Patents
Literature
112 results about "Tofacitinib" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
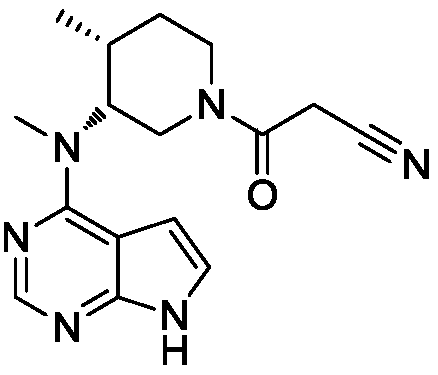
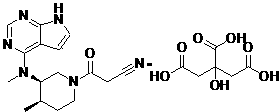
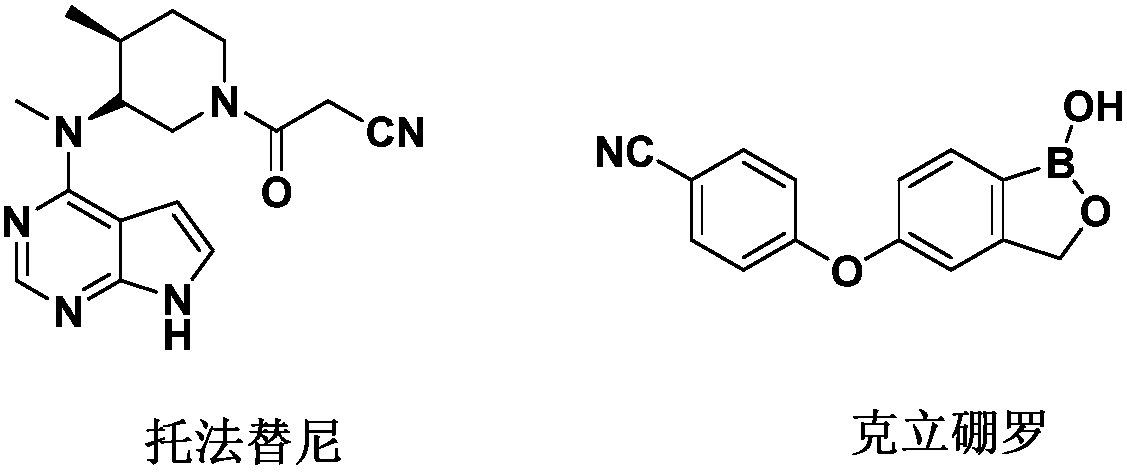
Tofacitinib, sold under the brand Xeljanz among others, is a medication used to treat rheumatoid arthritis, psoriatic arthritis and ulcerative colitis. Common side effects include diarrhea, headache, and high blood pressure. Serious side effects may include infections, cancer, and pulmonary embolism. In 2019 EMA’s safety committee began a review of tofacitinib and has recommended that doctors temporarily not prescribe the 10 mg twice-daily dose to people at high risk for pulmonary embolism. The US Food and Drug Administration (FDA) has also released warnings about the risk of blood clots.
Tofacitinib oral sustained release dosage forms
ActiveUS20140271842A1Safely ingestedMinimize biasBiocideNervous disorderSustained release drugTofacitinib
The present invention relates to oral sustained release formulations of tofacitinib and pharmaceutical acceptable salts thereof. The formulations described herein have desirable pharmacokinetic characteristics.
Owner:PFIZER INC
Preparation method of tofacitinib
InactiveCN103819474AHigh yieldSuitable for industrial productionOrganic chemistryAlkyl transferBenzoyl bromide
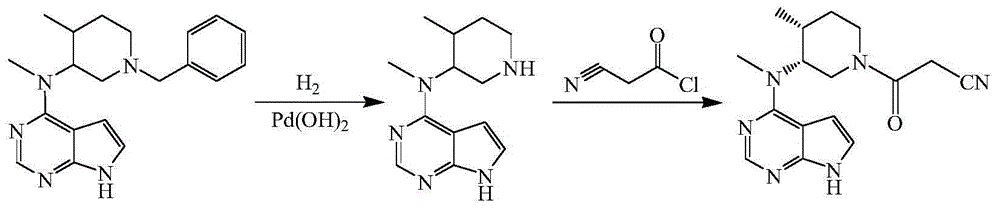
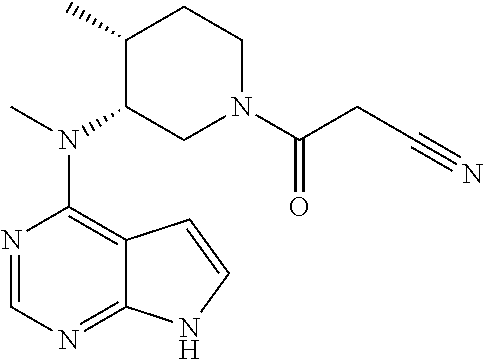
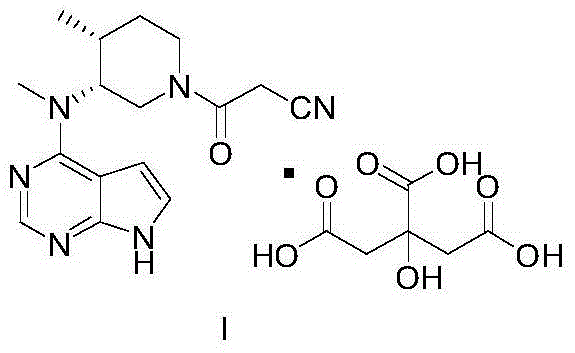
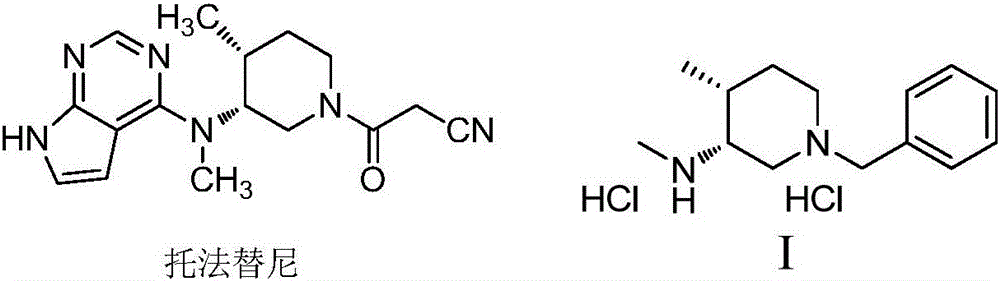
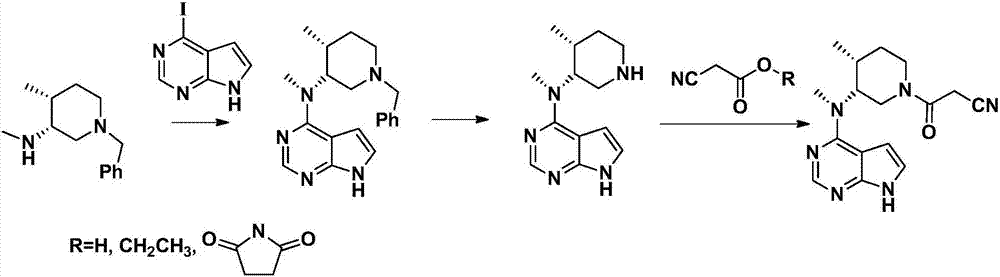
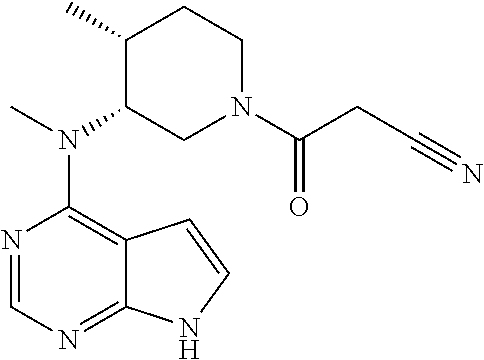
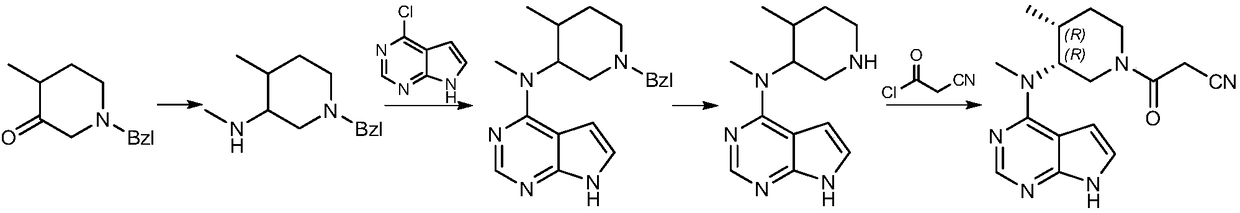
The invention discloses a preparation method of tofacitinib, namely 3-{(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d] pyrimidin-4-yl)amino]piperidin-1-yl}-3-oxopropanenitrile. According to the preparation method, an onium pyridine salt is formed by using benzyl bromide as a raw material; then reduction, selective oxidation, alkylation, deprotection and an acylation reaction are conducted to obtain a compound, namely 3-{(3R,4R)-4-methyl-3-[methyl(7H-pyrrolo[2,3-d] pyrimidin-4-yl)amino]piperidin-1-yl}-3-oxopropanenitrile shown as the formula I.
Owner:湖南华腾制药有限公司
Industrial production method applicable to citric acid tofacitinib
InactiveCN104788461AReduce manufacturing costHigh purityOrganic chemistryBulk chemical productionTofacitinibActive ingredient
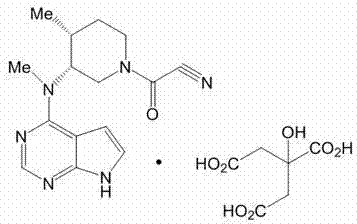
The invention relates to an industrial production method applicable to citric acid tofacitinib. The invention relates to a novel industrial production method of a high-purity orally taken JAK inhibitor and a medicine for treating rheumatoid arthritis. Raw materials are subjected to substitution, protecting group removal, reduction debenzylation, condensation and salifying to obtain a compound shown in a formula I, and the defects of a synthetic route reported in existing literatures that the impurity content in the prepared finished product is high, cost is high and yield is low are overcome. The novel industrial production method provided by the invention has the advantages that cost of a used reagent is low, environmental pollution is hardly caused, operation is easy, and short time is consumed, so that the novel industrial production method is applicable to industrial production; meanwhile, product quality is good, total impurity content is low, content of each individual impurity is controlled to be 0.1% or below, the finished product is controlled to be in a single stable crystal form, and medicinal-grade raw material requirements can be met.
Owner:NANJING CHENGONG PHARM CO LTD
Method for synthesizing citric acid tofacitinib
ActiveCN106146517AEliminate potential safety hazardsShort reaction timeCarboxylic acid salt preparation4-methylpiperidineTofacitinib
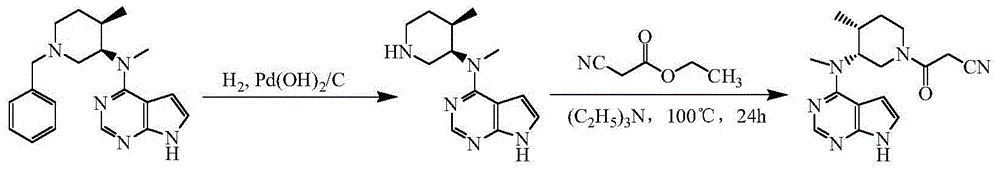
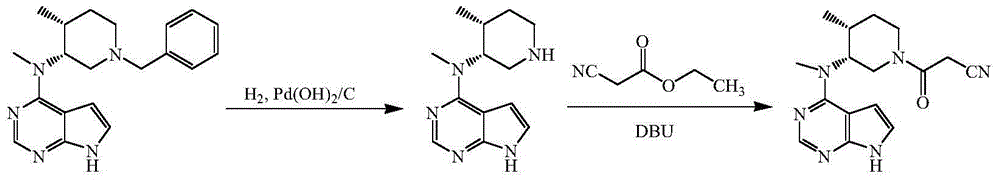
The invention discloses an efficient and safe method for synthesizing citric acid tofacitinib. N-[(3R,4R)-1-benzyl-4-methylpiperidine-3-base]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine is used as the raw material, Pd / C and HCOOH reduction debenzylation is conducted, condensation is conducted under the catalysis of an EDCI or EDCI, HOBT and triethylamine compound system with cyanoacetic acid, and salifying is conducted with citric acid in acetone to obtain citric acid tofacitinib. By the adoption of the synthesis method, potential safety hazards caused by hydrogen and ammonium formate are avoided, debenzylation reaction and amidation are thorough, no side reaction is caused basically, reaction time is shortened greatly, yield is high, aftertreatment is easy, and citric acid tofacitinib can be prepared efficiently and safely.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Tofacitinib tablet and preparation method thereof
InactiveCN104622827AIncrease disintegration speedImprove dissolution rateOrganic active ingredientsAntipyreticAdhesiveTofacitinib
The invention belongs to the field of medicine preparations, and particularly relates to a tofacitinib tablet and a preparation method thereof. Direct-pressed lactose and microcrystalline cellulose with good mobility and compression formability are selected by the tablet as a diluent and a drying adhesive. The tablet is prepared by direct powder compression method. The tablet is simple in technology, time-saving and energy-saving; the prepared tofacitinib composition is controllable in quality; and the stability of the product is ensured.
Owner:CHONGQING HUAPONT PHARMA
Crystalline and non-crystalline forms of tofacitinib, and a pharmaceutical composition comprising tofacitinib and a penetration enhancer
Owner:PFIZER INC
Preparation method of tofacitinib intermediate
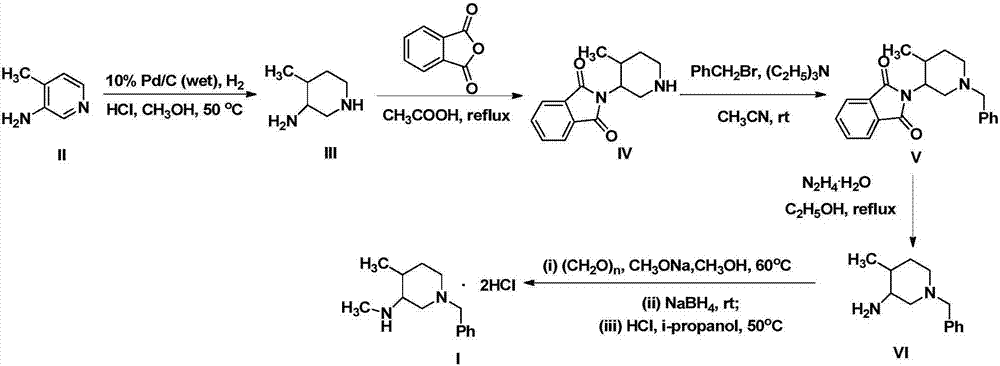
The invention relates to a novel preparation method of a tofacitinib intermediate and in particular to a preparation method of the tofacitinib intermediate (3R,4R)-1-benzyl-N-4-dimethyl piperidine-3-amine dihydrochloride. The preparation method comprises the following steps of: by taking 1-benzyl-4-methyl-1,2,3,6-tetrahydropyridine as an initial raw material, oxidizing olefin to form a ketone II by means of a one-step process; forming imine III with amine; applying asymmetric reduction imine to form amine; removing a trans isomer by recrystallization to obtain a cis-form structure IV; and finally, applying chiral resolution to obtain a final product (3R,4R)-1-benzyl-N-4-dimethyl piperidine-3-amine dihydrochloride I. The preparation method is creative in process, the process steps are shortened, and the synthetic yield of an asymmetric compound is greatly increased, thereby laying a solid foundation for industrial production.
Owner:苏州楚凯药业有限公司
Preparation method of JAKs inhibitor drug tofacitinib
InactiveCN105732641ALess side effectsReduce generationOrganic chemistryAntipyreticAcetic acidTofacitinib
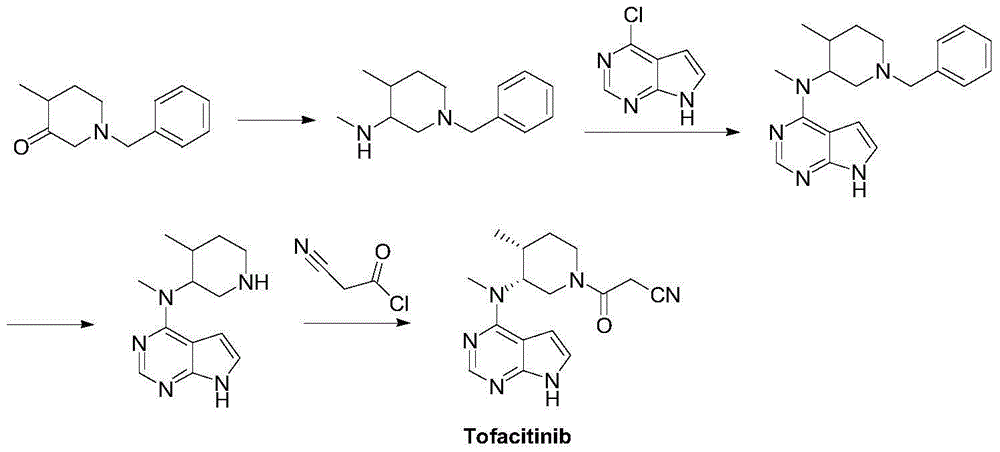
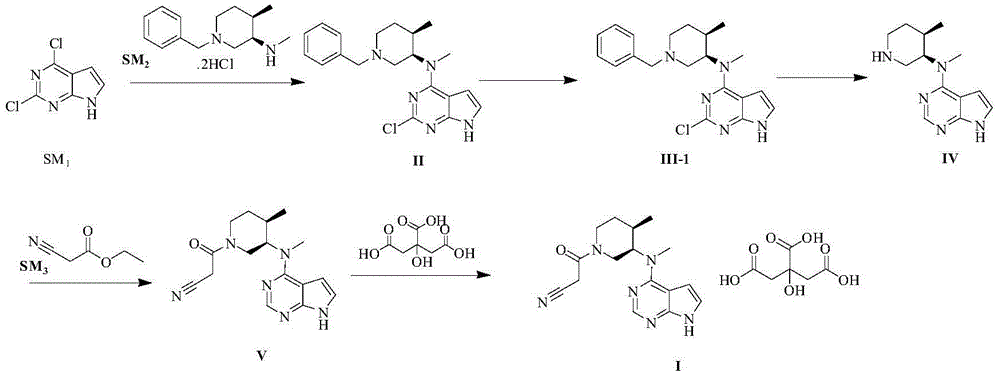
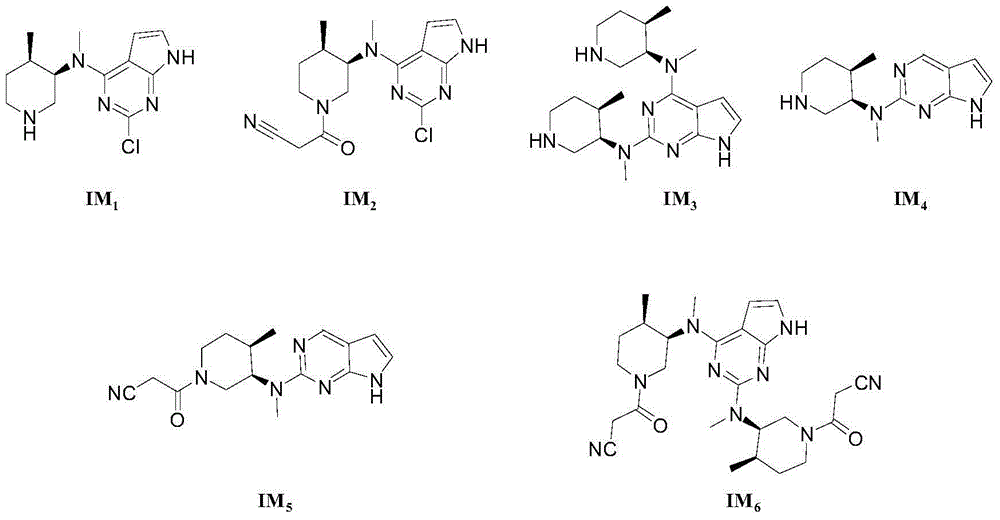
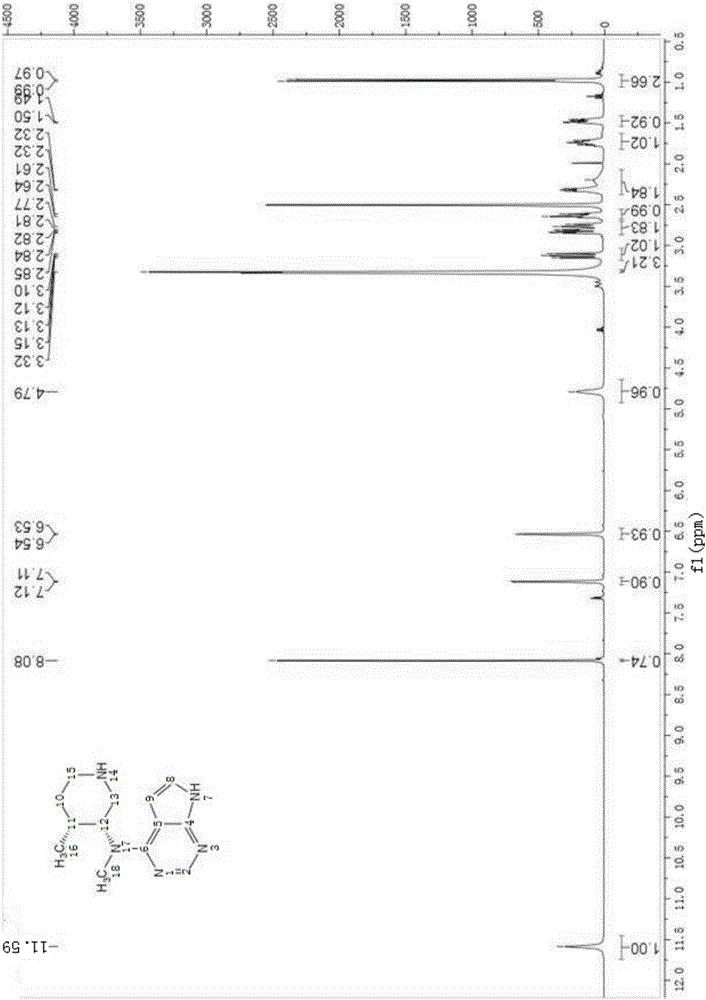
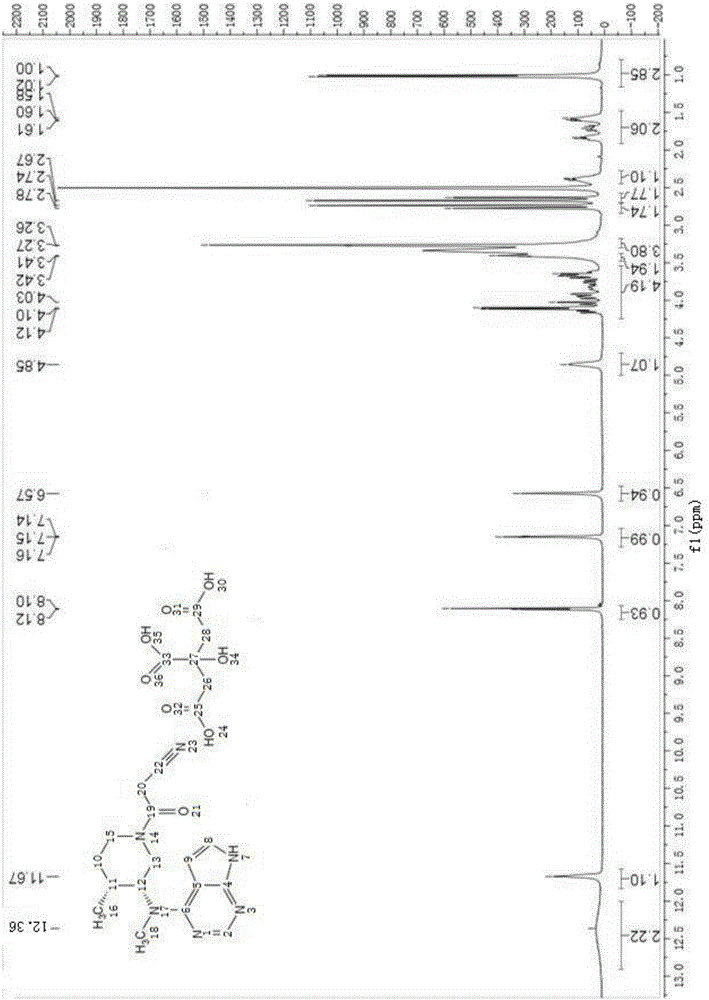
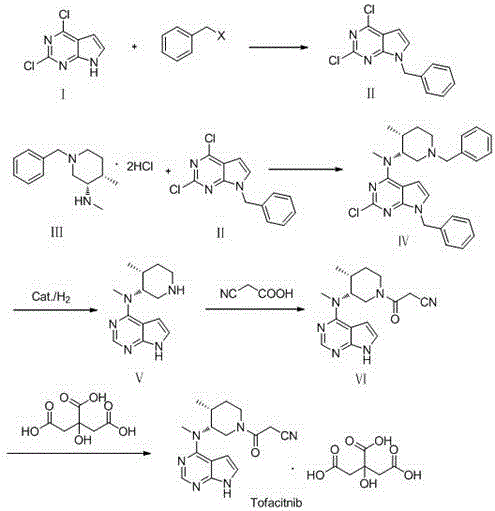
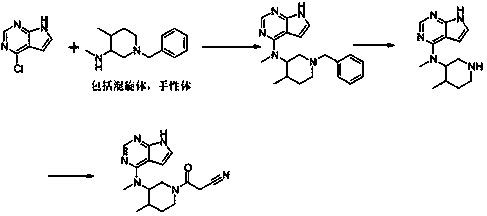
The invention discloses a preparation method of JAKs inhibitor drug tofacitinib, which is characterized in that it comprises the following steps: (1) using 2,4-dichloro-7H pyrrole [2,3-D] pyrimidine as a raw material, Under the action of base, react with halobenzyl to prepare compound II; (2) (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride React with 7-benzyl-2,4-dichloro-7H pyrrole [2,3-D] pyrimidine under the action of a base to prepare compound IV; (3) the compound IV obtained in step (2) is catalyzed under the action of a metal catalyst Hydrogenation reaction to obtain compound V; (4) Compound V obtained in step (3) is coupled and docked with cyanoacetic acid under the action of a condensing agent to obtain compound VI; (5) Compound VI obtained in step (4) is prepared by salting with citric acid Get tofacitinib. The synthesis route of the method is short, the reaction process of each step is easy to operate, the solvent can be recycled and used mechanically, the pollution is small, and it is suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Tofacitinib intermediate and preparation method thereof
InactiveCN104341422AEasy to operateMild conditionsOrganic chemistry methodsCarboxylic acid salt preparationTofacitinibChemical compound
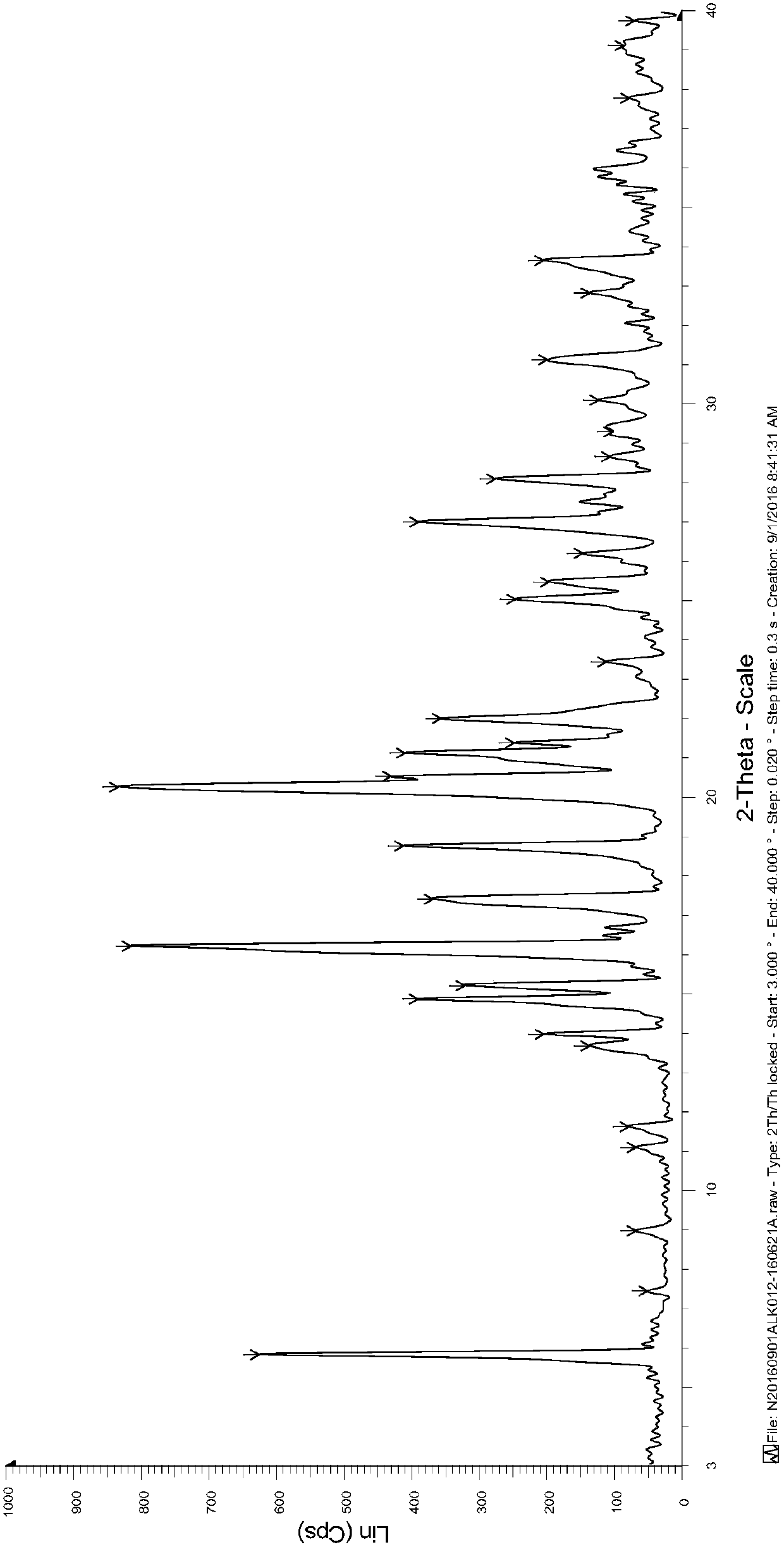
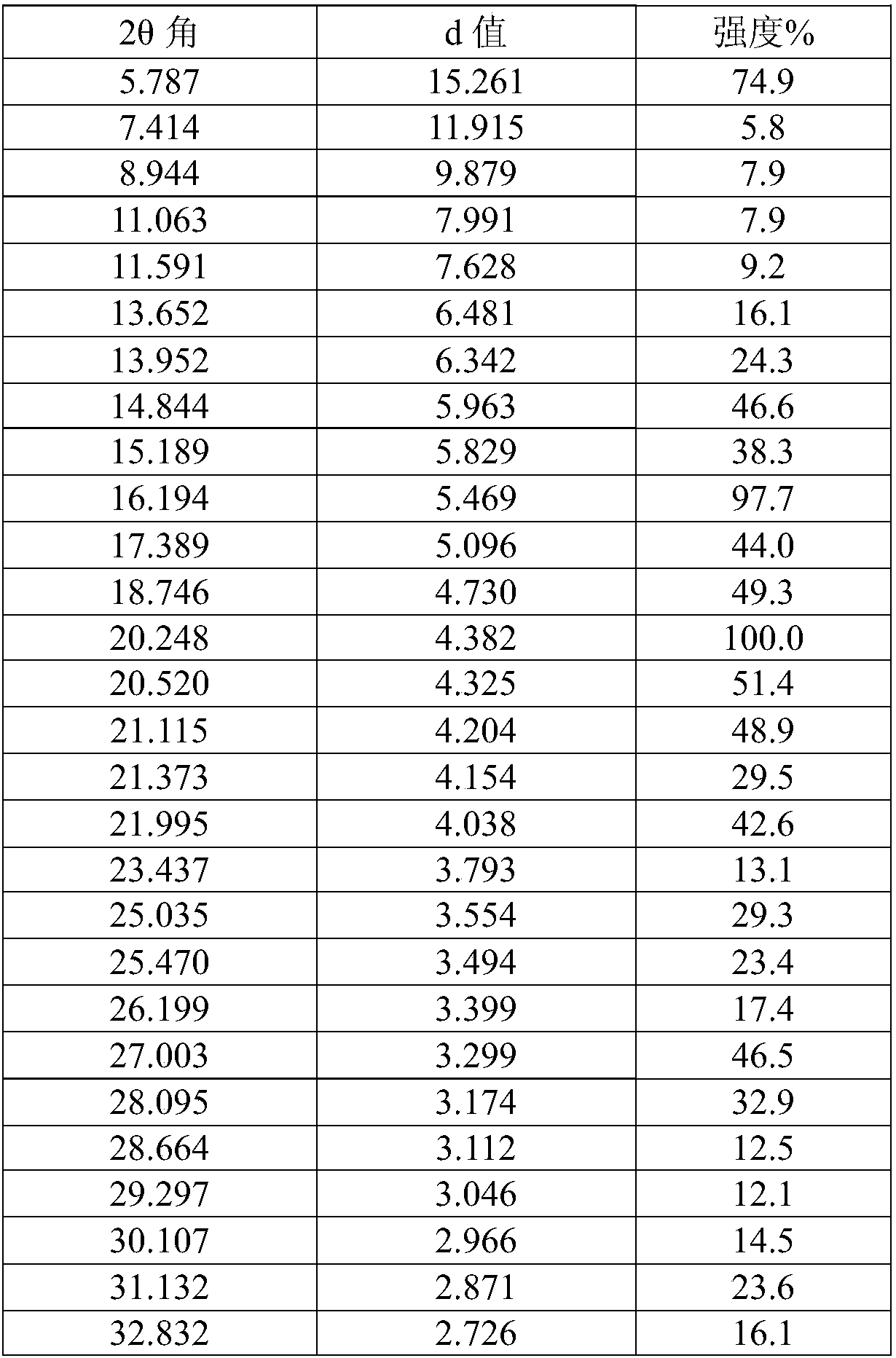
The invention discloses a tofacitinib intermediate, a crystal form and a preparation method thereof, the tofacitinib intermediate is a compound shown as a formula VI. The positions of characteristic diffraction peaks of the intermediate in an X-ray powder diffraction pattern when the reflection angle 2theta is about 7.4 DEG, 10.0 DEG, 11.8 DEG, 14.6 DEG, 16.3 DEG, 19.0 DEG, 19.3 DEG, 20.5 DEG, 23.8 DEG, 24.2 DEG, 25.2 DEG, 25.8 DEG, 26.0 DEG, 27.0 DEG, 28.0 DEG, 28.3 DEG, 28.9 DEG, 29.3 DEG, 31.2 DEG, 31.7 DEG, 32.1 DEG, 34.4 DEG and 38.2 DEG. The formula VI compound is stable and easy to store and transport.
Owner:CHONGQING PHARMA RES INST
Industrial production method of citric acid tofacitinib
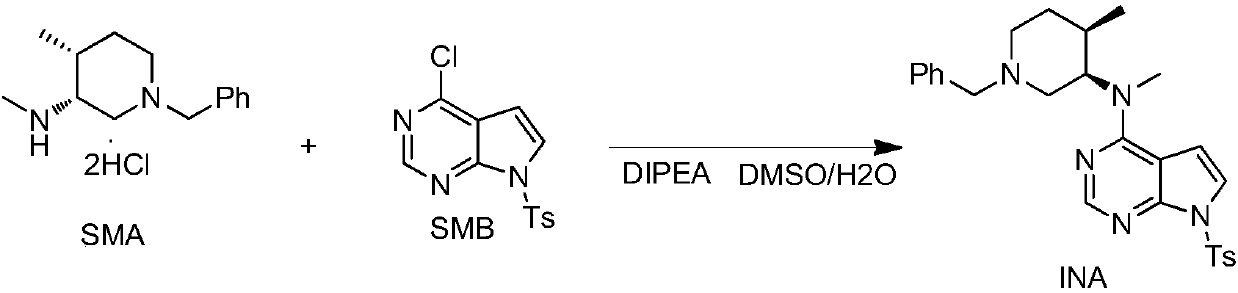
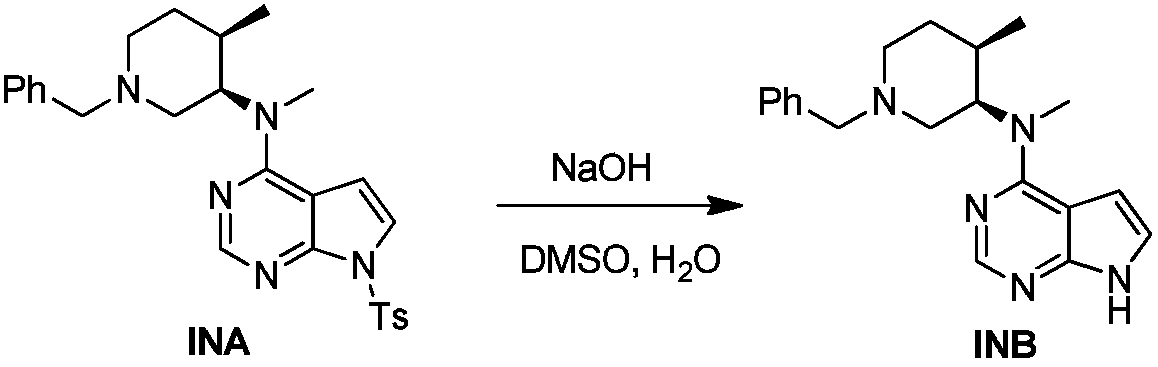
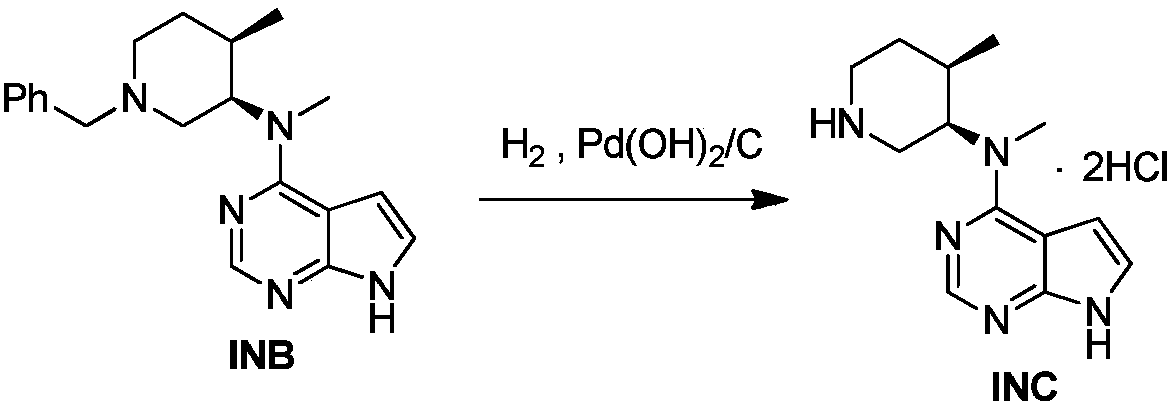
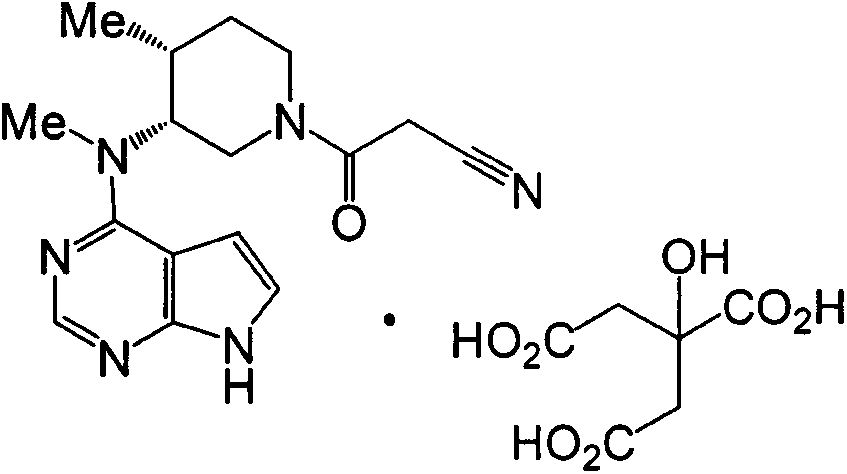
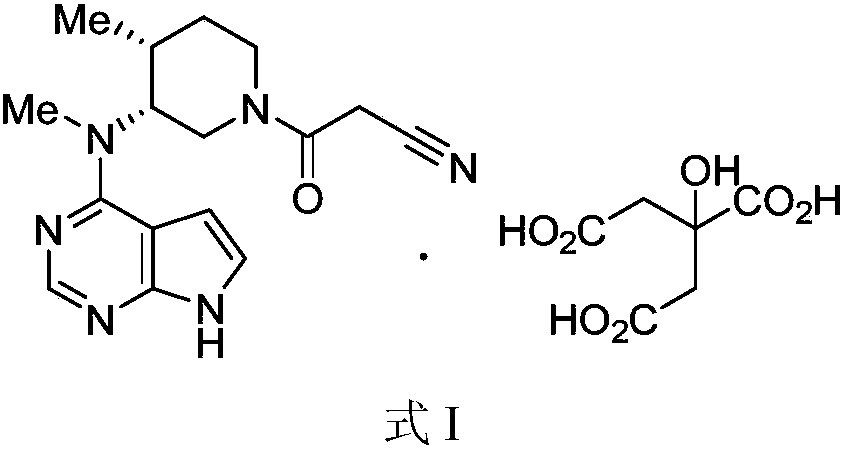
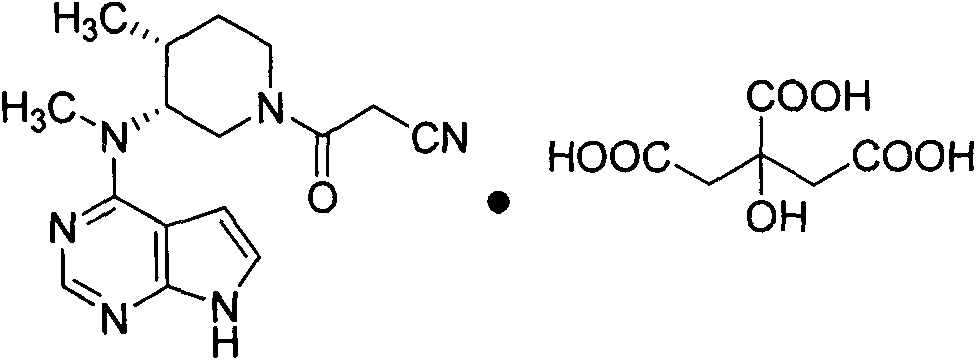
The invention discloses an industrial production method of citric acid tofacitinib. The method comprises the following steps: (1) adding a compound SMA and a compound SMB to a mixed solvent 1 which comprises DMSO and purified water, and catalyzing through DIPEA to react to obtain an intermediate INA; (2) carrying out a homogeneous reaction of the INA under an alkaline condition to obtain a rough intermediate INB; (3) refining the rough INB through low-level alcohol and water to obtain fine INB; (4) treating the fine INB by debenzylation and acidification to obtain an intermediate INC (salt form); (5) carrying out a reaction between the INC and SMC to obtain an intermediate IND which is tofacitinib free alkali; (6) preparing salt through the IND and citric acid monohydrate in purified waterto obtain citric acid tofacitinib. According to the method, the defects of preparation technologies in existing literatures can be solved; the used solvents are three types of solvents, so that the environmental pollution is low; the time consumption is small; the operation processes are simple; industrial production can be carried out. The formula is shown in the description.
Owner:YANGTZE RIVER PHARM GRP CO LTD
Tofacitinib tablet with excellent property
The invention discloses a tofacitinib coating tablet with excellent property. The coating tablet comprises a coating basic reagent, tofacitinib and an officinal additive, wherein the coating basic reagent is in a coating layer of the coating tablet and selected from polyvinyl alcohol and Pullulan; and the proportion (wt / wt) of the contents of the polyvinyl alcohol to the Pullulan in the coating tablet is 1: 1-1: 5. The coating tablet is excellent in mechanical property, high in stability, convenient in pharmacy, rapid in effect and efficient.
Owner:JIANGSU SEMPOLL PHARMA
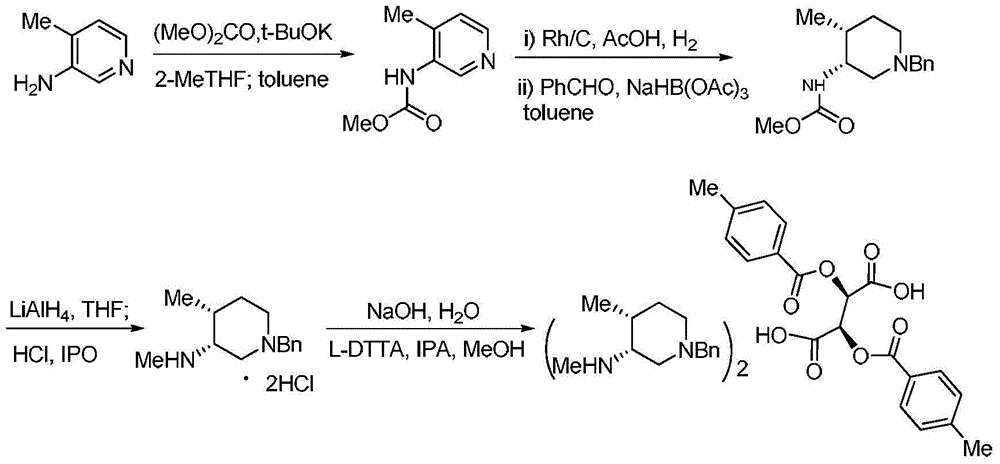
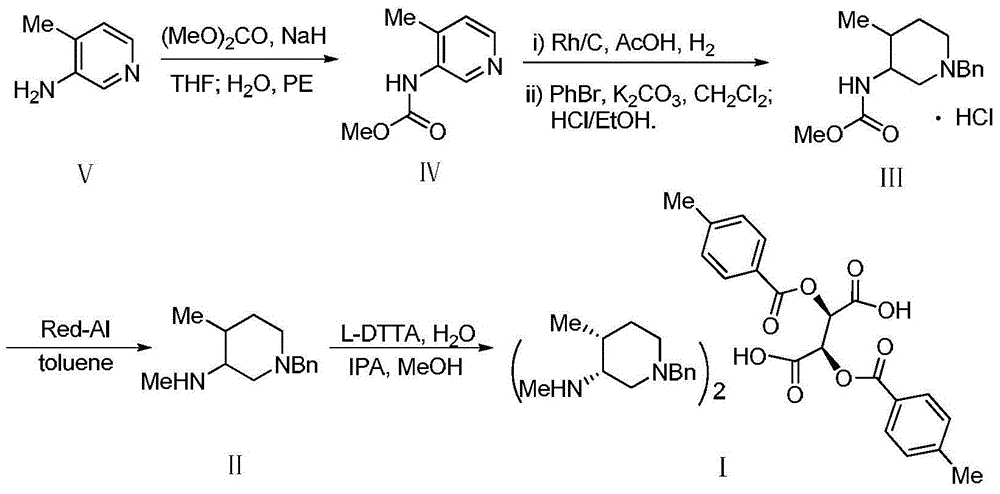
Bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate synthesis method
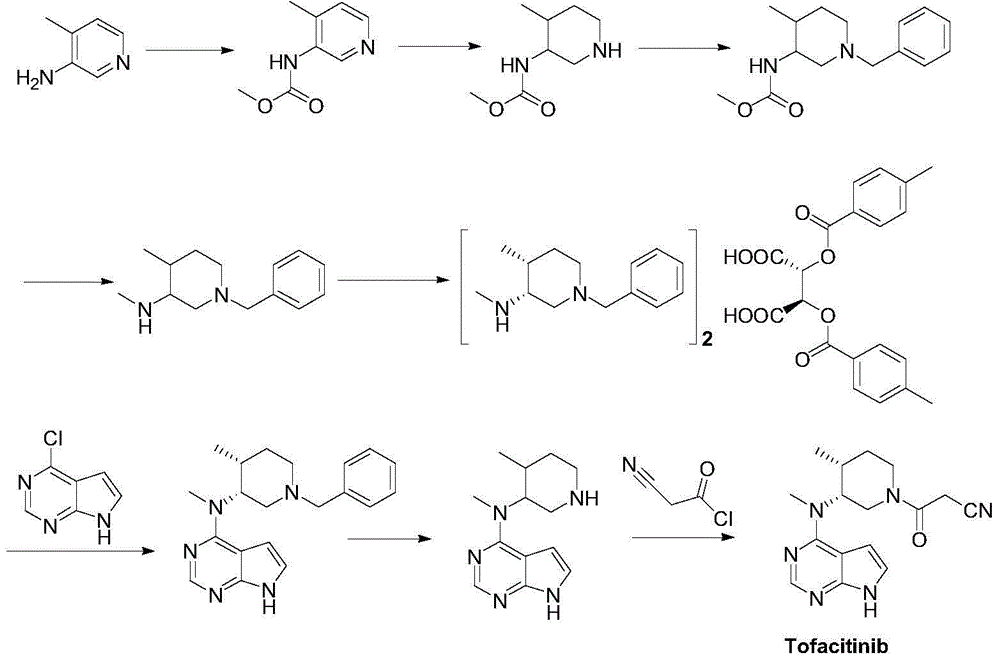
The present invention relates to a novel preparation method of a tofacitinib intermediate bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate. According to the preparation method, 3-amino-4-methyl piperidine is adopted as a starting raw material, is subjected to a N-methoxycarbonylation reaction under the sodium hydride effect, and is subjected to a catalytic hydrogenation reaction, a nucleophilic substitution reaction, an amide reduction reaction under the red aluminum effect, and L-di-p-toluyl tartaric acid (L-DTTA) chiral splitting so as to finally prepare the target compound bis-(3R,4R)-1-benzyl-N,4-dimethyl piperidin-3-amine L-di-p-toluyl tartrate. The process of the present invention has characteristics of further existing process improving, simple operation, easy post-treatment, high yield, and low cost.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
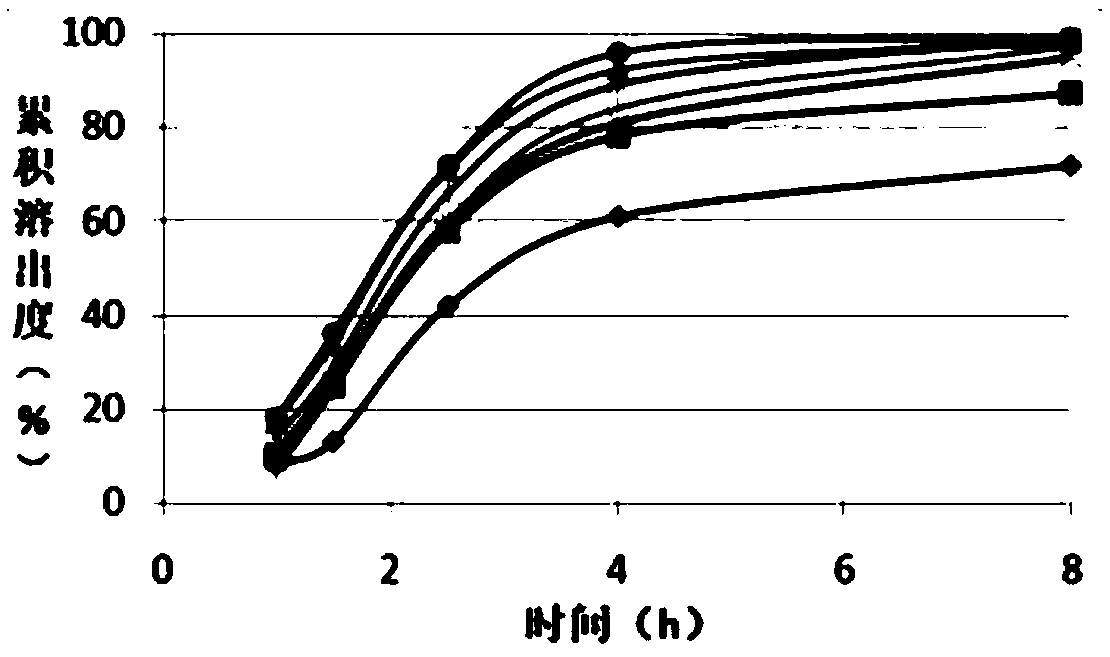
Tofacitinib controlled release tablet, preparation method and application of Tofacitinib controlled release tablet
ActiveCN111150711AReduce adverse effectsReduce manufacturing costOrganic active ingredientsNervous disorderOrganic solventTofacitinib
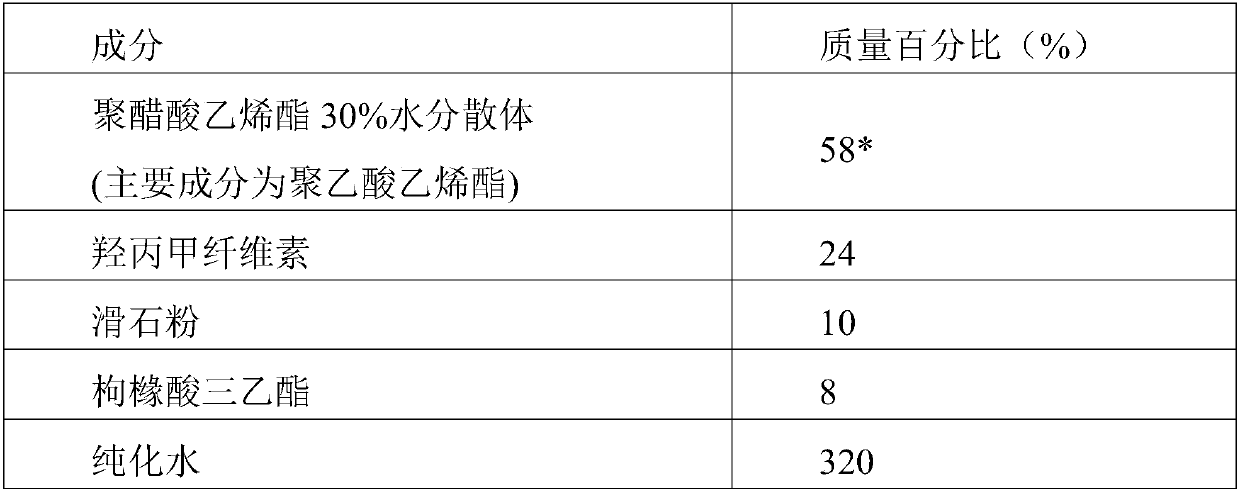
The invention discloses a tofacitinib controlled release tablet, a preparation method and application of the tofacitinib controlled release tablet. The tofacitinib controlled release tablet consists of a medicine-containing tablet core and a controlled release coating film, wherein the medicine-containing tablet core contains a tofacitinib raw material and an auxiliary material; and the controlledrelease coating film contains a film-forming material, a pore-forming agent and / or an excipient. The preparation process of the tofacitinib controlled release tablet requires no laser drilling or special precision equipment, and is simple, easy to implement, low in production cost and short in period, and no organic solvent is used in a coating liquid preparation process, so that adverse effectson the human body and the environment are avoided, and the preparation process is suitable for industrial production.
Owner:SHANGHAI BOCIMED PHARM RES CO LTD
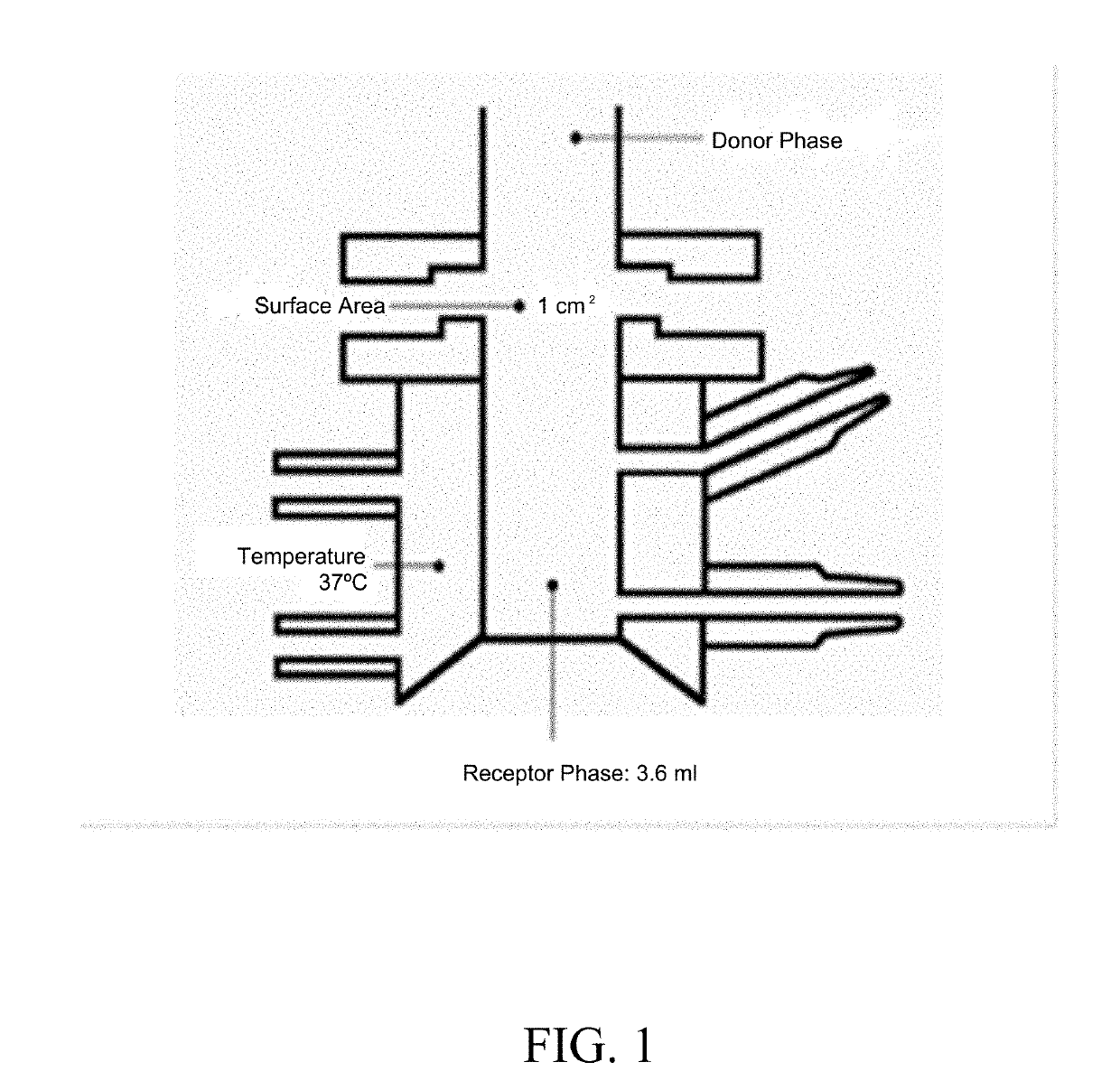
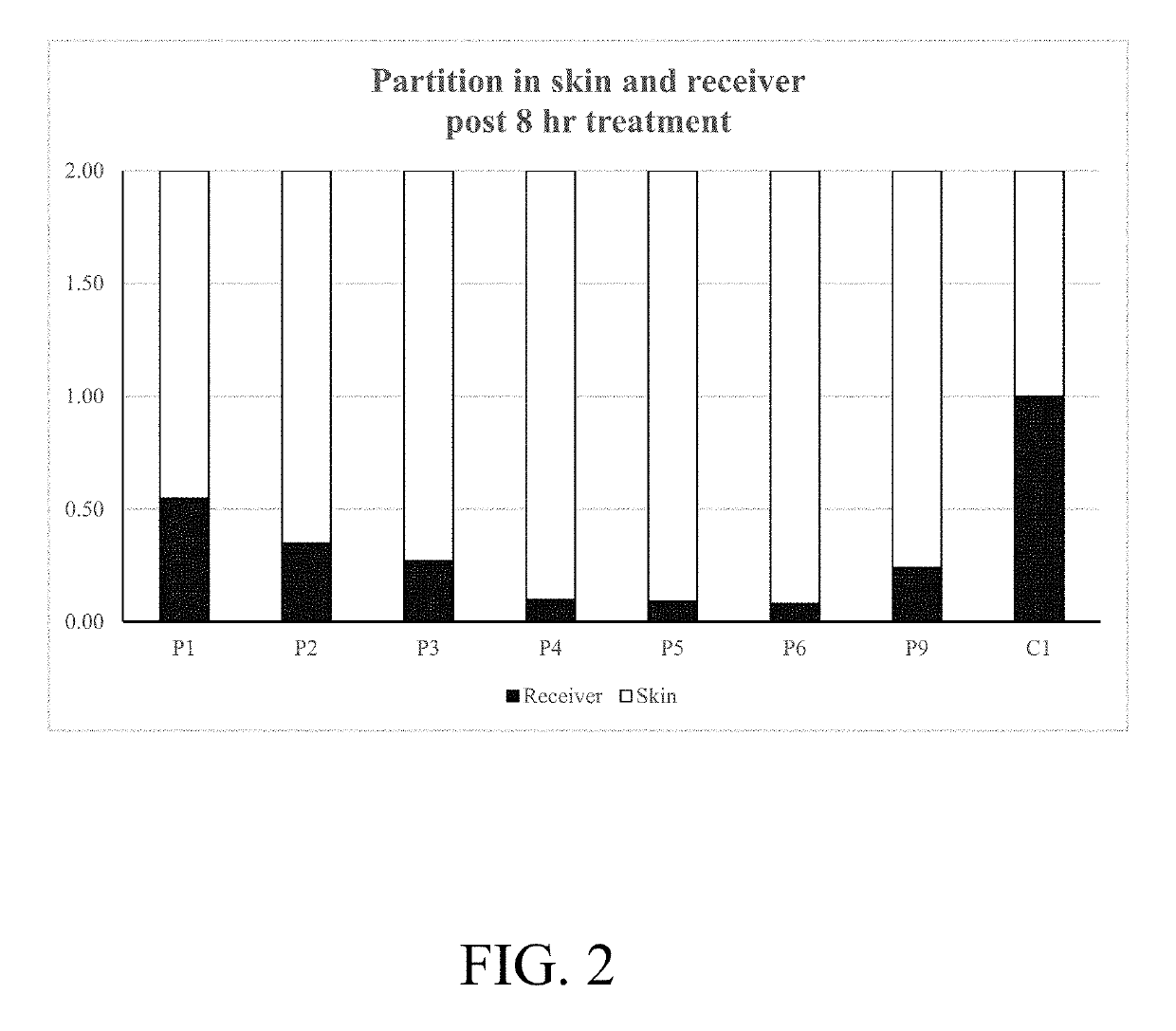
Topical formulations comprising tofacitinib
A topical formulation comprising (a) a therapeutically effective amount of tofacitinib; (b) at least one solvent; and (c) optionally one or more other pharmaceutically acceptable excipients is provided. Also provided is a method for treating and / or preventing autoimmune diseases in a subject administering said topical formulation.
Owner:TWI BIOTECH
Tofacitinib intermediate preparation method and method for preparing tofacitinib or its salt by using tofacitinib intermediate preparation method
InactiveCN104761555AOvercome the defects of complex preparation process and long production cycleEasy to handleOrganic chemistryPlatinumOrganic solvent
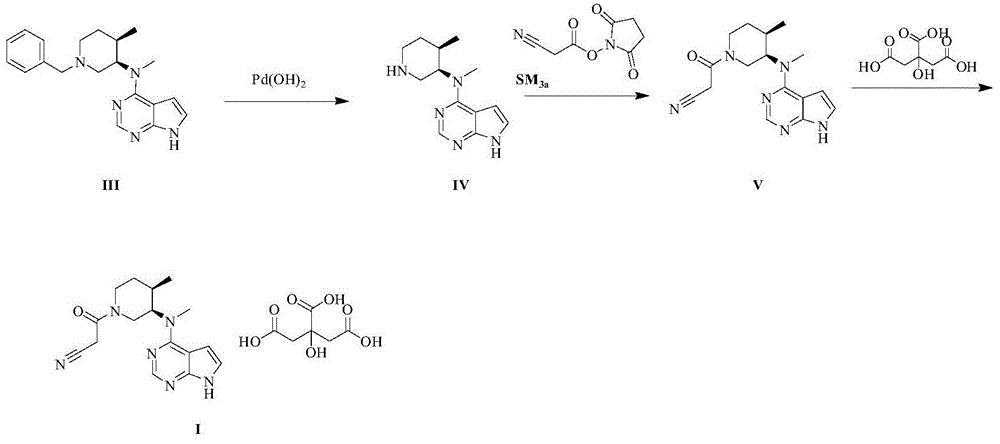
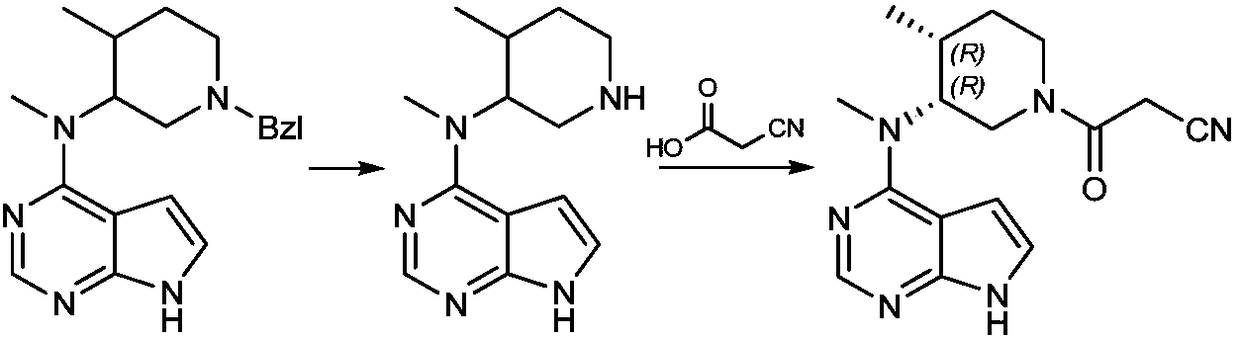
The invention provides a N-methyl-N((3R,4R)-4-methyl piperidine-3-group)-7H-pyrrolo[2,3-D]pyrimidine-4-amine (tofacitinib intermediate)preparation method and a method for preparing tofacitinib or its salt by using the tofacitinib intermediate preparation method. The tofacitinib intermediate preparation method can dissolve a compound in a formula (II) in a mixed solvent system of an acidic solvent and an organic solvent, and then a hydrogenation reaction is carried out under existence of palladium carbon or platinum carbon catalyst, thereby a compound in a formula (1) can be obtained. The method is simple to operate, and the tofacitinib intermediate yield is high. The invention also relates to the compound in the formula (1) prepared by the method and tofacitinib or its medicinal salt prepared by the compound.
Owner:HEBEI GUOLONG PHARMA CO LTD
Preparation method of tofacitinib intermediate
ActiveCN107056681ARaw materials are easy to getNew routeOrganic chemistryTofacitinibCombinatorial chemistry
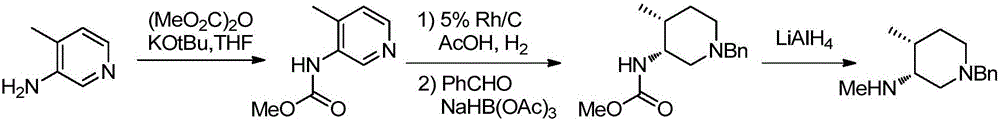
The invention discloses a preparation method of a tofacitinib intermediate compound-1-benzyl-3-methyl amino-4-methyl piperidine dihydrochloride. The method comprises the following steps: (1) carrying out selective deprotection on a compound as shown in a structural formula (V) to synthesize a compound-1-benzyl-3-amino-4-methyl piperidine as shown in a structural formula (VI); (2) enabling the compound as shown in the structural formula (VI) to have a primary amine monomethylation reaction to generate the target compound-1-benzyl-3-methyl amino-4-methyl piperidine dihydrochloride as shown in a structural formula (I). The method has the characteristics of being easy in obtaining of raw materials, novel in route, less in by-products, simple and convenient to operate, high in yield, and the like.
Owner:GUANGZHOU BAIYUSN GUANGHUA PHARMA
Tofacitinib oral sustained release dosage forms
ActiveUS9937181B2Minimize biasReduced activityNervous disorderAntipyreticSustained release drugTofacitinib
Owner:PFIZER INC
New method for preparing citric acid tofacitinib medicinal crystal form
InactiveCN107814802AImprove the purification effectEasy to operateOrganic chemistryTofacitinibCitric acid
The invention belongs to the field of medicine and particularly relates to a new method for preparing a citric acid tofacitinib medicinal crystal form. The method has the advantages of being simple inoperation, good in reproducibility, high in yield, good in purifying effect for API related substances, high in crystal form purity, suitable for industrialized mass production and the like, and overcomes the problems of complicated operation, low reproducibility, low yield, poor purifying effect for API related substances, low crystal form purify, unsuitability for industrialized production andthe like.
Owner:JIANGSU ALICORN PHARMATECH CO LTD
Synthesis method for tofacitinib
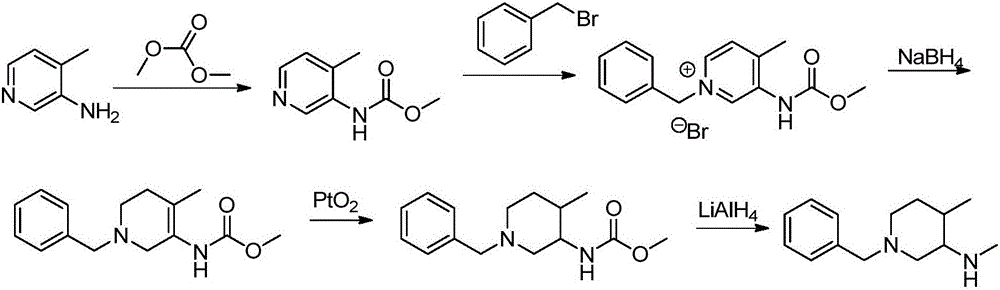
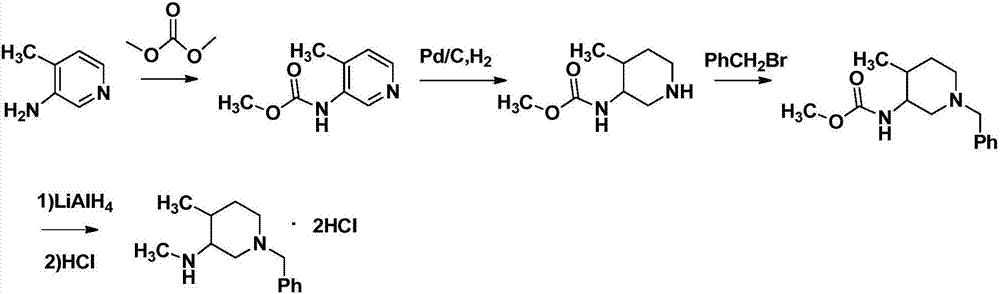
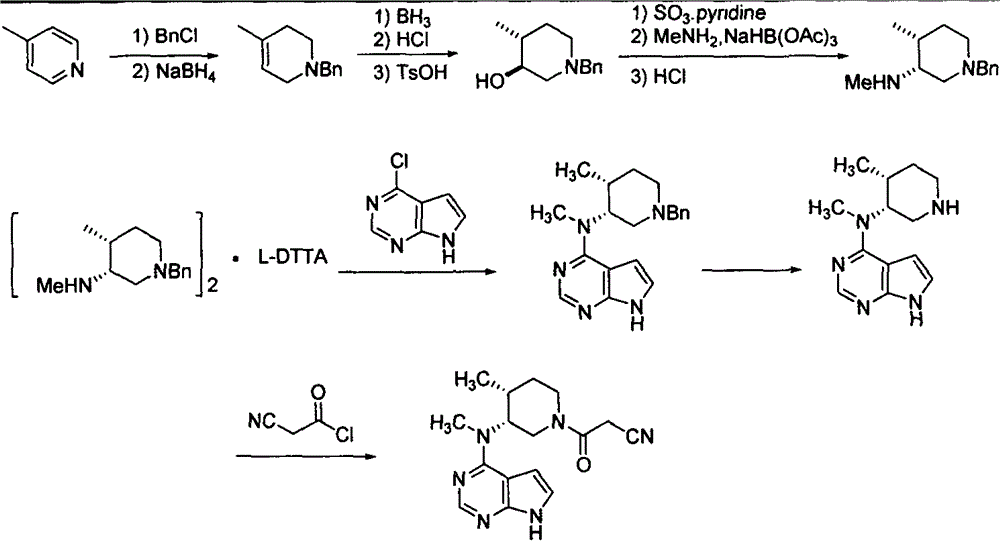
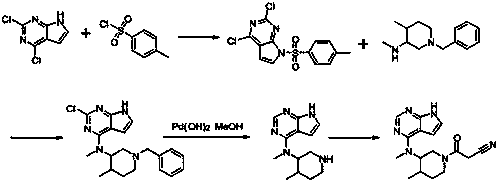
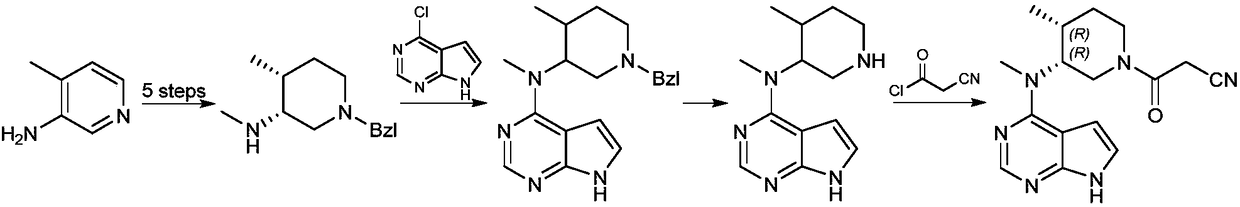
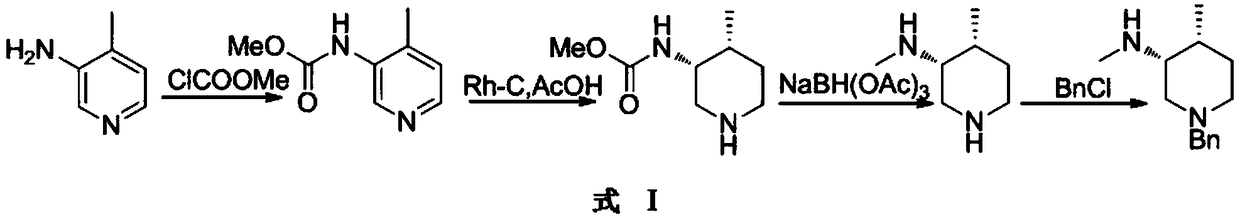
The invention relates to a synthesis method for tofacitinib serving as a JAK inhibitor. According to the method, the tofacitinib is prepared by taking (4-picoline-3-yl)methyl carbamate as a raw material, and performing catalytic hydrogenation, benzyl protection, reduction, salification, separation, deprotection and amidation salification. The method specifically comprises the following steps: (1) performing catalytic hydrogenation and reduction on (4-picoline-3-yl)methyl carbamate in sulfuric acid and Pd / C; (2) reacting cis-(4-picoline-3-yl)methyl carbamate and benzyl chloride to obtain cis-(1-benzyl-4-methylpiperidine-3-yl)methyl carbamate; (3) performing HOBT catalytic condensation on N-[(3R,4R)-4-methylpiperidine-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidine-4-amine and cyanoacetic acid to obtain tofacitinib free alkali. The preparation method provided by the invention has easily obtained raw materials, mild reaction conditions, easiness and convenience in operation and high yield, and is suitable for industrial production.
Owner:济南扬诺生物科技有限公司
Tofacitinib analogues
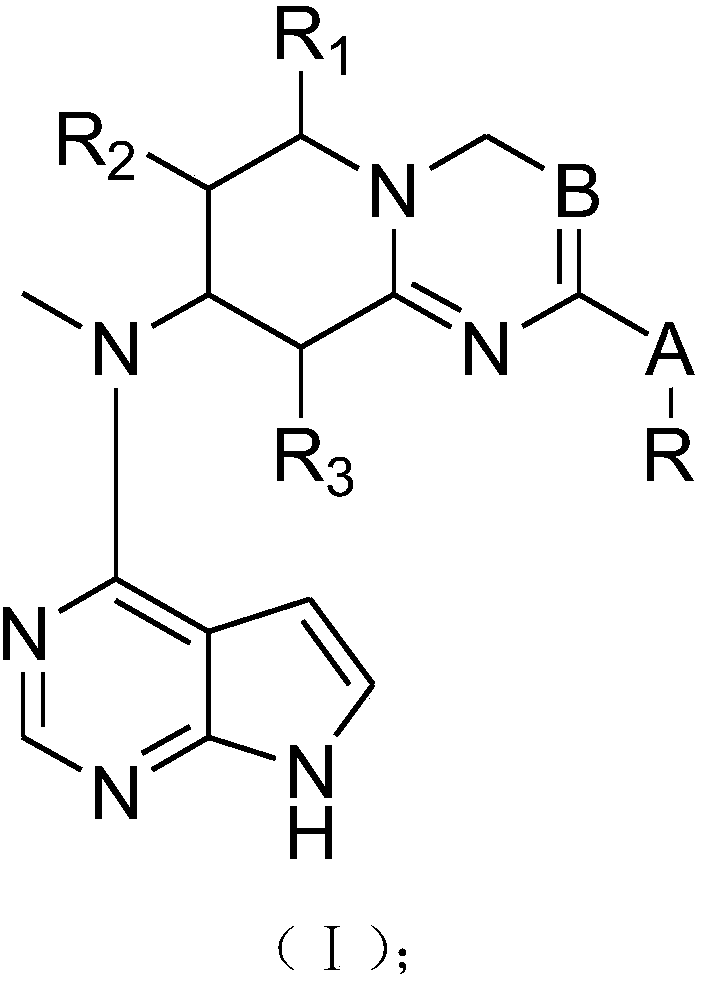
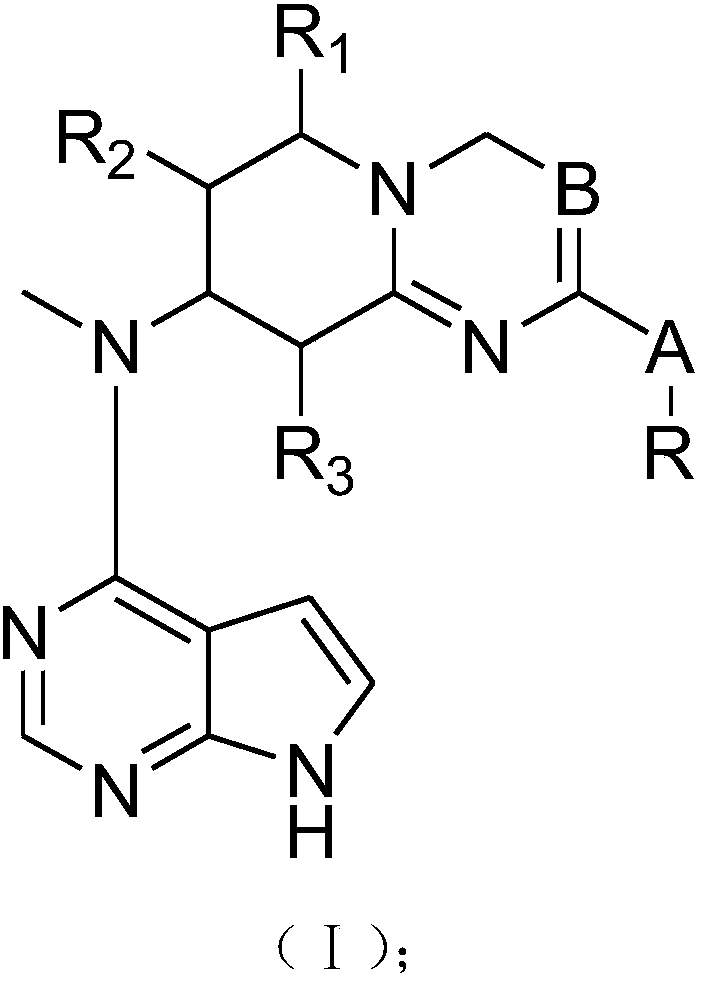
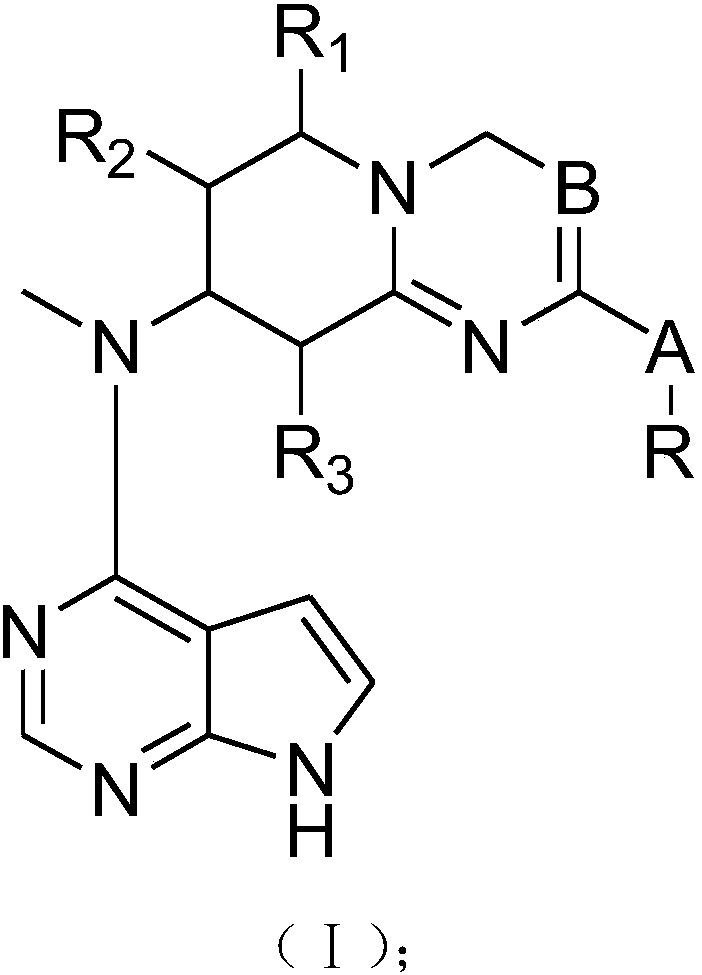
InactiveCN107778321AGood treatment effectOrganic active ingredientsOrganic chemistryTofacitinibStereochemistry
The present invention provides tofacitinib analogues. The analogues contain a compound represented by a formula (I) in the description or a pharmaceutically-acceptable salt of the compound. The invention also discloses a pharmaceutical composition containing the compound represented by the formula I and an application.
Owner:WUXI FORTUNE PHARMA
Method for preparing tofacitinib
ActiveCN104387392AEasy to purifyRaw materials are cheap and easy to getOrganic chemistryTofacitinibRaw material
The invention belongs to the technical field of medicines, and especially relates to a method for preparing tofacitinib. The method for preparing tofacitinib employs a compound with the structural formula shown as (I), and is characterized in that a compound with the structural formula shown as (III) is prepared from the compound I and a compound with the structural formula shown as (V) or a compound with the structural formula shown as (VI), and the compound III is subjected to a dewatering reaction under the condition of adding a dewatering agent, so that the tofacitinib product with the structural formula shown as (IV) is obtained. Tofacitinib is prepared through two new synthetic routines, the raw materials are cheap and easily obtained, the operation process is simple, the intermediate is easily purified, the total yield is high, the production cost is low, and the method is suitable for industrial amplified production.
Owner:SHANDONG WEIFANG PHARMA FACTORY
Hair growth inducer
InactiveCN106138058AReduces symptoms associated with shedding diseaseImprove regenerative abilityOrganic active ingredientsCosmetic preparationsDiseaseTofacitinib
The invention discloses a hair growth inducer, which comprises an active ingredient composed of tofacitinib and minoxidil with a mass percentage of 0.1:99.9-99.9:0.1. The hair growth inducing agent provided by the invention, its active ingredient comprises tofacitinib and minoxidil, wherein tofacitinib is a kind of JAK inhibitor, can interact with target cells (such as dermis, epidermis, dermal papilla cells or hair follicles) Cell) specific protein active site selectively binds, inhibits the transmission of cell pathway signals and the synthesis of characteristic cytokines, thereby reducing the symptoms associated with hair loss diseases; Minoxidil is a potassium ion channel opener that can directly relax Vascular smooth muscle has a powerful dilation effect on small arteries, reduces peripheral resistance, promotes the growth of hair follicle cells, and thus regenerates hair; the combination of these two substances synergistically acts on the target protein of target cells and vascular smooth muscle, and inhibits cytokines Synthesis and accelerated blood circulation, in order to achieve a significant effect of promoting hair regeneration.
Owner:颜晓丽
Compound pharmaceutical composition for treating inflammatory diseases of skin
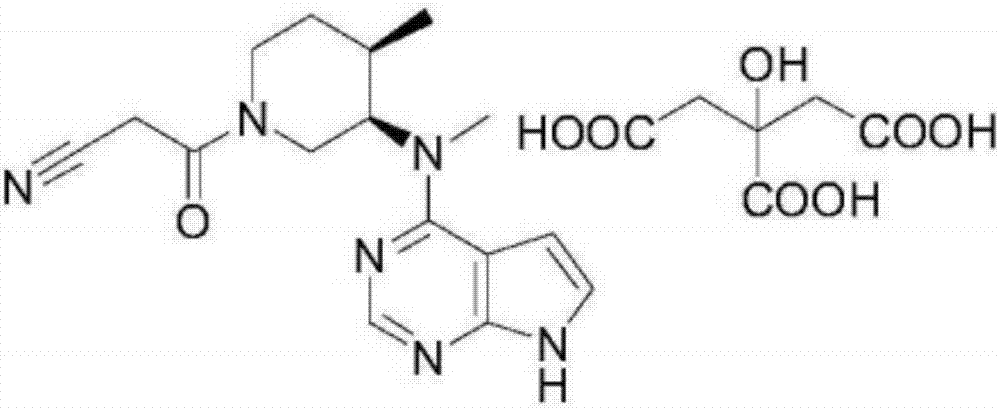
The invention relates to the technical field of medicines, in particular to compound pharmaceutical composition for treating inflammatory diseases of skin. The compound pharmaceutical composition is characterized in that active ingredients of the compound medicine comprise tofacitinib and crisaborole. The pharmaceutical composition has higher treatment effects and lower use dosage, has remarkablecollaborative treatment effects and can be used for treating inflammatory diseases of skin.
Owner:HEFEI IND PHARMA INST +1
Tofacitinib crystal form compound and preparation method thereof
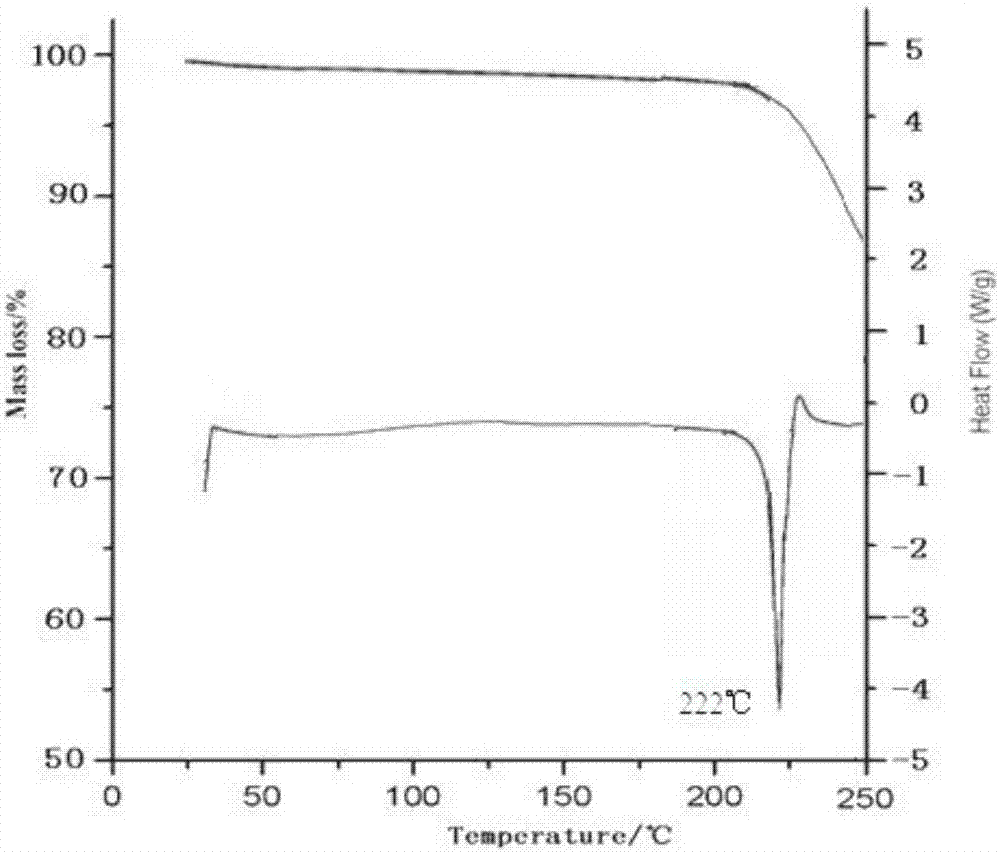
ActiveCN106967072AImprove liquidityImprove solubilityOrganic active ingredientsAntipyreticSolubilityTofacitinib
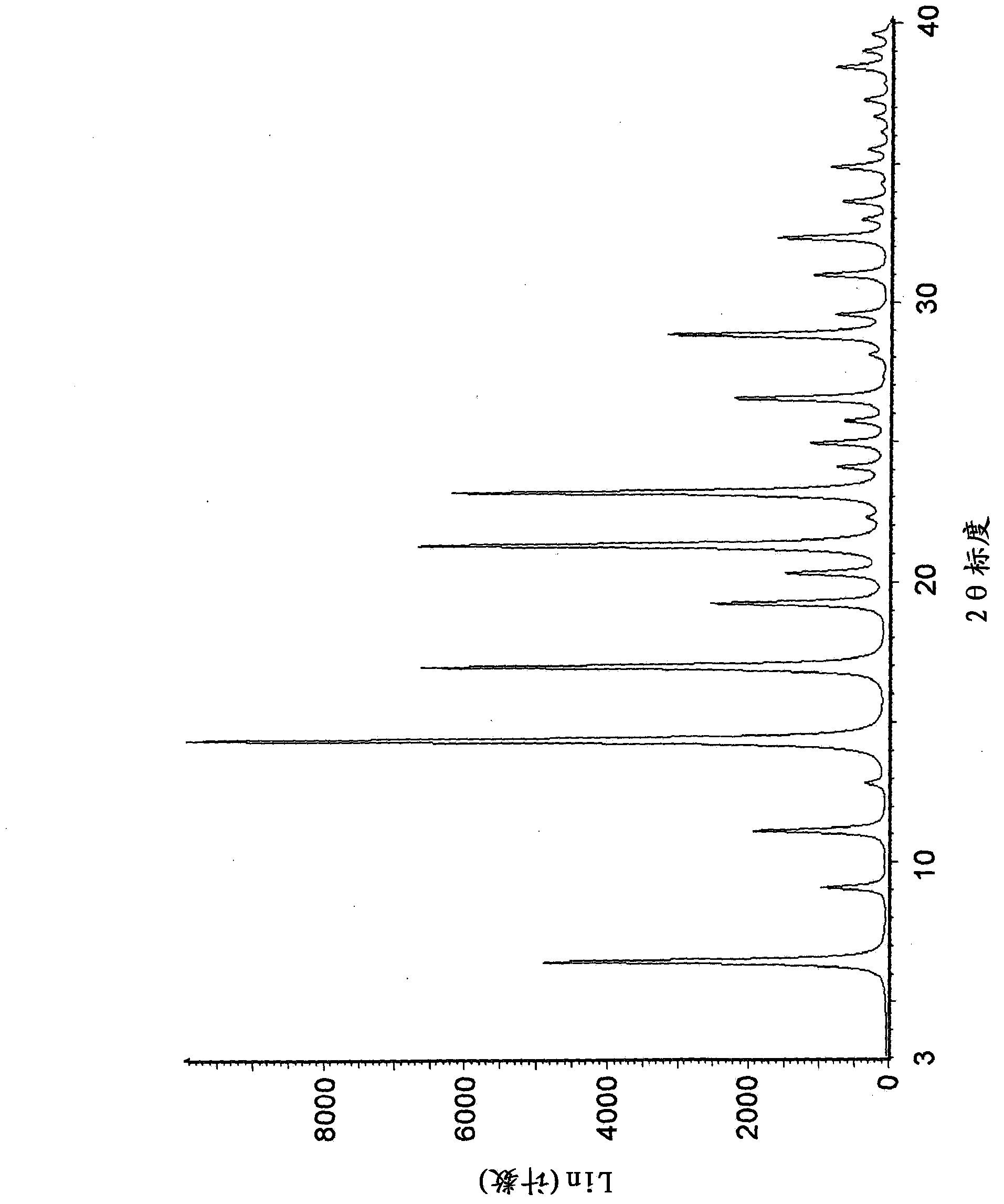
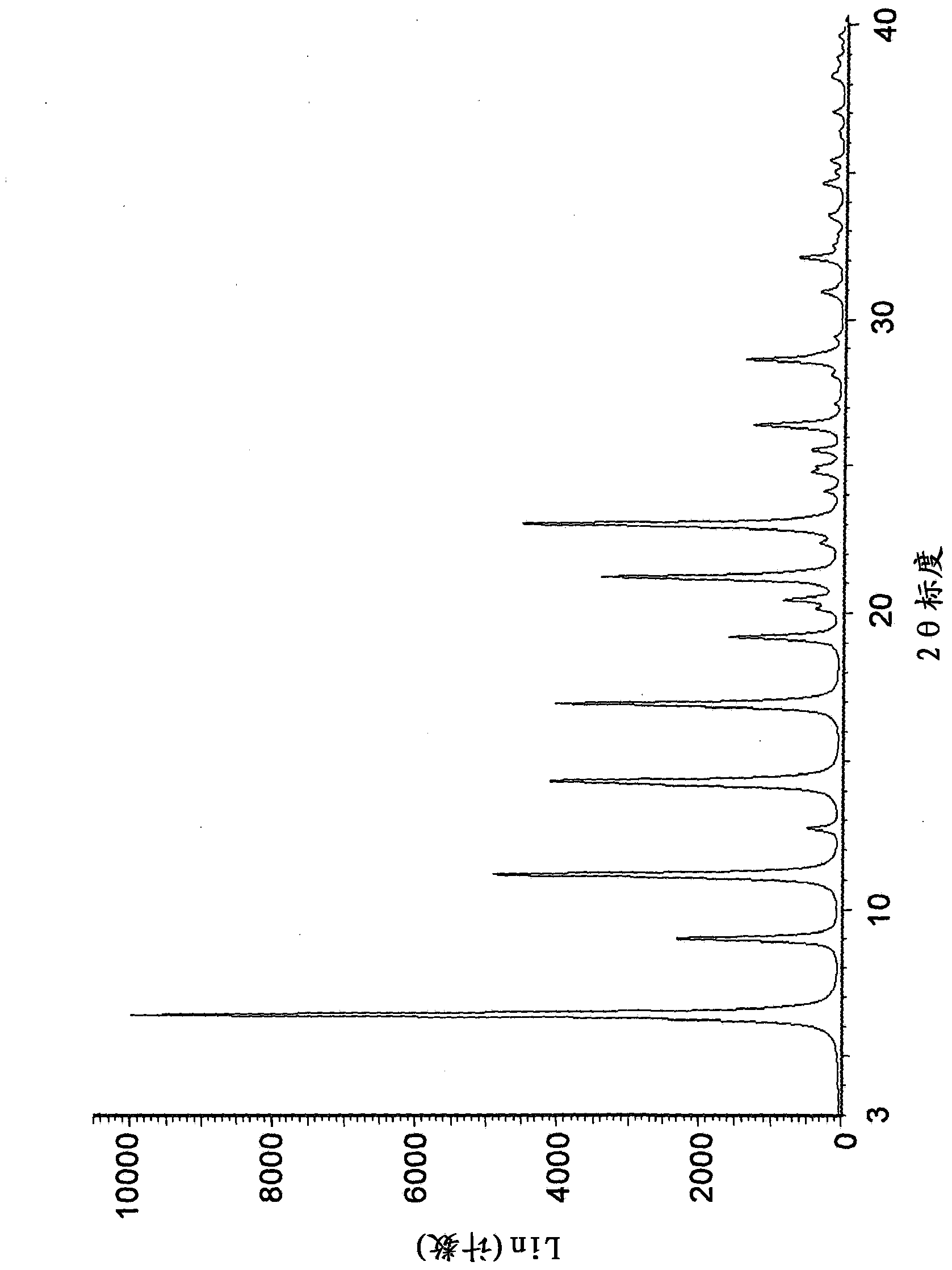
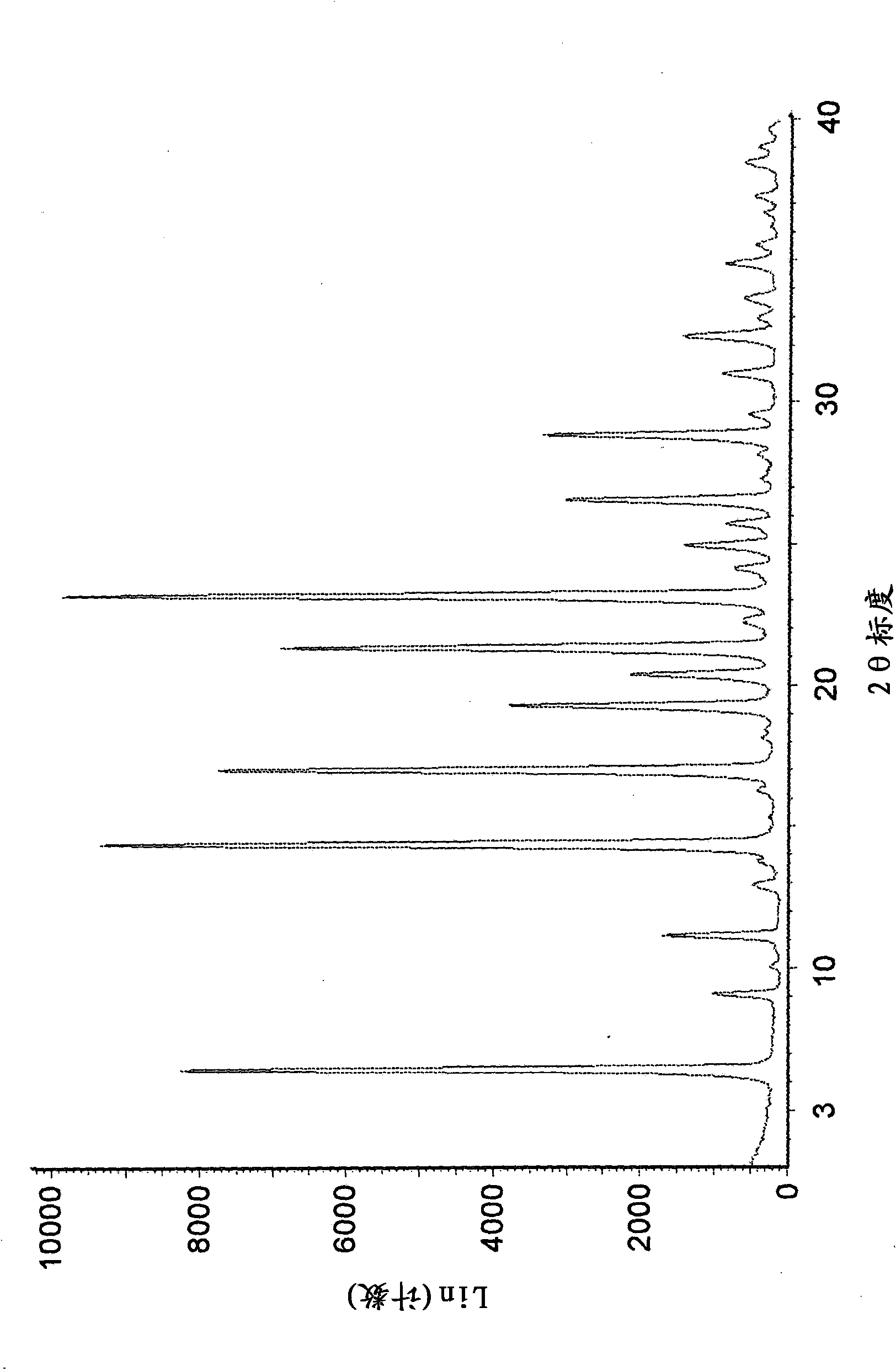
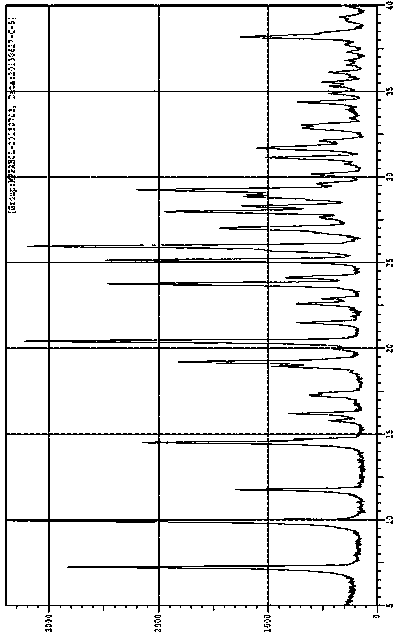
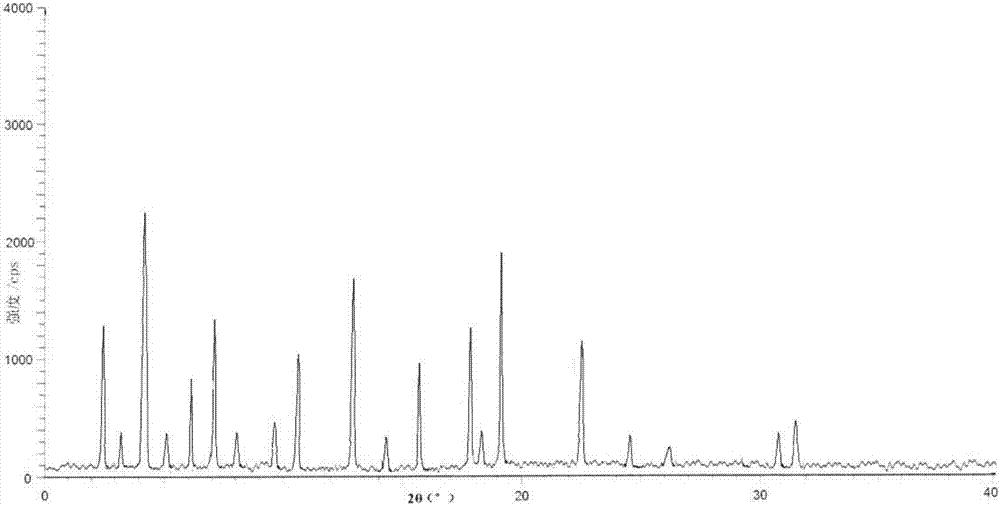
The invention belongs to the technical field of medicine and discloses a tofacitinib crystal form compound and a preparation method thereof. The X-ray powder diffraction spectrum shown by the 2 theta +-0.2 degree diffraction angle shows characteristic diffraction peaks at 2.42 degrees, 3.25 degrees, 4.23 degrees, 5.04 degrees, 6.12 degrees, 7.08 degrees, 8.02 degrees, 9.72 degrees, 10.63 degrees, 12.90 degrees, 14.35 degrees, 15.71 degrees, 17.82 degrees, 18.34 degrees, 19.24 degrees, 22.46 degrees, 24.56 degrees, 26.62 degrees, 30.75 degrees and 32.45 degrees, and the X-ray powder diffraction spectrum obtained by Cu-K alpha ray measurement and shown by the figure I is totally different from the prior art. The tofacitinib crystal form compound has better water solubility and high stability, the preparation method is simple and easy to operate, the medication safety is improved greatly after the compound is prepared into a drug composition, and the compound is very suitable for clinical application.
Owner:SHANDONG YUXIN PHARMA CO LTD
Preparation method of tofacitinib impurity
ActiveCN109336892AEasy to separate and purifyHigh purityOrganic chemistryQuality researchTofacitinib
The invention discloses a preparation method of tofacitinib impurity. A synthetic route is as shown in the specification. The preparation method provided by the invention has the advantages that the synthetic process is simple, the products are easy to separate and purify and the purity of the obtained products is as high as 98.0%. Therefore, the obtained compound I, II in the preparation method can be used for the quality research and process research of related products as impurity reference substances of tofacitinib raw medicinal materials and preparations.
Owner:珠海优润医药科技有限公司
Tofacitinib tablets and preparation method thereof
InactiveCN107441054AImprove stabilityImprove medication complianceOrganic active ingredientsAntipyreticWater insolubleTofacitinib
The invention provides tofacitinib tablets and a preparation method thereof. The tablets contain tofacitinib or pharmacologically acceptable salt, a corrigent, an excipient with good water wettability, water insoluble macromolecules and a disintegrant. The preparation method of the tablets comprises the following steps: mixing the tofacitinib or pharmacologically acceptable salt, the excipient with good water wettability, and water insoluble macromolecules to prill; and then adding the corrigent, the disintegrant and a lubricant; and mixing and tabletting the mixture to obtain the tablets. According to the tofacitinib tablets and the preparation method thereof provided by the invention, the tablets are convenient to medicate and quick and efficient to take effect, and can be prepared by means of conventional production equipment; no special demands are put on the equipment; and the preparation process is simple, the stability of the preparation can be guaranteed, and the tablets are conveniently transported and stored and suitable for scaled production.
Owner:FUJIAN INST OF MICROBIOLOGY
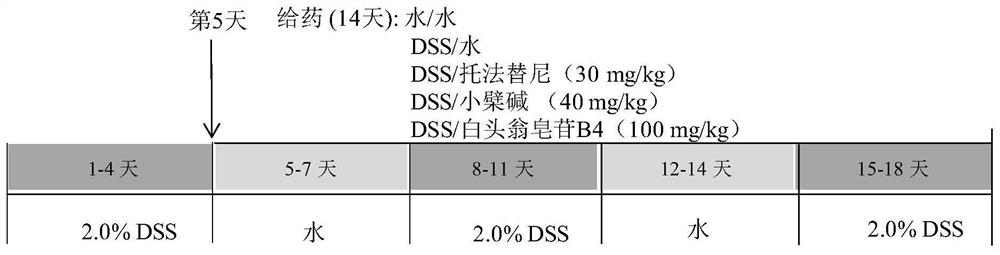
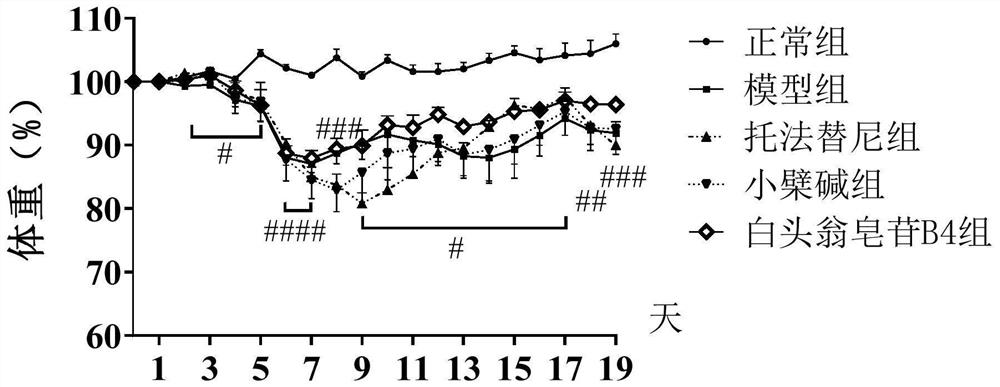
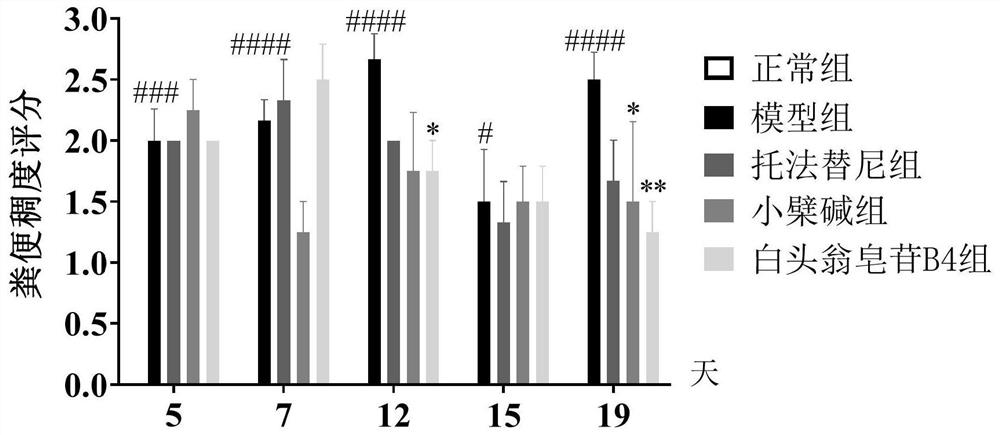
Application of anemoside B4 in preparation of drugs for treating ulcerative colitis
PendingCN112107586ARelief of symptoms in miceObvious effectOrganic active ingredientsDigestive systemSide effectTreatment effect
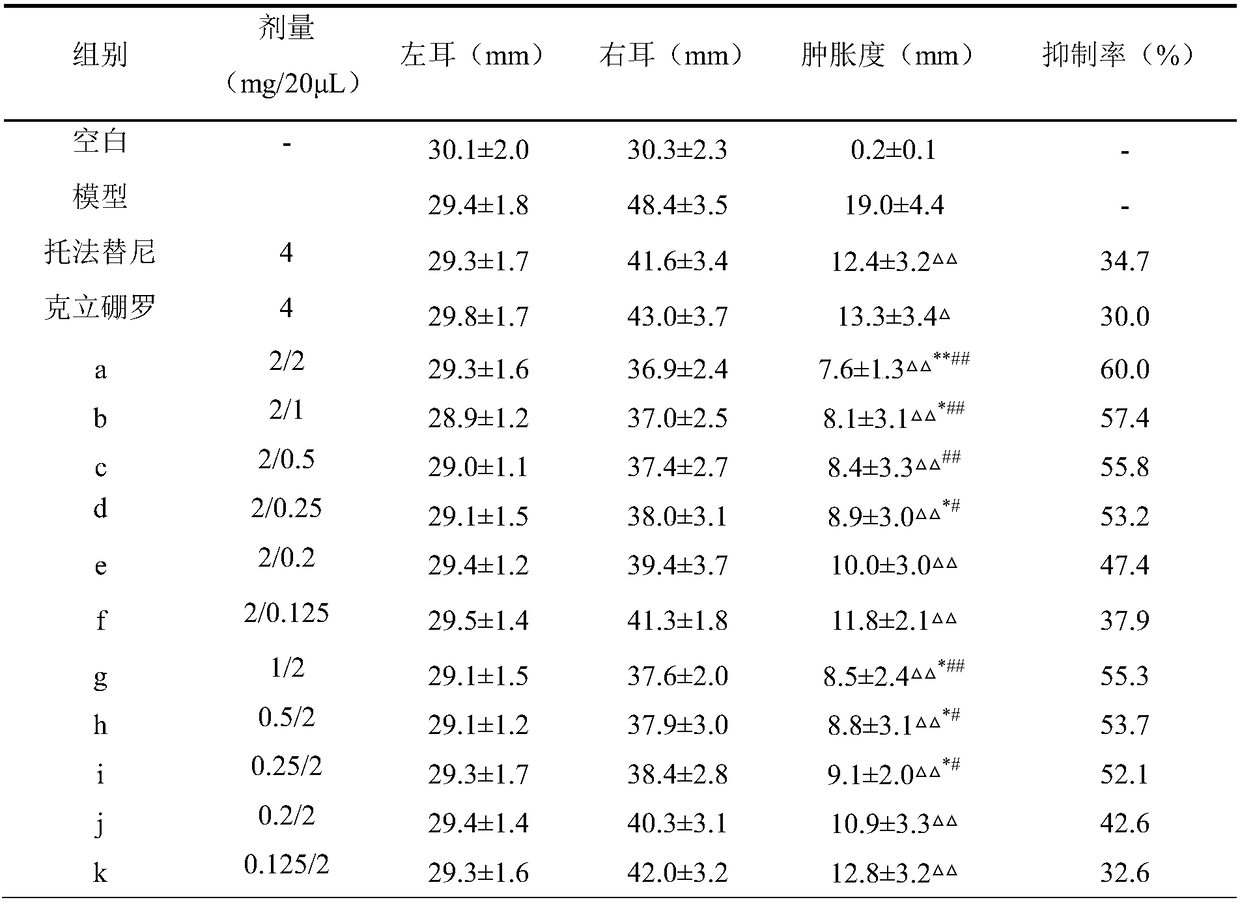
The invention discloses application of anemoside B4 in preparation of drugs for treating ulcerative colitis. The anemoside B4 can be used for remarkably relieving symptoms of a model mouse suffering from the ulcerative colitis, has an action superior to that of a latest UC Western medicine Tofacitinib and that of a traditional Chinese medicine monomer berberine having a relatively large treatmentprospect to the UC, cannot cause spleen swelling accompanying during treatment on the UC by the two kinds of medicines, has a certain relieving action on the spleen swelling and meanwhile is free of structural impairment to other internal organs. The anemoside B4 is definite in UC treatment effect, low in toxic or side effects and high in safety and is advantageously applied to clinical treatmenton the UC, and problems of the existing medicines that the treatment effect is insufficient, side effects are high, and the toxicity is high are solved.
Owner:SOUTH CHINA UNIV OF TECH
Synthesis method for preparing intermediates of tofacitinib
The invention provides a novel method for synthesizing primary ring cis-4-methyl-3-methylamino-1-benzylpiperidine bis-hydrochloride of tofacitinib. The primary ring cis-4-methyl-3-methylamino-1-benzylpiperidine bis-hydrochloride is made from 3-halogenation-picoline. The novel method includes steps of catalytic Ullman coupling, 3-halogenation-picoline and benzyl halide salifying, hydroboration reagent reduction, salifying and the like to obtain target compounds. The method has the advantages of simple conditions, easily available reagents and operational safety.
Owner:甘肃皓天医药科技有限责任公司
4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method
The invention discloses a 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method. A compound is an important intermediate for synthesizing ruxolitinib and tofacitinib as a JAK inhibitor for treating rheumatoid arthritis. The 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method comprises the following steps of by taking a compound I (4,6-dichloro-5-allyl pyrimidine) as a starting material, performing oxidation reaction on the compound I and ozone to produce a compound II; then performing nucleophilic substitution reaction on the compound II and triethyl orthoformate to produce a compound III; then performing nucleophilic substitution reaction on the compound III and ammonia gas to produce a compound IV; and finally, performing ring closing on the compound IV self in an acid environment to produce a compound V, i.e., 4-chloro-7H-pyrrolo[2,3-d]pyrimidine, wherein a synthetic route is shown as the following formula (described in the description). The synthetic method disclosed by the invention is cheap and available in raw materials, simple and short in synthetic route, low in cost, high in yield and easy in industrial production.
Owner:EAST CHINA NORMAL UNIVERSITY +1
Synthesis method of JAK inhibitor tofacitinib
The invention relates to a synthesis method of a JAK inhibitor tofacitinib. The method comprises the steps: with N-[(3R,4R)-1-benzyl-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine as a raw material, with formic acid, ammonium formate or hydrazine hydrate as a hydrogen donor, carrying out hydrogenation to remove benzyl to obtain N-[(3R,4R)-4-methylpiperidin-3-yl]-N-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine, and then carrying out a reaction with cyanoacetic acid to obtain tofacitinib. Compared with an original synthesis method, the method provided by the invention has the advantages of mild conditions, simple and convenient operation, high yield, low cost and the like, and is suitable for industrialized production.
Owner:JINAN TIANYU SURVEYING & MAPPING INSTR CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
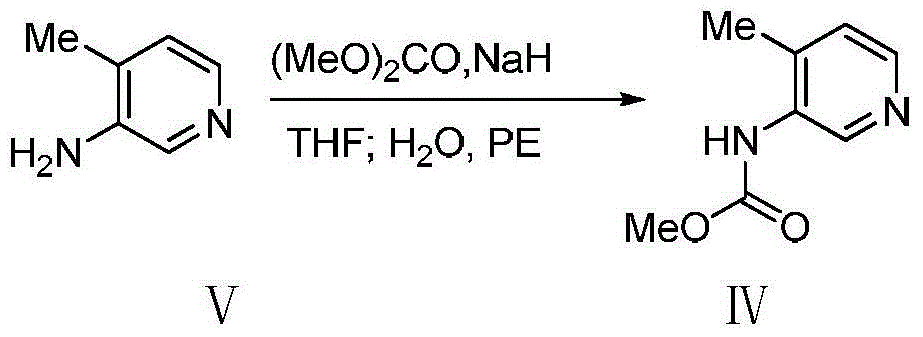
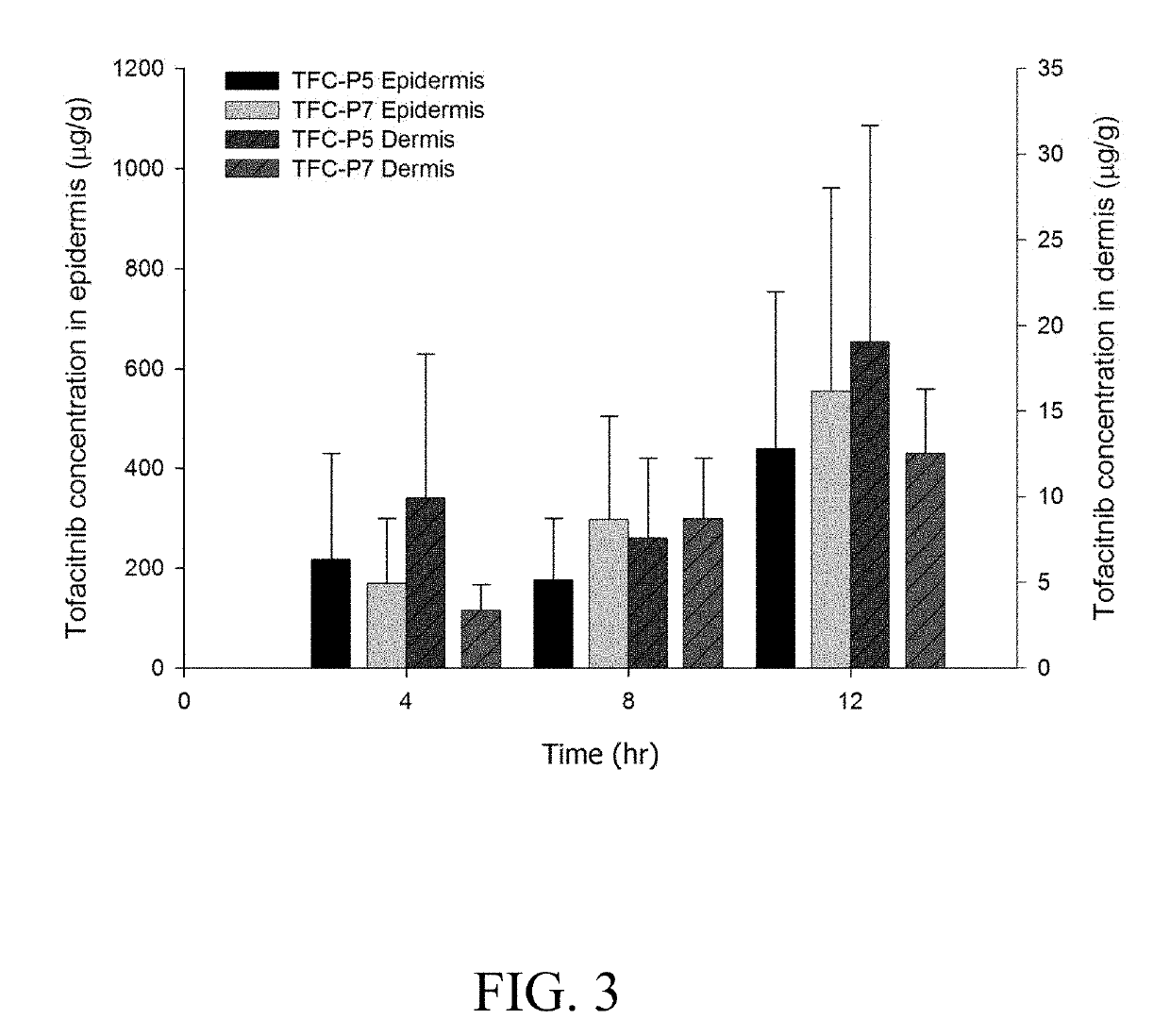

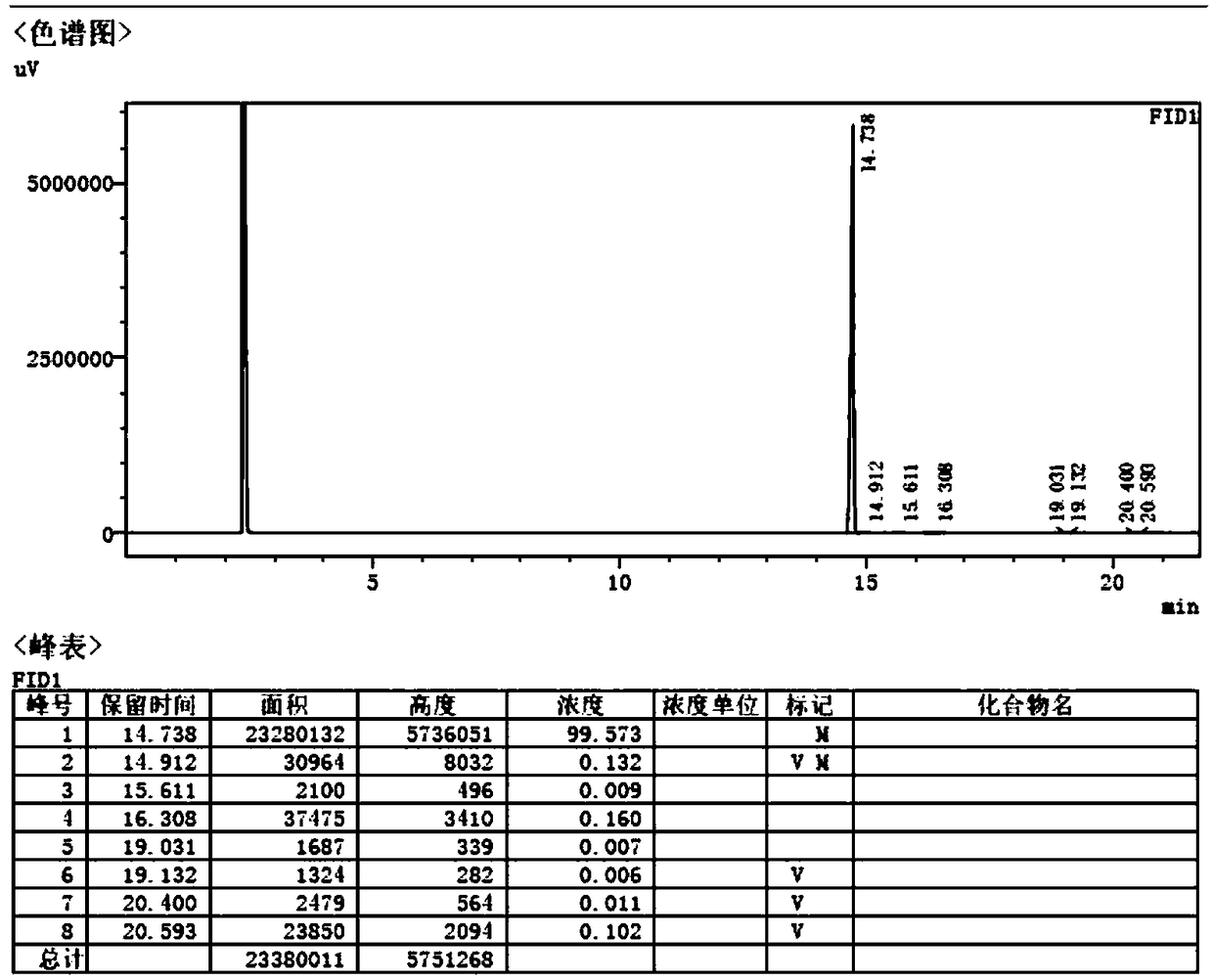
![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka.patsnap.com/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000011.png)
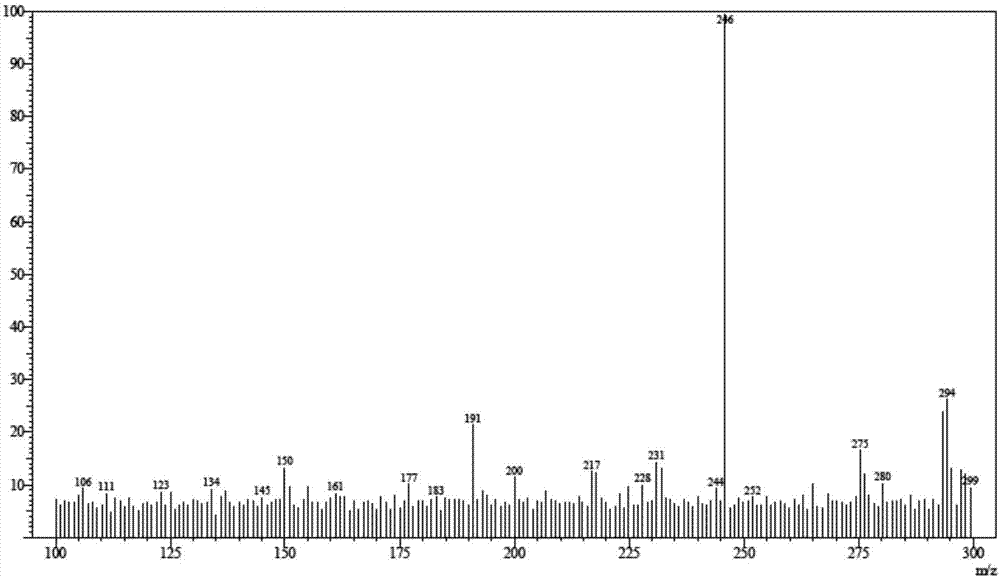
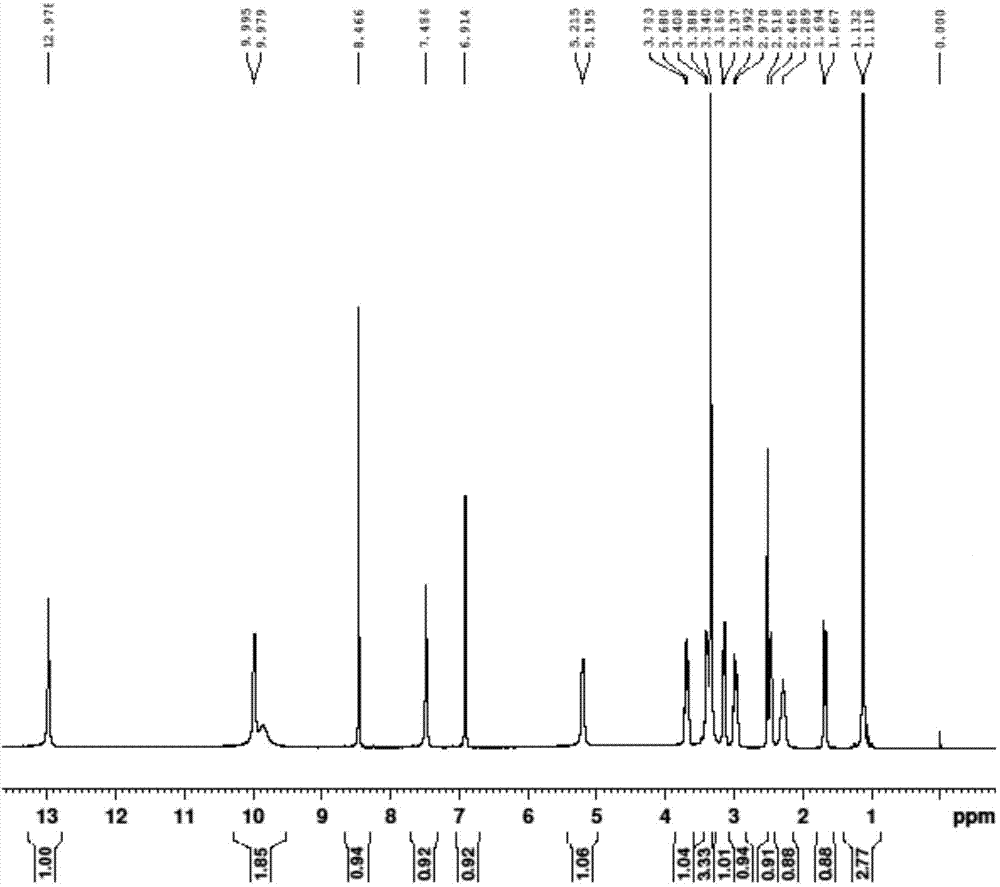
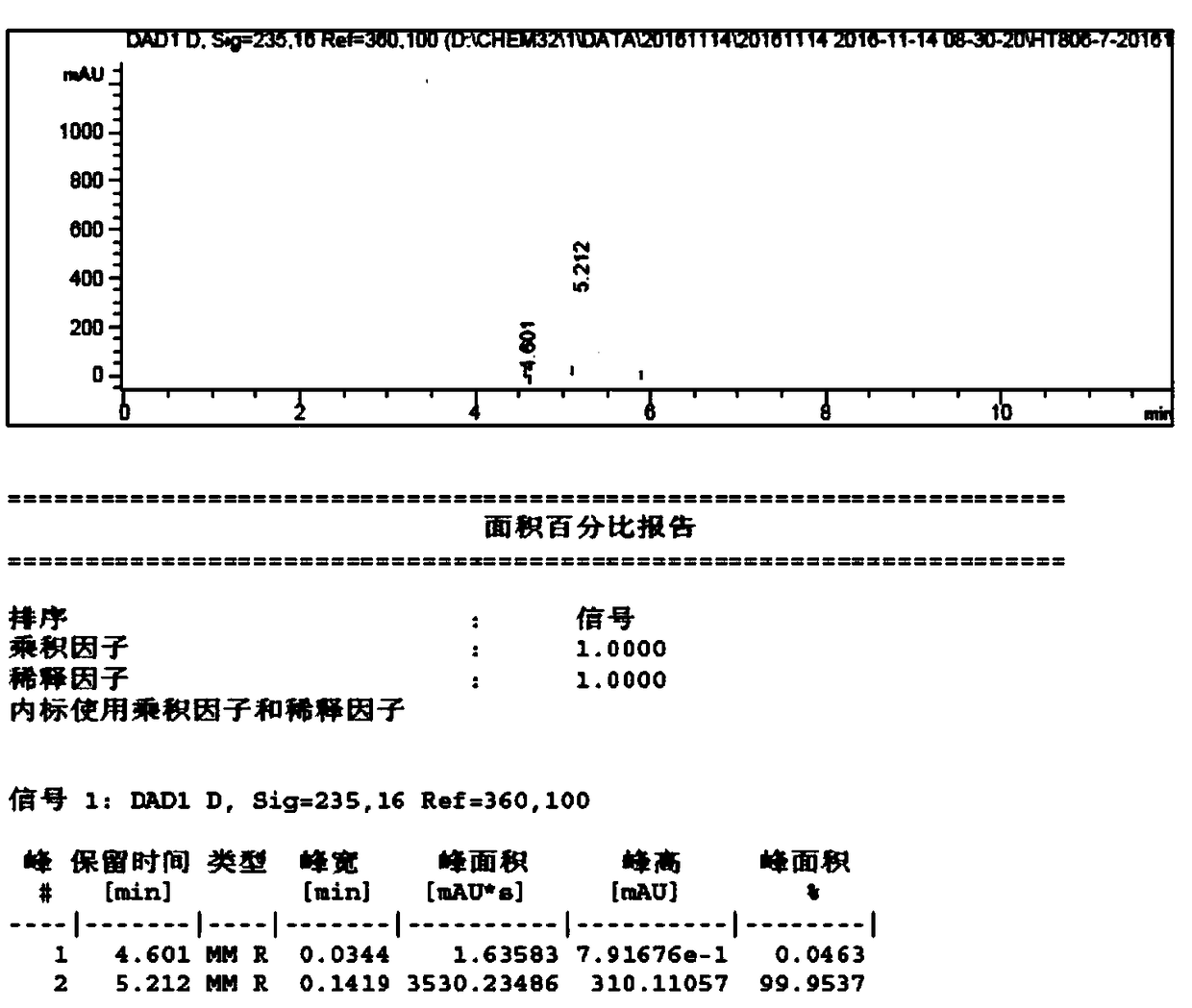
![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka.patsnap.com/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000021.png)
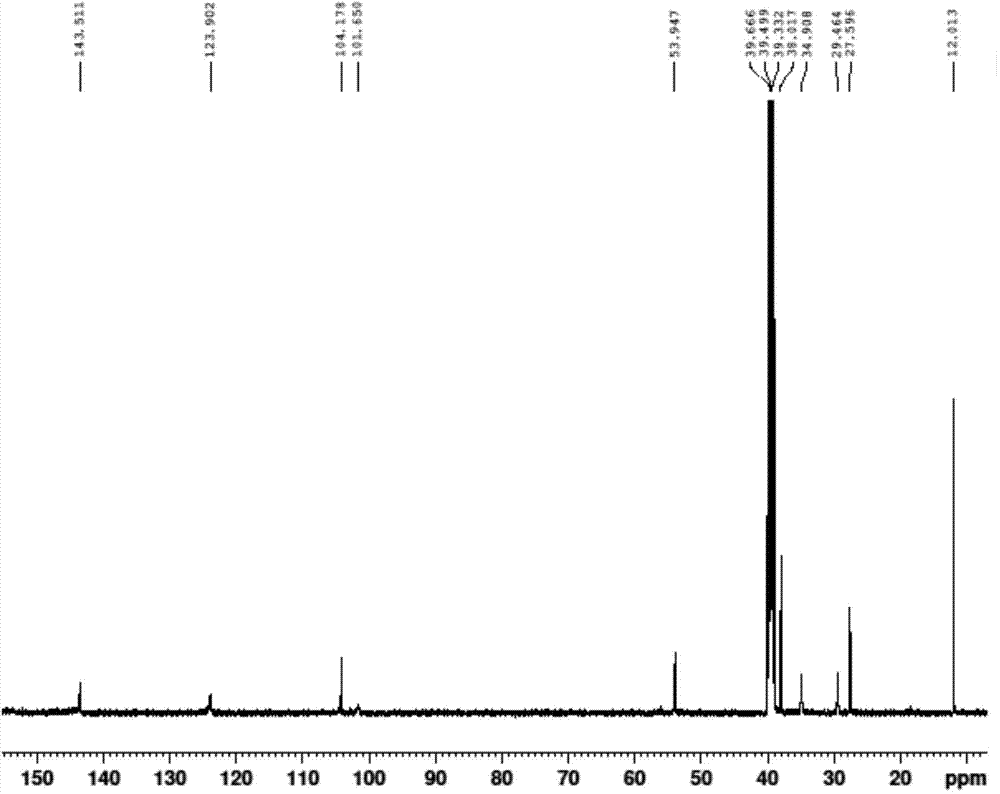
![4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method 4-chloro-7H-pyrrolo[2,3-d]pyrimidine synthetic method](https://images-eureka.patsnap.com/patent_img/7f09348c-cd6f-4a14-a89f-f5d475eecaf2/BDA0001621800940000022.png)