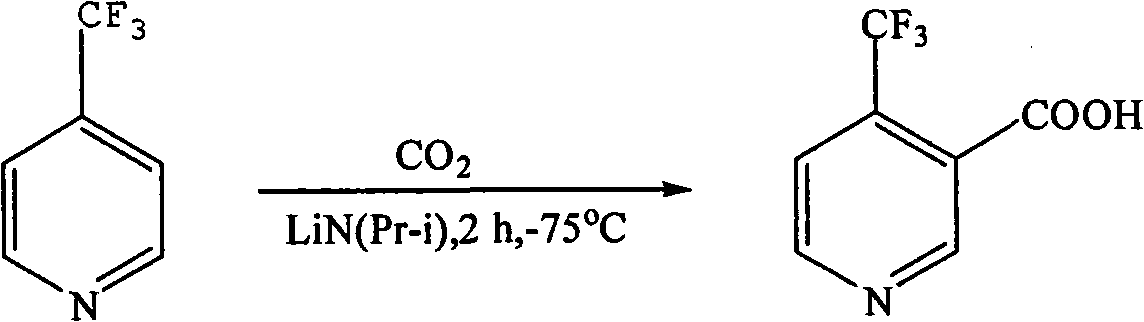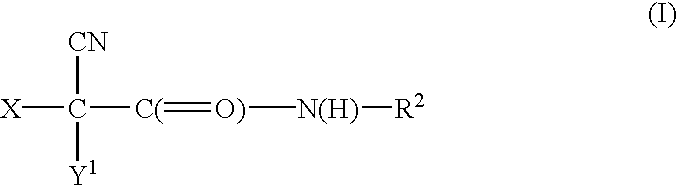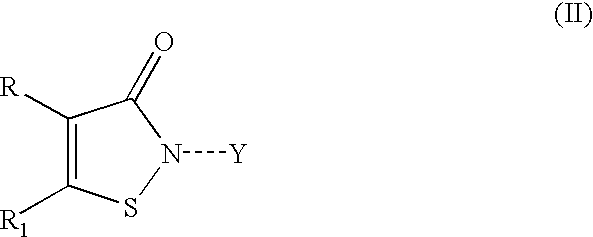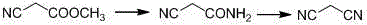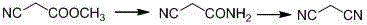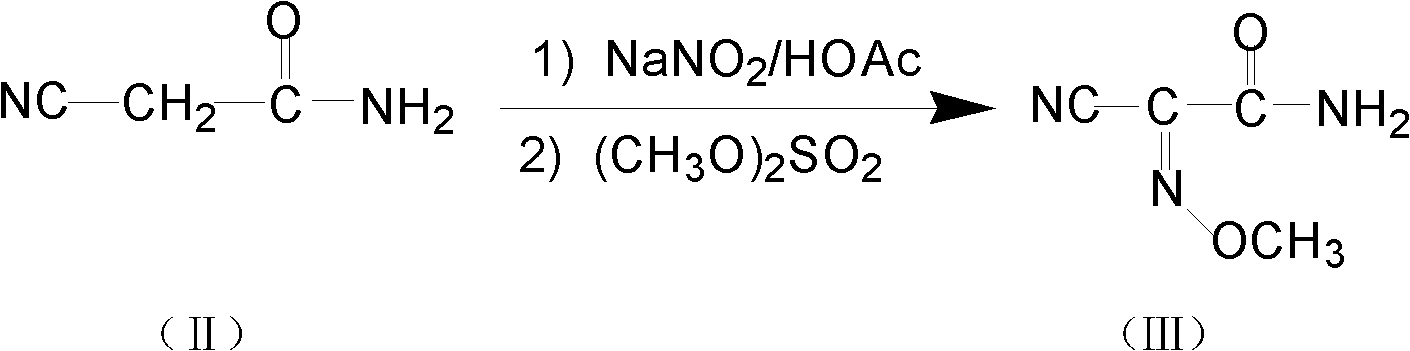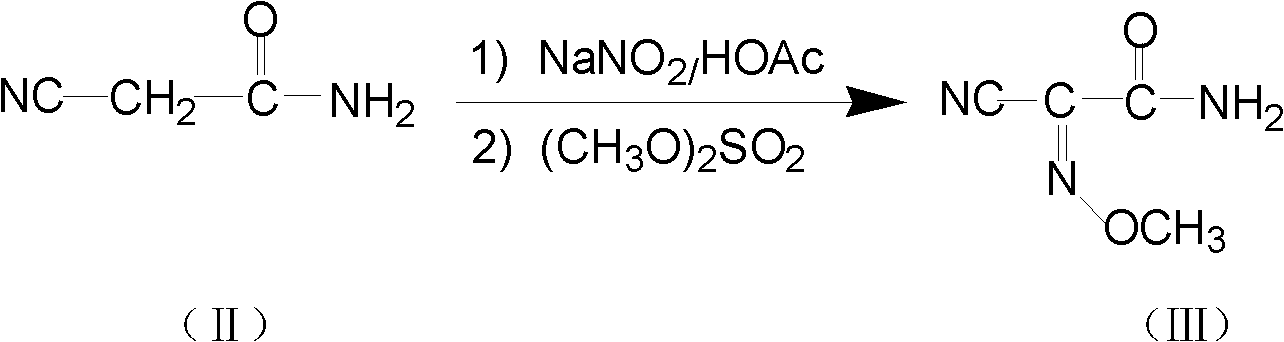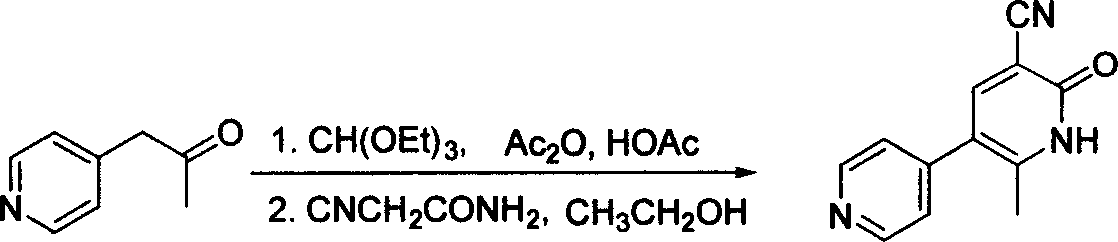Patents
Literature
128 results about "Cyanoacetamide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
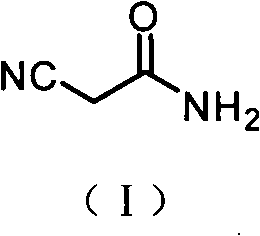
2-Cyanoacetamide is an organic compound. It is an acetic amide with a nitrile functional group.
Synthetic method of malononitrile
InactiveCN103044286AHigh purityImprove stabilityPreparation by carboxylic acid amide dehydrationPtru catalystDistillation
The invention discloses a synthetic method of malononitrile. Cyanoacetamide reacts with triphosgene in the presence of a catalyst to synthesize malononitrile, wherein the catalyst is any one substance or a mixture of multiple substances selected from N,N-dimethyl formamide, sodium chloride, pyridine and triethylamine. The method provided by the invention employs the triphosgene as a dehydrating agent, and the triphosgene is low in cost, available and high in stability, and facilitates storage and transportation; the reaction products of the cyanoacetamide and the triphosgene only include carbon dioxide and hydrogen chloride gases except for the malononitrile, without solid waste, and therefore, after the reaction is completed, the steps of removing the solid waste by means of filtering, centrifuging and the like are not needed; the reaction mixture is directly subjected to reduced pressure distillation after the solvent is recovered so that the malononitrile having the purity of higher than 98% can be obtained; and the posttreatment is simple.
Owner:CHONGQING UNISPLENDOUR CHEM
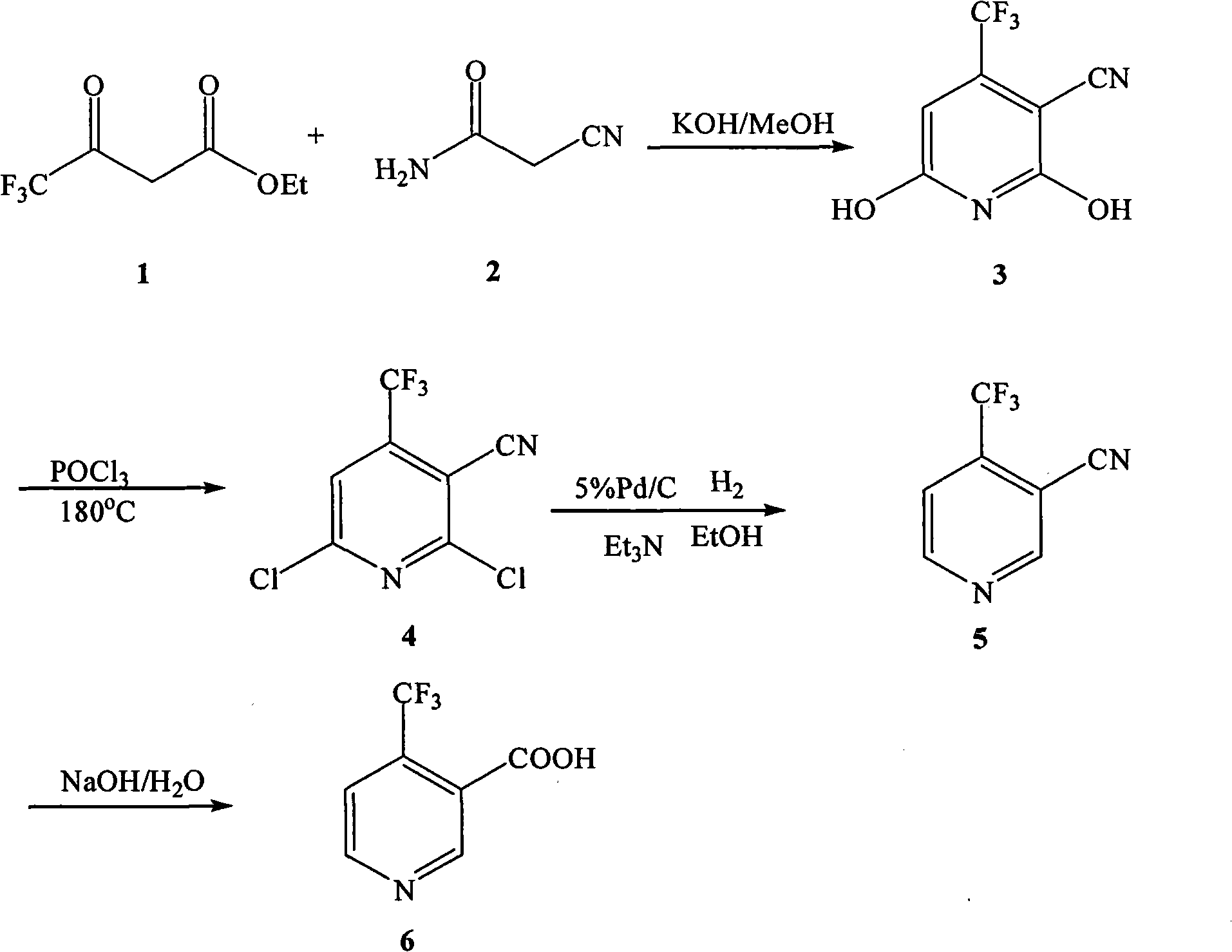
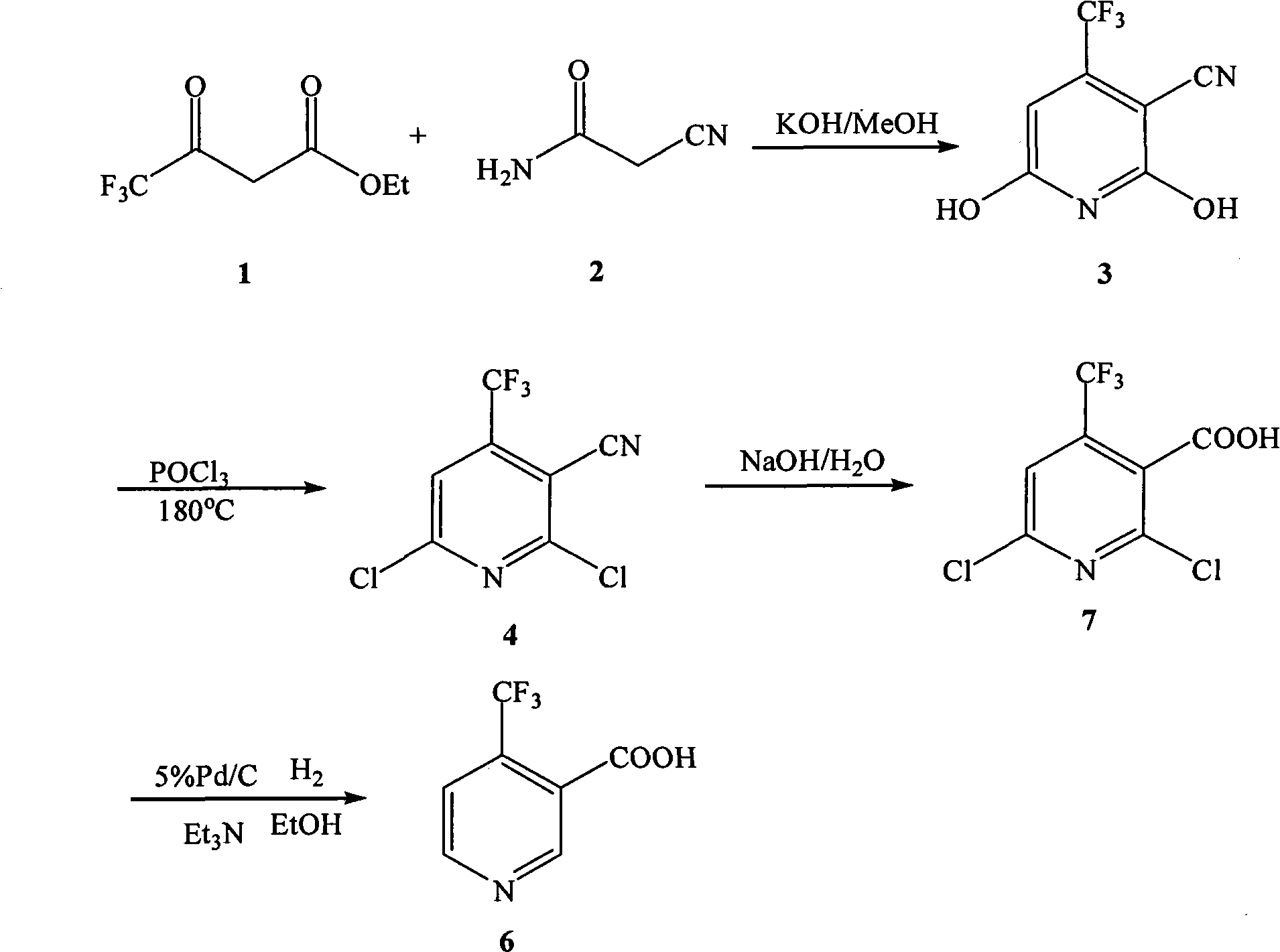
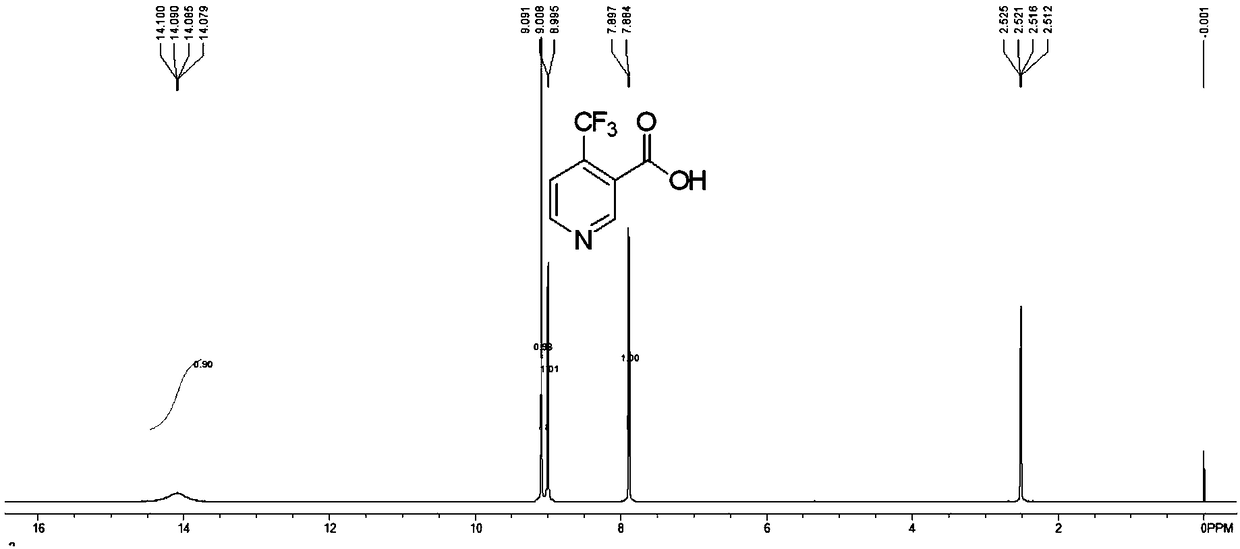
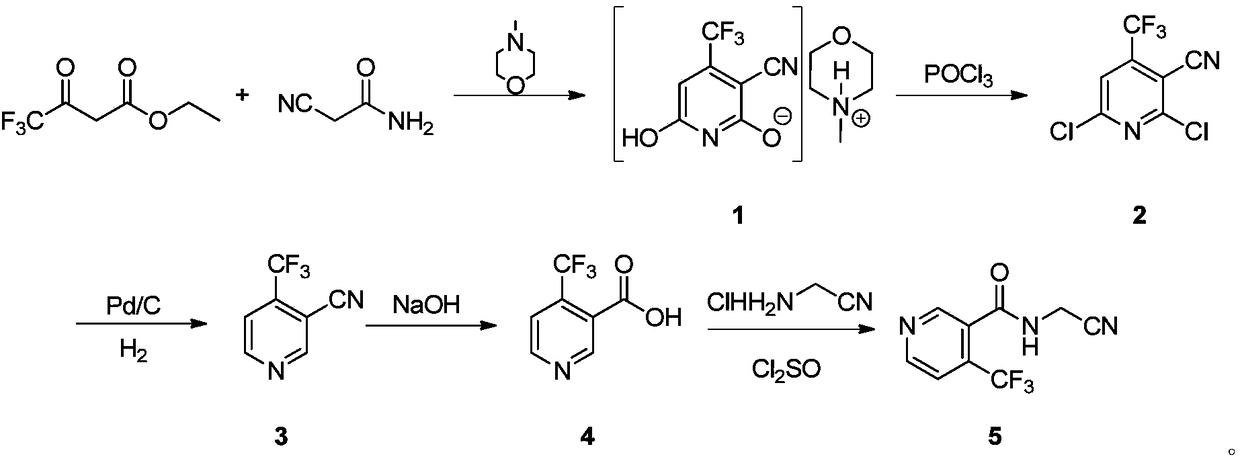
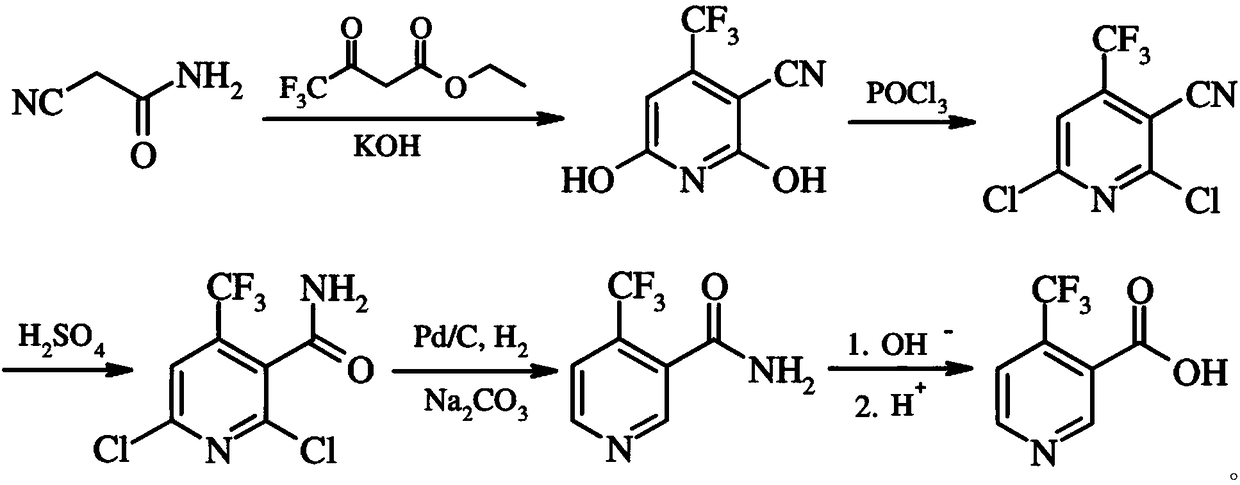
Preparation method of 4-trifluoromethyl nicotinic acid
InactiveCN101851193AEmission reductionImprove securityOrganic chemistryAcetic acidPotassium hydroxide
The invention discloses a preparation method of 4-trifluoromethyl nicotinic acid, and relates to a preparation method of a compound, which comprises the following steps: taking 4,4,4-trifluoroacetoacetate and cyanoacetamide as raw materials, taking potassium hydroxide as a catalyst to obtain 2,6-dihydroxy-3-cyano-4-trifluoromethyl pyridine by cyclization, then using POCl3 to chloridize to obtain 2,6-chloro-3-cyano-4-trifluoromethyl pyridine, and finally carrying out Pd / C catalytic hydrogenolysis or hydrogenolysis and then hydrolyzing or hydrolyzing and then carrying out Pd / C catalytic hydrogenolysis to obtain the target product 4-trifluoromethyl nicotinic acid. The invention provides a feasible and safe preparation method of the 4-trifluoromethyl nicotinic acid, which has certain economic advantages and can be applied to industrial production.
Owner:XIHUA UNIV
Halocyanoacetamide antimicrobial compositions
A stabilized halocyanoacetamide composition in the form of a solution or emulsifiable concentrate using selected ester solvents is disclosed. Preferred ester solvents include glyceryl triacetate, ethyl acetate and diethyl phthalate. Particularly preferred are stabilized compositions containing 2,2-dibromo-3-nitrilopropionamide, glyceryl triacetate and optional non-ionic surfactants or additional antimicrobial compounds, particularly 3-isothiazolone compounds.
Owner:GIRONDA KEVIN F +5
Process For Producing Flexible Polyurethane Foam
A composition and process useful to make flexible polyurethane foams and in particular flexible molded polyurethane foams is disclosed. The usage of dipolar aprotic liquids such as DMSO, DMI, sulfolane, N-methyl-acetoacetamide, N,N-dimethylacetoacetamide as well as glycols containing hydroxyl numbers OH#≦1100 as cell opening aides for 2-cyanoacetamide or other similar molecules containing active methylene or methine groups to make a polyurethane foam is also disclosed. The advantage of using cell opener aids results in a) no foam shrinkage; b) lower use levels of cell opener; c) foam performance reproducibility d) optimum physical properties. In addition, combining the acid blocked amine catalyst together with the cell opener and the cell opener aid results in a less corrosive mixture as well as provides a method that does not require mechanical crushing for cell opening.
Owner:EVONIK OPERATIONS GMBH
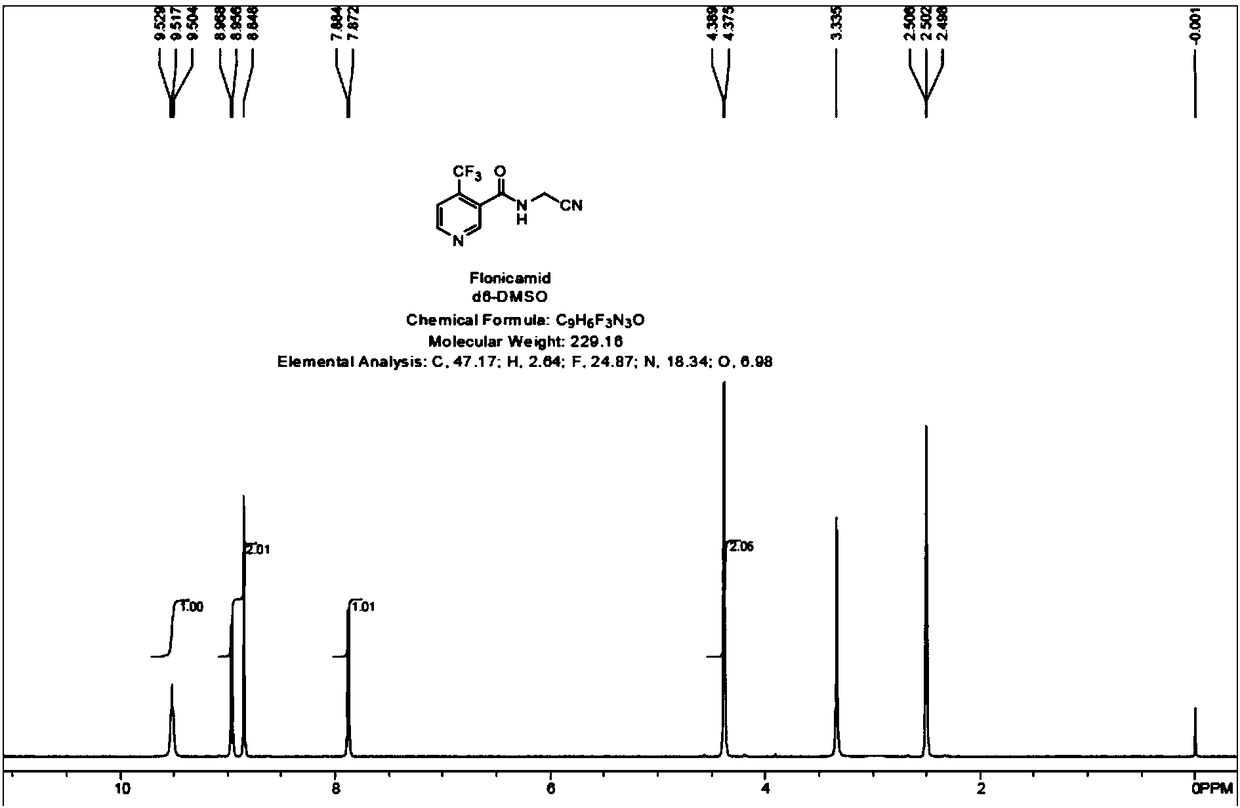
Preparation methods of flonicamid and intermediate 4-(trifluoromethyl)nicotinic acid thereof
The invention relates to preparation methods of flonicamid and an intermediate 4-(trifluoromethyl)nicotinic acid thereof. The preparation method of 4-(trifluoromethyl)nicotinic acid comprises the steps that cyanoacetamide, ethyl 4,4,4-trifluoroacetoacetate and organic alkali serve as the raw materials, 2,6-dyhydroxy-3-cyano-4-tirfluoromethylpyridine N-methylmorpholine salt is prepared first, and then phosphorus oxychloride chlorination, catalytic hydrogenation and hydrolyzation are conducted in sequence to obtain 4-(trifluoromethyl)nicotinic acid; the preparation method of the flonicamid comprises the steps that 4-(trifluoromethyl)nicotinic acid is prepared and then mixed with glycinonitrile hydrochloride, and after an amidation reaction is conducted, the flonicamid is obtained. Compared with the prior art, the total recovery of the prepared flonicamid is high, the cost is low, operation is easy, the equipment requirement is low, and the requirement of industrial production can be met;the technological process is simple, separation and purification are easy, and the reaction condition is mild.
Owner:SHANGHAI HETENG FINE CHEM
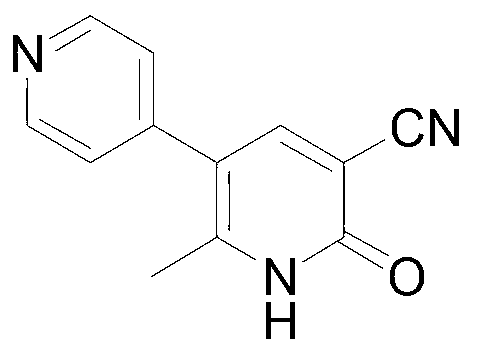
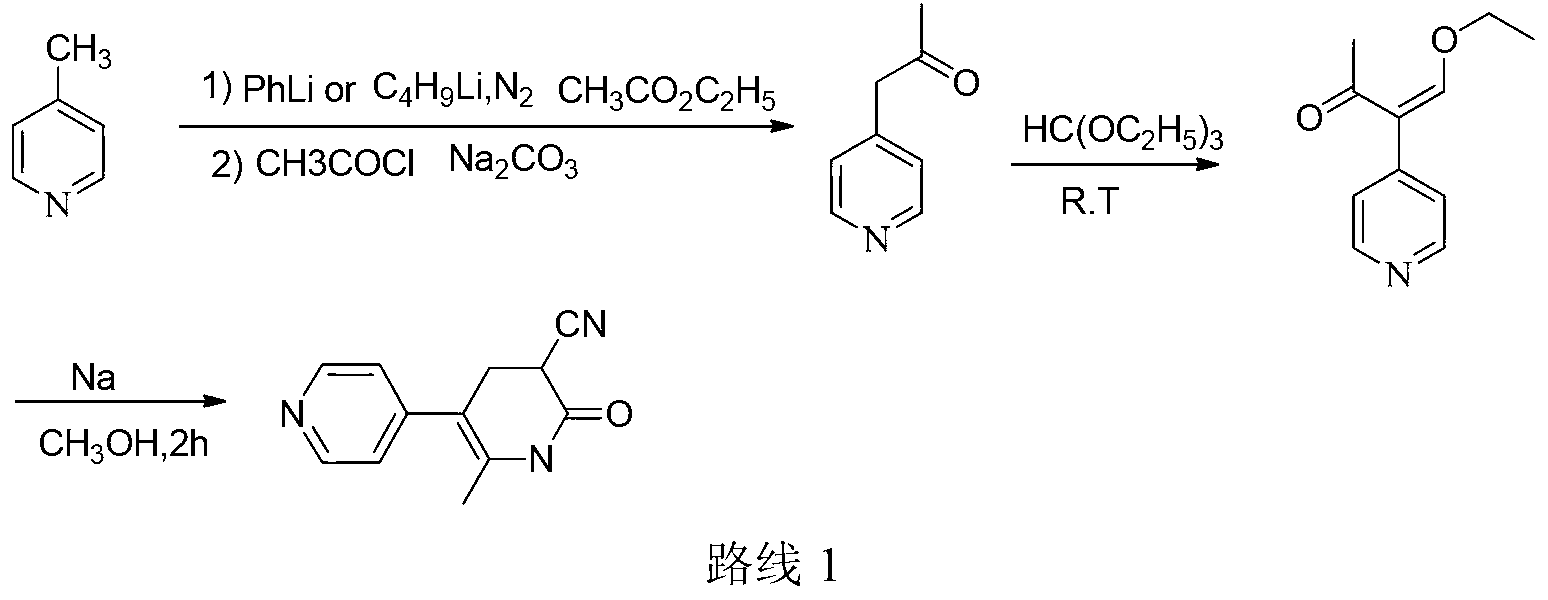
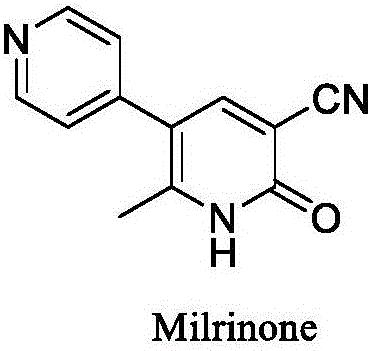
Method for synthesising milrinone
ActiveCN103288725ALow costThe number of times of extraction and decompression concentration is reducedOrganic chemistryAcetic anhydrideSodium bisulfate
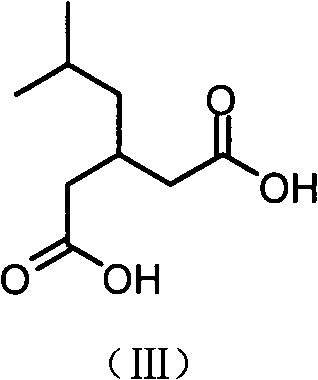
The invention discloses a method for synthesising milrinone. The method comprises the following steps of: mixing 4-methylpyridine with acetylchloride in a solvent at a temperature below 10 DEG C; heating to react with or without a catalyst, and then adjusting the pH value of the reaction solution to 7-8 by sodium hydroxide aqueous solution; then directly adding saturated sodium hydrogen sulfite aqueous solution, and reacting; adjusting the pH value of the water layer obtained by the reaction by sodium hydroxide, and reacting to obtain a compound in formula (III); mixing the compound in formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and then reacting at 50-100 DEG C to obtain a compound in formula (IV); cyclizing the compound in formula (IV) with alpha-cyanoacetamide in an alkaline condition to obtain a compound in formula (V). The process is more moderate in reaction conditions, simpler and more convenient to operate, and capable of greatly shortening the original reaction time, reducing cost and increasing yield simultaneously; the process is a preparation method for milrinone which is suitable for industrialized production.
Owner:NANJING KING FRIEND BIOCHEM PHARMA CO LTD
Preparation method for high-purity milrinone
ActiveCN104387320AQuality improvementImprove crystal effectOrganic chemistryAcetic anhydrideSingle crystal
The invention discloses a preparation method for high-purity milrinone (shown as a formula (I), 1,6-dihydro-2-methyl-6-oxo-3,4-bipyridine-5-carbonitrile), and belongs to the field of chemical medicines. The method comprises: employing 4-methylpyridine as a raw material and acetylating with acetyl chloride, and hydrolyzing after the reaction is finished, so as to obtain a compound of a formula (III); mixing the compound of the formula (III) with glacial acetic acid, acetic anhydride and triethyl orthoformate, and reacting at 35 DEG C-45 DEG C, so as to obtain a compound of a formula (IV); performing cyclization on the compound of the formula (IV) and alpha-cyanoacetamide, so as to obtain a crude product of a compound of the formula (I); and refining the crude product of the formula (I) compound through an ethanol-water system, so as to obtain a high-purity refined product with the maximum interplanar spacing d of 8.39 + / - 0.02 Angstrom. The technology is relatively mild in reaction conditions and relatively simple in operation, and is capable of preparing the milrinone product with high purity and a single crystal form. The obtained milrinone crystal form is relatively excellent in solubility in normal saline or glucose, and is beneficial for improvement of the preparation quality.
Owner:HUZHOU ZHANWANG PHARMA
Process For Producing Flexible Polyurethane Foam Using Natural Oil Polyols
ActiveUS20130197114A1Low usage levelLess corrosive mixtureOther chemical processesSulfolaneMechanical crushing
A composition and process useful to make flexible polyurethane foams and in particular flexible molded polyurethane foams is disclosed. The usage of dipolar aprotic liquids such as DMSO, DMI, sulfolane, N-methyl-acetoacetamide, N,N-dimethylacetoacetamide as well as glycols containing hydroxyl numbers OH#≦1100 as cell opening aides for 2-cyanoacetamide or other similar molecules containing active methylene or methine groups to make a polyurethane foam is also disclosed. The advantage of using cell opener aids results in a) no foam shrinkage; b) lower use levels of cell opener; c) foam performance reproducibility d) optimum physical properties. In addition, combining the acid blocked amine catalyst together with the cell opener and the cell opener aid results in a less corrosive mixture as well as provides a method that does not require mechanical crushing for cell opening.
Owner:EVONIK OPERATIONS GMBH
Viscosity stabilizer for oil-displacing polymer solution and preparation method of stabilizer
PendingCN110724513AGood viscosity stabilityEliminate the effect of viscosityDrilling compositionPropanoic acidAntioxidant
The invention discloses a viscosity stabilizer for an oil-displacing polymer solution and a preparation method of the viscosity stabilizer. The stabilizer composition includes a solvent and additives;the additives are at least two selected from the group consisting of an antioxidant, an anti-salt agent and a bactericide; the antioxidant is at least one selected from the group consisting of thioethanol, thiourea, thiodipropionic acid, sodium thiosulfate, potassium iodide, 2-hydroxyethylamine and acetone; the anti-salt agent is at least one selected from the group consisting of ethylenediaminetetraacetic acid or a salt thereof, 1-hydroxyethylidene-1,1-diphosphonic acid or a salt thereof, polyacrylic acid and sodium citrate; and the bactericide is at least one selected from the group consisting of N,N-dimethyldithiocarbamic acid sodium salt, methylene dithiocyanate, 2,2-dibromo-3-cyanoacetamide, 2-nitro-2-bromo-1,3-propanediol, glutaraldehyde and isothiazolinone. The stabilizer solves the problem of large viscosity loss of the polymer solution during polymer displacement.
Owner:DAQING PETROLEUM ADMINISTRATION +2
Malononitrile synthesis method
InactiveCN104945278AReduce dosageReduce pollutionPreparation by carboxylic acid amide dehydrationFiltrationSynthesis methods
The invention relates to a malononitrile synthesis method and belongs to the technical field of malononitrile synthesis. The malononitrile synthesis method comprises the following steps of 1, adding methanol or ethanol into a reactor, feeding ammonia gas into the reactor at a temperature of -20 to 50 DEG C, dropwisely adding methyl cyanoacetate or ethyl cyanoacetate into the reactor at a temperature of -20 to 50 DEG C, after dropwise addition, carrying out heat-preservation stirring, carrying out reduced pressure degasification until a temperature is reduced to below -10 DEG C and carrying out filtration and drying to obtain cyanoacetamide, and 2, mixing a dehydrant, a catalyst, cyanoacetamide and a solvent, heating the mixture for a reflux reaction lasting for 6h, carrying out cooling and filtration, removing the solvent to obtain a crude product, and carrying out reduced pressure rectification to obtain a malononitrile product. The preparation method has less reaction processes, can be operated simply, has a high yield and good product purity, utilizes cheap and easily available raw materials, greatly reduces a production cost, is free of an adsorbent, has a less phosphorous oxychloride use amount, greatly reduces three wastes, realizes easy recovery and recycle of the solvent, is suitable for industrial production and solves the problems of the existing a malononitrile synthesis method.
Owner:JINGZHOU HELE IND SCI & TECH CO LTD
Method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime
ActiveCN102093266AShort processReduce manufacturing costSulfonic acid esters preparationSulfonyl chlorideReaction intermediate
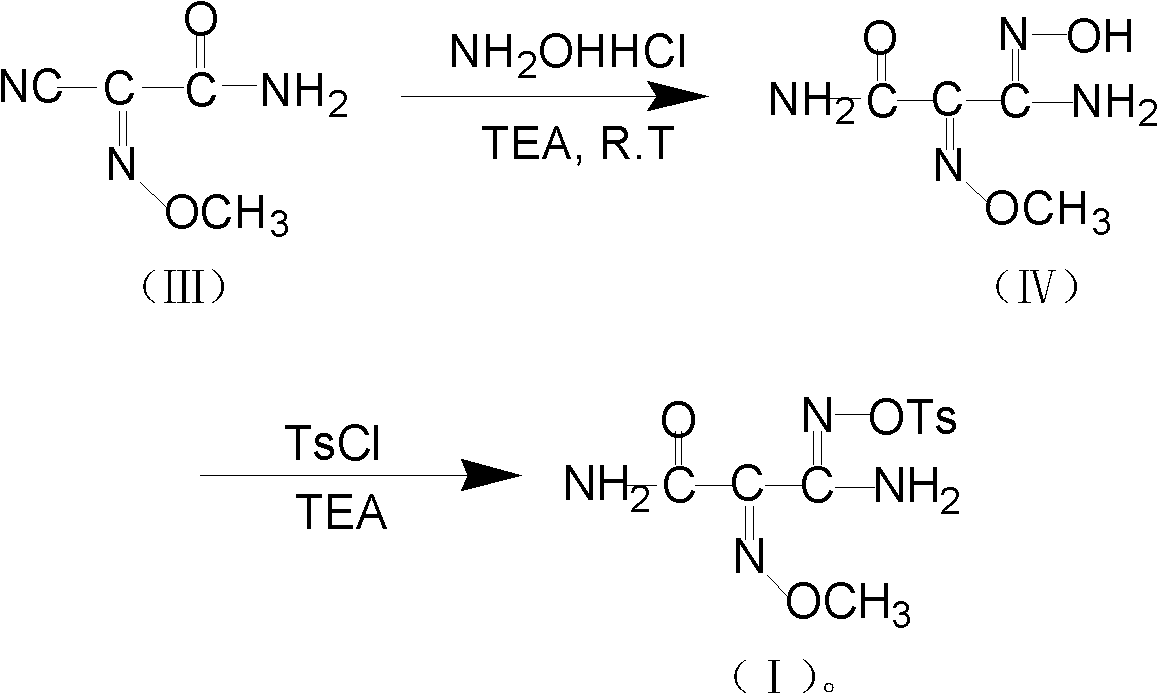
The invention relates to a method for preparing O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime, which comprises the following steps: (1) reacting cyanoacetamide, which is used as an initial raw material, with sodium nitrite in a nitrosation mode to obtain a reaction intermediate, and reacting the reaction intermediate with dimethyl sulfate in an esterification mode to obtain 2-cyano-2-methoxyl-imido-acetamide; and (2) under the action of hydroxylamine hydrochloride, hydrolyzing cyano groups in the 2-cyano-2-methoxyl-imido-acetamide obtained in the step (1) into amino groups, and reacting with p-methylbenzene sulfonyl chloride in an esterification mode to generate the O-tosyl-2-carbamoyl-2-methoxyl-imido-acetamido-oxime. The preparation method provided by the invention has the advantages of accessible raw materials, short technical process and high yield, and is suitable for industrial production.
Owner:BENGBU BBCA MEDICINE SCI DEV
Process for preparing milrinone
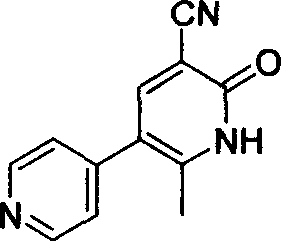
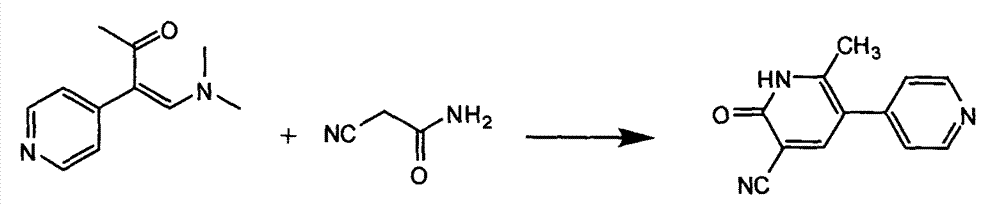
In accordance with the invention, 1-(4-pyridinyl)-propanone is used as raw material for condensation reaction with triethyl orthoformate, wherein the condensate directly reacts with the alpha-cyanoacetamide to obtain Milrinone without the need of purification. The prepared Milrinone is an important chemical product which can be used for preparing cardiotonic drugs.
Owner:LUNAN PHARMA GROUP CORPORATION
Preparation method of antiepileptic drug intermediate
InactiveCN102863326APromote precipitationEasy to operatePreparation from carboxylic acid esters/lactonesGlutaric acidCyanoacetic acid
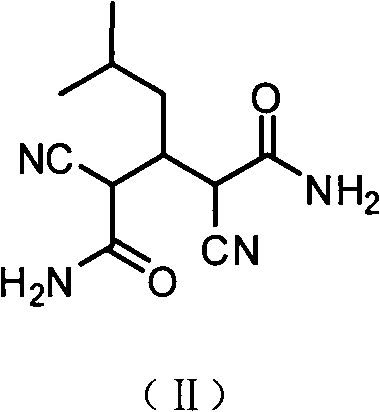
The invention relates to a preparation method of an antiepileptic drug intermediate 3-isobutyl glutaric acid. The preparation method includes that cyanoacetic acid ester ammonia is decomposed to obtain cyanoacetamide, condensation is performed on the cyanoacetamide and isovaleraldehyde to generate 2, 4-dicyan-3-isobutyl glutaric acid amide, the 2, 4-dicyan-3-isobutyl glutaric acid amide is hydrolysed and decarboxylated to obtain the 3-isobutyl glutaric acid, and total yield in the three steps can reach 77%. The preparation method of the antiepileptic drug intermediate is simple to operate, intermediate products and end products can be separated out easily from reaction, and the preparation method is low in cost and suitable for industrial production.
Owner:CHANGZHOU PHARMA FACTORY
3-aryl-2-cyanoacrylamide derivative as well as preparation method and application thereof
ActiveCN103965077AMild reaction conditionsEasy to operateCarboxylic acid nitrile preparationOrganic compound preparationBenzaldehydeSolvent
The invention discloses a 3-aryl-2-cyanoacrylamide derivative, a preparation method and application thereof, and belongs to the technical field of fluorescent material synthesis. The preparation method comprises the following steps: in an alcohols solvent, taking methoxyl substituted benzaldehyde as shown in formula (II) and cyanoacetamide as shown in formula (III) as raw materials, under catalysis of L-proline, performing a Knoevenagel condensation reaction to prepare the 3-aryl-2-cyanoacrylamide derivative. The preparation method has the advantages of mild reaction conditions, simple operation, convenient aftertreatment, low equipment requirements and high yield of the obtained product; the fluorescence color of the product after grinding can be changed, the color contrast ratio before and after change is high; in the meantime, the ground sample can be heated or suffocated with vapors of multiple organic solvents to recover the initial color, so that the 3-aryl-2-cyanoacrylamide derivative is high in reversible stimuli-responsive force-induced color changing performance, and can be applied to the sensor field, the anti-counterfeit field, the storage field, the display field, and the like.
Owner:山西鸿生化工股份有限公司
Preparation method and equipment of 4-trifluoromethyl-nicotinic acid
InactiveCN109232407AEasy to use raw materialsRaw materials are easy to getOrganic chemistryEthyl acetateHydrolysis
The invention discloses a preparation method of 4-trifluoromethyl-nicotinic acid. The preparation method comprises the following steps: taking cyanoacetamide, ethyl trifluoroacetoacetate and strong alkali as raw materials; firstly, preparing 2,6-dihydroxyl-3-cyano-4-tirfluoromethylpyridine; after carrying out chlorination through phosphorus oxychloride to obtain 2,6-dichloro-3-cyano-4-tirfluoromethylpyridine; then adding sulfuric acid to convert cyano into amino; and carrying out catalytic hydrogenation and hydrolysis to obtain the 4-trifluoromethyl-nicotinic acid. The preparation method has the advantages of high conversion rate and reduction of the cyano; the cyano is used as a strong electron suction group and has a carbon-nitrogen triple bond with relatively strong polarity; after thecyano is eliminated, the reduction rate can be remarkably reduced and the yield is improved; and the preparation method is worthy of being greatly popularized.
Owner:衢州凯沃化工有限公司
Additives for Improving Polyurethane Foam Performance
A composition and process useful to make flexible polyurethane foams and in particular flexible molded polyurethane foams is disclosed. The usage of dipolar aprotic liquids such as DMSO, DMI, sulfolane, N-methyl-acetoacetamide, N,N-dimethylacetoacetamide as well as glycols containing hydroxyl numbers OH#≦1100 as cell opening aides for 2-cyanoacetamide or other similar molecules containing active methylene or methine groups to make a polyurethane foam is also disclosed. The advantage of using cell opener aids results in a) no foam shrinkage; b) lower use levels of cell opener; c) foam performance reproducibility d) optimum physical properties. In addition, combining the acid blocked amine catalyst together with the cell opener and the cell opener aid results in a less corrosive mixture as well as provides a method that does not require mechanical crushing for cell opening.
Owner:EVONIK OPERATIONS GMBH
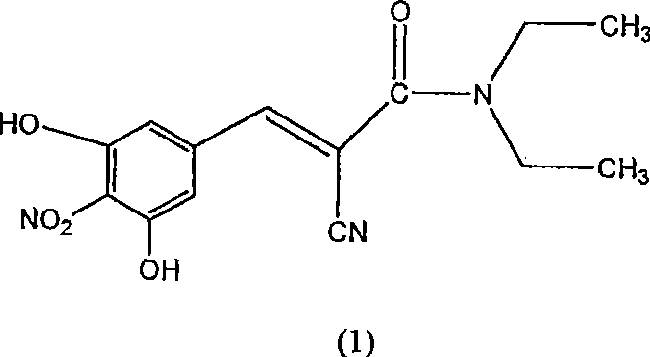
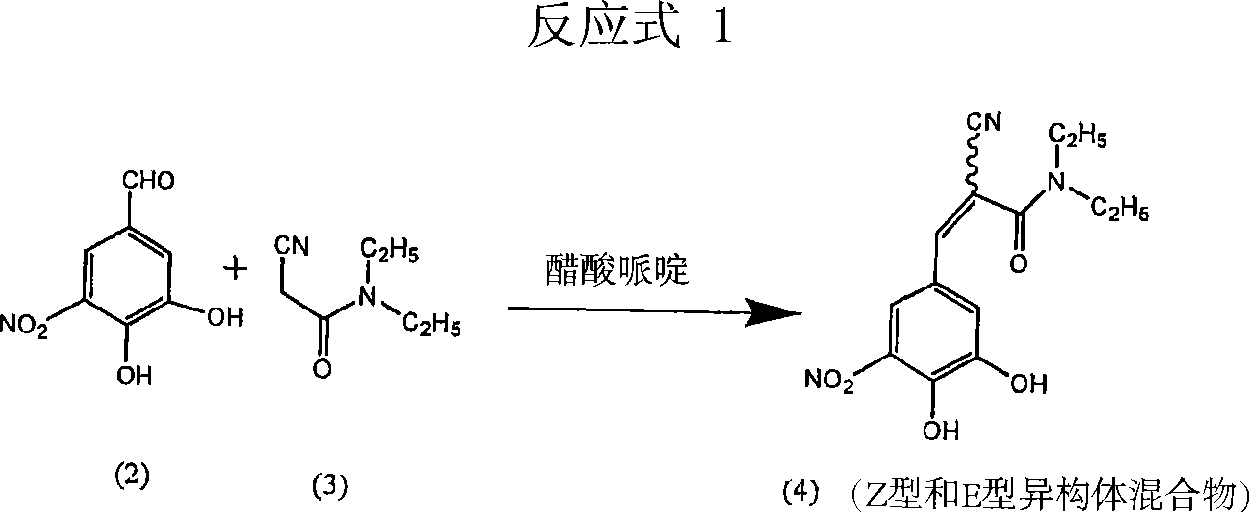
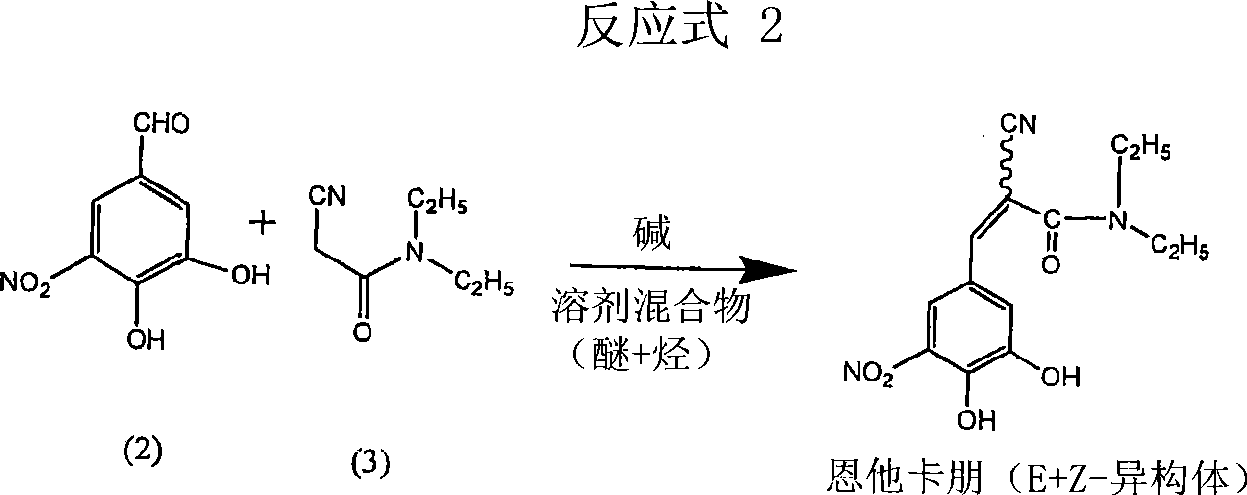
A process for the preparation of entacapone form-A
InactiveCN101460451ACarboxylic acid nitrile preparationOrganic compound preparationCorrosive acidSolvent
A process to prepare (2E) -2-cyano-3- (3 , 4-dihydroxy-5-nitrophenyl) -N, N-diethyl-2- propenamide (Entacapone) eliminating corrosive acids in the purification, with more than 99.5 % purity with a Z-isomer content of less than 0.1% comprising condensation of 3, 4-Dihydroxy{5-nitrobenzaldehyde with N,N-diethyl cyanoacetamide in the presence of a base selected from cyclic and acyclic secondary amines and a mixture of solvents, to obtain a crude product, stirring the crude product in a halogenated solvent, filtering and finally crystallization of polymorph A of Entacapone in a solvent.
Owner:ACTAVIS GRP PTC EHF
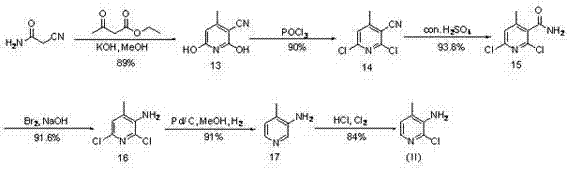
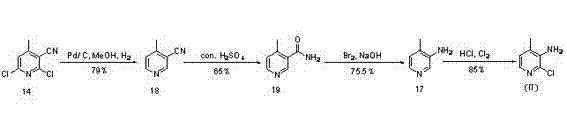
Novel synthesis method of Nevirapine key intermediate 2-chloro-3-amino-4-methylpyridine
InactiveCN102675193AWide variety of sourcesEasy to purifyOrganic chemistryMethyl palmoxirateAldehyde formation
The invention discloses a novel synthesis method of a Nevirapine key intermediate 2-chloro-3-amino-4-methyl pyridine and belongs to the pharmaceutical chemical field. The specific steps include that crotonic aldehyde and cyanoacetamide are subjected to ring closing reaction with the presence of strong alkaline reagents and oxygen to produce 2-hydroxy-3-cyano-4-methylpyridine; the 2-hydroxy-3-cyano-4-methylpyridine is reacted with phosphorus oxychloride to produce 2-chloro-3-amino-4-methylpyridine; the 2-chloro-3-amino-4-methylpyridine is hydrolyzed by concentrated sulfuric acid to obtain 2-chloro-3-formylamino-4-methylpyridine; and the 2-chloro-3-formylamino-4-methylpyridine is subjected to Hoffman amide degradation reaction to obtain the Nevirapine key intermediate 2-chloro-3-amino-4-methylpyridine. The technical scheme of the novel synthesis method of the Nevirapine key intermediate 2-chloro-3-amino-4-methyl pyridine has the advantages that sources of raw materials are wide, production cost is low, the reaction condition is mild, the operation is simple, the products are easy to purify, reaction steps are short, and the like, and the novel synthesis method is particularly suitable for large scale industrial production.
Owner:SHANGHAI SYNCORES TECH INC
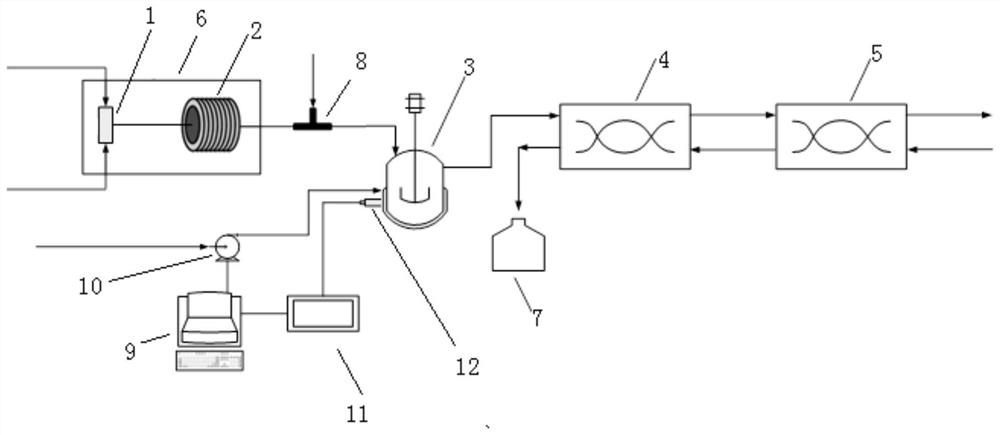
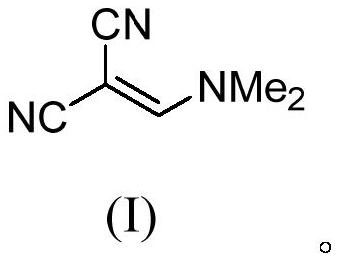
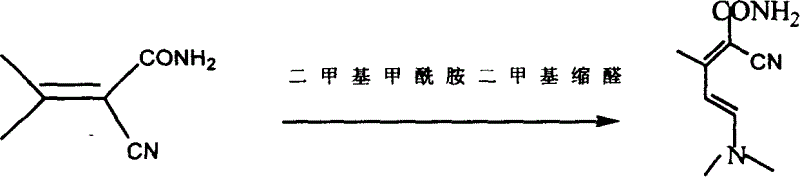
Method for continuously preparing (dimethylamine methylene)malononitrile by using micro-reaction system
ActiveCN112028791AHigh yieldShort reaction timeOrganic compound preparationChemical/physical/physico-chemical microreactorsPtru catalystMethyl palmoxirate
The invention discloses a method for continuously preparing (dimethylamine methylene)malononitrile by using a micro-reaction system. The method comprises the steps of respectively and simultaneously pumping a solution obtained by mixing cyanoacetamide, N,N-dimethylformamide and a catalyst and phosphorus oxychloride into a micro-reaction system comprising a first micro-mixer and a micro-channel reactor which are communicated with each other, and carrying out continuous catalytic dehydration condensation reaction; after the pH value of the crude product mixed solution is adjusted, carrying out continuous liquid-liquid extraction separation on the crude product mixed solution in a centrifugal extraction unit composed of a plurality of annular space type centrifugal extractors connected in series by using an organic solvent, collecting an extraction phase to obtain the target product (dimethylamine methylene)malononitrile. Compared with the prior art, the method has the advantages that thereaction can be safely carried out at normal temperature, the reaction time is short, the product yield is greater than 95%, the efficiency of the technological process is high, the energy consumption is low, and the method has a good industrial application prospect.
Owner:FUDAN UNIV
Process for preparing (+/-)-3-(Carbamoymethyl)-5-methylhexanoic acid
ActiveCN102964263AHigh yieldLow costOrganic compound preparationCarboxylic acid amides preparationAcetic anhydridePtru catalyst
The invention relates to a process for preparing (+ / -)-3-(Carbamoymethyl)-5-methylhexanoic acid. The process comprises the steps: (1) preparing 3-isobutylglutaric acid, namely, reacting cyanoacetamide with isovaleric aldehyde in the presence of a catalyst, and then adding concentrated sulfuric acid for reaction to obtain 3-isobutylglutaric acid; (2) reacting 3-isobutylglutaric acid with acetic anhydride to obtain 3-isobutylglutaric anhydride; and (3) reacting 3-isobutylglutaric anhydride with ammonia to produce (+ / -)-3-(Carbamoymethyl)-5-methylhexanoic acid. According to the process, the raw materials adopted by the process are available, the operation is simple, the yield of each reaction is higher than 80 percent, the total yield of the target product is high, and the cost is low.
Owner:太仓市茜泾化工有限公司
Method for preparing milrinone
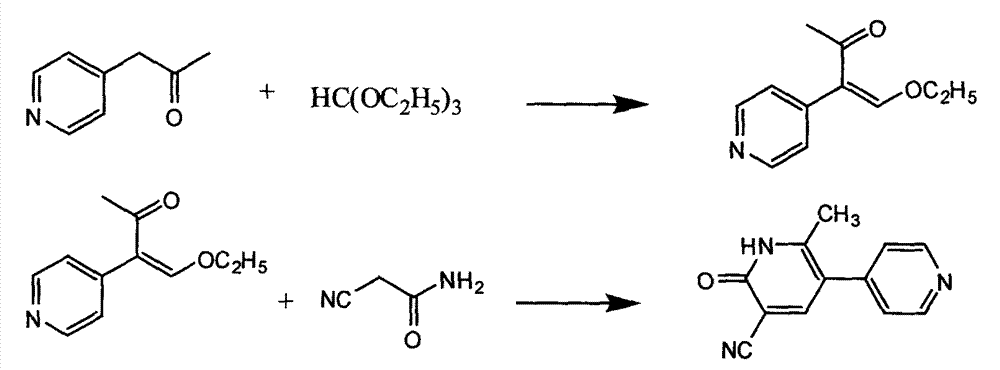
The invention provides a method for preparing milrinone, wherein the method comprises the following steps: with 4-methyl pyridine (SM) as a raw material, in ethyl acetate, generating 1-(4-pyridyl)-2-acetone (represented by the formula I); then under action of triethyl orthoformate, acetic acid and acetic anhydride, generating 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone (represented by the formula II); and finally, under an alkaline condition, carrying out a reaction of 1-ethoxy-2-(4-pyridyl)vinyl methyl ketone with cyanoacetamide to generate milrinone. The method has the advantages of simple and efficient operation, mild reaction conditions, strong safety, easy control and relatively high yield, and is suitable for industrialized production.
Owner:JIANGSU HAICI BIOLOGICAL PHARMA CO LTD OF YANGTZE RIVER PHARMA GRP
Synthesis method of malononitrile
ActiveCN107827777ALow costSimple processBicarbonate preparationPreparation by carboxylic acid amide dehydrationDistillationSynthesis methods
The invention relates to the technical field of malononitrile synthesis, and particularly discloses a synthesis method of malononitrile. The synthesis method of the malononitrile comprises the following steps: performing mixing treatment on a solvent, cyanoacetamide, phosphoryl chloride and solid phosgene to obtain suspension, heating to 50 to 90 DEG C, and performing heat-preservation reaction toobtain a malononitrile solution; performing heating distillation treatment on the malononitrile solution, recycling the solvent and the phosphoryl chloride to obtain a distillation residue; performing vacuum reduced pressure distillation treatment on the distillation residue to obtain the malononitrile. The synthesis method of the malononitrile has the advantages of simple process, convenient operation and low cost; the phosphoryl chloride and the solid phosgene are adopted as a mixed dehydrating agent, so that the yield and the quality of a product are greatly improved, and production of solid wastes is avoided.
Owner:HEBEI JIUTIAN MEDICINE CHEM CO LTD
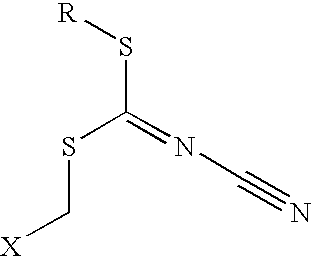
Microbicidal compositions including a cyanodithiocarbimate and a second microbicide, and methods of using the same
InactiveUS7772156B2Economical to useEasy to controlBiocideHydroxy compound active ingredientsAmmonium compounds2-Butene
Microbicidal compositions including (a) cyanodithiocarbimate and (b) an N-alkyl heterocyclic compound; a triazole compound or salt thereof or metal complex thereof; a microbicide with an activated halogen atom or a formaldehyde releasing compound; 1,4-bis(bromoacetoxy)-2-butene; 2-(thiocyanomethylthio)benzothiazole; a methylene-bis(thiocyanate); a halogenated acetophenone; a halopropynl compound; an iodosulfone; a phenol; a halocyanoacetamide compound and / or a quaternary ammonium compound are described. Components (a) and (b) can be present in a synergistically effective amount to control the growth of at least one microorganism. Methods for controlling the growth of microorganisms with the compositions are also disclosed.
Owner:BUCKMAN LAB INT INC
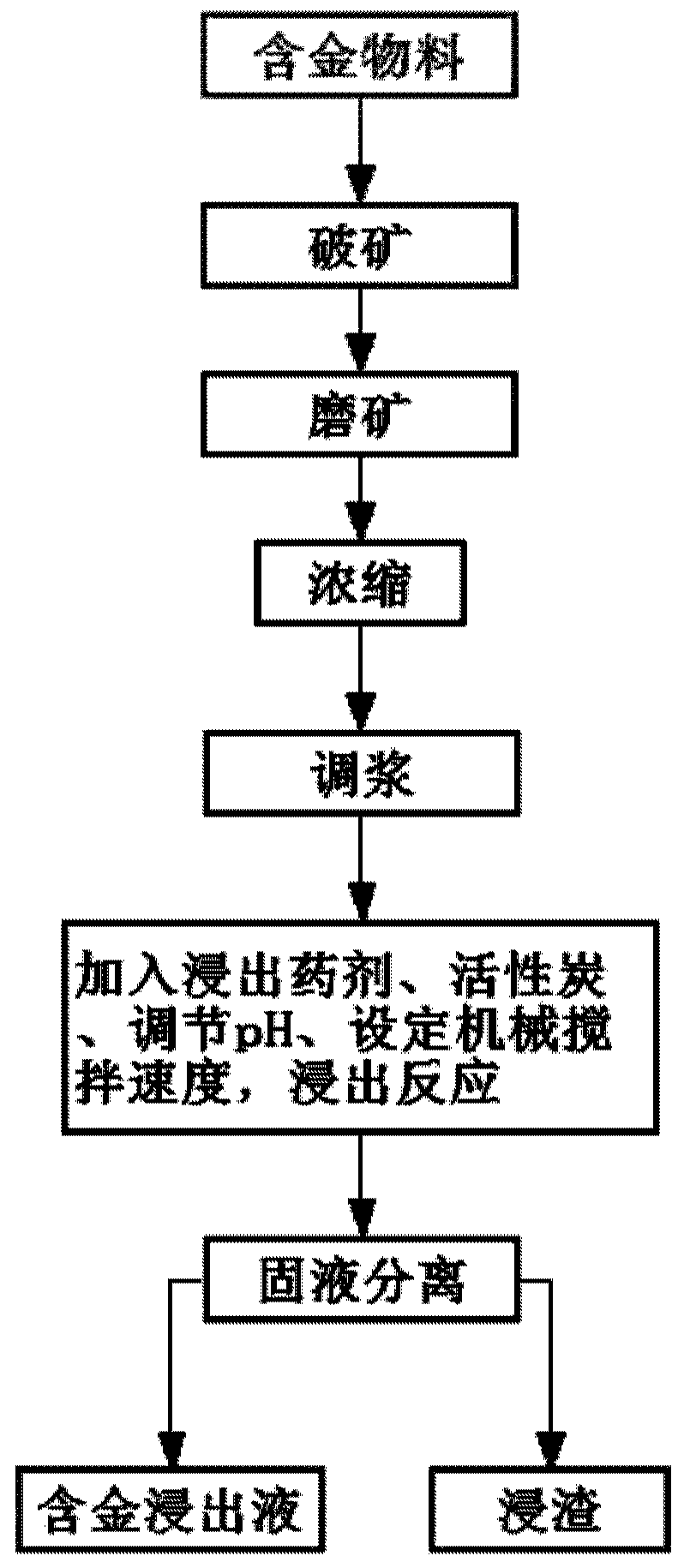
Gold extracting agent and gold extracting process using same
ActiveCN110184454ARapid responseQuick and efficient responseProcess efficiency improvementThioureaHydrometallurgy
The invention discloses a gold extracting agent and a gold extracting process using the same, and relates to the field of hydrometallurgy. The gold extracting agent comprises, by mass, 5-30 parts of leaching agents and 4-10 parts of auxiliary leaching agents, wherein the leaching agents comprise one or a combination of several of halogenated five-membered cyclic imine, five-membered cyclic imide,cyanoacetamide, and trichloroisocyanuric acid; and the auxiliary leaching agents comprise one or a combination of several of KI, NaI, KBr, NaBr, NaCl, KCl, EDTA-2Na, Na2CO3, CaCO3 and talc. Accordingto the gold extraction agent, halogen and various strong oxidizing free radicals are slowly released during reaction, so that the gold extraction agent has high activity, the reaction of gold oxide israpid and efficient, gold ions form a gold-containing coordination compound with high stability in the presence of an extractant and an auxiliary extractant, and compared with cyanide, thiourea, chlorine, bromine and other gold extraction agents, the gold extraction agent is nontoxic or extremely low in toxicity, so that the gold extraction rate reaches 90% or above
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
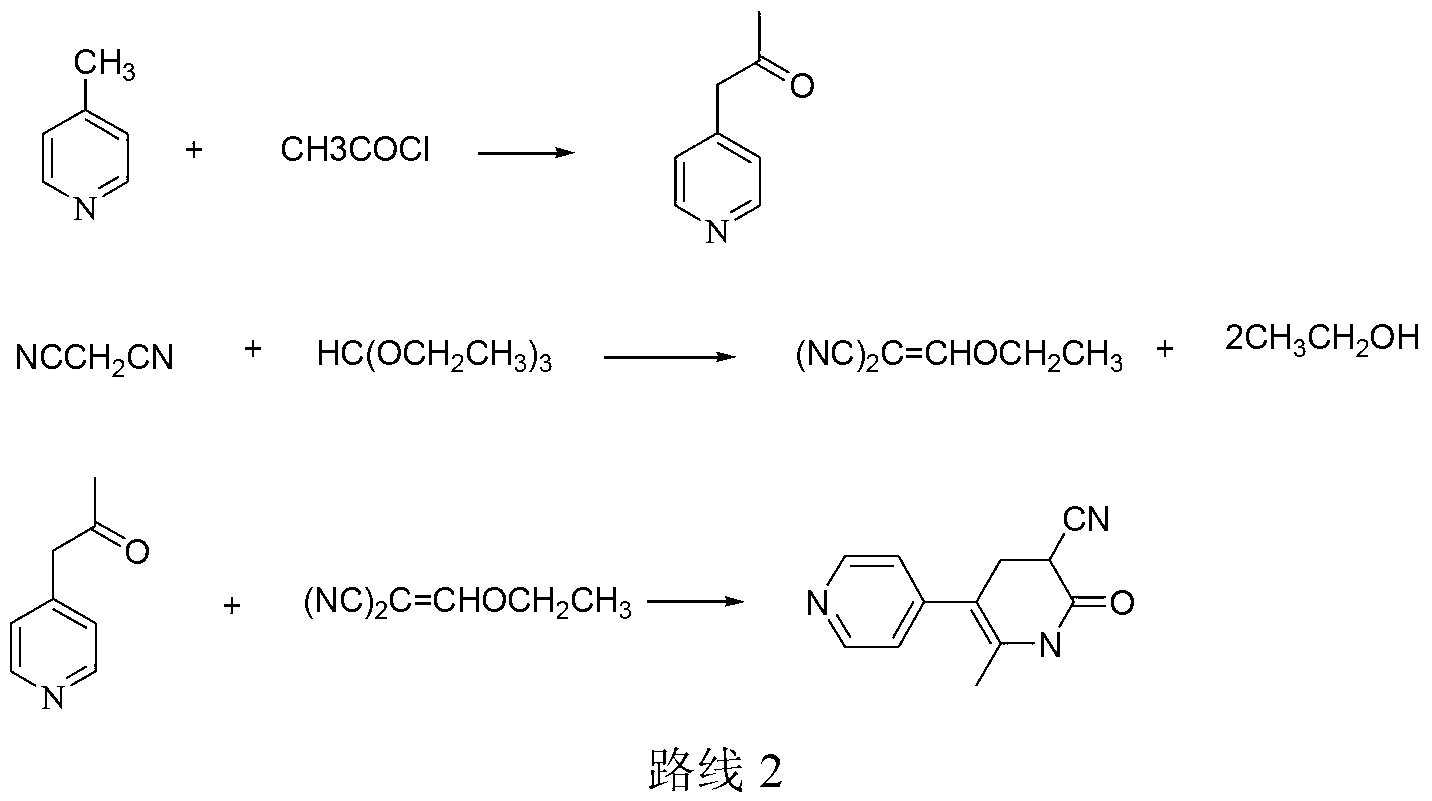
Preparation method of high-purity milrinone
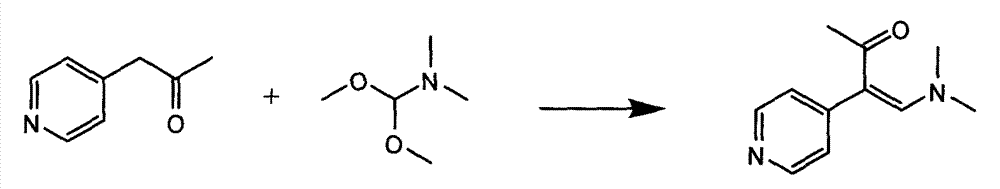
InactiveCN104326975AMild preparation conditionsSimple and fast operationOrganic chemistryDimethyl acetalMethanol
The invention relates to a preparation method of high-purity milrinone as shown in a formula (I) described in the specification. The preparation method comprises the following steps: carrying out reflux reaction on N, N-methyl formamide dimethyl acetal (DMF-DMA) by taking 1-(4-pyridyl) acetone as a starting material, concentrating after the reaction is ended, adding normal hexane into residues to separate out solids to obtain an intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone; carrying out reflux reaction on the intermediate 1-(4-pyridyl)-2-(dimethylamino) vinyl methyl ketone and cyanoacetamide in the presence of sodium methylate, and regulating the pH value after the reaction is ended to obtain crude milrinone; and refining the crude milrinone by methanol / sodium methylate and alcohol / water to obtain a qualified milrinone product. The preparation method disclosed by the invention is simple in process operation, relatively small in pollution and suitable for industrial production.
Owner:ZHENGZHOU SIHUAN MEDICINE ARTICLE CO LTD
Fluid-based cell and microorganism treating and preserving reagent and preparation method thereof
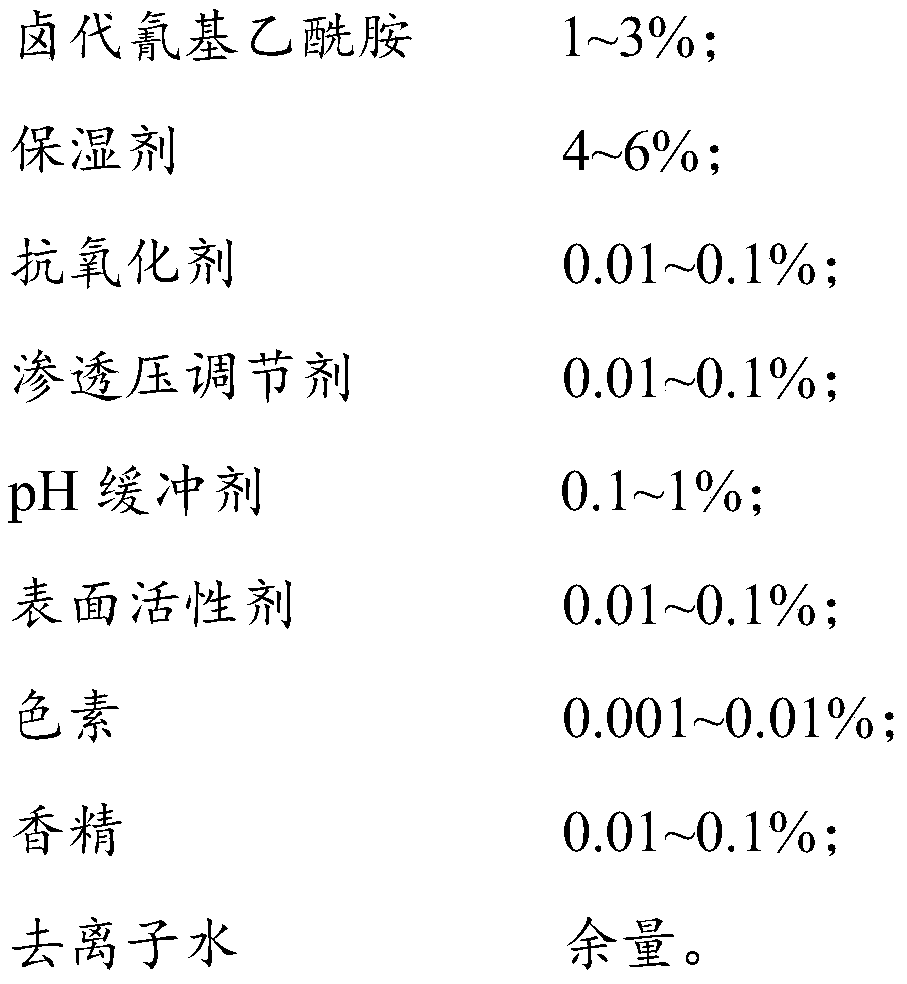
PendingCN110283726AGood effectStrong penetrating powerDead animal preservationMicroorganism preservationPolyvinyl alcoholAntioxidant
The invention discloses a fluid-based cell and microorganism treating and preserving reagent and a preparation method thereof. Halogenated cyanoacetamide, a moisturizing agent, a pH buffer agent, an osmotic pressure regulator, a surfactant, an antioxidant, pigment and essence are uniformly stirred, the obtained mixture is added to deionized water at the water temperature of 30-35 DEG C, and sodium hydroxide is added to adjust the pH value to 5.5-6.5 to obtain the fluid-based cell and microorganism treating and preserving reagent. The reagent is a fixing agent for fixing biological samples, contains no mercury or formaldehyde, can be compatible with different parasitism stages (such as sporocysts, eggs, larvae and trophozoites) and is effective under the condition that no polyvinyl alcohol or aldehyde (such as glyoxal and glutaraldehyde) is added.
Owner:孝感宏翔生物医械技术有限公司
A nitrogen-modified catalyst for preparing vinyl chloride and its preparation method
ActiveCN104289254BSimple preparation processLow costPreparation by hydrogen halide split-offOrganic-compounds/hydrides/coordination-complexes catalystsBarium saltEthane Dichloride
The invention discloses a nitrogen-modified catalyst applied to preparation of vinyl chloride and a preparation method of the nitrogen-modified catalyst. According to the nitrogen-modified catalyst, active carbon is used as a carrier, wherein the nitrogen-modified catalyst comprises a metal salt loaded compound and a nitrogen-containing compound; according to the total mass of the catalyst, the mass percentage of the metal salt compound is 0.01-10%, and the mass percentage of the nitrogen-containing compound is 0.01-10%. The metal salt compound is strontium salt or barium salt or mixture of the strontium salt and the barium salt; the nitrogen-containing compound is selected from at least one of guanidine hydrochloride, acetamidine hydrochloride, acrylamide, urea, methanesulfonamide and cyanoacetamide. The nitrogen-modified catalyst is applied to reaction of preparing vinyl chloride by catalytic cracking of 1,2-dichloroethane; not only is the cracking temperature reduced, and also the nitrogen-modified catalyst is high in conversion rate of 1,2-dichloroethane and selectivity of vinyl chloride, and is efficient, and has energy-saving and environment-friendly effects.
Owner:SHANGHAI ADVANCED RES INST CHINESE ACADEMY OF SCI
Synthesis method of 2-chloro-3-amido-4picoline from cyanoacetamide and acetone
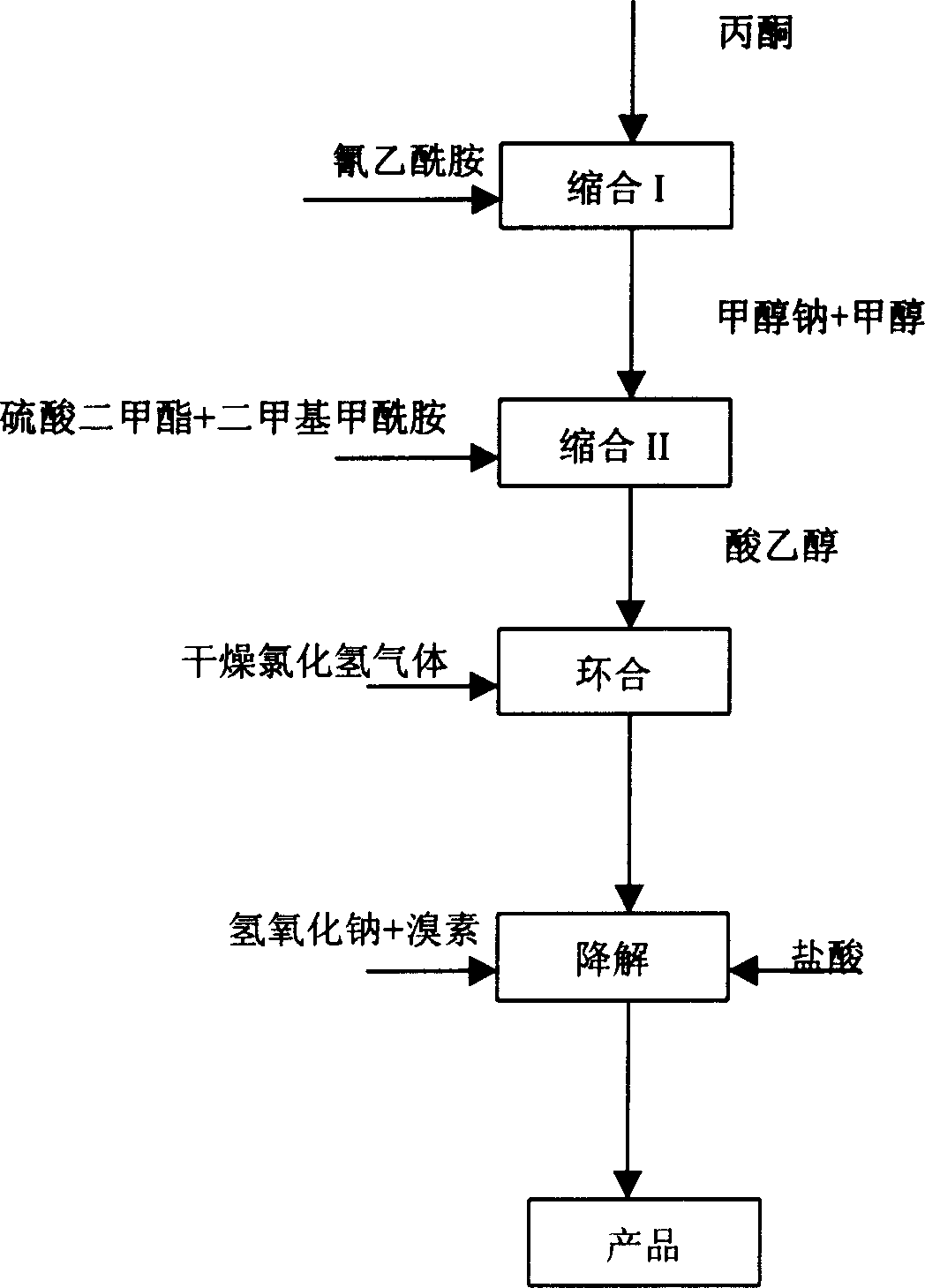
InactiveCN1763010AFew reaction stepsRelaxed reaction conditionsOrganic chemistrySynthesis methodsPyridine
The present invention relates to the process of synthesizing 2-chloro-3-amino-4-methyl pyridine as one kind of intermediate for AIDS medicine Nevirapine with cyanoacetamide and acetone. The present invention features that cyanoacetamide and acetone as initial materials are prepared into 2-chloro-3-amino-4-methyl pyridine through condensation, cyclization and degradation. Compared with available technology, the present invention has the outstanding features of less reaction steps, mild reaction condition, high selectivity, high product yield up to 37 %, product purity over 99 % and being suitable for industrial production.
Owner:江阴暨阳医药化工有限公司
Preparation method of high-purity milrinone
ActiveCN103965101ALess residueEasy to operate the machineOrganic chemistryMilrinoneMethylvinyl ketone
The invention discloses a preparation method of high-purity milrinone. The method comprises the steps of dissolving 1-ethoxyl-2-(4-pyridyl) methyl vinyl ketone, cyanoacetamide and alkali into a monohydric alcohol-water system, reacting for 15-18 hours at -5 DEG C to 10 DEG C, adding water into reaction liquid and diluting to 1.5 times of the original volume after completing reaction, then adding activated carbon, stirring for 15-25 minutes at room temperature, filtering, regulating the pH value of filtrate to be 7 by using a hydrochloric acid solution, filtering, washing a filter cake by using water until the filtrate is colorless, refluxing and dissolving the filter cake by using 50vt% ethanol, filtering, and stirring and crystallizing the filtrate at -5 DEG C to 10 DEG C to obtain white solid milrinone with the purity of more than 99.9%. The method disclosed by the invention is simple and fast in process, high in yield and pure in product, and is more suitable for industrial production.
Owner:合肥启旸生物科技有限公司
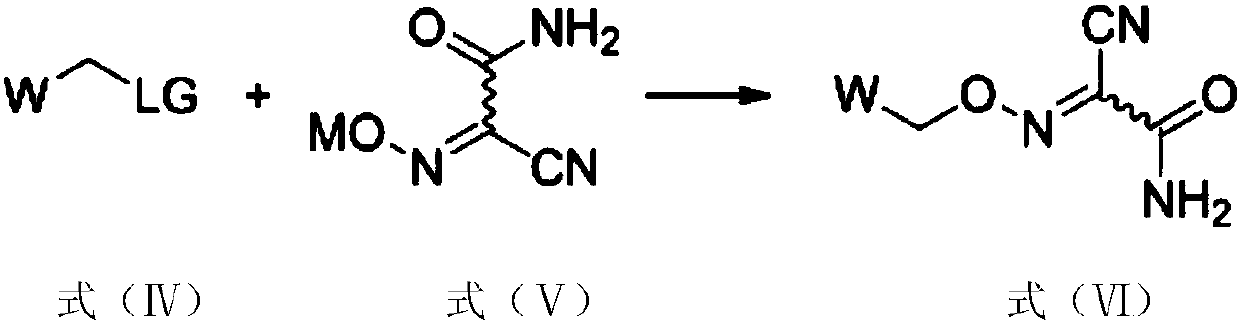
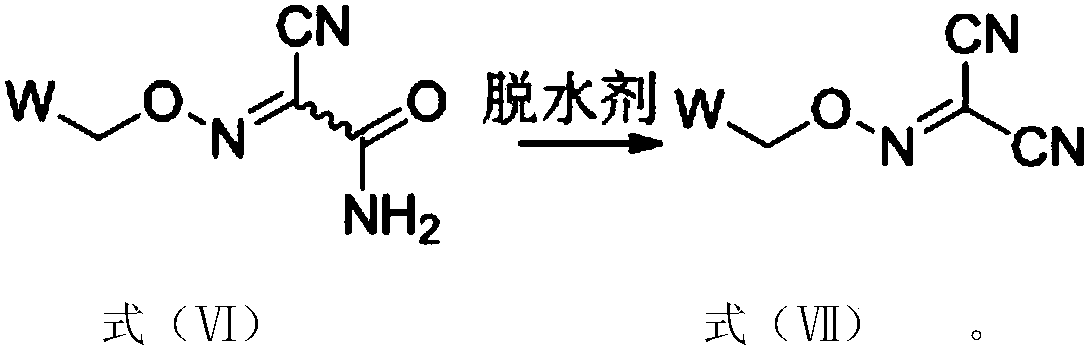
Method for preparing malononitrile oxime ether compound and intermediate compound
ActiveCN110713443AReduce processing costsHigh yieldOrganic compound preparationPreparation by carboxylic acid amide dehydrationArylPolymer science
The invention provides a method for preparing a malononitrile oxime ether compound and an intermediate compound. The malononitrile oxime ether compound has a structure as shown in a formula (VII), wherein W is selected from aryl or heteroaryl. The preparation method comprises the following steps: under the action of a first solvent and a catalyst, carrying out a reaction on a first raw material and a second raw material to obtain an intermediate compound, wherein the first raw material has a structure as shown in a formula (IV), and the second raw material has a structure as shown in a formula(V); and under the action of a second solvent, carrying out dehydration reaction on the intermediate compound shown in the formula (VI) and a dehydrating agent to obtain the malononitrile oxime ethercompound. In the preparation process of the intermediate, cheap cyanoacetamide is used as a raw material, the reaction conditions are mild, the yield of an intermediate compound is high, and the process cost is low. The required malononitrile oxime ether compound can be obtained only through one-step dehydration reaction. By adopting the preparation method, the yield of malononitrile oxime etheris increased, and the process cost is reduced.
Owner:SHENYANG SINOCHEM AGROCHEMICALS R&D CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com