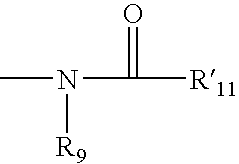
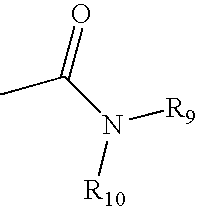
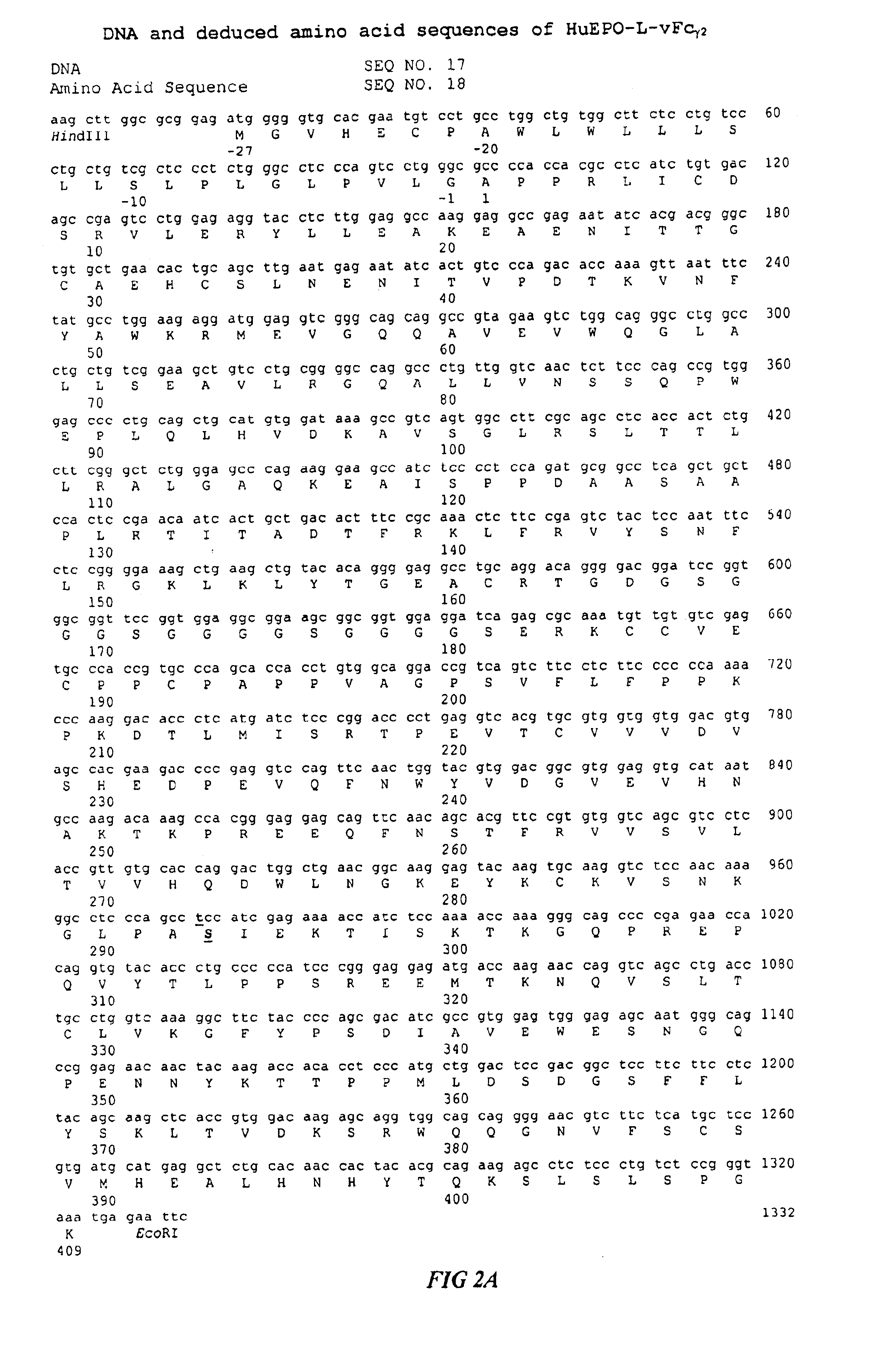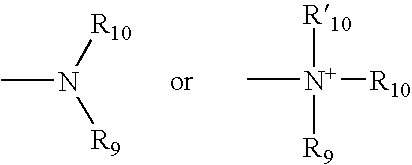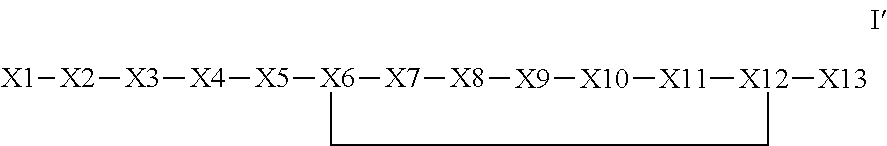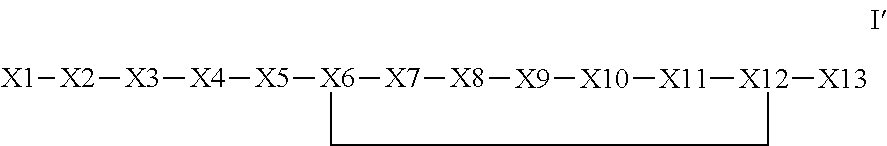Patents
Literature
8130results about How to "Improve biological activity" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
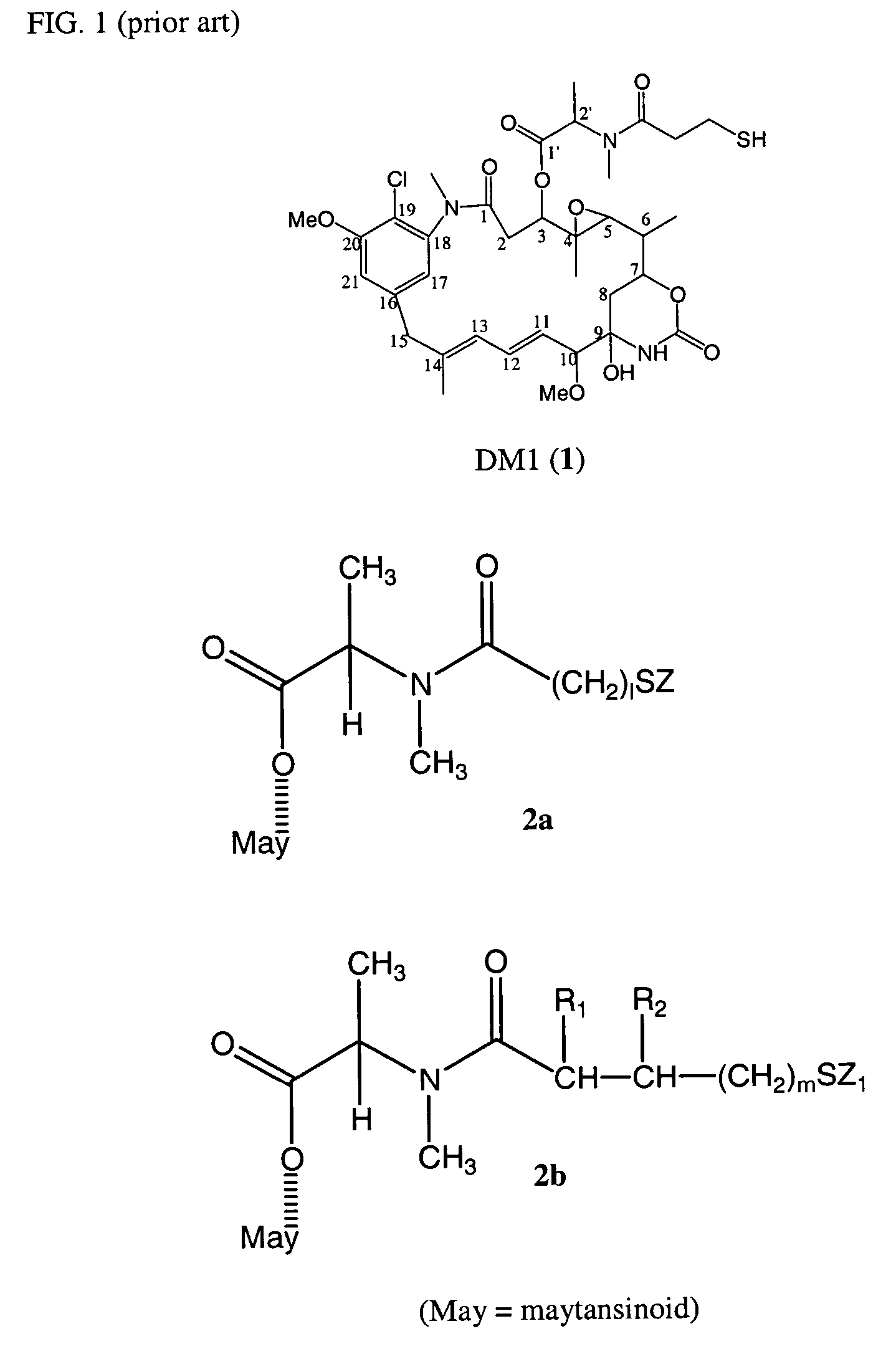
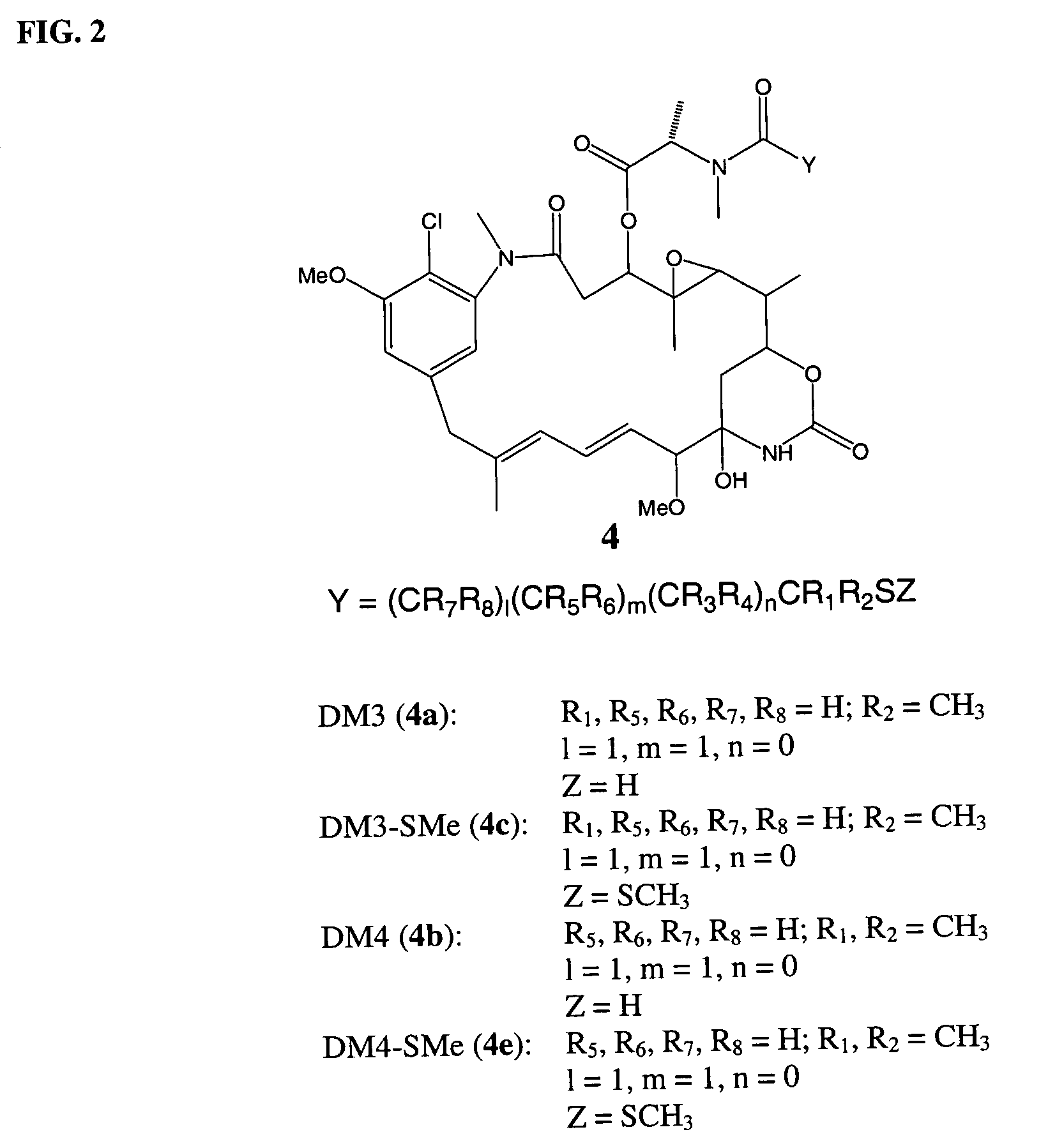
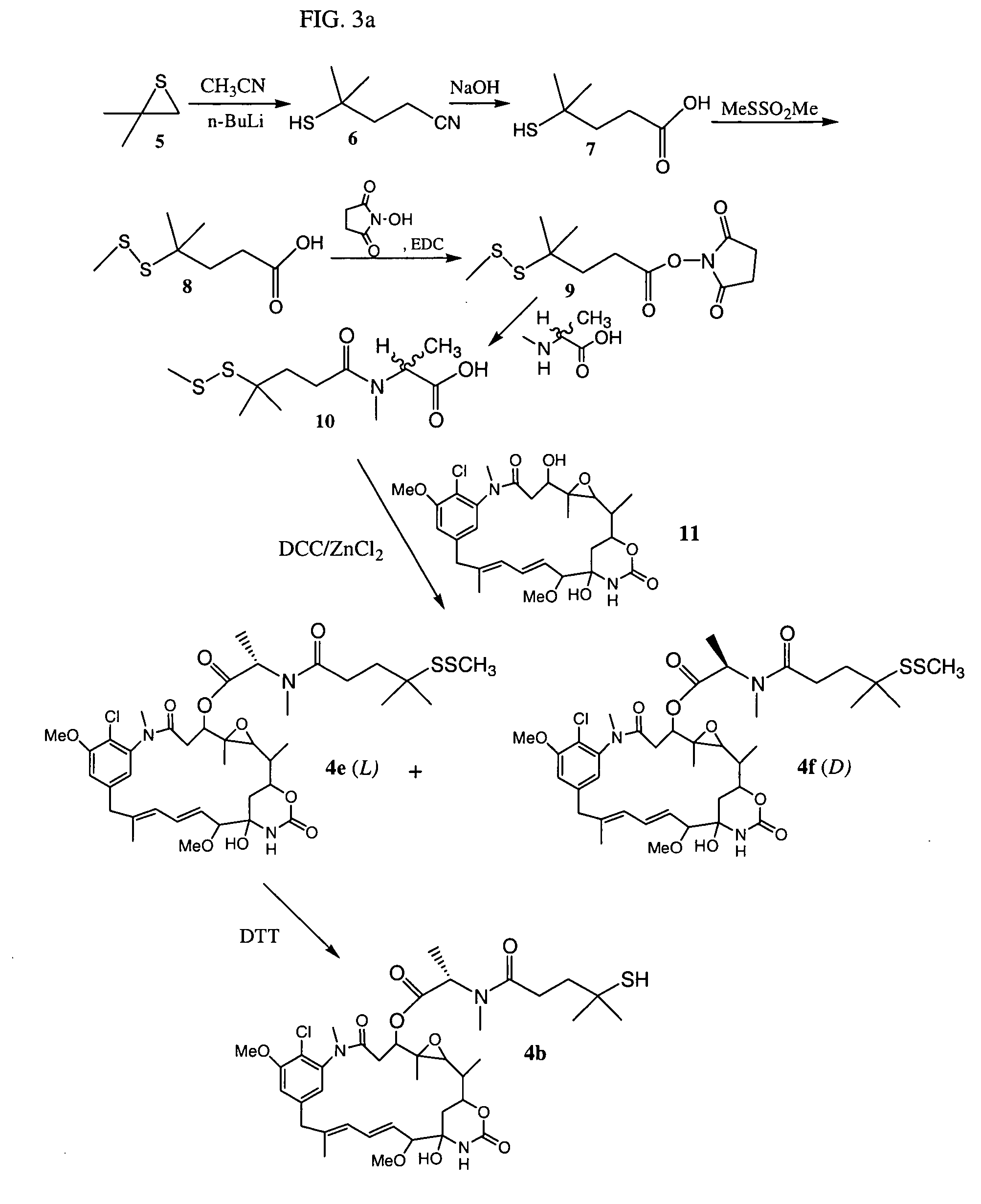
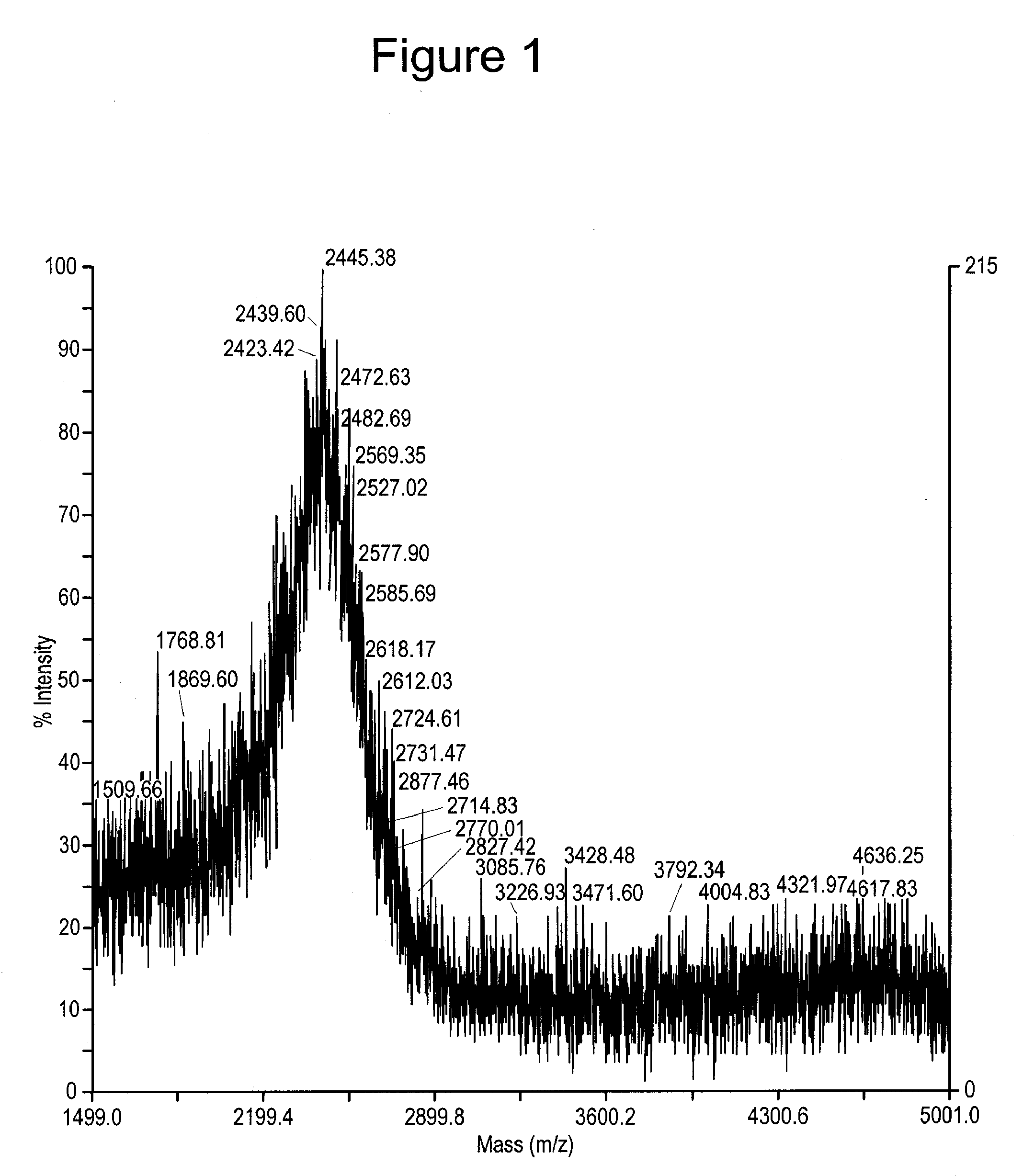
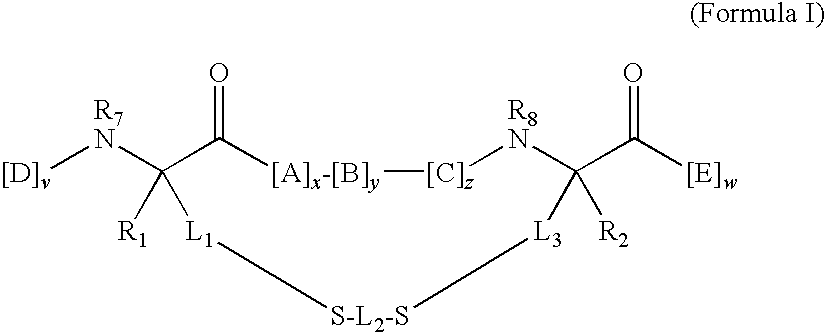
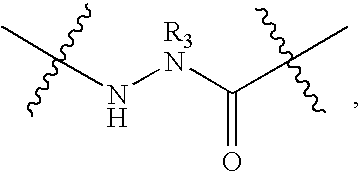
Cytotoxic agents comprising new maytansinoids
ActiveUS7276497B2Improve anti-tumor activityImprove biological activityOrganic active ingredientsOrganic chemistryAnimal tumorEfficacy
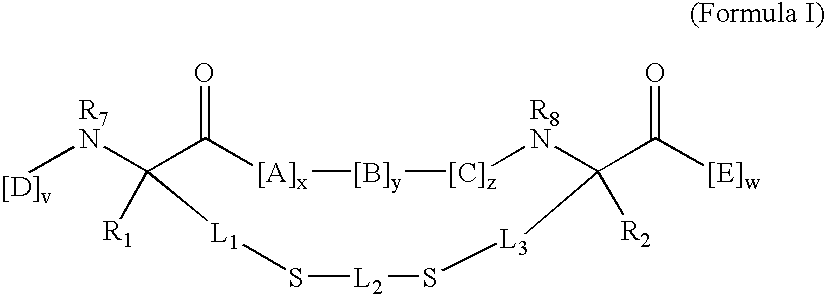
New thiol and disulfide-containing maytansinoids bearing a mono or di-alkyl substitution on the α-carbon atom bearing the sulfur atom are disclosed. Also disclosed are methods for the synthesis of these new maytansinoids and methods for the linkage of these new maytansinoids to cell-binding agents. The maytansinoid-cell-binding agent conjugates are useful as therapeutic agents, which are delivered specifically to target cells and are cytotoxic. These conjugates display vastly improved therapeutic efficacy in animal tumor models compared to the previously described agents.
Owner:IMMUNOGEN INC
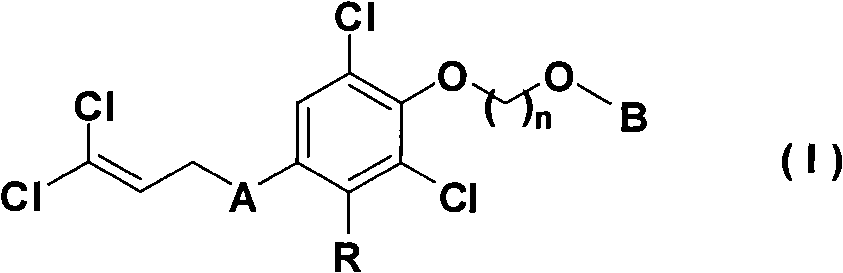
Nitrogen heterocyclic ring dichlorin allyl ether compounds with insecticidal activity
Owner:HUNAN CHEM RES INST
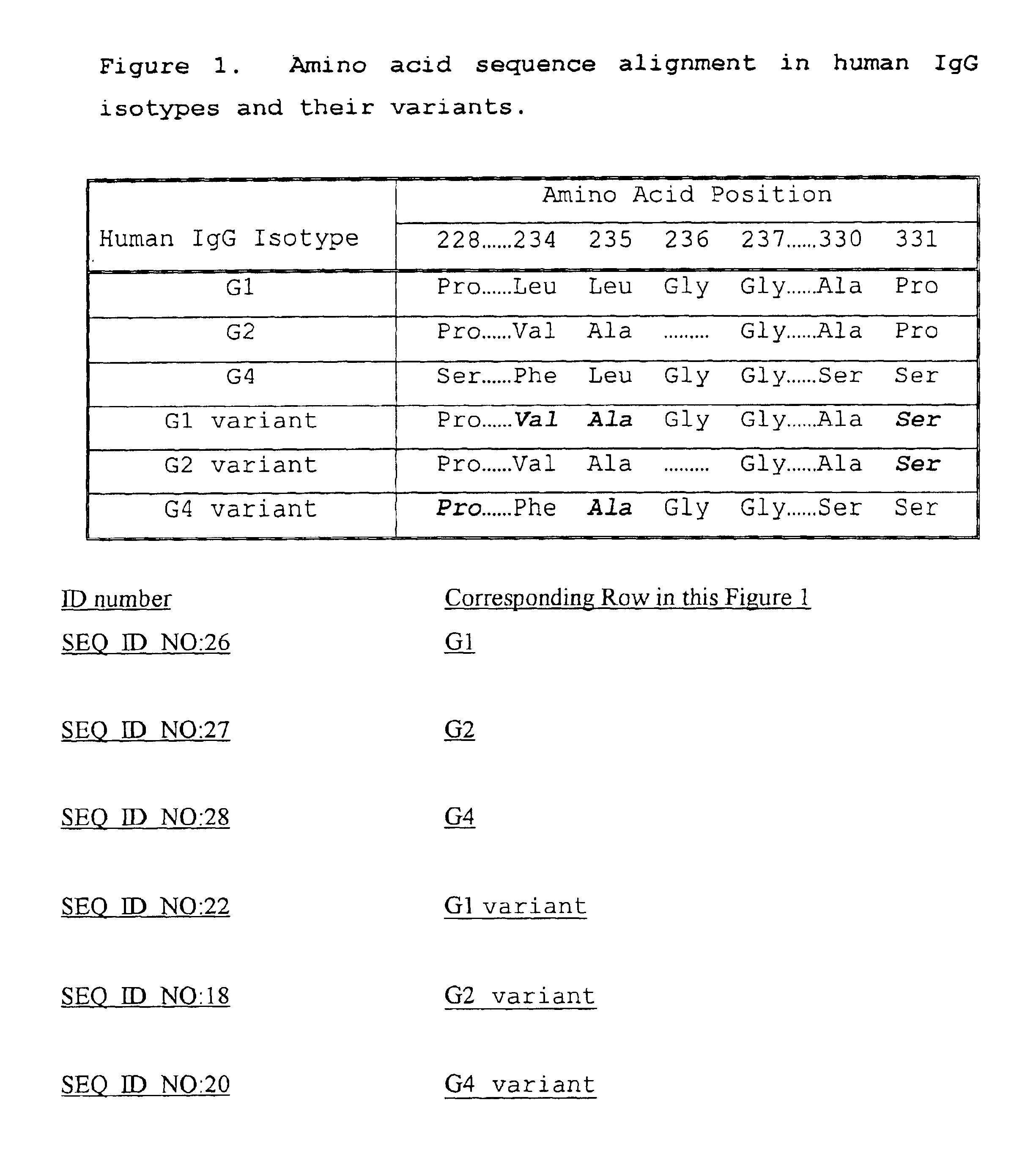
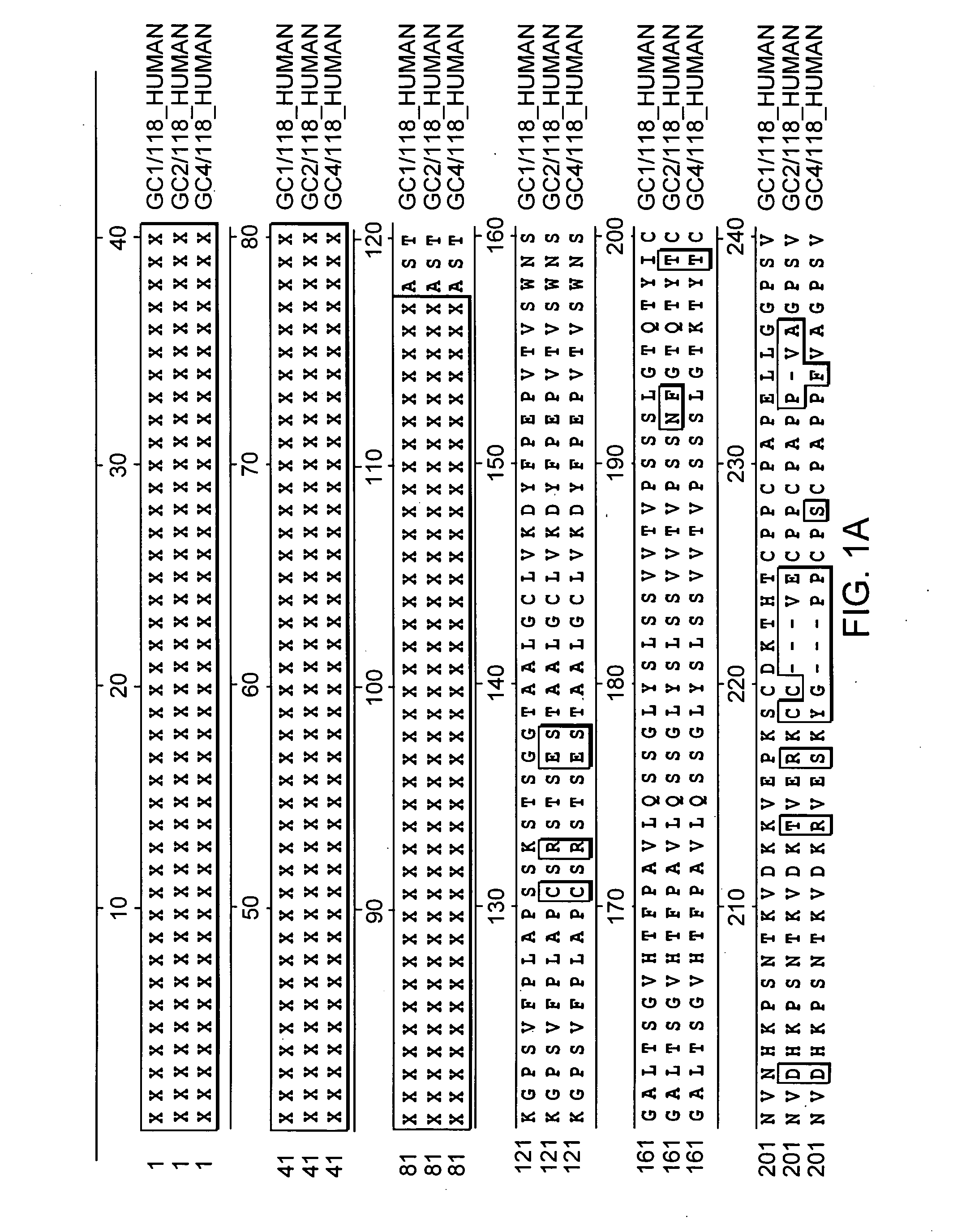
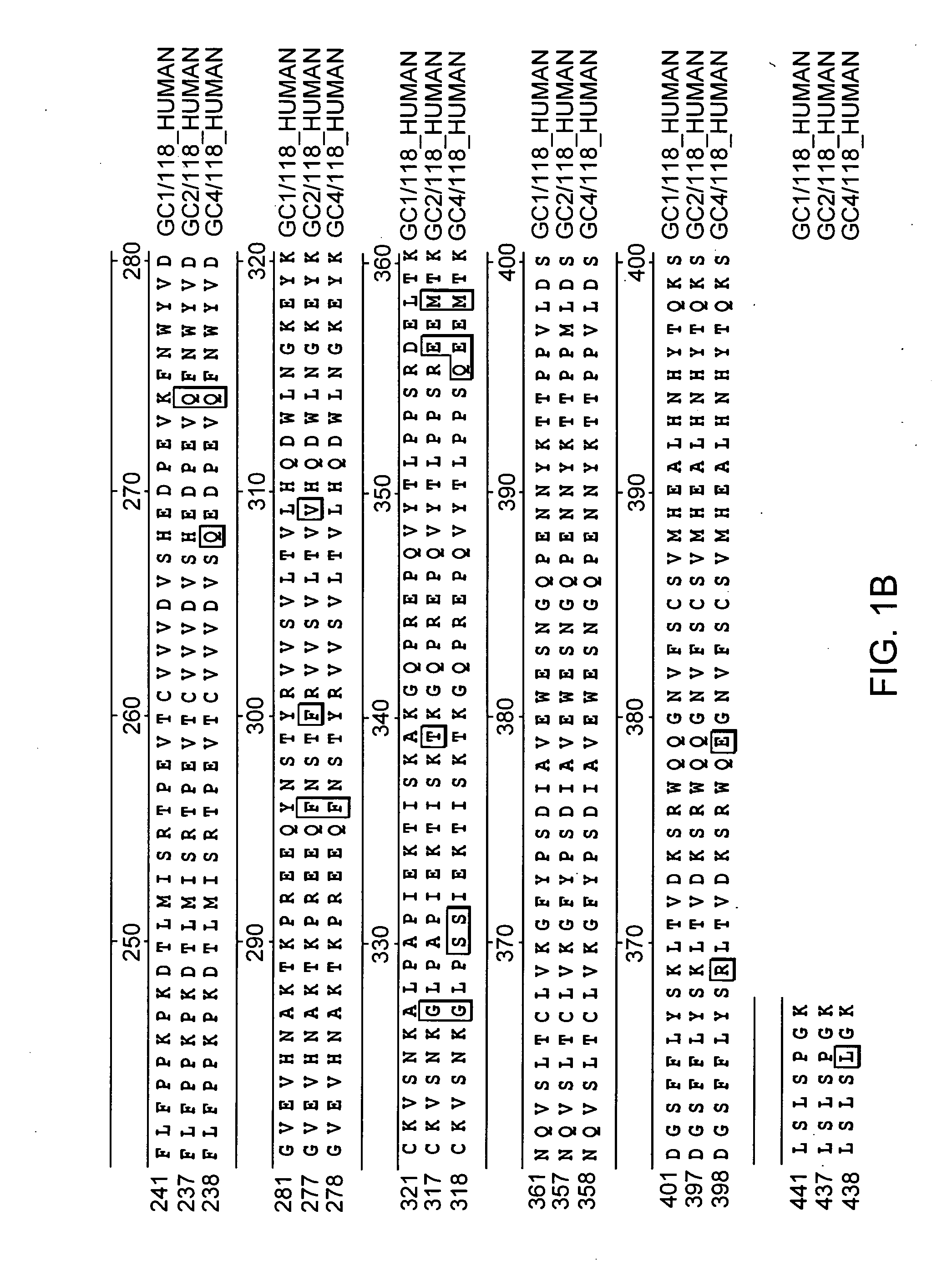
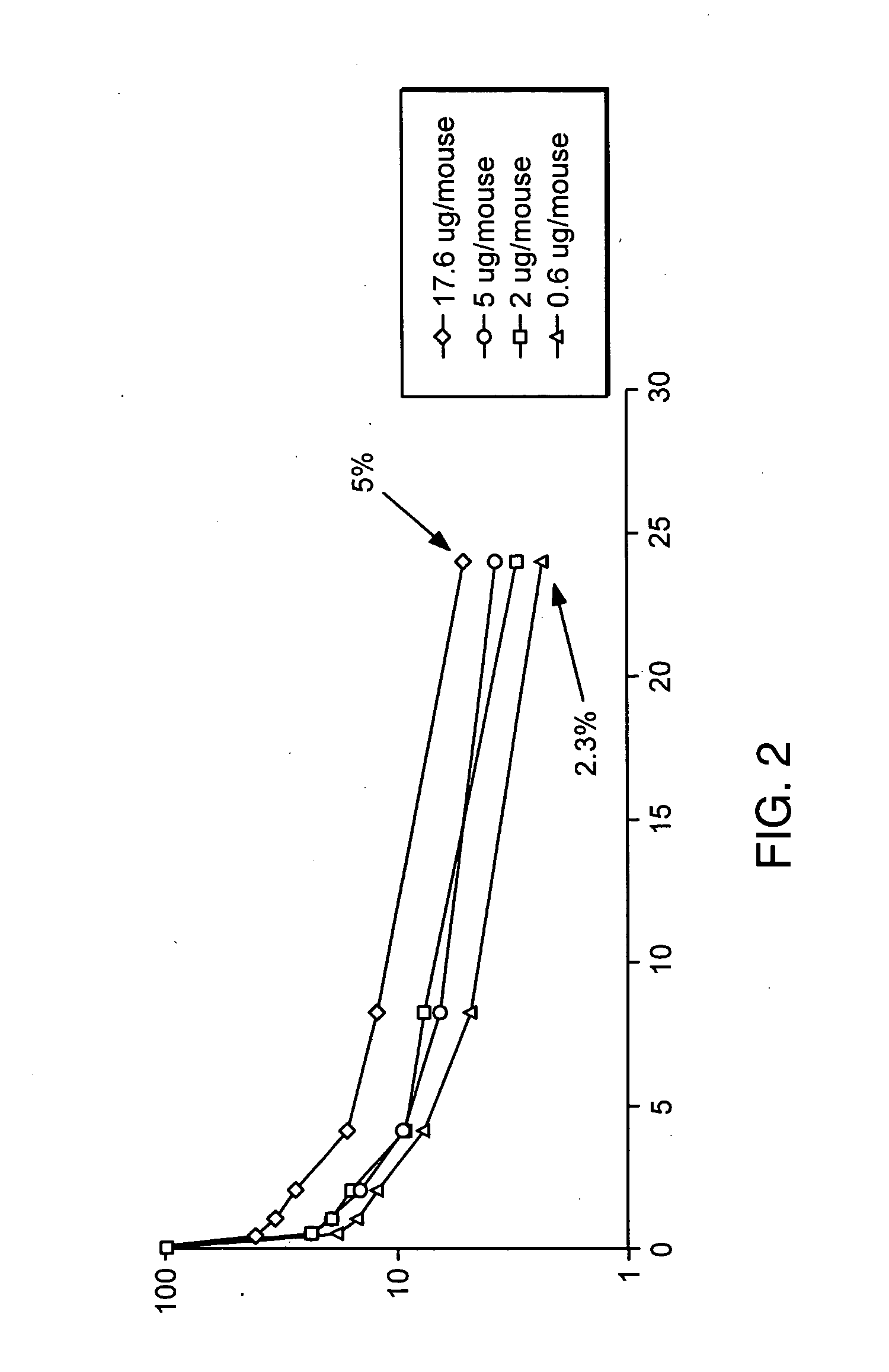
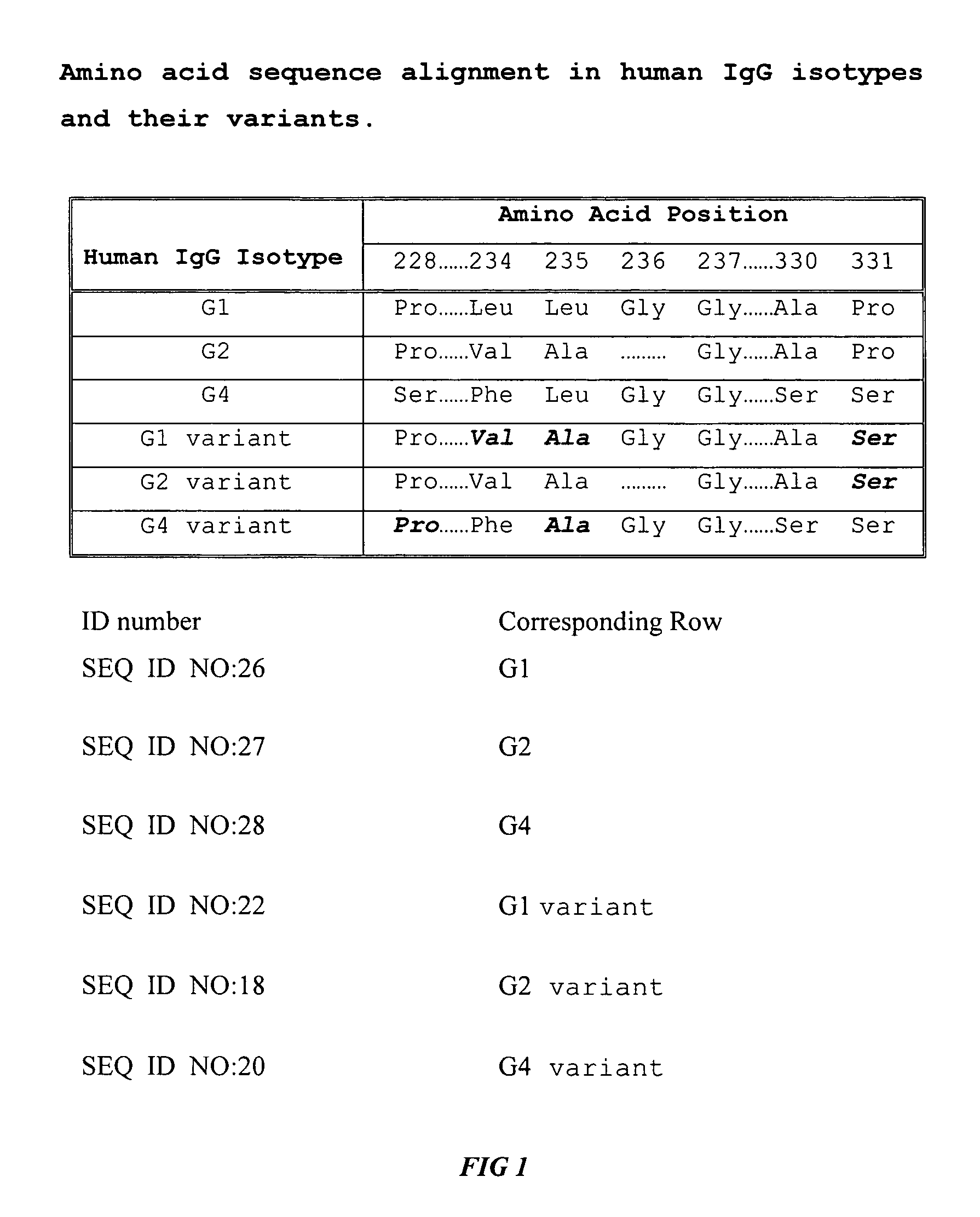
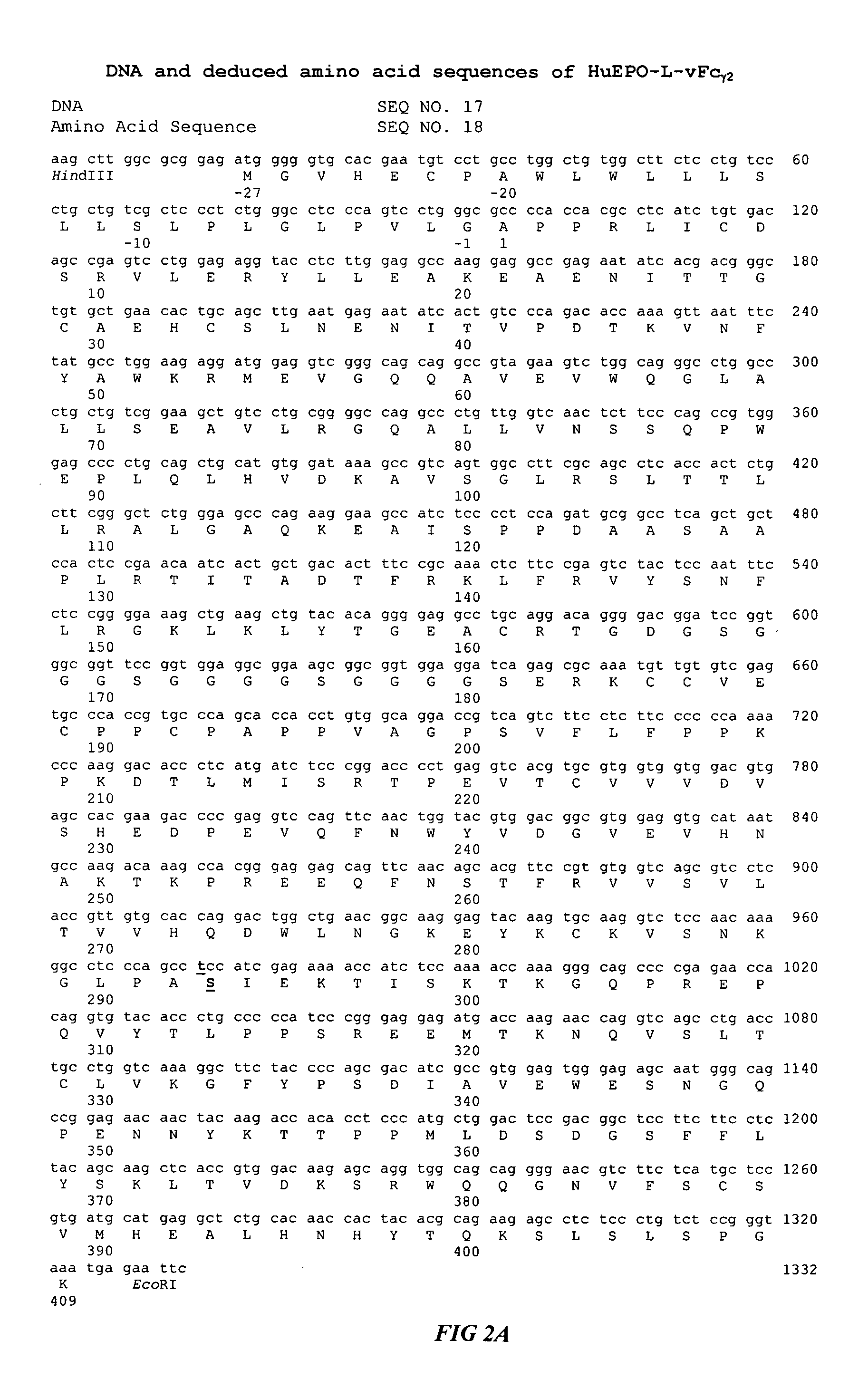
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
Fc-erythropoietin fusion protein with improved pharmacokinetics
InactiveUS20050192211A1Improve pharmacokineticsSimplify erythropoietin therapyPeptide/protein ingredientsAntibody mimetics/scaffoldsErythropoietinNucleic acid
The present invention provides Fc-erythropoietin (“Fc-EPO”) fusion proteins with improved pharmacokinetics. Nucleic acids, cells, and methods relating to the production and practice of the invention are also provided.
Owner:MERCK PATENT GMBH
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
Transdermal drug delivery compositions and topical compositions for application on the skin
InactiveUS20090053290A1Improve biological activityEliminate side effectsPowder deliveryCosmetic preparationsRadio frequencyOtic Agents
Transdermal delivery compositions and topical compositions for application to the skin are provided. The transdermal delivery composition includes at least two penetrants working synergistically but by disparate biochemical pathways. In one embodiment, the transdermal delivery system includes benzyl alcohol and lecithin organogel. The transdermal delivery compositions are used in a variety of topical compositions as a means of transdermally delivering and topically administering different drugs and agents, including compositions promoting collagen biosynthesis, retinoids and skin lighteners, chemical denervation agents such as BOTOX®, anti-fungal agents, anesthetics and non-steroidal anti-inflammatory drugs (NSAIDs). In addition, these topical compositions may be used in combination with non-ablative treatment modalities, such as microdermabrasion, laser-based skin remodeling and radio-frequency-based skin remodeling.
Owner:NUVIANCE
Process for producing fast-setting, bioresorbable calcium phosphate cements
InactiveUS6379453B1Improve biological activityOther chemical processesDentistry preparationsSeed crystalCalcium phosphate cement
Owner:CALCITEC
Pyrazole amide compound containing diphenyl ether, and application and pesticide composition of pyrazole amide compound
ActiveCN104557709AImprove biological activityGood prevention effectBiocideOrganic chemistryDiphenyl etherActive ingredient
Disclosed are a pyrazole amide compound containing diphenyl ether as represented by formula I and / or formula II, application thereof in the prevention and treatment of rice sheath blight and cucumber powdery mildew, and pesticide composition containing the compound. The pyrazole amide compound of the present invention containing diphenyl ether as represented by formula I and / or formula II, and a bactericide containing the pyrazole amide compound as an active ingredient have good prevention and treatment effects on rice sheath blight and cucumber powdery mildew.
Owner:燕化永乐(乐亭)生物科技有限公司 +1
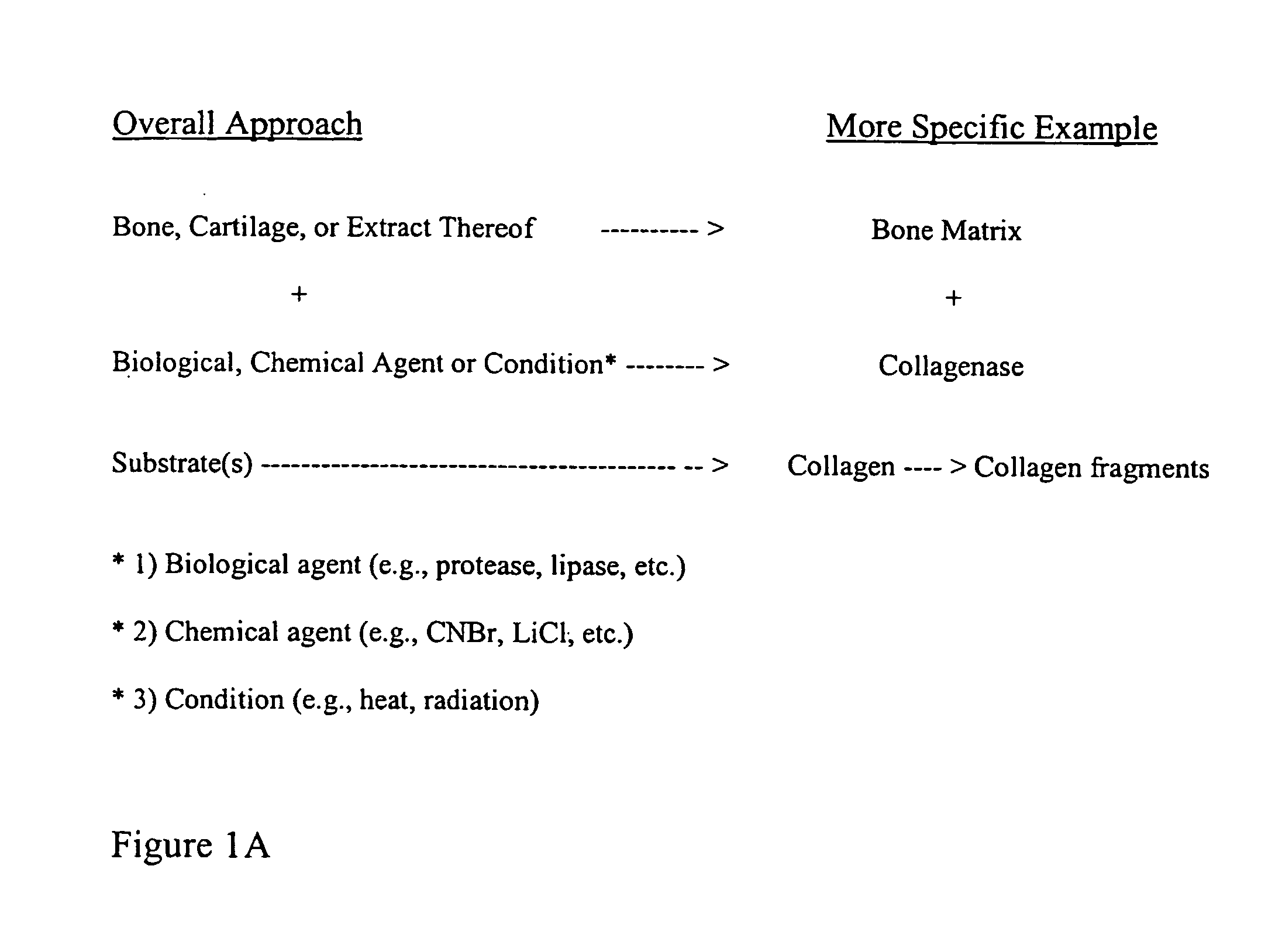
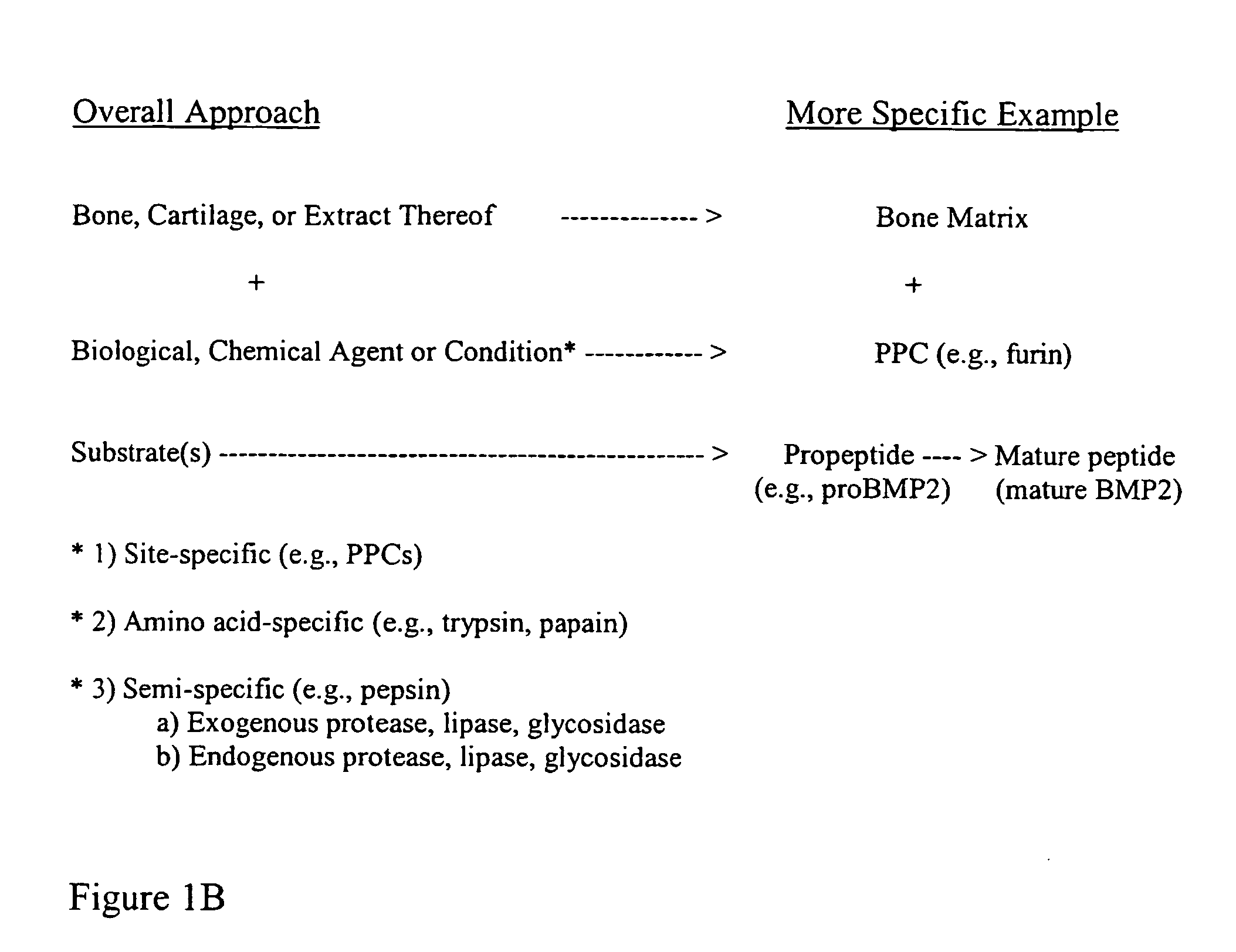
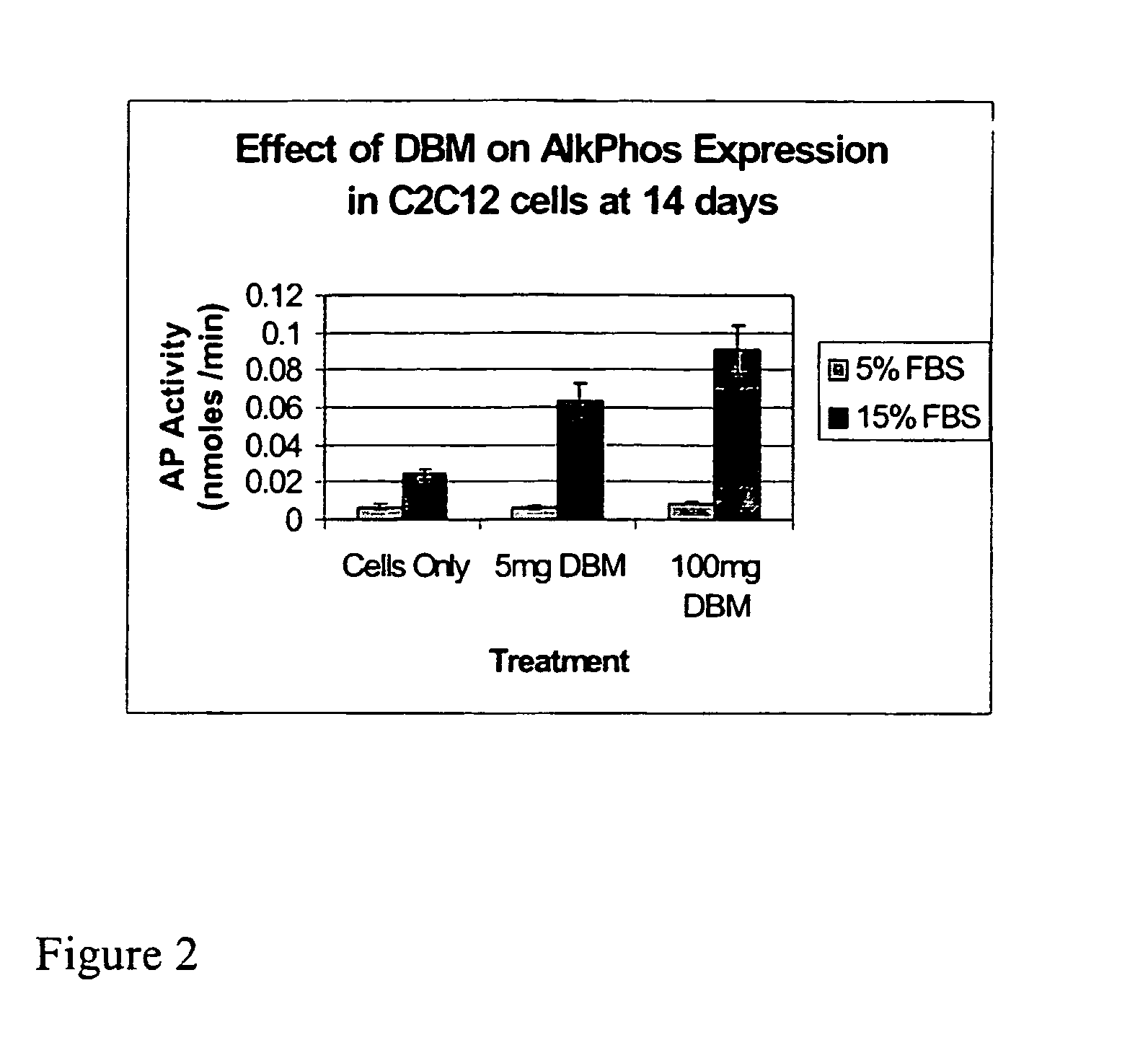
Bone matrix compositions and methods
ActiveUS20070154563A1Good osteoinductivityHigh activityHydrolysed protein ingredientsBone implantOsteoblastLine of therapy
The present invention provides methods of improving the osteogenic and / or chondrogenic activity of a bone matrix, e.g., a dermineralized bone matrix (DBM), by exposing the bone matrix to one or more treatments or conditions. In preferred embodiments the bone matrix is derived from human bone. The treatment or condition may alter the structure of the bone matrix and / or cleave one or more specific proteins. Cleavage may generate peptides or protein fragments that have osteoinductive, osteogenic, or chondrogenic activity. Preferred treatments include collagenase and various other proteases. The invention further provides improved bone and cartilage matrix compositions that have been prepared according to the inventive methods and methods of treatment using the compositions. The invention further provides methods of preparing, testing, and using the improved bone matrix compositions. Ona assay comprises exposing relatively undifferentiated mesenchymal cells to a bone matrix composition and measuring expression of a marker characteristic of osteoblast or chondrocyte lineage(s). Increased expression of the marker relative to the level of the marker in cells that have been exposed to a control matrix (e.g., an inactivated or untreated matrix) indicates that the treatment or condition increased the osteogenic and / or chondrogenic activity of the bone matrix. Suitable cells include C2C12 cells. A suitable marker is alkaline phosphatase. The inventive methods increase the osteogenic and / or chondrogenic activity of human DBM when tested using this assay system.
Owner:WARSAW ORTHOPEDIC INC
Bis-sulfhydryl macrocyclization systems
InactiveUS20090047711A1Good biological propertiesSimple structureApoptosis related proteinsCyclic peptide ingredientsCombinatorial chemistryAmino acid
The present invention provides novel peptidomimetic macrocycles and methods for their preparation and use, as well as amino acid analogs and macrocycle-forming linkers, and kits useful in their production.
Owner:AILERON THERAPEUTICS INC
Human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive
InactiveCN101804218AIncrease usageImprove usabilityAbsorbent padsBandagesDressing changeCurative effect
The invention discloses a human-body absorbable trauma dressing containing Yunnan white drug powder or Yunnan white drug powder extractive, which is a novel medicine-carried dressing or a novel formulation of Yunnan white drug powder. The invention has the following remarkable characteristics: (1) the dressing can be absorbed by human bodies to lessen the pain added by dressing change and reduce the treatment cost; (2) the dressing can be made into a film solid dressing or an aquagel dressing so as to expand the use modes, the scope of applications and the drug effect of the Yunnan white drug powder; and (3) the curative effect of the dressing is enhanced by selecting a carrier material, auxiliary medicaments and functional accessories and adjusting the microstructure structure. The novel absorbable Yunnan white drug powder dressing overcomes the defects of the traditional Yunnan white drug powder in use and has economic and social values.
Owner:王艳
Bis-Sulfhydryl Macrocyclization Systems
ActiveUS20090088553A1Good biological propertiesSimple structureCell receptors/surface-antigens/surface-determinantsPeptide preparation methodsCombinatorial chemistryAmino acid
The present invention provides novel peptidomimetic macrocycles and methods for their preparation and use, as well as amino acid analogs and macrocycle-forming linkers, and kits useful in their production.
Owner:AILERON THERAPEUTICS INC
Bis-sulfhydryl macrocyclization systems
ActiveUS20100184628A1Good biological propertiesSimple structureApoptosis related proteinsCyclic peptide ingredientsCombinatorial chemistryAmino acid
Owner:AILERON THERAPEUTICS INC
Conjugates comprising a biodegradable polymer and uses therefor
InactiveUS20050169968A1Improve biological activityProlong half-life in vivoPeptide/protein ingredientsPharmaceutical non-active ingredientsActive agentThrombosis prevention
Biologically active agents covalently linked to a polymer. The polymer is preferably a biodegradable polymer are provided. The biologically active agent is preferably a protein, such as an extracellular soluble protein, e.g., an extracellular enzyme. The enzyme can be an apyrase, e.g., NTPDase. Conjugates of the invention can be used as therapeutics in subjects. For example, a conjugate comprising an apyrase can be used for treating and preventing thrombosis, atherosclerotic plaque complications and vascular disorders.
Owner:MERSANA THERAPEUTICS INC +1
Casein formulations for the delivery of bioactive constituents
InactiveUS6652875B1Improve concentrationIncreases the rate that the phosphoprotein dissolvesBiocideCosmetic preparationsAdditive ingredientChemistry
The present invention provides a formulation for the delivery of bioactive constituents to biological surfaces, wherein said formulation comprises a suspensions or solution of at least one isolated and purified casein protein or salt thereof, in water, together with at least one bioactive constituent.
Owner:UNIVERSITY OF MELBOURNE
Peptide pharmaceutical compositions
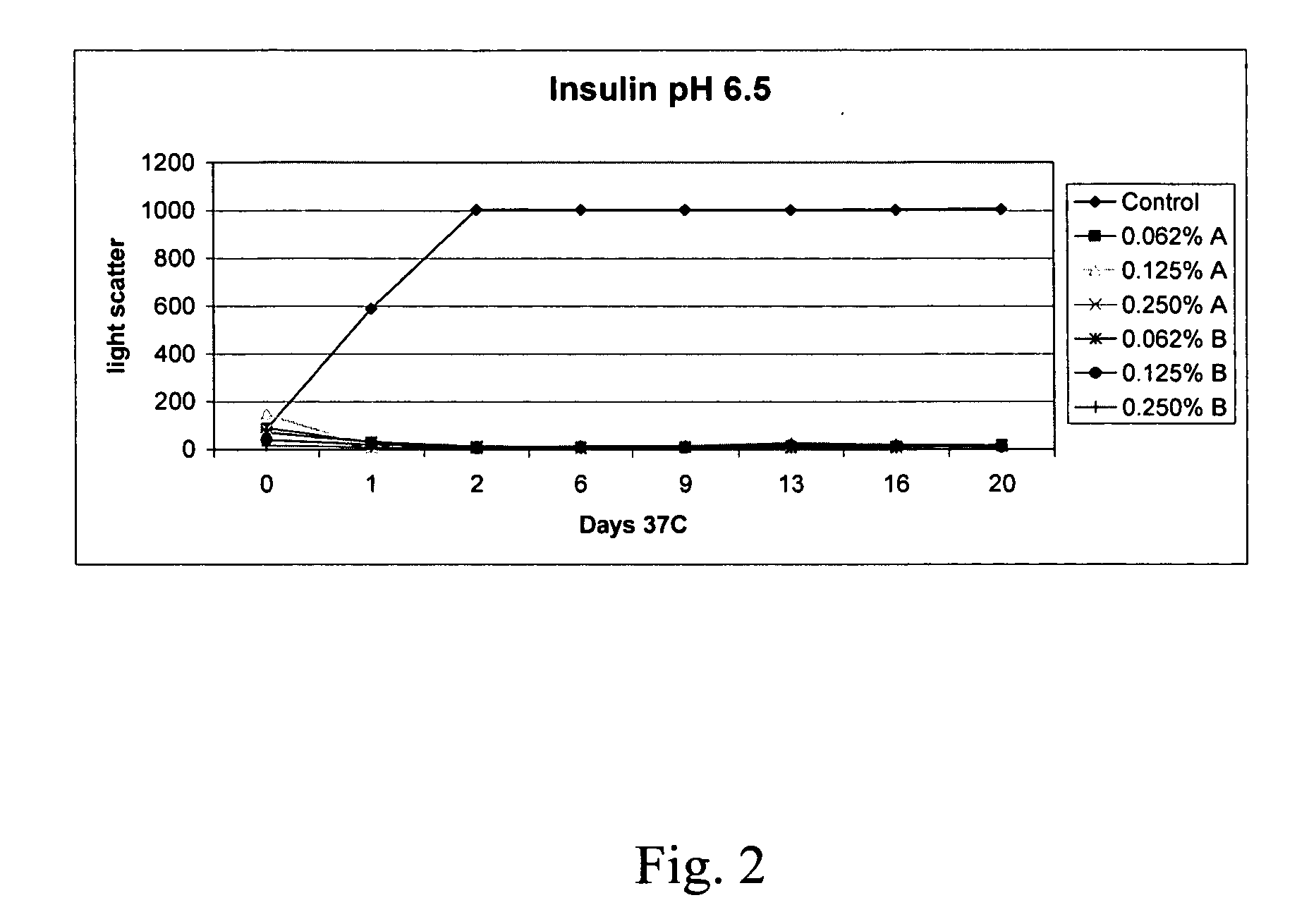
ActiveUS20070111938A1Improve biological activityReducing and preventing aggregationBiocidePeptide/protein ingredientsMedicinePeptide T
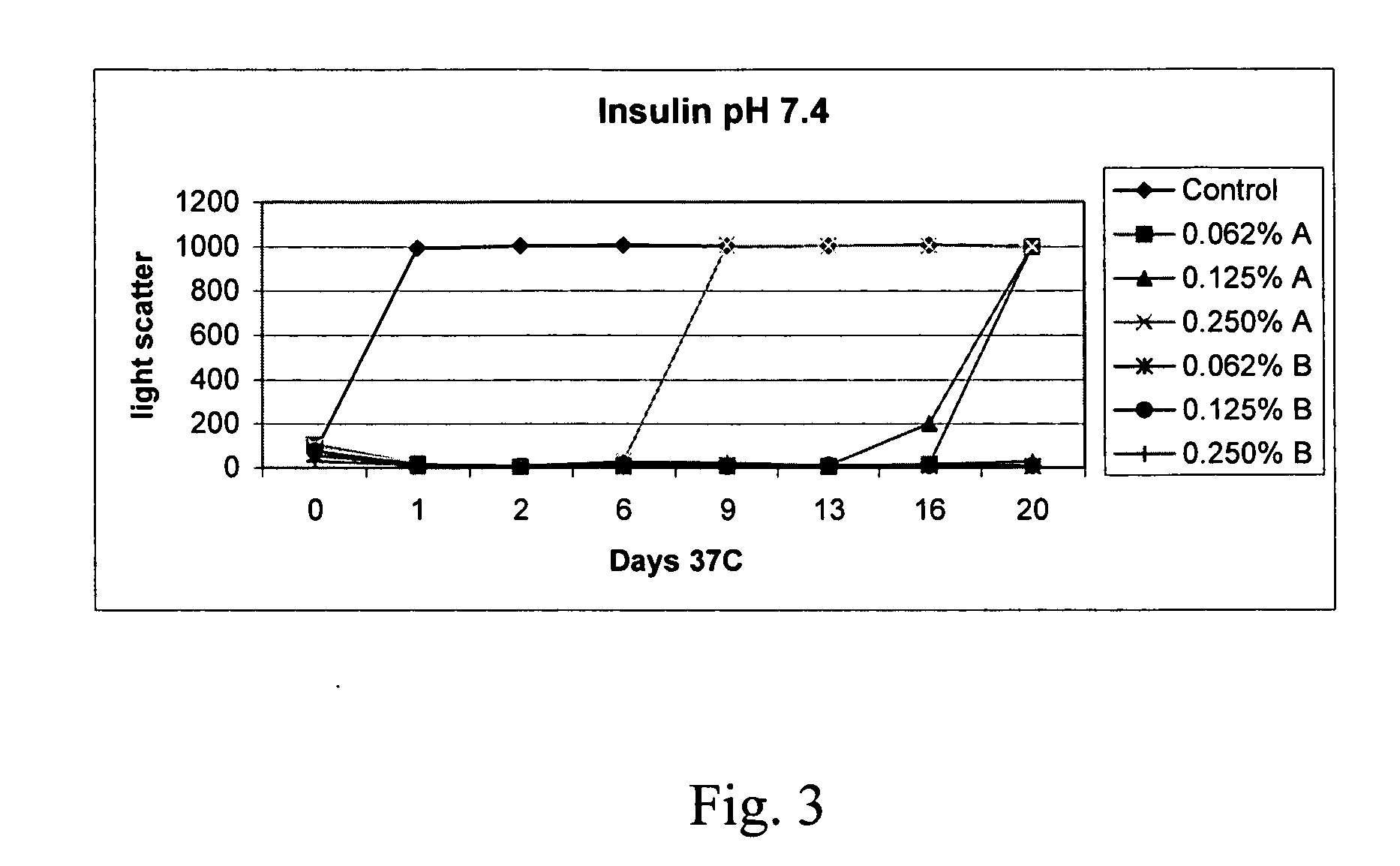
The present invention relates to alkylglycoside-containing compositions and methods for increasing the stability, reducing the aggregation and immunogenicity, increasing the biological activity, and reducing or preventing fibrillar formation of a peptide, polypeptide, or variant thereof, for example insulin and Peptide T or analog thereof.
Owner:PEPTIDE ACCOUNT LLC
Synthetic apelin mimetics for the treatment of heart failure
ActiveUS8673848B2Extended half-lifeIncrease constraintsNervous disorderSkeletal disorderCardiac fibrosisVentricular tachycardia
The invention provides a synthetic polypeptide of Formula I′:or an amide, an ester or a salt thereof, wherein X1, X2, X3, X4, X5, X6, X7, X8, X9, X10, X11, X12 and X13 are defined herein. The polypeptides are agonist of the APJ receptor. The invention also relates to a method for manufacturing the polypeptides of the invention, and its therapeutic uses such as treatment or prevention of acute decompensated heart failure (ADHF), chronic heart failure, pulmonary hypertension, atrial fibrillation, Brugada syndrome, ventricular tachycardia, atherosclerosis, hypertension, restenosis, ischemic cardiovascular diseases, cardiomyopathy, cardiac fibrosis, arrhythmia, water retention, diabetes (including gestational diabetes), obesity, peripheral arterial disease, cerebrovascular accidents, transient ischemic attacks, traumatic brain injuries, amyotrophic lateral sclerosis, burn injuries (including sunburn) and preeclampsia. The present invention further provides a combination of pharmacologically active agents and a pharmaceutical composition.
Owner:NOVARTIS AG
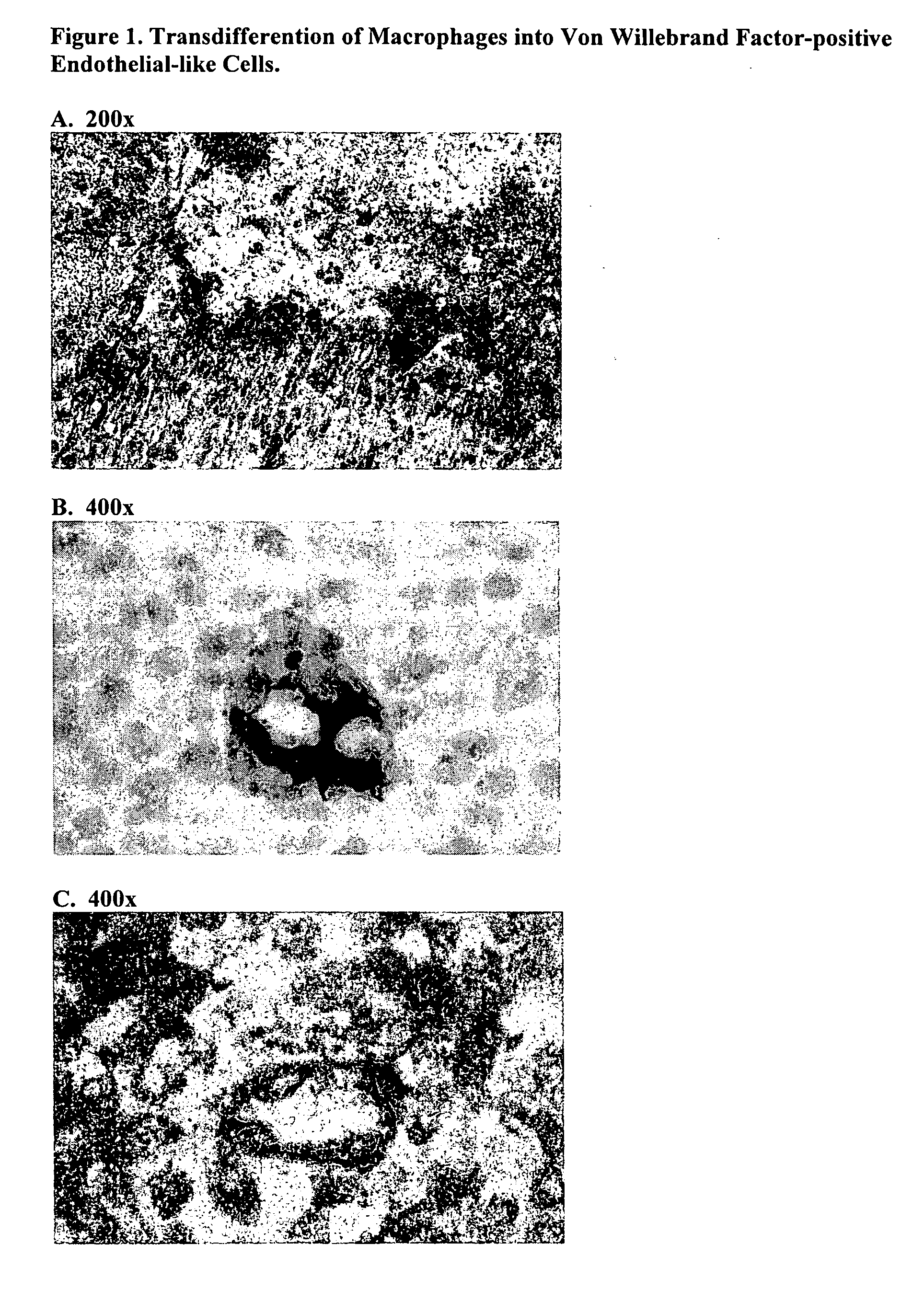
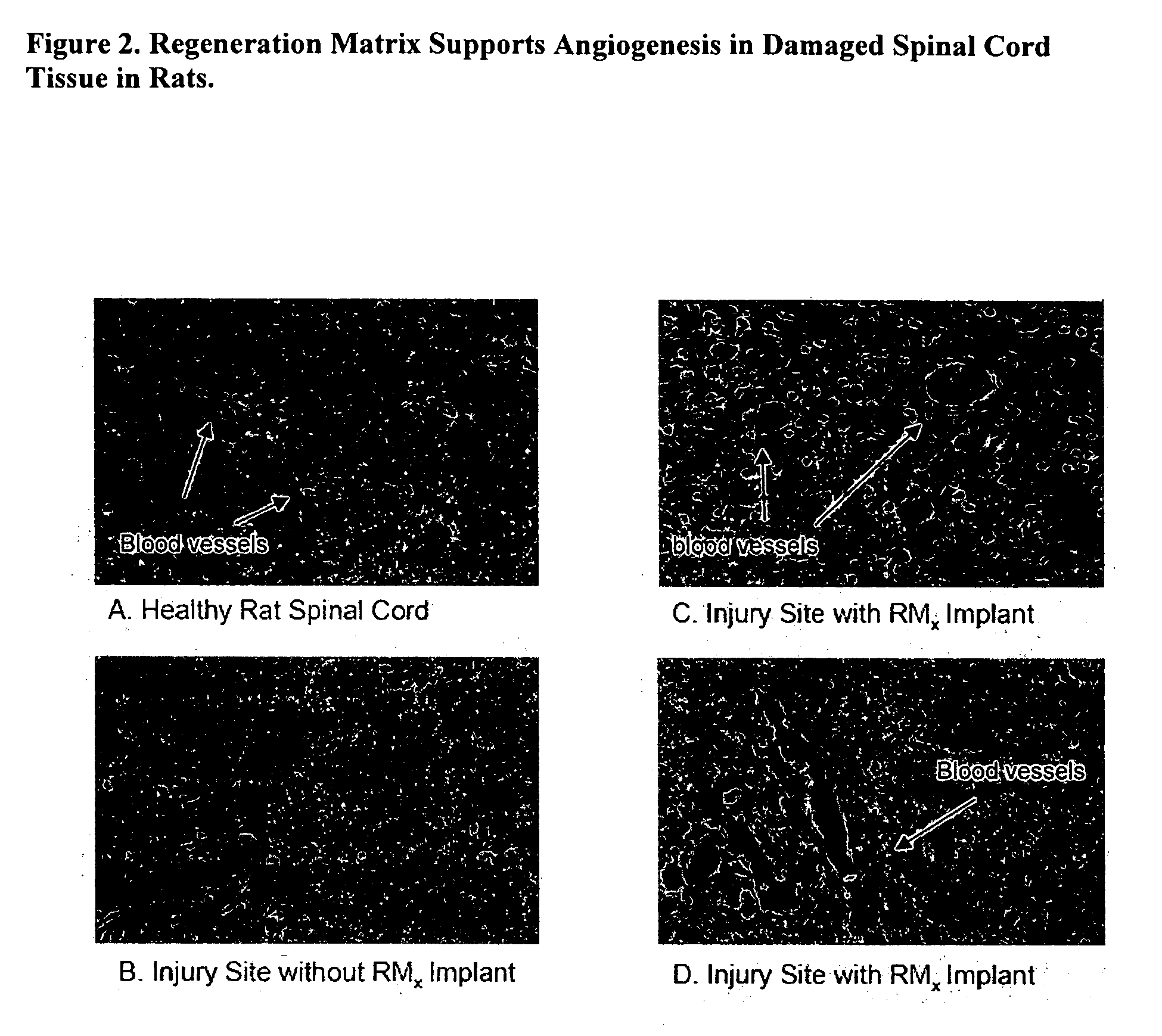
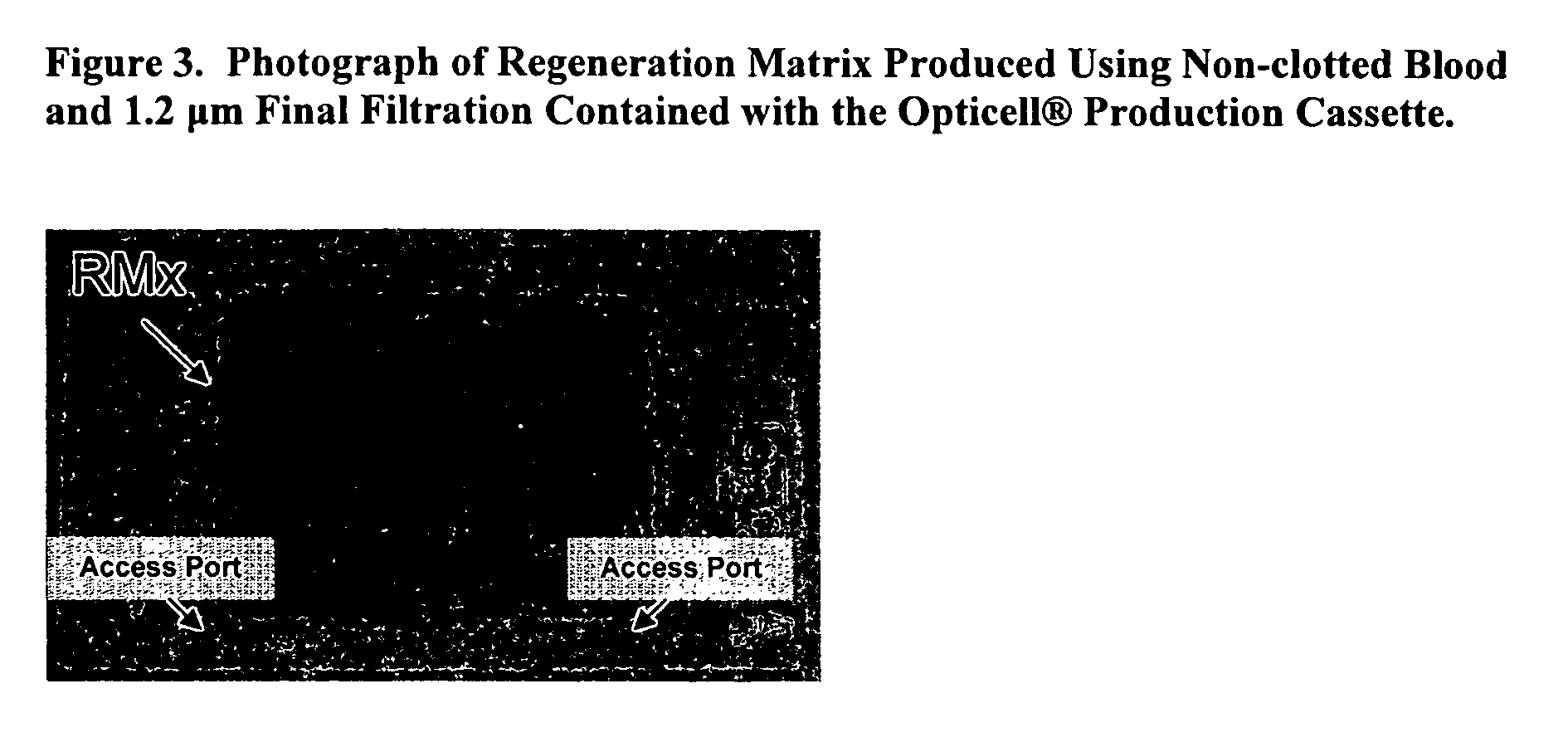
Acellular bioabsorbable tissue regeneration matrices
ActiveUS20070202189A1Lightweight productionPromote regenerationBiocideNervous disorderNerves tissuePathology
Owner:GENESIS TECH LTD
Lipoxin compounds and their use in treating cell proliferative disorders
InactiveUS6887901B1Increased in vive potencyHigh binding affinitySalicyclic acid active ingredientsBiocideHalf-lifeMedicine
Compounds having the active site of natural lipoxins, but a longer tissue half-life are disclosed. In particular, 15-epi-lipoxins and their use in ameliorating undesired cell proliferation, which characterizes diseases such as cancer, are also disclosed.
Owner:THE BRIGHAM & WOMEN S HOSPITAL INC
Slow-release fertilizer with effect of soil remediation and remediation method of soil polluted by polycyclic aromatic hydrocarbons
ActiveCN101928188AImprove repair efficiencyImprove biological activityContaminated soil reclamationFertilizer mixturesSolubilityPhytoremediation
The invention discloses slow-release fertilizer with the effect of soil remediation, which is prepared by polyhydroxyalkanoates which can be completely biodegraded, organic and inorganic nutrients and other substances, by slow-releasing the organic and inorganic nutrients, microorganism propagation and sustained and rapid growth of plants can be promoted, the bioactivity and water solubility of polycyclic aromatic hydrocarbons are quickened, and simultaneously, the defects of fertilizer loss and repeated fertilization can be avoided, thereby saving the cost and enhancing the soil remediation efficiency. The invention also discloses a soil pollution remediation method by utilizing the slow-release fertilizer, plants with the remediation effect are planted in the soil with the slow-release fertilizer, and efficient, environment-friendly and sustained remediation of the soil polluted by the polycyclic aromatic hydrocarbons (PAHs) can be realized by utilizing the microorganism-plant remediation technology.
Owner:SHENZHEN ECOMANN BIOTECH
Small intestinal submucosa (SIS) soft tissue repair patch and preparation method thereof
InactiveCN102462561AImprove surface porosityImprove biological activityProsthesisTissue repairSoft tissue.FNA
The invention relates to a method for preparing a small intestinal submucosa (SIS) soft tissue repair patch, and the soft tissue repair patch prepared by the method. The invention also relates to application of the soft tissue repair path to tissue repair.
Owner:BEIJING MED ZENITH MEDICAL SCI CORP LTD
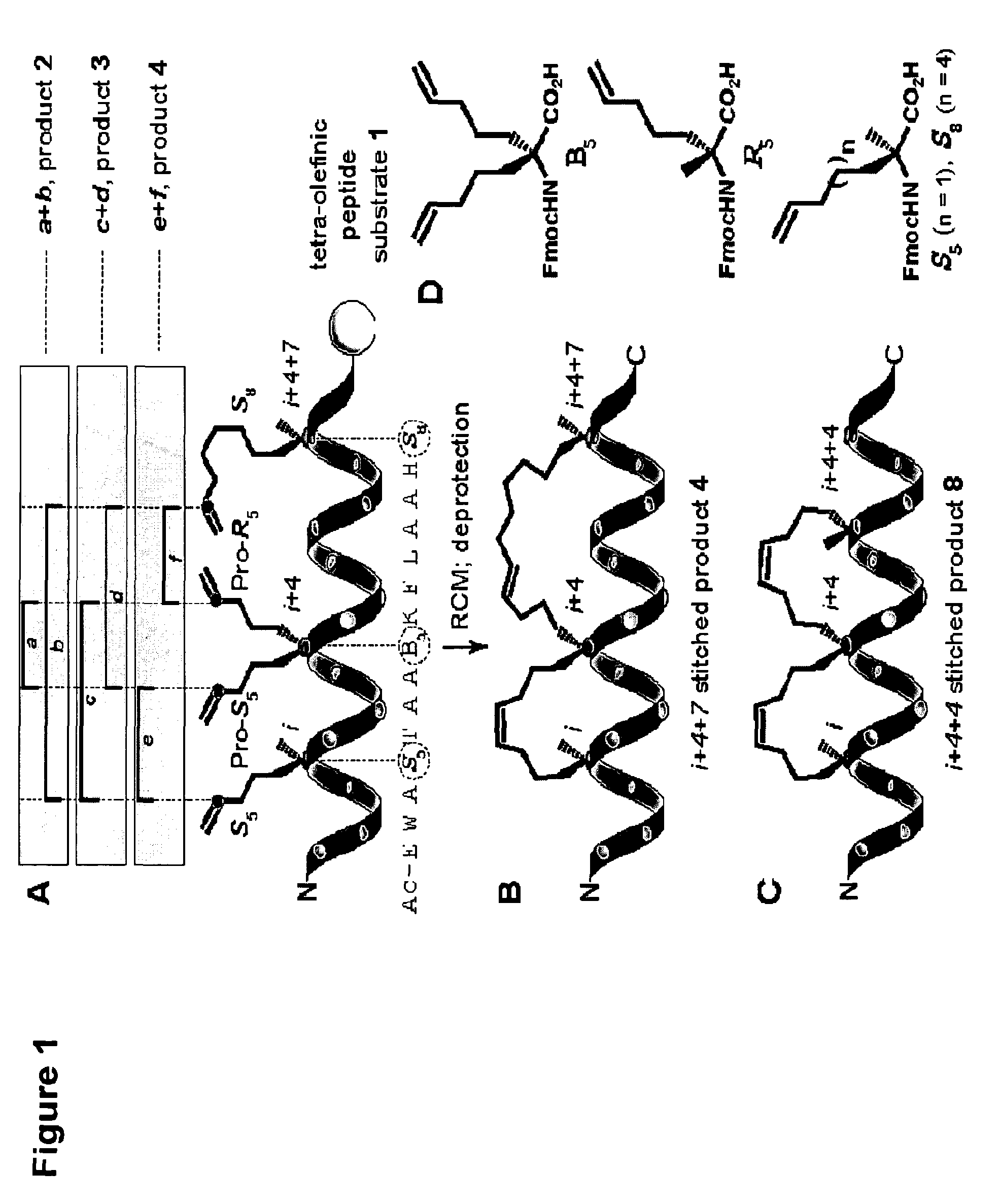
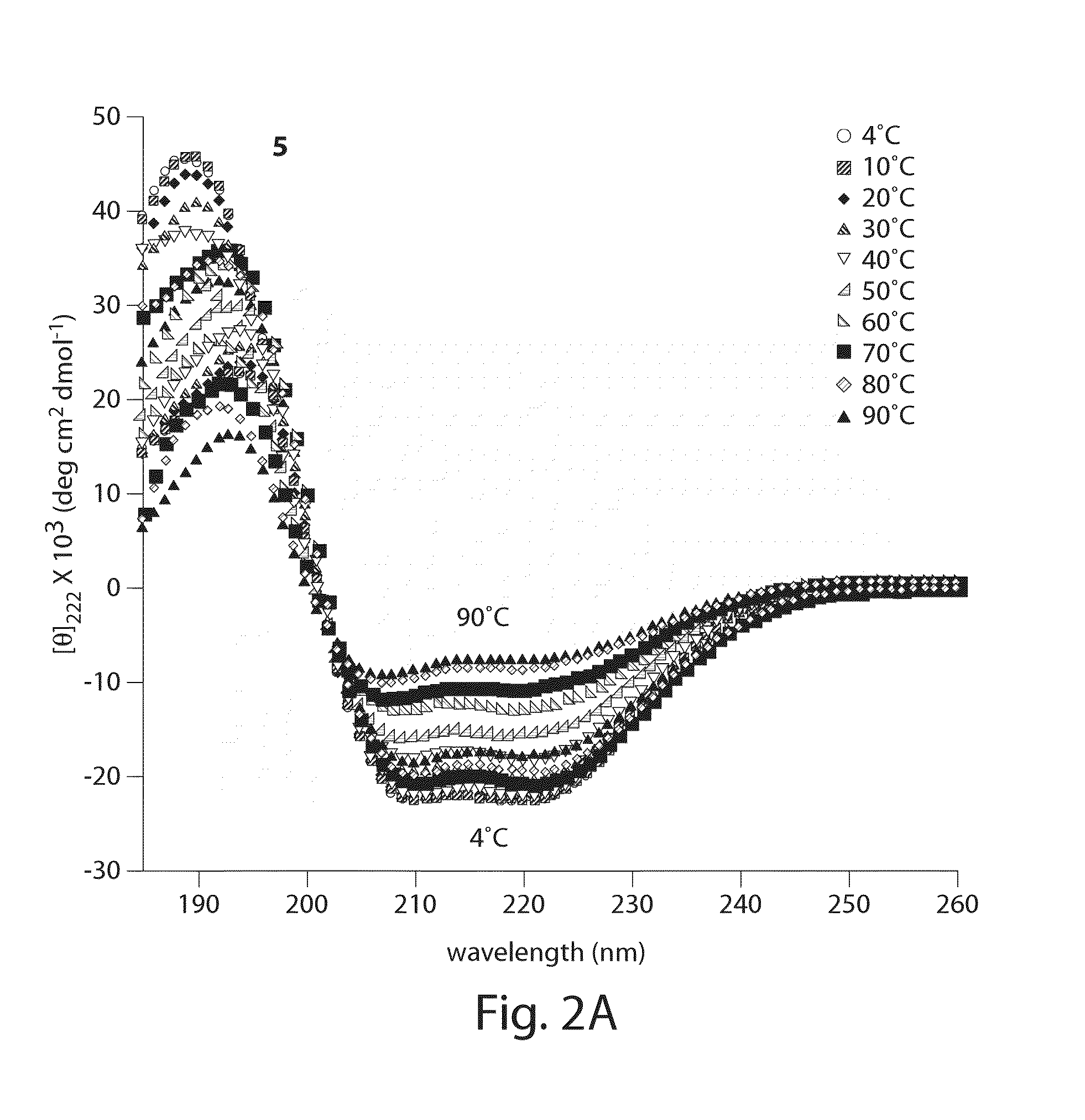
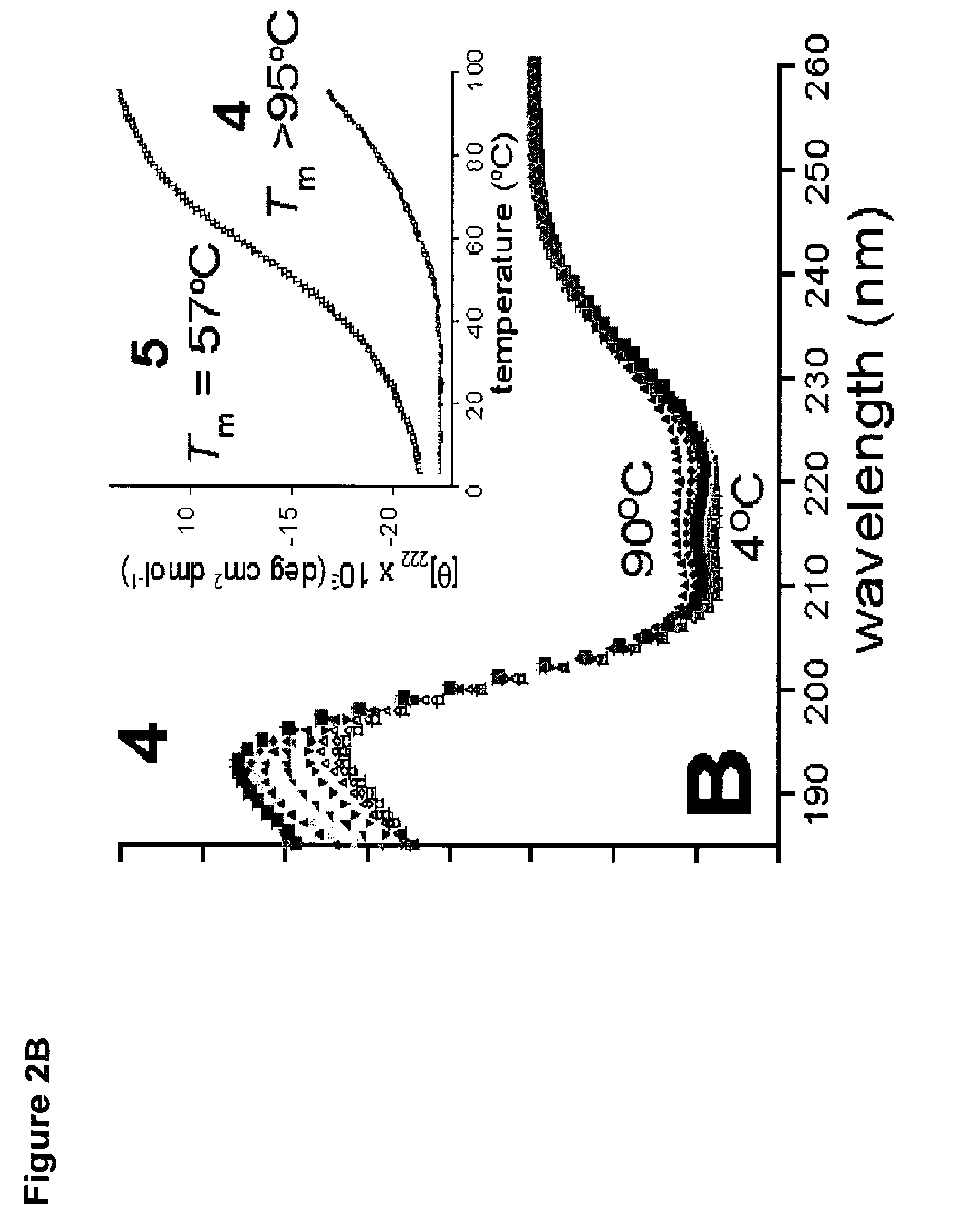
Stitched polypeptides
ActiveUS8592377B2Improve the immunityImprove permeabilityAntibacterial agentsSenses disorderBiochemistry
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Inhibiting GS-FDH to modulate NO bioactivity
InactiveUS20020128205A1Increasing NO bioactivityIncreased nitric oxide bioactivityBiocideAntipyreticPatient needNo donors
Patients needing NO donor therapy or inhibition of pathologically proliferating cells or increased NO bioactivity are treated with a therapeutically effective amount of an inhibitor of glutathione-dependent formaldehyde dehydrogenase.
Owner:DUKE UNIV
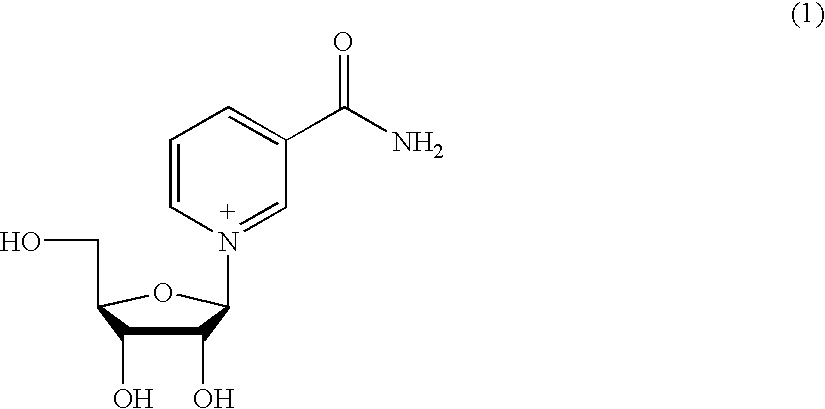
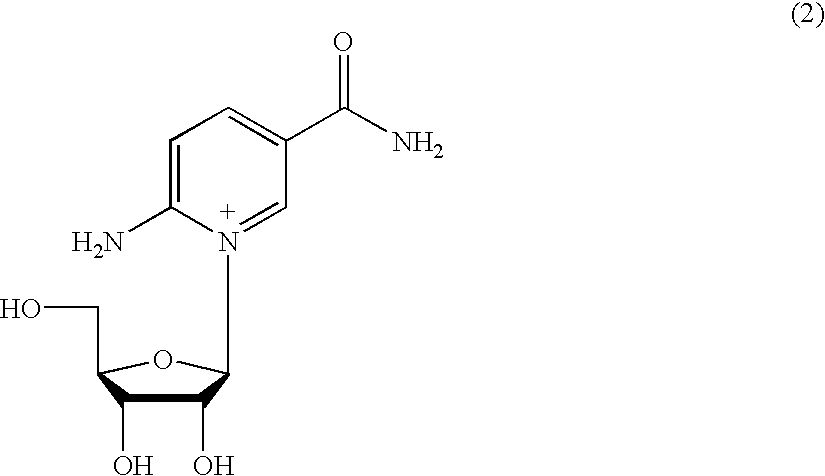
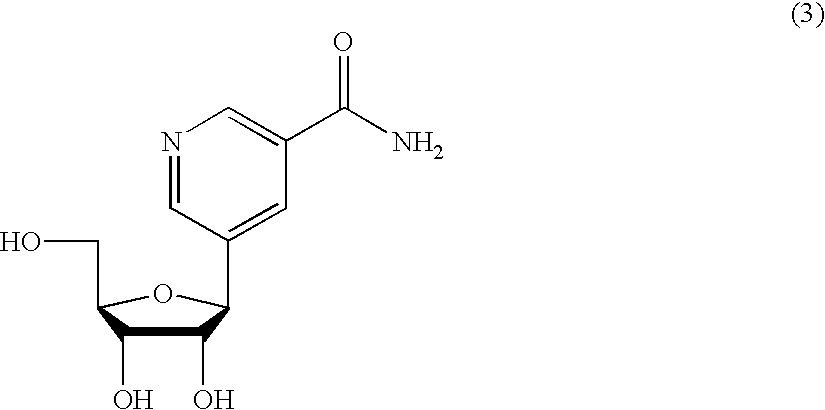
Molecules for transporting a compound across the blood-brain barrier
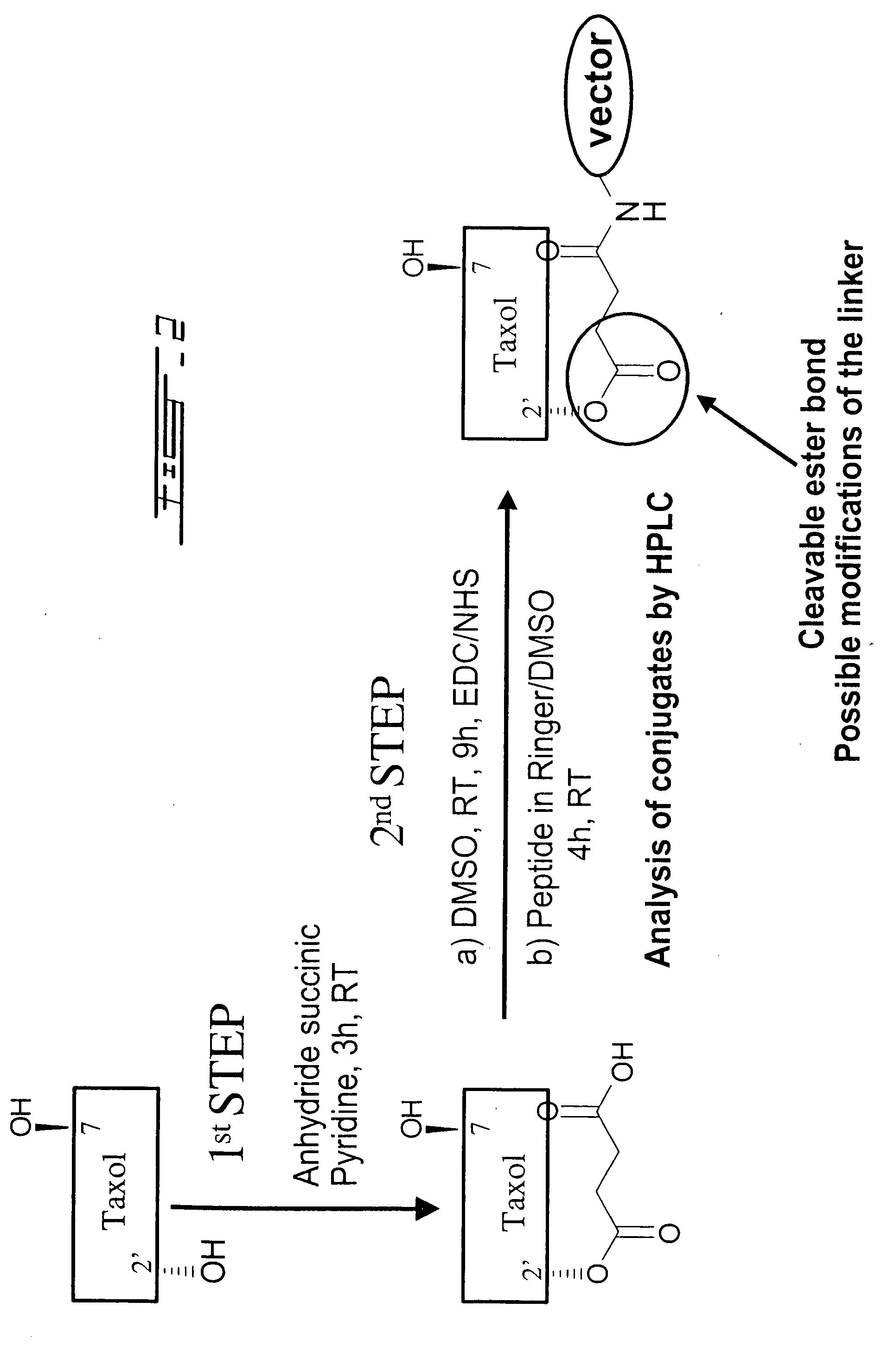
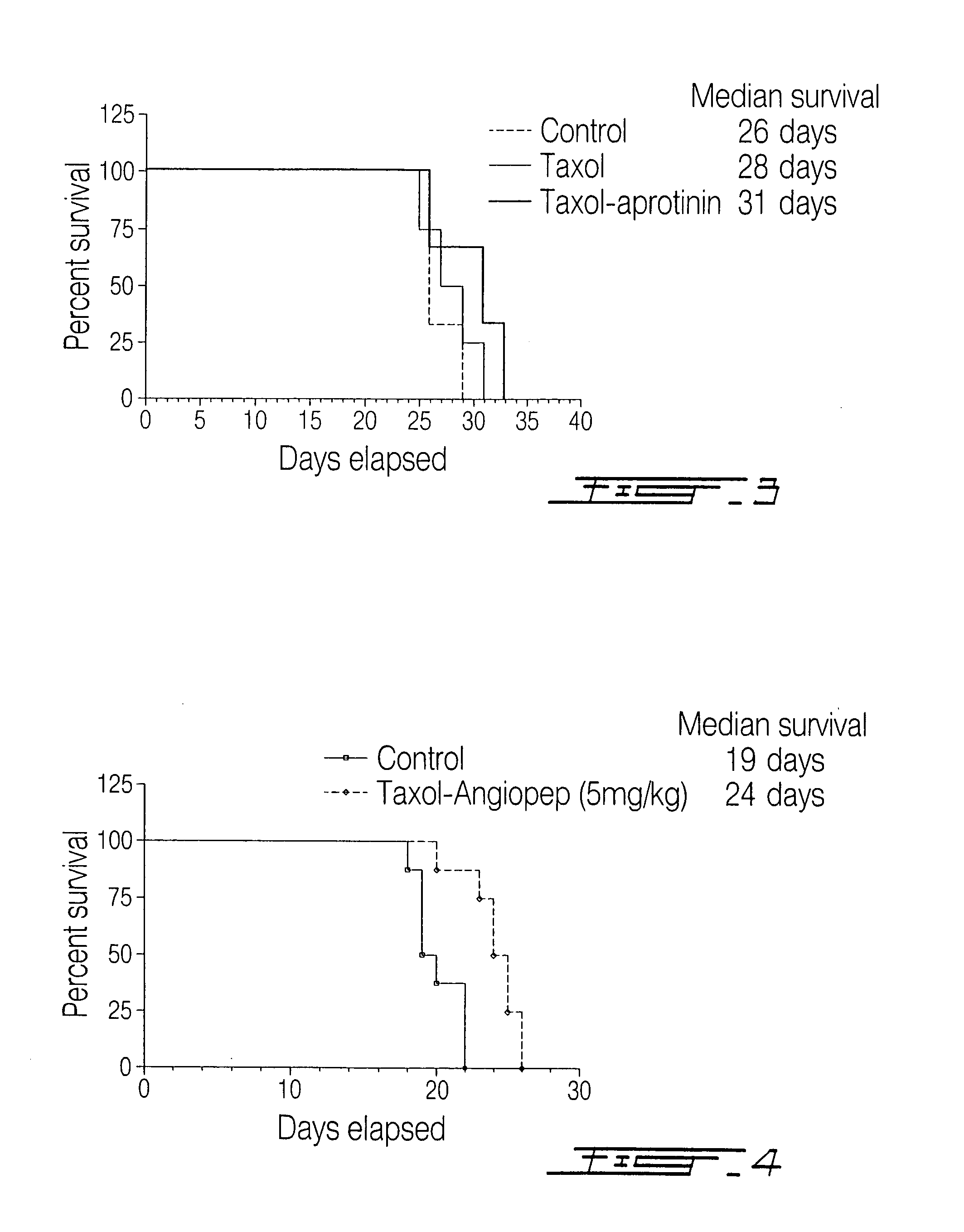
ActiveUS20060189515A1Non-invasiveImprove biological activityOrganic active ingredientsNervous disorderDisease causeProtein C
The invention relates to improvements in the field of drug delivery. More particularly, the invention relates to polypeptide derived from aprotinin and from aprotinin analogs as well as conjugates and pharmaceutical compositions comprising these polypeptides. The present invention also relates to the use of these polypeptide for transporting a compound or drug across the blood-brain barrier of an individual and in the treatment and diagnosis of neurological diseases.
Owner:UNIV DU QUEBEC A MONTREAL +1
Casein formulations for the delivery of bioactive constituents
InactiveUS20030206939A1Increase rate increaseImprove biological activityBiocideCosmetic preparationsAdditive ingredientChemistry
The present invention provides a formulation for the delivery of bioactive constituents to biological surfaces, wherein said formulation comprises a suspension or solution of at least one isolated and purified casein protein or salt thereof, in water, together with at least one bioactive constituent.
Owner:PACIFIC BIOLINK
Endogenous retrovirus up-regulated in prostate cancer
InactiveUS20060275747A1Avoid the needHigh sensitivityMicrobiological testing/measurementMaterial analysisEndogenous retrovirusNucleotide sequenc
A specific member of the HERV-K family located in chromosome 22 at 20.428 megabases (22q11.2) has been found to be preferentially and significantly up-regulated in prostate tumors. The invention provides methods for diagnosing prostate cancer, comprising the step of detecting in a patient sample the presence or absence of an expression product of the virus. The virus has five features not seen in other HERV-K members: (1) its own specific nucleotide sequence, and consequently amino acid sequences; (2) tandem 5′ LTRs; (3) a fragmented 3′ LTR; (4) an env gene interrupted by an alu insertion; and (5) unique gag sequences.
Owner:CHIRON CORP
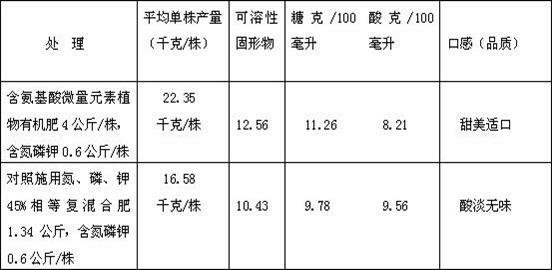
Compound amino acid organic fertilizer and production method of compound amino acid organic fertilizer
The invention disclose a compound amino acid organic fertilizer, which is characterized in that the compound amino acid organic fertilizer consists of the following ingredients in parts by weight: 5 to 10 of compound amino acid, 0.3 to 0.5 of microbial yeasts, 16 to 29 of inorganic fertilizers, 5 to 10 of humic acid fertilizers and 0.2 to 1 of trace elements. A production method of the compound amino acid organic fertilizer comprises the following steps that: firstly, compound amino acid water solution, the microbial yeasts and the humic acid fertilizers are respectively prepared, then, inorganic compounds containing boron, copper, iron, manganese and zinc are added into the compound amino acid water solution, and the chelation is carried out at a temperature being 80 to 110 DEG C so that the mixture becomes free-state chelated amino acid salt; after the metering, 10 to 50kg of raw materials are added into each ton of farmyard manure to be mixed, then, the mixed materials are placed into a warehouse for natural stacking and curing for 3 to 5 days and can be pelletized and packed to leave factories. The compound amino acid organic fertilizer can be used as a base fertilizer and an additional fertilizer and belongs to a compound organic fertilizer capable of improving the amino acid content of high-quality fragrant rice and fruit and vegetables, improving the fruit and vegetable quality and improving the sugar content of sugarcanes.
Owner:GUANGXI YUXIN BIOLOGICAL TECH
Organic waste water treatment process
InactiveCN1631818AAvoid toxicityHigh removal rateMultistage water/sewage treatmentHigh densityElectrolysis
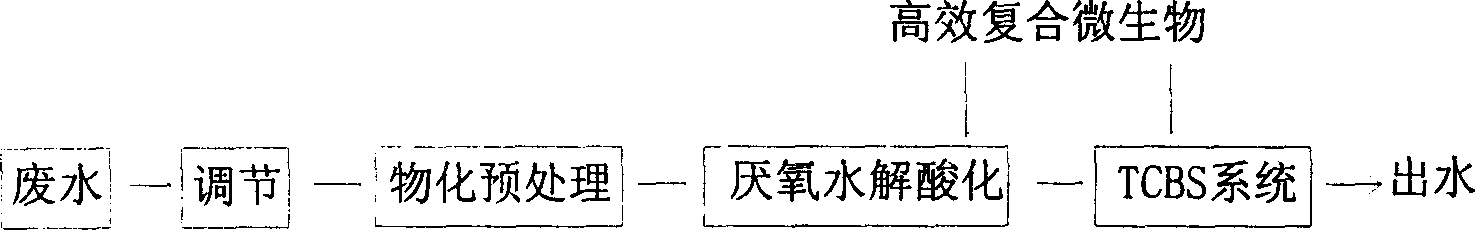
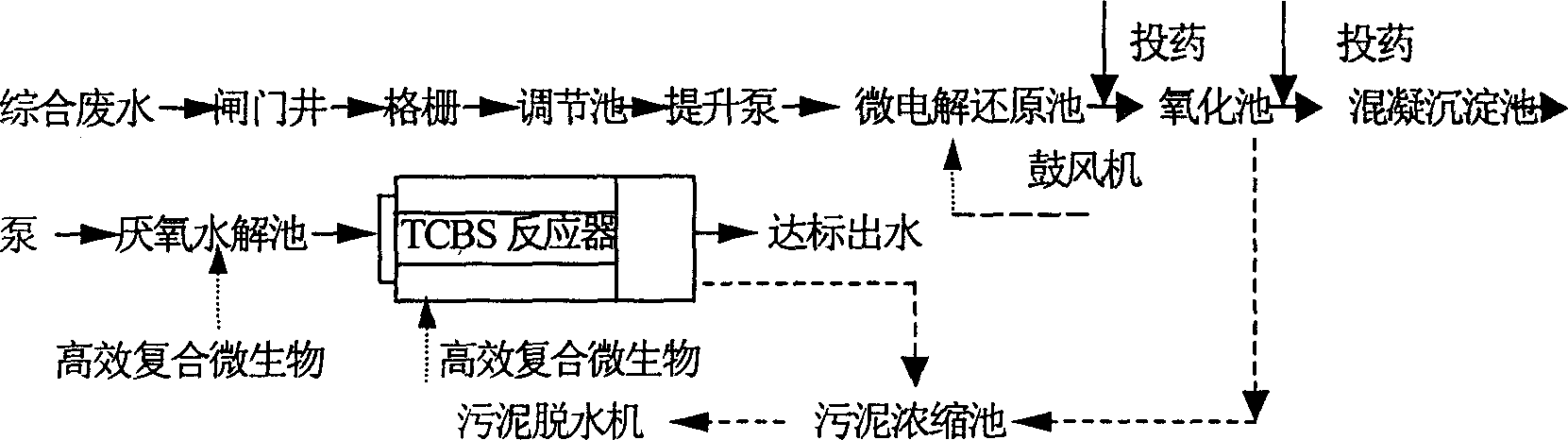
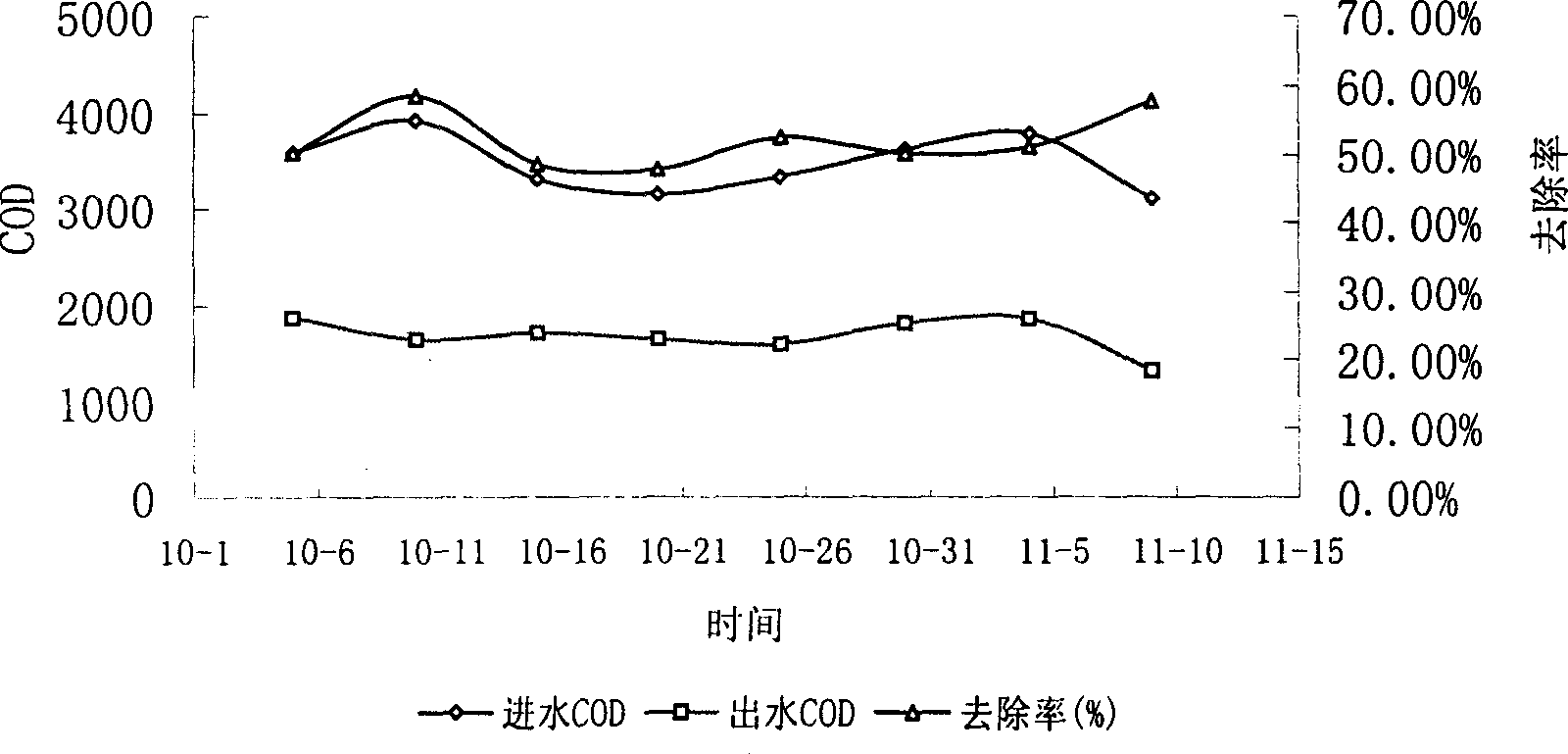
Disclosed is a technique for processing organic waste water, including the following steps: (a) preprocession: pour the waste water into the micro electrolytic reducing pool where the iron-carbon micro electrolytic reaction occurs under the agtatering effect, add hydrogen peroxide into it to have. Fento oxidation, then the water enters into coagulation pool into which add NaOH and PAM; (b) anaerobe hydrolysis oxidation procession: power the organic waste water preprocessed into high effective anaerobe hydrolysis oxidation pool in which add into the TCBS reactor in which add into high effective compound microbe, making the water mix with the flowing-back mud with high density after denitrification. The invention can increase the biochemical of organic waste water, strengthen its resistance to poison and impact as well as the biological denitrification funcation, and making the waste water reach the national environment protection requirement by reducing the polluting load by steps.
Owner:何义亮 +1
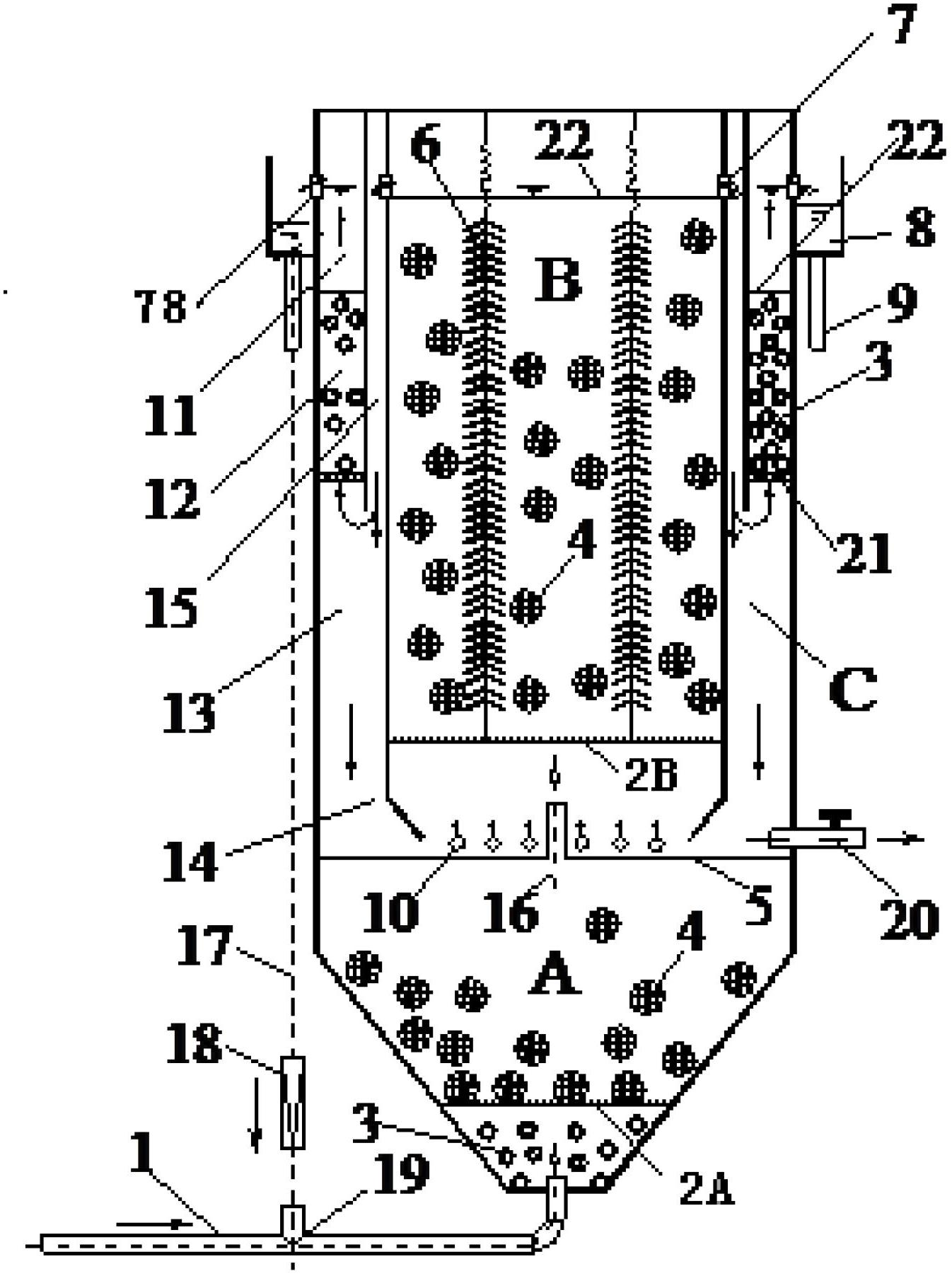
Integrated internal circulation type denitrification and decarburization bio-membrane reactor and operating method thereof
InactiveCN102659244AEfficient removalEfficient nitrogen and carbon removalTreatment with aerobic and anaerobic processesSludgeMembrane reactor
The invention provides an integrated internal circulation type denitrification and decarburization bio-membrane reactor and an operating method thereof. The main body of the reactor consists of an anoxic zone on the lower part, an aerobic zone on the upper part and a settling zone on the periphery of the aerobic zone; a composite filler is filled in the anoxic zone, and a water inlet pipe is communicated with the bottom of the anoxic zone; the aerobic zone is partitioned into a filler zone for adding a carrier on the upper part and a mud-water mixing zone on the lower part by adopting an orifice plate, the anoxic zone and the aerobic zone are partitioned through an impermeable partition plate, and a plurality of aeration pipes are arranged at the bottom of the aerobic zone and provided with air inlet pores; and the settling zone consists of three parts, namely a clean water zone, a contact settling zone and a sludge returning zone which are sequentially arranged from top to bottom, wherein the bottom of the sludge returning zone is provided with a sludge returning seam and communicated with the bottom of the aerobic zone. The reactor realizes zero power consumption in sludge returning, and is compact in structure; and a back flushing system is not required in the contact settling zone, and additional agents and carbon sources are not required, so that the reactor is low in running cost.
Owner:SUZHOU UNIV OF SCI & TECH
Effective plant component extracting process
InactiveCN1986029AChange microscopic propertiesEasy extractionSolid solvent extractionOrganic solventAlcohol
The effective plant component extracting process includes mechanically crushing the mixture of solid plant material and proper amount of chemical assistant, and the subsequent extraction of the effective component with water or alcohol. The mechanical crushing can produce micro shearing strain in the interface, break cell wall, expose fresh cut surface and produce mutual adsorption and other action between the grain clusters and molecular groups of the chemical assistant and the effective component, so as to alter the microscopic performance of the effective plant component, raise the leached amount by 80-400 %, short the extracting period greatly, raise the extracting efficiency obviously raise the extraction specificity and selectivity, minimize the organic solvent in use, lower the extraction cost and raise the bioavailability of the extracted matter.
Owner:DALIAN SEM BIOLOGICAL ENG TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com