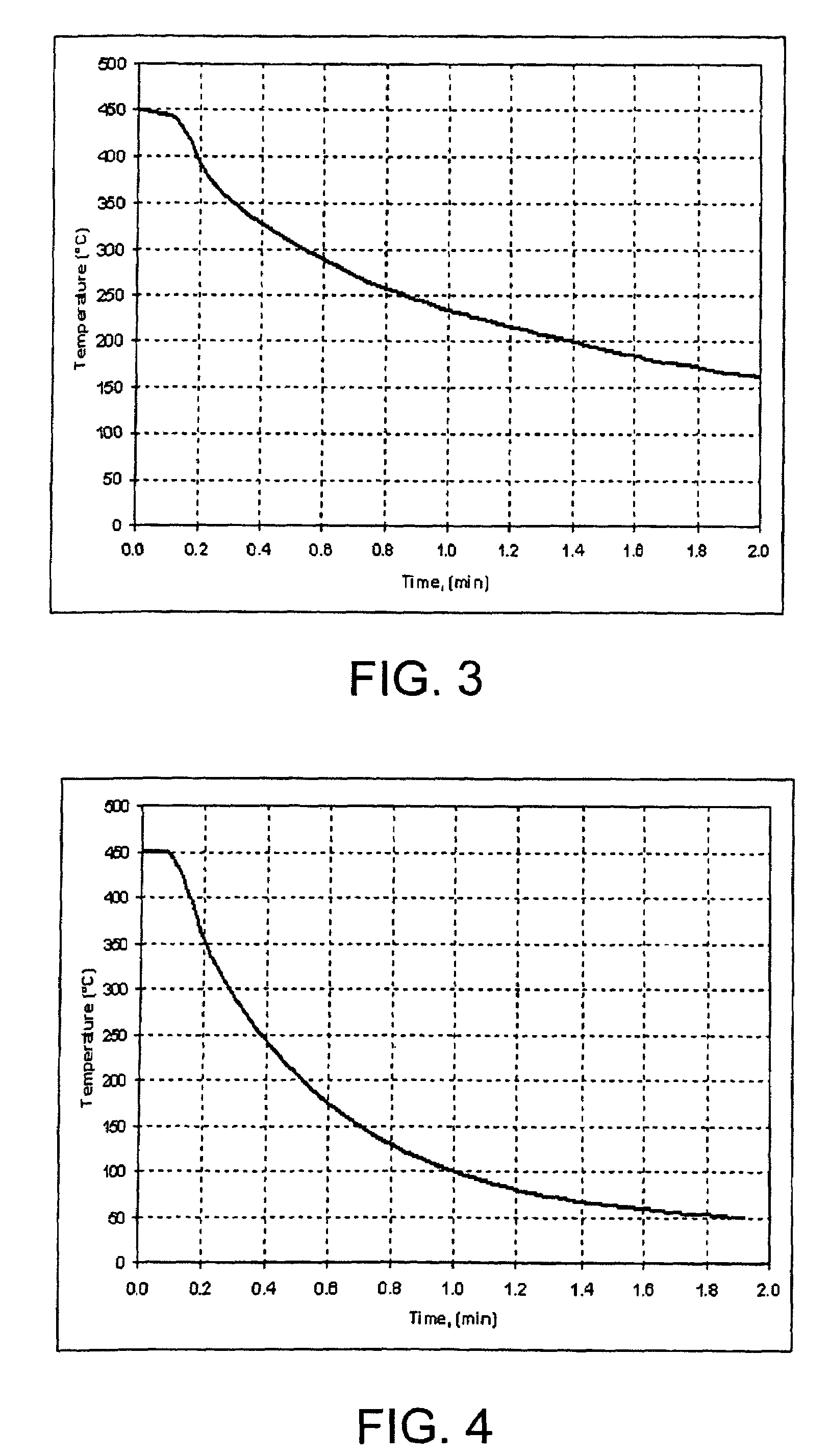
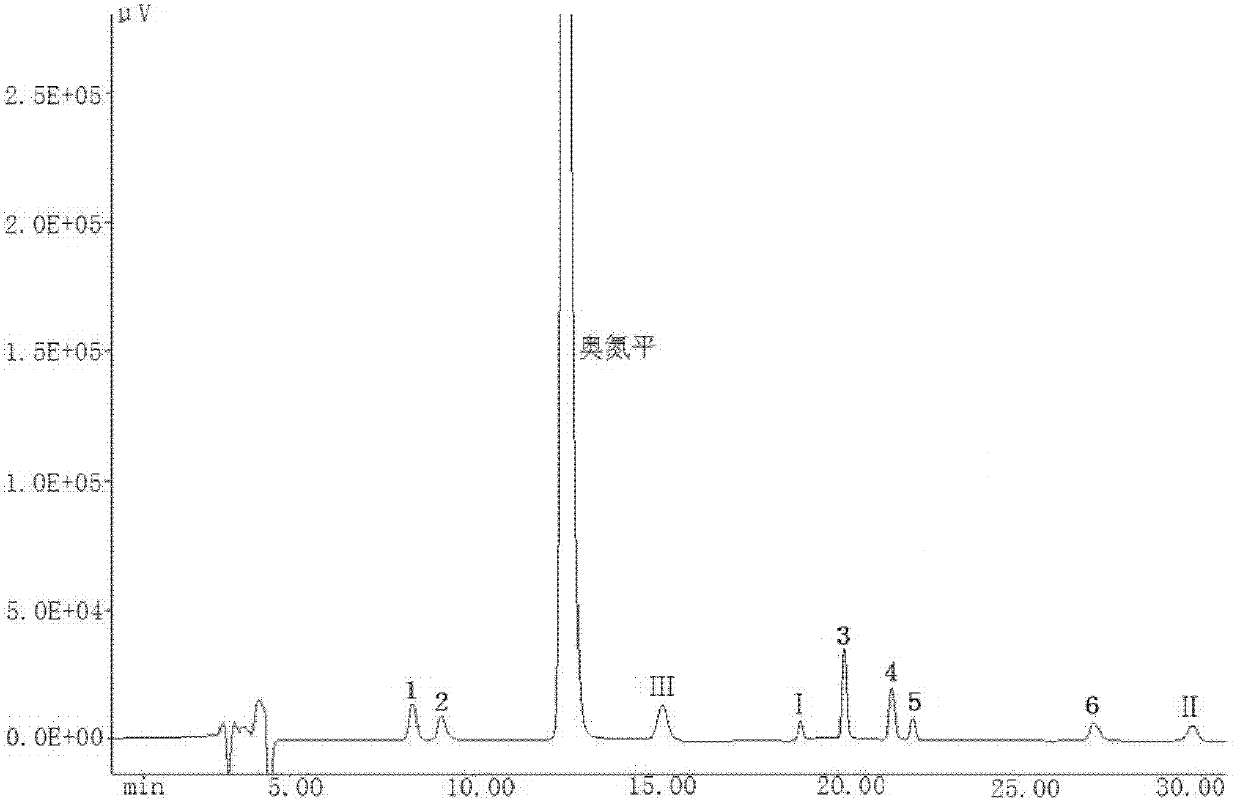
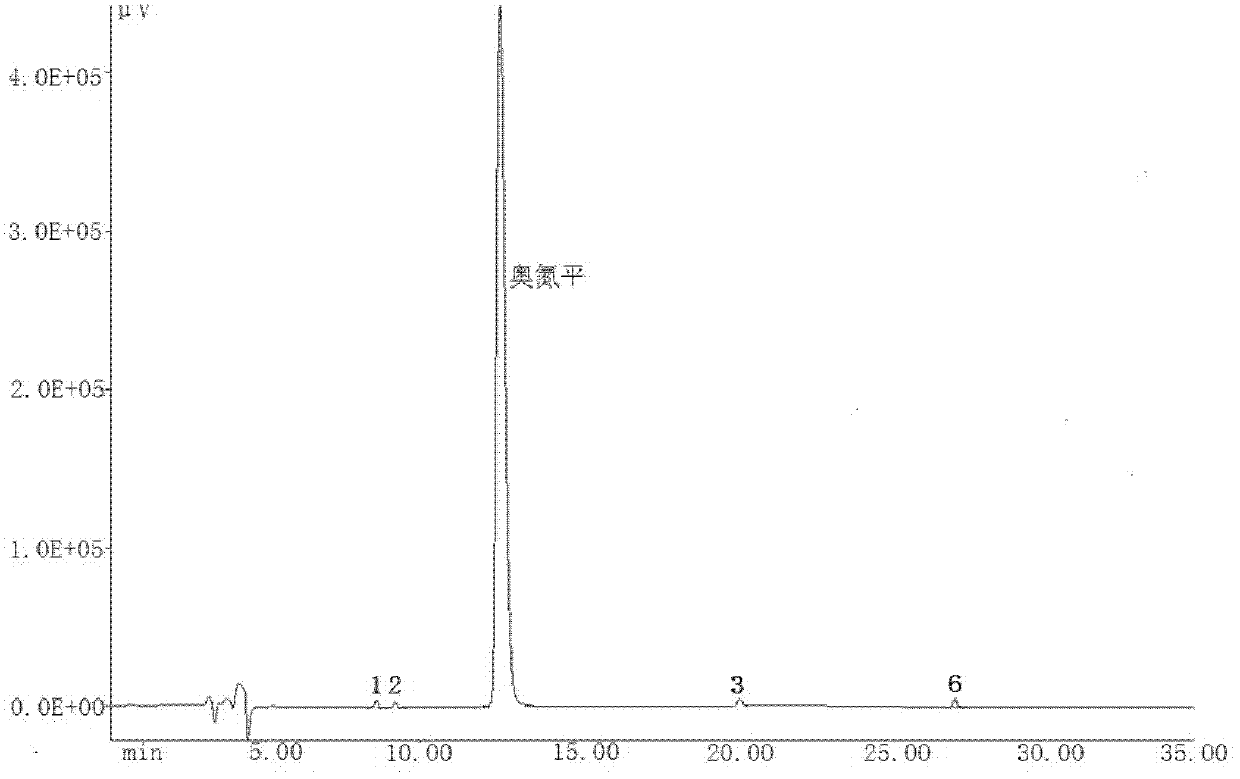
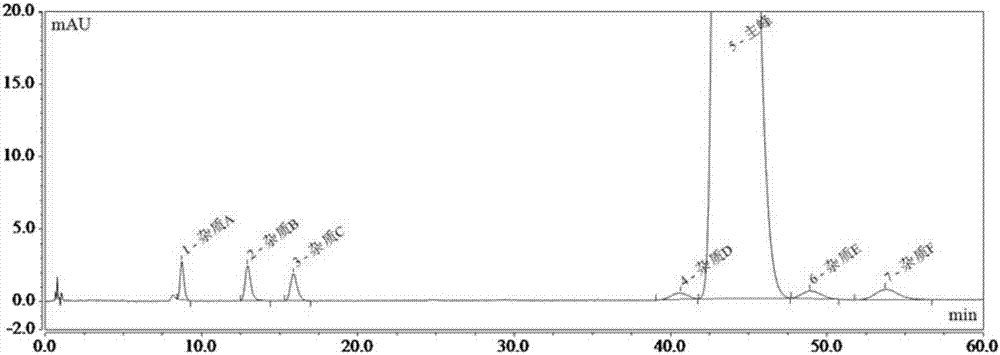
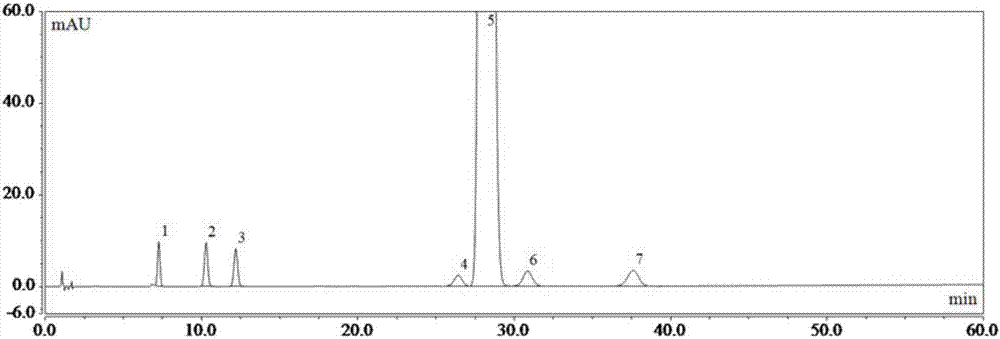
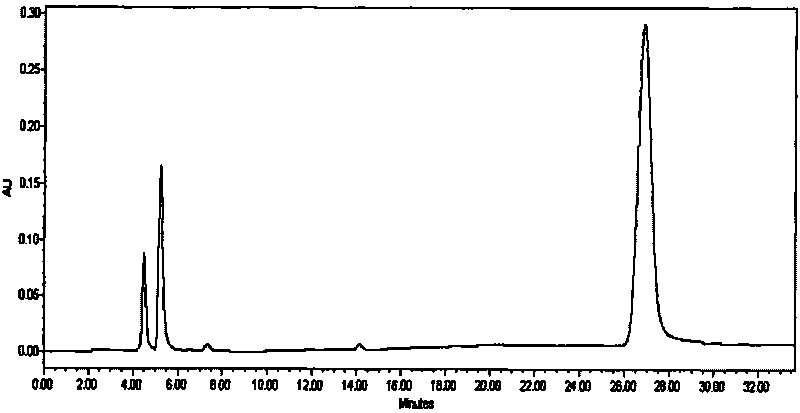
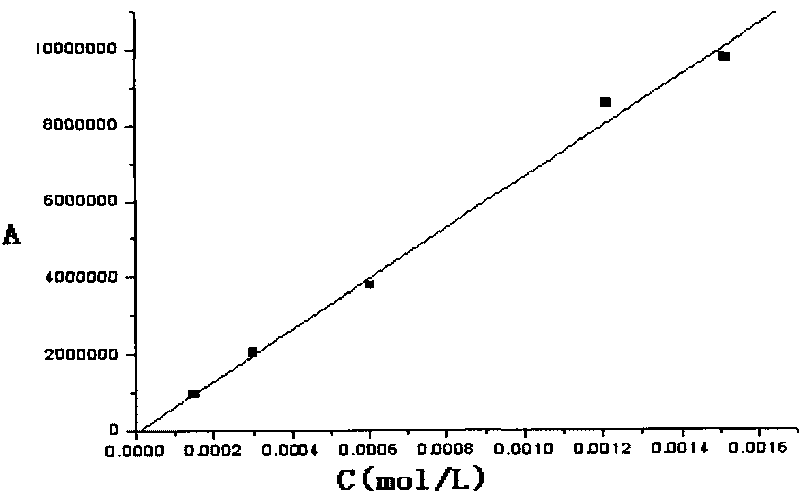
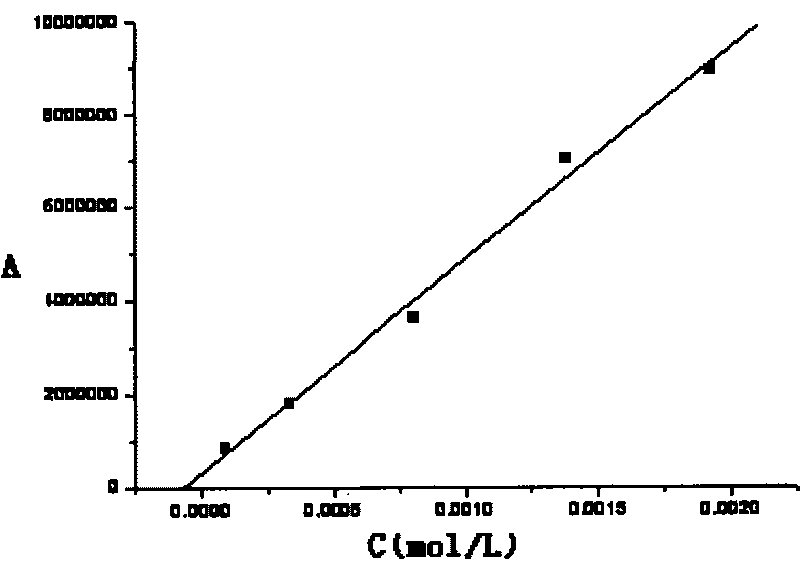
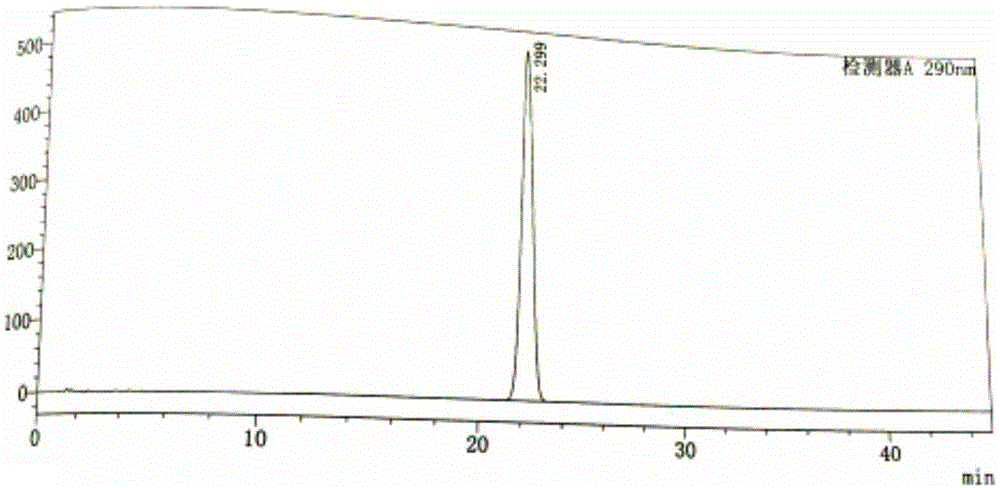
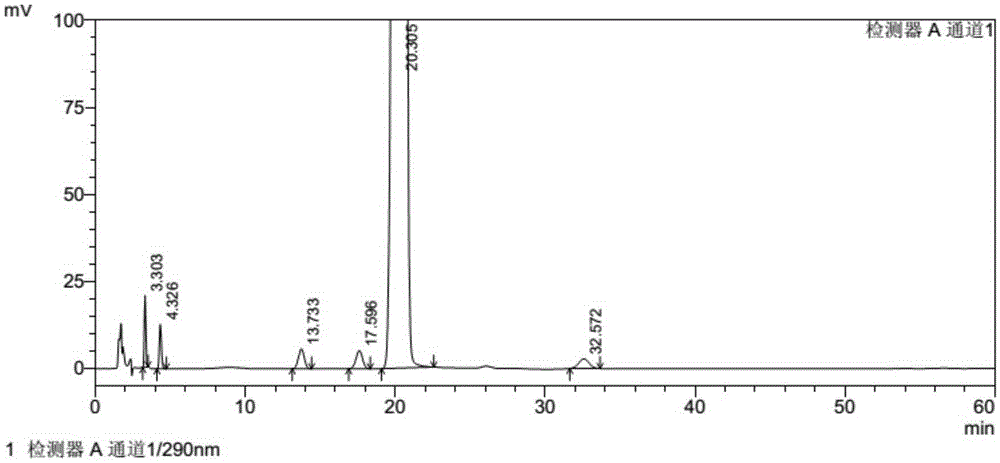
Patents
Literature
1463 results about "Column temperature" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
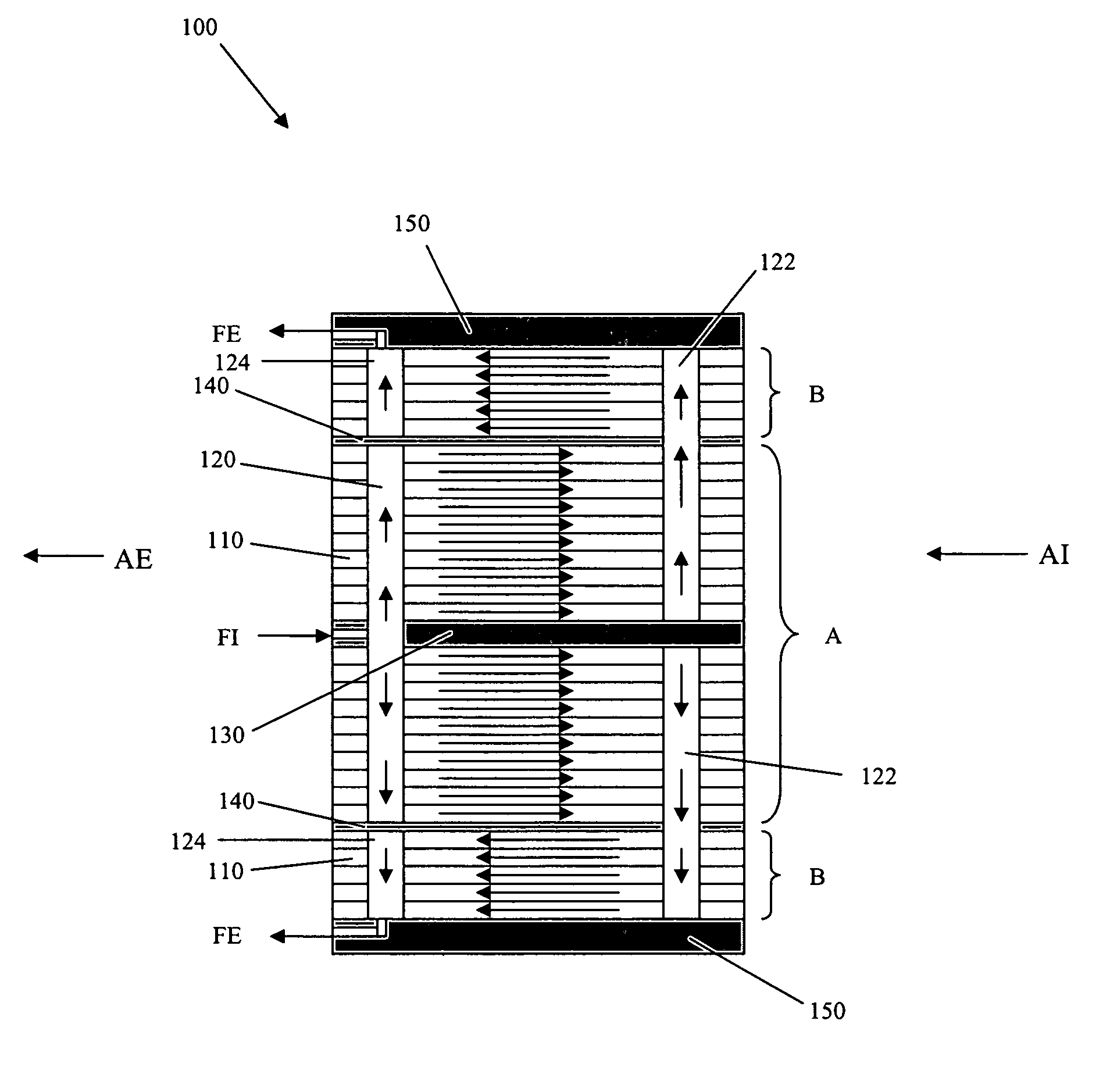
Solid oxide fuel cell column temperature equalization by internal reforming and fuel cascading
Owner:BLOOM ENERGY CORP
Water base dispersion of fluorinated polymer and process for producing the same
The object of the present invention is a fluoropolymer aqueous dispersion showing only a moderate viscosity increase upon temperature rise and having a low fluorine-containing anionic surfactant concentration as well as a method of producing such fluoropolymer aqueous dispersion. The present invention provides a fluoropolymer aqueous dispersion comprising a particle comprising a fluoropolymer dispersed in an aqueous medium in the presence of a nonionic surfactant, wherein a supernatant for assaying as obtained by subjecting the fluoropolymer aqueous dispersion to 30 minutes of centrifugation at 25° C. and at a gravitational acceleration of 1677 G, when subjected to high-performance liquid chromatography [HPLC] under the conditions of a flow rate of 1.0 ml / minute and a column temperature of 40° C. using an acetonitrile / 0.05 M aqueous solution of phosphoric acid (60 / 40% by volume) mixture as a developing solution, followed by detection at an absorption wavelength at which the nonionic surfactant can be identified, shows a ratio (A1 / A0), which is the ratio between the total area (A0) under the detected line and the area (A1) under the detected line over a retention time period shorter than 16 minutes, of not lower than 0.4 and the supernatant for assaying has a fluorine-containing anionic surfactant content of not higher than 100 ppm.
Owner:DAIKIN IND LTD
Method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S
InactiveCN104634891AGood repeatabilityReduce mistakesComponent separationChromatographic separationPhosphate
The invention discloses a method for rapidly, accurately and repeatedly determining foot-and-mouth disease vaccine antigen 146S. A size exclusion high-efficiency liquid-phase chromatographic column in a molecular weight separation range of 2*10<4> to 1*10<7>Da is adopted to carry out the chromatographic separation on a detected sample on a high-efficiency liquid-phase chromatography. The operation pressure of the chromatography is 1.0MPa to 2.5MPa, the flow rate in the chromatographic column is 0.5 to 1.0 ml / min, a flow phase is phosphate buffer (pH 7.0 to 7.5) containing 0.1M sodium sulfate, and the column temperature is 15 to 25 DEG C. An ultraviolet and laser detector is used for detecting an optical signal of effluent at an outlet of the size exclusion high-efficiency liquid-phase chromatographic column, and a peak area of a sample can be analyzed by virtue of a computer software system of the high-efficiency liquid-phase chromatography. A standard curve of the absorption peak area and 146S concentration is established by virtue of a relation between the ultraviolet absorption peak and the concentration of different 146S standard products of different concentrations. Chromatograph is carried out on the detected sample through the size exclusion high-efficiency liquid-phase chromatographic column. The ultraviolet absorption peak area is measured, and the concentration of 146S in the detected sample can be acquired according to the standard curve.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Method for establishing fingerprint spectrum of liver-enhancing medicine
ActiveCN102539553AEfficient separationEffective characterizationComponent separationColumn temperatureGradient elution
The invention provides a method for establishing a fingerprint spectrum of a liver-enhancing medicine. The liver-enhancing medicine is composed of oriental wormwood, isatis root, angelica, white paeony root, red-rooted salvia root, Radix curcumae, Astragalus mongholicus, Codonopsis pilosula, rhizoma alismatis, sealwort, rehmannia, yam, hawthorn, large-leaved gentian, liquorice and medicated leaven. The establishing method comprises the step of detecting paeoniflorin in the liver-enhancing medicine by using a high efficiency liquid chromatography method, wherein conditions are as follows: a chromatographic column takes octadecylsilane chemically bonded silica as a filling material; mobile phases comprise a mobile phase A which is acetonitrile and a mobile phase B which is an acidic water solution, and the mobile phases are subjected to gradient elution; the flow velocity is 1.0mL / min; the column temperature is 30 DEG C; the detection wavelength is 210nm to 400nm; and the number of theoretical plates is calculated according to the paeoniflorin peak and should not be less than 6000. The obtained fingerprint spectrum is a chromatographic peak which mainly takes active ingredients of the large-leaved gentian, the white paeony root, the liquorice, the red-rooted salvia root, the oriental wormwood and the astragalus mongholicus in raw materials of the liver-enhancing medicine as main parts. According to the establishing method provided by the invention, the fingerprint spectrum capable of comprehensively representing the active ingredients of the liver-enhancing medicine can be effectively obtained and has the characteristics of high precision, stability and repeatability. The obtained fingerprint spectrum can be used for guaranteeing the stability and consistency of the product quality, so that the safety and effectiveness of a product are guaranteed.
Owner:SHIJIAZHUANG DONGFANG PHARMA
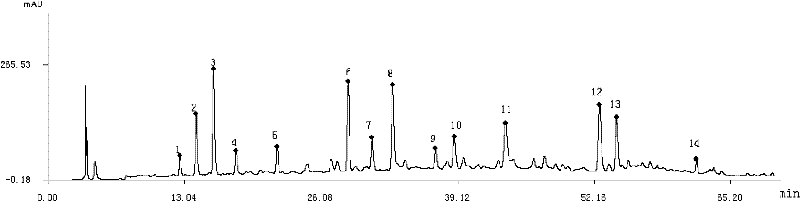
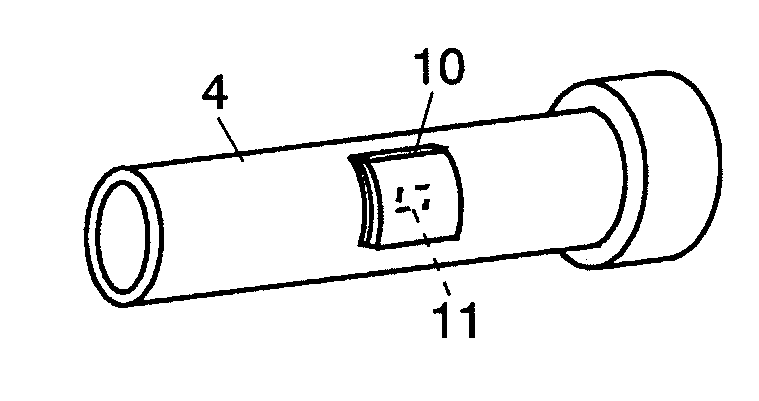
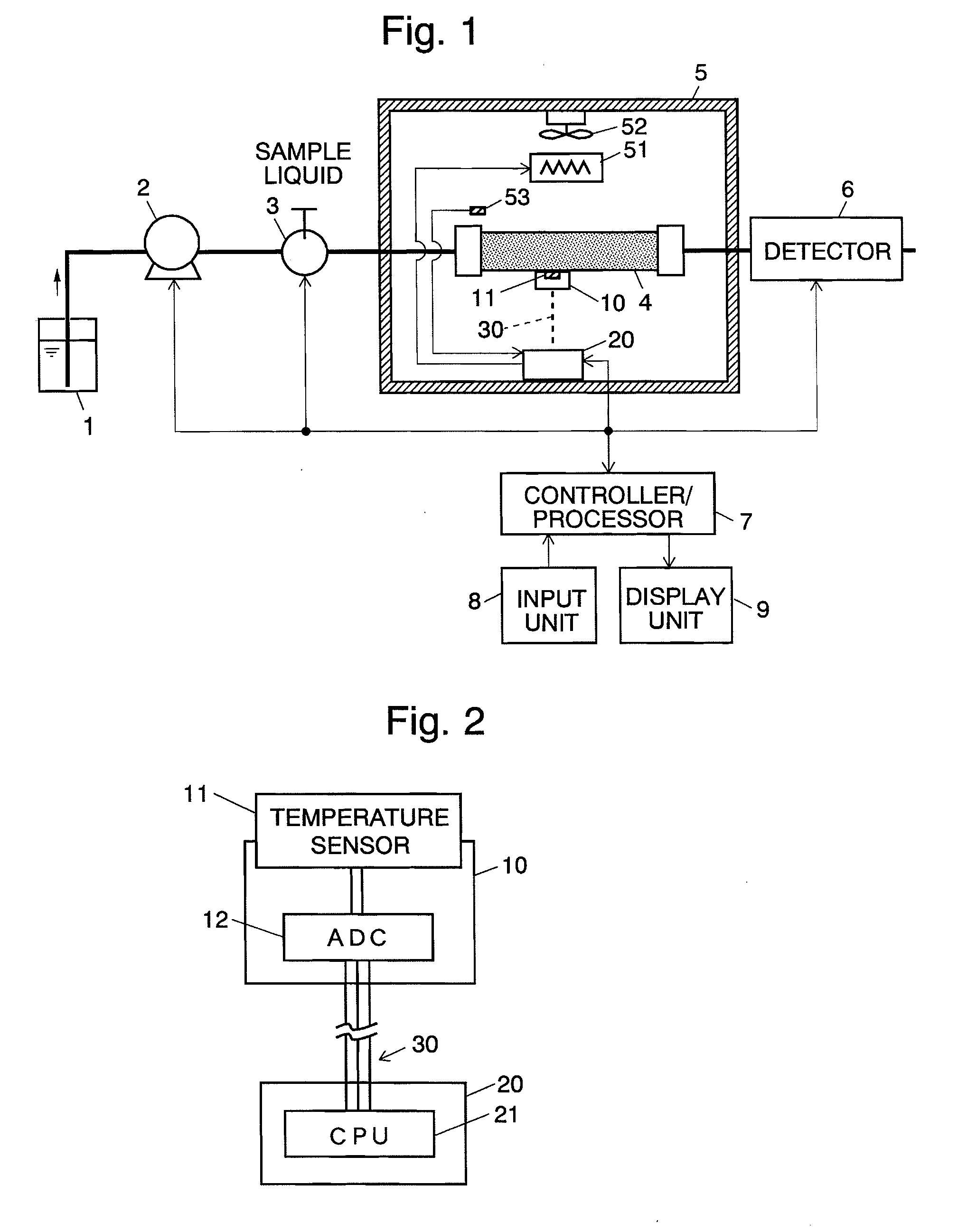
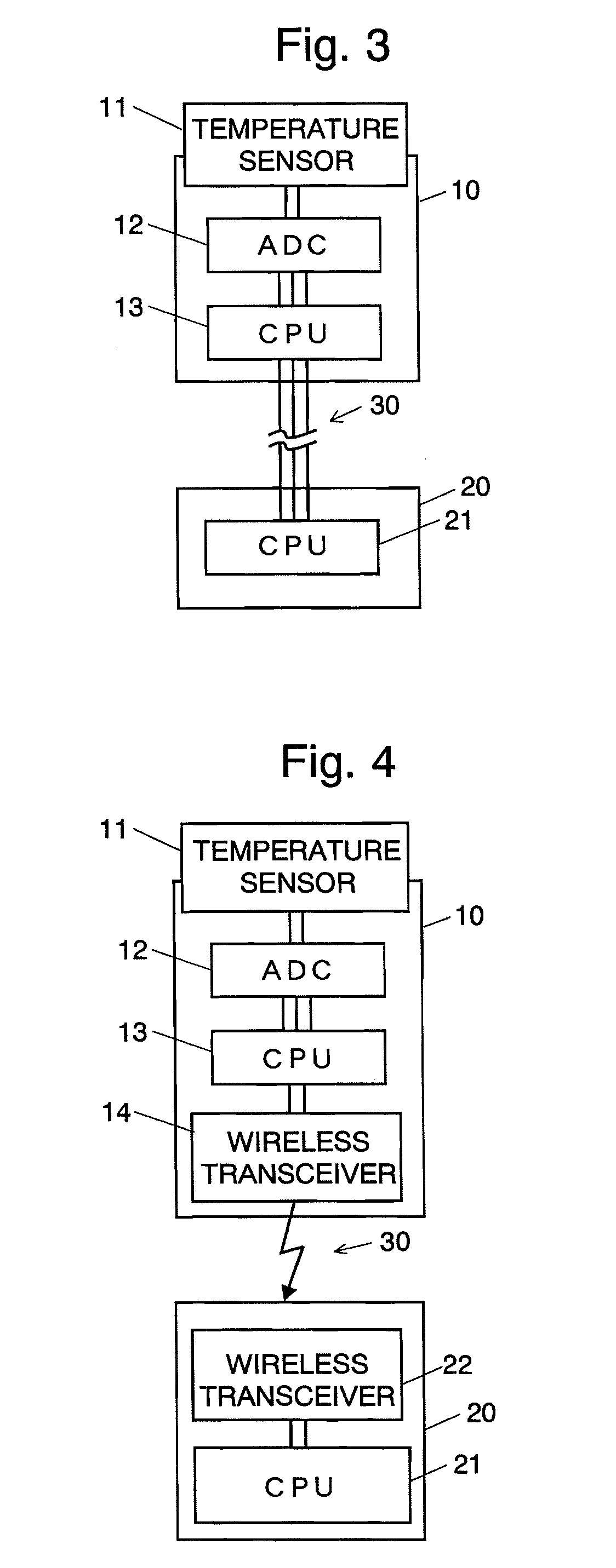
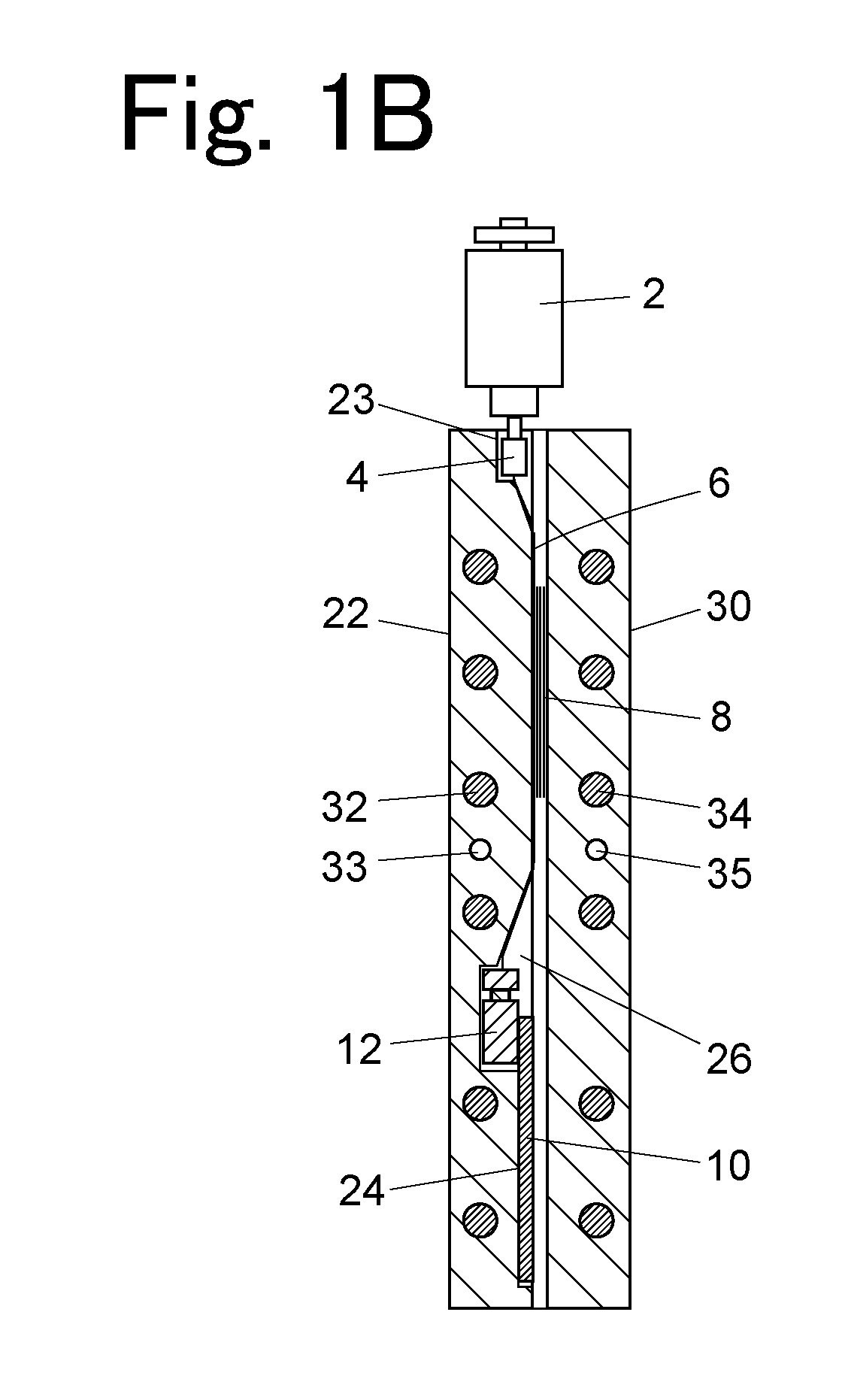
Column temperature monitoring apparatus and chromatographic apparatus
InactiveUS20100044288A1Accurate detectionPrecise temperature controlThermometer detailsComponent separationChromatographic separationTemperature control
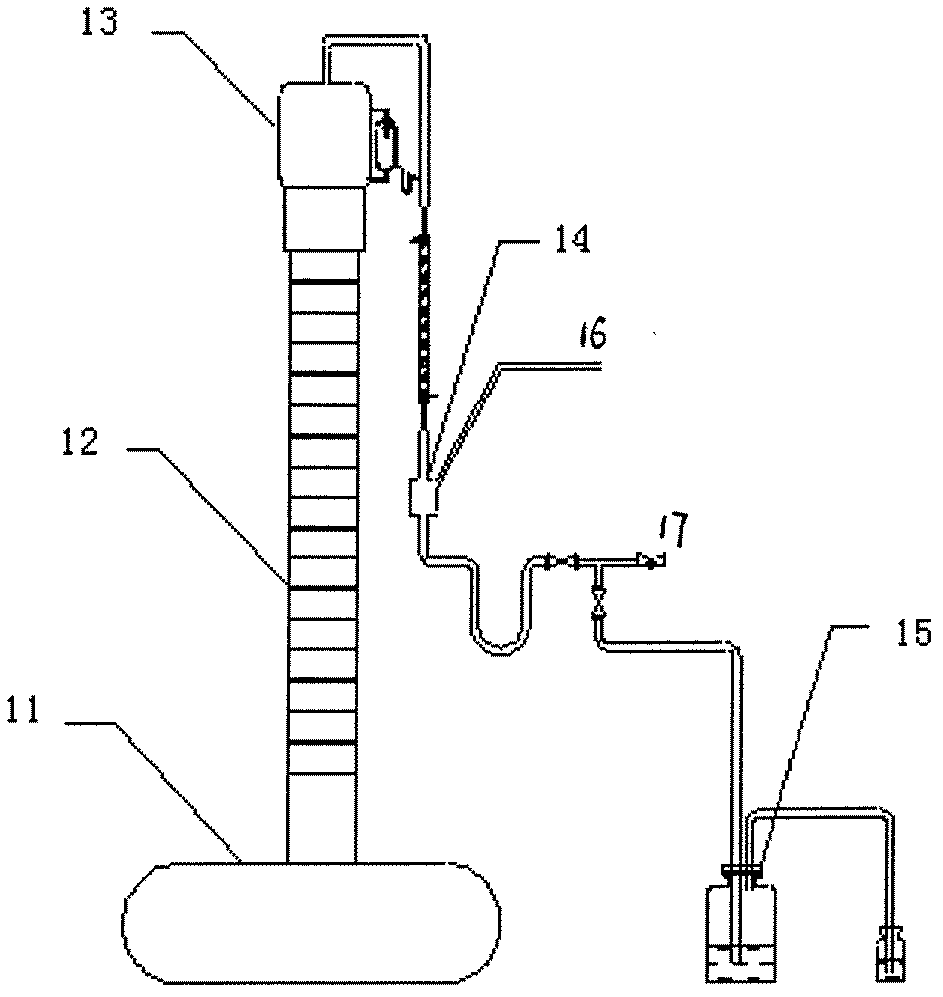
A column temperature monitoring apparatus is provided in which the analysis reproducibility and analysis accuracy are improved by increasing the accuracy of the control of a column temperature. A sensor unit 10 is attached to a column 4 in such a manner that a temperature sensor 11 touches the outer surface of the column 4, and the temperature data read by the temperature sensor 11 is provided to a temperature controlling / processing unit 20 provided in a constant temperature bath 5 via a wired or wireless communication path 30. The temperature controlling / processing unit 20 temperature-controls the column by controlling the heating current supplied to a heater 51 so that the actually obtained column temperature reaches the target temperature indicated from the controller / processor 7. In the column temperature monitoring apparatus according to the present invention, a temperature controlling is performed based not on the temperature of air inside the constant temperature bath or a heat block but on the temperature of the outer surface of the column, which enhances the accuracy of the column temperature's detection.
Owner:SHIMADZU CORP
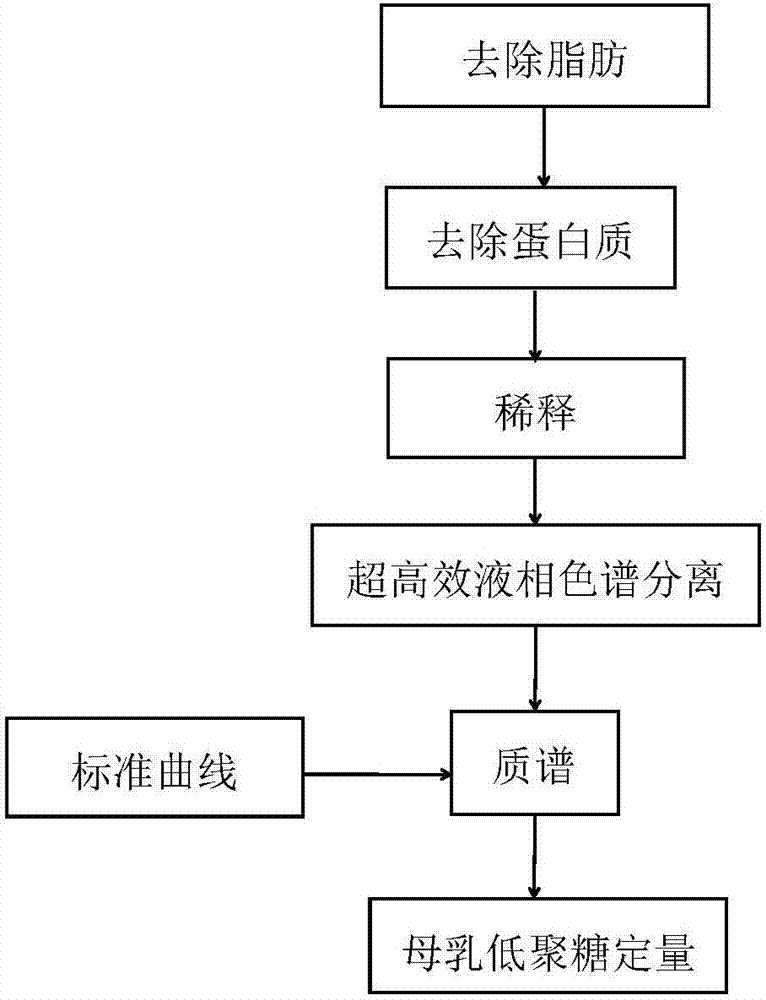
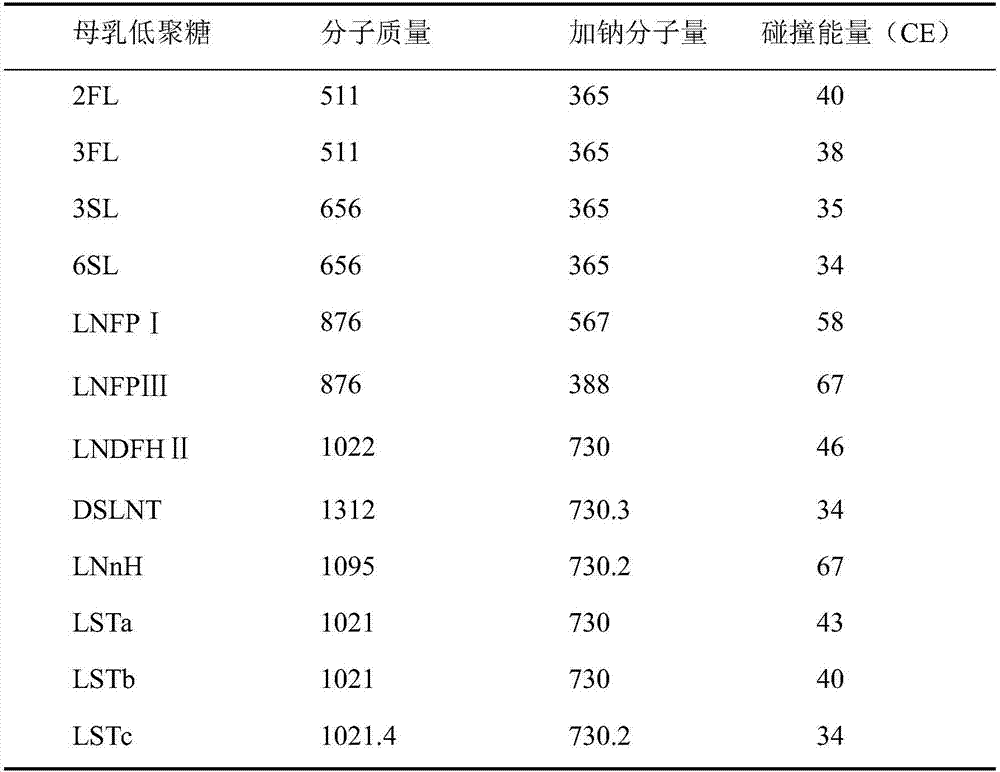
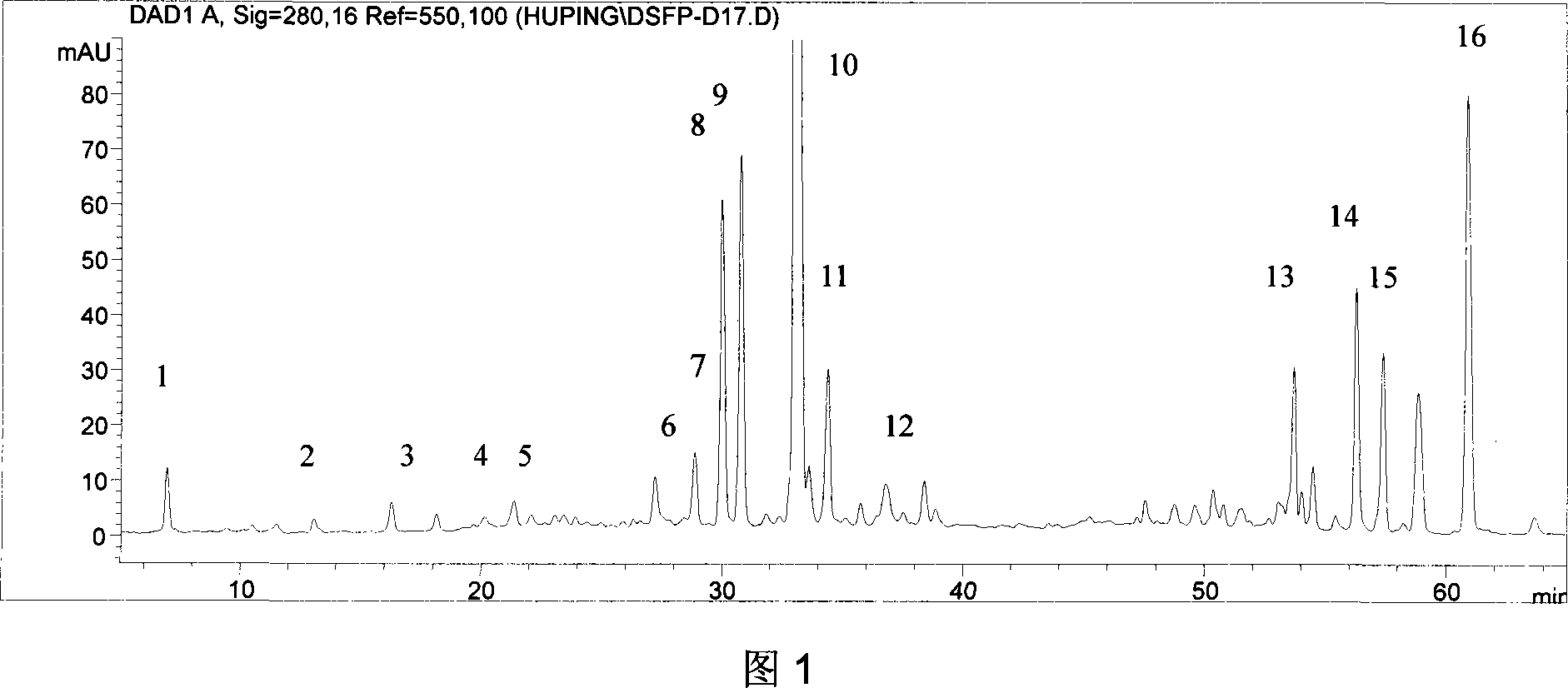
Quick qualitative and quantitative method for oligosaccharide in breast milk
ActiveCN107192771AAccurate detectionQuick checkComponent separationGradient elutionColumn temperature
The invention discloses a quick qualitative and quantitative method for oligosaccharide in breast milk. The quick qualitative and quantitative method mainly includes steps of 1, pretreating samples, to be more specific, removing fat and proteins from 150-250 micro-l of breast milk to obtain ultimate supernatant, adding ultra-pure water into the supernatant and diluting the supernatant to obtain the loaded samples; 2, establishing standard curves for standard substances by the aid of ultrahigh-performance liquid chromatography and mass spectrometry; 3, separating different components of the oligosaccharide in the breast milk in the loaded samples by the aid of ultrahigh-performance liquid mass spectrometry and carrying out quantitative analysis by the aid of mass spectrometry combined with the standard curves so as to obtain the content of the oligosaccharide in the breast milk. The ultrahigh-performance liquid chromatography is implemented by the aid of amino chromatographic columns with the sizes of 2.1*100 mm and 1.7 micrometers, 8-10 mmol / L of ammonium formate solution (A) and acetonitrile (B), and the ammonium formate solution (A) and the acetonitrile (B) are used as mobile phases; gradient elution programs are carried out by the aid of 95%-75% of B for 0-10 min or are carried out by the aid of 75% of B for 10-15 min or are carried out by the aid of 75%-65% of B for 15-20 min or are carried out by the aid of 65%-10% of B for 20-21 min or are carried out by the aid of 10% of B for 21-24 min or are carried out by the aid of 10%-95% of B for 24-25 min or are carried out by the aid of 95% of B for 25-35 min; the flow rates are 0.3 mL / min, and the column temperatures are 40-60 DEG C. The quick qualitative and quantitative method has the advantage that 12 types of oligosaccharide in the breast milk can be quickly detected by the aid of the quick qualitative and quantitative method and can be quantified by the aid of the quick qualitative and quantitative method.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Method of controlling the quality of salvia miltiorrhiza raw material fingerprint in the plant medicine for improving hemorheology
ActiveCN101040907AGuarantee normal implementationHigh sensitivityOrganic active ingredientsComponent separationTanshinone IIAColumn temperature
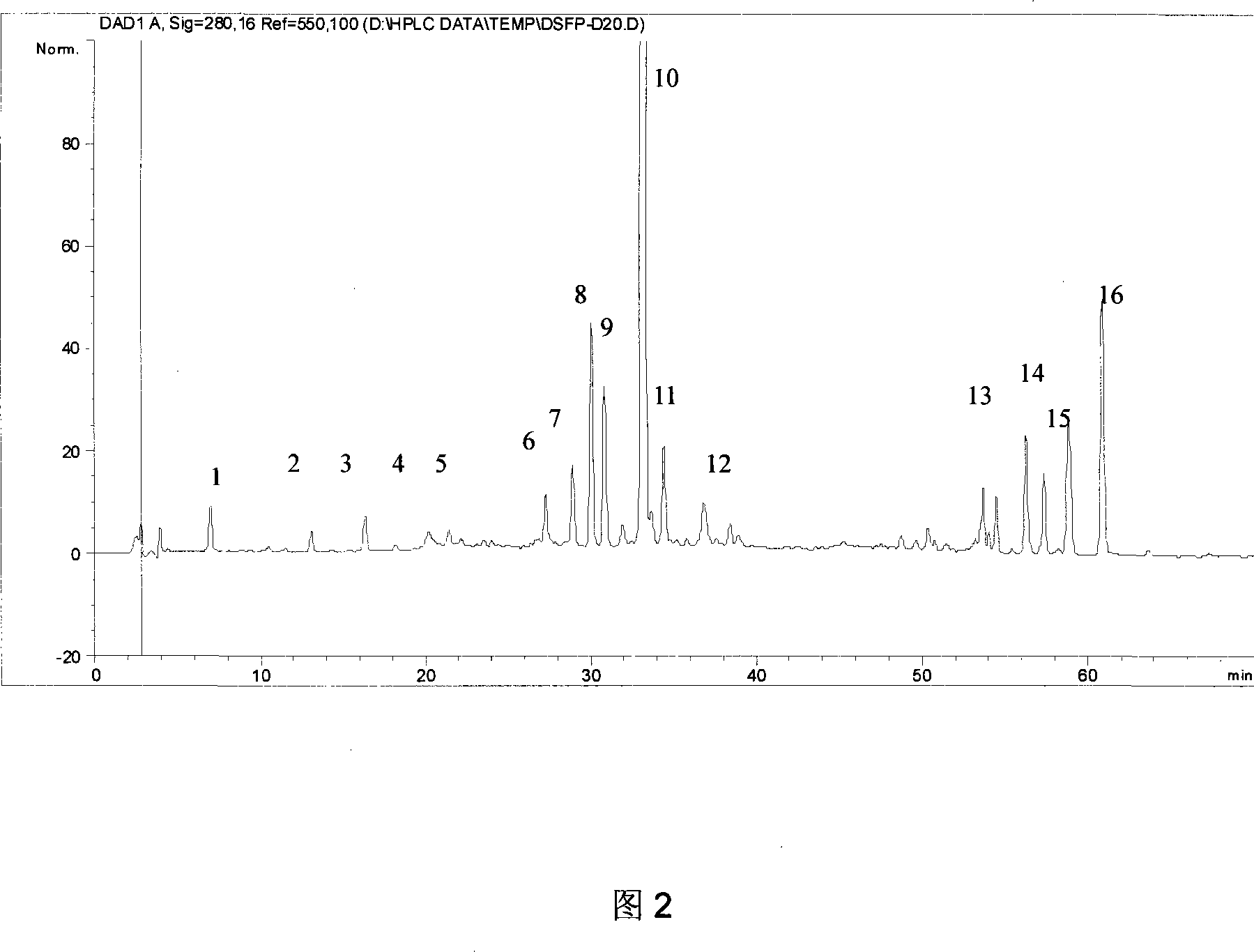
The invention relates to a DanShen fingerprint spectrum quality control method, comprising that (1), adding 1.0g DanShen powder into carbinol to extract via microwave, (2), washing flow phase gradient that the Alltima C18 column is 4.6mm, 250mm, and 5mum, checking wavelength is 280nm, flow speed is 1.0ml / min, the column temperature is 20-40Deg. C, and the sample amount is 10-20ul, (3), building standard fingerprint spectrum that the first peak is DanShen element, the eighth peak is rosmarinic acid, the ninth peak is alkannic acid, the tenth peak is phenolic acid B, the eleventh peak is phenolic acid B isomer, the fourteenth peak is cryptotanshinone and the fourteenth peak is tanshinone IIA, (4), controlling the quality of fingerprint spectrum that the check peak relative holding times are 0.21 of DanShen element, 0.91 of rosmarinic acid, 0.93 of alkannic acid, 1.00 of phenolic acid B, 1.04 of phenolic acid B isomer, 0.92 of cryptotanshinone and 1.00 of tanshinone IIA, (5), the DanShen planting collecting method. The invention can control the quality of materials to assure the stable quality of product.
Owner:ANHUI ZHIHETANG PHARMACY
Method for determining N-methylamino ammate in production of acesulfame
ActiveCN103018368AAccurate measurementRapid determinationComponent separationOrganic solventColumn temperature
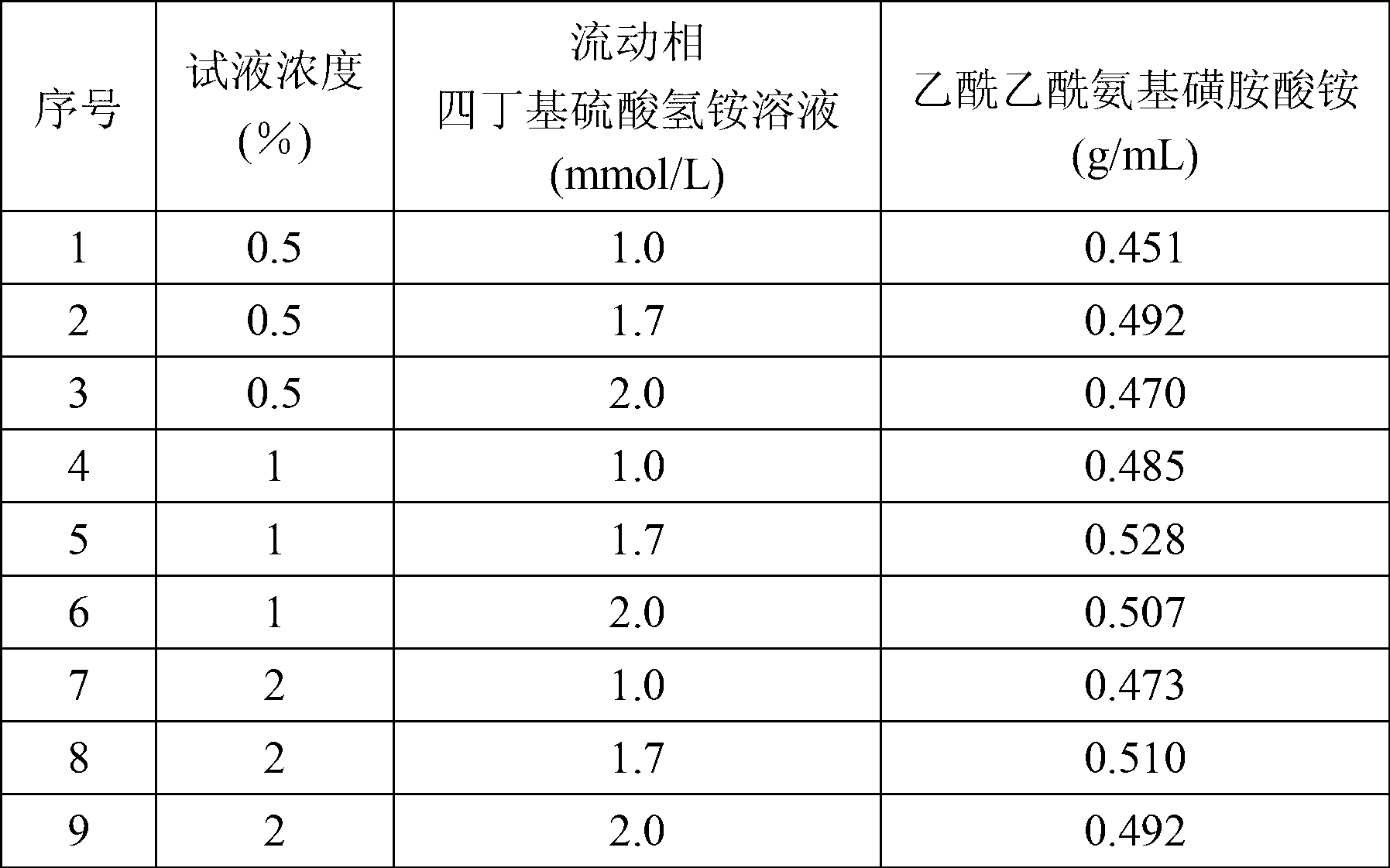
The invention discloses a method for determining N-methylamino ammate in production of acesulfame. The method mainly comprises the steps of: (1) selecting an analytical chromatographic condition, an ODS column (4.6mm*25cm) or other equivalent chromatographic columns, wherein the wavelength is 250nm, the flow velocity is 1ml / min, the column temperature is 25 DEG C, and the sample amount is 20mu l; (2) preparing tetrabutylammonium hydrogen sulfate solution from deionized water until the molar concentration is 1.0-2.0mmol / L, and preparing a mobile phase from the prepared tetrabutylammonium hydrogen sulfate solution and chromatographic grade methanol according to the volume ratio of 60 to 40; (3) diluting the sample to be tested by an organic solvent according to the volume ratio until the concentration is 0.5-2.0%, so as to prepare a test solution; (4) absorbing the test solution by a microinjector, injecting into an efficient liquid chromatograph, and analyzing by an area external standard method; and (5) recording the content of the N-methylamino ammate in the sample to be tested. By adopting the method disclosed by the invention, the content of the N-methylamino ammate can be accurately and rapidly determined.
Owner:南通宏信化工有限公司
Methods and systems for cooling a chromatographic column
InactiveUS20060278076A1Rapid coolingReduce the impactIon-exchange process apparatusComponent separationInlet pressureColumn temperature
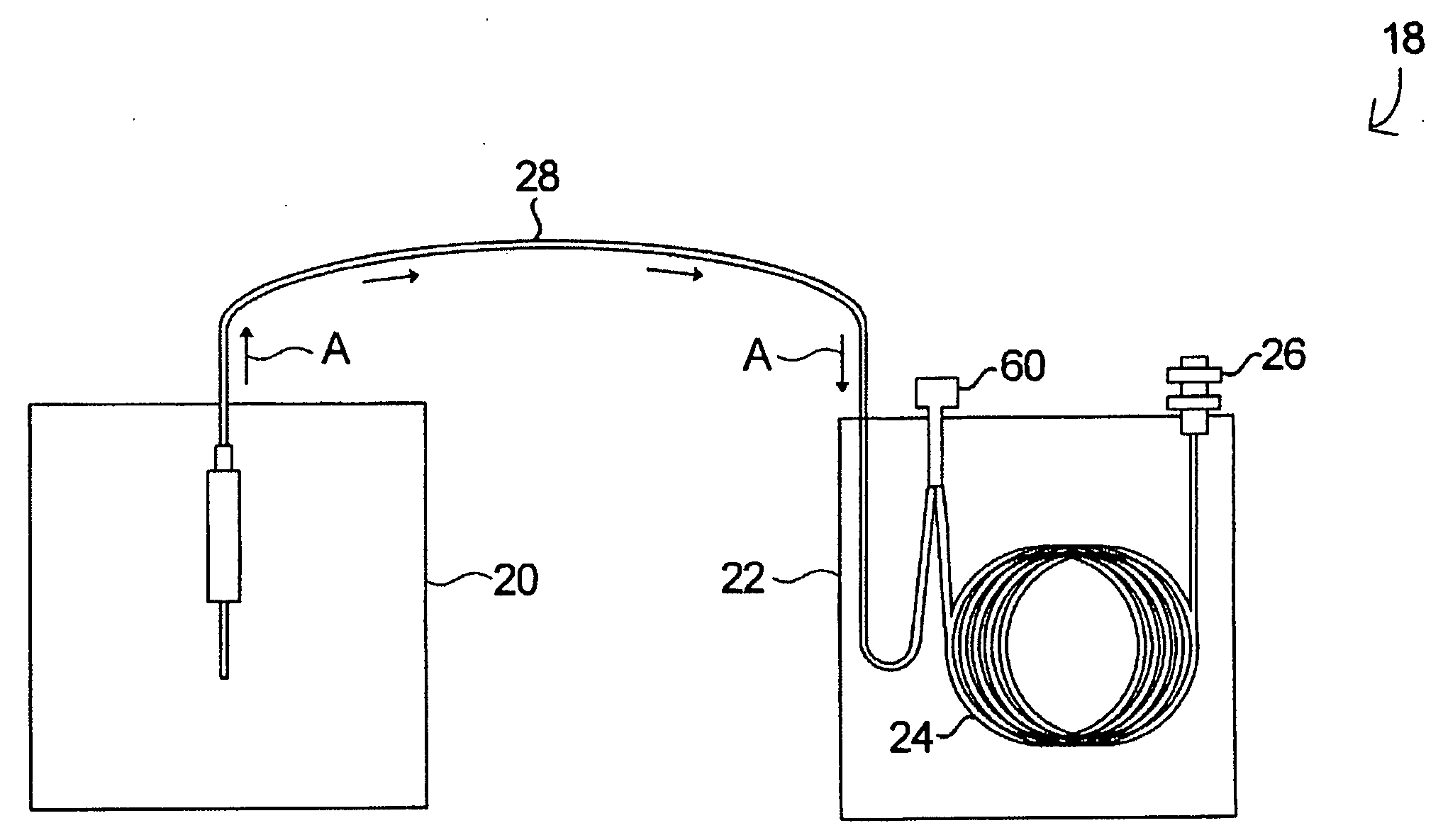
Systems and methods for cooling a chromatographic column is disclosed generally, comprising heating a chromatographic column, supplying fluid into the column via the inlet end of the column at an inlet pressure, decreasing the temperature of the column, thereby causing the fluid in the column to contract, and controlling the fluid in the column such that the rate at which the fluid in the column contracts does not exceed the flow rate of the fluid supplied to the column. In certain embodiments, the rate of change of the volume of the fluid in the column as the column temperature decreases is modeled, and the rate of contraction of the gas in the column is estimated therefrom. In some embodiments, the column temperature and / or inlet pressure are controlled by a programmable chromatographic oven.
Owner:PERKINELMER HEALTH SCIENCES INC
Methods and systems for cooling a chromatographic column
InactiveUS7534286B2Rapid coolingReduce impactIon-exchange process apparatusComponent separationPhysical chemistryColumn temperature
Systems and methods for cooling a chromatographic column is disclosed generally, comprising heating a chromatographic column, supplying fluid into the column via the inlet end of the column at an inlet pressure, decreasing the temperature of the column, thereby causing the fluid in the column to contract, and controlling the fluid in the column such that the rate at which the fluid in the column contracts does not exceed the flow rate of the fluid supplied to the column. In certain embodiments, the rate of change of the volume of the fluid in the column as the column temperature decreases is modeled, and the rate of contraction of the gas in the column is estimated therefrom. In some embodiments, the column temperature and / or inlet pressure are controlled by a programmable chromatographic oven.
Owner:PERKINELMER HEALTH SCIENCES INC
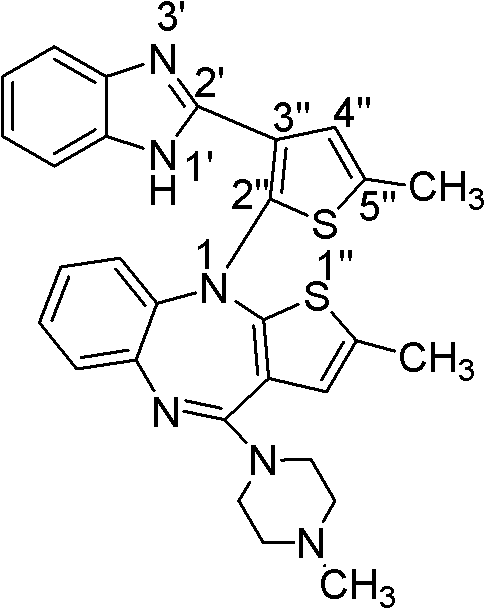
A kind of olanzapine related substance and its preparation method and high performance liquid chromatography analysis method
ActiveCN102276624ARich varietyHigh sensitivityOrganic chemistryComponent separationHplc methodGradient elution
The invention discloses an olanzapine related substance and a preparation method as well as a high-efficiency liquid-phase chromatographic analysis method thereof. The olanzapine related substance has a structural formula shown in the specification. The preparation method of the olanzapine related substance comprises the following steps of: concentrating olanzapine ethanol recrystallization mother liquor; and separating through silica gel column chromatography to prepare the olanzapine related substance. In addition, the invention provides the high-efficiency liquid-phase chromatographic analysis method of the olanzapine related substance. In the high-efficiency liquid-phase chromatographic analysis method, a reversed phase C18 chromatographic column is selected and used, the detection wavelength is 220-280nm, the flow velocity is 0.8-1.0ml / minute, the column temperature is 25-30 DEG C, acetonitrile and a 0.1-0.4-percent buffer solution of glacial acetic acid and triethylamine in equal proportion are used as a mobile phase to perform gradient elution; the olanzapine related substance and other eight related substances can be simultaneously detected; and thus, quality control of olanzapine and olanzapine-containing medicaments can be completely, scientifically, effectively and quickly realized.
Owner:DALIAN UNIV OF TECH
Method for measuring trace chloridion and sulfate radical in loprazolam samples by ion chromatography
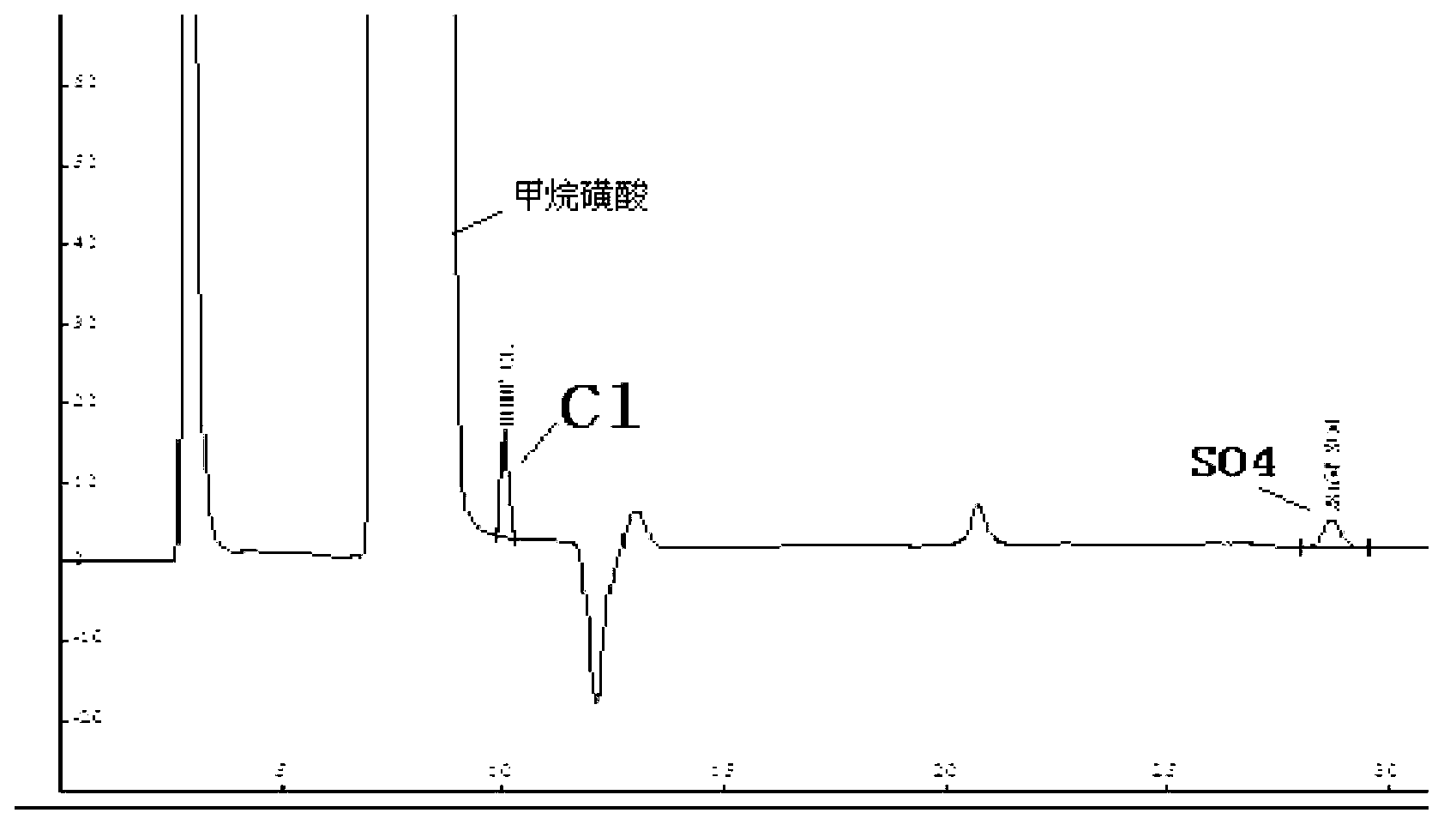
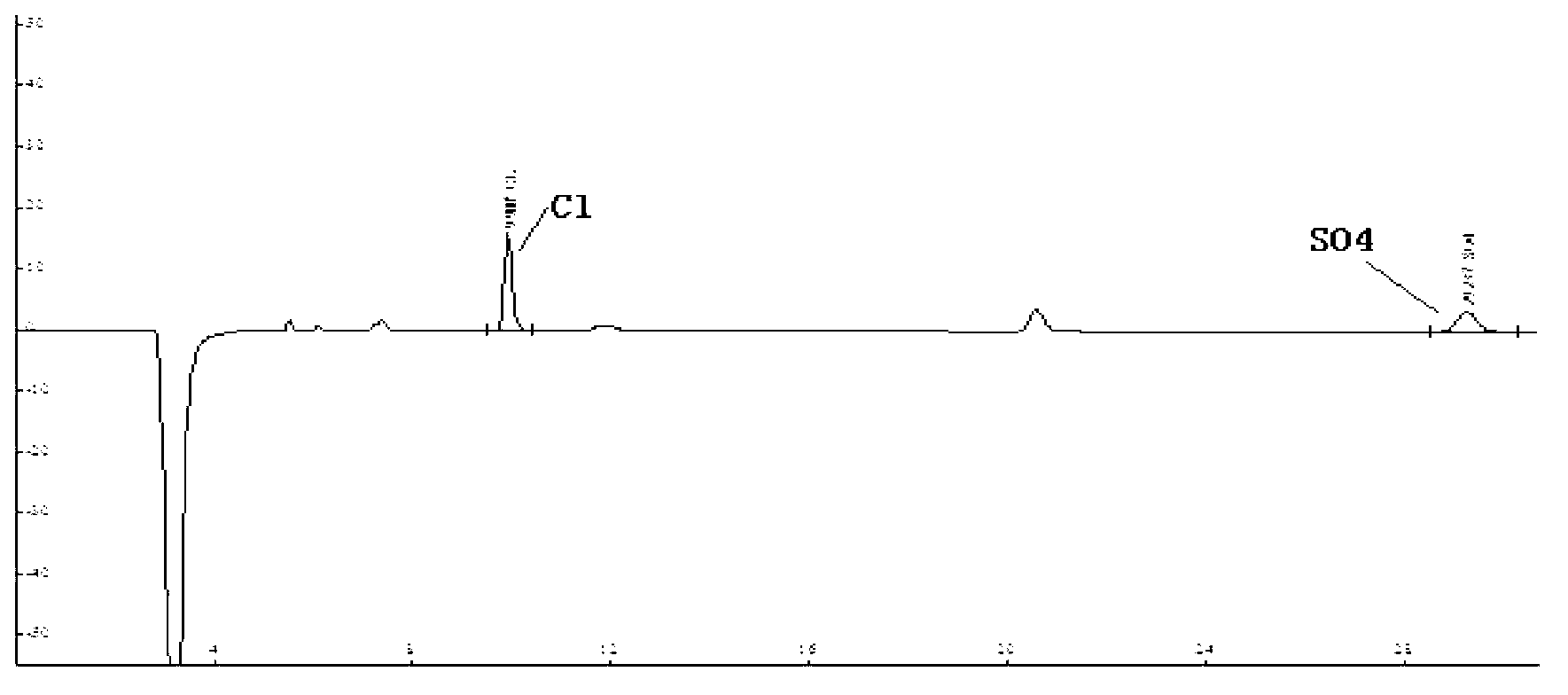
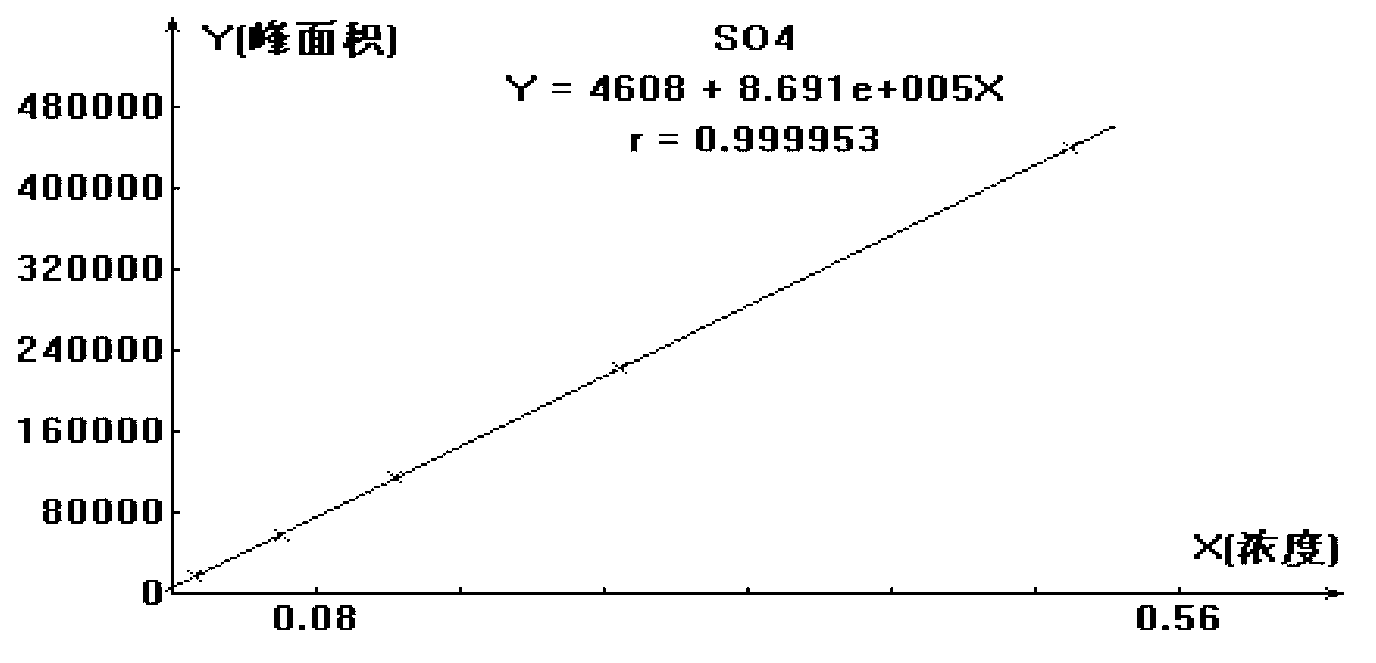
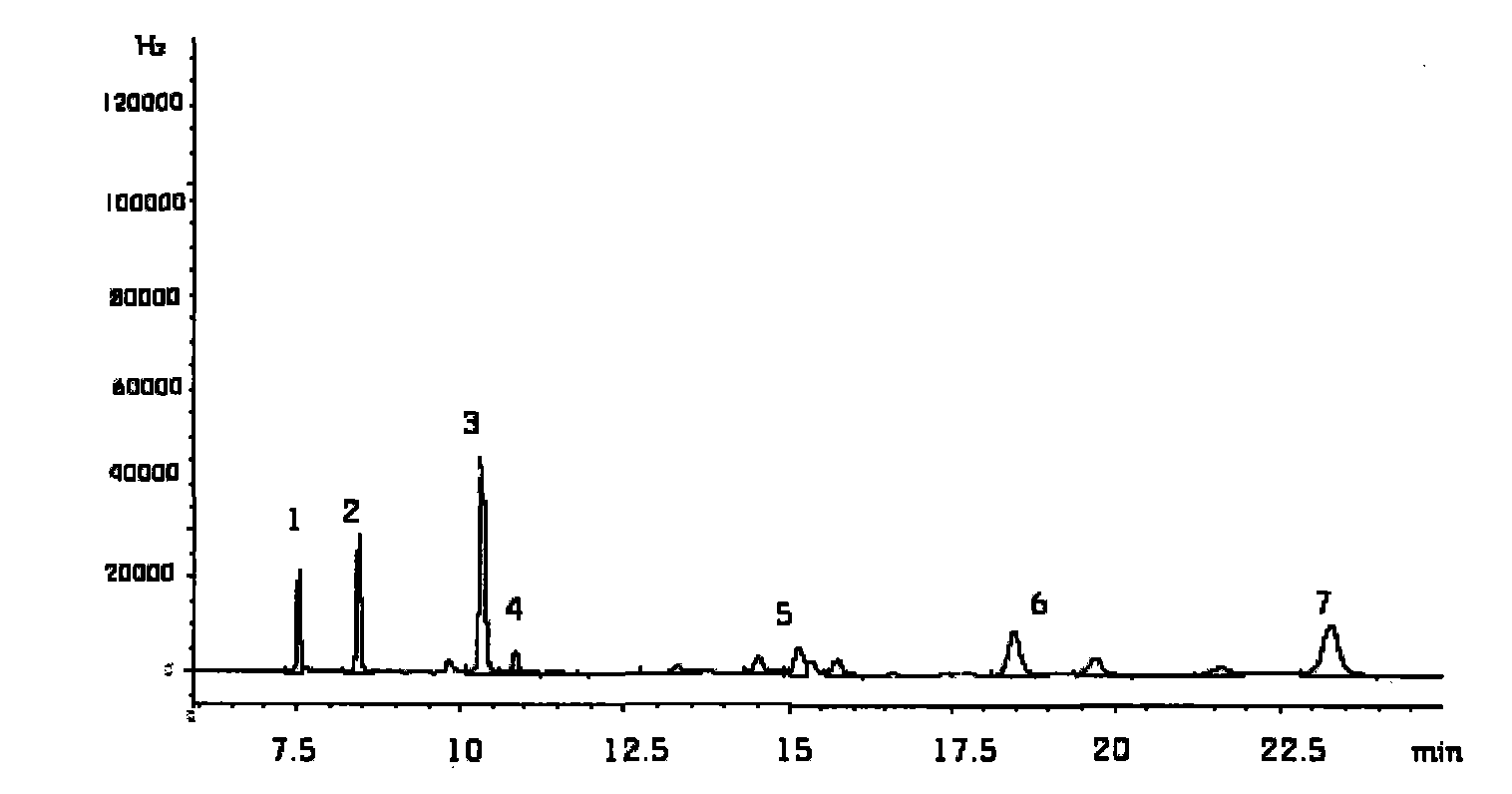
ActiveCN102914600AMeet testing needsImprove accuracyComponent separationSulfate radicalsIon chromatography
The invention discloses a method for measuring trace chloridion and sulfate radical in loprazolam samples by ion chromatography and belongs to the field of analytic chemistry. The method includes the followings steps of preparing and analyzing Cl- and SO42- mixed standard solution and obtaining a linear equation of concentration and peak area, pretreating the loprazolam samples, feeding the treated loprazolam samples to an ion chromatography by a disposable needle type filter, utilizing 3.6mmol / L sodium carbonate as leacheate and analyzing and measuring the content of the chlorodion and the sulfate radical according to the obtained linear equation, wherein a chromatographic column adopted in the ion chromatography is a high-capacity chromatographic column, the inner diameter of filler of the chromatographic column is 5 micrometers, the temperature of a column temperature box is set to be 45 DEG C, sampling input quantity is 100 microliters, applied curb current is 40mA, and flow velocity is 0.8ml / min. The method is quick and simple, high in accuracy, convenient and easy to operate and applicable to quality control in the process of production of loprazolam, the integral analyzing process can be completed within 25min, and the detection requirements of the fine chemical industry are met.
Owner:ANHUI WAYEE SCI & TECH CO LTD
Detection method of pyrethroid pesticide remained in rice
InactiveCN101915809AEasy to washHigh quantitative recoveryComponent separationAcetonitrileColumn temperature
The invention discloses a detection method of pyrethroid pesticide remained in rice. The method comprises the following steps of: fully extracting pyrethroid pesticide with acetonitrile for 4 h; vibrating with ultra-pure water and then standing still for 30 min so that the pyrethroid pesticide is separated very well; centrifugally splitting the phase of the pyrethroid pesticide for 6 min to have better split phases and higher extraction rate; leaching and cleaning with hexane and acetone (in a ratio of 90 to 10) to better remove the pyrethroid pesticide; and increasing a column temperature to 260 DEG C at a programmed temperature rise rate of 40 DEG C / min and keeping both a sampling temperature and a detector temperature at 260 DEG C. The gas chromatograph is simple and convenient to operate, finishes the separation and the detection of the pyrethroid pesticide in a shorter time and has simple and rapid gas chromatography conditions and more accurate measuring value. Accordingly, the detection method of the pyrethroid pesticide remained in rice has higher extraction rate, simple and rapid gas chromatography conditions, lower operation requirement and higher quantitative recovery rate.
Owner:王冬群 +2
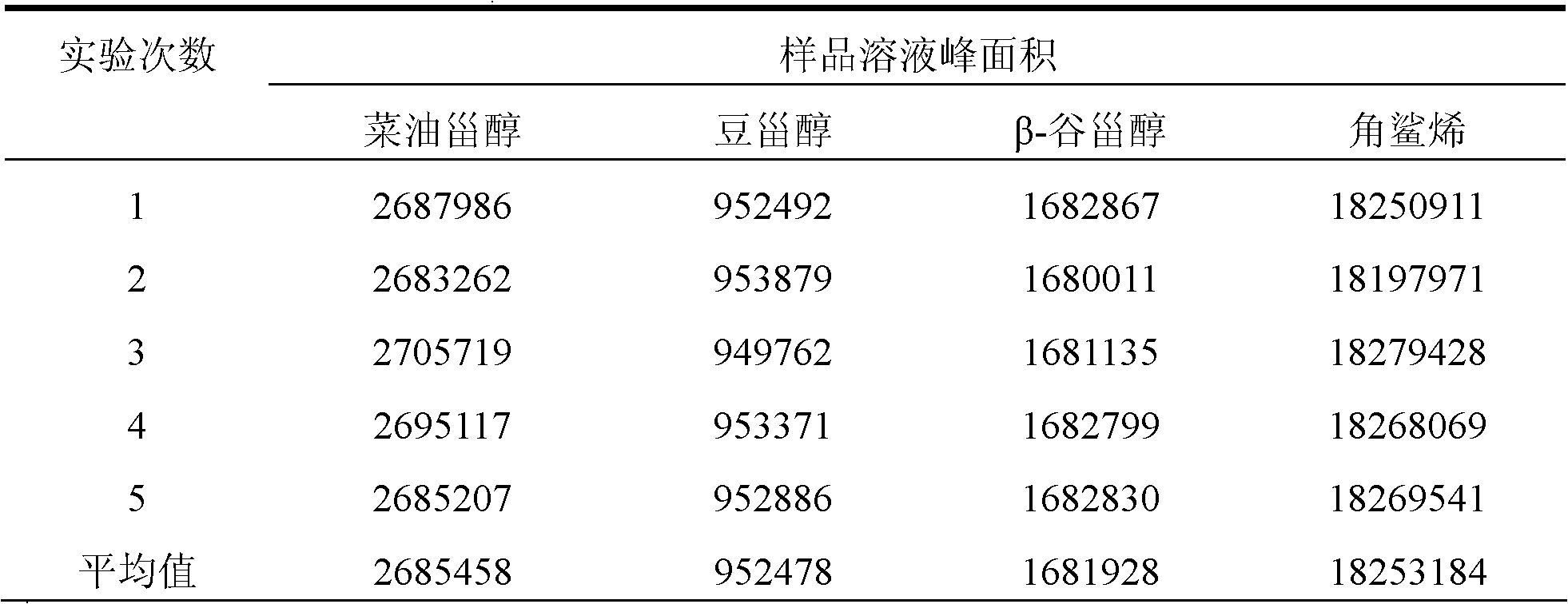
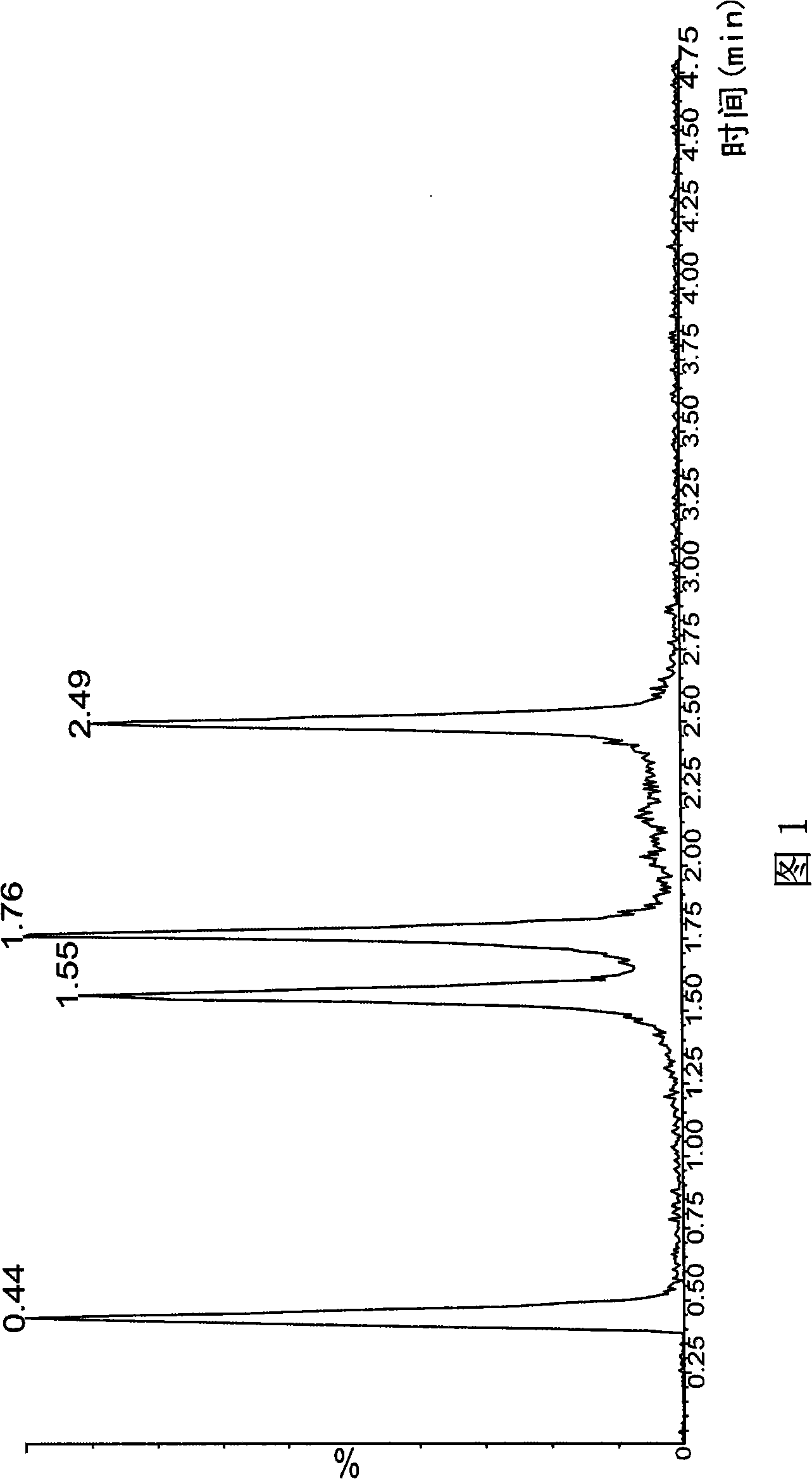
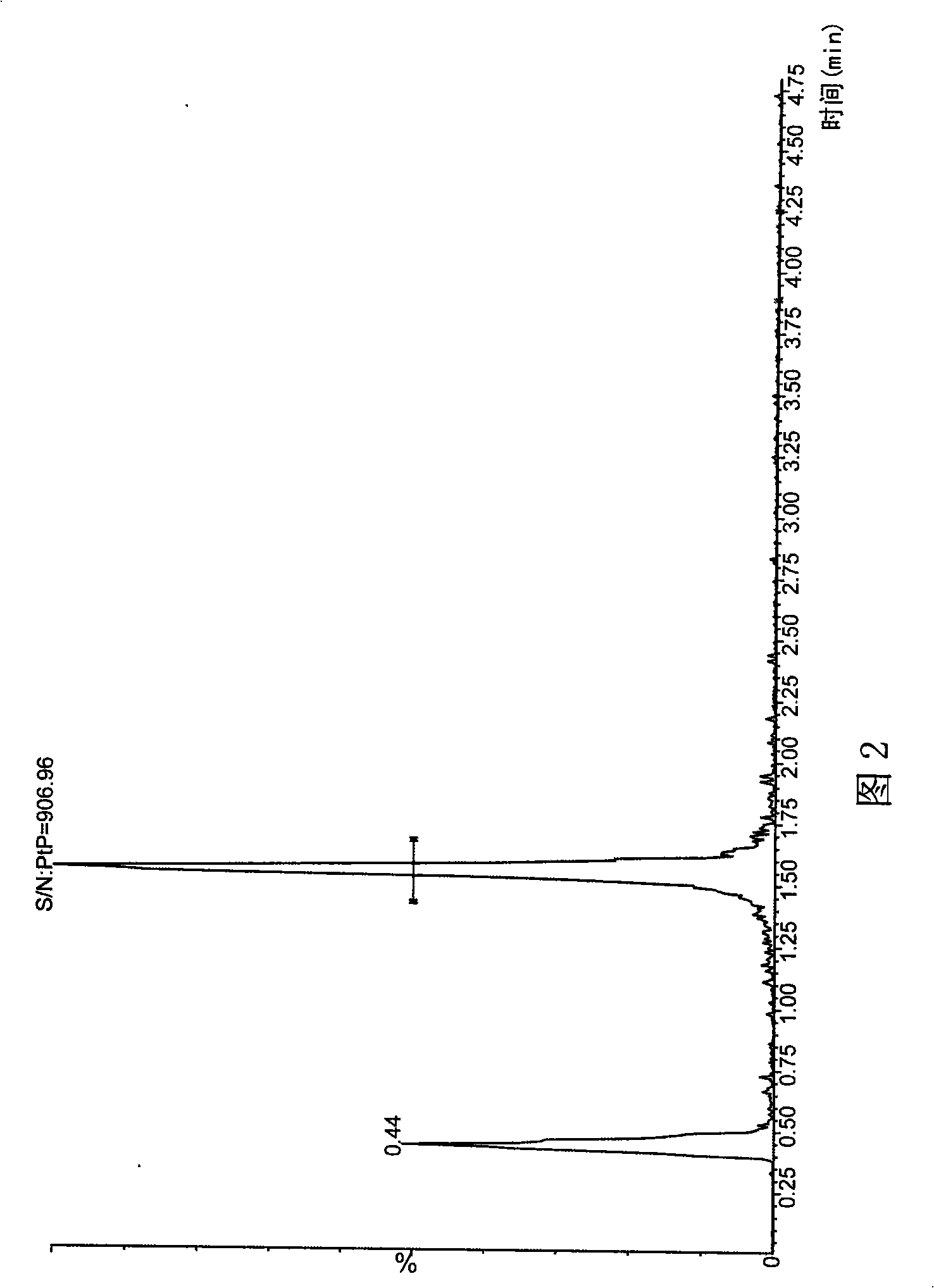
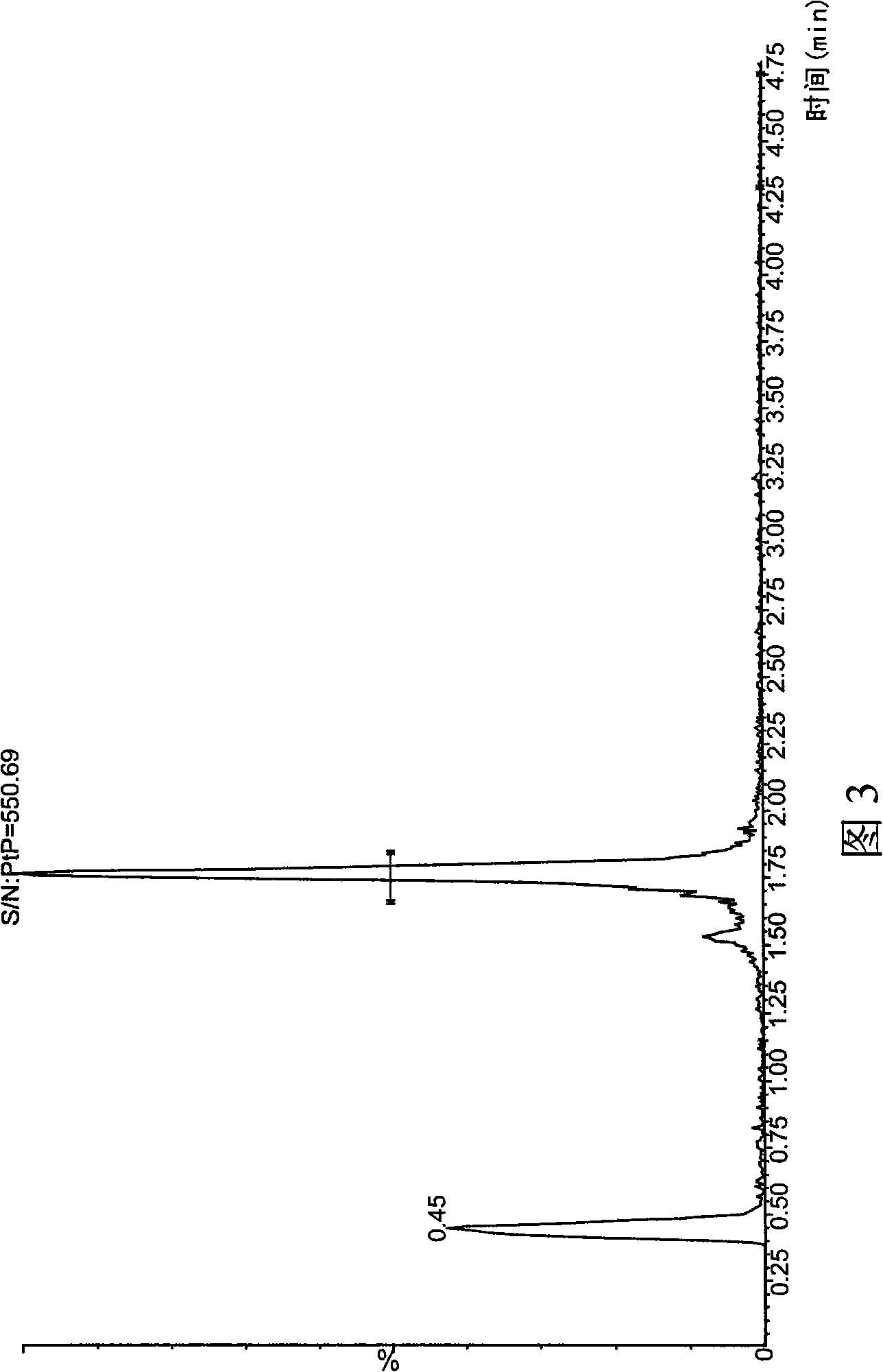
Method for simultaneously determining phytosterol and squalene in vegetable oil
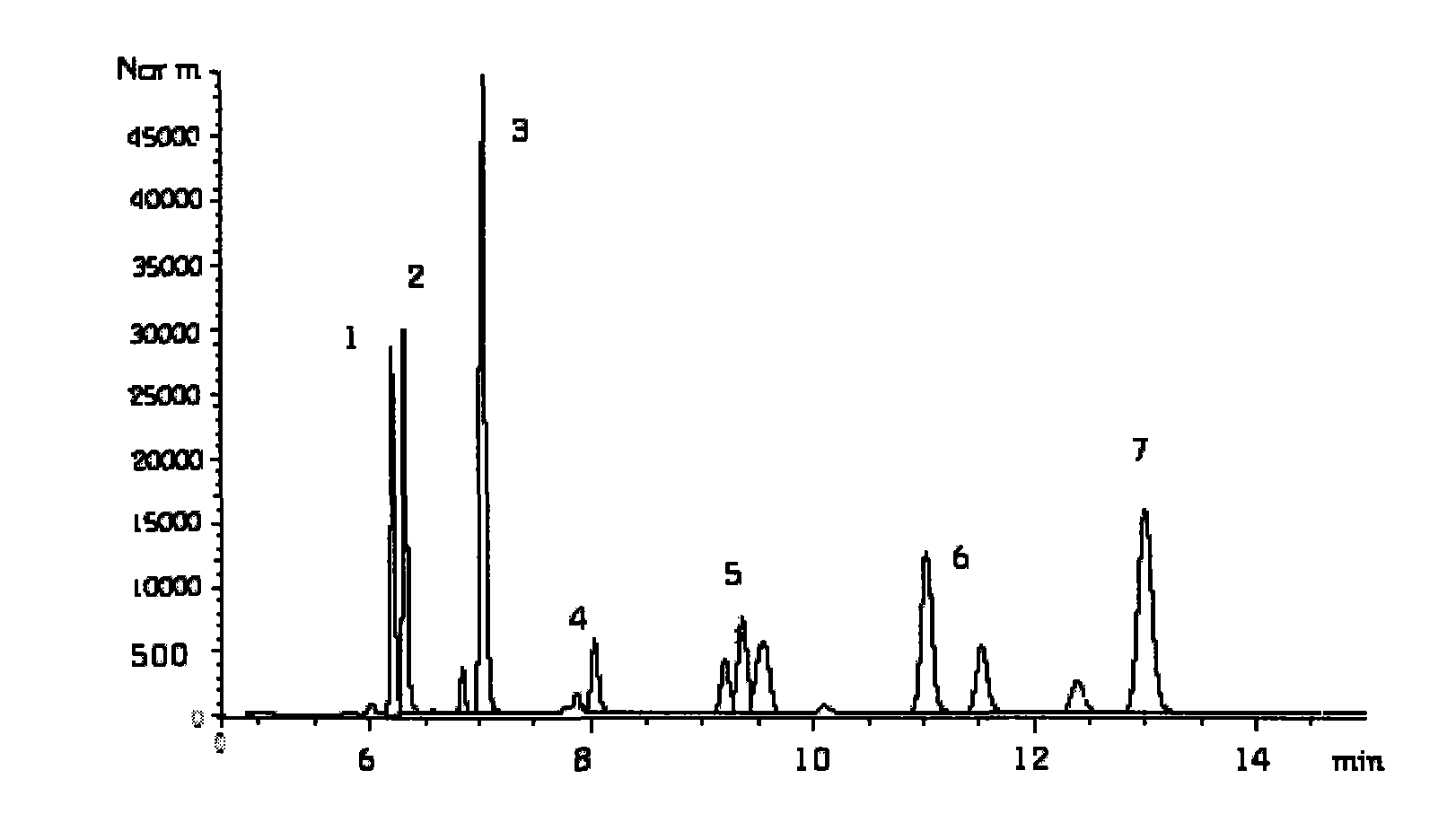
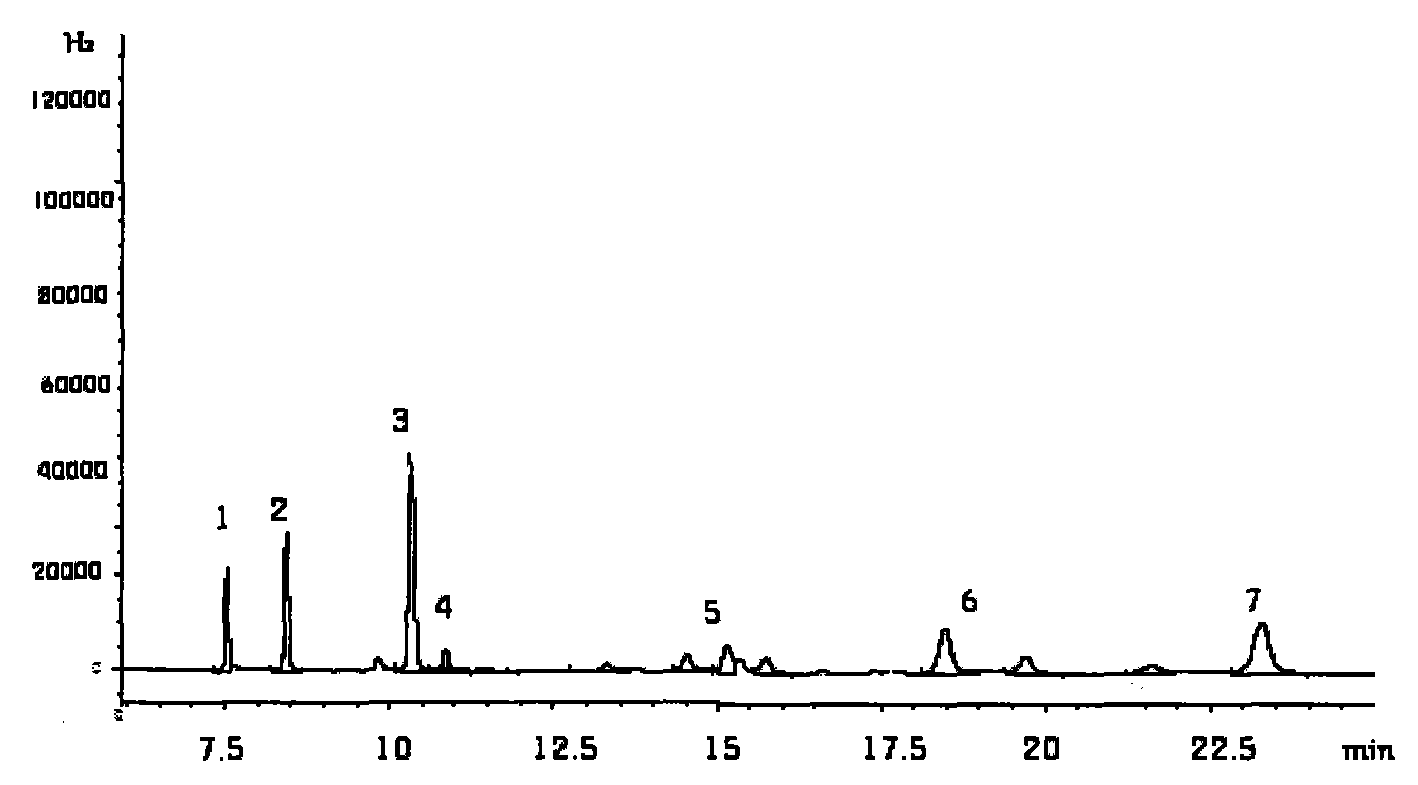
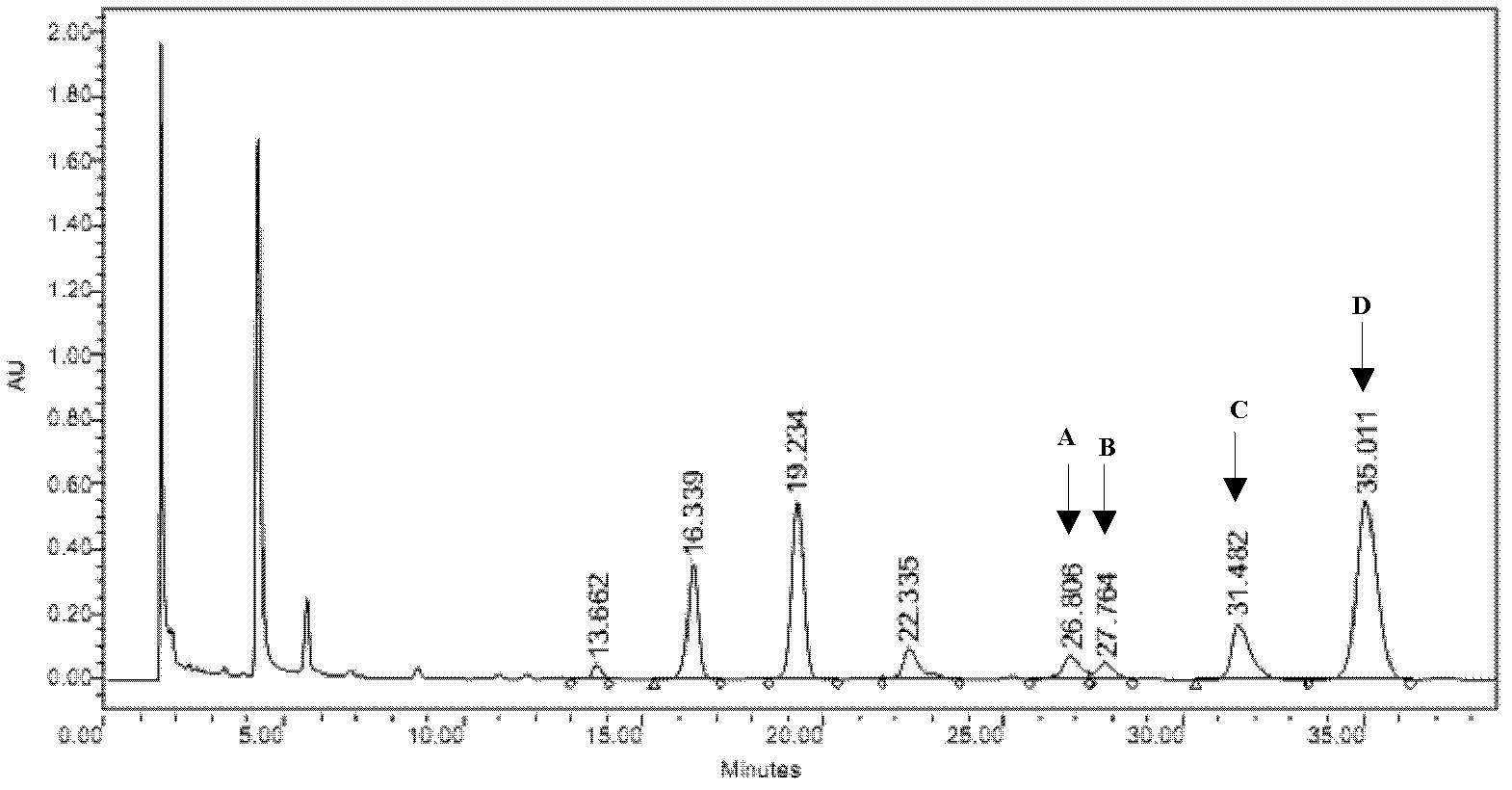
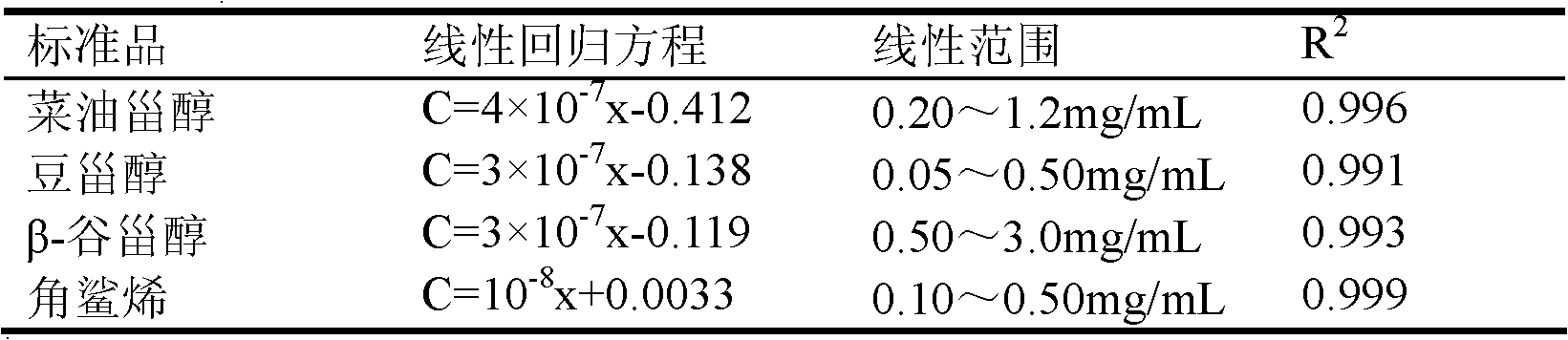
The invention discloses a method for simultaneously determining stigmasterol, campesterol, beta-sitosterol and squalene in vegetable oil. The method comprises the following steps of: 1) saponifying the vegetable oil, and extracting an unsaponifiable matter in a saponification solution by using an organic solvent; and 2) dissolving the unsaponifiable matter in the organic solvent, and determining by using high-performance liquid chromatography, wherein the high-performance liquid chromatography has the chromatographic conditions that a chromatographic column is a C18 column, a mobile phase is a solvent of which the volume ratio of acetonitrile to water is (90:10) to (100:0), an ultraviolet detection wavelength is 205 to 210 nm, a sample size is 10 to 20 mu L, flow speed is 1.0 to 1.5 mL / min and column temperature is 25 to 30 DEG C. By adoption of the method, effective separation of the campesterol, the stigmasterol, the beta-sitosterol and the squalene in the vegetable oil can be simultaneously realized, consumed time for separation is about 40 min, and components in a chromatogram are eluted in a sequence of the campesterol, the stigmasterol, the beta-sitosterol and the squalene.
Owner:INST OF AGRO FOOD SCI & TECH CHINESE ACADEMY OF AGRI SCI
Milk and milk product tetracycline antibiotic residual quantity checking method
ActiveCN101290306AShort detection timeHigh sensitivityComponent separationTesting medicinal preparationsTriple quadrupole mass spectrometryGradient elution
The invention relates to a method for detecting the residue amount of terracycline antibiotics in milk and dairy products. The method utilizes an ultra performance liquid chromatography - electrospray tandem triple quadrupole mass spectrometer to determine the residue amount of the terracycline antibiotics. The method is as follows: a sample is extracted from Na2EDTA-McIlvaine buffer solution (pH4.0); proteins are removed by trichloroacetic acids; a columella is extracted through an HLB solid phase and then purified and enriched; the sample is separated by a chromatographic column, with the column temperature of 30 DEG C; gradient elution is performed by utilization of water solution (v / v) which contains 0.1 percent of methanoic acids and acetonitrile as a moving phase; and quantitative detection is performed by adoption of the multi-reaction monitoring means. The detection limit of instruments is between 1.0 and 2.0 mu g / kg; a related coefficient r reaches over 0.999 within the linear range of between 1 and 100 mu g / kg; and the recovery rate is between 81.7 and 100.7 percent (the addition levels are 10 mu g / kg, 50 mu g / kg and 100 mu g / kg). The method has the advantages of quickness, accuracy, high sensitivity and wide application scope.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Method for fast and high-efficient determination of phenolic acids in grape wine
The invention relates to a method for fast and high-efficient determination of 13 phenolic acids in grape wine, belonging to the technical field of analysis of flavor substances in the grape wine. According to the method disclosed by the invention, ultra-performance liquid chromatography is utilized for performing qualitative and quantitative analysis on phenolic acid substances in the grape wine, and the method specifically comprises the following steps: selecting a BEHC18 chromatographic column, taking 2% acetic acid water and methanol as a mobile phase, setting the flow rate at 0.3mL / min, performing ultraviolet detection on wavelength to get the result that the wavelength of gentisic acid is 320nm and the wavelength of the remainder is 280nm, setting the column temperature at 50 DEG C, setting the sample injection quantity at 1 mu L, and quantifying by a peak area external reference method; and by adopting the method, the detection and the analysis can be simultaneously performed on the 13 phenolic acids in the grape wine within 10min. According to the method disclosed by the invention, any sample pretreatment is not required, and the loss caused by the pretreatment can be avoided; and compared with traditional HPLC (high-performance liquid chromatography), by adopting the method disclosed by the invention, more phenolic acid type compounds can be simultaneously detected, the separation effect is good, the analysis process is greatly shortened, convenient and easy to operate, the detection efficiency is greatly improved and the cost of the mobile phase is saved. The method disclosed by the invention has important significance for further system research of the flavor substances in the grape wine and improvement of the quality of the grape wine.
Owner:JIANGNAN UNIV
Preparation purification method of germanium tetrachloride for optical fiber
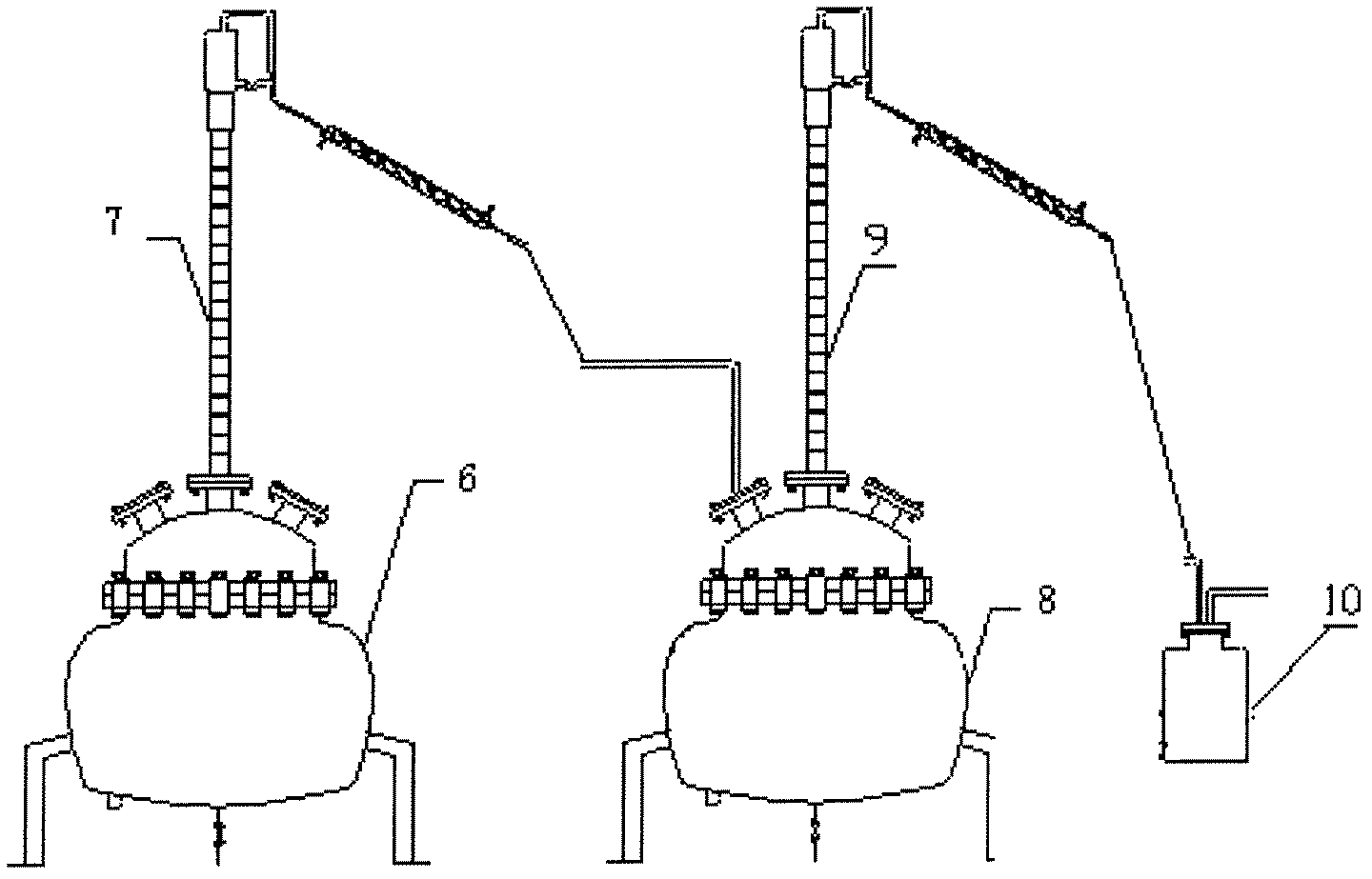
InactiveCN103183375AMeet the requirements of germanium tetrachlorideSuitable for mass production applicationsGermanium halidesPurification methodsDistillation
The invention provides a preparation purification method of germanium tetrachloride for optical fibers, which adopts germanium concentrate as a raw material; hydrochloric acid, sulfuric acid, and ferric trichloride are added into a chlorination reaction vessel according to a certain proportion for reaction to prepare crude germanium tetrachloride; the crude germanium tetrachloride is fully mixed with hydrochloric acid and manganese dioxide according to a certain proportion in a repeated distillation reaction vessel for distillation and purification to obtain preliminarily purified germanium tetrachloride; the germanium tetrachloride after repeated distillation purification is processed in a quartz rectifying tower in inert atmosphere by controlling a tower bottom temperature to be 75-95 DEG C, a tower column temperature to be 30-95 DEG C, and a reflux ratio of 4:1-14:1, so as to obtain high-purity germanium tetrachloride. The high-purity germanium tetrachloride prepared through three procedures of chlorination distillation, repeated distillation, and rectification purification, has a content of transition metal impurities of less than 3 ppb, a content of hydrogen-containing impurities of less than 1 ppm, and meets the requirements for perform rod germanium tetrachloride for optical fibers.
Owner:GRINM ELECTRO OPTIC MATERIALS
Detection method for dammarane type four-ring triterpenoid saponin
The invention relates to a method for detecting dammarane tetracyclic triterpenoid saponin and secondary aglycone. The inventive method uses high-efficiency liquid color spectrum-mass spectrum coupled device, wherein the conditions of high-efficiency liquid color spectrum comprises: color spectrum column is ODSC18 column; the column temperature is 20-38Deg. C; the conditions of mass spectrum comprise: the ion source is Turbo Ionspray source; the ejecting voltage is 3000-5000V; the Tem is 250-300Deg. C; the detector uses triplicate four-electrode serial mass spectrum detection and positive ion detection; the scanning method uses multi-reaction detection (MRM); CE is 17-36V; DP is 8-110V; the ion detection comprises: processing impact induce dissociation on the quasi-molecule ion peak of said saponin of first-stage scanning mass spectrum, to obtain the second-stage fragment ion; and uses multi-reaction detection (MRM) scanning method to analyze the ion reaction quantitatively, to represent the blood drug density of monomer saponin and secondary aglycone.
Owner:INST OF MEDICINAL PLANT DEV CHINESE ACADEMY OF MEDICAL SCI
Gas chromatography device
ActiveUS20150260694A1Improve responsivenessAverage power consumptionSamplingComponent separationGas liquid chromatographicColumn temperature
A gas chromatography device includes a sample injection part, a detector, a separation column, and a transfer line connecting between the sample injection part and the separation column and between the sample injection part and the detector. Furthermore, a column temperature adjustment part for adjusting the temperature of the separation column, and a line temperature adjustment part for adjusting the temperature of the transfer line are provided. The line temperature adjustment part is structured to include a heat block which includes a heating element and which is in contact with the transfer line from one side, and a holding member which presses the transfer line toward the heat block side by being in contact from the other side, and to sandwich the transfer line by the heat block and the holding member.
Owner:SHIMADZU CORP
High performance liquid chromatography-mass spectrometry (HPLC-MS) quantitative method for measuring 10 components of fructus evodiae formula granule
The invention relates to a high performance liquid chromatography-mass spectrometry (HPLC-MS) quantitative method for measuring 10 components of a fructus evodiae formula granule. The method is characterized in that a HPLC-MS technology is used; acetonitrile is taken as the A phase; acetic acid (0.12%)-ammonium acetate (1 mmol / L) is taken as the B phase; gradient elution with four scale transformations is performed; the flowing speed is 0.8 mL / min; the column temperature is 40 DEG C; in a multiple reaction monitoring (MRM) mode, positive ions and negative ions are monitored at the same time, the ion source is an ESI source, the atomized gas (GS1) is N2, the auxiliary gas (GS2) is N2; nitrogen gas is used during the whole process; the collision gas pressure is medium; the resolutions of Q1 and Q3 are both UNIT; the method can detect 10 components in the fructus evodiae formula granule, namely chlorogenic acid, caffeic acid, rutin, hyperin, hesperidin, dehydroevodiamine, limonin, evodiamine, rutaecarpin, and evocarpine; 10 components can be all detected in 22 minutes, and the method is rapid and accurate and is not disturbed.
Owner:HEBEI UNIV OF CHINESE MEDICINE +1
Detection method of parecoxib sodium genotoxicity impurity and application thereof
ActiveCN105372376AAchieve separationHigh sensitivityComponent separationPhosphateReversed-Phase Liquid Chromatography
The present invention provides a detection method of parecoxib sodium genotoxicity impurity and application thereof. The method uses a reversed-phase liquid chromatography method. The chromatography conditions are as follows: the chromatographic column comprise a C18 column, a C8 column, a phenyl column and a Hilic column; a mobile phase consists of water-acetonitrile, dilute phosphoric acid-acetonitrile, and phosphate-acetonitrile; a flow phase comprises an aqueous phase and an organic phase in the ratio of 90:10-10:90; the column temperature is 25-40 DEG C; the flow rate of the mobile phase is 0.2-2ml / min; detection wavelength is 205-290nm; a detector is a UV detector or a photodiode array (PDA) detector; and the sample size is 0.1-40 mul. The detection method of parecoxib sodium genotoxicity impurity achieves the separation of parecoxib sodium and three genotoxicity impurities in a short period of time, has high sensitivity and specificity, and simple operation, reaches the separation rate of the main component and genotoxicity impurities, and the separation rate of genotoxicity impurities both greater than 1.5; and the method can be used for quality control of parecoxib sodium, and has practical value.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
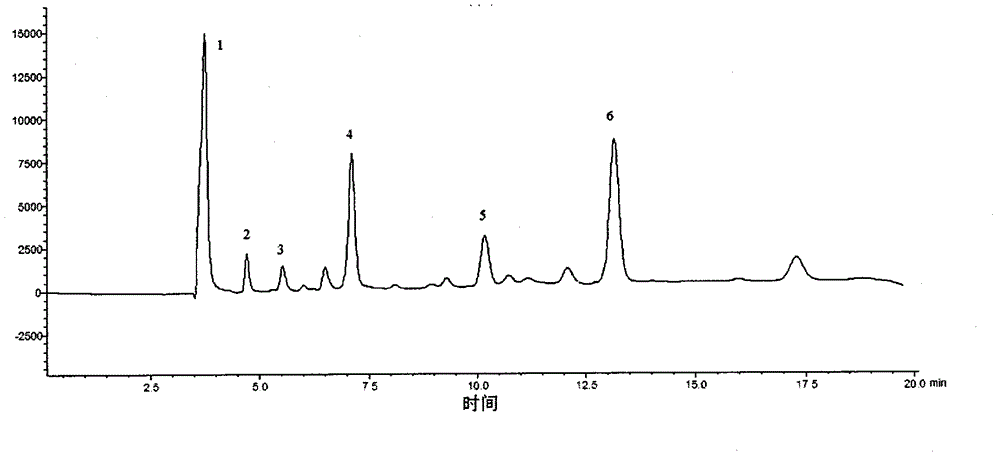
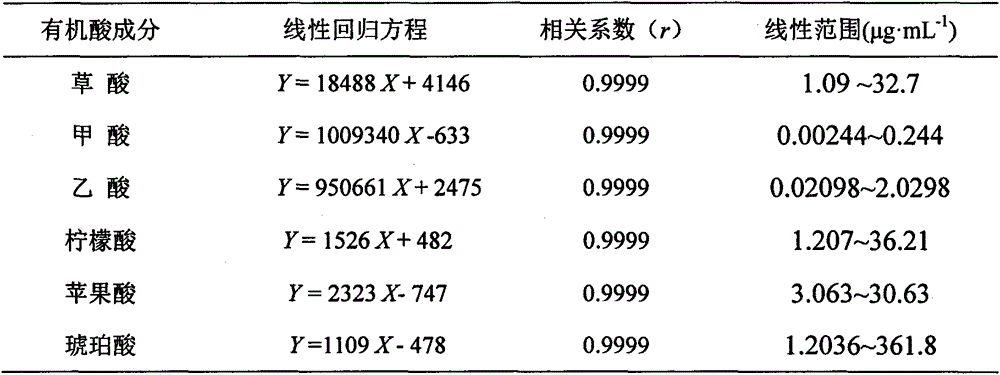
HPLC (High Performance Liquid Chromatography) method for simultaneously determining content of six organic acids in pinellia ternata
InactiveCN104597160AImprove linearityGood reproducibilityComponent separationHplc methodPhosphoric acid
The invention relates to an HPLC (High Performance Liquid Chromatography) method for simultaneously determining content of six organic acids in pinellia ternata. The method comprises the following steps: (1) preparing a sample solution; (2) preparing a comparison product solution; (3) determining by a high performance liquid chromatography: taking the comparison product solution and the sample solution and carrying out determination analysis under following chromatographic conditions: a chromatographic column is GL InterSustain-C18 (4.6mm*250mm, 5 microns), and a mobile phase is a phosphoric acid adjusted 0.03mol / L (NH4)H2PO4 buffering solution with the pH value of 2.0 and methanol; and the volume ratio is 97 to 3, the flow speed is 0.8mL / min, the column temperature is 30 DEG C and the detection wavelength is 210nm. Under the conditions of the method, oxalic acid, formic acid, malic acid, acetic acid, citric acid and succinic acid in pinellia ternata are effectively separated, and methodological results meet the analysis determination requirements. Compared with the traditional potentiometric titration method, the measurement for content of total organic acids is clear and objective by virtue of the HPLC method disclosed by the invention, and the content of the six organic acids in pinellia ternata can be effectively and rapidly determined and the quality of pinellia ternata can be comprehensively and accurately controlled.
Owner:CHONGQING MEDICAL UNIVERSITY
Preparation detection method for substances related to rosuvastatin calcium preparation
The invention relates to a preparation detection method for substances related to rosuvastatin calcium preparation. The preparation detection method comprises the following steps: preparing (3S,5S)-rosuvastatin calcium diastereoisomer, rosuvastatin-5S-lactone, rosuvastatin-5R-lactone, rosuvastatin-5-oxide, a rosuvastatin photodegradation product 1, a rosuvastatin photodegradation product 2, rosuvastatin photodegradation product 1-lactone and rosuvastatin photodegradation product 2-lactone, which are used as comparison products of the substances related the rosuvastatin calcium preparation; metering a test sample solution, a comparison product solution and a blank solution; and injecting the solutions into a liquid chromatograph respectively. The chromatographic conditions are as follows: a chromatographic column is a C18 chromatographic column; the column temperature is 30-40 DEG C, the flow speed is 0.6-1.0ml / min and the detection wavelength is 210-260nm. The content of the substances related to the rosuvastatin calcium preparation can be detected so as to be good for improving the quality standard of the rosuvastatin calcium preparation.
Owner:RUNZE PHARMACEUTICAL (SUZHOU) CO LTD
Method for determining fingerprint spectrum of traditional Chinese medicine for treating coronary heart disease
ActiveCN103760254AEffective symptomatic treatmentConducive to reflect the qualityComponent separationCoronary artery diseasePhosphoric acid
The invention relates to a method for determining fingerprint spectrum of traditional Chinese medicine for treating coronary heart disease. The method comprises the following steps: preparing a test solution, preparing a reference solution, and carrying out gradient elution under the chromatographic conditions that an Agilent ZORBAX Extend C18 chromatographic column is used as a filling agent, a mobile phase A is methanol and a mobile phase B is 0.4% phosphoric acid water solution; determining under conditions that the flow velocity is at 1.0ml / min, the column temperature is at 35 DEG C and the detection wavelength is 230-240nm, thus obtaining the fingerprint spectrum of traditional Chinese medicine. The method has the characteristics of being simple and convenient, good in reproducibility, more in characteristic peaks, accurate and reliable, and the like, and the quality of the traditional Chinese medicine preparation can be controlled effectively by the established fingerprint spectrum.
Owner:SHAANXI BUCHANG PHARMA
Method for detecting bufadienolide components in Liushen pill
InactiveCN106706812AQuality improvementImprove detection efficiencyComponent separationReference sampleColumn temperature
The invention discloses a method for detecting bufadienolide components in a Liushen pill. The method comprises the following steps: preparing a reference sample solution; preparing a test sample solution; and calculating the content through an HPLC assay determination method and a standard curve method. According to the invention, optimal chromatographic conditions such as mobile phase composition, a chromatographic column, an elution program, detection wavelength and column temperature are screened out through a large number of experiments, and the chromatographic conditions are optimized to obtain the optimal detection conditions. The detection method provided by the invention can realize quick, accurate and reliable determination, has high specificity and favorable reproducibility, can simultaneously detect 9 bufadienolide components in the Liushen pill, and can be used for quality control on the Liushen pill, thereby having important significance for drug quality control.
Owner:雷允上药业集团有限公司
Method for detecting related substances of ibuprofen and its sodium salt and preparation
The invention provides a method for detecting related substances of ibuprofen or its sodium salt and preparation. The related substances comprise impurities A, B, C, D, E and F. The method comprises (1) preparation of a test sample solution: taking an appropriate amount of ibuprofen or ibuprofen sodium raw materials, putting the materials into a certain volume of a container, dissolving the materials, diluting the solution until a desired volume marked by a scale, shaking the solution, and filtering the solution to obtain a certain concentration of the test sample solution, and (2) sample detection: pouring the test sample solution into a chromatographic instrument, and acquiring a chromatogram map of the related substances separated effectively under chromatographic conditions of use of a chromatographic column containing octadecyl silane chemically bonded silica as a filler having size of 250*4.6mm and pore sizes of 5 micrometers and having a column temperature of 20 to 40 DEG C, use of a mobile phase having a volume ratio of organic phase acetonitrile to a water phase phosphoric acid aqueous solution of 32% to 48% and phosphoric acid content of 0.01 to 0.1% in the phosphoric acid aqueous solution, a flowing rate of 1.0 to 2.3 ml / min, and a detection wavelength of 205 to 225nm. The method can realize effective separation of a variety of impurities.
Owner:CHINA PHARM UNIV
High performance liquid chromatographic analysis method for antioxidant 1076 content
ActiveCN101710108AAccurate contentAccurate measurementComponent separationColumn temperatureMethyl propionate
The invention discloses a high performance liquid chromatographic analysis method for antioxidant 1076, which adopts a reserved phase high performance liquid chromatography method. The reserved phase high performance liquid chromatography comprises the following conditions that: the chromatographic column is a C18 column and is of 5 mu m and 150*4.6 mm (I.D.); the mobile phase comprises the following components in a volume ratio with gradient conditions: 90 percent of methanol and 10 percent of water in 0 minute, 90 percent of methanol and 10 percent of water in 5 minutes, and 100 percent of methanol and 0 percent of water in 10 minutes; and the flow rate is 0.8 milliliter per minute, the column temperature is 25 DEG C, the detection wavelength is 220 nanometers, and the sampling volume is 20 microliters. The chromatographic analysis method of the invention accurately measures the main body content of the antioxidant 1076, further measures the contents of an intermediate beta-(3,5-tertbutyl-4-hydroxyphenyl) methyl propionate and a raw material 2,6-ditertbutyl phenol at the same time, and is suitable for quality control of the antioxidant 1076 in the industrialized production process.
Owner:SHANGHAI CHEM REAGENT RES INST
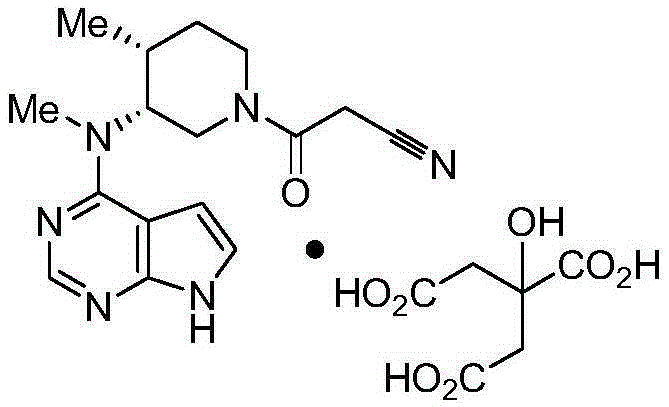
Determination method for content of tofacitinib citrate and related substances of tofacitinib citrate by reversed phase high-performance liquid chromatography
The invention discloses a determination method for the content of tofacitinib citrate and related substances of the tofacitinib citrate by reversed phase high-performance liquid chromatography. The determination method is characterized in that octadecylsilane chemically bonded silica is taken as filler; 0.02mol / L potassium dihydrogen phosphate solution and acetonitrile with volume ratio of 83:1 are taken as a mobile phase to perform isocratic elution, and the flow speed is 1.0ml / min; the 0.02mol / L potassium dihydrogen phosphate solution contains trimethylamine with mass concentration of 0.2 percent, and pH value is adjusted to 5.2 by using phosphoric acid; the detection wavelength is 290nm, and the column temperature is 30DEG C. According to the determination method disclosed by the invention, by adopting the isocratic elution, requirements on an instrument are reduced, as well as stable pressure and accurate and reliable experimental result are obtained; meanwhile, by adopting the method disclosed by the invention, the concentration of linear range is improved, and the preparation for a test solution during experiment is facilitated.
Owner:NINGBO LIWAH PHARM CO LTD
Method for detecting residual quantity of norfloxacin antibiotic in milk
InactiveCN101609073AShort detection timeHigh sensitivityComponent separationPreparing sample for investigationTriple quadrupole mass spectrometryColumn temperature
The invention relates to a method for detecting residual quantity of norfloxacin antibiotic in milk. The method utilizes an ultra-performance liquid chromatography (UPLC)-electrospray tandem triple quadrupole mass spectrometer to determine the residual quantity of norfloxacin antibiotic. A sample is extracted by Na2 EDTA-Mellvaine buffer solution (pH 4.0), subjected to protein removal by trichloroacetic acid, purified and concentrated by a PEP solid phase extraction columella, separated by adopting a chromatographic column with column temperature of 30 DEG C, subjected to gradient elution by adopting aqueous solution (v / v) containing 0.1% formic acid and acetonitrile as a flowing phase, and then quantitated by adopting a multiple reaction monitoring manner. The instrument detection limit is 1.0-2.0 mug / Kg; and within the linear range of 1-100 mug / Kg, the correlation coefficient r can reach more than 0.999, and the coefficient of recovery is 80%-105% (adding level of 10, 50 and 100 mug / Kg). The method has the advantages of rapidness, accuracy, high sensitivity and wide application scope.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Method for controlling the quality of schisandra raw material fingerprint in the plant medicine for improving hemorheology
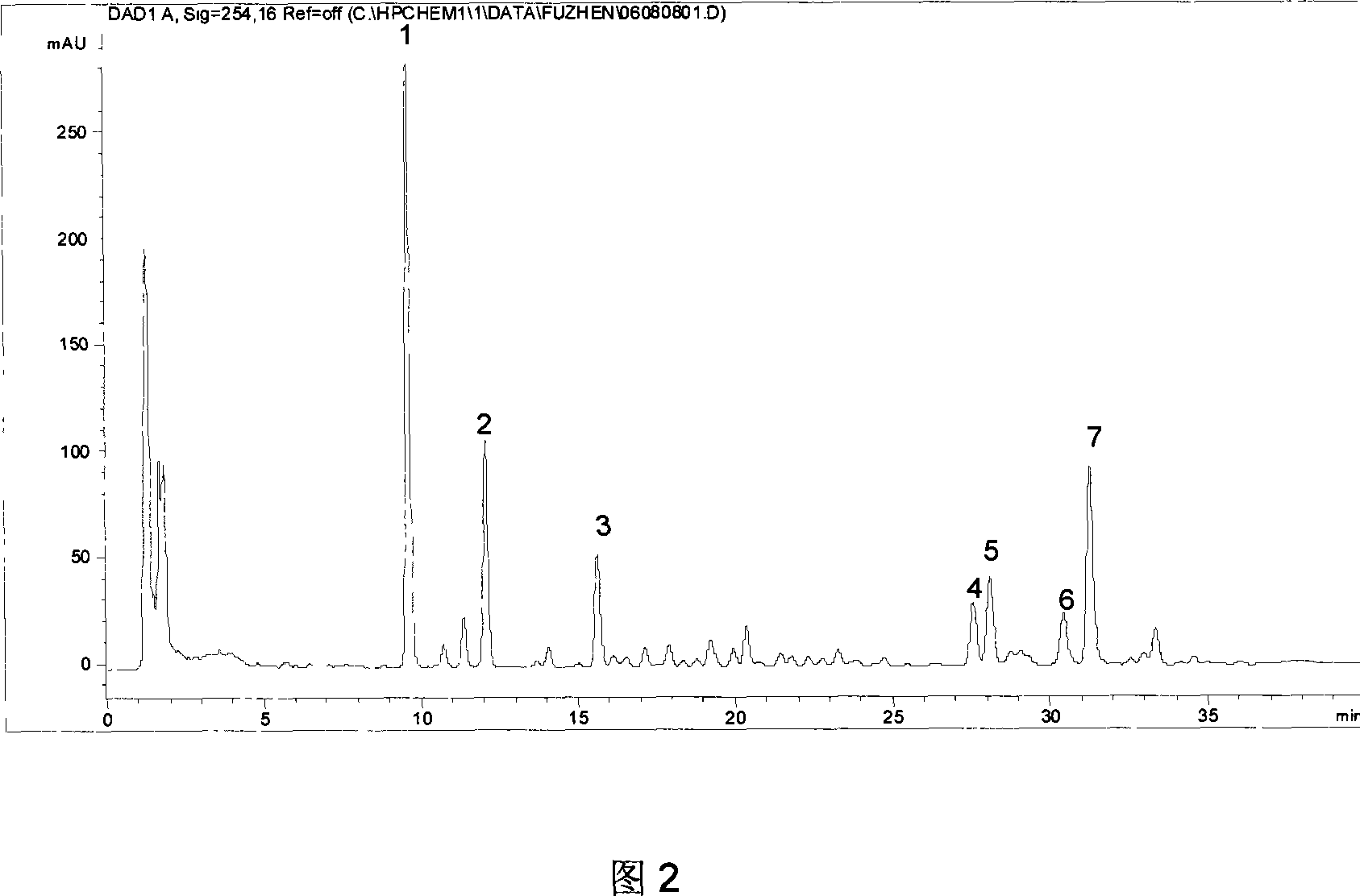
ActiveCN101040909AGuarantee normal implementationMeet the identificationComponent separationPlant ingredientsColumn temperaturePhase gradient
The invention relates to a schisandra fruit fingerprint spectrum quality control method, comprising that (1), adding 0.5g schisandra fruit to extract via microwave for 50min, filtering, (2), washing flow phase gradient that the spectrum column is Eclipse, as 0-40min, 60-20% A, 40-80% B, flow speed is 0.8-1.2ml / min, the wavelength is 210-280nm, the column temperature is 20-40Deg. C, and the sample amount is 10-20ul, (3), building standard fingerprint spectrum that the first peak is schisandra fruit alcohol 1, the third peak is schisandra fruit alcohol 2, the fourth peak is gemixin N / isomer, the sixth peak is schisandra fruit I element, the seventh and eighth peaks are gemixin N / isomer, the ninth peak is schisandra fruit II element, (4), controlling the quality of fingerprint spectrum that the check peak relative holding times are 1.00, 1.25, 1.63, 1.88, 3.18 and 3.27, (5), the schisandra fruit planting collecting method. The invention can control the quality of materials to assure the stable quality of product.
Owner:SHANGHAI MODERN CHINESE TRADITIONAL MEDICINE TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com