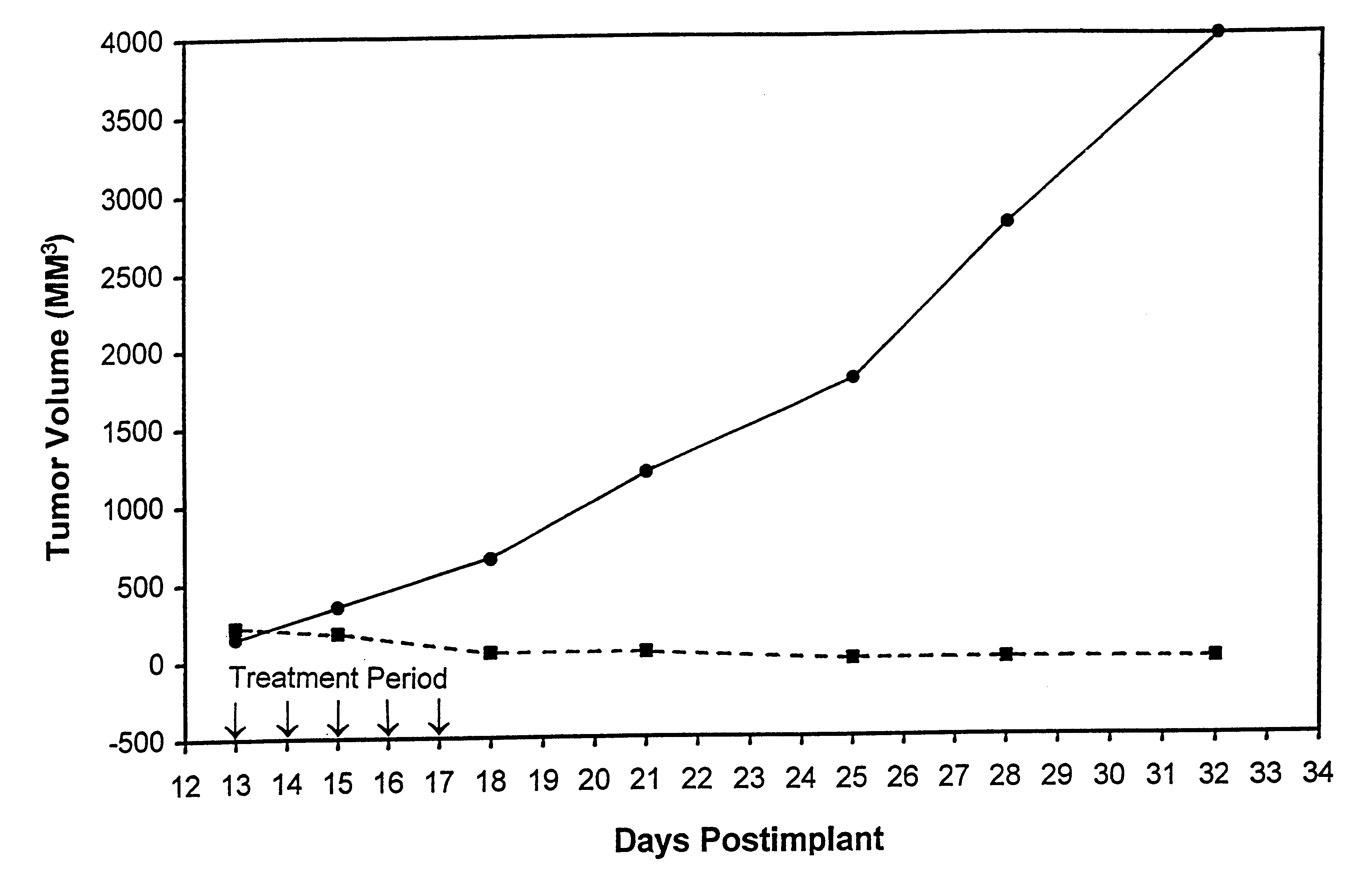
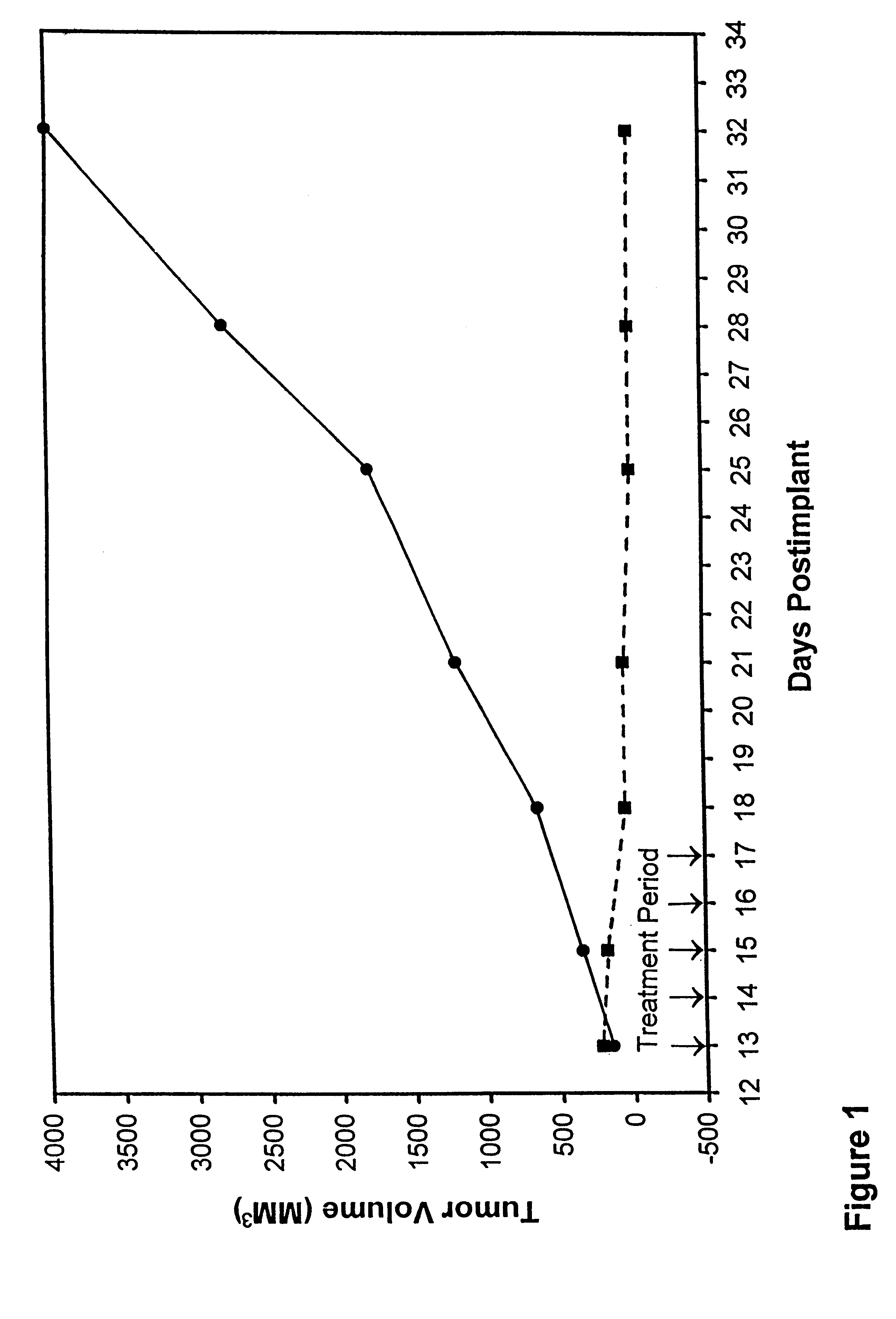
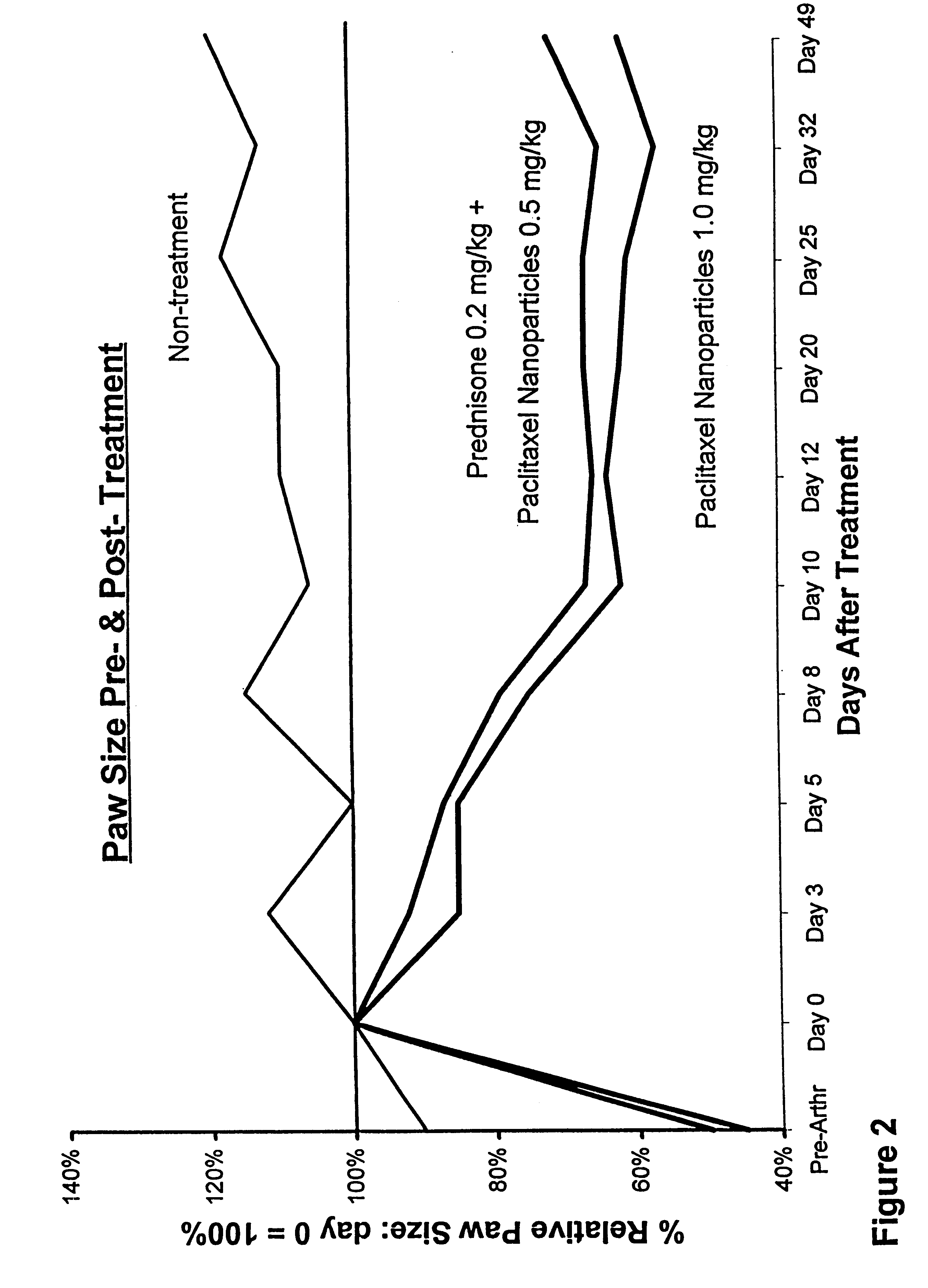
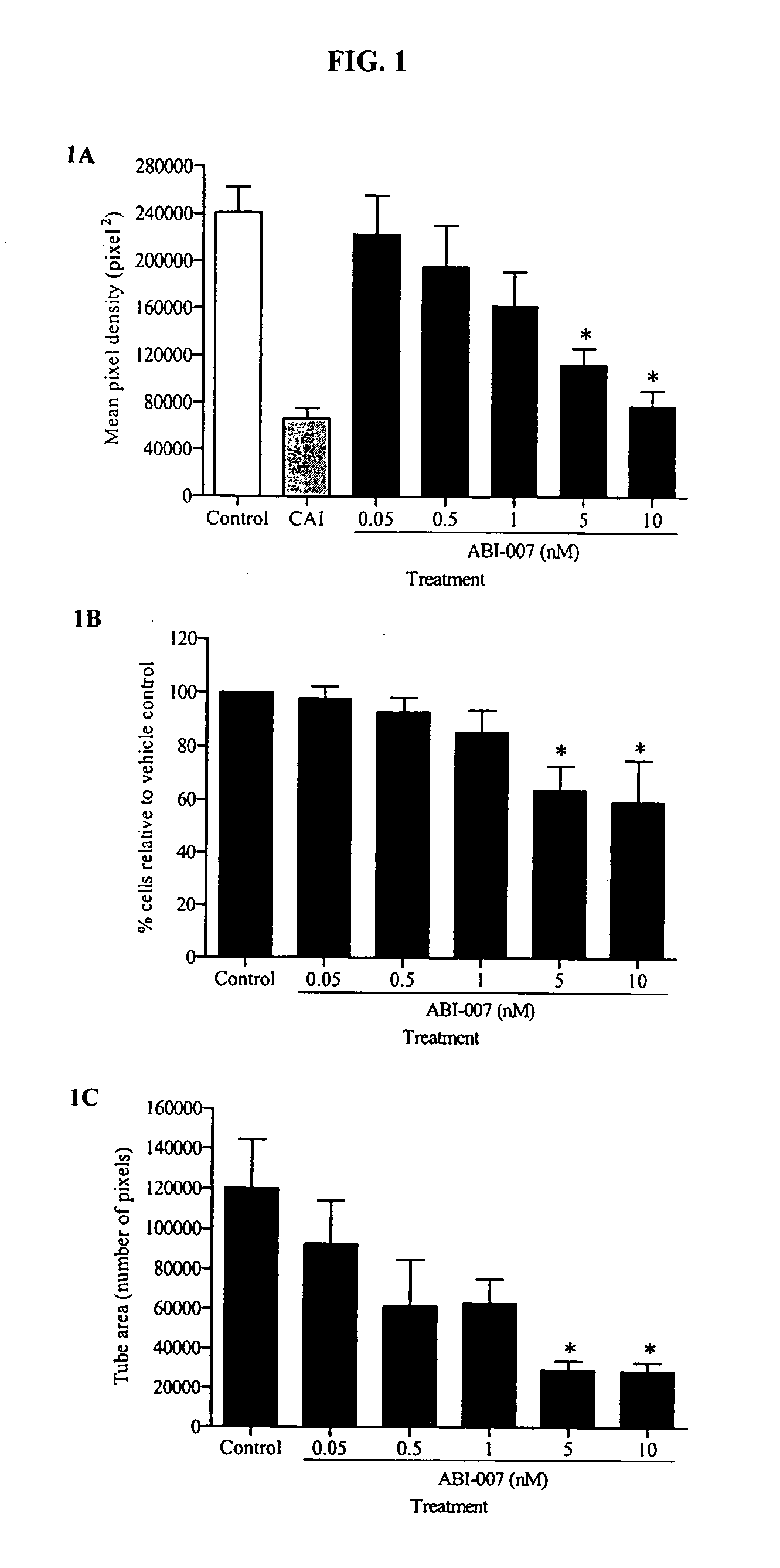
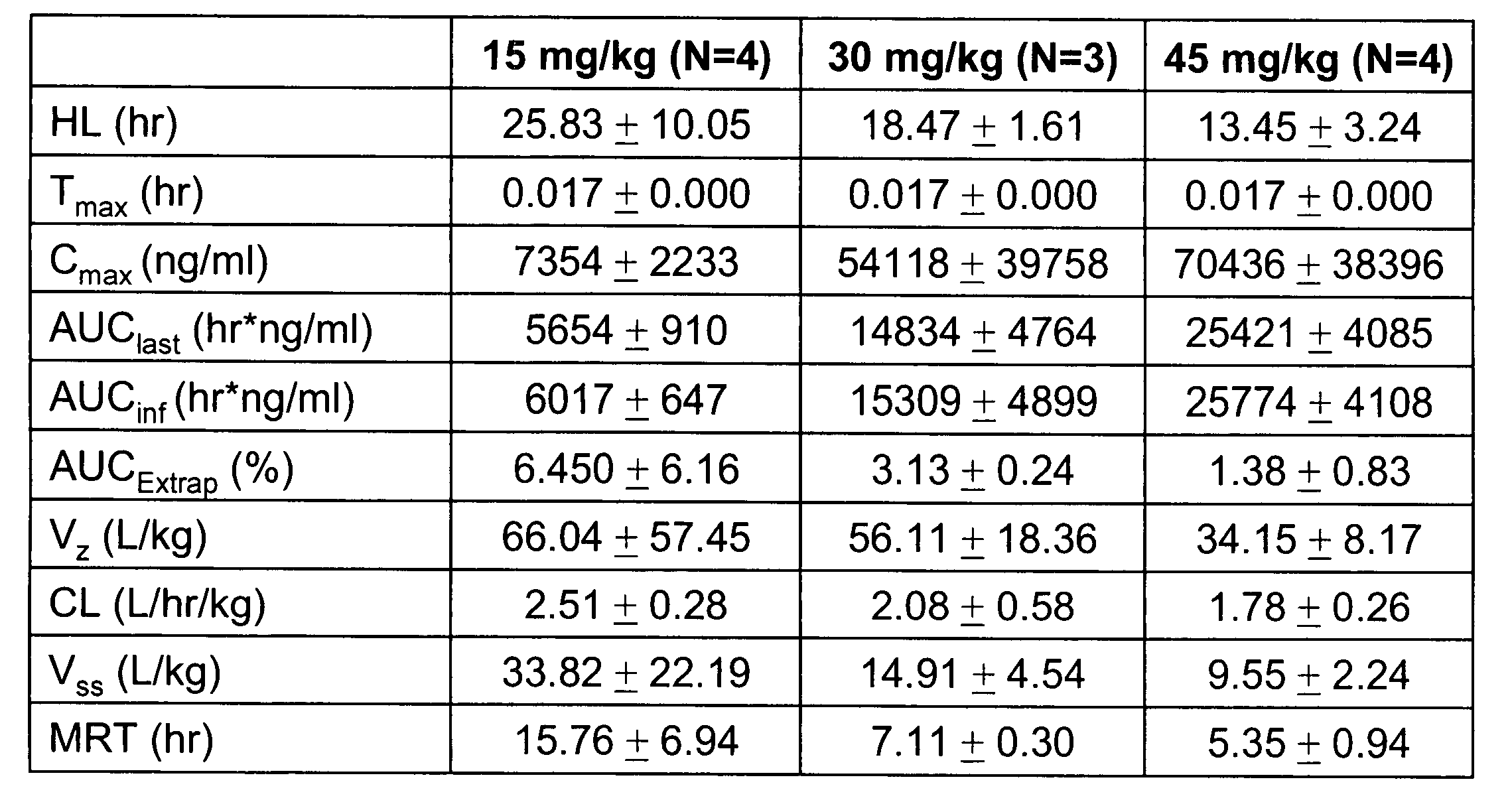
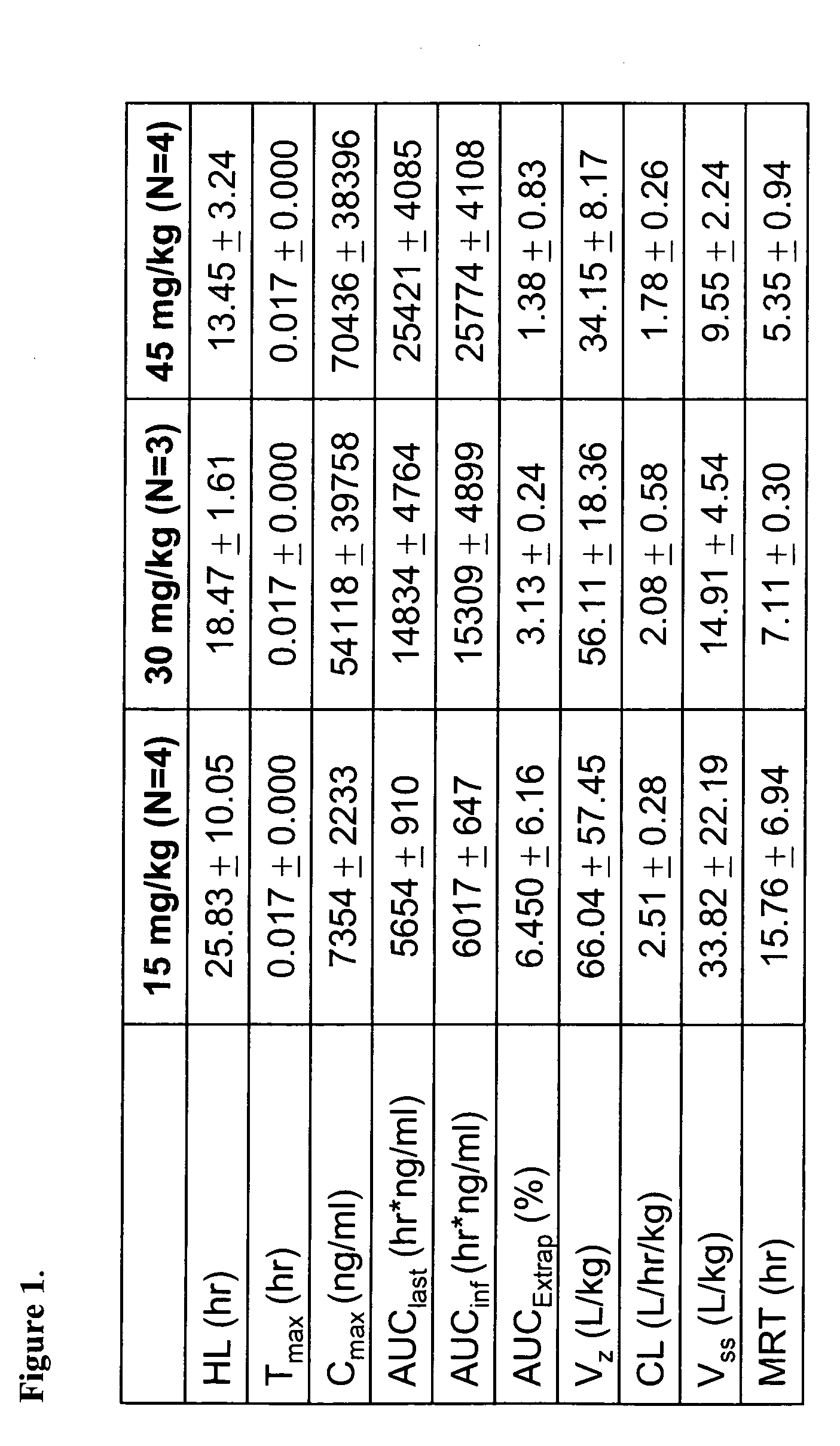
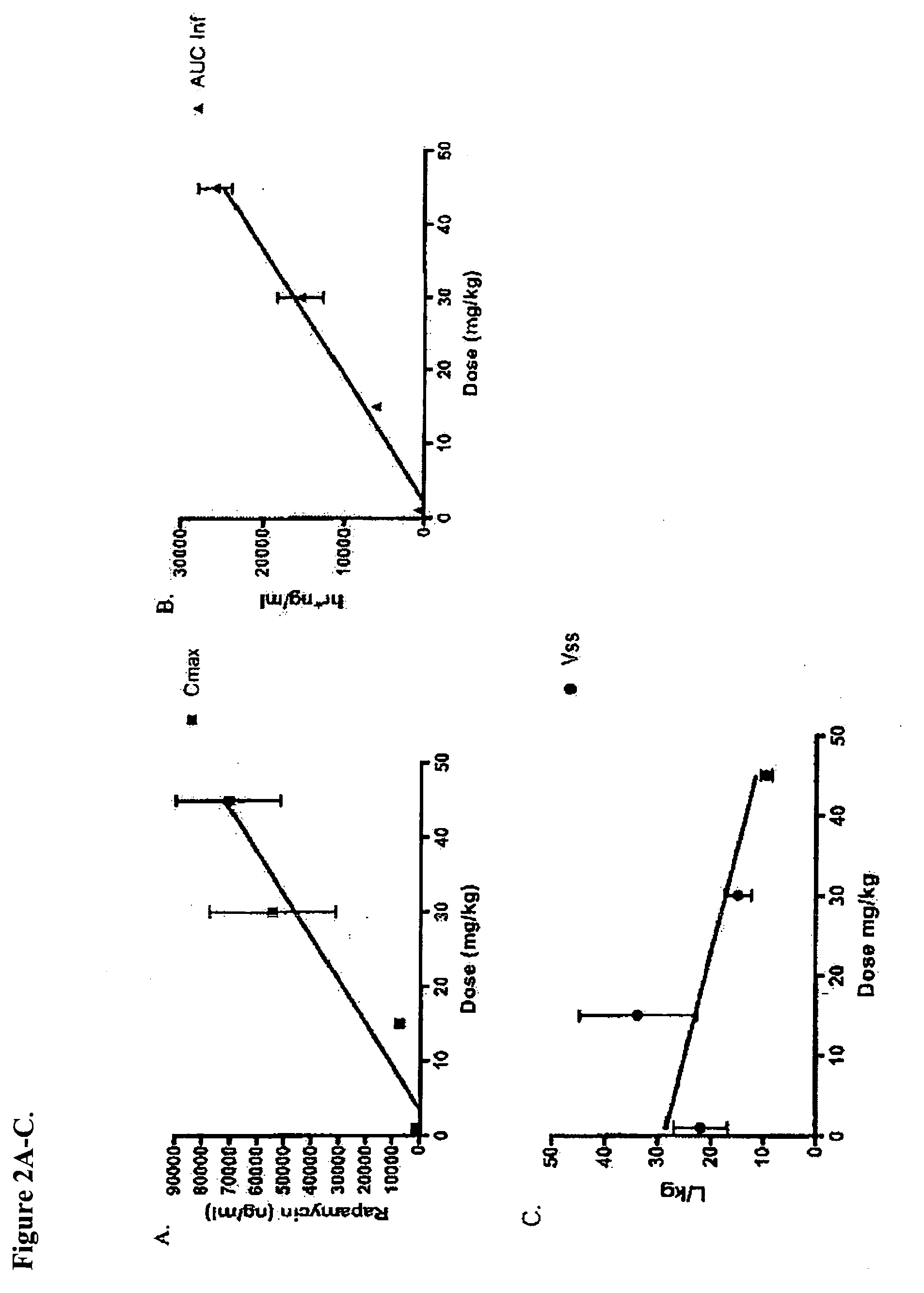
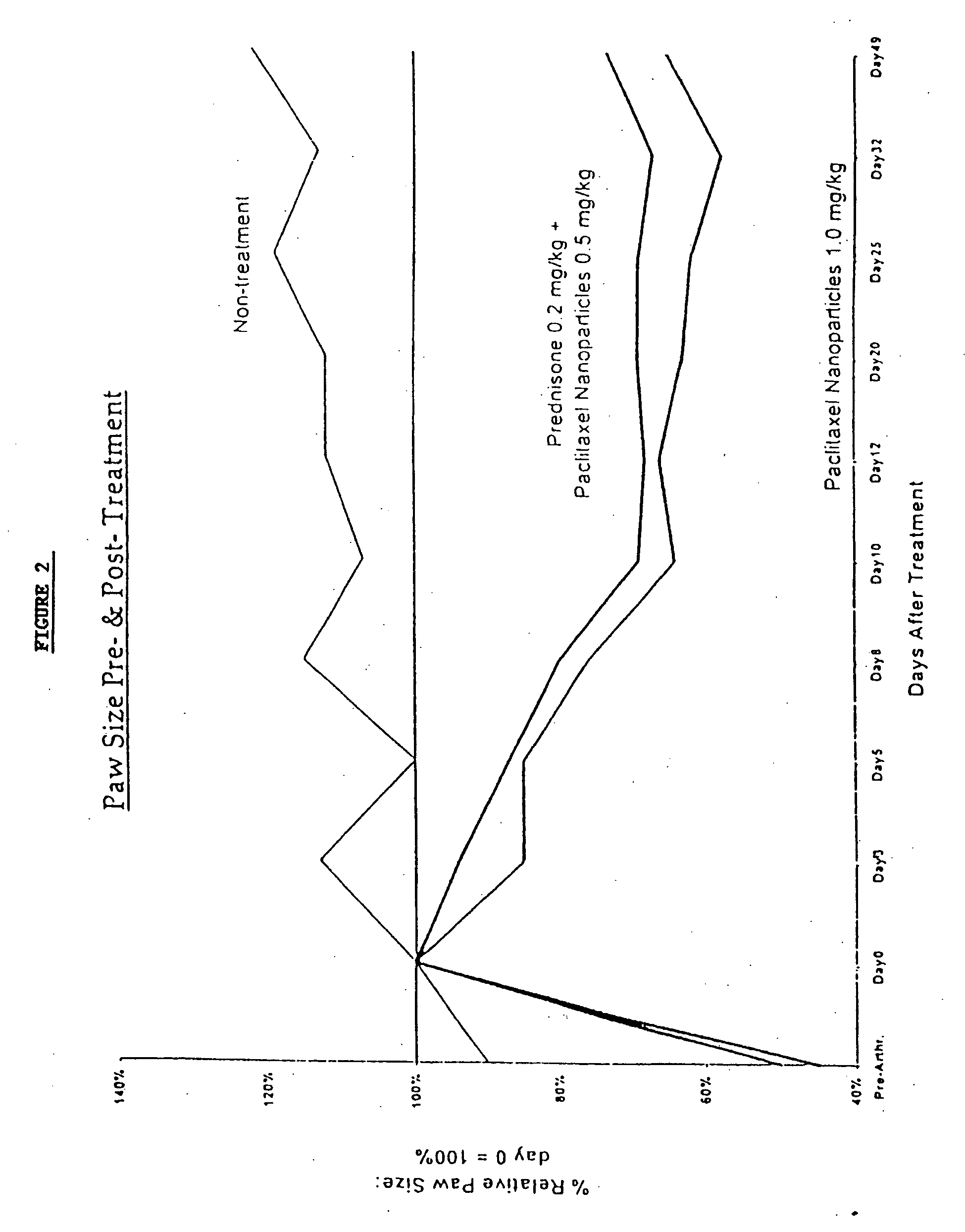
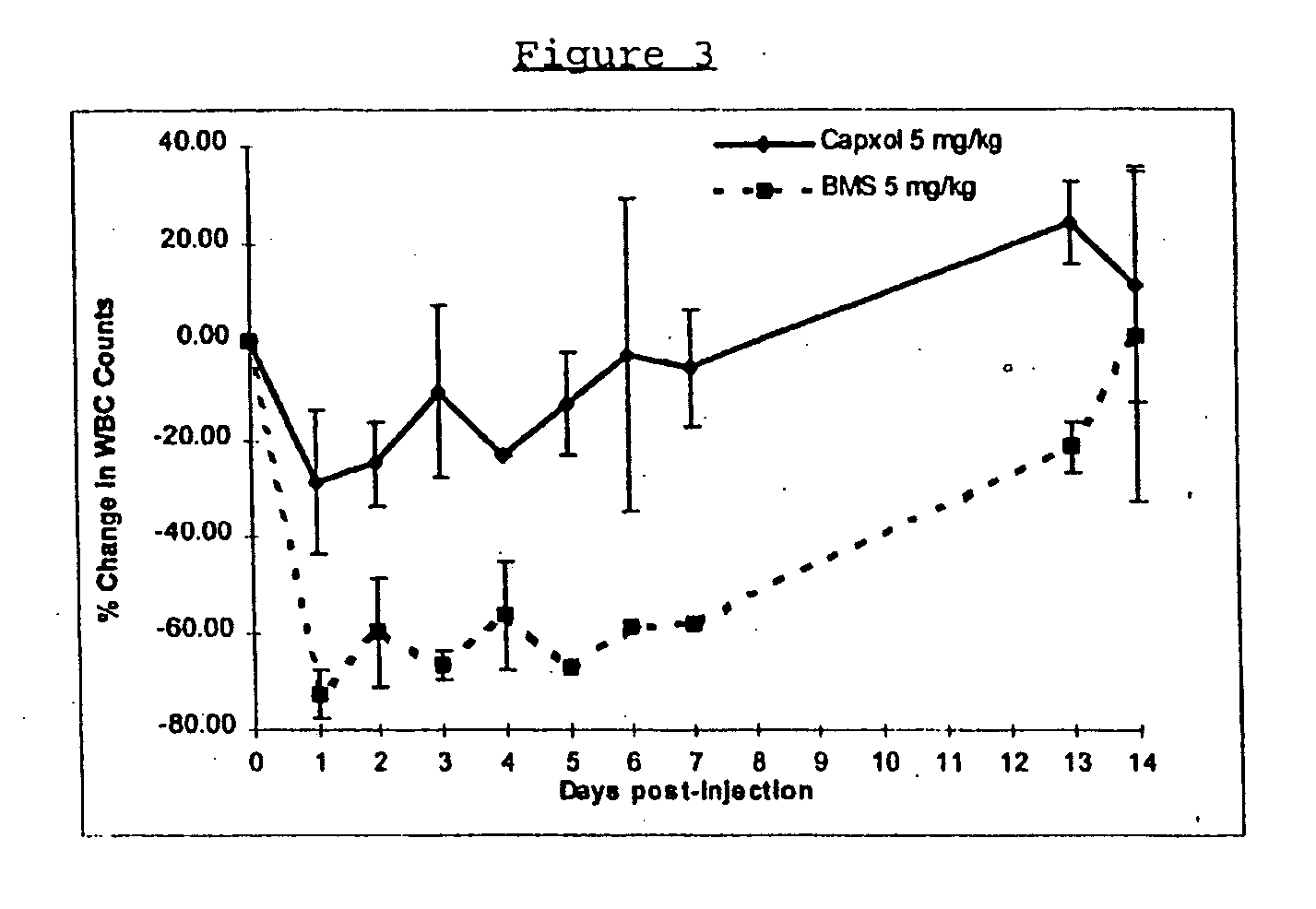
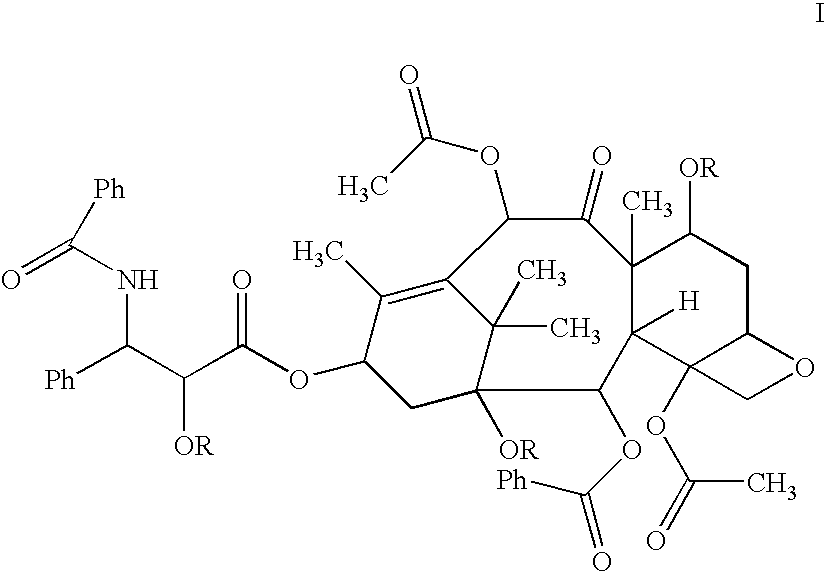
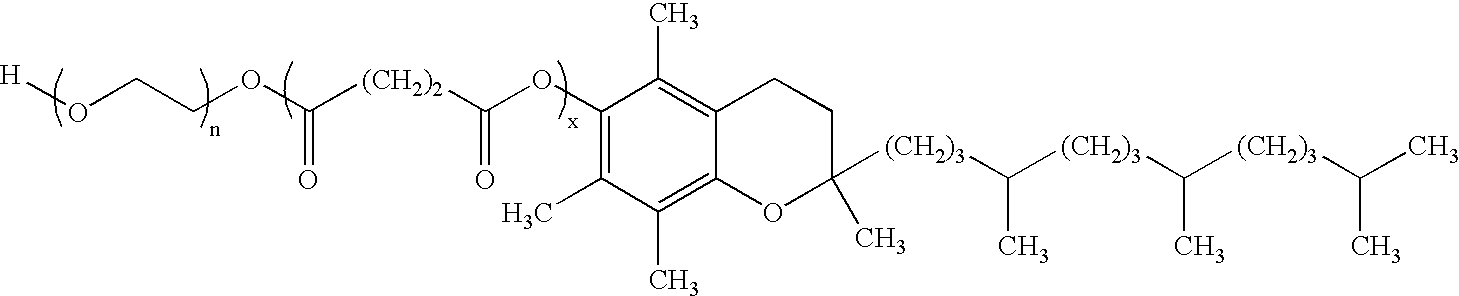
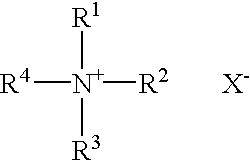Patents
Literature
2122 results about "Paclitaxel" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
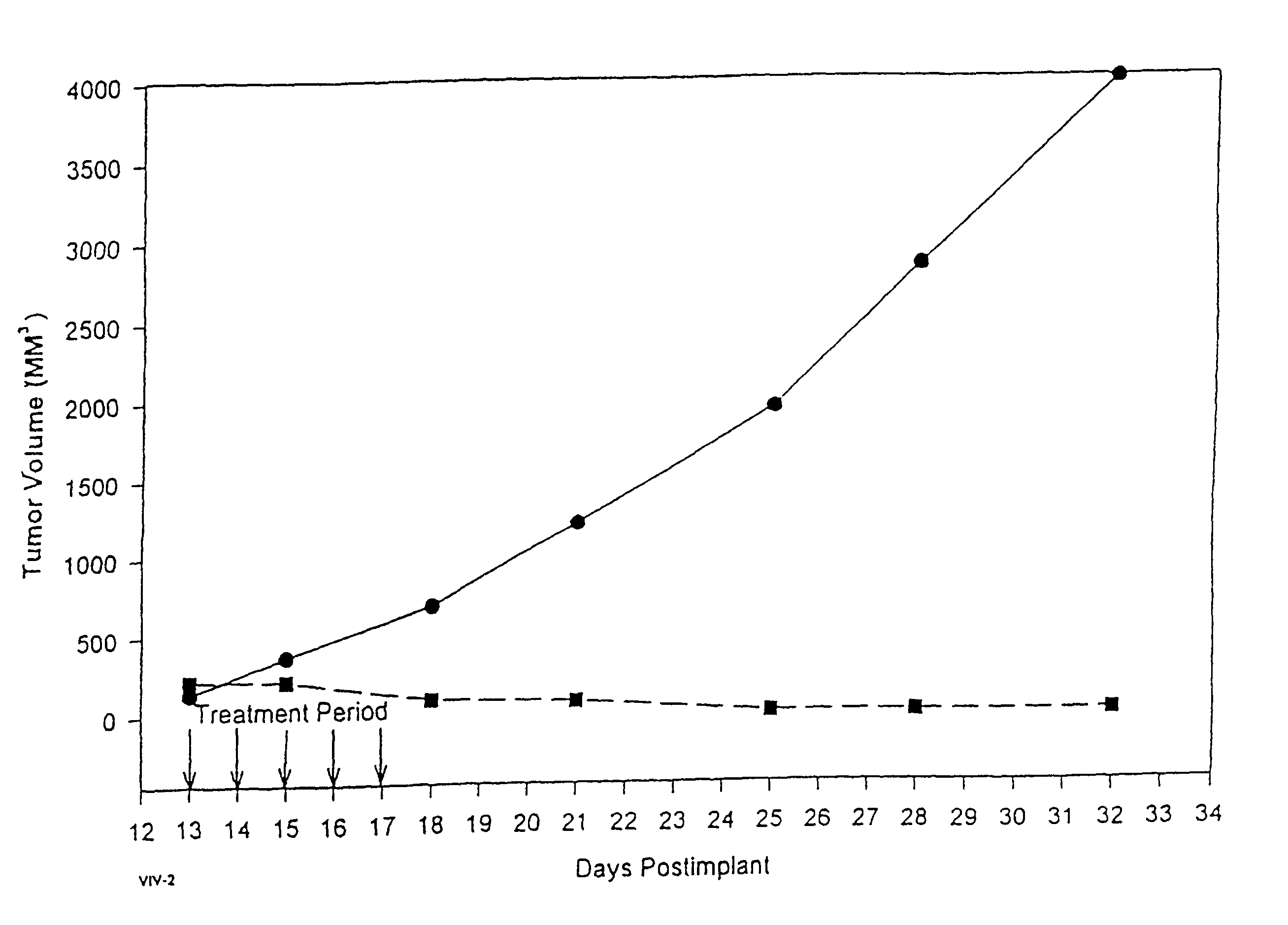
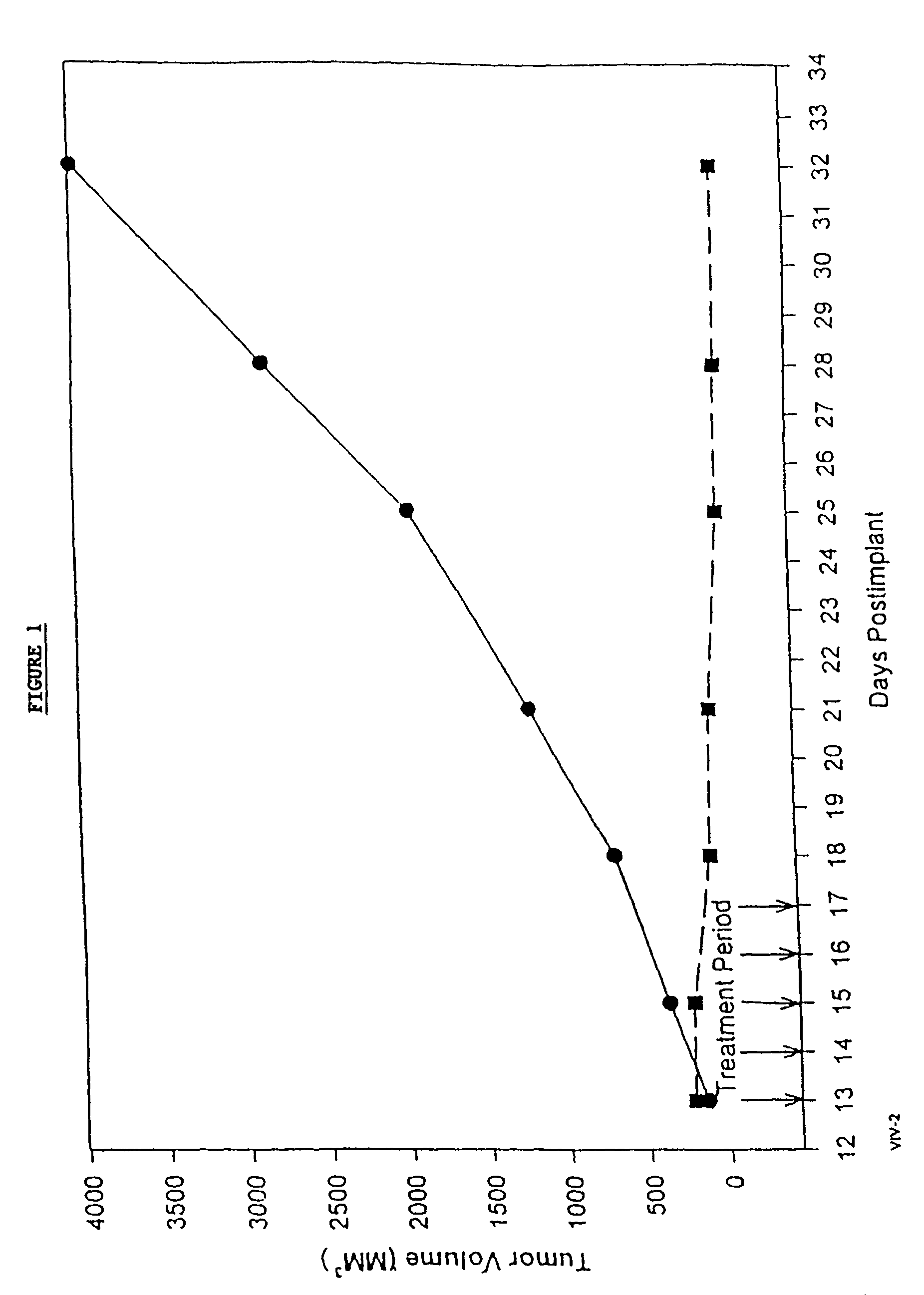
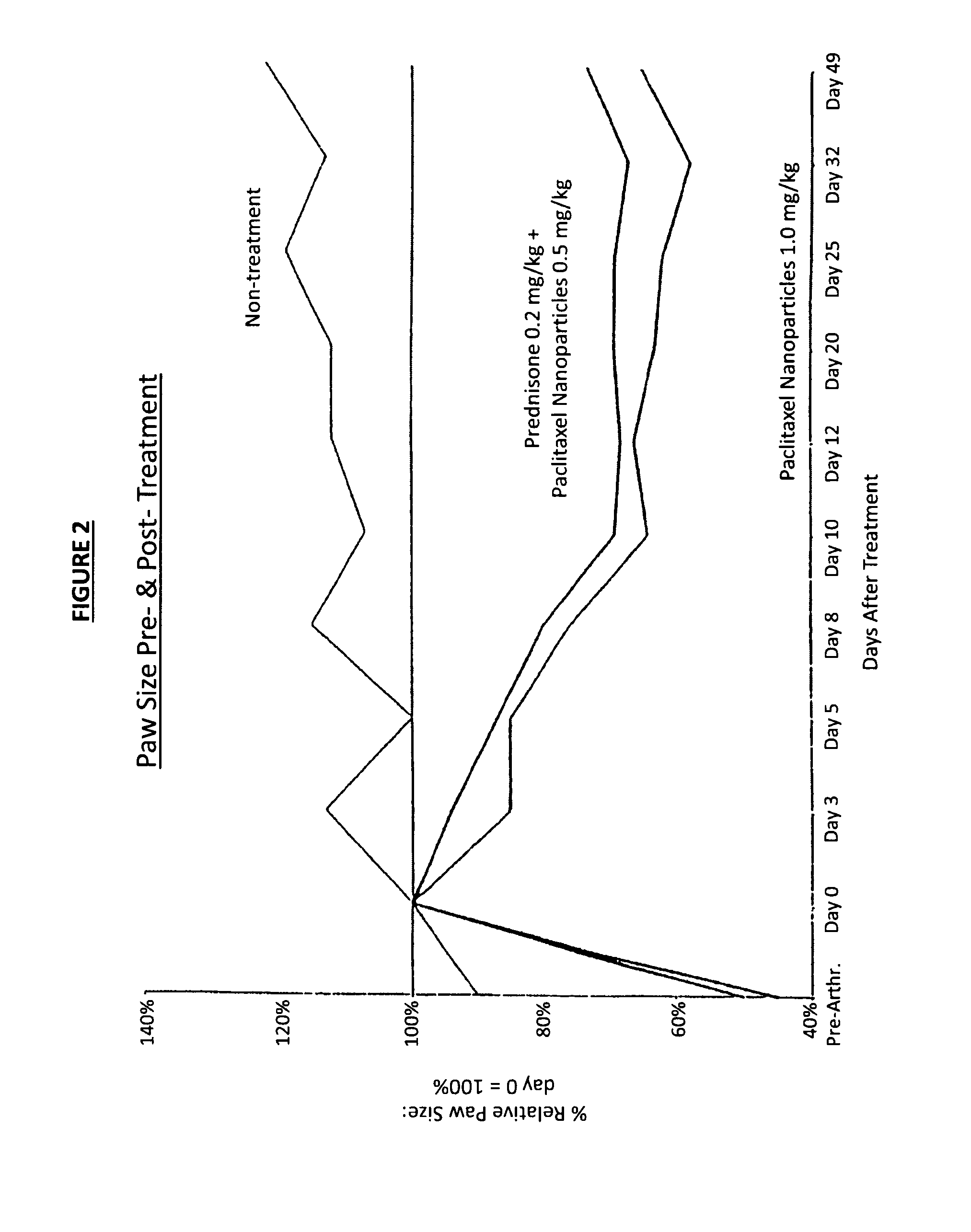
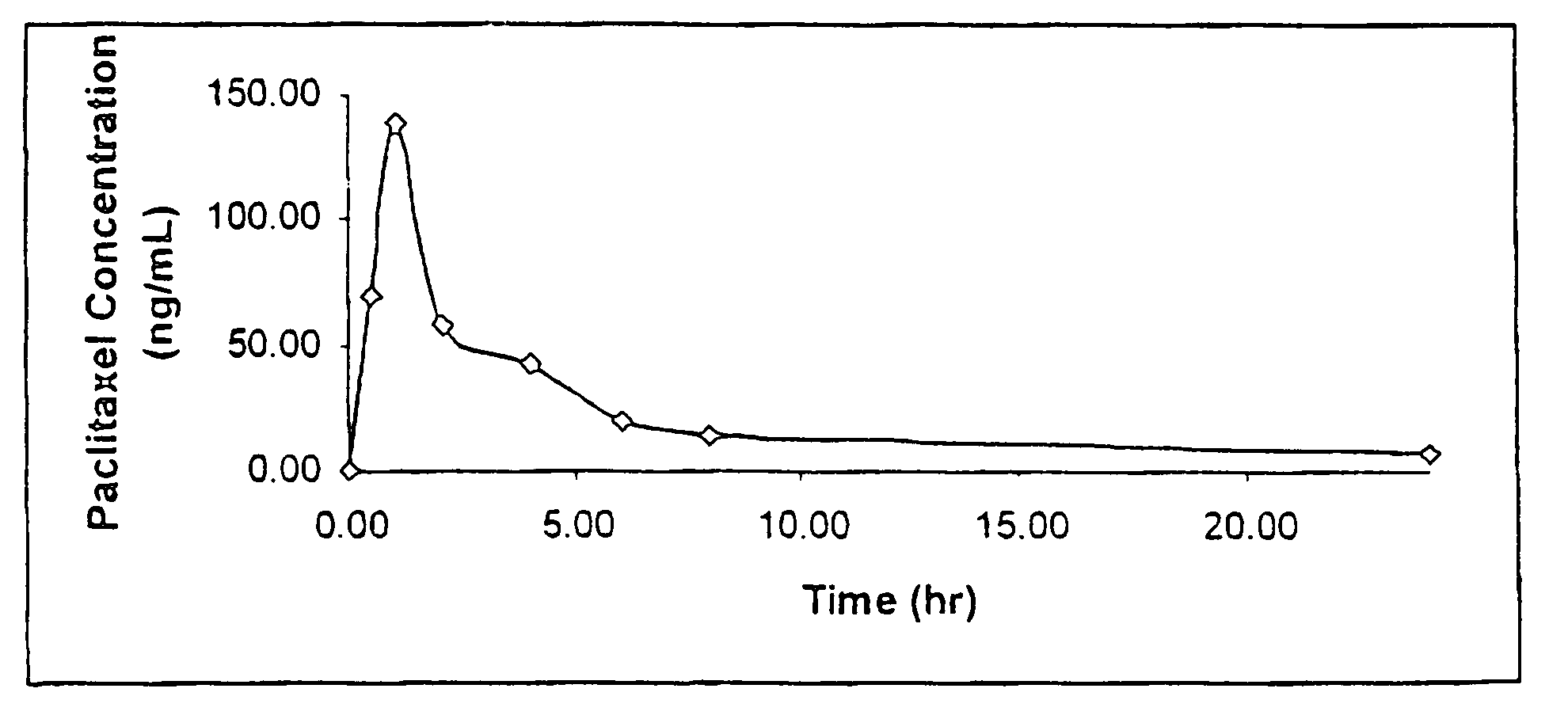
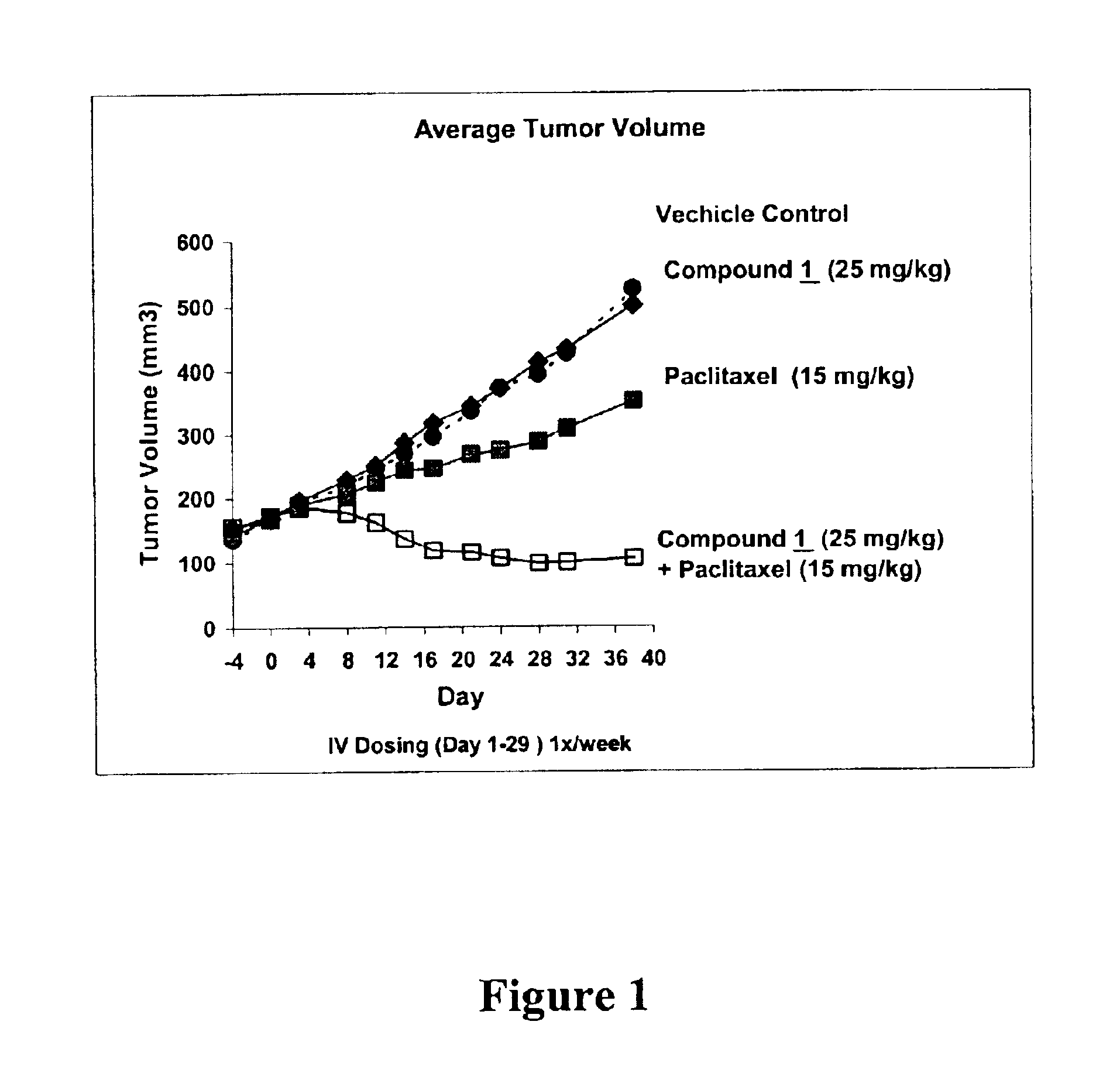
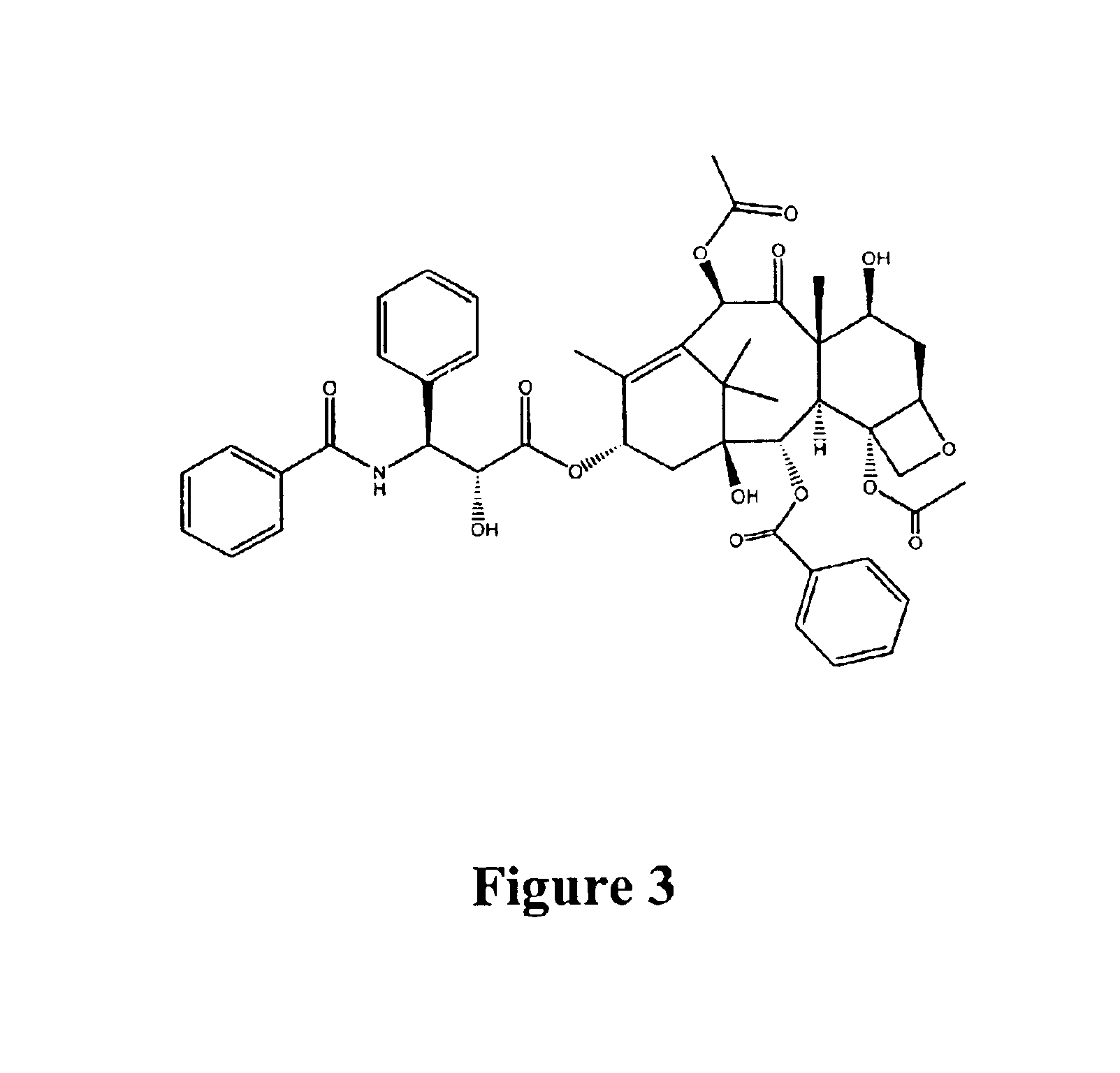
Paclitaxel is used to treat various types of cancer.
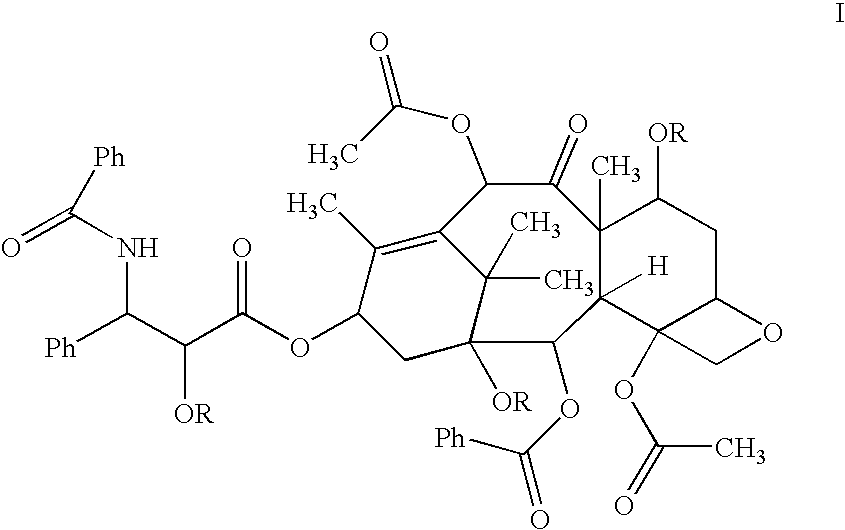
Protein stabilized pharmacologically active agents, methods for the preparation thereof and methods for the use thereof
InactiveUS6749868B1Low toxicityLong half-lifePowder deliveryEchographic/ultrasound-imaging preparationsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful election of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Nanoparticles of paclitaxel and albumin in combination with bevacizumab against cancer
InactiveUS20100112077A1Organic active ingredientsHeavy metal active ingredientsBevacizumab InjectionAnti vegf antibody
The present invention provides combination therapy methods of treating proliferative diseases (such as cancer) comprising a first therapy comprising administering to an individual an effective amount of a taxane in a nanoparticle composition, and a second therapy which may include, for example, radiation, surgery, administration of chemotherapeutic agents (such as an anti-VEGF antibody), or combinations thereof. Also provided are methods of administering to an individual a drug taxane in a nanoparticle composition based on a metronomic dosing regime.
Owner:ABRAXIS BIOSCI LLC
Sutures and surgical staples for anastamoses, wound closures, and surgical closures
ActiveUS20050038472A1Good storage stabilityRemove moistureSuture equipmentsPowder deliverySurgical stapleMicroparticle
The invention relates to sutures and surgical staples useful in anastomoses. Various aspects of the invention include wound closure devices that use amphiphilic copolymer or parylene coatings to control the release rate of an agent, such as a drug or a biological material, polymerizing a solution containing monomers and the agent to form a coating, using multiple cycles of swelling a polymer with a solvent-agent solution to increase loading, microparticles carrying the agent, biodegradable surgical articles with amphiphilic copolymer coatings, and sutures or surgical staples the deliver a drug selected from the group consisting of triazolopyrimidine, paclitaxol, sirolimus, derivatives thereof, and analogs thereof to a wound site.
Owner:MIRUS LLC
Nanoparticulate formulations of docetaxel and analogues thereof
InactiveUS20060188566A1Rapid dissolutionLow injection volumeBiocideOrganic active ingredientsDocetaxelDocetaxel-PNP
Owner:ELAN PHRMA INT LTD
Treatment of Asthma and Chronic Obstructive Pulmonary Disease With Anti-proliferate and Anti-inflammatory Drugs
InactiveUS20080175887A1Promote absorptionOrganic active ingredientsPowdered material dispensingDiseaseObstructive Pulmonary Diseases
Embodiments of the present invention provide a method for treatment of respiratory disorders such as asthma, chronic obstructive pulmonary disease, and chronic sinusitis, including cystic fibrosis, interstitial fibrosis, chronic bronchitis, emphysema, bronchopulmonary dysplasia and neoplasia. The method involves administration, preferably oral, nasal or pulmonary administration, of anti-inflammatory and anti-proliferative drugs (rapamycin or paclitaxel and their analogues).
Owner:LUTONIX INC
Methods and compositions for treating pulmonary hypertension
InactiveUS20100166869A1Pulmonary hypertension is delayedReduce pressureBiocidePowder deliveryNanoparticleCarrier protein
The present invention features methods for treating, stabilizing, preventing, and / or delaying pulmonary hypertension by administering nanoparticles that comprise rapamycin or a derivative thereof and / or nanoparticles that comprise a taxane (e.g., paclitaxel) or a derivative thereof. The invention also provides compositions (e.g., unit dosage forms) comprising nanoparticles that comprise a carrier protein and rapamycin or a derivative thereof and / or nanoparticles that comprise a carrier protein and a taxane (e.g. paclitaxel) or a derivative thereof.
Owner:ABRAXIS BIOSCI LLC
Medical implants with a combination of compounds
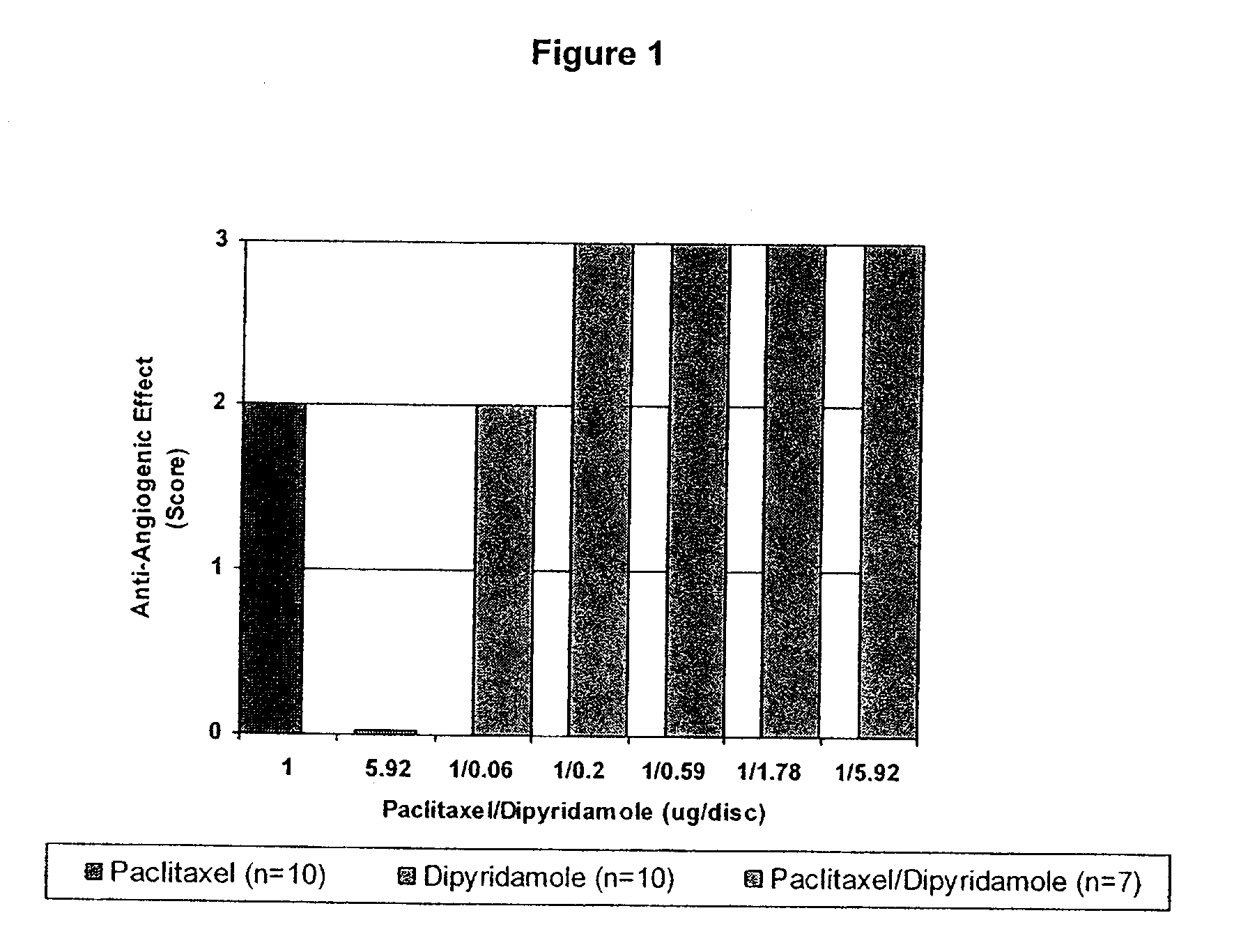
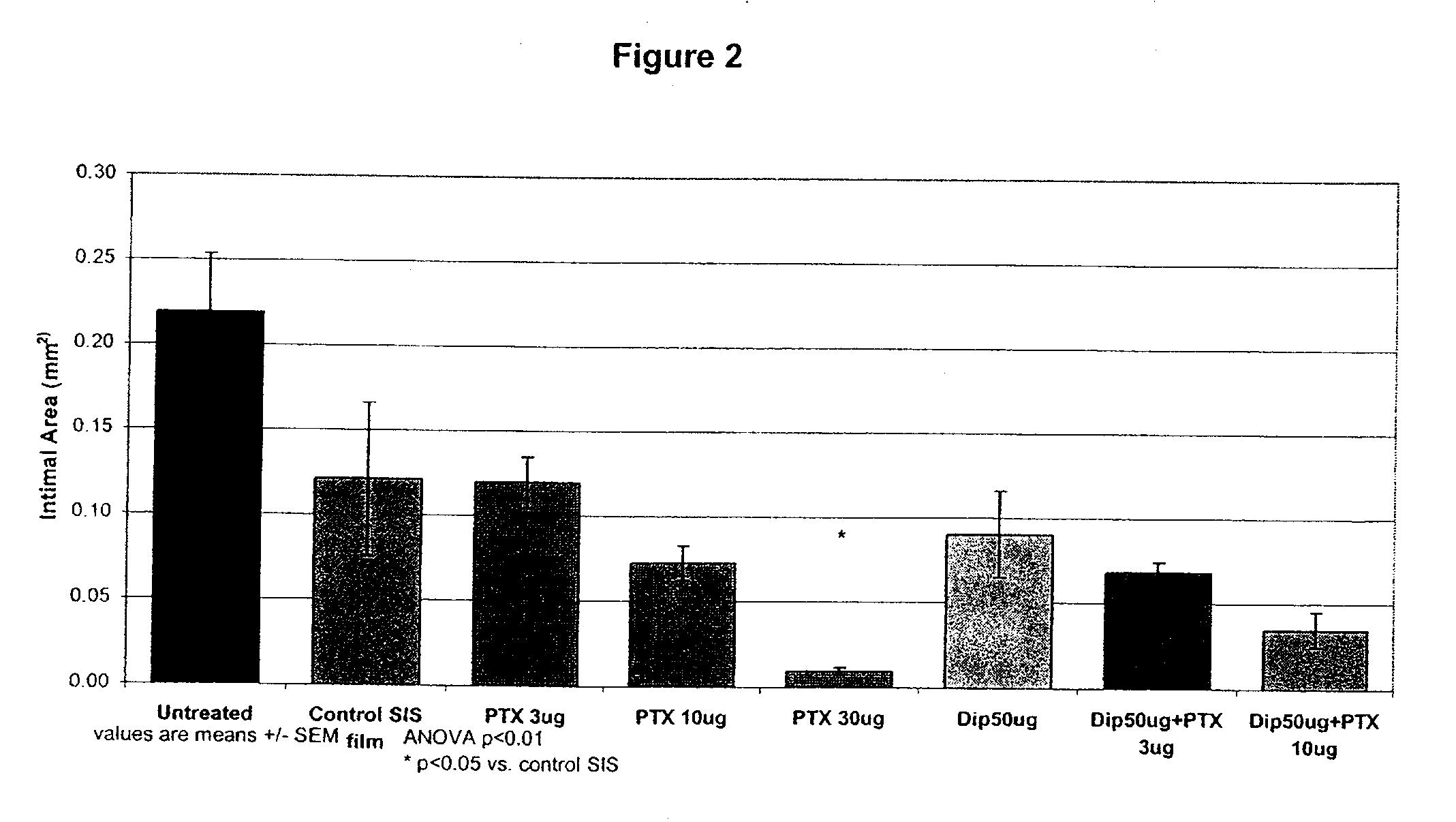
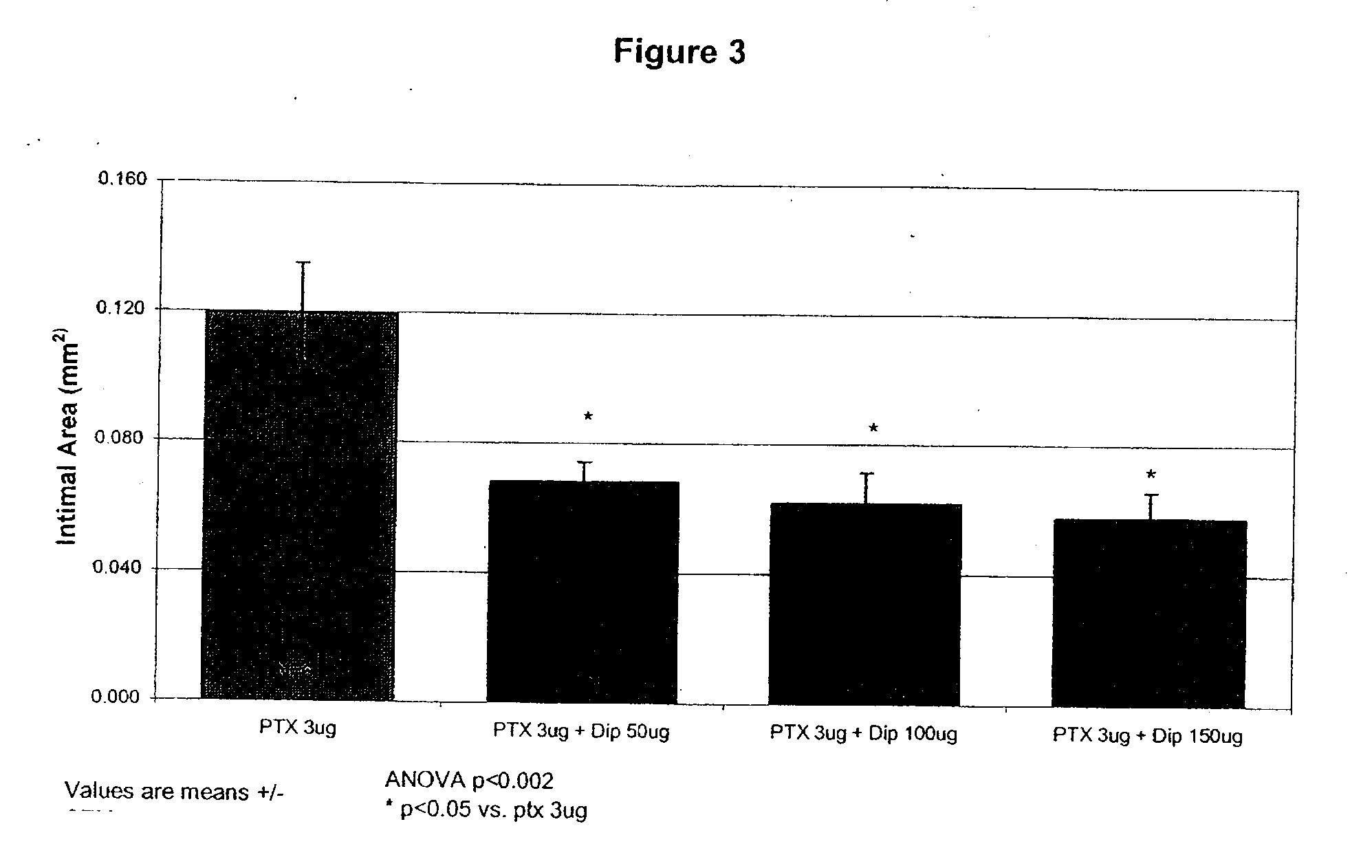
InactiveUS20100074934A1Minimize formationImprove biological effectOrganic active ingredientsBiocideDipyridamoleFibrosis
Implants are associated with a combination of paclitaxel or derivatives and dipyridamole or derivatives in order to inhibit fibrosis that may otherwise occur when the implant is placed within an animal. Exemplary implants include intravascular implants (e.g., coronary and peripheral vascular stents, catheters, balloons), non-vascular stents, pumps and sensors, vascular grafts, perivascular devices, implants for hemodialysis access, vena cava filters, implants for providing an anastomotic connection, electrical devices, intraocular implants, and soft tissue implants and fillers.
Owner:ANGIOTECH PHARMA INC
Formulation for paclitaxel
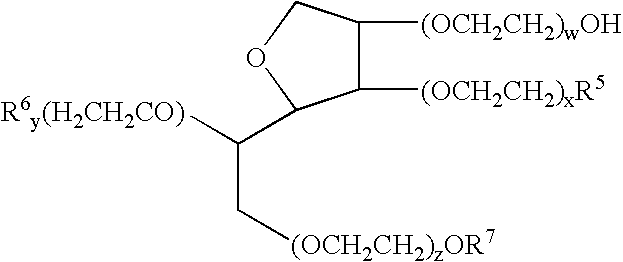
A pharmaceutical formulation is provided for delivering paclitaxel in vivo comprising: water and micelles comprising paclitaxel and a pharmaceutically-acceptable, water-miscible solubilizer forming the micelles, the solubilizer selected from the group consisting of solubilizers having the general structuresR1COOR2, R1CONR2, and R1COR2,wherein R1 is a hydrophobic C3-C50 alkane, alkene or alkyne and R2 is a hydrophilic moiety. The solubilizer is selected such that it does not have a pKa less than about 6.
Owner:SUPERGEN
Drug-eluting medical device
The present invention relates to a drug-eluting medical device, in particular a balloon for angioplasty catheters with drug elution to prevent the restenosis of the vessel subjected to angioplasty. More particularly, the present invention relates to a catheter balloon completely or partially coated with paclitaxel in hydrated crystalline form or in hydrated solvated crystalline form, having an immediate release and bioavailability of a therapeutically effective amount of paclitaxel at the site of intervention. The balloon can be made of a polyether-polyamide block copolymer, or a polyester amide, or polyamide-12.
Owner:INVATEC TECH CENT
Drug eluting coatings for medical implants
ActiveUS20040037886A1Minimizing restenosisMinimizing thrombosisSuture equipmentsBiocideEverolimusCyclosporins
Drug eluting coating compositions are composed of at least one therapeutic agent dispersed in modified, biologically active binders. The therapeutic agents included in the coating composition are paclitaxel, sirolimus, tacrolimus, everolimus, actinomycin-D, dexamethasone, mycophenolic acid, cyclosporins, estradiol, and derivatives and analogs thereof. These therapeutic agents are applied to the surface of the medical device by a modified, biologically active binders. By using these biologically active binders, the therapeutic agents can be applied to at least one surface of a medical implant without using inert polymer carriers.
Owner:BIOVENTION INC
Oral pharmaceuticals formulation comprising paclitaxel, derivatives and methods of administration thereof
InactiveUS20040092428A1Improve bioavailabilityImprove oral bioavailabilityOrganic active ingredientsCyclic peptide ingredientsOral medicationBioavailability
The invention concerns excipients or combinations thereof suitable for preparing an oral formulation containing a pharmaceutical agent. More particularly, the invention is directed to stable, efficacious and bioavailable oral pharmaceutical formulations comprising paclitaxel, derivatives of paclitaxel and pharmaceutically acceptable salts thereof. The formulations of the invention increase bioavailability of paclitaxel when dissolved in the gastrointestinal system. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts of such derivatives to patients in need thereof. The formulations of the invention are particularly suitable for oral administration to mammals including humans.
Owner:TRANSFORM PHARMACEUTICALS INC
Drug delivery systems, kits, and methods for administering zotarolimus and paclitaxel to blood vessel lumens
A system and compositions including zotarolimus and paclitaxel are disclosed, as well as methods of delivery, wherein the drugs have effects that complement each other. Medical devices are disclosed which include supporting structures that include at least one pharmaceutically acceptable carrier or excipient, which carrier or excipient can include one or more therapeutic agents or substances, with the carrier including at least one coating on the surface thereof, and the coating associated with the therapeutic substances, such as, for example, drugs. Supporting structures for the medical devices that are suitable for use in this invention include, but are not limited to, coronary stents, peripheral stents, catheters, arterio-venous grafts, by-pass grafts, and drug delivery balloons used in the vasculature. These compositions and systems can be used in combination with other drugs, including anti-proliferative agents, anti-platelet agents, anti-inflammatory agents, anti-thrombotic agents, cytotoxic drugs, agents that inhibit cytokine or chemokine binding, cell de-differentiation inhibitors, anti-lipaedemic agents, matrix metalloproteinase inhibitors, cytostatic drugs, or combinations of these and other drugs.
Owner:ABBOTT LAB INC
Tumor targeting drug-loaded particles
A composition for delivering a tumor therapeutic agent to a patient includes a fast-release formulation of a tumor apoptosis inducing agent, a slow-release formulation of a tumor therapeutic agent, and a pharmaceutically acceptable carrier. An apoptosis-inducing agent in a pharmaceutically acceptable carrier may be administered before or concomitantly therewith. Nanoparticles or microparticles (e.g., cross-linked gelatin) of the therapeutic agent (e.g., paclitaxel) also may be used. The nanoparticles or microparticles may be coated with a bioadhesive coating. Microspheres that agglomerate to block the entrance of the lymphatic ducts of the bladder to retard clearance of the microparticles through the lymphatic system also may be employed. This invention also uses drug-loaded gelatin and poly(lactide-co-glycolide) (PLGA) nanoparticles and microparticles to target drug delivery to tumors in the peritoneal cavity, bladder tissues, and kidneys.
Owner:AU JESSIE L S +1
Diterpene Glycosides as Natural Solubilizers
InactiveUS20110033525A1Improve solubilityRetain activityBiocideHydroxy compound active ingredientsItraconazoleCapsaicin
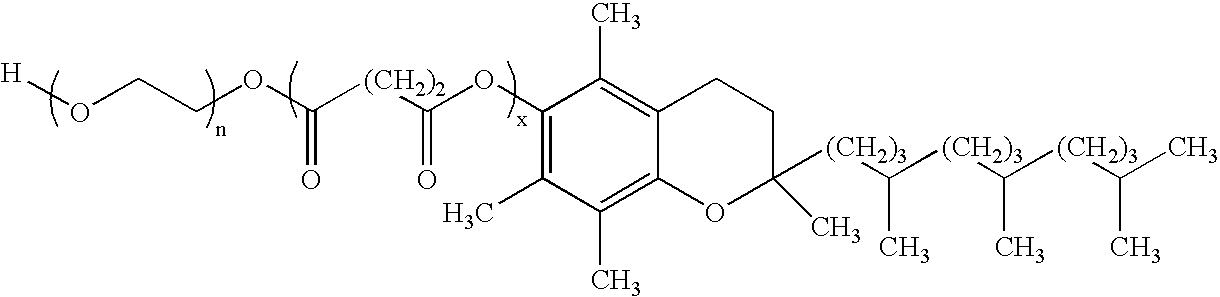
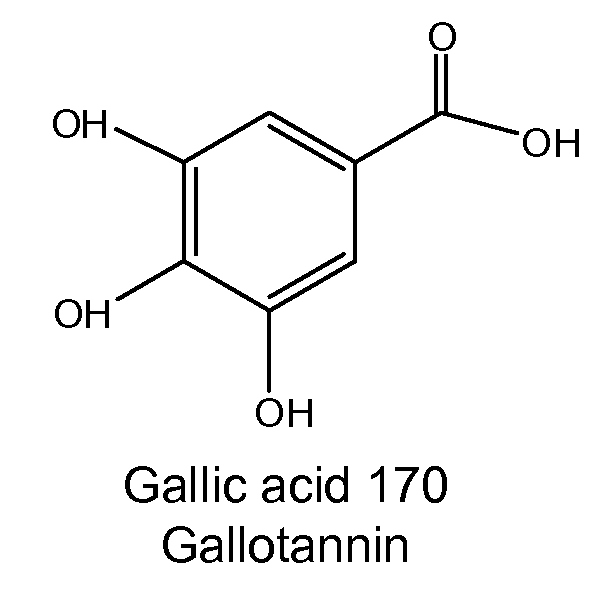
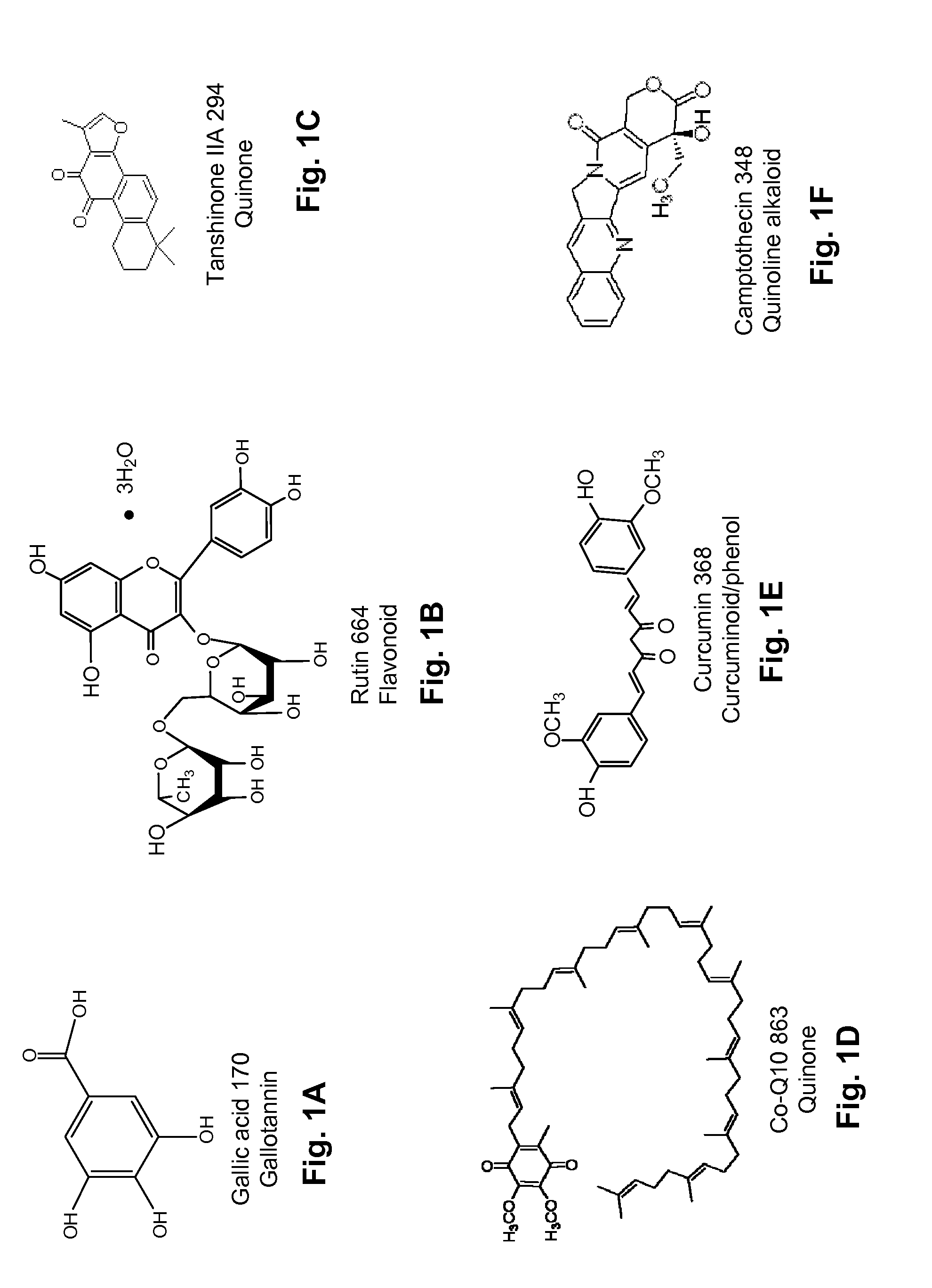
Several diterpene glycosides (e.g., rubusoside, rebaudioside, steviol monoside and stevioside) were discovered to enhance the solubility of a number of pharmaceutically and medicinally important compounds, including but not limited to, paclitaxel, camptothecin, curcumin, tanshinone HA, capsaicin, cyclosporine, erythromycin, nystatin, itraconazole, and celecoxib. The use of the diterpene glycoside rubusoside increased solubility in all tested compounds. The diterpene glycosides are a naturally occurring class of water solubility-enhancing compounds that are non-toxic and that will be useful as new complexing agents or excipients in the pharmaceutical, agricultural (e.g., solubilizing pesticides), cosmetic and food industries. Aqueous solutions by using rubusoside to increase the solubility of otherwise insoluble drugs will have several new routes of administration. In addition, aqueous solutions of therapeutic compounds with rubusoside were shown to retain the known pharmacological activity of the compounds.
Owner:BOARD OF SUPERVISORS OF LOUISIANA STATE UNIV & AGRI & MECHANICAL COLLEGE
Porous composites with high-aspect ratio crystals
ActiveUS20140271775A1Easy to attachHigh aspect ratioOrganic active ingredientsBiocideVascular diseaseMedicine
The present disclosure is directed toward composite materials comprising high aspect ratio habits of drug crystals which can be partially or fully extending into a substrate, and additionally, can be projecting from a substrate at an angle of about 20° to about 90°. The present disclosure is directed toward medical devices, such as medical balloons, comprising said composite and methods of using and making the same. The described composite can be used for the local treatment of vascular disease. The present disclosure is also directed toward paclitaxel crystals with a hollow acicular habit.
Owner:WL GORE & ASSOC INC
Formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS8853260B2Improve abilitiesPromote formationOrganic active ingredientsBiocideSuspended particlesWater insoluble
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Anticancer compositions
Pharmaceutical dosage forms for anticancer drugs, and paclitaxel in particular, are described in which the active drug is formulated as storage stable self-emulsifying preconcentrate.
Owner:RTP PHARMA
Process for preparation of paclitaxel trihydrate and docetaxel trihydrate
A process for converting paclitaxel or docetaxel to the respective trihydrate characterized by very high purity, comprises dissolving either paclitaxel or docetaxel in a mixture of alkane and chlorinated alkane to provide a crude product of 65-75% assay and dissolving the crude product in an alkyl ketone, followed by addition of an alkane to provide a product of increased chromatographic purity; dissolving the product of increased chromatographic purity in an aliphatic nitrile, with addition of water to precipitate taxane trihydrate.
Owner:DABUR PHARM LTD
Compositions and methods for preparation of poorly water soluble drugs with increased stability
The present invention provides stable pharmaceutical compositions of poorly water soluble pharmaceutical agents and stabilizing agents which function to increase stability of the compositions. The use of stabilizing agents provide extended stability of nanoparticle suspensions and other formulations of poorly water soluble pharmaceutical agents such as docetaxel under certain conditions, for example upon dilution for administration.
Owner:ABRAXIS BIOSCI LLC
Micelle composition of polymer and passenger drug
InactiveUS20060251710A1Improving micelle encapsulation efficiencyBiocidePowder deliverySolubilitySide effect
Hydrophobic drugs become more practical for treatments by being encapsulated in micelle compositions for increasing solubility. Micelle compositions may include an excipient tocopherol and / or prodrug formulations of the drug. Micelles extend the time period the drug remains in the micelles to improve drug circulation time and thereby drug delivery. Hydrophobic drugs for micelle encapsulation may include rapamycin, geldanamycin, and paclitaxel. Administration of these micelle compositions does not require Cremophor EL or Tween 80, avoiding serious side effects associated with these products which would previously accompany such drug administration.
Owner:WISCONSIN ALUMNI RES FOUND
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070087022A1Eliminate side effectsHigh anticancer activityOrganic active ingredientsBiocideSuspended particlesActive agent
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Treating cancer using electromagnetic fields in combination with other treatment regimens
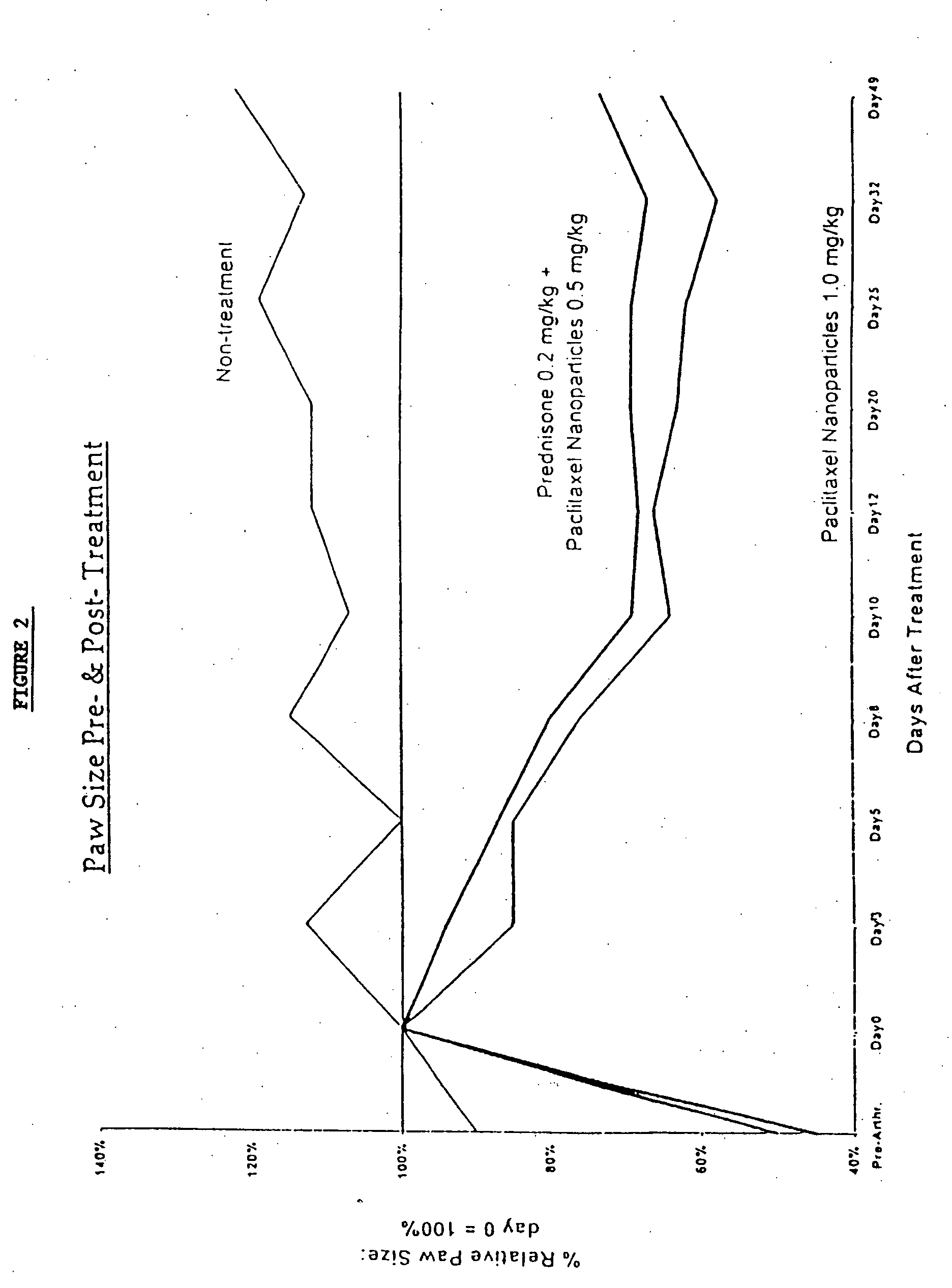
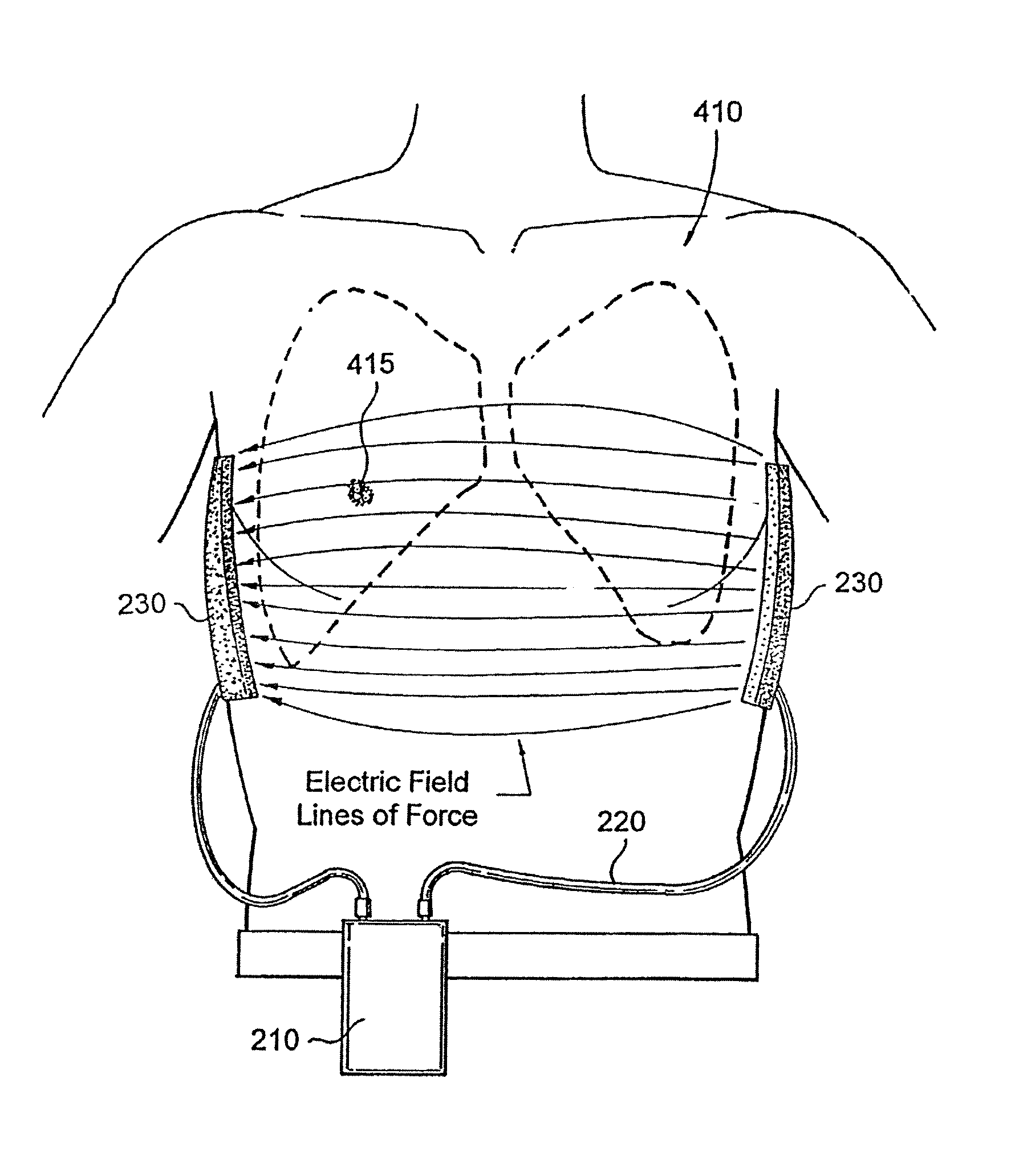
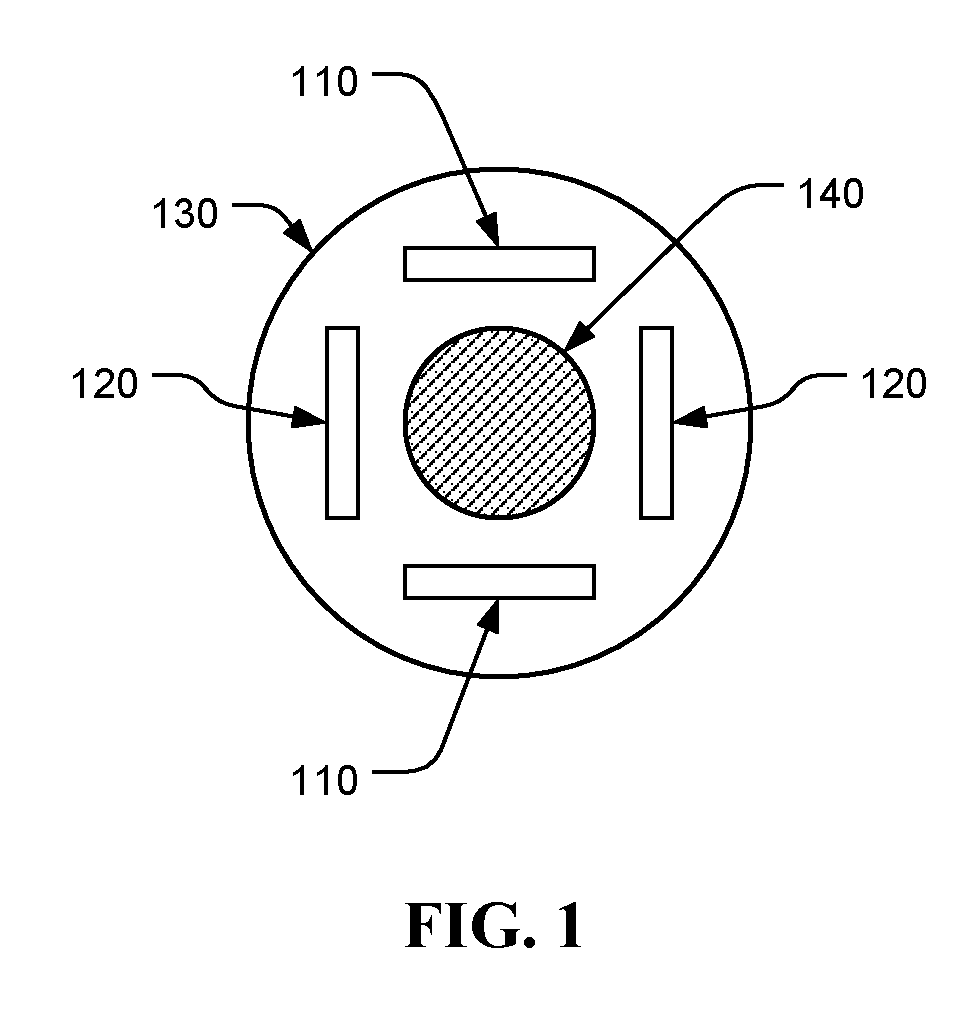
Chemotherapeutic treatment for certain cancers may be combined with low intensity, intermediate frequency alternating electric fields that are tuned to a particular type of target cell. When the tuned fields were combined with Paclitaxel, Doxorubicin or Cyclophosphamide, excellent results were obtained against human breast cancer cells (MDA-MB-231) and non-small cell lung (H1299) carcinomas in culture. More specifically, cell proliferation inhibition similar to that obtained by drug alone was reached by exposure to the combined treatment at drug concentrations between one and two orders of magnitude lower than for drug-only regimens of treatment.
Owner:NOVOCURE GMBH
Pharmaceutically acceptable composition comprising an aqueous solution of paclitaxel and albumin
InactiveUS20050282734A1Prevent restenosisFree of organic solventsBiocideOrganic active ingredientsOrganic solventSerum protein albumin
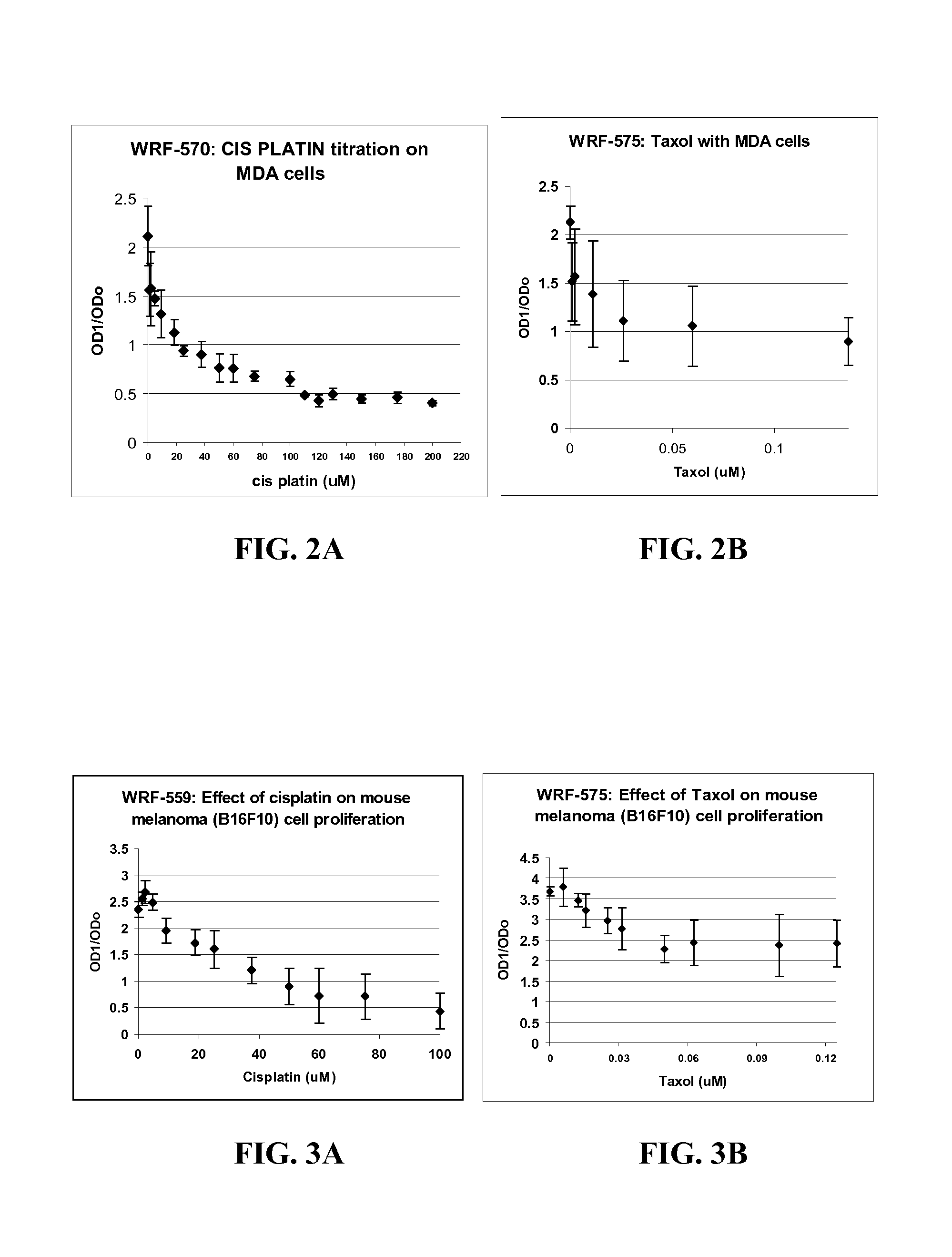
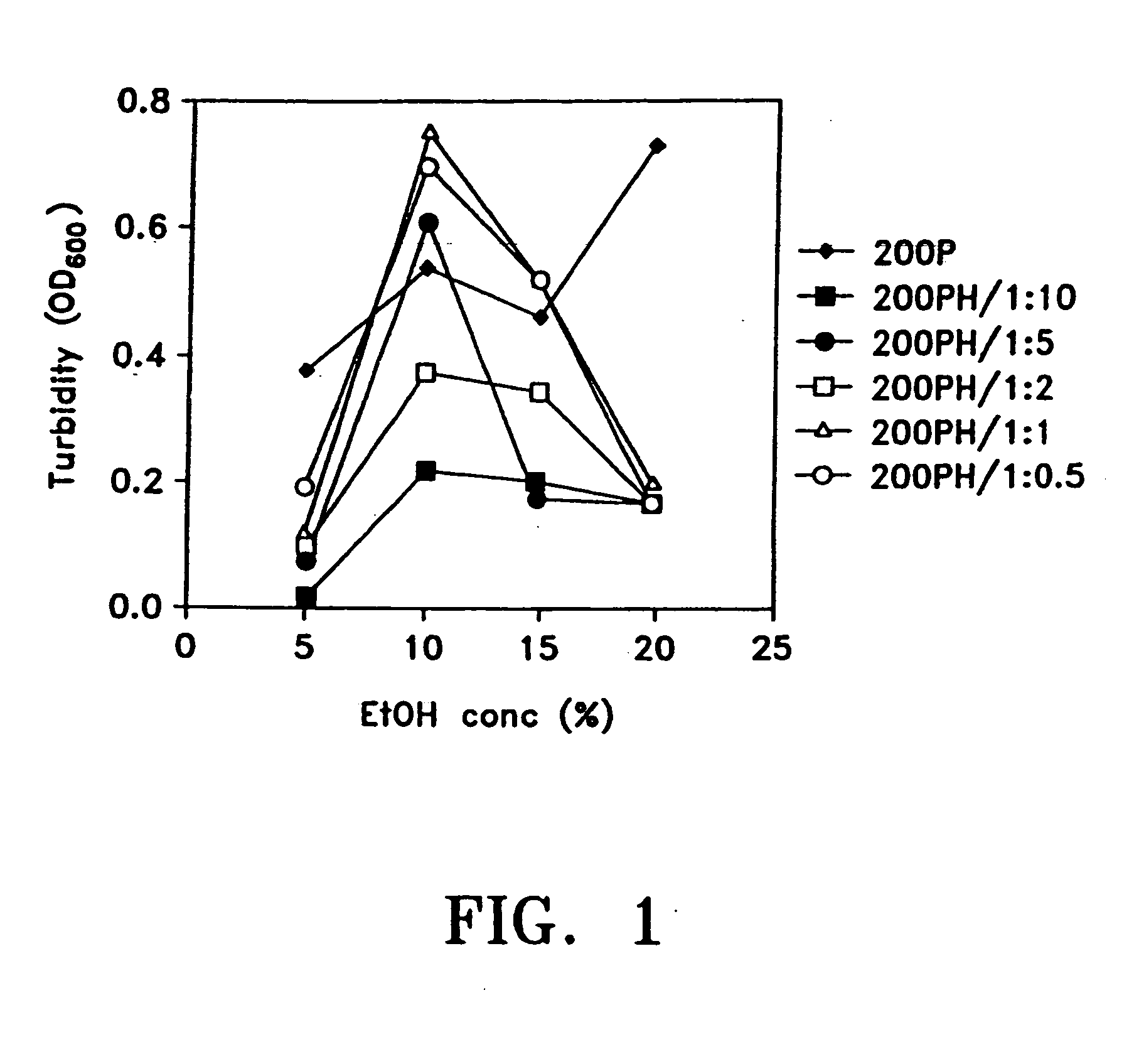
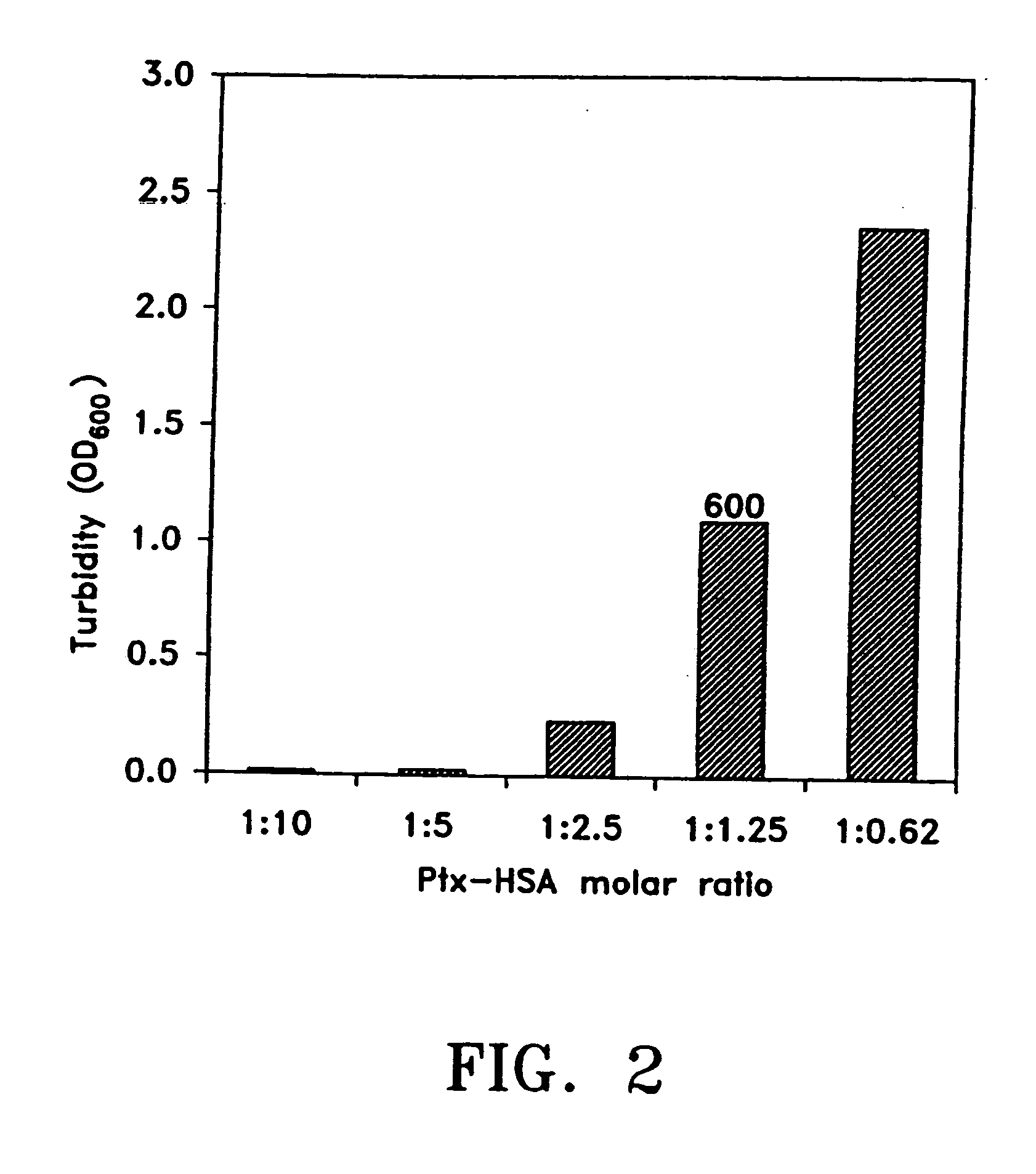
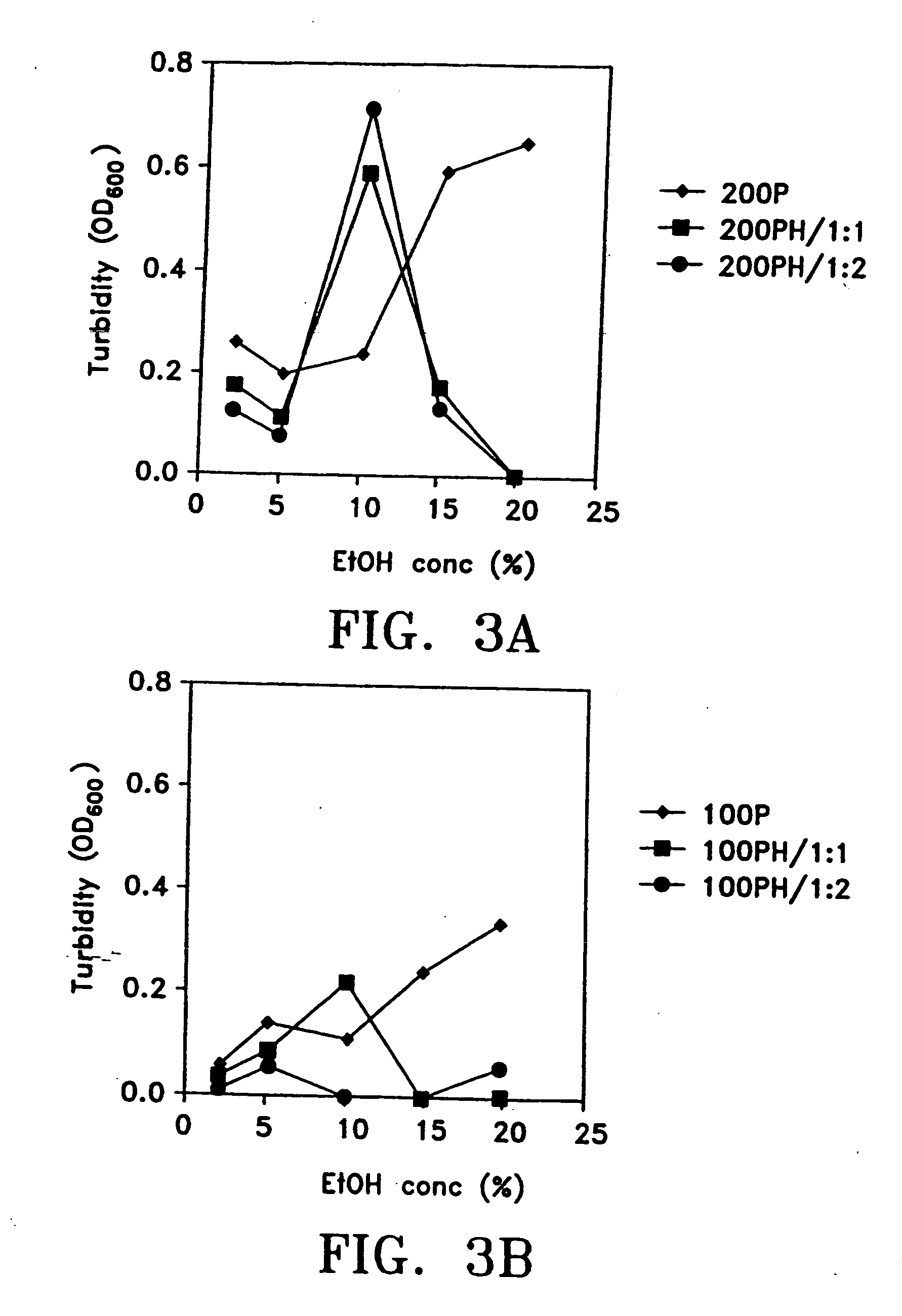
An optically clear, pharmaceutically acceptable aqueous composition comprising paclitaxel or a derivative thereof, serum albumin and a pharmaceutically acceptable vehicle, wherein the composition comprises no more than 10% organic solvent and has a pH of about 3.0 to about 4.8, is described. The serum albumin can be fatted or defatted, and the composition can optionally be lyophilized or optionally lyophilized and reconstituted. At least 70% of the paclitaxel is bound to serum albumin, the ratio of paclitaxel to albumin is at least about 1:5, and the concentration of paclitaxel is at least about 25 μg / ml. Methods of making and using this composition an also provided.
Owner:KADIMA TENSHUK A +6
Oral formulation for paclitaxel
A pharmaceutical formulation is provided for delivering paclitaxel in vivo comprising: water and micelles comprising paclitaxel and a pharrnaceutically-acceptable, water-miscible solubilizer forming the micelles, the solubilizer selected from the group consisting of solubilizers having the general structureswherein R1 is a hydrophobic C3-C50 alkane, alkene or alkyne and R2 is a hydrophilic moiety. The solubilizer is selected such that it does not have a pKa less than about 6.
Owner:SUPERGEN
Medicinal compositions for concomitant use as anticancer agent
InactiveUS20030215523A1Good synergyEliminate side effectsHeavy metal active ingredientsBiocideCarboplatinAnticarcinogen
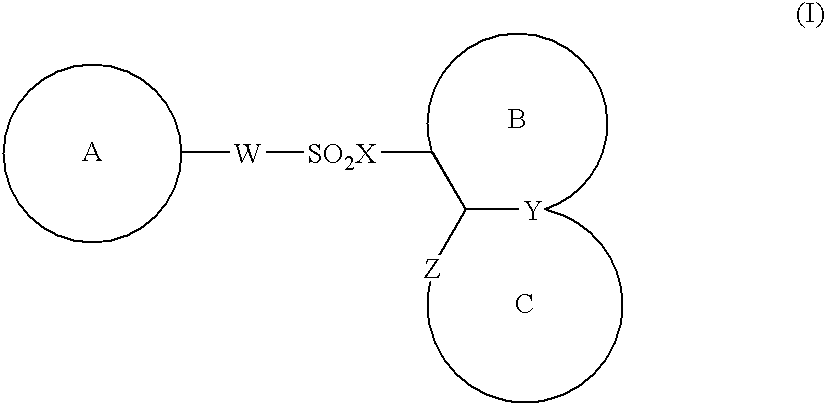
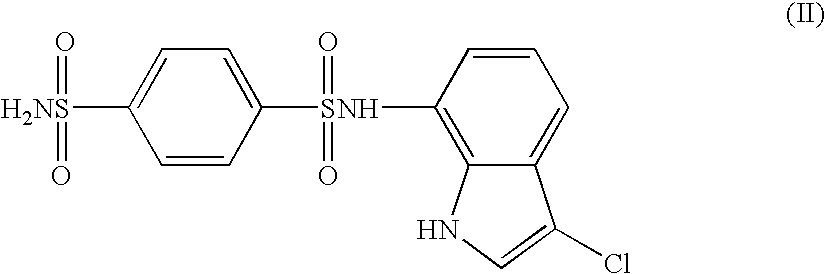
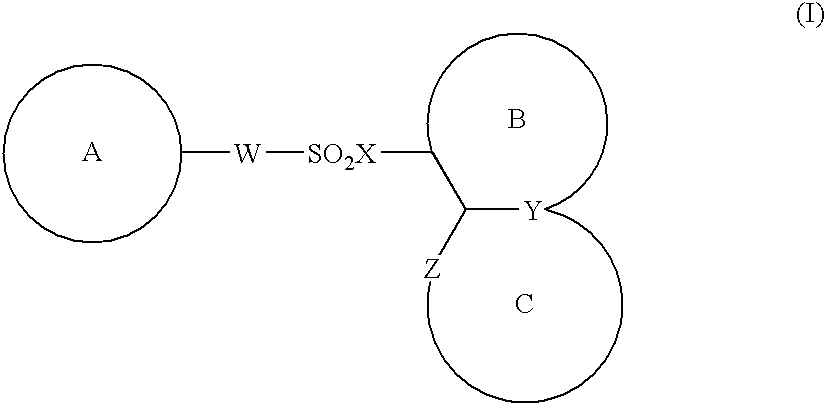
The present invention provides a medicinal composition having an excellent antitumor activity. That is, it provides a medicinal composition comprising a sulfonamide compound, a sulfonate compound or a salt of them, which is represented by the following formula: (wherein ring A represents an aromatic ring which may have a substituent group; ring B represents a 6-membered unsaturated hydrocarbon ring which may have a substituent group etc.; ring C represents a 5-membered hetero-ring containing one or two nitrogen atoms, and the ring C may have a substituent group; W represents a single bond or -CH=CH-; X represents -NH- etc.; and Y represents a carbon atom or a nitrogen atom; and Z represents -NH- etc.), particularly N-(3-chloro-1H-indol-7-yl)-4-sulfamoylbenzenesulfonamide or a salt thereof, combined with at least one substance selected from (1) irinotecan hydrochloride trihydrate; (2) mitomycin C; (3) 5-fluorouracil; (4) cisplatin; (5) gemcitabine hydrochloride; (6) doxorubicin; (7) taxol; (8) carboplatin; (9) oxaliplatin; (10) capecitabine; and (11) a salt of the above-mentioned (1) to (10).
Owner:EISIA R&D MANAGEMENT CO LTD
Novel formulations of pharmacological agents, methods for the preparation thereof and methods for the use thereof
InactiveUS20070093547A1Eliminate side effectsHigh anticancer activityBiocideOrganic active ingredientsSuspended particlesFree protein
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of substantially water insoluble pharmacologically active agents (such as the anticancer drug paclitaxel) in which the pharmacologically active agent is delivered in the form of suspended particles coated with protein (which acts as a stabilizing agent). In particular, protein and pharmacologically active agent in a biocompatible dispersing medium are subjected to high shear, in the absence of any conventional surfactants, and also in the absence of any polymeric core material for the particles. The procedure yields particles with a diameter of less than about 1 micron. The use of specific composition and preparation conditions (e.g., addition of a polar solvent to the organic phase), and careful selection of the proper organic phase and phase fraction, enables the reproducible production of unusually small nanoparticles of less than 200 nm diameter, which can be sterile-filtered. The particulate system produced according to the invention can be converted into a redispersible dry powder comprising nanoparticles of water-insoluble drug coated with a protein, and free protein to which molecules of the pharmacological agent are bound. This results in a unique delivery system, in which part of the pharmacologically active agent is readily bioavailable (in the form of molecules bound to the protein), and part of the agent is present within particles without any polymeric matrix therein.
Owner:ABRAXIS BIOSCI LLC
Pharmaceutical formulations comprising paclitaxel, derivatives, and pharmaceutically acceptable salts thereof
The invention concerns paclitaxel solubilizers and formulations thereof with a high propensity to dissolve paclitaxel. The formulations of the invention reduce or obviate the need for the disadvantageous excipient Cremophor(R) EL. The formulations of the invention are useful for administering paclitaxel, its derivatives, or pharmaceutically acceptable salts or such derivatives to patients in need thereof. The formulations of the invention are suitable for parenteral, oral, local, or transdermal administration to mammals including humans, particularly for intravenous delivery.
Owner:TRANSFORM PHARMACEUTICALS INC
Breast cancer therapy based on hormone receptor status with nanoparticles comprising taxane
ActiveUS20100048499A1BiocidePhosphorous compound active ingredientsPR - Progesterone receptorNanoparticle
The present invention relates to methods and kits for the treatment of breast cancer based on hormone receptor status of progesterone receptor and estrogen receptor comprising the administration of a taxane alone, in combination with at least one other and other therapeutic agents, as well as other treatment modalities useful in the treatment of breast cancer. In particular, the invention relates to the use of nanoparticles comprising paclitaxel and albumin (such as Abraxane®) either alone or in combination with other chemotherapeutic agents or radiation, which may be used for the treatment of breast cancer which does not express estrogen receptor and / or progesterone receptor.
Owner:ABRAXIS BIOSCI LLC
Taxol enhancer compounds
InactiveUS6924312B2High anticancer activityImprove efficiencyBiocideOrganic chemistryArylStructural formula
Disclosed is a compound represented by the Structural Formula (I): Y is a covalent bond, a phenylene group or a substituted or unsubstituted straight chained hydrocarbyl group. In addition, Y, taken together with both >C═Z groups to which it is bonded, is a substituted or unsubstituted aromatic group. Preferably, Y is a covalent bond or —C(R7R8)—.R1 and R2 are independently an aryl group or a substituted aryl group, R3 and R4 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R5-R6 are independently —H, an aliphatic group, a substituted aliphatic group, an aryl group or a substituted aryl group.R7 and R8 are each independently —H, an aliphatic or substituted aliphatic group, or R7 is —H and R8 is a substituted or unsubstituted aryl group, or, R7 and R8, taken together, are a C2-C6 substituted or unsubstituted alkylene group.Z is ═O or ═S.Also disclosed are pharmaceutical compositions comprising the compound of the present invention and a pharmaceutically acceptable carrier or diluent. Also disclosed is a method of treating a subject with cancer by administering to the subject a compound of Structural Formula (I) in combination with taxol or an analog of taxol.
Owner:SYNTA PHARMA CORP
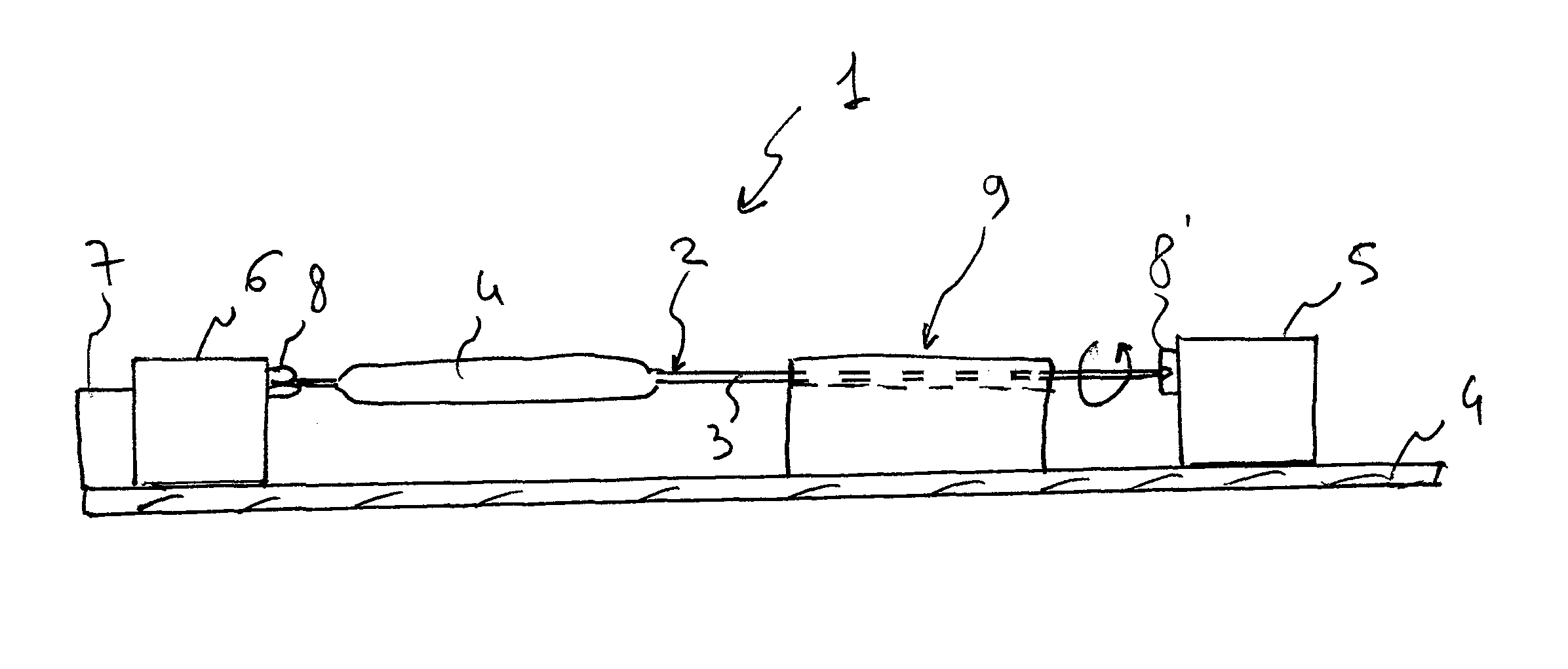
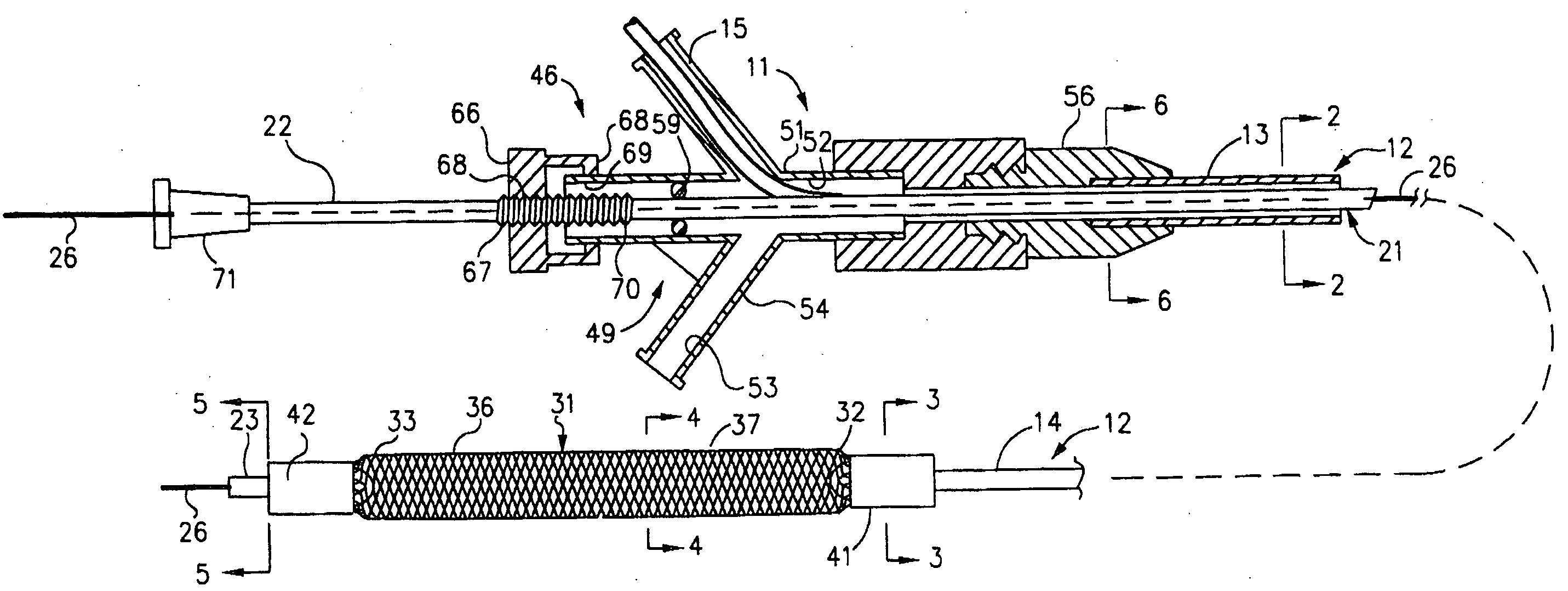
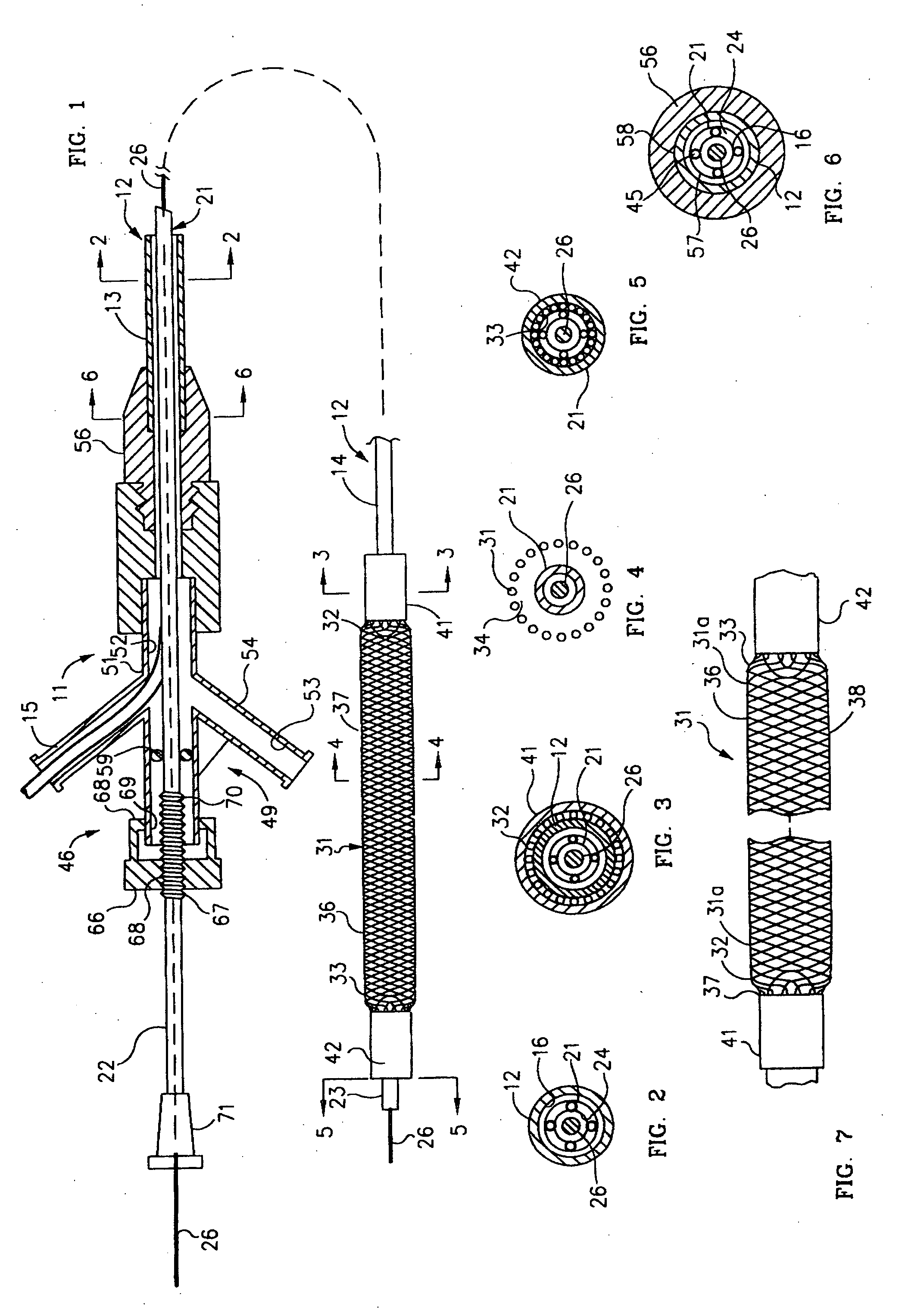
Mechanical apparatus and method for dilating and delivering a therapeutic agent to a site of treatment
InactiveUS20050043680A1Uniform exposureMinimal dangerElectrotherapyBalloon catheterPresent methodBiomedical engineering
A mechanical dilatation and medicament delivery device for enlarging a flow passage of a vessel by dilating and delivering a charged paclitaxel analogue therapeutic agent or medicament to an obstruction in the vessel. The present invention comprises a substantially cylindrically shaped expansion member and includes a means engaged to the expansion member for altering the distance between the proximal end and the distal end of the expansion member thereby transforming the expansion member between a diametrically contracted configuration to diametrically expanded configuration. A charged paclitaxel analogue therapeutic agent or medicament is coated on either the expansion member, or combined / incorporated into a substrate coated on the expansion member. The present method comprises the steps of advancing the coated expansion member to the obstruction in a vessel and applying opposed forces on said expansion member in an axial direction to move the expansion member to an expanded configuration wherein the expansion member dilates the obstruction and the expansion member either passively or actively delivers a charged paclitaxel analogue therapeutic agent or medicament to the obstruction.
Owner:BOSTON SCI SCIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com