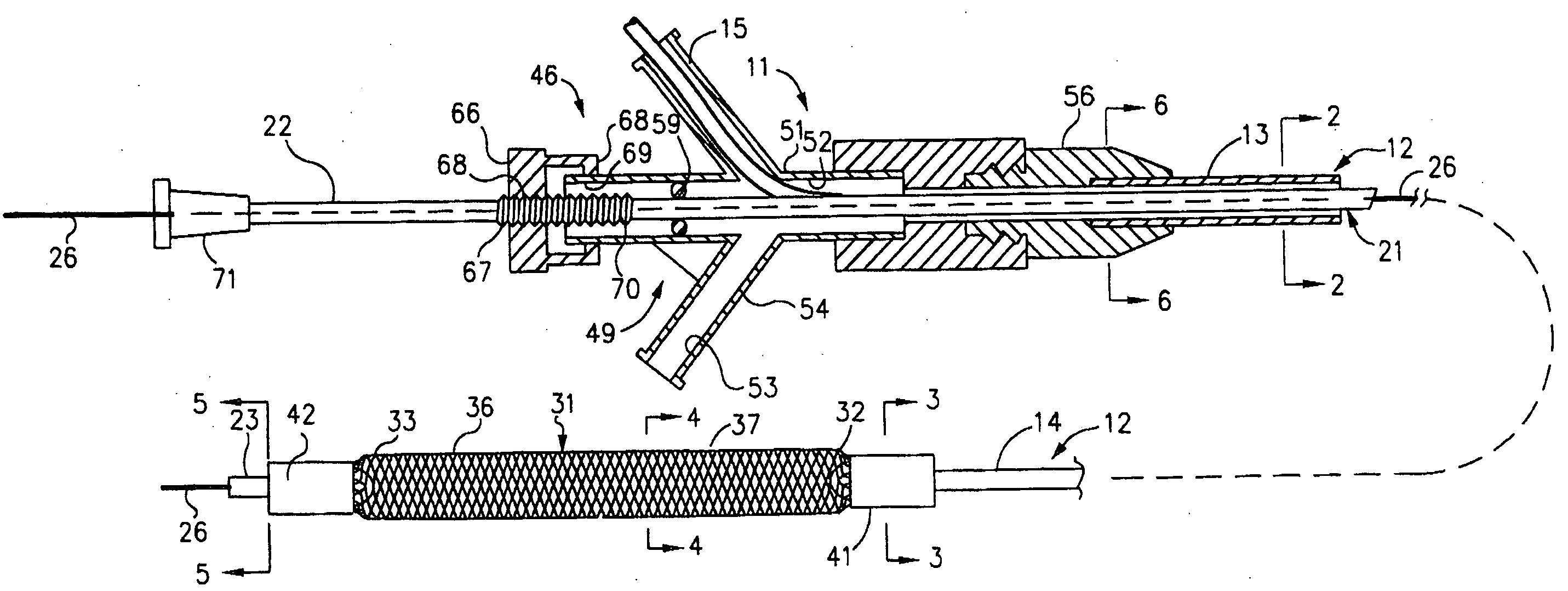
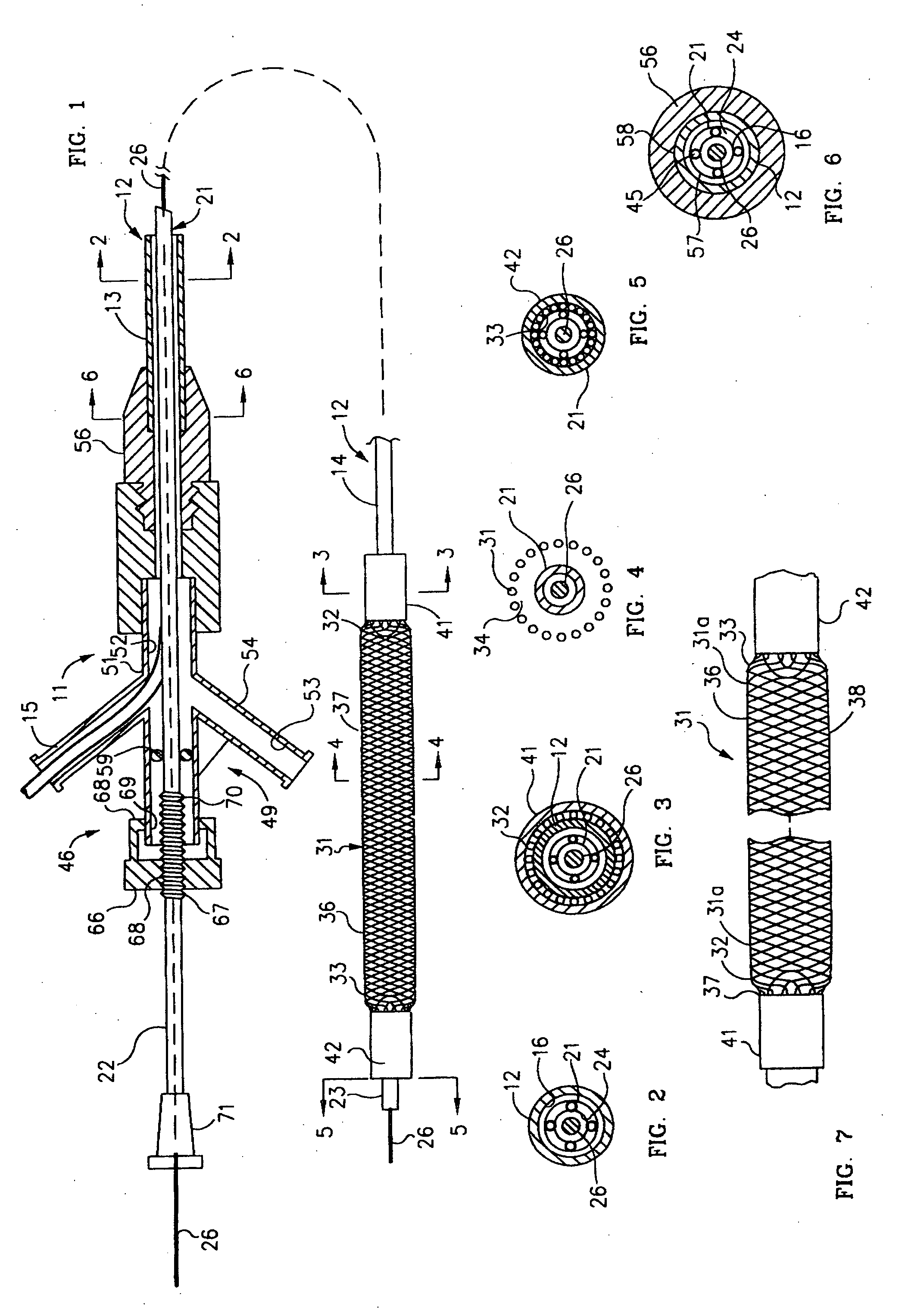
Mechanical apparatus and method for dilating and delivering a therapeutic agent to a site of treatment
a technology of mechanical apparatus and therapeutic agent, applied in the direction of balloon catheter, immunological disorders, therapy, etc., can solve the problems of tissue ischemia and necrosis, traumatic open heart surgery, and failure to maintain patency, etc., and achieve the effect of precise sizing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
Local Delivery of Paclitaxel
[0092] Paclitaxel is one of the most potent inhibitors of cellular proliferation in clinical use and has been shown to be efficacious in a large number of cancers. Paclitaxel is very lipophilic and essentially insoluble in water. Liposome or micelles were prepared by mixing 0.72 mg phosphatidylcholine and 0.8 mg of phosphatidylserine in a test tube with 800 microliters of chloroform. The solution was evaporated to dryness. Paclitaxel labeled with a fluorescent probe (Oregon Green) was dissolved in methanol to obtain a 20 1 mg / 1 ml solution. Twenty-five microliters of this solution was combined with 975 microliters of 8 mM CaCl2. The paclitaxel solution was added to the dried lipid mixture in small aliquots with constant stirring. The hydrogel-coated metal mesh catheter was placed in the paclitaxel / liposome or micelle mixture and then removed. In some cases, the hydrogel-coated mesh portion of the catheter is covered with a retractable sheath to prevent l...
example 3
In-vivo Delivery of a Charged Paclitaxel Analogue (TX-67)
[0093] Catheters loaded with a charged paclitaxel analog (TX-67) were created for In-vivo delivery. 75 mg of TX-67 were compounded into 2 g of a 5% polymer solution dissolved in an 85% Ethanol 15% water solution. The solution was vortex until the TX-67 was dissolved. Multiply coats of the resulting mix were applied directly to a metal mesh catheter and allowed to dry in a 40° C. oven.
[0094] The catheter was positioned at the target site of a study porcine animal and the mesh was expanded against it's arterial wall. Iontophoersis was performed by applying an electrical current to the catheter mesh. The circuit was completed by placing a conductive patch on the animal's skin that was connected to a current source. In this example the iontophoersis parameters were 10 mA for 10 minutes. The mesh was contracted and removed.
[0095] One hour post procedure the tissue was excised and placed on dry ice. The frozen tissue samples were...
example 4
Time Dependent In-vivo Studies with a Charged Paclitaxel Analogue (TX-67)
[0097] Several present invention catheters were used to treat four porcine femoral arteries. The catheters were coated with TX-67 incorporated in a hydrogel non-therapeutic substrate. The catheters were advanced to the femoral arteries of each porcine animal and the mesh expanded to make contact with the arterial wall. A percutaneous patch was placed over the site and continuous DC current of 2 mamp was administered with the negative electrode attached to the catheter and positive to the skin. Continuous blood flow was established through the mesh and documented on cine-angiography. The mesh was left expanded for a total of ten minutes and then the electrical current was discontinued and the mesh contracted in diameter and removed. The animals were sacrificed two at one hour and the other two at twenty four hours after treatment and the femoral arteries at the site of treatment removed. The frozen tissue sampl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com