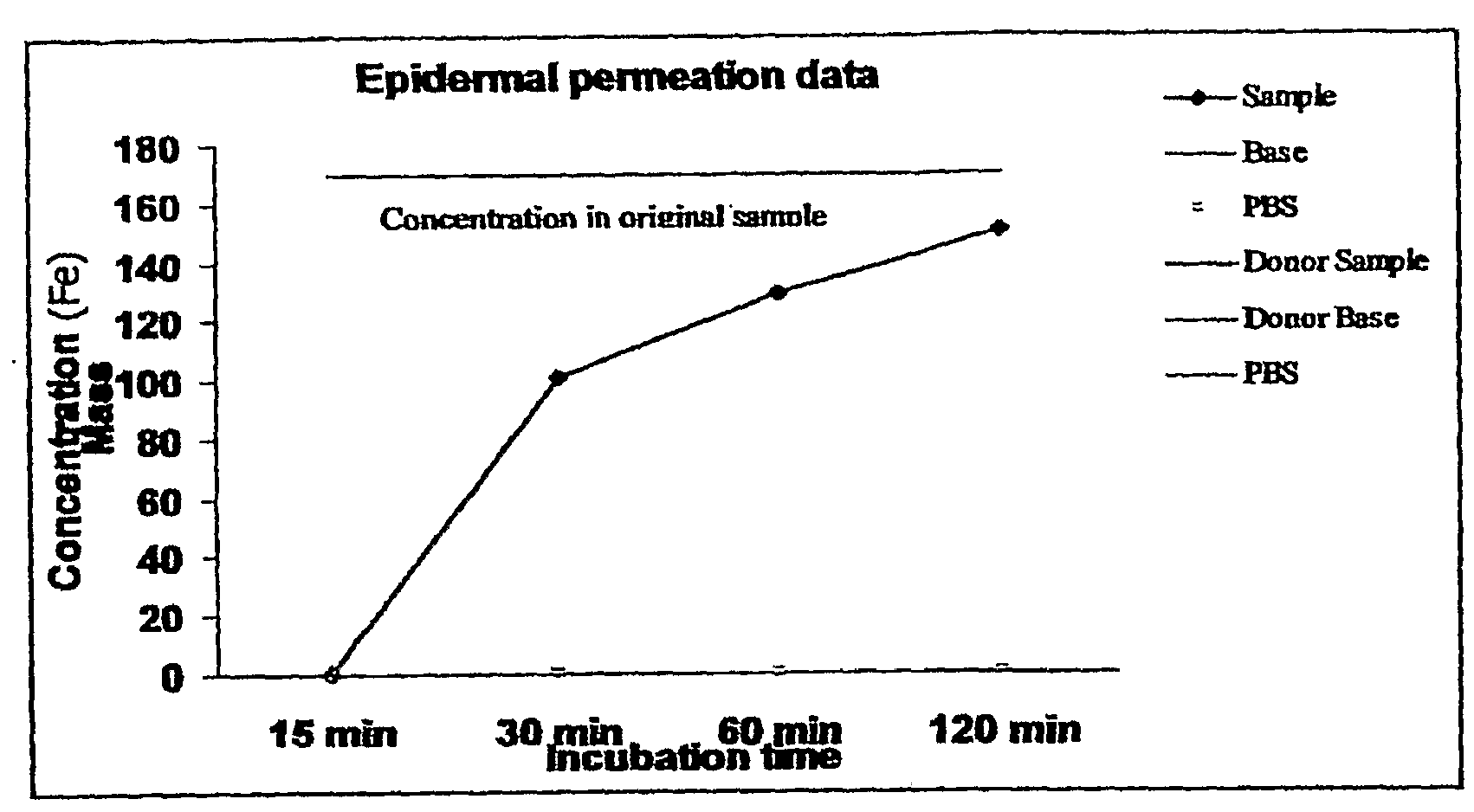
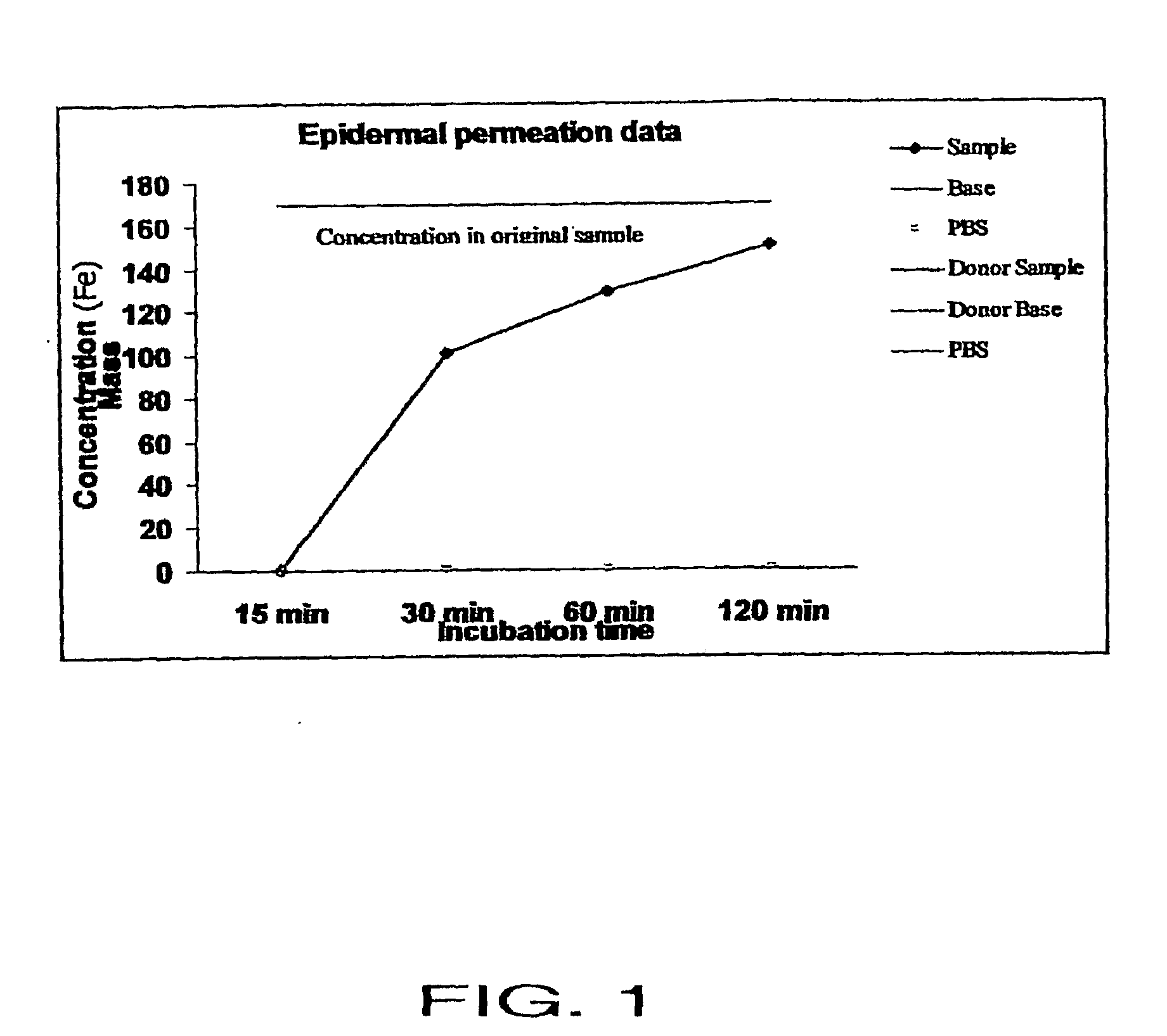
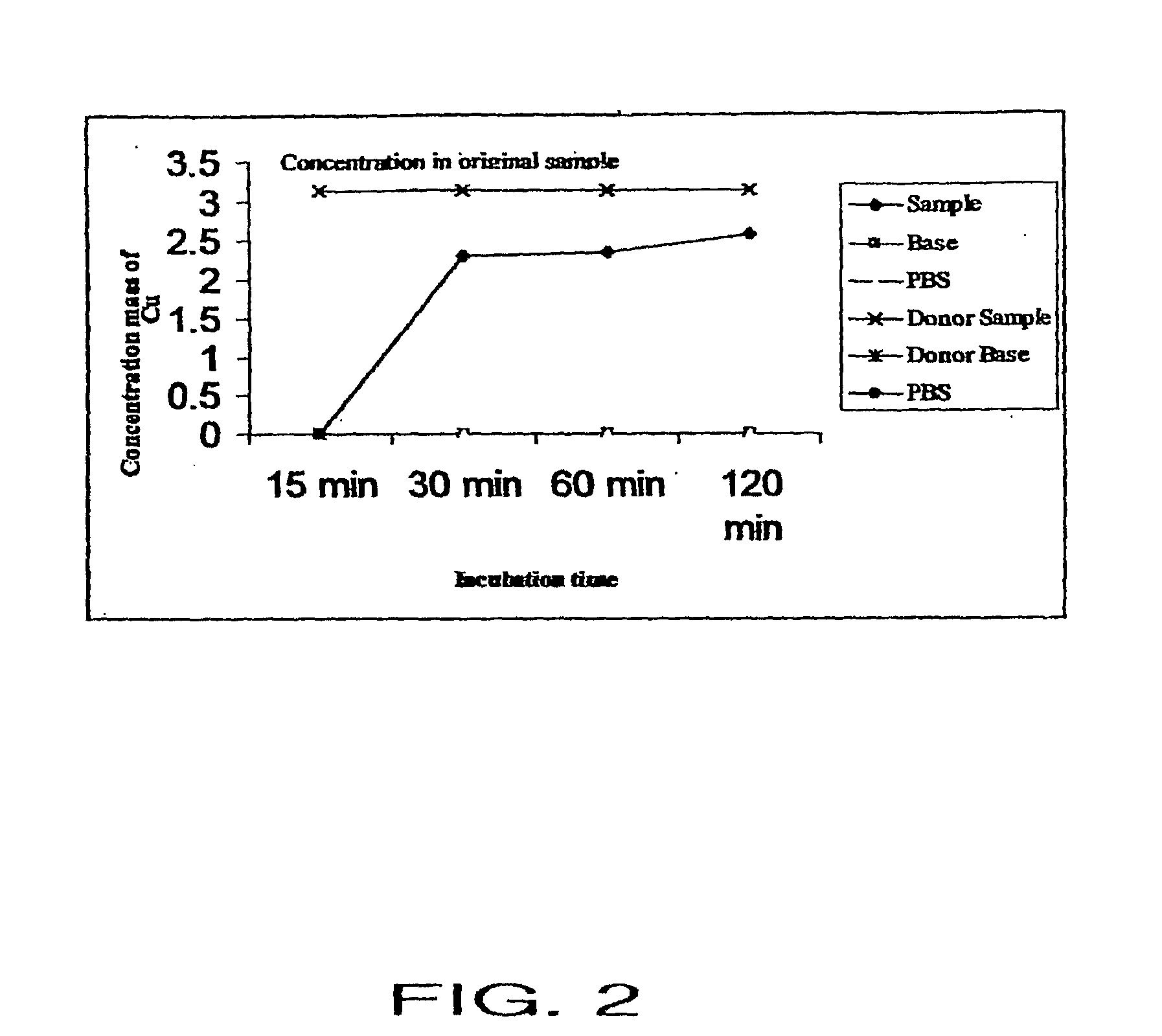
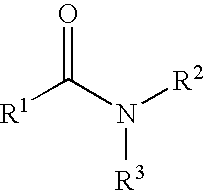
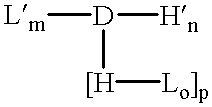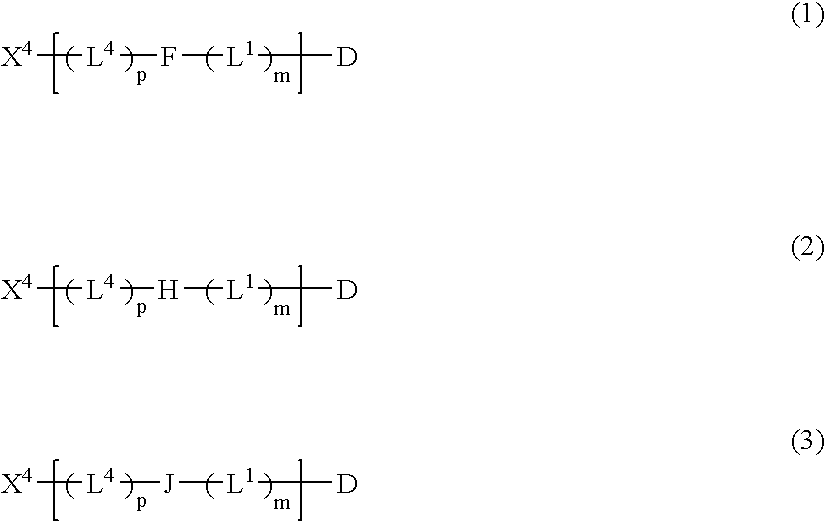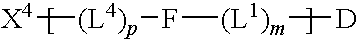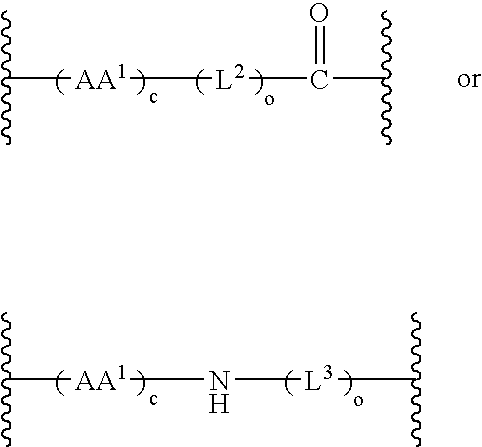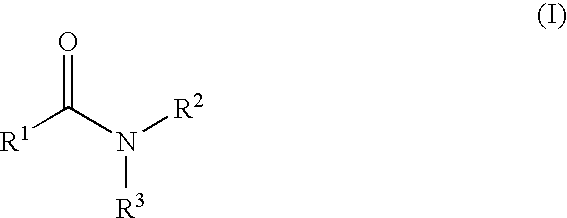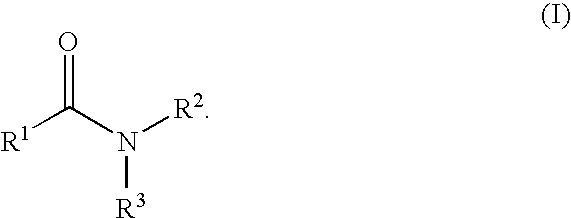Patents
Literature
32584 results about "Pharmaceutical Substances" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
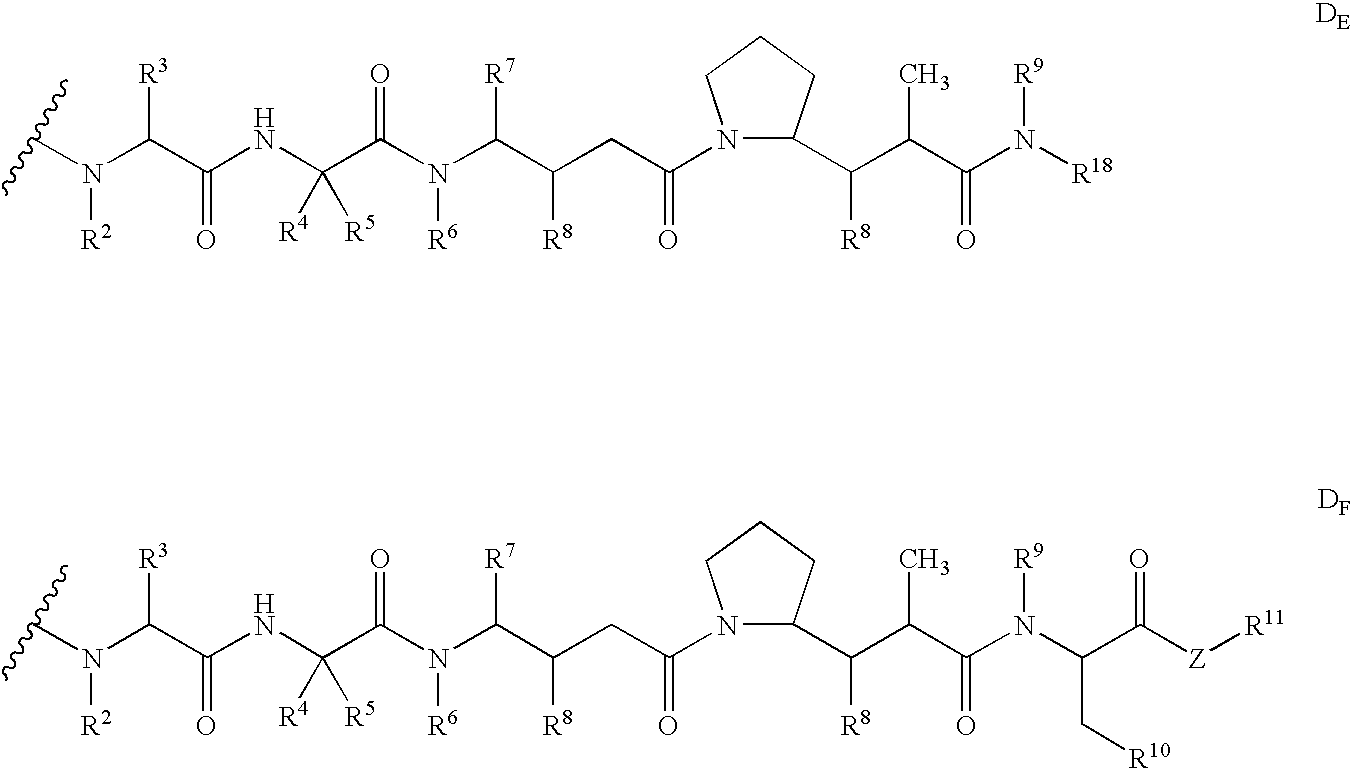
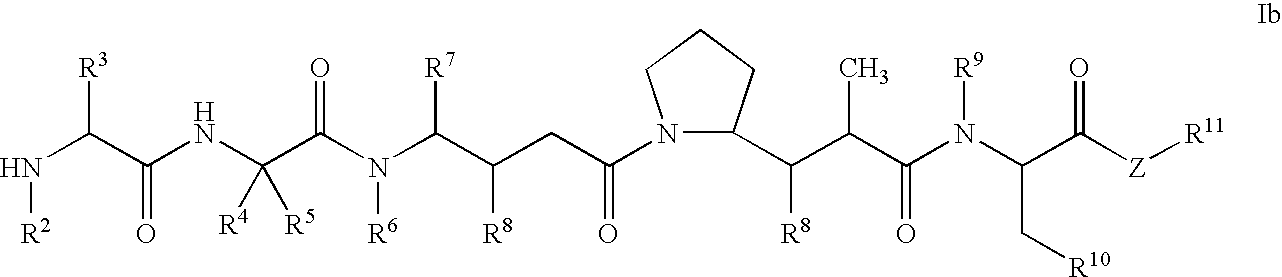
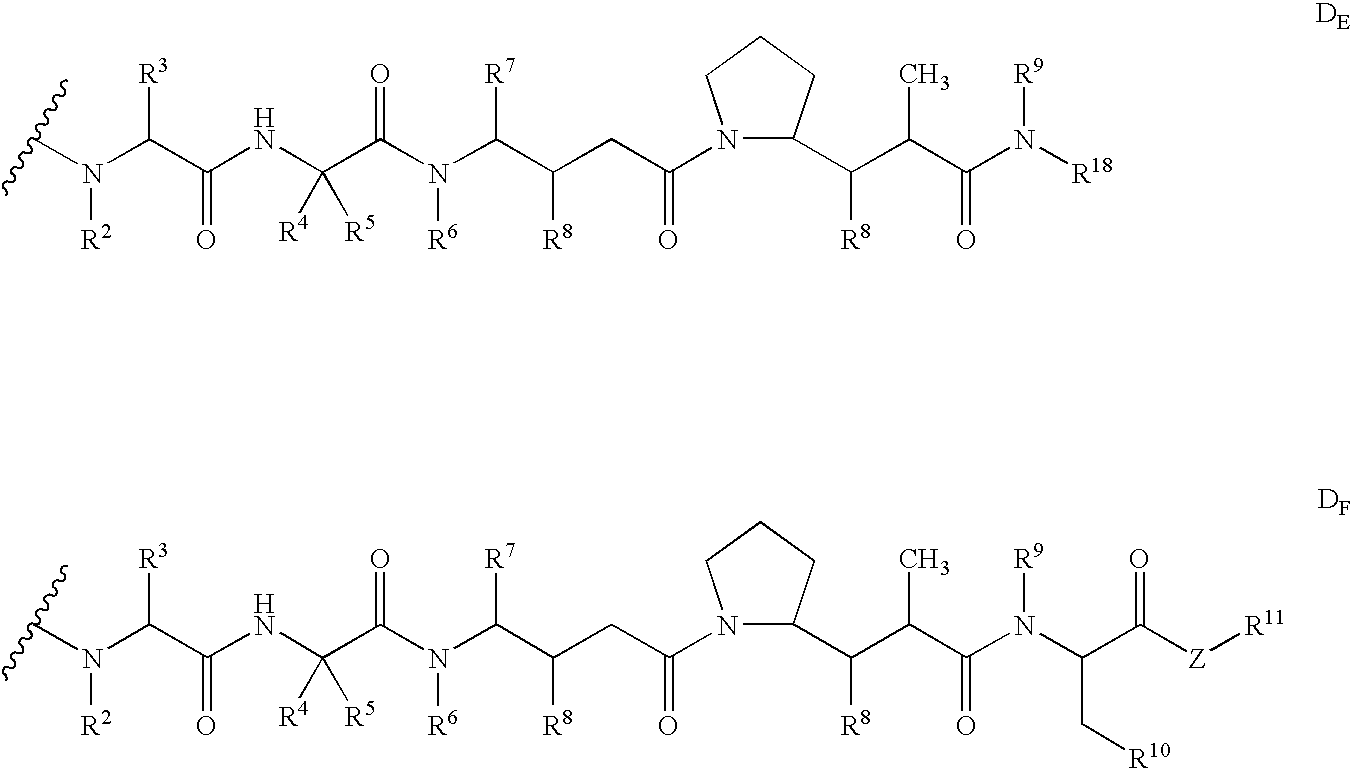
Monomethylvaline compounds capable of conjugation to ligands
ActiveUS20050238649A1Improve bioavailabilityImprove compoundAntibacterial agentsBiocideD-norephedrineBiochemistry
Owner:SEAGEN INC
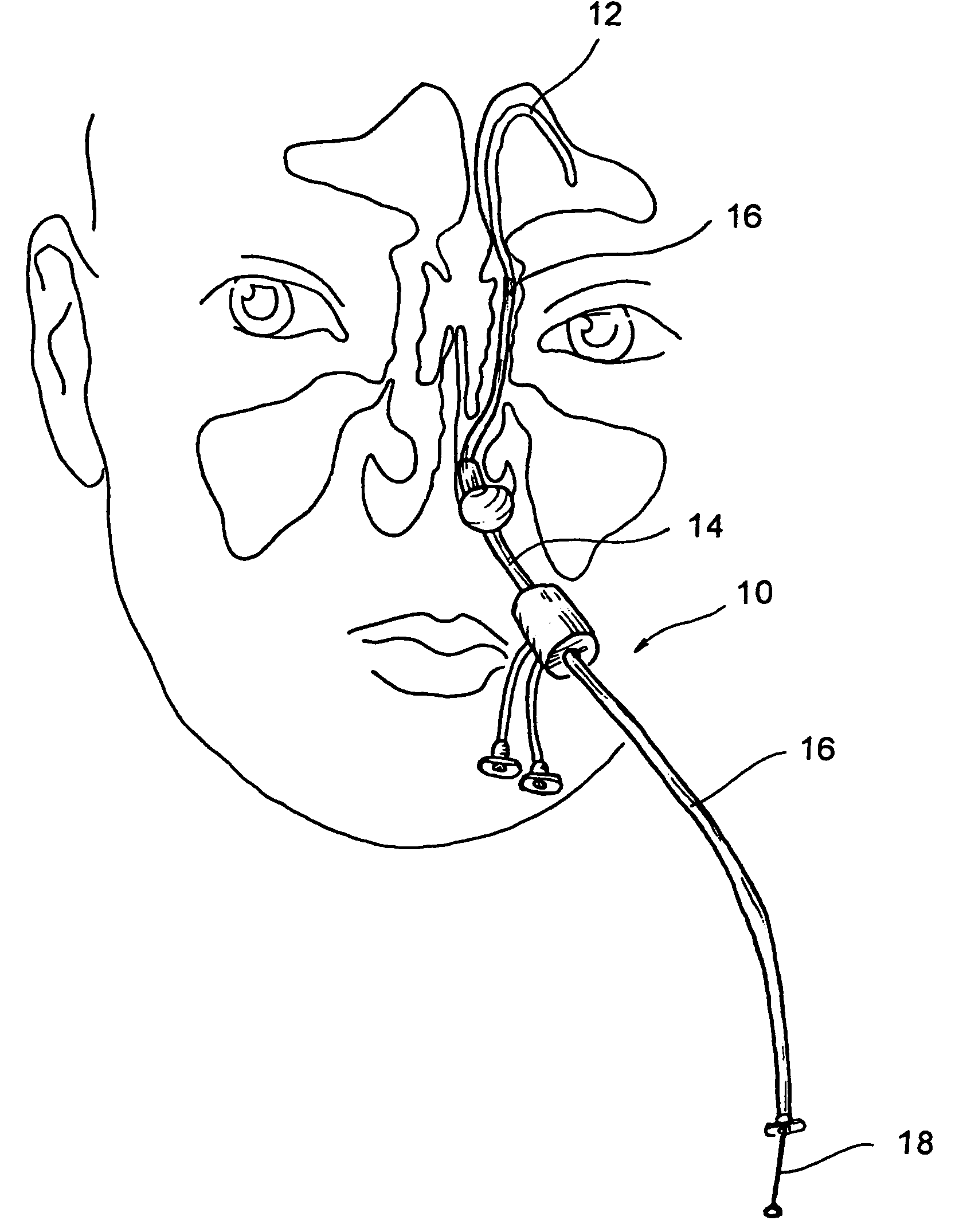
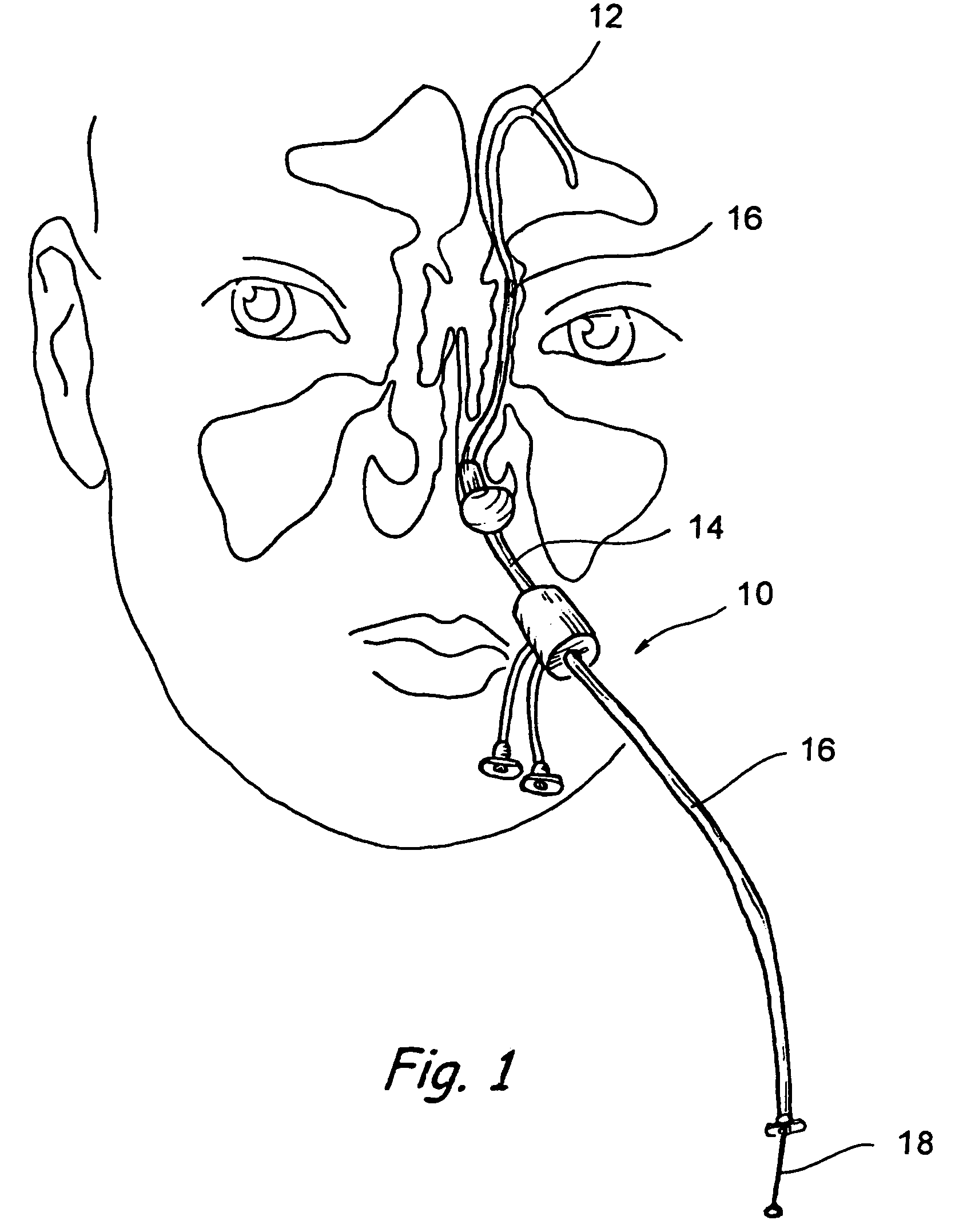
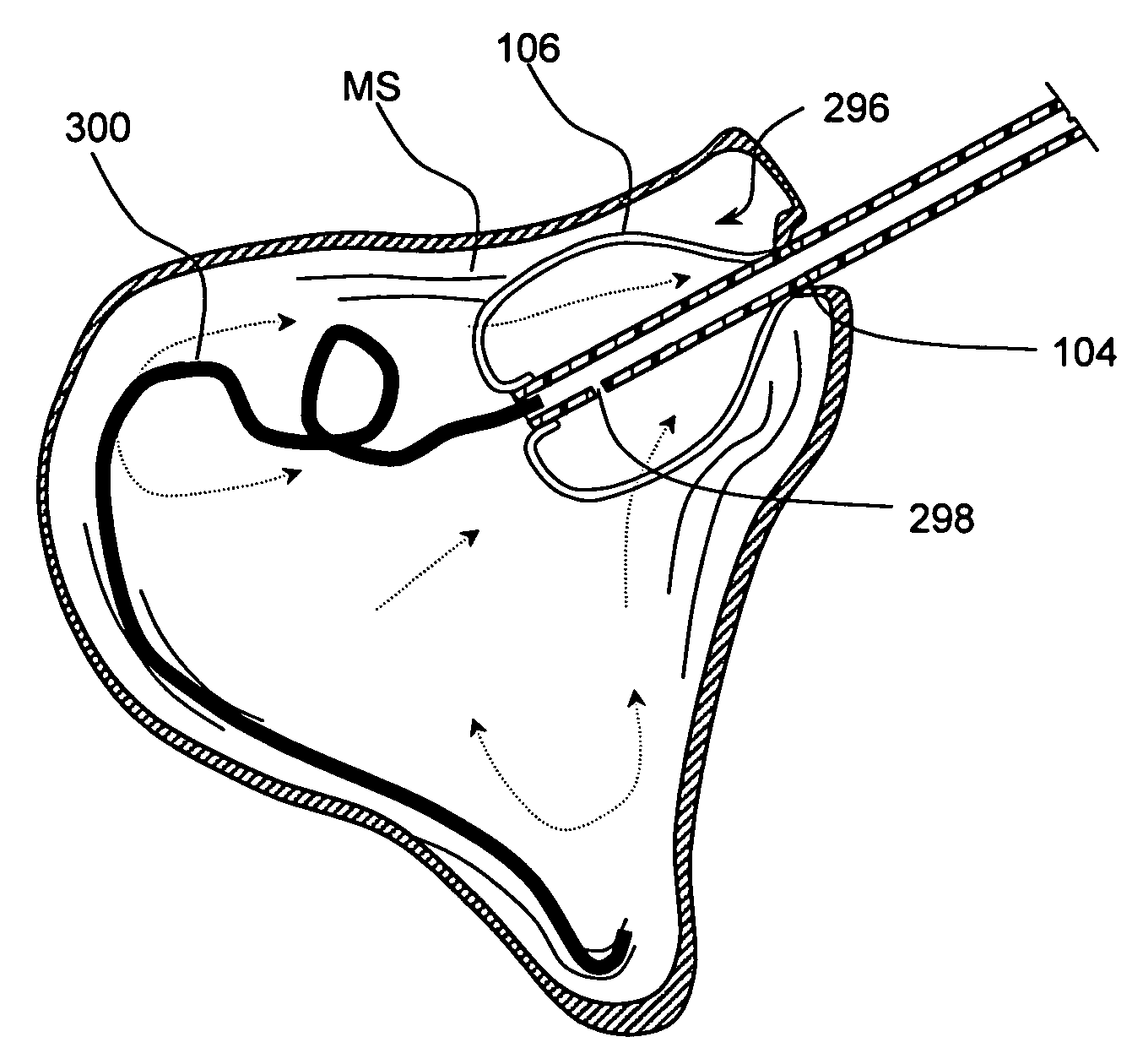
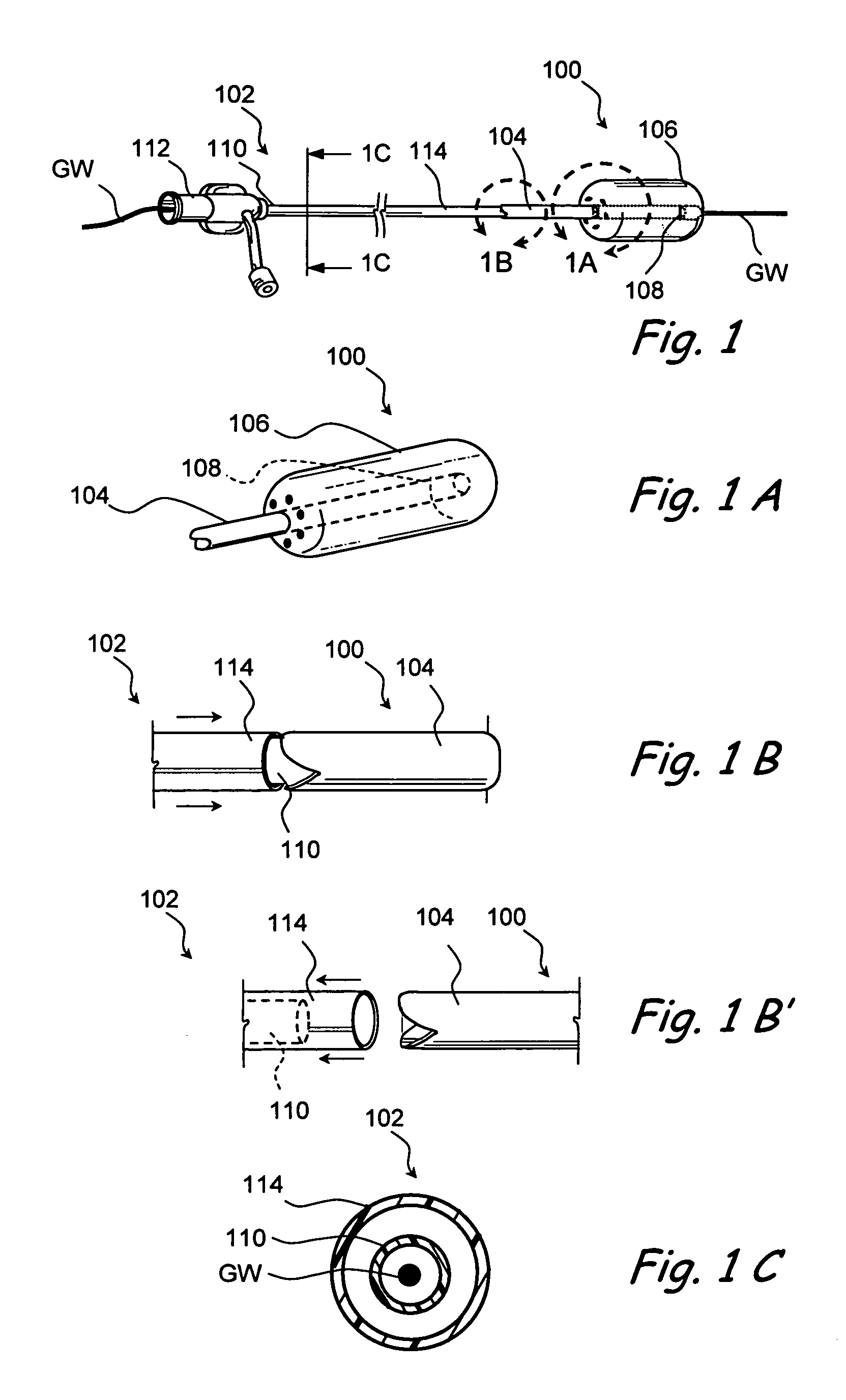
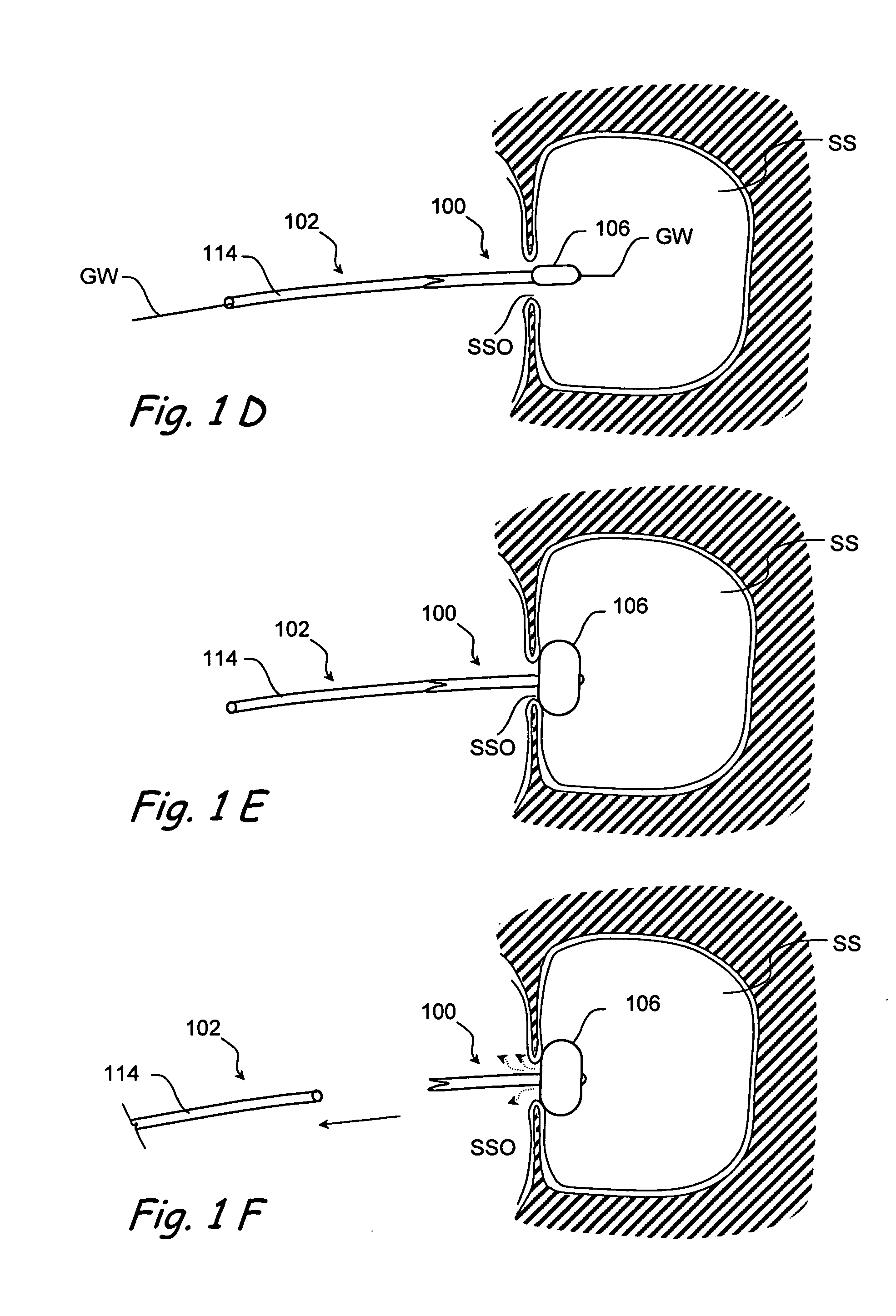
Implantable device and methods for delivering drugs and other substances to treat sinusitis and other disorders
Implantable devices and methods for delivering drugs and other substances to locations within the body of a human or animal subject to treat or diagnose sinusitis and a variety of other disorders. The invention includes implantable substance delivery devices that comprise reservoirs and barriers that control the rate at which substances pass out of the reservoirs. The delivery devices may be advanced into the body using guidewires, catheters, ports, introducers and other access apparatus. In some embodiments the delivery devices may be loaded with one or more desired substance before their introduction into the body. In other embodiments the delivery devices are loaded and / or reloaded with a desired substance after the delivery device has been introduced into the body.
Owner:ACCLARENT INC
Cyclodextrin polymers for carrying and releasing drugs
This invention discloses methods for preparing compositions of cyclodextrin polymers for carrying drugs and other active agents. Methods are also disclosed for preparing cyclodextrin polymer carriers that release drugs under controlled conditions. The invention also discloses methods for preparing compositions of cyclodextrin polymer carriers that are coupled to biorecognition molecules for targeting the delivery of drugs to their site of action. The advantages of the water soluble (or colloidal) cyclodextrin polymer carrier are: (1) Drugs can be used that are designed for efficacy without conjugation requirements. (2) It will allow the use of drugs designed solely for efficacy without regard for solubility. (3) Unmodified drugs can be delivered as macromolecules and released within the cell. (4) Drugs can be targeted by coupling the carrier to biorecognition molecules. (5) Synthesis methods are independent of the drug to facilitate multiple drug therapies.
Owner:KOSAK KENNETH M
Means to achieve sustained release of synergistic drugs by conjugation
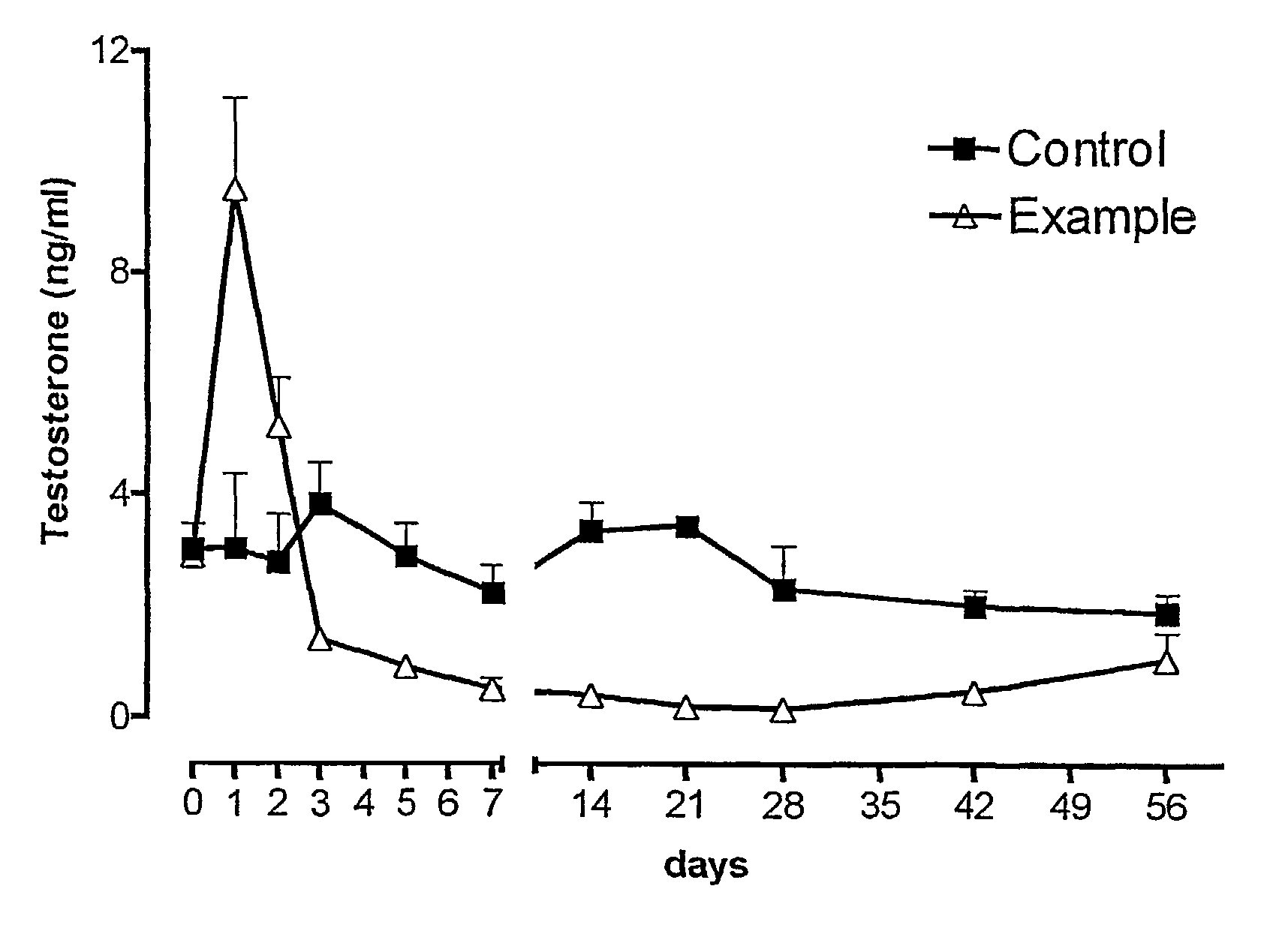
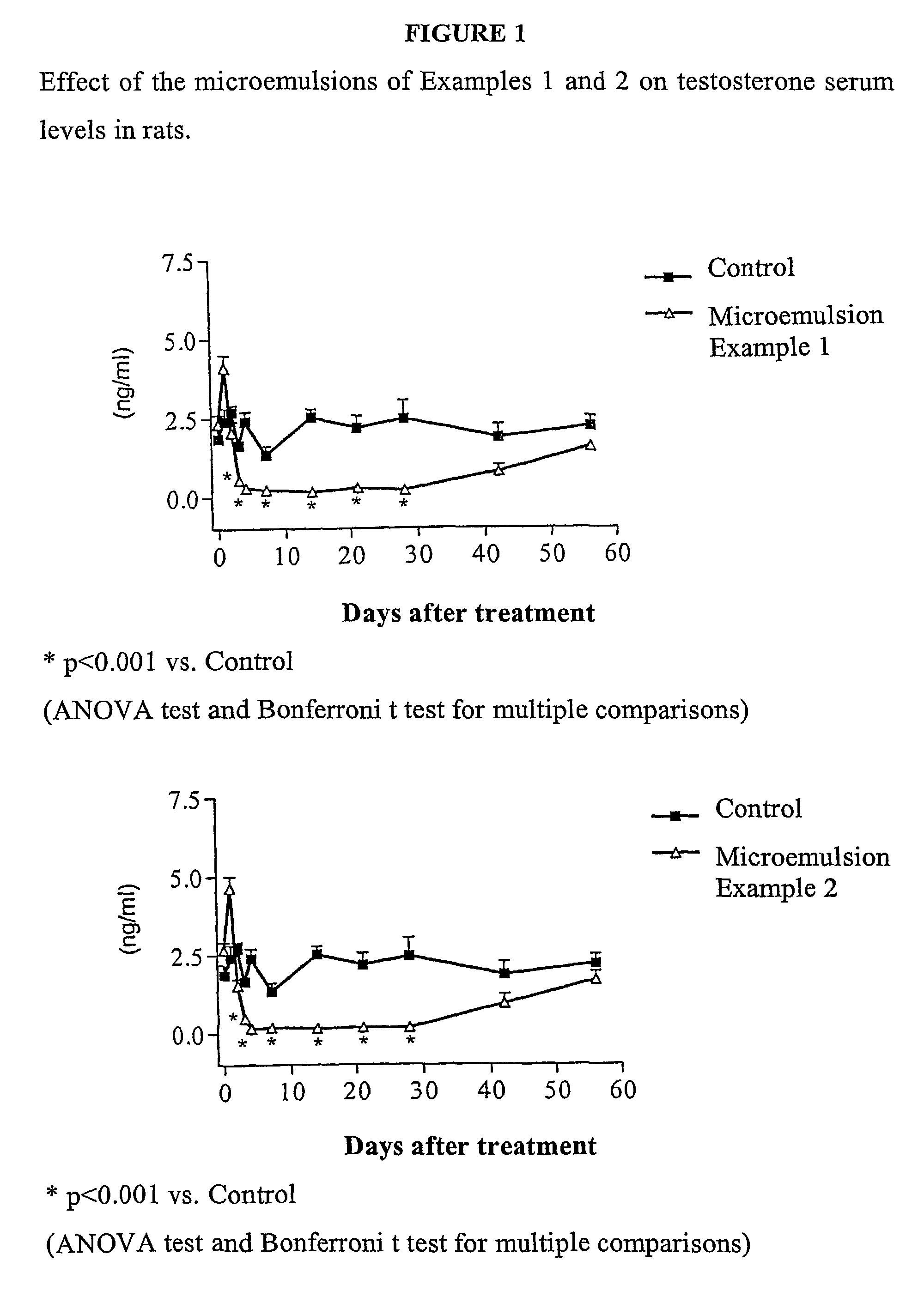
A codrug composition of at least two drug compounds covalently linked to one another via a labile bond to form a single codrug composition, or ionically linked to one another to form a single workings composition, and methods of use of the codrug for the treatment of various medical conditions. The codrug may be administered by itself or in the form of a bioerodible or nonbioerodible substance.
Owner:UNIVERSITY OF KENTUCKY +1
Chemical linkers and conjugates thereof
ActiveUS20060024317A1Increase solubilityDecrease aggregationMaterial nanotechnologyHybrid immunoglobulinsDrugToxin
The present disclosure provides drug-ligand conjugates that are potent cytotoxins, wherein the drug is linked to the ligand through either a peptidyl, hydrazine, or disulfide linker. The disclosure is also directed to compositions containing the drug-ligand conjugates, and to methods of treatment using them.
Owner:MEDAREX LLC
Oral transmucosal drug dosage using solid solution
InactiveUS6264981B1High dissolution rateEasy to usePharmaceutical non-active ingredientsPill deliverySolid solutionPharmaceutical formulation
The present invention is directed toward formulation and method for oral transmucosal delivery of a pharmaceutical. The invention provides a drug formulation comprising a solid pharmaceutical agent in solid solution with a dissolution agent. The formulation is administered into a patient's oral cavity, delivering the pharmaceutical agent by absorption through a patient's oral mucosal tissue. The formulation and method provide for improved oral mucosal delivery of the pharmaceutical agent.
Owner:CEPHALON INC
Methods and compositions useful for administration of chemotherapeutic agents
InactiveUS6096331AReduce morbidityLow toxicityPowder deliveryEchographic/ultrasound-imaging preparationsActive agentIn vivo
In accordance with the present invention, there are provided compositions and methods useful for the in vivo delivery of a pharmaceutically active agent, wherein the agent is associated with a polymeric biocompatible material.
Owner:ABRAXIS BIOSCI LLC
Mixed micellar drug deliver system and method of preparation
Pharmaceutical compositions comprising a macromolecular pharmaceutical agent in micellar form are disclosed. The micelles are formed from an alkali metal alkyl sulfate, and at least one additional micelle-forming compound as described in the specification. An alkali metal salicylate and a pharmaceutically acceptable edetate are also included in the composition. Micelle size ranges between about 1 and 10 nanometers. Methods for making and using the compositions are also disclosed.
Owner:GENEREX PHARMA
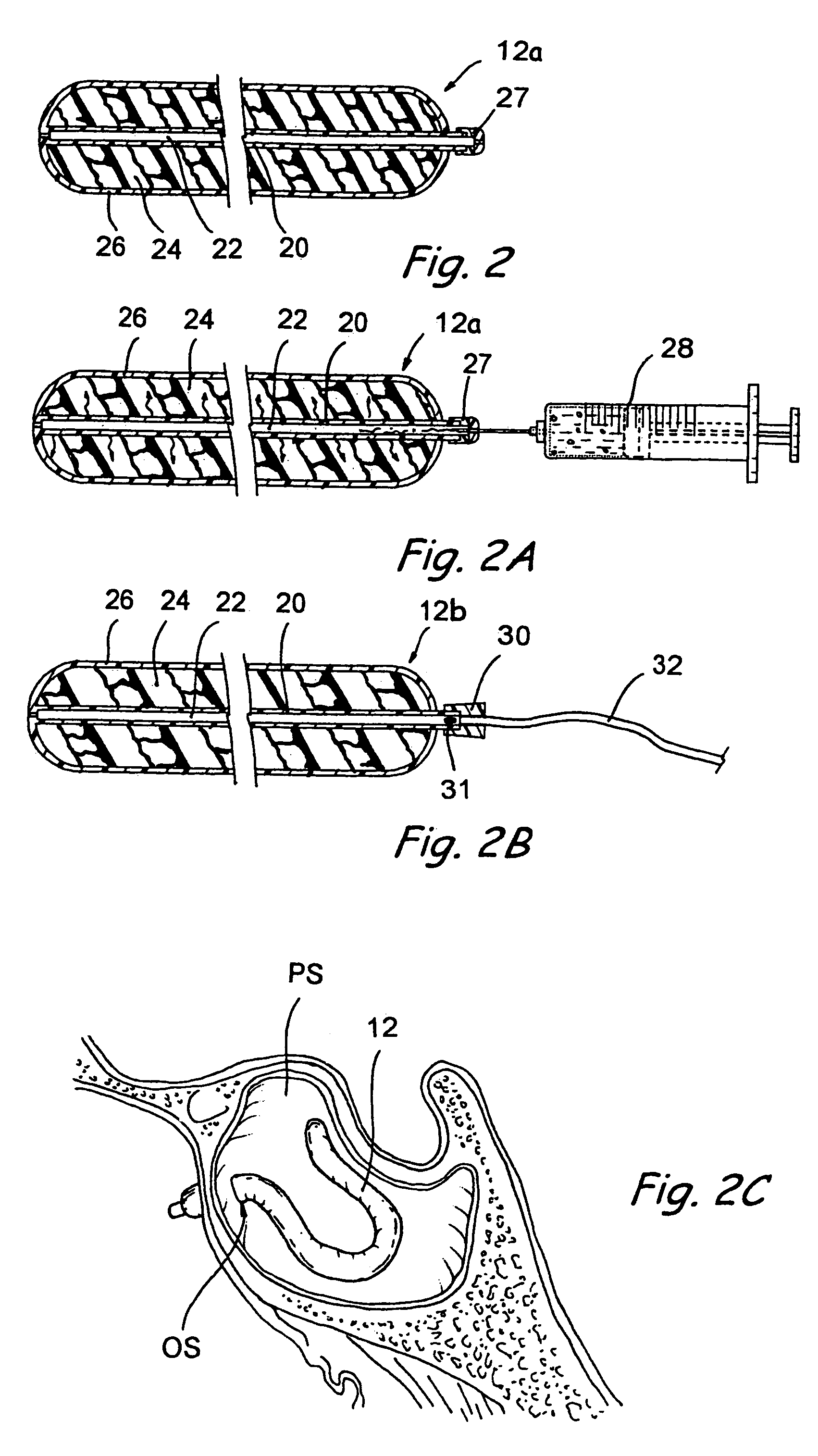
Devices and methods for delivering therapeutic substances for the treatment of sinusitis and other disorders
ActiveUS20060106361A1Surgical needlesPharmaceutical delivery mechanismDrugUnexpected therapeutic effect
Devices and methods for delivering drugs and other therapeutic or diagnostic substances to desired locations within the bodies of human or non-human animal subjects. An implantable delivery device comprising a reservoir is initianlly attached to a deliver catheter or delivery tool and is introduced into the body and positioned at a desired site. A therapeutic or diagnostic substance is then introduced into the reservoir and the delivery catheter or deliver tool is then removed, leaving the implantable delivery device implanted within the body. The substance is then delivered from the reservoir at a rate that causes the desire diagnostic or therapeutic effect. Also provided are substance eluting stents that elute substance from a selected surface of the stent (e.g., the outer surface) but not from another surface of the stent (e.g., the inner surface).
Owner:ACCLARENT INC
N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-4-cyanothiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
Nucleic Acid-Lipopolymer Compositions
InactiveUS20090042829A1Increase efficiency and dosing flexibilityEfficiently be lyophilizedSpecial deliveryPeptide/protein ingredientsCholesterolFiller Excipient
Compositions, methods, and applications that increase the efficiency of nucleic acid transfection are provided. In one aspect, a pharmaceutical composition may include at least about 0.5 mg / ml concentration of a nucleic acid condensed with a cationic lipopolymer suspended in an isotonic solution, where the cationic lipopolymer includes a cationic polymer backbone having cholesterol and polyethylene glycol covalently attached thereto, and wherein the molar ratio of cholesterol to cationic polymer backbone is within a range of from about 0.1 to about 10, and the molar ratio of polyethylene glycol to cationic polymer backbone is within a range of from about 0.1 to about 10. The composition further may include a filler excipient.
Owner:CLSN LAB
Non-gelatin substitutes for oral delivery capsules, their composition and process of manufacture
Gelatin-free capsule for use in oral administration of medicines, cosmetic or bath applications, or dietary supplements can be prepared from compositions comprisinga) 8-50% by weight of water-dispersible or water-soluble plasticizer,b) 0.5 to 12% by weight kappa-carrageenan,c) 0 to 60% dextrins, andd) 1% to 95% by weight water,with the kappa-carrageenan comprising at least 50% by weight of all gums forming or contributing to formation of thermoreversible gels in the composition. A capsule for oral administration or cosmetic application may comprise a fill material to be administered to a patient or subject and a capsule, the capsule comprising an aqueous based film comprisinga) water-dispersible or water-soluble plasticizer, andb) carrageenan,with the carrageenan comprising at least 50% or 75% by weight of kappa-carrageenan, and the carrageenan comprising at least 50% or 75% by weight of all gums which form or contribute to the formation of thermoreversible gels. A process for forming the capsules may comprise heating the composition, casting or extruding the composition into a film, gelling the composition by cooling, associating a fill material with the gelled composition (usually as a film) and sealing the film about the fill material.
Owner:PATHEON SOFTGELS INC
N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing them and their use in inhibiting dipeptidyl peptidase-IV
The invention discloses certain N-(substituted glycyl)-thiazolidines, pharmaceutical compositions containing said compounds as an active ingredient thereof, and the use of said compounds in inhibiting dipeptidyl peptidase-IV.
Owner:NOVARTIS AG
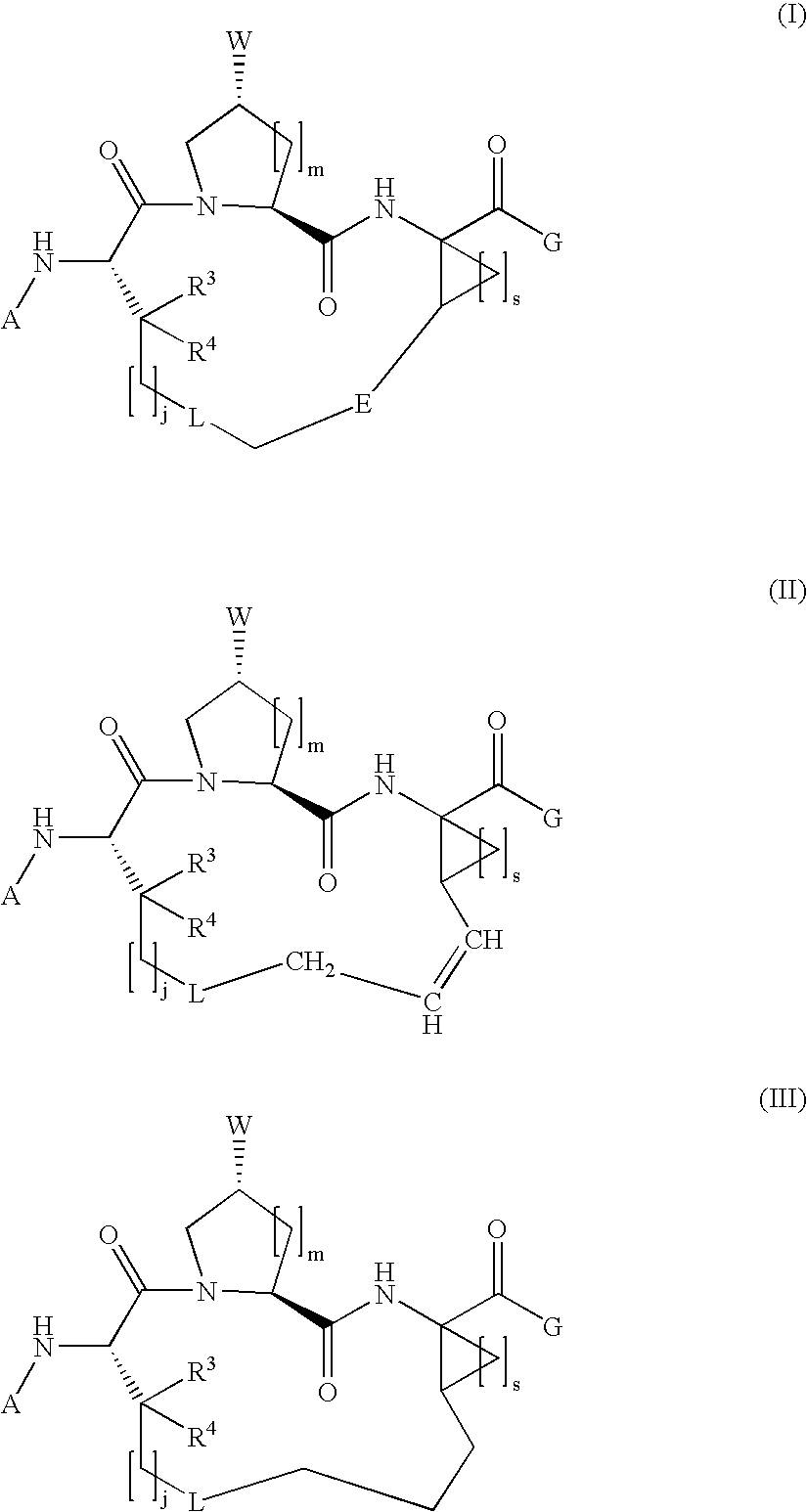
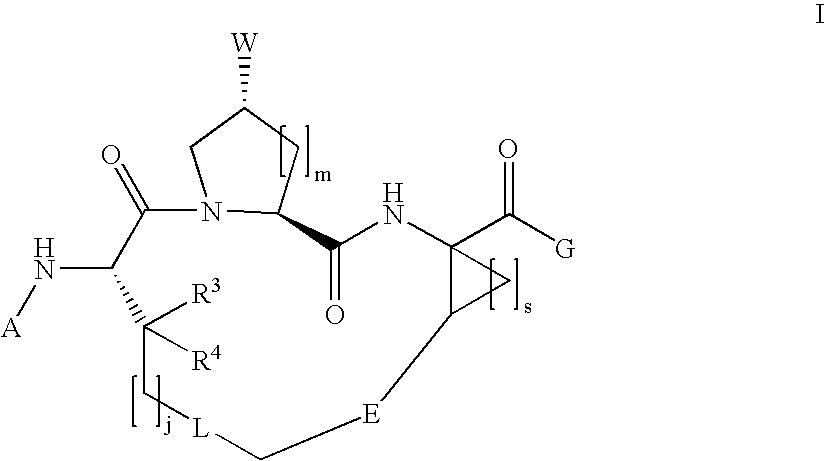
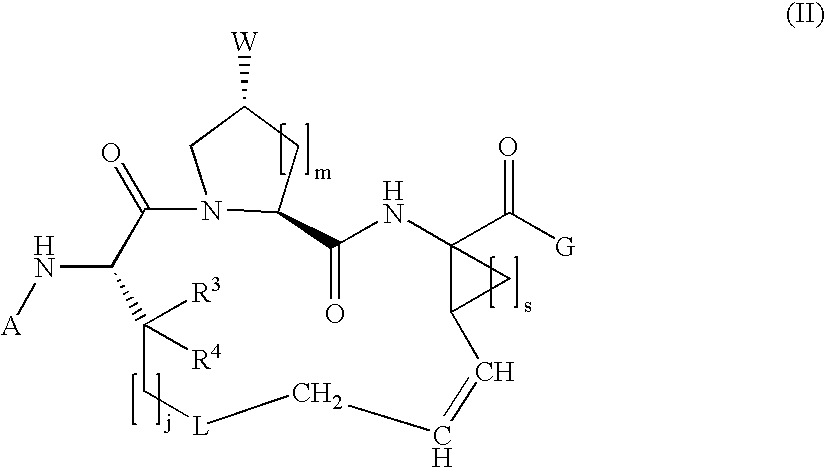
Macrocyclic hepatitis C serine protease inhibitors
The present invention relates to compounds of Formula I, II or Ill, or a pharmaceutically acceptable salt, ester, or prodrug, thereof: wherein W is a substituted or unsubstituted heterocyclic ring system. The compounds inhibit serine protease activity, particularly the activity of hepatitis c virus (HCV) NS3-NS4A protease. Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis c virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
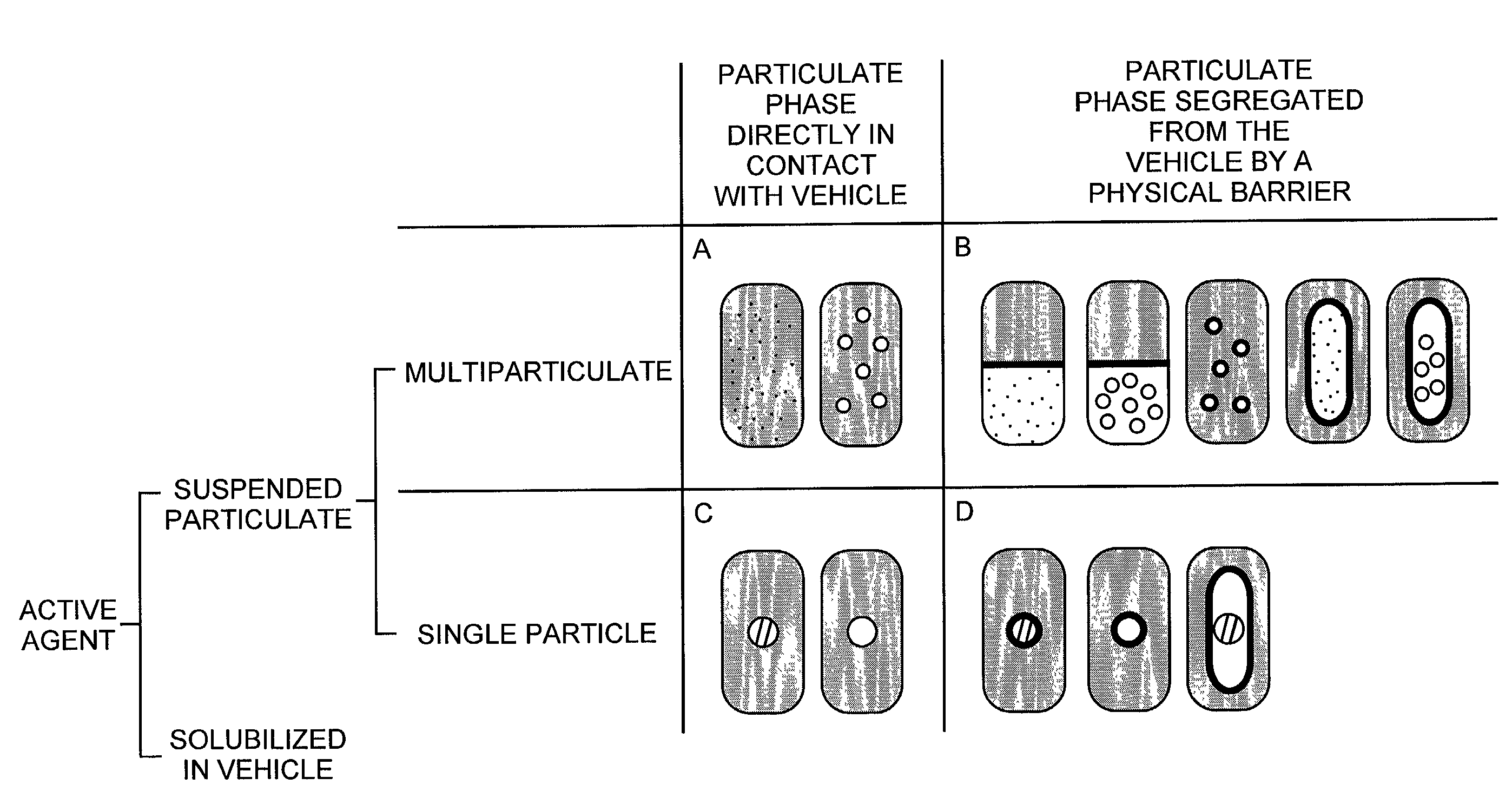
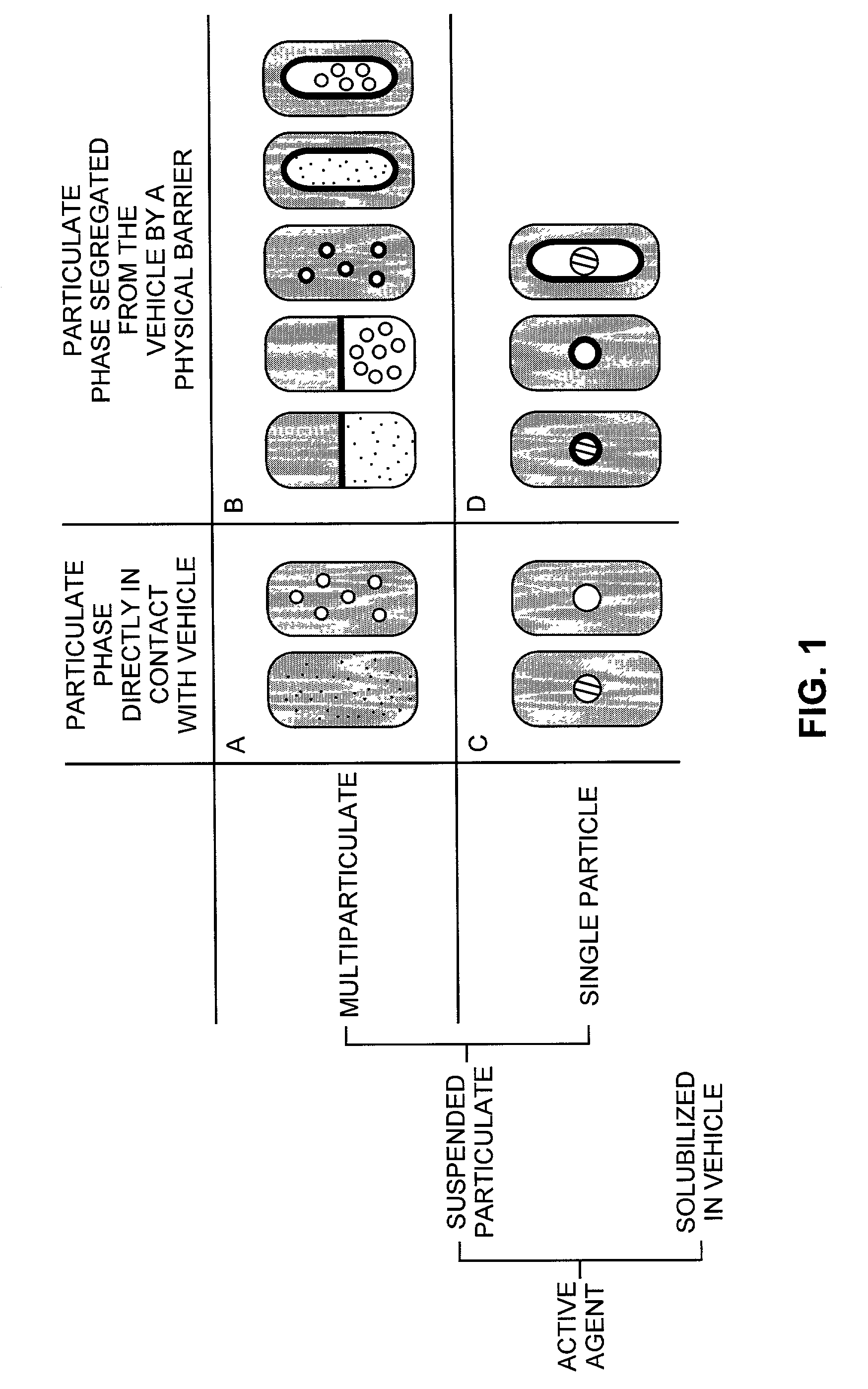
Pharmaceutical formulations and systems for improved absorption and multistage release of active agents
InactiveUS7374779B2Improve bioavailabilityLow variabilityPowder deliveryOrganic active ingredientsActive agentFast release
The present invention pertains to pharmaceutical formulations and systems for delivery of active agents, wherein a first fraction of an active agent is suspended in a vehicle and a second fraction of active agent is solubilized in the vehicle, with the suspended fraction representing about 5 wt. % to about 80 wt. % of the active agent and the second fraction representing about 20 wt. % to about 95 wt. % of the active agent. One or more additional active agents, which may be fully solubilized, partially solubilized, or suspended, may also be present. The first and second fractions of the active agent may or may not have different release profiles. Generally, a significant fraction of the solubilized drug will release rapidly, providing for rapid onset, while the suspended drug may be formulated for delayed and / or sustained release.
Owner:LIPOCINE
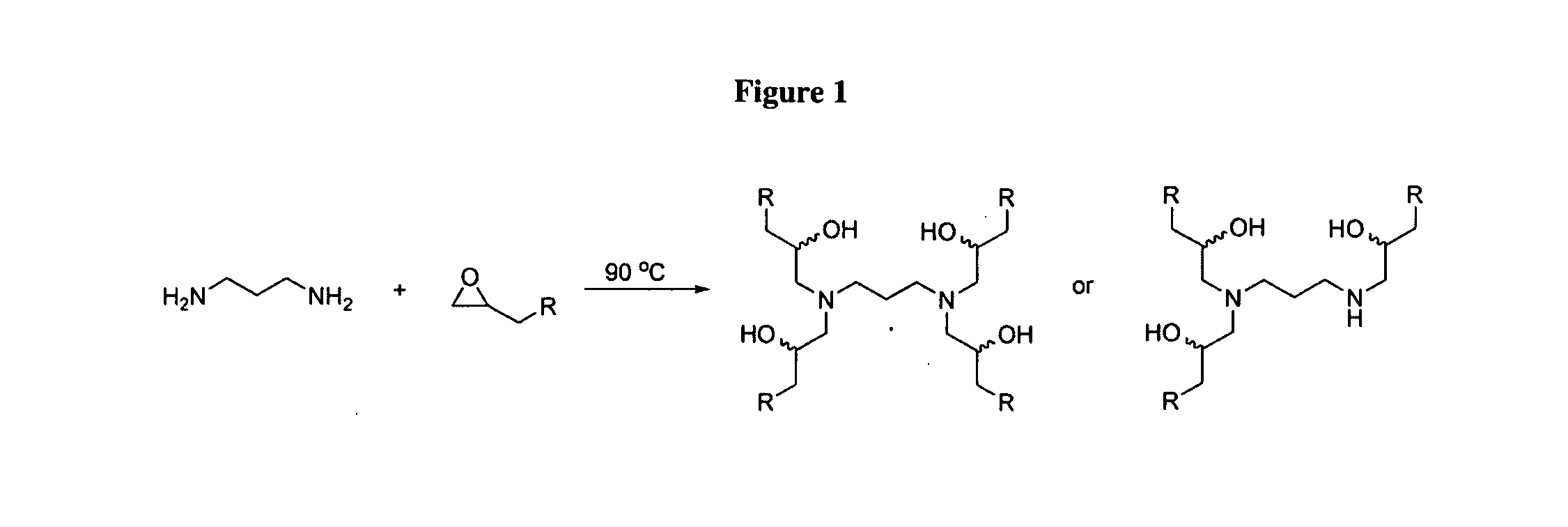
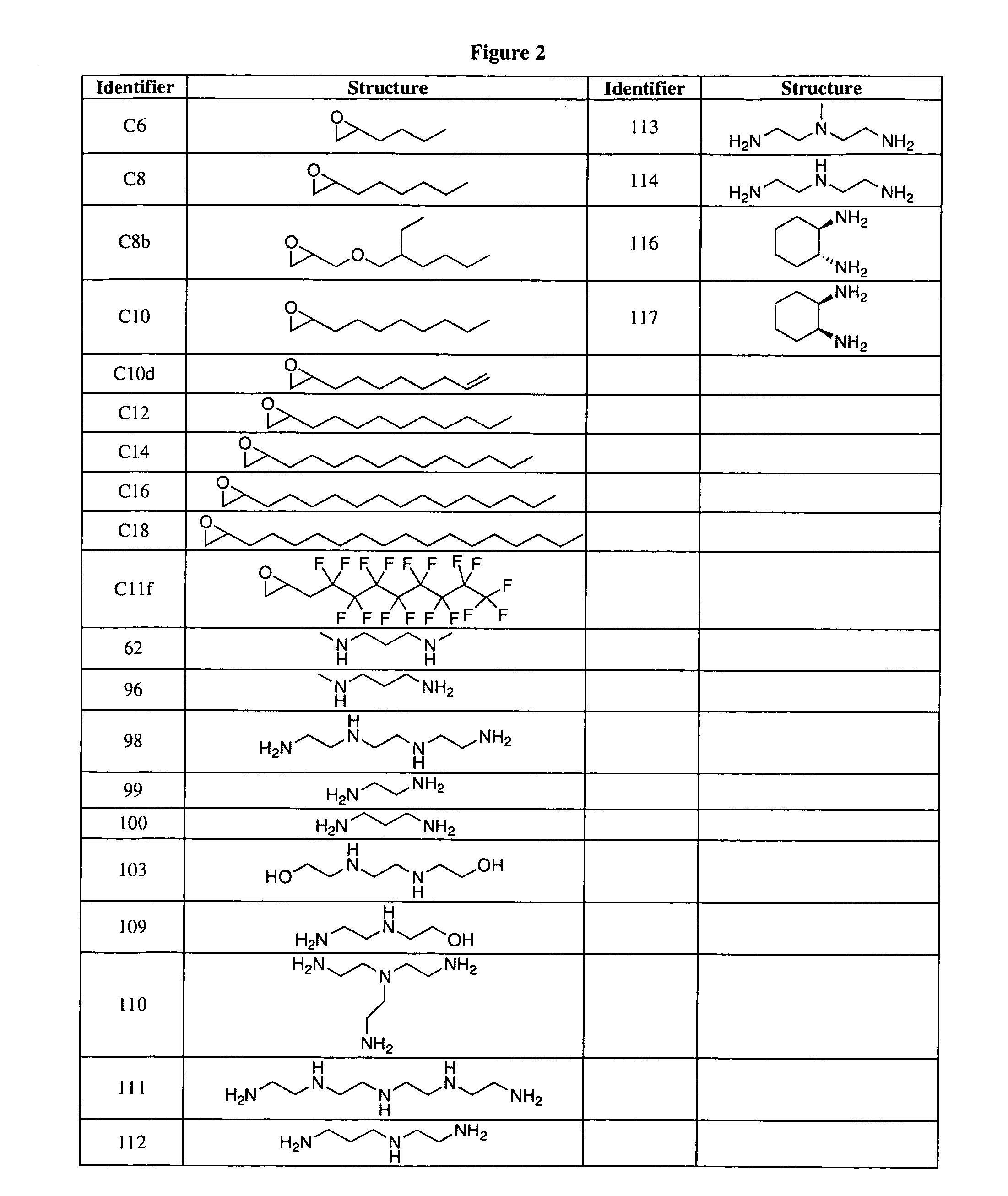
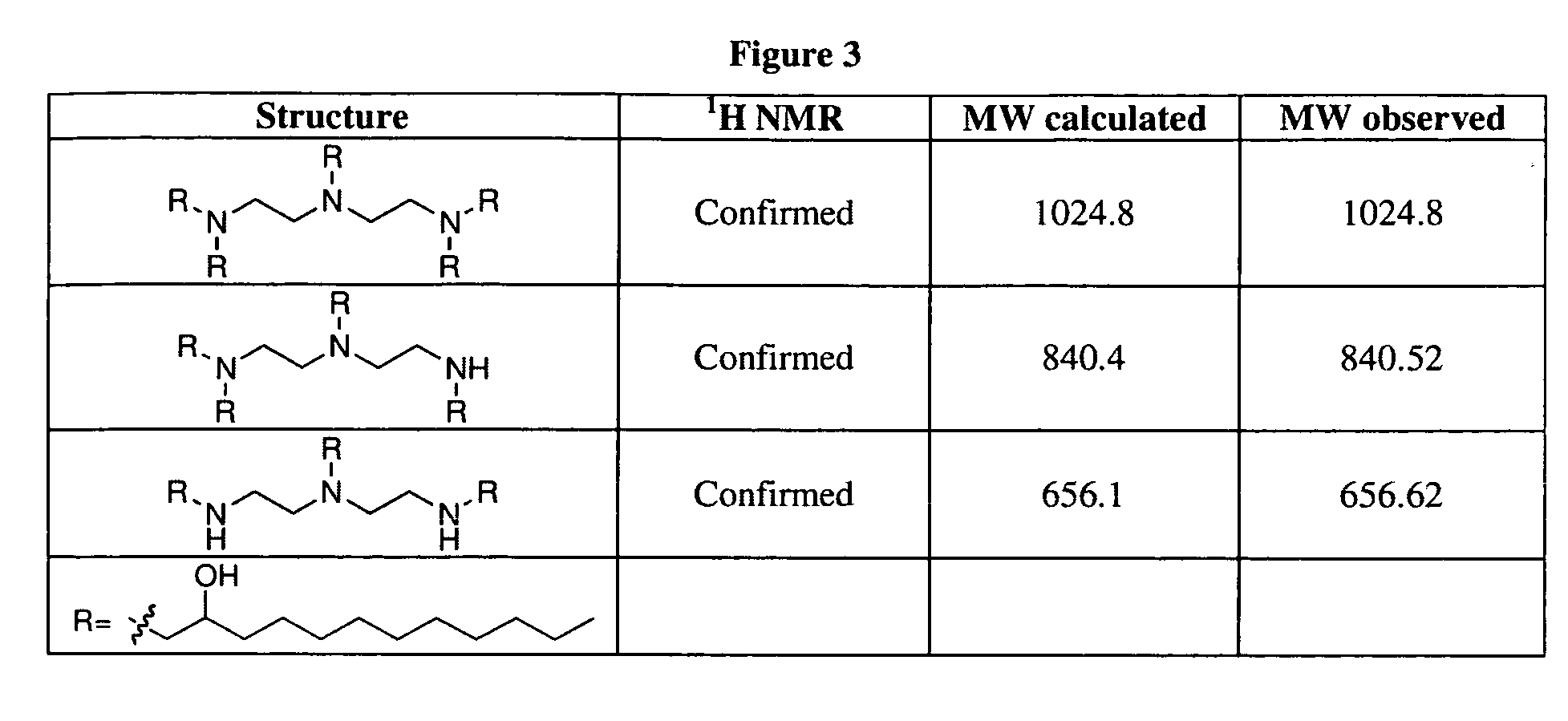
Aminoalcohol lipidoids and uses thereof
Aminoalcohol lipidoids are prepared by reacting an amine with an epoxide-terminated compound are described. Methods of preparing aminoalcohol lipidoids from commercially available starting materials are also provided. Aminoalcohol lipidoids may be prepared from racemic or stereochemically pure epoxides. Aminoalcohol lipidoids or salts forms thereof are preferably biodegradable and biocompatible and may be used in a variety of drug delivery systems. Given the amino moiety of these aminoalcohol lipidoid compounds, they are particularly suited for the delivery of polynucleotides. Complexes, micelles, liposomes or particles containing the inventive lipidoids and polynucleotide have been prepared. The inventive lipidoids may also be used in preparing microparticles for drug delivery. They are particularly useful in delivering labile agents given their ability to buffer the pH of their surroundings.
Owner:MASSACHUSETTS INST OF TECH
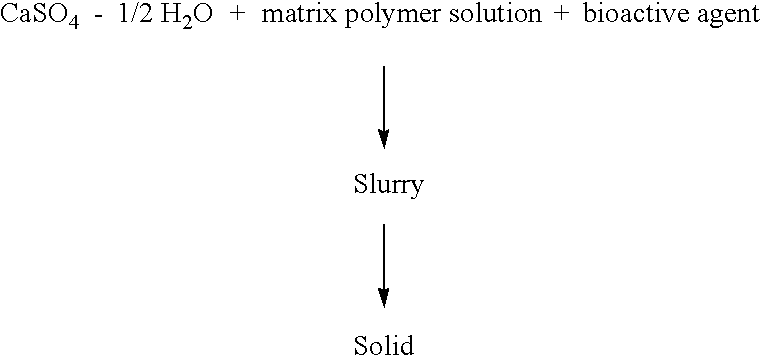
Resorbable matrices for delivery of bioactive compounds
This invention relates generally to the production and use of inorganic-conditioning agent complexes for the controlled release of compounds including medicinals. Advantageously, the inorganic used is calcium sulfate and the conditioning agent is calcium stearate.
Owner:ROYER BIOMEDICAL INC
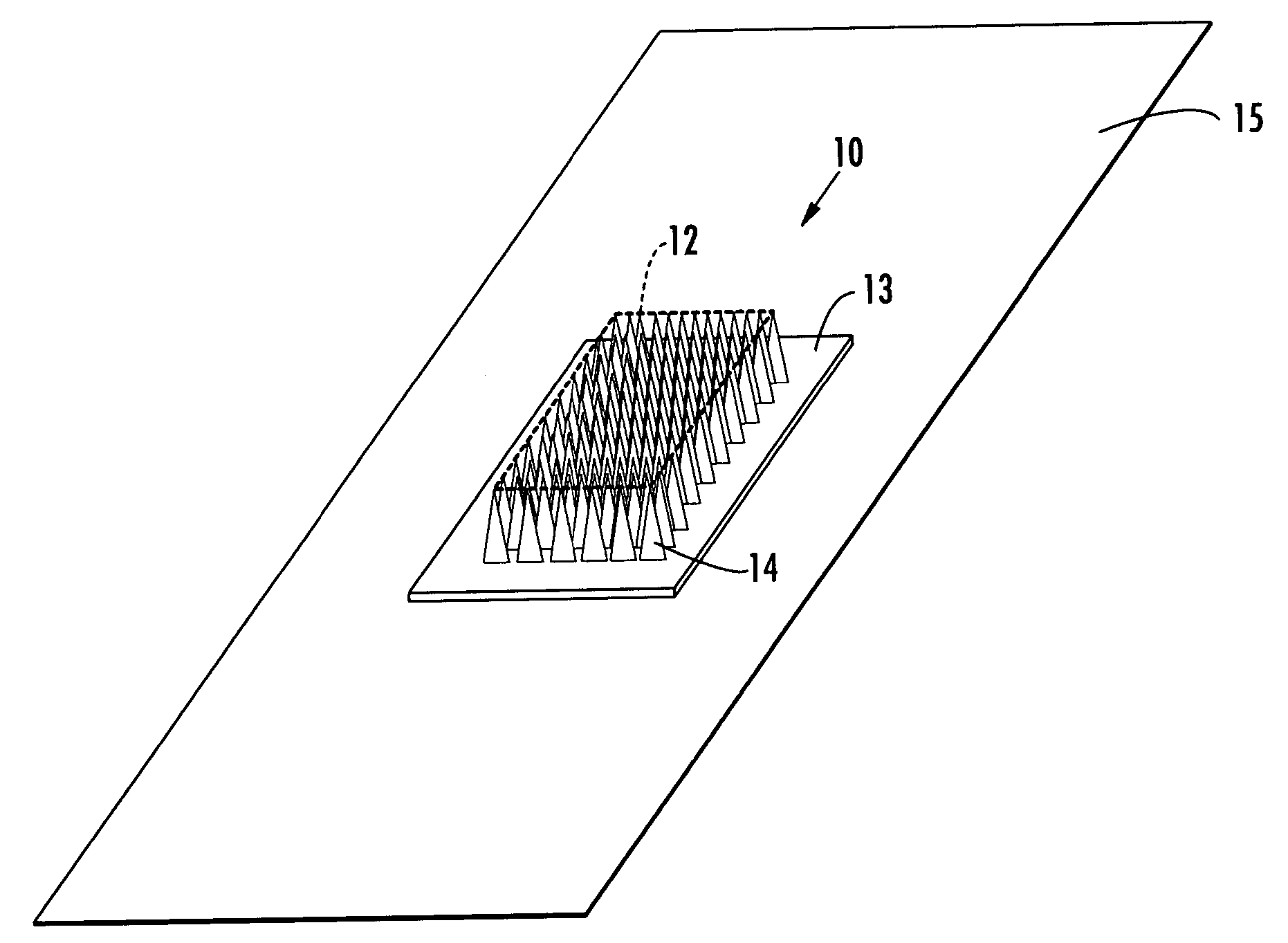
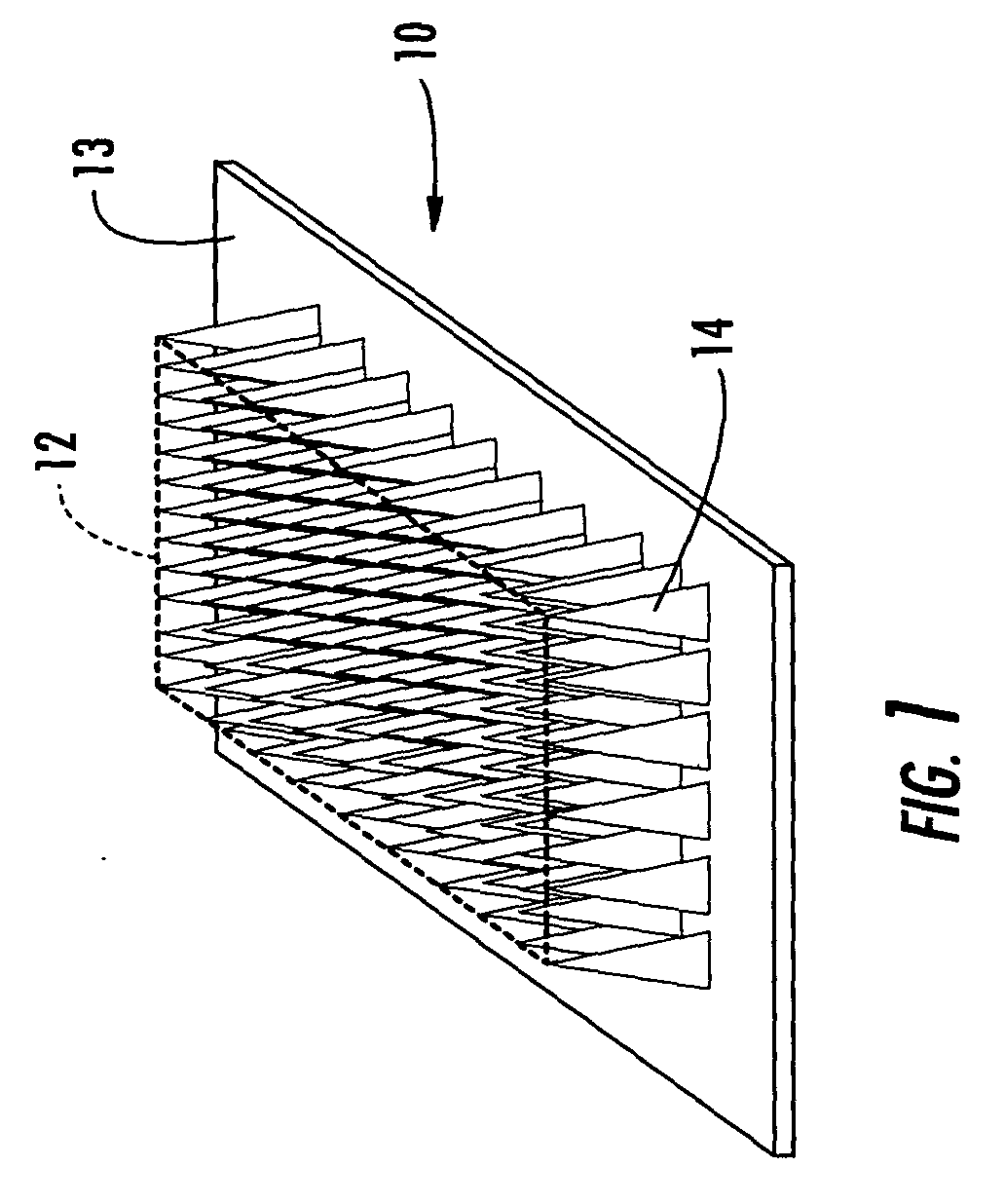
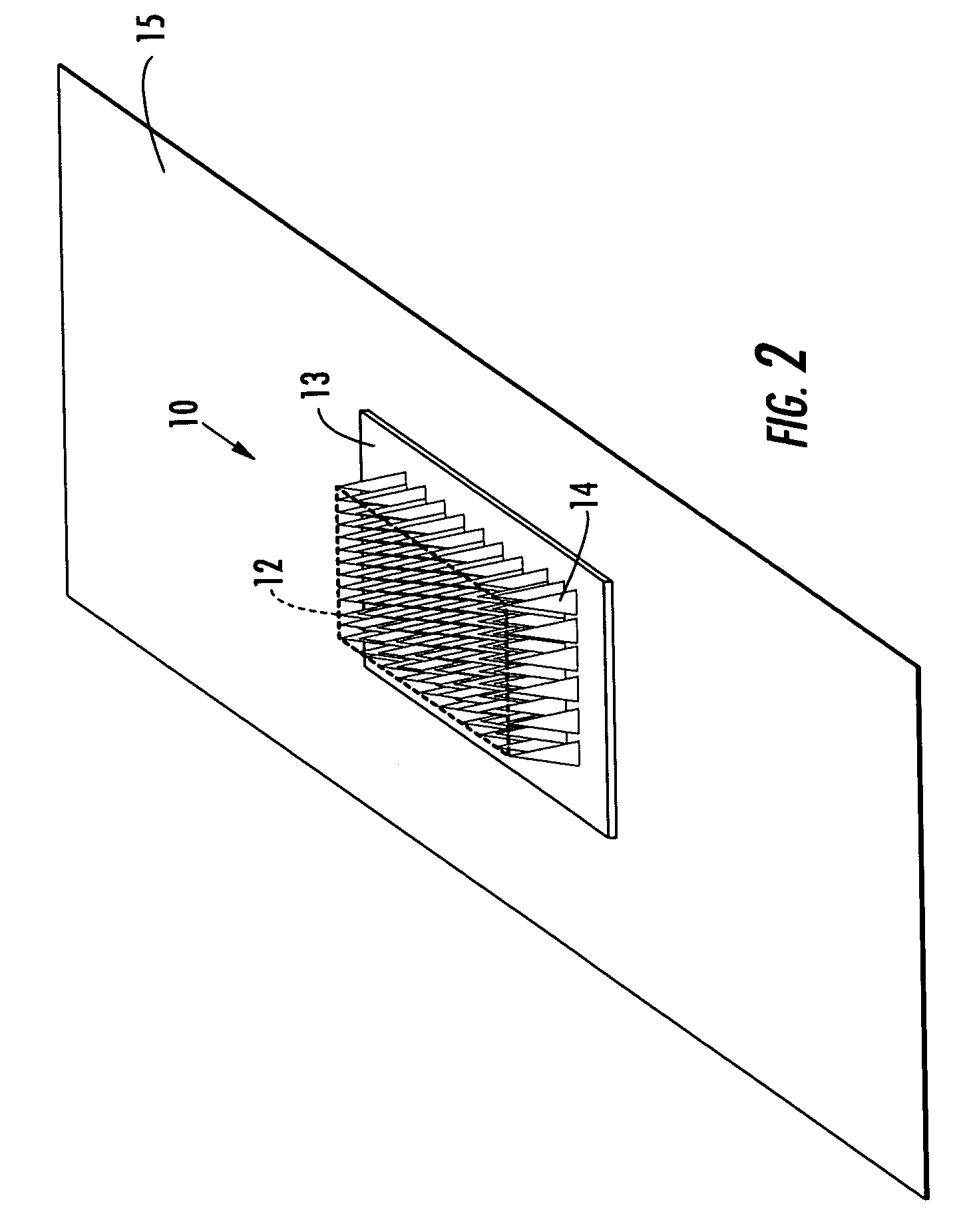
Applicator for applying functional substances into human skin
An applicator for applying functional substances, such as cosmetic powder, food color marking, India ink effect marks, or drugs into human skin, having a base, a plurality of microneedles fixed to and projecting from the base a distance only sufficient to penetrate into the stratum corneum or dermis, with the microneedles being of a material that is capable of disintegration and dispersion into the stratum corneum or dermis, such as maltose. The needles contain the functional substance for delivery into the stratum corneum or dermis. The microneedles are of a length approximately 0.5 to 500 μm when used to apply a functional substance to the stratum corneum, or are of a length of approximately 500 to 5,000 μm when used to apply a functional substance to the dermis.
Owner:TOBINAGA YOSHIKAZU +1
Peptides as NS3-serine protease inhibitors of hepatitis C virus
InactiveUS7012066B2Organic active ingredientsDigestive systemSerine Protease InhibitorsHepatitis C virus
The present invention discloses novel compounds which have HCV protease inhibitory activity as well as methods for preparing such compounds. In another embodiment, the invention discloses pharmaceutical compositions comprising such compounds as well as methods of using them to treat disorders associated with the HCV protease.
Owner:DENDREON PHARMA INC +1
Small Molecule Modulators of HIV-1 Capsid Stability and Methods Thereof
InactiveUS20130165489A1Inhibiting and suppressing and preventing viral infectionBiocideOrganic chemistryViral infectionVirology
The present invention includes a method of inhibiting, suppressing or preventing a viral infection in a subject, comprising administering to the subject a pharmaceutical composition comprising one or more of the compounds useful within the invention.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Foamable vehicle and pharmaceutical compositions thereof
A hygroscopic pharmaceutical composition includes at least one hygroscopic substance at a concentration sufficient to provide an Aw value of at least 0.9 and an antiinfective agent. A foamble pharmaceutical carrier includes about 50% to about 98% of a polar solvent selected from the group consisting of a polyol and PEG; 0% to about 48% of a secondary polar solvent; about 0.2% to about 5% by weight of a surface-active agent; about 0.01% to about 5% by weight of at least one polymeric agent; and a liquefied or compressed gas propellant at a concentration of about 3% to about 25% by weight of the total composition.
Owner:VYNE THERAPEUTICS INC
Delivery of oral drugs
InactiveUS20010020147A1Comfortable and convenient motionComfortable and convenient feelPowder deliveryLiquid surface applicatorsMean diameterHuman patient
Disclosed is a system for delivery of a drug comprising a multiple unit dosing device comprising a housing and an actuator, said device containing multiple doses of multiparticulates comprising drug particles, said device upon actuation delivering a unit dose of said multiparticulates, said drug particles having a mean diameter of greater than 10 mum to about 1 mm such that an effective dose of said drug cannot be delivered into the lower lung of a human patient. Also disclosed are novel methods, devices and dosage forms for delivering a drug.
Owner:PHARMAKODEX LTD
Transdermal drug delivery compositions and topical compositions for application on the skin
InactiveUS20090053290A1Improve biological activityEliminate side effectsPowder deliveryCosmetic preparationsRadio frequencyOtic Agents
Transdermal delivery compositions and topical compositions for application to the skin are provided. The transdermal delivery composition includes at least two penetrants working synergistically but by disparate biochemical pathways. In one embodiment, the transdermal delivery system includes benzyl alcohol and lecithin organogel. The transdermal delivery compositions are used in a variety of topical compositions as a means of transdermally delivering and topically administering different drugs and agents, including compositions promoting collagen biosynthesis, retinoids and skin lighteners, chemical denervation agents such as BOTOX®, anti-fungal agents, anesthetics and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, these topical compositions may be used in combination with non-ablative treatment modalities, such as microdermabrasion, laser-based skin remodeling and radio-frequency-based skin remodeling.
Owner:NUVIANCE
Chemical linkers and conjugates thereof
The present disclosure provides drug-ligand conjugates that are potent cytotoxins, wherein the drug is linked to the ligand through either a peptidyl, hydrazine, or disulfide linker. The disclosure is also directed to compositions containing the drug-ligand conjugates, and to methods of treatment using them.
Owner:ER SQUIBB & SONS INC
Sustained release pharmaceutical compositions for the parenteral administration of hydrophilic compounds
InactiveUS7157099B2Easy to prepareOrganic active ingredientsPeptide/protein ingredientsParenteral nutritionBULK ACTIVE INGREDIENT
Owner:ITALFARMACO SPA
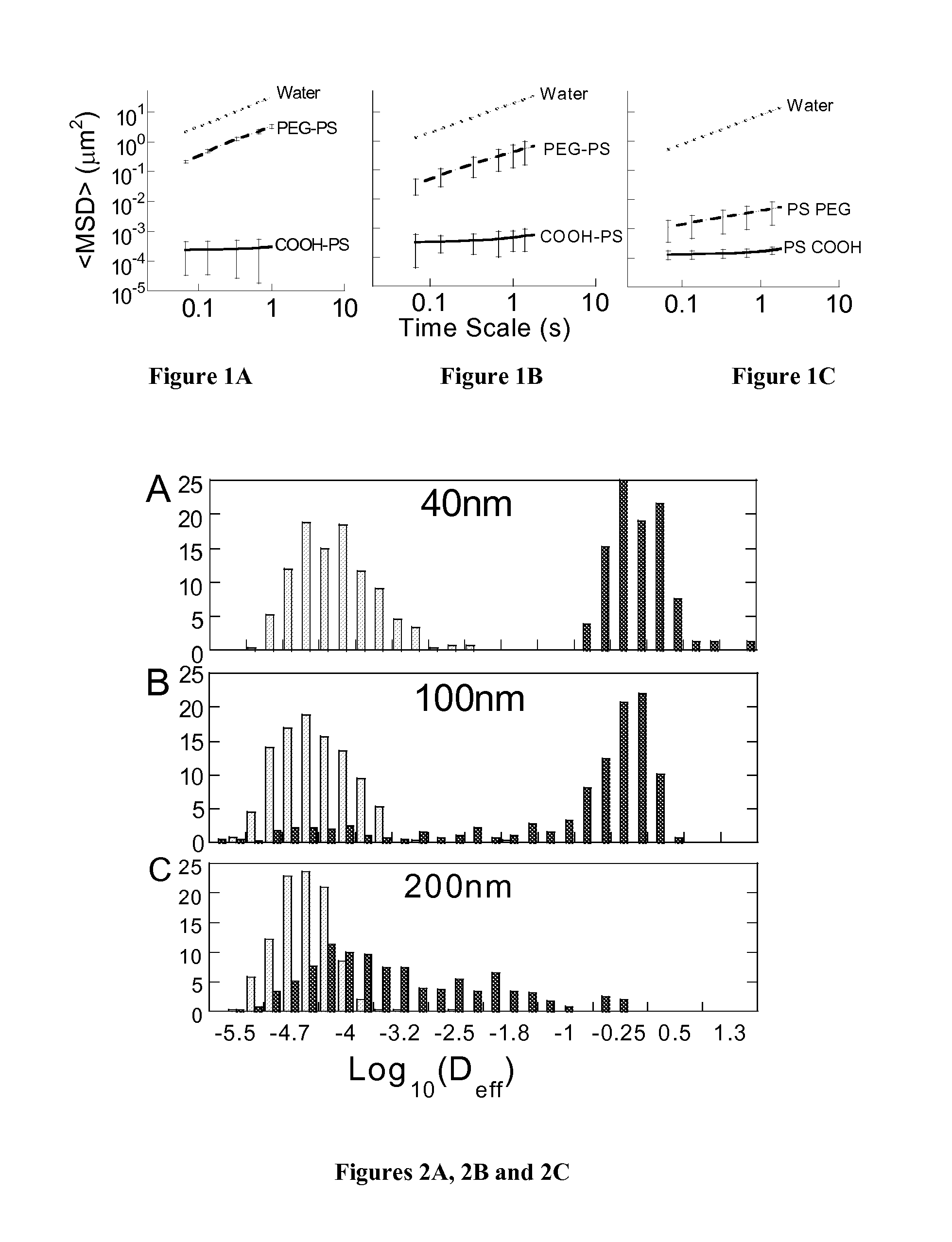
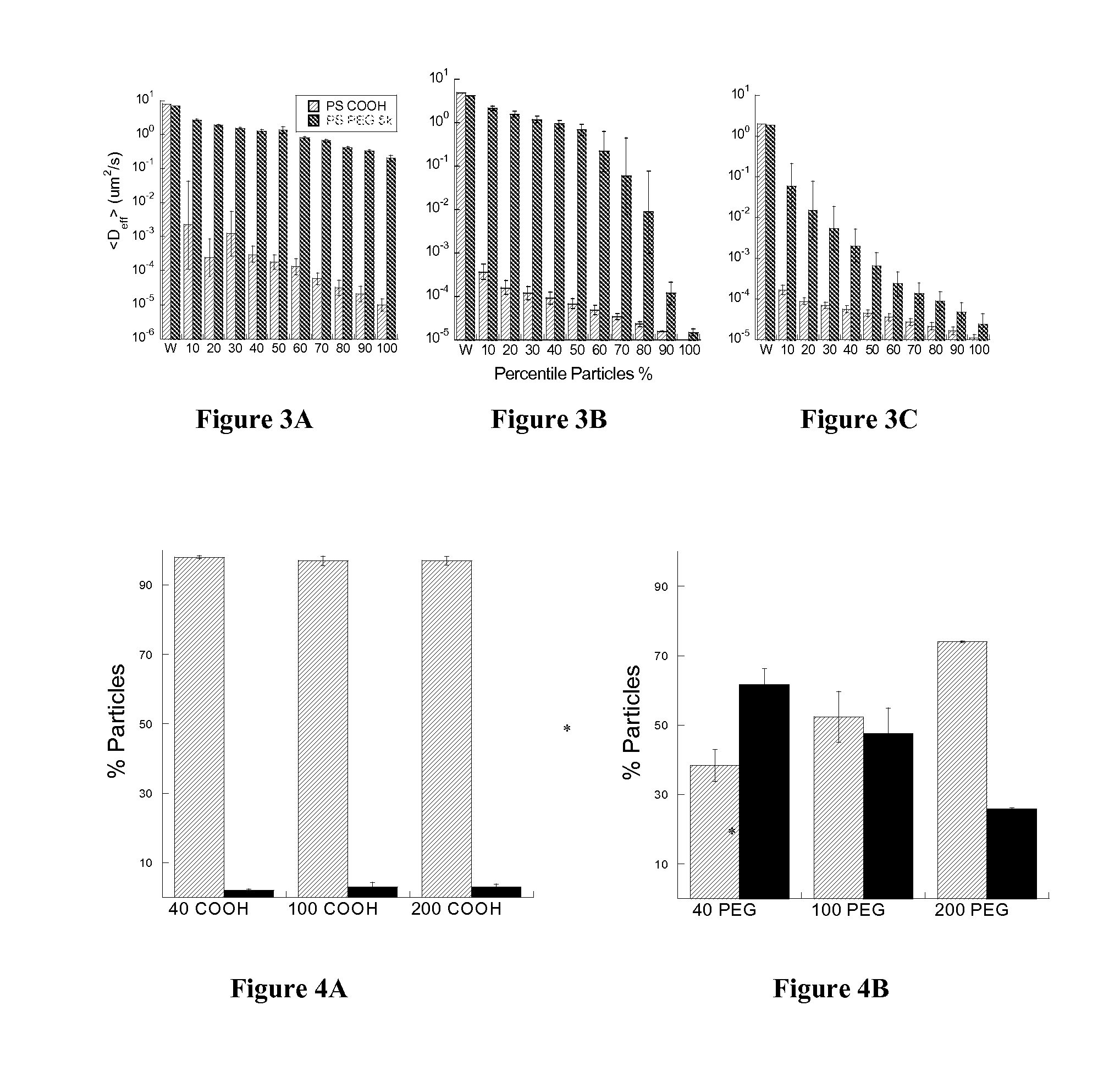
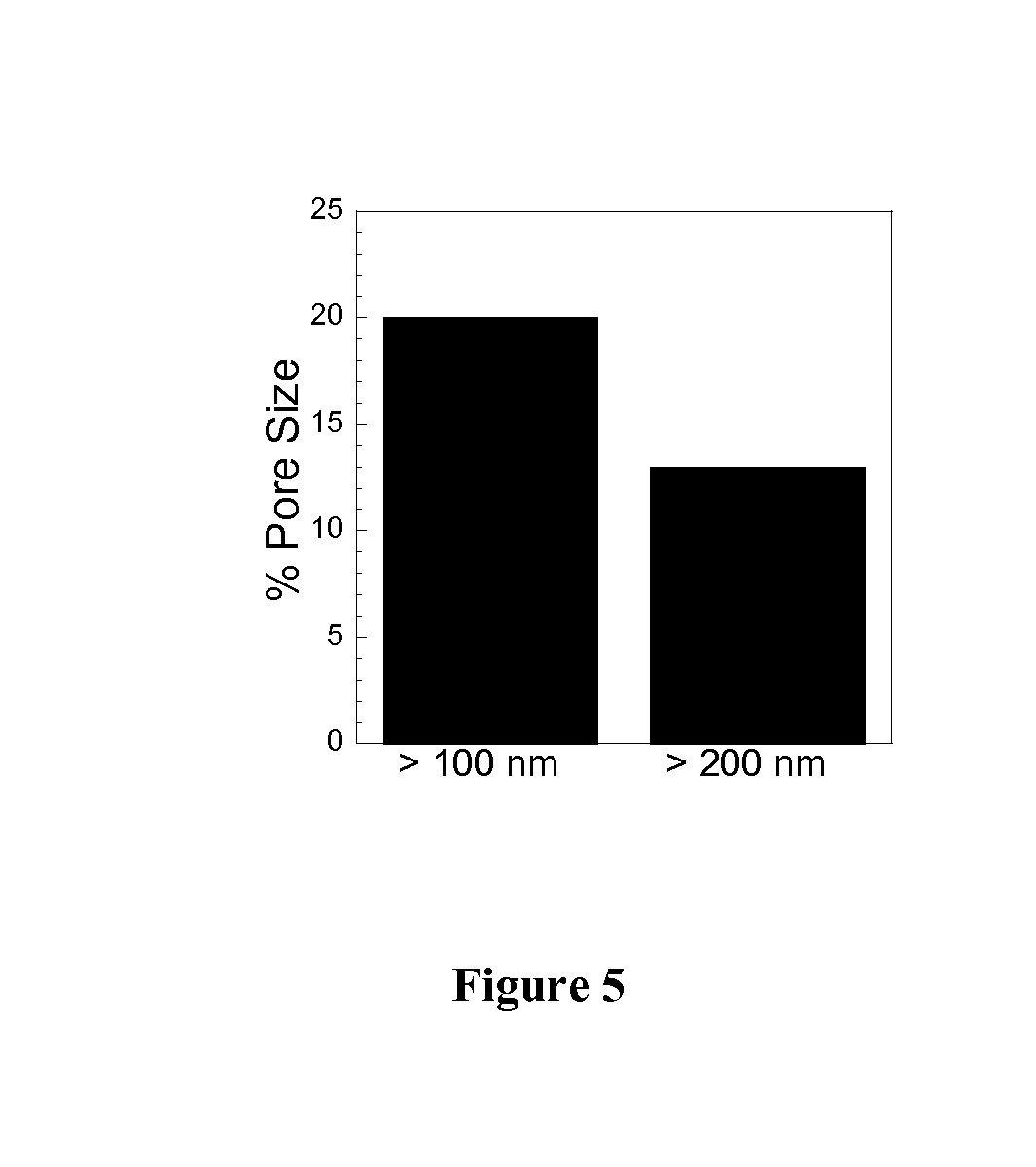
Rapid Diffusion of Large Polymeric Nanoparticles in the Mammalian Brain
ActiveUS20130183244A1Reduce deliveryHigher drug payloadPowder deliveryNervous disorderGene deliveryHydrophilic coating
Non-adhesive particles as large as 110 nm can diffuse rapidly in the brain ECS, if coated with hydrophilic coatings such as PEG coatings and preferably having neutral surface charge. The ability to achieve brain penetration with larger particles will significantly improve drug and gene delivery within the CNS since larger particles offer higher drug payload, improved drug loading efficiency, and significantly longer drug release durations.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
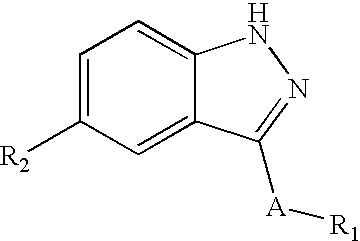
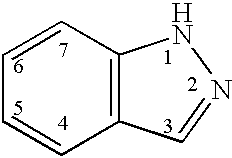
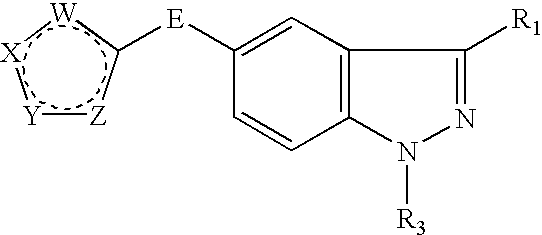
Indazole derivatives as JNK inhibitors and compositions and methods related thereto
Compounds having activity as selective inhibitors of JNK are disclosed. The compounds of this invention are indazole derivatives having the following structure: wherein R1, R2 and A are as defined herein. Such compounds have utility in the treatment of a wide range of conditions that are responsive to JNK inhibition. Thus, methods of treating such conditions are also disclosed, as are pharmaceutical compositions containing one or more compounds of the above compounds.
Owner:SIGNAL PHARMA LLC
Novel flavors, flavor modifiers, tastants, taste enhancers, umami or sweet tastants, and/or enhancers and use thereof
ActiveUS20050084506A1Enhancing savory tasteIncrease sweet tasteBiocideCosmetic preparationsMonosodium glutamateSalty taste
The present invention relates to the discovery that certain non-naturally occurring, non-peptide amide compounds and amide derivatives, such as oxalamides, ureas, and acrylamides, are useful flavor or taste modifiers, such as a flavoring or flavoring agents and flavor or taste enhancer, more particularly, savory (the “umami” taste of monosodium glutamate) or sweet taste modifiers,—savory or sweet flavoring agents and savory or sweet flavor enhancers, for food, beverages, and other comestible or orally administered medicinal products or compositions.
Owner:SENOMYX INC
Topical Nutraceutical Compositions with Selective Body Slimming and Tone Firming Antiaging Benefits
InactiveUS20040146539A1Cut skinReduction in signCosmetic preparationsToilet preparationsPimpleWrinkle skin
I have discovered cosmetic or topical pharmaceutical compositions for external body part or organ slimming, firming, cellulite reduction, fat-reduction, and obesity control benefits that are in synergistic combination with benefits for the treatment of skin aging, skin wrinkles reduction, skin exfoliating, treatment of acne, treatment of rosacea, age-spots reduction, skin surface whitening, skin surface brightening striae distensae (stretch marks) reduction, treatment of pimples, treatment of skin infections and lesions, spider veins reduction, blood microcirculation (venous insufficiency) improvement, UVA / UVB protection of skin, and skin redness reduction. These compositions thus provide multiple combinations of skin and external body part or organ enhancement benefits that can be selective and specific for external body parts and organs such as face, chin, cheeks, arms, "love handles" in abdomen area, eye lids and eye zone, neck, breasts, thighs, and hips. These compositions include a body beneficial composition selected from certain nutraceutical, cosmetic, and pharmaceutical ingredients, a composition to promote collagen and elastin synthesis in the skin, and a cosmetically or pharmaceutically acceptable delivery system.
Owner:GUPTA SHYAM K
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com