Patents
Literature
1093results about How to "Short synthetic route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
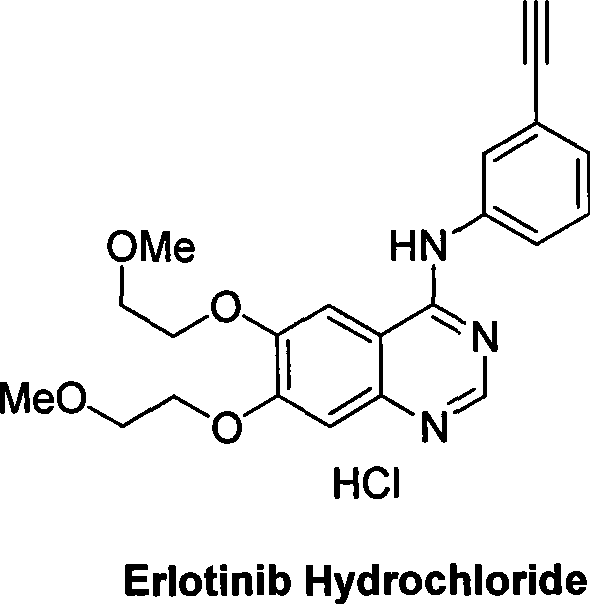
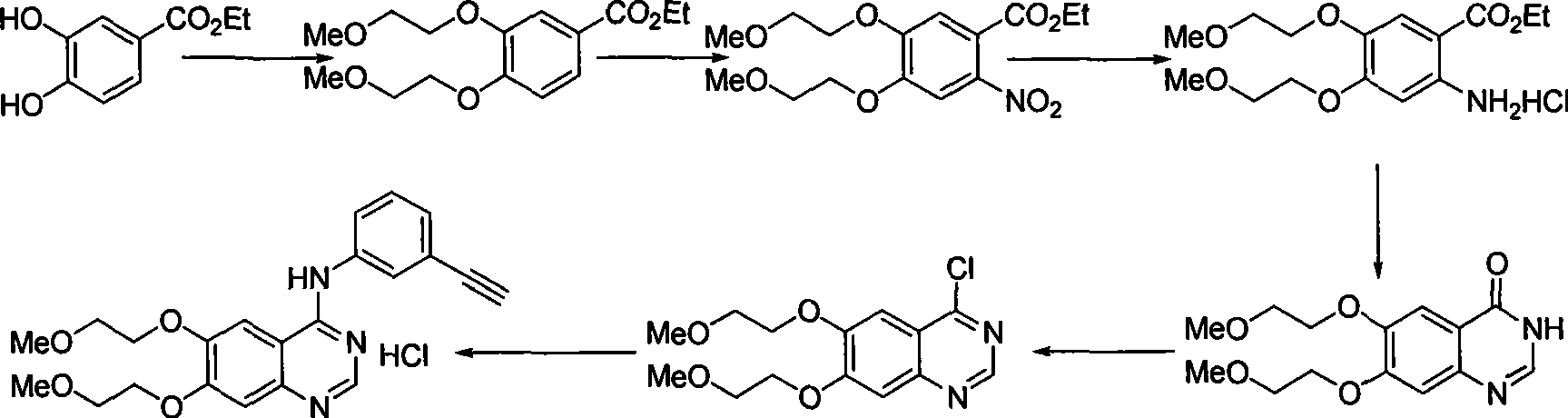
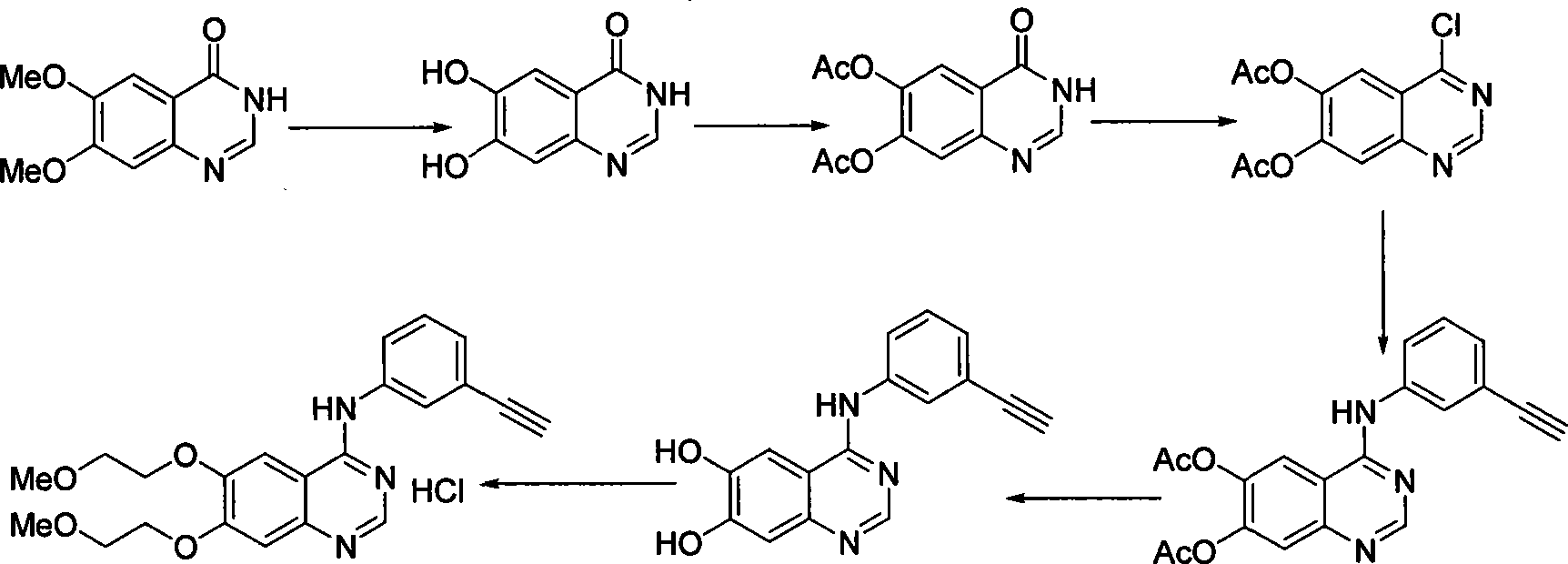
Preparation of erlotinid hydrochloride
ActiveCN101463013AShort synthetic routeAvoid excessive hydrolysisOrganic chemistryDimethylquinazoloneMedicinal chemistry
The invention discloses a preparation method of erlotinib hydrochloride. The method is characterized by taking 3,4-dihydroxybenzaldehyde as a raw material, synthesizing to obtain 6,7-dimethoxyquinazoline-4-one, directly chloridizing to obtain a product, allowing the product to react with meta-ethynylaniline to obtain the erlotinib hydrochloride. The method has mild reaction condition and is applicable to industrialized production.
Owner:FUJIAN SOUTH PHARMA CO LTD
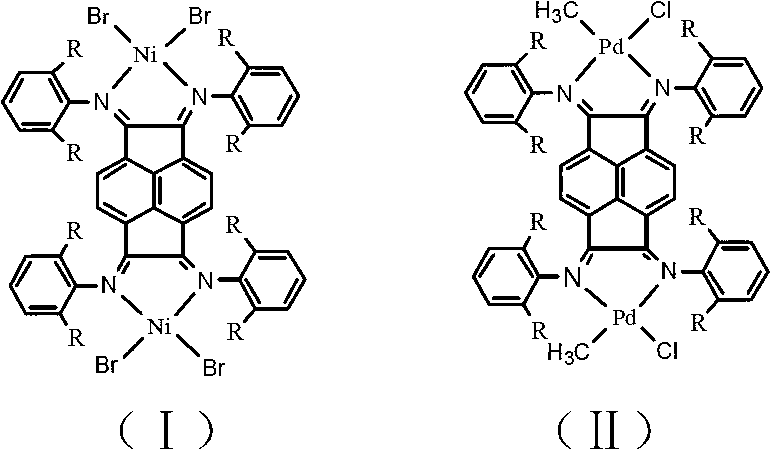
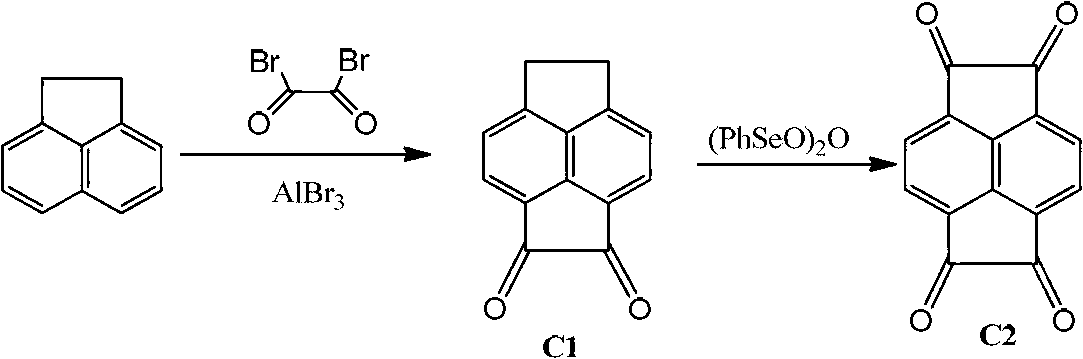
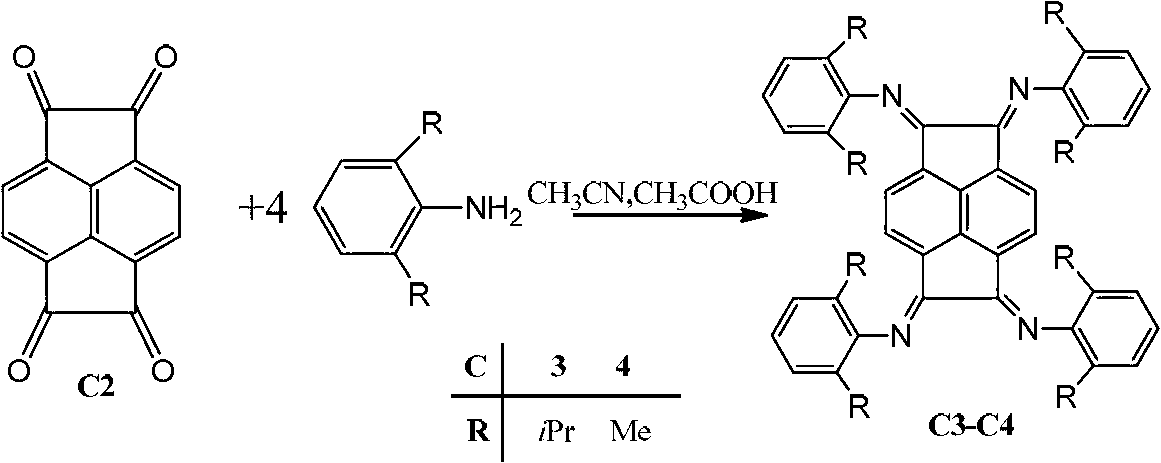
Binuclear acenaphthene (alpha-diimine) nickel/palladium catalysts for olefins, and preparation method and application thereof
ActiveCN102827311AHigh activityImprove stabilityNickel organic compoundsBulk chemical productionNickel catalystDiimine
The invention discloses a preparation method for binuclear acenaphthene (alpha-diimine) nickel / palladium catalysts for olefins and application of the catalysts in catalysis of polymerization of olefins. The binuclear acenaphthene (alpha-diimine) nickel / palladium catalysts for olefins in the invention have structural formulas as represented by formula (I) and formula (II) in the specification. The binuclear acenaphthene (alpha-diimine) palladium catalyst for polymerization of olefins has high activity and good stability and can be used for preparing high-molecular-weight hyperbranched polyethylene with bimodal distribution; the binuclear acenaphthene (alpha-diimine) nickel catalyst for polymerization of olefins has high activity and good stability and can be used for preparing polypropylene with a weight-average molecular weight of more than 100,000 and thermoplastic elastomers with elasticity and a high molecular weight. The preparation method for the synthesized catalysts used for polymerization of olefins has the advantages of a simple process, a short synthetic route, low cost, high yield and easy industrialization.
Owner:ZHEJIANG UNIV
Method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis
ActiveCN102559520AHigh excessLess side effectsFungiMicroorganism based processesPhosphateReaction temperature
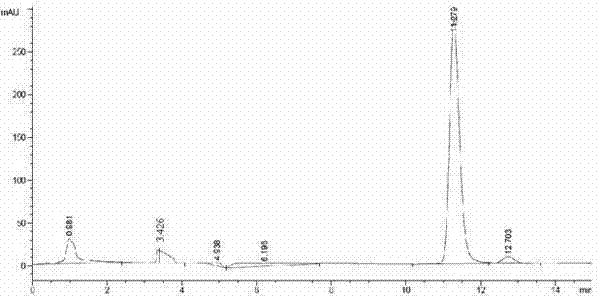
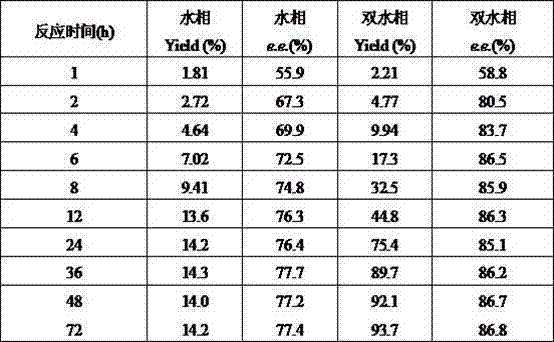
The invention relates to a method for preparing (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol by utilizing microbial catalysis, and belongs to the technical field of biological catalysis. According to the method, 4-chlorphenyl-(pyridine-2-yl)-ketone is subjected to asymmetrical reduction by utilizing kluyveromycessp (CCTCCM 2011385) whole cells to synthesize the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol. The method comprises the following steps of: sieving a microbe which has high-stereoselectivity carbonyl reductase activity on a prochiral ketone substrate, determining a series of conditions of asymmetrical reduction reaction, such as reaction temperature, pH, the concentration of cells, the concentration of the substrate and reaction time and additives (including various secondary solvents, polyethyleneglycol (PEG), phosphates and the like), wherein the enantiomer excess value and yield of the (S)-(4-chlorphenyl)-(pyridine-2-yl)-methanol serving as a product can reach 86.7 percent e.e and 92.1. The product is separated and extracted initially by silicagel column chromatography, so that the purity of the separated product is 99.2 percent, and the extraction yield is 56.7 percent.
Owner:JIANGNAN UNIV
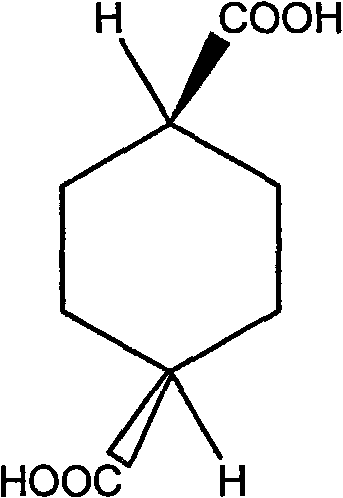
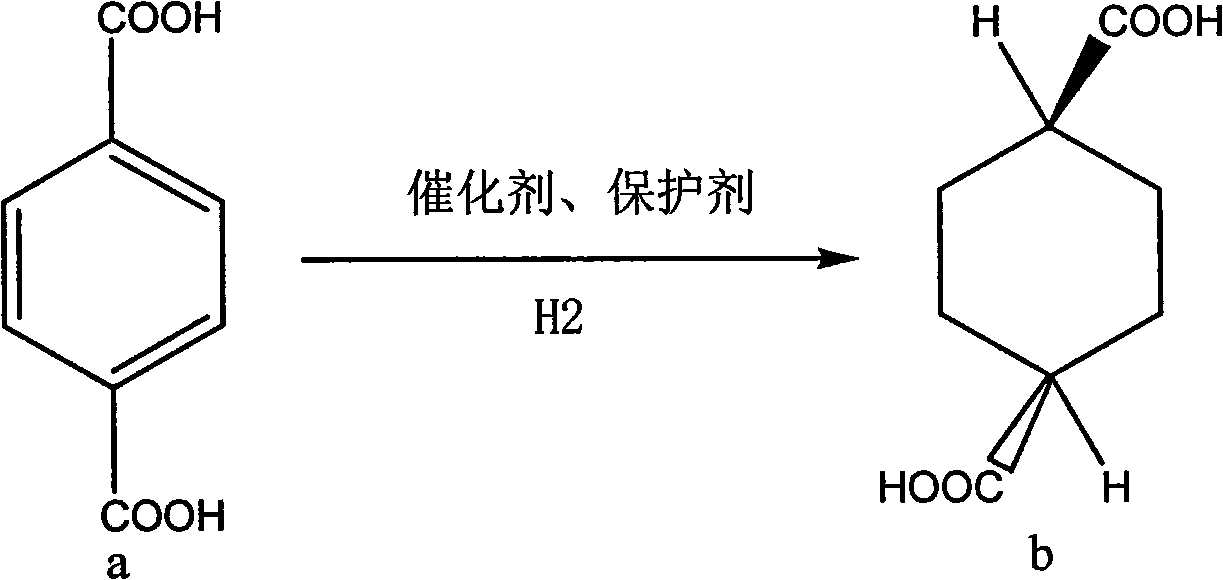
Synthesizing method for trans-1,4-cyclohexanedicarboxylic acid
InactiveCN101591237ARaw materials are easy to getShort synthetic routeCarboxylic preparation by ozone oxidationHydrogenNitrogen gas
The invention discloses a synthesizing method for trans-1,4-cyclohexanedicarboxylic acid, which comprises the following steps: adding terephthalic acid, catalysts, protective agents and water into an autoclave; introducing hydrogen gas after introducing nitrogen gas for displacement; continuously adding hydrogen at a temperature of between 100 and 120 DEG C; and after the reaction is finished, sequentially performing overheat filtering, cooling and filtering to obtain the product. The synthesizing method is adopted to prepare the trans-1,4-cyclohexanedicarboxylic acid through one-step reaction by taking the terephthalic acid as an initial raw material, and has the advantages of easily-obtained raw material, short synthesis route, safe and simple process, low cost, over 95 percent of yield and easy realization of industrialized production.
Owner:JIANGSU KANGHENG CHEM
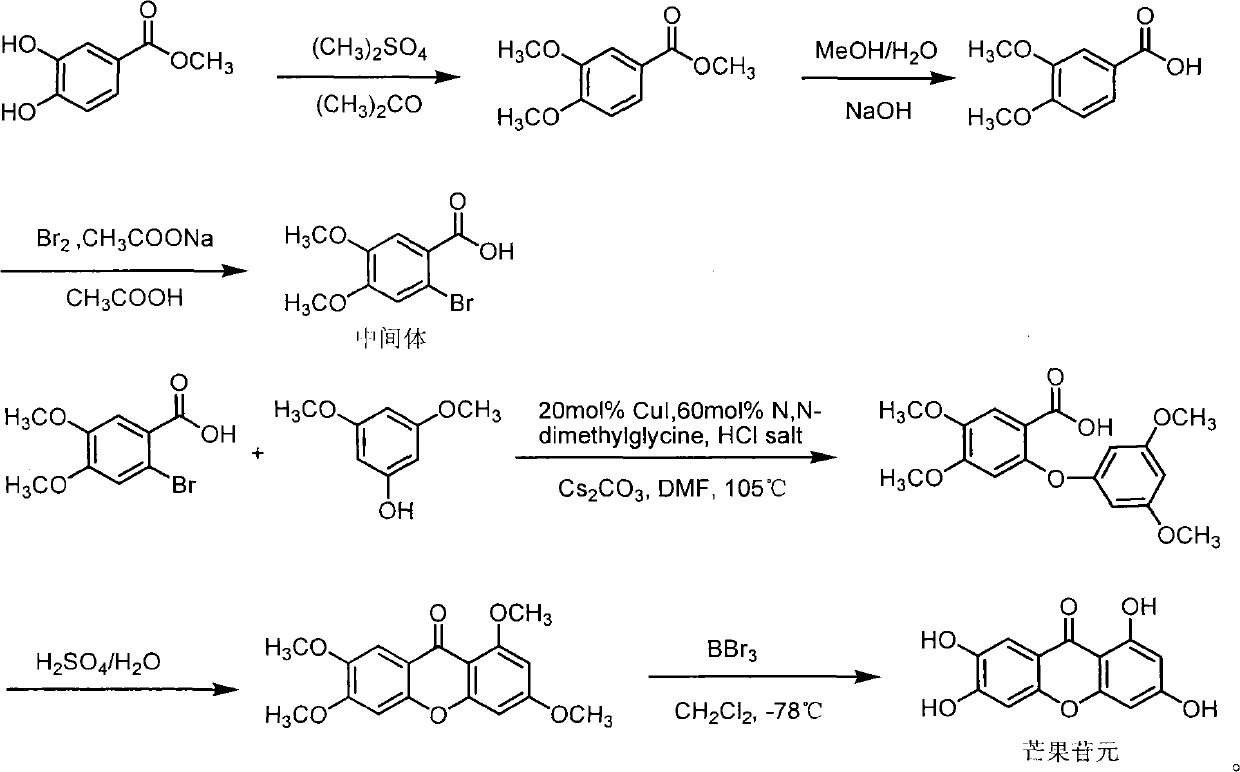
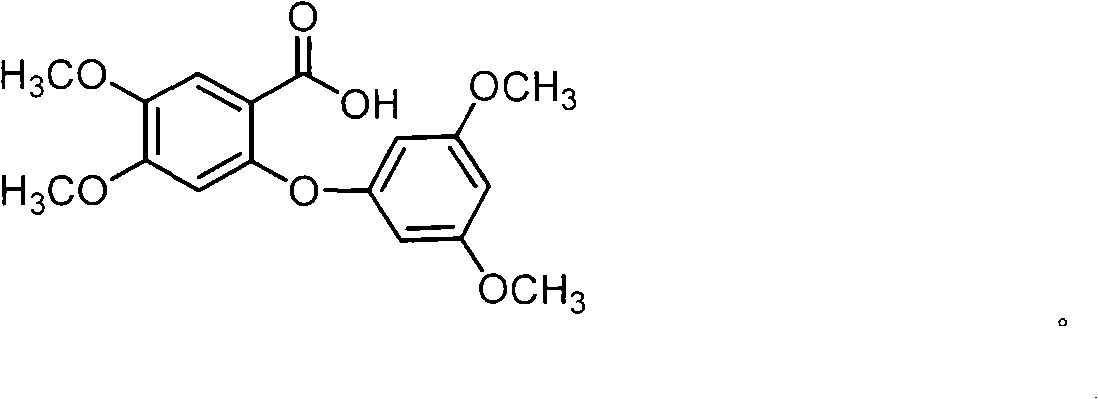
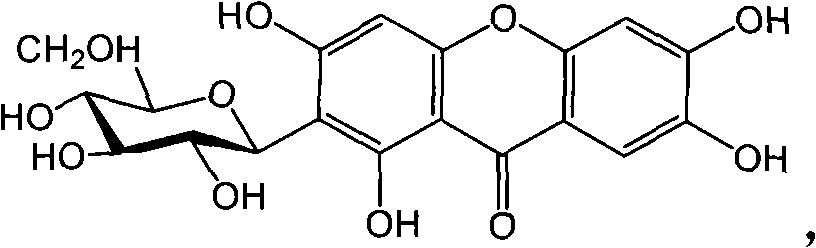
Full chemical synthesis method for mangiferin aglycones
InactiveCN102002031AShort synthetic routeSynthetic reaction conditions are mildOrganic chemistryChemical synthesisChemical reaction
The invention discloses a full chemical synthesis method for mangiferin aglycones. In a process of full chemical synthesis, the mangiferin aglycones are prepared from 3,4-methyl dihydroxybenzoate serving as an initiative raw material through six chemical reactions and steps. The synthetic process of the full chemical synthesis of the mangiferin aglycones has the advantages of short synthetic routes, mild condition of a synthetic reaction and high yield, and can be used for large-scale production.
Owner:KUNMING MEDICAL UNIVERSITY
Vanadium dioxide intelligent temperature control film and preparation method thereof
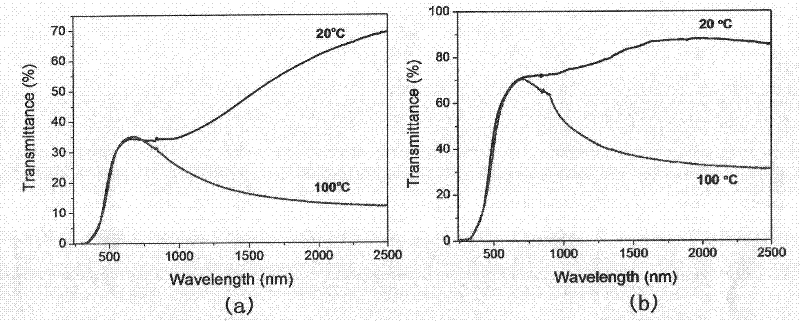
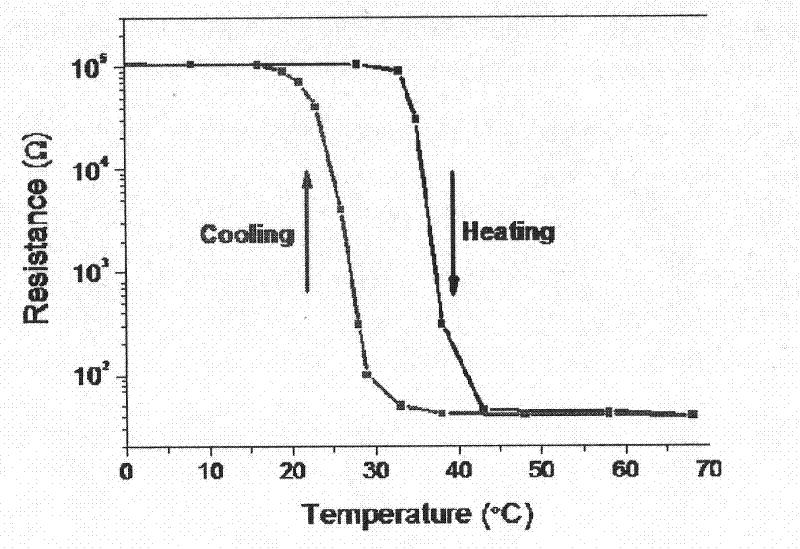
The invention relates to a vanadium dioxide intelligent temperature control film and a preparation method thereof. The preparation method comprises the following steps of: uniformly dispersing and dissolving vanadic oxide powder in an organic solvent with weak reductibility by adopting a wet chemical solution method; adding PVP (Polyvinyl Pyrrolidone) or PEG (polyethylene glycol) and a metal salt to prepare a doped VOx film (x is more than 2.0 and less than 2.5); and performing thermal treatment to form a doped porous hypovanadic oxide (VO2) film. In a better embodiment, the preparation method comprises the following steps of: adding polyvinyl pyrrolidone and a wolfram salt into a system consisting of vanadic oxide powder, benzyl alcohol and isopropyl alcohol to prepare a wolfram-doped VOx film; and annealing in hydrogen / argon atmosphere at the temperature 410 DEG C for 3 hours to prepare a wolfram-doped porous VO2 film, wherein the metal-insulator phase-transition temperature of the wolfram-doped porous VO2 film can be adjusted between 30 DEG C and 68 DEG C according to doping amount of wolfram, the penetration rate of a visible light region is 70 percent, the difference between the penetration rate before phase transition and the penetration rate after phase transition at the position of which the wavelength is 2,500 nanometers is 62 percent, 3-4 orders of magnitude of specific resistance is changed, and higher practical value is achieved.
Owner:SHANGHAI INST OF CERAMIC CHEM & TECH CHINESE ACAD OF SCI
Preparation method for 2-amino sulfonyl-4-methylsalfonamido methyl benzoic acid methyl ester
ActiveCN103755603AShort synthetic routeSuitable for industrial productionSulfonic acid amide preparationBenzoic acidOrganic solvent
The invention discloses a preparation method for 2-amino sulfonyl-4-methylsalfonamido methyl benzoic acid methyl ester. According to the preparation method, sulfonamide methyl toluene is used as raw material and subjected to chlorosulfonation-ammonolysis reaction, oxidation reaction and methanolysis reaction to prepare the2-amino sulfonyl-4-methylsalfonamido methyl benzoic acid methyl ester, wherein in the chlorosulfonation reaction, sulfonamide methyl toluene and organic solvents are added to a reaction device to be cooled to minus 10 DEG C-0 DEG C via an ice bath, then chlorosulfonation reagents are dropwise added, next the mixtures are heated till reflux occurs and the reaction is complete. The preparation method disclosed by the invention can prepare 2-amino sulfonyl-4-methylsalfonamido methyl benzoic acid methyl ester by using sulfonamide methyl toluene as raw material via three reaction steps, greatly shortens the synthetic process, and is quite suitable for commercial process. The overall yield can reach above 50% by optimizing the chlorosulfonation-ammonolysis reaction condition.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
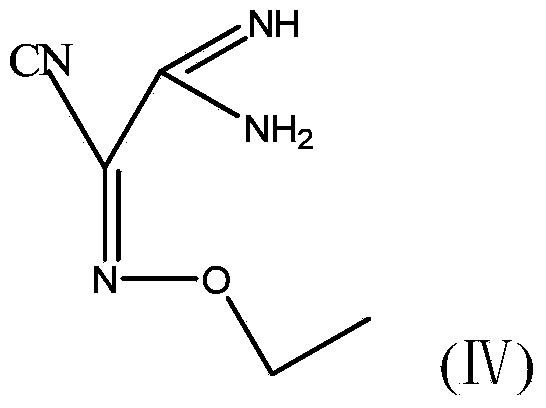
Method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid
ActiveCN103804321AShort synthetic routeFew synthetic stepsOrganic chemistryAcetic acidPotassium thiocyanate
The invention discloses a method for preparing (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method comprises steps of firstly, carrying out oximation reaction on malononitrile and sodium nitrite under the action of acetic acid so as to obtain a compound A, then carrying out Williamson synthesis on the compound A and bromoethane or diethyl sulfate under alkaline condition, so as to obtain a compound B, then carrying out amidine reaction on the compound B and ammonia water, so as to obtain a compound C, carrying out cyclization reaction on the compound C and potassium rhodanide for ring closing so as to obtain a compound D, and finally, hydrolyzing the compound D under the action of a strong base, so as to obtain (Z)-5-amino-alpha-(ethoxy imino group)-1, 2, 4-thiadiazole-3-acetic acid. The method has few synthesis steps, has low cost, uses the materials which are cheap and easy to obtain, is beneficial to industrial production, and has light contamination; the purity of the product can reach 99%, so that synthesis of high-purity ceftaroline fosamil in the subsequent step is guaranteed.
Owner:山西海泰电子材料有限公司
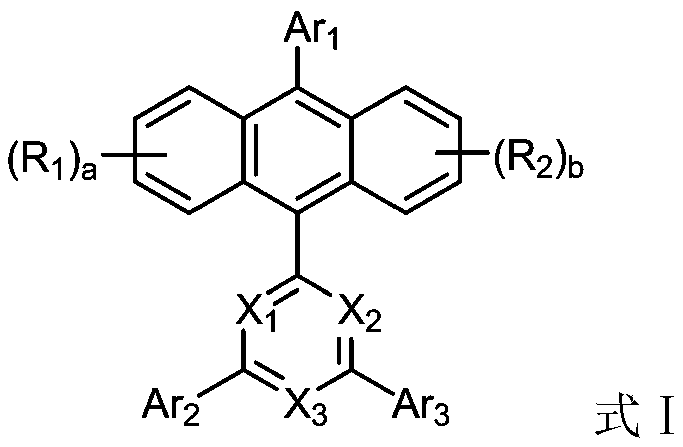
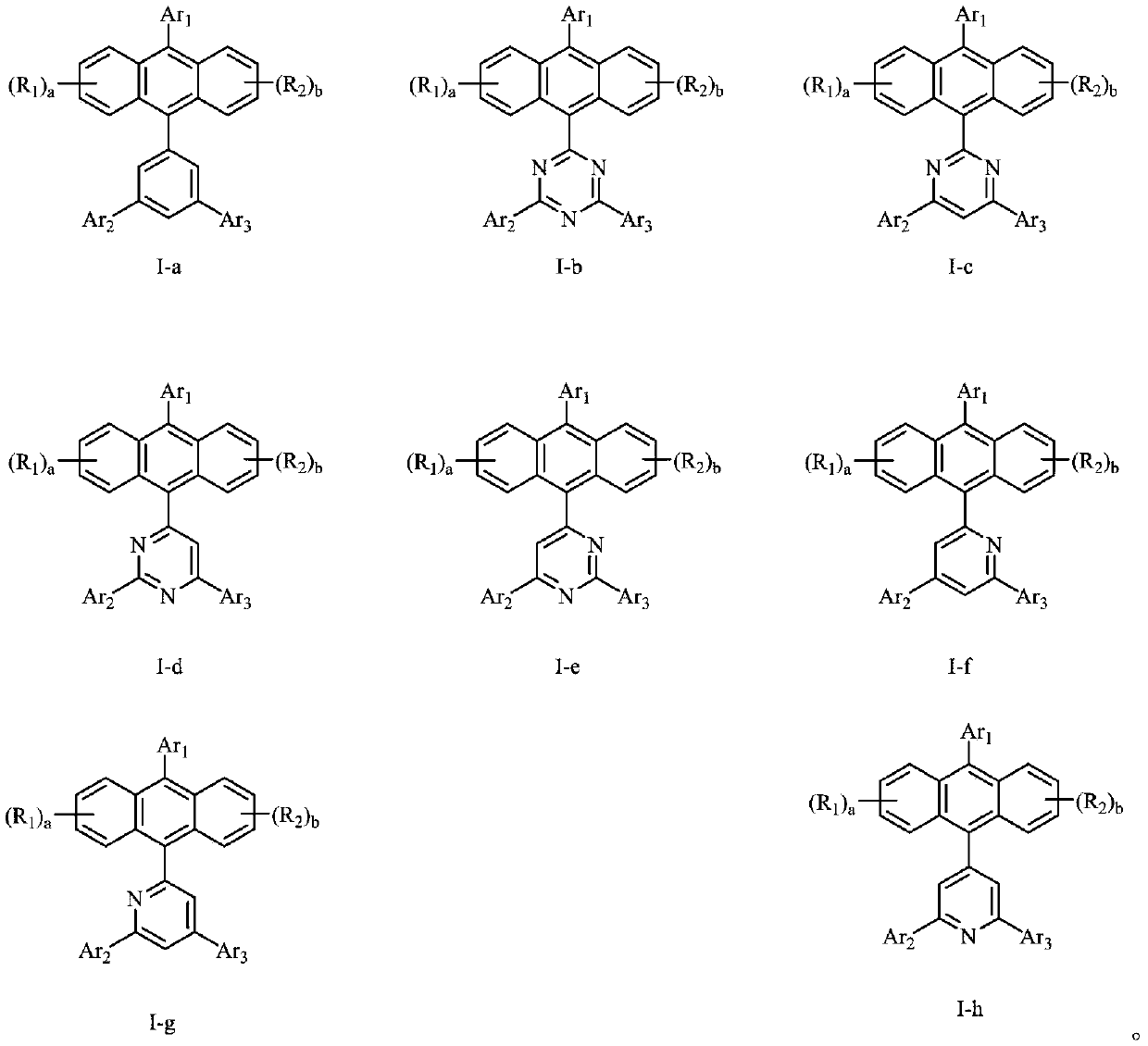
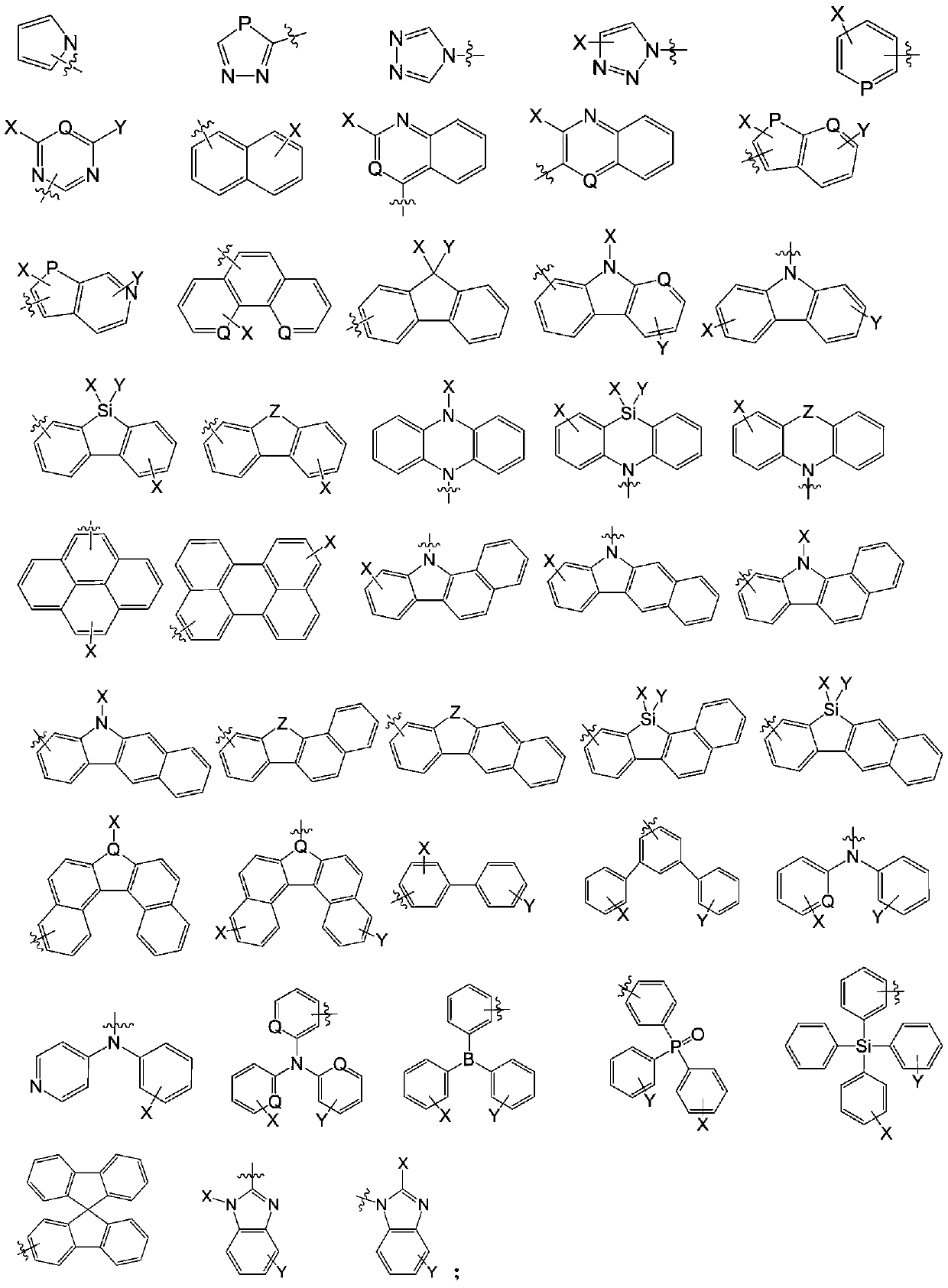
Anthracene organic luminescence compound, and preparation method and application thereof
PendingCN109879812AShort synthetic routeSimple processSilicon organic compoundsGroup 5/15 element organic compoundsAnthraceneOrganic electroluminescence
The invention relates to the technical field of organic optoelectronic materials and relates to an anthracene organic luminescence compound, a preparation method thereof and an organic electroluminescence device. The anthracene organic luminescence compound has a structure shown as a formula I: the formula 1 is shown in the description. Compared with other electron transfer layer materials or luminescent layer materials, the novel anthracene organic luminescence compound provided by the invention used as an electron transfer layer material or luminescent layer material of the organic electroluminescence device has the advantage that the luminous efficiency of the device prepared from the material is obviously promoted, and service life is obviously prolonged. The anthracene organic luminescence compound has the advantages of relatively short synthetic route, simple process, easily acquired raw materials, low cost and suitability for industrial production.
Owner:JILIN OPTICAL & ELECTRONICS MATERIALS
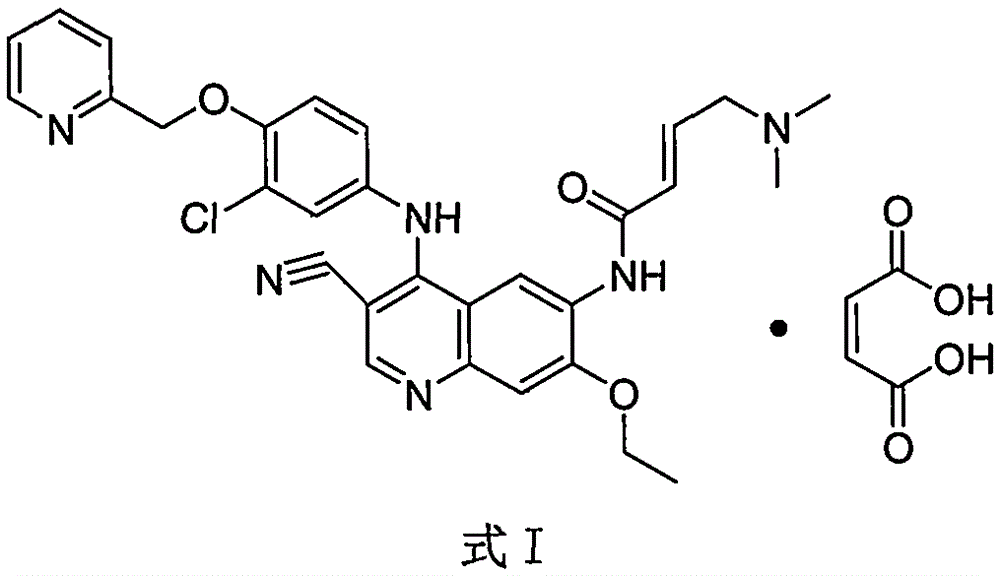
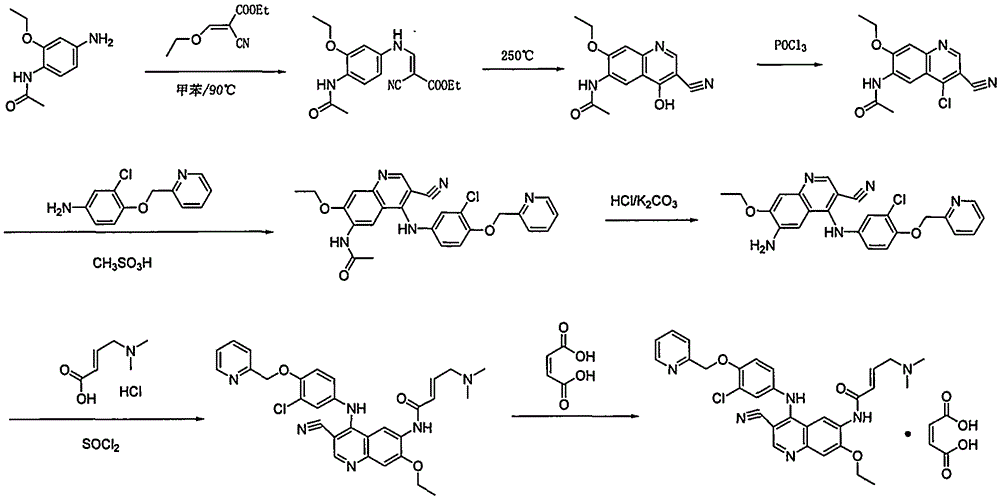
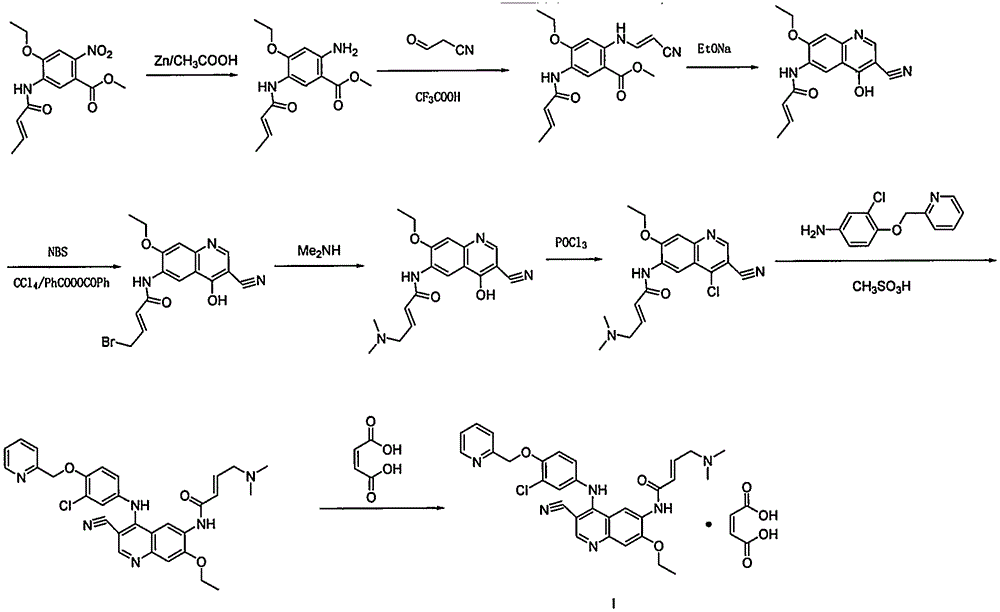
Preparation method of antineoplastic drug maleic acid neratinib
ActiveCN105330646AWide variety of sourcesShort synthetic routeCarboxylic acid salt preparationNitro compoundMedicinal chemistry
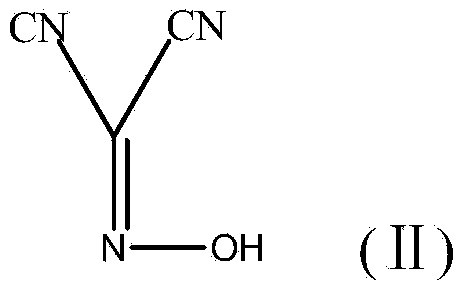
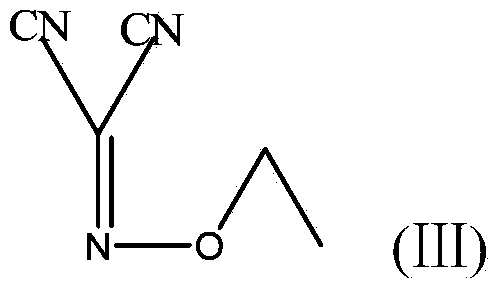
The invention provides a preparation method of antineoplastic drug maleic acid neratinib. The defects in the prior art are overcome. The preparation method comprises the steps that the formula II and the formula III are coupled to form the formula IV under the effect of a catalyst; a nitro-compound IV is reduced under the effect of a reduction system to form a formula V; an amino compound V and a formula VI are condensed to obtain neratinib VII, and then the neratinib VII and maleic acid form a salt to obtain the maleic acid neratinib I. By the adoption of the technical route, the preparation method has the advantages that the synthetic route is short, reaction conditions are mild, the yield is high, raw materials are wide in source, and environmental protection is achieved.
Owner:SHANGHAI XUNHE PHARMA TECH CO LTD
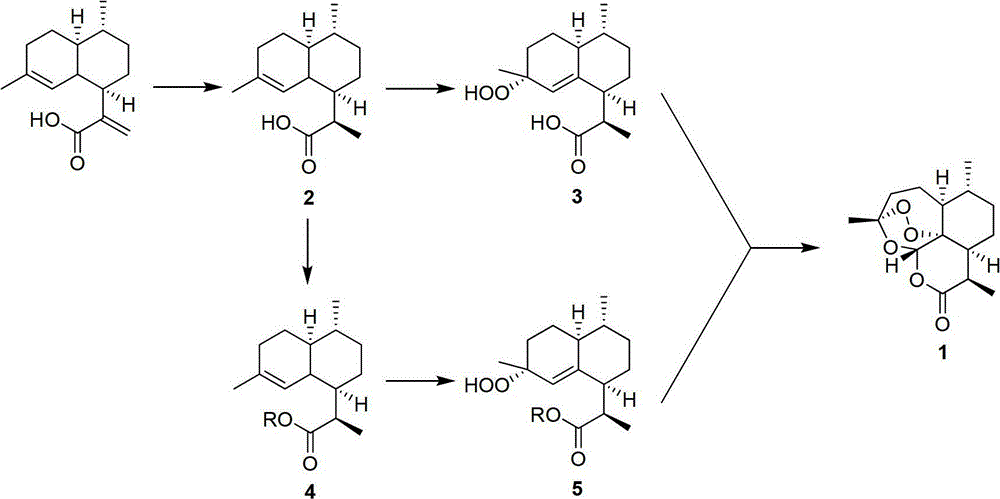
Method for preparing artemisinin through arteannuic acid
The invention discloses a method for preparing artemisinin through arteannuic acid. The method comprises the steps that first the arteannuic acid is processed to obtain a dihydroartemisinic acid under the effect of a reducing agent such as sodium borohydride / nickel chloride or a hydrogen / metal catalyst, and then the dihydroartemisinic acid is oxidized into a peroxided dihydroartemisinic acid through peroxide in the presence of the catalyst, and finally the target product artemisinin can be obtained with high yield under the catalyzing of the acid and the effect of oxygen; or a dihydroartemisinic acid derivative can be obtained from the dihydroartemisinic acid based on the protection on carboxyl, and the dihydroartemisinic acid derivative is oxidized into a relevant peroxided dihydroartemisinic acid derivative through the peroxide in the presence of the catalyst, and then the target product artemisinin can be obtained with high yield under the catalyzing of the acid and the effect of the oxygen. Compared with the prior art, the method for preparing the artemisinin through the arteannuic acid has the advantages as follows: the used agent has low cost, and is easy to obtain; the synthetic route is short; the reaction selectivity is high; the preparation process is environmental-friendly; the operation and post-processing are simple; the total yield is high; and the method for preparing artemisinin through the arteannuic acid is applied to industrial production.
Owner:SHANGHAI JIAO TONG UNIV
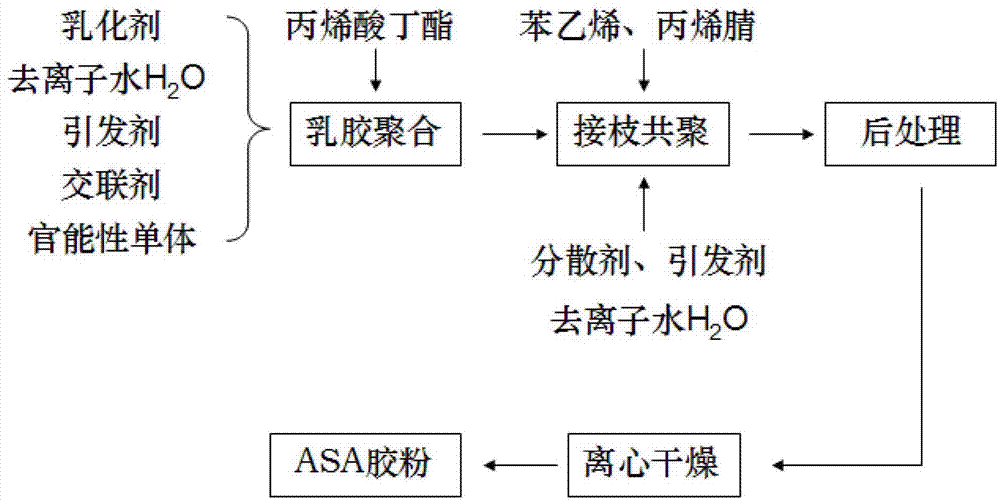
ASA (acrylonitrile styrene acrylate) graft copolymerization resin rubber powder and preparation method thereof
The invention provides an ASA (acrylonitrile styrene acrylate) graft copolymerization resin rubber powder. The ASA graft copolymerization resin rubber powder comprises the following components by parts by weight: 5-30 parts of butyl acrylate, 50-70 parts of styrene, 20-25 parts of acrylonitrile, 0.08-0.35 part of initiator, 0.05-0.5 part of emulgator, 150-500 parts of deionized water H2O, 0.025-0.15 part of diacrylic acid poly butylene terephthalate, 0.025-0.15 part of functionality monomer, 0.002-0.02 part of 1-dodecanethiol, 0.25-0.5 part of dispersing agent, and 0.01-0.05 part of sodium hydrosulfite. According to the ASA graft copolymerization resin rubber powder and the preparation method, the comprehensive properties of the resin can be remarkably improved, steps of amplifying grain size of latex and condensing are omitted, dosage of additives and water is reduced, the technological processes are shortened, the production efficiency is improved, and the manufacture cost is lowered by 30% compared with that of similar foreign products.
Owner:HANGZHOU HUACHUANG IND
Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole
ActiveCN102993102AEasy to operateHigh yieldOrganic compound preparationCarboxylic acid amides preparationAcetic acidEthylene oxide
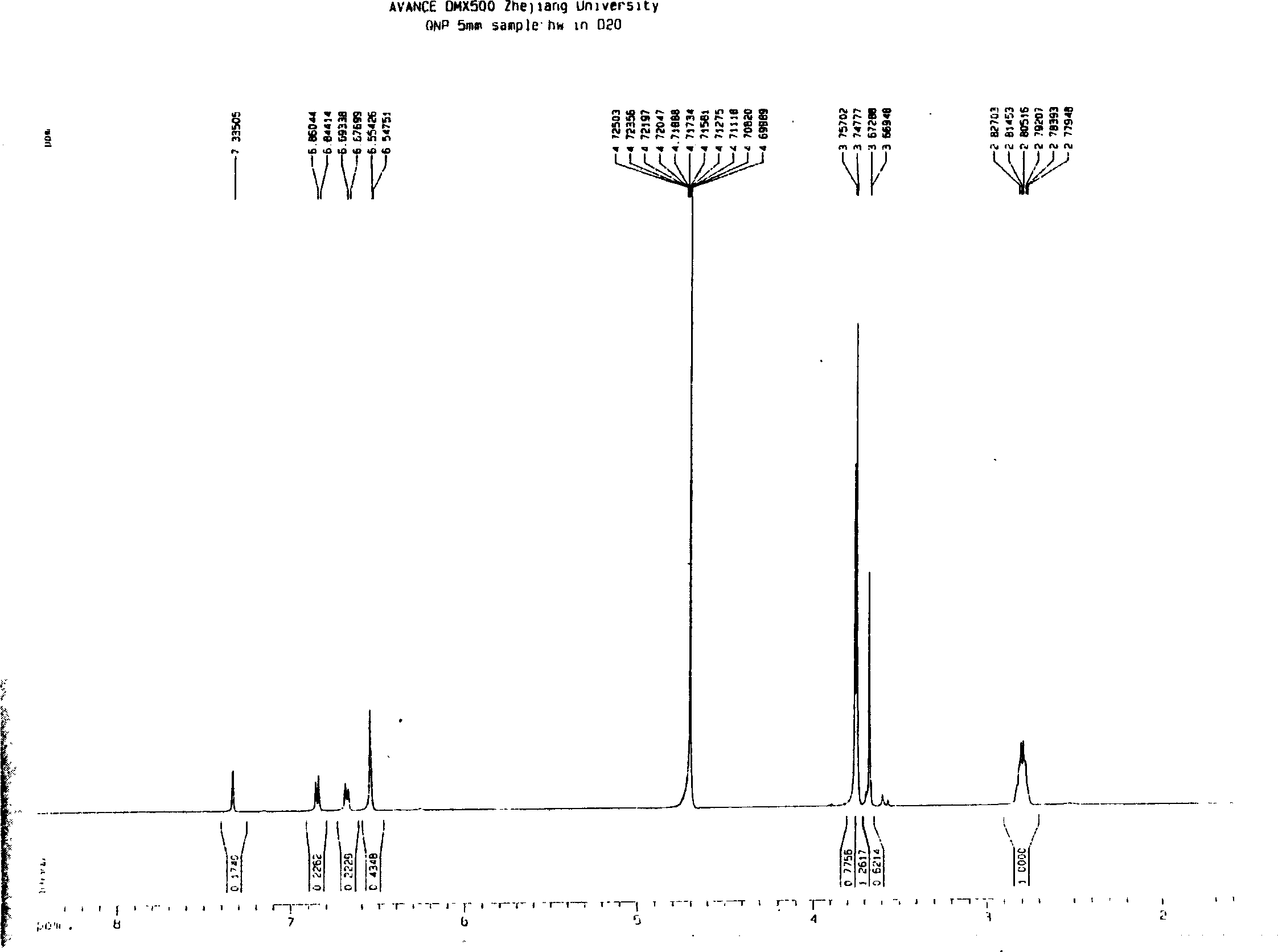
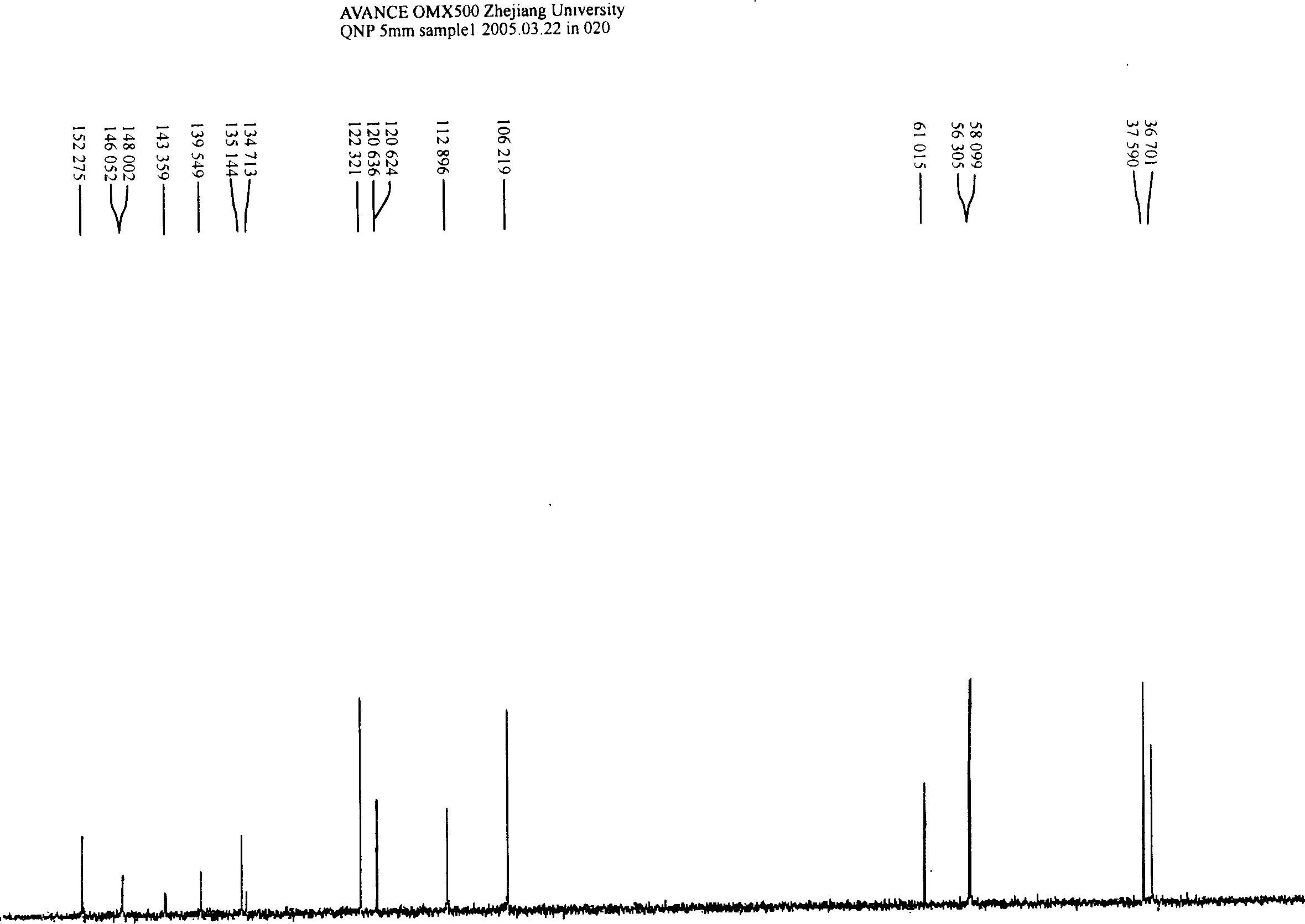
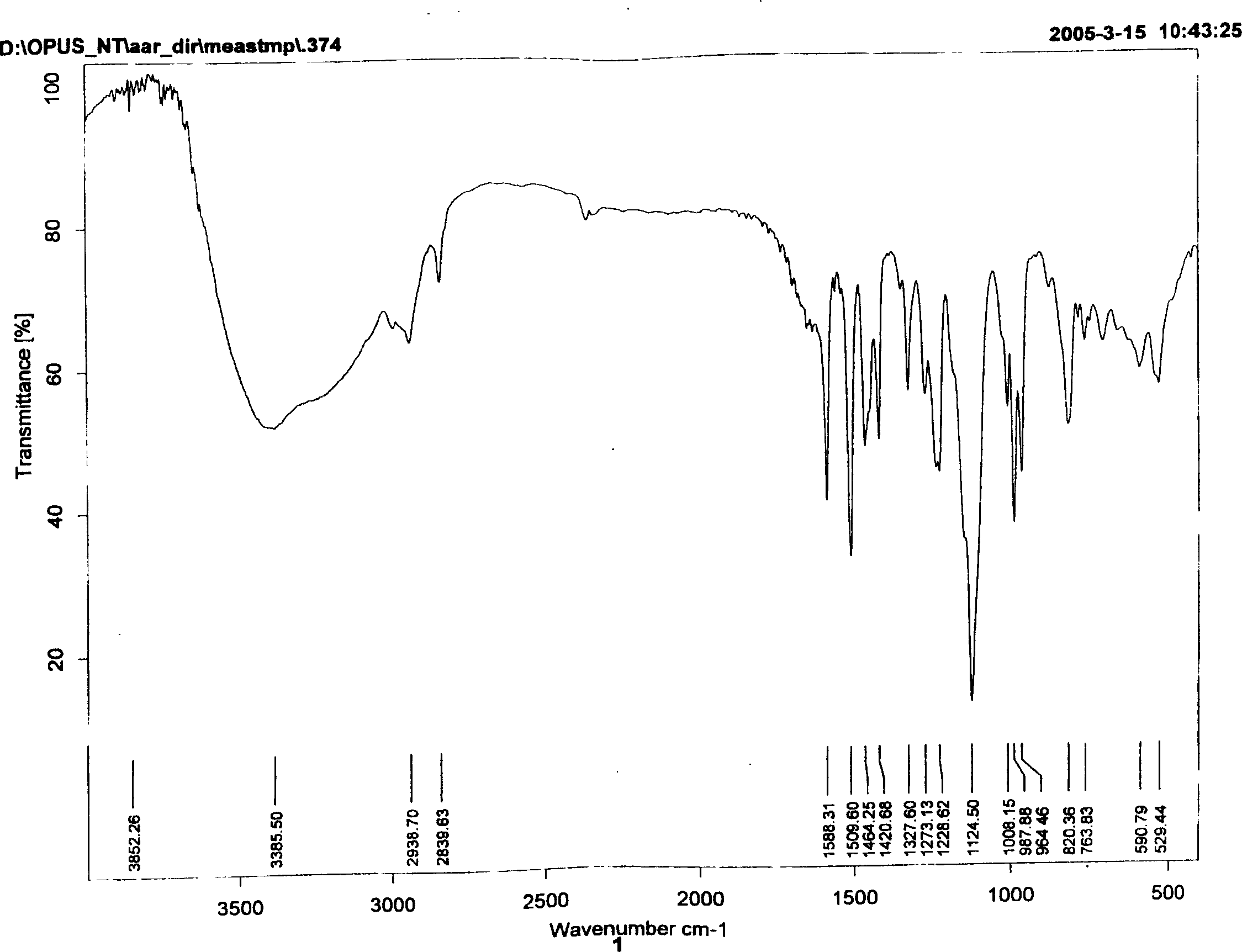
The invention discloses a synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole. NL-101, namely [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole is prepared from chloroacetic acid replacing hypertoxic low-boiling ethylene oxide as a raw material by multi-reactions in one pot. The separating and purifying step is cancelled, the synthetic route is short, and the reaction environment in particular including temperature, pressure and the like can be safely controlled and the method is green and environment-friendly. The invention also discloses a series of intermediate compounds obtained in the reaction of synthesizing NL-101. The purity of NL-101 prepared by the synthetic route through analysis and characterization reaches 95%, and the purity after recrystallization and refining is greater than 99%. The method is high in product yield, easy to operate, and beneficial for industrialized production in a large scale.
Owner:HANGZHOU TINO PHARMA CO LTD
Medicinal composition for stabilizing delotadine in preparation
InactiveCN1552324ASolve the defect of brown-red substanceGuaranteed stabilityOrganic active ingredientsImmunological disordersLactoseActive ingredient
A composite medicine in which desloratadine is stabilized features that after desloratadine is mixed with medicinal auxiliaries, an antioxidizing agent is added to the mixture.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Prepn process of 1-fluoronaphthalene
ActiveCN1887833AShort synthetic routeMild conditionsHalogenated hydrocarbon preparationNitriteNitrous acid ester
The preparation process of 1-fluoronaphthalene includes the following steps: (1) reacting 1-naphthylamine with nitrous acid, nitrous acid ester or nitrite in acid medium to obtain diazo salt; (2) reacting the diazo salt with fluoroboric acid or its salt or fluorophosphoric acid or its salt to obtain diazo fluoroborate or diazo fluorophosphate; and (3) heating the diazo fluoroborate or diazo fluorophosphate to decompose to obtain 1-fluoronaphthalene. The process has short synthesis path, less side products, mild reaction condition, easy control, relatively low cost, great production capacity, high product purity and other advantages, and the product is used as medicine intermediate.
Owner:SHANGHAI CHEMSPEC CORP
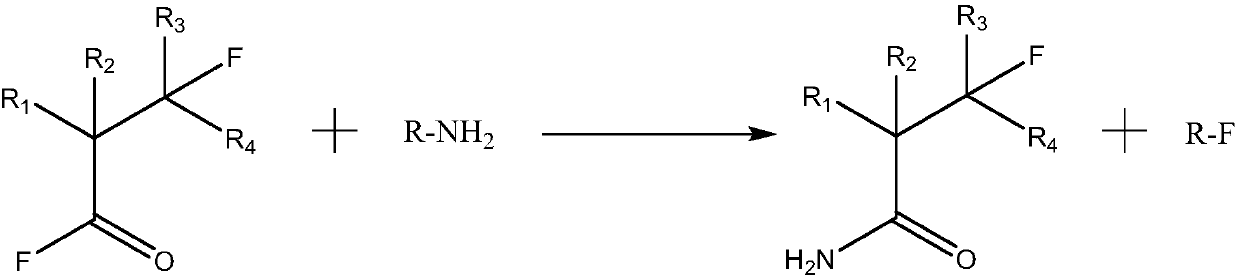
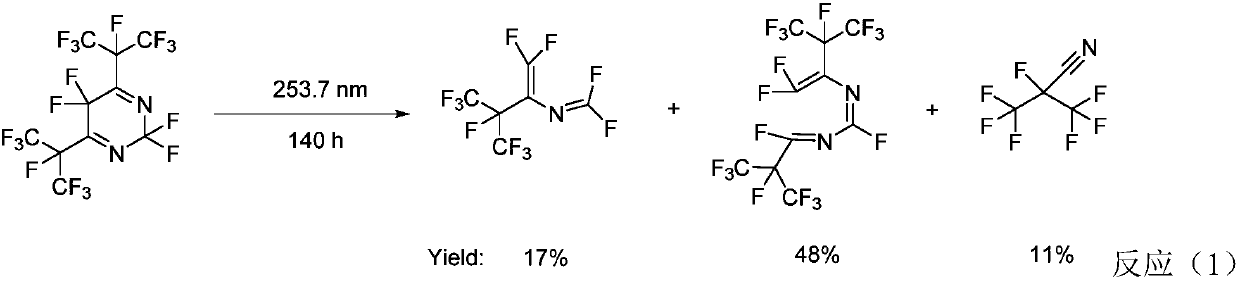
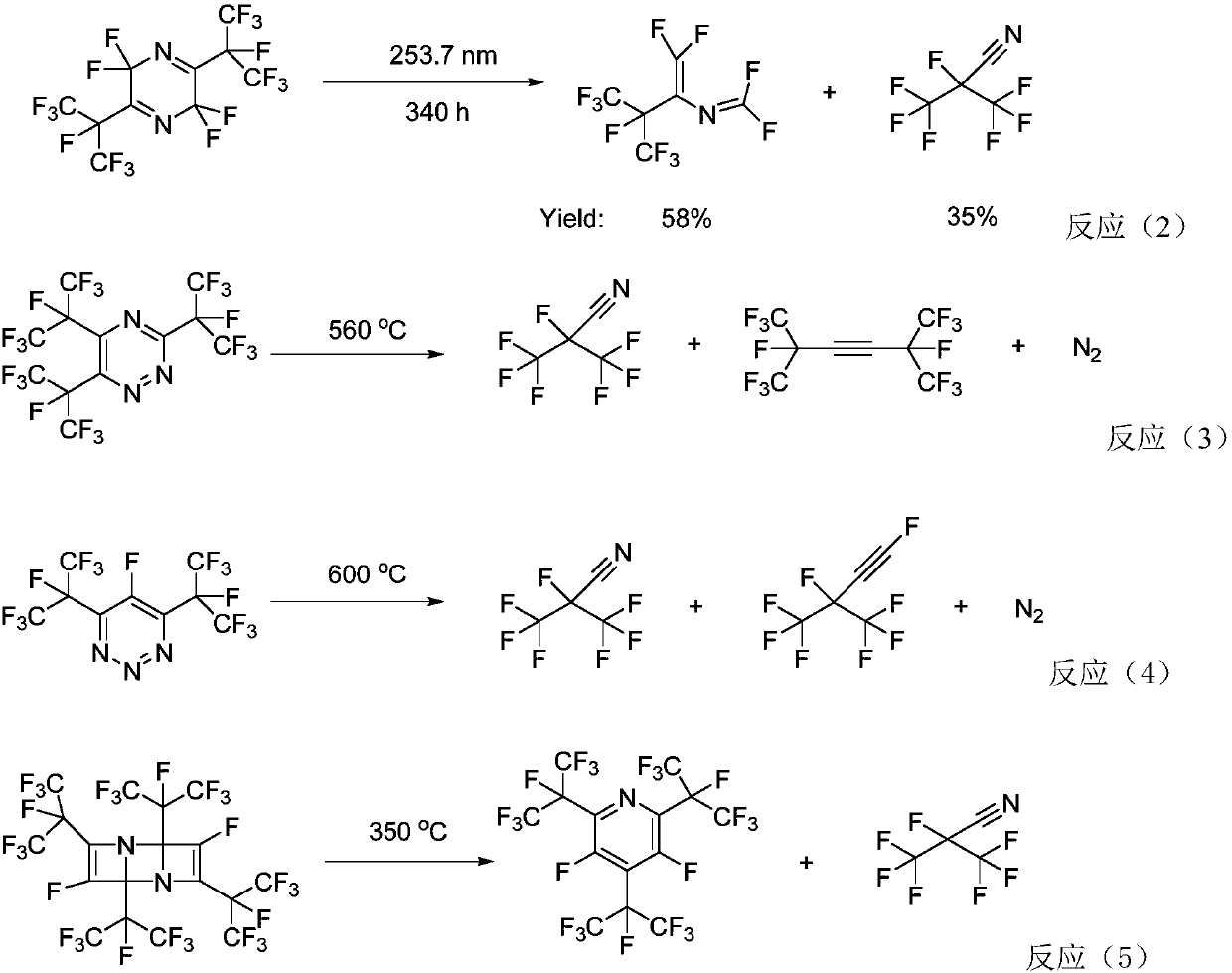
Perfluoro nitrile preparation method
ActiveCN107935884ALow priceImprove solubilityOrganic compound preparationCarboxylic acid amides preparationCarbonyl fluorideMetallole
The invention discloses a perfluoro nitrile preparation method. The perfluoro nitrile preparation method comprises the following steps: an addition reaction is performed on perfluoroolefine R1R2C=CR3R4 and carbonyl fluoride to obtain acyl fluoride R1R2(COF)C-CFR3R4(the general formula of R1,R2,R3 and R4 is -CnF(2n+1) group, and n is a nonnegative integer set); b, performing a reaction on acyl fluoride R1R2(COF)C-CFR3R4, acyl fluoride and an alkali metal amide or an amino compound R-NH2(R is Li, Na, K, Rb, Cs or -CmH(2m+1) group, and m is a nonnegative integer set) to obtain amide R1R2(CONH2)C-CFR3R4; and c, performing a dehydration reaction on amide R1R2(CONH2)C-CFR3R4 to obtain perfluoro nitrile R1R2(CN)C-CFR3R4. The perfluoro nitrile preparation method has a short reaction path, can easily obtain perfluoroolefine and carbonyl fluoride, is low in cost and high in overall yield of perfluoro nitrile, and the reaction path is easy to industrialize.
Owner:泉州宇极新材料科技有限公司
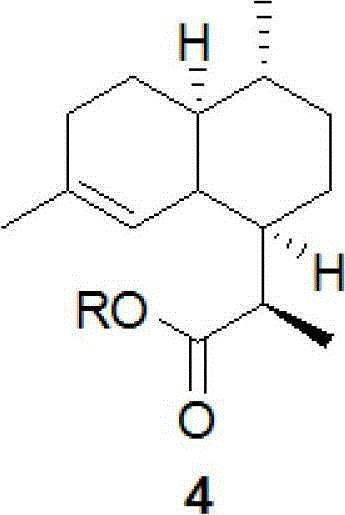
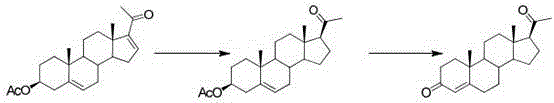
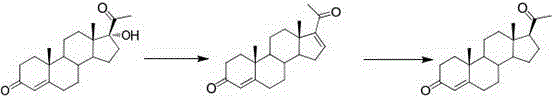
Method for preparing progesterone and derivatives thereof
InactiveCN106589037AReduce manufacturing costEasy to operateSteroidsProgesteronesRearrangement reaction
The invention discloses a method for preparing progesterone and derivatives thereof. A compound 1 serves as a starting material, and through an oxidation reaction and a rearrangement reaction, the progesterone and derivatives thereof, namely, compounds 3 are obtained, wherein the reaction formula is defined in the description. By means of the method, the finished product progesterone and derivatives thereof are obtained with the total yield being 75wt% or above; the method is low in cost, environmentally friendly and suitable for industrial production.
Owner:ZHEJIANG XIANJU PHARMA
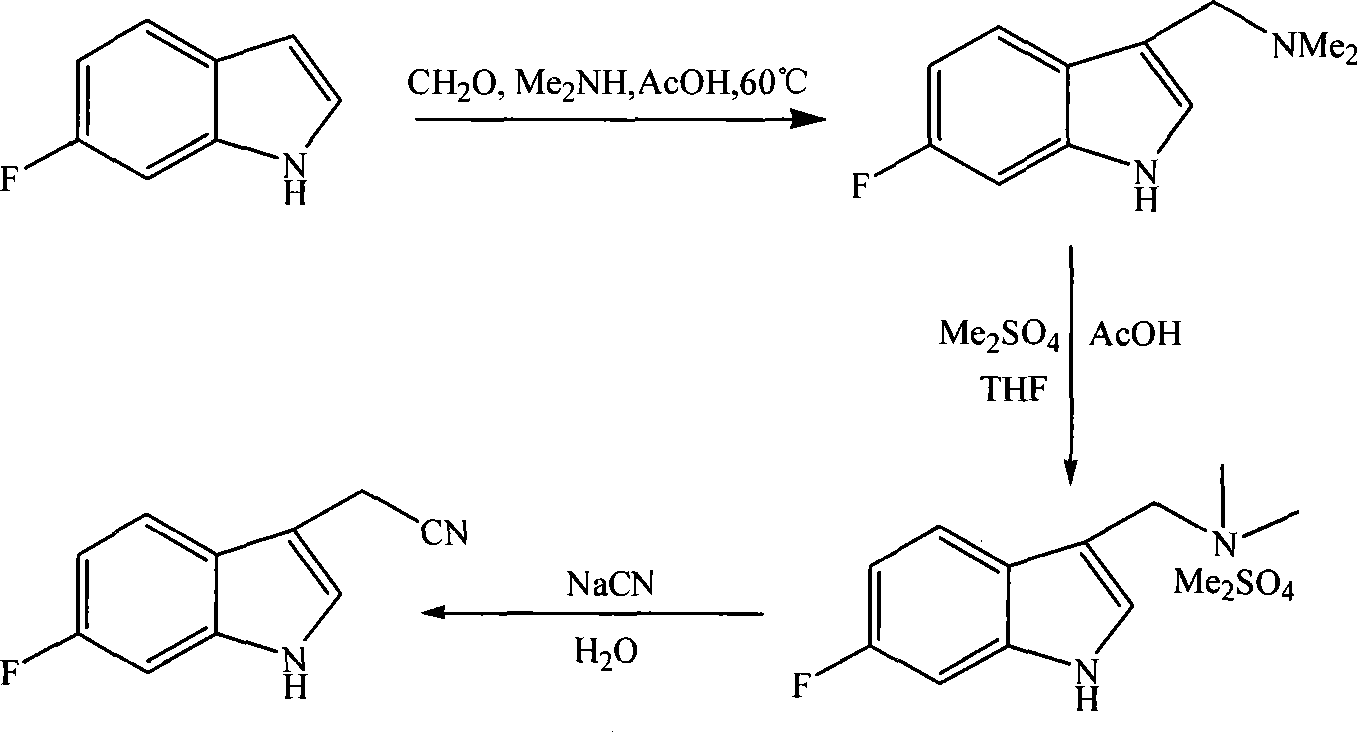
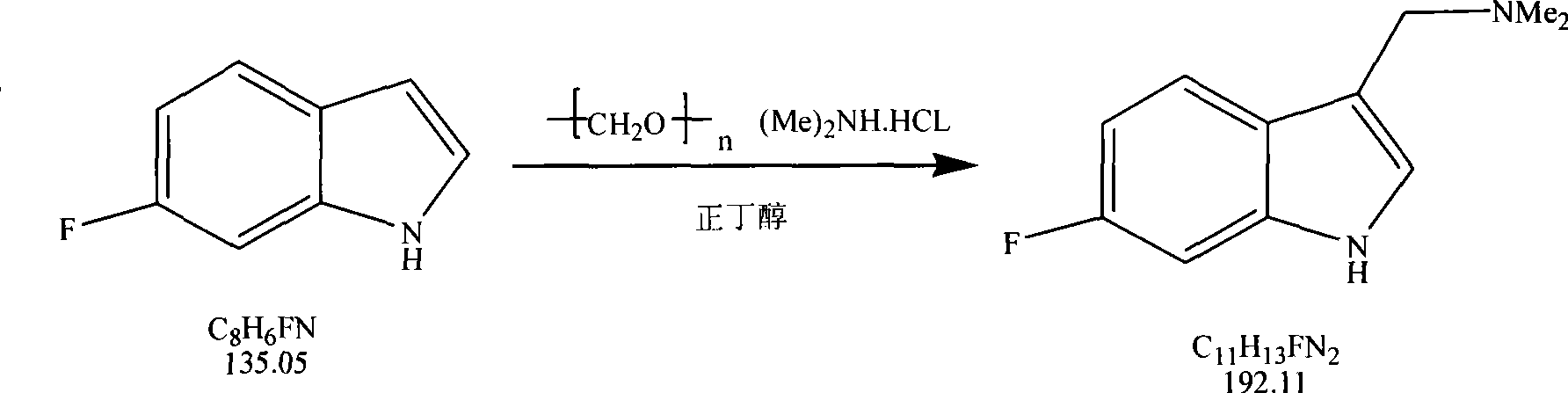
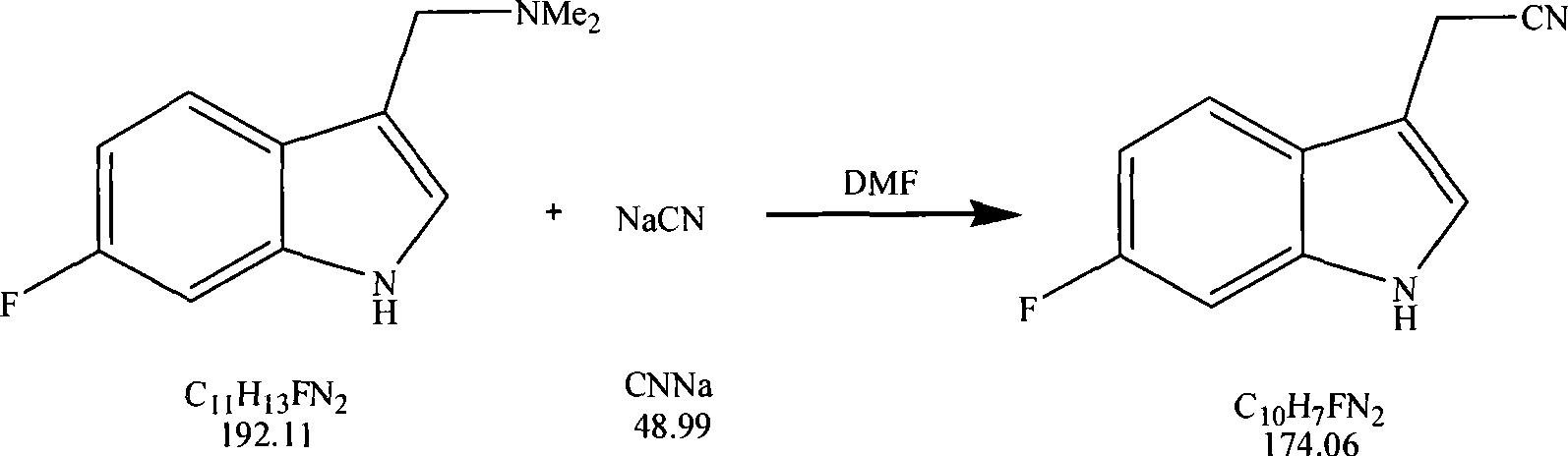
Method for synthesizing 6-fluoroindole-3-acetonitrile
The invention relates to the synthesis of organic compounds, in particular to a method for synthesizing 6-fluoroindole-3-acetonitrile, which comprises the following steps: step one, synthesis of 6-fluorogramine: blending raw materials of 6-fluoroindole, dimethylamine hydrochloride and paraformaldehyde according to the mol ratio of 1:1-1.5:1-2, mixing the raw materials evenly and then adding the mixture to an organic solvent, stirring continuously the mixture during the reaction, and heating and refluxing the mixture to prepare the 6-fluorogramine for stand by services; and step two, synthesis of the 6-fluoroindole-3-acetonitrile: blending the raw materials of the 6-fluorogramine prepared in the step one and sodium cyanide according to the mol ratio of 1:1-2, mixing the raw materials into the organic solvent, refluxing the mixture and evaporating out the solvent, and extracting an organic layer to obtain the 6-fluoroindole-3-acetonitrile. The method has the advantages that the short synthetic route is short and can be completed in just two steps, the raw materials are readily available, the substitution of the third position of indole is accurate, the control is convenient with low cost, the reaction yield is high, and a prepared high-purity medicament intermediate product is applicable to industrial production.
Owner:大连凯飞精细化工有限公司
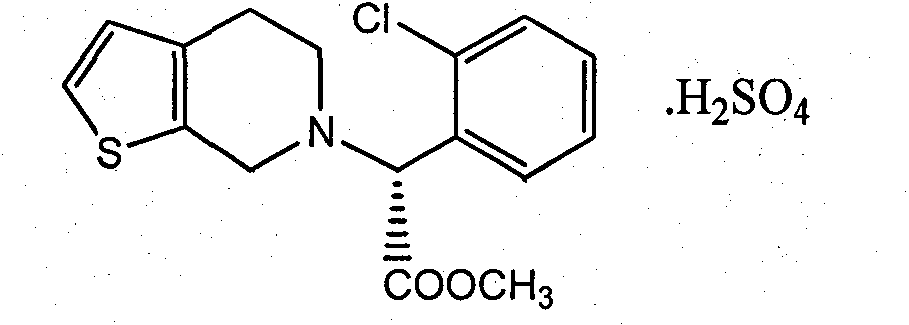
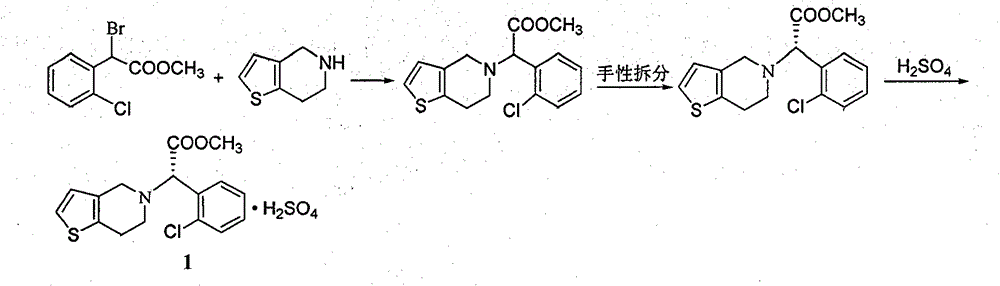
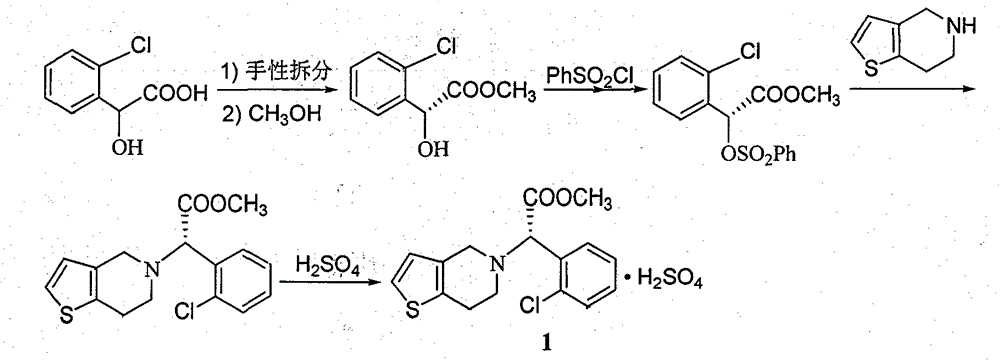
Clopidogrel hydrogen sulfate preparation method
ActiveCN104370935AShort synthetic routeRaw materials are easy to getOrganic chemistryOrganic reactionAcetaldehyde
The present invention relates to a clopidogrel hydrogen sulfate preparation method. According to the clopidogrel hydrogen sulfate preparation method, 2-thiophene aldehyde is adopted as a basic starting material, the 2-thiophene aldehyde and o-chlorophenylglycine methyl ester are subjected to a condensation reaction to produce the corresponding imine intermediate, the imine intermediate is reduced into the corresponding secondary amine intermediate by adopting sodium borohydride or by directly adopting sodium cyanoborohydride intermediate, the secondary amine intermediate and formaldehyde are subjected to reaction ring closure to obtain clopidogrel, and the clopidogrel is acidified with sulfuric acid to obtain the target compound clopidogrel hydrogen sulfate. According to the present invention, the provided synthesis route is short, the related reactions are classical organic reactions, the reaction conditions are mild, the operation is simple, the total yield is high, the cost is low, and the preparation method is suitable for industrial production.
Owner:HENAN PURUI PHARMA TECH CO LTD
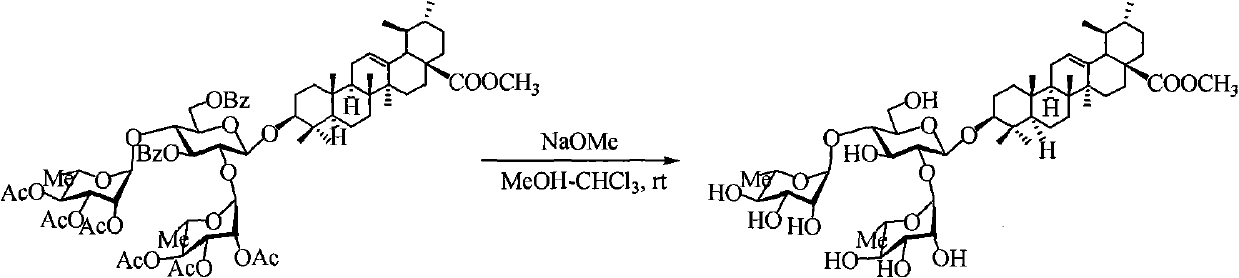
Ursolic acid saponin, preparation method thereof and application in resisting highly pathogenic H5N1 influenza virus
InactiveCN101941996AFast preparationShort synthetic routeOrganic active ingredientsSugar derivativesAnti virusHighly pathogenic
The invention discloses ursolic acid saponin, a preparation method thereof and application in resisting highly pathogenic H5N1 influenza virus, and specifically relates to ursolic acid saponin compounds shown in general formula (I). A series of ursolic acid saponin is prepared from natural product ursolic acid serving as raw material by introducing an ester group into the C-28 site through structural modification at first, then introducing beta-glucosyl into the C-3 site, selectively protecting 3,6-OHs of the glucose by using BBTZ and introducing glycosyl into 2,4-OHs of the glucose. Pharmacological test shows that the ursolic acid saponin compounds have obvious inhibition effect on the invasion process of the H5N1 highly pathogenic influenza viruses on host cells and can be used as a medicament for preventing or treating the influenza viruses. The invention also discloses a pharmaceutical composition containing the ursolic acid saponin compounds and the combination of the compounds of the invention and other anti-virus medicines.
Owner:INST OF MATERIA MEDICA AN INST OF THE CHINESE ACAD OF MEDICAL SCI +1
Synthesis method for N-Boc-3-piperidone
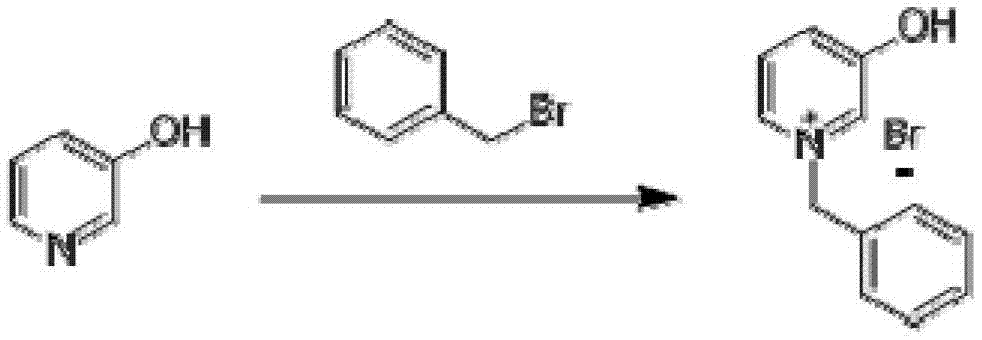
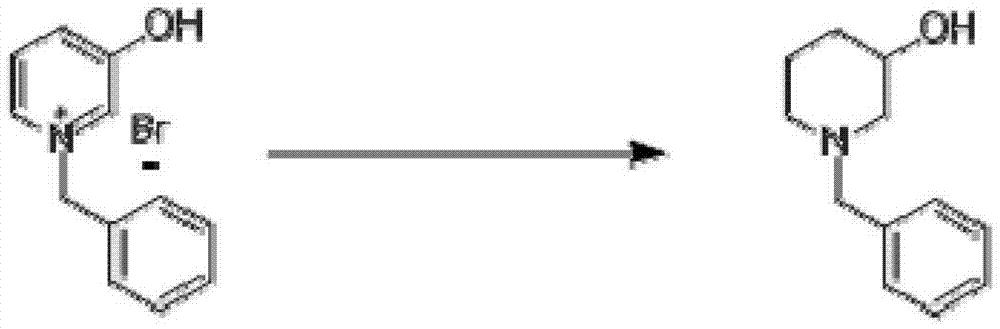
InactiveCN103204801AEasy to separate and purifyShort synthetic routeOrganic chemistryPalladium on carbonBenzoyl bromide
The invention discloses a synthesis method for N-Boc-3-piperidone. The synthesis method comprises the following steps of: reacting 3-hydroxyl pyridine with benzyl bromide in an organic solvent to obtain an N-benzyl-3-hydroxyl pyridine quaternary ammonium salt; reducing the N-benzyl-3-hydroxyl pyridine quaternary ammonium salt by sodium borohydride to obtain N-benzyl-3-hydroxyl piperidine; reacting N-benzyl-3-hydroxyl piperidine with di-tert-butyl dicarbonate ester to obtain N-Boc-3-hydroxyl piperidine under hydrogen protection and the catalysis of a palladium-carbon catalyst; and reacting N-Boc-3-hydroxyl piperidine with the mixed oxidant of dimethyl sulfoxide and oxalyl chloride to obtain N-Boc-3-piperidone under the action of an organic base. Compared with the existing synthesis method, the synthesis method disclosed by the invention is shorter in synthesis route, and easier for separation and purification of reactants, thus reducing the production cost, the energy consumption and the pollution; and the total productivity of N-Boc-3-piperidone can achieve more than 42%, and the purity thereof is greater than 98%.
Owner:甘肃天骄商贸有限公司
Setoglaucine salt, preparation method thereof and medicinal composition containing the same
InactiveCN1907989AGood water solubilityImprove bioavailabilityPhosphorus organic compoundsAntineoplastic agentsOrganic acidSolubility
The invention discloses a chaetocin salt and preparing method and drug composition with chaetocin salt, which possesses structure formula (I) on the right, wherein R is salt of inorganic oxyacid or organic monobasic acid group and metal, ammonium salt or organic amine. The invention improves solubility greatly, which prevents tumour effectively.
Owner:ZHEJIANG TIANHUANG MEDICINAL PLANT PHARMA
Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds
ActiveCN103708988AReduce manufacturing costLow costOrganic substitutionHalogenated hydrocarbon preparationTetrafluoroethyleneOrganic solvent
The invention discloses a synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds. The method comprises: (i) a step of subjecting halogenobenzene to a Grignard reaction in an organic solvent to form a Grignard reagent having the following formula, wherein X is a chlorine atom or a bromine atom; and (ii) a step of subjecting the Grignard reagent and tetrafluoroethene, in an organic solvent, to an [alpha],[beta],[beta]-trifluorostyrene synthetic reaction to prepare the [alpha],[beta],[beta]-trifluorostyrene or substituted [alpha],[beta],[beta]-trifluorostyrene, with the molar ratio of the tetrafluoroethene to the Grignard reagent being not less than 1.
Owner:SHANGHAI 3F NEW MATERIAL TECH CO LTD +1
Synthesis method of 4-hydroxy-8-bromoisoquinoline
ActiveCN105693607AShort synthetic routeHigh overall yieldOrganic chemistry5-hydroxytryptamine receptorsDisease
The invention discloses a synthesis method of 4-hydroxy-8-bromoisoquinoline. The method comprises the following steps: mixing bromobenzylamine with a toluene solution, then adding p-toluenesulfonic acid and glyoxylic acid, heating, refluxing, dehydrating and condensing to generate a 2-bromobenzene imidoacetic acid crude product, adding polyphosphoric acid, simultaneously heating and stirring, pouring the product into water for filtering after the reaction is completed, washing filter cake with ethyl ether, then drying the filter cake. According to the synthesis method of 4-hydroxy-8-bromoisoquinoline, bromobenzylamine is used as the raw material; the synthesis route is simple; the process selection is reasonable; the raw material is simple and easily available; the operation and after-treatment are convenient; the total yield reaches up to 76%; the 4-hydroxy-8-bromoisoquinoline is easy to magnify and high in biological activity, can be used as a 5-hydroxytryptamine receptor, and has a strong effect of treating dementia and schizophrenia diseases.
Owner:SUZHOU KANGRUN PHARMA
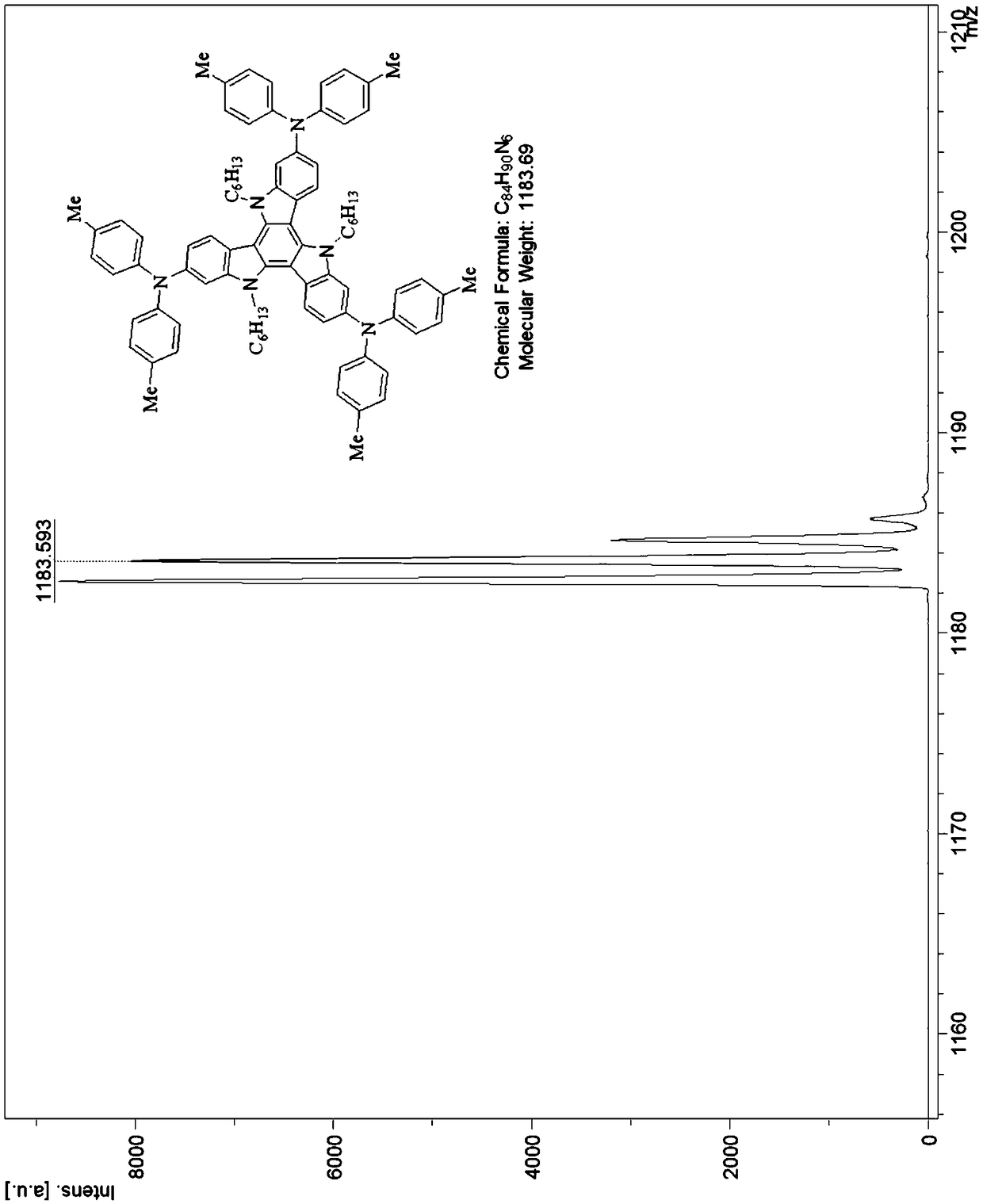
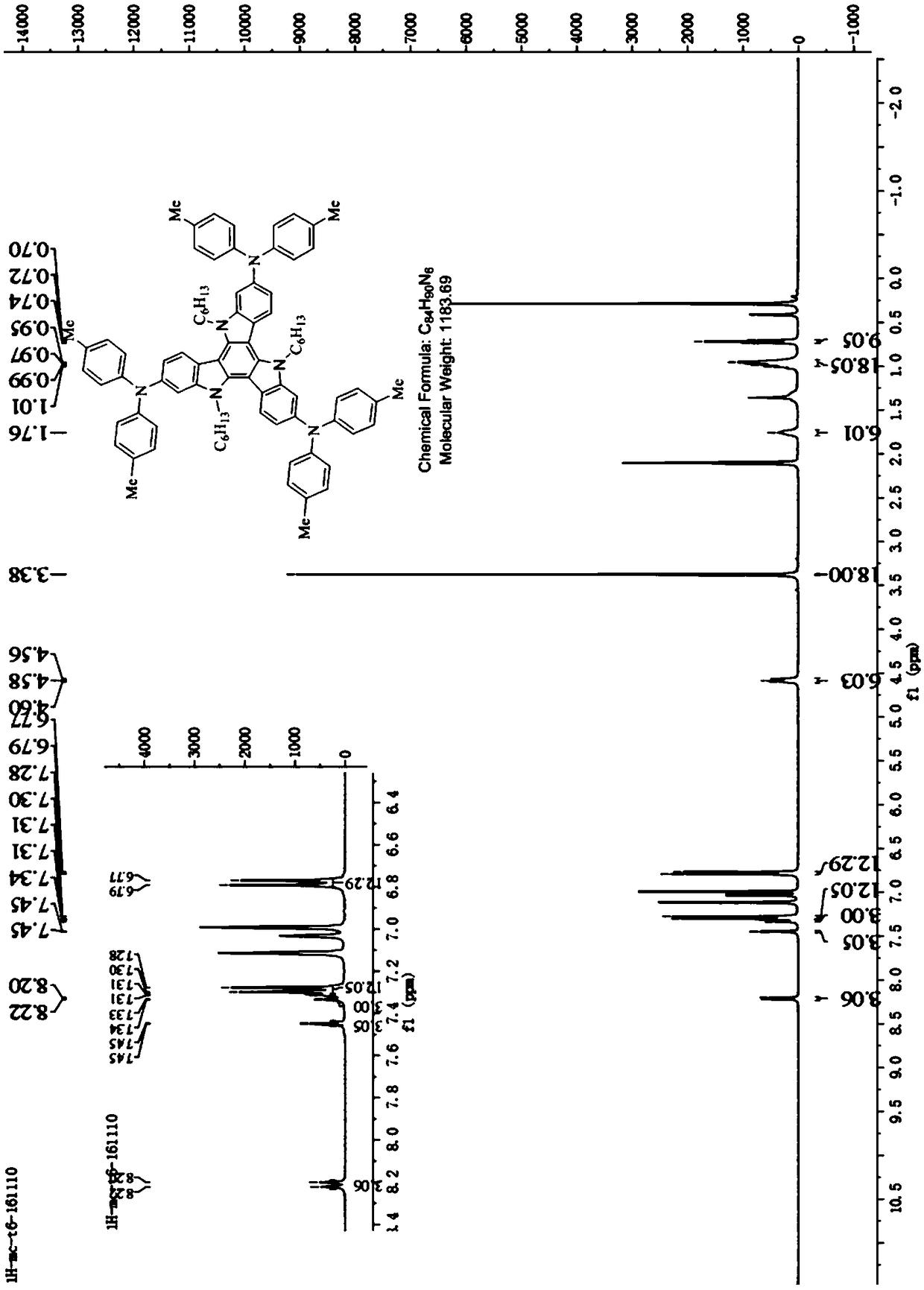
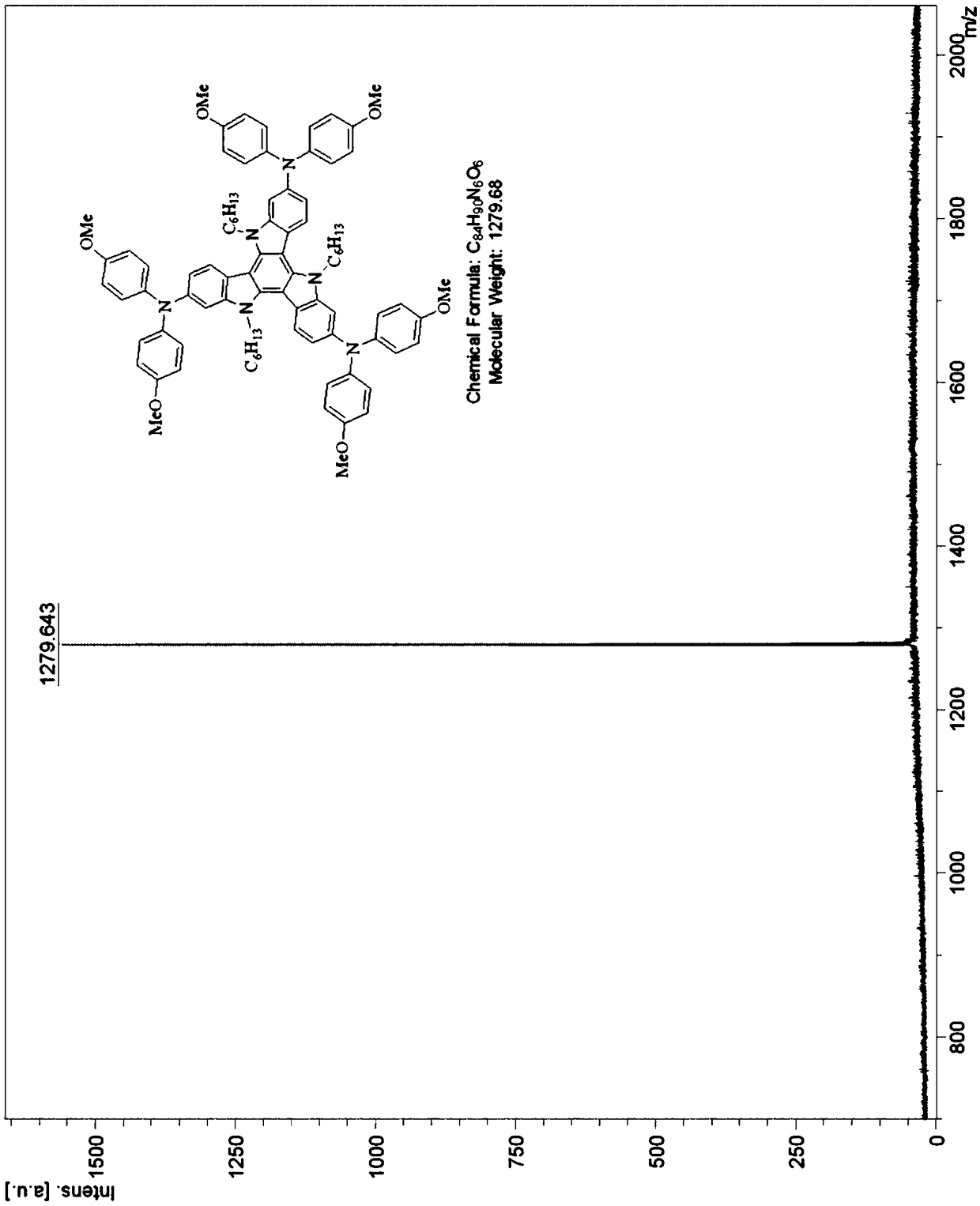
Tricarbazole-aromatic amine derivative cavity transmission material and preparation method and application thereof
InactiveCN108794494AShort synthetic routeReaction raw materials are cheapOrganic chemistrySolid-state devicesOrganic solar cellOrganic field-effect transistor
The invention discloses a tricarbazole-aromatic amine derivative cavity transmission material and a preparation method and application thereof. The tricarbazole-aromatic amine derivative cavity transmission material is a star-like micromolecule compound which uses tricarbazole as the core and uses aromatic amide derivative as a modifying group; a formula structure is shown in a formula I in the attached figure, wherein R is selected from one of C1 to C30 straight and branched alkyl or alkyl chains. The tricarbazole-aromatic amine derivative cavity transmission material has the advantages thatthe synthesis route is simple and short, the price of the reaction raw materials is low, the reaction process is easy to control, the separation is easy, the purity is high, and the yield rate is high; the cavity transfer rate is higher, and the dissolving property is good; especially, when the cavity transmission material is applied to a rovskite solar battery, the excellent photoelectric conversion efficiency is obtained; the novel cavity transmission material with excellent property and low cost can be applied to organic electroluminescent devices, organic solar batteries, perovskite solarbatteries or organic field effect transistor devices, and the important application potential is realized.
Owner:NANJING UNIV OF POSTS & TELECOMM
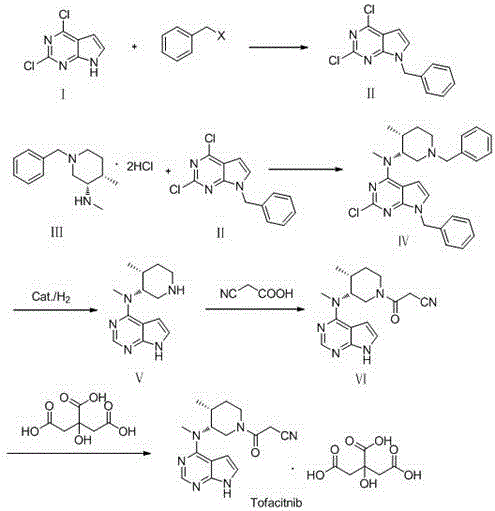
Preparation method of JAKs inhibitor drug tofacitinib
InactiveCN105732641ALess side effectsReduce generationOrganic chemistryAntipyreticAcetic acidTofacitinib
The invention discloses a preparation method of JAKs inhibitor drug tofacitinib, which is characterized in that it comprises the following steps: (1) using 2,4-dichloro-7H pyrrole [2,3-D] pyrimidine as a raw material, Under the action of base, react with halobenzyl to prepare compound II; (2) (3R,4R)-N,4-dimethyl-1-(phenylmethyl)-3-piperidinamine hydrochloride React with 7-benzyl-2,4-dichloro-7H pyrrole [2,3-D] pyrimidine under the action of a base to prepare compound IV; (3) the compound IV obtained in step (2) is catalyzed under the action of a metal catalyst Hydrogenation reaction to obtain compound V; (4) Compound V obtained in step (3) is coupled and docked with cyanoacetic acid under the action of a condensing agent to obtain compound VI; (5) Compound VI obtained in step (4) is prepared by salting with citric acid Get tofacitinib. The synthesis route of the method is short, the reaction process of each step is easy to operate, the solvent can be recycled and used mechanically, the pollution is small, and it is suitable for large-scale industrial production.
Owner:NANTONG CHANGYOO PHARMATECH CO LTD
Preparation method for 3-aminomethyltetrahydrofuran
ActiveCN106366056AReduce pollutionStarting materials are cheap and readily availableOrganic chemistryAlcoholAcrylonitrile
The invention discloses a preparation method for 3-aminomethyltetrahydrofuran. The preparation method comprises the following steps: with acrylonitrile as a starting material, carrying out an addition reaction with 2-halogenated ethyl alcohol so as to obtain an intermediate 2-haloethyl-2-nitrile ethyl ether; then subjecting the intermediate 2-haloethyl-2-nitrile ethyl ether to cyclic condensation so as to obtain an intermediate 3-nitrile tetrahydrofuran; and finally subjecting the intermediate 3-nitrile tetrahydrofuran to catalytic hydrogenation so as to obtain 3-aminomethyltetrahydrofuran. The preparation method provided by the invention has the advantages of cheap and easily-available starting material, short synthetic route, simple process operation, low production cost, little pollution to the human body and the environment, good yield and applicability to large-scale industrial production.
Owner:CHANGZHOU SUNLIGHT PHARMA
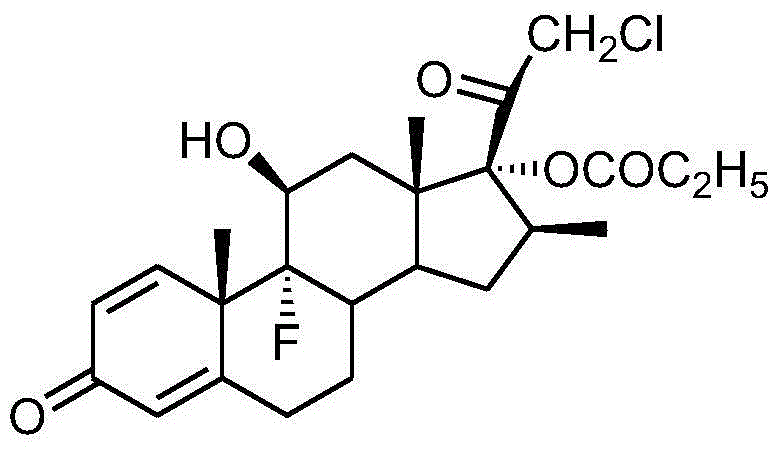
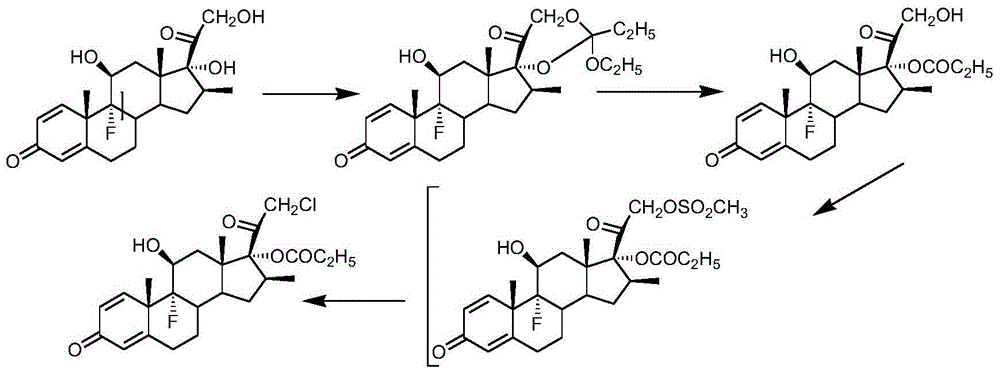
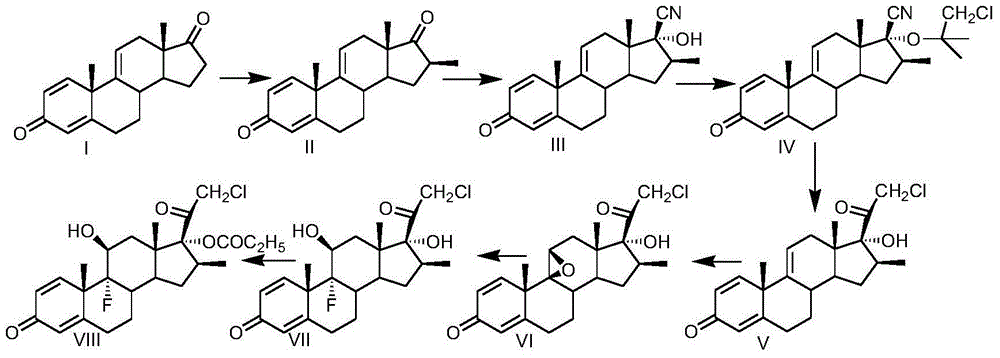
Preparation method of clobetasol and preparation method of clobetasol propionate
The invention discloses a preparation method of clobetasol and the preparation method of clobetasol propionate. The preparation method of the clobetasol comprises the following steps: by taking a compound I, namely 1,4,9(11)-triene androstane-3,17-diketone as an initial raw material, performing a methylation reaction, a cyan substitution reaction, a siloxy protection reaction, an intramolecular nucleophilic substitution reaction, a bromoepoxy reaction and a fluorination reaction to prepare a compound VII which is clobetasol. The compound VII is subjected to a propyl esterification reaction to prepare a compound VIII which is clobetasol propionate. According to the preparation method disclosed by the invention, since relatively basic initial raw materials which are cheap are used, each step of reaction is relatively easy to implement and high yield is achieved; the operation of multi-step protection and deprotection is simplified; moreover, 21 sites of fluorine are directly arranged in one step during arrangement of a side chain, and multiple steps of reaction for arranging the 21 sites of fluorine in the prior art are directly avoided, so that the synthetic route is greatly shortened, the total yield is increased, the product quality is improved and the production cost is greatly lowered.
Owner:江西赣亮医药原料有限公司
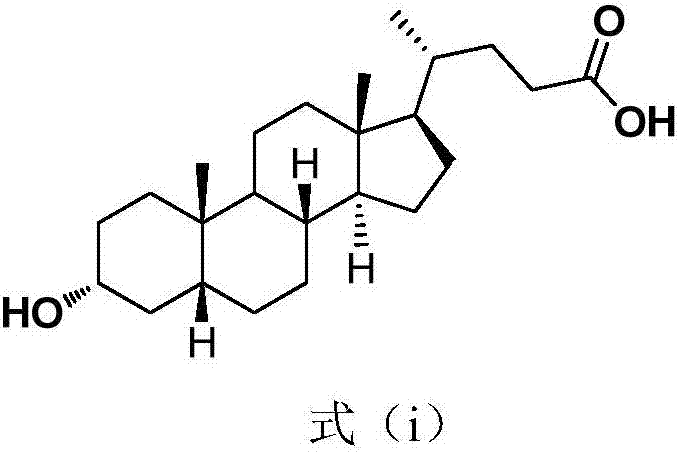
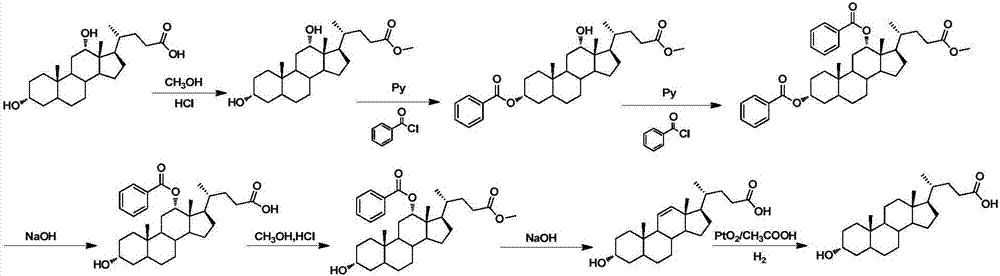
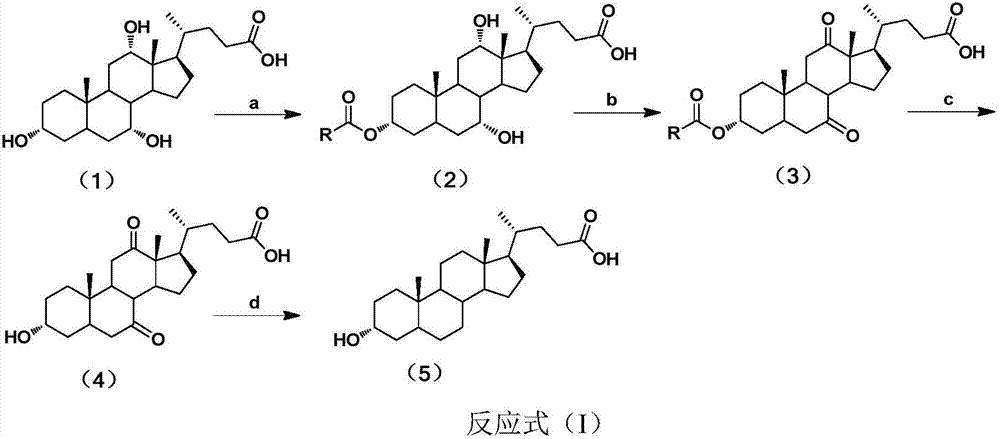
Method for synthesizing lithocholic acid from cholic acid
The invention discloses a method for synthesizing lithocholic acid from cholic acid. According to the method, cholic acid is used as a starting raw material, and lithocholic acid is synthesized through the following four steps of reaction: selective protection of 3alpha-OH; oxidation of 7alpha-OH and 12alpha-OH into carbonyl groups; hydrolysis; and Huang Min-lon reduction. The raw material used in the method is easily available and cheap, and the method is few in synthesis steps and side reactions, simple in after-treatment, high in overall yield and suitable for industrial production.
Owner:JIANGSU JIAERKE PHARMA GRP CORP
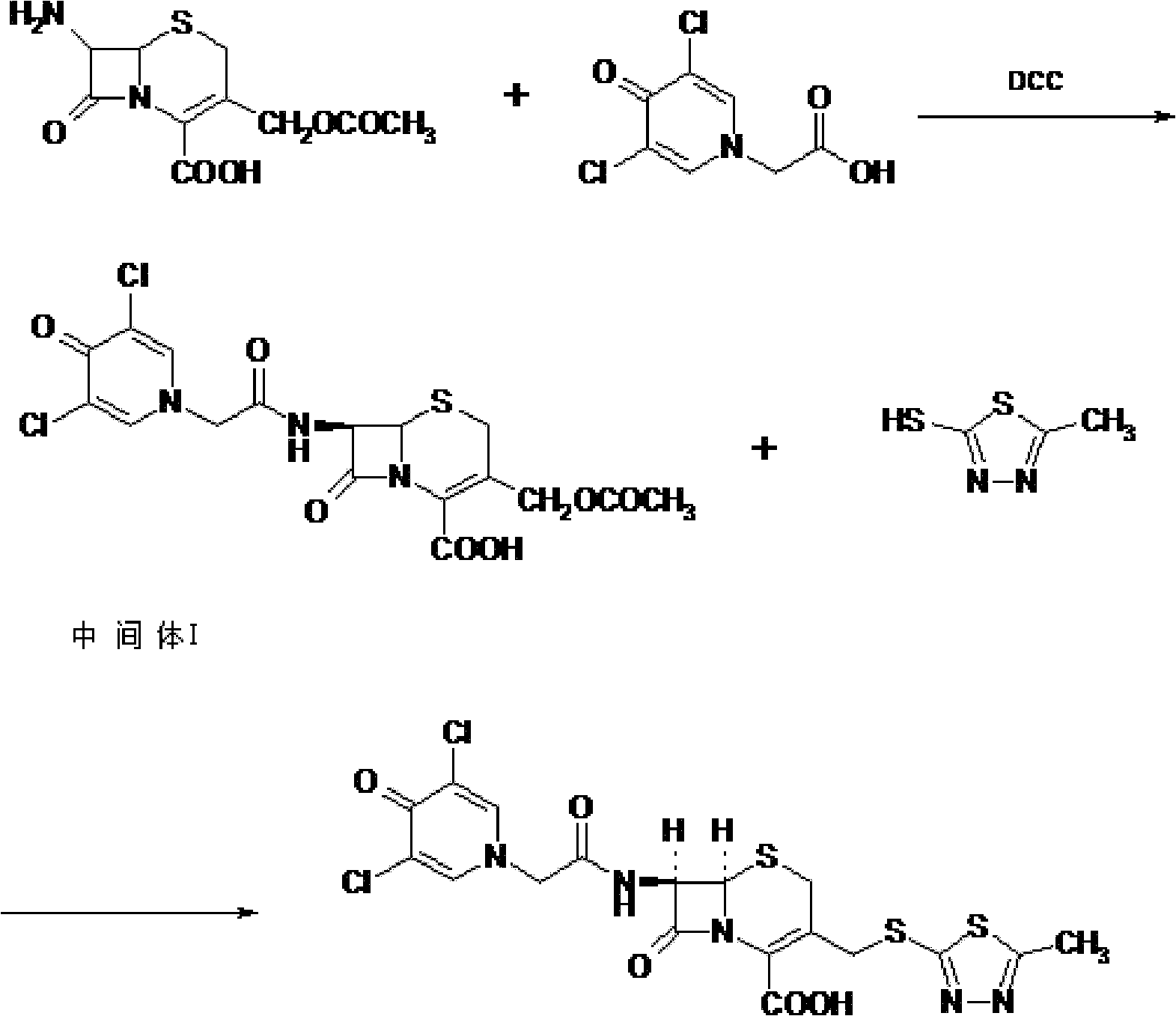
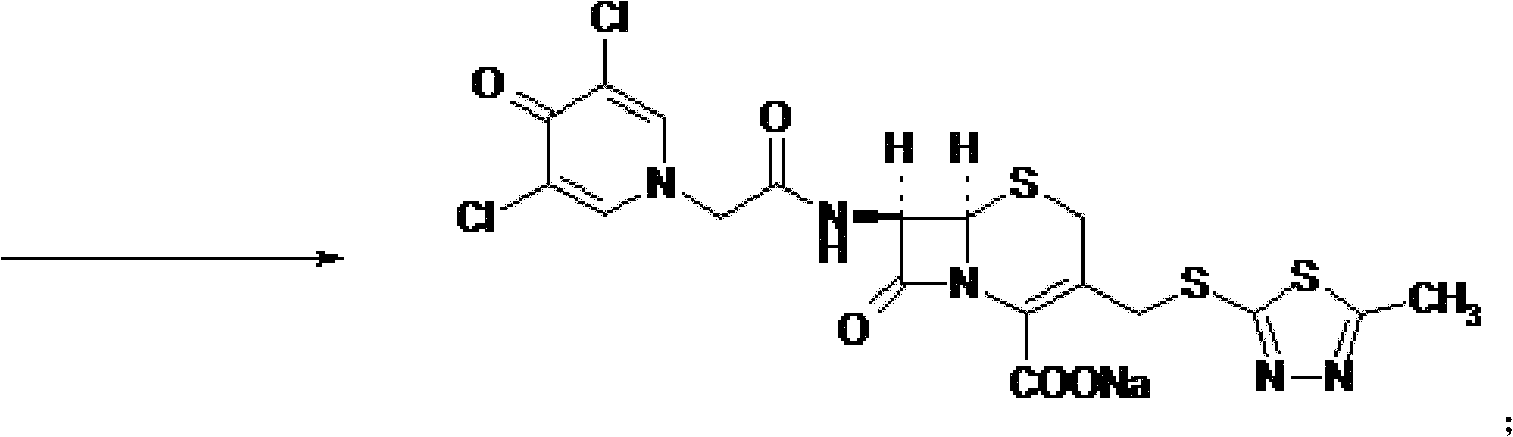
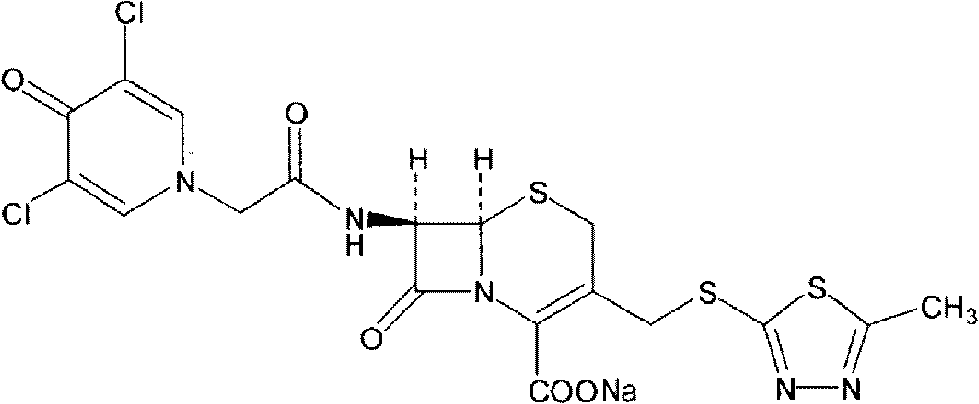
Cefazedone sodium medicament powder injection and method for synthesizing raw medicine of Cefazedone sodium
ActiveCN101584671BSingle componentImprove solubilityAntibacterial agentsOrganic active ingredientsSodium bicarbonateNitrogen gas
The present invention provides a cefazedone sodium medicament powder injection composed of 100% of cefazedone sodium. The cefazedone sodium is prepared by a method as follows: (1) 7-ACA and 3, 5-dichloro pyridine acetic acid react with each other with the action of an anhydrating agent, a mixture after the reaction is post-processed to obtain an intermediate product I; (2) the intermediate productI and 2-mercapto-5-methyl-1, 3, 4-thiadiazoles react with each other with the protection of nitrogen at a temperature of 50 to 90 DEG C, a mixture after the reaction is purified to obtain a water solution which is added with an inorganic acid to regulate pH value to be equal to 1 to 3, a precipitation is extracted from the water solution and is post-processed to obtain cefazedone; (3) the cefazedone and sodium hydrogen carbonate react with each other in water to obtain a cefazedone sodium solid body after an aftertreatment. The powder injection has single component and perfect dissolution performance, the raw medicine has a short synthetic route, the aftertreatment of the intermediate product or final product are all simple, and the yield and purity of the whole reaction process are all high.
Owner:SHANDONG LUOXIN PHARMA GRP CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole](https://images-eureka.patsnap.com/patent_img/699fb17d-fcea-43e3-9b32-2956095f09ea/BDA0000091868740000021.PNG)
![Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole](https://images-eureka.patsnap.com/patent_img/699fb17d-fcea-43e3-9b32-2956095f09ea/BDA0000091868740000022.PNG)
![Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole Synthetic method of [1-methyl-2-(8'-octyl hydroxamic acid group)-5-N,N-bi(2'-chloroethyl)]-1H-benzimidazole](https://images-eureka.patsnap.com/patent_img/699fb17d-fcea-43e3-9b32-2956095f09ea/BDA0000091868740000041.PNG)


![Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds](https://images-eureka.patsnap.com/patent_img/06b35e3d-080d-42f4-b08a-9d67604c56fa/BDA00002218509300021.PNG)
![Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds](https://images-eureka.patsnap.com/patent_img/06b35e3d-080d-42f4-b08a-9d67604c56fa/BDA00002218509300031.PNG)
![Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds Synthetic method of [alpha],[beta],[beta]-trifluorostyrene type compounds](https://images-eureka.patsnap.com/patent_img/06b35e3d-080d-42f4-b08a-9d67604c56fa/BDA00002218509300041.PNG)