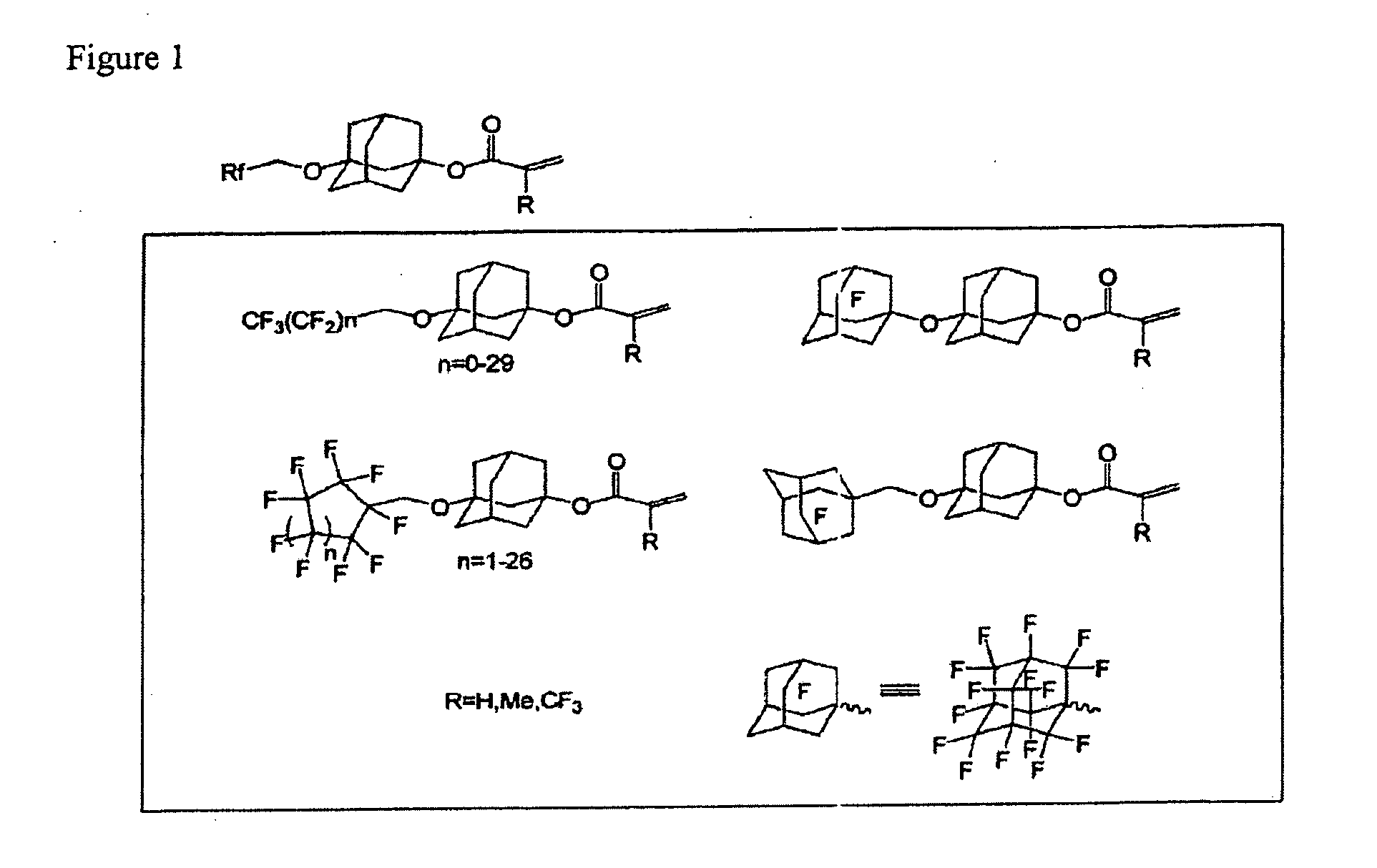
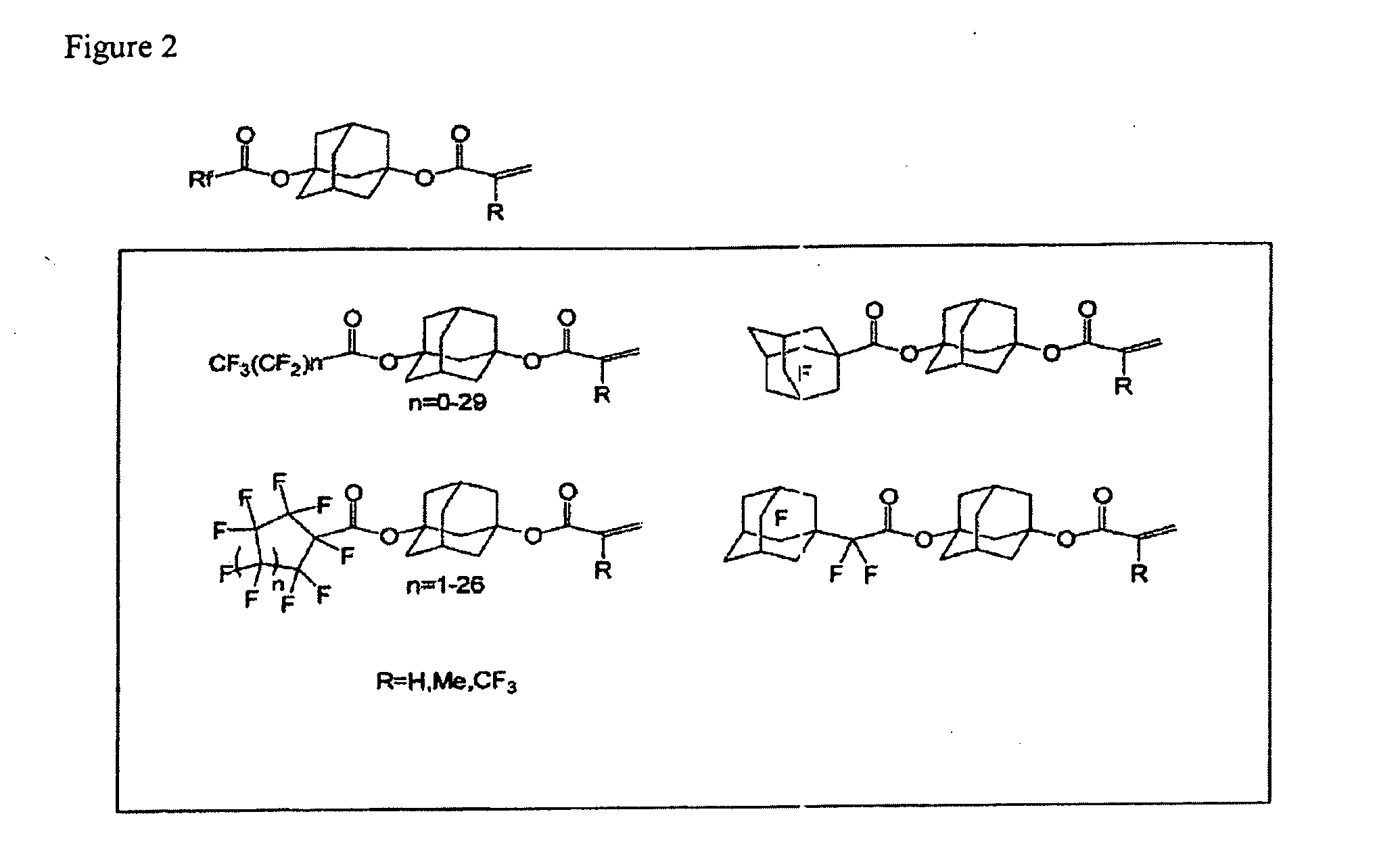
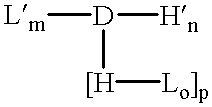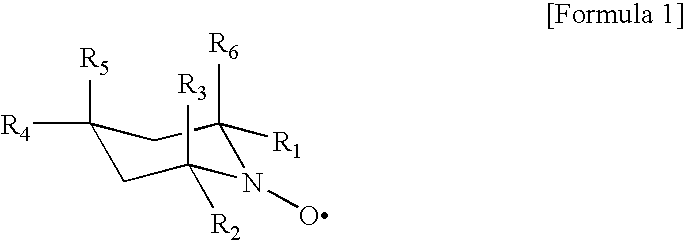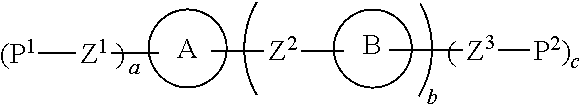Patents
Literature
966 results about "Adamantane" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
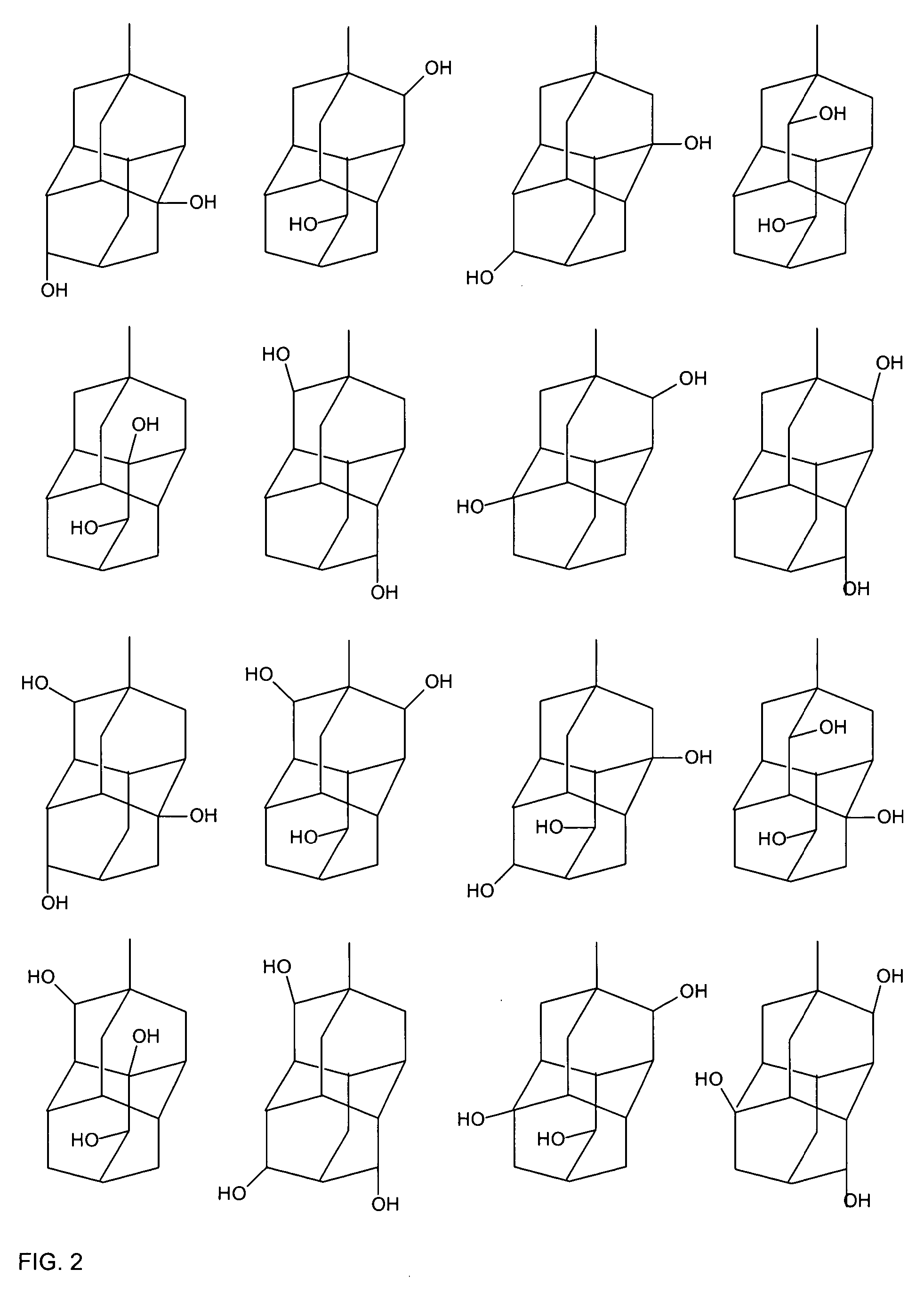
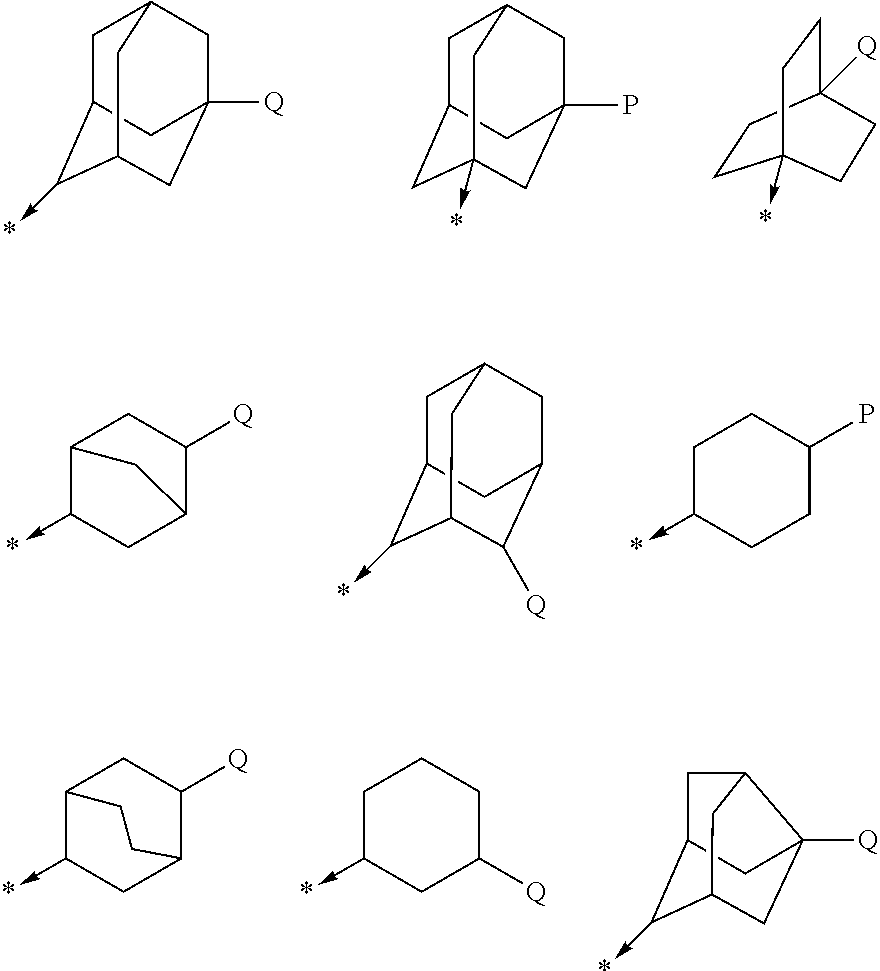
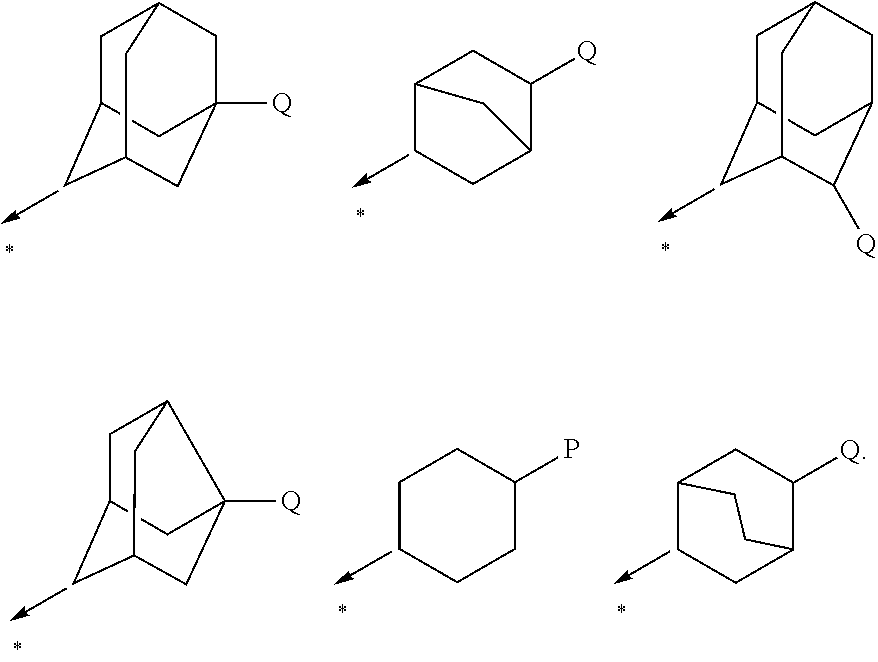
Adamantane is a colorless, crystalline chemical compound with a camphor-like odor. With a formula C₁₀H₁₆, it is a cycloalkane and also the simplest diamondoid. Adamantane molecules consists of three connected cyclohexane rings arranged in the "armchair" configuration. It is unique in that it is both rigid and virtually stress-free. Adamantane is the most stable among all the isomers with formula C₁₀H₁₆, which include the somewhat similar twistane. The spatial arrangement of carbon atoms in the adamantane molecule is the same as in the diamond crystal. This motivates the name adamantane, which is derived from the Greek adamantinos (relating to steel or diamond).
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
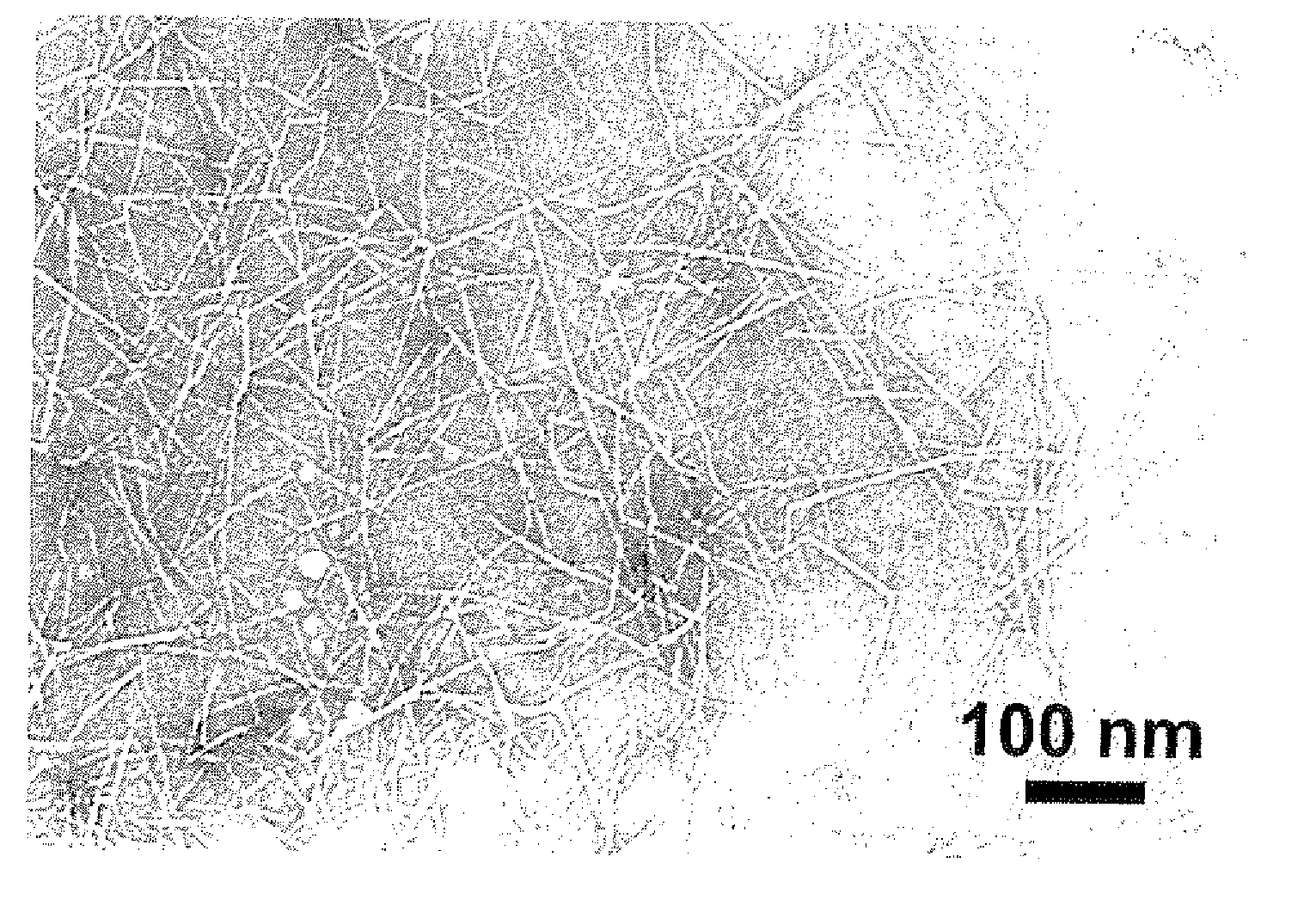
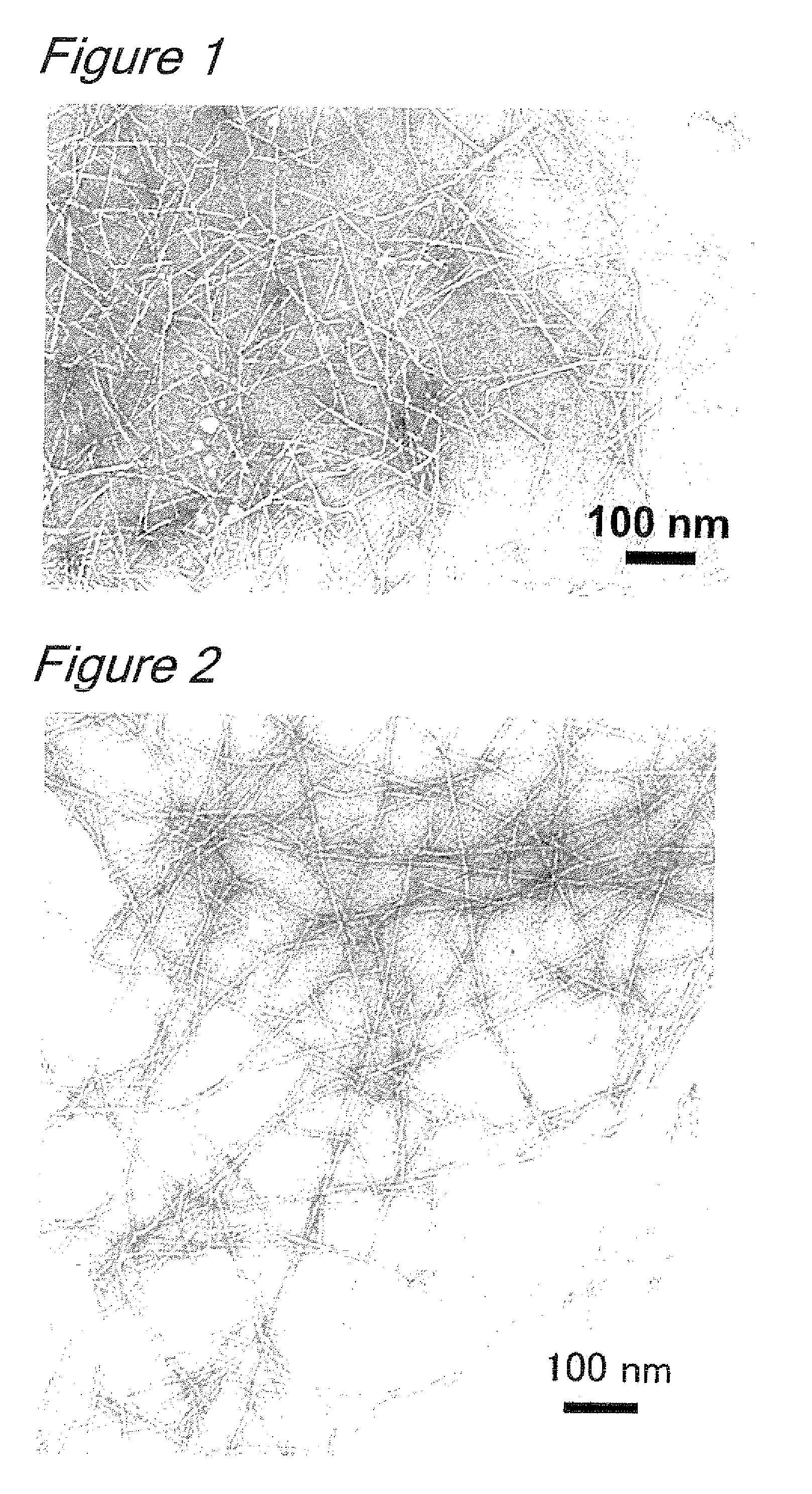
Processes for producing cellulose nanofibers, cellulose oxidation catalysts and methods for oxidizing cellulose
The present invention aims to provide a process for producing cellulose nanofibers using a 4-hydroxy TEMPO derivative less expensive than TEMPO and a process capable of rapidly producing homogeneous cellulose nanofibers. Wood cellulose can be efficiently converted into nanofibers by a process for producing cellulose nanofibers comprising treating a cellulosic material with an oxidizing agent in water in the presence of a cellulose oxidation catalyst comprising an N-oxyl compound represented by formula 1 below:wherein R1 and R2 are each independently hydrogen or a C1-C6 straight or branched alkyl group; and(i) one of R4 or R5 is —OR, —OCOR or —OSO2R wherein R is a straight or branched carbon chain having 4 or less carbon atoms, and the other of R4 or R5 is hydrogen, and R3 and R6 are methyl, or(ii) R4 is hydrogen, and R5, R3 and R6 are taken together with a piperidine ring to form an aza-adamantane compound having formula 2 below:or a mixture thereof, and a compound selected from the group consisting of bromides, iodides and mixtures thereof to prepare oxidized cellulose, and microfibrillating the oxidized cellulose to convert it into nanofibers.
Owner:NIPPON PAPER IND CO LTD
Aromatic polycarbonate resin, process for producing the same, optical-part molding material, and optical part
InactiveUS7112644B2Increase resistanceHigh transparencyOrganic chemistryRecord information storageHeat resistancePolycarbonate
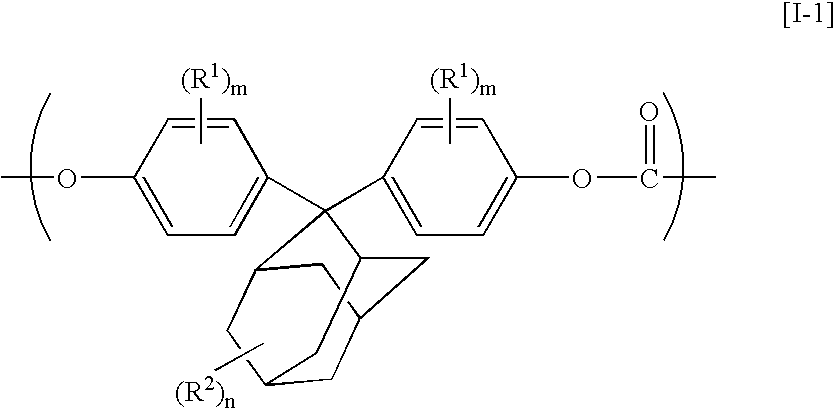
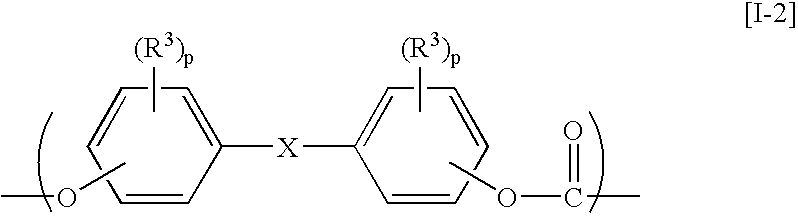
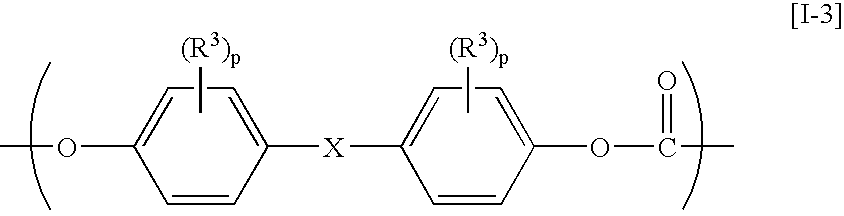
The present invention relates to an aromatic polycarbonate resin which comprises repetitive units originating in residual groups of a 2,2-bis(4-hydroxyphenyl)adamantane compound and a 1,3-bis(4-hydroxyphenyl)adamantane compound having a substituent on an aromatic ring and which is excellent in a transparency, a heat resistance and a mechanical strength and has a good moldability and a production process for the aromatic polycarbonate resin described above in which the adamantane compounds described above are reacted with a carbonic ester-forming compound. Further, it relates to an aromatic polycarbonate resin which is excellent in an optical characteristic and an optical part prepared by molding the same.
Owner:IDEMITSU KOSAN CO LTD
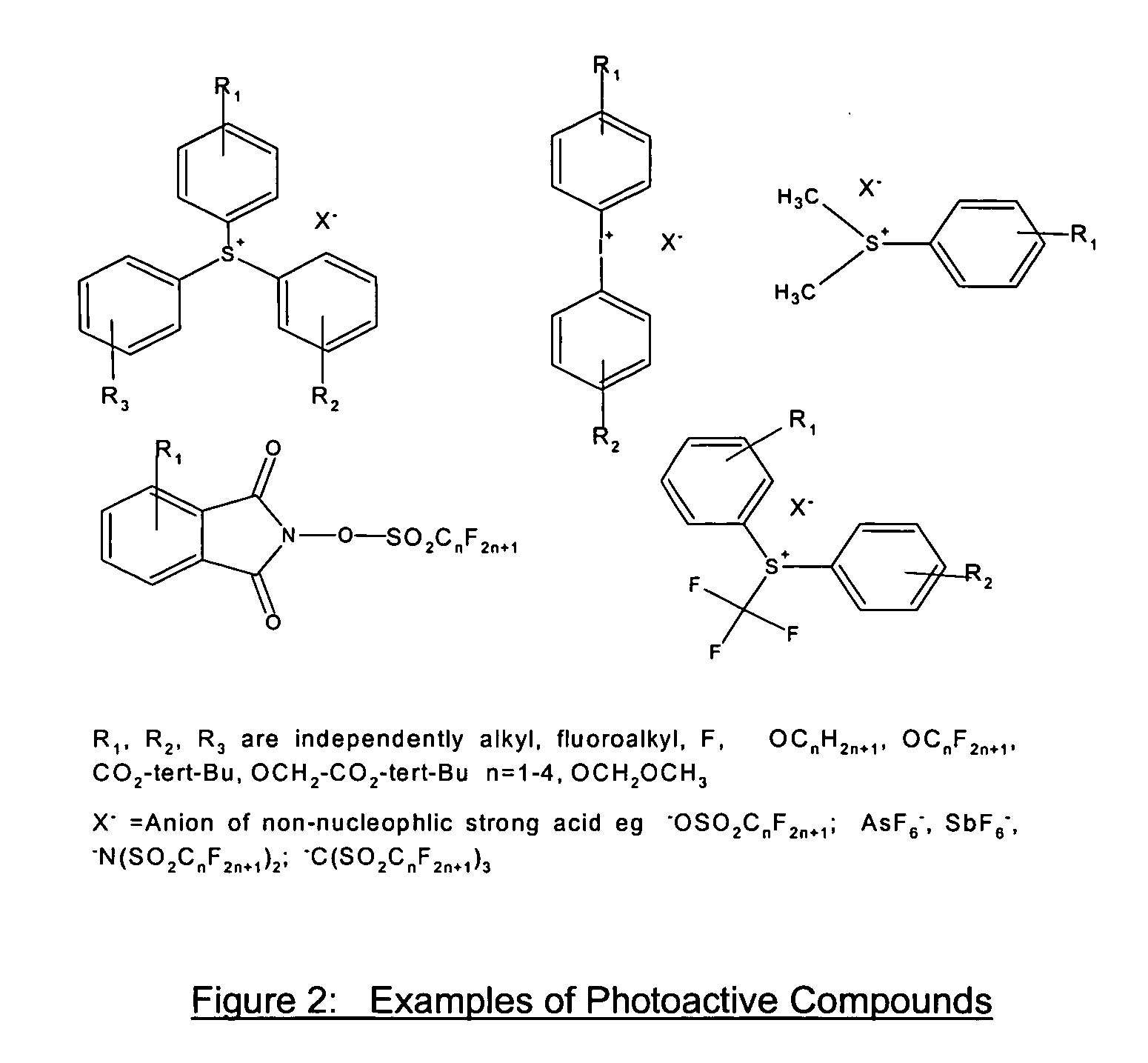
Positive photosensitive composition and pattern-forming method using the same
InactiveUS20070054217A1Positive photosensitive compositionIncrease exposurePhotosensitive materialsPhotomechanical apparatusMethacrylateActinic Rays
A positive photosensitive composition comprises: (A) a compound capable of generating an acid upon irradiation with actinic ray or radiation; and (B) a resin having a group capable of decomposing by action of an acid to increase solubility of the group in an alkali developer, wherein the resin (B) comprises: at least one methacrylate repeating unit; at least one acrylate repeating unit; and at least one repeating unit (Ba) having a diamantane structure.
Owner:FUJIFILM CORP +1
Acetal compound, polymer, resist composition, and patterning process
ActiveUS20110236831A1High resolutionEfficient use ofOrganic chemistryOrganic compound preparationPolymer scienceOrganosolv
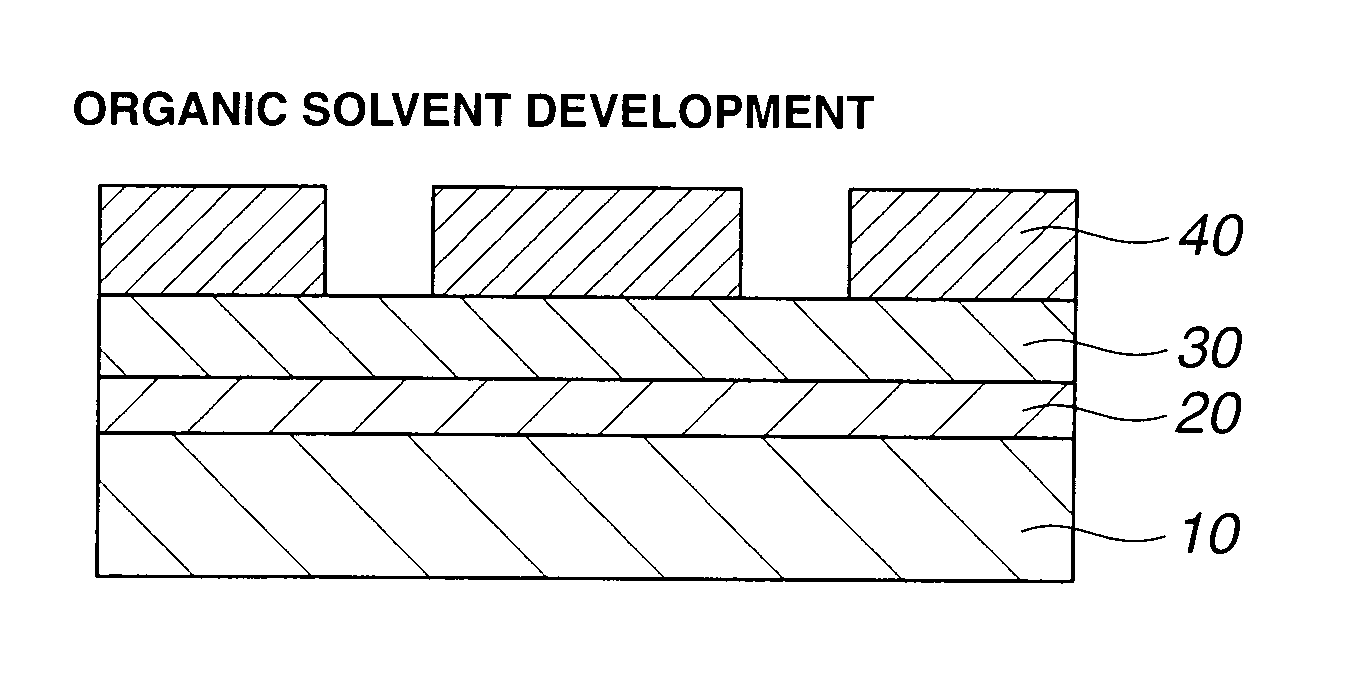
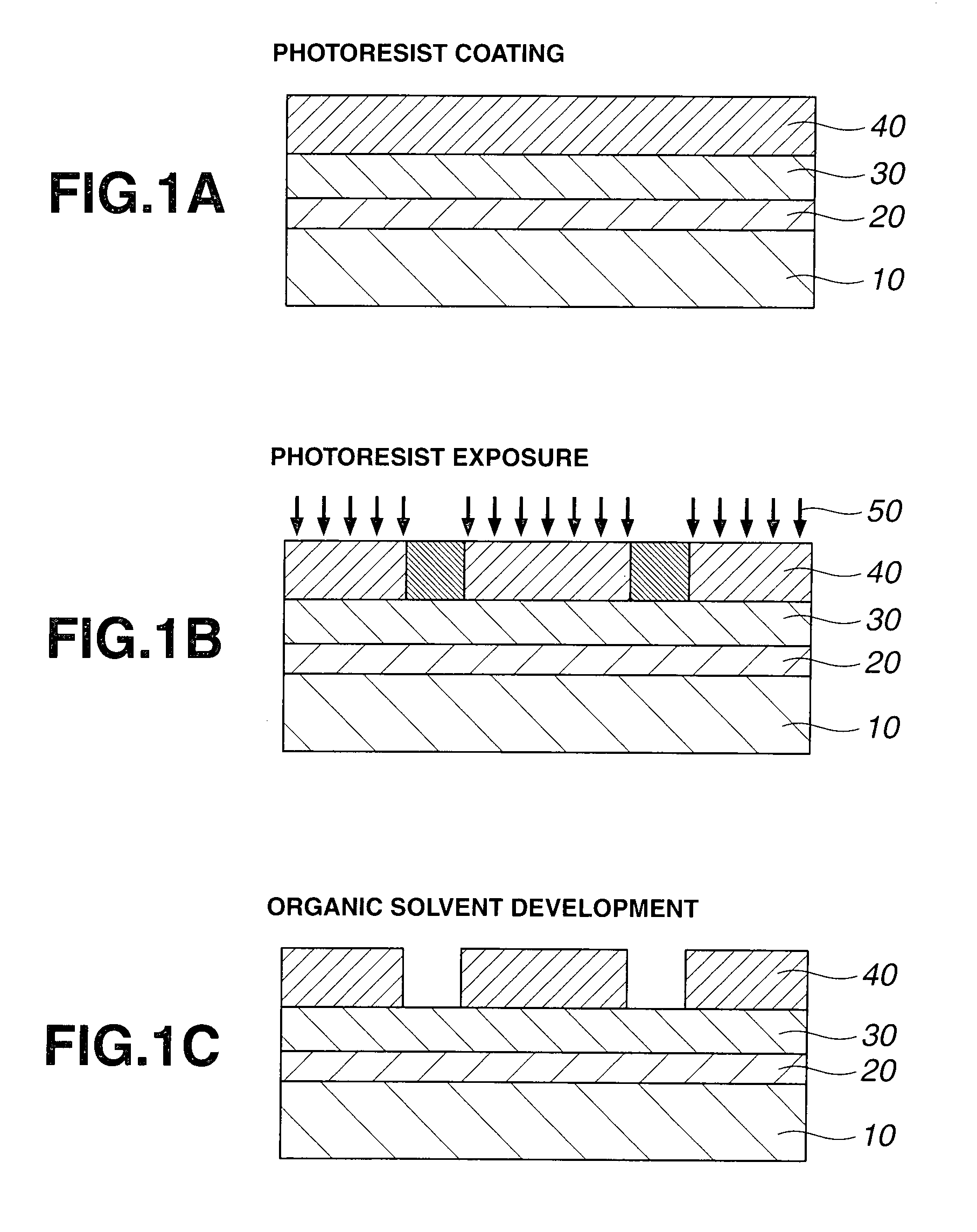
The invention provides an acetal compound containing an adamantane ring having an alcoholic hydroxyl group which is protected with an acetal group having a carbonyl moiety of branched structure. A photoresist film comprising a polymer comprising recurring units derived from the acetal compound and an acid generator is characterized by a high dissolution contrast when it is subjected to exposure and organic solvent development to form an image via positive / negative reversal.
Owner:SHIN ETSU CHEM IND CO LTD
Polycarbocyclic derivatives for modification of resist, optical and etch resistance properties
InactiveUS20050026068A1Suitable absorption propertyExcellent etch resistanceEsterified saccharide compoundsSugar derivativesResistCarbon chain
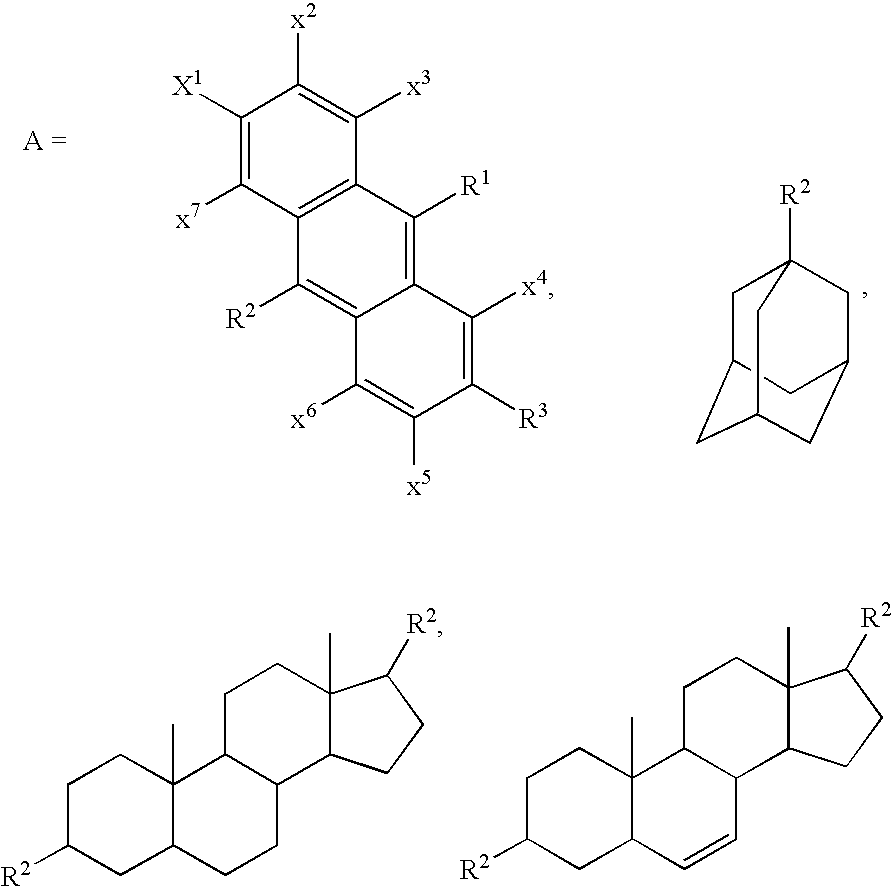
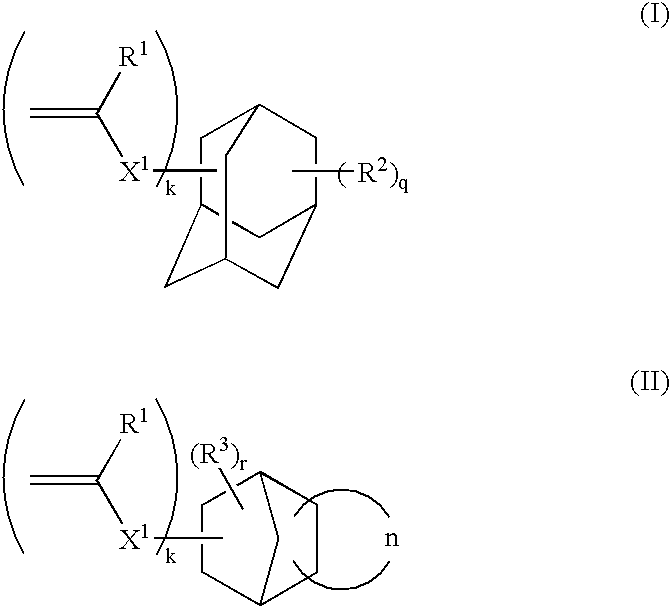
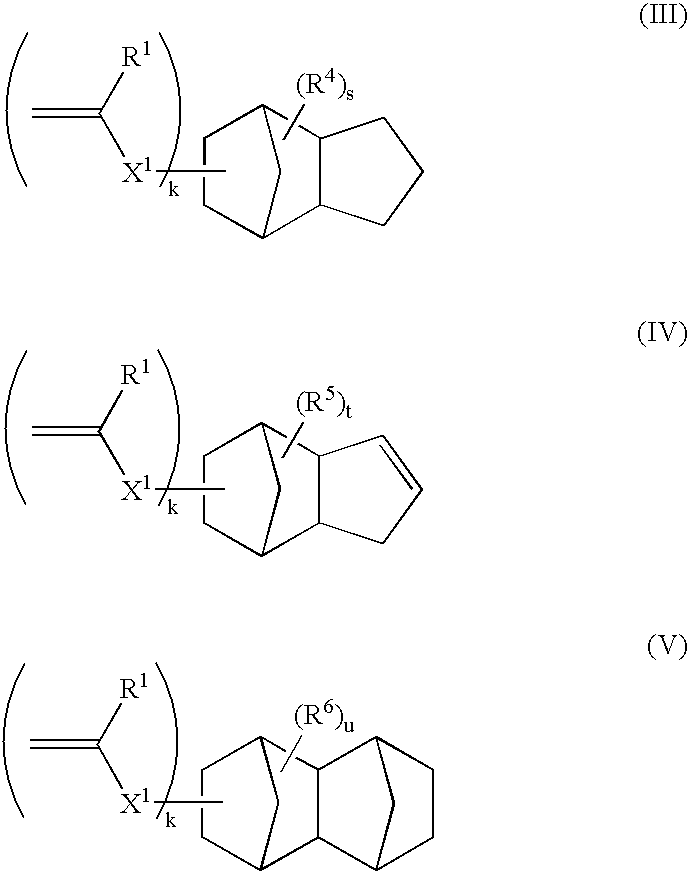
Mixed carbocycle derivatives containing at least two carbocycles per molecule from the group of anthracenes, adamantanes and steroids with functionalized carbon chains are synthesized and used as modifiers of resist properties and especially etch resistance enhancement and absorption characteristics. These derivatives are characterized by formulas I-V, where A and R may be an anthryl- and / or an adamantyl- and / or a steroid moiety. Methods for the preparation of the above compounds are disclosed.
Owner:GOGOLIDES EVANGELOS +5
Photoresist compositions comprising diamondoid derivatives
InactiveUS20050074690A1Improve hydrophilicityMore solubleOrganic compound preparationRadiation applicationsResistDiamantane
Novel positive-working photoresist compositions are disclosed. The monomers of the base resin of the resist contain diamondoid-containing pendant groups higher than adamantane in the polymantane series; for example, diamantane, triamantane, tetramantane, pentamantane, hexamantane, etc. The diamondoid-containing pendant group may have hydrophilic-enhancing substituents such as a hydroxyl group, and may contain a lactone group. Advantages of the present compositions include enhanced resolution, sensitivity, and adhesion to the substrate.
Owner:CHEVROU USA INC
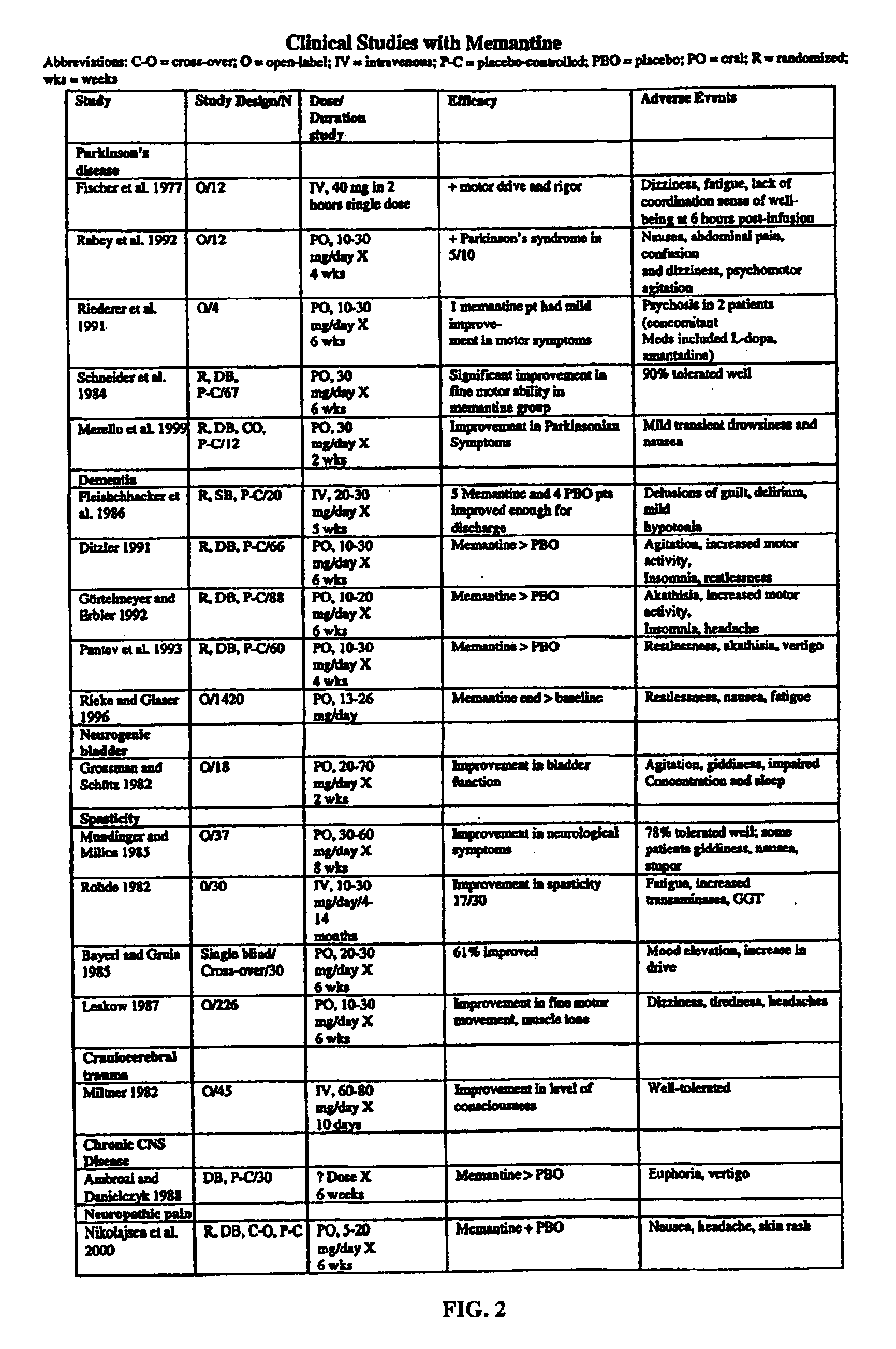
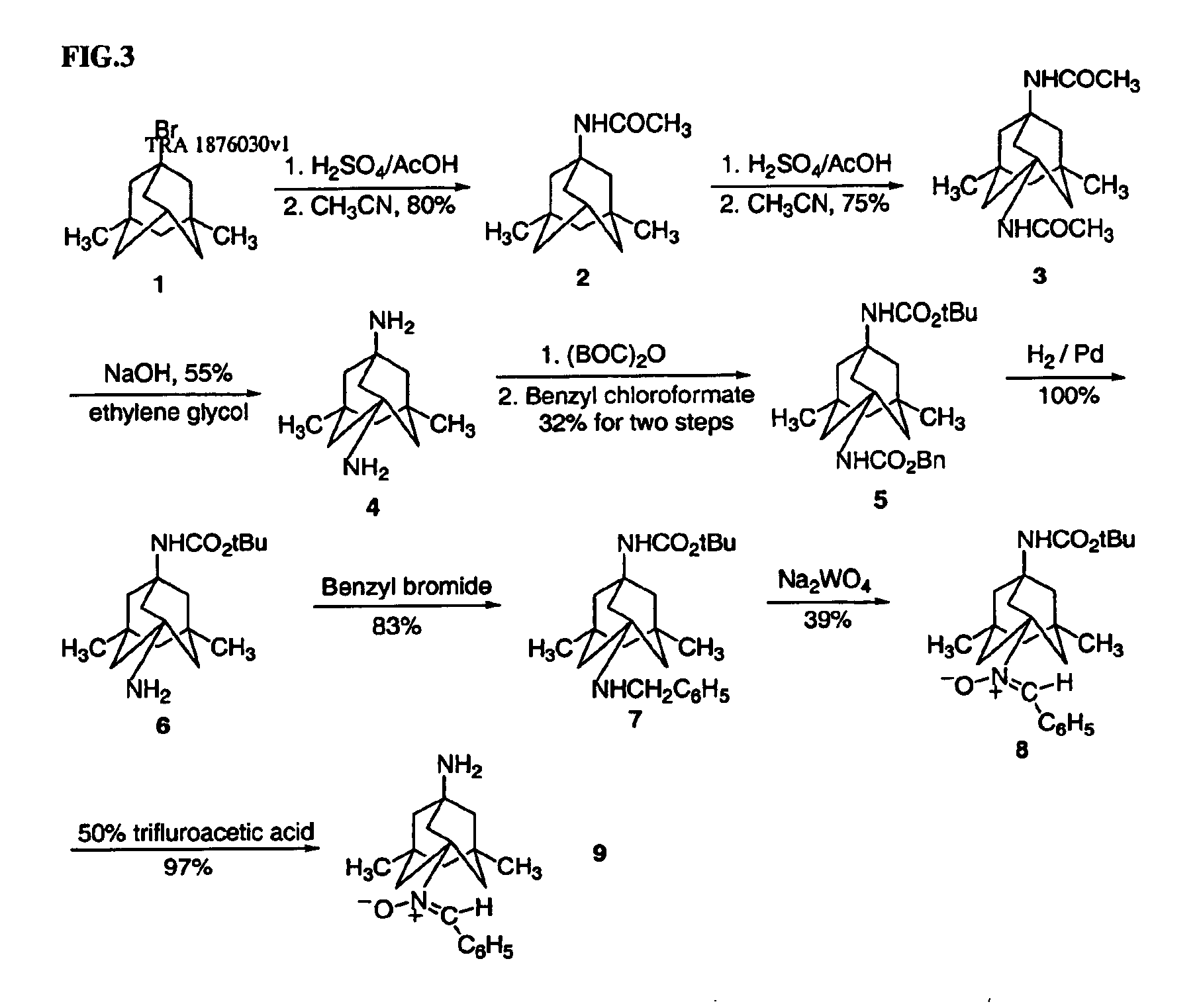
Preparation method of memantine hydrochloride
InactiveCN1400205AGet stableOperational securityNervous disorderOrganic compound preparationMemantine HydrochlorideSolvent
The preparation method of medicine for curing dementia, N-methyl-D-aspartic acid (NMDA) receptor antagonist memantine hydrochloride includes the following steps: using polybasic alcohol as solvent; making 1-bromo-3,5-dimethyl adamantane and urea according to the mole ratio of 1:0.25-10 react for 0.5-48 hr. at 20-200 deg.C; after the reaction is completed, adding sodium hydroxide into the reactionsolution according to that the mole ratio of 1-bromo-3,5-dimethyl adamantane and sodium hydroxide is 1:0.1-10, making alcoholysis at 50-200 deg.C, chloroform extraction, concentration, acidification with hydrochloric acid and salt-forming so as to obtain the invented menantine hydrochloride. It features safe and simple operation and low cost, etc.
Owner:GUANGZHOU INST OF GEOCHEMISTRY - CHINESE ACAD OF SCI
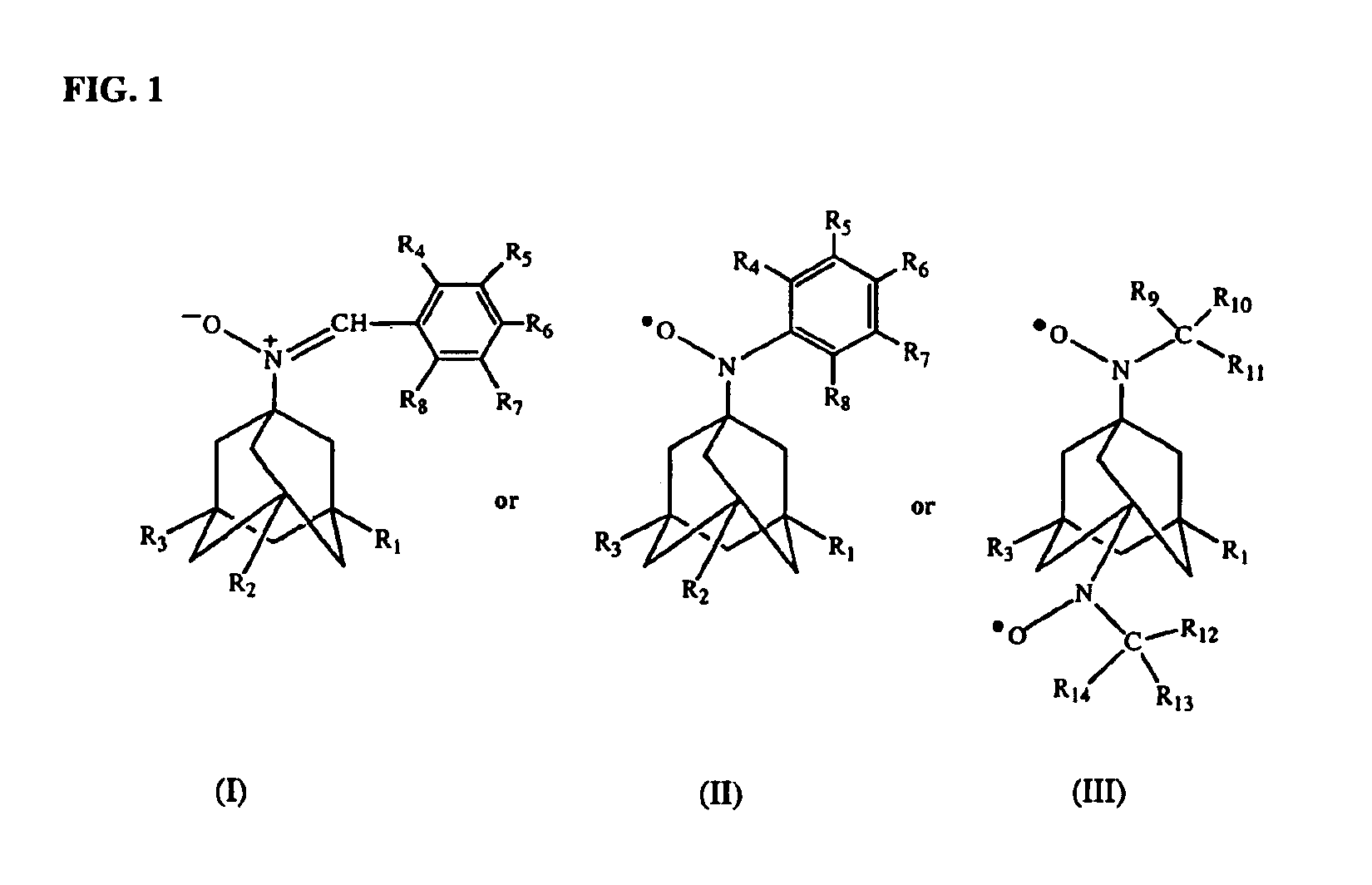
Antioxidant nitroxides and nitrones as therapeutic agents
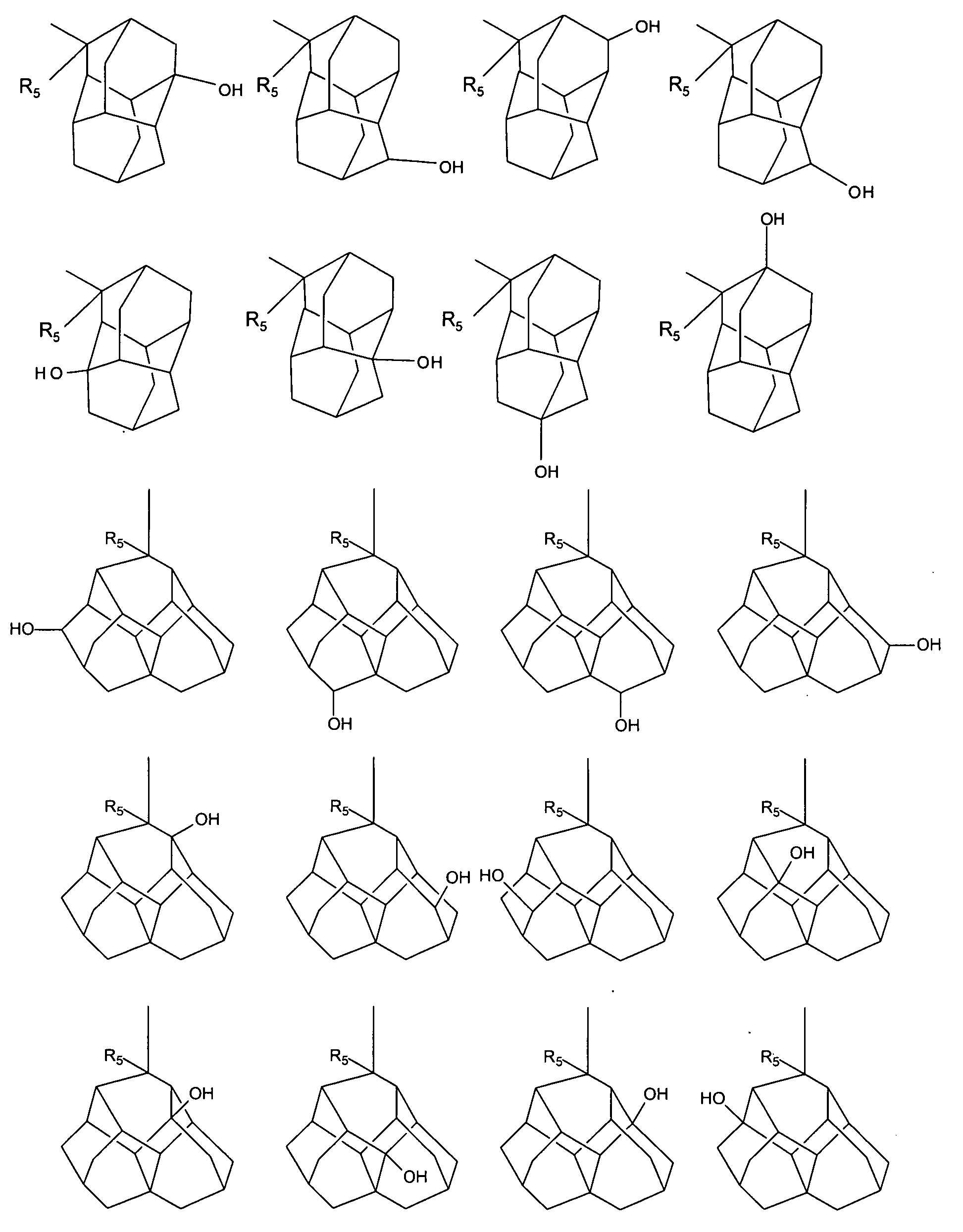
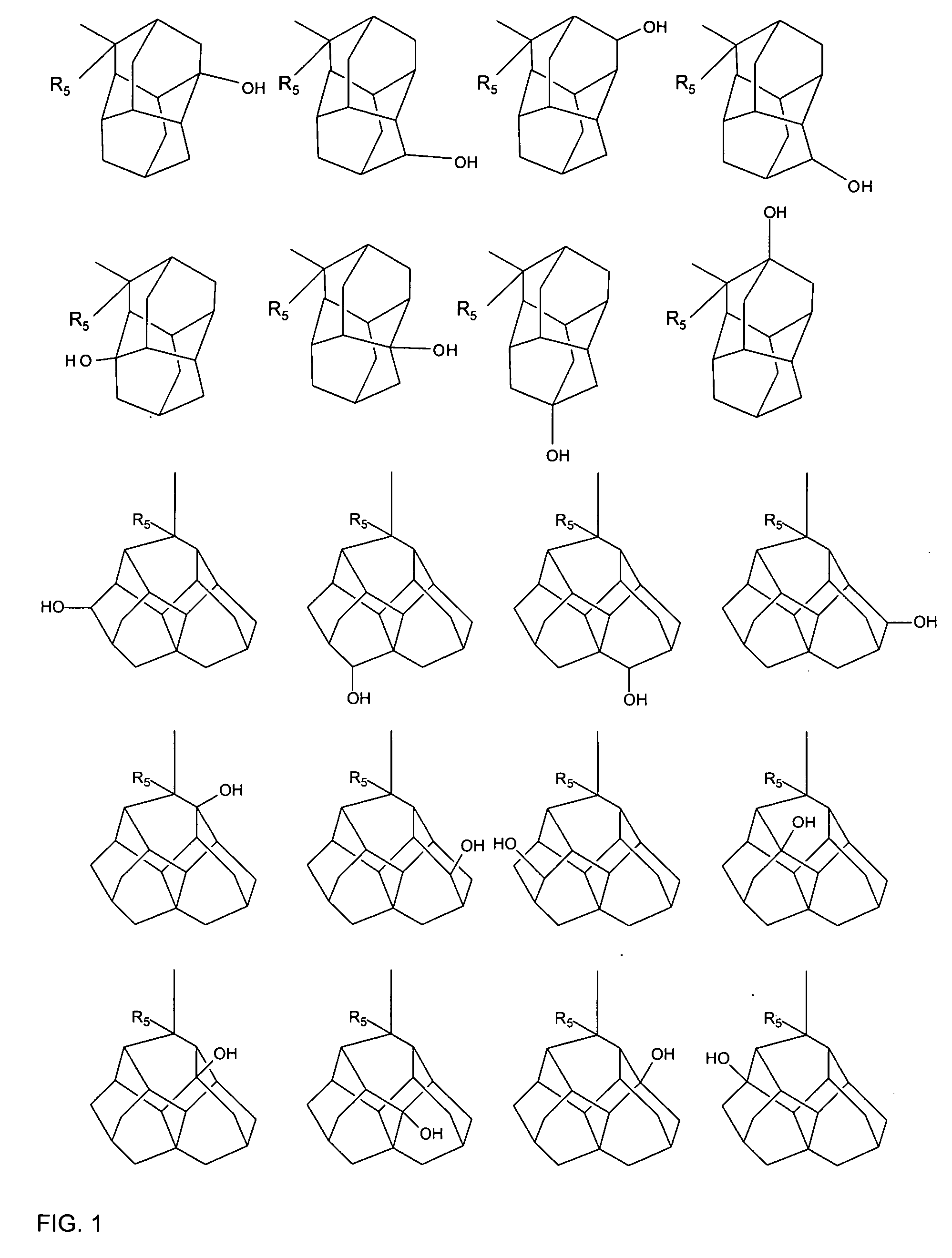
The invention provides novel adamantane compounds having one of the following formulas: wherein:R1 and R3 are H, OH, alkyl, cycloalkyl, amino or aryl, and can be the same or different;R2 is H, NH2, alkyl, OH, COOH, amino, amide, or carbamate;R4-R8 are H, OH, NH2, alkyl, OH, COOH, ester, amino, amide, or alkyloxy, and can be the same or different;R9-R14 are H, alkyl, or phenyl, and can be the same or different;and wherein when any of R1-R8 is amino, the compounds are the free bases and their acid addition salts.The present invention also relates to compositions and methods for treating and / or preventing neurological and inflammatory disorders in a patient by administering a therapeutically effective amount of the compounds of formula (I), (II) or (III) and a pharmaceutically acceptable carrier.
Owner:PANORAMA RES INC +1
Photoresist composition
The present invention relates to a photosensitive composition useful at wavelengths between 300 nm and 10 nm which comprises a polymer containing a substituted or unsubstituted higher adamantane.
Owner:MERCK PATENT GMBH
Novel organic electroluminescent compounds and organic electroluminescent device using the same
InactiveUS20090230852A1Excellent backboneImprove luminous efficiencyGroup 5/15 element organic compoundsGroup 8/9/10/18 element organic compoundsPolymer scienceHeteroatom
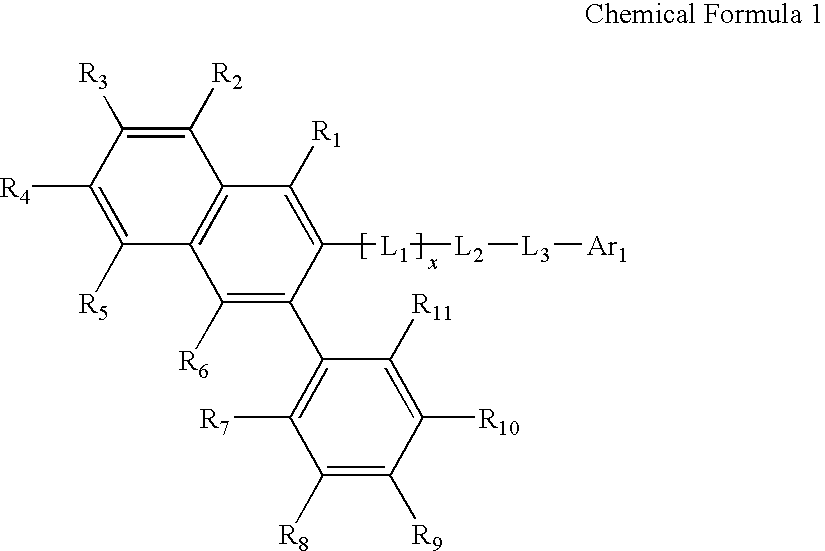
The organic electroluminescent compounds according to the present invention are represented by Chemical Formula (1):wherein, L1 represents (C6-C60)arylene or (C3-C60)heteroarylene containing one or more heteroatom(s) selected from N, O and S, or a bivalent group selected from the following structures:L2 and L3 independently represent a chemical bond, or (C1-C60)alkyleneoxy, (C1-C60)alkylenethio, (C6-C60)aryleneoxy, (C6-C60)arylenethio, (C6-C60)arylene or (C3-C60)heteroarylene containing one or more heteroatom(s) selected from N, O and S;Ar1 represents NR41R42, (C6-C60)aryl, (C3-C60)heteroaryl containing one or more heteroatom(s) selected from N, O and S, a 5- or 6-membered heterocycloalkyl containing one or more heteroatom(s) selected from N, O and S, (C3-C60)cycloalkyl, adamantyl, (C7-C60)bicycloalkyl, or a substituent selected from the following structures:and x is an integer from 1 to 4.
Owner:GRACEL DISPLAY INC
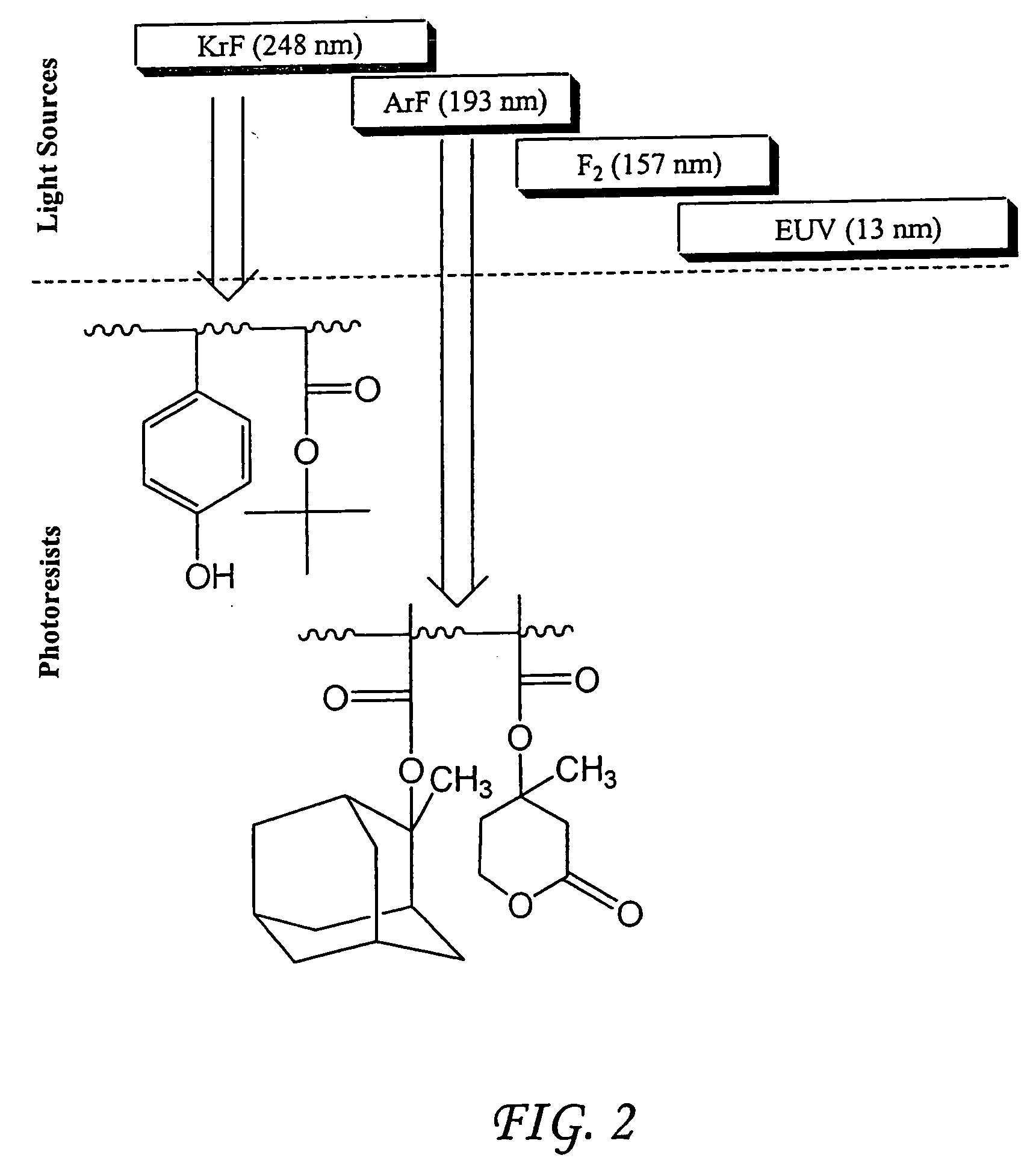
Photoresist composition for deep UV and process thereof
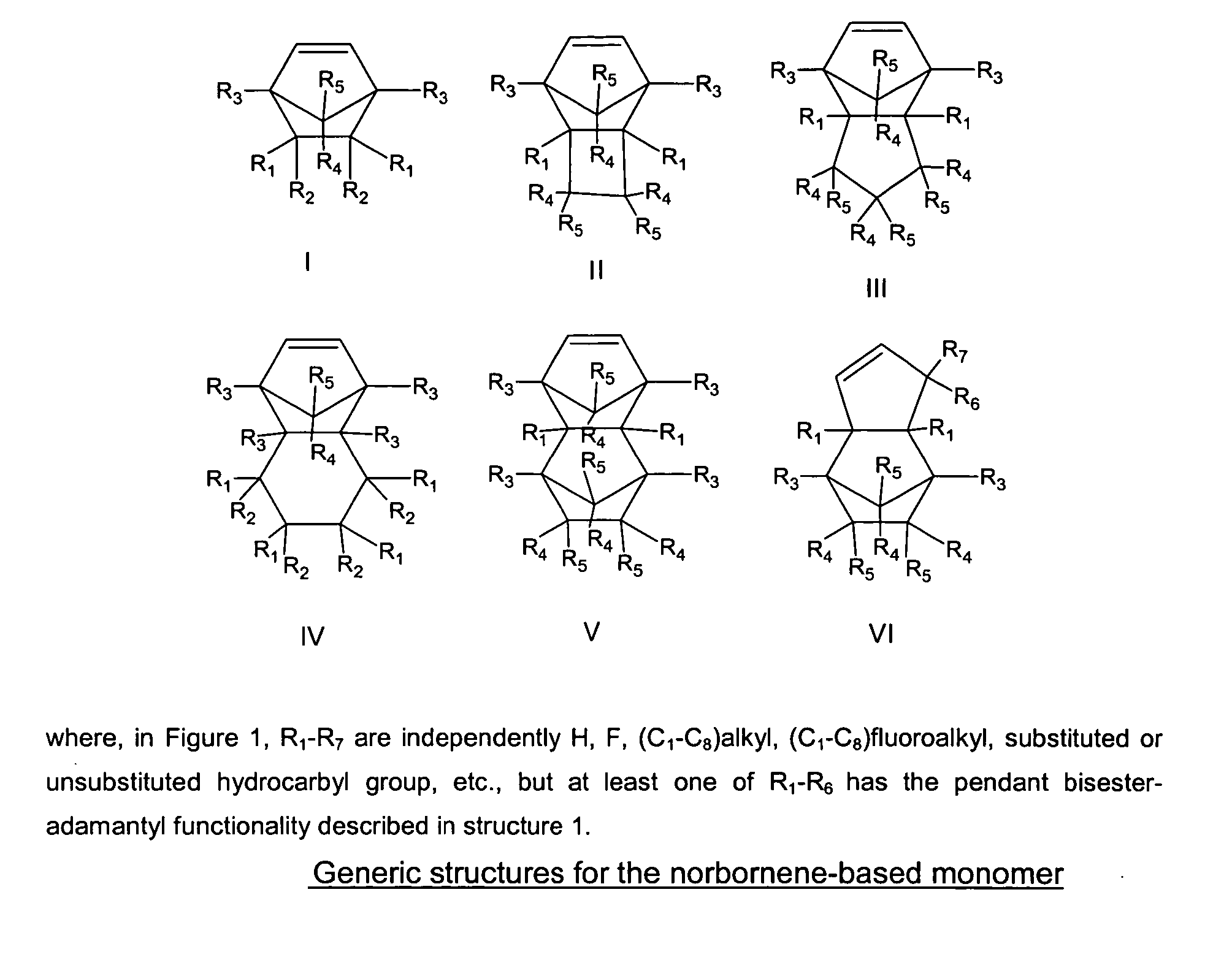
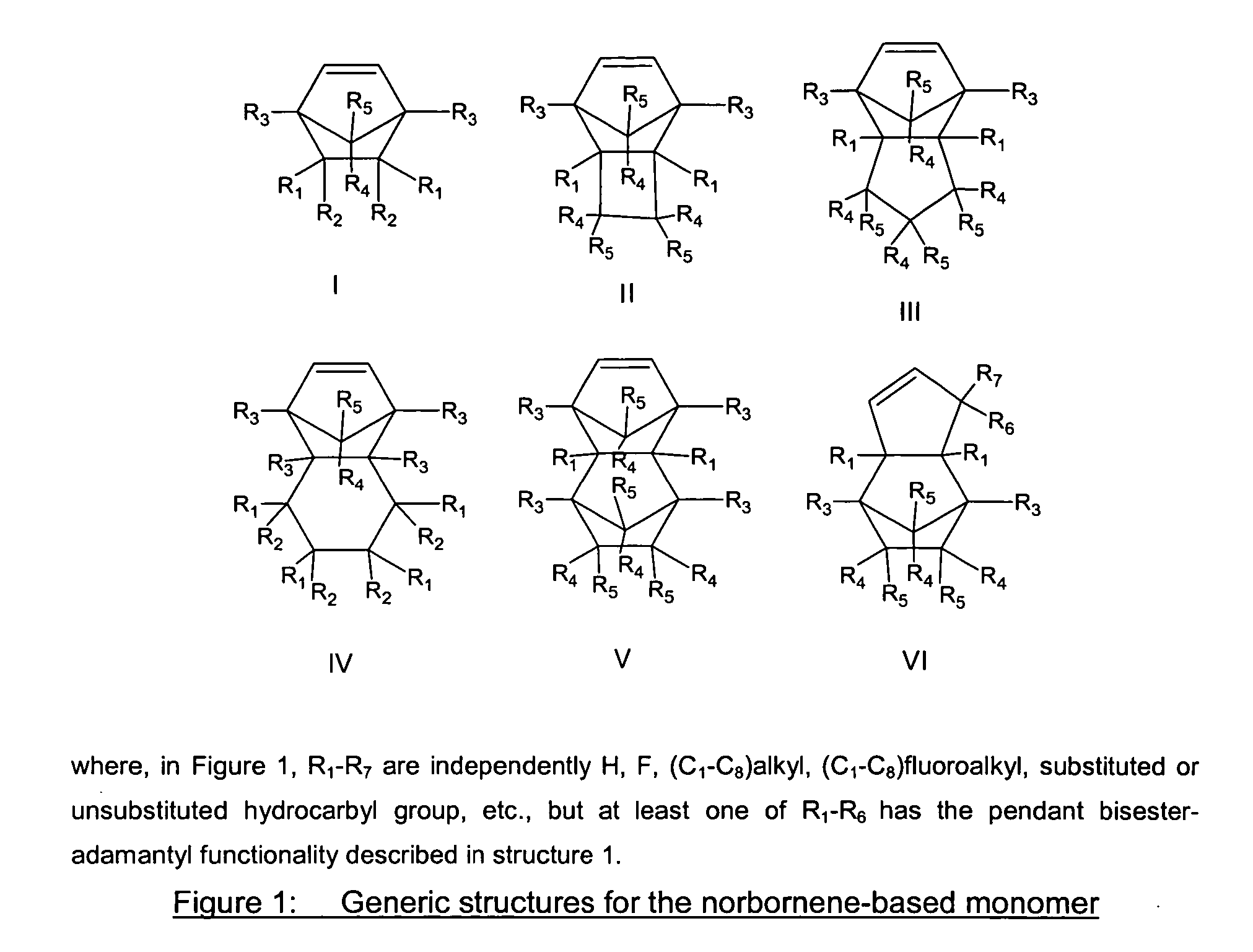
The present invention relates to a novel photoresist composition sensitive to radiation in the deep ultraviolet and a process for imaging the composition. The photoresist composition comprises a) a novel polymer that is insoluble in an aqueous alkaline solution and comprises at least one acid labile group, and b) a compound capable of producing an acid upon irradiation. The novel polymer of the present invention comprises at least one unit with a bisester group, (—C(O)OWC(O)O—), attached on one side to a polymer backbone unit (A) comprising an aliphatic group, and attached on the other side to an adamantyl group. The invention also relates to the novel polymer and a novel monomer for obtaining the novel polymer.
Owner:MERCK PATENT GMBH
Ink composition, inkjet recording method, printed material, and process for producing lithographic printing plate
InactiveUS20080008966A1Improve curing effectGood flexibilityPhotosensitive materialsPretreated surfacesPhotochemistryCarbon atom
Owner:FUJIFILM CORP
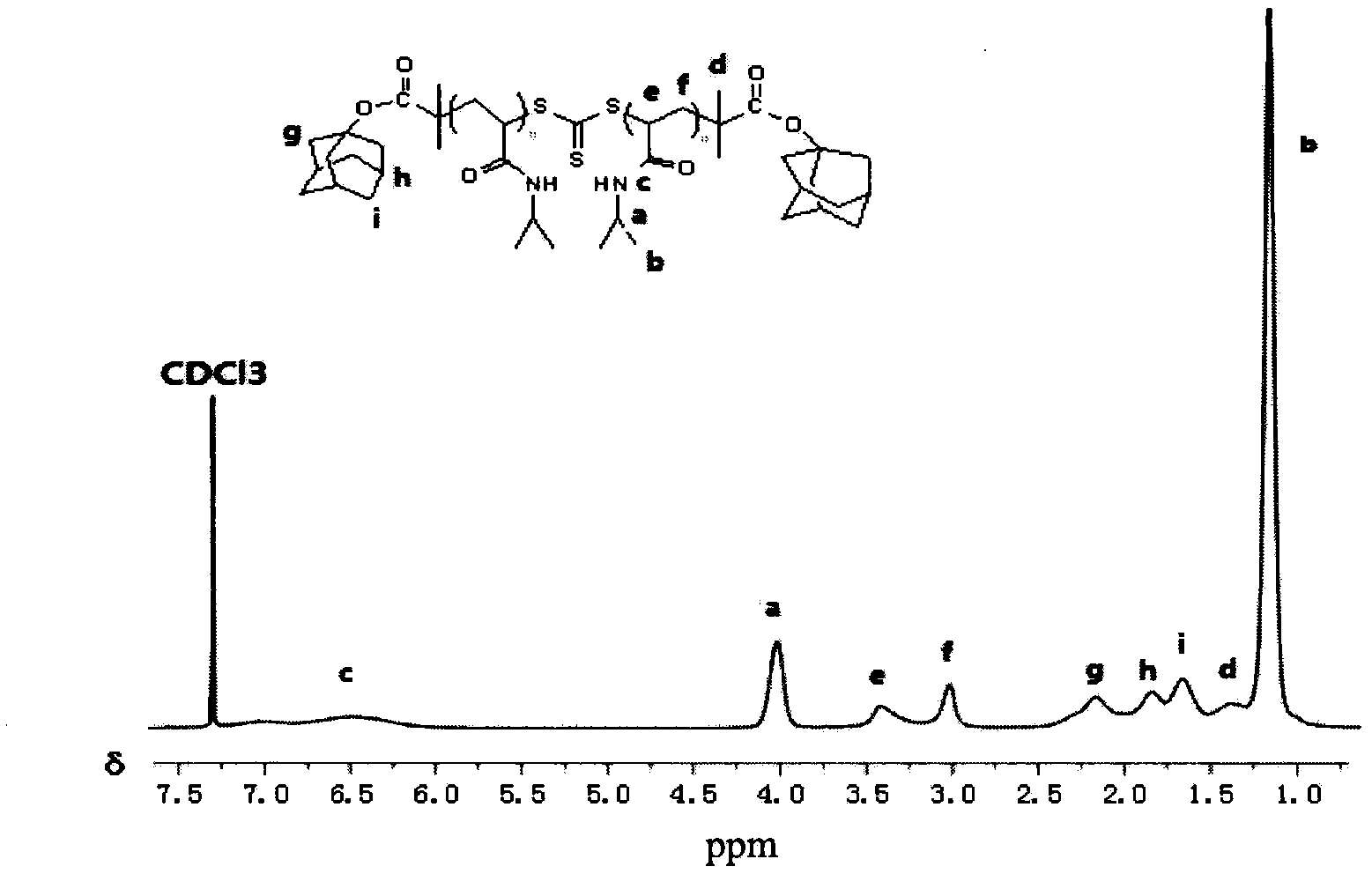
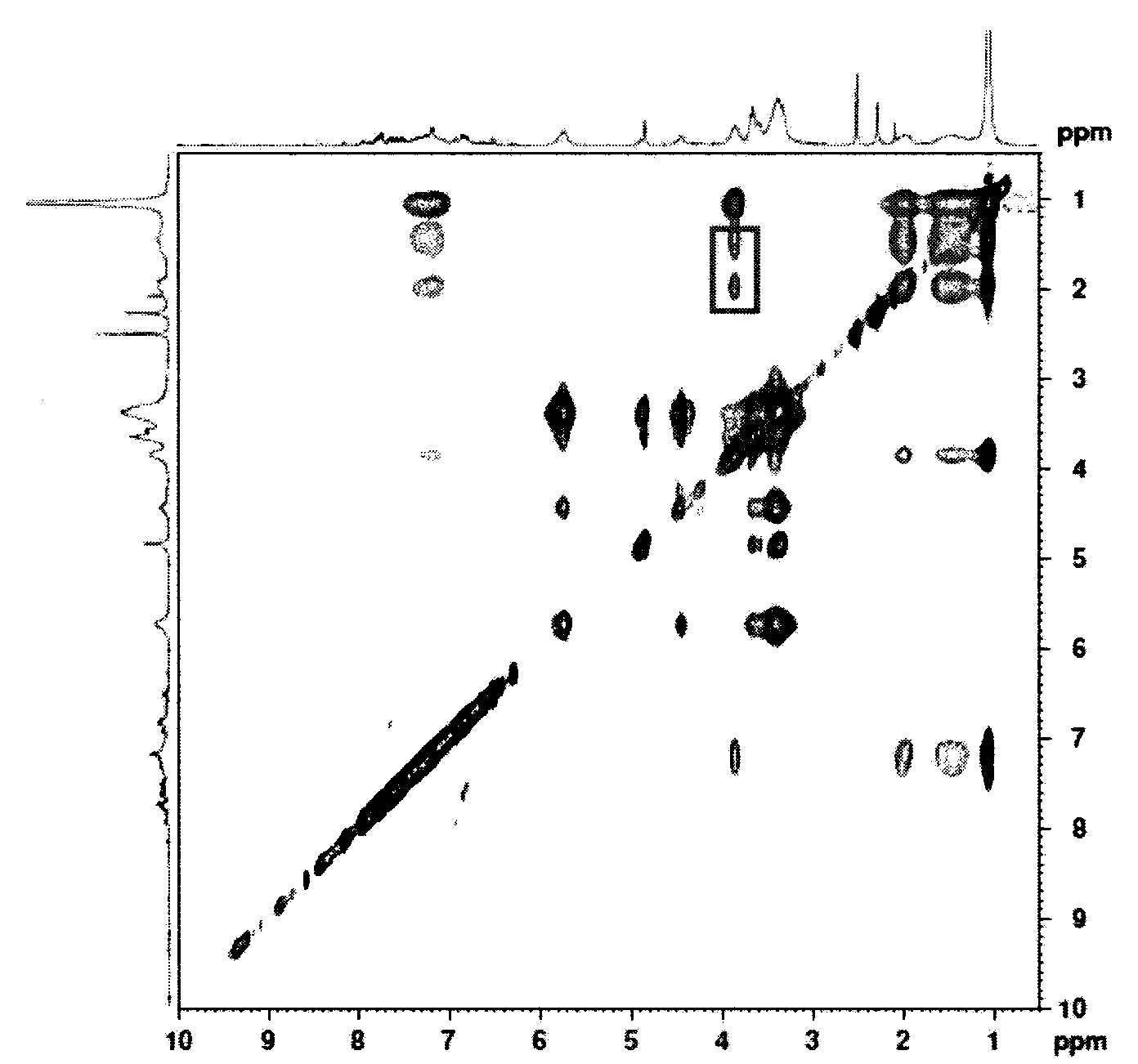
Preparation method of double-sensitivity cyclodextrin supermolecule aggregate
The invention discloses a preparation method of a double-sensitivity cyclodextrin supermolecule aggregate, belonging to the technical field of functional materials. The preparation method comprises the following steps of preparing a host molecule-photosensitive 4-hydroxycinnamic acid-cyclodextrin (4HCA-CD) by using 4-hydroxycinnamic acid (4HCA) to modify beta-cyclodextrin (beta-CD); using trithioester with adamantine (AD) at tail end as a chain transfer agent, and preparing temperature-sensitive object polymer-double-arm adamantine-poly(N-isopropyl acrylamide)-adamantine (AD-PNIPAM-AD) by using a reversible addition-fragmentation chain transfer free radical polymerization (RAFT) method; constructing a double-sensitivity supermolecule inclusion complex 4HCA-CD / AD-PNIPAM-AD by utilizing comprehensive performance of a beta-CD dewatering cavity and AD; and self-assembling the 4HCA-CD / AD-PNIPAM-AD to form the supermolecule aggregate which is capable of realizing reversible conversion in shape and size by changing light and temperature. The supermolecule aggregate prepared by the preparation method disclosed by the invention has good light / temperature double sensitivities and is capable of carrying out smart response onto external stimulus, so that the supermolecule aggregate has a wide application prospect in the fields of drug loading, controlled release, and the like.
Owner:JIANGNAN UNIV
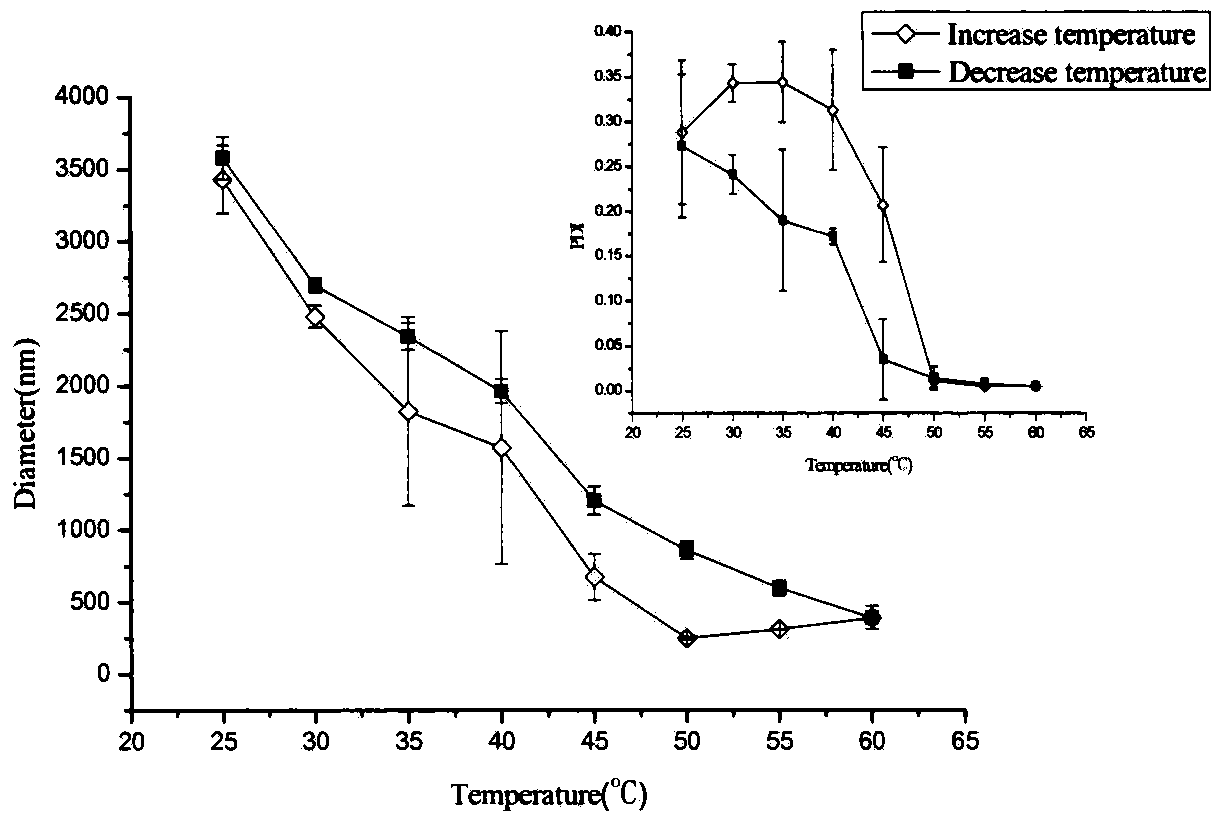
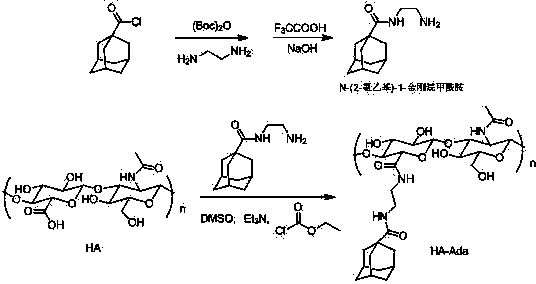
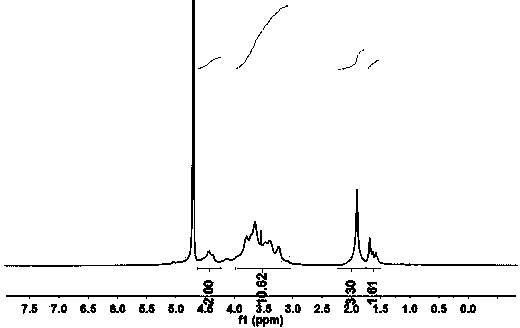
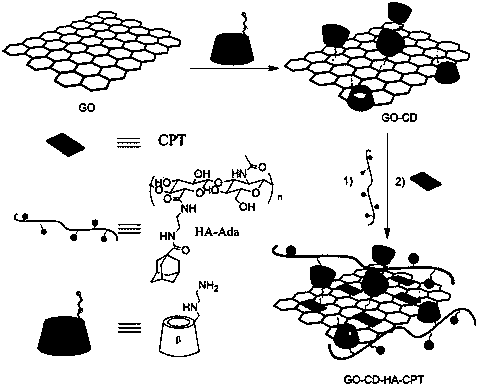
Graphene/hyaluronic acid assembly taking cyclodextrin as medium and preparation method thereof
InactiveCN103920160AImprove stabilityGood biocompatibilityOrganic active ingredientsMacromolecular non-active ingredientsCancer cellBiocompatibility Testing
The invention discloses a graphene / hyaluronic acid assembly taking cyclodextrin as a medium. The graphene / hyaluronic acid assembly is a nano supermolecule assembly synthesized based on beta-cyclodextrin-modified graphene and adamantine-modified hyaluronic acid, wherein graphene is modified by beta-cyclodextrin; by virtue of strong host-guest interaction between beta-cyclodextrin and adamantine, graphene and hyaluronic acid are combined together to form the supermolecule assembly. The graphene / hyaluronic acid assembly has the advantages that the supermolecule assembly greatly improves stability and biocompatibility of the cyclodextrin-modified graphene under physiological conditions; by utilizing targeted recognition action of hyaluronic acid on tumor cells, the supermolecule assembly can selectively kill the cancer cells, and has anti-cancer activity higher than that of pure drug camptothecin; the targeted drug transmission system is simple in preparation process, easy to implement and low in material cost, and has potential application prospect in clinic treatment of cancers.
Owner:NANKAI UNIV
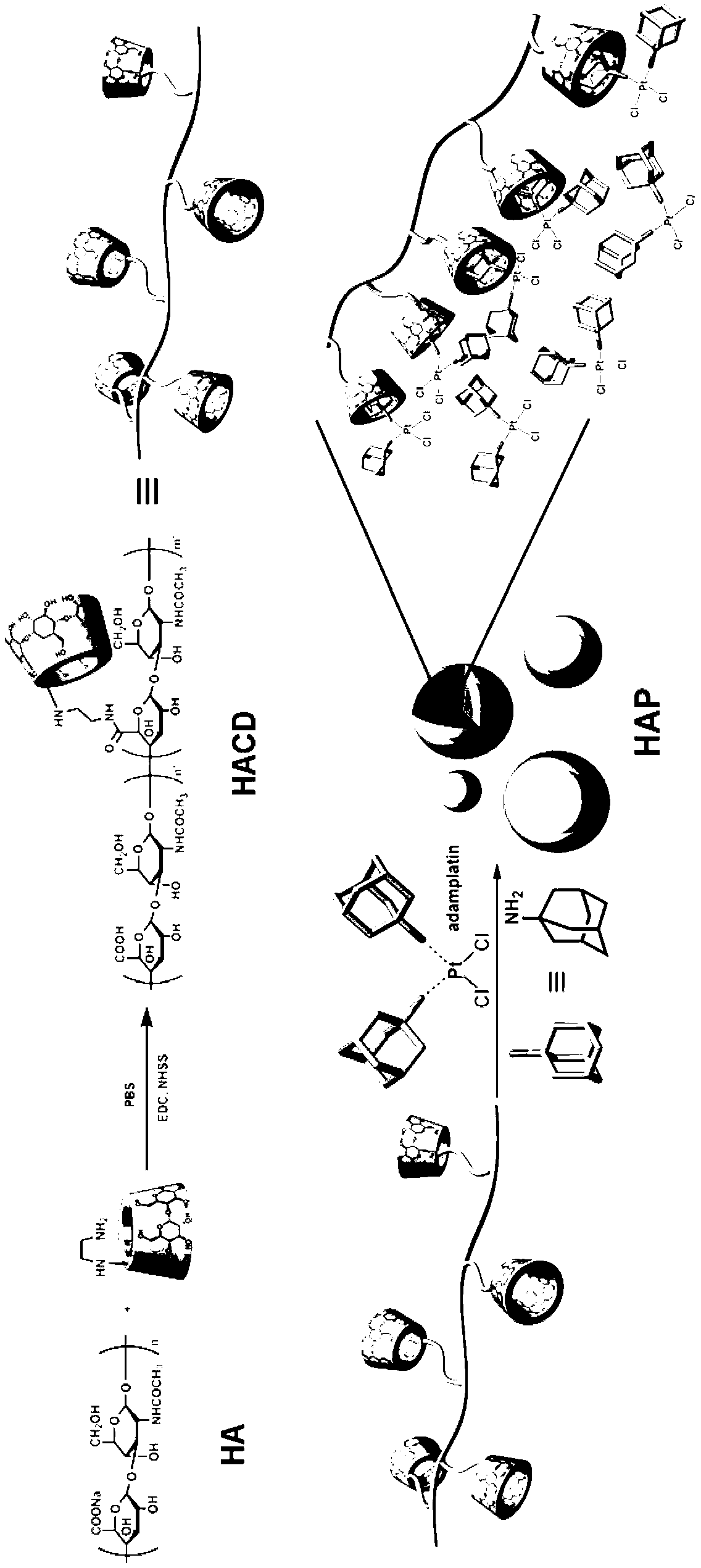
Supramolecule assembly of targeting-delivery anticancer adamplatin and preparation of supramolecule assembly
InactiveCN102698286AAchieve selective killingSmall toxicityHeavy metal active ingredientsPharmaceutical non-active ingredientsSide effectCancer cell
The invention discloses a supramolecule assembly of targeting-delivery anticancer adamplatin. The supramolecule assembly is a binary supramolecule assembly which is synthesized on the basis of cyclodextrin-decorated hyaluronic acid and adamplatin. A preparation method of the supramolecule assembly is characterized in that the cyclodextrin-decorated hyaluronic acid and the adamplatin are respectively synthesized, and through the strong non-covalent interaction of cyclodextrin and adamantine and the amphiphilic action of molecules, a supermolecule nano particle which takes the hydrophilic hyaluronic acid as a shell and the adamplatin as a core is formed. The supramolecule assembly disclosed by the invention has the advantages that the supramolecule assembly of the targeting-delivery anticancer adamplatin has a simple synthetic route, is low in preparation cost and high in productivity, and is suitable for amplification synthesis and practical production application; and through endocytosis in which a malignant cell surface hyaluronic acid receptor serves as a medium, the supramolecule assembly (HAP) is brought in cancer cells in a target manner, so that the protection of normal cells and the targeting selective killing of cancer cells are realized, the anti-cancer activity is obviously improved, and toxic and side effects are obviously reduced.
Owner:NANKAI UNIV
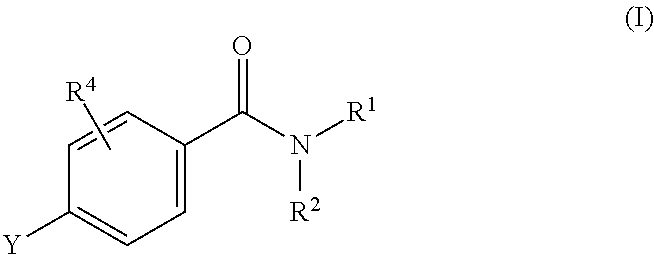
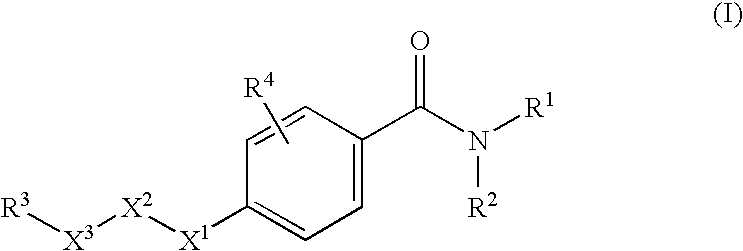
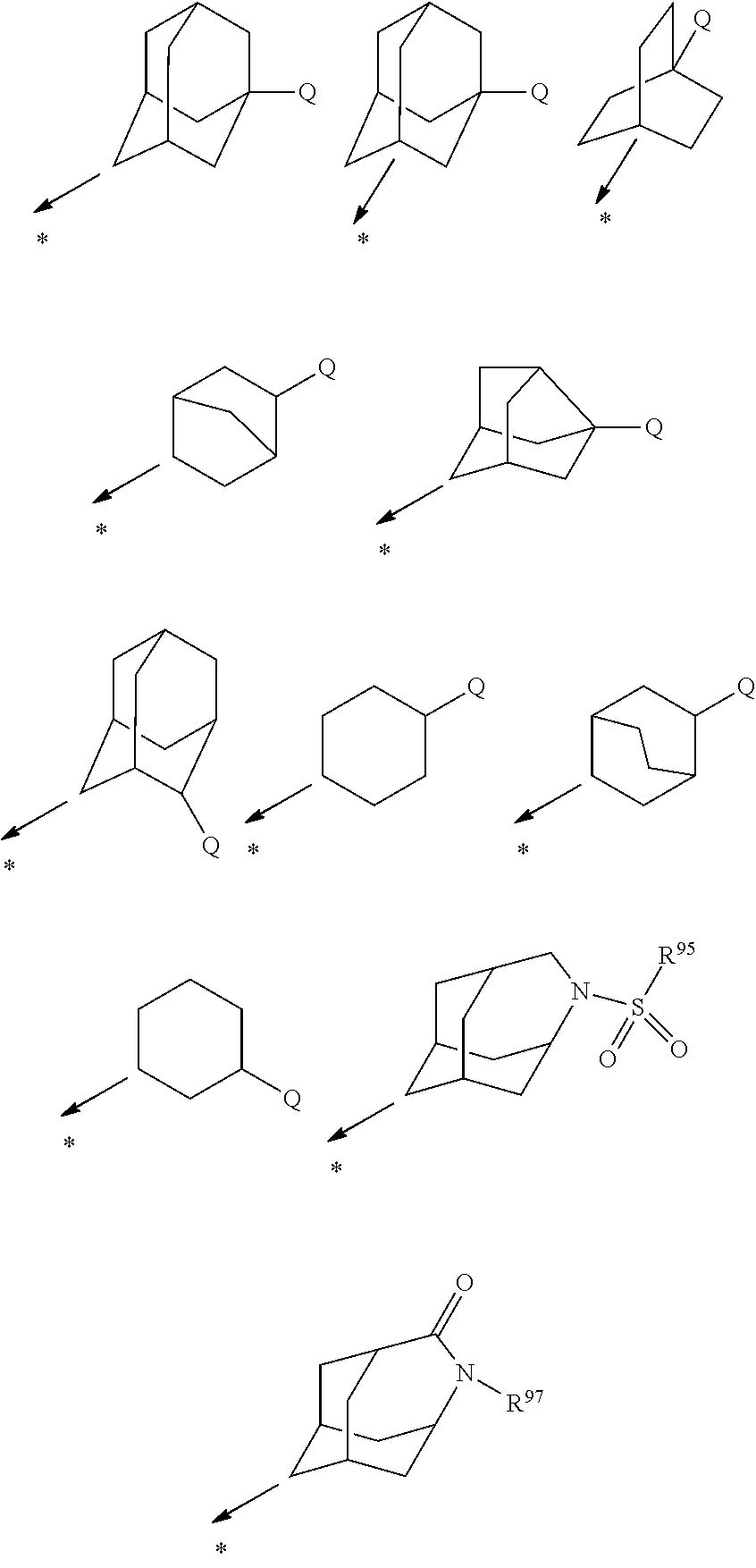
N-adamantyl benzamides as inhibitors of 11-beta-hydroxysteroid dehydrogenase
InactiveUS20110003856A1Decreased intracellular concentrationImpaired glucose toleranceBiocideSenses disorderDisease11-beta-Hydroxysteroid Dehydrogenases
Novel substituted benzamide based inhibitors, their use in therapy, pharmaceutical compositions comprising the compounds, the use of said compounds in the manufacture of medicaments, and therapeutic methods comprising the administration of said compounds are described. The present compounds modulate the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and are accordingly useful in the treatment of diseases in which such a modulation is beneficial, such as the metabolic syndrome.
Owner:HIGH POINT PHARMA
N-adamantyl benzamides as inhibitors of 11-beta-hydroxysteroid dehydrogenase
Novel substituted benzamide based inhibitors, their use in therapy, pharmaceutical compositions comprising the compounds, the use of said compounds in the manufacture of medicaments, and therapeutic methods comprising the administration of said compounds are described. The present compounds modulate the activity of 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) and are accordingly useful in the treatment of diseases in which such a modulation is beneficial, such as the metabolic syndrome.
Owner:HIGH POINT PHARMA
Acid-sensitive compound and resin composition for photoresist
InactiveUS20030180662A1Improve adhesionGood reproducibilityOrganic chemistryPhotosensitive materialsResistAlicyclic Hydrocarbons
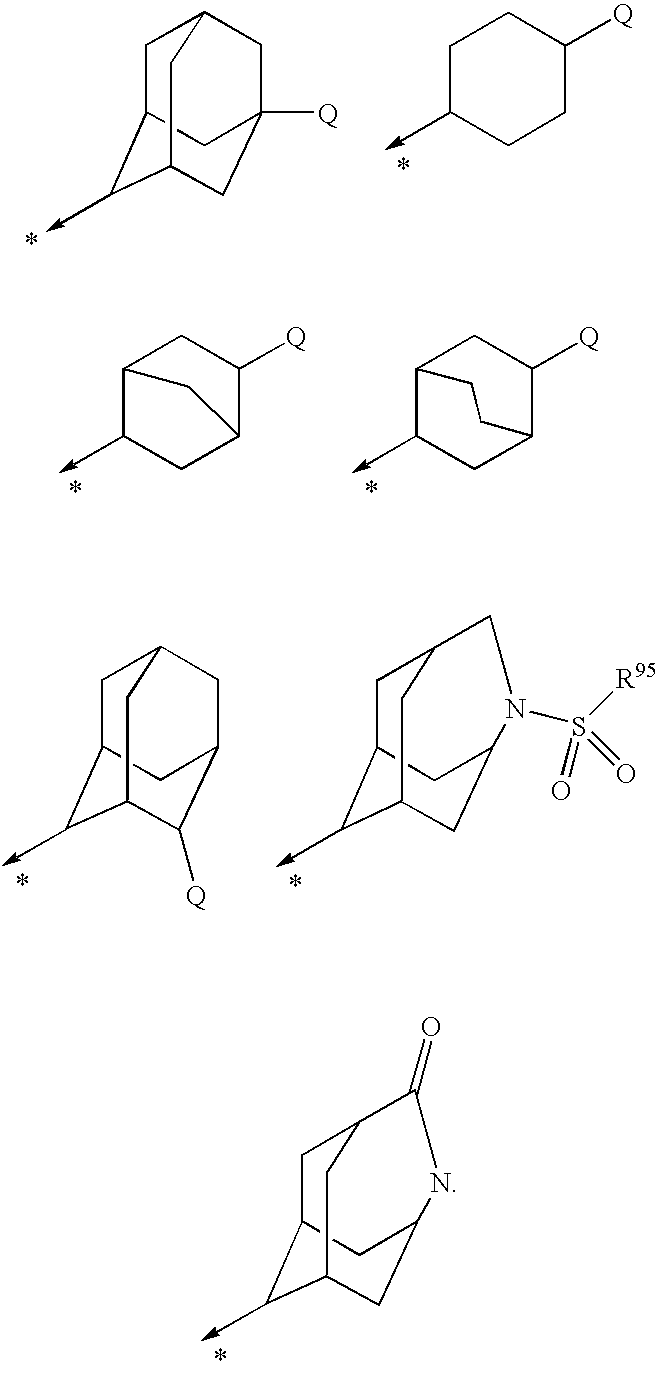
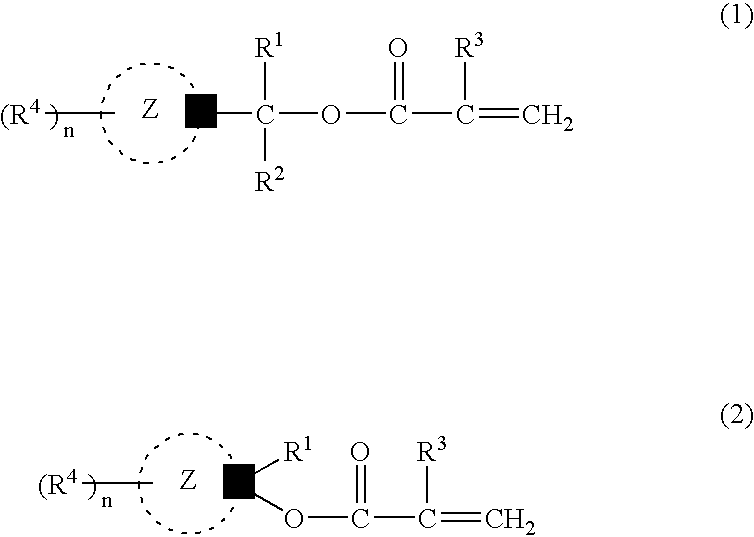
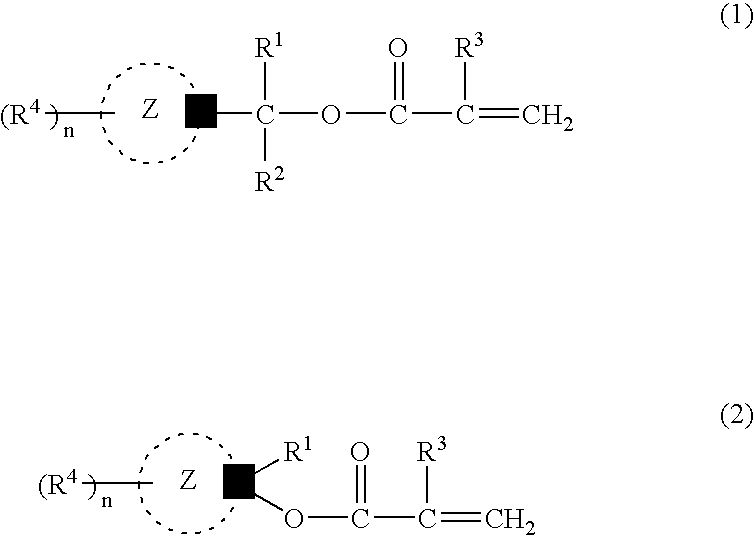
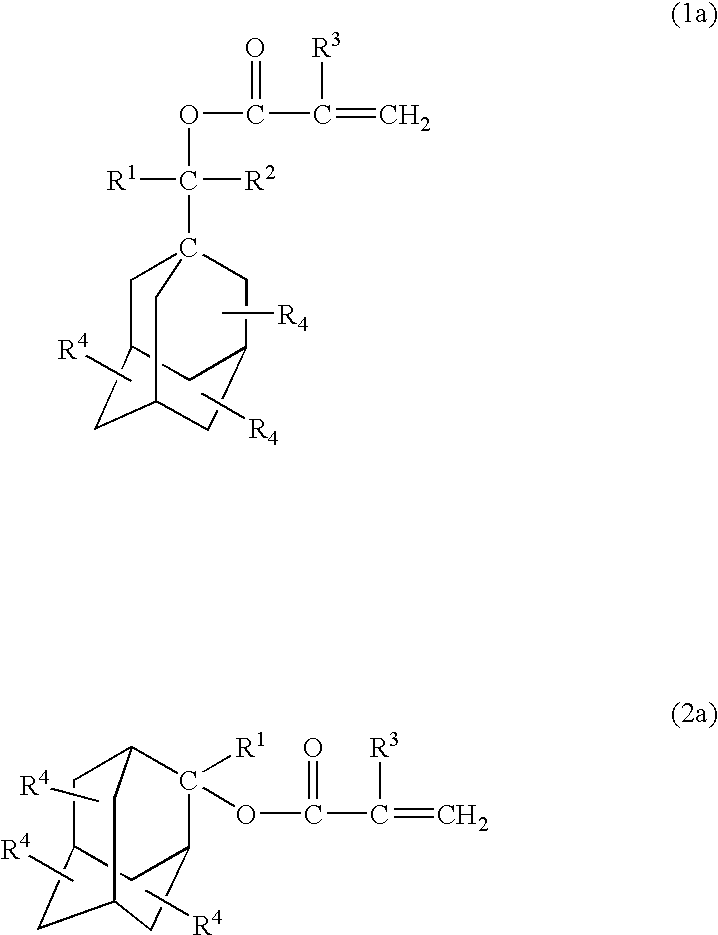
The photoresist resin composition comprises a polymer containing an acid-responsive compound unit of the following formula (e.g. an adamantane skeleton) and a photoactive acid precursor. R<1 >may be an alkyl group having a tertiary carbon atom in the 1-position and the Z ring is a bridged-ring hydrocarbon ring comprising 2 to 4 rings. wherein R<1 >and R<2 >are the same or different from each other and each represents a hydrogen atom, an alkyl group or a cycloalkyl group; R<3 >represents a hydrogen atom or a methyl group; R<4 >represents a hydrogen atom, a halogen atom, an alkyl group, an oxygen-containing group, an amino group or an N-substituted amino group; the Z ring represents a monocyclic or polycyclic alicyclic hydrocarbon ring; n represents an integer of not less than 1; with proviso that R<4 >does not concurrently represent a hydrogen atom, and may be different over n occurrences; in formula (1), R<1 >and R<2 >may, jointly and together with the adjacent carbon atom, form an alicyclic hydrocarbon ring. The above photoresist resin composition is high in etching resistance, can be solubilized by irradiation, and is capable of providing a finer line pattern.
Owner:DAICEL CHEM IND LTD
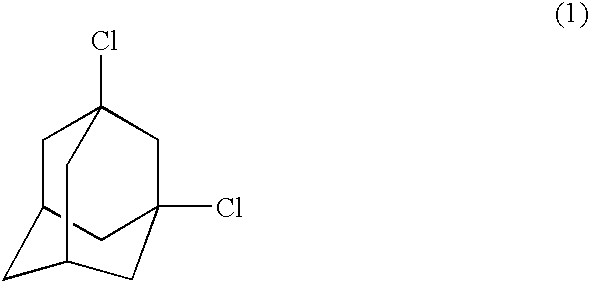
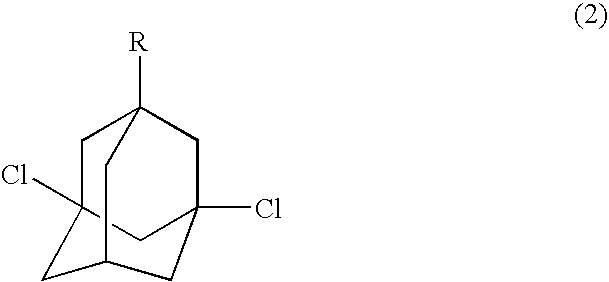
Process for preparing dihalogenated adamantanes
InactiveUS6878853B2High yieldHalogenated hydrocarbon separation/purificationOrganic solventPhotochemistry
This invention discloses a process for preparing a dihalogenated adamantane by reacting an adamantane optionally substituted with alkyl at 1-position with a halosulfonic acid, comprising the first stage of monohalogenation conducted at −5 to 15° C. and then the second stage of dihalogenation conducted at 17 to 35° C., preferably in the absence of an organic solvent.
Owner:TOKUYAMA CORP
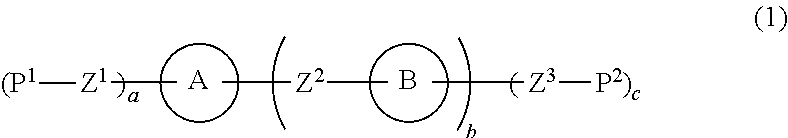
Liquid crystal composition and liquid crystal display device
ActiveUS20150129801A1Improve solubilitySmall viscosityLiquid crystal compositionsOrganic chemistryCrystallographySolubility
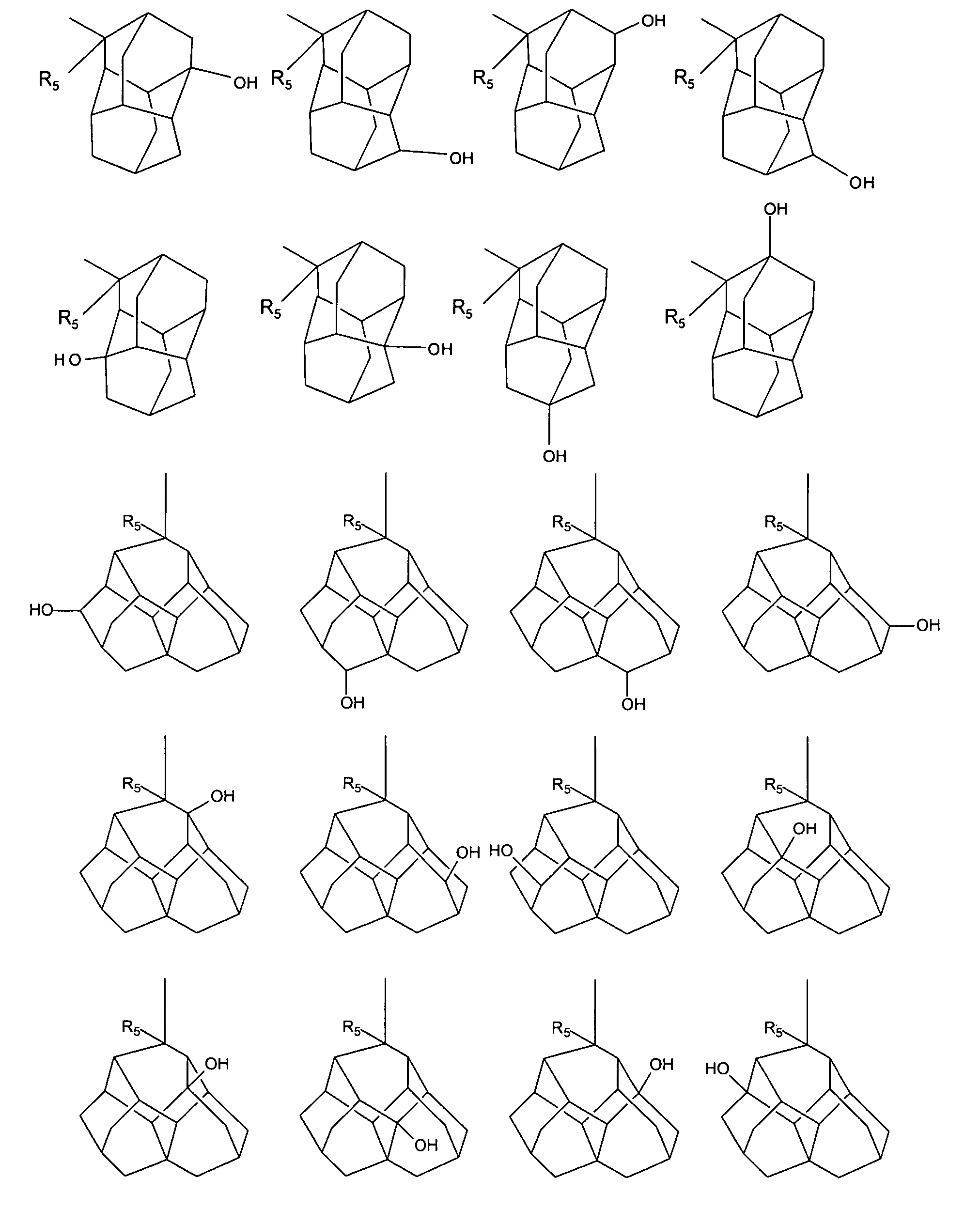
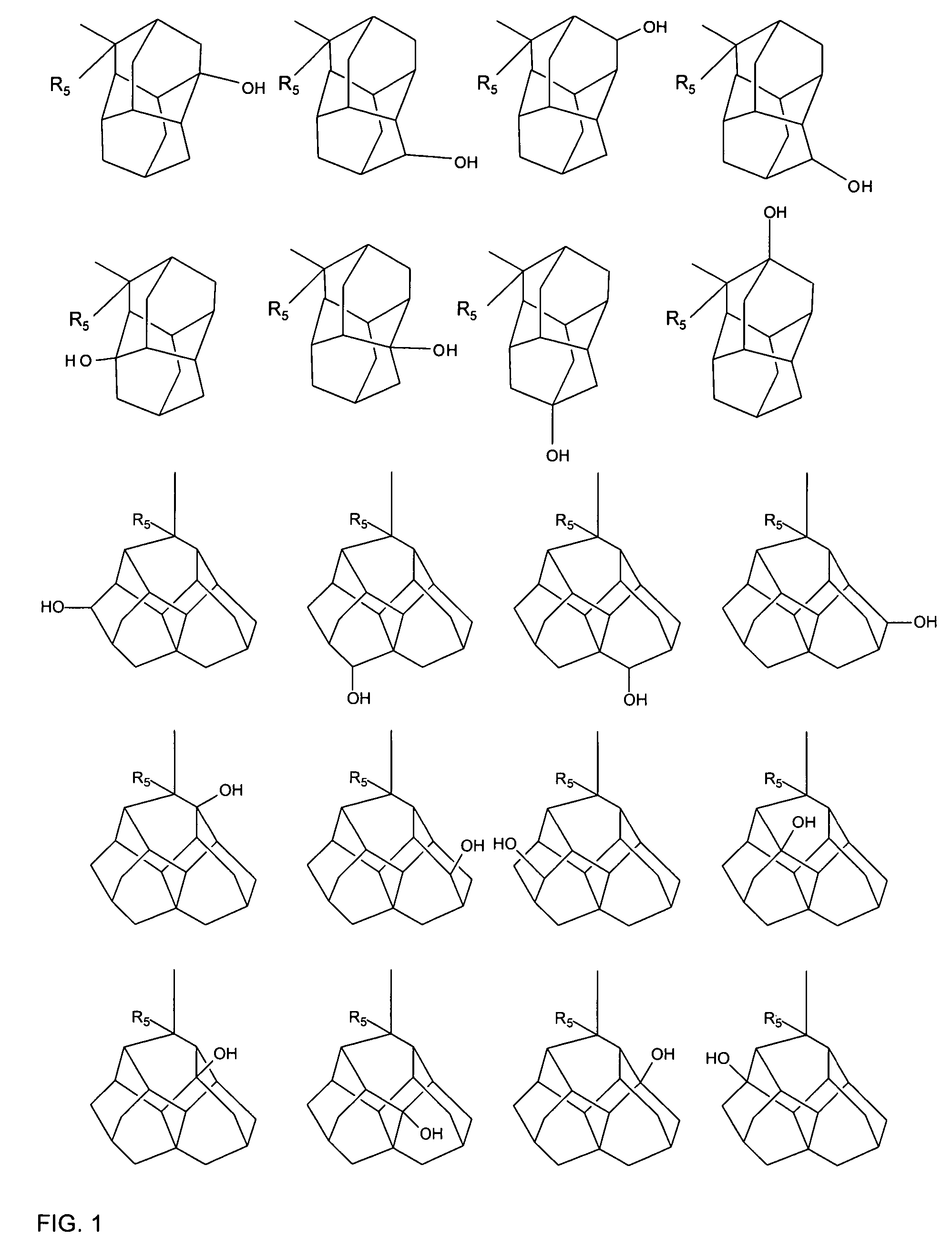
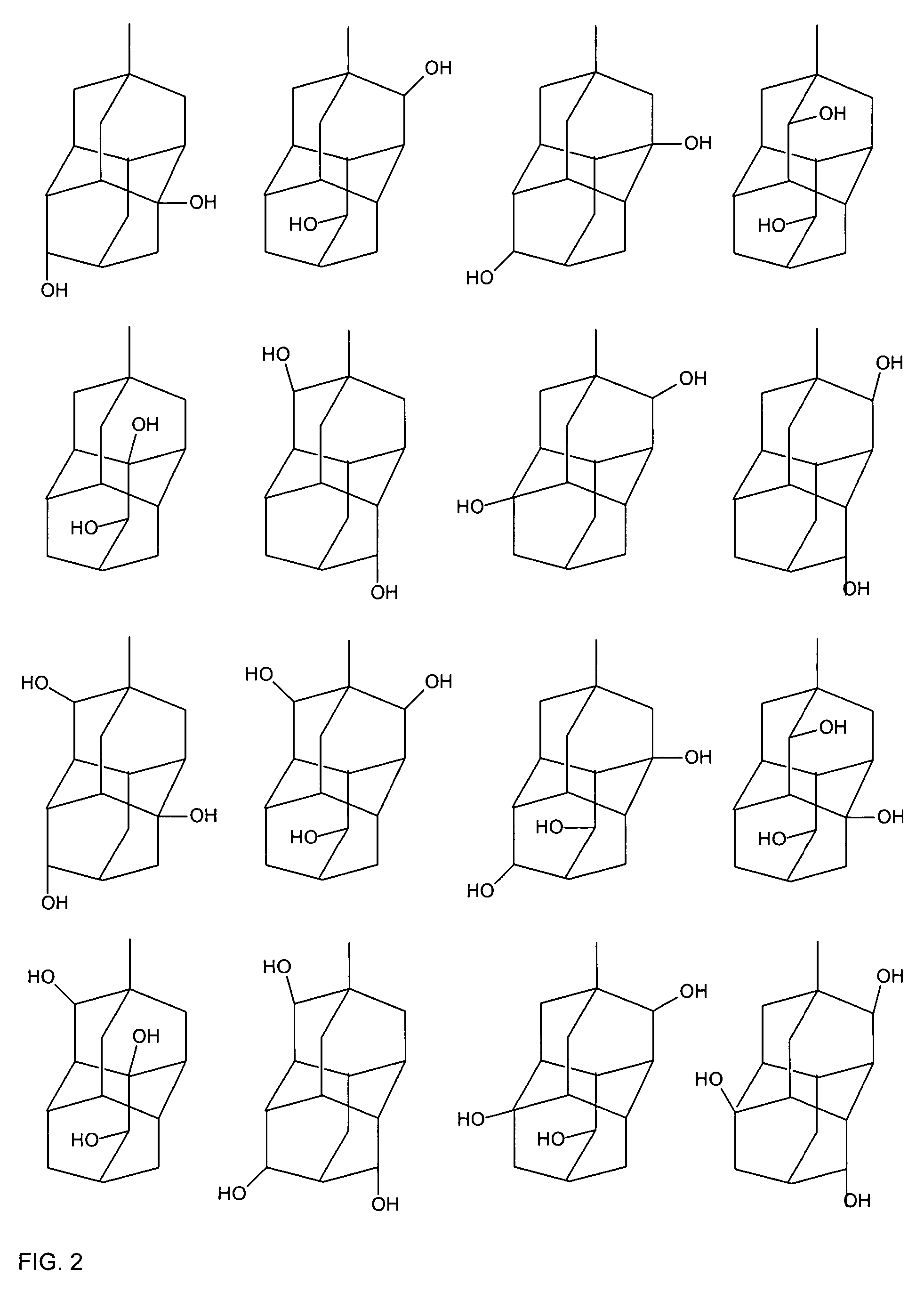
A liquid crystal composition that includes a polymerizable compound having a high solubility, a composition obtained by polymerizing the polymerizable compound in the liquid crystal composition, and an AM device including any of the compositions are described. The liquid crystal composition has negative dielectric anisotropy, and includes at least one compound selected from the group of compounds represented by formula (1):wherein P1 and P2 are polymerizable groups, and, for example, ring A and ring B are independently an adamantane ring or a noradamantane ring, Z1, Z2 and Z3 are a single bond, and a, b and c are 1.
Owner:JNC CORP +1
Acid-sensitive, developer-soluble bottom Anti-reflective coatings
ActiveUS20100213580A1Photosensitive materialsSemiconductor/solid-state device detailsVinyl etherAnti-reflective coating
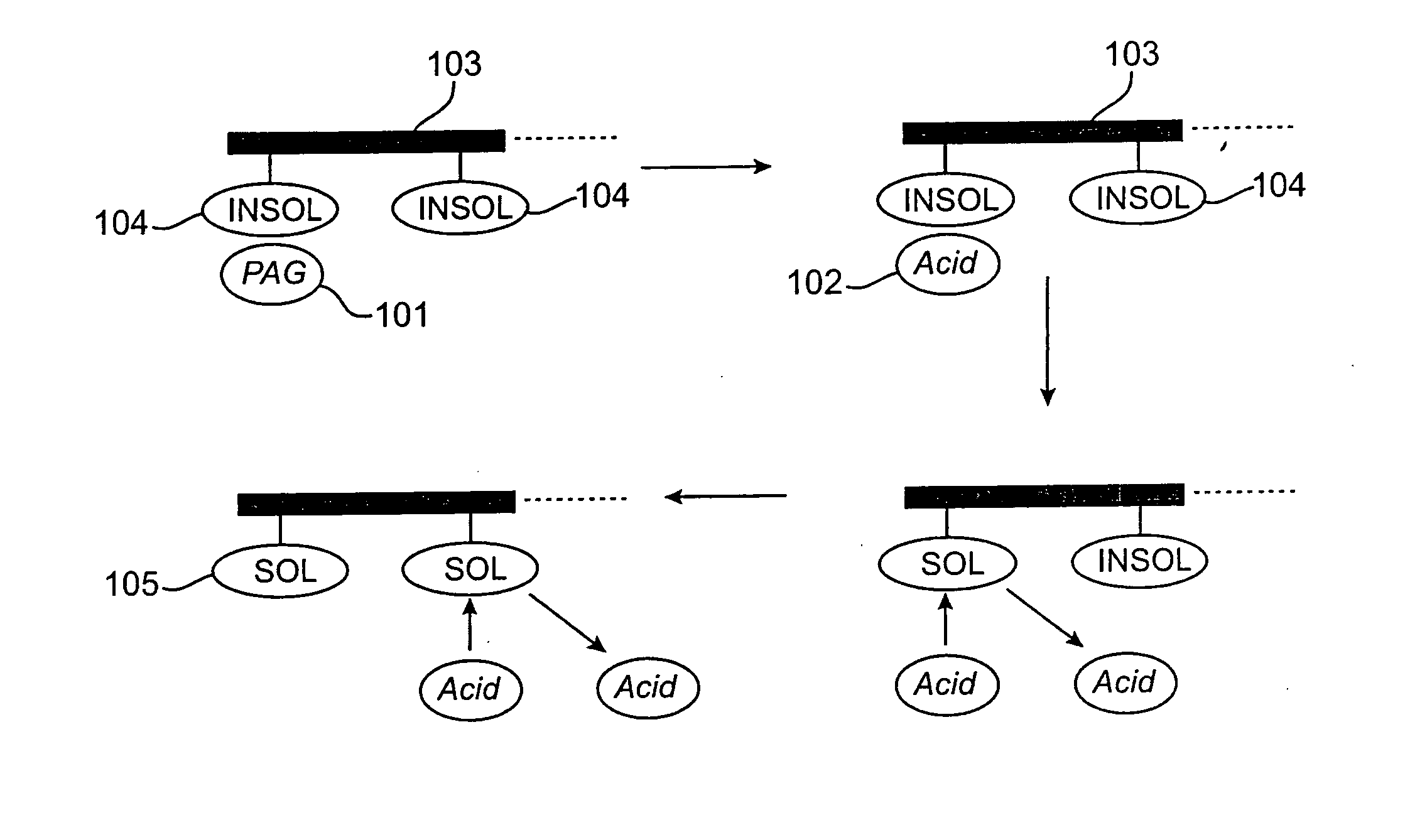
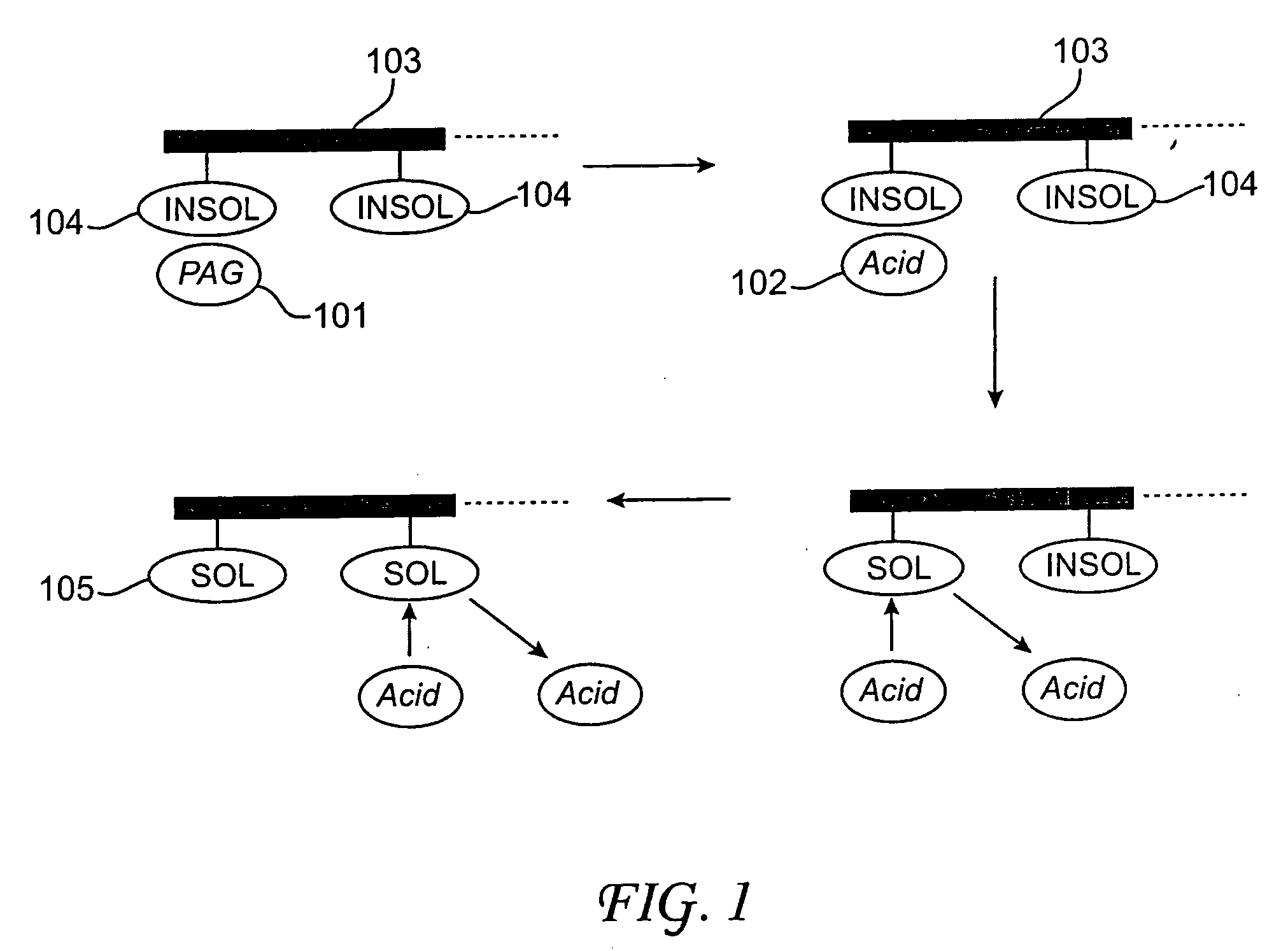
Acid-sensitive, developer-soluble bottom anti-reflective coating compositions are provided, along with methods of using such compositions and microelectronic structures formed thereof. The compositions preferably comprise a crosslinkable polymer dissolved or dispersed in a solvent system. The polymer preferably comprises recurring monomeric units having adamantyl groups. The compositions also preferably comprise a crosslinker, such as a vinyl ether crosslinking agent, dispersed or dissolved in the solvent system with the polymer. In some embodiments, the composition can also comprise a photoacid generator (PAG) and / or a quencher. The bottom anti-reflective coating compositions are thermally crosslinkable, but can be decrosslinked in the presence of an acid to be rendered developer soluble.
Owner:BREWER SCI
Chemiluminescence immunity analysis detecting myocardium calcium protein T hypersensitization method for acridine ester and alkaline phosphatase
InactiveCN101226200AChemiluminescene/bioluminescenceBiological testingBiotin-streptavidin complexCalcium protein
The invention relates to a chemical illumination immunity analysis method for checking human cTnT, which uses acridiniumester and / or alkaline phosphatase as label. The immunity reaction uses two-site immunoassay and / or competition law. The immunity reaction can use streptavidin-biotin two-site immunoassay to improve sensitivity. The invention uses NaOH and H2O2 as acridiniumester illumination initiating agent, uses 1, 2-dioxo cyclohexane derivative (adamantine derivative) as the illumination substrate of alkaline phosphatase, and uses fluorescein derivative and surface activator as illumination renforcing agent. The solid carrier is orifice plate, macromolecule polymer tube (ball) and ferriferrous oxide magnetic particles or the like.
Owner:天津天美生物技术有限公司
Low dielectric constant materials with improved thermo-mechanical strength and processability
InactiveUS6987147B2Synthetic resin layered productsSemiconductor/solid-state device manufacturingPolymer networkDiamantane
A polymeric network comprises a plurality of monomers that include a cage compound with at least three arms, wherein at least one of the arms has two or more branches, and wherein each of the branches further comprises a reactive group. Monomers in contemplated polymeric networks are covalently coupled to each other via the reactive groups. Particularly contemplated cage compounds include adamantane and diamantane, and especially contemplated branched arms comprise ortho-bis(phenylethynyl)phenyl. Especially contemplated polymeric networks have a dielectric constant of no more than 3.0, and are formed on the surface of a substrate.
Owner:HONEYWELL INT INC
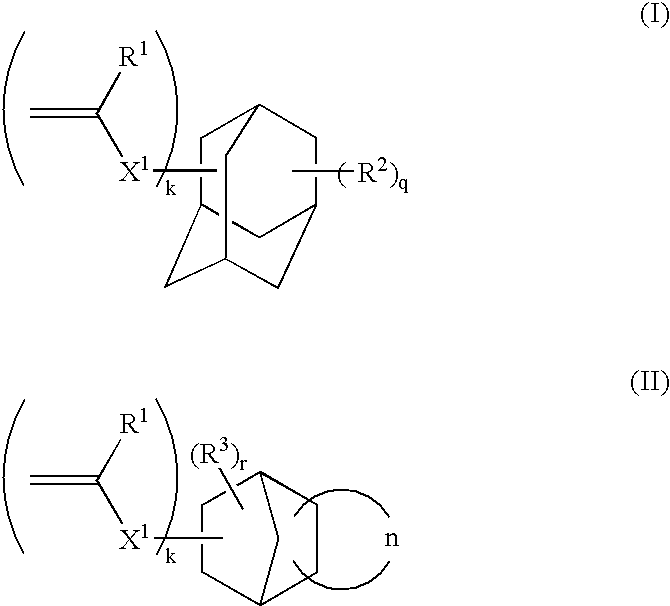
Polymerizable compound having adamantane structure, process for production of the same, and resin composition
InactiveUS20100022730A1Improve immersionImprove the immunityOrganic compound preparationCarboxylic acid esters preparationPhotolithographyDry etching
A polymerizable compound having a fluorinated substituent (Z) represented by the general formula (1), an adamantane structure and a polymerizable group (A) having the structure represented by the general formula (1), a production method thereof, and a photoresist composition, a thermocurable resin composition and a photocurable resin composition containing a polymer obtained using the polymerizable compound are provided. Use of the polymerizable compound with the adamantane structure and a resin composition thereof in the present invention provides in the field of photolithography the effect of preventing a liquid immersion medium from penetration and improving dry etching resistance in a liquid immersion exposure method as well as reducing adhesion to a mold and improving dry etching resistance in a nanoimprint method.
Owner:IDEMITSU KOSAN CO LTD
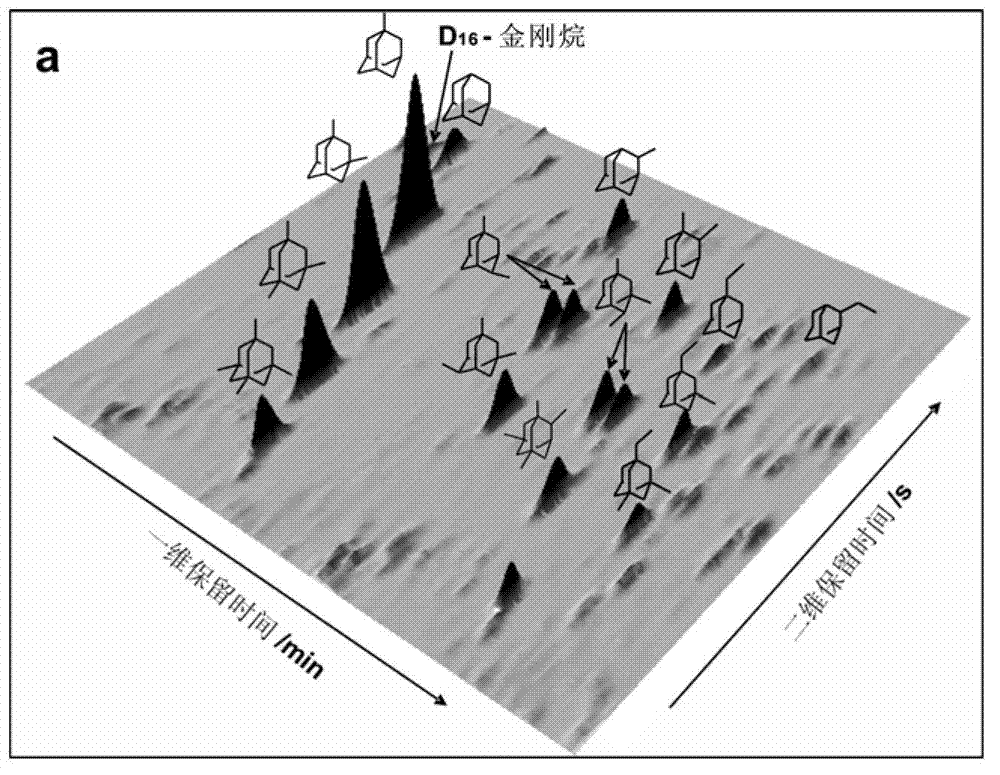
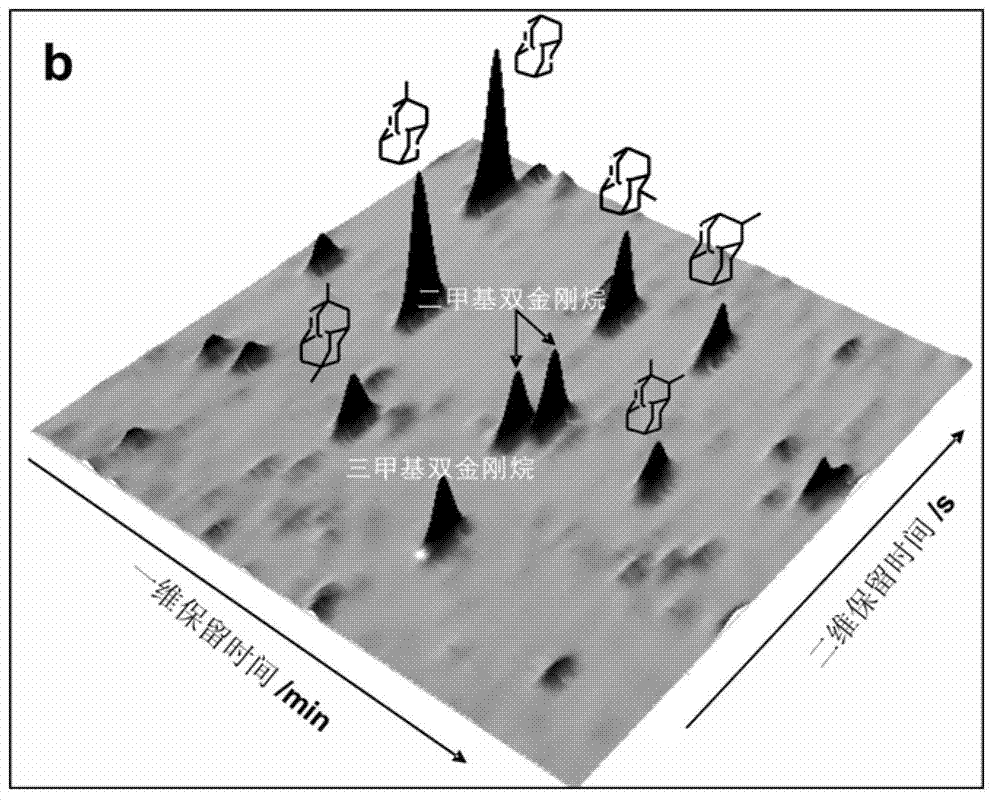
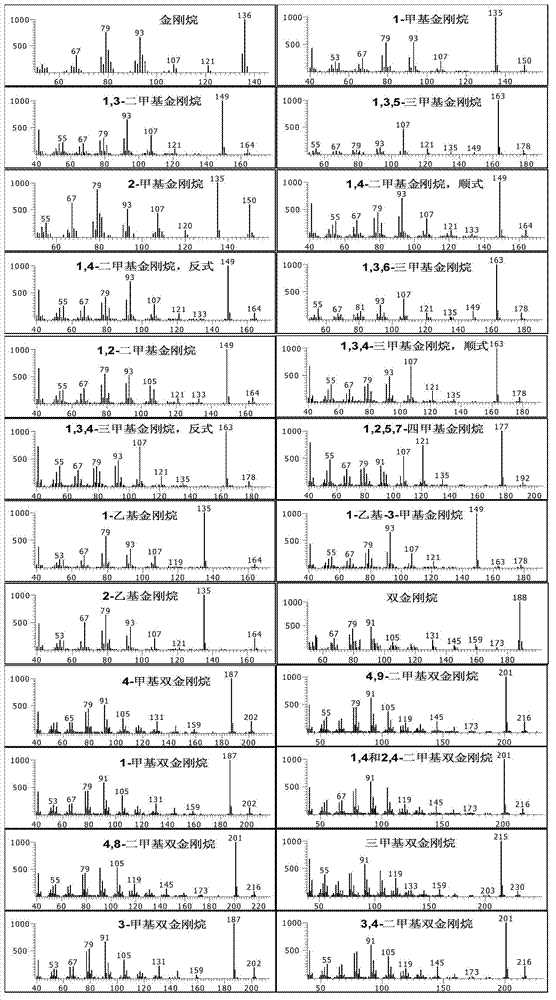
Comprehensive two-dimensional gas chromatography method for quantitatively determining diamondoid hydrocarbons in petroleum sample
ActiveCN102768256AHigh resolutionLarge peak capacityComponent separationGas phaseComprehensive two-dimensional gas chromatography
The invention relates to a comprehensive two-dimensional gas chromatography method for quantitatively determining diamondoid hydrocarbons in petroleum sample. The method comprises the steps of (1) preparing to-be-detected sample; (2) analyzing the to-be-detected sample with comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry (GC*GC-TOFMS) to obtain peak information of diamondoid hydrocarbons in the to-be-detected sample; (3) analyzing the to-be-detected sample with comprehensive two-dimensional gas chromatography-hydrogen flame ionization detector (GC*GC-FID) to obtain GC*GC-FID spectrogram of the to-be-detected sample; according to the peak information obtained in step (2), determining peak positions of diamondoid hydrocarbons and D16-adamantane in the GC*GC-FID spectrogram, and obtaining their peak area integration results; and (5) calculating according to internal standard method to obtain quantitative results of diamondoid hydrocarbons. The method is suitable for quantitative determination of diamondoid hydrocarbons in all crude oil and rock extract samples.
Owner:PETROCHINA CO LTD
Method for synthesizing Cu-SSZ-13 molecular sieve catalyst by one step
InactiveCN106238092AOptimizing Hydrothermal Aging PropertiesHigh solid phase yieldMolecular sieve catalystsCrystalline aluminosilicate zeolitesAlkali metalChemistry
The invention relates to a method for synthesizing Cu-SSZ-13 molecular sieve catalyst by one step. The method is characterized by comprising the following steps: (1) mixing an organic amine template agent and deionized water; adding or not adding an NaOH aqueous solution; adding Cu-TEPA clathrate, and stirring evenly; adding an aluminum source and a silicon source, and stirring until all materials are mixed evenly; (2) placing a mixed solution obtained in step (1) into a stainless reaction still with a tetrafluoroethylene or para-polyphenylene lining; crystallizing the reaction still for 12 hours to 6 days at 100 to 200 DEG C.; (3) washing a crystallized product for a plurality of times until the solution is neutral; drying the obtained solid product in a drying oven for 5 to 12 hours at 60 to 150 DEG C; heating the dried solid to 500 to 600 DEG C. and roasting for 3 to 10 hours so as to obtain the Cu-SSZ-13 molecular sieve catalyst product. By adopting the method disclosed by the invention, the use amount of the N,N,N-trimethyl-1-ammonium adamantane template agent is greatly reduced, and the alkali metal ion content of the product is controlled, so that the hydrothermal aging property of the catalyst is optimized.
Owner:WUXI WEIFU ENVIRONMENT PROTECTION CATALYST
Hyaluronic acid- cyclodextrin-adamantane polyethylene glycol carrier as well as preparation method and application thereof
ActiveCN103611165AImprove controllabilityAdjustable sizeOrganic active ingredientsAntipyreticTumor targetPolyethylene glycol
The invention belongs to a novel pharmaceutical preparation form and the novel technical field, specifically relates to a hyaluronic acid- cyclodextrin-adamantane polyethylene glycol carrier as well as a preparation method and application thereof, and simultaneously provides a nano drug delivery system which is self-assembled by taking hyaluronic acid-cyclodextrin-adamantane polyethylene glycol as the carrier and utilizing clathration force of the cyclodextrin to cover hydrophobic guest molecules. The hyaluronic acid- cyclodextrin-adamantane polyethylene glycol carrier has the advantages that the nano drug administration system not only can passively transmit drugs to tumor tissues by virtue of an EPR (Ethylene-Propylene Rubber) effect, but also can improve an anti-tumor effect of the drugs by virtue of a tumor-targeting effect of the hyaluronic acid. PEG (Polyethylene Glycol) molecules on the surface can be effectively prevented from being absorbed by a reticuloendothelial system, and in-vivo circulating time can be prolonged.
Owner:SHENYANG PHARMA UNIVERSITY
Method for producing amine series and quaternary ammonium salts with adamantane framework
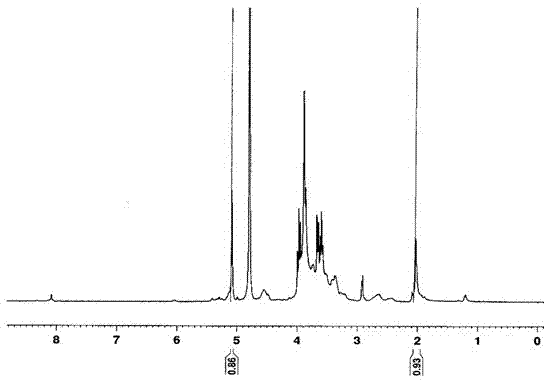
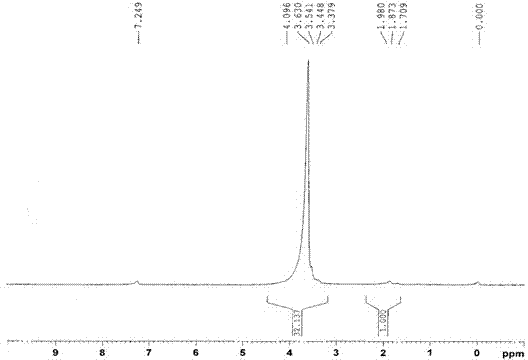
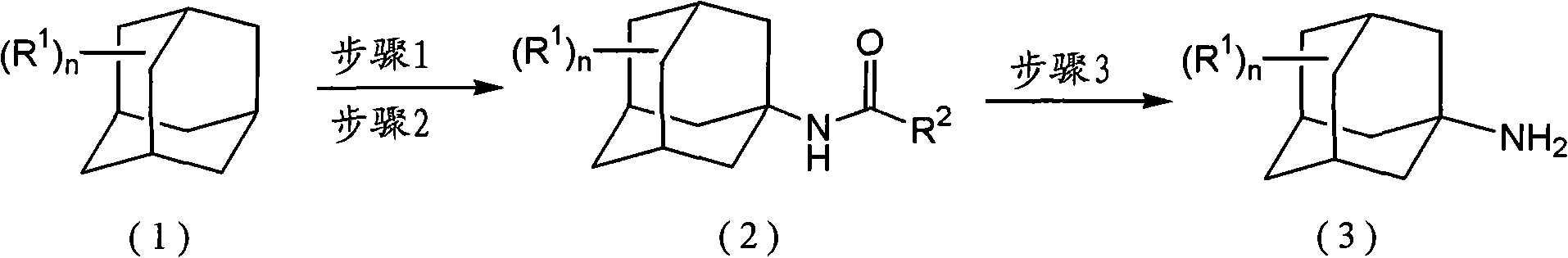
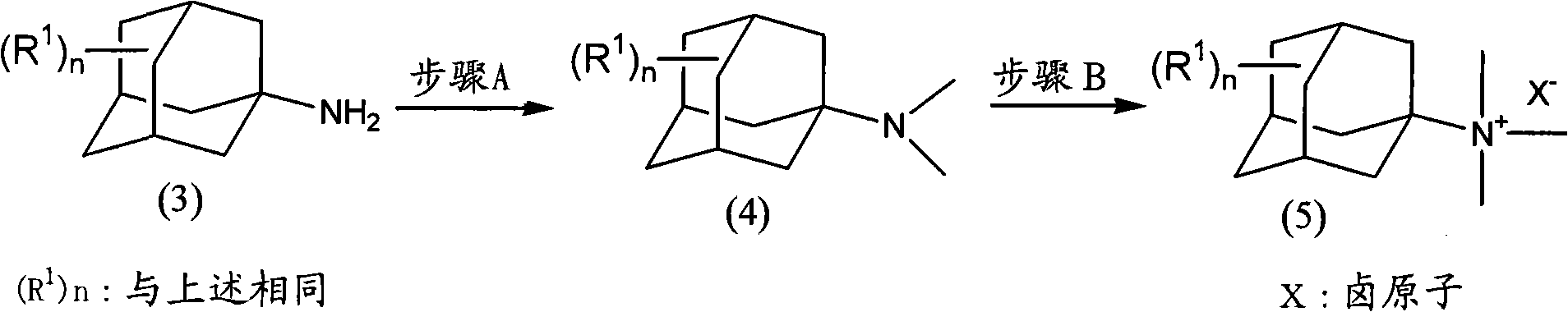
InactiveCN101993377AEfficient manufacturingHigh purityAmino preparation from aminesOrganic compound preparationHalomethaneOleum
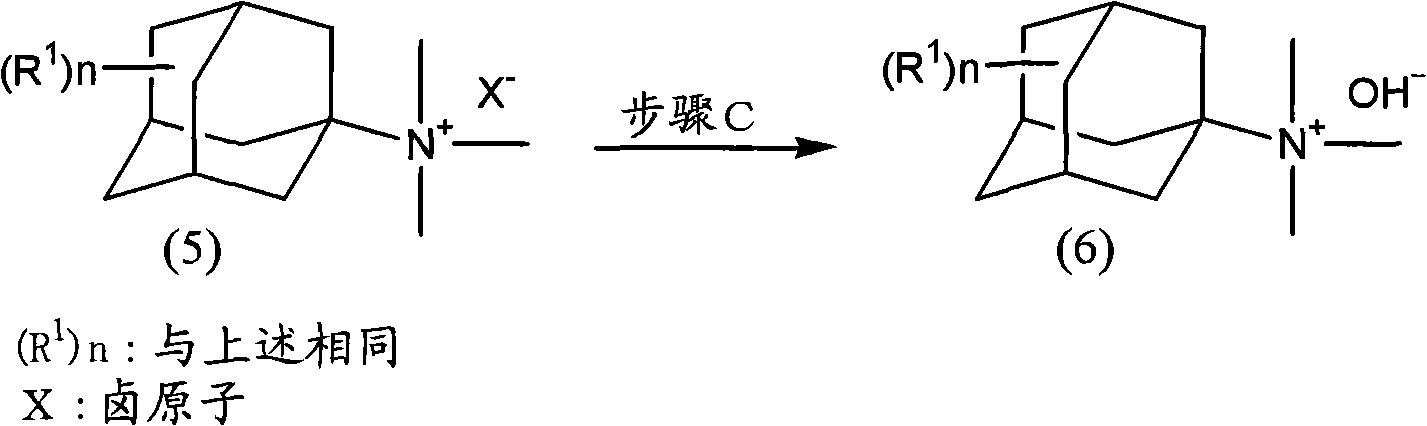
The present invention provides a method for producing amine series with an adamantane framework, including the following steps: step 1: reacting adamantane series in a mixed liquid containing oleum and organic nitrile compounds; step 2: performing hydrolyzing treatment to a reaction liquid obtained in the step 1 to form acidamide series with the adamantane framework; step 3: performing alkali treatment to the acidamide series obtained in the step 2 to form the amine series with the adamantane framework. A method for producing quaternary ammonium salts with the adamantane framework in sequence includes the following steps: step A: reacting the amine series with the adamantane framework dissolved in a dissolvant a with aminic acid and methylene oxide or paraformaldehyde, to form dimethylamine series with the adamantane framework; step B: dissolving the dimethylamine series with the adamantane framework obtained in the step A in a dissolvant b to react with halomethane so as to form quaternary ammonium salt halogenide with the adamantane framework; step C: performing ion exchange to the quaternary ammonium salt halogenide with the adamantane framework to form quaternary ammonium salt hydroxides with the adamantane framework.
Owner:IDEMITSU KOSAN CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com