Stent-valves for valve replacement and associated methods and systems for surgery
a valve replacement and valve valve technology, applied in the field of stent valves, can solve the problems of increasing the risks, death, and limits of patients' activities, and achieve the effect of facilitating a surgical approach and reducing risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
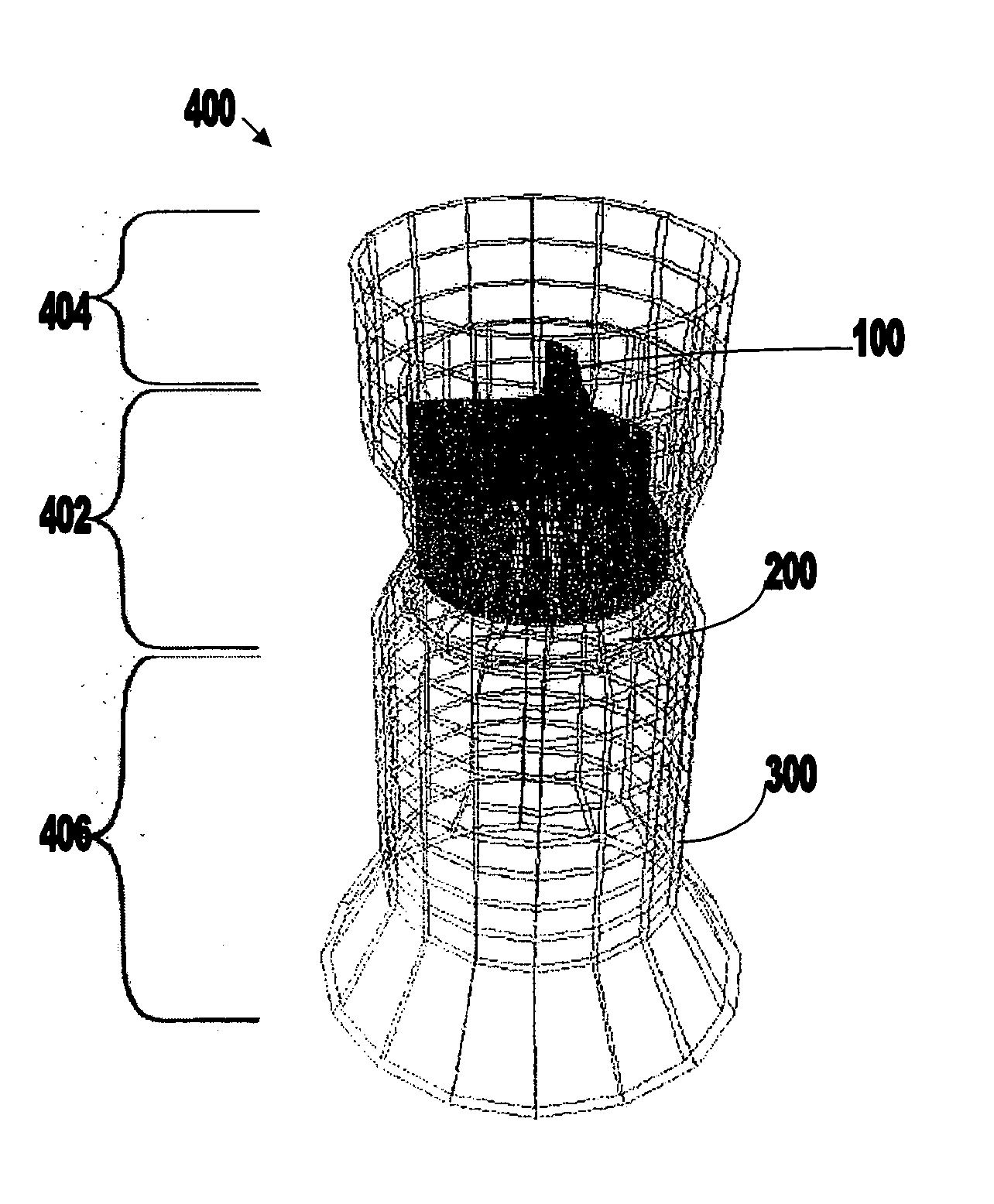
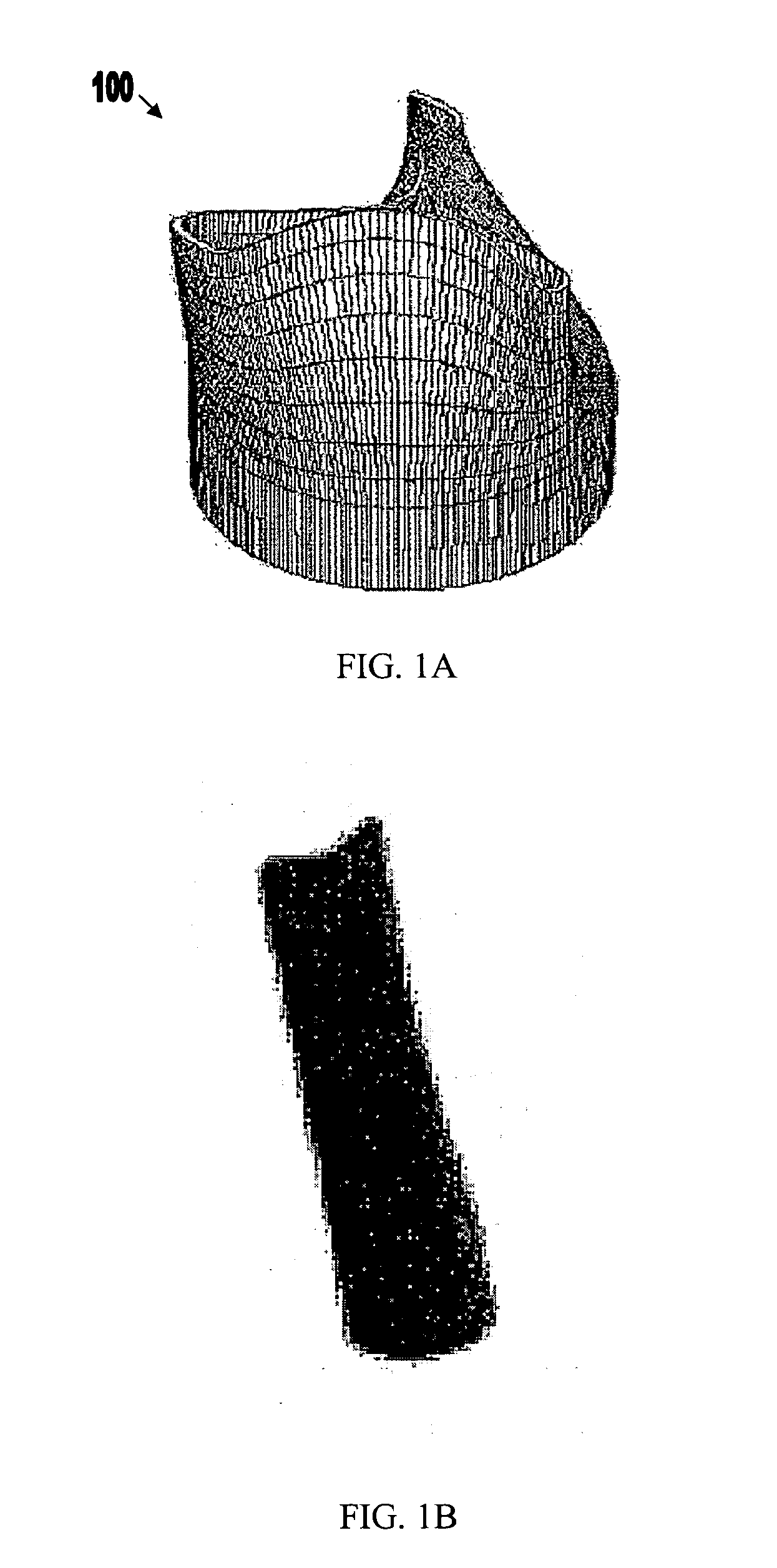
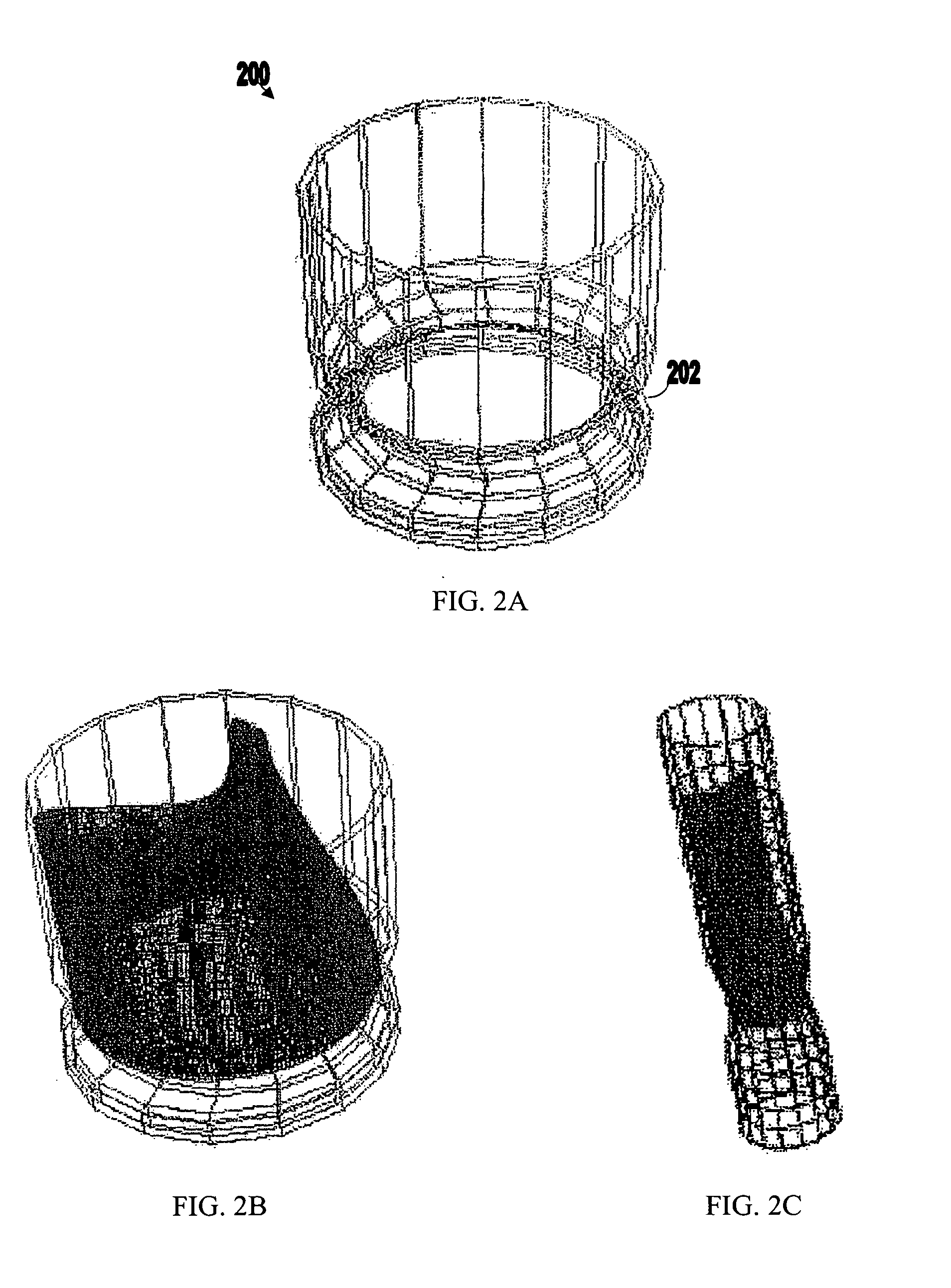
[0061]FIGS. 1A-3B show components 100, 200, and 300 for use in replacing, for example, a failed (e.g., degenerated) aortic valve, mitral valve, or pulmonary cardiac valve (e.g., in a pediatric patient) in accordance with some embodiments of the present invention. More particularly, FIGS. 1A and 1B show a valve component 100. FIGS. 2A-2C show a stent component 200 for housing valve component 100. FIGS. 3A and 3B show a stent component 300 for housing stent component 200 and valve component 100. A device that includes components 100 and 200 may be referred to as a single-stent-valve. A device that additionally includes component 300 may be referred to as a double-stent-valve.
[0062]FIG. 4 shows a double-stent-valve 400 that includes valve component 100, stent component 200, and stent component 300 in accordance with some embodiments of the present invention. Double-stent-valve 400 may replace a failed native or artificial valve. As used herein, a “native valve” refers to a valve natur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com