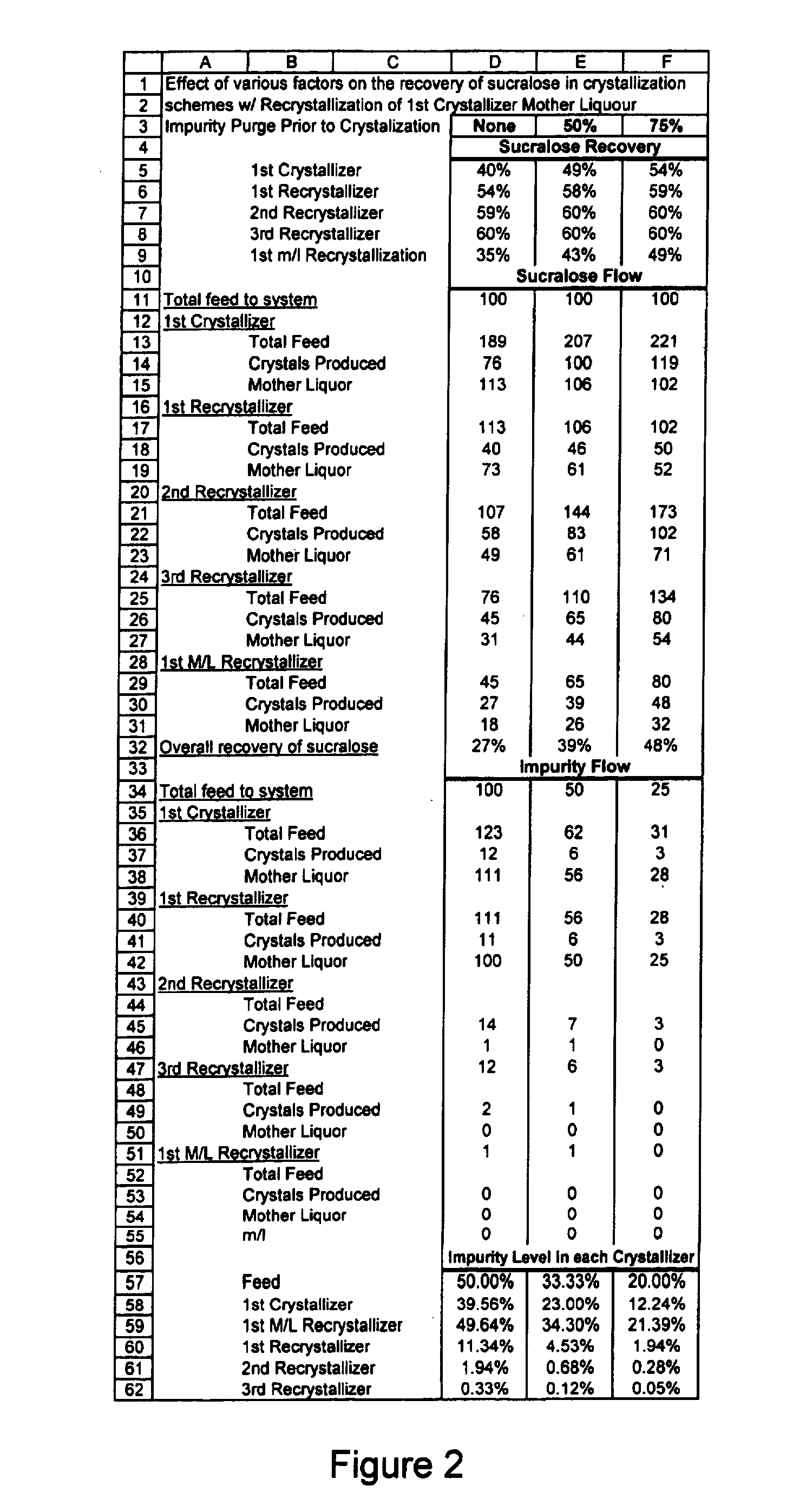
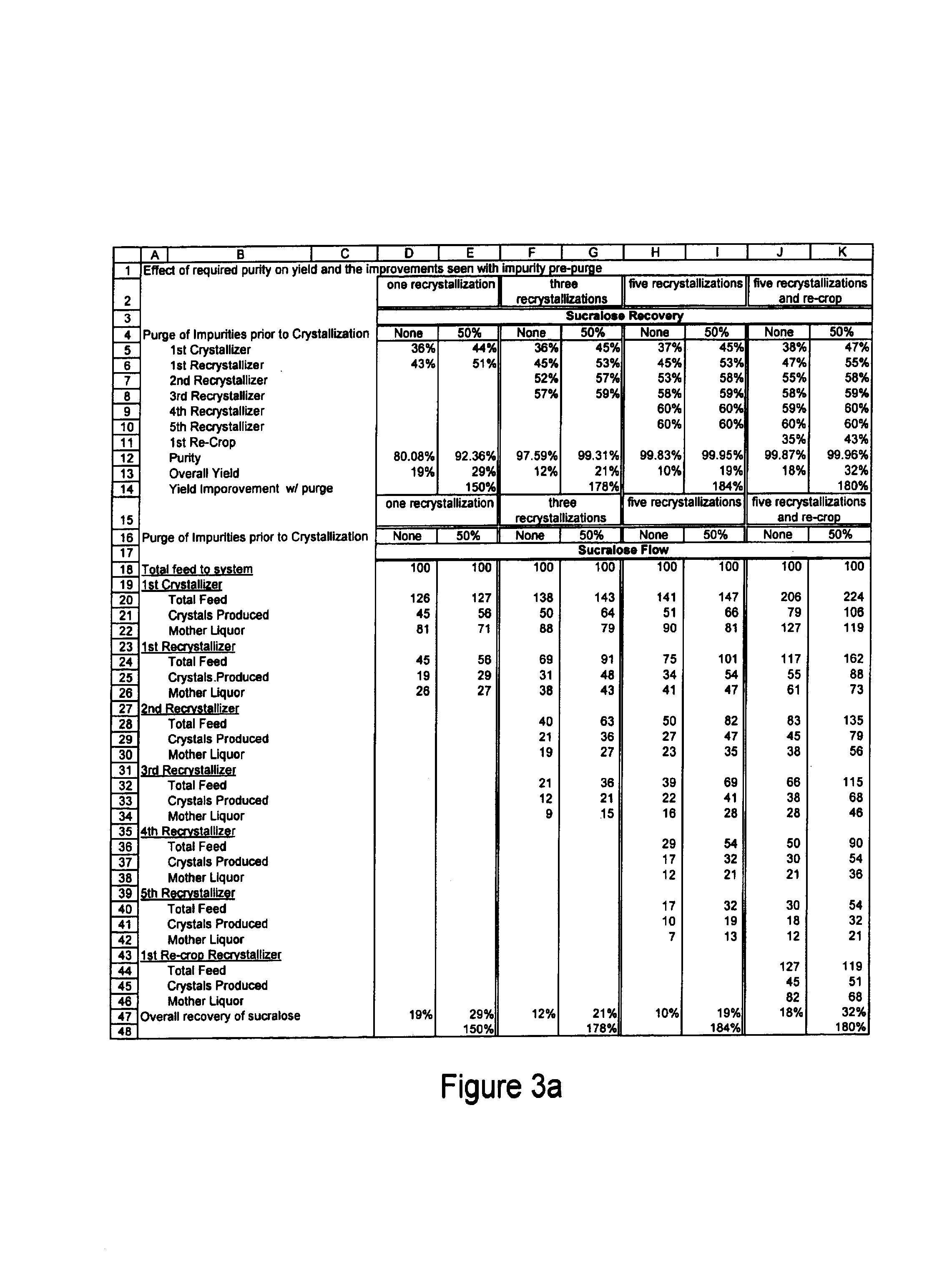
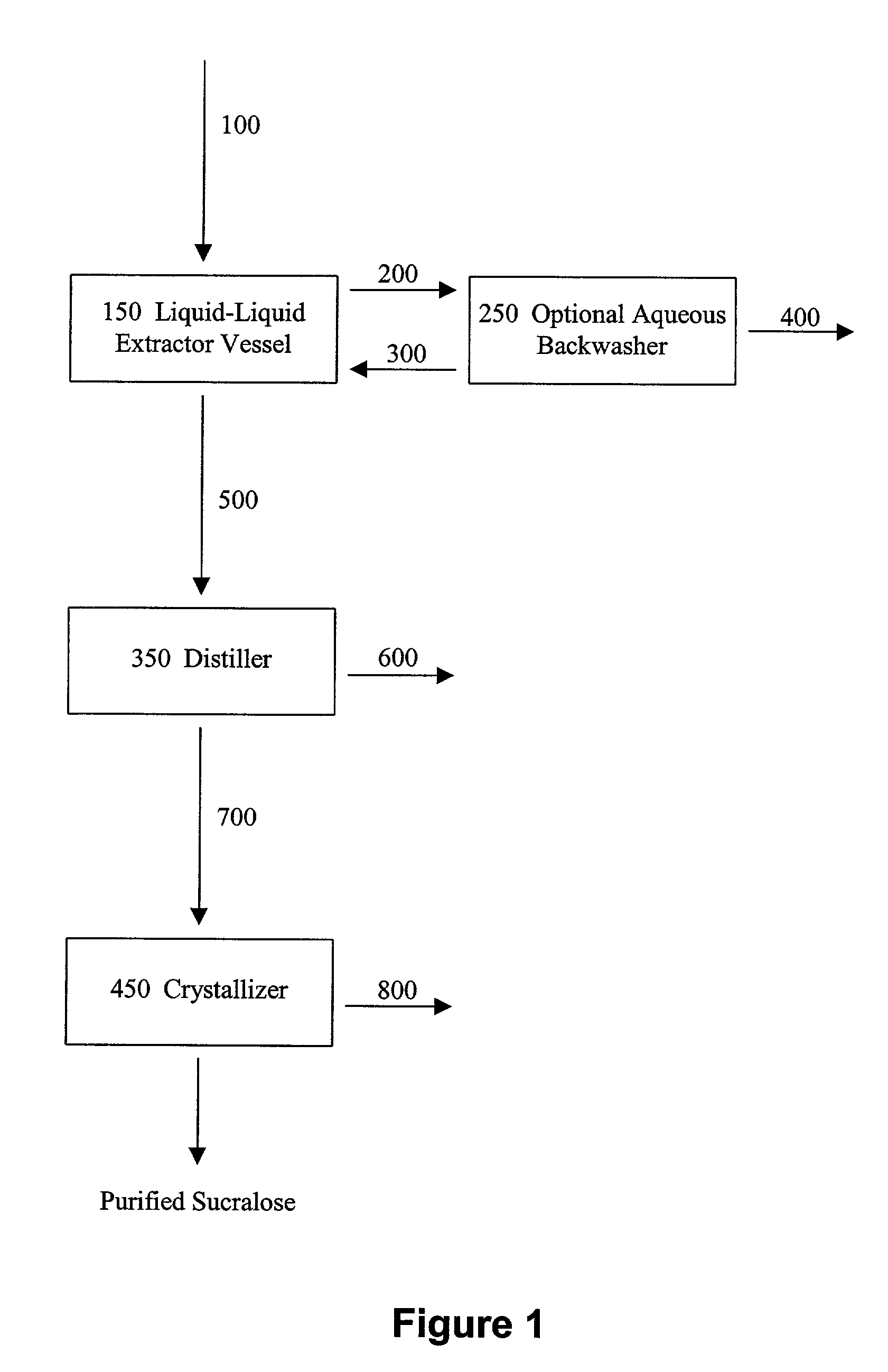
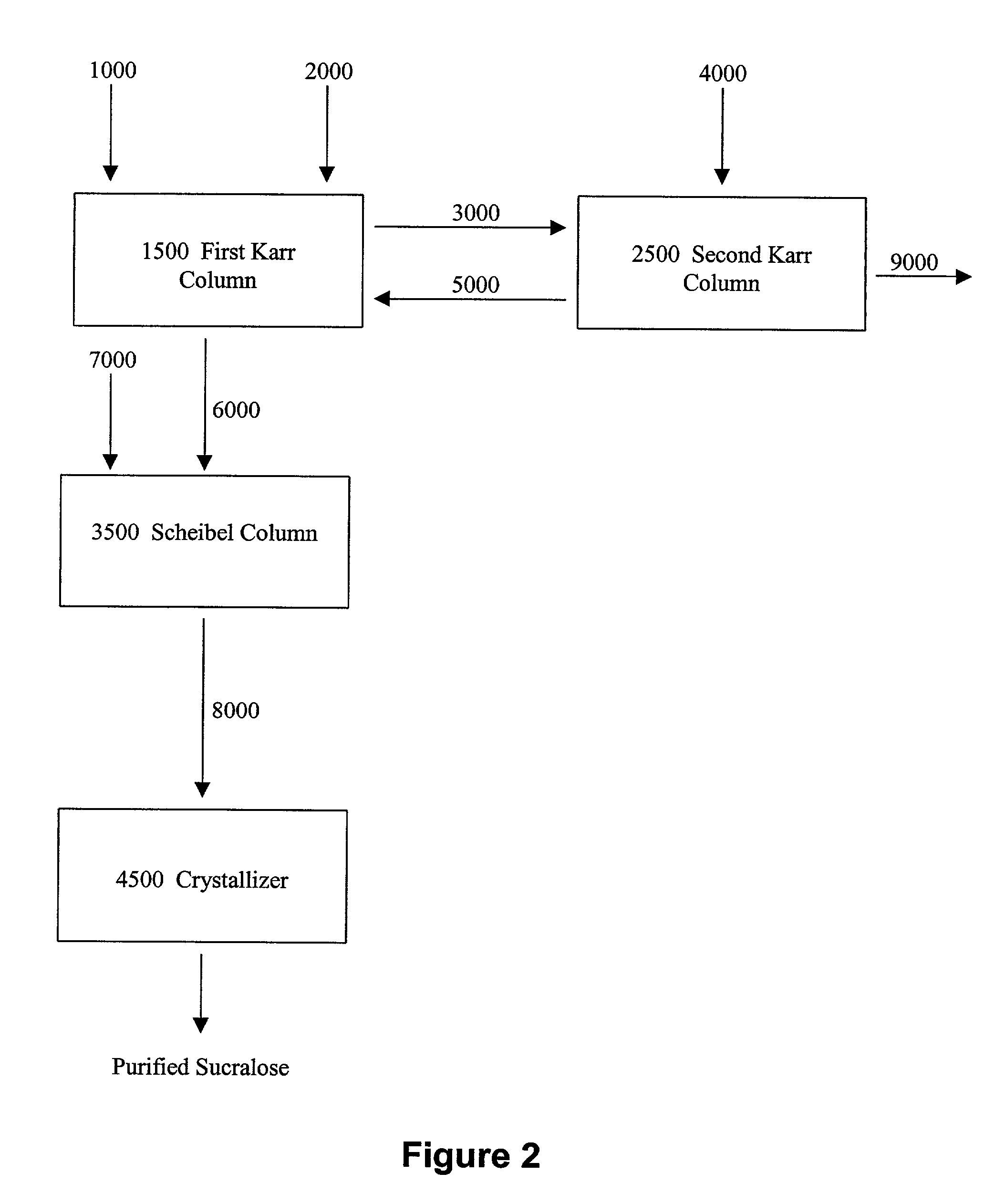
Process for improving sucralose purity and yield
InactiveUS6998480B2Improve efficiencyHigh purityEsterified saccharide compoundsSugar derivativesSucraloseCrystallization Purification
This invention relates to processes for purifying sucralose by the use of an initial non-crystallization purification procedure followed by three or more sequential crystallization steps and recycle of the mother liquor remaining from each crystallization step to the feed of another crystallization or purification step. This invention also relates to sucralose compositions as well as compositions comprising the sucralose compositions of the present invention. These compositions may be highly pure and have a superior taste profile.
Owner:TATE & LYLE TECH LTD
Electrotransport device having a reservoir housing having a flexible conductive element
ActiveUS20050004506A1Esterified saccharide compoundsOrganic active ingredientsElectricityEngineering
This invention relates to an electrotransport device, which incorporates a flexible conductive element within the reservoir housing of the device, which permits electrical communication from within the housing to outside of the housing without the use of opening, which require various methods of sealing the opening against leaks and moisture.
Owner:ALZA CORP
Cytoprotective compounds, pharmaceutical and cosmetic formulations, and methods
InactiveUS20030073712A1Organic active ingredientsSenses disorderMedicineCongestive heart failure chf
Cytoprotective compounds, many of which are phenolic derivatives characterized by a substituted phenol having certain conjugated bonds, are useful in the treatment of certain ischemic or inflammatory conditions, including but not limited to stroke, myocardial infarction, congestive heart failure, and skin disorders characterized by inflammation or oxidative damage. They are also useful in the manufacture of pharmaceutical and cosmetic formulations for the treatment of such conditions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Process for the preparation of sucralose
ActiveUS20070227897A1Economical and efficientHigh yieldEsterified saccharide compoundsElectrolysis componentsSucroseElectrolysis
A process for preparing sucrose-6-ester is provided, which comprises electrolyzing an electrolyte solution containing sucrose, an acylating reagent and a halide catalyst. Also disclosed is a process for preparing sucralose, which involves the preparation and chlorination of sucrose-6-ester followed by deacylation of the molecule. The process of the invention can be more readily performed with a higher yield than those in the art.
Owner:TECHNO (FUJIAN) FOOD INGREDIENTS CO LTD
Preparation of nucleosides ribofuranosyl pyrimidines
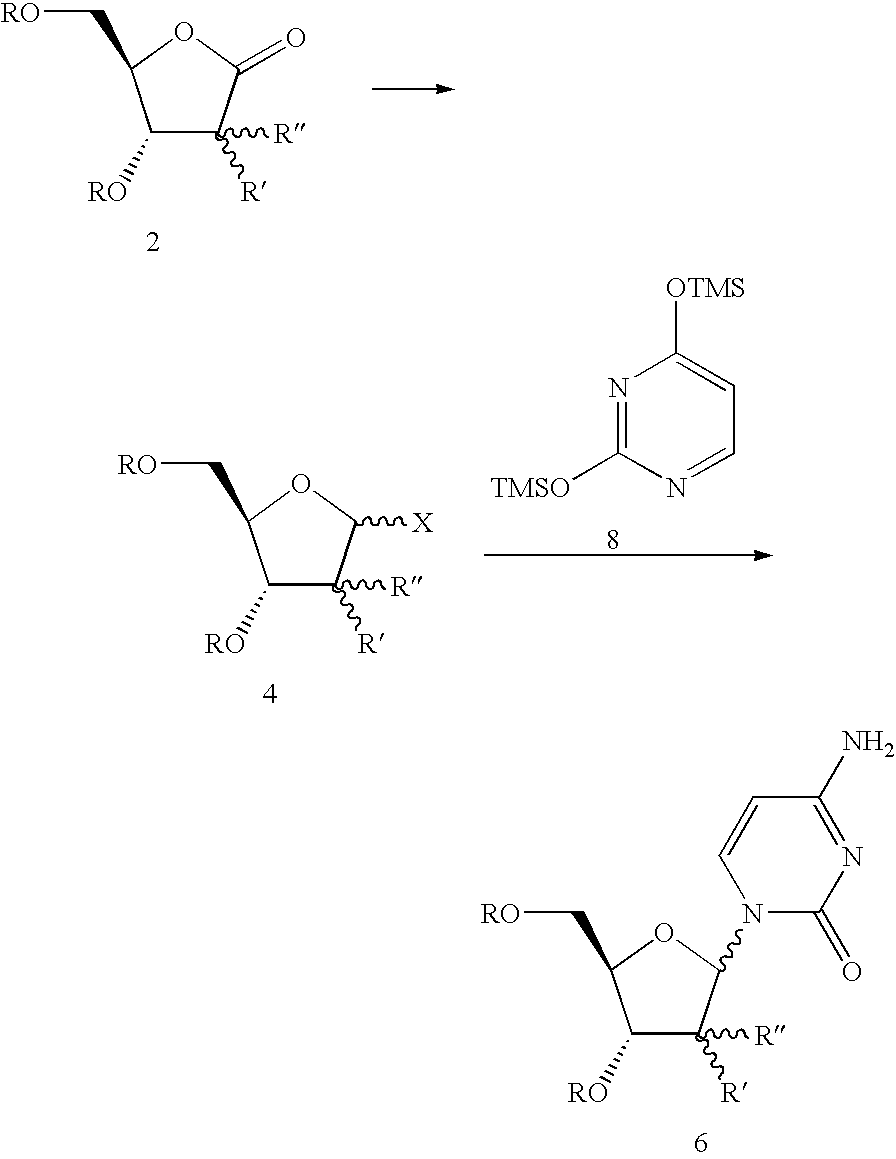
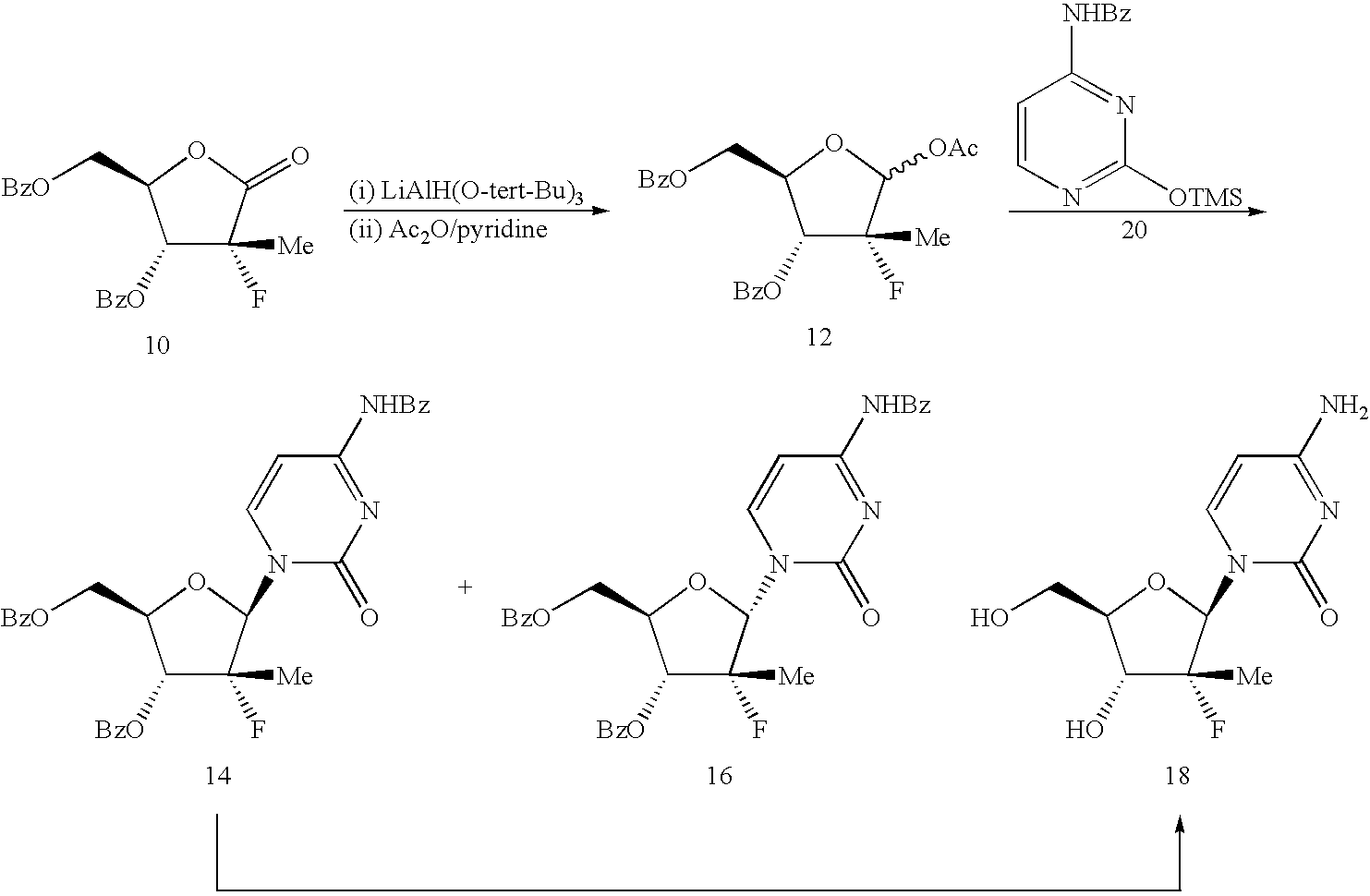
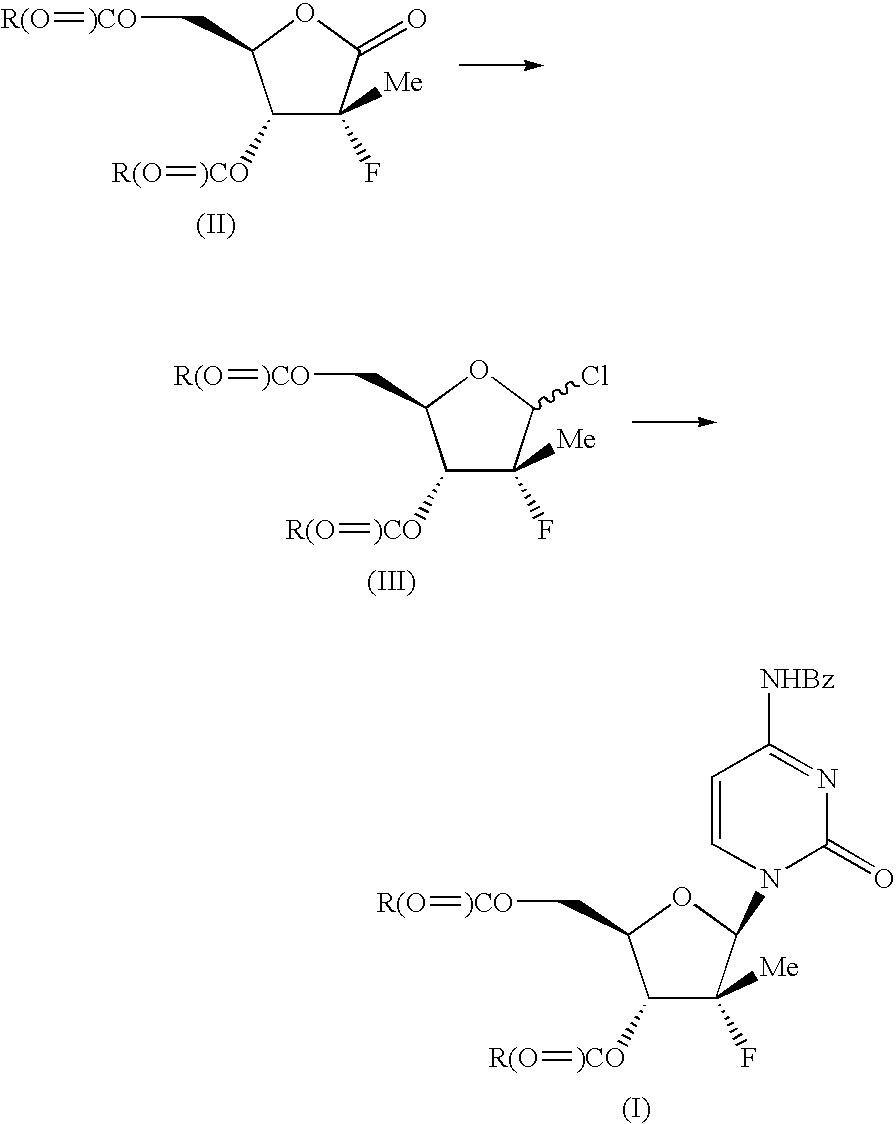
InactiveUS20080139802A1Efficient scalable processEasy to handleEsterified saccharide compoundsSugar derivativesCombinatorial chemistrySynthesis of nucleosides
The present process provides an improved method for converting 2′-deoxy-2′-fluoro-2′-methyl-D-ribonolactones derivatives to 3-fluoro-3-methyl-2-chlorofuran compounds which are useful for the synthesis of nucleosides and improved processes for the synthesis of the D-ribonolactone compounds.
Owner:PHARMASSET +1
Lower alkyl ester recycling in polyol fatty acid polyester synthesis
InactiveUS6465642B1Quality improvementReduce side effectsEsterified saccharide compoundsSugar derivativesPolyesterPolyol
Process for synthesizing polyol fatty acid polyesters which includes the steps of reacting an excess of lower alkyl ester with polyol to esterify hydroxyl groups thereof and form polyol fatty acid polyester, separating at least a portion of the unreacted lower alkyl ester from the polyol fatty acid polyester, and recycling the separated unreacted lower alkyl ester for further reaction with polyol or partially esterified polyol. The recycled lower alkyl ester is substantially free of lower alkyl ester degradation reaction products, such as carbonyls and free fatty acids.
Owner:THE PROCTER & GAMBLE COMPANY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com