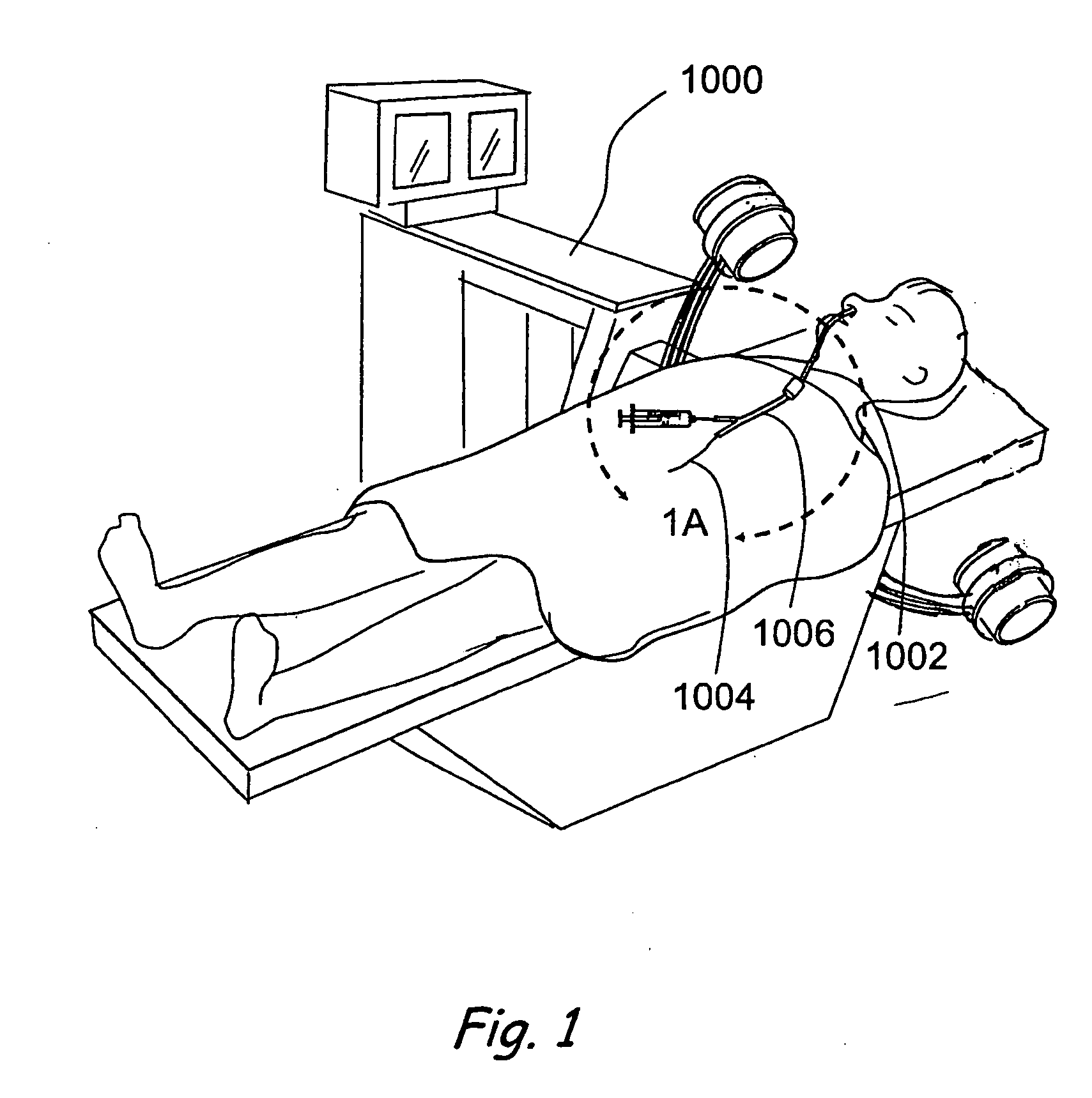
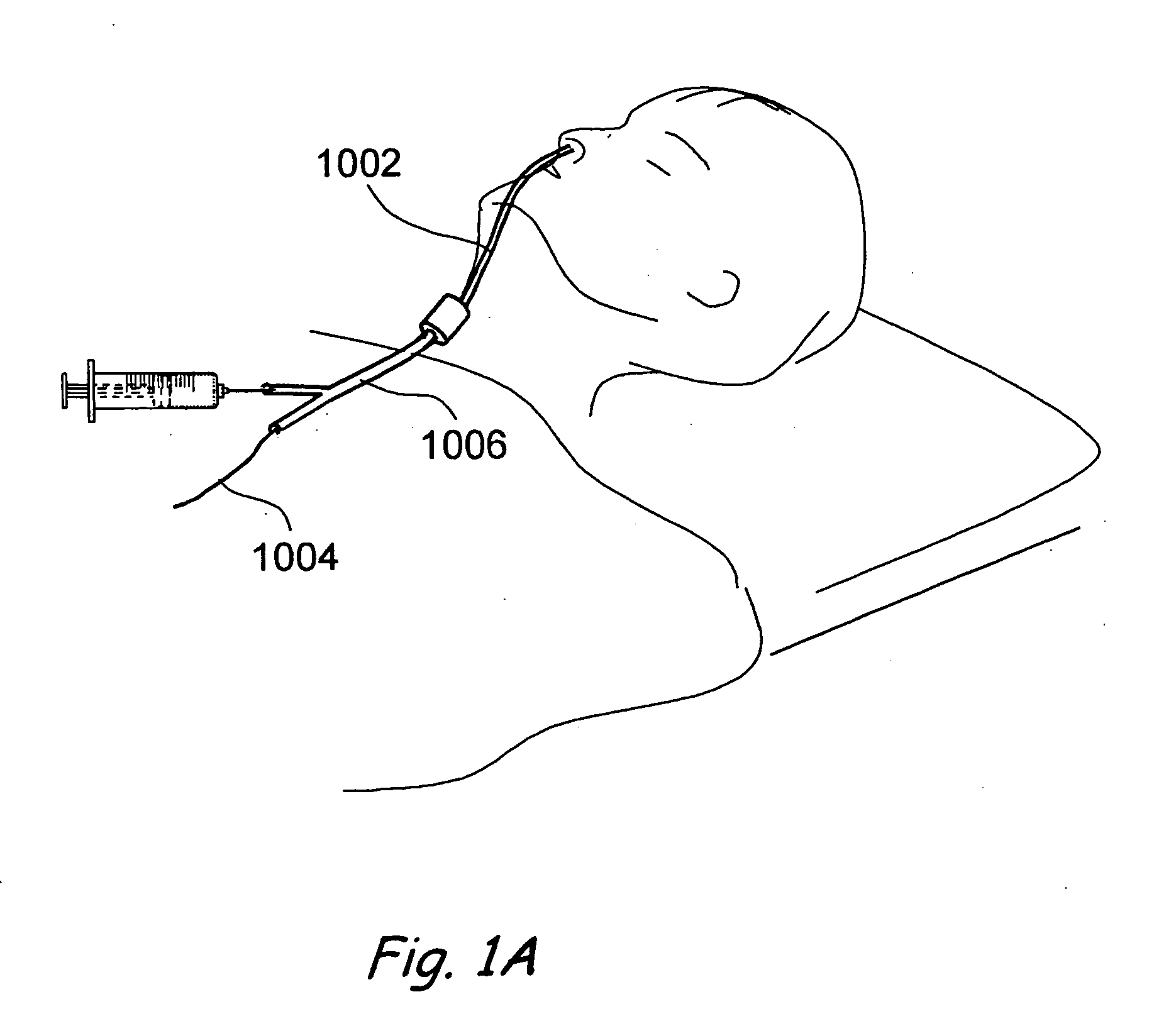
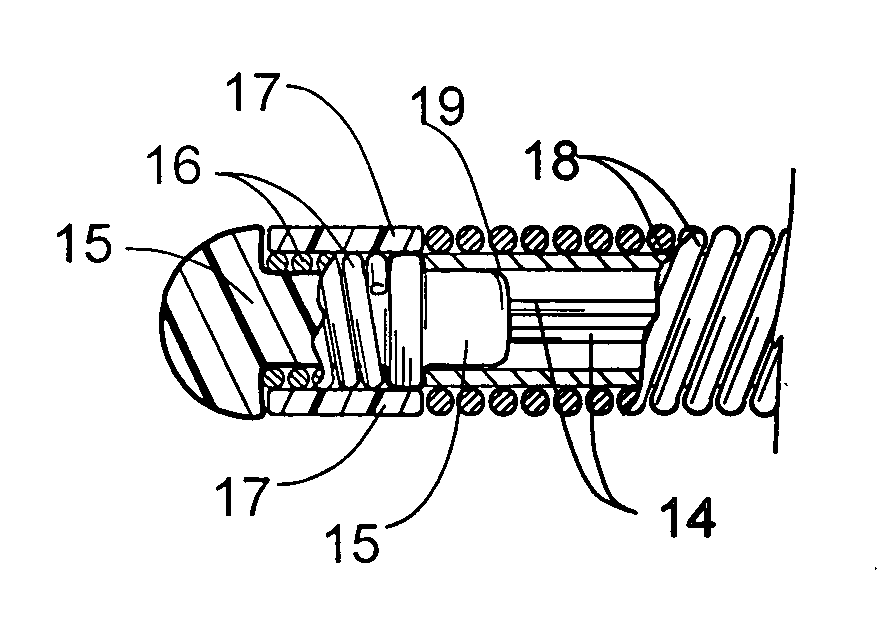
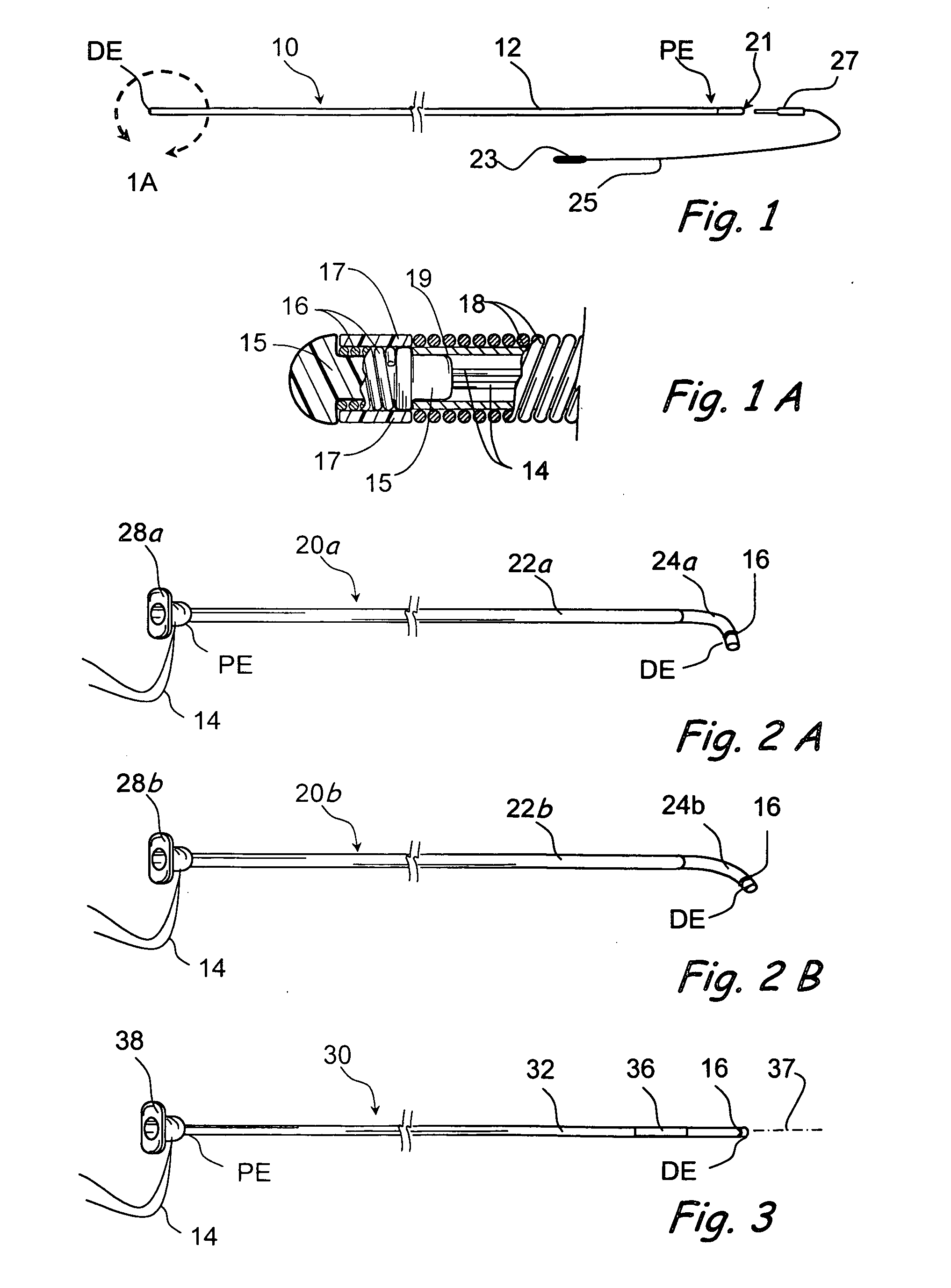
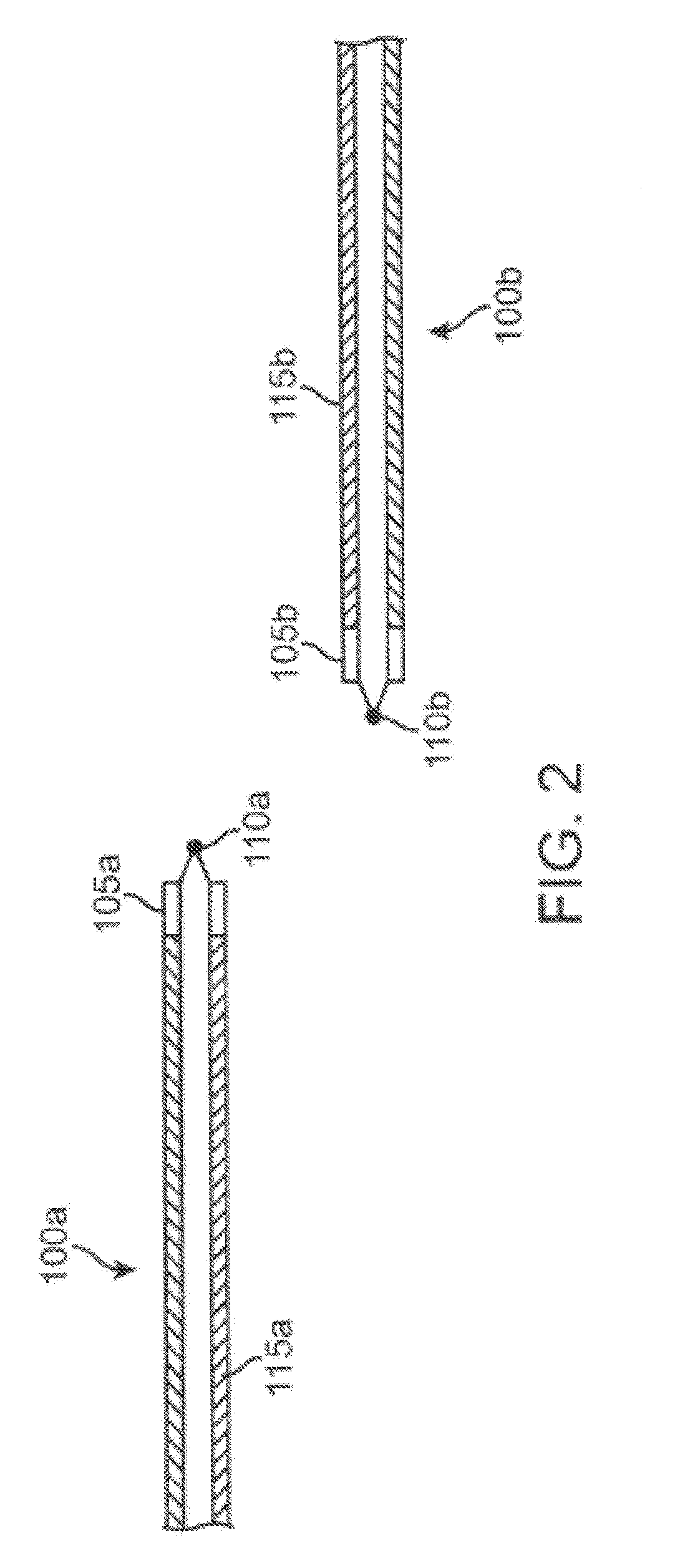
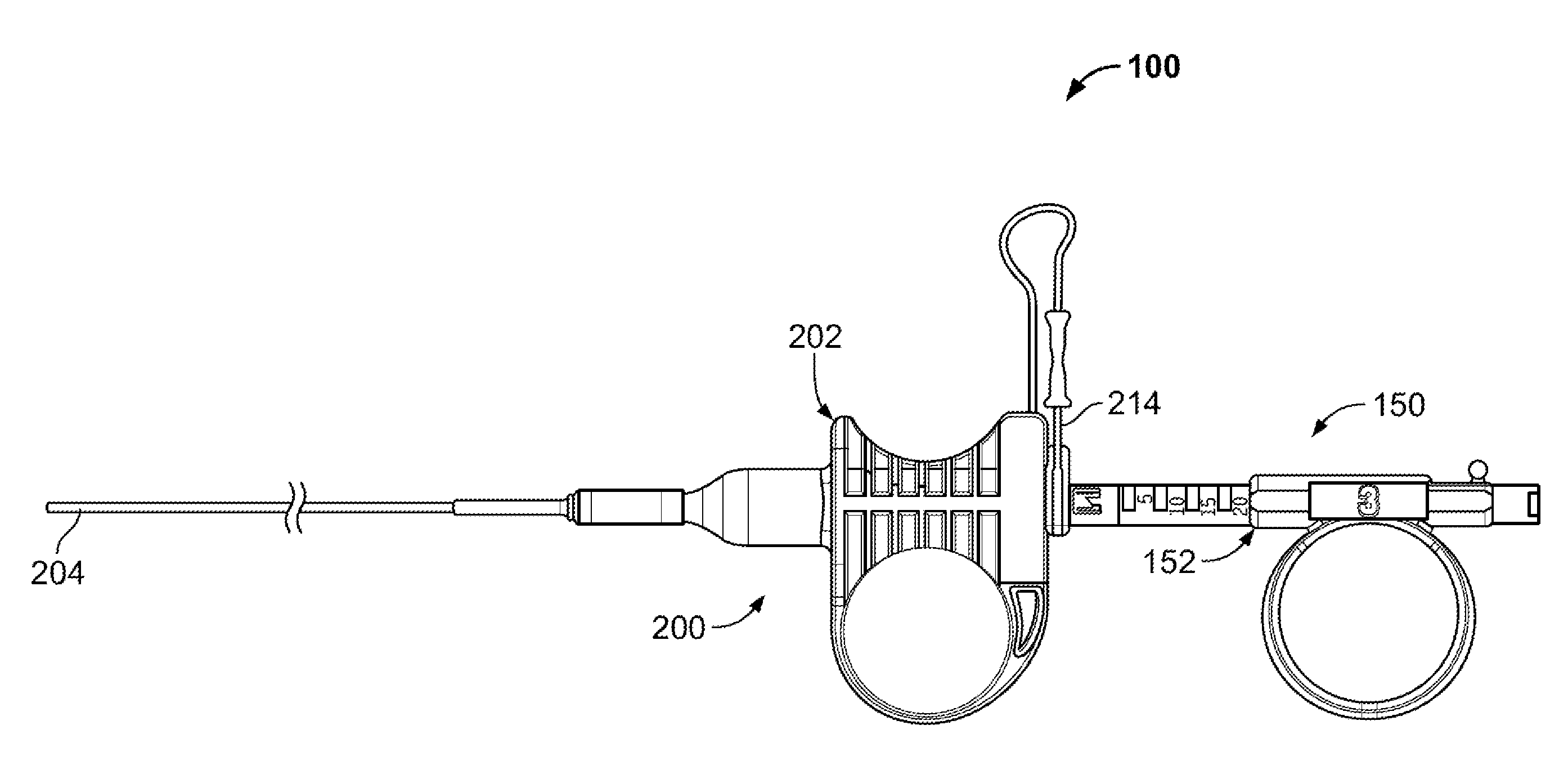
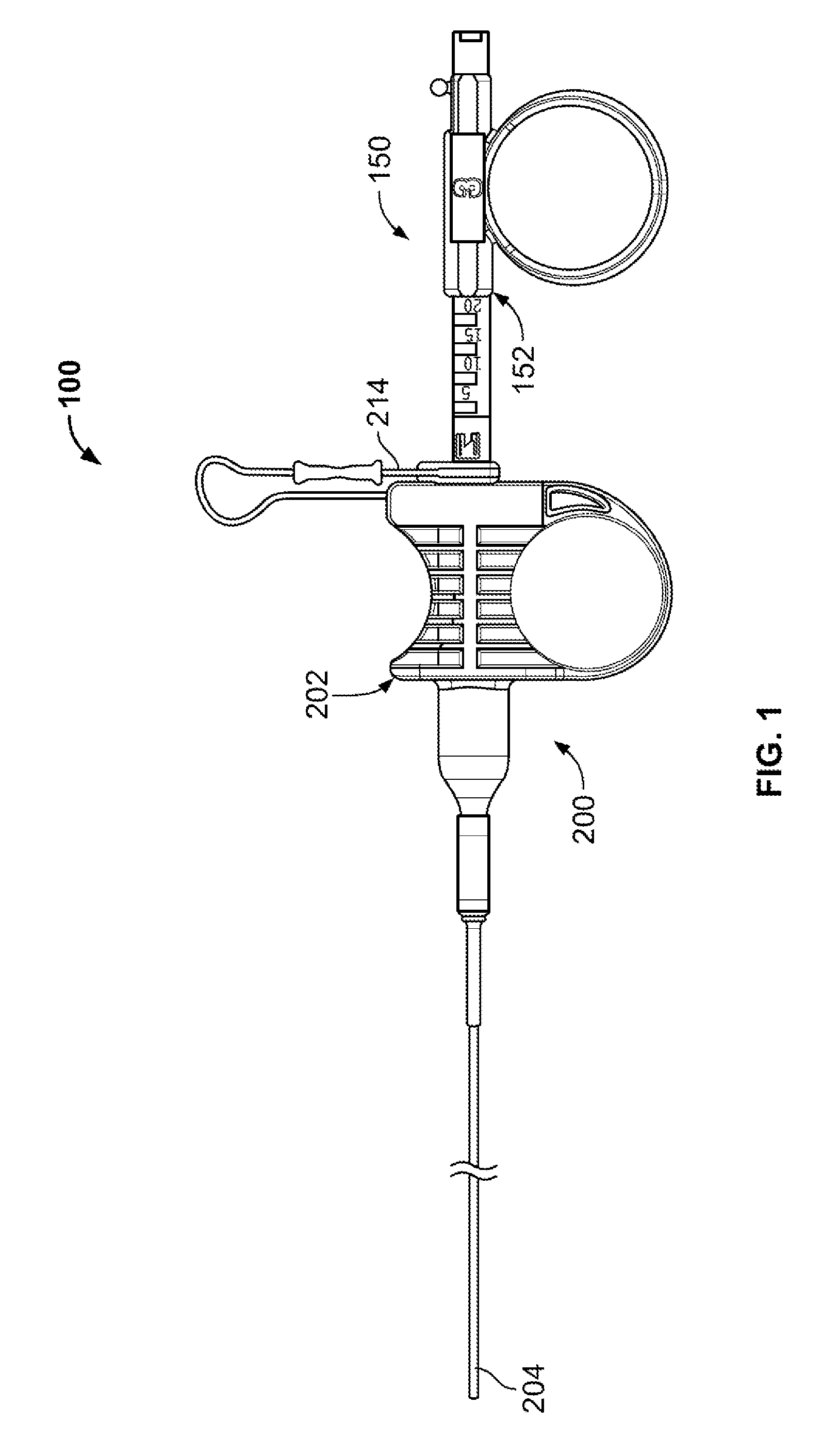
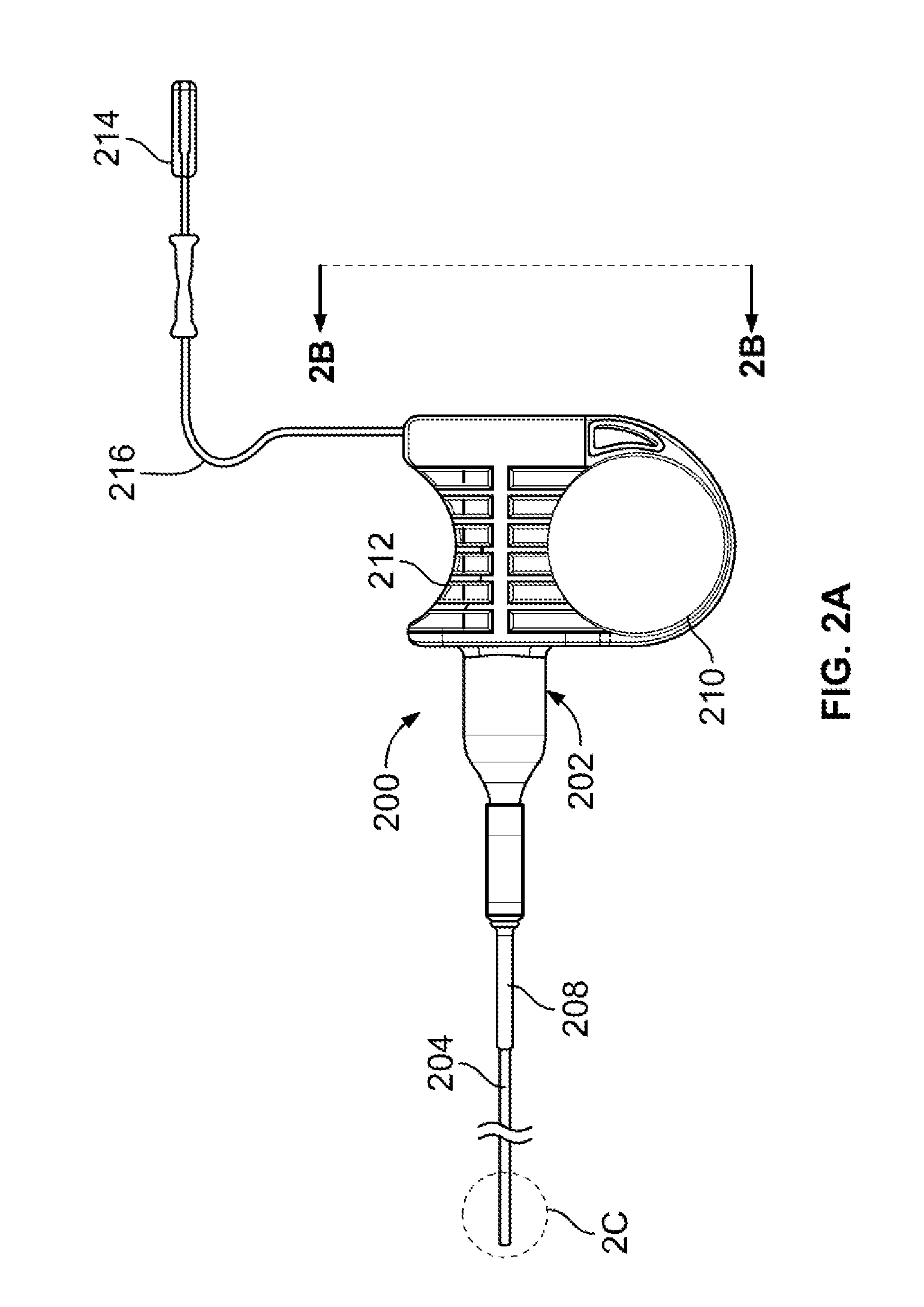
Patents
Literature
3523results about "Guide wires" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
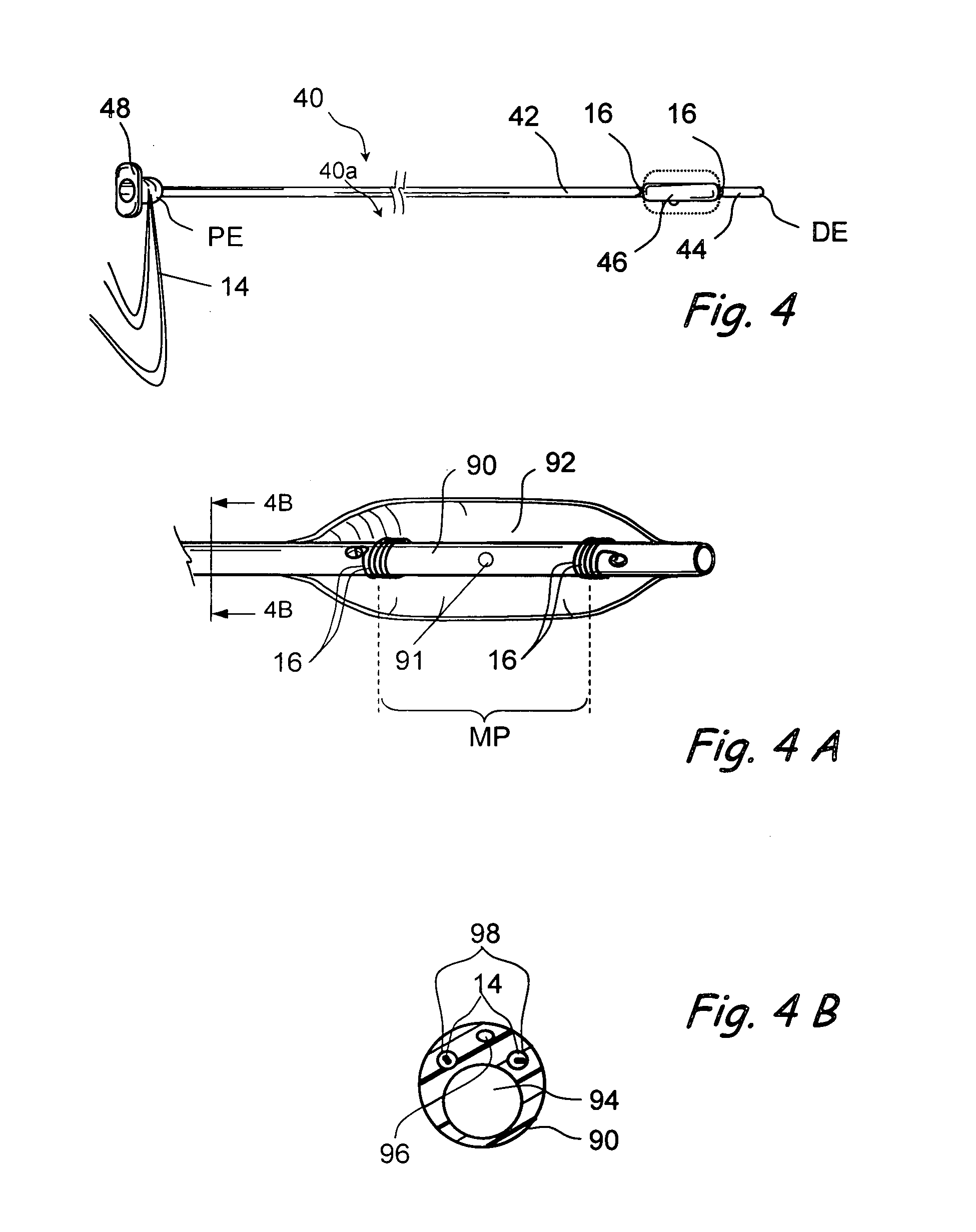
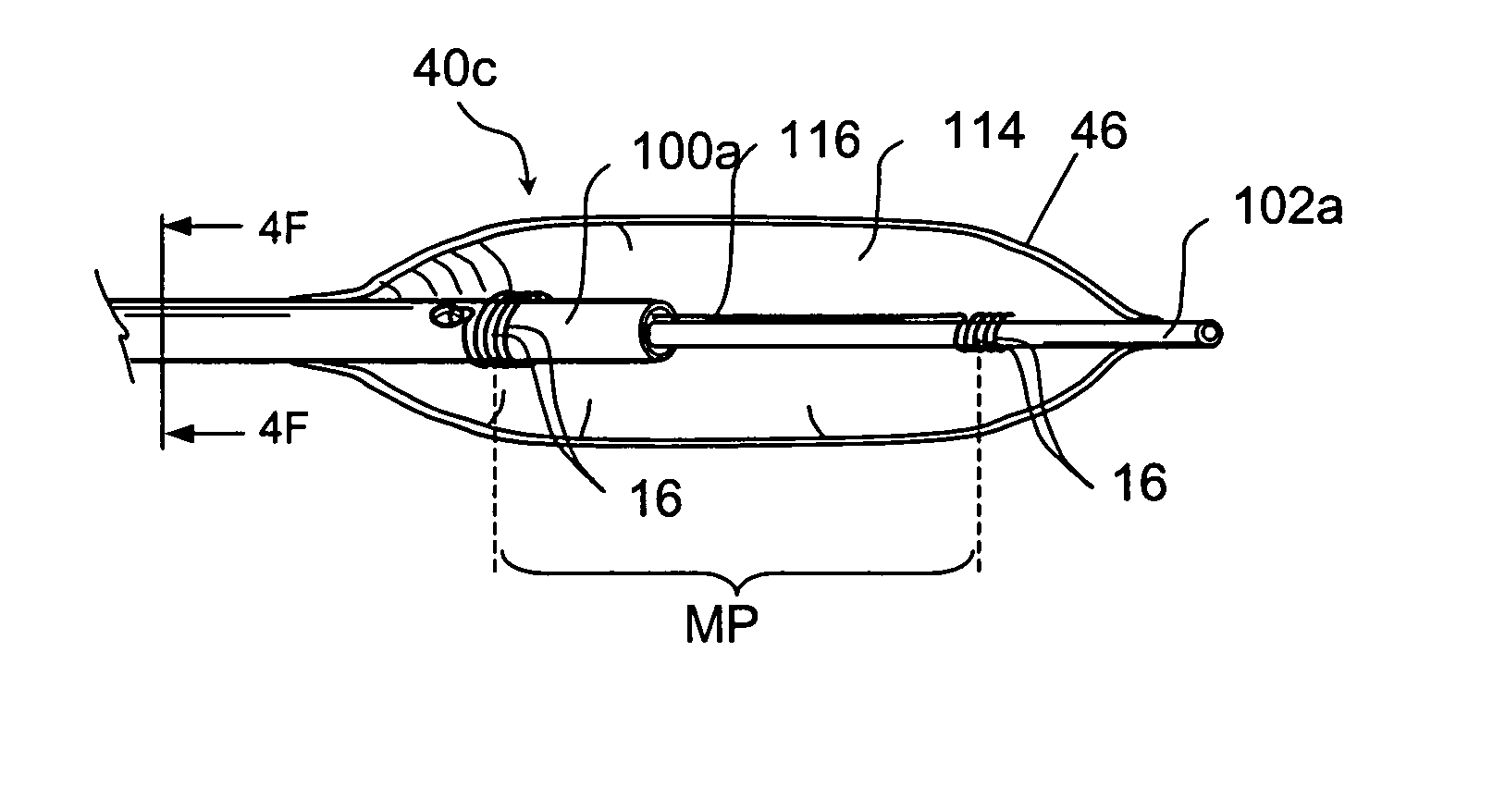
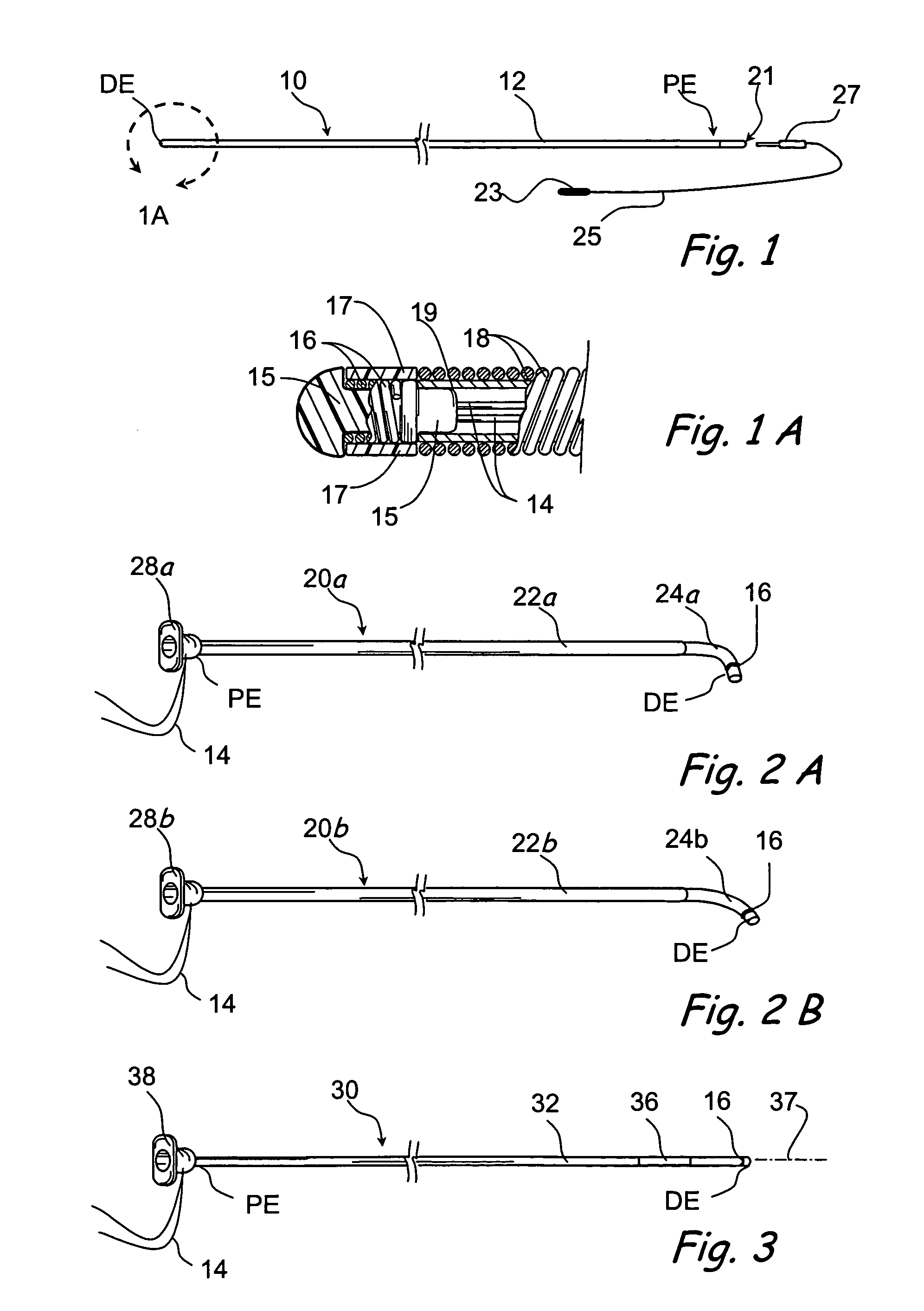
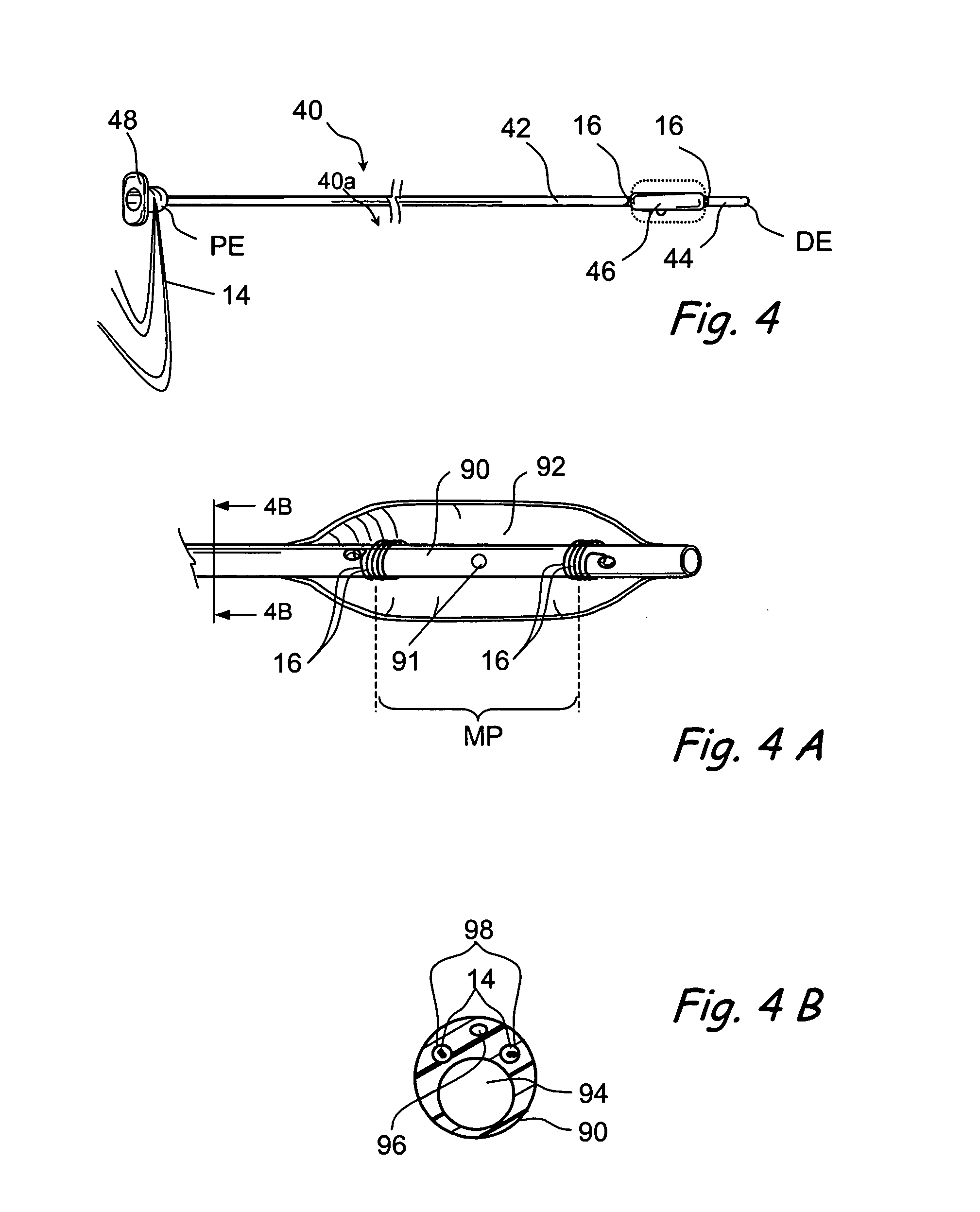
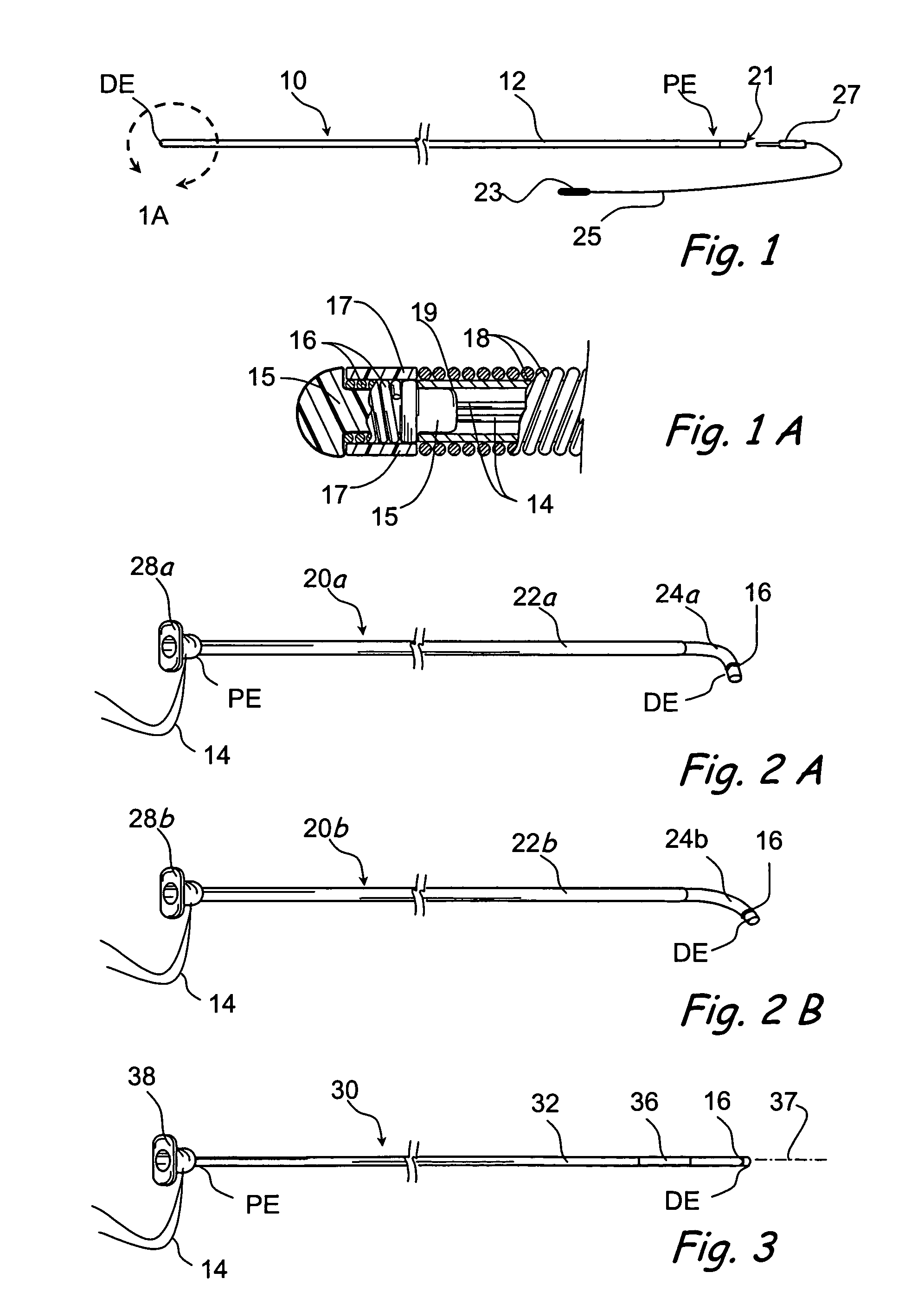
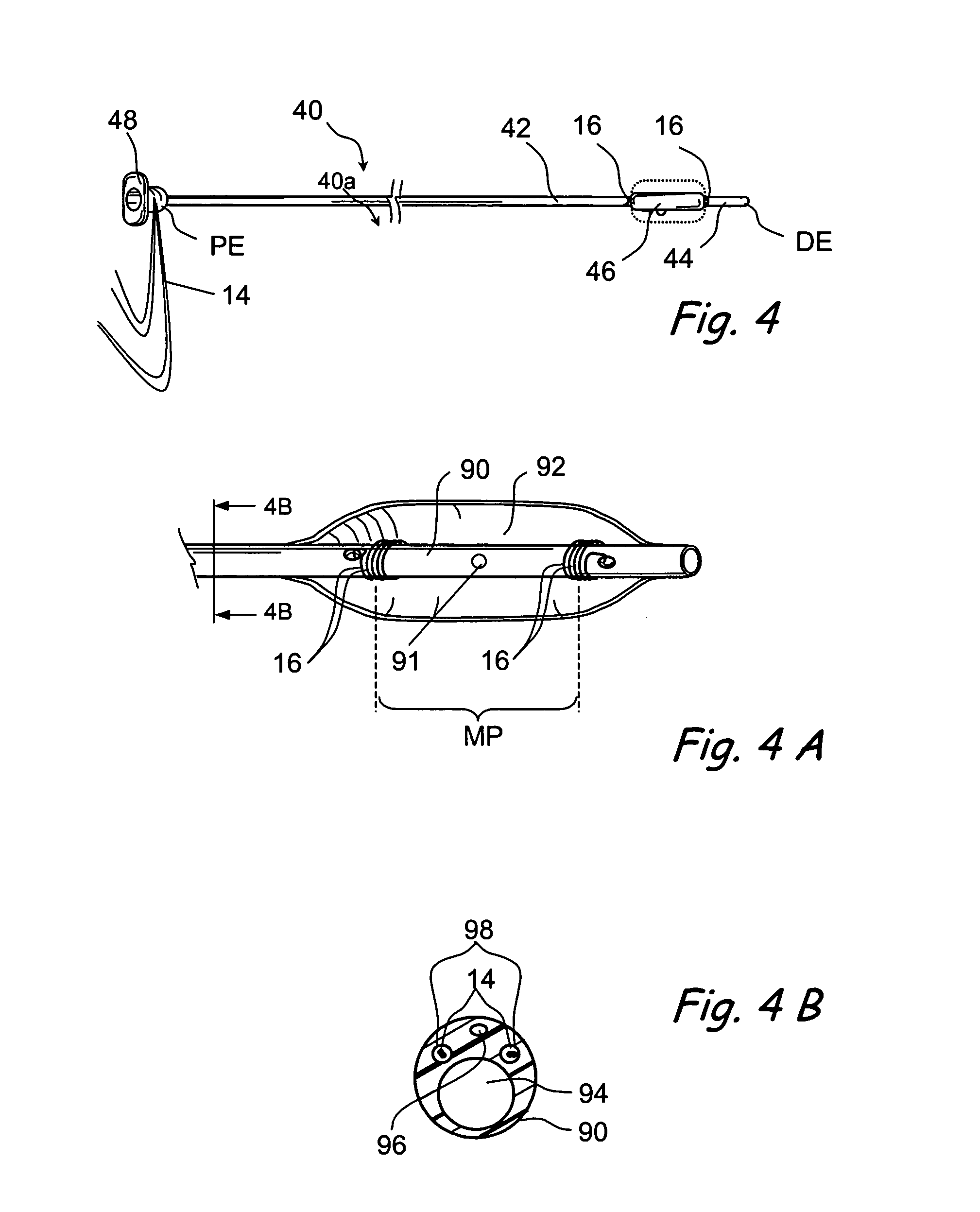
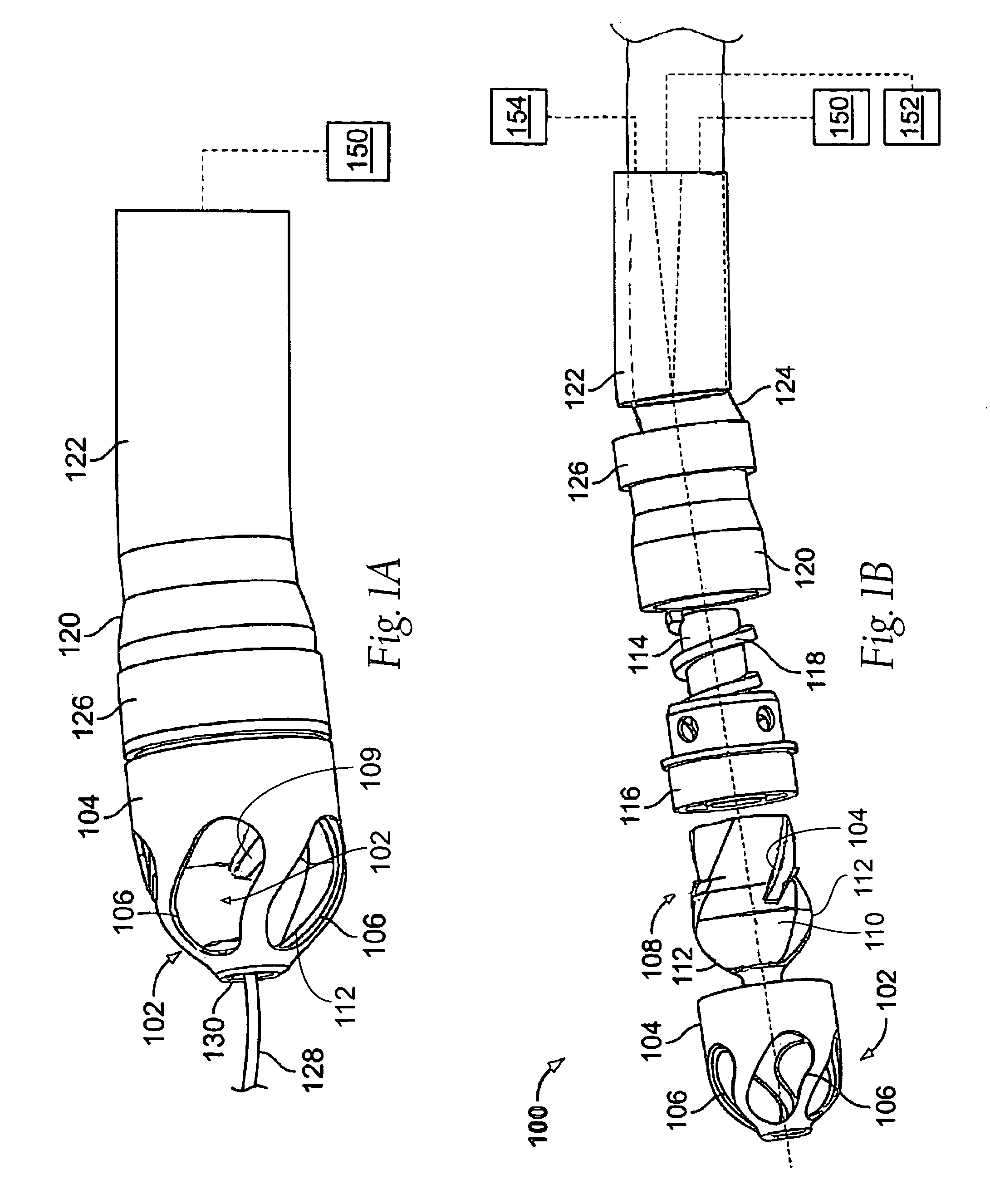
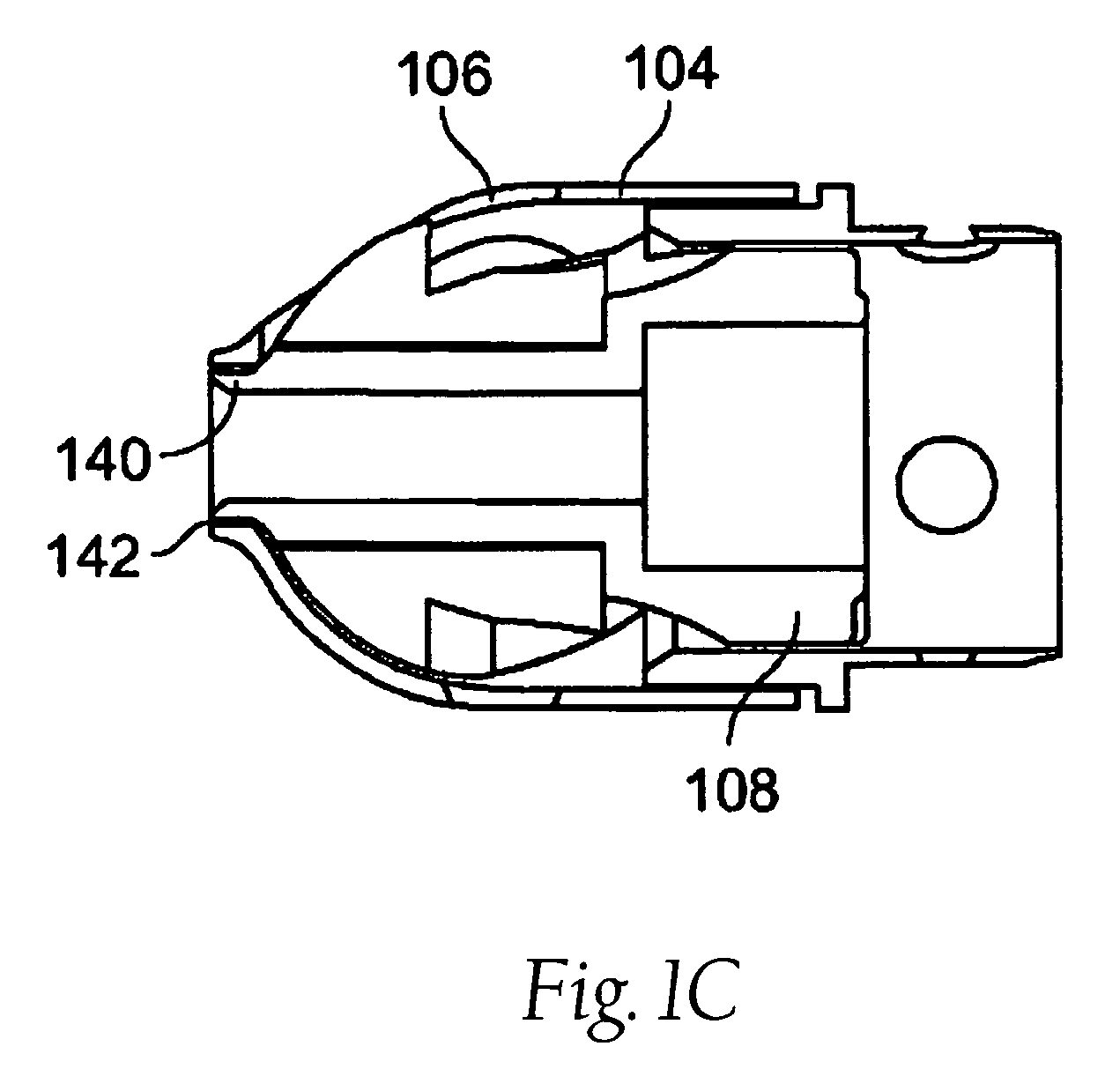
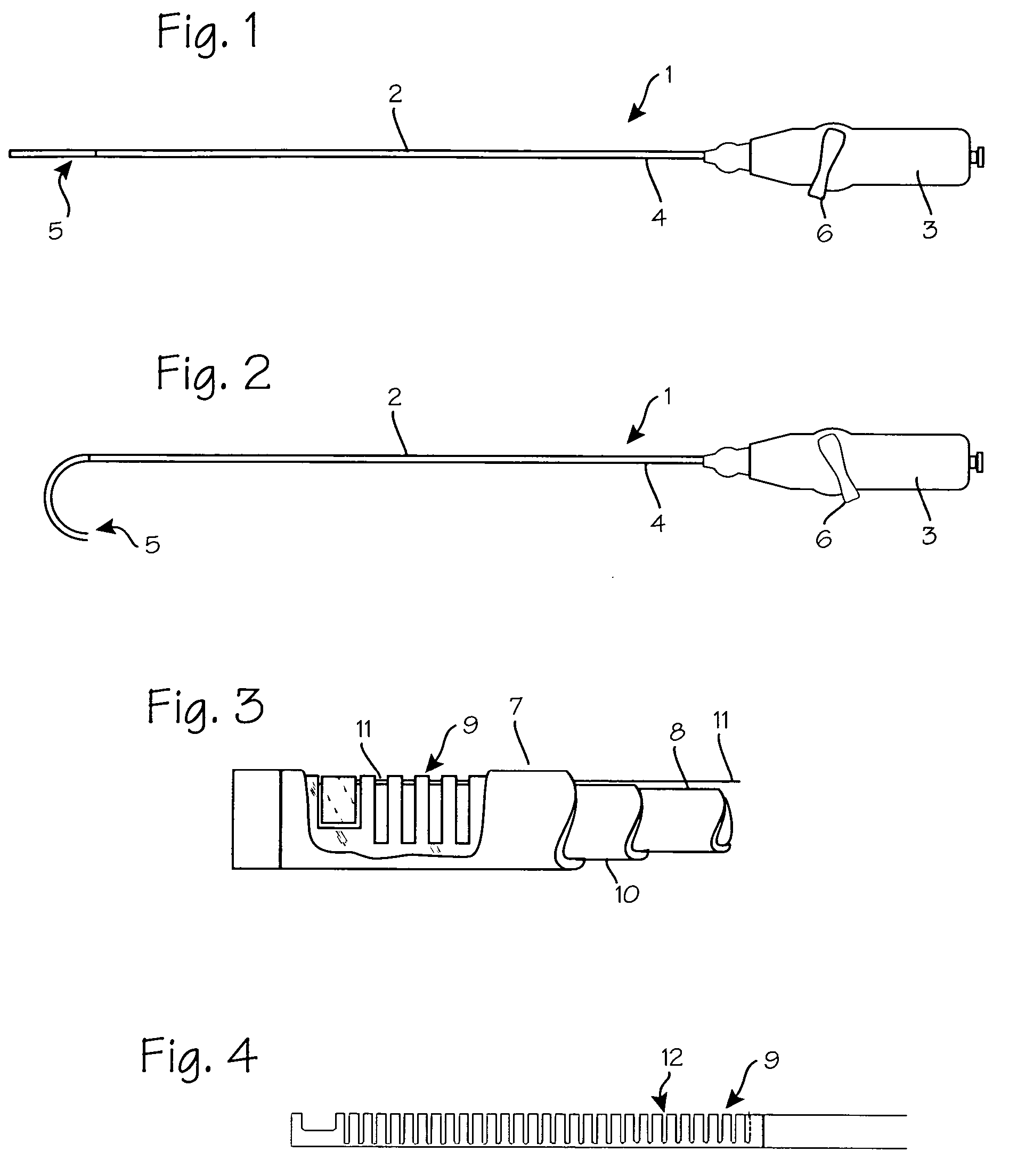
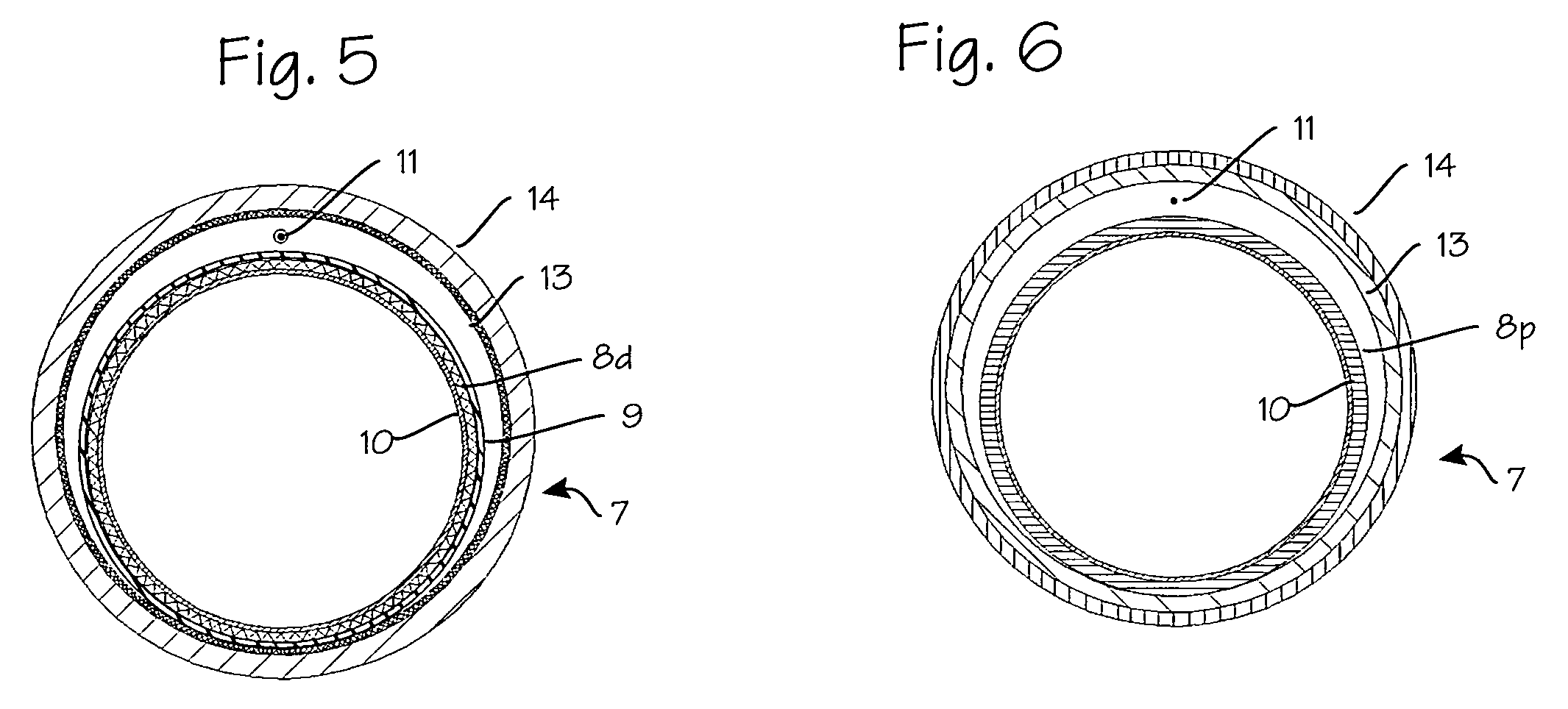
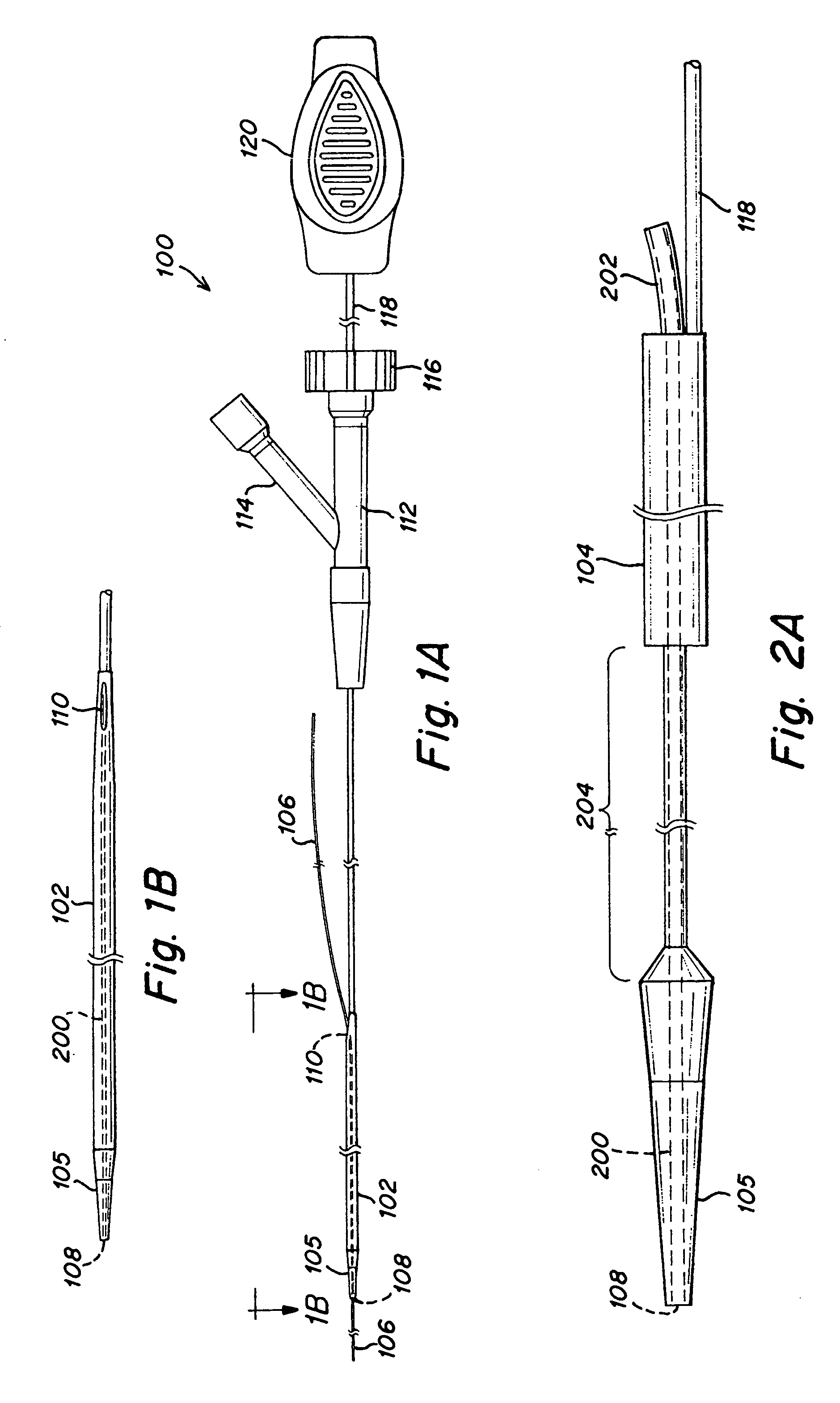
Tissue access guidewire system and method
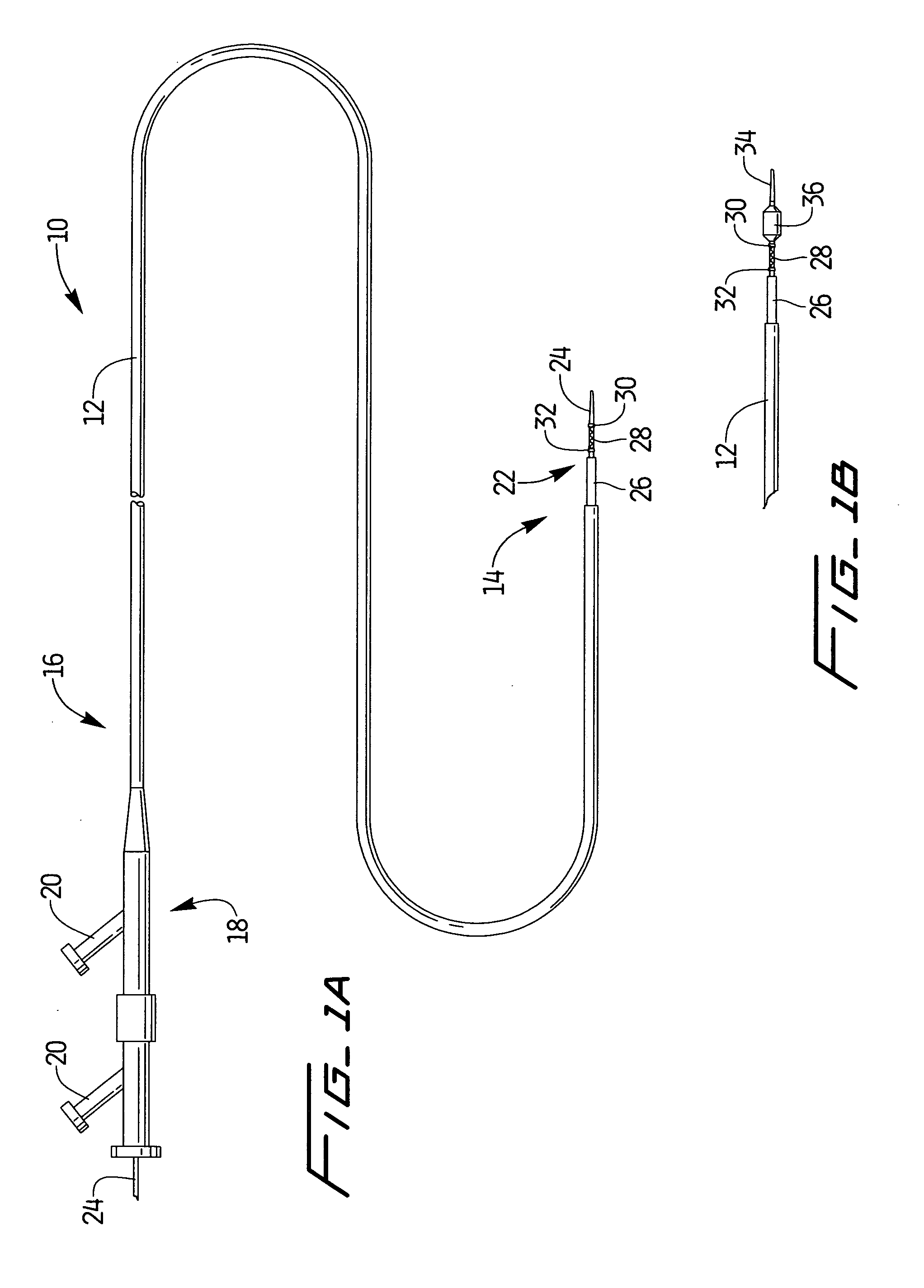
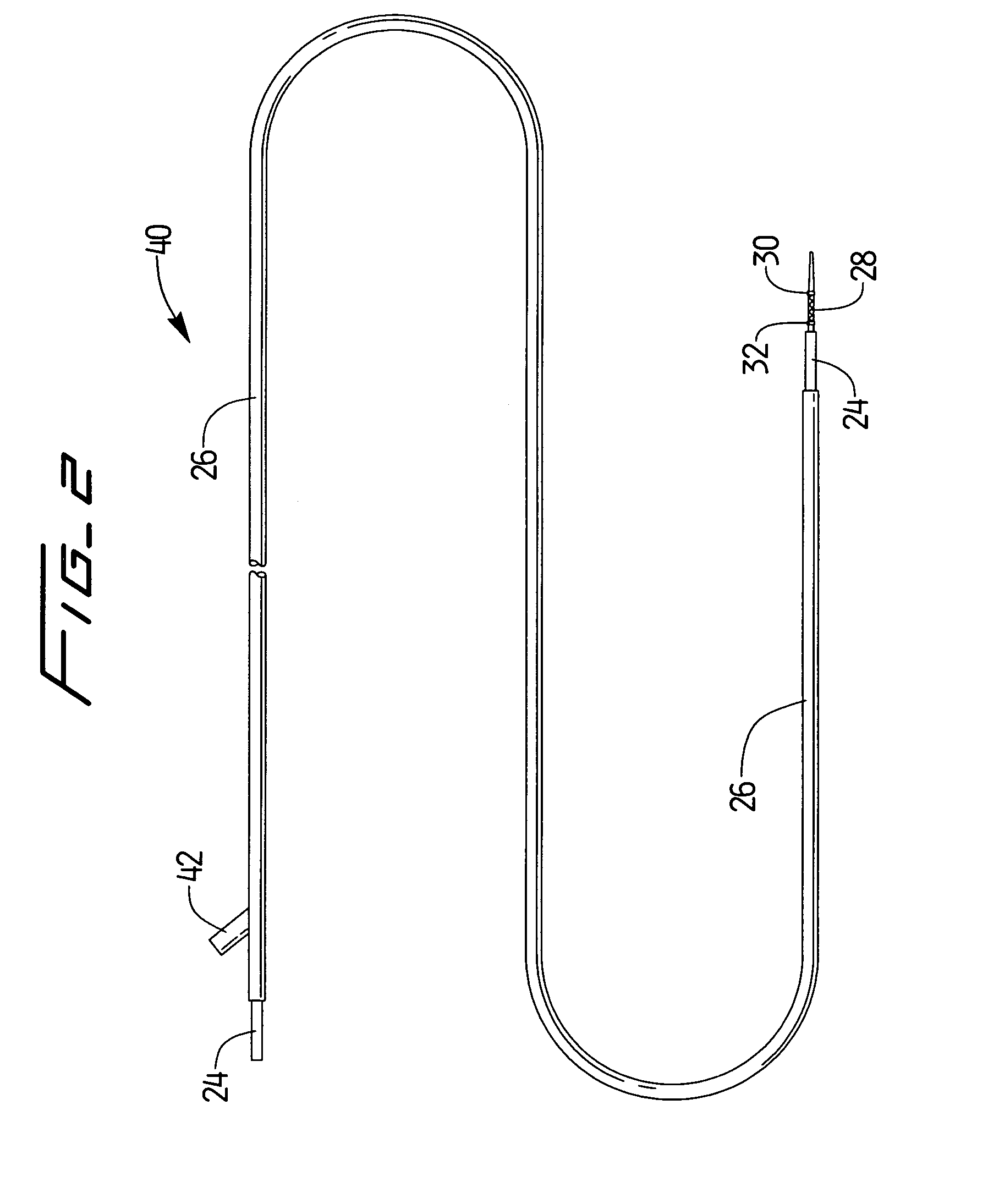
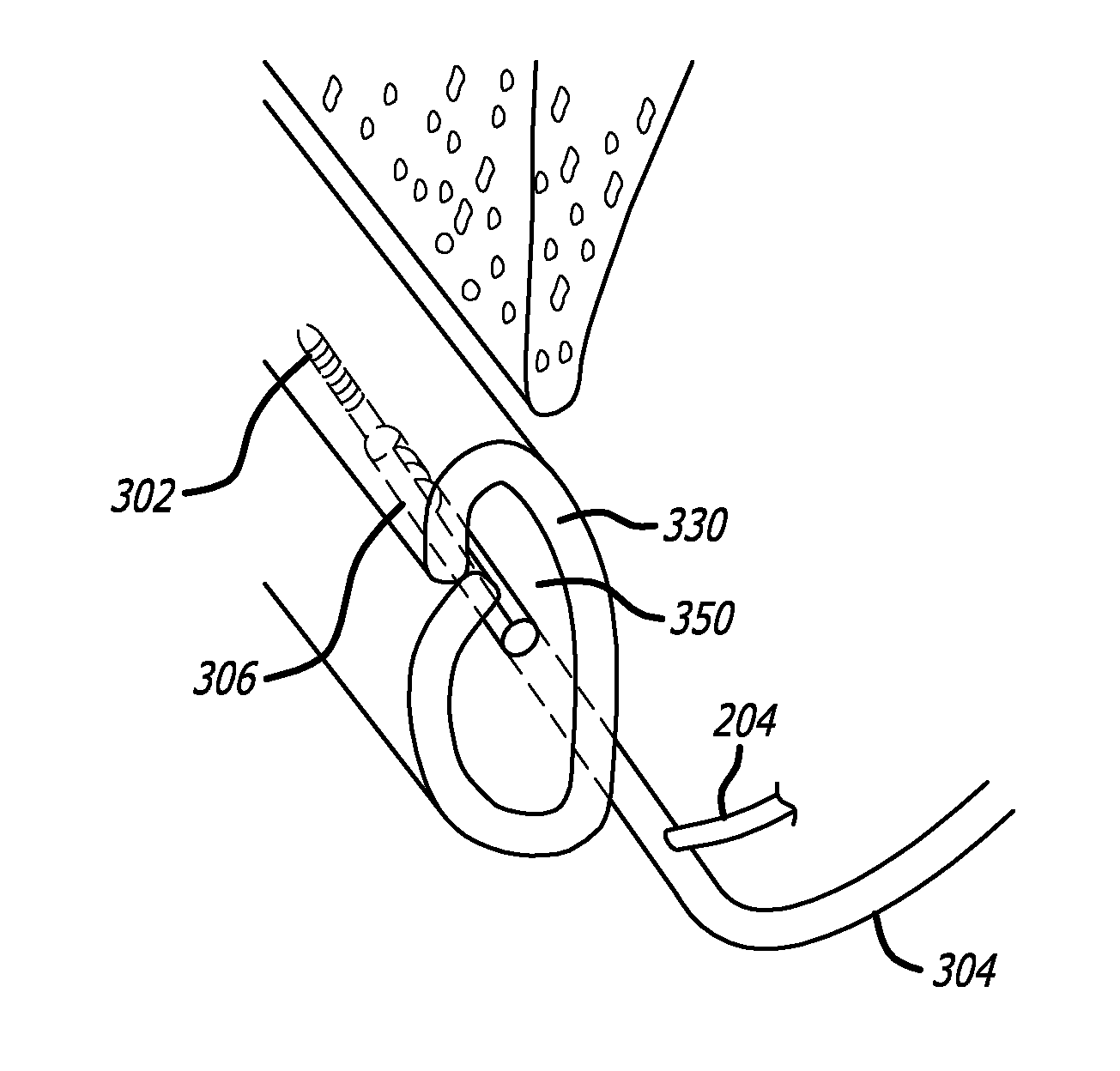
A method and system for guiding at least a portion of a surgical device to a desired position between two tissues in a patient's body involves coupling a guidewire to the device and pulling the distal end of the guidewire to guide at least a portion of the surgical device to a desired position between the two tissues. The surgical device generally includes one or more guidewire coupling members and may comprise a tissue access device. A system may include a guidewire and a surgical device. In some embodiments, a guidewire, a tissue access device, and one or more additional devices to use with the access device may be provided. Methods, devices and systems may be used in open, less-invasive or percutaneous surgical procedures, in various embodiments.
Owner:MIS IP HLDG LLC +1
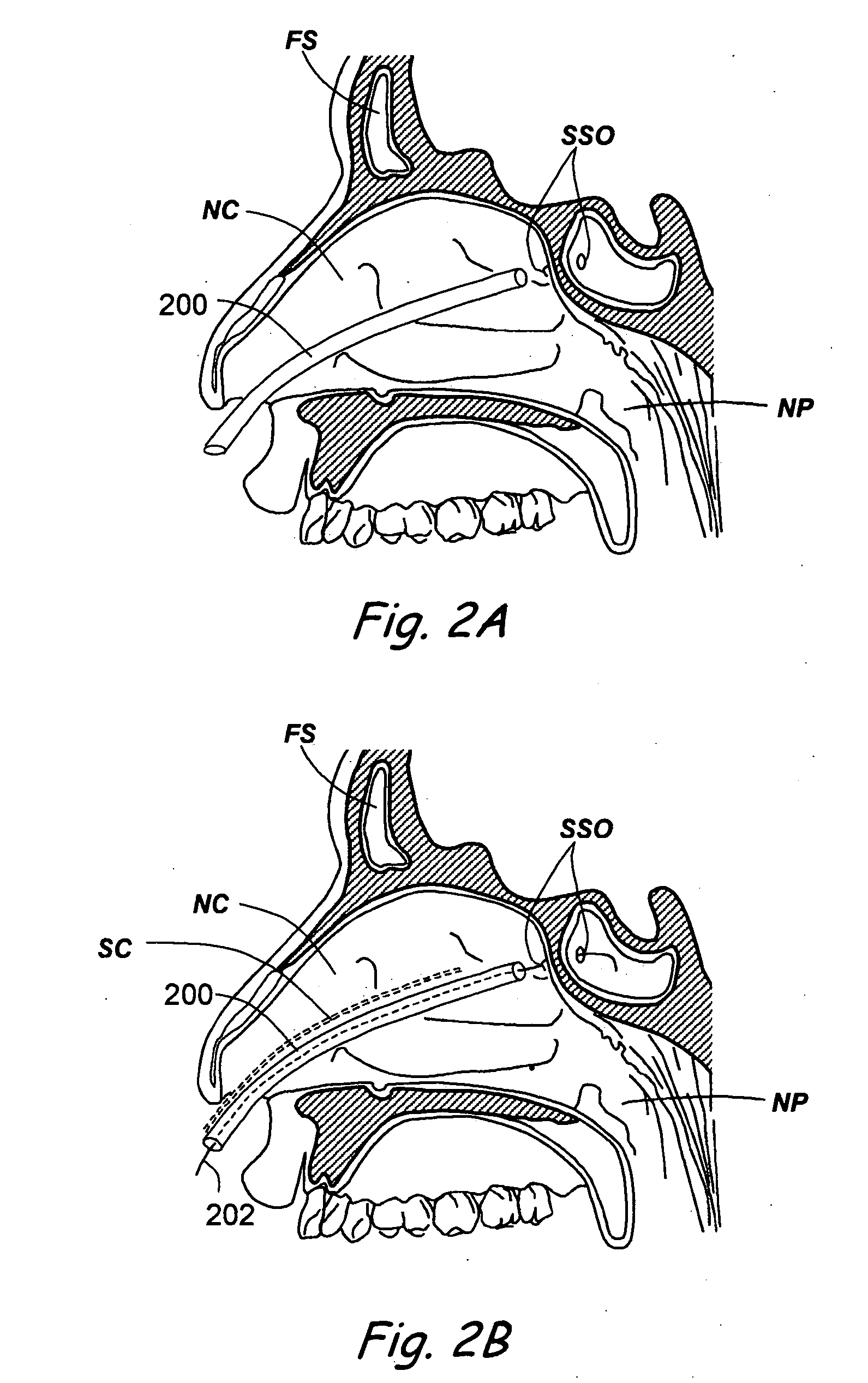
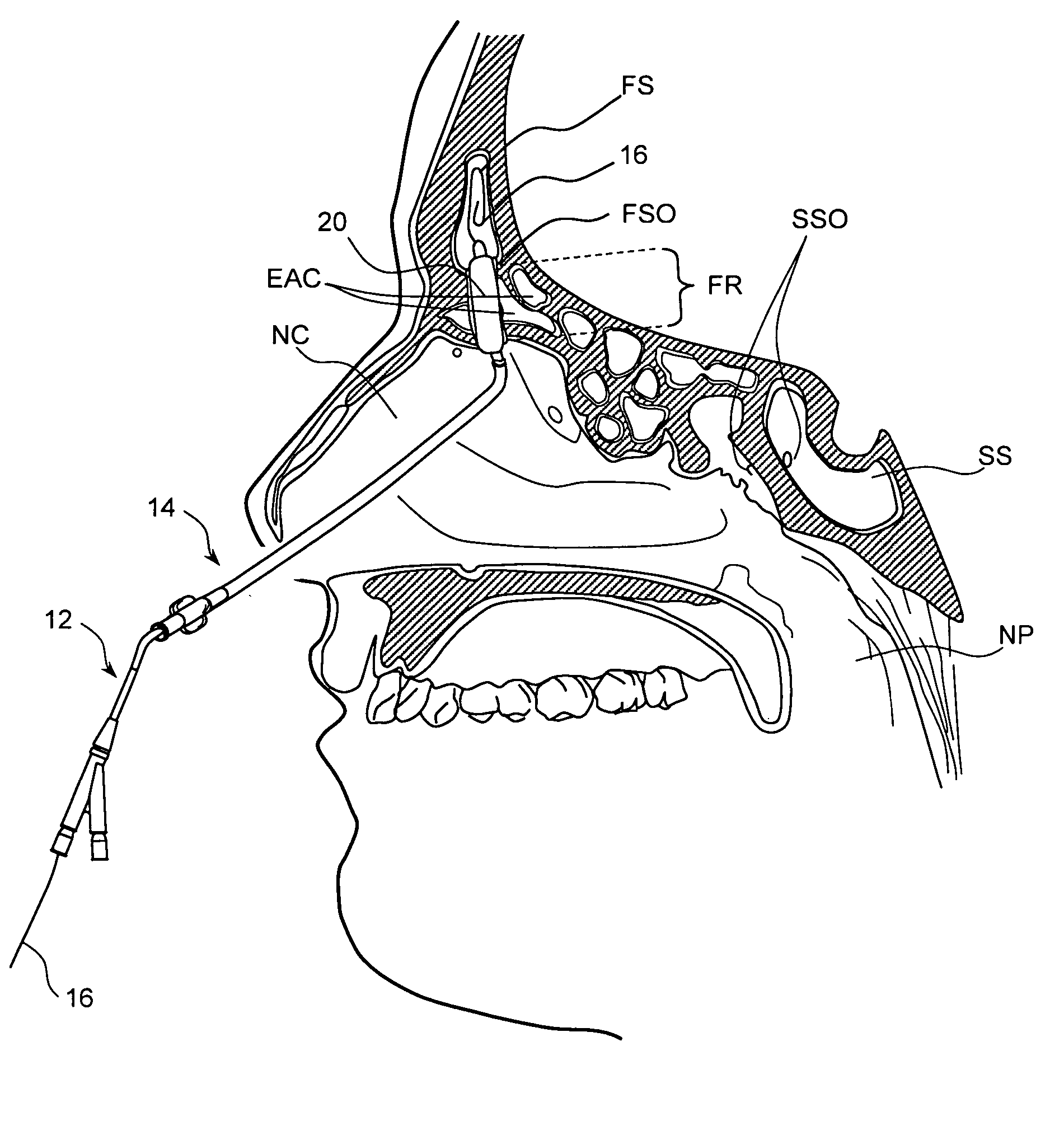
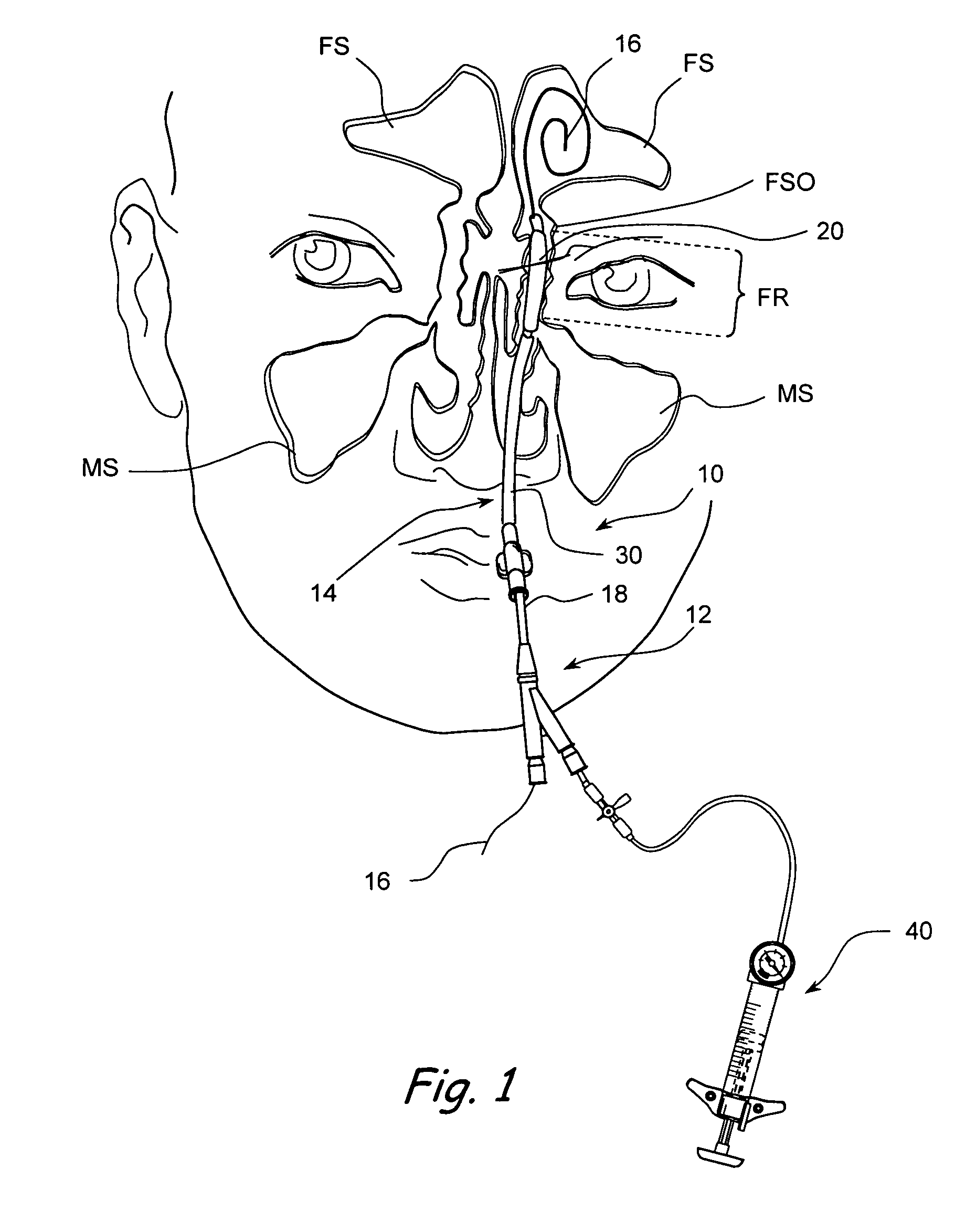
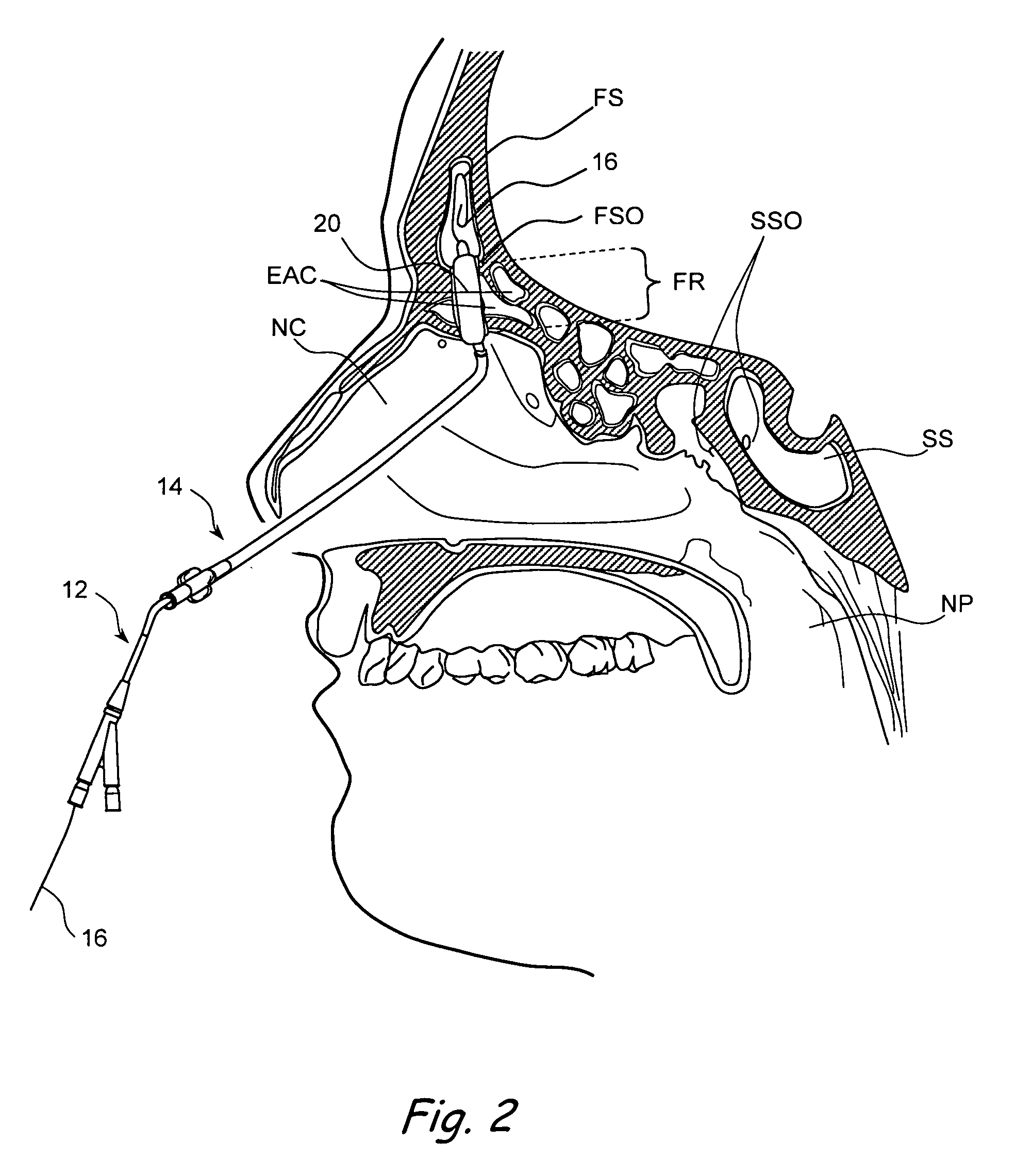
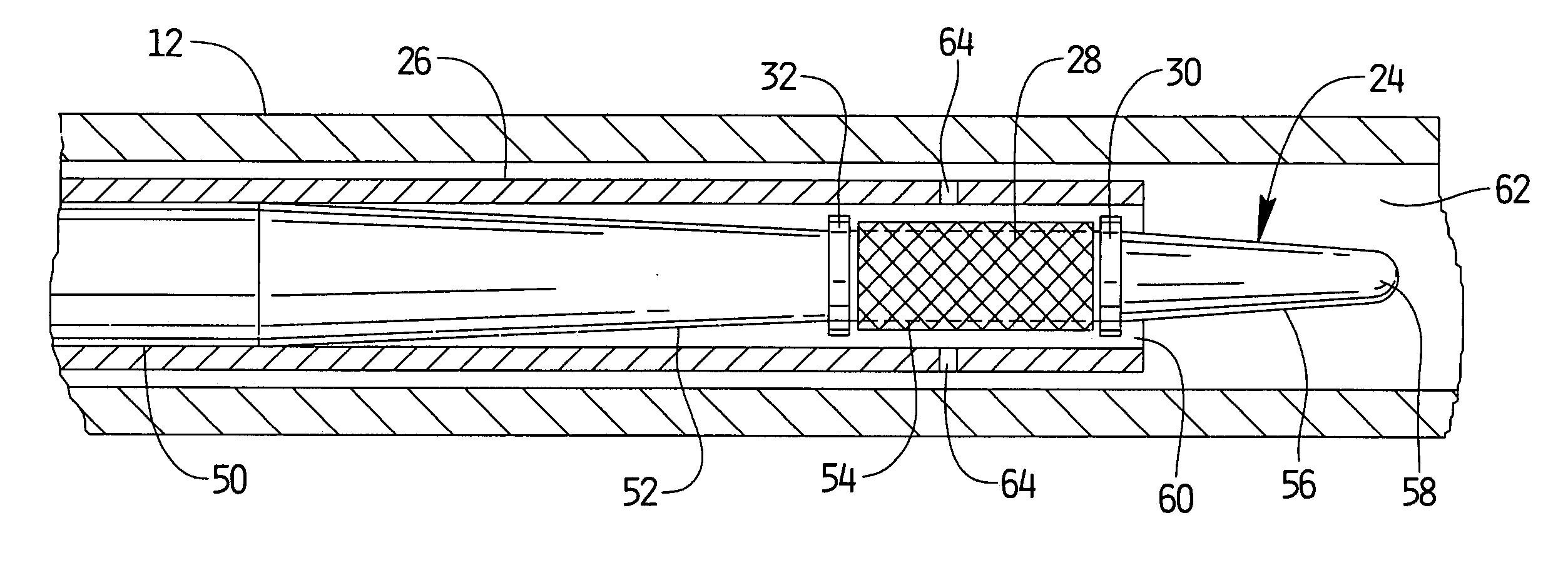
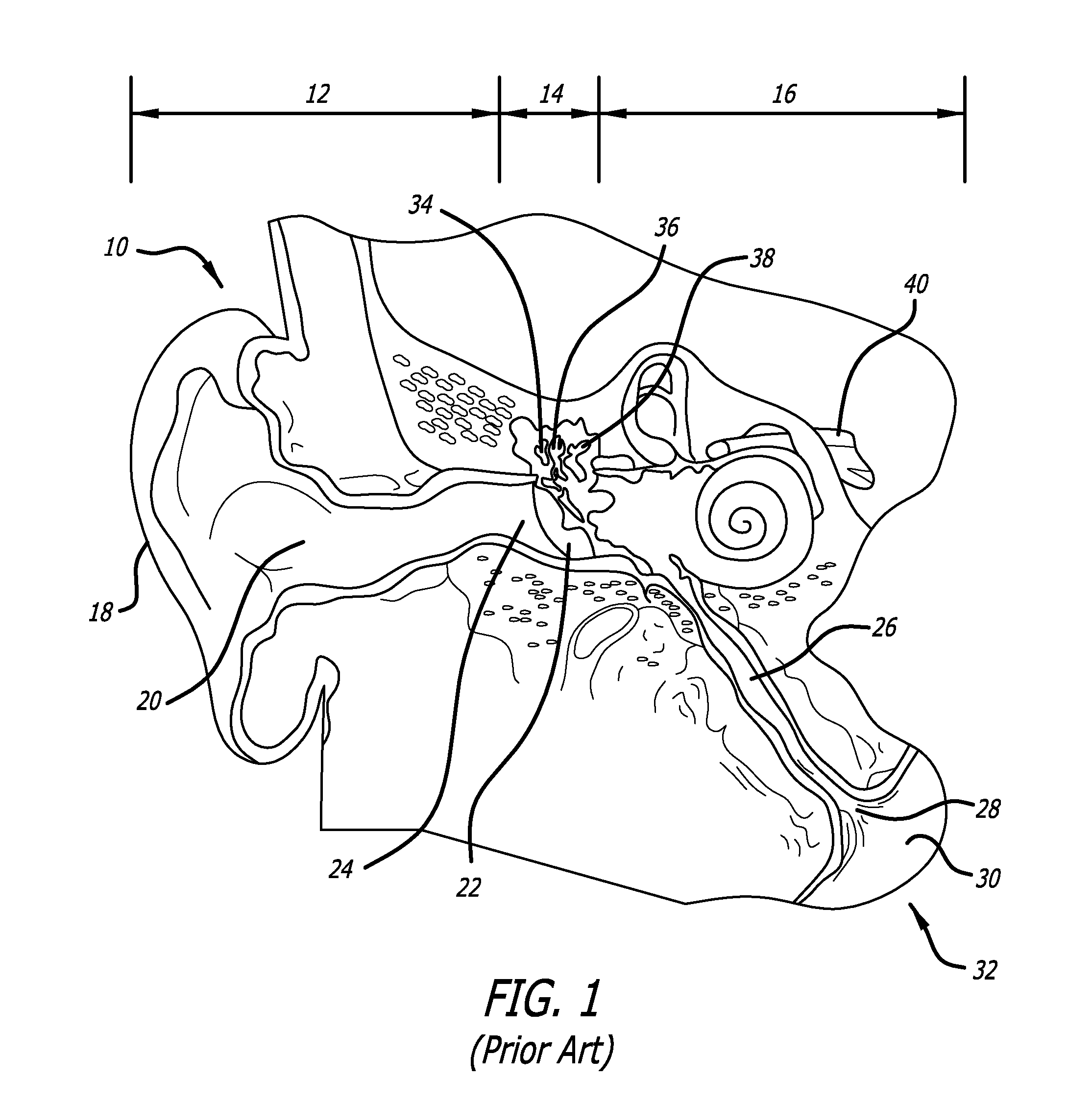
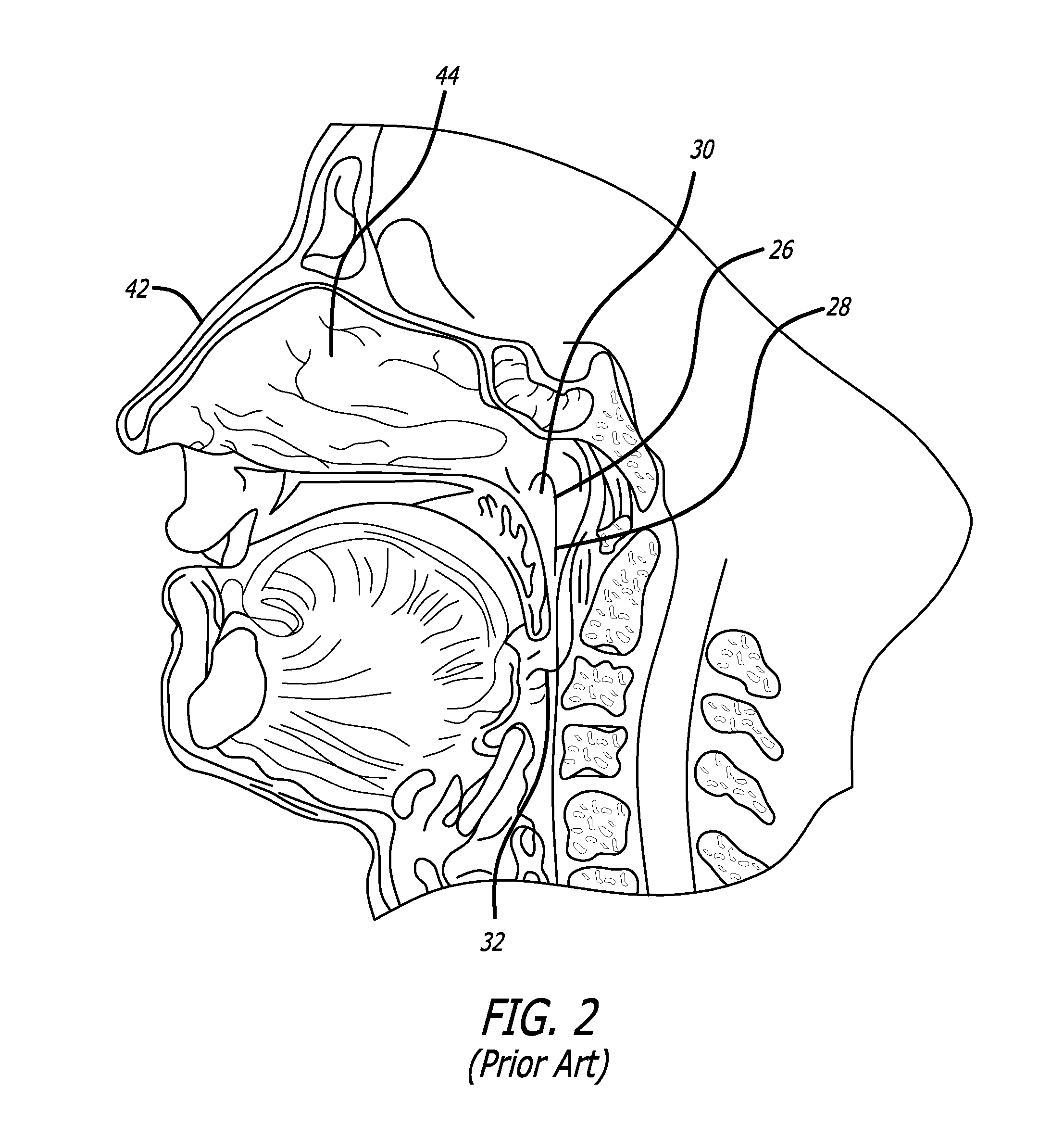
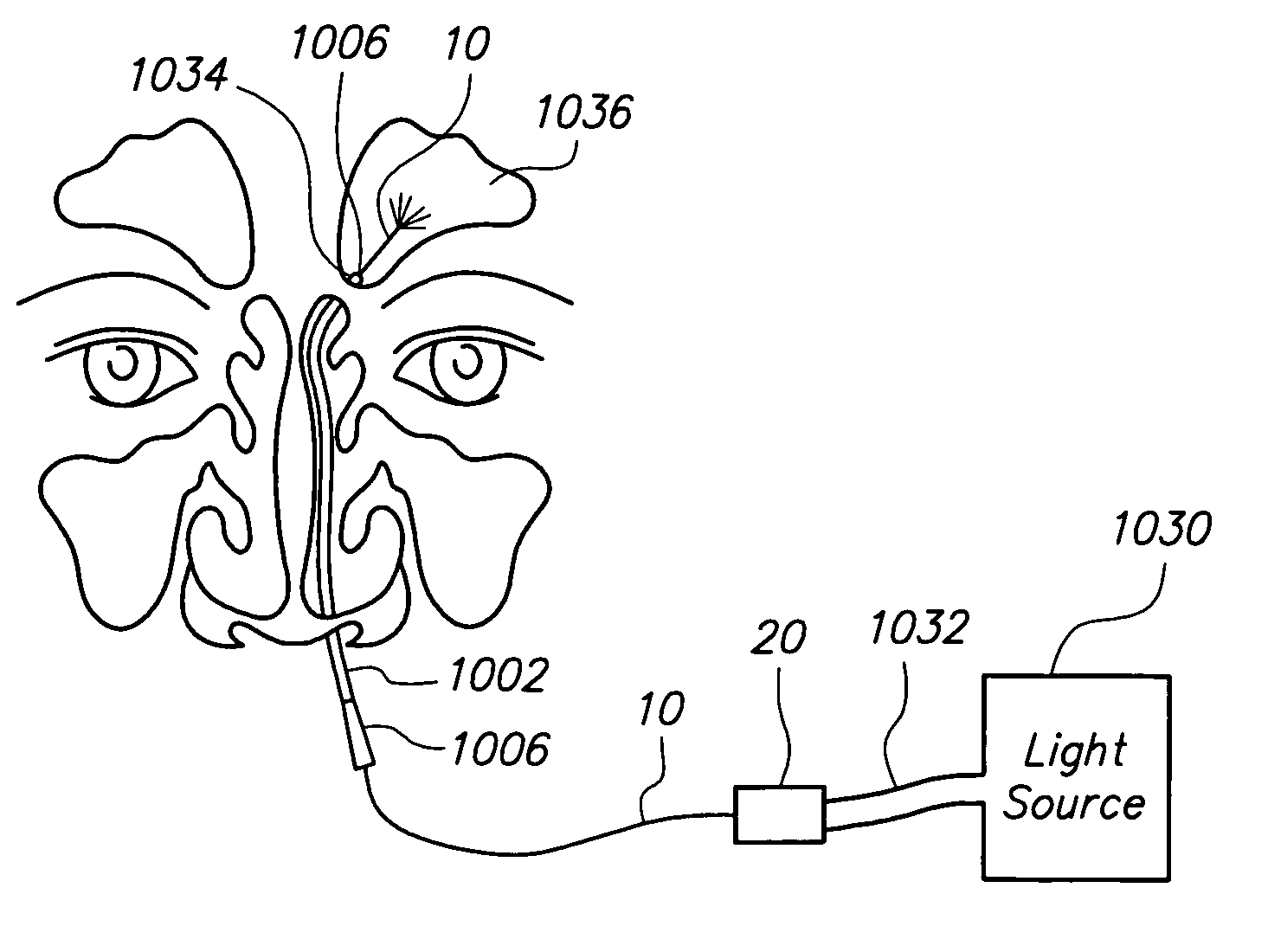
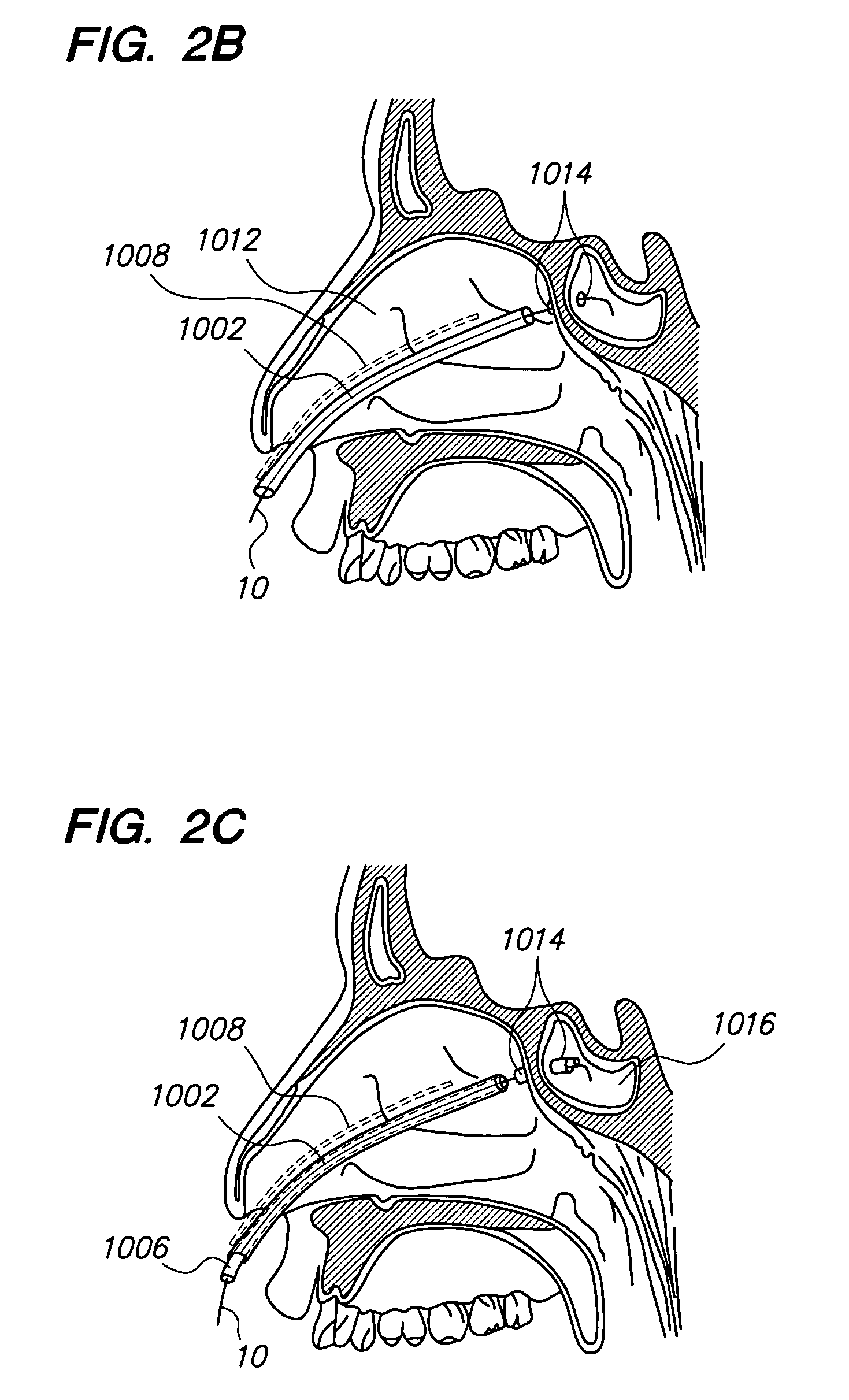
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
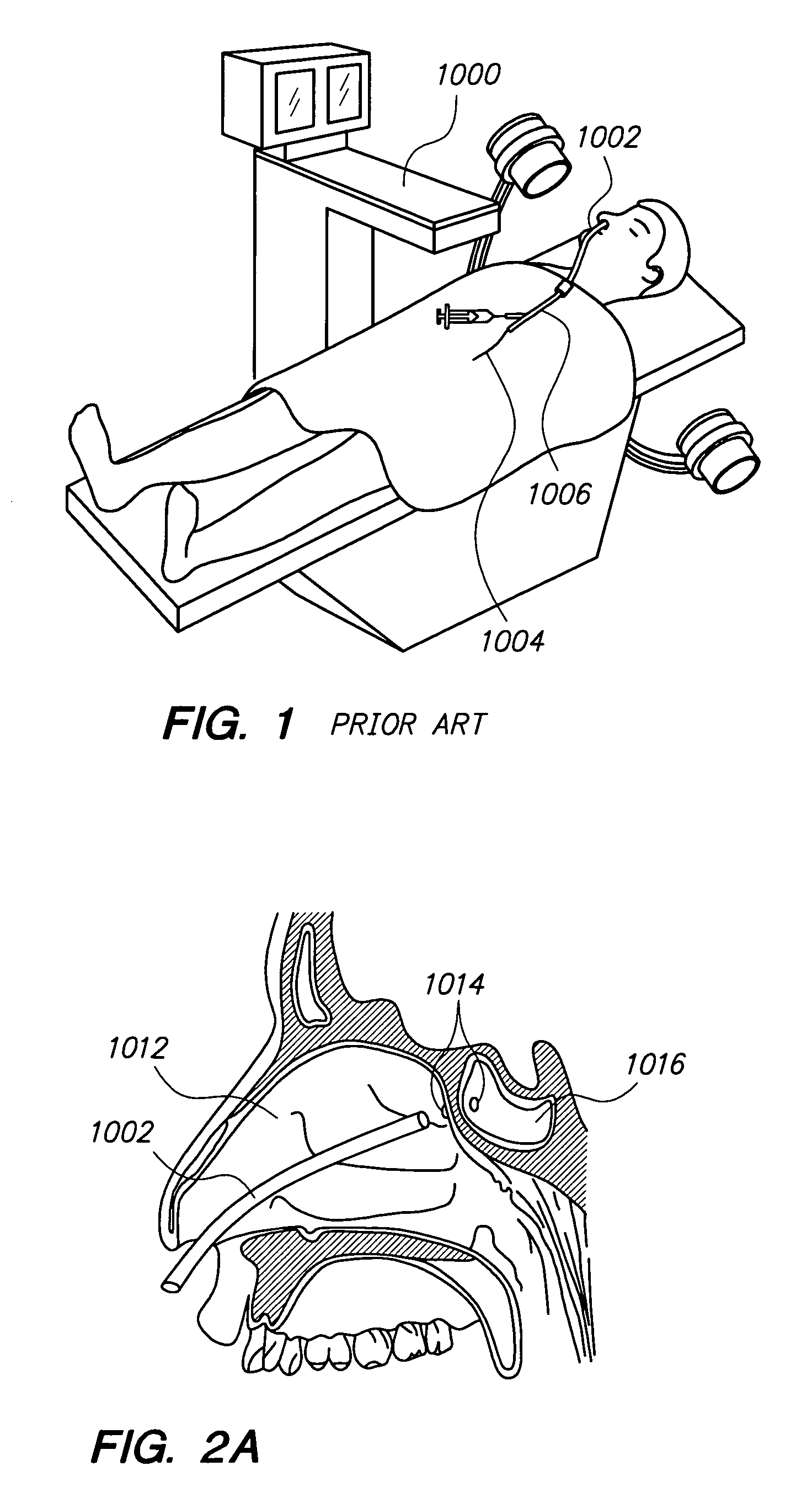
Systems and methods for performing image guided procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
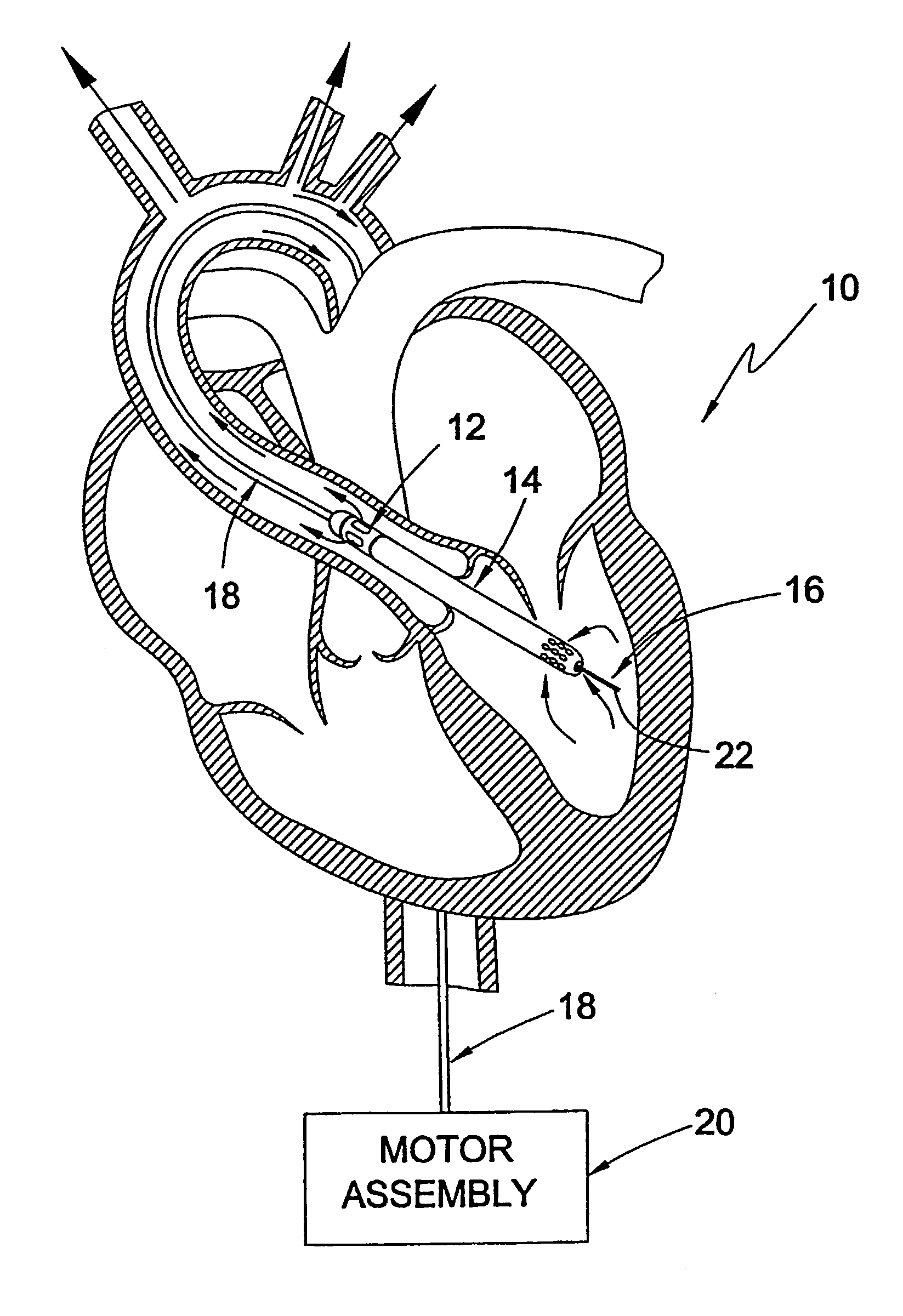
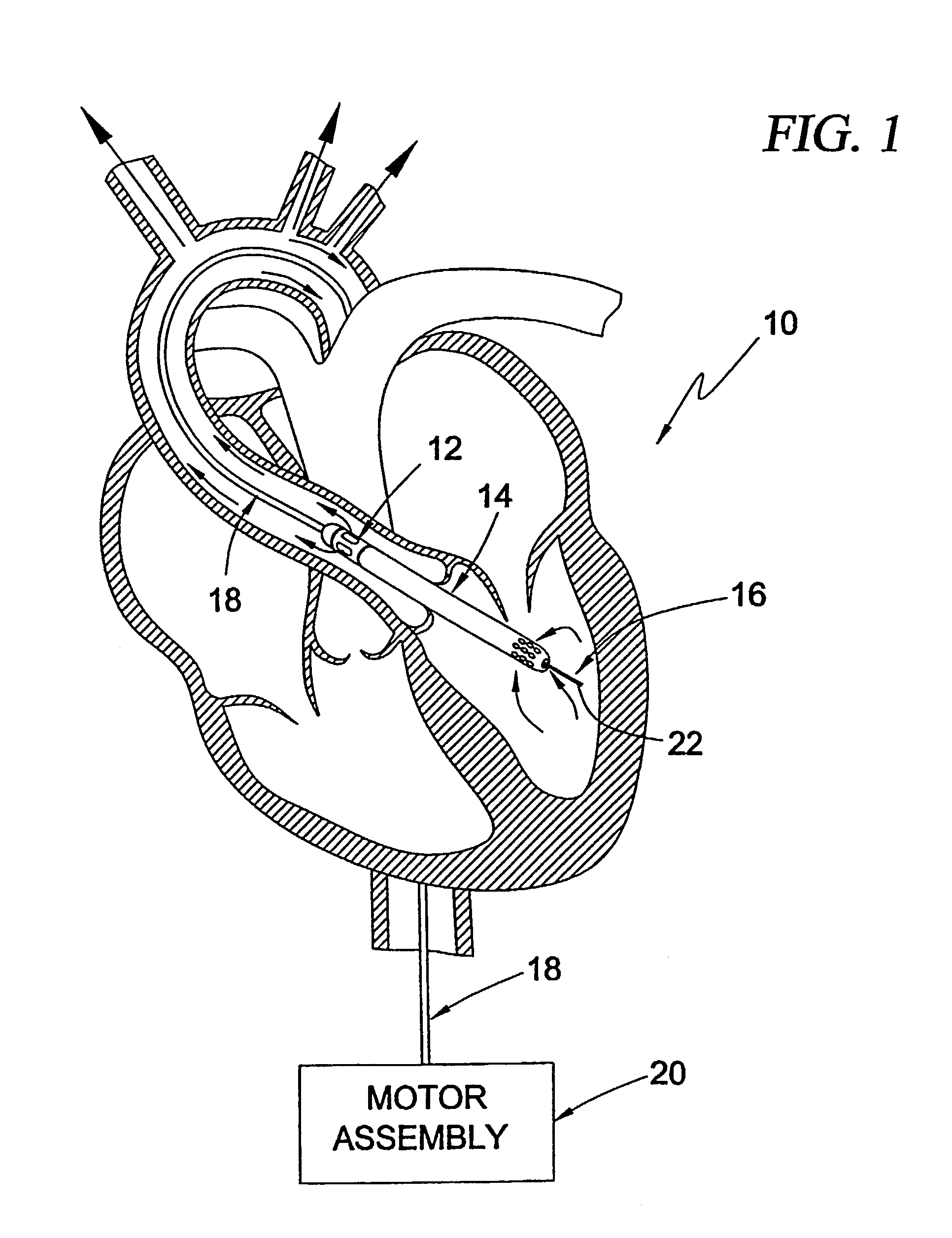
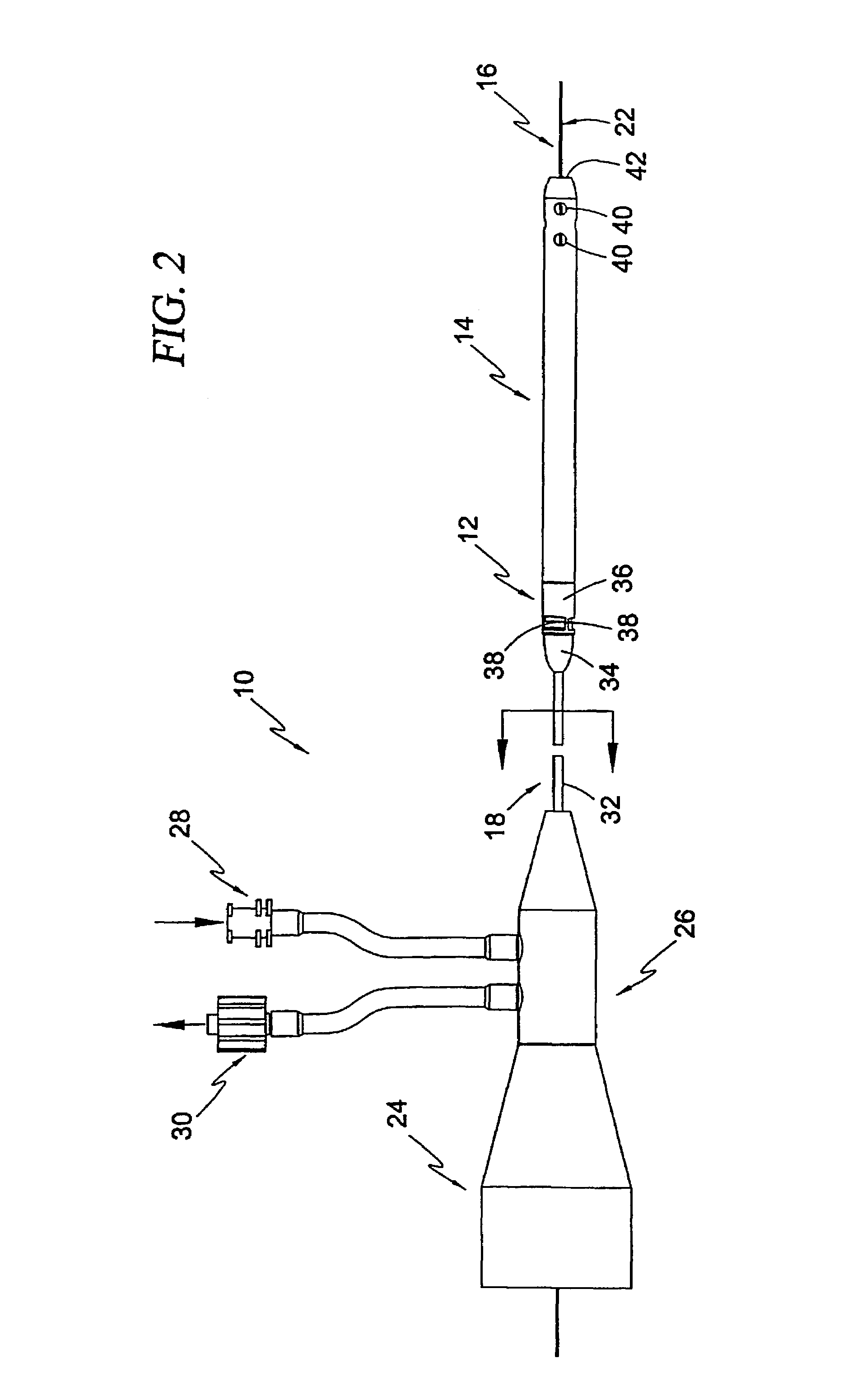
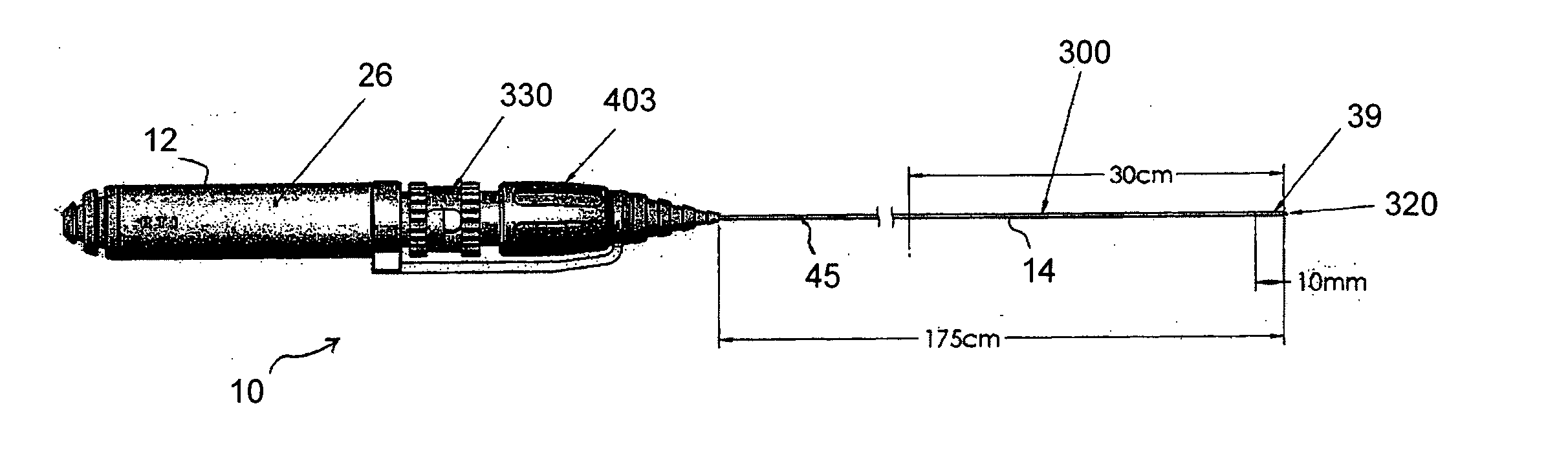
Guidable intravascular blood pump and related methods
InactiveUS7022100B1Integration of featureEliminate needGuide wiresControl devicesBlood pumpBlood vessel
An improved intravascular blood pump system (10) and related methods involving the broad inventive concept of equipping the intravascular blood pump (12) with guiding features such that the intravascular blood pump can be selectively positioned at a predetermined location within the circulatory system of a patient.
Owner:MAQUET CARDIOVASCULAR LLC
Guidewire for crossing occlusions or stenoses
InactiveUS20060074442A1Easy to controlFacilitate occlusionCannulasGuide wiresCoronary arteriesThrombus
A deflectable and torqueable hollow guidewire device is disclosed for removing occlusive material and passing through occlusions, stenosis, thrombus, plaque, calcified material, and other materials in a body lumen, such as a coronary artery. The hollow guidewire generally comprises an elongate, tubular guidewire body that has an axial lumen. A mechanically moving core element is positioned at or near a distal end of the tubular guidewire body and extends through the axial lumen. Actuation of the core element (e.g., oscillation, reciprocation, and / or rotation) creates a passage through the occlusive or stenotic material in the body lumen.
Owner:BOSTON SCI SCIMED INC
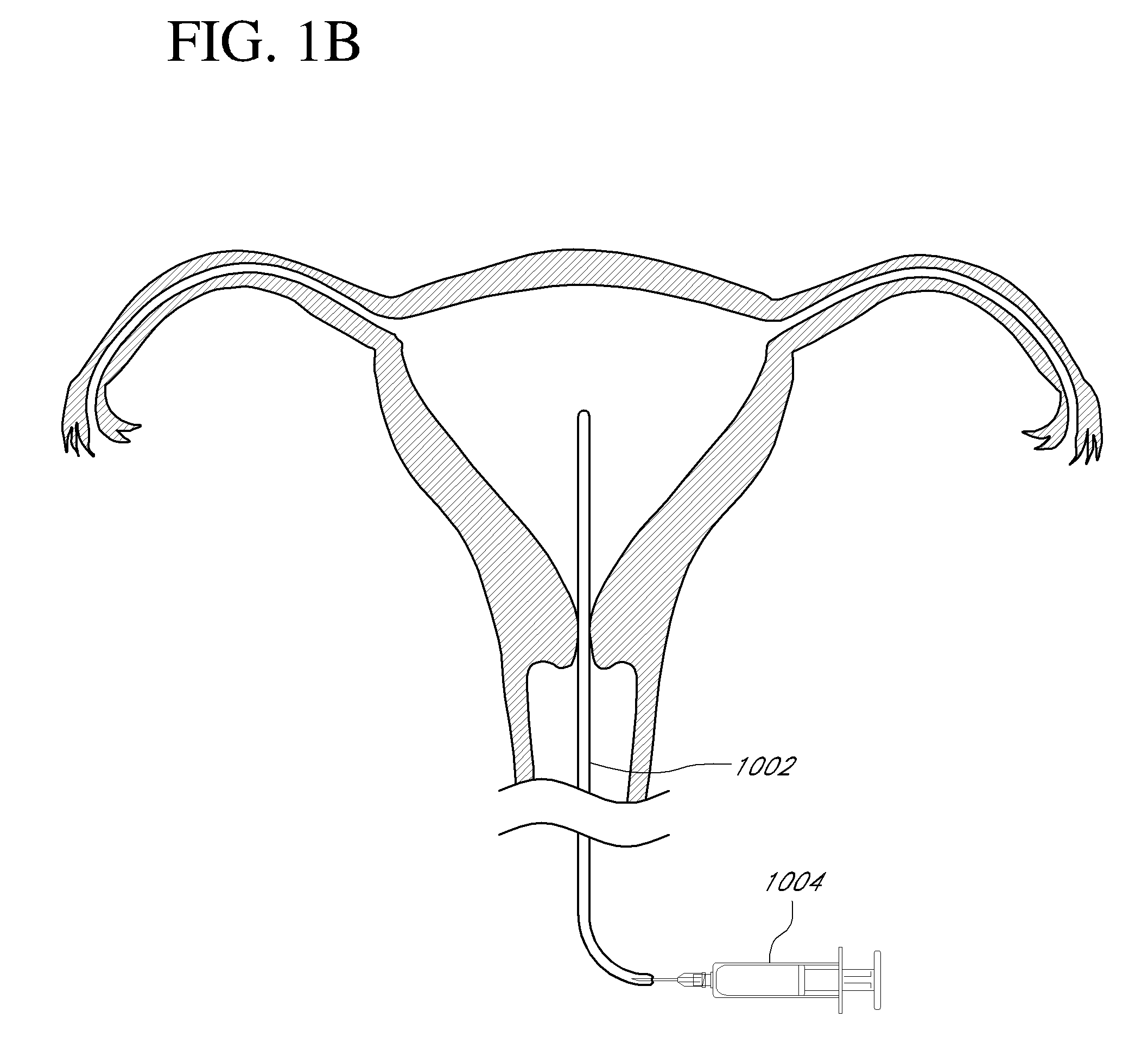
Systems, methods and devices for performing gynecological procedures
ActiveUS20080245371A1Prevent painful and potentially destructive dilation of the cervix is disclosedPrevent painful and potentially destructive dilationMedical devicesEndoscopesProcedural PainGynecology
Systems, methods, apparatus and devices for performing improved gynecologic and urologic procedures are disclosed. The system and devices provide simplified use and reduced risk of adverse events. Patient benefit is achieved through improved outcomes, reduced pain, especially peri-procedural pain, and reduced recovery times. The various embodiments enable procedures to be performed outside the hospital setting, such as in a doctor's office or clinic.
Owner:HOLOGIC INC
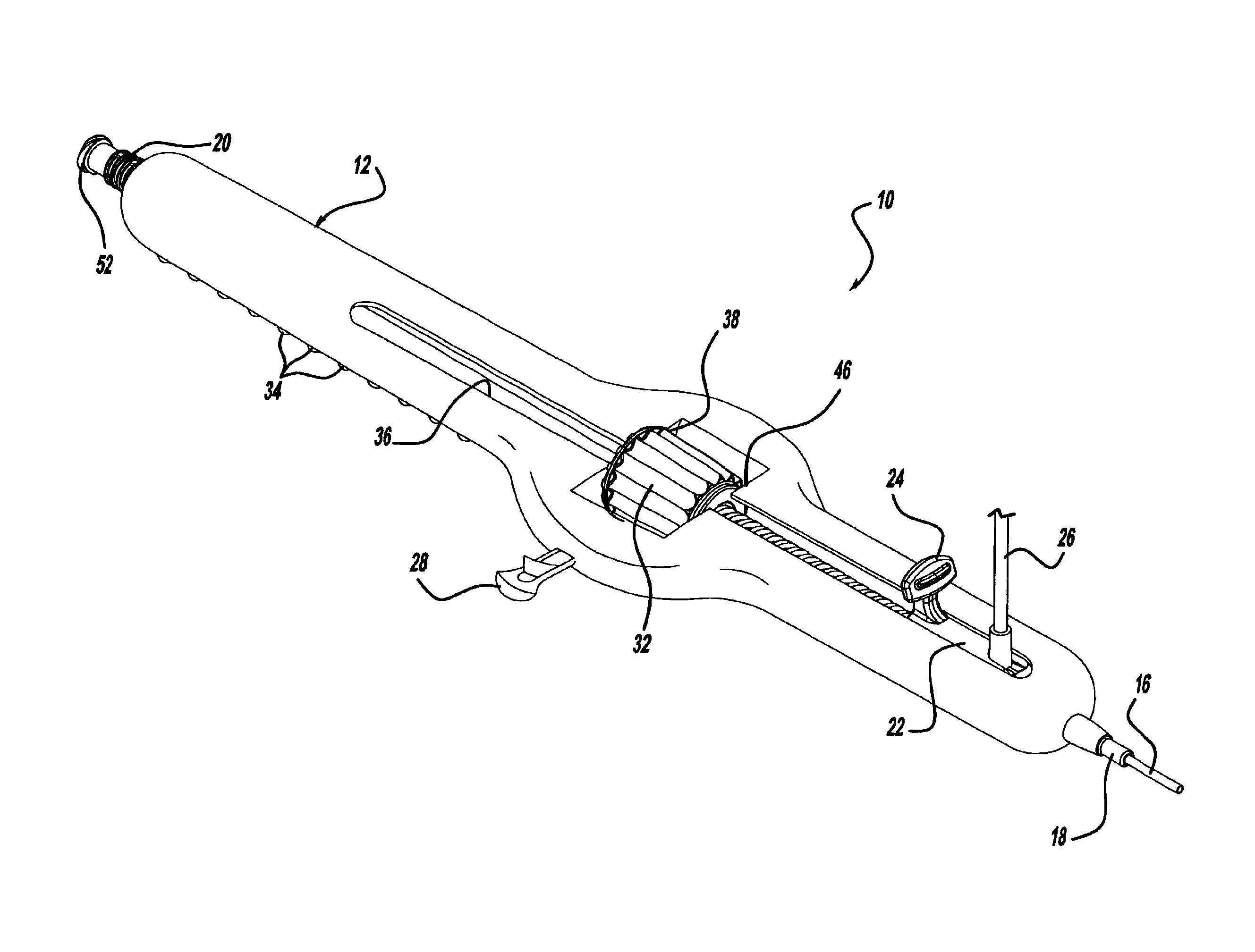
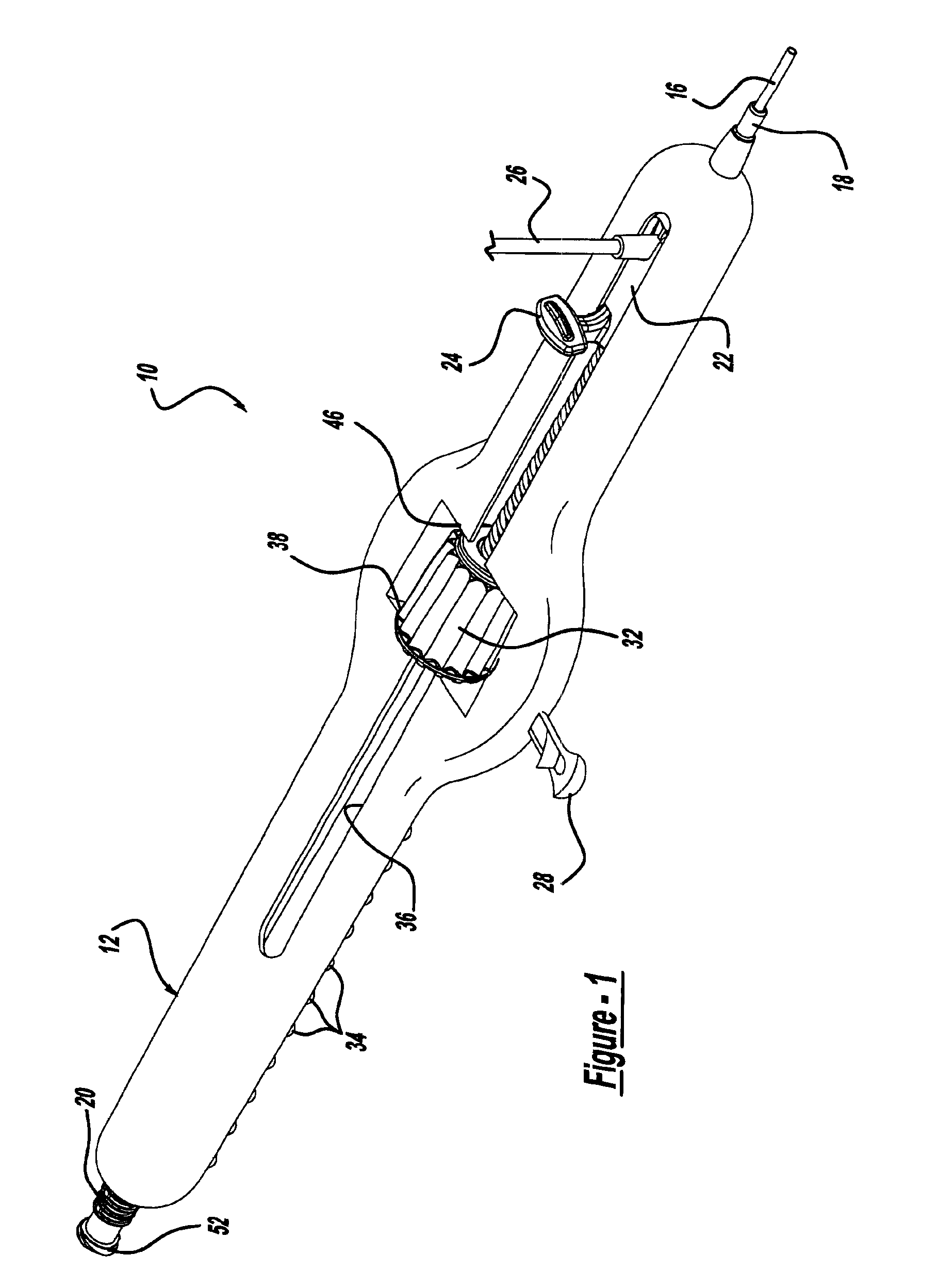
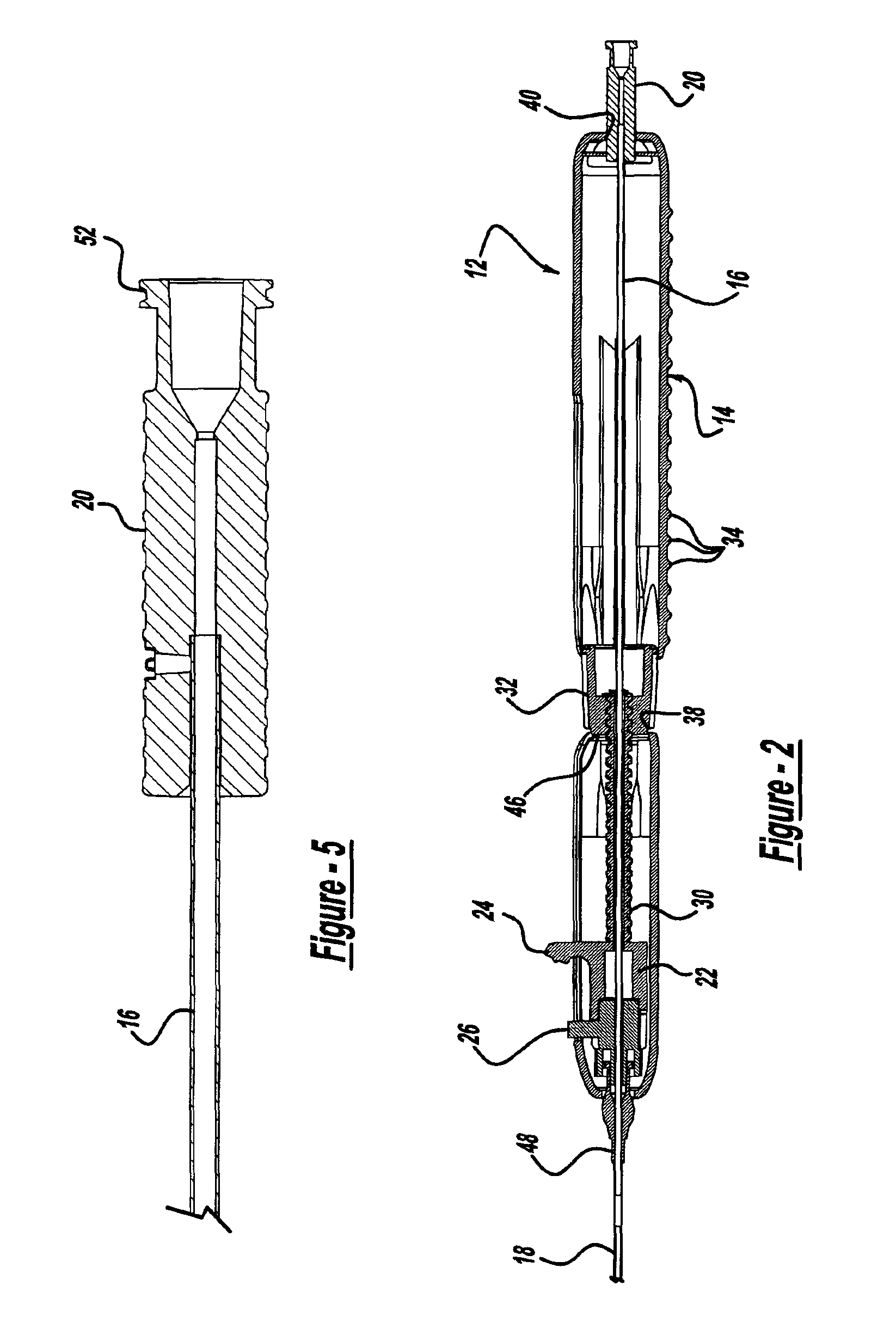
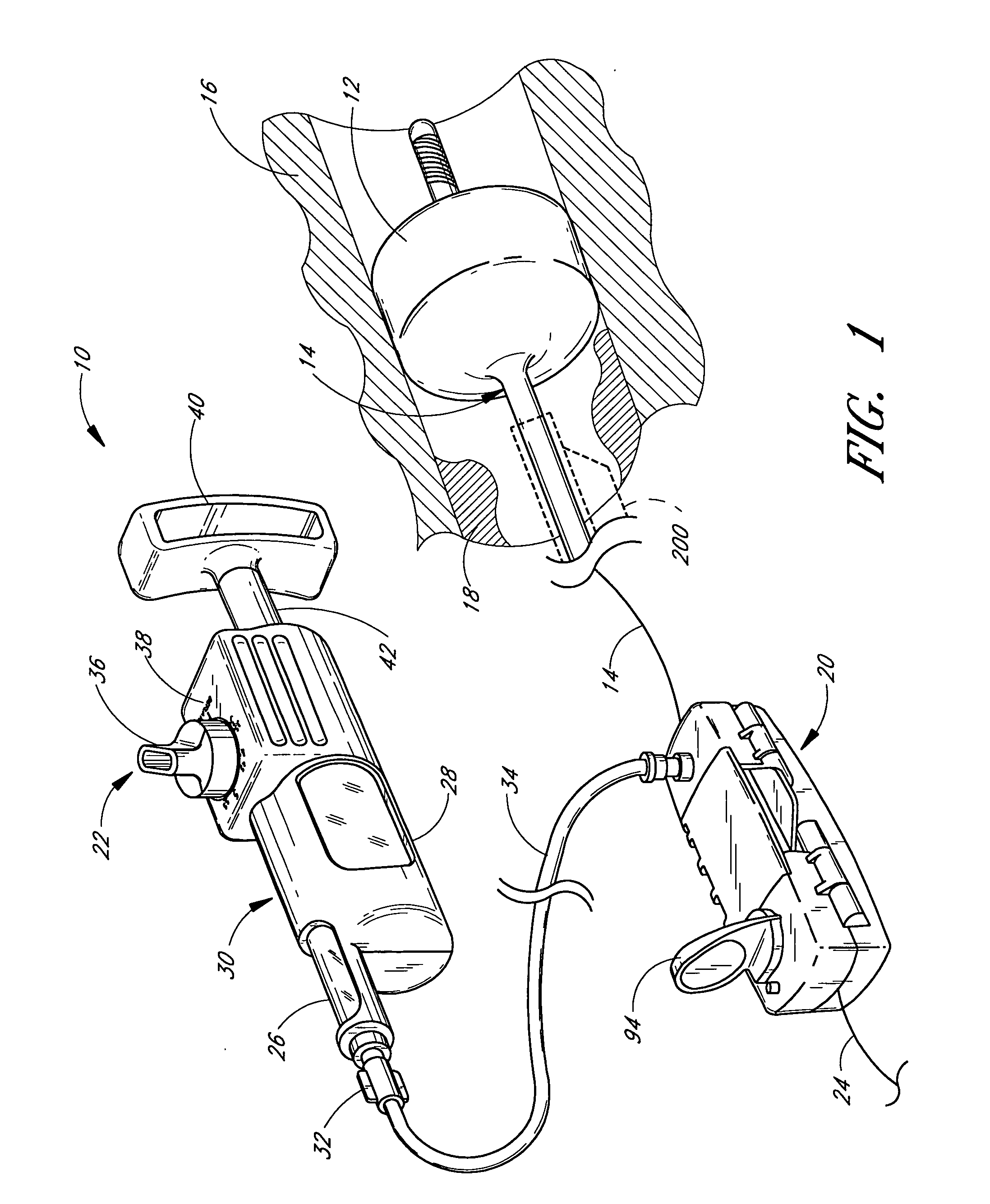
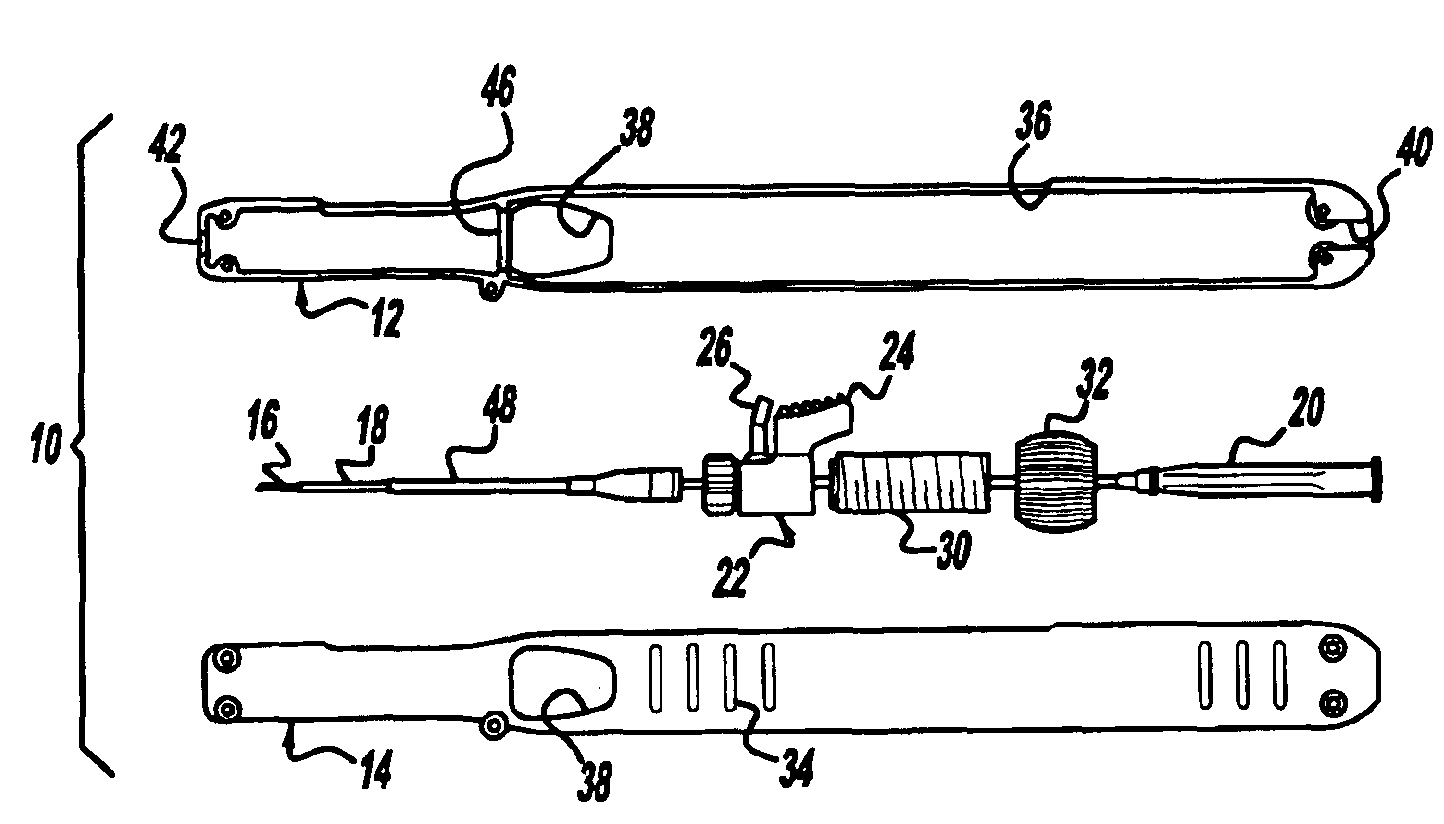
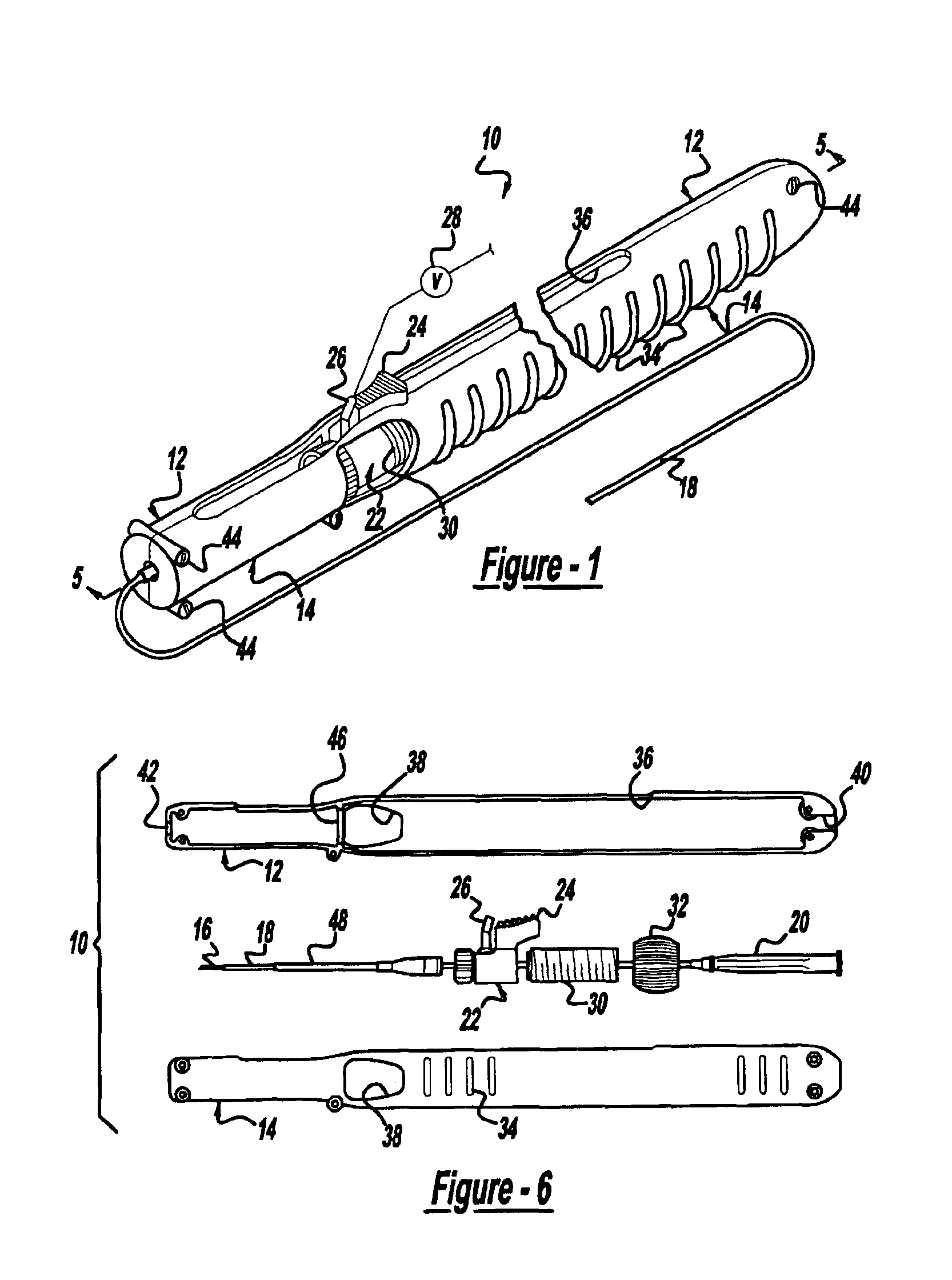
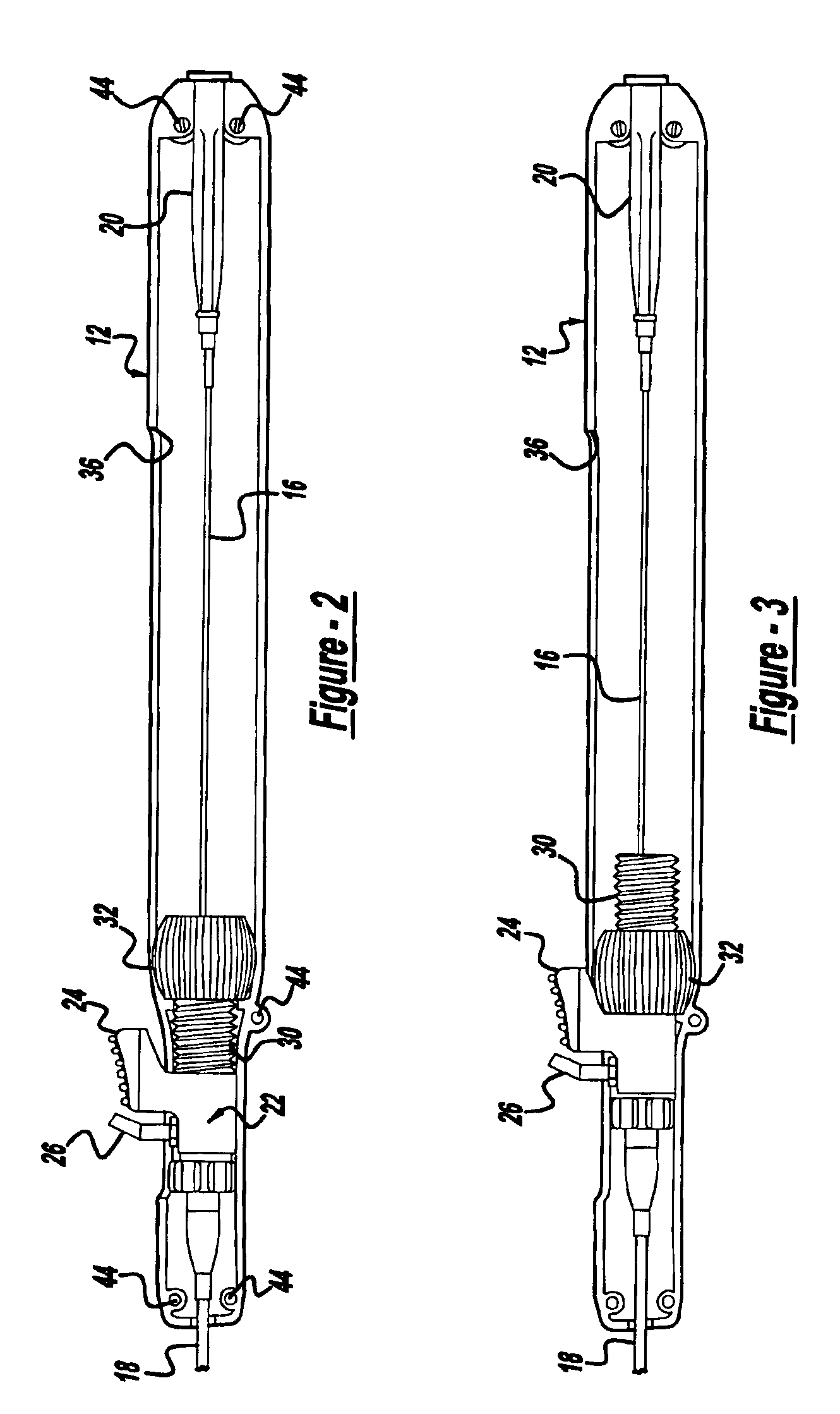
Locking handle deployment mechanism for medical device and method
InactiveUS6866669B2Easy and accurate deployment and positioningEasy to operateStentsEar treatmentLocking mechanismMedical device
A system for delivering at least one medical device to a desired location for treatment, and then selectively deploy it in position, includes an improved handle. One of the possible features of the handle may be to selectively hold the delivery system components at any desired configuration during deployment and positioning of the medical device. Another possible feature of the handle may be more than one mode of operation, in which the deployment of the medical device can selectively proceed at more than one speed. Yet another possible feature of the handle may be a locking mechanism that resists inadvertent or accidental movement or retraction of the stent delivery system components during packaging, sterilization, shipping, storage, handling and preparation of the stent delivery system.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Devices, systems and methods useable for treating sinusitis
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat. Specific devices (e.g., tubular guides, guidewires, balloon catheters, tubular sheaths) are provided as are methods for manufacturing and using such devices to treat disorders of the ear, nose or throat.
Owner:ACCLARENT INC
Method and apparatuses for treating an intravascular occlusion
InactiveUS20060200191A1Minimize timeReduce amountBalloon catheterMulti-lumen catheterThree vesselsExternal carotid artery
Methods for an intravascular occlusion are provided. A guidewire having an occlusive device such as balloon or a filter at one end is advanced across the occlusion using a guide catheter, and the occlusive device is expanded distal to the occlusion to occlude the blood vessel. The guide catheter may also have an occlusive device to occlude the vessel proximal to the occlusion. In a treatment method for the carotid arteries, occlusive devices may be provided in the external carotid artery, in the internal carotid artery, and in the common carotid artery.
Owner:ZADNO AZIZI GHOLAM REZA
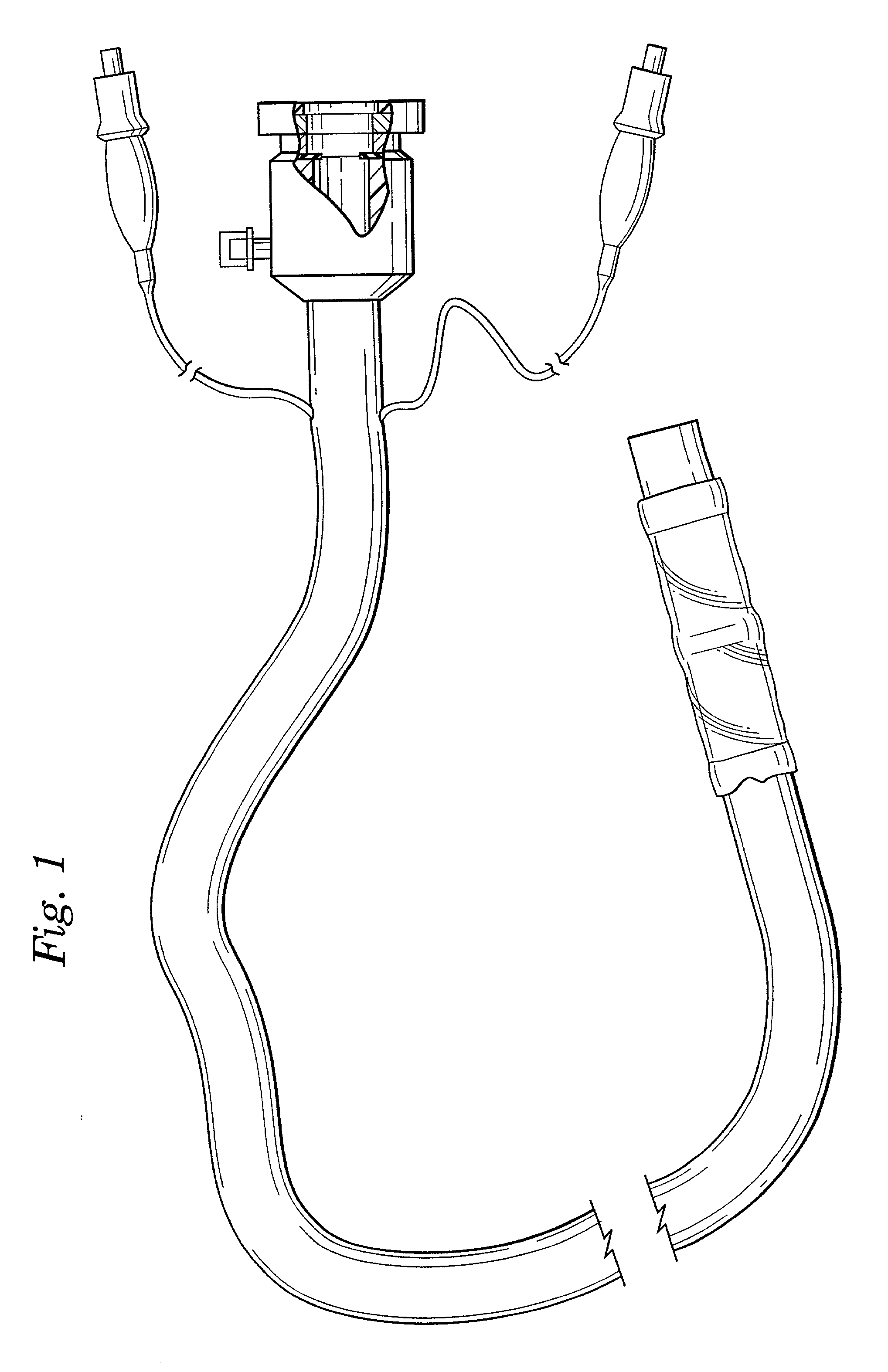
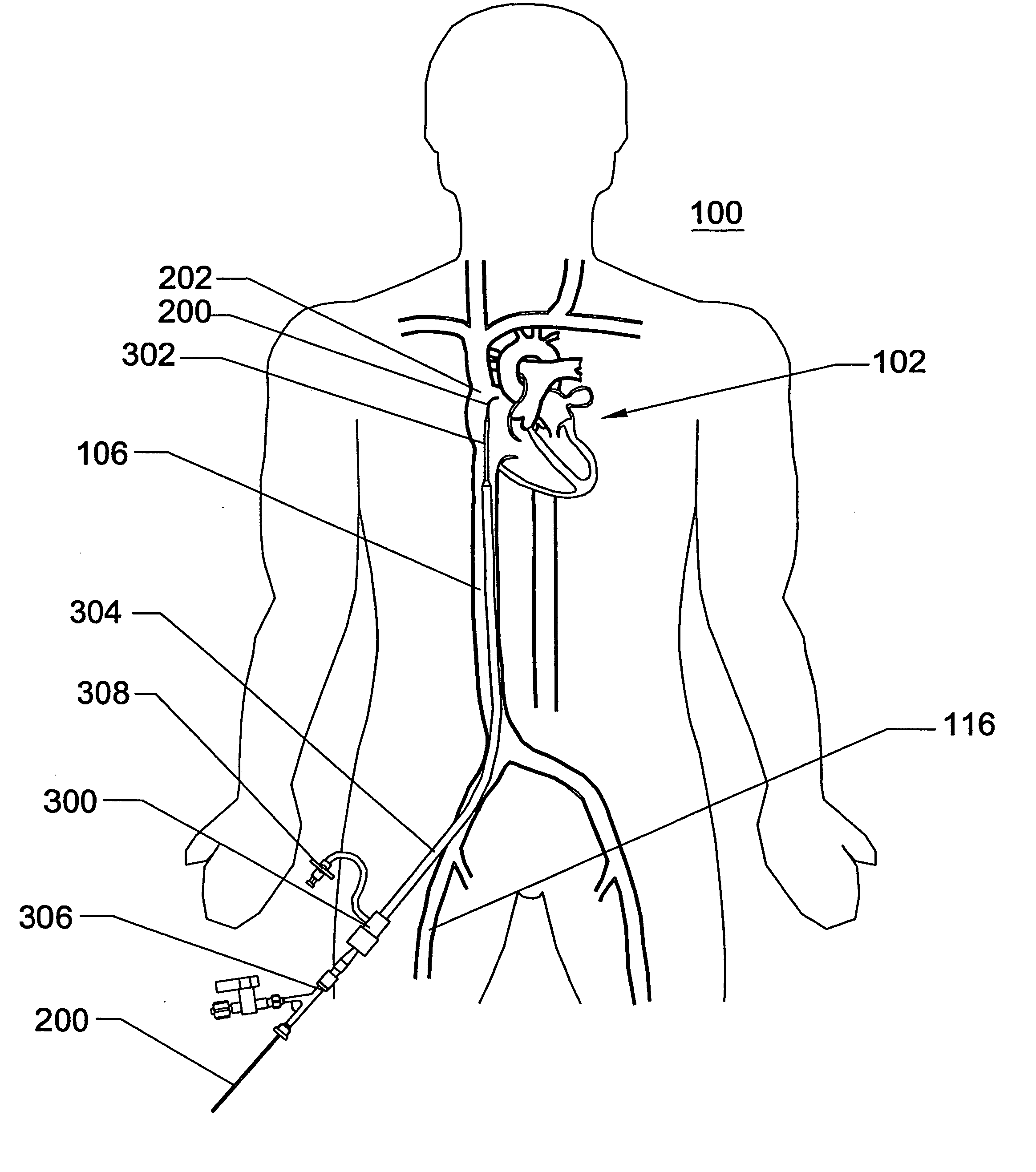
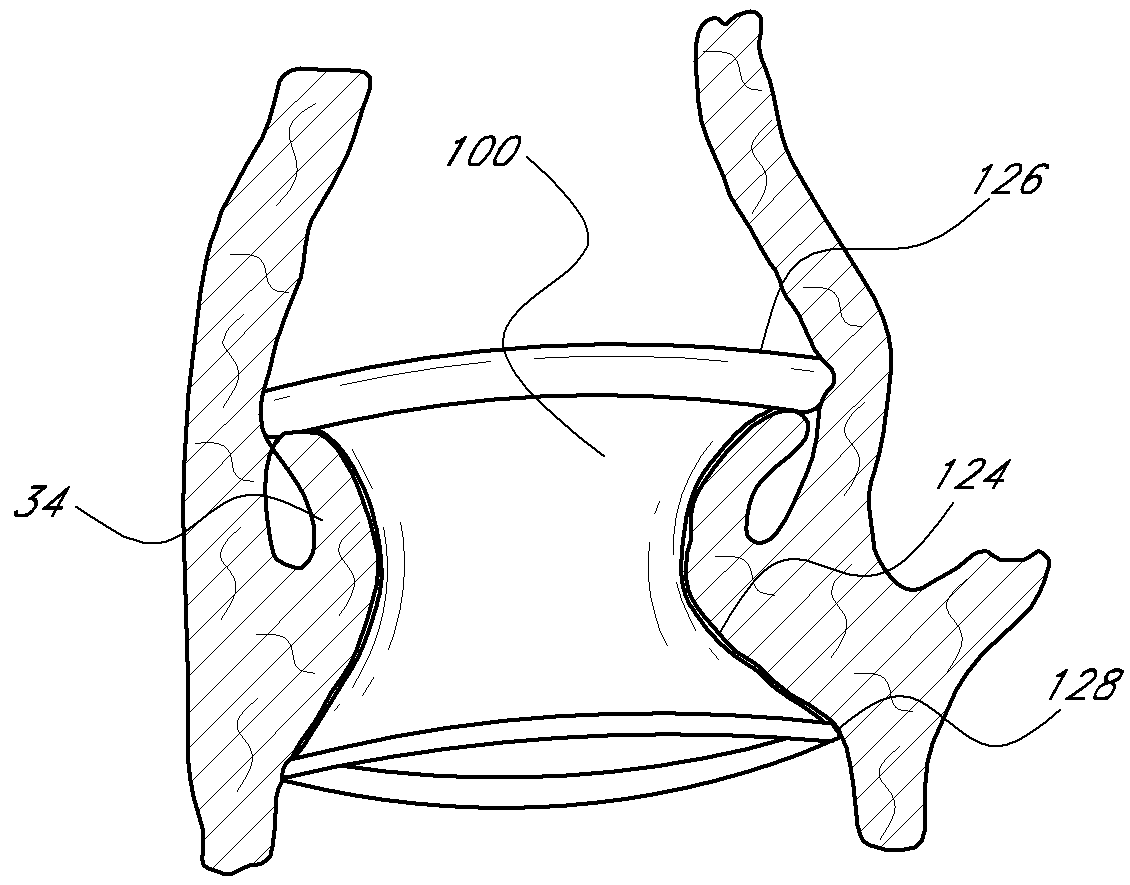
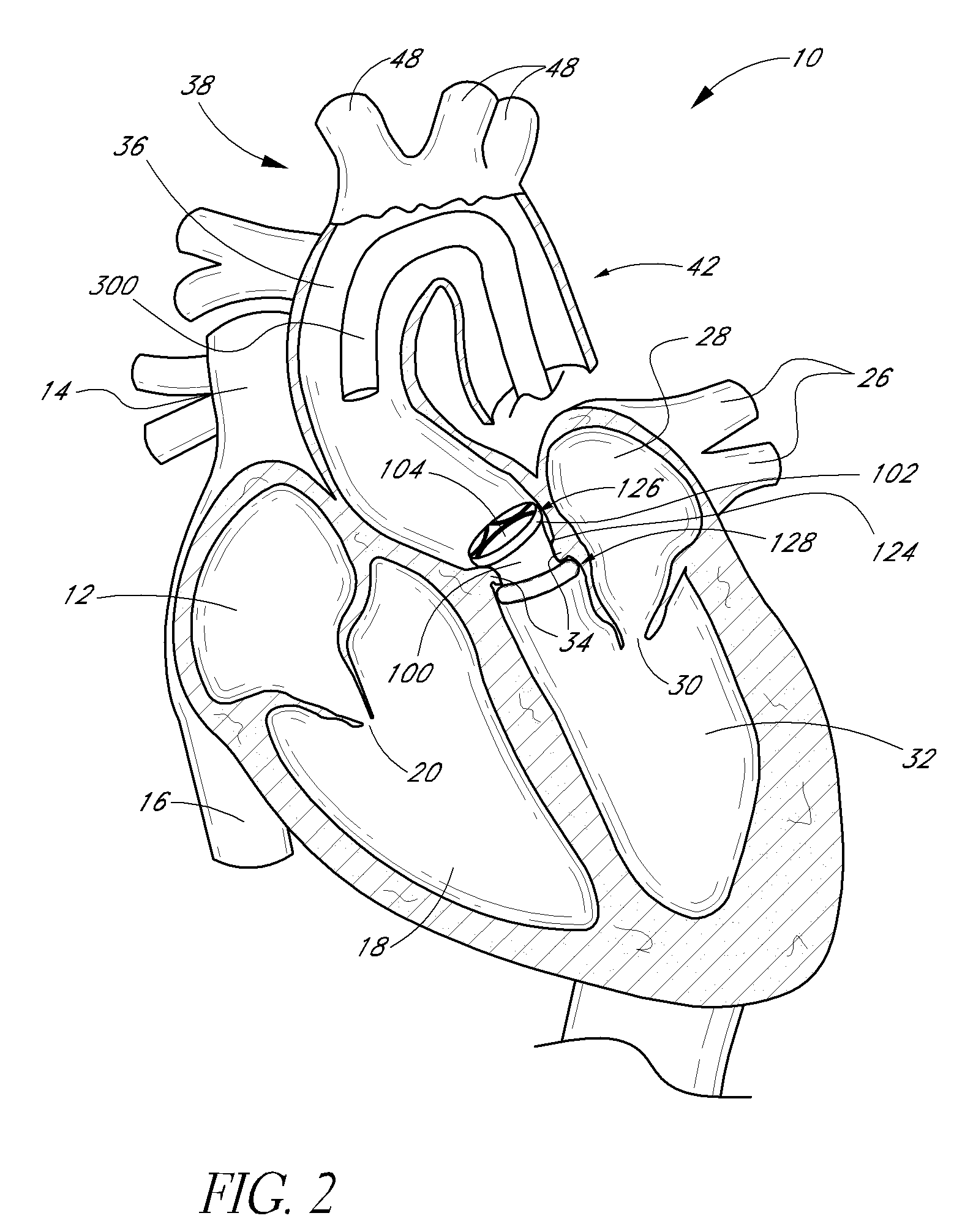
Expandable trans-septal sheath
InactiveUS20060135962A1Inhibit bindingAvoid interferenceGuide needlesEar treatmentAccess routeDilator
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement through the atrial septum into the left atrium. The distal end of the sheath is expanded using a radial dilator. In one application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of atrial implants, valve repair, or the like.
Owner:ONSET MEDICAL CORP
Atherectomy devices and methods
ActiveUS8070762B2Prevent accidental cuttingIncrease blockingEar treatmentCannulasBiomedical engineeringBlood vessel
Owner:ATHEROMED
Guidewire having deployable sheathless protective filter
InactiveUS20050021075A1Reduce the potential for damageAvoid damageGuide wiresSurgeryFiberEngineering
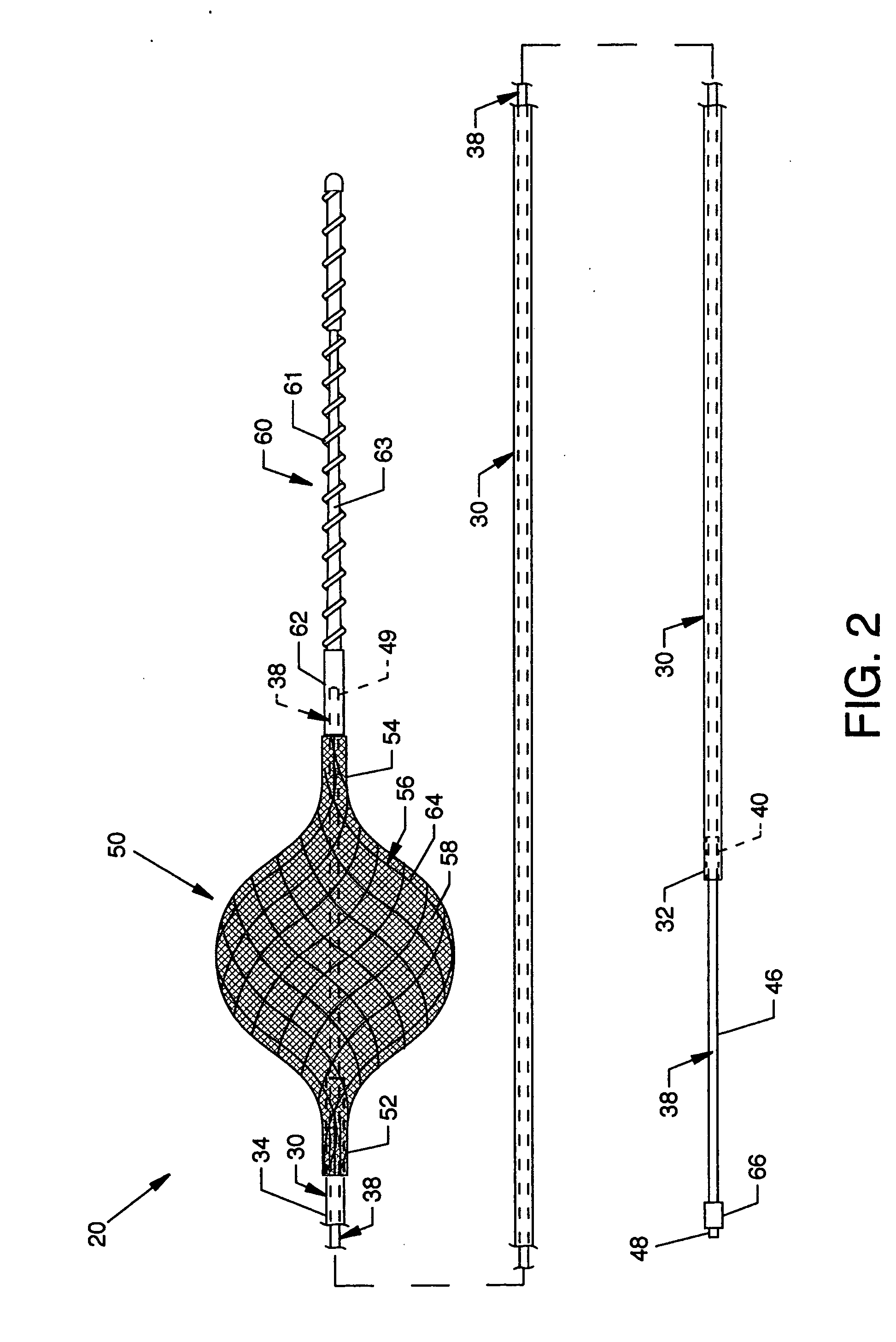
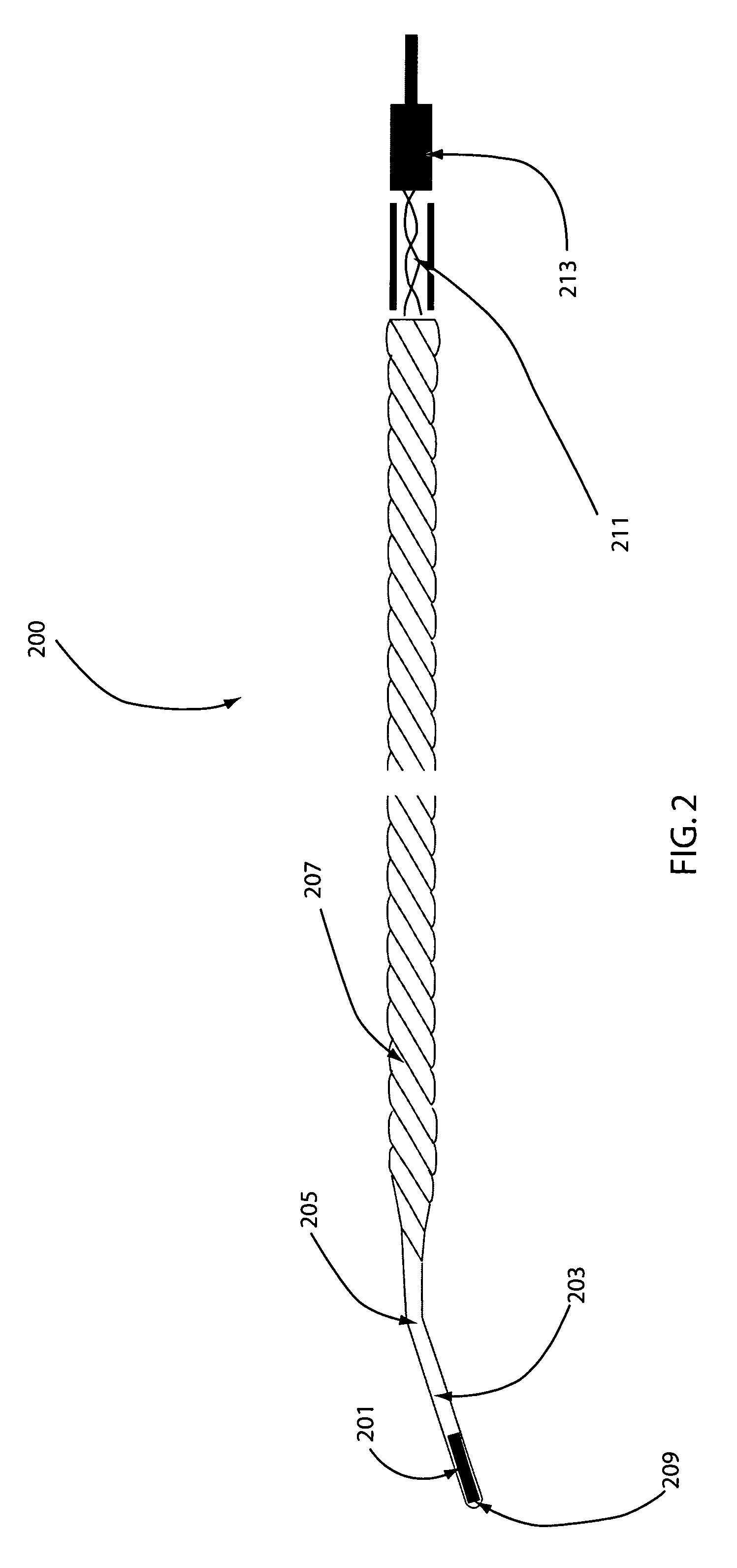
A protective system or apparatus for use in vascular procedures includes a tubular guidewire, a control cable slidable within the tubular guidewire, and a sheathless filter. The control cable is attached to a distal end of the sheathless filter and the tubular guidewire is attached to a proximal end of the sheathless filter. Selective displacement of the control cable radially expands the sheathless filter to create a proximal exterior convex primary filter surface that is positionable downstream from a site of a vascular procedure. The sheathless filter also presents a distal interior concave secondary filter surface. Preferably, the sheathless filter is constructed of a braided wire framework in the form of a tube over which woven polymer fibers or strands are applied to create a filter mesh having a softer filter surface.
Owner:MEDRAD INC.
Integrated mechanical handle with quick slide mechanism
A delivery system includes a sheath and a handle. The handle includes: a slide shaft having a threaded outer surface; and a hub assembly coupled to the sheath. The hub assembly includes: an inner slider having a thread tooth pivot support; a thread tooth pivotably mounted to the thread tooth pivot support; and a sleeve having a thread tooth press member pressing on the thread tooth, where motion of the sleeve relative to the inner slider pivots the thread tooth on the thread tooth pivot support to engage and disengage the hub assembly with the threaded outer surface.
Owner:MEDTRONIC VASCULAR INC
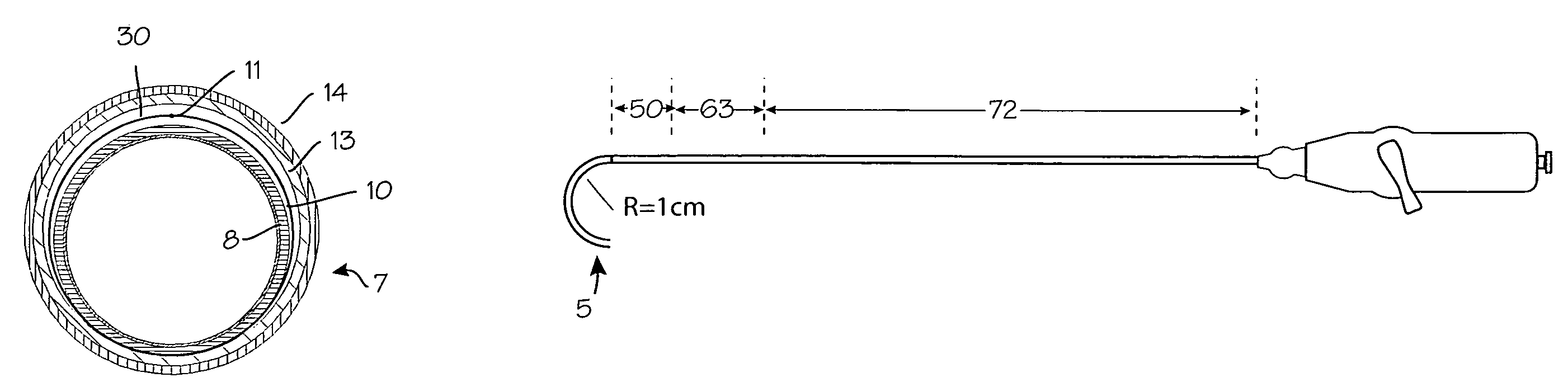
Devices, systems and methods useable for treating frontal sinusitis
Devices, systems and methods wherein a dilator, such as a balloon or other expandable member, is positionable within the frontal sinus ostium and adjacent frontal recess and useable to dilate the frontal sinus ostium and substantially all of the frontal sinus recess without requiring repositioning and repeated re-expansion of the dilator. One balloon catheter device of the invention comprises a catheter body that is less than about 50 cm in length (and in some embodiments less than 25 cm in length and a semi-compliant or non-compliant balloon on the catheter body. The balloon may have a working length of about 12 mm to about 30 mm and a width at its widest point when fully inflated of about 2 mm to about 7 mm. Such balloon may be constructed to withstand inflation pressures of about 12 atmospheres. In some embodiments, the dilator is advanced through or over a guide (e.g., guidewire or guide catheter) that has a preformed shape.
Owner:ACCLARENT INC
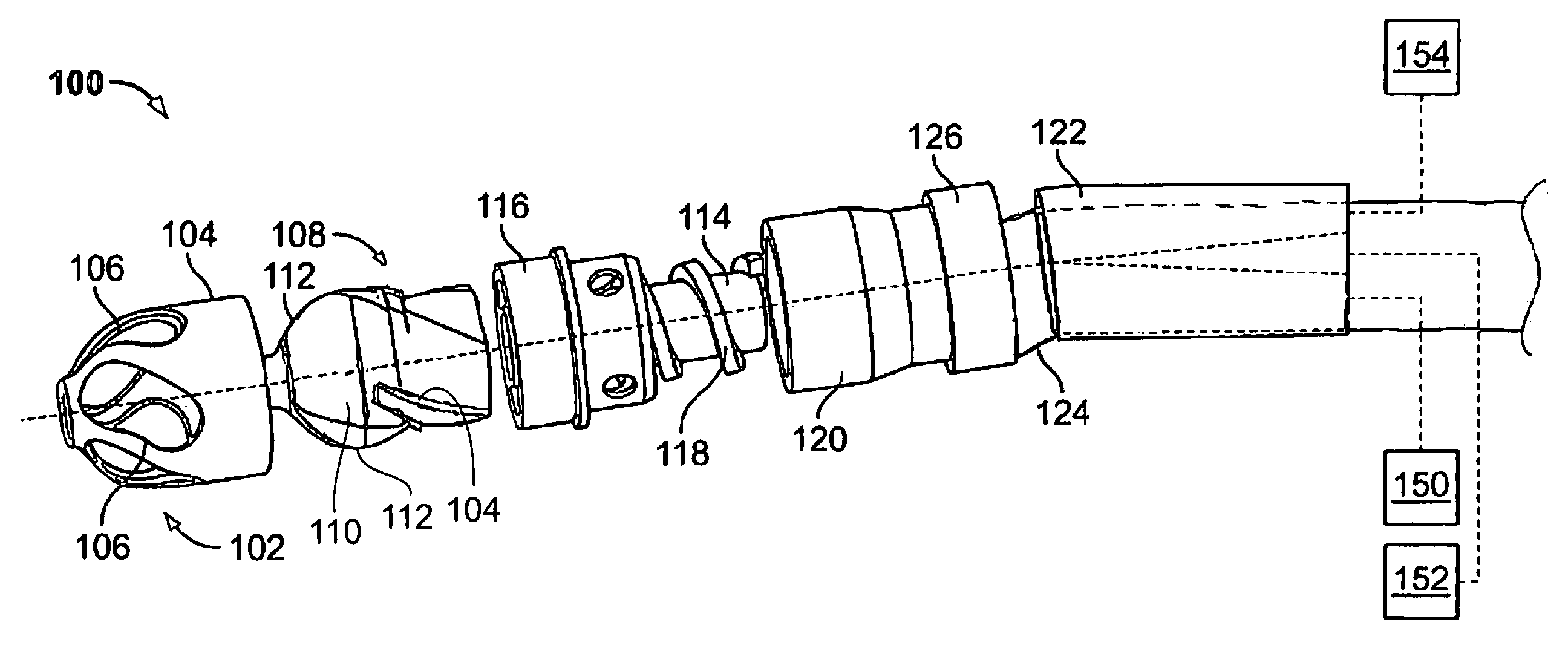
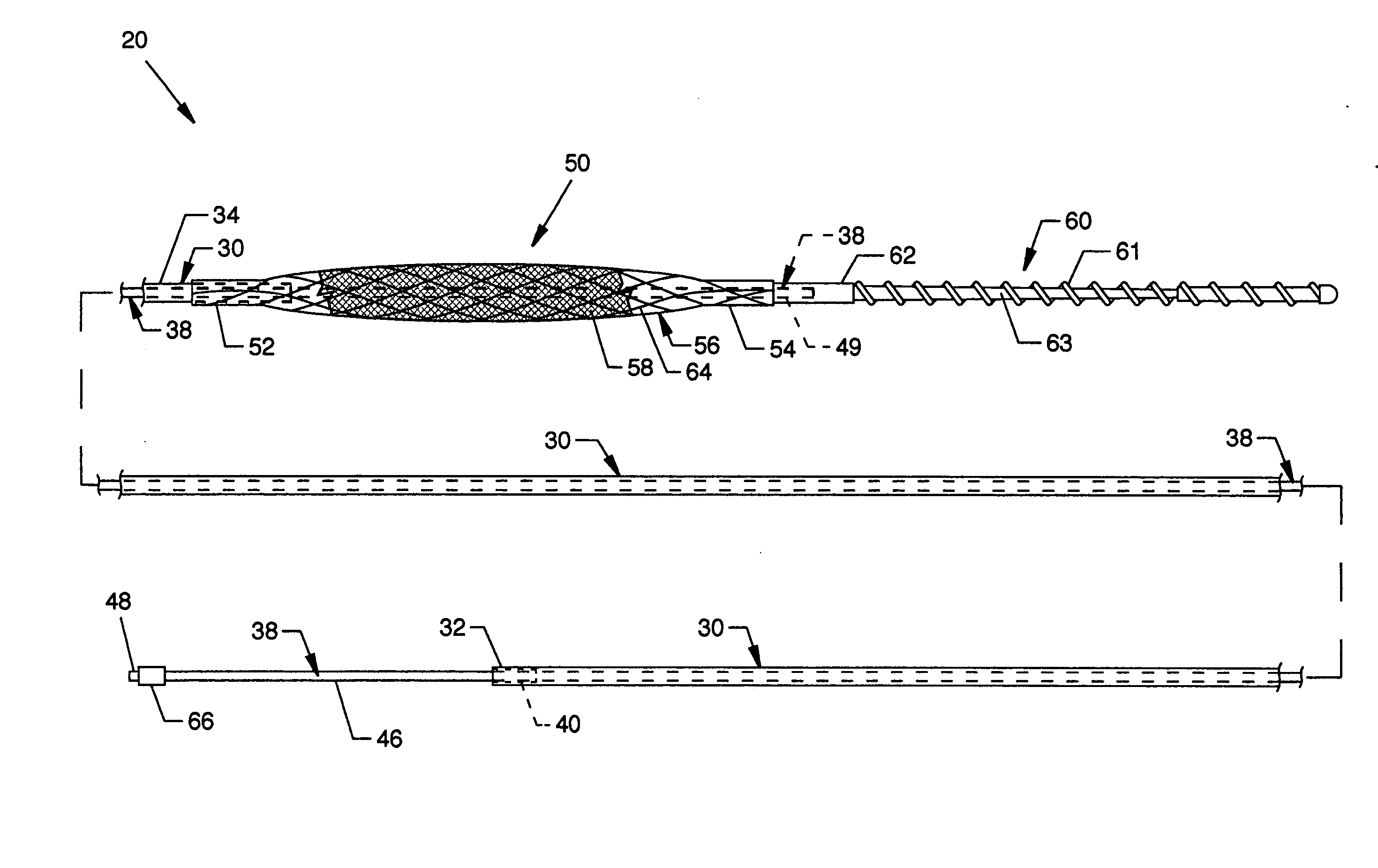
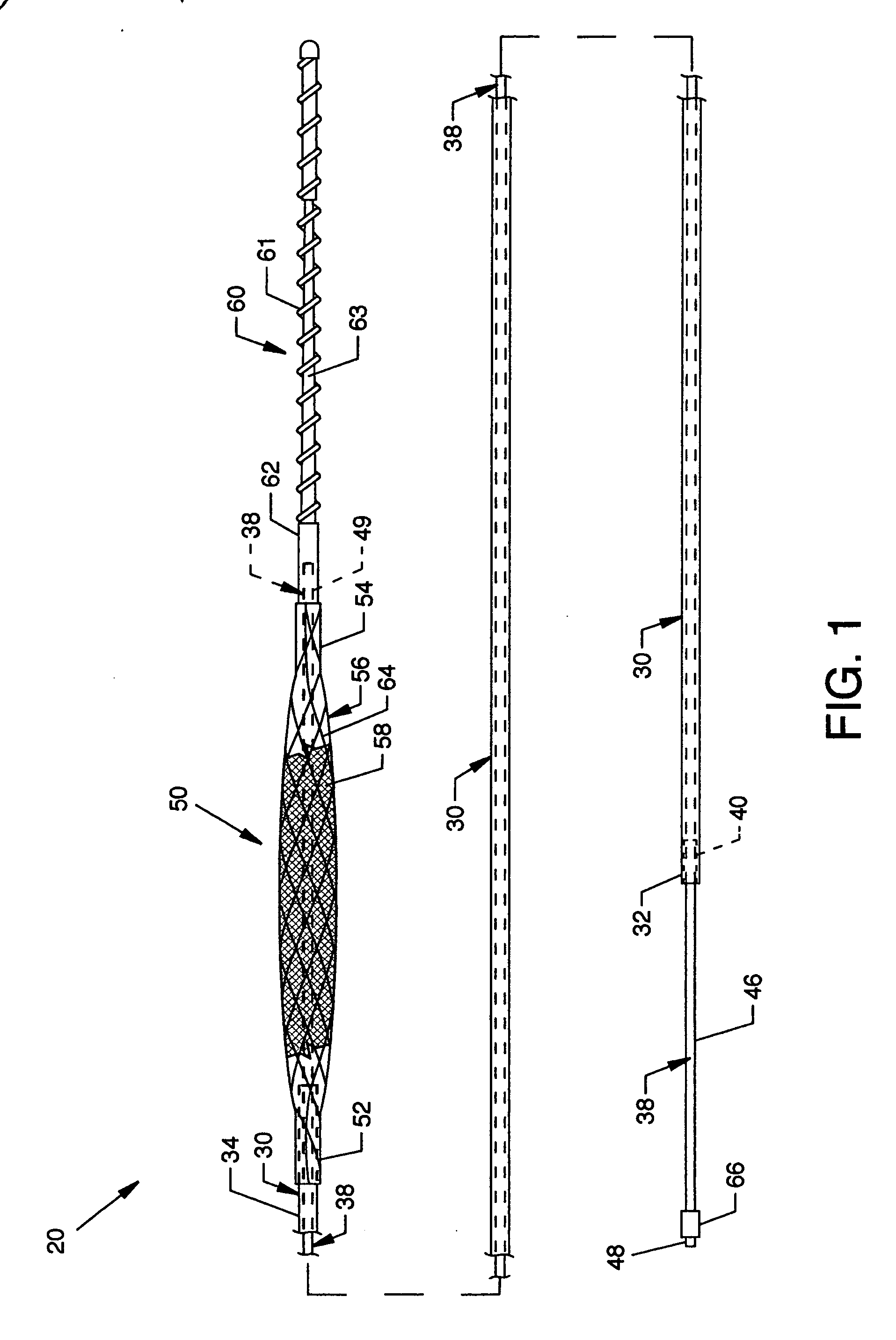
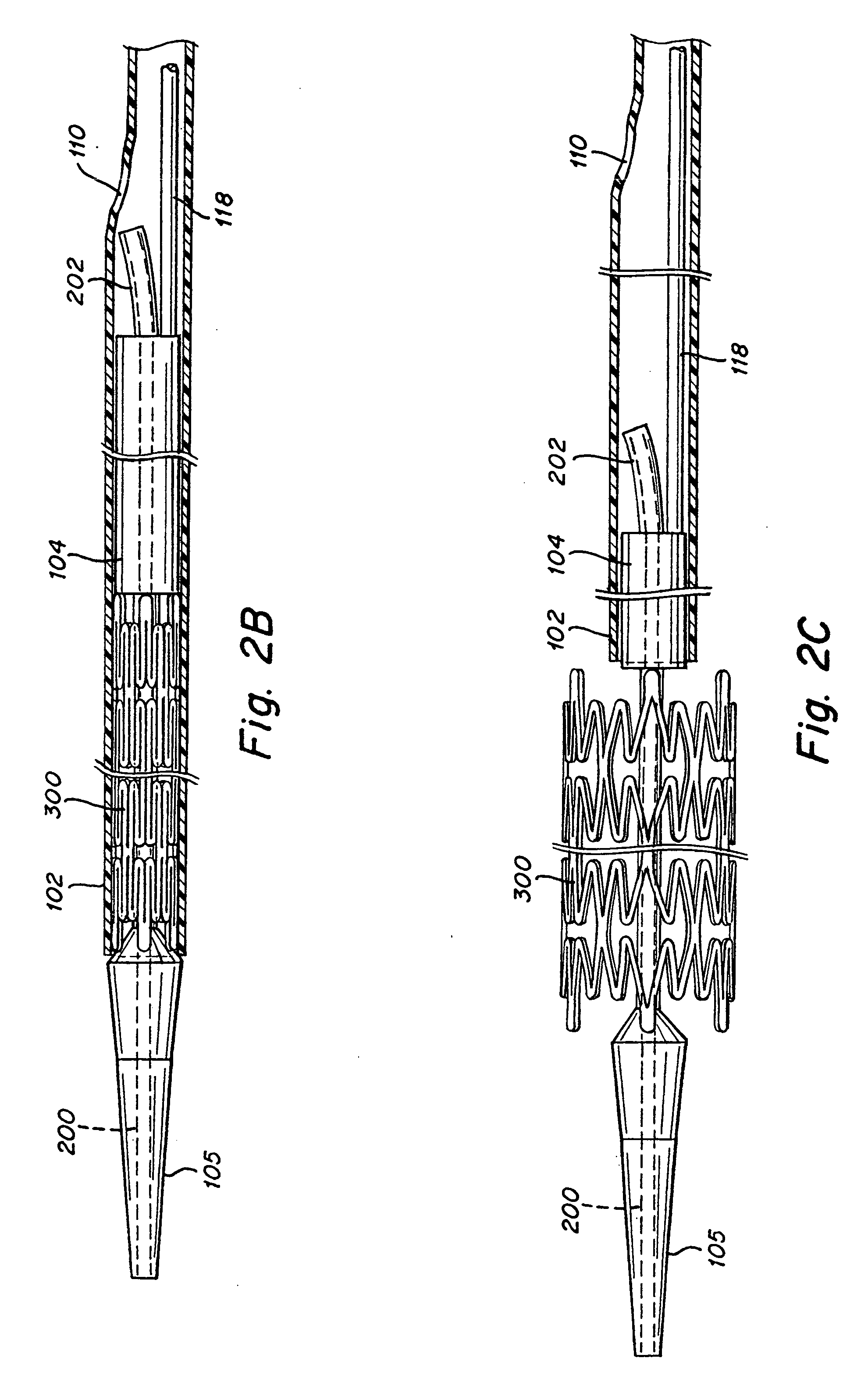
Guidewire loaded stent for delivery through a catheter
A guidewire loaded stent for delivery through a catheter is described herein. The stent delivery assembly can deliver and place a stent within tortuous regions of the body which are accessible to guidewires but inaccessible to stenting catheters. The assembly comprises a guidewire covered in part by a retractable sheath and a radially expandable stent near or at the distal end of the guidewire. The whole assembly is advanced through conventional catheters or it may be used alone. In either case, when the stent is adjacent to a treatment site within the body, the sheath is retracted proximally to expose the stent for radial expansion into contact with the vessel wall. Radio-opaque marker bands are optionally located on either side or both sides of the stent on the guidewire body to aid in visual placement. The assembly can optionally include an expandable balloon on the guidewire for different treatment modalities.
Owner:BACK BAY MEDICAL
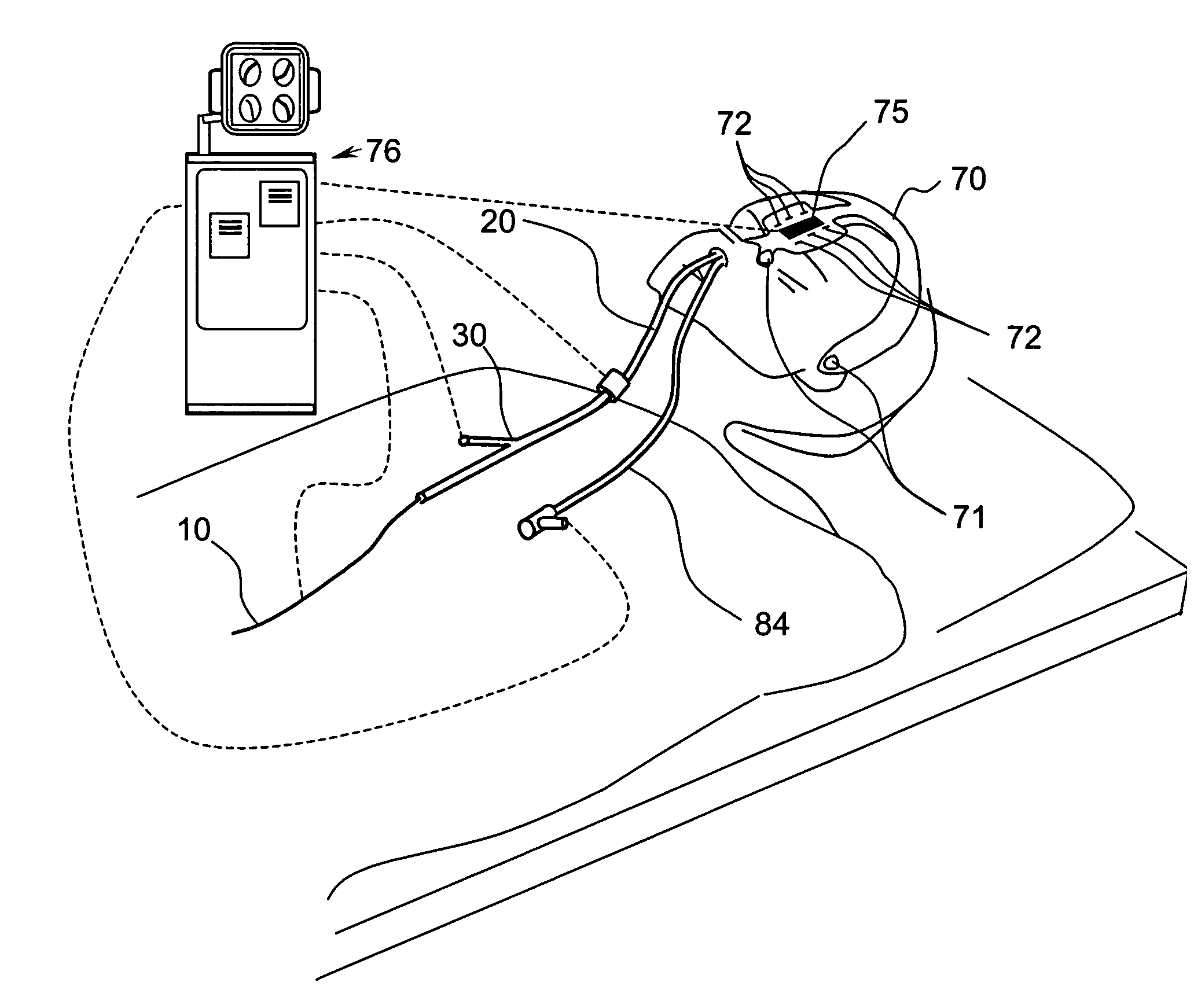
Method and System for Treating Target Tissue Within the Eustachian Tube
InactiveUS20100274188A1Reduce edemaPromote wettingElectrotherapyEar treatmentNasal passageNasal passages
A method for dilating a Eustachian tube of a patient is disclosed. In one embodiment, the method may involve advancing a dilation device through a nasal passage of the patient to position a dilator of the device at least partially in a Eustachian tube of the patient, expanding the dilator to an expanded configuration to dilate a portion of the Eustachian tube, collapsing the dilator, and removing the dilation device from the patient. The dilated portion of the Eustachian tube remains at least partially dilated after removal of the device.
Owner:ACCLARENT INC
Guidewire apparatus for temporary distal embolic protection
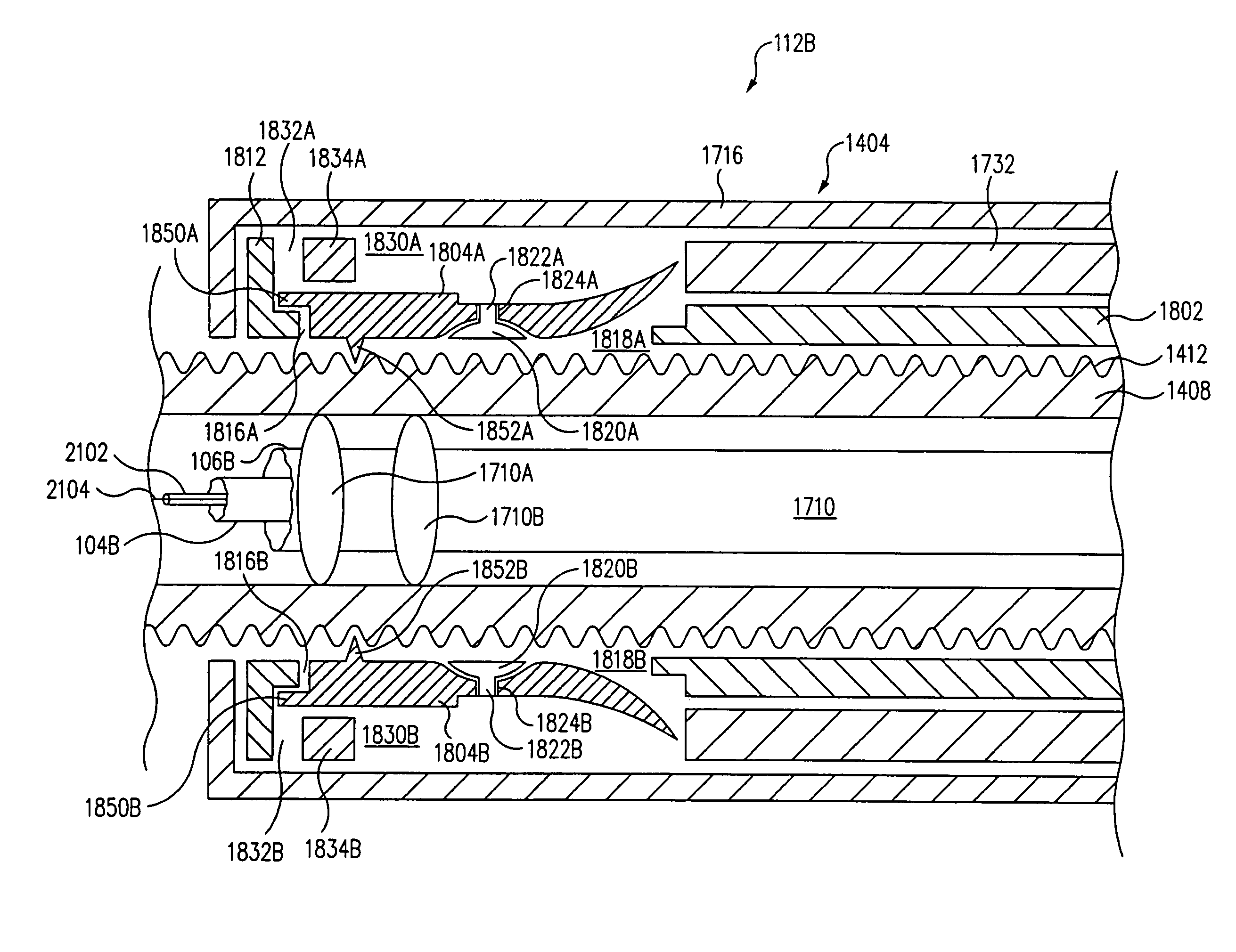
A guidewire apparatus for use during percutaneous catheter interventions, such as angioplasty or stent deployment. A protection element comprising a filter or an occluder is mounted near the distal end of a steerable guidewire, which guides a therapeutic catheter. The guidewire apparatus comprises a hollow shaft movably disposed about a core wire and, optionally, a slippery liner interfitted there between. The shaft and core wire control relative displacement of the ends of the protection element, causing transformation of the protection element between a deployed configuration and a collapsed configuration.
Owner:MEDTRONIC VASCULAR INC
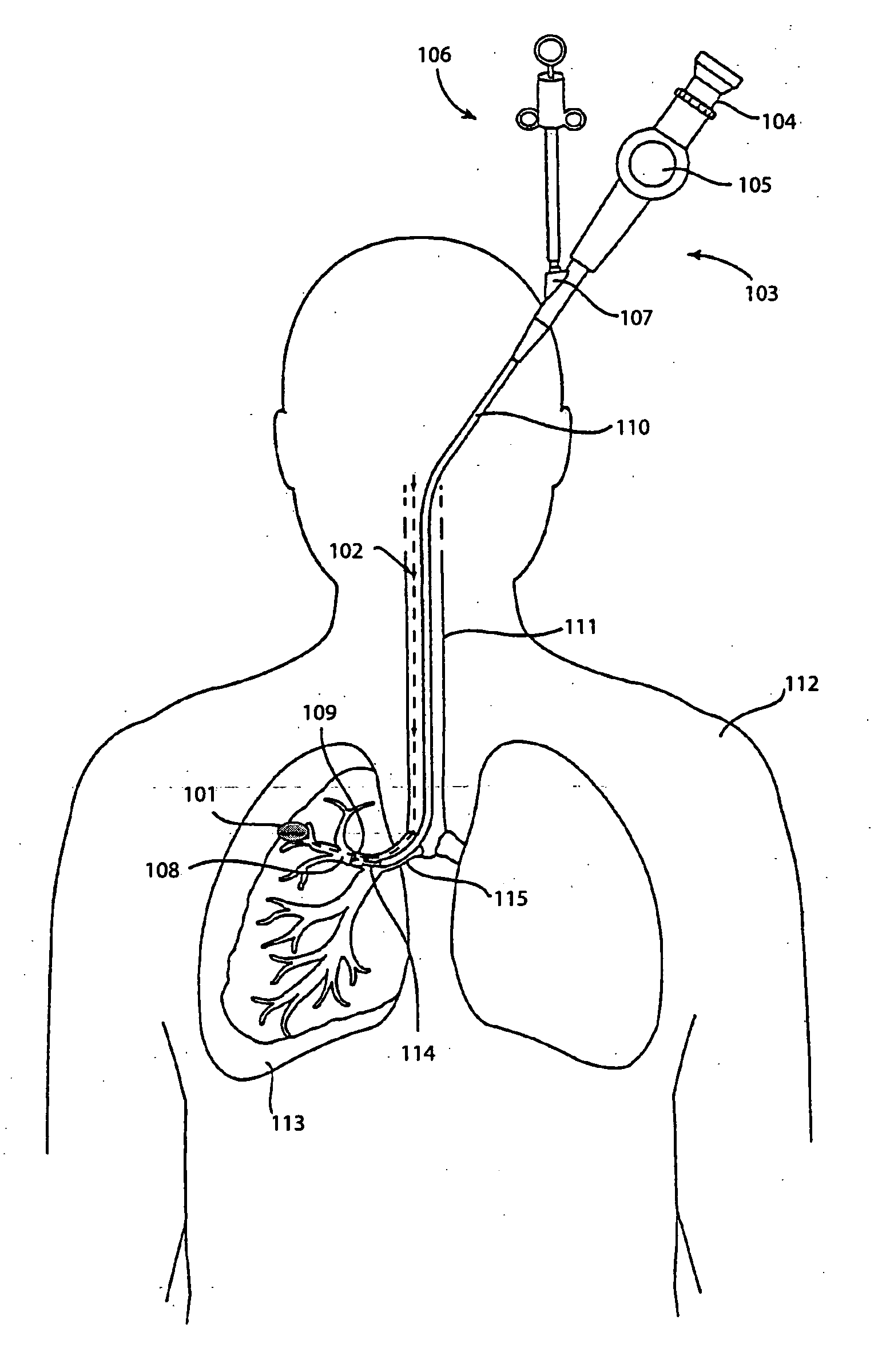
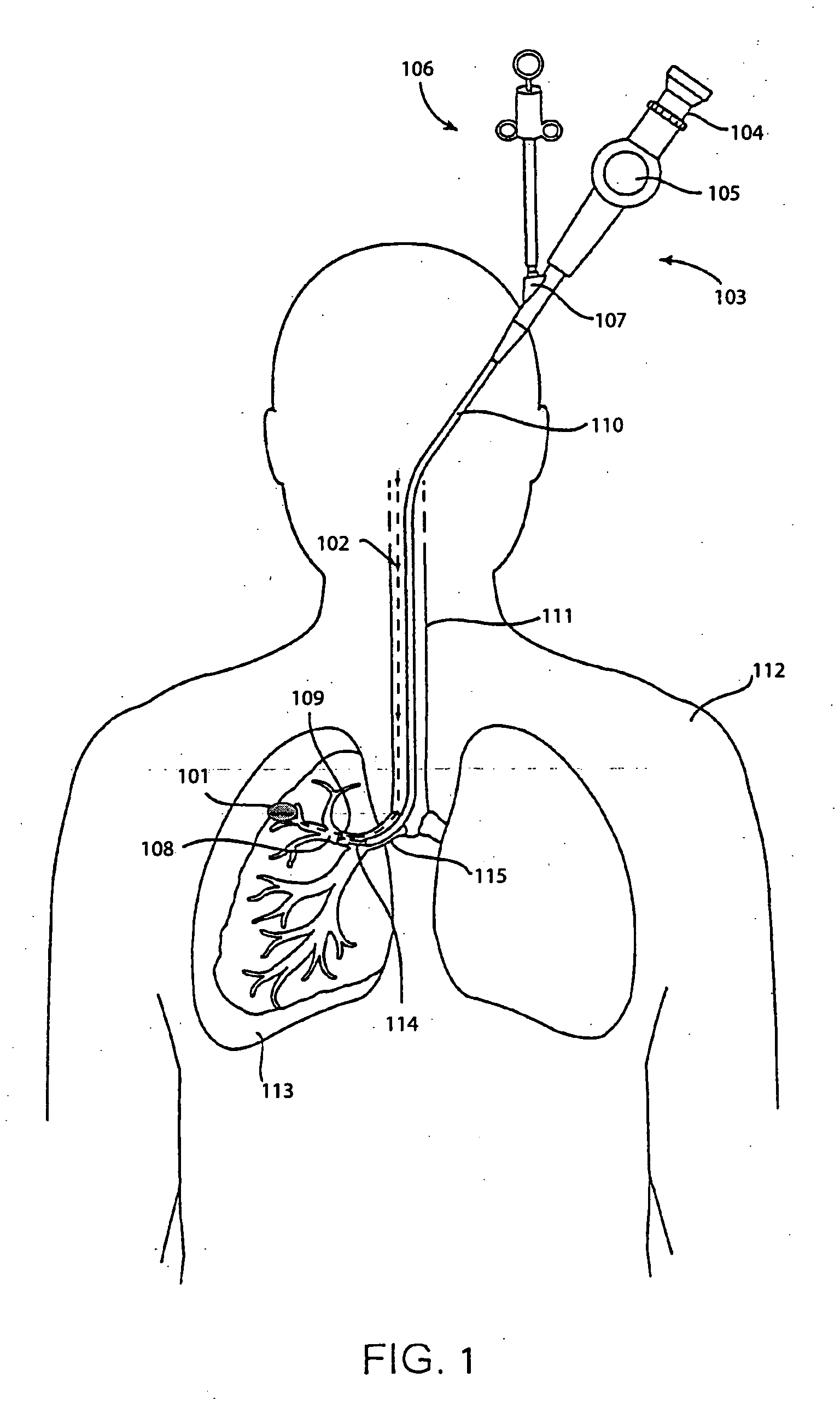
Method and apparatus for guiding an instrument to a target in the lung
ActiveUS20060184016A1Precise positioningImprove accuracyBronchoscopesGuide wiresMarine navigationMedical treatment
The invention provides methods and apparatus for navigating a medical instrument to a target in the lung. In one embodiment, the invention includes inserting a bronchoscope into the lung, inserting a catheter into the lung through the working channel of the bronchoscope, inserting a tracked navigation instrument wire into the lung through the catheter, navigating the tracked navigation instrument through the lung to the target, advancing the catheter over the tracked navigation instrument to the target, removing the tracked navigation instrument from the catheter, and inserting a medical instrument into the catheter, thus bringing the medical instrument in proximity to the target.
Owner:PHILIPS ELECTRONICS LTD
Steerable guide catheters and methods for their use
ActiveUS7402151B2Optimize catheter shape and steerabilityImprove navigabilityGuide needlesEar treatmentAccess routeGuide tube
Methods for easy, atraumatic access to areas of the vasculature that are otherwise difficult to access, using steerable guide catheters constructed with components that are selected to provide optimal navigability, torque transfer, and push ability for a variety of typical percutaneous access routes. The catheter wall thickness in the deflecting segment of the guide catheter is about 1 French (⅓ mm) or less, and includes a slotted deflection tube, and this construction allows a very tight turning radius which in turn enables guide catheter access to regions of the vasculature that are otherwise inaccessible.
Owner:BIOCARDIA
Methods and devices for facilitating visualization in a surgical environment
ActiveUS7559925B2Avoid insufficient lengthSufficient lightingMedical devicesEndoscopesMedicineLight emitting device
Owner:ACCLARENT INC
Translumenally implantable heart valve with formed in place support
A cardiovascular prosthetic valve, the valve comprising an inflatable cuff comprising at least one inflatable channel that forms, at least in part, an inflatable structure, and a valve coupled to the inflatable cuff, the valve configured to permit flow in a first axial direction and to inhibit flow in a second axial direction opposite to the first axial direction, the valve comprising a plurality of tissue supports that extend generally in the axial direction and that are flexible and / or movable throughout a range in a radial direction.
Owner:DIRECT FLOW MEDICAL INC
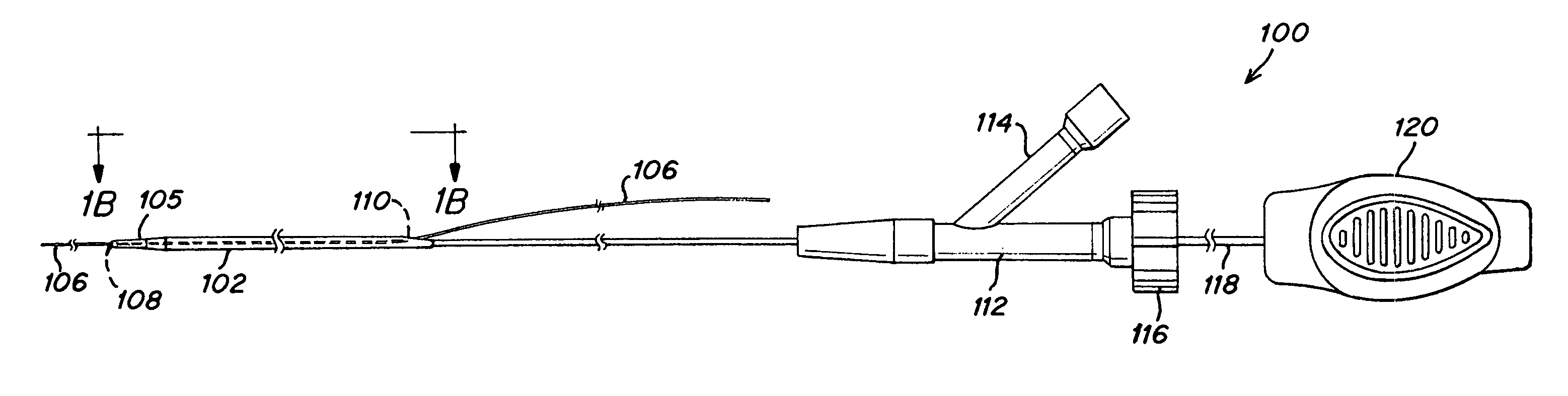
Handle deployment mechanism for medical device and method
InactiveUS6939352B2Easy and accurate deployment and positioningStentsEar treatmentMedical deviceBiomedical engineering
A system for delivering at least one medical device to a desired location for treatment, and then selectively deploy it in position, includes an improved handle. One of the possible features of the handle may be to selectively hold the delivery system components at any desired configuration during deployment and positioning of the medical device. Another possible feature of the handle may be more than one mode of operation, in which the deployment of the medical device can selectively proceed at more than one speed.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Implant delivery system with interlocked RX port orientation
A medical implant delivery system maintains an orientation between a guidewire lumen of an inner member of the system and a rapid-exchange port in an outer member. The medical device is disposed intermediate the inner and outer members and in friction or pressure-fit contact with the outer member. Once the guidewire lumen of the inner member and the rapid exchange port of the outer member are oriented, the friction or pressure-fit operates to maintain the orientation until deployment of the medical implant. Orientation is further maintained by a telescoping coupling of the guide wire lumen with the rapid exchange port.
Owner:TYCO HEALTHCARE GRP LP
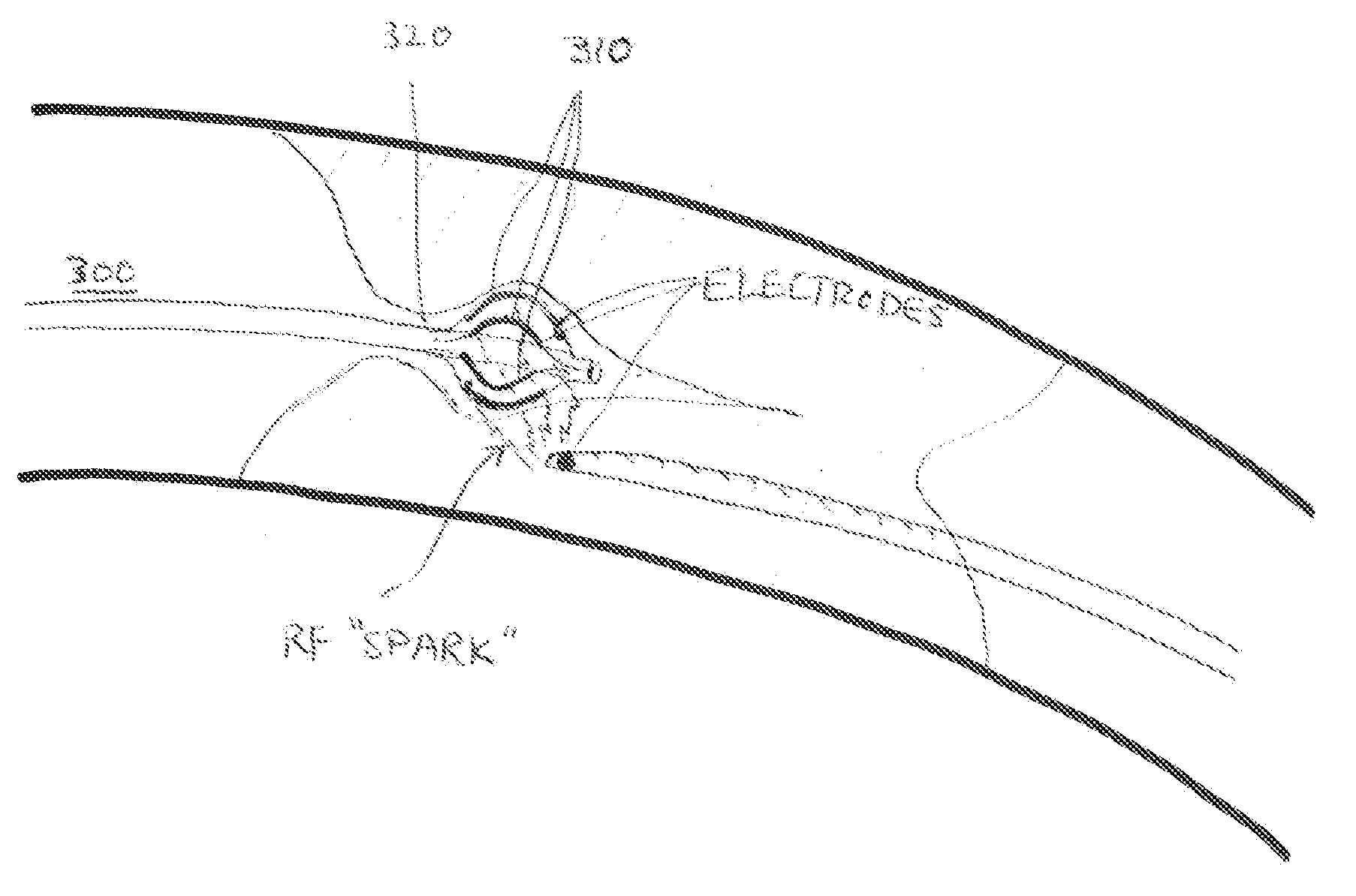
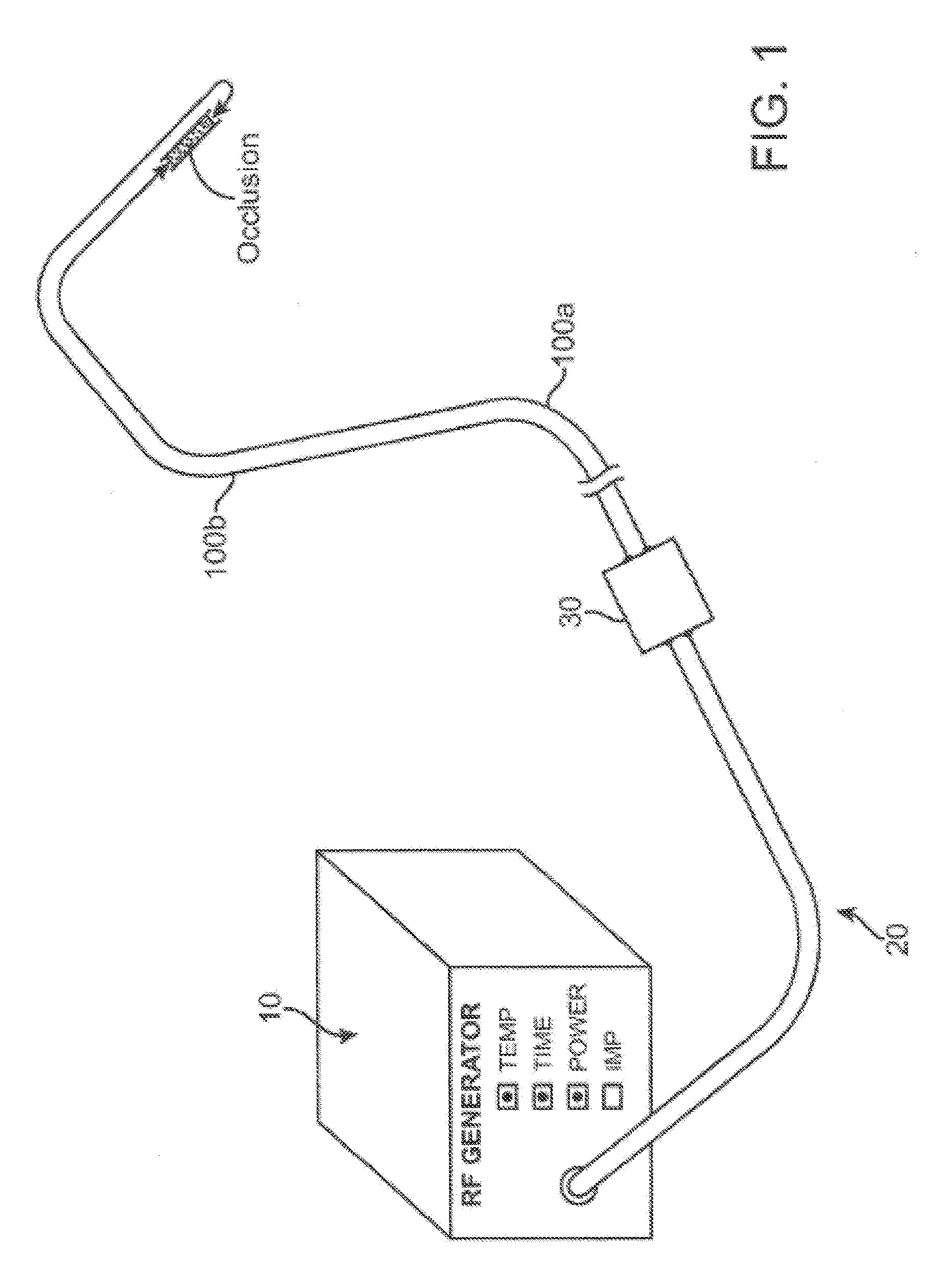
Recanalizing occluded vessels using radiofrequency energy
A method and systems for treating chronic total occlusions, particularly those that are difficult to treat, is disclosed. In this approach, recanalizing the CTO is achieved using a combined antegrade and retrograde approach. The proximal end of the occlusion is penetrated using an antegrade wire, using a traditional approach. Using collateral vessels, the distal end of the occlusion is crossed in a retrograde fashion. By appropriately maneuvering each member and applying radiofrequency energy between the proximal and distal ends of the occlusion, a continuous channel is created.
Owner:ASAHI MEDICAL TECH INC
Tissue sampling devices, systems and methods
ActiveUS20100312141A1Minimize unintended injuryHigh strengthBronchoscopesGuide wiresNeedle penetrationMedicine
Methods, devices, and systems are described herein that allow for improved sampling of tissue from remote sites in the body. A tissue sampling device comprises a handle allowing single hand operation. In one variation the tissue sampling device includes a blood vessel scanning means and tissue coring means to excise a histology sample from a target site free of blood vessels. The sampling device also includes an adjustable stop to control the depth of a needle penetration. The sampling device may be used through a working channel of a bronchoscope.
Owner:BRONCUS MEDICAL
Navigation system for cardiac therapies using gating
InactiveUS20100030061A1Accurate identificationReduce exposureUltrasonic/sonic/infrasonic diagnosticsElectrotherapyEcg signalDisplay device
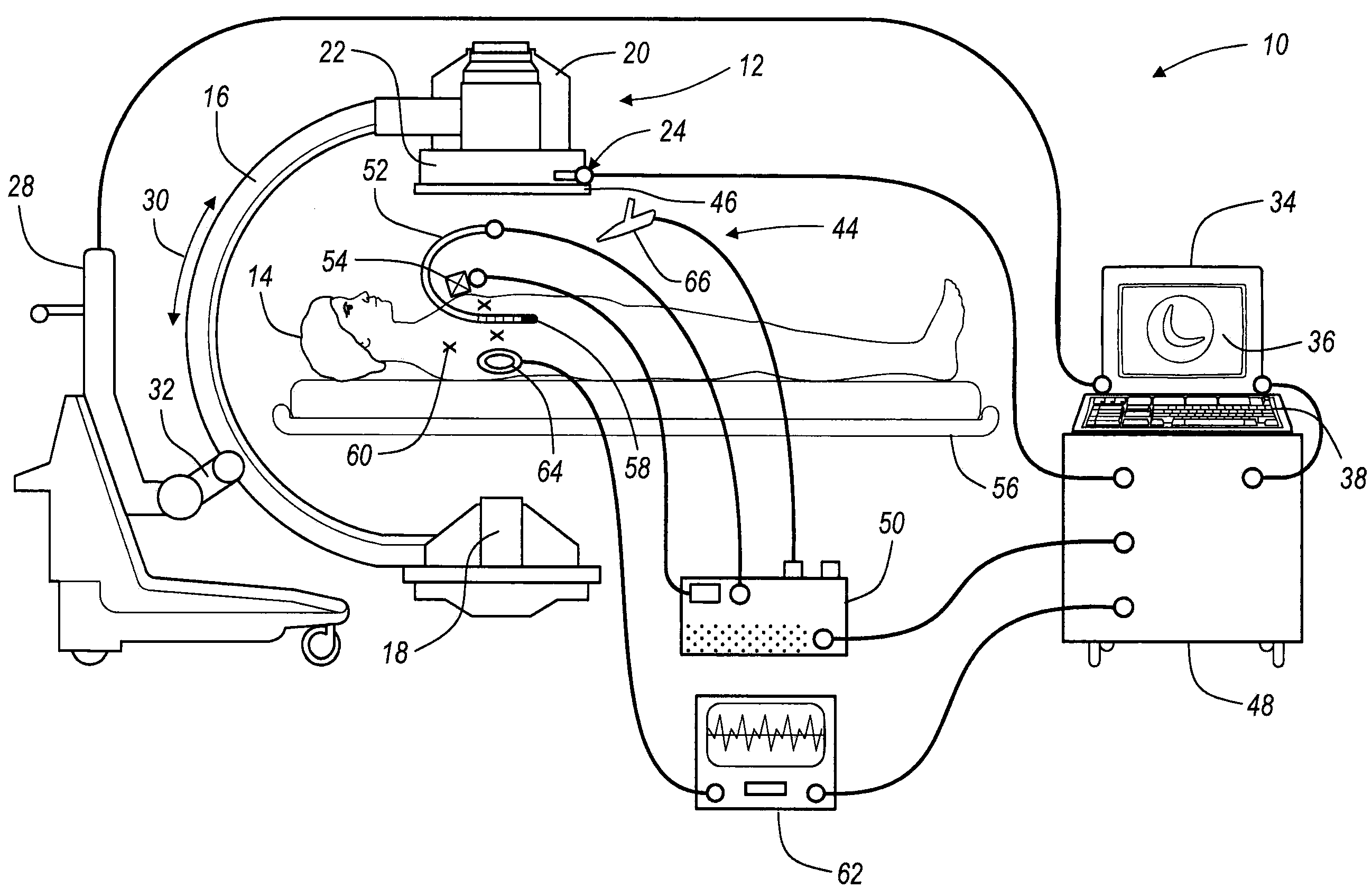
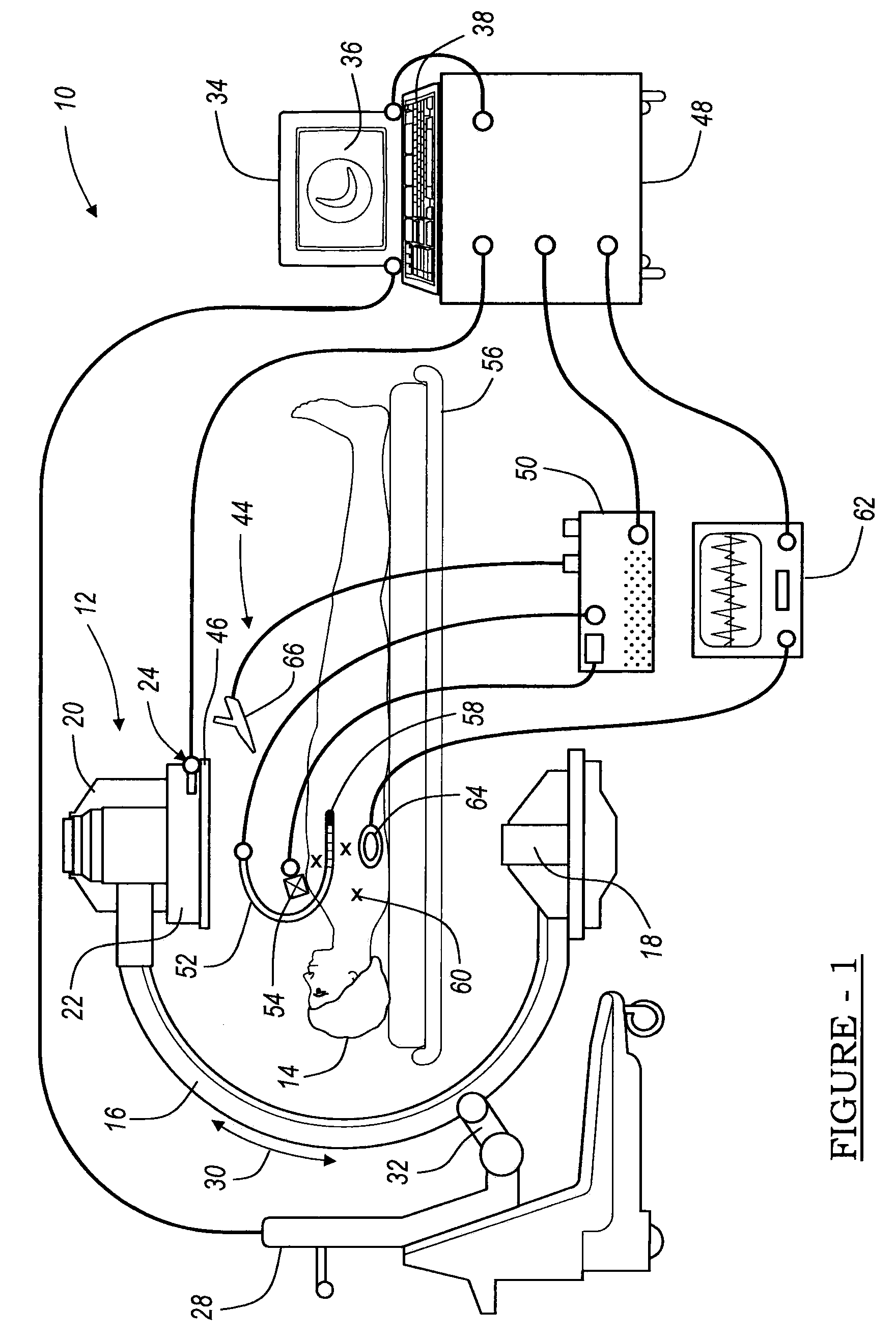
An image guided navigation system for navigating a region of a patient which is gated using ECG signals to confirm diastole. The navigation system includes an imaging device, a tracking device, a controller, and a display. The imaging device generates images of the region of a patient. The tracking device tracks the location of the instrument in a region of the patient. The controller superimposes an icon representative of the instrument onto the images generated from the imaging device based upon the location of the instrument. The display displays the image with the superimposed instrument. The images and a registration process may be synchronized to a physiological event.
Owner:MEDTRONIC INC
Prevention of myocardial infarction induced ventricular expansion and remodeling
ActiveUS20050080402A1Prevent further deteriorationInhibit swellingSuture equipmentsPowder deliveryCardiac muscleTherapeutic treatment
A method for direct therapeutic treatment of myocardial tissue in a localized region of a heart having a pathological condition. The method includes identifying a target region of the myocardium and applying material directly and substantially only to at least a portion of the myocardial tissue of the target region. The material applied results in a physically modification the mechanical properties, including stiffness, of said tissue. Various devices and modes of practicing the method are disclosed for stiffening, restraining and constraining myocardial tissue for the treatment of conditions including myocardial infarction or mitral valve regurgitation.
Owner:MYOMEND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com