Patents
Literature
858 results about "Treating Site" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
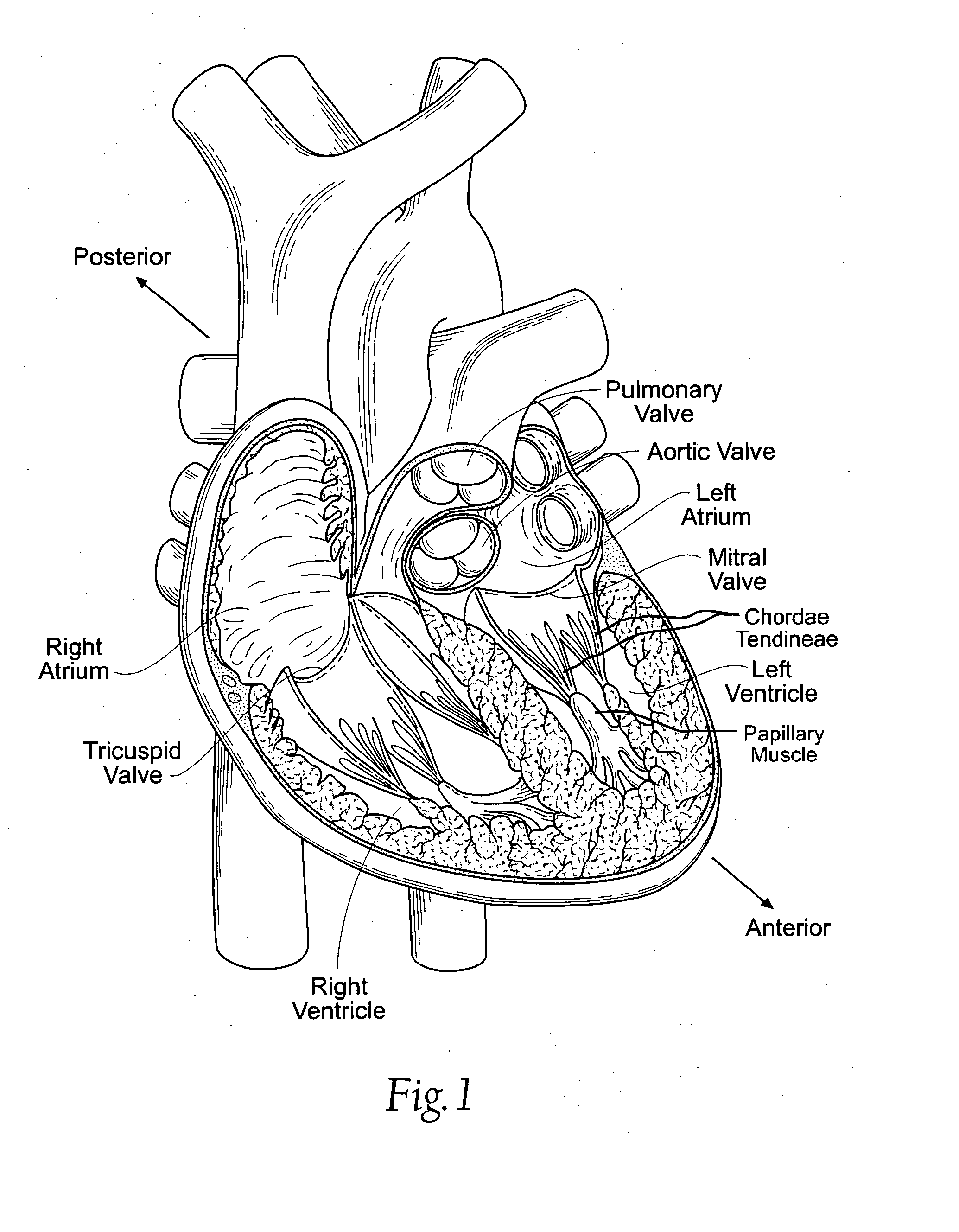
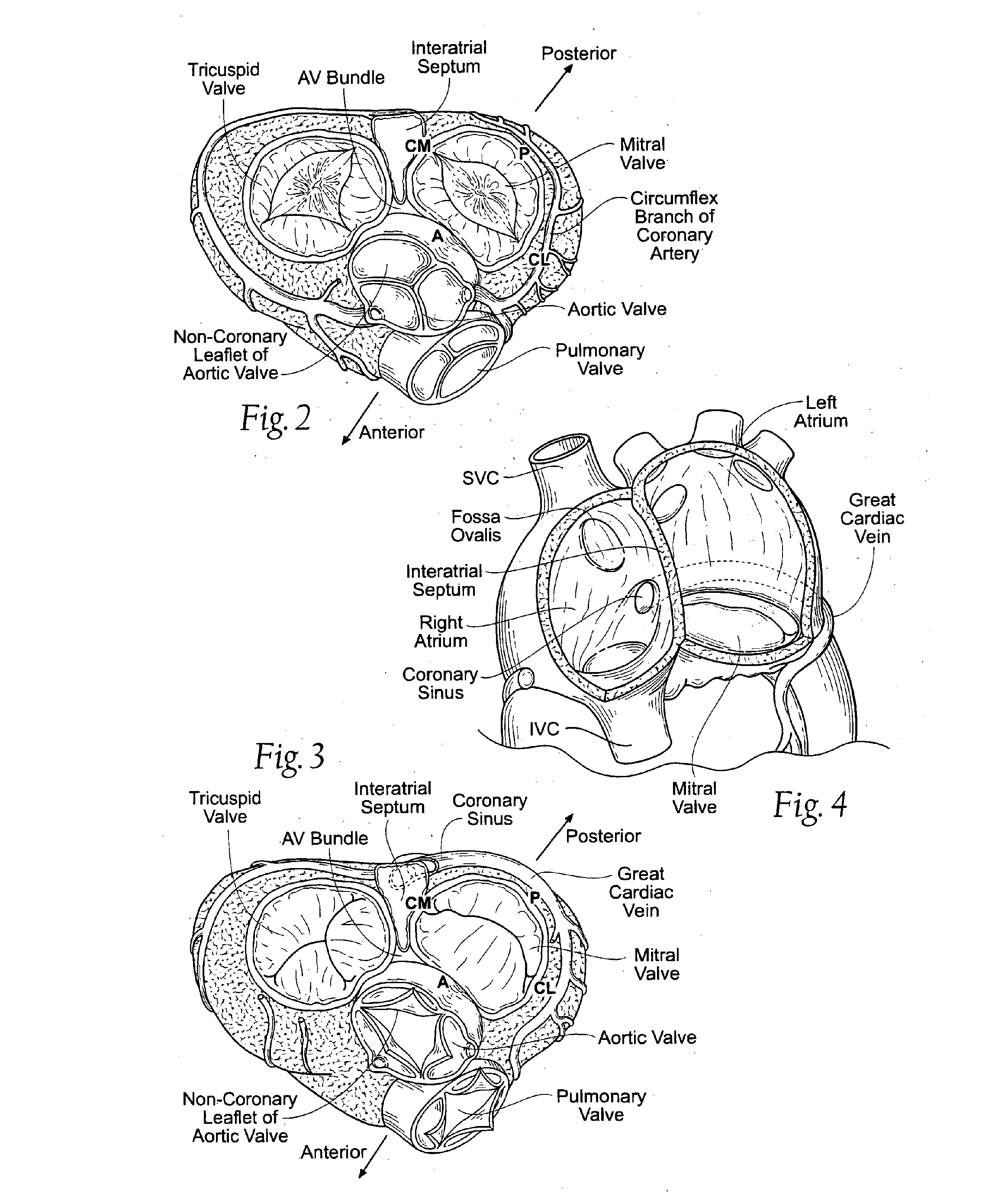
Any healthcare facility where care is provided as part of a cooperative group. EXAMPLE(S): A cancer center that is part of the North Central Cancer Treatment Group (NCCTG). NOTE(S): There are two ways a TreatingSite can be associated to a CooperativeGroup: either directly or through a CooperativeGroupMember.
Hemostatic compositions for arresting blood flow from an open wound or surgical site
A hemostatic composition for stopping or decreasing blood flow from an open wound or medical or surgical procedure. Compositions of the invention comprise a mixture of a cationic polymer and a cation exchange material. In one embodiment, the composition comprises a mixture: (1) a high molecular weight copolymer of diallyl dimethyl ammonium chloride (DADMAC) and acrylamide [DADMAC copolymer], and (2) the hydrogen form of a crosslinked, sulfonated polystyrene (hydrogen resin). In an exemplified embodiment, a composition of the invention comprises the mixture of DADMAC copolymer and hydrogen resin provided in a dry powdered form. The compositions of the invention may be applied directly to a wound or treatment site, or they may be incorporated into a wound dressing, such as a bandage. The seal formed at a wound or treatment site treated with the present invention is adhesive and exhibits considerable toughness.
Owner:BIOLIFE
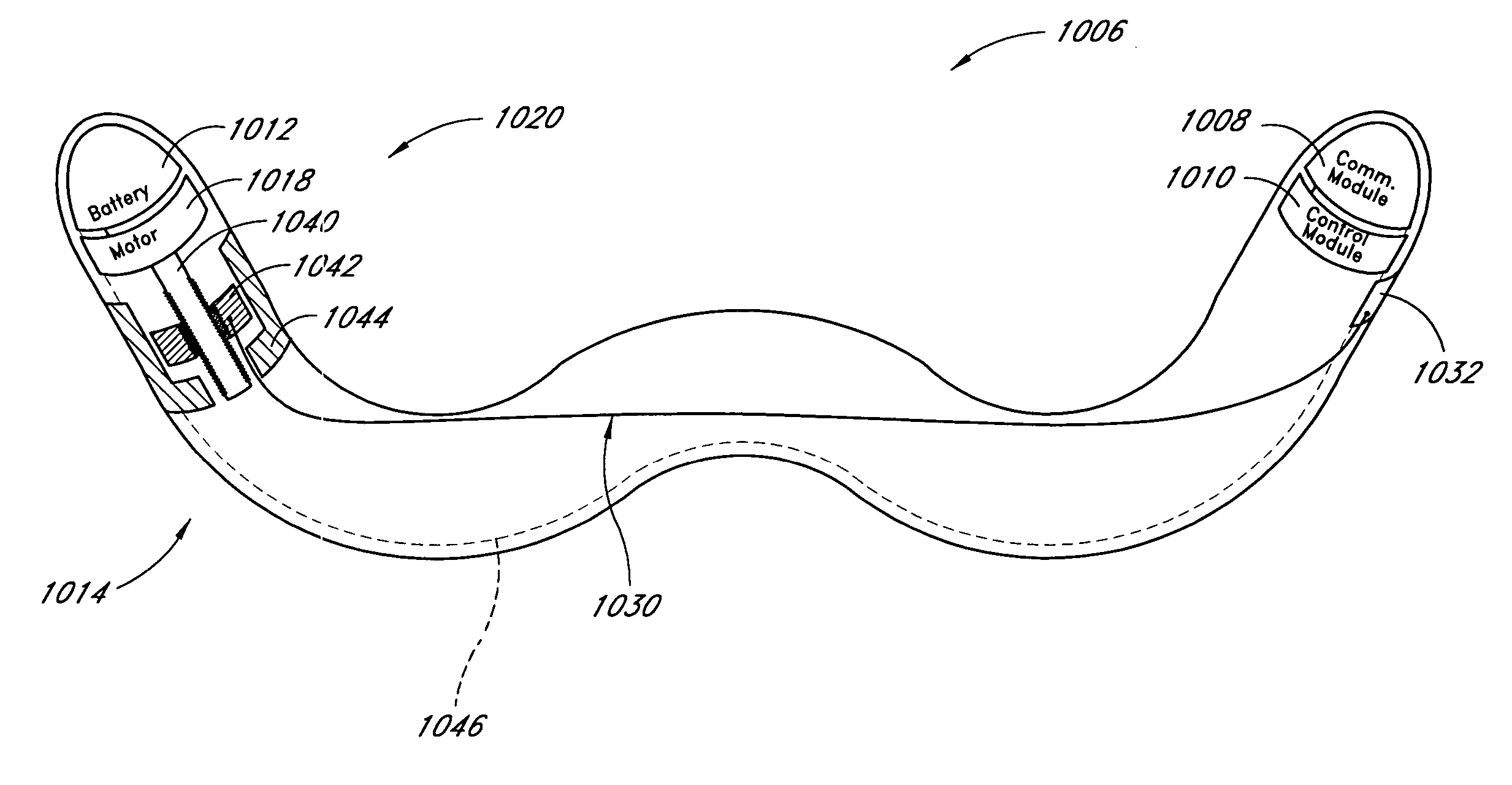
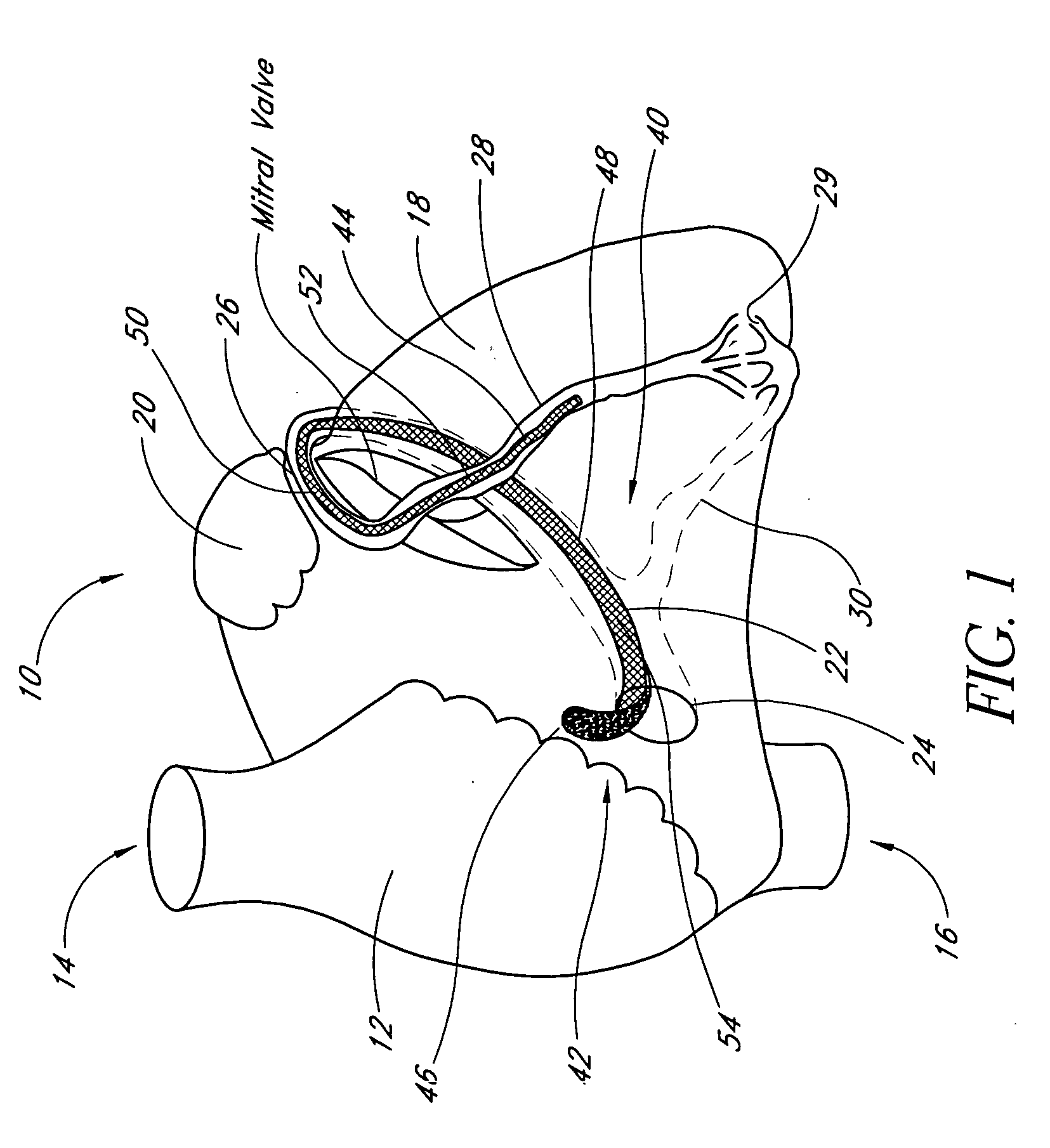
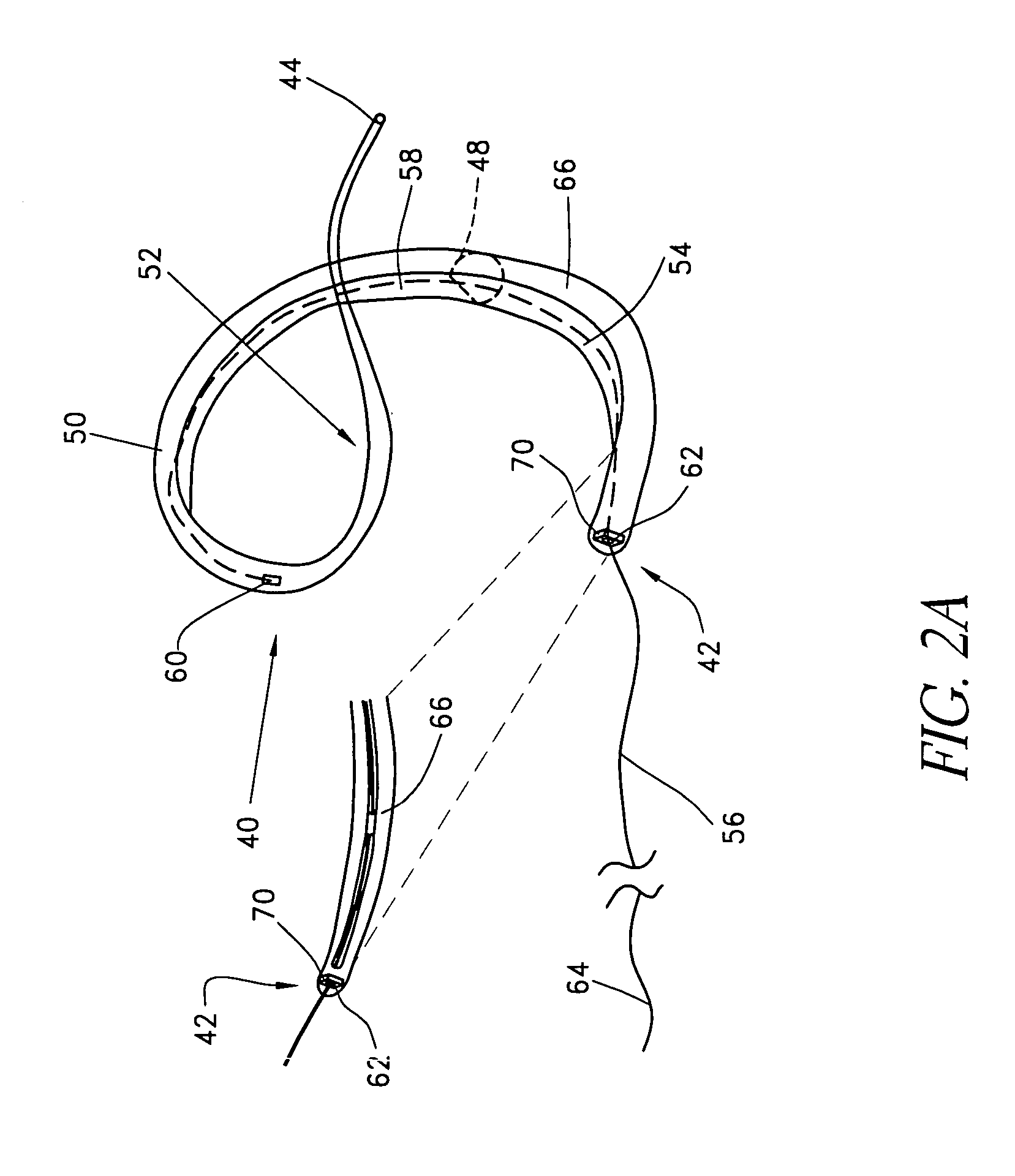
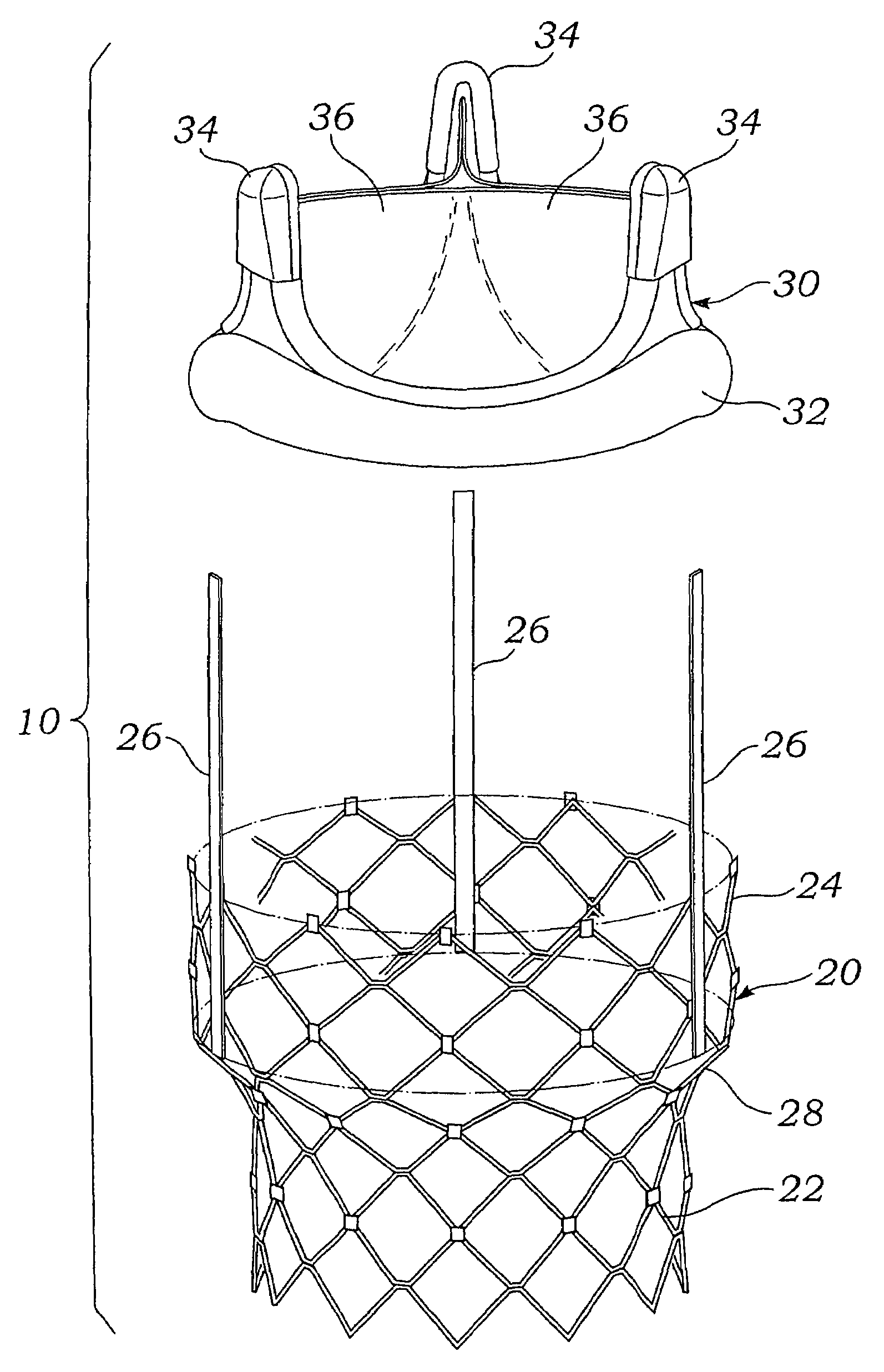
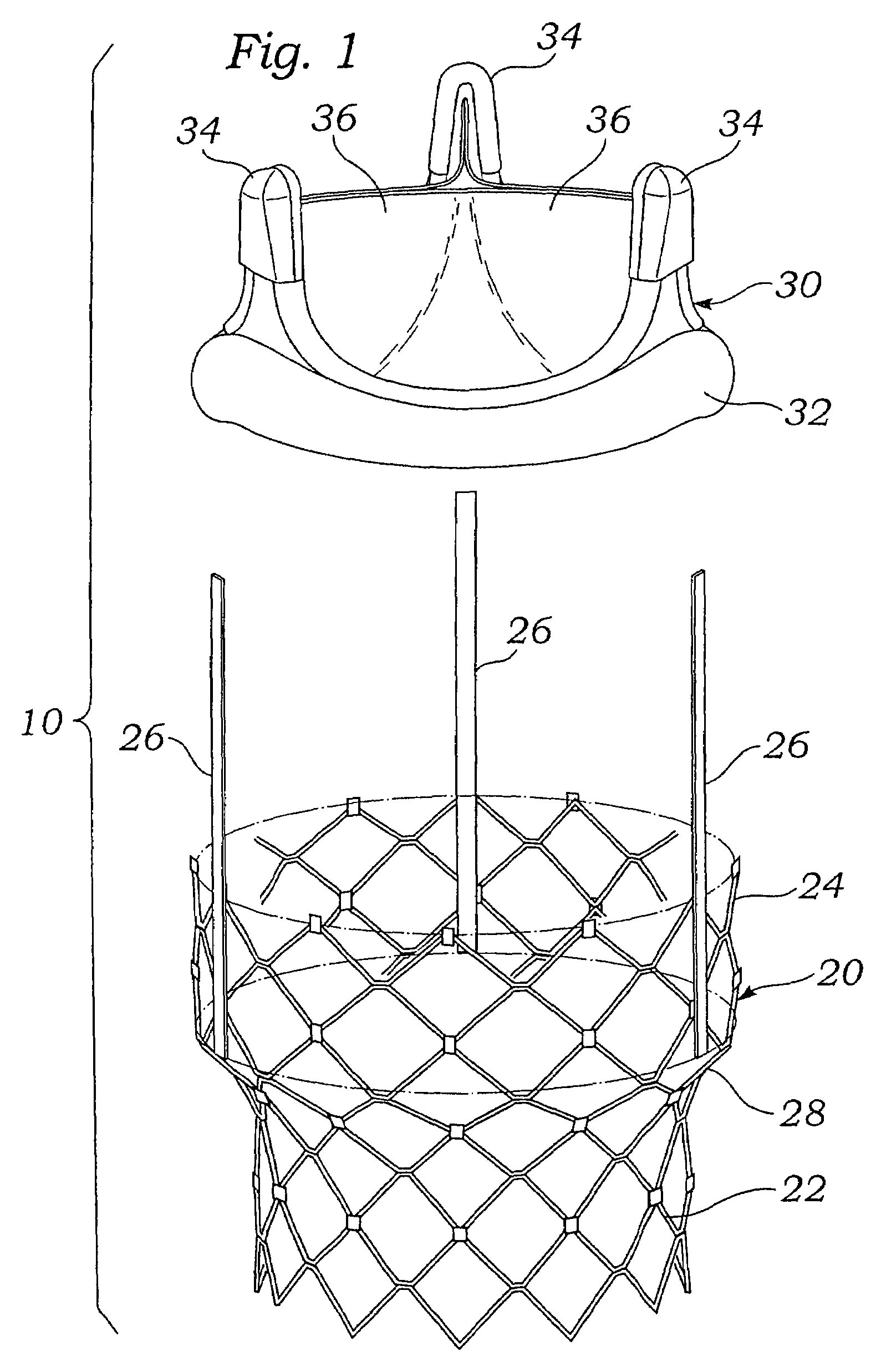
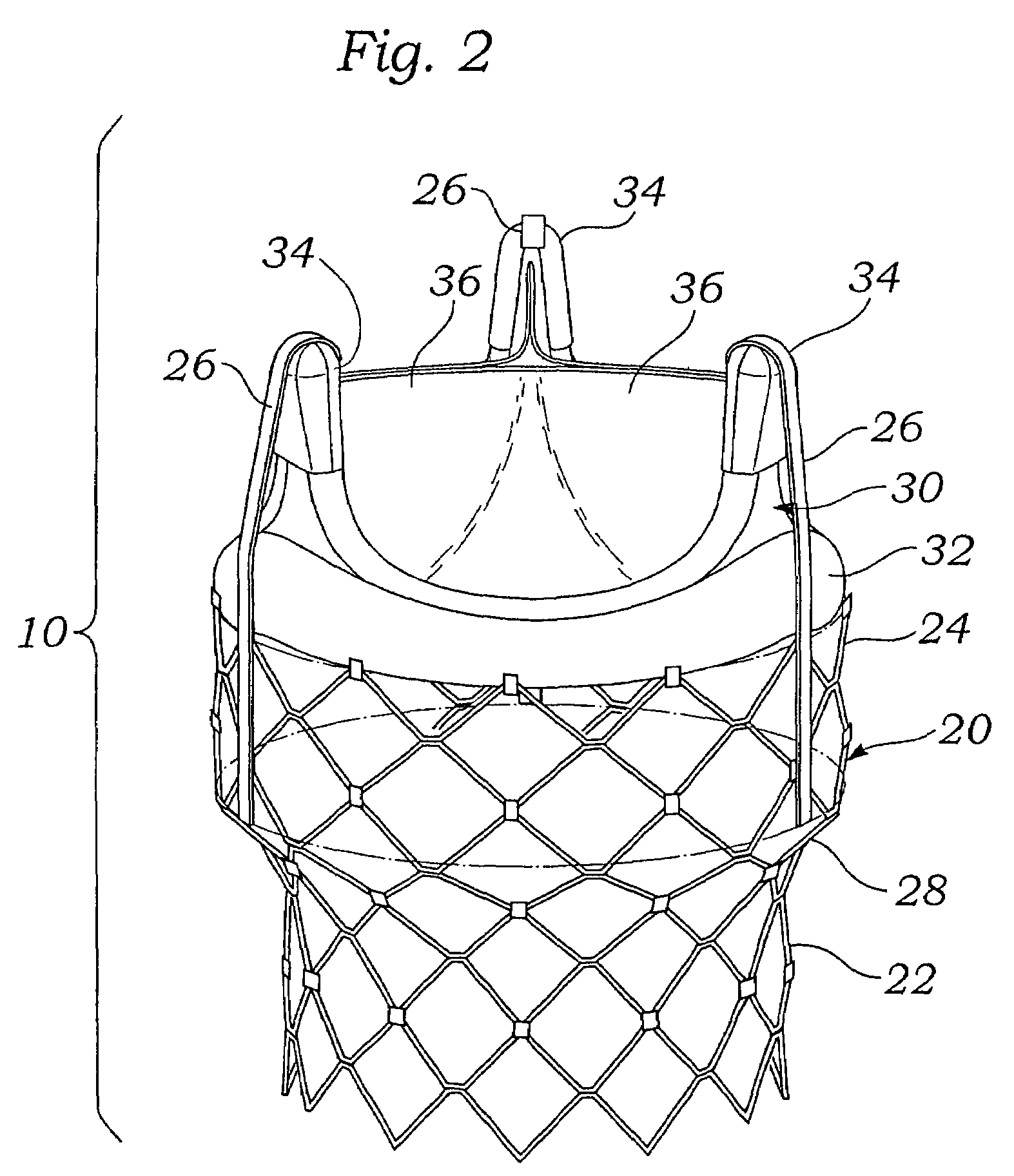
Remotely activated mitral annuloplasty system and methods
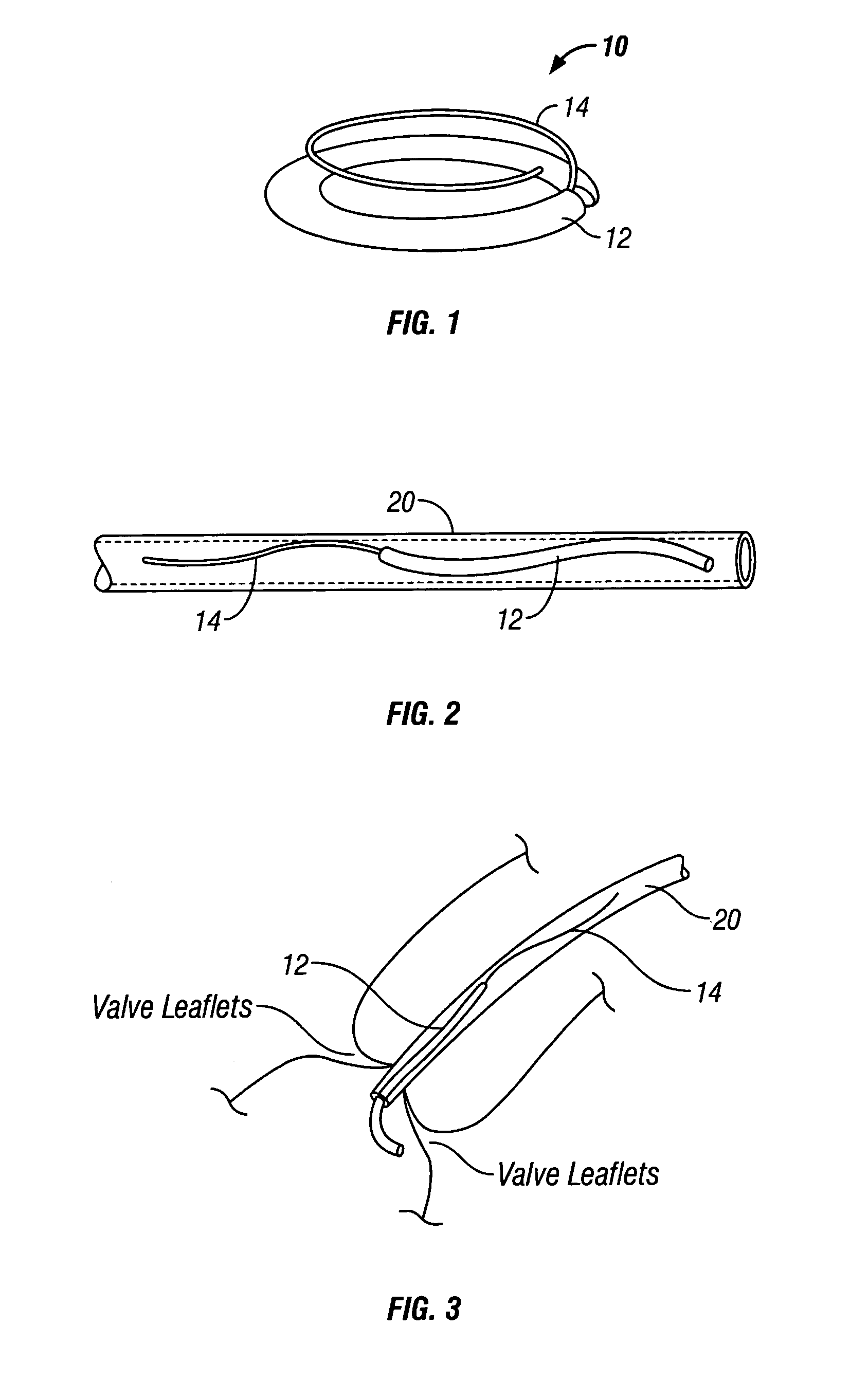
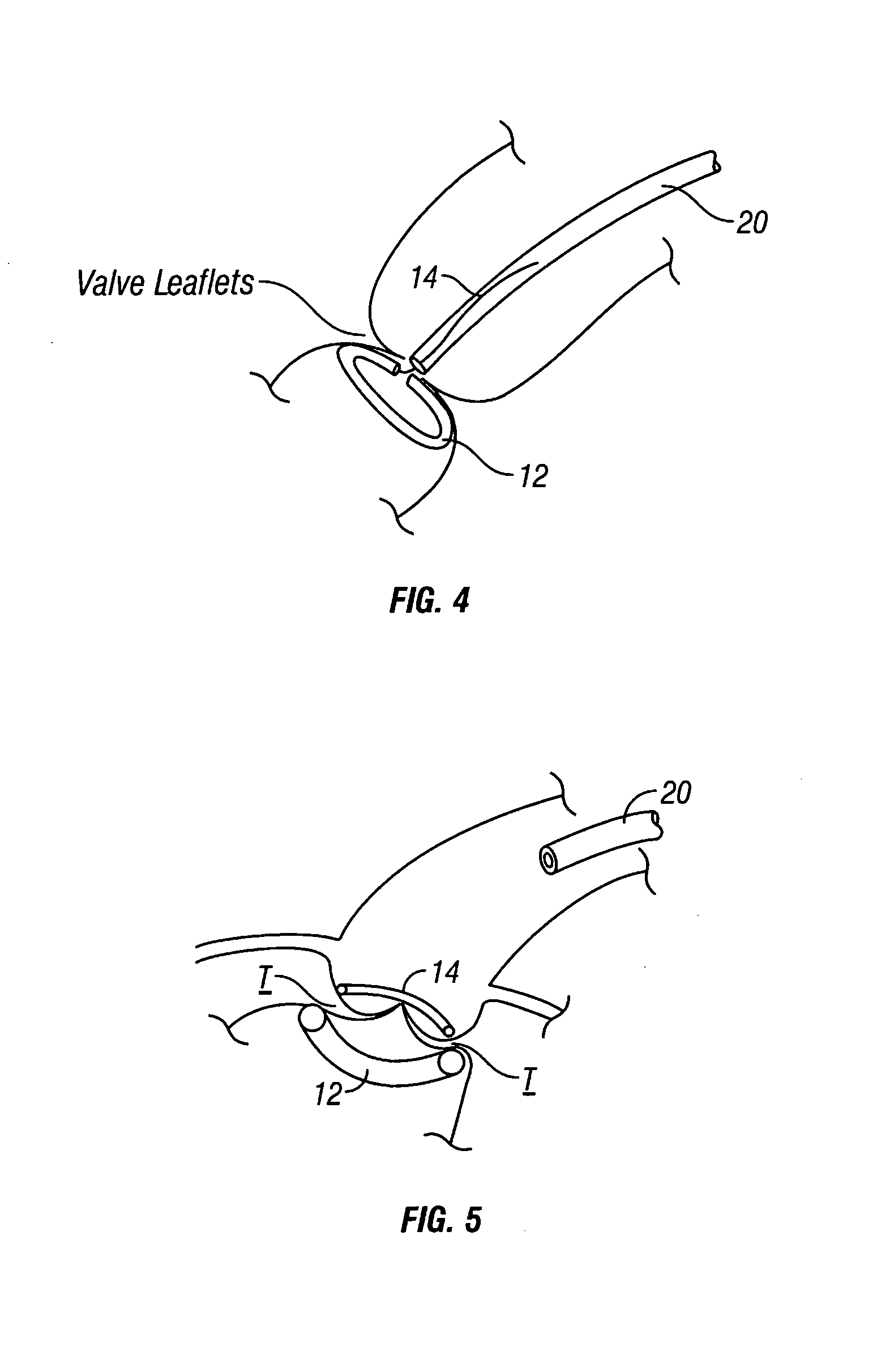
Disclosed are implants and methods for remote remodeling of a mitral valve annulus. The implant comprises a body transformable from a flexible configuration for navigation to a treatment site, to a remodeling configuration for, in one application, applying pressure to the posterior leaflet of the mitral valve. On board electronics allow post deployment adjustment of the implant.
Owner:EDWARDS LIFESCIENCES AG
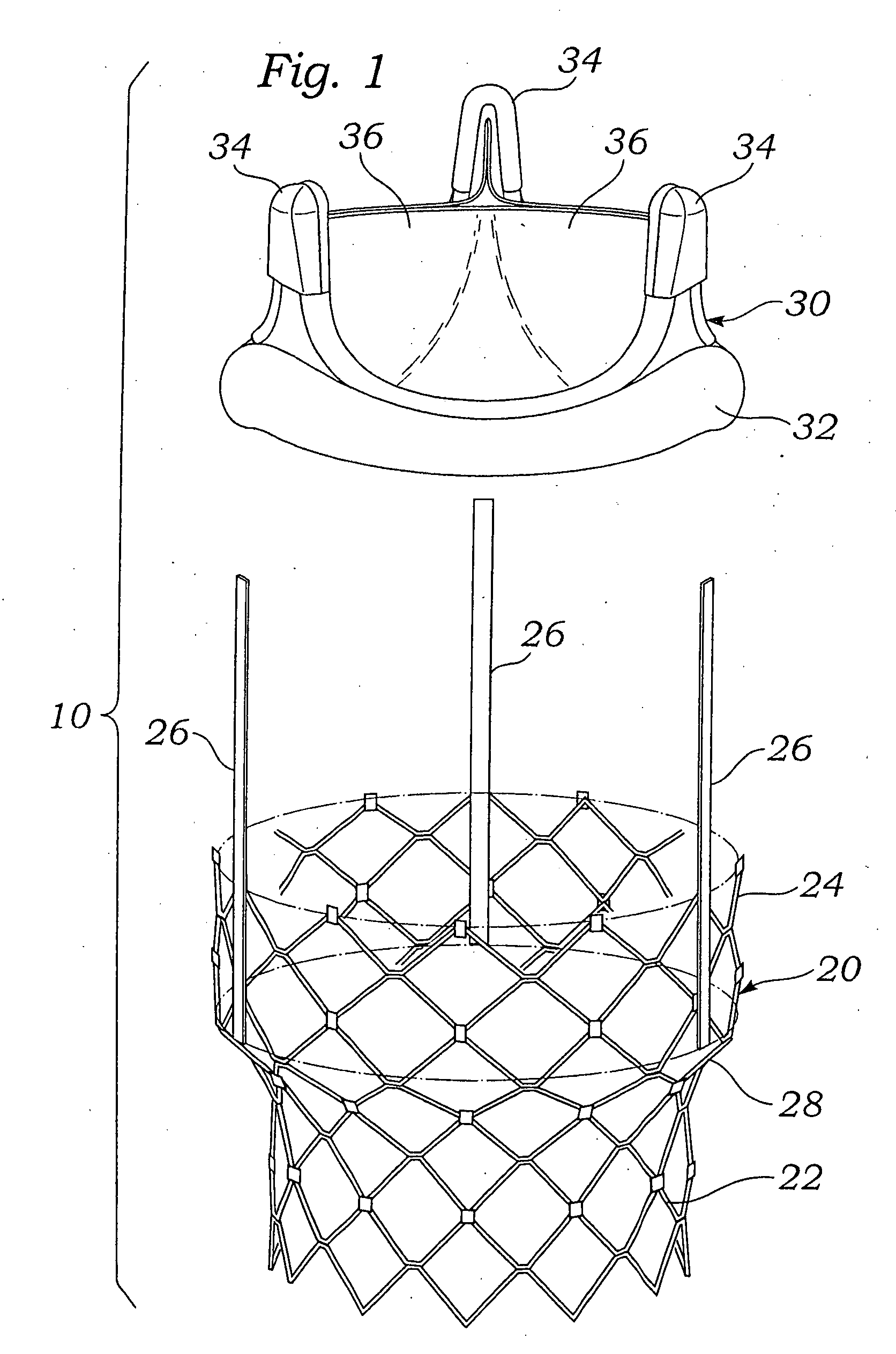
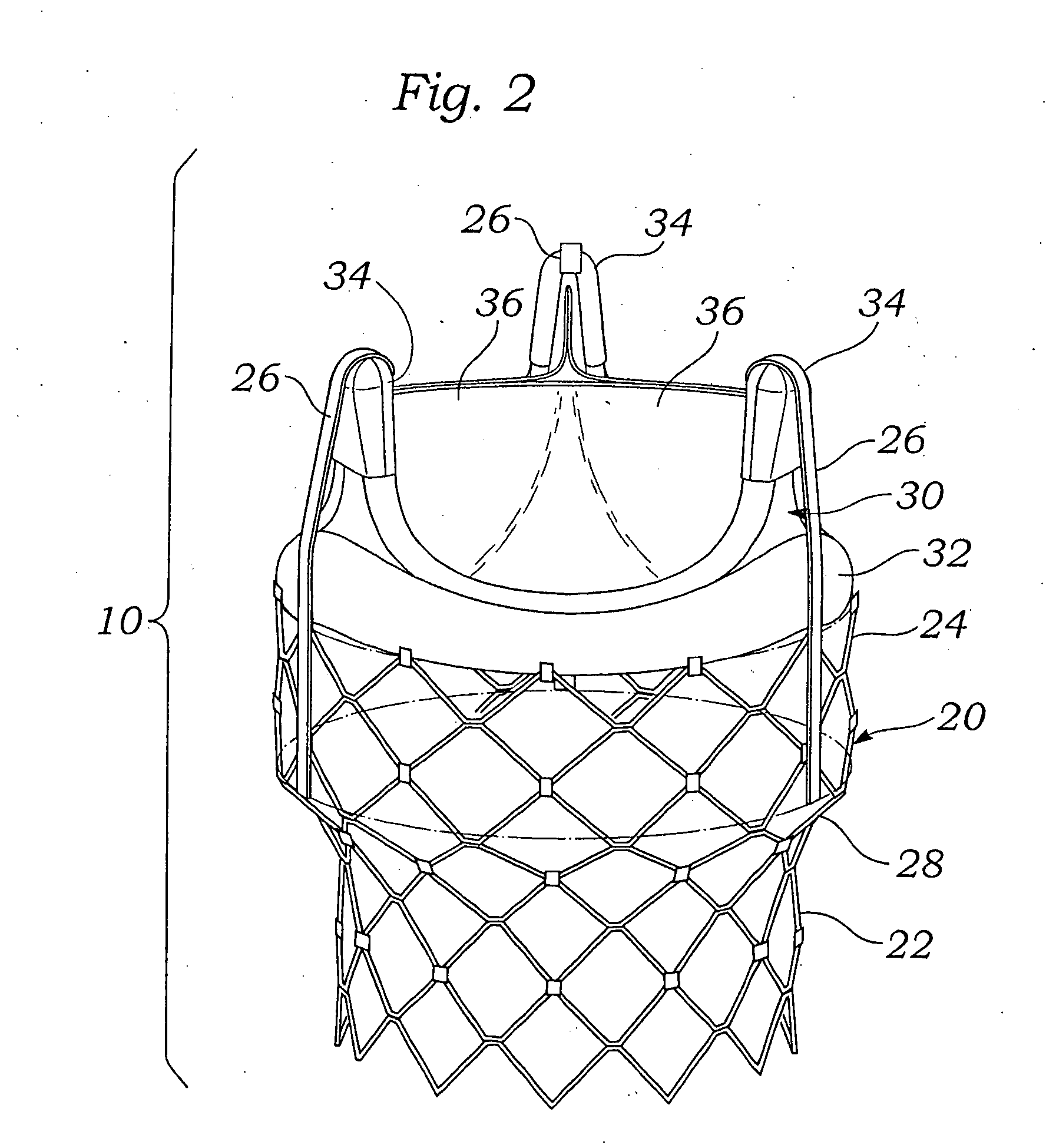
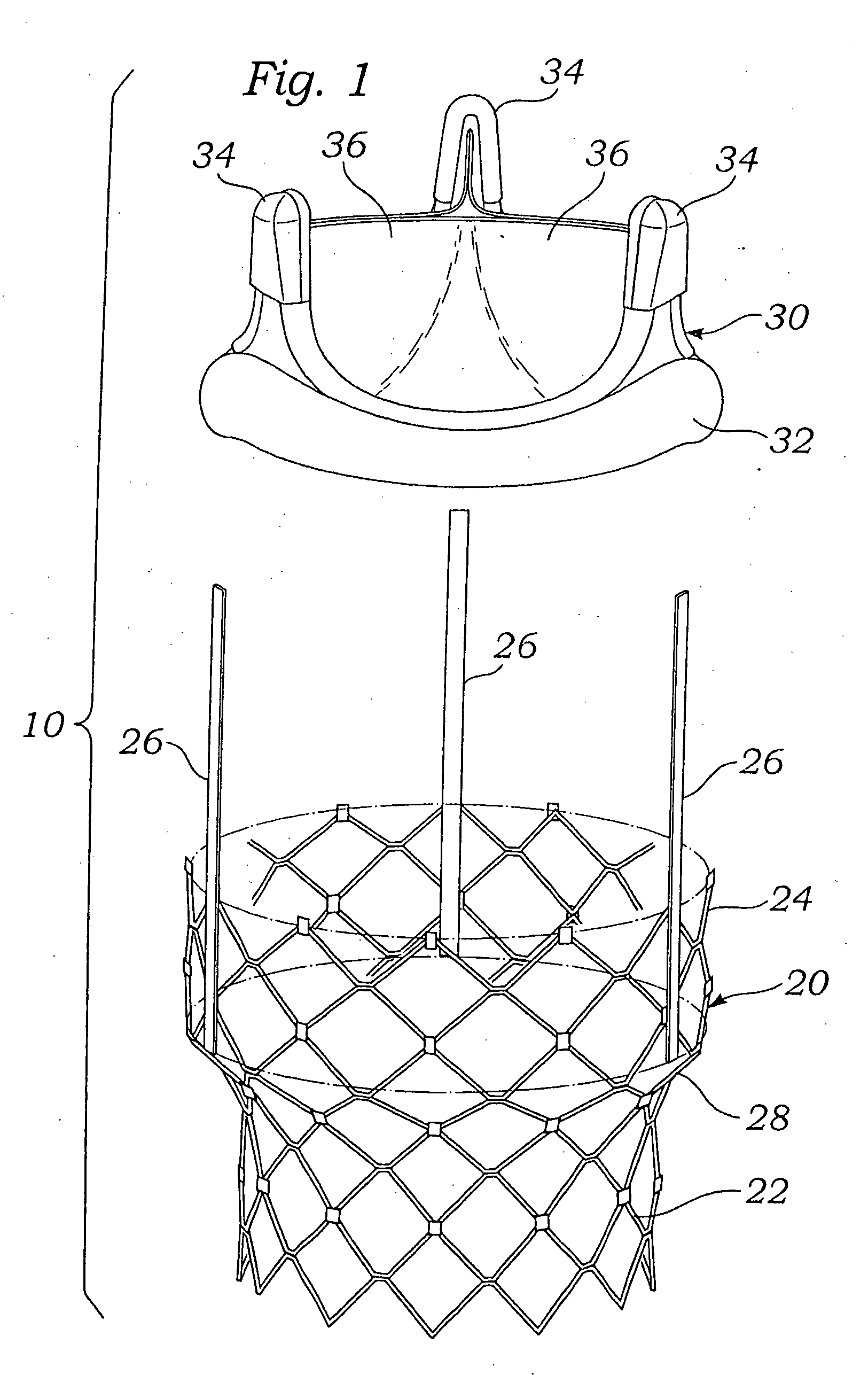
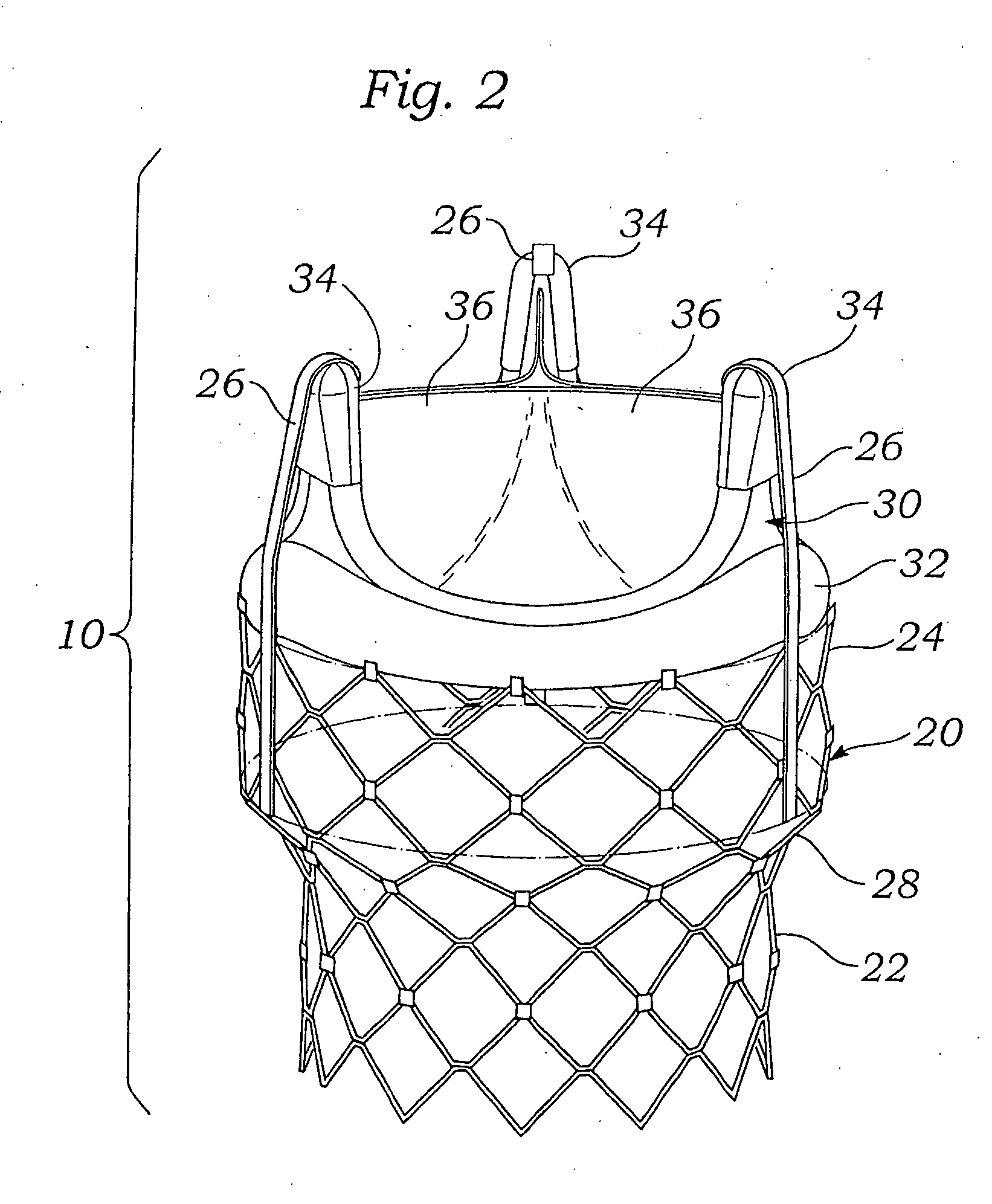
Methods for rapid deployment of prosthetic heart valves
ActiveUS20060287717A1Quickly and easily replacingUse minimizedStentsAnnuloplasty ringsInsertion stentCoupling
A two-stage or component-based valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve comprises a support structure that is deployed at a treatment site. The prosthetic valve further comprises a valve member configured to be quickly connected to the support structure. The support structure may take the form of a stent that is expanded at the site of a native valve. If desired, the native leaflets may remain and the stent may be used to hold the native valve open. In this case, the stent may be balloon expandable and configured to resist the powerful recoil force of the native leaflets. The support structure is provided with a coupling means for attachment to the valve member, thereby fixing the position of the valve member in the body. The valve member may be a non-expandable type, or may be expandable from a compressed state to an expanded state. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment.
Owner:EDWARDS LIFESCIENCES CORP
Medical treatment system with energy delivery device for limiting reuse
InactiveUS7118564B2Without disabling functionalityControlling energy of instrumentEye treatmentClosed loopLaser surgery
The present invention provides an energy delivery device for use with a medical treatment system for the more efficacious treatment of patients during laser surgery which limits the number of uses or prevents reuse of the energy delivery device after a certain threshold limit has been reached. The energy delivery device comprises a diffusing optical fiber and a memory device having data programmed therein and being operatively connected to an energy generator the optical fiber includes a temperature sensor for generating a temperature signal in a closed loop manner. The data stored in the memory device includes a multiplicity of use parameters, usage limits, usage counts, and count limits all relating to the properties of the medical treatment system. The use parameters may include an elapsed time, a total treatment time, and a number of treatment sites. A main processor is also included for calculating a temperature from the temperature signal and for updating the use parameters in response to data received by the main processor. The main processor is also used to compare the use parameters to their corresponding usage limits. The main processor can create and increment a usage count when at least one of the use parameters exceeds its corresponding usage limit. Thereafter, the main processor compares the usage count to the count limit and disables the energy delivery device when the usage count exceeds a predetermined count limit.
Owner:CILAG GMBH INT
Method and apparatus for valve repair
InactiveUS7125421B2Minimize traumaLow costAnnuloplasty ringsTubular organ implantsLinear configurationBiomedical engineering
A tissue connection device is provided for use on a patient at a treatment site. The device comprises an elongate member having a distal end and a proximal end. The elongate member has a first, substantially linear configuration during delivery through an elongate delivery device, wherein the first configuration is sufficient to allow said member to be delivered percutaneously into the patient to the treatment site. The elongate member has a second, substantially circular configuration when said member disengages from the delivery device, wherein the second configuration is sufficient to support tissue at the treatment site. The elongate member in the second configuration defines a single ring.
Owner:MITRAL INTERVENTIONS INC
Methods for rapid deployment of prosthetic heart valves
ActiveUS7708775B2Quickly and easily replacingUse minimizedStentsAnnuloplasty ringsCouplingProsthetic heart
A two-stage or component-based valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve comprises a support structure that is deployed at a treatment site. The prosthetic valve further comprises a valve member configured to be quickly connected to the support structure. The support structure may take the form of a stent that is expanded at the site of a native valve. If desired, the native leaflets may remain and the stent may be used to hold the native valve open. In this case, the stent may be balloon expandable and configured to resist the powerful recoil force of the native leaflets. The support structure is provided with a coupling means for attachment to the valve member, thereby fixing the position of the valve member in the body. The valve member may be a non-expandable type, or may be expandable from a compressed state to an expanded state. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment.
Owner:EDWARDS LIFESCIENCES CORP
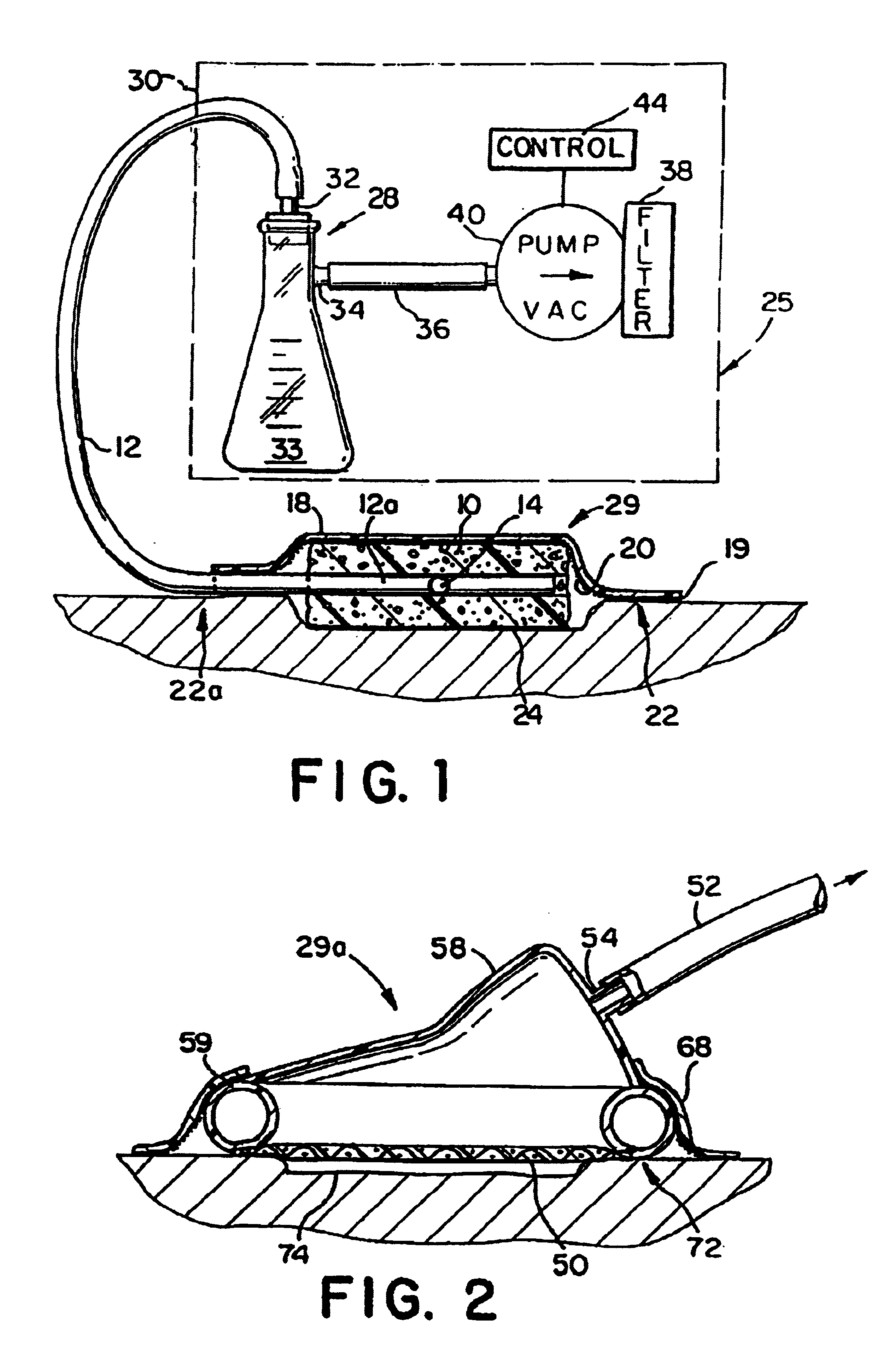
Wound treatment employing reduced pressure
A method of treating tissue damage comprises applying a negative pressure to a wound sufficient in time and magnitude to promote tissue migration and thus facilitate closure of the wound. The method is applicable to wounds, burns, infected wounds, and live tissue attachments. A wound treatment apparatus is provided in which a fluid impermeable wound cover is sealed over a wound site. A screen in the form of an open-cell foam screen or a rigid porous screen is placed beneath the wound cover over the wound. A vacuum pump supplies suction within the wound cover over the treatment site.
Owner:WAKE FOREST UNIV HEALTH SCI INC
Rapid deployment prosthetic heart valve
ActiveUS20060287719A1For quick replacementMinimize timeStentsAnnuloplasty ringsCouplingProsthetic heart
A two-stage or component-based valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve comprises a support structure that is deployed at a treatment site. The prosthetic valve further comprises a valve member configured to be quickly connected to the support structure. The support structure may take the form of a stent that is expanded at the site of a native valve. If desired, the native leaflets may remain and the stent may be used to hold the native valve open. In this case, the stent may be balloon expandable and configured to resist the powerful recoil force of the native leaflets. The support structure is provided with a coupling means for attachment to the valve member, thereby fixing the position of the valve member in the body. The valve member may be a non-expandable type, or may be expandable from a compressed state to an expanded state. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment.
Owner:EDWARDS LIFESCIENCES CORP
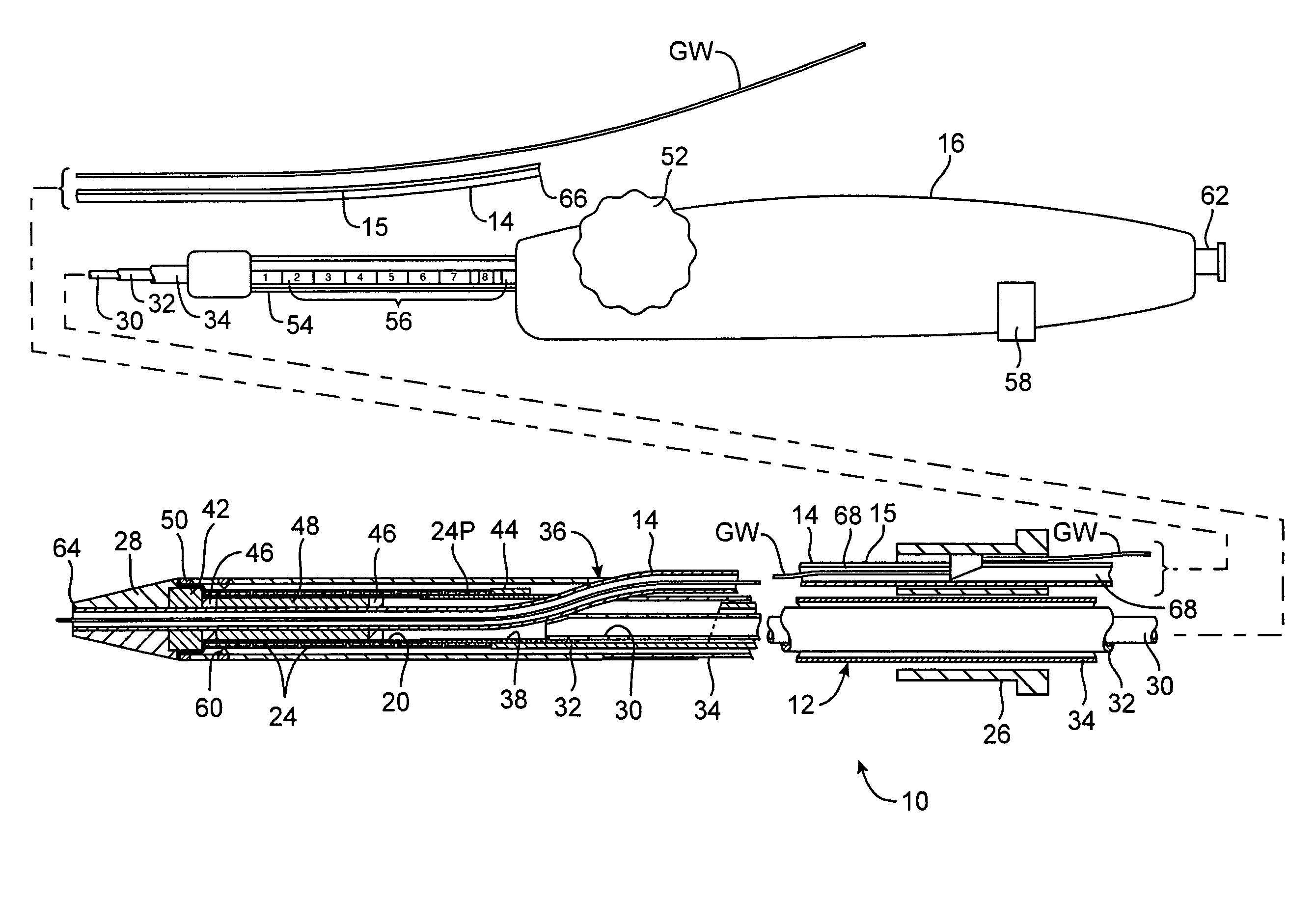
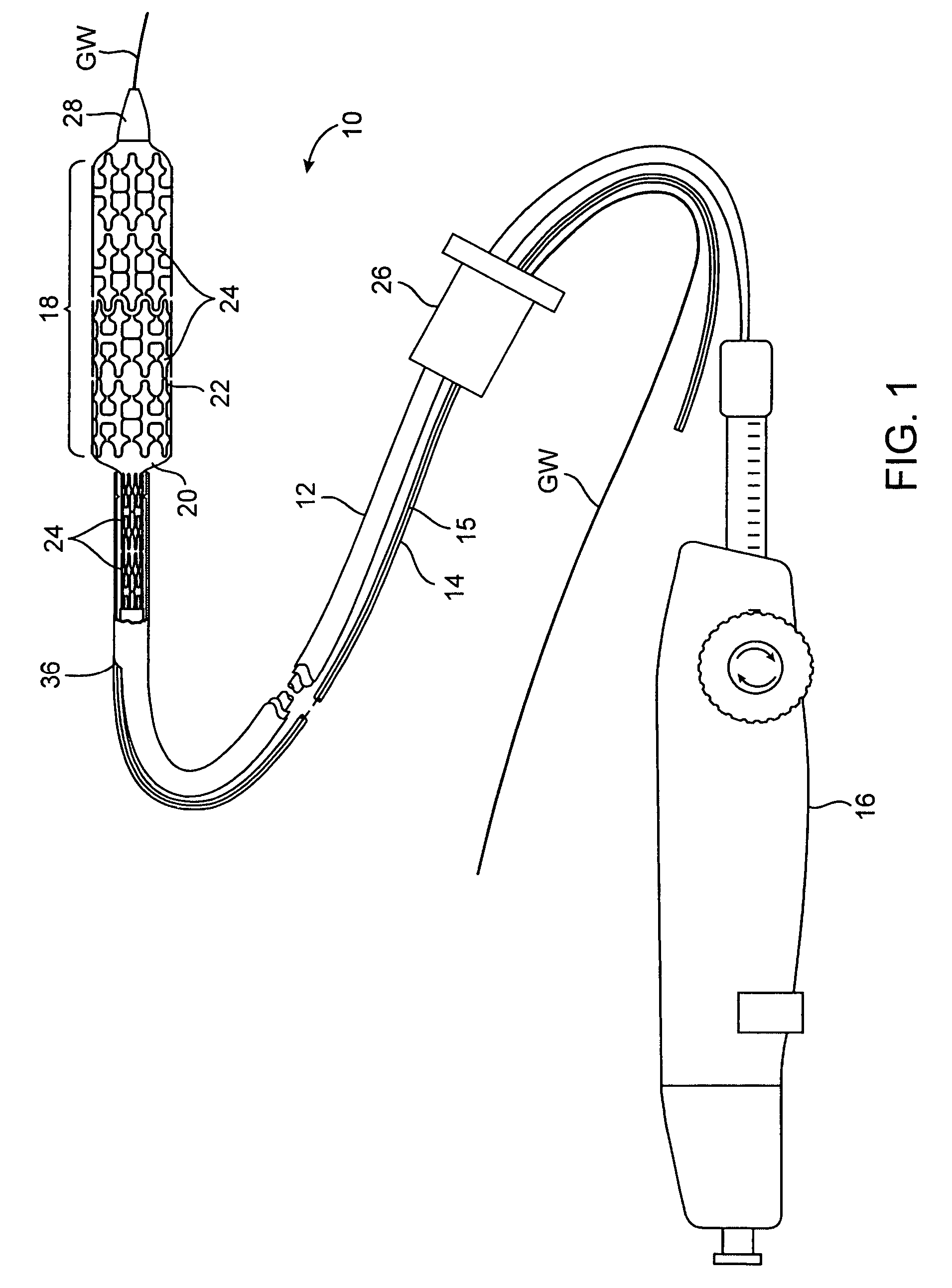
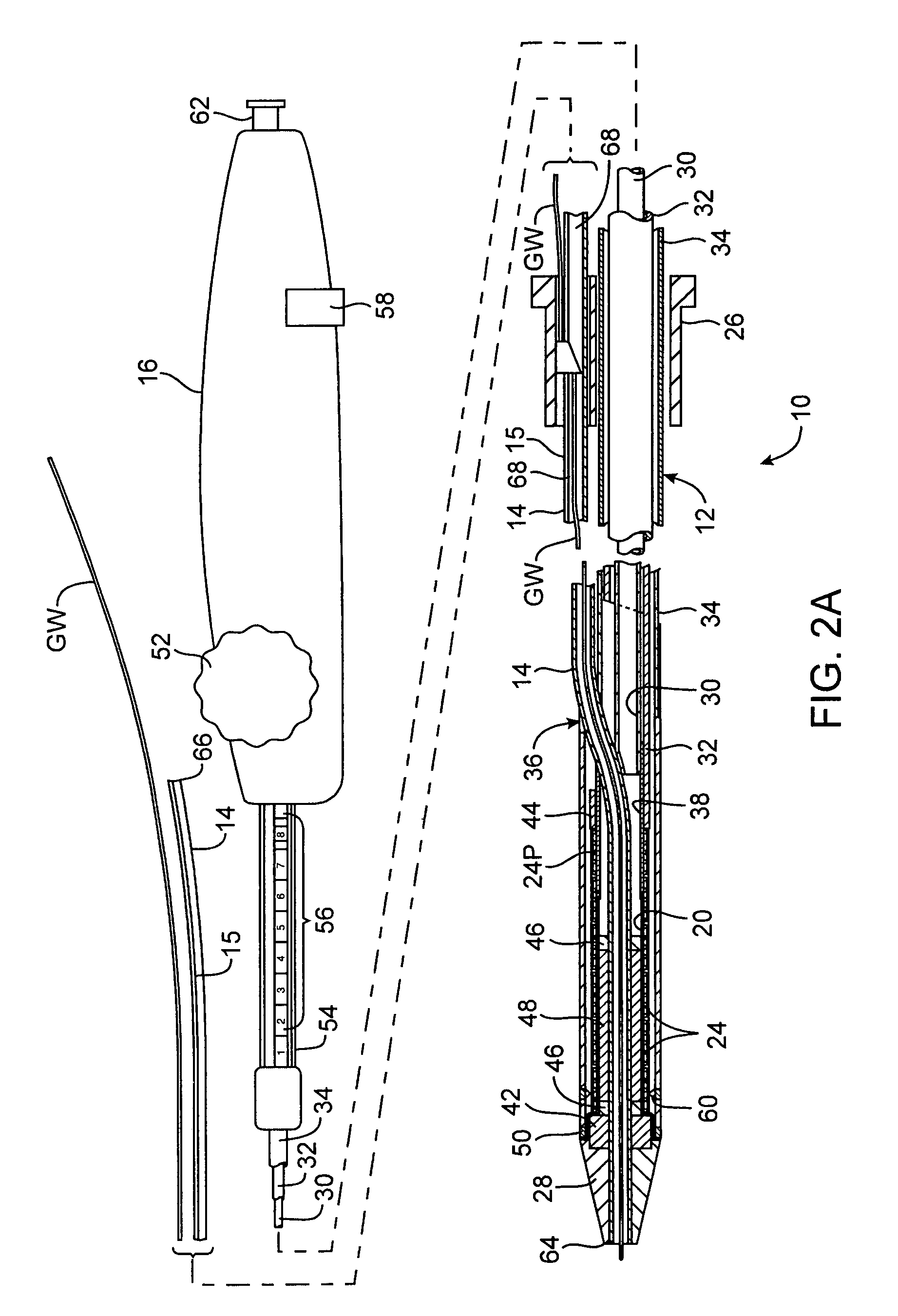
Rapid exchange interventional devices and methods
Wire-guided interventional devices and methods are provided which enable faster and easier catheter exchanges. The interventional devices include a catheter shaft and a guidewire tube wherein the catheter shaft and the guidewire tube each have a length sufficient to extend to the vascular penetration when the interventional device is positioned at the treatment site. In some embodiments, a collar is disposed around the catheter shaft and guidewire tube that automatically inserts or removes the guidewire from the guidewire tube or automatically collapses or extends the guidewire tube as the catheter is introduced or withdrawn.
Owner:JW MEDICAL SYSTEMS LTD
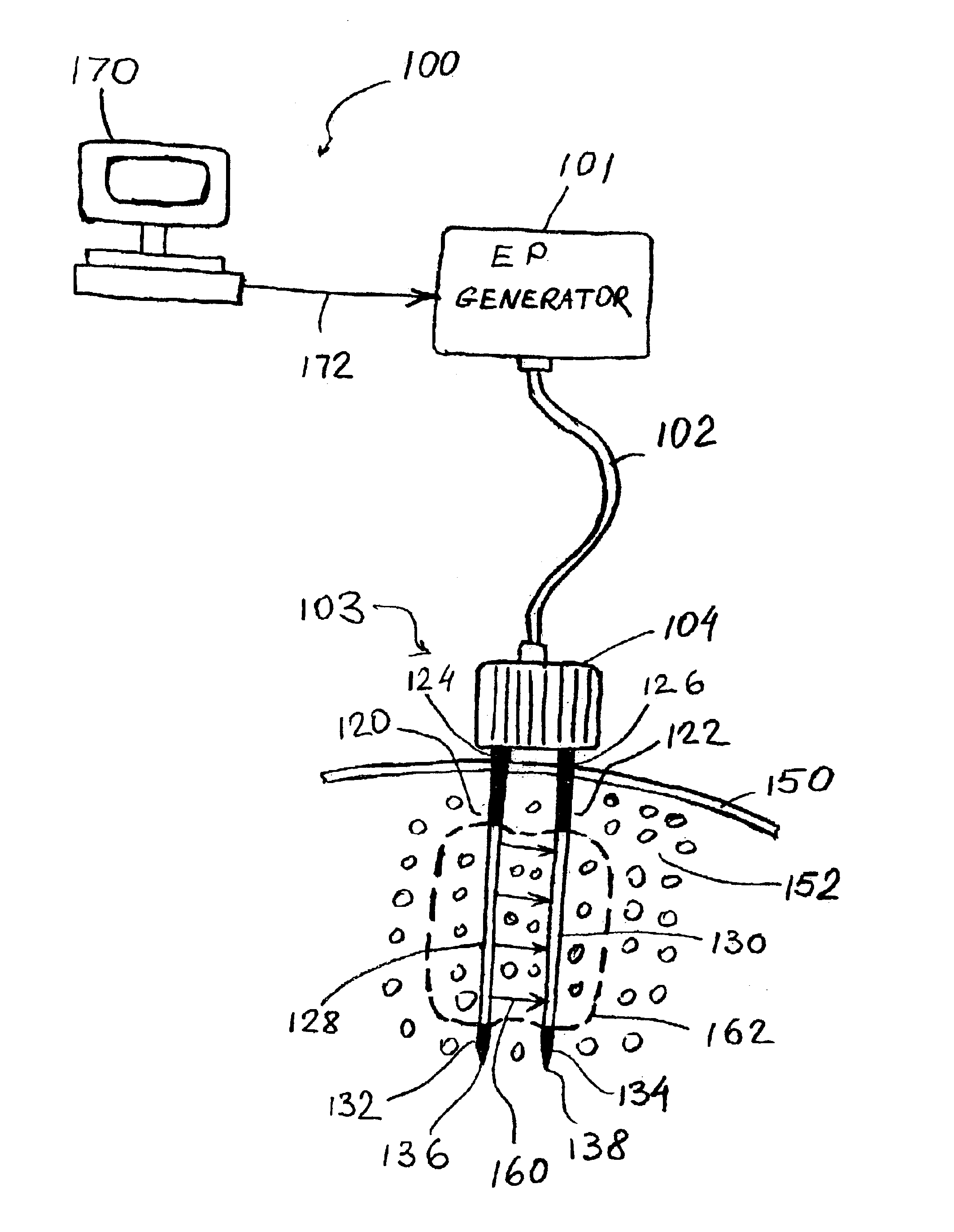
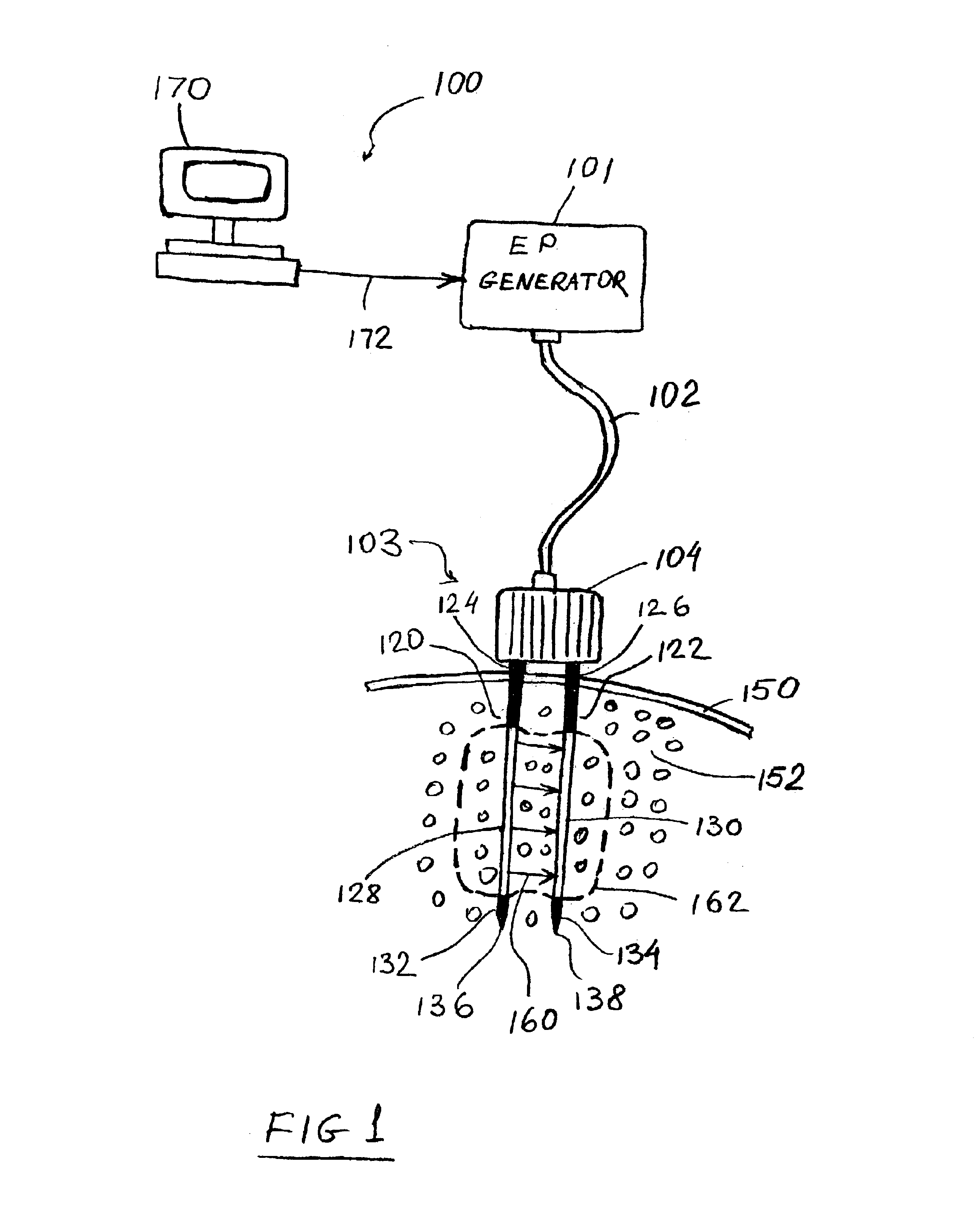
Apparatus and method for reducing subcutaneous fat deposits by electroporation
InactiveUS6795728B2Surgical instrument detailsSkin piercing electrodesReduced subcutaneous fatInvasive treatments
An apparatus and method for minimally invasive treatment of deep subcutaneous fat deposits in lieu of cosmetic surgery is disclosed. The apparatus comprises a high voltage pulse generator connected to two or more needle electrodes at least one of which is configured for placement deeply under the skin in a treatment site of the patient's body. High voltage pulses, delivered to the electrodes, create an electric field that kills subcutaneous fat cells.
Owner:ANGIODYNAMICS INC
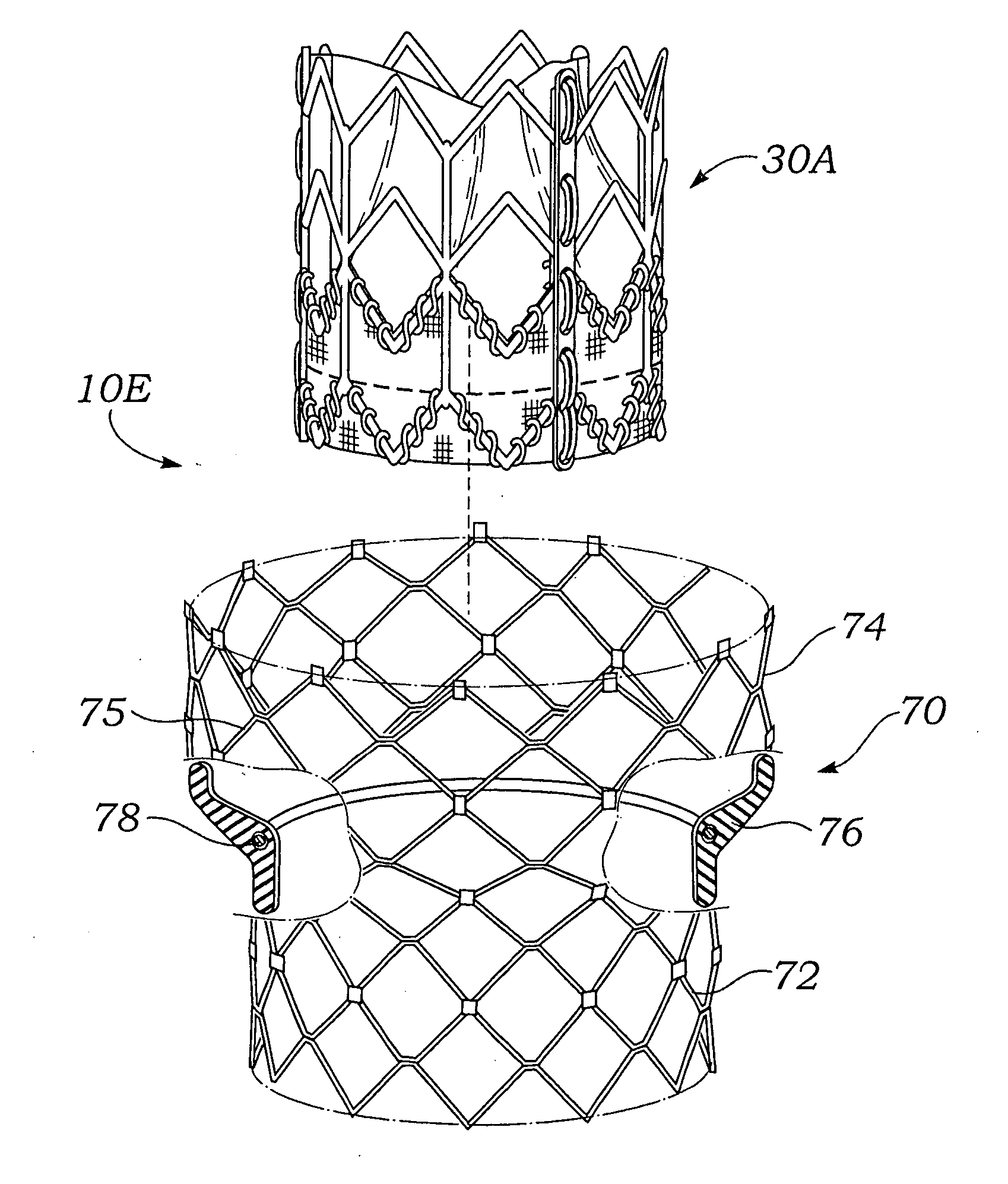
Reinforced Surgical Conduit for Implantation of a Stented Valve Therein
A pulmonary valve replacement system having a vascular conduit and a prosthetic valve device including a valve operably connected to a support structure. The prosthetic valve device is positioned within the vascular conduit. A conduit support includes a substantially circular cross-section. The conduit support is positioned adjacent to and reinforces the vascular conduit. In one embodiment, the pulmonary valve replacement system includes a catheter and an inflatable member operably attached to the catheter. The prosthetic valve device is disposed on the inflatable member. The invention provides a method for replacing a pulmonary valve including providing a vascular conduit positioned at a treatment site. The vascular conduit includes a conduit support positioned adjacent the vascular conduit. A prosthetic valve device is deployed within the vascular conduit via catheter. The prosthetic valve device includes a valve operably connected to a support structure. The vascular conduit is supported with the conduit support.
Owner:MEDTRONIC VASCULAR INC
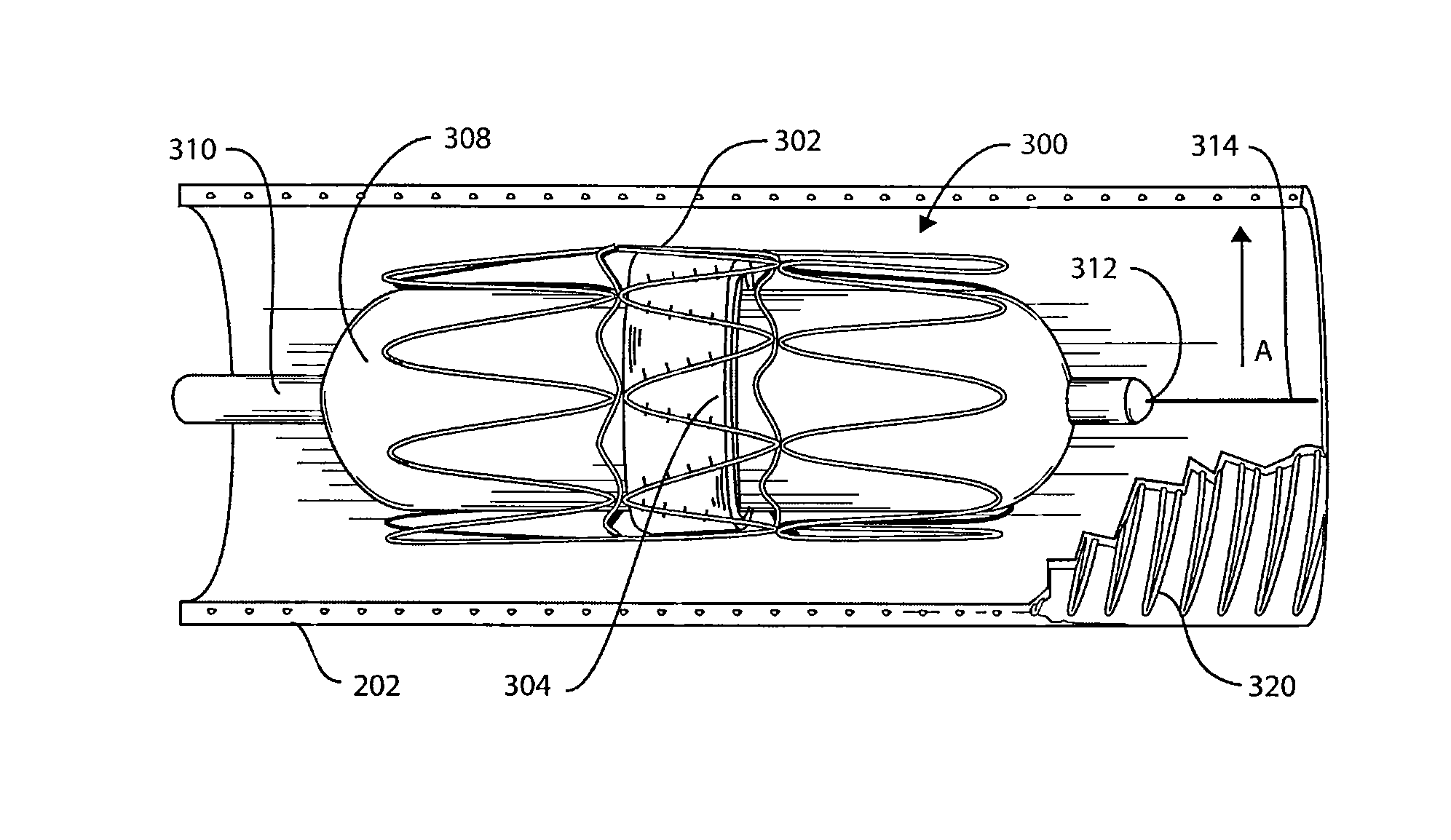
Self-constrained segmented stents and methods for their deployment
InactiveUS20060069424A1Precise positioningChallenge can be overcomeStentsBlood vesselsInsertion stentEngineering
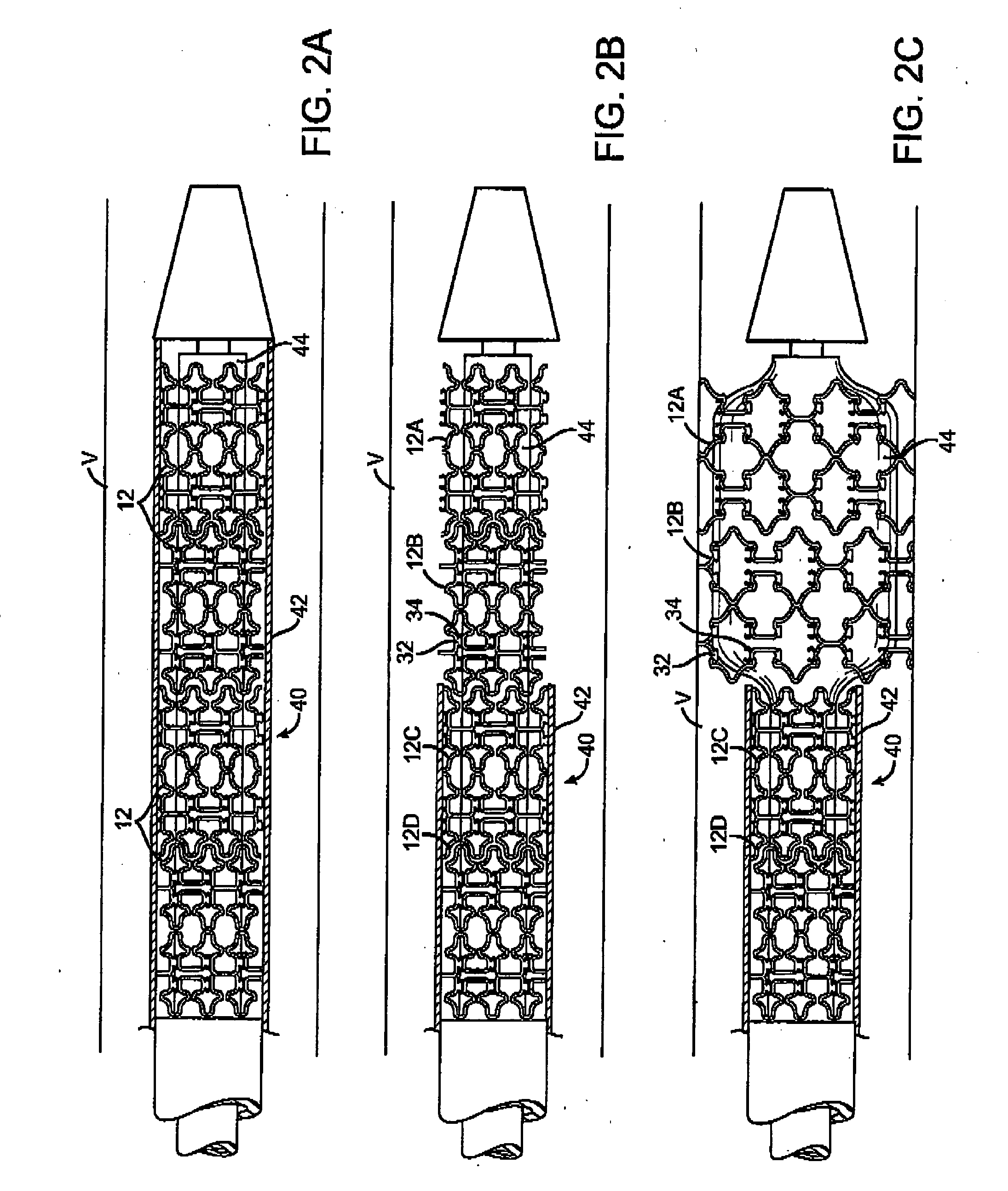
A self-expanding stent includes a plurality of segments having a collapsed configuration and an expanded configuration. Preferably, the segments are unconnected to each other in at least the expanded configuration. The segments include restraining structures that temporarily restrain them from expansion until activated. This allows the user to position the desired number of segments at a treatment site and to deploy them simultaneously, thereby avoiding misalignment, overlap, and excessive spacing between segments. In preferred embodiments, multiple segmented stents of user-selectable length may be deployed at multiple locations in a single intervention.
Owner:XTENT INC
Stent-graft-membrane and method of making the same
A braided self-expandable stent-graft-membrane made of elongated members forming a generally tubular body. A membrane layer and graft layer are disposed on a endoprosthesis such as a stent. The membrane layer is substantially impermeable to fluids. The outermost layer is biocompatible with the body tissue. The innermost layer is biocompatible with the fluid in the passage. An embodiment includes a graft layer disposed on the inside of a stent and a membrane layer disposed on the outside of the stent. The innermost layer is biocompatible with the fluid in the passage. The stent-graft-membrane is used at a treatment site in a body vessel or organ where it is desirous to exclude a first fluid located outside the endoprosthesis from reaching a second fluid located in the lumen. The membrane may be made of silicone or polycarbonate urethane. The graft may be braided, woven, spun or spray-cast PET, PCU, or PU fibers. The layers may include ePTFE or PTFE.
Owner:LIFEPORT SCI
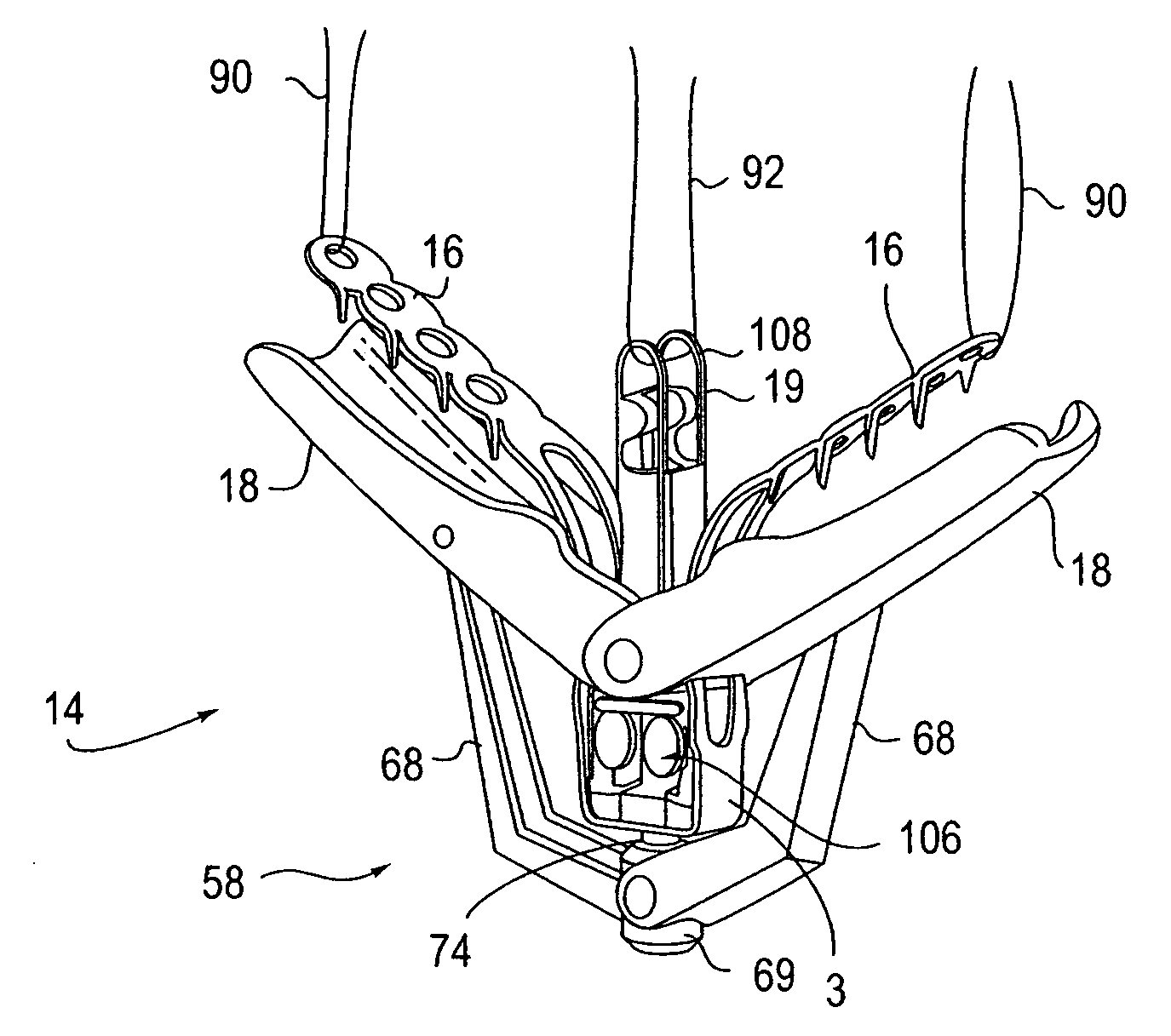
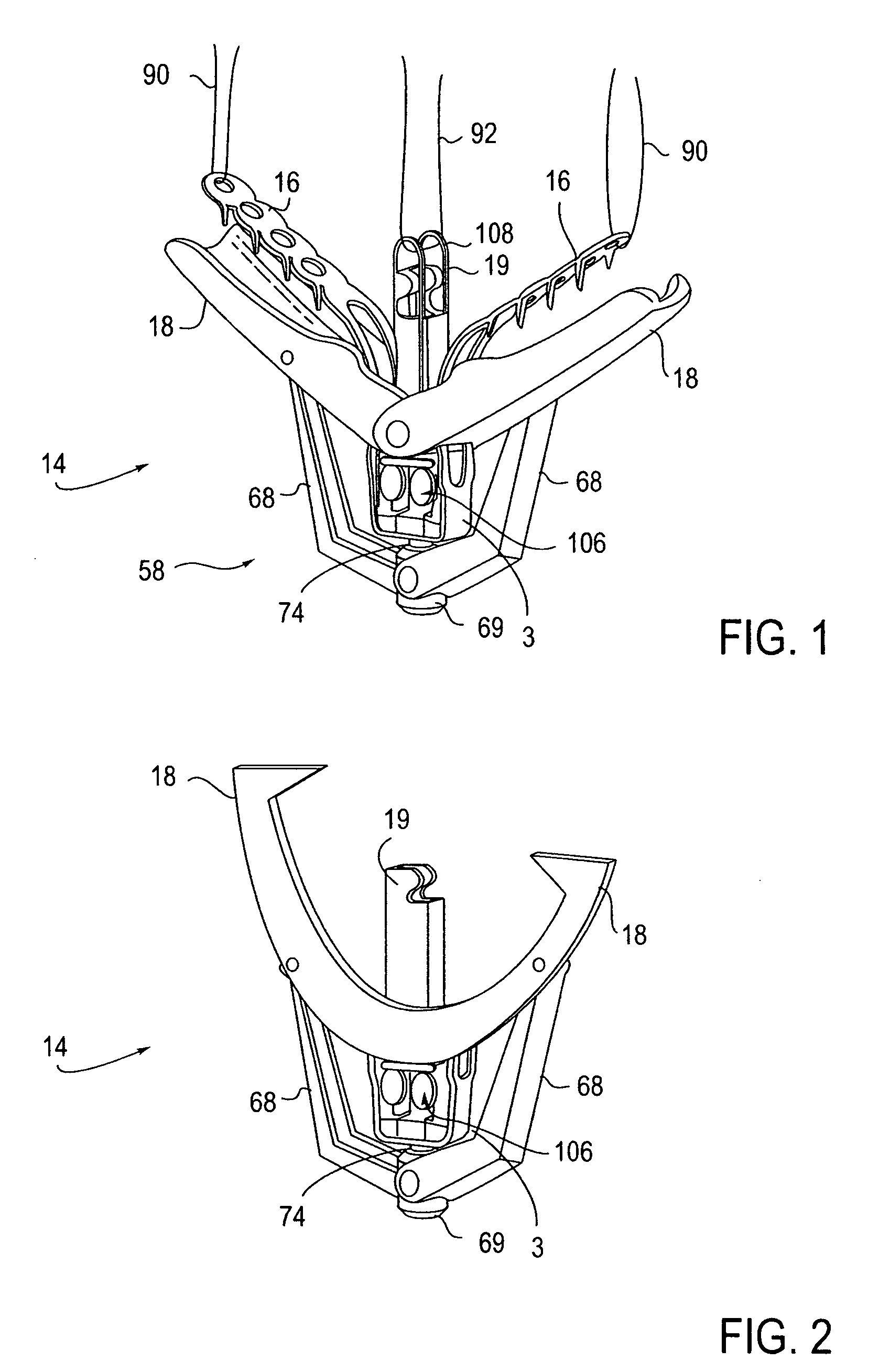
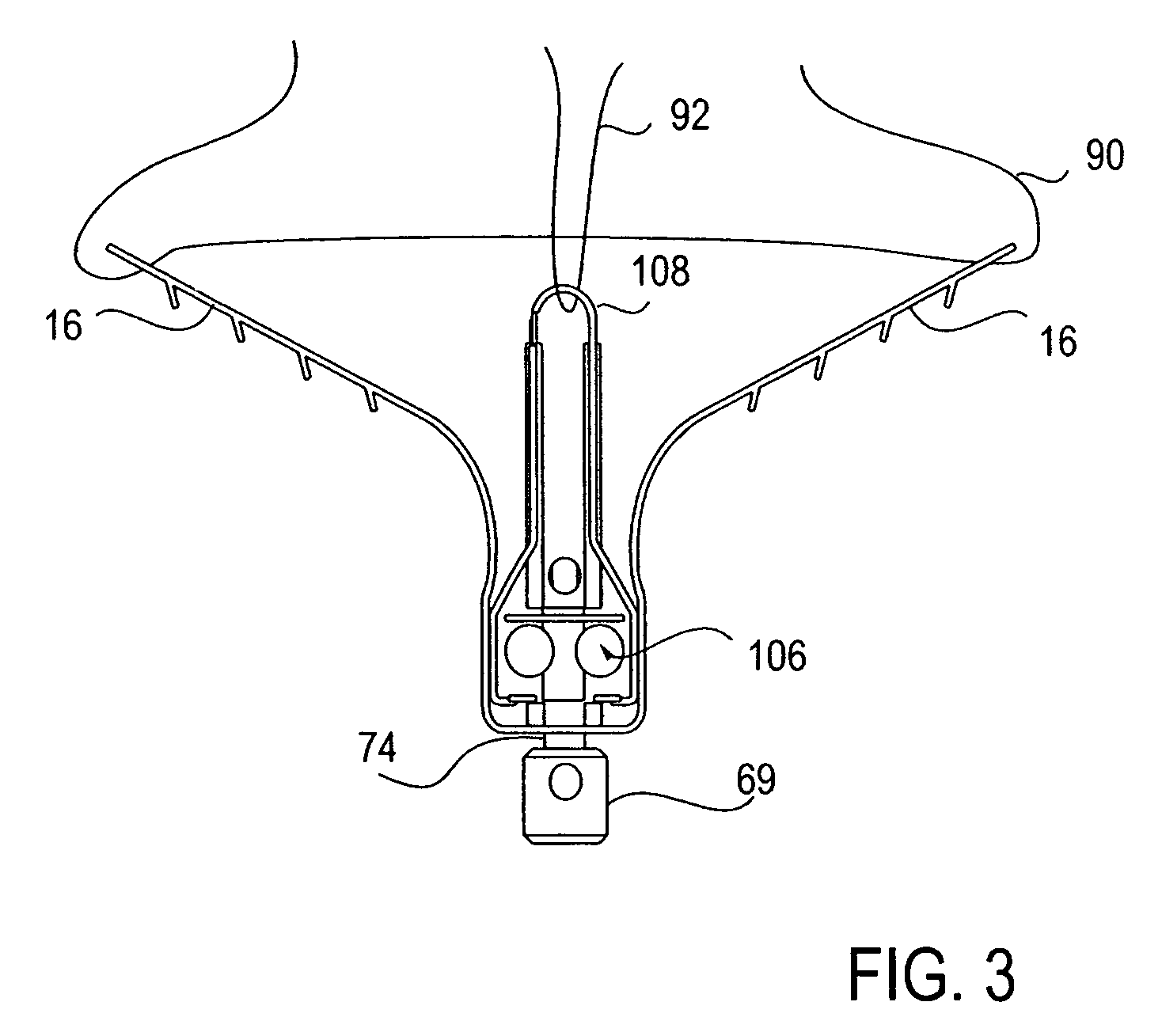
Locking mechanisms for fixation devices and methods of engaging tissue
InactiveUS20060020275A1Reduce frictional contactRestrict movementSuture equipmentsAnnuloplasty ringsEngineeringThoracic cavity
Devices, systems and methods are provided for tissue approximation and repair at treatment sites. In particular, fixation devices are provided comprising a pair of elements each having a first end, a free end opposite the first end, and an engagement surface therebetween for engaging the tissue, the first ends being moveable between an open position wherein the free ends are spaced apart and a closed position wherein the free ends are closer together with the engagement surfaces generally facing each other. The fixation devices also include a locking mechanism coupled to the elements for locking the elements in place. The devices, systems and methods of the invention will find use in a variety of therapeutic procedures, including endovascular, minimally-invasive, and open surgical procedures, and can be used in various anatomical regions, including the abdomen, thorax, cardiovascular system, heart, intestinal tract, stomach, urinary tract, bladder, lung, and other organs, vessels, and tissues. The invention is particularly useful in those procedures requiring minimally-invasive or endovascular access to remote tissue locations, where the instruments utilized must negotiate long, narrow, and tortuous pathways to the treatment site.
Owner:EVALVE
Device, system, and method for treating cardiac valve regurgitation
ActiveUS20070250160A1Great coaptionChange shapeHeart valvesSurgeryValvular regurgitationTherapeutic Area
A device, a system and a method for treating heart valve regurgitation. The annulus reshaping device comprises a base and a plurality of legs radially arranged there upon. The device can transform from a delivery configuration wherein it is deliverable by catheter to a treatment site, into a deployment configuration, and then a treatment configuration for treating valvular regurgitation. The device is implanted into the annulus of a heart valve, and the legs of the device can be telescopically withdrawn to apply an inward force to the annulus. The system comprises a device slidably received within a catheter. The method of treatment comprises delivering a device to a treatment area, via catheter, releasing the device from the catheter, positioning the legs of the device on a valve annulus, and applying an inward force to the annulus to reduce the regurgitation.
Owner:MEDTRONIC VASCULAR INC
Locking mechanisms for fixation devices and methods of engaging tissue
InactiveUS7604646B2Prevent movementReduce frictional contactSuture equipmentsAnnuloplasty ringsEngineeringSurgical department
Owner:EVALVE
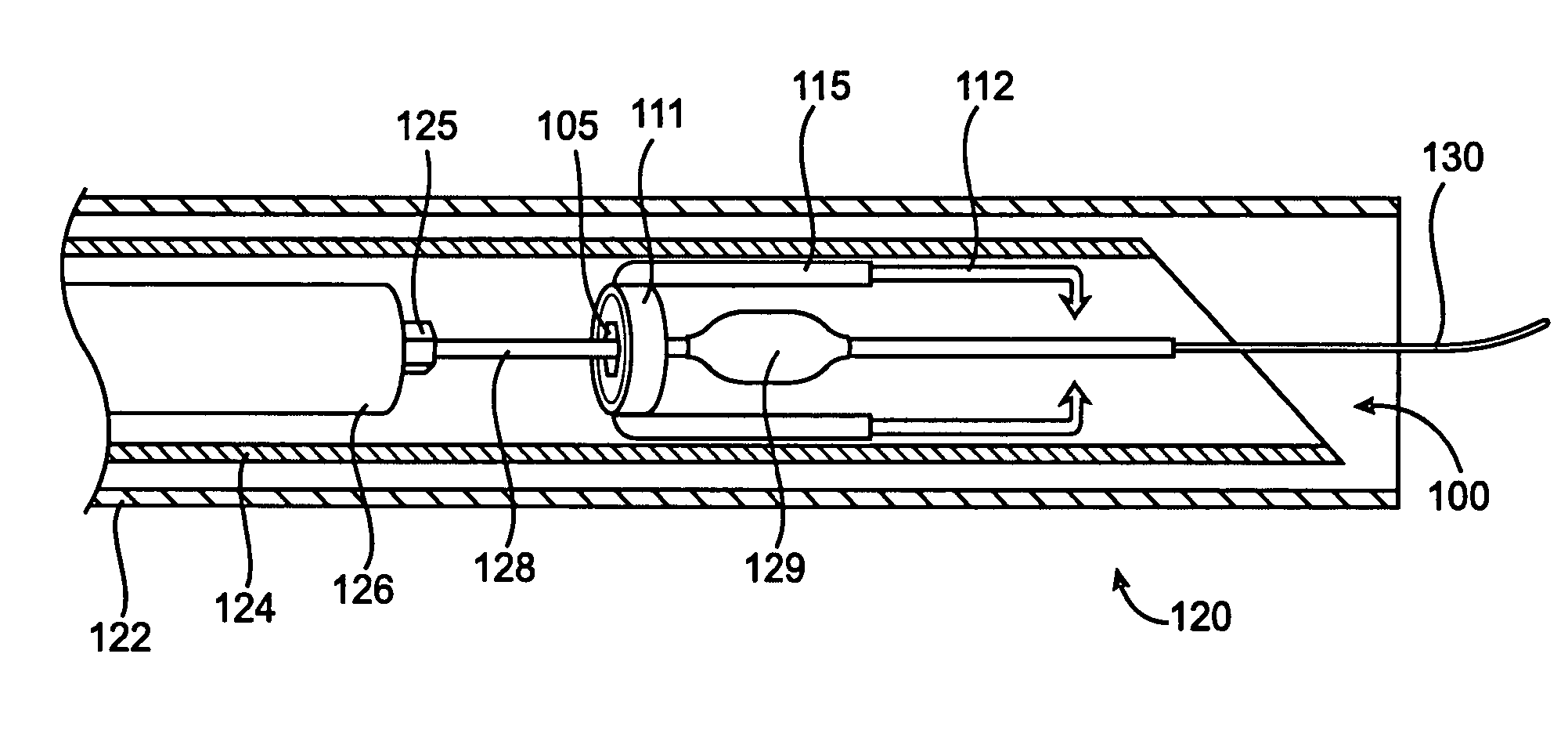
Intralumenal contact sensor
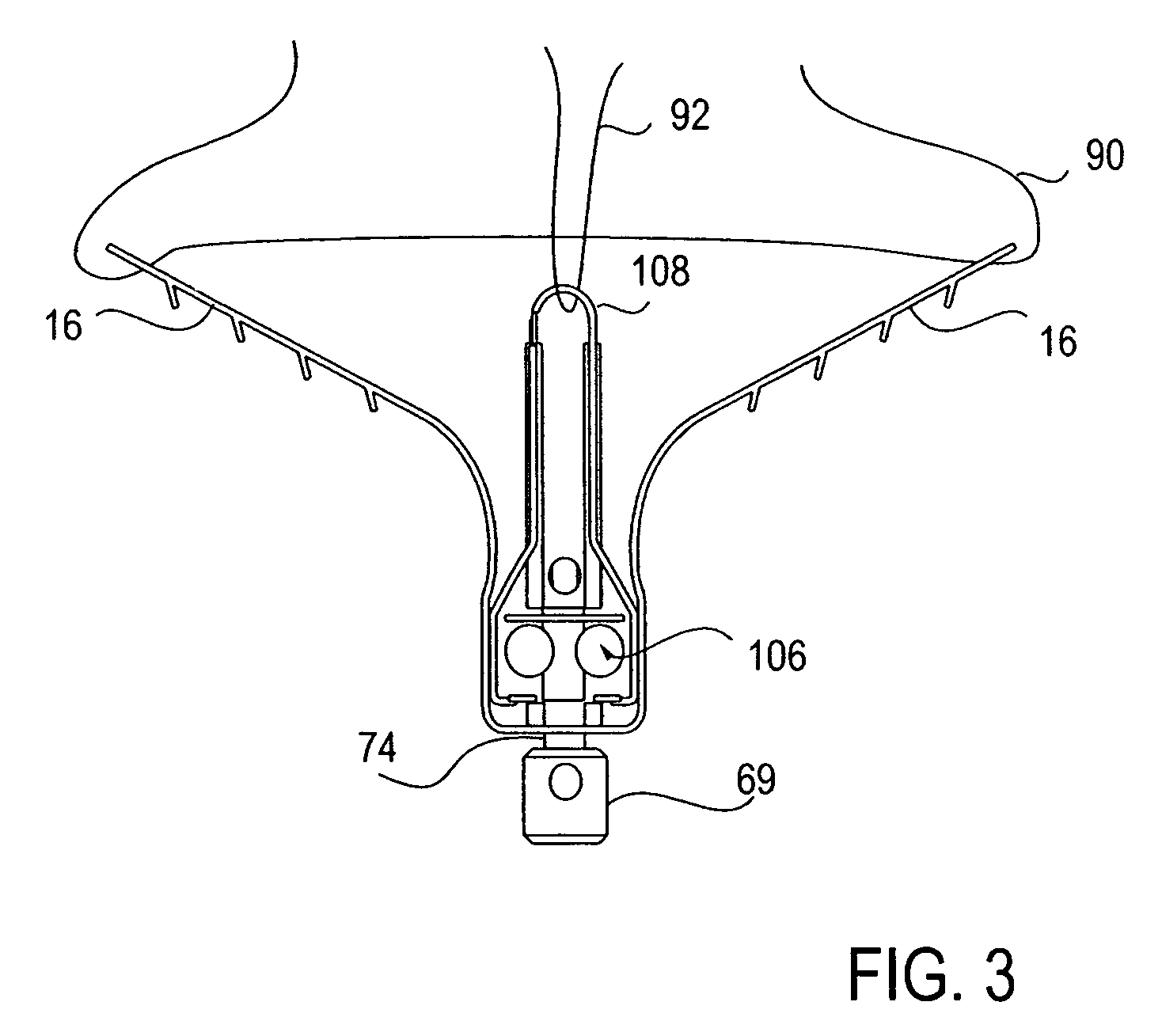
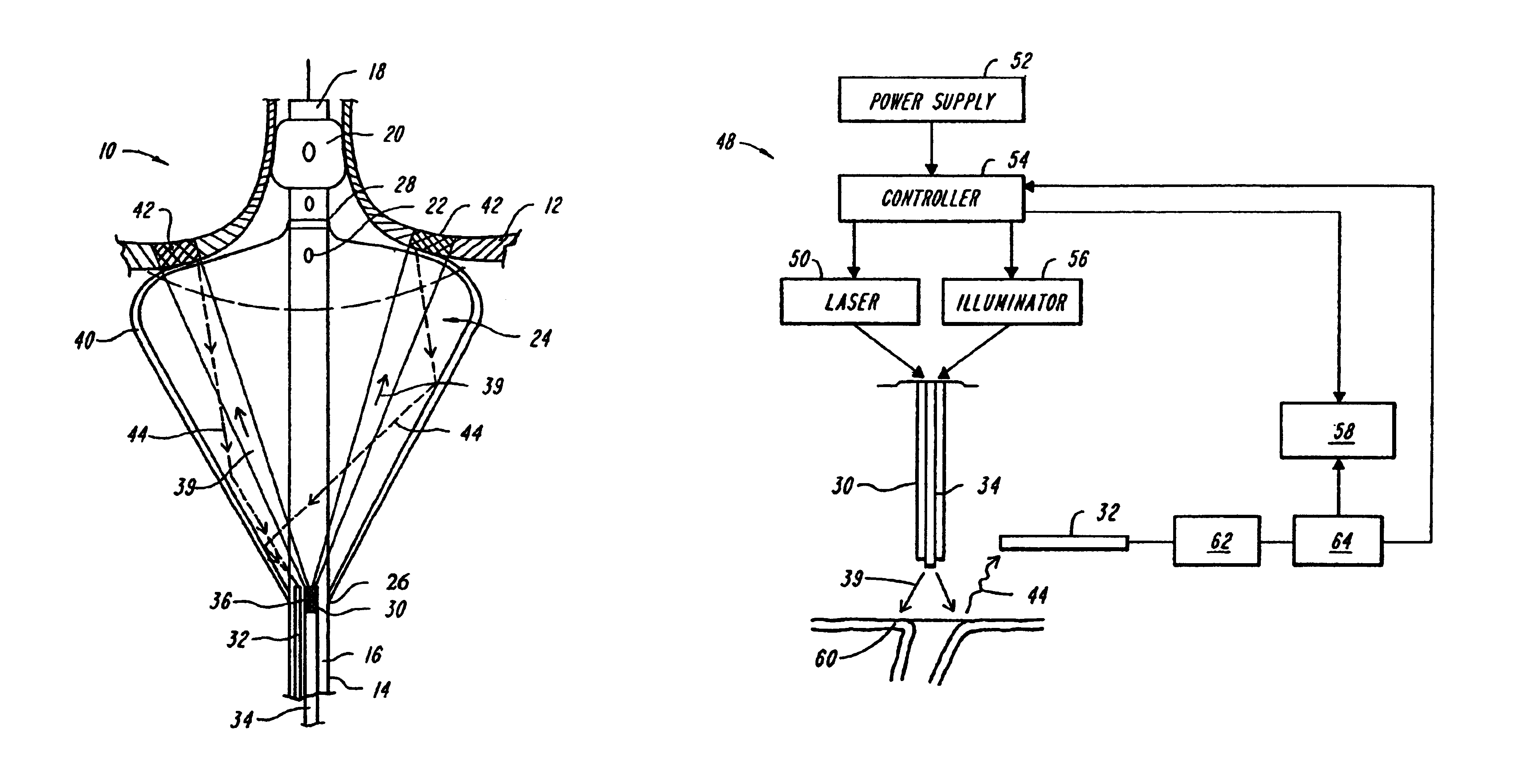
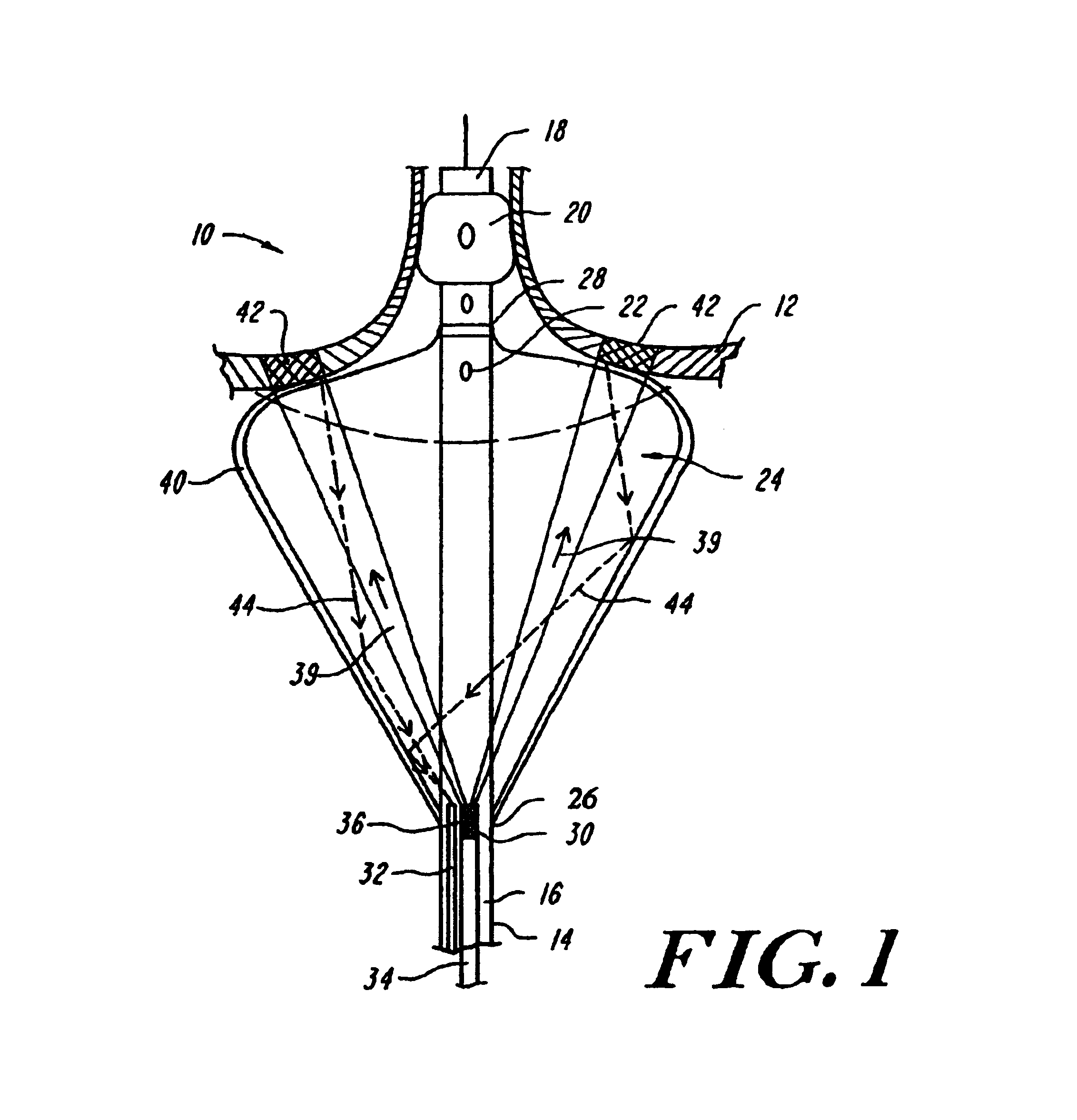
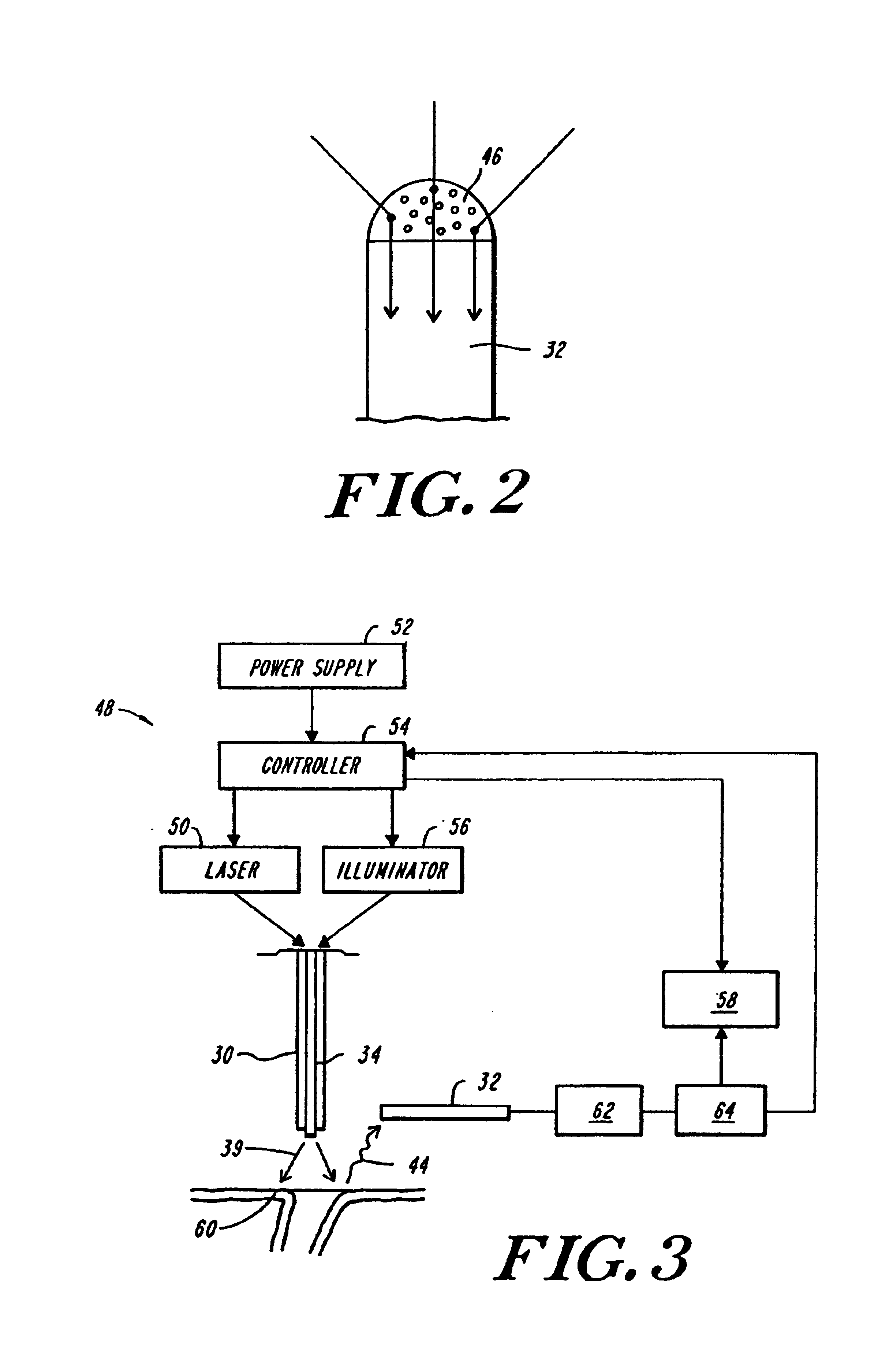
InactiveUS6942657B2Eliminate aberrant wave conductionReduce lossDiagnosticsCatheterLight treatmentLaser light
An apparatus and method for phototherapy are described in which laser light or other radiation is projected from within a catheter, through a balloon member, and toward the surface of tissue. The light reflected from body fluids or the tissue surface is captured by a collecting device located within the catheter, e.g., within the balloon member, and the intensity of the reflected light is ascertained. The apparatus and method provides for accurately positioning the apparatus against the tissue treatment site.
Owner:CARDIOFOCUS INC
Emboli protection devices and related methods of use
ActiveUS7374560B2Improve visualizationStopping normal blood flowStentsDilatorsRetrograde FlowEmbolization material
An evacuation sheath assembly and method of treating occluded vessels which reduces the risk of distal embolization during vascular interventions is provided. The evacuation sheath assembly includes an elongated tube defining an evacuation lumen having proximal and distal ends. A proximal sealing surface is provided on a proximal portion of the tube and is configured to form a seal with a lumen of a guided catheter. A distal sealing surface is provided on a distal portion of the tube and is configured to form a seal with a blood vessel. Obturator assemblies and infusion catheter assemblies are provided to be used with the evacuation sheath assembly. A method of treatment of a blood vessel using the evacuation sheath assembly includes advancing the evacuation sheath assembly into the blood vessel through a guide catheter. Normal antegrade blood flow in the blood vessel proximate to the stenosis is stopped and the stenosis is treated. Retrograde blood flow is induced within the blood vessel to carry embolic material dislodged during treating into the evacuation sheath assembly. If necessary to increase retrograde flow, the coronary sinus may be at least partially occluded. Alternatively, antegrade flow may be permitted while flow is occluded at the treatment site.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
Quick-connect prosthetic heart valve and methods
Owner:EDWARDS LIFESCIENCES CORP
Guidewire loaded stent for delivery through a catheter
A guidewire loaded stent for delivery through a catheter is described herein. The stent delivery assembly can deliver and place a stent within tortuous regions of the body which are accessible to guidewires but inaccessible to stenting catheters. The assembly comprises a guidewire covered in part by a retractable sheath and a radially expandable stent near or at the distal end of the guidewire. The whole assembly is advanced through conventional catheters or it may be used alone. In either case, when the stent is adjacent to a treatment site within the body, the sheath is retracted proximally to expose the stent for radial expansion into contact with the vessel wall. Radio-opaque marker bands are optionally located on either side or both sides of the stent on the guidewire body to aid in visual placement. The assembly can optionally include an expandable balloon on the guidewire for different treatment modalities.
Owner:BACK BAY MEDICAL
Curable media for implantable medical device
InactiveUS6875212B2Large implantsProcedure can be minimizedInternal osteosythesisJoint implantsOrthopedic fixation devicesMedical device
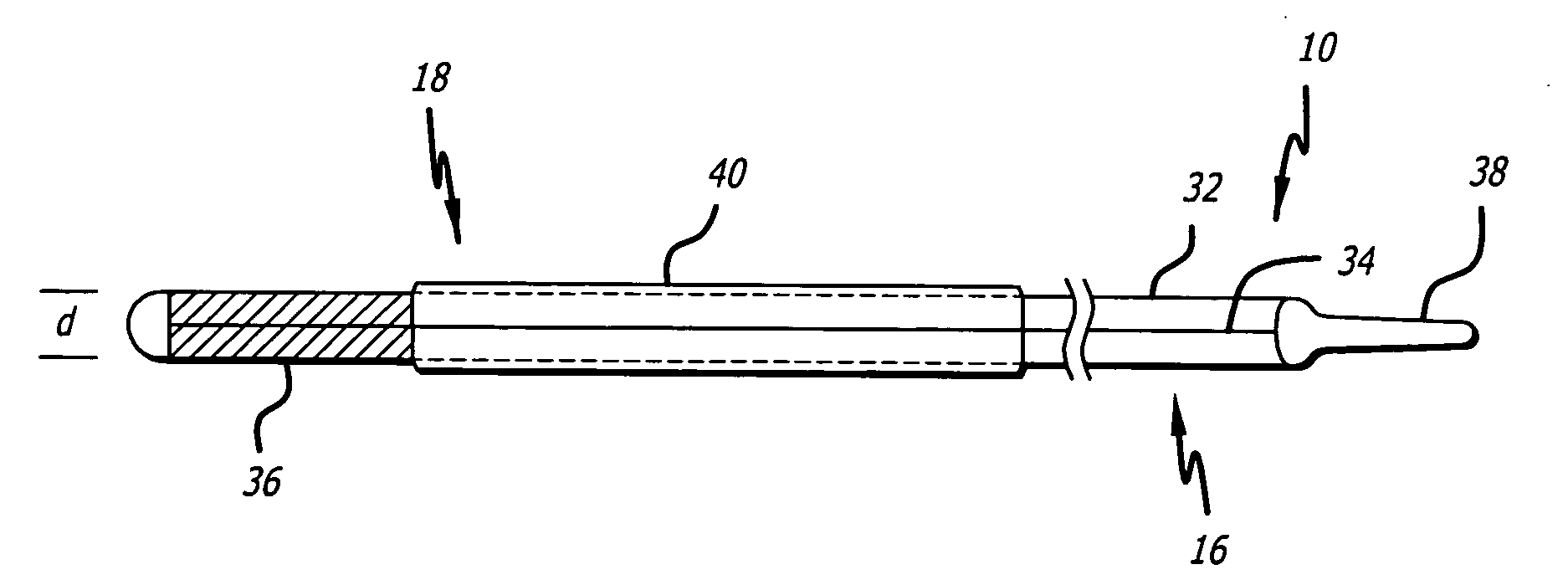
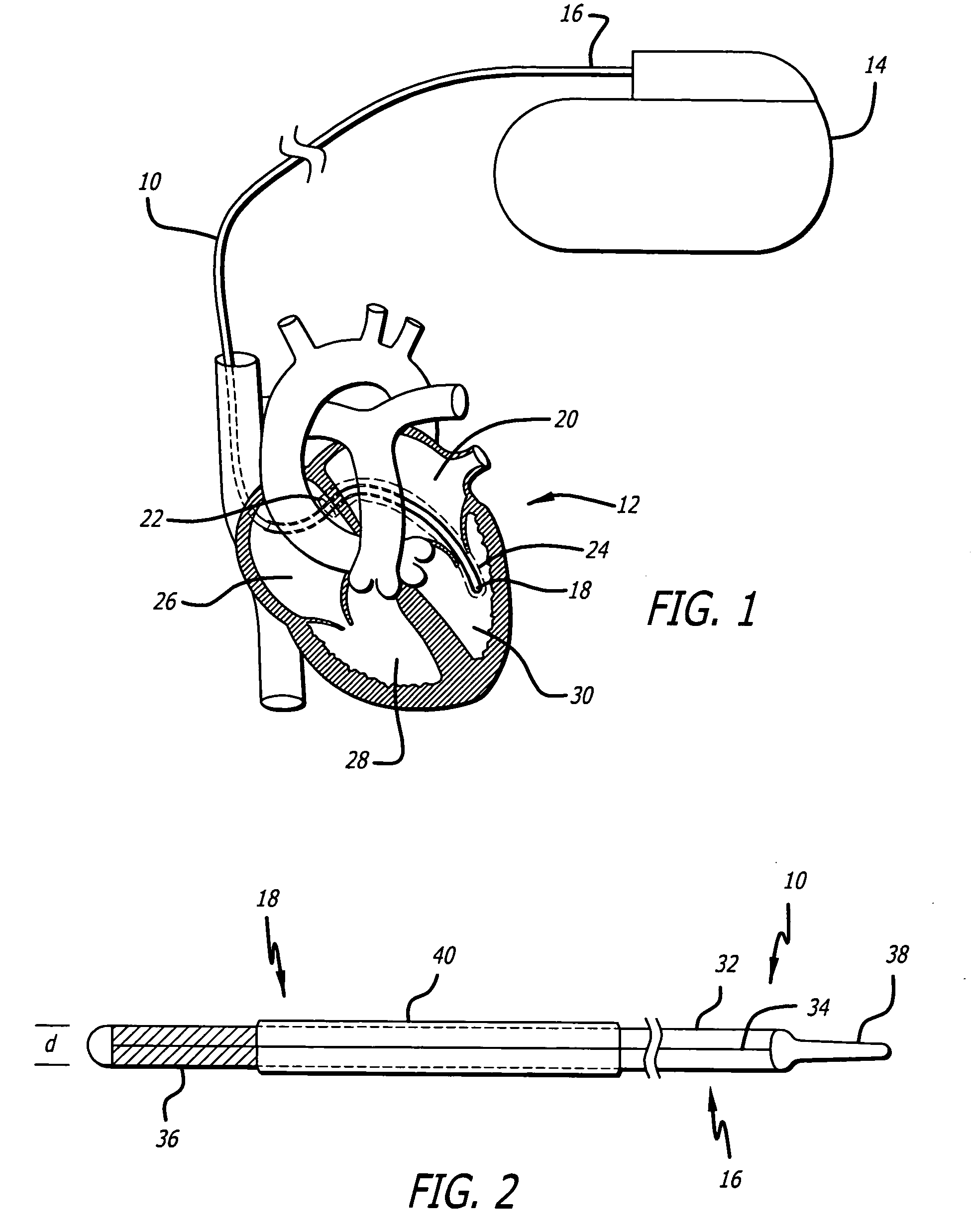
A subcutaneously formed in place orthopedic fixation device is provided, such as for fixation of the spine or other bone or bones. The device comprises an inflatable member, such as a tubular balloon. The balloon is positioned at a treatment site in the body while in a flexible, low crossing profile configuration. The balloon is thereafter inflated with a hardenable epoxy media comprising one or more epoxy compounds and one or more amine curing compounds that cures rapidly in place with low to moderate exotherm. Methods and delivery structures are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Formable orthopedic fixation system
An implantable inflatable orthopedic device is provided. The device comprises a flexible wall, defining an interior cavity, a reinforcing element exposed to the cavity, an inflation pathway in communication with the cavity, and a valve, for closing the pathway. A delivery catheter is also provided for removably carrying the orthopedic device to the treatment site.
Owner:WARSAW ORTHOPEDIC INC
Method and apparatus for temperature control of biologic tissue with simultaneous irradiation
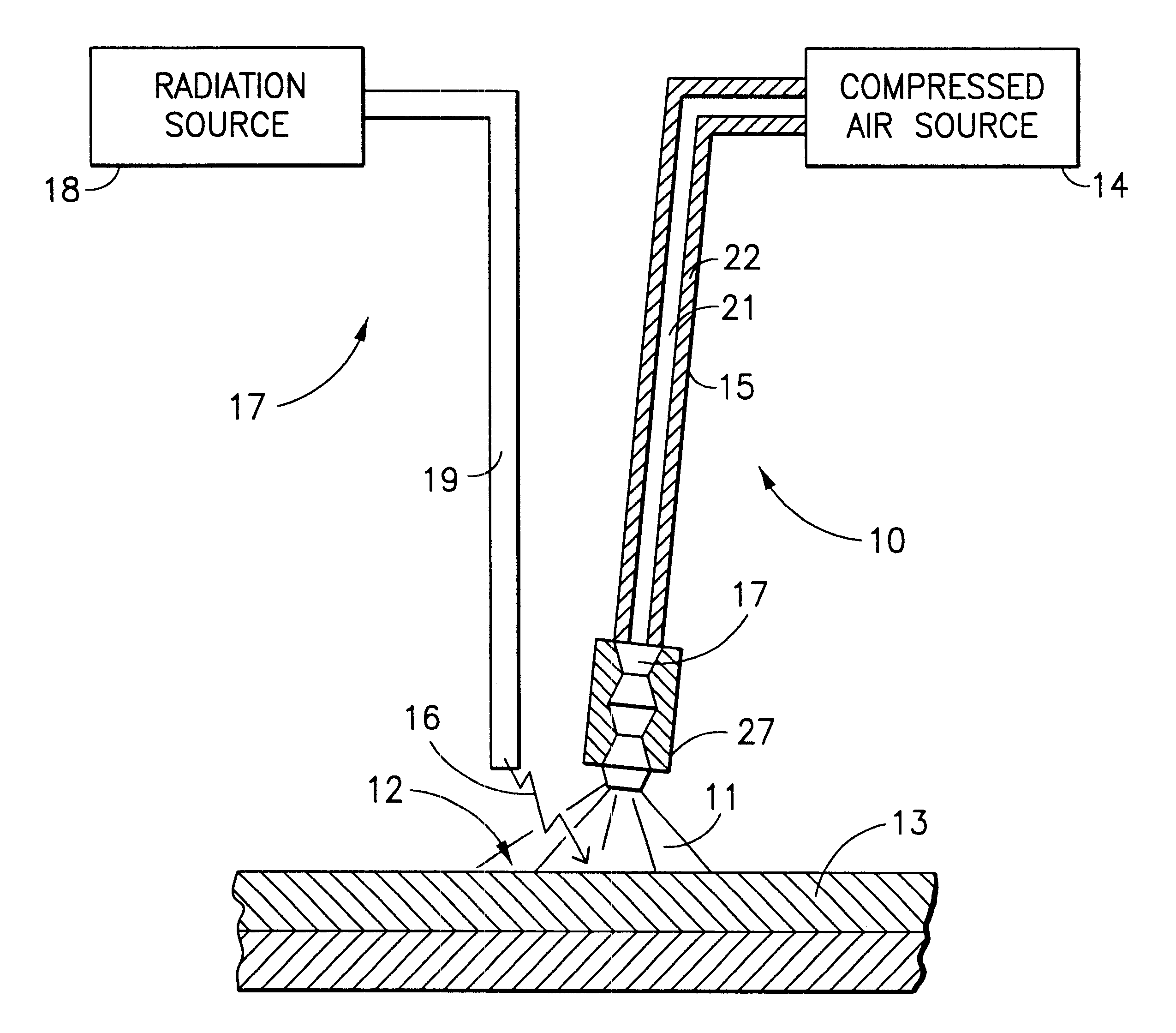
InactiveUS6475211B2Reduce usageLow costDiagnosticsSurgical instrument detailsTemperature controlTemperature modulation
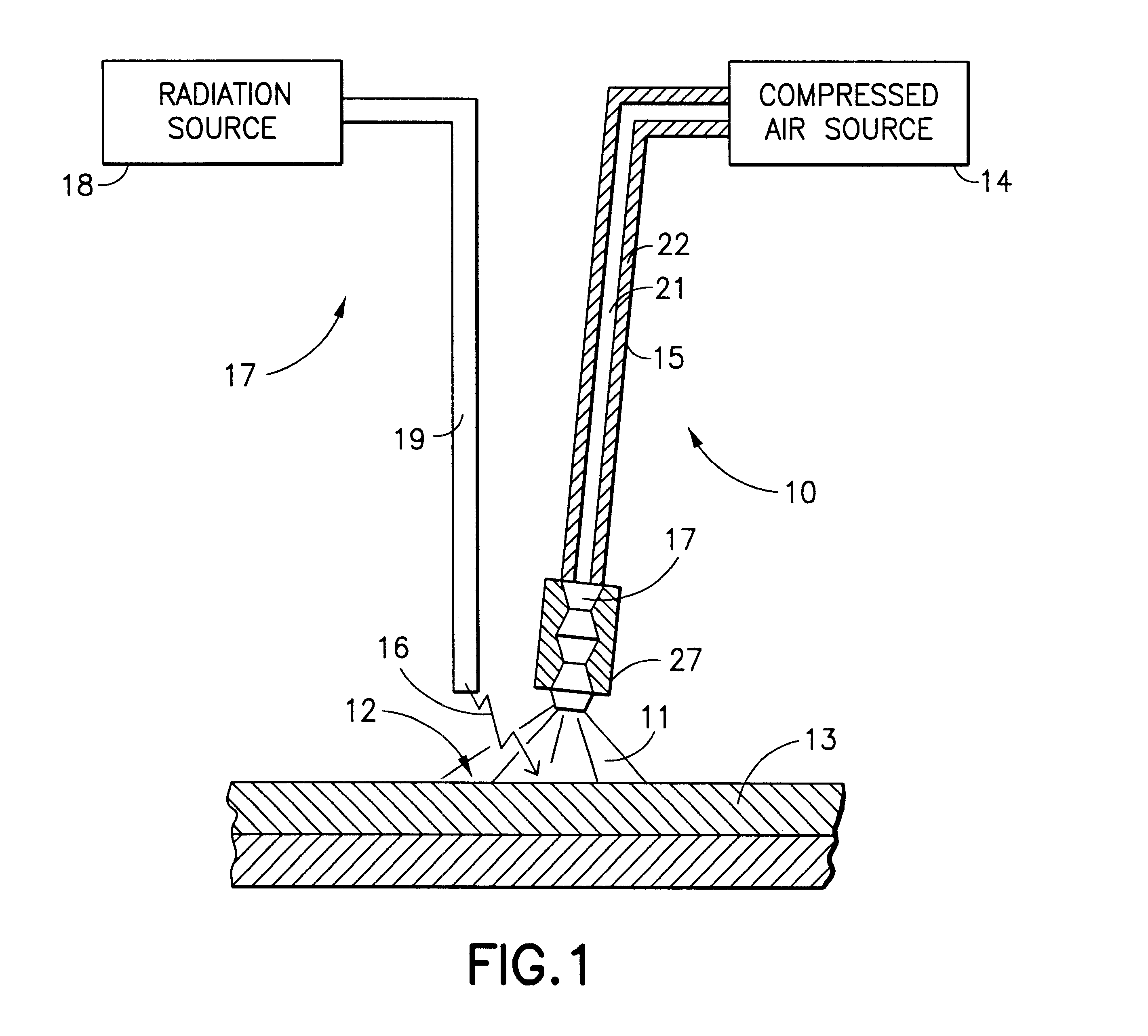
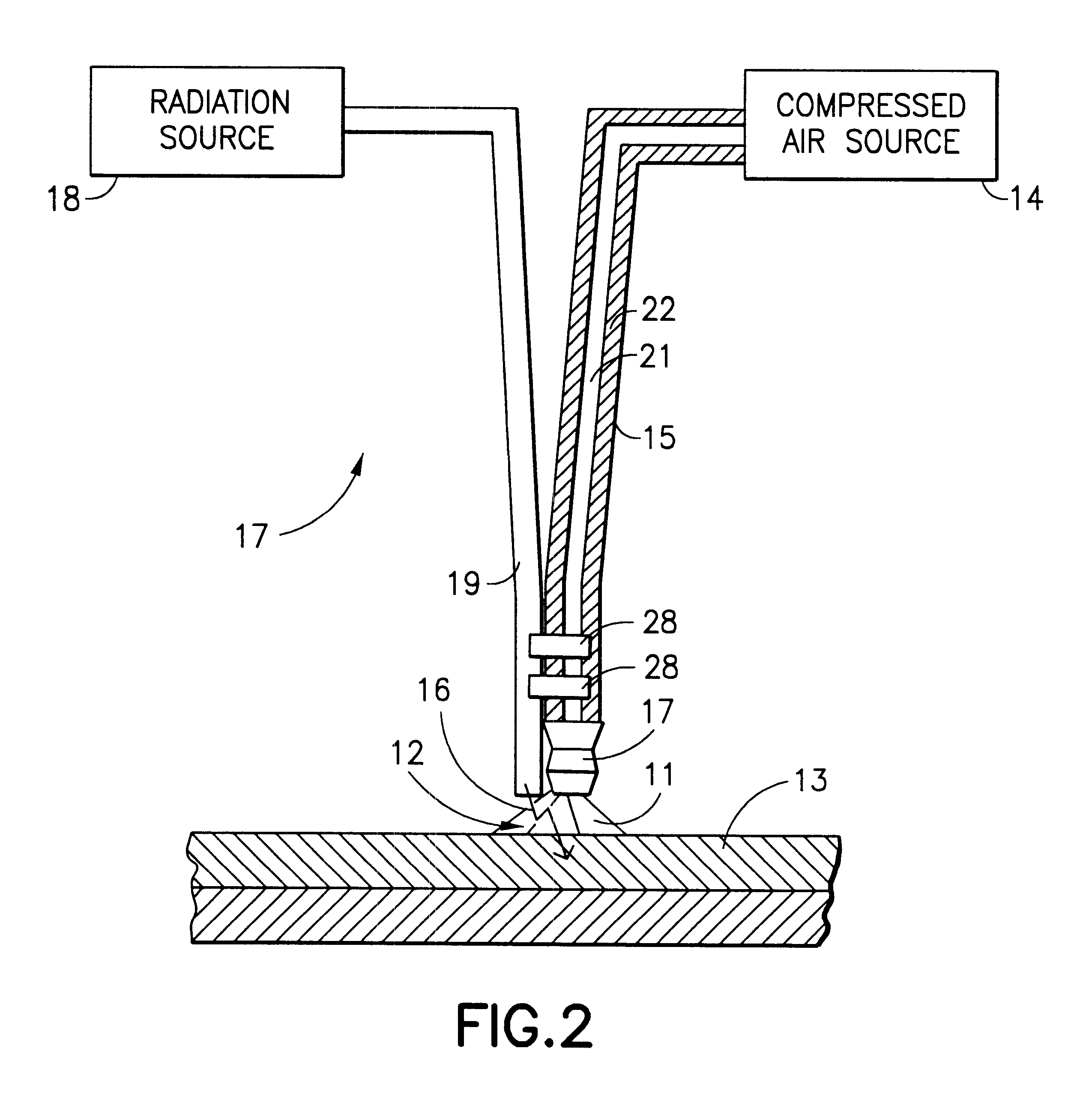
A method and apparatus for treatment of the skin or other biologic tissue includes the ability to subject said skin or other tissue to temperature modulation and radiation, simultaneously. The apparatus that delivers warm or cold material to the treatment site to effect this modulation of temperature may be attached to the apparatus that delivers radiation or it may be a separate entity, that could be utilized with a variety of radiation generating equipment.
Owner:COOL LASER OPTICS
Drug eluting implants to prevent cardiac apoptosis
Implantable devices are configured to be positioned in or near the heart and to carry and deliver an anti-apoptotic drug to a treatment site in or near the heart. The implantable devices include, but are not limited to, leads, stents, heart valves, atrial septal defect devices, cardiac patches and ventricular restraint devices. Depending on the composition of the device, the drug may be carried by the device through a coating applied to the device, or may be included in the device during the device manufacturing process. The drug may also be included in microparticles, such a microspheres, that are delivered locally through a conduit, such as a catheter.
Owner:CARDIAC PACEMAKERS INC
Curable media for implantable medical device
A subcutaneously formed in place orthopedic fixation device is provided, such as for fixation of the spine or other bone or bones. The device comprises an inflatable member, such as a tubular balloon. The balloon is positioned at a treatment site in the body while in a flexible, low crossing profile configuration. The balloon is thereafter inflated with a hardenable epoxy media comprising one or more epoxy compounds and one or more amine curing compounds that cures rapidly in place with low to moderate exotherm. Methods and delivery structures are also disclosed.
Owner:SDGI HLDG
Double-walled stent system
A double walled stent system particularly suited for treating abnormalities of the right ventricular outflow tract is disclosed having an exterior stent component and an interior stent component. The exterior stent component is secured to the interior stent component in a non-fixed, sliding relationship. The exterior stent component includes a plurality of longitudinally-extending connectors such as straight or sinusoidal bands. The interior stent component has a generally tubular cylindrical body and is centered within the exterior stent component. The stent system has a contracted delivery configuration and a radially expanded configuration for contacting the vessel wall. When deployed, the longitudinally-extending connectors of the exterior stent component come in contact with the vessel wall and fix the stent system to the treatment site. The interior stent component also radially expands but remains centered inside the exterior component and makes little to no contact with the vessel wall.
Owner:MEDTRONIC VASCULAR INC
Intravascular deliverable stent for reinforcement of vascular abnormalities
InactiveUS20070168019A1Avoid interactionReduce overall outer diameterStentsCatheterVascular Skin TumorSaphenous veins
A catheter deliverable stent / graft especially designed to be used in a minimally invasive surgical procedure for treating a variety of vascular conditions such as aneurysms, stenotic lesions and saphenous vein grafts, comprises an innermost tubular structure and at least one further tubular member in coaxial arrangement. In one embodiment, the innermost tubular structure is of a length (L1) and is formed by braiding a relatively few strands of highly elastic metallic alloy. The pick and pitch of the braid are such as to provide relative large fenestrations in the tubular wall that permit blood flow through the wall and provide the primary radial support structure. A portion of the innermost tubular structure of a length L1 is surrounded by a further braided tubular structure having relatively many strands that substantially inhibit blood flow through the fenestrations of the innermost tubular structure. The composite structure can be stretched to reduce the outer diameter of the stent / graft, allowing it to be drawn into a lumen of a delivery catheter. The catheter can then be advanced through the vascular system to the site of treatment and then released, allowing it to self-expand against the vessel wall. Various optional embodiments are disclosed that allow one skilled in the art to tailor the design to the specific application.
Owner:ST JUDE MEDICAL CARDILOGY DIV INC
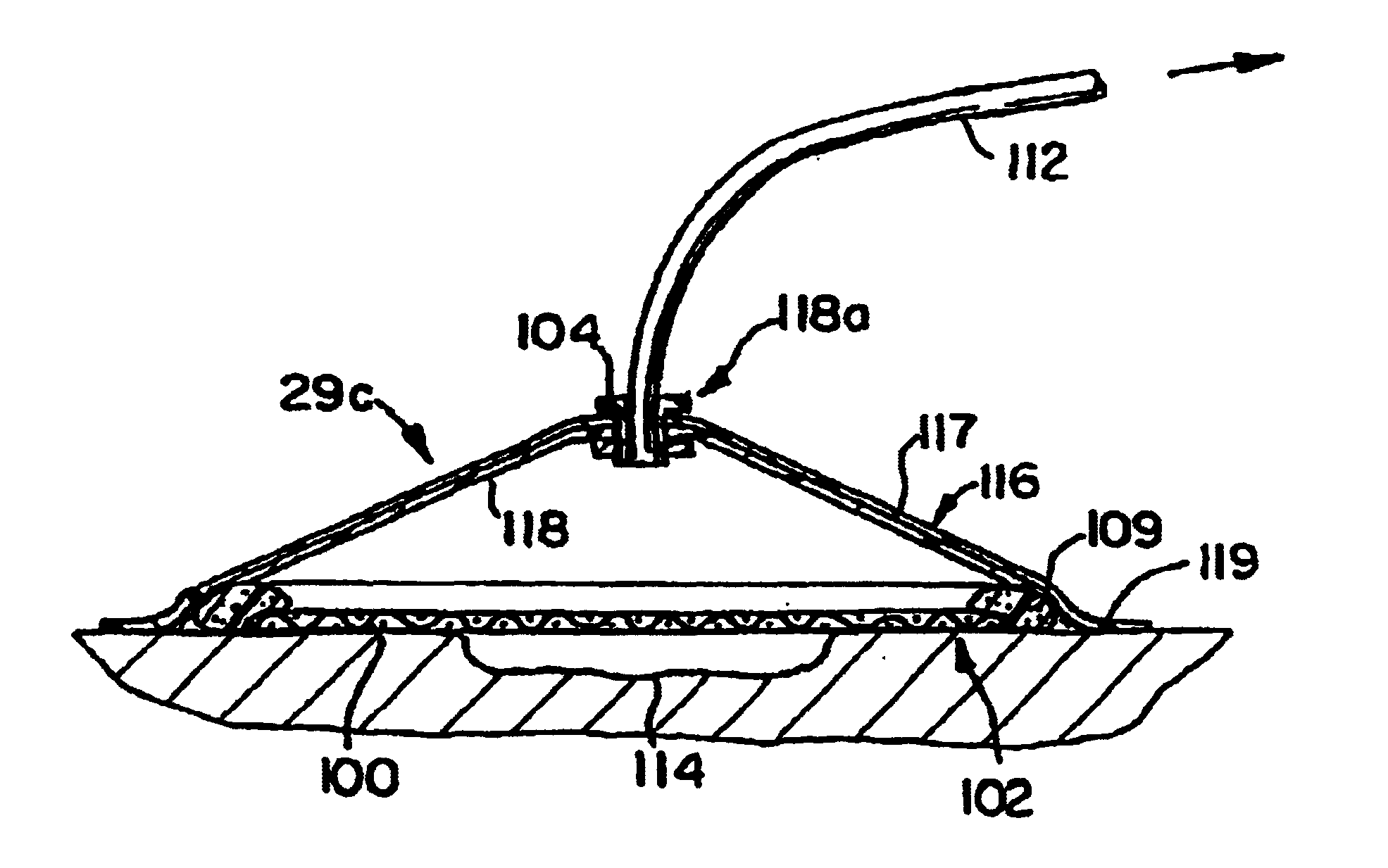
Slidable Fixation Device for Securing a Medical Implant
A fixation device for retaining a leadless medical implant to tissue includes an array of elongate tines having self-expanding distal portions. The fixation tines may be advanced between an implant body and an outer jacket to deploy the tines from a delivery configuration in which the tines are constrained by the outer jacket to an expanded configuration in which the distal end portions of the tines are released from the outer jacket. The implant and fixation device are contained within a sheath for delivery to the treatment site and a pusher within the sheath advances the fixation device relative to the implant body and deploys the tines. A distal end of the implant having an electrode may form a distal tip of the delivery system, and a potential implantation site may be tested prior to deployment of the fixation device to allow for easy repositioning of the implant.
Owner:MEDTRONIC VASCULAR INC
Fixation devices for variation in engagement of tissue
InactiveUS20060089671A1Robust graspReduce refluxHeart valvesSurgical veterinaryBiomedical engineeringBlood vessel
Owner:EVALVE
Formed in place fixation system with thermal acceleration
InactiveUS20050234453A1High normal sclerosisInternal osteosythesisDilatorsOrthopedic fixation devicesThermal acceleration
A subcutaneously formed in place orthopedic fixation device is provided, such as for fixation of the spine or other bone or bones. The device comprises an inflatable member, such as a tubular balloon. A heat source is provided in thermal communication with the interior of the balloon. The balloon is positioned at a treatment site while in a flexible, low crossing profile configuration. The balloon is thereafter inflated with a hardenable media, and heated to accelerate hardening of the media. Methods and delivery structures are also disclosed.
Owner:WARSAW ORTHOPEDIC INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com