Patents
Literature
151 results about "Stress incontinence" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stress incontinence occurs when your bladder leaks urine during physical activity or exertion. It may happen when you cough, lift something heavy, change positions, or exercise.
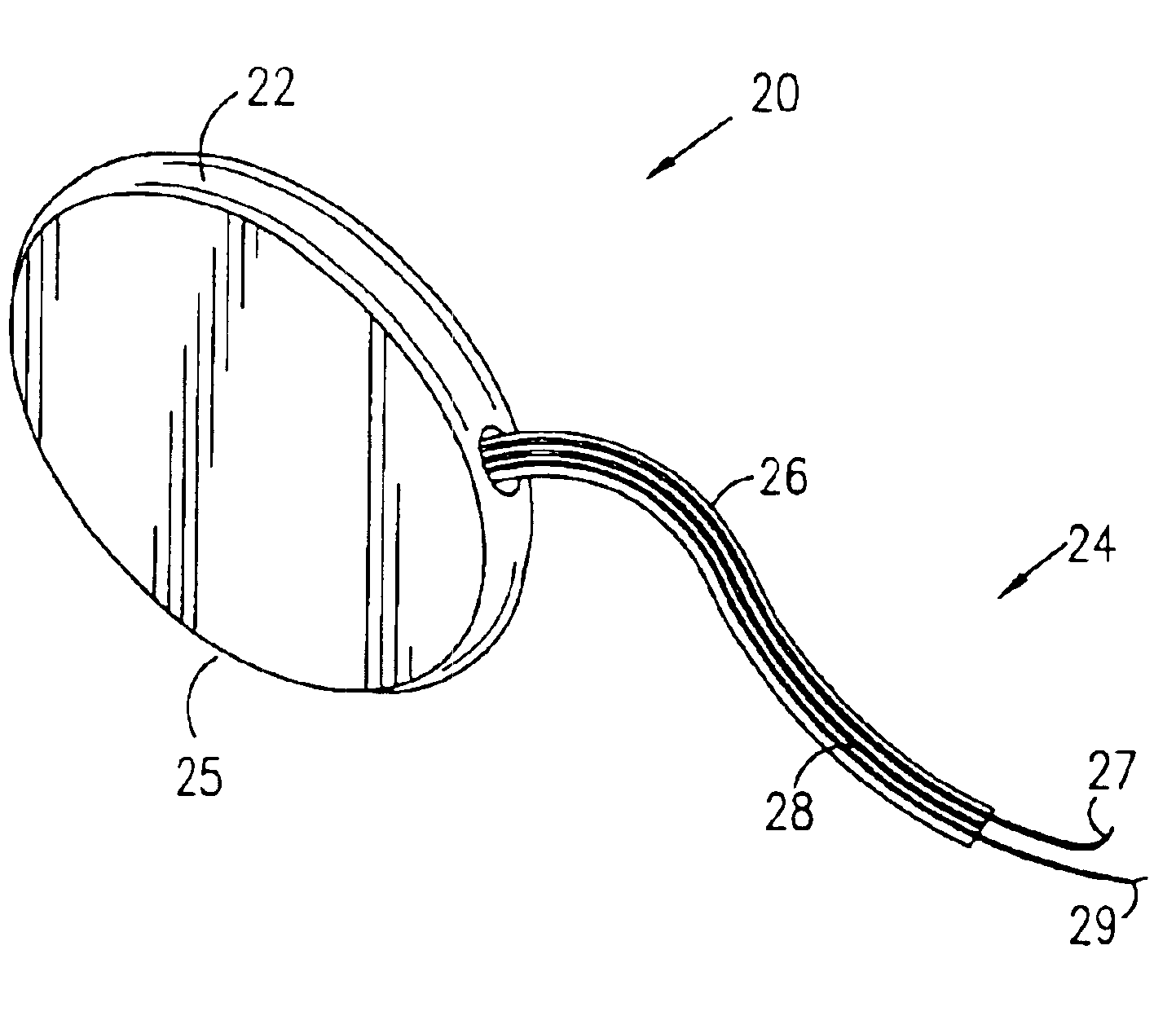
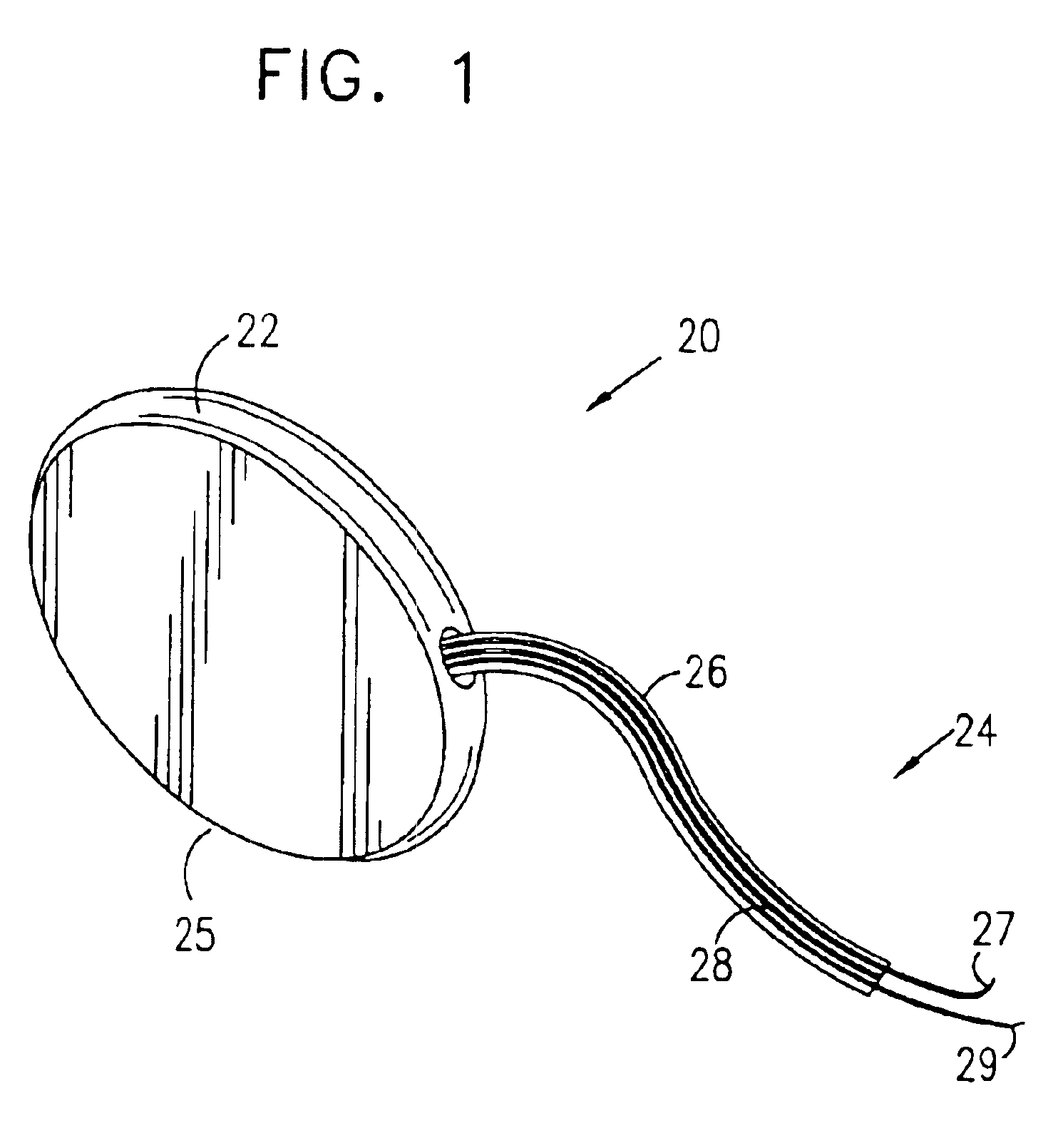
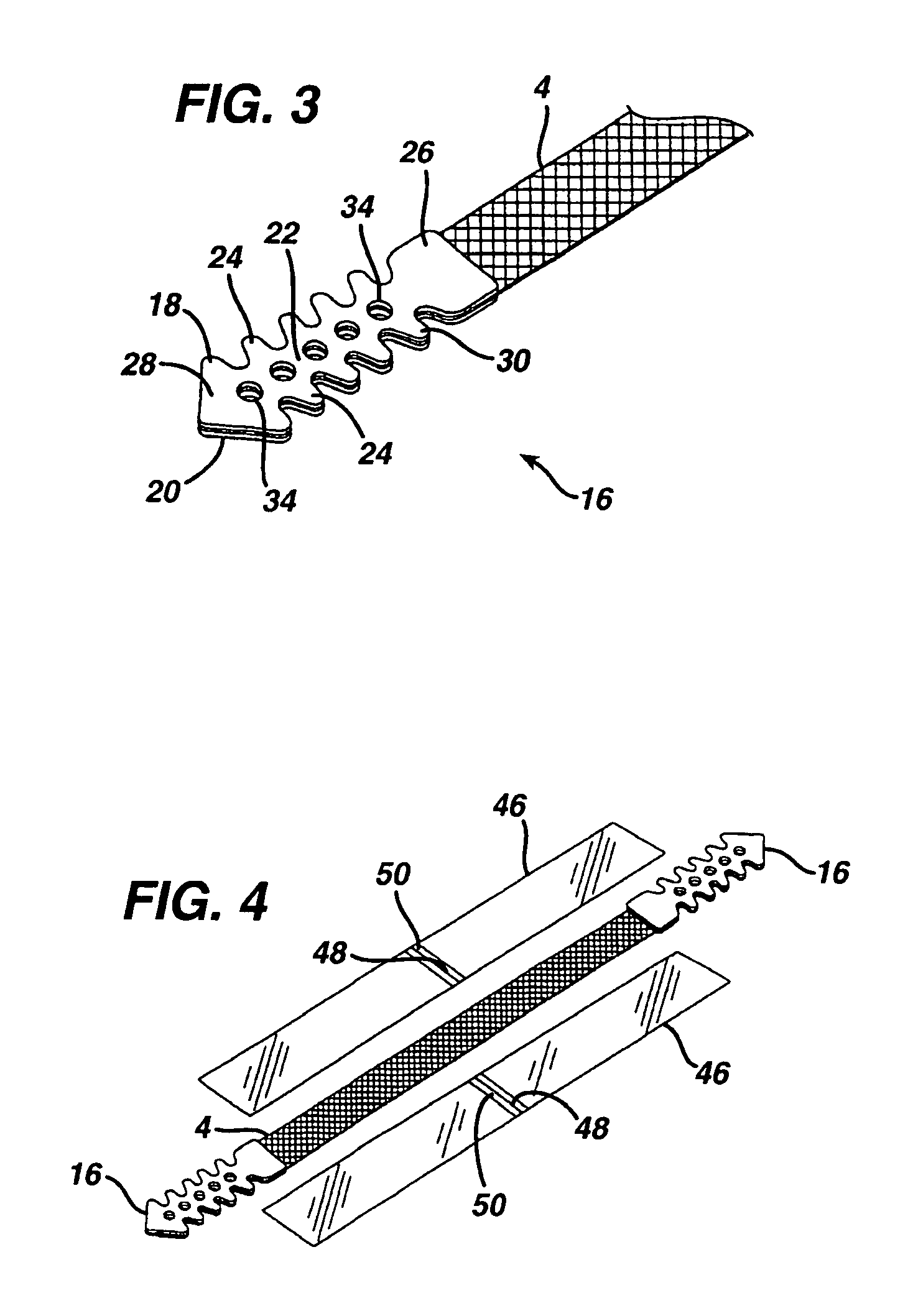
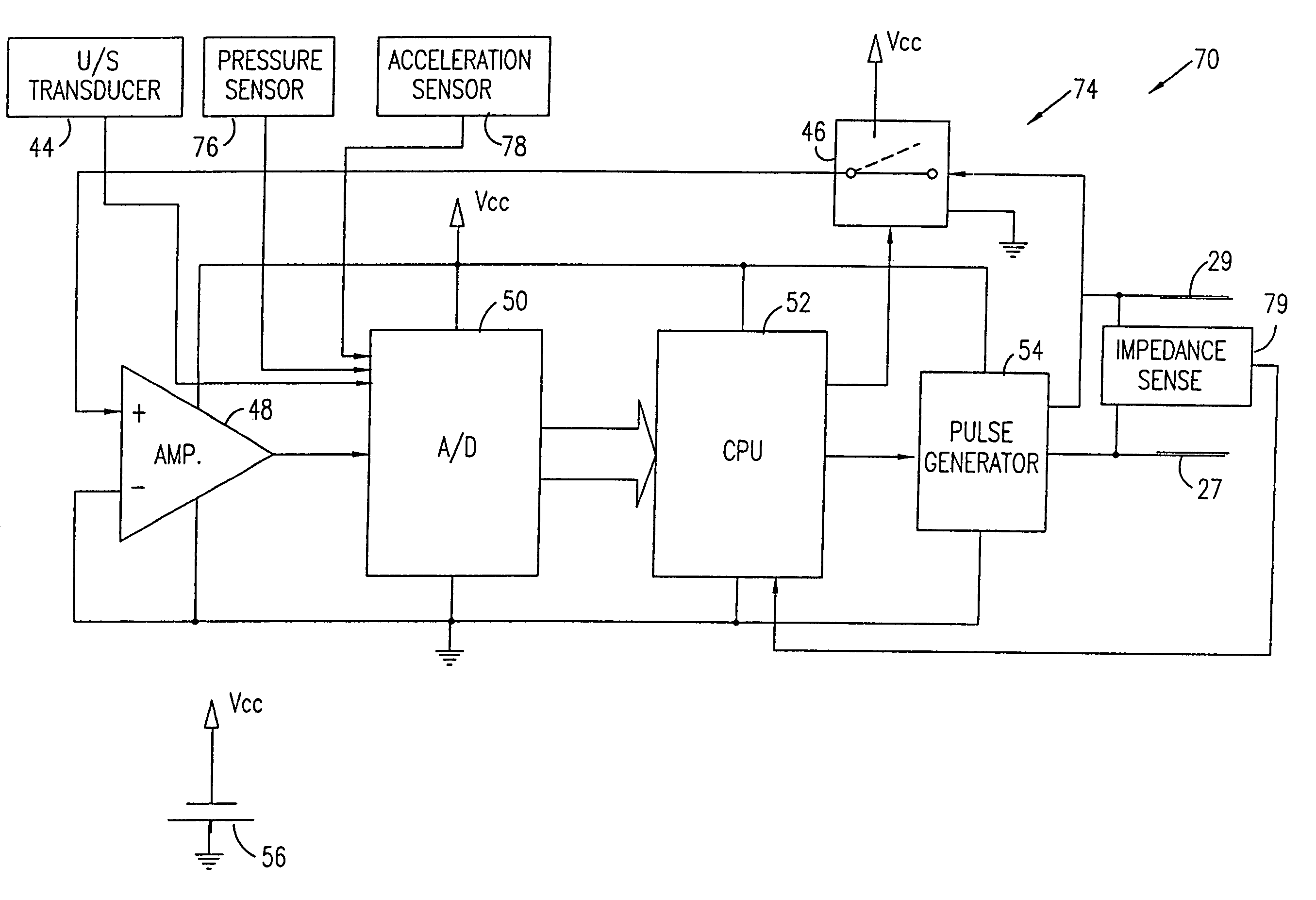
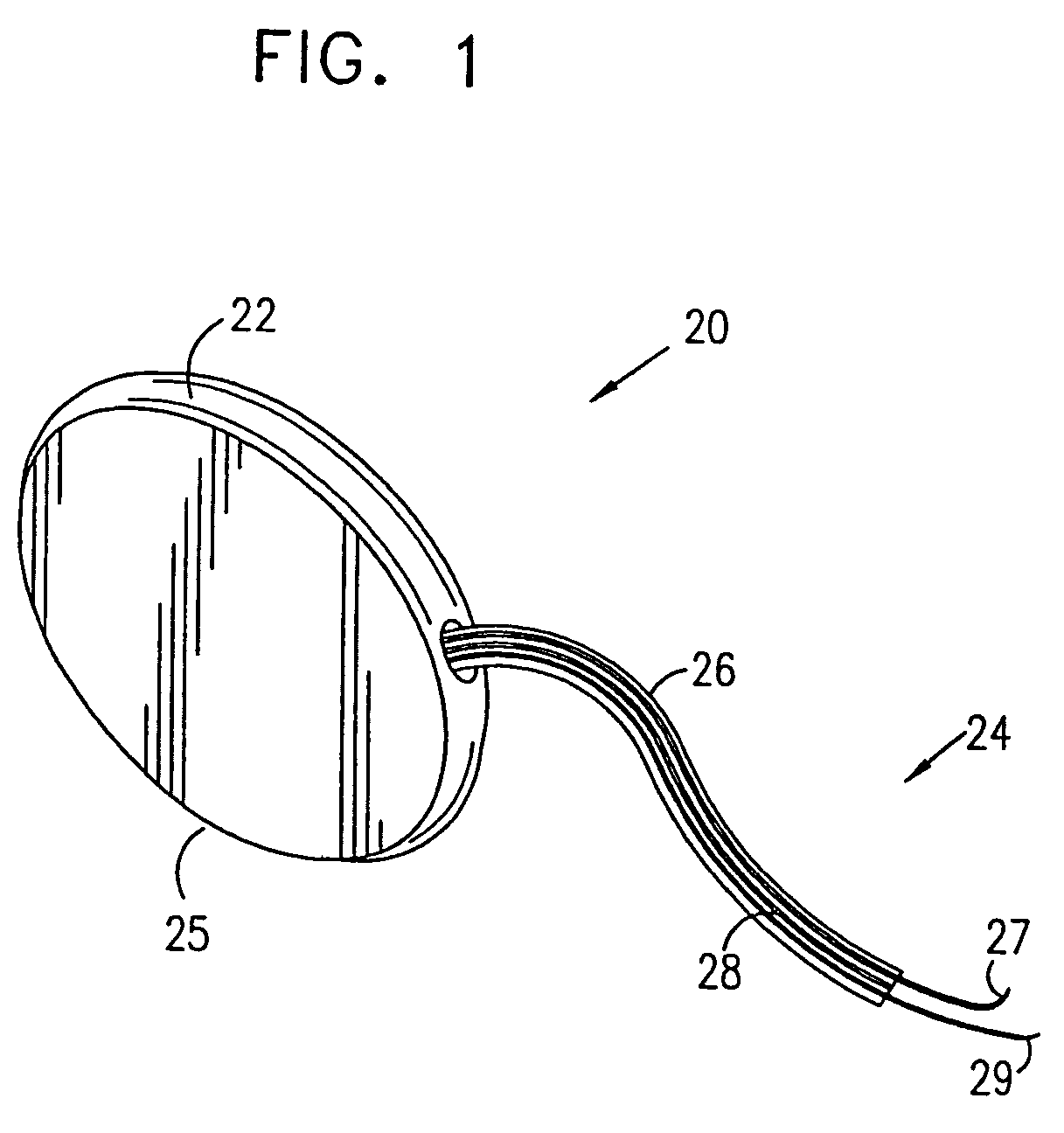
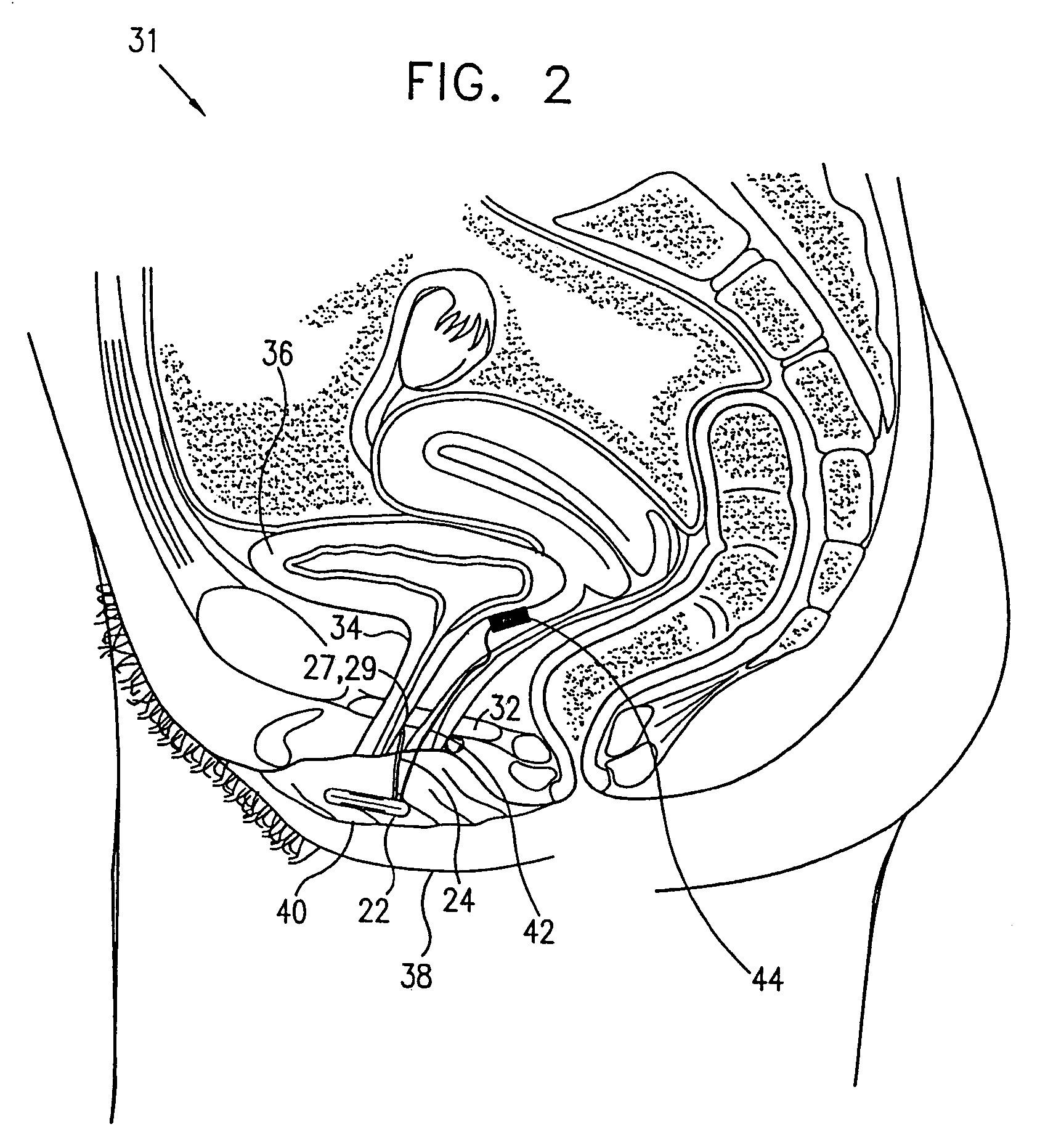
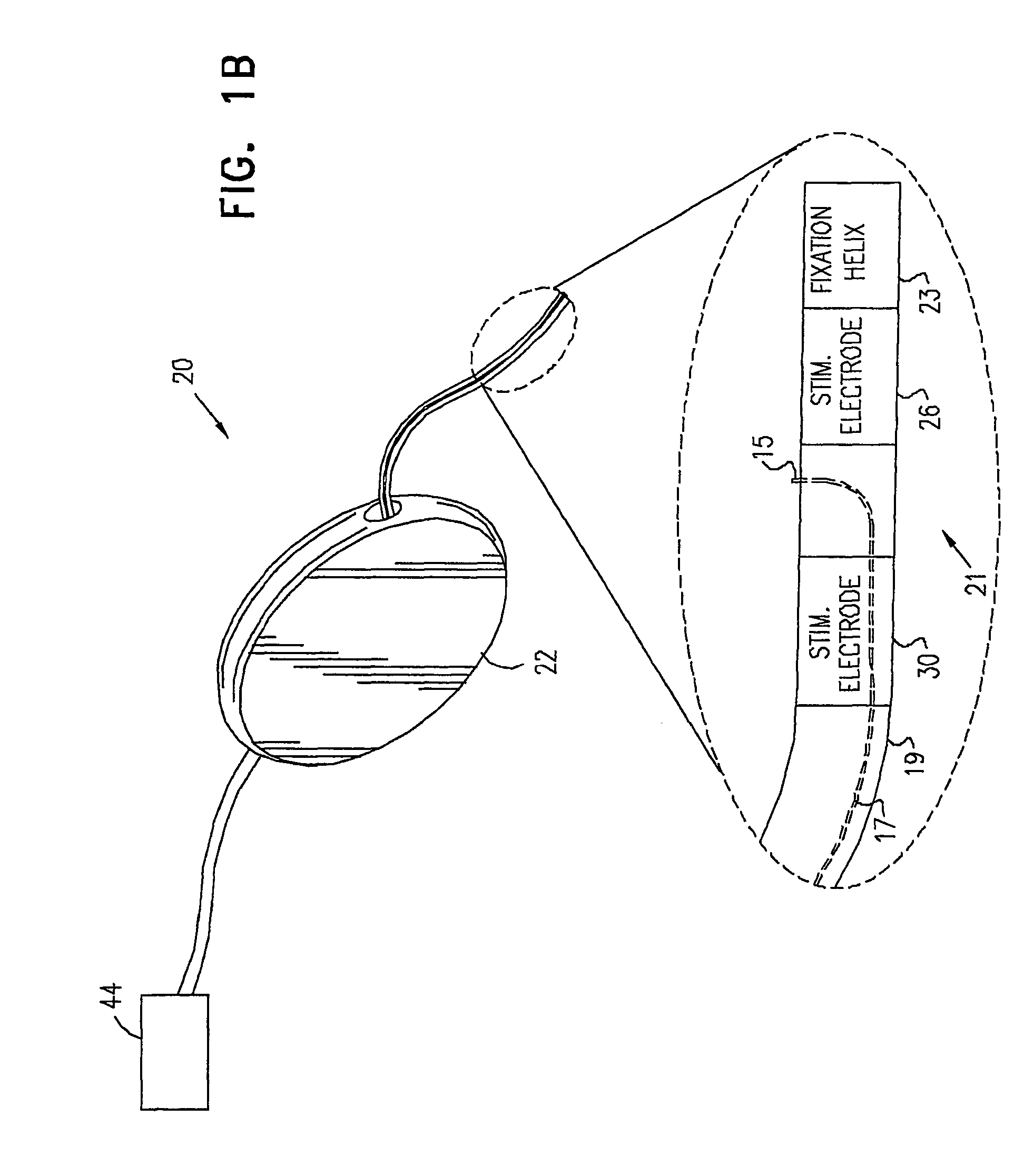
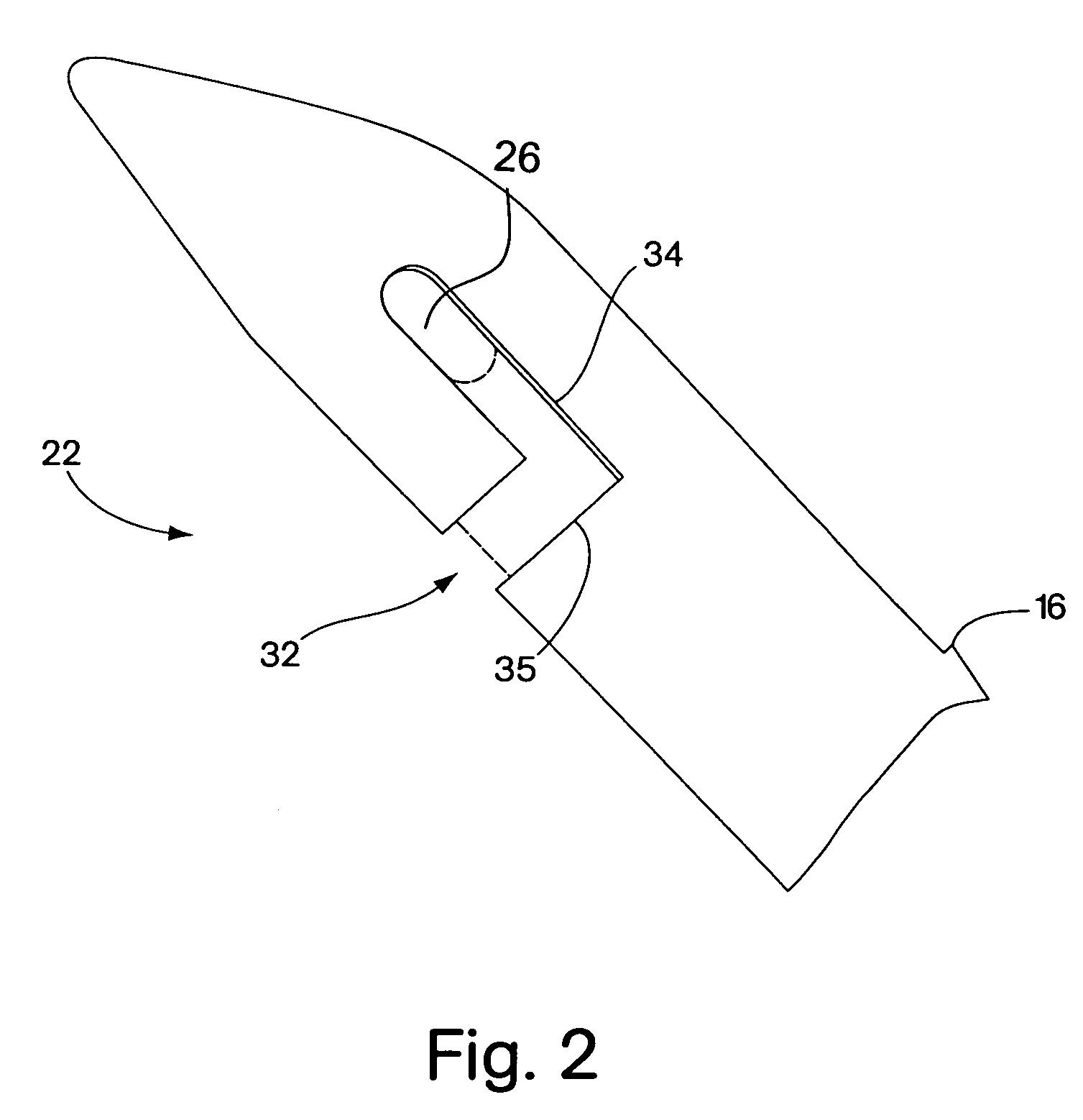
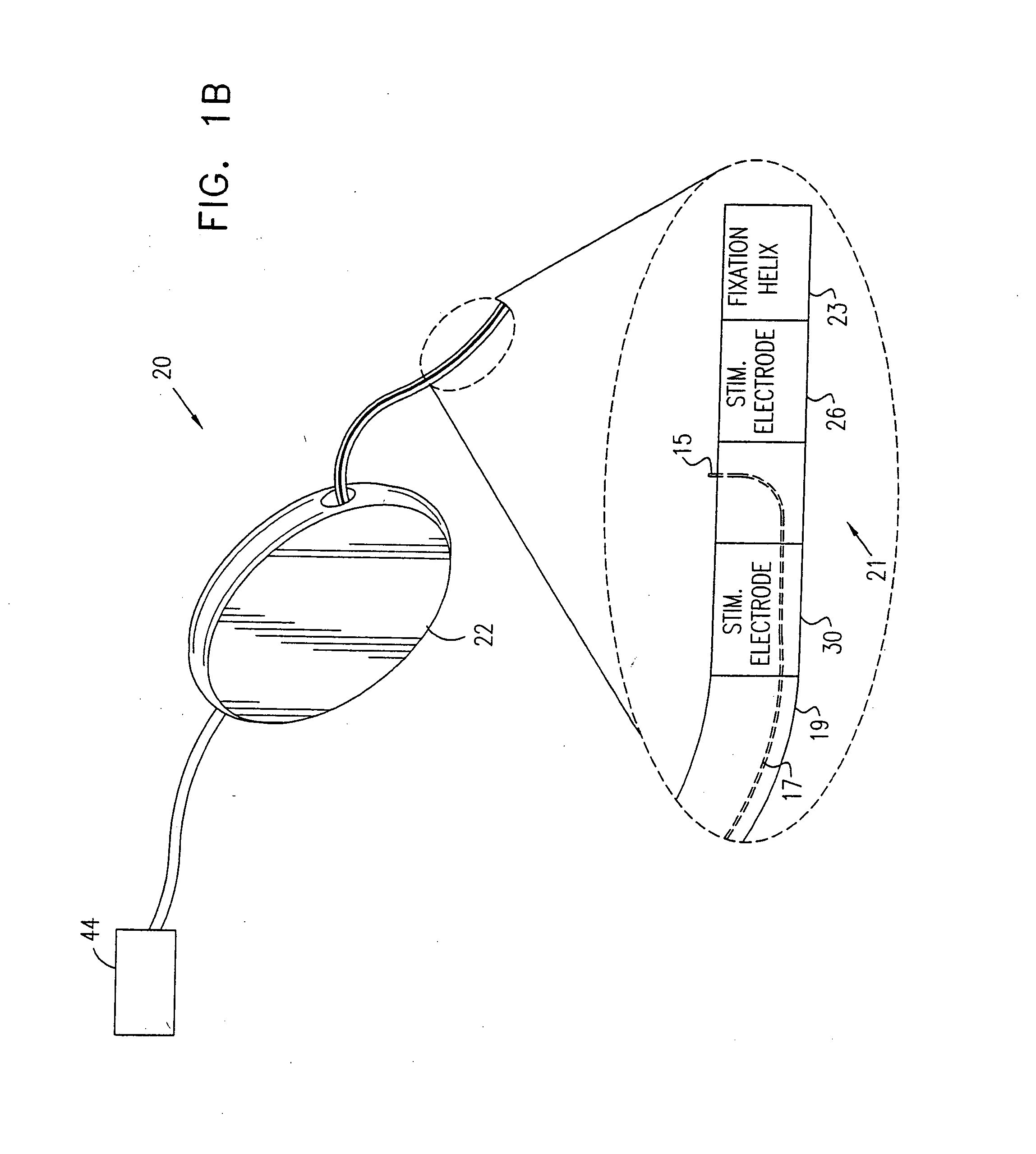
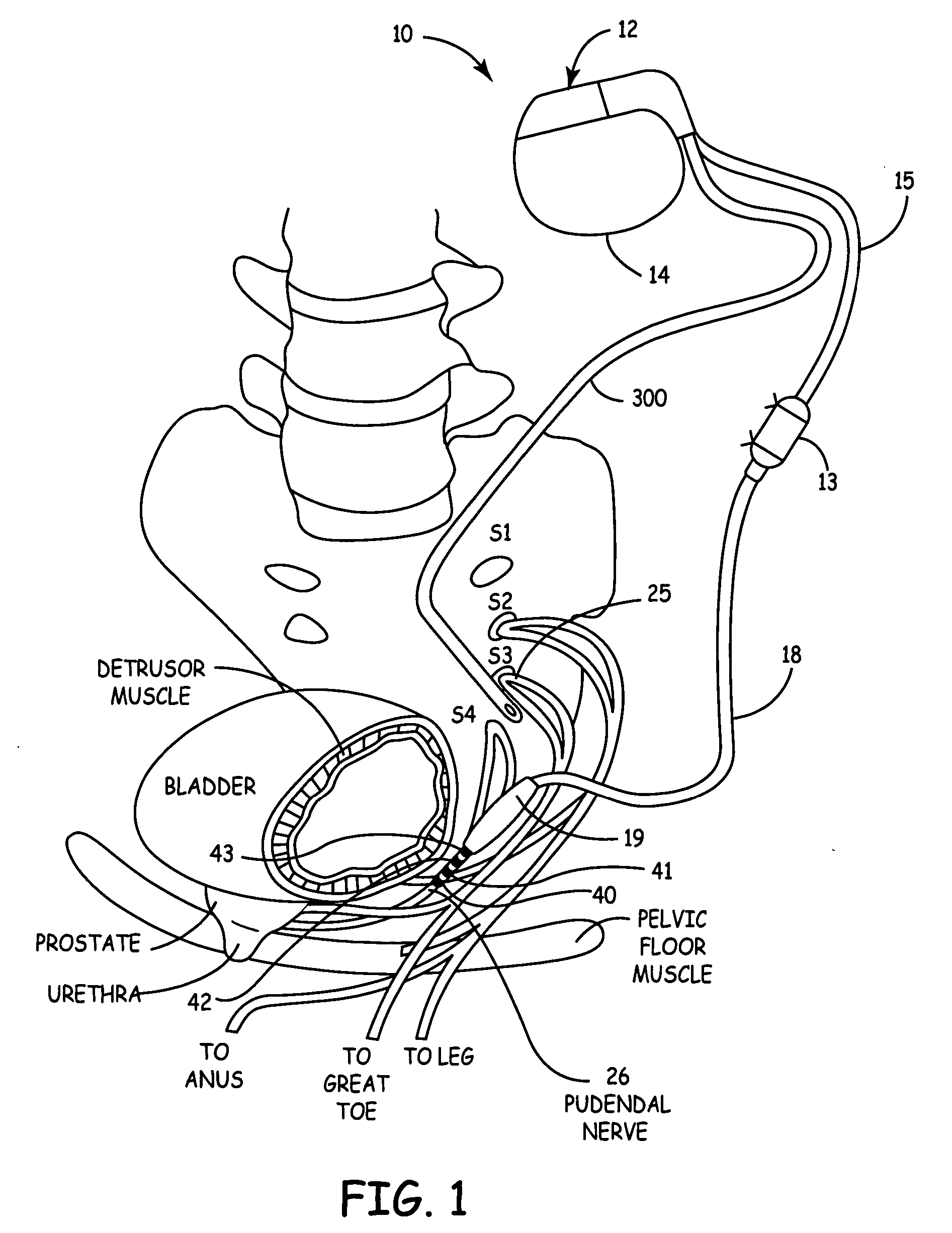
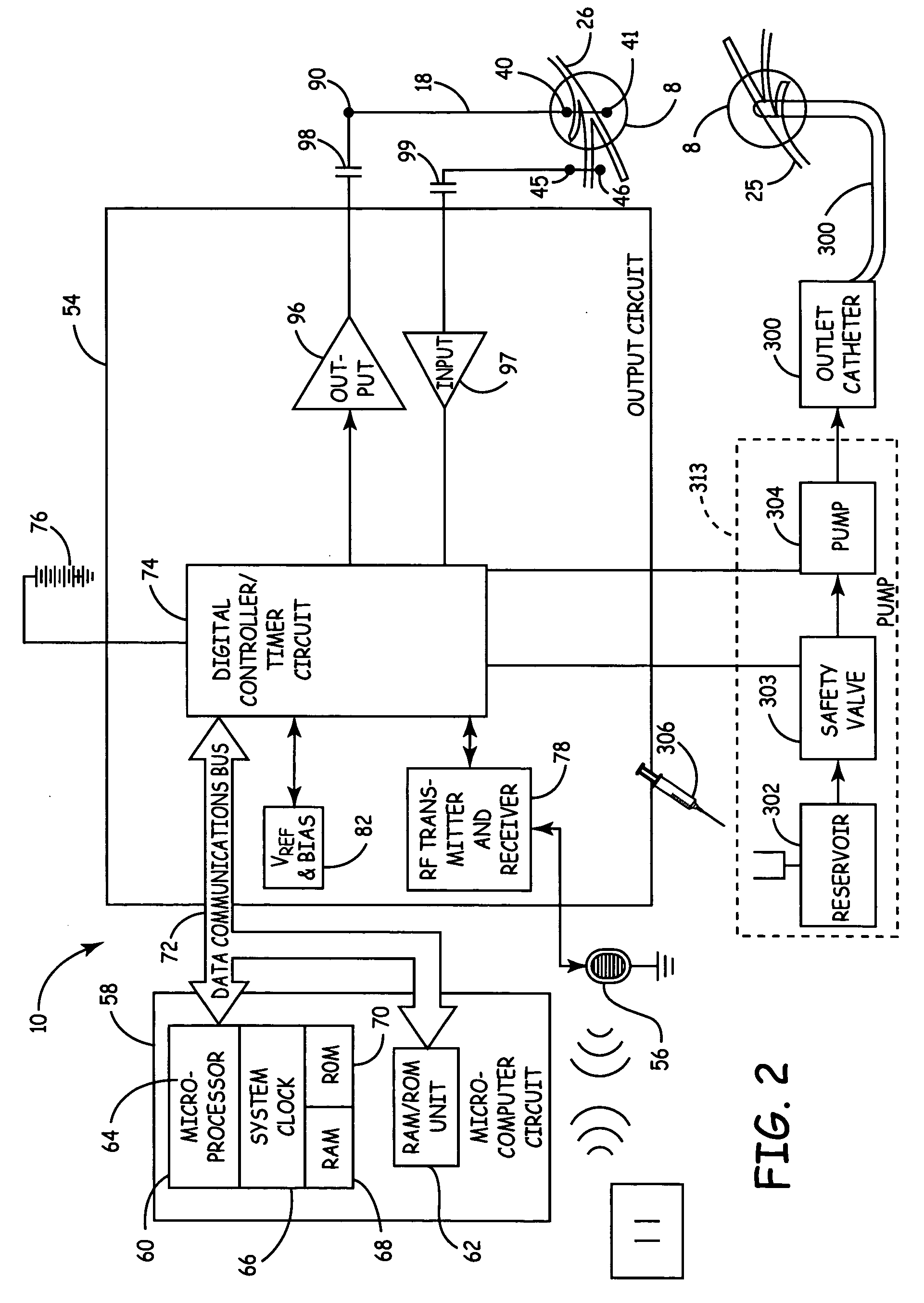
Mechanical and electrical sensing for incontinence treatment
InactiveUS6896651B2Inhibit involuntary urine flowFunction increaseElectrotherapyAnti-incontinence devicesUrethraStress incontinence
A device and method for treatment of urinary stress incontinence. At least one electrode is implanted in a pelvic muscle of a patient. A control unit receives signals indicative of abdominal stress in the patient and responsive thereto applies an electrical waveform to the electrode which stimulates the muscle to contract, so as to inhibit involuntary urine flow through the patient's urethra due to the stress.
Owner:ASTORA WOMENS HEALTH +1
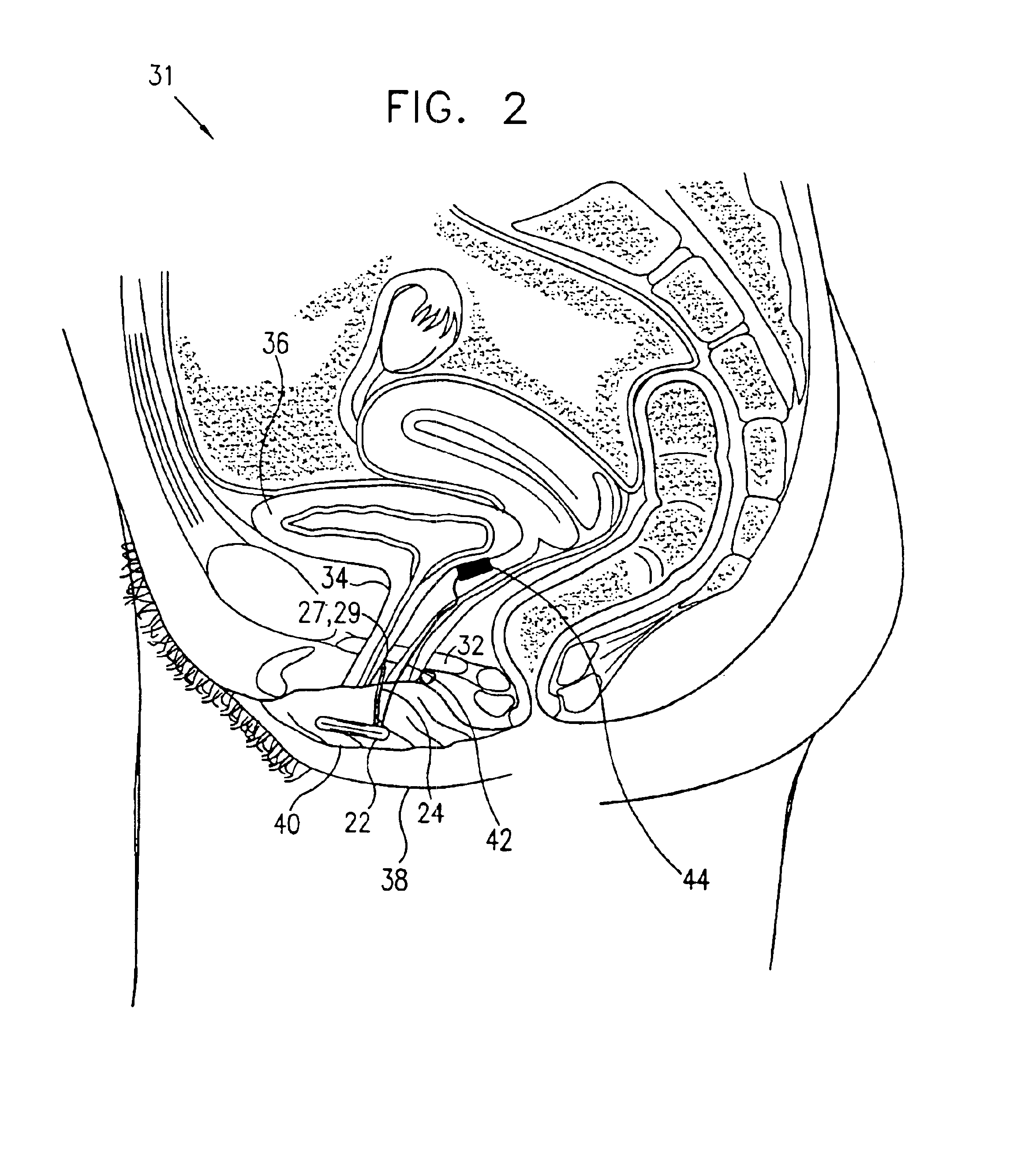
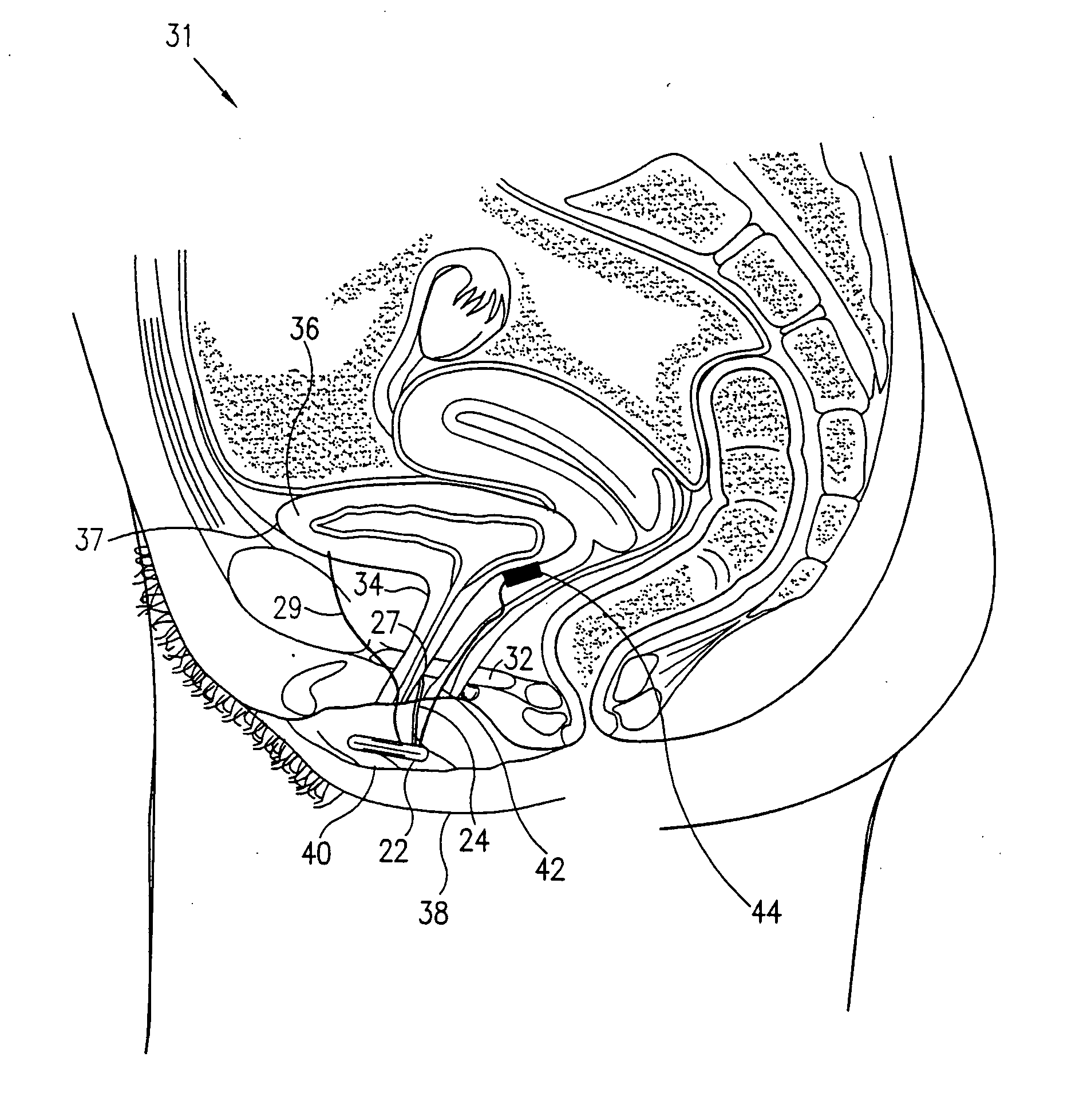
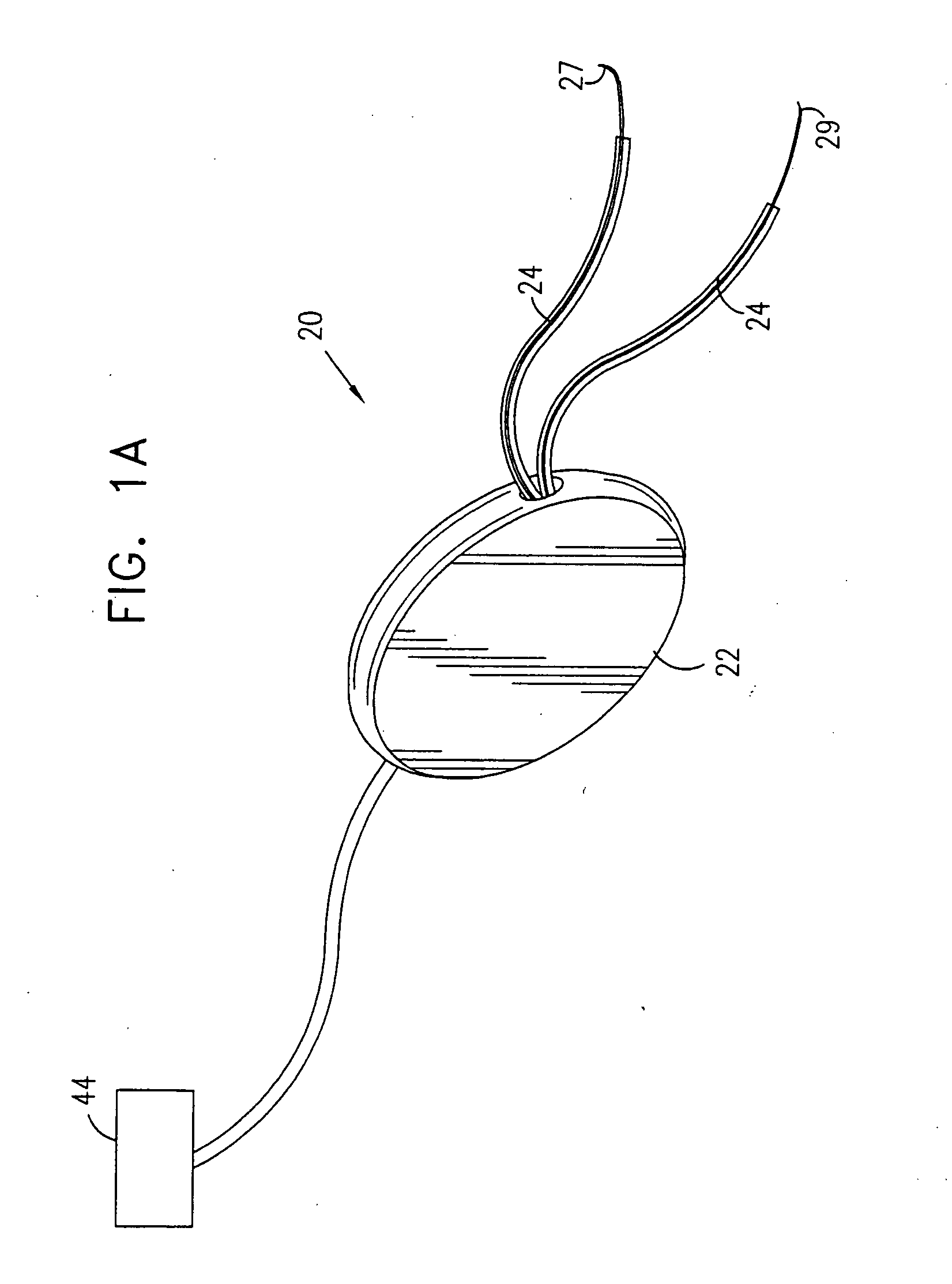
Pelvic disorder treatment device
InactiveUS6862480B2Reduce decreaseRelieving pelvic painUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
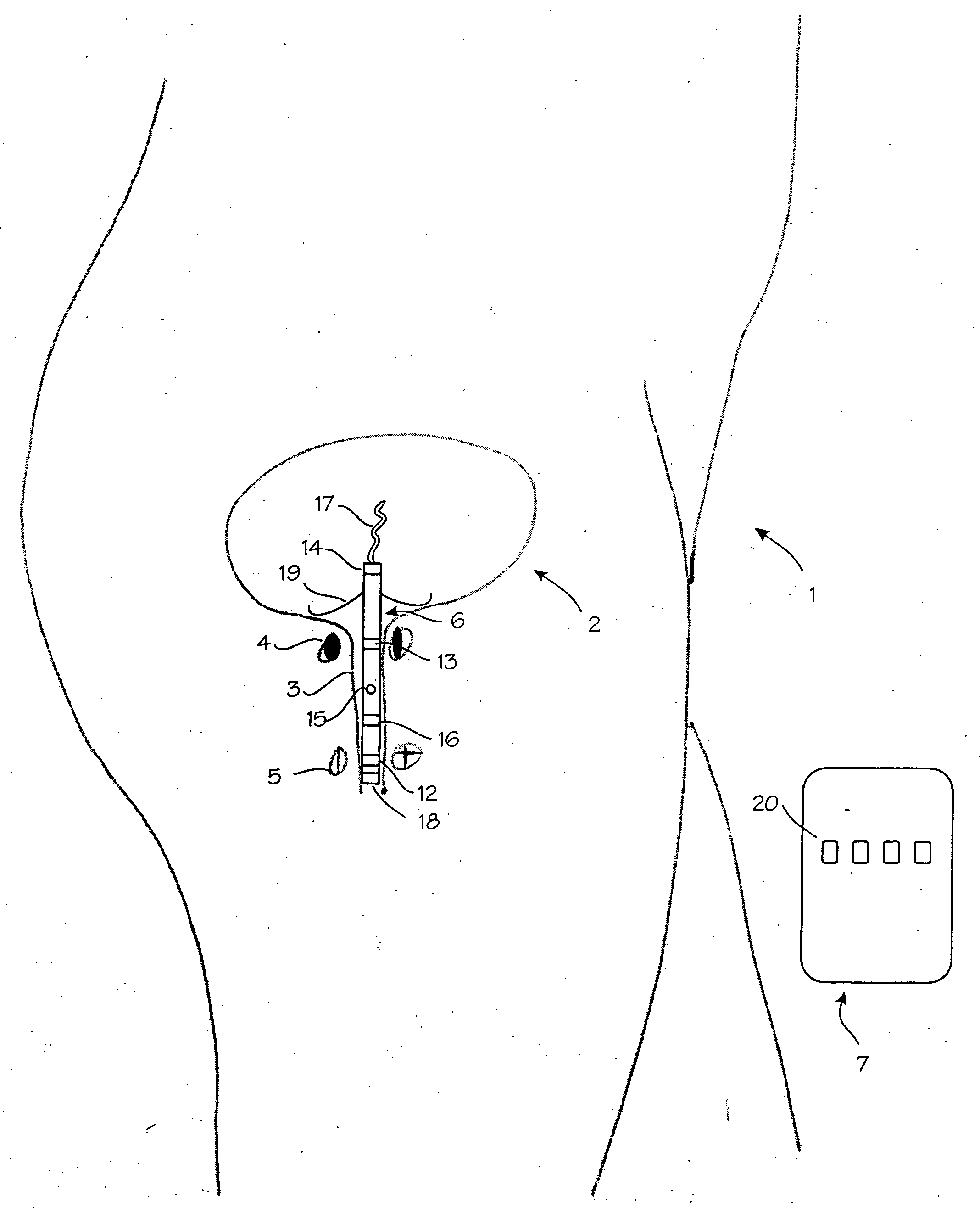
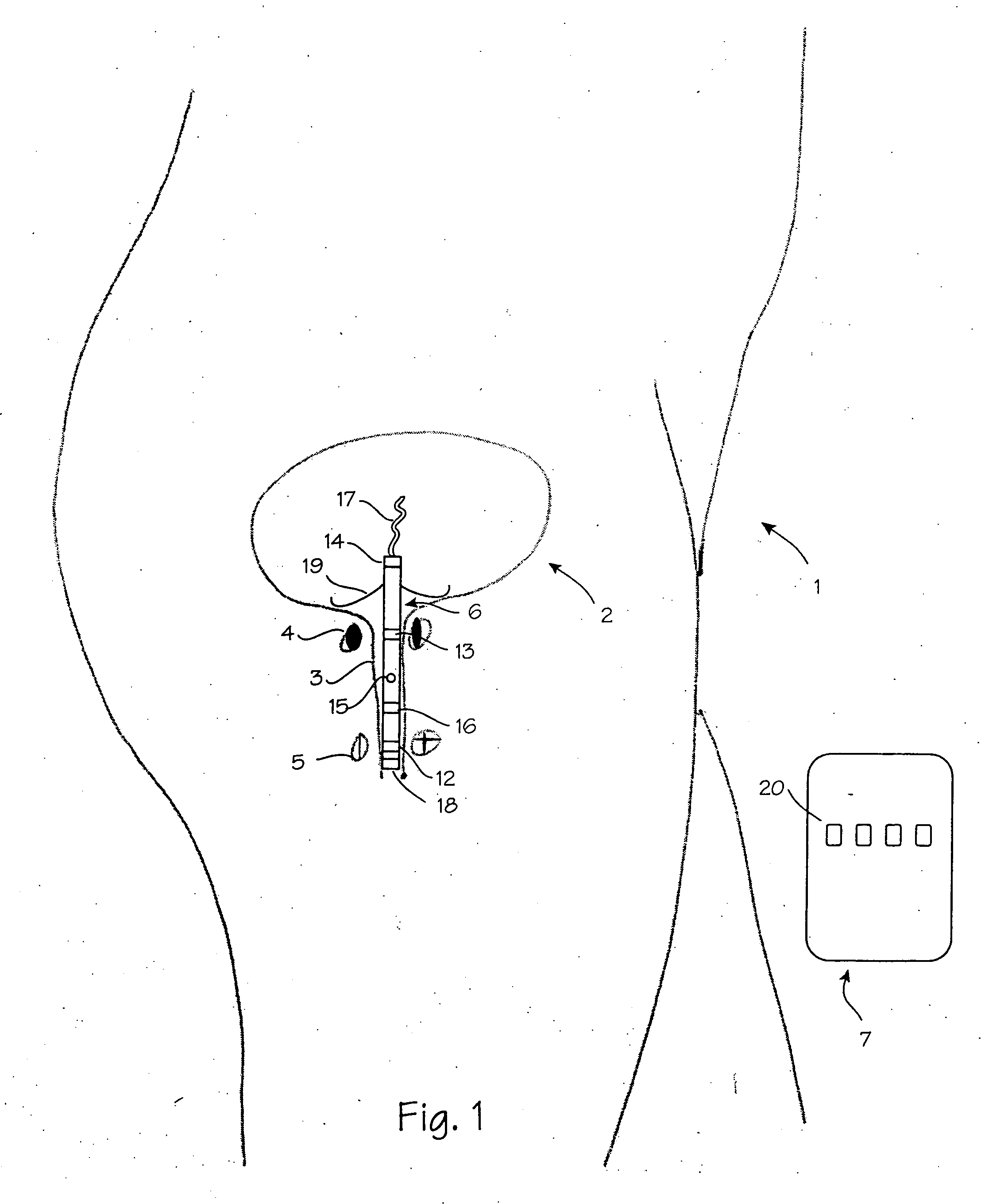
A device for treating a medical condition is provided, and a surgical procedure for implanting the device is disclosed. The device includes a sensor, which is adapted to generate a signal responsive to a state of a patient, and at least one electrode, which is adapted to be coupled to a pelvic site of the patient. A control unit is adapted to receive the signal, to analyze the signal so as to distinguish between an imminent stress incontinence event and an imminent urge event, and, responsive to analyzing the signal, to apply an electrical waveform to the at least one electrode. In various configurations, the device may be used alternatively or additionally to treat fecal incontinence, interstitial cystitis, chronic pelvic pain, or urine retention.
Owner:ASTORA WOMENS HEALTH
Minimally invasive medical implant and insertion device and method for using the same
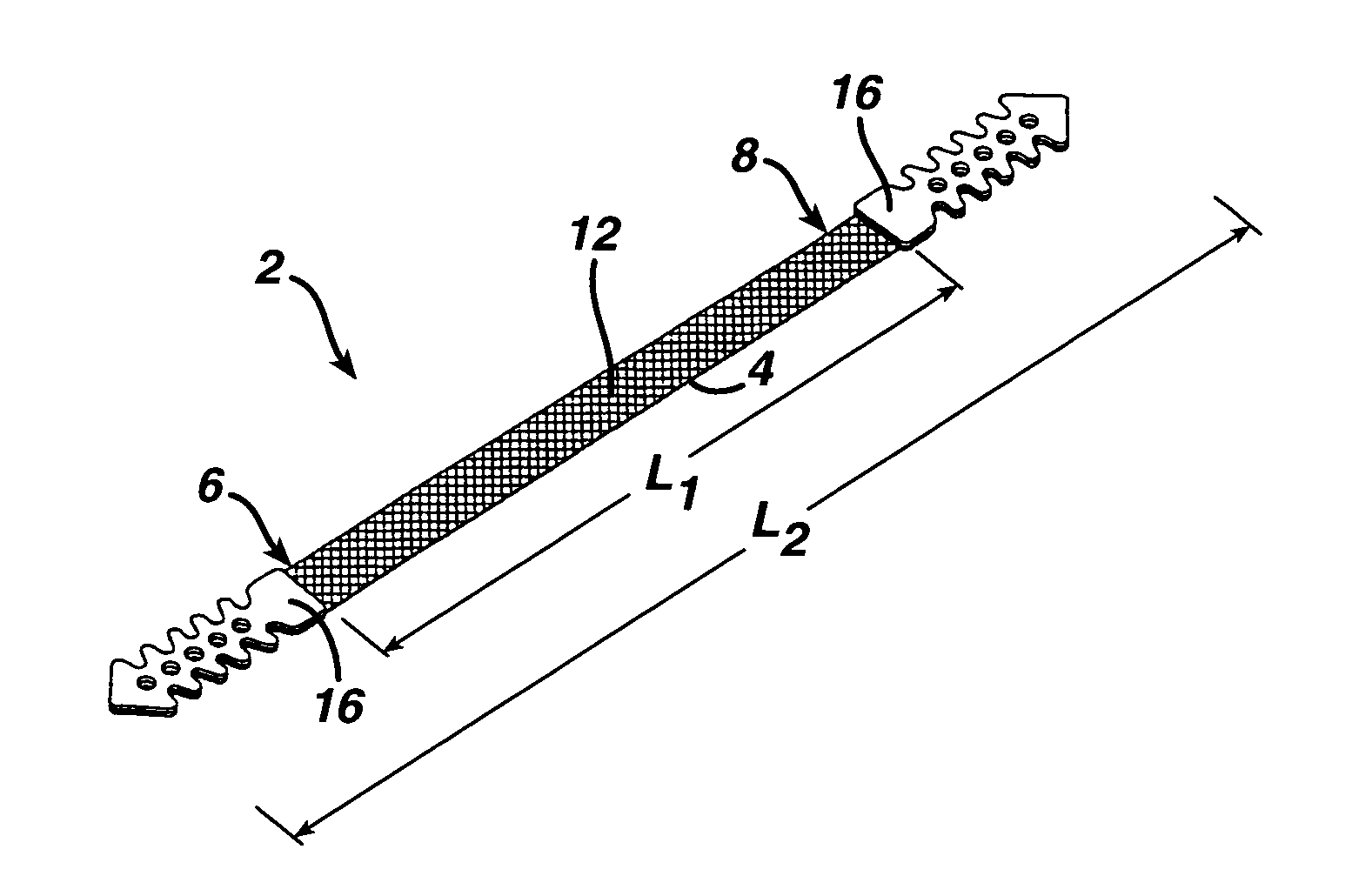
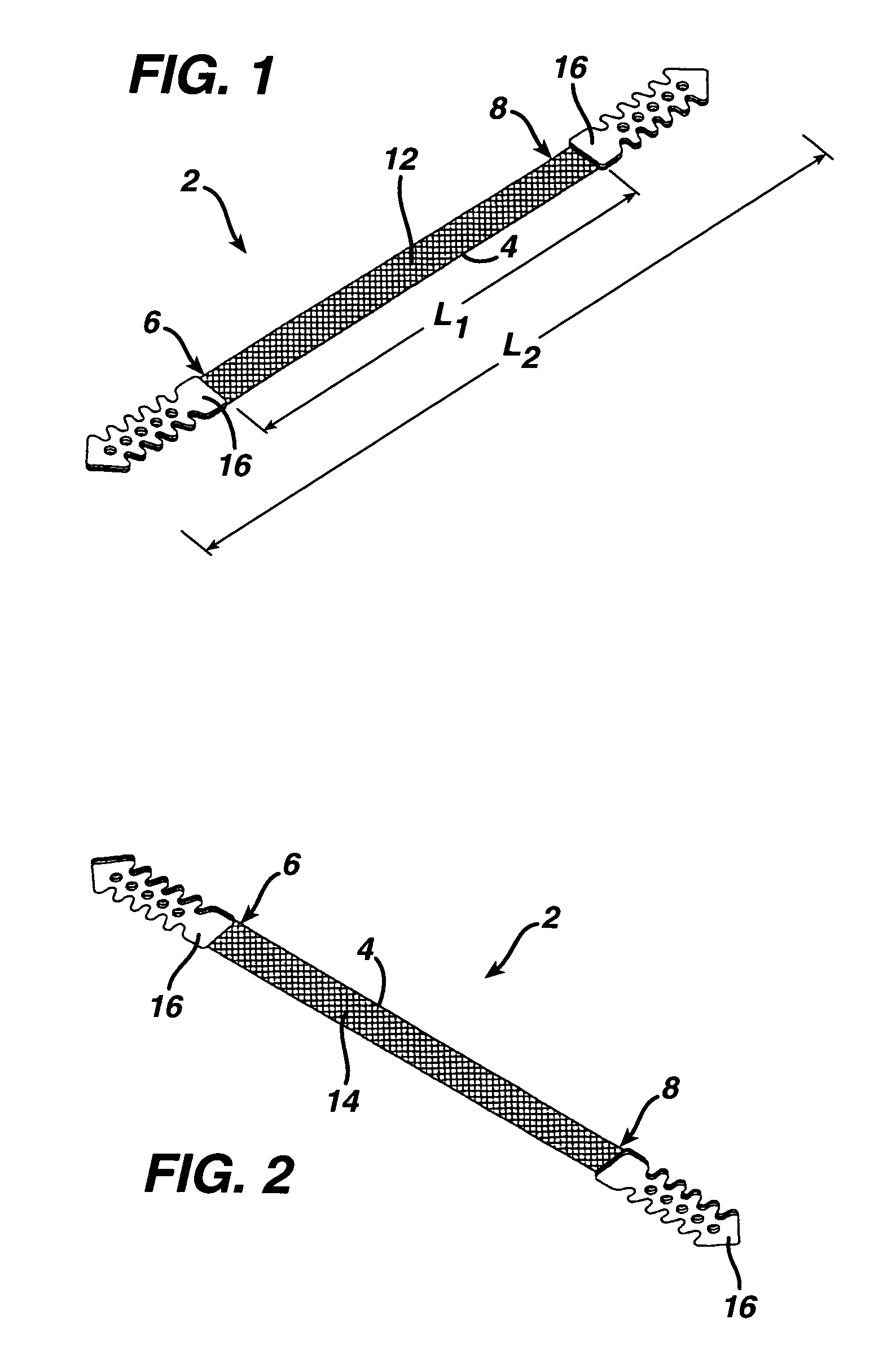
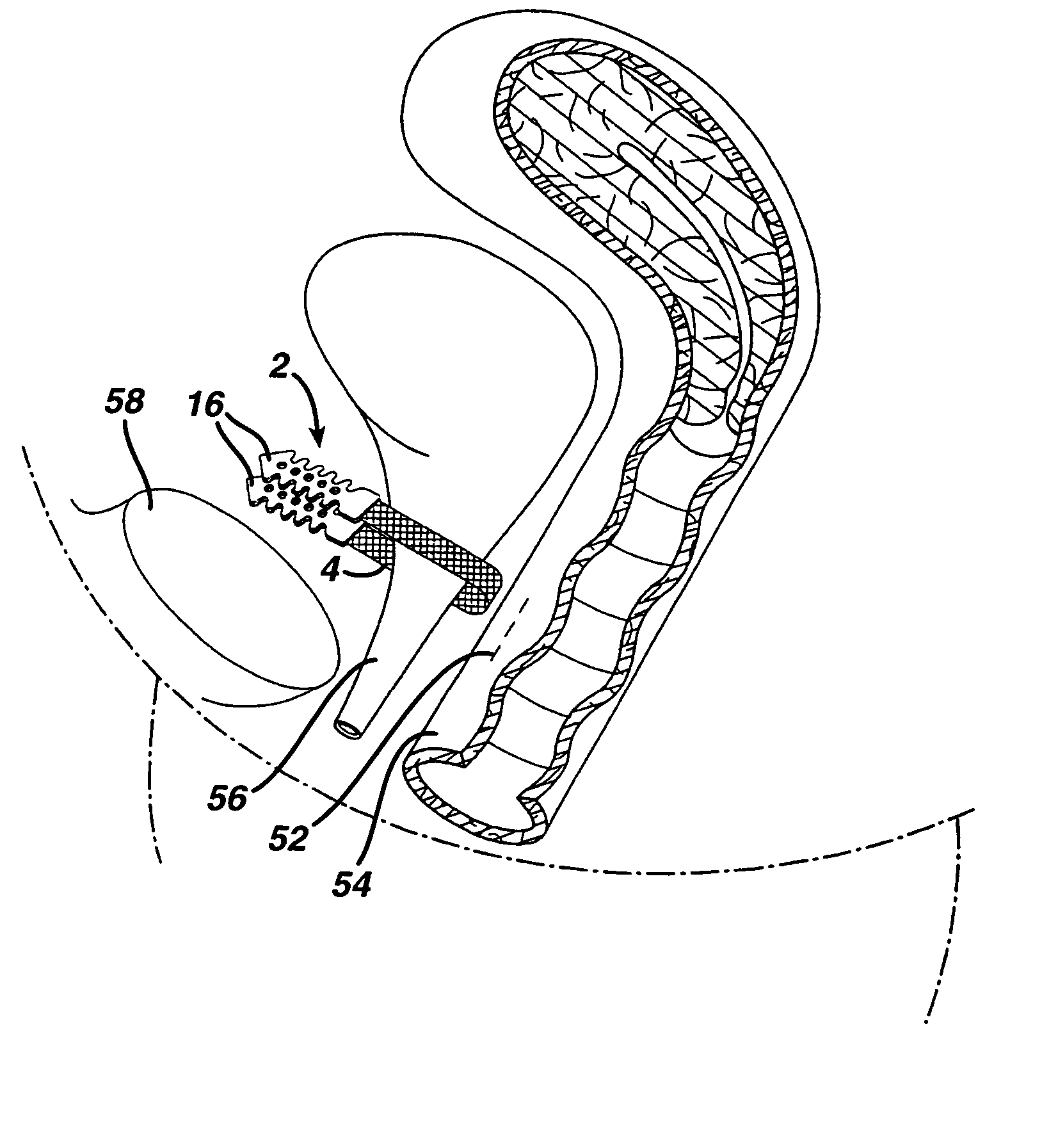
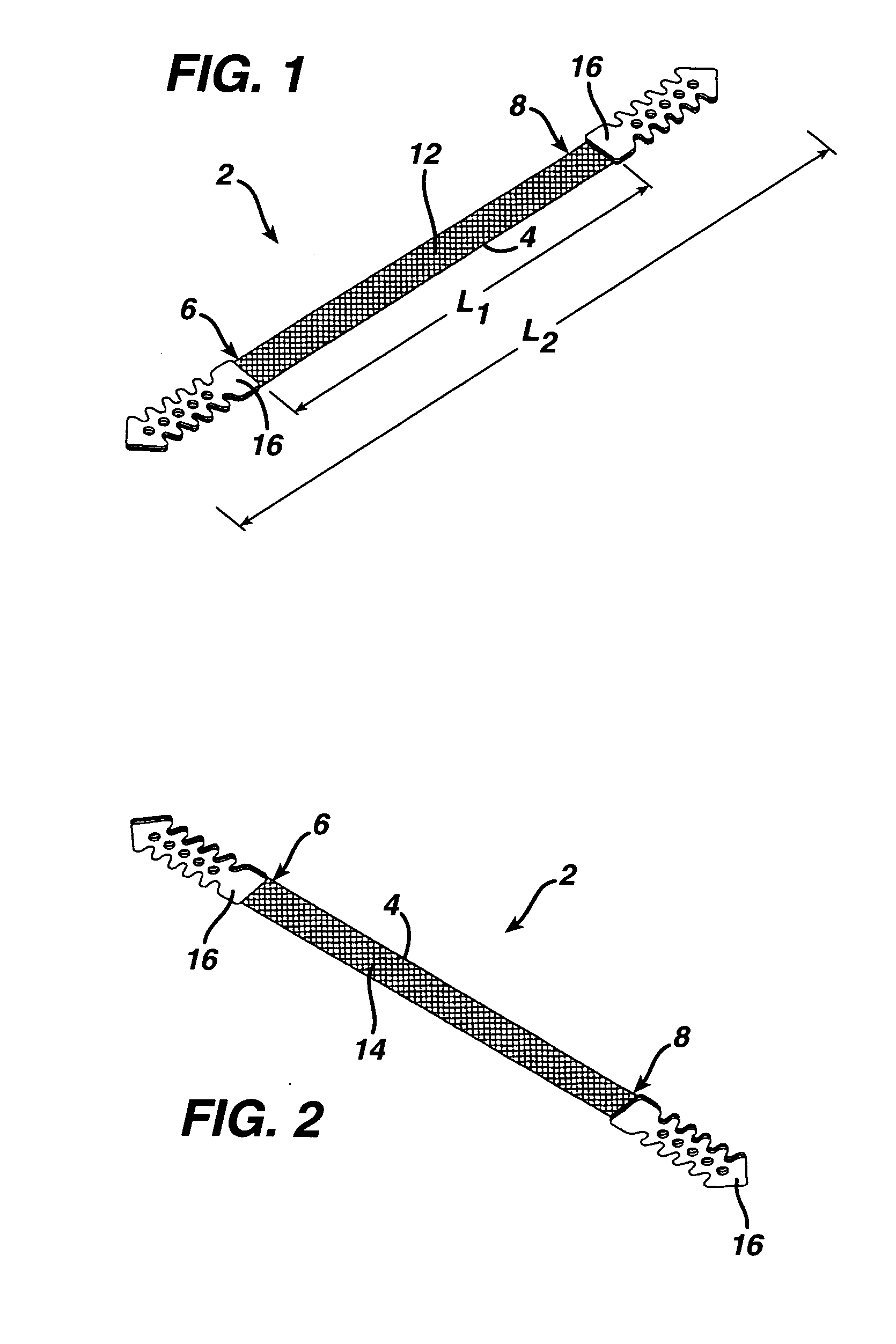
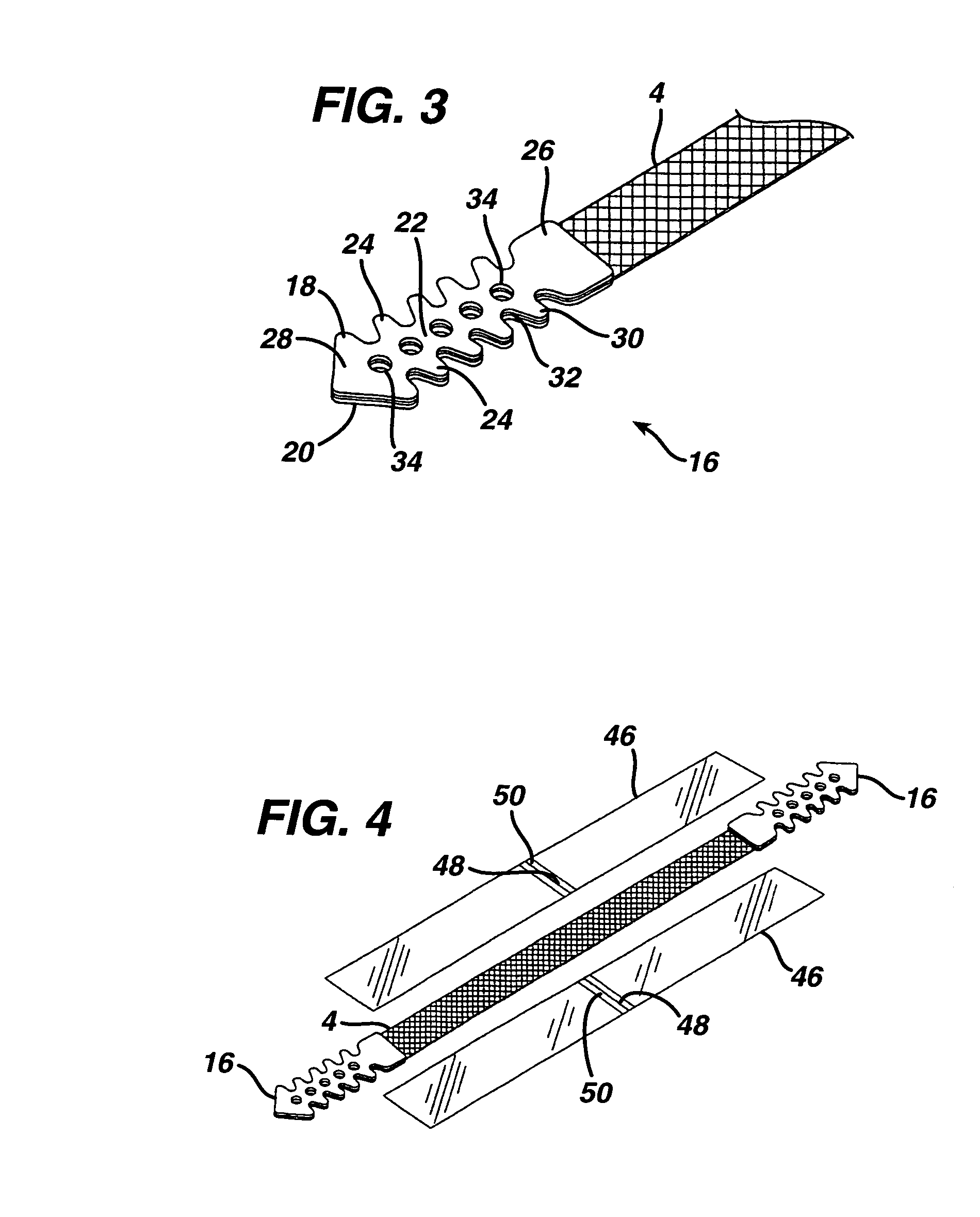
A medical implant and method for implantation of the same. One embodiment is an implant for use in the treatment of stress urinary incontinence that includes an implantable, elongated tape having a multiplicity of openings formed through the thickness thereof, the tape having a first end region and a second end region longitudinally opposite the first end region, and first and second bio-compatible fixation elements attached to the first and second end regions of the tape respectively. Each bio-compatible fixation element has a tissue adherence property greater than that of the tape.
Owner:ETHICON INC
Incontinence treatment device
InactiveUS7387603B2Function increaseAvoid flowElectrotherapyAnti-incontinence devicesUrethraStress incontinence
A device and method for treatment of urinary stress incontinence. At least one electrode is implanted in a pelvic muscle of a patient. A control unit receives signals indicative of abdominal stress in the patient and responsive thereto applies an electrical waveform to the electrode which stimulates the muscle to contract, so as to inhibit involuntary urine flow through the patient's urethra due to the stress.
Owner:ASTORA WOMENS HEALTH
Minimally invasive medical implant and insertion device and method for using the same
ActiveUS20060025649A1Anti-incontinence devicesSurgical needlesStress incontinenceBiomedical engineering
A medical implant and method for implantation of the same. One embodiment is an implant for use in the treatment of stress urinary incontinence that includes an implantable, elongated tape having a multiplicity of openings formed through the thickness thereof, the tape having a first end region and a second end region longitudinally opposite the first end region, and first and second bio-compatible fixation elements attached to the first and second end regions of the tape respectively. Each bio-compatible fixation element has a tissue adherence property greater than that of the tape.
Owner:ETHICON INC
Control of urge incontinence
InactiveUS7582053B2Function increasePrevent incontinenceElectrotherapyAnti-incontinence devicesFecal incontinenceStress incontinence
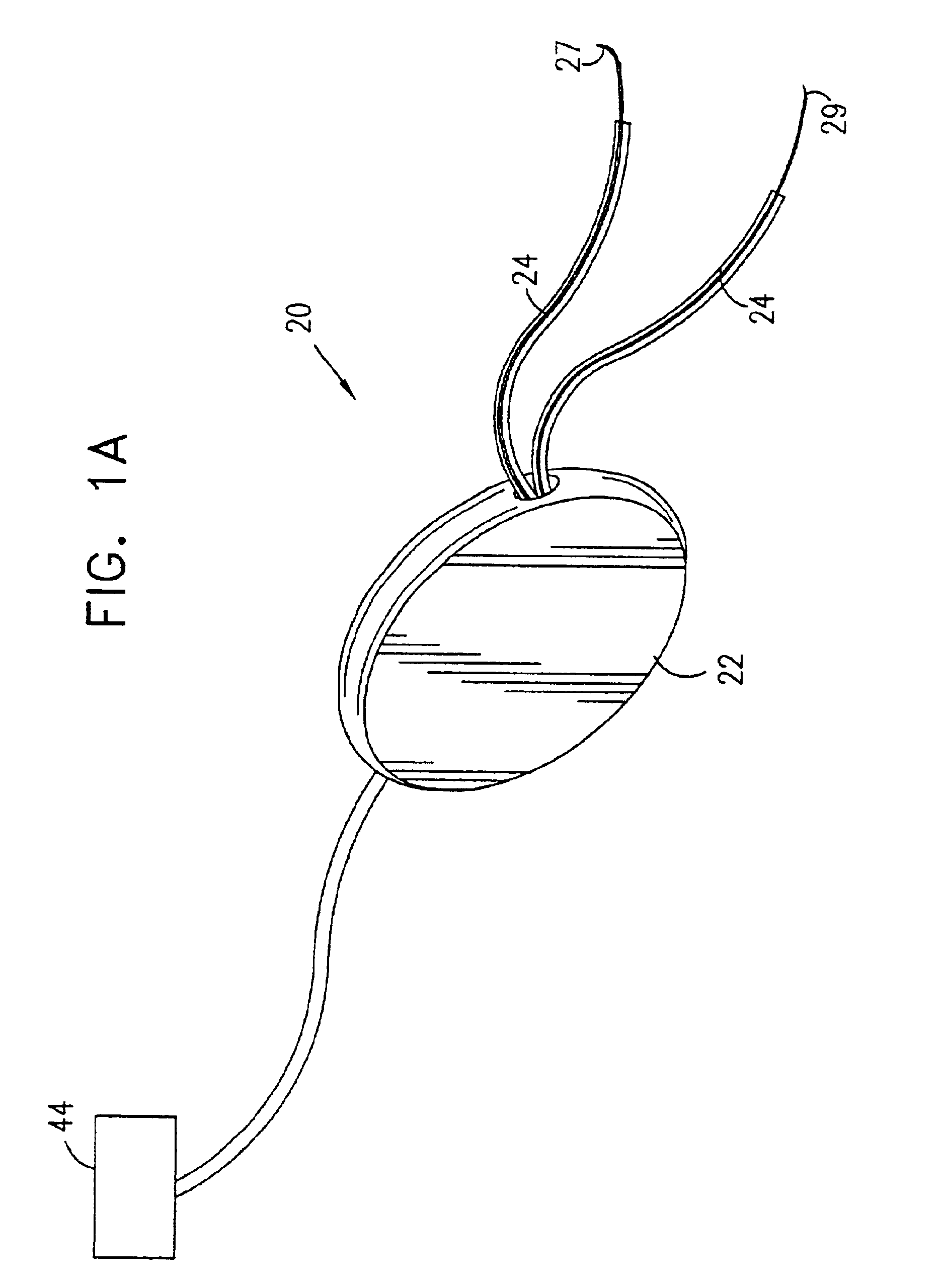
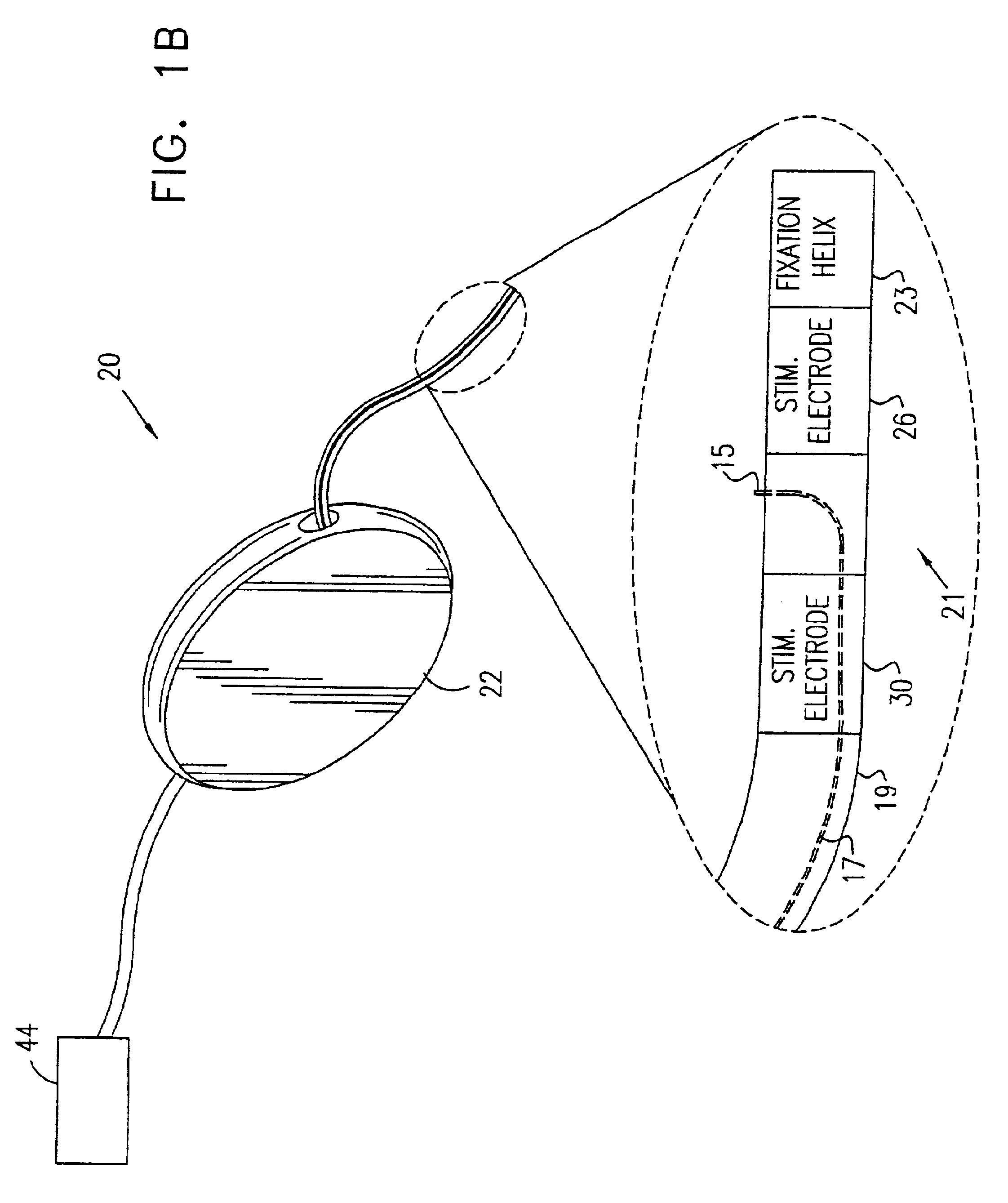
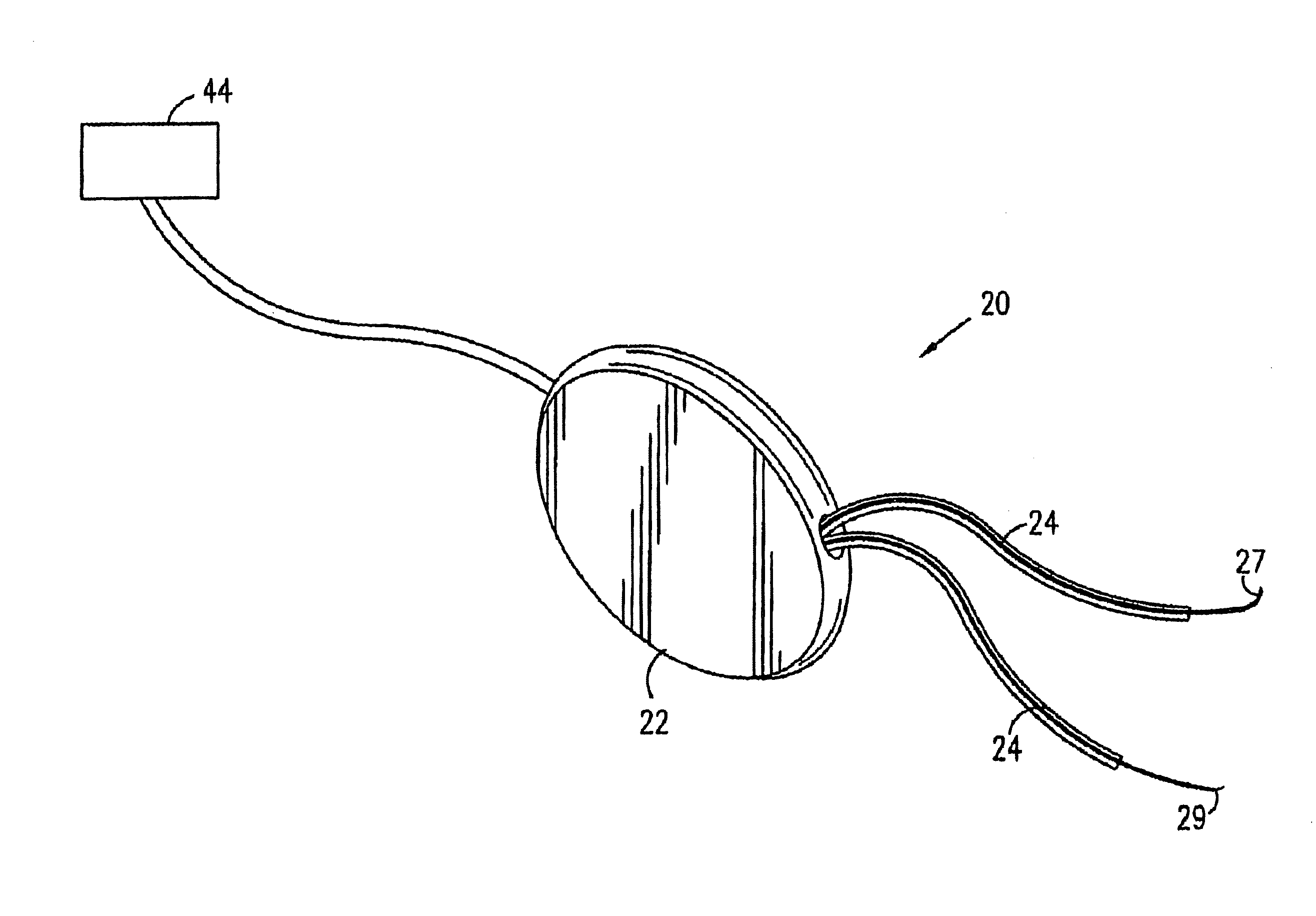
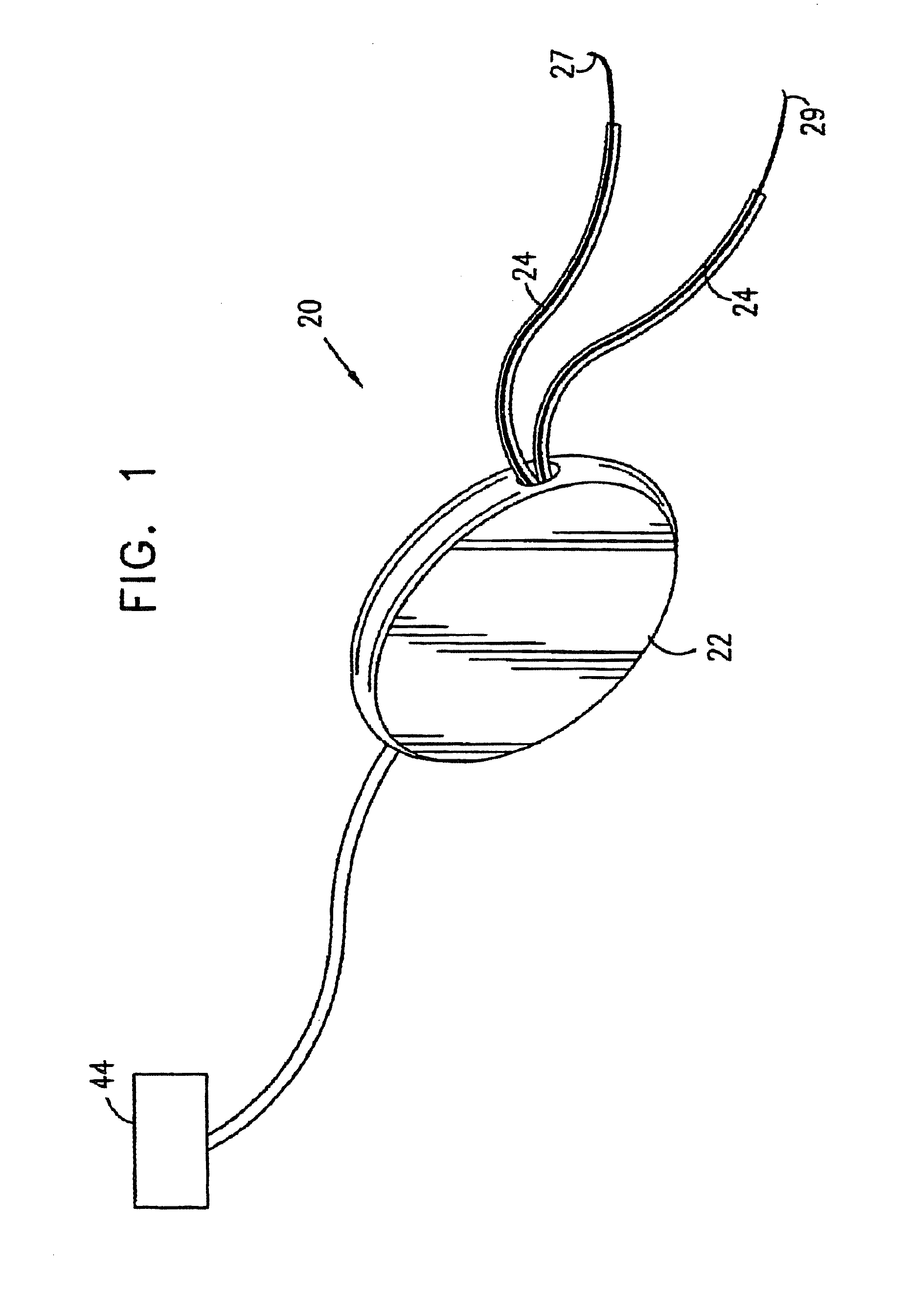
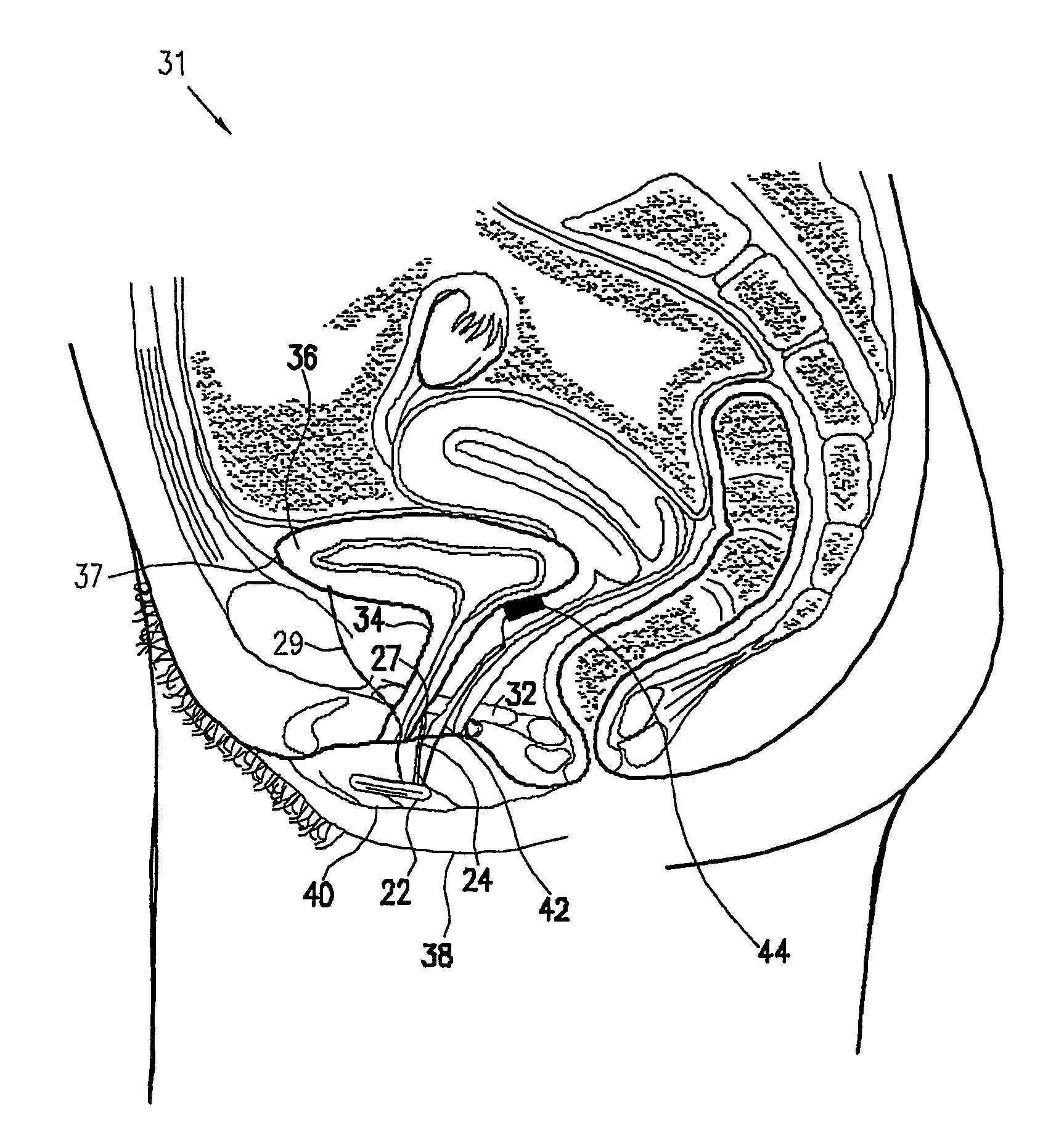
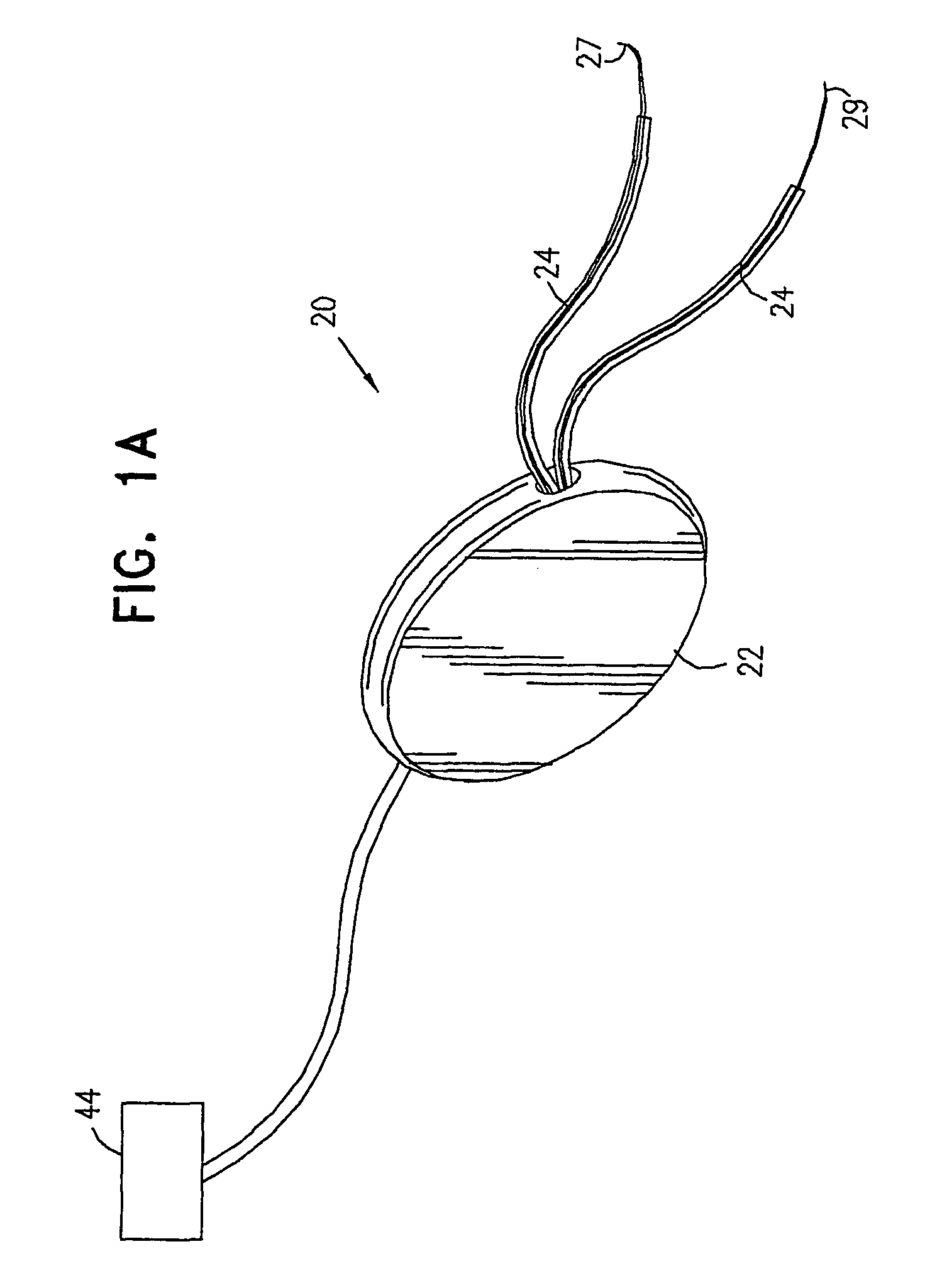
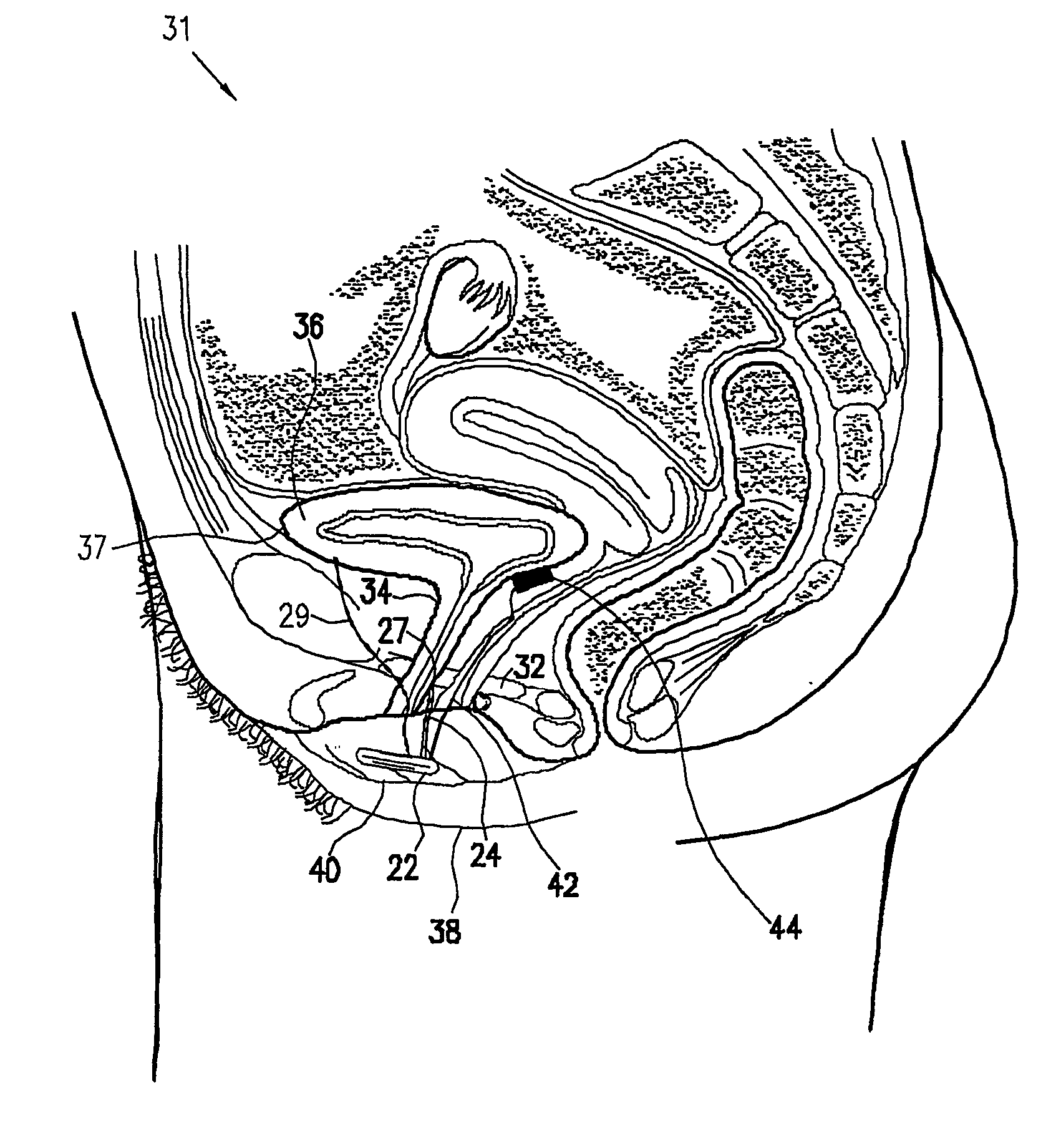
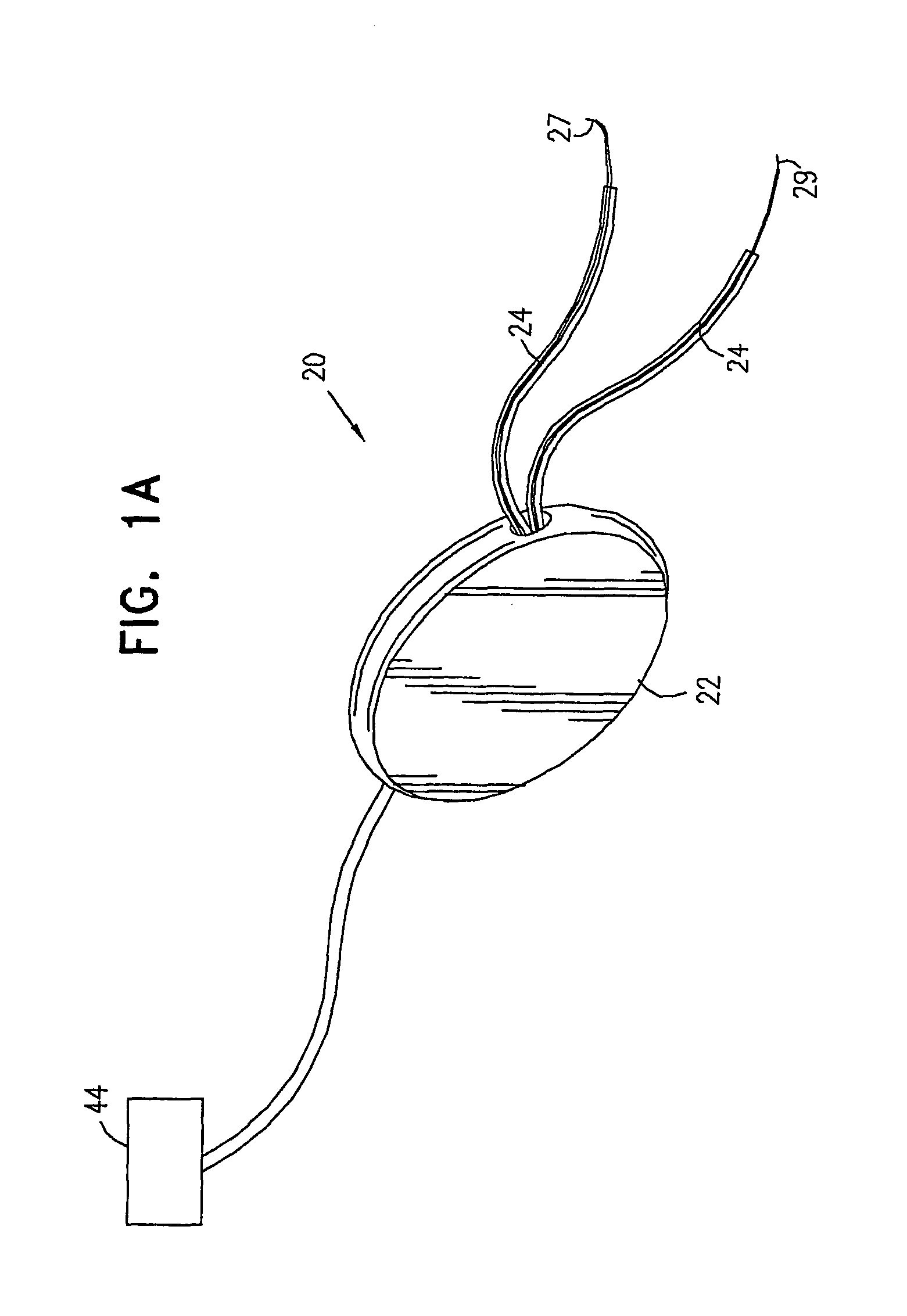
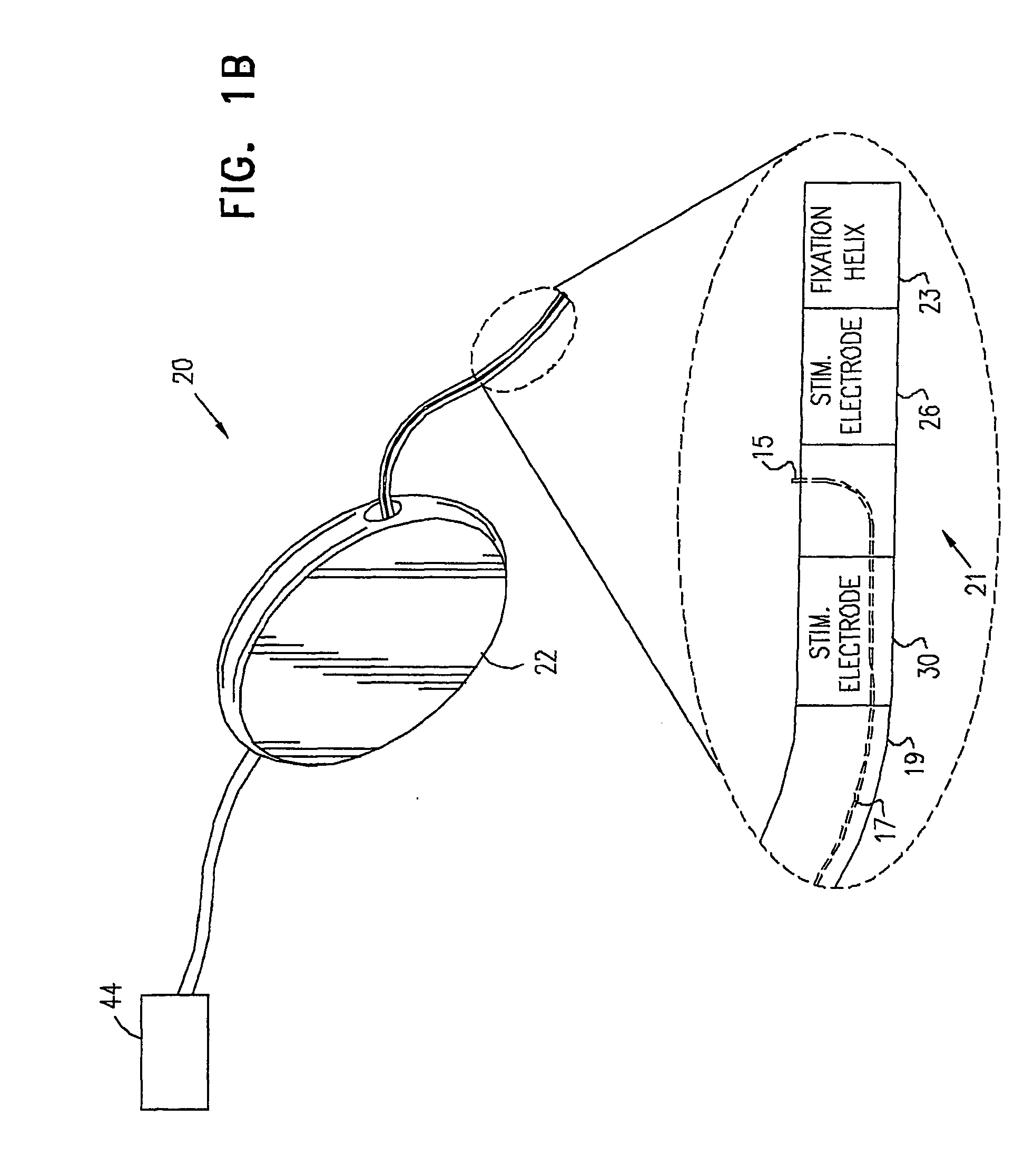
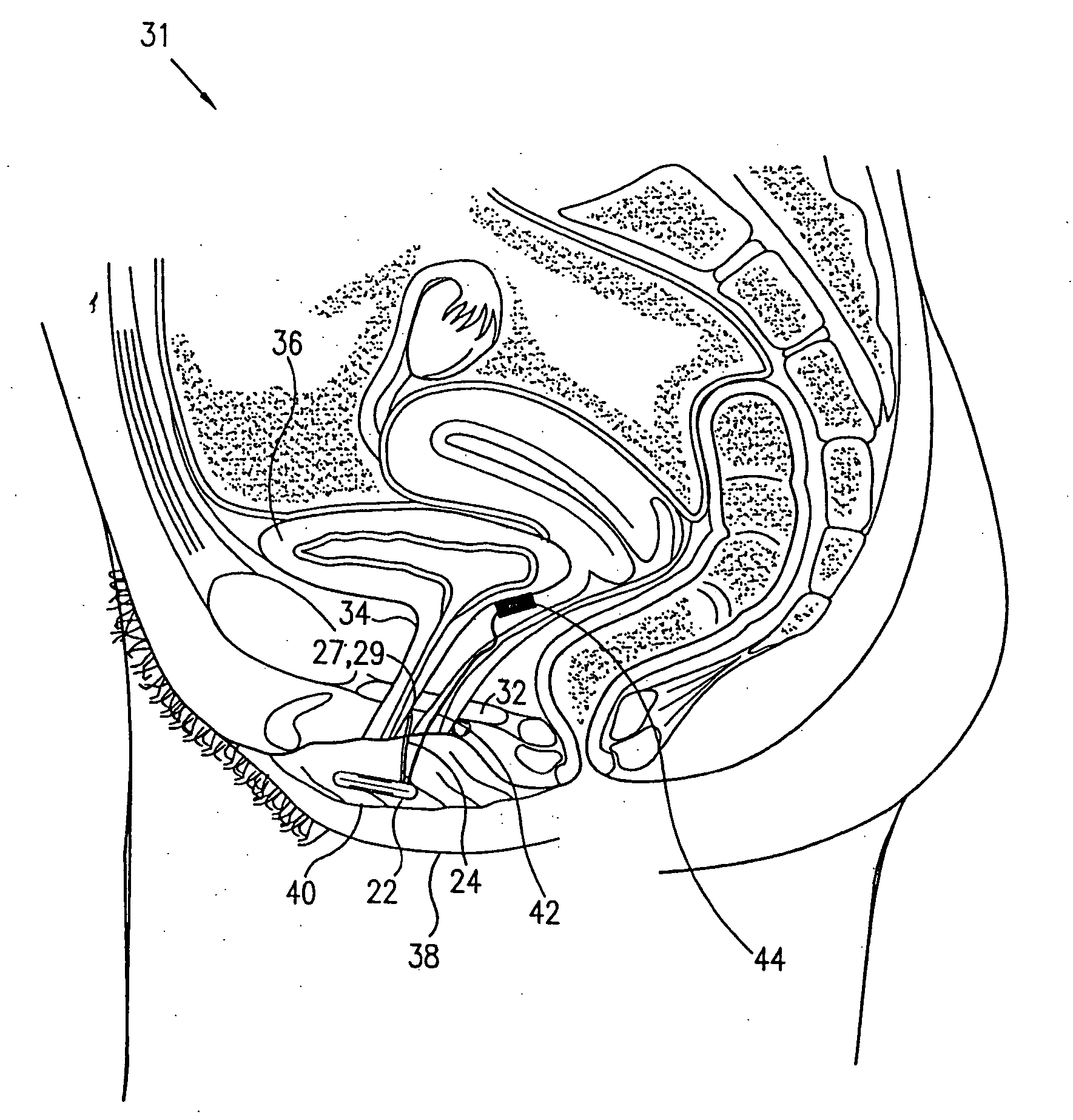
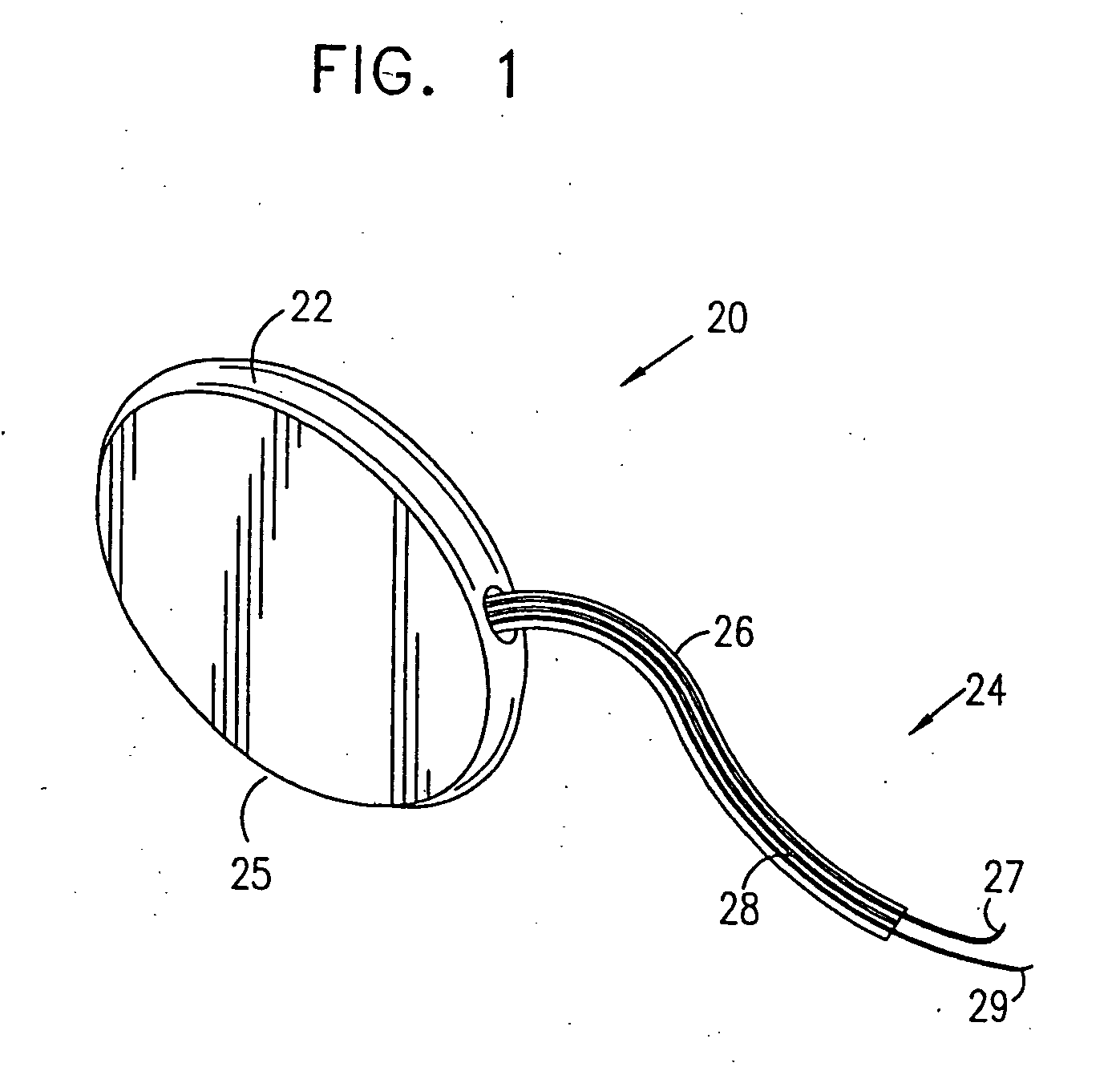
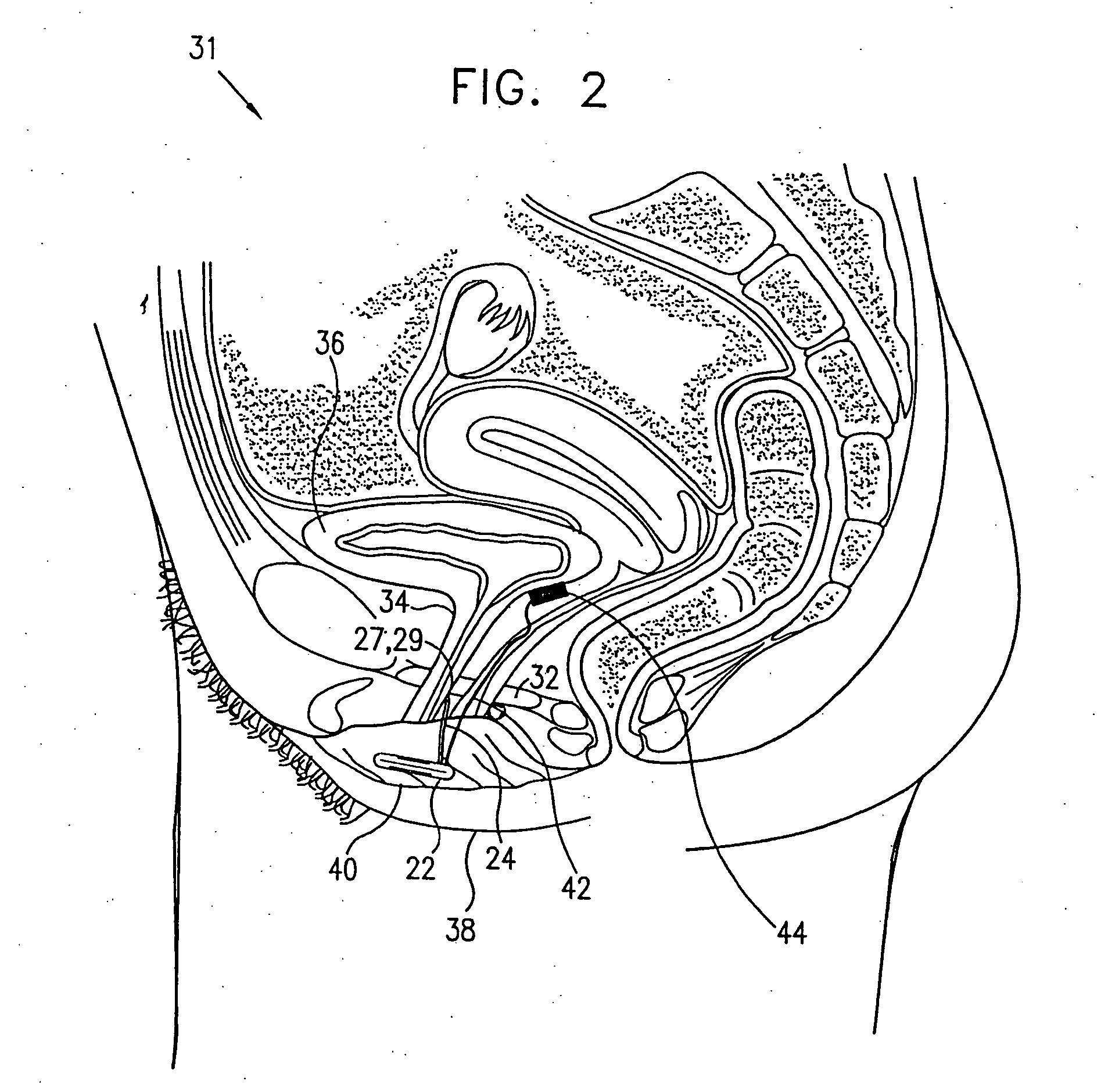
A device (20) for treatment of a patient's urinary incontinence, including a sensor, (44), which generates a signal responsive to a physiological characteristic indicative of a likelihood of incontinence. A control unit (22) receives the signal from the sensor. At least one electrode (29) is preferably implanted in the patient. The electrode is coupled to cause contraction of a pelvic muscle of the patient responsive to application of electrical energy to the electrode. Responsive to the signal, the control unit applies an electrical waveform to the electrode, so as to inhibit the incontinence.
Owner:MEDTRONIC INC +1
Pelvic disorder treatment device
ActiveUS7613516B2Relieving pelvic painReliable controlUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
Owner:MEDTRONIC INC +1
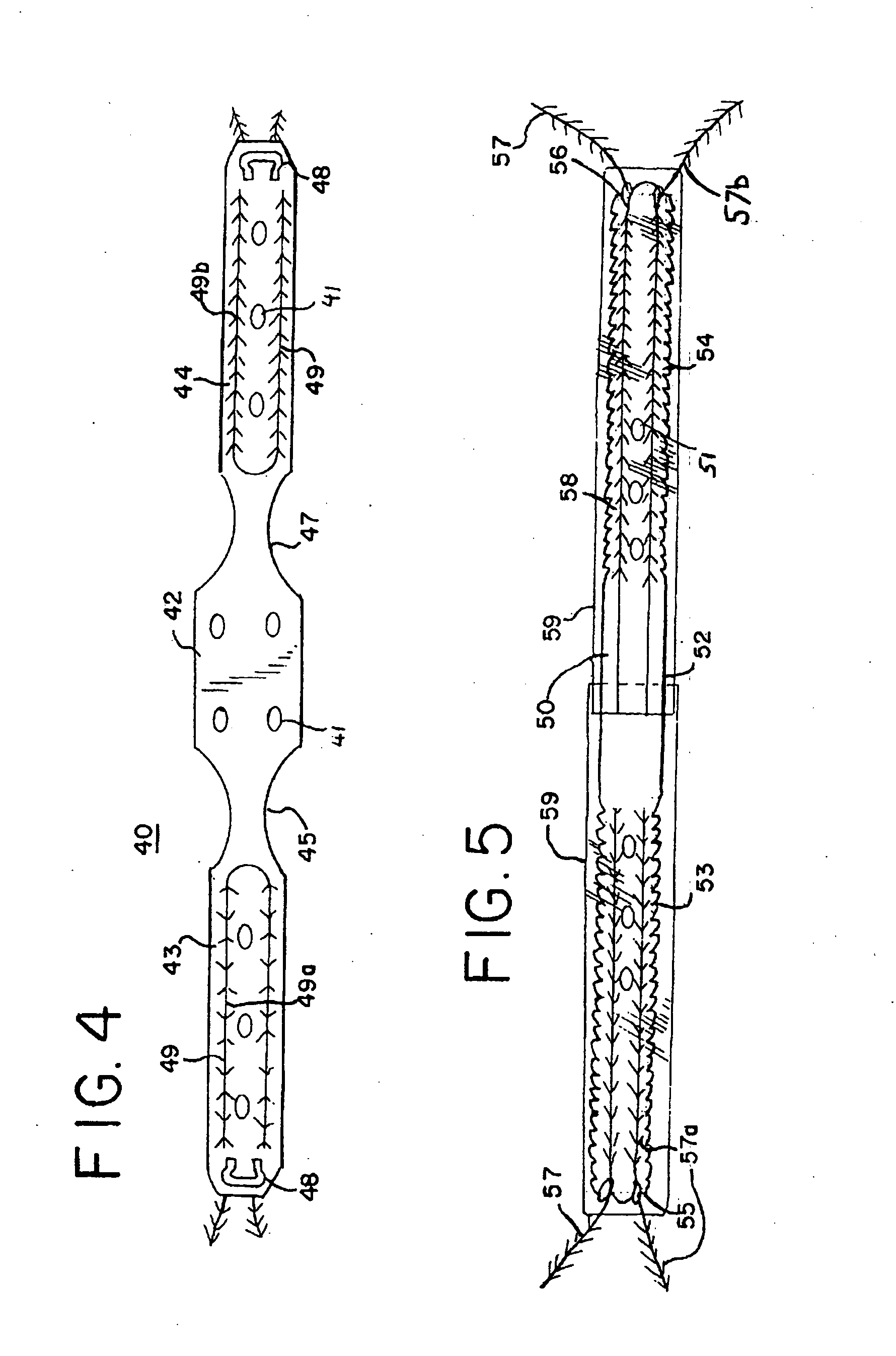
Method and articles for treatment of stress urinary incontinence
ActiveUS20100010631A1Minimal complicationMinimal painAnti-incontinence devicesMedical devicesUrethraPelvic diaphragm muscle
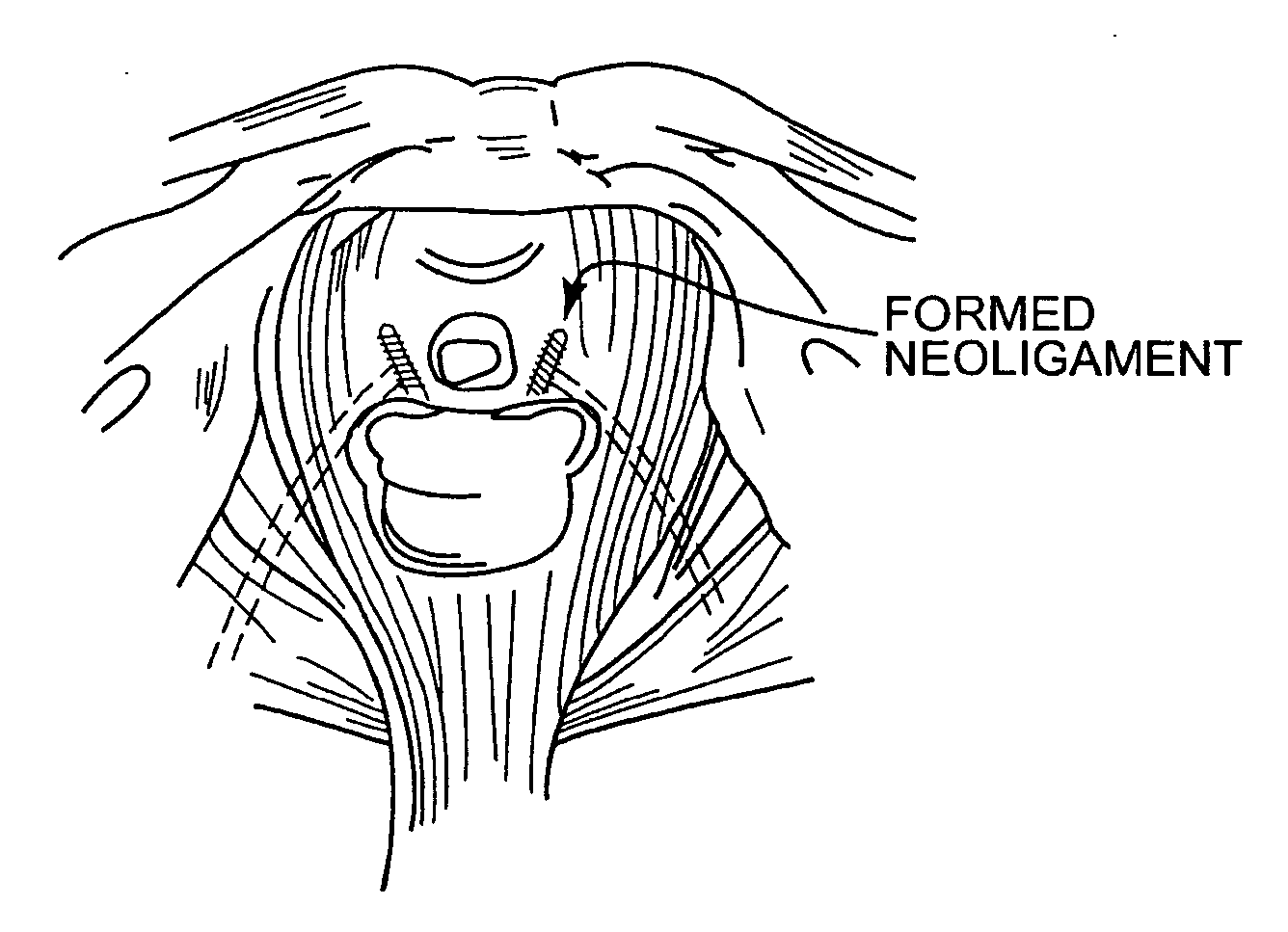
Improved methods and apparatuses for treatment of incontinence or pelvic organ prolapse are provided. A neo ligament for increasing the supportive function of the tissues supporting the urethra, bladder neck, or rectum is disclosed. Methods of using such a neoligament to treat incontinence are disclosed. Additionally, a self tensioning elastic tissue bolster for support of the tissues that support the urethra, rectum, and bladder neck are disclosed. A method of treating incontinence by injecting proliferative agents into supportive structures is disclosed. Methods and apparatus for transvaginal and transperitieai pelvic floor bolster treatment of incontinence and bladder pillar systems are also disclosed.
Owner:BOSTON SCI SCIMED INC
Drug Delivery Methods and Devices for Treating Stress Urinary Incontinence
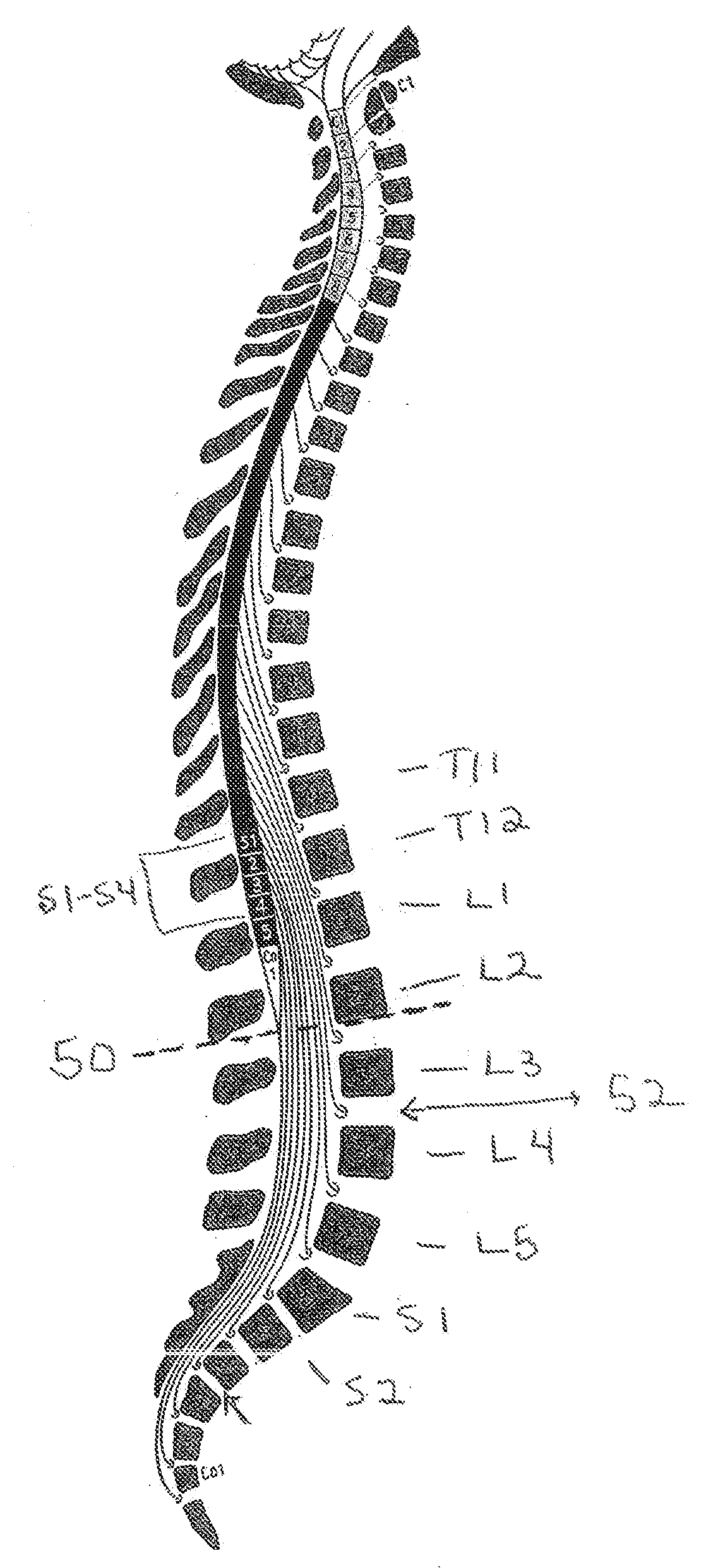
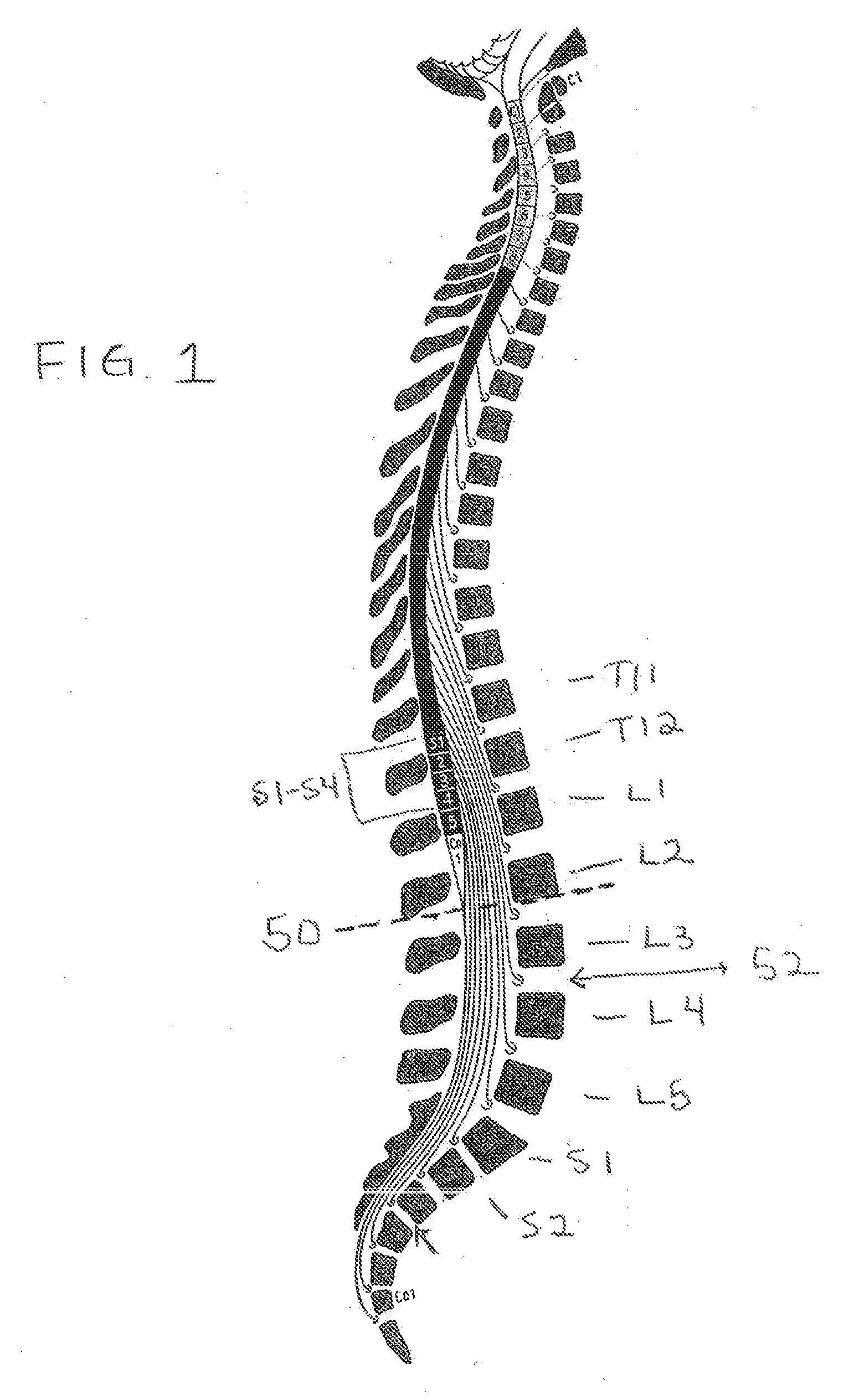
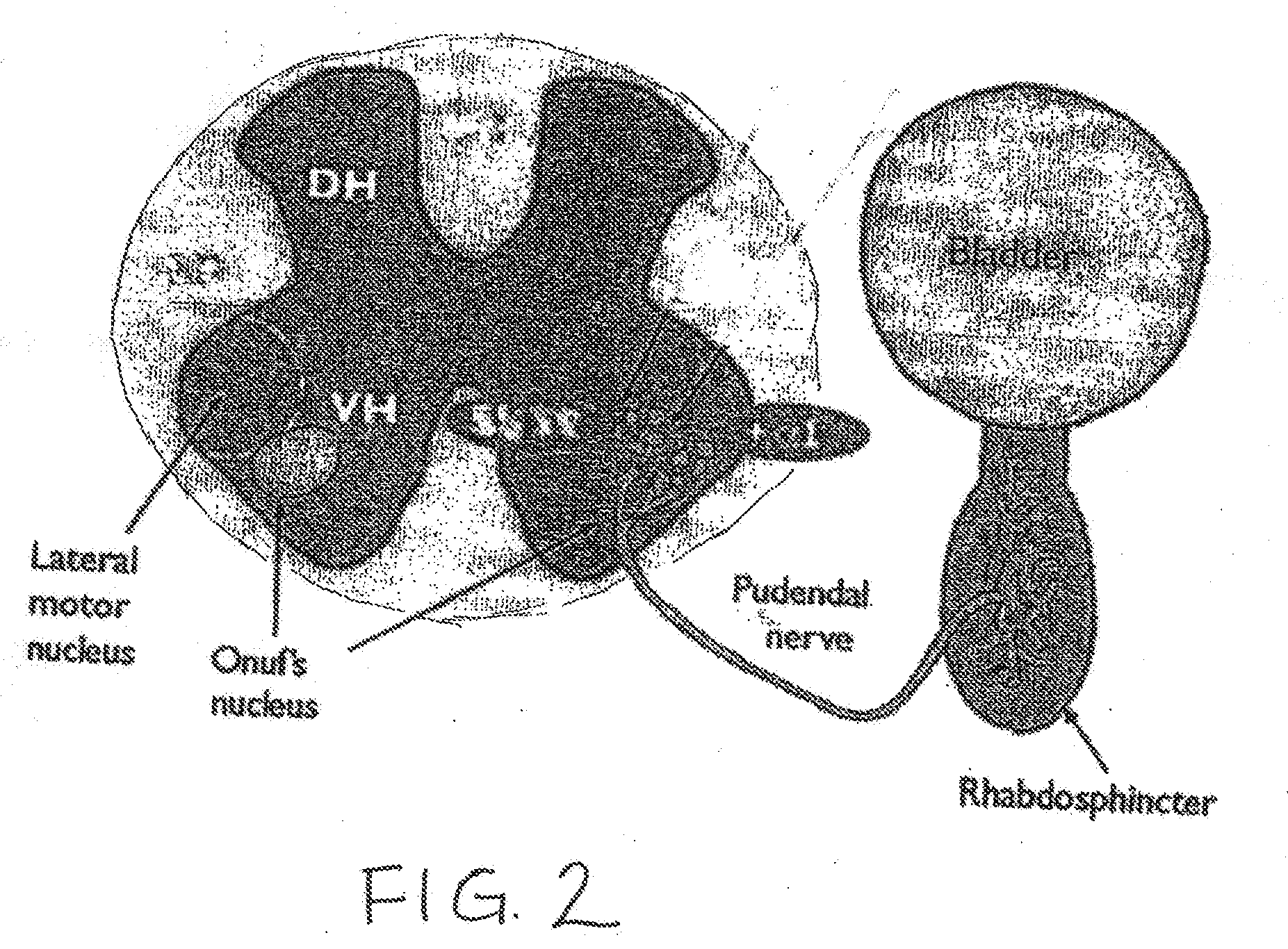
InactiveUS20070253995A1Chronic pelvic painBiocidePharmaceutical delivery mechanismStress incontinenceOnuf's nucleus
The disclosure describes a method and system for delivering a drug to the Onuf's nucleus of a patient for the treatment or prevention of stress urinary incontinence. The system includes drug delivery devices that deliver one or more drugs to a site located adjacent to, around or within the Onuf's nucleus stress incontinence alleviation. A depot configured to release a therapeutically effective amount of a stress incontinence-reducing drug is one drug delivery device disclosed. Other drug delivery devices include implantable infusion pumps.
Owner:MEDTRONIC INC
Apparatus and method for the treatment of stress urinary incontinence
An apparatus and method for the treatment of stress urinary incontinence. The apparatus includes a suburethral sling having an adjustment member for adjusting the tension of the sling both during the procedure and post-procedure. The method includes using a needle to simultaneously implant the sling and to deliver a local anesthetic in the groin area while implanting the sling.
Owner:BOSTON SCI SCIMED INC
Methods and devices for the treatment of urinary incontinence
InactiveUS7527633B2Reduce riskEasy accessSuture equipmentsSurgical needlesBlunt dissectionStress incontinence
Methods and devices for treating female stress urinary incontinence are disclosed. The methods include transvaginally accessing the pelvic cavity and introducing a suburethral sling into the retropubic space. In some embodiments the ends of the sling are attached to an anatomical support structure. In other embodiments, the ends of the suburethral sling are not attached to an anatomical support structure. The devices include a surgical instrument for blunt dissection of the pelvic cavity which includes a curved shaft and a blunt distal end. A hook deployment device may optionally be attached to the surgical instrument.
Owner:BOSTON SCI SCIMED INC
Tension free pelvic floor repair
A kit and method for supporting a pelvic floor of a person with stress urinary incontinence or other symptoms. The method involves using barbed sutures as part of a sling for supporting the pelvic floor or urethra of a person experiencing stress urinary incontinence or other symptoms. A surgeon implants the sling along with the barbed sutures, using one of several known methods. The barbed sutures may be interwoven with the sling and the supports for the sling, so that once the sling and its supports are placed into the patient, barbs of the sutures will resist movement. The sutures may also be woven into the supports to resist movement.
Owner:COOK MEDICAL TECH LLC
Pelvic disorder treatment device
ActiveUS20050216069A1Reduce inconvenienceMinimize discomfortUltrasonic/sonic/infrasonic diagnosticsElectrotherapyFecal incontinenceInterstitial cystitis
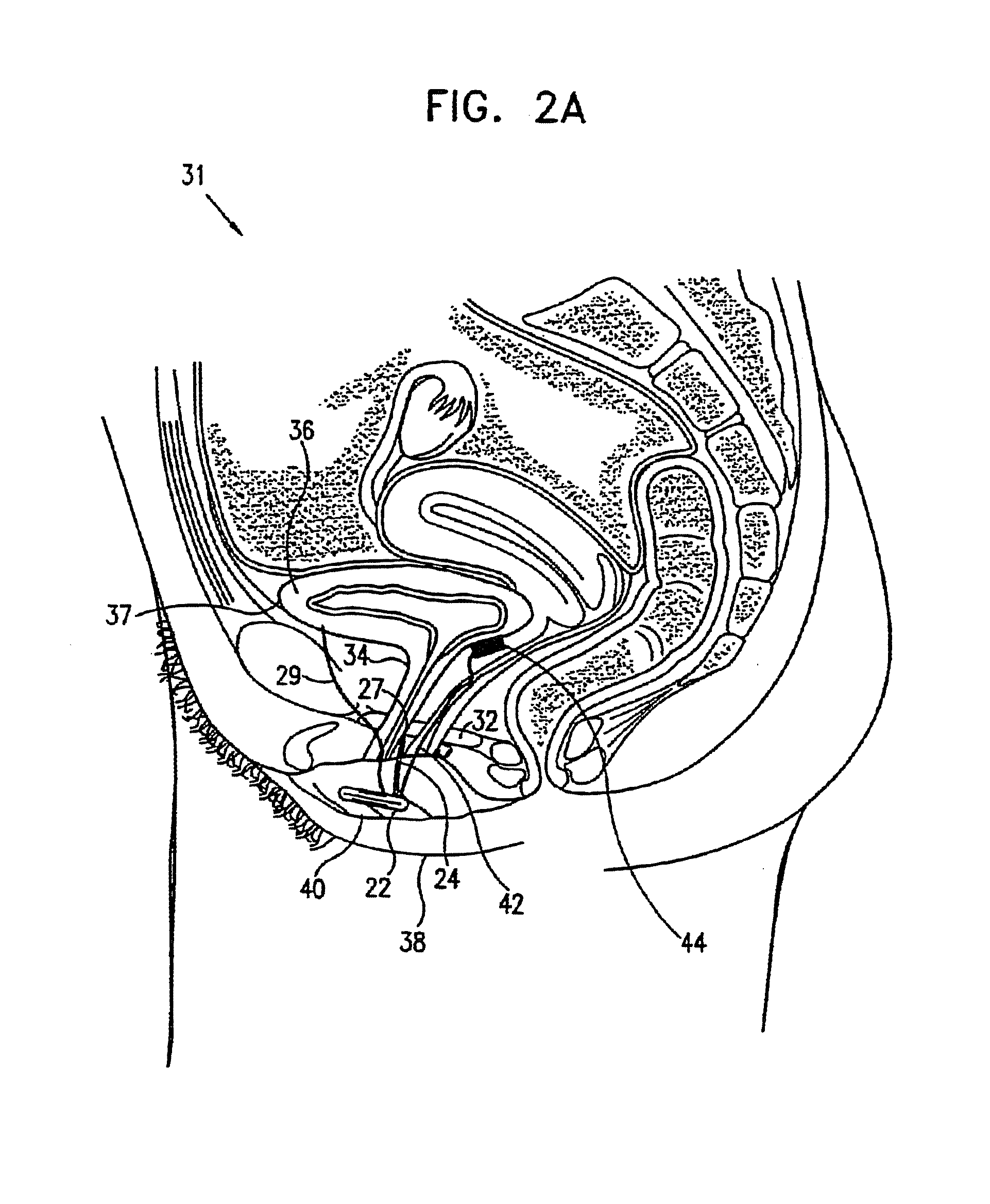
A device (20) for treating a medical condition is provided, and a surgical procedure for implanting the device is disclosed. The device (20) includes a sensor (44), which is adapted to generate a signal responsive to a state of a patient, and at least one electrode (27), which is adapted to be coupled to a pelvic site of the patient. A control unit (22) is adapted to receive the signal, to analyze the signal so as to distinguish between an imminent stress incontinence event and an imminent urge event, and, responsive to analyzing the signal, to apply an electrical waveform to the at least one electrode (27). In various configurations, the device (20) may be used alternatively or additionally to treat fecal incontinence, interstitial cystitis, chronic pelvic pain, or urine retention.
Owner:MEDTRONIC INC +1
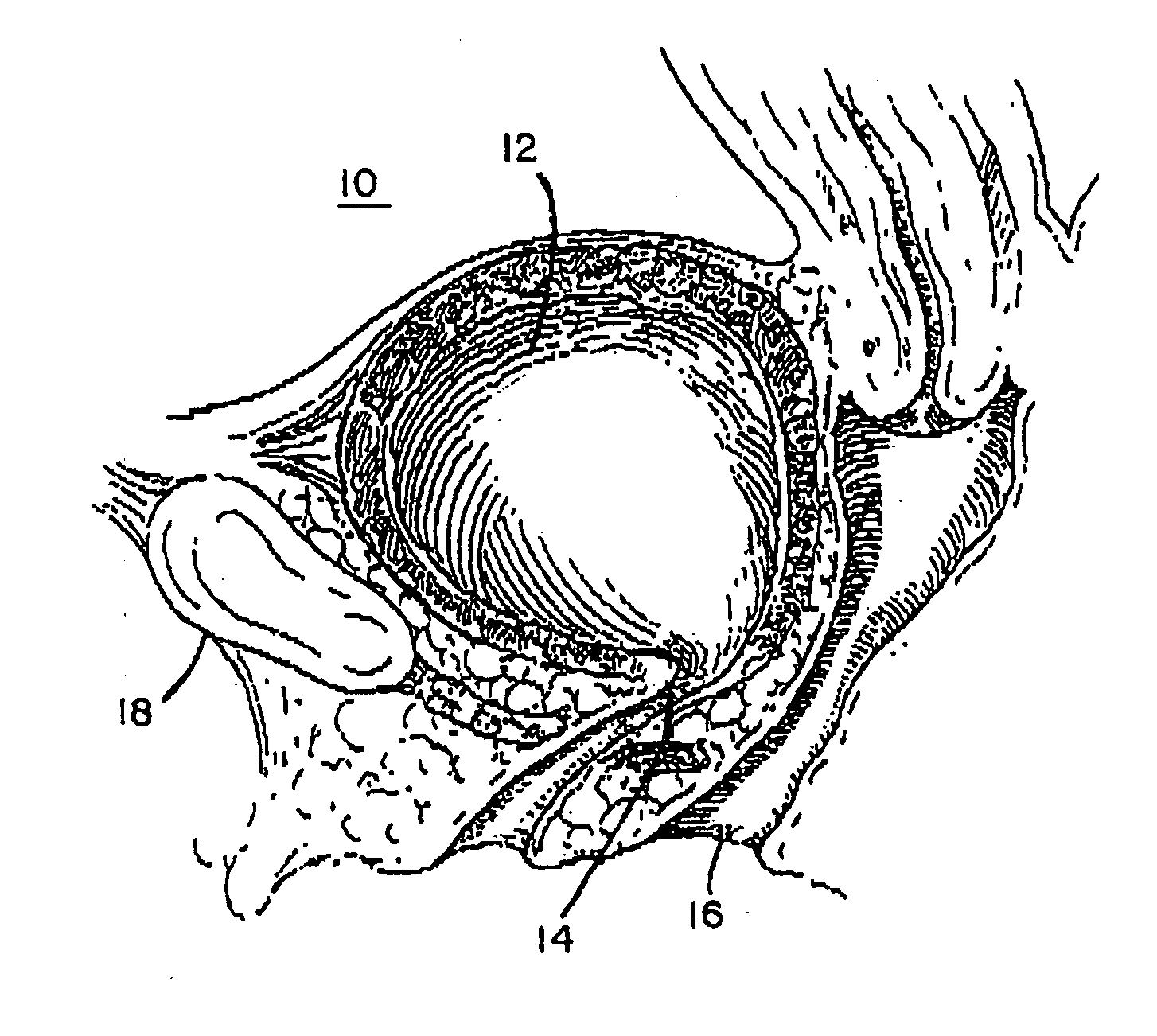
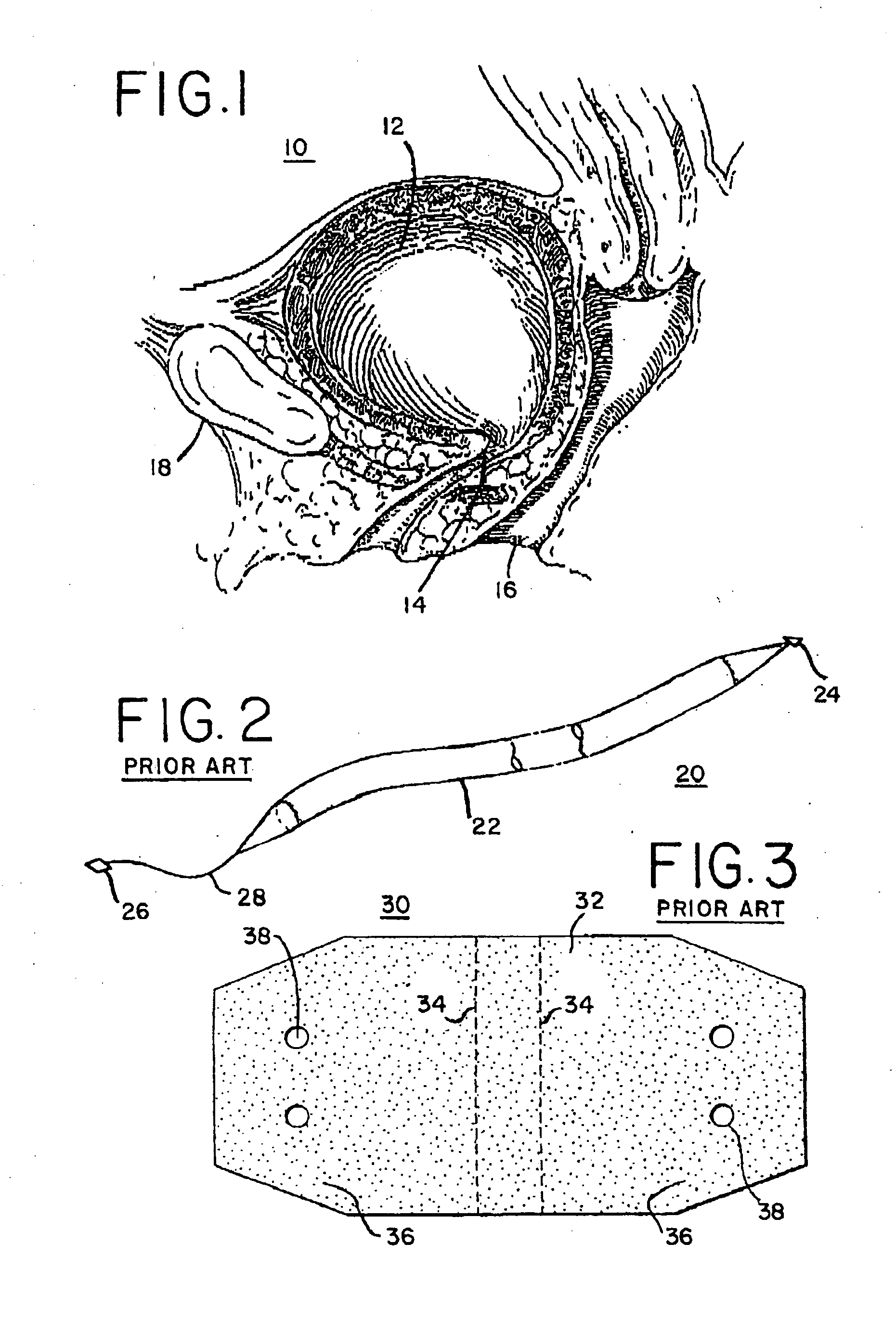
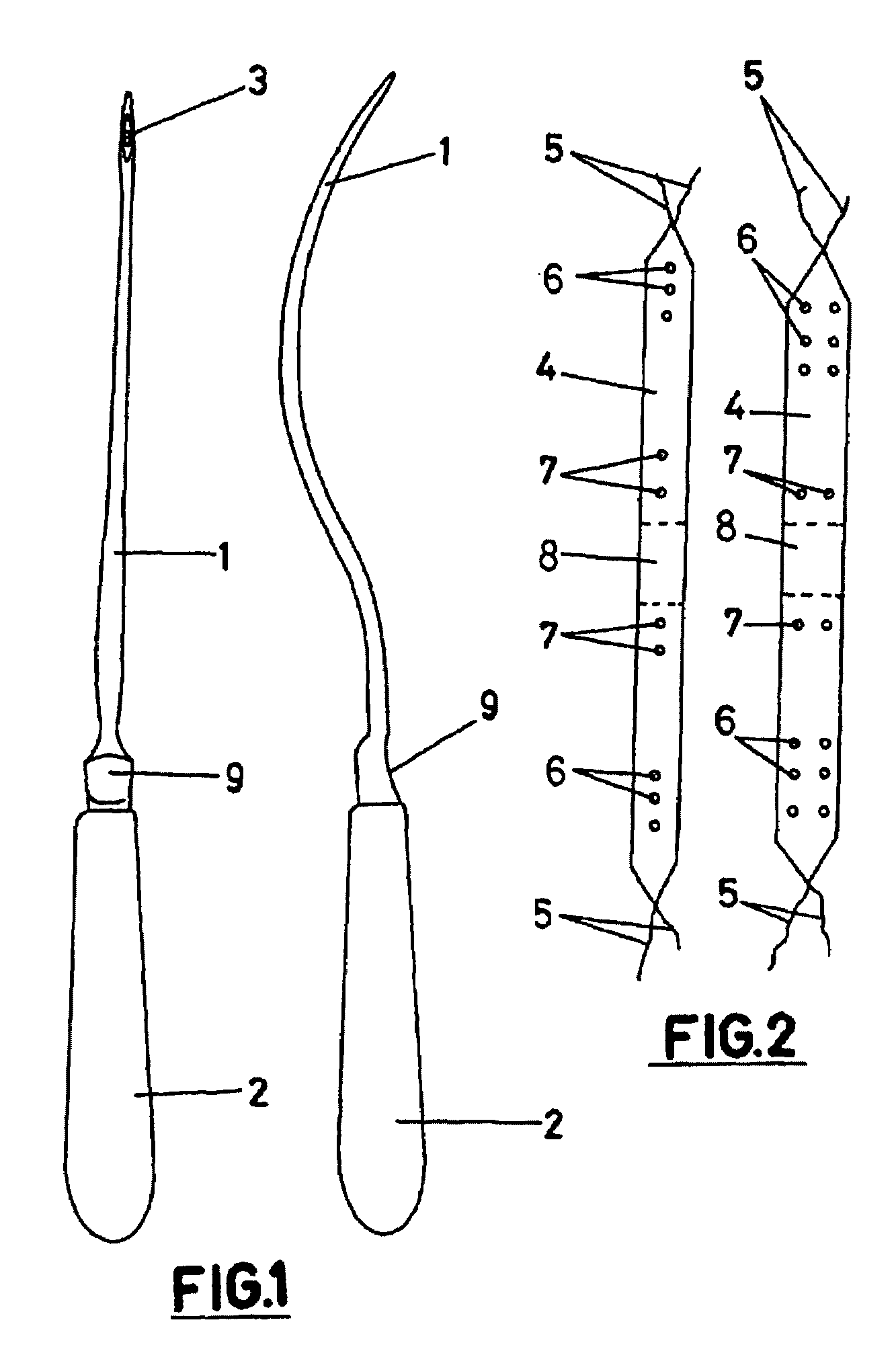
Implantable prosthesis for correcting urinary stress incontinence in women
A prosthesis for correcting urinary stress incontinence in women including right and left para-urethral hemi-prostheses, each of the hemi-prostheses formed of a biocompatible material and in the form of a strip, one end of the strip having a bulged portion and another end of which is adapted to be attached to the aponeurosis of the rectus muscle of the abdomen, and means for attaching the another end to the aponeurosis of the rectus muscle of the abdomen.
Owner:EUROPLAK
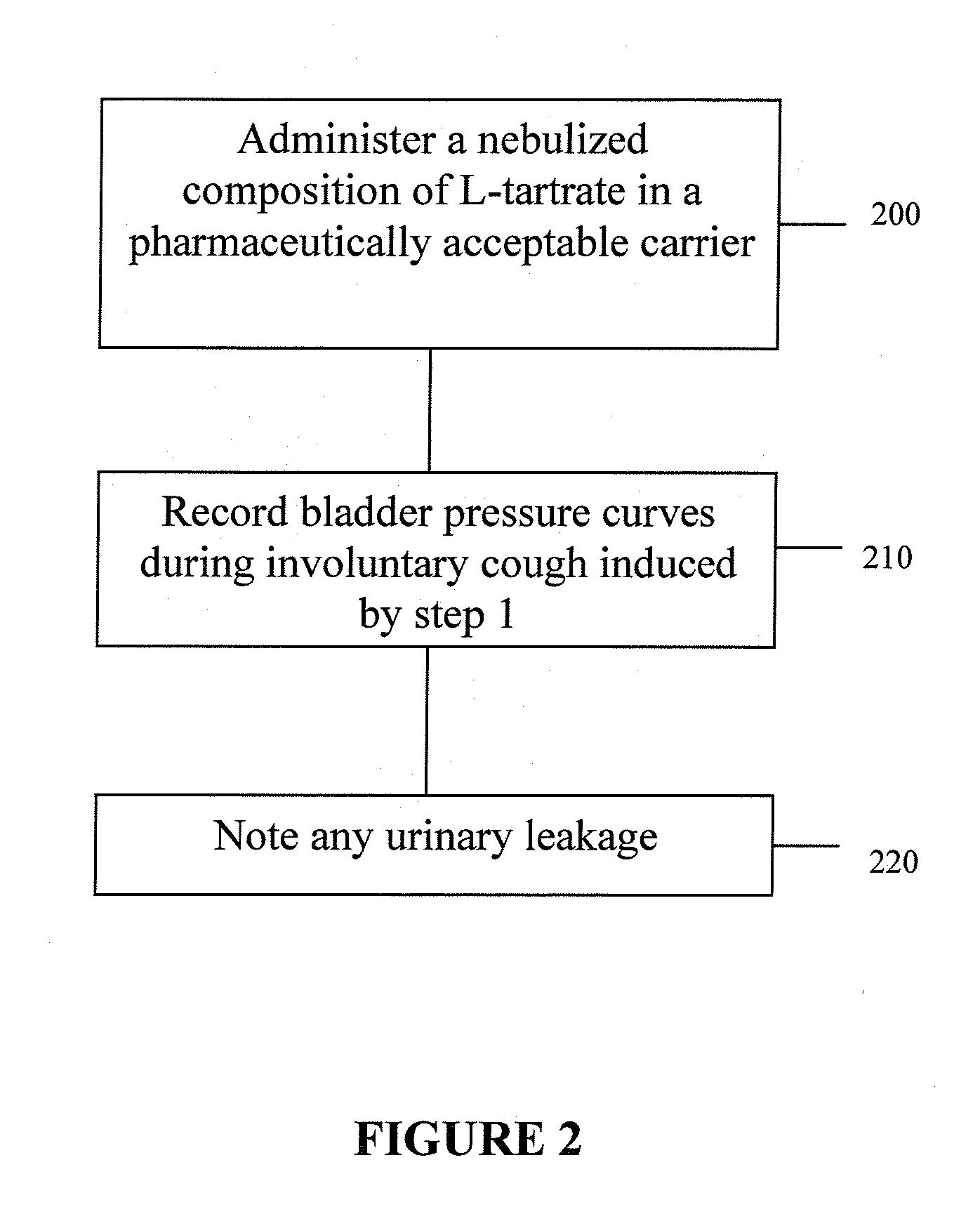
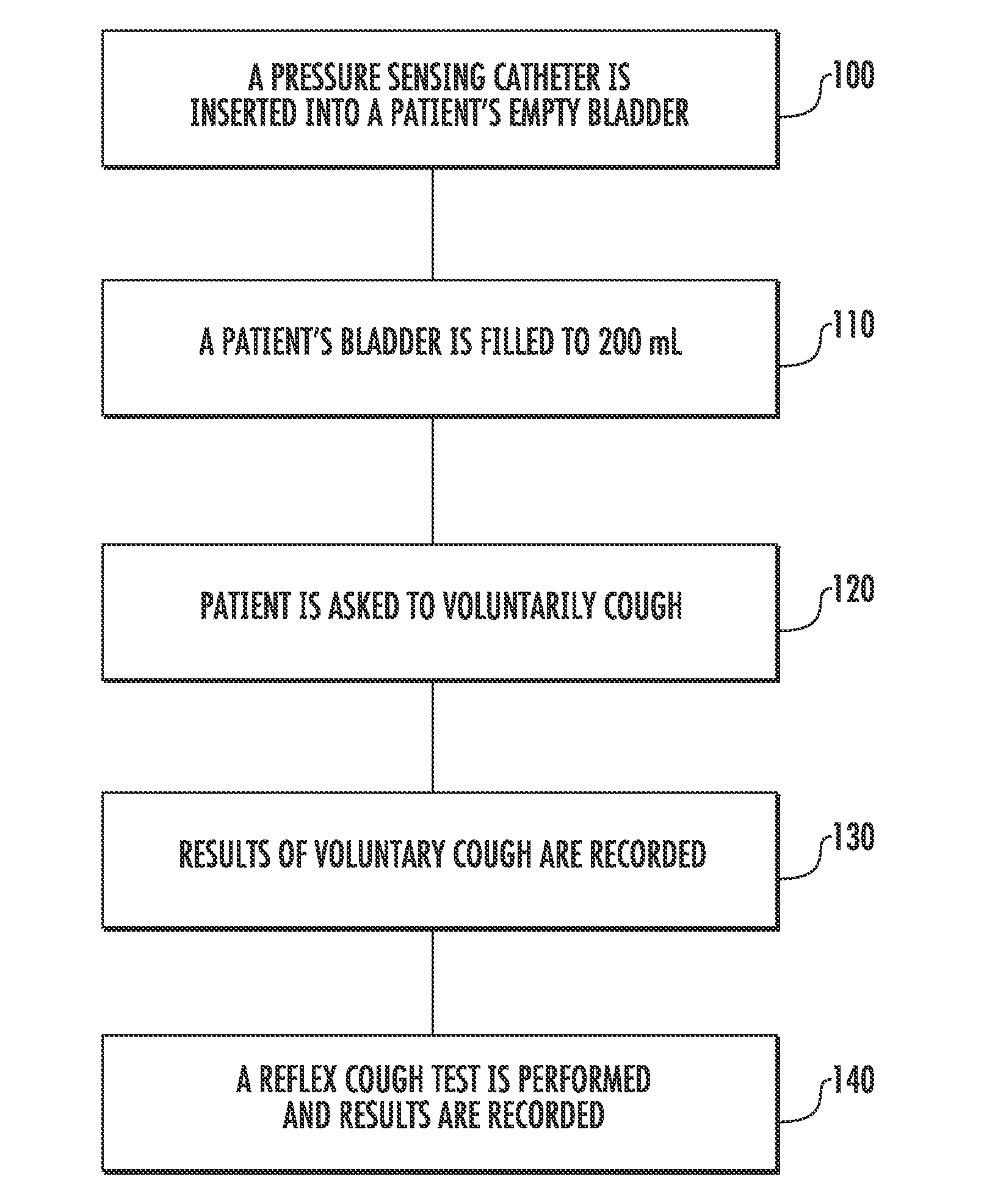
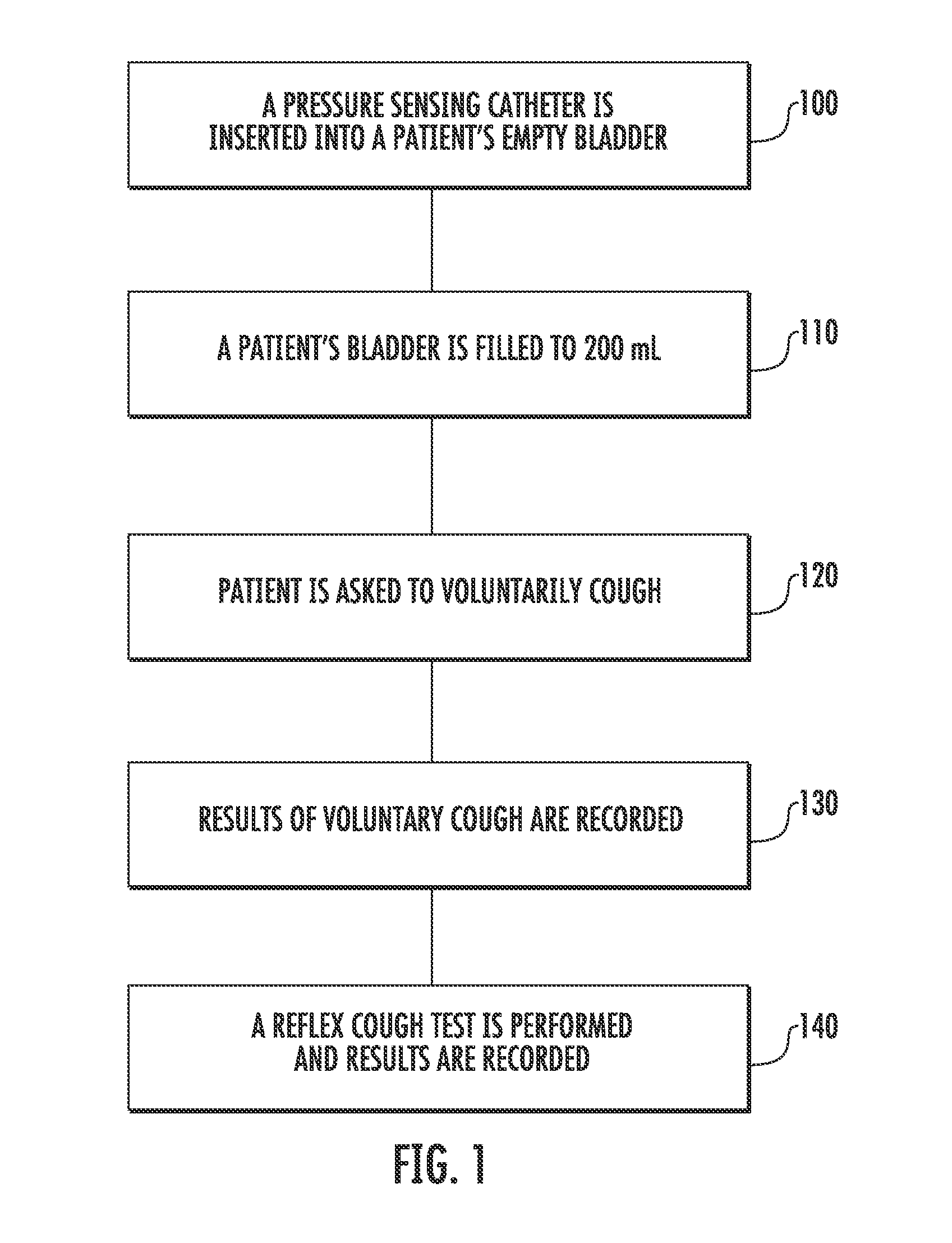
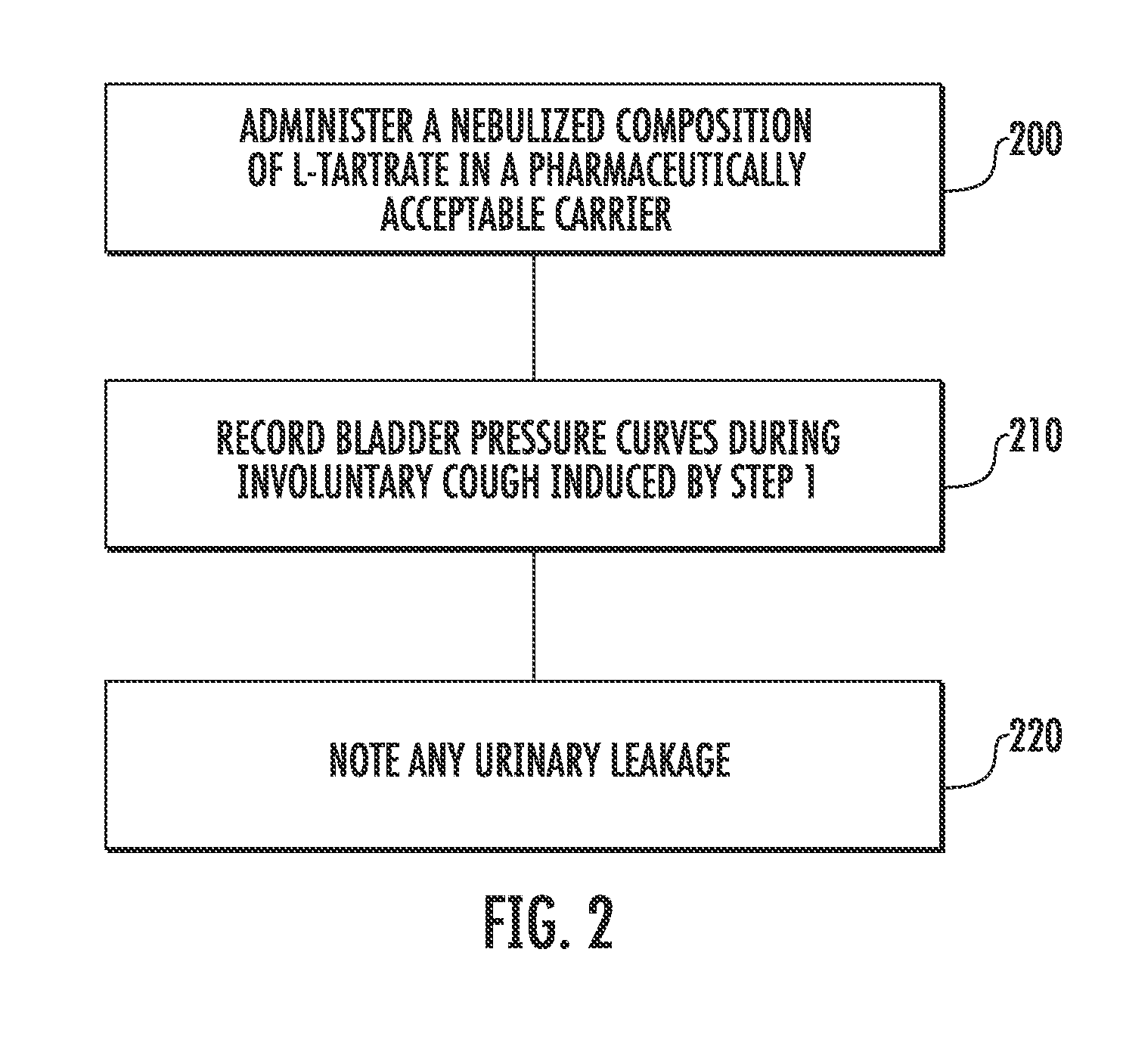
Techniques for Evaluating Urinary Stress Incontinence
ActiveUS20070255090A1Reliable and reliableOvercome problemsDiagnostic recording/measuringSensorsUrine leakagePressure sense
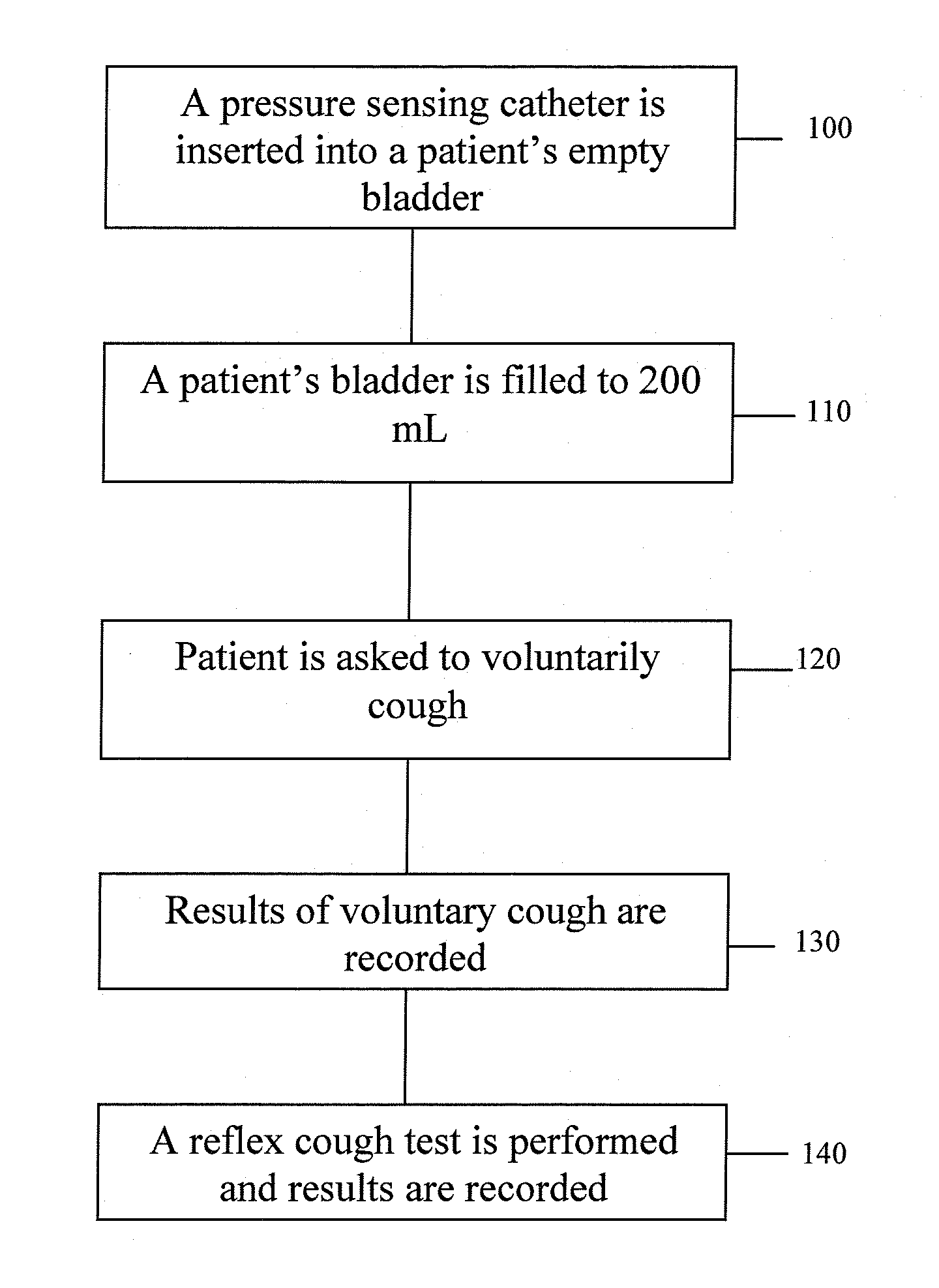
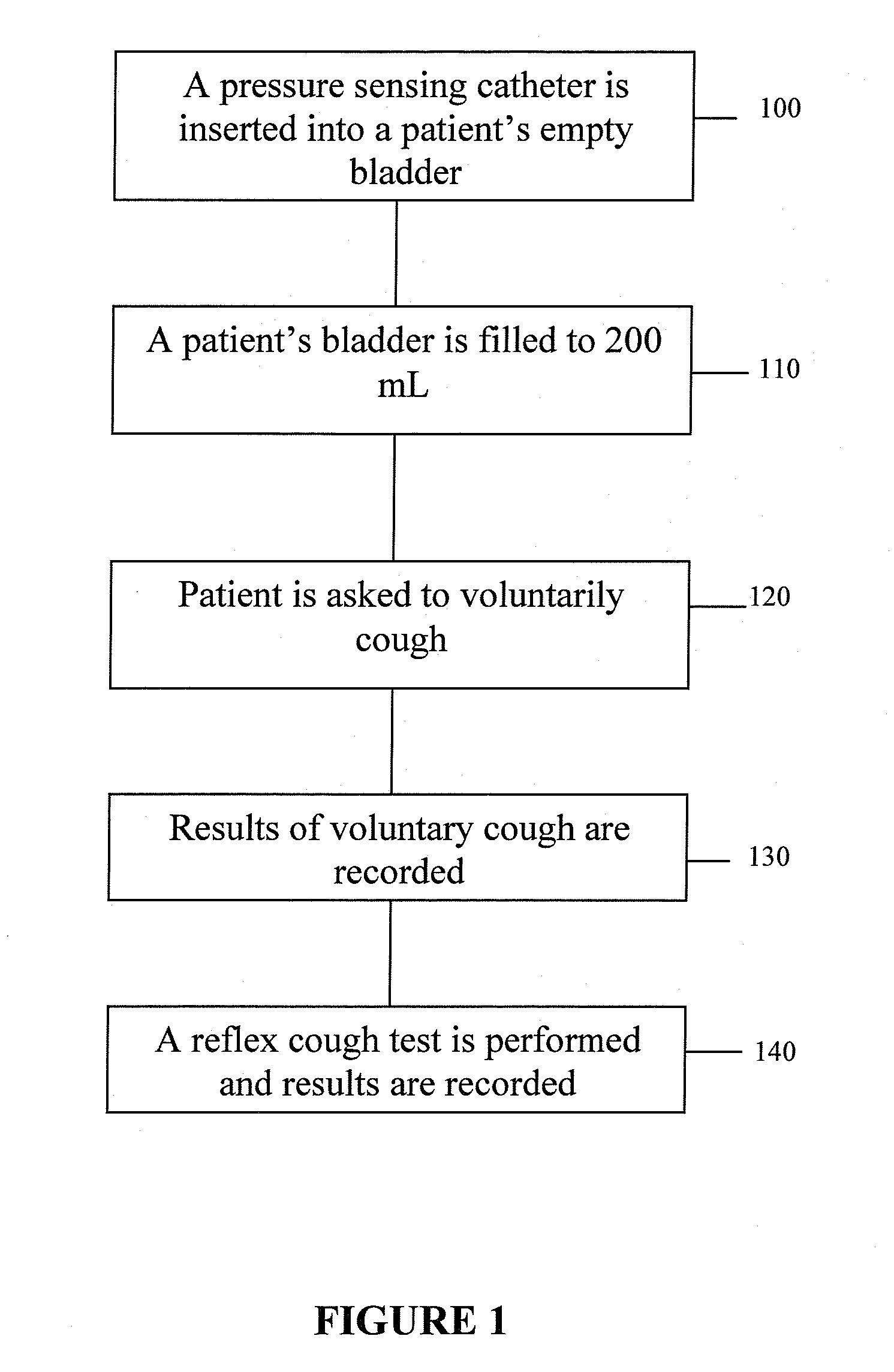
Techniques for detecting stress urinary incontinence use a pressure sensing catheter the electrical indications of which are applied to a processing unit for detecting pressure levels generated during involuntary coughs. The involuntary coughs are induced preferentially by using a nebulized composition of L-tartrate in a pharmaceutically acceptable carrier. The area under the curve generated from pressure samples is calculated and used in conjunction with the detection of urine leakage to determine the existence of stress urinary incontinence.
Owner:PNEUMOFLEX SYST
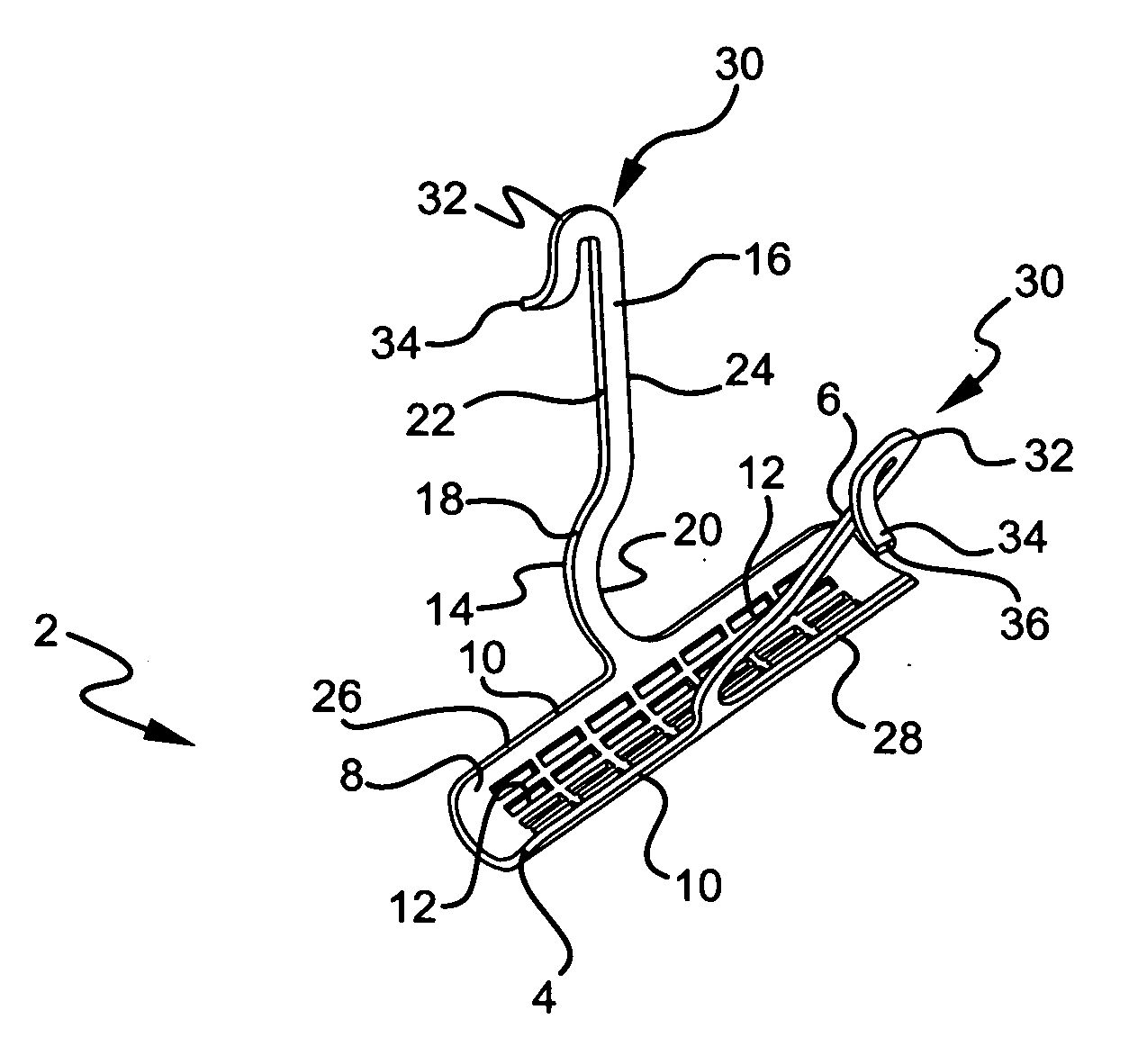
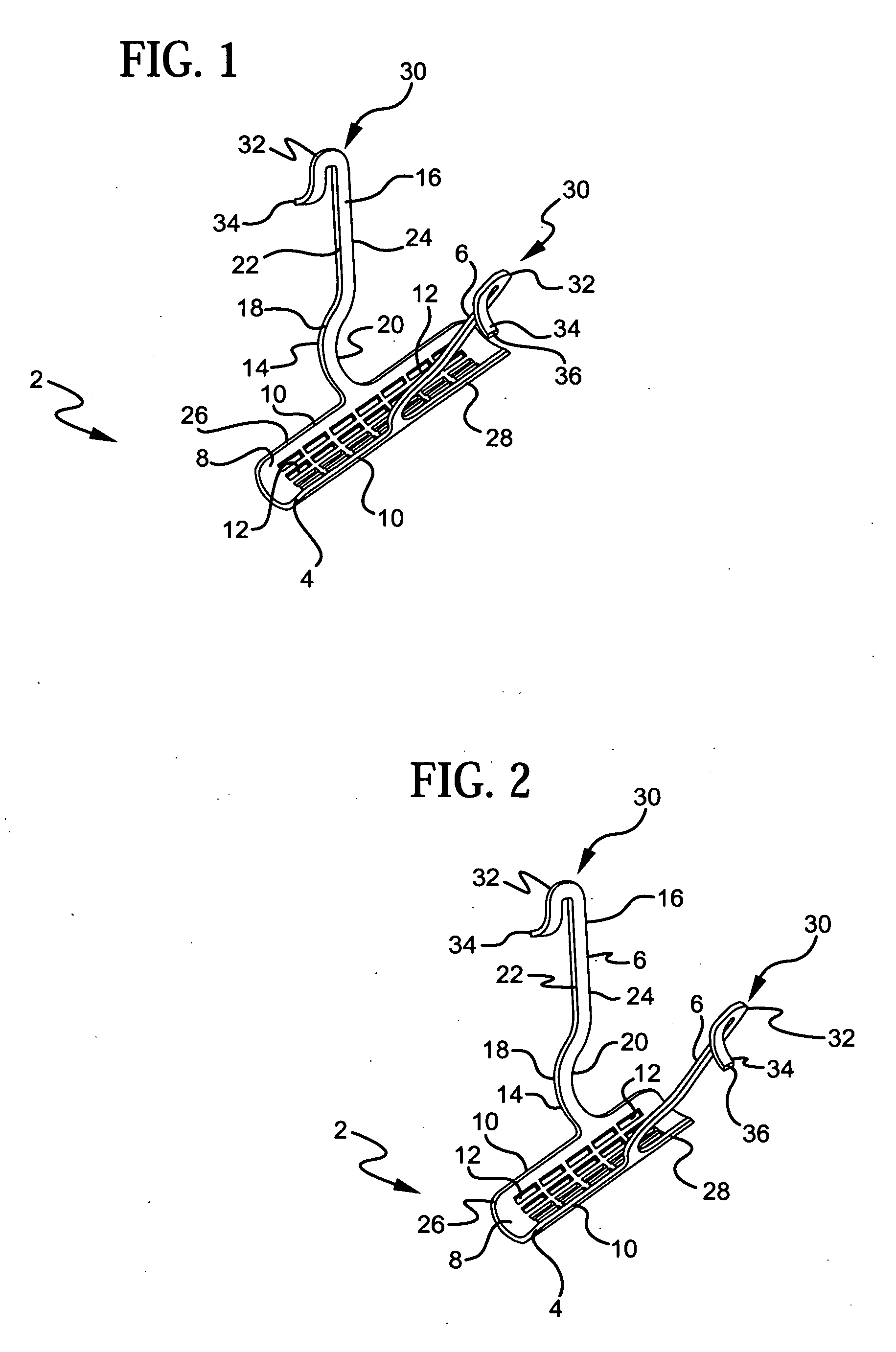
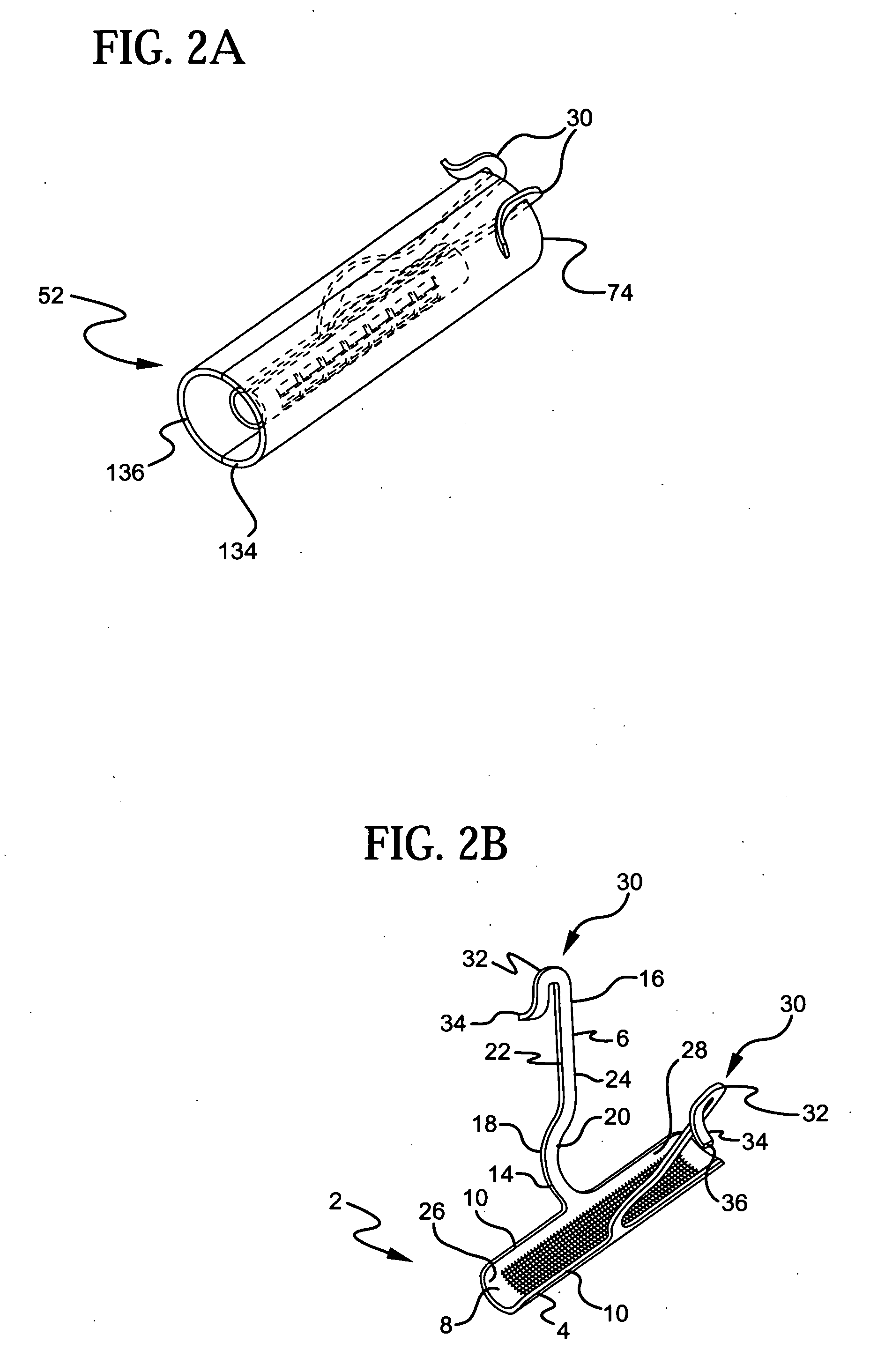
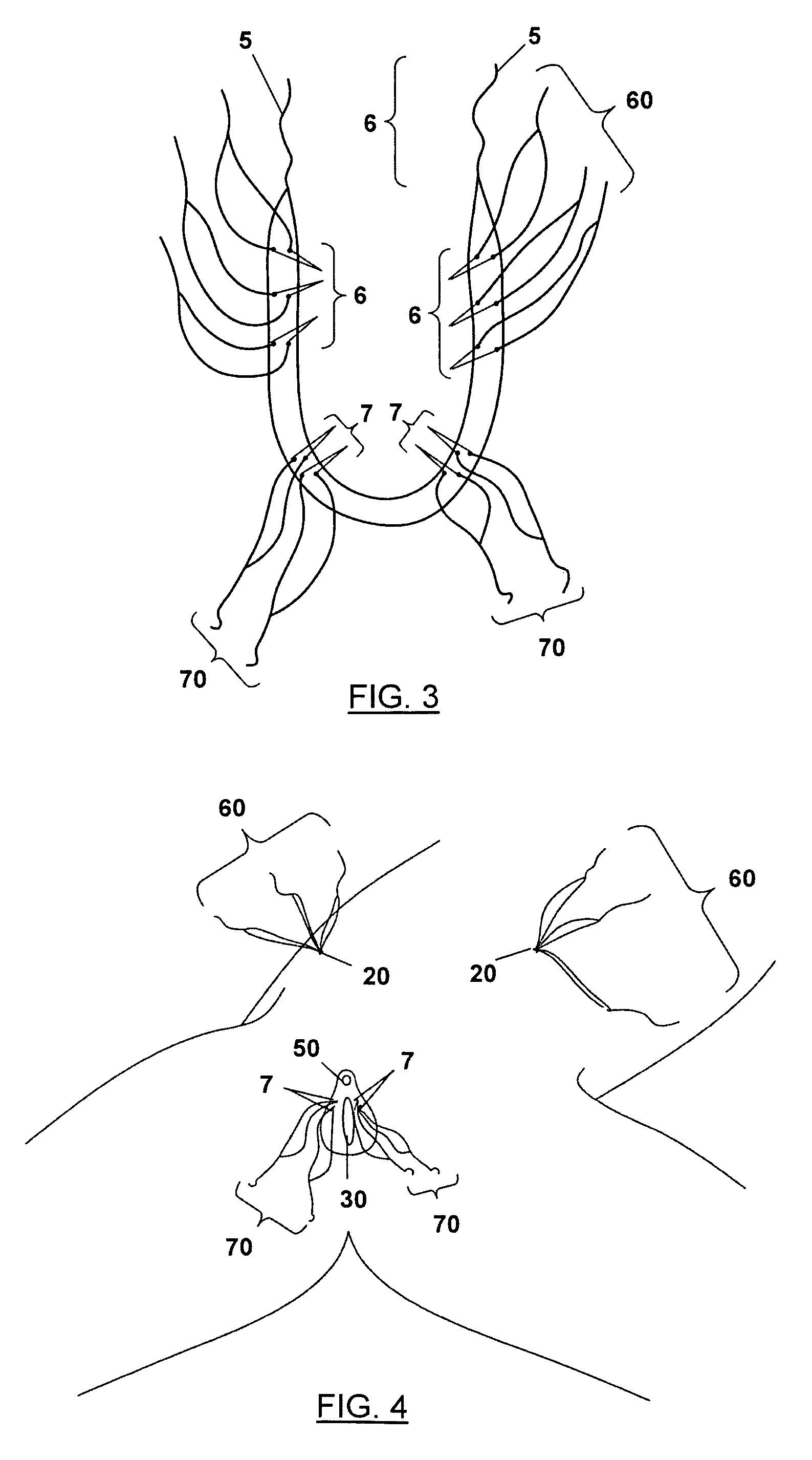
Stress urinary incontinence implant and device for deploying same
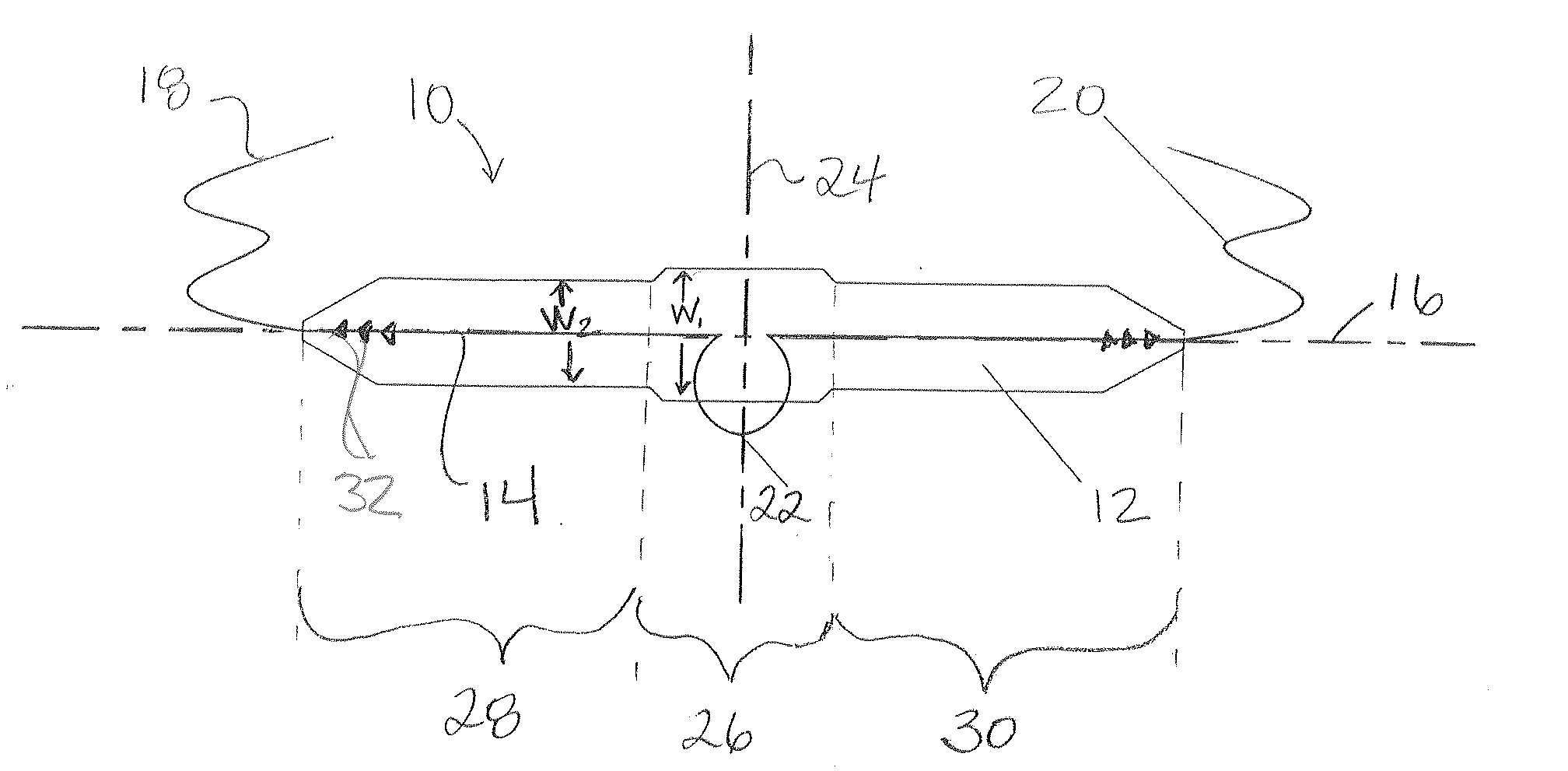
InactiveUS20070156012A1Facilitates and shortens surgical procedureAnti-incontinence devicesSurgical needlesUrethraVaginal canal
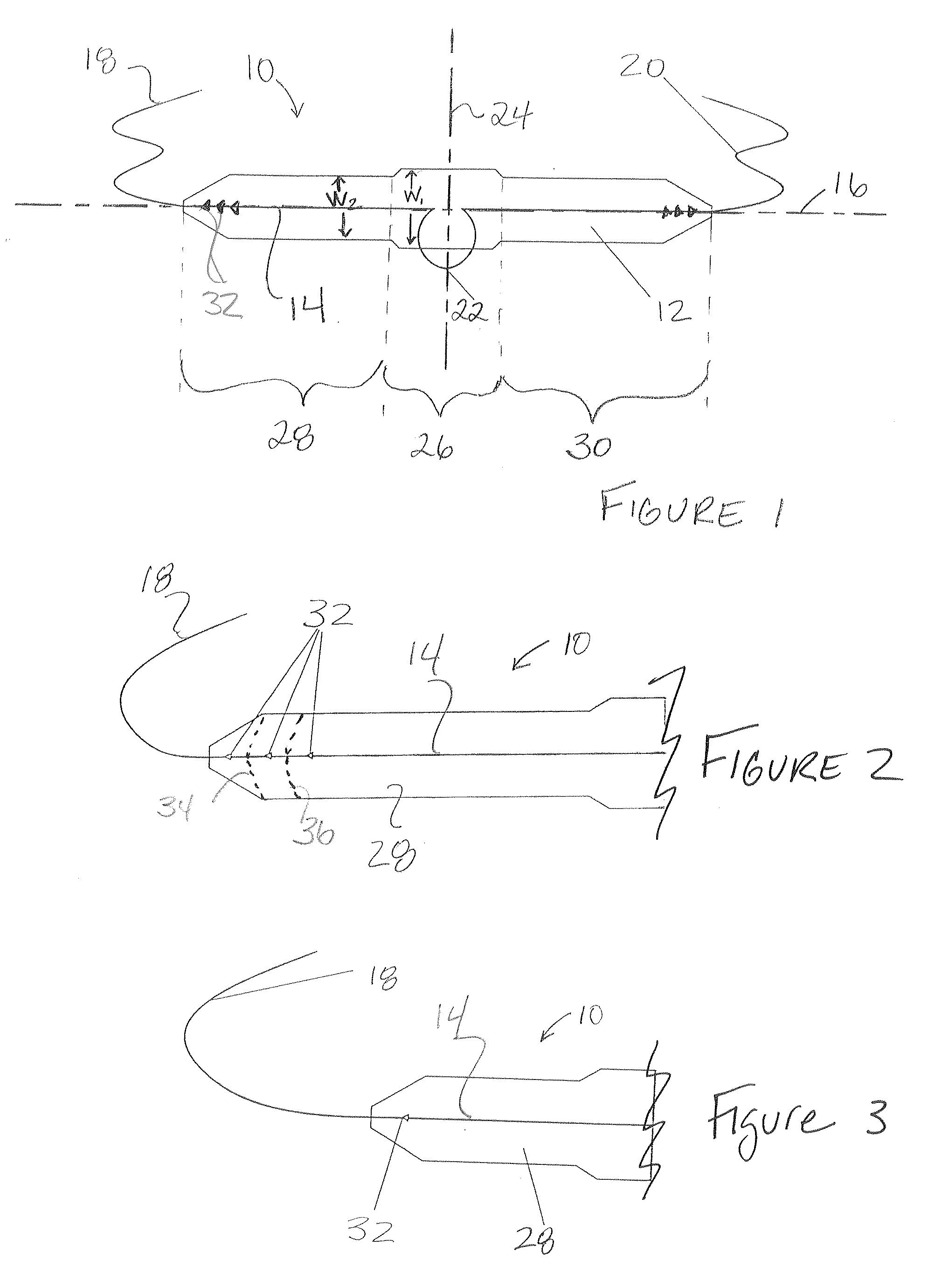
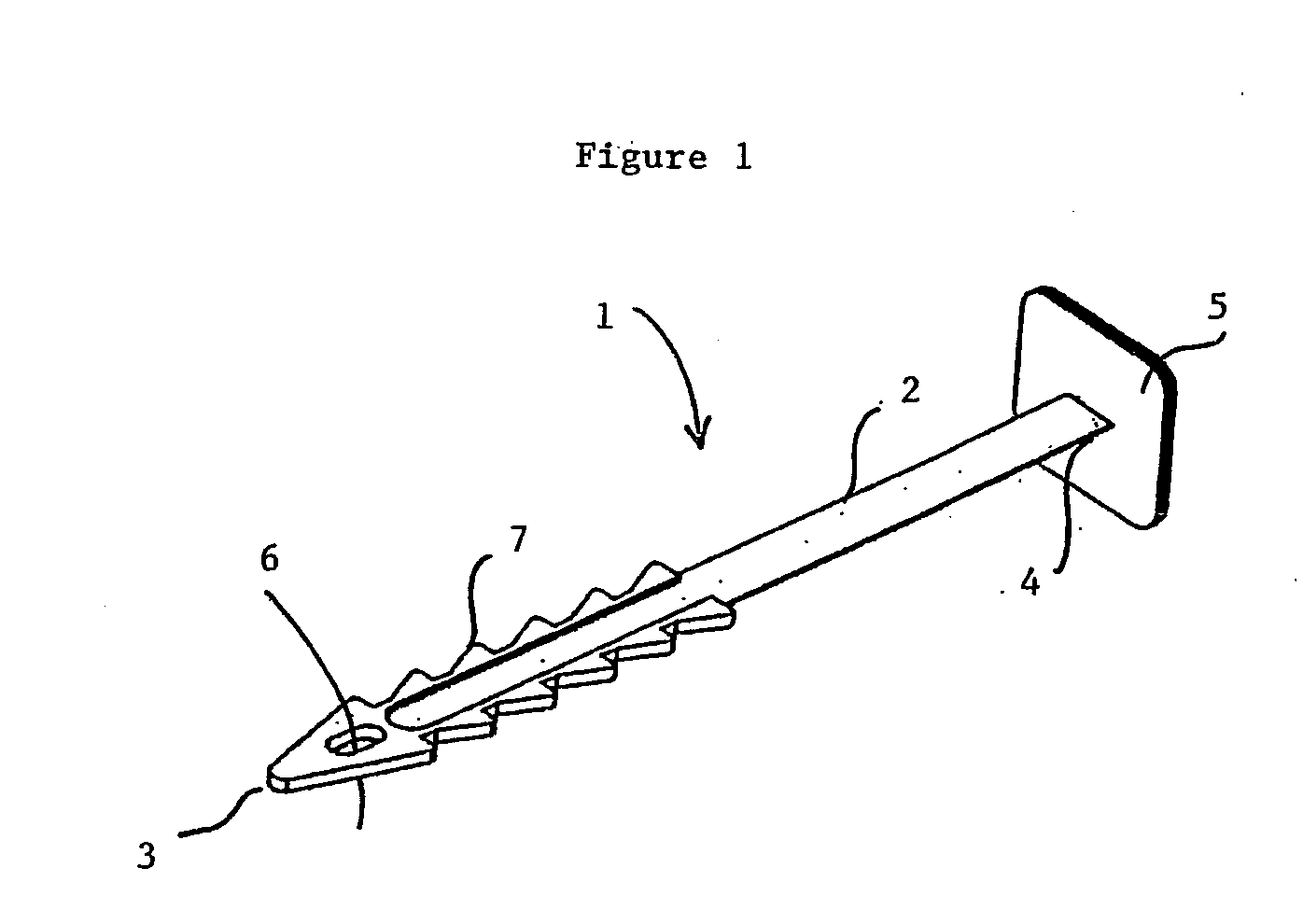
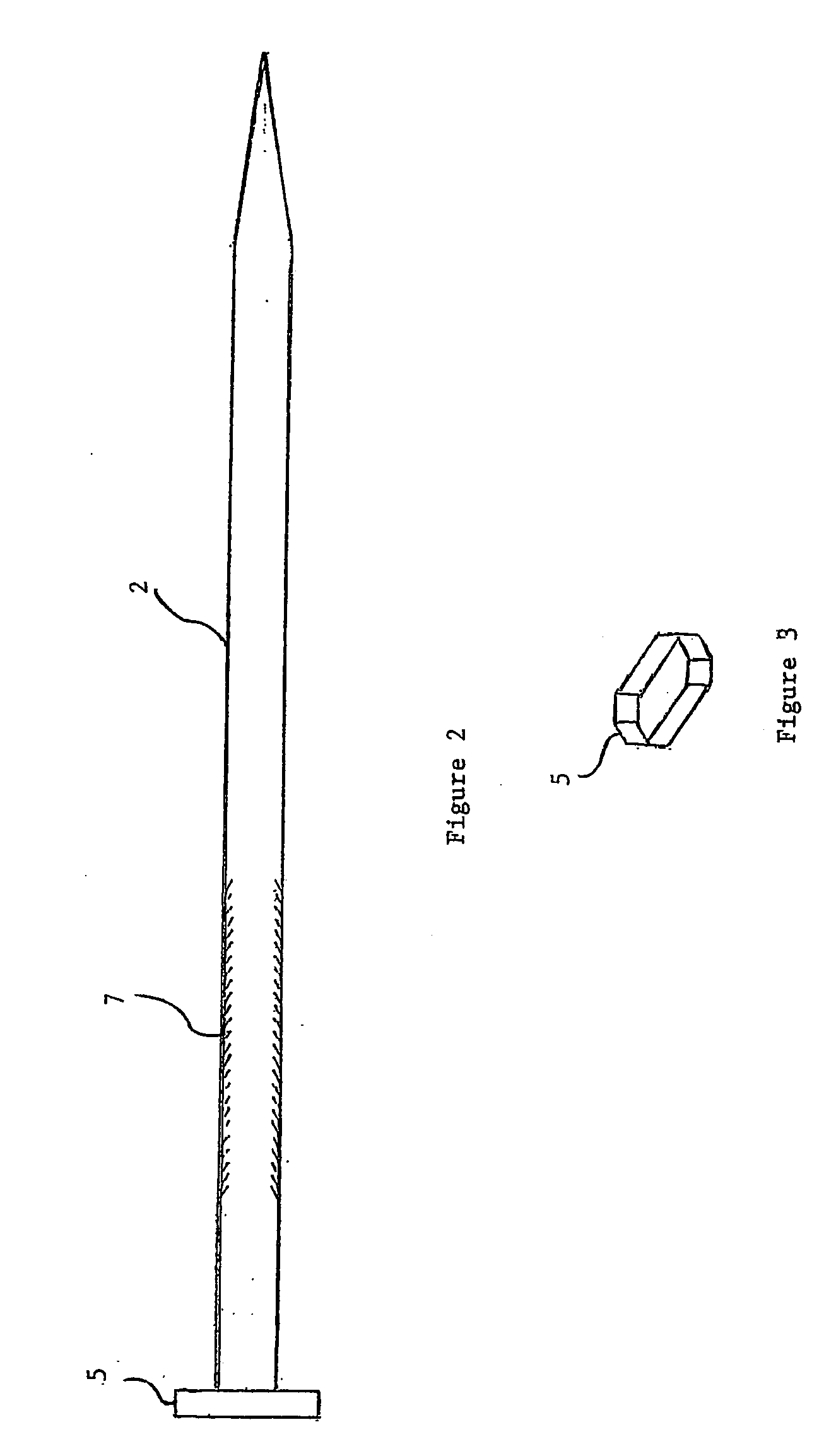
A suburethral implant for treating stress urinary incontinence (SUI) includes an elongated plate-like member which is slightly curved in transverse cross-section, and a pair of spaced apart arms having hooked ends or barbs which extend from the plate-like member. A device for deploying the suburethral implant for treating SUI includes a vaginal probe for insertion in the vaginal canal of a patient, and a urethra locator probe for the simultaneous insertion in the urethra of a patient. The urethra probe is spaced apart from and overlies the vaginal probe. The vaginal probe includes a wall in which is formed an exit port. A suburethral implant introducer assembly is extendable and retractable through the exit port on the vaginal probe. The introducer assembly has a distal end on which is removably mounted a suburethral implant. The introducer assembly is extendable through the exit port to pierce the vaginal canal wall of the patient in order to position the suburethral implant in proximity to the patient's urethra. The hooked ends or barbs on the arms of the suburethral implant engage the tissue surrounding the patient's urethra and are affixed thereto during deployment of the implant. The introducer assembly is then retracted into the vaginal probe, whereby the implant is released from the introducer assembly and remains affixed to tissue in proximity to the patient's urethra.
Owner:ETHICON INC
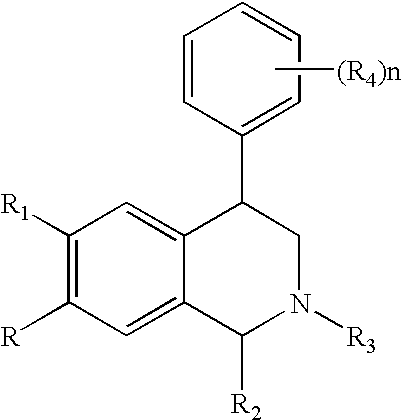
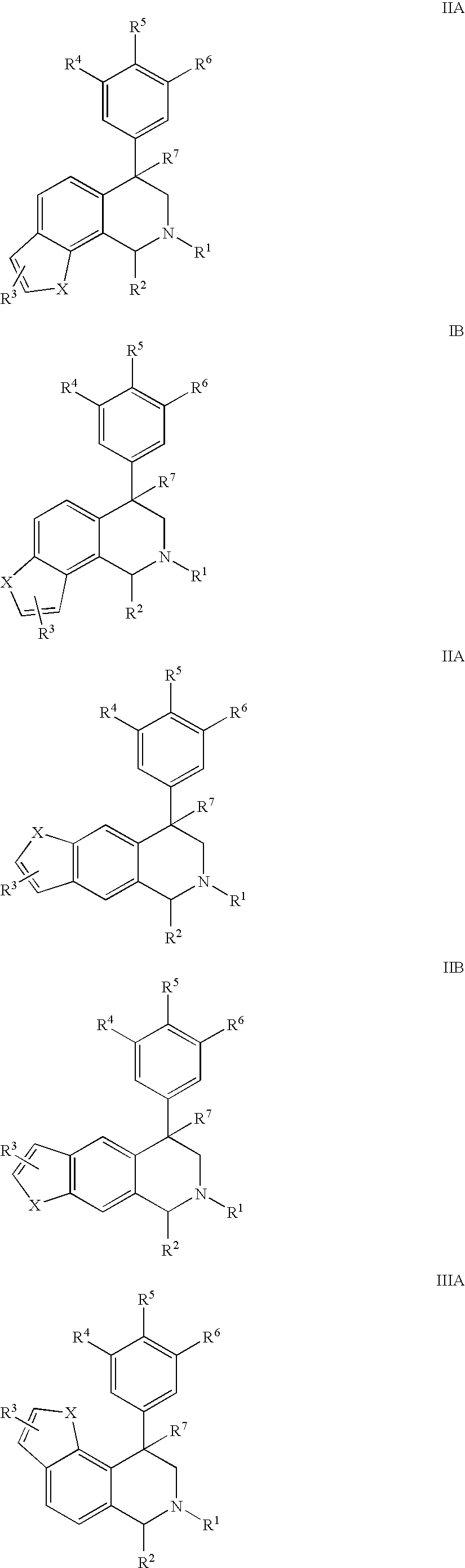
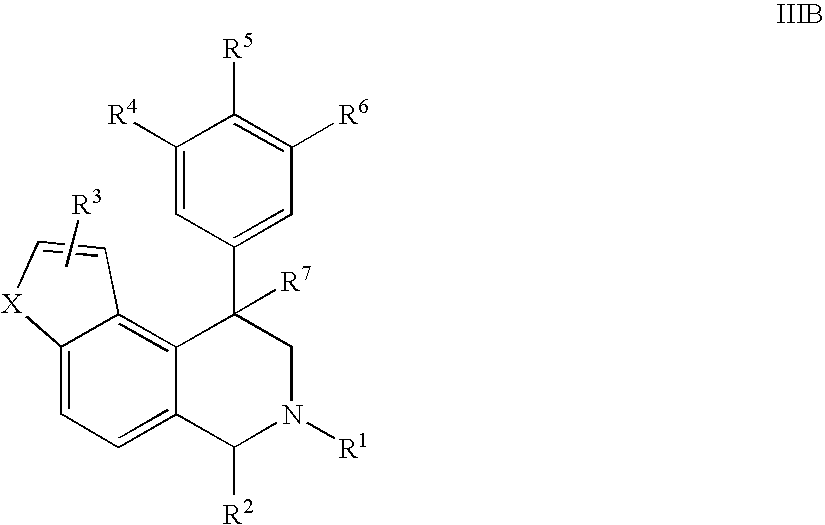
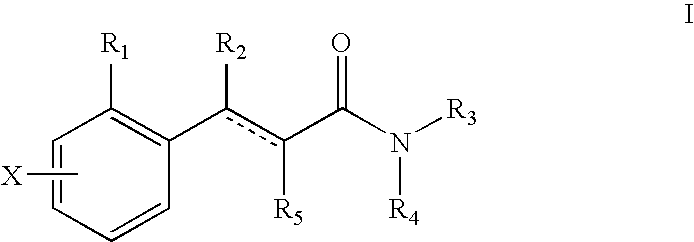
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
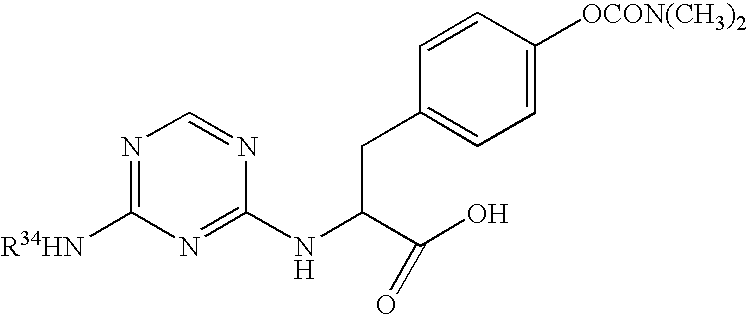
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
System for the treatment of stress urinary incontinence
InactiveUS8016743B2Small caliberLess aggressiveAnti-incontinence devicesSurgical needlesUrethraStress incontinence
The inventive system includes an elongated band, which includes a urethral area at a central part of the band, the band including a first set of holes, one of the first set of holes located on one side of the central part and another of the first set of holes located on the other side of the central part along the length of the band, first threads passing through the first set of holes and configured to permit loosening of the band following the placement of the band and the conclusion of the operation, and a second set of holes located closer to distal ends of the band than the first set of holes, and second threads passing through the second set of holes and configured to permit tightening of the band following the placement of the band and the conclusion of the operation.
Owner:JESUS ROMERO MAROTO
Pelvic disorder treatment device
InactiveUS20050049648A1Relieving pelvic painReduce painUltrasonic/sonic/infrasonic diagnosticsElectrotherapyInterstitial cystitisFecal incontinence
Owner:MEDTRONIC INC +1
Techniques for evaluating stress urinary incontinence (SUI) using involuntary reflex cough test
ActiveUS20100137737A1Overcome problemsElectromyographyMedical automated diagnosisBacteriuriaUrine leakage
A system and method evaluates a patient for stress urinary incontinence. An involuntary reflex cough event is induced within the patient that activates the nucleus ambiguous and medial motor cell column of the patient and stimulates involuntary cough activated paraspinal muscles in the pelvis of the patient. And elecromyogram (EMG) is obtained from the involuntary cough activated paraspinal muscles and its duration determined. Any urine leakage time that occurs during the involuntary reflex cough event is identified and correlated within a processor together with the urine leakage time and EMG and duration of cough event to determine stress urinary incontinence.
Owner:PNEUMOFLEX SYST
Implant Inserted Without Bone Anchors For Treatment of Urge Incontinence
InactiveUS20090112052A1Relieve symptomsLess effectSuture equipmentsDiagnosticsFecal incontinenceCombined use
The present invention discloses an implant for placement in the retropubic space of a patient. Novel methods and assemblies for use in conjunction with the implant are also described, which include mechanical positioning of the sling, placement of a mechanical implant underneath the urethra or mechanical vibration (intermittent) under the urethra or other incontinence lumen.
Owner:AMS RES CORP
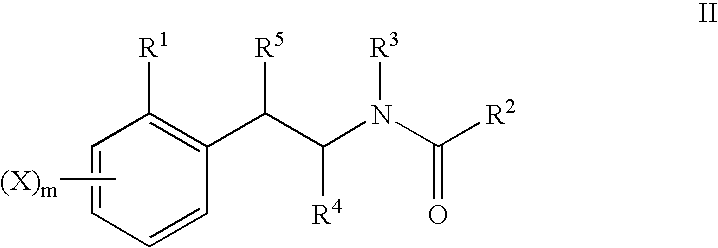
Phenyl or heteroaryl amino alkane derivatives as ip receptor antagonist
InactiveUS20060089371A1Excellent IP receptor antagonistic activitySuitable for productionBiocideOrganic active ingredientsVisceral painHeadaches
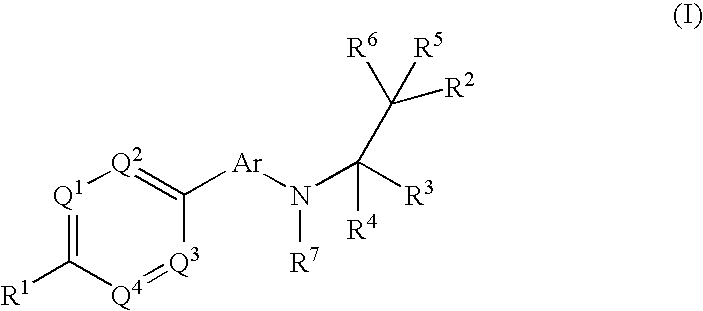
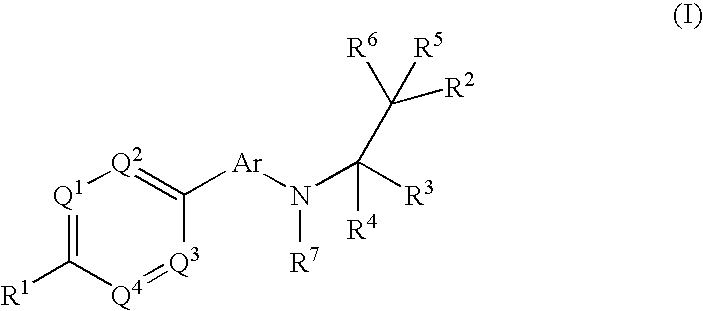
The present invention relates to phenyl or heteroaryl amino alkane derivatives of formula (I) in which the groups Q1-Q4, Ar, and R1-R7 are as defined in the specification and claims. These materials are useful as active ingredients of pharmaceutical preparations. The phenyl or heteroaryl amino alkanes of the present invention have IP receptor antagonistic activity, and can be used for the prophylaxis and treatment of diseases associated with IP receptor antagonistic activity. Such diseases include urological diseases or disorders as follows: bladder outlet obstruction, overactive bladder, urinary incontinence, detrusor hyper-reflexia, detrusor instability, reduced bladder capacity, frequency of micturition, urge incontinence, stress incontinence, bladder hyperreactivity, benighn prostatic hypertrophy (BPH), prostatitis, urinary frequency, nocturia, urinary urgency, pelvic hypersensitivity, urethritis, pelvic pain syndrome, prostatodynia, cystitis, or idiophatic bladder hypersensitivity. The compounds of the present invention are also useful for treatment of pain including, but not limited to inflammatory pain, neuropathic pain, acute pain, chronic pain, dental pain, premenstrual pain, visceral pain, headaches, and the like; hypotension; hemophilia and hemorrhage; and inflammation, since these diseases also are alleviated by treatment with an IP receptor antagonist. The application claims the compounds, pharmaceutical compositions containing them, and methods of treatment using them.
Owner:BAYER HEALTHCARE AG
Combination of serotonin reuptake inhibitors and norephinephrine reuptake inhibitors
InactiveUS20050014848A1Prevent relapseIncreasing and improving neuronal processBiocideAmine active ingredientsStress inducedNorepinephrine reuptake inhibitor
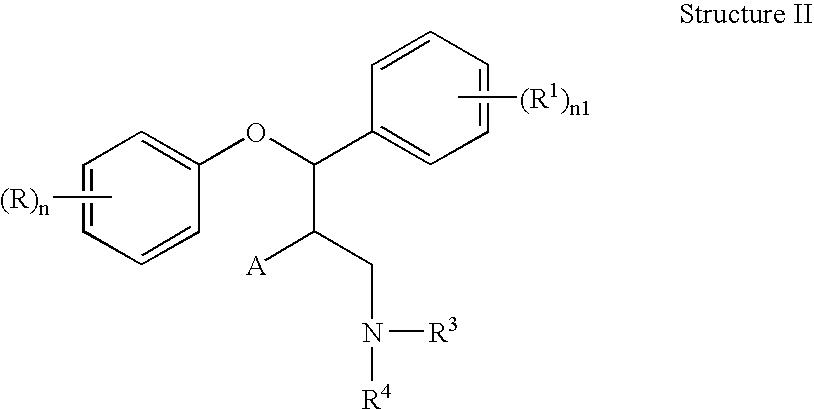
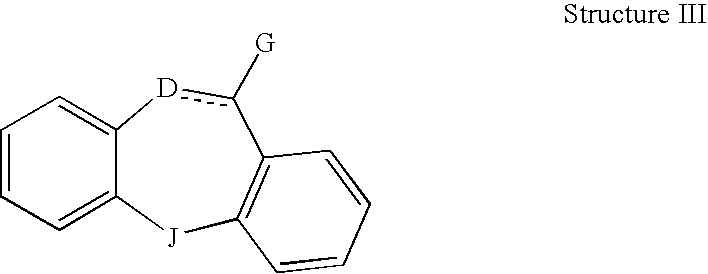
This invention is directed to pharmaceutical compositions and methods for treating a disorder or condition selected from the group consisting of depression, anxiety disorders, phobias, avoidant personality disorder, eating disorders, chemical dependencies, Parkinson's diseases, obsessive-compulsive disorder, negative symptoms of schizophrenia, cognitive dysfunction related to schizophrenia, premenstrual syndrome, stress-induced incontinence, headache, neuropathic pain, chronic pain, urinary incontinence, post-traumatic stress disorder, chronic stress, acute stress, fibromyalgia, depression comorbid with fibromyalgia, obesity, migraine and a combination thereof in a mammal. The methods in one embodiment comprise administering to a mammal in need of treatment for the disorder or condition: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined in the specification; and (iii) a pharmaceutically acceptable carrier. The pharmaceutical compositions and methods of the invention are also useful for preventing a relapse associated with one of the foregoing disorders or conditions, and for treating a symptom associated with one of the foregoing disorders or conditions, wherein the symptom is selected from the group consisting of cognitive dysfunctions and somatic complaints.
Owner:PFIZER INC
Incontinence treatment device
InactiveUS20050113881A1Function increaseAvoid flowElectrotherapyAnti-incontinence devicesMuscle contractionUrethra
A device and method for treatment of urinary stress incontinence. At least one electrode is implanted in a pelvic muscle of a patient. A control unit receives signals indicative of abdominal stress in the patient and responsive thereto applies an electrical waveform to the electrode which stimulates the muscle to contract, so as to inhibit involuntary urine flow through the patient's urethra due to the stress.
Owner:ASTORA WOMENS HEALTH
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
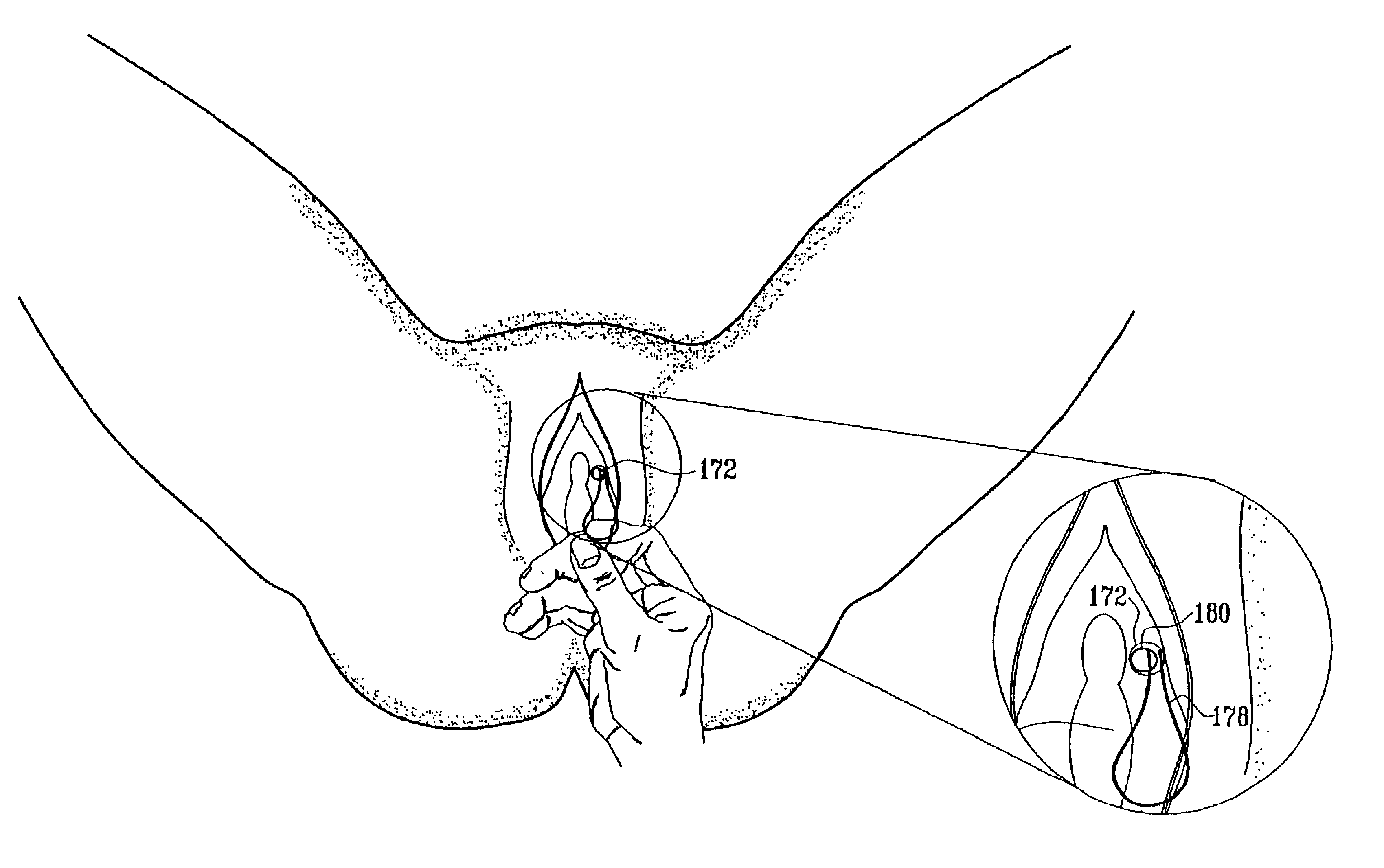
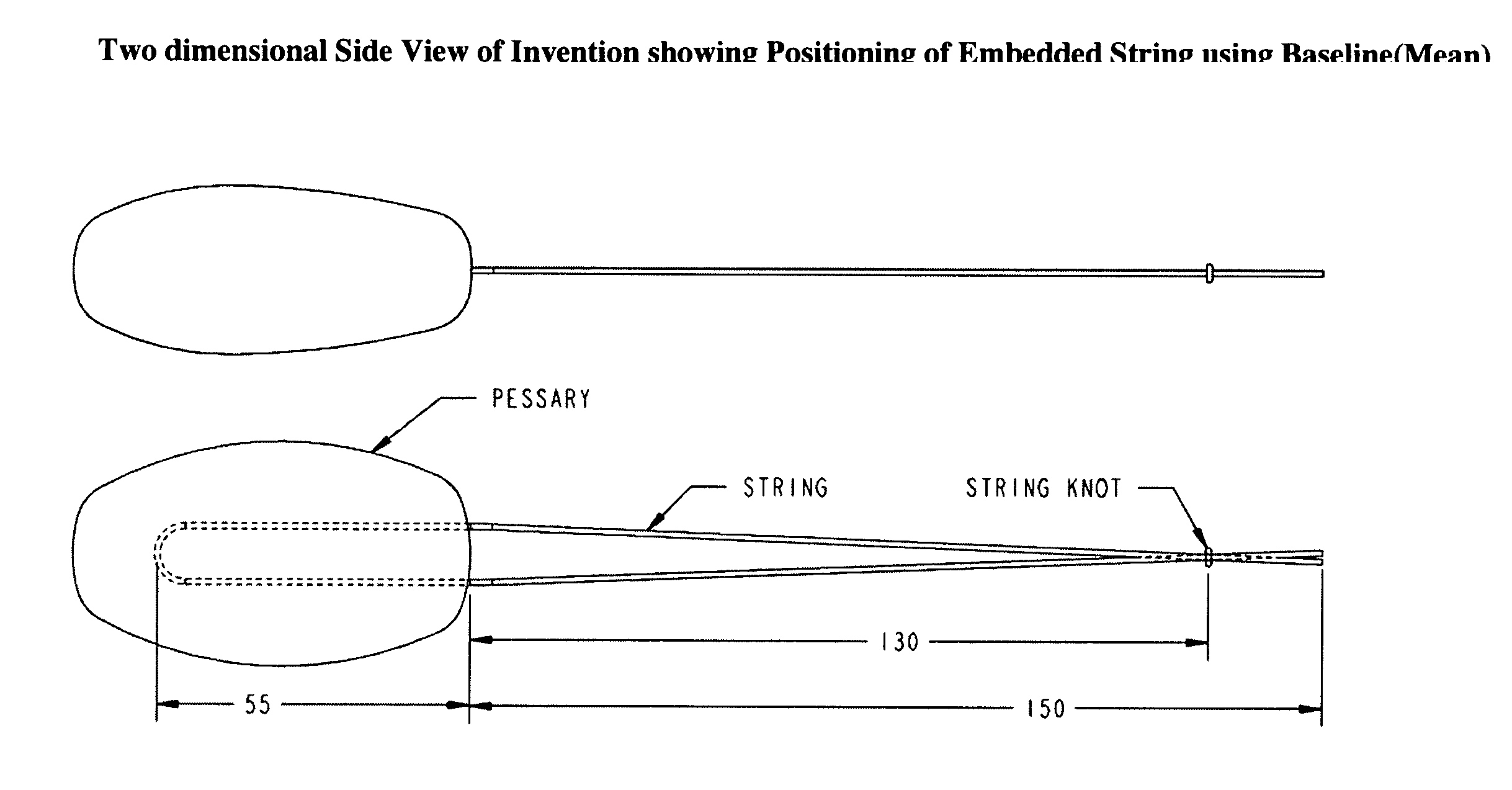
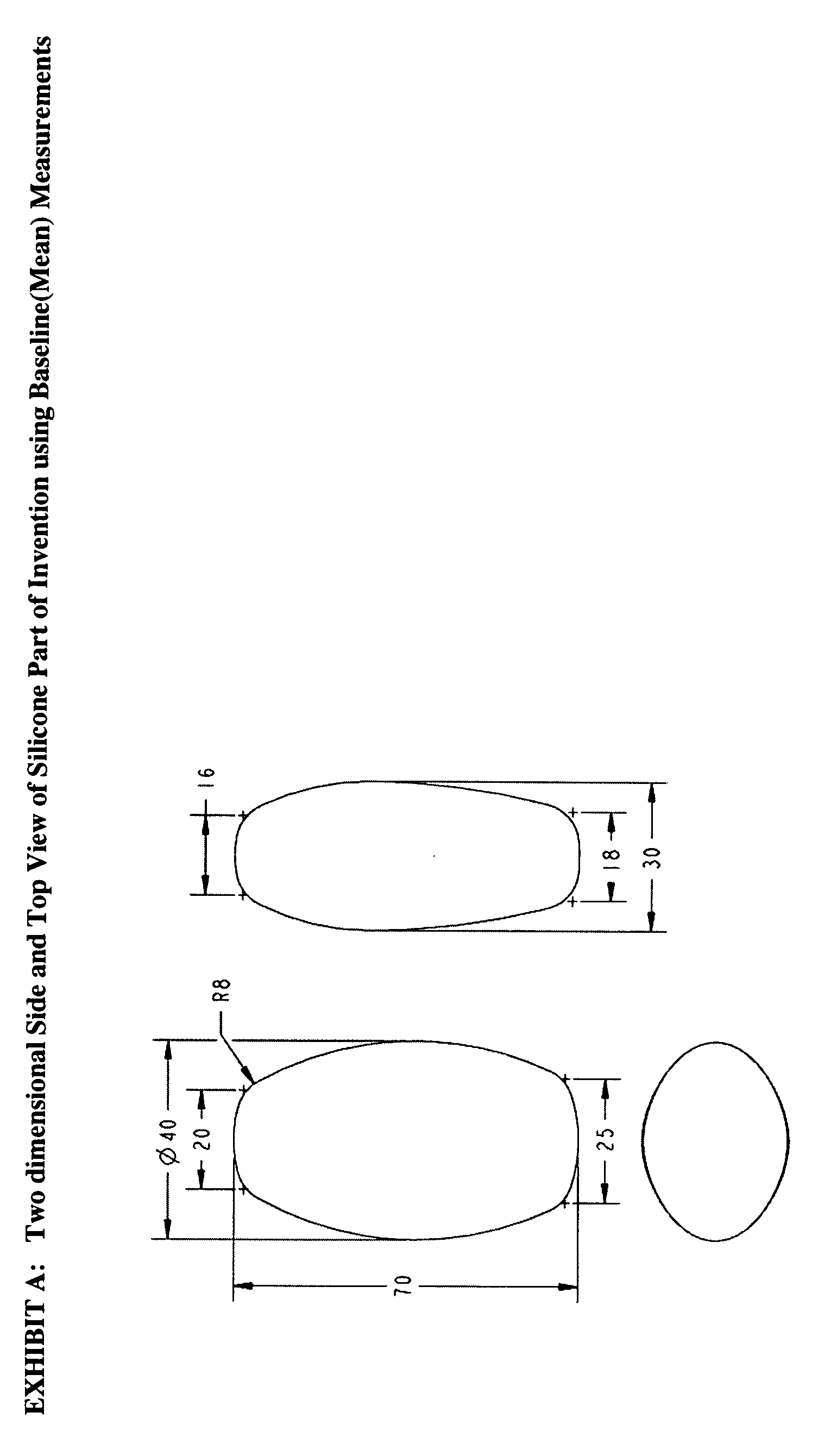
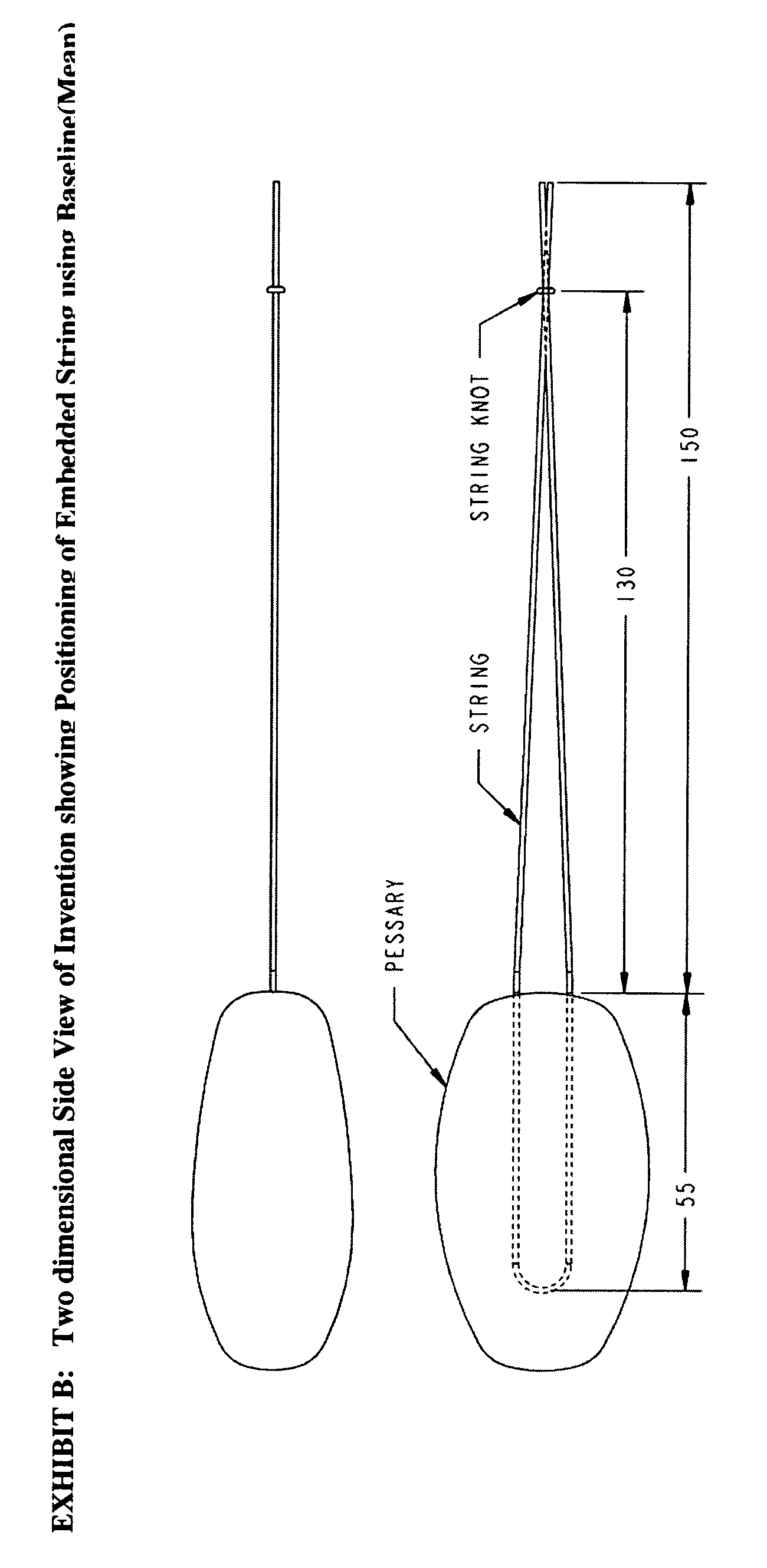
Vaginal pessary for the management of stress incontinence
InactiveUS20090095304A1Relieve pressureReduce needAnti-incontinence devicesPessariesUrine leakageBacteriuria
The invention is an intra-vaginal pessary device constructed of bio-compatible, medical grade, non-latex silicone, to manage and diagnose mild to moderate stress incontinence in women by providing a user-friendly, non-surgical, occasional, non-invasive option for mid-urethral support. The invention is an approximately oval, non-absorbent device with an embedded pull-string for removal when not in use. The pessary is self-inserted into the vagina by a woman, using the same technique she would use if she were inserting a tampon. The pressure the pessary provides against the vaginal wall has the effect of closing the urethra and thereby preventing urine leakage.
Owner:RICHARDSON SHARON ANN +1
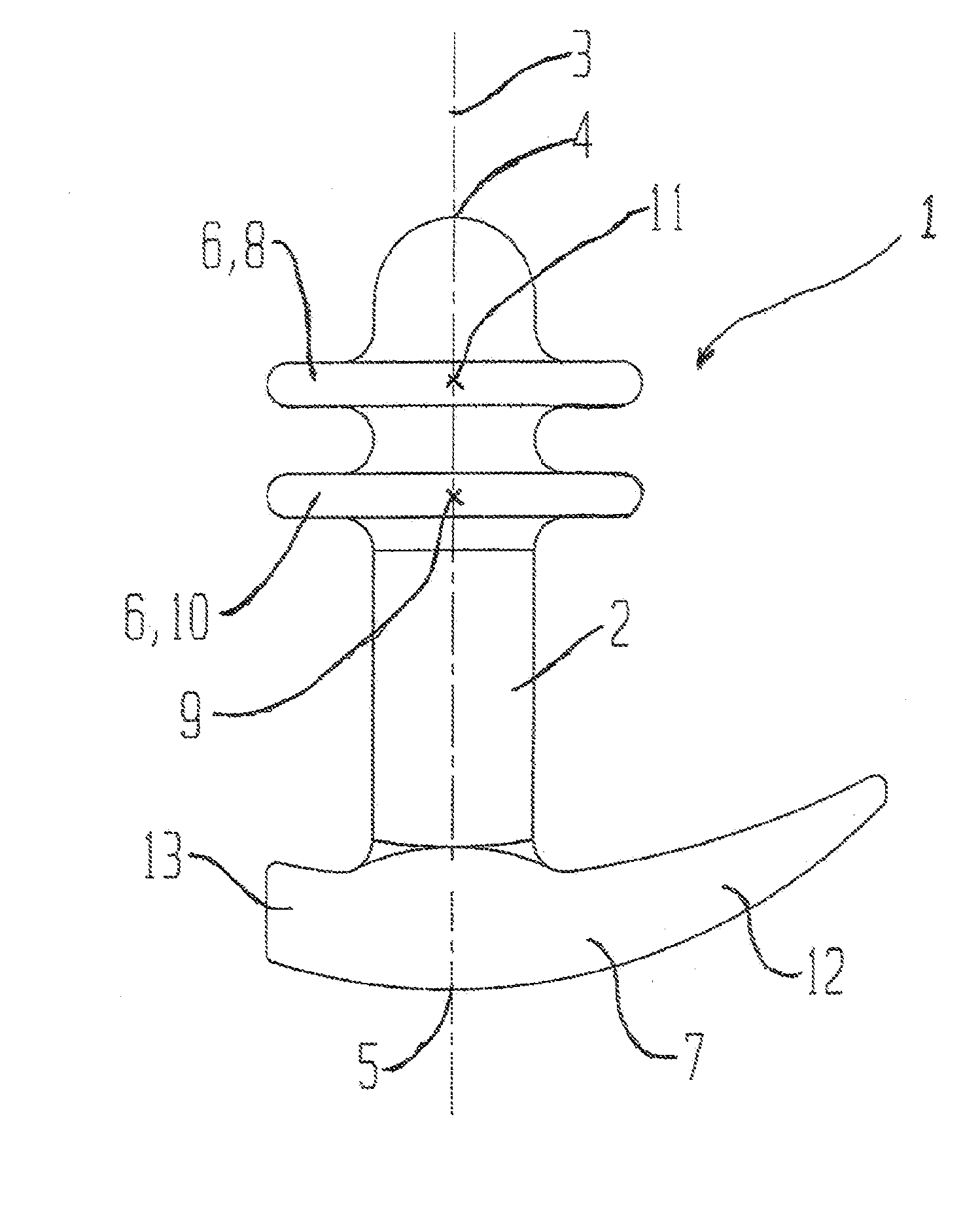
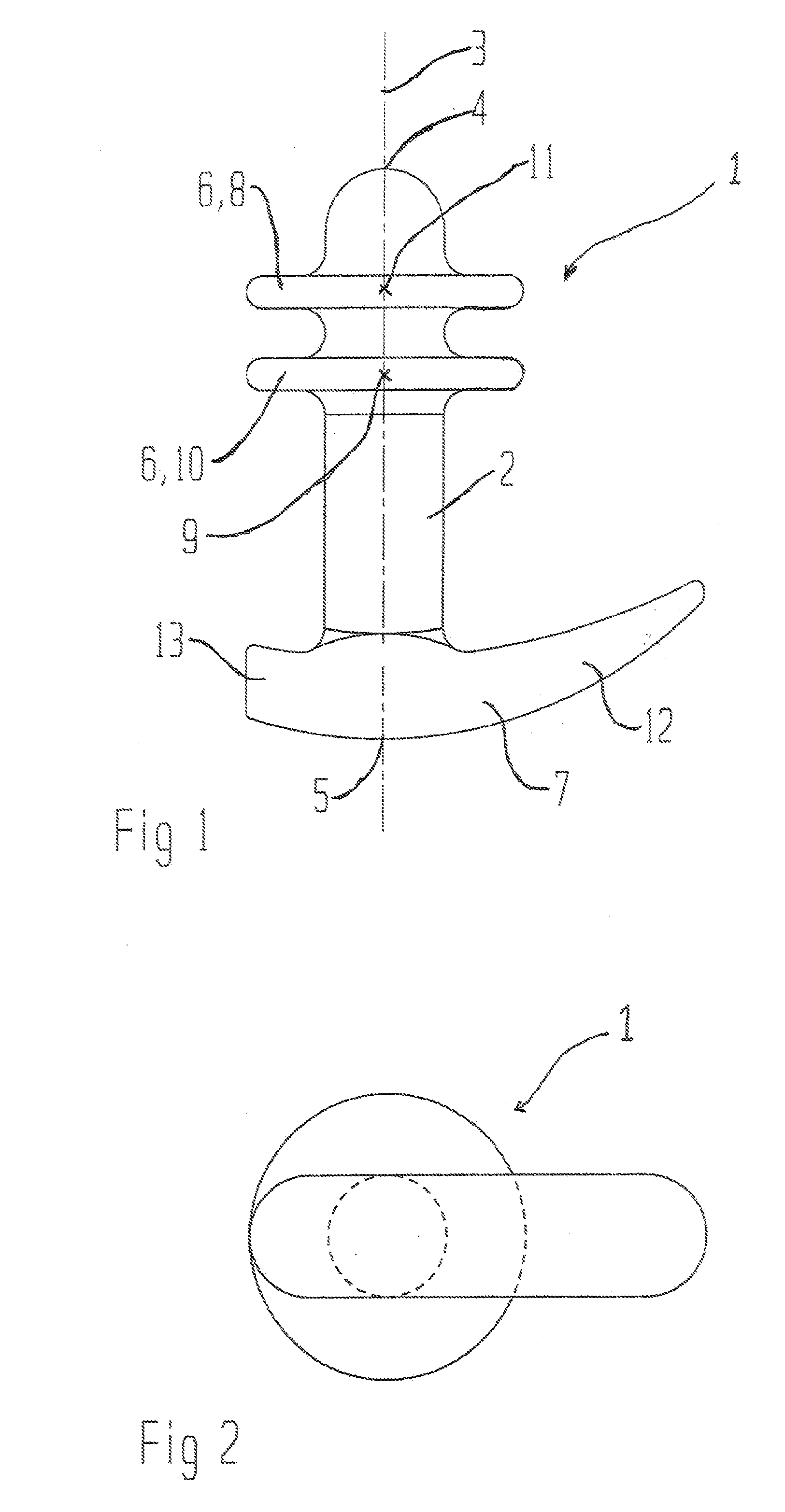
Vaginal device
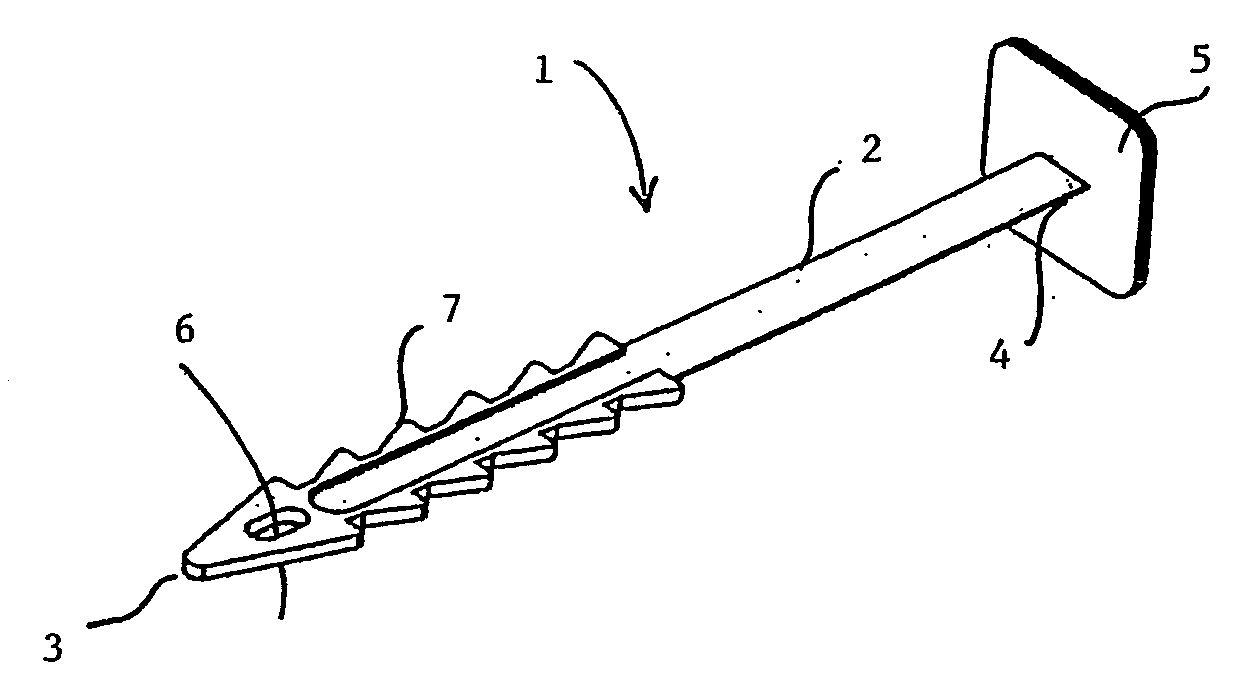
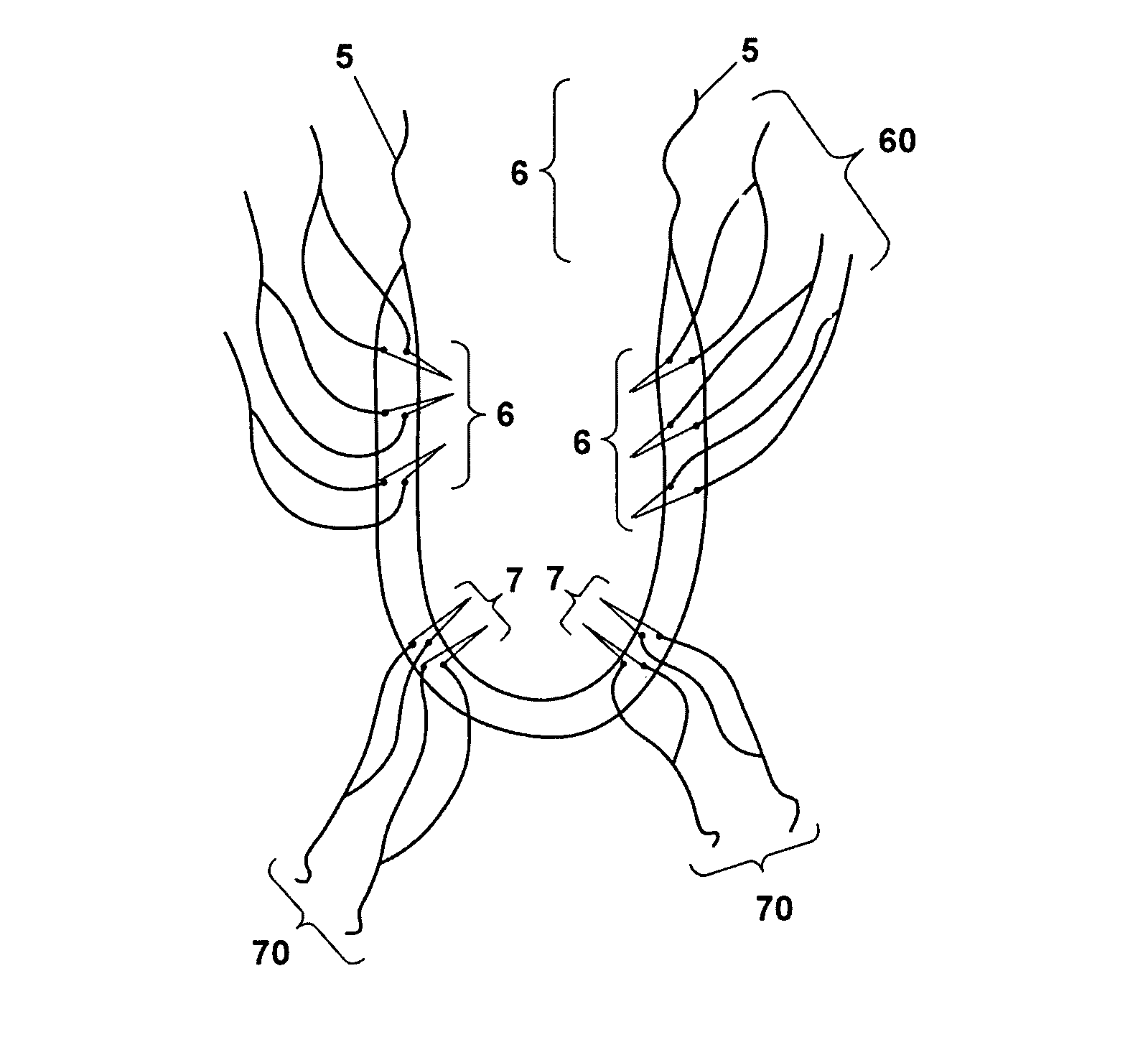
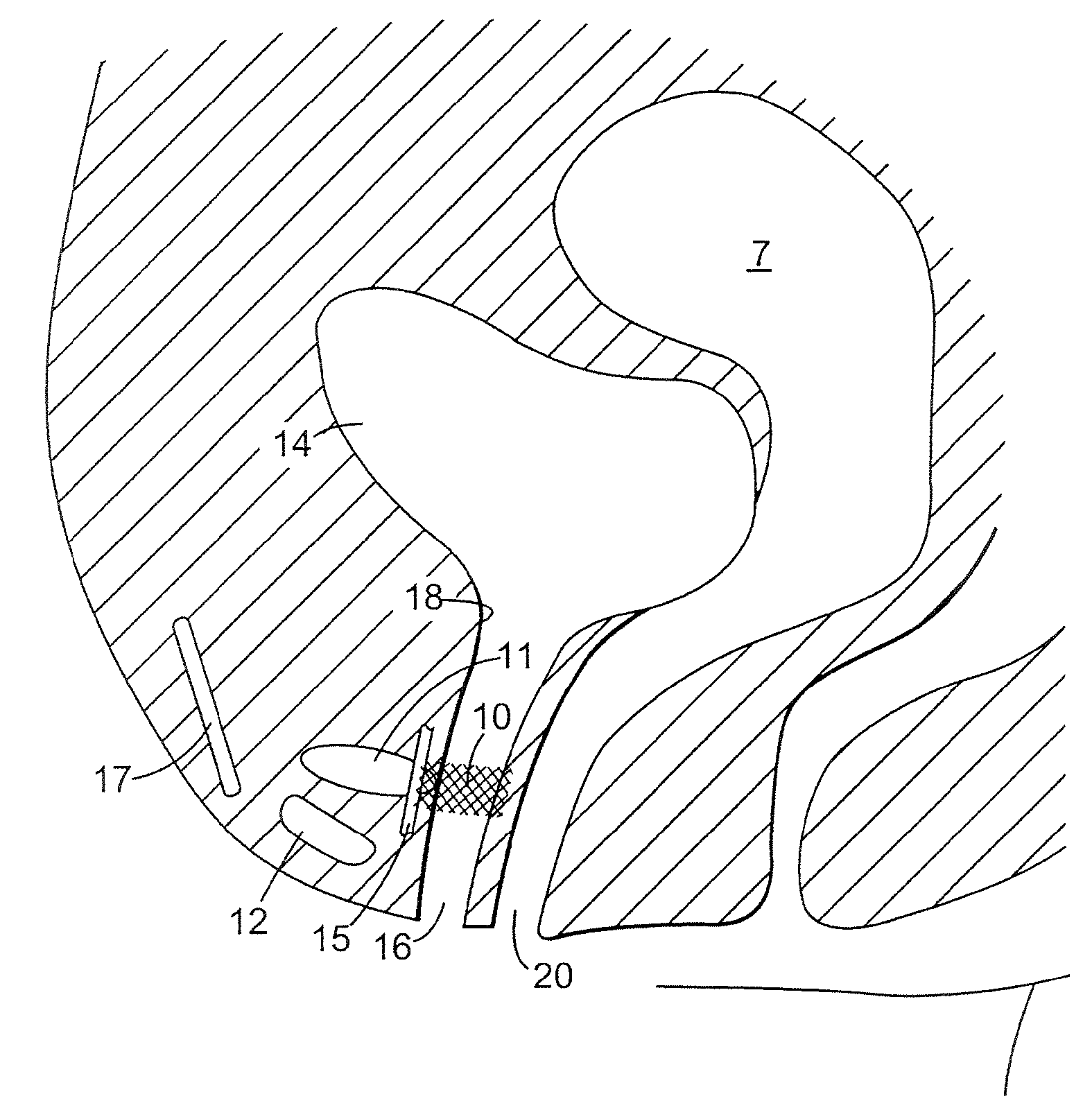
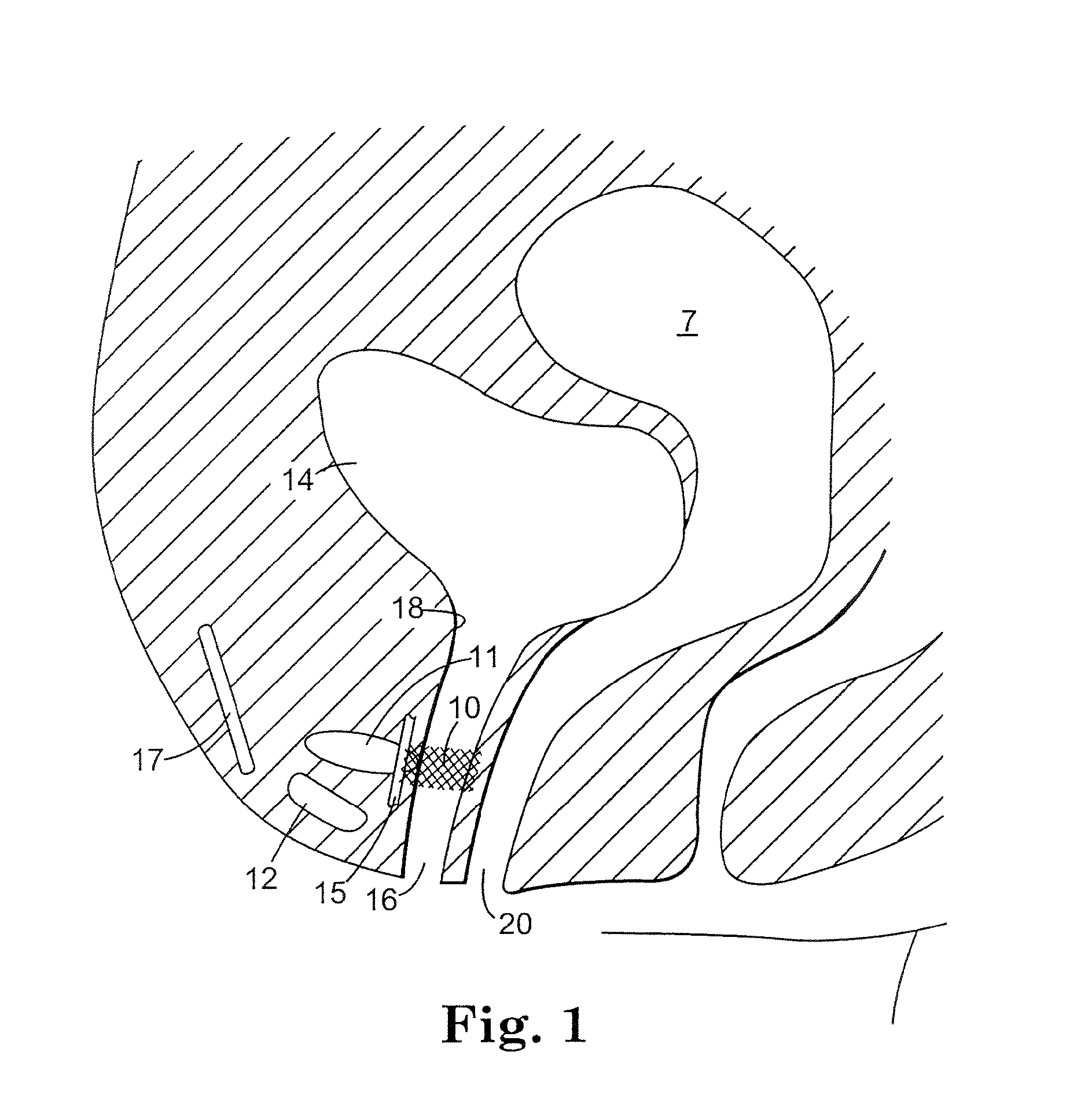
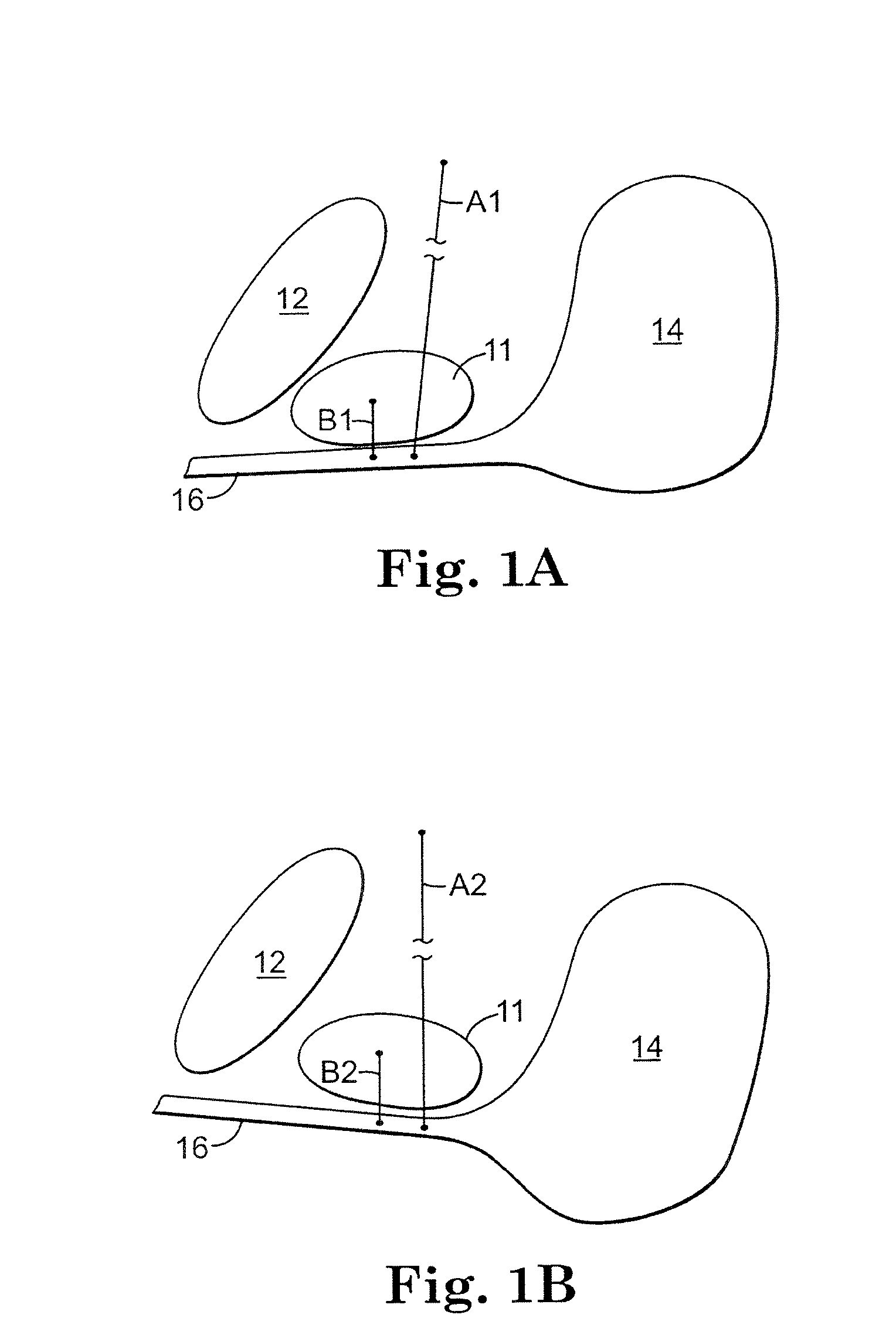
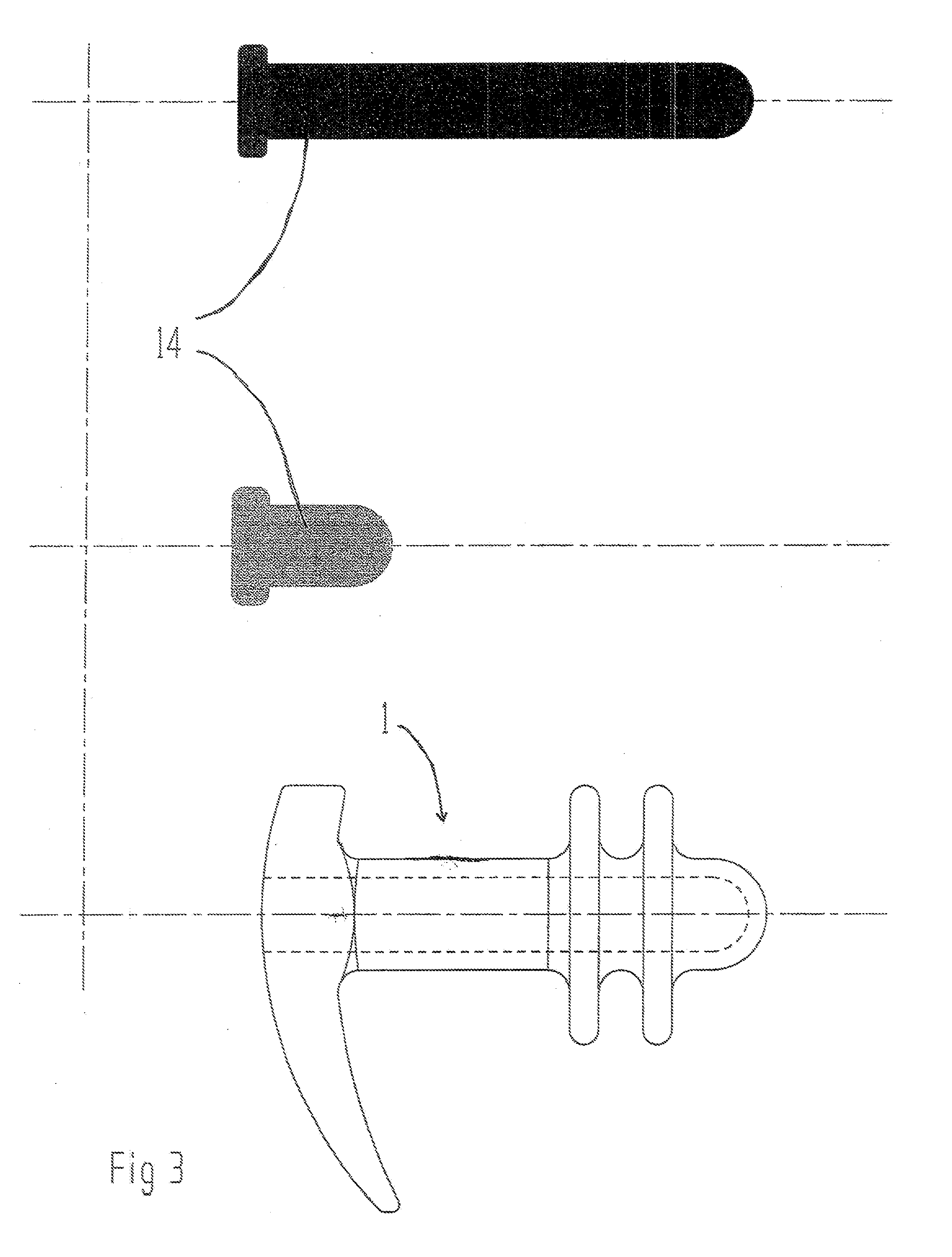
The present invention refers to a vaginal device 1 of an elastic material, said vaginal device 1 comprising a longitudinal portion 2 having a geometrical centre line 3, an upper end 4 and a lower end 5 and which vaginal device 1 is intended to be inserted securely fixated inside of a vagina, the upper end 4 being the innermost of the vaginal device 1 in the vagina during use, to support against the urethra, through the vaginal wall, to prevent urinary stress incontinence in a woman, wherein the vaginal device 1 comprises at least one supporting portion 6 protruding from the longitudinal portion 2 and a reference member 7 protruding from the longitudinal portion 2 at the lower end 5, which reference member 7 during use is fixated against the vaginal introitus, holding the vaginal device 1 securely fixated inside of the vagina and ensuring said at least one supporting portion 6 to support against the urethra.
Owner:INVENT MEDIC SWEDEN AB
Wireless urinary incontinence monitoring system
InactiveUS20070225616A1Convenient diagnosis and treatmentCost of treatment can be reducedElectromyographyPerson identificationMonitoring systemStress incontinence
A system and method for ambulatory monitoring of the urinary tract for diagnosis of urinary stress incontinence.
Owner:ALPINE BIOMED CORP
Method, system and device for treating disorders of the pelvic floor by electrical stimulation of and the delivery of drugs to the left and right pudendal nerves
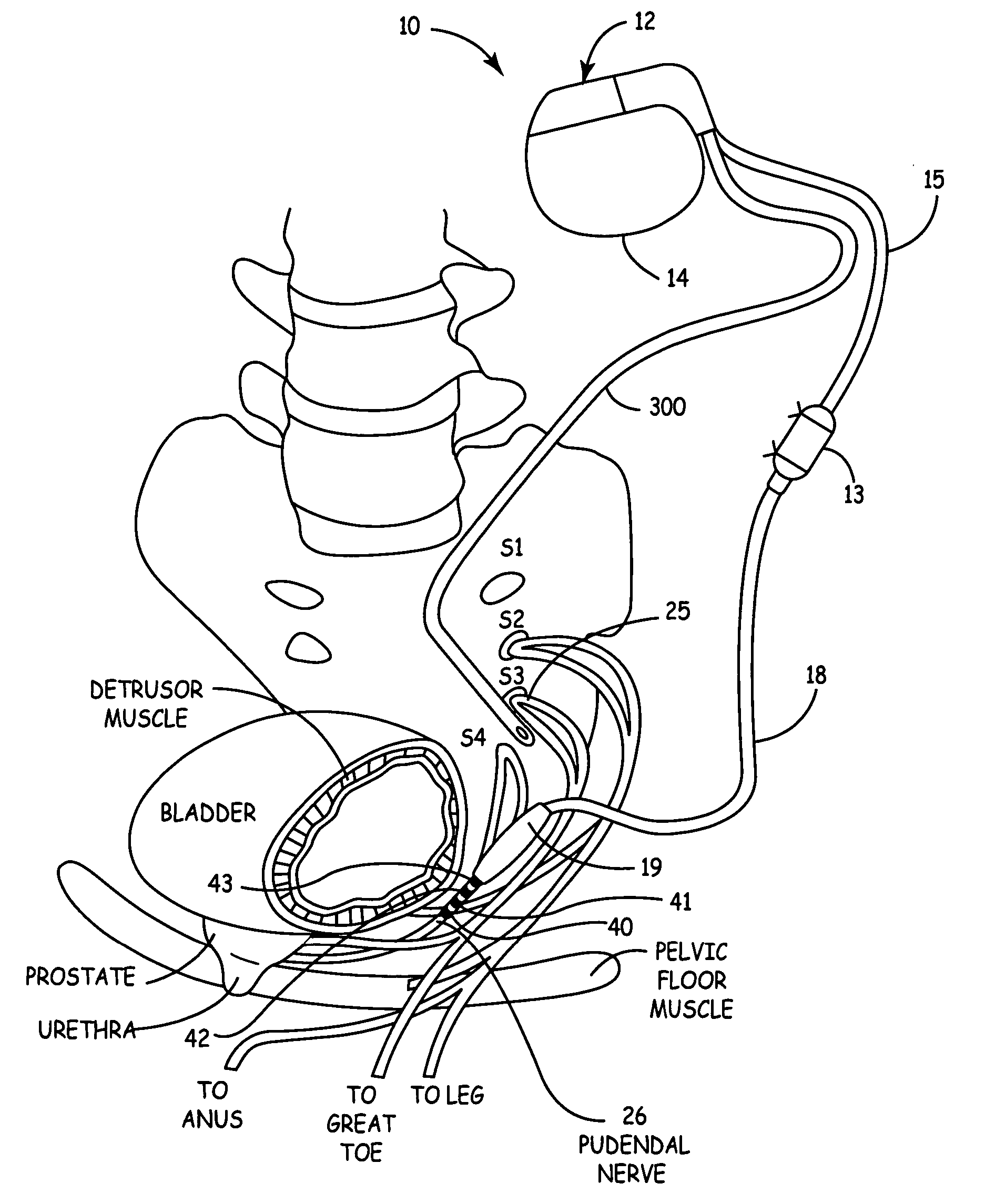
InactiveUS20060122659A9Strong specificityLower medical costsSpinal electrodesPharmaceutical delivery mechanismDiseaseProstatalgia
Described are devices and methods for treating various disorders of the pelvic floor by means of electrical stimulation of the pudendal nerves, and means for delivering one or more drugs in association with such electrical stimulation therapies. One or more electrical stimulation signals are applied, and one or more drugs are infused, injected or otherwise administered, to appropriate portions of a patient's pelvic floor and the pudendal nerves or portions thereof in an amount and manner effective to treat a number of disorders, including, but not limited to, urinary voiding dysfunction, fecal voiding dysfunction, constipation, stress incontinence, urge incontinence, urinary retention disorder, sexual dysfunction, orgasmic dysfunction, erectile dysfunction, pelvic pain, prostatitis, prostatalgia and prostatodynia.
Owner:MEDTRONIC INC
Pelvic floor repairing sheet
The invention provides a pelvic floor repairing sheet. The repairing sheet comprises a fiber membrane, wherein the fiber membrane at least comprises a first fiber layer with directed orientation and a second fiber layer without orientation. The pelvic floor repairing sheet has excellent biocompatibility, excellent softness and excellent tissue compliance, is beneficial to tissue penetration to form firm repairing, and further has the properties of anti-infection and hemostasis. The pelvic floor repairing sheet can be used for manufacturing a tension-free vaginal tape for treating stress incontinence.
Owner:MEDPRINSHENZHEN REGENERATIVEA MEDICAL TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com