Patents
Literature
14904 results about "Spectrometer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
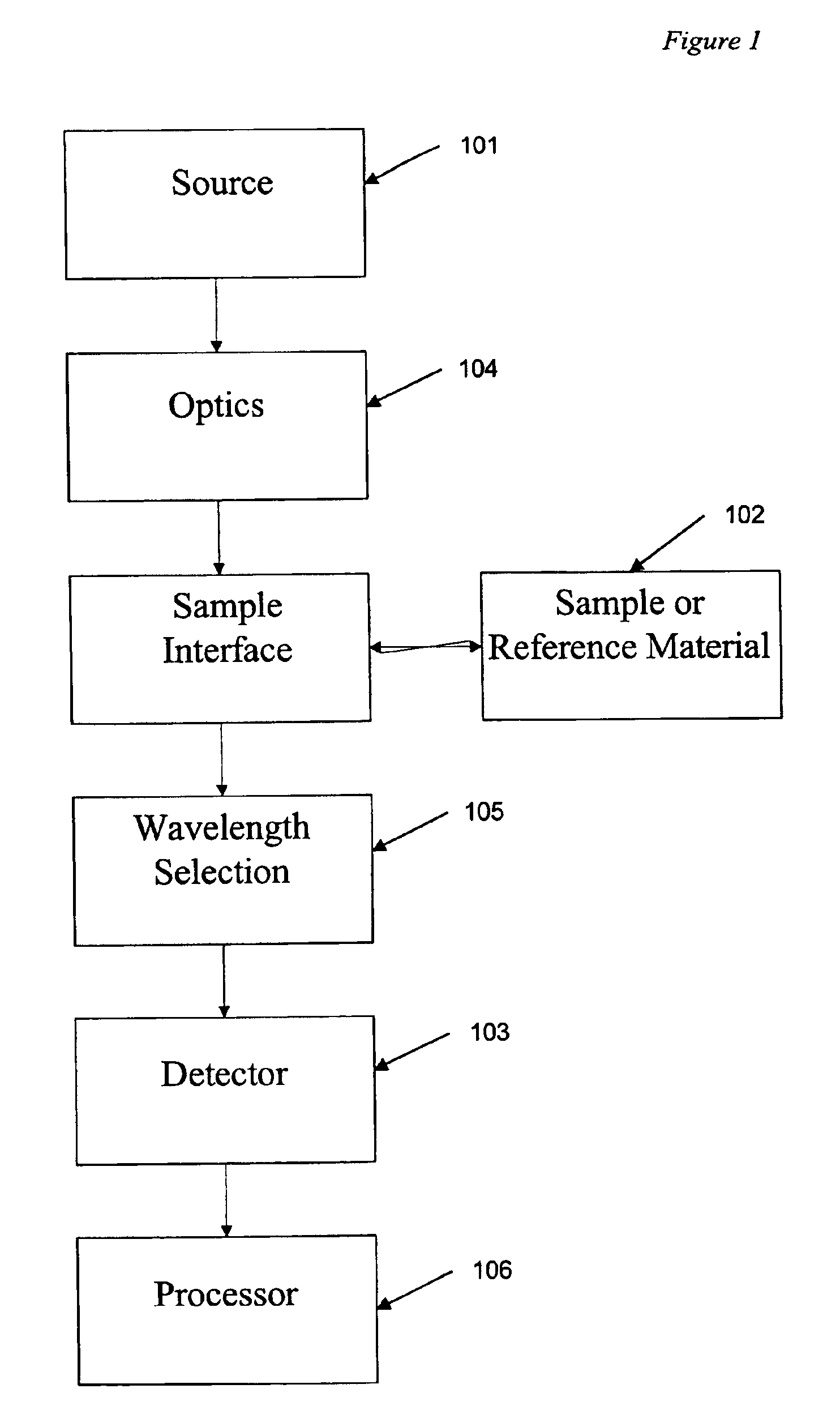
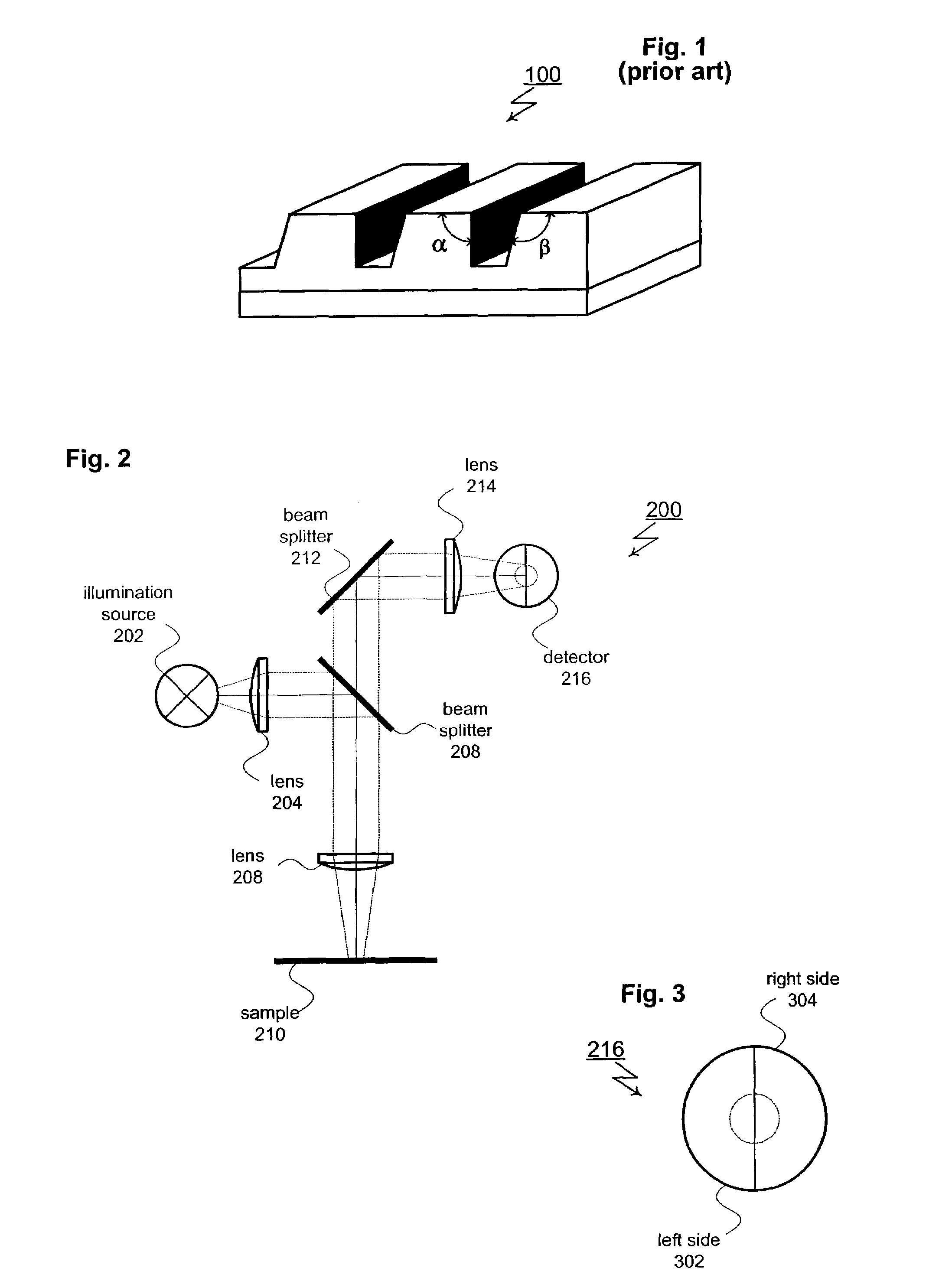
A spectrometer (/spɛkˈtrɒmɪtər/) is a scientific instrument used to separate and measure spectral components of a physical phenomenon. Spectrometer is a broad term often used to describe instruments that measure a continuous variable of a phenomenon where the spectral components are somehow mixed. In visible light a spectrometer can separate white light and measure individual narrow bands of color, called a spectrum. A mass spectrometer measures the spectrum of the masses of the atoms or molecules present in a gas. The first spectrometers were used to split light into an array of separate colors. Spectrometers were developed in early studies of physics, astronomy, and chemistry. The capability of spectroscopy to determine chemical composition drove its advancement and continues to be one of its primary uses. Spectrometers are used in astronomy to analyze the chemical composition of stars and planets, and spectrometers gather data on the origin of the universe.
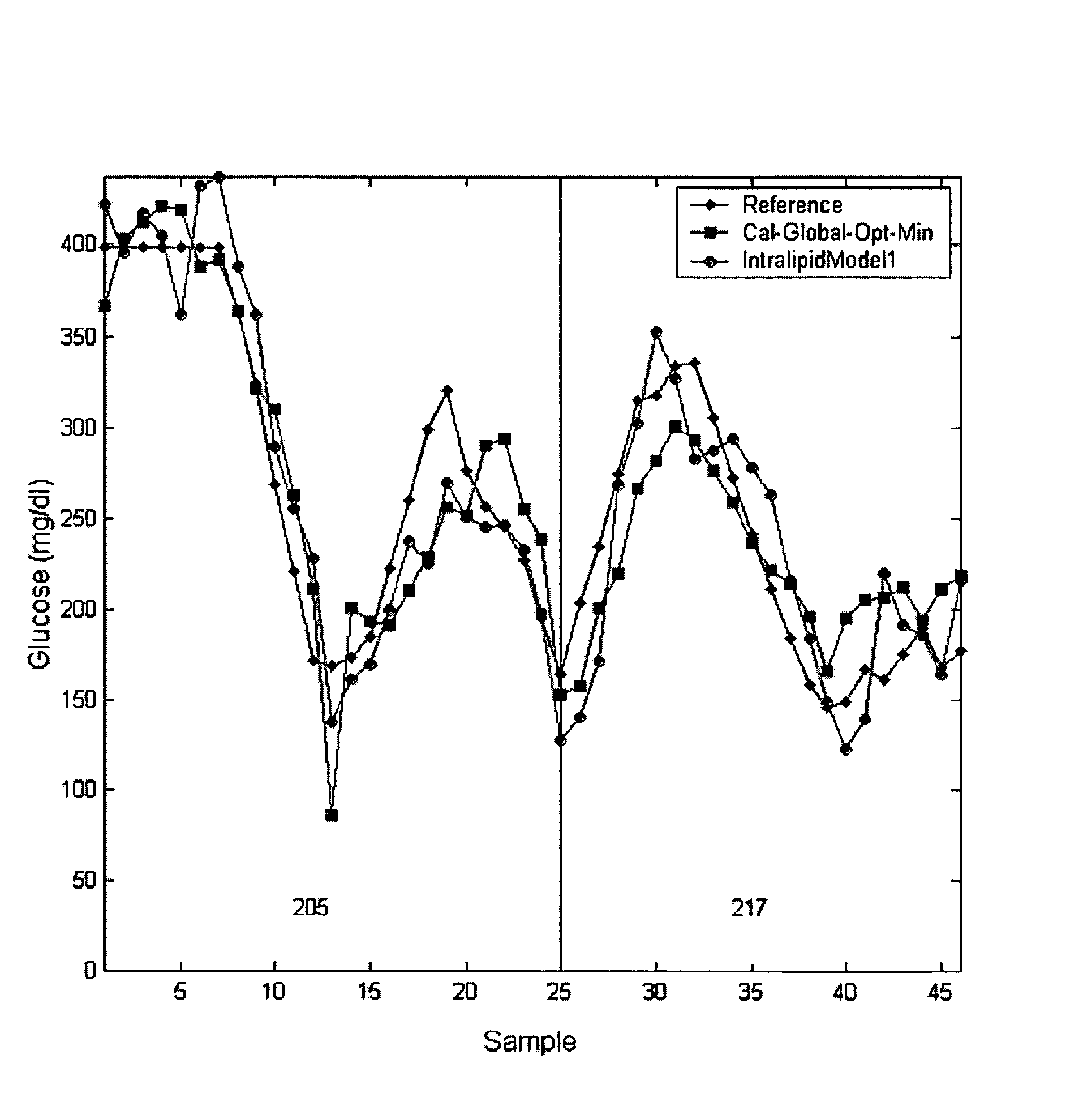
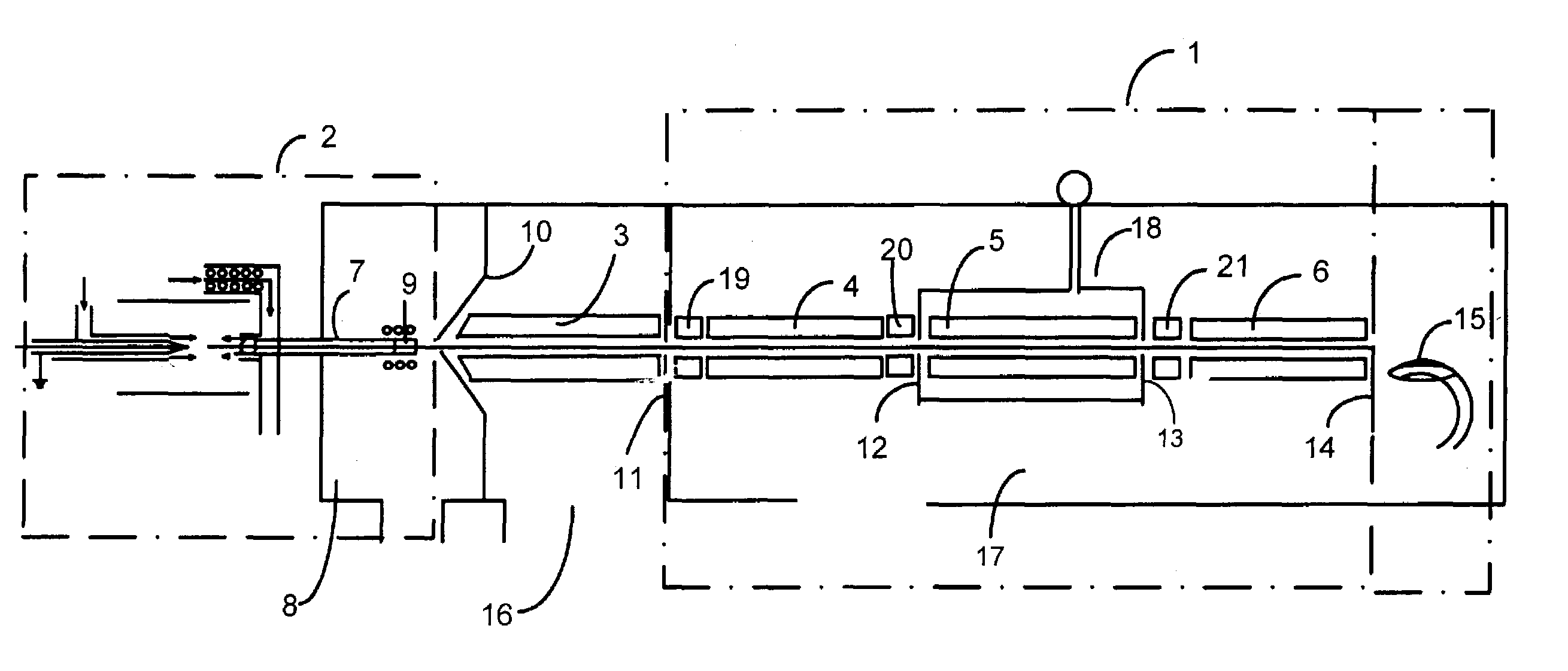
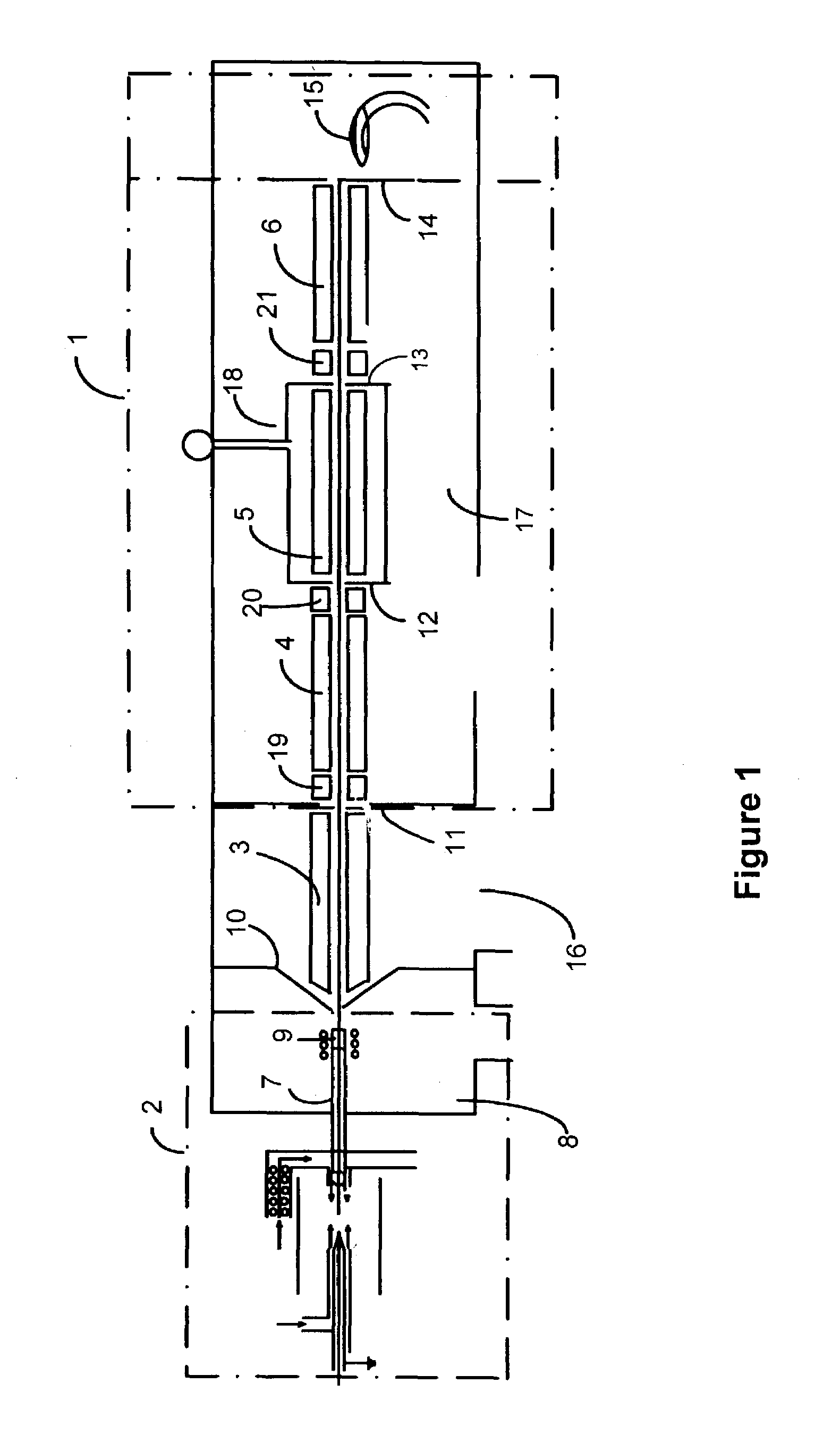
Method of adapting in-vitro models to aid in noninvasive glucose determination
InactiveUS7317938B2Easy to controlStrict controlDiagnostic recording/measuringSensorsConcentrations glucoseNon invasive
Owner:GLT ACQUISITION
Barrier materials for display devices
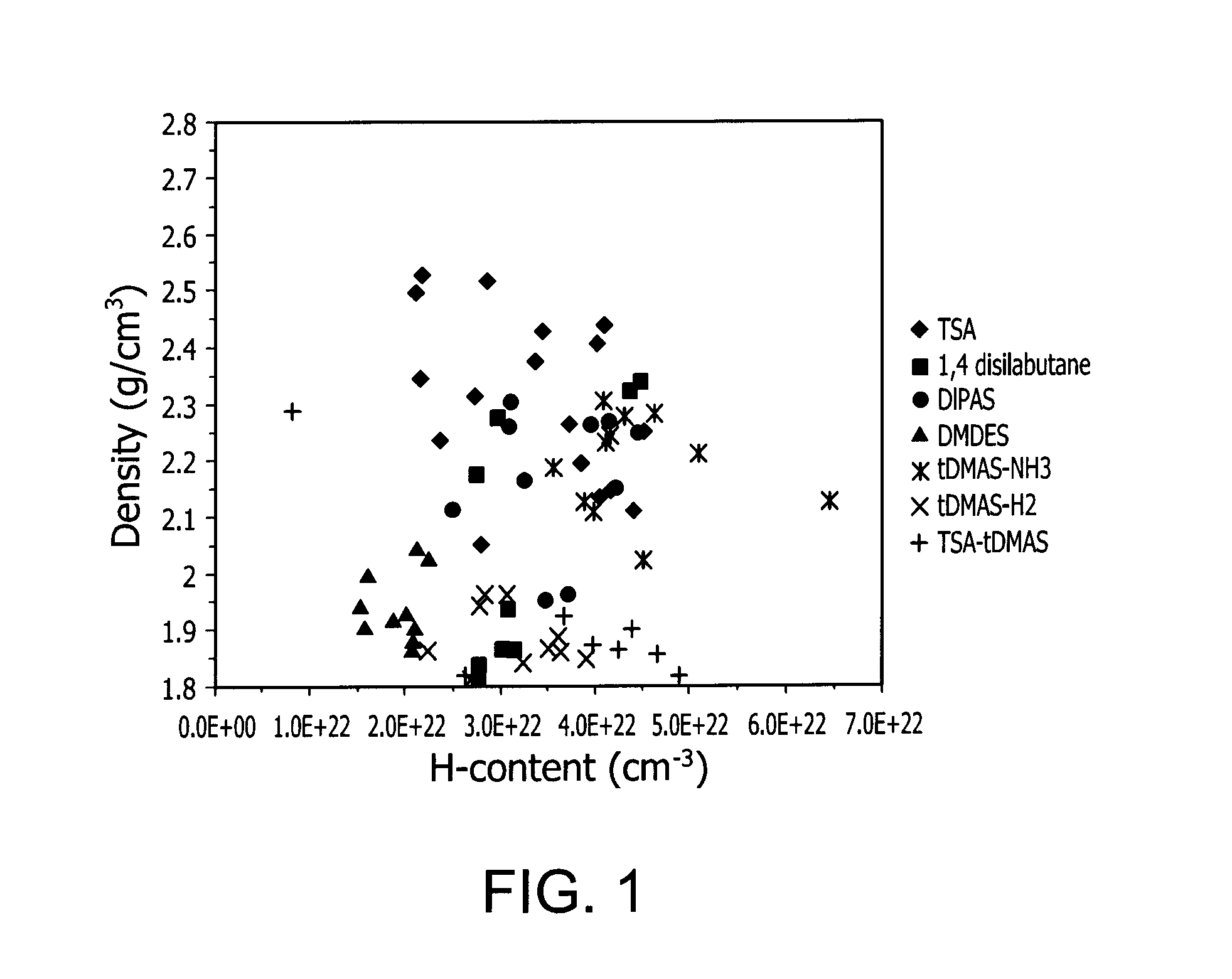
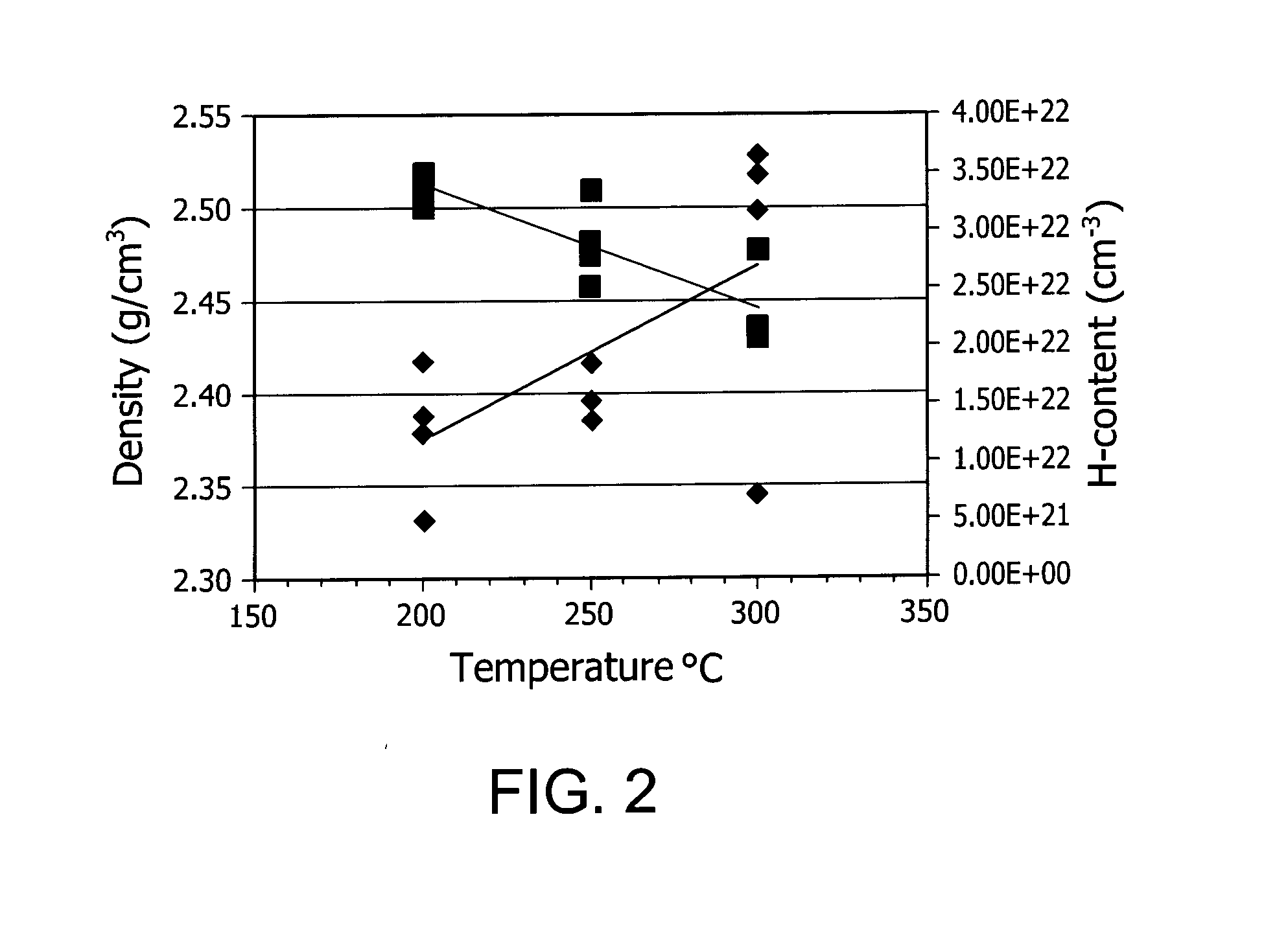
ActiveUS20150021599A1TransistorSemiconductor/solid-state device manufacturingHydrogen contentDisplay device
Described herein are apparatus comprising one or more silicon-containing layers and a metal oxide layer. Also described herein are methods for forming one or more silicon-containing layers to be used, for example, as passivation layers in a display device. In one particular aspect, the apparatus comprises a transparent metal oxide layer, a silicon oxide layer and a silicon nitride layer. In this or other aspects, the apparatus is deposited at a temperature of 350° C. or below. The silicon-containing layers described herein comprise one or more of the following properties: a density of about 1.9 g / cm3 or greater; a hydrogen content of about 4×1022 cm−3 or less, and a transparency of about 90% or greater at 400-700 nm as measured by a UV-visible light spectrometer.
Owner:VERSUM MATERIALS US LLC
Method and apparatus for chemical monitoring
The present invention relates to monitoring chemicals in a process chamber using a spectrometer having a plasma generator, based on patterns over time of chemical consumption. The relevant patterns may include a change in consumption, reaching a consumption plateau, absence of consumption, or presence of consumption. In some embodiments, advancing to a next step in forming structures on the workpiece depends on the pattern of consumption meeting a process criteria. In other embodiments, a processing time standard is established, based on analysis of the relevant patterns. Yet other embodiments relate to controlling work on a workpiece, based on analysis of the relevant patterns. The invention may be either a process or a device including logic and resources to carry out a process.
Owner:LIGHTWIND CORP
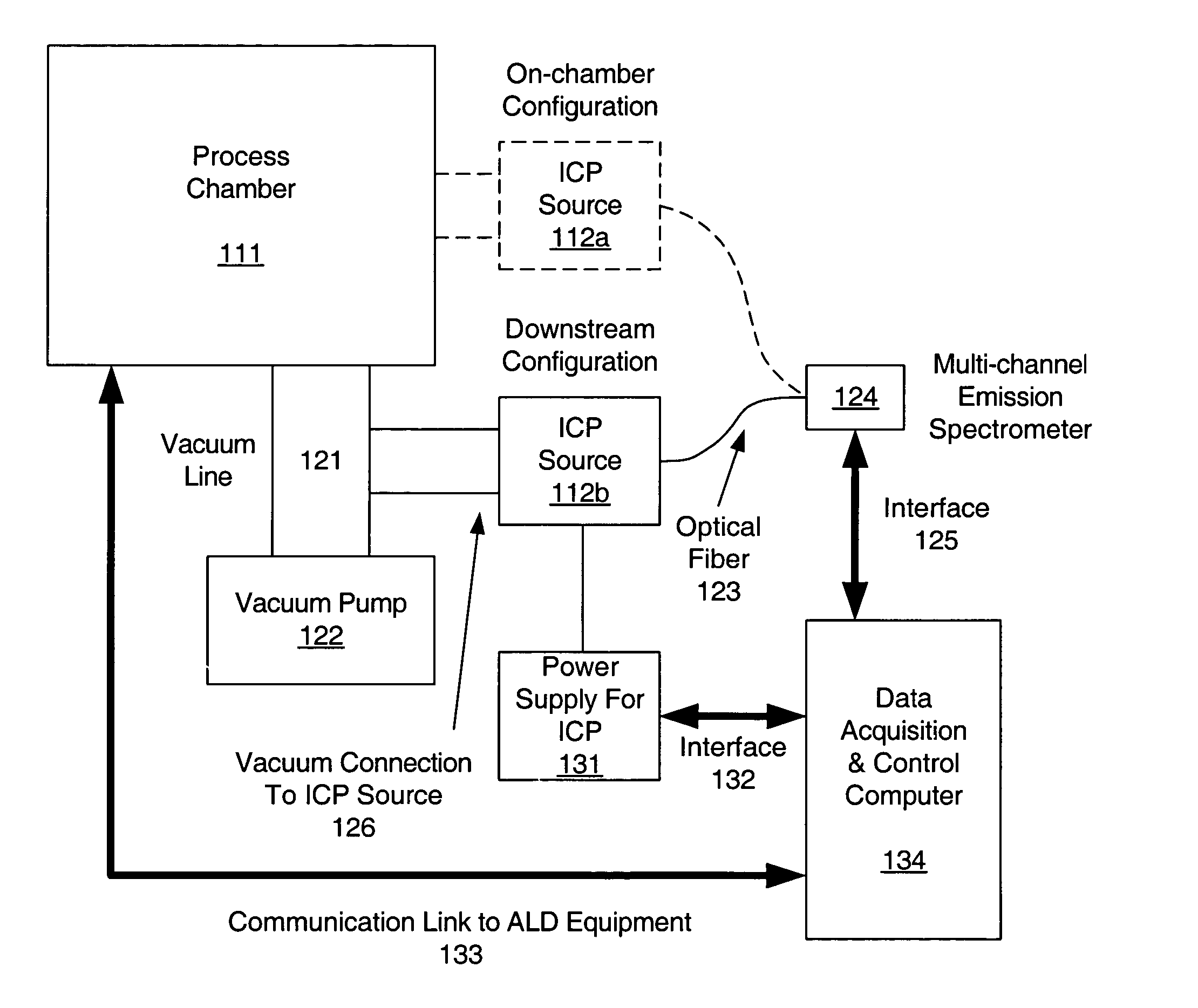
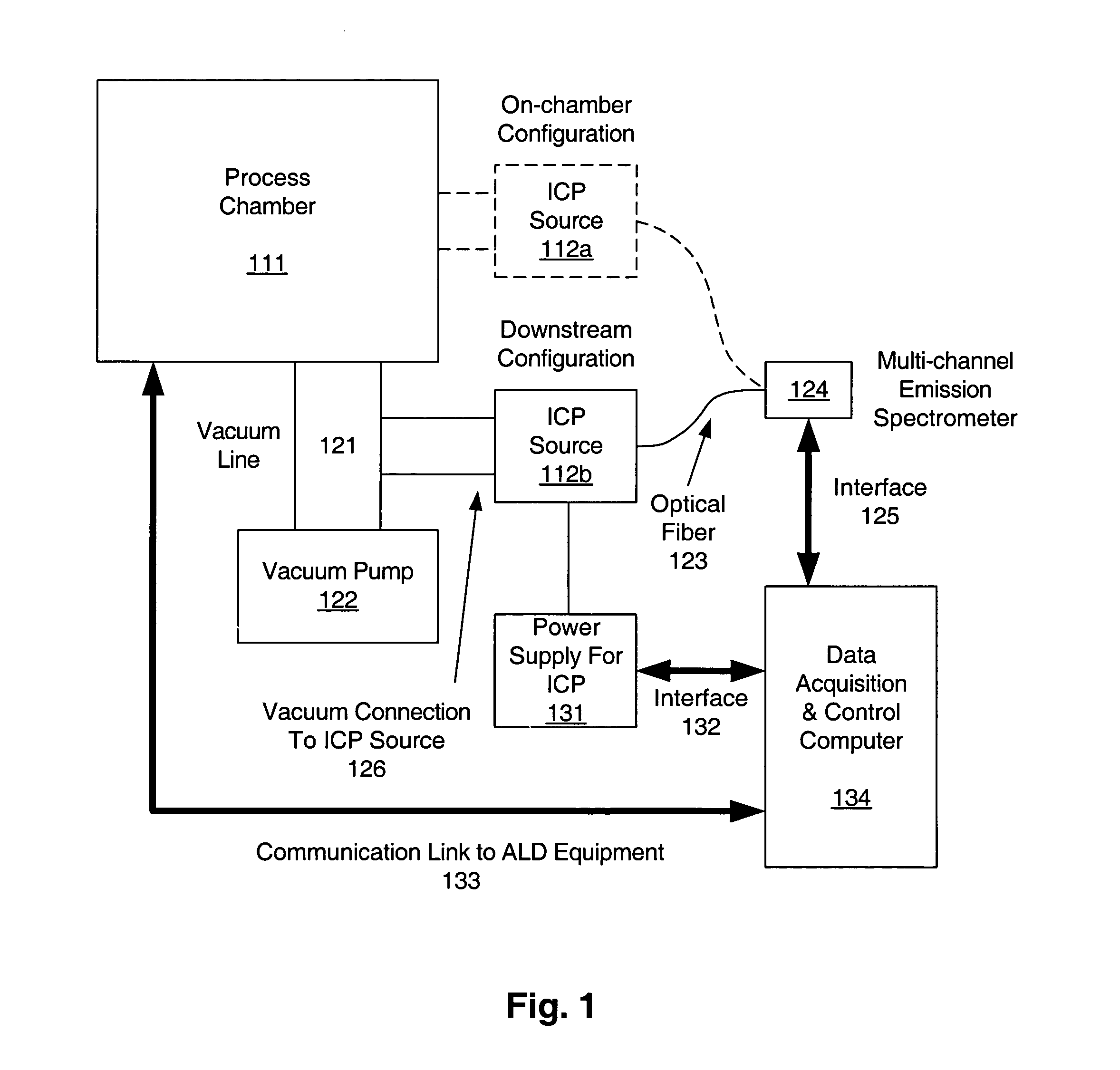
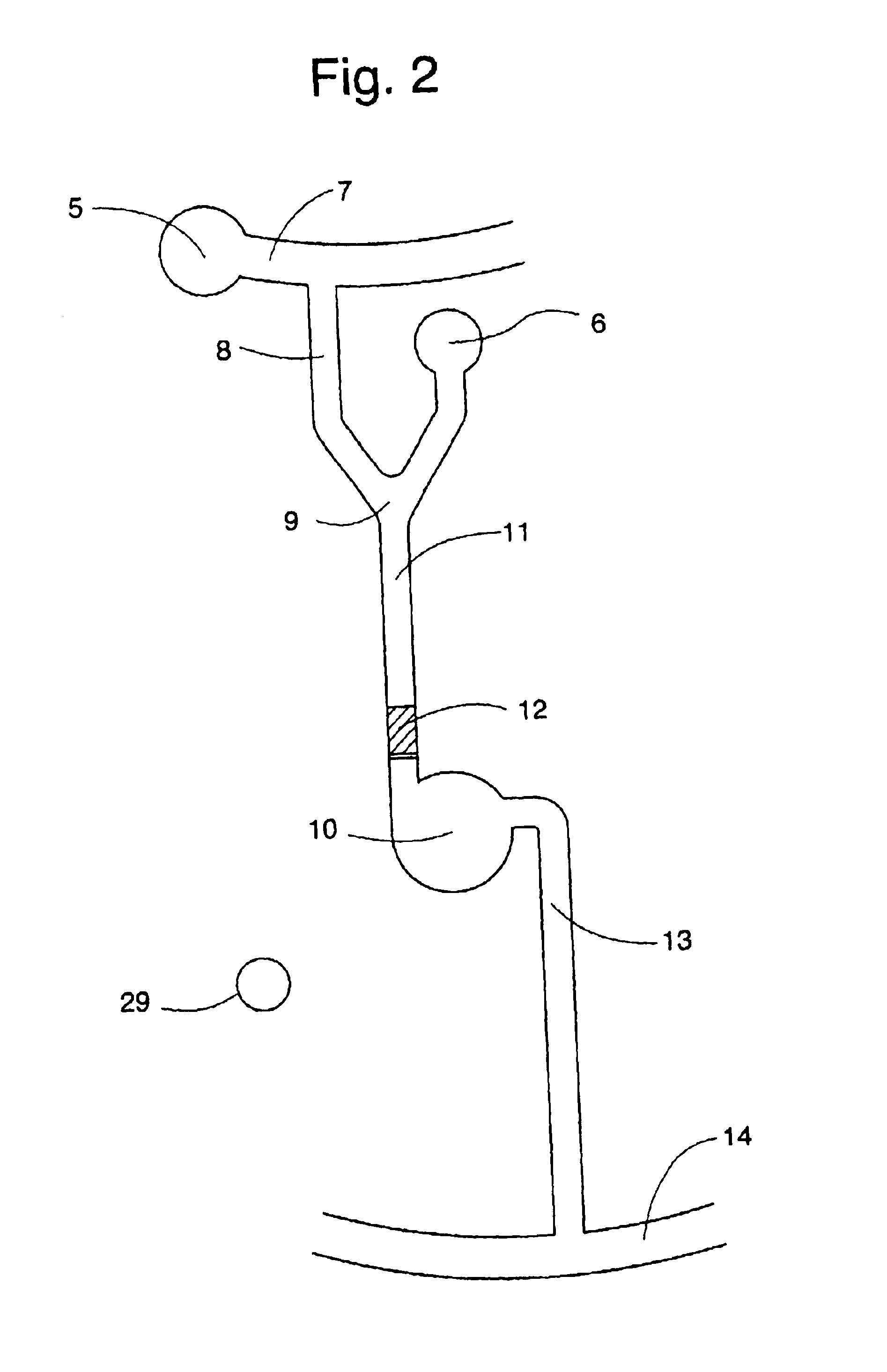
Inductively coupled plasma spectrometer for process diagnostics and control
InactiveUS6867859B1High sensitivitySimple reactor designEmission spectroscopyRadiation pyrometryOptical radiationInductively coupled plasma
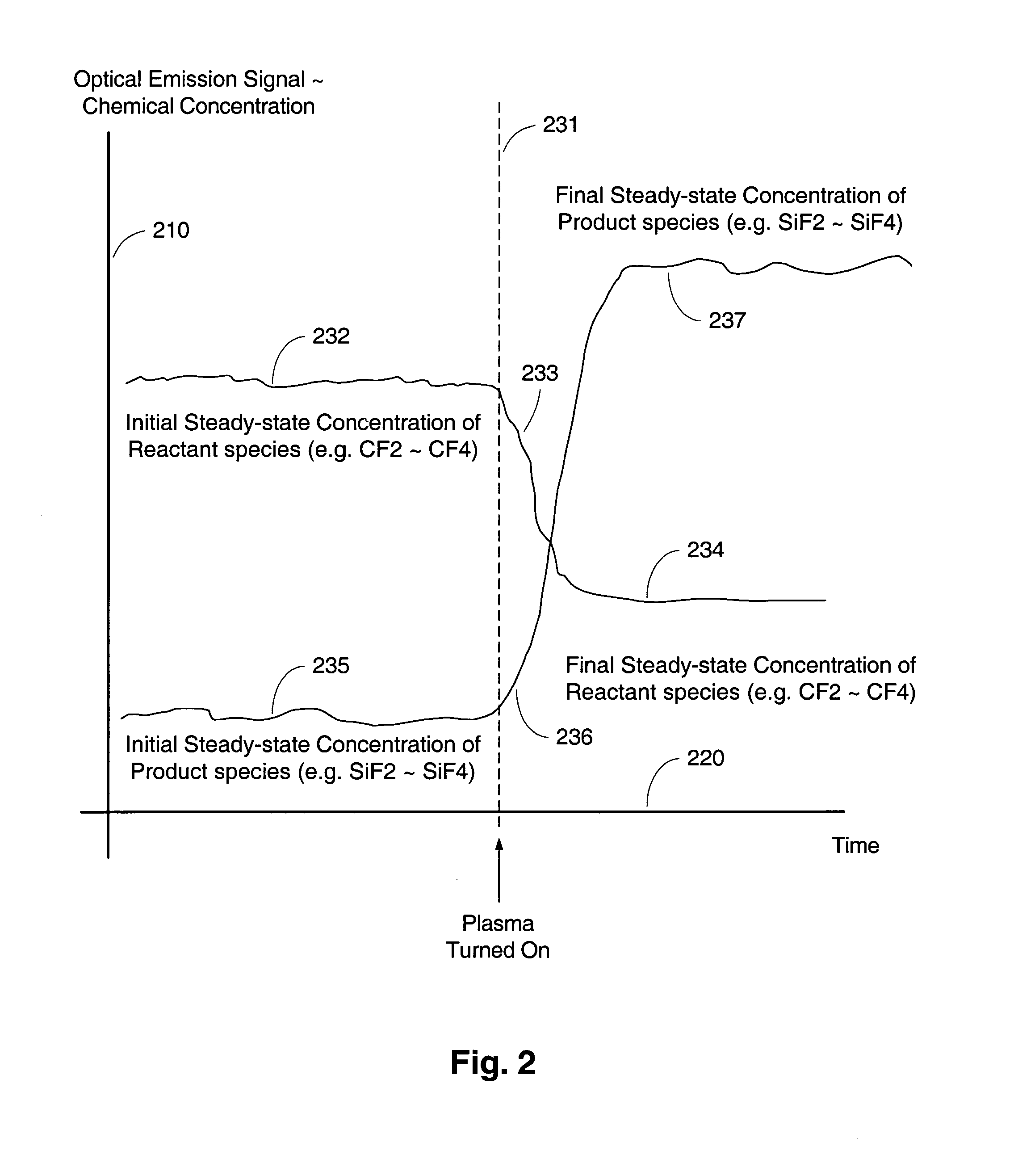
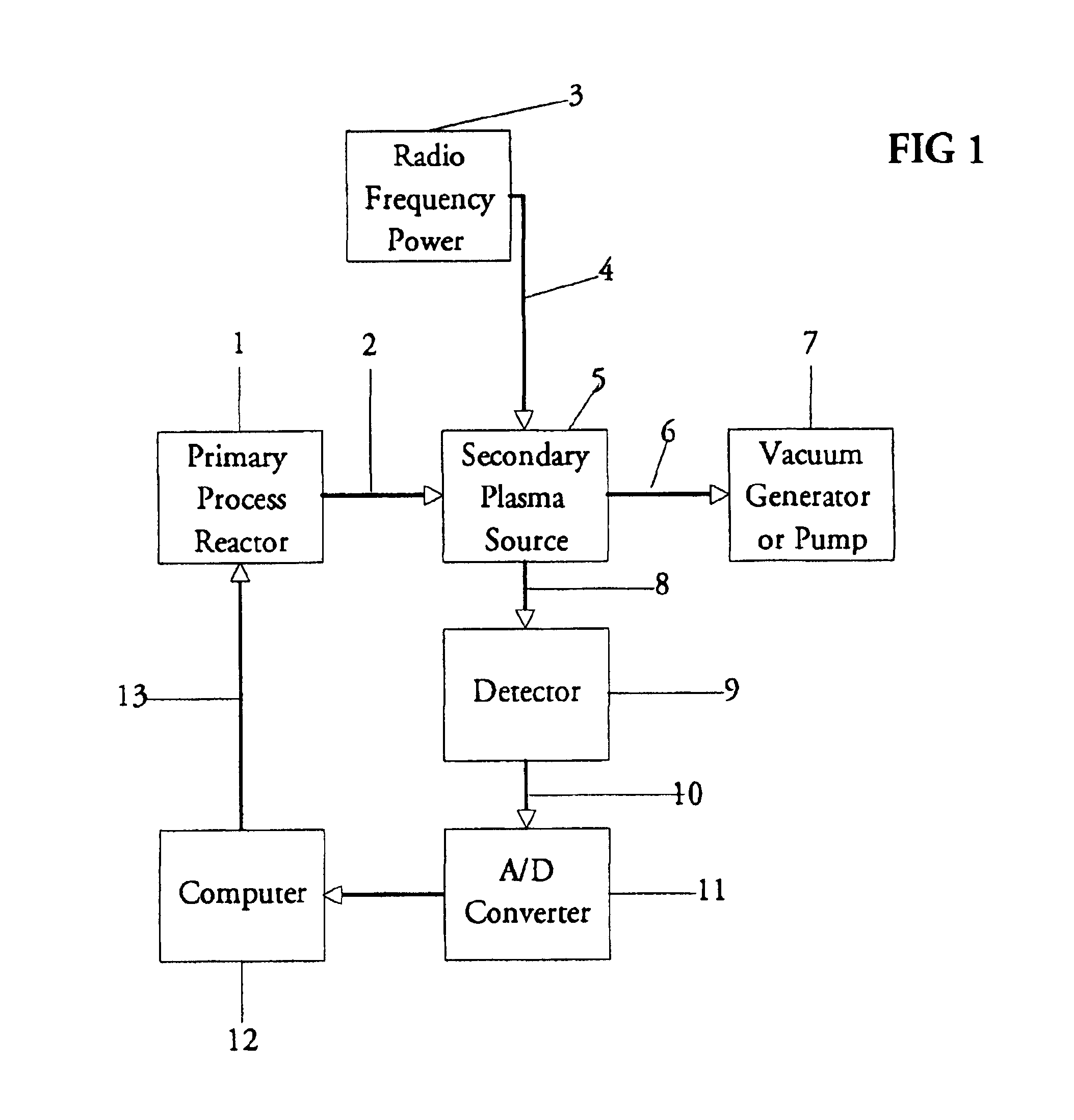
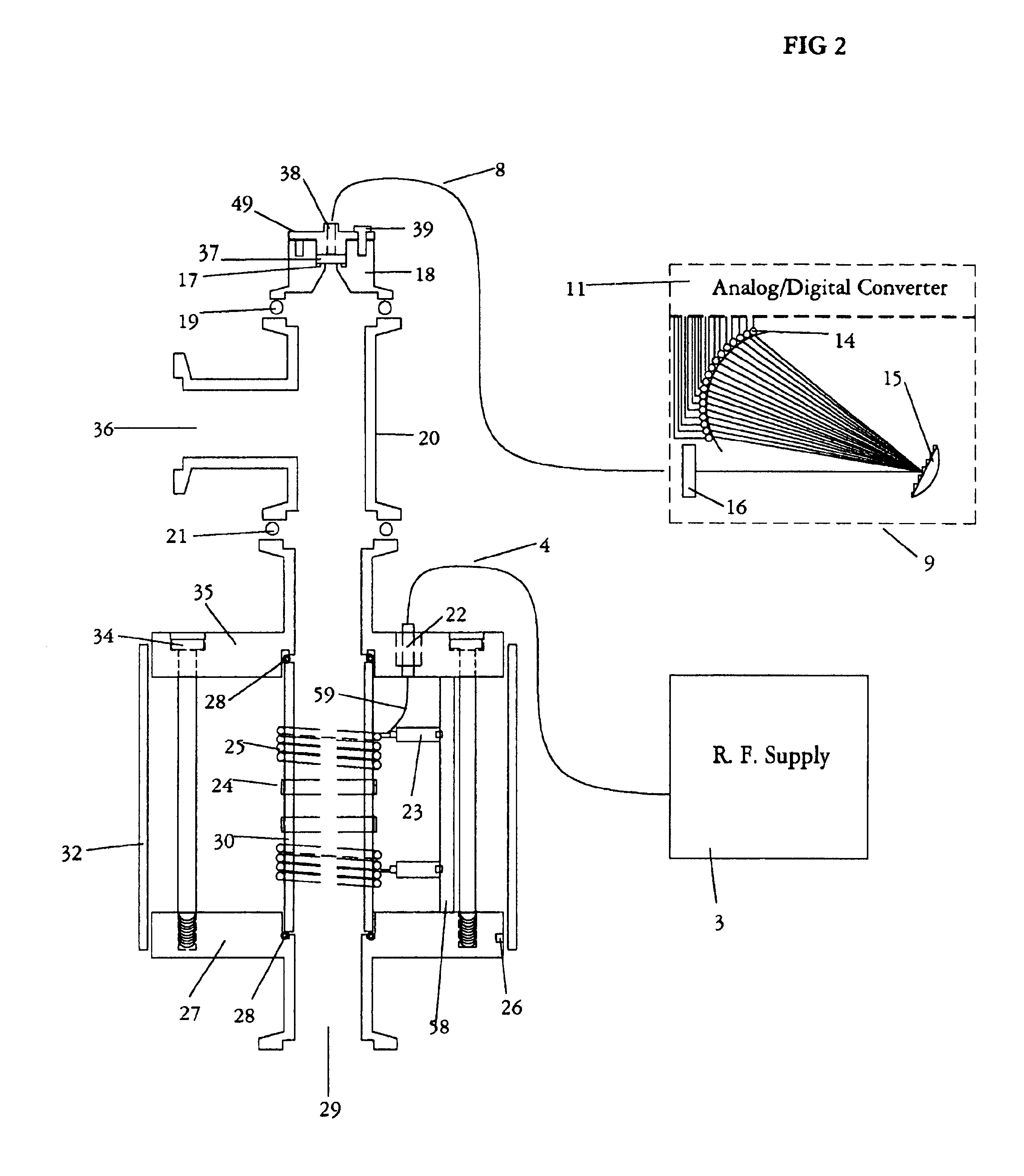
The present invention relates to an apparatus and method for forming a plasma in the exhaust line of a primary process reactor. The plasma is generated in an inductive source (5) to examine the chemical concentrations of the waste or exhaust gas in vacuum lines that are below atmospheric pressure. The optical radiation emitted by the plasma is analyzed by an optical spectrometer (9) and the resulting information is used to diagnose, monitor, or control operating states in the main vacuum vessel.
Owner:LIGHTWIND CORP
Compact spectrometer device
InactiveUS6057925ARugged in constructionEasy to manufactureRadiation pyrometrySpectrum investigationDetector arrayLight beam
A color measuring sensor assembly includes an optical filter such as a linear variable filter, and an optical detector array positioned directly opposite from the optical filter a predetermined distance. A plurality of lenses, such as gradient index rods or microlens arrays, are disposed between the optical filter and the detector array such that light beams propagating through the lenses from the optical filter to the detector array project an upright, noninverted image of the optical filter onto a photosensitive surface of the detector array. The color measuring sensor assembly can be incorporated with other standard components into a spectrometer device such as a portable calorimeter having a compact and rugged construction suitable for use in the field.
Owner:VIAVI SOLUTIONS INC
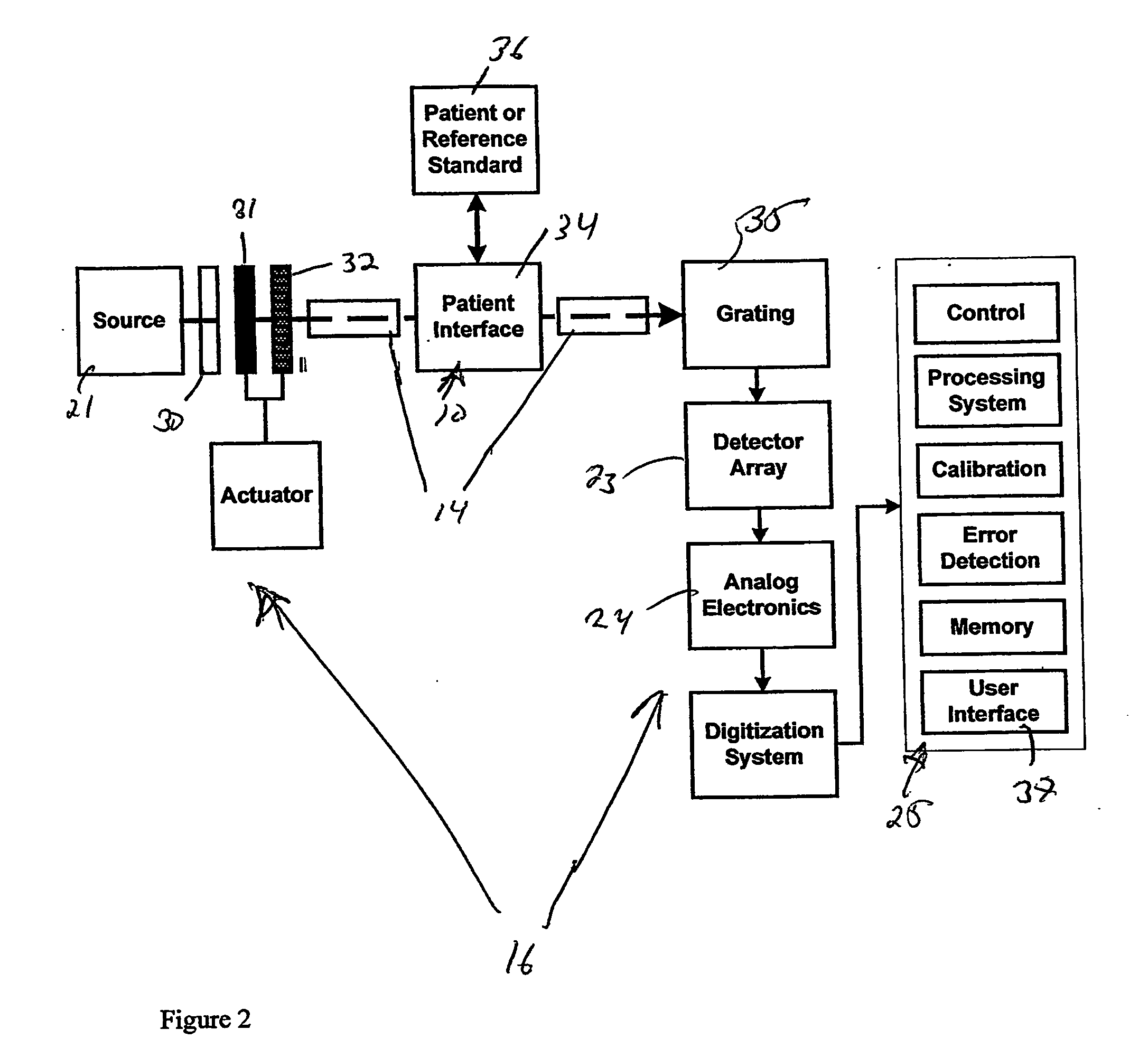
System for non-invasive measurement of glucose in humans
InactiveUS6865408B1Efficient collectionMaximize glucose net analyte signal-to-noise ratioRadiation pyrometryDiagnostics using spectroscopyData acquisitionNon invasive
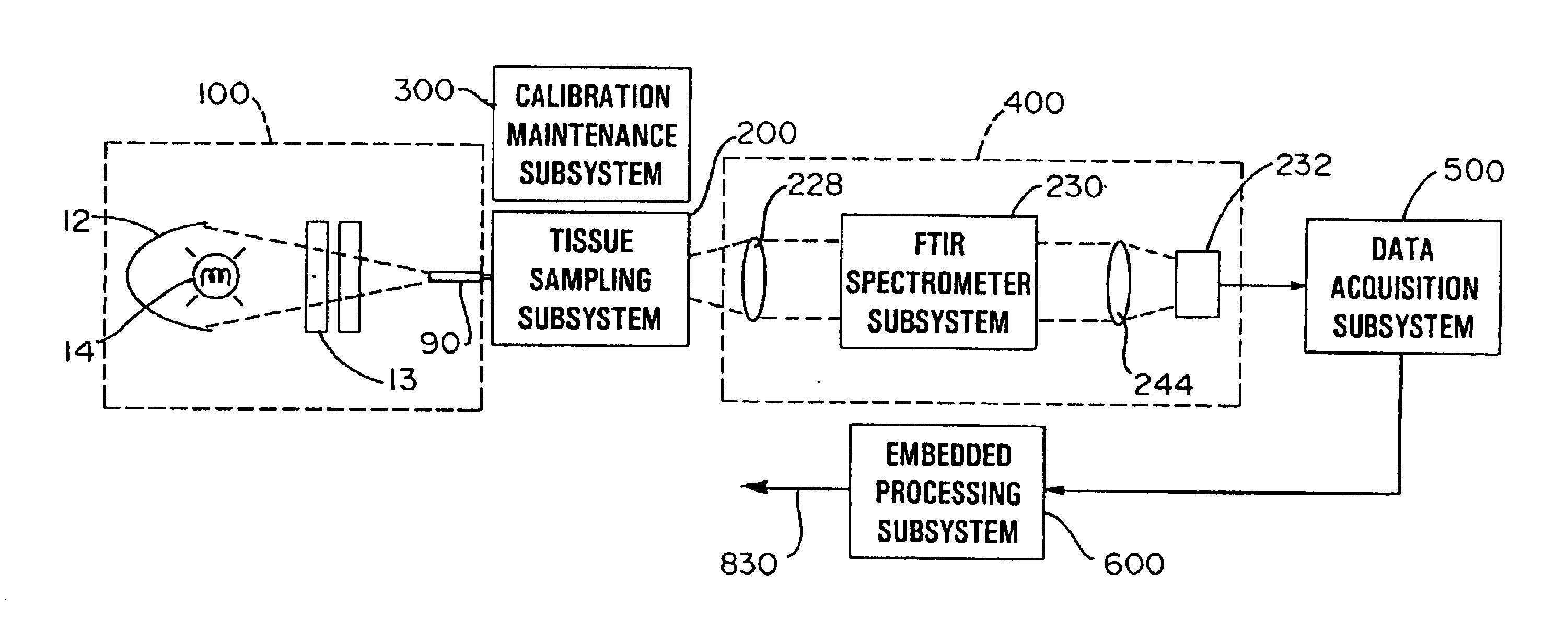
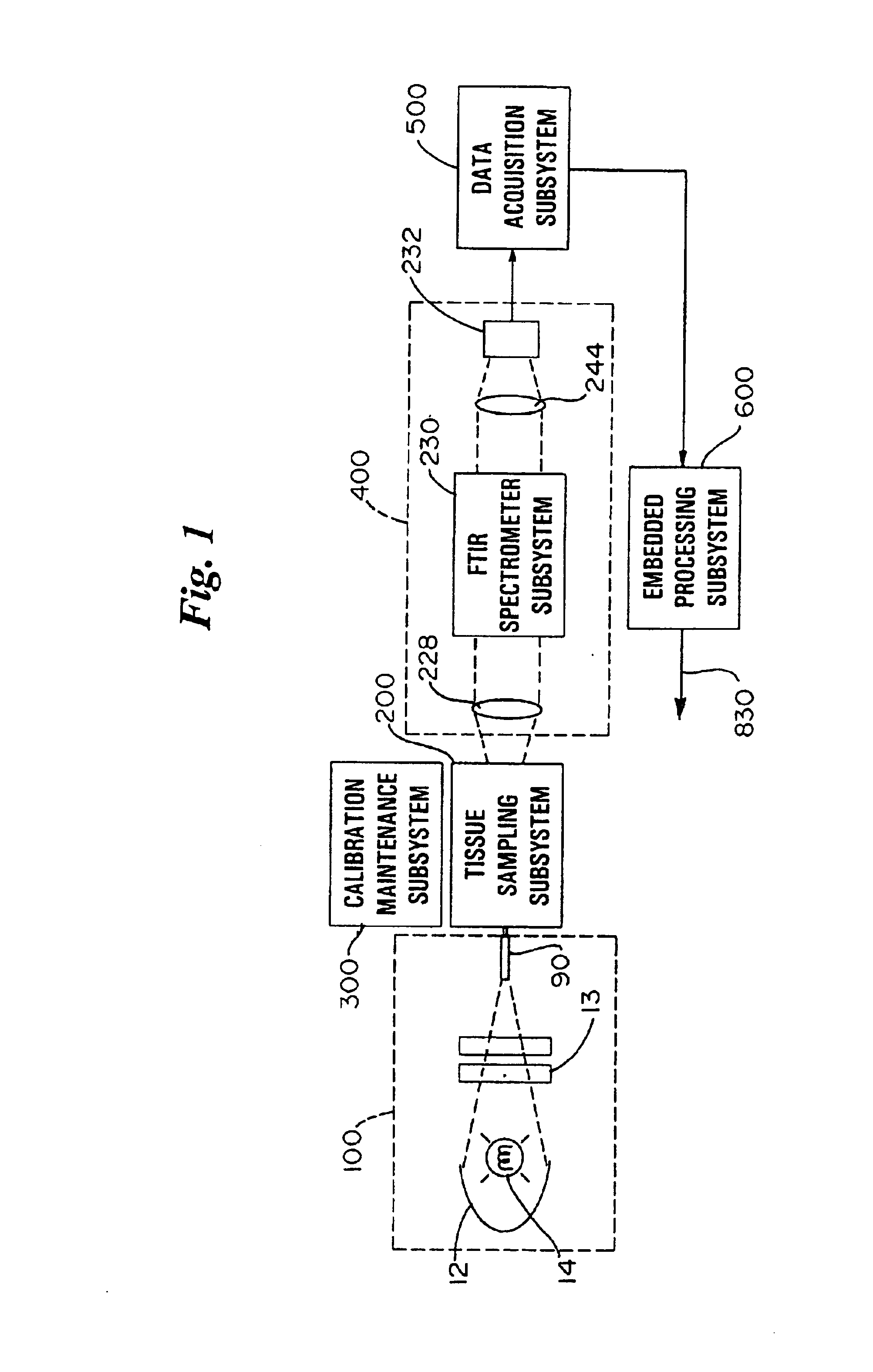
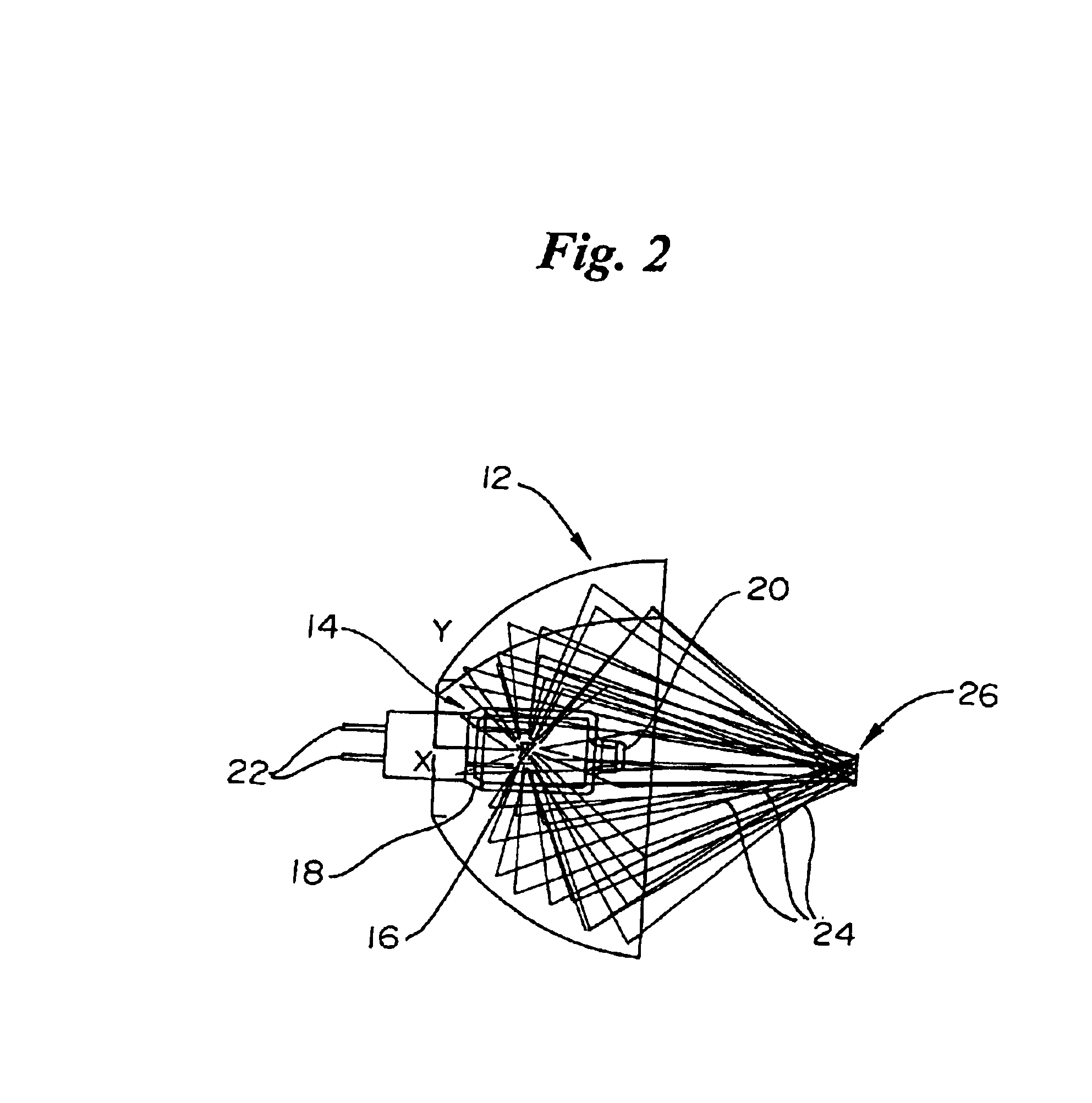
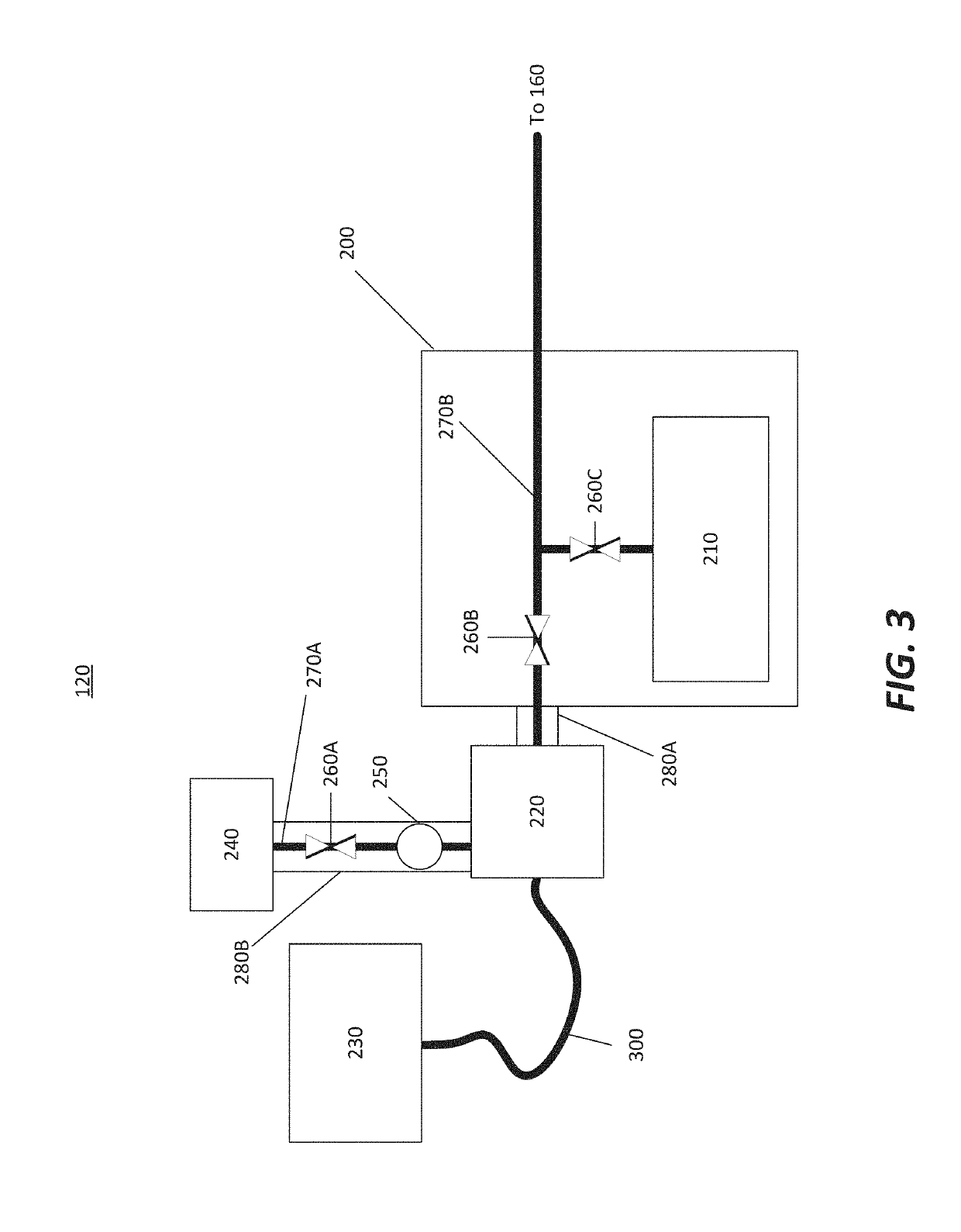
An apparatus and method for non-invasive measurement of glucose in human tissue by quantitative infrared spectroscopy to clinically relevant levels of precision and accuracy. The system includes six subsystems optimized to contend with the complexities of the tissue spectrum, high signal-to-noise ratio and photometric accuracy requirements, tissue sampling errors, calibration maintenance problems, and calibration transfer problems. The six subsystems include an illumination subsystem, a tissue sampling subsystem, a calibration maintenance subsystem, an FTIR spectrometer subsystem, a data acquisition subsystem, and a computing subsystem.
Owner:INLIGHT SOLUTIONS
Additive manufacturing apparatus and method
ActiveUS20160236279A1Sufficient reflectivityAdditive manufacturing apparatusSpectrum investigationManufactured apparatusFeedback control
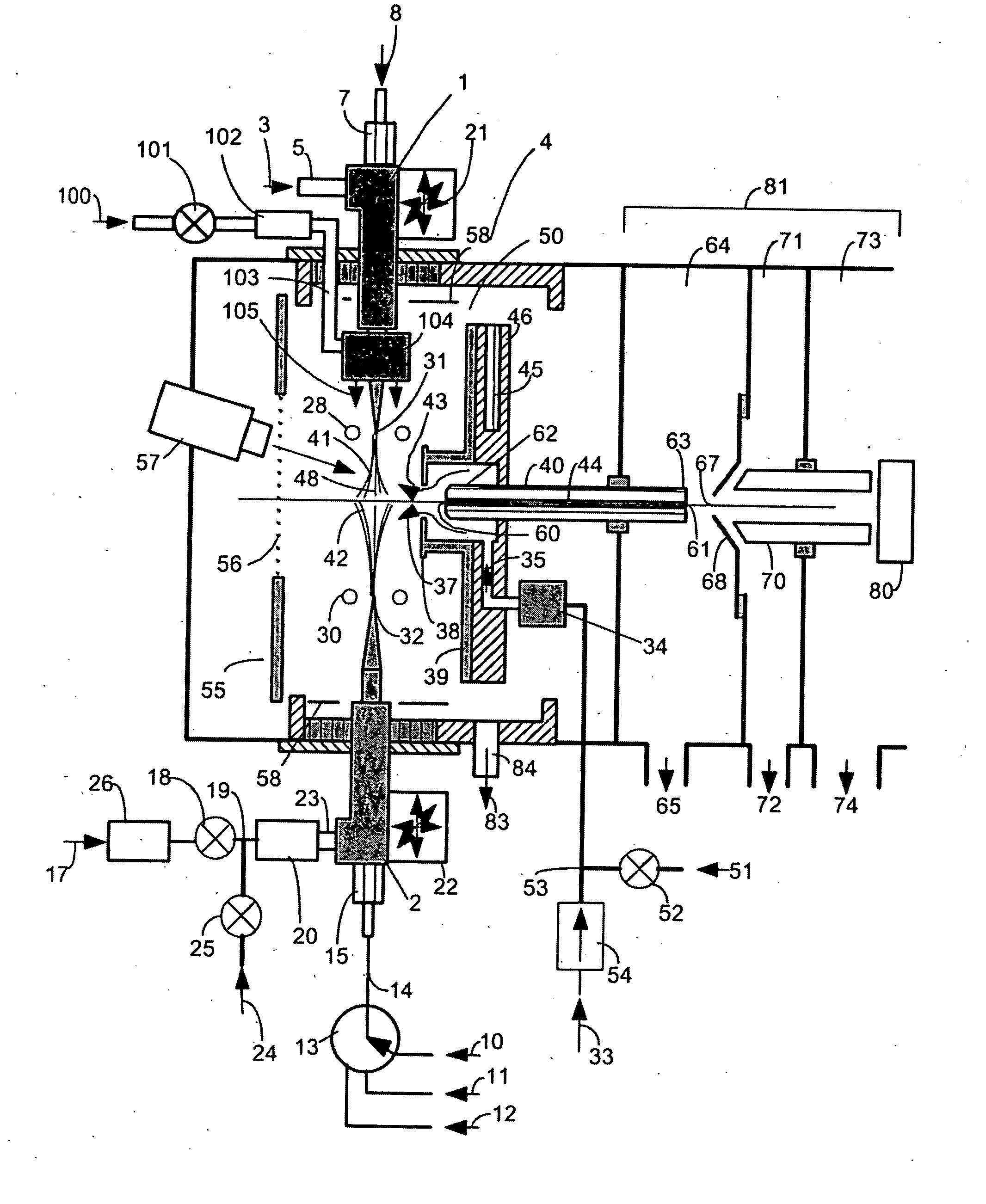
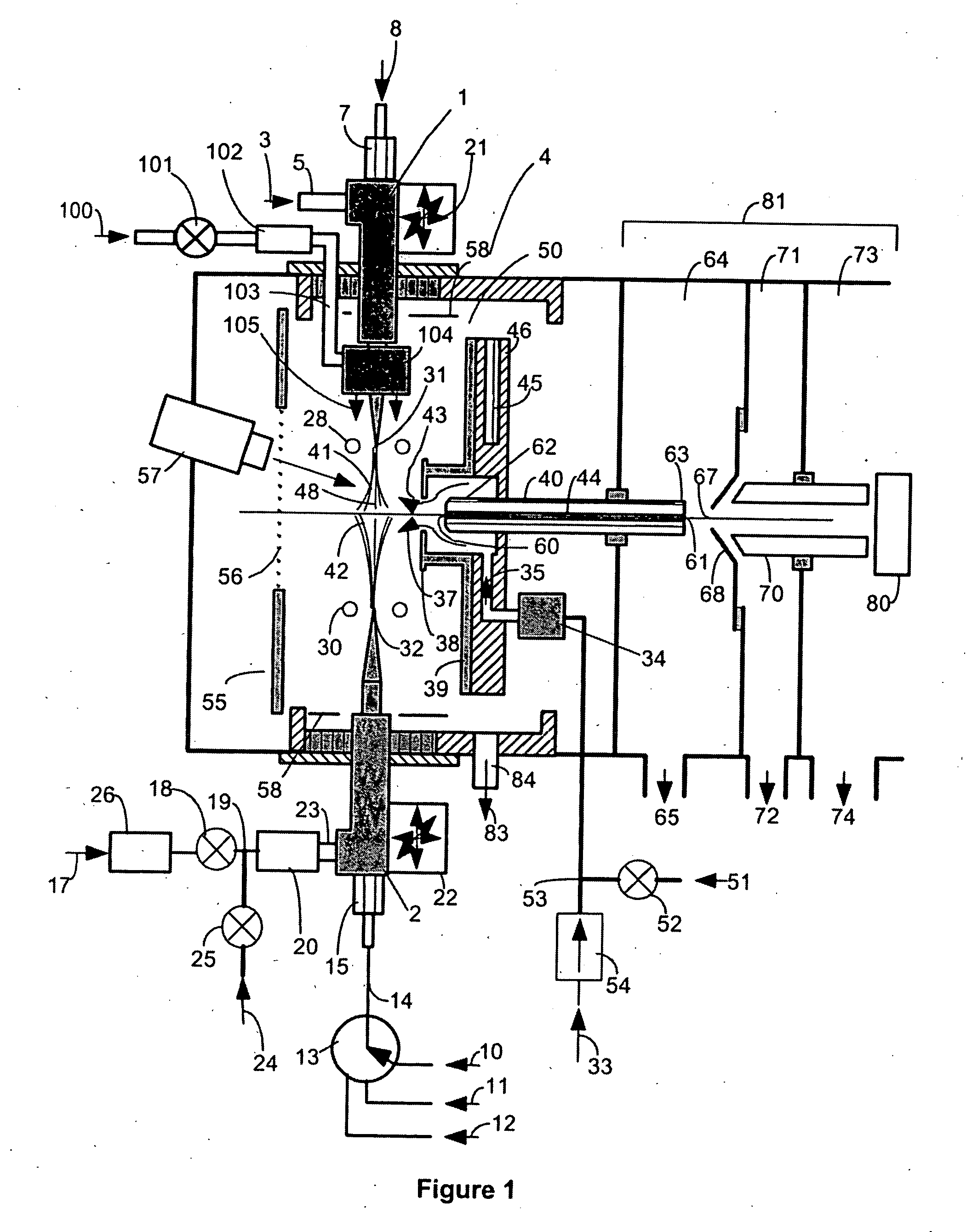
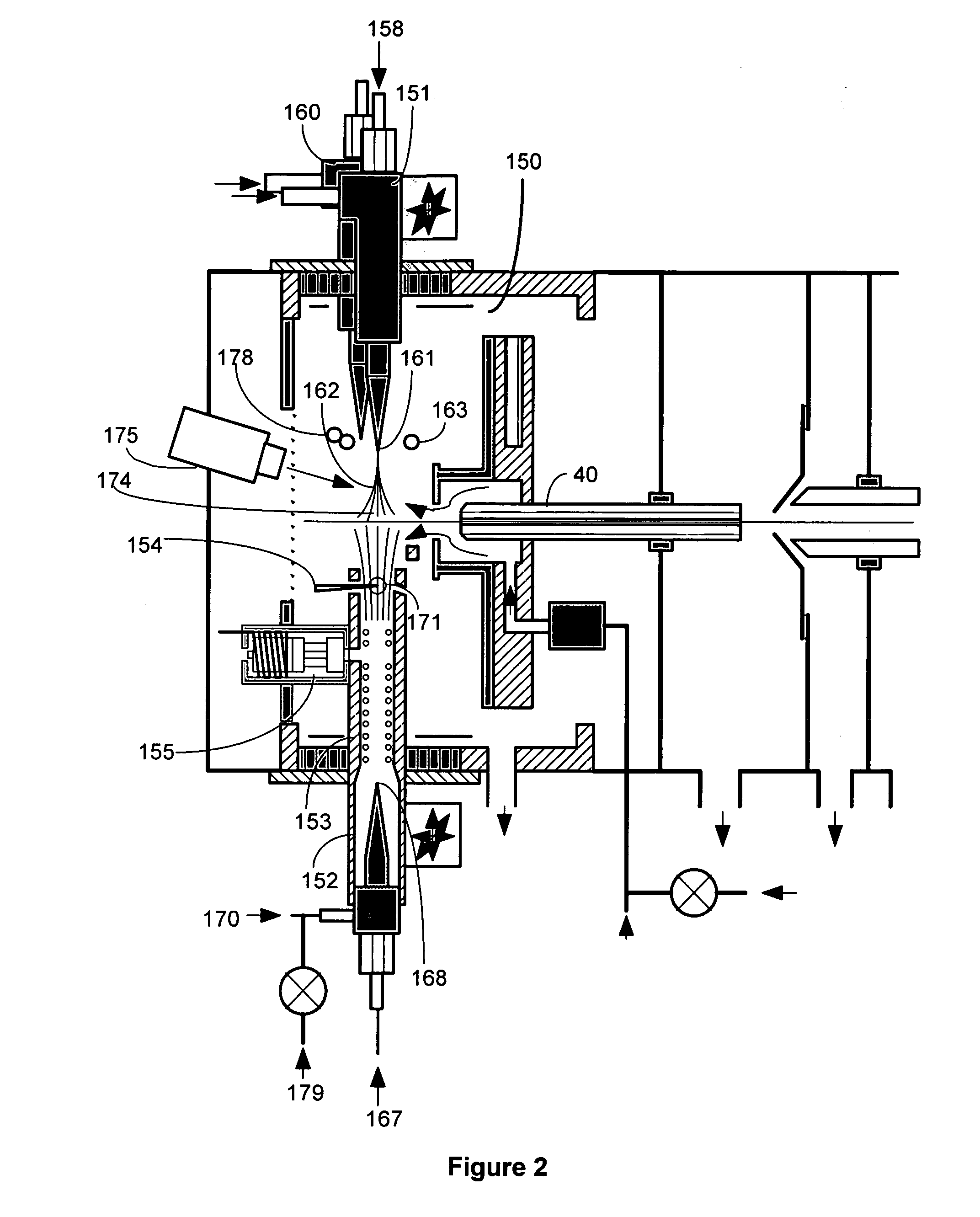
This invention concerns a laser solidification apparatus for building objects by layerwise solidification of powder material. The apparatus including a build chamber containing a build platform, a device for depositing layers of powder material on to the build platform, an optical unit for directing a laser beam to selectively solidify areas of each powder layer and a spectrometer for detecting characteristic radiation emitted by plasma formed during solidification of the powder by the laser beam. The invention also relates to a spectrometer for detecting characteristic radiation generated by interaction of the metal with the or a further laser beam. The spectra recorded using the spectrometer may be used for feedback control during the solidification process.
Owner:RENISHAW PLC
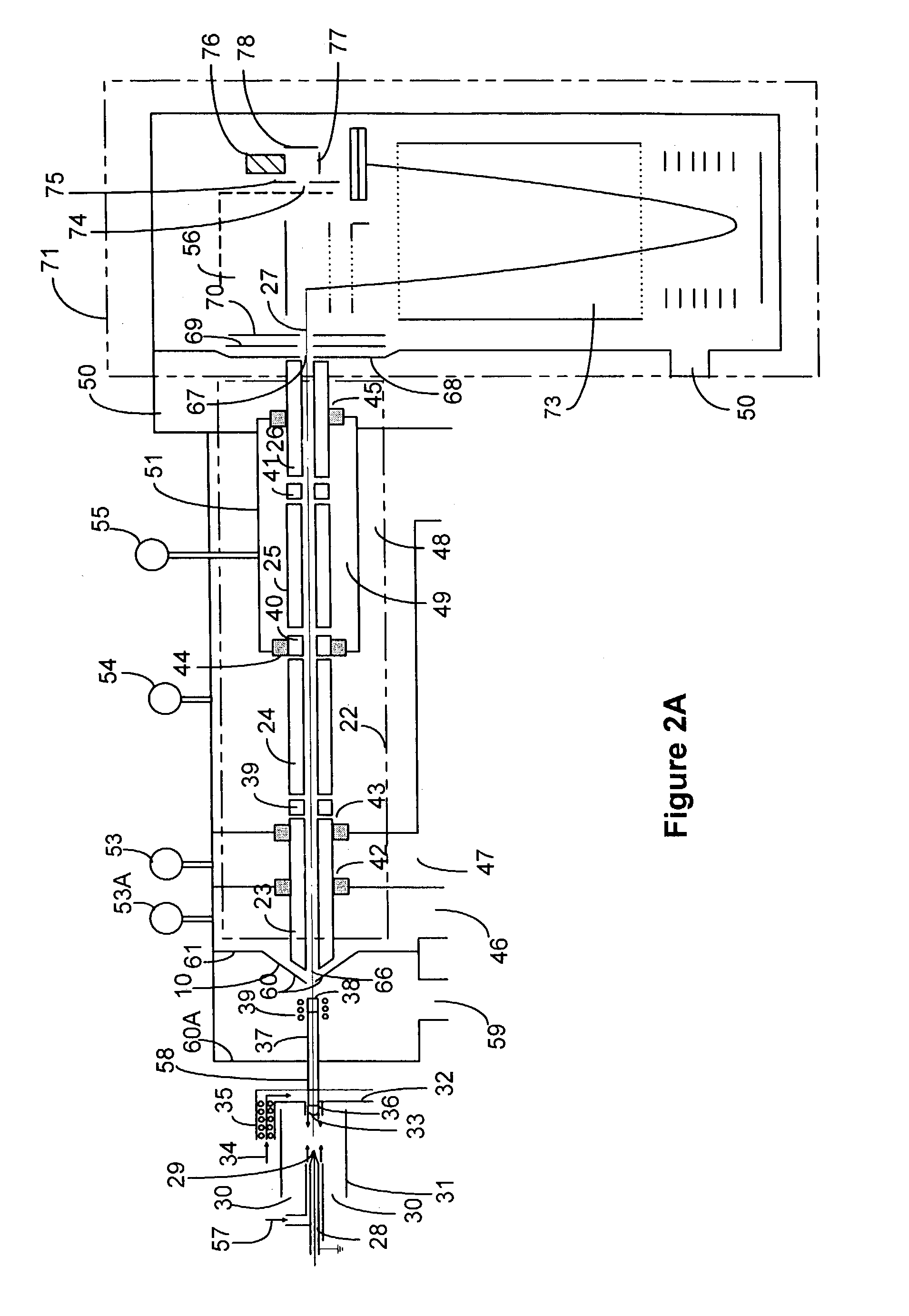
Compact apparatus for noninvasive measurement of glucose through near-infrared spectroscopy
ActiveUS20050010090A1Diagnostics using spectroscopyScattering properties measurementsPhysiologyD-Glucose
The invention involves the monitoring of a biological parameter through a compact analyzer. The preferred apparatus is a spectrometer based system that is attached continuously or semi-continuously to a human subject and collects spectral measurements that are used to determine a biological parameter in the sampled tissue. The preferred target analyze is glucose. The preferred analyzer is a near-IR based glucose analyzer for determining the glucose concentration in the body.
Owner:GLT ACQUISITION
Mass spectrometry with segmented RF multiple ion guides in various pressure regions
InactiveUS7034292B1Reduce lossesEliminate and reduce numberIsotope separationSpectrometer combinationsFourier transform on finite groupsMass analyzer
A mass spectrometer is configured with individual multipole ion guides, configured in an assembly in alignment along a common centerline wherein at least a portion of at least one multipole ion guide mounted in the assembly resides in a vacuum region with higher background pressure, and the other portion resides in a vacuum region with lower background pressure. Said multipole ion guides are operated in mass to charge selection and ion fragmentation modes, in either a high or low pressure region, said region being selected according to the optimum pressure or pressure gradient for the function performed. The diameter, lengths and applied frequencies and phases on these contiguous ion guides may be the same or may differ. A variety of MS and MS / MSn analysis functions can be achieved using a series of contiguous multipole ion guides operating in either higher background vacuum pressures, or along pressure gradients in the region where the pressure drops from high to low pressure, or in low pressure regions. Individual sets of RF, + / −DC and resonant frequency waveform voltage supplies provide potentials to the rods of each multipole ion guide allowing the operation of ion transmission, ion trapping, mass to charge selection and ion fragmentation functions independently in each ion guide. The presence of background pressure maintained sufficiently high to cause ion to neutral gas collisions along a portion of each multiple ion guide linear assembly allows the conducting of Collisional Induced Dissociation (CID) fragmentation of ions by axially accelerating ions from one multipole ion guide into an adjacent ion guide. Alternatively ions can be fragmented in one or more multipole ion guides using resonant frequency excitation CID. A multiple multipole ion guide assembly can be configured as the primary mass analyzer in single or triple quadrupole mass analyzers with or without mass selective axial ejection. Alternatively, the multiple multipole ion guide linear assembly can be configured as part of a hybrid Time-Of-Flight, Magnetic Sector, Ion Trap or Fourier Transform mass analyzer.
Owner:PERKINELMER U S LLC
Apparatus for detecting or monitoring for a chemical precursor in a high temperature environment
ActiveUS20190264324A1Emission spectroscopyElectric discharge tubesOptical Emission SpectrometerSmall sample
An apparatus and method are disclosed for monitoring and / or detecting concentrations of a chemical precursor in a reaction chamber. The apparatus and method have an advantage of operating in a high temperature environment. An optical emissions spectrometer (OES) is coupled to a gas source, such as a solid source vessel, in order to monitor or detect an output of the chemical precursor to the reaction chamber. Alternatively, a small sample of precursor can be periodically monitored flowing into the OES and into a vacuum pump, thus bypassing the reaction chamber.
Owner:ASM IP HLDG BV
Collapsible noninvasive analyzer method and apparatus
The invention relates generally to a noninvasive spectroscopic based analyzer. More particularly, a collapsible spectrometer and or deployable subject interface for an analyzer, such as a noninvasive glucose concentration analyzer, is described.
Owner:GLT ACQUISITION
Device for capturing thermal spectra from tissue
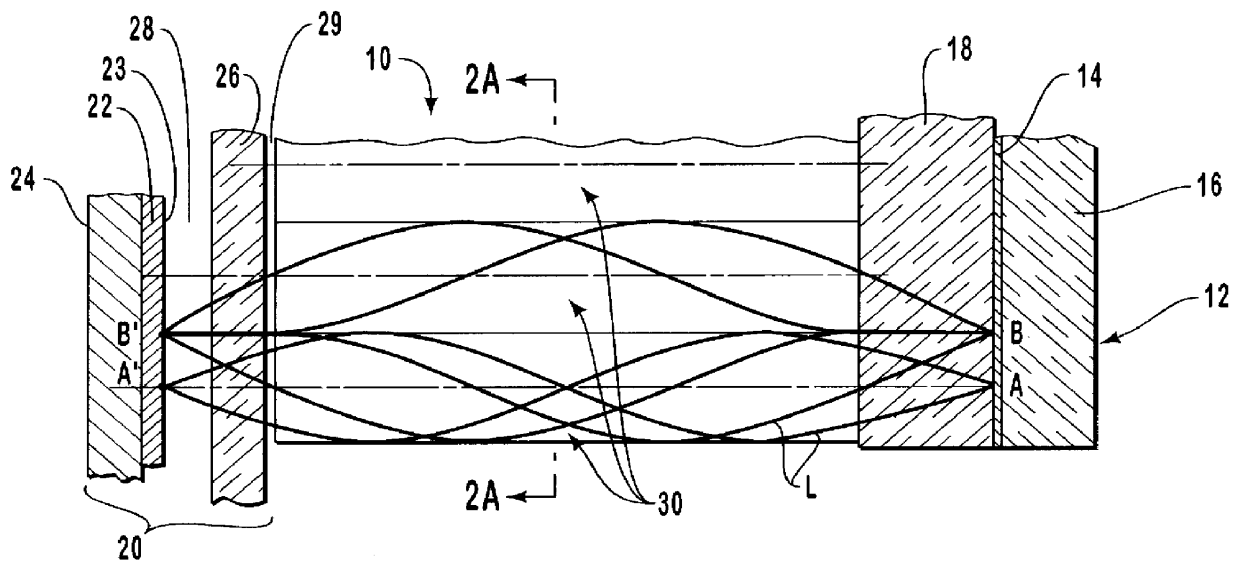
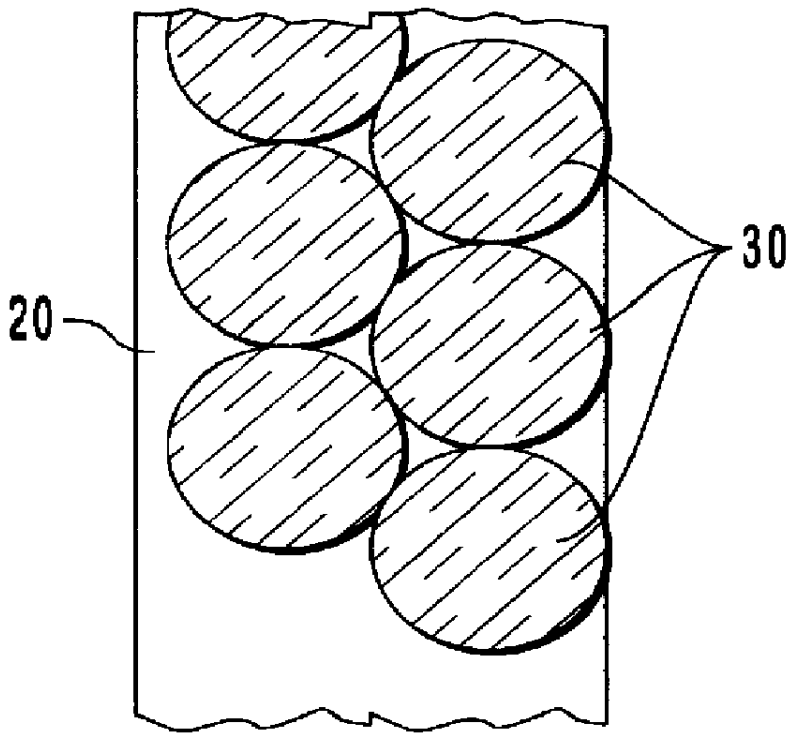
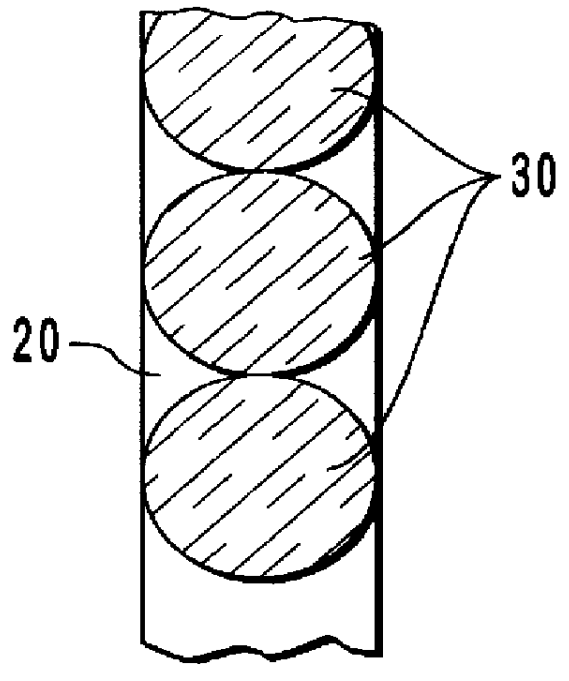
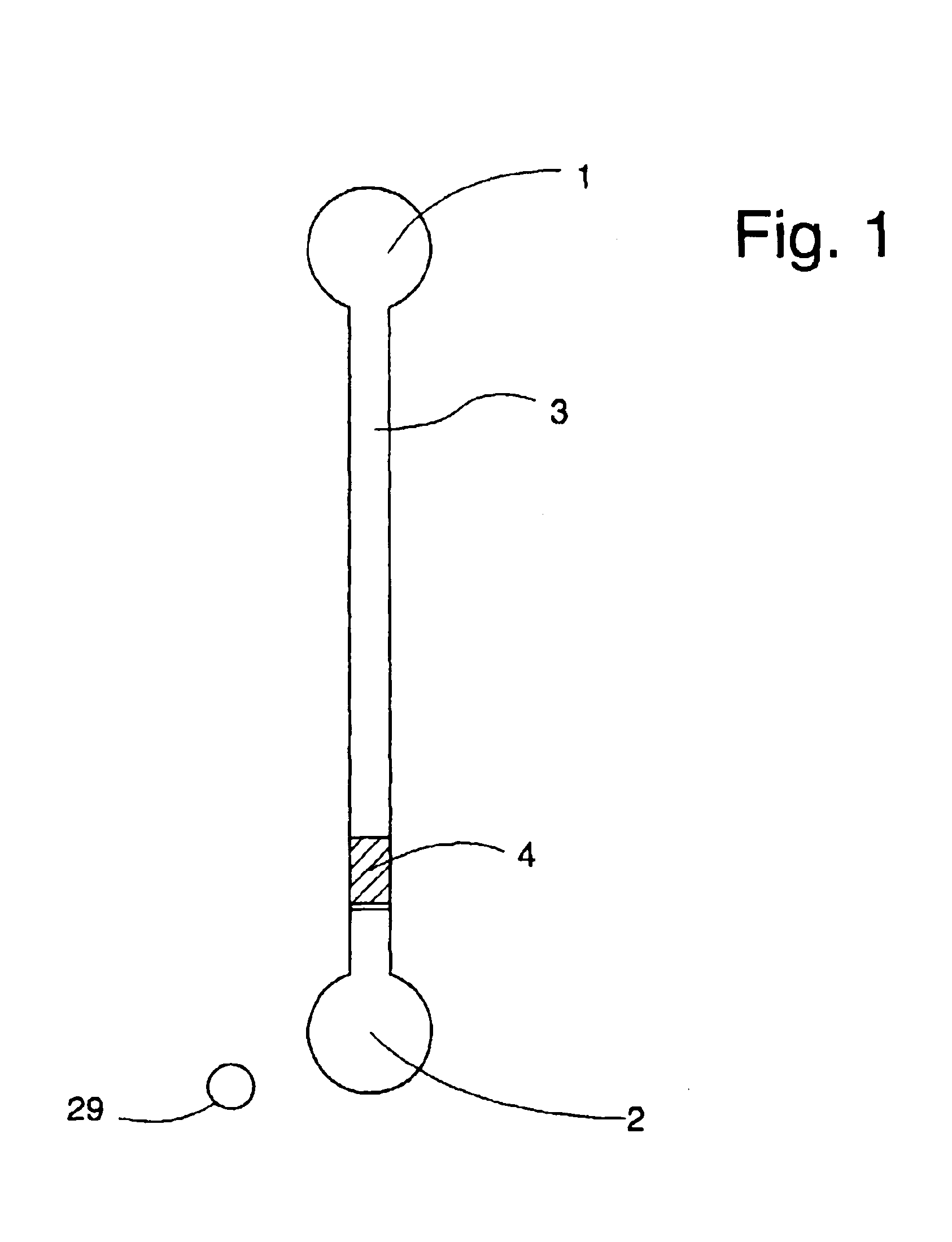
InactiveUS6959211B2Improve determinationIsolating measurementDiagnostic recording/measuringColor/spectral properties measurementsAnalyteGlucose polymers
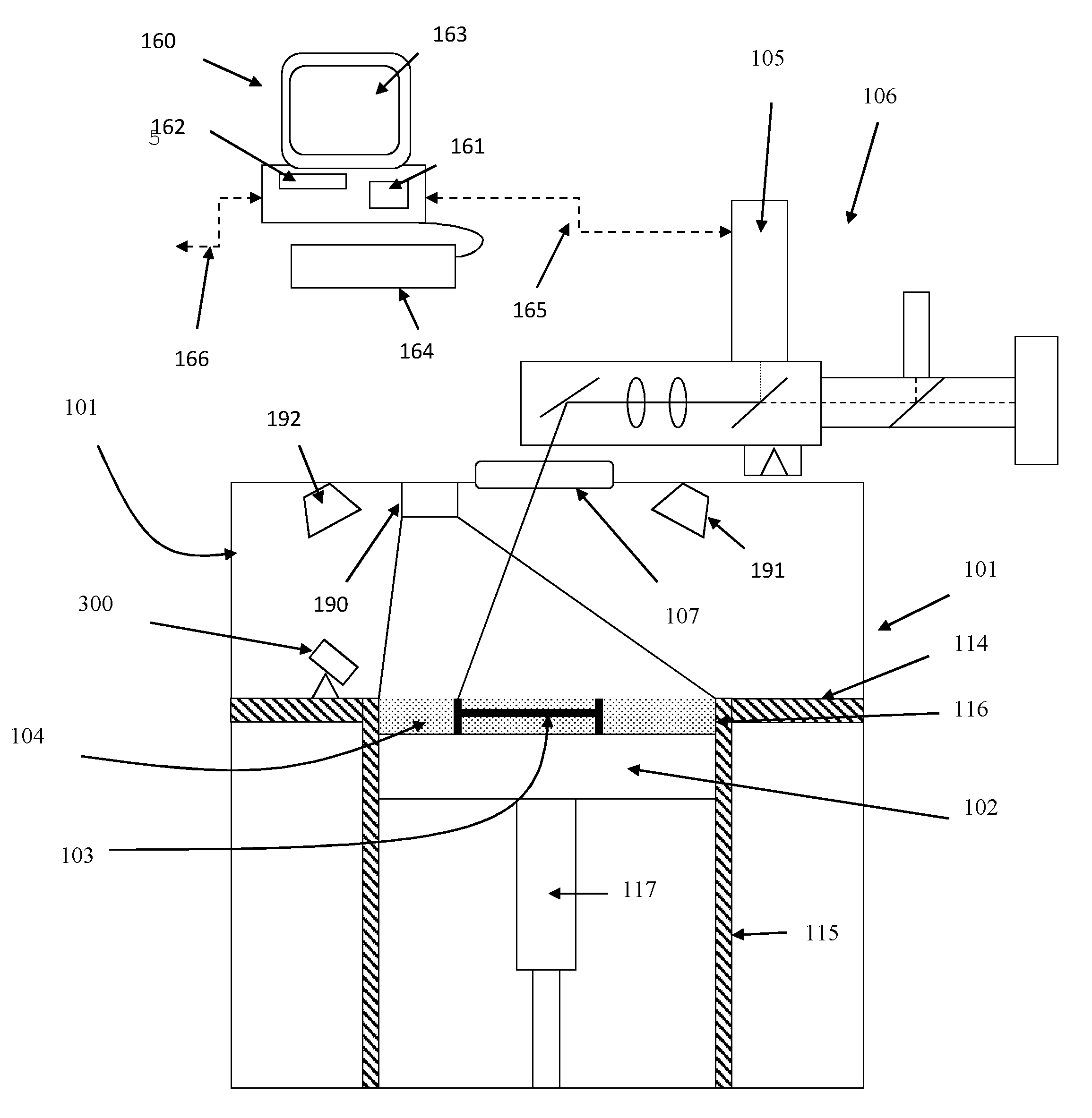
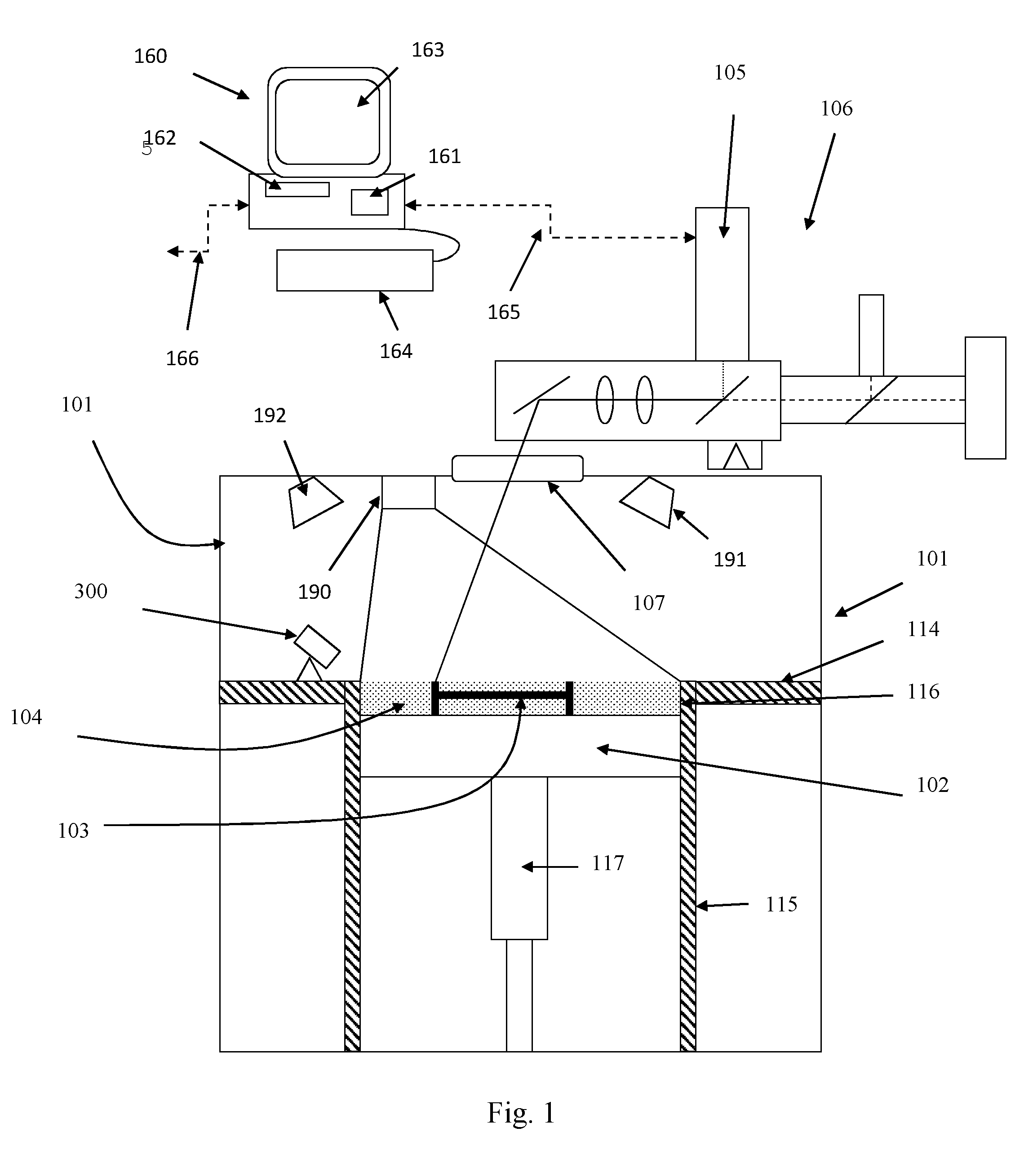
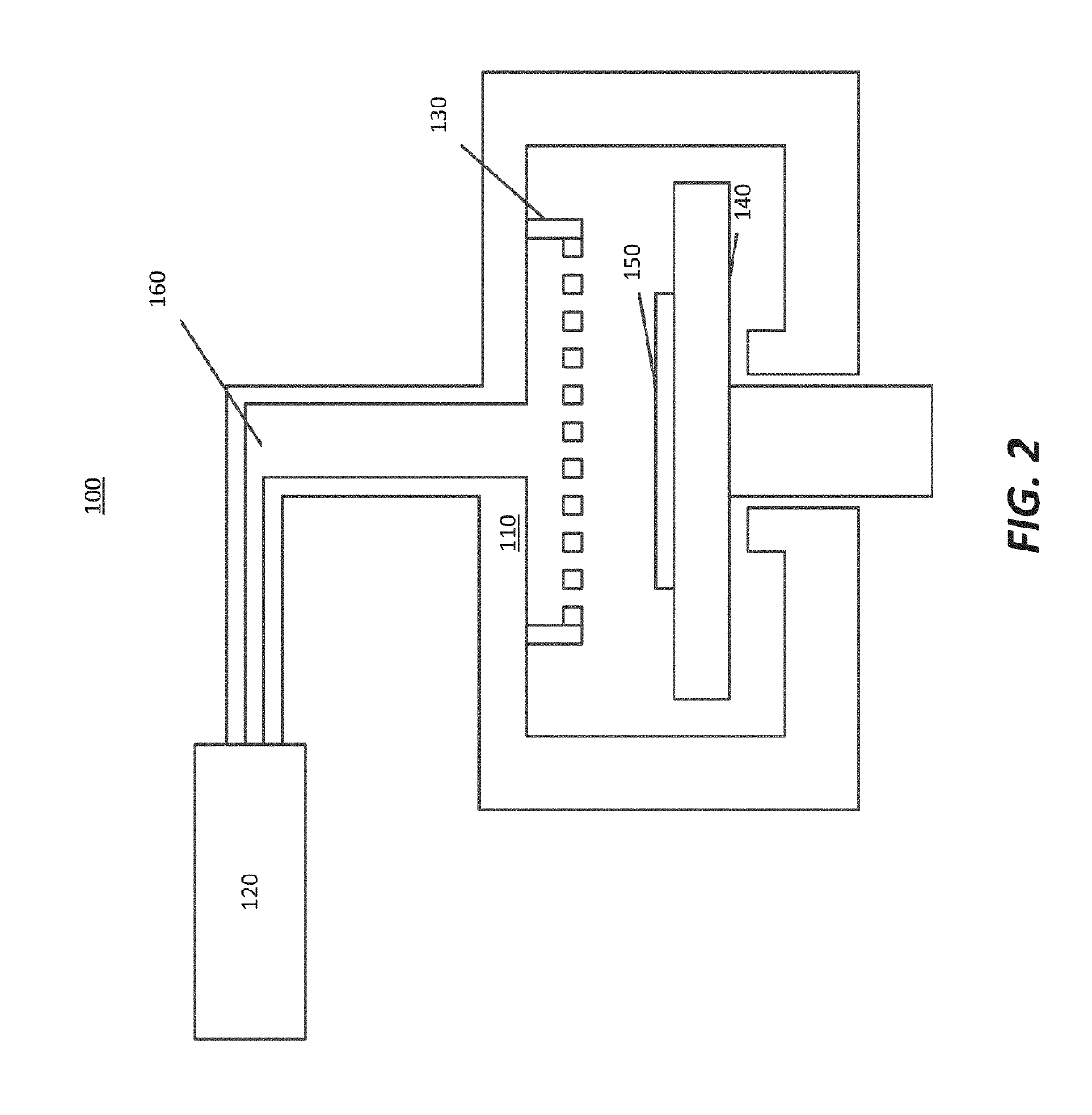
A device and method are provided for use with a noninvasive optical measurement system, such as a thermal gradient spectrometer, for improved determination of analyte concentrations within living tissue. In one embodiment, a wearable window is secured to a patient's forearm thereby isolating a measurement site on the patient's skin for determination of blood glucose levels. The wearable window effectively replaces a window of the spectrometer, and thus forms an interface between the patient's skin and a thermal mass window of the spectrometer. When the spectrometer must be temporarily removed from the patient's skin, such as to allow the patient mobility, the wearable window is left secured to the forearm so as to maintain a consistent measurement site on the skin. When the spectrometer is later reattached to the patient, the wearable window will again form an interface between the spectrometer and the same location of skin as before.
Owner:OPTISCAN BIOMEDICAL
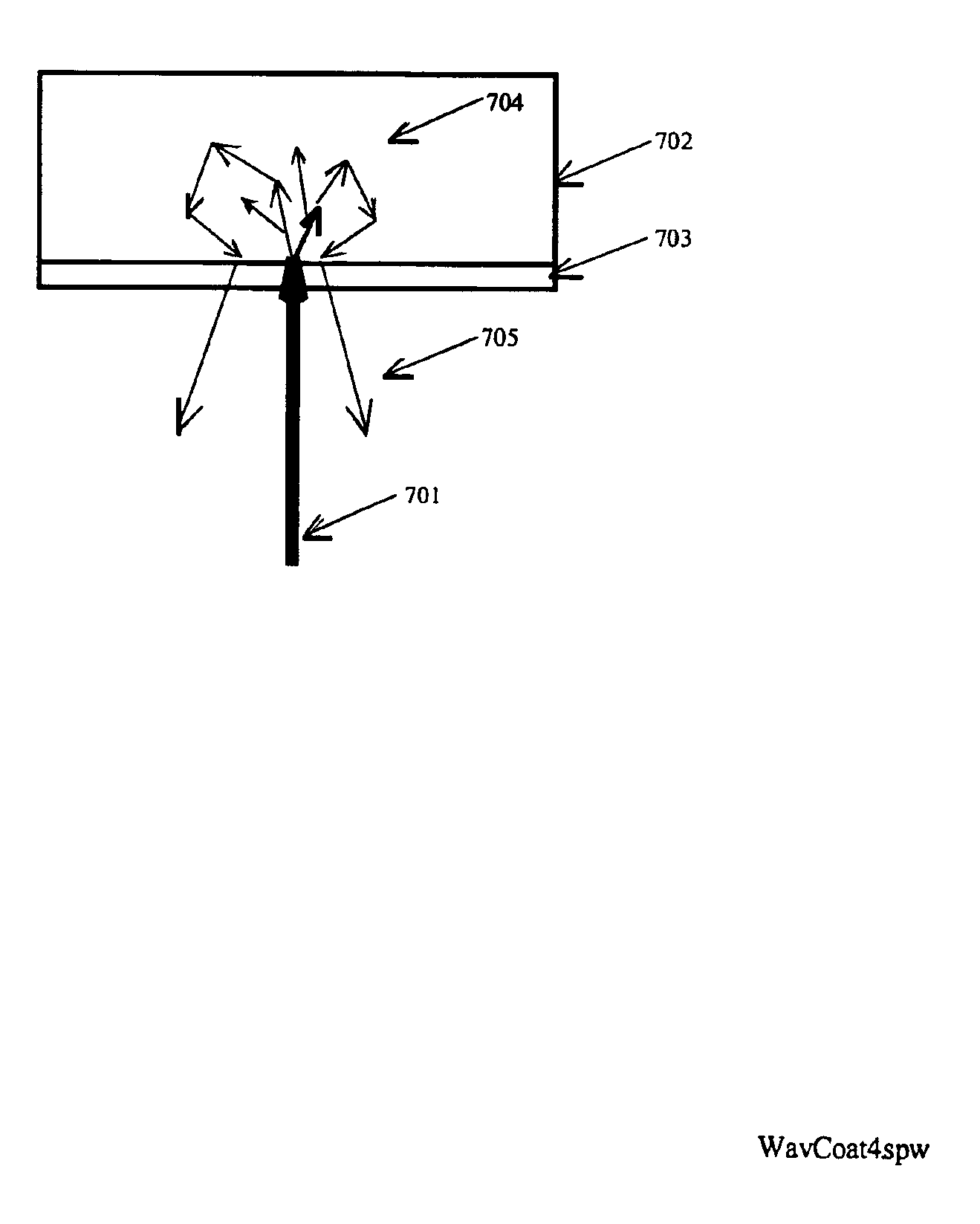
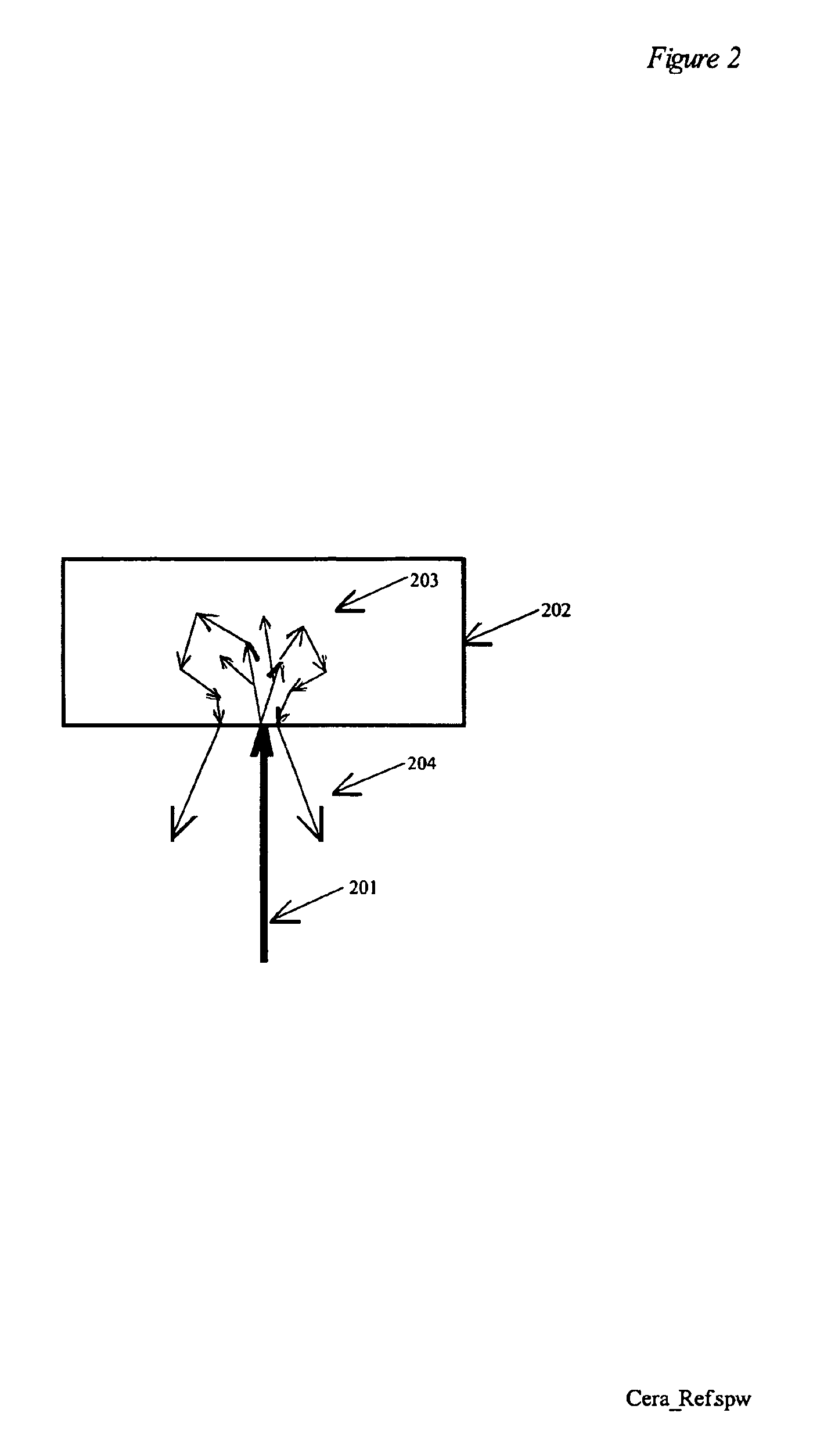
Spectroscopic system and method using a ceramic optical reference
A ceramic reference in conjunction with a spectrometer, a metallized ceramic material, and a method of utilizing a ceramic material as a reference in the ultraviolet, visible, near-infrared, or infrared spectral regions are presented. The preferred embodiments utilize a ceramic reference material to diffusely reflect incident source light toward a detector element for quantification in a reproducible fashion. Alternative embodiments metallize either the incident surface or back surface of to form a surface diffuse reflectance standard. Optional wavelength reference layers or protective layers may be added to the ceramic or to the metallized layer. The reference ceramic is used to provide a measure of optical signal of an analyzer as a function of the analyzers spatial, temporal, and environmental state.
Owner:GLT ACQUISITION
Microfludic system (EDI)
InactiveUS6717136B2Minimal loss of precious materialCheap and disposableIon-exchange process apparatusMaterial nanotechnologyAnalyteEngineering
A microfluidic device comprising an MS-analyte presentation unit for a EDI-MS apparatus, said unit comprising an essentially planar support plate which on one side has one, two or more ports (MS-ports) comprising an area (EDI area) for presenting the MS-analyte to a mass spectrometer. The EDI area comprises a layer I of conducting material. The characteristic feature of the device is that layer (I) has a conductive connection and / or that there is a calibrator area in the proximity of the MS-port.
Owner:GYROS
Microfluidic sample delivery devices, systems, and methods
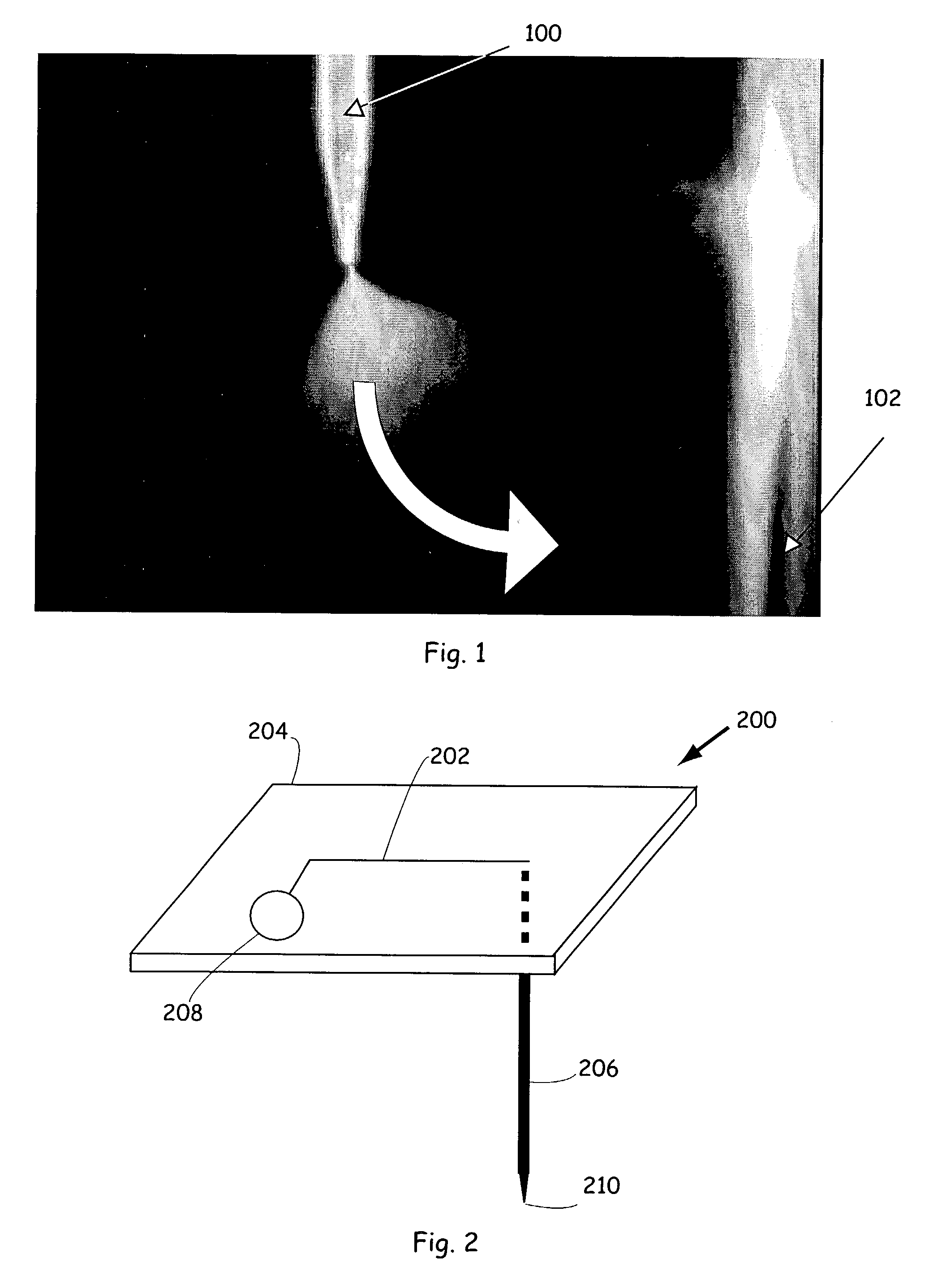
ActiveUS7303727B1Improve throughputResidue reductionParticle separator tubesComponent separationSpray nozzleMass spectrometry
Methods and apparatus for delivering fluidic materials to sample destinations, including mass spectrometers for analysis are provided. In preferred embodiments, sample aliquots are electrosprayed from tapered spray tips of capillary elements into the orifices of mass spectrometric inlet systems. In certain embodiments, fluidic samples are orthogonally sprayed from capillary elements or other fluid conduits, whereas in other embodiments samples are sprayed after devices are rotated or otherwise translocated from sample sources to sample destinations. In still other embodiments, samples are sprayed from flexed or deflected capillary elements at selected sample destinations.
Owner:CAPLIPER LIFE SCI INC
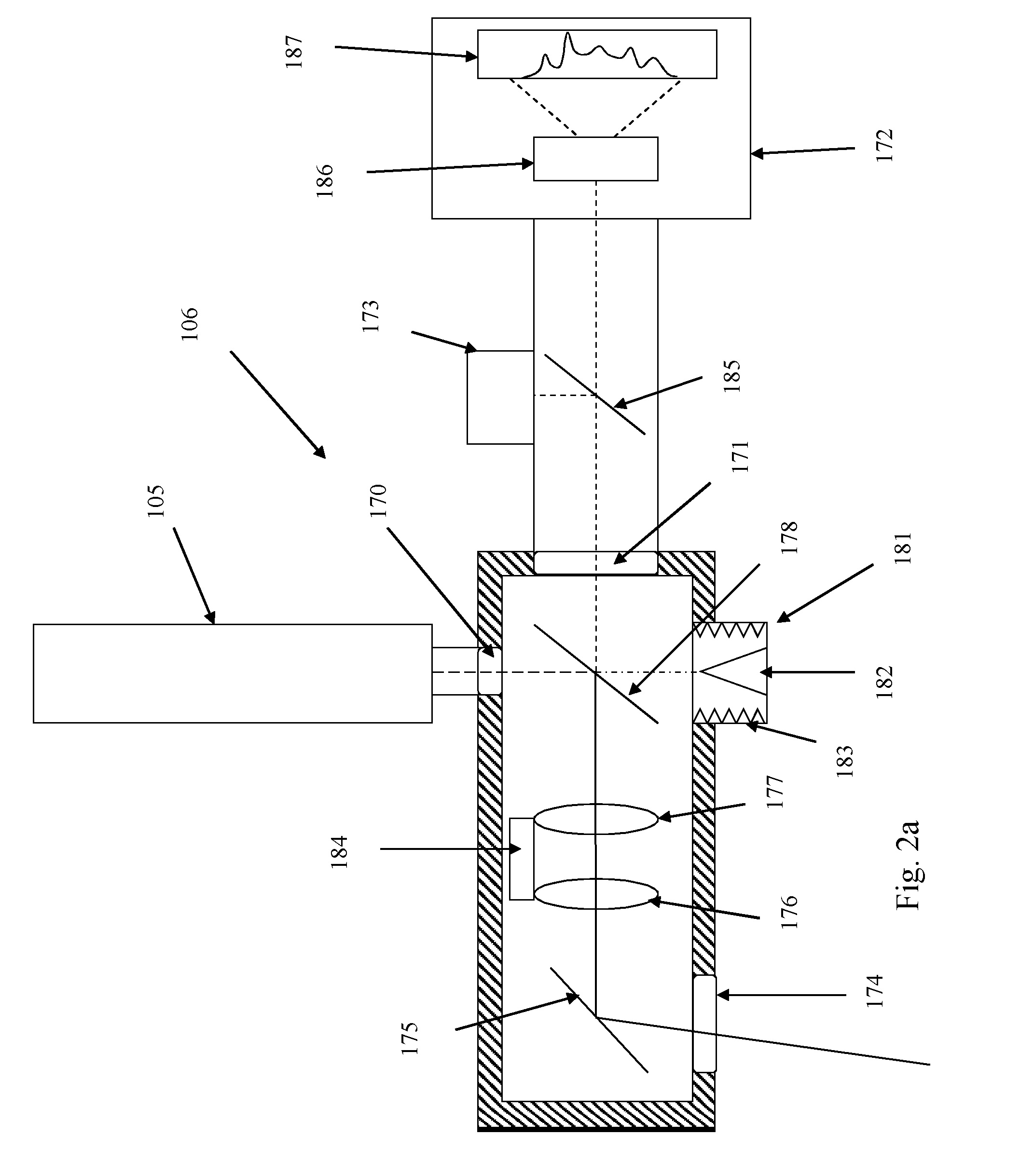
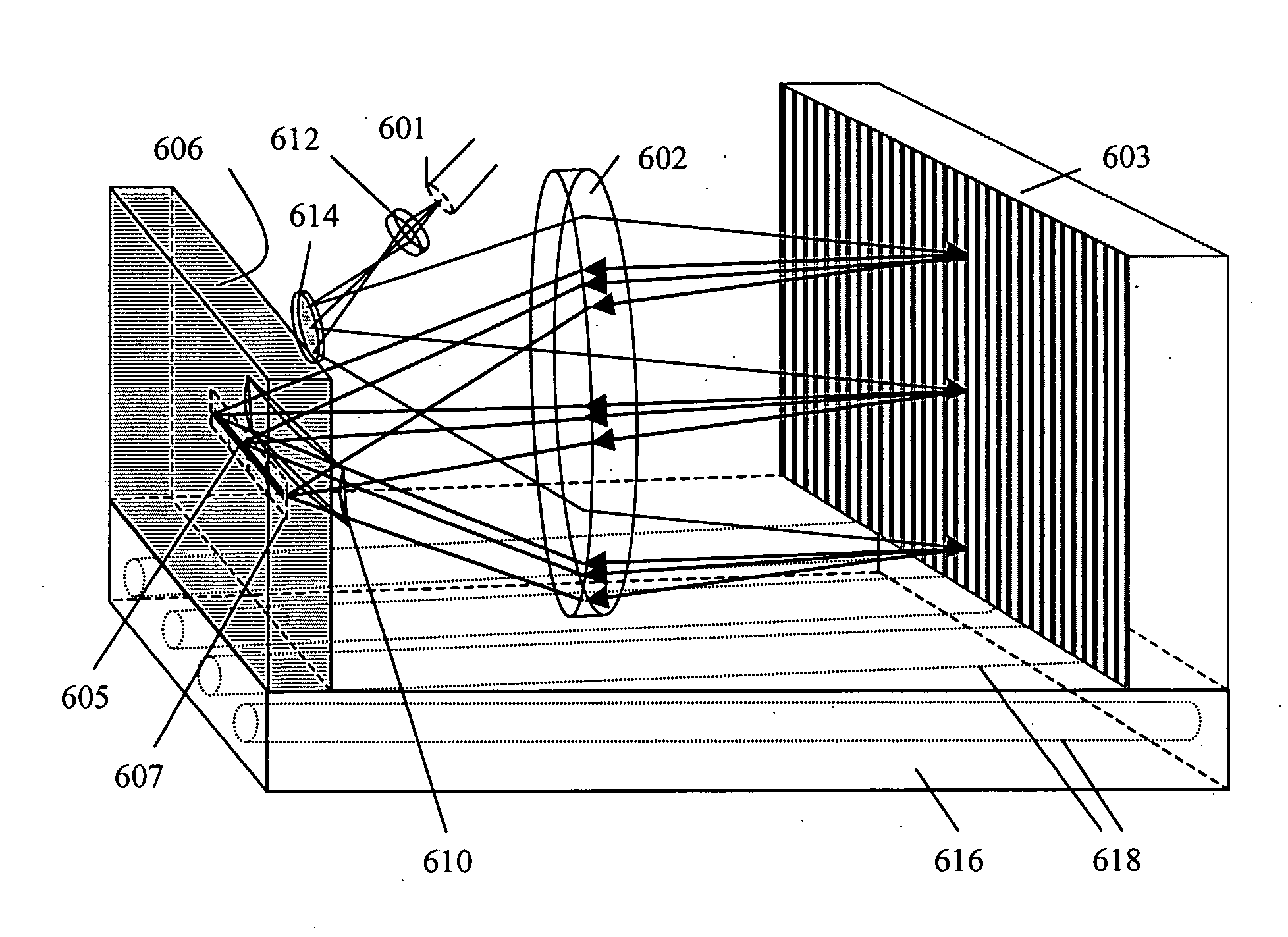
Cross-dispersed spectrometer in a spectral domain optical coherence tomography system
InactiveUS7342659B2Eliminating spatial order overlapReduce non-linearityRadiation pyrometrySpectrum investigationTwo dimensional detectorGrating
Owner:CARL ZEISS MEDITEC INC
Atmospheric pressure ion source for mass spectrometry
InactiveUS20060255261A1Low costIncrease speedIsotope separationMass spectrometersGas phaseCorona discharge
A multiple function atmospheric pressure ion source interfaced to a mass spectrometer comprises multiple liquid inlet probes configured such that the sprays from two or more probes intersect in a mixing region. Gas phase sample ions or neutral species generated in the spray of one probe can react with reagent gas ions generated from one or more other probes by such ionization methods as Electrospray, photoionization, corona discharge and glow discharge ionization. Reagent ions may be optimally selected to promote such processes as Atmospheric Pressure Chemical Ionization of neutral sample molecules, or charge reduction or electron transfer dissociation of multiply charged sample ions. Selected neutral reagent species can also be introduced into the mixing region to promote charge reduction of multiply charged sample ions through ion-neutral reactions. Different operating modes can be performed alternately or simultaneously, and can be rapidly turned on and off under manual or software control.
Owner:WHITEHOUSE CRAIG +3
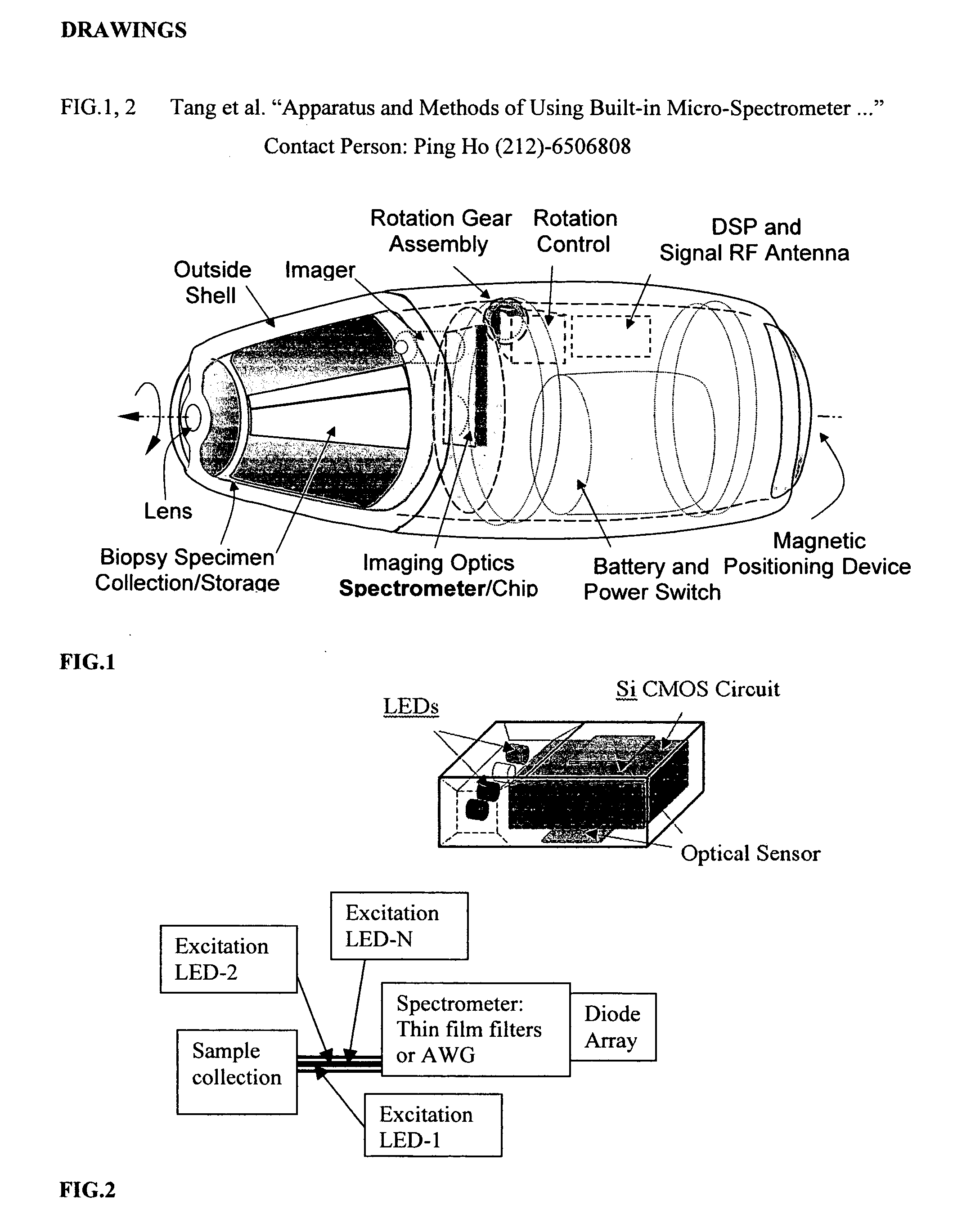
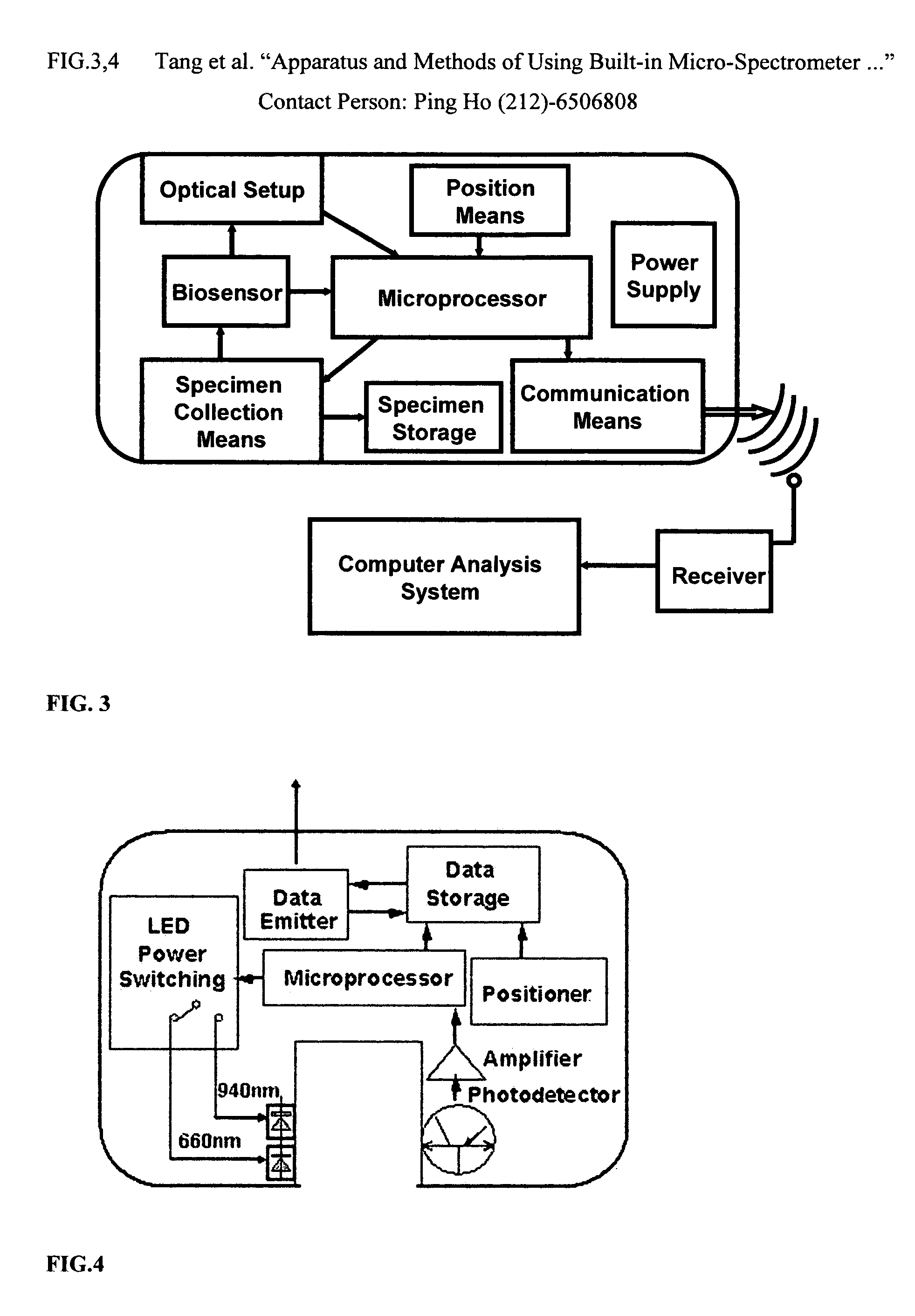
Positioning devices and methods for in vivo wireless imaging capsules
A wireless capsule as a disease diagnosis tool in vivo can be introduced into a biological body by a native and / or artificial open, or endoscope, or an injection. The information obtained from a micro-spectrometer, and / or an imaging system, or a micro-biosensor, all of which are built-in a wireless capsule, can be transmitted to the outside of the biological body for medical diagnoses. In addition, a real-time specimen collection device is integrated with the diagnostic system for the in-depth in vitro analysis
Owner:WANG LEMING +4
Cross-dispersed spectrometer in a spectral domain optical coherence tomography system
InactiveUS20060164639A1Eliminating spatial order overlapReduce non-linearityRadiation pyrometrySpectrum investigationTwo dimensional detectorGrating
A spectral-domain optical coherence tomography system using a cross-dispersed spectrometer is disclosed. The interfered optical signal is dispersed by a grating into several orders of diffraction, and these orders of diffraction are separated by an additional dispersive optical element. The spectral interferogram is recorded by a set of linear detector arrays, or by a two-dimensional detector array.
Owner:CARL ZEISS MEDITEC INC
Corona discharge ion source for analytical instruments
InactiveUS6225623B1Time-of-flight spectrometersMaterial analysis by electric/magnetic meansDopantCorona discharge
An ion mobility spectrometer comprises an ion mobility cell (10) into which molecules of a sample to be analysed are introduced. The ion mobility cell (10) is doped with ions produced by a corona discharge ionisation source (40). In one mode of operation, the corona discharge ionisation source (40) operates to produce a continual dopant stream, and in a second mode of operation, the corona discharge ionisation source (40) produces dopant ions selectively. In the non-continuous mode of operation, the ion mobility cell (10) may be doped with chemical dopant ions instead, switching between the two dopant regimes being accomplished very rapidly. The ion mobility spectrometer is particularly suitable for the detection of explosive compounds and narcotics, the ion mobility spectrum of explosives doped with ions from the corona discharge ionisation source differing from the ion mobility spectrum of such explosive compounds doped with chemical dopants.
Owner:SMITHS DETECTION WATFORD LTD
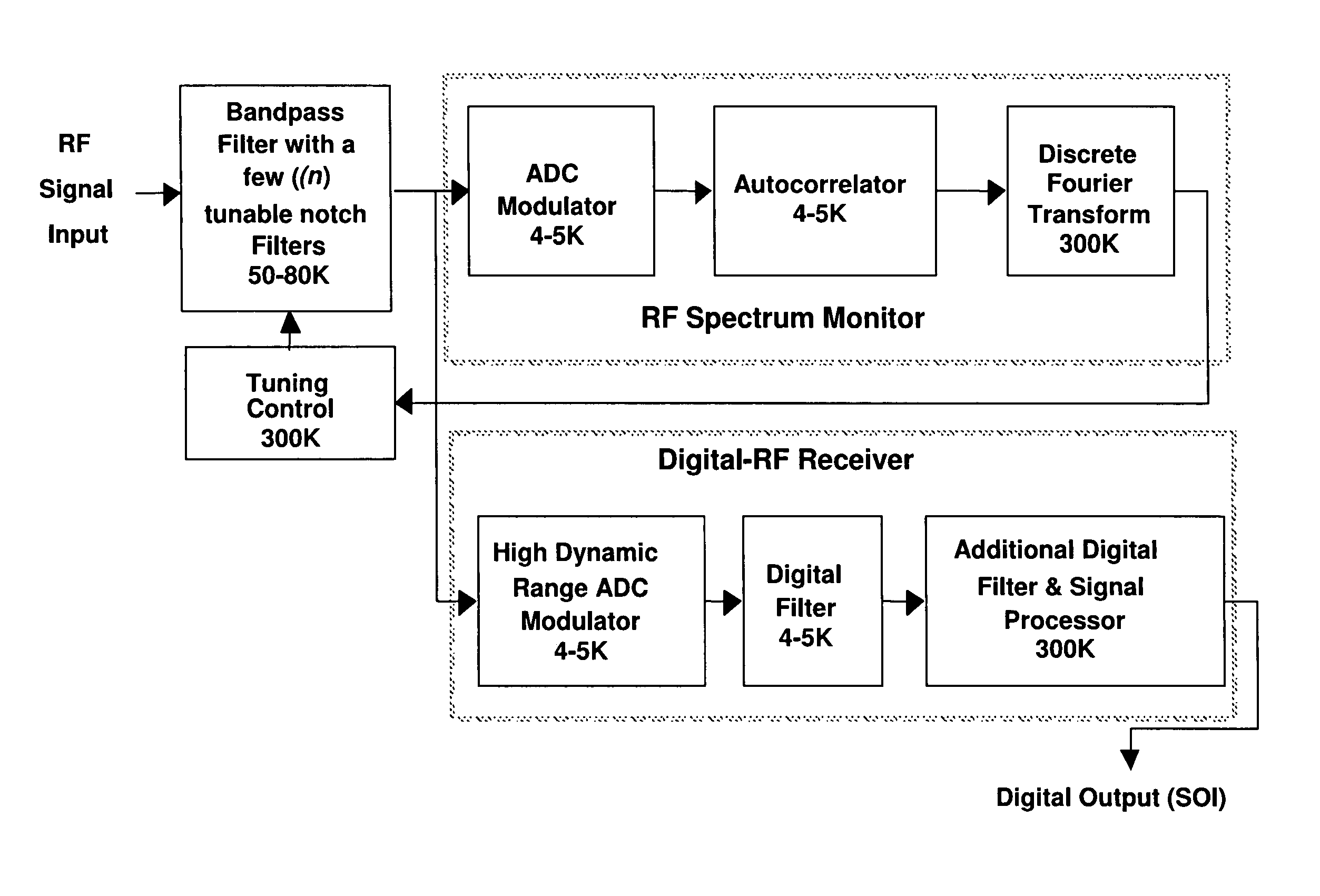
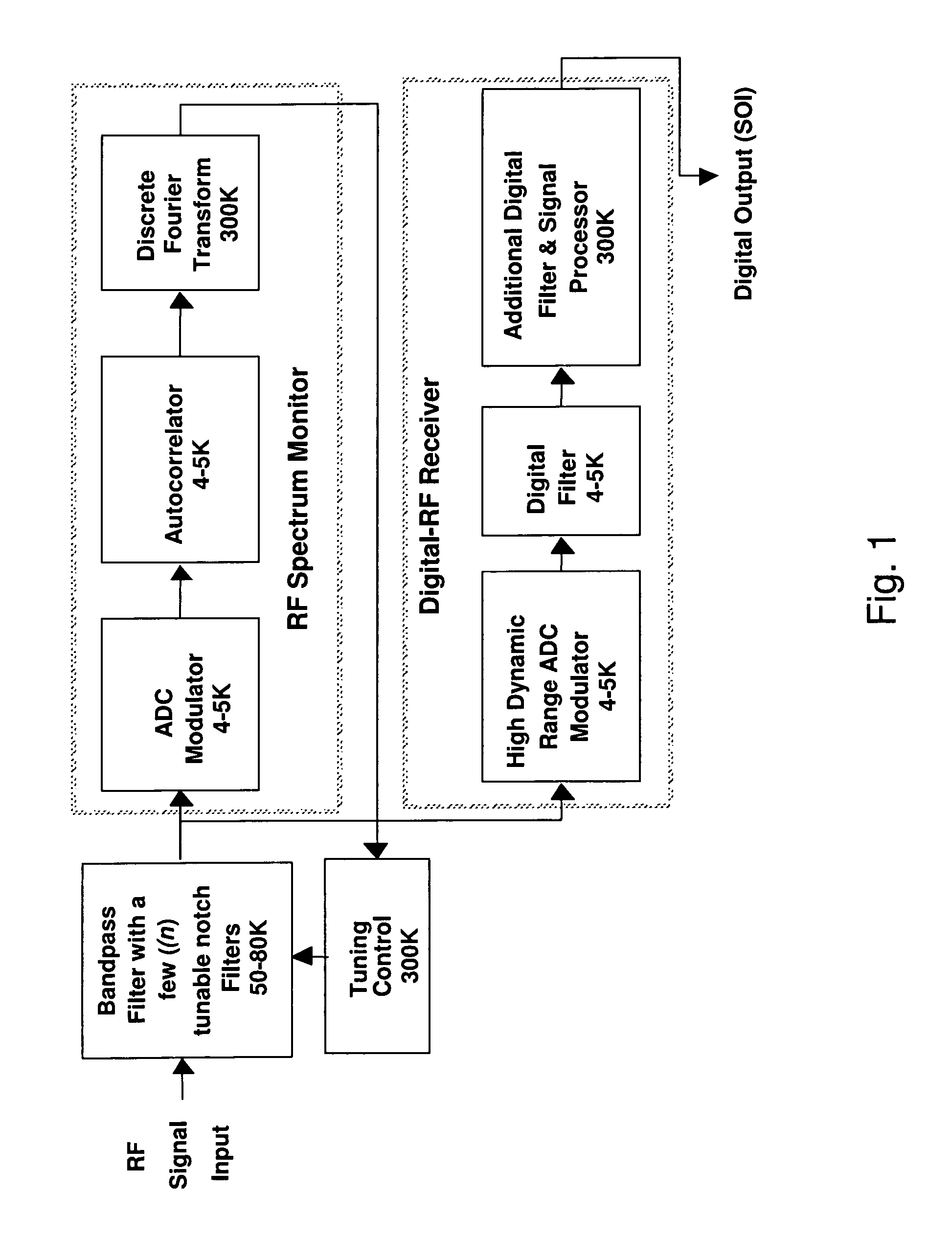
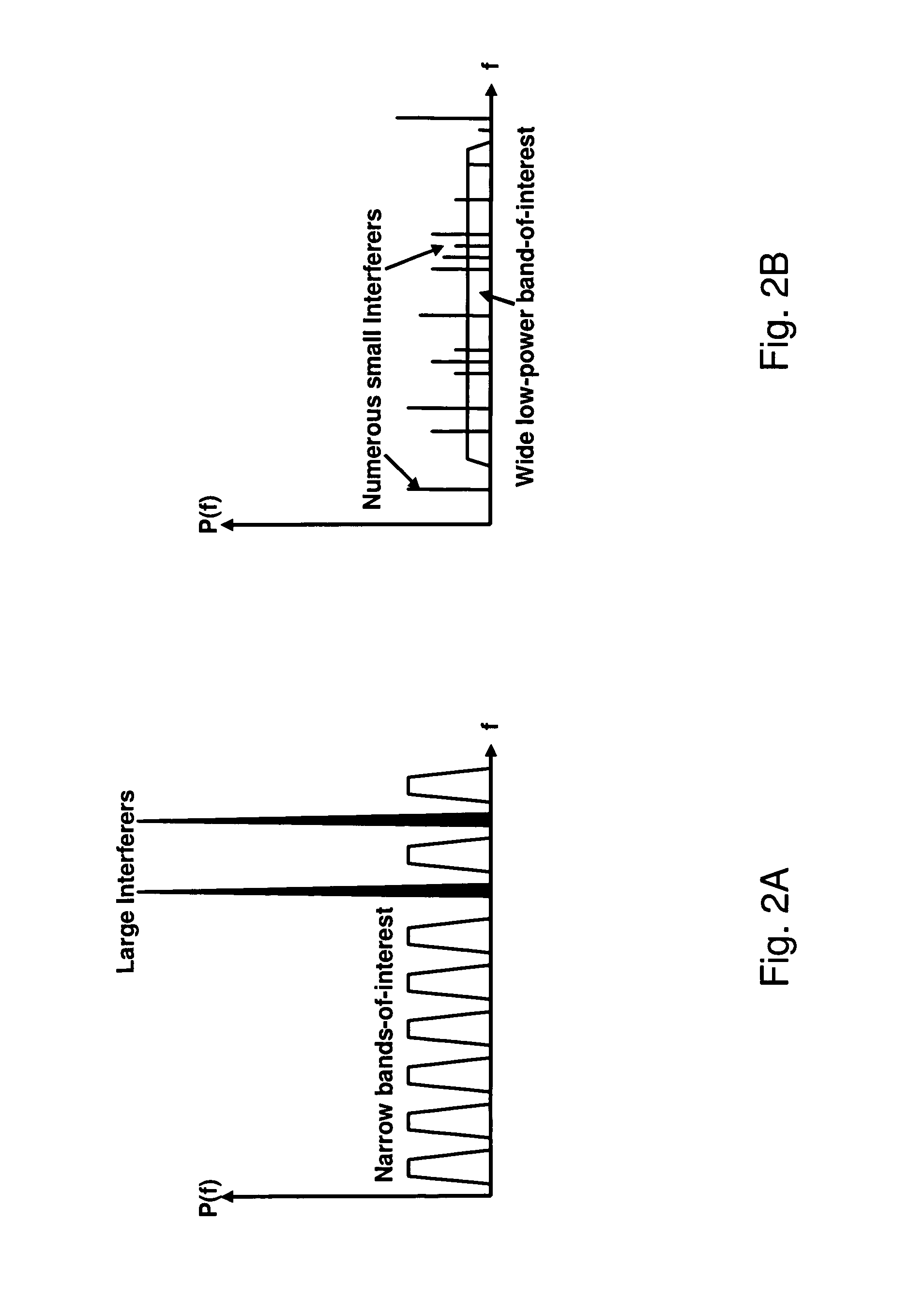
Wideband digital spectrometer
ActiveUS8045660B1Improve linearityIncrease speedTransmissionNonlinear distortionDigital signal processing
A method for reducing interference, comprising receiving a wideband signal, having at least one large amplitude component; adaptively modifying the wideband signal with respect to at least one high intensity component without substantial introduction of non-linear distortion, while reducing a residual dynamic range thereof; digitizing the modified wideband signal to capture information describing at least the high intensity signal at a sampling rate sufficient to extract modulated information present within the wideband signal at an upper limit of the band. The system analyzes a spectral characteristic of the wideband signal; and extracts adaptation parameters for the adaptive filtering. The system therefore provides both large net dynamic range and wideband operation. Preferably, the digitizer and filter, and part of the spectral characteristic analyzer is implemented in using superconducting circuit technology, with, for example low temperature superconducting digital signal processing components, and high temperature superconducting analog filtering components.
Owner:HYPRES
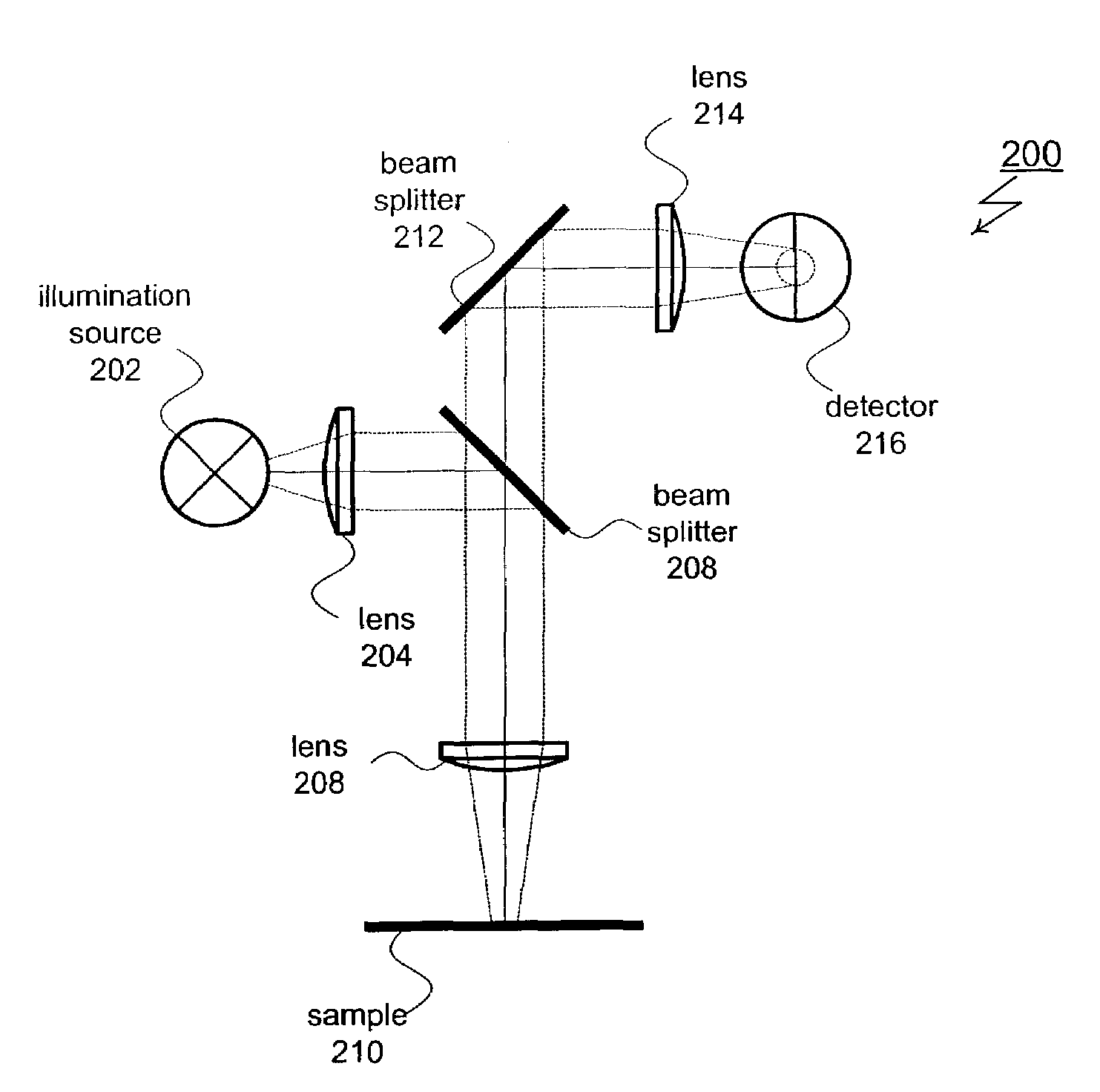
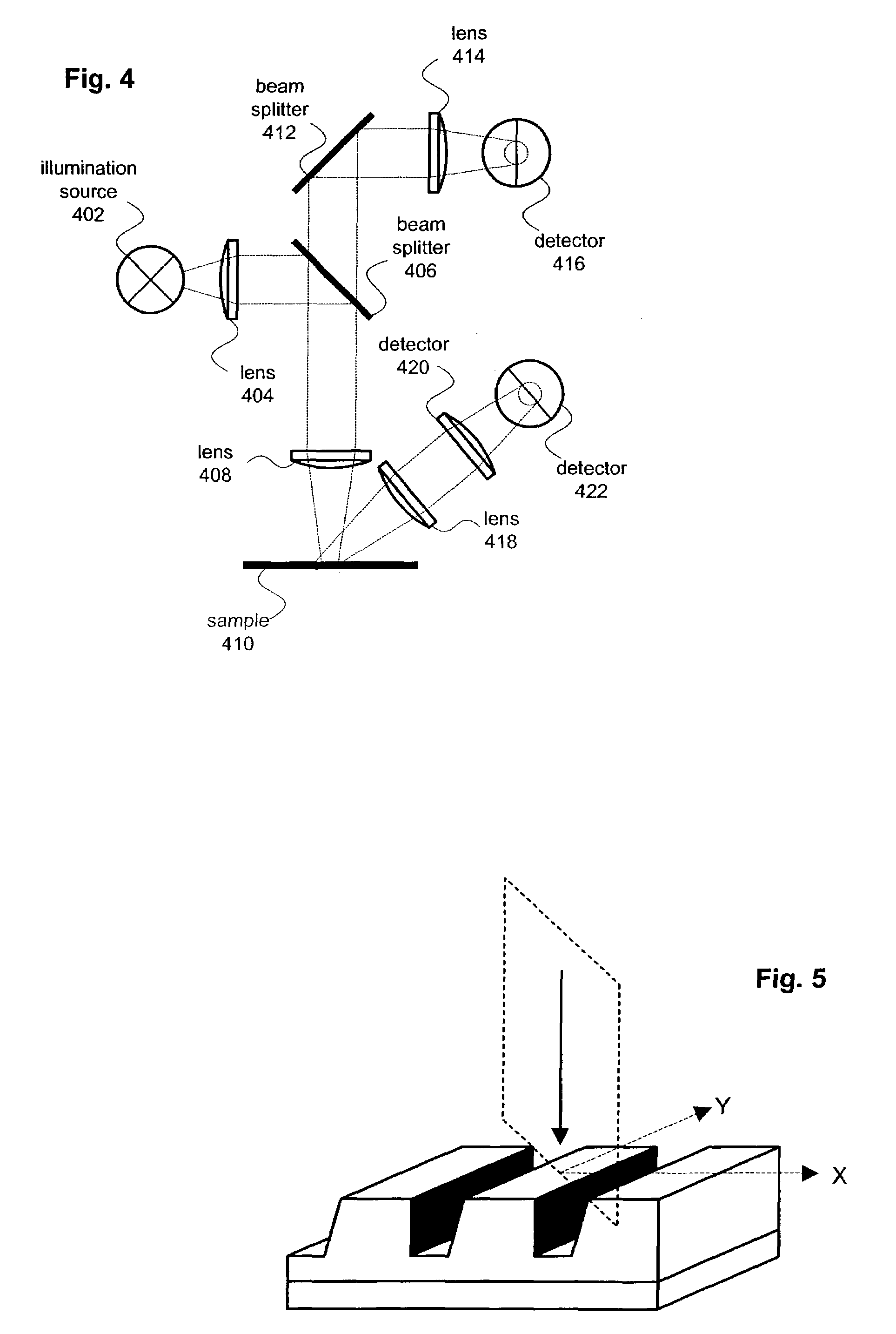
Optical scatterometry of asymmetric lines and structures
A method for analyzing asymmetric structures (including isolated and periodic structures) includes a split detector for use in a broadband spectrometer. The split has detector has separate right and left halves. By independently measuring and comparing the right and left scattered rays, information about asymmetries can be determined.
Owner:THERMA WAVE INC +1
Volatile matrices for matrix-assisted laser desorption/ionization mass spectrometry
InactiveUS6104028AEasy to spreadReduce formationSamples introduction/extractionWithdrawing sample devicesThermal ionization mass spectrometryRoom temperature
A sample preparation method is disclosed for volatilization and mass spectrometric analysis of nonvolatile high molecular weight molecules. Photoabsorbing molecules having significant sublimation rates at room temperature under vacuum, and preferably containing hydroxy functionalities, are disclosed for use as matrices in matrix-assisted laser desorption / ionization mass spectrometry. The samples are typically cooled in the mass spectrometer to temperatures significantly below room temperature.
Owner:AGENA BIOSCI
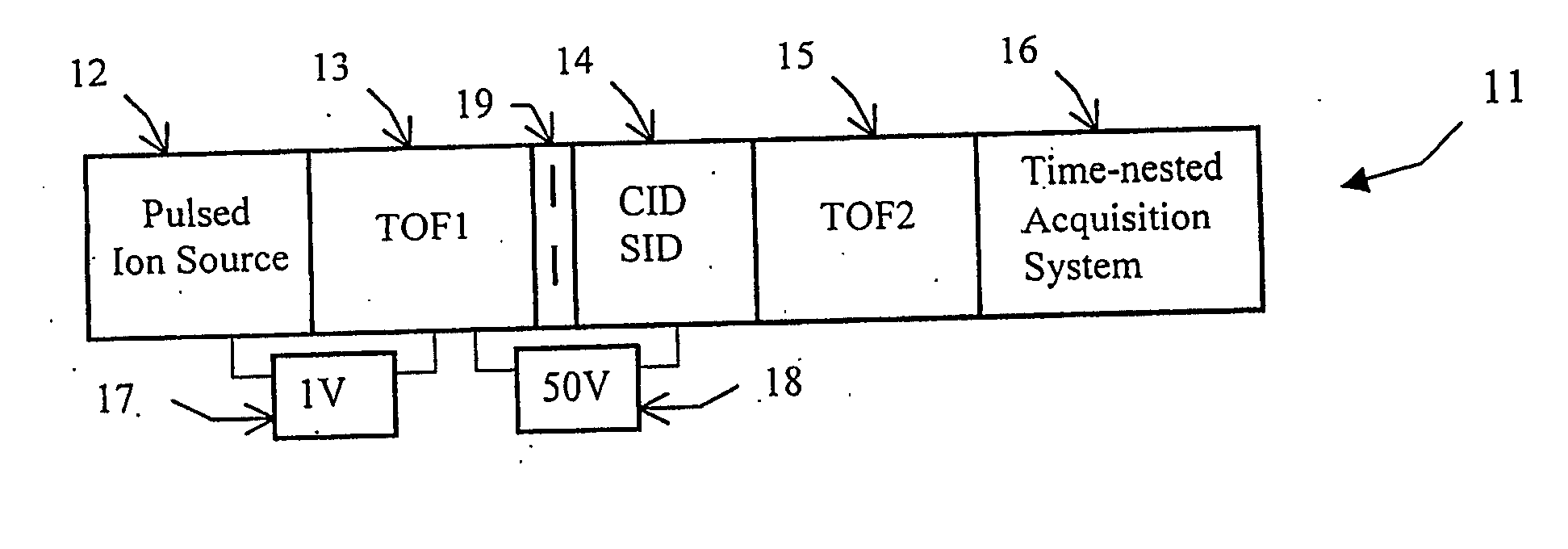
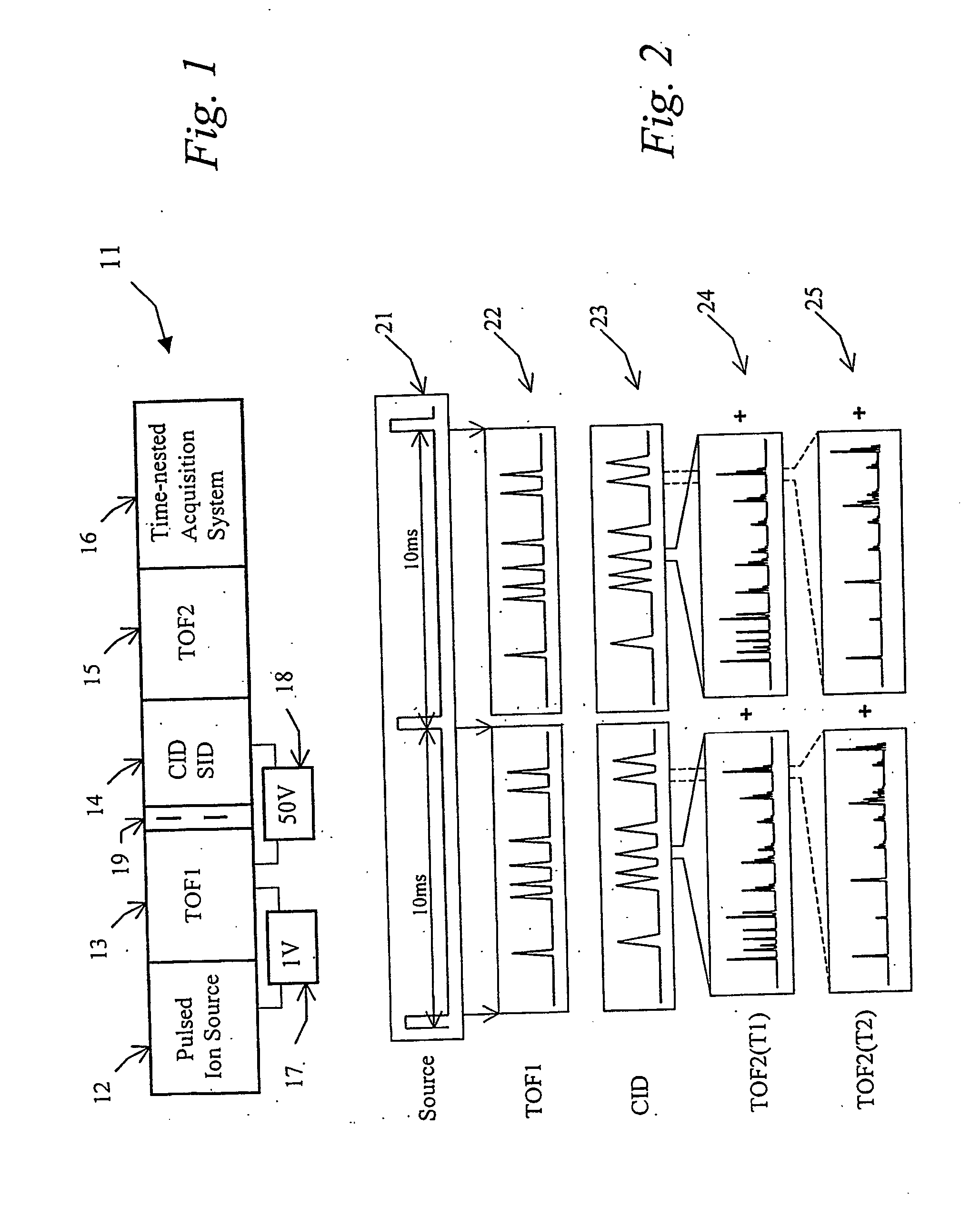
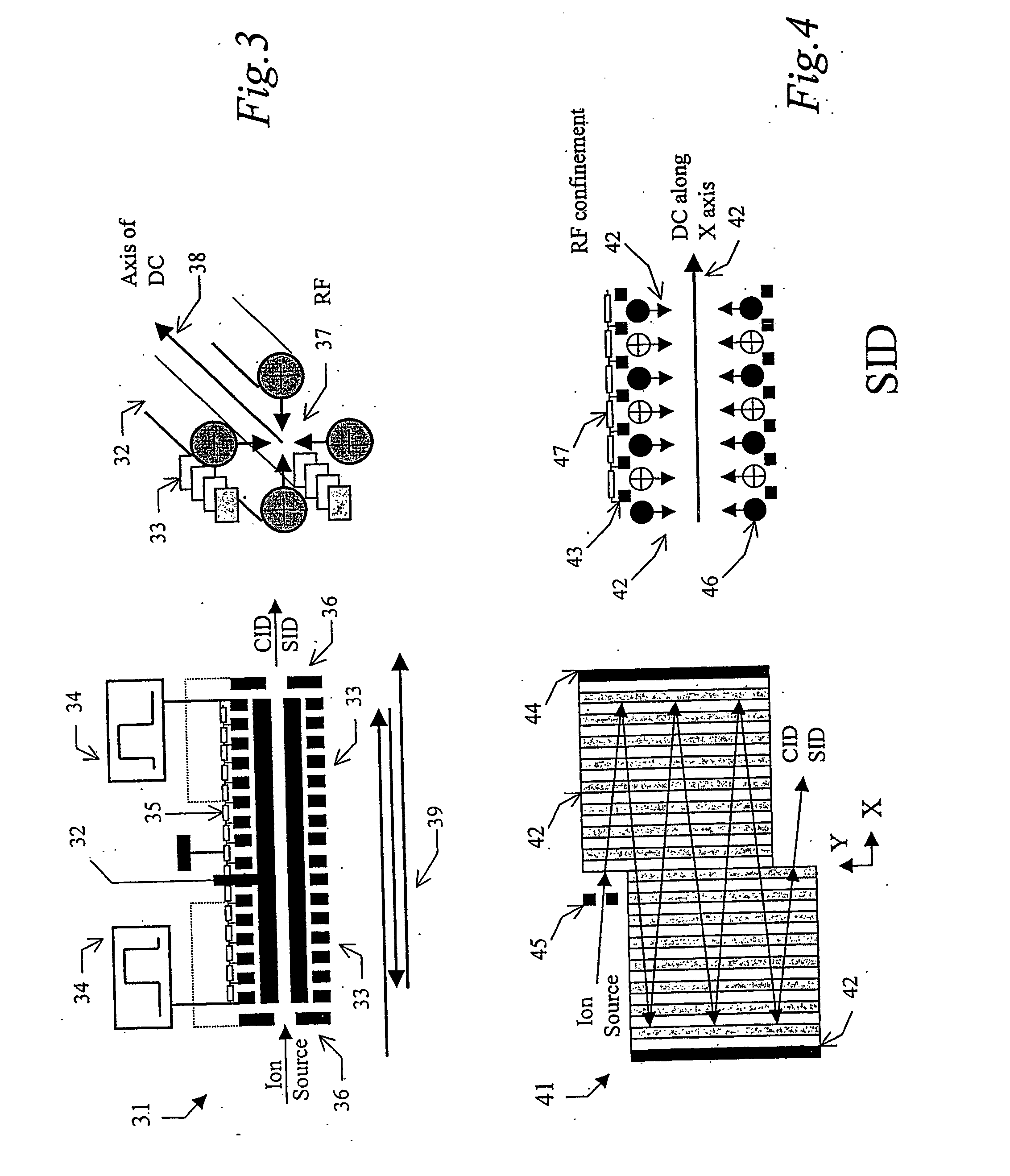
Tandem time of flight mass spectrometer and method of use
InactiveUS20050242279A1Rapid MS-MS analysisEasy to separateTime-of-flight spectrometersIsotope separationRelative energyMass analyzer
To provide comprehensive (i.e. rapid and sensitive) MS-MS analysis, the inventor employs a time-nested separation, using two time-of-flight (TOF) mass spectrometers. Parent ions are separated in a slow and long TOF1, operating at low ion energy (1 to 100 eV), and fragment ions are mass analyzed in a fast and short TOF2, operating at much higher keV energy. Low energy fragmentation cell between TOF1 and TOF2 is tailored to accelerate fragmentation and dampening steps, mostly by shortening the cell and employing higher gas pressure. Since separation in TOF1 takes milliseconds and mass analysis in TOF2—microseconds, the invention provides comprehensive MS-MS analysis of multiple precursor ions per single ion pulse. Slow separation in TOF1 becomes possible with an introduction of novel TOF1 analyzers. The TOF-TOF could be implemented using a static TOF1, here described on the examples of spiratron, planar and cylindrical multi-pass separators with griddles spatial focusing ion mirrors. Higher performance is expected with the use of novel hybrid TOF1 analyzers, combining radio frequency (RF) and quadratic DC fields. RF field retains low-energy ions within TOF1 analyzer, while quadratic DC field improves resolution by compensate for large relative energy spread.
Owner:LECO CORPORATION
Mass spectrometer
InactiveUS6906319B2High sensitivityIncrease delay timeStability-of-path spectrometersTime-of-flight spectrometersIon trap mass spectrometryMass analyzer
A mass spectrometer is disclosed wherein ions having a particular desired charge state are selected by operating an ion mobility spectrometer in combination with a quadrupole mass filter. Precursor ions are fragmented or reacted to form product ions in a collision cell ion trap and sent back upstream to an upstream ion trap. The fragment or product ions are then passed through the ion mobility spectrometer wherein they become temporally separated according to their ion mobility. Fragment or product ions are then re-trapped in the collision cell ion trap before being released therefrom in packets. A pusher electrode of a time of flight mass analyzer is energized a predetermined period of time after a packet of ions is released from the collision cell ion trap. Accordingly, it is possible to select multiply charged precursor ions from a background of singly charged ions, fragment them, and mass analyze the fragment ions with a near 100% duty cycle across the whole mass range.
Owner:MICROMASS UK LTD
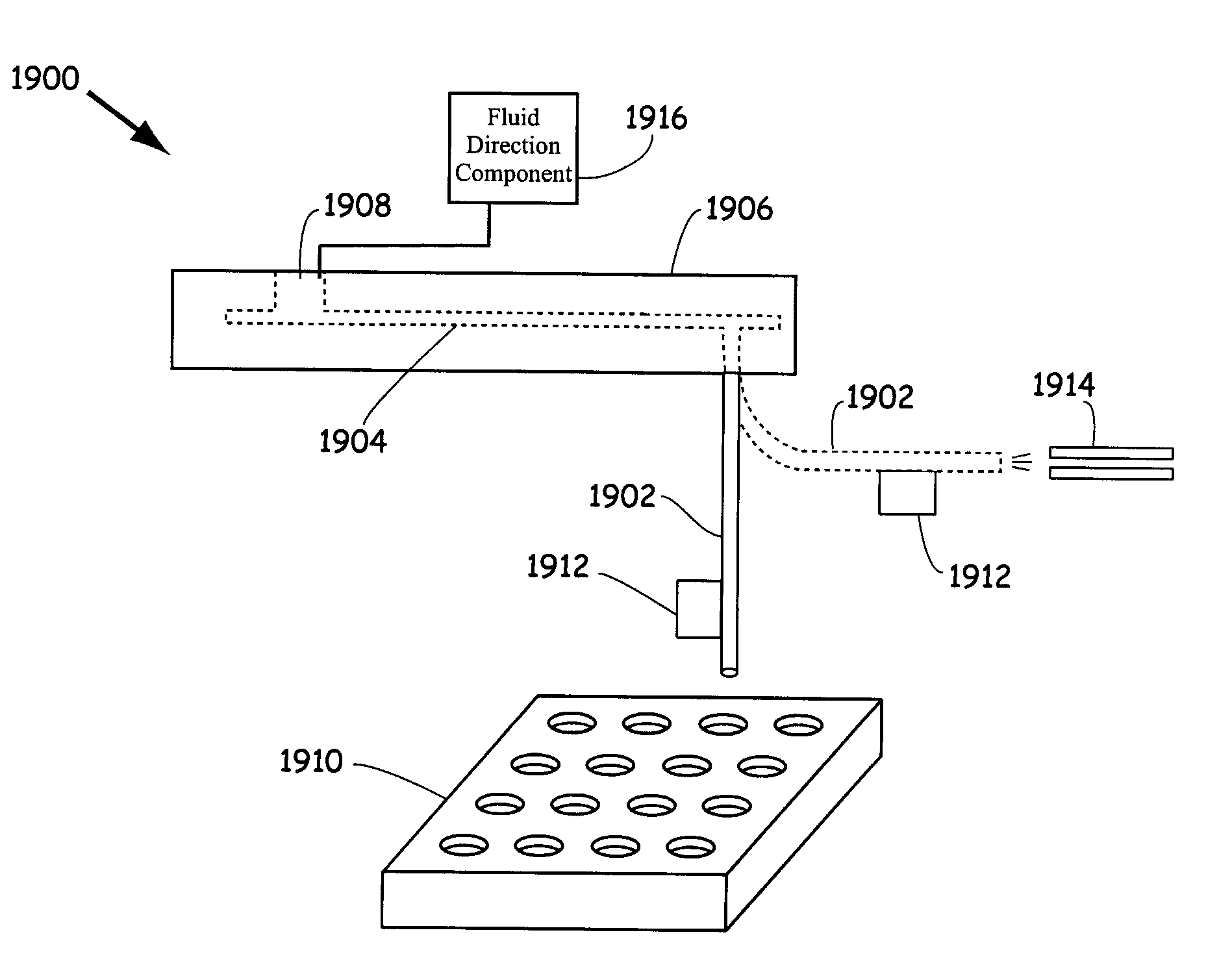
Systems and methods for preparing and analyzing low volume analyte array elements
InactiveUS7285422B1Rapid productionLess-expensive to employPeptide librariesSequential/parallel process reactionsAnalyteMass Spectrometry-Mass Spectrometry
The invention provides methods for dispensing tools that can be employed to generate multi-element arrays of sample material on a substrate surface. The substrates surfaces can be flat or geometrically altered to include wells of receiving material. The tool can dispense a spot of fluid to a substrate surface by spraying the fluid from the pin, contacting the substrate surface or forming a drop that touches against the substrate surface. The tool can form an array of sample material by dispensing sample material in a series of steps, while moving the pin to different locations above the substrate surface to form the sample array. The invention then passes the prepared sample arrays to a plate assembly that disposes the sample arrays for analysis by mass spectrometry. To this end, a mass spectrometer is provided that generates a set of spectra signal which can be understood as indicative of the composition of the sample material under analysis.
Owner:AGENA BIOSCI
Mass spectrometer system
ActiveUS20050063864A1Reduce adverse effectsSamples introduction/extractionIsotope separationStructure analysisSpectroscopy
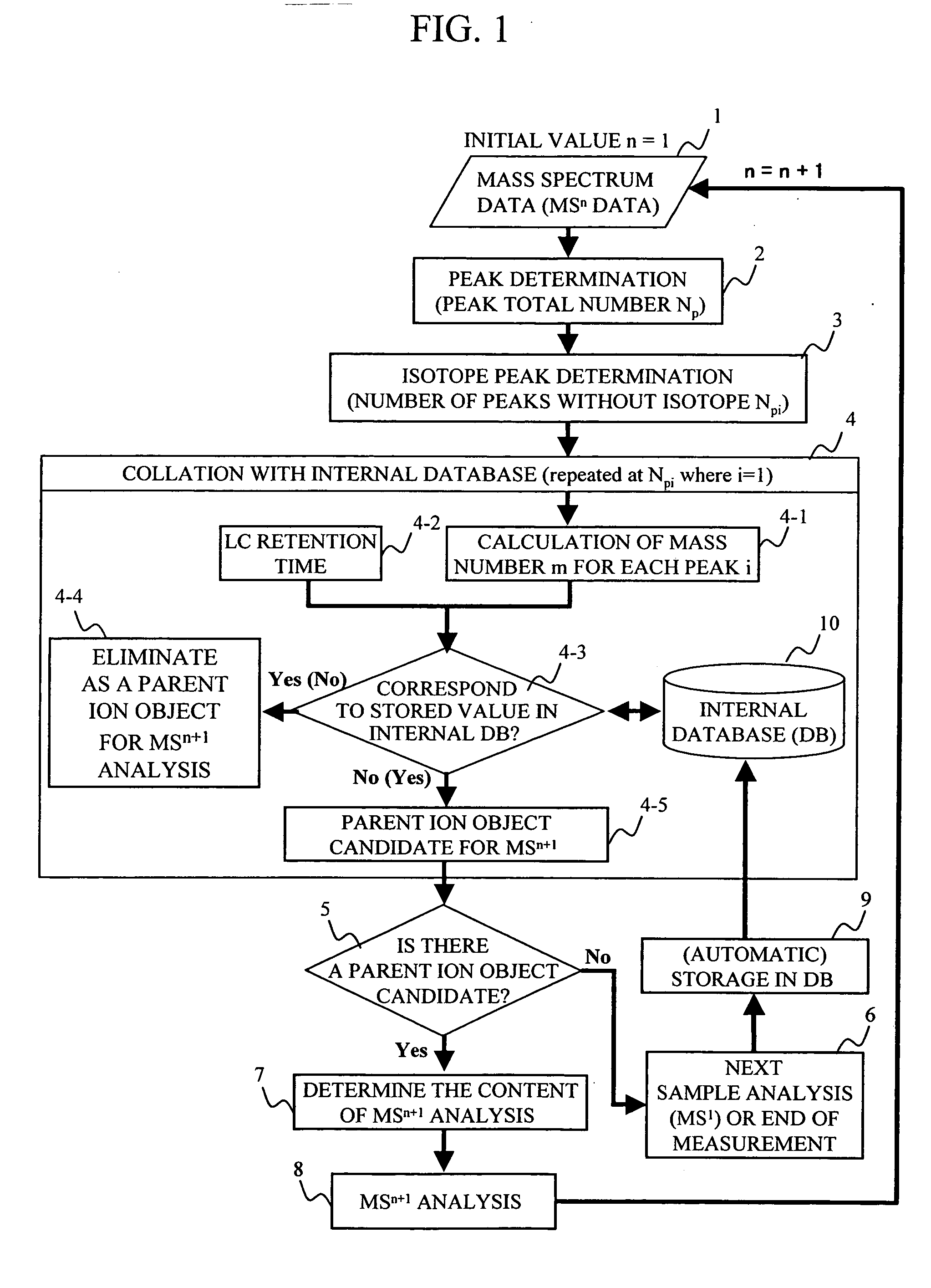
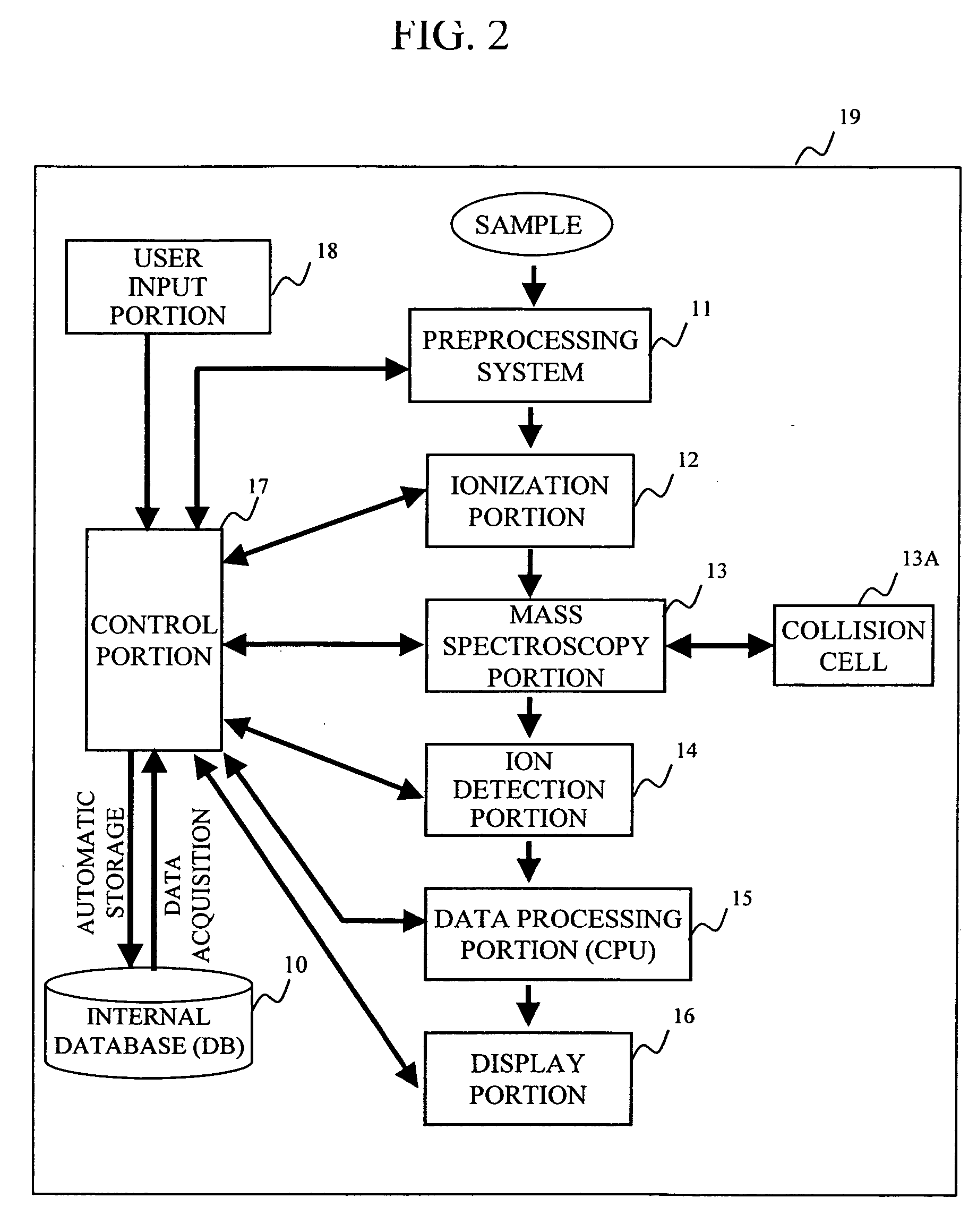
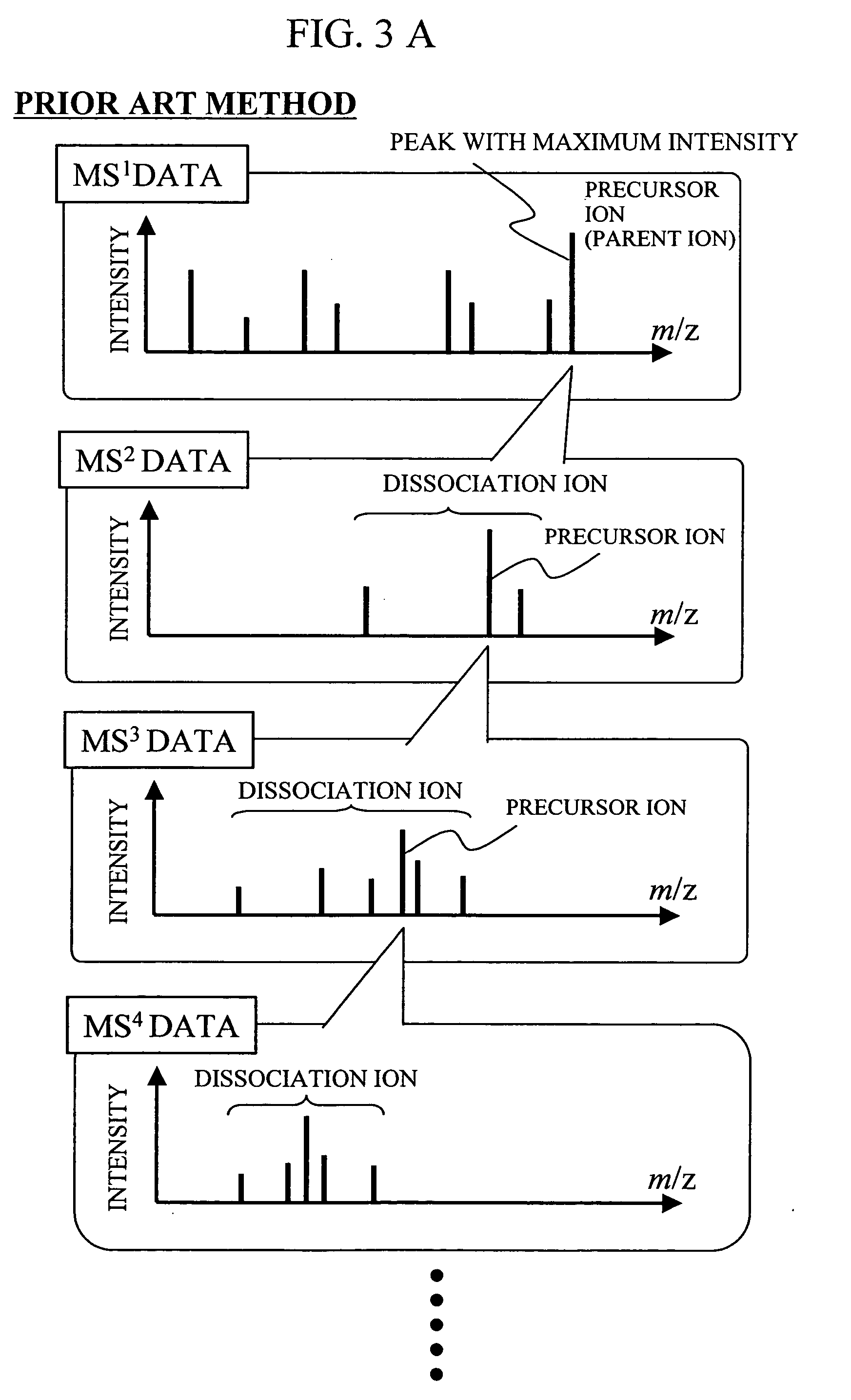
During the structural analysis of a protein or peptide by tandem mass spectroscopy, a peptide ion derived from a protein that has already been measured and that is expressed in great quantities is avoided as a tandem mass spectroscopy target. A peptide derived from a minute amount of protein, which has heretofore been difficult to analyze, can be automatically determined as a tandem mass spectroscopy target within the real time of measurement. Data concerning a protein that has already been measured and a peptide derived from the protein is automatically stored in an internal database. The stored data is collated with measured data with high accuracy to determine an isotope peak. In this way, the process of selecting a peptide peak that has not been measured as the target for the next tandem analysis can be performed within the real time of measurement and a redundant measurement of peptides derived from the same protein can be avoided. The information contained in the MSn spectrum is effectively utilized in each step of the MSn involving a multi-stage dissociation and mass spectroscopy (MSn), so that the flows for the determination of the next analysis content and the selection of the parent ion for the MSn+1 analysis, for example, can be optimized within the real time of measurement and with high efficiency and accuracy. Thus, a target of concern to the user can be subjected to tandem mass spectroscopy without wasteful measurement.
Owner:HITACHI HIGH-TECH CORP
Multi-reflecting time-of-flight mass spectrometer with isochronous curved ion interface
ActiveUS20060214100A1Time-of-flight spectrometersIsotope separationIon trap mass spectrometryIon transfer
The present invention relates generally to a multi-reflecting time-of-flight mass spectrometer (MR TOF MS). To improve mass resolving power of a planar MR TOF MS, a spatially isochronous and curved interface may be used for ion transfer in and out of the MR TOF analyzer. One embodiment comprises a planar grid-free MR TOF MS with periodic lenses in the field-free space, a linear ion trap for converting ion flow into pulses and a C-shaped isochronous interface made of electrostatic sectors. The interface allows transferring ions around the edges and fringing fields of the ion mirrors without introducing significant time spread. The interface may also provide energy filtering of ion packets. The non-correlated turn-around time of ion trap converter may be reduced by using a delayed ion extraction from the ion trap and excessive ion energy is filtered in the curved interface.
Owner:LECO CORPORATION
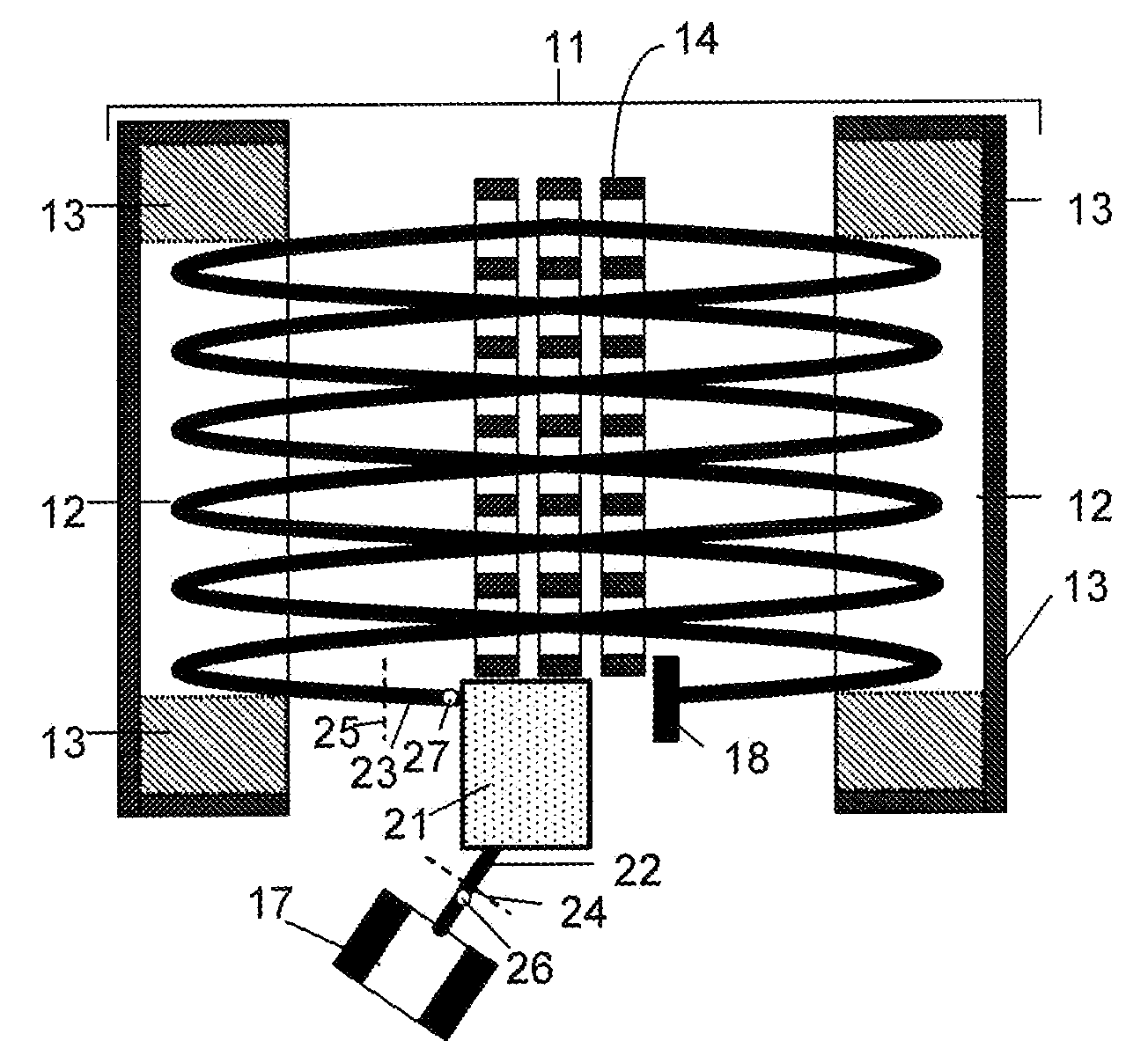
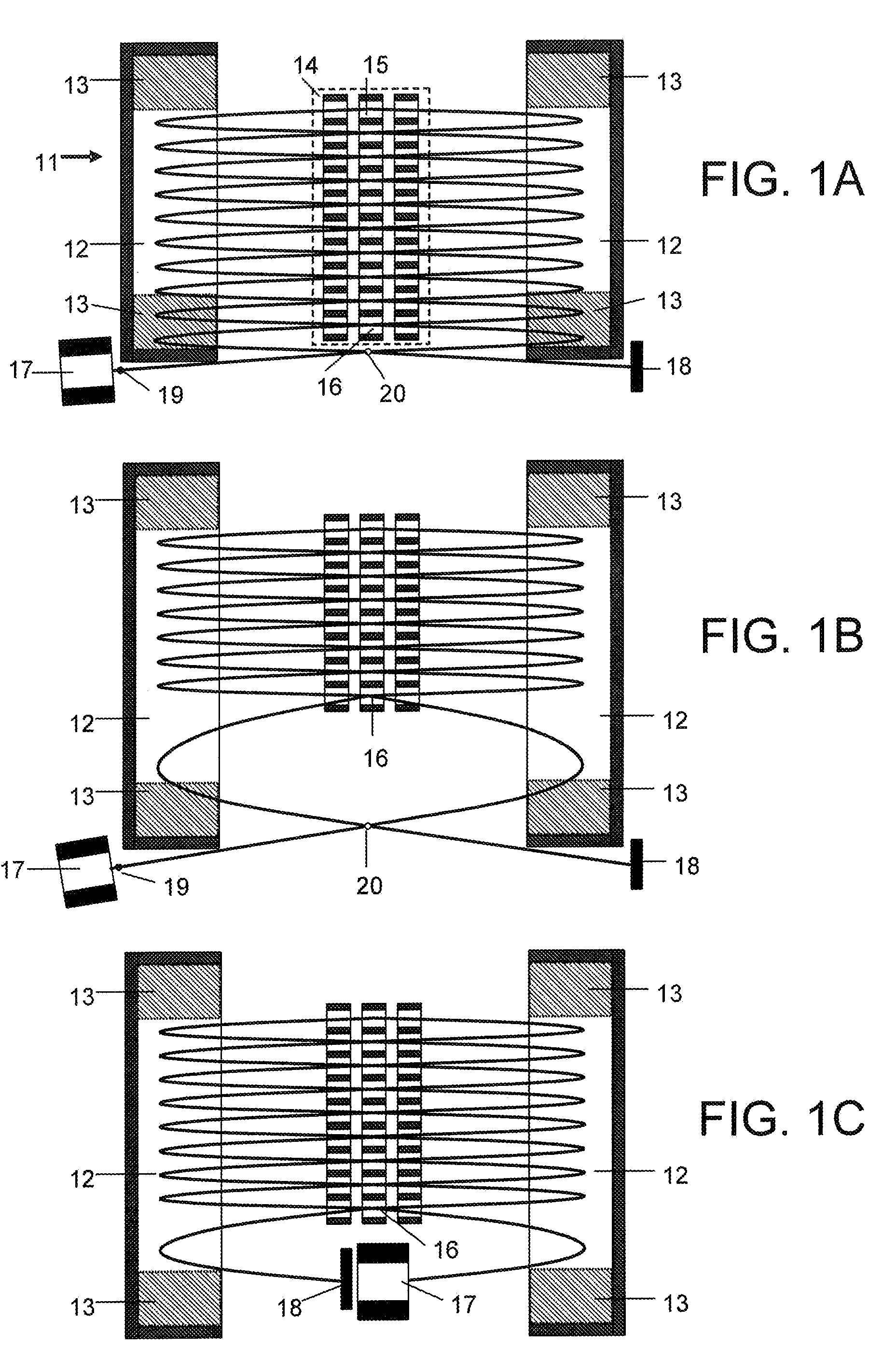
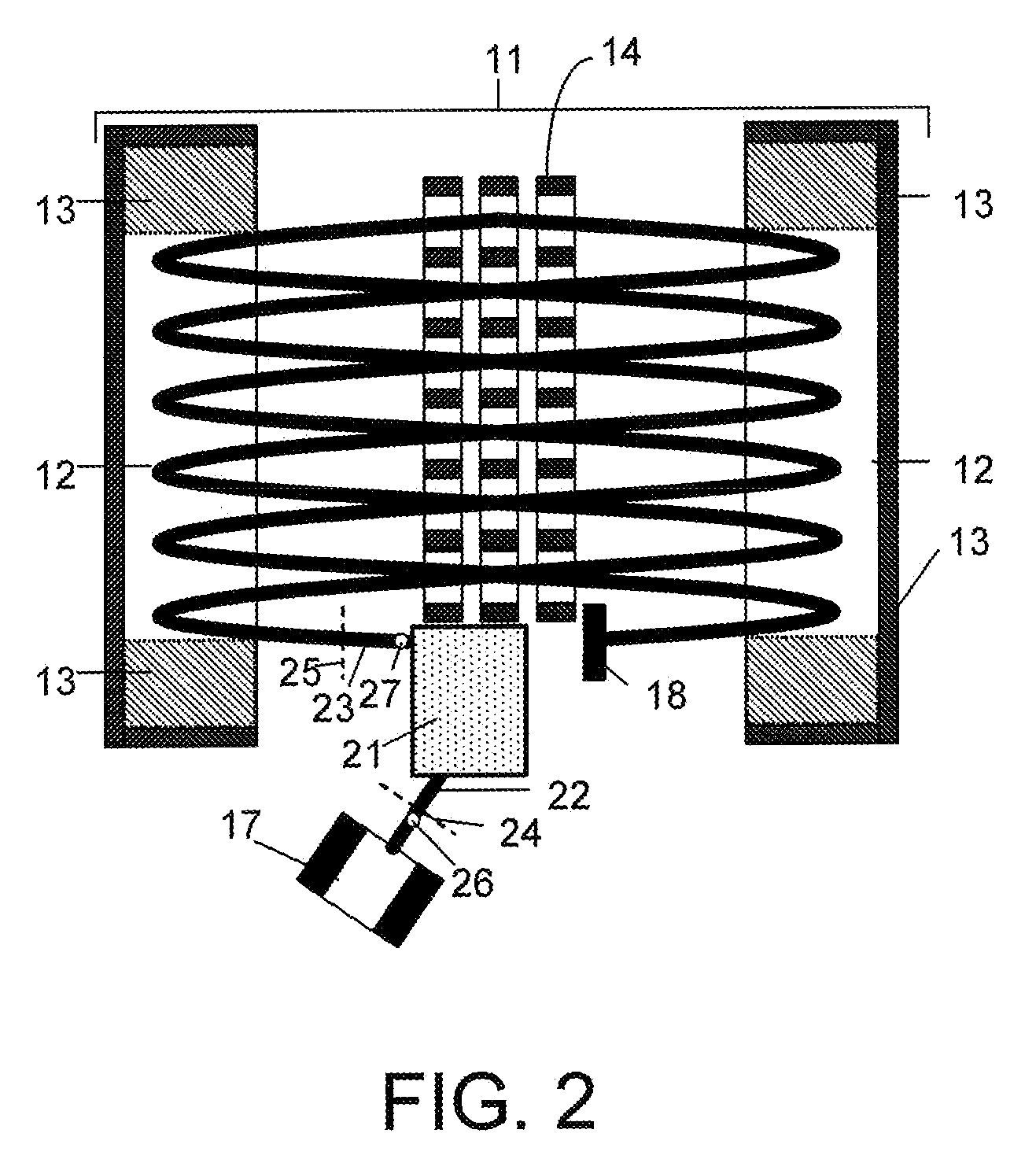
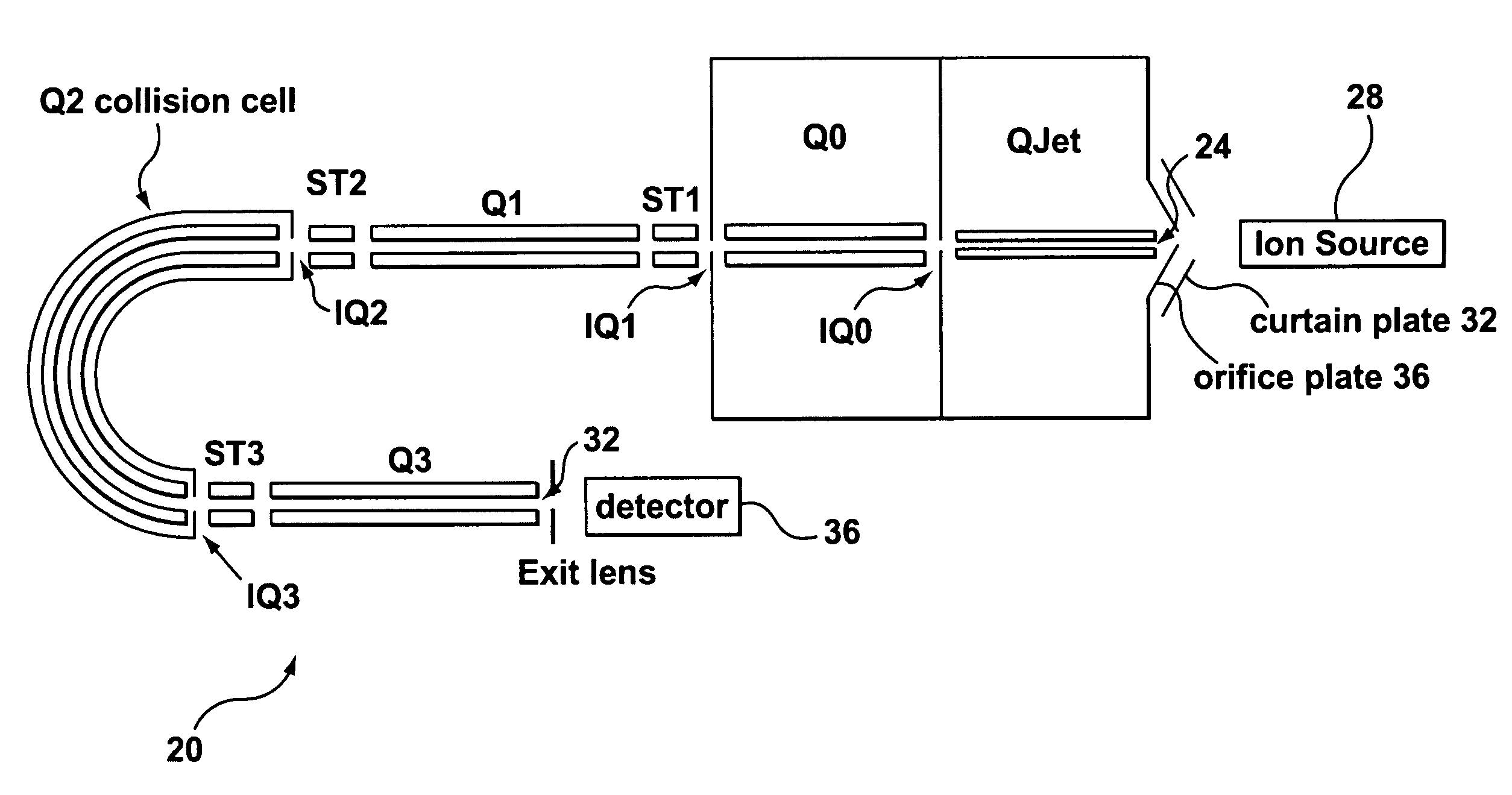
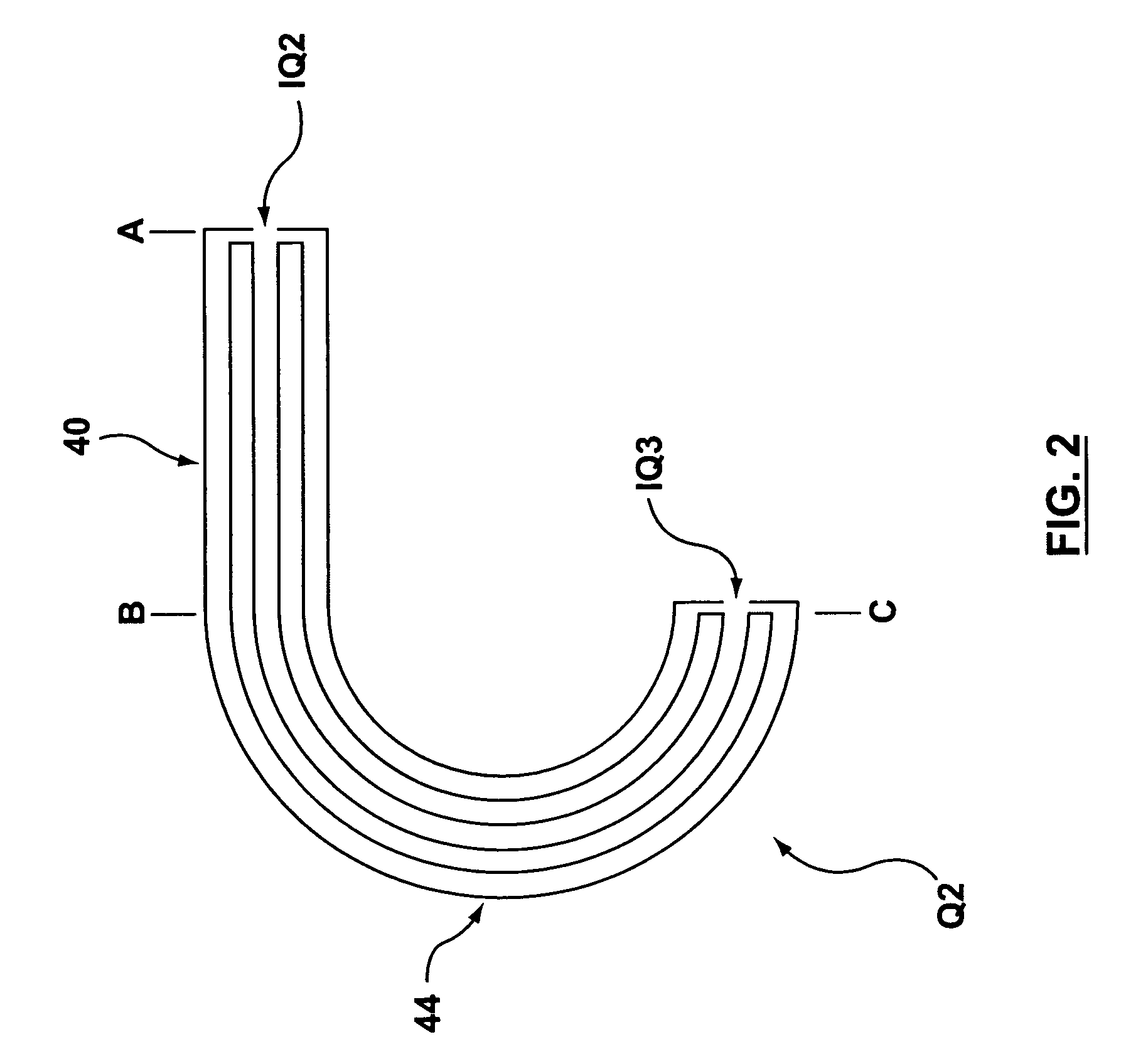
Collision cell for mass spectrometer
A novel curved collision cell for a mass spectrometer is described. The collision cell includes a straight section having a length that is selected to cause a precursor ion entering the straight section to lose a desired amount of kinetic energy such that when the precursor ion enters the curved section of the collision cell the precursor ion will tend to neither escape nor contact the collision cell, and thereby tending to survive its passage within the curved portion.
Owner:DH TECH DEVMENT PTE
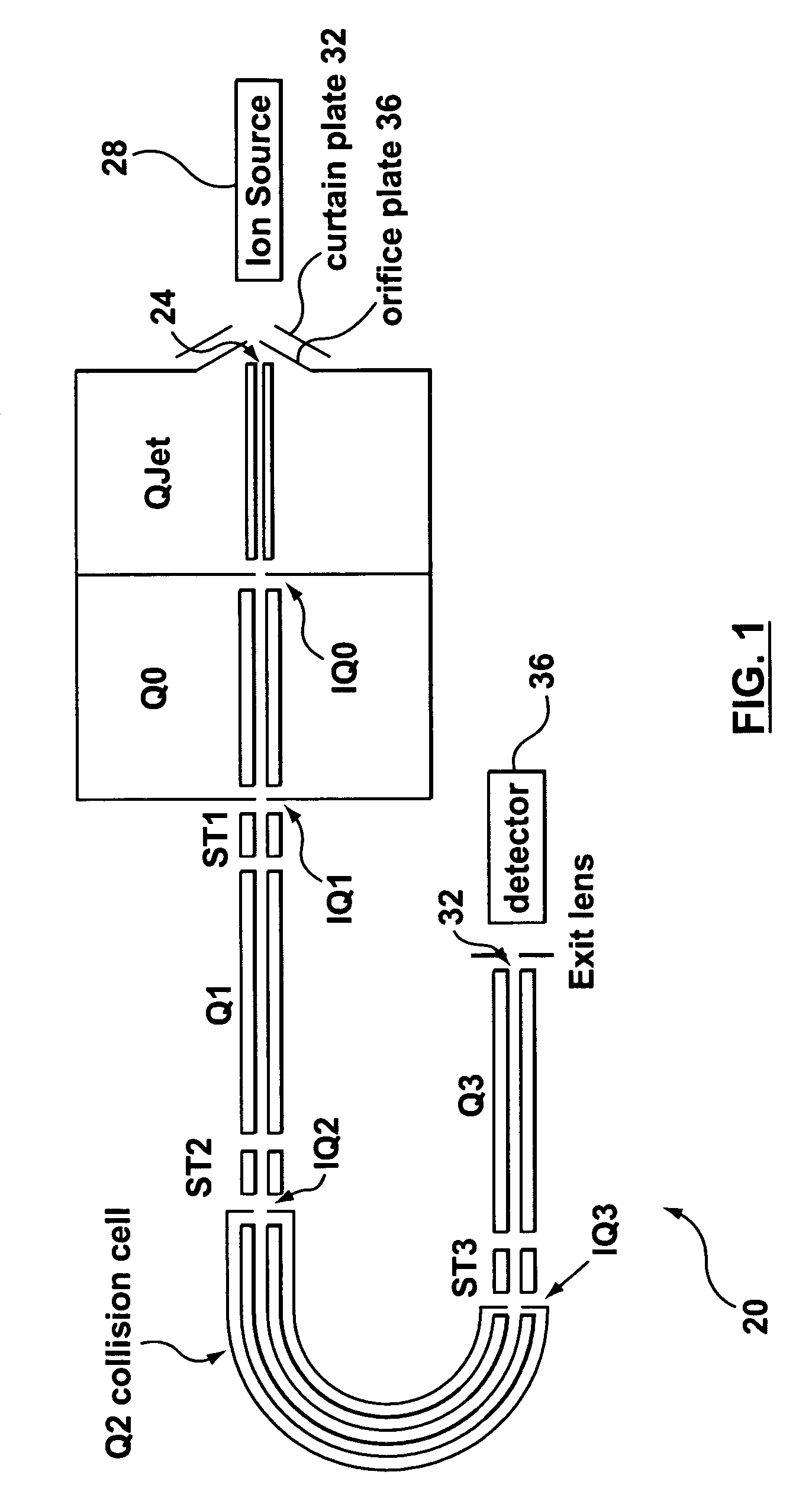
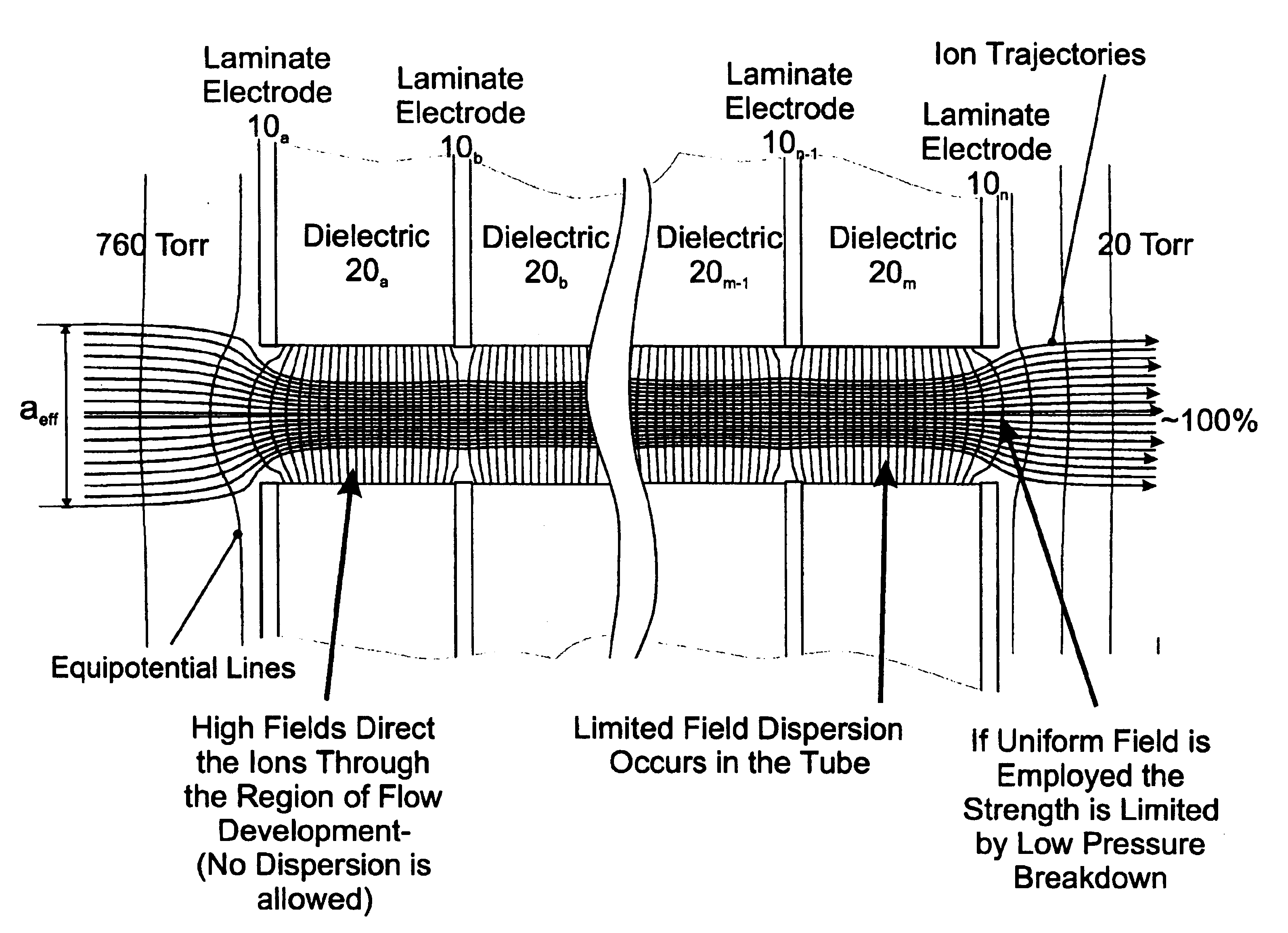
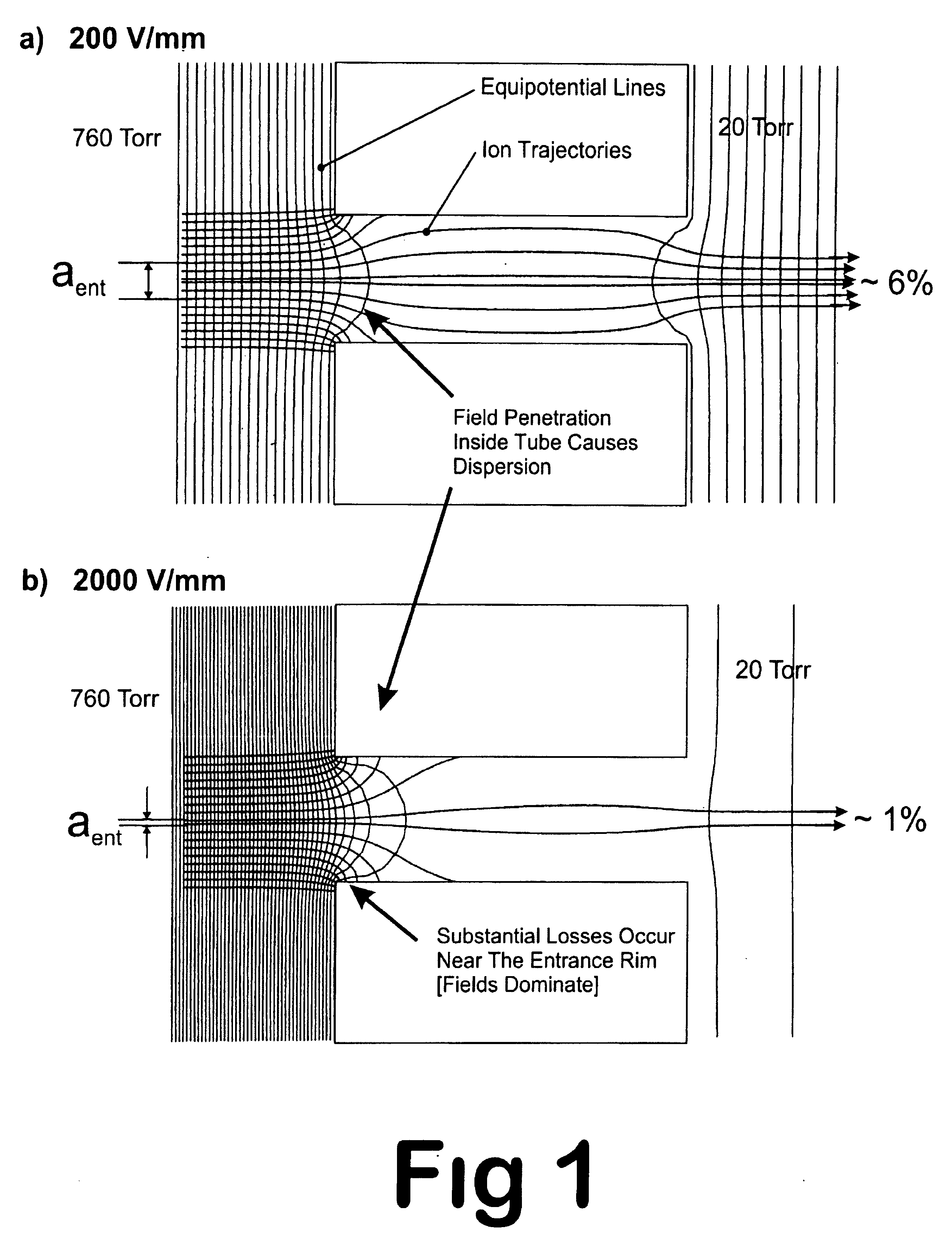
Laminated tube for the transport of charged particles contained in a gaseous medium
InactiveUS6943347B1Reduce gas loadControl flowElectron/ion optical arrangementsIsotope separationGas phaseMass Spectrometry-Mass Spectrometry
An improved tube for accepting gas-phase ions and particles contained in a gas by allowing substantially all the gas-phase ions and gas from an ion source at or greater than atmospheric pressure to flow into the tube and be transferred to a lower pressure region. Transport and motion of the ions through the tube is determined by a combination of viscous forces exerted on the ions by the flowing gas molecules and electrostatic forces causing the motion of the ions through the tube and away from the walls of the tube. More specifically, the tube is made up of stratified elements, wherein DC potentials are applied to the elements so that the DC voltage on any element determines the electric potential experience by the ions as they pass through the tube. A precise electrical gradient is maintained along the length of the stratified tube to insure the transport of the ions. Embodiments of this invention are methods and devices for improving the sensitivity of mass spectrometry or ion mobility spectrometers when coupled to atmospheric and above atmospheric pressure ionization sources. An alternate embodiment of this invention applies an AC voltage to one or more of the conducting elements in the laminate.
Owner:CHEM SPACE ASSOIATES
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com