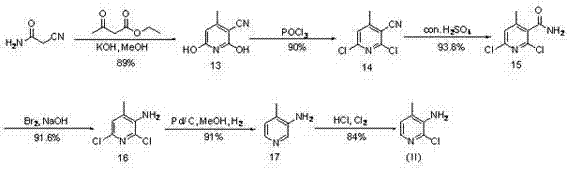
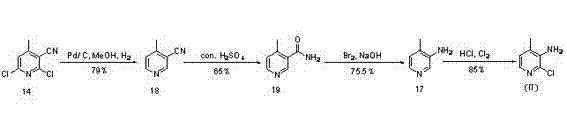
Novel synthesis method of Nevirapine key intermediate 2-chloro-3-amino-4-methylpyridine
A technology of picoline and nevirapine, applied in the synthesis of pharmaceutical intermediates, the synthesis field of nevirapine key intermediate 2-chloro-3-amino-4-methylpyridine, can solve the problem of high equipment requirements, long reaction steps and dangerous and other problems, to achieve the effect of simple operation, short reaction steps and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Example 1: Add 7.0 g (1.0 eq) of crotonaldehyde and 9.2 g (1.1 eq) of cyanoacetamide to 210 mL of dimethyl sulfoxide, then add 22.4 g (4.0 eq) of potassium tert-butoxide to the reaction in one go , the reaction temperature rises. Air was blown in, and the reaction was carried out at room temperature (about 15°C) overnight. At this time, LC-MS was used to monitor, and the target product was formed. The reaction was poured into water, the pH was adjusted to pH=5 with 2N hydrochloric acid, extracted 3 times with ethyl acetate, the organic layers were combined, dried, and concentrated to obtain 8.52g of the target product 2-hydroxyl-3-cyano-4methylpyridine ( 27 ) solid, yield 60%.
[0062] 1 HNMR (DMSO); ppm: 2.4 (s,3H); 3.5 (bs,1H); 6.26 (m,1H).
Embodiment 2
[0063] Example 2: 7.0 g (1.0 eq) of crotonaldehyde and 18.4 g (2.0 eq) of cyanoacetamide were added to 210 mL of dimethyl sulfoxide, and then sodium tert-butoxide (4.0 eq) was added to the reaction at one time, The reaction temperature rises. Infuse air and react overnight at room temperature. At this time, LC-MS was used to monitor, and the target product was formed. The reaction was poured into water, the pH was adjusted to pH=5 with 2N hydrochloric acid, extracted 3 times with ethyl acetate, the organic layers were combined, dried, and concentrated to obtain 7.77g of the target product 2-hydroxyl-3-cyano-4methylpyridine ( 27 ) solid, yield 58%.
[0064] 1 HNMR (DMSO); ppm: 8.03 (d, J=2 Hz, 1H), 7.6 (d, J=2 Hz, 1H), 2.5 (s, 3H).
Embodiment 3
[0065] Example 3: 7.0 g (1.0 eq) of crotonaldehyde and 9.2 g (1.1 eq) of cyanoacetamide were added to 210 mL of methanol, and then 22.4 g (4.0 eq) of potassium tert-butoxide was added to the reaction at one time, and the reaction The temperature rises. Air was blown in and reacted overnight at 45°C. At this time, LC-MS was used to monitor, and the target product was formed. Spin the reaction system to dryness, add water and adjust the pH to pH=5 with 2N hydrochloric acid, extract 3 times with ethyl acetate, combine the organic layers, dry and concentrate to obtain 5.86g of the target product 2-hydroxy-3-cyano-4methyl Pyridine ( 27 ) solid, yield 40%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com