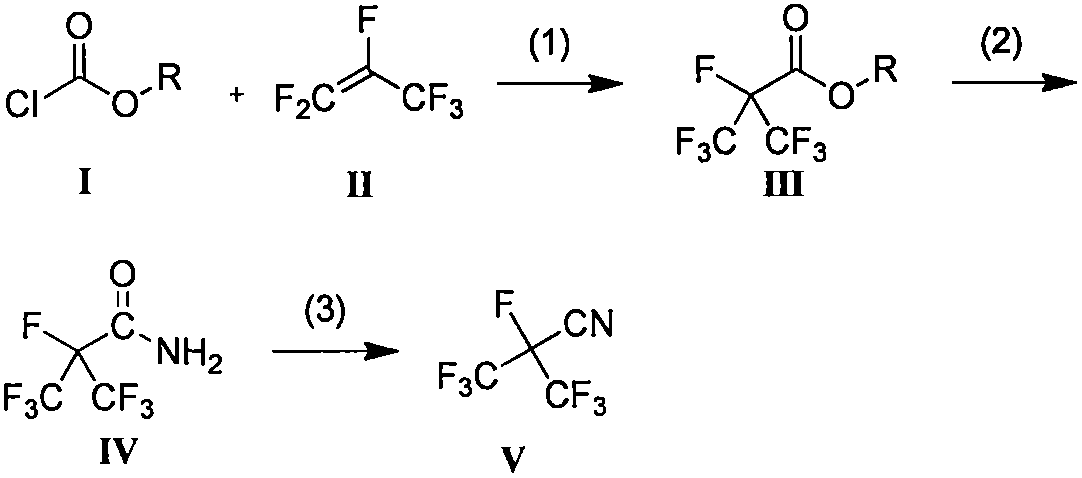
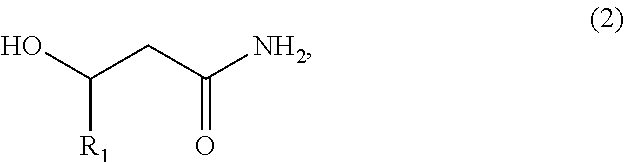
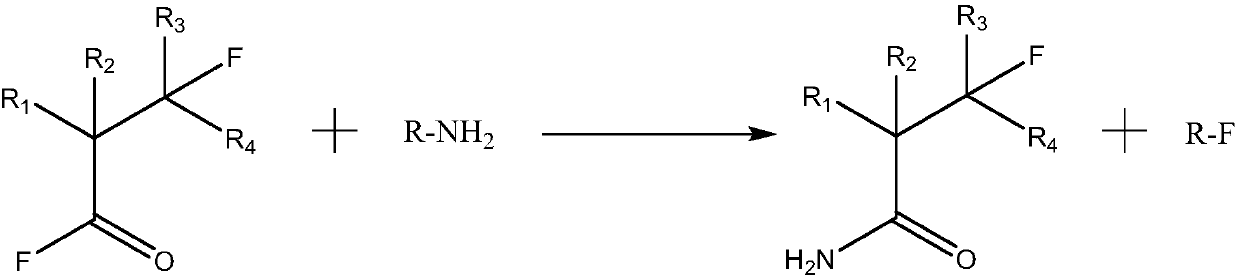
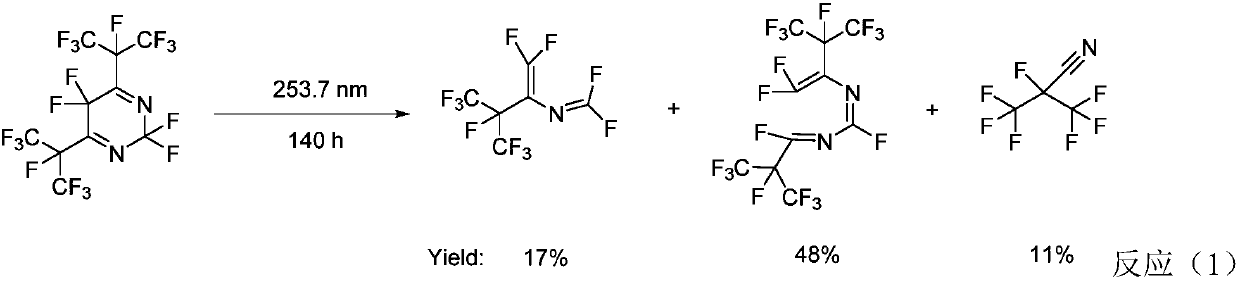
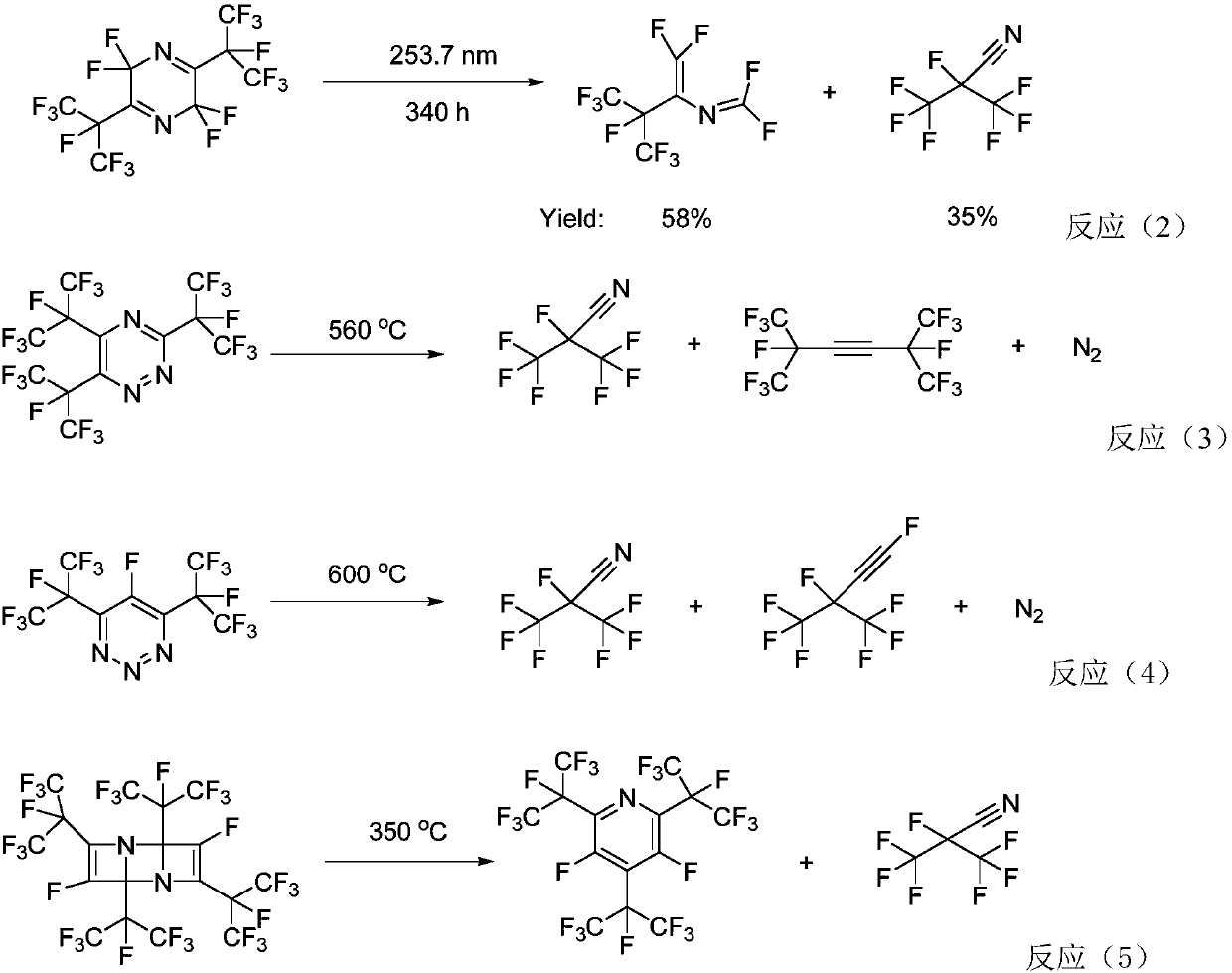
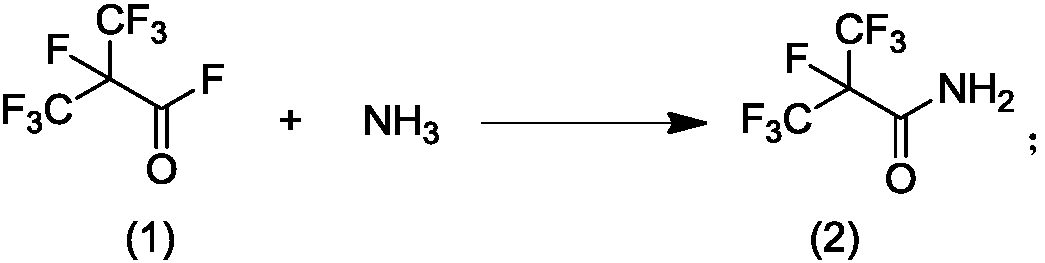
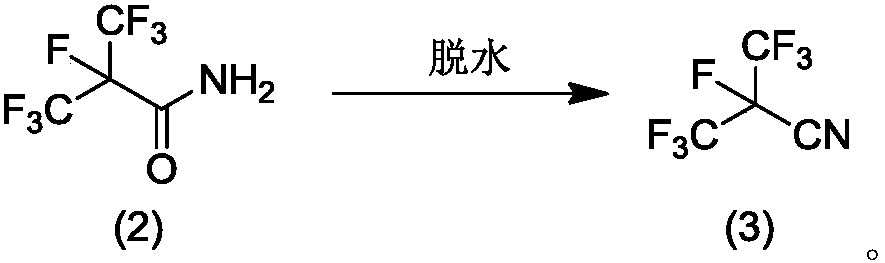
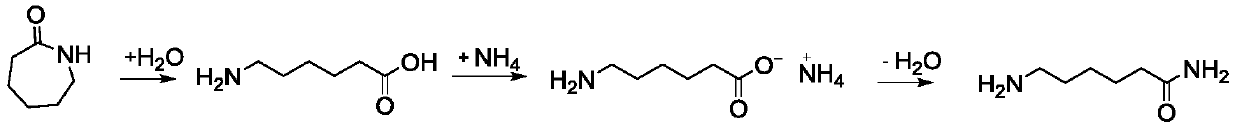
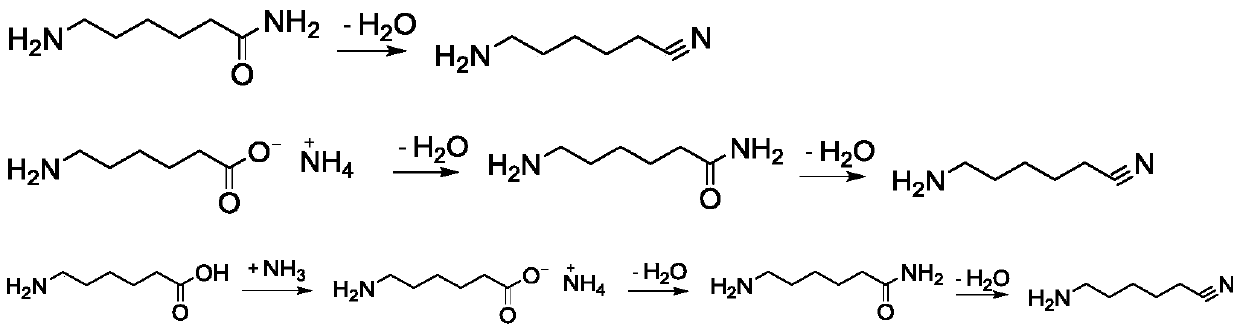
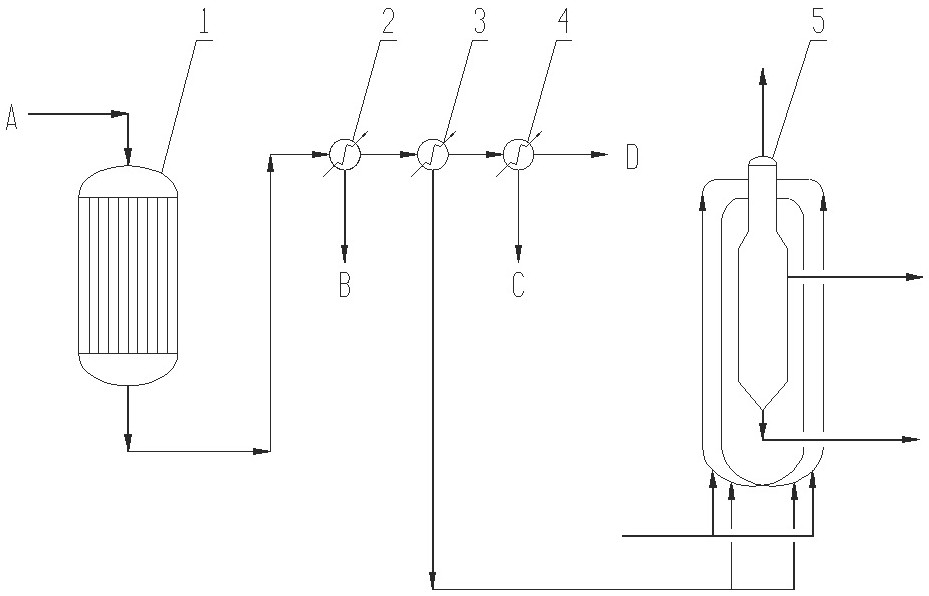
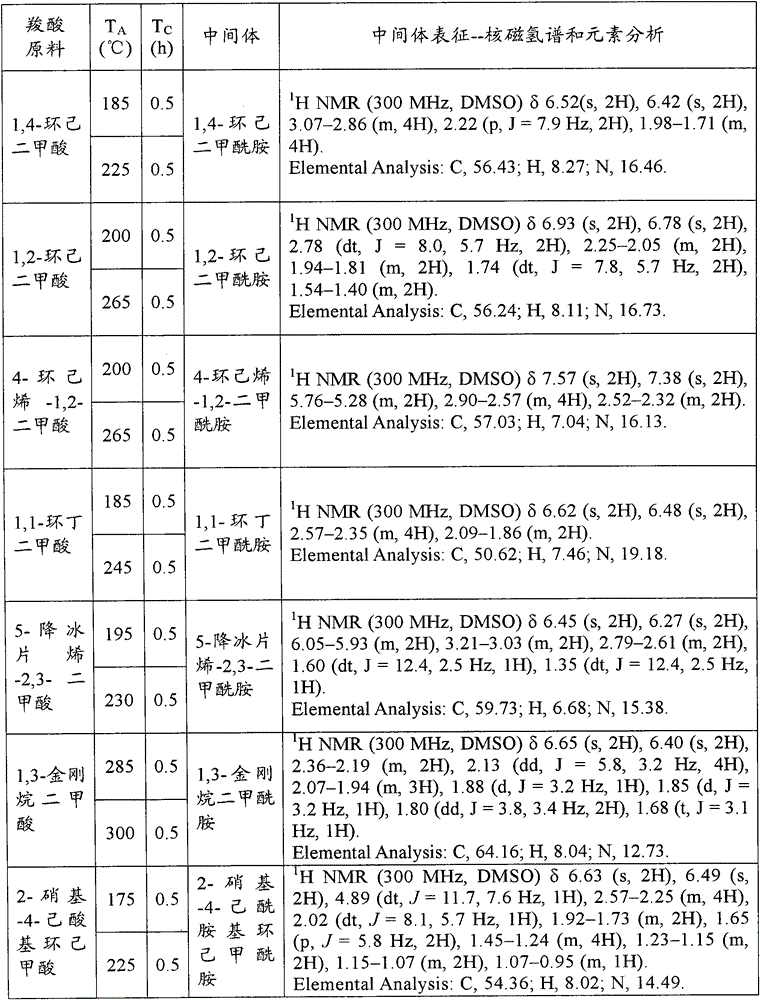
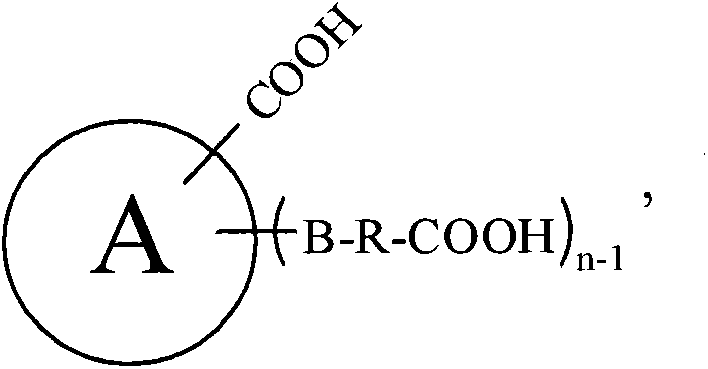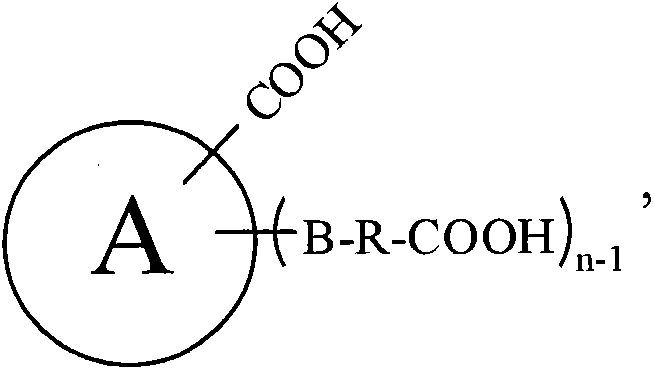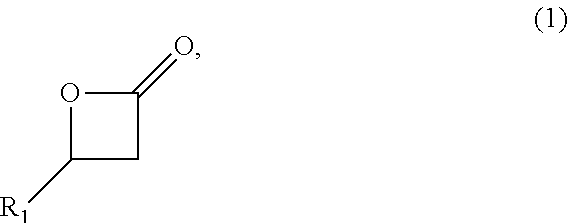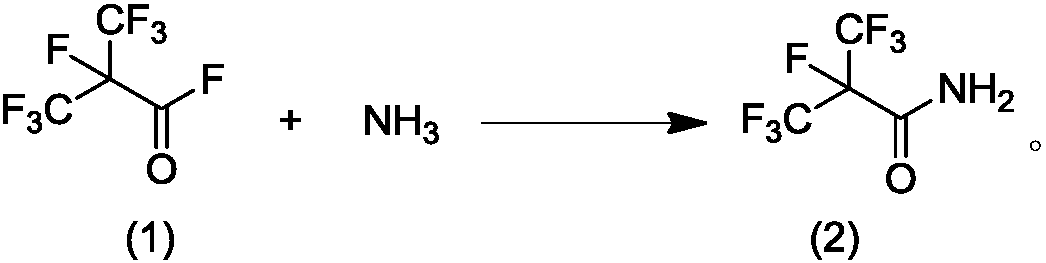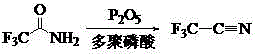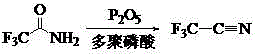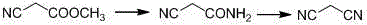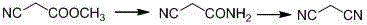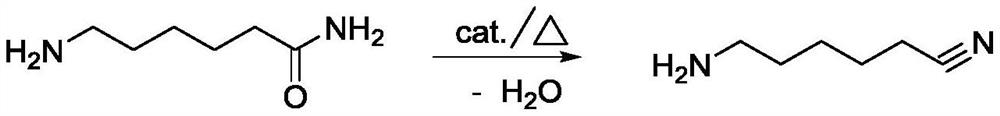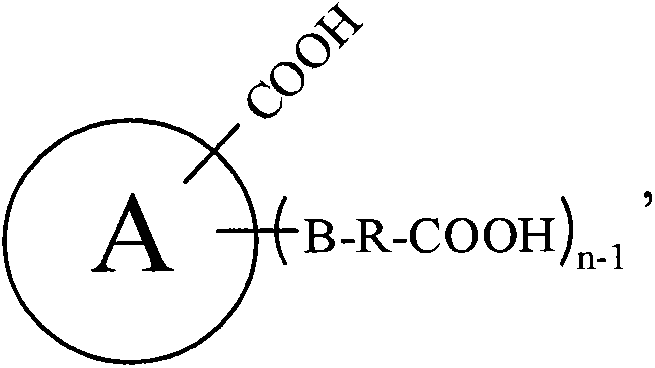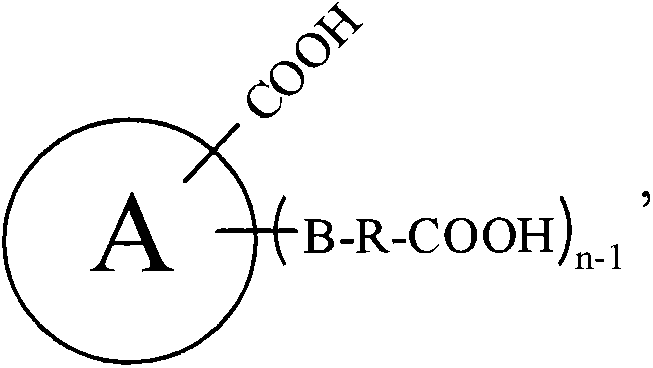Patents
Literature
270results about "Preparation by carboxylic acid amide dehydration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
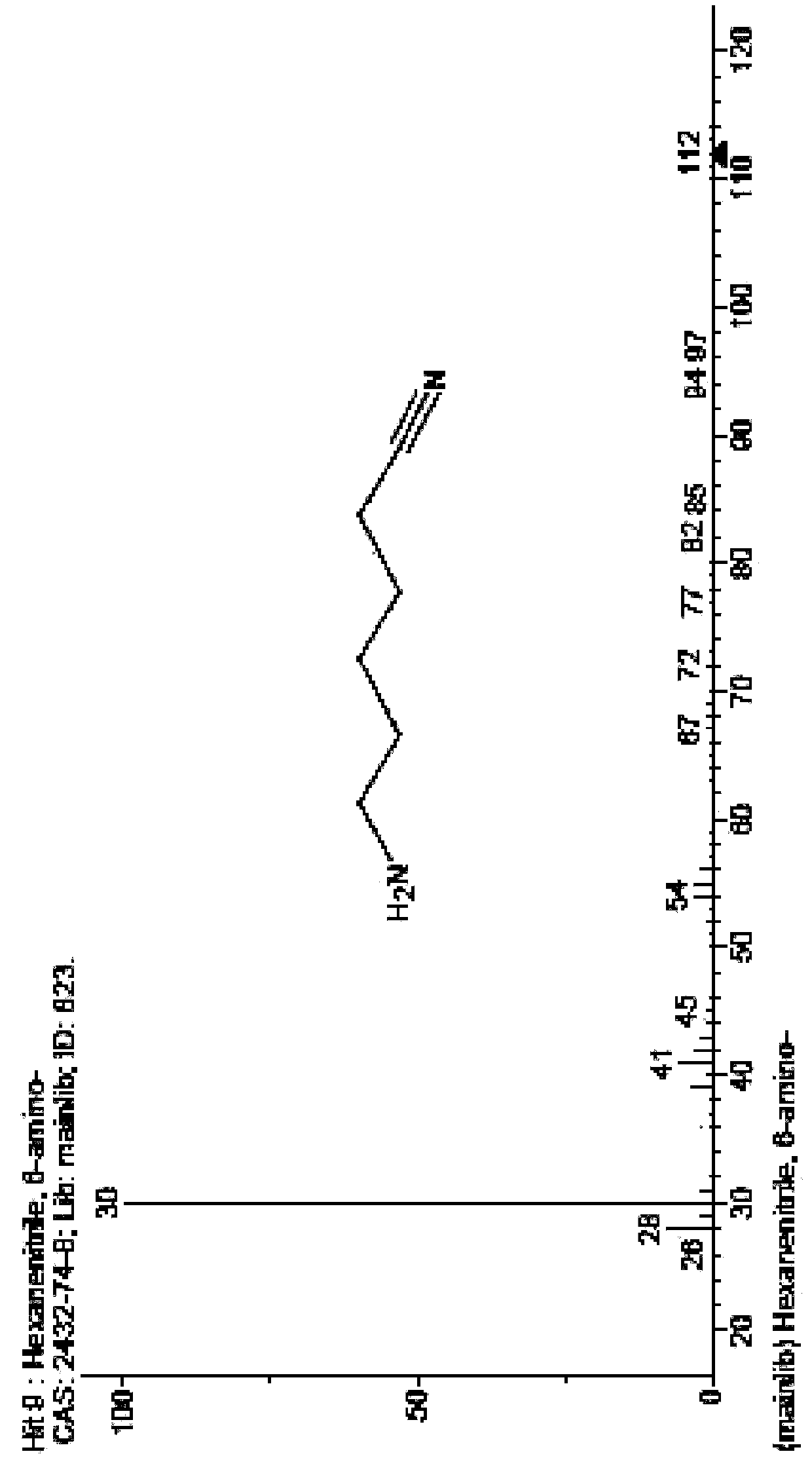
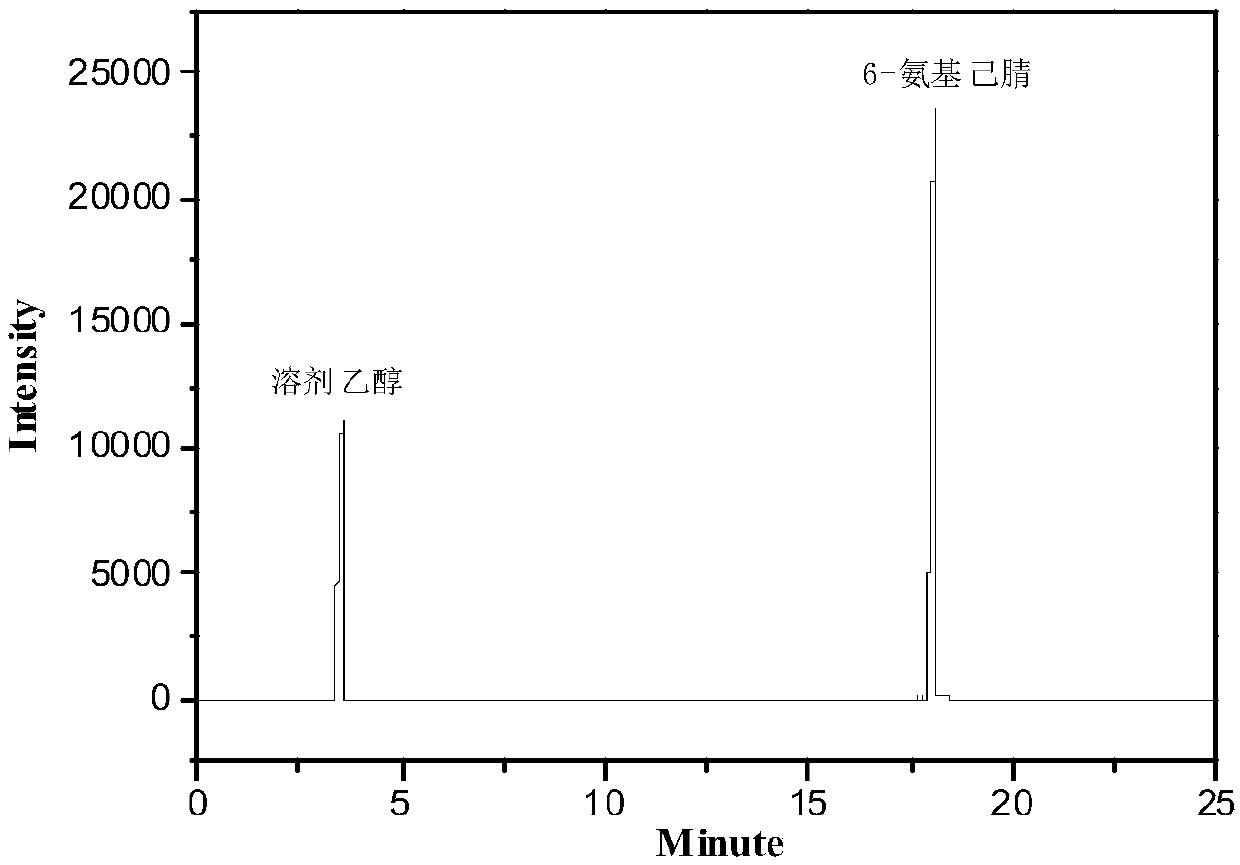
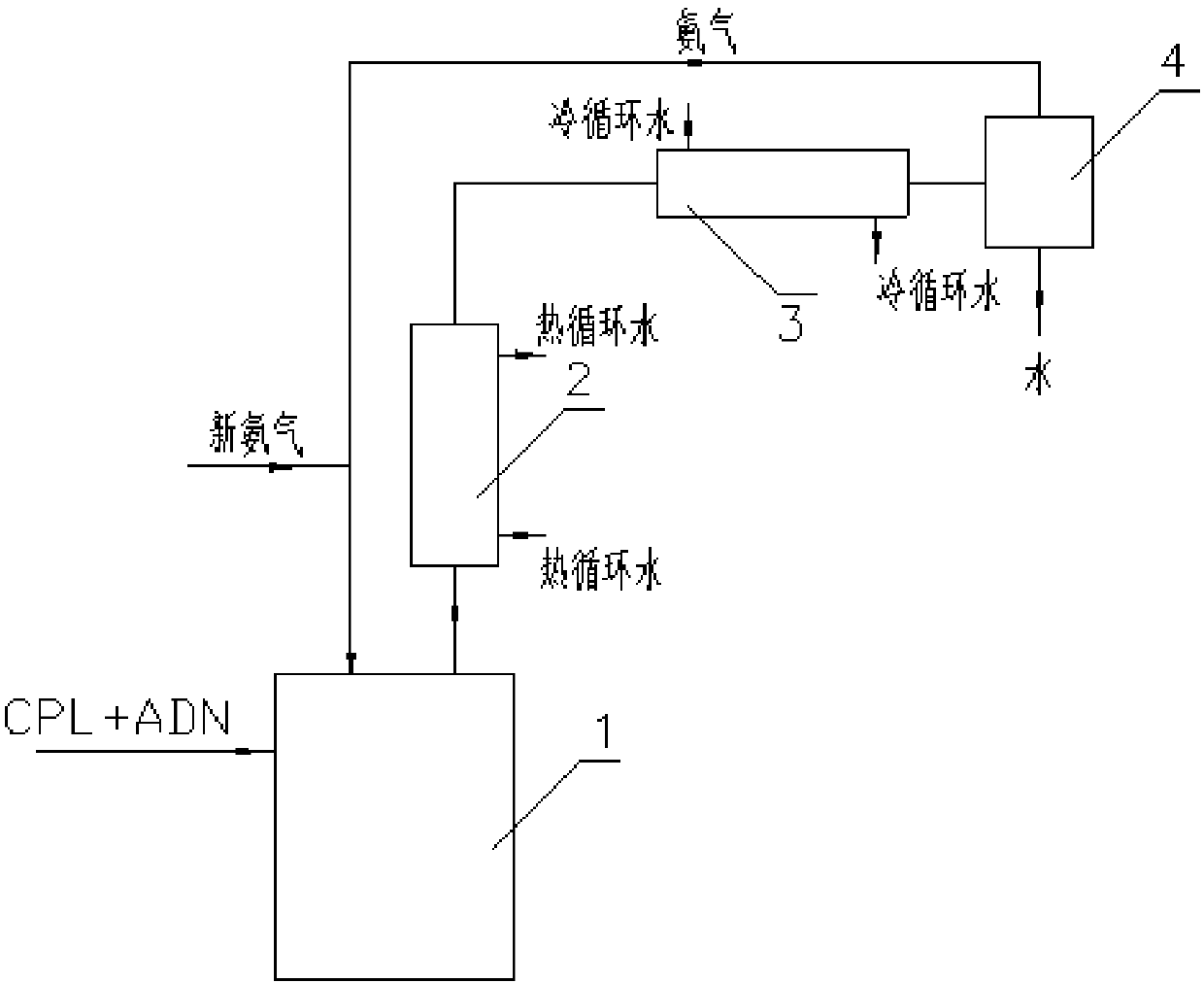
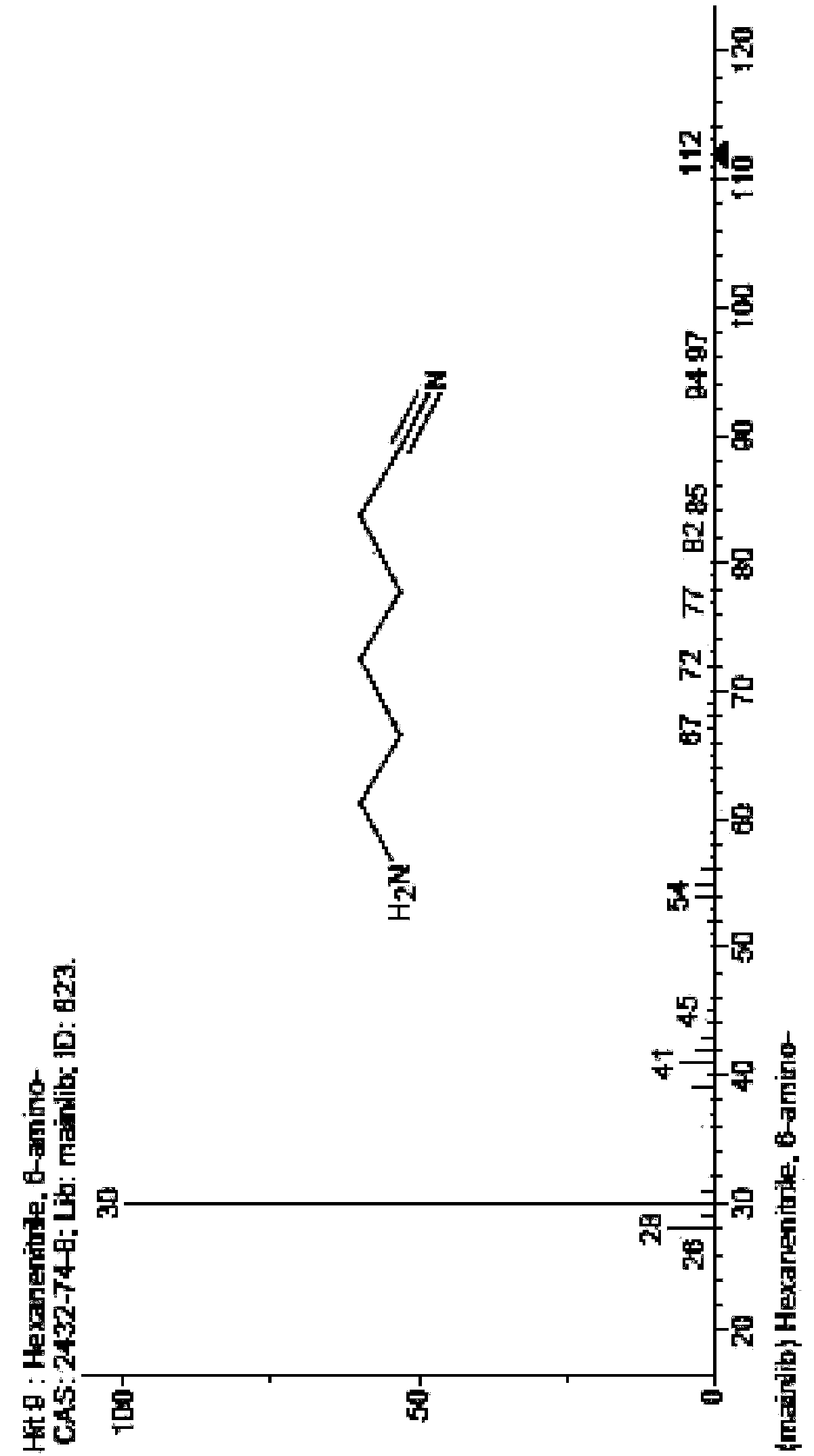
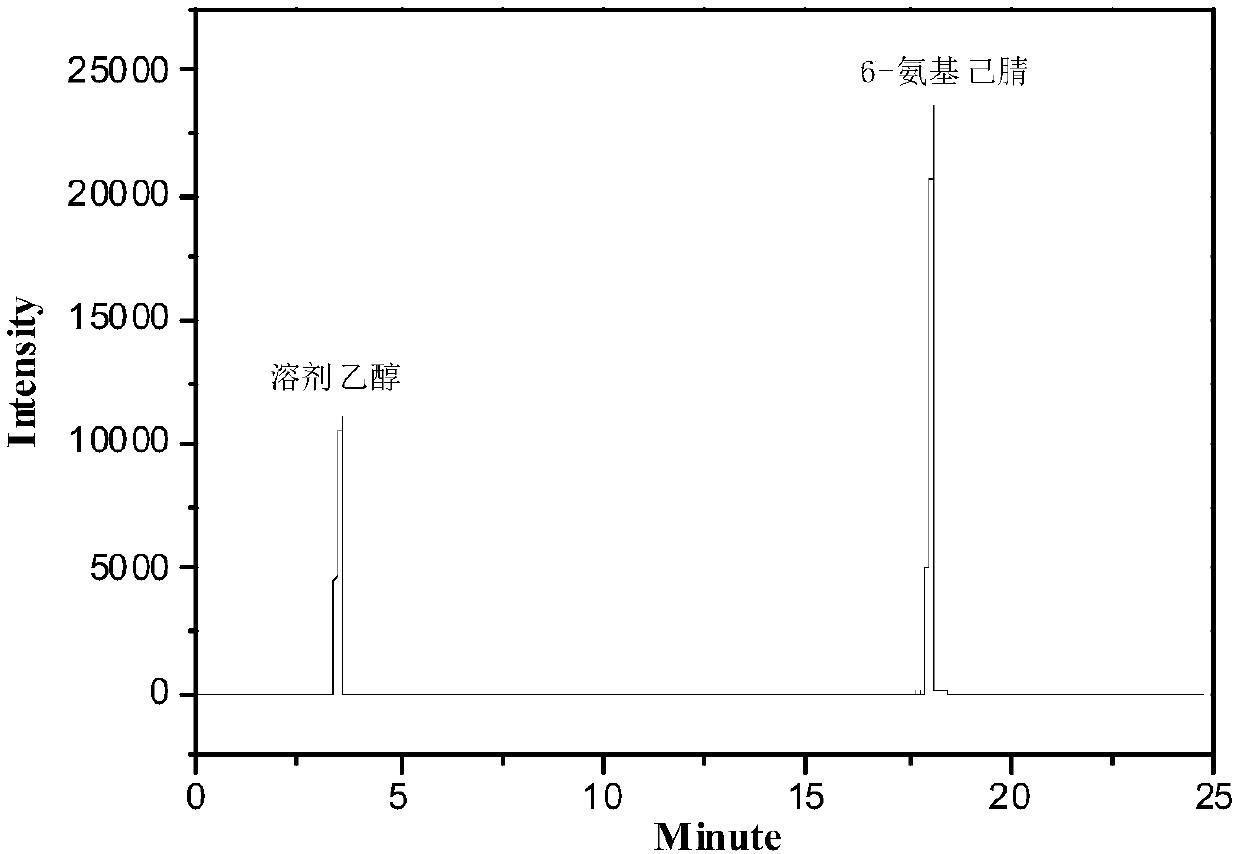
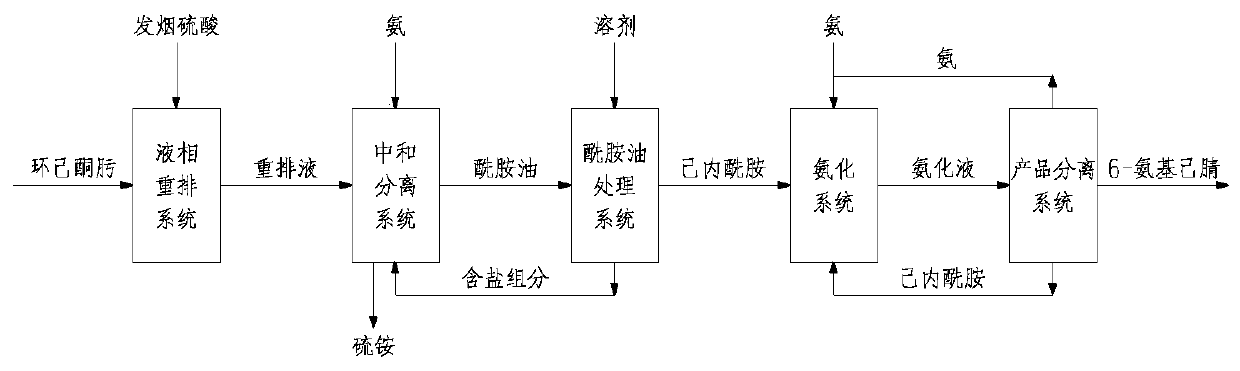
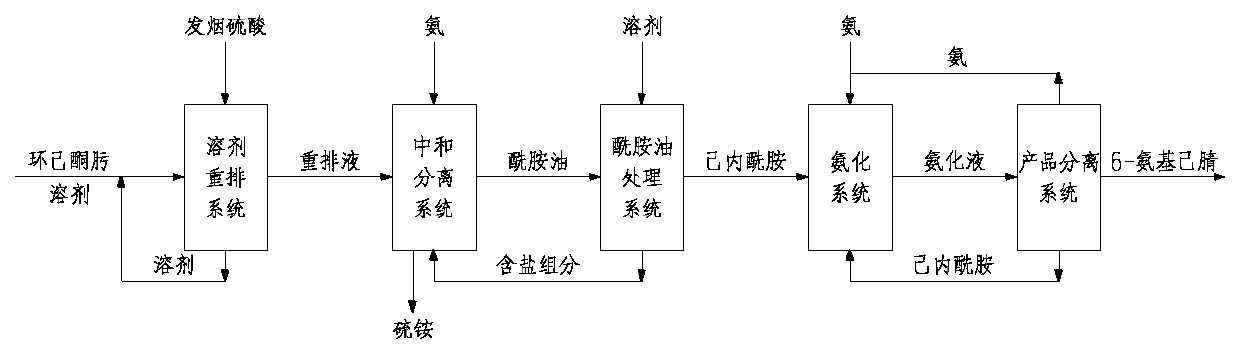
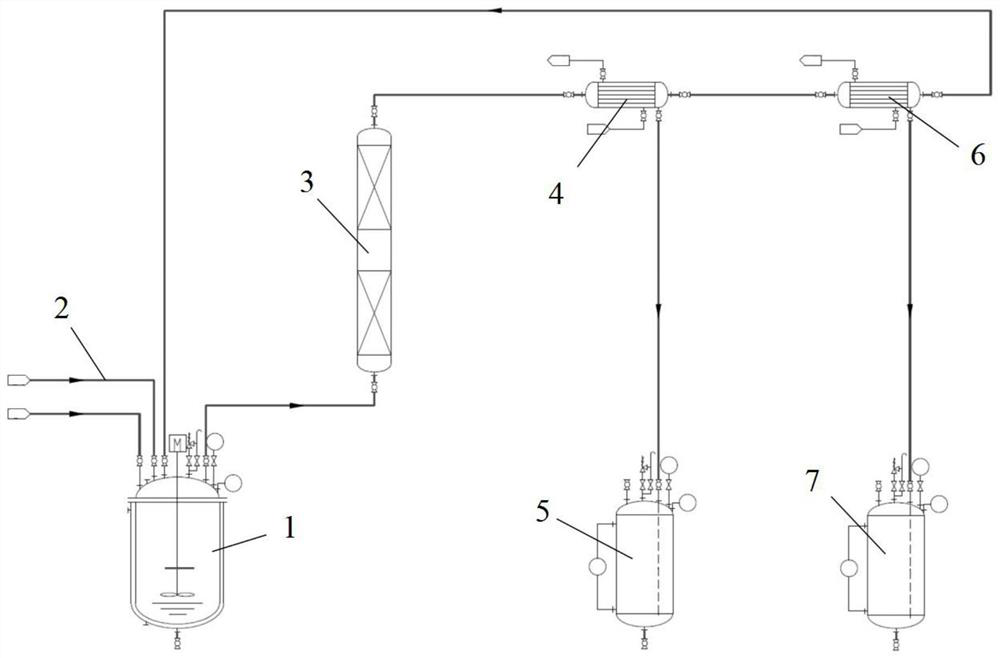
Method and device for preparing 6-amino-capronitrile with caprolactam liquid phase method
InactiveCN107739318AHigh reaction conversion rateGood choicePreparation by carboxylic acid amide dehydrationOrganic solventCaprolactam
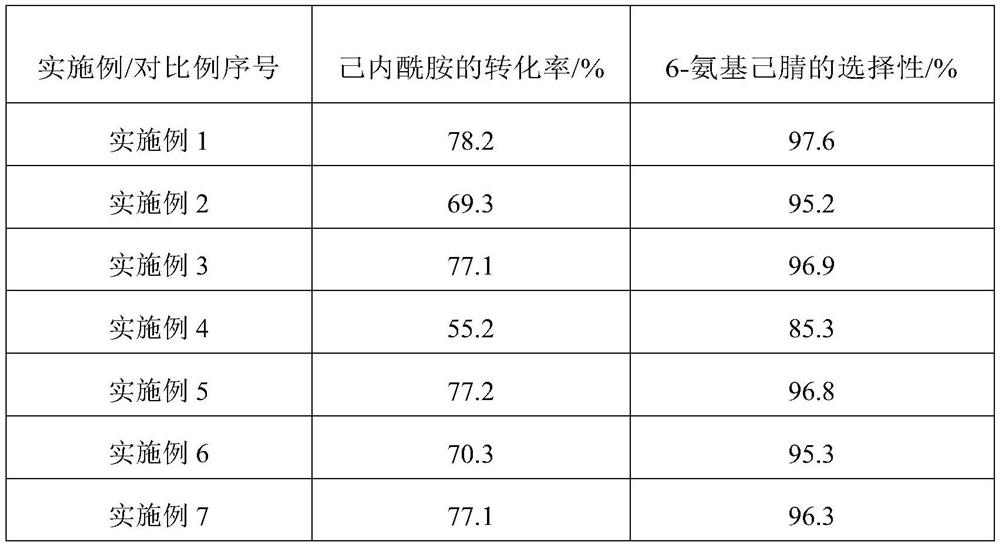
The invention provides a method and device for preparing 6-amino-capronitrile from caprolactam by adopting a liquid phase method. In the method for preparing the 6-amino-capronitrile, the caprolactamis taken as a raw material; the method for preparing the 6-amino-capronitrile comprises the following step: S1: mixing the caprolactam, an organic solvent and a catalyst at a certain mass ratio, so asto obtain a mixed solution, adding the mixed solution into a reaction kettle and stirring and heating the mixed solution; S2: when the mixed solution in step S1 reaches certain temperature, introducing ammonia gas into the mixed solution for a reaction; S3, after the reaction in step S2 is ended, rectifying and purifying a reaction product, so as to obtain pure 6-amino-capronitrile. In the methodfor preparing the 6-amino-capronitrile, the caprolactam is taken as the raw material, the reaction conversion rate is comparatively high, and the preparation process is simple.
Owner:CHINA TIANCHEN ENG +1
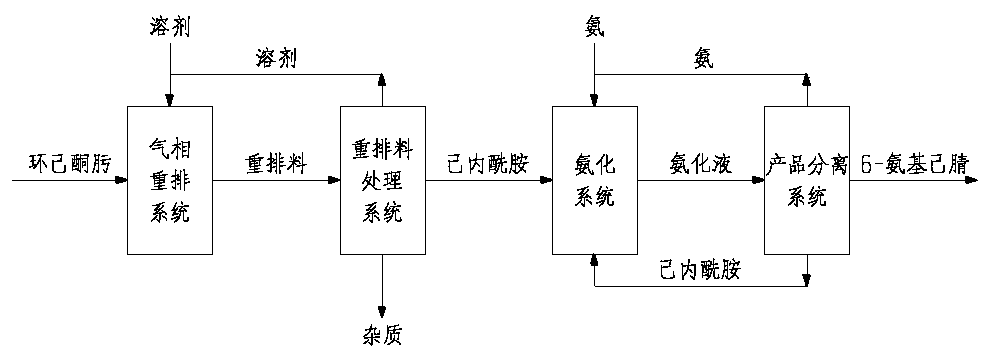
Method for preparing 6-aminocapronitrile by gas phase method
InactiveCN107602416AEasy to makeHigh reaction conversion ratePreparation by carboxylic acid amide dehydrationGas phasePhase method
The invention provides a method for preparing 6-aminocapronitrile by a gas phase method. The method uses caprolactam as a raw material, and comprises the following steps of S1, mixing caprolactam vapor with an hot ammonia gas according to a certain mass ratio; S2, subjecting a mixture of the caprolactam vapor and the hot ammonia gas, which is obtained in the step S1, to ammoniation and dehydrationreaction in a condition that a catalyst exists, so as to obtain ammoniated efflux; S3, separating and purifying the ammoniated efflux obtained in the step S2, so as to obtain the pure 6-aminocapronitrile. The method for preparing the 6-aminocapronitrile by the gas phase method, which is provided by the invention, is relatively high in reaction conversion ratio which can be up to 96 percent or above, and is simple in preparation process.
Owner:CHINA TIANCHEN ENG +1
Aromatic diamine with phthalonitrile pendant group, preparation method thereof and polyimides or polyamide prepared therefrom
InactiveCN101307013ARegulating processabilityRegulatory usabilityPreparation by carboxylic acid amide dehydrationFiberSide chain
The invention discloses an aromatic diamine containing o-phthalonitrile side group. The structure formula of the aromatic diamine is shown as the right formula. The invention also discloses a method for preparing the aromatic diamine and polyimide and daiamid which are prepared by taking the aromatic diamine containing the o-phthalonitrile side group as one of raw materials. The o-phthalonitrile side group in the aromatic diamine is positioned on the side chain, thereby adjusting the degree of crosslinking with adjusting the polymer molecular weight and widening the application of the polyimide and the daiamid in a thick-wall composite material or a composite material element with a complicated shape and the fields of membrane material and fiber.
Owner:SICHUAN UNIV
Preparation methods of nitrile and corresponding amine
ActiveCN105016944AReduce dosageIncrease profitOrganic compound preparationPreparation by carboxylic acid amide dehydrationAmmoniaNitrile
The invention relates to a preparation method of nitrile. Compared with the prior art, the preparation method has the characteristics of obvious reduction of the usage amount of ammonia sources, low environmental pressure, low energy consumption, low production cost, high purity and yields of nitrile products, and the like, and can be used for obtaining nitrile with a more complex structure. The invention also relates to a method for preparing corresponding amine with nitrile.
Owner:SINOPEC YANGZI PETROCHEM +1
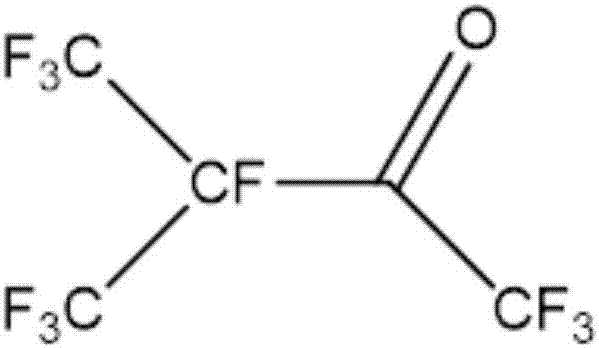
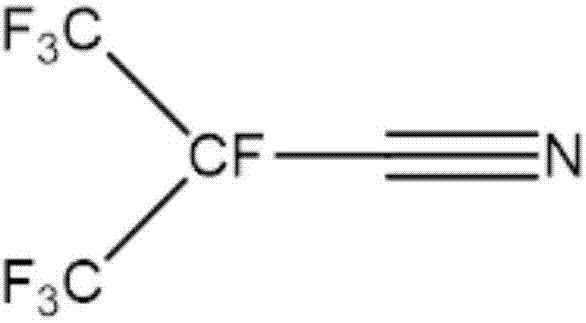
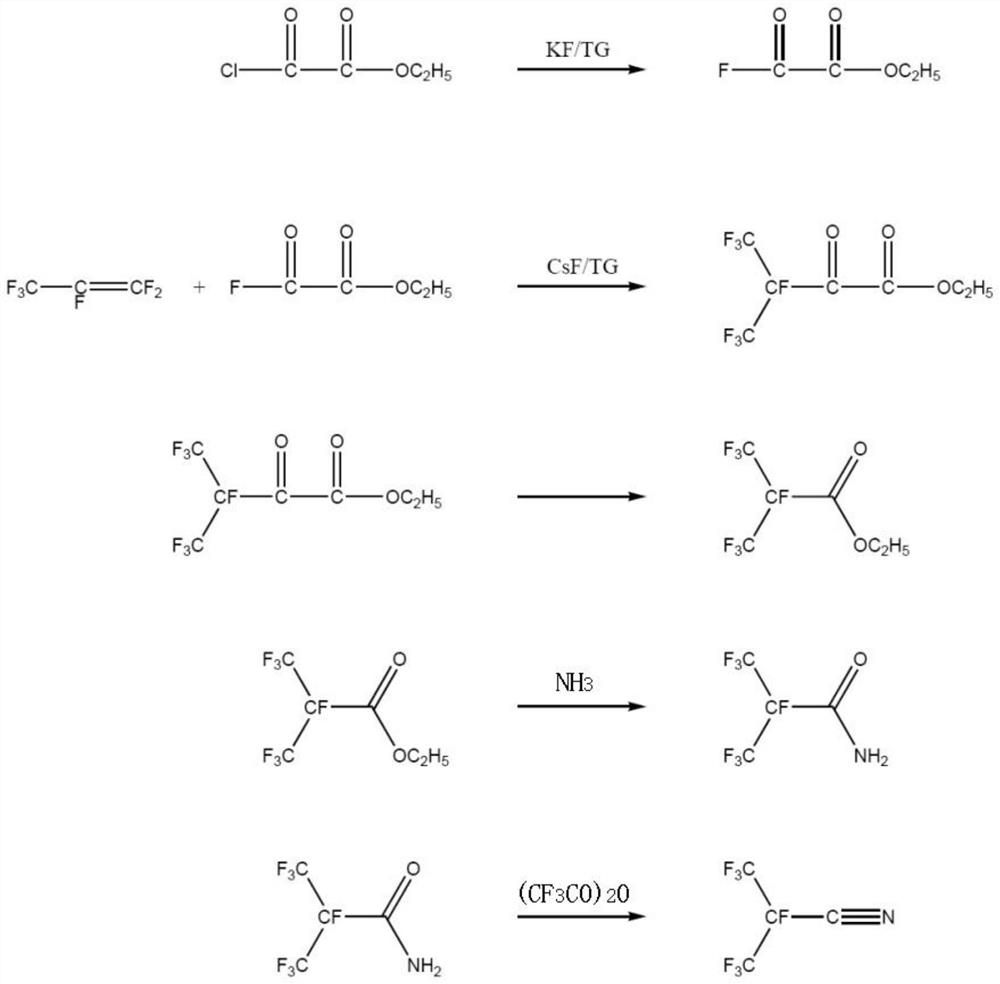
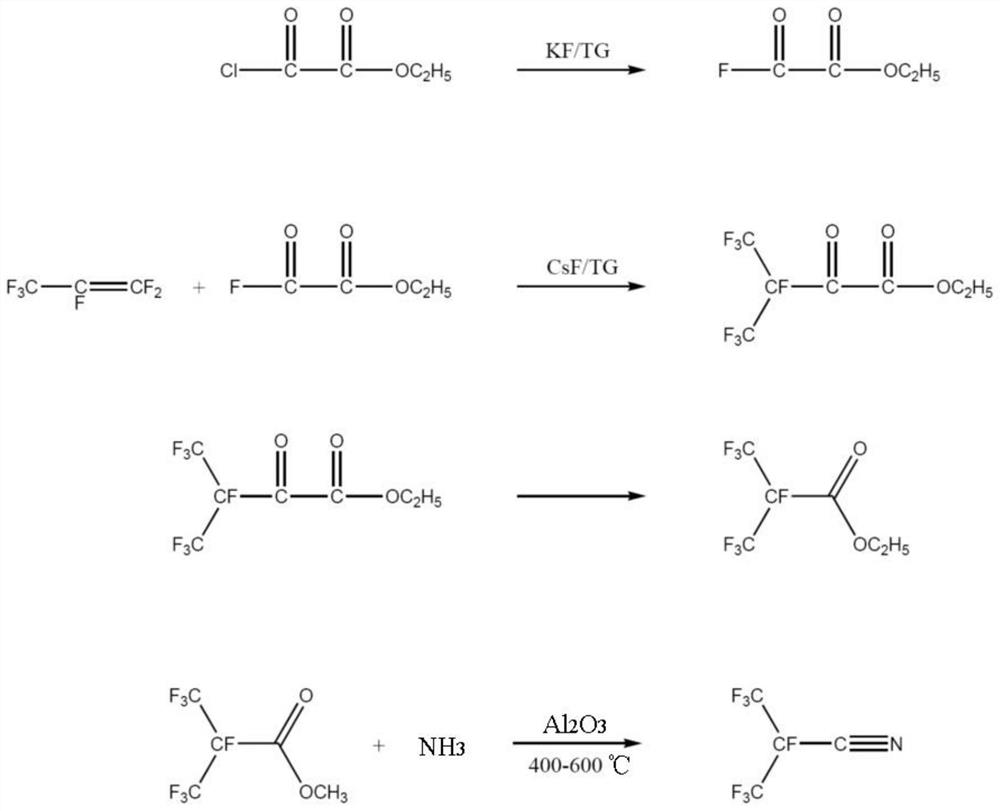
Preparation method of perfluoronitrile
ActiveCN108424375ALow priceHigh yieldOrganic compound preparationCarboxylic acid amides preparationLithiumCarbonyl fluoride
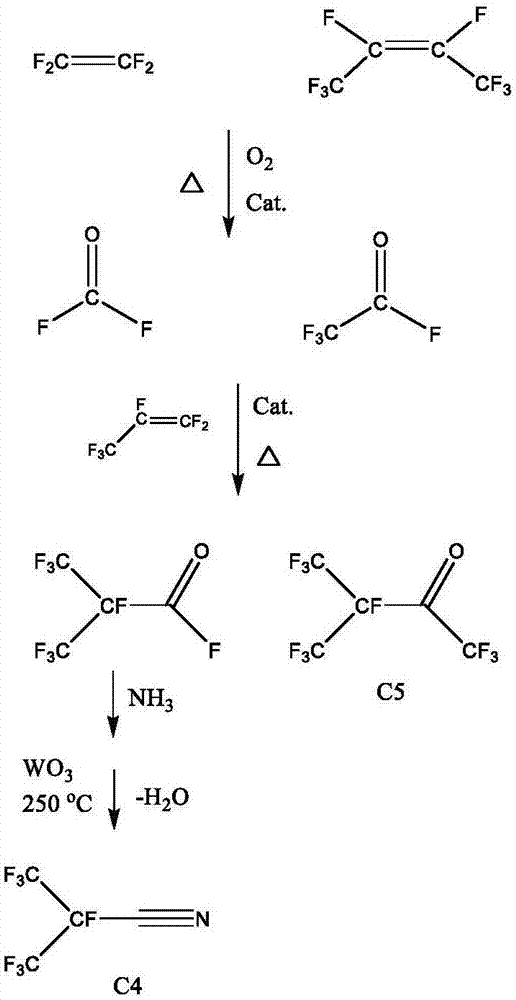
The invention discloses a preparation method of perfluoronitrile. The preparation method comprises the following steps: a, performing a gas-phase addition reaction on perfluoroolefin R1R2C=CR3R4 and carbonyl fluoride to obtain acyl fluoride R1R2(COF)C-CFR3R4 (R1, R2, R3 and R4 have a general formula of a -CnF2n+1 group and n is a nonnegative integer set); b, aminating and dehydrating the acyl fluoride R1R2(COF)C-CFR3R4, acyl fluoride and alkali metal amide or an amine compound R-NH2 (R is lithium, sodium, potassium, rubidium, cesium or a -CmH2m+1 group and m is a nonnegative integer set) to obtain the perfluoronitrile R1R2(CN)C-CFR3R4. By the preparation method, a reaction route is short, the perfluoroolefin and the carbonyl fluoride are easy to obtain and low in price, the overall yield of the perfluoronitrile is high and the route is easy to industrialize.
Owner:泉州宇极新材料科技有限公司
Synthetic method of malononitrile
InactiveCN103044286AHigh purityImprove stabilityPreparation by carboxylic acid amide dehydrationPtru catalystDistillation
The invention discloses a synthetic method of malononitrile. Cyanoacetamide reacts with triphosgene in the presence of a catalyst to synthesize malononitrile, wherein the catalyst is any one substance or a mixture of multiple substances selected from N,N-dimethyl formamide, sodium chloride, pyridine and triethylamine. The method provided by the invention employs the triphosgene as a dehydrating agent, and the triphosgene is low in cost, available and high in stability, and facilitates storage and transportation; the reaction products of the cyanoacetamide and the triphosgene only include carbon dioxide and hydrogen chloride gases except for the malononitrile, without solid waste, and therefore, after the reaction is completed, the steps of removing the solid waste by means of filtering, centrifuging and the like are not needed; the reaction mixture is directly subjected to reduced pressure distillation after the solvent is recovered so that the malononitrile having the purity of higher than 98% can be obtained; and the posttreatment is simple.
Owner:CHONGQING UNISPLENDOUR CHEM
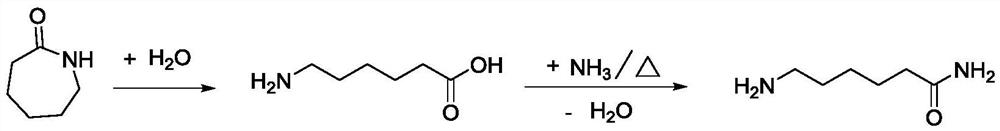
Method for preparing 6-aminocapronitrile from cyclohexanone-oxime
InactiveCN110835311AReduce consumptionReduce processing complexityLactams preparationLactams separation/purificationDistillationIon exchange
The invention discloses a method for preparing 6-aminocapronitrile from cyclohexanone-oxime. The method comprises the following steps: carrying out a rearrangement reaction on cyclohexanone-oxime to obtain a rearranged reaction material; and treating the rearranged reaction material to obtain caprolactam, carrying out an ammoniation dehydration reaction on the caprolactam to obtain an ammoniated dehydrated reaction material, and performing separation to obtain the 6-aminocapronitrile. Compared with the prior art, the method of the invention reduces complex separation and refining processes such as back extraction, ion exchange, hydrogenation, evaporation and distillation required after liquid phase rearrangement of the cyclohexanone-oxime, reduces the processes of crystallization, solventrefining and recovery needed after gas phase rearrangement, and organically combines the caproamide evaporation and rearrangement treatment processes in the ammoniation dehydration reaction process. The steam consumption can be reduced by 2.0-3.0 t / t 6-aminocapronitrile, and the cyclohexanone-oxime consumption is reduced by 20-50 kg / t 6-aminocapronitrile.
Owner:HUNAN BAILI ENGINEERING SCIENCE AND TECHNOLOGY CO LTD
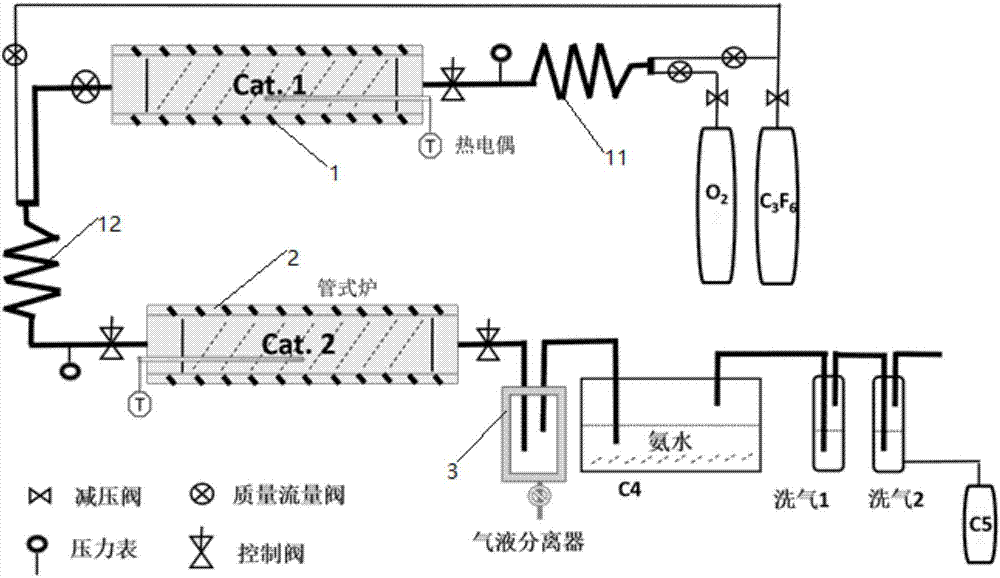
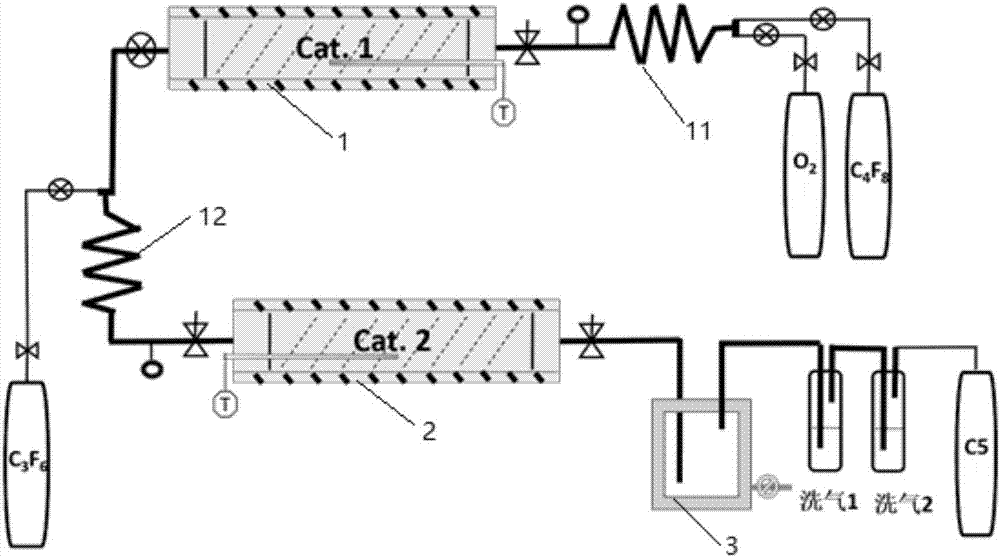
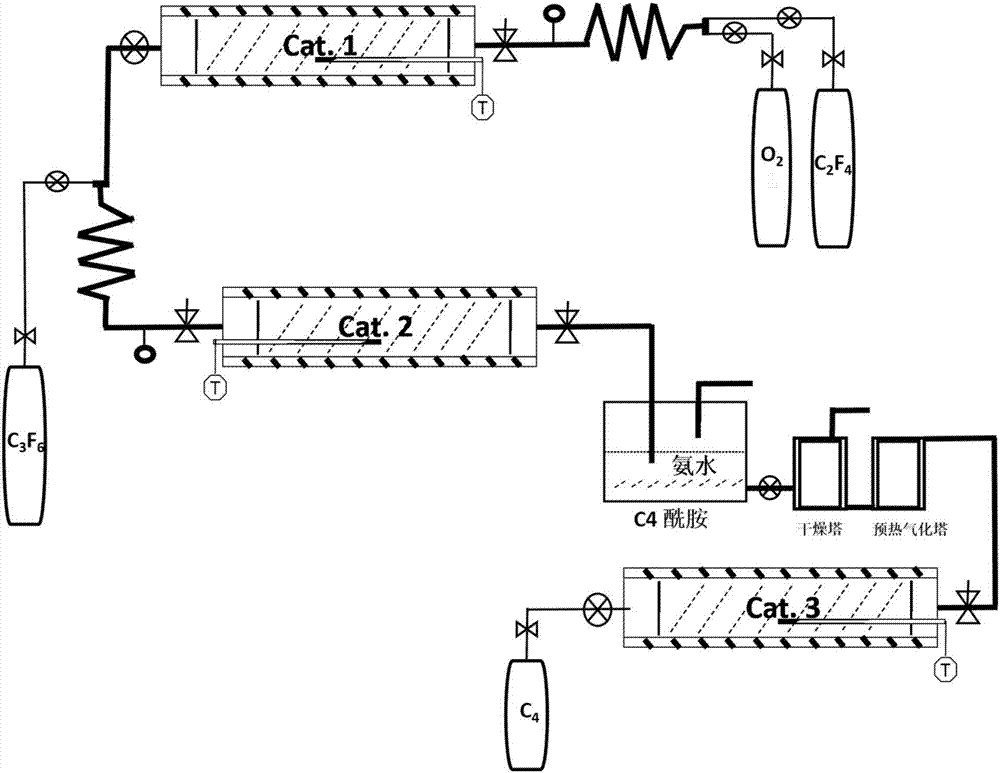
Environmentally-friendly insulating gas combined production technology and industrial production device thereof
ActiveCN106946669AReduce manufacturing costReduce the discharge of three wastesOrganic compound preparationCarboxylic acid amides preparationFixed bedProcess engineering
The invention discloses an environmentally-friendly insulating gas combined production technology and an industrial production device thereof. The combined production technology comprises the following steps: 1, carrying out a contact reaction on oxygen and HFP in a first fixed bed reactor under a catalyst condition or a catalyst-free condition; 2, mixing a gas product flowing out of the first fixed bed reactor with the HFP, allowing the gas product and the HFP to enter a second fixed bed reactor, and carrying out a contact reaction in the second fixed bed reactor under the action of a catalyst; and 3, carrying out separation on the effluent of the second fixed bed reactor to prepare a C5 product and a C4 product. Combined production of two novel environmentally-friendly insulating gases is realized from cheap raw materials comprising perfluoropropylene and oxygen at an atom economy of 80-90%, so the production cost is greatly reduced, and the discharge of three wastes in the production process is reduced.
Owner:STATE GRID CORP OF CHINA +3
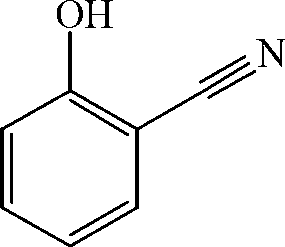
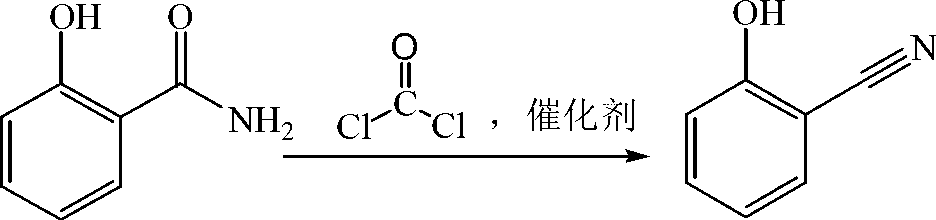
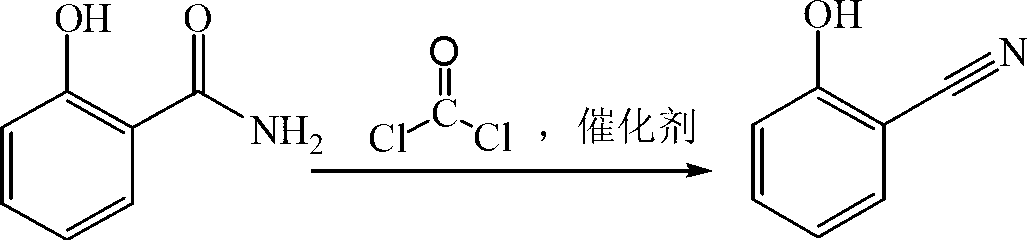
Preparation method of salicylonitrile
ActiveCN103012205AImprove qualityHigh yieldPreparation by carboxylic acid amide dehydrationChlorobenzeneSlag
The invention discloses a method for synthesizing salicylonitrile by using ortho-hydroxybenzamide as a raw material, dioxane or tetrahydropyrane and tetrahydrofuran as catalysts, and dimethylbenzene or benzene, methylbenzene and chlorobenzene as solvents through introducing phosgene at a refluxing temperature. The reaction formula is described in the specification. The invention relates to a method for preparing the salicylonitrile without generating waste water and waste slag. Compared with the prior art, the method has the advantages of less reaction byproducts, high quality, high yield, simplicity and safety in operation, low price and easily available raw material, the content of the salicylonitrile is not less than 98 percent (liquid chromatogram, external standard method), and the yield of the salicylonitrile is not less than 95 percent (in the terms of o-hydroxybenzamide).
Owner:湖南海利常德农药化工有限公司
Separation method of ammoniation dehydration product of caprolactam and synthesis method of hexamethylenediamine
ActiveCN111574400ARealize separation and purificationThe separation method is simpleOrganic compound preparationCarboxylic acid amides preparationHexamethylenediamineProcess engineering
The invention provides a separation method of an ammoniation dehydration product of caprolactam and a synthesis method of hexamethylenediamine. The separation method comprises the following steps: S1,carrying out ammoniation and dehydration reaction on caprolactam and ammonia gas to obtain a product system; S2, carrying out primary condensation treatment on the product system to obtain a caprolactam-containing fraction and a primary gas-phase component; and S3, carrying out secondary condensation treatment on the primary gas-phase component to obtain a 6-aminocapronitrile fraction and a secondary gas-phase component, wherein the temperature of the secondary condensation treatment is lower than that of the primary condensation treatment. According to the separation method, the product system obtained through the reaction is directly subjected to condensation treatment, secondary heating is not needed, continuous operation of the reaction and product separation can be achieved, waste heat can be fully utilized, the separation efficiency is high, the production intensity is high, the separation energy consumption is low, and therefore the whole process is more energy-saving.
Owner:JIANGSU YANGNONG CHEM GROUP +2
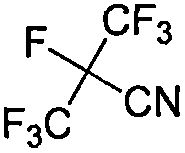
Method for synthesizing perfluoroisobutyronitrile
ActiveCN108395382ALow costHigh purityOrganic compound preparationCarboxylic acid esters preparationHexafluoropropyleneChloroformate
The invention discloses a method for synthesizing perfluoroisobutyronitrile. According to the method, industrialized products including hexafluoropropylene and chloroformic ester are used as raw materials to prepare a product; a reaction route comprises the following steps: synthesizing heptafluoroisobutyrate through the hexafluoropropylene and the chloroformic ester under the action of fluoride through a one-pot method; taking the heptafluoroisobutyrate and ammonia to react to obtain heptafluoroisobutyramide; dehydrating the heptafluoroisobutyramide through a dehydrating agent, and rectifyingand purifying to obtain the perfluoroisobutyronitrile (2,3,3,3-tetrafluoro-2-trifluoromethylpropionitrile). According to the method disclosed by the invention, the used raw materials are commerciallyavailable; the main raw materials including the chloroformic ester and the hexafluoropropylene have low cost and can be abundantly supplied; reaction conditions are moderate and the reaction conversion rate and the yield are high; the product of each step is easy to separate and the purity of the product is high; the method has the advantages of convenience and safety in operation of a technologyand easiness for realizing industrial production.
Owner:昊华气体有限公司 +1
Biobased Carbon Fibers and Carbon Black and Methods of Making the Same
ActiveUS20190002293A1Transportation safetyOrganic compound preparationCarboxylic acid amides preparationFiberCarbon fibers
Bio-based materials, e.g., epoxide starting material, a beta-lactone starting material and / or a beta-hydroxy amide starting material, may be used as feedstocks in processes for making and using acrylonitrile and acrylonitrile derivatives to produce, among other products, carbon fibers and carbon black.
Owner:NOVOMER INC
Perfluoro nitrile preparation method
ActiveCN107935884ALow priceImprove solubilityOrganic compound preparationCarboxylic acid amides preparationCarbonyl fluorideMetallole
The invention discloses a perfluoro nitrile preparation method. The perfluoro nitrile preparation method comprises the following steps: an addition reaction is performed on perfluoroolefine R1R2C=CR3R4 and carbonyl fluoride to obtain acyl fluoride R1R2(COF)C-CFR3R4(the general formula of R1,R2,R3 and R4 is -CnF(2n+1) group, and n is a nonnegative integer set); b, performing a reaction on acyl fluoride R1R2(COF)C-CFR3R4, acyl fluoride and an alkali metal amide or an amino compound R-NH2(R is Li, Na, K, Rb, Cs or -CmH(2m+1) group, and m is a nonnegative integer set) to obtain amide R1R2(CONH2)C-CFR3R4; and c, performing a dehydration reaction on amide R1R2(CONH2)C-CFR3R4 to obtain perfluoro nitrile R1R2(CN)C-CFR3R4. The perfluoro nitrile preparation method has a short reaction path, can easily obtain perfluoroolefine and carbonyl fluoride, is low in cost and high in overall yield of perfluoro nitrile, and the reaction path is easy to industrialize.
Owner:泉州宇极新材料科技有限公司
Method for preparing perfluoro-isobutyronitrile and intermediates thereof
InactiveCN109748814AHigh yieldEasy to operateOrganic compound preparationCarboxylic acid amides preparationOrganic solventWastewater
The invention discloses a method for preparing perfluoro-isobutyronitrile. The method includes carrying out reaction on perfluoro-isobutyryl fluoride and ammonia solution or ammonia gas in water or organic solvents to generate perfluoro-isobutyramide at first; dehydrating the perfluoro-isobutyramide by dehydrating agents to obtain the perfluoro-isobutyronitrile. The method has the advantages of novel raw materials and routes, simplicity in reaction operation, high product yield and low waste gas, wastewater and industrial residue quantities.
Owner:ZHEJIANG LANTIAN ENVIRONMENTAL PROTECTION HI TECH +1
Process for preparing nitriles by elimination reactions
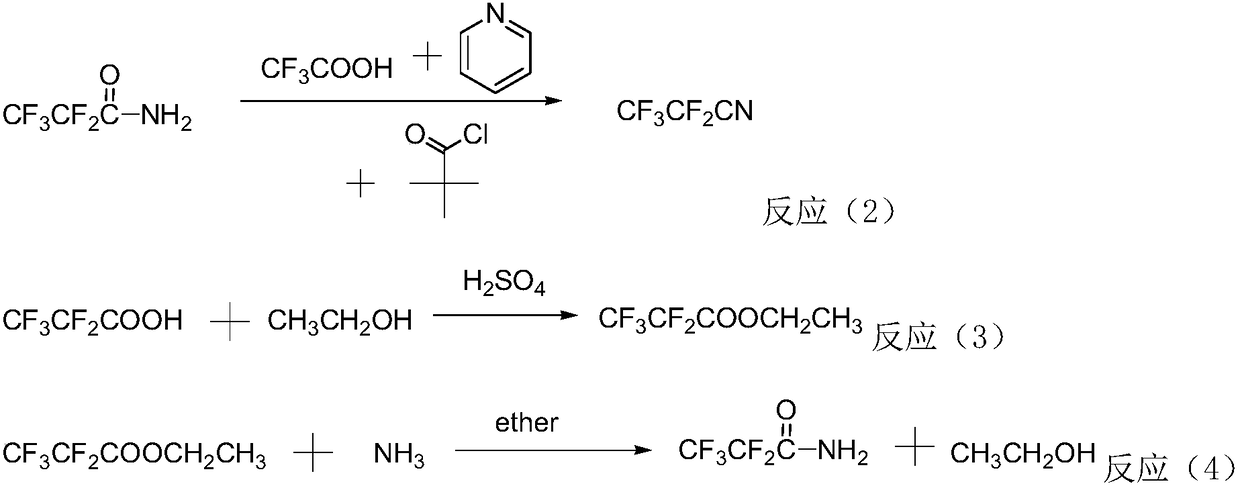
ActiveUS7939688B2Readily water-solubleSimple phase separationOrganic compound preparationPreparation by carboxylic acid amide dehydrationArylOrganic solvent
Process for preparing nitriles by reacting N-alkylcarboxamides (RCO—NHR1) or ammonium salts of carboxylic acids (RCOO—NH3R1+) or carboxylic acids in the presence of alkylamines or ammonium salts (RCOOH+NH2R1, RCOOH+NH3R1+), respectively, R being an arbitrarily substituted linear or branched C1-C12-alkyl radical, a C3-C12 cycloalkyl radical or an alkenyl, alkynyl or aryl or heteroaryl radical and R1 being an arbitrary substituted, linear or branched C2-C1 alkyl radical, a C3-C12 cycloalkyl radical or an alkenyl or alkynyl radical, with phosphonic anhydrides in the presence of an optional base in an organic solvent at a temperature in the range from −30 to 180° C. In advantageous embodiments, the phosphonic anhydride is a 2,4,6-substituted 1,3,5,2,4,6-trioxatriphosphinane 2,4,6-trioxide of the formula (I)
Owner:EUTICALS
Process for the preparation of 2-hydroxybenzonitrile
InactiveUS6248917B1Physical/chemical process catalystsOrganic compound preparationGas phaseOrganic chemistry
2-Hydroxybenzonitrile is prepared by passing 2-hydroxybenzamide in the gas phase over a heterogeneous catalyst thereby effecting dehydration of the 2-hydroxybenzamide to produce 2-hydroxybenzonitrile.
Owner:ALZCHEM TROSTBERG
Preparation method of trifluoroacetonitrile
InactiveCN102746190AReduce pollutionHigh selectivityPreparation by carboxylic acid amide dehydrationHeat transmissionSolid reaction
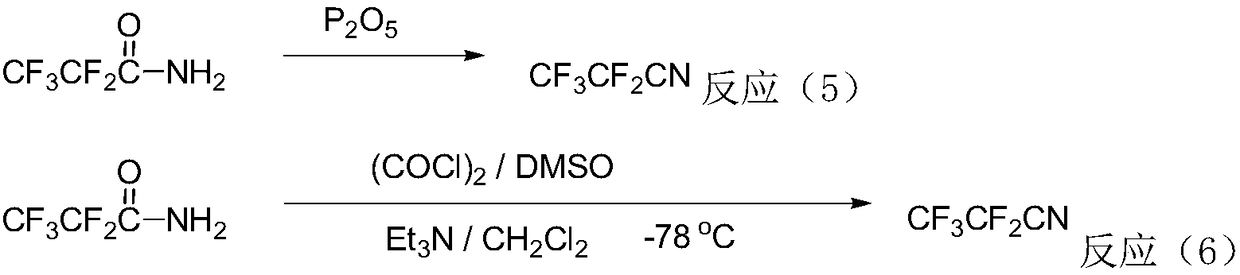
The invention relates to a preparation method of the trifluoroacetonitrile which is an important chemical intermediate with wide usages. The preparation method includes trifluoroacetamide serves as a raw material, a polyphosphoric acid is chosen as a solvent under the action of a dehydrating agent of phosphorus pentoxide, and the trifluoroacetonitrile is obtained through dehydration reaction. According to the preparation method of the trifluoroacetonitrile, problems of heat transmission and the like in solid-solid reaction are solved, the process is simplified and the cost is reduced by choosing an appropriate dehydrating agent, the resultant yield and the resultant purity are high, and the method is suitable for industrial production.
Owner:JIANGSU TETRA NEW MATERIAL TECH
Environment friendly method for preparing DMF (Dimethyl Formamide) solution of 2-hydroxy-benzonitril, DMF solution of 2-hydroxy-benzonitril and application thereof
ActiveCN102516122AReduce manufacturing costOrganic compound preparationPreparation by carboxylic acid amide dehydrationInorganic saltsWastewater
The invention discloses an environment friendly method for preparing a DMF (Dimethyl Formamide) solution of 2-hydroxy-benzonitril, which is characterized in that triphosgene and salicylamide are respectively added into DMF to be fully reacted. With the preparation method disclosed by the invention, more than 98% of 2-hydroxy-benzonitril (excluding solvent) can be obtained, the chemical conversion and the selectivity are both greater than 98%, and no problems exist if the 2-hydroxy-benzonitril is directly applied for the next step of reaction. In the process of production, no polluting waste gas and waste water are discharged, scraps are inorganic salts which can directly used or organic amine hydrochlorides which can be recycled, the comprehensive production cost of the 2-hydroxy-benzonitril is greatly reduced, and the production process is environment friendly.
Owner:上海禾本药业股份有限公司
Method for producing hexamethylenediamine key intermediate 6-aminocapronitrile by continuous gas-phase two-step method
ActiveCN111574401AReduced Catalytic PolymerizationReduce polymerizationOrganic compound preparationCarboxylic acid amides preparationAminocapronsäurePtru catalyst
The invention provides a method for producing a hexamethylenediamine key intermediate 6-aminocapronitrile by a continuous gas-phase two-step method. The method comprises the following steps: S1, mixing caprolactam and water in a gas phase state to carry out continuous hydrolysis or mixing caprolactam and ammonia water in a gas phase state to carry out continuous hydrolysis ammoniation reaction toobtain a first product system containing 6-aminocaproic acid, 6-aminocaproic acid ammonium salt and / or 6-aminocaproamide; and S2, carrying out continuous gas-phase catalytic ammoniation and dehydration reaction on the first product system and ammonia gas to obtain a second product system containing 6-aminocapronitrile. According to the patent of the invention, the problem that water generated by reaction in a one-step process promotes polymerization reaction of the raw material caprolactam is reduced from the source, and the selectivity of aminocapronitrile is improved; and the problems of catalyst deactivation and pressure drop rise caused by polymerization and coking of caprolactam in the reactor are effectively reduced, the stability of the device is improved, and the service life of the catalyst is prolonged.
Owner:JIANGSU YANGNONG CHEM GROUP +2
Production technology for environment-friendly insulating gas and industrial production device
ActiveCN106986757AImprove economyReduce manufacturing costOrganic compound preparationPreparation by carboxylic acid amide dehydrationChemistryFluorocarbon
The invention discloses a production technology for an environment-friendly insulating gas and an industrial production device. The production technology comprises the following steps: 1) performing contact reaction on oxygen and symmetric perfluoroolefine under a catalyst condition or catalyst-free condition in a first fixed bed reactor; 2) mixing the gas product flowing from the first fixed bed reactor with gaseous olefin, and then entering into a second fixed bed reactor and performing contact reaction under the catalyst effect in the fixed bed reactor, thereby generating fluoroketone compounds or perfluorocarbon fluorocarbons; and 3) scrubbing the fluoroketone compounds, thereby acquiring a corresponding fluoroketone environment-friendly insulating gas; performing ammonolysis treatment on the perfluorocarbon fluorocarbons and then converting into a perfluorinated nitrile environment-friendly insulating gas under the effect of a dewatering agent or the catalyst in a third fixed bed reactor. The production technology disclosed by the invention is characterized by simple reaction condition, high atom economical efficiency, low cost and continuous and easy large-scale production.
Owner:STATE GRID CORP OF CHINA +3
Synthesis method for pesticide intermediate trifluoroacetonitrile
InactiveCN103804231AHigh yieldEase of industrial productionPreparation by carboxylic acid amide dehydrationSynthesis methodsSilicon tetrachloride
The invention provides a synthesis method for a pesticide intermediate trifluoroacetonitrile. The synthesis method comprises the following steps: adding trifluorofluoroacetamide, carbon tetrachloride and trifluoroacetic anhydride into a gas reaction kettle; raising the temperature to 150-180 DEG C; dehydrating and reacting; and cooling to obtain the trifluoroacetonitrile. A reaction formula is as follows: CF3CONH2+PPh3+CCl4->CF3C(Gl)=NH+O=PPh3+CHCl3CF3C(Cl)=NH+PPH3->CF3CN+PPh3+HCl.
Owner:JIANGSU INST OF ECOMONES
Method for preparing perfluorinated nitrile through gas phase catalysis
ActiveCN109320436AHigh yieldReduce industrial synthesis costsOrganic compound preparationCarboxylic acid amides preparationGas phaseEthylene oxide
The invention discloses a method for preparing perfluorinated nitrile through gas phase catalysis. The method comprises the following steps: a, in the absence of a catalyst, enabling acyl fluoride R1COF or perfluor substituted ethylene oxide Cyclo-CF2-CR2R3-O- to perform a gas phase amination reaction with an ammonia gas or a primary amine compound, to obtain amide of which a general formula is R1CONH2 or CR2R3FCONH2, wherein general formulas of R1, R2 and R3 are CnF[2n+1], CxF[2x+1], and CyF[2y+1], wherein x and y are non-negative integer sets, x+y=n, and n is a positive integer set; and b, in the presence of the catalyst, dehydrating the amide R1CONH2, to obtain the perfluorinated nitrile R1CN. The method is short in reaction route, and the perfluor substituted ethylene oxide or the acylfluoride is easily obtained. A total yield of the perfluorinated nitrile is high, and the route is easy for continuous industrialization.
Owner:陕西宇极新材料科技有限公司
Catalyst, preparation method thereof and method for synthesizing hexamethylenediamine intermediate
PendingCN111659374AHigh catalytic activityHigh selectivityOrganic compound preparationCatalyst activation/preparationPtru catalystHexamethylenediamine
The invention provides a catalyst, a preparation method thereof and a method for synthesizing a hexamethylenediamine intermediate. The catalyst is of a core-shell structure, the core of the core-shellstructure is a carrier, the carrier is selected from any one or more of magnesium oxide, silicon dioxide, calcium oxide and titanium oxide, and the shell layer of the core-shell structure is of a porous structure and comprises any one or more of aluminum oxide, zinc oxide, copper oxide and iron oxide. The core of the core-shell structure provides a loading effect for the shell layer; the shell layer of the core-shell structure comprises the active metal oxide component; therefore, the catalyst with the specific core-shell structure can simultaneously and respectively carry out catalytic reaction on the core and the shell layer and the thus catalytic activity of catalyst is improved, especially for a continuous step-by-step catalytic reaction system, the catalyst with the core-shell structure is beneficial to respectively carrying out different catalytic steps of the catalytic reaction system on the core and the shell of the catalyst so that the mutual influence among different catalytic steps is reduced, and byproducts of the catalytic reaction system are reduced.
Owner:JIANGSU YANGNONG CHEM GROUP +3
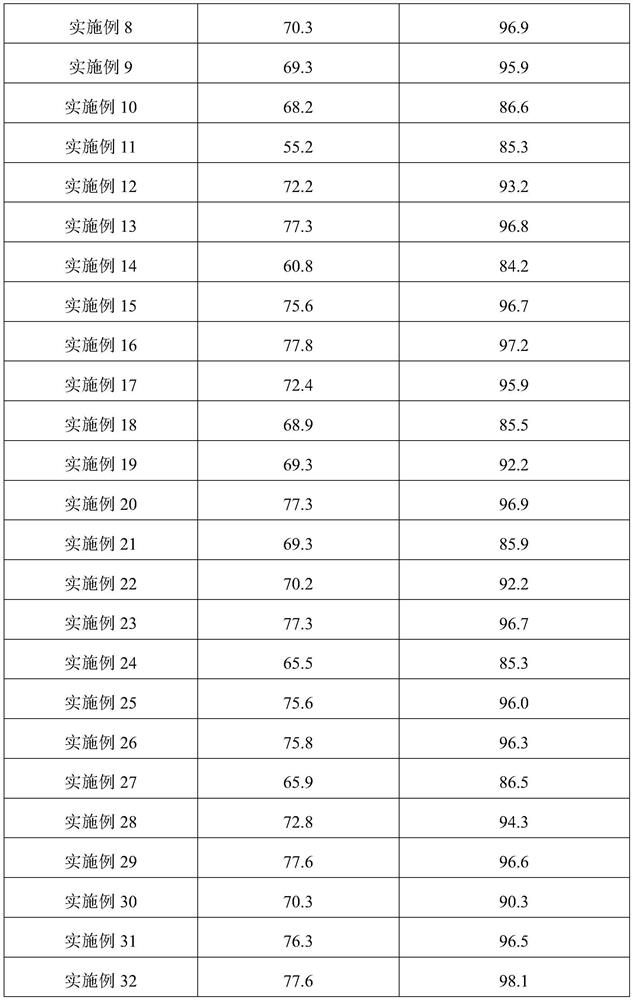
Process for producing p-phenyl cyanophenyl
InactiveCN101429137AHigh purityHigh yieldPreparation by carboxylic acid amide dehydrationAminolysisChloride
The invention provides a method for preparing terephthalic benzonitrile. The method comprises the steps of taking biphenyl and trichloro-acetic chloride as raw materials and preparing theterephthalic benzonitrile through acylation, aminolysis and dehydration reaction. The method overcomes the disadvantages that the prior art is expensive in raw materials, high in intermediate toxicity, harsh in reaction conditions, low in reaction yield, poor in purity and the like, provides a terephthalic benzonitrile synthesizing process which is easy to industrialize, convenient to operate, high in yield and high in product purity, and provides a key raw material intermediate for producing the pigment red 264 with bright color and high quality.
Owner:CINIC CHEM SHANGHAI
Manufacturing method of nitrile and corresponding amine thereof
ActiveCN104557357AReduce dosageIncrease profitOrganic compound preparationPreparation by carboxylic acid amide dehydrationAmmoniaNH3 compound
The invention relates to a manufacturing method of nitrile. Compared with the prior art, the manufacturing method has the characteristics of significantly reduced using amount of an ammonia source, low environmental pressure, low energy consumption, low production cost, high purity and yield of a nitrile product and the like, and nitrile with a more complex structure can be obtained. The invention also relates to a method for manufacturing corresponding amine from nitrile.
Owner:SINOPEC YANGZI PETROCHEM +1
Malononitrile synthesis method
InactiveCN104945278AReduce dosageReduce pollutionPreparation by carboxylic acid amide dehydrationFiltrationSynthesis methods
The invention relates to a malononitrile synthesis method and belongs to the technical field of malononitrile synthesis. The malononitrile synthesis method comprises the following steps of 1, adding methanol or ethanol into a reactor, feeding ammonia gas into the reactor at a temperature of -20 to 50 DEG C, dropwisely adding methyl cyanoacetate or ethyl cyanoacetate into the reactor at a temperature of -20 to 50 DEG C, after dropwise addition, carrying out heat-preservation stirring, carrying out reduced pressure degasification until a temperature is reduced to below -10 DEG C and carrying out filtration and drying to obtain cyanoacetamide, and 2, mixing a dehydrant, a catalyst, cyanoacetamide and a solvent, heating the mixture for a reflux reaction lasting for 6h, carrying out cooling and filtration, removing the solvent to obtain a crude product, and carrying out reduced pressure rectification to obtain a malononitrile product. The preparation method has less reaction processes, can be operated simply, has a high yield and good product purity, utilizes cheap and easily available raw materials, greatly reduces a production cost, is free of an adsorbent, has a less phosphorous oxychloride use amount, greatly reduces three wastes, realizes easy recovery and recycle of the solvent, is suitable for industrial production and solves the problems of the existing a malononitrile synthesis method.
Owner:JINGZHOU HELE IND SCI & TECH CO LTD
Synthetic method of perfluoroisobutyronitrile
PendingCN111825568ASynthetic process safetyEfficient synthesis processOrganic compound preparationCarboxylic acid esters preparationOxalyl fluoridePtru catalyst
Owner:福建省漳平市九鼎氟化工有限公司
Method for preparing hexamethylenediamine key intermediate 6-aminocapronitrile by two-step method
ActiveCN111662210AAvoid cokingImprove conversion rateOrganic compound preparationCarboxylic acid amides preparationPtru catalystHexamethylenediamine
The invention provides a method for preparing a hexamethylenediamine key intermediate 6-aminocapronitrile by a two-step method. The method comprises the following steps: step S1, in a first reactor, after caprolactam is subjected to a hydrolysis reaction in an aqueous solution, introducing first hot ammonia gas into the first reactor to obtain an aminocaproamide crude product, the temperature of the first hot ammonia gas being 150-250 DEG C; S2, performing catalytic dehydration on the aminocaproamide crude product and second hot ammonia gas in a second reactor to obtain aminocapronitrile, wherein the temperature of the second hot ammonia gas ranges from 200 DEG C to 300 DEG C. According to the invention, the polymerization of the hydrolysate is effectively controlled in the step S1 so thatpolymerization of the polymer on the surface of the catalyst when the aminocaproamide crude product participates in the catalytic dehydration reaction in the step S2 is effectively relieved, coking of the catalyst is avoided, the service life of the catalyst is prolonged, and then lasting high-conversion-rate and high-selectivity preparation of 6-aminocapronitrile from caprolactam is guaranteed.
Owner:JIANGSU YANGNONG CHEM GROUP +2
Method and device for producing hexamethylenediamine from caprolactam
ActiveCN112812020AHydrogenation benefitsControl not to be hydrogenatedAmino compound purification/separationOrganic compound preparationPtru catalystHexamethylenediamine
The invention discloses a method and a device for producing hexamethylenediamine from caprolactam, ammonia gas and caprolactam are subjected to ammoniation and dehydration reaction under the action of a catalyst, and the obtained ammoniation and dehydration reaction product is subjected to three-stage condensation separation; caprolactam obtained through secondary condensation and 6-aminocapronitrile condensate are subjected to a hydrogenation reaction under the action of a catalyst, a mixture containing hexamethylenediamine and caprolactam obtained through the reaction is separated to obtain hexamethylenediamine, and water accounting for 5%-500% of the total weight of the condensate is added in the hydrogenation reaction process to serve as a hydrogenation reaction diluent. According to the method, the caprolactam aqueous solution is used as the 6-aminocapronitrile hydrogenation reaction diluent, ethanol does not need to be added as the diluent, and generation of by-products N-Et-HMD, BHT and tar is reduced. The ammoniation dehydration reaction liquid is not subjected to rectification separation, so that the energy consumption is reduced.
Owner:宁波迦尔新材料技术有限公司
Preparation methods of nitrile and corresponding amine
ActiveCN105016942AReduce dosageIncrease profitOrganic compound preparationPreparation by carboxylic acid amide dehydrationAmmoniaNitrile
The invention relates to a preparation method of nitrile. Compared with the prior art, the preparation method has the characteristics of obvious reduction of the usage amount of ammonia sources, low environmental pressure, low energy consumption, low production cost, high purity and yields of nitrile products, and the like, and can be used for obtaining nitrile with a more complex structure. The invention also relates to a method for preparing corresponding amine with nitrile.
Owner:SINOPEC YANGZI PETROCHEM +1
Popular searches
Amino group formation/introduction Amino compound preparation Cyano group formation/introduction Carboxylic acid halides preparation Carbonyl compound preparation Carboxylic acid nitrile purification/separation Carbon preparation/purification Wet spinning methods Fibre chemical features Pigment treatment with organosilicon compounds
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com