Patents
Literature
12594results about "Suction devices" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
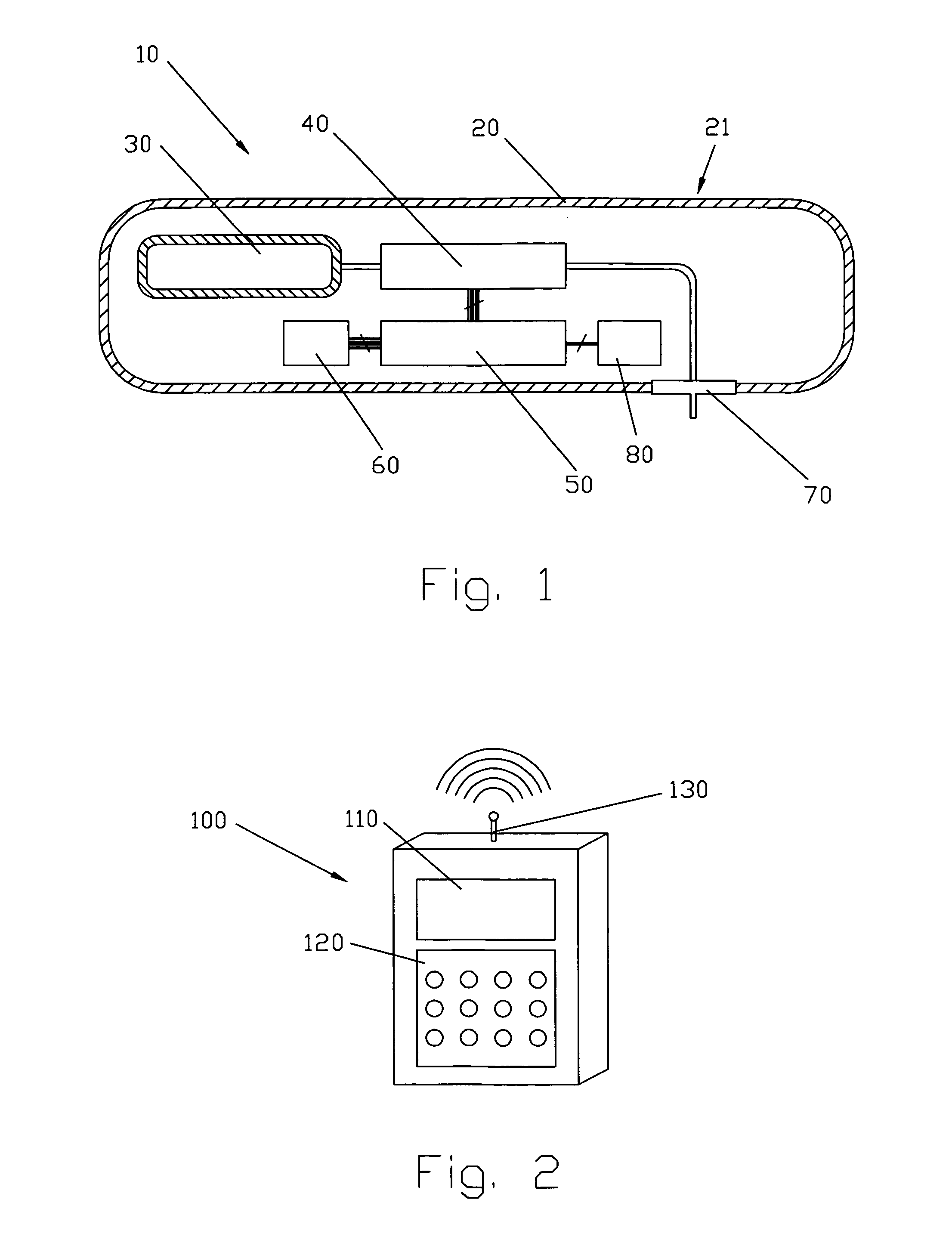
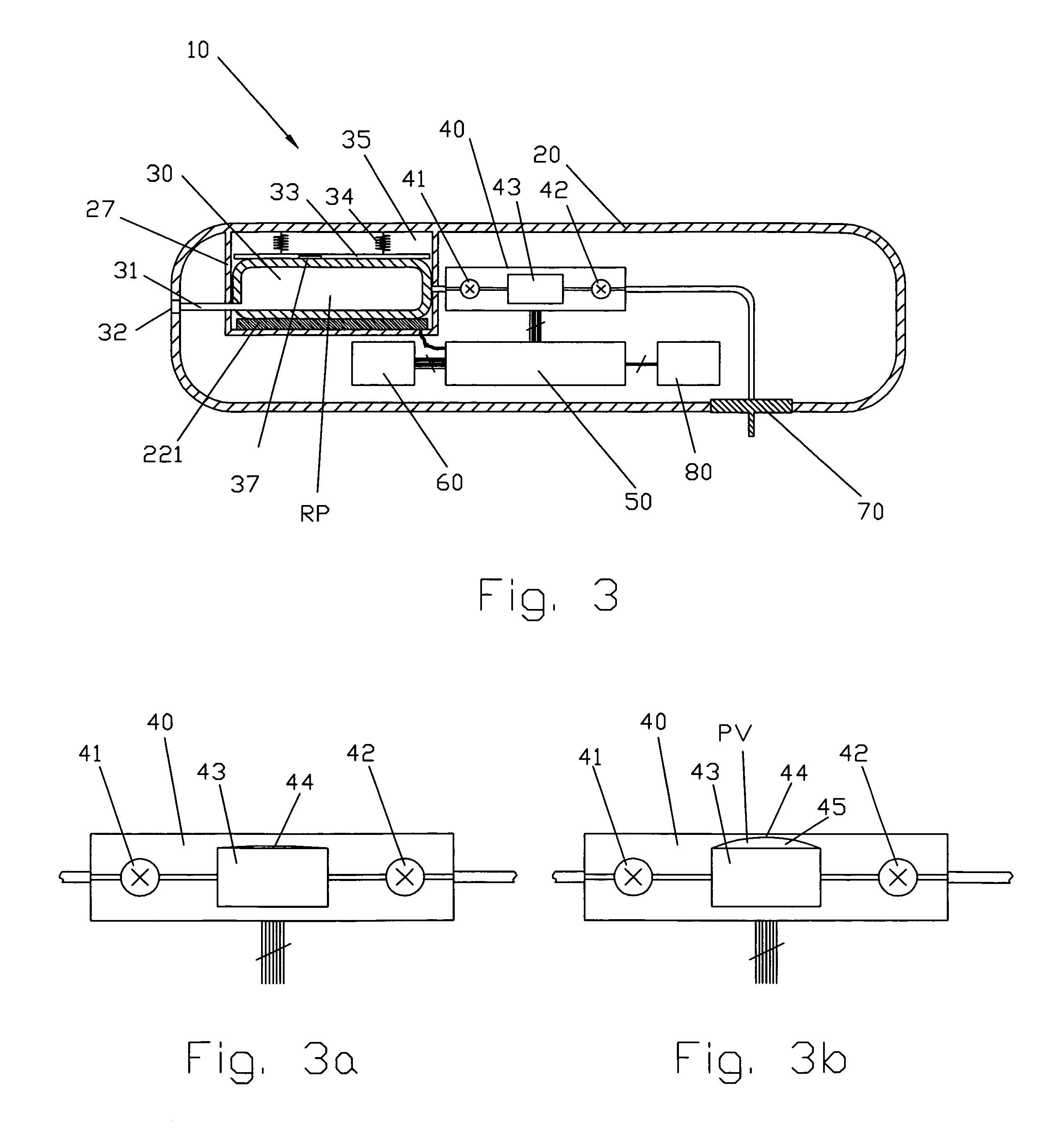
Smart recognition apparatus and method
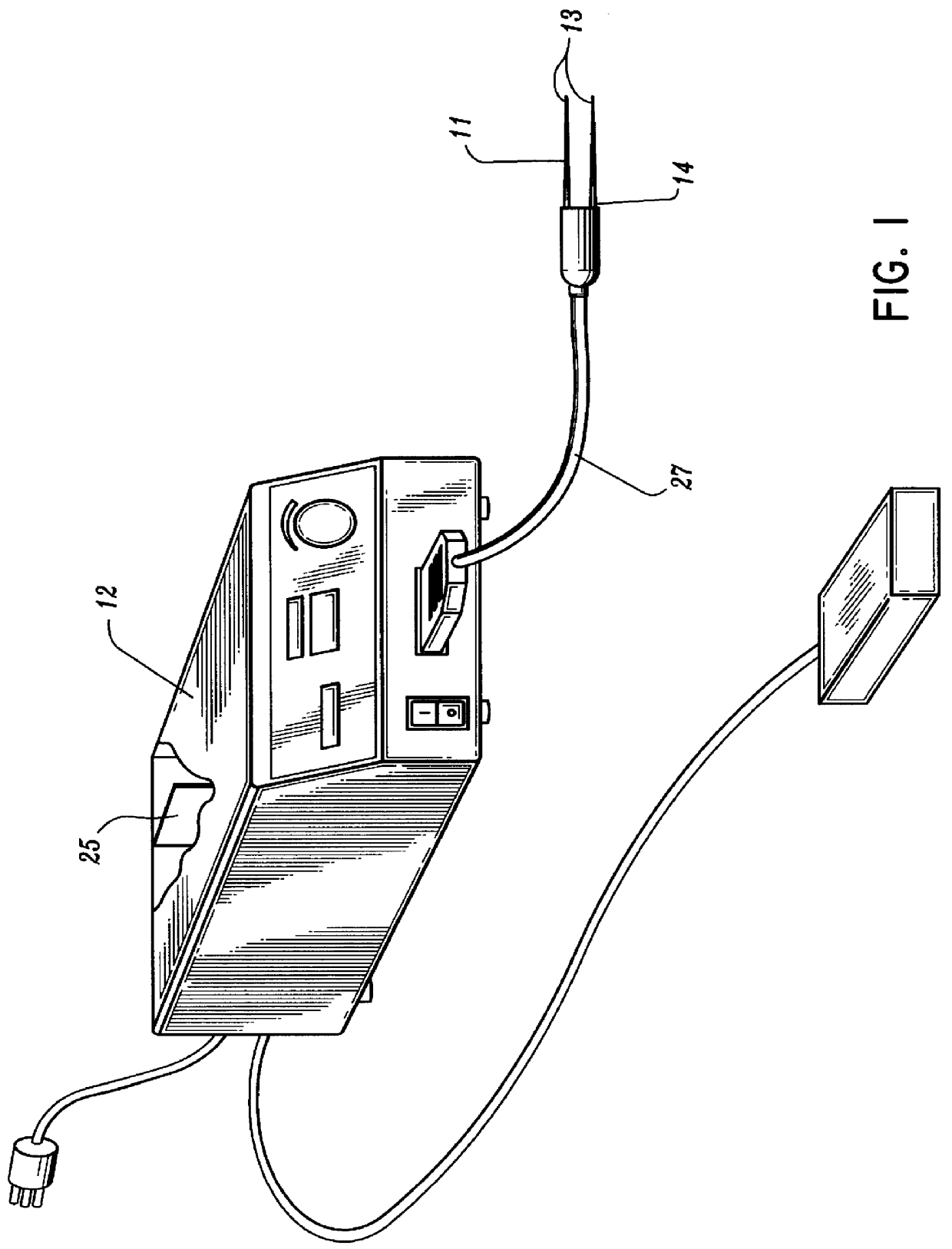
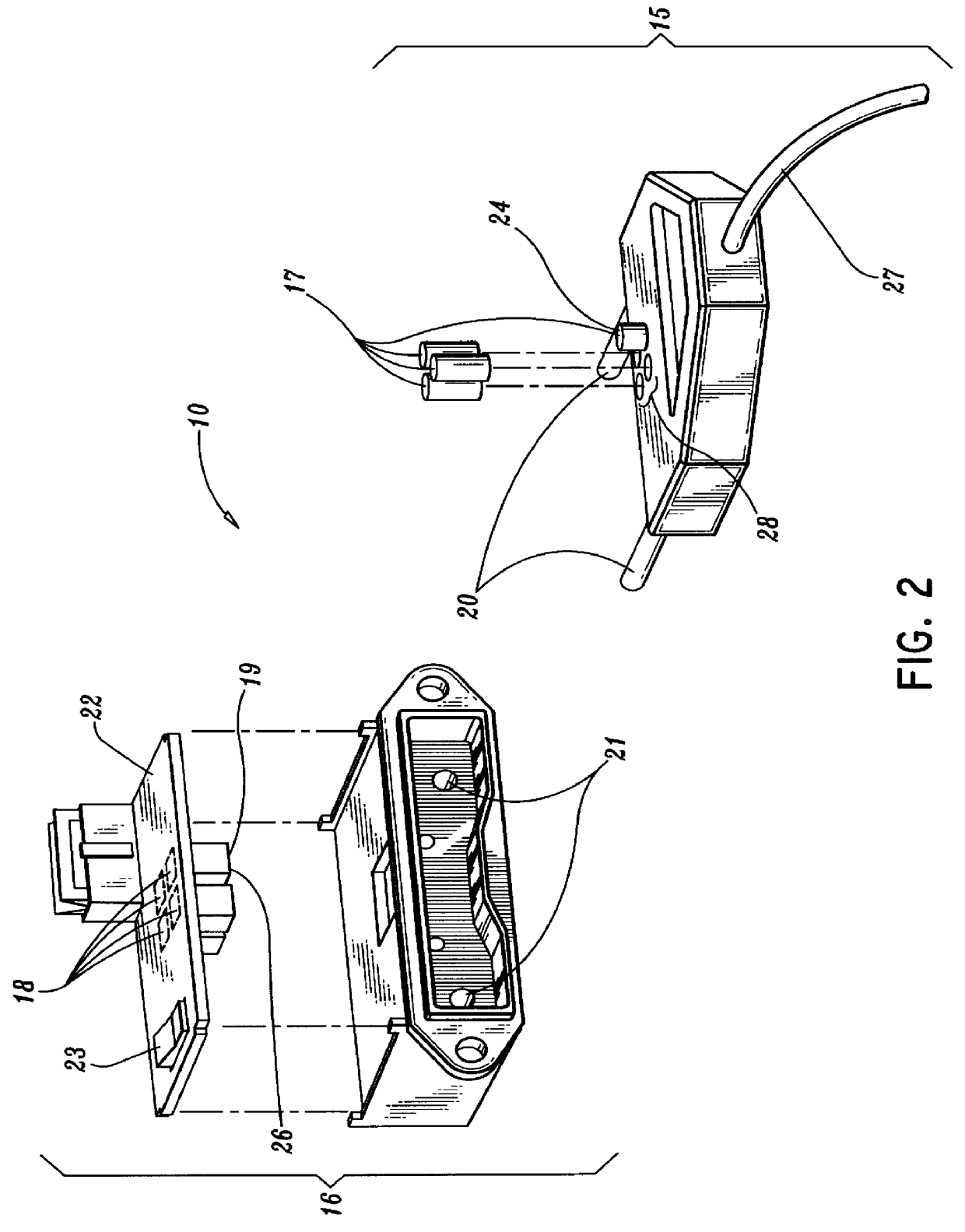
A qualifying connection for an instrument attaches to a source of electrosurgery energy to and the instrument and has first and second parts coupled to the instrument and the source, respectively. Optical couplings on the connection transmit invisible energy to identify the instrument and are proximate on the first and second parts. A light modifier on the first part is proximal to the second part for modification of radiation in the infrared wavelengths so infrared transmitters encode signals and non contact coded proximity detectors on the second part are the coupled detectors. Non contact coded proximity detectors respond to modified infrared light establishing an Nth bit identification code. An infrared light supply in the source pass from the transmitters across the communicating couplings for encoding signals by modification of the infrared light with a light modifier. Mechanical attachments include conjugating male and female portions physically extending between the parts for mating engagement. The attachments juxtaposition the parts when the attachments geometrically conjugate to geographically positioning the couplings proximate for communicating. The attachments have one or more conductors for delivery of high frequency energy from the source to the instrument. A cable fits between the first part of the connection and the instrument and has electrical conductors for carrying energy passing through the first part of the connection from the source to the instrument. An identifying circuit couples to the second part and responds to invisible light optically communicated across the couplings for verifying the type of instrument connected by the cable to the source.
Owner:COVIDIEN AG
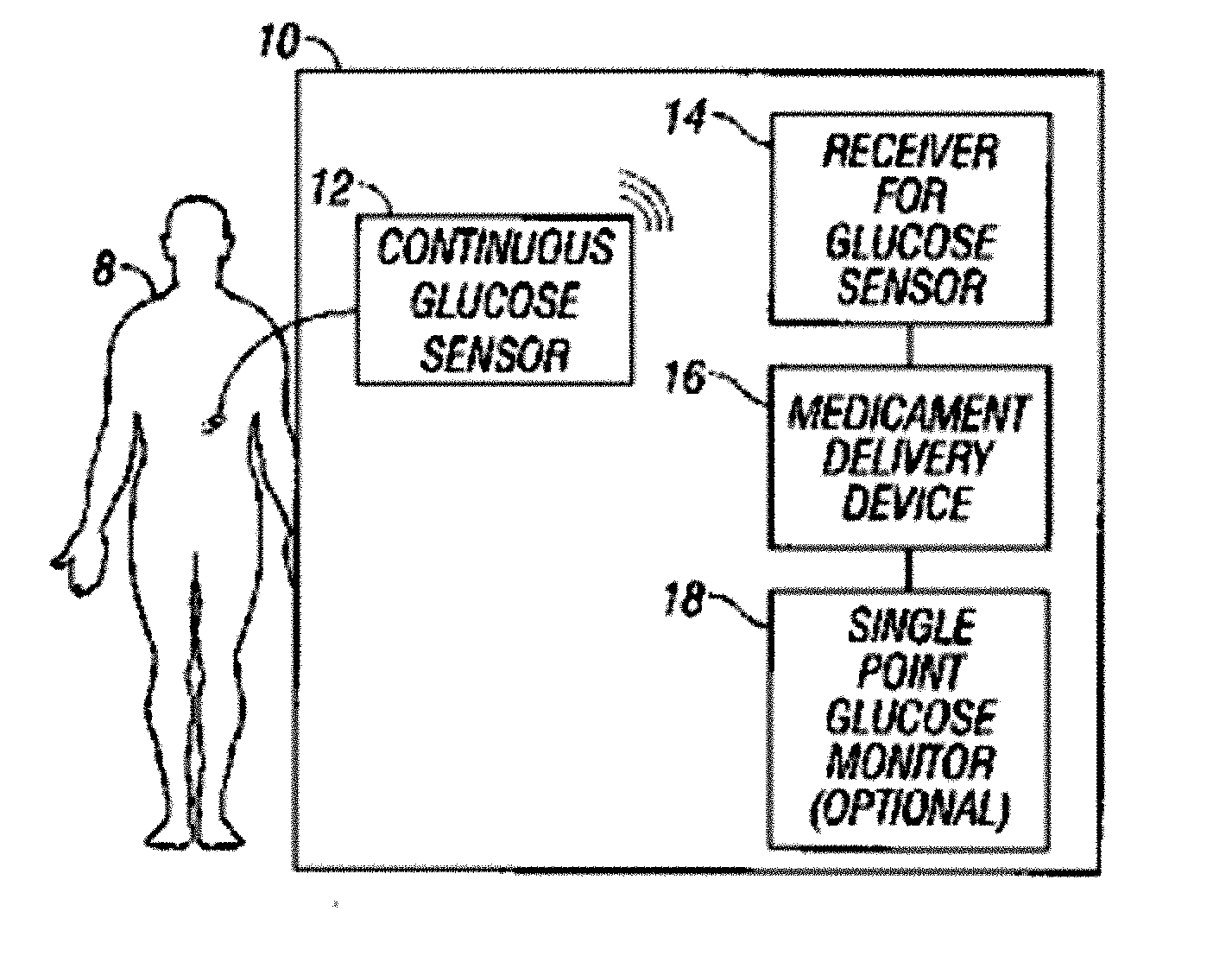
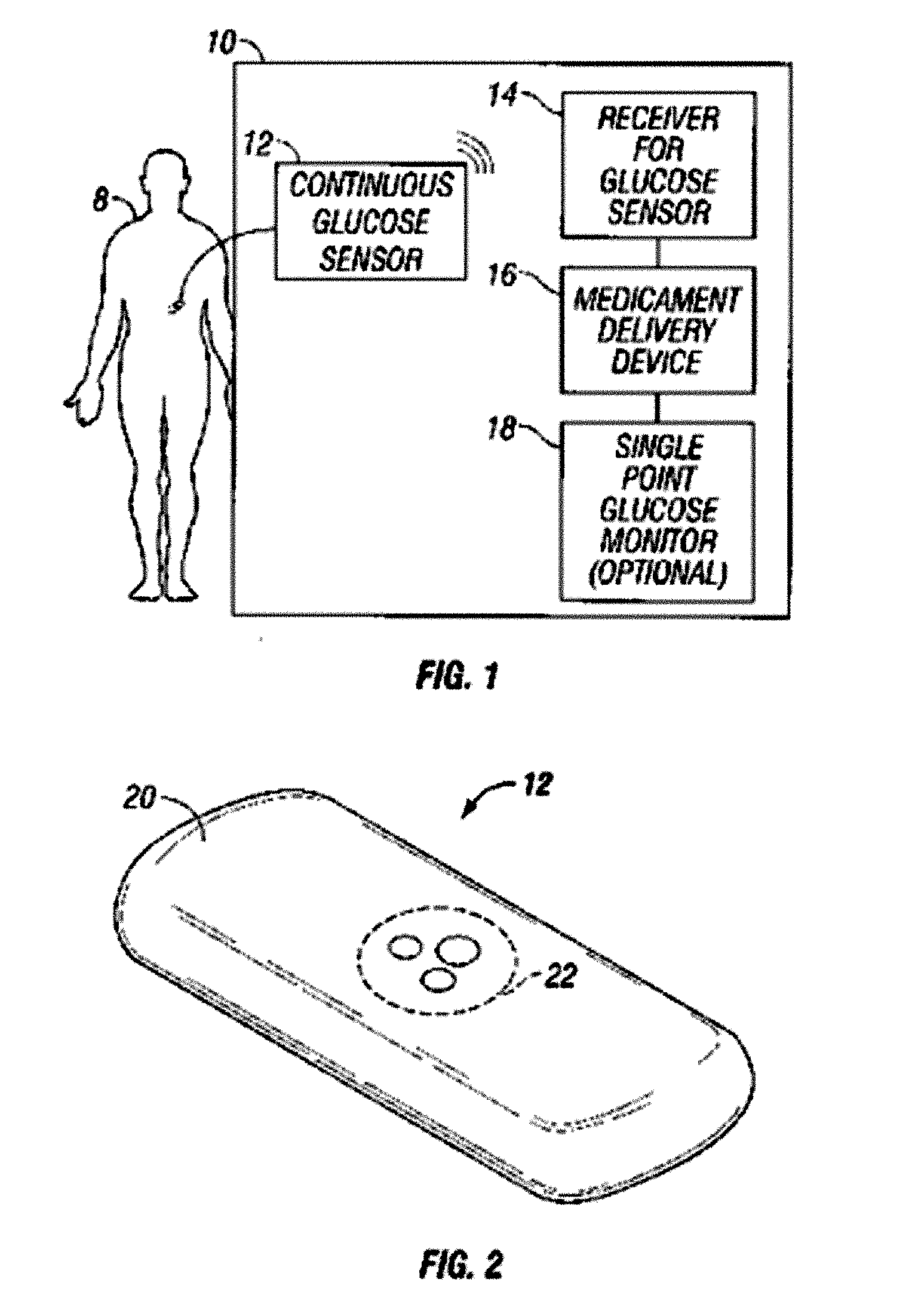
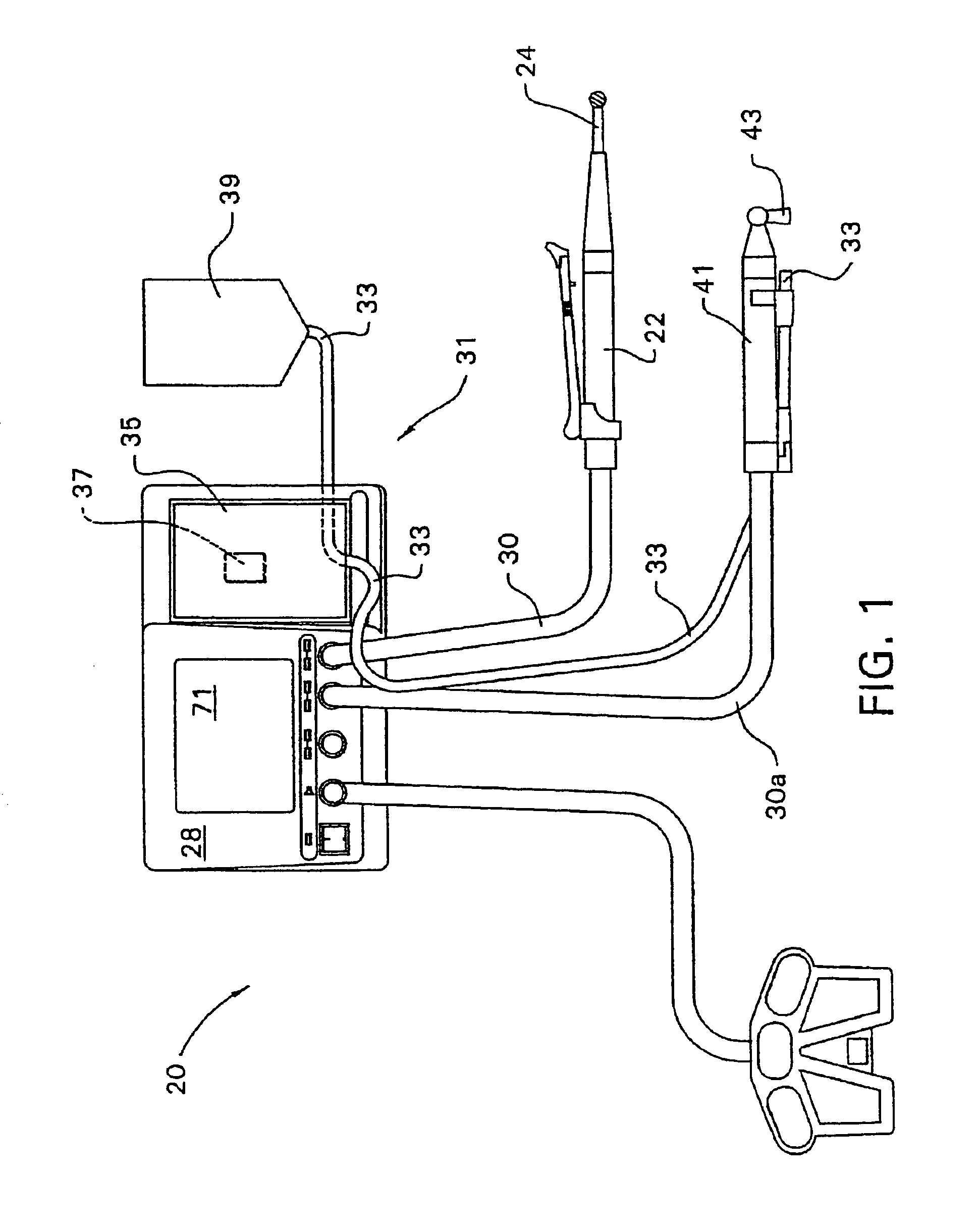
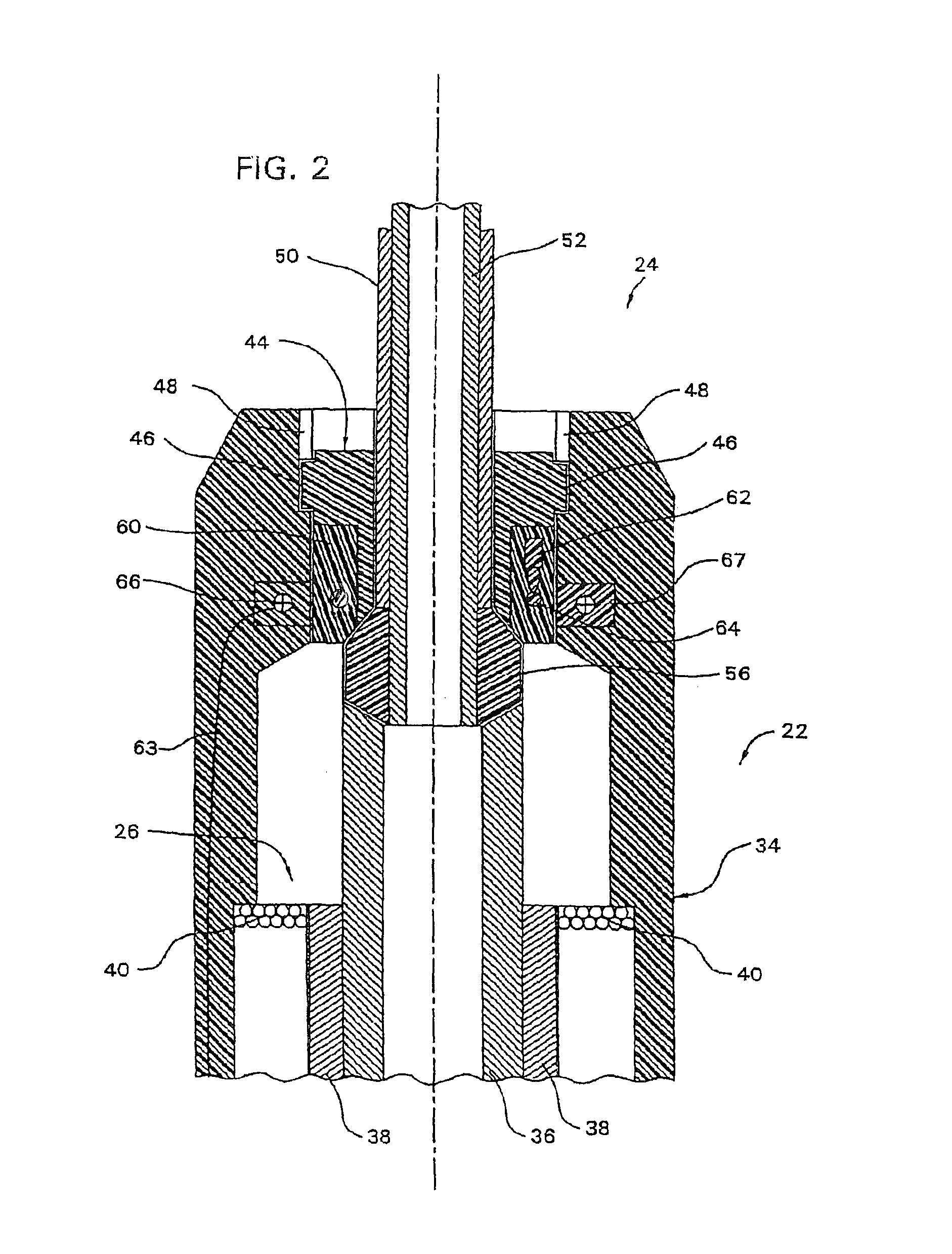
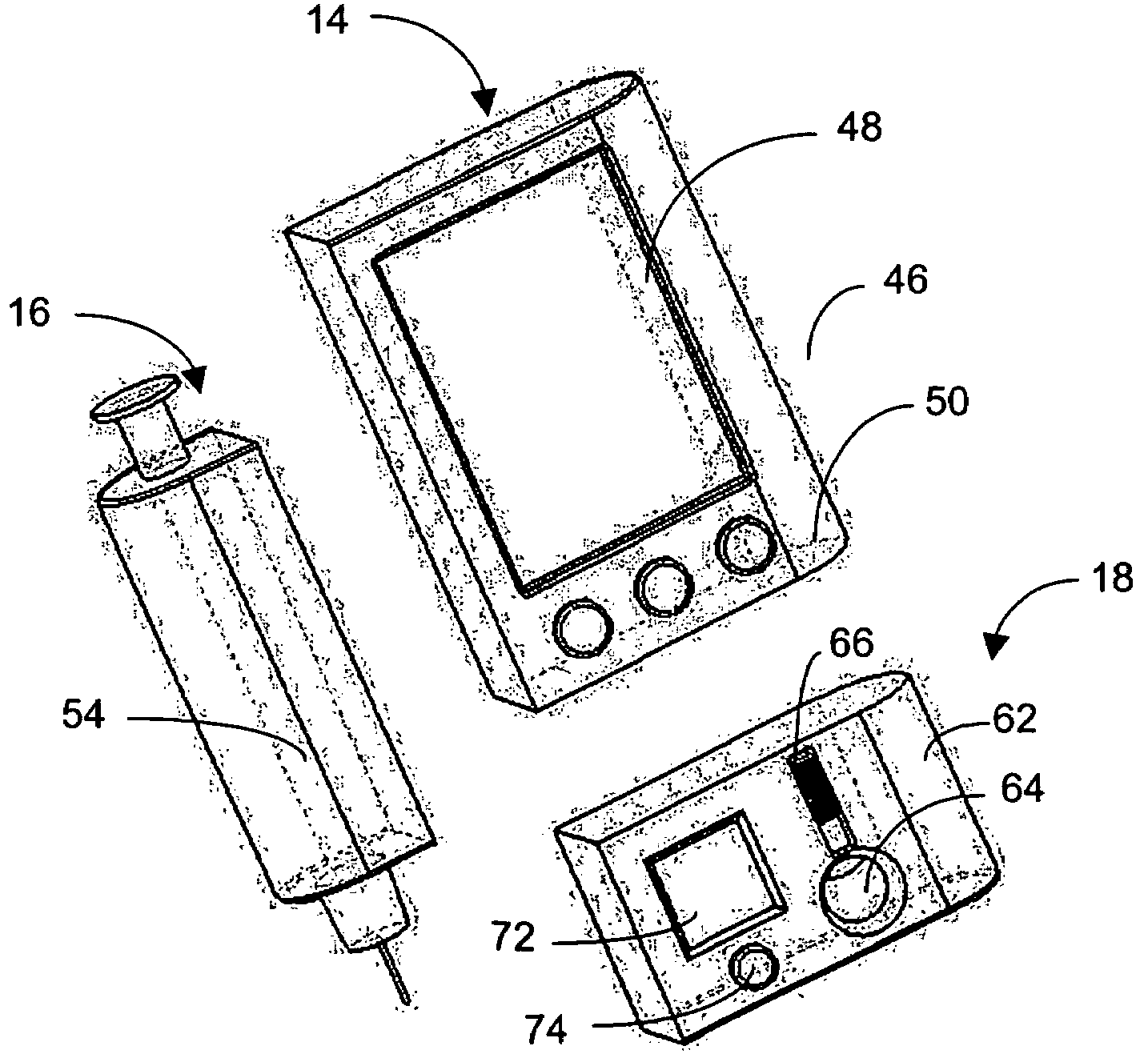
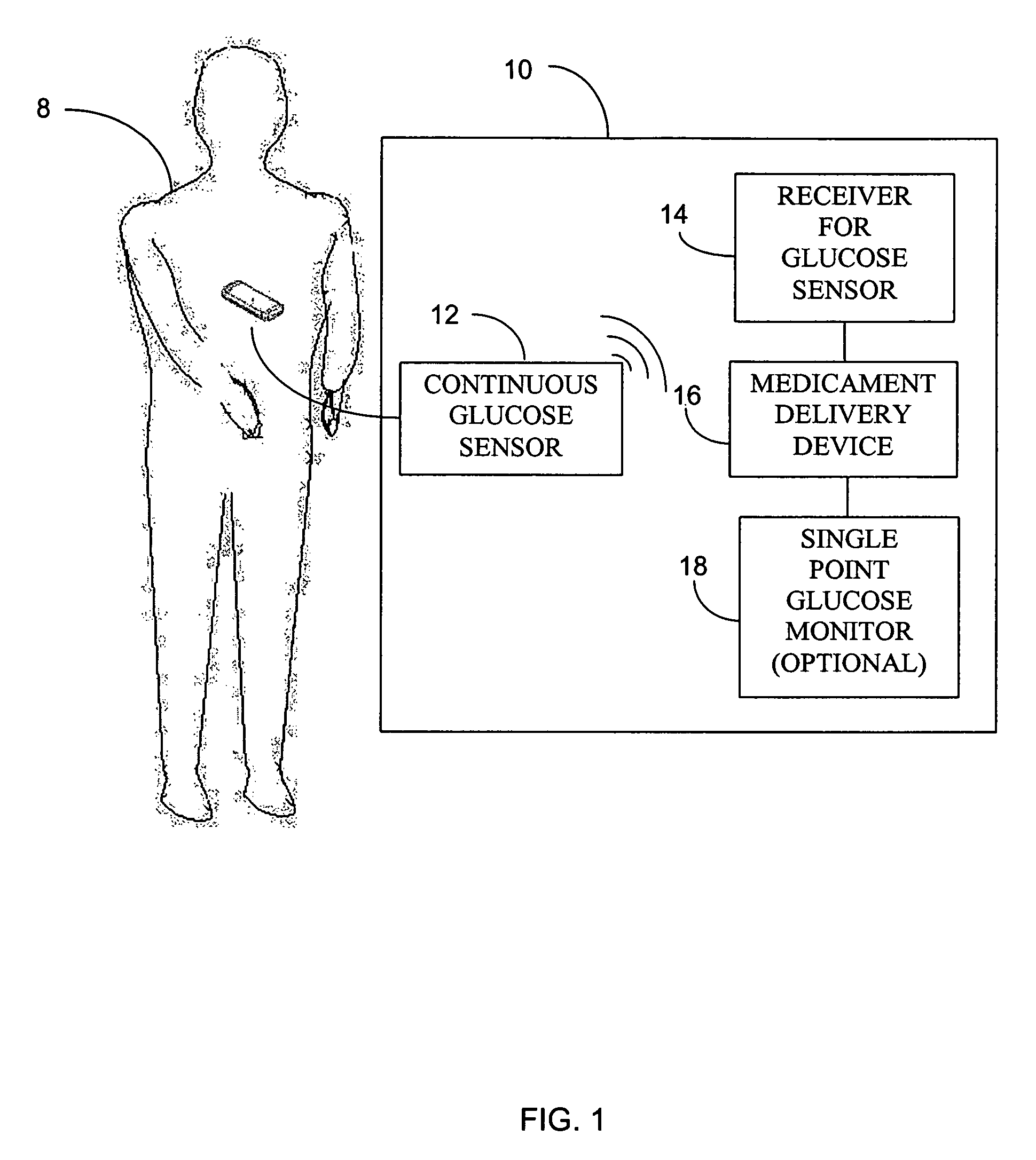
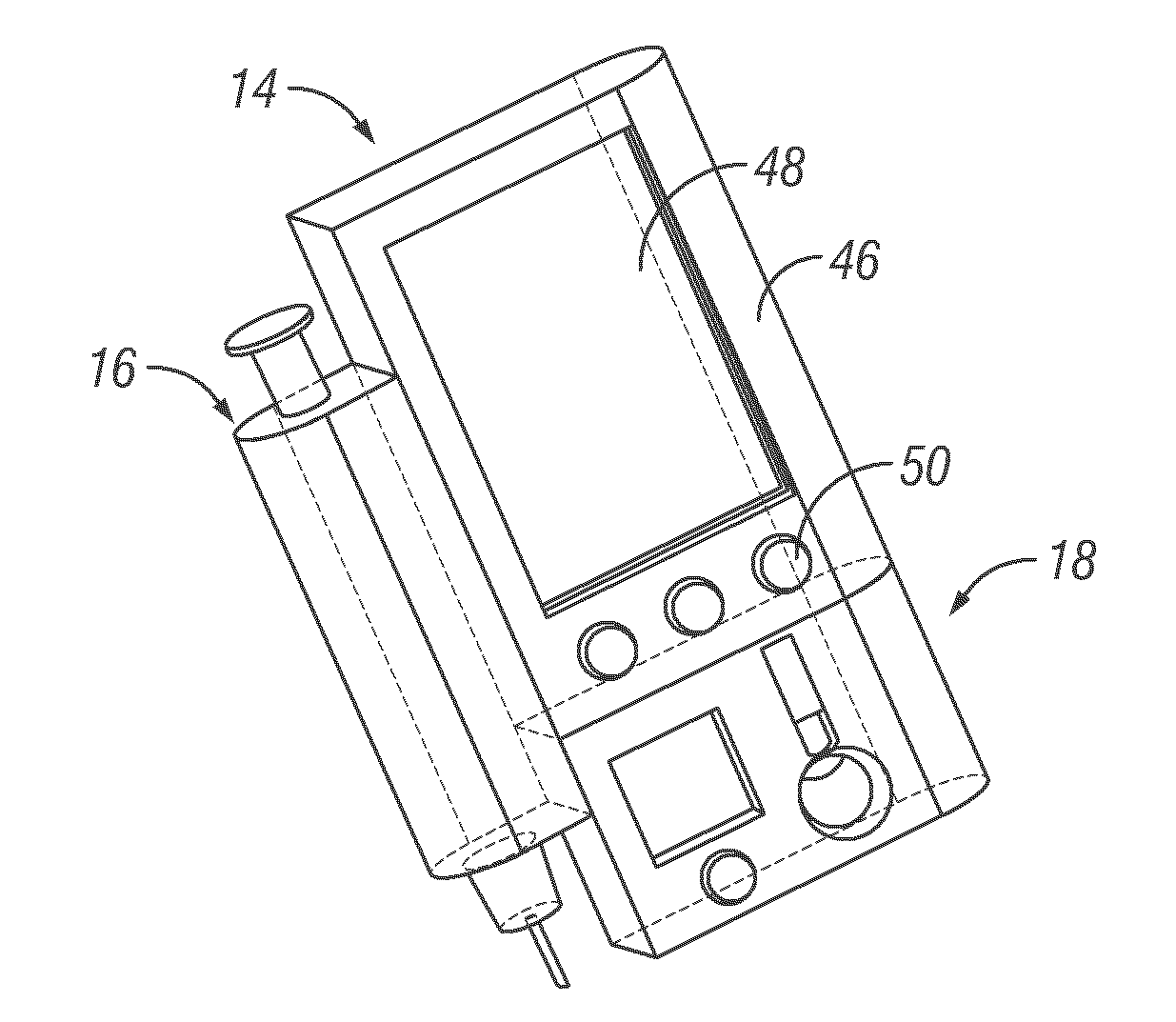
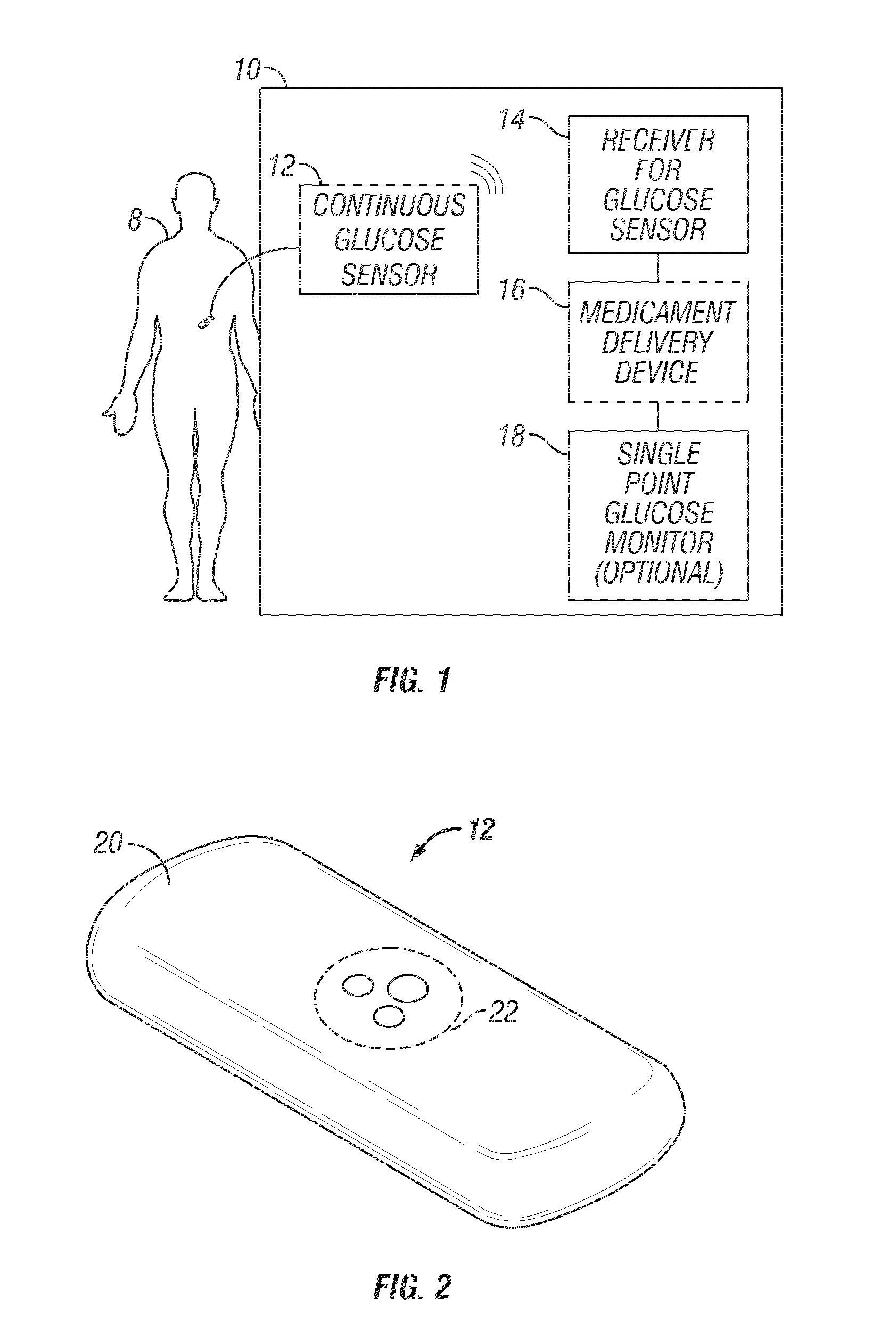
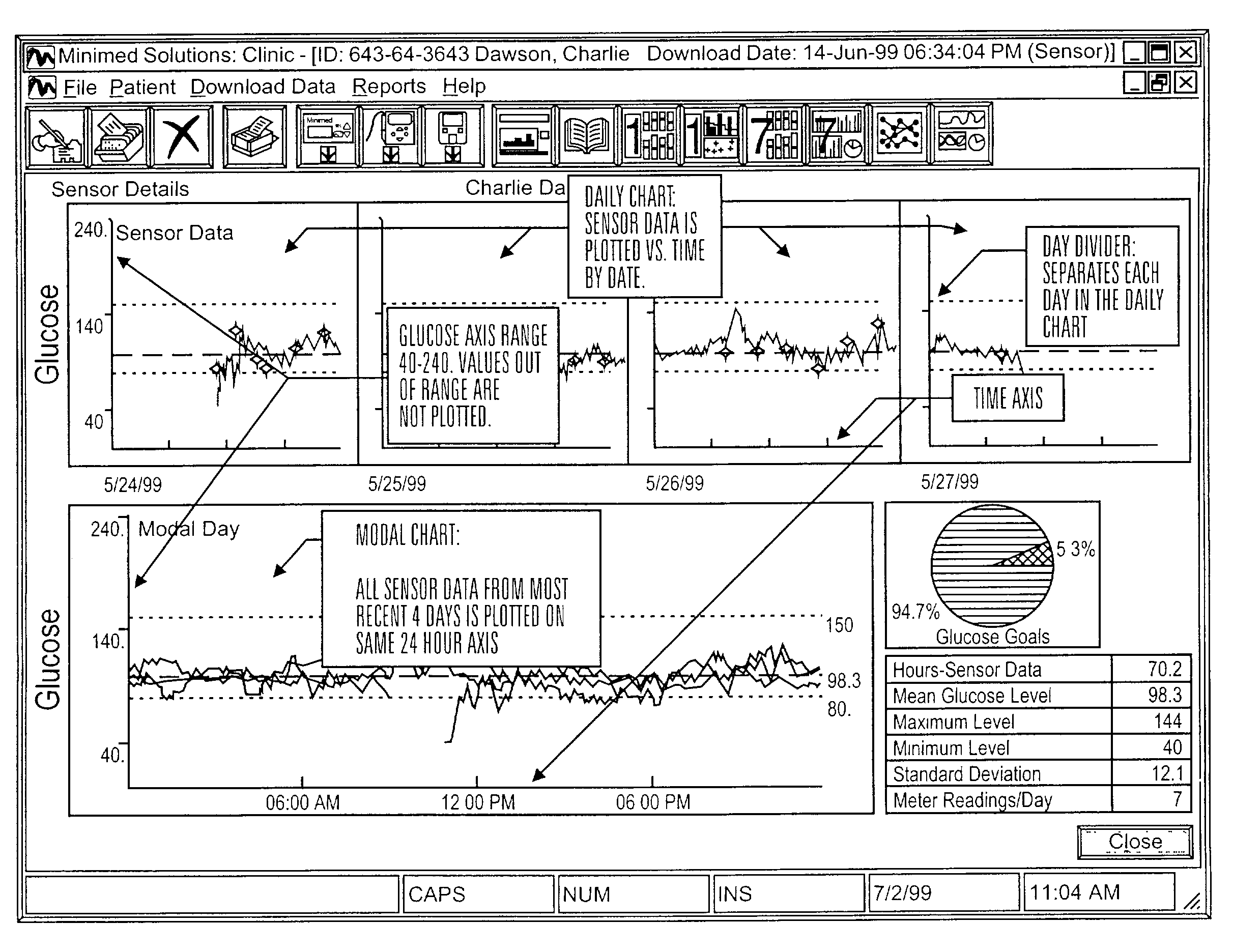
Integrated delivery device for continuous glucose sensor
Abstract of the DisclosureSystems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
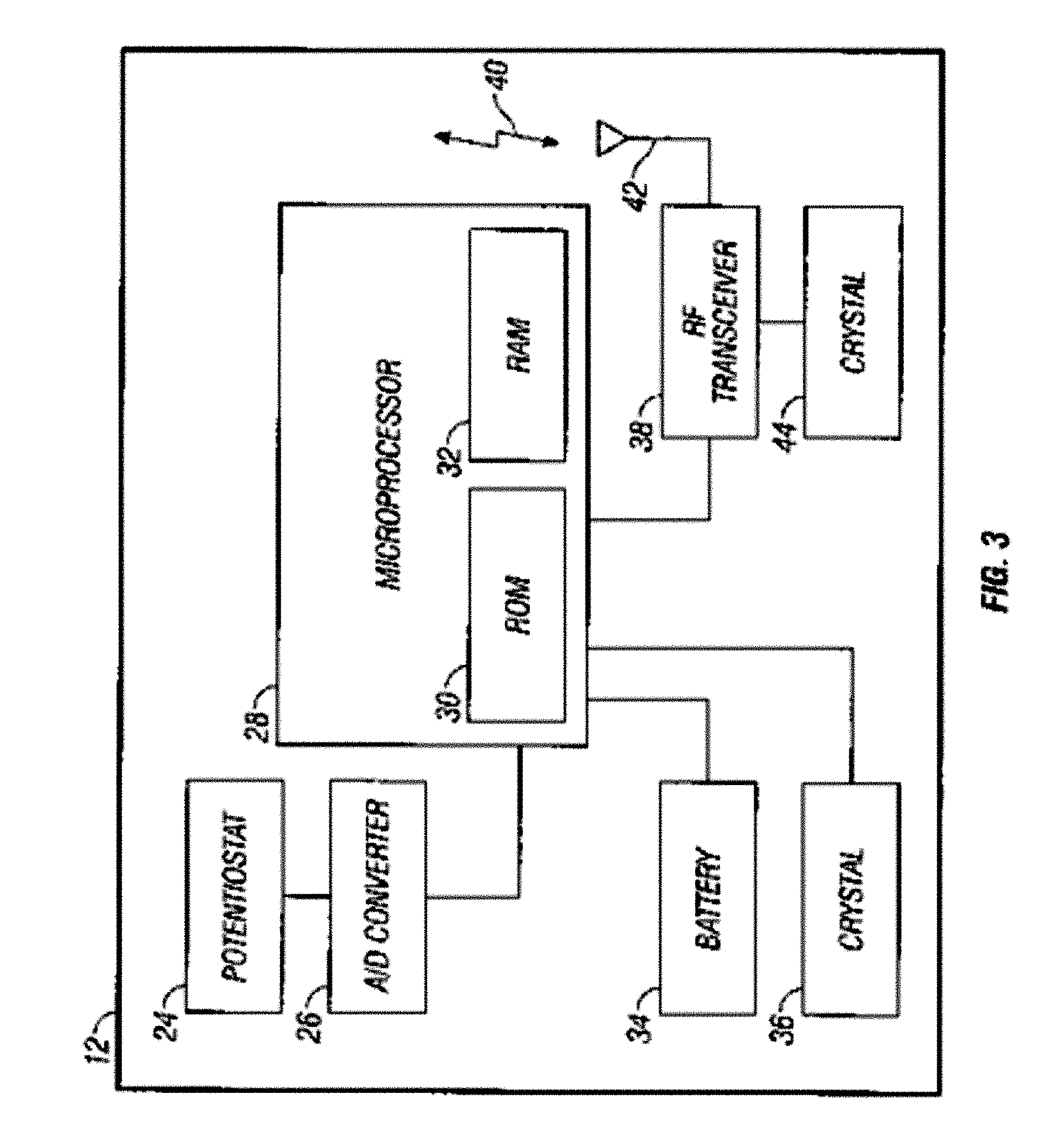
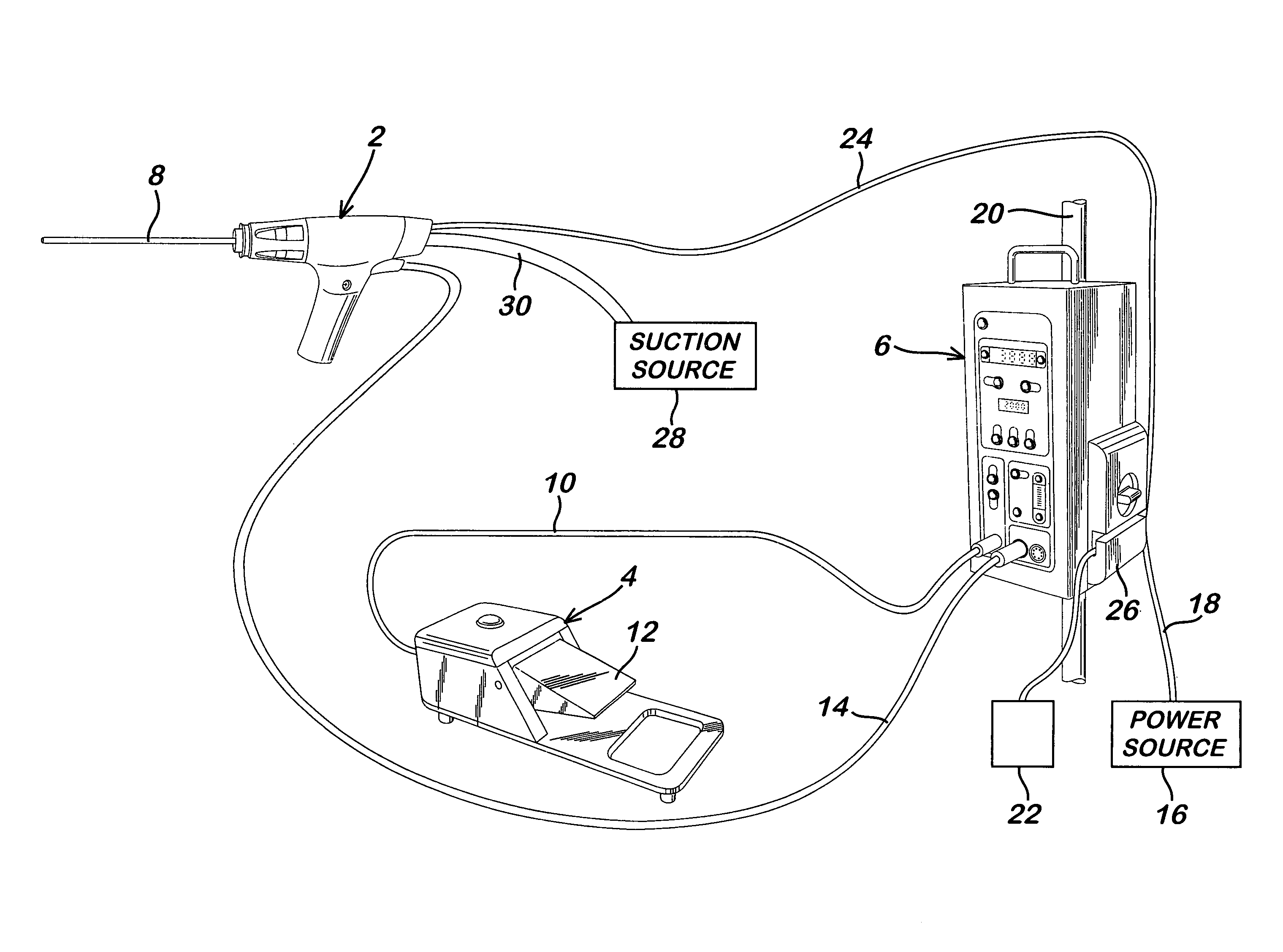
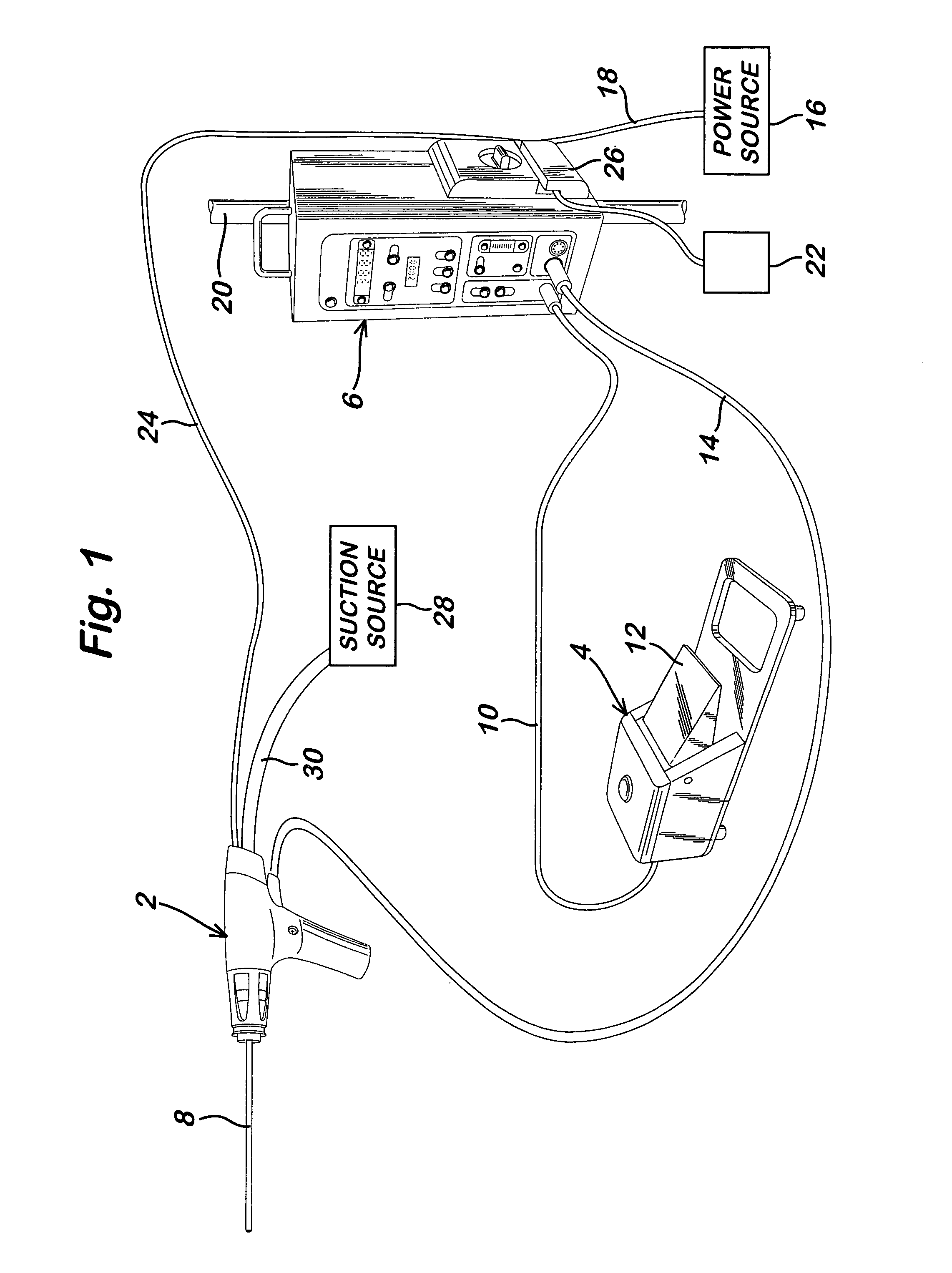
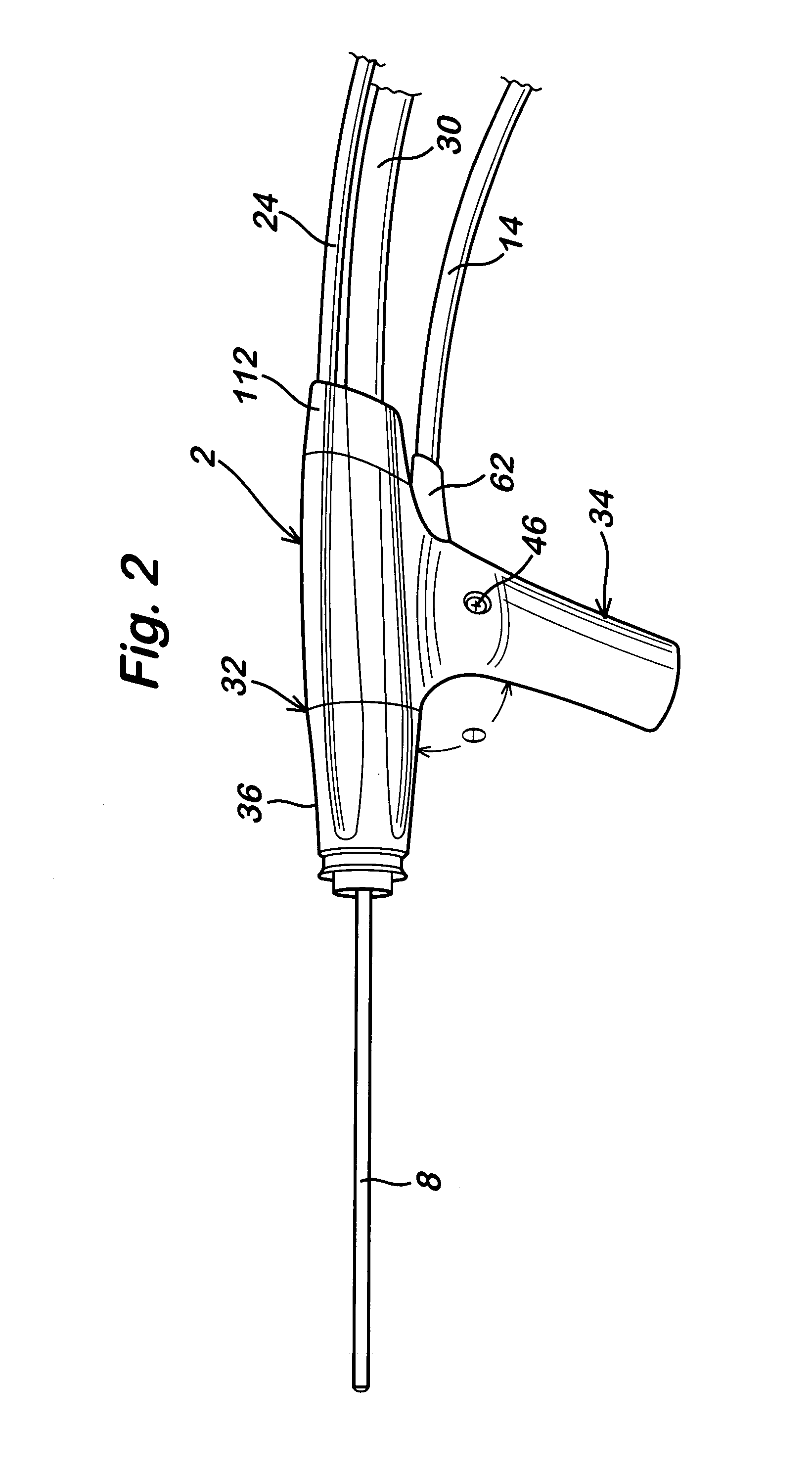
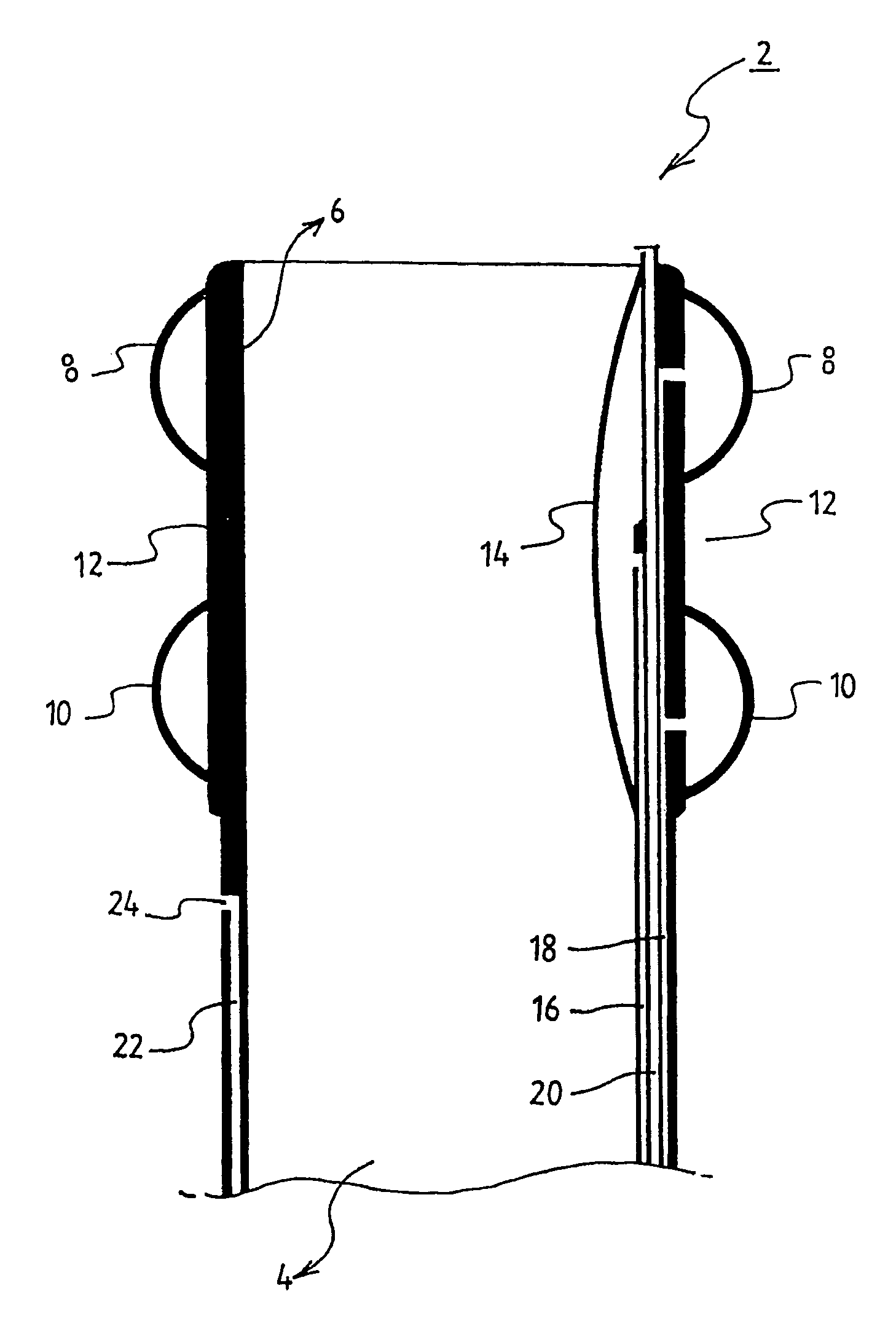
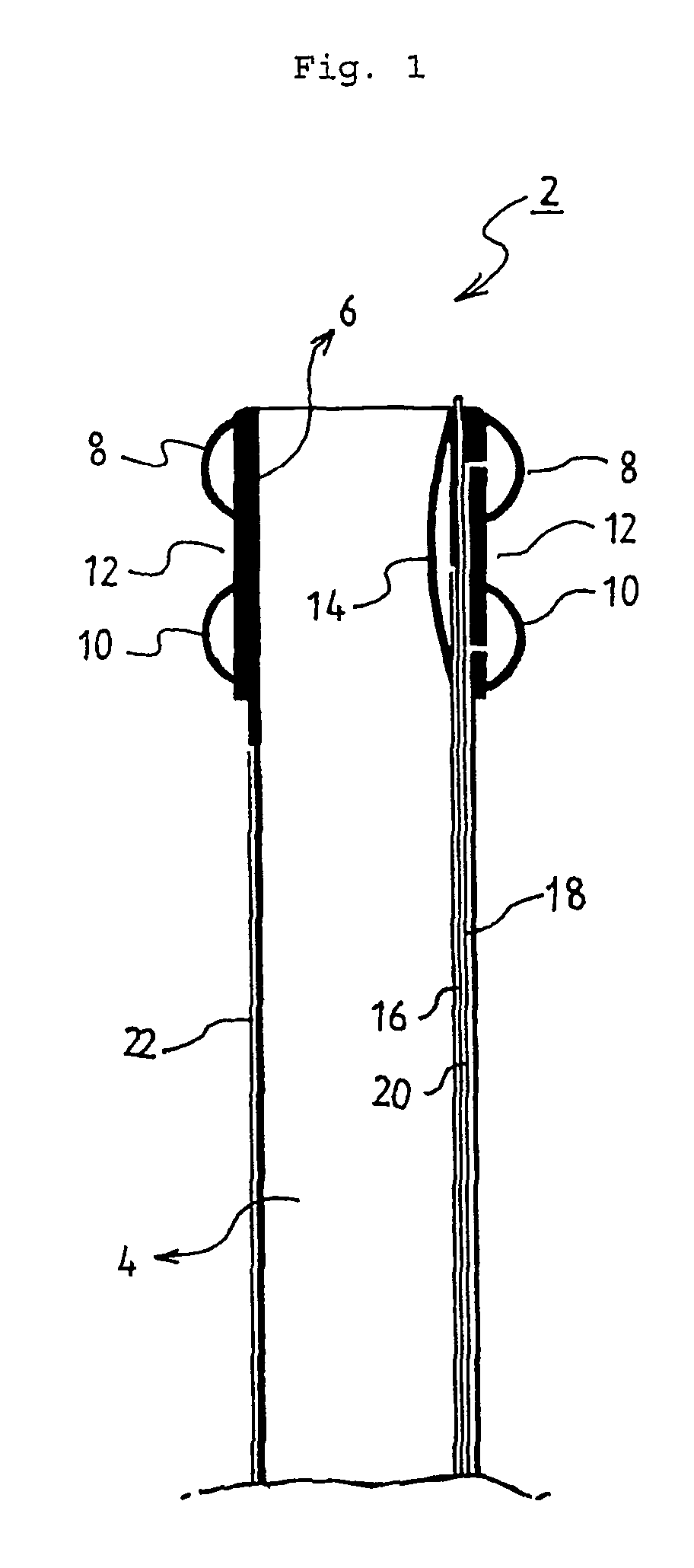
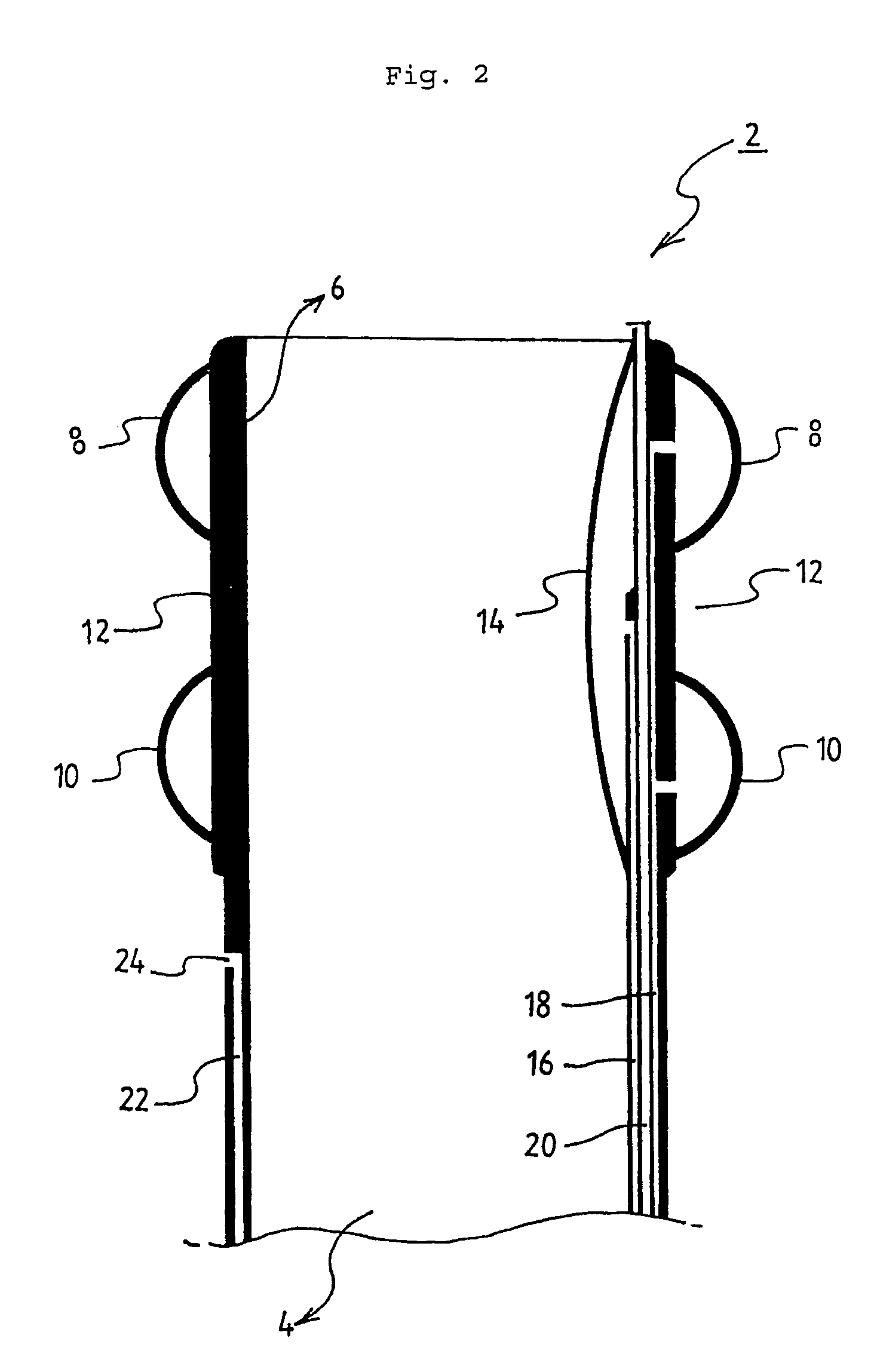
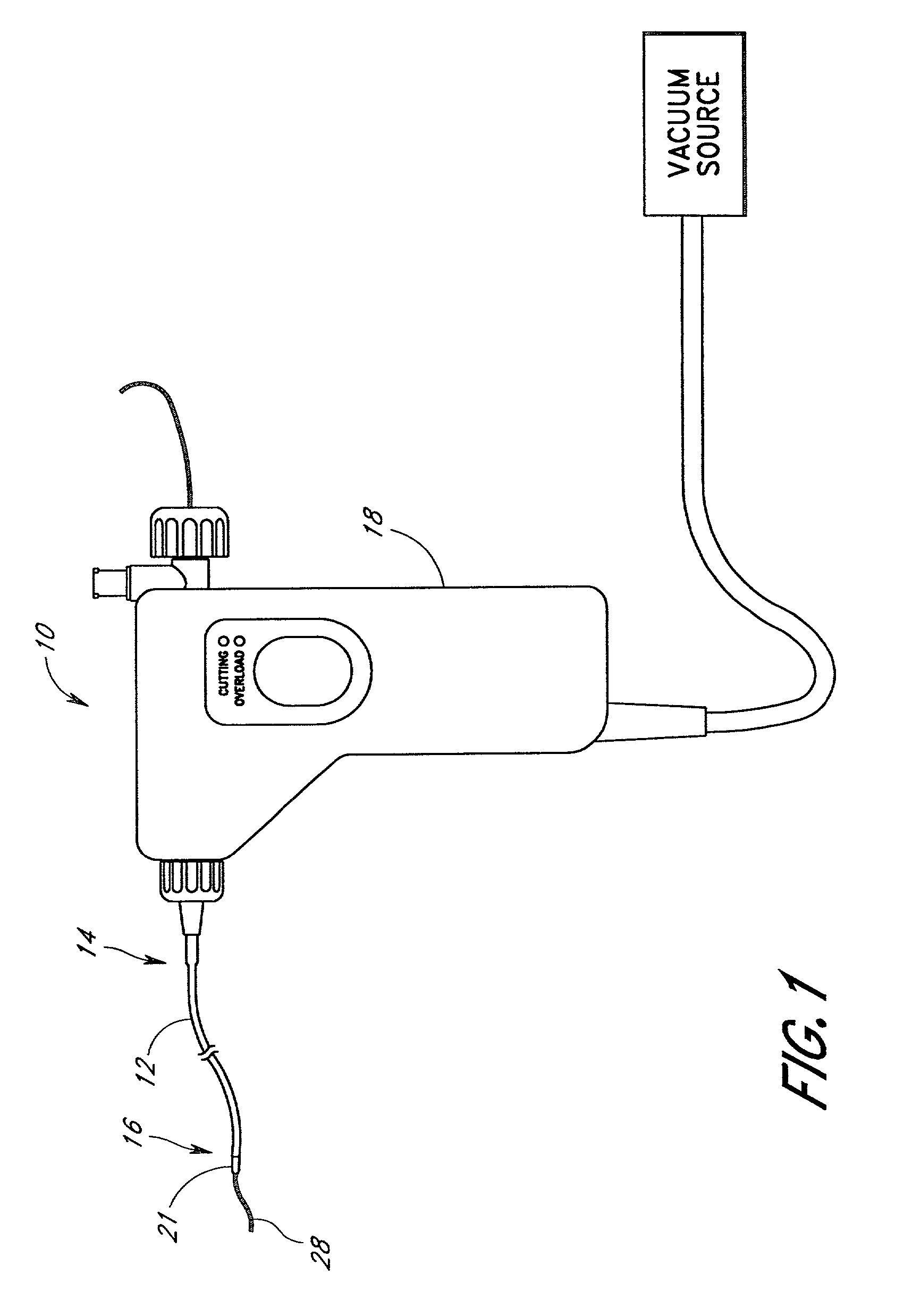
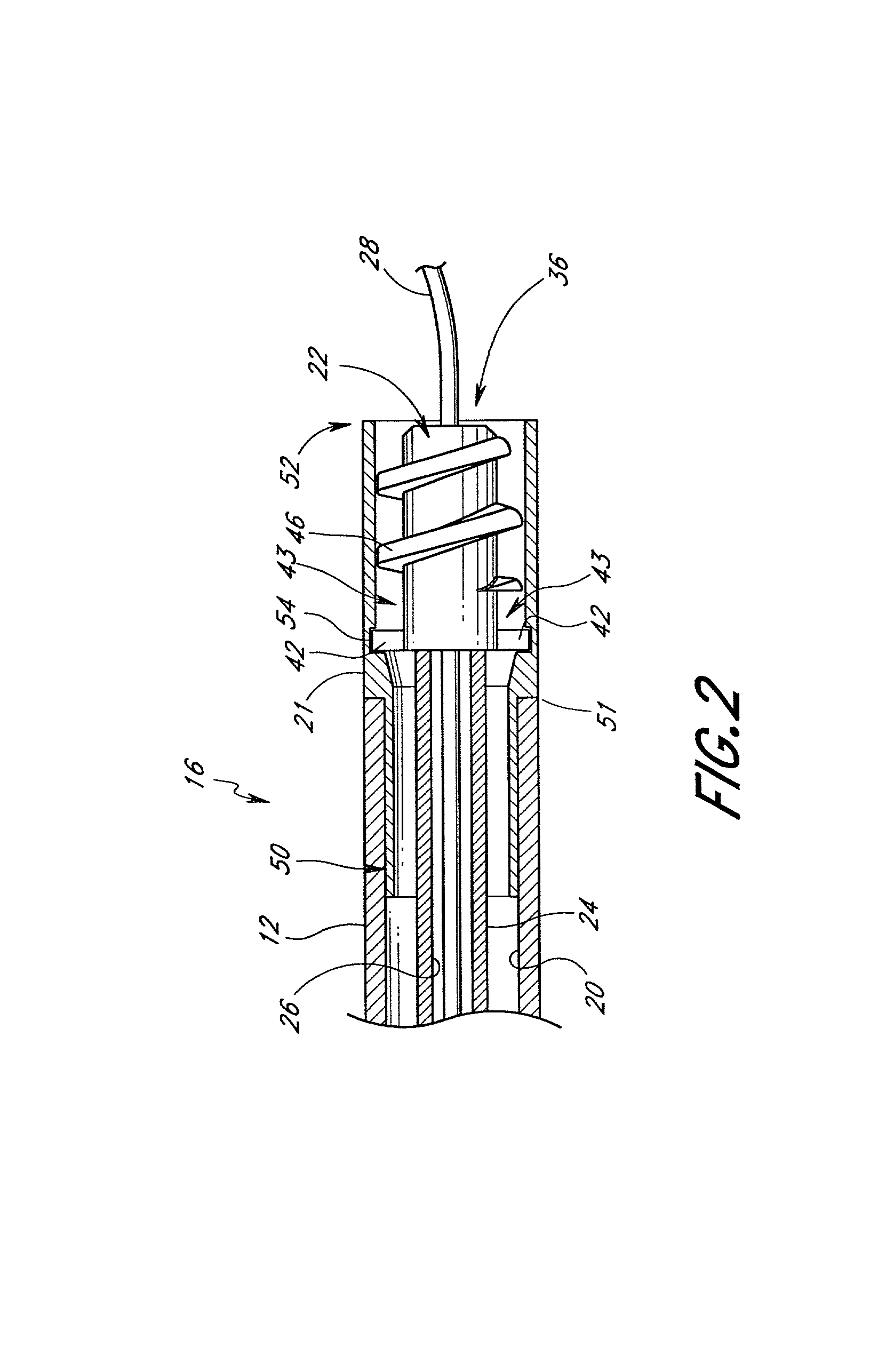
Powered surgical apparatus, method of manufacturing powered surgical apparatus, and method of using powered surgical apparatus
ActiveUS7247161B2Promotes its utilizationFacilitate and promote ease and effectiveness of cleaning and sterilizationSleeve/socket jointsIncision instrumentsEngineeringIrrigation fluids
A powered surgical apparatus can be used with a source of irrigation fluid and a source of suction. The powered surgical apparatus can include a cutting blade assembly and a handle. The handle can include an upper portion defining a distal section connectable to the cutting blade assembly and a lower portion extending downwardly from the upper portion. The handle can be connectable to the source of irrigation fluid and the source of suction. The system can also include a manually actuable input device that provides at least one signal relevant to at least one operation of the system, and a controller that receives the at least one input signal and provides an output signal to perform the at least one operation of the system.
Owner:GYRUS ACMI INC (D B A OLYMPUS SURGICAL TECH AMERICA) +1
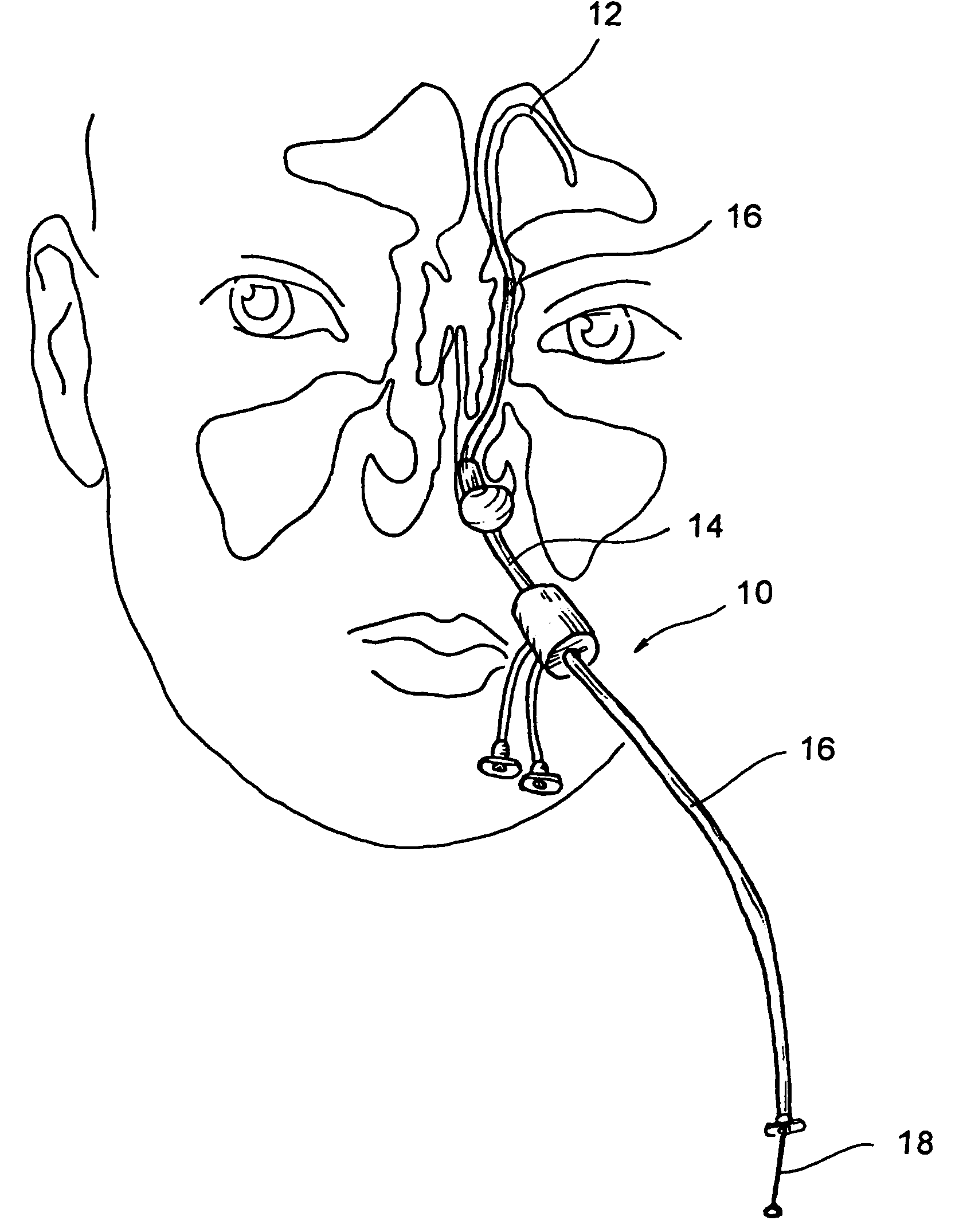
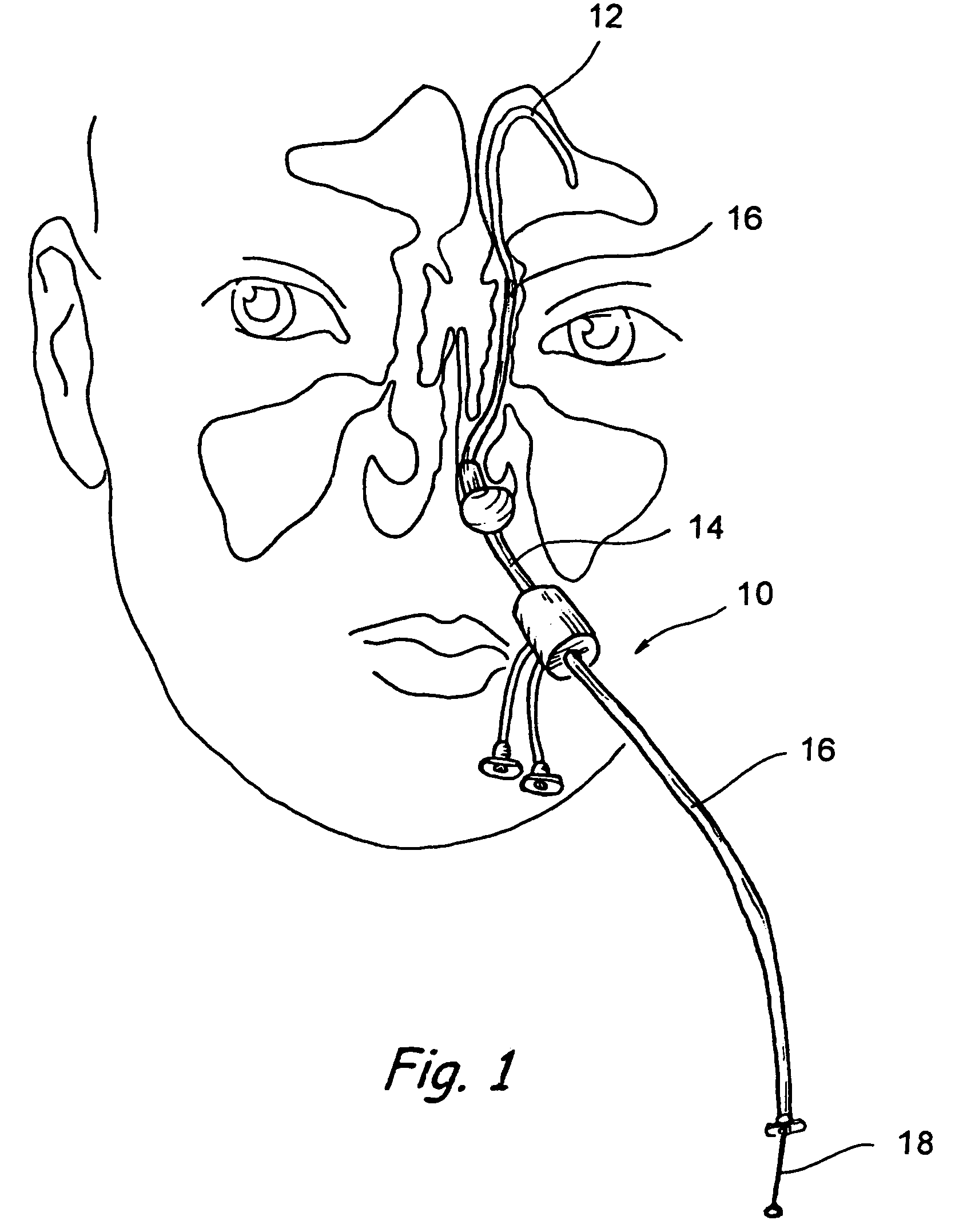
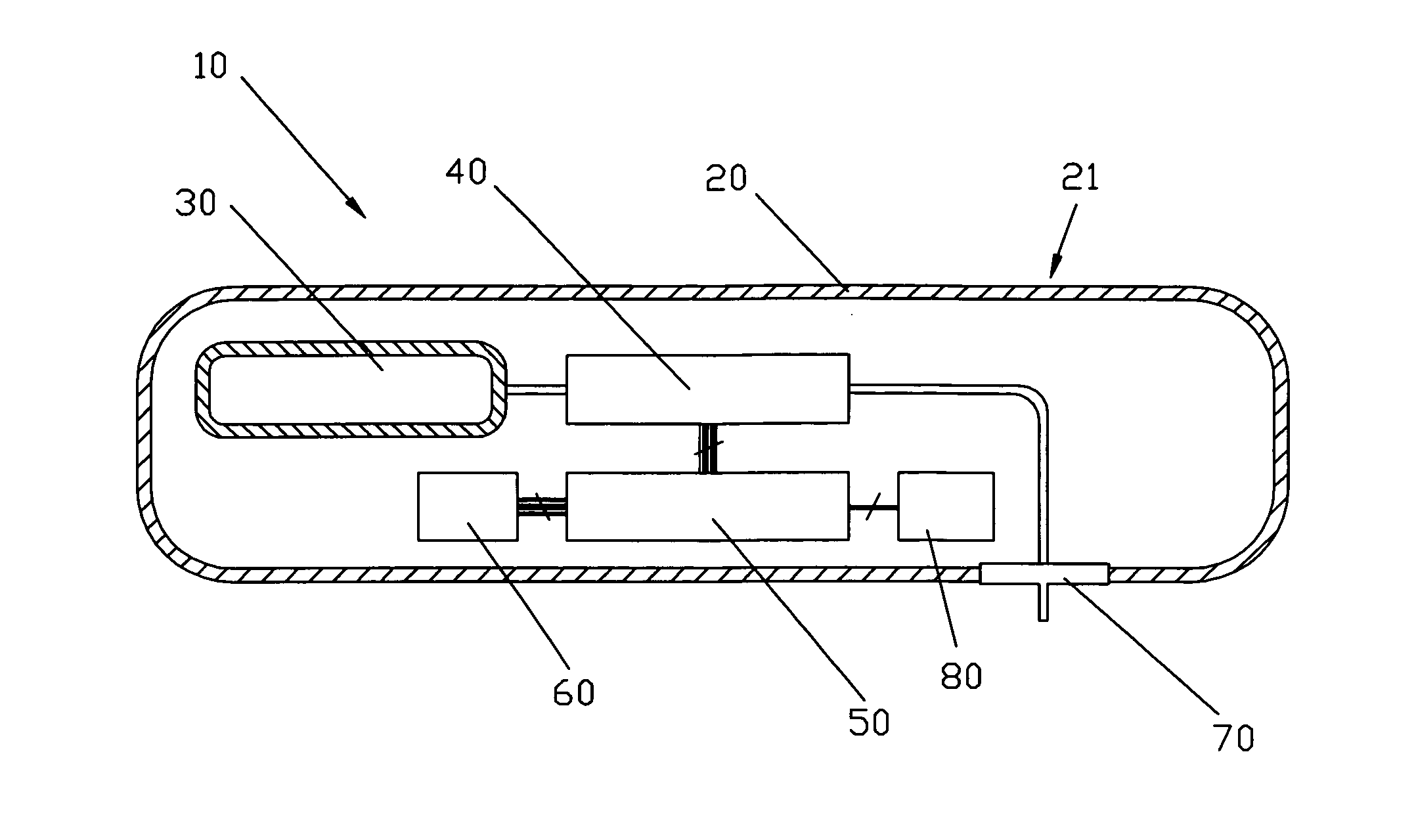
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
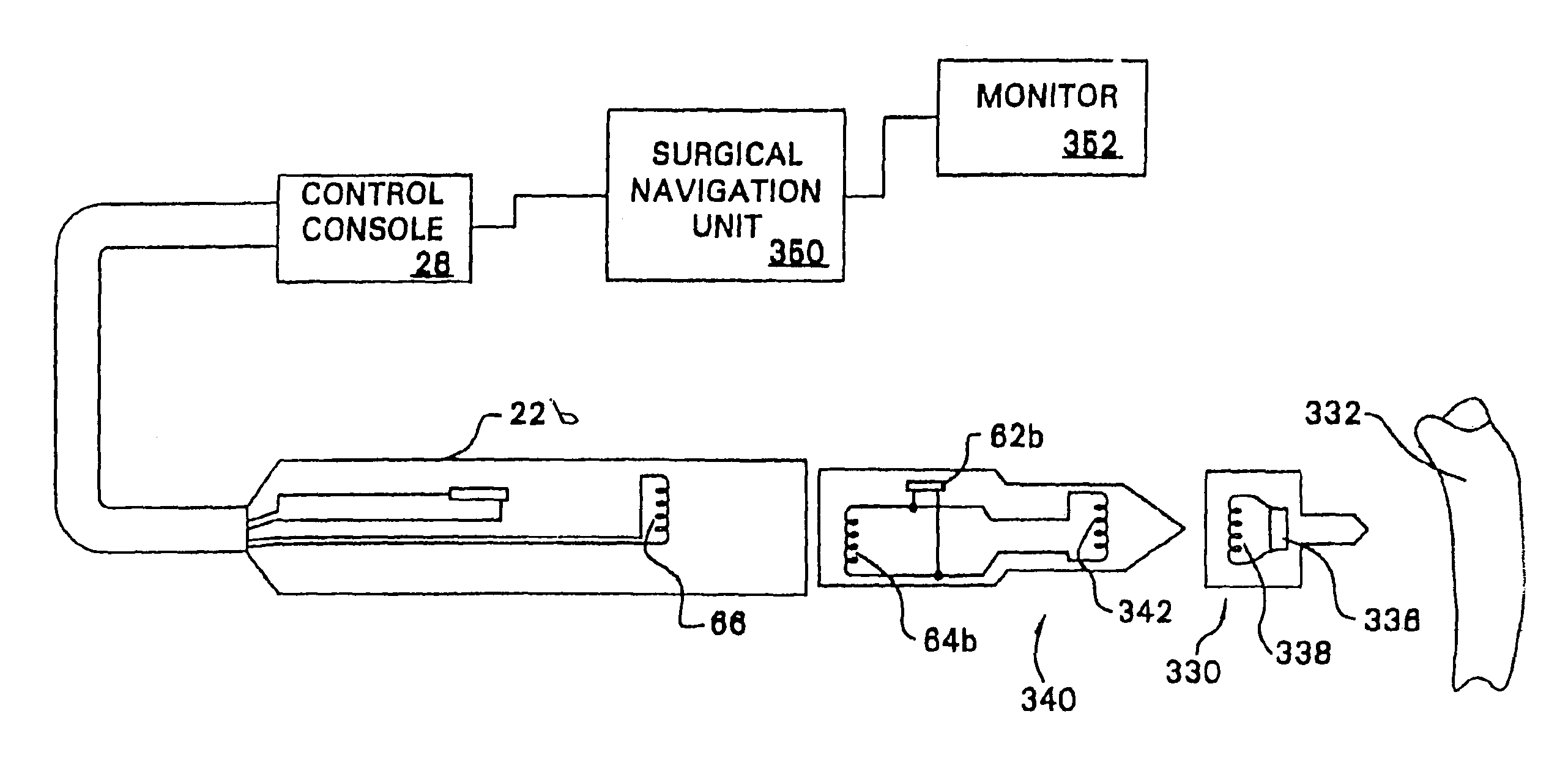
Method for assembling, identifying and controlling a powered surgical tool assembly assembled from multiple components
A surgical tool system comprising a control console, a powered surgical device, an intermediate attachment removably connected to the surgical device and a cutting accessory removably connected to the intermediate attachment. Internal to the cutting accessory is an identification device that contains data specific to the operation of the accessory. The control console, through the transfer of signals through the powered surgical device and the intermediate attachment reads the data in the cutting accessory. Based on these data, the control console selectively actuates the powered surgical device. In some versions of the invention, the identification device may be an RFID chip. Signals are exchanged between the surgical device and the intermediate attachment and between the intermediate attachment and cutting accessory by inductive coupling. An identification device internal to the intermediate attachment provides the control console with data describing the intermediate attachment.
Owner:STRYKER CORP
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
Analyte sensor
Systems and methods of use for continuous analyte measurement of a host's vascular system are provided. In some embodiments, a continuous glucose measurement system includes a vascular access device, a sensor and sensor electronics, the system being configured for insertion into communication with a host's circulatory system.
Owner:DEXCOM INC
Electric treatment system
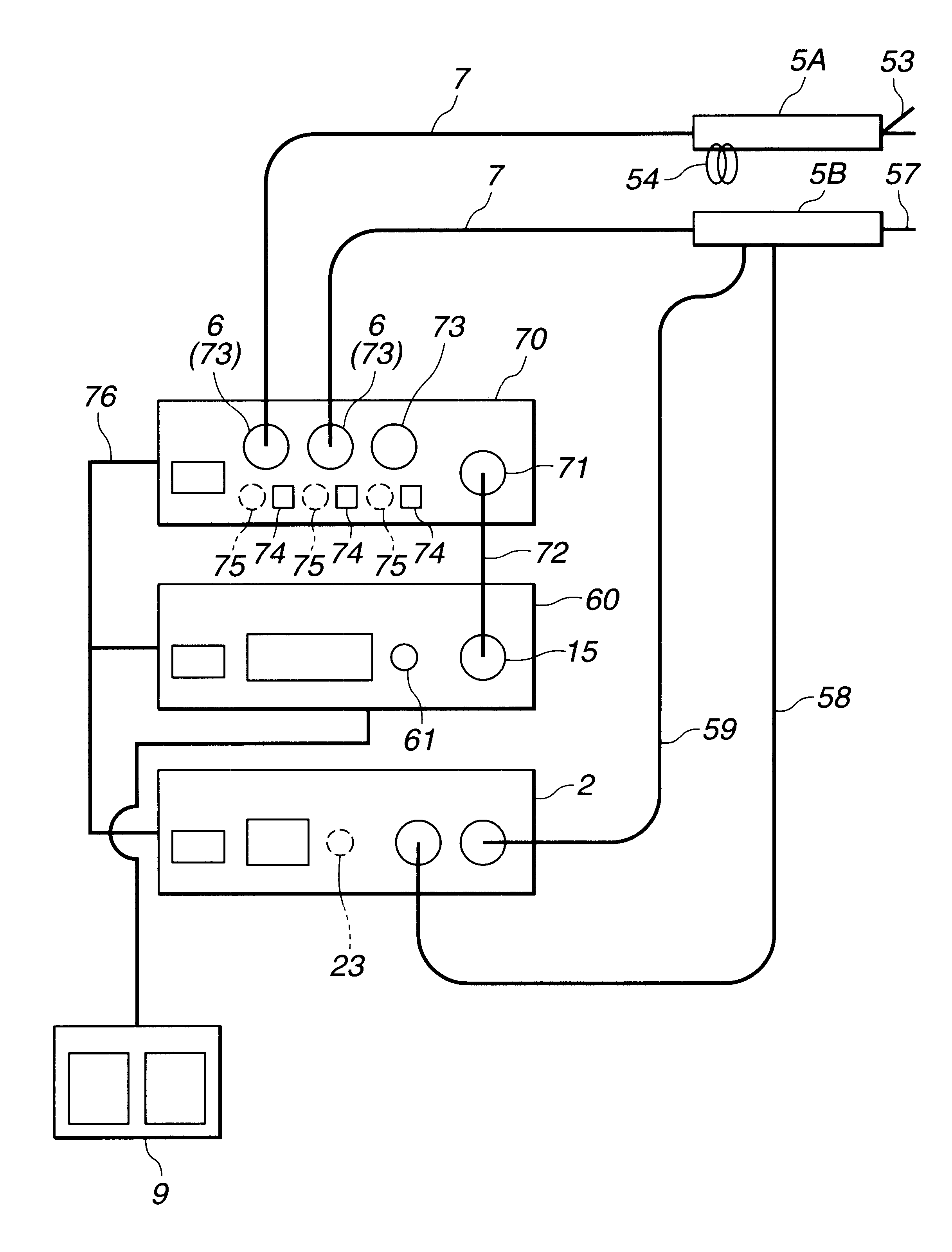
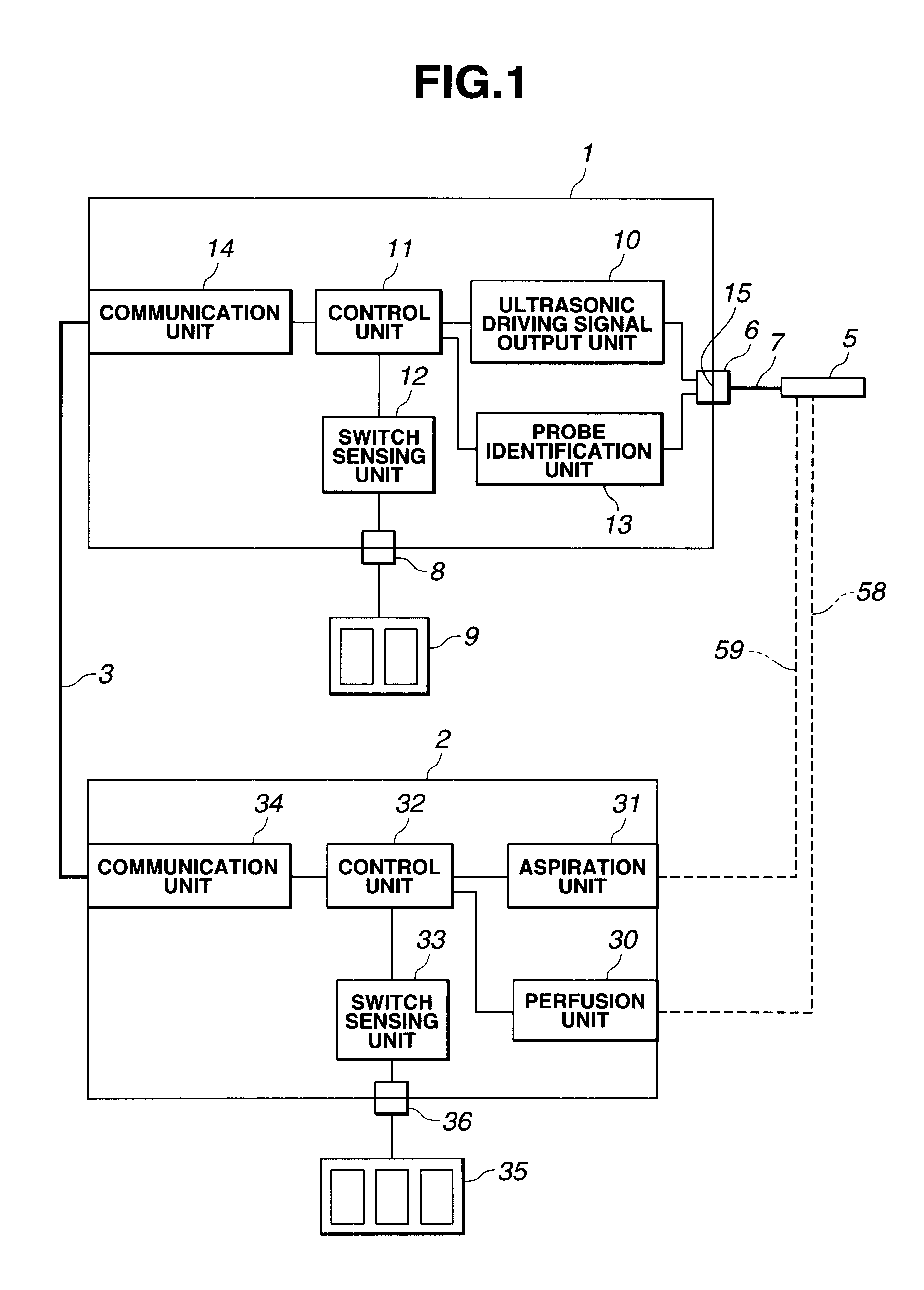
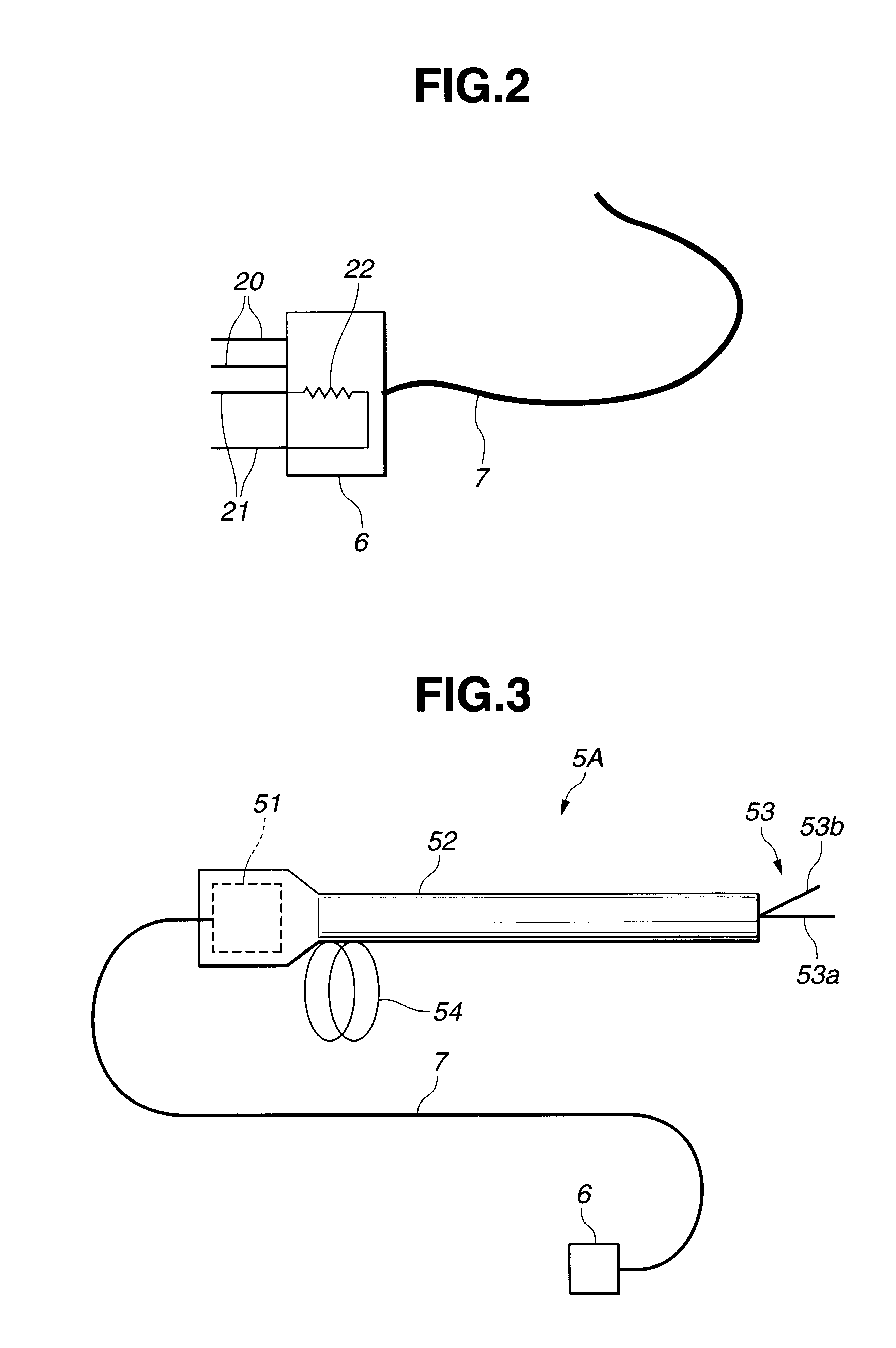
InactiveUS6666860B1Work lessAvoid uneven performanceSurgical instruments for heatingSuction devicesElectricityEngineering
A treatment tool for performing a treatment for a curative procedure with ultrasonic waves, high frequency current and so on is provided with an identifier formed of a resistor or the like for indicating the type of the treatment tool. A medical instrument such as an ultrasonic wave output apparatus discriminates the type of a treatment tool connected thereto from the identifier to automatically set operating parameters such as an output value suitable for the treatment tool and to associatively operate an ancillary medical instrument such as a perfusion / aspiration apparatus depending on the treatment tool, thereby reducing extra work such as manual setting to allow for a smooth treatment.
Owner:OLYMPUS CORP
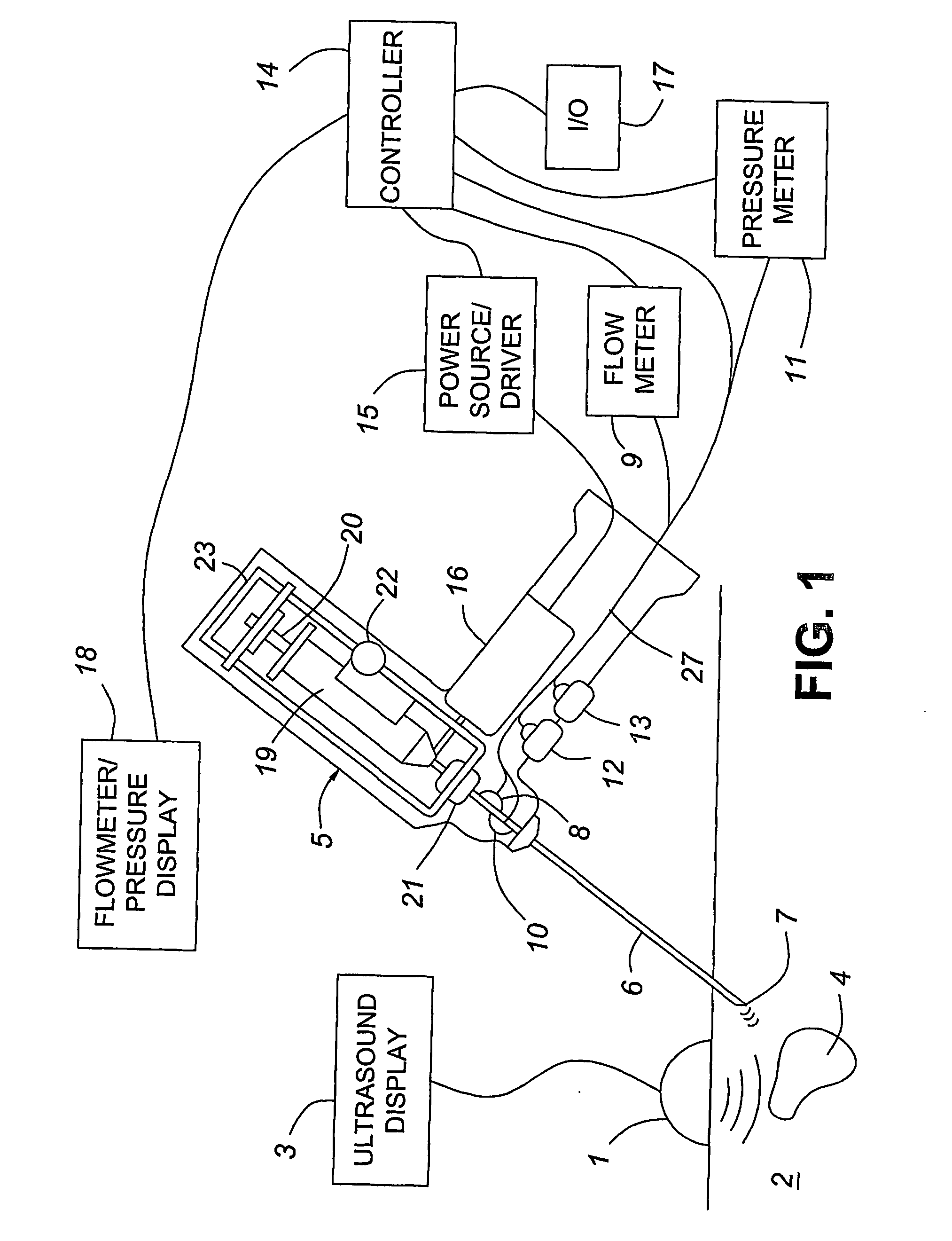
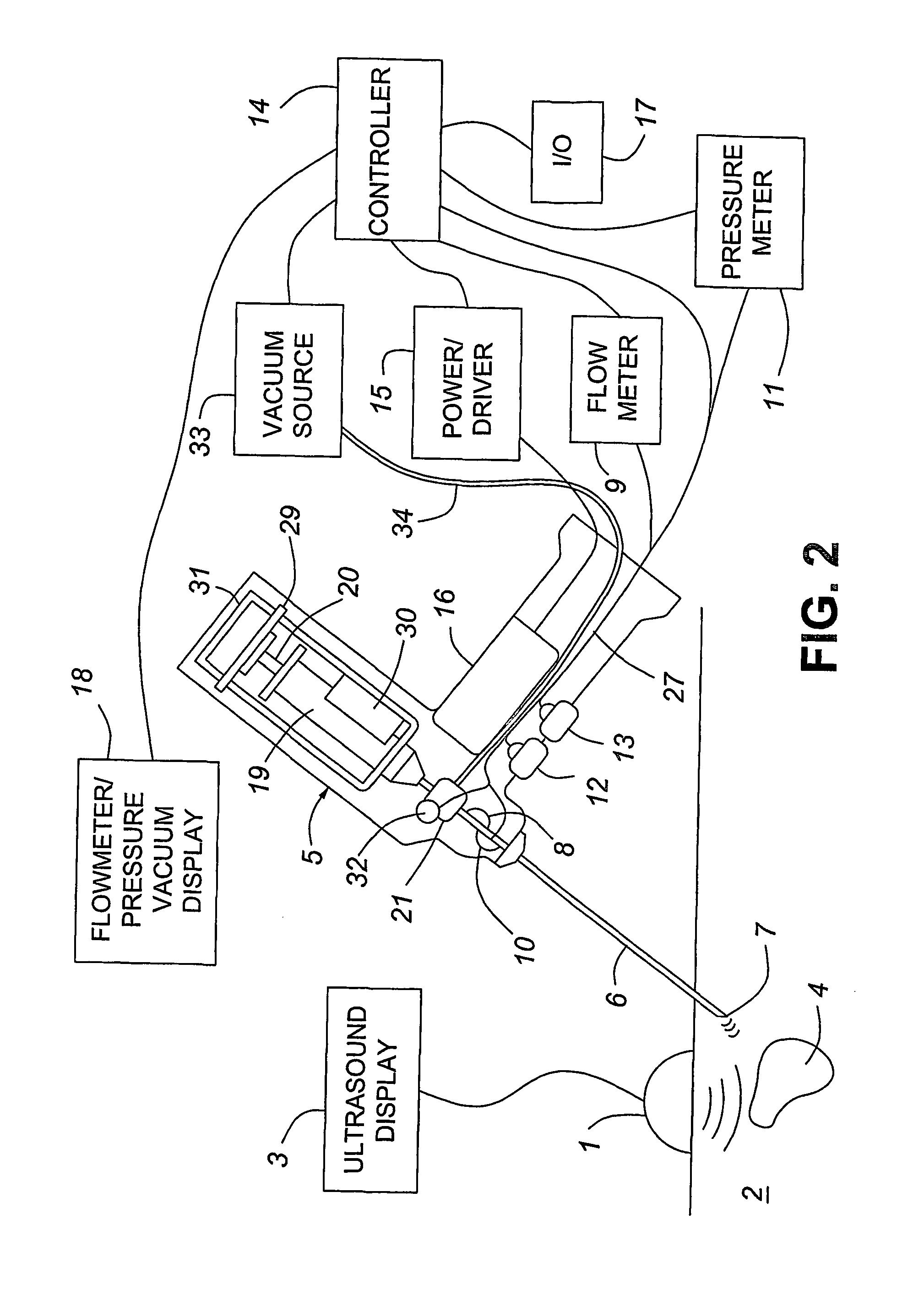
Medical devices with enhanced ultrasonic visibilty
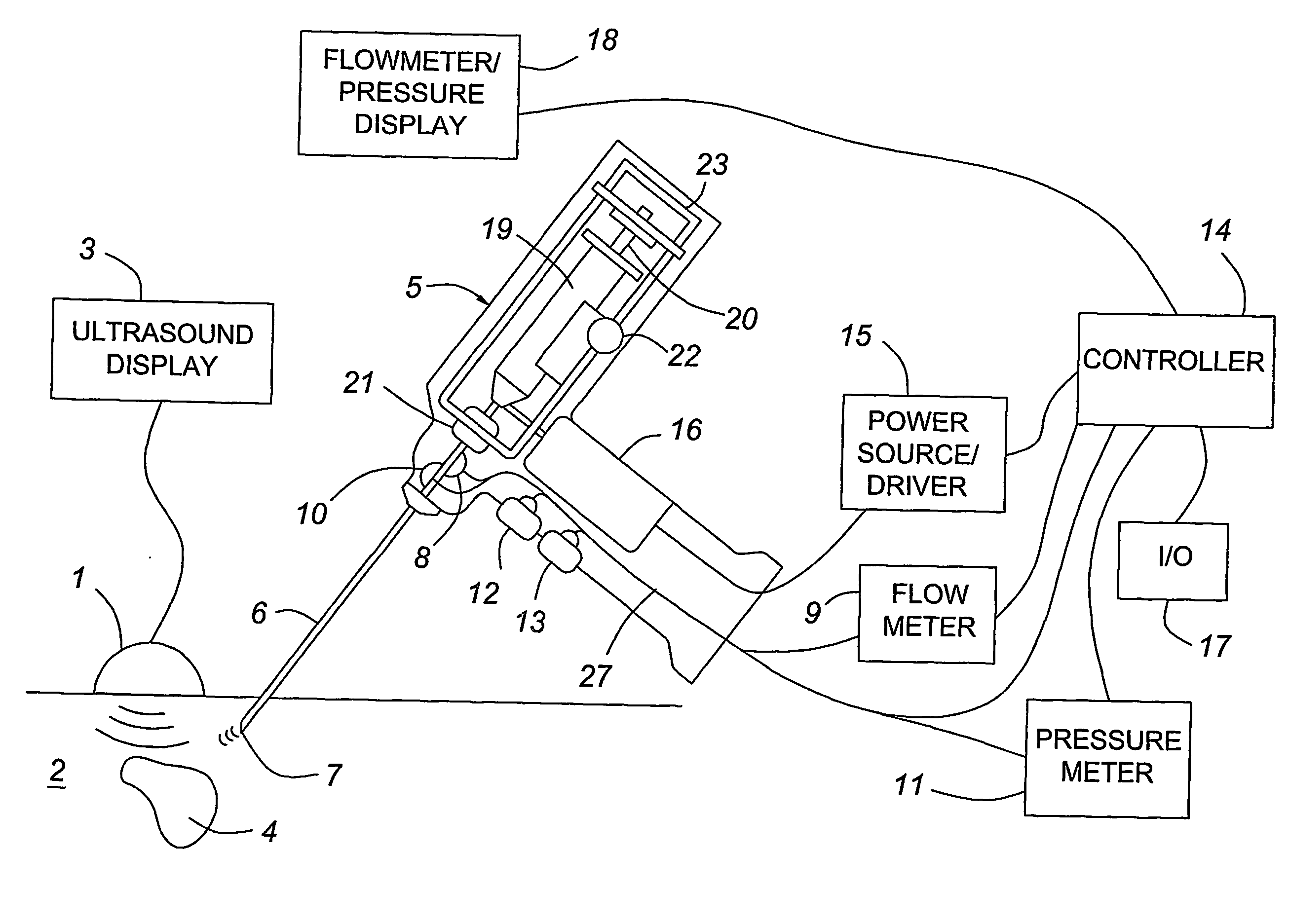
InactiveUS20070197954A1Ultrasonic/sonic/infrasonic diagnosticsElectrotherapyTip positionSolid tissue
A medical device having enhanced ultrasonic visibility is provided. The device permits localized drug delivery, probe positioning, fluid drainage, biopsy, or ultrasound pulse delivery, through the real-time ultrasound monitoring of the needle tip position within a patient. The device permits controlled dispersion of a drug into solid tissue, the lodging of particles into solid tissue, and drug delivery into specific blood vessels. As a needle is inserted, a fluid that contrasts echogenically with the organ environment is injected into the patient. The fluid travels a brief distance before being slowed and stopped by the patient's tissue and this fluid flow will be detectable by ultrasound. The needle position during insertion will be monitored using ultrasound until it is at the desired point of action. A therapeutic drug is then delivered or a probe inserted
Owner:ARTENGA
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM INC
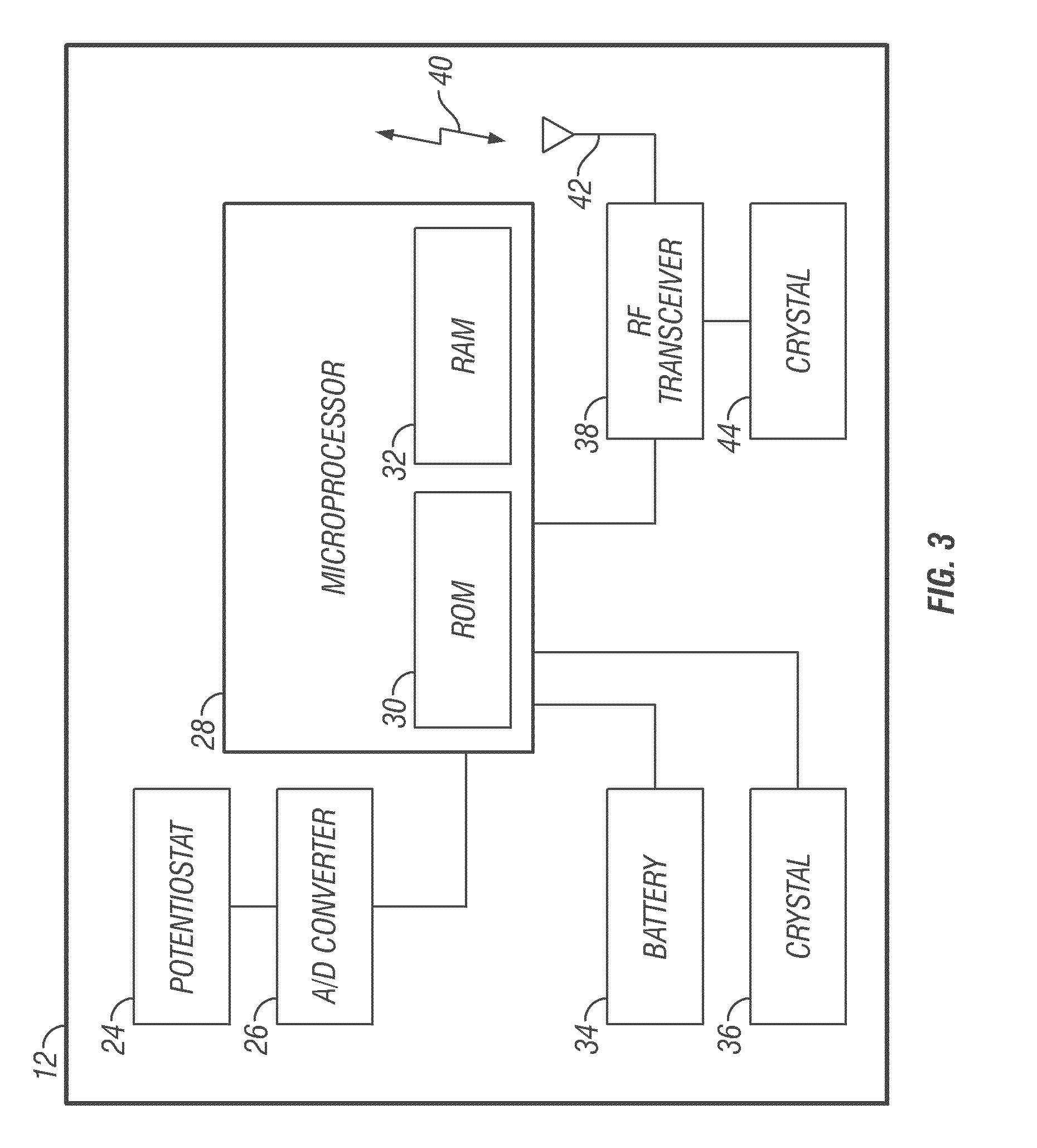
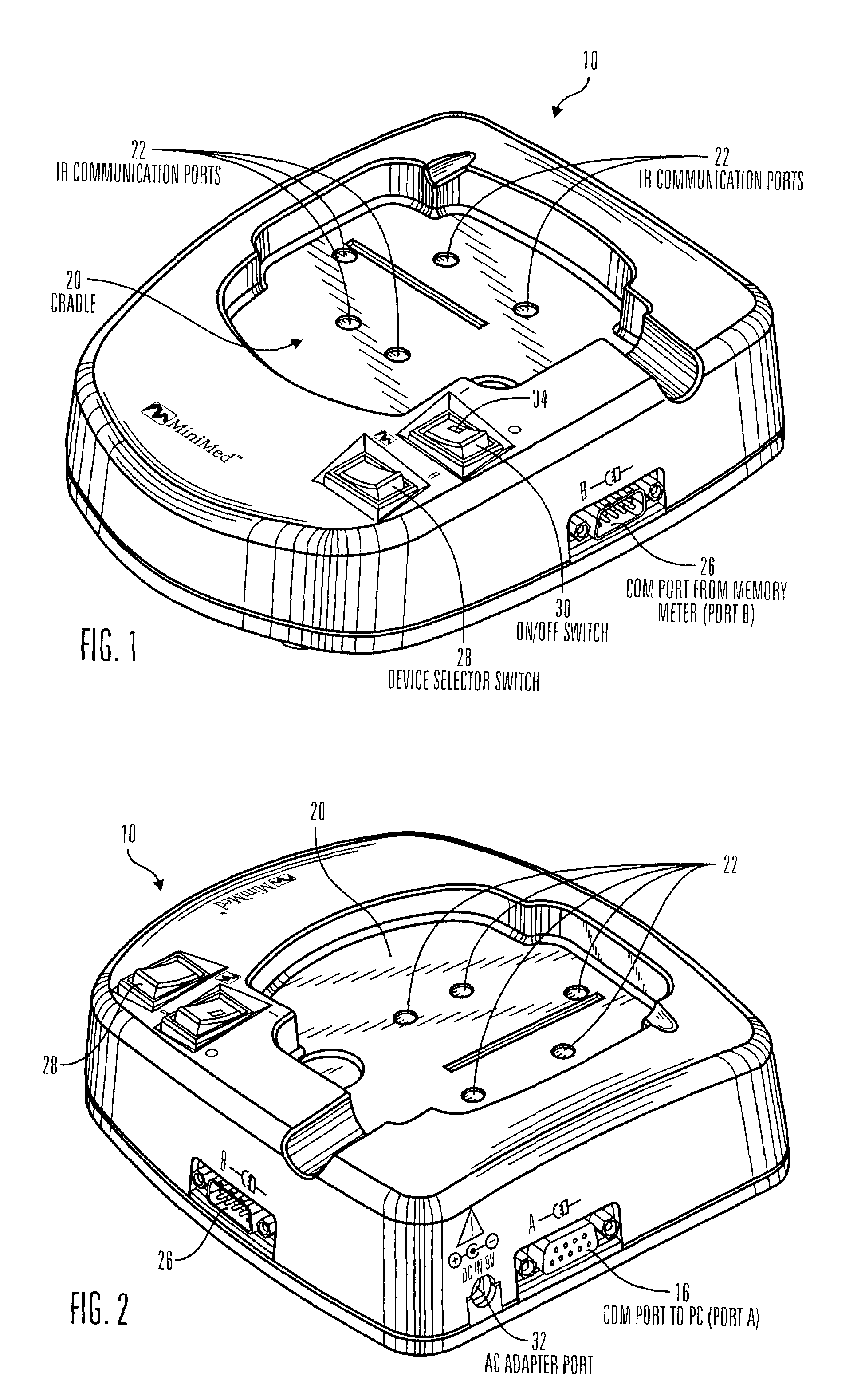
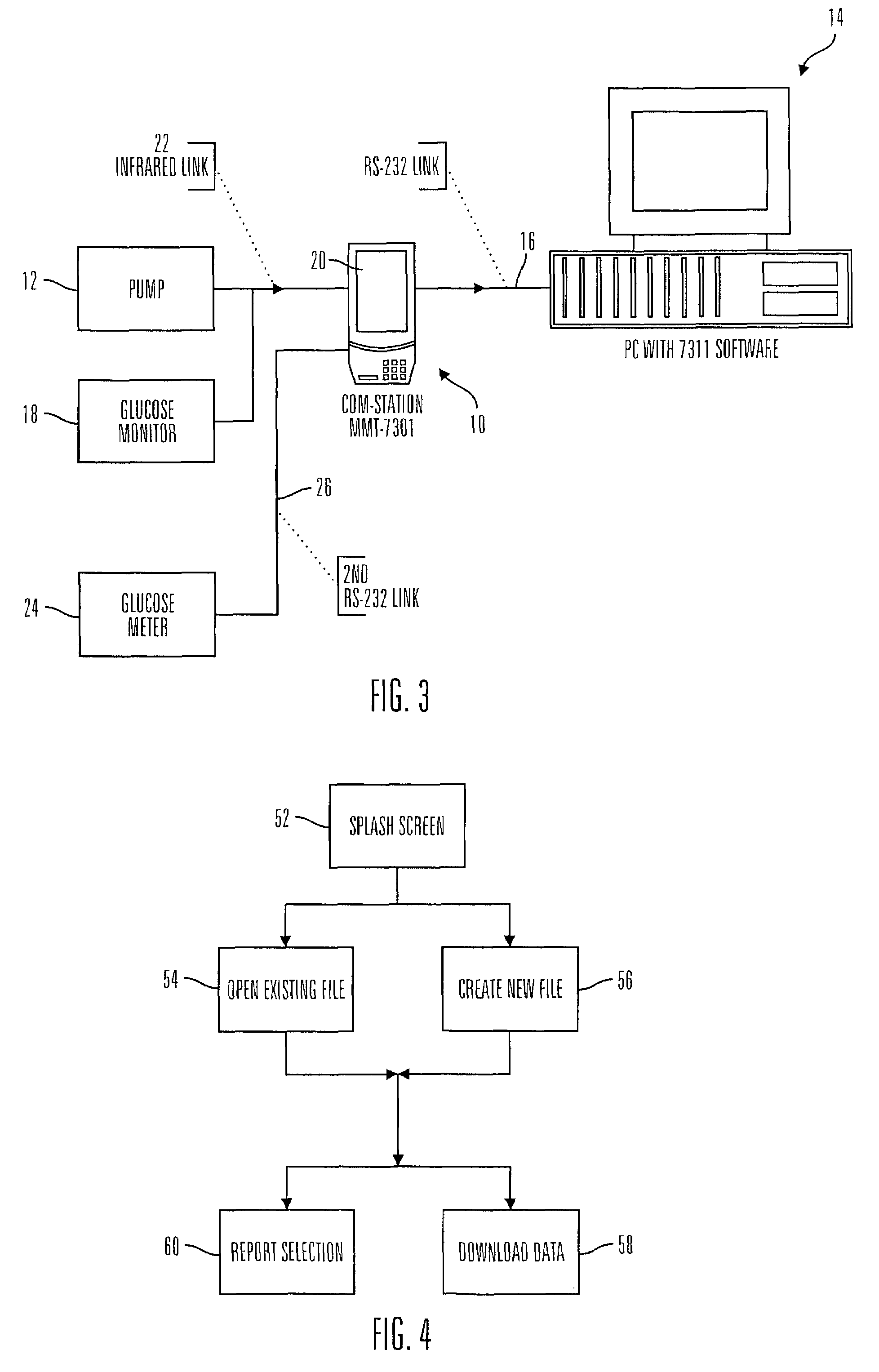
Communication station and software for interfacing with an infusion pump, analyte monitor, analyte meter, or the like
InactiveUS7647237B2Peptide/protein ingredientsDigital data processing detailsAnalyteSystem combination
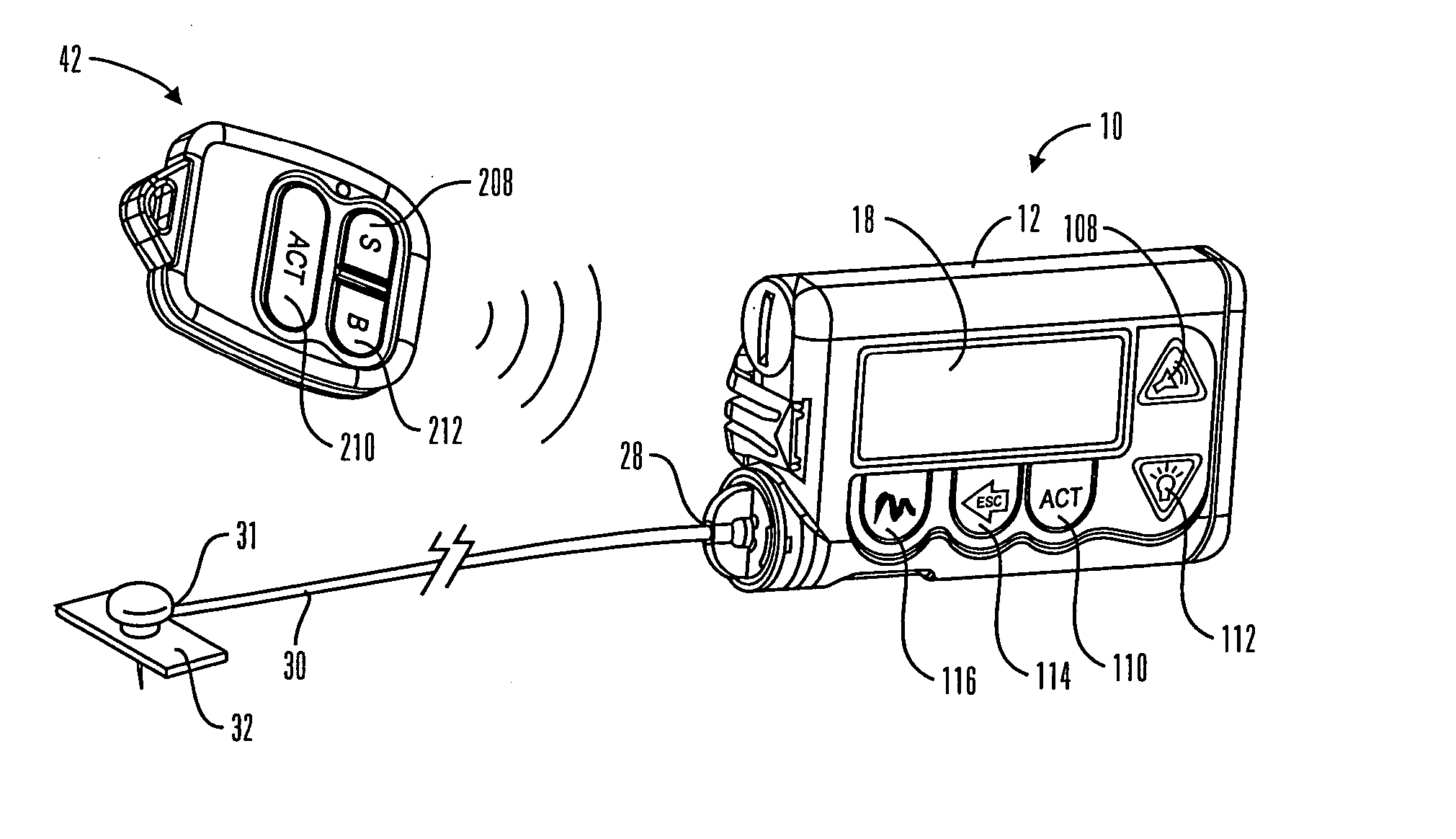
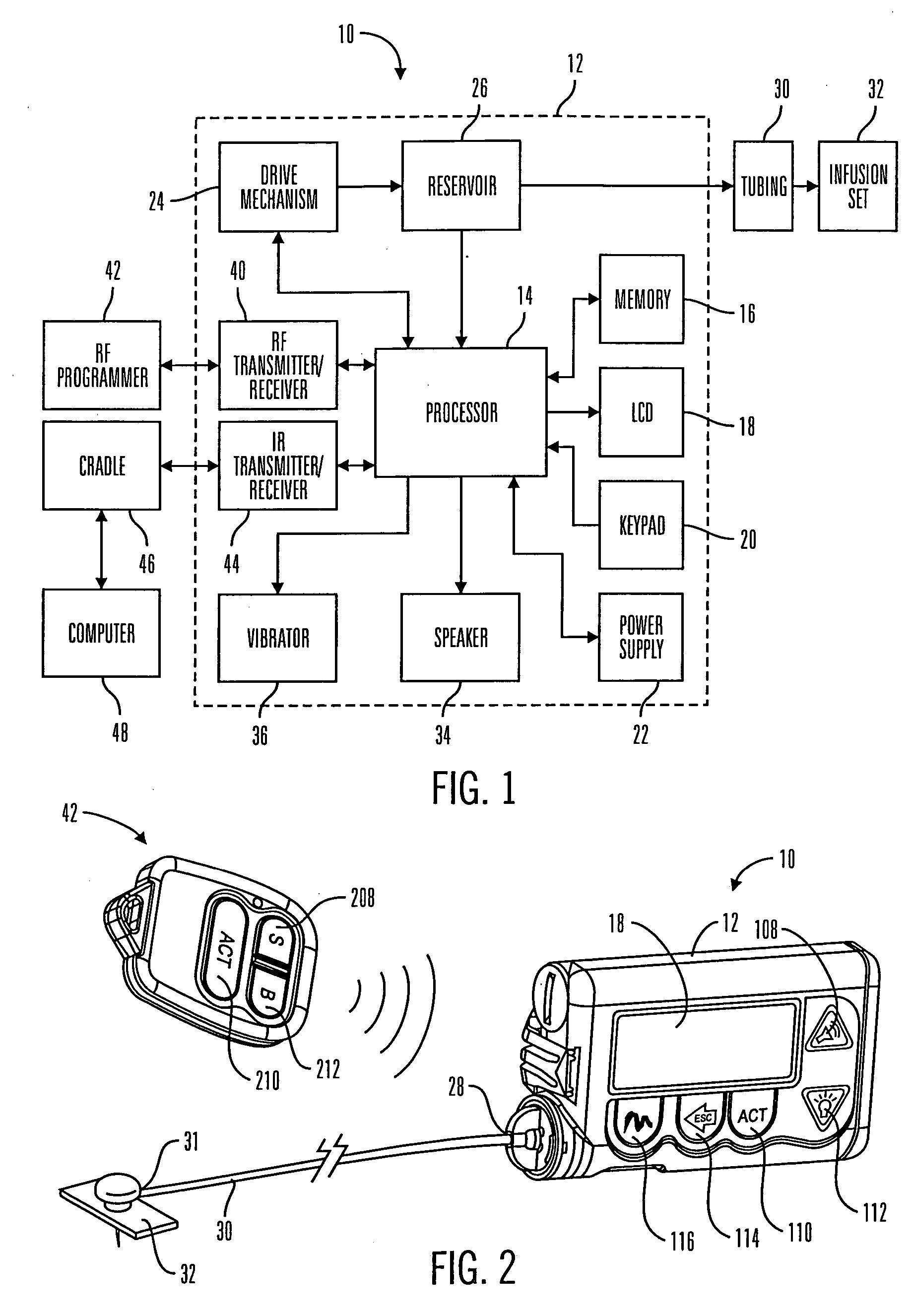
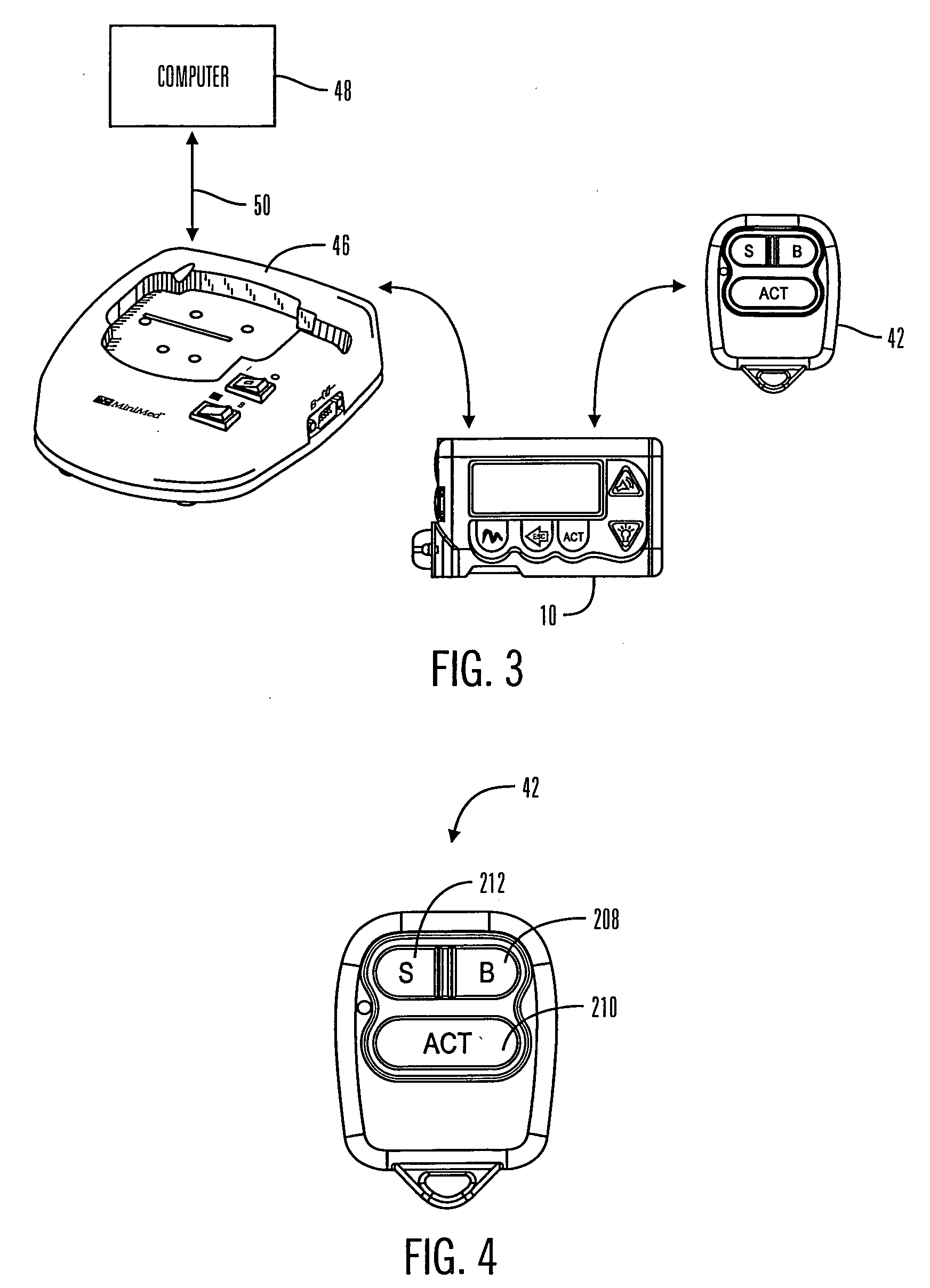
A communication station is for use with a medical device (such as an infusion pump) and a processing device (such as a computer). The communication station includes a housing, a medical device interface coupled to the housing, a processing device interface coupled to the housing and a processor coupled to the housing. The device interface interfaces with the medical device, and the processing device interface interfaces with the processing device. The processor provides a communication path between the medical device and the processing device such that programming and instructions may be communicated from the processing device to the medical device and data may be transferred from the medical device to the processing device. The communication station may be combined with a system that is capable of generating reports either locally or remotely. In addition, the medical device interface may be a cradle that is configurable to attach to different shaped medical devices.
Owner:MINIMED
Indwelling fecal diverting device
ActiveUS8398669B2Improve securityEasy to installBalloon catheterAnti-incontinence devicesFecesSurgery
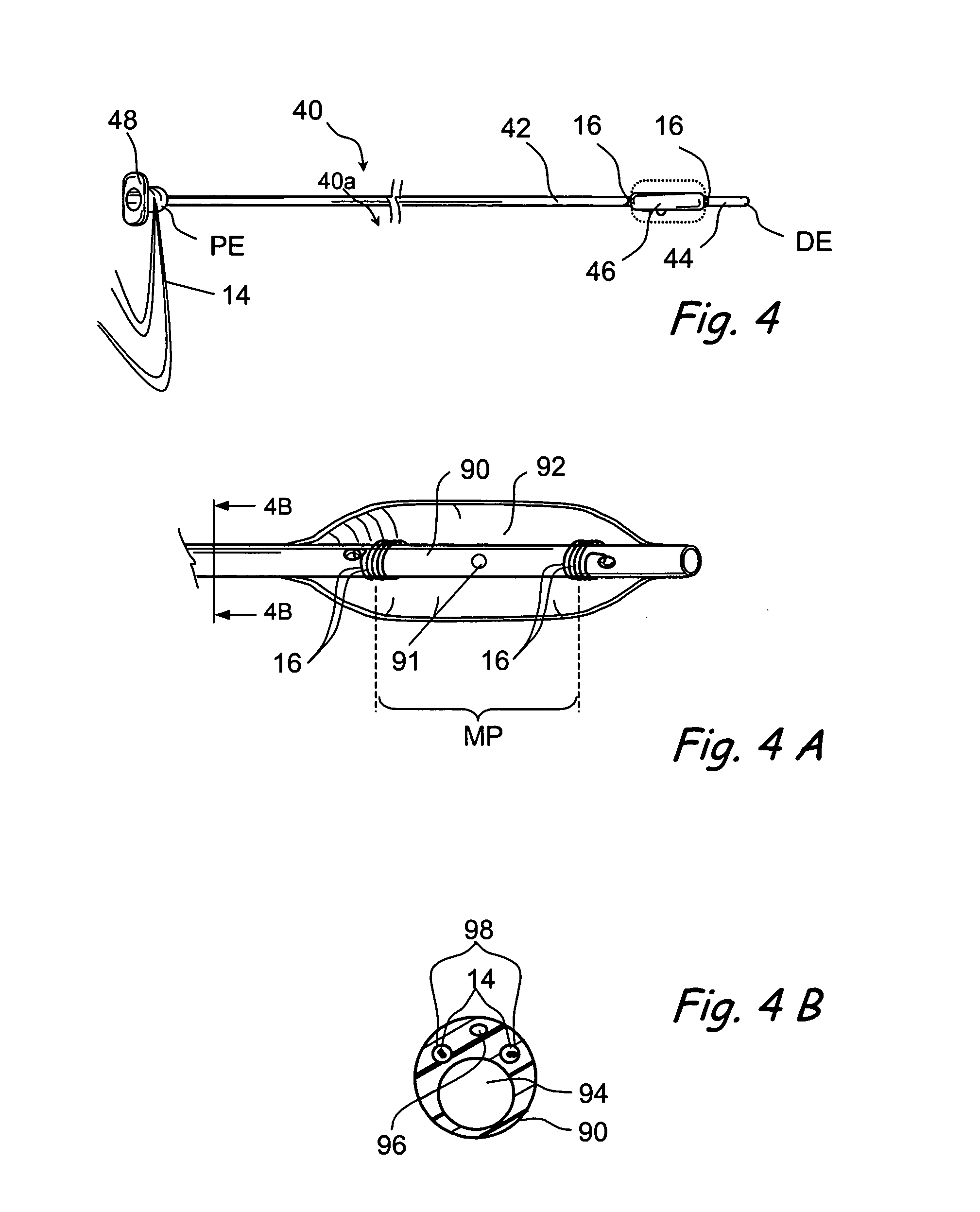
Disclosed is an indwelling fecal diverting device. The device comprises an elongate tube formed, at an upper end thereof, with a tubular body part; a pair of fixing balloons attached up and down to an outer surface of the tubular body part such that a clamping portion is defined between the fixing balloons; and a tube opening and closing balloon attached to an inner surface of the tubular body part. An injection passage is defined in the tube so that a remedial liquid can be injected through the injection passage to the outside of the tube to medically treat an anastomosed portion of an intestinal tract of a patient. The indwelling fecal diverting device is fitted into the intestinal tract of the patient, air is supplied into the fixing balloons to inflate them, and the intestinal tract is clamped around the clamping portion using a clamping band.
Owner:YUSHIN MEDICAL +1
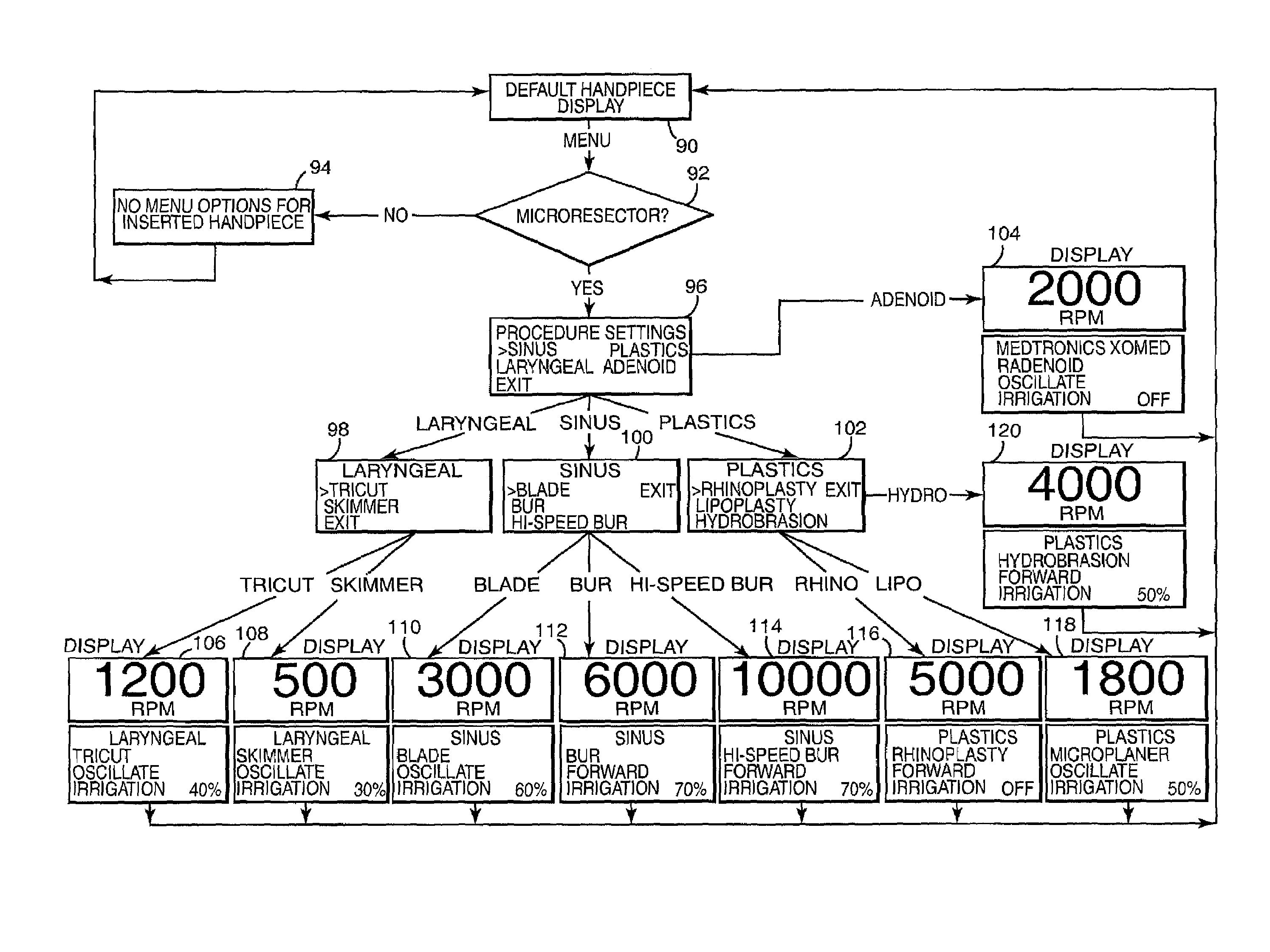
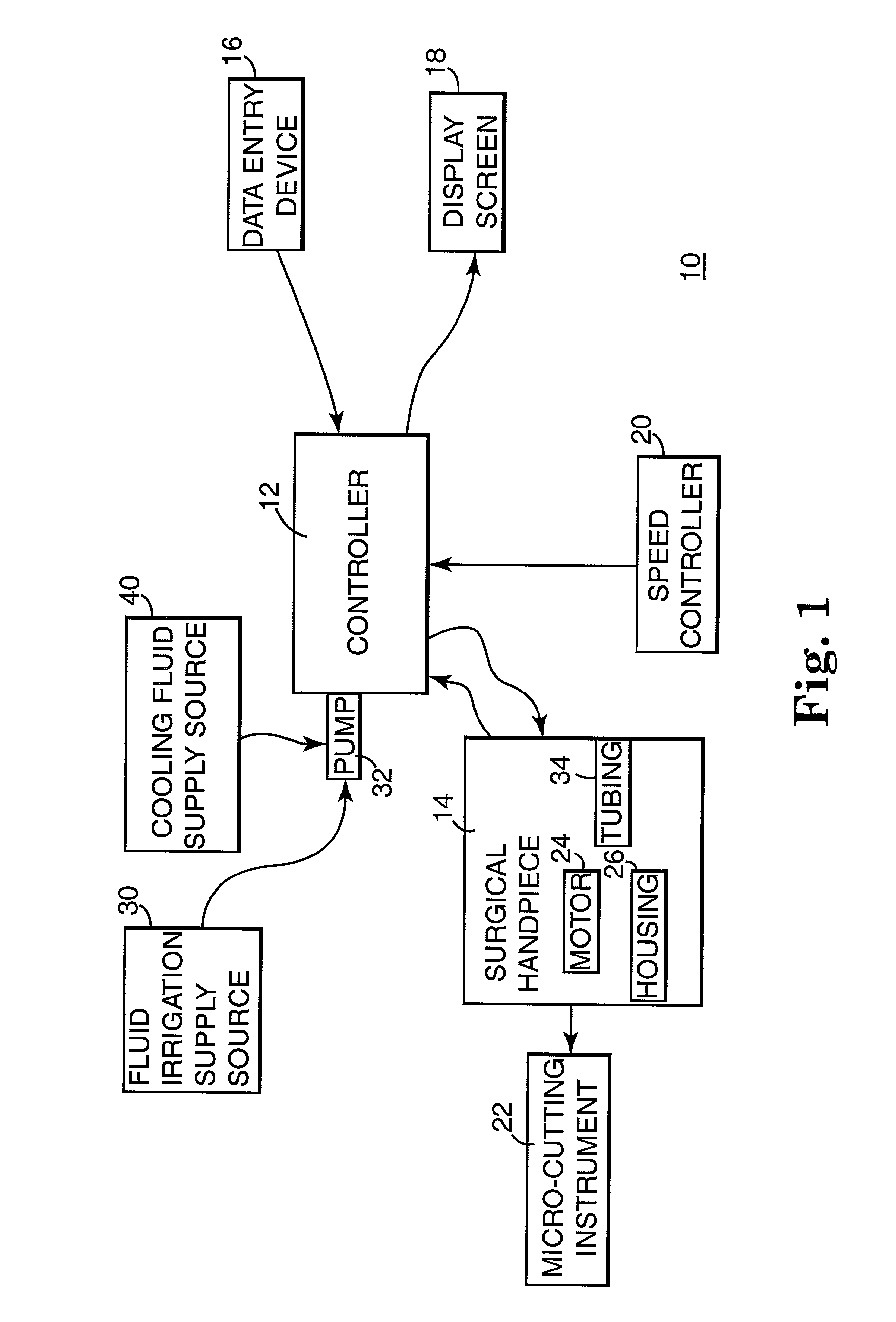
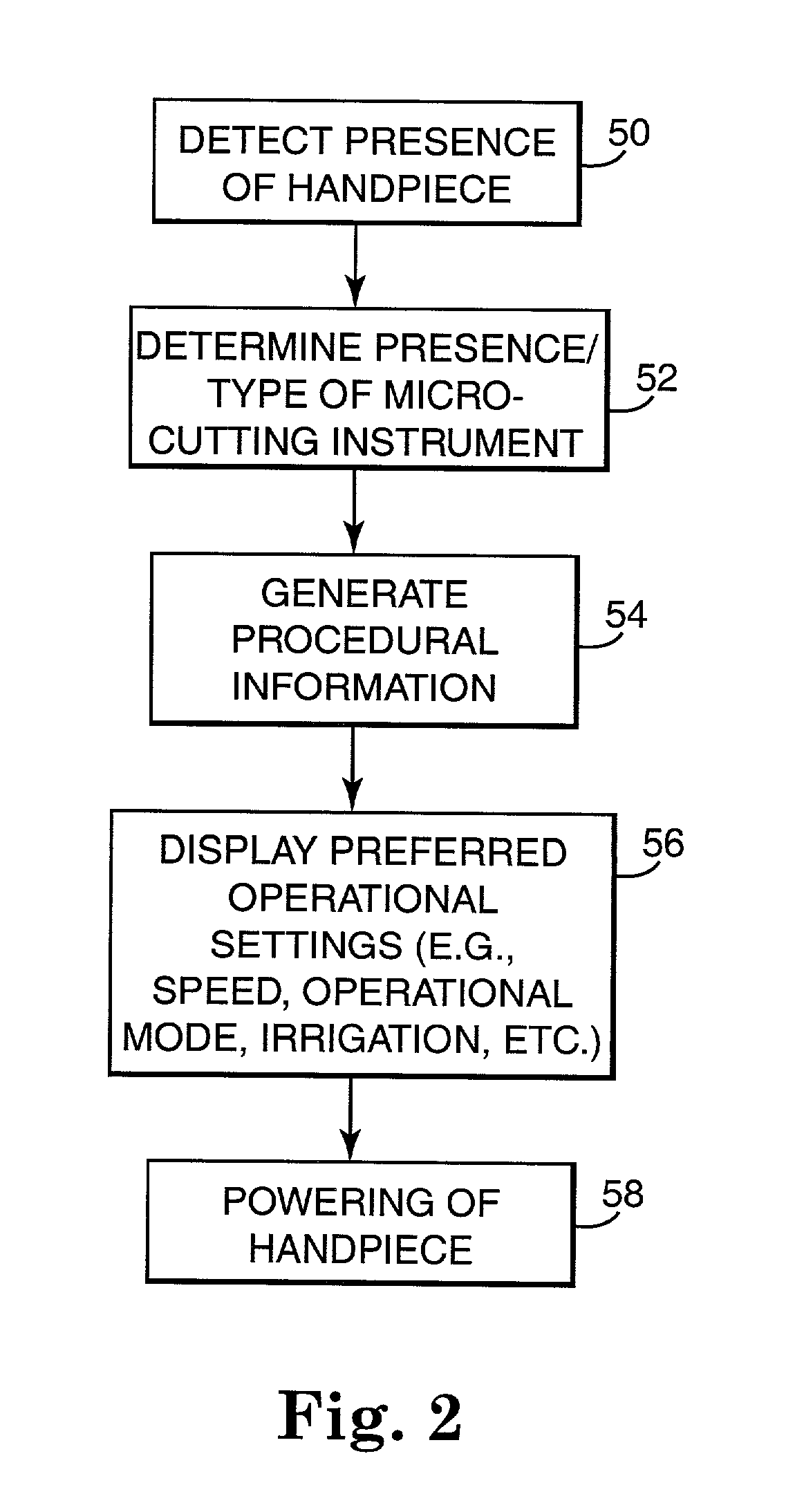
Motor control system for a surgical handpiece
A system and method for powered surgical handpiece capable of powering various micro-cutting instruments is described. The system is comprised of a controller adapted for controlling / interfacing with a powered surgical handpiece based upon user-defined procedural information. A data entry device is used for entering the user-defined procedural information used by the controller for configuring and operating the motor control system.
Owner:MEDTRONIC INC
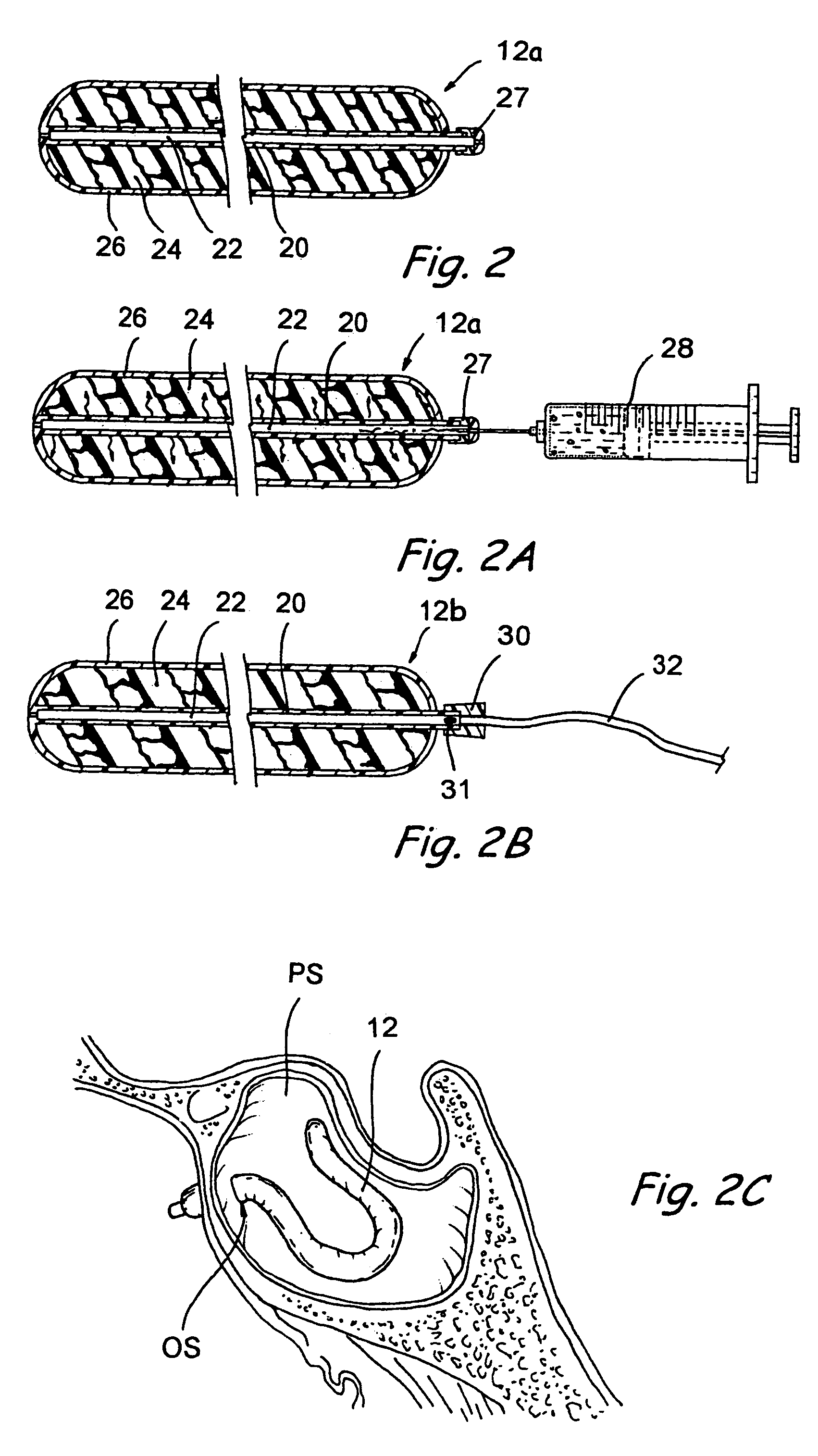
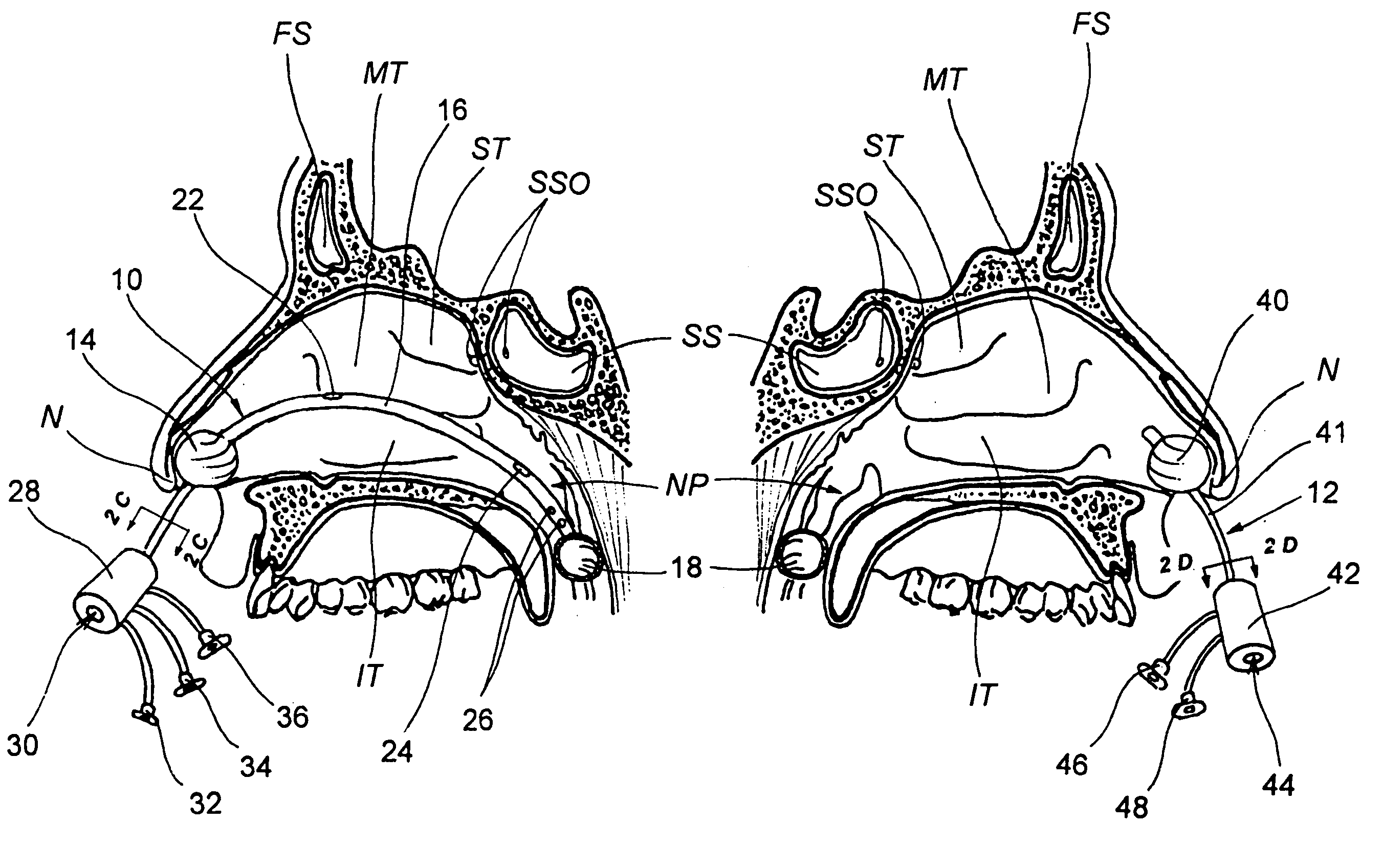
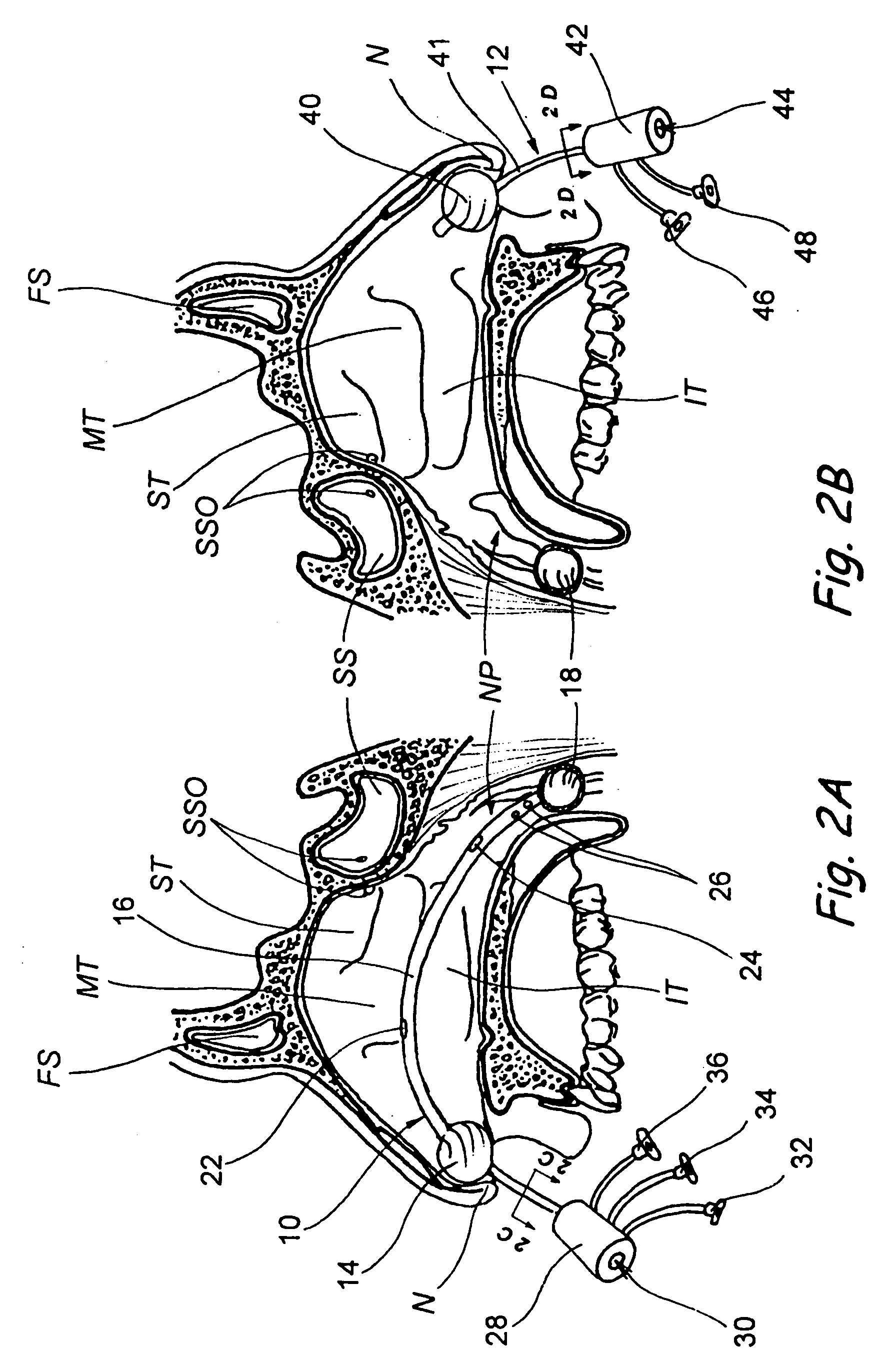
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Wound dressing and method of use
ActiveUS20110282309A1Reduce decreaseExtended service lifeNon-adhesive dressingsPlastersWound.exudateMoisture
A system, method, and apparatus are disclosed for dressing a wound. The apparatus comprises a liquid and gas permeable transmission layer, an absorbent layer for absorbing wound exudate, the absorbent layer overlying the transmission layer, a gas impermeable cover layer overlying the absorbent layer and comprising a first orifice, wherein the cover layer is moisture vapor permeable.
Owner:SMITH & NEPHEW INC
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
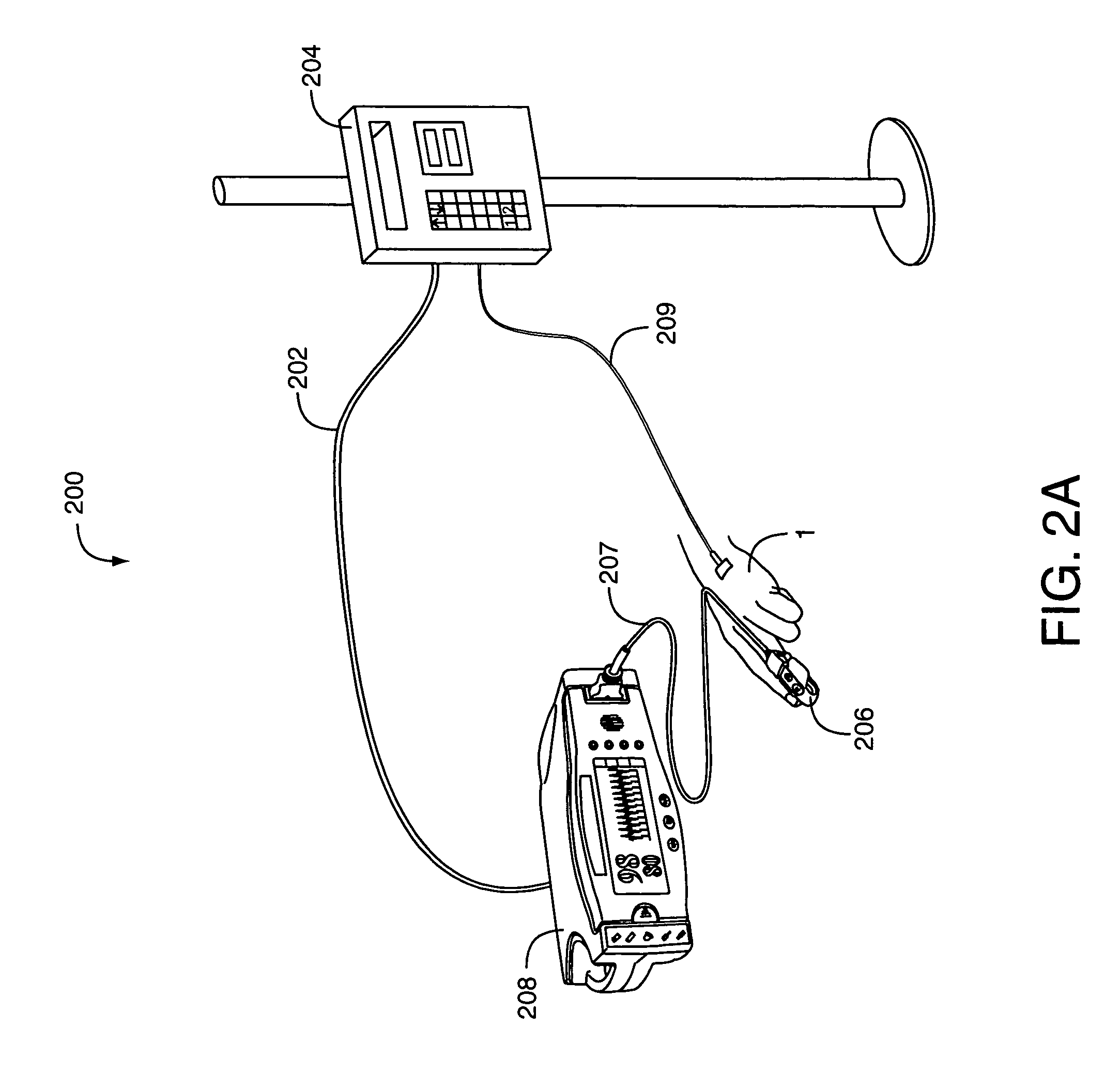
Devices, systems and methods for patient infusion
InactiveUS7029455B2Easy to carryReduce financial burdenDrug and medicationsPharmaceutical delivery mechanismUser inputRemote control
A device for delivering a fluid to a patient, including an exit port, a dispenser for causing fluid from a reservoir to flow to the exit port, a local processor programmed to cause a flow of fluid to the exit port based on flow instructions from a separate, remote control device, and a wireless receiver connected to the local processor for receiving the flow instructions. The device also includes a housing free of user input components for providing flow instructions to the local processor, in order to reduce the complexity and costs of the device so that the device lends itself to being disposable in nature. A system and a kit are also described that include the fluid delivery device, a separate, remote control device, and accessories for transcutaneous delivery of fluid medications. Methods of utilizing the fluid delivery device to infuse fluid medications are additionally disclosed.
Owner:INSULET CORP
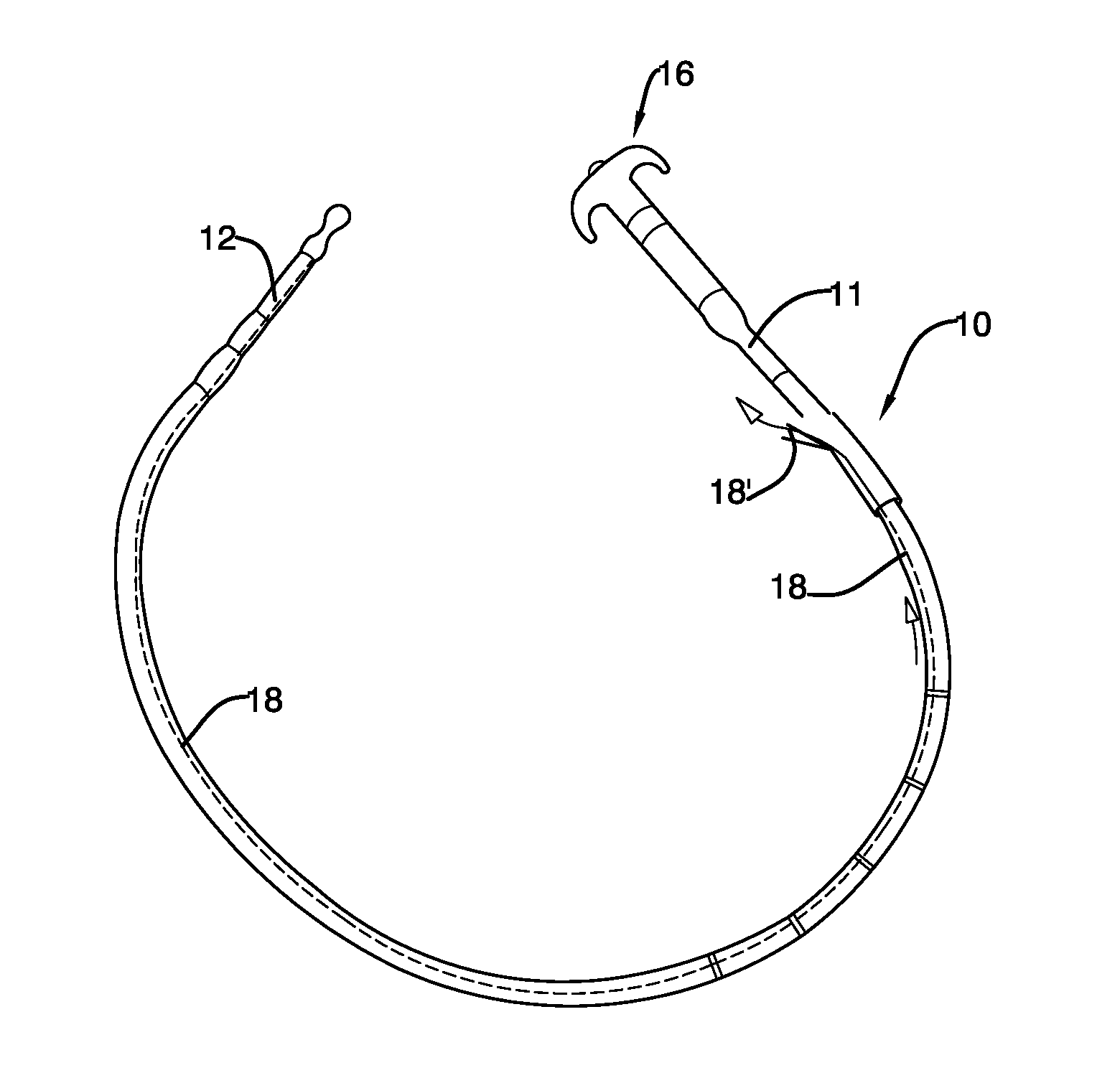
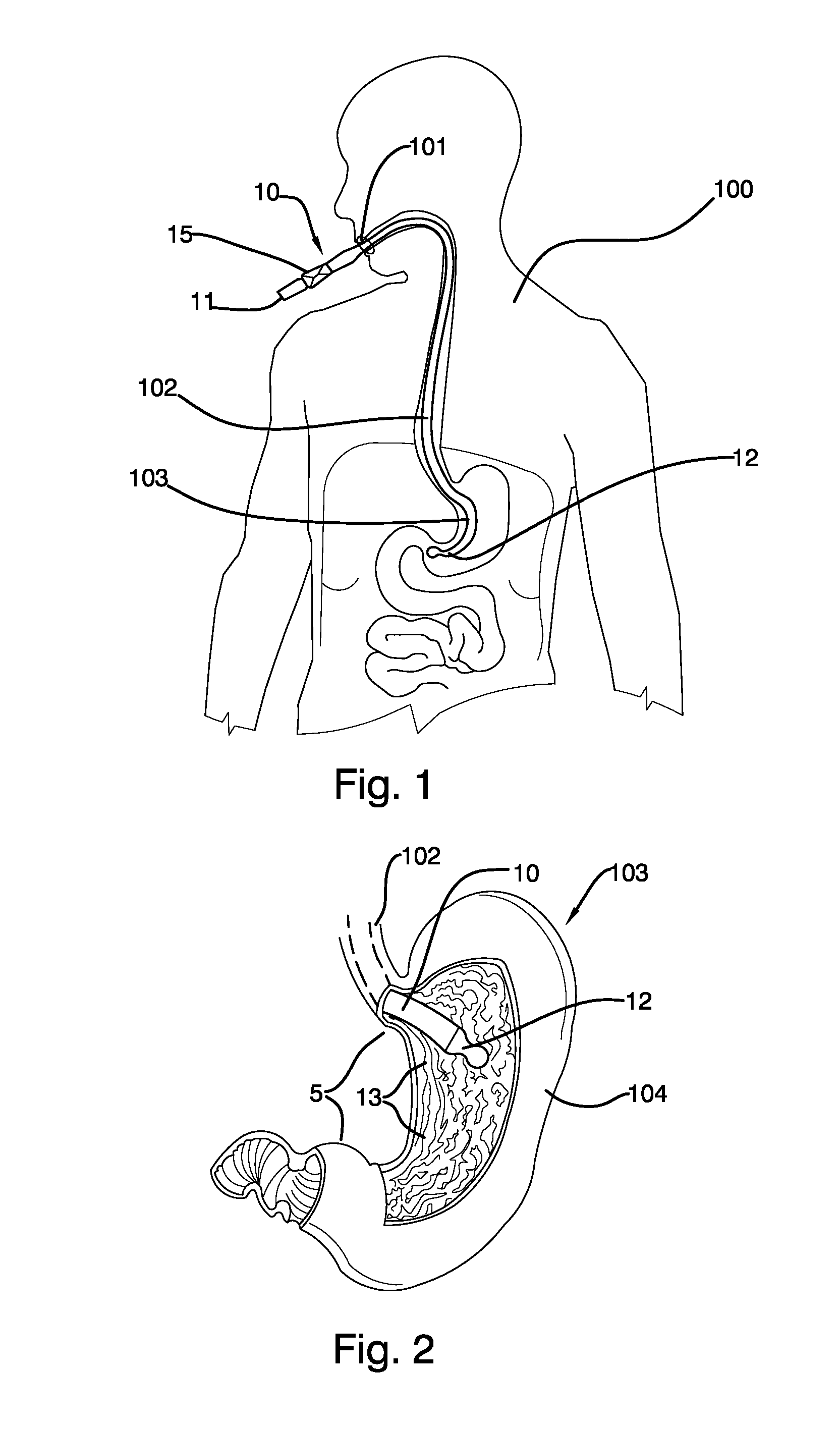
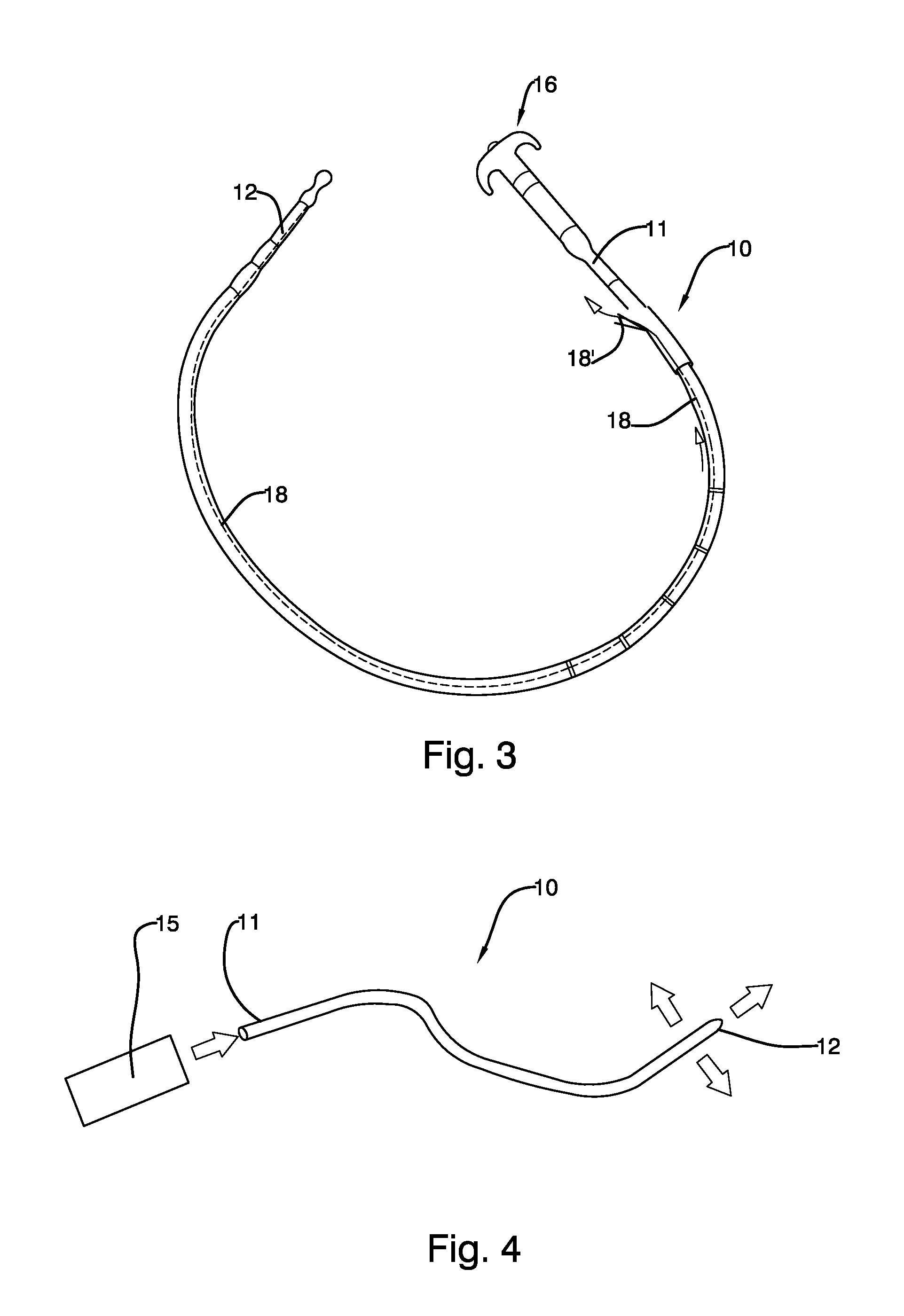
Mechanically-guided transoral bougie
The present invention is referred to a mechanically-guided transoral bougie, comprising an elongated body with an external end and a distal end; said external end includes a guiding mechanism mechanically connected to said distal end to allow the surgeon to move said distal end in any direction once the bougie is inserted into the stomach. Said guiding mechanism includes a manually operated guiding control.
Owner:JACOBS MOISES
Biocompatible wound dressing
InactiveUS7070584B2Promote cell growthPrevent vacuum leakageWound drainsMedical applicatorsWound dressingWound site
A biocompatible wound dressing comprised of a pad for insertion substantially into a wound site and a wound drape for sealing enclosure of the foam pad at the wound site. The pad, comprised of a foam or other like material having relatively few open cells in contact with the areas upon which cell growth is to be encouraged so as to avoid unwanted adhesions, but having sufficiently numerous open cells so that drainage and negative pressure therapy may continue unimpaired, is placed in fluid communication with a vacuum source for promotion of fluid drainage, as known in the art. The pad is further comprised of an ultra-low density fused-fibrous ceramic, or a bioabsorbable branched polymer, or cell growth enhancing matrix or scaffolding.
Owner:KCI LICENSING INC
Flow restriction system and method for patient infusion device
InactiveUS7018360B2Precise deliveryAvoid flowMedical devicesPressure infusionEngineeringFlow limitation
A device for delivering fluid, such as insulin for example, to a patient. The device includes a flow path having an exit port assembly adapted to connect to a transcutaneous patient access tool, and a reservoir connected to the exit port assembly. The device also includes a flow restriction system having an air removal filter communicating with the flow path and allowing air to exit the flow path and preventing fluid from exiting the flow path, and a flow restrictor positioned within the flow path between the air removal filter and the exit port assembly. Among other features and advantages, the flow restriction system of the present invention allows the flow path of the fluid delivery device to be purged of air, or “primed” prior to operation, such that desired volumes of fluid can be accurately delivered by the device.
Owner:INSULET CORP
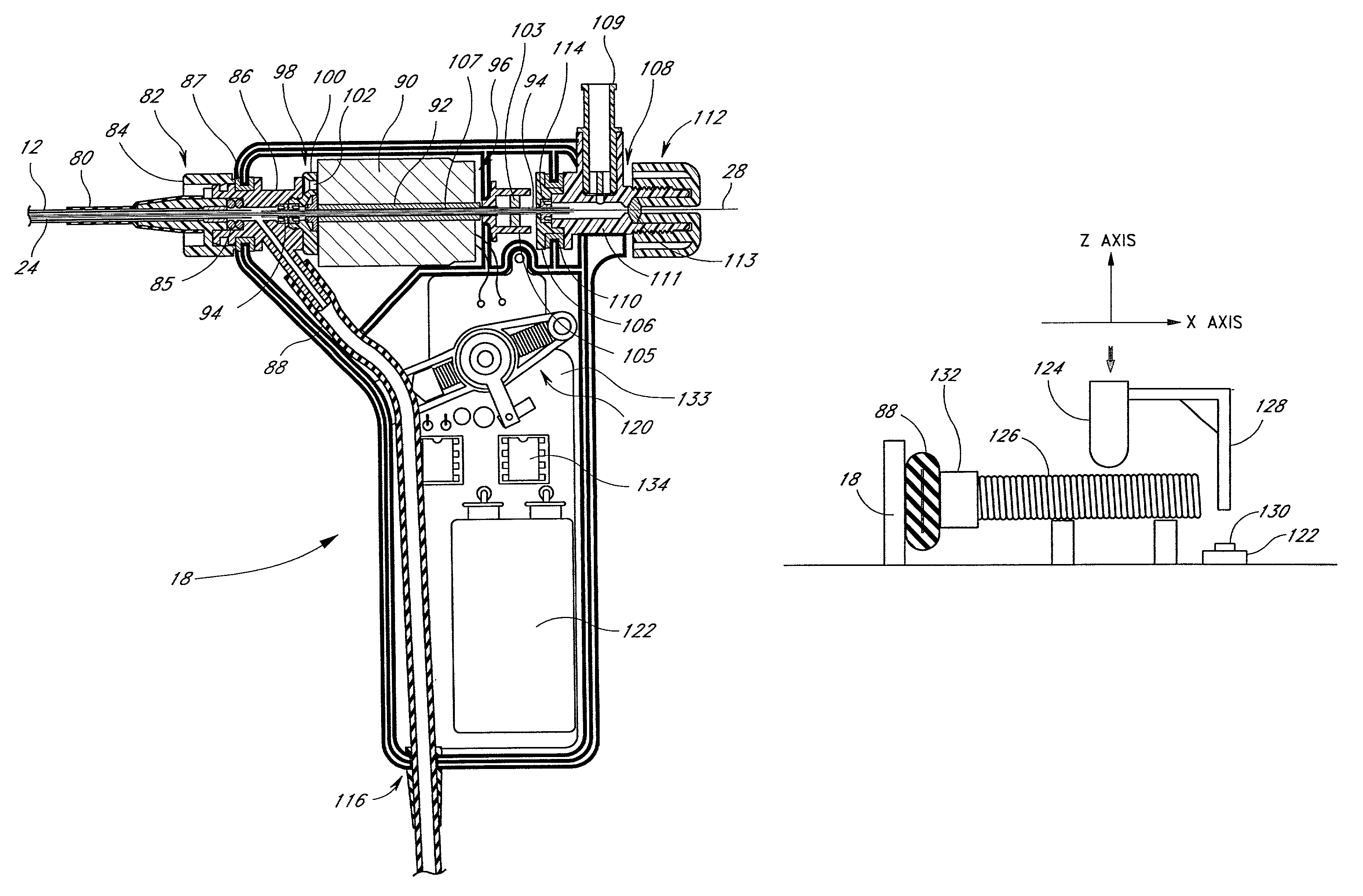
Infusion pump with a sealed drive mechanism and improved method of occlusion detection
InactiveUS20020128594A1Error minimizationImprove accuracyFlexible member pumpsSurgeryOcclusion detectionImproved method
A piston-type infusion pump is provided having an improved method of occlusion detection. The infusion pump includes processing circuitry for controlling the drive mechanism to infuse medication to a patient, including a sensor to track the position of the syringe plunger, thereby metering the amount of medication dispensed to the patient. The processing circuitry also includes a force sensor for providing signals indicative of the presence of occlusions along the infusion path. The operation of the drive mechanism causes delivery of medication to the patient. The infusion pump is constructed to be watertight.
Owner:LIFESCAN IP HLDG LLC
Methods for targeted electrosurgery on contained herniated discs
InactiveUS7179255B2Reduce pressureReduced neckingEnemata/irrigatorsHeart valvesFibrous ringCorneal ablation
Apparatus and methods for treating an intervertebral disc by ablation of disc tissue. A method of the invention includes positioning at least one active electrode within the intervertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. In other embodiments, a curved or steerable probe is guided to a specific target site within a disc to be treated, and the disc tissue at the target site is ablated by application of at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s). A method of making an electrosurgical probe is also disclosed.
Owner:ARTHROCARE
Infusion device and driving mechanism and process for same with actuator for multiple infusion uses
InactiveUS6932584B2Small thickness dimensionMinimize traumaIntravenous devicesPiston pumpsEngineeringActuator
Owner:MEDTRONIC MIMIMED INC
Drug administration controller
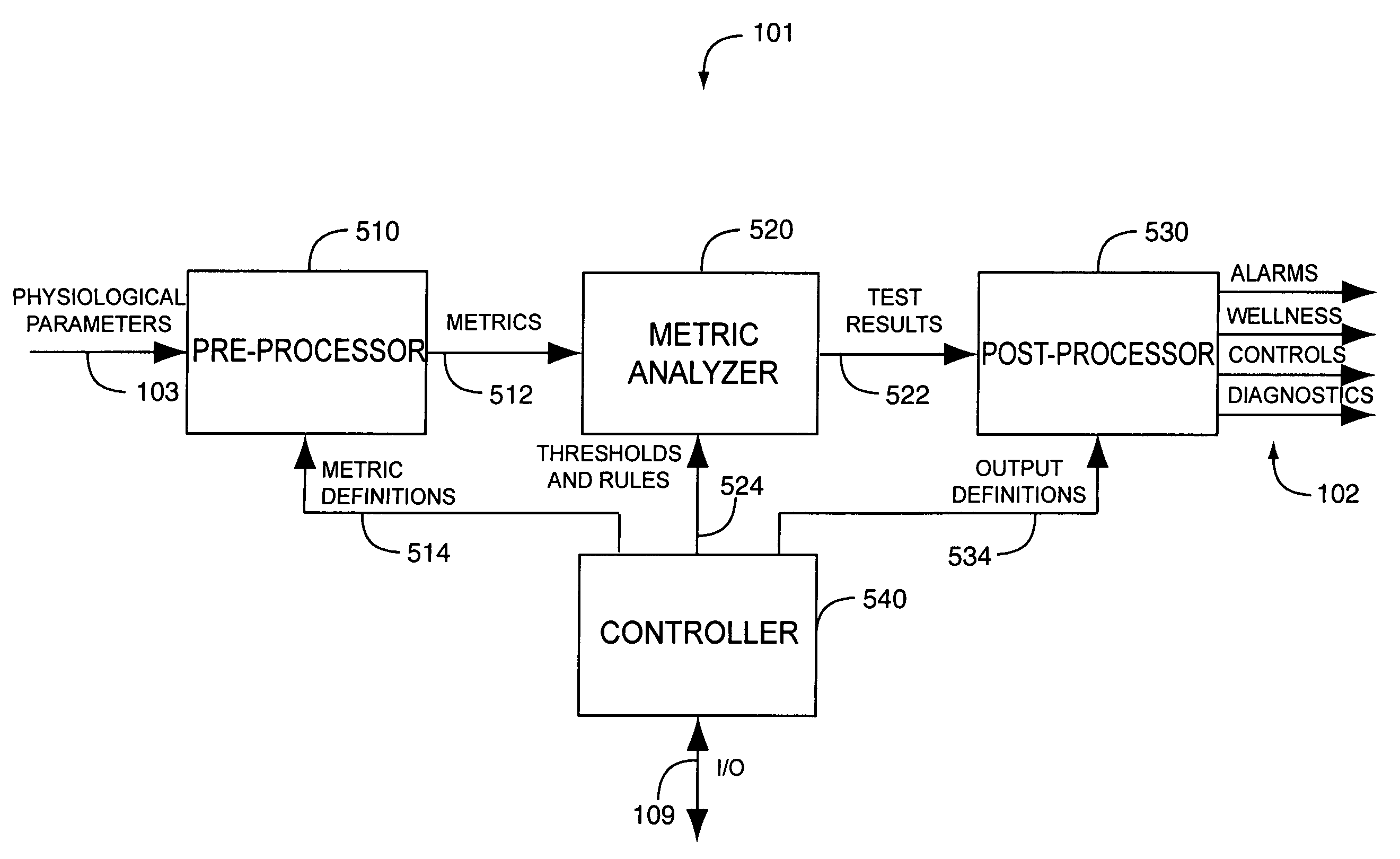
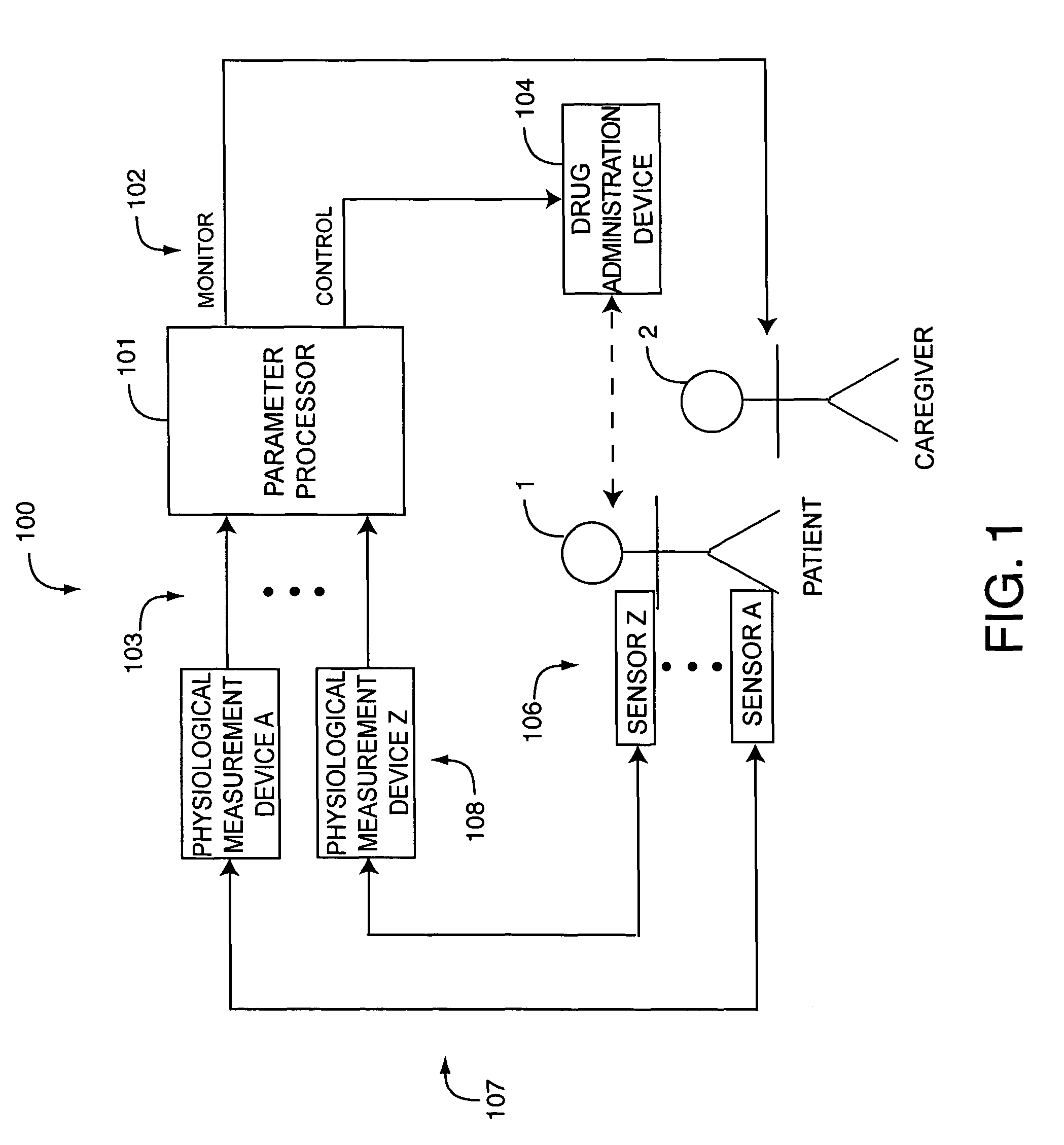
A drug administration controller has a sensor that generates a sensor signal to a physiological measurement device, which measures a physiological parameter in response. A control output responsive to the physiological parameter or a metric derived from the physiological parameter causes a drug administration device to affect the treatment of a person, such as by initiating, pausing, halting or adjusting the dosage of drugs administered to the person.
Owner:JPMORGAN CHASE BANK NA
Methods and devices for performing procedures within the ear, nose, throat and paranasal sinuses
Devices, systems and methods for performing image guided interventional and surgical procedures, including various procedures to treat sinusitis and other disorders of the paranasal sinuses, ears, nose or throat.
Owner:ACCLARENT INC
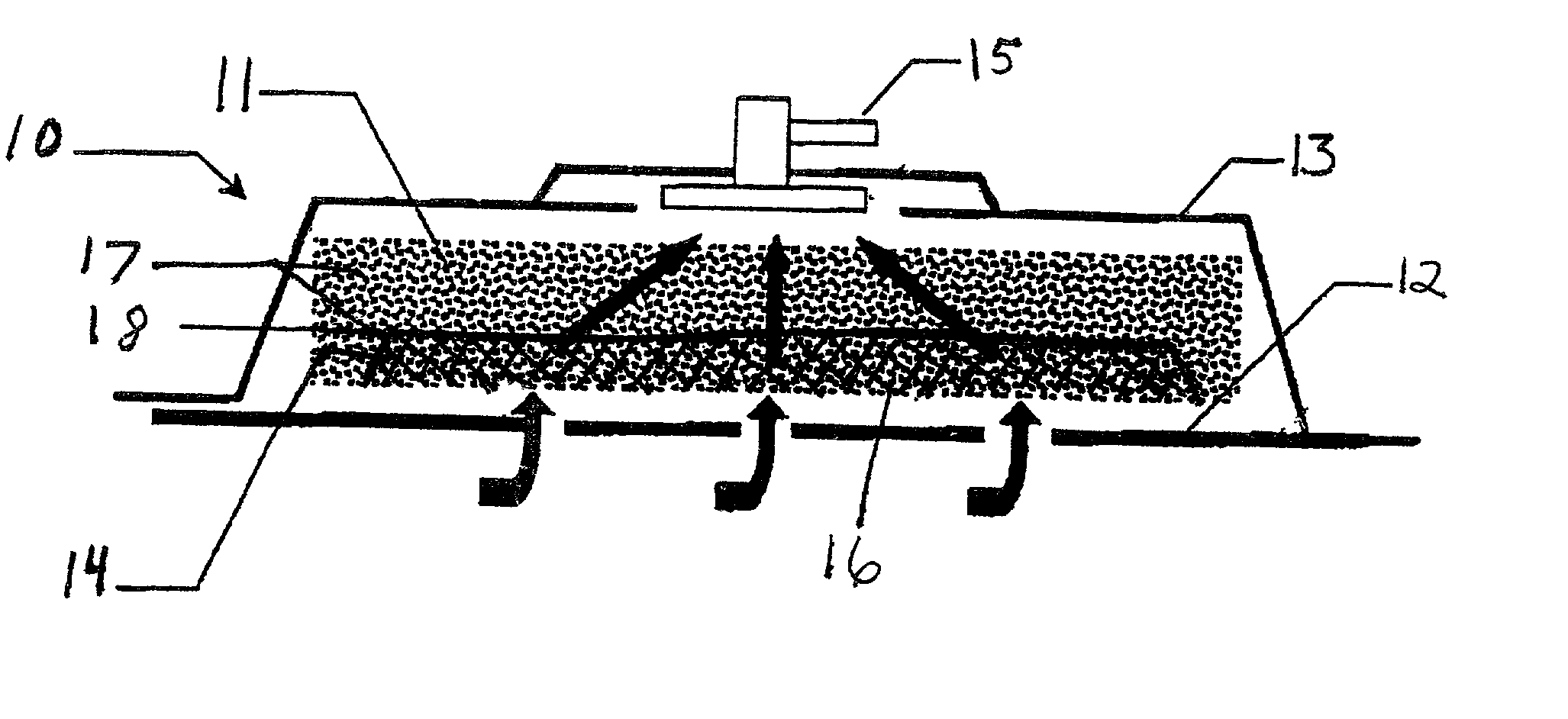
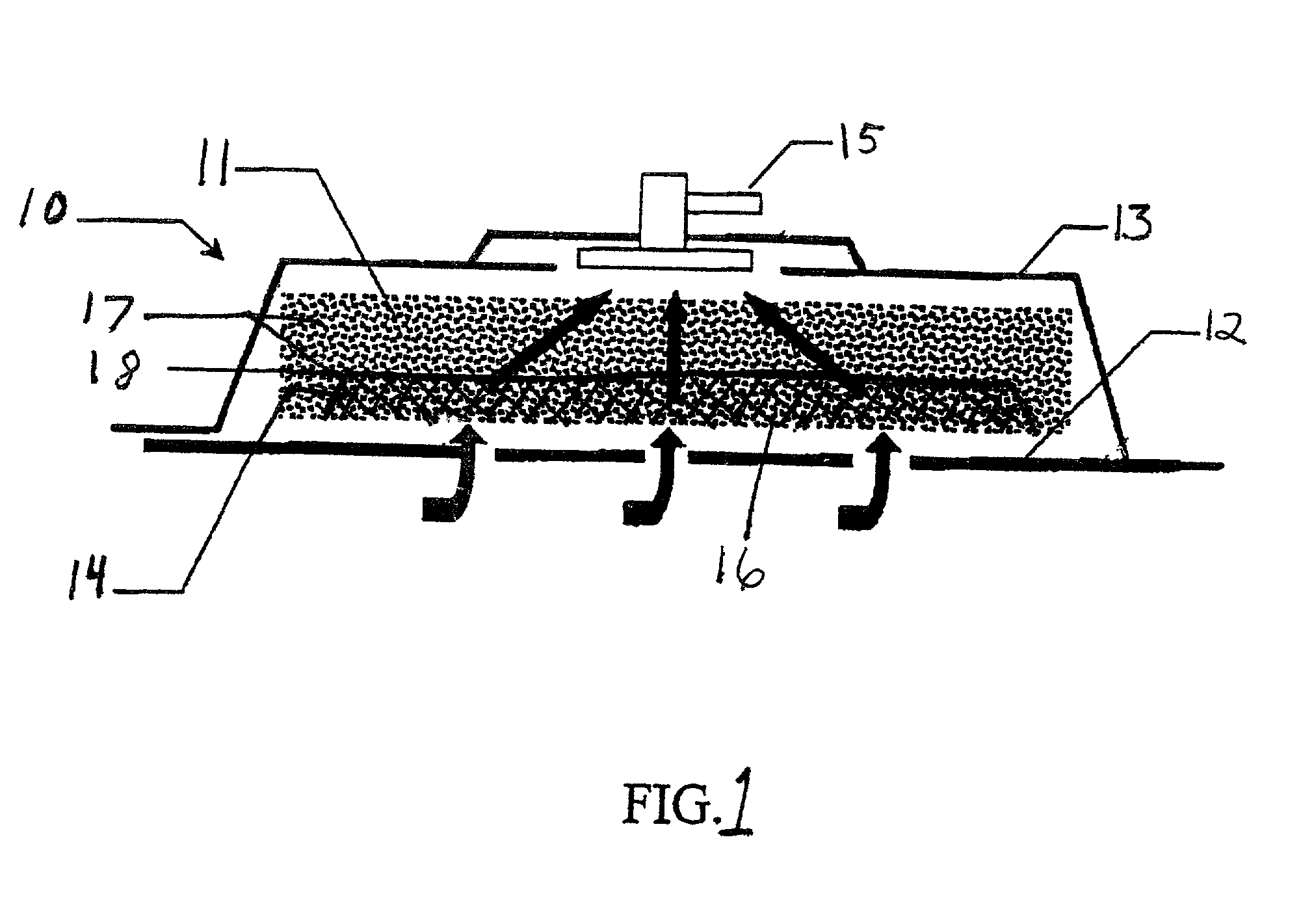
Removable wound closure
InactiveUS7381859B2Promote wound healingMinimizes adhesion formationNon-adhesive dressingsWound drainsElastomerPorosity
A system and method for the temporary closure of a wound, especially an abdominal wound, to facilitate re-entry, final closure, and long term healing of the wound. An abdominal wound dressing and methods of use are described that enable the application of negative pressure to the wound site in a site healing promoting manner while also limiting the formation of adhesions that would prevent the removal of the dressing. The dressing comprises a layer of porous foam material (36) enclosed by sheets of elastomeric material (38) punctuated by a number of appropriately placed holes (34). Multiple layers of porous foam may also be used. A suction tube connector (16) is provided on an upper surface of a layer of foam (12) for connection to a negative pressure source. At least one layer of foam is enclosed in elastomeric material and is placed in direct contact with the tissue within the open wound. Fluids are drawn by negative pressure through the holes positioned in the elastomeric envelope, and through the foam. If multiple foam layers are employed, the lower layer(s) of foam are of a finer porosity while the upper layer of foam is coarse. An adhesive elastomeric sheet (14) covers the entire wound dressing and seals the edges to the skin surrounding the wound. An appropriate vacuum device is attached to the suction tube connector.
Owner:KCI LICENSING INC
Infusion device menu structure and method of using the same
InactiveUS20050137530A1Drug and medicationsAutomatic syringesDisplay deviceHuman–computer interaction
A portable infusion system that is programmable by an individual for delivering fluid from a reservoir into a user includes a drive mechanism, an input device, a processor, and a display. The drive mechanism forces the fluid out of the reservoir, and the input device accepts one or more inputs. The processor uses one or more of the one or more inputs to control the drive mechanism. The display receives information from the processor and visually displays one or more screens containing the information. At least one of the one or more screens includes a menu with at least two menu items, and the input device is used to select one menu item from amongst the at least two menu items.
Owner:MINIMED
Rotational atherectomy device
Owner:TYCO HEALTHCARE GRP LP
Biocompatible wound dressing
InactiveUS20070185426A1Reduce pressureReduced tissue treatmentNon-adhesive dressingsAdhesive dressingsWound dressingContact layer
A multi-layer reduced pressure delivery apparatus is provided for applying reduced pressure tissue treatment to a tissue site. The multi-layer apparatus includes a tissue contact layer, a release layer, and a manifold layer. The tissue contact layer includes a scaffold adapted to contact the tissue site, the release layer includes a hydrogel-forming material and a plurality of flow channels, and the manifold layer includes a distribution manifold. The release layer is positioned between the tissue contact layer and the manifold layer to allow easy release of the manifold layer from the tissue contact layer following the administration of reduced pressure tissue treatment.
Owner:KCI LICENSING INC
Methods for repairing damaged intervertebral discs
InactiveUS7318823B2Reduce internal pressureReduce moistureBiocideOrganic chemistryIntervertebral discActive electrode
Apparatus and methods for treating an intervertebral disc by ablation of disc tissue. A method of the invention includes positioning at least one active electrode within the intervertebral disc, and applying at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s), wherein the volume of the nucleus pulposus is decreased, pressure exerted by the nucleus pulposus on the annulus fibrosus is reduced, and discogenic pain of a patient is alleviated. In other embodiments, a curved or steerable probe is guided to a specific target site within a disc to be treated, and the disc tissue at the target site is ablated by application of at least a first high frequency voltage between the active electrode(s) and one or more return electrode(s). A method of making an electrosurgical probe is also disclosed.
Owner:ARTHROCARE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com