Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
a paranasal sinus and ostia technology, applied in the field of minimal invasive devices, systems and methods for treating sinusitis and other ear, nose & throat disorders, can solve the problems of damage to the epithelium that lines the sinuses, blocked passageways which drain through the paranasal sinuses, and mucosal congestion within the paranasal sinuses, so as to facilitate the insertion of working devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0104] The following detailed description, the accompanying drawings and the above-set-forth Brief Description of the Drawings are intended to describe some, but not necessarily all, examples or embodiments of the invention. The contents of this detailed description do not limit the scope of the invention in any way.
[0105] A number of the drawings in this patent application show anatomical structures of the ear, nose and throat. In general, these anatomical structures are labeled with the following reference letters:
Nasal CavityNCNasopharynxNPFrontal SinusFSEthmoid SinusESEthmoid Air CellsEACSphenoid SinusSSSphenoid Sinus OstiumSSOMaxillary SinusMSMucoceleMC
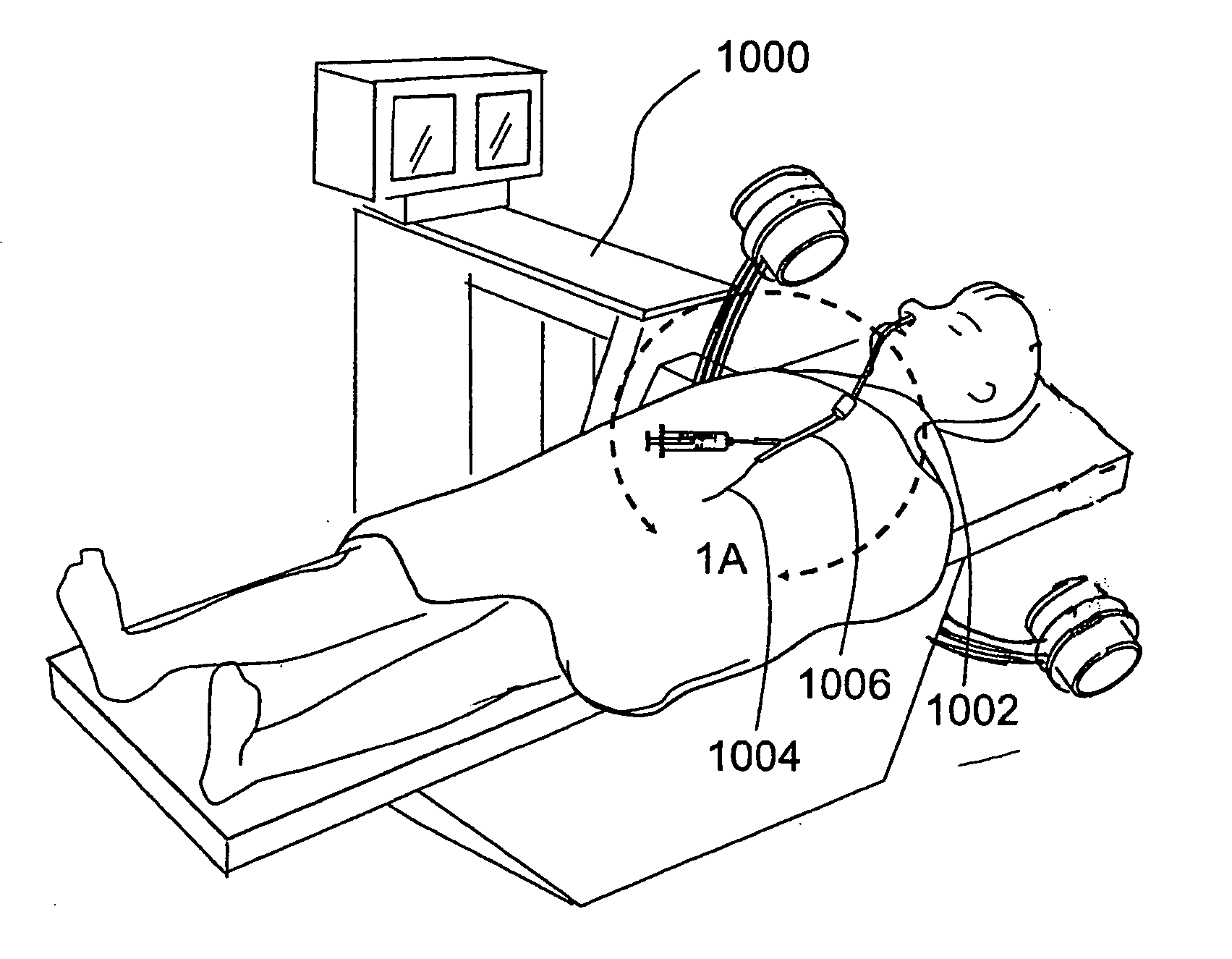
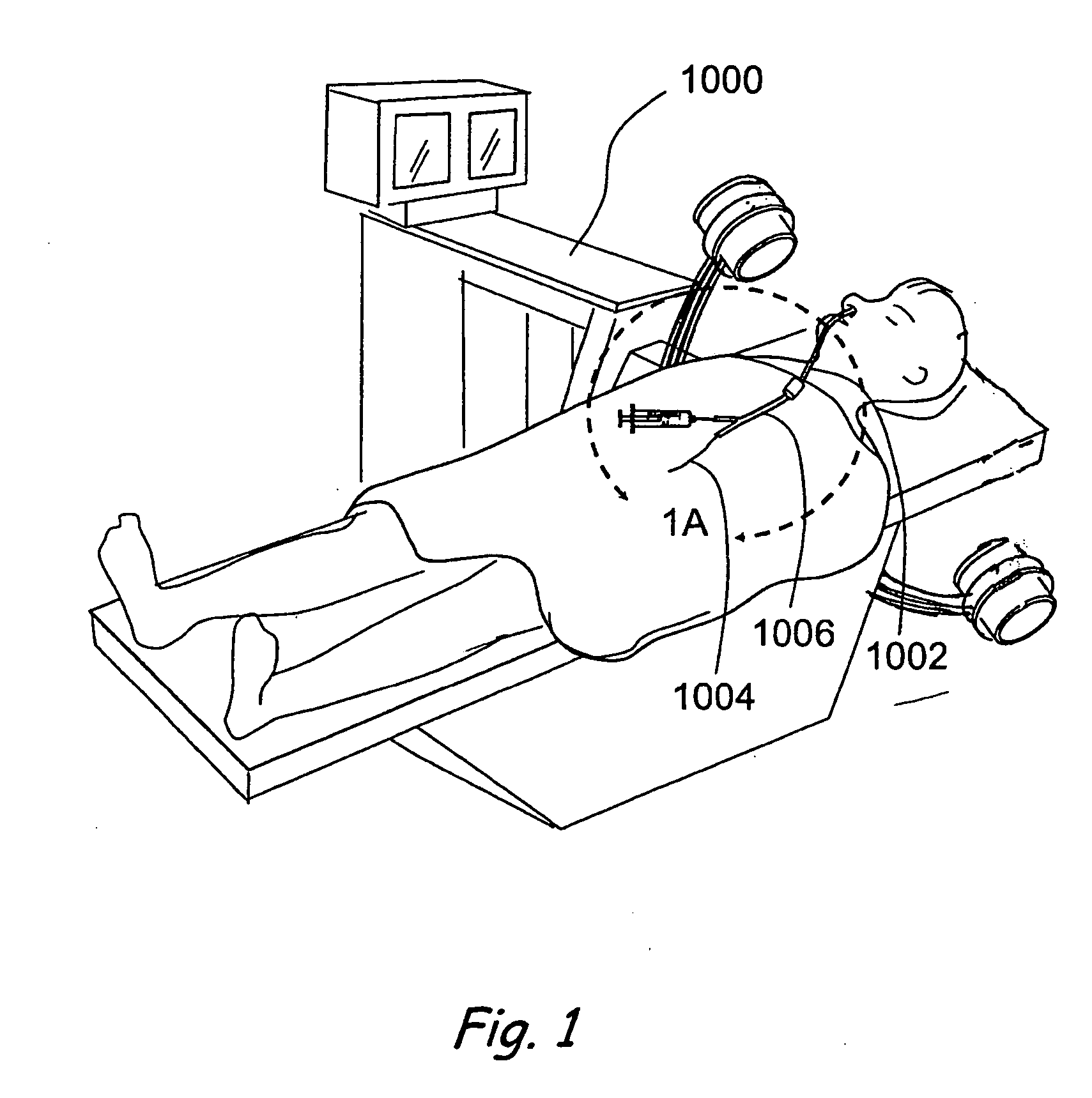
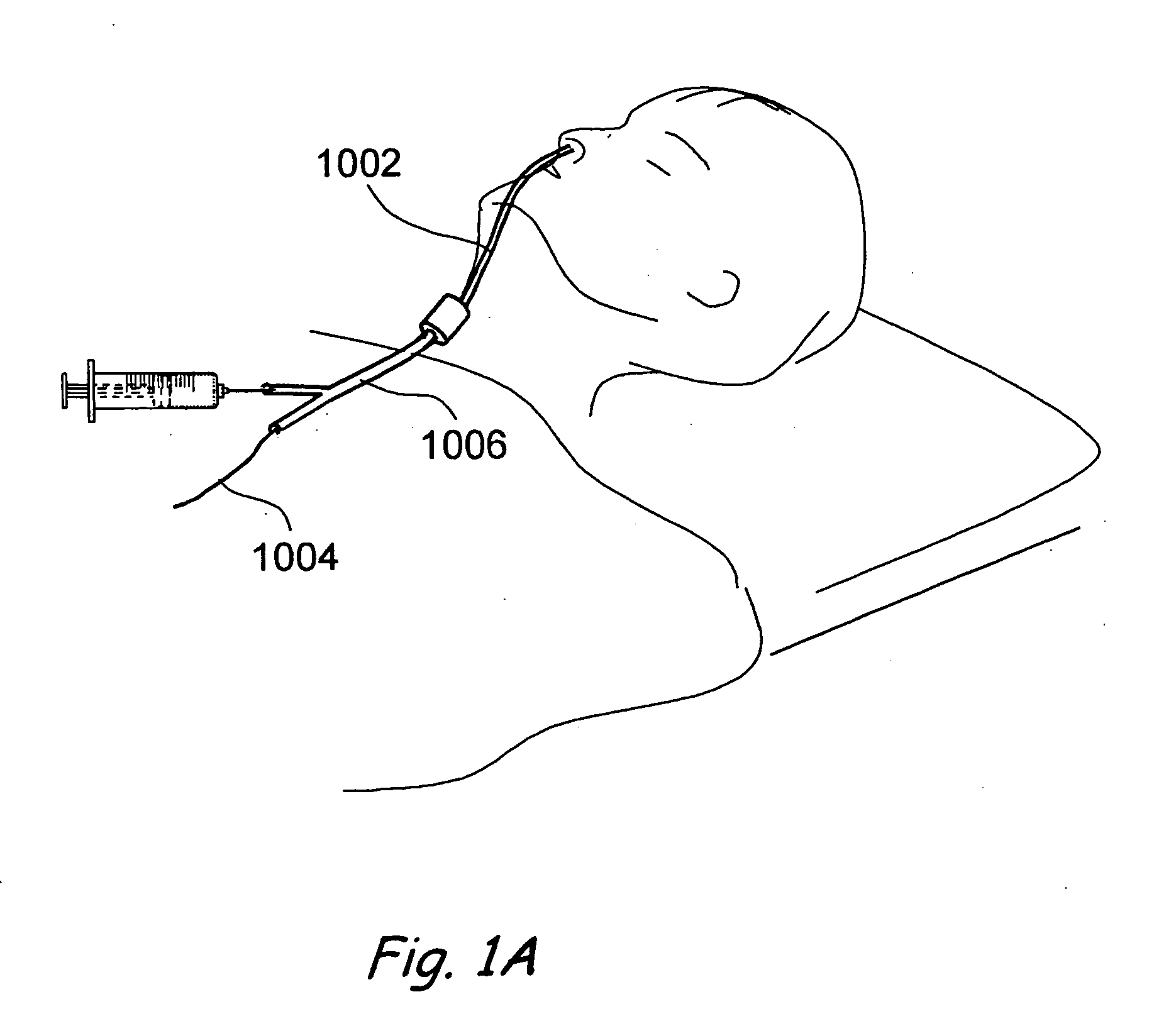
[0106]FIGS. 1 and 1A provide a general showing of a minimally invasive surgery system of the present invention comprising a C-arm fluoroscope 1000 that is useable to visualize a first introducing device 1002 (e.g., a guide catheter or guide tube), a second introducing device 1004 (e.g., a guidewire or elongate probe) and a wor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com