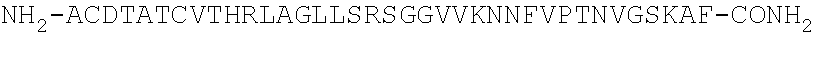Patents
Literature
132 results about "Interstitial cystitis" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
A chronic, painful bladder condition where increased urinary urgency and frequency is observed.
Pelvic disorder treatment device
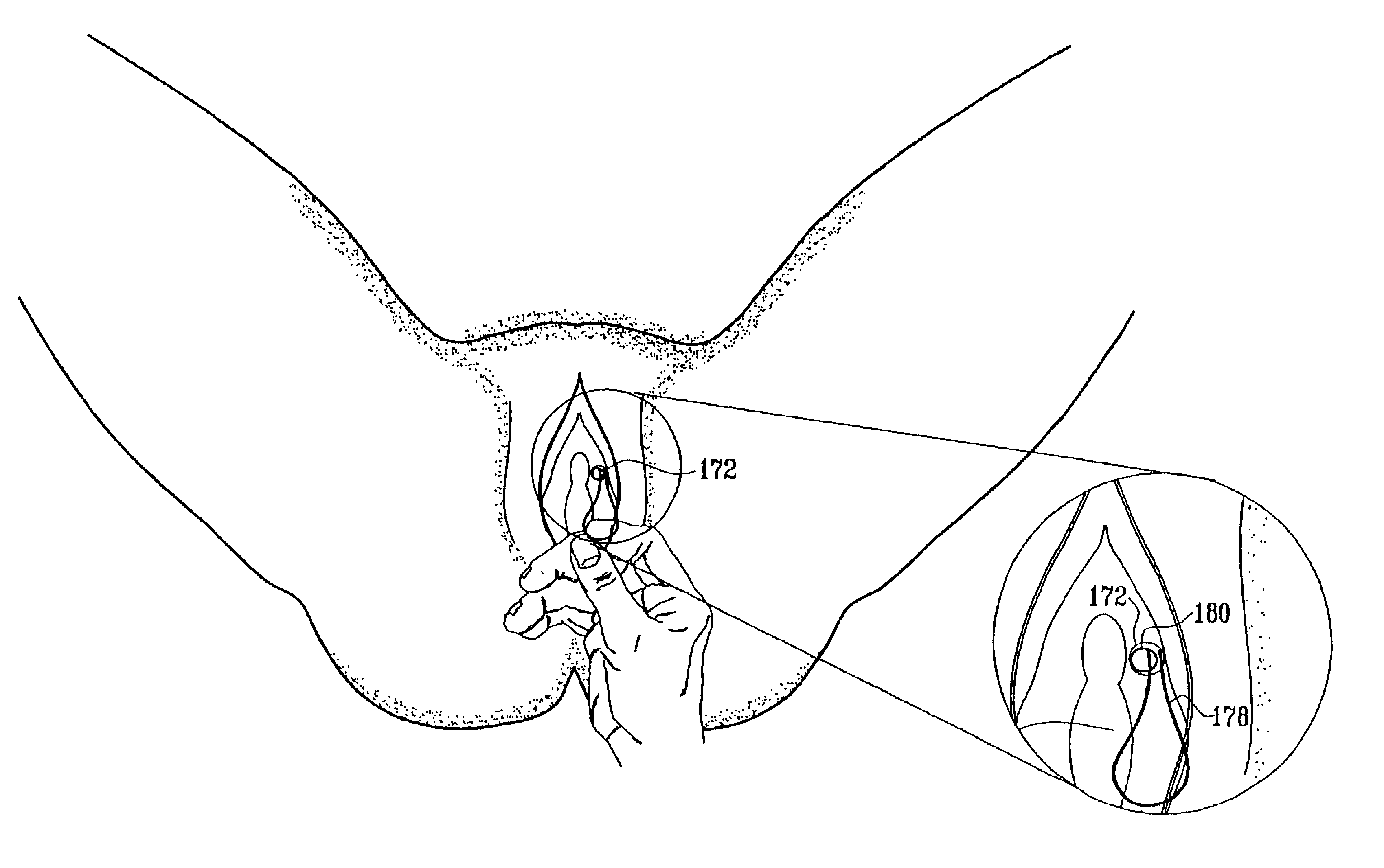
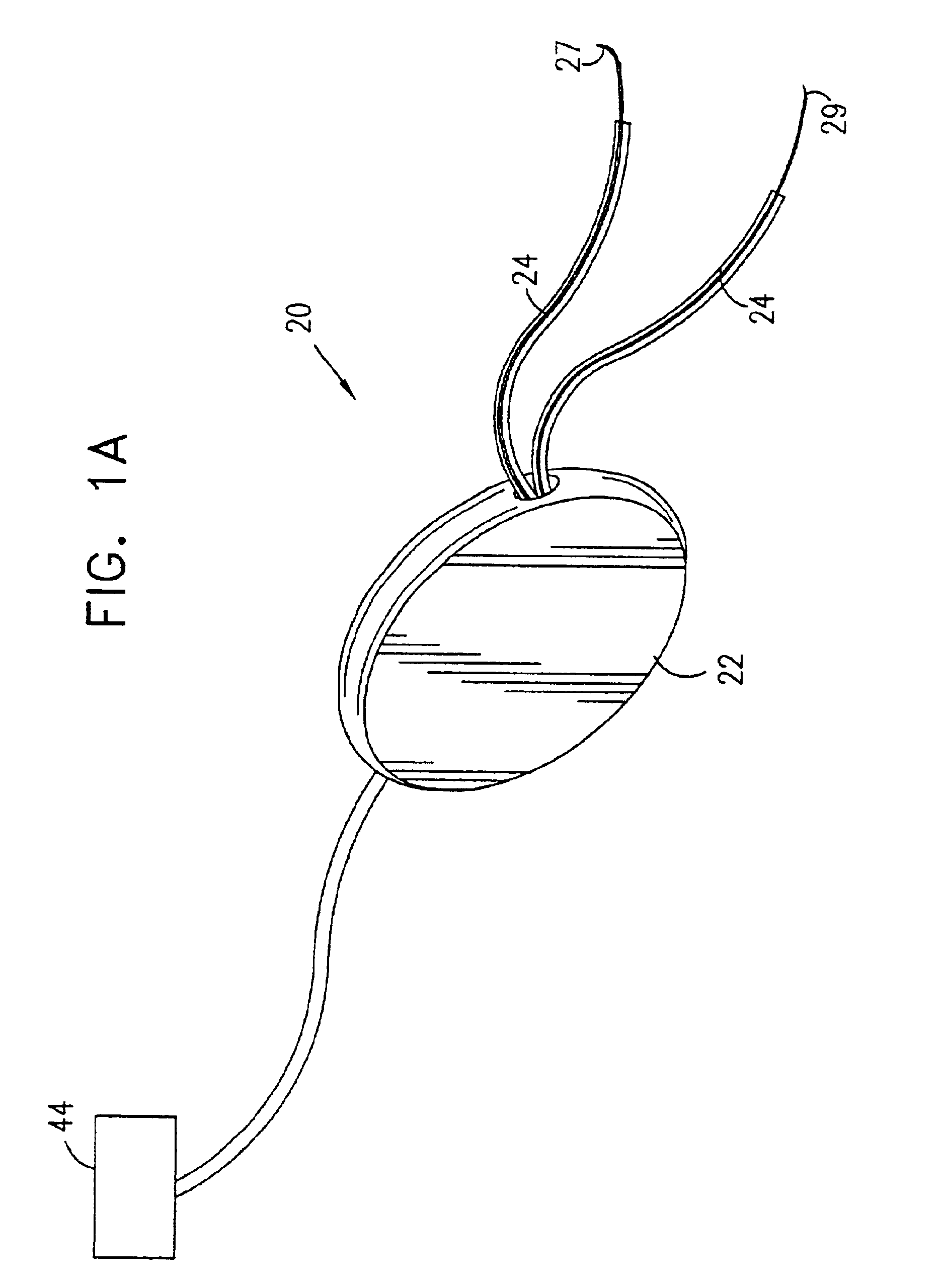
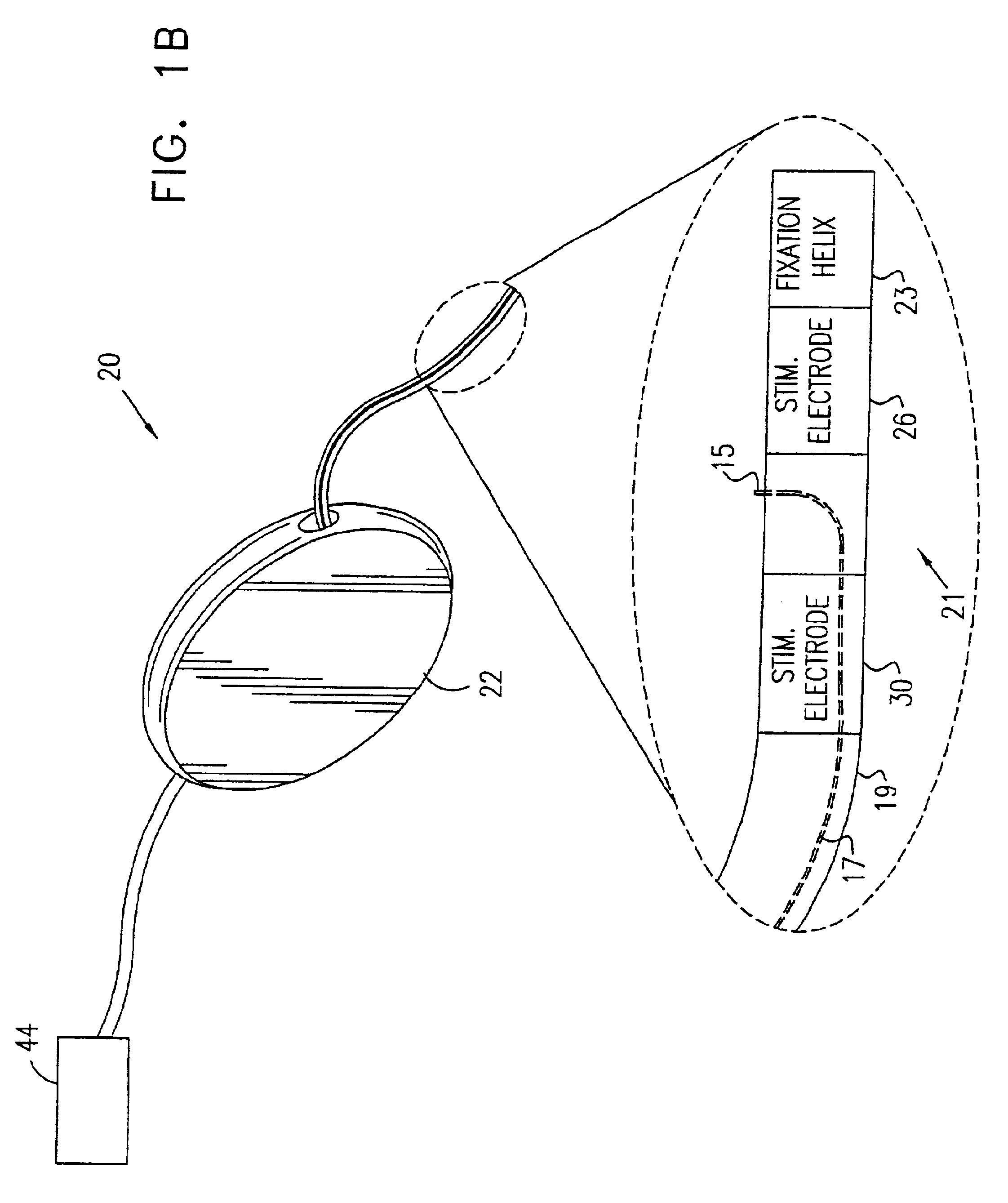
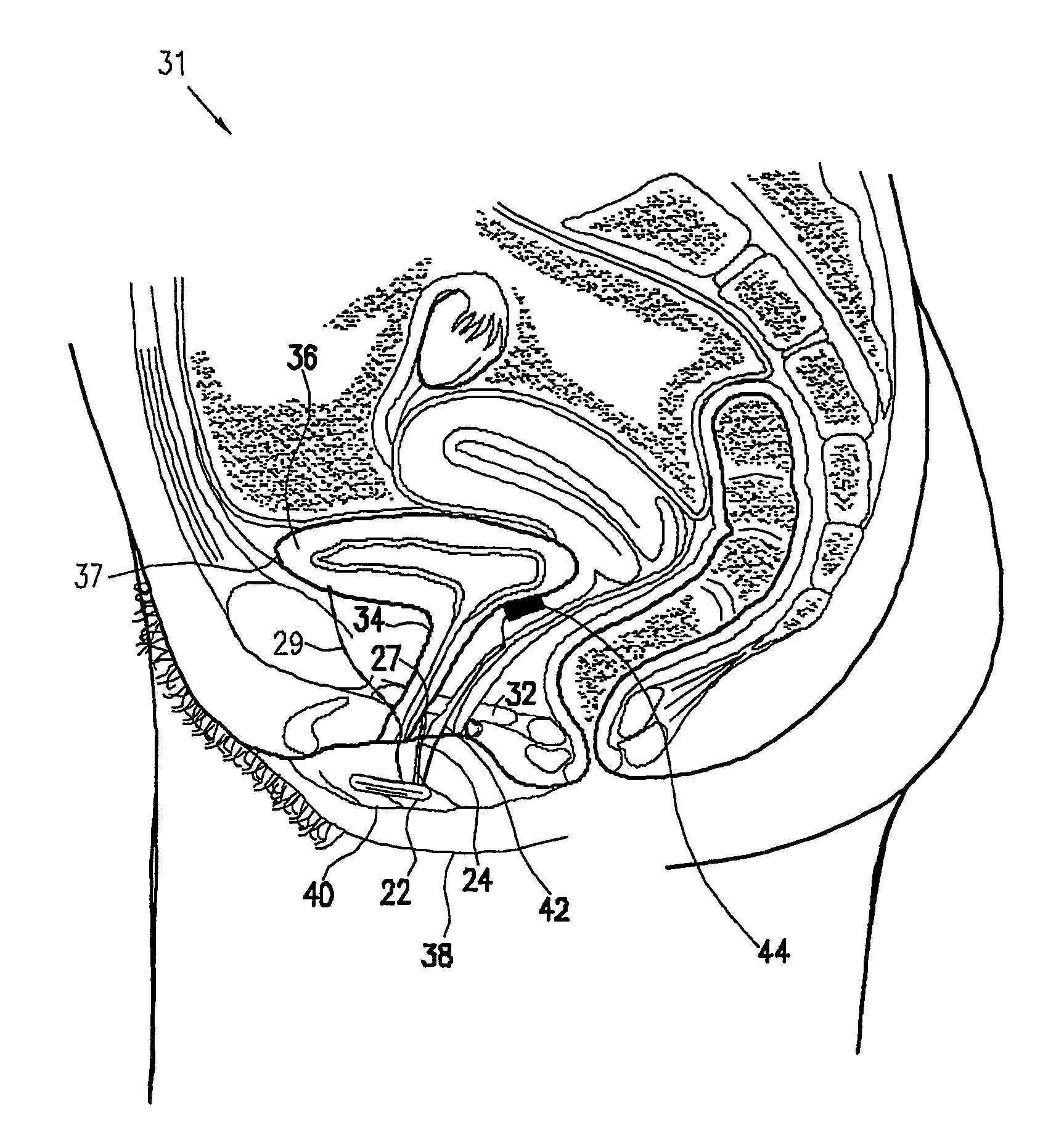
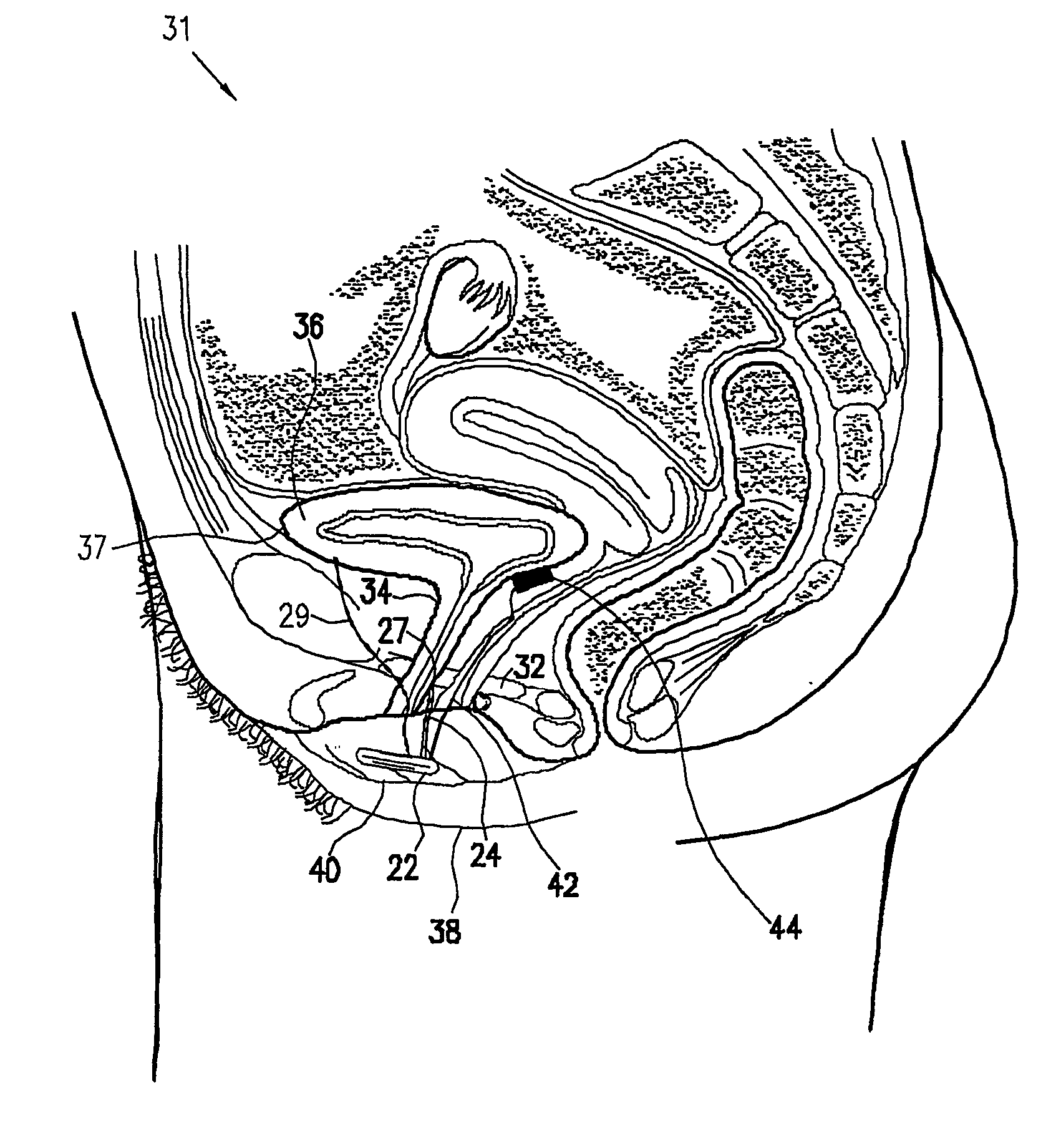
InactiveUS6862480B2Reduce decreaseRelieving pelvic painUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
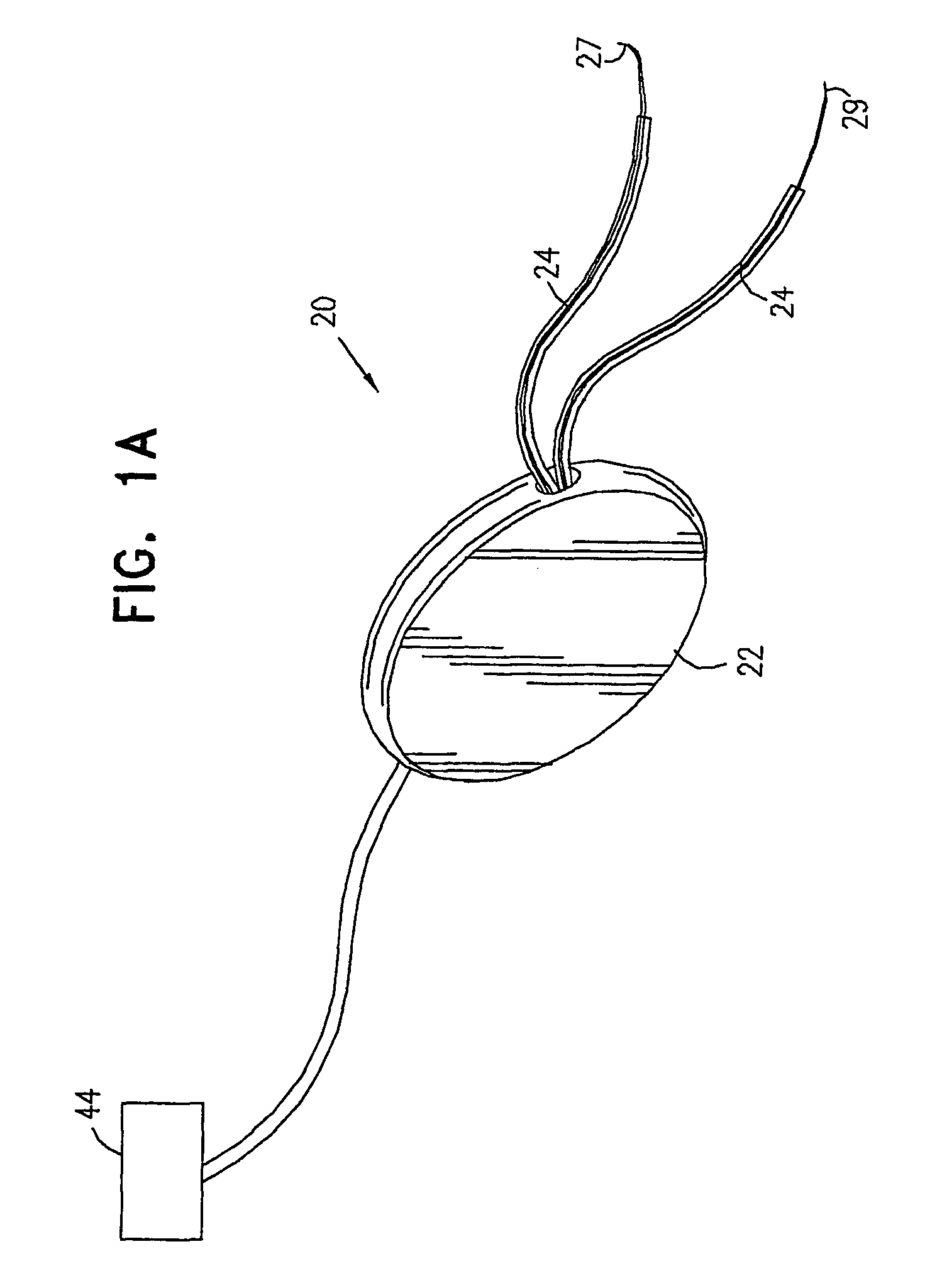
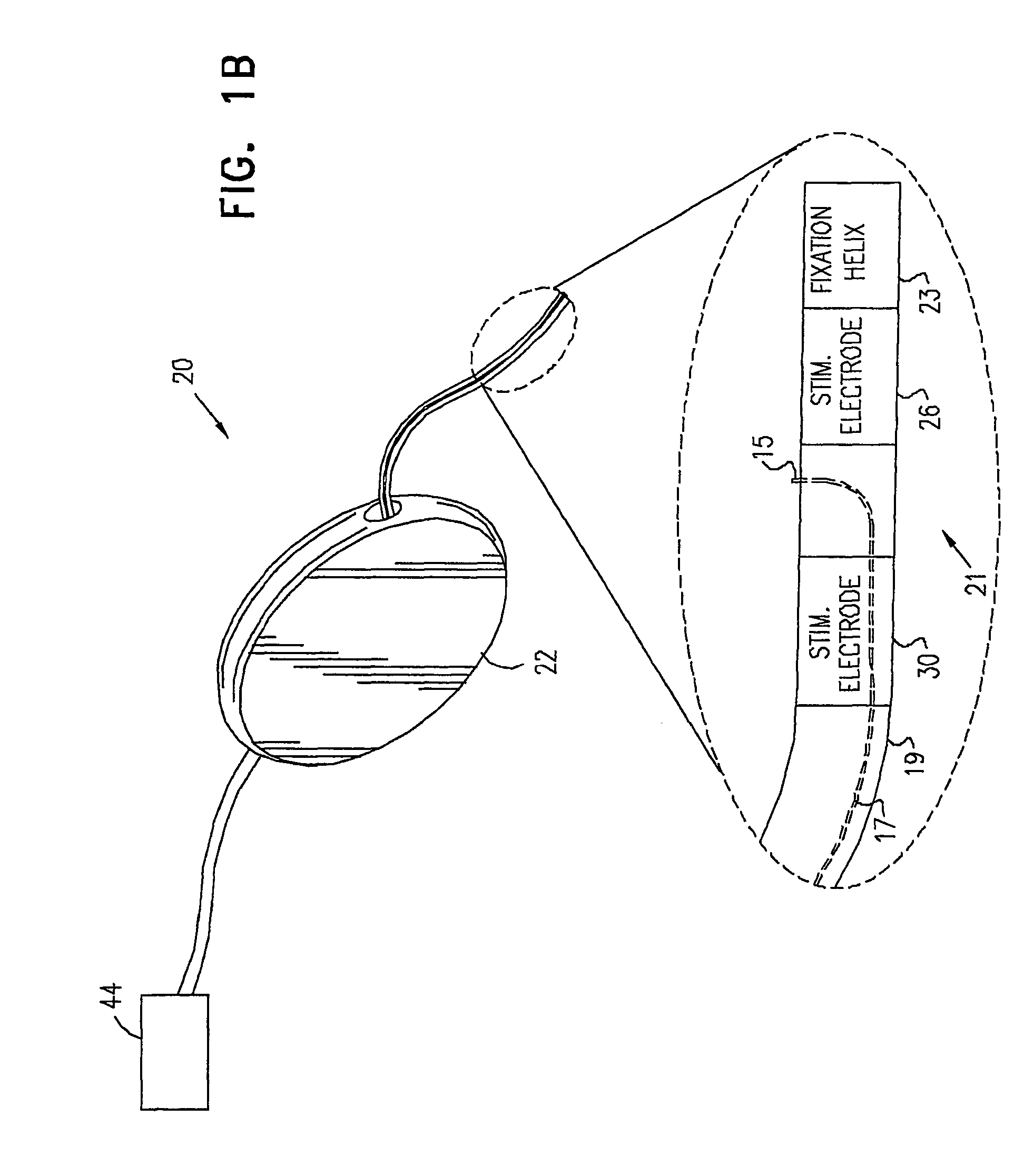
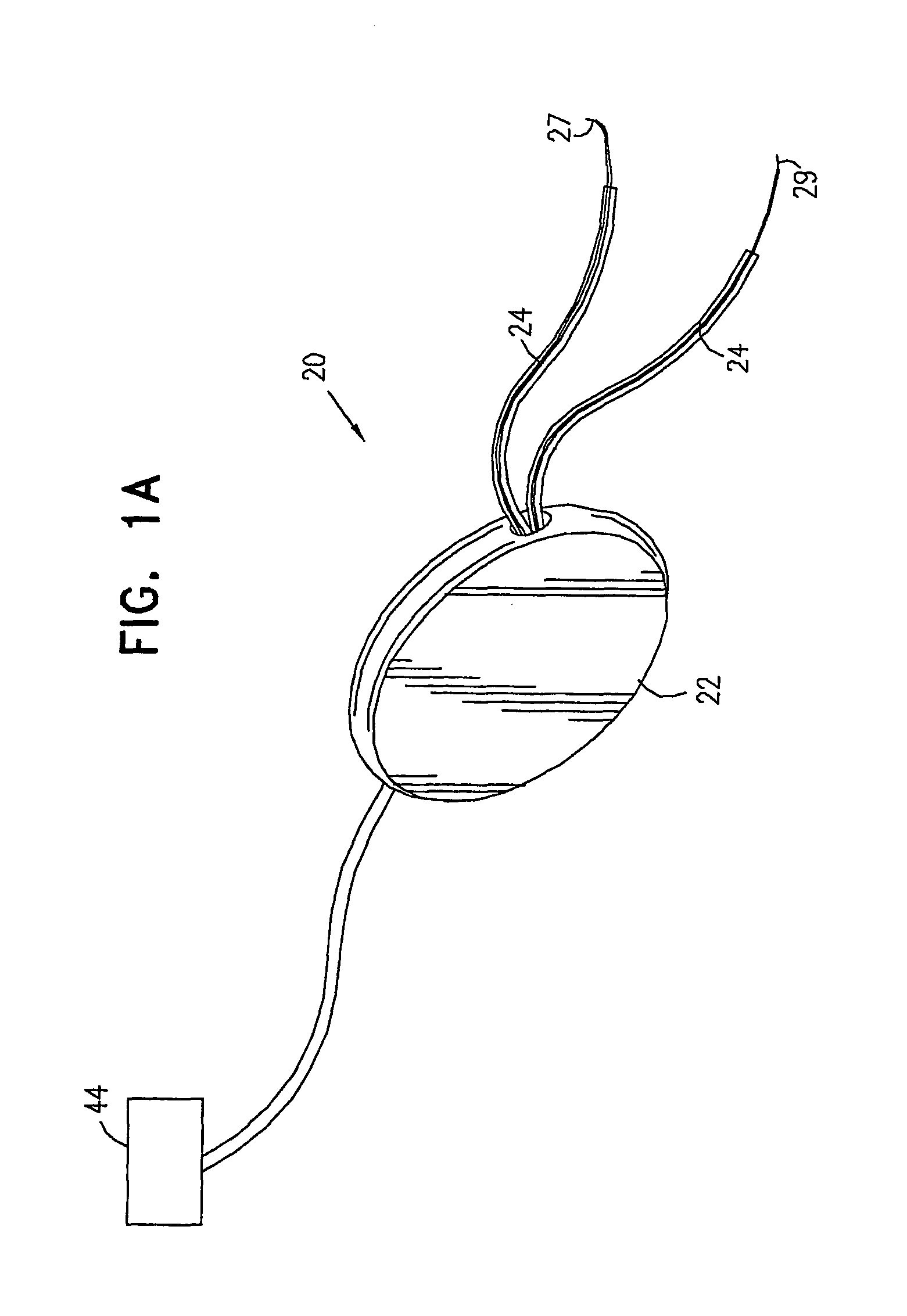
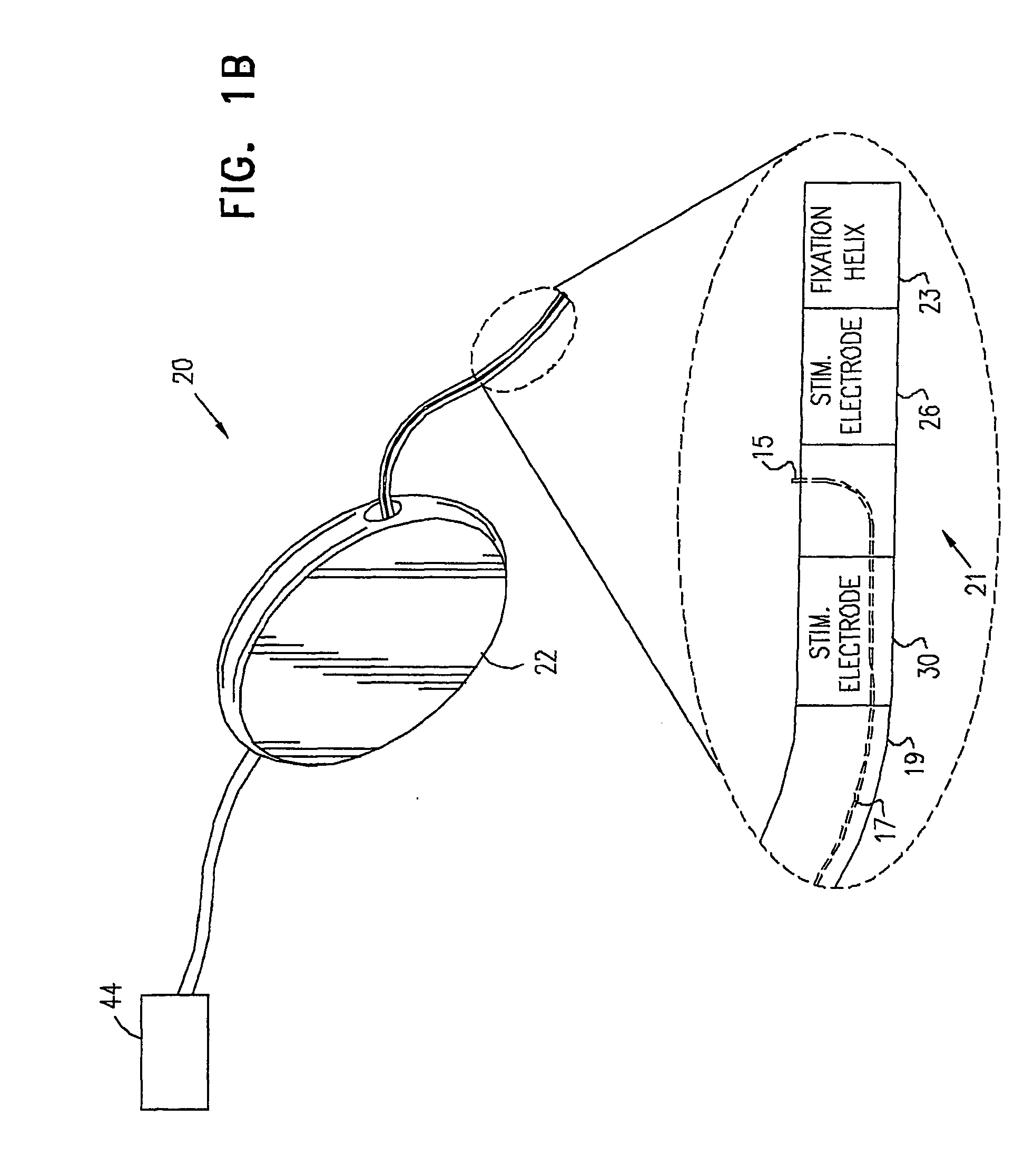
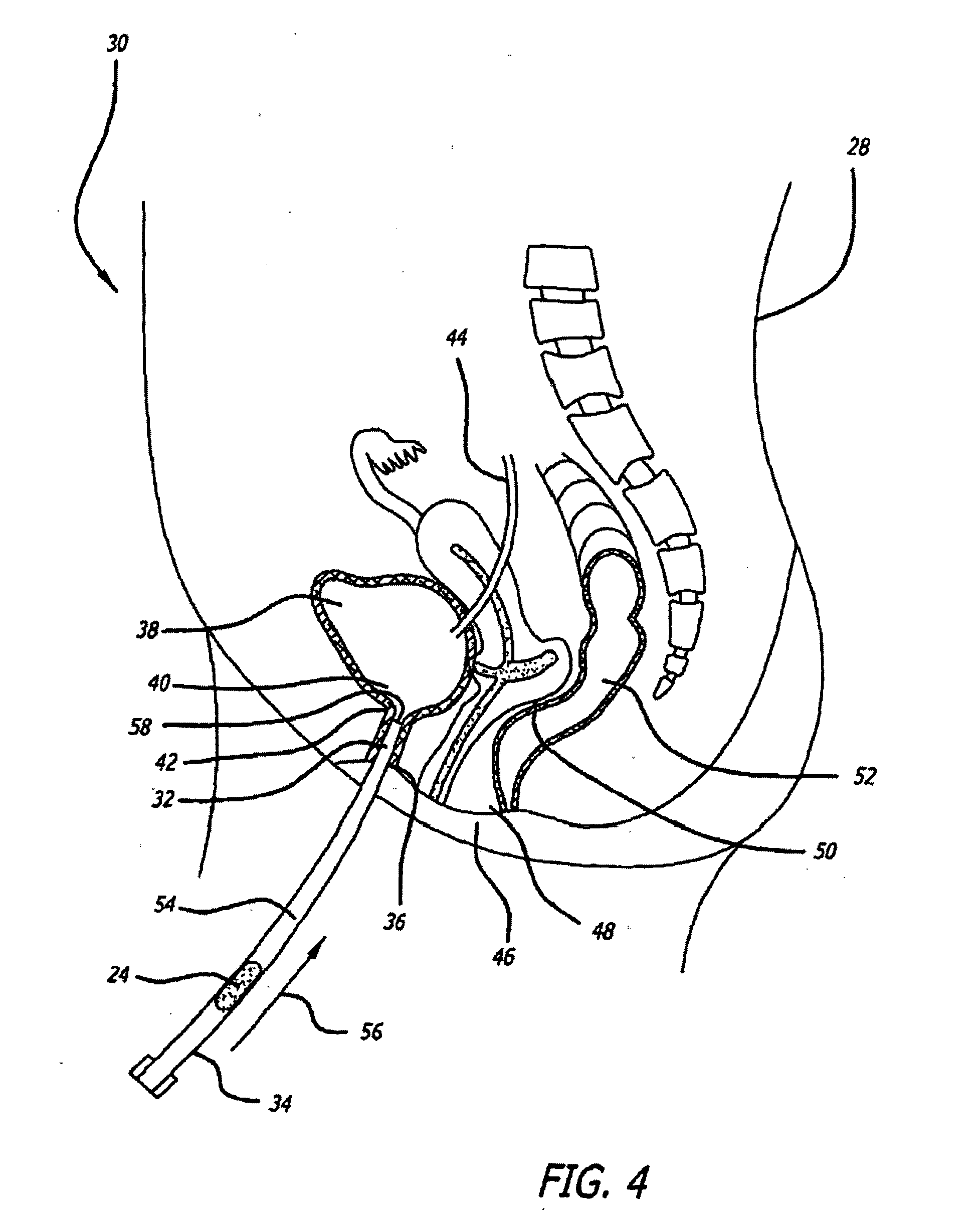
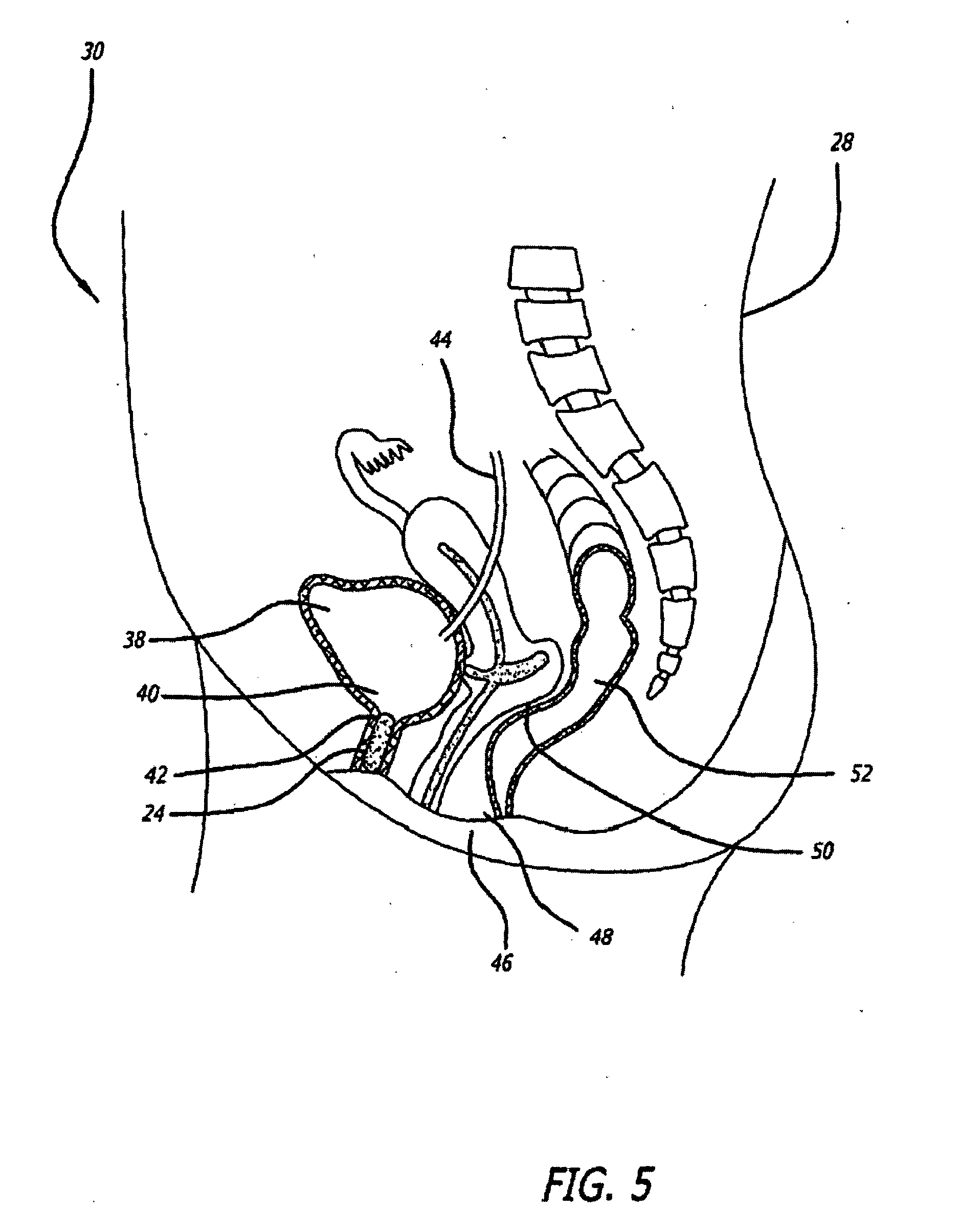
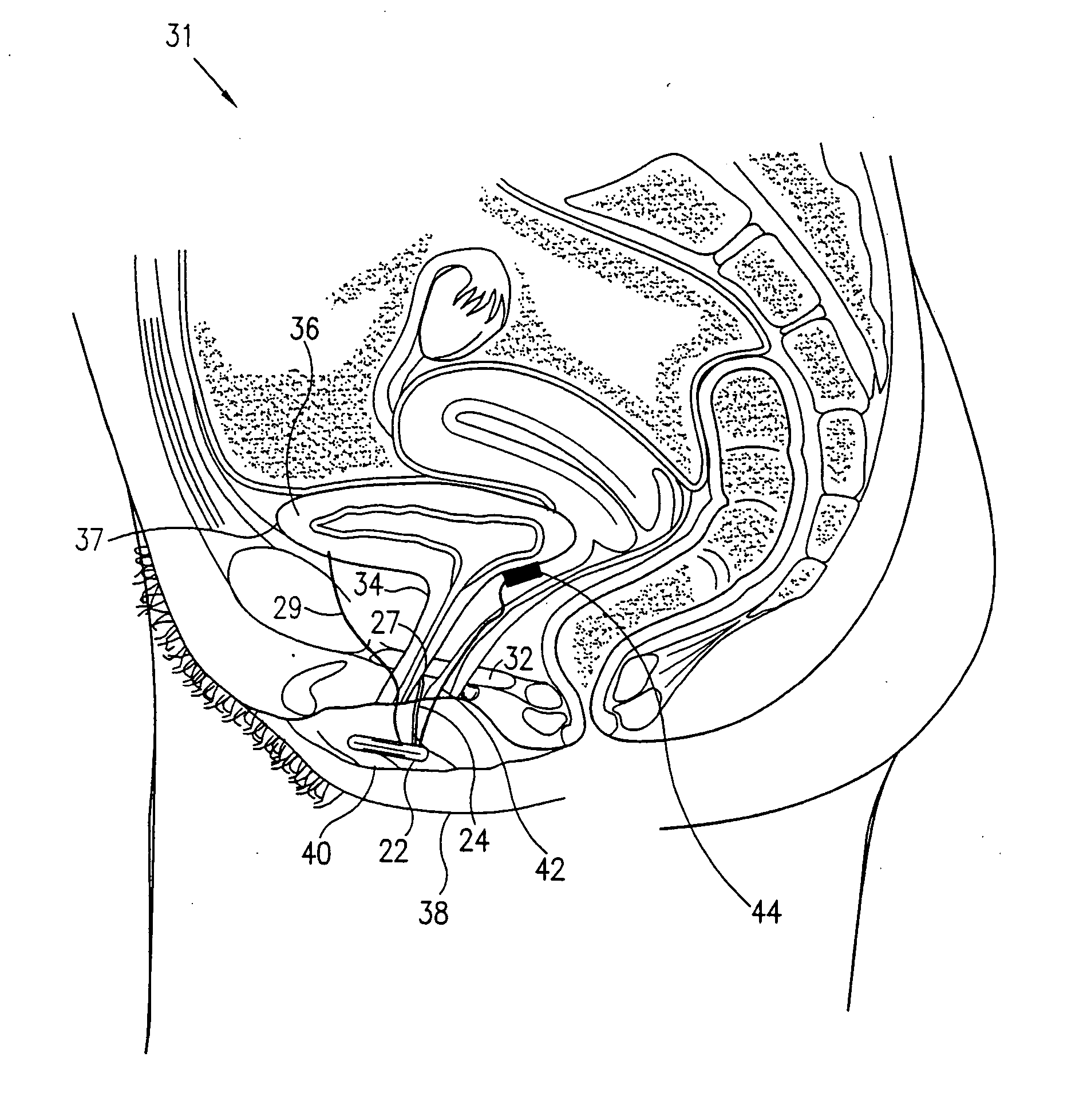
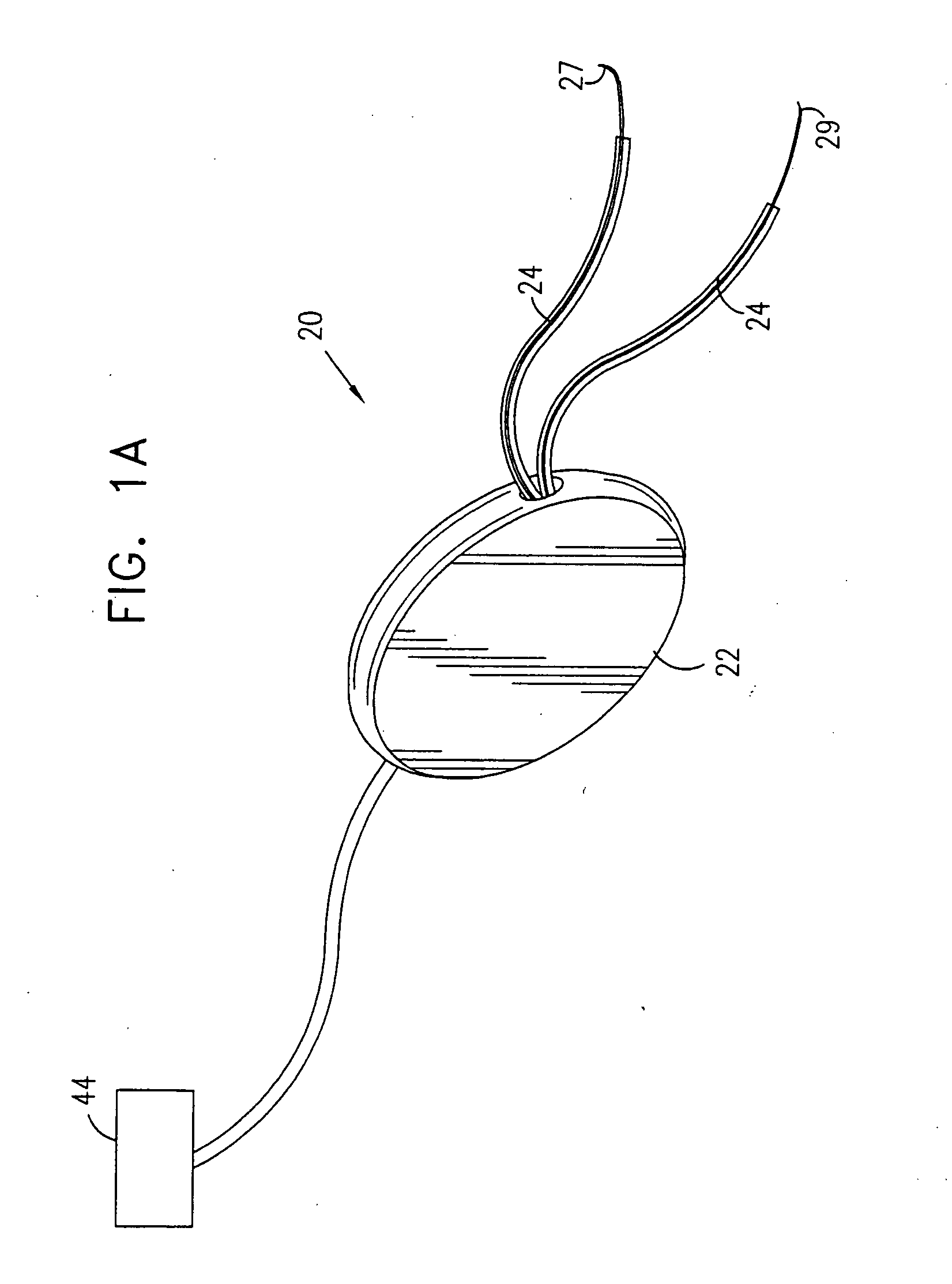
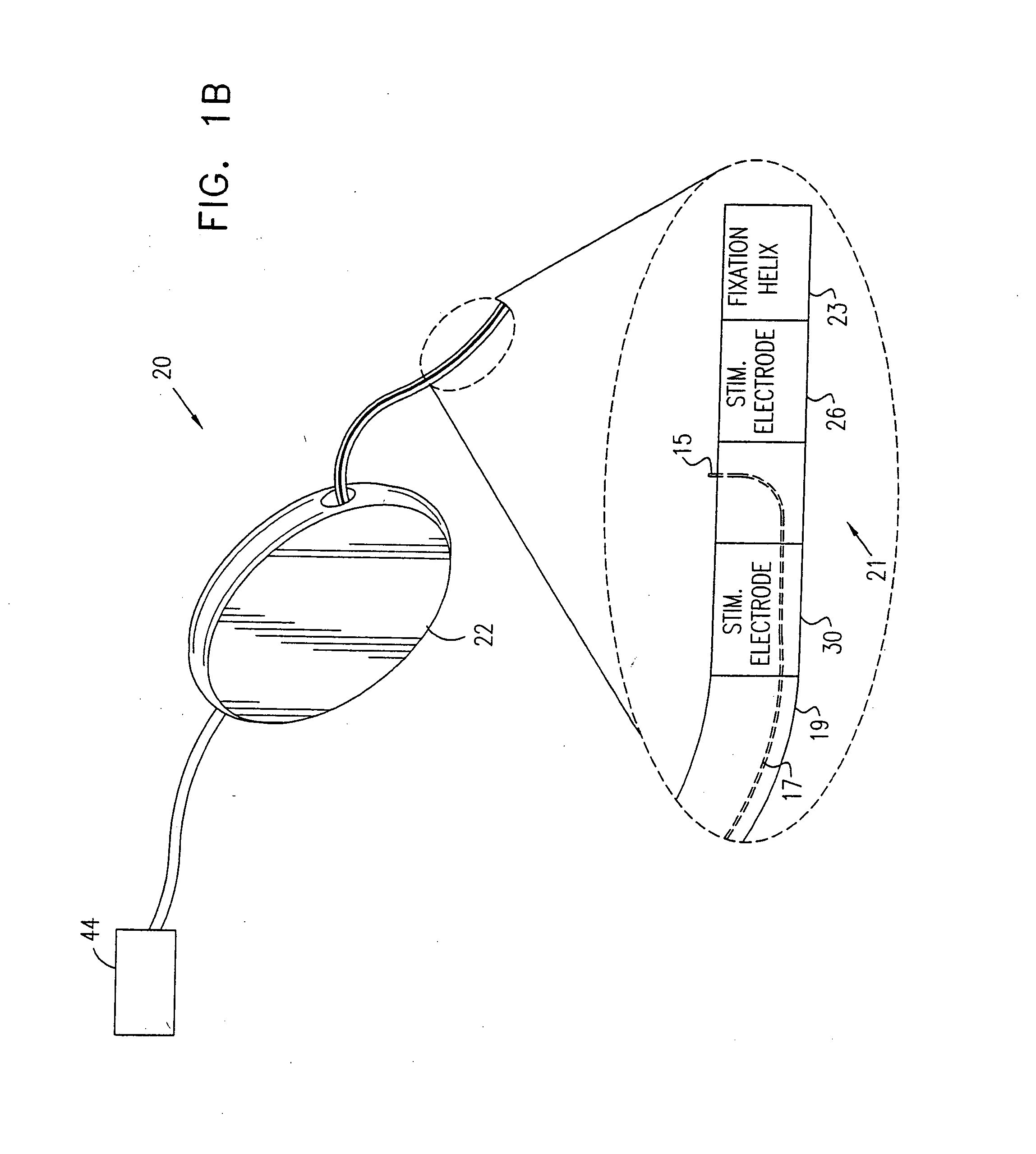
A device for treating a medical condition is provided, and a surgical procedure for implanting the device is disclosed. The device includes a sensor, which is adapted to generate a signal responsive to a state of a patient, and at least one electrode, which is adapted to be coupled to a pelvic site of the patient. A control unit is adapted to receive the signal, to analyze the signal so as to distinguish between an imminent stress incontinence event and an imminent urge event, and, responsive to analyzing the signal, to apply an electrical waveform to the at least one electrode. In various configurations, the device may be used alternatively or additionally to treat fecal incontinence, interstitial cystitis, chronic pelvic pain, or urine retention.
Owner:ASTORA WOMENS HEALTH
Pelvic disorder treatment device
ActiveUS7613516B2Relieving pelvic painReliable controlUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
Owner:MEDTRONIC INC +1
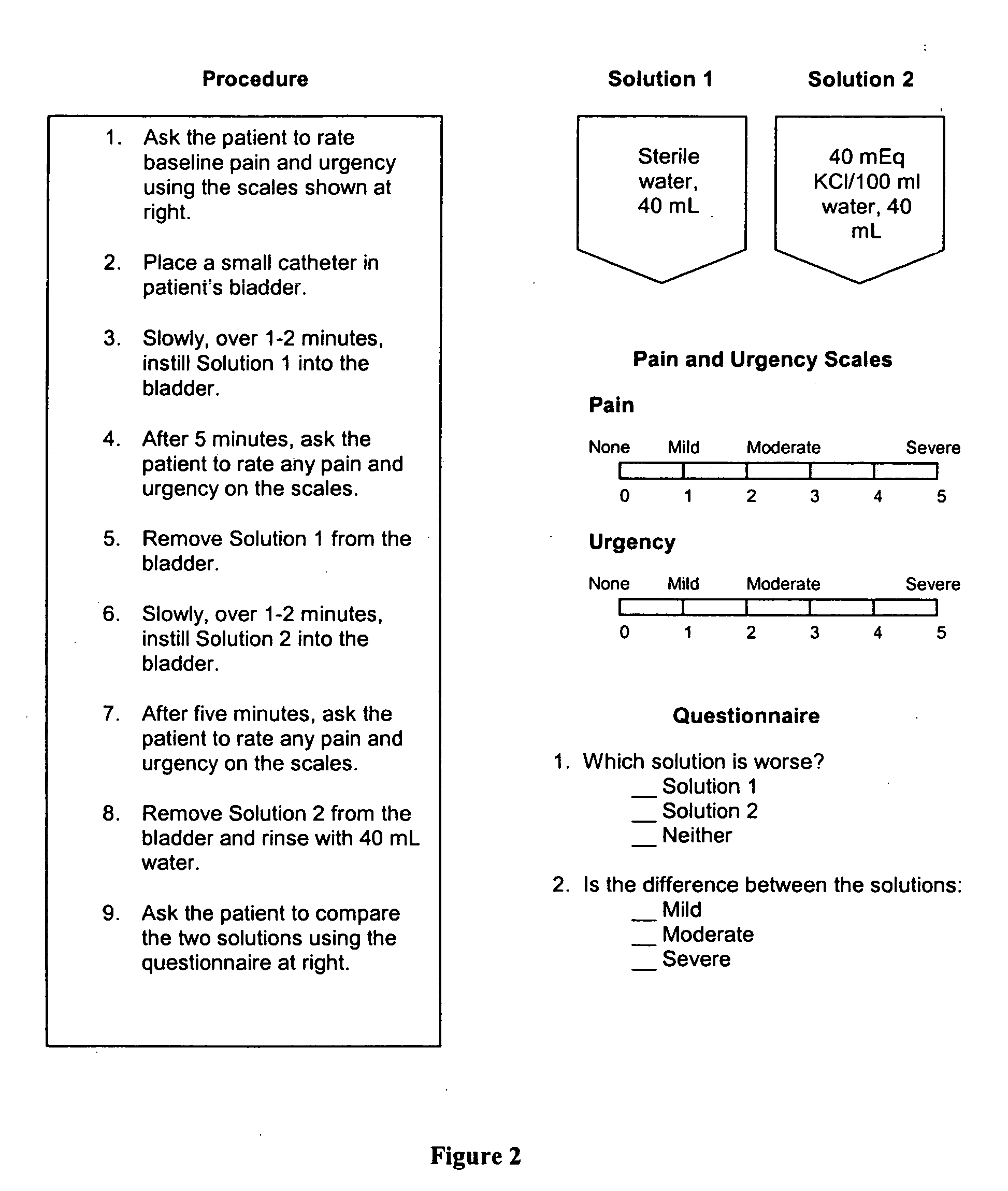
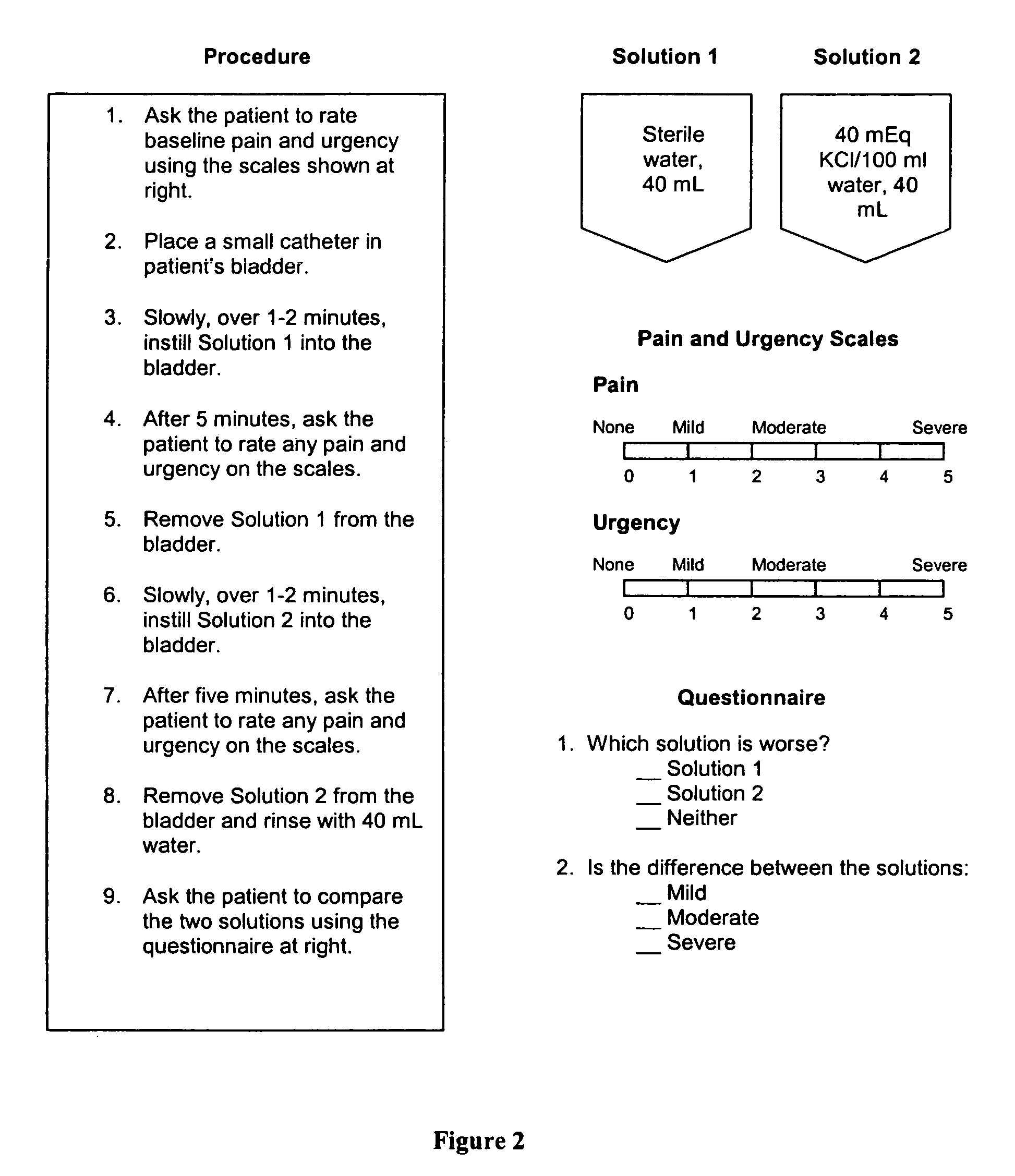
Treatment of cystitis-like symptoms with chondroitin sulfate following administration of a challenge solution
Cystitis of the bladder and urinary tract, particularly interstitial cystitis, are treated using effective unit doses of chondroitin sulfate. Further, cystitis patients are screened for their response to a given cystitis treatment using a method in which patients are first challenged with an irritant and then treated with a selected cystitis therapeutic. Candidates for further treatment are identified as those patients who on receiving the selected therapeutic, report relief from at least one symptom elicited with the irritant. Also provided are kits comprising solutions for carrying out this screening method.
Owner:STELLAR INT
Treatment of disease states and adverse physiological conditions utilizing anti-fungal compositions
InactiveUS20060177424A1Effectively ameliorateEffective regulationBiocideBacteria material medical ingredientsSinusitisVaginal Yeast Infections
A method for treatment or prophylaxis of a disease state or other physiological condition, e.g., autism, delayed development, acid reflux disease, vaginal yeast infections, impaired hearing, chronic ear infections, seasonal allergies, Fibromyalgia syndrome, Crohn's disease, colitis, irritable bowel syndrome, interstitial cystitis, acne, sinusitis, rheumatoid arthritis, chronic fatigue syndrome, asthma, attention deficit disorder, attention deficit / hyperactivity disorder, rosacea, multiple sclerosis, hyperglycemia, or Ménière's disease, by administration of an anti-fungal composition that includes at least one of the bacilli (1) Bacillus subtilis, (2) Lactobacillus sporogenes, and (3) Streptococcus faecalis.
Owner:COBB AND CO
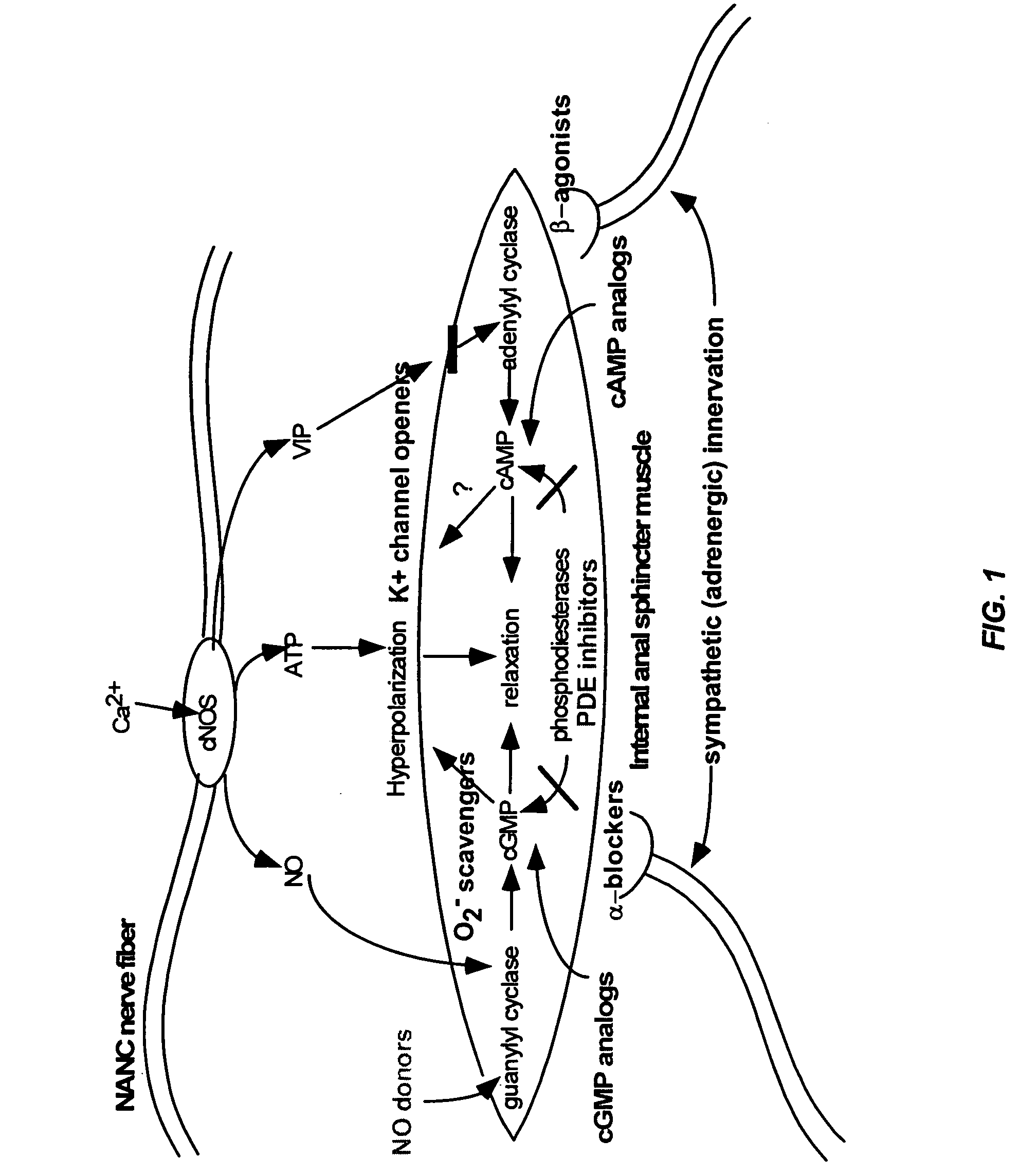
Compounds and methods for the treatment of urogenital disorders
InactiveUS6987129B2Reduce painLess discomfortBiocidePeptide/protein ingredientsDiseaseFemale Sexual Arousal Disorder
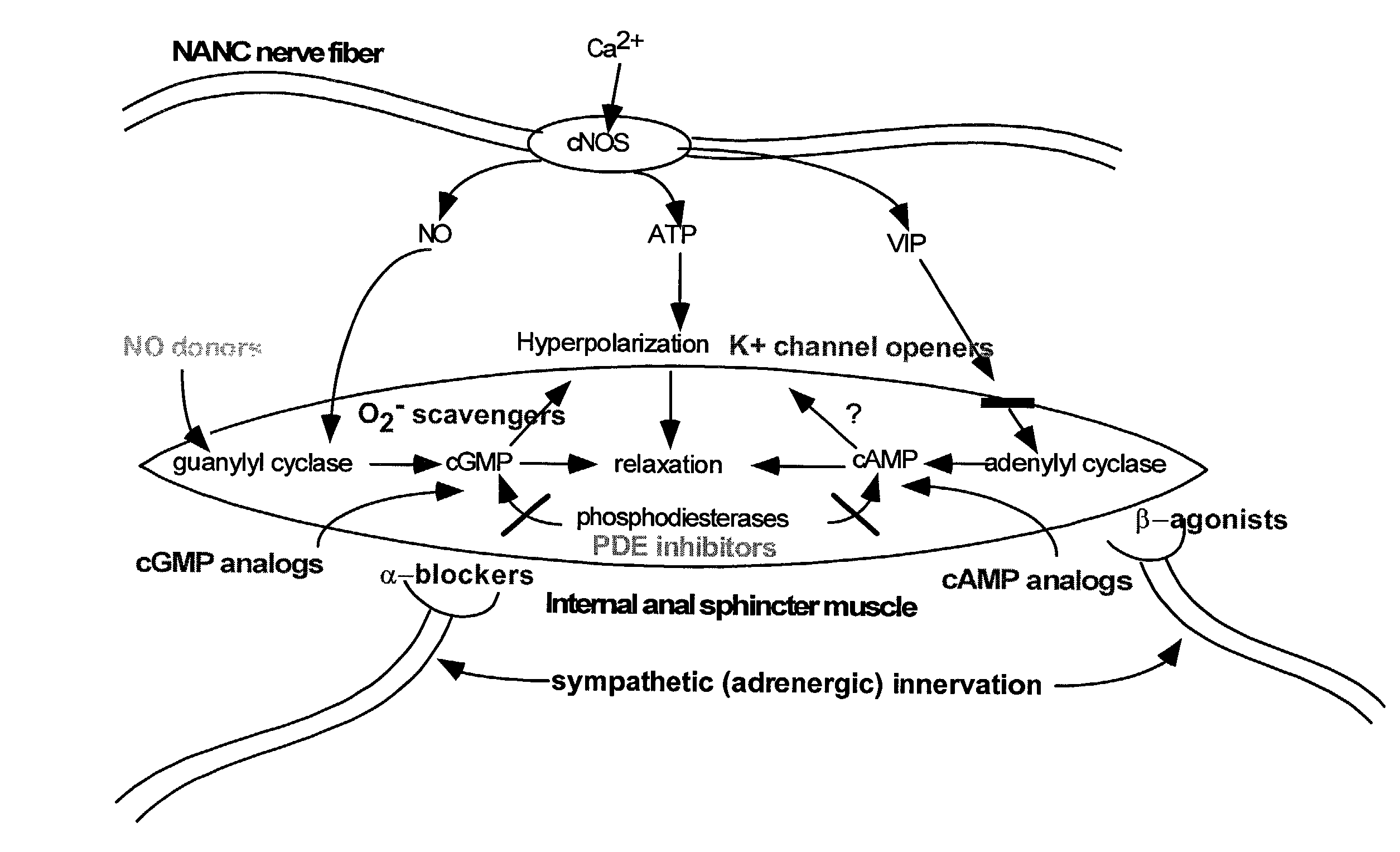
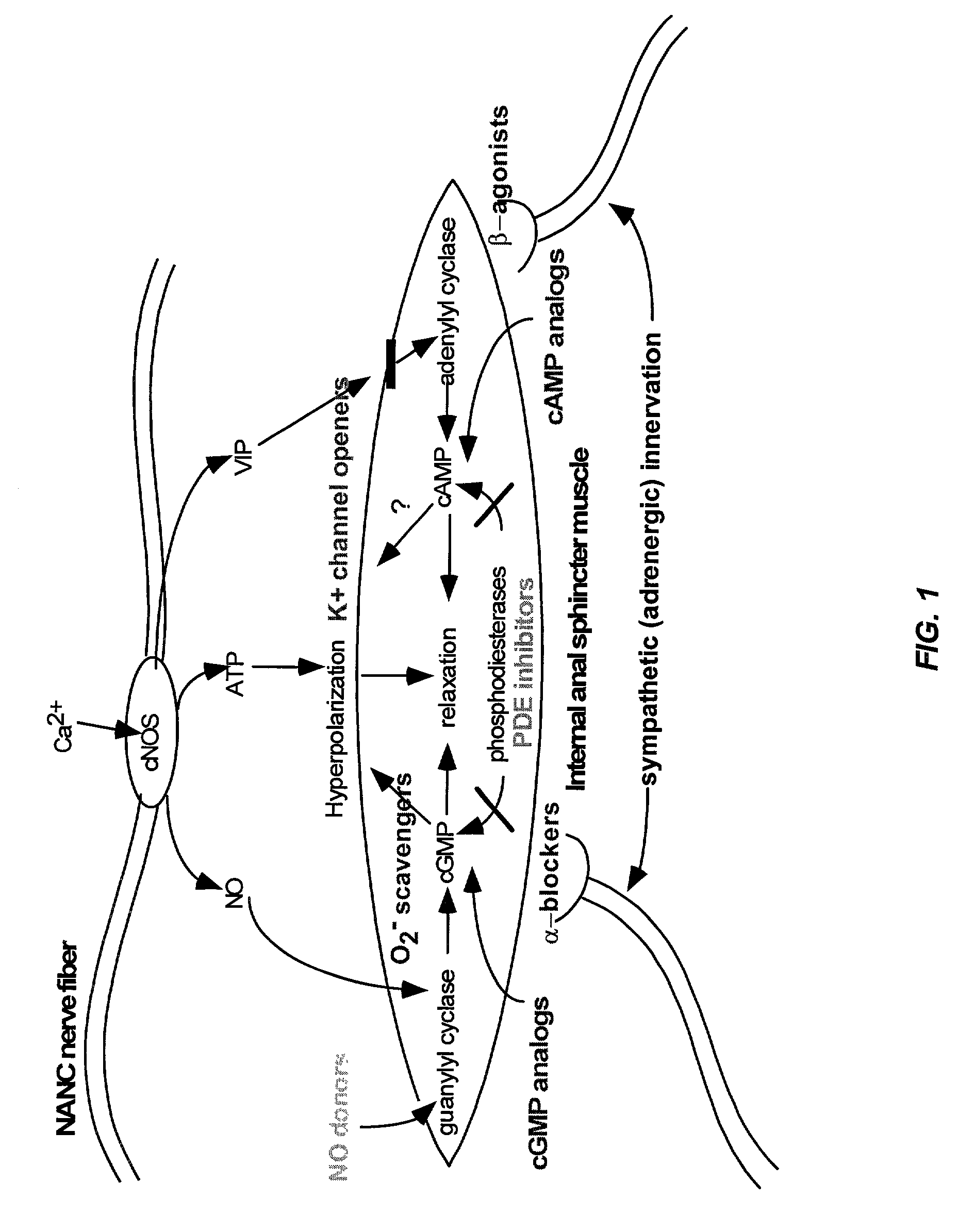
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
Pelvic disorder treatment device
ActiveUS20050216069A1Reduce inconvenienceMinimize discomfortUltrasonic/sonic/infrasonic diagnosticsElectrotherapyFecal incontinenceInterstitial cystitis
A device (20) for treating a medical condition is provided, and a surgical procedure for implanting the device is disclosed. The device (20) includes a sensor (44), which is adapted to generate a signal responsive to a state of a patient, and at least one electrode (27), which is adapted to be coupled to a pelvic site of the patient. A control unit (22) is adapted to receive the signal, to analyze the signal so as to distinguish between an imminent stress incontinence event and an imminent urge event, and, responsive to analyzing the signal, to apply an electrical waveform to the at least one electrode (27). In various configurations, the device (20) may be used alternatively or additionally to treat fecal incontinence, interstitial cystitis, chronic pelvic pain, or urine retention.
Owner:MEDTRONIC INC +1
Method of treating mast cell activation-induced diseases with a proteoglycan
InactiveUS6689748B1Decrease in urinaryBiocidePeptide/protein ingredientsInflammatory Bowel DiseasesInterstitial cystitis
The invention provides a method for preventing and treating the harmful biological effects of biochemicals secreted from activated mast cells in the organism of warm blooded animals and more especially human beings, said effects being associated with allergy (including but not limited to allergic conjunctivitis, allergic rhinitis, allergic otitis, asthma, allergic uticaria, food allergy and atopic dermatitis), hyperproliferative diseases such as leukemia and systemic mastocytosis, interstitial cystitis, inflammatory bowel disease, irritable bowel syndrome, osteoporosis and scleroderma. The method consists in administering to said animals and especially to human beings an effective amount of a proteoglycan such as chondroitin sulfate with mast cell secretion inhibitory activity, alone or in combination with one or more synergistic adjuvants such those belonging to the class of flavonoids or compounds with histamine-1 receptor antagonist activity.
Owner:THETA BIOMEDICAL CONSULTING & DEVMENT
Selective opioid compounds
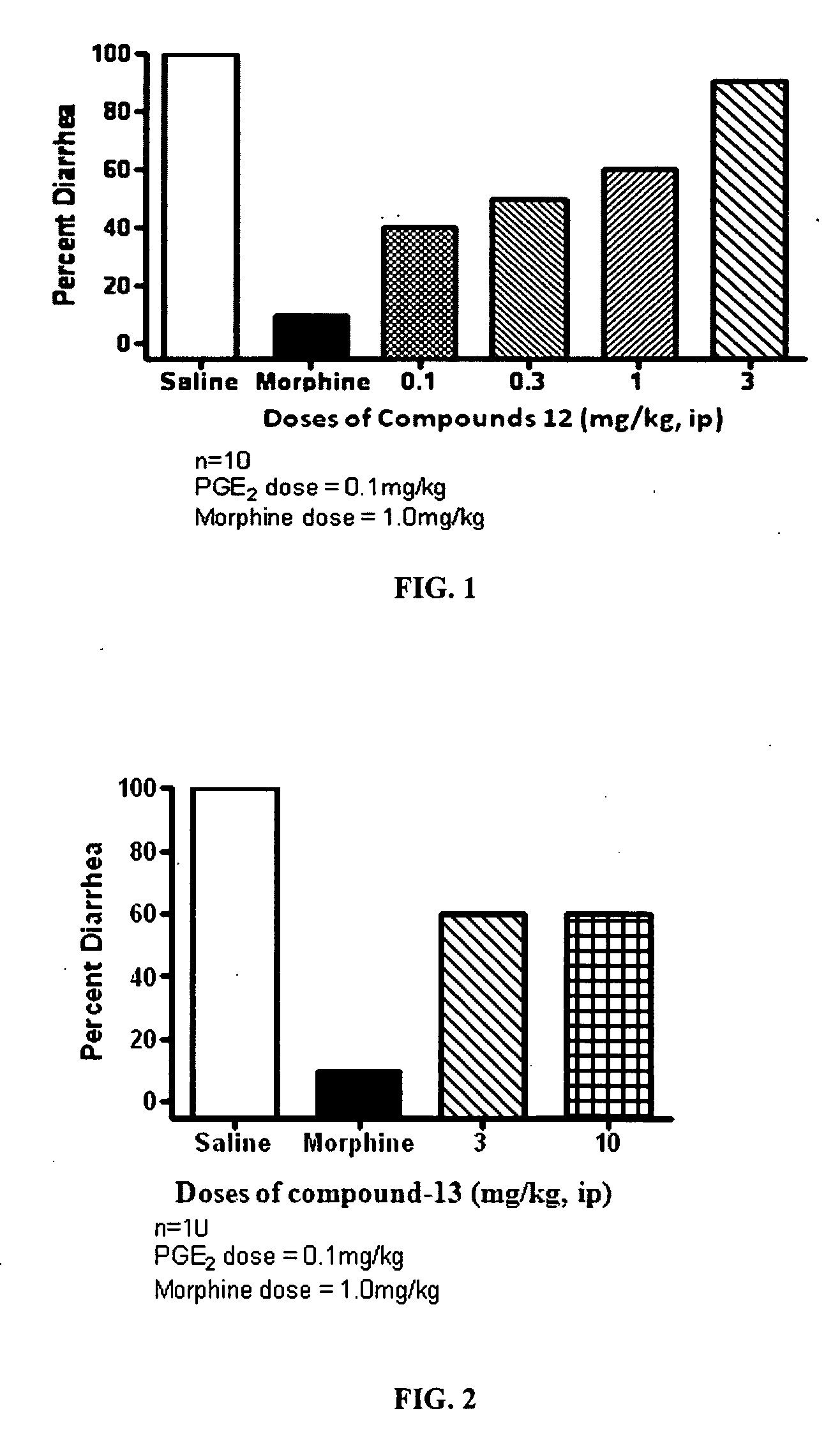
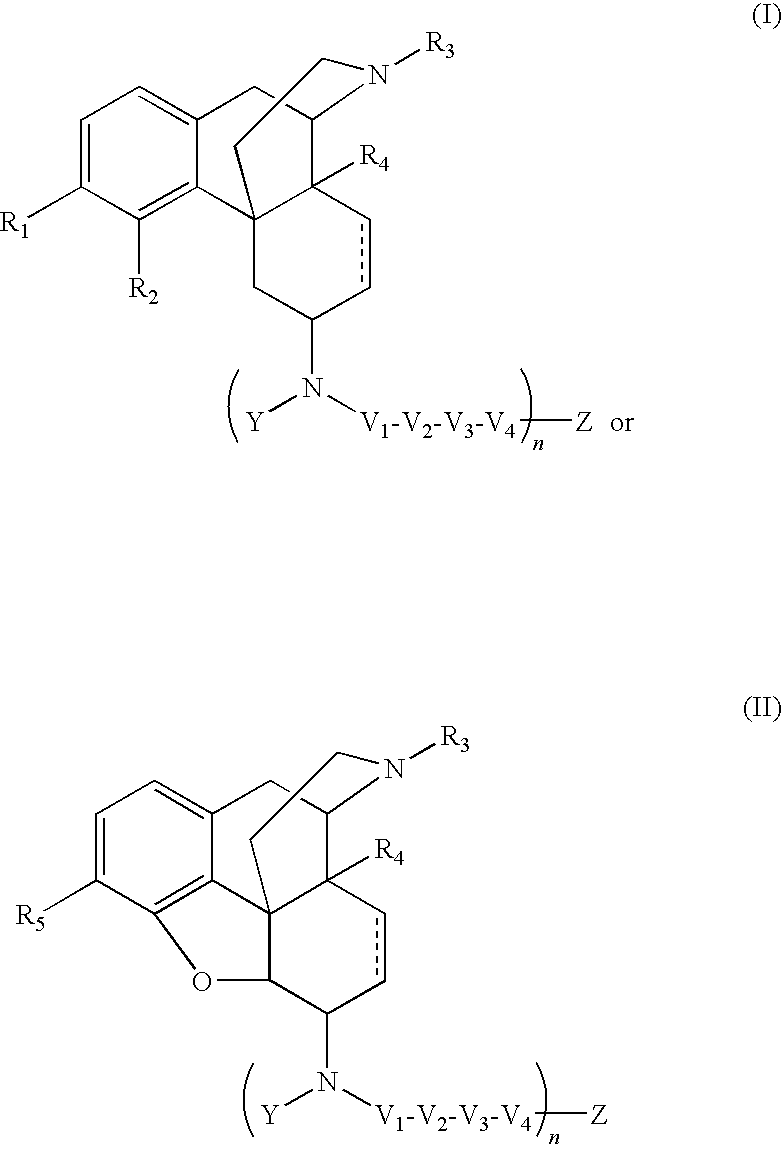
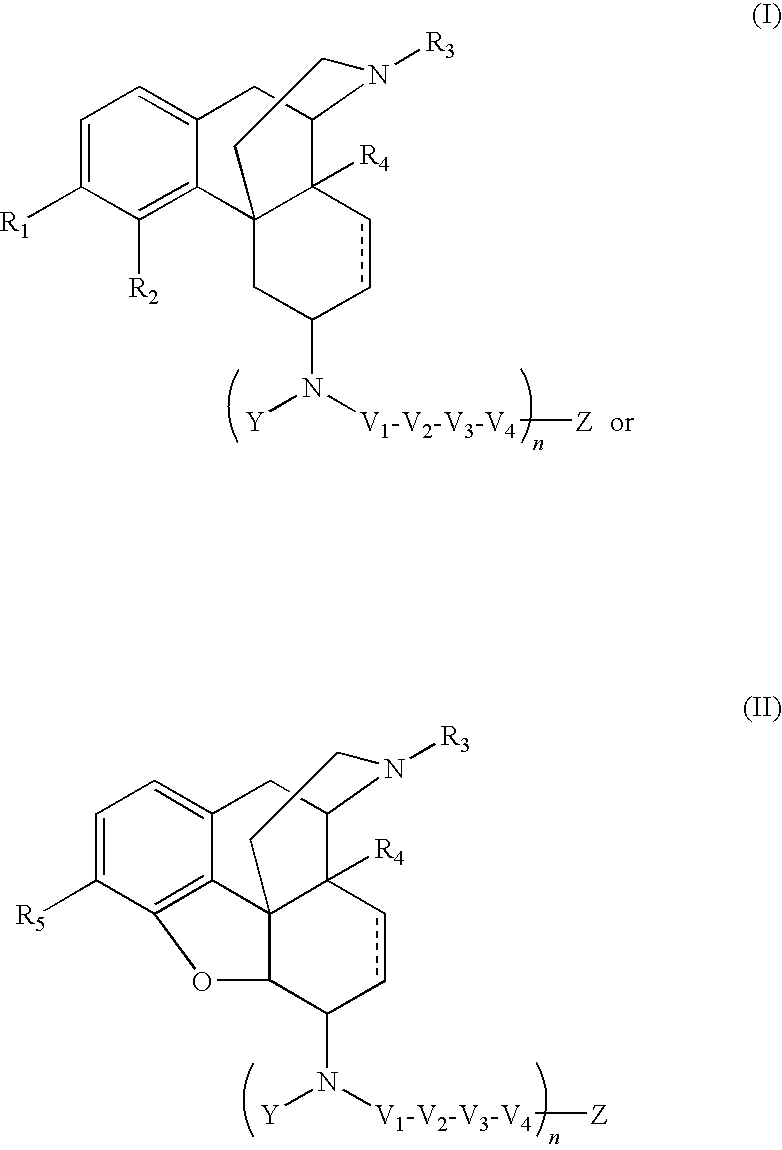
ActiveUS20090209569A1Reducing lipid permeability of drugReduce penetrationAntibacterial agentsBiocideDiseaseInterstitial cystitis
The present invention relates to compounds of Formula I or II, or pharmaceutically acceptable salts, esters, or prodrugs thereof:which relates to morphinan compounds useful as μ, δ, and / or κ receptor opioid compounds and pharmaceuticals containing same that may be useful for mediating analgesia, combating drug addiction, alcohol addiction, drug overdose, mental illness, bladder dysfunctions, neurogenic bladder, interstitial cystitis, urinary incontinence, premature ejaculation, inflammatory pain, peripherally mediated and neuropathic pain, cough, lung edema, diarrhea, cardiac disorders, cardioprotection, depression, and cognitive, respiratory, diarrhea, irritable bowel syndrome and gastro-intestinal disorders, immunomodulation, and anti-tumor agents.
Owner:ALKERMES INC
Novel interstitial therapy for immediate symptom relief and chronic therapy in interstitial cystitis
ActiveUS20050234013A1Useful sizeSmall sizeBiocidePharmaceutical delivery mechanismDiseaseInterstitial cystitis
The present invention relates to a disorder of the lower urinary tract, and in particular, reducing the symptoms (including treatment) of interstitial cystitis in vivo. In a preferred embodiment, the present invention relates to treatment formulations and methods for reducing interstitial cystitis in patients.
Owner:RGT UNIV OF CALIFORNIA
Transluminal drug delivery methods and devices
Transluminal drug delivery method and device embodiments can include a urethral suppository formulated to prevent or treat diseases of the urethra and surrounding organs, such as interstitial cystitis or urethritis, by enhancing the absorption of a therapeutic agent of the suppository into body tissues without adversely affecting the natural defense mechanisms of these tissues. Adverse effects on the glycosaminoglycan (GAG) barrier can be mitigated or eliminated by the presence of a suitable polysaccharide in the suppository.
Owner:KALIUM
Novel antiproliferative factor and methods of use
InactiveUS20050096263A1Improved and facilitatedAssist in resistanceAntipyreticAnalgesicsInterstitial cystitisCompound (substance)
A novel antiproliferative factor comprising a glycopeptide is disclosed. In specific embodiments, the novel antiproliferative factor is associated with the bladder. Compositions, diagnostic kits and reagents, and methods of using the compounds for identifying and / or treating interstitial cystitis and cancer are disclosed.
Owner:HEALTH & HUMAN SERVICES THE GOVERNMENT OF THE US SEC THE DEPT OF +1
Pelvic disorder treatment device
InactiveUS20050049648A1Relieving pelvic painReduce painUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
Owner:MEDTRONIC INC +1
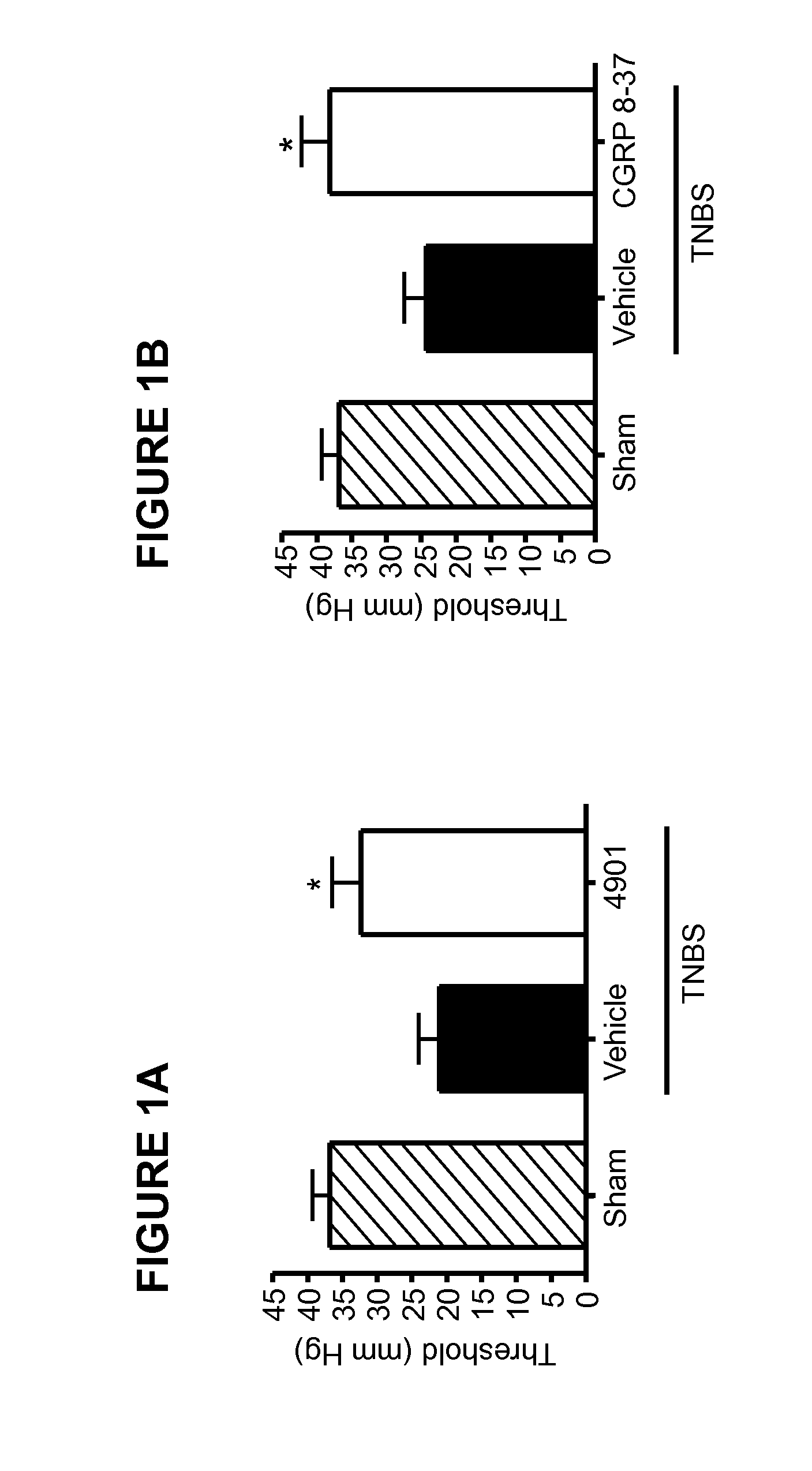
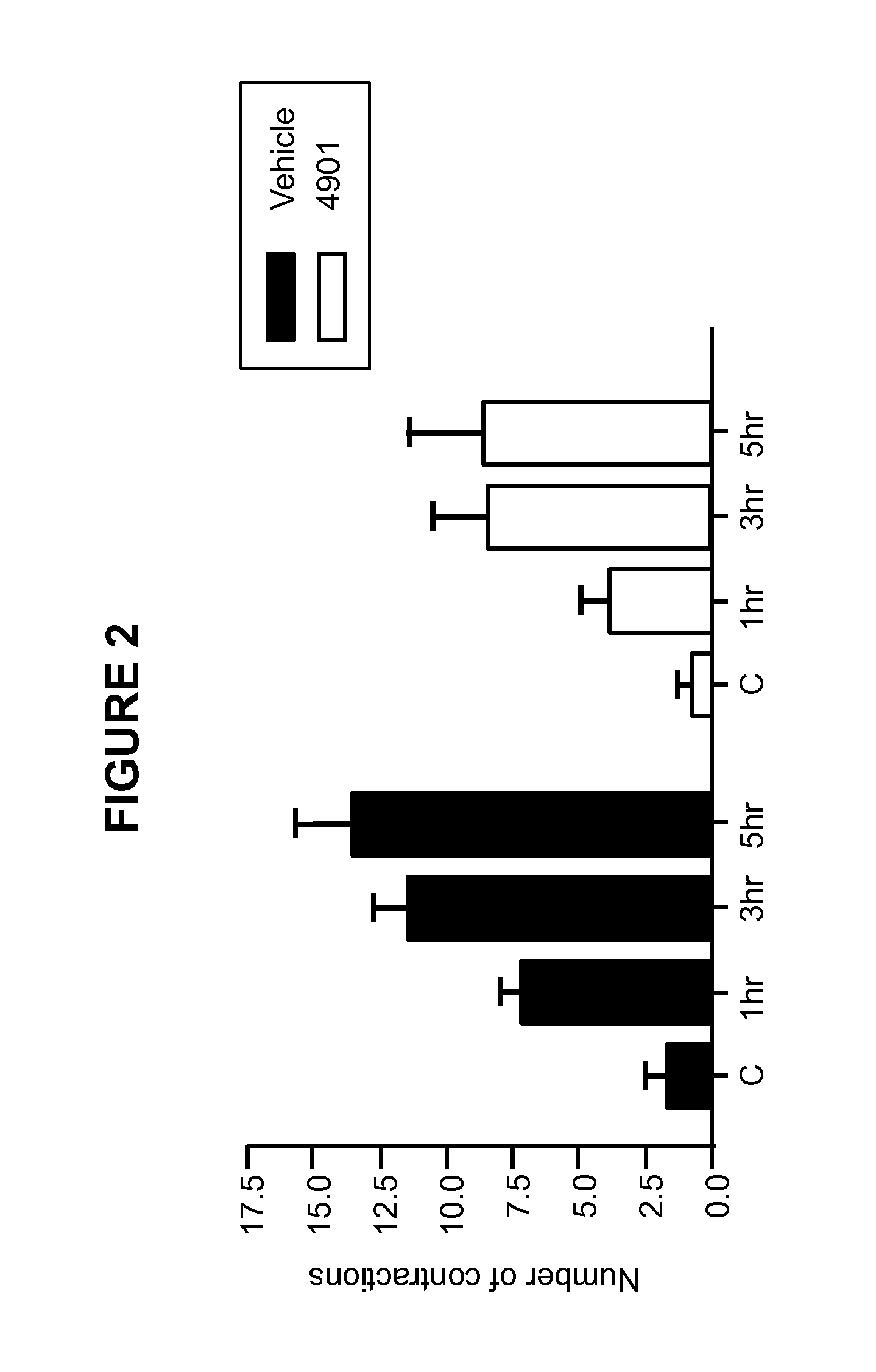
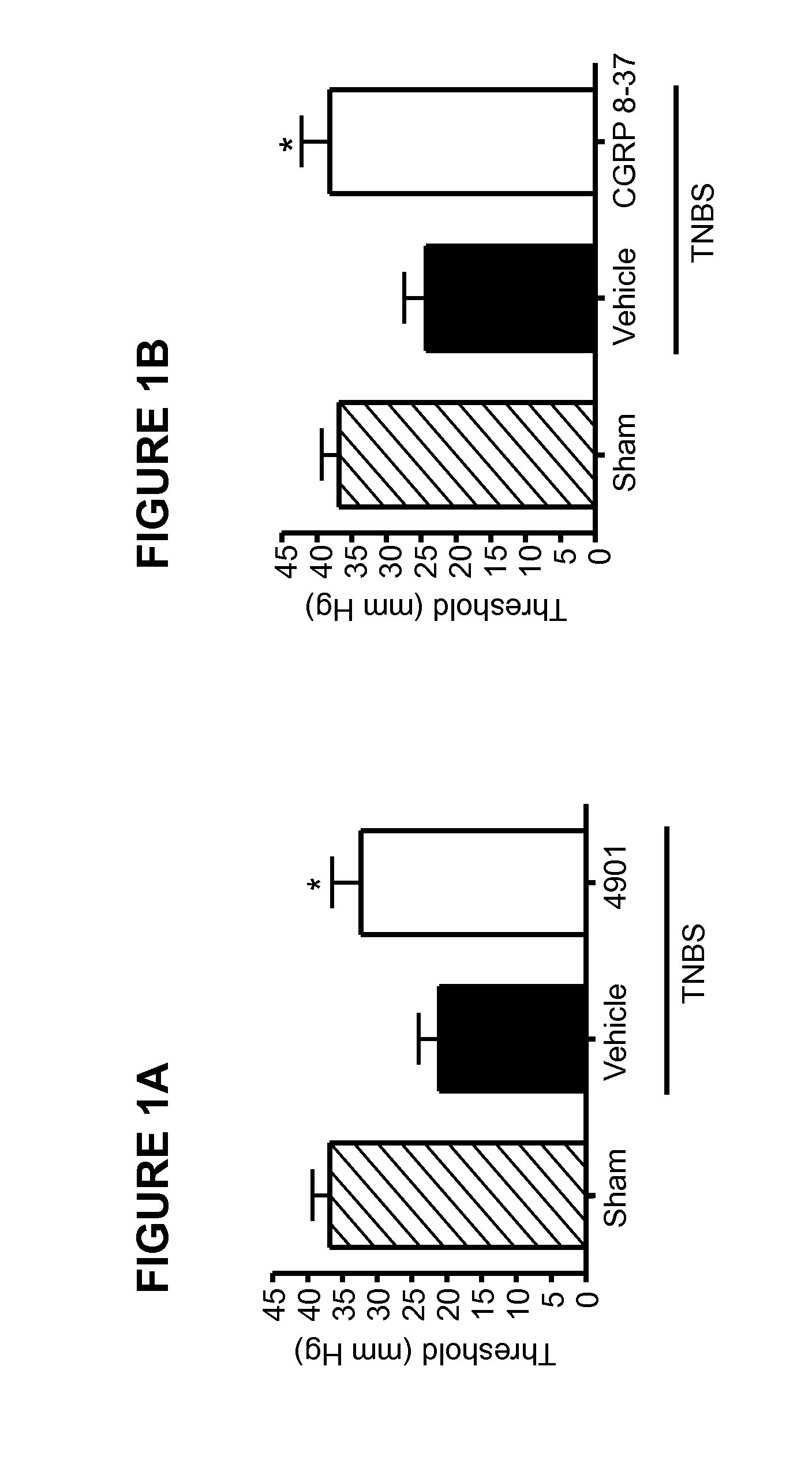
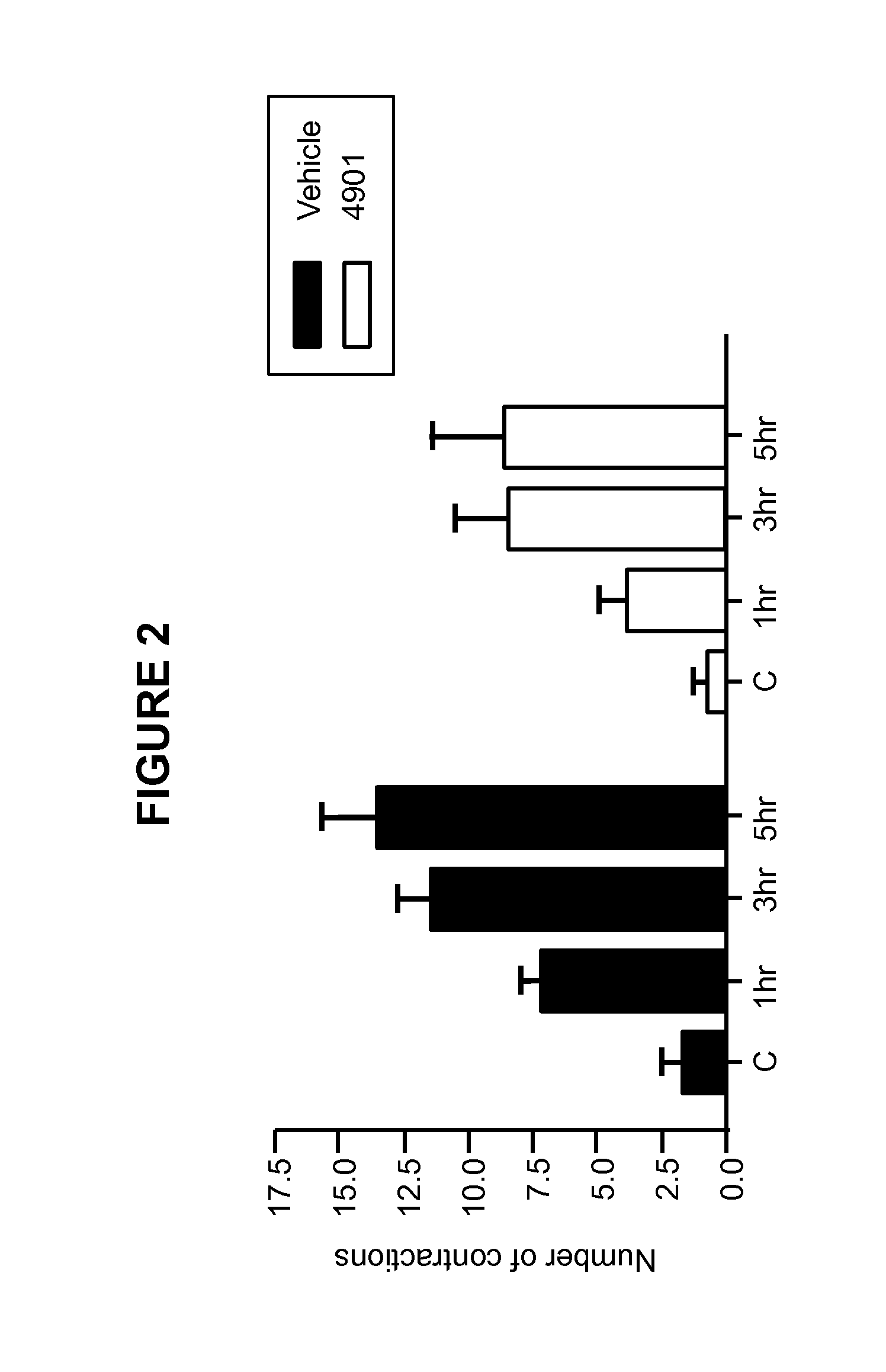
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Antiproliferative factor
InactiveUS20020016443A1Sugar derivativesPeptide/protein ingredientsBladder cancerInterstitial cystitis
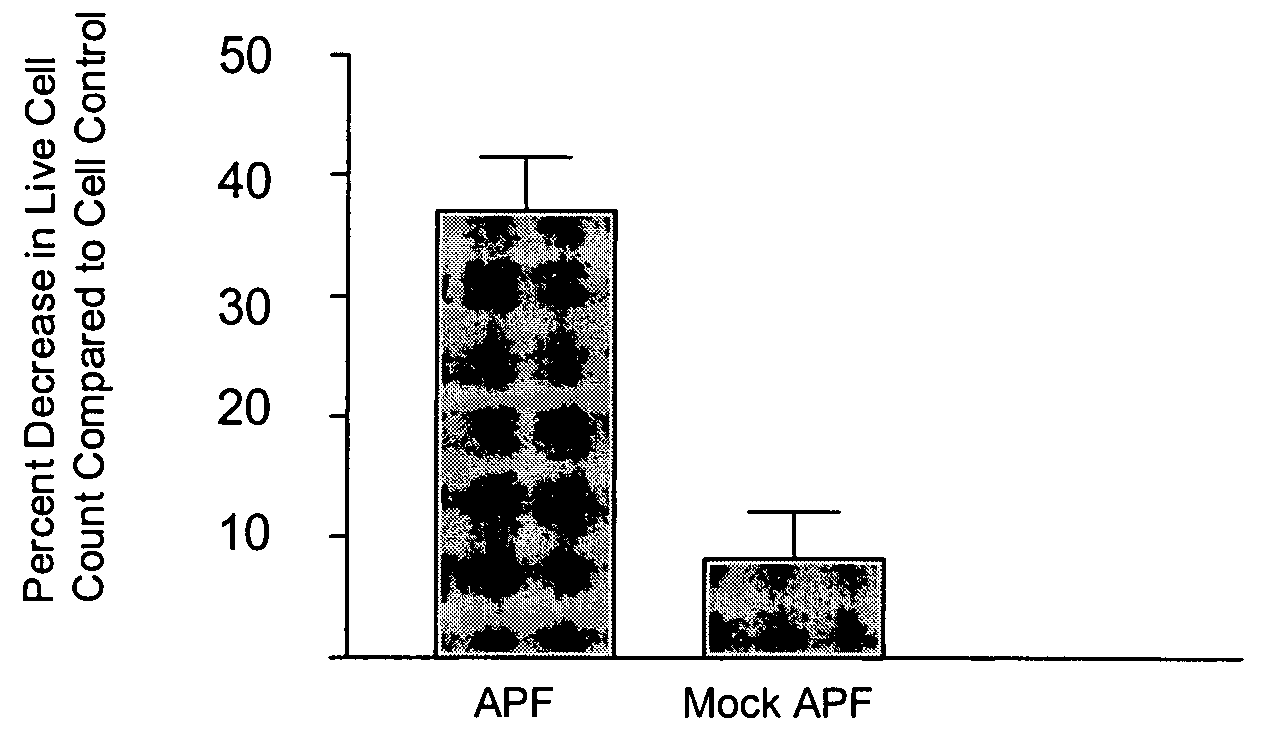
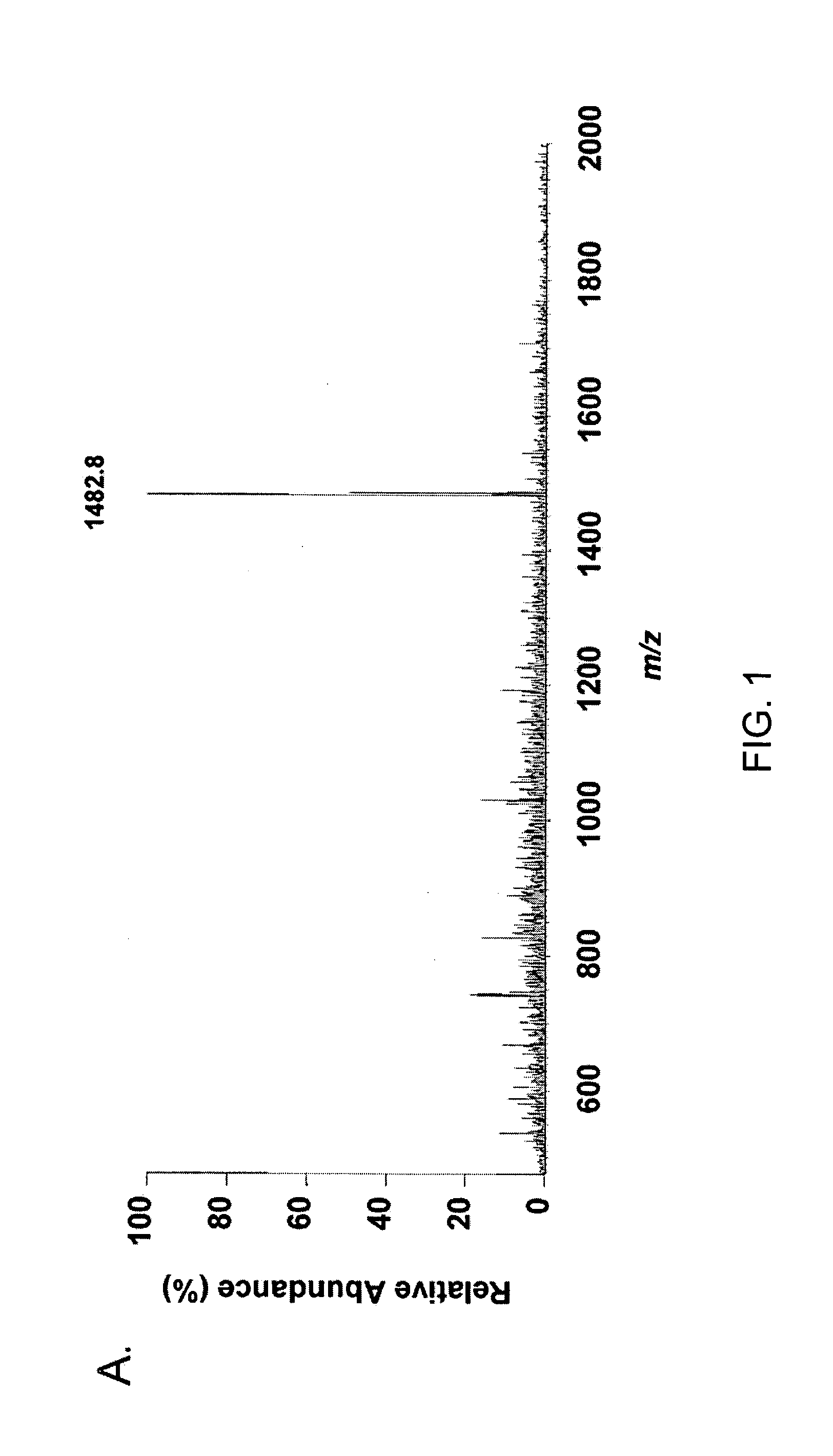
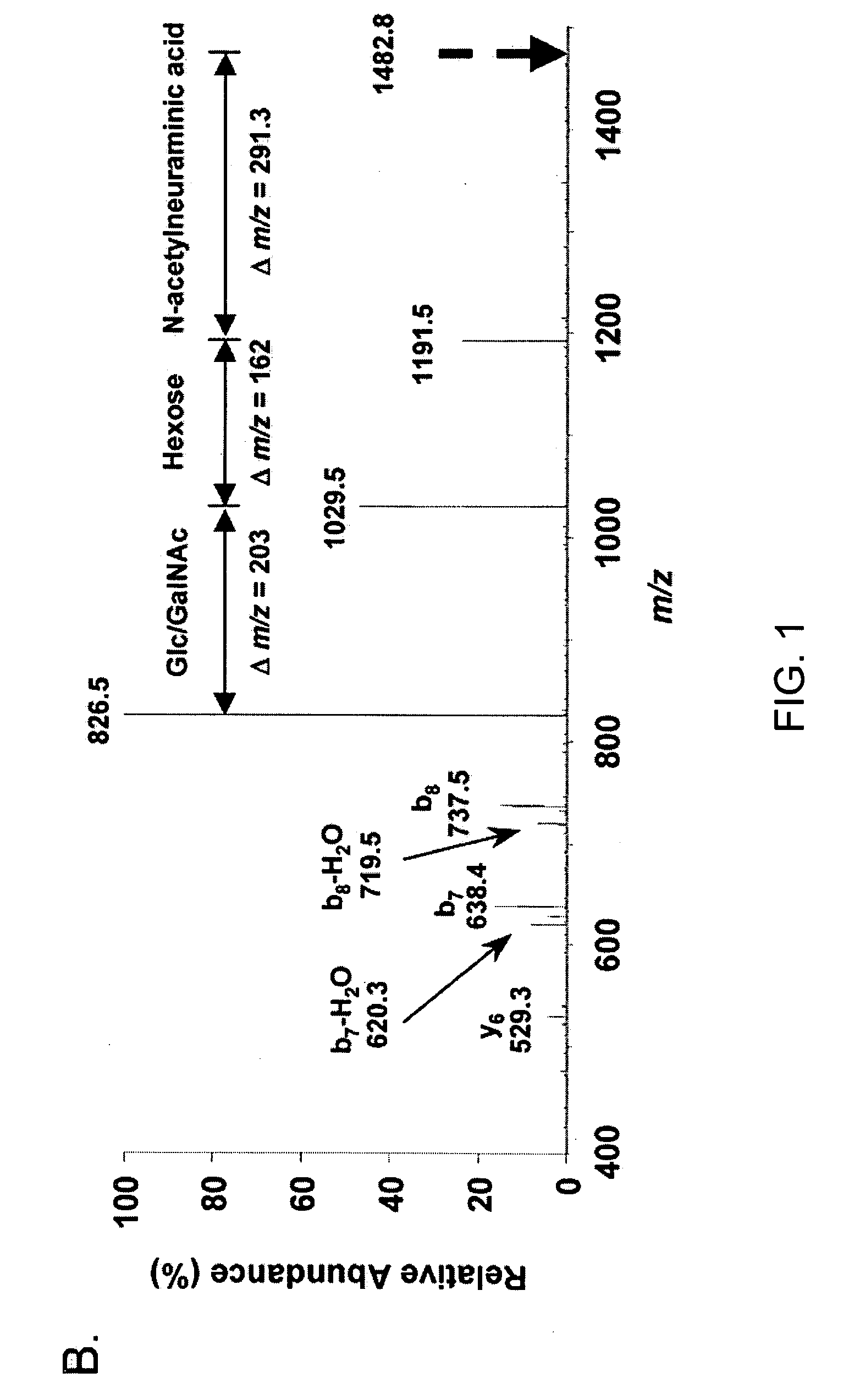
The invention relates to a novel antiproliferative factor (APF) present in urine of patients with interstitial cystitis (IC). APF is useful as a marker for disease activity and its antagonists are useful as therapeutic medicaments for IC and other conditions associated with elevated APF. APF and its agonists are useful in the treatment of diseases associated with cell proliferation, such as bladder cancer.
Owner:KEAY SUSAN K +3
Methods for treating visceral pain by administering antagonist antibodies directed against calcitonin gene-related peptide
The invention features methods for preventing or treating visceral pain, including pain associated with functional bowel disorder, inflammatory bowel disease and interstitial cystitis, by administering an anti-CGRP antagonist antibody.
Owner:TEVA PHARMACEUTICALS INTERNATIONAL GMBH
Glycosaminoglycan composition and method for treatment and prevention of interstitial cystitis
ActiveUS20060234978A1Simple compositionTreatment and/or prevention of interstitial cystitisBiocideCarbohydrate active ingredientsN Acetyl D GlucosamineWhole body
The invention provides compositions and methods useful for the treatment and / or prevention of interstitial cystitis and / or a related urinary tract condition in man or in animals. Specifically, provided are compositions specially formulated for direct instillation into the bladder and / or parenteral use in the treatment and / or prevention of interstitial cystitis. Compositions adapted for direct instillation into the bladder and / or for systemic administration are provided comprised of therapeutic amounts of: chondroitin sulfate in combination with hyaluronan (hyaluronic acid) are provided. Compositions adapted for direct instillation into the bladder and / or for systemic administration are also provided comprised of therapeutic amounts of: chondroitin sulfate, hyaluronan (hyaluronic acid) and N-acetyl D-glucosamine.
Owner:ARTHRODYNAMIC HLDG LLC
Compounds and methods for the treatment of urogenital disorders
The present invention provides methods for treating a variety of urogenital disorders, such as, for example, vaginismus, dyspareunia, vulvodynia (including vulvar vestibulitis), interstitial cystitis, nonspecific urethriris (i.e., nonspecific pain and / or burning of the urinary tract) and sexual dysfunctions, such as, for example, female sexual arousal disorders and female sexual orgasmic disorders, using a variety of compounds, including, but not limited to, NO donors, calcium channel blockers, cholinergic modulators, α-adrenergic receptor antagonists, β-adrenergic receptor agonists, phosphodiesterase inhibitors, cAMP-dependent protein kinase activators (e.g., cAMP mimetics), superoxide scavengers, potassium channel activators, estrogen-like compounds, testosterone-like compounds, benzodiazepines, adrenergic nerve inhibitors, antidiarrheal agents, HMG-CoA reductase inhibitors, smooth muscle relaxants, adenosine receptor modulators, adenylyl cyclase activators, endothelin receptor antagonists, bisphosphonates and cGMP-dependent protein kinase activators (e.g., cGMP mimetics).
Owner:STREHKEHN INT LTD
EphA2, hypoproliferative cell disorders and epithelial and endothelial reconstitution
InactiveUS20050049176A1Convenient treatmentReduces and avoids unwanted effectPeptide/protein ingredientsAntipyreticCell layerInflammatory Bowel Diseases
The present invention relates to methods and compositions designed for the treatment, management, or prevention of a hypoproliferative cell disorder, especially those disorders relating to the destruction, shedding, or inadequate proliferation of epithelial and / or endothelial cells, particularly interstitial cystitis (IC) and lesions associated with inflammatory bowel disease (IBD). The methods of the invention comprise the administration of an effective amount of one or more agents that are antagonists of EphA2. In certain embodiments, the EphA2 antagonistic agent of the invention decreases EphA2-endogenous ligand binding, upregulates EphA2 gene expression and / or translation, increases EphA2 protein stability or protein accumulation, decreases EphA2 cytoplasmic tail phosphorylation, promotes EphA2 kinase activity (other than autophosphorylation or ligand-mediated EphA2 signaling), increases proliferation of EphA2 expressing cells, increases survival of EphA2 expressing cells, and / or maintains / reconstitutes epithelial and / or endothelial cell layer integrity. The invention also provides pharmaceutical compositions comprising one or more EphA2 antagonistic agents of the invention either alone or in combination with one or more other agents useful for therapy for a hypoproliferative cell disorder. Diagnostic methods and methods for screening for therapeutically useful agents are also provided.
Owner:MEDIMMUNE LLC
Methods and compositions for the treatment of urinary incontinence
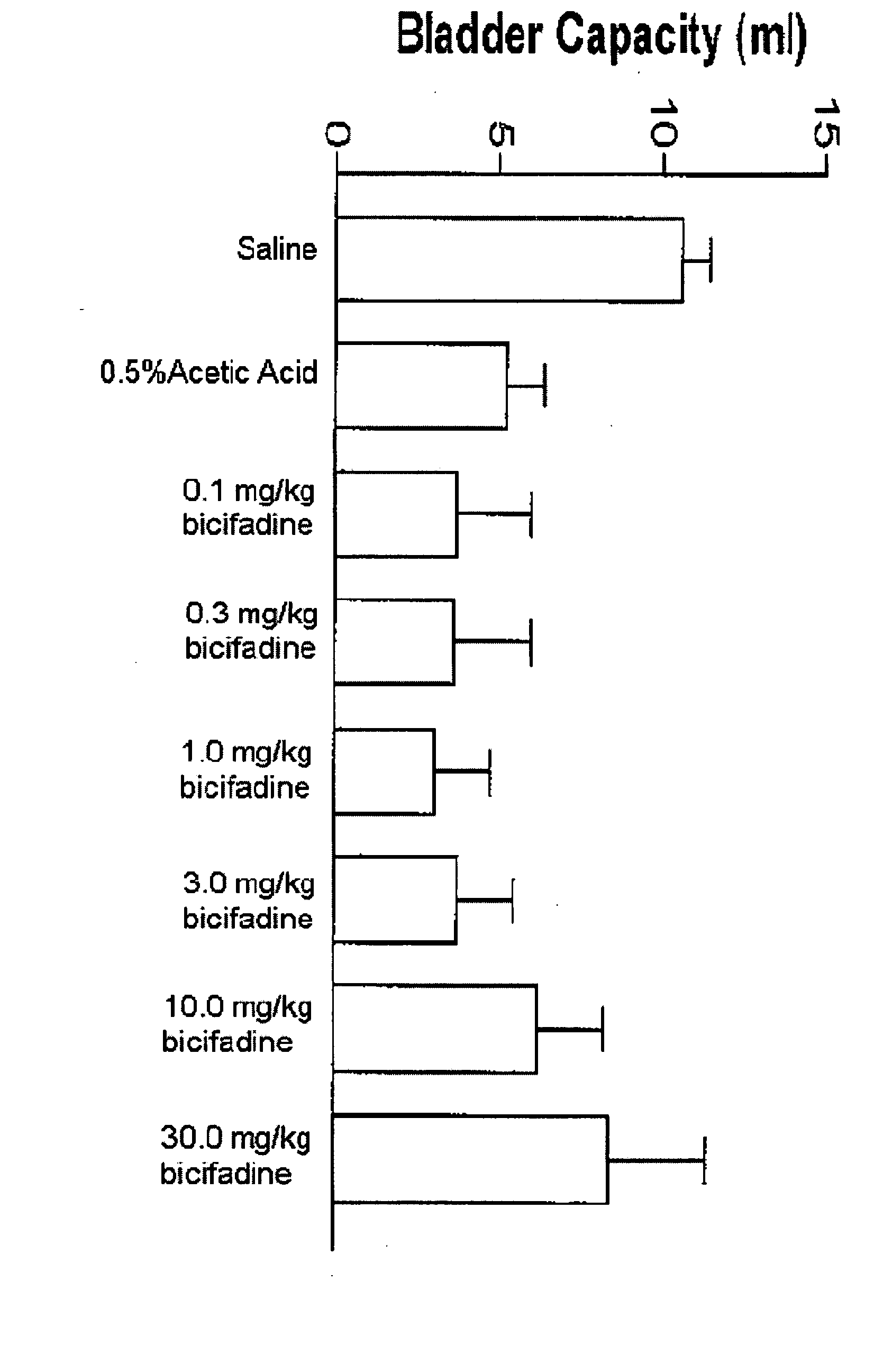
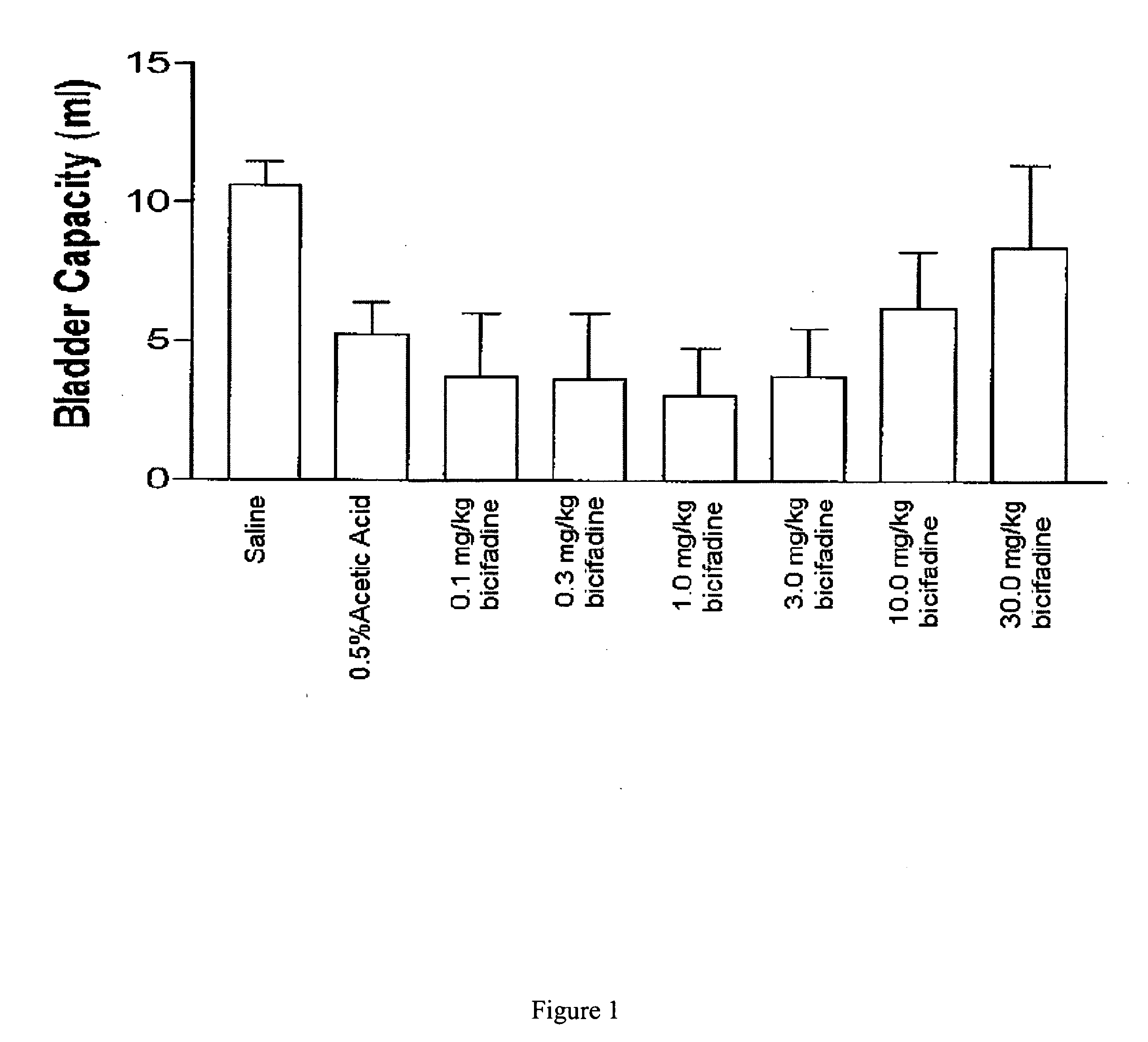
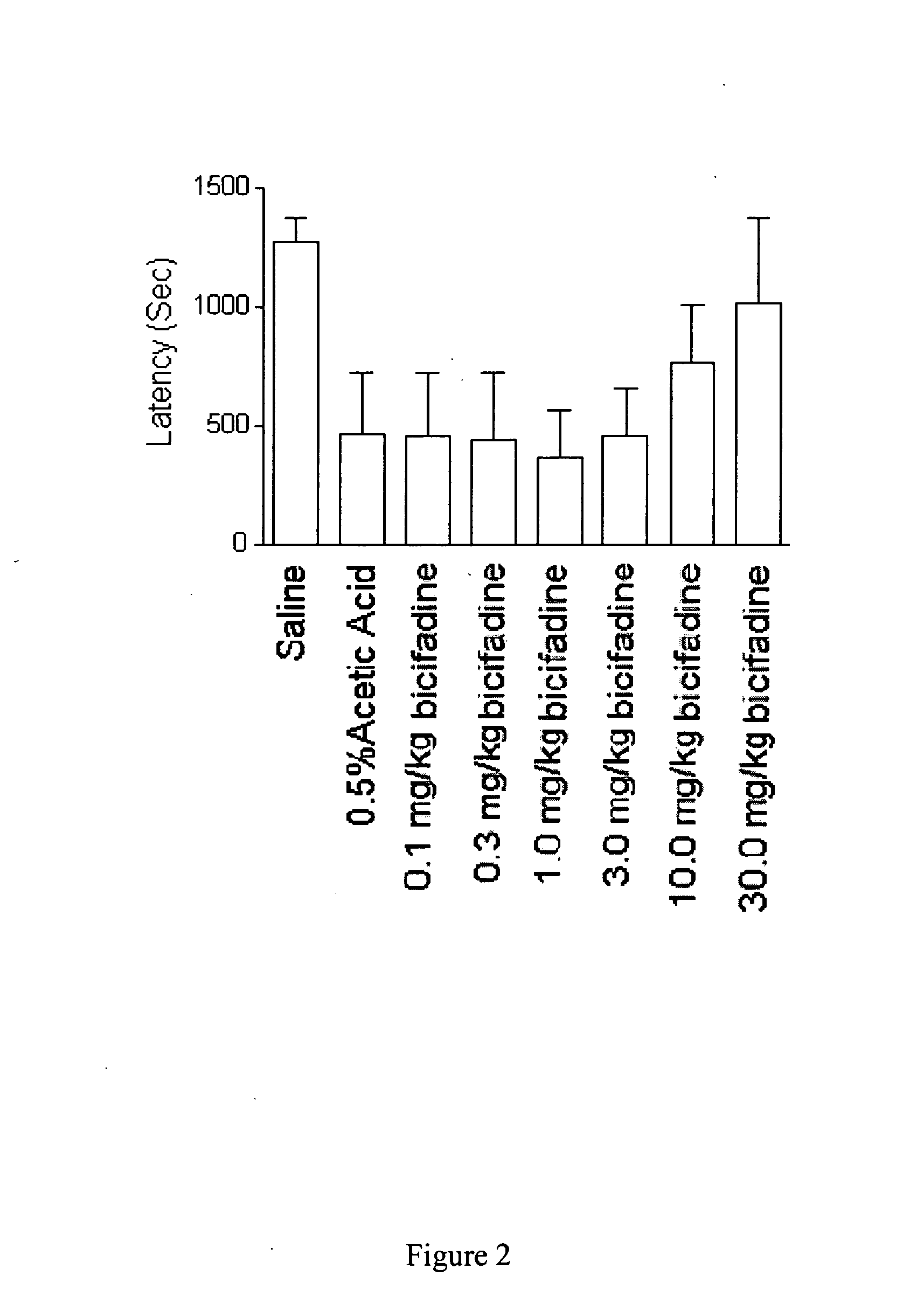
InactiveUS20080009538A1Lower urinary tract disordersBiocideUrinary disorderBicifadineNeuropathic bladder
Methods and compositions containing bicifadine are provided for the prevention and treatment of lower urinary tract disorders in mammalian subjects. The methods and compositions may be used to prevent or treat urinary incontinence, urinary urgency, nocturia, and enuresis associated with neurogenic and non-neurogenic overactive bladder, interstitial cystitis, prostatitis, prostadynia, and benign prostatic hyperplasia, among other conditions. Additional compositions and methods are provided which employ bicifadine in combination with a second anti-incontinence agent, or a different therapeutic agent to yield more effective anti-incontinence treatment tools, and / or dual activity therapeutic methods and formulations useful to prevent or reduce urinary incontinence and one or more additional symptoms such as urinary urgency, overflow, frequency, or pain in mammalian subjects.
Owner:DOV PHARMA
Interstitial therapy for immediate symptom relief and chronic therapy in interstitial cystitis
Owner:RGT UNIV OF CALIFORNIA
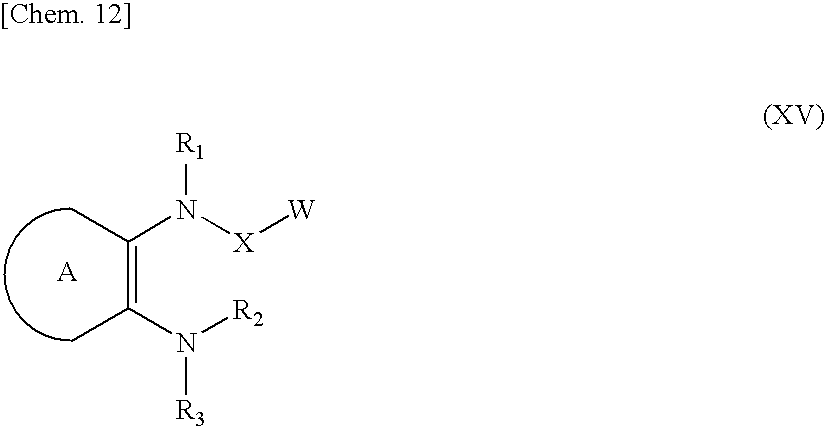
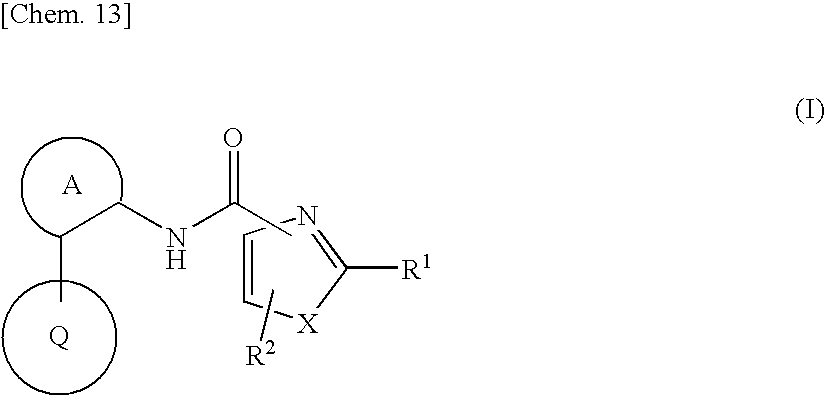
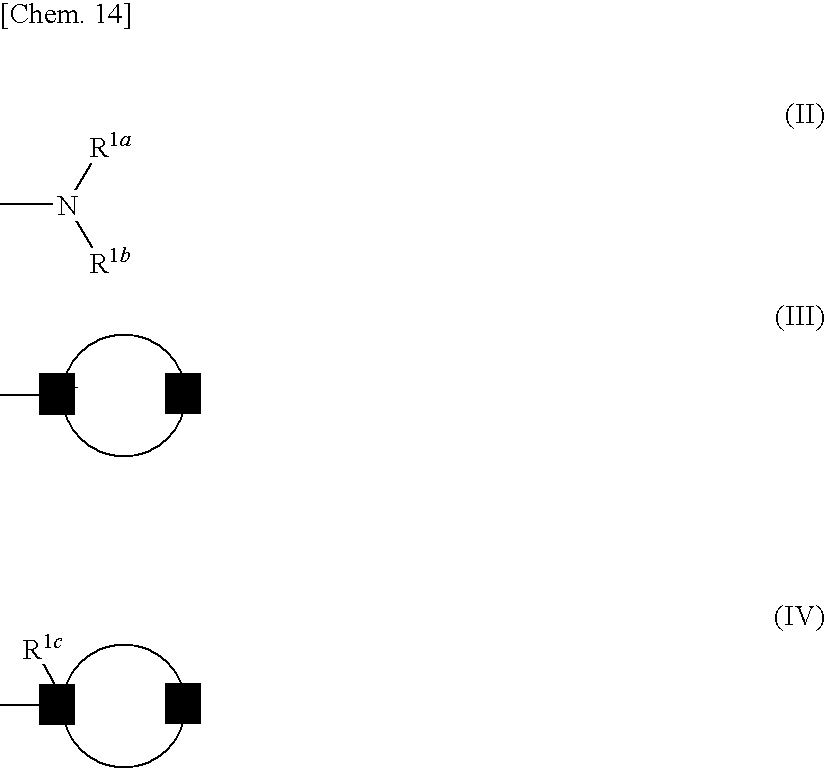
Azolecarboxamide derivative
InactiveUS20090286766A1Potent activityImprove actionBiocideNervous disorderDiseaseInterstitial cystitis
Provided is an agent for treating or preventing urinary frequency, urinary urgency and urinary, incontinence which are associated with overactive bladder, a lower urinary tract disease such as interstitial cystitis and chronic prostatitis accompanied by lower urinary tract pain, and various diseases accompanied by pain. A novel azolecarboxamide derivative in which an azole ring such as thioazole or oxazole is bonded to a benzene ring, pyridine ring or pyrimidine ring through carboxamide was confirmed to have a potent trkA receptor-inhibitory activity and found to be an agent for treating or preventing lower urinary tract disease and various diseases accompanied by pain, which is excellent in efficacy and safety, and thus the present invention was accomplished.
Owner:ASTELLAS PHARMA INC
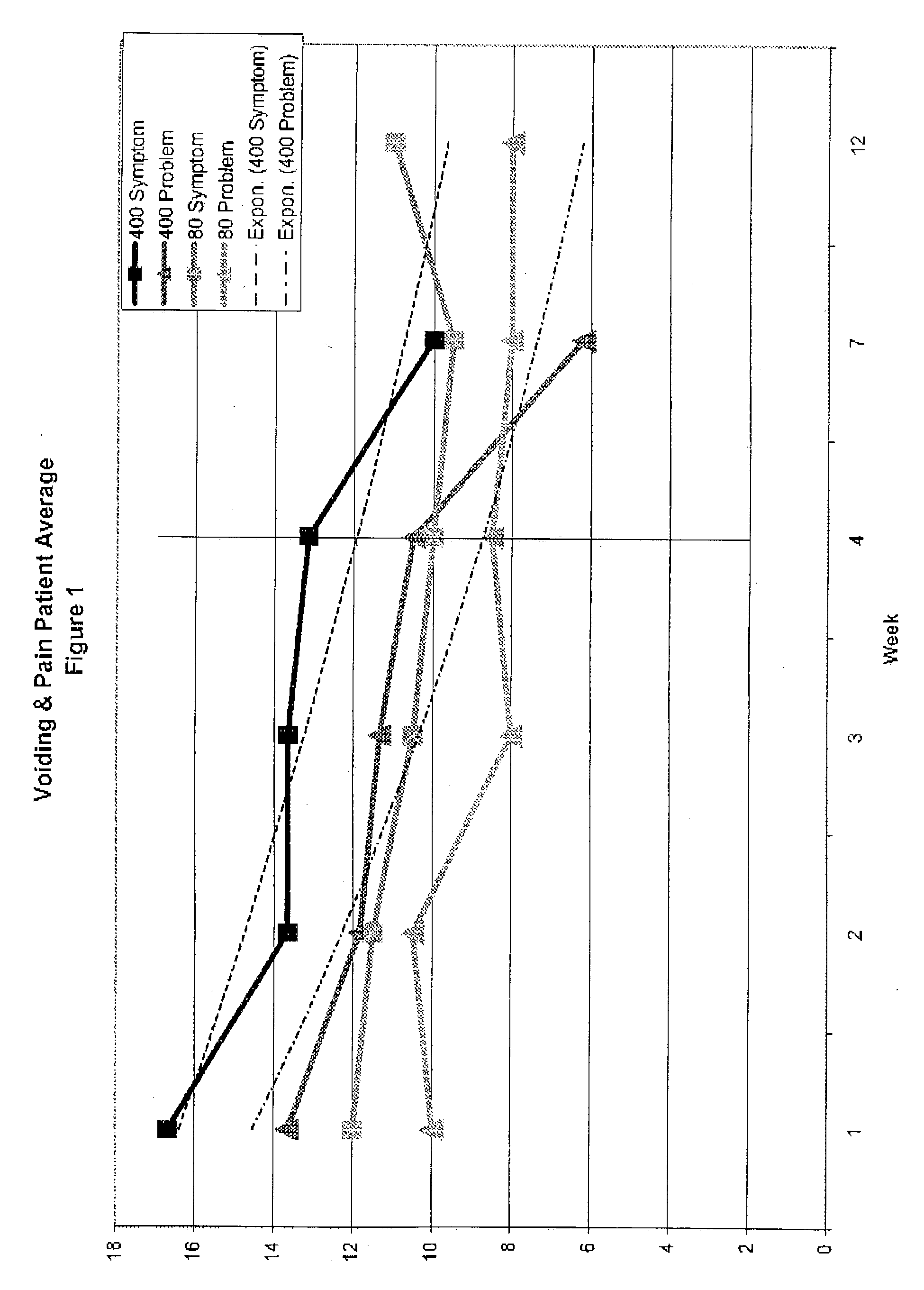
Cystitis treatment with high dose chondroitin sulfate
Interstitial cystitis and related GAG-deficient conditions of the bladder and urinary tract are treated by instillation of high dose chondroitin sulfate, such as 400 mg / 20 mL. The higher dose of chondroitin is effective for the rapid reduction of symptoms, particularly in patients with severe and otherwise recalcitrant cystitis.
Owner:STELLAR INT
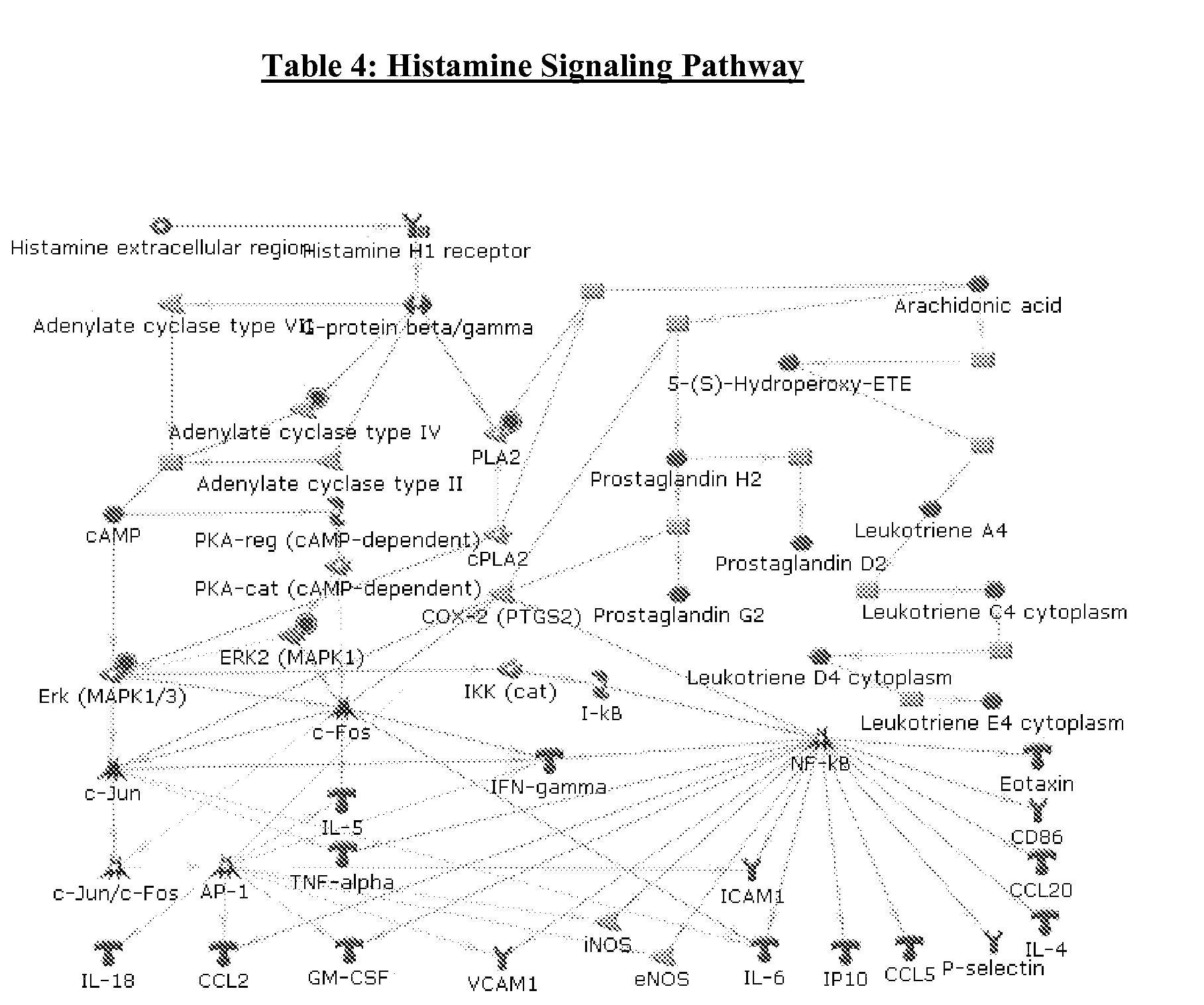
Antioxidant-Containing Food Composition For Use In Inhibiting Histamine Pathways In Companion Animals
InactiveUS20090156658A1Inhibition capacityInhibiting the deterioration of the mental capacity of an agedBiocideOrganic active ingredientsInterstitial cystitisHistamine Releases
The invention encompasses compositions for inhibiting histamine release pathways in a companion animal, for example, felines and in treating or preventing idiopathic cystitis or interstitial cystitis. The compositions of the invention include an amount of lipoic acid that is effective in inhibiting histamine release pathways in a companion animal, for example, felines and in treating or preventing idiopathic cystitis or interstitial cystitis.
Owner:HILLS PET NUTRITION INC
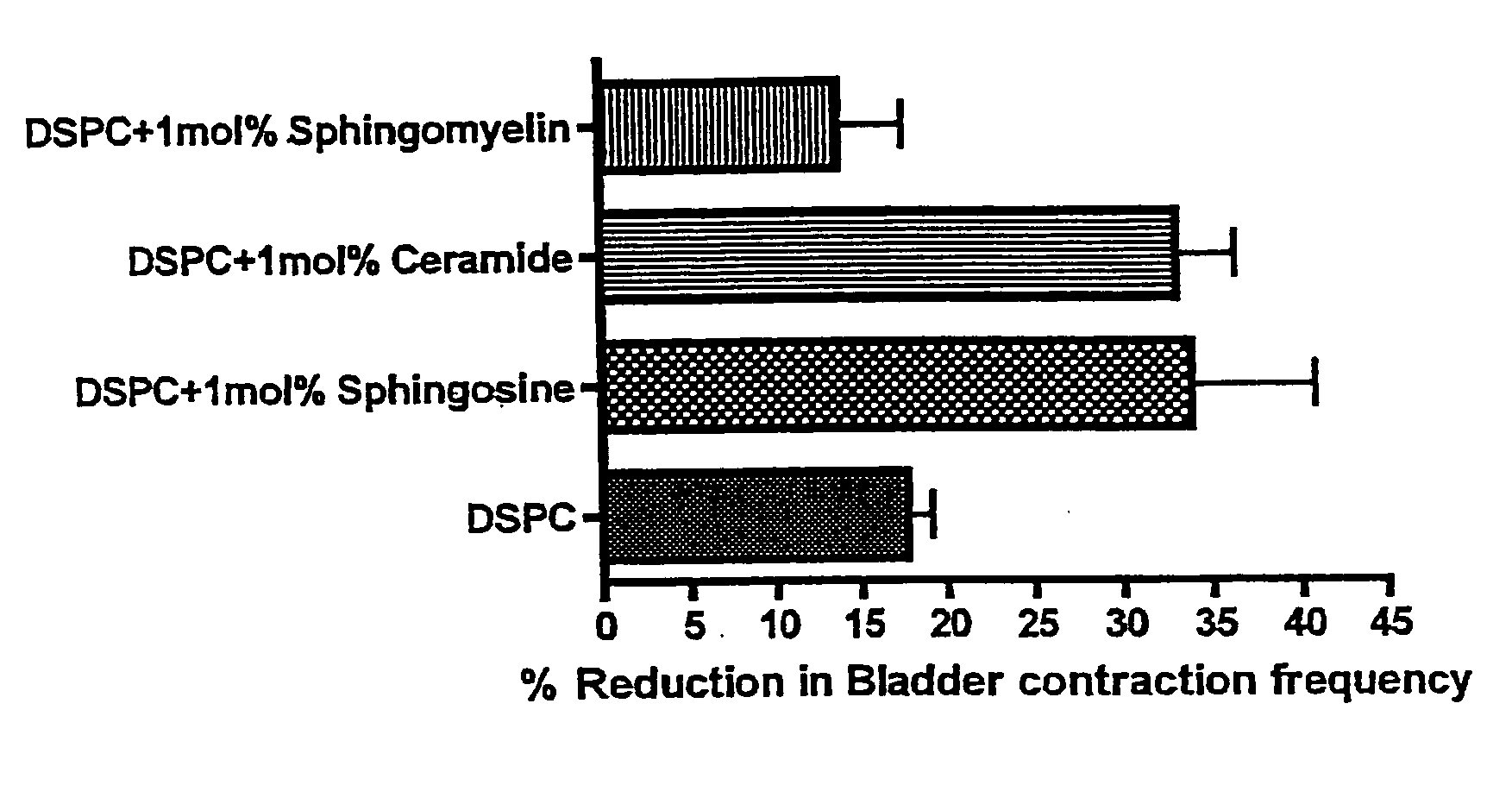
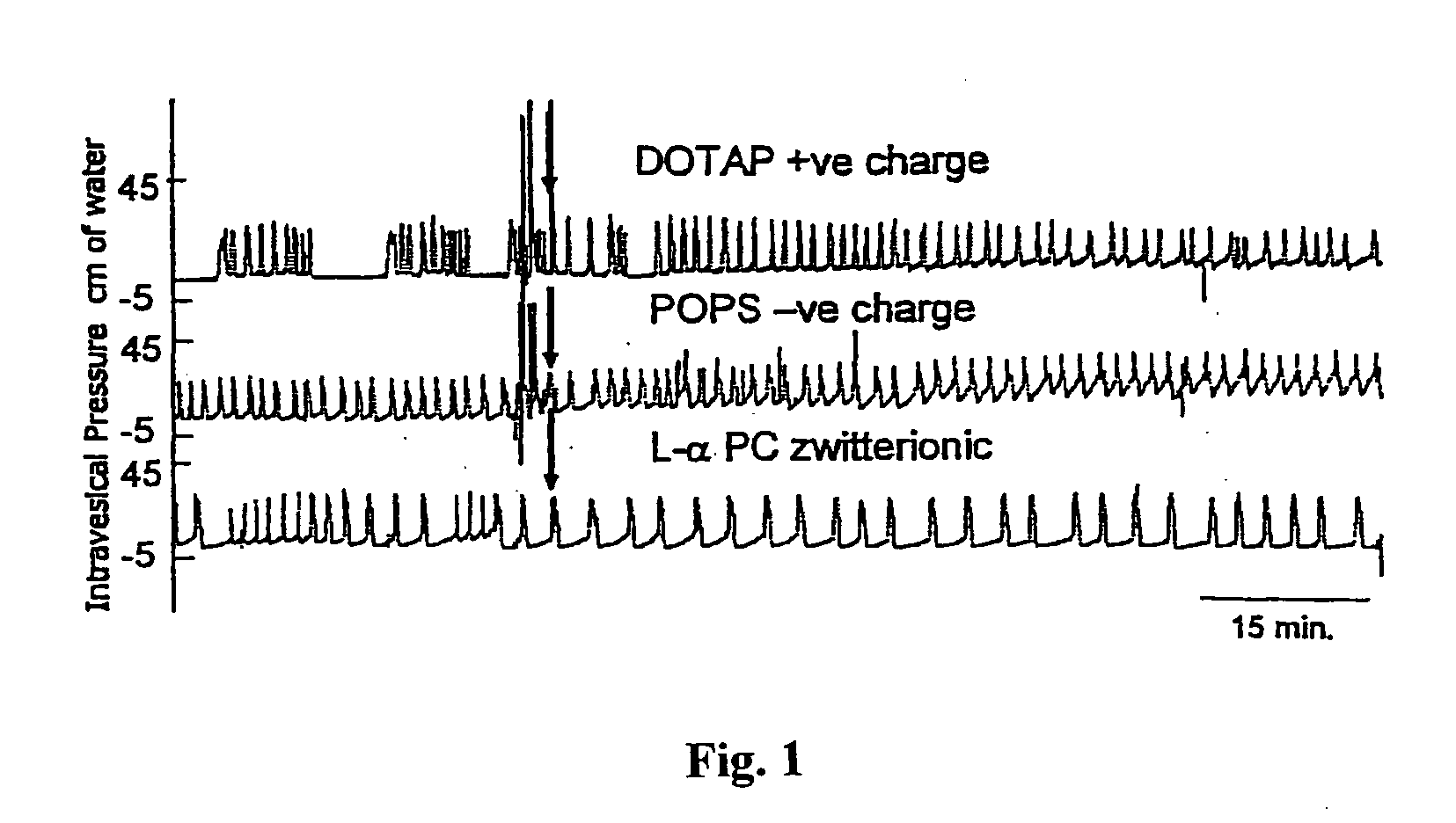
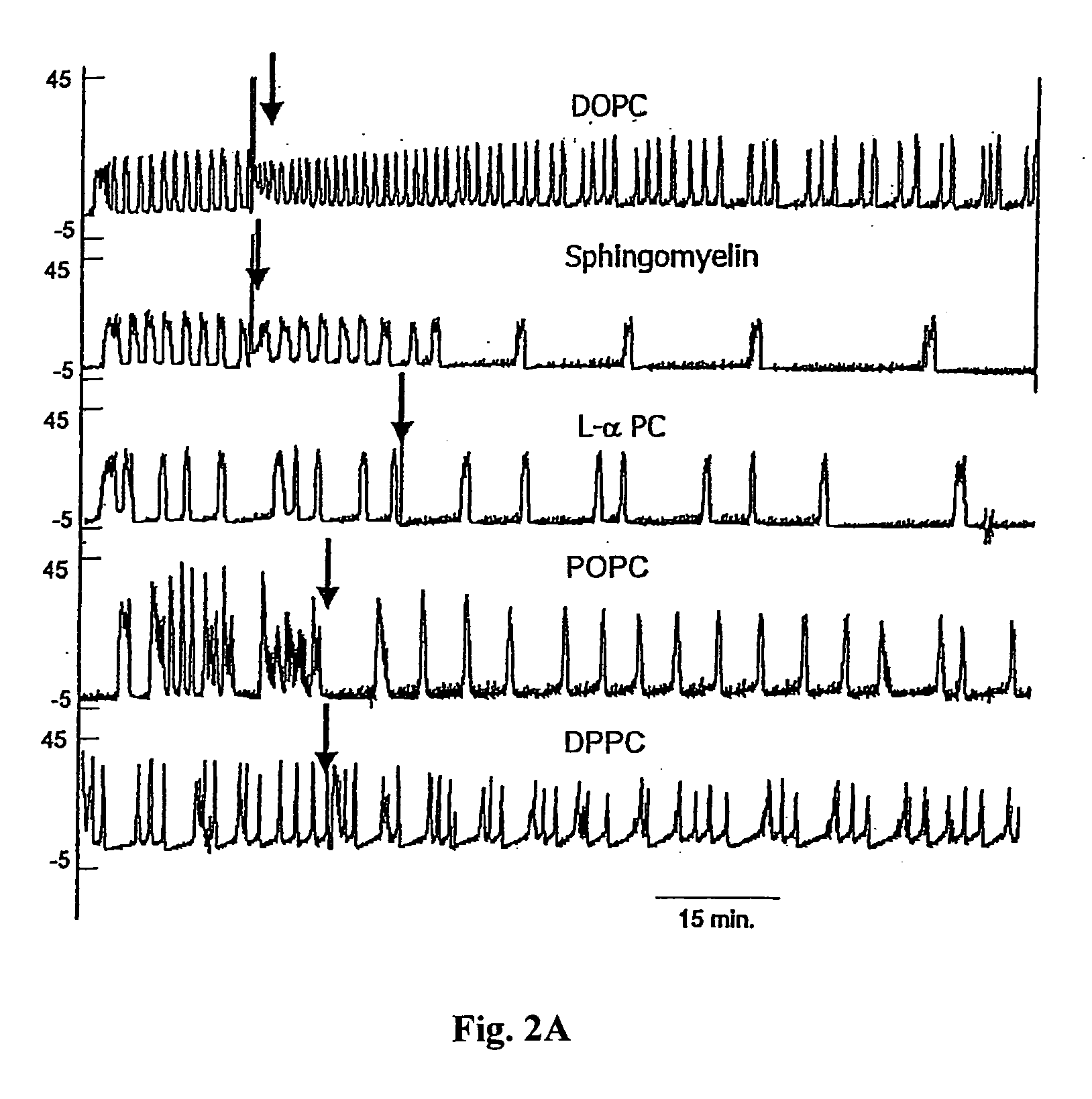
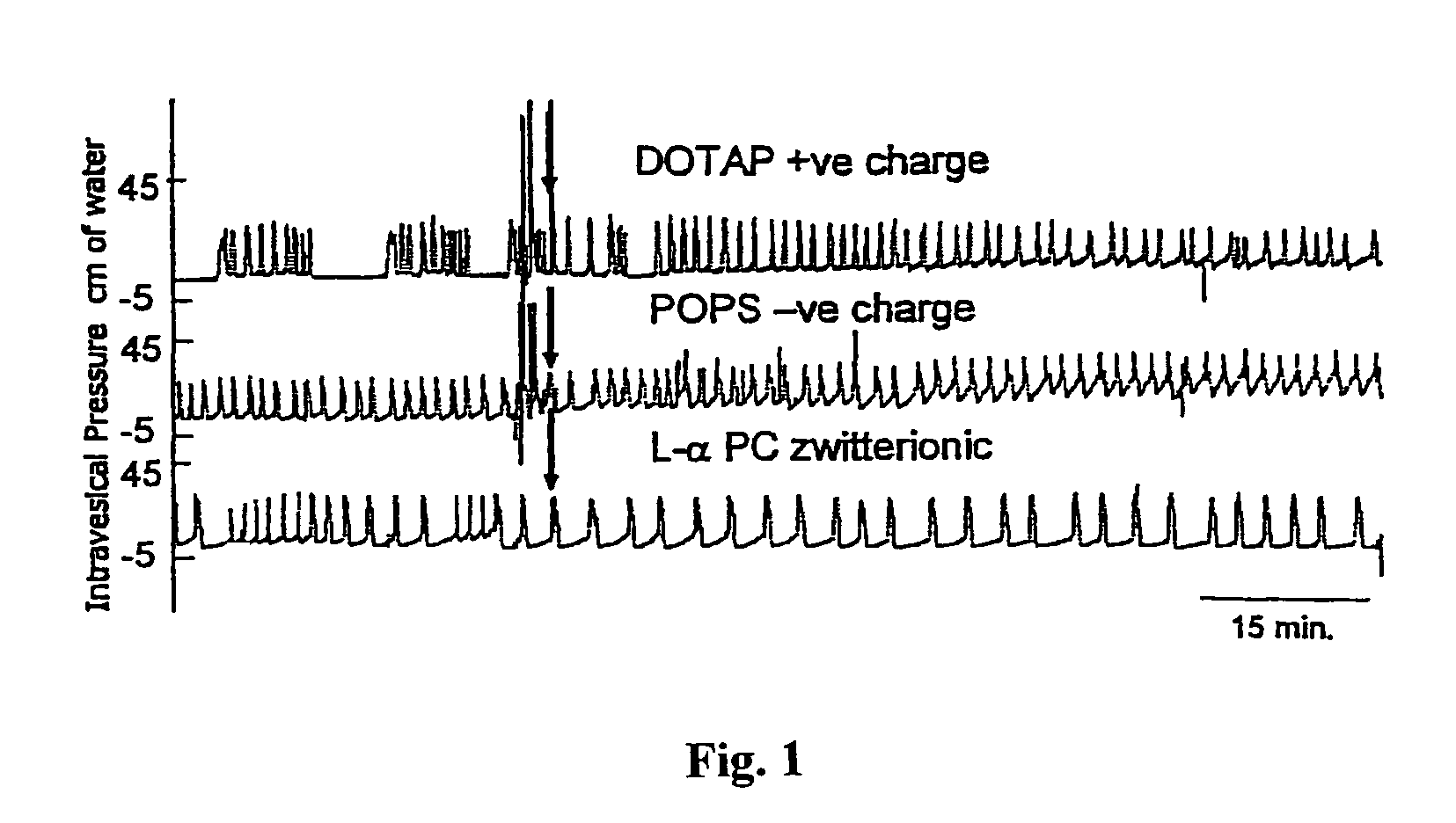
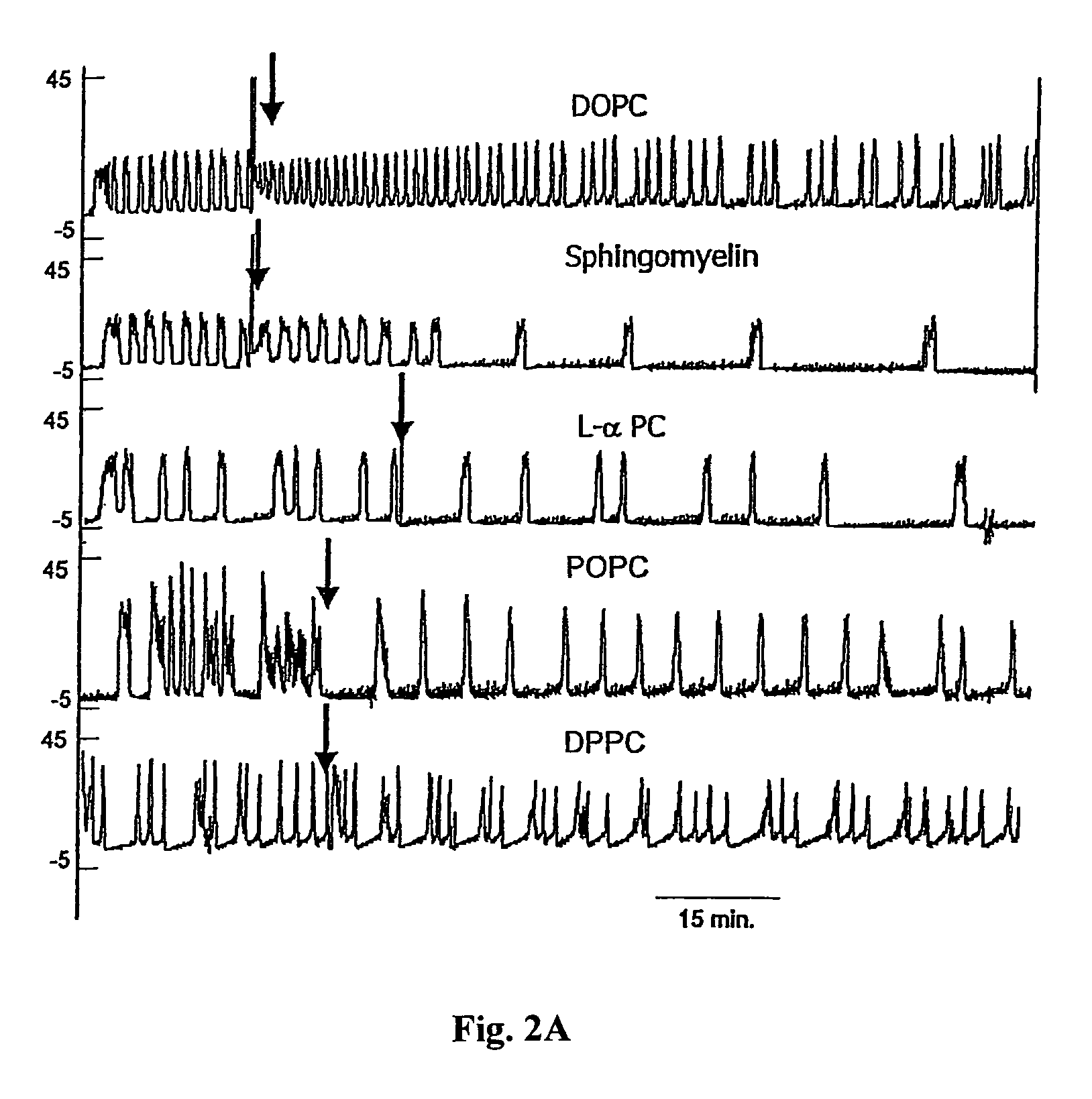
Sphingomyelin liposomes for the treatment of hyperactive bladder disorders
InactiveUS20070122466A1Reduce and prevent antibody-mediated resistanceIncrease stimulationLiposomal deliveryAgainst vector-borne diseasesDiseaseLipid formation
The present invention provides pharmaceutical compositions and methods for the instillation of lipid vehicles comprised of liposomes containing sphingomyelin or sphingomyelin metabolites to prevent, manage, ameliorate and / or treat disorders involving neuropathic pain and aberrant muscle contractions, such as what occurs in bladder hyperactivity disorders such as interstitial cystitis (IC) in animals or humans in need thereof. Also provided is a liposome-based delivery of drugs, e.g., antibiotics, pain treatments and anticancer agents, to the bladder, genitourinary tract, gastrointestinal system, pulmonary system and other organs or body systems. In particular, liposome-based delivery of vanilloid compounds, such as resiniferatoxin, capsaicin, or tinyatoxin and toxins, such as botulinum toxin is provided for the treatment of bladder conditions, including pain, inflammation, incontinence and voiding dysftunction.
Owner:UNIVERSITY OF PITTSBURGH
Phenoxyalkycarboxylic acid derivatives in the treatment of inflammatory diseases
A method of treating interstitial cystitis, irritable bowel syndrome, ulcerative colitis, and other inflammatory conditions, comprising: administering to a patient in need thereof an effective amount of a compound selected from compound 1, its metabolite 2 and pharmaceutically acceptable salts or prodrugs thereof:
Owner:MEDICINOVA INC
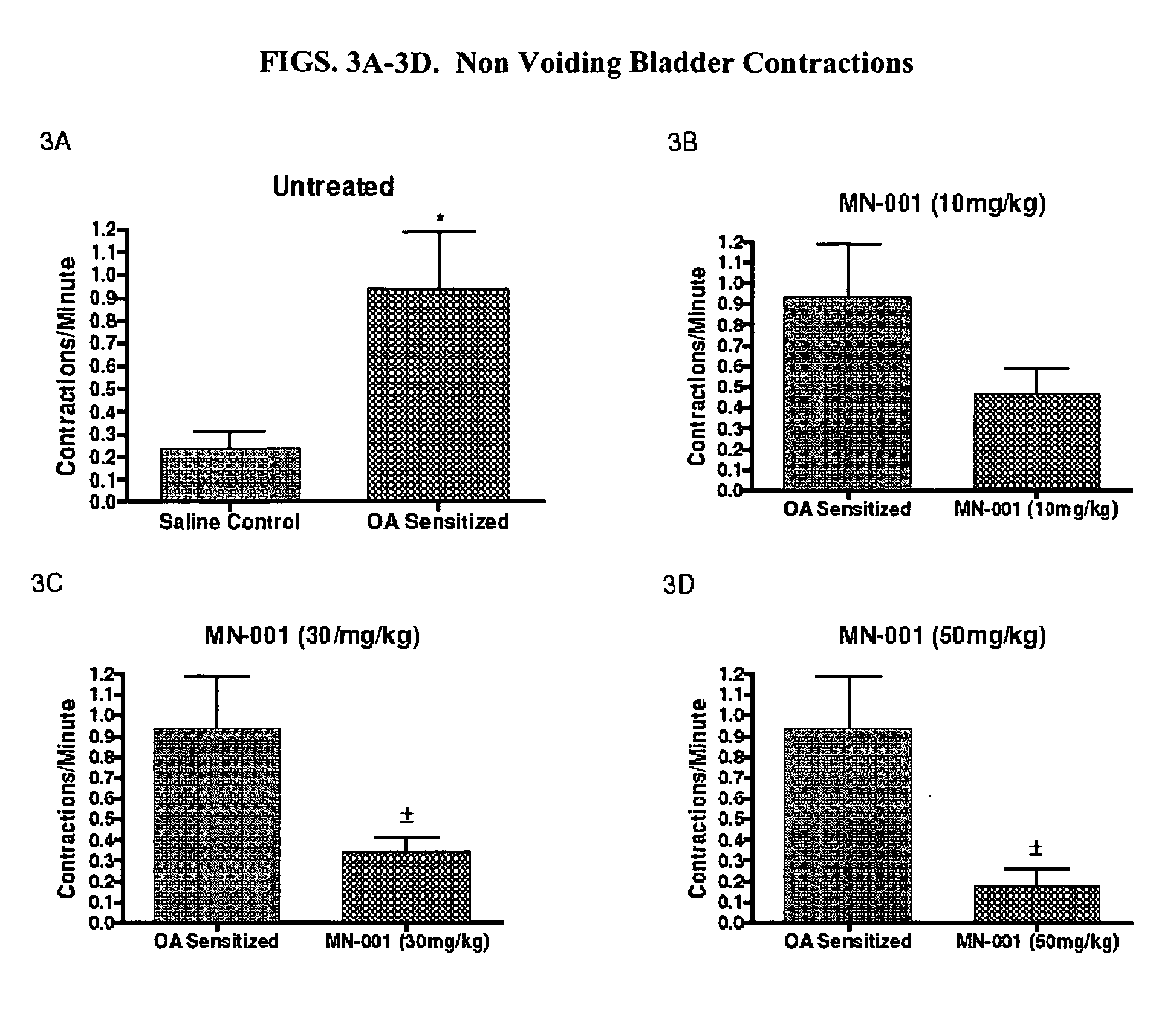
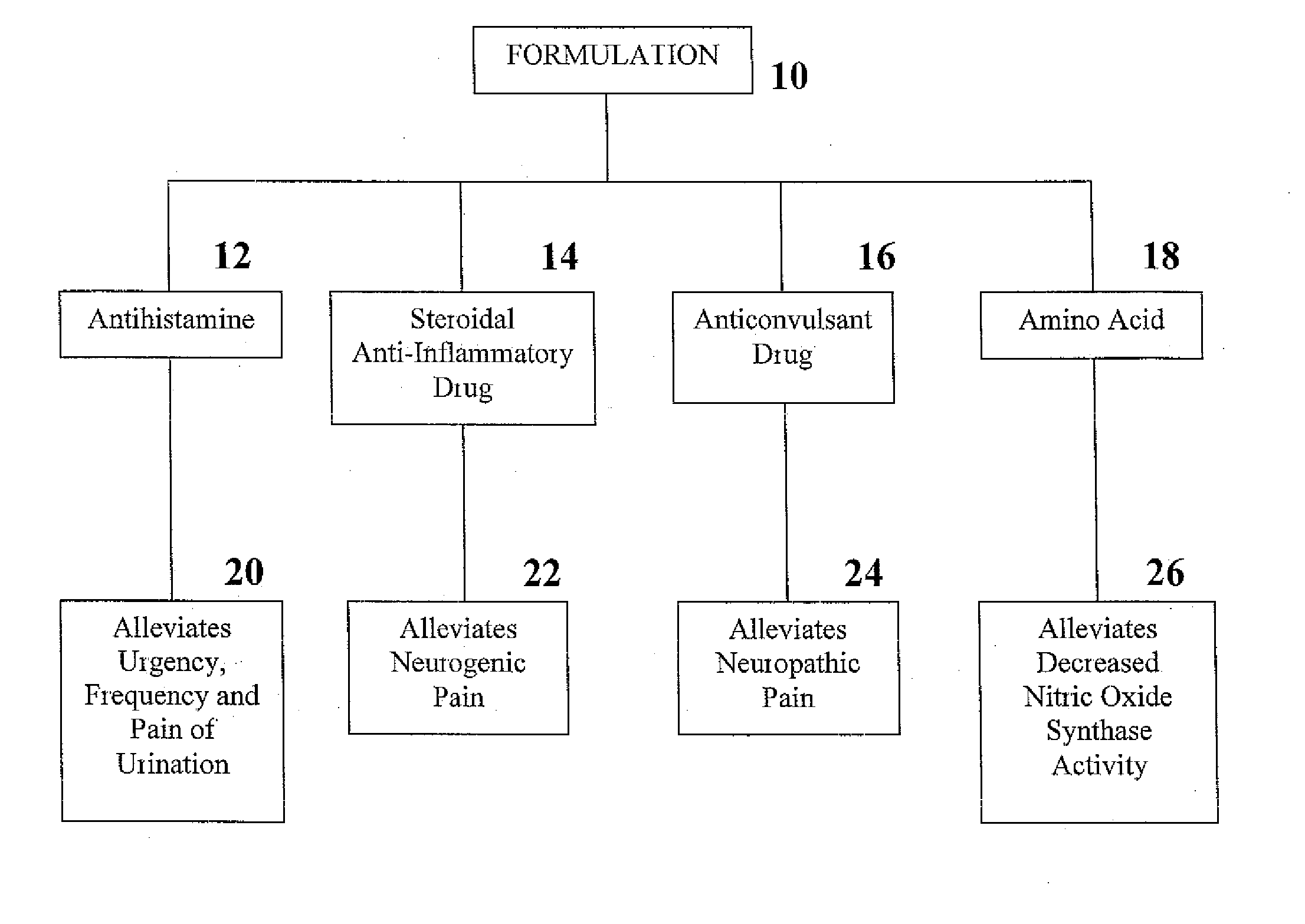
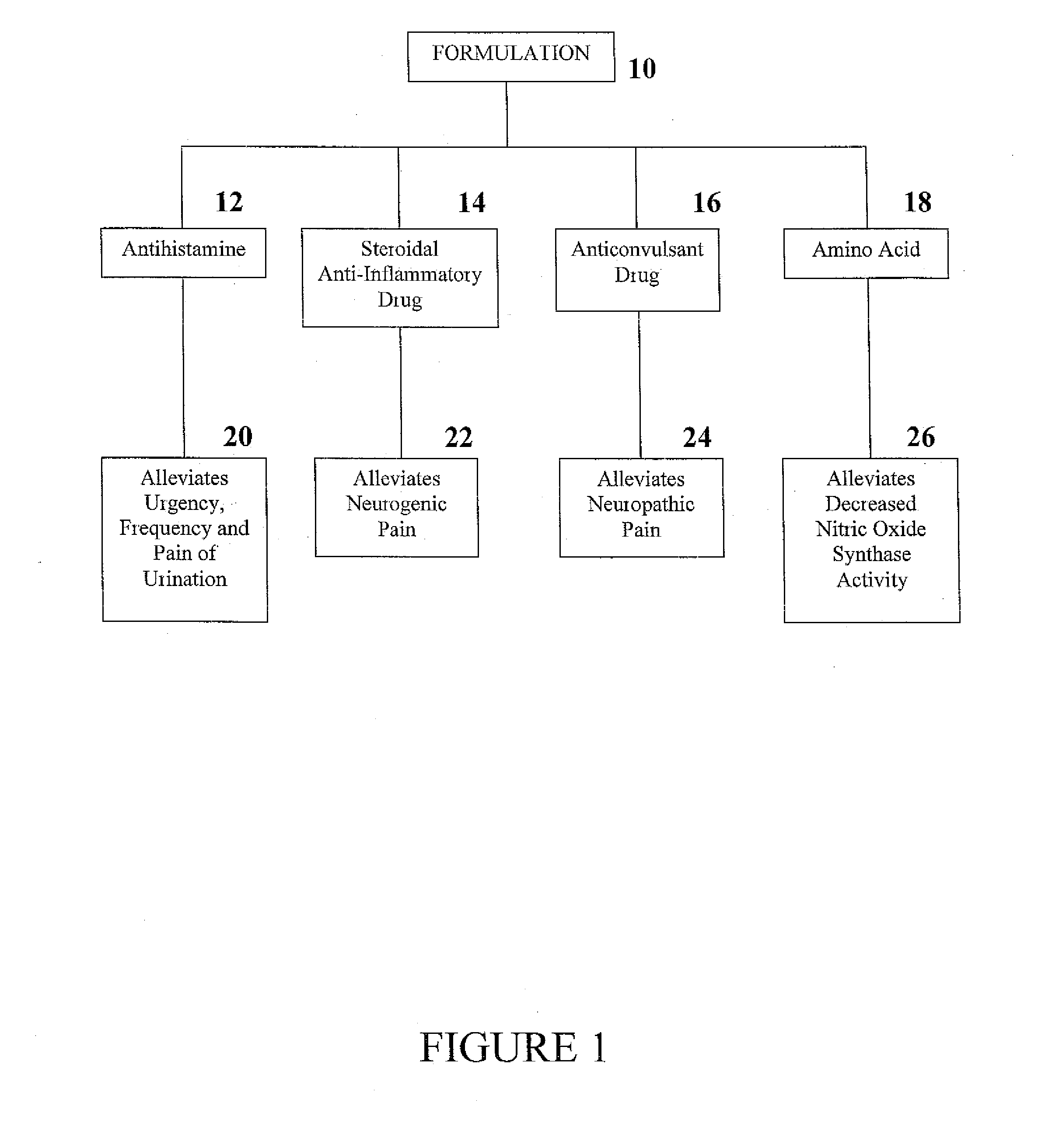
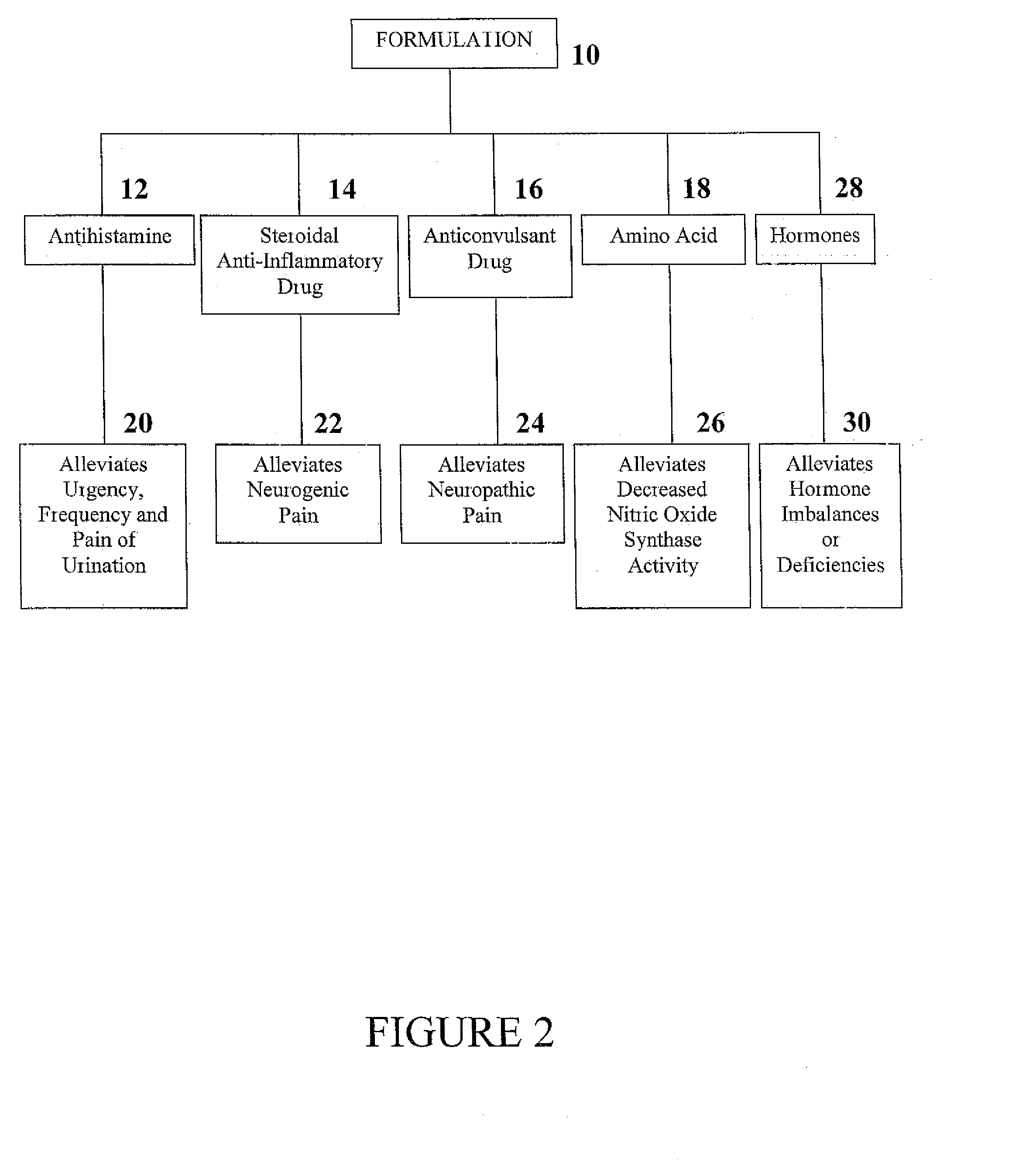
Formulation and Method for Treating Interstitial Cystitis and Related Bladder Conditions
InactiveUS20080275015A1Effective treatmentCombination novelOrganic active ingredientsBiocideDiseaseInterstitial cystitis
The invention of this application relates to a formulation and method of using the formulation for the treatment of interstitial cystitis and other similar conditions of the bladder. The formulation of this invention is a highly specialized, compounded pharmaceutical that contains a combination of numerous therapeutically different medications in a unique delivery vehicle and system. The individual components of the formulation work synergistically to restore and repair each of the different issues associated with interstitial cystitis. The individual components of the formulation exist in an aqueous vehicle to facilitate drug contact with the bladder wall. The formulation is instilled directly into the bladder, commonly in a physician's office.
Owner:POTTER JEFFREY A
Glycosaminoglycan composition and method for treatment and prevention of interstitial cystitis
ActiveUS7504387B2Treatment and/or prevention of interstitial cystitisRelieve symptomsBiocideCarbohydrate active ingredientsDiseaseN Acetyl D Glucosamine
The invention provides compositions and methods useful for the treatment and / or prevention of interstitial cystitis and / or a related urinary tract condition in man or in animals. Specifically, provided are compositions specially formulated for direct instillation into the bladder and / or parenteral use in the treatment and / or prevention of interstitial cystitis. Compositions adapted for direct instillation into the bladder and / or for systemic administration are provided comprised of therapeutic amounts of: chondroitin sulfate in combination with hyaluronan (hyaluronic acid) are provided. Compositions adapted for direct instillation into the bladder and / or for systemic administration are also provided comprised of therapeutic amounts of: chondroitin sulfate, hyaluronan (hyaluronic acid) and N-acetyl D-glucosamine.
Owner:ARTHRODYNAMIC HLDG LLC
Prg4 treatment for interstitial cystitis
ActiveUS20120321693A1Reducing urinary frequencyExtended stayAntipyreticDigestive systemInterstitial cystitisIn vivo
The present invention relates to a disorder of the lower urinary tract, and in particular, reducing the symptoms (including treatment) of interstitial cystitis in vivo. In a preferred embodiment, the present invention relates to treatment formulations and methods for reducing interstitial cystitis in patients via administration of a therapeutically effective concentration of PRG4.
Owner:LUBRIS
Sphingomyelin liposomes for the treatment of hyperactive bladder disorders
InactiveUS8110217B2Reduce and prevent antibody-mediated resistanceIncrease stimulationLiposomal deliveryAgainst vector-borne diseasesDiseaseMetabolite
Owner:UNIVERSITY OF PITTSBURGH
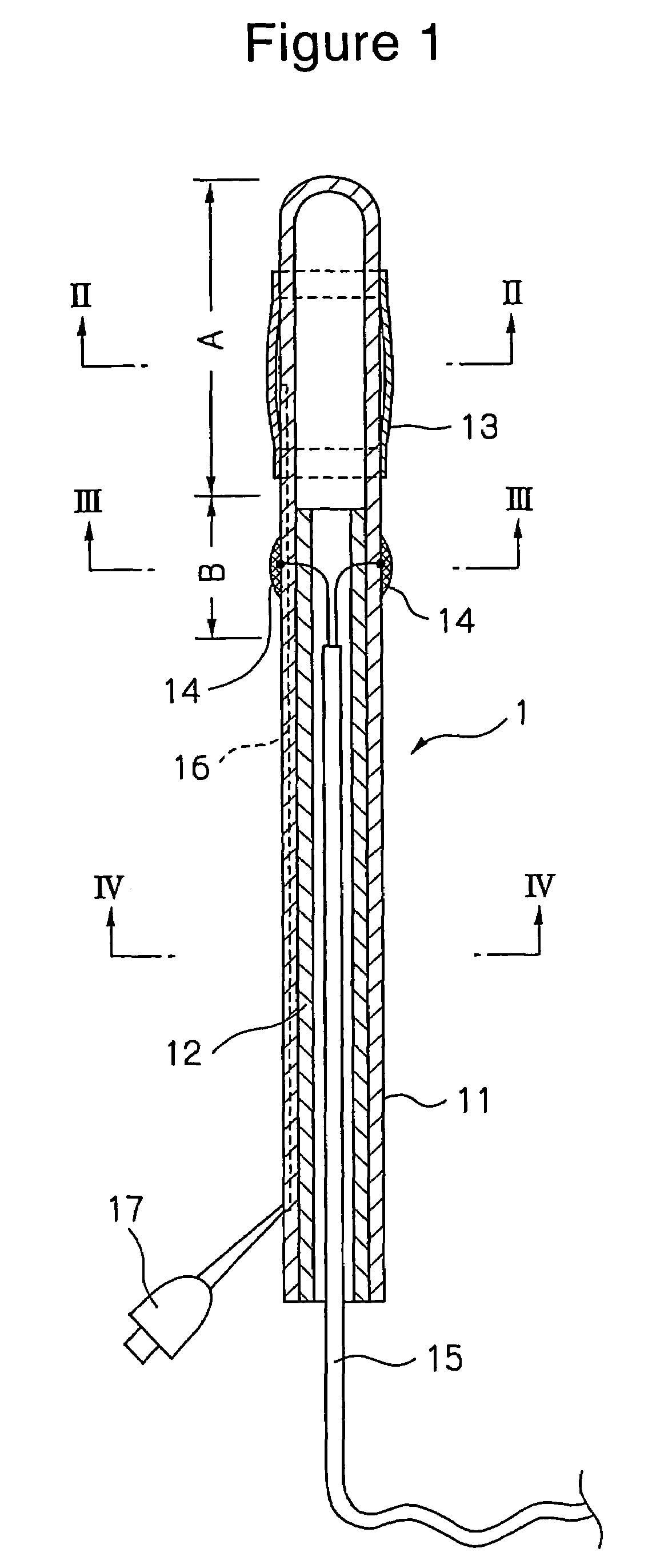
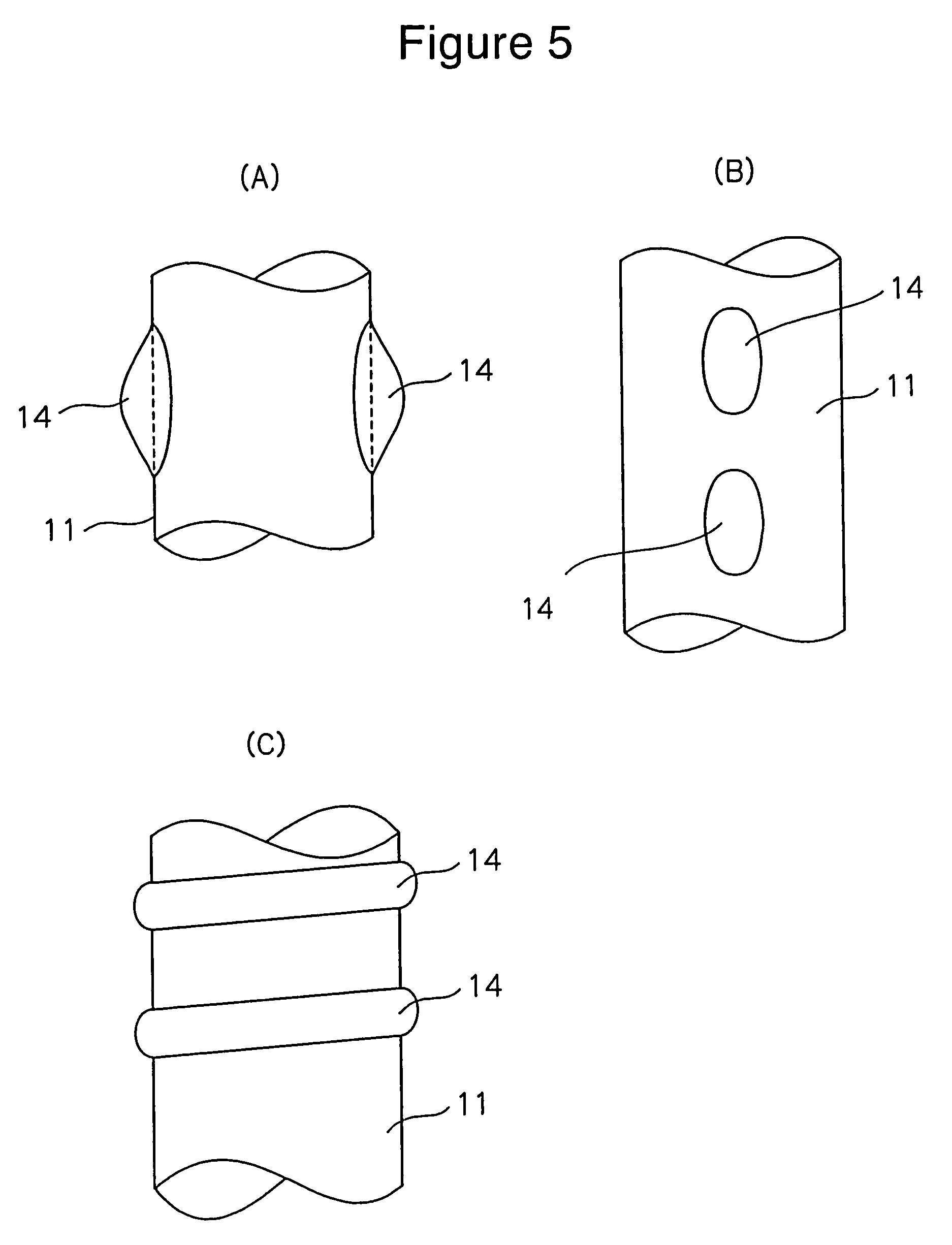
Diagnosis catheter for interstitial cystitis
A diagnosis catheter 1 for an interstitial cystitis is connected to a current perception threshold inspection apparatus 4. A catheter body 11 is made of a soft flexible material and includes a bladder-dwelling distal end section A and a diagnosis section B adjacent the distal end section A at a proximal end side of the body 11. A core member 12 is made of a hard flexible material and is inserted into the diagnosis section B in the catheter body 11. An inflatable balloon 13 is mounted on an outer periphery around the bladder-dwelling distal end section A of the catheter body 11. A fluid supply passage 16 is provided in the catheter body 11 so that an end of the passage 16 is communicated to the balloon 13 and a proximal end of the passage 16 is communicated to an injection part 17.
Owner:TSUKADA MEDICAL RES CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com