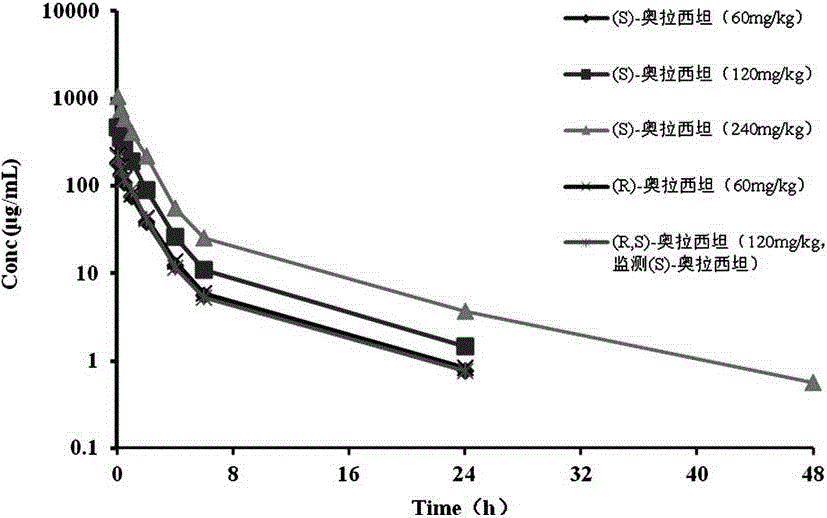
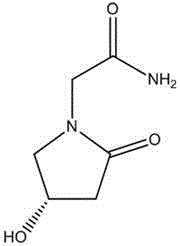
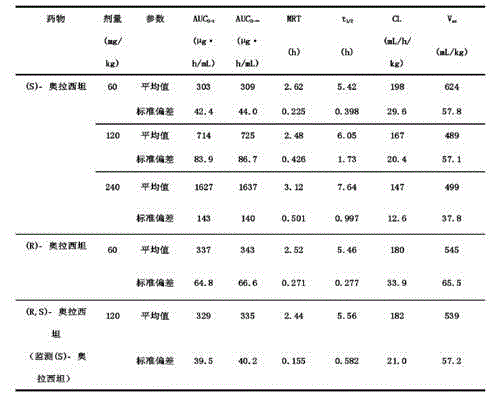
Patents
Literature
4831 results about "Efficacy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Efficacy is the ability to get a job done satisfactorily. The word comes from the same roots as effectiveness, and it has often been used synonymously, although in pharmacology a distinction is now often made between efficacy and effectiveness. The word efficacy is used in pharmacology and medicine to refer both to the maximum response achievable from a pharmaceutical drug in research settings, and to the capacity for sufficient therapeutic effect or beneficial change in clinical settings.
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
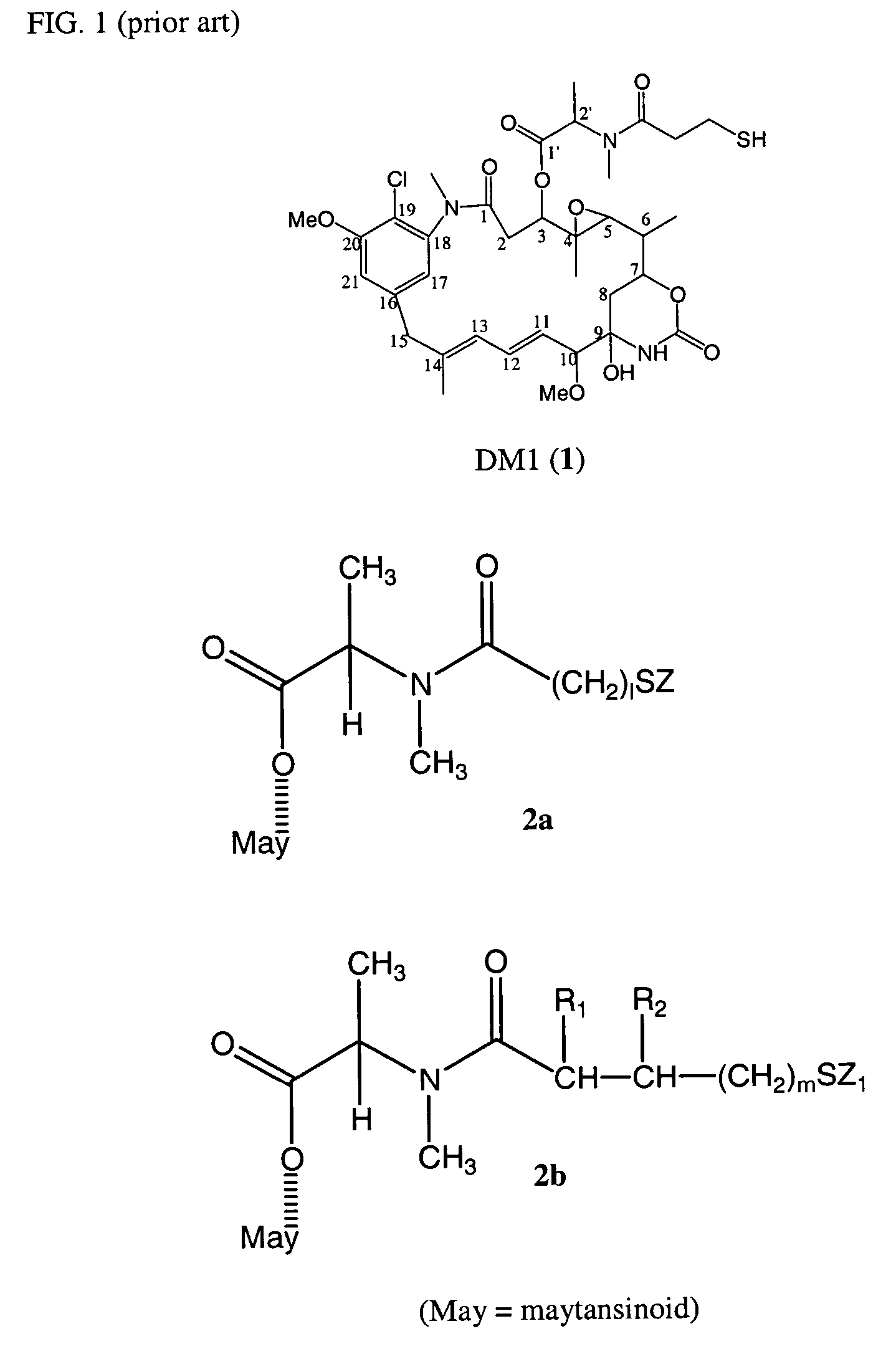
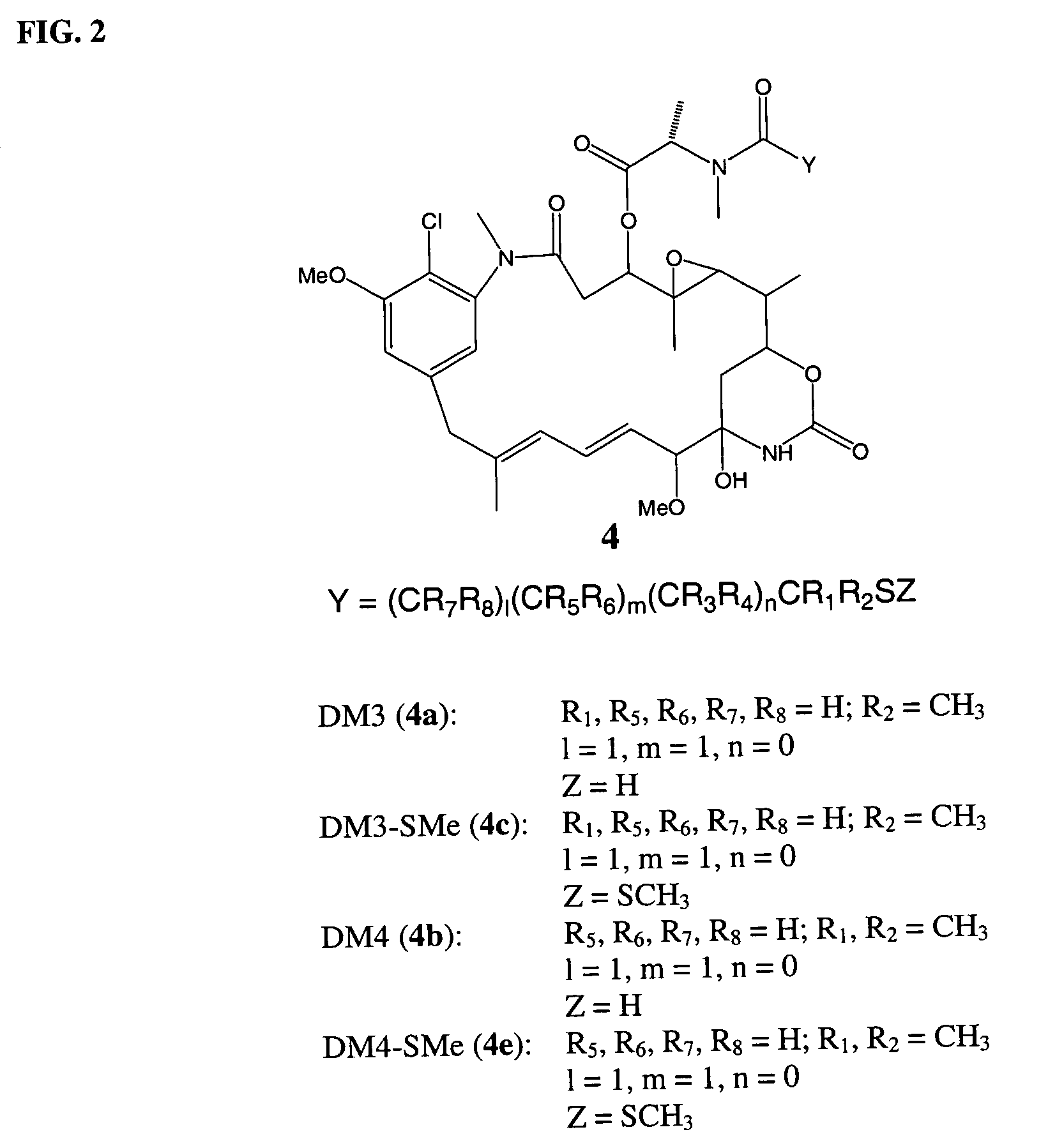
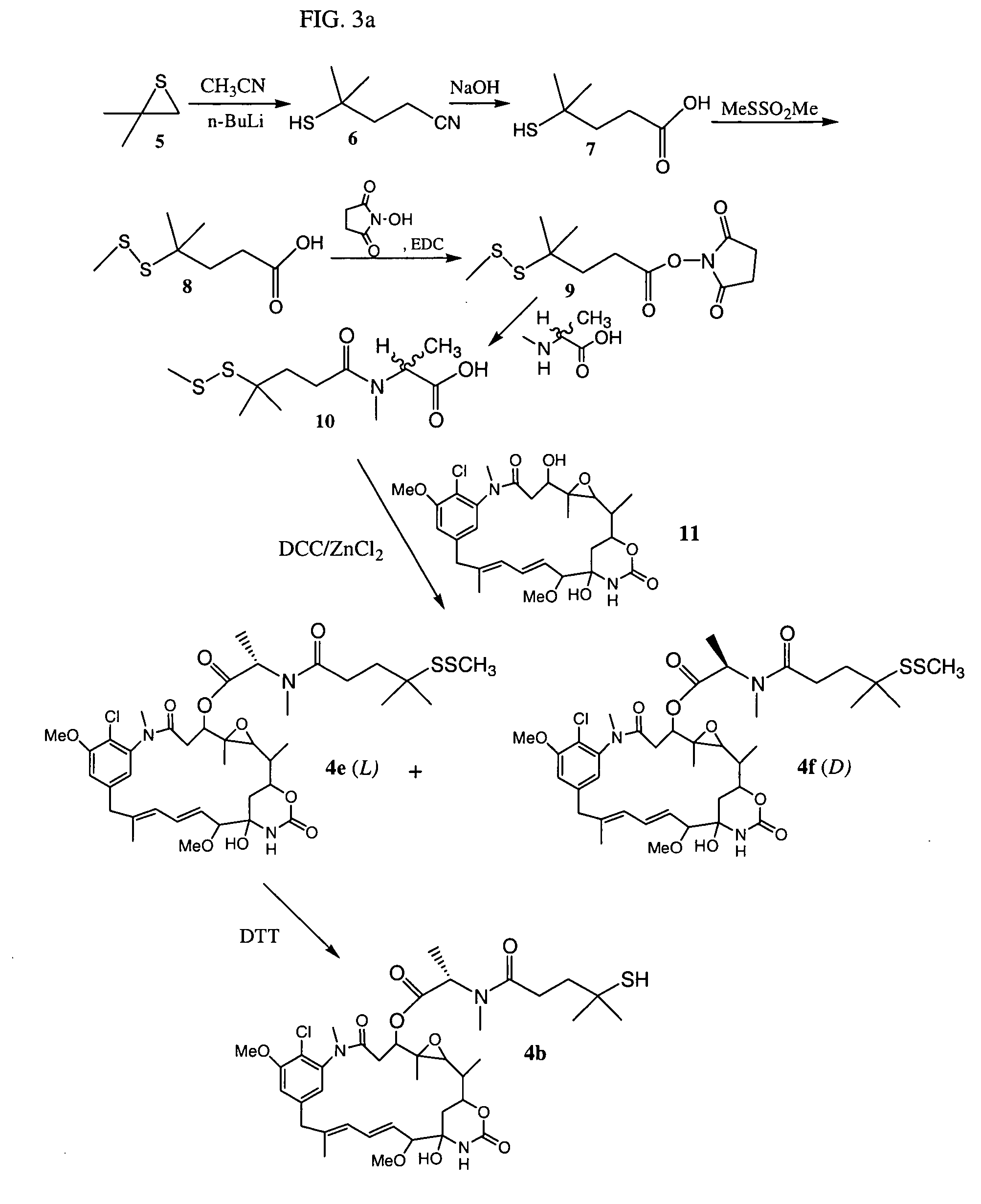
Cytotoxic agents comprising new maytansinoids
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
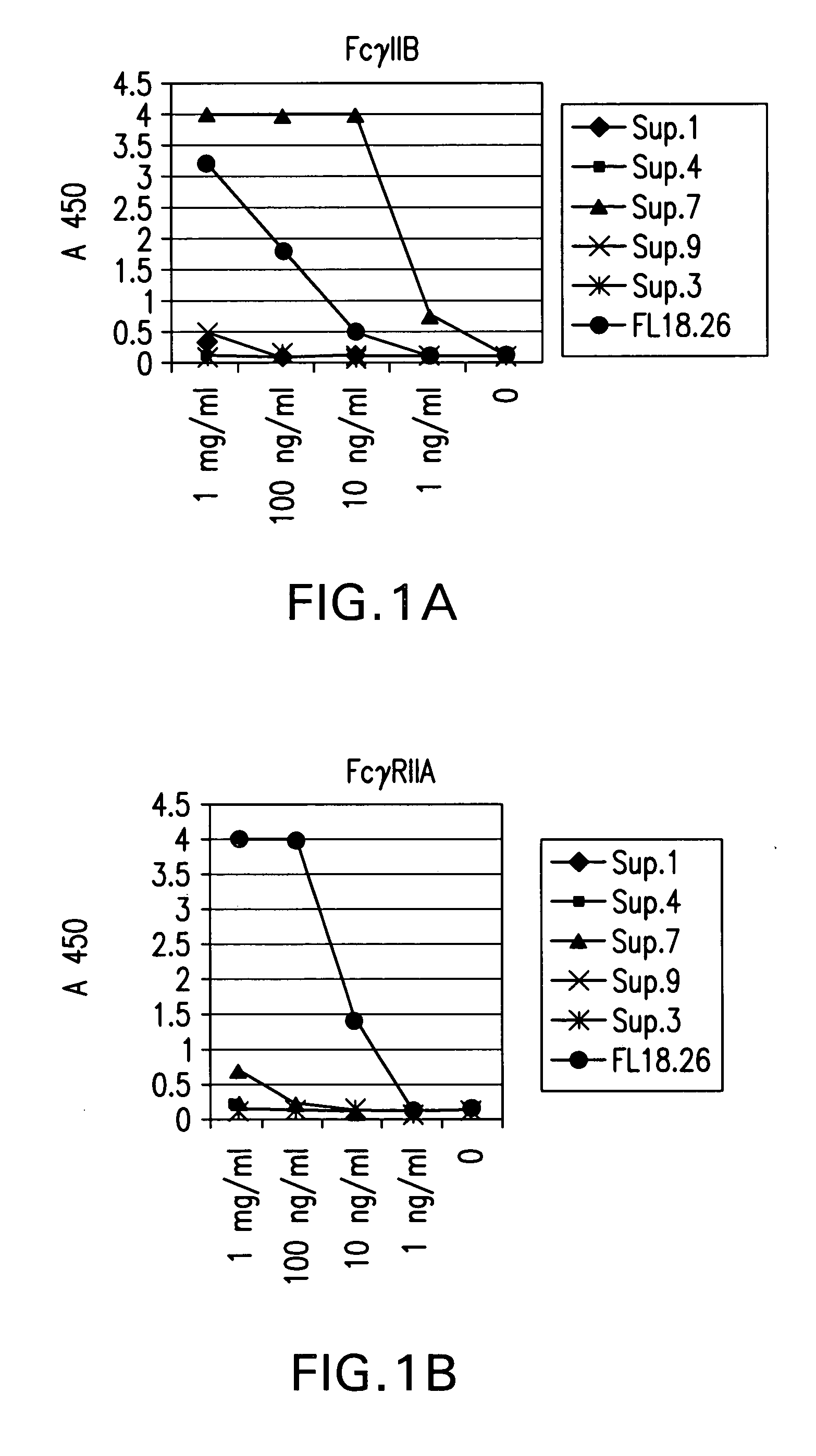
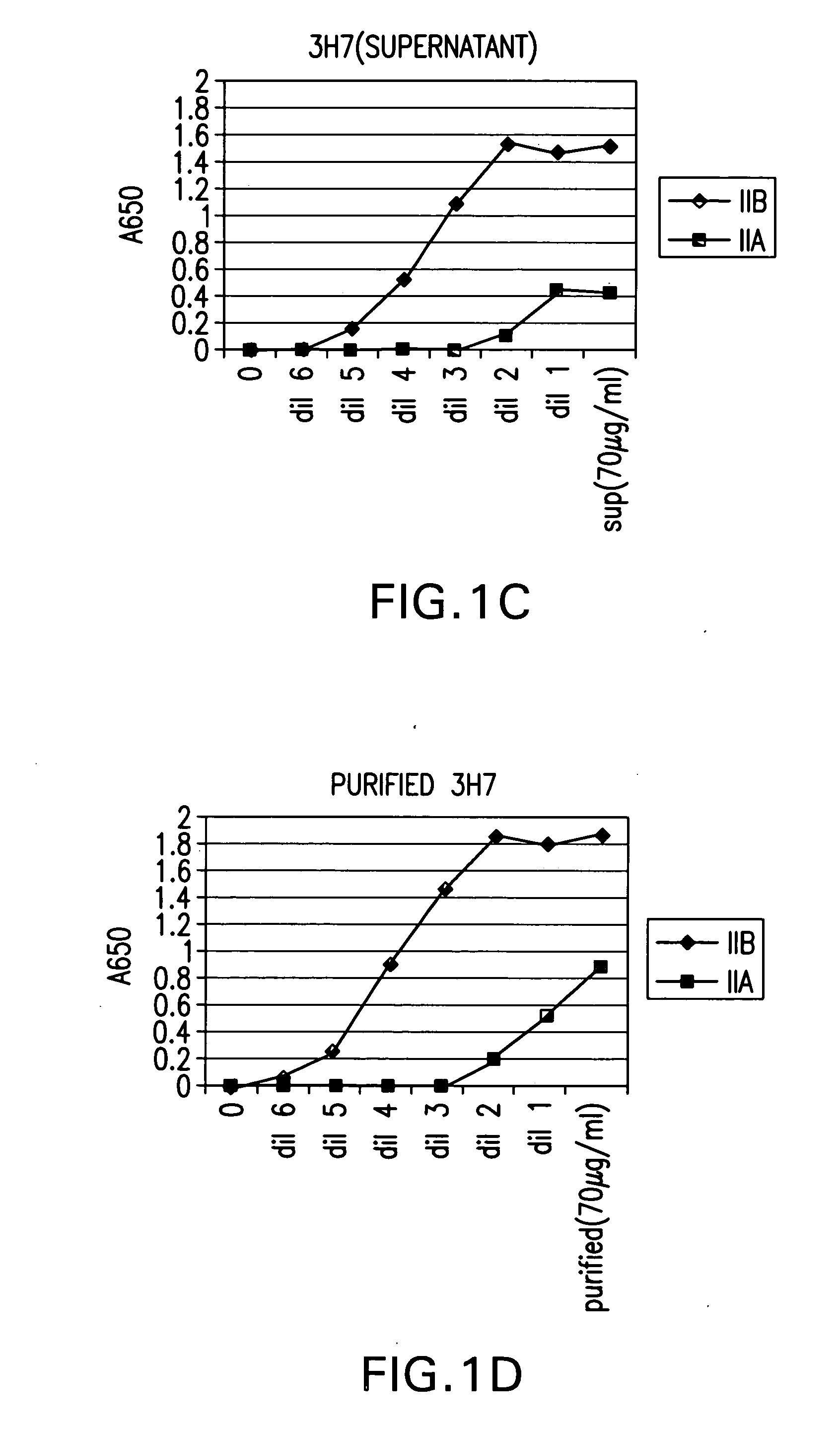
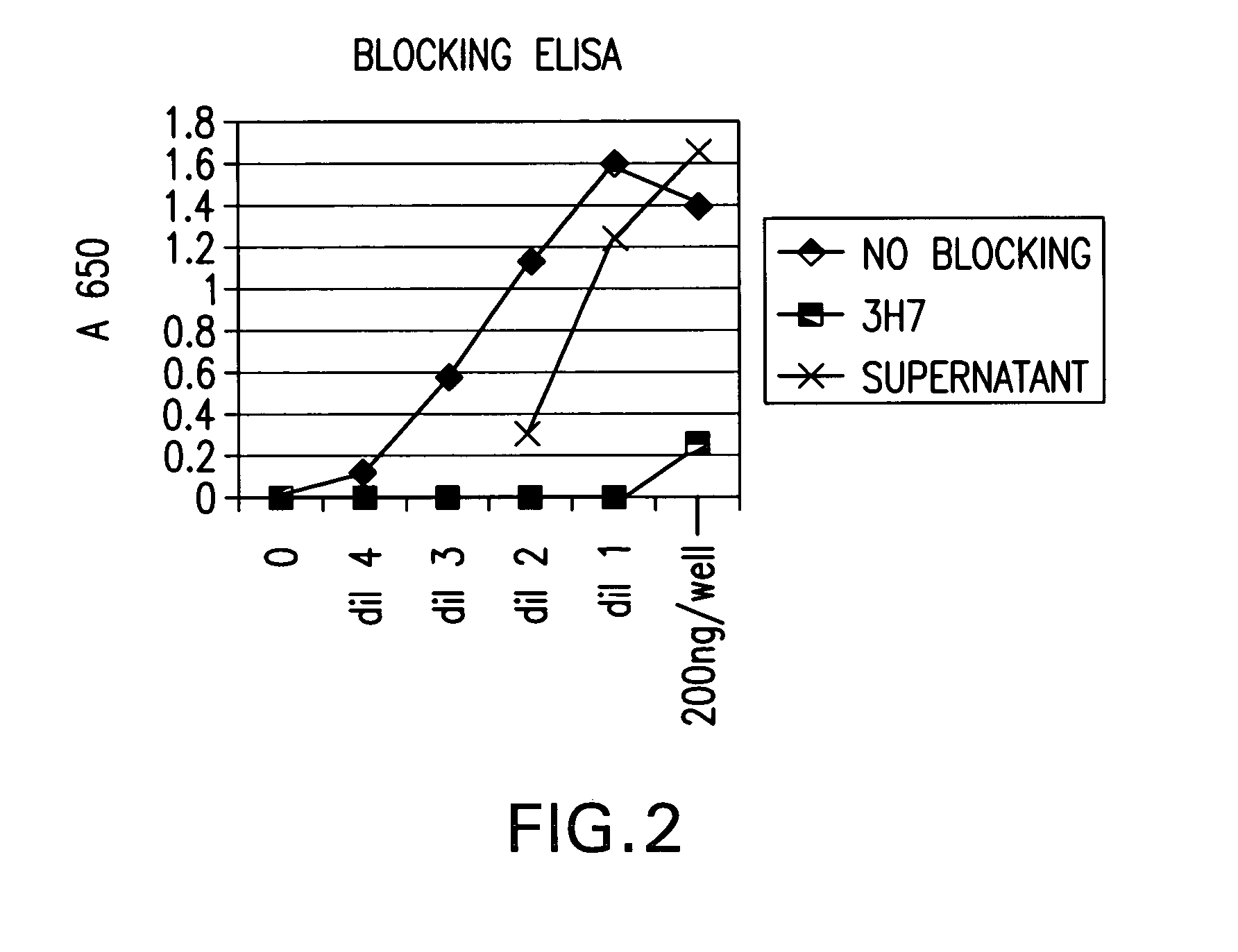
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS20050037000A1High affinityAltered affinityAntibacterial agentsSenses disorderTherapeutic antibodyWild type
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcgammaRIIA and / or FcgammaRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcgammaR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MARCOGENICS INC +1
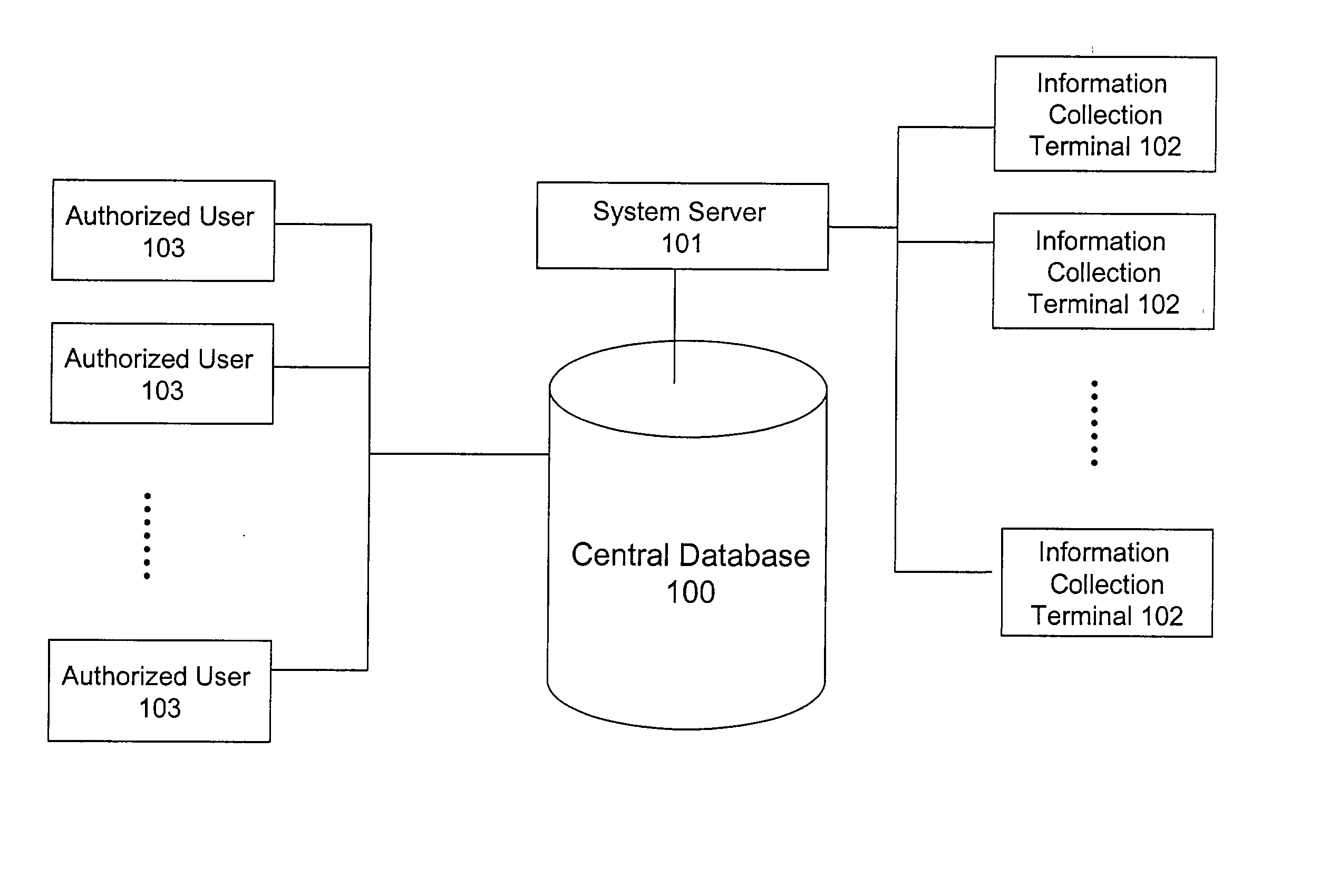
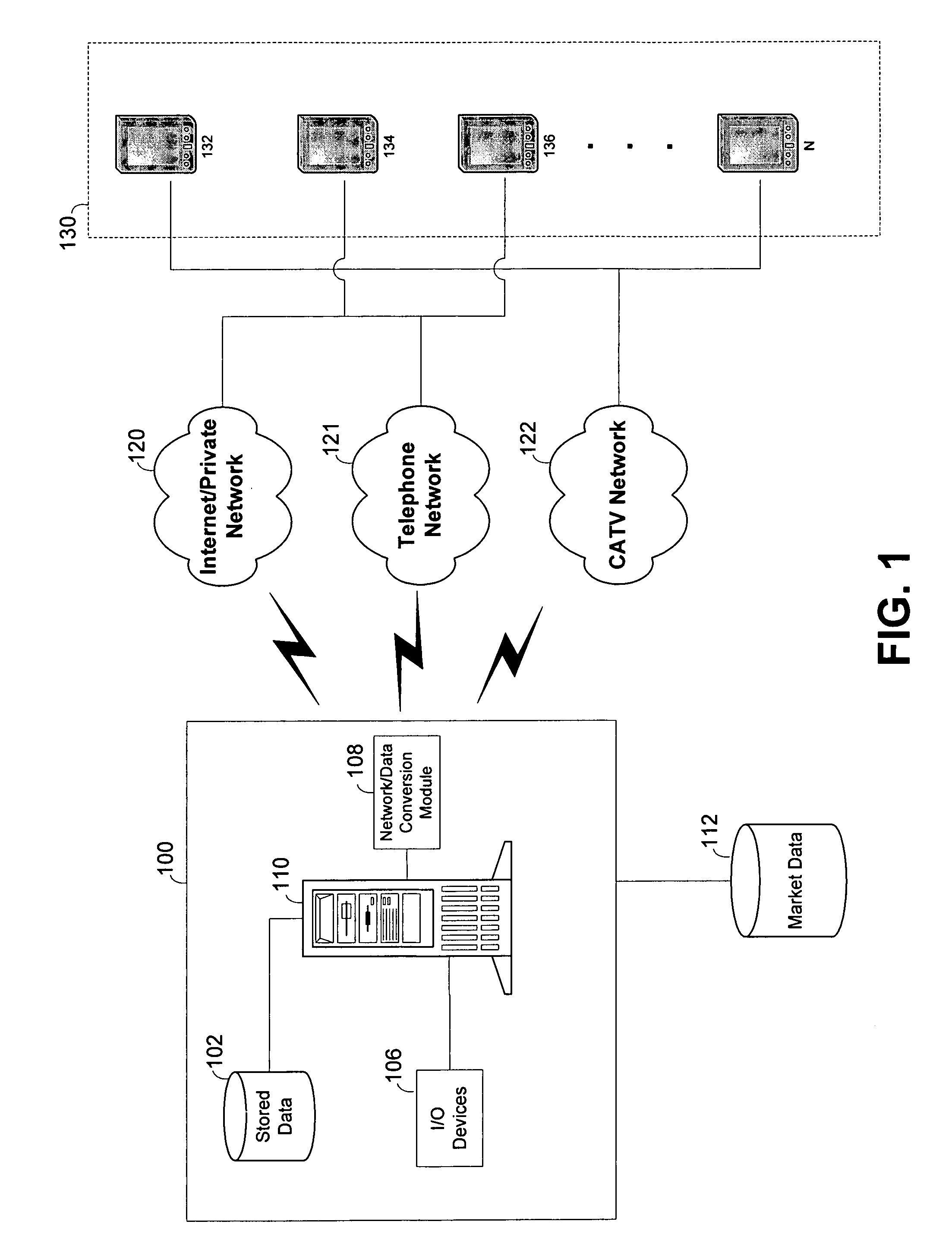
System and method for building and manipulating a centralized measurement value database
InactiveUS20020186818A1Low penetrationEasy to aimImage enhancementImage analysisMarket penetrationEfficacy
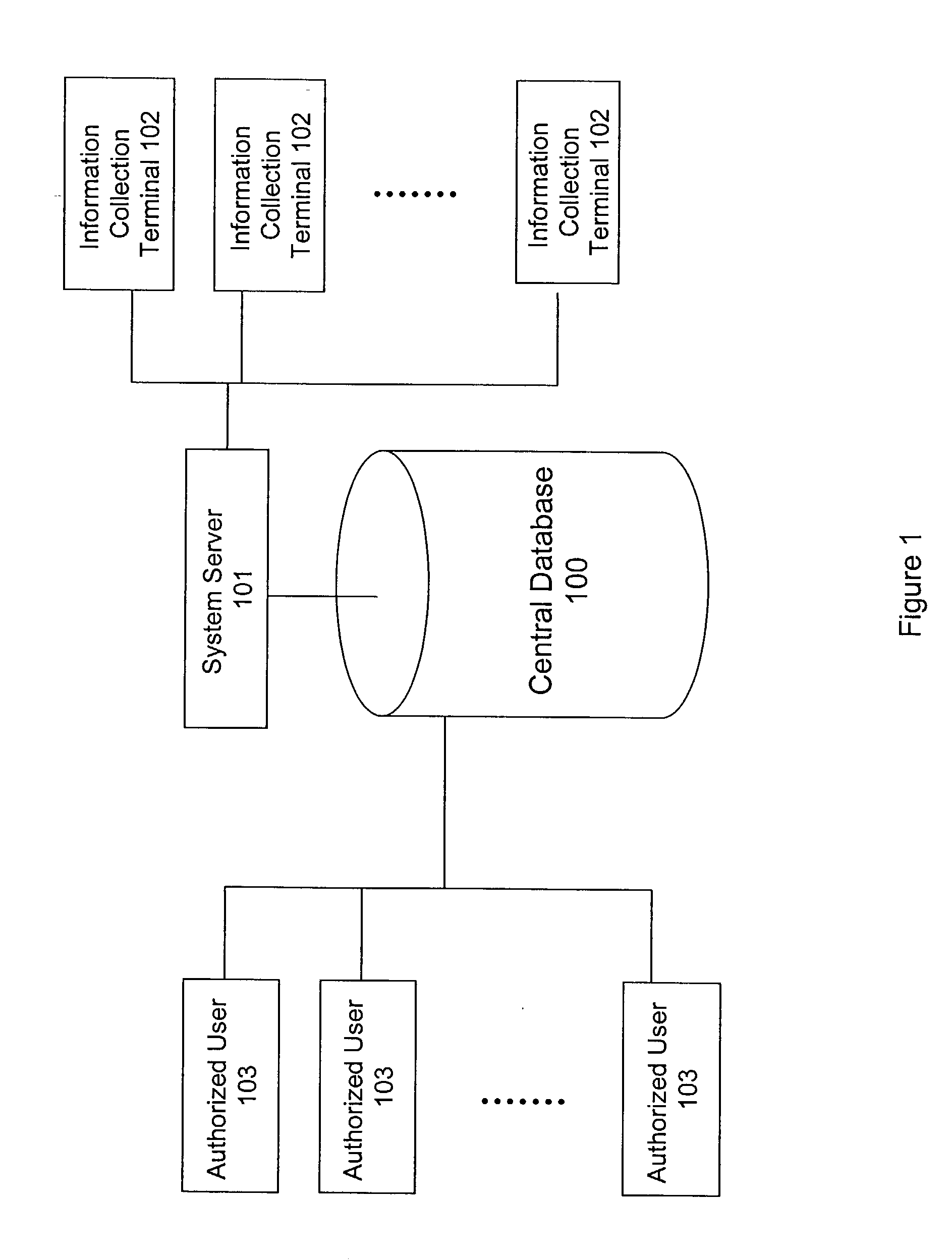
A system and method for building and / or manipulating a centralized medical image quantitative information database aid in diagnosing diseases, identifying prevalence of diseases, and analyzing market penetration data and efficacy of different drugs. In one embodiment, the diseases are bone-related, such as osteoporosis and osteoarthritis. Subjects' medical images, personal and treatment information are obtained at information collection terminals, for example, at medical and / or dental facilities, and are transferred to a central database, either directly or through a system server. Quantitative information is derived from the medical images, and stored in a central database, associated with subjects' personal and treatment information. Authorized users, such as medical officials and / or pharmaceutical companies, can access the database, either directly or through the central server, to diagnose diseases and perform statistical analysis on the stored data. Decisions can be made regarding marketing of drugs for treating the diseases in question, based on analysis of efficacy, market penetration, and performance of competitive drugs.
Owner:IMAGING THERAPEUTICS +1
Uses and compositions for treatment of rheumatoid arthritis
ActiveUS20080166348A1Relieve symptomsInhibit progressSsRNA viruses negative-senseBiocideAntigen bindingRheumatoid arthritis
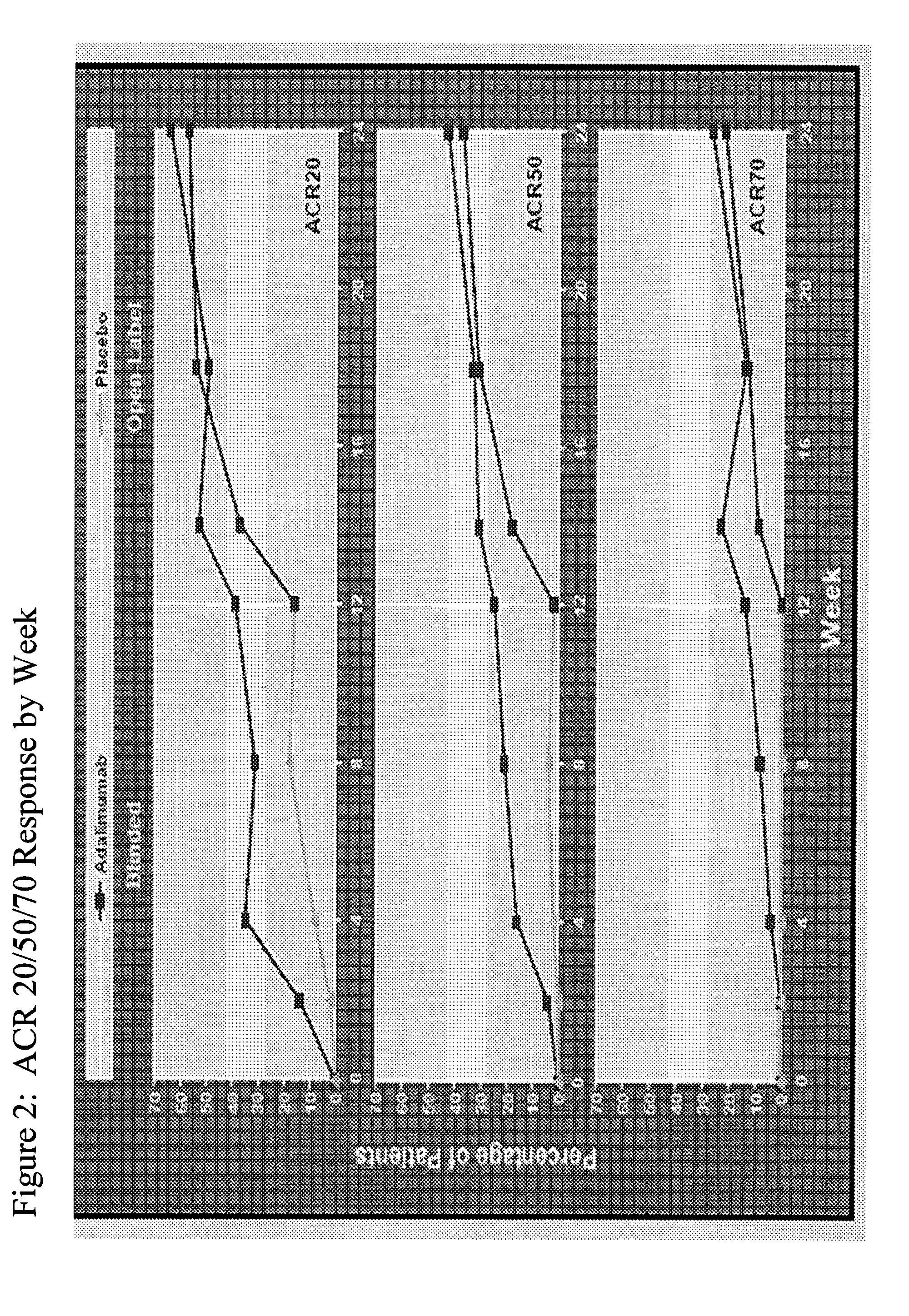
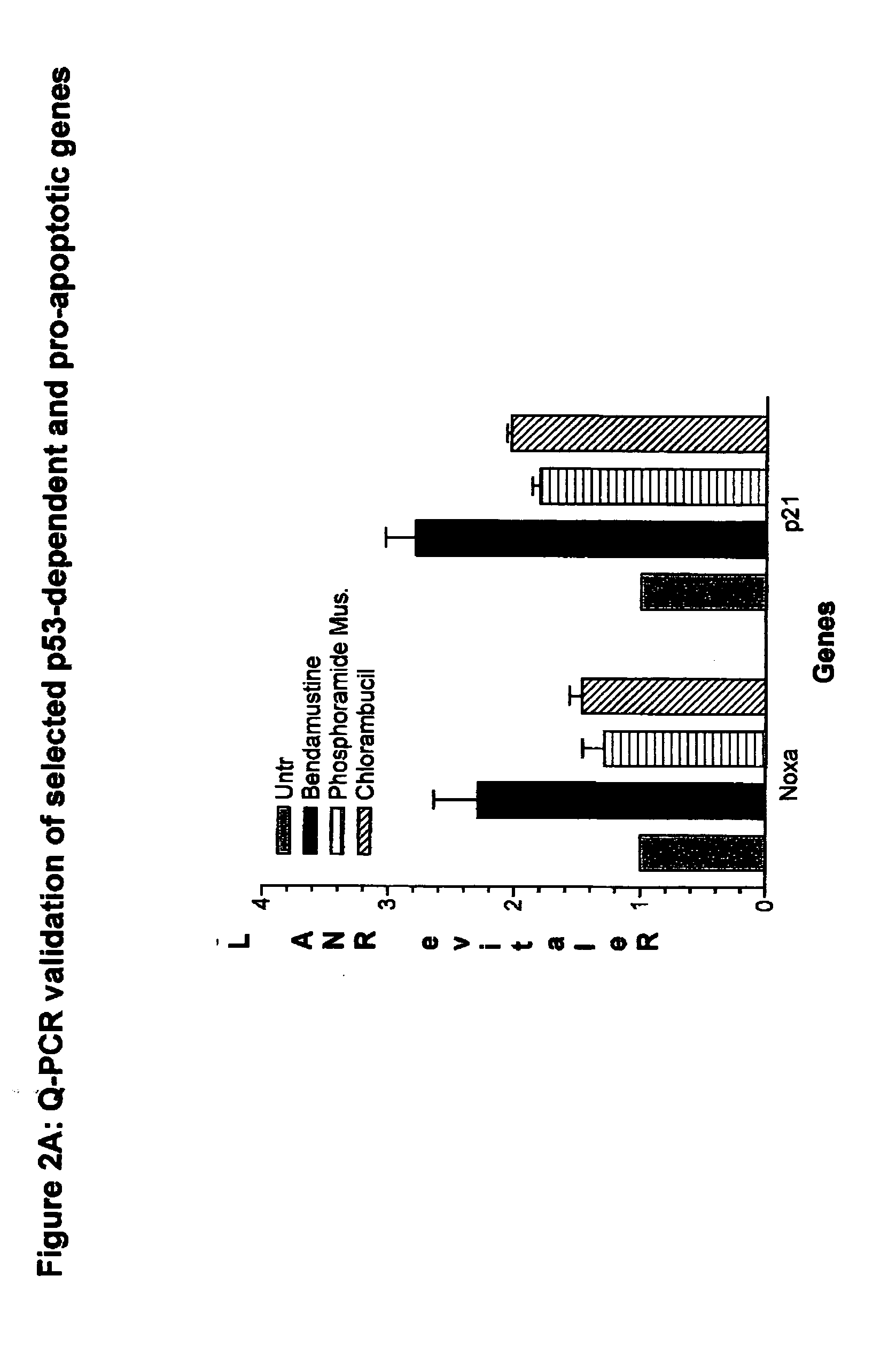
The invention provides methods, uses and compositions for the treatment of rheumatoid arthritis. The invention describes methods and uses for treating rheumatoid arthritis wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of rheumatoid arthritis in a subject.
Owner:ABBVIE BIOTECHNOLOGY LTD
System and method for recording patient history data about on-going physician care procedures
InactiveUS6154726AConvenient recordingOptimize schedulingData processing applicationsComputer-assisted medical data acquisitionStaff timeEfficacy
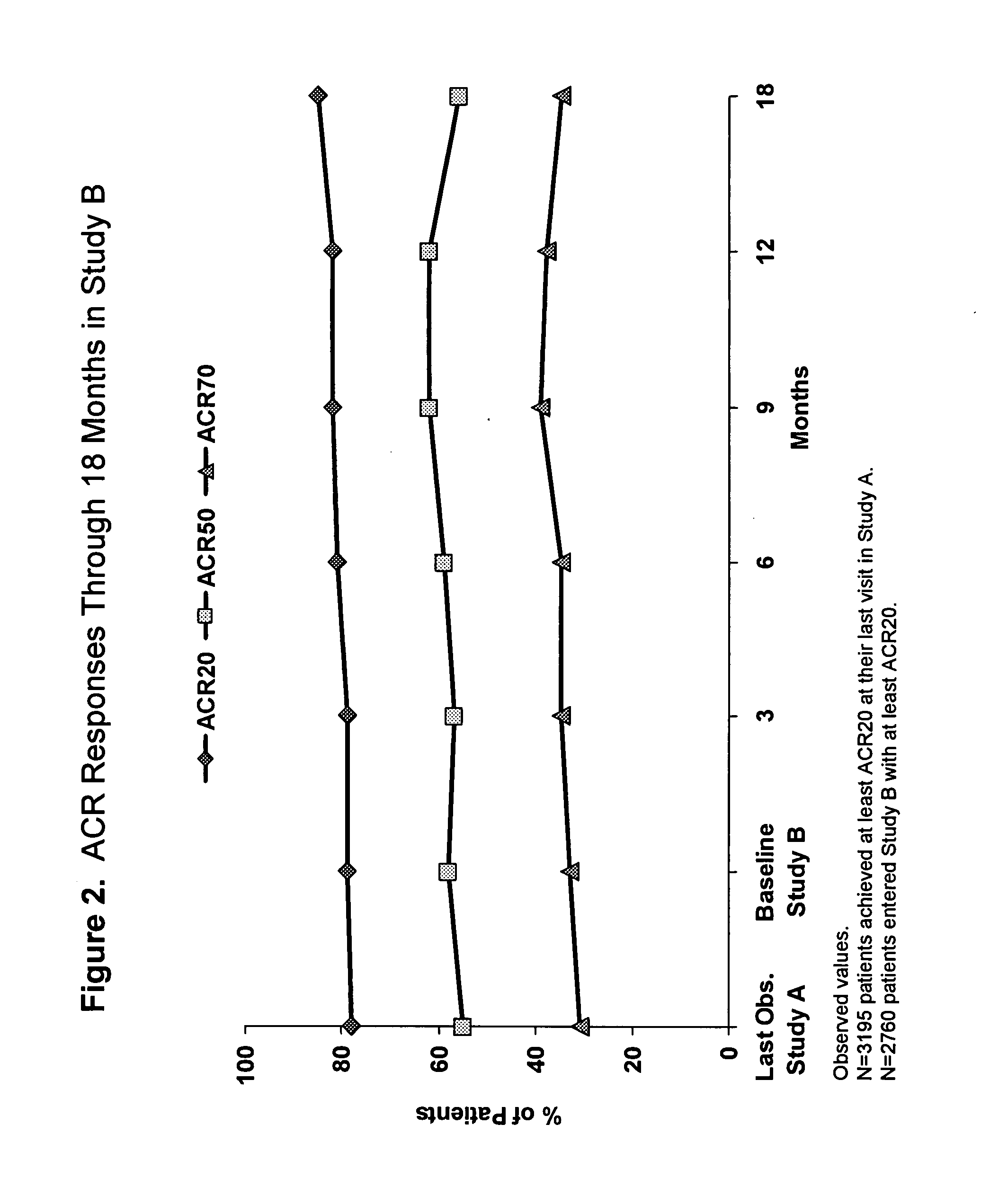
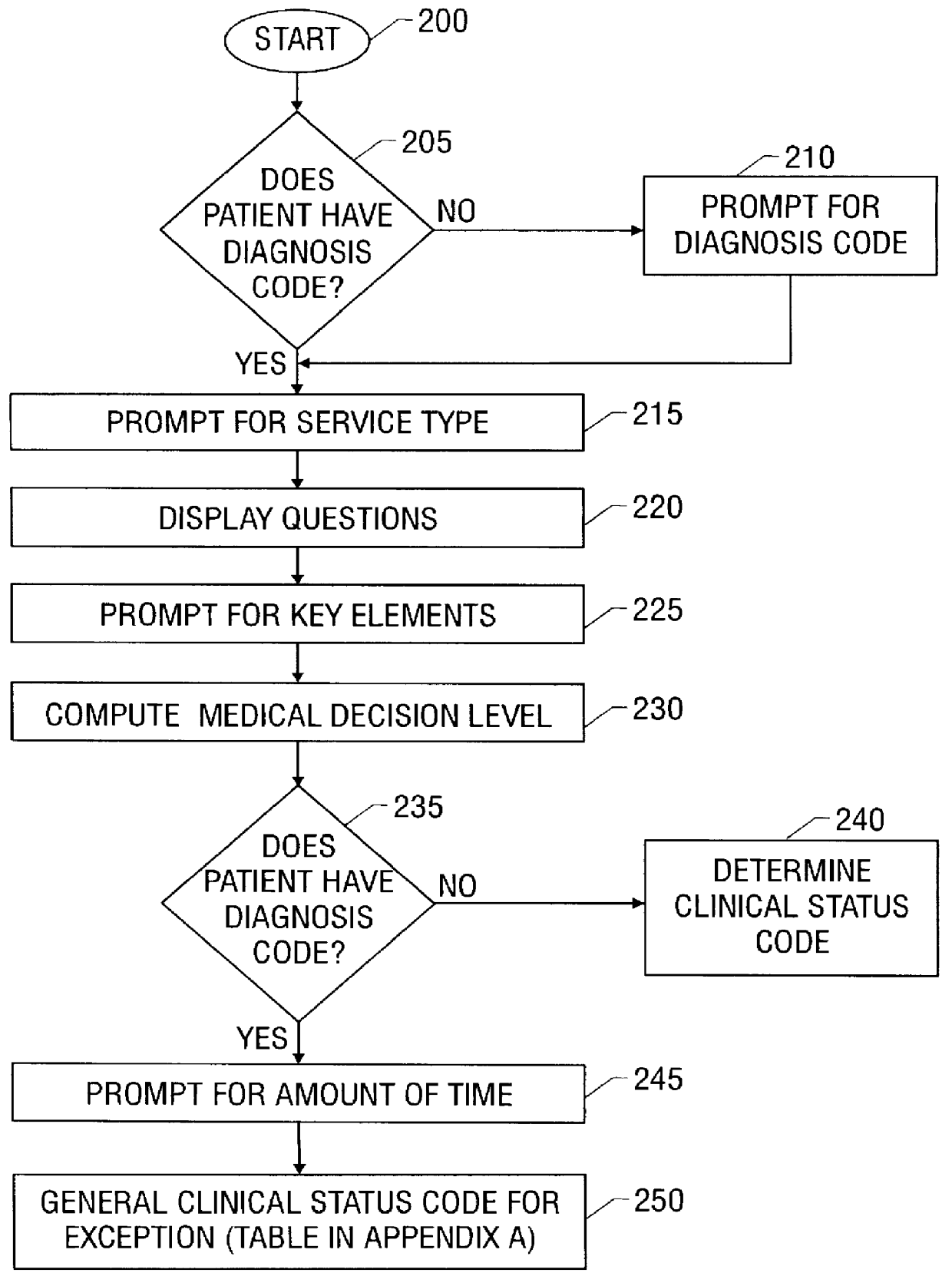
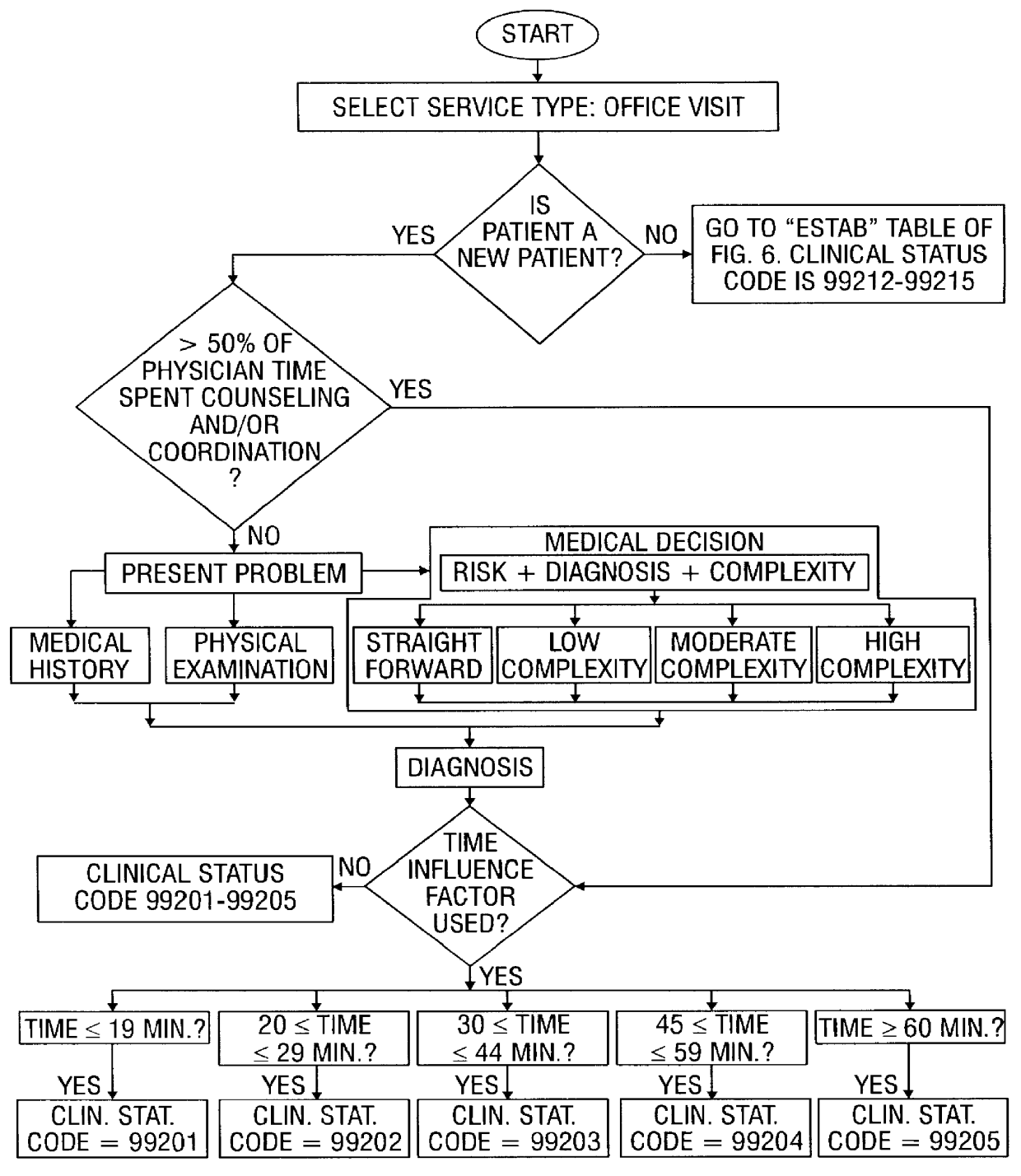
A system and method for processing patient data permits physicians and other medical staff personnel to record, accurately and precisely, historical patient care information. An objective measure of a physician's rendered level of care, as described by a clinical status code, is automatically generated. Data elements used in the determination of the generated clinical status code include a level of history of the patient, a level of examination of the patient, a decision-making process of the physician treating the patient, and a "time influence factor." The quantity and quality of care information for a particular patient is enhanced allowing future care decisions for that patient to be based on a more complete medical history. Enhanced care information can be used in outcome studies to track the efficacy of specific treatment protocols. Archiving of patient information is done in a manner which allows reconstruction of the qualitative aspects of provided medical services. The medical care data can be recorded, saved, and transferred from a portable system to a larger stationary information or database system. Considerable physician and staff time are saved and precision and accuracy are significantly enhanced, by generating these clinical status codes automatically (at the point of service by the care-provider without any intermediary steps) from information recorded simultaneously with the provision of services.
Owner:RENSIMER ENTERPRISES
Fcgamma riib specific antibodies and methods of use thereof
InactiveUS20050215767A1Enhance immune responseImprove responseSenses disorderAntipyreticTherapeutic antibodyAntiendomysial antibodies
The present invention relates to antibodies or fragments thereof that specifically bind FcγRIIB, particularly human FcγRIIB, with greater affinity than said antibodies or fragments thereof bind FcγRIIA, particularly human FcγRIIA. The invention provides methods of enhancing the therapeutic effect of therapeutic antibodies by administering the antibodies of the invention to enhance the effector function of the therapeutic antibodies. The invention also provides methods of enhancing efficacy of a vaccine composition by administering the antibodies of the invention.
Owner:MACROGENICS INC
Process for discriminating between biological states based on hidden patterns from biological data
The invention describes a process for determining a biological state through the discovery and analysis of hidden or non-obvious, discriminatory biological data patterns. The biological data can be from health data, clinical data, or from a biological sample, (e.g., a biological sample from a human, e.g., serum, blood, saliva, plasma, nipple aspirants, synovial fluids, cerebrospinal fluids, sweat, urine, fecal matter, tears, bronchial lavage, swabbings, needle aspirantas, semen, vaginal fluids, pre-ejaculate.), etc. which is analyzed to determine the biological state of the donor. The biological state can be a pathologic diagnosis, toxicity state, efficacy of a drug, prognosis of a disease, etc. Specifically, the invention concerns processes that discover hidden discriminatory biological data patterns (e.g., patterns of protein expression in a serum sample that classify the biological state of an organ) that describe biological states.
Owner:ASPIRA WOMENS HEALTH INC +1
Intelligent Drug and/or Fluid Delivery System to Optimizing Medical Treatment or Therapy Using Pharmacodynamic and/or Pharamacokinetic Data
ActiveUS20130296823A1Improve securityStrengthen security controlData processing applicationsDrug and medicationsPharmacodynamic StudyDelivery system
Owner:XHALE ASSURANCE INC +1
Uses and compositions for treatment of psoriatic arthritis
InactiveUS20080311043A1Improve the quality of lifeTreatment safetyCompounds screening/testingPeptide/protein ingredientsAntigen bindingPsoriasis arthropathy
The invention provides methods, uses and compositions for the treatment of psoriatic arthritis. The invention describes methods and uses for treating psoriatic arthritis, wherein a TNFα inhibitor, such as a human TNFα antibody, or antigen-binding portion thereof, is used to psoriatic arthritis in a subject. Also described are methods for determining the efficacy of a TNFα inhibitor for treatment of psoriatic arthritis in a subject.
Owner:HOFFMAN REBECCA S +7
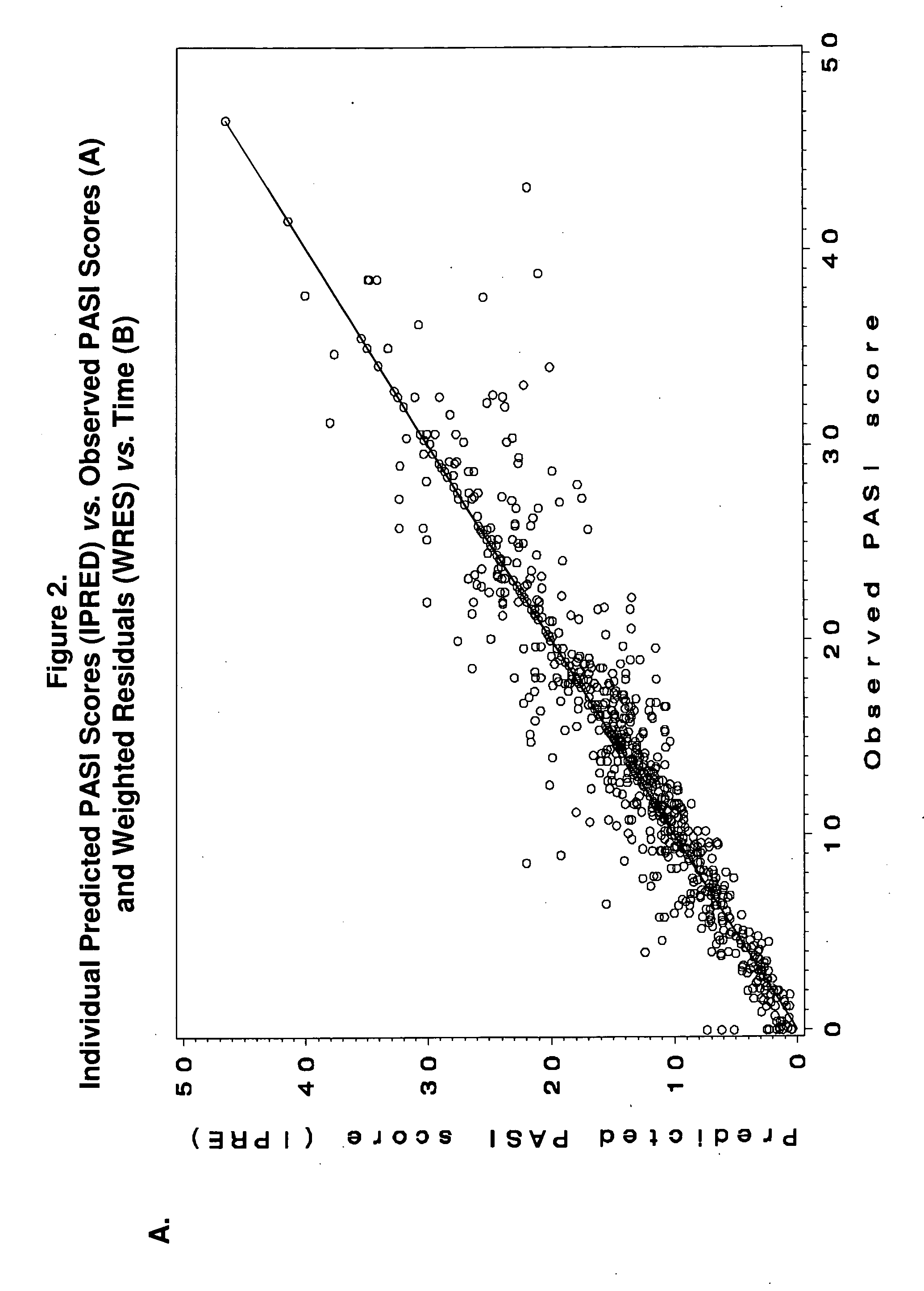
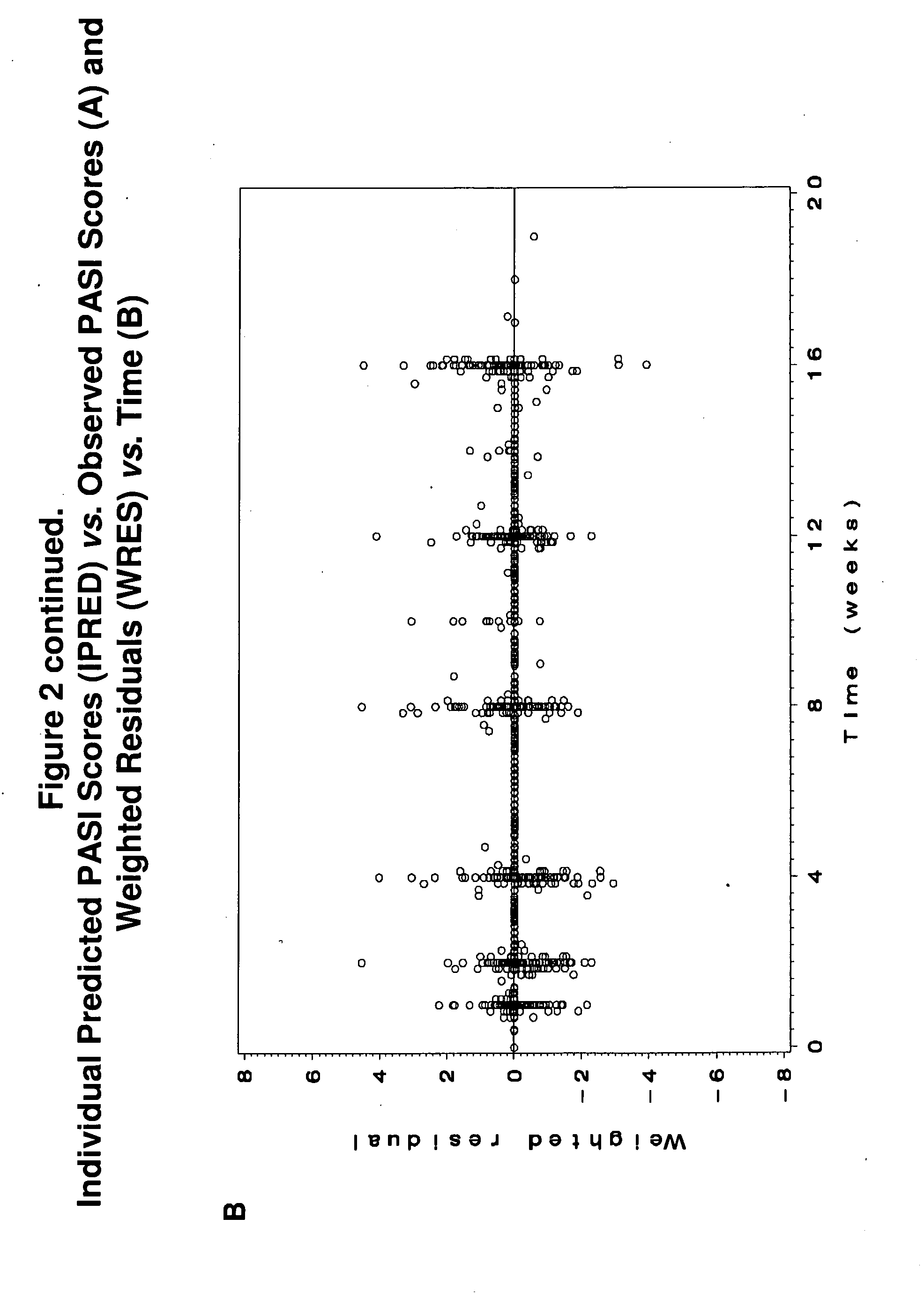
Predicting long-term efficacy of a compound in the treatment of psoriasis
InactiveUS20090271164A1Accurate curative effectLost responsivenessChemical property predictionAnalogue computers for chemical processesMedicineCurative effect
Owner:ABBVIE BIOTECHNOLOGY LTD
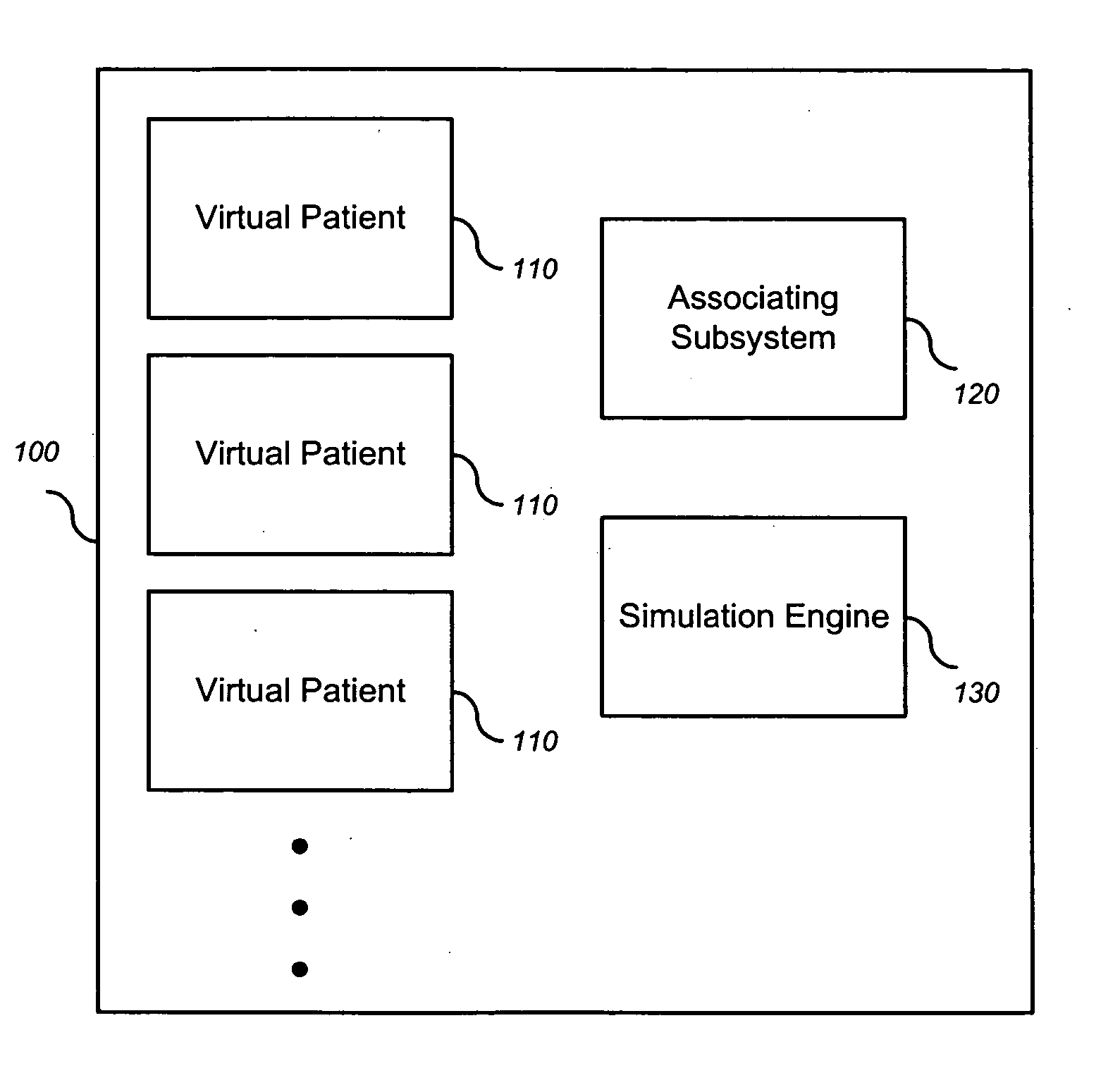
Simulating patient-specific outcomes
InactiveUS20050131663A1Timing is simpleMedical simulationAnalogue computers for chemical processesEfficacyBiomarker (petroleum)
The invention encompasses systems, methods, and apparatus for predicting and monitoring an individual's response to a therapeutic regimen. The invention includes multiple virtual patients, an associating subsystem operable to associate the subject with one or more of the virtual patients, and a simulation engine operable to apply one or more experimental protocols to the one or more virtual patients identified with the subject to generate a set of outputs. The set of outputs can represent therapeutic efficacy, identify biomarkers for monitoring therapeutic efficacy, or merely report the status of the biological system as it represents a particular individual
Owner:ENTELOS HLDG
Cancer treatments
InactiveUS20060128777A1Promoting bendamustine useReduce spreadOrganic active ingredientsBiocideTreatment effectCancer therapy
Methods and compositions for treating cancers characterized by death-resistant cancer cells are described. In general, such methods involve administration of a therapeutically effective amount of a compound that induces mitotic catastrophe in the some, and preferably most or all, of the cancerous cells. Methods for assessing the efficacy of such treatments are also provided.
Owner:CEPHALON INC
Methods and compositions for treatment of skin disorders
ActiveUS20110171227A1Undesirable effectImproving disease reductionSnake antigen ingredientsAntibody ingredientsEtanerceptPrior biologic therapy
The invention provides methods and compositions for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis. The invention includes methods for treating a skin disorder associated with detrimental TNFα activity, such as psoriasis, in a subject who has failed or lost response to prior biologic therapy, such as prior administration of etanercept. The invention further provides methods for determining the efficacy of a human TNFα antibody, or antigen-binding portion thereof, for the treatment of a skin disorder associated with detrimental TNFα activity, such as psoriasis.
Owner:ABBVIE BIOTECHNOLOGY LTD
Application of levo-oxiracetam in preparation of medicine for treating memory and intelligence disturbance
The invention relates to new use of levo-oxiracetam in pharmaceutical field, and in particular relates to application of levo-oxiracetam in preparation of a medicine for treating memory and intelligence disturbance. The experiment result shows that the levo-oxiracetam is a main active ingredient for playing efficacy in oxiracetam, the clinical dosage can be greatly reduced by singly using the levo-oxiracetam, and the potential toxic and side effect is reduced. According to the invention, the levo-oxiracetam is a single active ingredient, the raw material purity is greater than 99.5% so as to effectively avoid the toxicity risk caused by other impurities in the medicine, the pharmacy is safer, the medicine quality is more controllable, and the curative effect is more precise.
Owner:NANJING YOUKE BIOLOGICAL MEDICAL RES +2
Cyclodextrin polymer compositions for use as drug carriers
Owner:KOSAK KENNETH M
Compositions and Methods for Immunomodulation in an Organism
ActiveUS20120177598A1Long half-lifeGood treatment effectPolypeptide with localisation/targeting motifPeptide/protein ingredientsVaccinationHalf-life
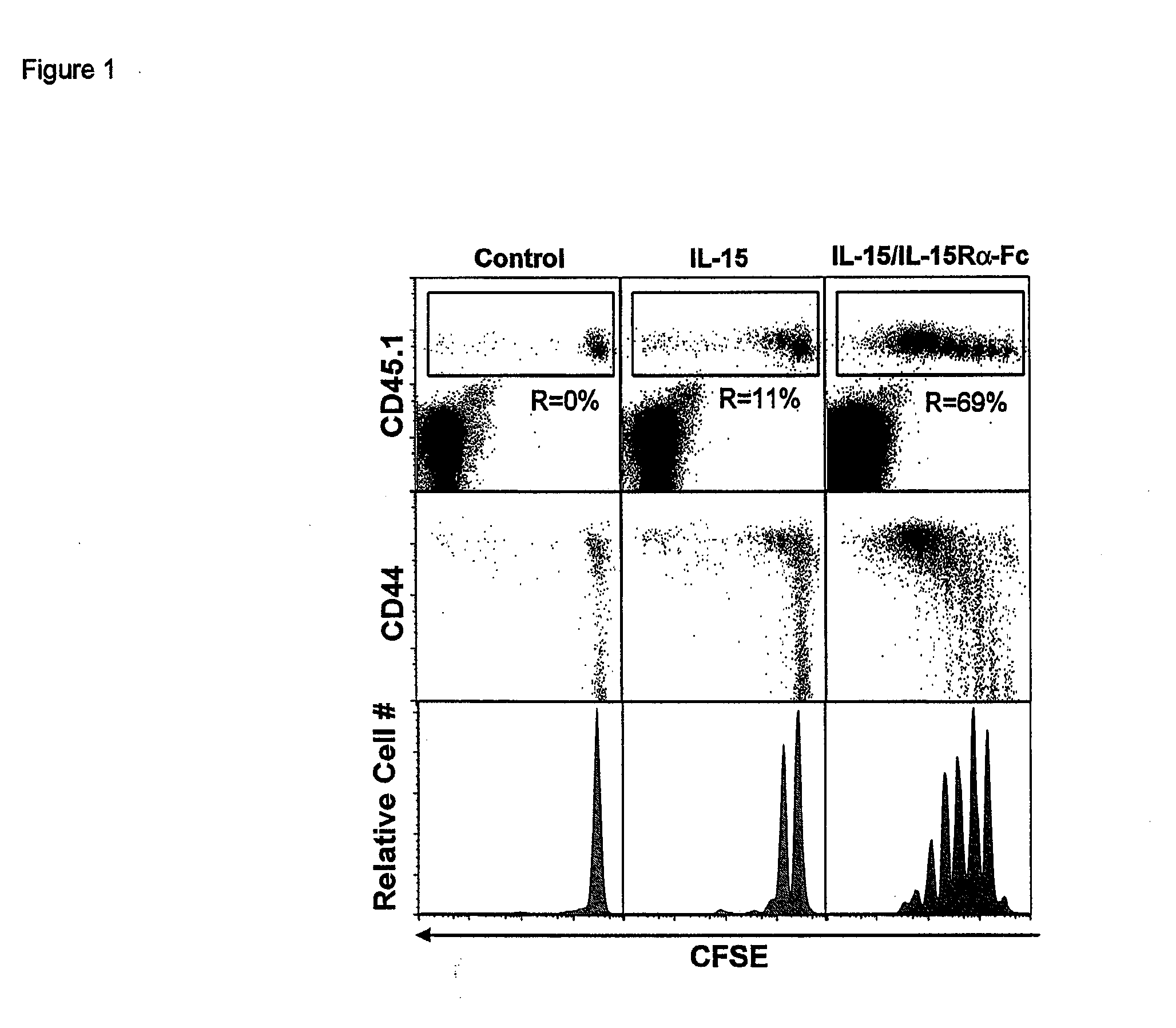
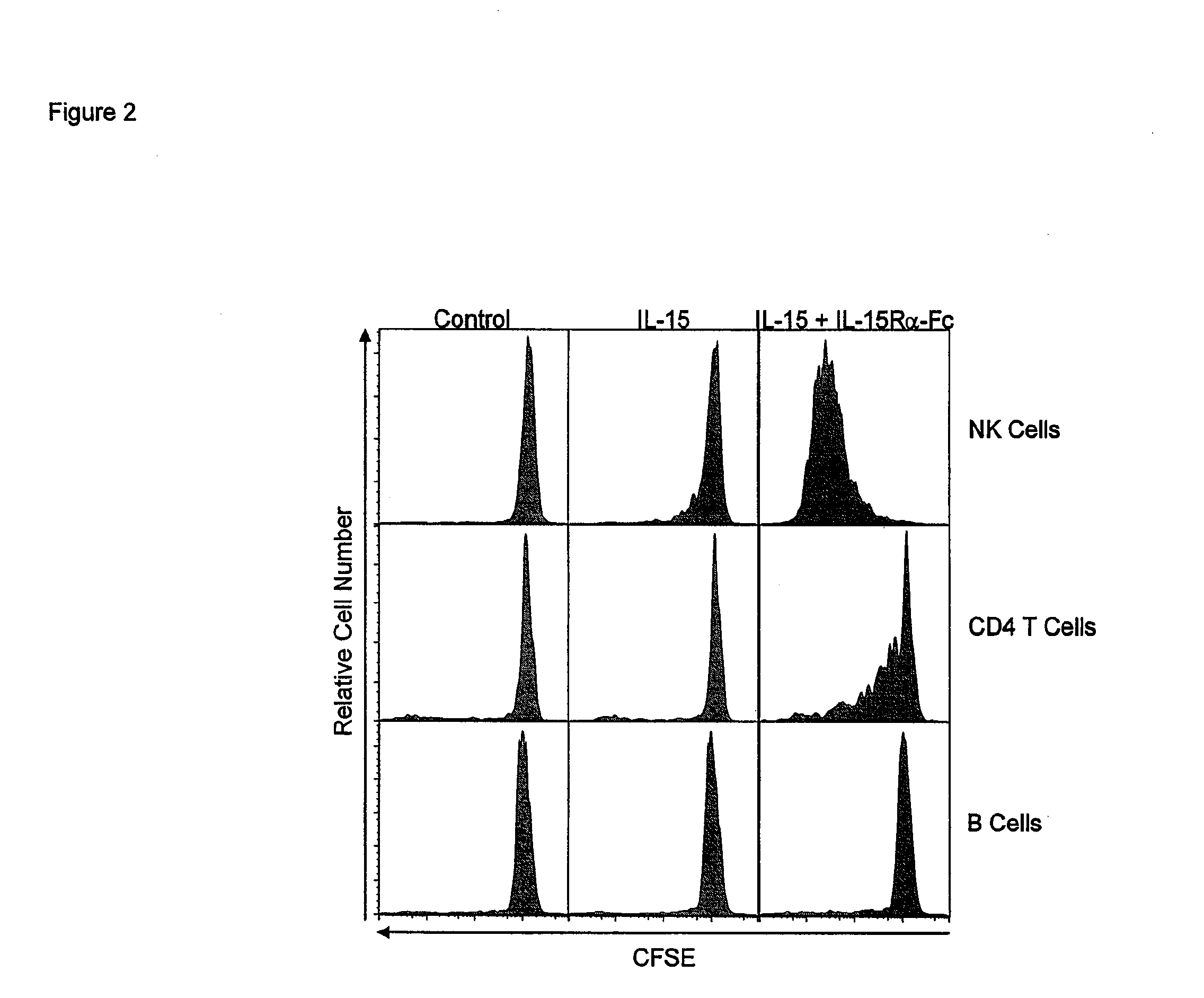
The present invention relates to a therapeutic polypeptide and methods for its creation and use for modulating an immune response in a host organism in need thereof. In particular, the invention relates to the administration to an organism in need thereof, of an effective amount of a pre-coupled polypeptide complex comprising a lymphokine polypeptide portion, for example IL-15 (SEQ ID NO: 5, 6), IL-2 (SEQ ID NO: 10, 12) or combinations of both, and an interleukin receptor polypeptide portion, for example IL-15Ra (SEQ ID NO: 7, 8), IL-2Ra (SEQ ID NO: 9, 11) or combinations of both, for augmenting the immune system in, for example, cancer, SCID, AIDS, or vaccination; or inhibiting the immune system in, for example, rheumatoid arthritis, or Lupus. The therapeutic complex of the invention surprisingly demonstrates increased half-life, and efficacy in vivo.
Owner:UNIV OF CONNECTICUT
Identification and engineering of antibodies with variant Fc regions and methods of using same
ActiveUS8217147B2High affinityLow affinityAntibody mimetics/scaffoldsImmunoglobulinsDiseaseTherapeutic antibody
The present invention relates to molecules, particularly polypeptides, more particularly immunoglobulins (e.g., antibodies), comprising a variant Fc region, wherein said variant Fc region comprises at least one amino acid modification relative to a wild-type Fc region, which variant Fc region binds FcγRIIIA and / or FcγRIIA with a greater affinity, relative to a comparable molecule comprising the wild-type Fc region. The molecules of the invention are particularly useful in preventing, treating, or ameliorating one or more symptoms associated with a disease, disorder, or infection. The molecules of the invention are particularly useful for the treatment or prevention of a disease or disorder where an enhanced efficacy of effector cell function (e.g., ADCC) mediated by FcγR is desired, e.g., cancer, infectious disease, and in enhancing the therapeutic efficacy of therapeutic antibodies the effect of which is mediated by ADCC.
Owner:MACROGENICS INC
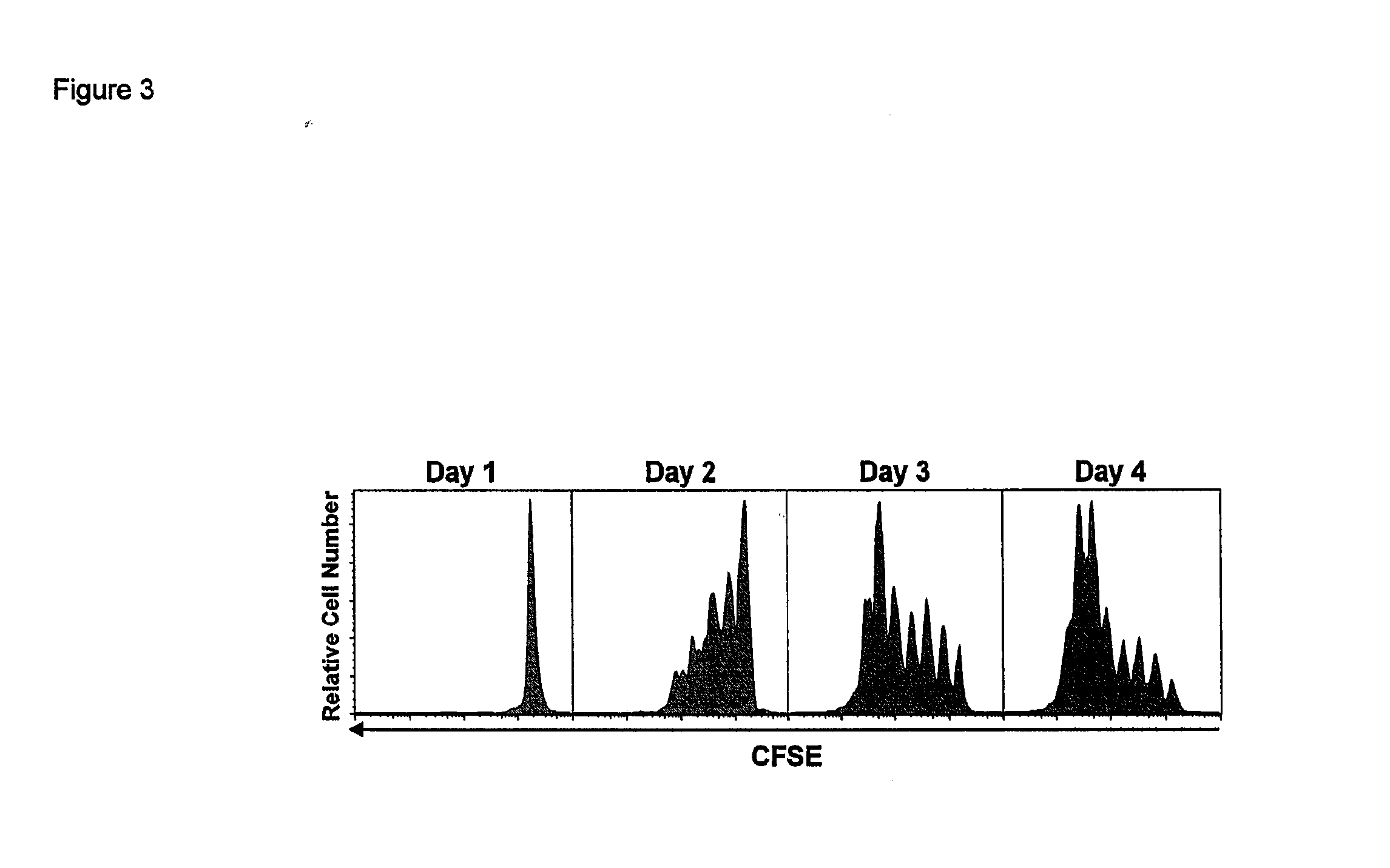
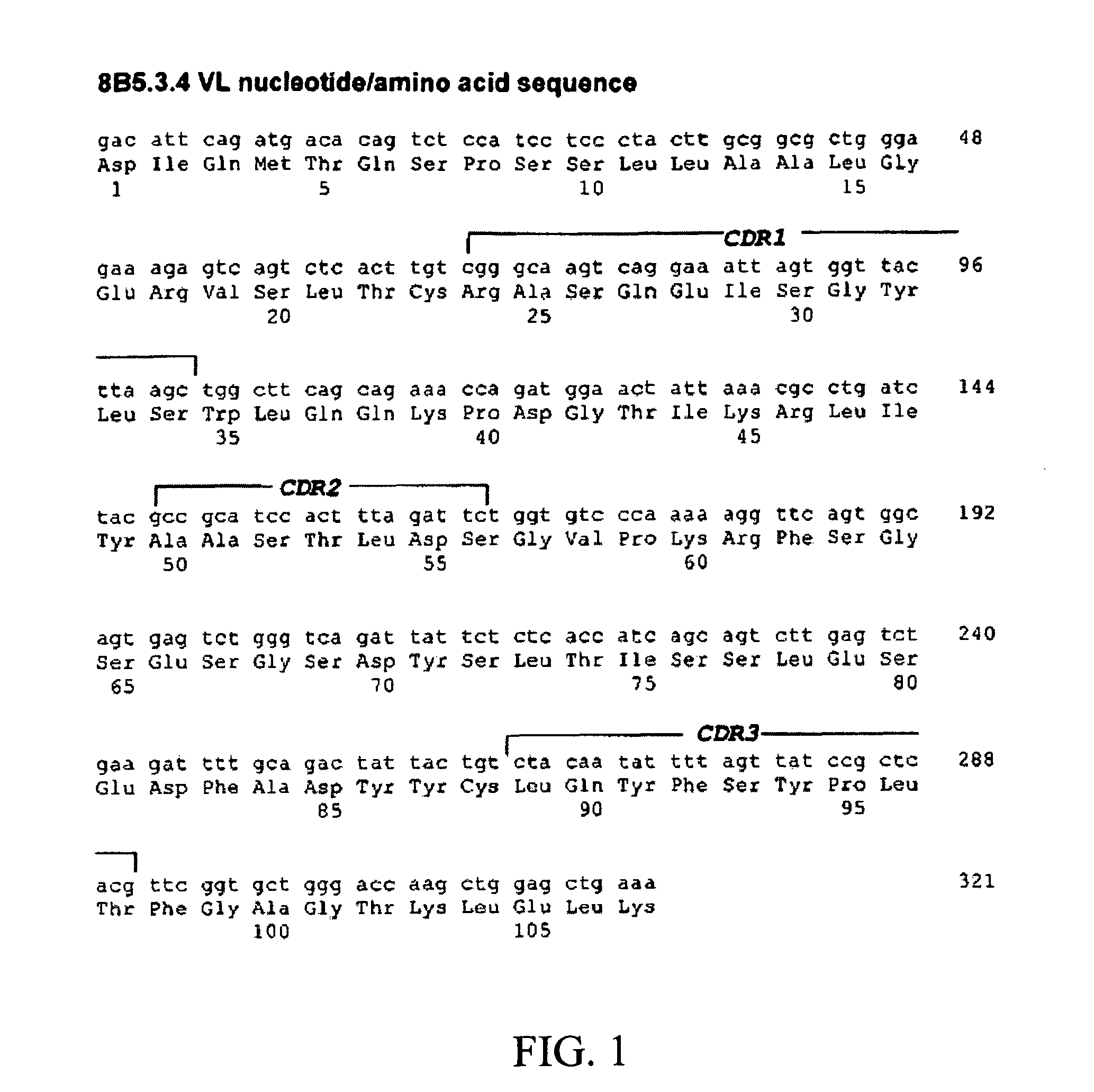
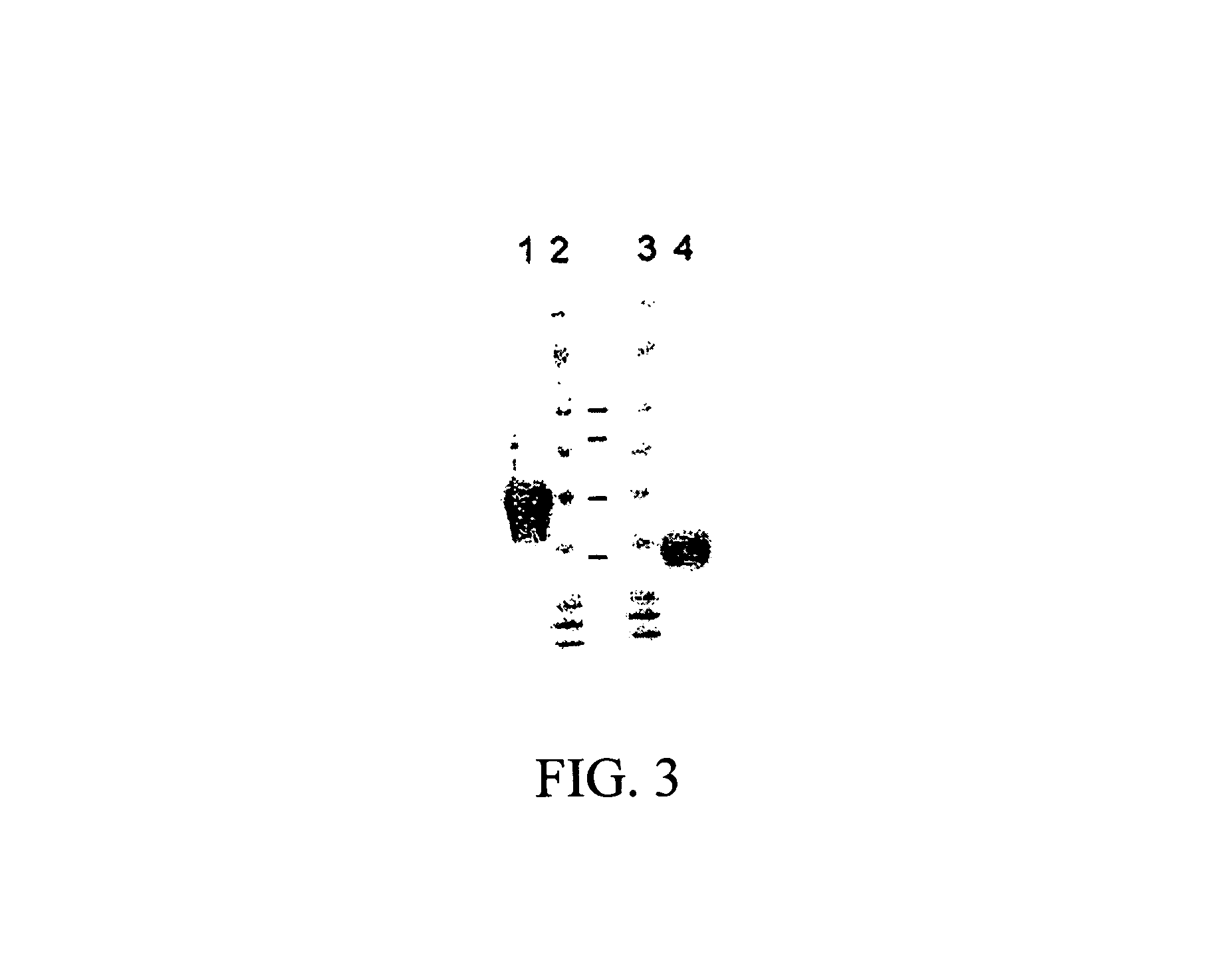
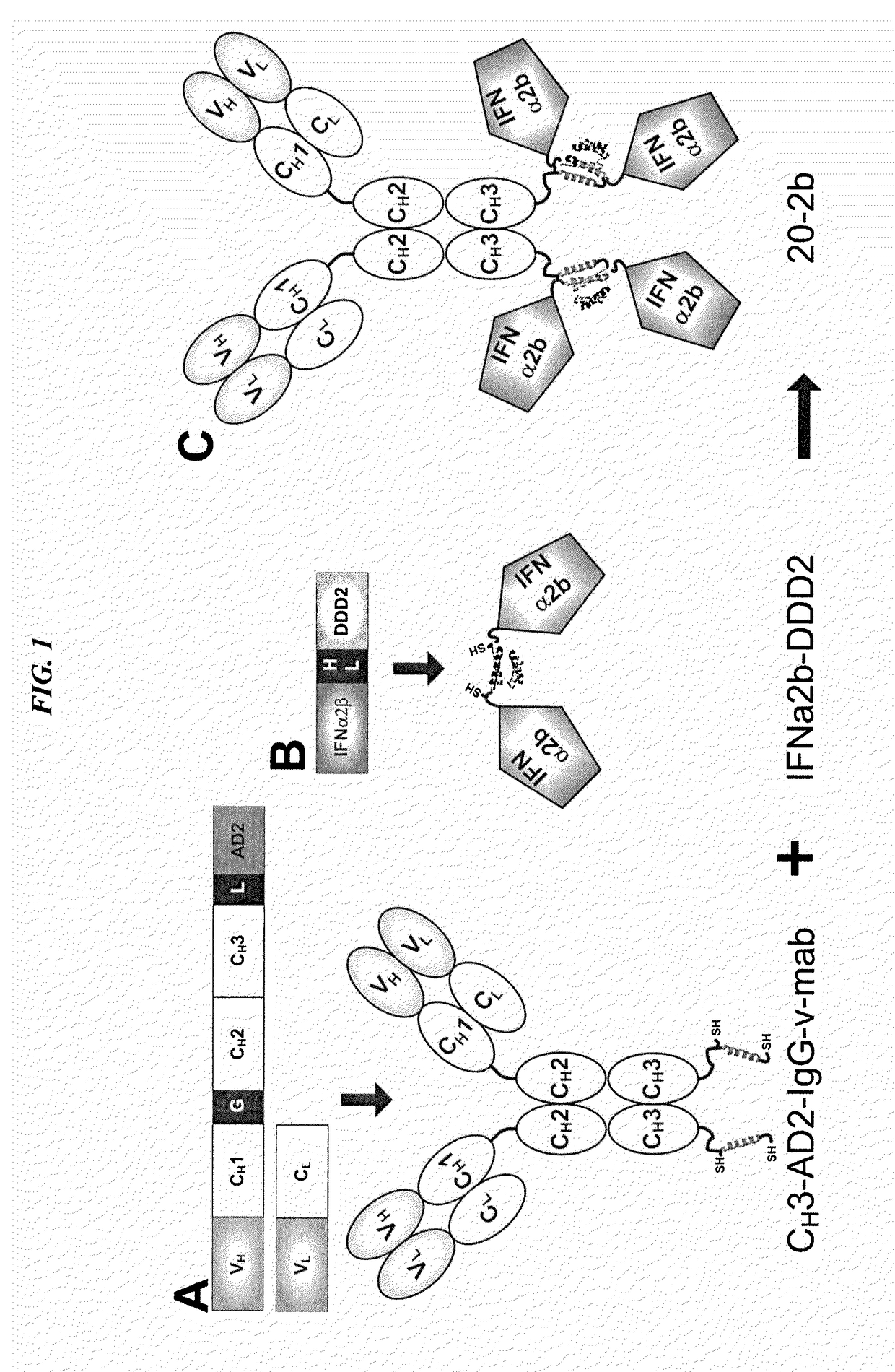
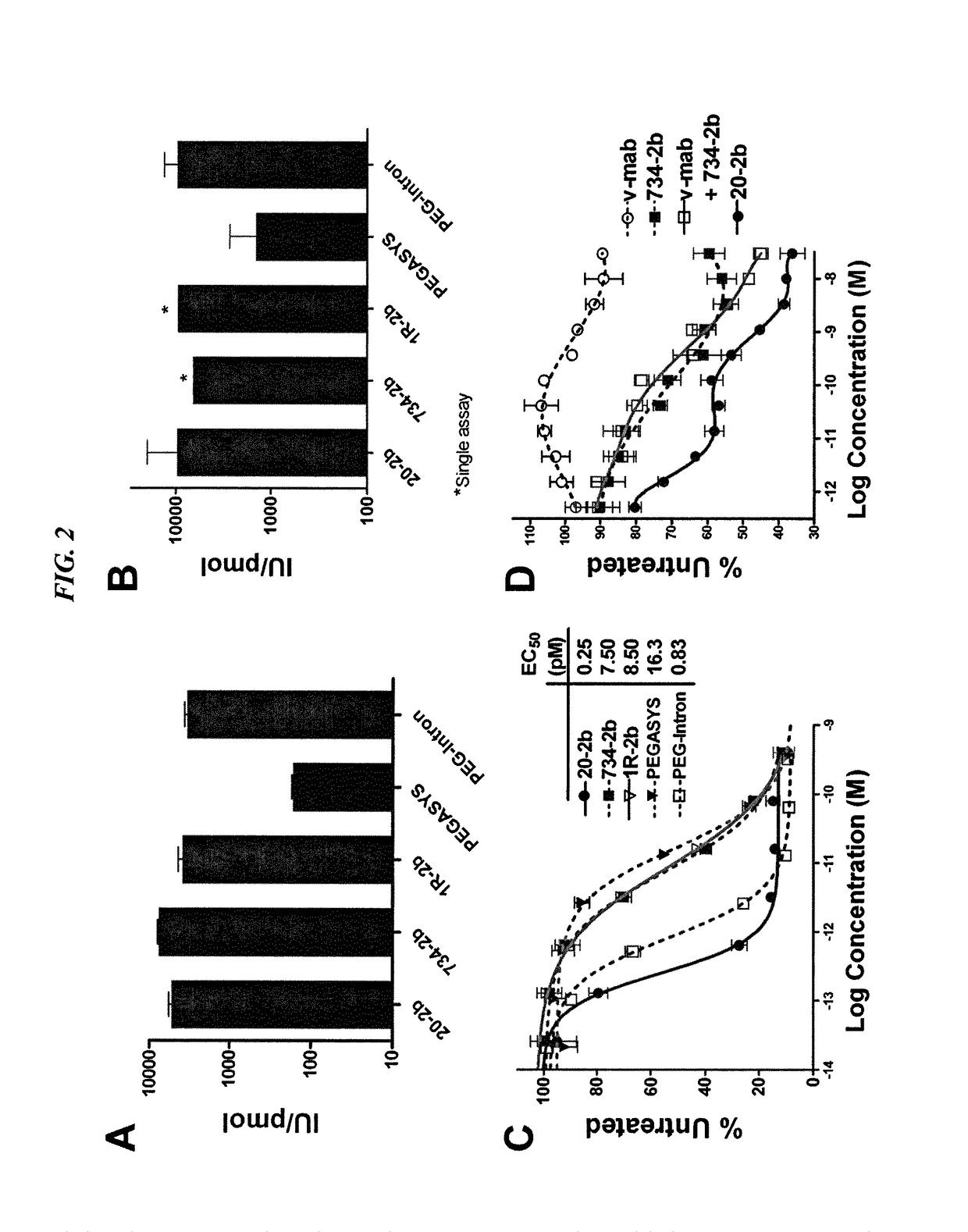
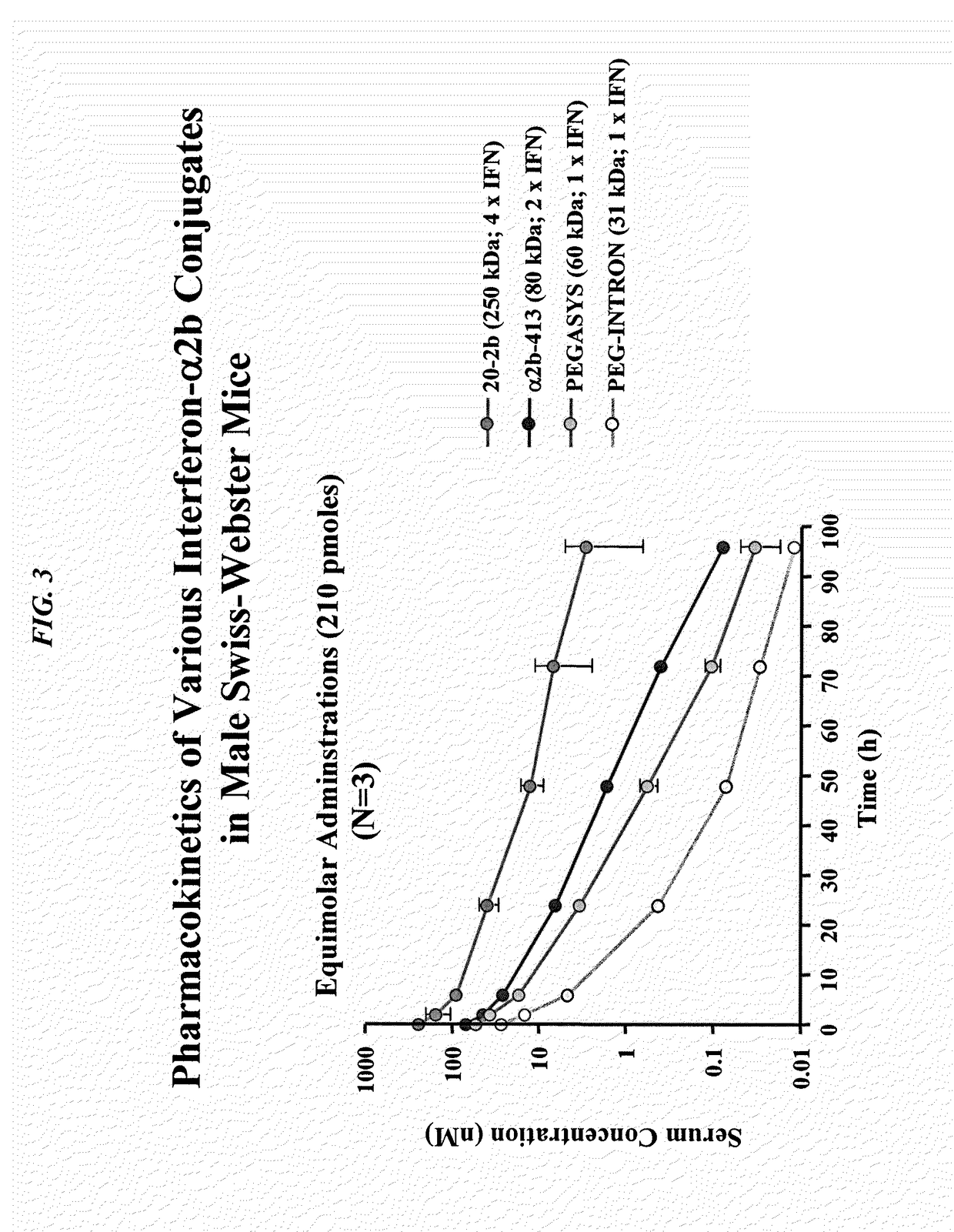
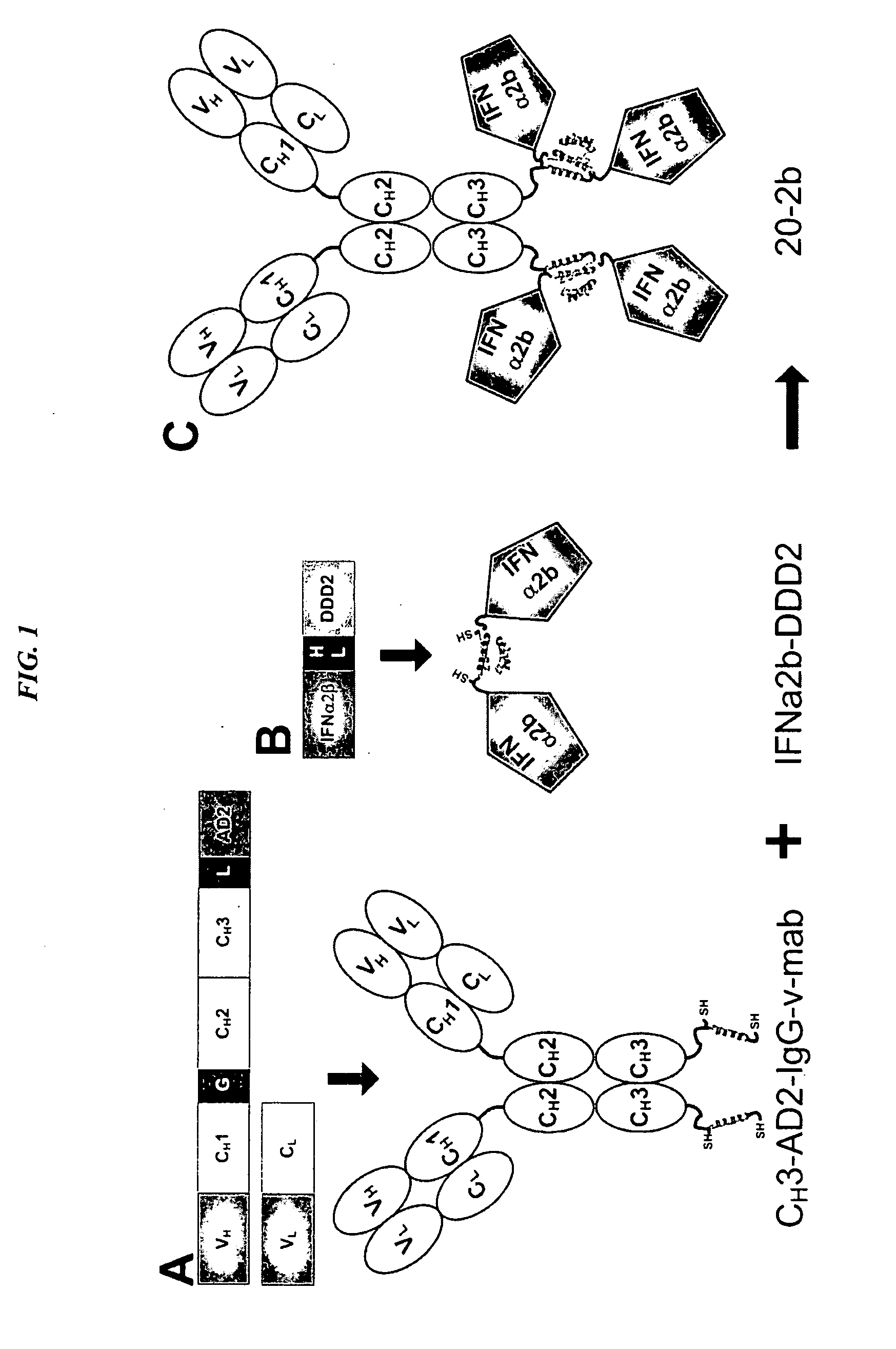
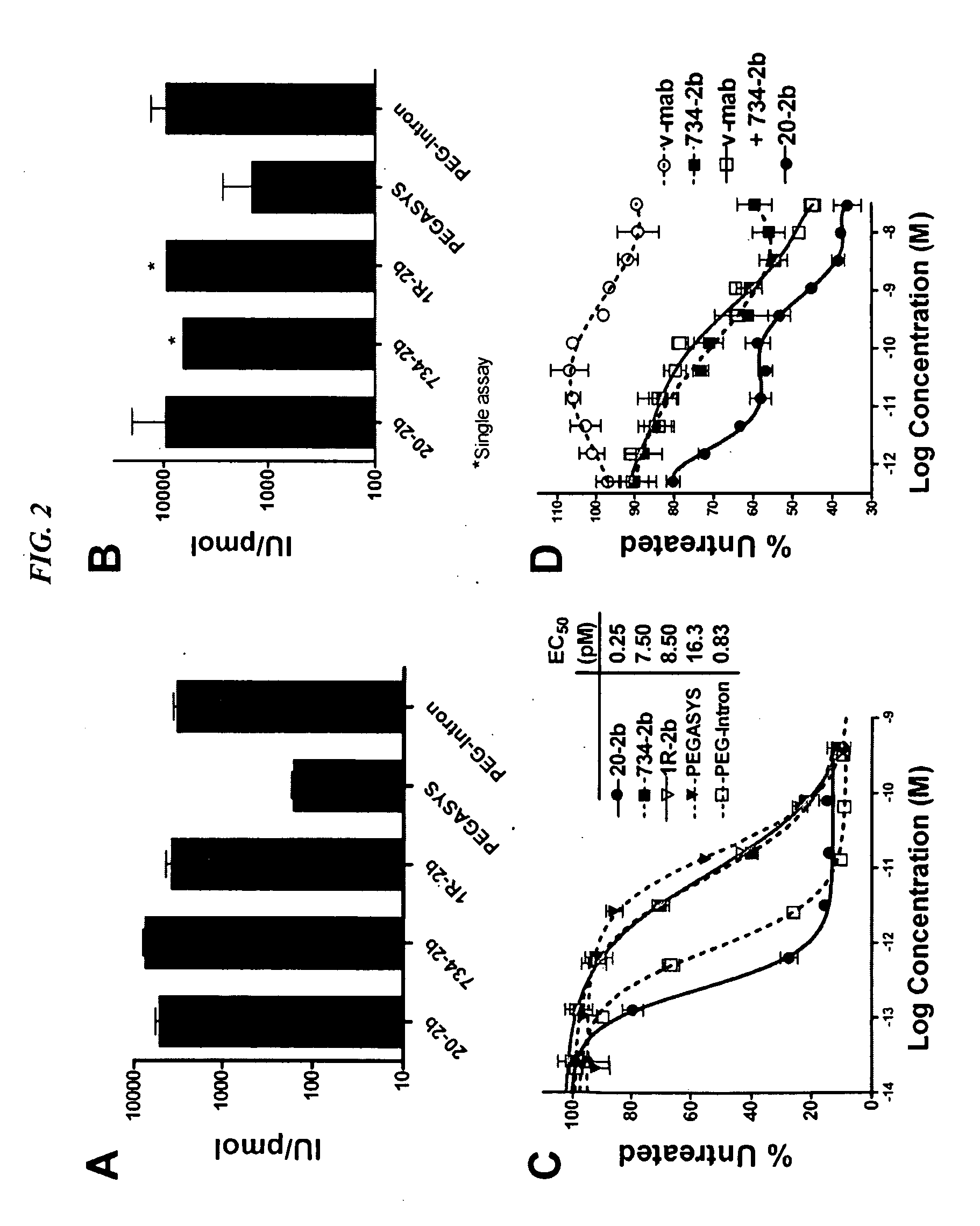
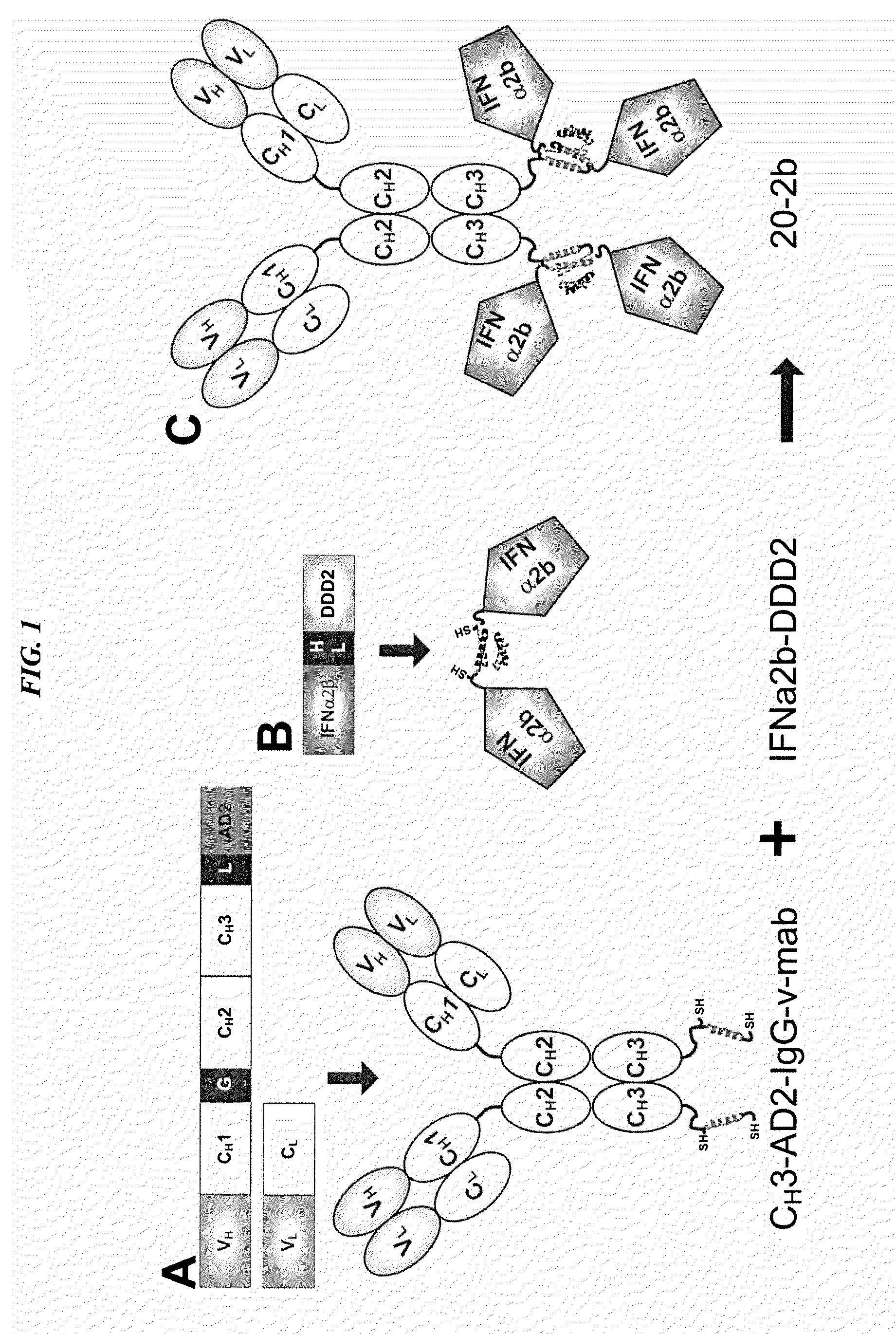
Modular method to prepare tetrameric cytokines with improved pharmacokinetics by the dock-and-lock (DNL) technology
The present invention concerns methods and compositions for forming cytokine-antibody complexes using dock-and-lock technology. In preferred embodiments, the cytokine-MAb DNL complex comprises an IgG antibody attached to two AD (anchor domain) moieties and four cytokines, each attached to a DDD (docking and dimerization domain) moiety. The DDD moieties form dimers that bind to the AD moieties, resulting in a 2:1 ratio of DDD to AD. The cytokine-MAb complex exhibits improved pharmacokinetics, with a significantly longer serum half-life than either naked cytokine or PEGylated cytokine. The cytokine-MAb complex also exhibits significantly improved in vitro and in vivo efficacy compared to cytokine alone, antibody alone, unconjugated cytokine plus antibody or cytokine-MAb DNL complexes incorporating an irrelevant antibody. In a most preferred embodiment the complex comprises an anti-CD20 IgG antibody conjugated to four IFN-α2b moieties, although other antibodies and cytokines have been used to form effect DNL complexes.
Owner:IBC PHARMACEUTICALS INC
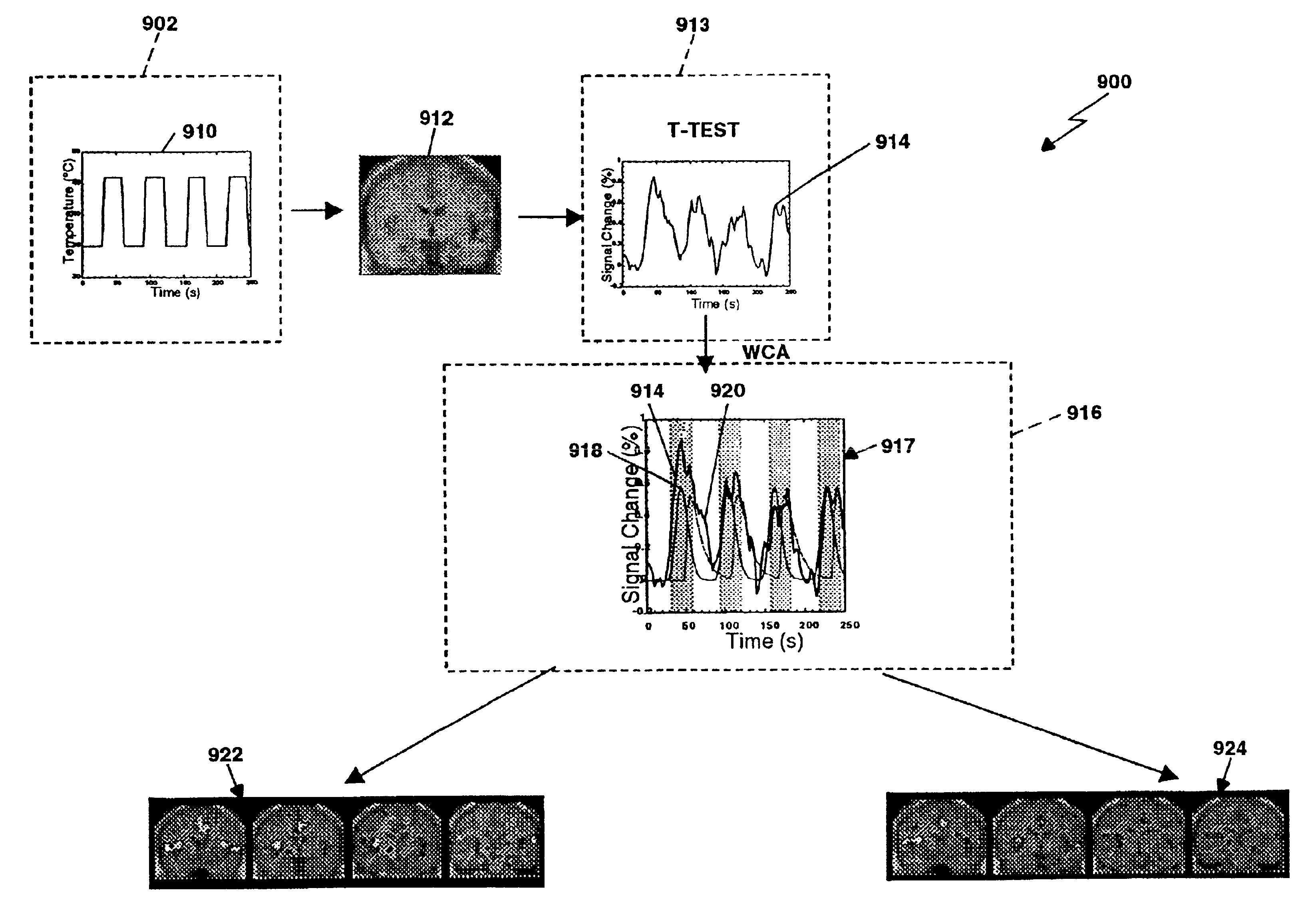
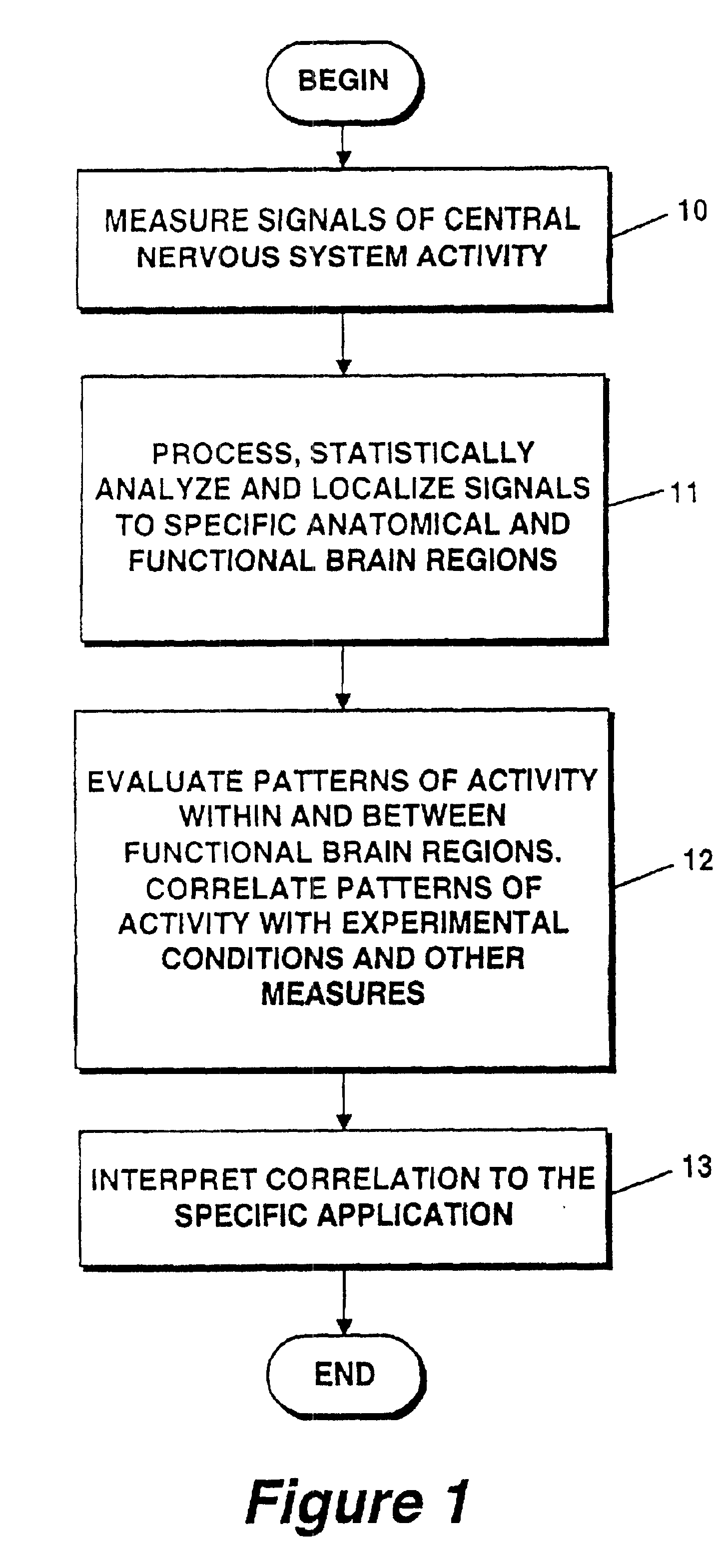
Method and apparatus for objectively measuring pain, pain treatment and other related techniques
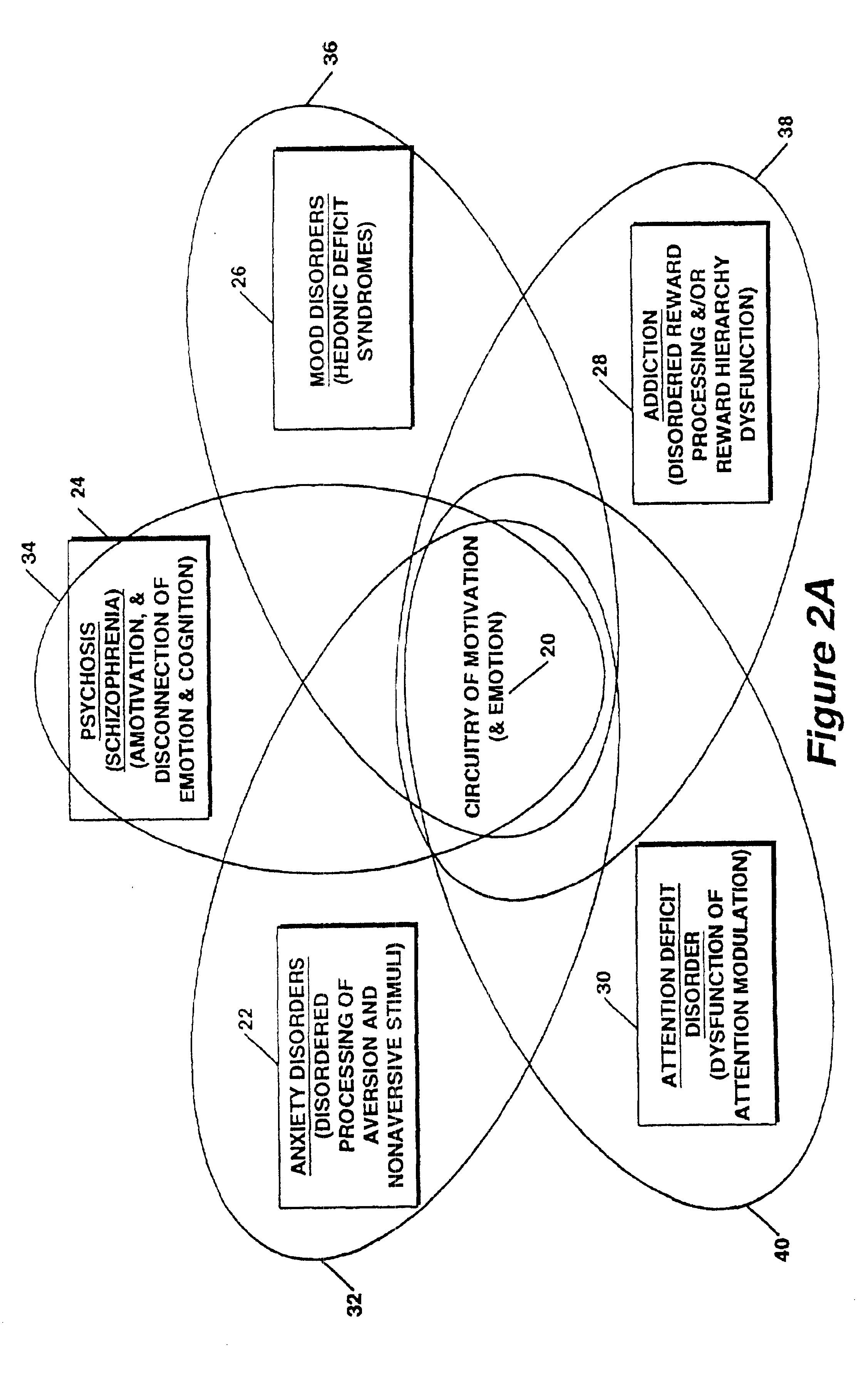
A method for measuring indices of brain activity includes non-invasively obtaining signals of central nervous system (CNS) activity, localizing signals to specific anatomical and functional CNS regions, correlating the signals from pain and reward brain regions, and interpreting the correlation results. The results of interpreting the correlation results can be used for objectively measuring, in individual humans or animals, their responses to motivationally salient stimuli including but not limited to stimuli which are internal or external, conscious or non-conscious, pharmacological or non-pharmacological therapies, and diseased based processes. This method for measuring brain activity in reward / aversive central nervous system regions, can further be used to determine the efficacy of compounds.
Owner:THE GENERAL HOSPITAL CORP
Modular Method to Prepare Tetrameric Cytokines with Improved Pharmacokinetics by the Dock-and-Lock (DNL) Technology
The present invention concerns methods and compositions for forming cytokine-antibody complexes using dock-and-lock technology. In preferred embodiments, the cytokine-MAb DNL complex comprises an IgG antibody attached to two AD (anchor domain) moieties and four cytokines, each attached to a DDD (docking and dimerization domain) moiety. The DDD moieties form dimers that bind to the AD moieties, resulting in a 2:1 ratio of DDD to AD. The cytokine-MAb complex exhibits improved pharmacokinetics, with a significantly longer serum half-life than either naked cytokine or PEGylated cytokine. The cytokine-MAb complex also exhibits significantly improved in vitro and in vivo efficacy compared to cytokine alone, antibody alone, unconjugated cytokine plus antibody or cytokine-MAb DNL complexes incorporating an irrelevant antibody. In a most preferred embodiment the complex comprises an anti-CD20 IgG antibody conjugated to four IFN-α2b moieties, although other antibodies and cytokines have been used to form effect DNL complexes.
Owner:IBC PHARMACEUTICALS INC
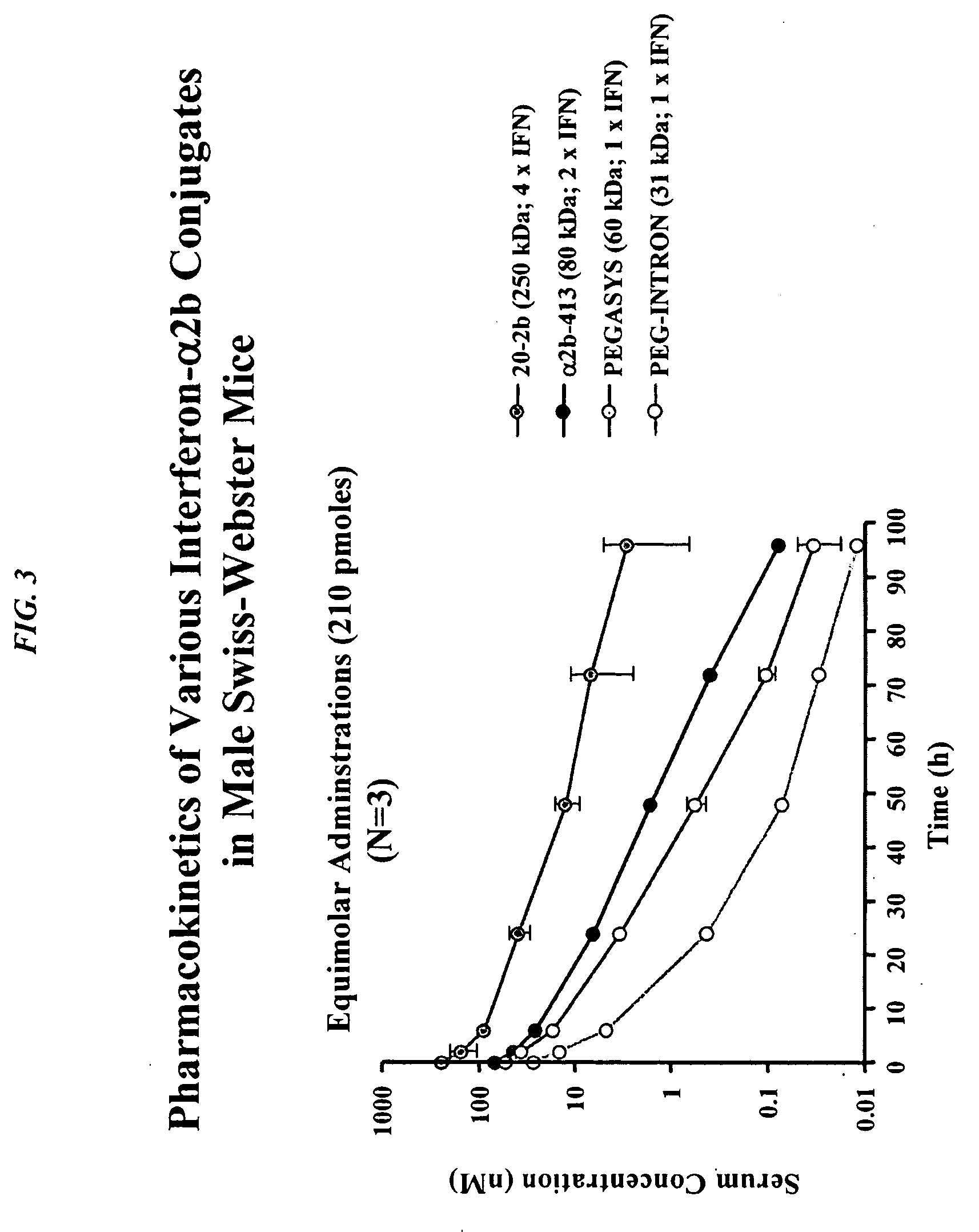
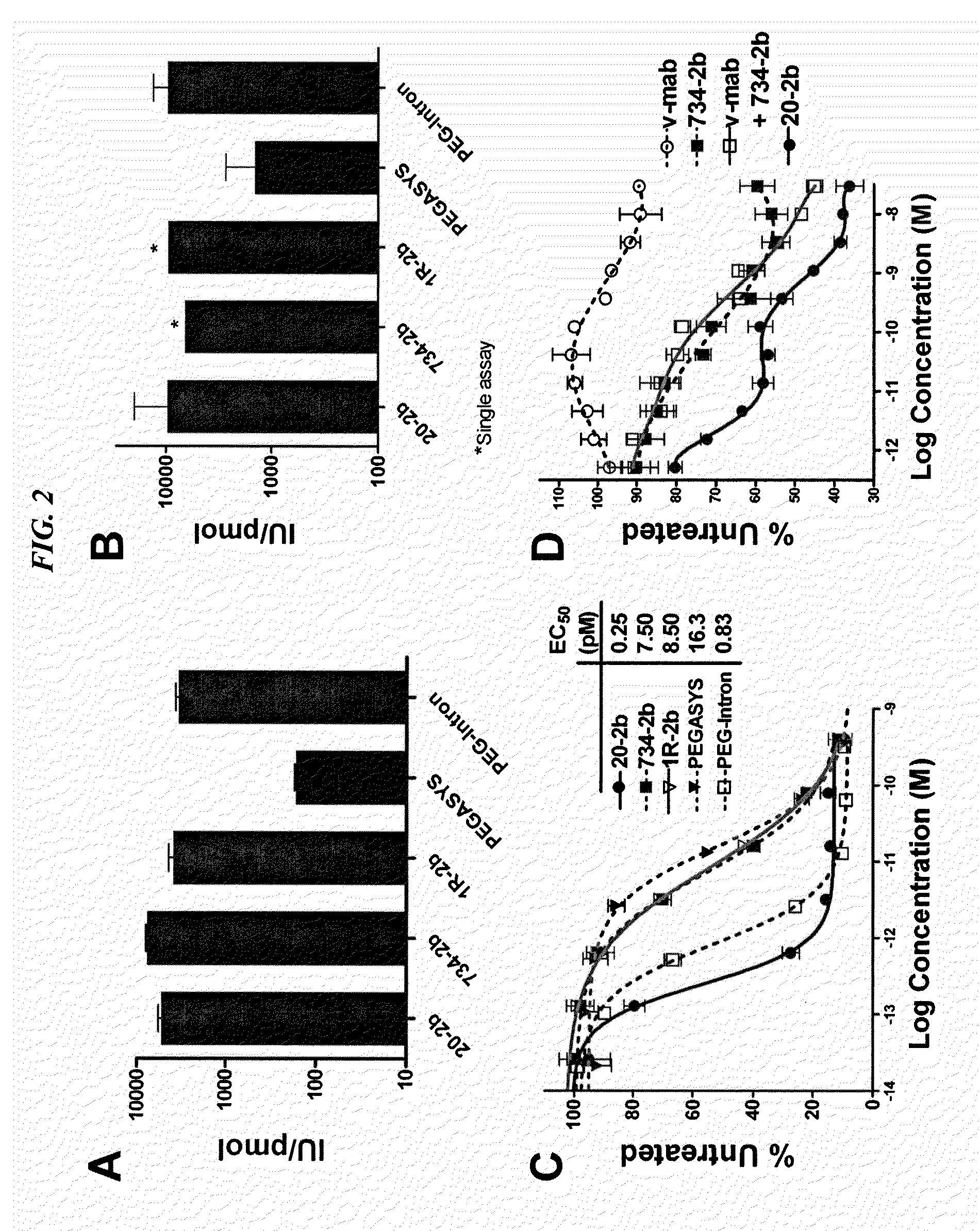
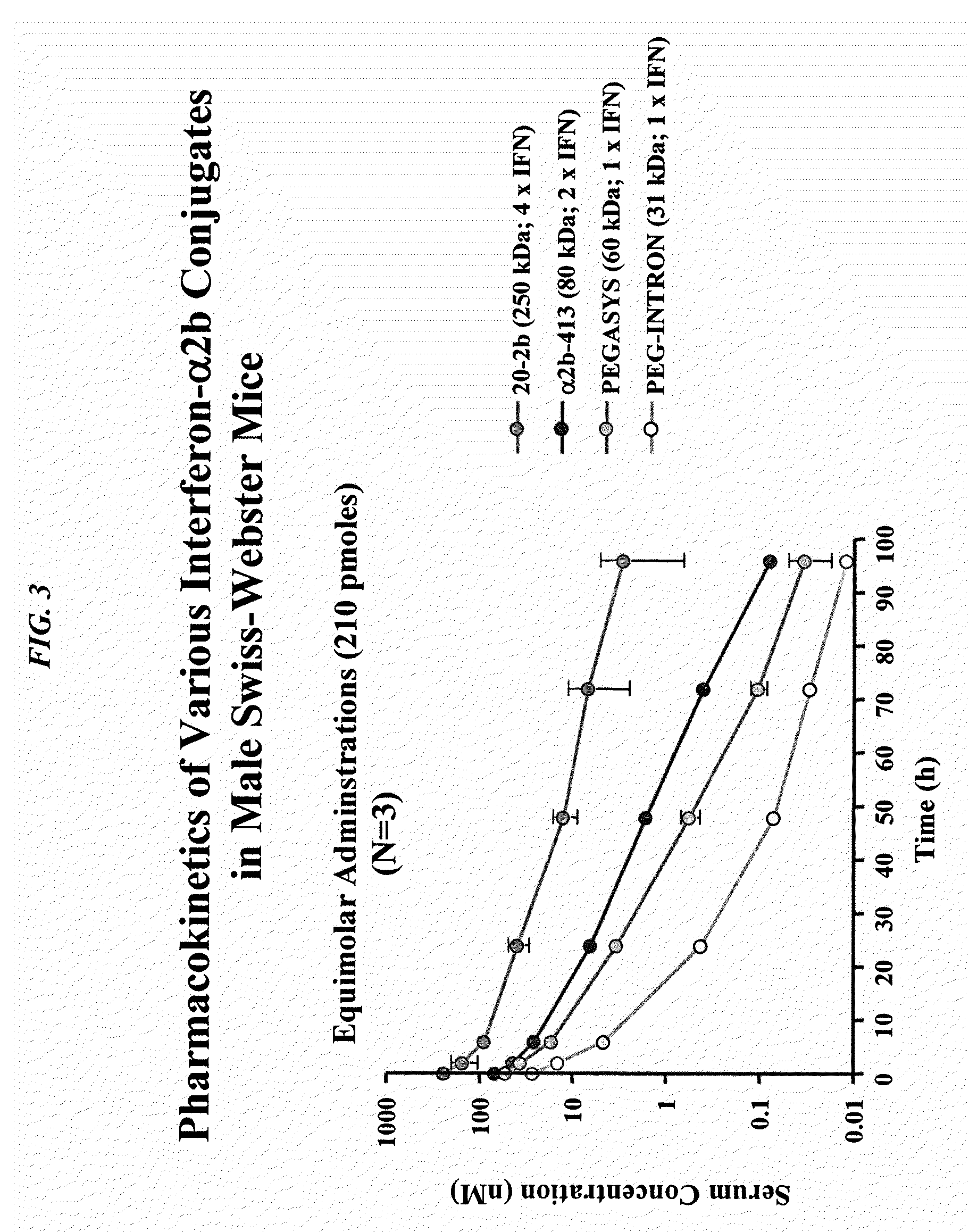
Dimeric alpha interferon pegylated site-specifically shows enhanced and prolonged efficacy in vivo
The present invention concerns methods and compositions for forming PEGylated complexes of defined stoichiometry and structure. In preferred embodiments, the PEGylated complex is formed using dock-and-lock technology, by attaching a therapeutic agent to a DDD sequence and attaching a PEG moiety to an AD sequence and allowing the DDD sequence to bind to the AD sequence in a 2:1 stoichiometry, to form PEGylated complexes with two therapeutic agents and one PEG moiety. In alternative embodiments, the therapeutic agent may be attached to the AD sequence and the PEG to the DDD sequence to form PEGylated complexes with two PEG moieties and one therapeutic agent. In more preferred embodiments, the therapeutic agent may comprise any peptide or protein of physiologic or therapeutic activity, preferably a cytokine, more preferably interferon-α2b. The PEGylated complexes exhibit a significantly slower rate of clearance when injected into a subject and are of use for treatment of a wide variety of diseases.
Owner:IBC PHARMACEUTICALS INC
Use of three-dimensional microfabricated tissue engineered systems for pharmacologic applications
ActiveUS20060019326A1Additive manufacturing apparatusMicrobiological testing/measurementExperimental drugSide effect
The present invention generally relates to a combination of the fields of tissue engineering, drug discovery and drug development. It more specifically provides new methods and materials for testing the efficacy and safety of experimental drugs, defining the metabolic pathways of experimental drugs and characterizing the properties (e.g., side effects, new uses) of existing drugs. Preferably, evaluation is carried out in three-dimensional tissue-engineered systems, wherein drug toxicity, metabolism, interaction and / or efficacy can be determined.
Owner:CHARLES STARK DRAPER LABORATORY +1
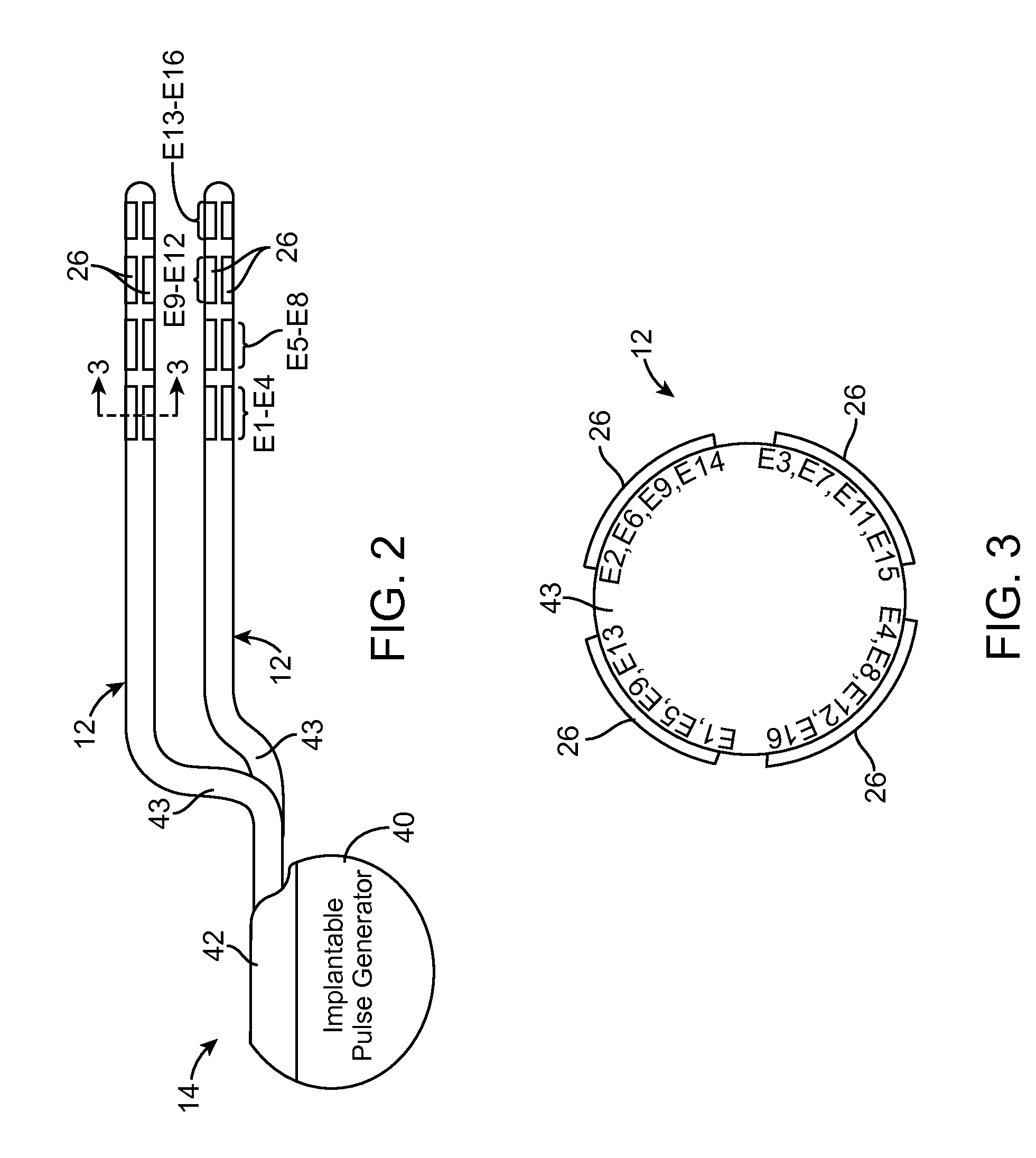
Neurostimulation system for selectively estimating volume of activation and providing therapy
An external control device, neurostimulation system, and method of programming a neurostimulator. A volume of tissue activation for each of a first one or more candidate stimulation parameter sets is simulated without conveying electrical stimulation energy into the tissue. One of the first candidate stimulation parameter set(s) is selected based on each simulated volume of tissue activation. Electrical stimulation energy is conveyed into the tissue in accordance with a second one or more candidate stimulation parameter sets, wherein the initial one of the second candidate stimulation parameter set(s) is the selected one of the first candidate stimulation parameter set(s). One of the second candidate stimulation parameter set(s) is selected based on a therapeutic efficacy of the electrical stimulation energy conveyed into the tissue. The neurostimulator is programmed with the selected one of the second candidate stimulation parameter set(s).
Owner:BOSTON SCI NEUROMODULATION CORP
Dilating and support apparatus with disease inhibitors and methods for use
A dilating and support apparatus with disease inhibitors and methods for use is disclosed that is particularly useful for repairing and / or serving as a conduit for body passageways that require reinforcement, dilatation, disease prevention or the like. Such apparatuses are utilized to deliver a therapy, that therapy being from a family of devices, drugs, or any of a variety of other elements to a specific location within the body. The instant disclosure provides a system of combining a novel radial deployment and / or drug delivery therapy with existing balloon dilatation therapy into one device. This combination will yield a significant decrease in cost to the healthcare system as well as providing a therapy to the patient with increased safety and efficacy. Further, the instant invention provides a novel and improved platform for synthetic / tissue interface between the device and the body.
Owner:ETHICON ENDO SURGERY INC
Tetrameric cytokines with improved biological activity
ActiveUS8034352B2Keep for a long timeLow toxicityMaterial nanotechnologyPeptide/protein ingredientsSerum igeHalf-life
The present invention concerns methods and compositions for forming cytokine-antibody complexes using dock-and-lock technology. In preferred embodiments, the cytokine-MAb DNL complex comprises an IgG antibody attached to two AD (anchor domain) moieties and four cytokines, each attached to a DDD (docking and dimerization domain) moiety. The DDD moieties form dimers that bind to the AD moieties, resulting in a 2:1 ratio of DDD to AD. The cytokine-MAb complex exhibits improved pharmacokinetics, with a significantly longer serum half-life than either naked cytokine or PEGylated cytokine. The cytokine-MAb complex also exhibits significantly improved in vitro and in vivo efficacy compared to cytokine alone, antibody alone, unconjugated cytokine plus antibody or cytokine-MAb DNL complexes incorporating an irrelevant antibody. In more preferred embodiment the cytokine is G-CSF, erythropoietin or INF-α2b.
Owner:IBC PHARMACEUTICALS INC
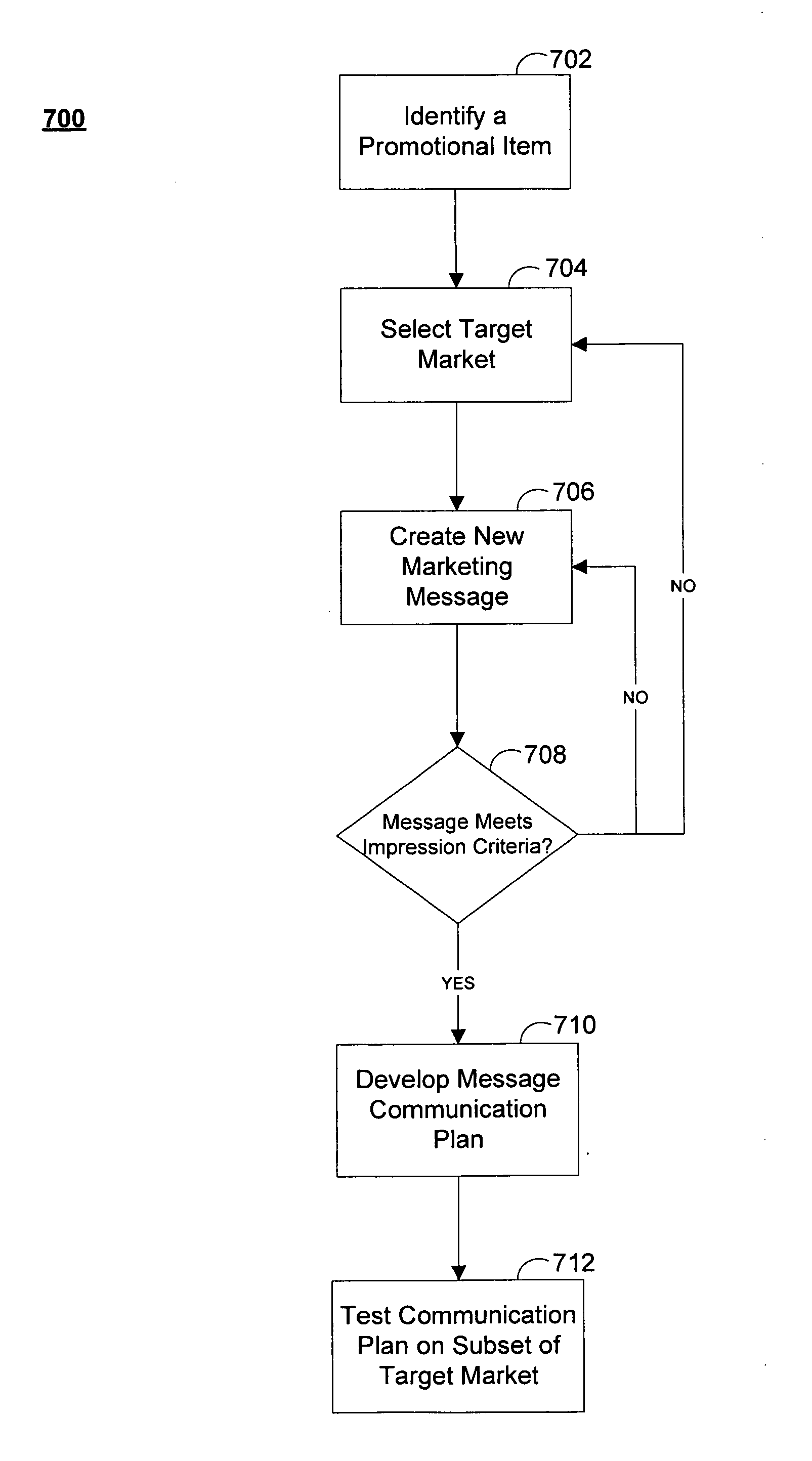
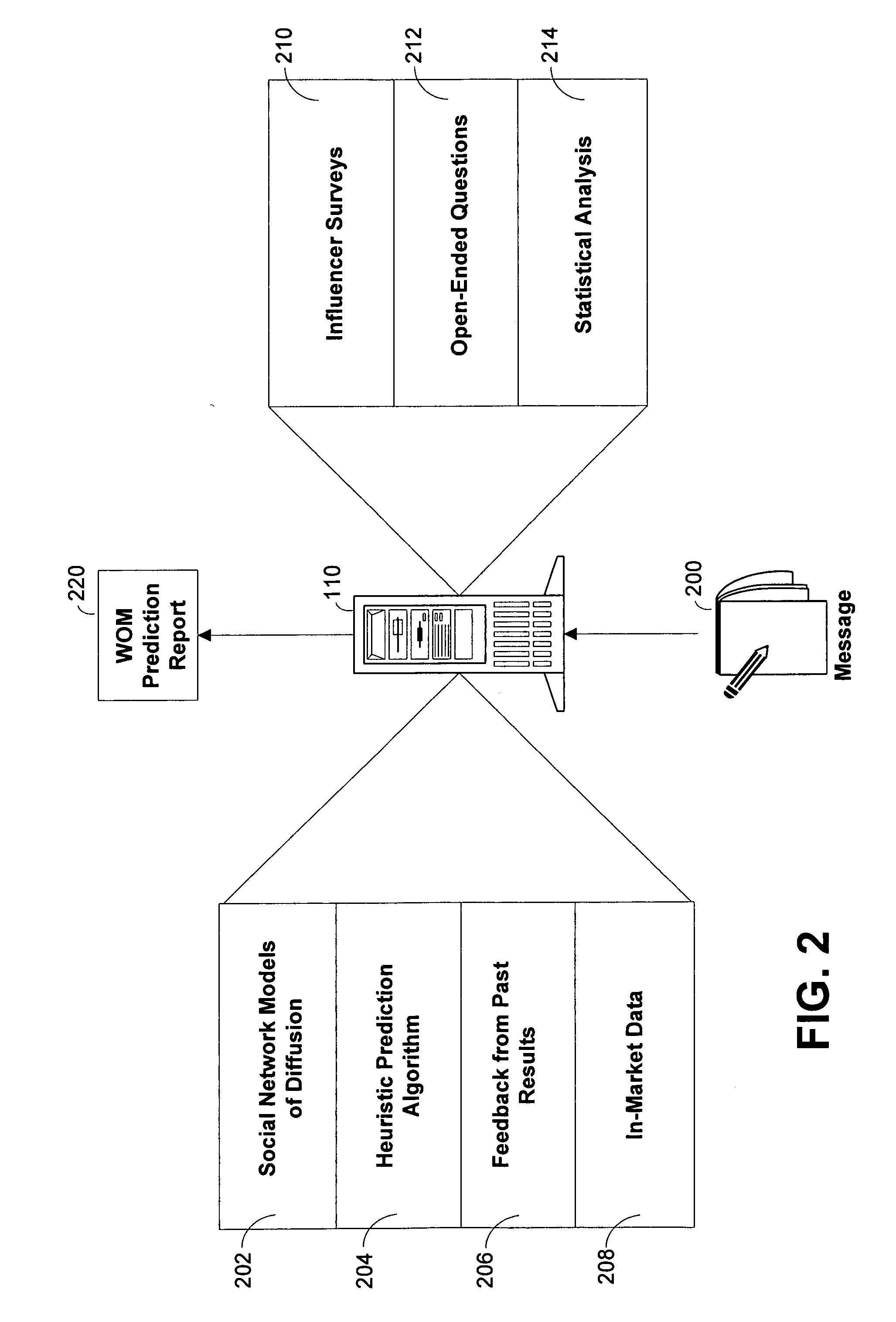
Systems and methods for predicting the efficacy of a marketing message
Systems and methods for predicting the efficacy of a marketing message to generate word of mouth (WOM) are disclosed. A prediction server may generate and distribute surveys to a subset of a target market group. The prediction server may then analyze the survey responses and compare the responses to past responses for marketing messages in similar product or service categories. One or more scores relating to the message's ability to generate WOM may then be computed. These scores may reflect purchase intent, message advocacy, and message amplification of the marketing message. The marketing message may then be refined based on the value of the one or more scores. The prediction server may also access in-market data in order to project volume build or equity build of a promotional item associated with the marketing message.
Owner:THE PROCTER & GAMBLE COMPANY
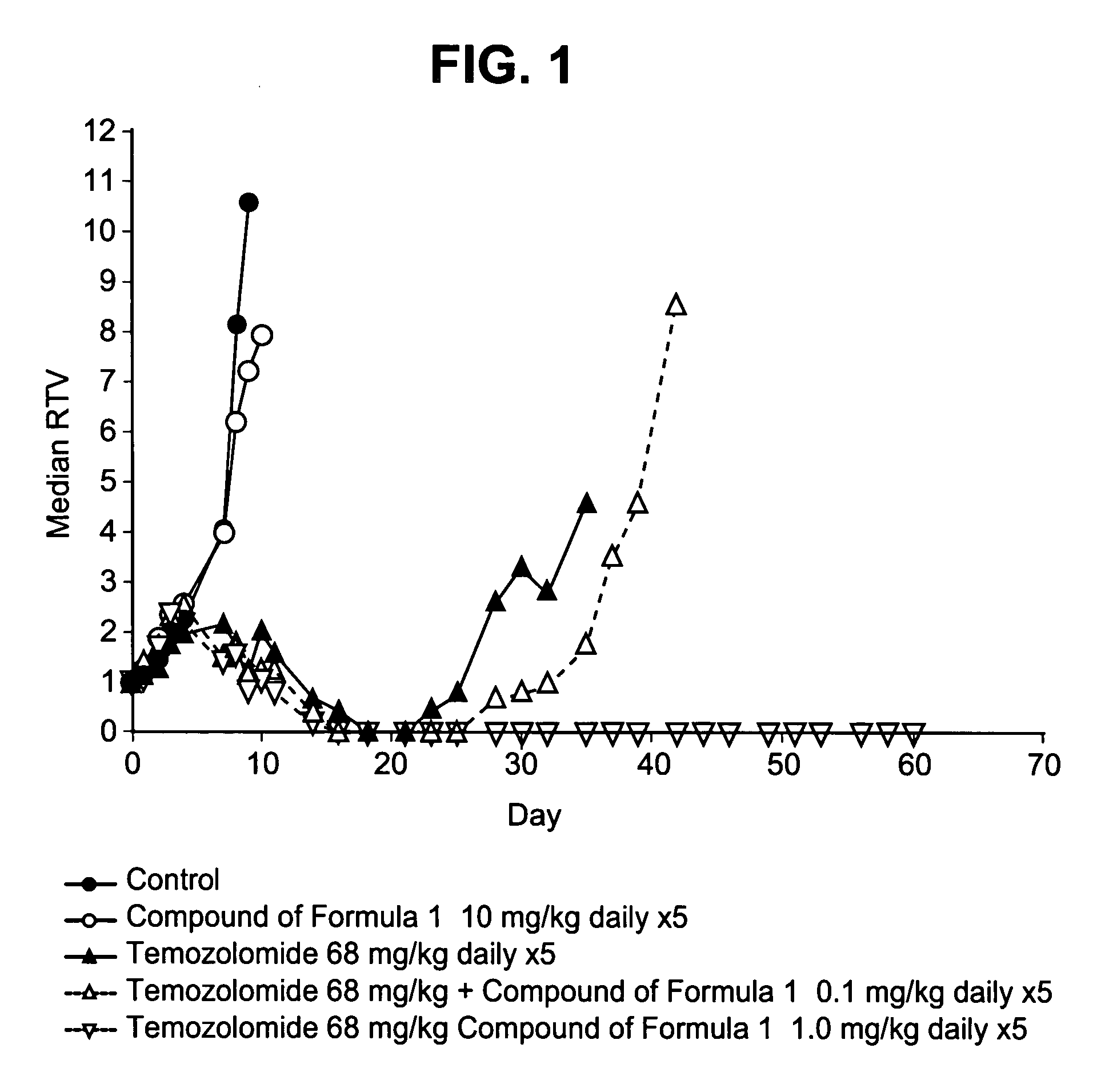
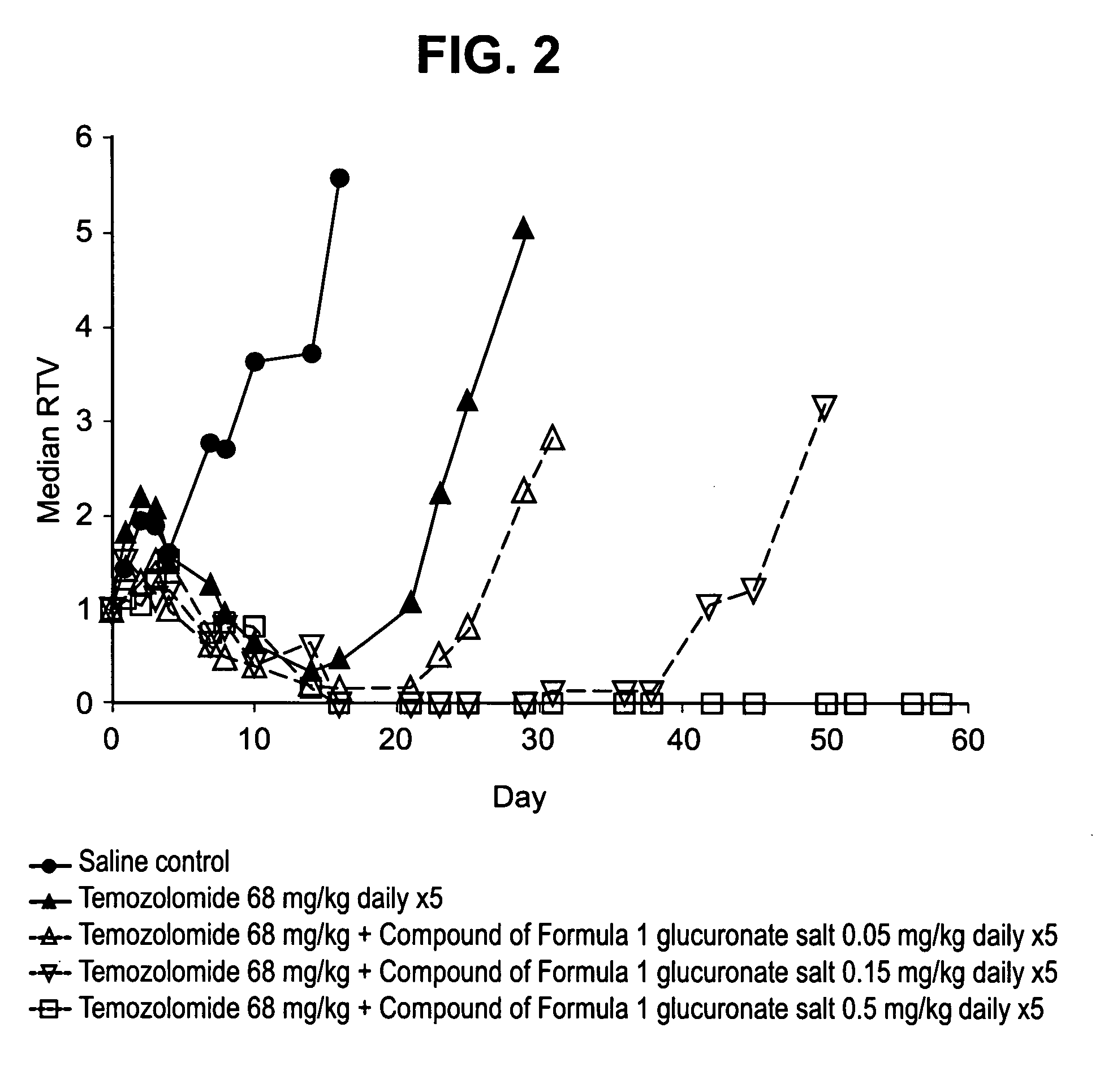
Therapeutic combinations comprising poly (ADP-ribose) polymerases inhibitor
This invention generally relates to use of 8-fluoro-2{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one represented by formula 1 as a chemosensitizer that enhances the efficacy of cytotoxic drugs or radiotherapy. This invention provides pharmaceutical combinations of 8-fluoro-2{4-[(methylamino)methyl]phenyl}-1,3,4,5-tetrahydro-6H-azepino[5,4,3-cd]indol-6-one, or a pharmaceutically acceptable salt thereof and at least one additional therapeutic agent, kits containing such combinations and methods of using such combinations to treat subjects suffering from diseases such as cancer.
Owner:AGOURON PHARMA INC +1
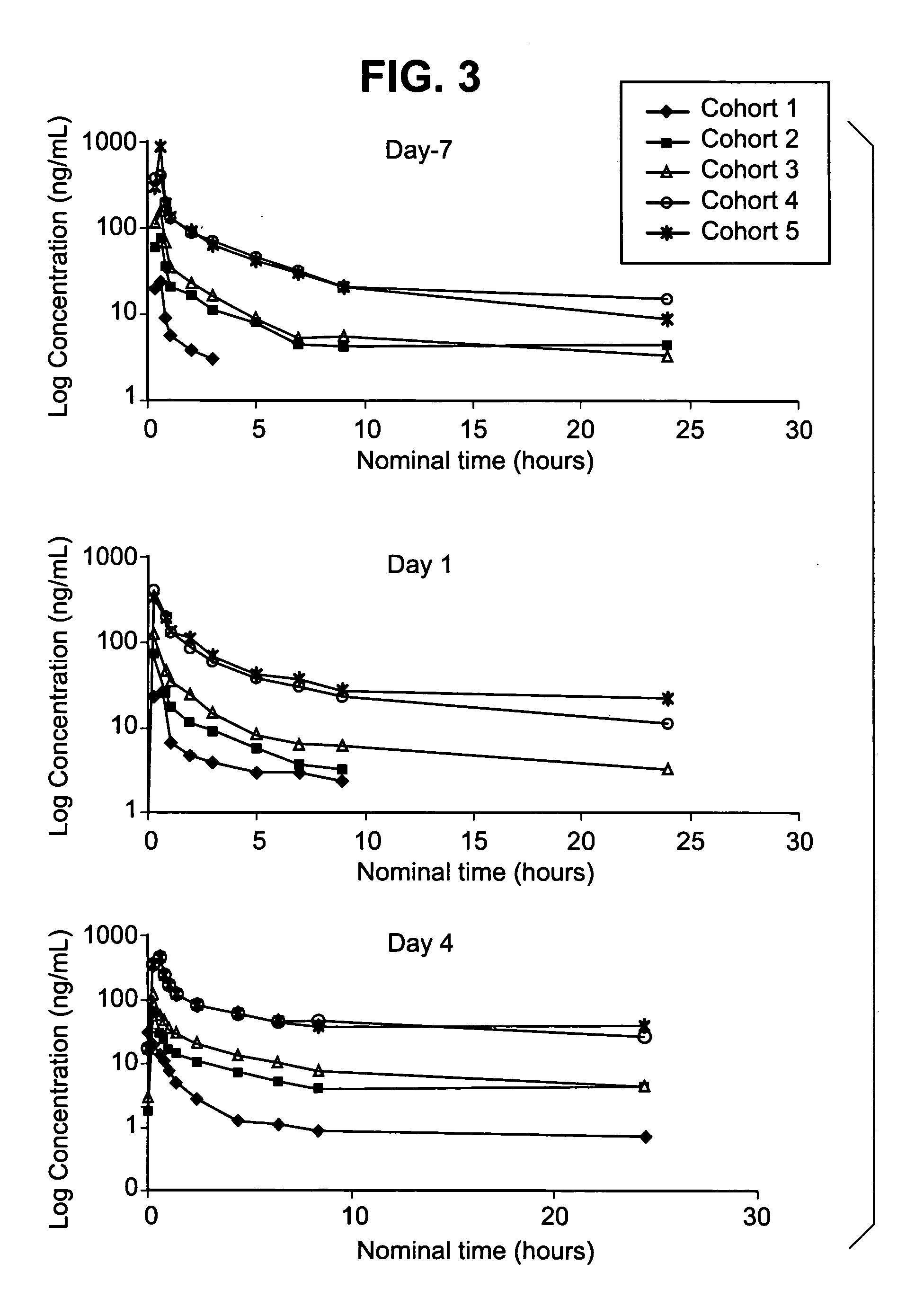
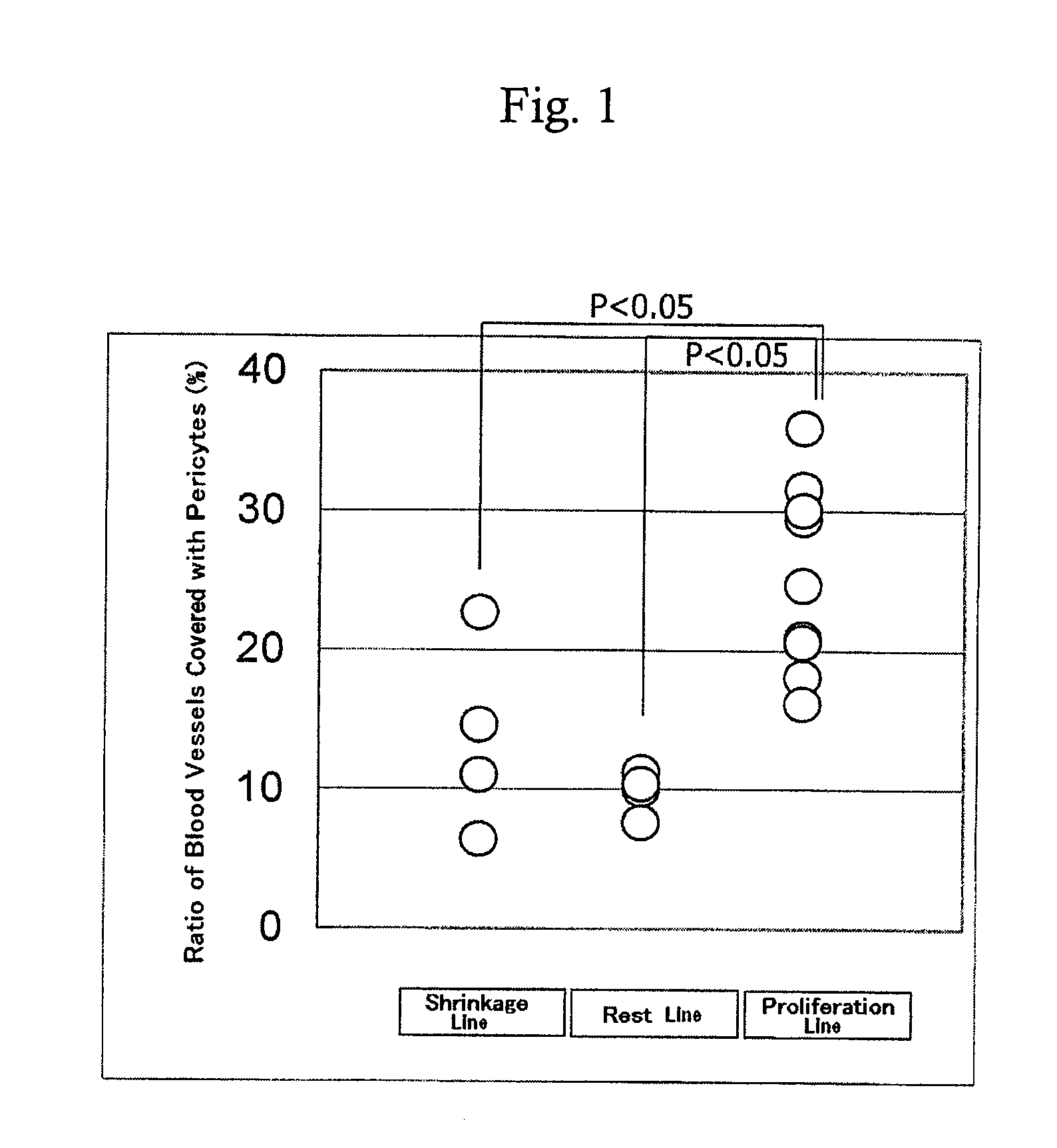
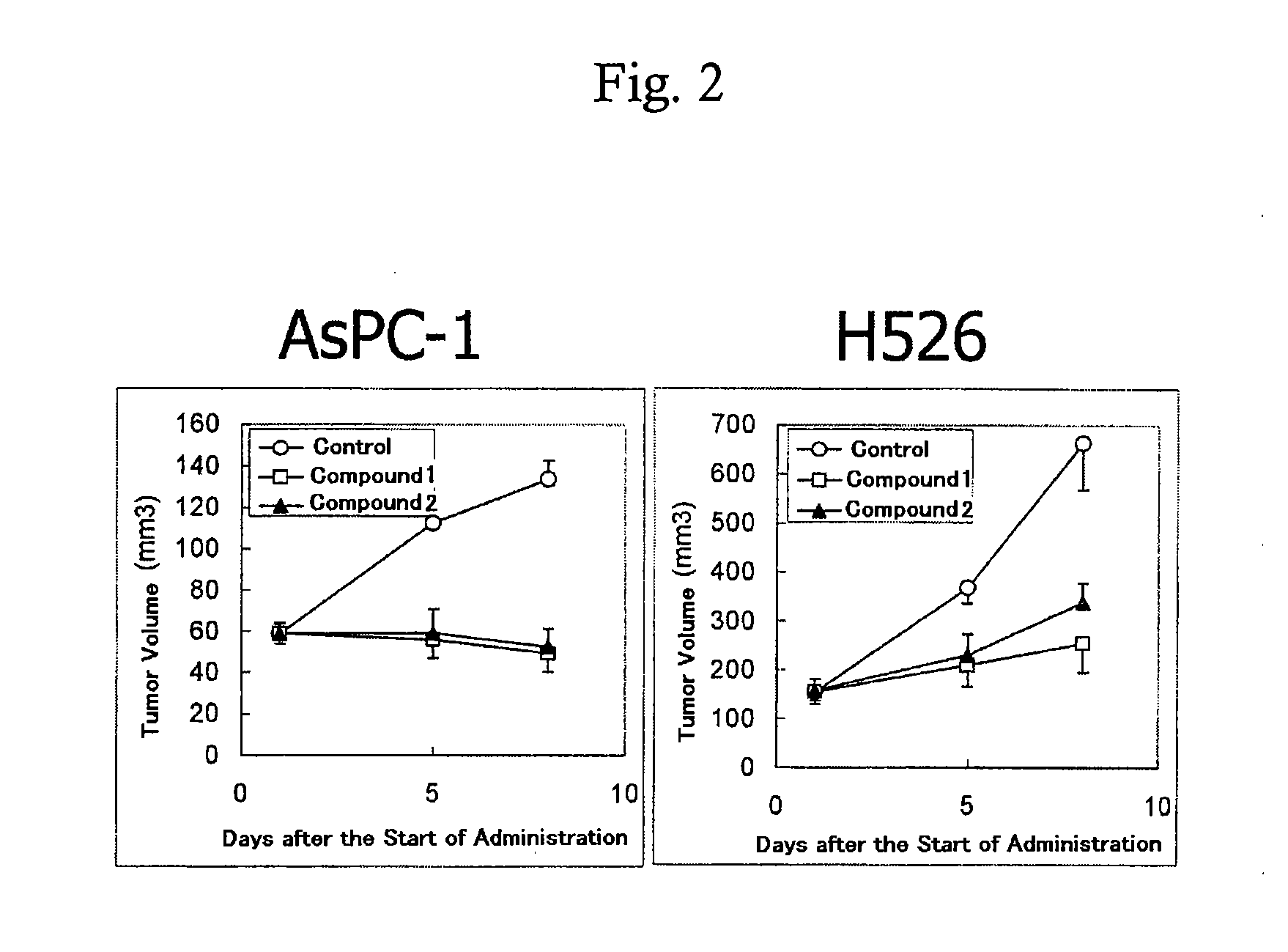
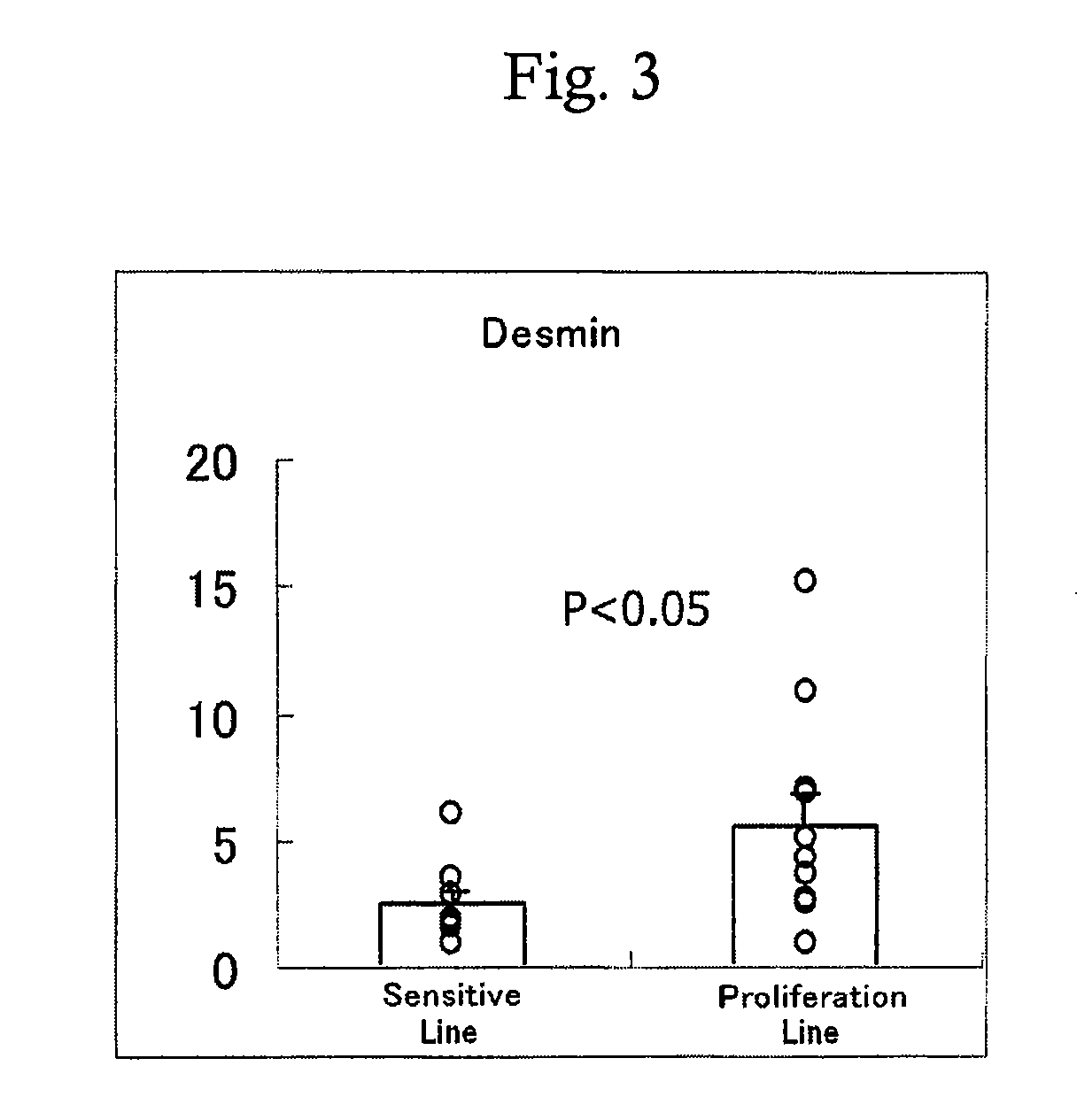
Method for prediction of the efficacy of vascularization inhibitor
InactiveUS20100105031A1Predict antitumor effectImprove anti-tumor effectMicrobiological testing/measurementDisease diagnosisAbnormal tissue growthCancer research
Disclosed is a method for the prediction of the efficacy of a vascularization inhibitor. In the method, the anti-tumor effect of a vascularization inhibitor can be predicted by measuring the number of blood vessels surrounded by pericytes in a tumor and using the measurement value as a measure for the anti-tumor effect.
Owner:EISIA R&D MANAGEMENT CO LTD
MiRNA with cell corpuscule as vector and preparation research approach thereof and application
InactiveCN101386848AEasy to storeWide detection rangeNervous disorderGenetic material ingredientsEfficacyDisease complication
The invention discloses micro ribonucleic acids (microRNA, miRNA) carried by cell microparticles (Microparticle, MP), a method for preparing the same, and application thereof in the technical field of biotechnological pharmacy. The invention provides a combination of the micro ribonucleic acids for evaluating the physiological and / or pathological states of a participant, and the combination contains all the micro ribonucleic acids which exist stably in serum / plasma particles of the participant and are detectable. At the same time, the invention provides an experimental method for preparing the cell microparticles containing specific micro ribonucleic acids and using the cell microparticles to perform gene-level regulation and control as well as modification on other cells and tissues. The combination and the method can be used for detecting and treating various diseases, including the aspects of the diagnosis and the differential diagnosis of various tumors, various acute and chronic infectious diseases and other acute and chronic diseases, the prediction and the curative effect evaluation of the occurrences of disease complications and the recurrences of malignant diseases, as well as the active ingredient screening, the efficacy evaluation and the judicial authentication of drugs, the detection of prohibited drugs and the like; besides, the combination and the method have the advantages of wide detection pedigree, high sensitivity, low detection cost, convenient available material, easy storage of samples and the like.
Owner:NANJING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com