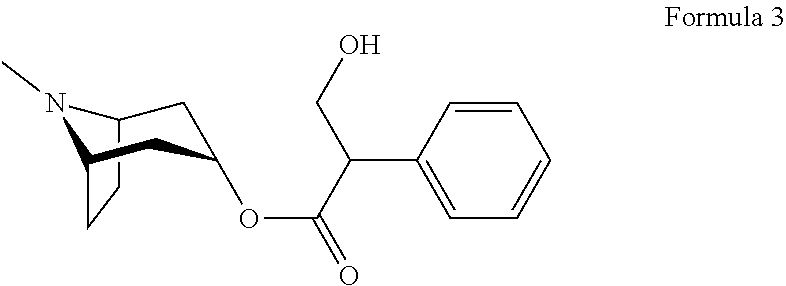
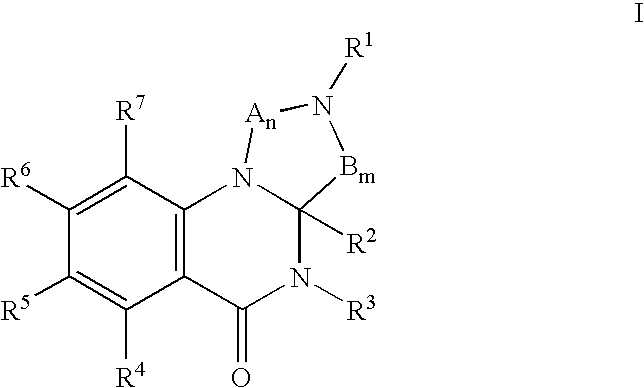
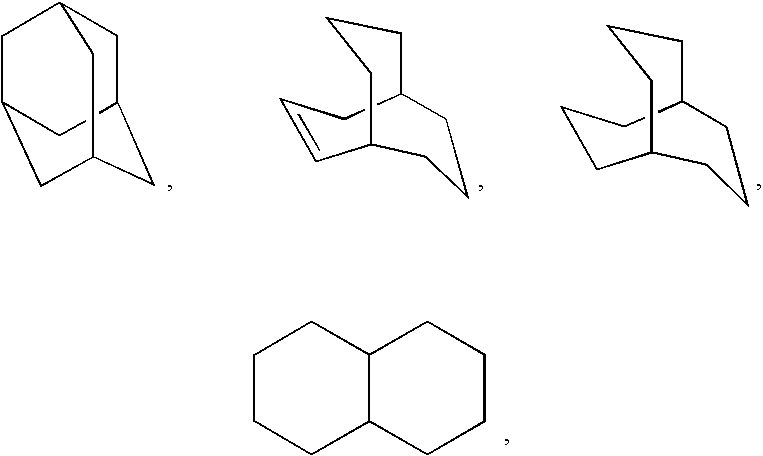
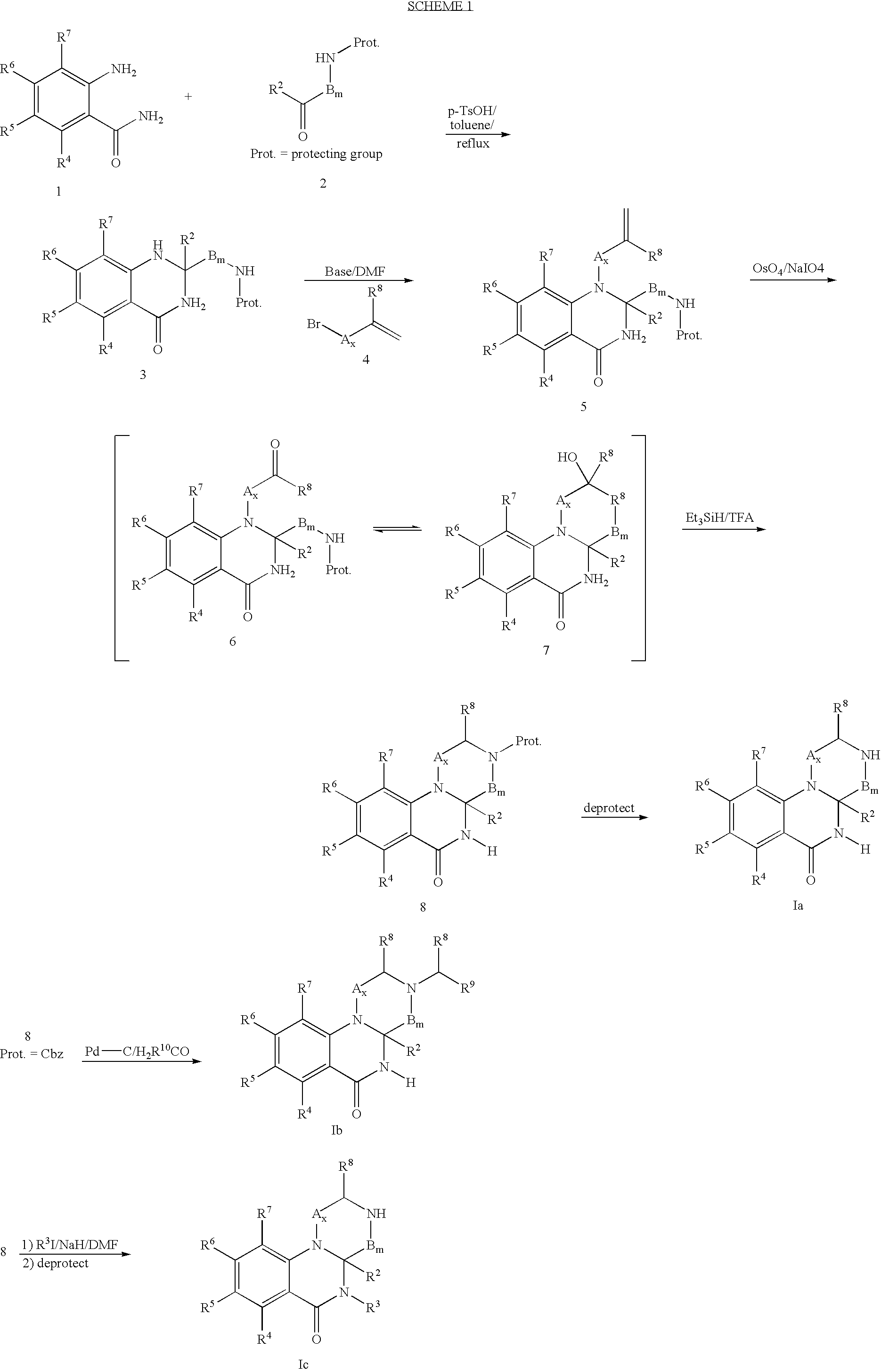
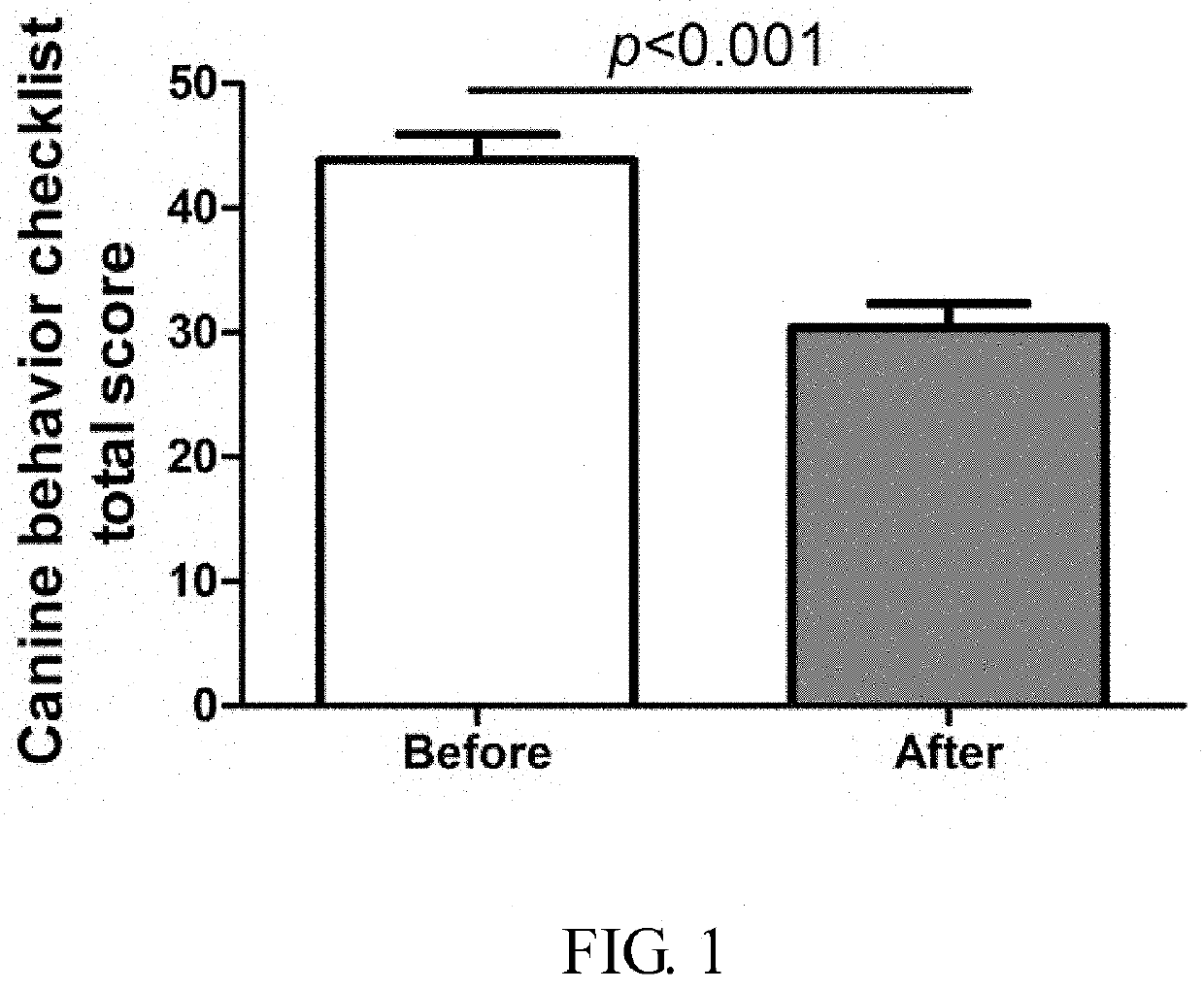
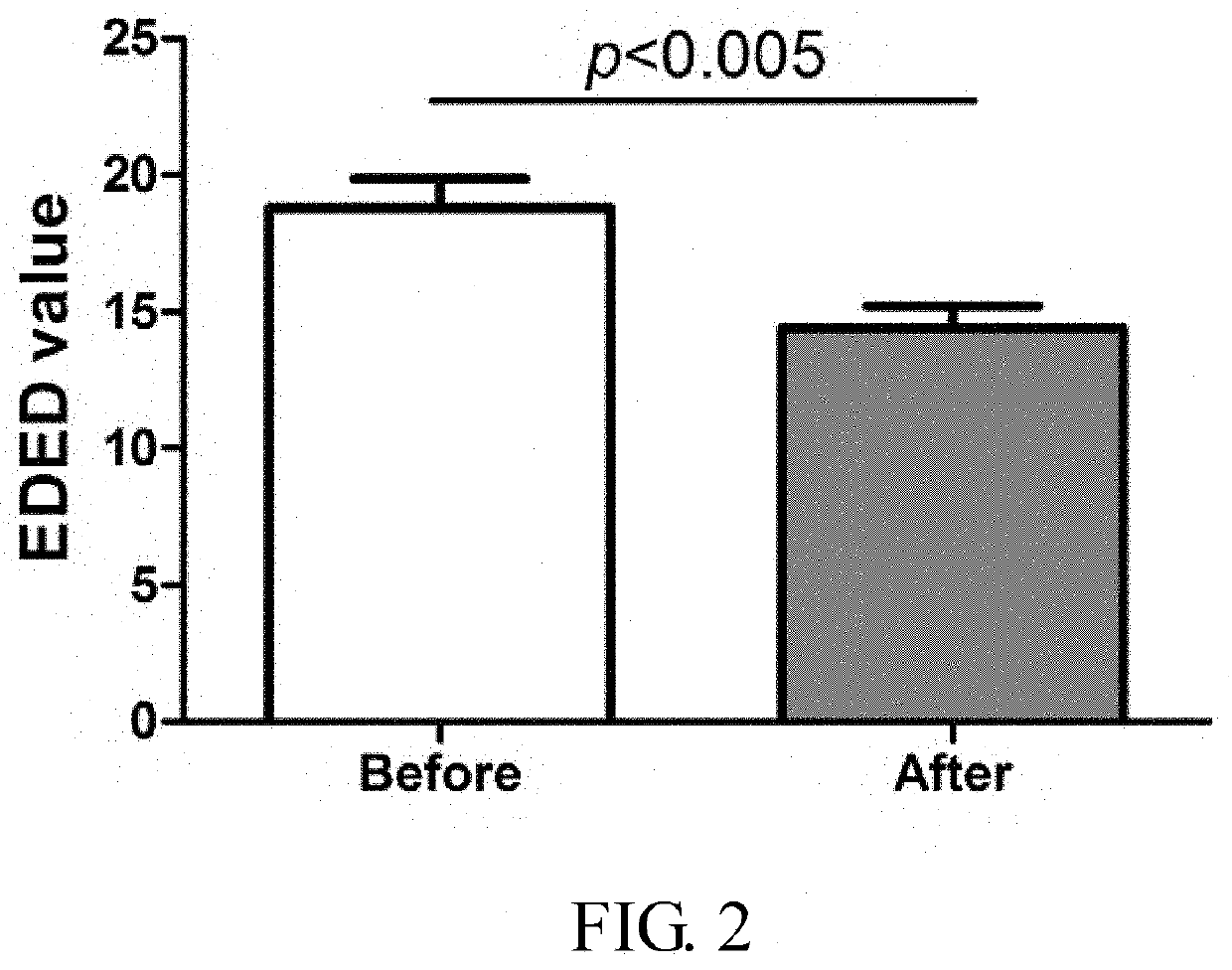
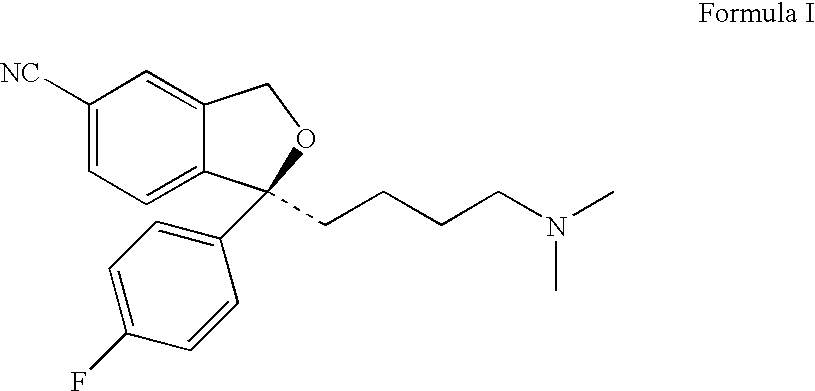
Patents
Literature
31 results about "Phobias" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Excessive, extreme, irrational, fear or panic reaction about a situation, living creature, place or object.
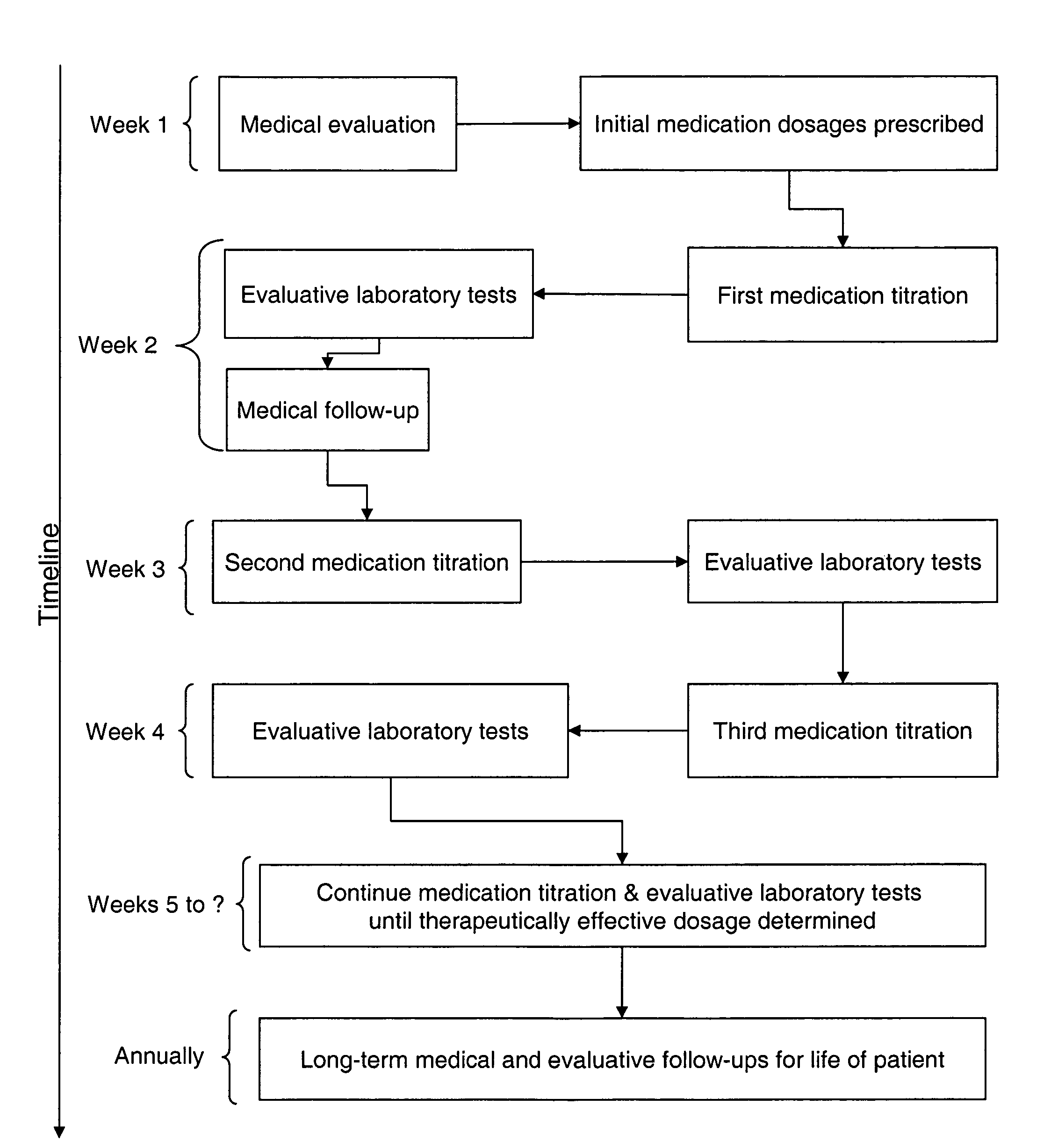
Prolonged administration of NMDA antagonist drug and safener drug to create improved stable neural homeostasis
InactiveUS20050222270A1Stable and lasting improved neural homeostasisBiocideOrganic active ingredientsDiseaseNervous system
An NMDA antagonist (such as ketamine) is administered with a safener (such as clonidine) in patients suffering from neurologic disorders other than pain. The ketamine is adminsitered at a dosage that causes slurred speech, for a span of several days. This treatment enables a patient's nervous system to return to a healthy “set point”, also called an improved stable neural homeostasis, in a manner similar to a broken bone healing itself if protected from jostling and reinjury by a cast. In at least some patients, this treatment can ease problems such as addictions to illegal or pain-killing drugs, nicotine, or alcohol, compulsive or criminal behavioral problems, severe depression, obsessive-compulsive disorders, phobias, etc. It may also provide some relief in some patients for problems such as chronic fatigue, chemical sensitivities, allergies, autoimmune disorders, and diabetes.
Owner:OLNEY JOHN W +3
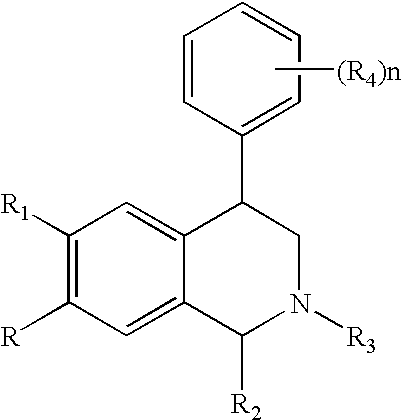
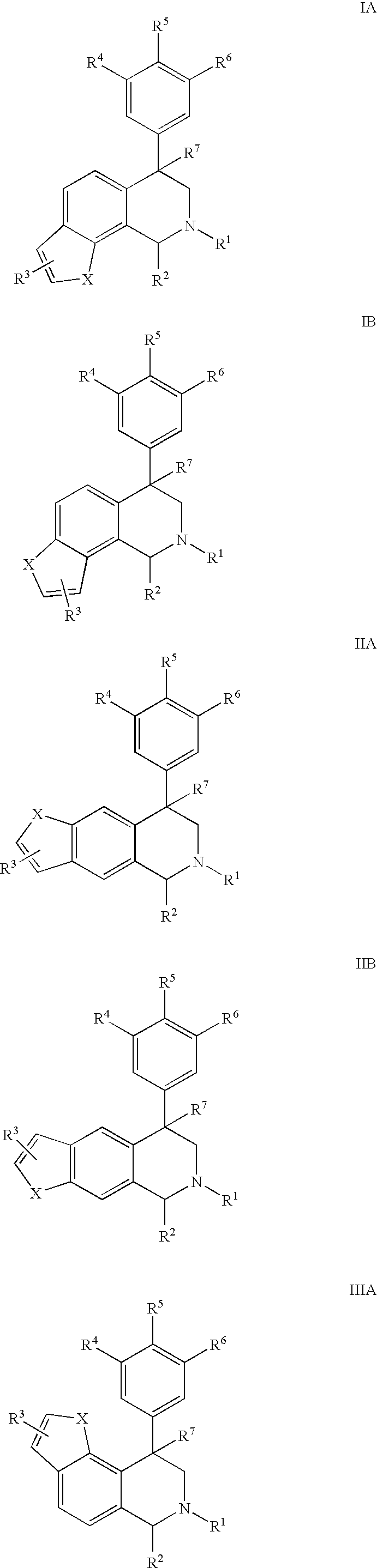
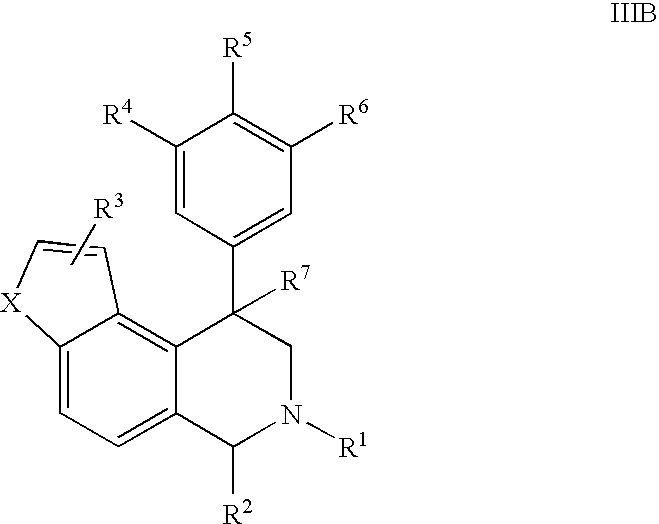
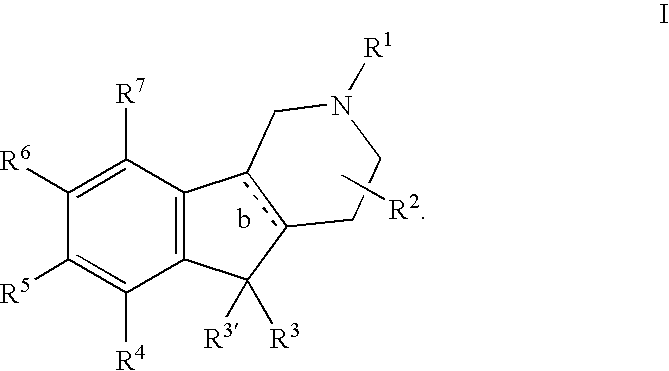
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
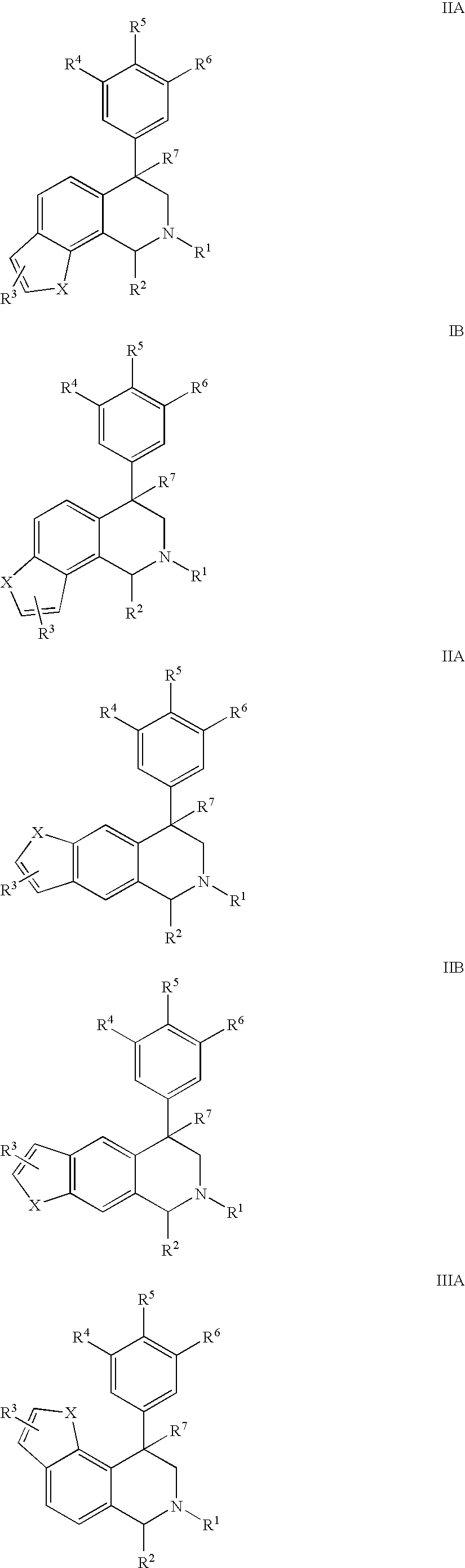
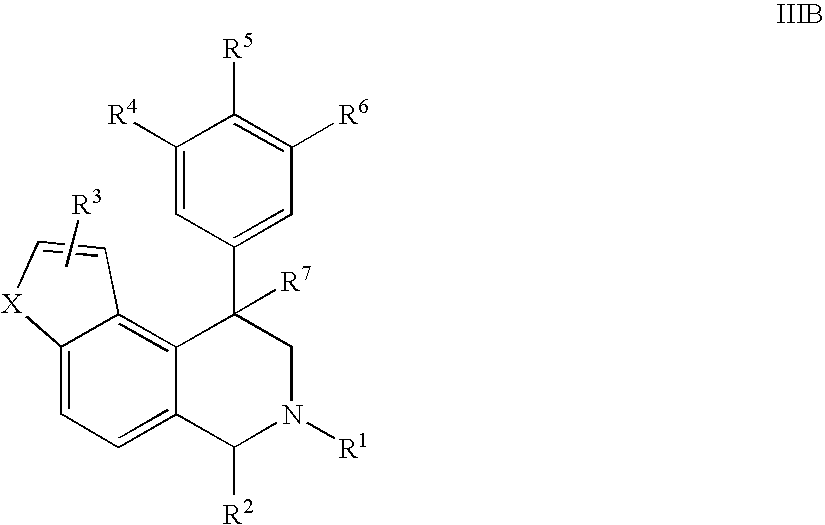
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
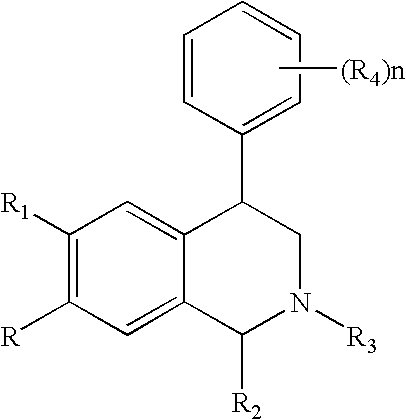
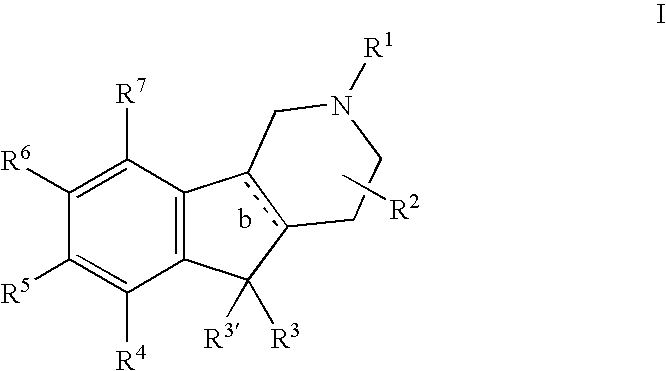
Novel 4-phenyl substituted tetrahydroisoquinolines and therapeutic use thereof
The present invention relates to a method of treating disorders including cognition impairment, generalized anxiety disorder, acute stress disorder, social phobia, simple phobias, pre-menstrual dysphoric disorder, social anxiety disorder, major depressive disorder, eating disorders, obesity, anorexia nervosa, bulimia nervosa, binge eating disorder, substance abuse disorders, chemical dependencies, nicotine addiction, cocaine addiction, alcohol addiction, amphetamine addiction, Lesch-Nyhan syndrome, neurodegenerative diseases, late luteal phase syndrome, narcolepsy, psychiatric symptoms anger, rejection sensitivity, movement disorders, extrapyramidal syndrome, Tic disorder, restless leg syndrome, tardive dyskinesia, sleep related eating disorder, night eating syndrome, stress urinary incontinence, migraine, neuropathic pain, diabetic neuropathy, fibromyalgia syndrome, chronic fatigue syndrome, sexual dysfunction, premature ejaculation, and male impotence. This method involves administering to a patient in need of such treatment a therapeutically effective amount of a disclosed compound. Such compounds are 4-phenyl substituted tetrahydroisoquinolines having the Formula IA, IB, IIA, IIB, IIIA or IIIC as set forth herein.
Owner:ALBANY MOLECULAR RESEARCH INC
Modulators of serotonin receptors
The present invention provides modulators of serotonin receptors, pharmaceutical compositions containing such modulators and methods for treating various diseases, conditions and disorders associated with modulation of serotonin receptors such as, for example: metabolic diseases, which includes but is not limited to obesity, diabetes, diabetic complications, atherosclerosis, impared glucose tolerance and dyslipidemia; central nervous system diseases which includes but is not limited to, anxiety, depression, obsessive compulsive disorder, panic disorder, psychosis, schizophrenia, sleep disorder, sexual disorder and social phobias; cephalic pain; migraine; and gastrointestinal disorders using such compounds and compositions.
Owner:BRISTOL MYERS SQUIBB CO
Combination of serotonin reuptake inhibitors and norephinephrine reuptake inhibitors
InactiveUS20050014848A1Prevent relapseIncreasing and improving neuronal processBiocideAmine active ingredientsStress inducedNorepinephrine reuptake inhibitor
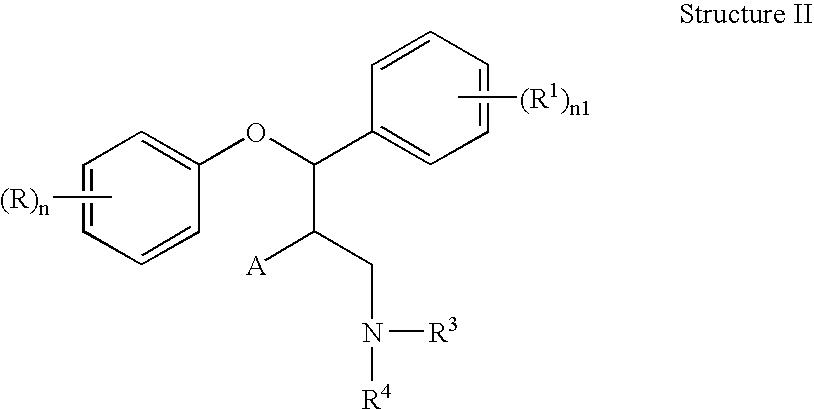
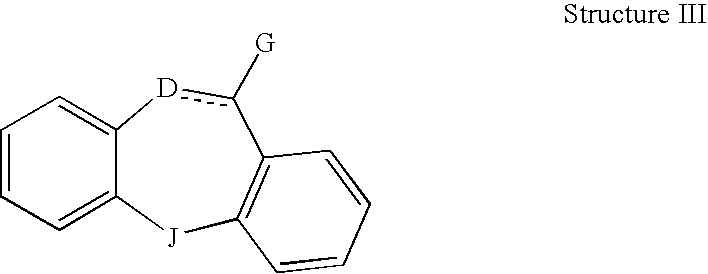
This invention is directed to pharmaceutical compositions and methods for treating a disorder or condition selected from the group consisting of depression, anxiety disorders, phobias, avoidant personality disorder, eating disorders, chemical dependencies, Parkinson's diseases, obsessive-compulsive disorder, negative symptoms of schizophrenia, cognitive dysfunction related to schizophrenia, premenstrual syndrome, stress-induced incontinence, headache, neuropathic pain, chronic pain, urinary incontinence, post-traumatic stress disorder, chronic stress, acute stress, fibromyalgia, depression comorbid with fibromyalgia, obesity, migraine and a combination thereof in a mammal. The methods in one embodiment comprise administering to a mammal in need of treatment for the disorder or condition: (i) at least one serotonin reuptake inhibitor or pharmaceutically acceptable salt thereof; (ii) at least one norepinephrine reuptake inhibitor or pharmaceutically acceptable salt thereof, wherein the norepinephrine reuptake inhibitor is selected from the group consisting of Structure II, Structure III, and Structure IV as defined in the specification; and (iii) a pharmaceutically acceptable carrier. The pharmaceutical compositions and methods of the invention are also useful for preventing a relapse associated with one of the foregoing disorders or conditions, and for treating a symptom associated with one of the foregoing disorders or conditions, wherein the symptom is selected from the group consisting of cognitive dysfunctions and somatic complaints.
Owner:PFIZER INC
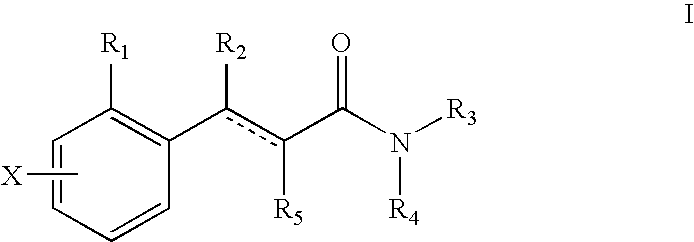
Substituted tricyclic heterocycles as serotonin receptor agonists and antagonists
The present application describes compounds, including all pharmaceutically acceptable salts, prodrugs, solvates and stereoisomers thereof, according to Formula I, pharmaceutical compositions, comprising at least one compound according to Formula I and optionally at least one additional therapeutic agent and methods of treating various diseases, conditions and disorders associated with modulation of serotonin receptors such as, for example: metabolic diseases, which includes but is not limited to obesity, diabetes, diabetic complications, atherosclerosis, impared glucose tolerance and dyslipidemia; central nervous system diseases which includes but is not limited to, anxiety, depression, obsessive compulsive disorder, panic disorder, psychosis, schizophrenia, sleep disorder, sexual disorder and social phobias; cephalic pain; migraine; and gastrointestinal disorders using compounds according to Formula I
Owner:BRISTOL MYERS SQUIBB CO
Combination of atypical antipsychotics and 5HT-1B receptor antagonists
InactiveUS20050256112A1Reduce morbidityDifferent recognizableNervous disorderMetabolism disorderDiseaseHeadaches
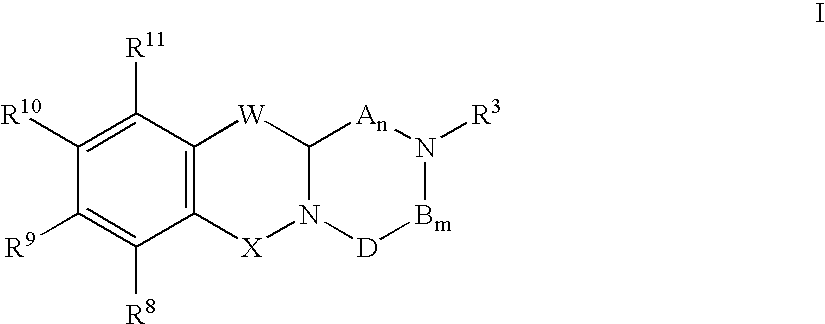
The present invention relates to a pharmaceutical composition for treating, for example, a disorder or condition selected from the group consisting of hypertension, depression, generalized anxiety disorder, phobias, posttraumatic stress disorder, avoidant personality disorder, sexual dysfunction, eating disorders, obesity, chemical dependencies, cluster headache, migraine, pain, Alzheimer's disease, obsessive-compulsive disorder, panic disorder, memory disorders, Parkinson's diseases, endocrine disorders, cerebellar ataxia, gastrointestinal tract disorders, negative symptoms of schizophrenia, premenstrual syndrome, Fibromyalgia Syndrome, stress incontinence, Tourette syndrome, trichotillomania, kleptomania, male impotence, cancer, chronic paroxysmal hemicrania and headache in a mammal, preferably a human, comprising (i) an atypical antipsychotic or a pharmaceutically acceptable salt thereof, (ii) a 5-HT1B receptor antagonist or a pharmaceutically acceptable salt thereof, wherein the 5-HT1B receptor antagonist is selected from the group consisting of (A) a compound of the formula I as described in the specification and (B) a compound of the formula II as described in the specification, and optionally (iii) a pharmaceutically acceptable carrier.
Owner:PFIZER INC
Compositions and Methods for Treating Social Anxiety
InactiveUS20110218215A1Preventing social phobiaStop formationBiocideNervous disorderAtropine sulfateMedicine
The disclosure provides a pharmaceutical composition for treating social anxiety, performance anxiety, and social phobia comprising a therapeutic amount for die treatment of a patient of a β-adrenergic receptor antagonist, an anti-diarrheal compound, and an optional anticholinergic compound. The β-adrenergic receptor antagonist may be the lipophilic β-blocker propranolol HCl, the anti-diarrheal compound may be the opioid diphenoxylate HCl, and the optional anticholinergic compound may be atropine sulfate. The composition for treating performance anxiety and social phobia can further include a pharmaceutically acceptable carrier. A method of preventing or treating social anxiety, performance anxiety, and social phobia in a patient is also provided, comprising administering a composition of the disclosure to a patient in need of such treatment. The composition administered in the present method comprises a therapeutic amount of a β-adrenergic receptor antagonist, an anti-diarrheal compound, and an optional anticholinergic compound.
Owner:HOLLY BENJAMIN D
Dihydroquinazolinones as 5HT modulators
The present application provides modulators of serotonin receptors, pharmaceutical compositions containing such modulators and methods for treating various diseases, conditions and disorders associated with modulation of serotonin receptors such as, for example: metabolic diseases, which includes but is not limited to obesity, diabetes, diabetic complications, atherosclerosis, impared glucose tolerance and dyslipidemia; central nervous system diseases which includes but is not limited to, anxiety, depression, obsessive compulsive disorder, panic disorder, psychosis, schizophrenia, sleep disorder, sexual disorder and social phobias; cephalic pain; migraine; and gastrointestinal disorders using such compounds and compositions.
Owner:BRISTOL MYERS SQUIBB CO
Use of enantiomeric pure escitalopram
The present invention relates to the use of anatiomeric pure escitalopram and / or of low dose medicaments thereof for the improved treatment of depression, in particular major depression disorder, neurotic disorders, acute stress disorder, eating disorders such as bulimia, anorexia and obesity, phobias, dysthymia, pre-mentrual syndrome, cognitive disorders, impulse control disorders, attention deficit hyperactivity disorder or drug abuse. The medicaments may also be used in the treatment of major depression disorder in "treatment resistant" patients.
Owner:H LUNDBECK AS
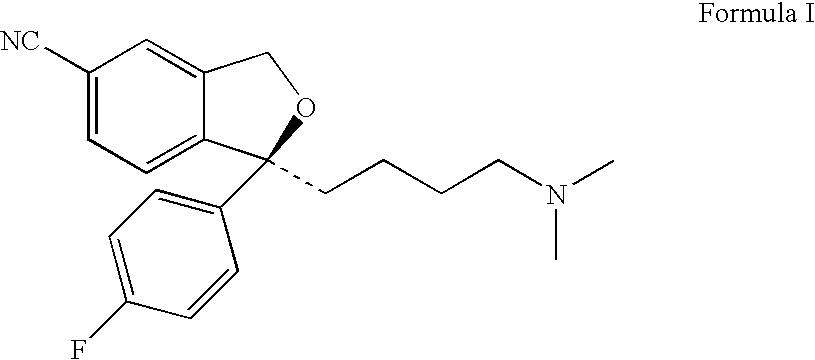
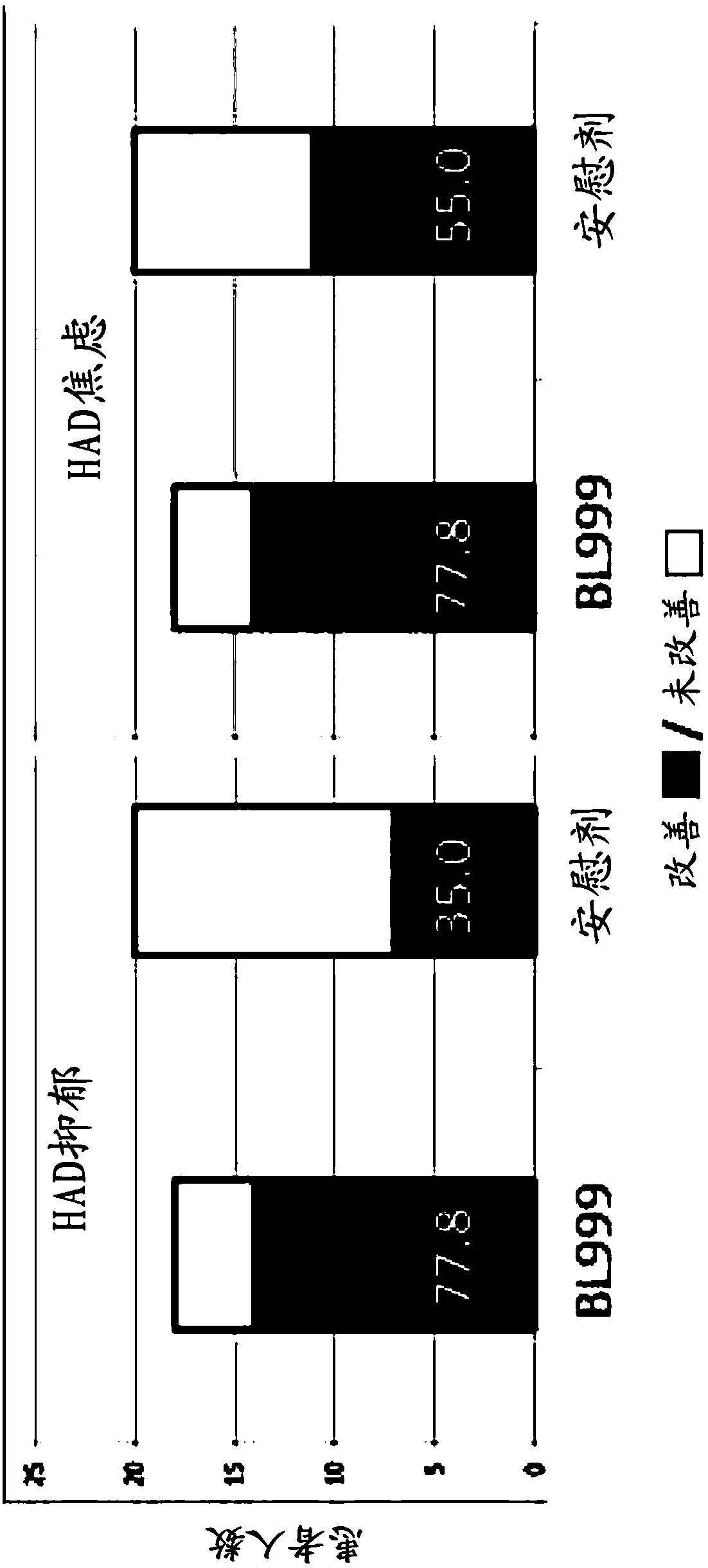
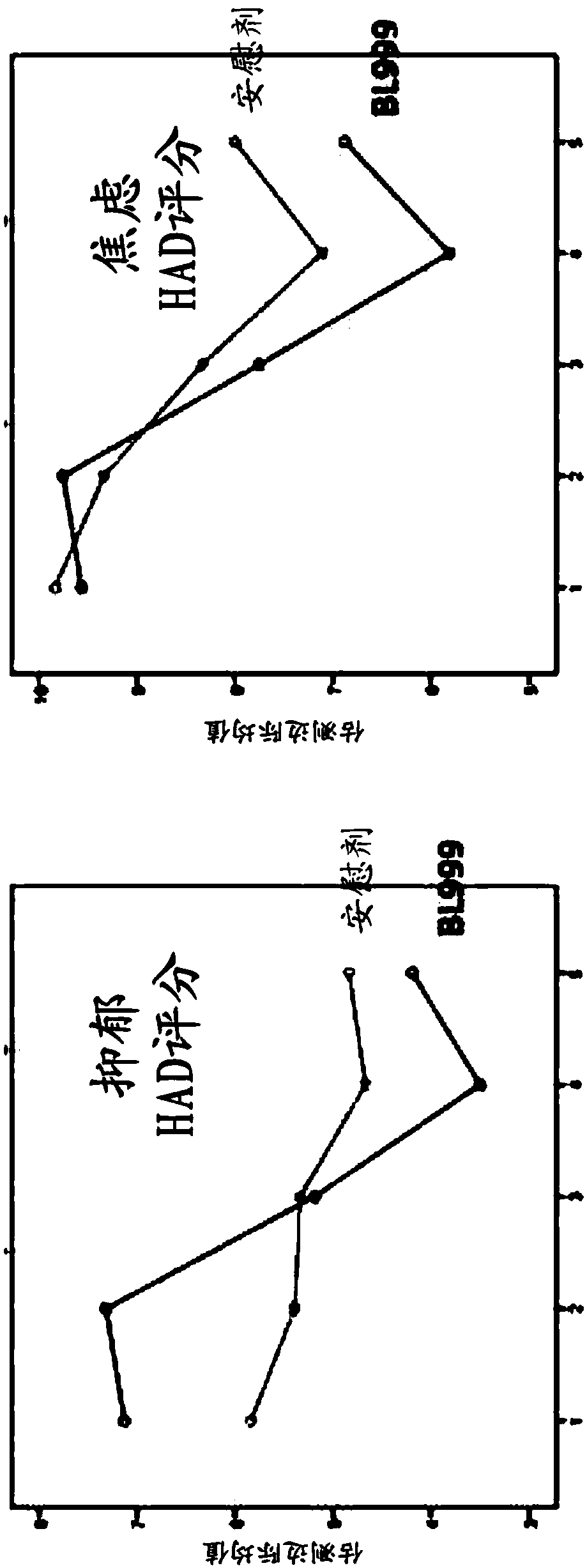
Methods and compositions using bifidobacterium longum to treat or prevent depressive symptoms
PendingCN108367033AApplication securityImprove securityNervous disorderUnknown materialsPhobiasDepression prevention
Compositions and methods use Bifidobacterium longum NCC3001 (ATCC BAA-999) to treat or prevent a depressive symptom. Prolonged anti-depressive effects can continue after administration of the compositions is ended. Non-limiting examples of a depressive symptom which can be treated or prevented include depressed mood; sadness; anxiety; "empty" feelings; loss of interest or pleasure; irritability; restlessness; changes in appetite or weight; sleep disturbances; lack of or decreased energy; feelings of worthlessness; guilt; helplessness; anger and hostility; difficulty in thinking, concentrating,or making decisions; hopelessness; tiredness; fatigue; memory difficulties; tearfulness; brooding; phobias; excessive worry over physical health; sexual dysfunction; persistent physical symptoms thatdo not respond to treatment; and combinations thereof. These depressive symptoms can be associated with a depressive state or depressive disorder or can be found sub-clinically or not associated withthese depressive states / disorders (e.g., not part of a syndrome or psychiatric disorder).
Owner:SOC DES PROD NESTLE SA
Treatment for Parkinson's Disease-combination high dose serotonergic synaptic reuptake inhibitor with phosphodiesterase inhibitor
A new treatment methodology and pharmacological composition for the treatment and remission of Parkinson's Disease and other neurological diseases are provided. The medication and treatment are based on the use of a combination of a phosphodiesterase inhibitor medication, commonly used to treat male erectile dysfunction, and a high-dose of serotonergic synaptic reuptake inhibitor medication, commonly used to treat depression, anxiety disorders, obsessive compulsive disorder and various panic phobias.The treatment regime is based upon the discovery that the primary cause of PD and various other related neurological conditions is dysfunction in the serotonergic pathways involving the brainstem, nucleus of Raphe, and various projecting serotonergic fibers. It has been determined that this dysfunction can be overcome by increasing the levels of the ligands and neurotransmitters cyclic-GMP and serotonin and the consequential increased binding of these ligands and neurotransmitters to efferent neuron receptors in the synapse. Testing indicates that the inventive treatment changes Parkinson's Disease from a debilitating, progressive, frightening, and previously untreatable pre-morbid condition to one that is rapidly reversible.
Owner:HELD JERRY M
Spiro imidazoline compounds
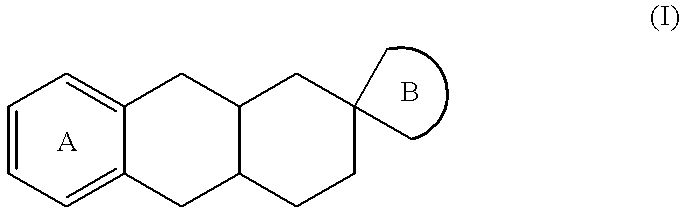
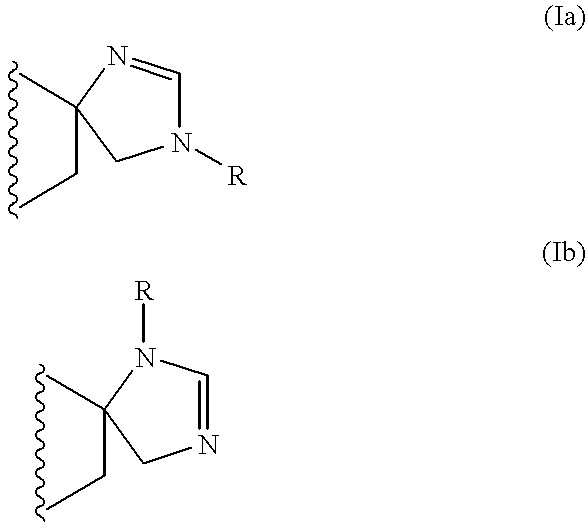
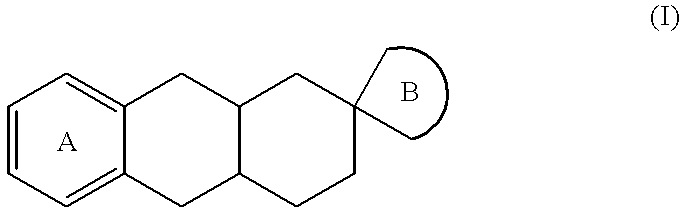
The invention relates to a compound of formula (I):wherein:A represents an optionally substituted benzene ring,B represents an imidazoline ring of formula (Ia) or (Ib):and medicinal products containing the same / are useful in treating or in preventing depression, obesity, panic attacks, anxiety, obsessive-compulsive disorders, cognitive disorders, phobias, impulsive disorders associated with the abuse of drugs and withdrawal therefrom, sexual dysfunctions, and Parkinson's disease.
Owner:LES LAB SERVIER
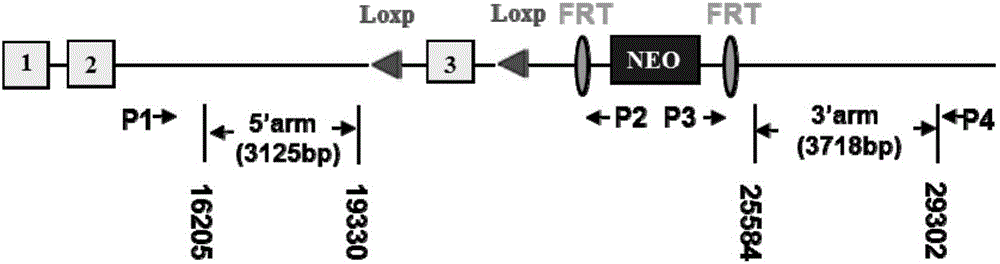
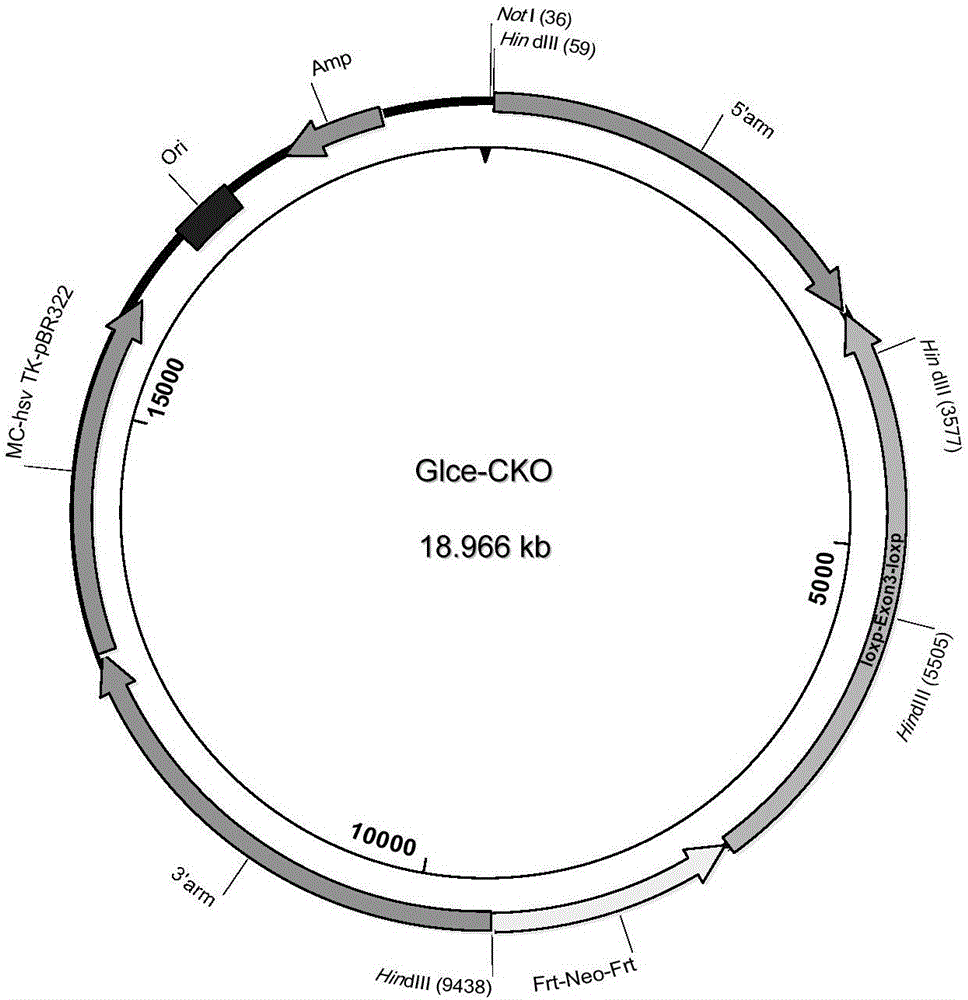
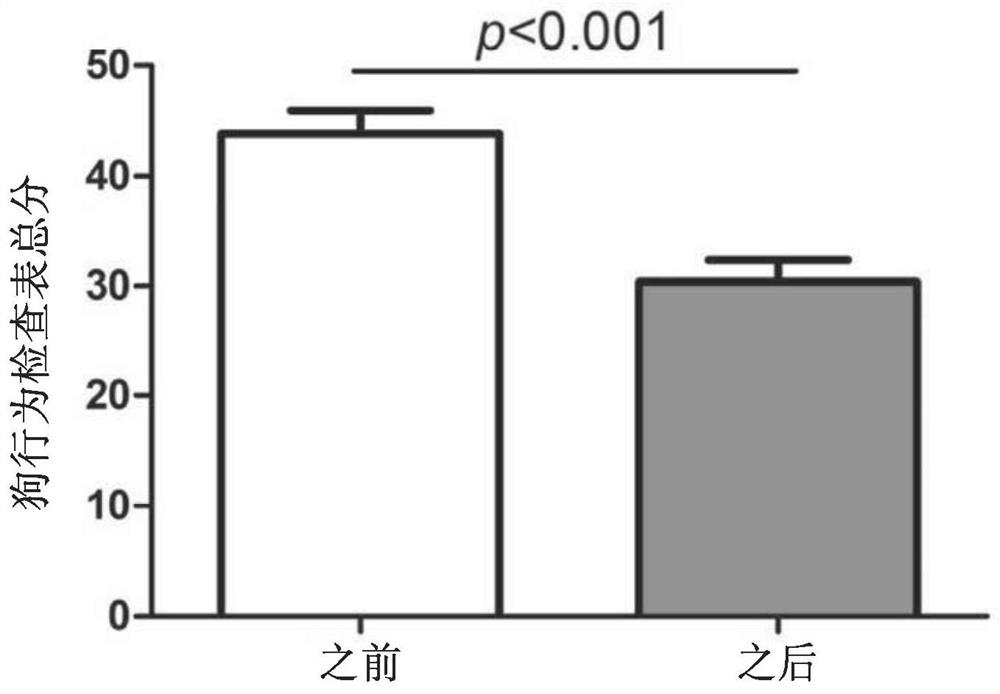
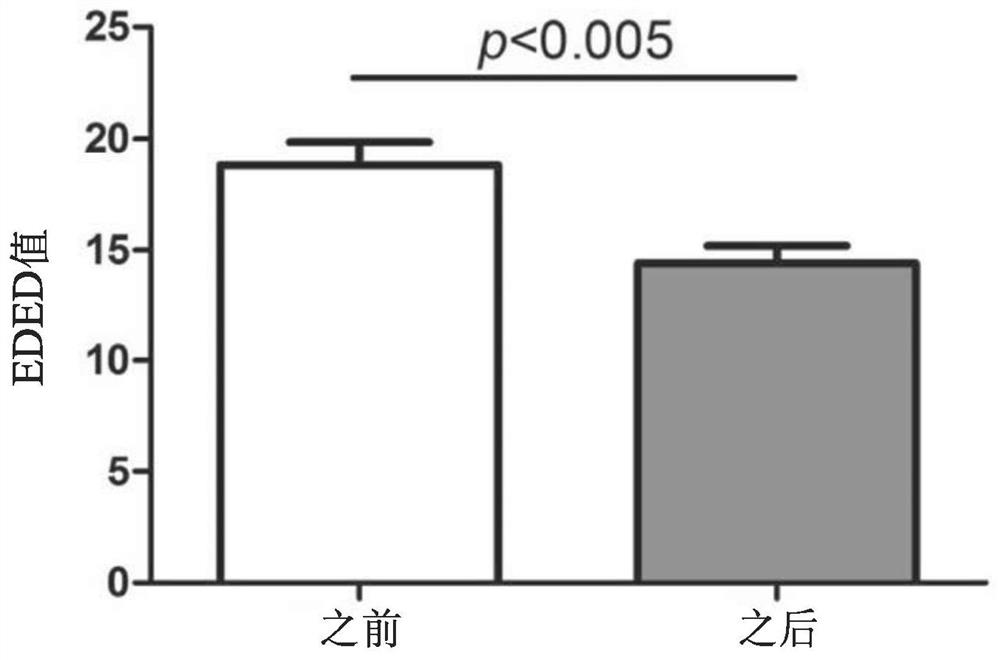
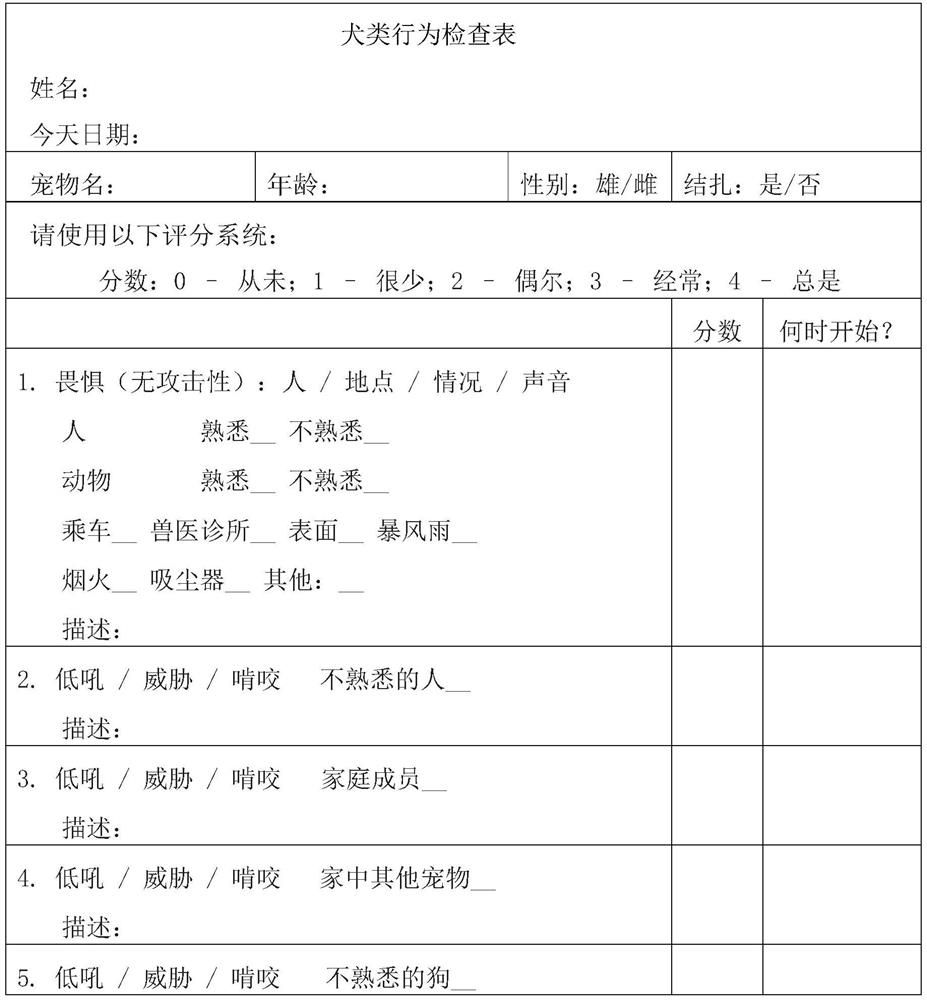
Establishment method of animal model for phobia or phobia-relevant diseases of non-human mammals and application of the animal model
The invention provides an establishment method of an animal model for phobia or phobia-relevant diseases of non-human mammals and application of the animal model and particularly provides a preparation method of the animal model. The preparation method comprises the following steps: (a) providing non-human mammal cells, and inactivating Glce genes in the cells, so as to obtain the non-human mammal cells with the inactivated Glce genes; and (b) preparing the animal model with the inactivated Glce genes for the phobia or the phobia-relevant diseases by virtue of the cells with the inactivated Glce genes prepared in the step (a). The animal model is an effective animal model for the phobia or the phobia-relevant diseases, can be used for researching diseases of the phobia, social phobia, topophobia and the like and further can be applied to the screening and tests of specific drugs.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Modulators of serotonin receptors
The present invention provides modulators of serotonin receptors, pharmaceutical compositions containing such modulators and methods for treating various diseases, conditions and disorders associated with modulation of serotonin receptors such as, for example: metabolic diseases, which includes but is not limited to obesity, diabetes, diabetic complications, atherosclerosis, impared glucose tolerance and dyslipidemia; central nervous system diseases which includes but is not limited to, anxiety, depression, obsessive compulsive disorder, panic disorder, psychosis, schizophrenia, sleep disorder, sexual disorder and social phobias; cephalic pain; migraine; and gastrointestinal disorders using such compounds and compositions.
Owner:BRISTOL MYERS SQUIBB CO
Psychological intensive phobia patient testing device
PendingCN113693595ADetermine cleaning effectEasy to seeFlexible article cleaningCleaning using toolsPhysical medicine and rehabilitationPhysical therapy
Owner:范黑龙
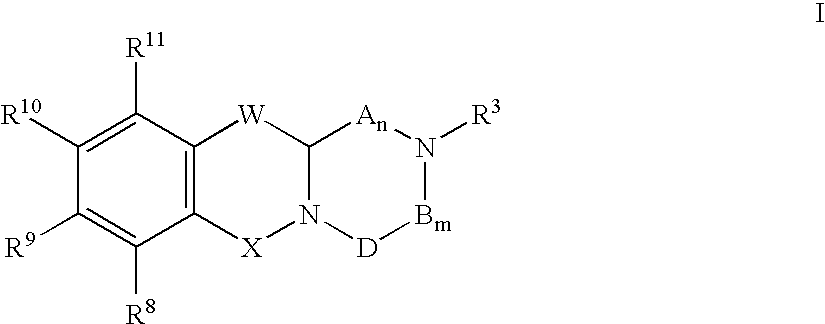
Use of d-serine derivatives for the treatment of anxiety disorders
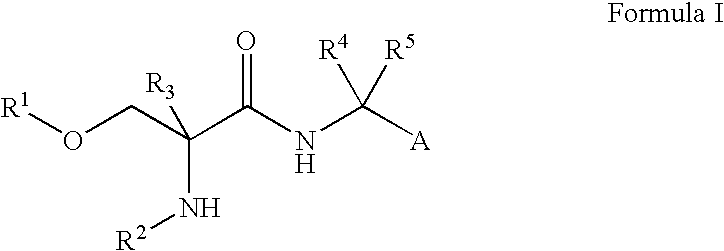
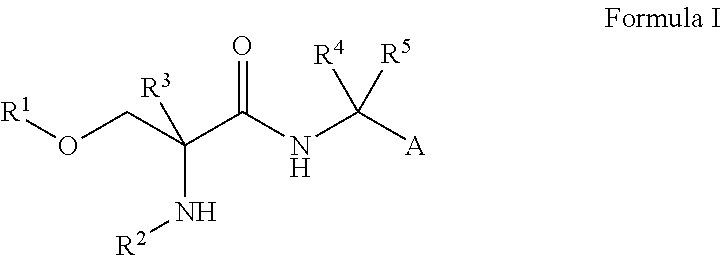
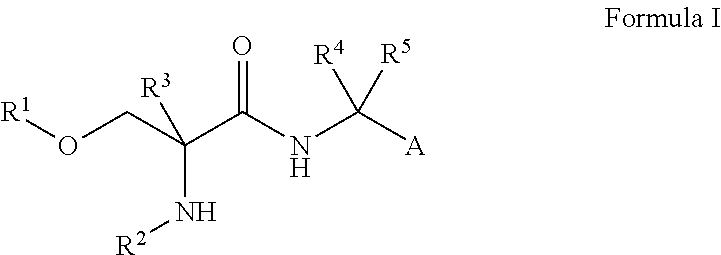
Compounds of Formula I are useful for the treatment of anxiety disorders such as generalized anxiety disorder (GAD), panic attack, post traumatic stress disorder (PTSD), obsessive compulsive disorder (OCD) and social phobias. wherein: A is chosen from: aryl or heteroaryl, A being optionally substituted with up to 5 independently-selected groups R8; R1 is chosen from: alkyl or haloalkyl; R2 is chosen from: H, C(O)R6, C(O)OR6, SO2R6 or C(O)NR6R7; R3, R4 and R5 are independently chosen from: H or alkyl; R6 and R7 are independently chosen from: H or alkyl; and R8 is chosen from: OH, CN, halo, alkyl, alkoxy, haloalkyl, haloalkoxy, C(O)R6, C(O)OR6, SO2R6 or C(O)NR6R7.
Owner:NPS PHARM INC
Therapy system and methods
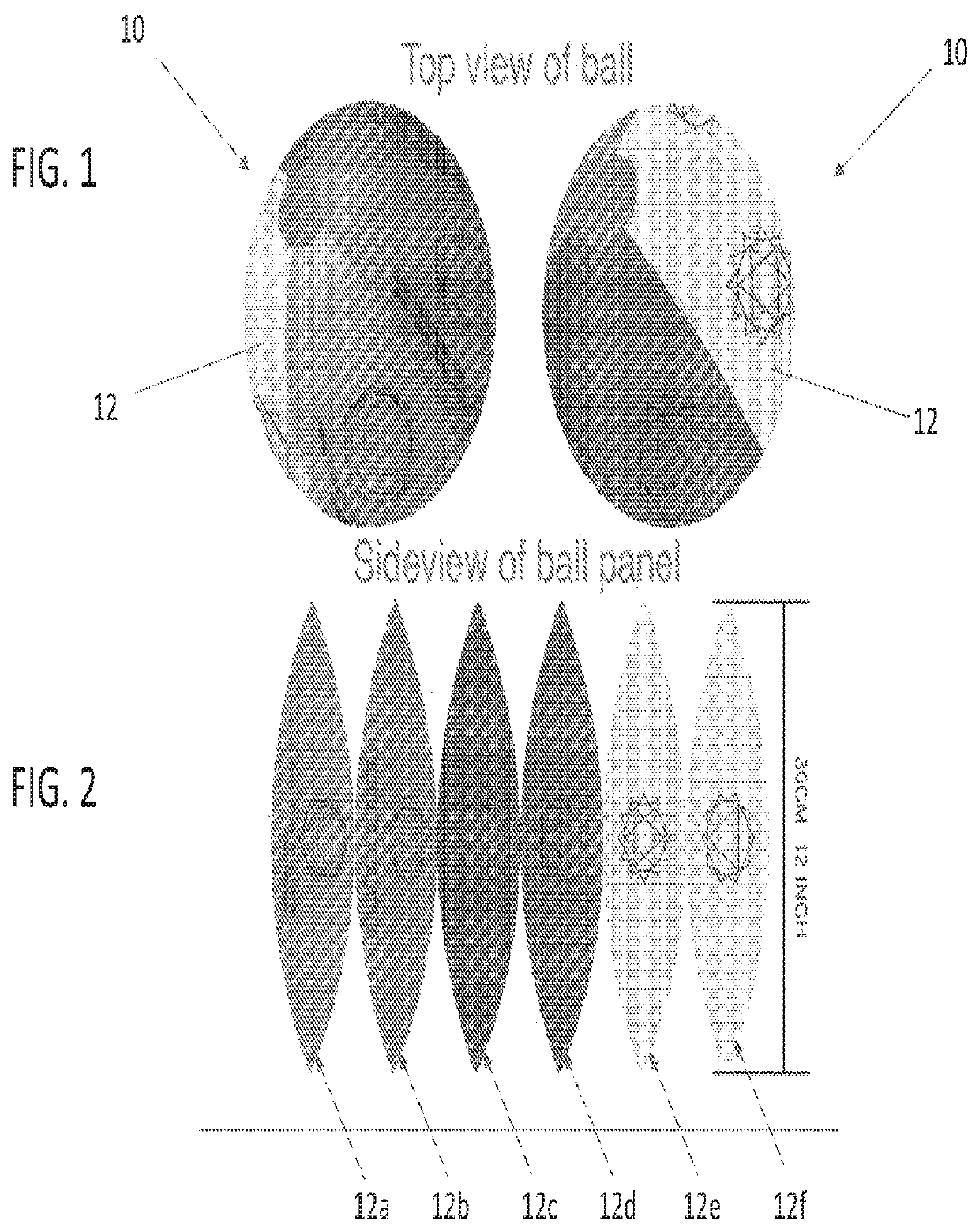
ActiveUS11033710B1Minimizing and alleviating target issueMinimizing and/or alleviating the target issueHollow inflatable ballsGymnastic exercisingPhysical medicine and rehabilitationTreatment issues
System and methods for therapy involving the use of three-dimensional articles of manufacture having printed media at predetermined locations thereon to overcome a therapeutic issue, such as (but not necessarily limited to) fear, phobias, trauma, addiction, grief, depression, feelings of loss, physical pain and diabetic neuropathy. By using the system and methods of the present invention while focusing on emotional and / or physical pain associated with a therapeutic issue, both hemispheres of the brain are activated and random stimulation via place or grid cells and their associated complex circuits occur, which collectively causes or creates new neural connections in the brain of the patient to thereby diminish or even resolve the emotional and / or physical pain associated with the therapeutic issue.
Owner:AVALON BIMM LLC
Crf2 ligands in combination therapy
This invention relates to antisense oligonucleotides directed against the mRNA of the corticotropin releasing factor subtype-2 (CRF2) receptor which substantially reduce expression of CRF2 receptors in the rodent brain and the use of antisense oligonucleotides in in vivo CNS studies of gene function and to treat a wide range of psychiatric disorders including anxiety, obsessive-compulsive disorder, panic disorders, post-traumatic stress disorder, phobias and depression.
Owner:BRISTOL MYERS SQUIBB PHARMA CO
Method for preventing or treating abnormal emotion or behavior in non-human animal by lactic acid bacterium
PendingUS20210353692A1Improve scoreReduce the valueNervous disorderBacteriaBiotechnologyLactic acid bacterium
Provided is a method for preventing or treating an emotional or behavioral problem in a non-human animal, such as fears, phobias, anxieties and aggression, by administering a lactic acid bacterium the non-human animal in need thereof
Owner:BENED BIOMEDICAL CO LTD
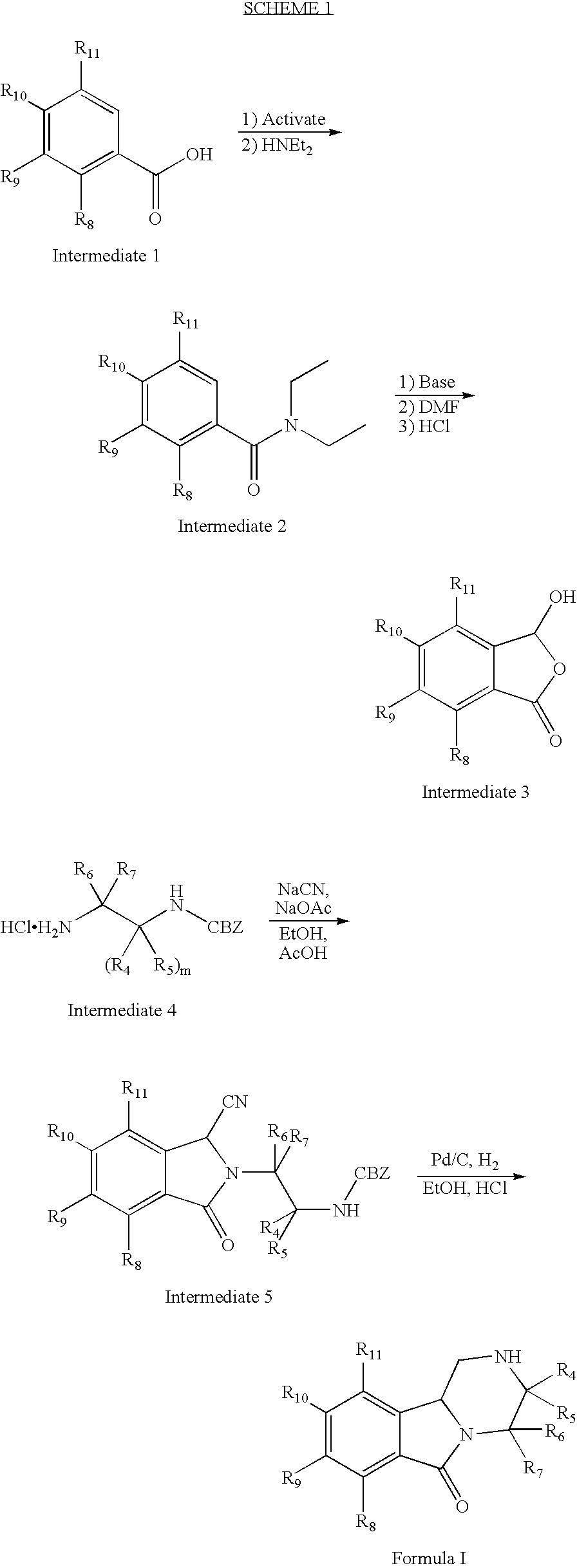
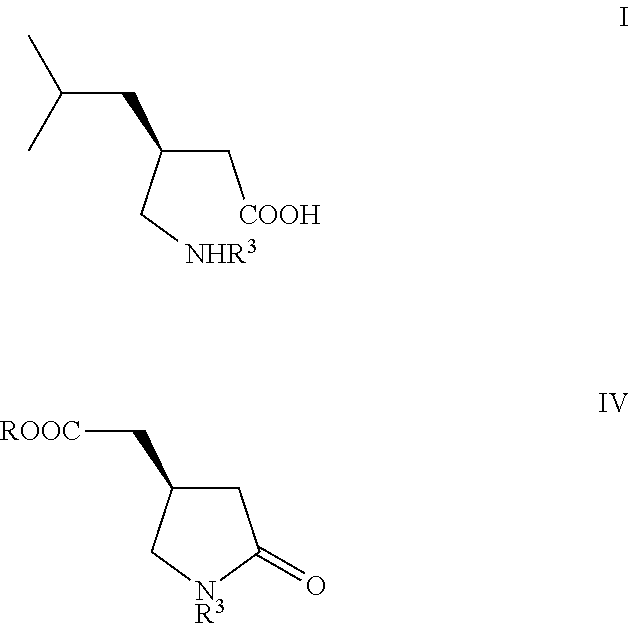
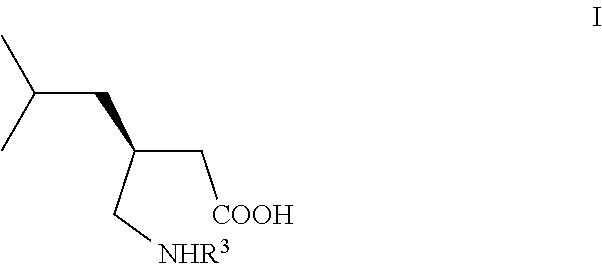
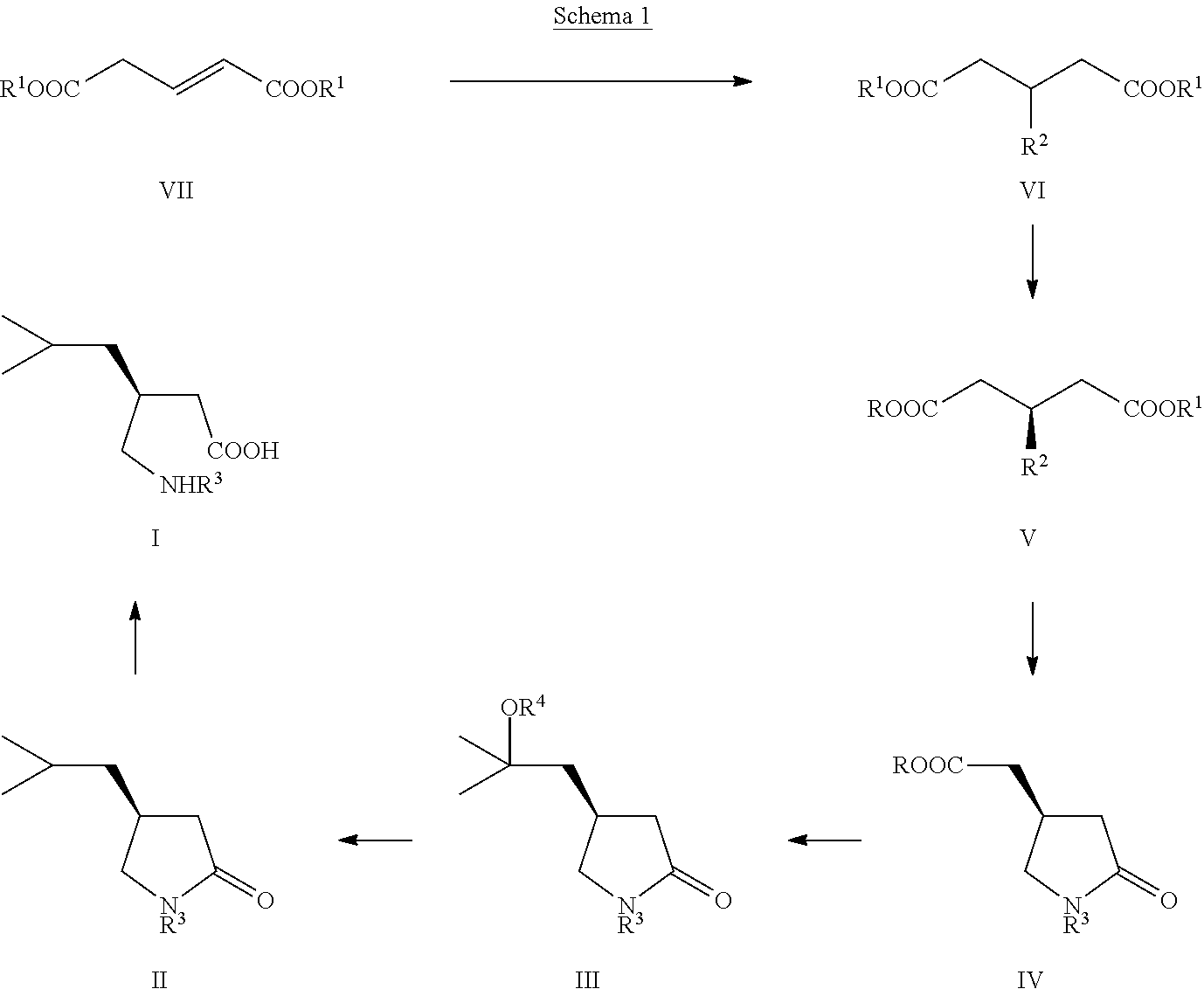
Manufacturing process for (S)-Pregabalin
The present invention relates to a novel manufacturing process and novel intermediates useful in the synthesis of pharmaceutically active compounds of general formula I used for treatment of epilepsy, neuropathic pain, anxiety and social phobia. The invention describes preparation of enantiomerically pure (S)-Pregabalin from chiral pyrrolidin-2-one of formula IV.
Owner:SOUKUP MILAN
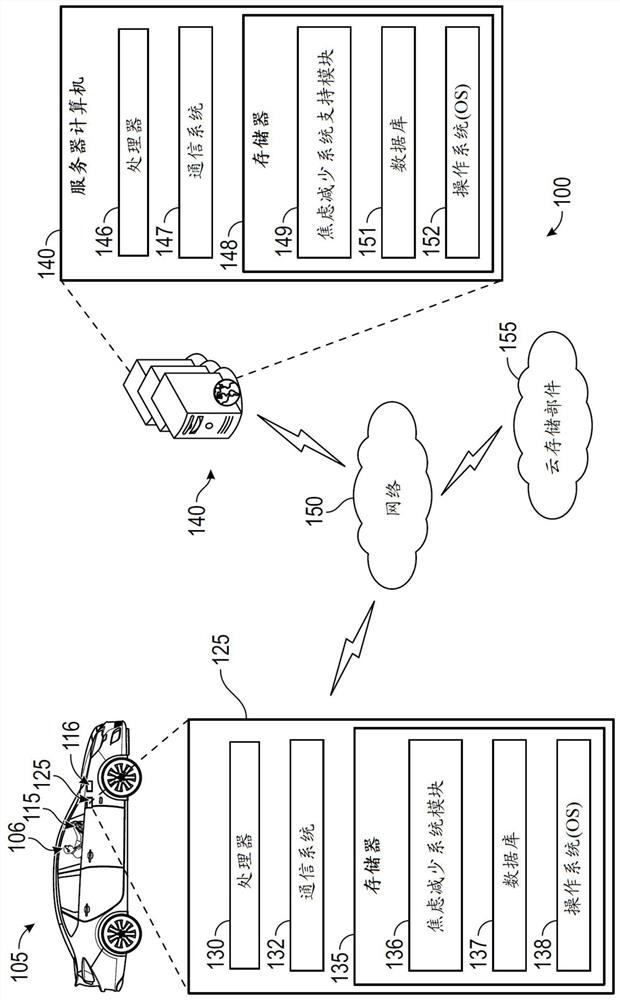
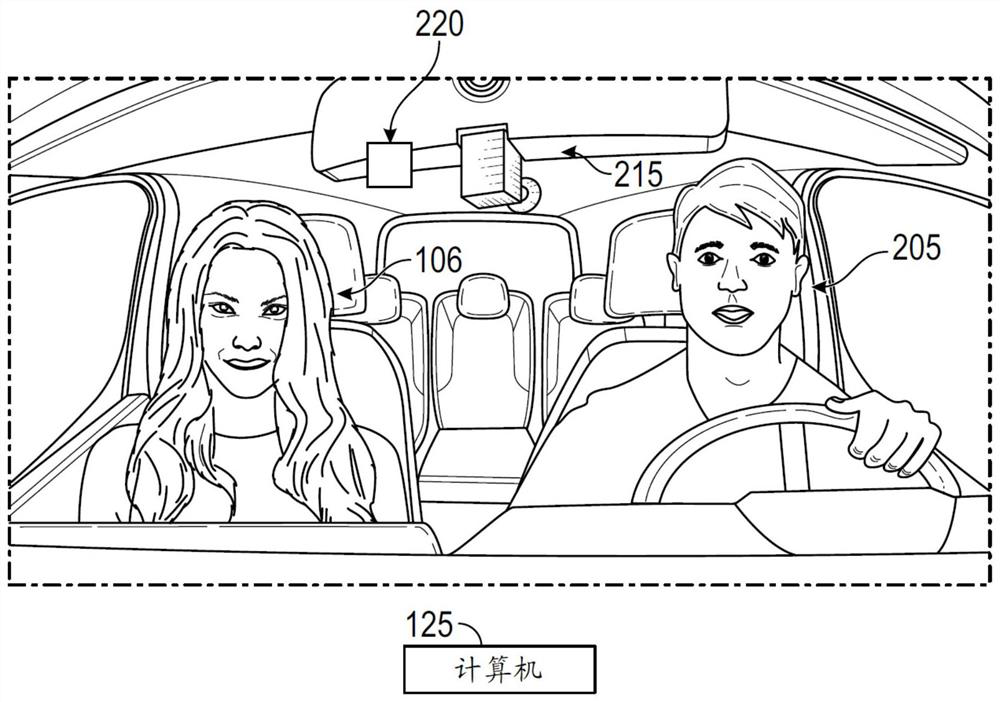
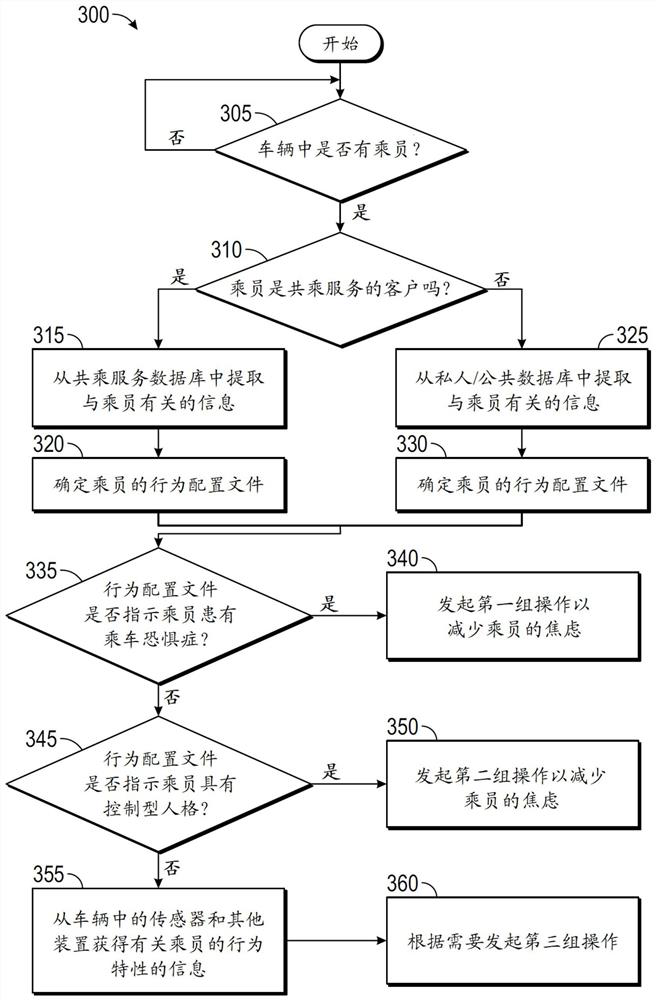
Systems and methods for reducing anxiety in occupant of vehicle
The disclosure provides systems and methods for reducing anxiety in an occupant of a vehicle. Exemplary embodiments described in the disclosure are generally directed to systems and methods for reducing anxiety in an occupant of a vehicle. In one exemplary method, a computer that is provided in a vehicle receives from a source such as a server computer, information that is used to determine a behavioral profile of an occupant of the vehicle. The computer utilizes the behavioral profile to identify a behavior characteristic of the occupant. The behavior characteristic is matched by the computerto one of various behavior categories such as an amaxophobia sufferer category or a controlling personality category. The computer then executes one or more operations in the vehicle based on the behavior category to which the occupant belongs. If the occupant belongs to the amaxophobia sufferer category, the computer may, for example, play an audio track, display a video clip, and / or change a driving characteristic of the vehicle.
Owner:FORD GLOBAL TECH LLC
Treatment for Parkinson's disease—combination high dose serotonergic synaptic reuptake inhibitor with phosphodiesterase inhibitor
A new treatment methodology and pharmacological composition for the treatment and remission of Parkinson's Disease and other neurological diseases are provided. The medication and treatment are based on the use of a combination of a phosphodiesterase inhibitor medication, commonly used to treat male erectile dysfunction, and a high-dose of serotonergic synaptic reuptake inhibitor medication, commonly used to treat depression, anxiety disorders, obsessive compulsive disorder and various panic phobias. The treatment regime is based upon the discovery that the primary cause of PD and various other related neurological conditions is dysfunction in the serotonergic pathways involving the brainstem, nucleus of Raphe, and various projecting serotonergic fibers. It has been determined that this dysfunction can be overcome by increasing the levels of the ligands and neurotransmitters cyclic-GMP and serotonin and the consequential increased binding of these ligands and neurotransmitters to efferent neuron receptors in the synapse. Testing indicates that the inventive treatment changes Parkinson's Disease from a debilitating, progressive, frightening, and previously untreatable pre-morbid condition to one that is rapidly reversible.
Owner:HELD JERRY M
Pharmaceutical formulation for use in the treatment of depressive and anxiety disorders
The present invention is aimed at a novel composition for use as a medicament; it is also aimed at said composition for use in the treatment of depressive and anxiety syndromes. In particular, said composition is for use in the treatment of: major depression, generalized anxiety disorder, social phobia, panic disorder, mixed depression and anxiety disorder, somatoform disorder, treatment-resistant depression, obsessive-compulsive disorder. The invention also relates to a process for the preparation of said pharmaceutical composition.
Owner:TURRI MILO
Method for preventing or treating abnormal emotion or behavior in non-human animal by lactic acid bacterium
PendingCN113302281AEvaluation value loweredLower scoreNervous disorderBacteriaBiotechnologyLactic acid bacterium
Provided is a method for preventing or treating an emotional or behavioral problem in a non-human animal, such as fears, phobias, anxieties and aggression, by administering a lactic acid bacterium to the non-human animal in need thereof.
Owner:BENED BIOMEDICAL CO LTD
Use of enantiomeric pure escitalopram
The present invention relates to the use of anantiomeric pure escitalopram and / or of low dose medicaments thereof for the improved treatment of depression, in particular major depression disorder, neurotic disorders, acute stress disorder, eating disorders such as bulimia, anorexia and obesity, phobias, dysthymia, pre-menstrual syndrome, cognitive disorders, impulse control disorders, attention deficit hyperactivity disorder or drug abuse. The medicaments may also be used in the treatment of major depression disorder in “treatment resistant” patients.
Owner:H LUNDBECK AS
Combination therapy wherein a serotonin reuptake inhibitor is used
The invention relates to the use of a compound, which is a serotonin reuptake inhibitor, and another compound, which is a GABAB receptor antagonist, inverse agonist or partial agonist for the preparation of a pharmaceutical composition for the treatment of depression, anxiety disorders and other affective disorders, such as generalized anxiety disorder, panic anxiety, obsessive compulsive disorder, acute stress disorder, post traumatic stress disorder and social anxiety disorder, eating disorders such as bulimia, anorexia and obesity, phobias, dysthymia, premenstrual syndrome, cognitive disorders, impulse control disorders, attention deficit hyperactivity disorder, drug abuse or any other disorder responsive to serotonin reuptake inhibitors.
Owner:H LUNDBECK AS
Compounds and pharmaceutical compositions for treating disorders associated with the 5-HT1A and 5-HT2A receptors
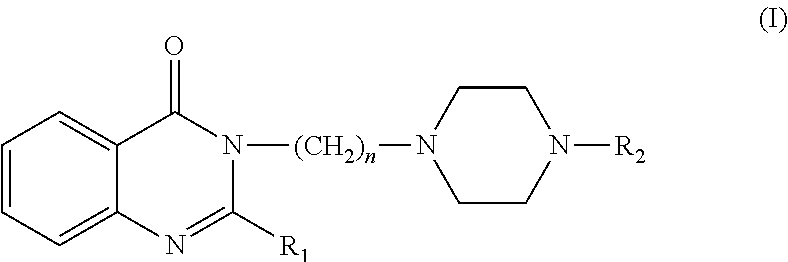
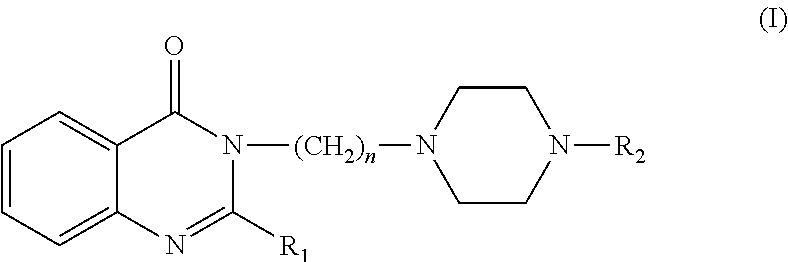
This invention is related to the alkyl-piperazine-phenyl 4 (3H)-quinazolinones general formula (I) compounds, pharmacologically active and able to act on the 5-HT1A and 5-HT2A serotonin receptors in a manner that promotes the control, relief or cure of disorders associated with these receptors, and pharmaceutical compositions containing the compounds for the treatment of disorders associated with these receptors. These compounds and their pharmaceutical compositions are useful in the treatment of conditions such as depression, anxiety, phobias, addictions, aggressiveness, impulsiveness, panic, psychotic, eating and sleep disorders, obsessive-compulsive disorder and female sexual dysfunctions, among other disorders associated with these receptors.
Owner:ACHE LAB FARM +1
Medicine for treating social anxiety disorder
InactiveCN105214011AGood treatment effectNervous disorderInanimate material medical ingredientsPhobiasBlood vessel
The invention relates to a medicine for treating social anxiety disorder, belongs to the technical fields of medicines and aims at solving the social anxiety disorder problem of some patients suffering from the social anxiety disorder. The social anxiety disorder is analyzed, besides psychological and historical reasons of the patients, physique reasons comprise liver fire and phlegm heat, internal disturbance of evil and wind and obstruction of blood vessels and orifices, and a split vision phobia is treated by combining the following traditional Chinese medicines with the effects of clearing liver fire, purging heat and reducing phlegm, regulating qi and removing stasis as well as tranquilizing mind: 10 parts of rhizoma pinellinae praeparata, 15 parts of curcuma aromatica, 10 parts of rhizoma typhonii, 10 parts of dragon bone, 15 parts of mongolian snake gourd, 9 parts of Chinese rhubarb, 10 parts of cape jasmine, 15 parts of indigo naturalis, 15 parts of raw peach kernel, 10 parts of fructus aurantii, 15 parts of uncaria, 12 parts of compound of glauber-salt and liquorice, 10 parts of bamboo shavings, 9 parts of vermiculite schist seu and 4 parts of angelica root. The medicine for treating the social anxiety disorder can suit the remedy to pathogenesis and has an obvious treatment effect.
Owner:蓝本祥
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com