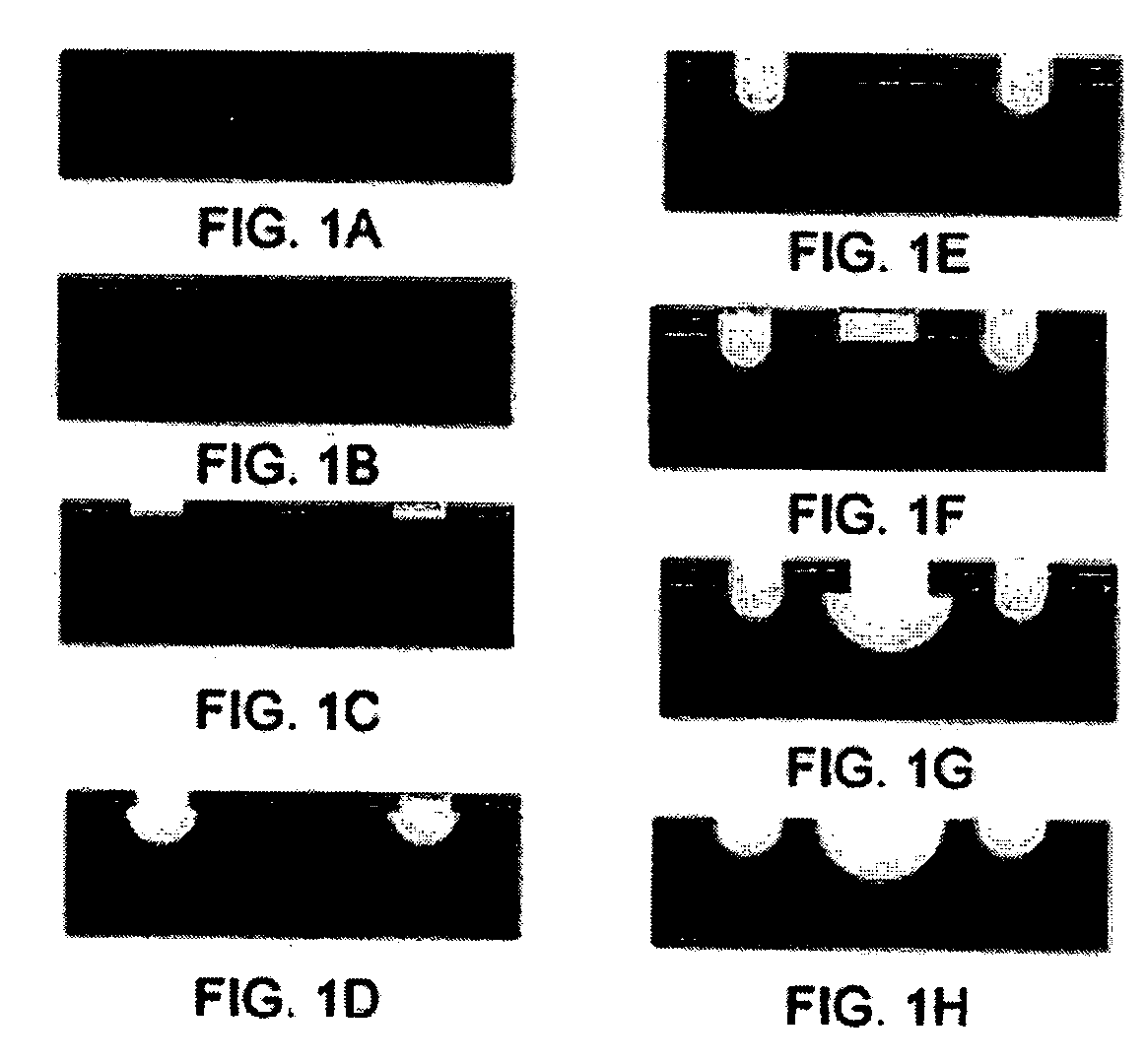
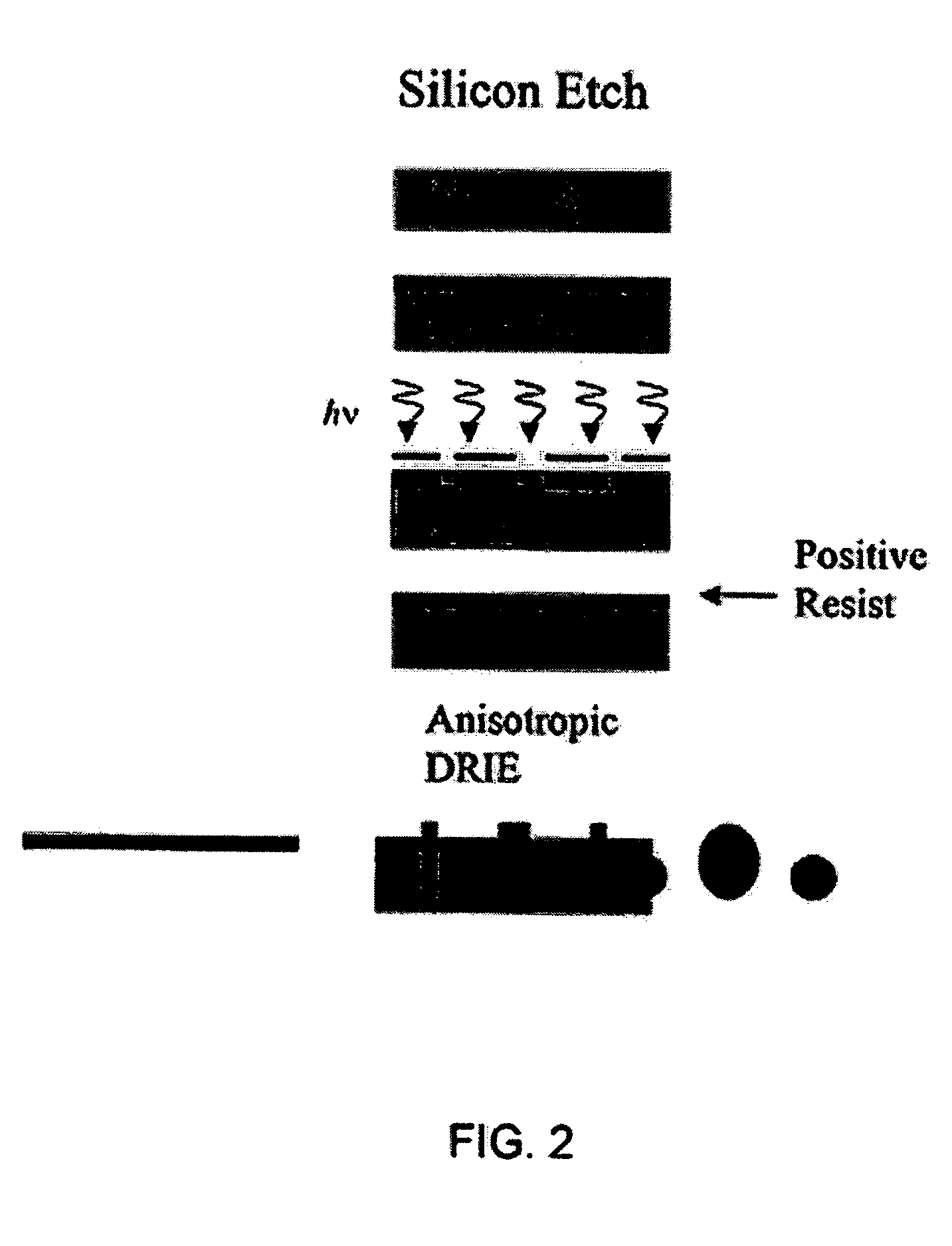
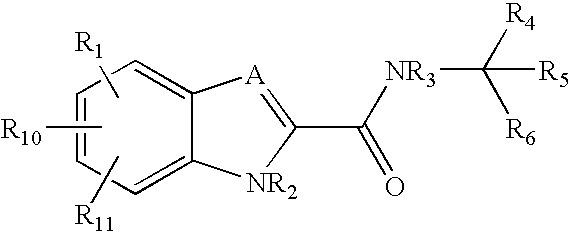
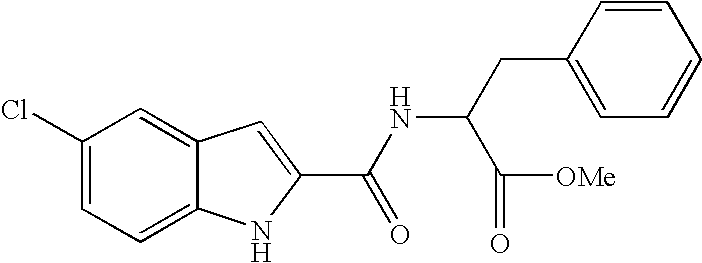
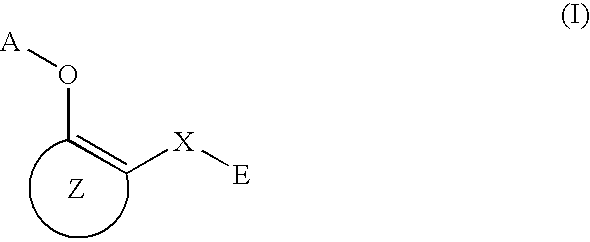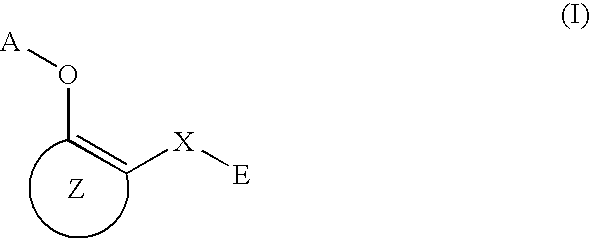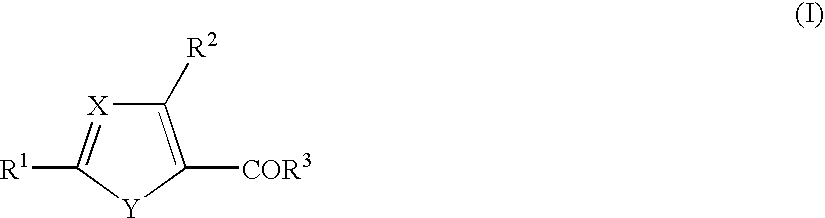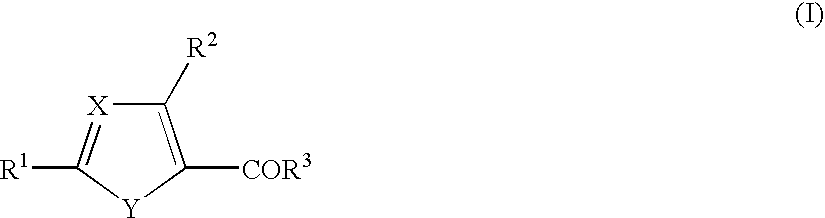Patents
Literature
1634 results about "Pharmacometrics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Pharmacometrics is mathematical models of biology, pharmacology, disease, and physiology used to describe and quantify interactions between xenobiotics and patients (human and non-human), including beneficial effects and adverse effects. It is normally applied to quantify drug, disease and trial information to aid efficient drug development, regulatory decisions and rational drug treatment in patients.
Apolipoprotein A-I agonists and their use to treat dyslipidemic disorders
The present invention provides peptides and peptide analogues that mimic the structural and pharmacological properties of human ApoA-I. The peptides and peptide analogues are useful to treat a variety of disorders associated with dyslipidemia.
Owner:DASSEUX JEAN LOUIS +5
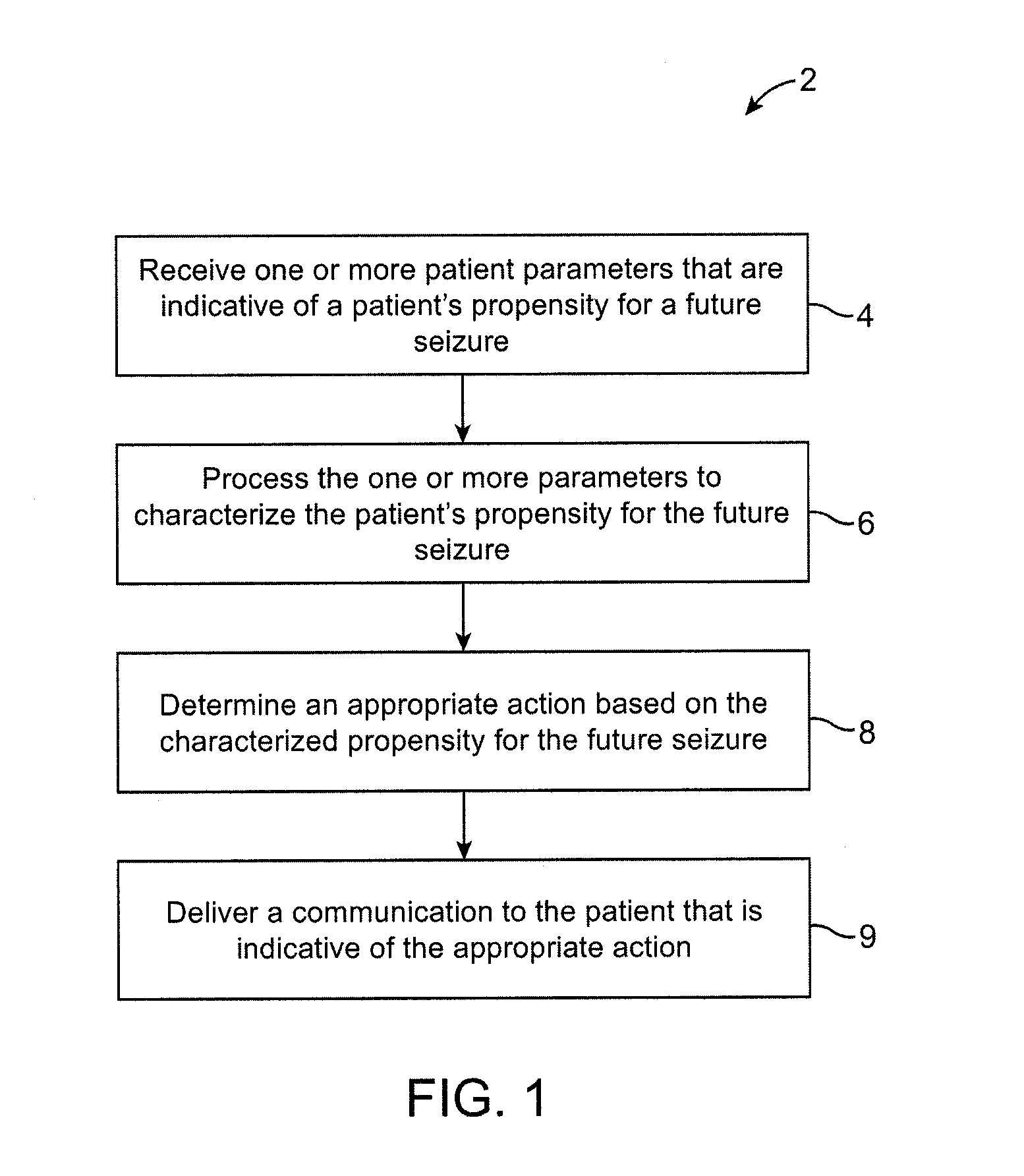
Methods and systems for administering an appropriate pharmacological treatment to a patient for managing epilepsy and other neurological disorders
InactiveUS20070287931A1Reduced magnitudeShorten the durationElectroencephalographyBiocidePharmacometricsNeurological disorder
The present invention provides systems and methods for managing epilepsy. In one embodiment, a method of the present invention characterize a patient's propensity for a future epileptic seizure and facilitates administration of a pharmacological agent. The dosage of the pharmacological agent is typically a function of at least one of the patient's propensity for the future epileptic seizure and time period to seizure.
Owner:CYBERONICS INC
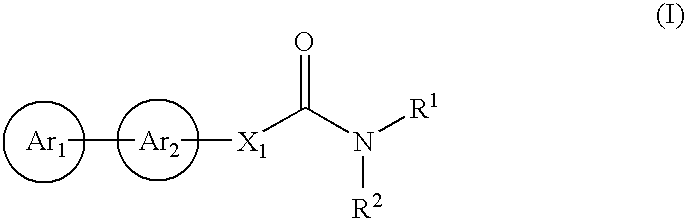
Cinnamide compound
The present invention relates to a compound represented by Formula (I): (wherein Ar1 represents an imidazolyl group which may be substituted with 1 to 3 substituents; Ar2 represents a pyridinyl group, a pyrimidinyl group, or a phenyl group which may be substituted with 1 to 3 substituents; X1 represents (1) —C≡C— or (2) a double bond etc. which may be substituted; R1 and R2 represent, for example, a C1-6 alkyl group or C3-8 cycloalkyl group which may be substituted) or a pharmacologically acceptable salt thereof and to the use thereof as pharmaceutical agents. The object of the present invention is to find a therapeutic or preventive agent for diseases caused by Aβ. According to the present invention, a therapeutic or preventive agents for diseases caused by Aβ can be provided.
Owner:EISIA R&D MANAGEMENT CO LTD
Implantable device for penetrating and delivering agents to cardiac tissue
InactiveUSRE37463E1Avoid damageEliminate the effects ofTransvascular endocardial electrodesDiagnostic recording/measuringElectrical conductorCardiac wall
An implantable devices for the effective elimination of an arrhythmogenic site from the myocardium is presented. By inserting small biocompatible conductors and / or insulators into the heart tissue at the arrhythmogenic site, it is possible to effectively eliminate a portion of the tissue from the electric field and current paths within the heart. The device would act as an alternative to the standard techniques for the removal of tissue from the effective contribution to the hearts electrical action which require the destruction of tissue via energy transfer (RF, microwave, cryogenic, etc.). This device is a significant improvement in the state of the art in that it does not require tissue necrosis.In one preferred embodiment the device is a non conductive helix that is permanently implanted into the heart wall around the arrhythmogenic site. In variations on the embodiment, the structure is wholly or partially conductive, the structure is used as an implantable substrate for anti arrhythmic, inflammatory, or angiogenic pharmacological agents, and the structure is deliverable by a catheter with a disengaging stylet. In other preferred embodiments that may incorporate the same variations, the device is a straight or curved stake, or a group of such stakes that are inserted simultaneously.
Owner:BIOCARDIA
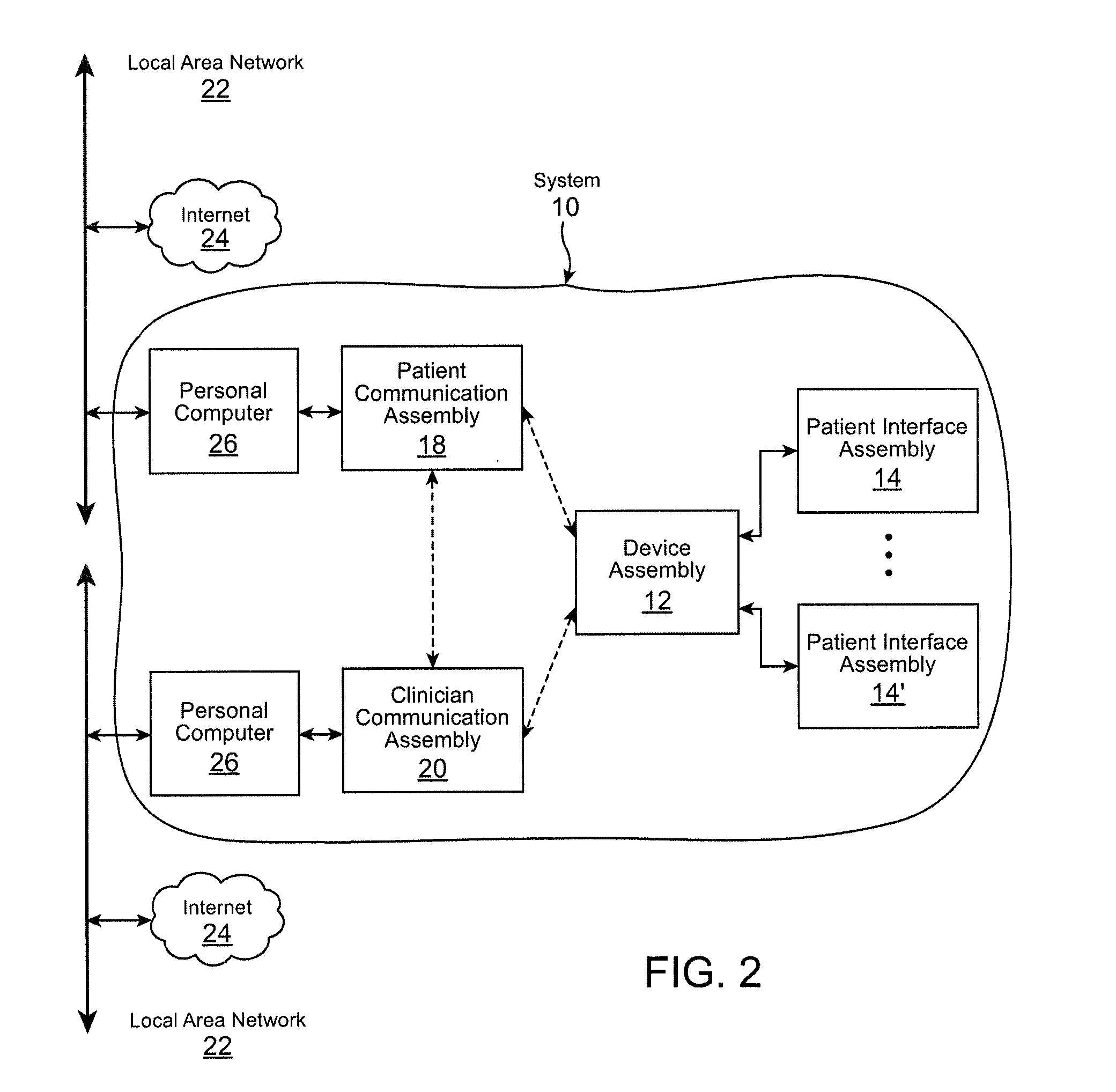
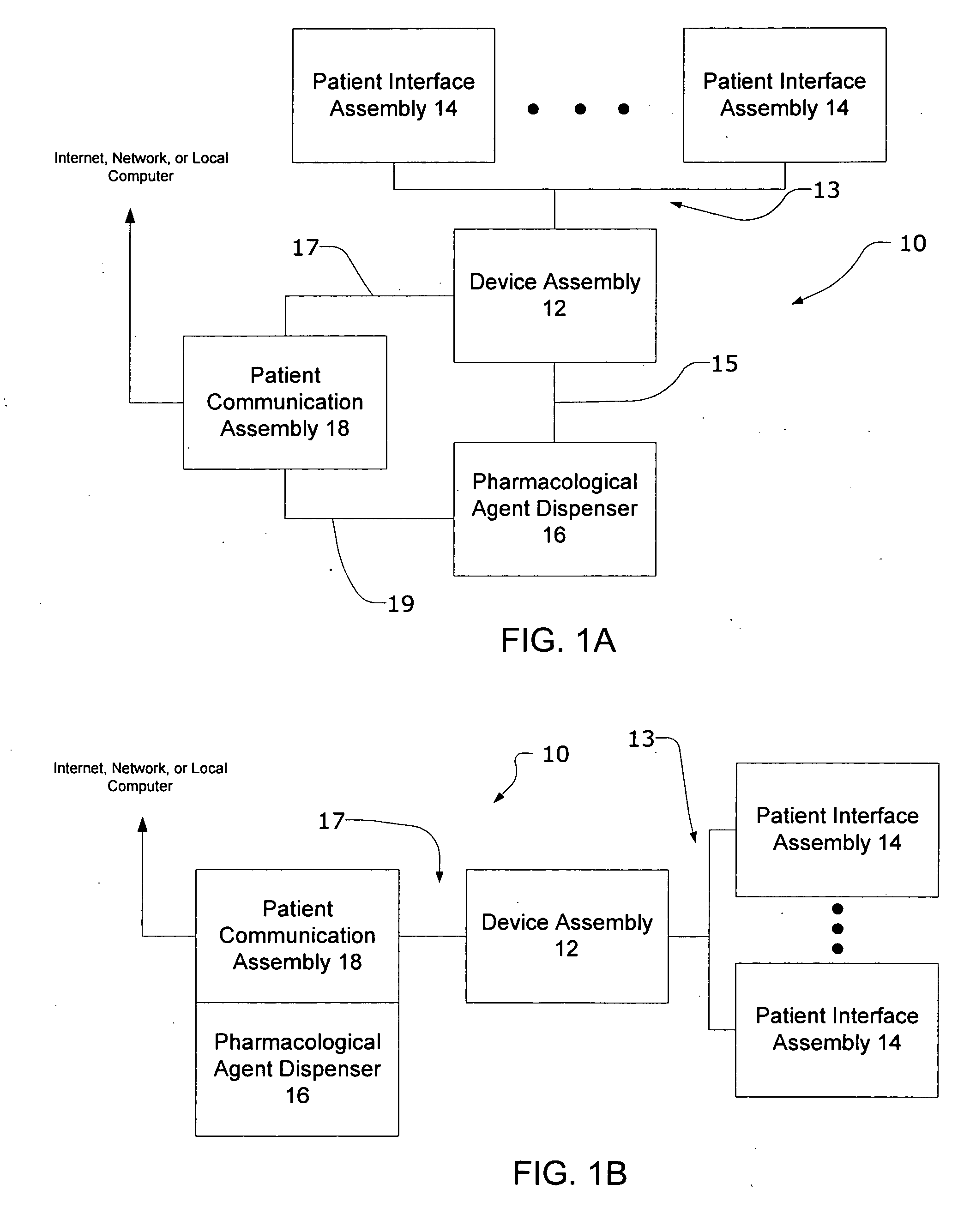
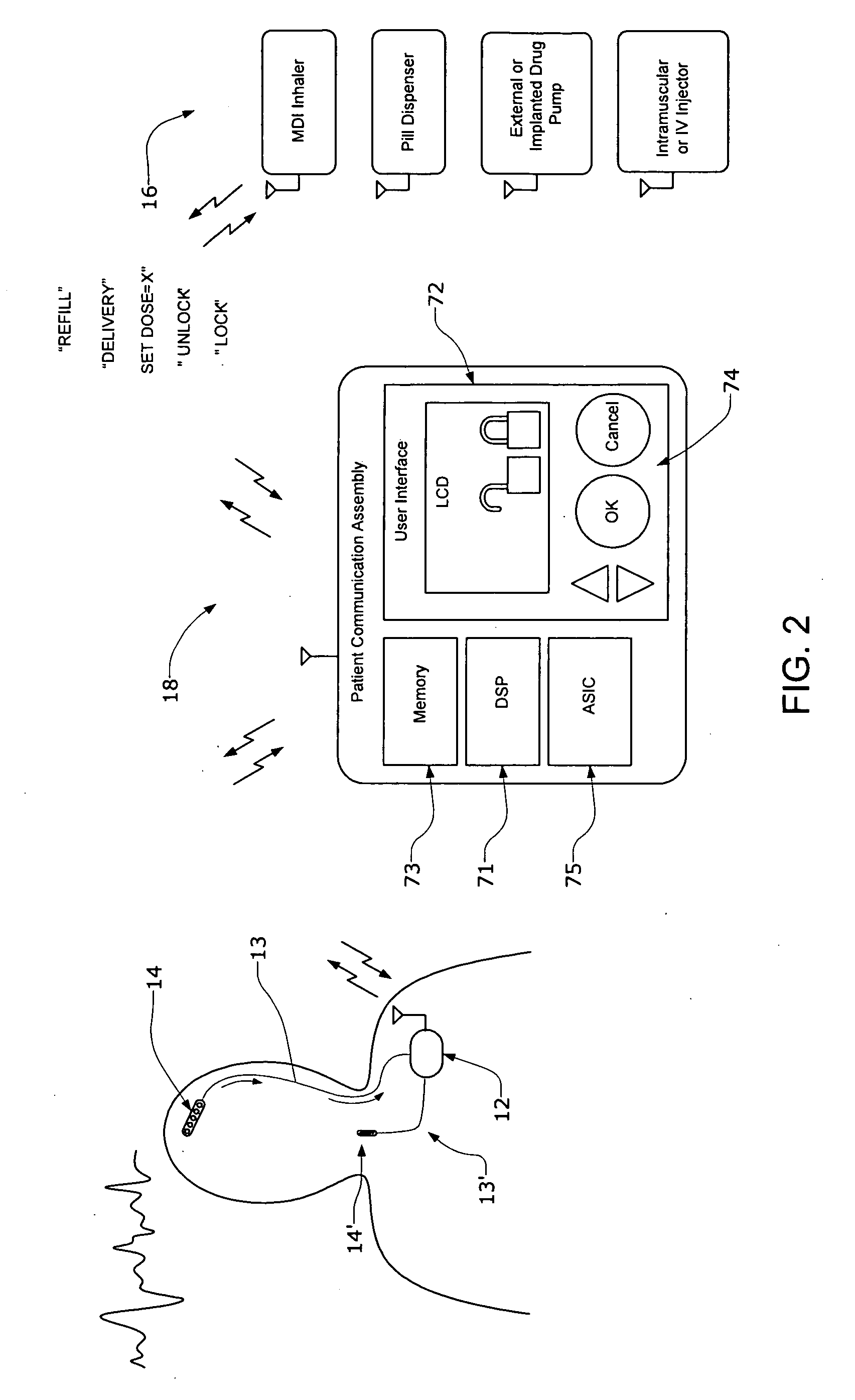
Systems and methods for characterizing a patient's propensity for a neurological event and for communicating with a pharmacological agent dispenser
InactiveUS20070149952A1Selectively limit accessSelectively limit to administrationDrug and medicationsTelemedicinePharmacometricsAntiepileptic drug
The present invention provides systems and methods for managing intake of a pharmacological agent. In one method of the present invention, the systems and methods are for controlling intake of an anti-epileptic drug. In such embodiments, one or more signals from a patient are processed to predict an onset of a seizure. Upon the prediction of the seizure, the patient is allowed to access the pharmacological agent in a pharmacological agent dispenser.
Owner:CYBERONICS INC
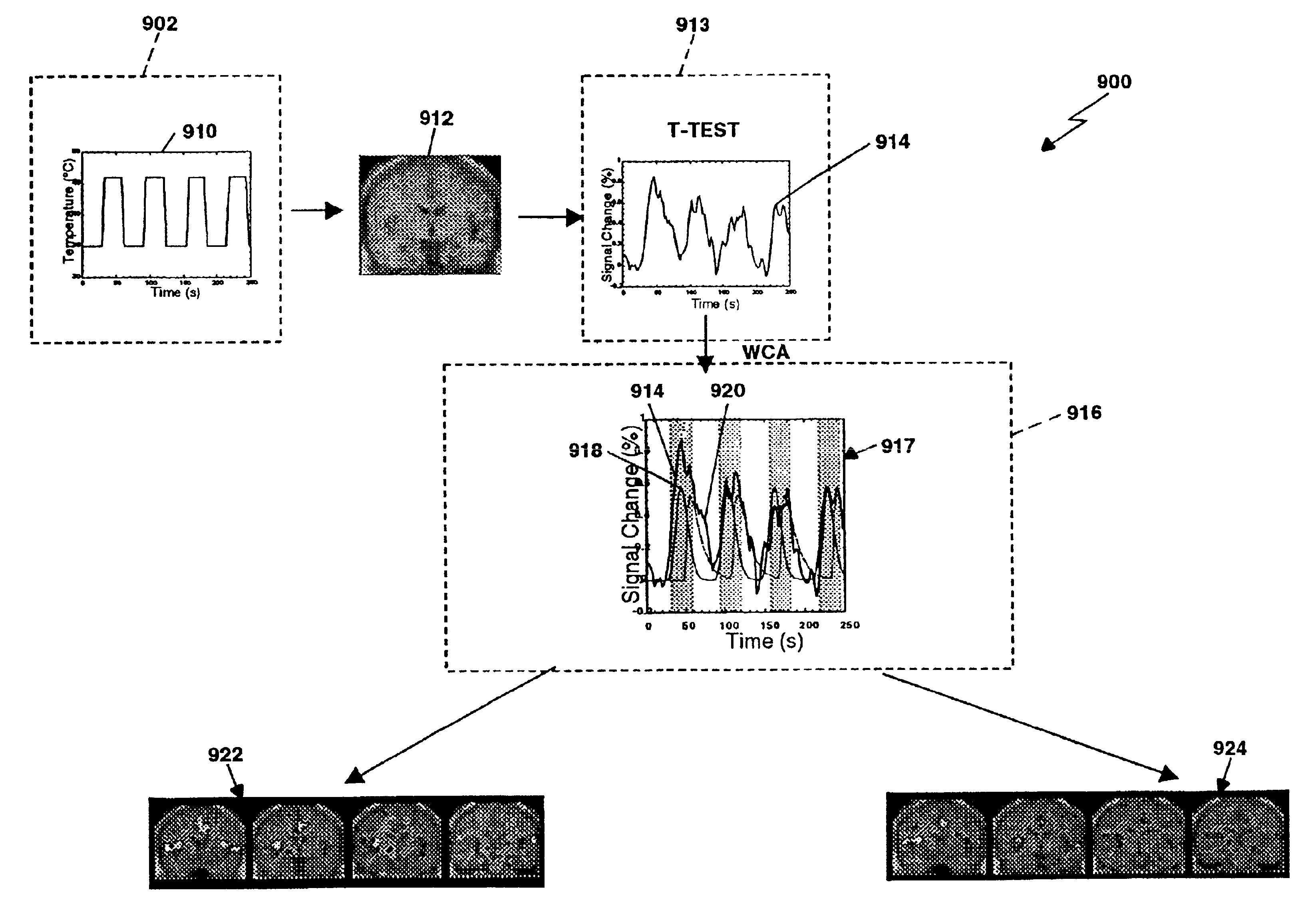
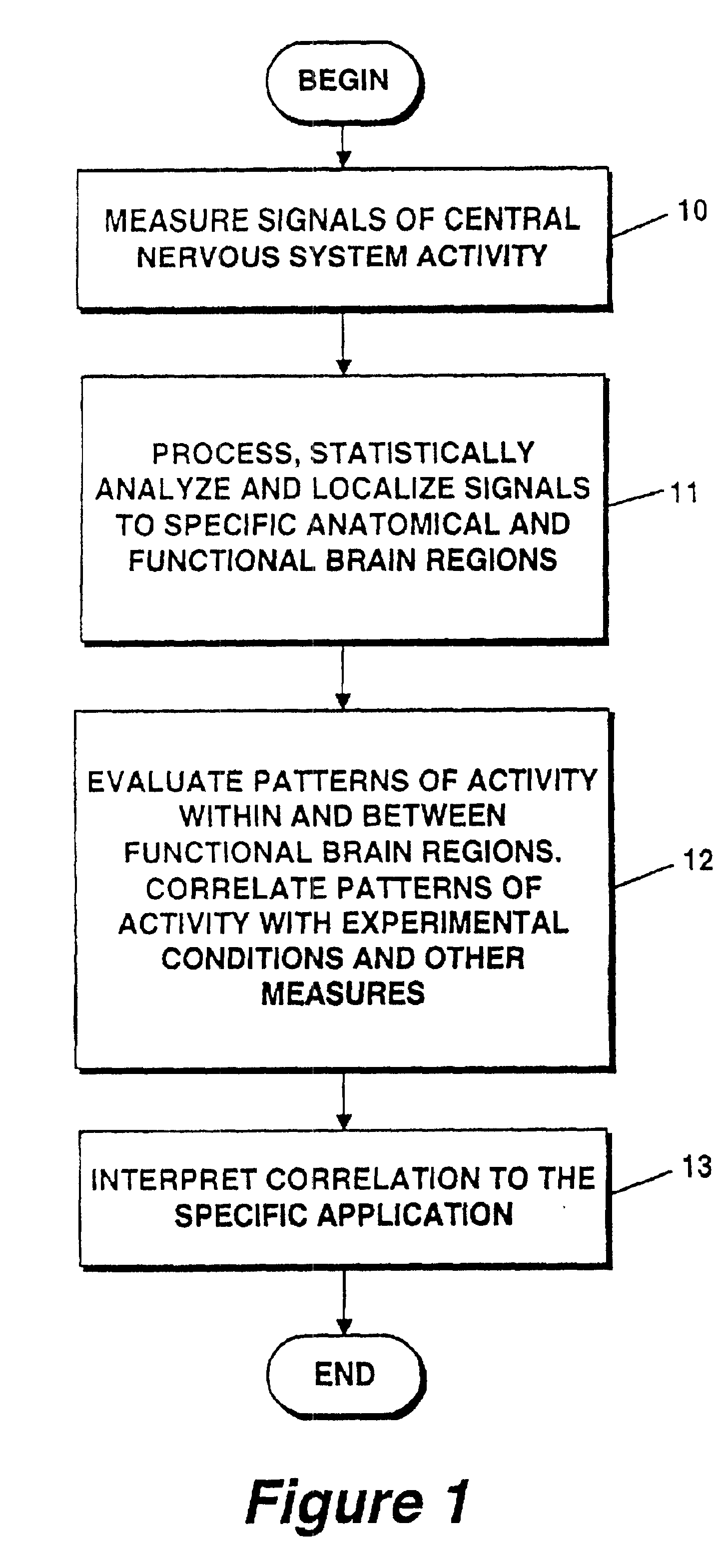
Method and apparatus for objectively measuring pain, pain treatment and other related techniques
A method for measuring indices of brain activity includes non-invasively obtaining signals of central nervous system (CNS) activity, localizing signals to specific anatomical and functional CNS regions, correlating the signals from pain and reward brain regions, and interpreting the correlation results. The results of interpreting the correlation results can be used for objectively measuring, in individual humans or animals, their responses to motivationally salient stimuli including but not limited to stimuli which are internal or external, conscious or non-conscious, pharmacological or non-pharmacological therapies, and diseased based processes. This method for measuring brain activity in reward / aversive central nervous system regions, can further be used to determine the efficacy of compounds.
Owner:THE GENERAL HOSPITAL CORP
Mucoadhesive drug delivery devices and methods of making and using thereof
ActiveUS20050196440A1Improve drug absorptionPromote resultsPowder deliveryOrganic active ingredientsActive agentSolvent
The present invention relates to mucoadhesive drug delivery devices and their methods of preparation and use. More specifically the present invention relates to mucoadhesive drug delivery devices comprising one or more biocompatible purified proteins combined with one or more biocompatible solvents and one or more mucoadhesive agents. The mucoadhesive drug delivery devices may also include one or more pharmacologically active agents. The drug delivery devices of the present invention adhere to mucosal tissue, thereby providing a vehicle for delivery of the pharmacologically active agent(s) through such tissue.
Owner:PETVIVO HLDG INC
Use of three-dimensional microfabricated tissue engineered systems for pharmacologic applications
ActiveUS20060019326A1Additive manufacturing apparatusMicrobiological testing/measurementExperimental drugSide effect
The present invention generally relates to a combination of the fields of tissue engineering, drug discovery and drug development. It more specifically provides new methods and materials for testing the efficacy and safety of experimental drugs, defining the metabolic pathways of experimental drugs and characterizing the properties (e.g., side effects, new uses) of existing drugs. Preferably, evaluation is carried out in three-dimensional tissue-engineered systems, wherein drug toxicity, metabolism, interaction and / or efficacy can be determined.
Owner:CHARLES STARK DRAPER LABORATORY +1
High throughput correlation of polymorphic forms with multiple phenotypes within clinical populations
A computer-assisted method of looking for pharmacologic targets, in which large numbers of persons are enrolled in drugh clinical trials, they are medically examined and documented, tissue samples are taken, the tissue samples are genotyped, and an examination is made of the genotypes to try to ascertain associations between the genotypes and the documented disease phenotypes of the patients.
Owner:SMITHKLINE BECKMAN CORP
Engineering absorption of therapeutic compounds via colonic transporters
Methods of modifying therapeutic compounds such as drugs to be substrates for active transporters expressed in epithelial cells lining the lumen of the human colon are disclosed. The transporters expressed in the human colon include the sodium dependent multi-vitamin transporter (SMVT), and monocarboxylate transporters 1 and 4 (MCT 1 and MCT 4). The modified compounds can themselves be pharmacologically active, or upon cleavage of a chemical moiety after uptake from the colon, can be metabolized to form a compound that is pharmacologically active (e.g., a prodrug). The modified compounds disclosed herein are suitable for use in extended release oral dosage forms, particularly those that release drug over periods of greater than about 2-4 hours following administration.
Owner:XENOPORT
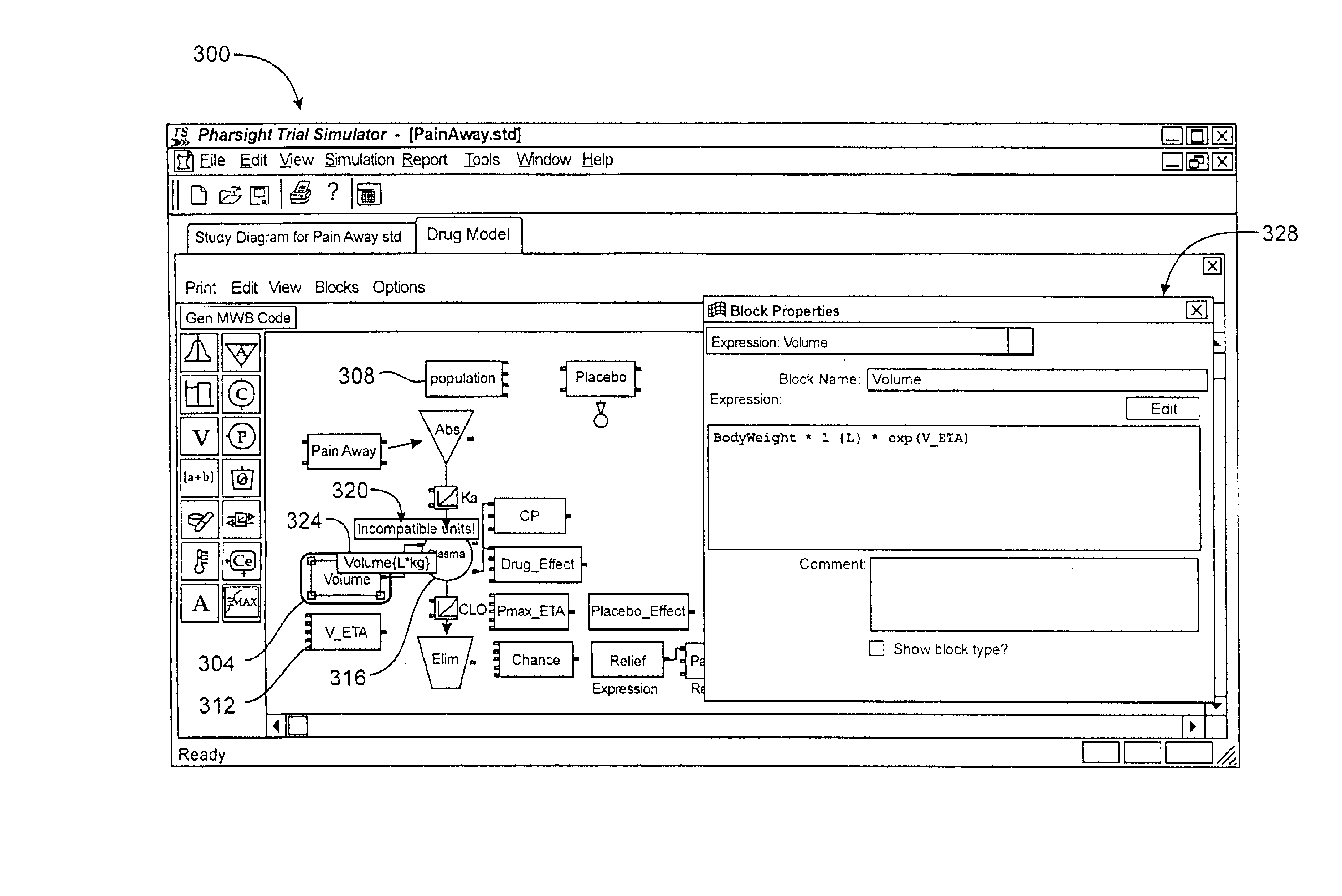
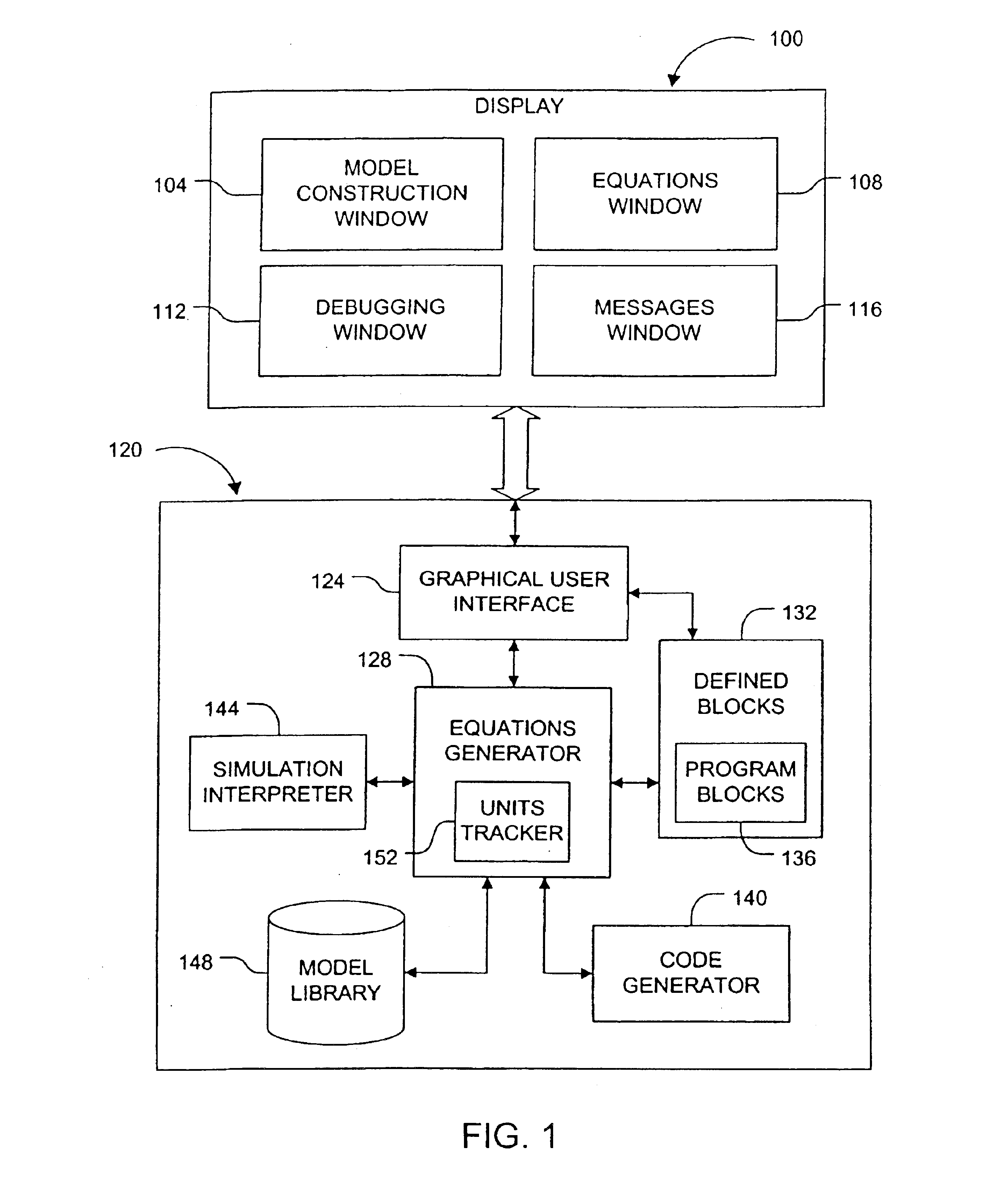
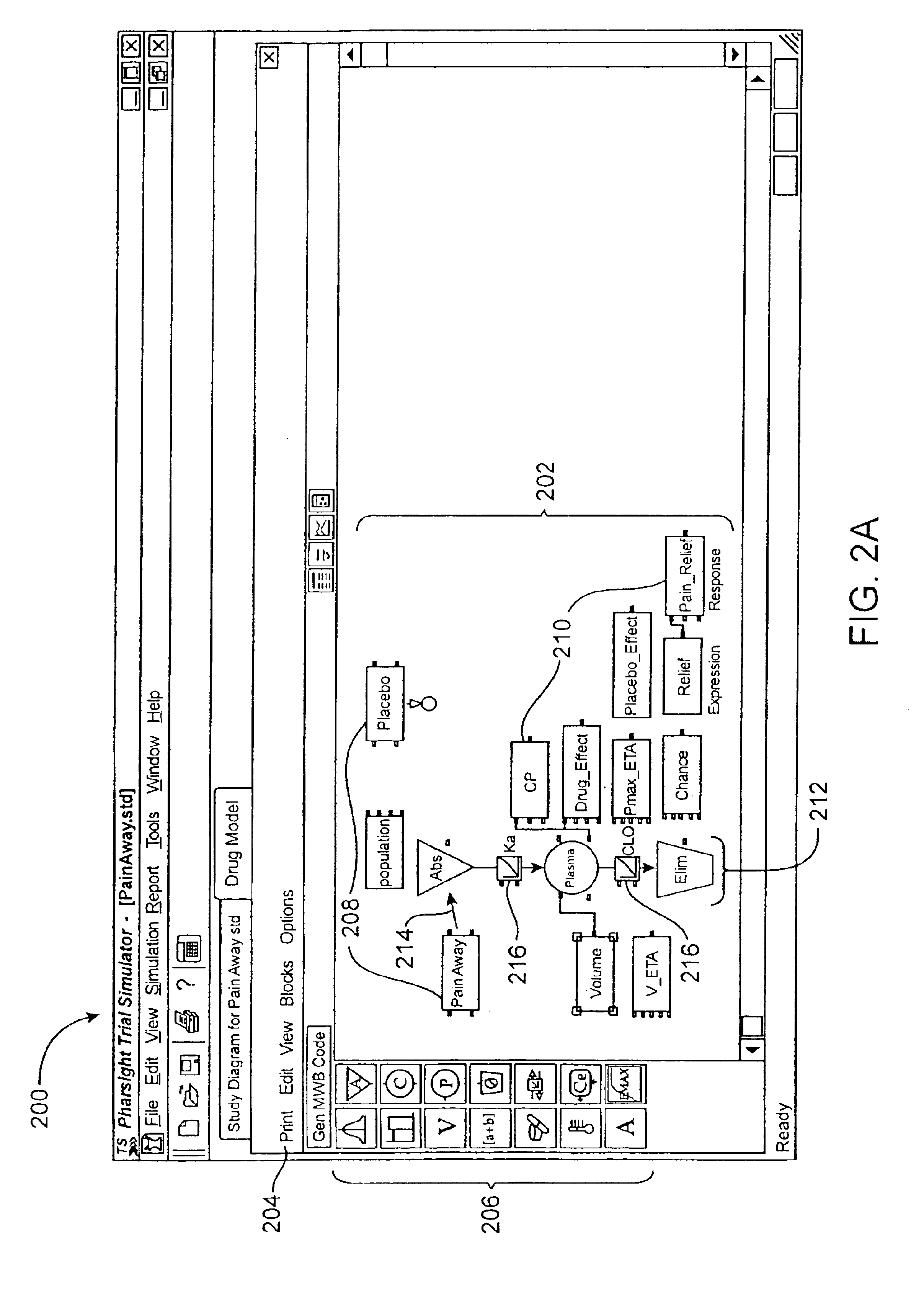
Unit tracking and notification in a graphical drug model editor
InactiveUS6937257B1Accelerating model buildingIncrease the verification processData visualisationAnalogue computers for chemical processesGraphicsGraphical user interface
A method for maintaining consistent unit relationships during graphical pharmacological computational model construction is disclosed. A graphical user interface is presented through which a user may place and connect objects representing pharmacokinetic and pharmacodynamic elements. The user may specify units definitions for variables and constants using unit expressions. As the objects are converted into an internal format representing the statements of the corresponding computational model, the unit expressions are included in this internal format as multidimensional data type information. This multidimensional data type information is regularly and automatically propagated for each statement in the internal format to identify inconsistent units. When such inconsistent units are identified, a warning message is generated to notify the user, substantially immediately after the inconsistent units are created.
Owner:CERTARA
Diagnosis, prognosis and identification of potential therapeutic targets of multiple myeloma based on gene expression profiling
InactiveUS20080293578A1Microbiological testing/measurementAnalogue computers for chemical processesGene targetsGene model
Provided herein is a method for gene expression profiling multiple myeloma patients into distinct subgroups via DNA hybridization and hierarchical clustering analysis of the hybridization data where the results may further be used to identify therapeutic gene targets. Also provided is a method for controlling bone loss in an individual via pharmacological inhibitors of DKK1 protein. In addition provided herein is a method for diagnosing multiple myeloma using a 15-gene model that classifies myeloma into groups 1-7.
Owner:BIOVENTURES LLC
Method of treating contrast-induced nephropathy
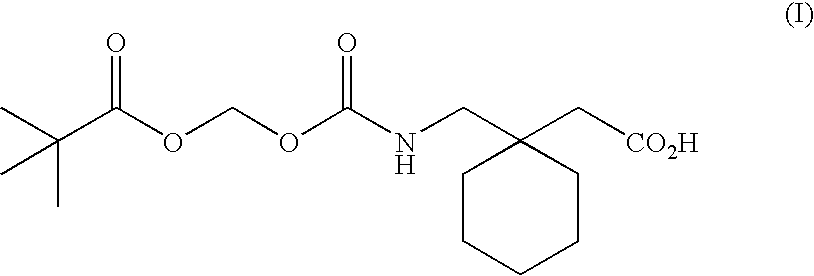
InactiveUS20120122844A1Treating and preventing and ameliorating contrast-induced nephropathyImprove the level ofBiocideOrganic chemistryNephrosisActive agent
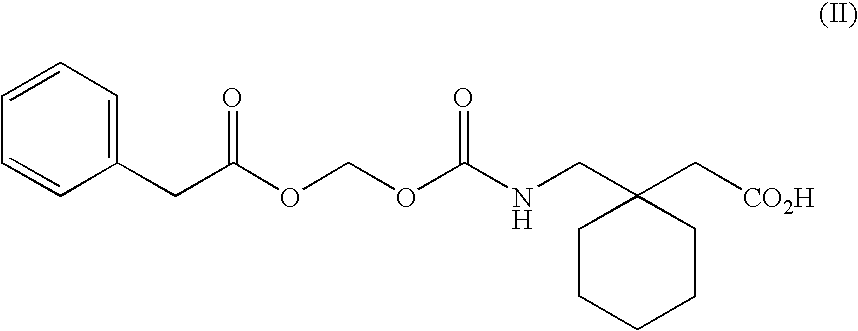
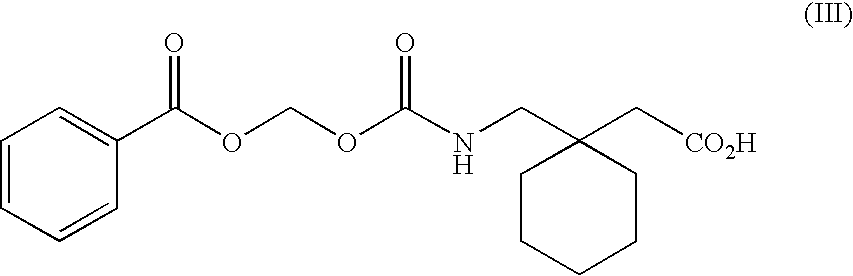
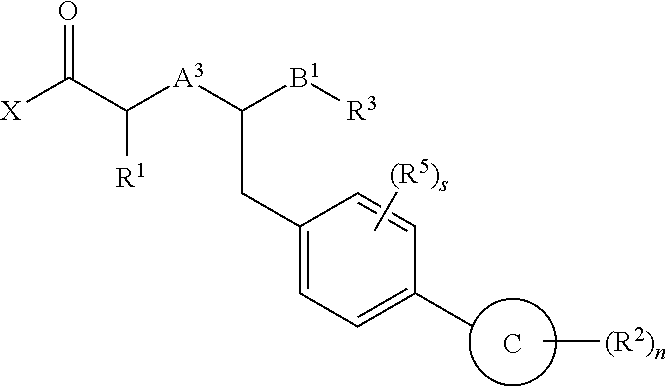
The present invention provides the use of a neutral endopeptidase inhibitor, in the manufacture of a medicament for the treatment, amelioration and / or prevention of contrast-induced nephropathy. The invention also relates to the use of a compound of Formula I:wherein R1, R2, R3, R5, X, A3, B1, s and n are defined herein, for the treatment, amelioration and / or prevention of contrast-induced nephropathy. The present invention further provides a combination of pharmacologically active agents for use in the treatment, amelioration and / or prevention of contrast-induced nephropathy.
Owner:NOVARTIS AG
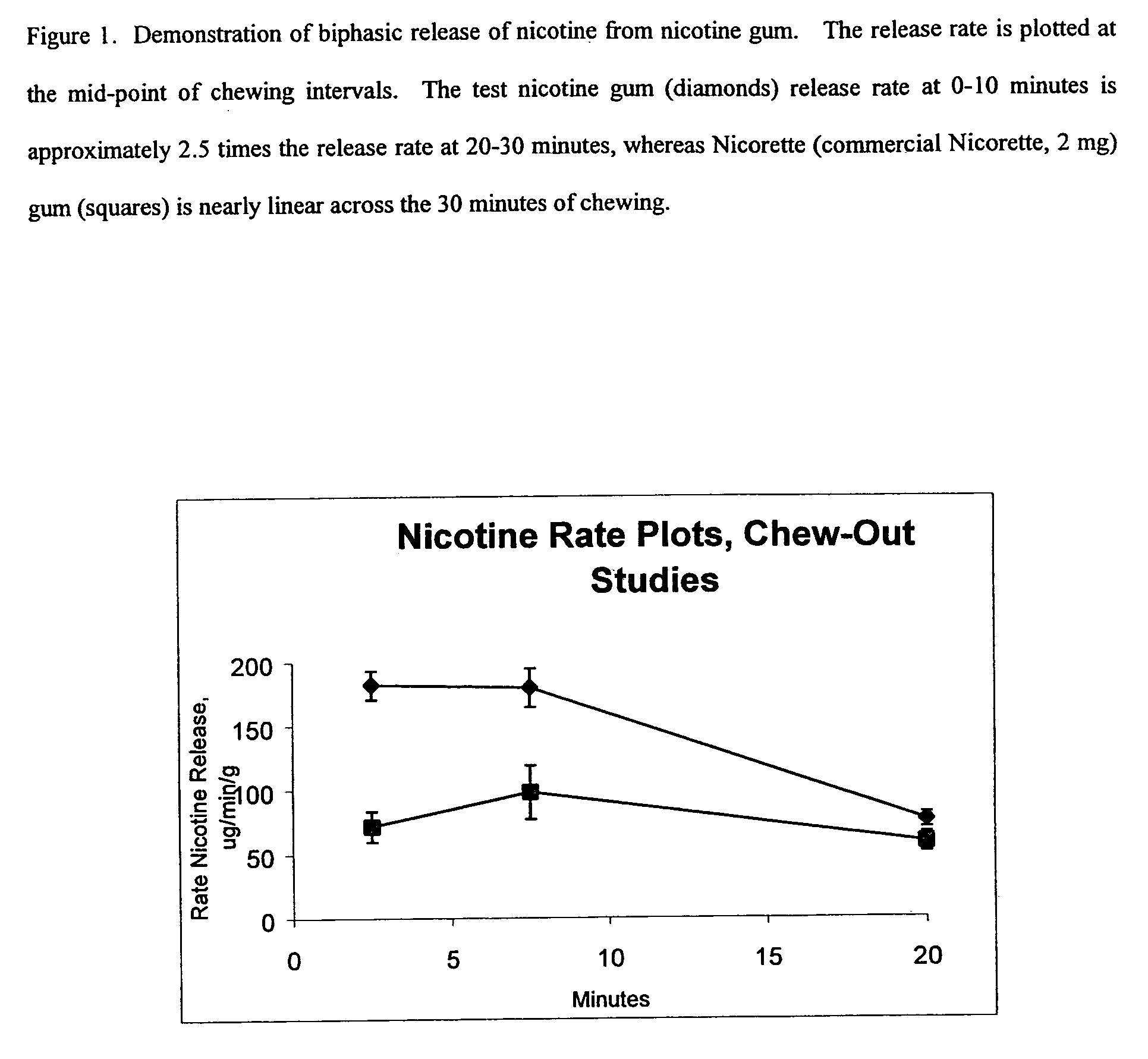
Chewing gums, lozenges, candies, tablets, liquids, and sprays for efficient delivery of medications and dietary supplements
InactiveUS20060073189A1Relieve symptomsLower of oral cavityInorganic non-active ingredientsChewing gumDietary supplementTherapeutic effect
The present invention relates to gums, lozenges, candies, tablets, liquids and spray compositions that contain orally administered medications and dietary supplements that are released in the oral cavity. The medications and dietary supplements contained therein may be delivered in a multi-phase mode. The compositions may also contain buffer systems that facilitate oral absorption. A rapid release is followed by slower release of medicant(s) and dietary supplements. The buffer system is released simultaneously with the medicant(s) and dietary supplements, thereby facilitating transmucosal and buccal absorption of active ingredient(s). The invention delivers, first, rapidly an initial pharmacologically effective dose of medicine and dietary supplements and, second, a prolonged pharmacologically sufficient dose for longer-term relief of symptoms or provision of therapeutic effect.
Owner:JSR NTI
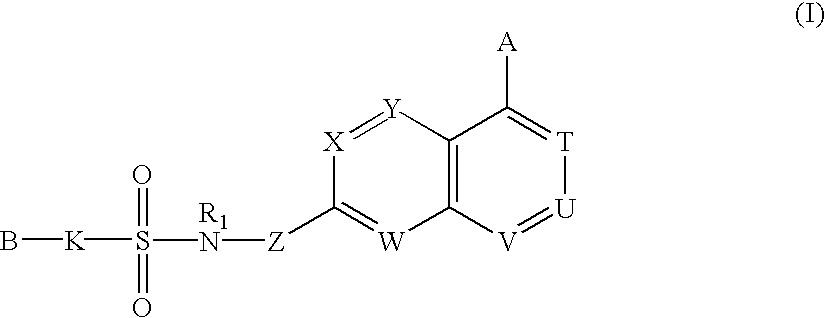
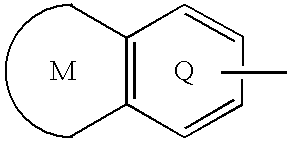
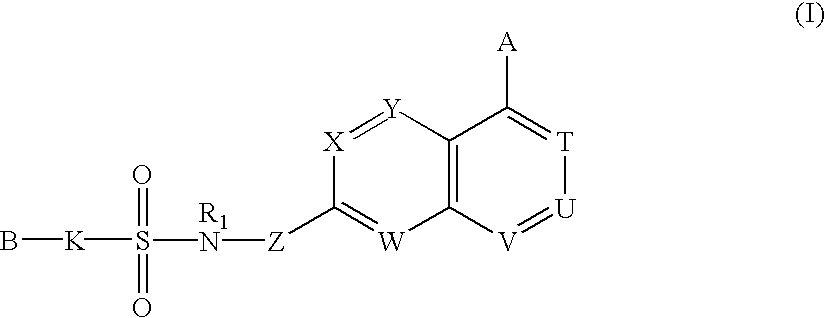
Sulfonamide-containing heterocyclic compounds
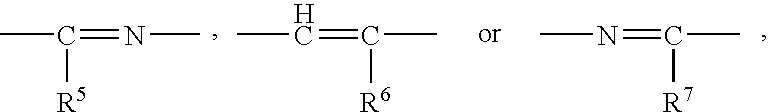
The present invention provides a sulfonamide- or sulfonylurea-containing heterocyclic compounds. Specifically, it provides a heterocyclic compound represented by the formula (I), a pharmacologically acceptable salt thereof or a hydrate of them.In the formula, A is hydrogen atom, a halogen atom, a C1-C4 alkyl or alkoxy group which may be substituted with a halogen atom, or cyano group; B is an optionally substituted aryl group or monocyclic heteroaryl group, or:(wherein, the ring Q is an aromatic ring which may have nitrogen atom; and the ring M is a ring sharing a double bond with the ring Q, which ring may have a heteroatom; and the rings Q and M may share nitrogen atom); K is a single bond; T, W, X and Y are the same as or different from each other and each is =C(D)- (wherein, D is hydrogen or a halogen atom) or nitrogen atom; U and V are the same as or different from each other and each is =C(D)-, nitrogen atom, -CH2-, oxygen atom or -CO-; Z is a single bond or -CO-NH-; and R1 is hydrogen atom, etc.
Owner:EISIA R&D MANAGEMENT CO LTD
Pyrazole compounds and their use as antidiabetes agents
The present invention provides a pyrazole compound that has liver glycogen phosphorylase inhibitory activity and is useful as a therapeutic or prophylactic agent for diabetes, the pyrazole compound represented by the following general formula (I): wherein Ring Q represents an aryl or heteroaromatic group, R1 represents a hydrogen atom, a halogen atom, a C1-6 alkyl group or a C1-6 alkoxy group, R2 represents a halogen atom, a C1-6 alkyl group, a C1-6 alkoxy group or an azido group, R3 represents a halogen atom, a hydroxyl group, a C1-6 alkyl group, a halo C1-6 alkyl group, a C1-6 alkoxy group, an azido group, an amino group, an acylamino group or a C1-6 alkylsulfonylamino group, R4 and R5 are identical with or different from each other and represent a hydrogen atom, a substituted or unsubstituted C1-6 alkyl group, a C3-8 cycloalkyl group, a substituted or unsubstituted saturated heterocyclic group, a substituted or unsubstituted aryl group, a C7-14 aralkyl group, a heteroaromatic group, or the like, or a pharmacologically acceptable salt thereof.
Owner:JAPAN TOBACCO INC
Hydrogel particle formulation
InactiveUS7022313B2Avoid lostEasy to customizePowder deliveryPeptide/protein ingredientsDiagnostic agentParticle injection
New compositions formed from the combination of an active substance with a hydrogel carrier moiety are provided. The compositions are suitable for use in high-velocity transdermal particle injection techniques. Methods of providing the new compositions are also provided. In addition, methods for administering pharmacologically active agent to a subject are provided. These methods are useful for delivering drugs, biopharmaceuticals, vaccines and diagnostics agents.
Owner:POWDERJECT RES LTD OXFORD (GB)
Compound, especially marker-dye on the basis of polymethines
InactiveUS20040260093A1Improve light resistanceLong storage periodMethine/polymethine dyesOrganic chemistryQuantum yieldFluorophore
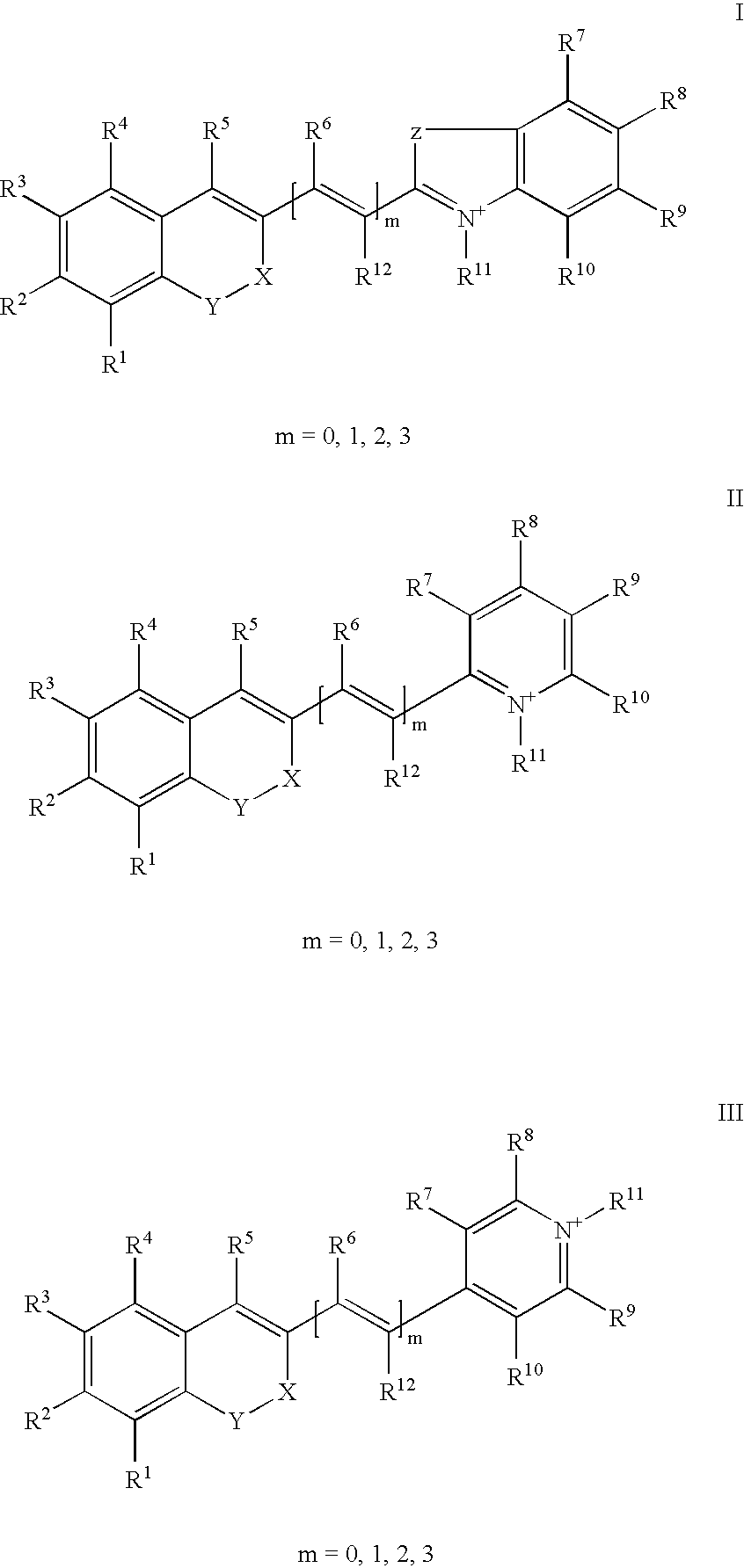
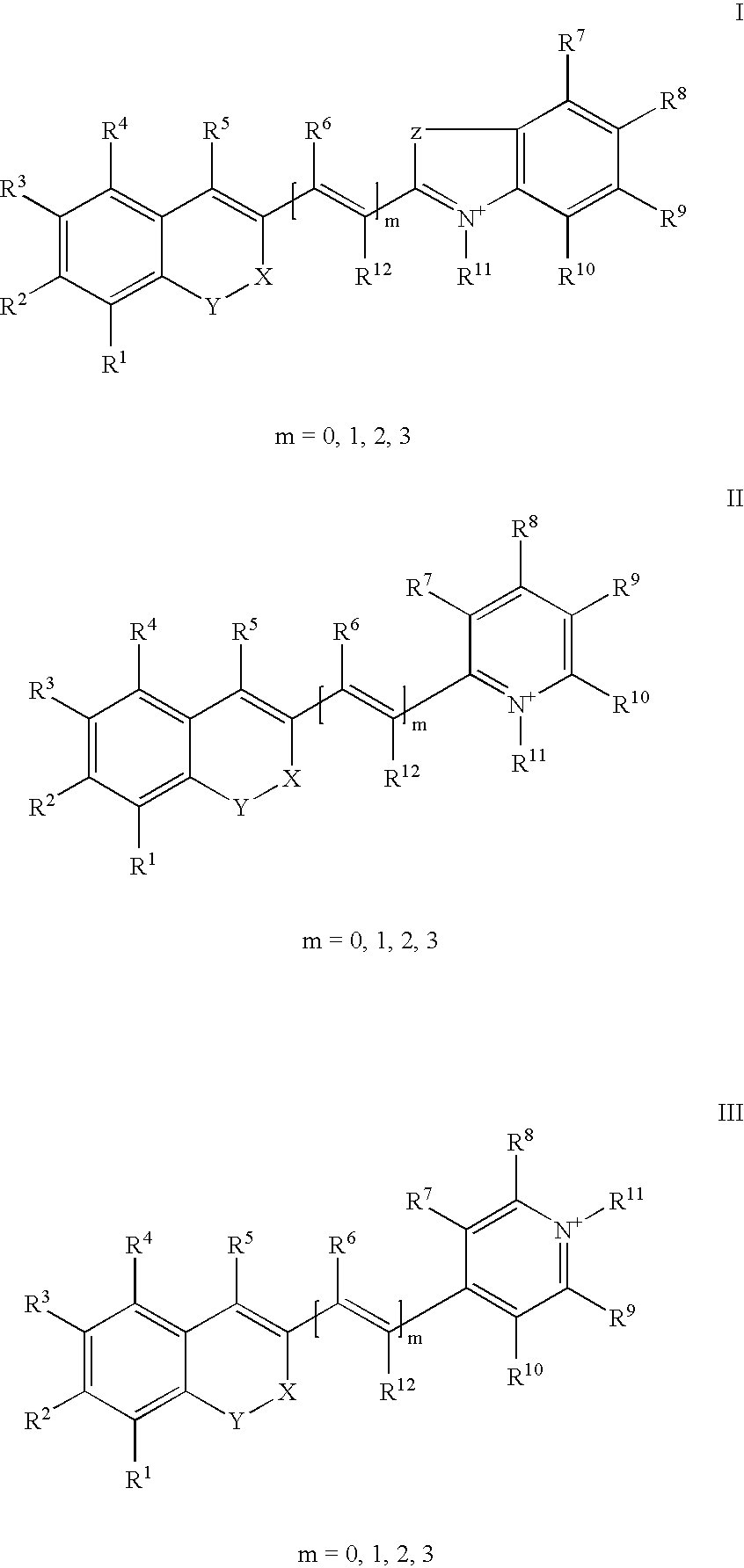
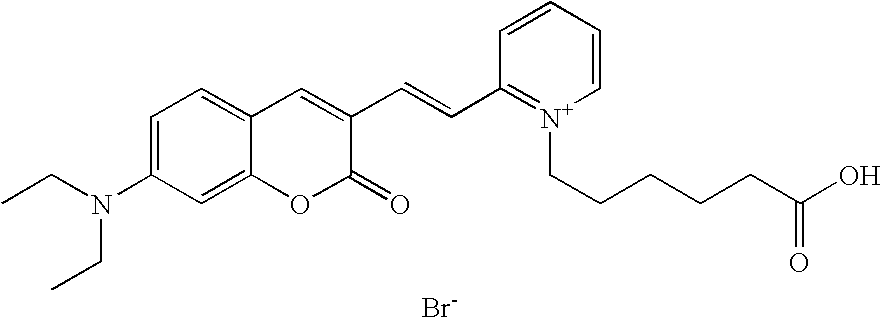
The invention relates to fluorescent dyes (fluorophores) based on polymethines for use in optical measurement and detection procedures, in particular those employing fluorescence, for example in medicine, in pharmacology and in the biological, materials and environmental sciences. The objective was to create fluorophores based on polymethines that have a large Stokes shift, high photostability, long storage life and a high fluorescent quantum yield, and that can be excited in the simplest possible manner by white-light sources or laser radiation in the UV, visible or NIR spectral region. According to the invention dyes on the basis of polymethines having the general formulas I, II or III are employed.
Owner:DYOMICS
Pyrimidine derivatives
InactiveUS20060293343A1Potent PDE inhibitory actionLow toxicityBiocideOrganic active ingredientsPyrimidineSide effect
Owner:ASAHI KASEI PHARMA
Method and system for screening compounds for muscular and/or neurological activity in animals
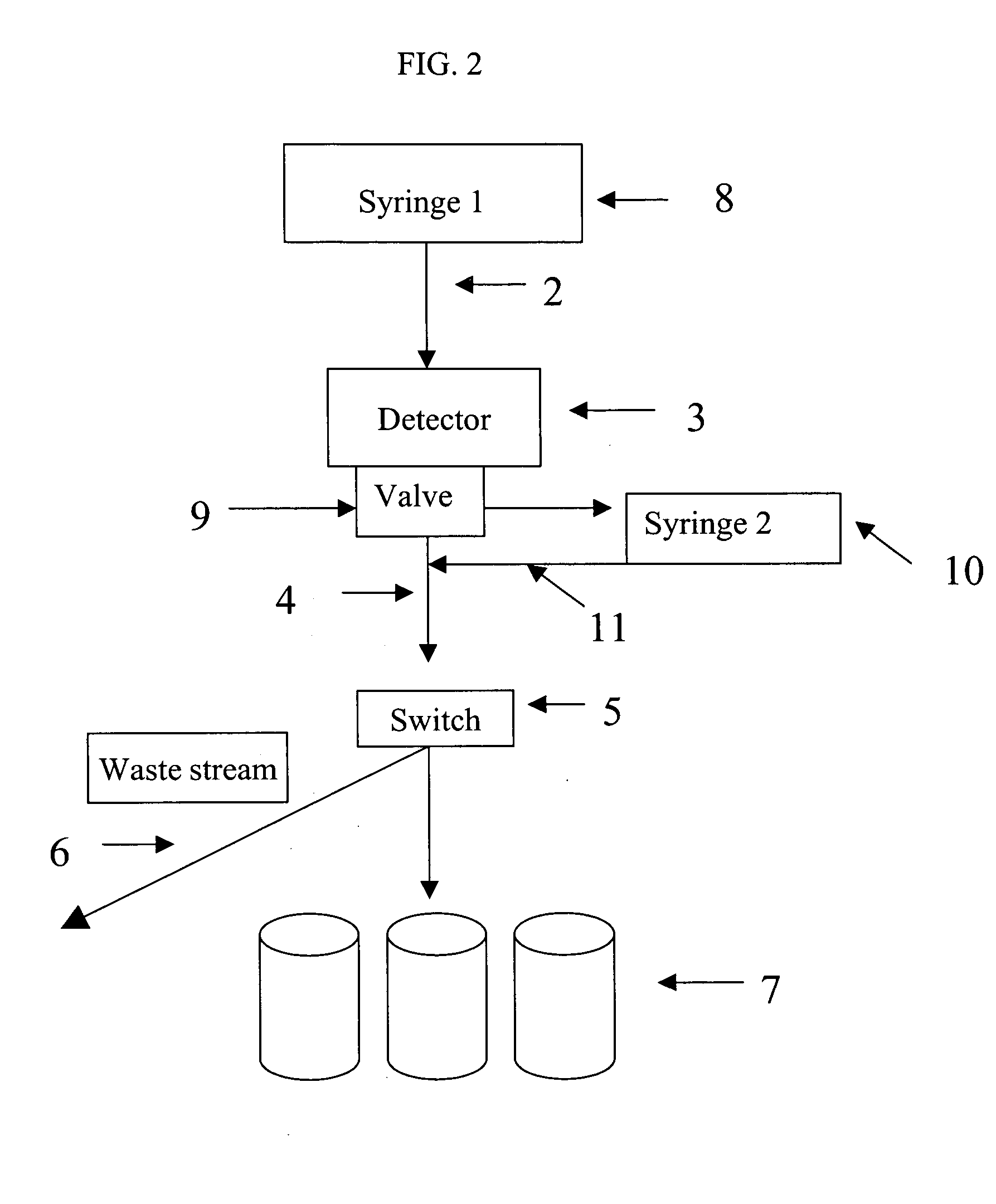
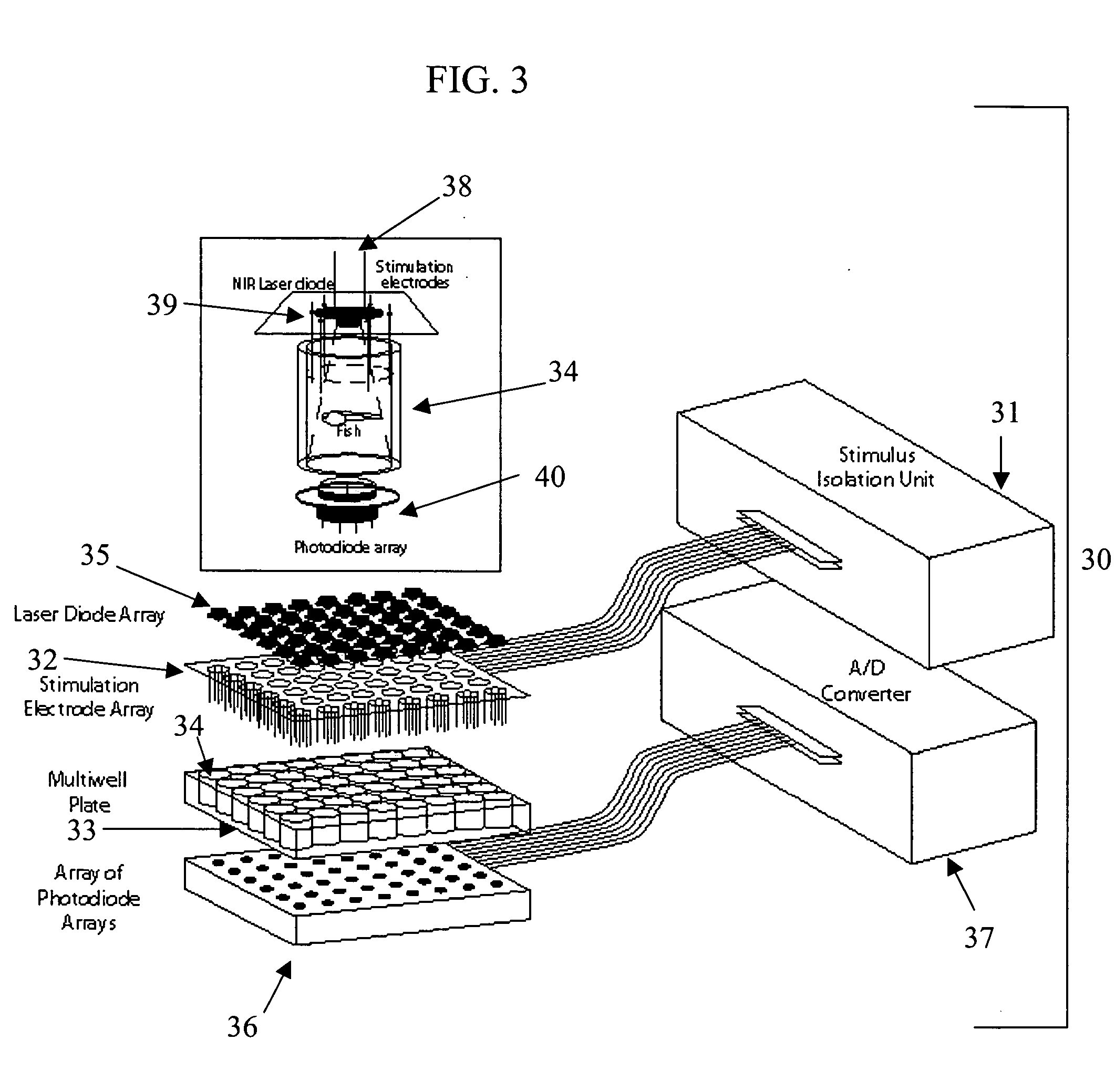
Screening methods and instrumentation for candidate pharmacological agents are applied to discover compounds with muscular and / or neurological activity. The method comprises the use of teleost fish, such as the medaka (Oryzias latipes), which may be stimulated with chemical agents or an electric field to produce, for example, seizure activity and / or convulsive activity. The convulsive behavior may be recorded optically and electrically. Antagonism of the convulsive behavior is produced by application of candidate pharmacological agents to the well containing the fish. The method may include stimulation and antagonism in a plurality of sample wells with a repetitive or simultaneous application of threshold electric fields. The methods and instrumentation can be applied to the study of other serious neurological diseases such as neuropathic pain. In addition the process of assaying the protection of animals to convulsant agents the assay measures pharmacological safety parameters including sedation and cognitive impairment.
Owner:MURPHY RANDALL +1
Medicament for treatment of dermal pigmentation
InactiveUS20070042997A1Inhibit dermal pigmentationLow toxicityBiocideOrganic chemistryPharmacometricsBackbone chain
Owner:INST OF MEDICINAL MOLECULAR DESIGN
Gel compositions for topical administration
Pharmaceutical gel compositions containing pharmacologically active agent for topical administration, as well as a method of making the same, are disclosed.
Owner:APTALIS PHARMA
Preventives or remidies for alzheimer's disease or amyloid protein fibrosis inhibitors containing nitrogen-containing heteroaryl compounds
InactiveUS20050054732A1Powerful activityImprove securityBiocideNervous disorderFibrosisBULK ACTIVE INGREDIENT
The present invention relates to preventives or remedies for Alzheimer's disease, or to amyloid protein fibril-formation inhibitors, which include as an active ingredient a compound of general formula (I) below or a pharmacologically permitted salt thereof; and also to nitrogen-containing heteroaryl derivatives having specific substituents, or pharmacologically permitted salts thereof, which are valuable as preventives or remedies for Alzheimer's disease, or as amyloid protein fibril-formation inhibitors: (where, R1 and R2 are H or alkyl; Z1 and Z2 are H, alkyl, alkoxy, haloalkyl or halogeno; Z3 is alkoxy, SH, alkylthio, NH2, mono- or di-alkylamino, OH or halogeno; Z4 and Z5 are H or halogeno; and A is 4,6-pyrimidine-1,3-diyl, 1,3,5-triazine-2,6-diyl, etc).
Owner:BTG INT LTD
Excipient removal from pharmacological samples
InactiveUS20140319077A1Easy to separateSolvent extractionWater/sewage treatment with mechanical oscillationsPharmaceutical drugPharmacometrics
Active pharmaceutical ingredients can be separated from their excipients by dissolving a pharmaceutical product (e.g. tablet, pill) into a solvent, then running the solution through an acoustophoretic device. Standing waves are used to separate the excipient from the active ingredient dissolved in the solvent.
Owner:FLODESIGN SONICS
Cyclic compounds
InactiveUS20040142930A1Strong inhibitory activityLess side effectsBiocideGroup 5/15 element organic compoundsArylAlkoxy group
1. A cyclic compound of the formula (I) or a pharmacologically acceptable salt thereof, wherein X is =CH- or =N-, Y is -NH-, -NR<4>-, -S-, -O-, -CH=N-, -N=CH-, -N=N-, -CH=CH-, etc., R<1 >is a lower alkoxy group, an amino group, a heterocyclic ring containing N atom(s), or a hydroxy group substituted by a heterocyclic ring containing N atom(s) (each of which is optionally substituted), R<2 >is a lower alkylamino group which is optionally substituted by an aryl group, a lower alkoxy group which is optionally substituted by an aryl group, a lower alkoxy group substituted by an aromatic heterocyclic ring containing N atom(s), R<3 >is an aryl group, a heterocyclic ring containing N atom(s), a lower alkyl group, a lower alkoxy group, a cyclo lower alkoxy group, a hydroxy group substituted by a heterocyclic ring containing N atom(s), or an amino group (each of which is optionally substituted), and R<3 >and a substituent in Y may be combined to form a lactone ring. The compound of the present invention has excellent selective PDE V inhibitory activity and therefore, is useful as a therapeutic or prophylactic drug for treating various diseases due to functional disorders on cGMP-signaling.
Owner:MITSUBISHI TANABE PHARMA CORP
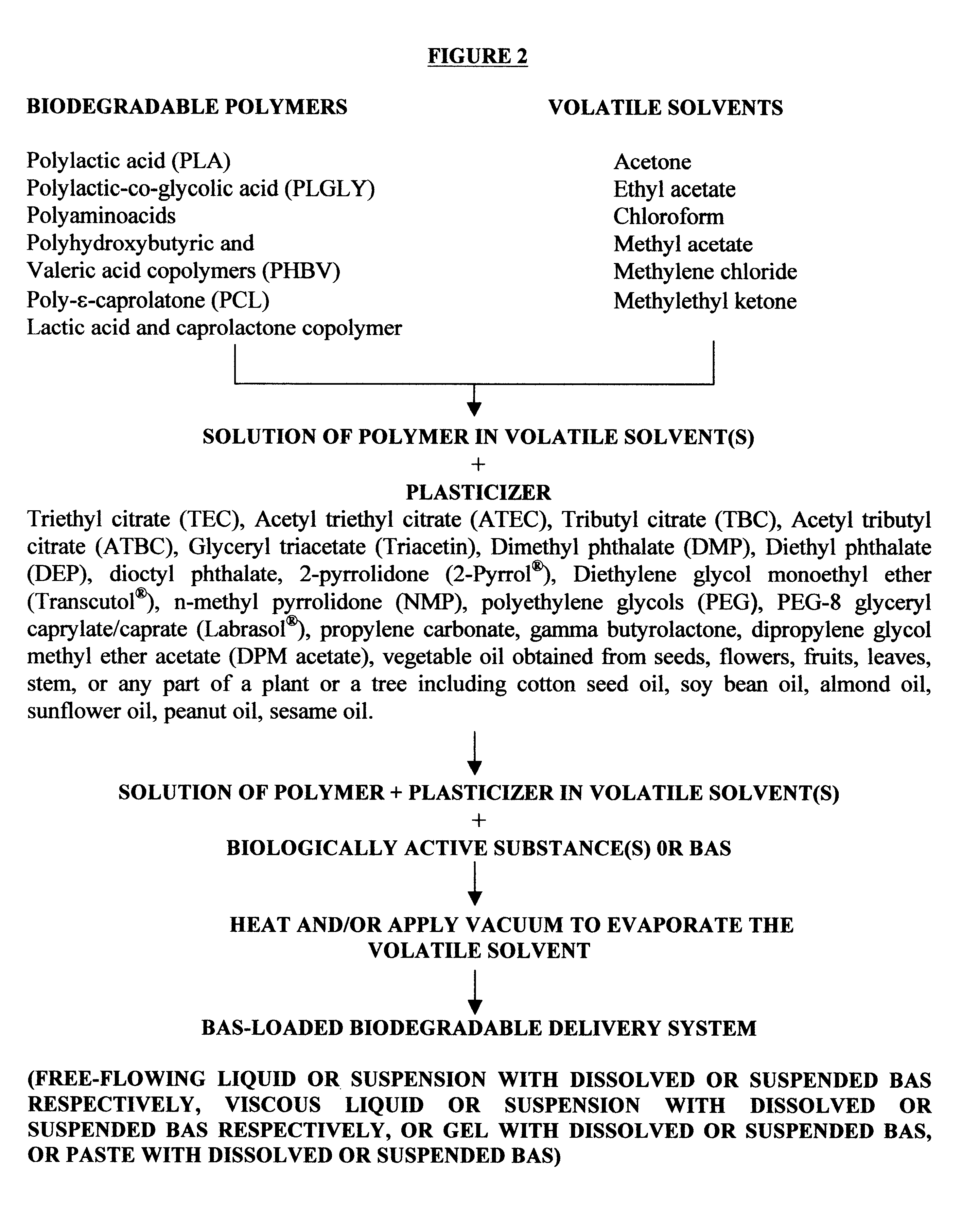
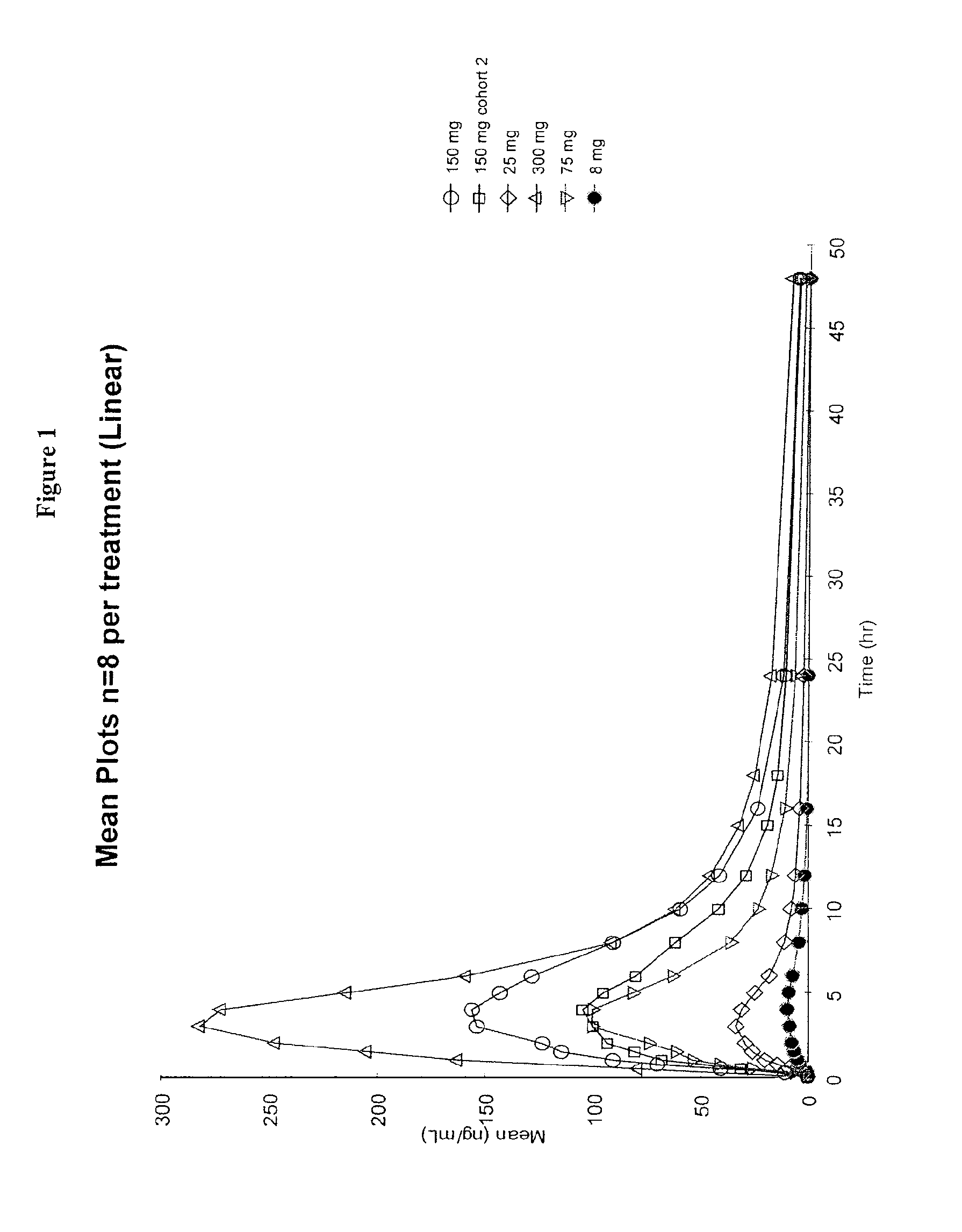
Biodegradable delivery systems of biologically active substances
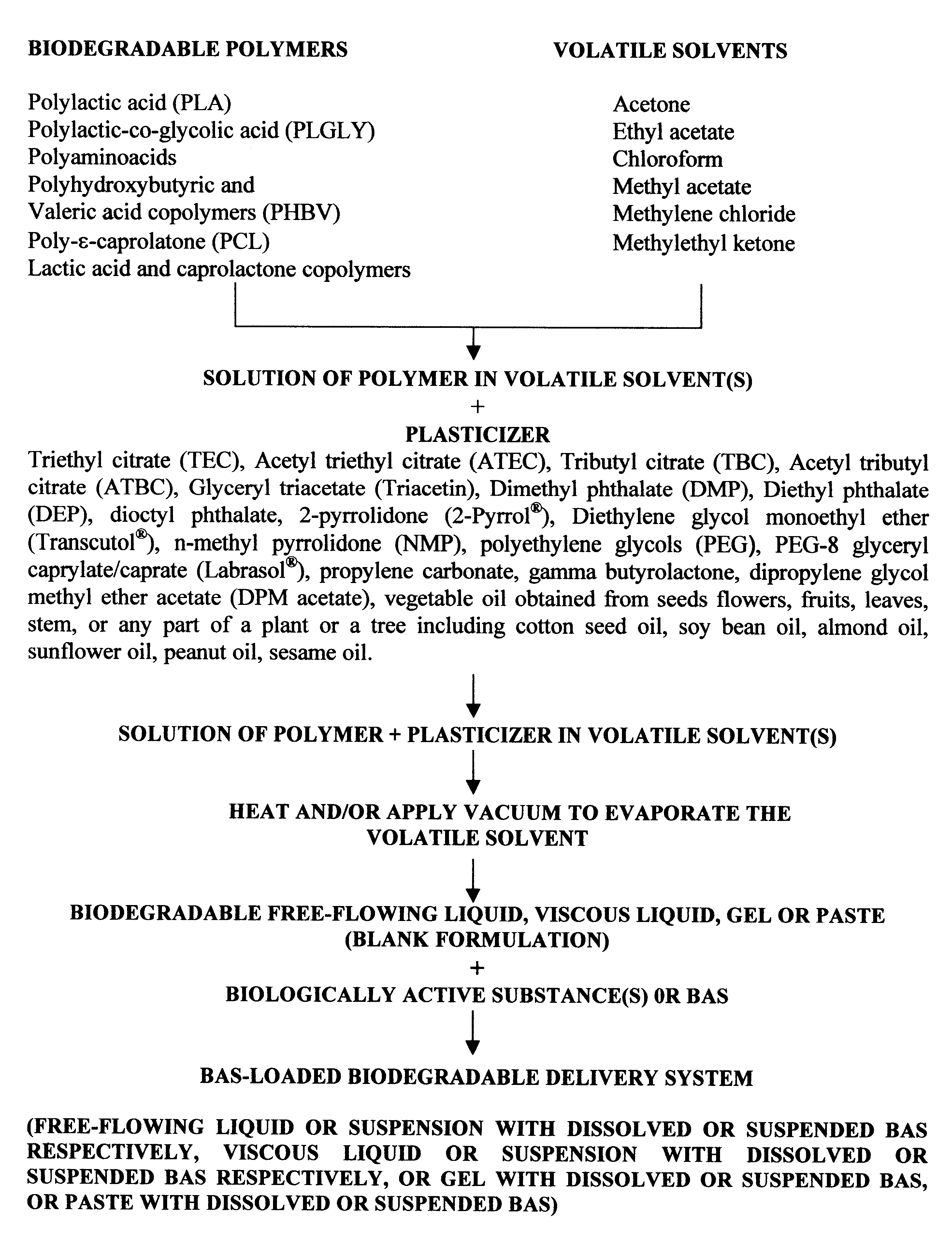
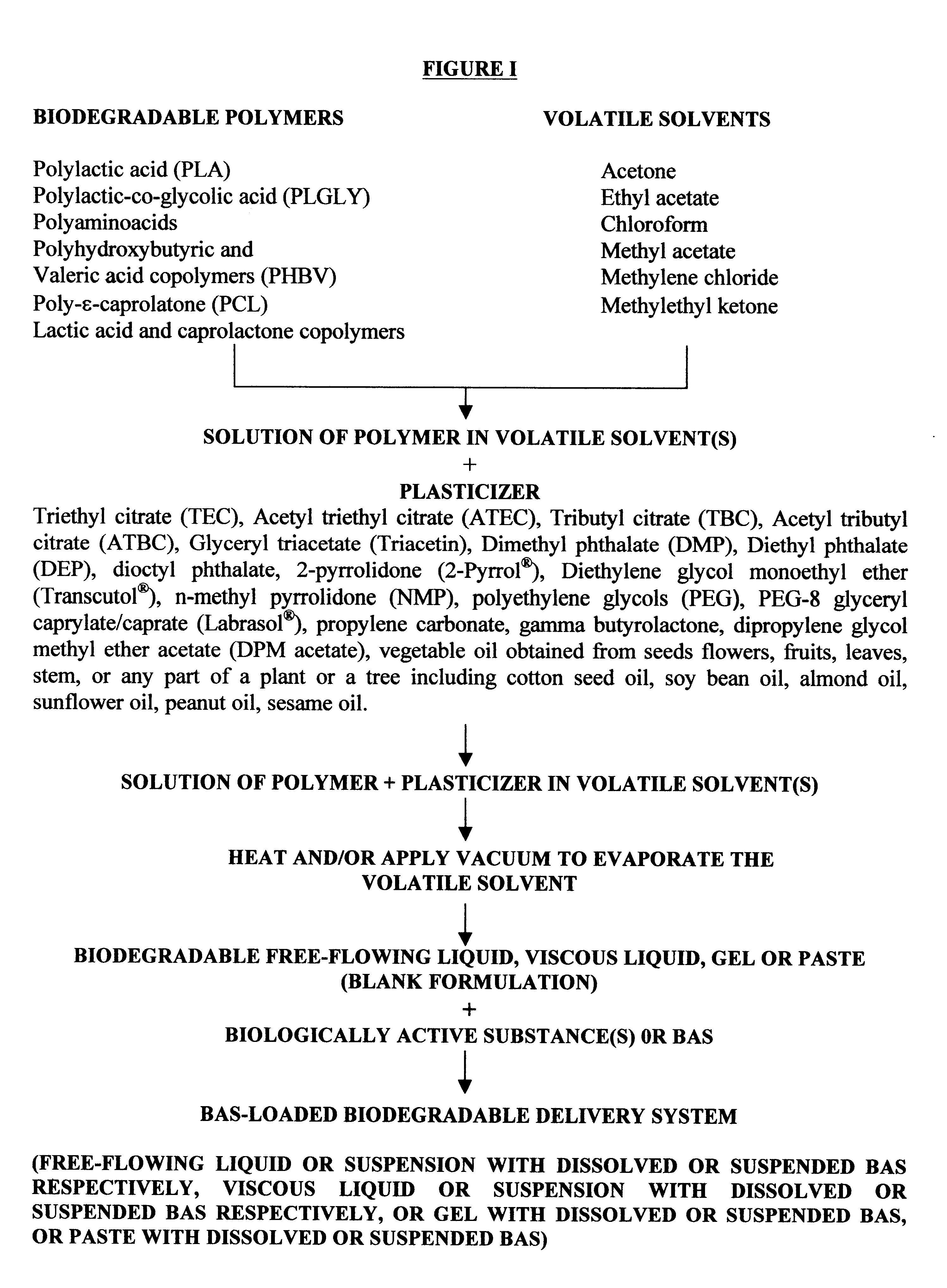
InactiveUS6193991B1High drug loadingHigh gradientOrganic active ingredientsPharmaceutical delivery mechanismControlled releaseSolvent evaporation
Biodegradable delivery systems of physiologically, pharmacologically and biologically active substance(s) (BAS) are provided. These systems are obtained by incorporating the BAS into a blend of biodegradable polymers and plasticizers using a novel solvent evaporation method. This method involves dissolving the biodegradable polymer or copolymer and a plasticizer into a volatile solvent. The BAS may then be added to this mixture. The volatile solvent is removed using vacuum or at an elevated temperature or using a combination of both vacuum and elevated temperature. The resultant mixture is a BAS-loaded formulation which when injected, implanted or applied in vivo in an animal or human, provides controlled release of the BAS over the desired period of time. Alternatively, a blank formulation may be first prepared by the aforementioned methodology without incorporating the BAS in the formulation. An appropriate quantity of BAS is then added to this formulation to yield a BAS-loaded formulation which may control the release of the BAS for the desired length of time.
Owner:SHUKLA ATUL J
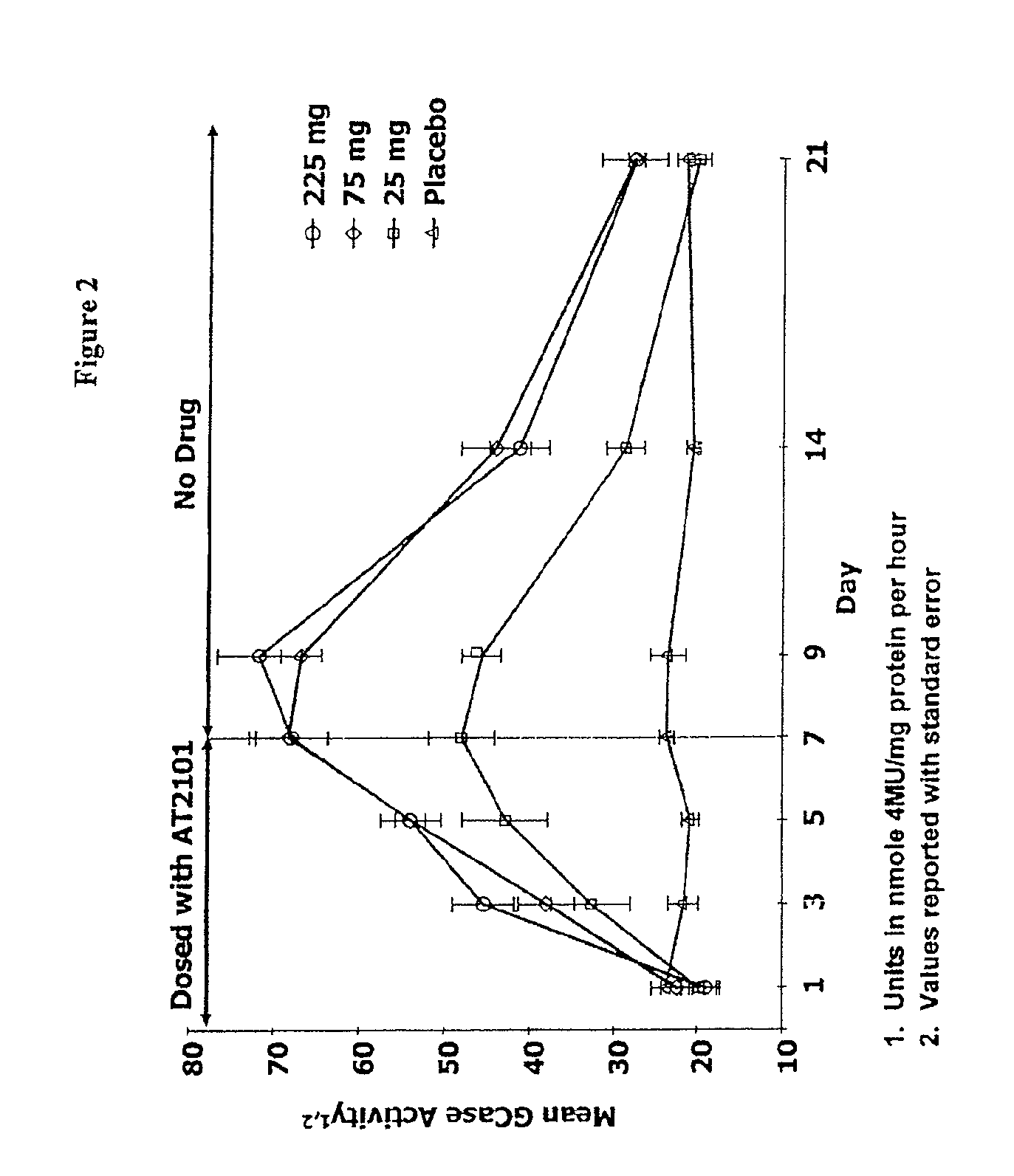
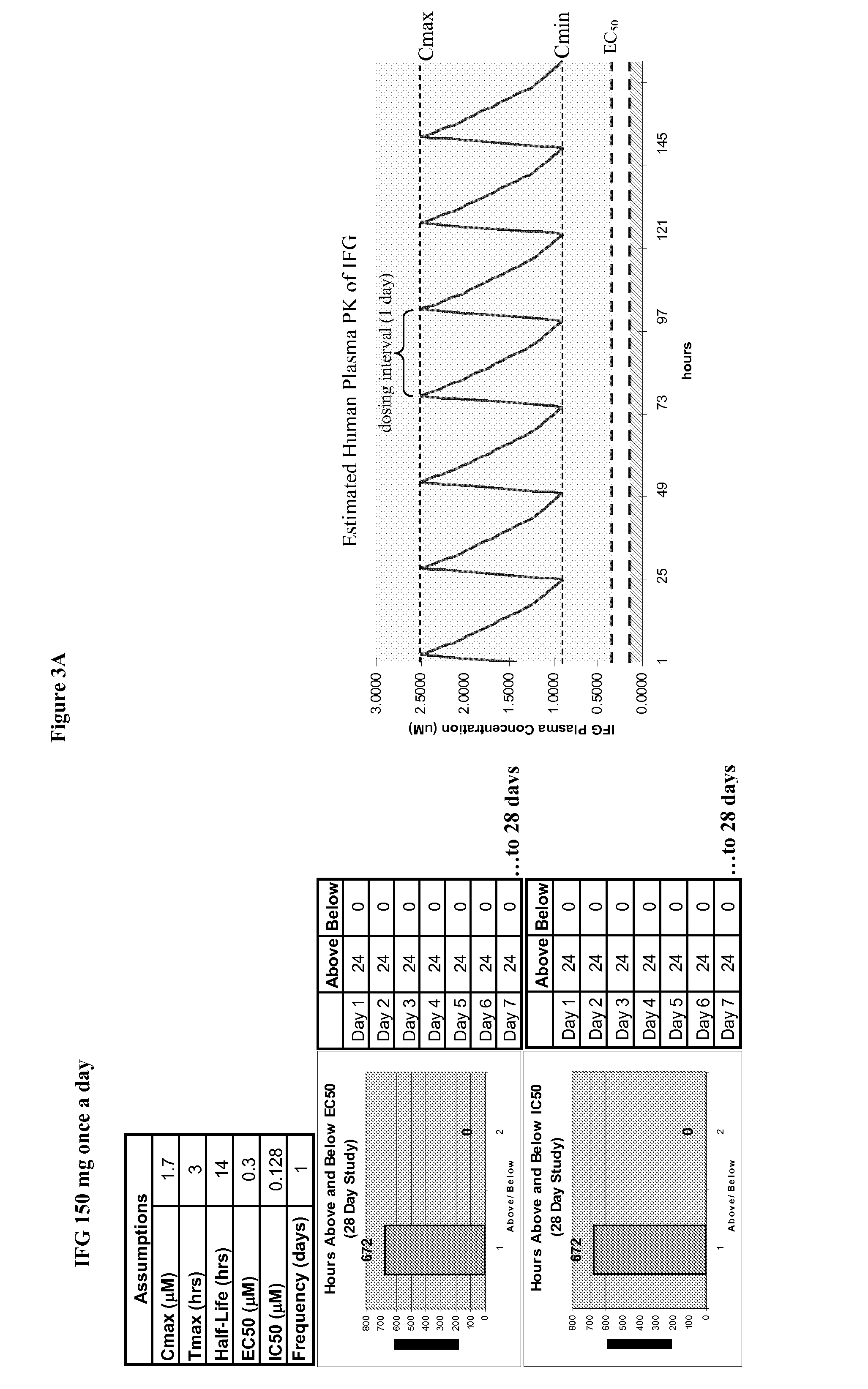
Dosing regimens for the treatment of lysosomal storage diseases using pharmacological chaperones
The present invention provides dosing regimens for administering pharmacological chaperones to a subject in need thereof. The dosing regimens can be used to treat disorders caused by improper protein misfolding, such as lysosomal storage disorders.
Owner:AMICUS THERAPEUTICS INC
Highly concentrated stable meloxicam solutions for needleless injection
Aqueous cyclodextrin-free solution of meloxicam suitable for administration by needleless injection, containing a pharmacologically acceptable meloxicam salt of an organic or inorganic base and one or more suitable excipients, the content of dissolved meloxicam salt being from 35 to 100 mg / ml. The formulation according to the invention has a shelf-life of up to 24 months or more.
Owner:FOLGER MARTIN ANDREAS +3
Triphenylalkene derivatives and their use as selective estrogen receptor modulators
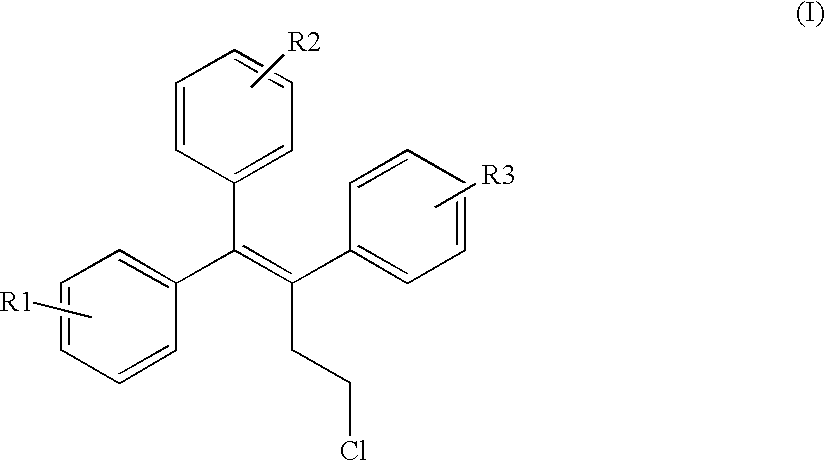
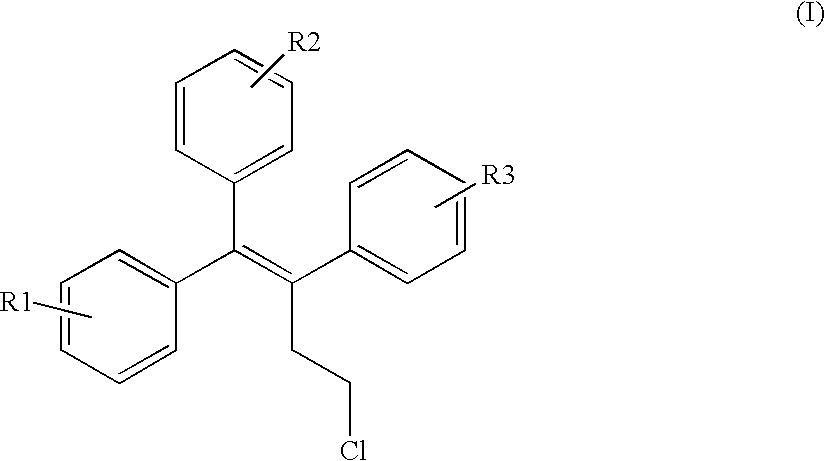
InactiveUS6576645B1Efficient productionSufficient amountBiocideNervous disorderHalogenStereoisomerism
The invention provides novel selective estrogen receptor modulator compounds of the general formula:wherein R1 and R2, which are the same or different area) H, halogen, OCH3, OH; or where X is O, NH or S; and n is an integer from 1 to 4; and R4 and R5, which are the same or different, are a 1 to 4 carbon alkyl, H, -CH2C=CH or -CH2CH2OH; or R4 and R5 form an N-containing five- or six-membered ring or heteroaromatic ring; orc) -Y-(CH2)nCH2-O-R6where Y is O, NH or S and n is an integer from 1 to 4; and R6 is H, -CH2CH2OH, or -CH2CH2Cl; ord) 2,3-dihydroxypropoxy, 2-methylsulfamylethoxy, 2-chloroethoxy, 1-ethyl-2-hydroxyethoxy, 2,2-diethyl-2-hydroxyethoxy or carboxymethoxy; andR3 is H, halogen, OH or -OCH3;stereoisomers thereof and their non-toxic pharmaceutically acceptable salts and esters and mixtures thereof, which compounds exhibit valuable pharmacological properties.
Owner:FORENDO PHARMA LTD
Nanoparticle compositions comprising liquid oil cores
InactiveUS20110195030A1Improved physical characteristic and stabilityWithout adversely size and functionOrganic active ingredientsBiocideParticle compositionIsopropylene glycol
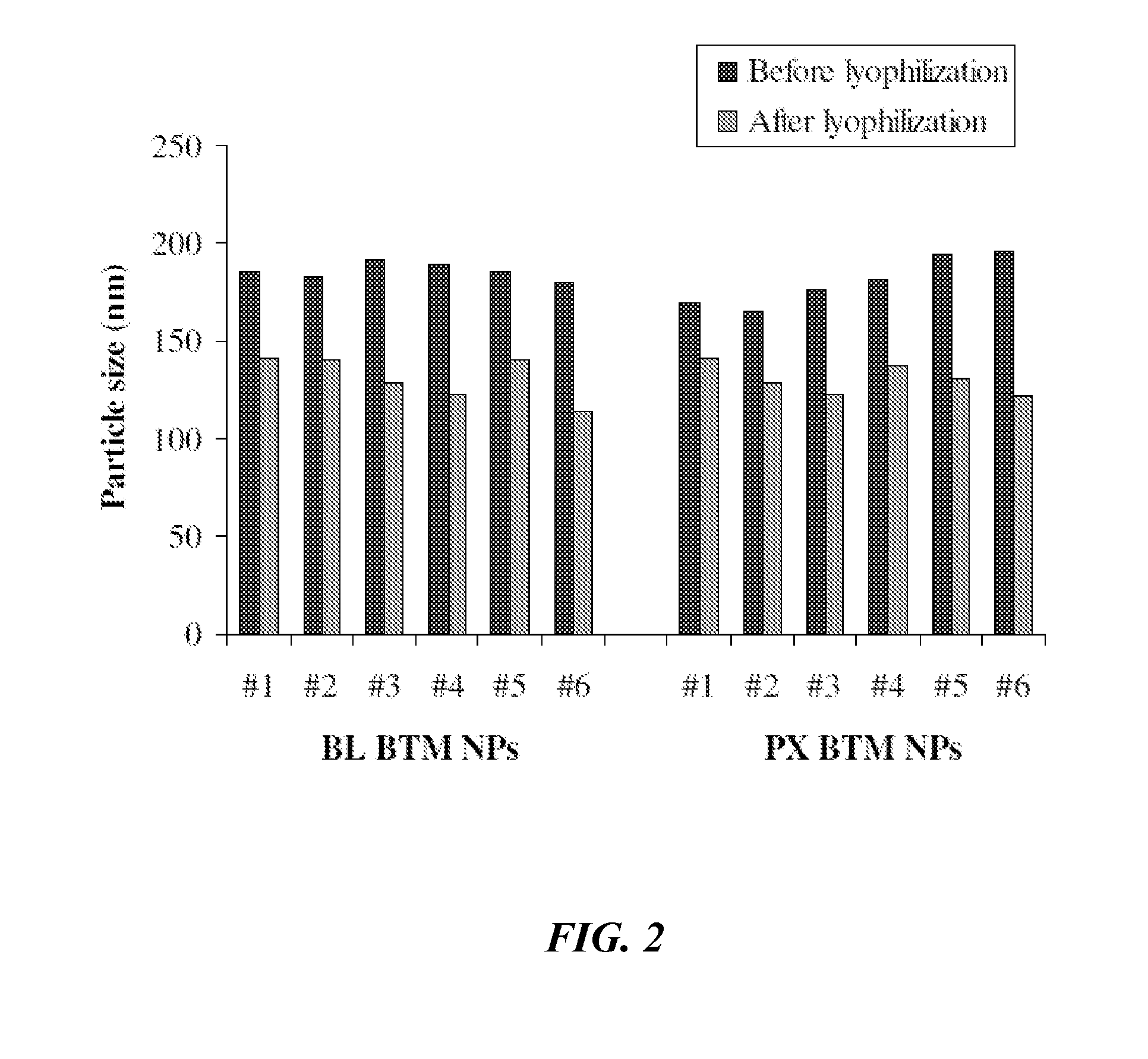
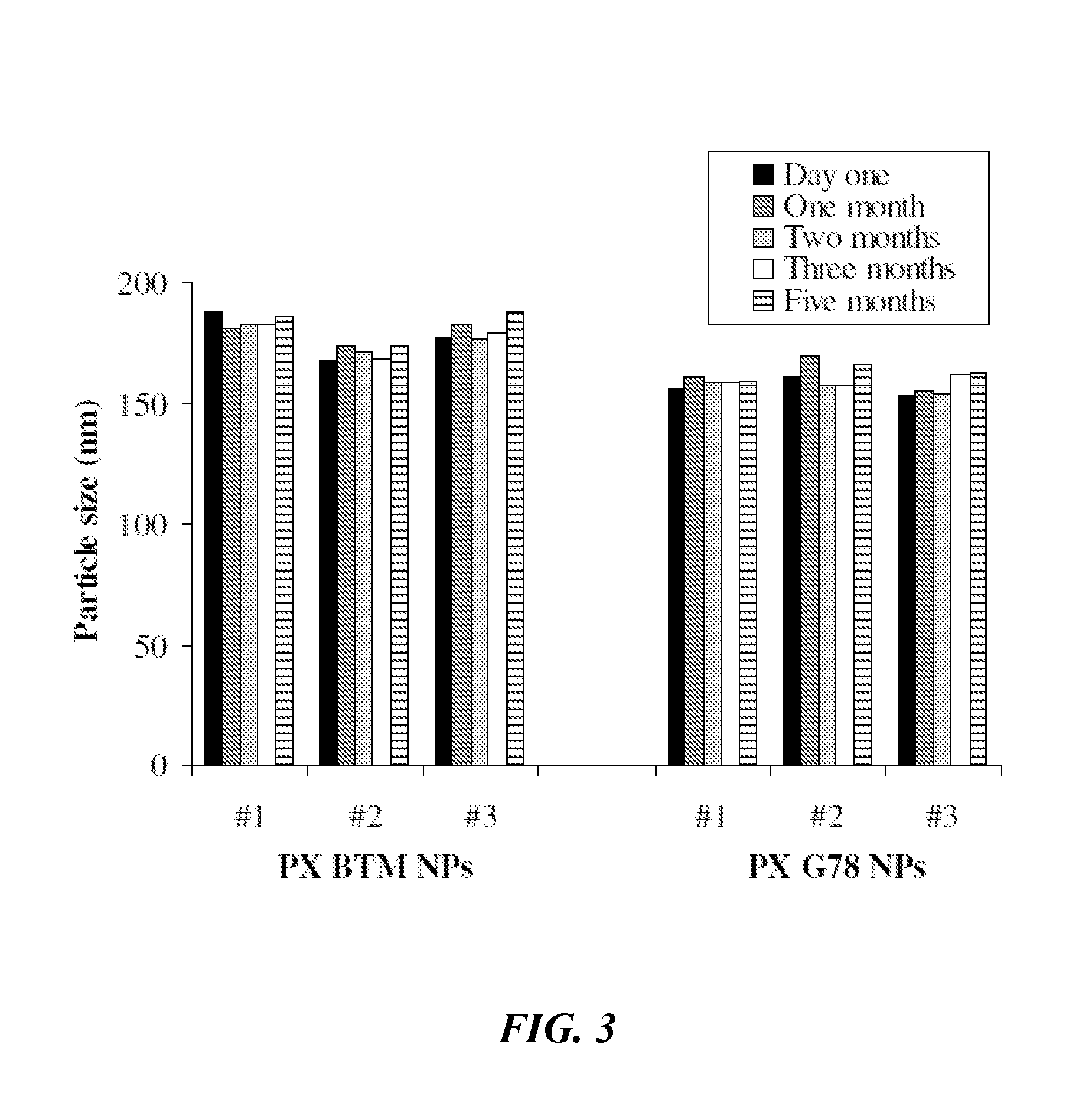
Nanocapsule and nanoemulsion particle compositions having improved physical and pharmacological properties are provided. The nanocapsule or nanoemulsion particle composition can comprise a pharmaceutically acceptable liquid oil phase, a surfactant, and optionally a co-surfactant. The liquid oil phase can comprise a monoglyceride, a diglyceride, a triglyceride, a propylene glycol ester, or a propylene glycol diester. In certain embodiments, the nanocapsule or nanoemulsion particle composition can be lyophilized and subsequently re-hydrated without increasing the mean particle size and / or adversely affecting the potency or efficacy of a therapeutic agent (e.g., paclitaxel) present in the nanocapsules or nanoemulsion particles.
Owner:THE UNIV OF NORTH CAROLINA AT CHAPEL HILL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com