Patents
Literature
7384 results about "Insertion stent" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
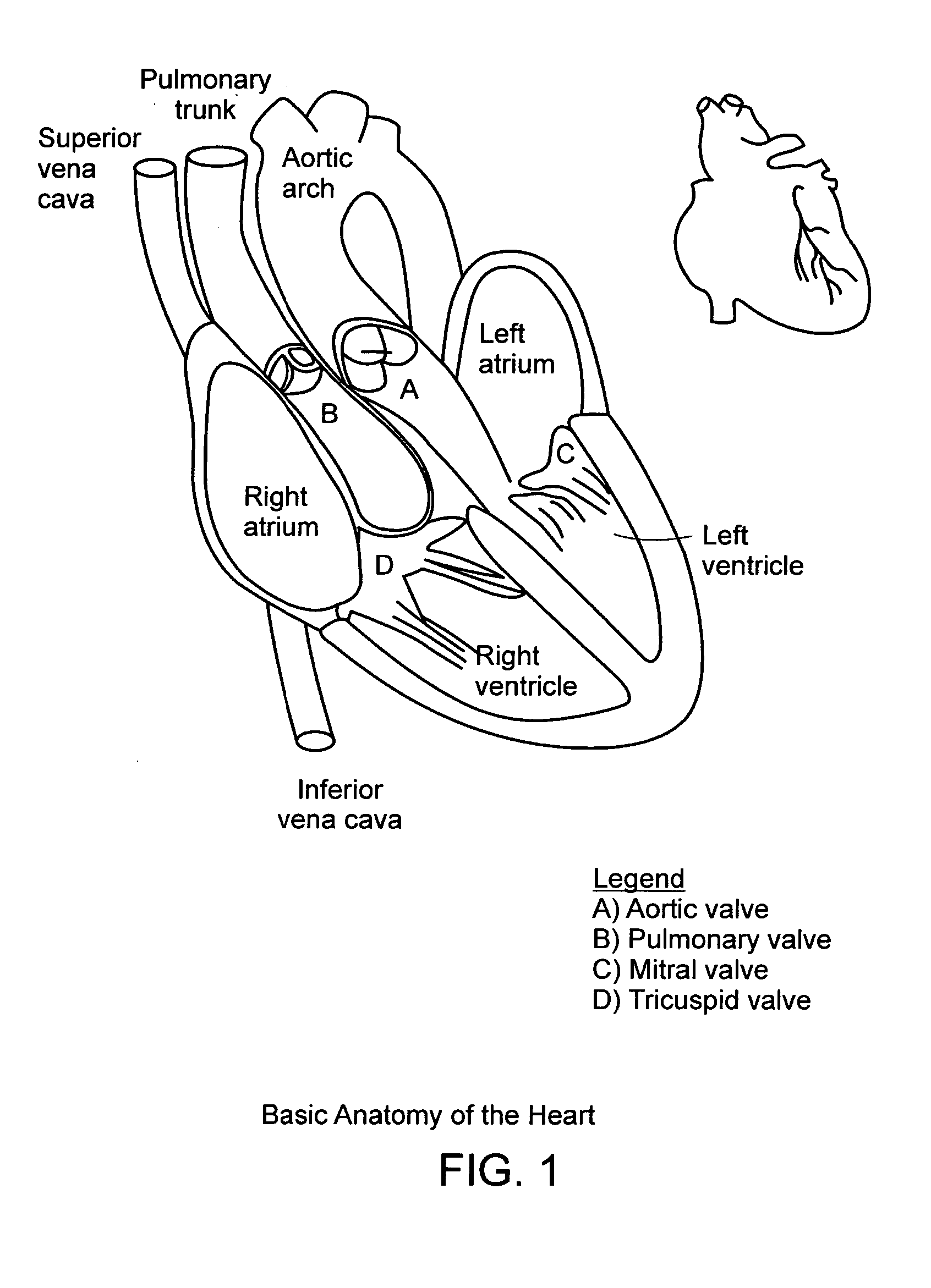
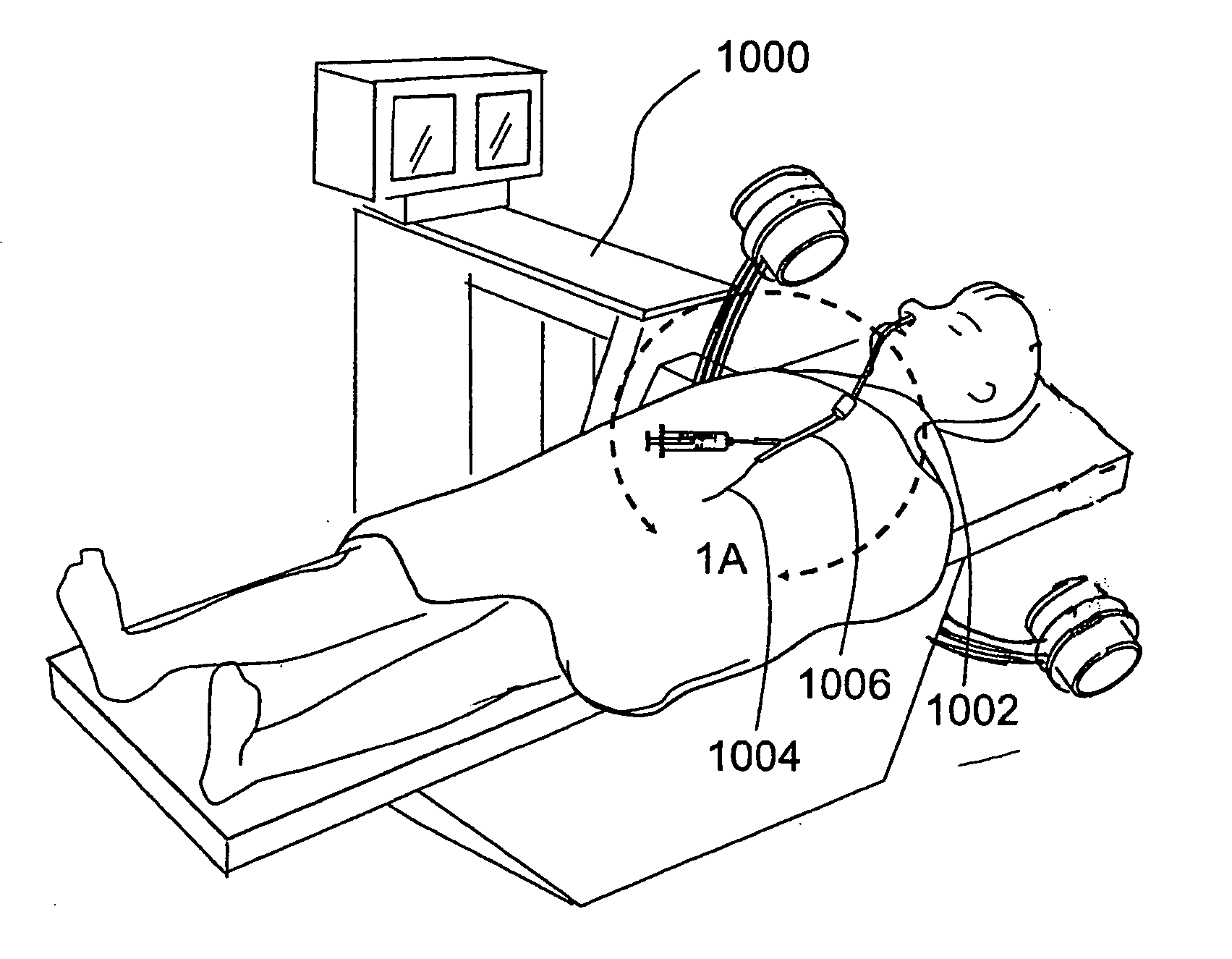
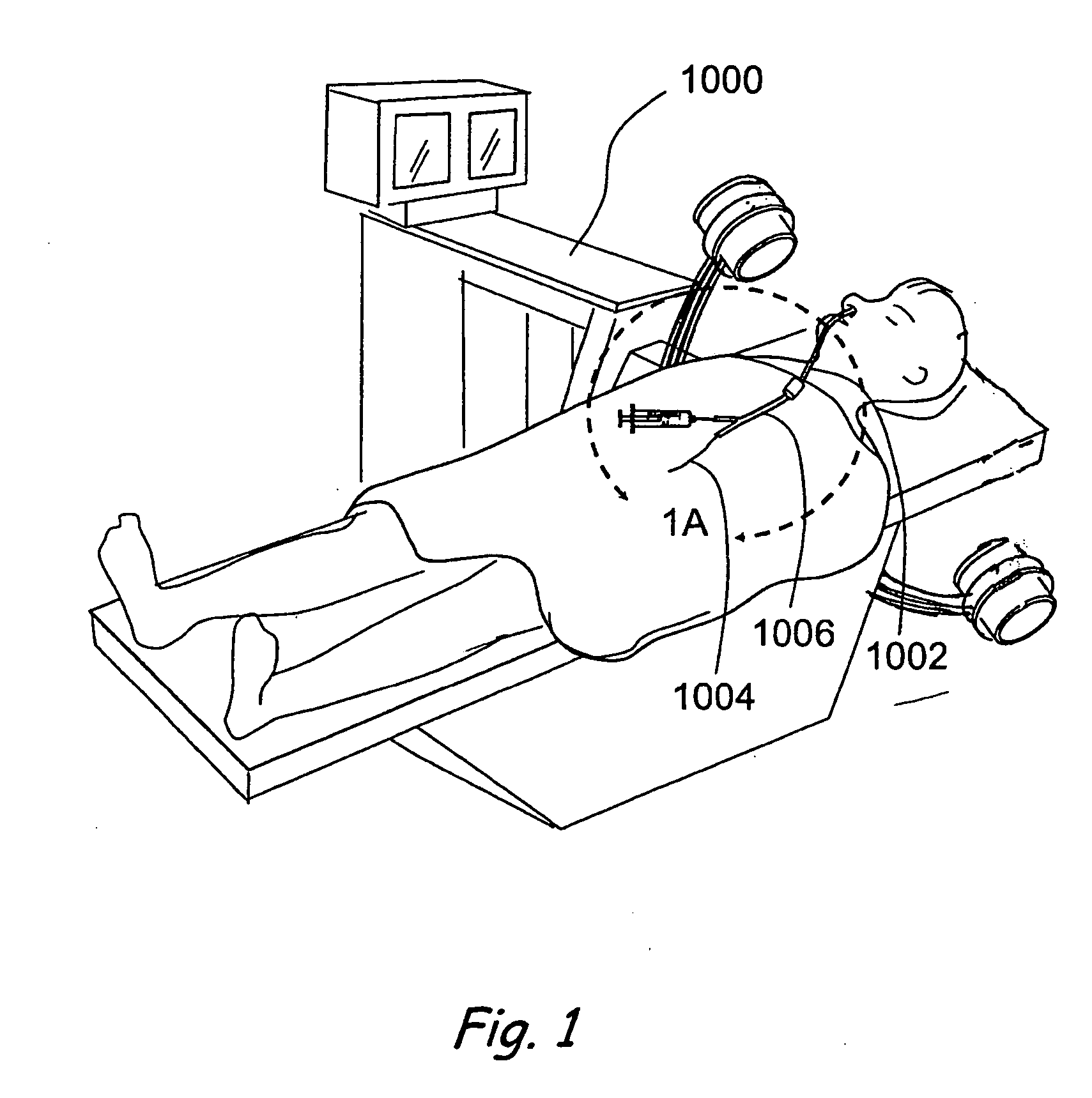
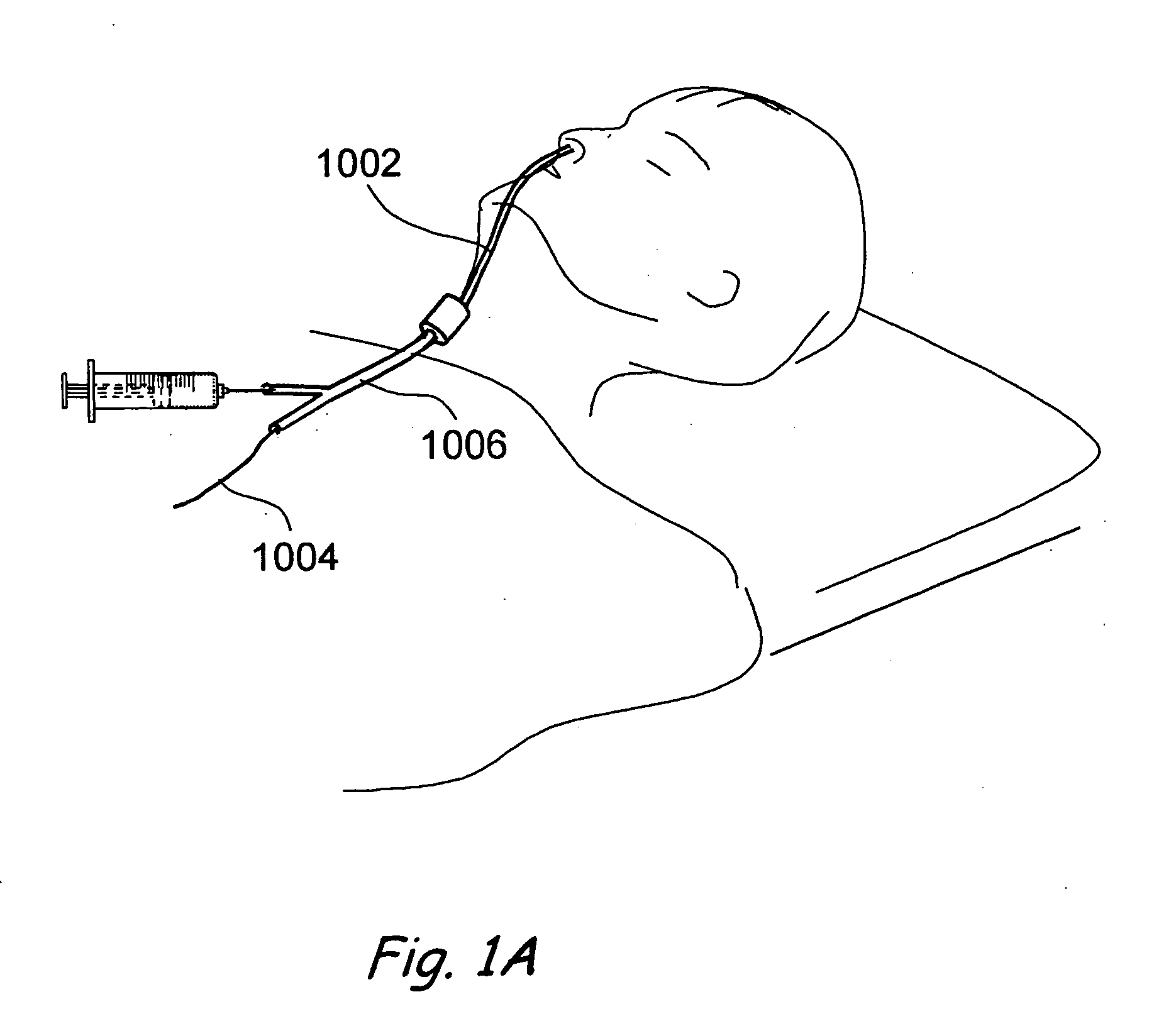
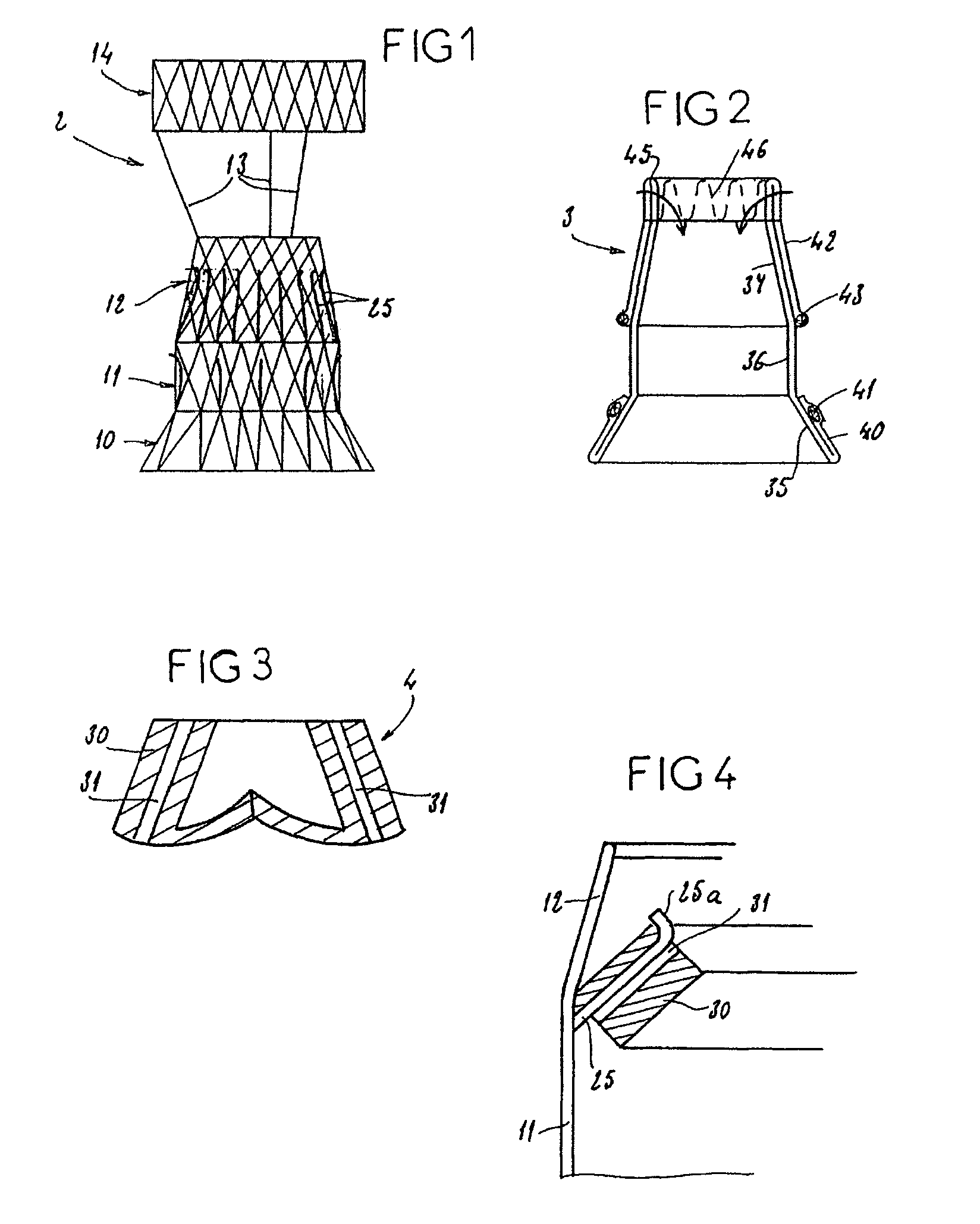
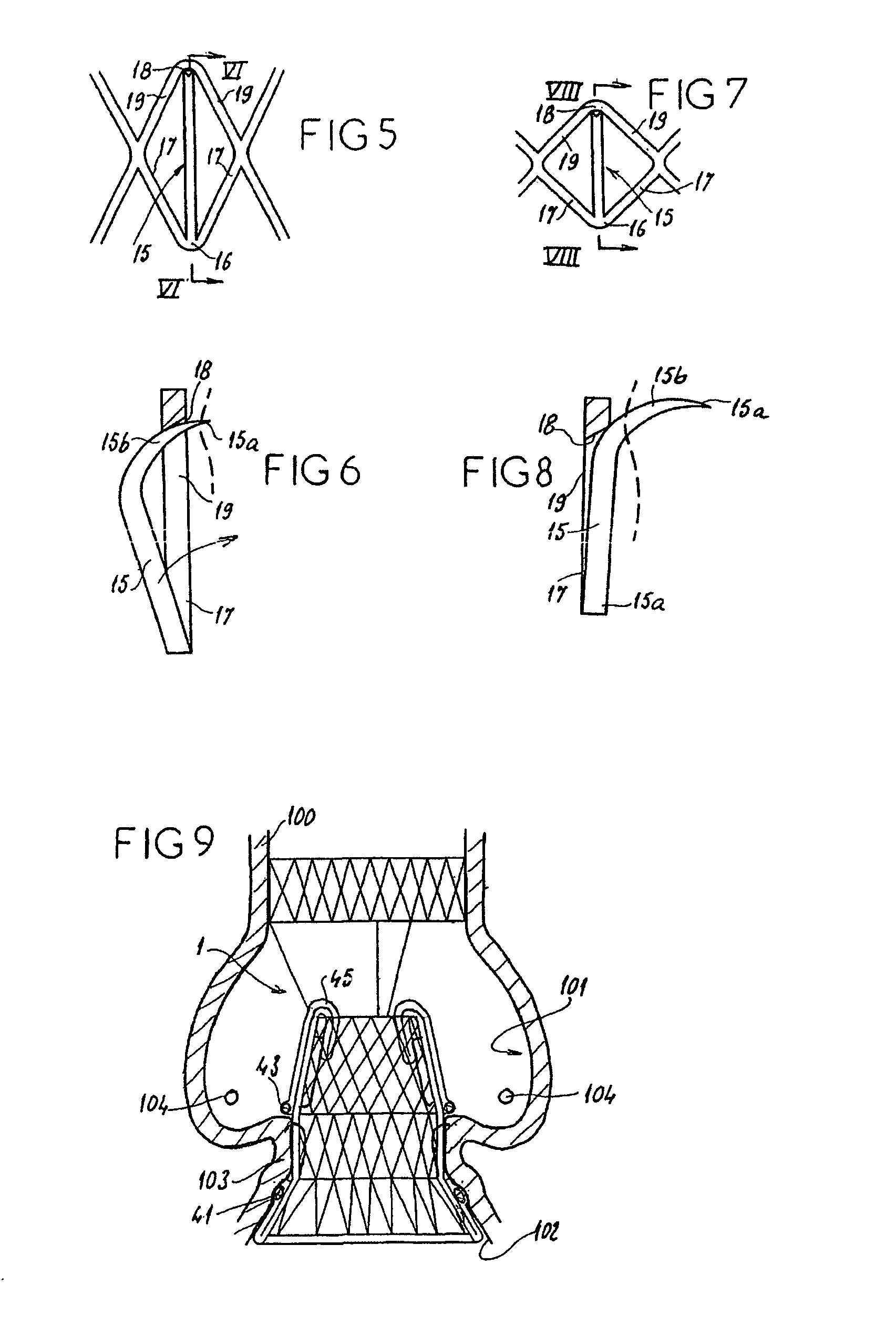
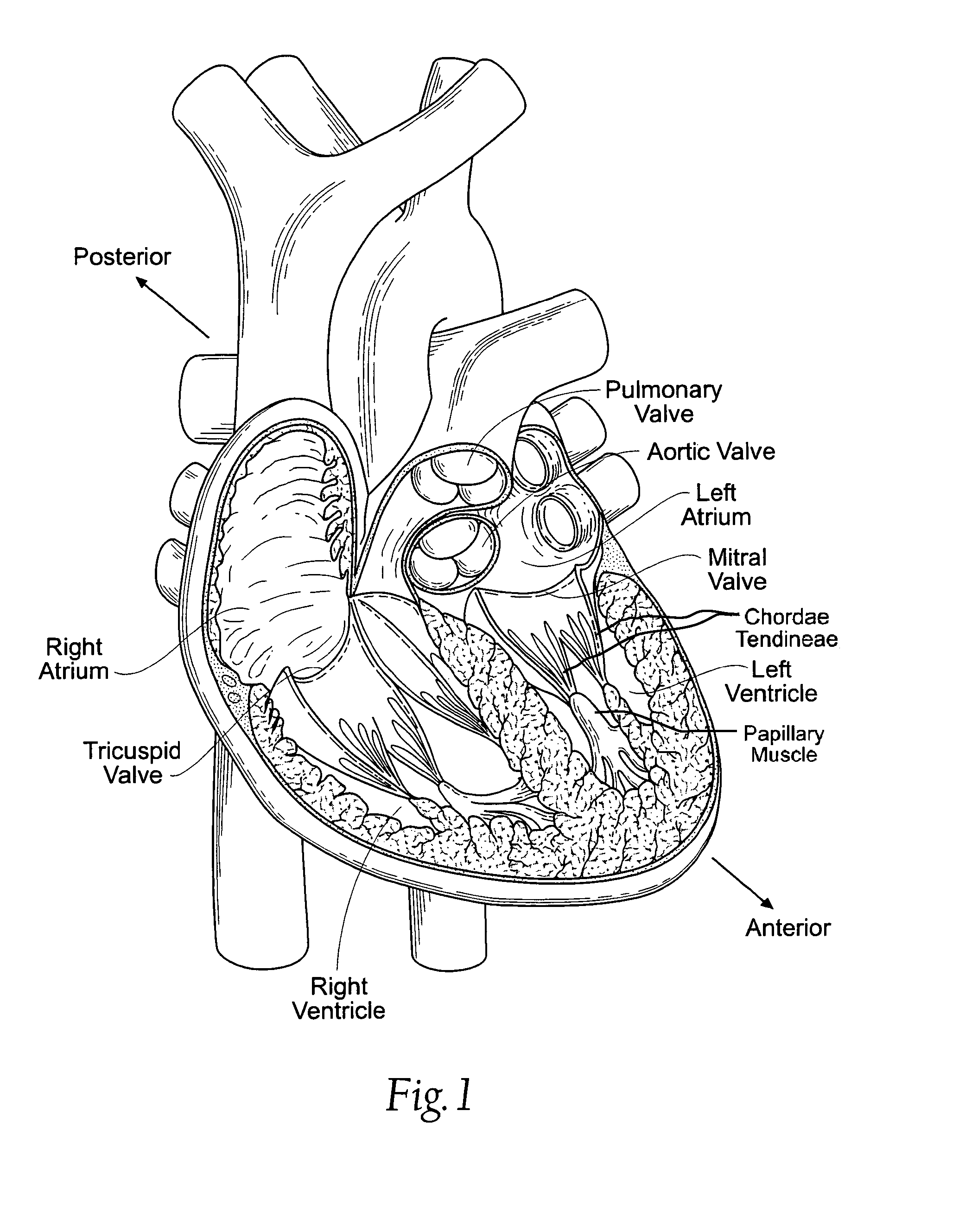
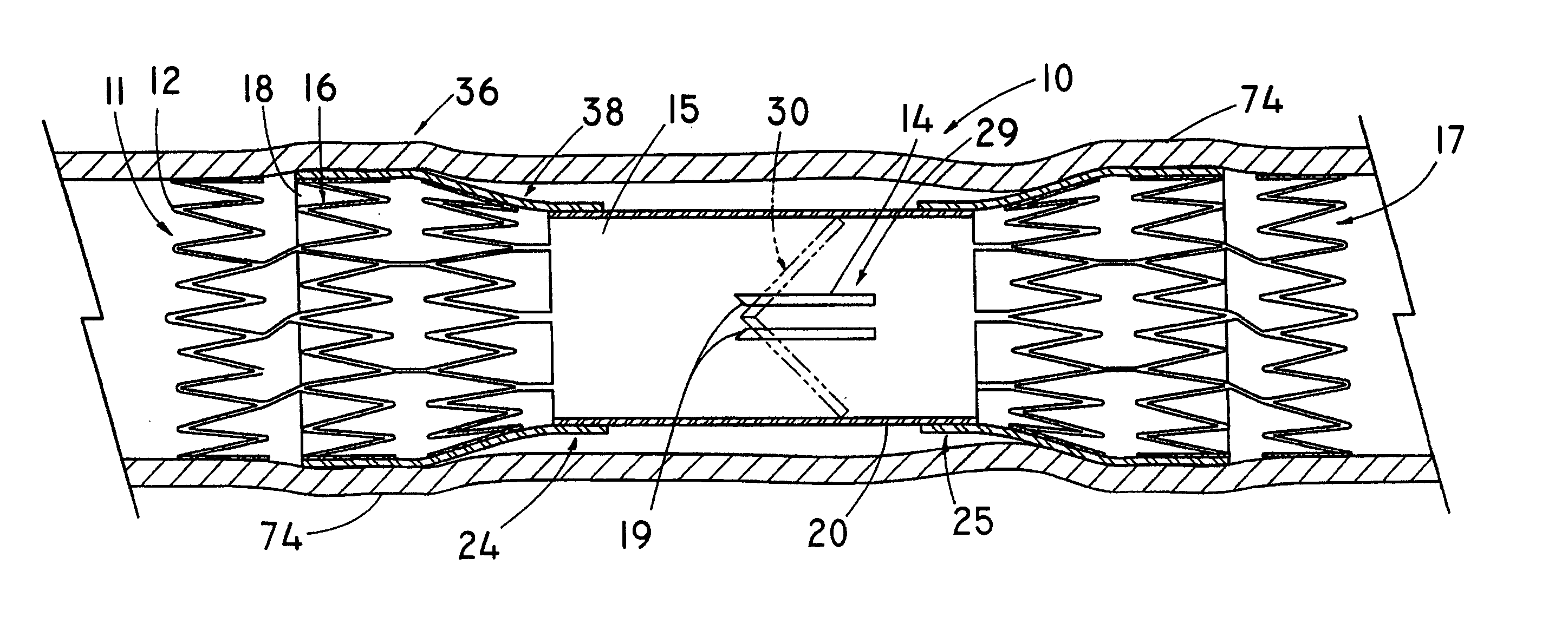
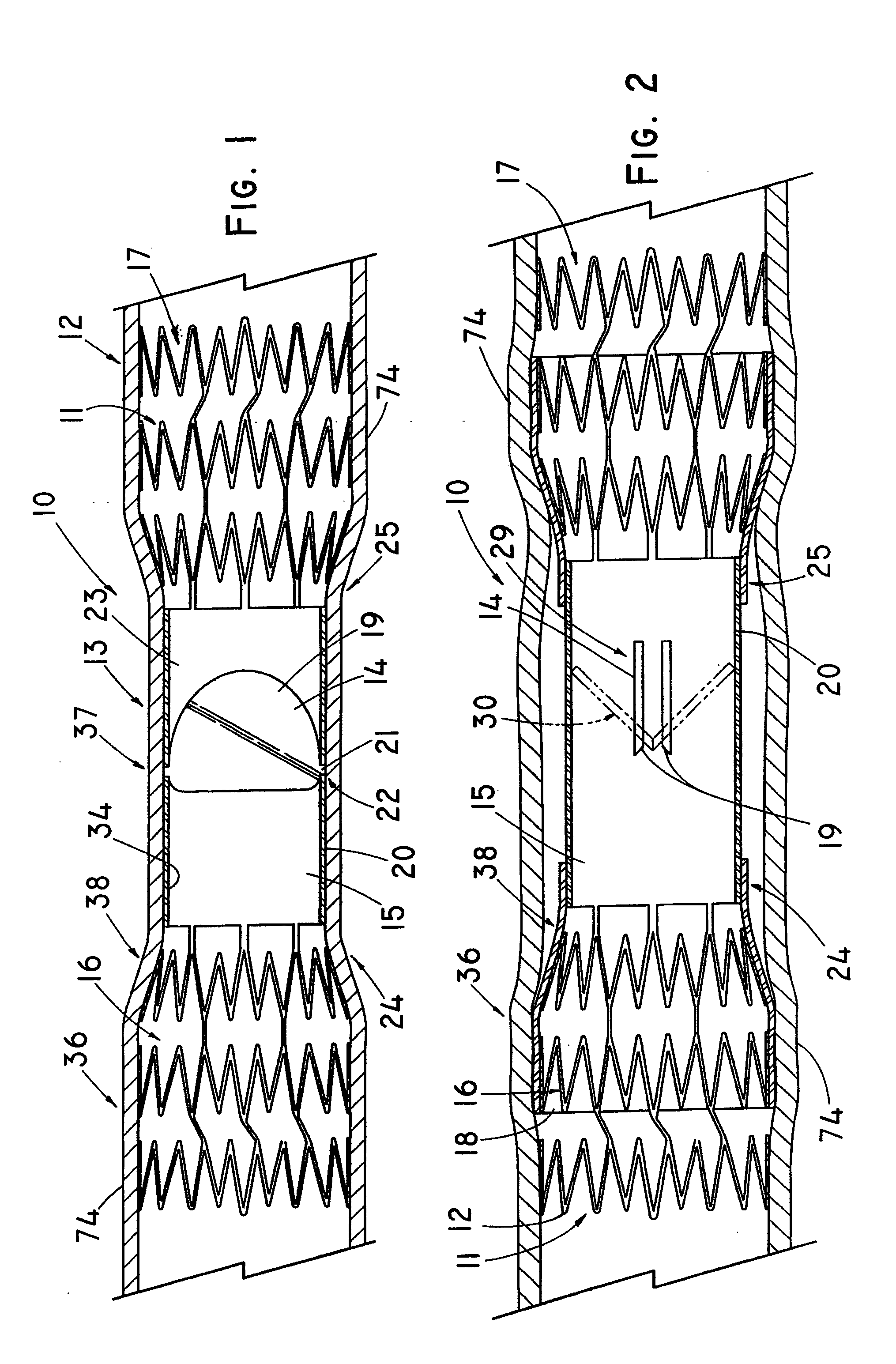
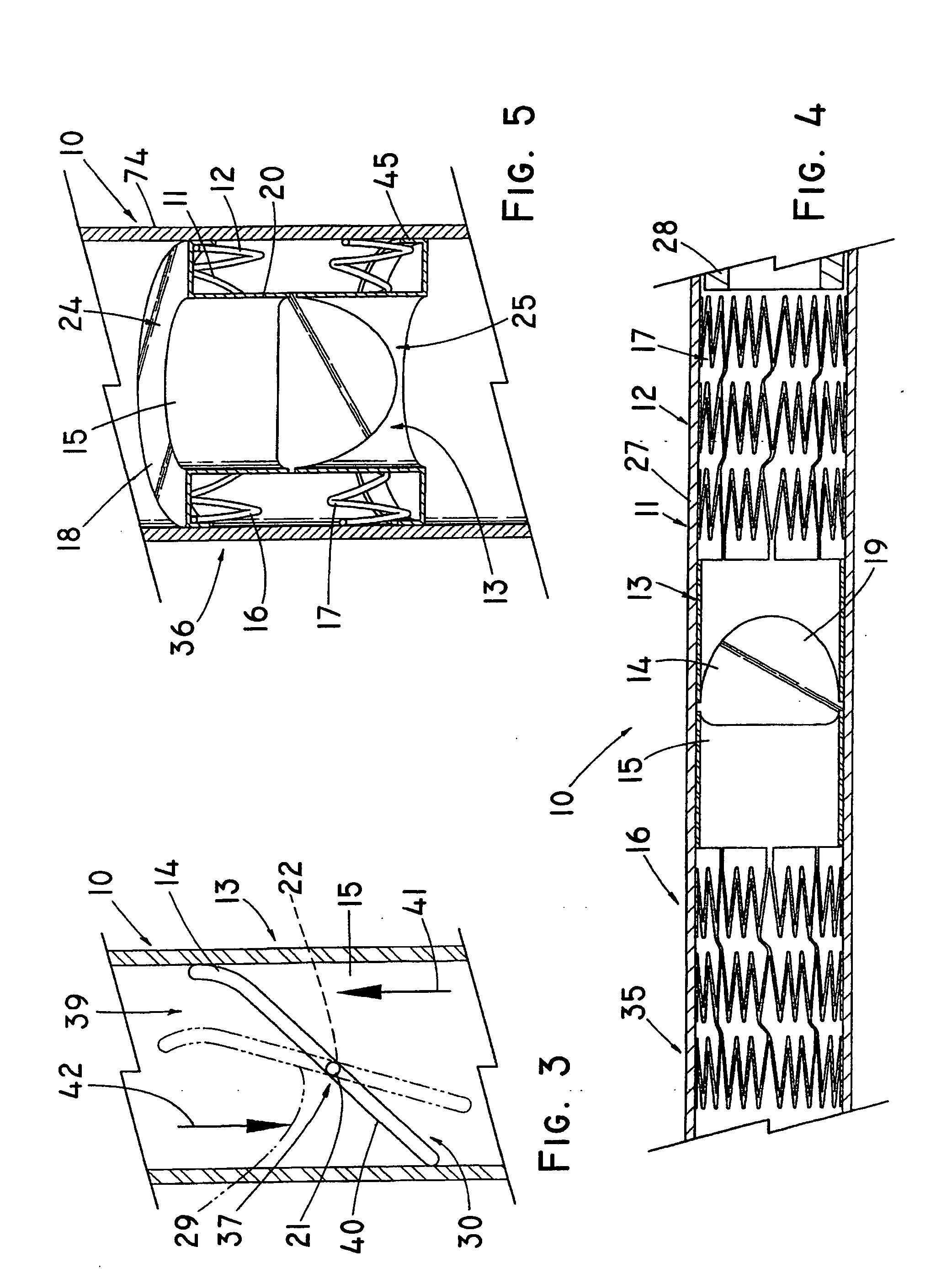
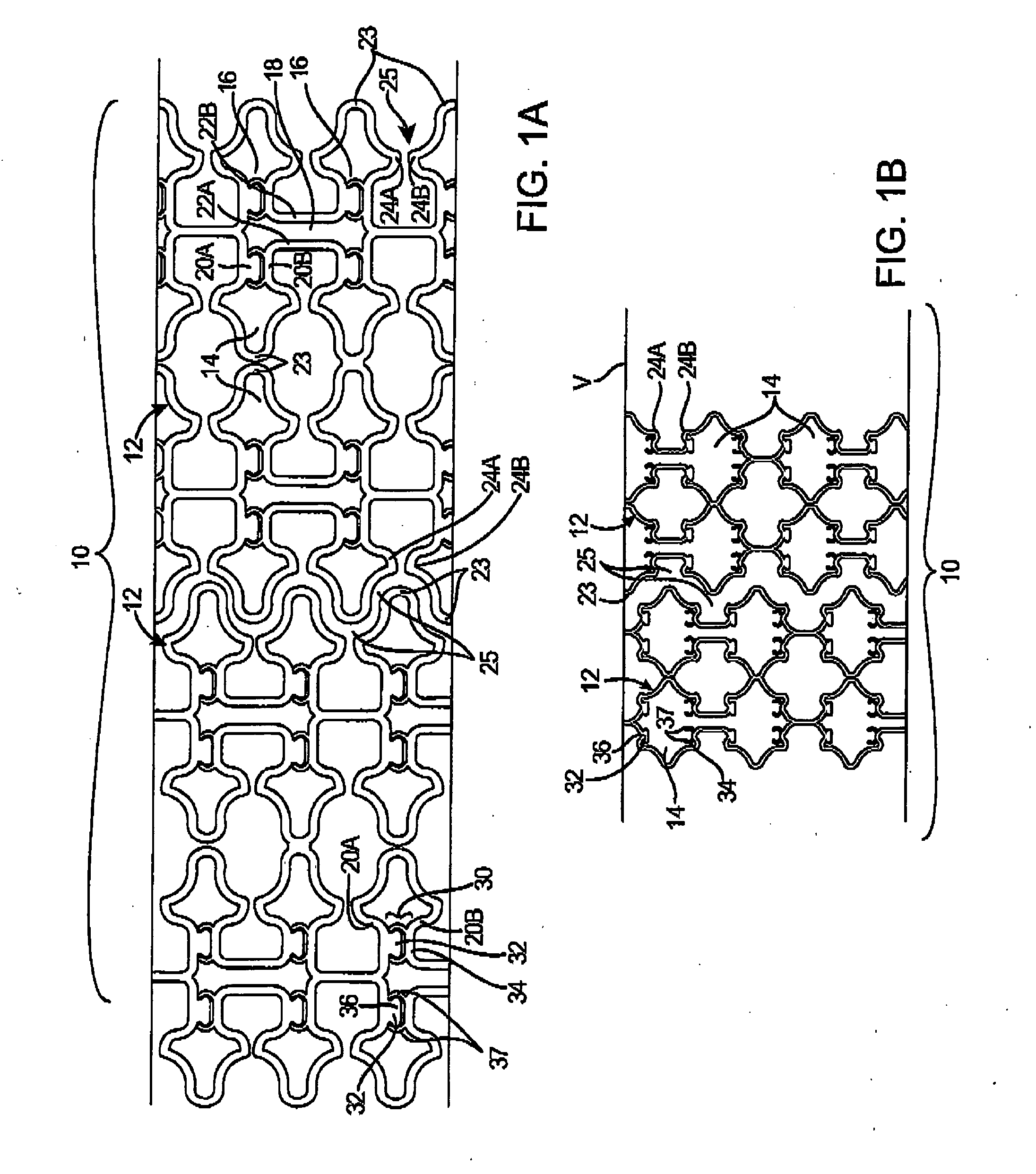
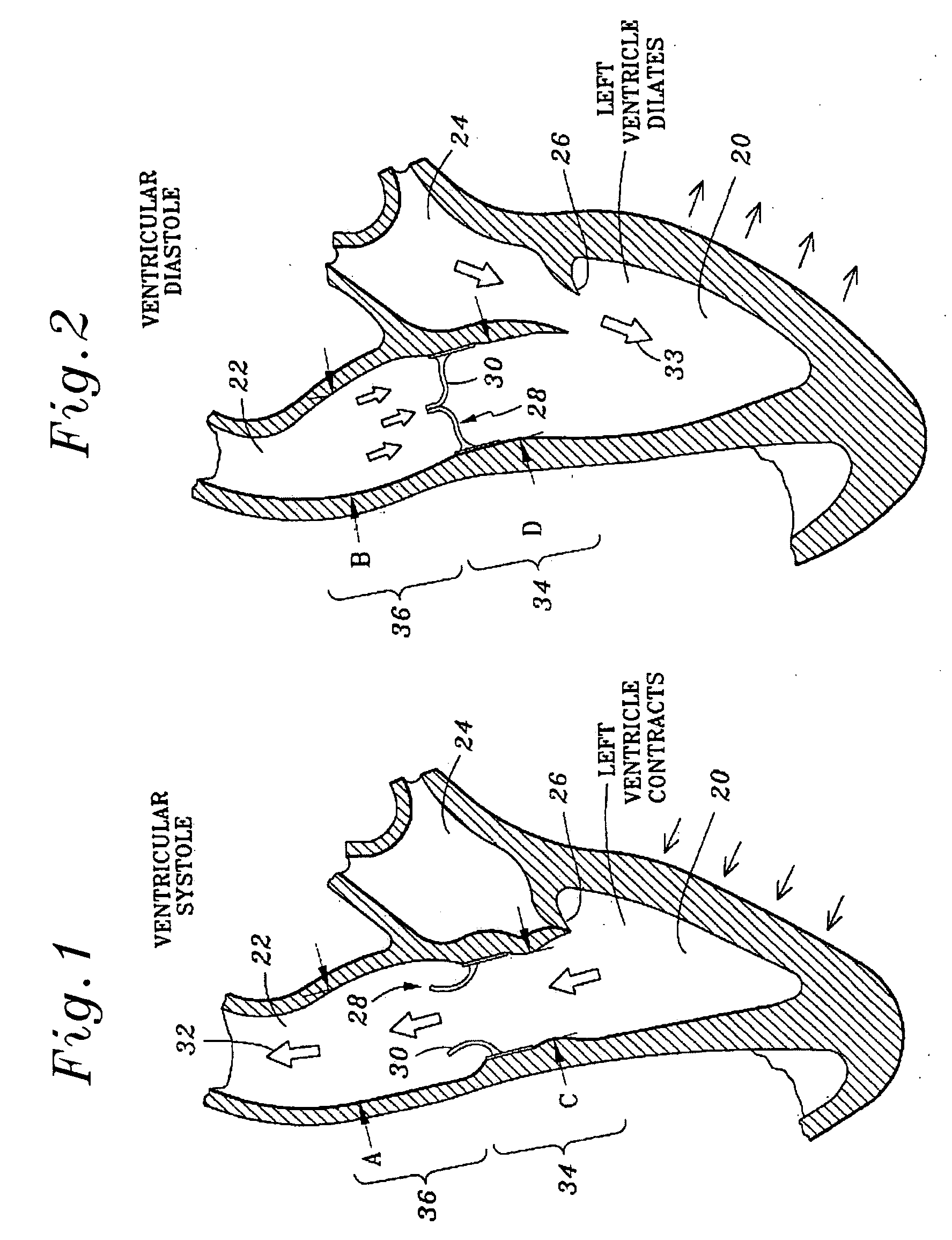
In medicine, a stent is a metal or plastic tube inserted into the lumen of an anatomic vessel or duct to keep the passageway open, and stenting is the placement of a stent.
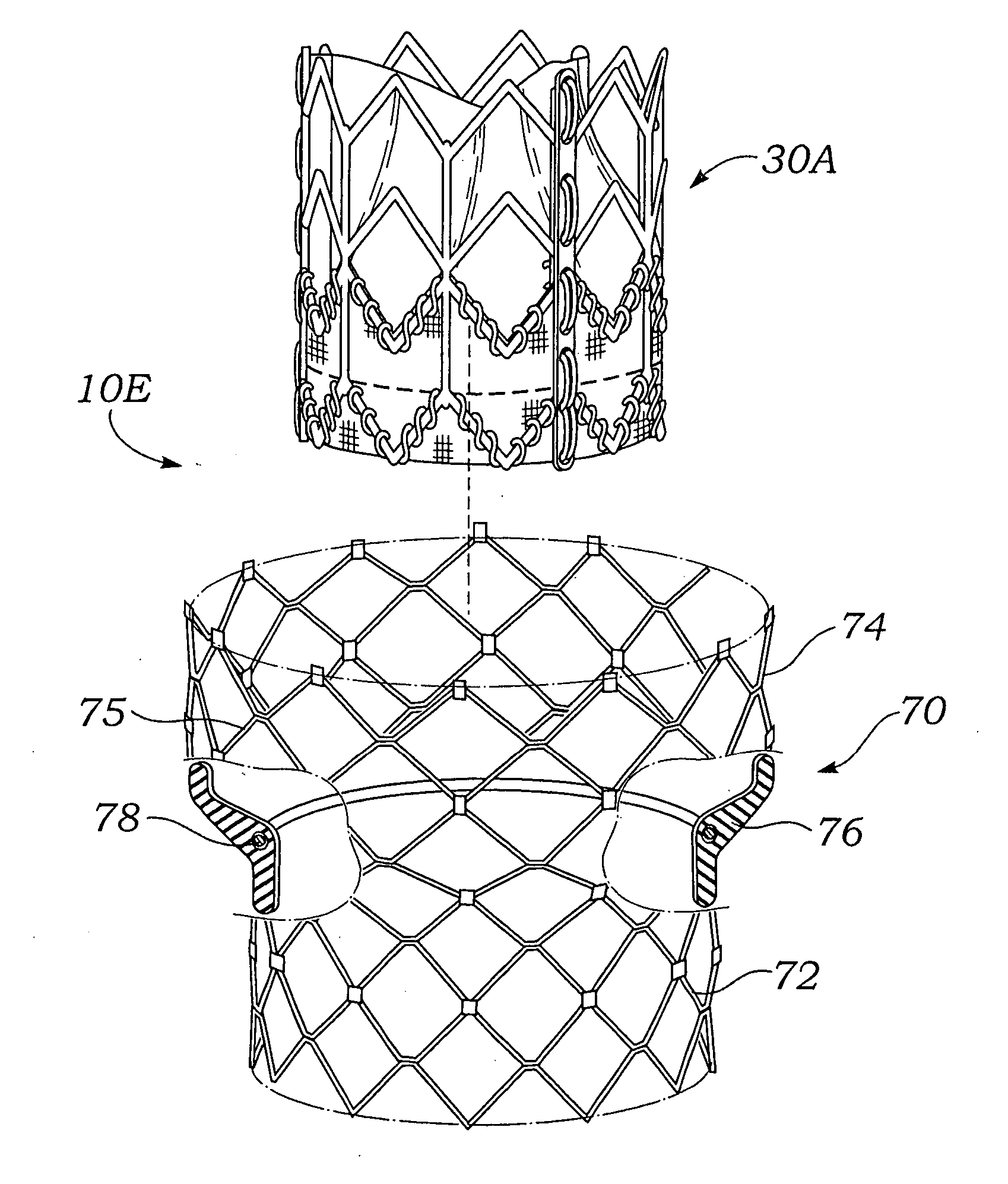
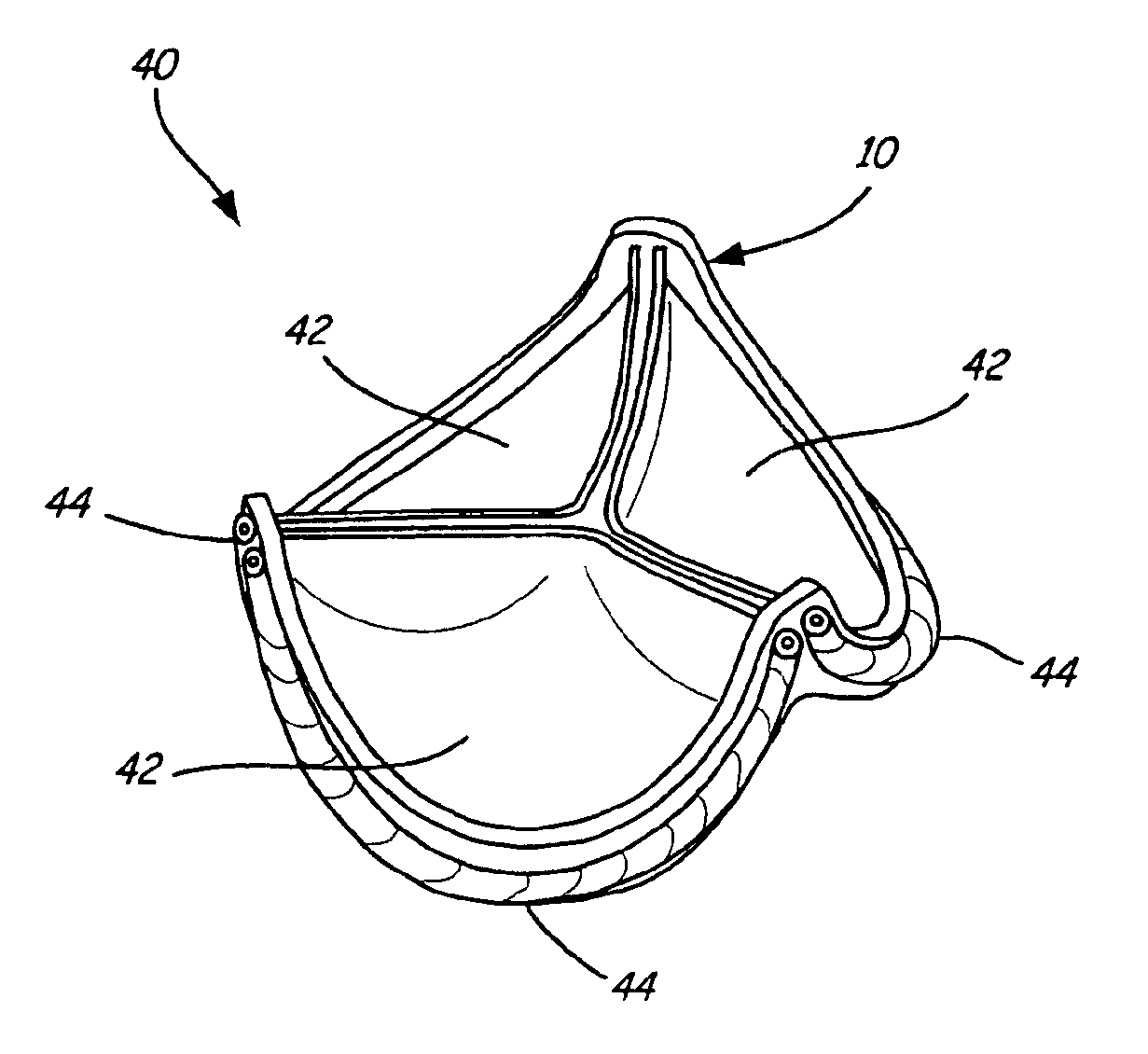
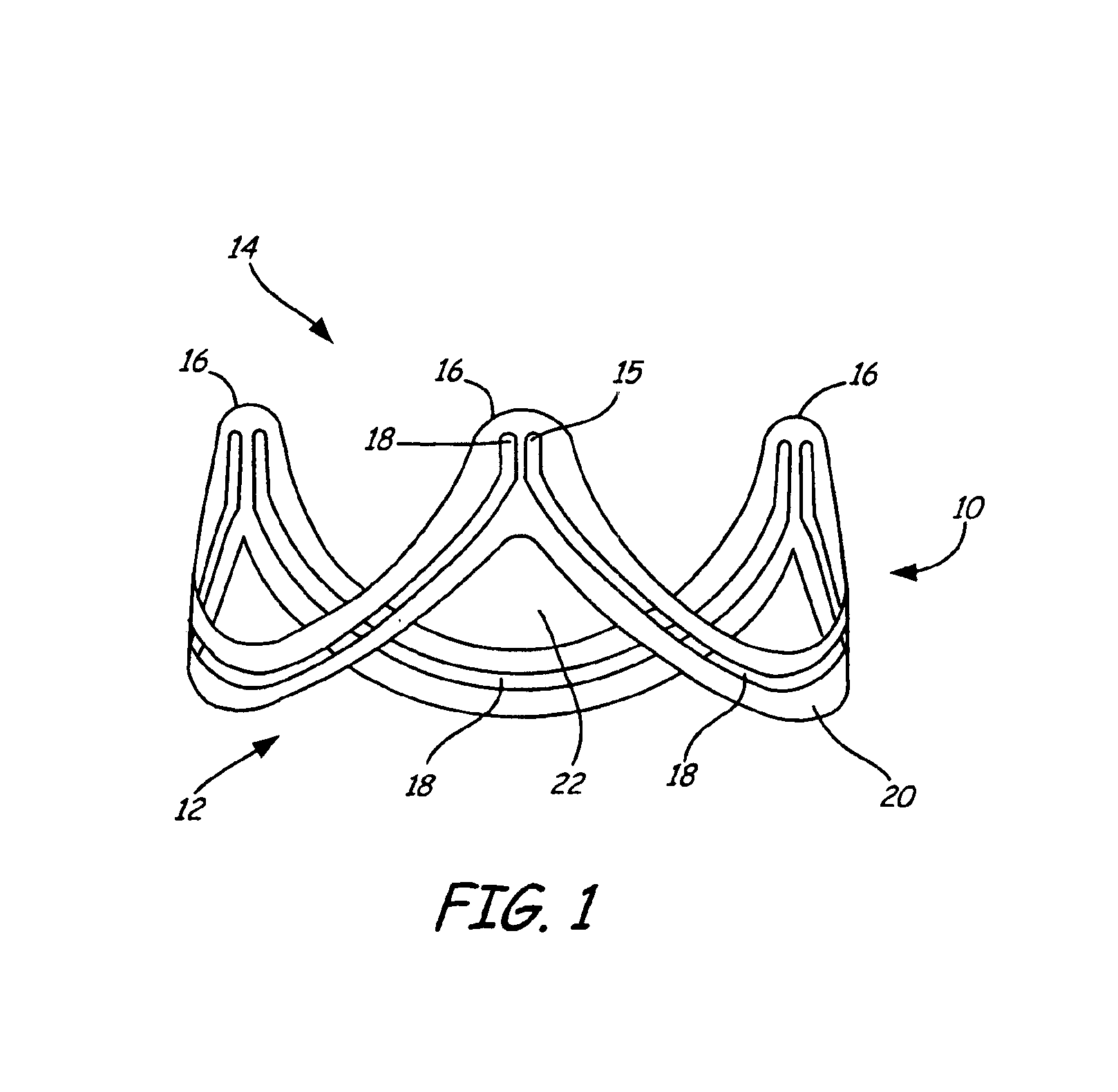
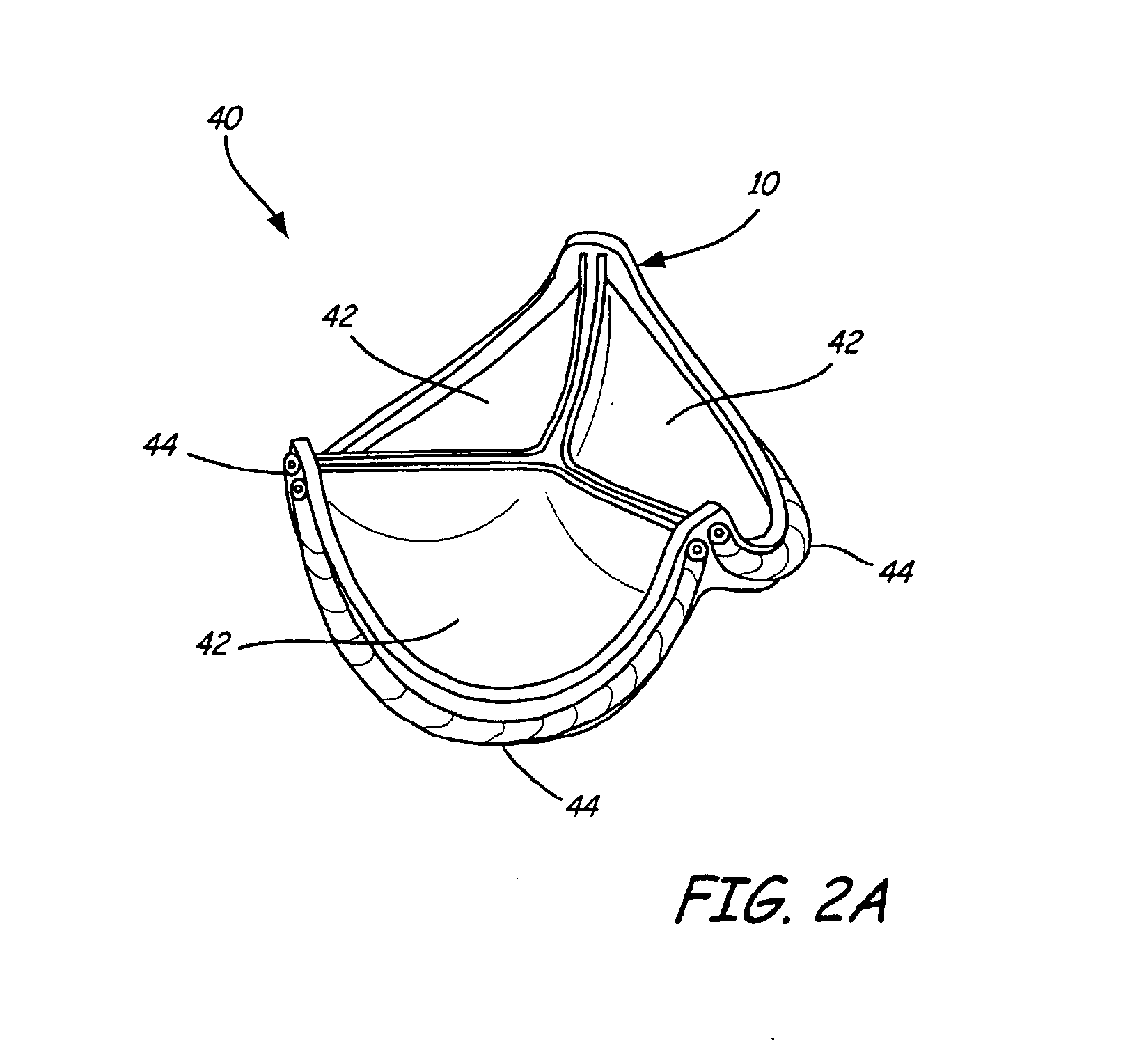
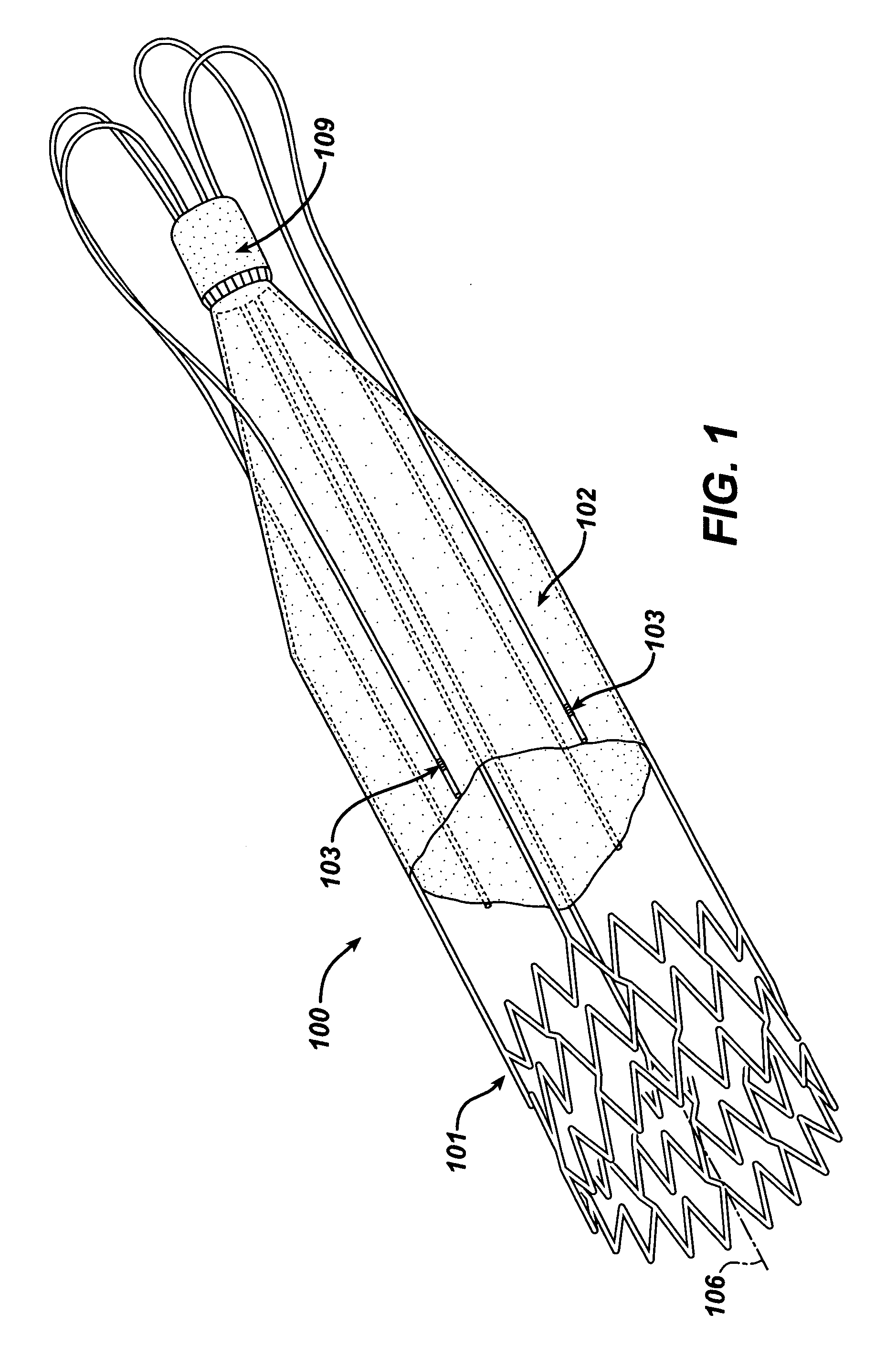
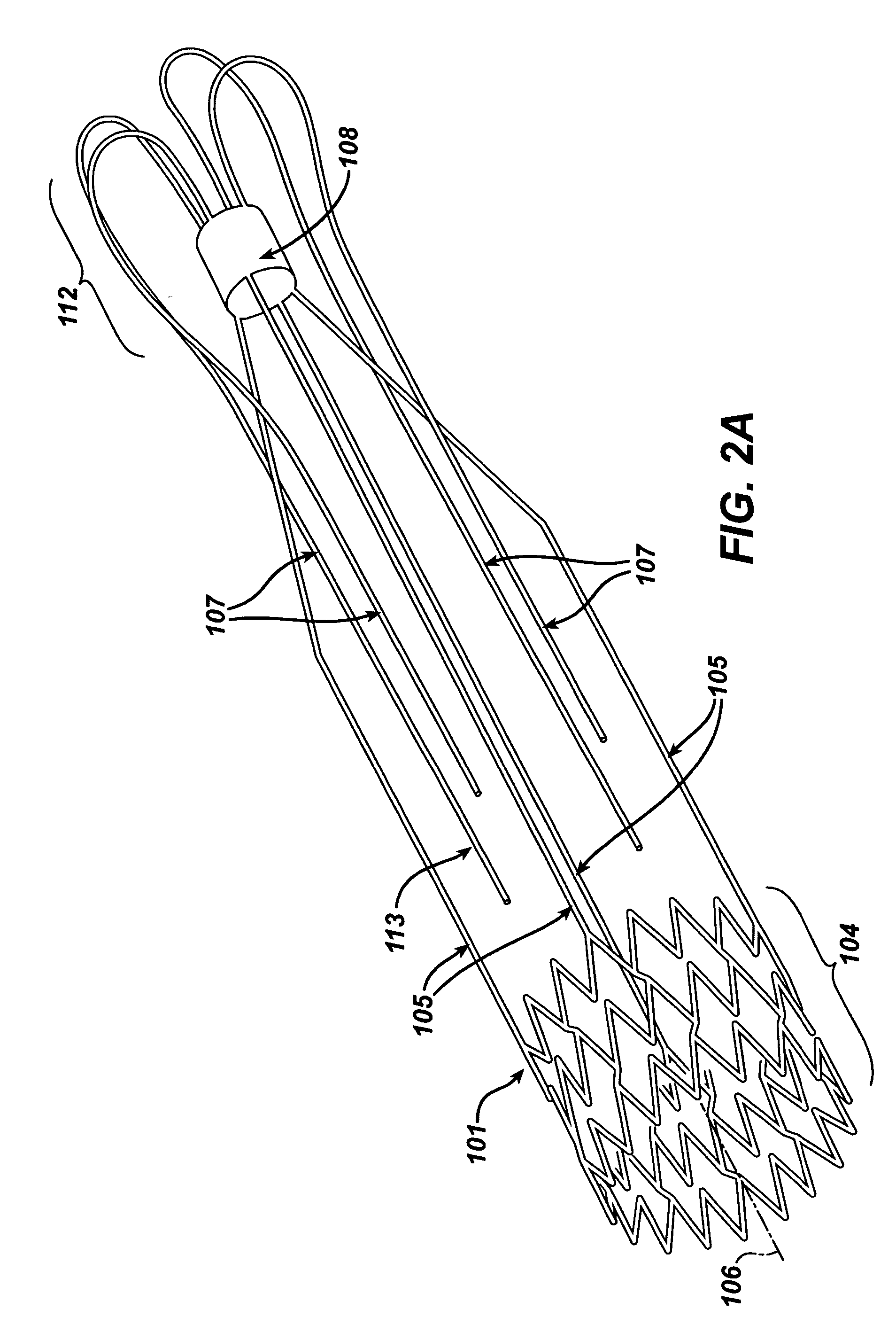
Stent-valves for valve replacement and associated methods and systems for surgery
InactiveUS20070213813A1Simple methodReduce riskStentsBalloon catheterLess invasive surgeryInsertion stent
Stent-valves (e.g., single-stent-valves and double-stent-valves), associated methods and systems for their delivery via minimally-invasive surgery, and guide-wire compatible closure devices for sealing access orifices are provided.
Owner:SYMETIS
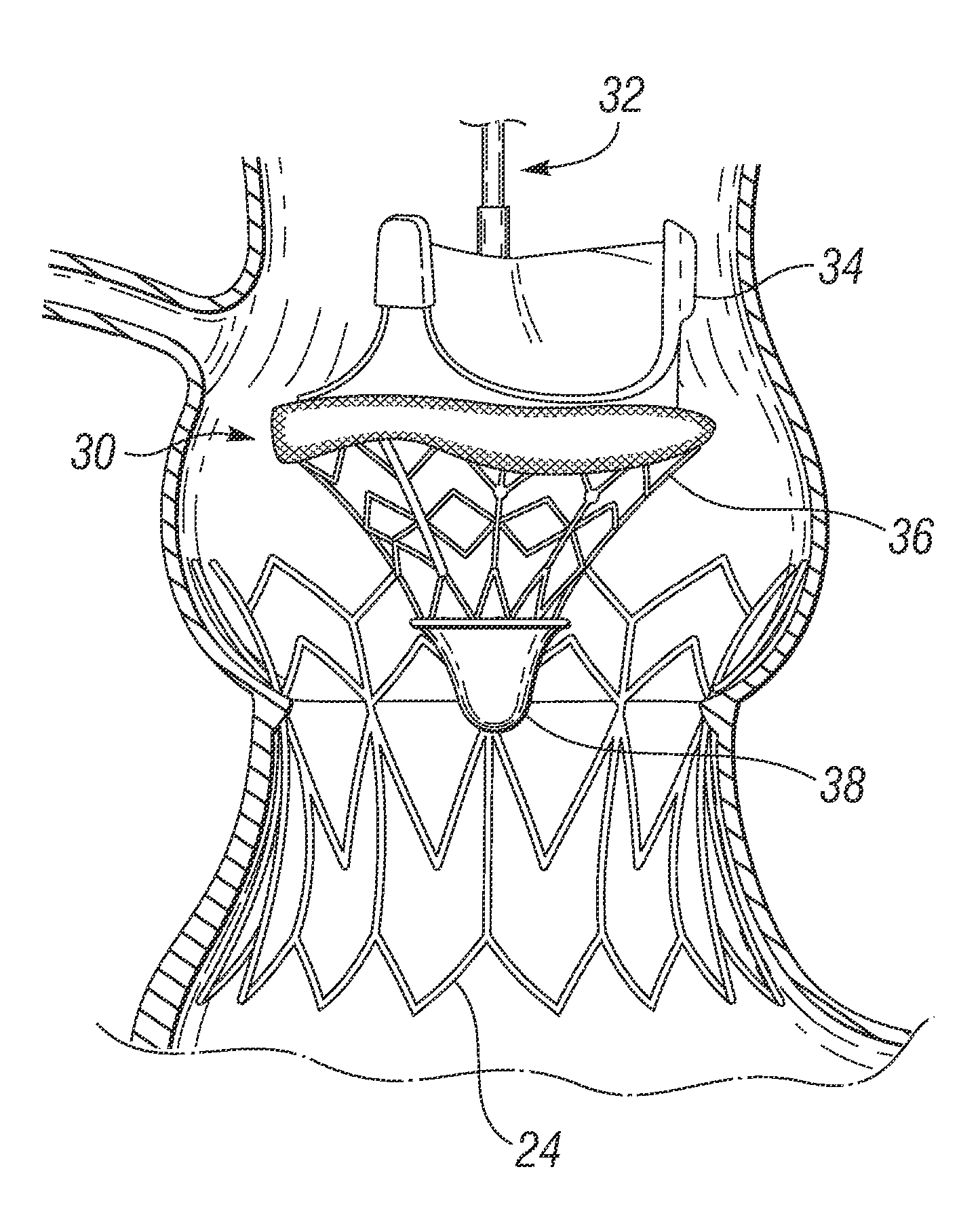
Transcatheter delivery of a replacement heart valve
A replacement heart valve apparatus. The heart valve apparatus includes a stent and a valve frame having a substantially cylindrical body defining a lumen. The valve frame includes a plurality of curved wire pairs attached to the substantially cylindrical body. Each curved wire pair includes an inner curved wire and an outer curved wire. The wire frame further having a plurality of leaflets. Each leaflet is attached to a respective inner curved wire and extends over a respective outer curved wire, so as to position the body of the leaflet within the lumen of the valve frame.
Owner:CHILDRENS MEDICAL CENT CORP
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
InactiveUS20050043790A1Destruction damageRisk of destructionHeart valvesBlood vesselsProsthetic valveInsertion stent
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
System and method for implanting a two-part prosthetic heart valve
Expandable heart valves for minimally invasive valve replacement surgeries are disclosed. In a first embodiment, an expandable pre-assembled heart valve includes a plastically-expandable annular base having plurality of upstanding commissure posts. A tubular flexible member including a prosthetic section and a fabric section is provided, with the prosthetic section being connected to the commissure posts and defining leaflets therebetween, and the fabric section being attached to the annular base. In a second embodiment, an expandable heart valve includes an annular tissue-engaging base and a subassembly having an elastic wireform and a plurality of leaflets connected thereto. The annular base and subassembly are separately stored and connected just prior to delivery to the host annulus. Preferably, the leaflet subassembly is stored in its relaxed configuration to avoid deformation of the leaflets. The expandable heart valves maybe implanted using a balloon catheter. Preferably, the leaflets of the heart valves are secured to the commissure regions of the expandable stents using a clamping arrangement to reduce stress.
Owner:EDWARDS LIFESCIENCES CORP
Apparatus and methods for dilating and modifying ostia of paranasal sinuses and other intranasal or paranasal structures
Sinusitis and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches with flexible or rigid instruments. Various methods and devices are used for remodeling or changing the shape, size or configuration of a sinus ostium or duct or other anatomical structure in the ear, nose or throat; implanting a device, cells or tissues; removing matter from the ear, nose or throat; delivering diagnostic or therapeutic substances or performing other diagnostic or therapeutic procedures. Introducing devices (e.g., guide catheters, tubes, guidewires, elongate probes, other elongate members) may be used to facilitate insertion of working devices (e.g. catheters e.g. balloon catheters, guidewires, tissue cutting or remodeling devices, devices for implanting elements like stents, electrosurgical devices, energy emitting devices, devices for delivering diagnostic or therapeutic agents, substance delivery implants, scopes etc.) into the paranasal sinuses or other structures in the ear, nose or throat.
Owner:ACCLARENT INC
Methods for rapid deployment of prosthetic heart valves
ActiveUS20060287717A1Quickly and easily replacingUse minimizedStentsAnnuloplasty ringsInsertion stentCoupling
A two-stage or component-based valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve comprises a support structure that is deployed at a treatment site. The prosthetic valve further comprises a valve member configured to be quickly connected to the support structure. The support structure may take the form of a stent that is expanded at the site of a native valve. If desired, the native leaflets may remain and the stent may be used to hold the native valve open. In this case, the stent may be balloon expandable and configured to resist the powerful recoil force of the native leaflets. The support structure is provided with a coupling means for attachment to the valve member, thereby fixing the position of the valve member in the body. The valve member may be a non-expandable type, or may be expandable from a compressed state to an expanded state. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment.
Owner:EDWARDS LIFESCIENCES CORP
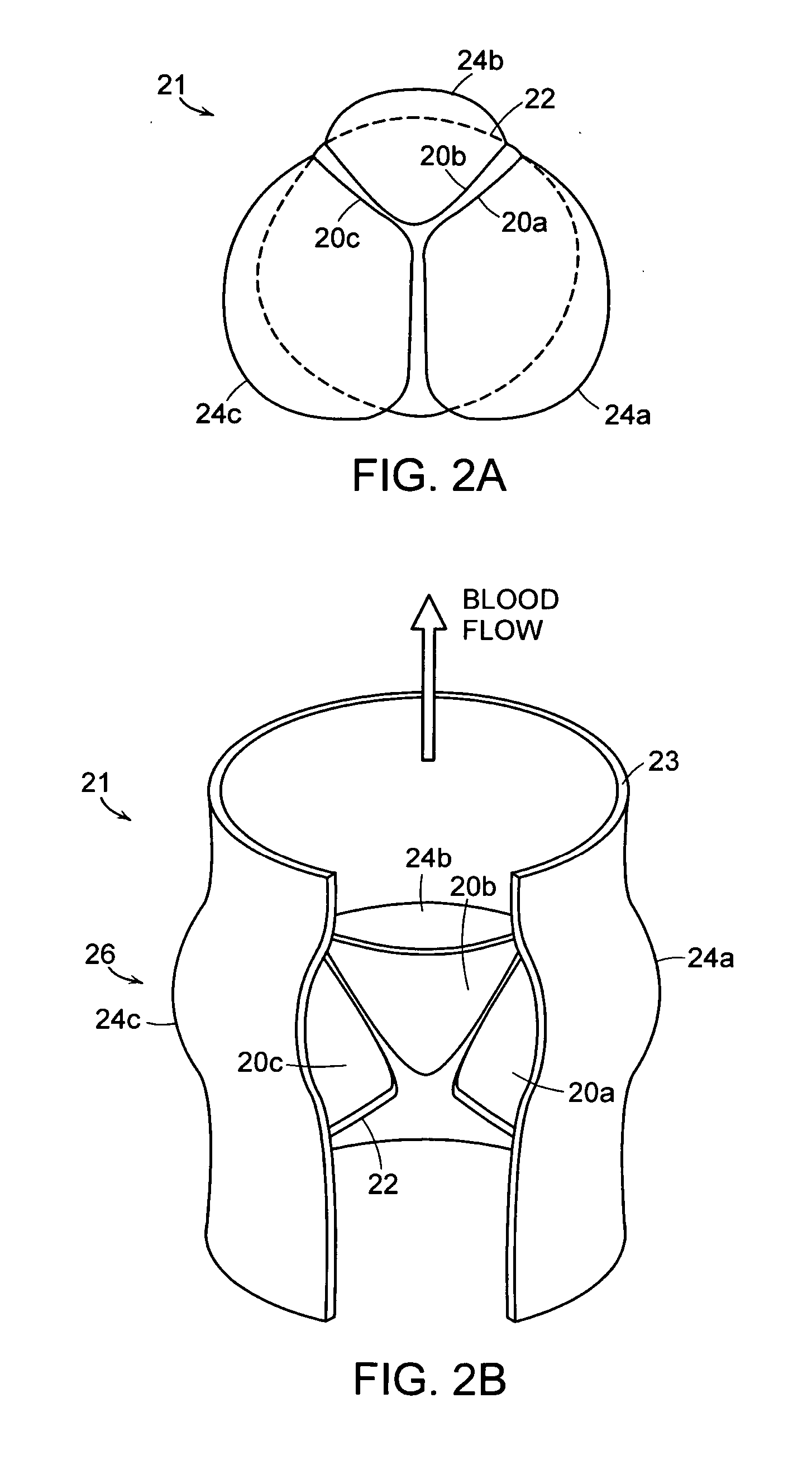
Stent mounted valve
ActiveUS20050137682A1Prevent backflowSmall amount of regurgitationHeart valvesBlood vesselsProsthetic valveInsertion stent
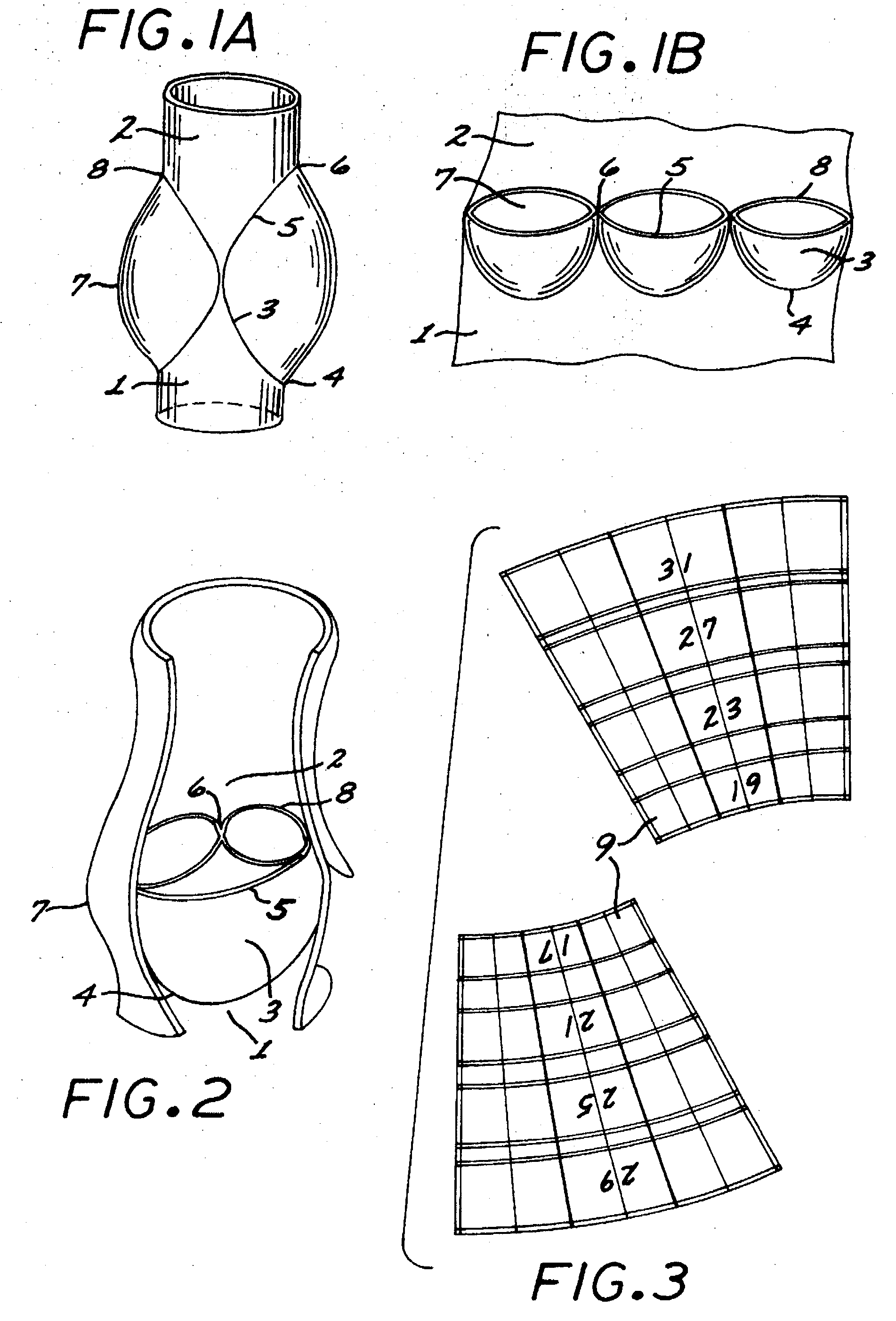
There is described a prosthetic valve to be inserted into a body lumen, the valve having leaflets that are spread apart during forward flow of fluid to create an orifice, and the leaflets coming into contact with each other during reverse flow of fluid, thereby impeding the reverse flow of fluid, the valve comprising: a hollow, cylindrical stent having an inner surface and an outer surface, and having a first and a second open end; and valve means formed from a single tubular membrane, the membrane mounted to the stent, the membrane having a graft portion internally folded and bonded to itself at a plurality of points to form pouches such that the leaflets extend from the pouches, and a sleeve portion on an outer surface of the stent to secure the membrane thereto.
Owner:JUSTINO HENRI
Devices, systems and methods for diagnosing and treating sinusitus and other disorders of the ears, nose and/or throat
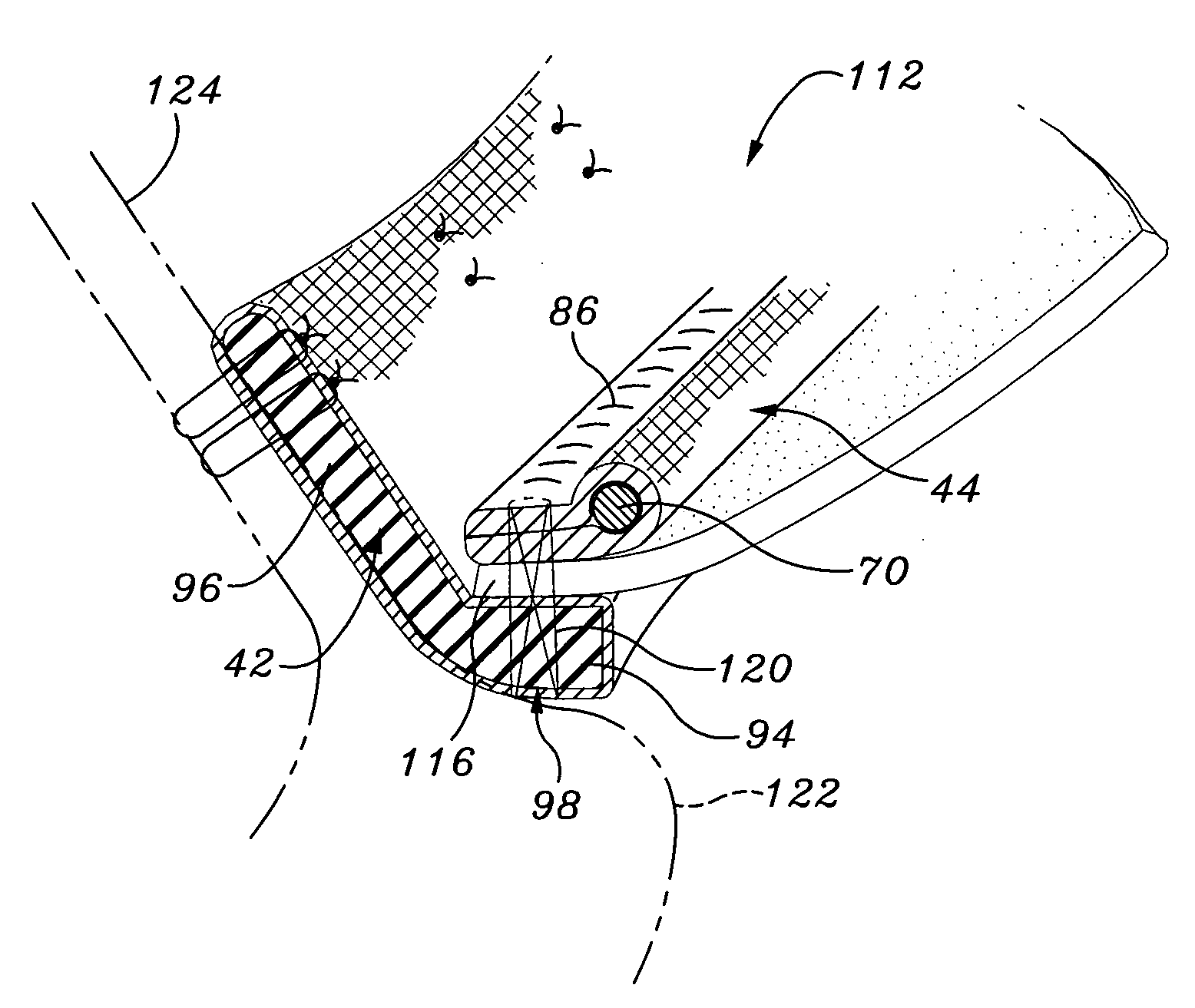
Sinusitis, enlarged nasal turbinates, tumors, infections, hearing disorders, allergic conditions, facial fractures and other disorders of the ear, nose and throat are diagnosed and / or treated using minimally invasive approaches and, in many cases, flexible catheters as opposed to instruments having rigid shafts. Various diagnostic procedures and devices are used to perform imaging studies, mucus flow studies, air / gas flow studies, anatomic dimension studies, endoscopic studies and transillumination studies. Access and occluder devices may be used to establish fluid tight seals in the anterior or posterior nasal cavities / nasopharynx and to facilitate insertion of working devices (e.g., scopes, guidewires, catheters, tissue cutting or remodeling devices, electrosurgical devices, energy emitting devices, devices for injecting diagnostic or therapeutic agents, devices for implanting devices such as stents, substance eluting devices, substance delivery implants, etc.
Owner:ACCLARENT INC
Vascular implant and delivery system
ActiveUS20100298931A1Reliable and controlled mannerStentsHeart valvesVascular implantInsertion stent
A vascular implant for replacing a native heart valve comprises a self expanding stent supporting a valve body having leaflets. The stent preferably comprises an anchoring structure configured to prevent the implant from passing through the valve annulus. For delivery, the implant is compacted within a delivery device and secured at one end. During delivery the implant is partially released from the delivery device, and positioning of the implant is verified prior to full release. The implant can be at least partially resheathed and repositioned if desired.
Owner:EDWARDS LIFESCI CARDIAQ
Kit enabling a prosthetic valve to be placed in a body enabling a prosthetic valve to be put into place in a duct in the body
The present invention is an assembly comprising a prosthetic valve to be implanted; a radially expandable stent comprising at least one zone intended to be expanded to allow the stent, in the expanded state, to bear against the wall of the body duct to be fitted with the valve, this bearing making it possible to immobilize this stent with respect to this wall; and means for mounting the valve with respect to the stent, making it possible to connect the valve to the stent in such a way that the placement of the stent allows the valve to be mounted in the body duct, and expansion means such as a balloon catheter being provided to trigger expansion of the stent at the implantation site. According to the invention, the valve and the stent are designed in such a way that, at the moment when the stent is expanded, the valve is situated outside the zone or zones of the stent that are subjected to said expansion means. The invention thus consists in separating the valve and said zone or zones to be expanded, so that the expansion of the stent can be effected with an expansion force suitable for perfect anchoring of this stent in the wall of the body duct to be fitted with the valve, and without any risk of destruction or damage of the valve.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
Sensors for detecting substances indicative of stroke, ischemia, or myocardial infarction
ActiveUS20060079740A1Thickness minimizationTransport of glucose to the sensor is not altered over timeStentsCatheterMetaboliteCitrulline
A sensor is disclosed, for implantation within a blood vessel to monitor a substance in or property of blood. In one embodiment, the sensor detects nitric oxide or a nitric oxide metabolite. In another embodiment, other substances such as glutamate, aspartate, arginine, citrulline, acetylcholine, calcium, potassium, or dopamine are monitored. The sensor may be attached to a support structure such as a stent, guidewire, or catheter. In a further embodiment, a catheter is disclosed that extracts patient fluid to a sensor outside the body for monitoring a substance or property of the patient fluid. Methods are also disclosed.
Owner:SILVER JAMES H +1
Prosthetic valve devices and methods of making such devices
Prosthetic valve devices for implantation in body vessels, and methods of making same, are provided. The device has a main body with first and second ends and defining a lumen, with the second end being inverted into the lumen. A valve is disposed at the second end. In multiple valve devices, valves are disposed at the first and second ends, and individually may be inverted into the lumen. The prosthetic valve devices may further include a support structure such as a stent. Methods of making a prosthetic valve device include providing a main body having first and second ends and defining a lumen, forming a valve at the second end, and inverting the second end into the lumen. Methods may further comprise forming multiple valves and may also include attaching a support structure.
Owner:COOK MEDICAL TECH LLC
Noncylindrical stent deployment system for treating vascular bifurcations
A device and method for treating pathological narrowing of fluid-carrying conduits of the human body (such as blood vessels) in an area of a bifurcation is disclosed. In particular, a stent system carries a self expandable noncylindrical stent, which is particularly suited for treating a widened portion of a blood vessel immediately proximal to a bifurcation. A stent delivery system is also disclosed, for delivering the stent such that a larger expanded diameter end of the stent faces the bifurcation, and a smaller expanded diameter end of the stent faces proximally in the main vessel.
Owner:BIOSENSORS INT GROUP
Self-expandable medical instrument for treating defects in a patient's heart
Owner:JENAVALVE TECH INC
Woven stent/graft structure
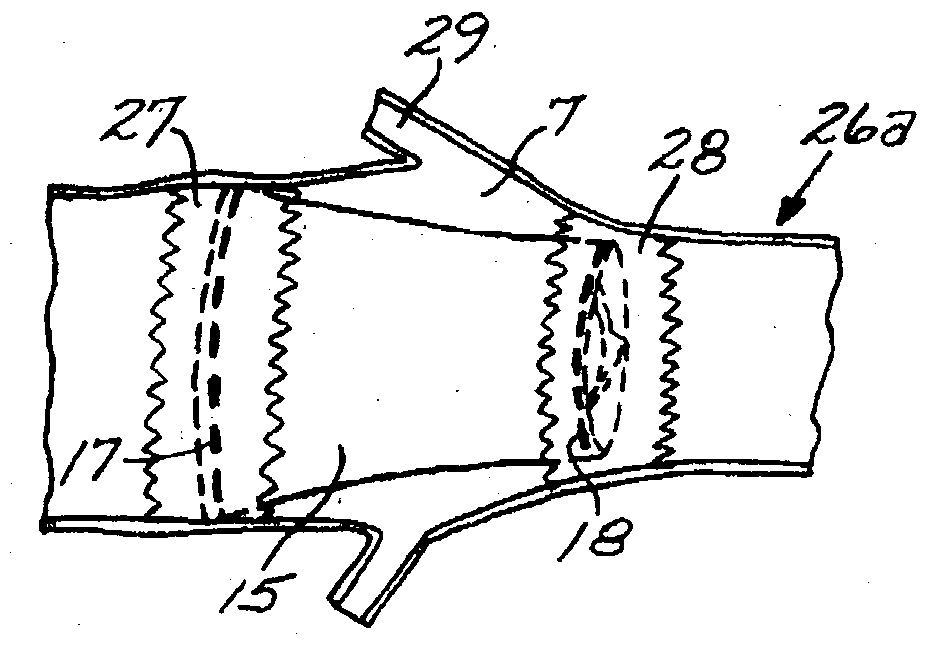
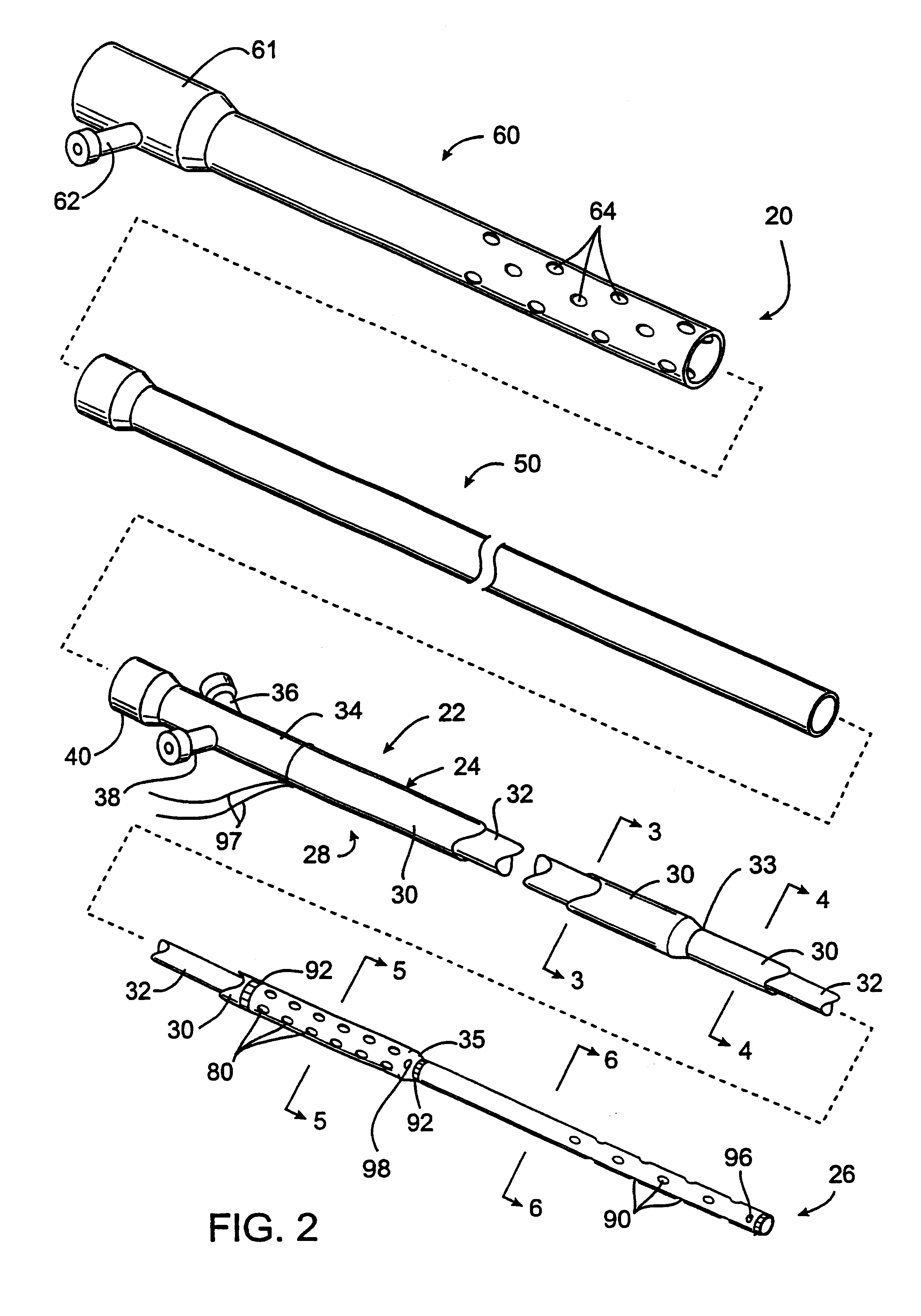
A combined stent / graft structure for repair of a body tube in a living body. The structure includes a textile graft adapted to enhance fluid integrity of the body tube and a stent expandable between a first position permitting easy insertion of the stent into the body tube and a second position wherein the stent presses securely against the inside surface of the body tube. The stent includes a first elongate wire-shaped stent member with both a stent member global axis and a stent member local axis, and is integrally secured to the graft by at least one graft yarn of which the graft is formed. Substantial portions of the first stent member global axis form a non-orthogonal angle with the graft main portion axis when projected into a plane containing the graft main portion axis. A woven textile is also part of the invention as is a method of manufacturing such a woven textile, which can be used to produce stent / graft structures according to the invention.
Owner:SECANT MEDICAL
Prosthetic Valve for Transluminal Delivery
InactiveUS20100004740A1Preventing substantial migrationEliminate the problemBalloon catheterHeart valvesVenous accessImplantation Site
A prosthetic valve assembly for use in replacing a deficient native valve comprises a replacement valve supported on an expandable valve support. If desired, one or more anchors may be used. The valve support, which entirely supports the valve annulus, valve leaflets, and valve commissure points, is configured to be collapsible for transluminal delivery and expandable to contact the anatomical annulus of the native valve when the assembly is properly positioned. Portions of the valve support may expand to a preset diameter to maintain coaptivity of the replacement valve and to prevent occlusion of the coronary ostia. A radial restraint, comprising a wire, thread or cuff, may be used to ensure expansion does not exceed the preset diameter. The valve support may optionally comprise a drug elution component. The anchor engages the lumen wall when expanded and prevents substantial migration of the valve assembly when positioned in place. The prosthetic valve assembly is compressible about a catheter, and restrained from expanding by an outer sheath. The catheter may be inserted inside a lumen within the body, such as the femoral artery, and delivered to a desired location, such as the heart. A blood pump may be inserted into the catheter to ensure continued blood flow across the implantation site during implantation procedure. When the outer sheath is retracted, the prosthetic valve assembly expands to an expanded position such that the valve and valve support expand at the implantation site and the anchor engages the lumen wall. Insertion of the catheter may optionally be performed over a transseptally delivered guidewire that has been externalized through the arterial vasculature. Such a guidewire provide dual venous and arterial access to the implantation site and allows additional manipulation of the implantation site after arterial implantation of the prosthetic valve. Additional expansion stents may be delivered by venous access to the valve.
Owner:MEDTRONIC COREVALVE
Prosthetic heart valve with slit stent
A valve prosthesis includes a plurality of flexible leaflets and a stent having a central lumen. A slit in the stent extends from the central lumen to an outer surface. An occluding portion of the flexible leaflets extends across the central lumen and an attachment portion extends from the central lumen through the slit and forms a sewing cuff for attachment to a patient's tissue. The attachment portion forms a sewing cuff for attachment to native heart tissue.
Owner:ST JUDE MEDICAL
Device for the implantation and fixation of prosthetic valves
ActiveUS20070100440A1High positioning accuracyImprove mobilityStentsBalloon catheterProsthetic valveProsthetic heart
A device for the transvascular implantation and fixation of prosthetic heart valves having a self-expanding heart valve stent (10) with a prosthetic heart valve (11) at its proximal end is introducible into a patient's main artery. With the objective of optimizing such a device to the extent that the prosthetic heart valve (11) can be implanted into a patient in a minimally-invasive procedure, to ensure optimal positioning accuracy of the prosthesis (11) in the patient's ventricle, the device includes a self-expanding positioning stent (20) introducible into an aortic valve positioned within a patient. The positioning stent is configured separately from the heart valve stent (10) so that the two stents respectively interact in their expanded states such that the heart valve stent (10) is held by the positioning stent (20) in a position in the patient's aorta relative the heart valve predefinable by the positioning stent (20).
Owner:JENAVALVE TECH INC
Anti-Paravalvular Leakage Components for a Transcatheter Valve Prosthesis
A valve prosthesis includes one or more anti-paravalvular leakage components coupled to a stent. The anti-paravalvular leakage component may encircle the stent and include a radially expandable control ring coupled to an unattached edge of a flexible skirt which extends the unattached skirt edge outwardly away from the stent and against the native heart valve to form an open-ended annular pocket around the stent. The anti-paravalvular leakage component may encircle the perimeter of the stent and include a flexible skirt having opposing edges coupled to the stent to form one or more enclosed compartments around the stent. Each compartment includes a one-way valve which allows for blood flow into the compartment but prevents blood flow out of the compartment. The anti-paravalvular leakage component may be at least one flap that is coupled to an inner surface of the stent and formed of a flexible material moveable by blood flow.
Owner:MEDTRONIC ARDIAN LUXEMBOURG SARL
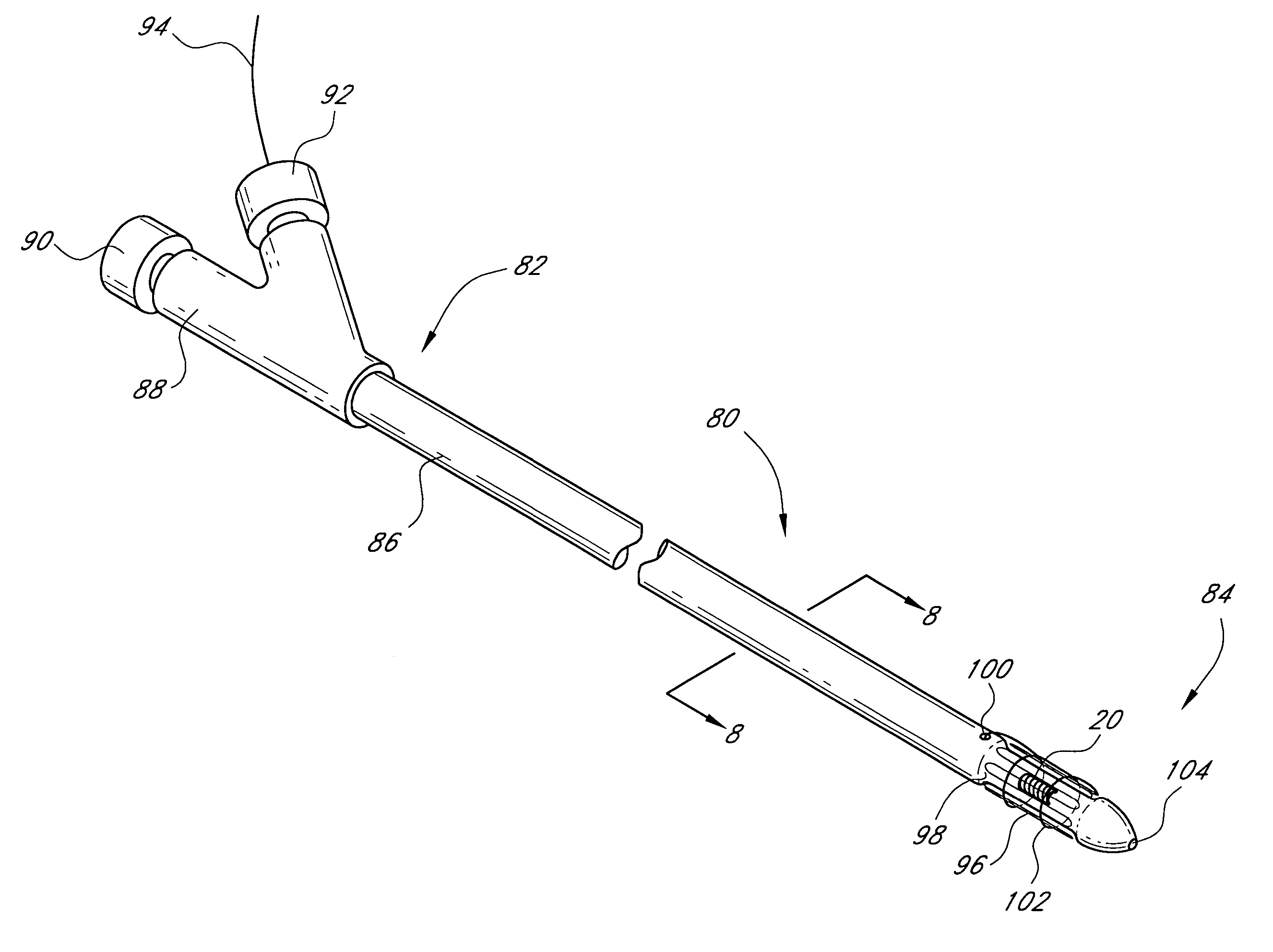
Devices and methods for operating and controlling interventional apparatus
Devices and methods are provided for operating and controlling an interventional element on an interventional catheter. The interventional element may be a stent or series of stents, a balloon, or any other interventional element for which length control is necessary or desirable. A handle member includes an elongated body and an actuator knob that rotates around the longitudinal axis of the body. Rotational movement of the actuator knob is translated to rotational movement of one or more lead screws by a system of gears, rollers, or combinations of gears and rollers. Each of one or more axially moveable members is positioned on a lead screw and attached to a portion of the catheter shaft in order to provide the ability to advance or retract the portion of the cathether shaft.
Owner:JW MEDICAL SYSTEMS LTD
Sigmoid valve and method for its percutaneous implantation
A multi-leaflet valve adapted to serve as a prosthesis for diseased native valve of a mammal is incorporated in self-expandable or inflatable endovascular stents or stents to form a combination which is introduced on a catheter with a guide wire into the circulatory system of the mammal to replace the diseased native valve. Once the combination is at the desired location the stent is caused to expand and affix itself to the patient's vessel wall. The prosthetic valve has the shape of a truncated cone that has an inflow and an outflow orifice with leaflets forming the outflow orifice and forming a plurality of commissures. A first flexible circular support is affixed in a substantially circular fashion around the truncated cone in proximity of the inflow orifice, and a second flexible circular support is affixed at the location of the commissures to form a circle around the truncated cone in proximity of the outflow orifice. The circular supports maintain the shape of the valve during the surgical implantation procedure and thereafter.
Owner:THE INT HEART INST OF MONTANA FOUND
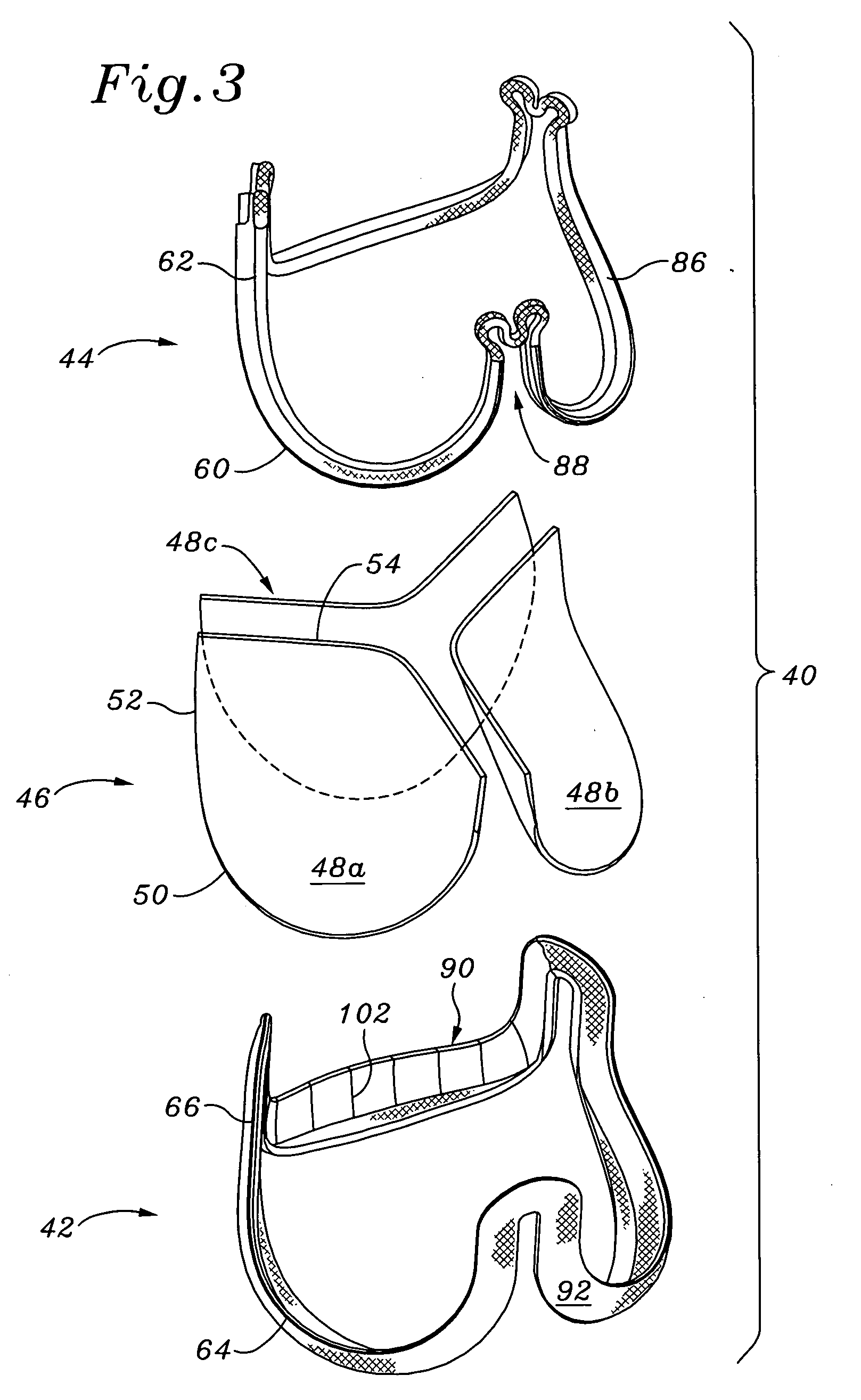
Implantable valvular prosthesis
The present invention relates to a medical device, and in particular, to a stent-based valve. The valve includes a radially expandable structural frame including an anchor structure having a first and a second open end, a connecting member having a first and a second end, and a cantilever valve strut having a first and a second end. The first end of the connecting member is attached to the second end of the anchor structure. The first end of the cantilever valve strut is cooperatively associated with the second end of the connecting member. The prosthetic valve further includes a biocompatible membrane assembly having a substantially tubular configuration disposed longitudinally about at least a portion of the connecting member. The membrane assembly has a first end having a first diameter and a second end having a second diameter, wherein the first diameter is greater than the second diameter. The first end of the membrane assembly is attached along the second end of the cantilever valve strut.
Owner:CARDINAL HEALTH SWITZERLAND 515 GMBH
Quick-connect prosthetic heart valve and methods
ActiveUS8308798B2Quickly and easily replacingUse minimizedBalloon catheterHeart valvesCouplingInsertion stent
A heart valve prosthesis that can be quickly and easily implanted during a surgical procedure is provided. The prosthetic valve has a base stent that is deployed at a treatment site, and a valve component configured to quickly connect to the base stent. The base stent may take the form of a self- or balloon-expandable stent that expands outward against the native valve with or without leaflet excision. The valve component has a non-expandable prosthetic valve and a self- or balloon-expandable coupling stent for attachment to the base stent, thereby fixing the position of the valve component relative to the base stent. The prosthetic valve may be a commercially available to valve with a sewing ring and the coupling stent attaches to the sewing ring. The system is particularly suited for rapid deployment of heart valves in a conventional open-heart surgical environment. A catheter-based system and method for deployment is provided.
Owner:EDWARDS LIFESCIENCES CORP
Chronically-implanted device for sensing and therapy
InactiveUS7236821B2Easy to solvePrevent restenosisStentsEndoradiosondesElectricityTherapeutic Devices
Systems, devices and methods are provided for providing sensing and therapy functions using a chronically-implanted device. According to one embodiment, the device includes a structure adapted to be chronically placed within a biosystem, and further includes sensing circuitry and therapy-providing circuitry attached to the structure. The sensing circuitry is adapted to sense mechanical parameters in the biosystem. The therapy-providing circuitry is adapted to provide therapy to the biosystem. According to various embodiments of the device, the sensing circuitry is further adapted to sense electrical and / or chemical parameters in the biosystem, and / or is adapted to provide electrical therapy and / or to provide drug-eluting therapy. One embodiment of the device includes a stent-like structure adapted to be chronically placed intravascularly in the biosystem.
Owner:CARDIAC PACEMAKERS INC
Percutaneously placed prosthesis with thromboresistant valve portion
InactiveUS20050182483A1Prevent and limit refluxAvoid poolingBall valvesVenous valvesVenous ValvesBlood flow
A venous valve prosthesis having a substantially non-expandable, valve portion comprising a valve-closing mechanism, such as a pair of opposing leaflets; and an anchoring portion, such as one or more self-expanding frames or stents that are expandable to anchor the prosthesis at the implantation site. In one embodiment, the rigid valve portion includes a deposition of material such as pyrolitic carbon to reduce the thrombogenecity of the blood-contacting surfaces. The anchoring portions preferably include a covering, such as a tubular construct of synthetic or collagen-derived material (such as a bioremodelable ECM material), which attaches about the support structure such that blood flow is directed through the valve mechanism as it transitions from the larger diameter anchoring portion to the intermediate, smaller-diameter portion of the prosthesis. In another embodiment, the valve support housing and valve-closing elements are delivered in a collapsed, folded, and / or dissembled state sized for delivery, then manipulated in situ to the second expanded configured following deployment.
Owner:COOK INC
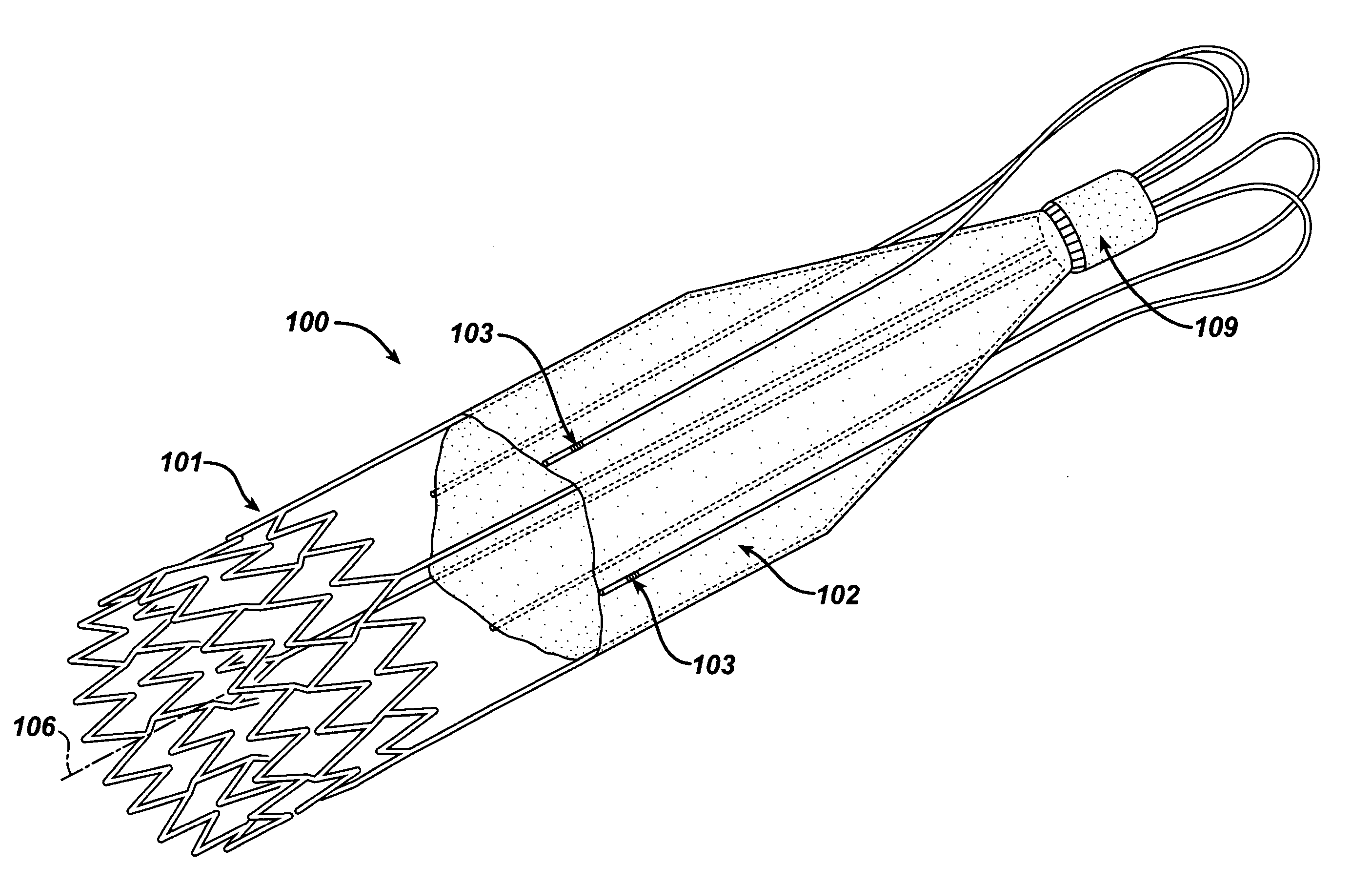
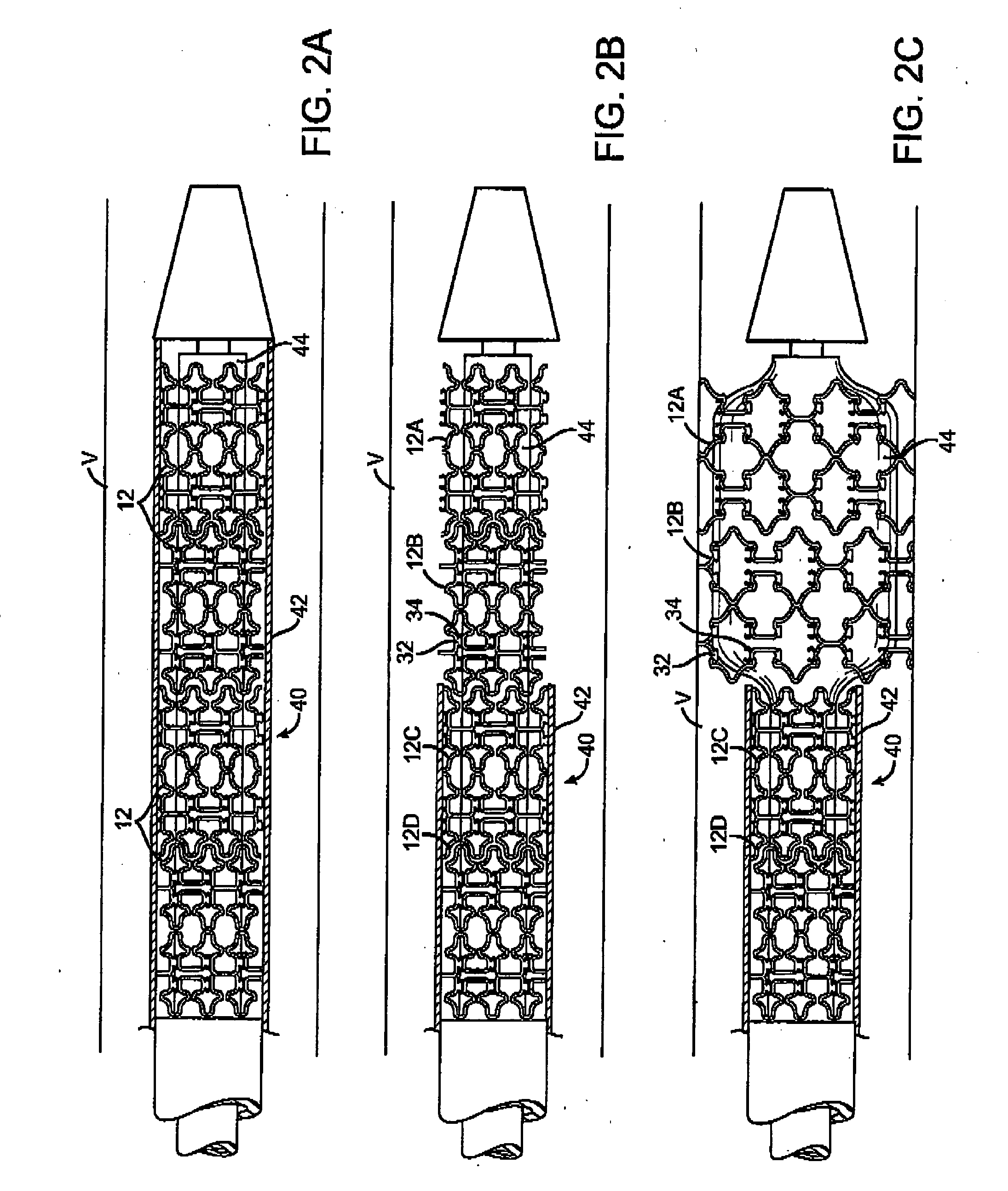
Self-constrained segmented stents and methods for their deployment
InactiveUS20060069424A1Precise positioningChallenge can be overcomeStentsBlood vesselsInsertion stentEngineering
A self-expanding stent includes a plurality of segments having a collapsed configuration and an expanded configuration. Preferably, the segments are unconnected to each other in at least the expanded configuration. The segments include restraining structures that temporarily restrain them from expansion until activated. This allows the user to position the desired number of segments at a treatment site and to deploy them simultaneously, thereby avoiding misalignment, overlap, and excessive spacing between segments. In preferred embodiments, multiple segmented stents of user-selectable length may be deployed at multiple locations in a single intervention.
Owner:XTENT INC
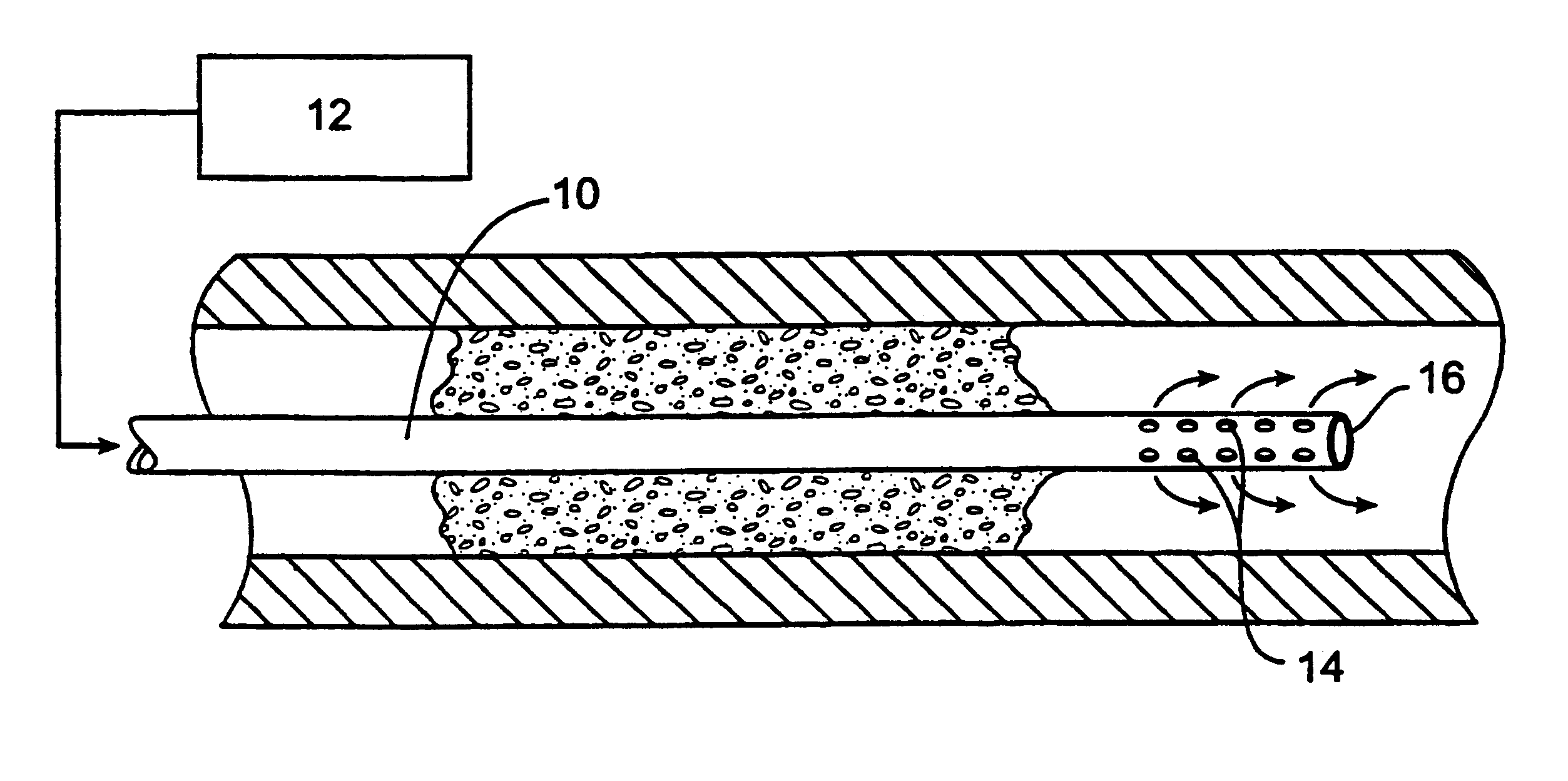
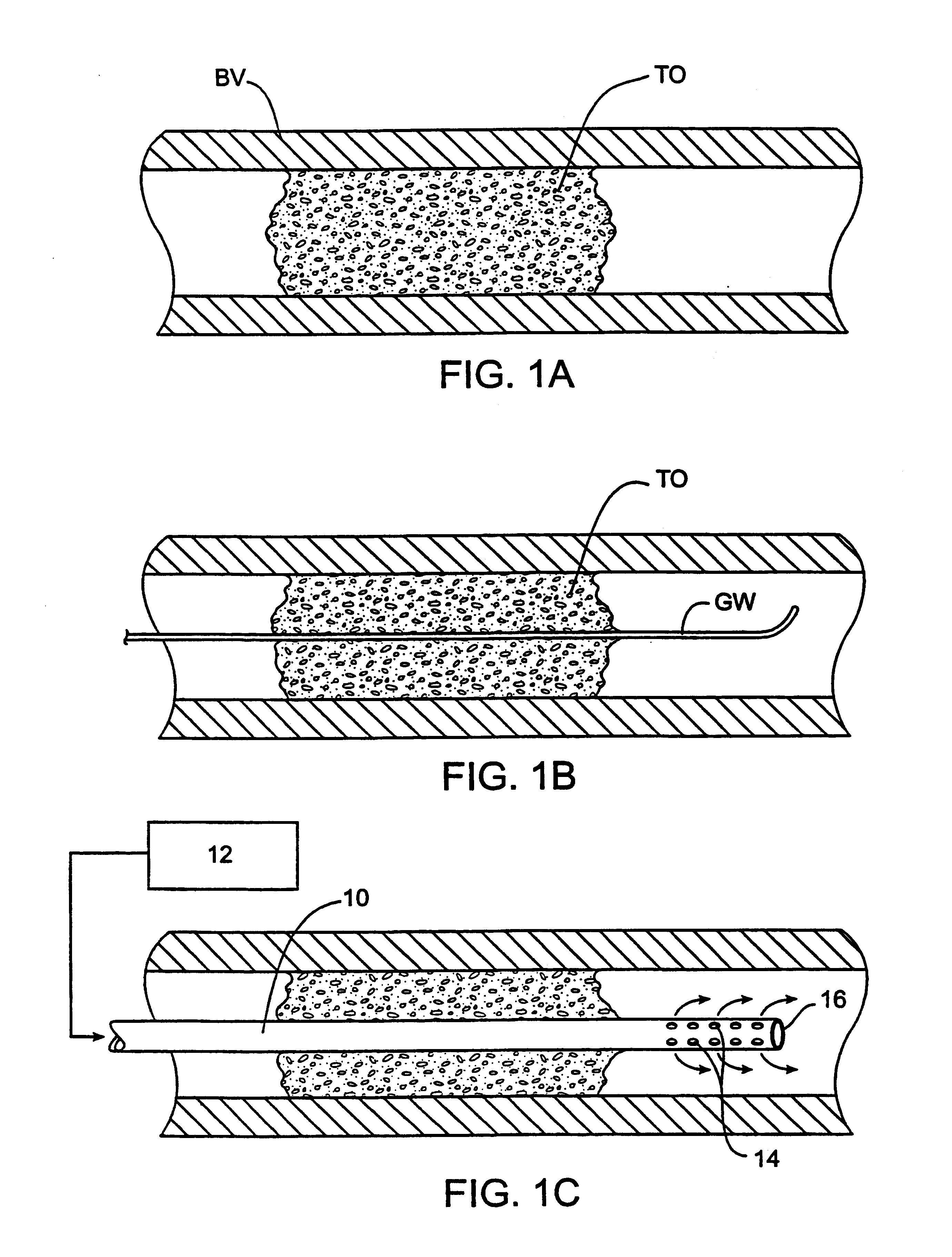
Methods and systems for treating ischemia
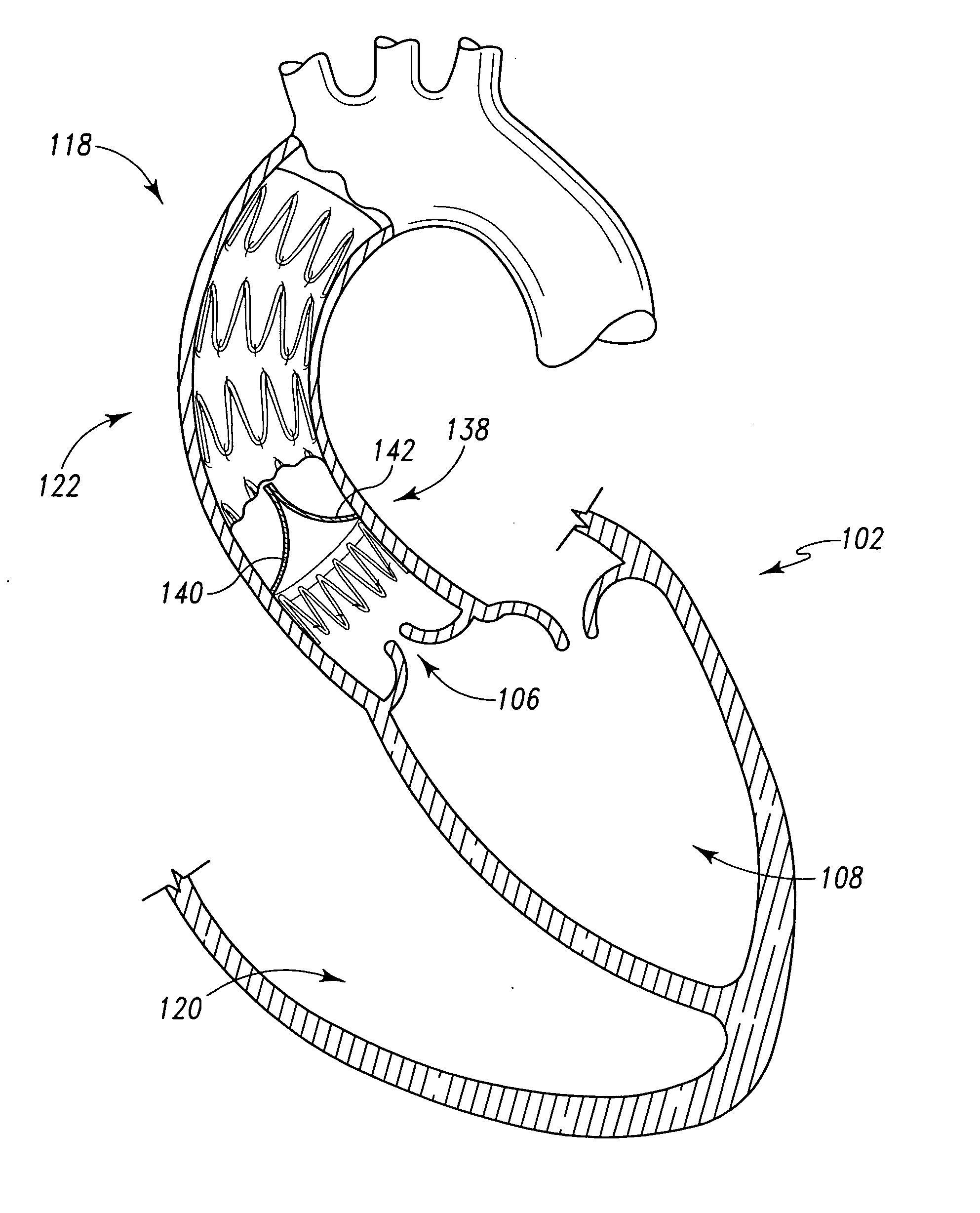
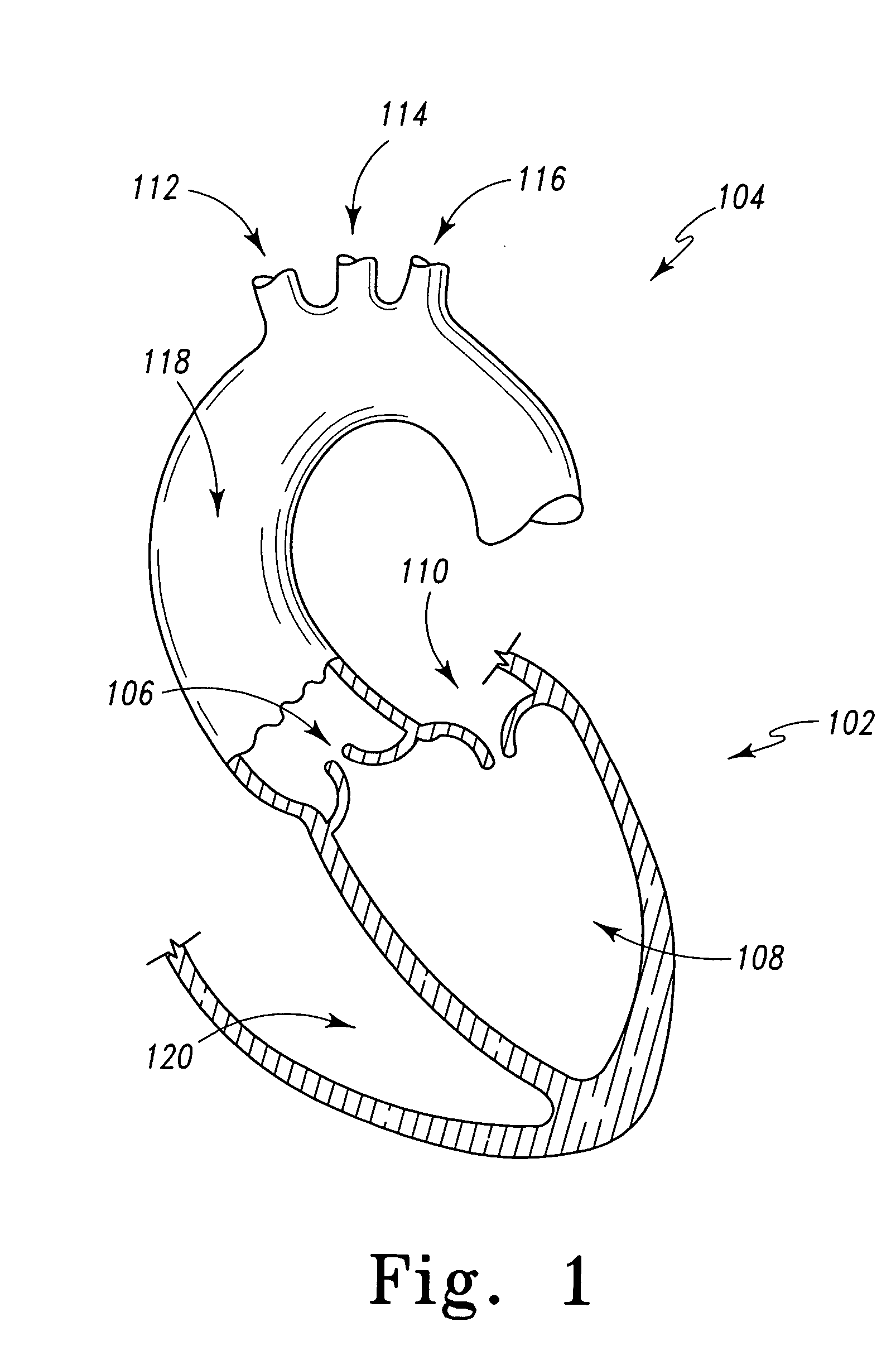
InactiveUS6295990B1Promote dissolution and removalMinimize and prevent ischemiaStentsBalloon catheterControl mannerDecreased mean arterial pressure
Methods for treating total and partial occlusions employ a perfusion conduit which is penetrated through the occlusive material. Oxygenated blood or other medium is then perfused through the conduit in a controlled manner, preferably at a controlled pressure below the arterial pressure, to maintain oxygenation and relieve ischemia in tissue distal to the occlusion. In another aspect, interventional devices, such as stents or balloon catheters, are passed through the perfusion catheter to remove obstructions. Optionally, the occlusion may be treated while perfusion is maintained, typically by introducing a thrombolytic or other agent into the occlusive material using the perfusion conduit or by employing mechanical means to remove the obstruction. Such methods are particularly suitable for treating acute stroke to prevent damage to the cerebral tissue.
Owner:SALIENT INTERVENTIONAL SYST
Highly flexible heart valve connecting band
ActiveUS20060229719A1Facilitates supra-annular attachmentIncrease flexibilityHeart valvesInsertion stentTissues types
A connecting band for a highly flexible tissue-type heart valve having a stent with cusps and commissures that are permitted to move radially. The connecting band follows the cusps and commissures and extends outwardly. The valve is connected to the natural tissue along the undulating connecting band using conventional techniques, such as sutures. The connecting band may be a cloth-covered inner suture-permeable member and attaches to the underside of the valve at the cusps to provide support to the stent and to the outer side of the valve at the commissures. The connecting band includes commissure portions defining generally axial gaps that help permit flexing of the valve. The inner member may include one or more slits along the cusps to enhance flexibility. The inner member may further include a continuous outwardly projecting sewing ridge around its periphery which includes a series of ribs separated by grooves around the inflow edge of the cusps. The sewing ridge enables supra-annular implant of a valve constructed with the connecting band.
Owner:EDWARDS LIFESCIENCES CORP
Endoluminal graft with a prosthetic valve
InactiveUS20050222674A1Restricted fluid flowControl flowStentsHeart valvesProsthetic valveInsertion stent
An endoluminal prosthesis is provided for restricting fluid flow in a lumen. The endoluminal prosthesis has a tubular graft having a flexible body with at least one stent coupled thereto. The endoluminal prosthesis also includes a prosthetic valve coupled to the inside the tubular graft. The prosthetic valve can be made of a synthetic or an organic material, such as an extracellular matrix, and can operate to limit fluid flow in one direction, but allow fluid flow in the other direction.
Owner:MED INST INC
Actively Controllable Stent, Stent Graft, Heart Valve and Method of Controlling Same
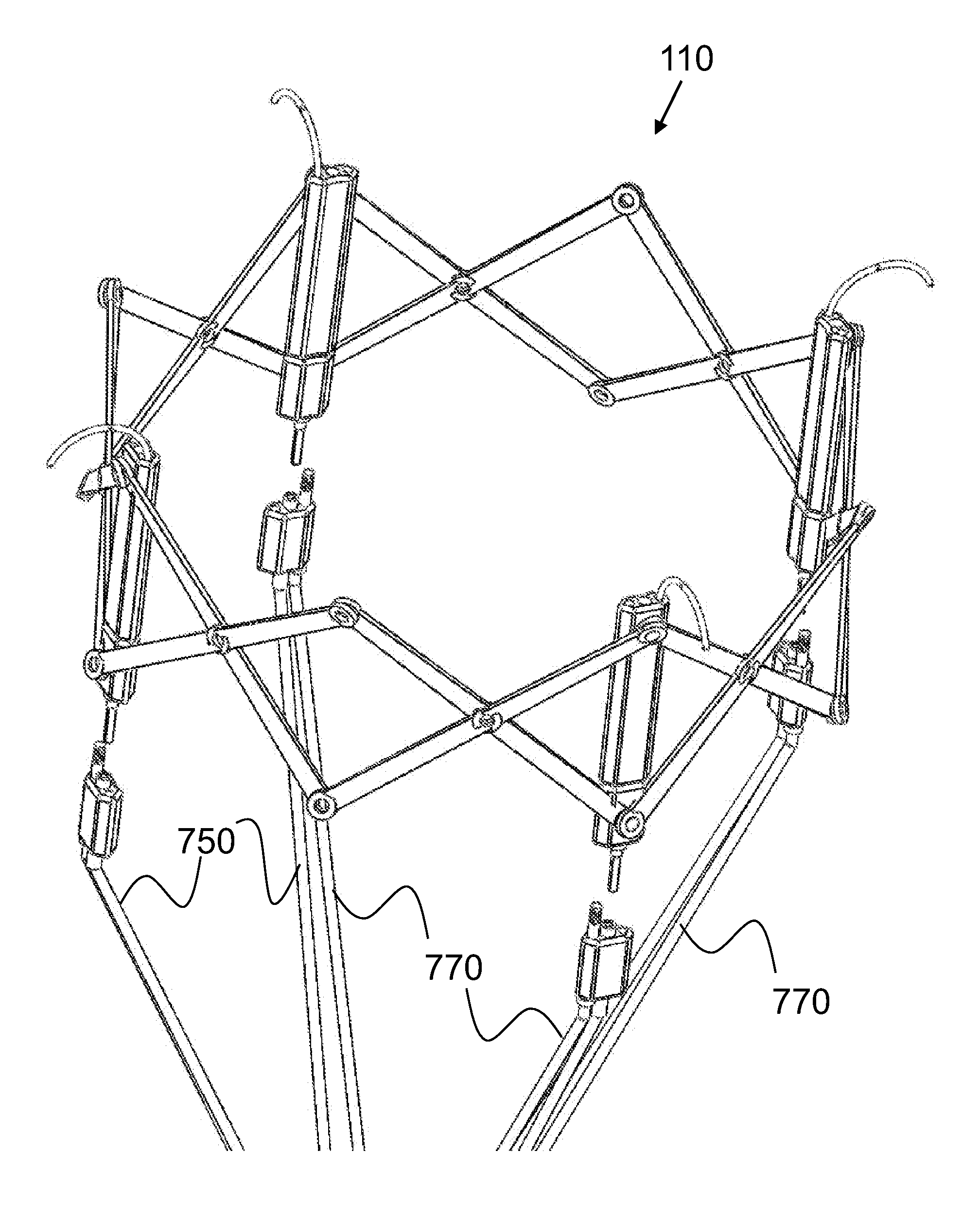
Sealable and repositionable implant devices are provided to increase the ability of endovascular grafts and valves to be precisely deployed or re-deployed, with better in situ accommodation to the local anatomy of the targeted recipient anatomic site, and with the ability for post-deployment adjustment to accommodate anatomic changes that might compromise the efficacy of the implant. A surgical implant includes a self-expanding stent of a shape-memory material set to a given shape. The stent has a wall with a portion having a first thickness and a second portion having a thickness greater than the first. The second portion defines a key-hole shaped longitudinal drive orifice. The implant includes a selectively adjustable assembly having adjustable elements and being operable to force a configuration change in at least a portion of the self-expanding stent. The adjustable elements have a part rotatably disposed within the longitudinal drive orifice.
Owner:EDWARDS LIFESCI CARDIAQ
Features
- R&D
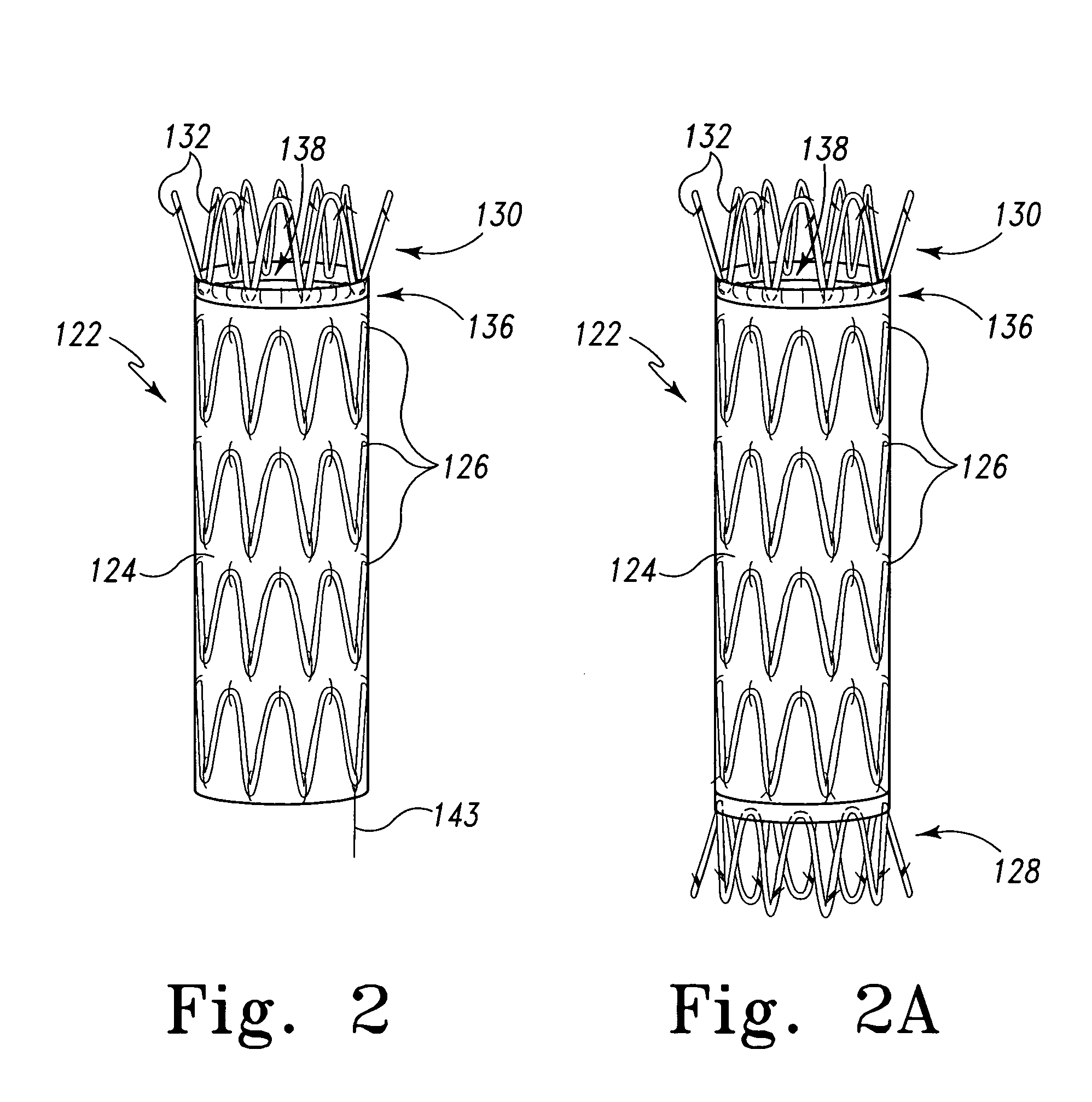
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com