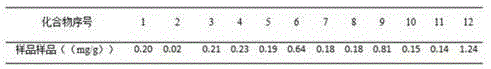
Patents
Literature
120 results about "Calycosin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
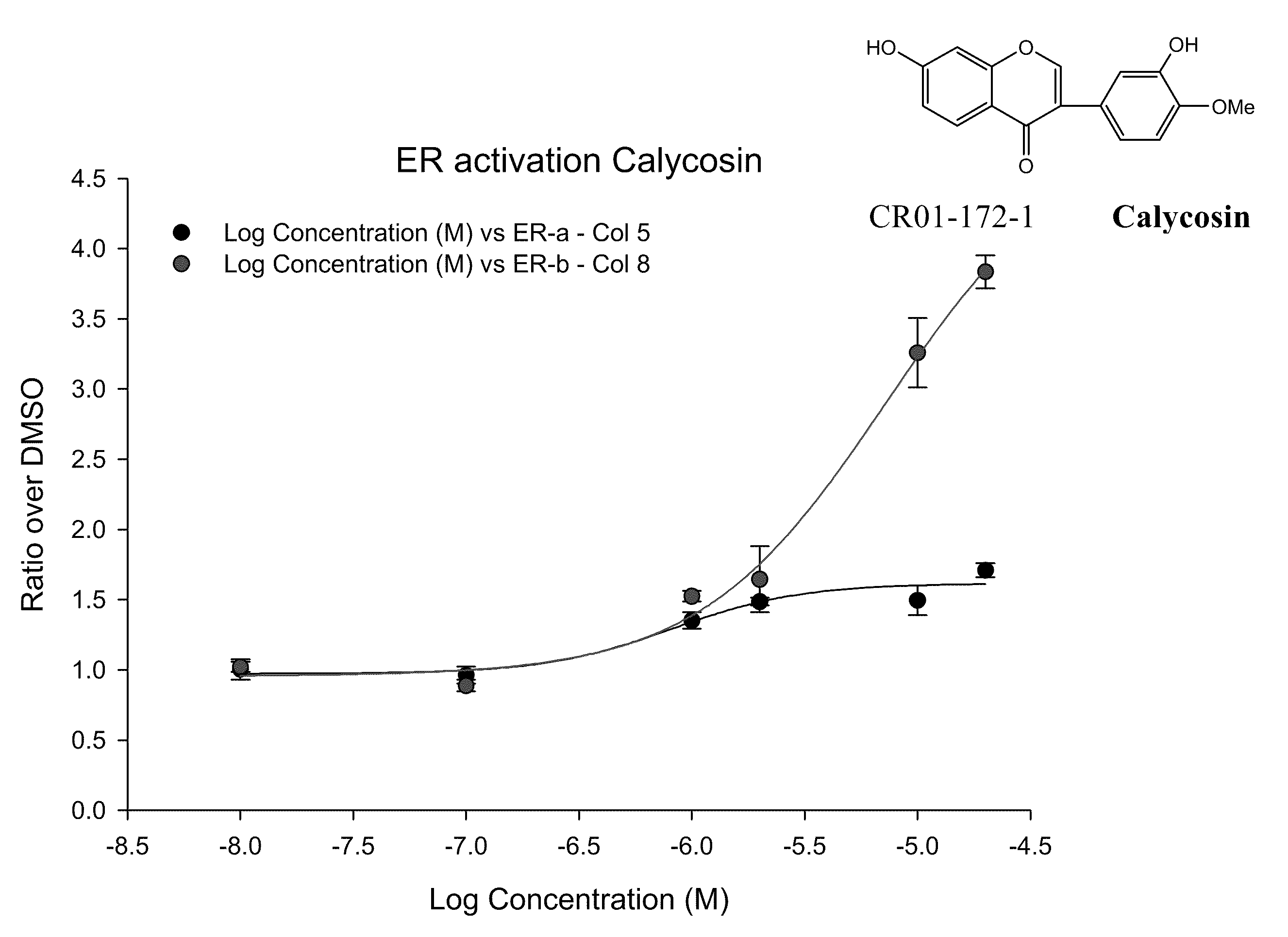
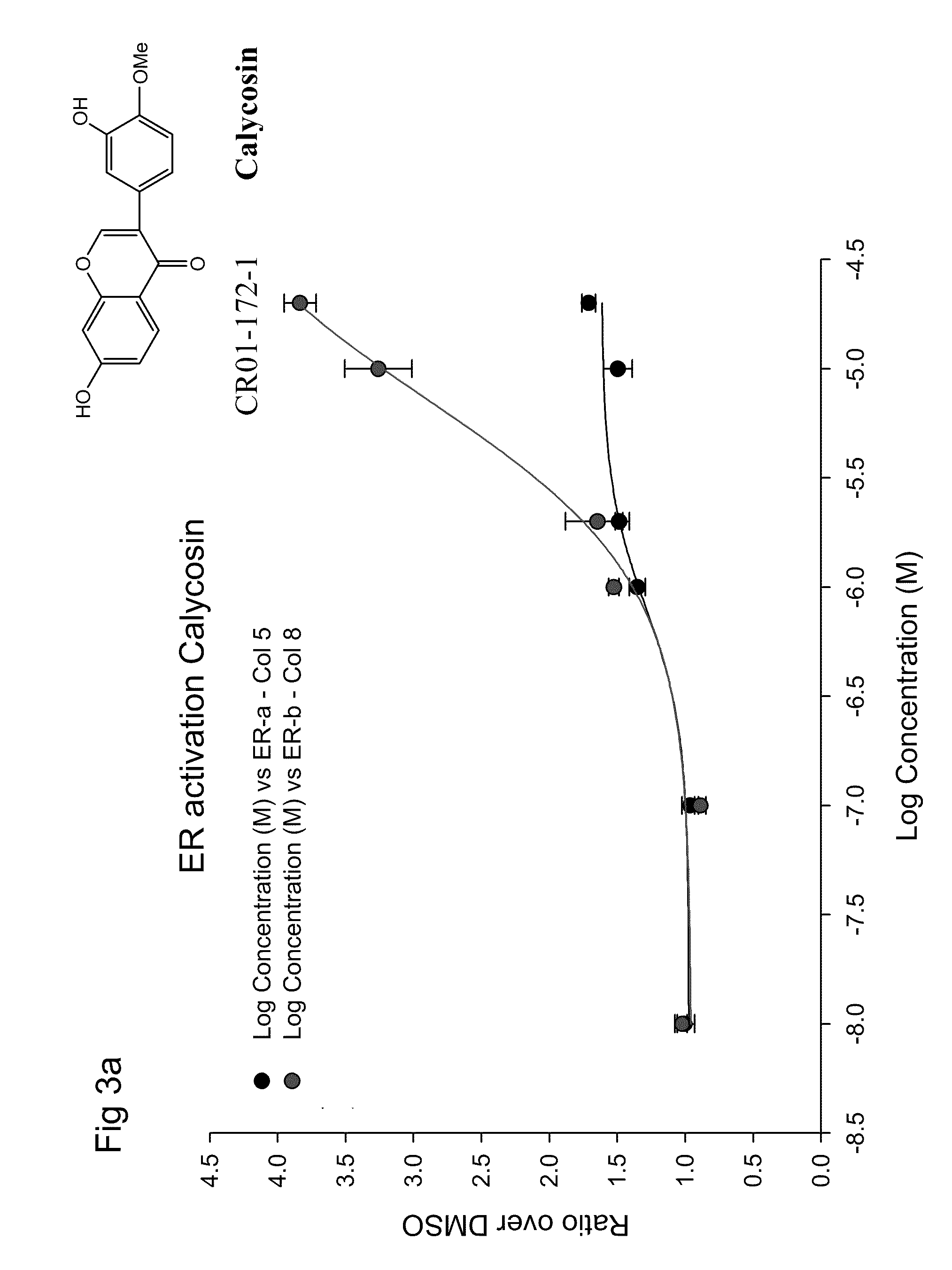
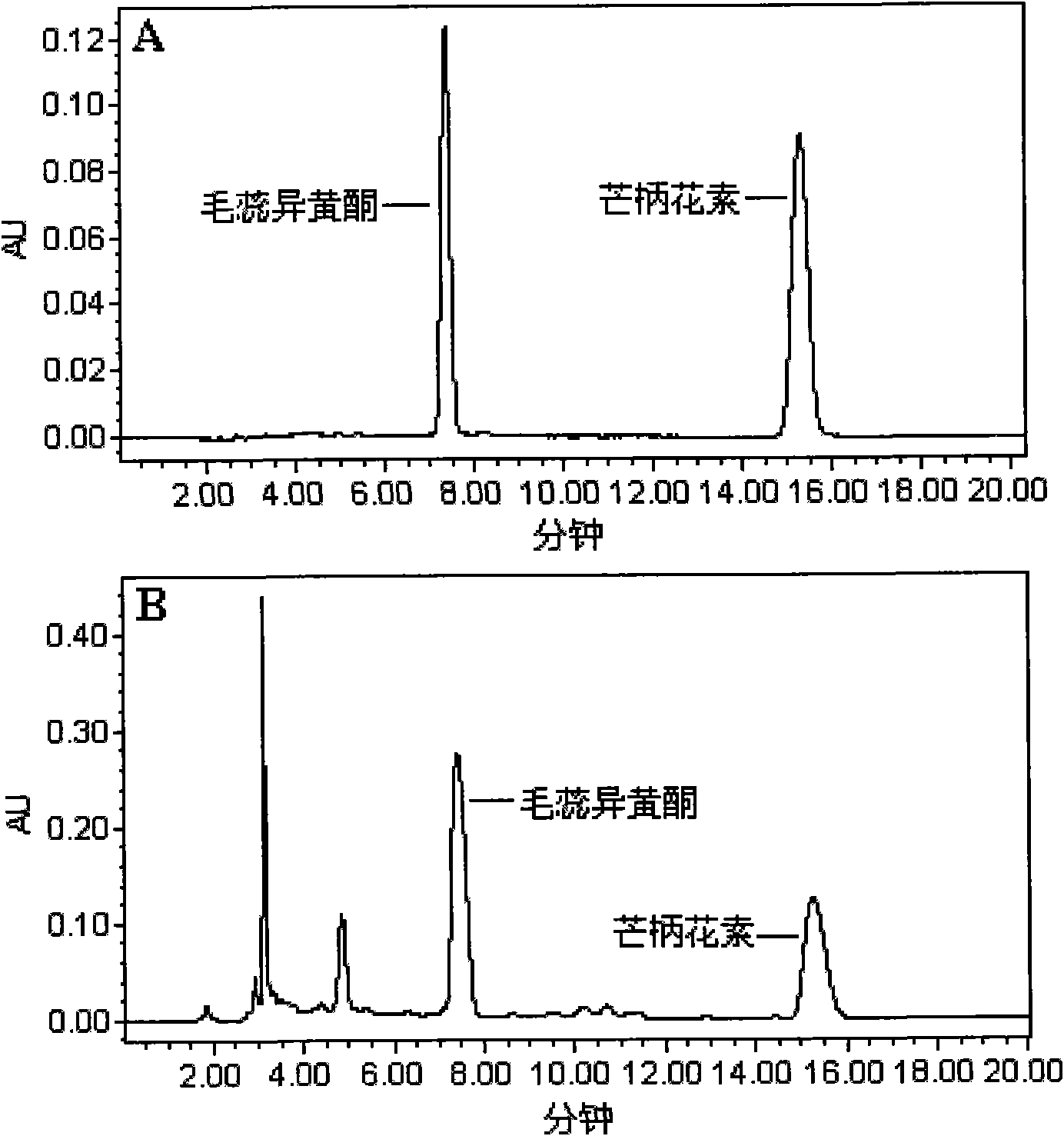
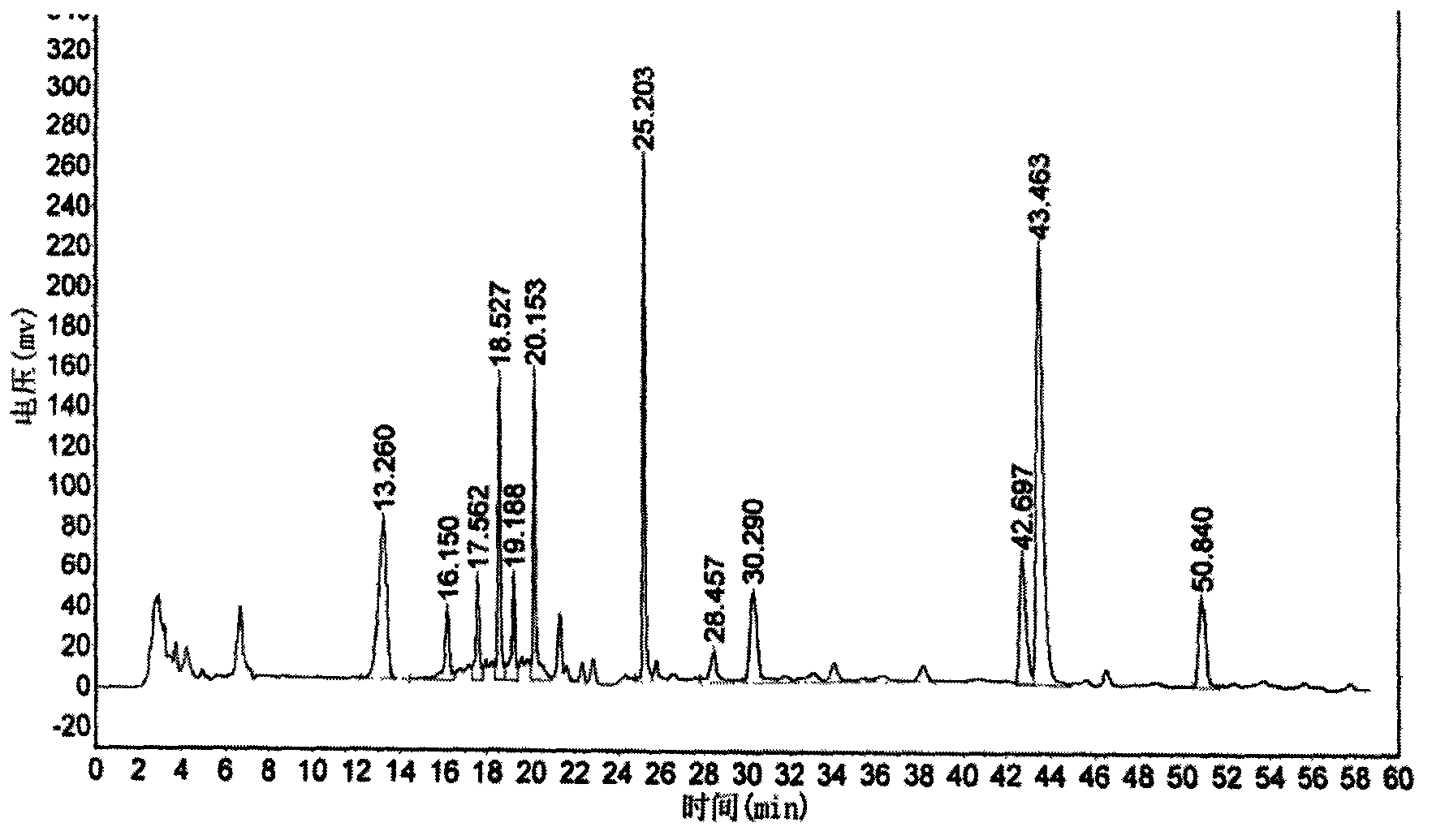
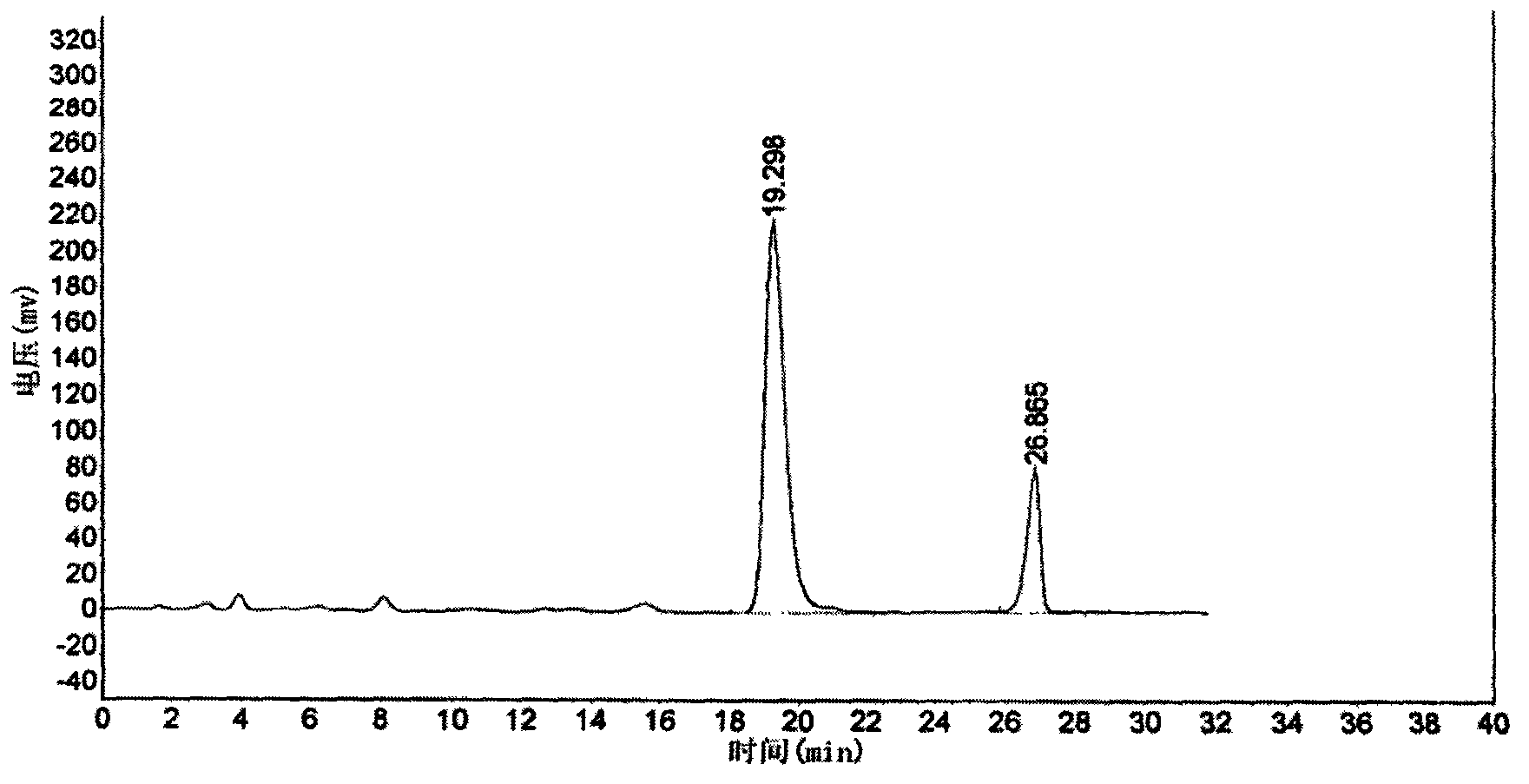
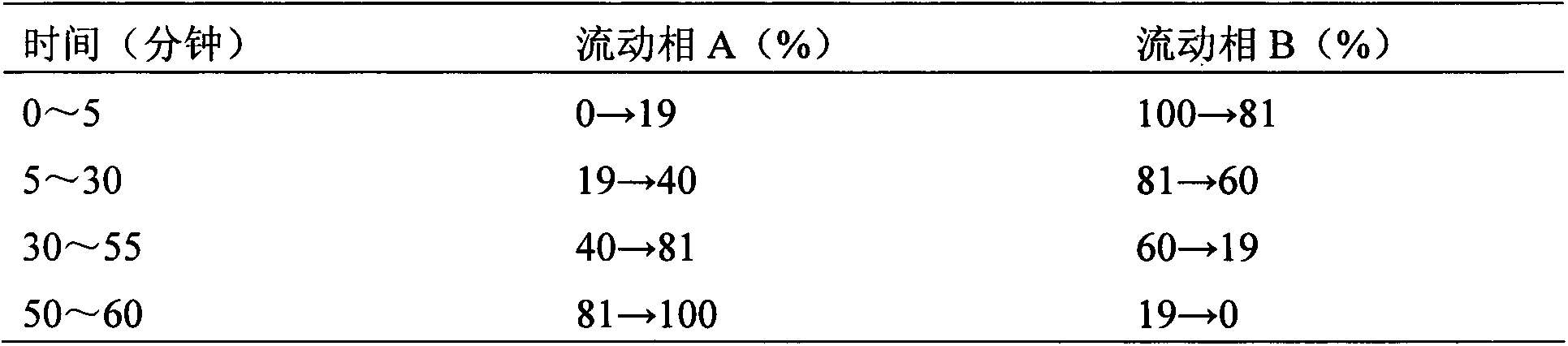
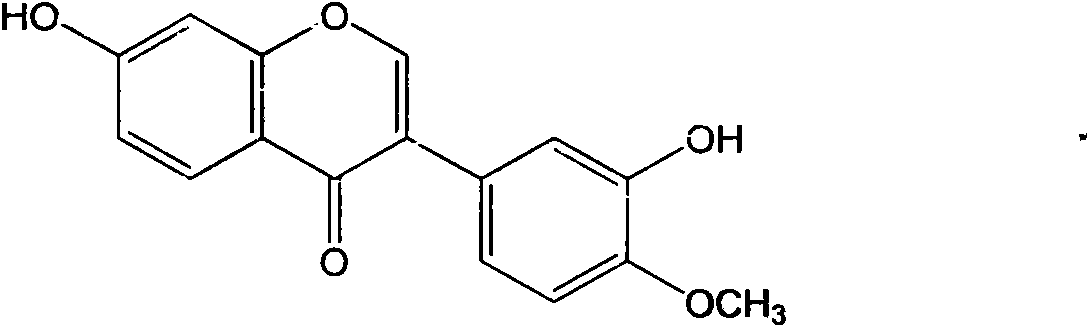
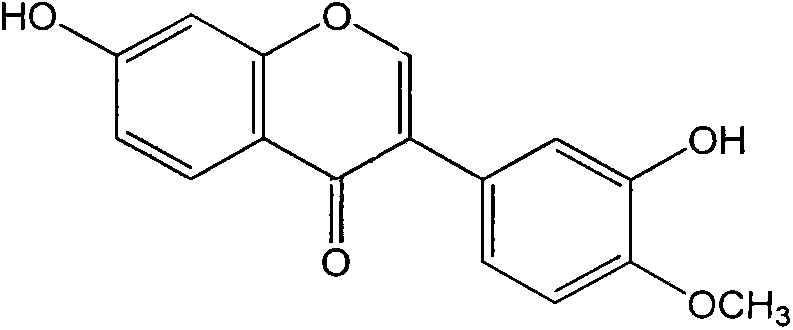
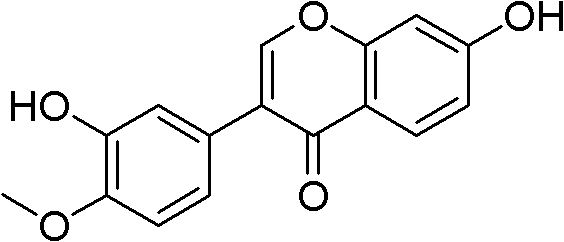
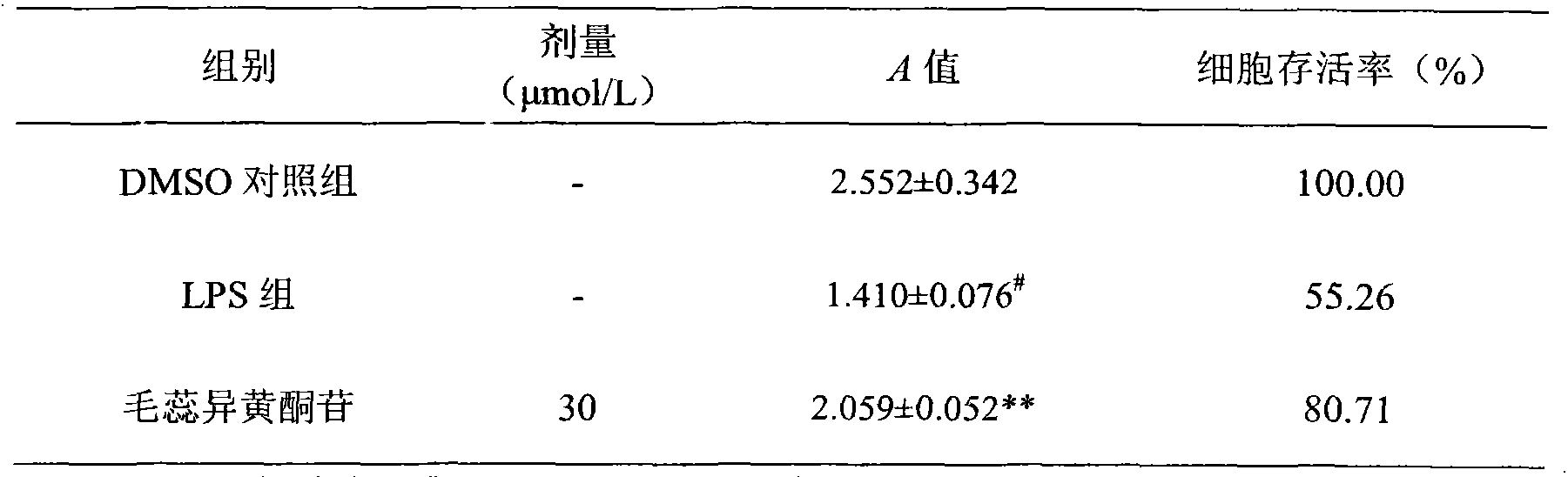
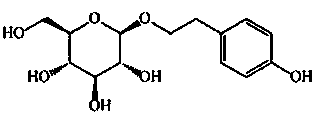
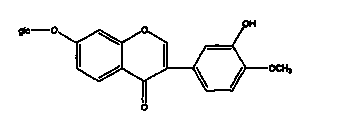
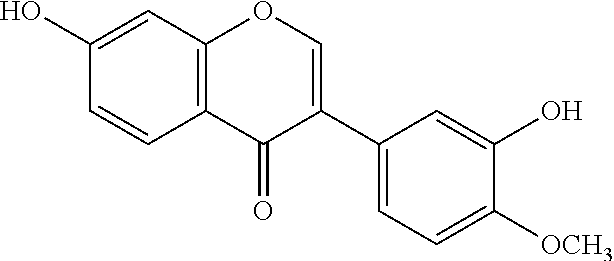
Calycosin is an O-methylated isoflavone. It can be isolated from Astragalus membranaceus Bge. var. mongholicus and Trifolium pratense L. (red clover).
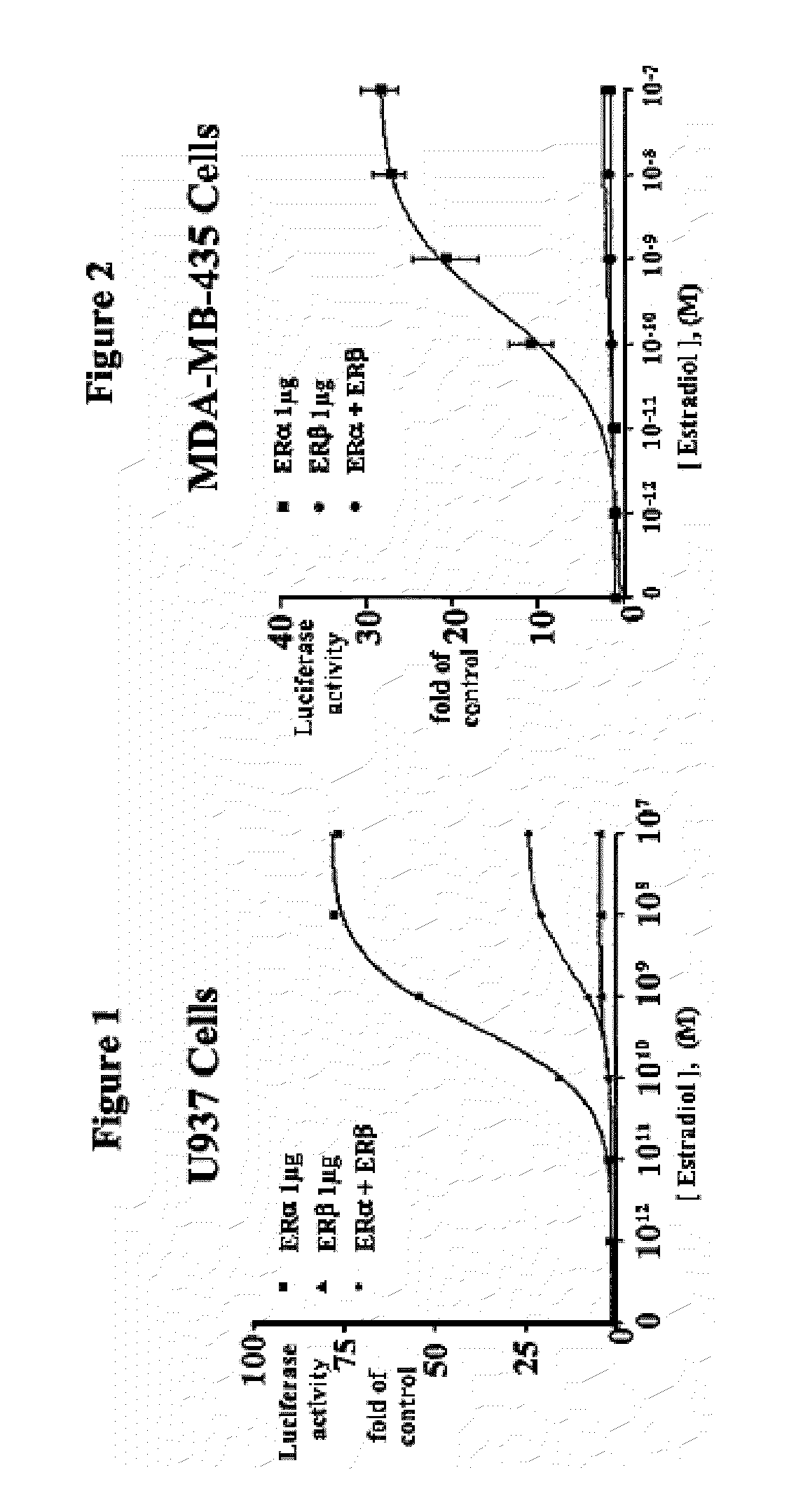
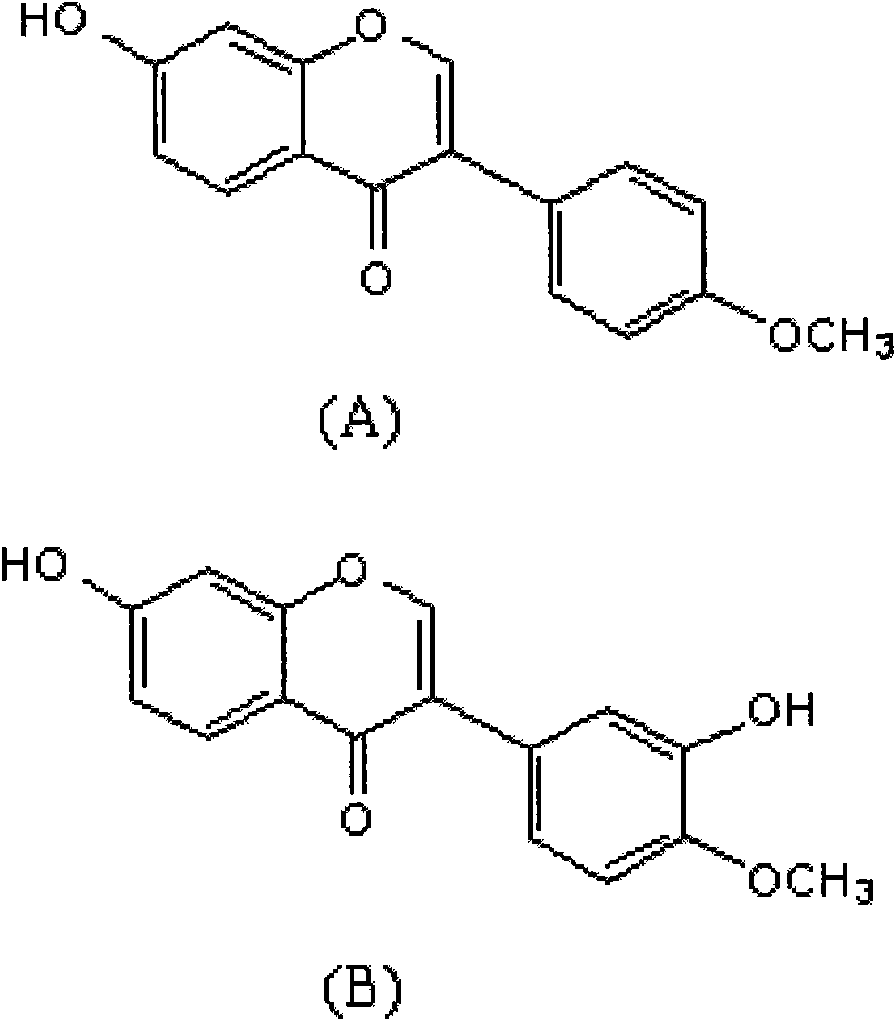
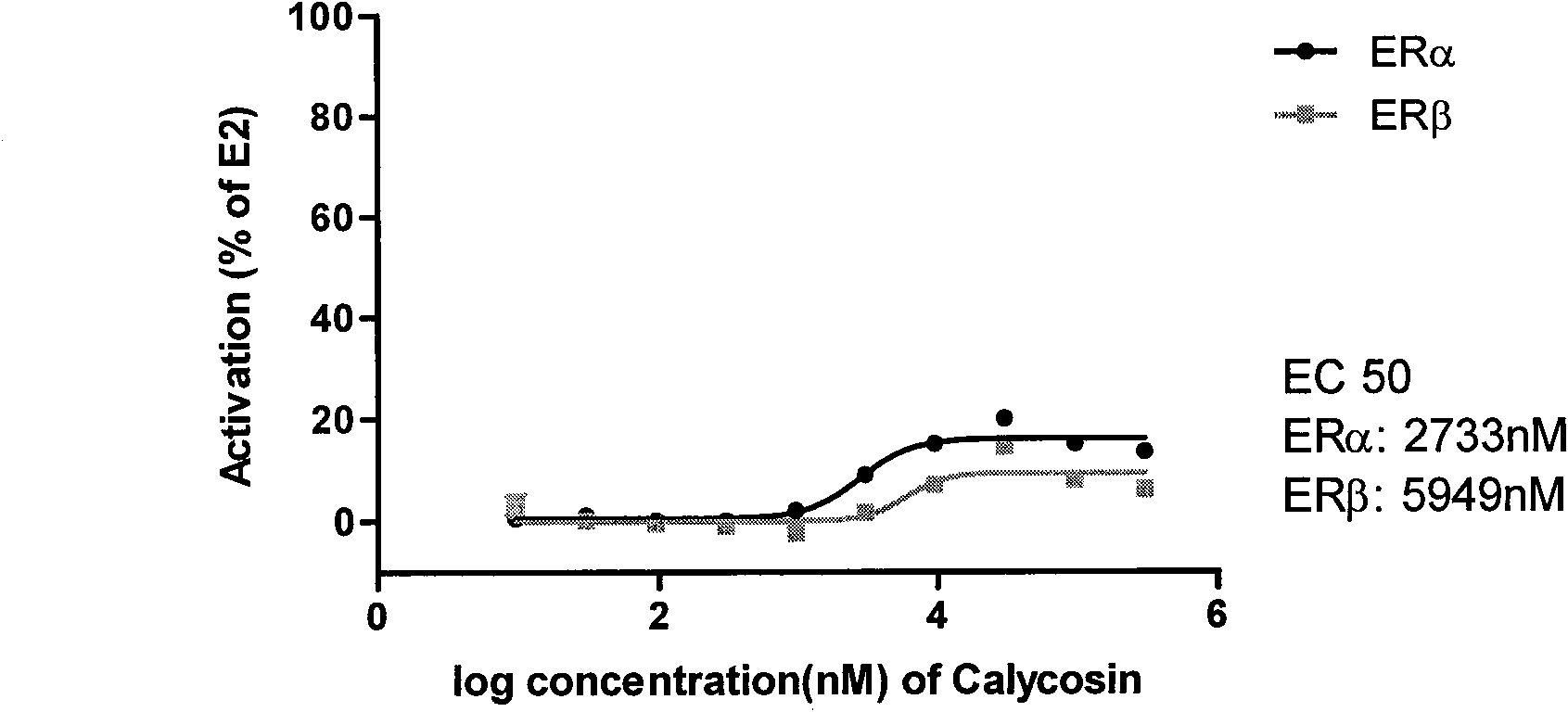
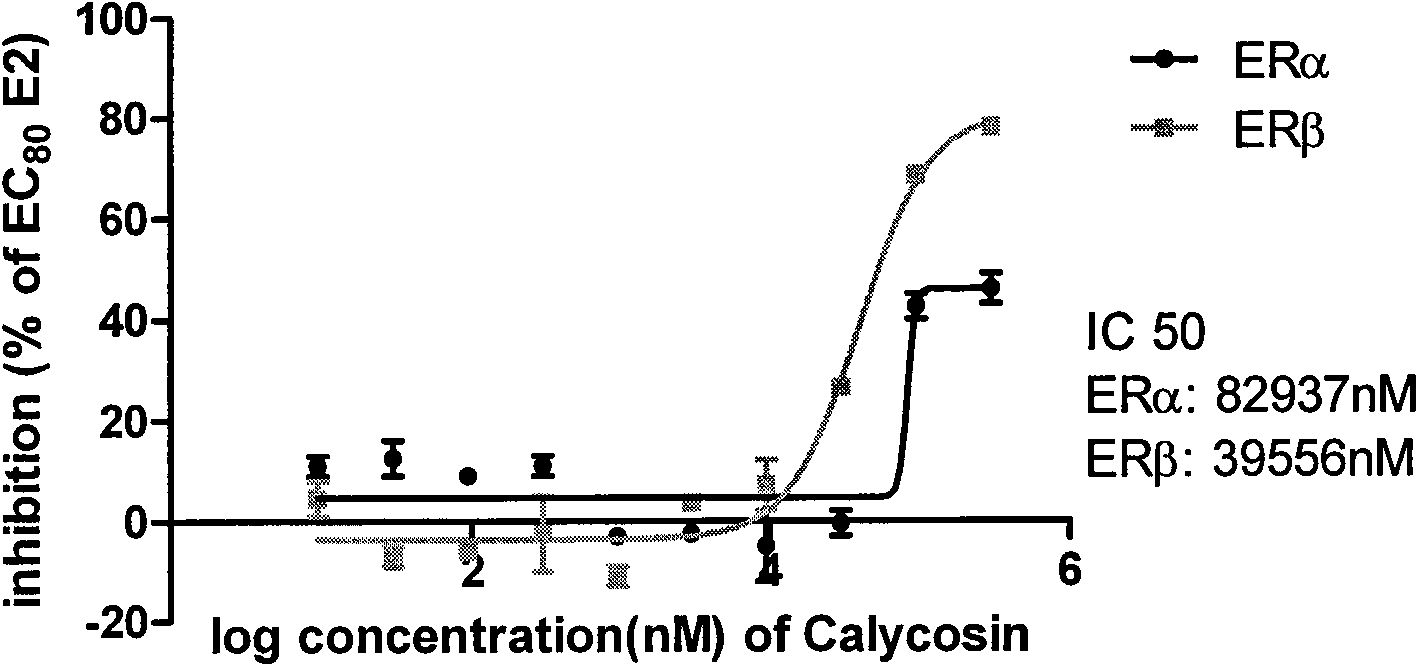
Calycosin and analogs thereof for the treatment of estrogen receptor beta-mediated diseases
InactiveUS20090258942A1Reduce riskReduced likelihoodBiocideNervous disorderDiseaseEstrogenic Effects
Estrogenic compositions comprising calycosin and analogs thereof are provided. Also provided are methods of using said extracts to achieve an estrogenic effect, especially in a human, e.g. a female human. In some embodiments, the methods include treatment of climacteric symptoms. In some embodiments, the methods include treatment of estrogen receptor positive cancer, such as estrogen responsive breast cancer. In some embodiments, the methods include treatment or prevention of osteoporosis.
Owner:BIONOVO
Method for extracting, separating and purifying formononetin and calycosin from Astragalus mongholicus waste residue
InactiveCN101775418AReduce pollutionEfficient and reasonable useOrganic chemistryFermentationDevitrificationCavitation
The invention provides a method for extracting, separating and purifying formononetin and calycosin from Astragalus mongholicus waste residue. The technical scheme adopted in the invention is as follows: taking waste residue after the production and process of astragalus injection as a raw material; adopting a series of original and efficient technologies for extracting, separation and purifying such as homogenate extraction-mixing enzyme induction biotransformation technology, negative pressure cavitation extract technology, liquid-liquid extraction technology, macroporous absorption resin enrichment technology, normal phase silica gel medium pressure column chromatography technology and devitrification at a low temperature, recrystallization technology and the like to obtain the formononetin and calycosin with high purity, wherein the purity thereof can be more than 95%, and the yield is 80-95%. The raw material used in the invention is the waste residue in the industry production, and the process of the method is simple and practicable, has small pollution to the environment; the obtained formononetin and calycosin have low production cost, high purity and yield, good repeatability, high utilization effect of Astragalus mongholicus resources and the like; and the method is suitable to scaled industrial production.
Owner:NORTHEAST FORESTRY UNIVERSITY +1
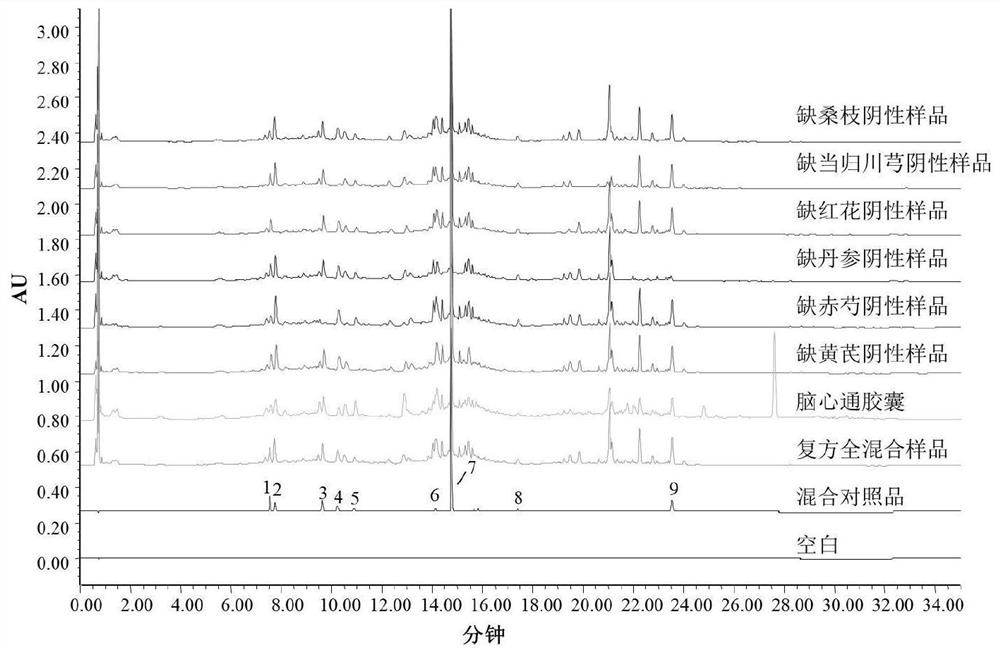
Six-flavor hematinic capsule, its quality control method and application thereof
ActiveCN103381217ARealize authenticationAccurate measurementSenses disorderComponent separationBlurred visionLithospermum
The invention discloses a six-flavor hematinic capsule, its quality control method and an application thereof. The six-flavor hematinic capsule is prepared by adding auxiliary materials into raw materials consisting of Chinese angelica, Ligusticum wallichii, Radix Astragali, prepared rehmannia root, lithospermum and white peony root. According to the quality control method of the six-flavor hematinic preparation, whether the six-flavor hematinic capsule contains white peony root, Chinese angelica, Radix Astragali, prepared rehmannia root, lithospermum, Ligusticum wallichii and components is identified by thin layer chromatography; by high-performance liquid chromatography, in vitro dissolution behavior of the six-flavor hematinic capsule is determined, and effective component groups are identified by fingerprint; verbascoside and calycosin glucoside are used as reference substances to simultaneously determine contents in the six-flavor hematinic capsule; and the content of a volatile component ligustilide in the six-flavor hematinic capsule is determined by a gas chromatographic method. The method provided by the invention is simple to operate, is accurate and advanced, has good linear relation, reappearance, precision, stability and recovery rate, can be adopted to effectively control product quality and guarantee curative effect of the product. The invention also provides an application in the preparation of a medicine for treating eye damage and blurred vision.
Owner:GUANGDONG GUOYUAN GUOYAO PHARMA CO LTD
Method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products
The invention relates to a method for determining fingerprint chromatography of radix astragali and ligusticum wallichii extract products, which comprises the following steps: 1)taking Radix Astragali and Ligusticum wallichii extract product fine powder, preciously weighing, adding methanol, performing ultrasonic extraction, filtering and drying a filtrate, dissolving residue by ethanol and metering volume, filtering by a filter membrane, taking a subsequent filtrate to obtain a tested object solution; 2)taking a ferulic acid reference substance, a senkyunolide I reference substance, a calycosin glucoside reference substance, an ononin reference substance, a calycosin reference substance and a fermlononetin reference substance, preciously weighing, respectively adding methanol to prepare a reference substance solution; and 3)respectively and preciously absorbing the tested object solution and the reference substance solution, and injecting a high efficiency liquid chromatography for determining to obtain the fingerprint chromatography of radix astragali and ligusticum wallichii extract products. The method has active effect for guiding clinical medication and effective feeding guidance to the bulk drugs during the production process, and ensuring the reliable quality; the method has the advantages of convenient and fast operation, so that similarity result can be obtained, the quality of the traditional Chinese medicinal materials radix astragali and ligusticum wallichii extract products can be evaluated, and the result is objective and accurate.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV +1
Determining method for contents of twelve components in traditional Chinese medicine composition preparation
ActiveCN104914199AQuality improvementGood repeatabilityComponent separationBiotechnologyAstragaloside
The present invention discloses a UPLC-MS quantitative method for the contents of twelve components in a traditional Chinese medicine composition preparation, specifically determination of the contents of calycosin-7-O-beta-D-glucoside (1), isoquercitrin (2), narirutin (3), hesperidin (4), ginsenoside Re (5), ginsenoside Rg1(6), periplocoside (7), ginsenoside Rf (8), ginsenoside Rb1 (9), astragaloside (10), ginsenoside Rd (11) and periplocin H1 (12) in the traditional Chinese medicine composition, and belongs to the field of traditional Chinese medicine composition preparation component detection. The determining method of the present invention has characteristics of short period, good reproducibility and high sensitivity.
Owner:HEBEI YILING MEDICINE INST
Method for detecting radix astragali
InactiveCN104198600AImprove accuracyThe method is simple and accurateComponent separationAstragalosidePesticide residue
The invention belongs to the field of detection of the quality of traditional Chinese medicinal materials and in particular relates to a method for detecting the quality of radix astragali. The method comprises the steps of determining the content of astragaloside and calycosin contained in radix astragali and determining the pesticide residues of radix astragali. The method conduces to solving the technical problems that identification methods of traditional physicochemical properties are complex to operate and have limitation and the quality is difficult to regulate and control, has high degree of accuracy, is simple, convenient and fast to operate, is low in cost and has good application prospect and economic benefits.
Owner:LONGXI ZHONGTIAN PHARM CO LTD
Method for rapidly detecting index composition in Chinese herbal medicine compound preparation
The invention relates to the field of detection on traditional Chinese medicine quality, relates to a method for rapidly detecting index composition in Chinese herbal medicine compound preparation, and especially relates to a rapid detection method for index composition in a Chinese herbal medicine compound preparation radix-stephaniae-tetrandrae radix-astragali soup. According to the method, methanol with the concentration of 50% is employed for ultrasonic extraction, and an HPLC-DAD process is applied to simutaneously determine the content of fangchinoline, tetrandrine, calycosin-7-glucoside, liquiritin and glycyrrhizic acid in radix stephaniae tetrandrae, radix astragali and radix glycyrrhizae in the radix-stephaniae-tetrandrae radix-astragali soup at one time. The method solves the problem that quality control is simultaneously performed on stephaniae tetrandrae, radix astragali and radix glycyrrhizae in the radix-stephaniae-tetrandrae radix-astragali soup, and avoids time and reagent waste caused by multiple detection on the five index compositions. The method is simple in operation and high in working efficiency and precision.
Owner:LONGHUA HOSPITAL SHANGHAI UNIV OF TRADITIONAL CHINESE MEDICINE
Content detection method for determining effective components in longshengzhi capsule by HPLC-QQQ/MS method
ActiveCN109307721AEasy to separateQuick analysisComponent separationBenzoylpaeoniflorinGradient elution
The invention provides a content detection method for determining effective components in a longshengzhi capsule by an HPLC-QQQ / MS method. The chromatograph condition is that the chromatographic column takes a octadecyl-bonded silica gel column as a filling agent, 0.1% formic acid-water as a mobile phase A, 0.1% formic acid-acetonitrile as a mobile phase B, and the volume ratio of the mobile phaseA and the mobile phase B is 80% to 0%:20 to 100%, so that gradient elution is performed. According to the content detection method for determining the effective components in the longshengzhi capsuleby the HPLC-QQQ / MS method, the effective substance components of traditional Chinese medicines such as astragaloside A, hydroxysafflor yellow A, calycosin 7-o-glucoside, calycosin, ferulic Acid, syringin, n-butylphthalide, ligustilide, senkyunolide A, senkyunolide I, senkyunolide H, ligustrazine, isofraxidin, dehydrocostus lactone, amygdalin, syringin E, paeoniflorin, oxypaeoniflora, benzoylpaeoniflorin, etc., in the longshengzhi capsule can be determined. The method has the advantages of being simple in operation method, high in sensitivity and accurate in content.
Owner:SHAANXI BUCHANG PHARMA
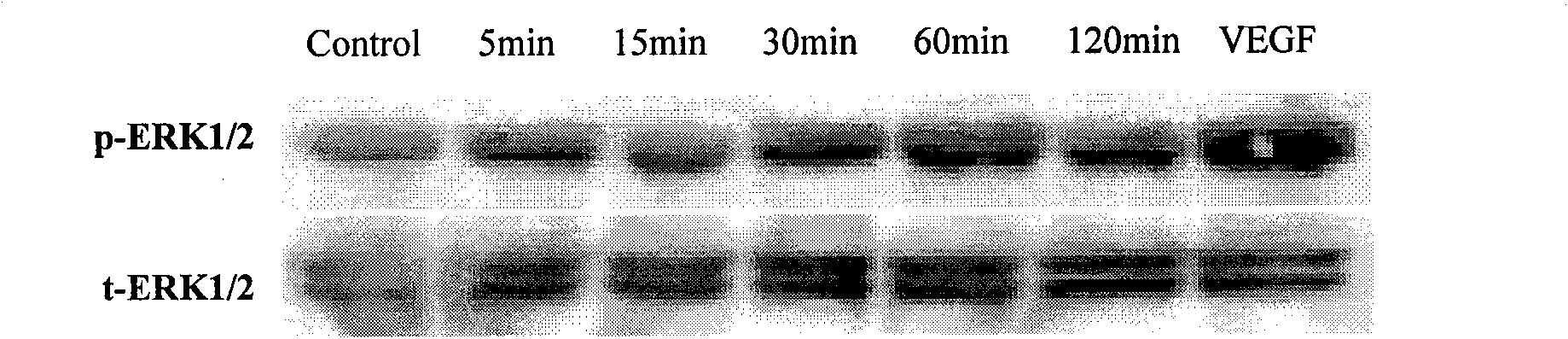
Application of calycosin in radix astragali in preparing medicament for vascular protection and angiogenesis promotion
The invention discloses an application of calycosin in radix astragali in preparing a medicament for vascular protection. Angiogenesis is promoted by vascular endothelial cell proliferation, , and further a good vascular protection effect is generated; the prepared medicament used as a selective estrogen modulator can replace estrogen, promote the angiogenesis, and protect the vascular endothelialcells, thereby treating cardiovascular diseases (such as atherogenesis and the like).
Owner:UNIVERSITY OF MACAU
Quality inspection method of Tongkang tablets
The invention discloses a quality inspection method of Tongkang tablets. The Tongkang tablet is prepared from the following raw materials: 80.5g of astragalus membranaceus, 53.5g of white atractylodes rhizome, 27g of divaricate saposhnikovia root, 80.5g of Chinese yam, 6.5g of dried tangerine or orange peel, and 80.5g of oyster. The quality inspection method comprises the following steps of morphological identification, inspection, fingerprint determination and content determination, wherein the identification is characterized by adopting a thin layer chromatography to identify the astragalus membranaceus, the white atractylodes rhizome, the divaricate saposhnikovia root and the dried tangerine or orange peel in the Tongkang tablet; the fingerprint determination is characterized by determining a fingerprint of a hesporidin component in the Tongkang tablet through a high performance liquid chromatography; the content determination is characterized by adopting the high performance liquid chromatography to carry out content determination on prim-o-glucosylcimifugin, calycosin-7-glucoside, 5-O-methylvisammioside and hesporidin in the Tongkang tablet at the same time. The quality inspection method provided by the invention has the advantages of quickness, stability, accuracy and the like, provides a standard inspection method for production and use of the Tongkang tablets, and can effectively ensure the quality of the Tongkang tablets during the production and use processes, so that the pharmaceutical effectiveness is ensured.
Owner:广州康和药业有限公司
Method for measuring content of eighteen ingredients of Huangqi Jianzhong Wan (astragalus mongholicus pill for invigorating stomach and regulating middle warmer)
InactiveCN109828062AEasy to handleGood reproducibilityComponent separationAstragalosideAdditive ingredient
The invention provides a method for measuring content of eighteen ingredients of Huangqi Jianzhong Wan (astragalus mongholicus pill for invigorating the stomach and regulating the middle warmer). Themethod includes the steps of subjecting the Huangqi Jianzhong Wan to ultrasonic extraction and centrifugation, extracting the liquid supernate, detecting the liquid supernate by an ultra performance liquid chromatography-tandem mass spectrometry positive and negative ion mode, preparing a mixed standard product solution from standard products of the eighteen ingredients, building a standard curve,and carrying out the methodological study. The method can realize measuring the eighteen ingredients of the Huangqi Jianzhong Wan, namely ononin, formononetin, calycosin, calycosin-7-glucoside, pterocarpan, astraisoflavan-7-O-beta-D-glucoside, isoflavane, isoliquiritin, liquiritigenin, liquiritin, glycyrrhetinic acid, cinnamic acid, isomucronulatol 7-O-beta-glucoside, astragaloside I, astragaloside II, astragaloside III, astragaloside IV and gallic acid. In the method, the sample pre-treatment process is simple and accordingly time for measurement is short; the method has the advantages of high specificity, high flexibility, good reproducibility and short analysis time, and can be applied to monitoring of production quality of the Huangqi Jianzhong Wan.
Owner:SHANXI UNIV
Method for simultaneously detecting multiple index components in vigor-preserving decoction preparation
The invention provides a method for simultaneously detecting multiple index components in a vigor-preserving decoction preparation. The vigor-preserving decoction preparation comprises the following index components: ginsenoside Rg1, ginsenoside Re, ginsenoside Rb1, calycosin-7-glucoside, calycosin, liquiritin, glycyrrhizic acid, isoliquiritin apioside, isoliquiritin and cinnamic acid. Detection is conducted according to the following high performance liquid chromatography conditions: a chromatographic column is an octadecyl bonded silica gel chromatographic column; the detection wavelength ofa DAD detector is 201-205 nm, 258-262 nm, 288-292 nm and 358-362 nm; and the mobile phase A is acetonitrile, the mobile phase B is an ammonium acetate buffer solution, and gradient elution is conducted. According to the provided detection method, effective separation of the multiple index components can be realized, and the provided method is found to be sensitive, accurate and relatively good inreproducibility through methodology research and verification of specificity, sensitivity and the like.
Owner:HEBEI SHINEWAY PHARMA +1
Building method for fingerprint of radix astragali seu hedysari pill with effect of reinforcing middle warmer
ActiveCN106950324AScientific Evaluation QualityAvoid one-sidednessComponent separationRadix Astragali seu HedysariGradient elution
The invention provides a building method for a fingerprint of a radix astragali seu hedysari pill with the effect of reinforcing middle warmer. The building method comprises the following steps: 1) preparing a reference methanol solution from a proper amount of calycosin, fermlononetin and paeoniflorin; 2) preparing a test article methanol solution from the radix astragali seu hedysari pill with the effect of reinforcing middle warmer; 3) injecting 10muml of the reference methanol solution and the test article methanol solution into an ultra performance liquid chromatograph, and performing gradient elution under the condition of ultra performance liquid chromatography (UPLC) to obtain a fingerprint, wherein the operating conditions of the liquid chromatograph are as follows: a moving phase is prepared from acetonitrile and a 0.1-percent methanoic acid aqueous solution, the flow speed of the moving phase is 0.3ml / min, the column temperature is 30 DEG C, and the detection wavelength is 254nm. The built fingerprint can clearly reflect the peak outlet positions of feature peaks of effective components in the radix astragali seu hedysari pill with the effect of reinforcing middle warmer, and can be used for controlling the quality of the radix astragali seu hedysari pill with the effect of reinforcing middle warmer.
Owner:SHANXI UNIV
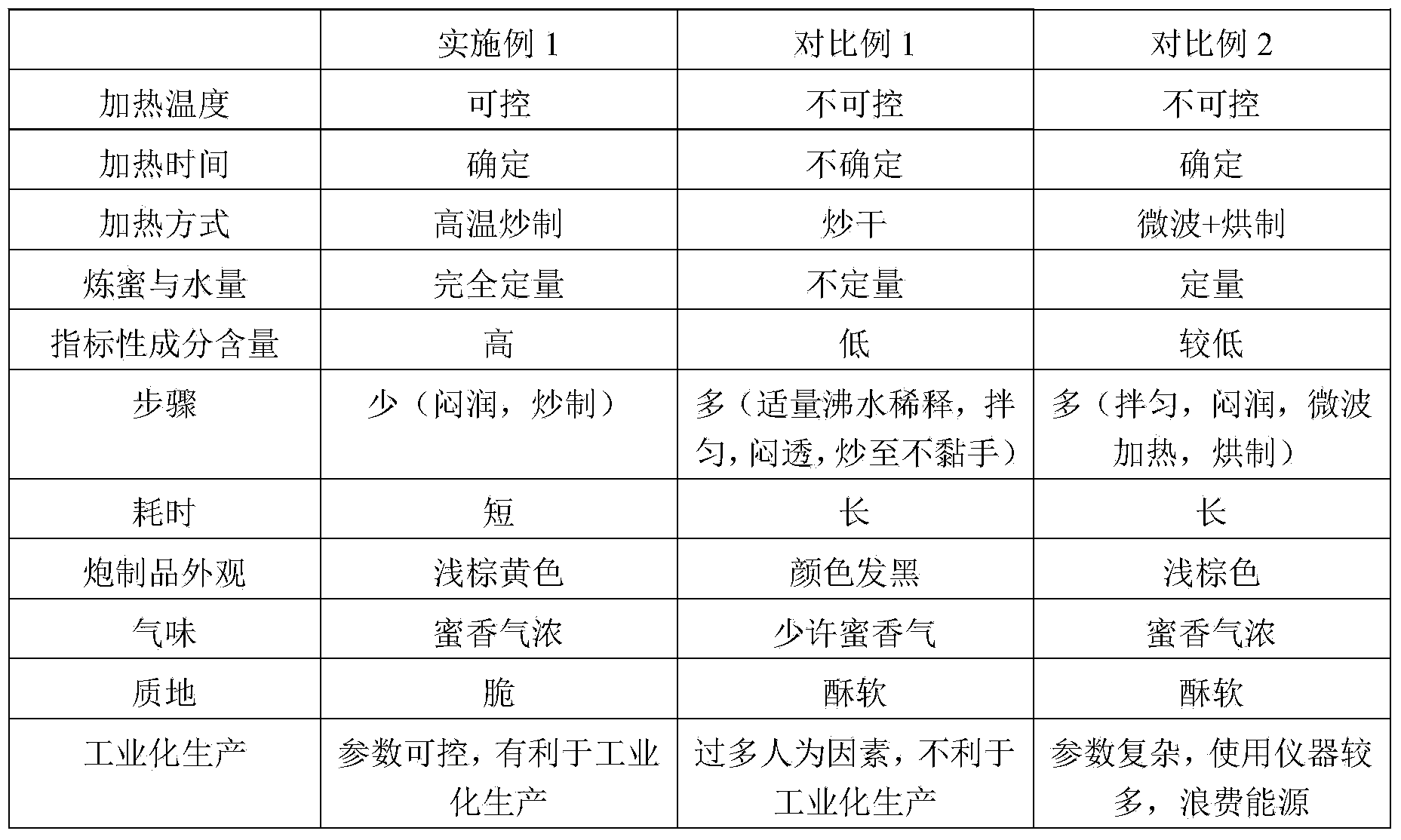
Method for preparing milkvetch root fried with honey
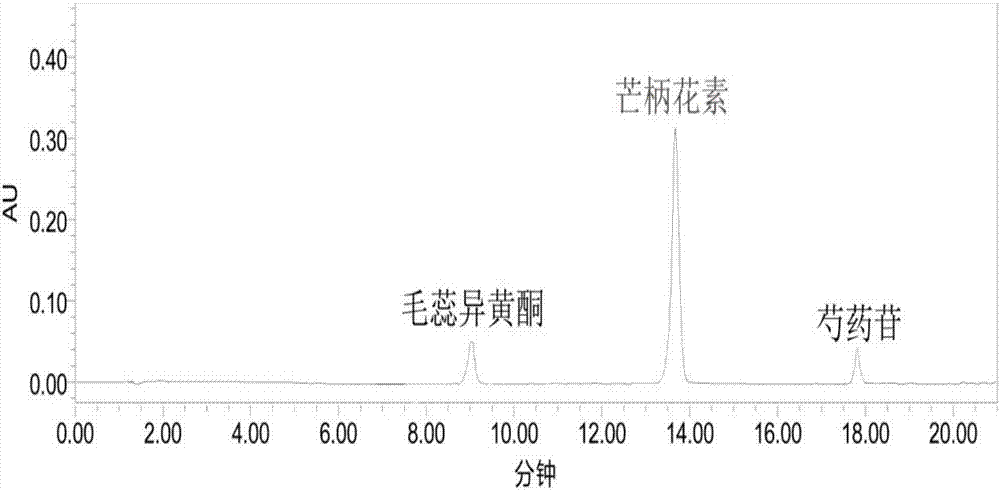
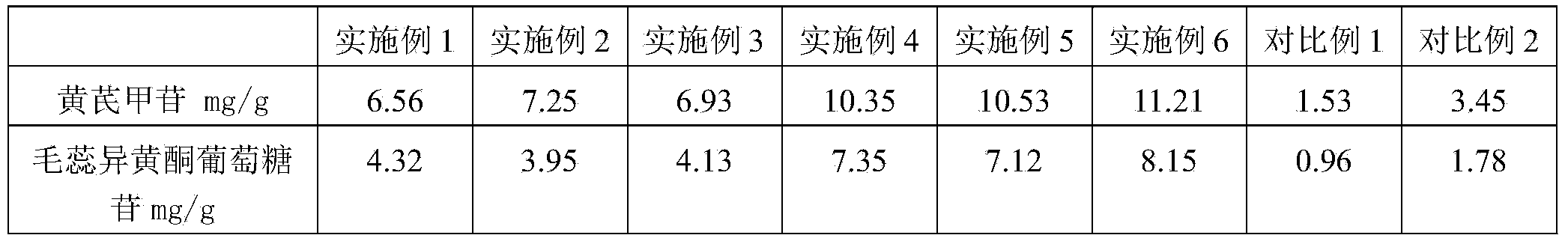
The invention relates to a method for preparing milkvetch root fried with honey. The method comprises the following steps: moistening raw milkvetch root and honey until the honey water is totally absorbed by the raw milkvetch root, frying the moistened milkvetch root at the temperature of 220-240 DEG C, thereby obtaining the milkvetch root fried with honey. According to the preparation method provided by the invention, the milkvetch root is moistened and fried at a high temperature, so that the heating temperature and time of the milkvetch root can be controlled, and the preparation process is controllable. According to the milkvetch root fried with honey prepared by the method, the content of astragaloside is 6.56-11.21mg / g, the content of calycosin-7-glucoside is 3.95-8.15mg / g, and the content of the components is far higher than that in the prior art.
Owner:SUZHOU TIANLING CHINESE TRADITIONAL MEDICINE SLICE
Method for measuring content of traditional Chinese medicine composition
Contents of effective components, such as calycosin-7-glucoside, specnuezhenide, palmatine hydrochloride, berberine hydrochloride and rosmarinic acid, of a traditional Chinese medicine composition are measured at the same time by means of the UPLC method, the method is simple and quick and suitable for quality control of the traditional Chinese medicine composition, and reference is provided for dynamic real-time monitoring in continuous production of the traditional Chinese medicine composition.
Owner:SHIJIAZHUANG YILING PHARMA
Applications of isoflavone compound
InactiveCN102302484AImprove therapeutic indexGood inhibitory effectOrganic active ingredientsAntiviralsAntiviral drugAdditive ingredient
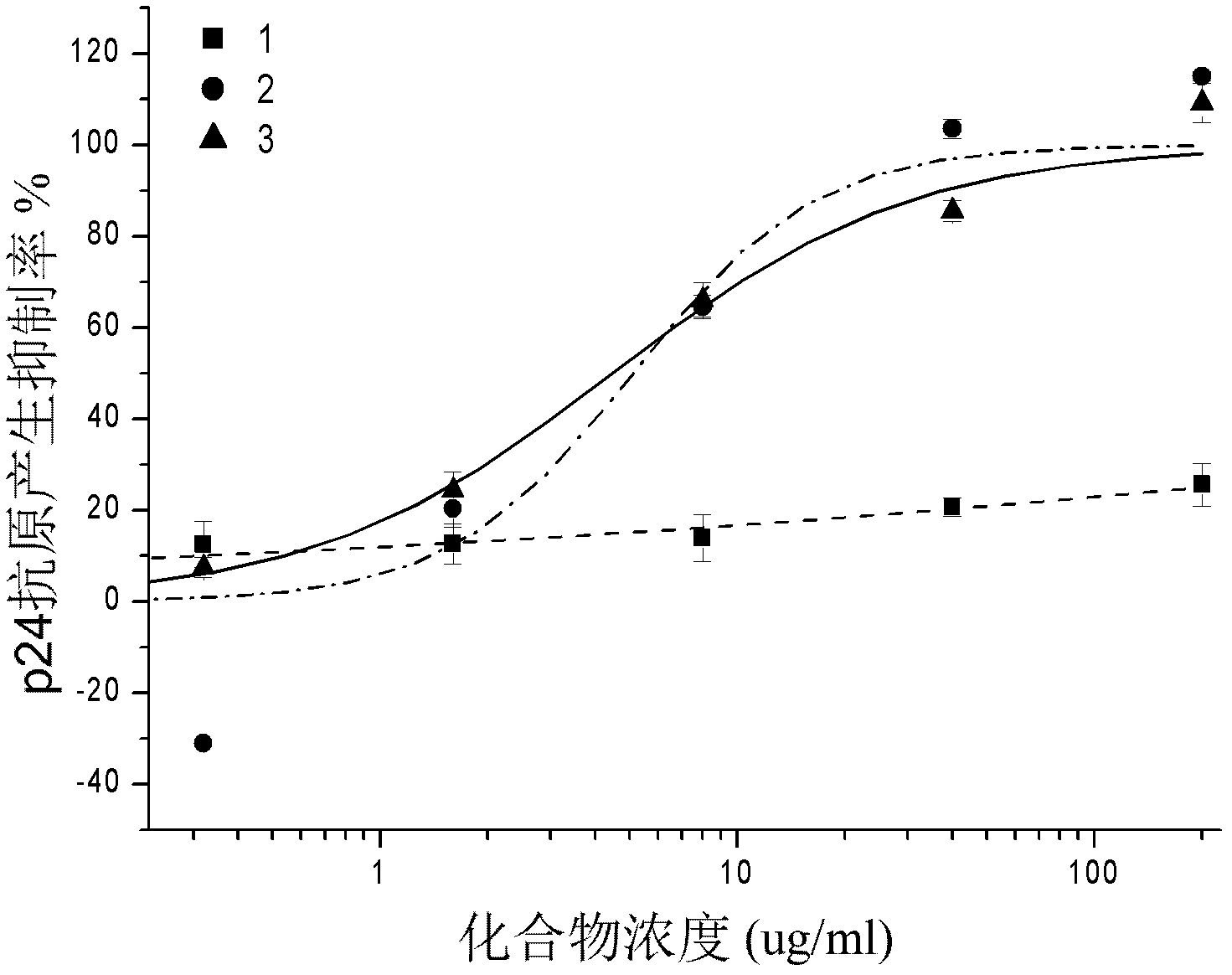
The invention provides applications of an isoflavone compound in preparing antiviral medicaments. The isoflavone compound provided by the invention is calycosin, and has obvious AIDS (Acquired Immune Deficiency Syndrome) virus and coxsakie virus resistant activities. The isoflavone compound provided by the invention as an active component is used for preparing medicaments for preventing or treating viral diseases.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI +1
Application of calycosin in preparation of immunity inhibitors
InactiveCN104107175AHas inhibitory effectOrganic active ingredientsImmunological disordersLymphocyte proliferationCalycosin
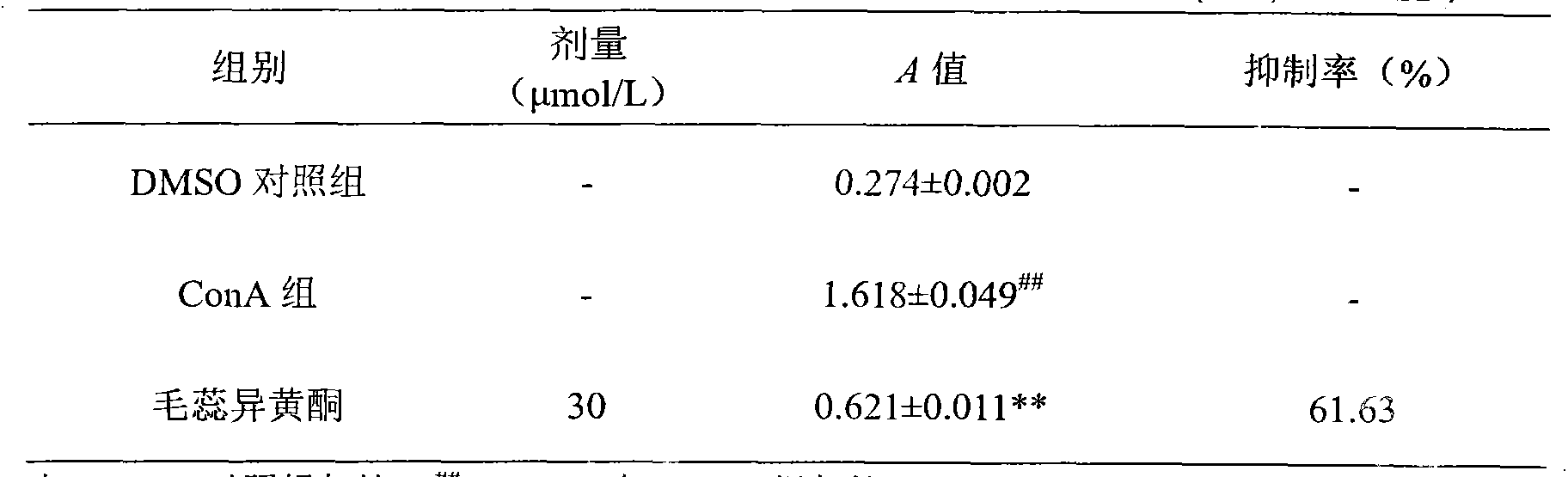
Through the experiments and researches on calycosin, it has been found for the first time that calycosin can prominently inhibit the T lymphocyte proliferation induced by concanavalin A (Con A), has an immunity inhibiting effect, and is advantageously used to prepare immunity inhibitors.
Owner:PEKING UNIV
High efficient sewage cleaning agent
InactiveCN105417729AEfficient sewage degradationReduce COD valueWater treatment compoundsTreatment with anaerobic digestion processesSodium BentoniteNitrifying bacteria
The invention discloses a high efficient sewage cleaning agent, which comprises the following components in parts by weight: 40 to 60 parts of diatomite, 15 to 25 parts of montmorillonite, 5 to 15 parts of bentonite, 1 to 3 parts of nutrients, 6 to 10 parts of microbial bacteria, and 0.4 to 0.8 part of calycosin; wherein the microbial bacteria comprise ammonifying bacteria, nitrifying bacteria, denitrifying bacteria, phosphor eating bacteria, and actinomycetes; and the nutrients comprise chicken manure and horse manure. The provided high efficient sewage cleaning agent can degrade organic substances in sewage high efficiently and is capable of prominently reducing the COD value. The cleaning agent comprises calycosin, which can prominently promote the degradation of organic substances by microbial bacteria and obviously improves the cleaning effect.
Owner:叶君芝
Method for preparing high-purity calycosin
ActiveCN102030735AHigh purityOvercome irreversible adsorptionOrganic chemistryCountercurrent chromatographyCalycosin
The invention relates to a method for separating high-purity calycosin from a leguminous plant astragalus root by using high-speed countercurrent chromatography, comprising the following steps of: (1) preparing an appropriate solvent system, standing and delaminating to obtain a top phase and a bottom phase; (2) selecting the top phase as a fixed phase and the bottom phase as a mobile phase, firstly filling a countercurrent chromatograph column by using the fixed phase, regulating the rotating speed of a principal machine at 600-1000 rpm, pumping the mobile phase into the countercurrent chromatograph column at the flow rate of 0.5-4 ml / minute, and sampling through a sampling valve after the integral system establishes dynamic balance; and (3) receiving a target component according to an ultraviolet spectrum of a detector, and concentrating and crystallizing to obtain the calycosin. The preparation method is suitable for various types of high-speed countercurrent chromatographs and the preparation of various contents of the calycosin and has large separation amount, high recovery rate and easy and convenient operation.
Owner:SHANGHAI TAUTO BIOTECH CO LTD
Determination method of content of calycosin glucoside in stable pulvis for strengthening body resistance and detoxifcation
The invention discloses a determination method of the content of calycosin glucoside in stable pulvis for strengthening body resistance and detoxification; an acetonitrile-0.2% formic acid solution (20:80) is used as a mobile phase; a detection wavelength is 260 nm; calycosin glucoside is used as a control; a sample is treated by methanol reflux, filtration, drying by distillation, and dissolution; and the content of calycosin glucoside in the pulvis for strengthening body resistance and detoxification is detected by a high performance liquid chromatography-ultraviolet detector. The method has good linear relation, good precision and reproducibility, can be used as a determination method of the content of calycosin glucoside in pulvis for strengthening body resistance and detoxification, and is simple, rapid, and easy to operate.
Owner:HENAN KANGXING PHARMA
Pharmaceutical composition with anti-aging effect
ActiveCN104523733AExtend your lifeHas anti-aging effectsOrganic active ingredientsNervous disorderDiabetes mellitusAge related disease
The invention relates to a pharmaceutical composition with an anti-aging effect. The pharmaceutical composition is prepared from the following monomer compounds in parts by weight: 12-18 parts of 7,2'-dyhydroxy-3',4'-dimethoxyisofiavanone, 9-14 parts of (6aR, 11aR)-9,10-dimethoxy pterocarpan-3-oxo-beta-D-glucoside, 6-10 parts of formononetin, 7-11 parts of formononetin-7-oxo-beta-D-glucoside, 20-30 parts of calycosin and 20-30 parts of calycosin-7-oxo-beta-D-glucoside. Compared with the prior art, the pharmaceutical composition disclosed by the invention has the effects of significantly prolonging life, can be used for preventing and treating aging-related diseases such as senile dementia, cardiovascular diseases and diabetes, and can be applied to the field of health foods.
Owner:TONGJI UNIV
Application of calycosin-7-glucoside in preparation of immunity inhibitors and anti-inflammatory drugs
Through the experiments and researches on calycosin-7-glucoside, it has been found for the first time that calycosin-7-glucoside can prominently inhibit the T lymphocyte proliferation induced by concanavalin A (Con A) and reduce the macrophage toxicity induced by lipopolysaccharide (LPS), has immunity inhibiting and anti-inflammatory effects, and is advantageously used to prepare immunity inhibitors and anti-inflammatory drugs.
Owner:PEKING UNIV
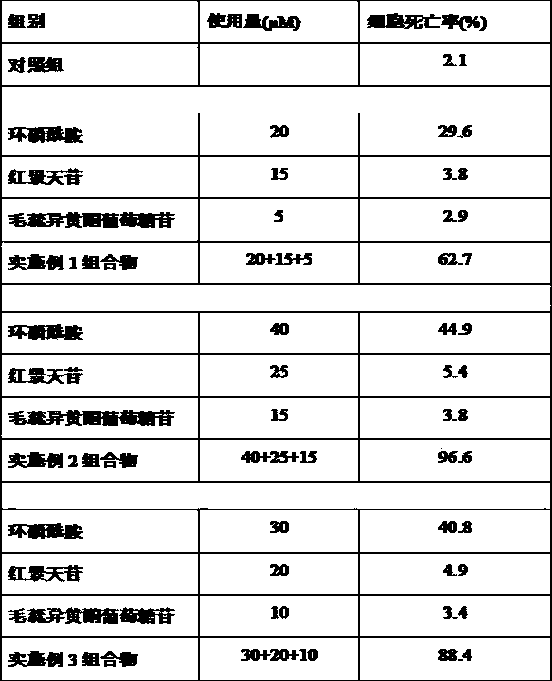
Cyclophosphamide-containing pharmaceutical composition and application in treatment for breast cancer
The invention belongs to the field of medicines, and particularly relates to a cyclophosphamide-containing pharmaceutical composition, and an application thereof in treatment for cancer, in particular for breast cancer. The pharmaceutical composition is composed of cyclophosphamide, salidroside and calycosin-7-oxy-beya-D-glucopyranoside, wherein the molar ratio of cyclophosphamide to salidroside to calycosin-7-oxy-beya-D-glucopyranoside is (20-40): (15-25): (5-15). The pharmaceutical composition disclosed by the invention is capable of reducing the toxic and side effects of cyclophosphamide and remarkably enhancing the curative effect, provides a new thought and strategy for treatment of breast cancer, and has important clinical significance and wide application prospect.
Owner:徐鹏
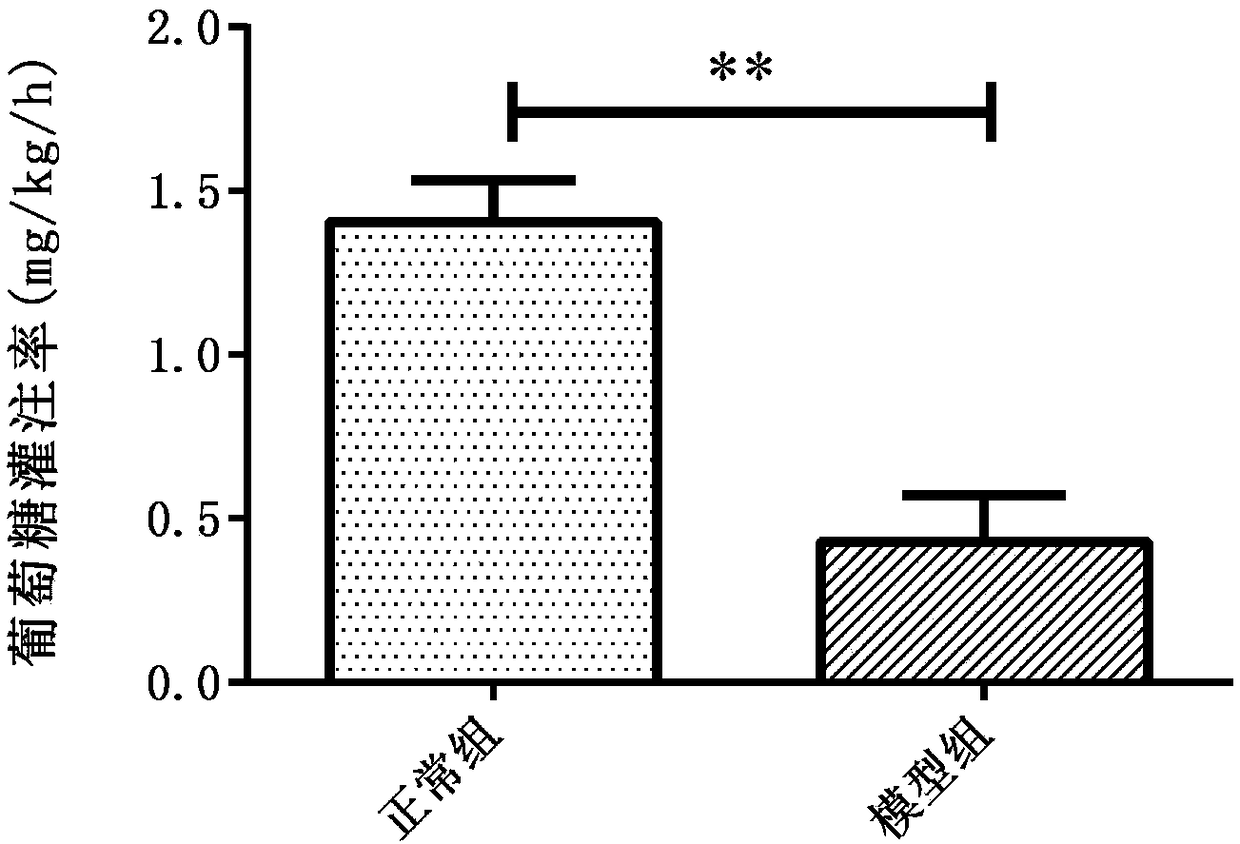
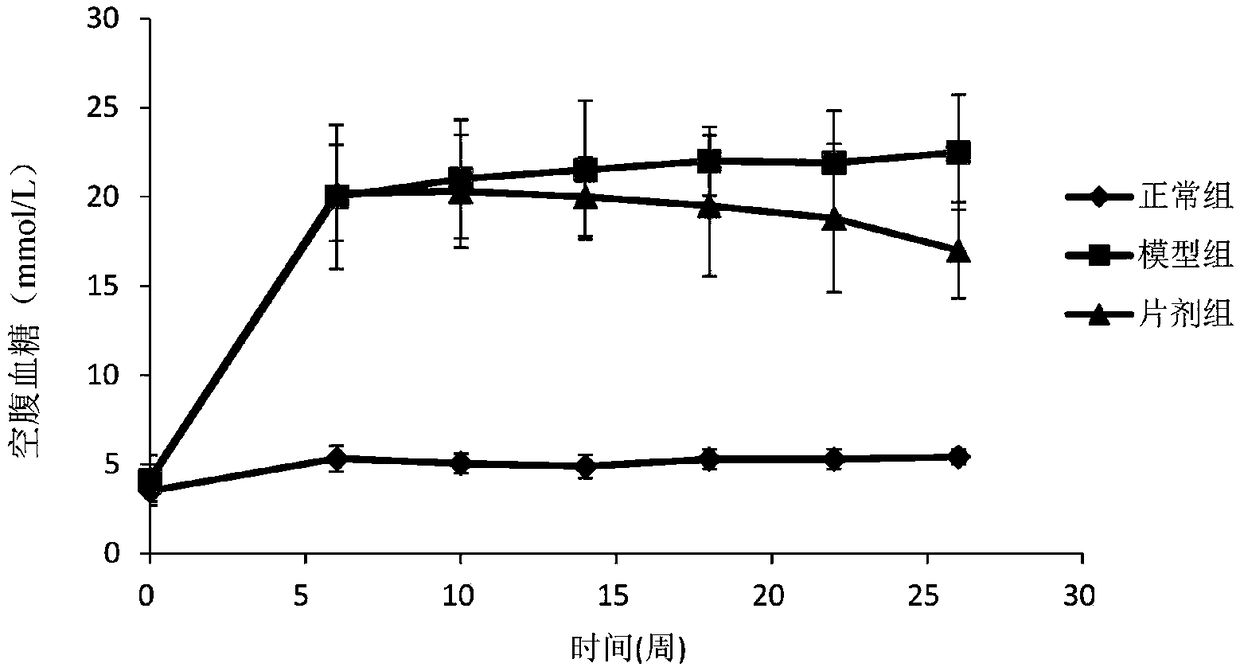
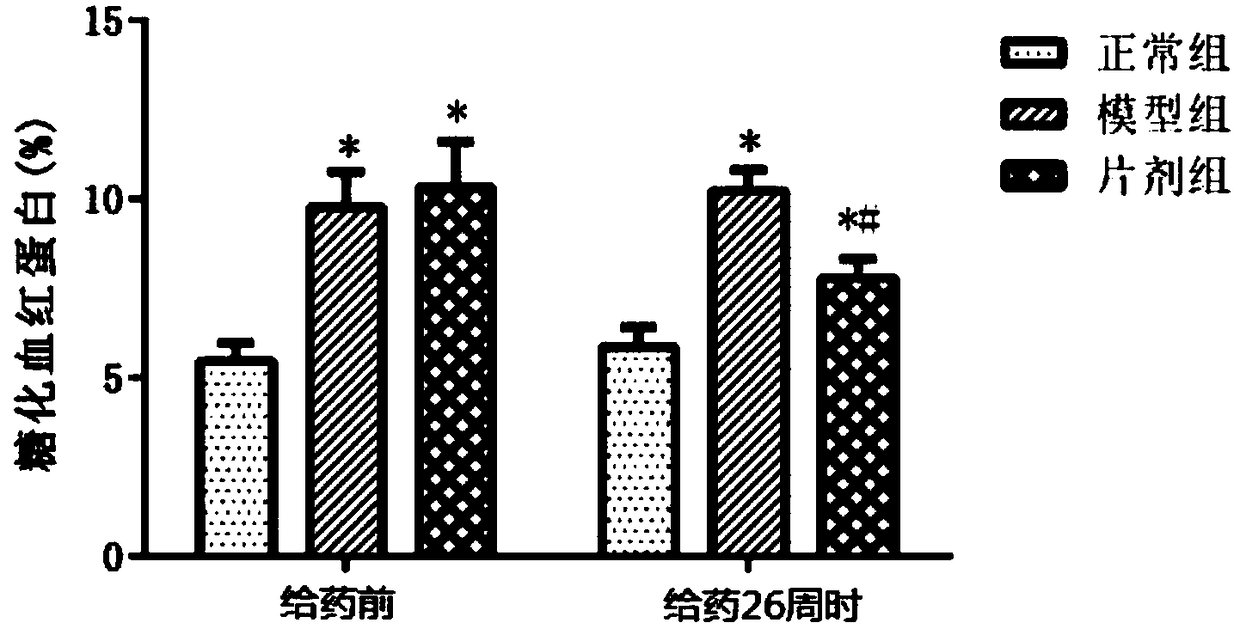
Drug composite for preventing diabetic nephropathy and preparation method thereof
ActiveCN101780069AGood control effectClear structureOrganic active ingredientsSugar derivativesCalycosinDiabetes-Related Complications
The invention discloses a drug composite for preventing diabetic nephropathy and application to preparing medicines for treating diabetes-related complications; the drug composite is composed of one or two of active ingredients-calycosin and calycosin-7-0-D-glucopyranpside, and a carrier which is acceptable in pharmacy; the content of the active ingredients is 0.1-99.5 percent; the drug composite is mainly applied to preparing medicines for treating diabetes-related complications; the drug composite has obvious prevention function to the diabetic nephropathy, the usage is convenient, the quality is controllable, so as to provide new drug candidate for treatment.
Owner:广州康臣药业有限公司
Pharmaceutical Composition For Preventing And Treating Diabetic Nephropathy And The Preparation Method Thereof
The present invention relates to a pharmaceutical composition for preventing and treating diabetic complications, mainly referring to diabetic nephropathy, and the pharmaceutical composition comprises one or both of calycosin and calycosin-7-O-β-D-glucoside as 0.1˜99.5% by weight based on the total weight of the composition, and the conventional drug carrier. The pharmaceutical composition could significantly prevent and treat diabetic nephropathy, with the convenience of quality control and administration, which provides a new drug candidate for patients with diabetic nephropathy.
Owner:GUANGZHOU CONSUN MEDICINE R & D CO LTD
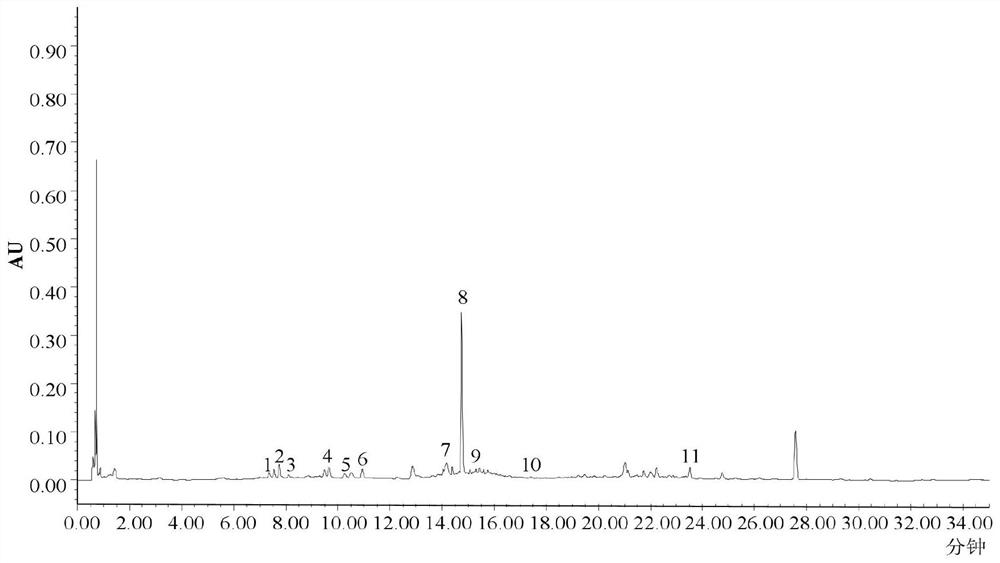
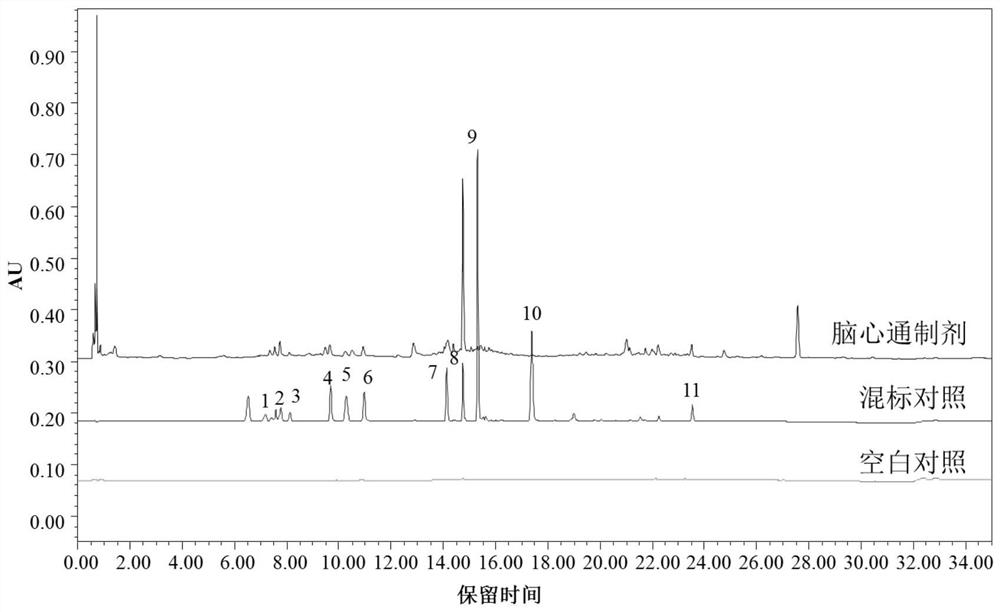
Fingerprint spectrum detection method of medicinal preparation
PendingCN111999395AImprove quality controlImprove detection efficiencyComponent separationSalvianolic acid BPharmaceutical formulation
The invention provides a fingerprint spectrum detection method of a medicinal preparation. According to the detection method, an HPLC-DAD wavelength switching method is adopted to simultaneously determine a plurality of effective components such as mulberroside A, hydroxysafflor yellow A, paeoniflorin, ferulic acid, calycosin-7-glucoside, rosmarinic acid, salvianolic acid B, formononetin and the like in the medicinal preparation; the sensitivity and the accuracy of the detection method are greatly enhanced, so that the comprehensiveness of quality evaluation of the medicinal preparation is guaranteed.
Owner:SHAANXI BUCHANG PHARMA
Pharmaceutical composition for treating diabetes mellitus in earlier stage
ActiveCN108186636AImprove securityFormulation types are flexible and diverseOrganic active ingredientsMetabolism disorderAstragalosideOnonin
The invention discloses a pharmaceutical composition for treating diabetes mellitus in earlier stage. The pharmaceutical composition is prepared from the following raw materials in parts by weight: 5-15 parts of epiberberine, 10-20 parts of coptisine, 15-25 parts of palmatine, 20-35 parts of berberine, 10-15 parts of astragaloside, 0-10 parts of astragalus saponin II, 0-15 parts of astragalus saponin III, 0-10 parts of astragalus saponin I, 20-30 parts of calycosin-7-glucoside, 0-20 parts of formononetin and 0-18 parts of ononin. The pharmaceutical composition provides a new choice for the treatment of the diabetes mellitus in the earlier stage, changes the status quo of the lack of effective prevention and treatment medicines of the diabetes mellitus in the earlier stage, can significantly improve the clinical symptoms of the diabetes mellitus in the earlier stage, significantly reduces blood glucose and glycosylated hemoglobin levels, regulates glucose metabolism, and significantly prevents or delays the progress of the diabetes mellitus in the earlier stage.
Owner:BEIJING SHIJITAN HOSPITAL CAPITAL MEDICAL UNIVERSTY
Method for analyzing all components of angelica sinensis six-yellow decoction
PendingCN112229934AAccurate analysisEasy to analyzeComponent separationAstragalosideChlorogenic acid
The invention provides a method for analyzing all components of an angelica sinensis six-yellow decoction, and belongs to the technical field of pharmaceutical analysis. An ultra-high performance liquid chromatography tandem quadrupole time-of-flight mass spectrometry (UPLC-Q-TOF-MS) technology is adopted to detect an angelica sinensis six-yellow decoction sample, and 15 chemical reference substances are adopted at the same time: baicalin, wogonoside, berberine hydrochloride, epiberberine hydrochloride, coptisine hydrochloride, palmatine hydrochloride, phellodendrine hydrochloride, astragaloside, calycosin-7-glucoside, ferulic acid, chlorogenic acid, ligustilide, verbascoside, baicalein and wogonin are adopted to confirm a detection result of the angelica sinensis six-yellow decoction andclearly identify 68 components; comprehensive analysis of the components of the angelica sinensis six-yellow decoction is realized, and the reliability of quality control is improved.
Owner:山东宏济堂制药集团股份有限公司
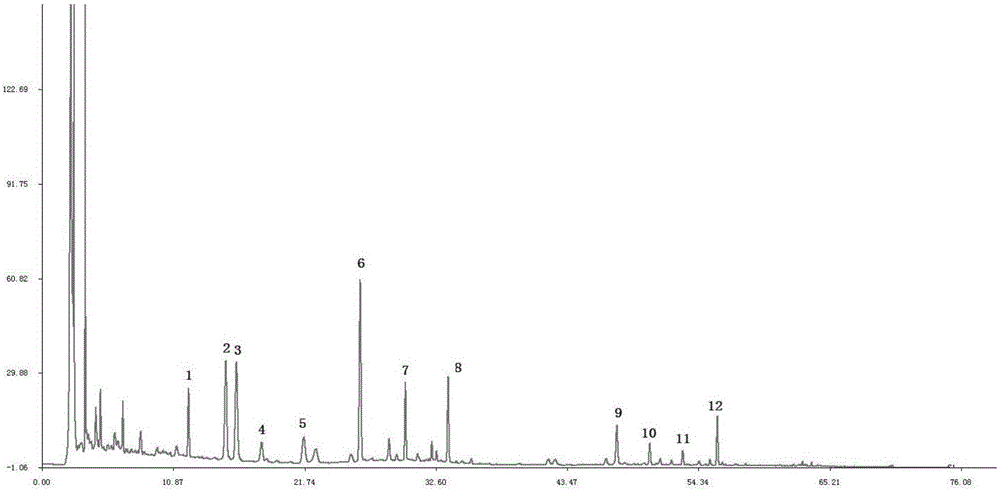
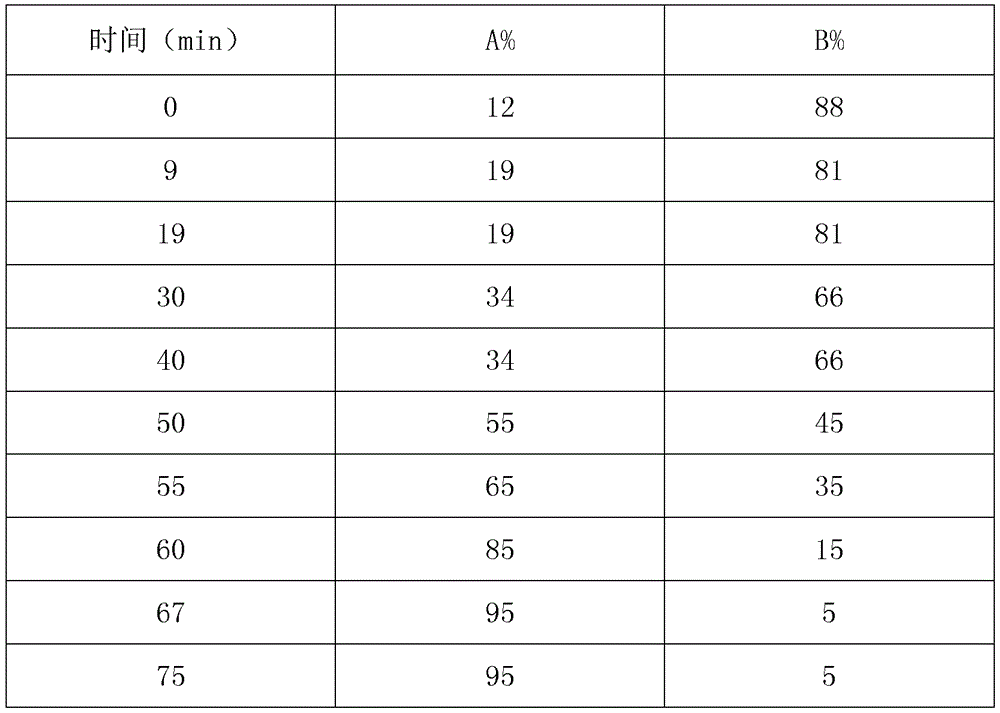
Fingerprint construction method and quality detection method for Radix Astragali and Chinese date oral liquid
ActiveCN107367567AReasonable and reliable quality control methodComprehensive, accurate and fast detectionComponent separationCalycosinPhosphoric acid
The invention relates to a fingerprint construction method and a quality detection method for a Radix Astragali and Chinese date oral liquid. The fingerprint construction method comprises the following steps: taking protocatechuic acid, calycosin-7-glucoside, ononin, calycosin, genistein and formononetin as reference substances, and carrying out gradient elution with 50% aqueous acetonitrile solution-0.2% aqueous phosphoric acid solution as a mobile phase; and carrying out segmented wavelength detection, and recording and obtaining the fingerprint. The quality detection method comprises the following steps: constructing standard fingerprints of 10 or more batches of the Radix Astragali and Chinese date oral liquid through the fingerprint construction method, and carrying out consistence comparison on the standard fingerprints and the fingerprint of a sample to be detected in order to detect the quality of the sample to be detected. Compared with the prior art, the methods have the advantages of high specificity and stability, realization of full, accurate and quick detection of the quality of the finished or semi-finished product of the Radix Astragali and Chinese date oral liquid, and suitableness for large-scale production and application.
Owner:中山市恒生药业有限公司
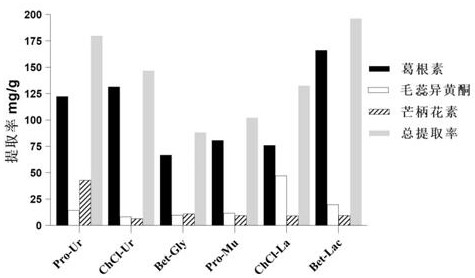
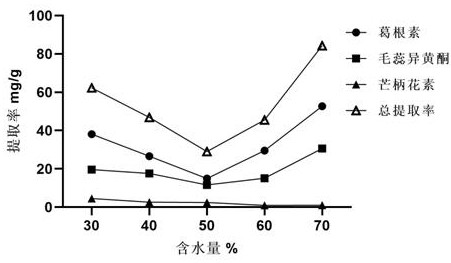
Method for extracting flavonoid components in prescription for nourishing yin, tonifying qi and activating blood by using natural deep-eutectic solvent and process optimization method thereof
ActiveCN113354610AReduce usageLow costOrganic chemistryComponent separationFluid phaseOrganic solvent
The invention provides a method for extracting the flavonoid components in a prescription for nourishing yin, tonifying qi and activating blood by using a natural eutectic solvent and a process optimization method thereof. According to the method, the total extraction rate of puerarin, calycosin and formononetin in a prescription for nourishing yin, tonifying qi and activating blood is taken as an evaluation index, a test scheme is determined by adopting Box-Benhnken Design on the basis of a single-factor test, high performance liquid chromatography is used for measuring the three flavonoid components, the total extraction rate is calculated, extraction process parameter optimization is performed in combination with the response surface model, and test verification is performed. Compared with a traditional organic solvent, the natural eutectic solvent adopted by the method is more efficient, economical and environmentally friendly, can be used for extracting puerarin, calycosin and formononetin in the prescription for nourishing yin, tonifying qi and activating blood, is a sustainable extraction medium, and provides a new reference for the extraction process of other prescriptions at the same time.
Owner:ZHEJIANG CHINESE MEDICAL UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com