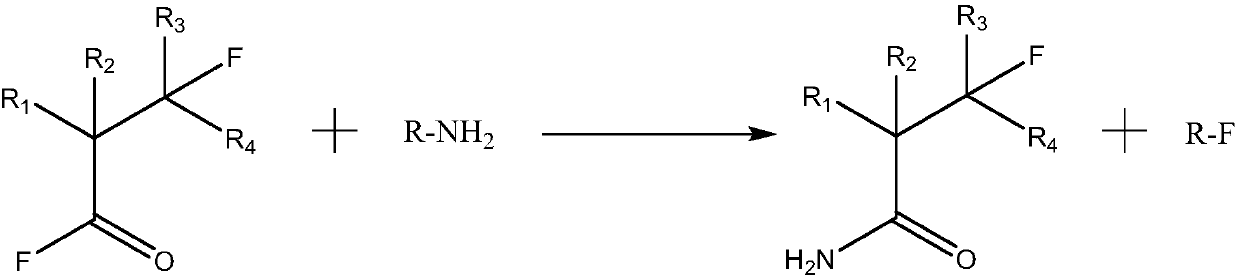
Perfluoro nitrile preparation method
A technology of fluoronitrile and perfluoroalkene, which is applied in the field of preparation method of perfluoronitrile, can solve the problems of unavailable raw materials, low yield of heptafluoroisobutyronitrile, high cost, etc., achieves shortened synthetic route, novel synthetic route, The effect of increasing the conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] In a 1-liter, 316-material autoclave, first add 0.5 mol of potassium fluoride and 0.1 mol of 18-crown-6, then evacuate, pass through anhydrous acetonitrile, and then put it under stirring conditions at -100°C , quickly feed 1 mol of hexafluoropropylene and 1 mol of carbonyl fluoride, after the feed is completed, keep the temperature at 15°C, and the reaction time is 10 hours. After the reaction, use a 200mL small steel cylinder made of 316 to collect the gas phase in the reaction system The product heptafluoroisobutyryl fluoride was 207.8 g, the purity was 99.7% (GC analysis), and the yield of heptafluoroisobutyryl fluoride was 95.9%.
Embodiment 2
[0053] In a 1-liter, 316-material autoclave, first add 0.5 mol of sodium fluoride and 0.1 mol of 15-crown-5, then evacuate, pass in anhydrous acetonitrile, and then put it under stirring conditions at -100°C , quickly feed 1 mol of hexafluoropropylene and 1 mol of carbonyl fluoride, after the feed is completed, keep the temperature at 15°C, and the reaction time is 10 hours. After the reaction, use a 200mL small steel cylinder made of 316 to collect the gas phase in the reaction system The product heptafluoroisobutyryl fluoride was 203.5 g, the purity was 98.7% (GC analysis), and the yield of heptafluoroisobutyryl fluoride was 93.0%.
Embodiment 3
[0055] In a 1-liter, 316-material autoclave, after vacuuming, 0.5 mol of heptafluoroisobutyryl fluoride and 1.5 mol of methylamine CH 3 NH 2 , after the feed is completed, keep the temperature at 15 ° C, and the reaction time is 20 hours. After the reaction is over, the valve is opened to release the gas in the reaction system to obtain 105.6 grams of solid heptafluoroisobutyramide, with a melting point of 46-51 °C, the purity is 99.5% (GC analysis), and the yield is 98.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com