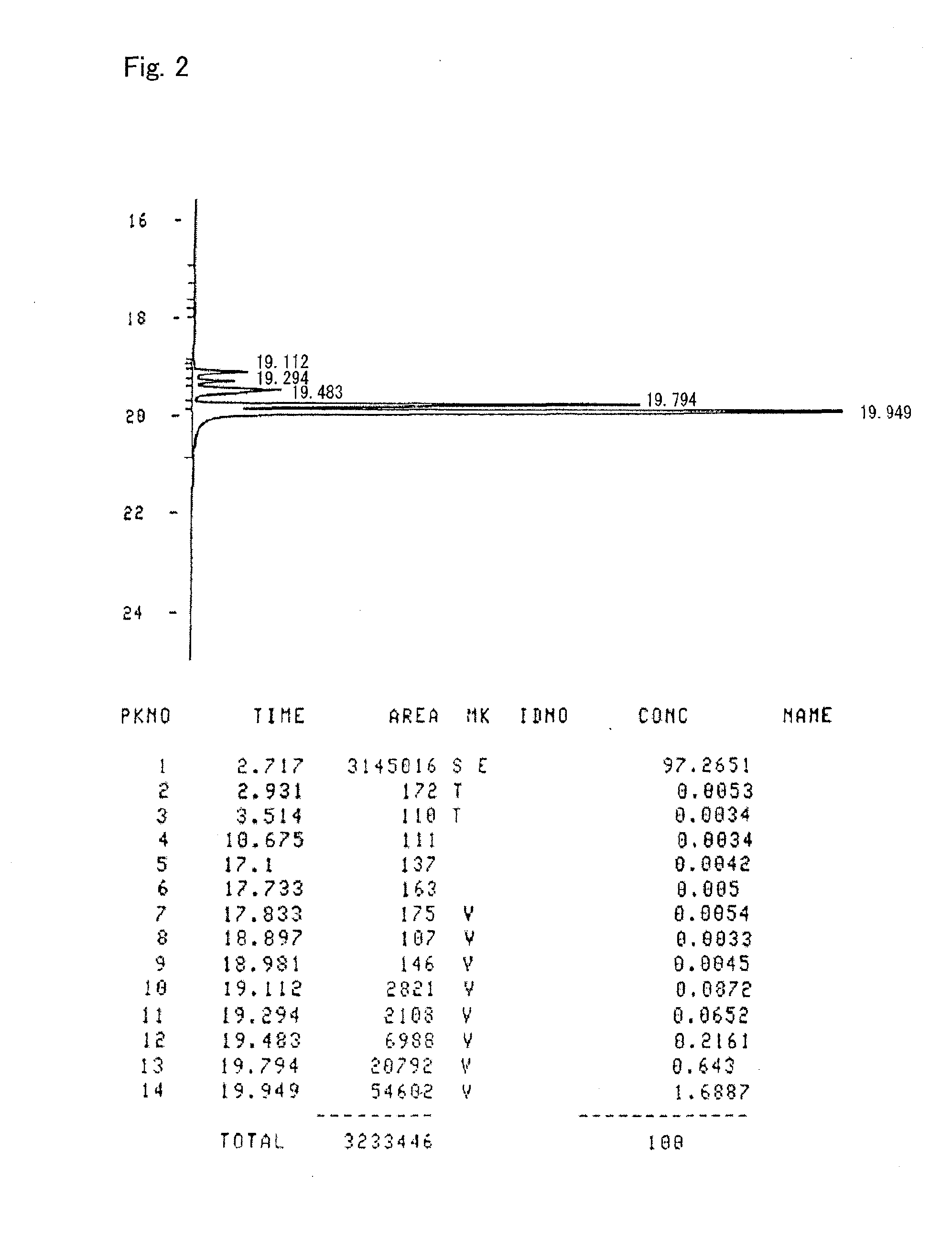
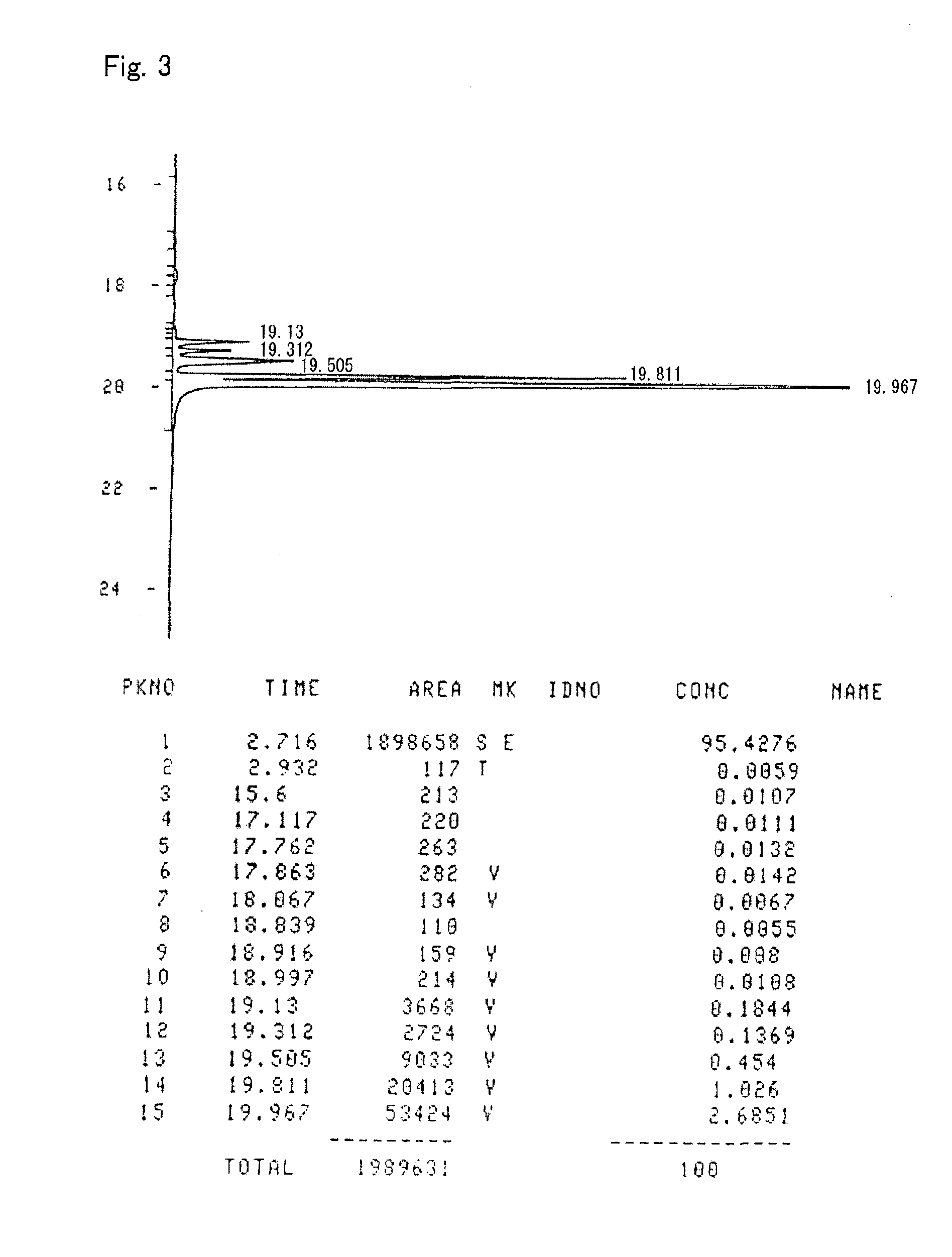
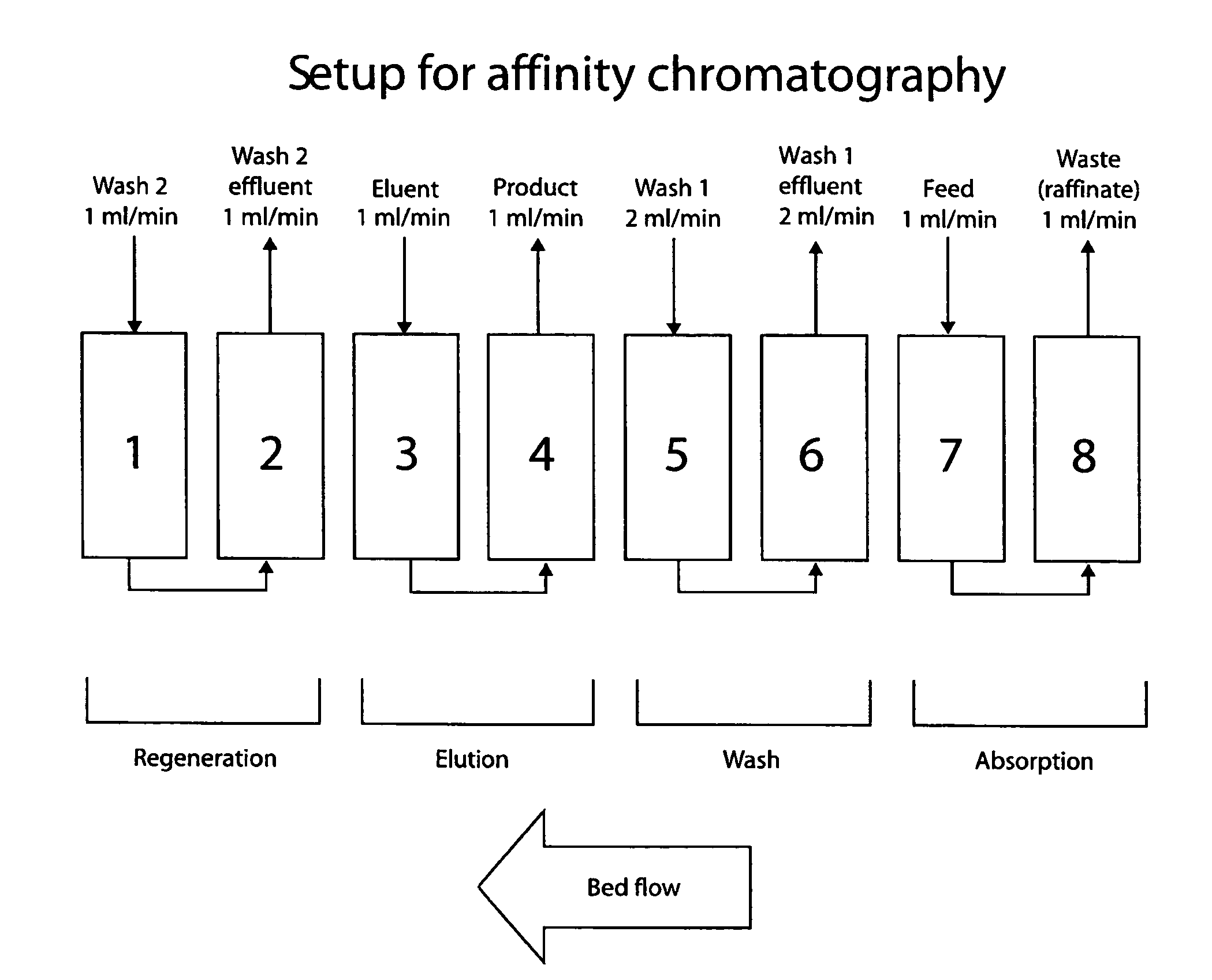
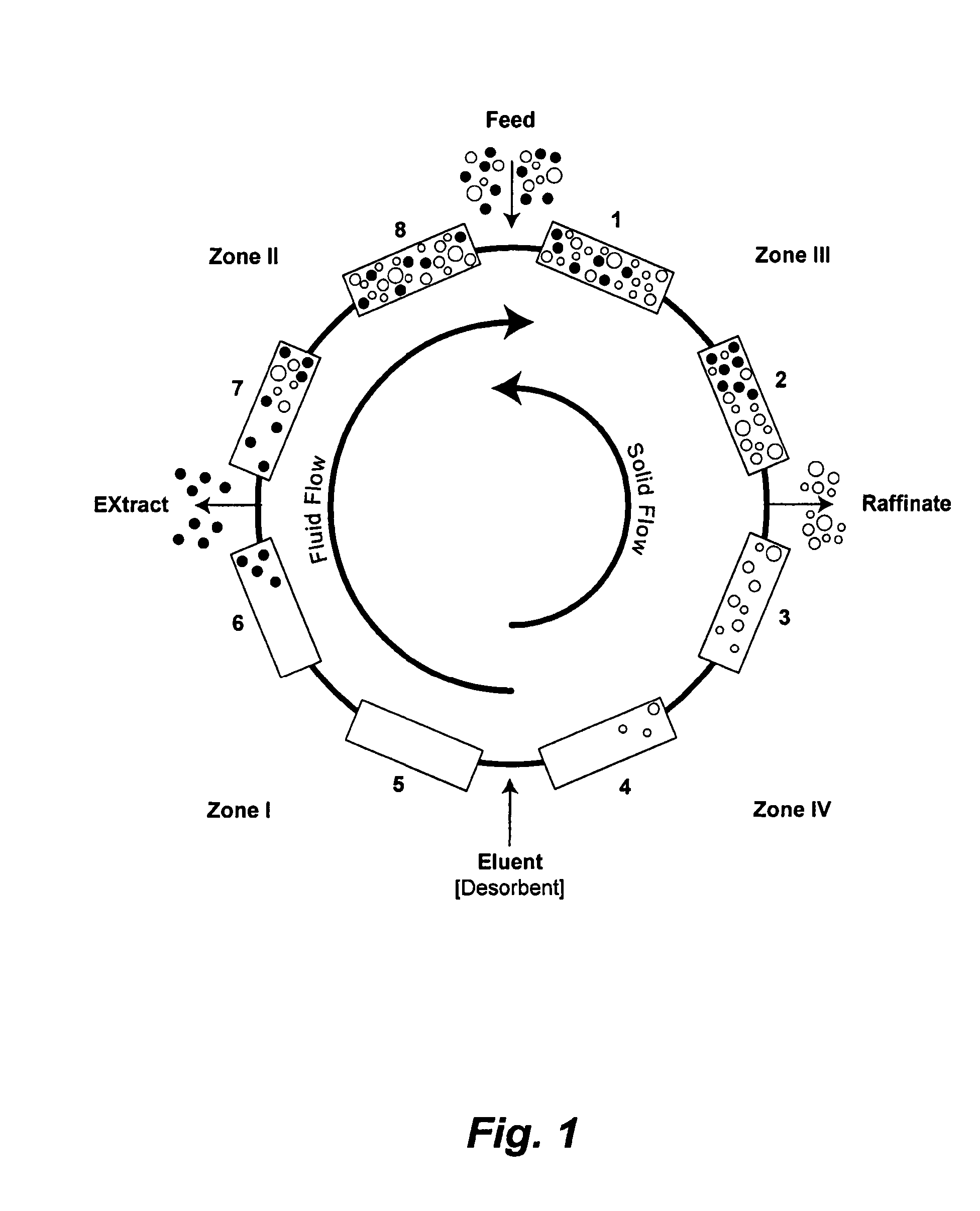
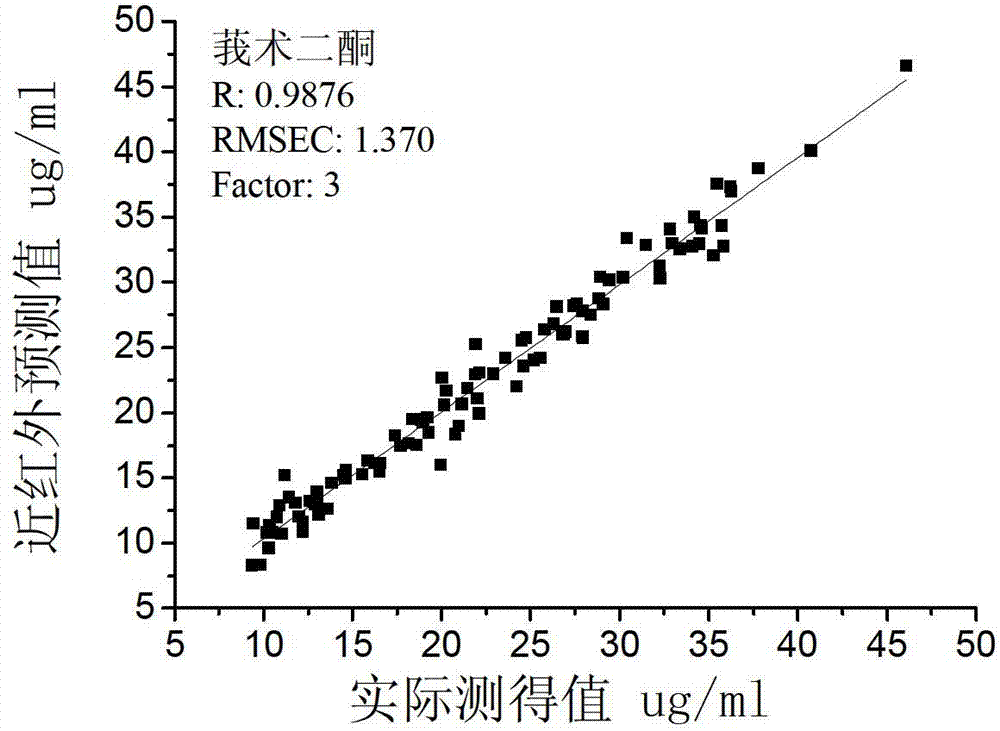
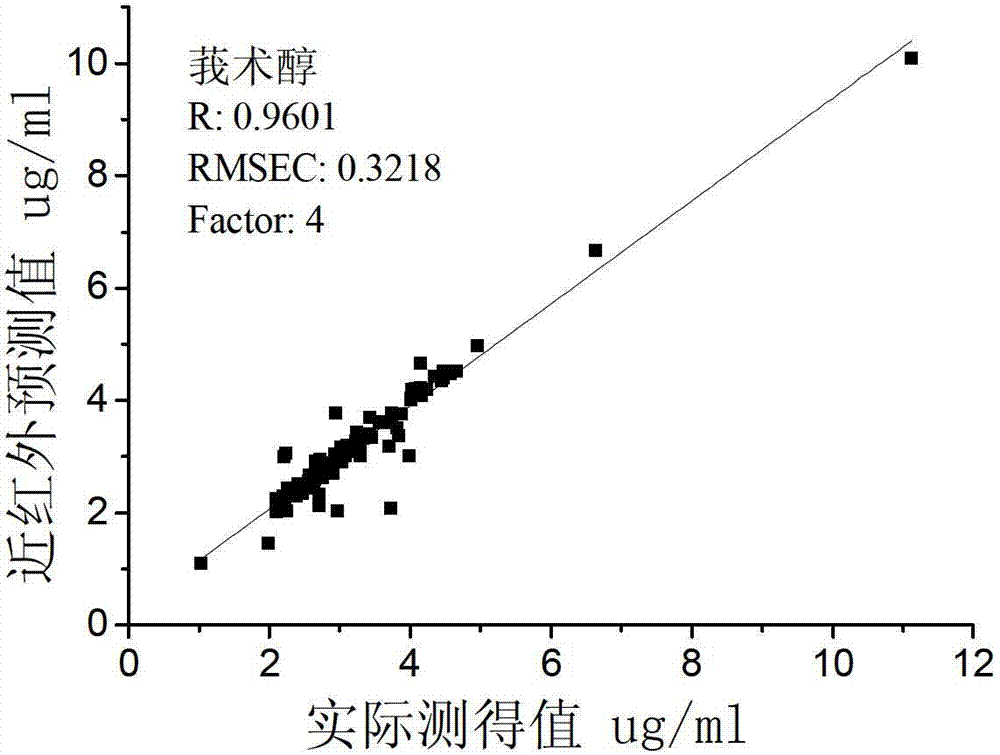
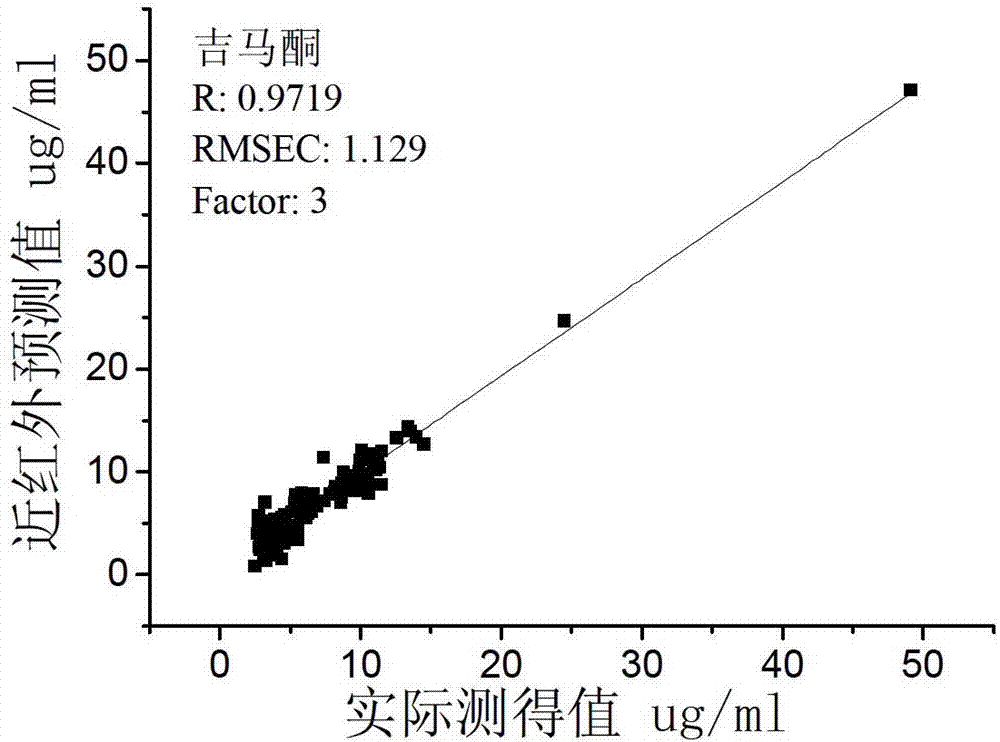
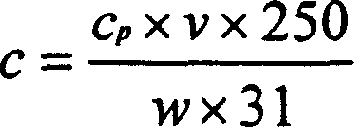Patents
Literature
536 results about "Gas chromatography" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
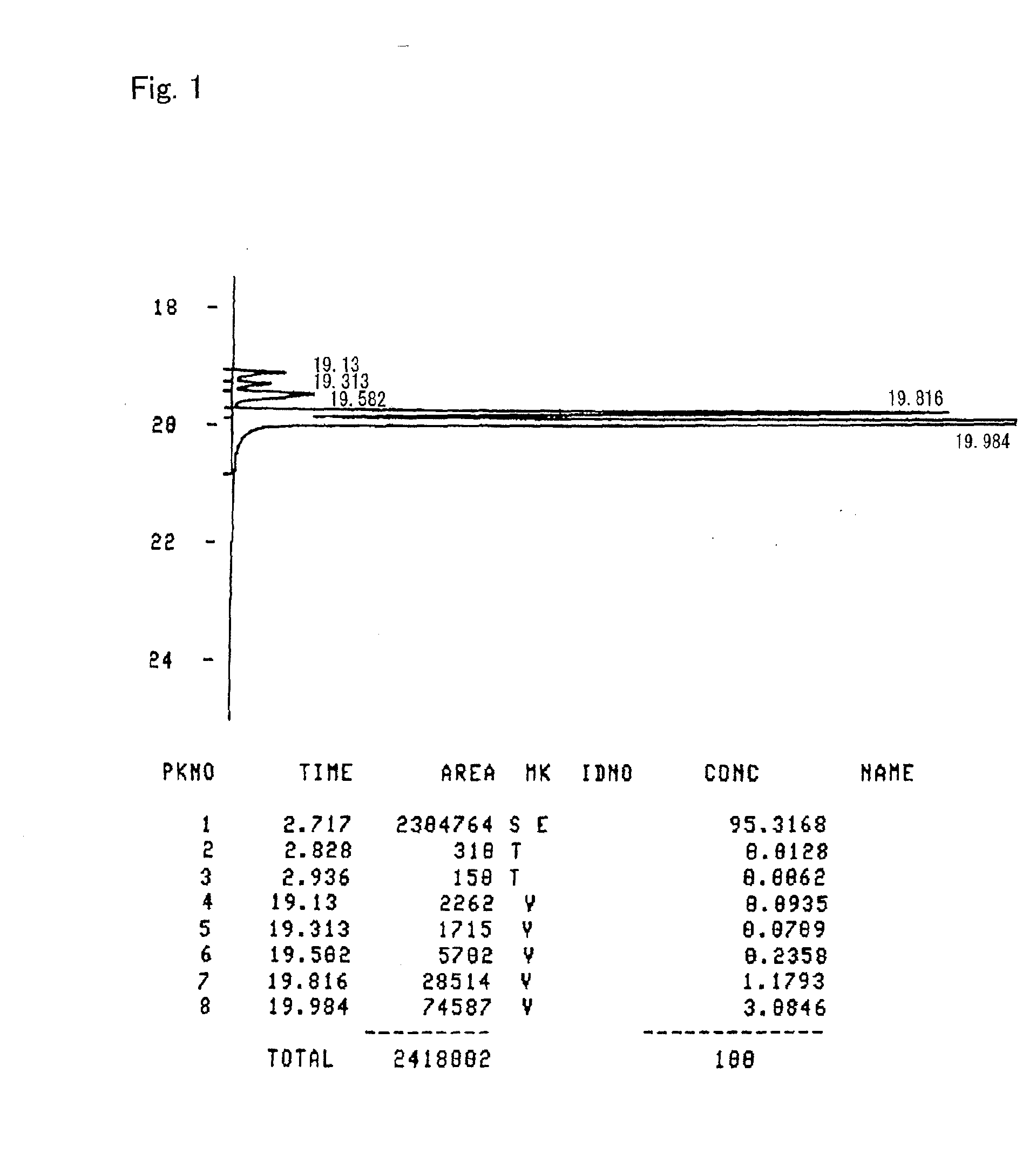
Gas chromatography (GC) is a common type of chromatography used in analytical chemistry for separating and analyzing compounds that can be vaporized without decomposition. Typical uses of GC include testing the purity of a particular substance, or separating the different components of a mixture (the relative amounts of such components can also be determined). In some situations, GC may help in identifying a compound. In preparative chromatography, GC can be used to prepare pure compounds from a mixture.
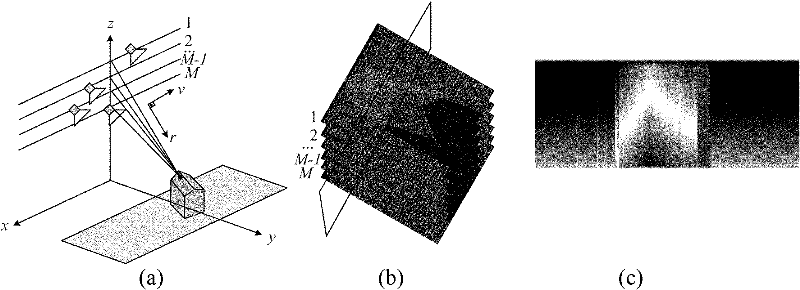
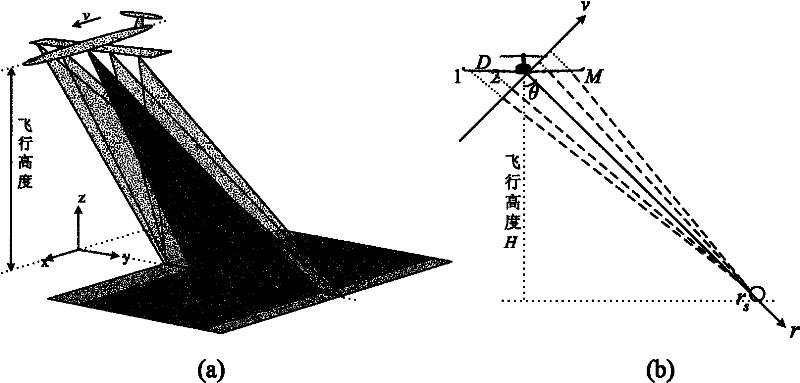
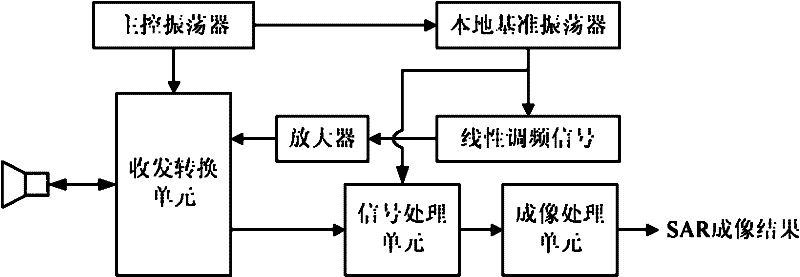
Airborne multi-antenna SAR chromatography three dimensional imaging system and imaging method thereof
InactiveCN102221697AControl load3D Imaging RealizationRadio wave reradiation/reflectionSynthetic aperture radarDimensional modeling
Provided is an airborne multi-antenna SAR chromatography three dimensional imaging system and an imaging method thereof. The invention is directed to the field of electronic signal processing technology and particularly relates to an SAR (Synthetic Aperture Radar) chromatography three dimensional imaging technology. According to the invention, a multi-antenna system structure is used to realize an SAR chromatography three dimensional imaging characterized by low flight risk, high resolution and large range. A multi-antenna working mode of single reception and multiple transmissions is adopted to reduce a loader load; meanwhile the possible imaging height scope is enlarged twice. An imaging method based on signal sparse expression is employed to realize a single flight super-resolution three dimensional imaging of the multi-antenna system. After the multi-antenna system structure is adopted, it requires less flight times to realized the three dimensional imaging. Meanwhile, the design of each flight locus is more flexible. Spaces are expanded for the application of SAR three dimensional imaging technologies in the airborne platform.
Owner:UNIV OF ELECTRONIC SCI & TECH OF CHINA
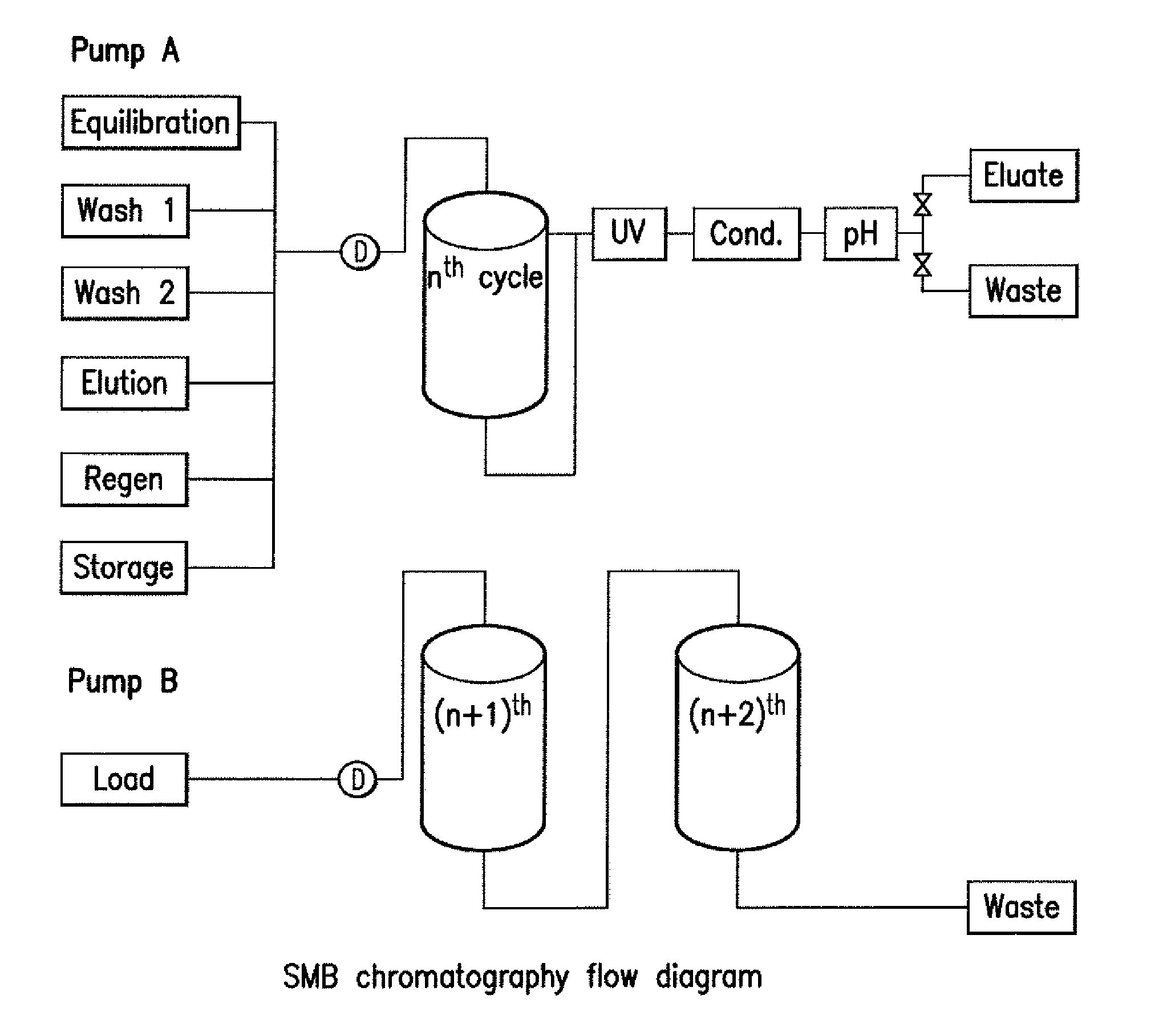
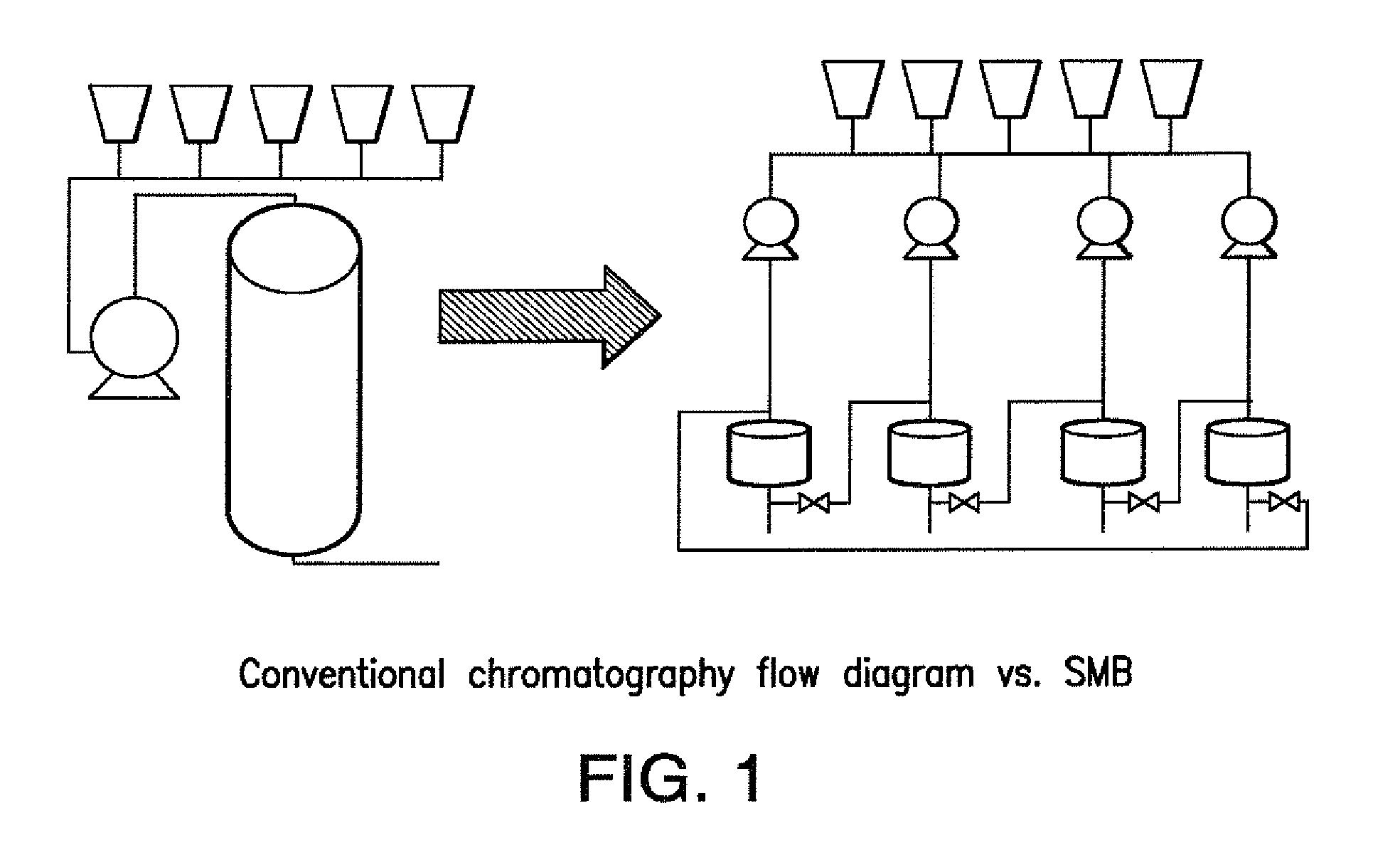
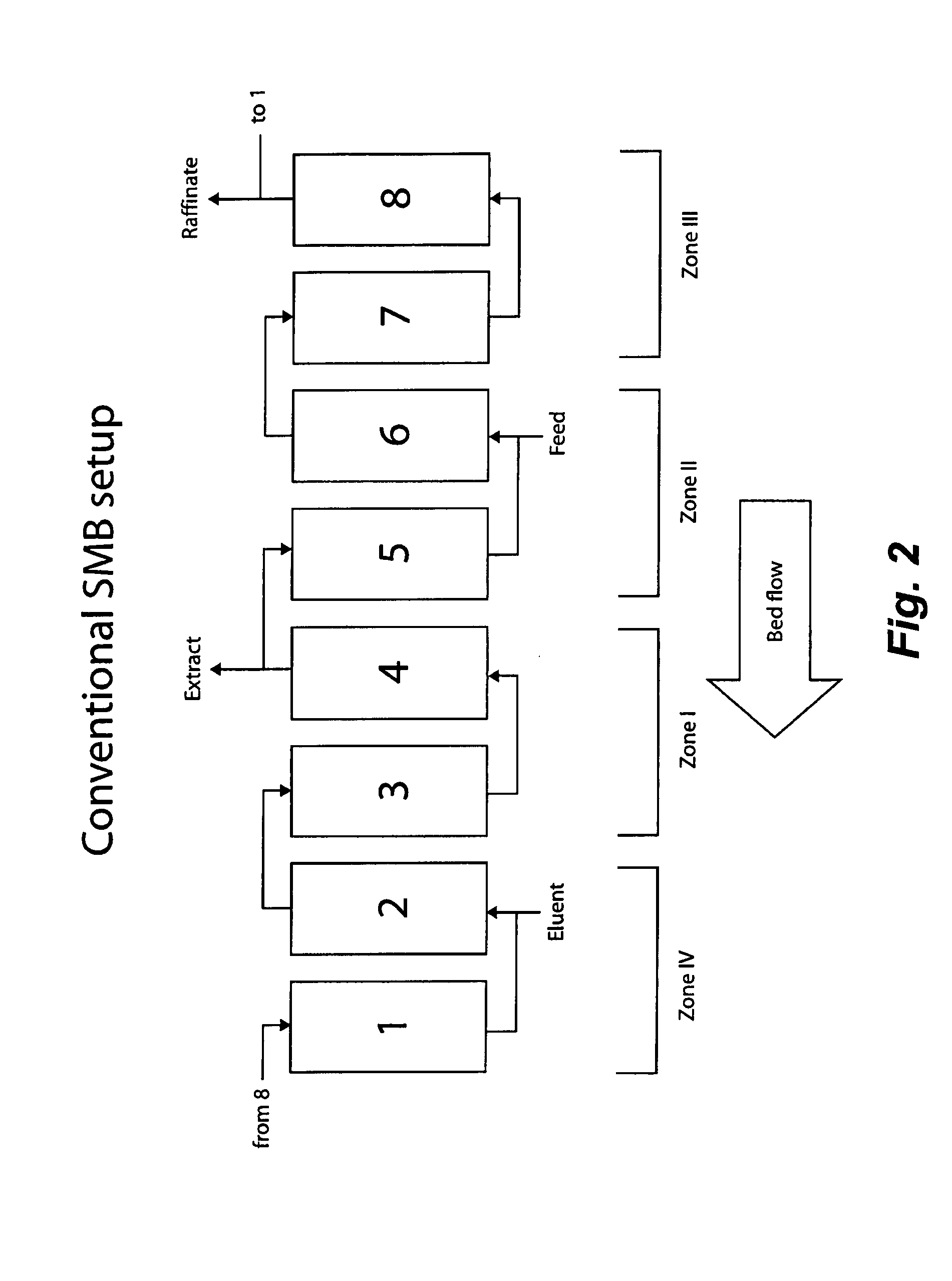
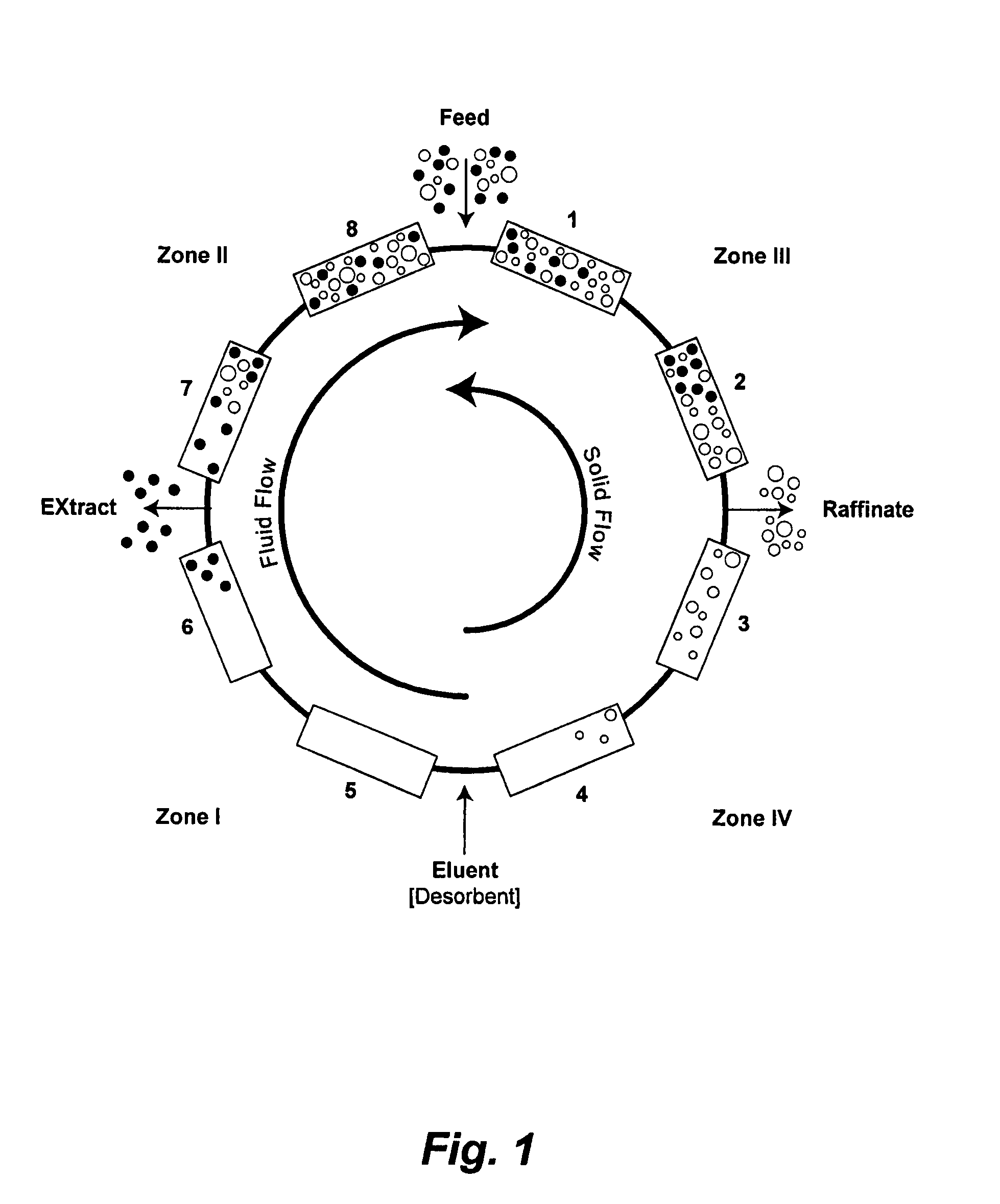
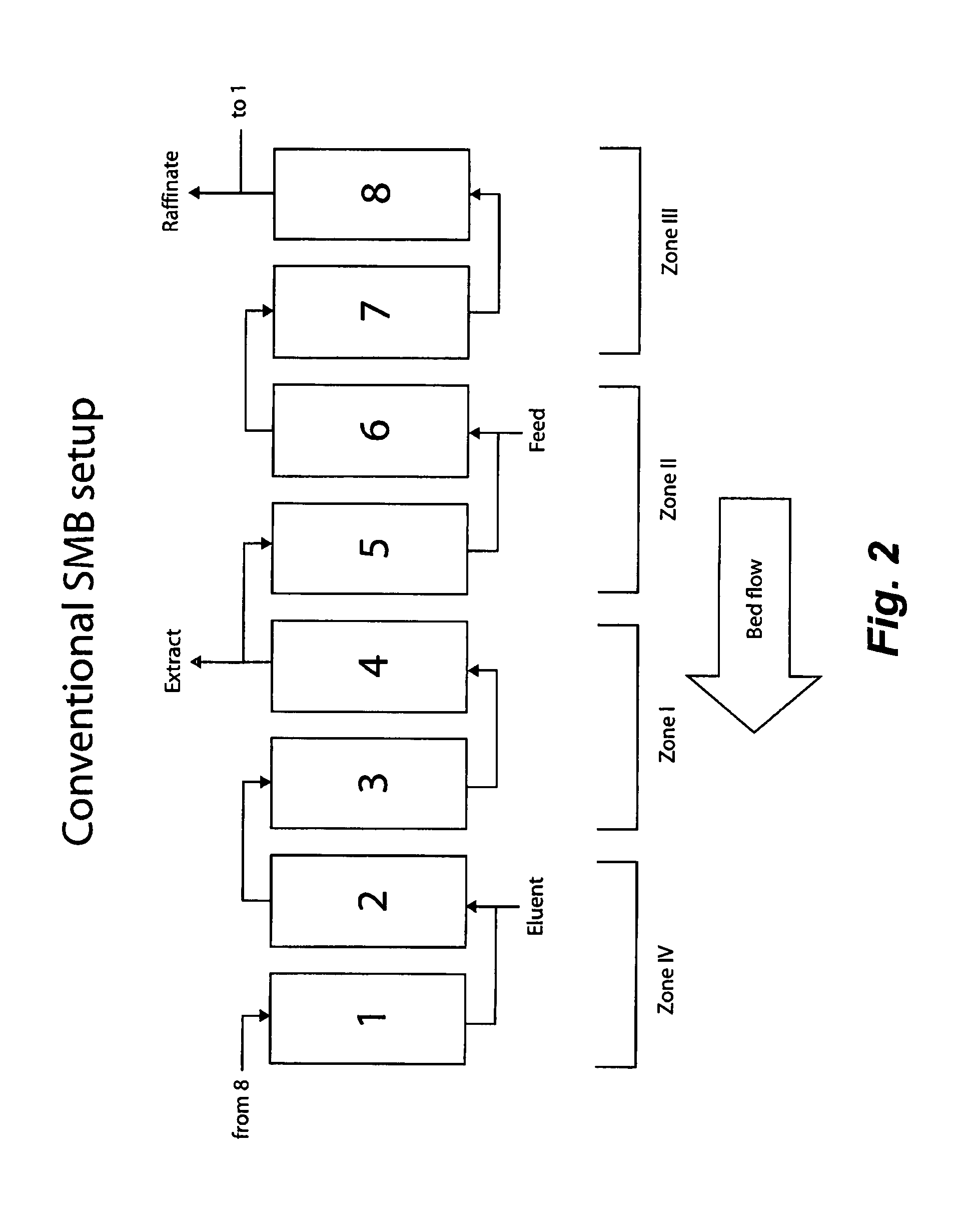
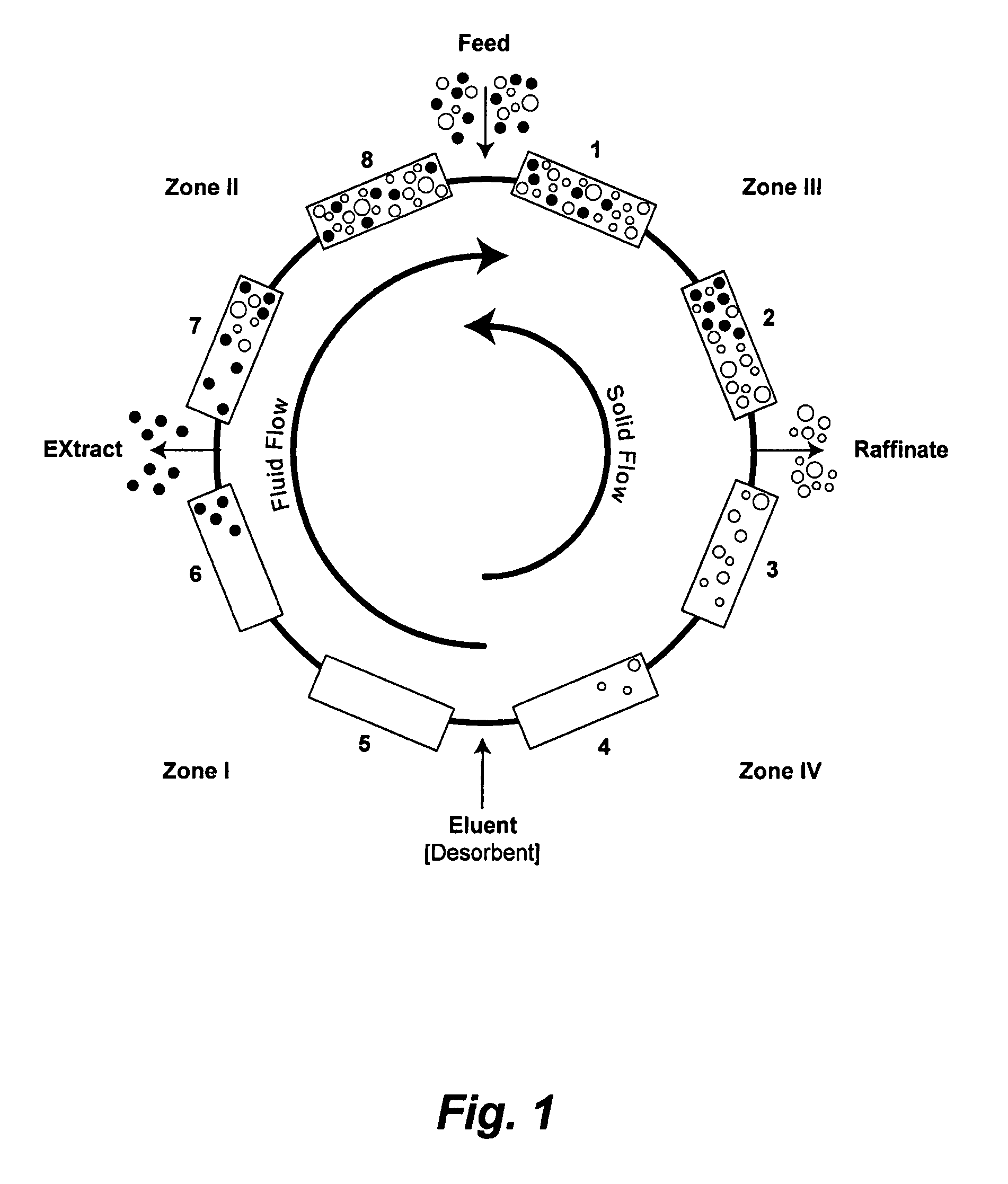
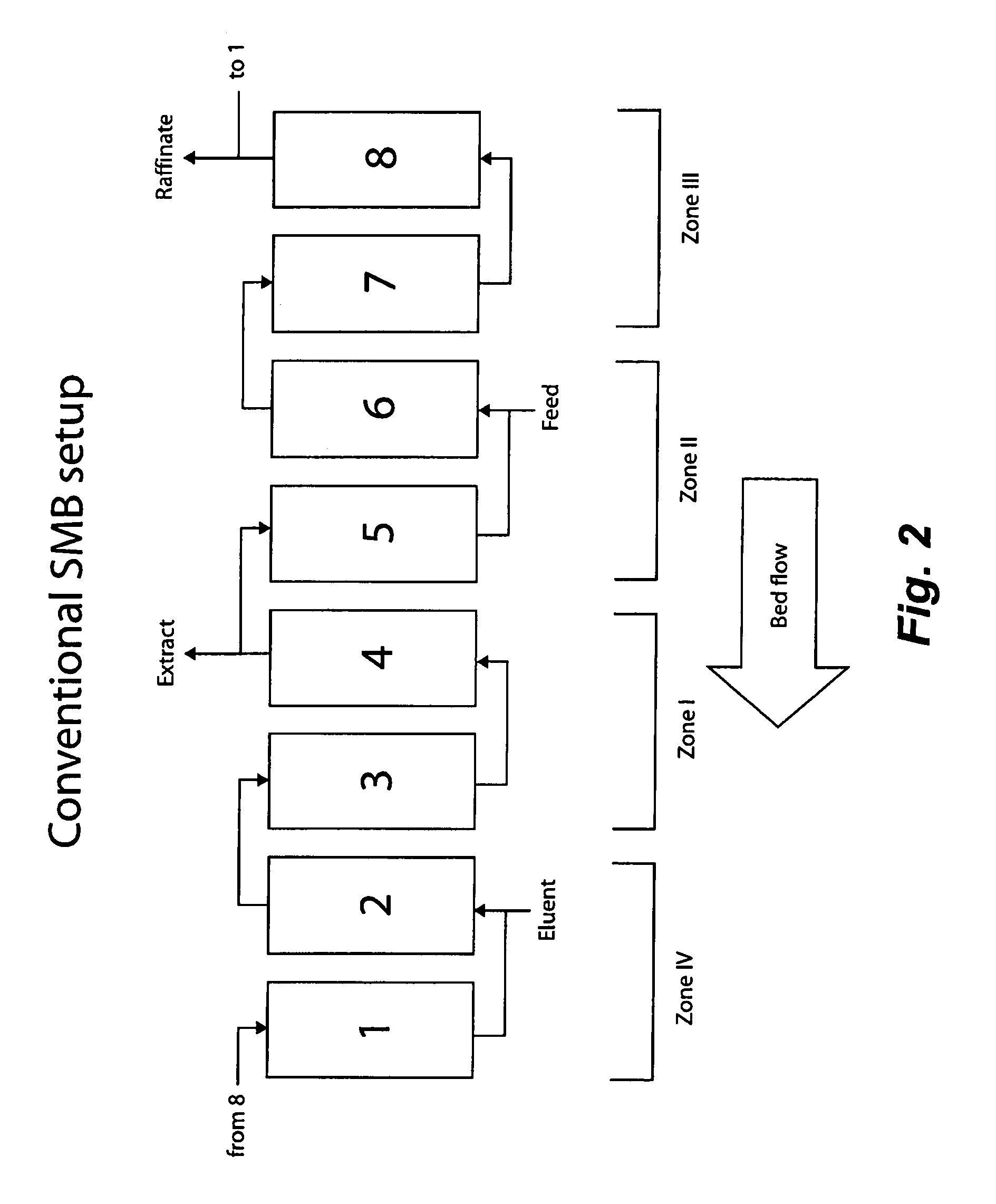
Method of purifying polypeptides by simulated moving bed chromatography
Provided are methods of separating an immunoreactive compound from at least one immaterial component, using a simulated moving bed (“SMB”) system and a SMB apparatus for use in these methods. Also provided are purified immunoreactive compounds prepared using the SMB methods and apparatus and methods of treatment with the purified immunoreactive compounds.
Owner:BIOGEN INC
Purification of antibodies using simulated moving bed chromatography
InactiveUS20120122076A1Hormone peptidesPeptide/protein ingredientsSimulated moving bedMonoclonal antibody
The present invention relates to compositions and methods for the chromatographic purification of antibodies, such as monoclonal antibodies, employing improved simulated moving bed separation strategies and, in certain embodiments, Raman spectroscopy.
Owner:ABBVIE INC
Aqueous dispersion for chemical mechanical polishing and chemical mechanical polishing method
InactiveUS20110053462A1Reduce polishing rateHigh polishing rateOther chemical processesDecorative surface effectsIon contentIon chromatography
A chemical mechanical polishing aqueous dispersion including (A) silica particles, and (B1) an organic acid, the sodium content, the potassium content, and the ammonium ion content of the silica particles (A) determined by ICP atomic emission spectrometry, ICP mass spectrometry, or ammonium ion quantitative analysis using ion chromatography having a relationship in which the sodium content is 5 to 500 ppm and at least one of the potassium content and the ammonium ion content is 100 to 20,000 ppm.
Owner:JSR CORPORATIOON
Enhanced purification of antibodies and antibody fragments by apatite chromatography
Methods are disclosed for use of apatite chromatography, particularly without reliance upon phosphate gradients, for purification or separation of at least one intact non-aggregated antibody, or at least one immunoreactive antibody fragment, from an impure preparation. Integration of such methods into multi-step procedures with other fractionation methods are additionally disclosed.
Owner:BIO RAD LAB INC
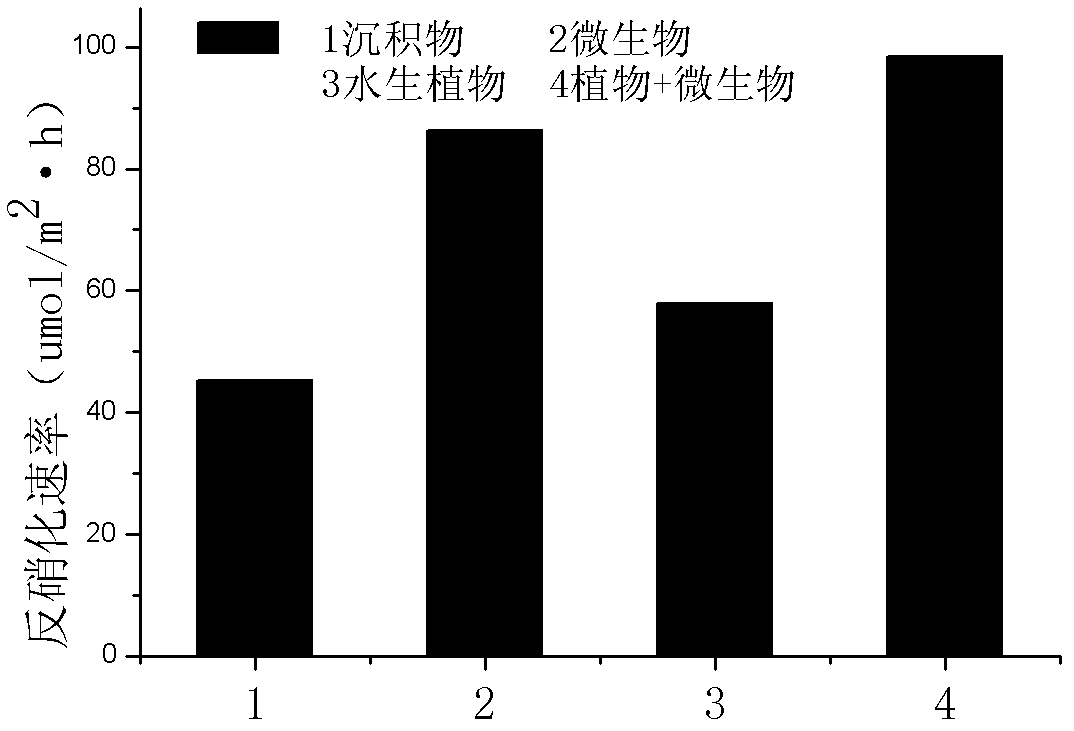
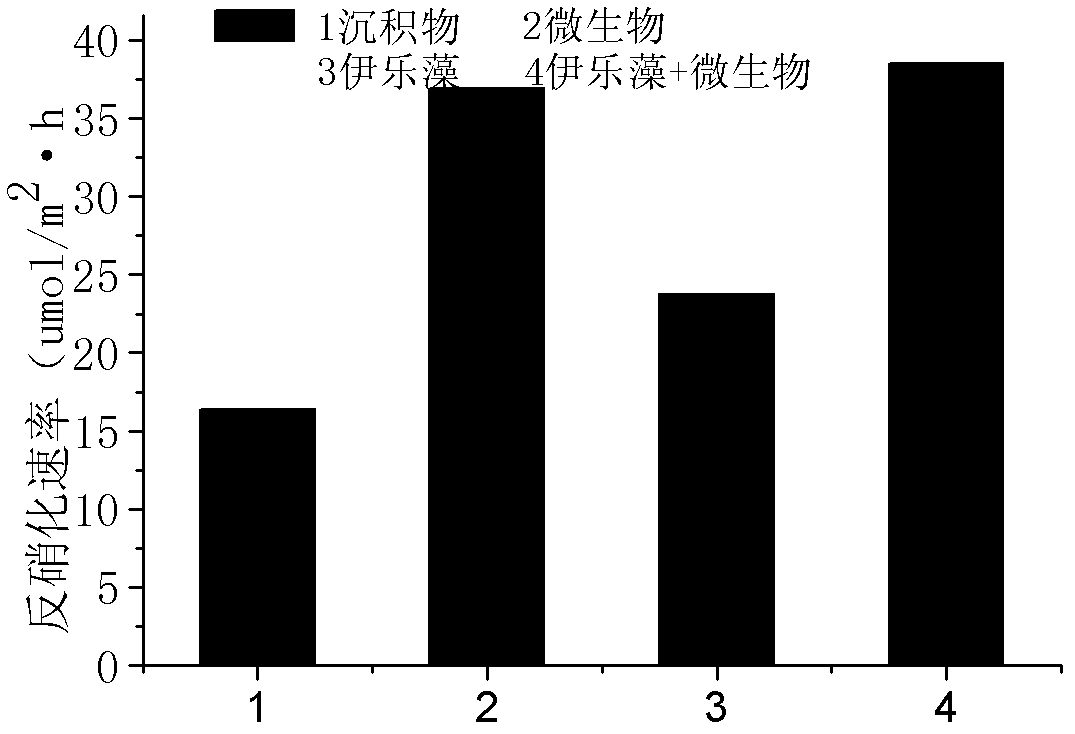
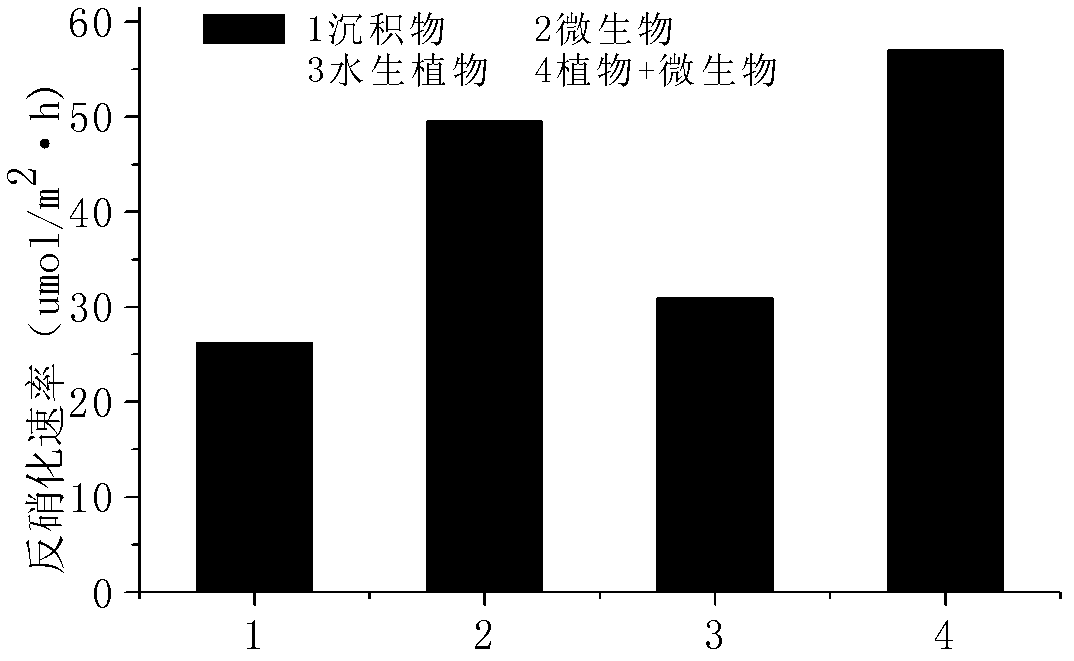
Precise quantization method for nitrogen cycle of lake ecosystem
A precise quantization method for nitrogen cycle of a lake ecosystem includes 1), applying an isotope pairing method to quantitatively research nitrogen migration and conversion of a sediment-water interface of the lake ecosystem; 2), determining release of nitrogen of a water-gas interface by measuring N2O yield by the aid of a closed chamber-gas chromatograph method; 3), measuring biomass of aquatic plants and nitrogen content in bodies of the aquatic plants by the aid of a Dumas method and determining nitrogen absorbed quantity of the aquatic plants; 4), measuring biomass of microorganisms in the ecosystem by the aid of an MPN (most probable number) method, and measuring the effect of the microorganisms in a nitrogen conversion process and conversion quantity of the microorganisms to the nitrogen; and 5), applying the stable isotope technology to measure assimilation of aquatic animals to the nitrogen, and realizing precise quantization of the nitrogen, namely, measuring a food web of the aquatic animals and a trophic-level structure to determine migration and conversion of the nitrogen in the food web. Procedures of the precise quantization method are realized in the same simulation ecosystem.
Owner:NANJING UNIV
Phenyl alkoxy silane prepared by sodium condensation method
InactiveCN101077877AReasonable designThorough responseGroup 4/14 element organic compoundsCondensation processChlorobenzene
The present invention relates to sodium condensation process for synthesizing phenyl alkoxyl silane. Chlorobenzene and MeaSiXb(OR)4-(a+b), where, X is Cl or Br, R is Me or Et, a is 0 or1 and b is 1, 2, 3 or 4, are made to produce sodium condensation reaction in solvent at 98-130 deg.c for 0.5-8 hr. The process has complete reaction of metal sodium, simple operation, short reaction period, benzene converting rate up to 70-80 %, and MePhSi(OR)2 selectivity up to 50 %.
Owner:盐城市华业医药化工有限公司
Alicyclic diepoxy compound, epoxy resin composition comprising the same, and cured article therefrom
Disclosed is an alicyclic diepoxy compound which gives a cured article suffering from no deterioration in properties even when used in hot and humid surroundings or used under such conditions as to give a strong acid, which is highly reactive upon curing, and which gives a cured article superior typically in thermal stability. Specifically, the alicyclic diepoxy compound includes a 3,4,3′,4′-diepoxybicyclohexyl compound represented by following Formula (1):wherein R1 to R18 each represent a hydrogen atom, a halogen atom, a hydrocarbon group which may have an oxygen atom or a halogen atom, or a substituted or unsubstituted alkoxy group, in which the alicyclic diepoxy compound contains isomers of the 3,4,3′,4′-diepoxybicyclohexyl compound in a content of less than 20% based on the total of the 3,4,3′,4′-diepoxybicyclohexyl compound and the isomers thereof in terms of peak area ratio as determined by gas chromatography.
Owner:DAICEL CHEM IND LTD
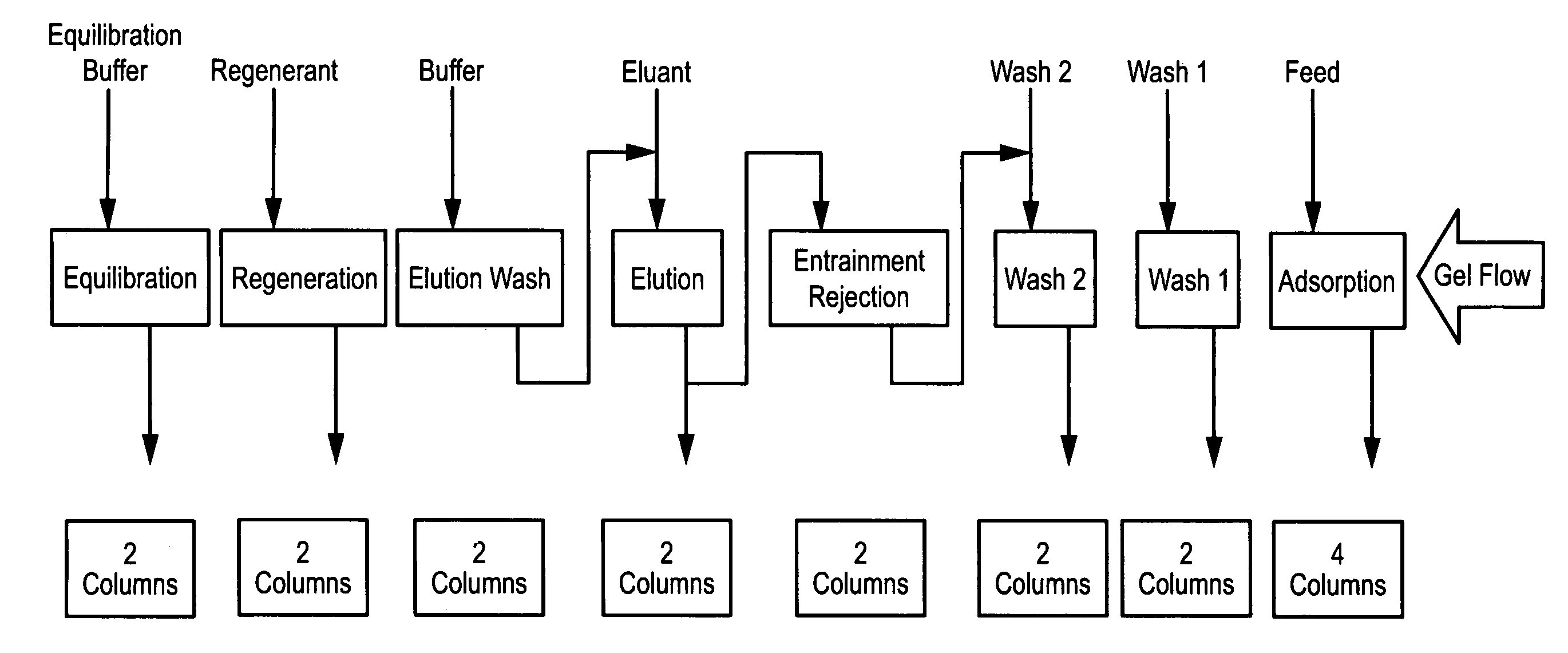
Continuous isocratic affinity chromatography
ActiveUS7790040B2Simple and easily programmable controlPromote repairIon-exchange process apparatusIon-exchanger regenerationSimulated moving bedMoving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Method for measuring ammonia content in cigarette smoke through ion chromatography
ActiveCN103472159AImprove stabilityAccurate measurementComponent separationParticulatesIon chromatography
The invention discloses a method for measuring ammonia content in cigarette smoke through ion chromatography. The method comprises the steps that an F319-04 filter leaf and an absorption bottle gather ammonia in main stream smoke, extract liquor is added to extract particulate matter in the F319-04 filter leaf, moderate extract liquor is mixed with absorption liquid in the absorption bottle, volume metering is conducted, an aqueous phase filter is used for filtering the mixed liquid, and an ion chromatograph is introduced to conduct measuring, wherein the extract liquor is aldehyde with strong acid. Filter leaf extraction is conducted by the aldehyde solvent with the strong acid, and therefore the stability of ammonia detection liquid can be effectively improved. The method for measuring the ammonia content in the cigarette smoke through the ion chromatography can measure the ammonia content in the sample solution accurately and fast through the ion chromatography, and is suitable for measurement of the ammonia content in large numbers of cigarette samples, good in practicability and beneficial to deep harm-reduction and tar-reduction research.
Owner:CHINA TOBACCO JIANGSU INDAL
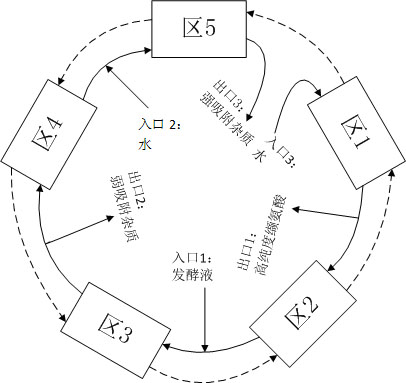
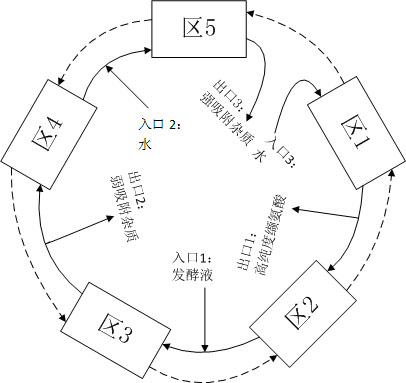
Method for continuously separating and purifying valine in fermentation liquor by using simulated moving bed chromatography
InactiveCN101948399AHigh purityFully cleanedOrganic compound preparationSolid sorbent liquid separationSimulated moving bedMoving bed
The invention discloses a method for continuously separating and purifying valine in fermentation liquor by using simulated moving bed chromatography, and belongs to the technical field of biological product processing. In the method, a simulated moving bed chromatography separating device suitable for the composition characteristic of valine fermentation liquor is designed, water is taken as a mobile phase, and the previously invented resin special for valine separation (disclosed in the Chinese patent 200910231735.4) is taken as a stationary phase. The method can completely remove impurities of alanine and leucine from the valine fermentation liquor, achieves product purity of over 99.7 percent to meet the medicinal standard, is suitable for continuous production, has higher recovery rate and production strength, and overcomes the defects of low purity, low efficiency, high pollution and the like in the conventional valine separation and purification process.
Owner:JIANGNAN UNIV
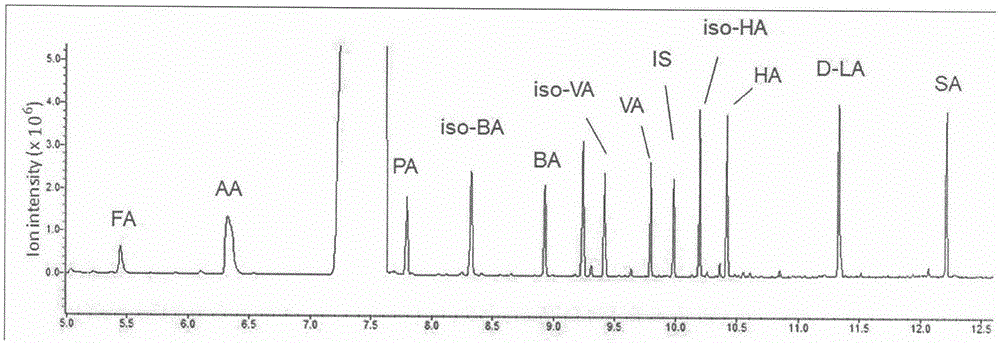
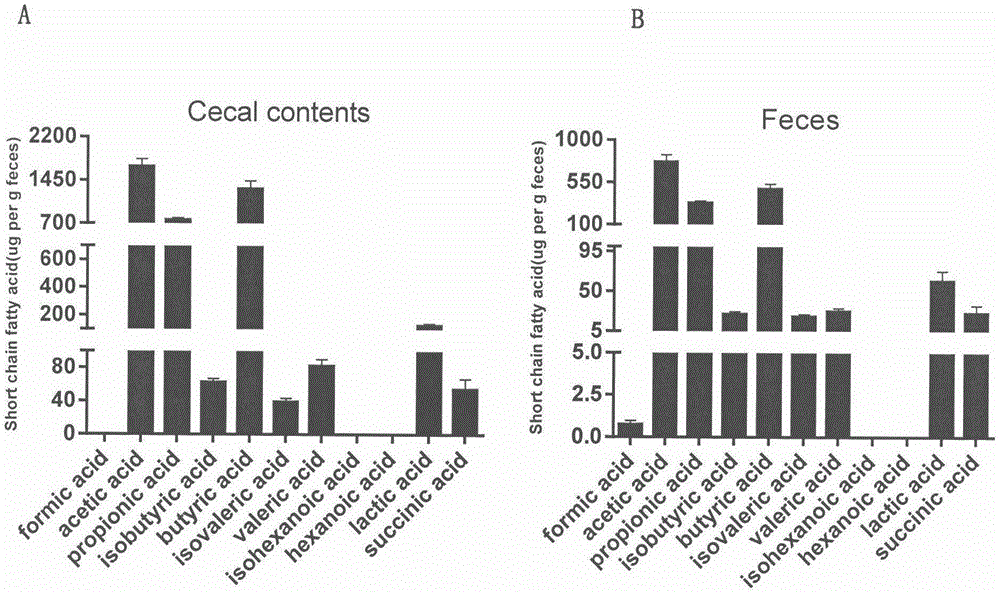
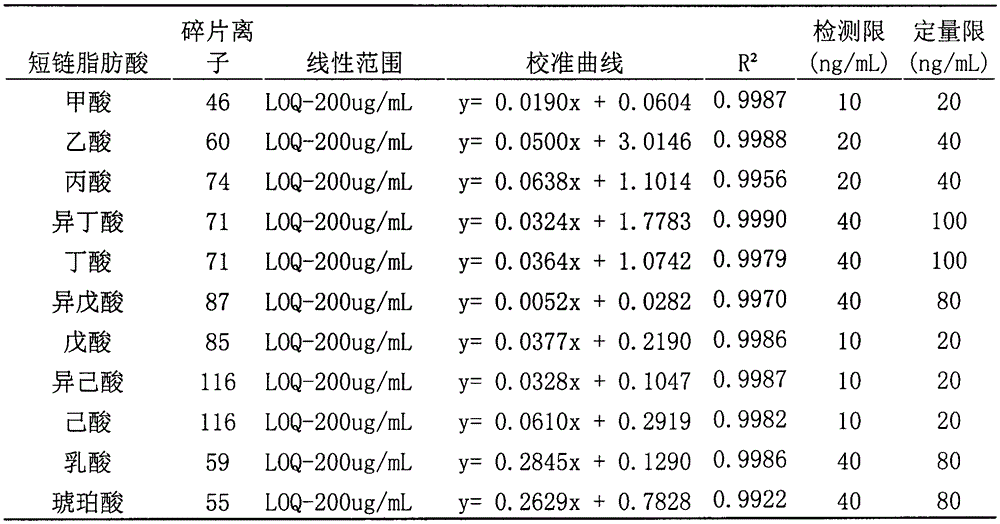
GC-MS (gas chromatography-mass spectrometer)-based method for quantifying eleven types of short-chain fatty acids in intestinal contents and fecal samples
InactiveCN105651908AHigh extraction recoveryImprove securityComponent separationGas phasePotential biomarkers
The invention belongs to the technical field of endogenous substance detection and discloses a GC-MS (gas chromatography-mass spectrometer)-based method for quantifying eleven types of short-chain fatty acids in intestinal contents and fecal samples. By adoption of a hydrochloric acid solution prepared from saturated salt water for acidification, high-stability ethyl acetate for extraction and N-tert-butyldimethylsilyl-N-methyl trifluoroacetamide MTBSTFA for derivatization, the eleven short-chain fatty acids in mouse intestinal contents and fecal samples are quantified by a gas chromatography-mass spectrometer. The method is high in accuracy and precision and low in detection limit, can be used for quantitative detection of eleven types of short-chain fatty acids and can meet detection requirements on determination of major short-chain fatty acids in the mouse intestinal contents and the fecal samples. Moreover, the method is conducive to analysis and comparison of metabolic disposition laws of the short-chain fatty acids under normal and enteritis conditions and observation whether changes of the short-chain fatty acids can represent dynamic conditions of enteritis or not, thereby providing significant bases for potential biomarkers under the enteritis condition.
Owner:CHINA PHARM UNIV
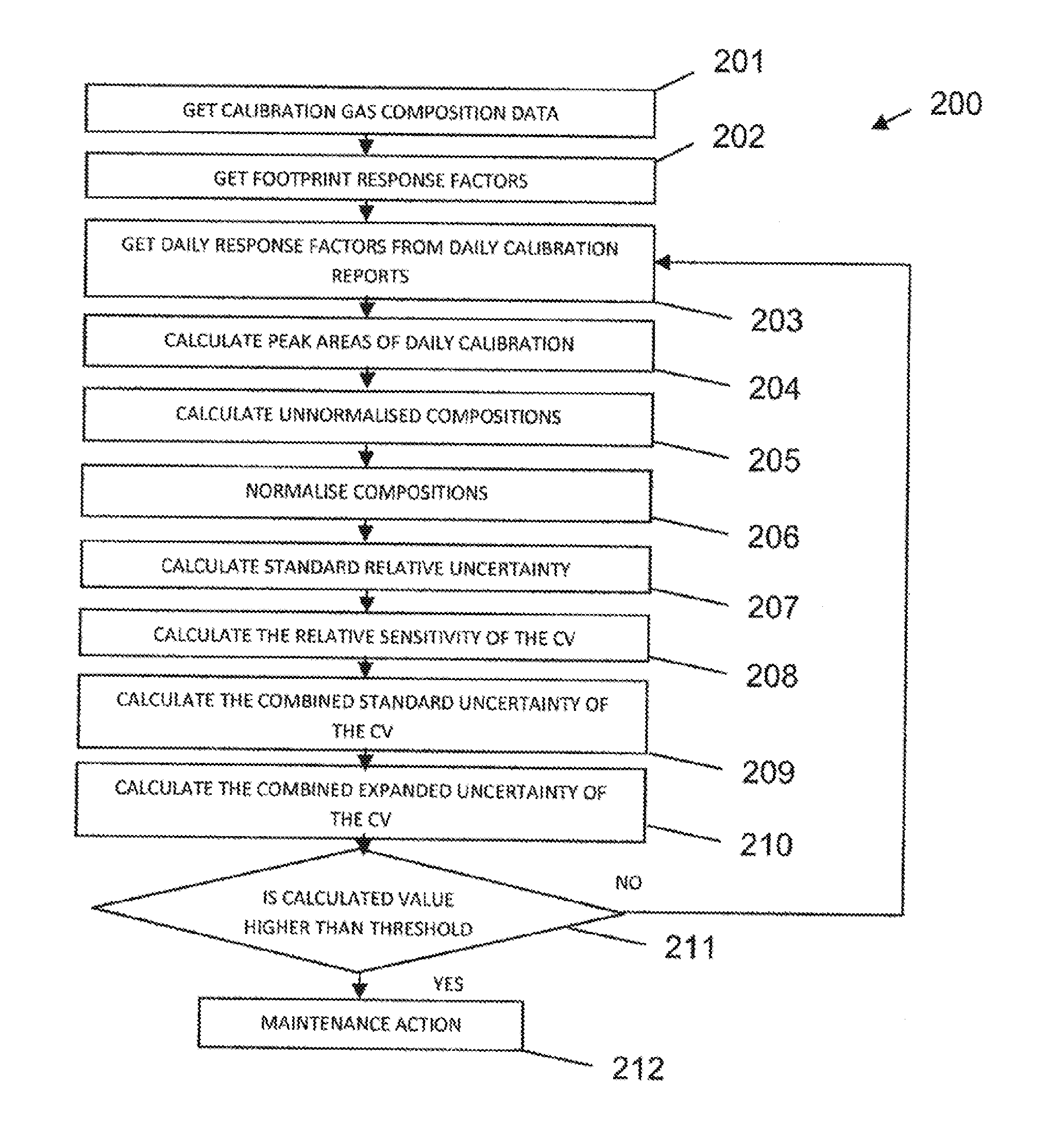
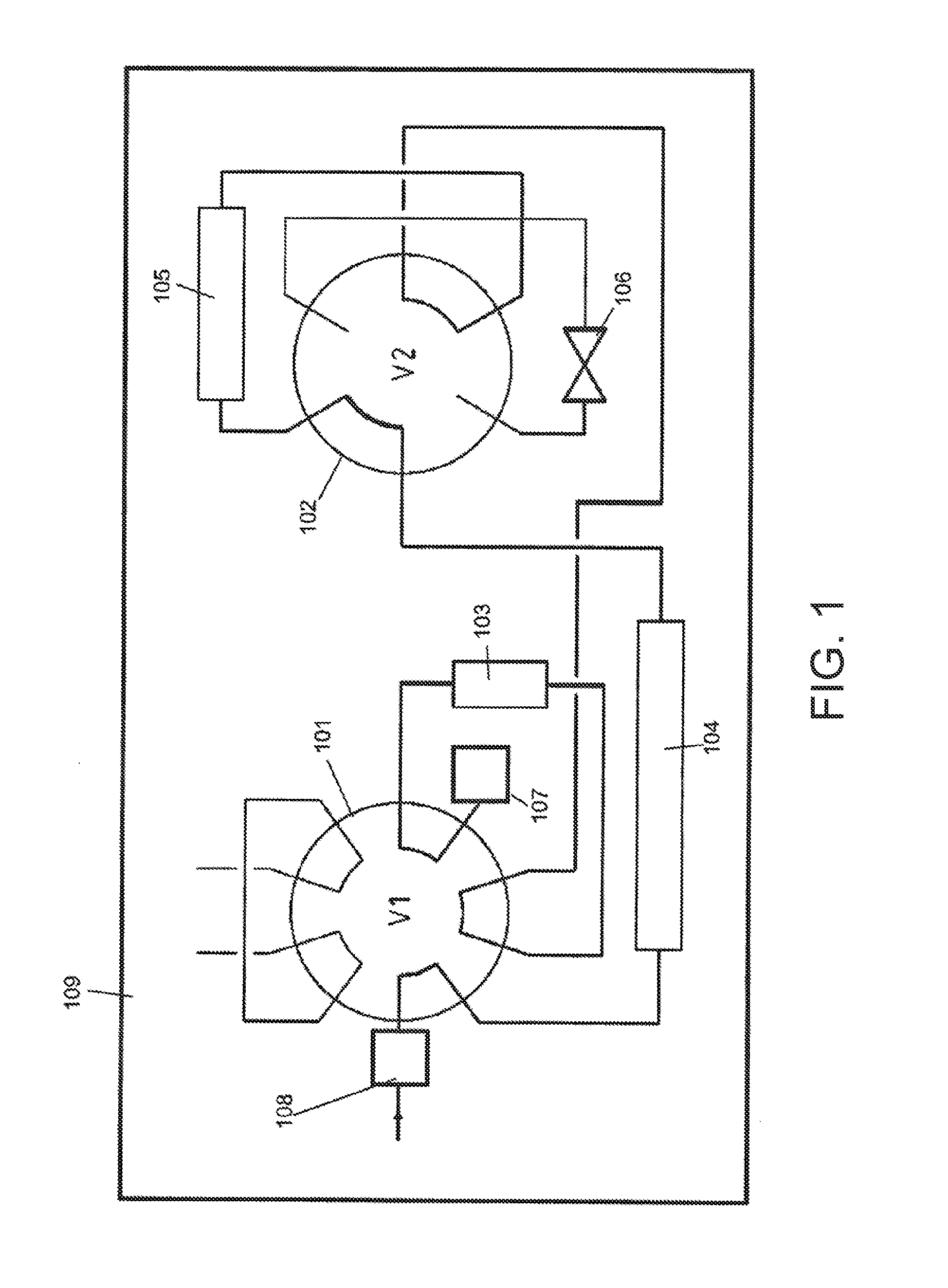
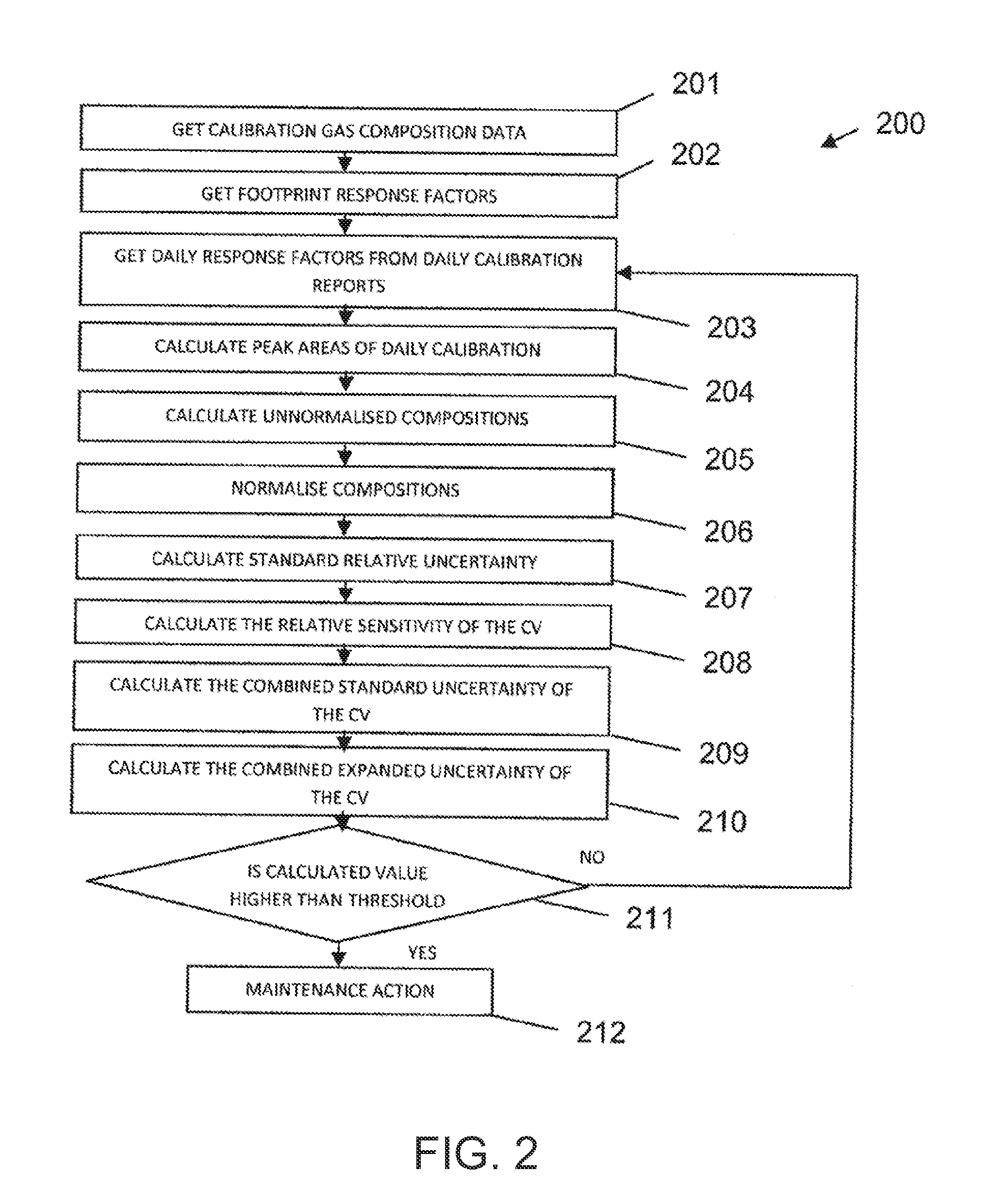
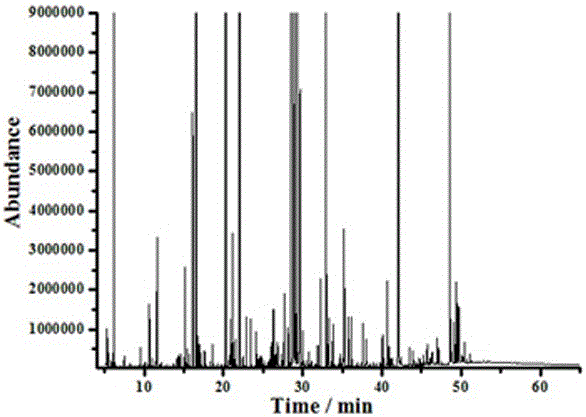
Improved method of analysing gas chromatography data
ActiveUS20130304393A1Economical resourceWaste of time economicalComponent separationSpecial data processing applicationsResponse factorData set
A method of analysing gas chromatography data is described. The method, a first response factor data set acquired from a gas chromatograph (GC) apparatus during a procedure on a calibration or reference gas sample at a first time is received. One or more additional response factor data sets acquired from the gas chromatograph apparatus during a procedure on a calibration or reference gas sample from one or more later times are received. The method comprises calculating a measure of uncertainty for at least one compound of the reference gas sample from the first and additional response factor data sets. The one or more later times are during an operational period of the gas chromatograph apparatus. The measure of uncertainty may be used to, for example, identify the necessity to perform a maintenance action in the GC or to assess whether the GC is in a healthy or unhealthy condition.
Owner:I VIGILANT TECH
Rapid determination method for organophosphorus pesticide residue in vegetable and fruit
InactiveCN1546988AEasy to buyFast measurementPreparing sample for investigationColor/spectral properties measurementsSorbentSolvent
The invention refers to a kind of measuring method for organic phosphorus agricultural chemicals residues in garden stuff. The invention uses organic solvent to extract the organic phosphorus agricultural chemicals in fruits and vegetables, uses absorbing agent to eliminate the phosphorus extraction material of inorganic phosphorus agricultural chemicals in the extraction liquid, in odder to eliminate the disturbance. The invention uses air or nitrogen to blow off the solvent in the extraction liquid, adds in potassium sulfate solvent in the residual, decomposes, converts the organic phosphorus agriculture chemicals into orthophosphate, finally, uses molybdenum antimony splitting resisting luminosity method to measure the orthophosphate density in the decomposed liquid, thus works out the whole residue quantity of organic phosphorus agricultural chemicals in the fruits and vegetables. The speed is fast, it is accurate, stable, and reliable.
Owner:XIAMEN UNIV
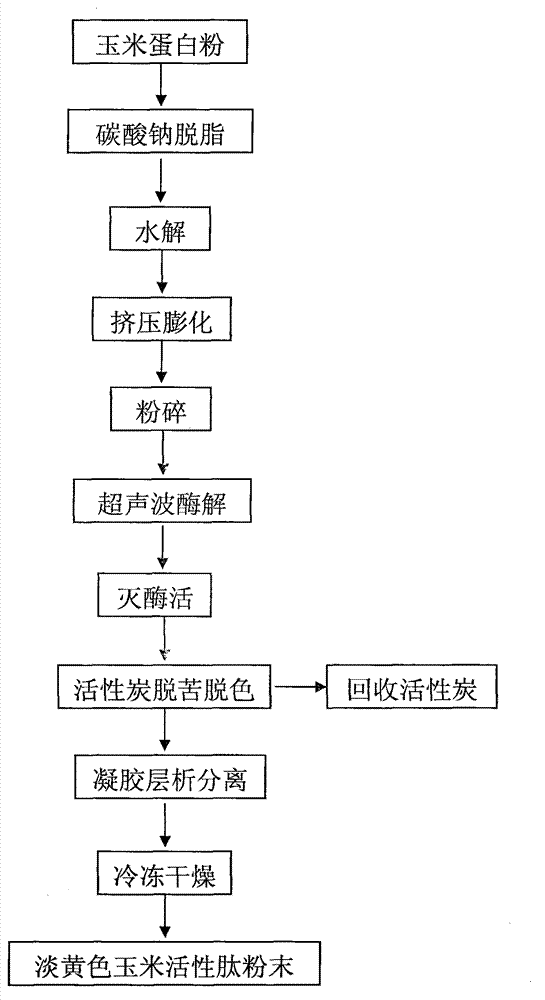
Preparation method of corn active peptides
InactiveCN102864201ALow priceImprove food safetyPeptide preparation methodsFermentationFreeze-dryingDry basis
The invention discloses a preparation method of corn active peptides. The preparation method includes subjecting corn protein powder to steps of pretreating, extrusion puffing, ultrasonic enzymolysis, enzyme deactivation, debittering, decoloring, gel chromatography, freeze-drying and the like. The preparation method is simple in process and low in cost. Content (by dry basis) of prepared corn active peptides reaches more than 85%, yield reaches more than 72%, and a feasible process for large-scale production is provided.
Owner:SHAANXI TONGZHENG TECH CO LTD
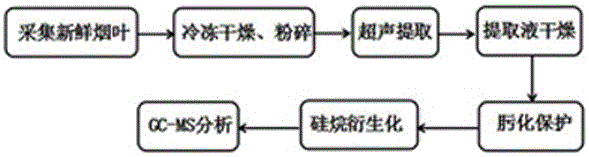
Method for detecting primary metabolites and secondary metabolites in fresh tobacco leaves with GC-MS (gas chromatography-mass spectrometer)
The invention provides a method for detecting primary metabolites and secondary metabolites in fresh tobacco leaves with GC-MS (gas chromatography-mass spectrometer). The method comprises steps as follows: the fresh tobacco leaves are rapidly collected and quick-frozen with liquid nitrogen on site, the quick-frozen tobacco leaves are transferred to dry ice and transported to a laboratory at low temperature, moisture is removed by a freeze dryer with a low-temperature freeze drying method, the tobacco leaves are pulverized by a pulverizer and screened by a 40-mesh sieve, the primary metabolites and the secondary metabolites of the pulverized tobacco leaves are extracted ultrasonically with a solvent, a supernatant of an extracting solution is subjected to nitrogen blow-drying, blow-dried extracts are subjected to an oximation reaction and a silane derivatization reaction, and GC-MS analysis is performed finally. The method has the advantages as follows: a tobacco leaf collecting method can really reflect growth and development periods, collecting parts, varieties and production places of the tobacco leaves; a tobacco leaf extracting and detecting method can cover the primary and secondary metabolites such as sugar, amino acid, organic acid, sterol, alkaloid, polyphenol and the like of the fresh tobacco leaves and has high-flux detection characteristics, and a selective ion monitoring method can realize accurately qualitative and quantitative analysis of 199 primary and secondary metabolites in the fresh tobacco leaves, and 135 metabolites are required to be verified in a standard substance.
Owner:ZHENGZHOU TOBACCO RES INST OF CNTC
Control system for simulated moving bed chromatography
ActiveUS7806137B2Simple and easily programmable controlPromote repairSolid sorbent liquid separationWater/sewage treatmentControl systemSimulated moving bed
Owner:TOSOH BIOSCIENCE LLC
Rapid center control testing method of Xingnaojing injection
ActiveCN102928352AImprove quality controlRapid determinationColor/spectral properties measurementsInfraredDiketone
Owner:WUXI JIMIN KEXIN SHANHE PHARMA +1
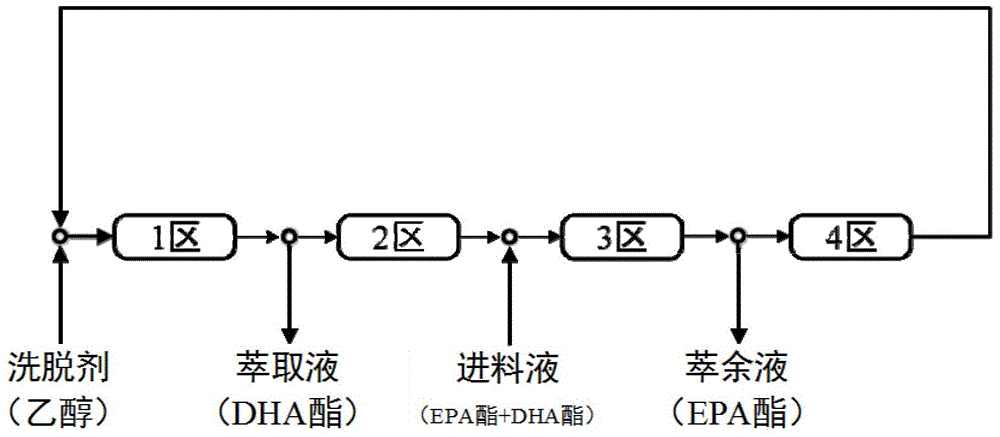
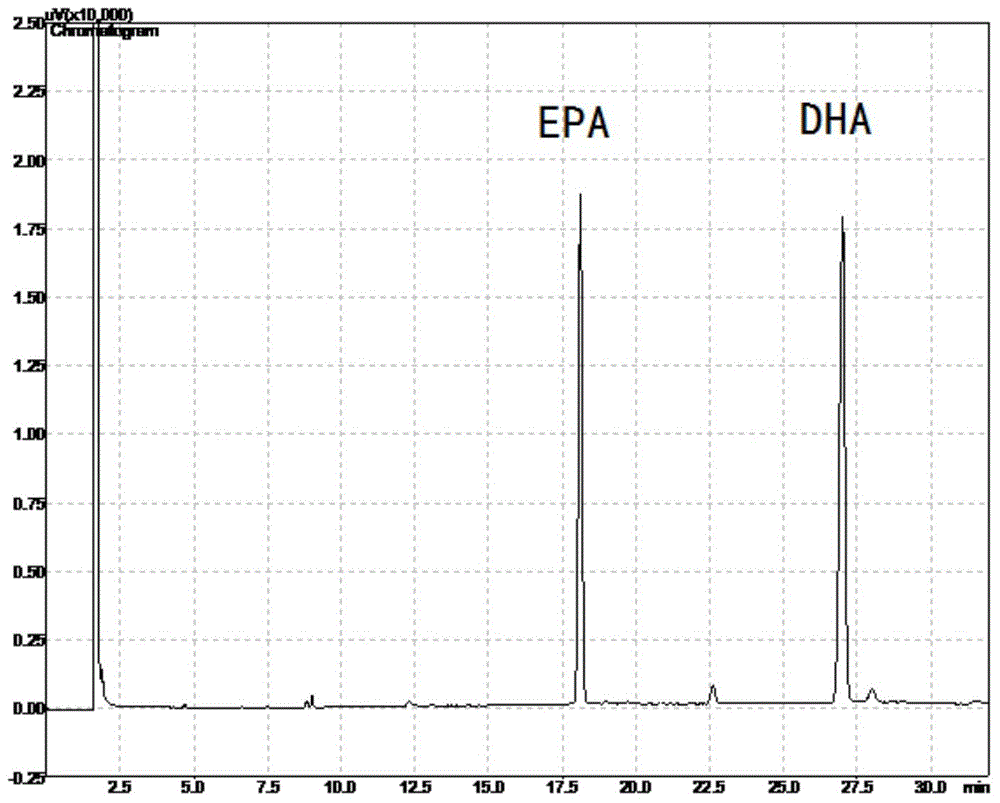
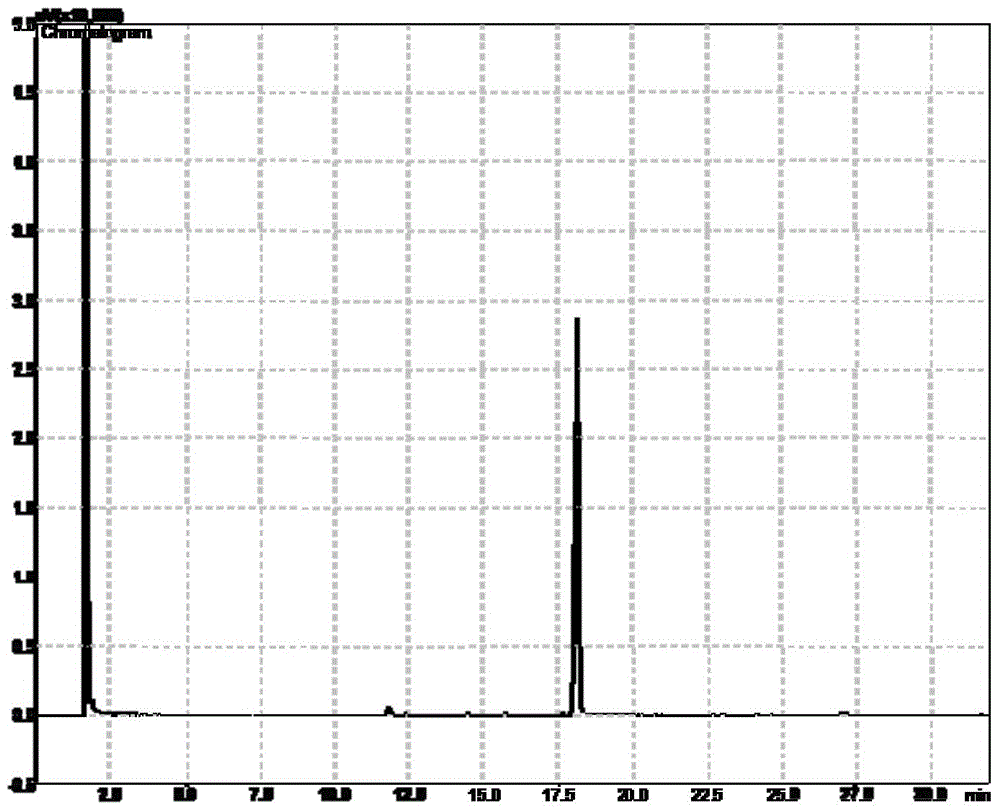
Method for preparing high-purity EPA ester and DHA ester monomers by virtue of simulated moving bed chromatography
ActiveCN104529772AEasy to recycleChemically stableOrganic compound preparationCarboxylic acid esters separation/purificationDocosahexaenoic acidEicosapentaenoic acid
The invention discloses a method for separating an eicosapentaenoic acid ester (EPA) ester from a docosahexaenoic acid (DHA) ester by virtue of simulated moving bed chromatography. According to the method, a polystyrene / divinylbenzene type reverse-phase resin is taken as a stationary phase and pure ethanol is taken as an eluent; mixed poly-unsaturated fatty acid esters are continuously separated by virtue of 4-area simulated moving bed chromatography; the ethanol solution of the DHA ester is continuously collected from an extract outlet, while the ethanol solution of the EPA ester is continuously collected from a raffinate outlet; finally, the monomers of the EPA ester and the DHA ester are obtained by virtue of vacuum concentration. The method has the characteristics of high yield, low solvent consumption and no toxic solvent.
Owner:ZHEJIANG UNIV
Taurolidine quality checking method
InactiveCN101285813AAvoid product qualityGuarantee product qualityWeighing by removing componentComponent separationGas phaseTaurolidine
The invention provides a quality detection method for Taurolidine. The method uses thin-layer chromatography, gas phase chromatography and titering process to detect the quality of the Taurolidiene. The quality detection method for Taurolidine has the advantages of rapidness, accuracy, convenient operation and excellent reproduction quality, and ensures the product quality of the Taurolidine.
Owner:CHANGCHUN MAILING BIOLOGICAL ENG CO LTD
Preparation of shaolin plaster for rheumatism and wound and quality control method
The invention relates to a preparation and quality control method of a Shaolin rheumatism trauma paste. The original production process of the Shaolin rheumatism trauma paste has the problems that (1) power consumption of plastication is great; (2) temperature rises quickly in the plastication, which results in easy aging and loss of cohesive force; (3) the menthol, menthyl salicylate and borneol in the prescription are once tried for soaking rubber to carry out the plastication, which leads to excessive loss of the components in finished products; when petrolatum is used for replacing the components to soak the rubber for carrying out the plastication, serious problem of oil permeation occurs in the finished products; (4) the relative density of the extract of medical material specified by the standards can not be controlled. The invention aims at providing the preparation and quality control method of the Shaolin rheumatism trauma paste, proposing a reasonable prescription of base materials and a production technique to ensure that finished products have clean and smooth appearance and good cohesive force and reducing the loss of active components of the finished products, particularly the volatile components such as menthol, menthyl salicylate and borneol. The quality standards essentially comprises thin layer identification of angelica, forsythia fruit, rhubarb root, root of common peony and resina draconis, limit tests on adhesion ability and aconitine and determination of the content of menthol, menthyl salicylate and borneol of the products by gas chromatography.
Owner:张会林
Glossy ganoderma spore oil and its supercritical prepn process
The present invention discloses one kind of glossy ganoderma spore oil, and belongs to the field of Chinese medicine technology. The glossy ganoderma spore oil is measured to contain total triterpene in 25-35 % and unsaturated fatty acids in 60-80 %, and has density of 0.85-1.0, refractive index at 25 deg.c of 1.45-1.50, and acid value of 15-25. The present invention also discloses the supercritical preparation process of the glossy ganoderma spore oil, and the preparation process has high extracting rate, and is favorable to the stability of the glossy ganoderma spore oil and suitable for industrial production.
Owner:吴逸芳
Method for determining alkaloids in tea leaves by using GC-MS (Gas Chromatography-Mass Spectrometer) method
InactiveCN105467055AOptimize detection conditionsShort detection timeComponent separationNornicotineRelative standard deviation
The invention belongs to the technical field of physiochemical detection of tea leaves and in particular relates to a method for determining alkaloids in tea leaves by using a GC-MS (Gas Chromatography-Mass Spectrometer) method. The method comprises the following step of determining nicotine and minor alkaloids (including nicotine, nornicotine, myosmine, neonicotine, neonicotine and cotinine) in tea leaf powder. The method is a method for extracting nicotine substances from the tea leaves by using a centrifugal tube filled with 0.01% triethylamine / tert-butyl methyl ether solution and analyzing the nicotine substances in the tea leaves by using a gas chromatography-mass spectrometer (GC-MS) method. When the method provided by the invention is used for detecting the content of the nicotine in the tea leaves, the method is rapid and effective, the pre-treatment is simple, an average relative standard deviation is smaller than 10 percent, and the average recycling rate of each index is 86.2 percent to 92.1 percent. The method has the advantages of rapidness and accuracy, high sensitivity and good repeatability.
Owner:CHINA NAT TOBACCO QUALITY SUPERVISION & TEST CENT
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
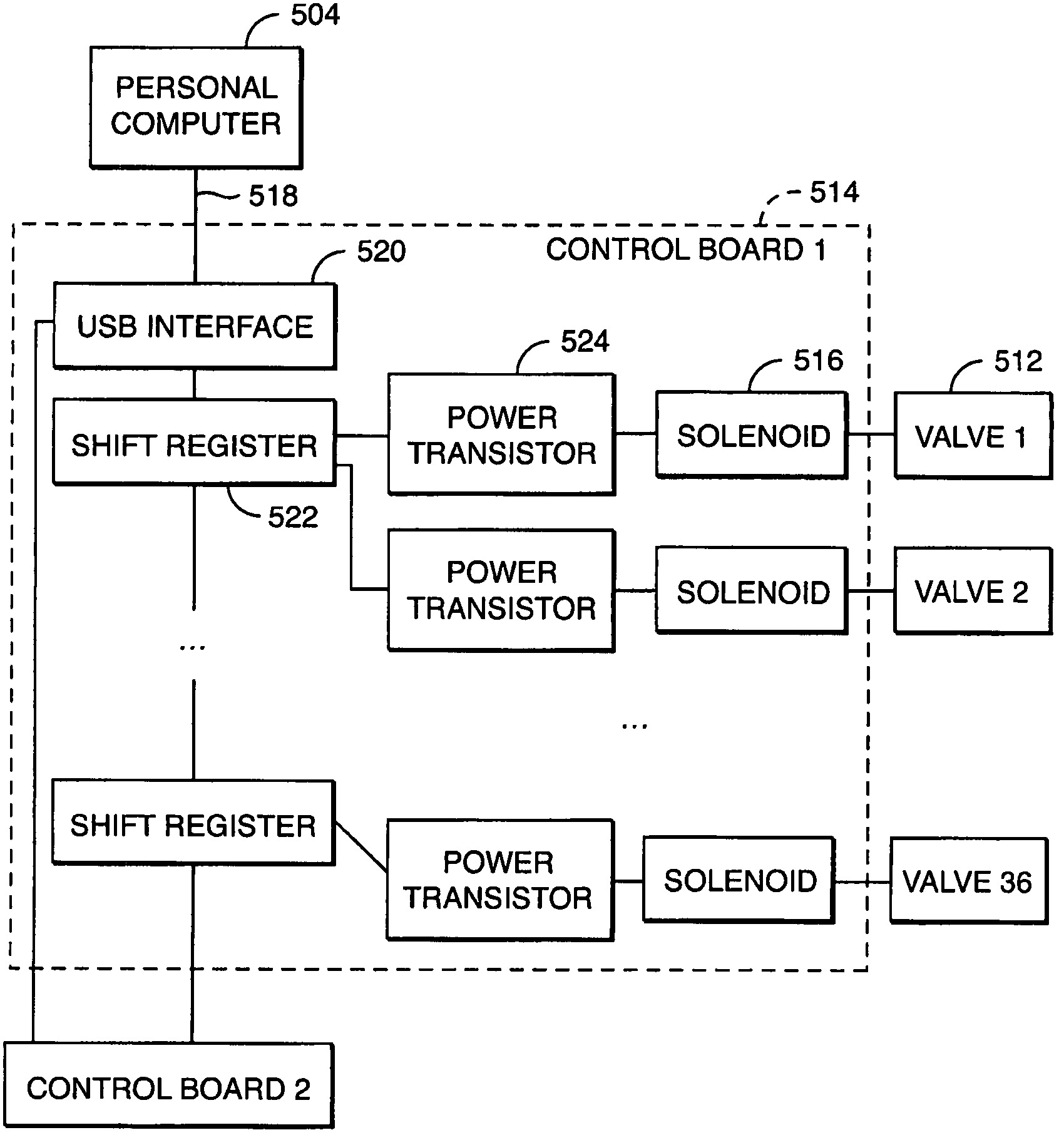
Valve module and methods for simulated moving bed chromatography
ActiveUS8807164B2Simple and easily programmable controlPromote repairOperating means/releasing devices for valvesComponent separationSimulated moving bedMoving bed
The present invention provides devices and methods for micro-scale simulated moving bed chromatography (SMB) for continuous preparation of analytic quantities of highly pure fractions of target molecules. The present apparatus and method of the invention is adapted in a preferred embodiment to separations by affinity chromatography involving three discontinuous liquid flow loops. An alternative embodiment of affinity chromatography utilizes standard SMB operating under isocratic conditions.
Owner:TOSOH BIOSCIENCE LLC
Medicine for treating infantile anorexia, its preparing method and quality control method
A Chinese medicine for treating anorexia of baby is prepared from 12 Chinese-medicinal materials including tuckahoe, tangerine peel, haw, rhubarb, etc through decocting, filtering, concentrating, mixing, etc. Its quality control method including thin-layer chromatography and high-effect liquid-phase chromatography is also disclosed.
Owner:GUANGDONG ZHONGSHENG PHARMA
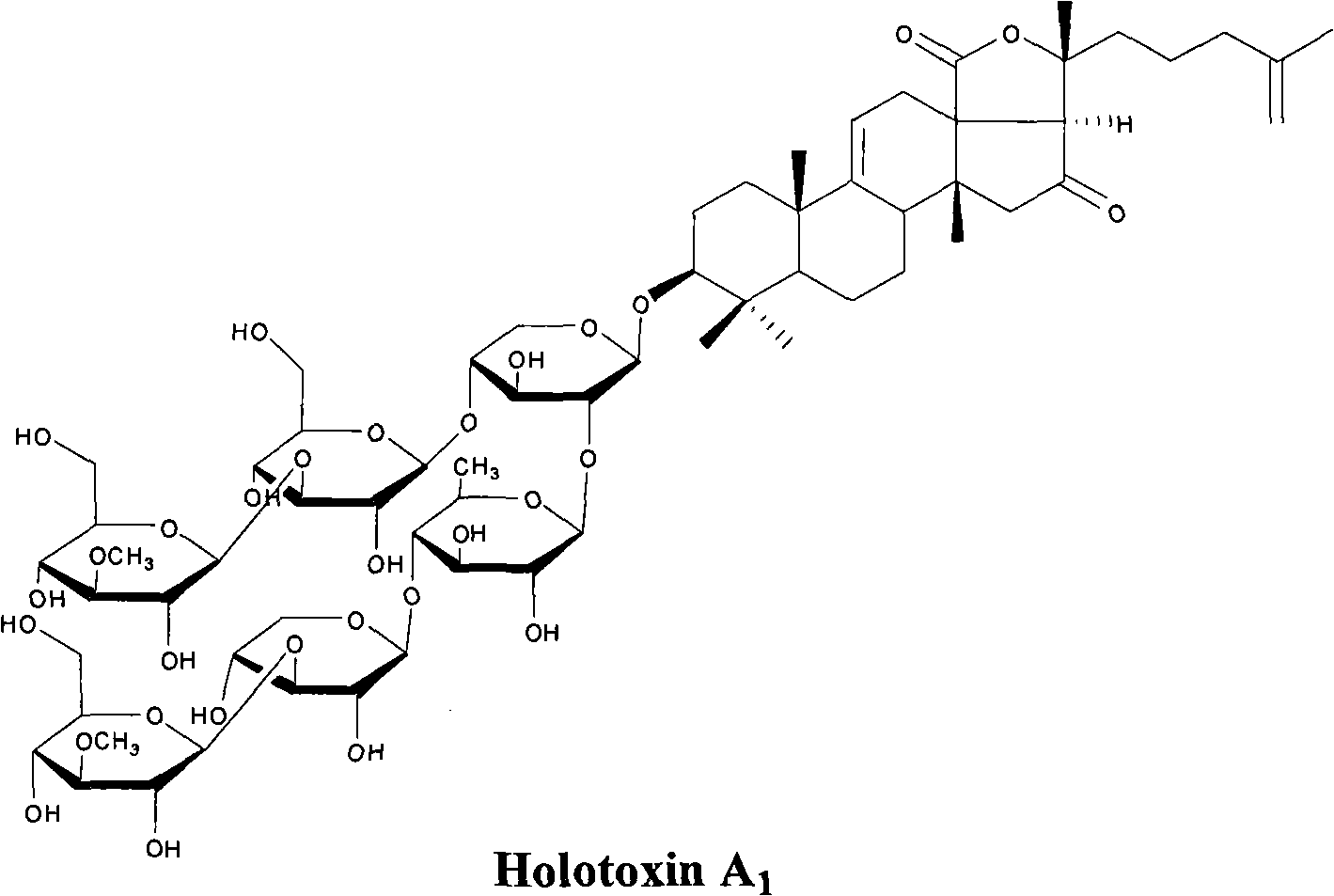
Technology method for preparing sea cucumber saponin Holotoxin A1 comparison product by utilizing fresh sea cucumber processing waste liquid
The invention relates to a technology method for preparing a sea cucumber saponin Holotoxin A1 comparison product by utilizing fresh sea cucumber processing waste liquid. The technology method is characterized by comprising the following steps of: (1) concentrating the fresh sea cucumber processing waste liquid at reduced pressure and carrying out alcohol precipitation, and drawing supernate; (2) leading the supernate to flow through a macropore adsorbent resin column, washing and collecting eluent after absorption until reaching saturation, and concentrating the eluent at reduced pressure to obtain extract; (3) mixing the extract with silica gel powder, and carrying out silicagel column chromatography by using a solution system consisting of methyl alcohol, methylene, ethyl acetate and water; using a system in which the ratio of methyl alcohol to methylene to ethyl acetate to water is 2:2:4:1 as an expanding system, taking a lower layer to carry out thin-layer chromatography (TLC), collecting components with silicagel column chromatography mobility of 0.2-0.3, concentrating the components at reduced pressure, and carrying out vacuum drying on the concentrated components to obtain white powder; and (4) dissolving the white powder with acetonitrile aqueous solution, carrying out ultraviolet absorption by using high performance liquid chromatography (HPLC) at the wavelength of 203nm, carrying out purification by utilizing acetonitrile aqueous solution as a flow phase, collecting main peaks, and carrying out the vacuum drying to obtain the sea cucumber saponin Holotoxin A1 comparison product. The technology method is mainly used for preparing sea cucumber saponin Holotoxin A1.
Owner:BIOLOGY INST OF SHANDONG ACAD OF SCI
Preparation method for oxytocin deamidation impurity
ActiveCN106749539APrevent elutionSuitable for continuous productionOxytocins/vasopressinsPeptide preparation methodsDesalinationReversed-Phase Liquid Chromatography
The invention discloses a preparation method for an oxytocin deamidation impurity. The preparation method comprises the following step of: successively subjecting a solution of a crude oxytocin deamidation impurity precursor to reversed-phase cyclization, reversed-phase purification and reversed-phase desalination by adopting high-performance liquid-phase reversed-phase chromatography, wherein a filling material for the high-performance liquid-phase reversed-phase chromatography is silica gel C18; and the crude oxytocin deamidation impurity precursor contains two free sulfhydryl groups. The preparation method provided by the invention innovatively adopts a reversed-phase adsorption method for cyclization, purification and desalination, solves the problems of cyclization, purification and desalination once for all, optimizes the production process, and is applicable to continuous industrial production.
Owner:SPH NO 1 BIOCHEM & PHARMA CO LTD
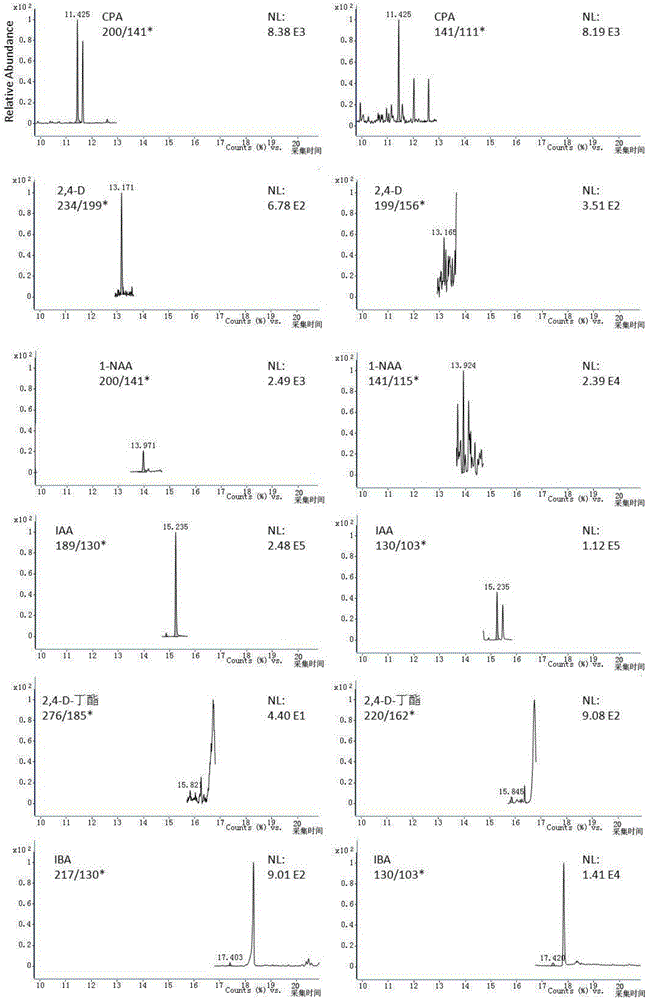
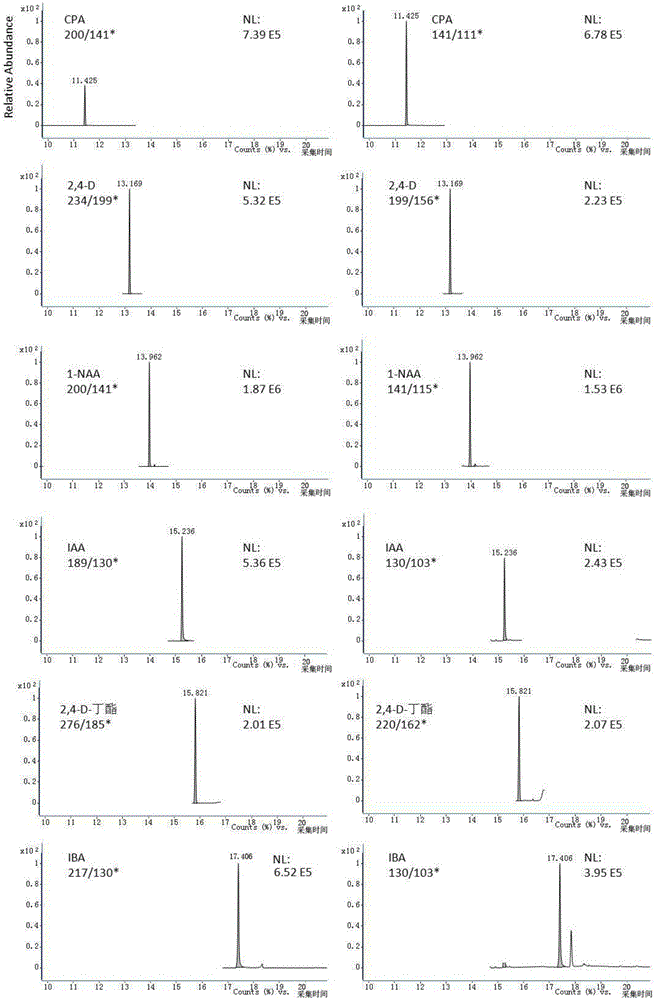
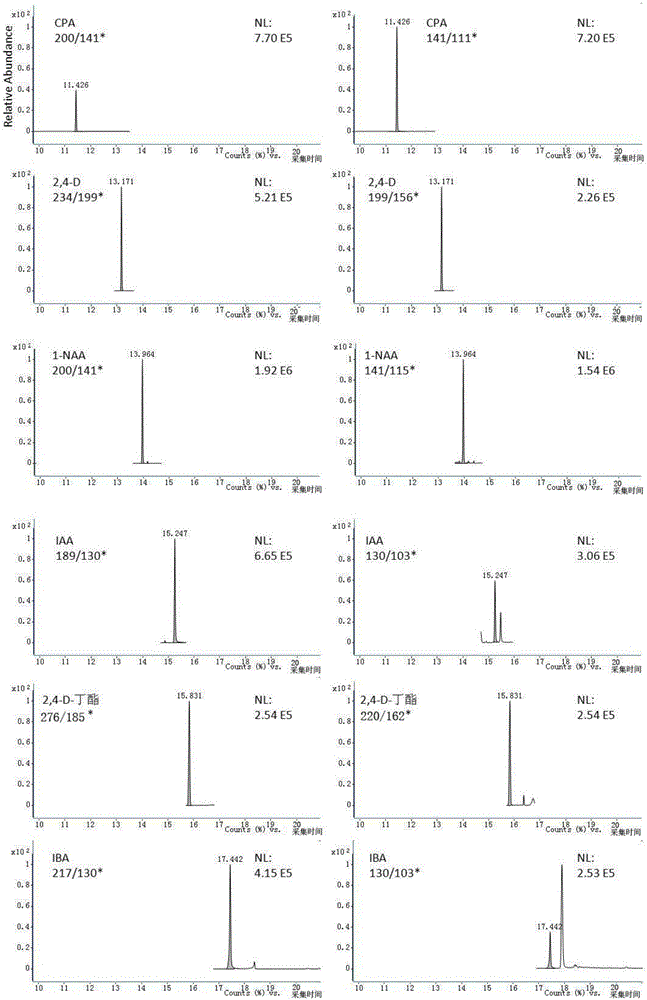
Method for simultaneous determination of six residual plant-growth regulators in bean sprout by using gas chromatography-tandem mass spectrometry
ActiveCN106404972ASimple methodDetermination is accurate and reliableComponent separationGas phasePlant growth
The invention relates to a determination method for six residual plant-growth regulators in a bean sprout, especially to a method for simultaneous determination of six residual plant-growth regulators in the bean sprout by using gas chromatography-tandem mass spectrometry. Six plant-growth regulators involved in the invention are composed of 4-chlorophenoxyacetic acid, 2,4-dichlorophenoxyacetic acid, naphthylacetic acid, indoleacetic acid, 2,4-D-butyl ester and indolebutyric acid. The method comprises the following steps: 1) sample treatment; 2) determination, wherein gas chromatography-tandem mass spectrometry is employed; 3) drafting of a standard curve; 4) blank test; and 5) result calculation and description. The determination method employs gas chromatography-tandem mass spectrometry for simultaneous determination of the six residual plant-growth regulators in bean sprout; and the method is simple, convenient, rapid, accurate and reliable, and the lower determination limit, recovery rate and precision of the method all accord with requirements.
Owner:ZHEJIANG ACAD OF SCI & TECH FOR INSPECTION & QUARANTINE
Method for detecting atomization homogeneity of electronic cigarette atomizer
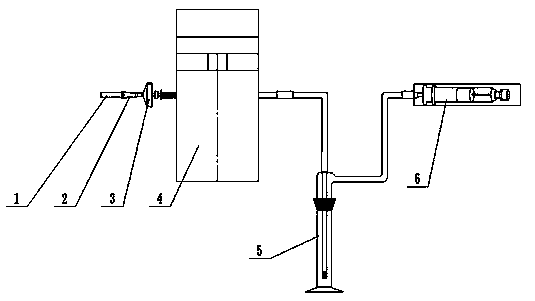
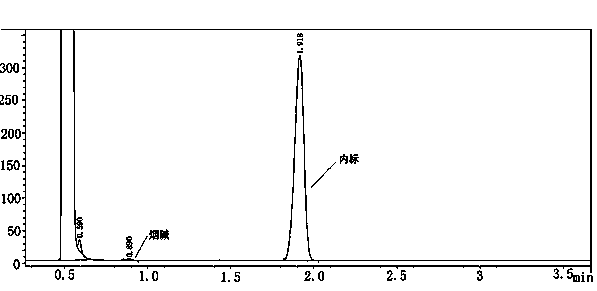
ActiveCN103940602AThe inspection method is accurate and reliableHuman factors are smallMachine part testingComponent separationNebulizerInternal standard
The invention relates to a method for detecting atomization homogeneity of an electronic cigarette atomizer. A linear smoking machine is adopted to smoke electronic cigarettes of the same specification and the same type in an ISO smoking mode, 44 mm Cambridge filters are used for trapping total particulate matter of smoke release matter after an electronic cigarette is smoked by 50 times continuously, an absorption bottle is connected between a catcher and a smoking needle cylinder, and 20 mL isopropyl alcohol extraction agents with 0.2-0.5 mg / mL n-heptadecane as internal standard substance is accurately added in the absorption bottle. After smoking, the absorption liquid in the absorption bottle is used for extracting nicotine in the Cambridge filters, and the gas chromatographic method is adopted to measure the nicotine content in the extraction agents according to the GB / T 23355-2009. The nicotine content in the smoke release matter of all electronic cigarette samples is calculated to obtain the SD, and the value of the SD responds the atomization homogeneity of the electronic cigarette atomizer in the batch. The method can quantitatively research the atomization homogeneity of the electronic cigarette atomizer, and is a practical and scientific method for estimation on the atomization homogeneity of the electronic cigarette atomizer.
Owner:CHINA TOBACCO YUNNAN IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com