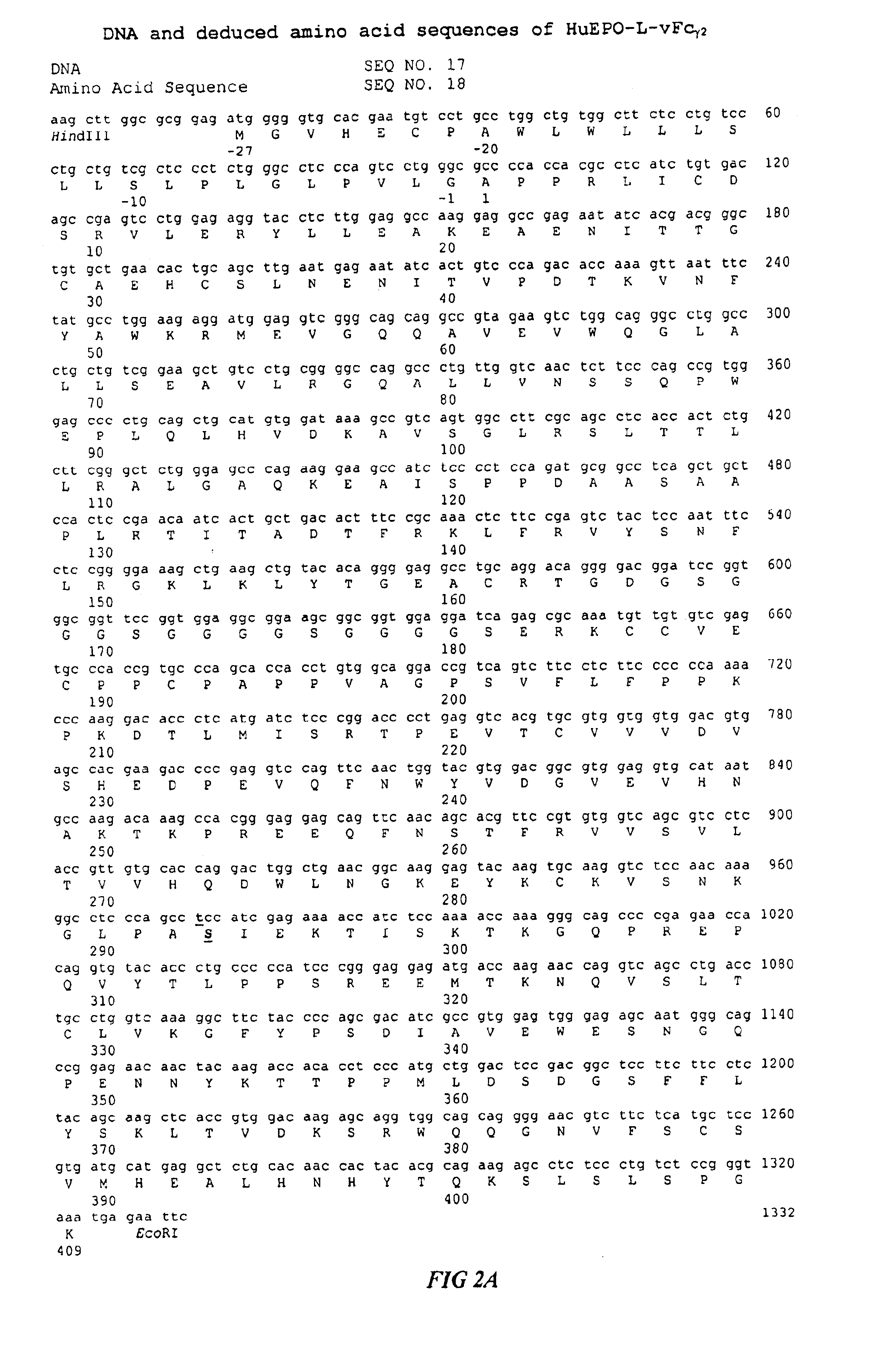Patents
Literature
361results about How to "Improve pharmacokinetics" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
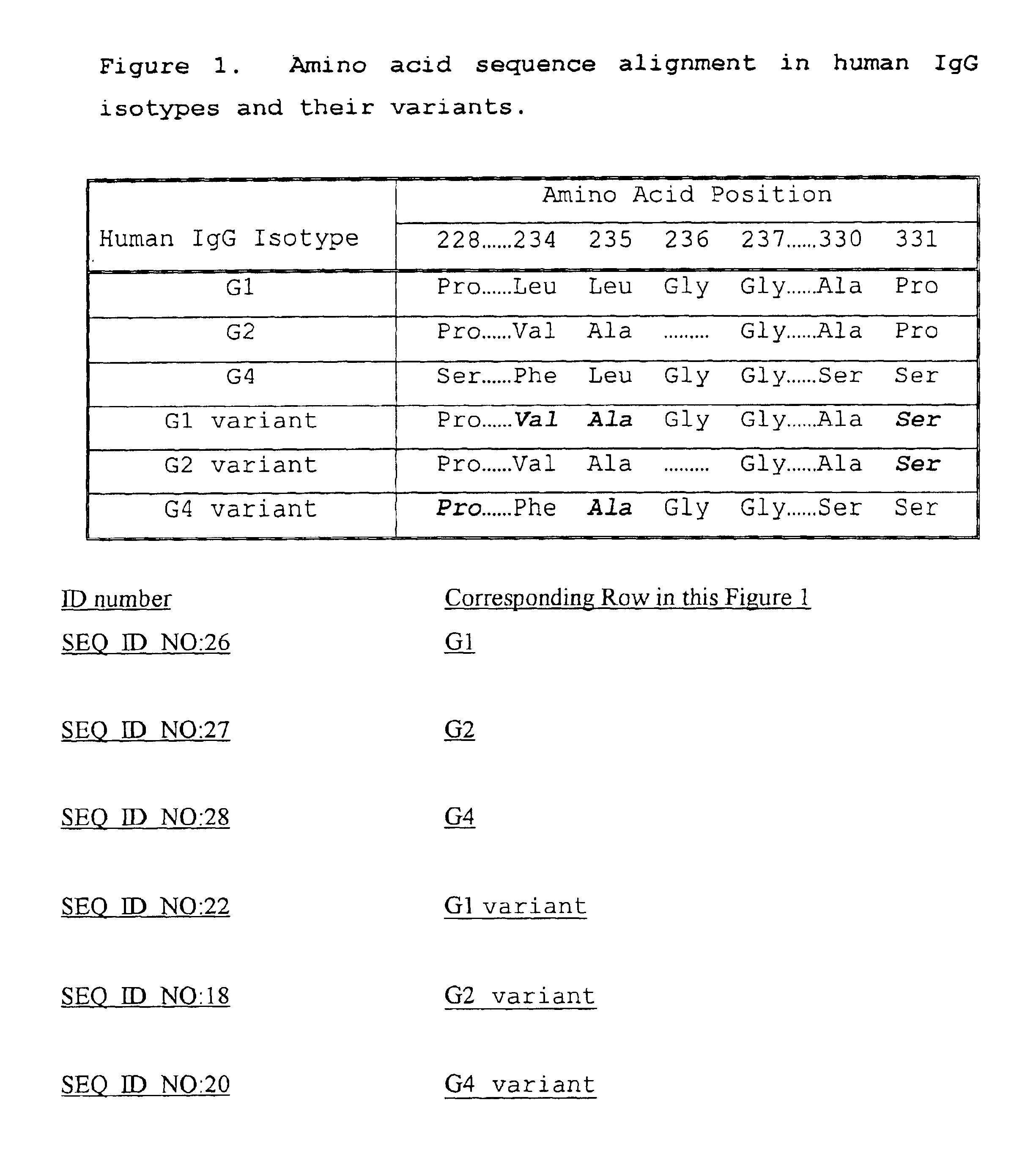
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS6900292B2Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:LONGBIO PHARM (SUZHOU) CO LTD
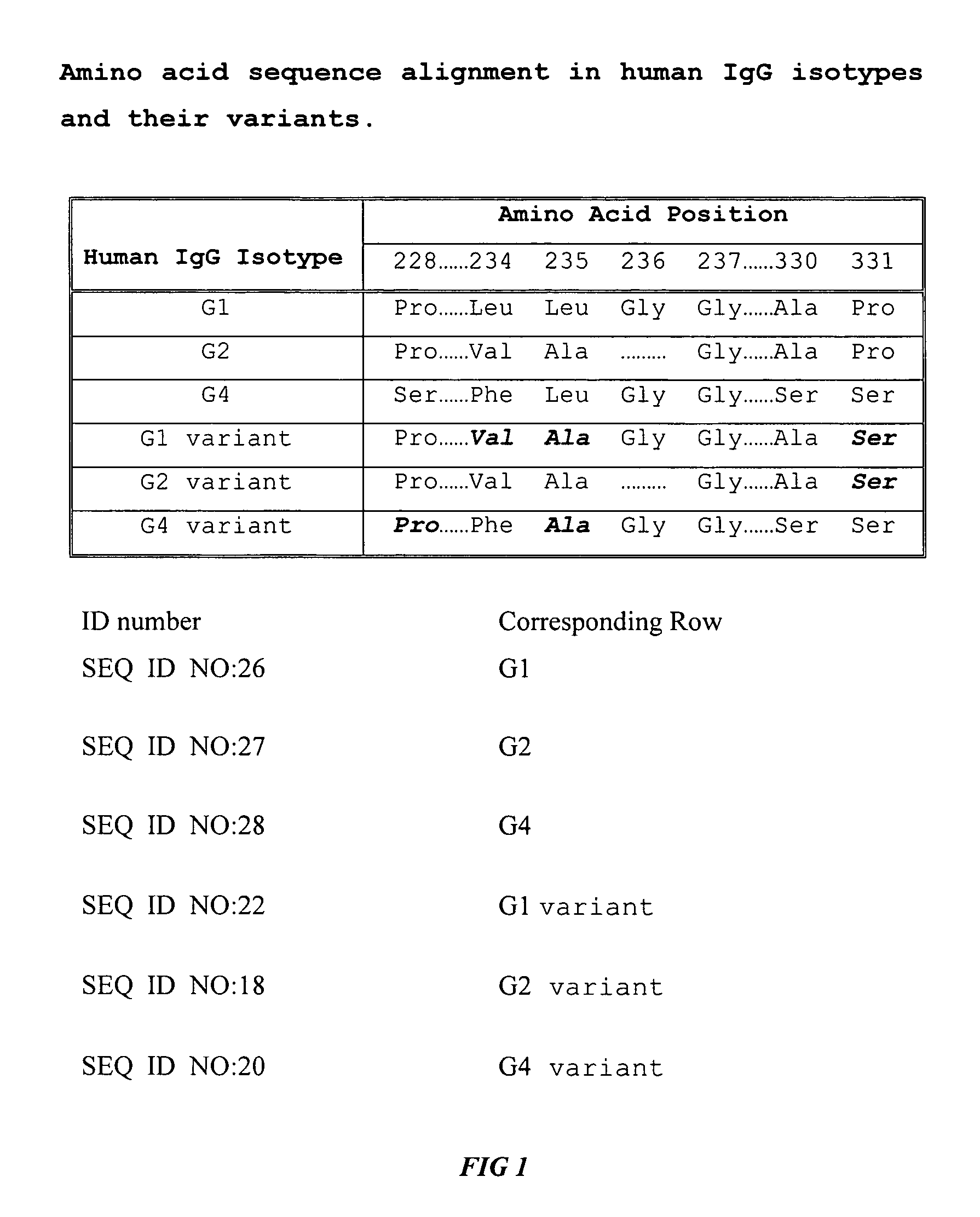
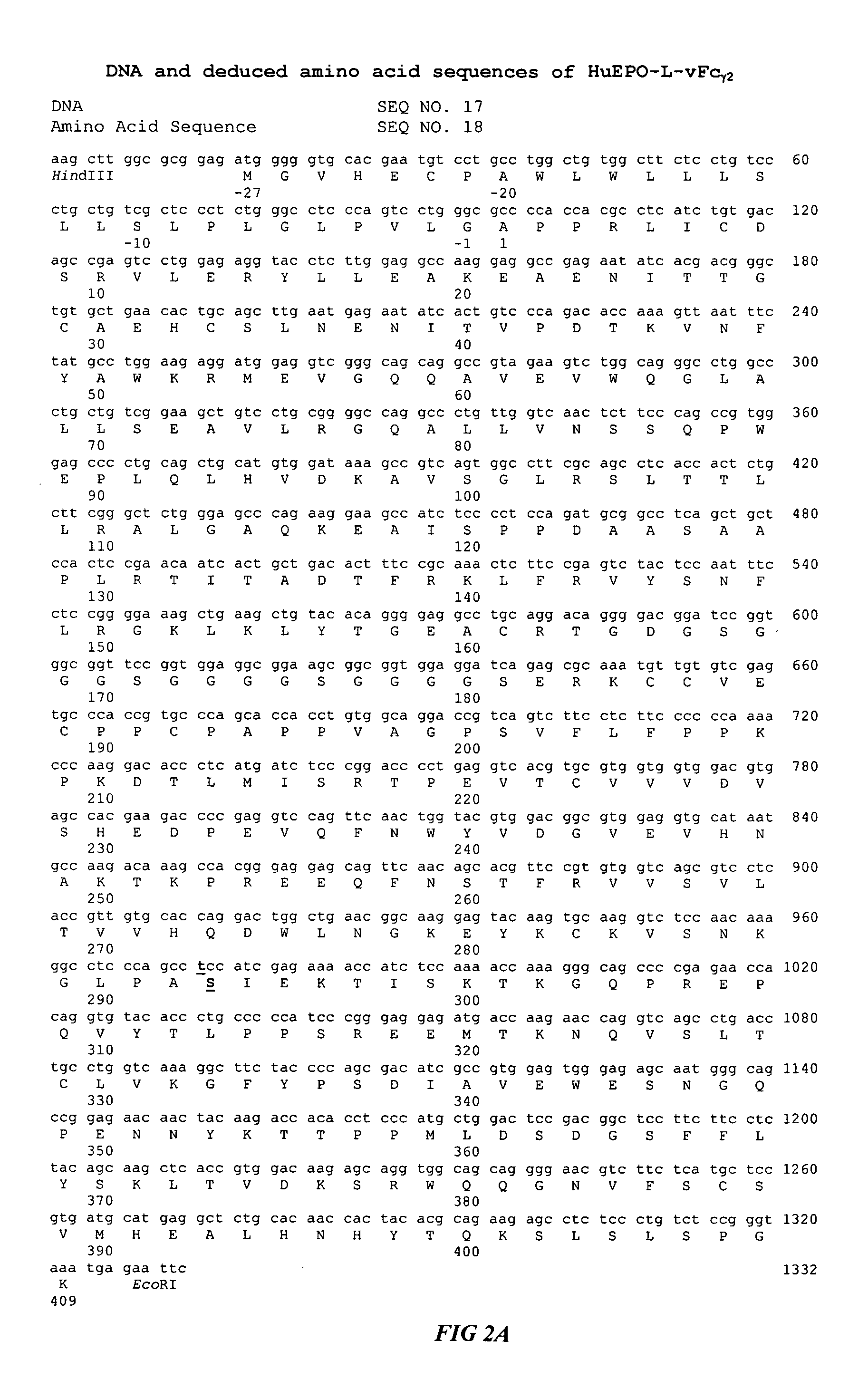
Fc fusion proteins of human erythropoietin with increased biological activities
InactiveUS20050124045A1Improve biological activityExtended serumPeptide/protein ingredientsAntibody mimetics/scaffoldsSide effectHalf-life
Fc fusion proteins of human EPO with increased biological activities relative to rHuEPO on a molar basis are disclosed. The HuEPO-L-vFc fusion protein comprises HuEPO, a flexible peptide linker of about 20 or fewer amino acids, and a human IgG Fc variant. The Fc variant is of a non-lytic nature and shows minimal undesirable Fc-mediated side effects. A method is also disclosed to make or produce such fusion proteins at high expression levels. Such HuEPO-L-vFc fusion proteins exhibit extended serum half-life and increased biological activities, leading to improved pharmacokinetics and pharmacodynamics, thus fewer injections will be needed within a period of time.
Owner:SUN LEE HWEI K +2
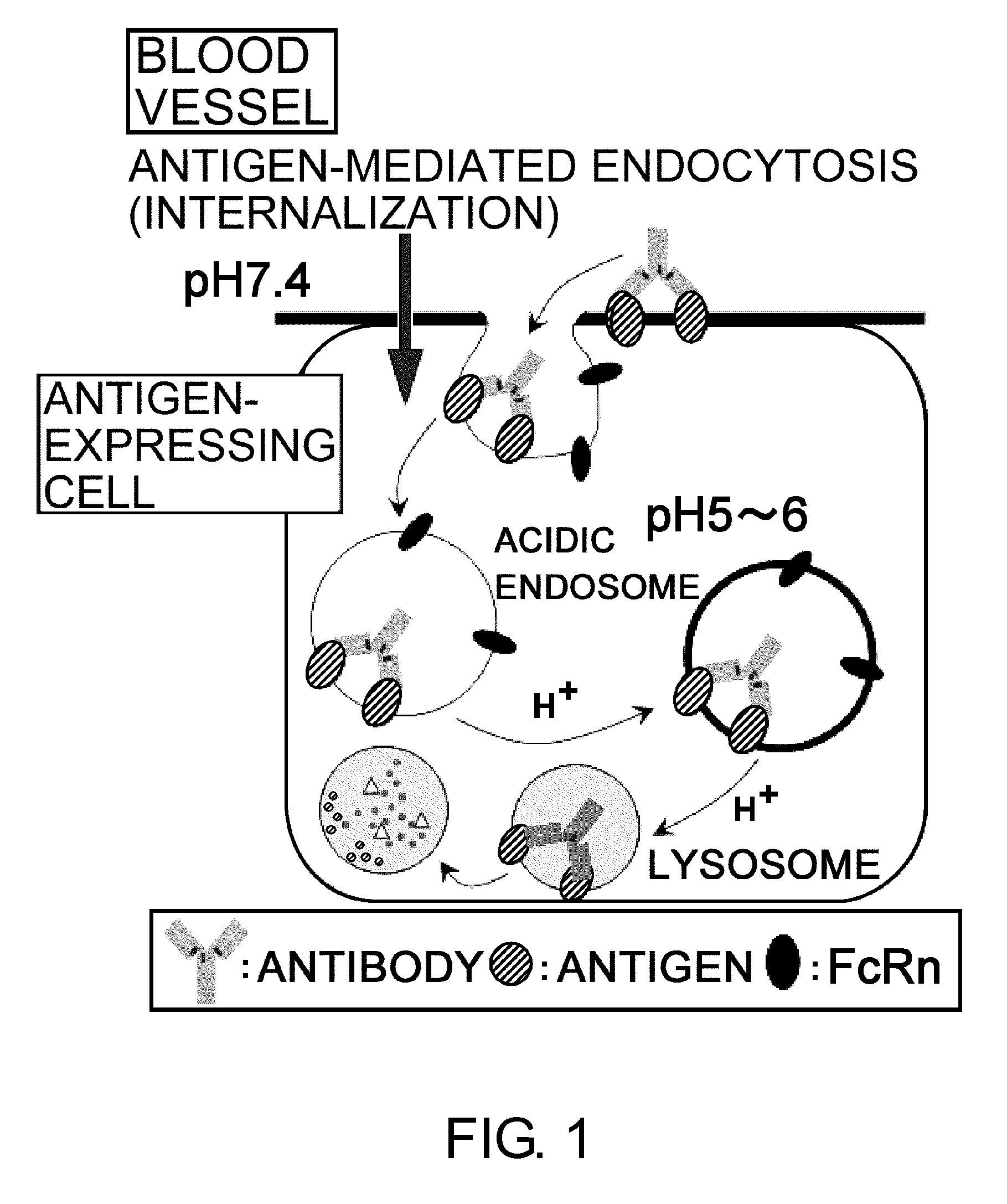
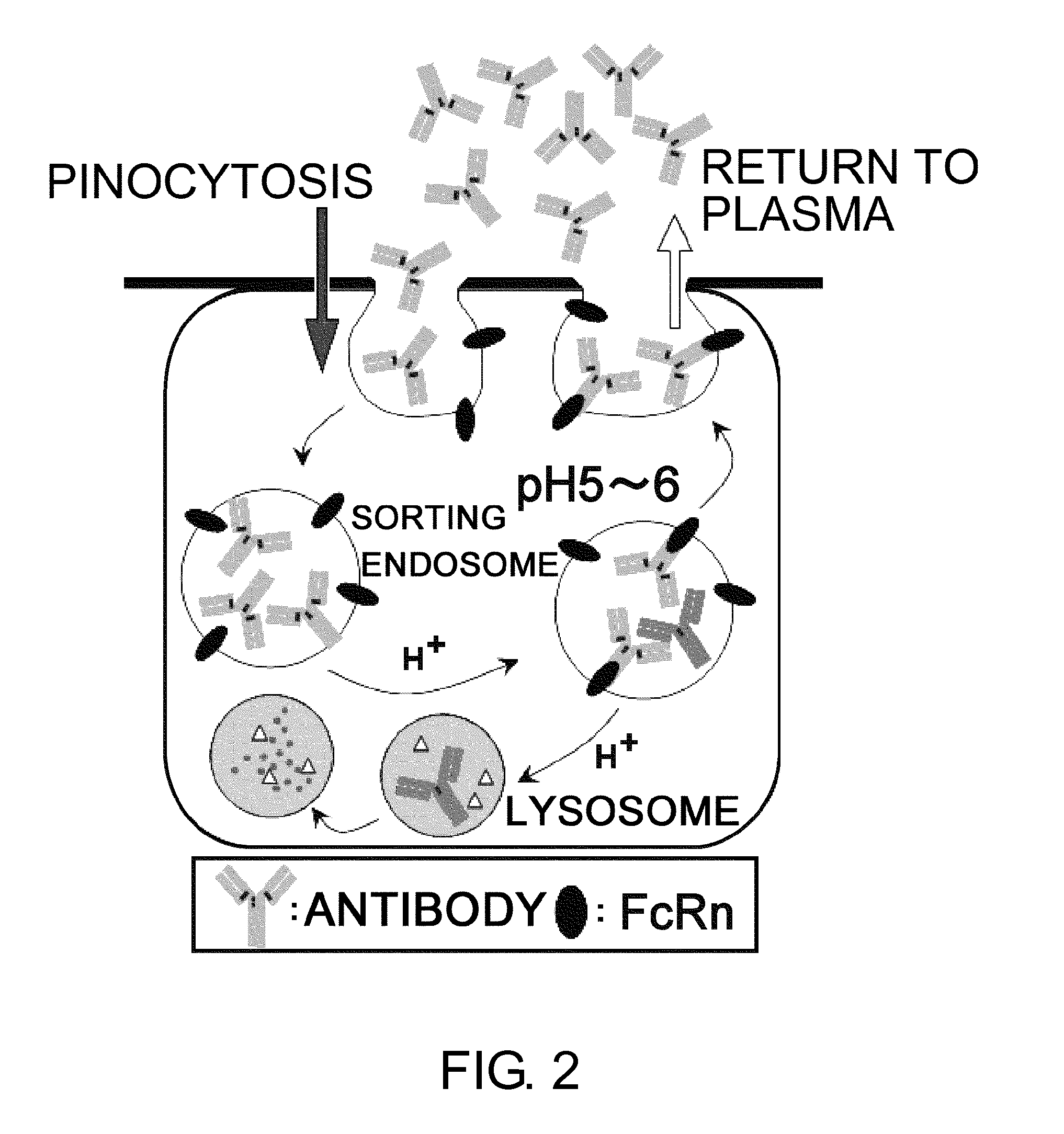
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20110111406A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
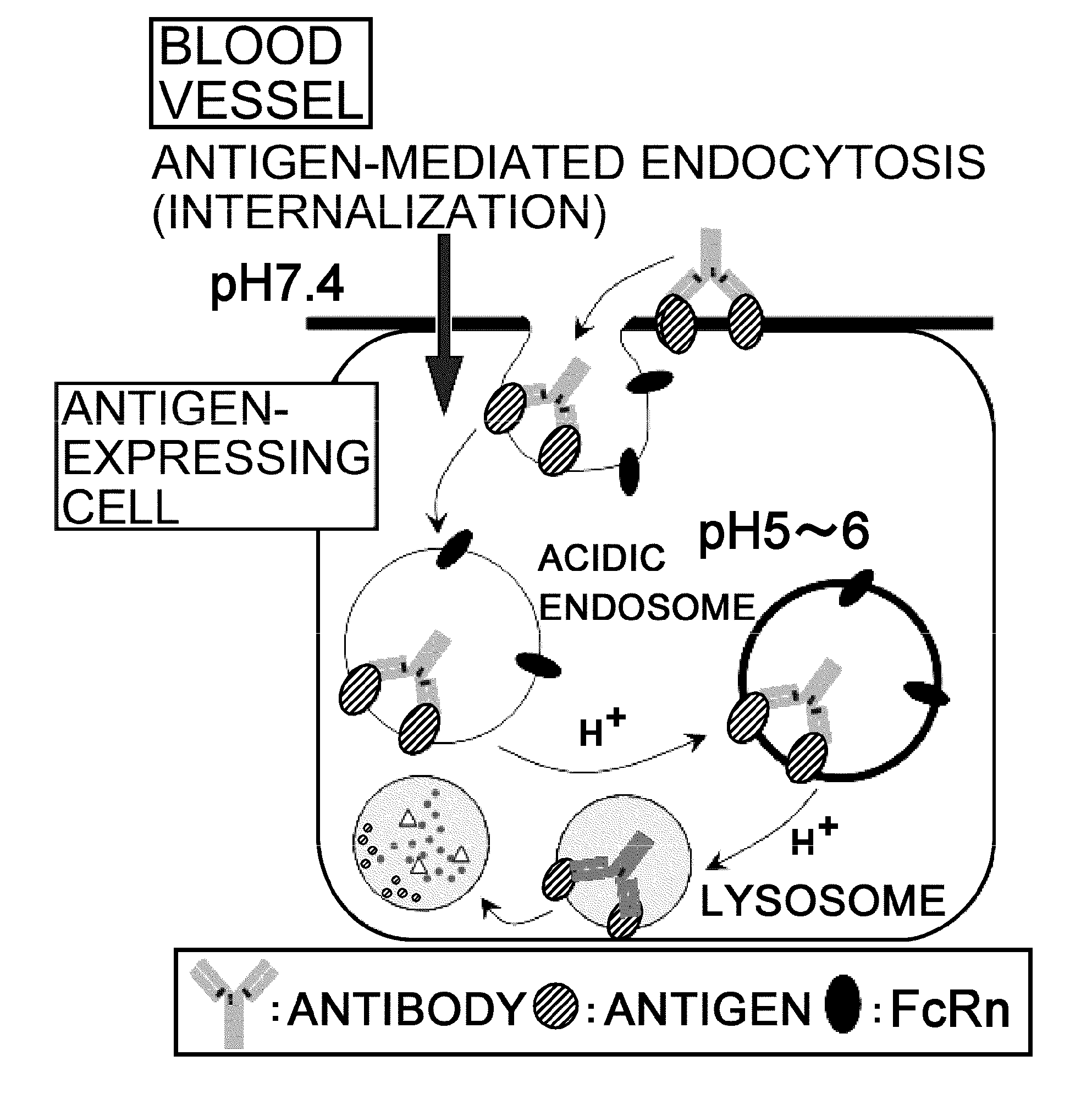
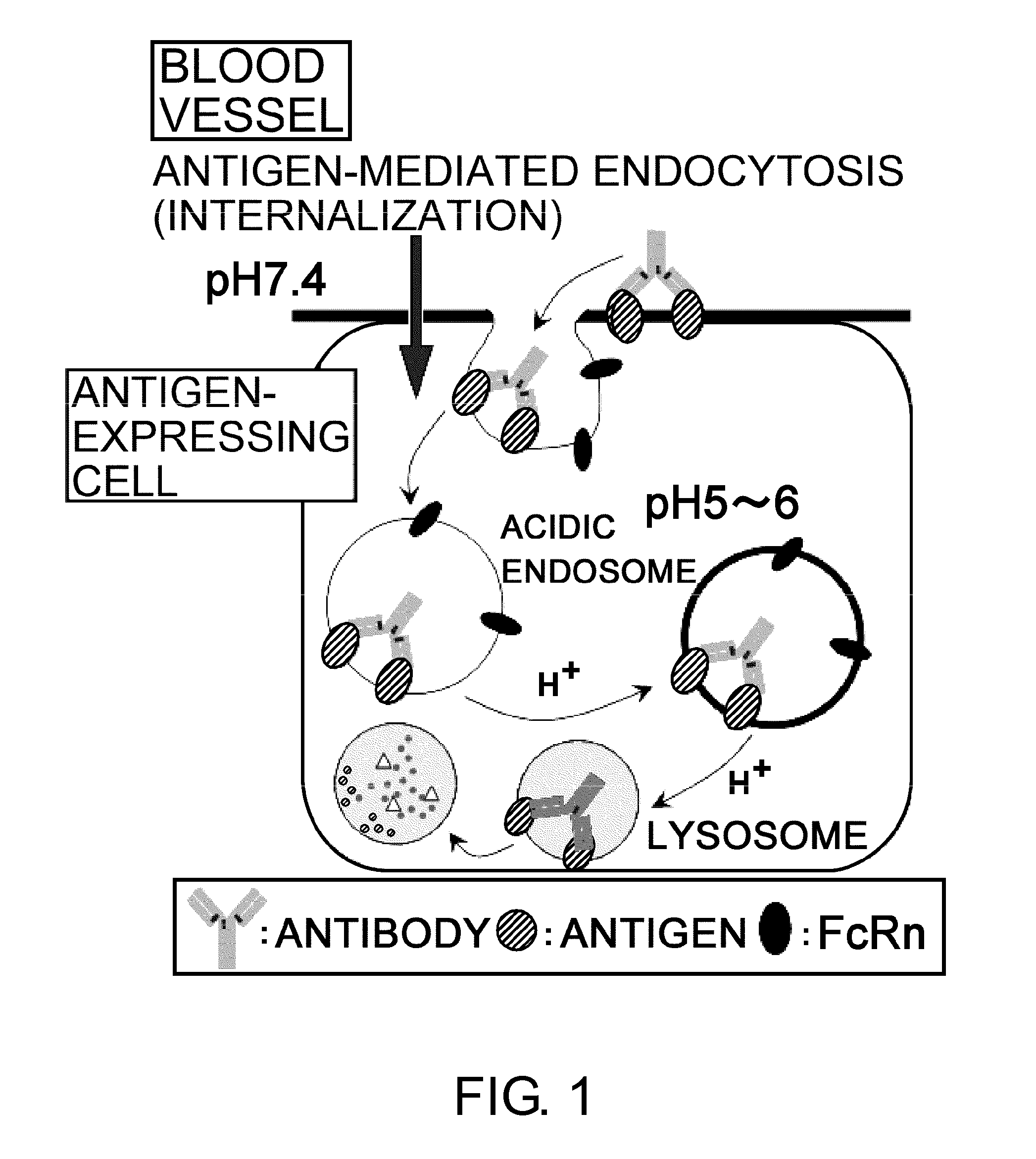
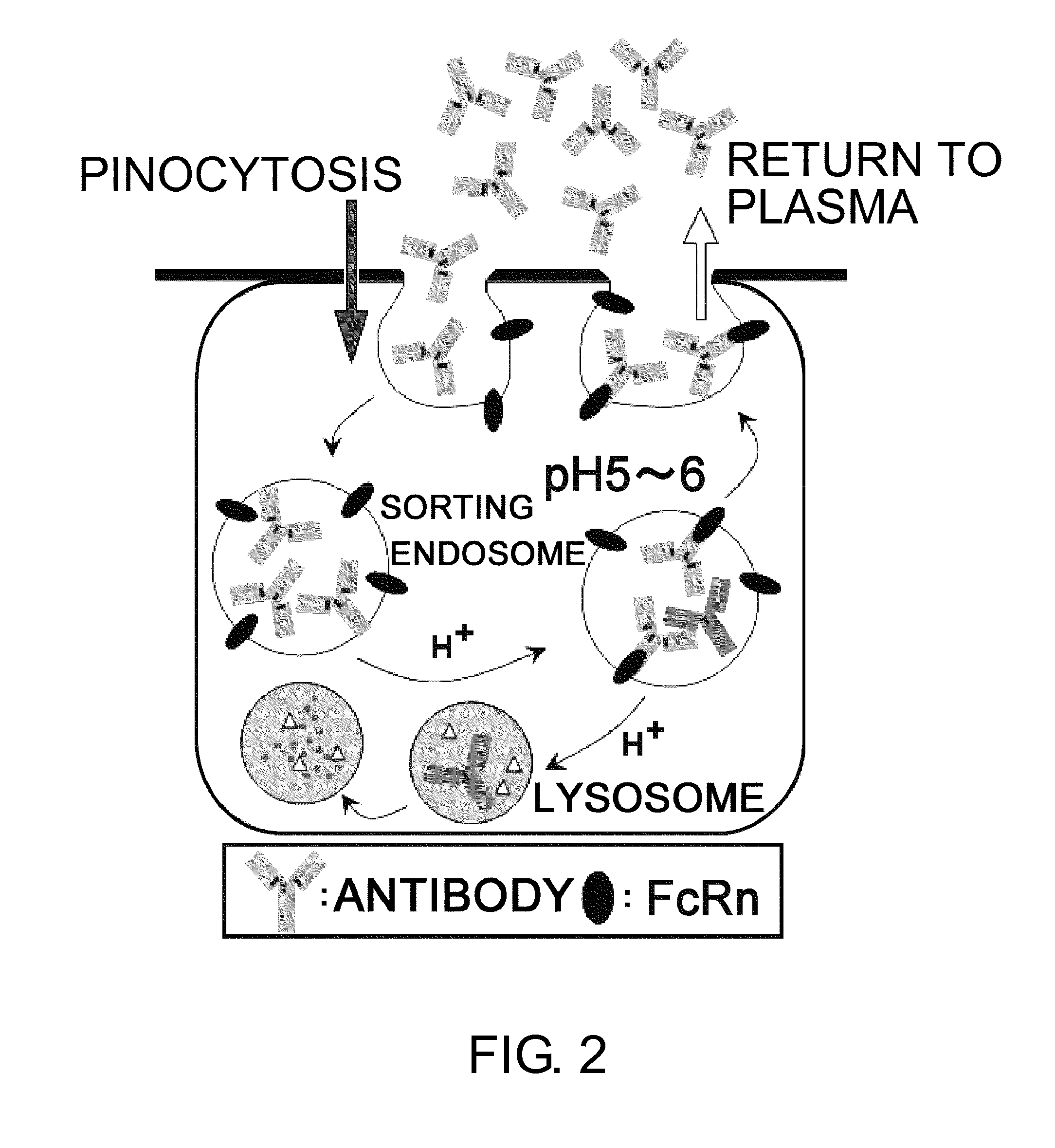
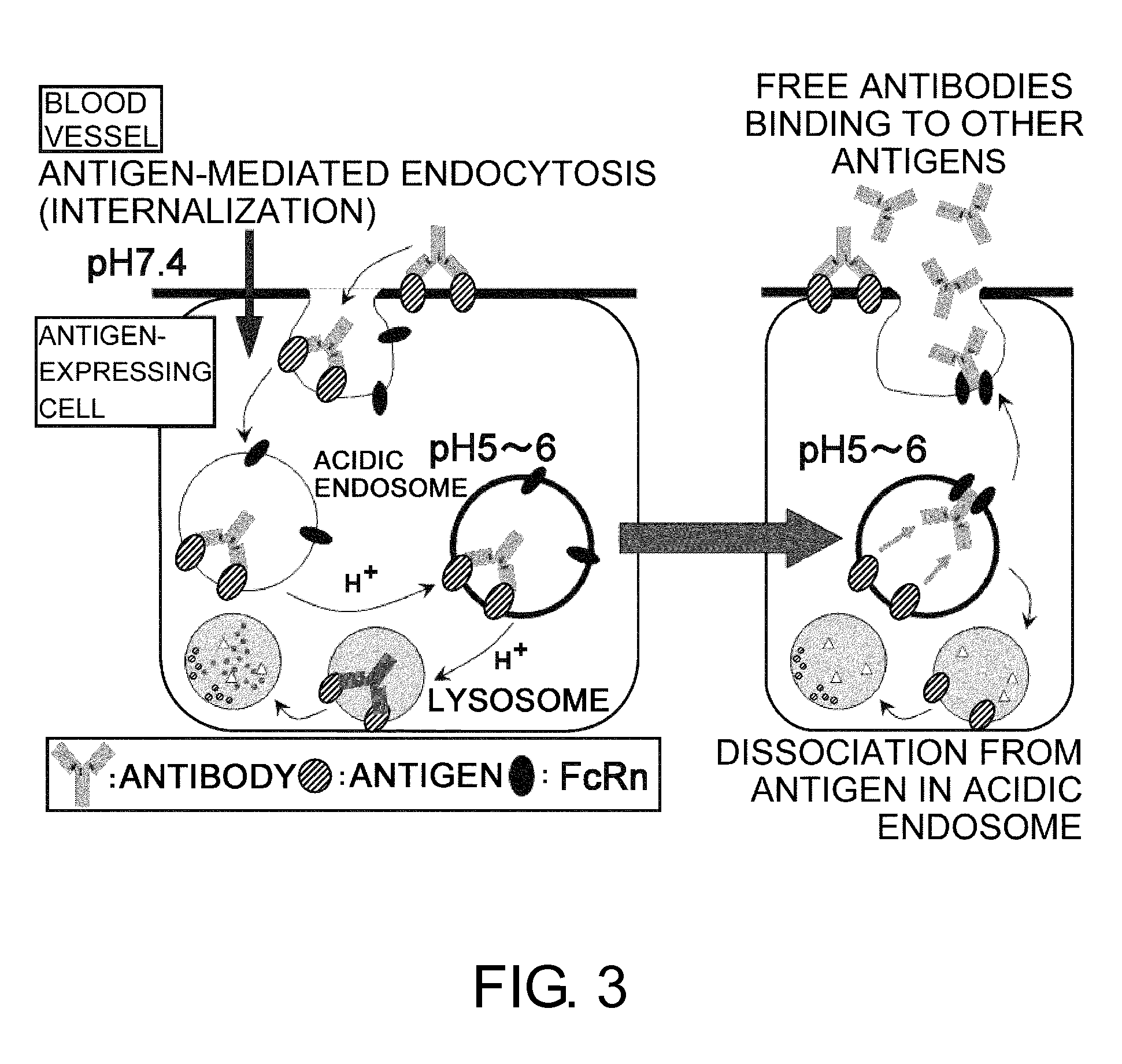
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Medical Device Applications of Nanostructured Surfaces
ActiveUS20070282247A1Increase heightPrevent/reduce bio-foulingNanotechElectrotherapyMedicineNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, a method of administering a composition to a patient is disclosed which comprises providing a composition-eluting device, said composition-eluting device comprising at least a first surface and a plurality of nanostructures attached to the first surface, and introducing the composition-eluting device into the body of the patient.
Owner:RGT UNIV OF CALIFORNIA +1
Methods and devices for improving delivery of a substance to skin
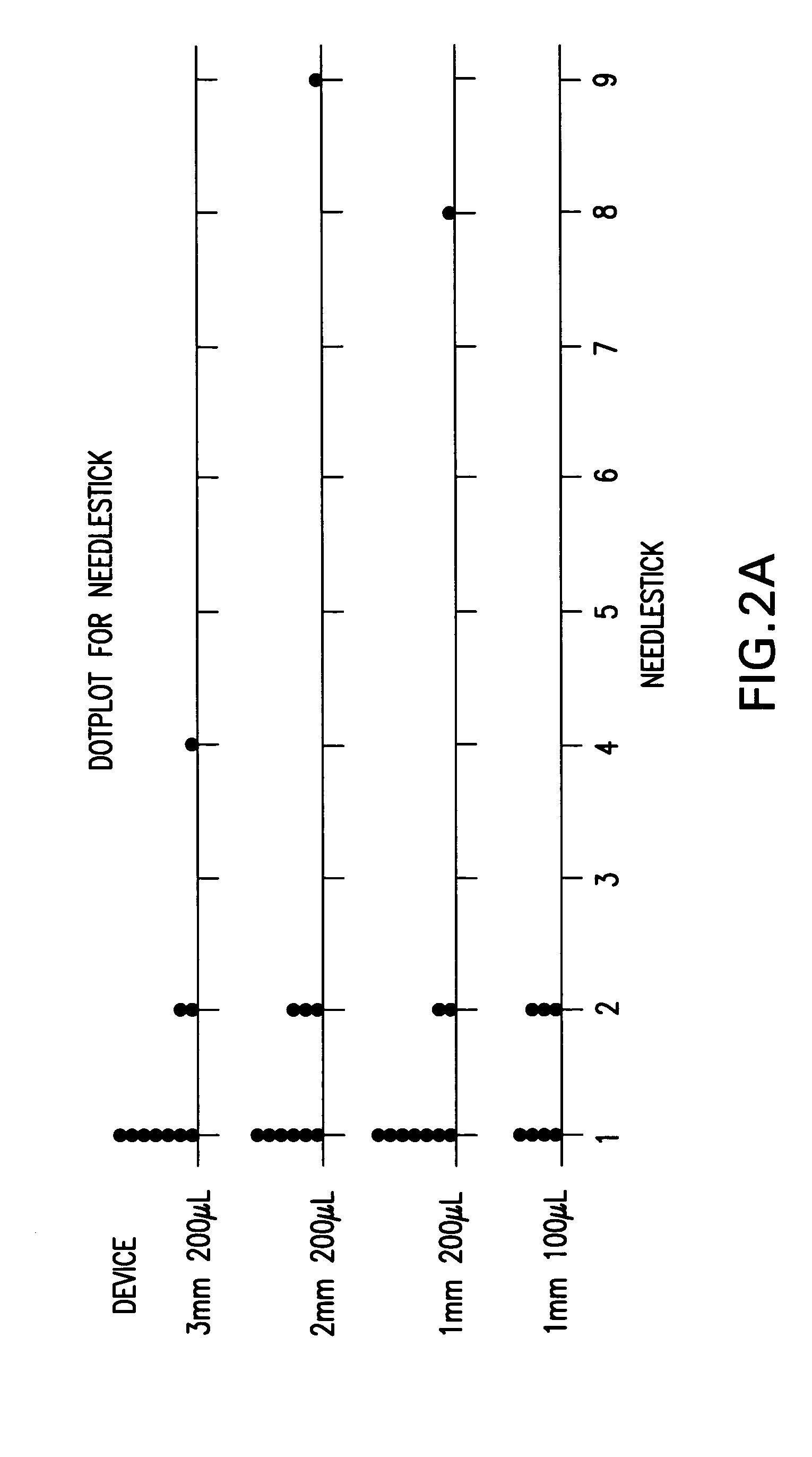
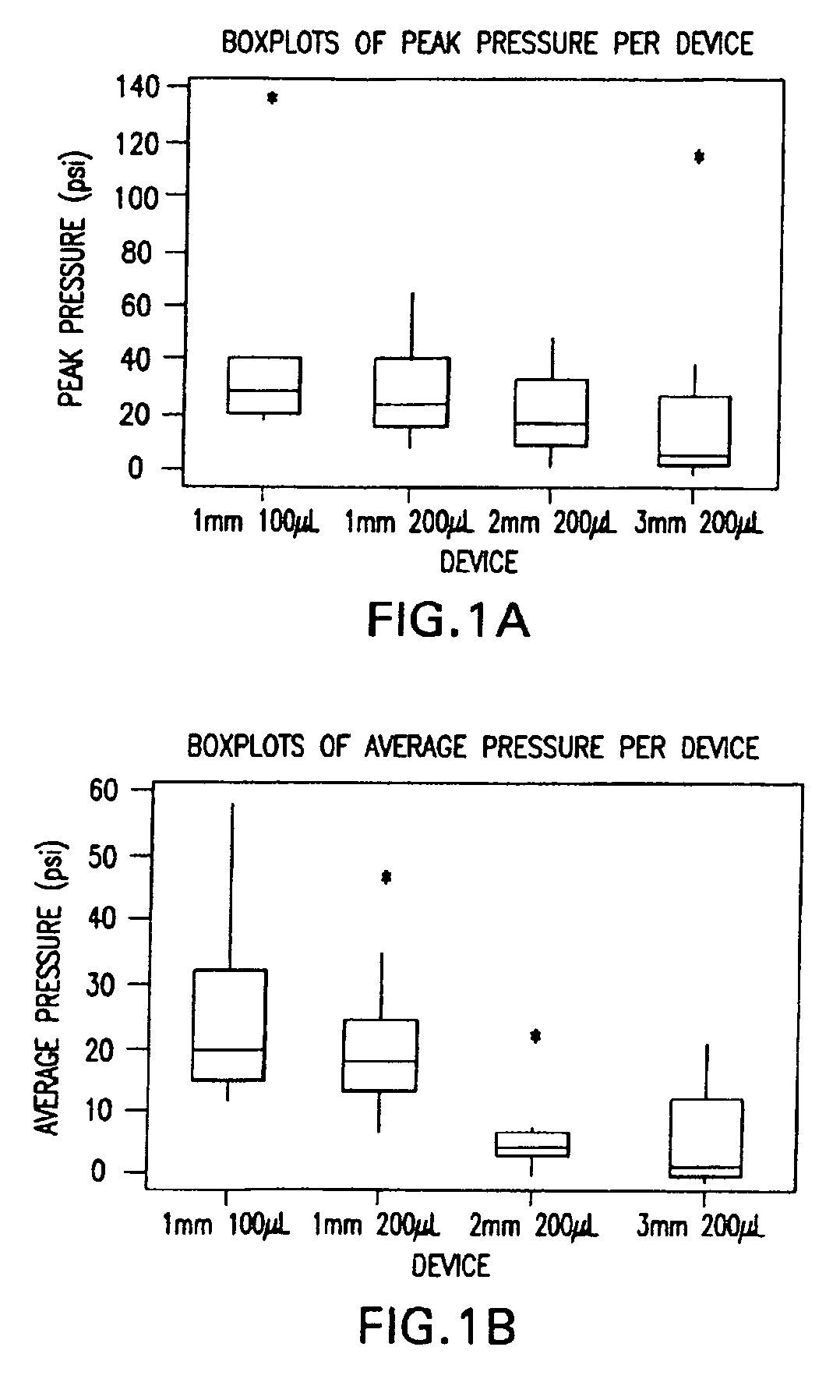
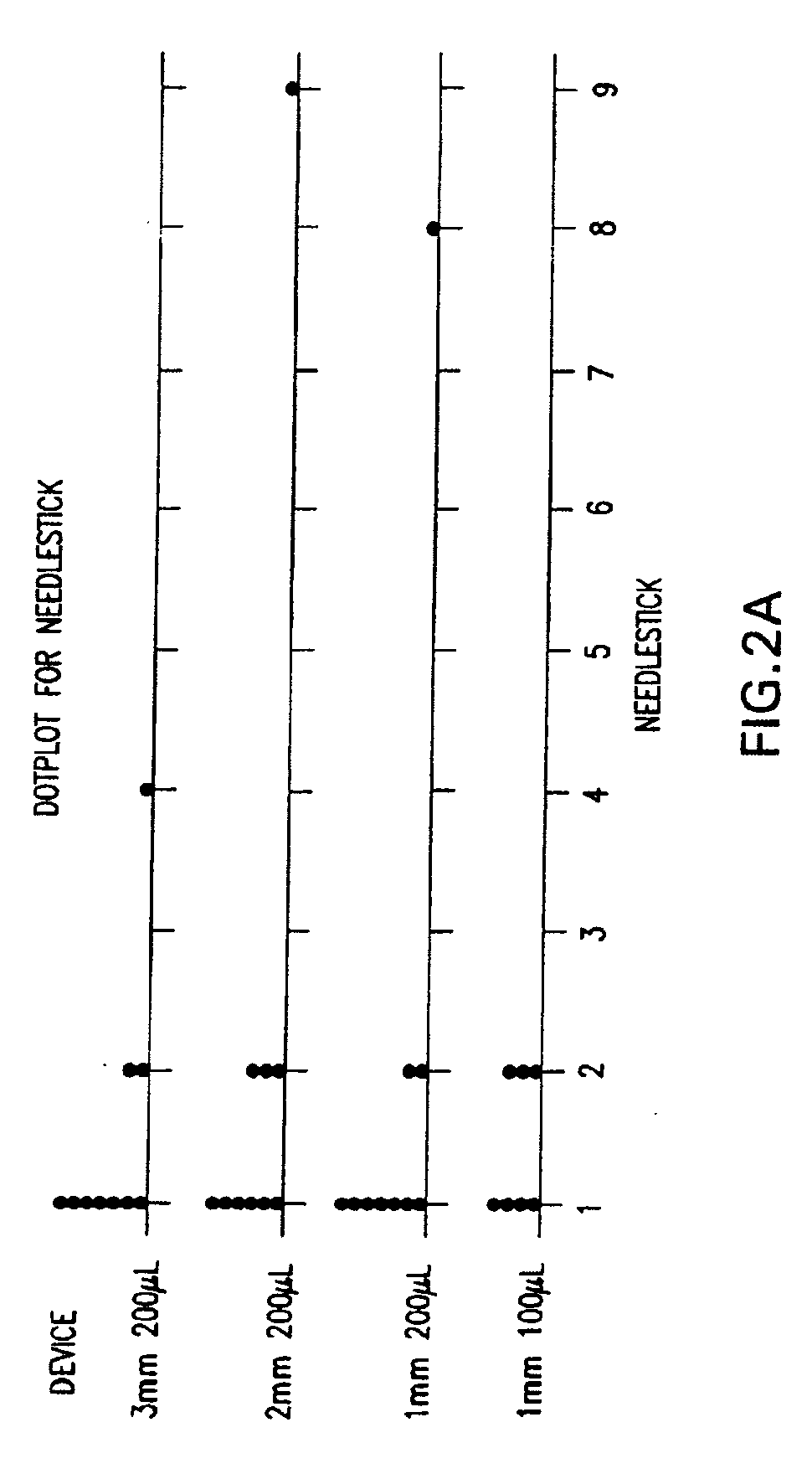
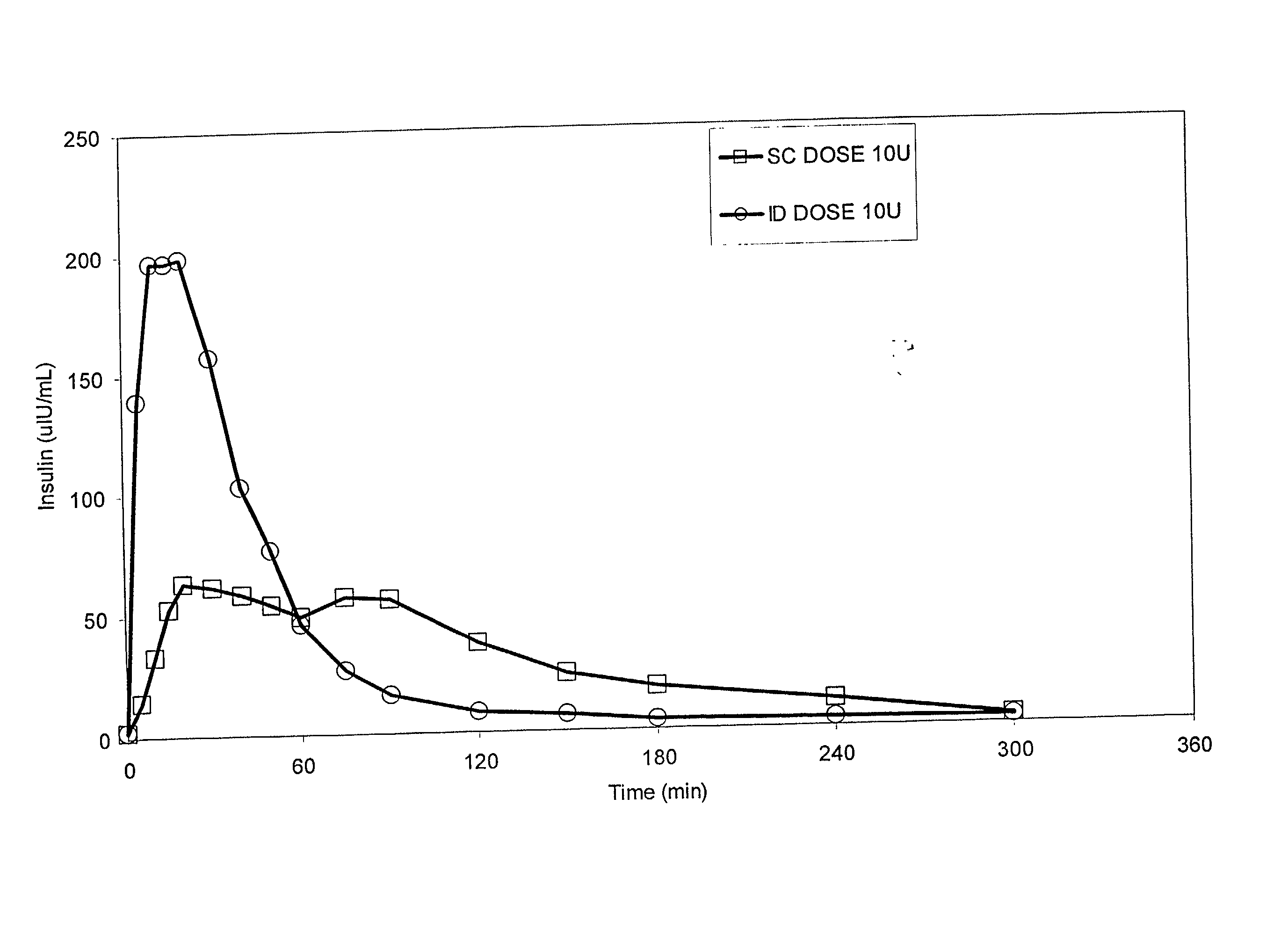
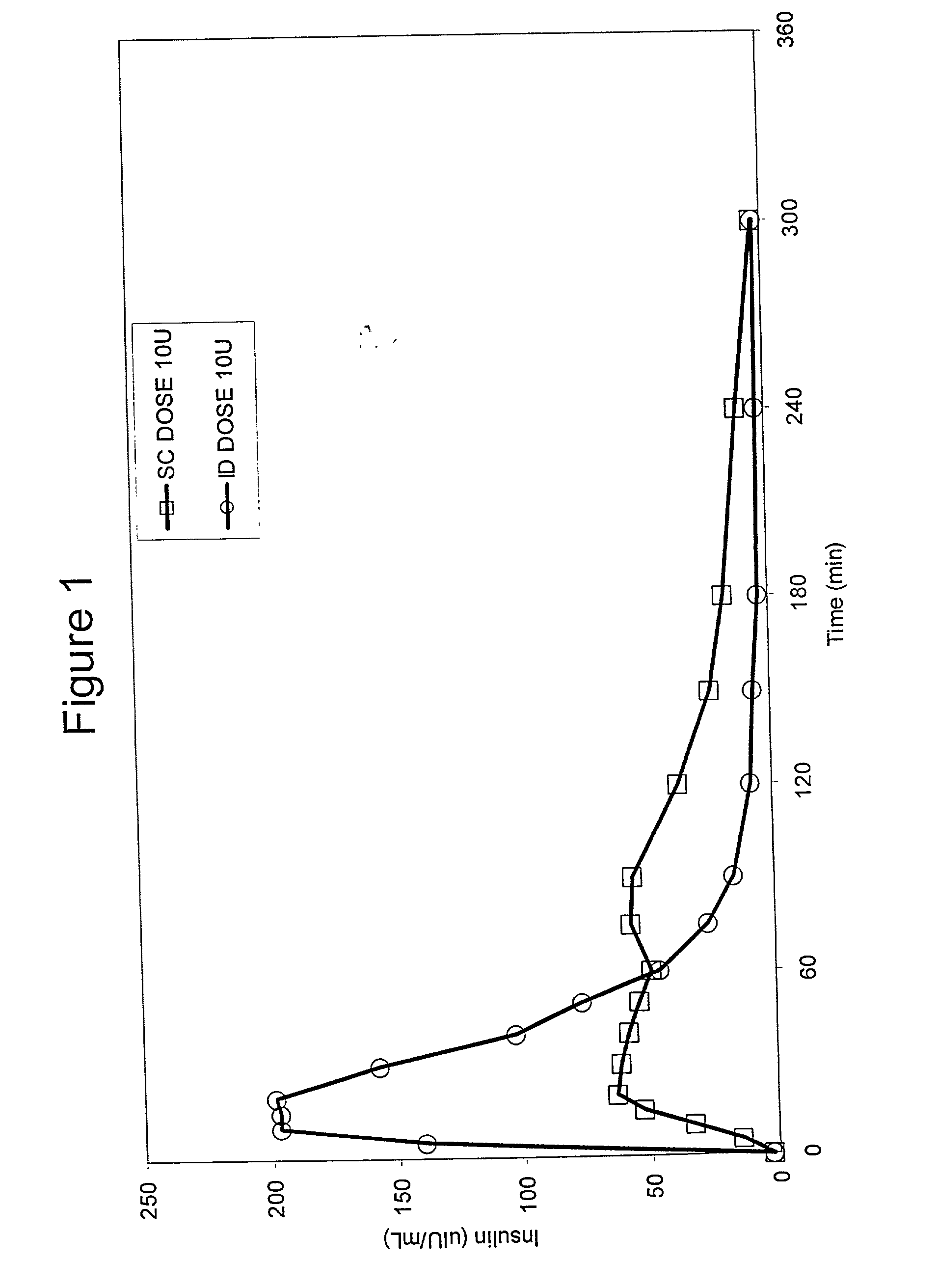
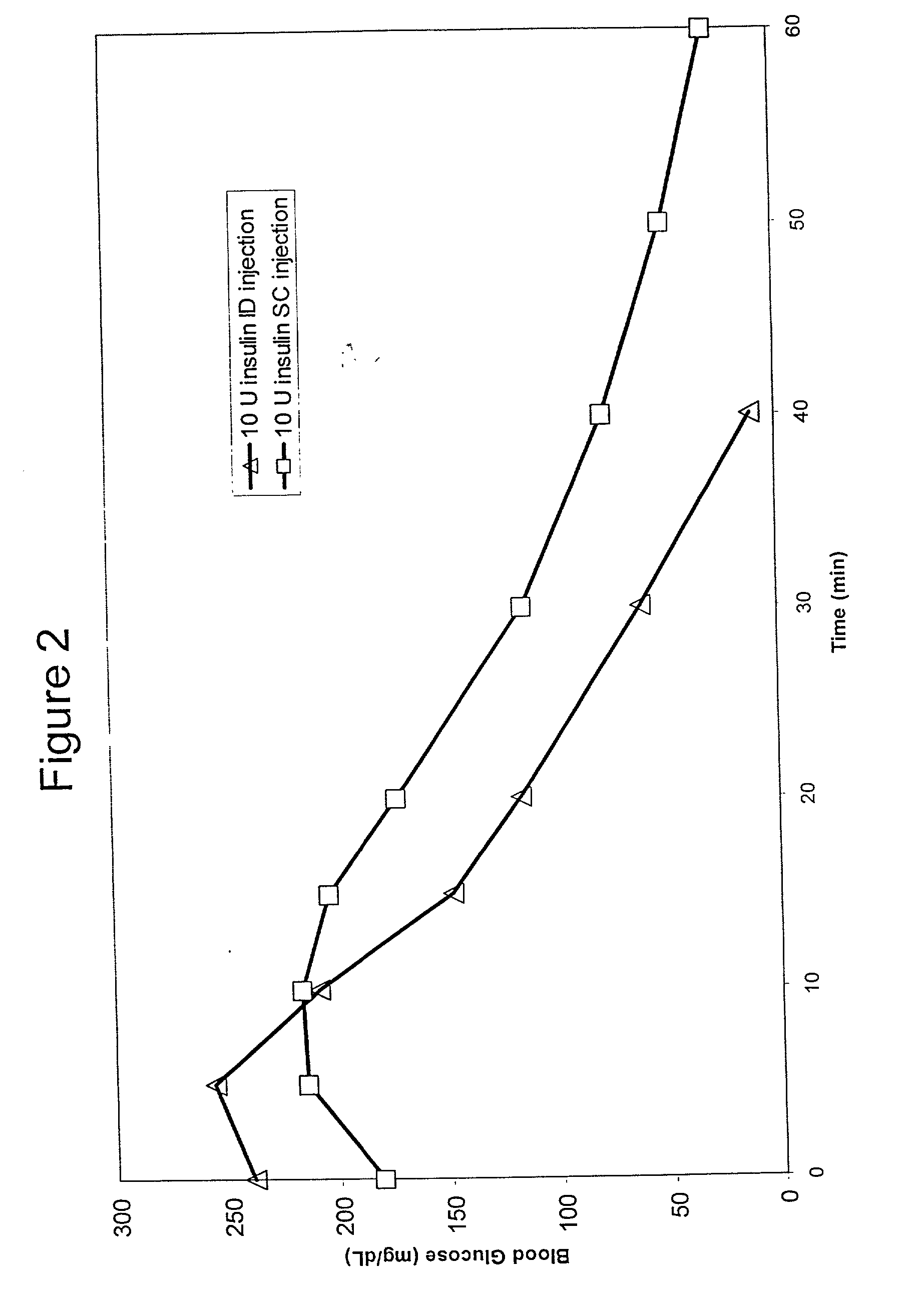
InactiveUS20050256499A1Efficacy is alteredImprove delivery capabilitiesMedical devicesPressure infusionDelivery PerformanceInjection site
A method of delivery of a substance to a human subject's skin comprising deposition into a specific compartment of the skin, wherein the delivery occurs at a controlled rate and pressure. The methods of the invention provide accurate deposition of s pre-selected volume of the substance, e.g., greater than 90% of the pre-selected volume. The methods of the invention encompass varying one or more parameters including but not limited to configurations of the delivery device, volume, pressure, and flow rate of delivery, to enhance the efficacy of delivery of the substance to the human skin. Substances delivered in accordance with the methods of the invention result in a more efficacious deposition of the substance into the targeted compartment, improved delivery performance, i.e., completeness of delivery as measured by quantification of the substance not delivered or the amount of the substance leaked out from the injection site, and enhanced safety as measured by the occurrence of minimal adverse cutaneous events at the site of injection.
Owner:BECTON DICKINSON & CO
Compositions and methods for intraocular delivery of fibronectin scaffold domain proteins
InactiveUS20080220049A1Easy to optimizeImprove bioavailabilitySenses disorderPeptide/protein ingredientsMedicineFibronectin
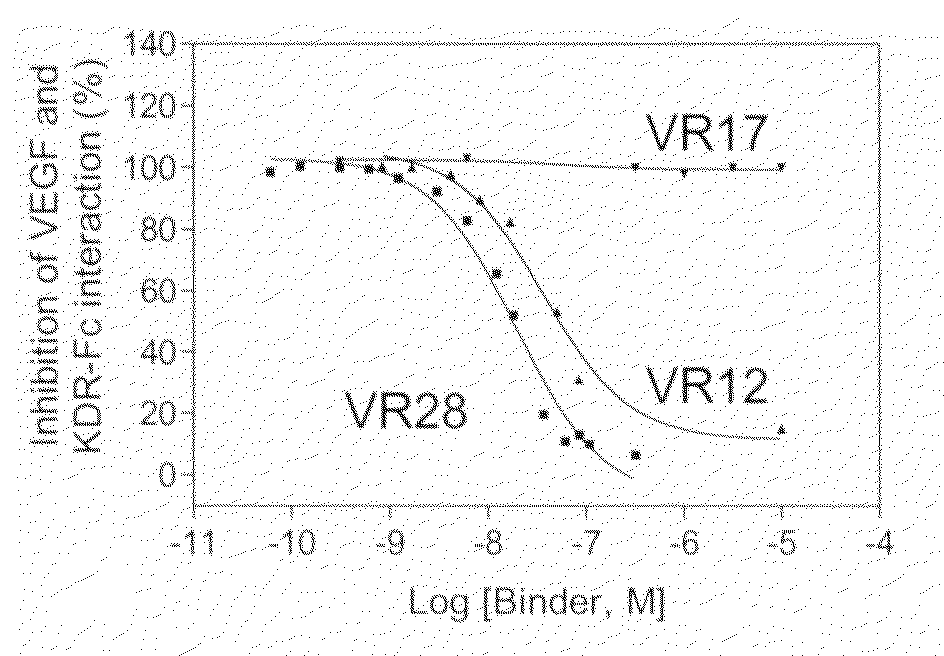
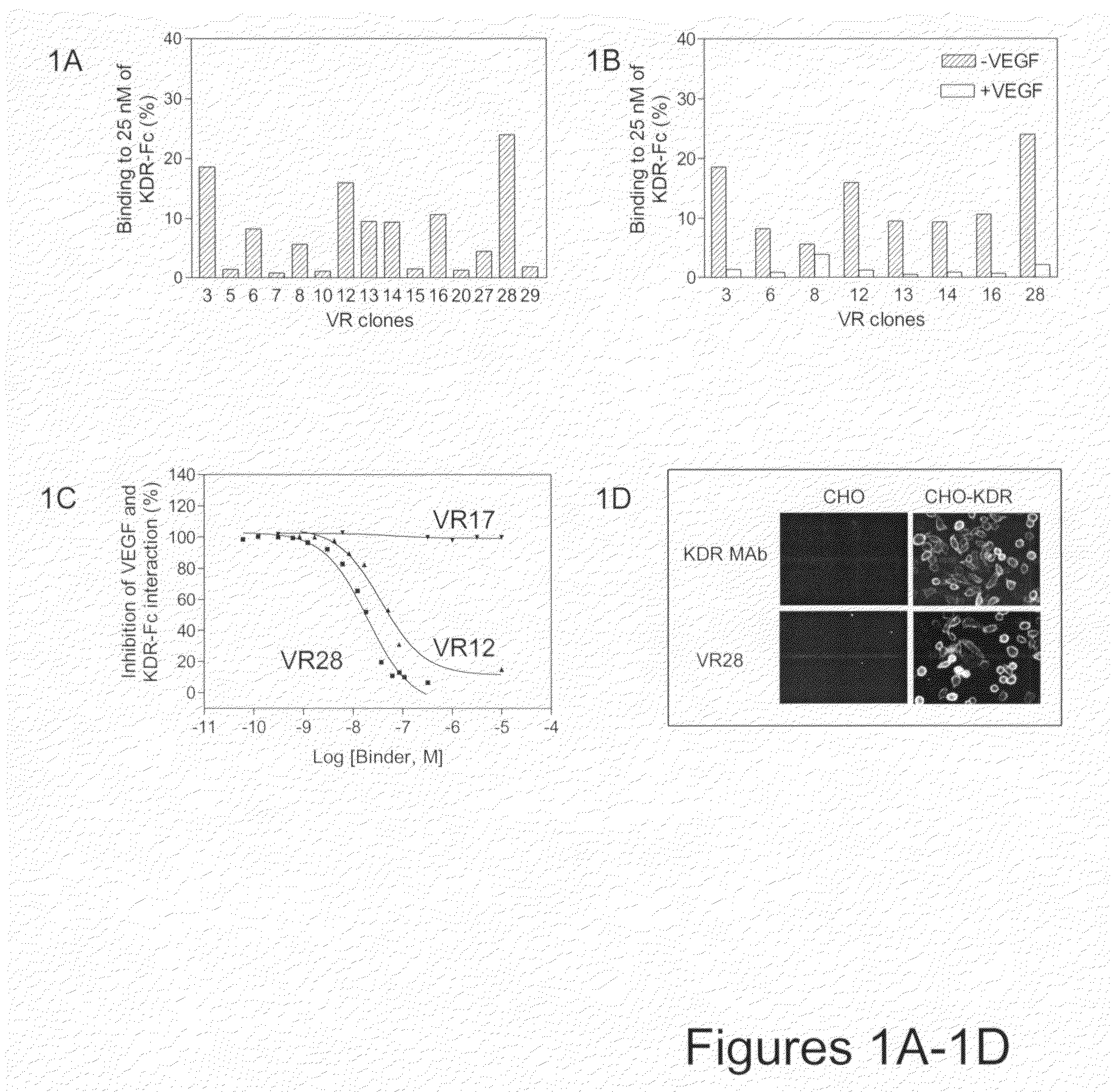
The present disclosure relates to novel sustained-release intraocular drug delivery systems and improvements in the treatment of retinopathies. In particular, fibronectin scaffold domain proteins that selectively inhibit VEGFR-2 are contemplated.
Owner:BRISTOL MYERS SQUIBB CO
Anti-IL-6 Receptor Antibody
InactiveUS20110245473A1Enhanced antigen-neutralizing activity and pharmacokineticsGood treatment effectCompound screeningApoptosis detectionHigh concentrationHinge region
The present inventors succeeded in discovering specific amino acid mutations in the variable region, framework region, and constant region of TOCILIZUMAB, and this enables to reduce immunogenicity risk and the heterogeneity originated from disulfide bonds in the hinge region, as well as to improve antigen binding activity, pharmacokinetics, stability under acidic conditions, and stability in high concentration preparations.
Owner:CHUGAI PHARMA CO LTD
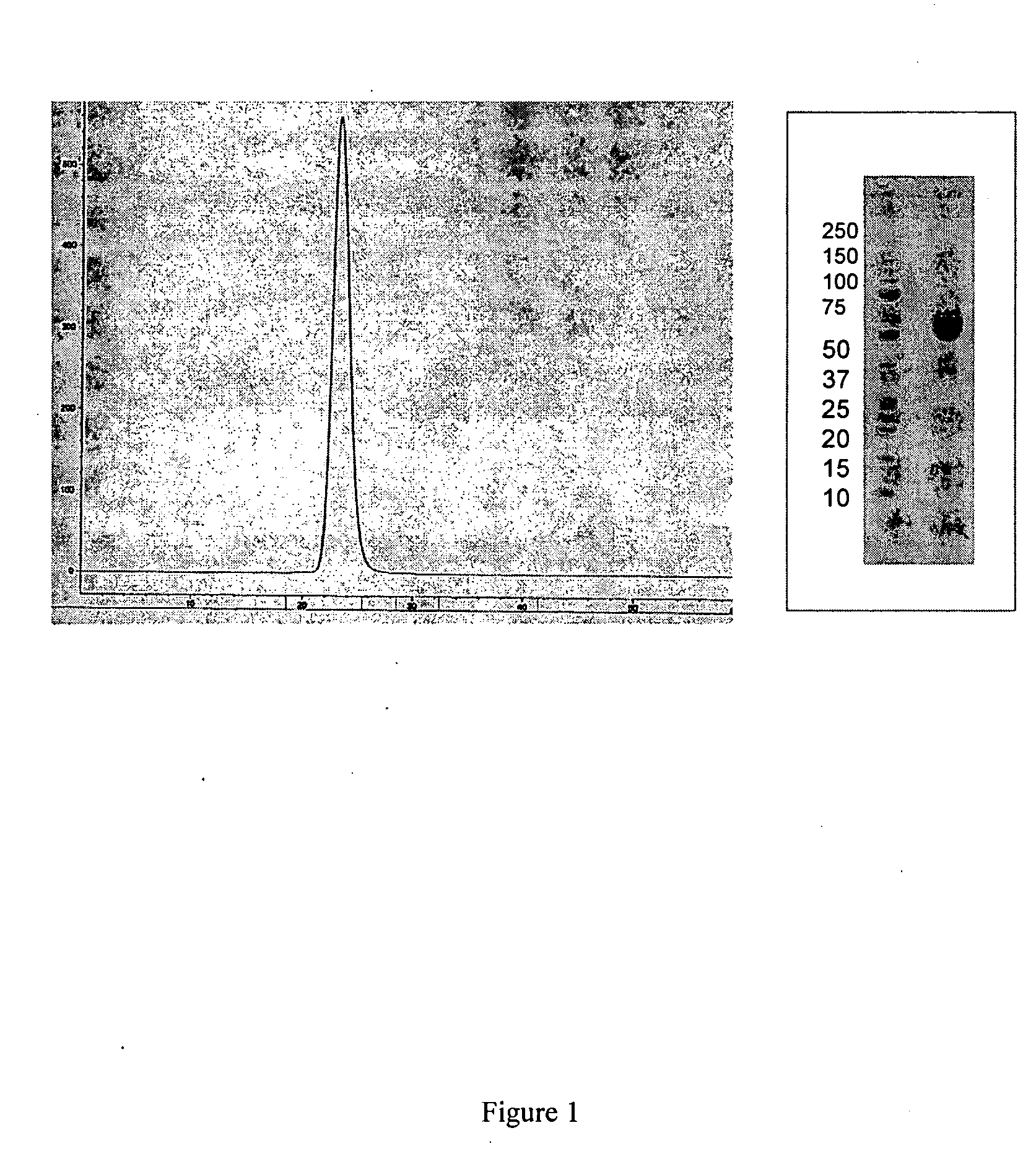
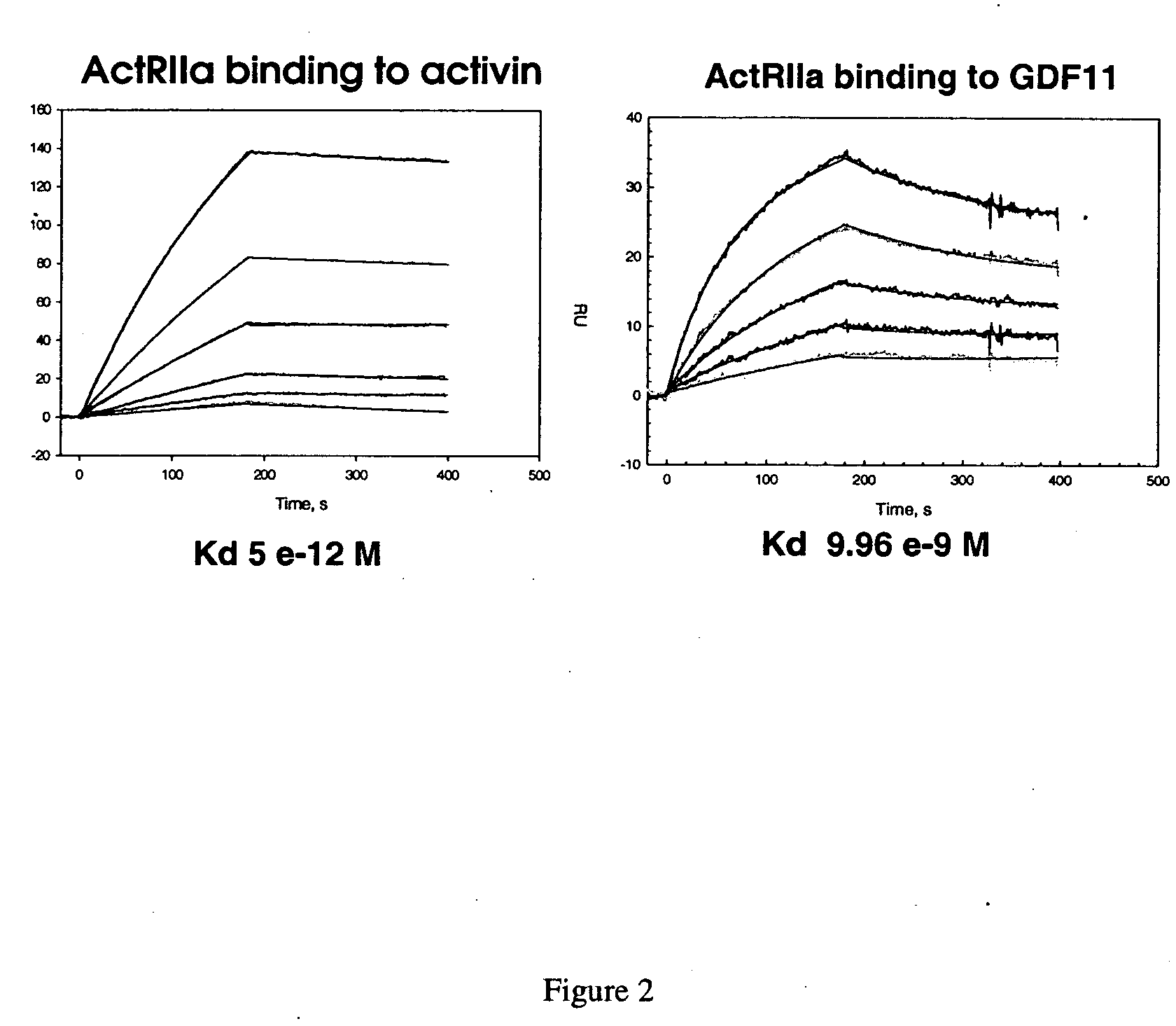
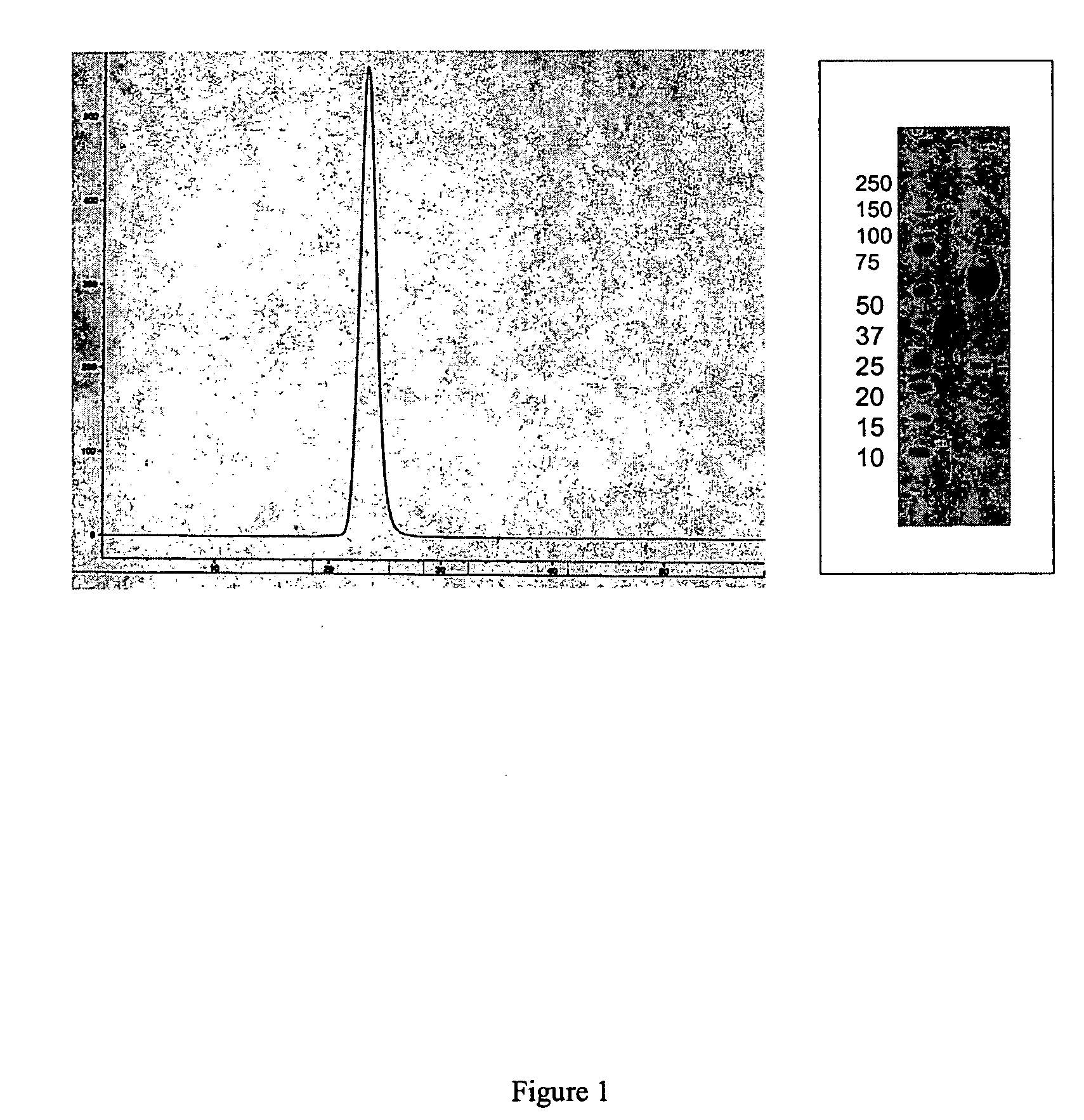
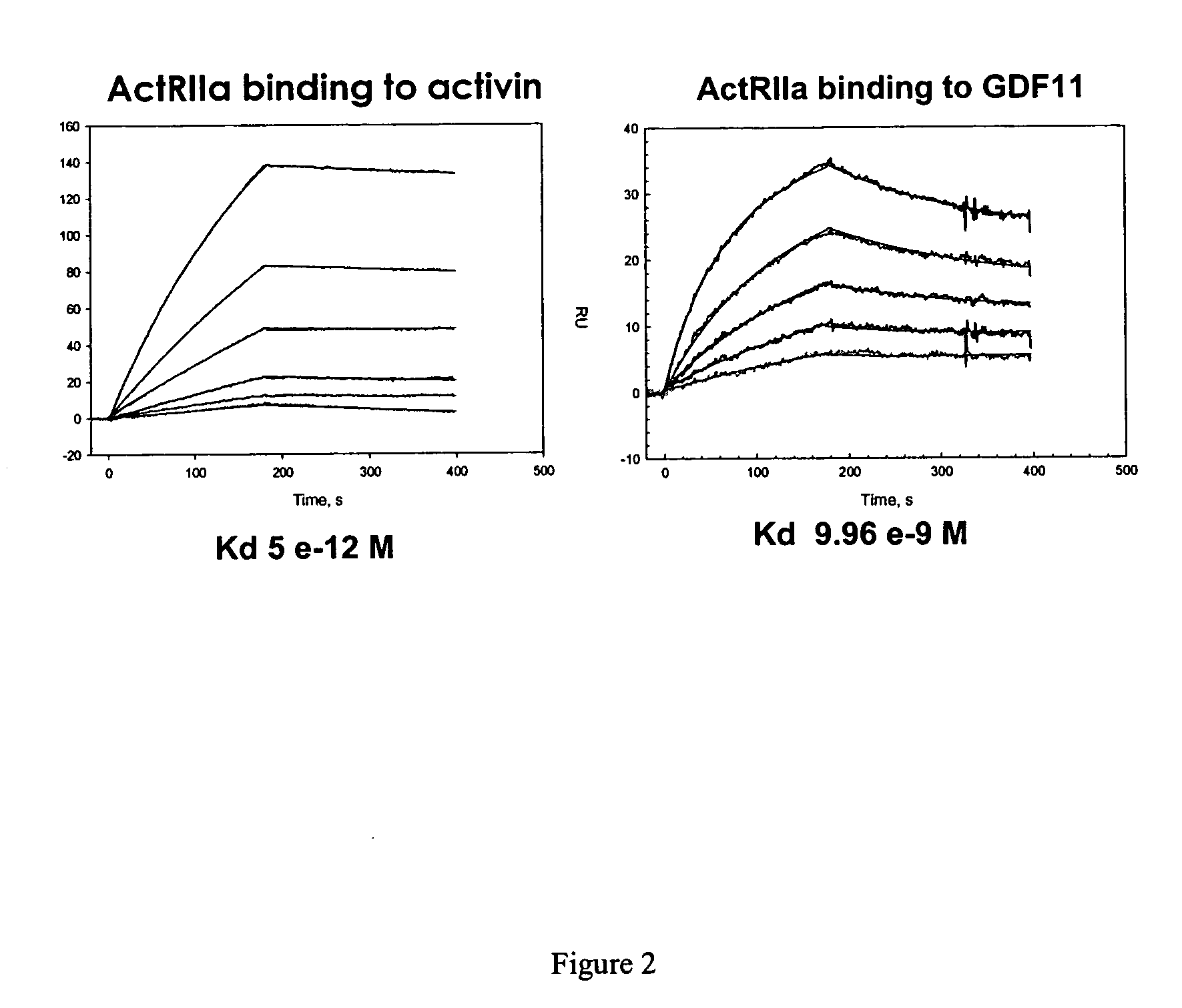
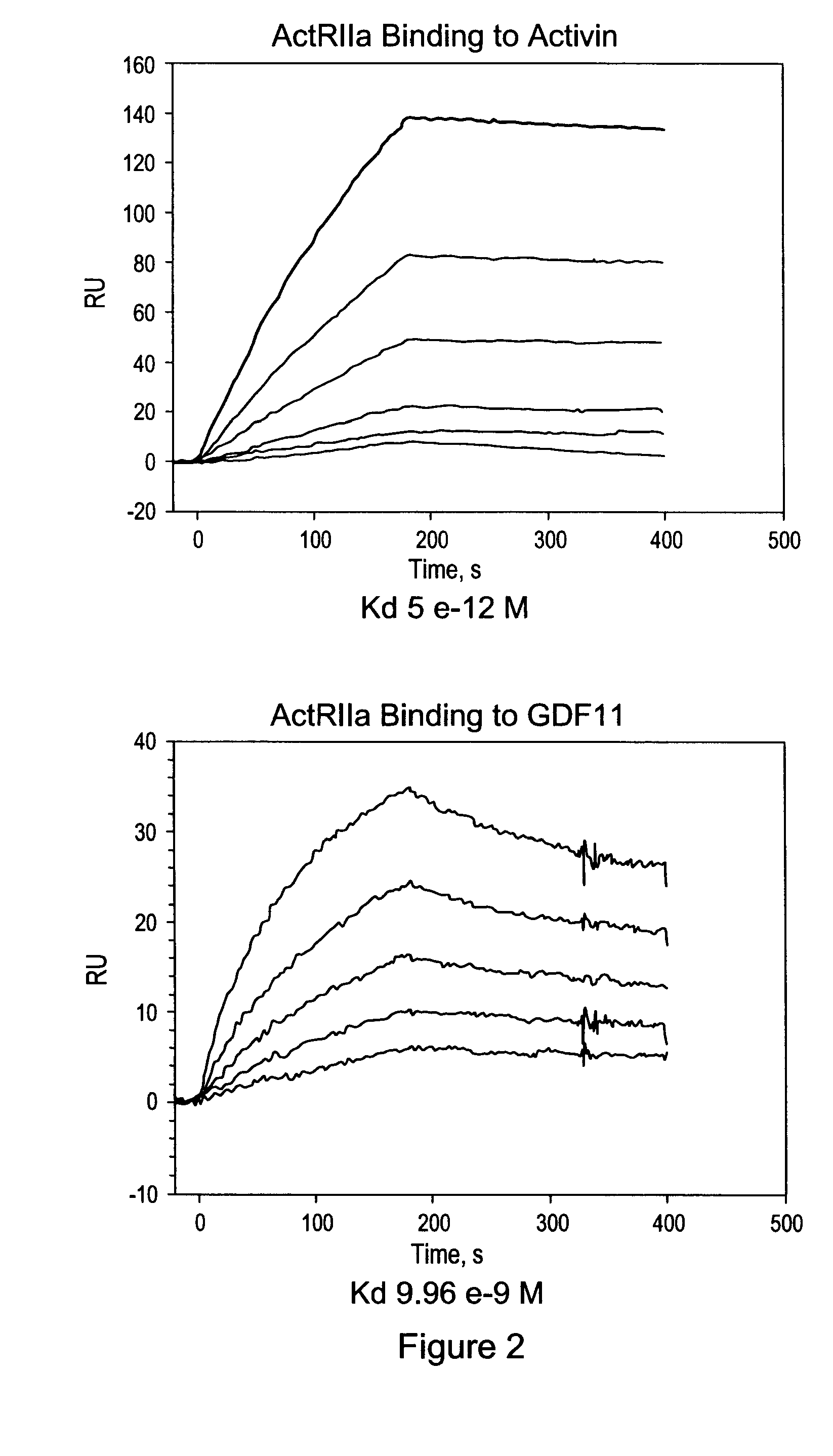
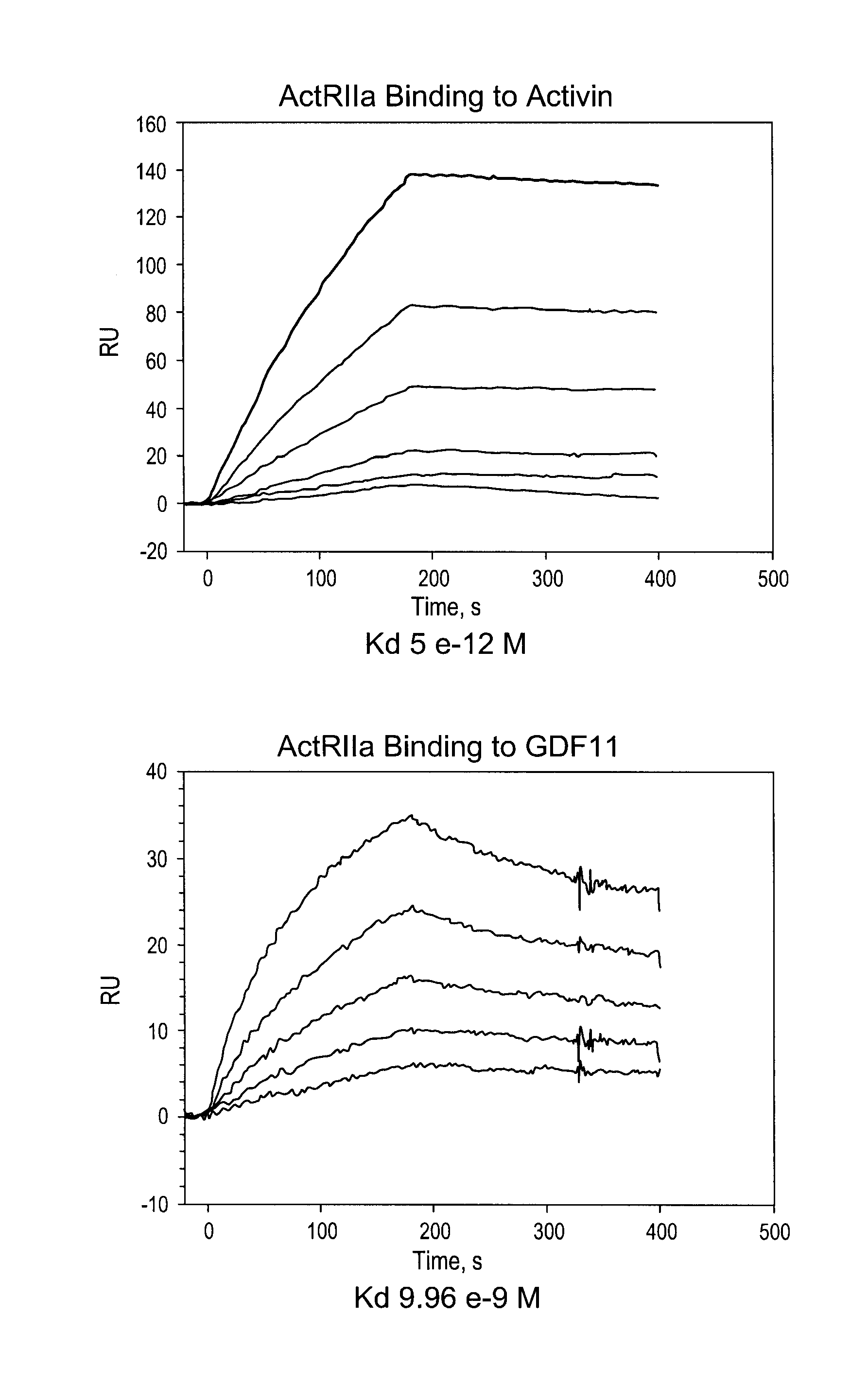
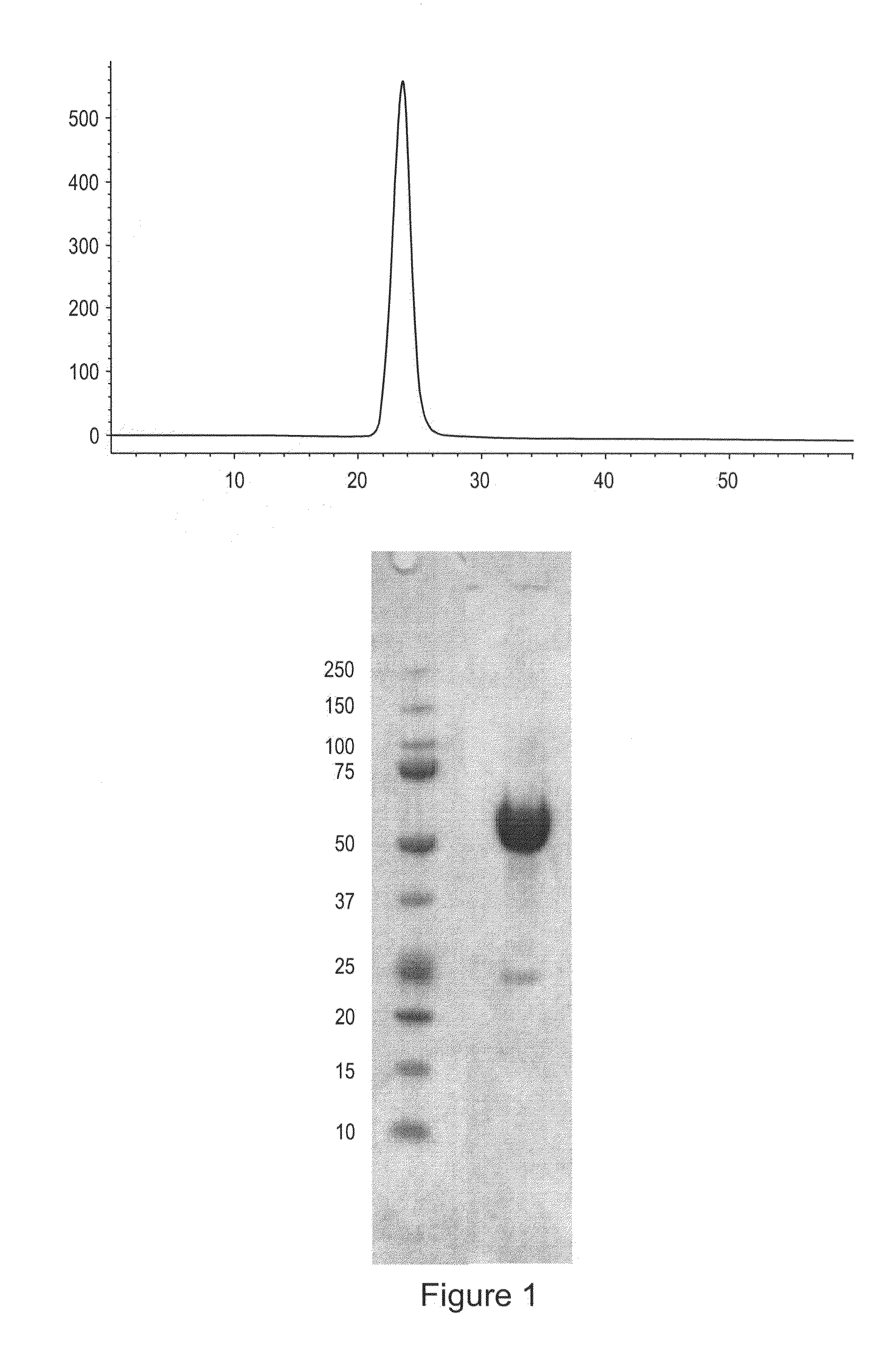
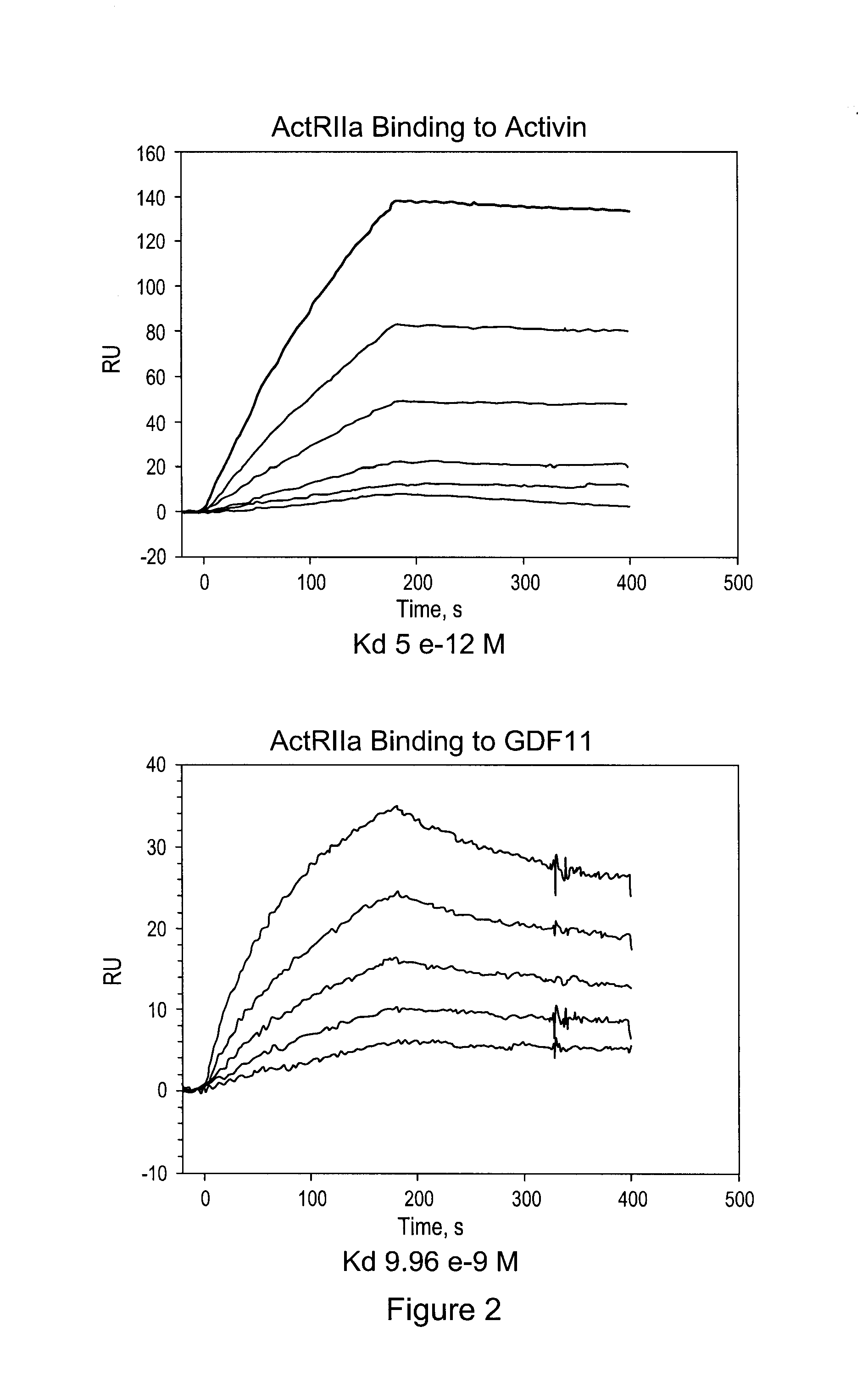
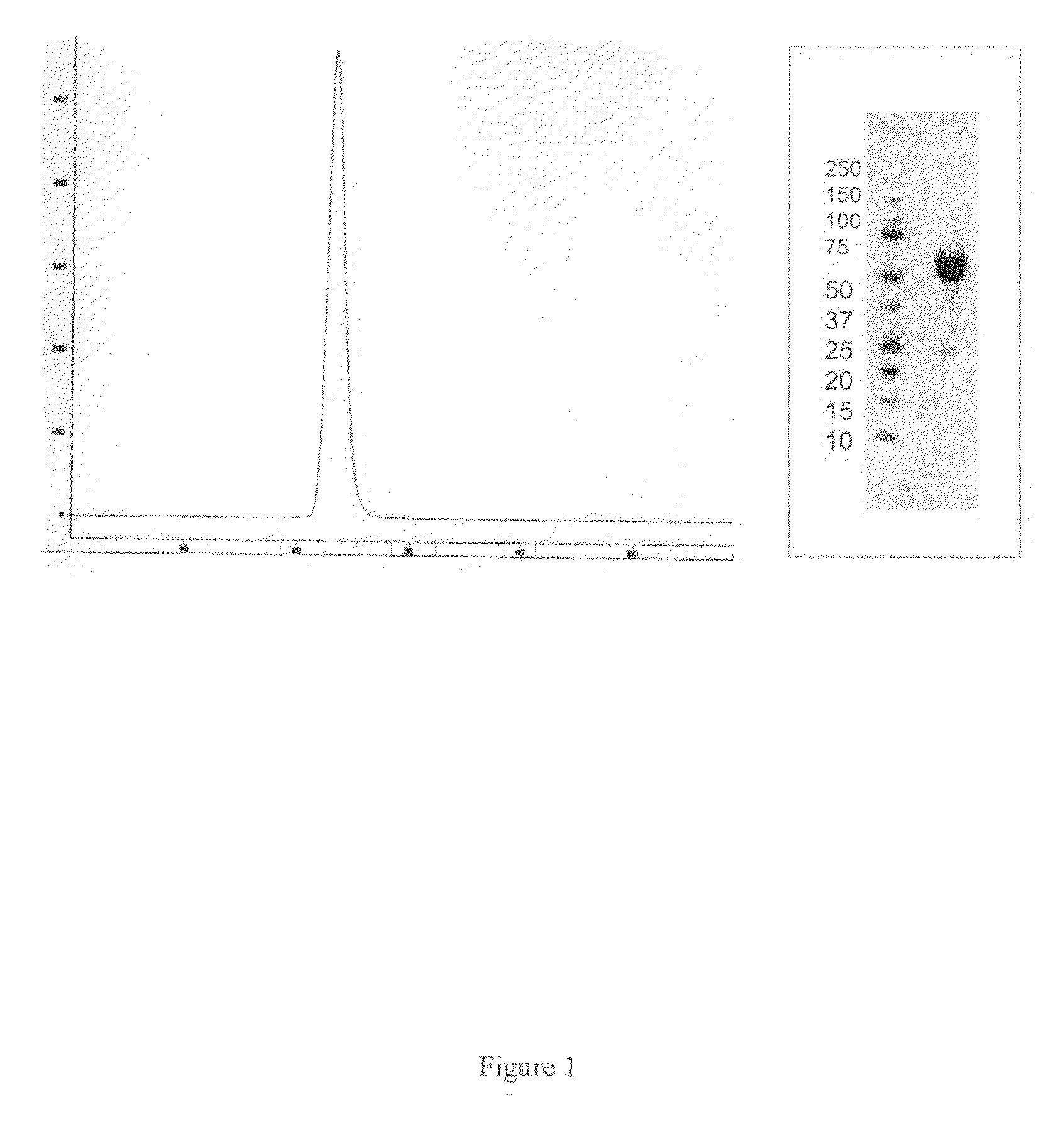
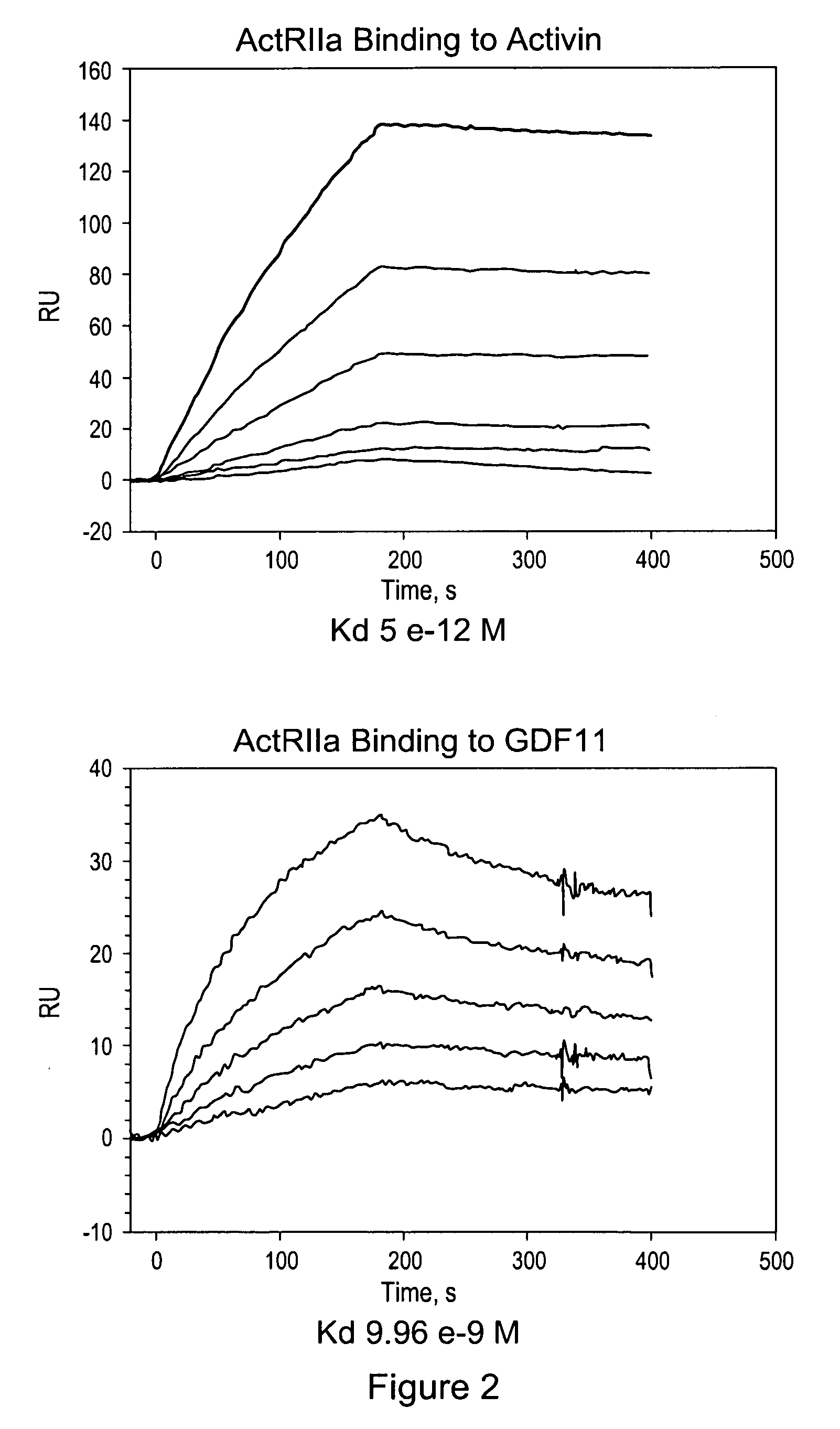
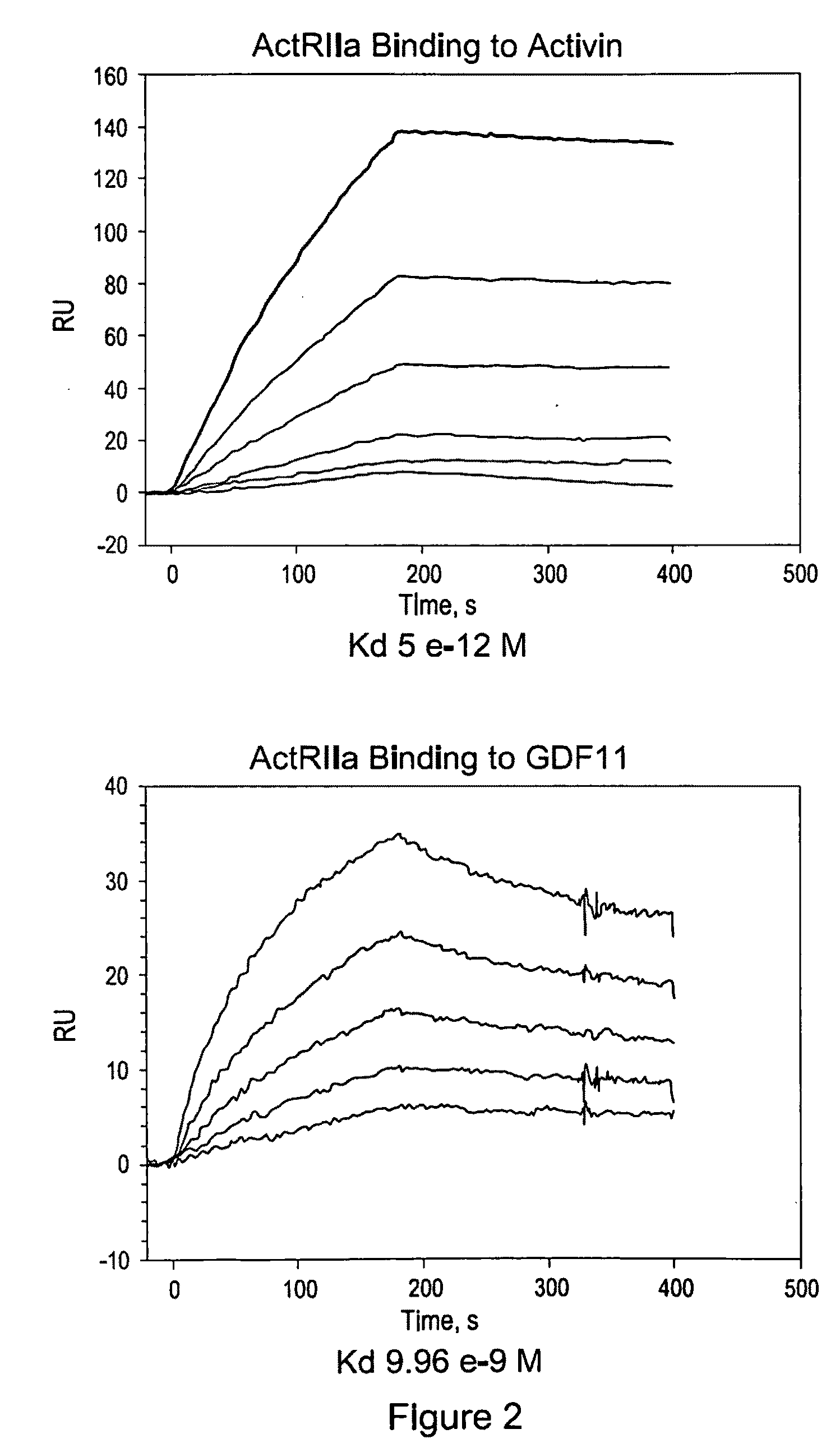
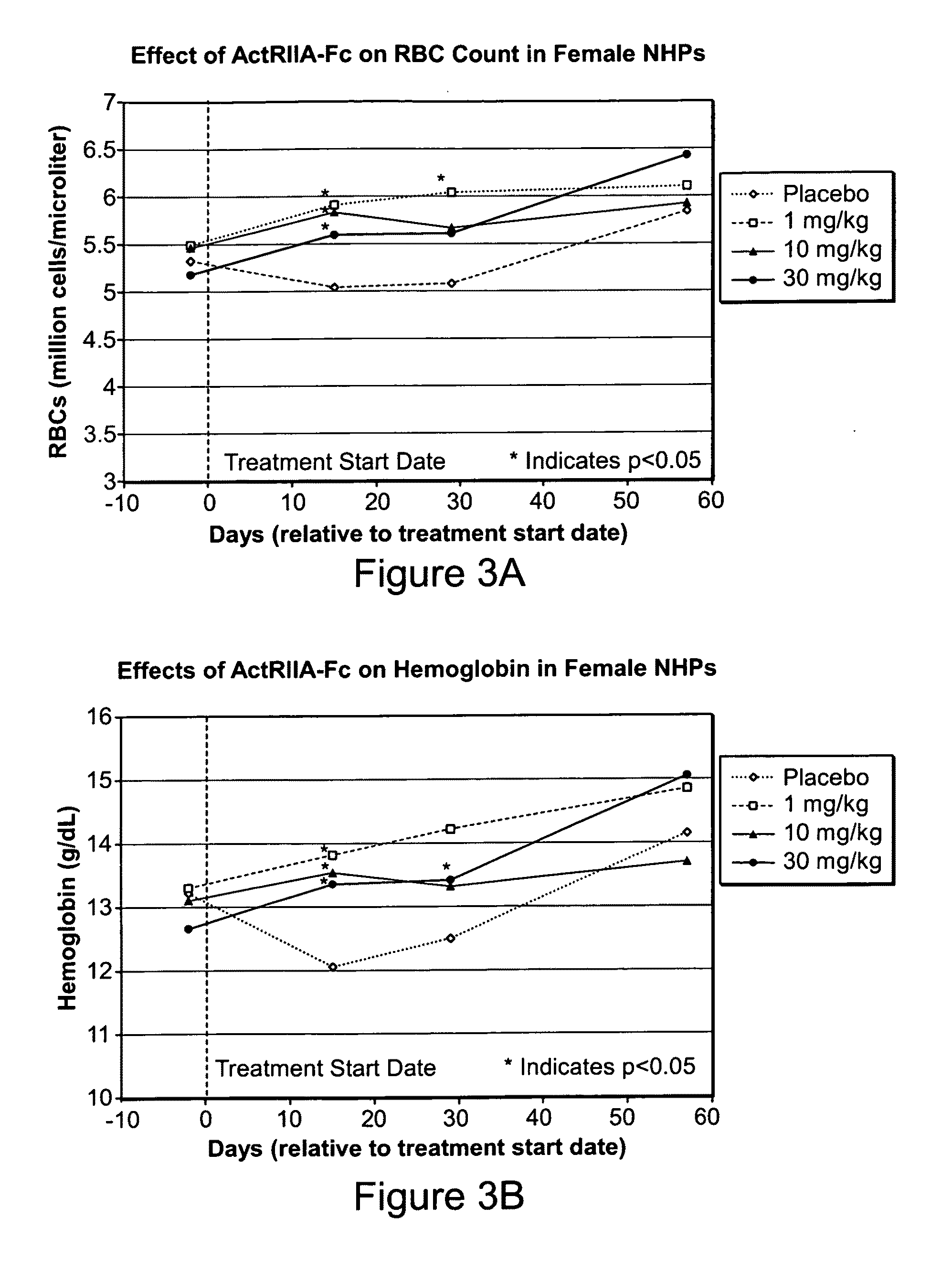
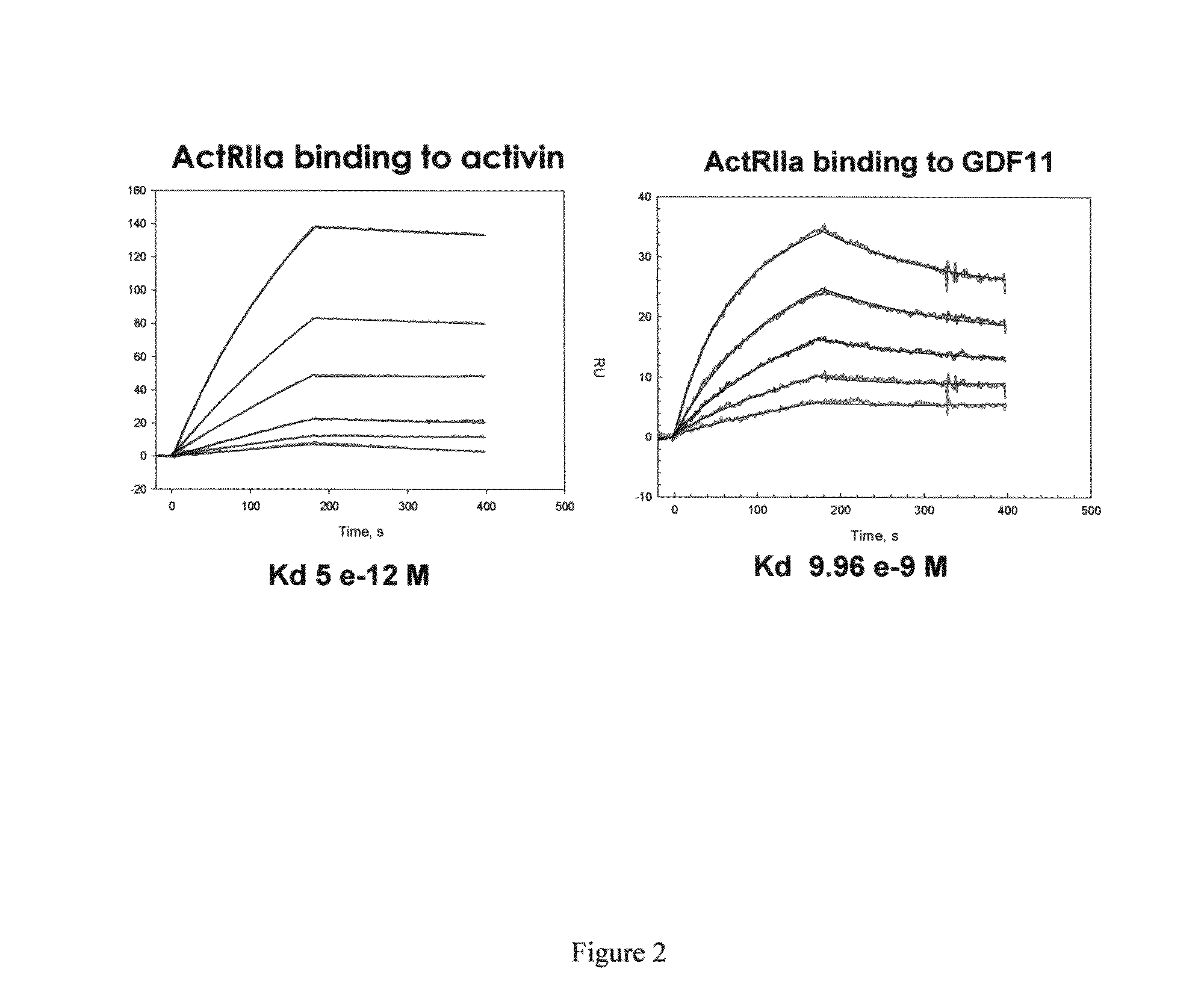
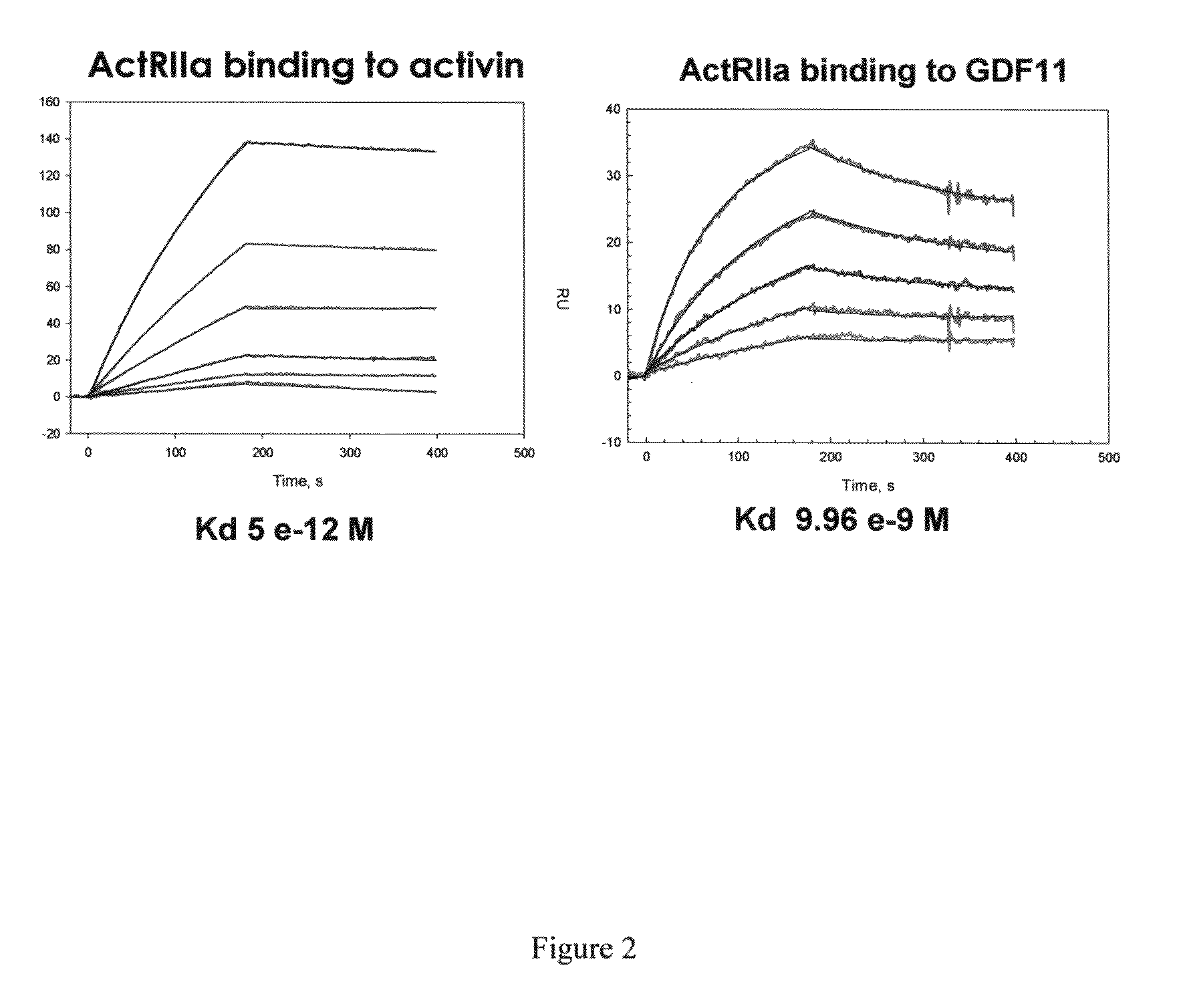
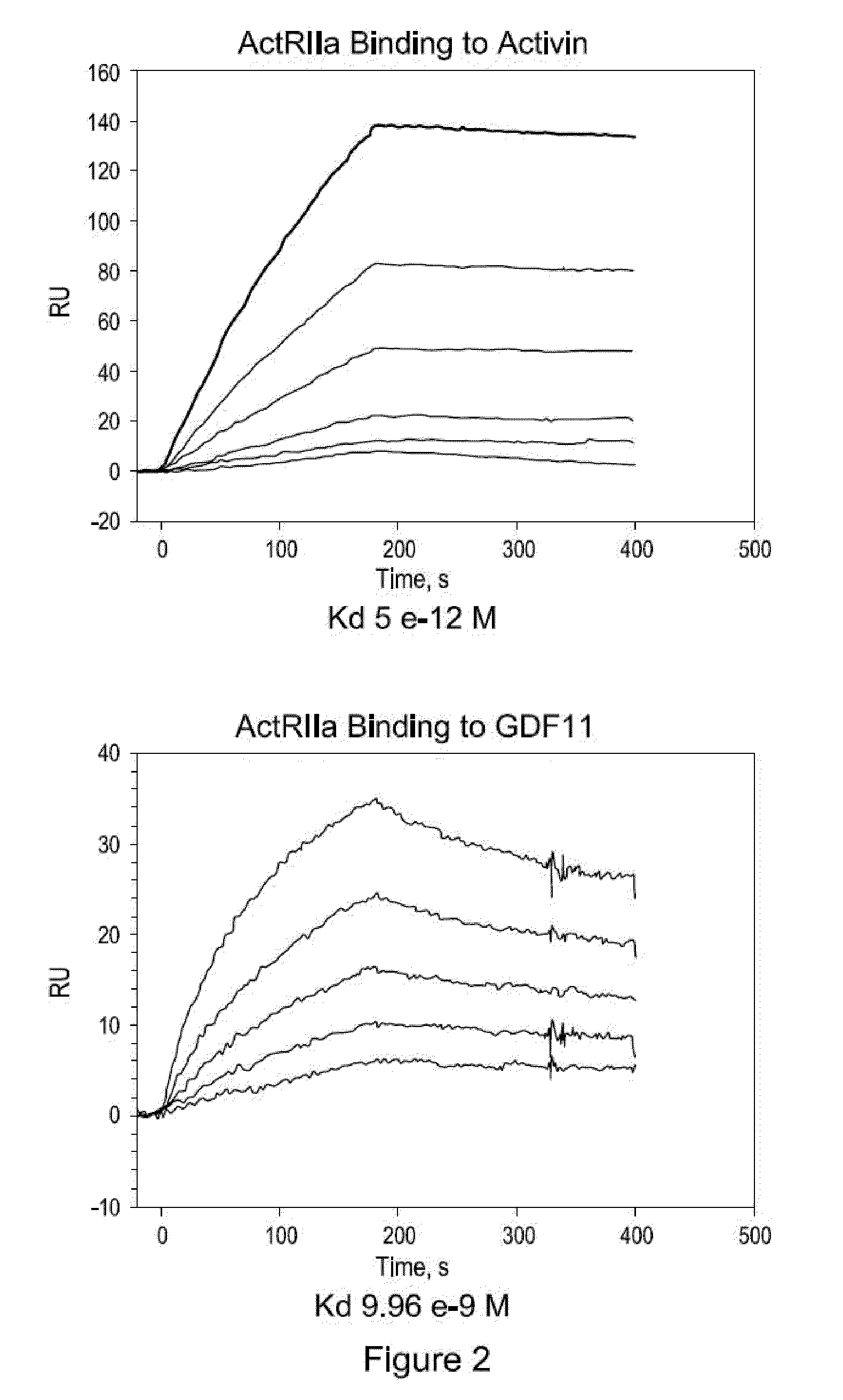
Activin-ActRIIa antagonists and uses for promoting bone growth
ActiveUS20070249022A1Increase in mineralizationReduce expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsCancer researchAntagonist
In certain aspects, the present invention provides compositions and methods for promoting bone growth and increasing bone density.
Owner:ACCELERON PHARMA INC
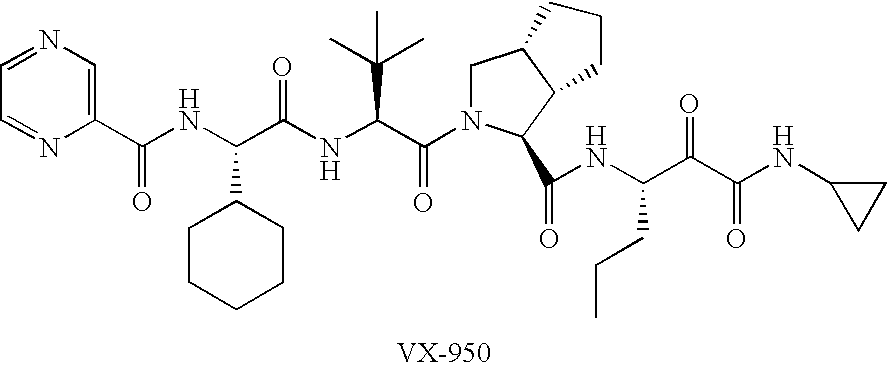
Combinations for HCV treatment
InactiveUS20060003942A1Improve pharmacokineticsImproving the pharmacokinetics of Hepatitis C NS3/4ABiocideOrganic active ingredientsHcv treatmentMonooxygenase
The present invention relates to co-administering a Hepatitis C virus NS3 / 4A protease inhibitor and a cytochrome P450 monooxygenase inhibitor. The combination acts by interfering with the life cycle of the hepatitis C virus and is therefore useful as an antiviral therapy. As such, the combination may be used for treating or preventing Hepatitis C infections in patients. The invention also relates to compositions comprising the combination of inhibitors. The invention also relates to kits and pharmaceutical packs comprising a Hepatitis C virus NS3 / 4A protease inhibitor and a cytochrome P450 monooxygenase inhibitor. The invention also relates to processes for preparing these compositions, combinations, kits, and packs.
Owner:VERTEX PHARMA INC
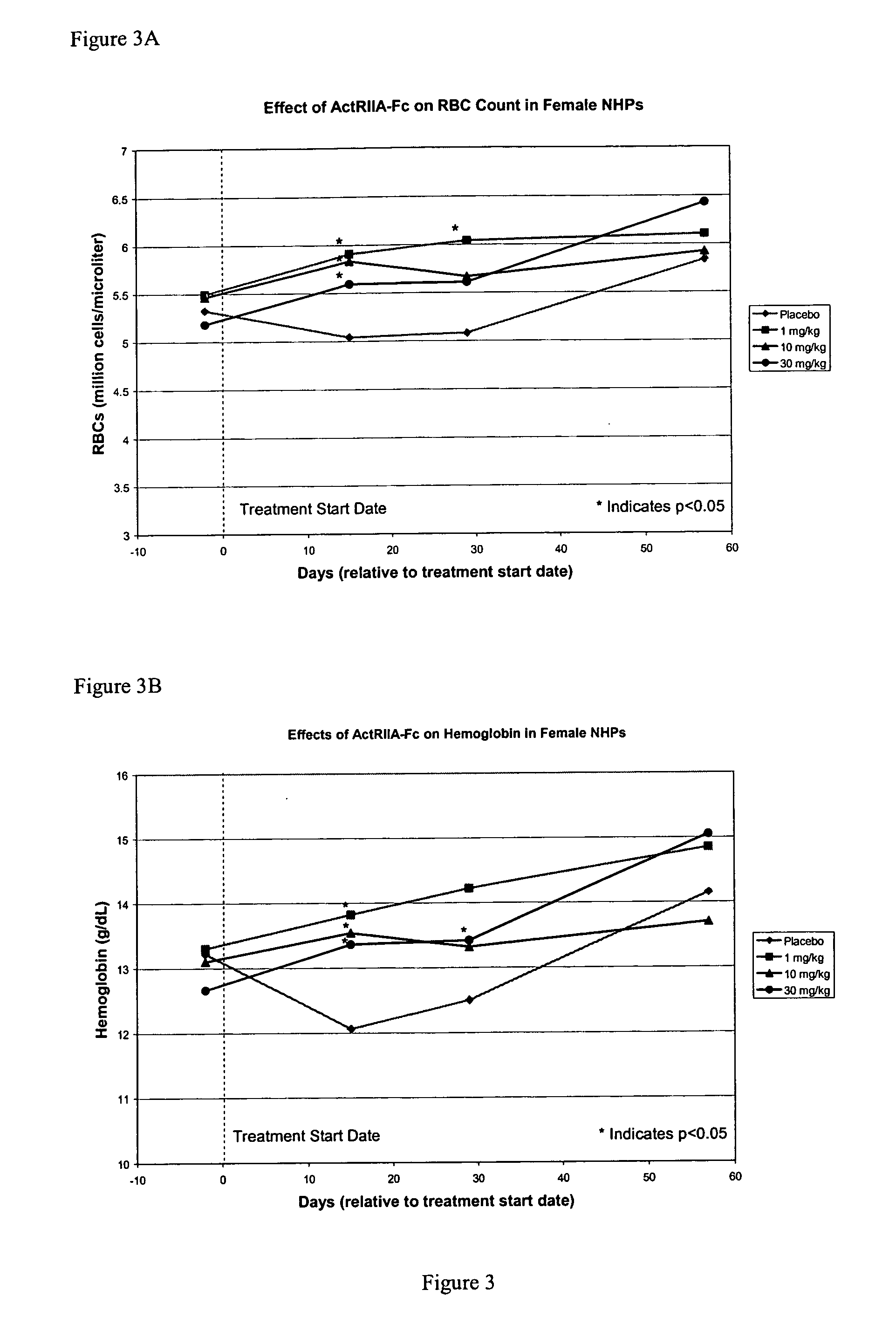
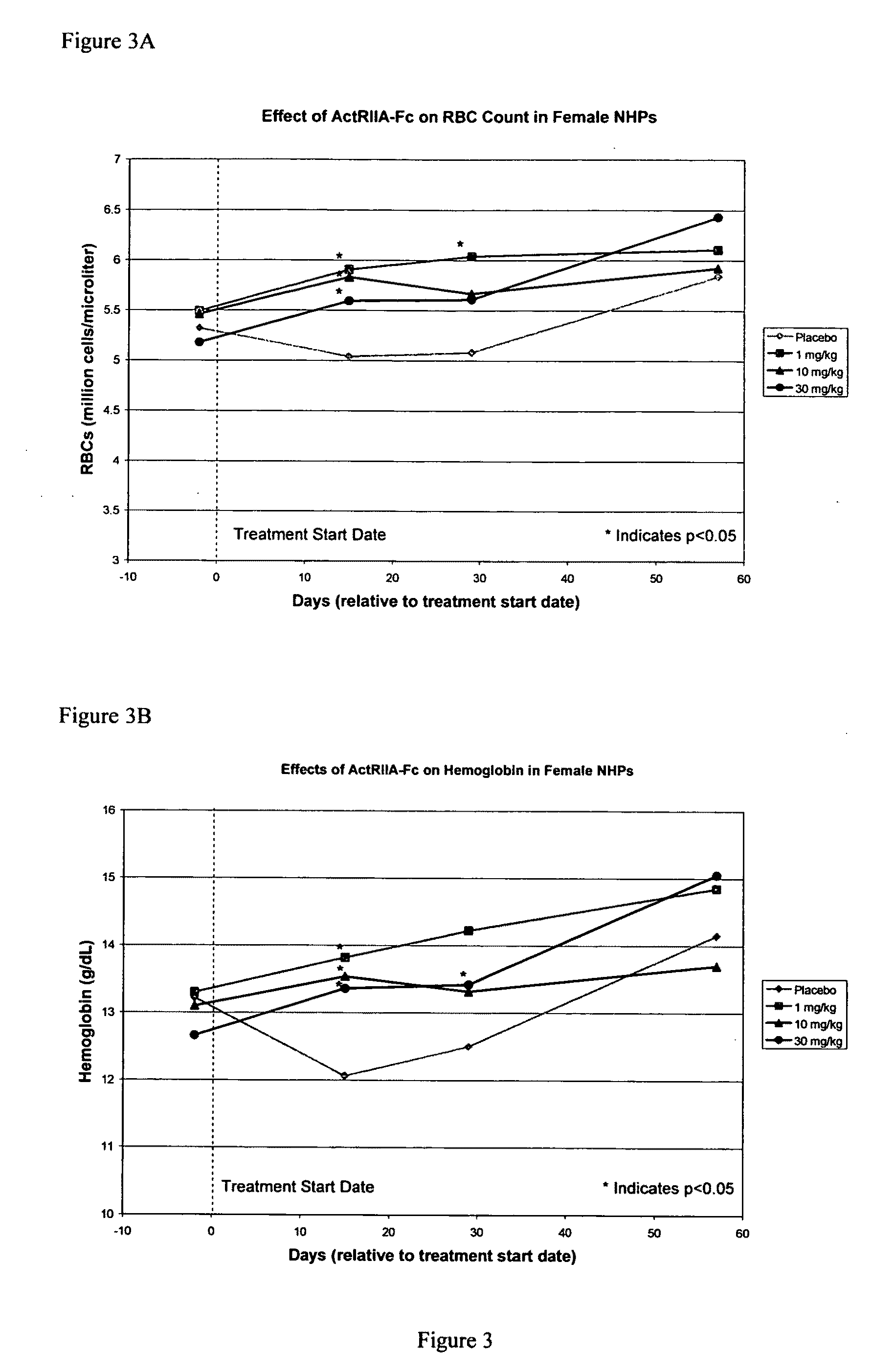
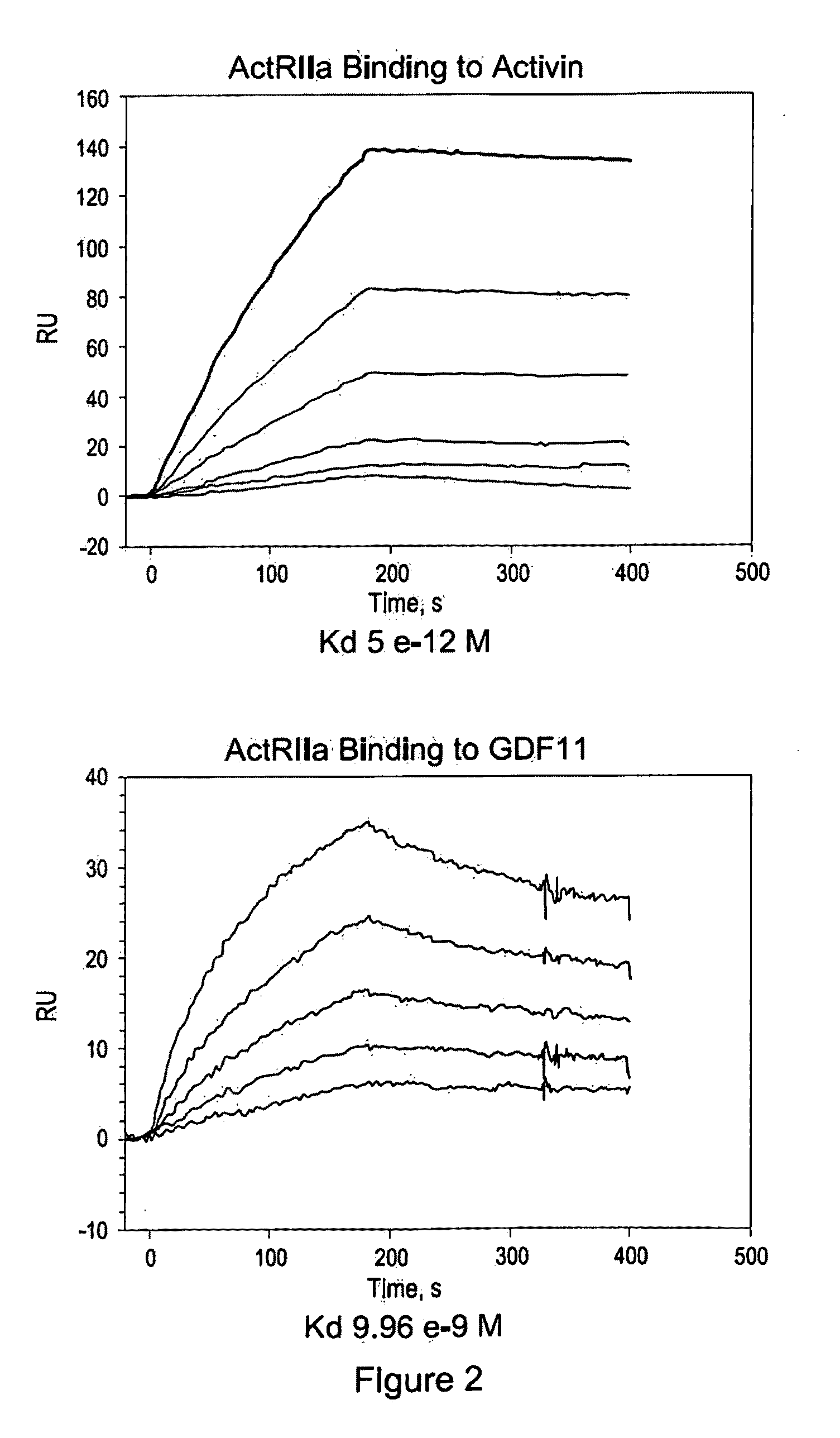
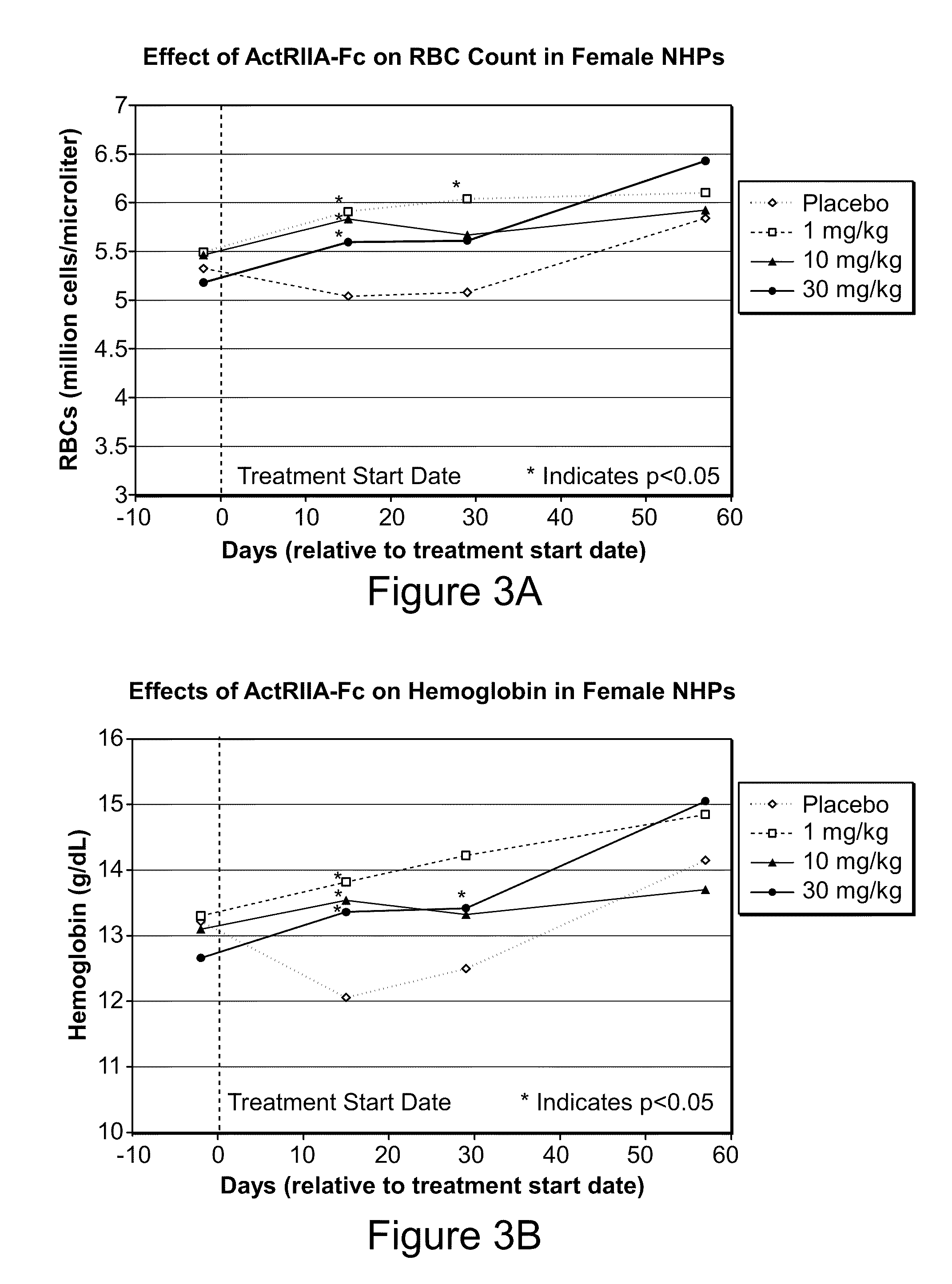
Activin-ActRII antagonists and uses for increasing red blood cell levels
ActiveUS20090047281A1Easy to produceReduce expressionCompound screeningApoptosis detectionPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Methods and devices for improving delivery of a substance to skin
InactiveUS20090124997A1Efficacy is alteredImprove delivery capabilitiesMedical devicesPressure infusionDelivery PerformanceHuman skin
A method of delivery of a substance to a human subject's skin comprising deposition into a specific compartment of the skin, wherein the delivery occurs at a controlled rate and pressure. The methods of the invention provide accurate deposition of s pre-selected volume of the substance, e.g., greater than 90% of the pre-selected volume. The methods of the invention encompass varying one or more parameters including but not limited to configurations of the delivery device, volume, pressure, and flow rate of delivery, to enhance the efficacy of delivery of the substance to the human skin. Substances delivered in accordance with the methods of the invention result in a more efficacious deposition of the substance into the targeted compartment, improved delivery performance, i.e., completeness of delivery as measured by quantification of the substance not delivered or the amount of the substance leaked out from the injection site, and enhanced safety as measured by the occurrence of minimal adverse cutaneous events at the site of injection.
Owner:BECTON DICKINSON & CO
Activin-actriia antagonists and uses for decreasing or inhibiting FSH secretion
ActiveUS20090118188A1Inhibit growthAvoid survivalHormone peptidesPeptide/protein ingredientsAndrologyPlant Germ Cells
In certain aspects, the present invention provides compositions and methods for decreasing FSH levels in a patient. The patient may, for example, be diagnosed with an FSH-related disorder or desire to delay or inhibit germ cell maturation.
Owner:ACCELERON PHARMA INC
Activin-actriia antagonists and uses for treating or preventing breast cancer
ActiveUS20090074768A1Inhibit growthAvoid survivalPeptide/protein ingredientsAntibody mimetics/scaffoldsGynecologyAntagonist
In certain aspects, the present invention provides compositions and methods for treating or preventing breast cancer in humans.
Owner:ACCELERON PHARMA INC
Activin-actriia antagonists and uses for promoting bone growth in cancer patients
ActiveUS20090142333A1Promote growthStimulates of mineralizationPeptide/protein ingredientsAntibody mimetics/scaffoldsIncreased Bone DensityBone growth
In certain aspects, the present invention provides compositions and methods for promoting bone growth and increasing bone density, as well as for the treatment of multiple myeloma.
Owner:ACCELERON PHARMA INC
Antigen-binding molecule capable of binding to two or more antigen molecules repeatedly
InactiveUS20130011866A1Pharmacokinetics of the antigen-binding molecule can be improvedGood effectSsRNA viruses negative-senseCompound screeningHalf-lifeAntigen binding
The present inventors discovered that antibodies having weaker antigen-binding activity at the early endosomal pH in comparison with that at the pH of plasma are capable of binding to multiple antigen molecules with a single antibody molecule, have long half-lives in plasma, and have improved durations of time in which they can bind to antigen.
Owner:CHUGAI PHARMA CO LTD
Methods for increasing red blood cell levels and treating anemia using a combination of GDF traps and erythropoietin receptor activators
ActiveUS8216997B2Increased formationImprove the level ofOrganic active ingredientsPeptide/protein ingredientsPrimatePhysiology
Owner:ACCELERON PHARMA INC
Activin-actrii antagonists and uses for increasing red blood cell levels
ActiveUS20090163417A1Reduce expressionIncrease redCompound screeningApoptosis detectionPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Method of increasing red blood cell levels or treating anemia in a patient
ActiveUS8058229B2Low affinityReduce impactPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Antagonists of activin-actriia and uses for increasing red blood cell levels
ActiveUS20100028331A1Easy to produceReduce expressionPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Activin-ActRIIa antagonists and uses for promoting bone growth
ActiveUS20090098113A1Reduce expressionPromote bone growthPeptide/protein ingredientsAntibody mimetics/scaffoldsIncreased Bone DensityBone growth
In certain aspects, the present invention provides compositions and methods for promoting bone growth and increasing bone density.
Owner:ACCELERON PHARMA INC
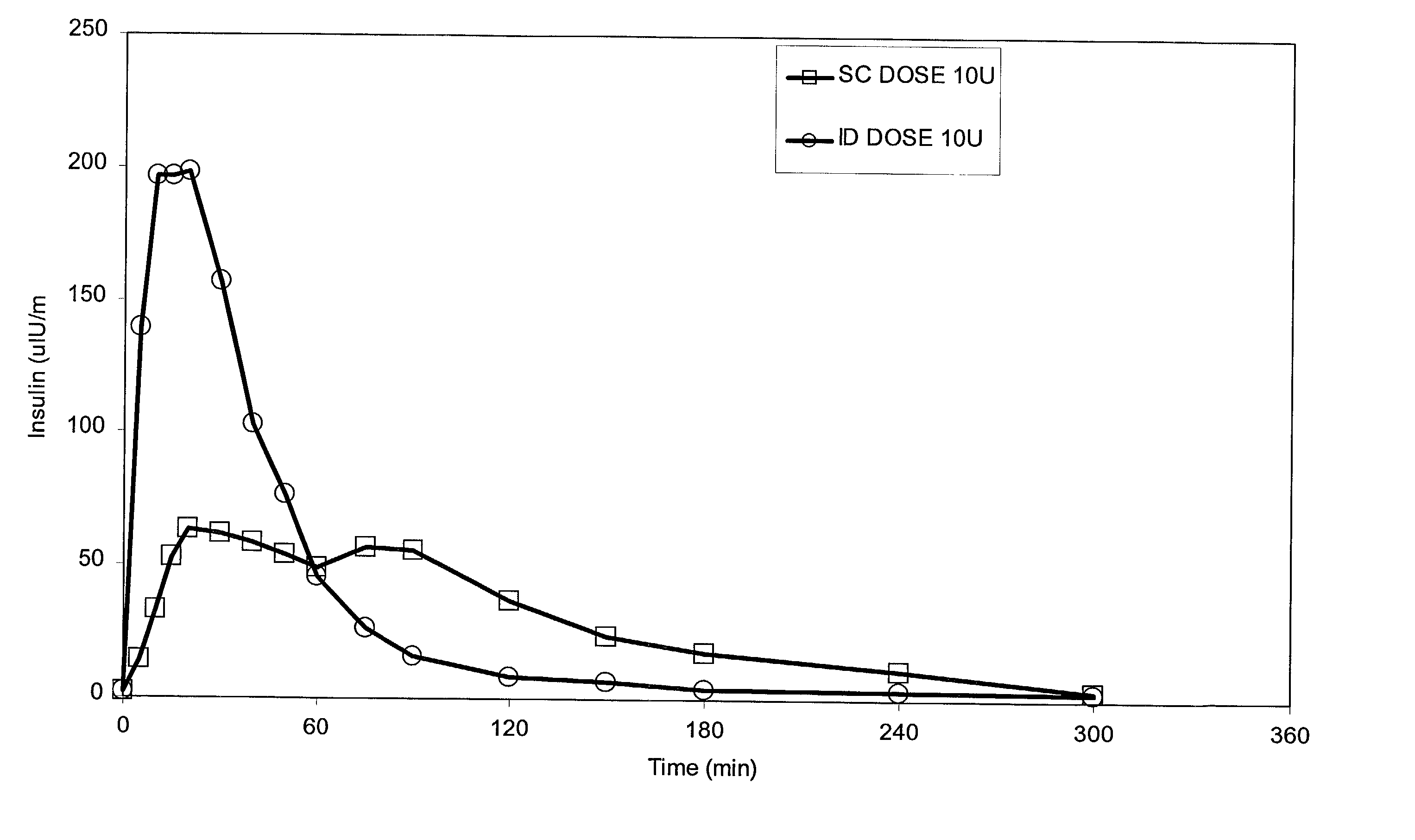
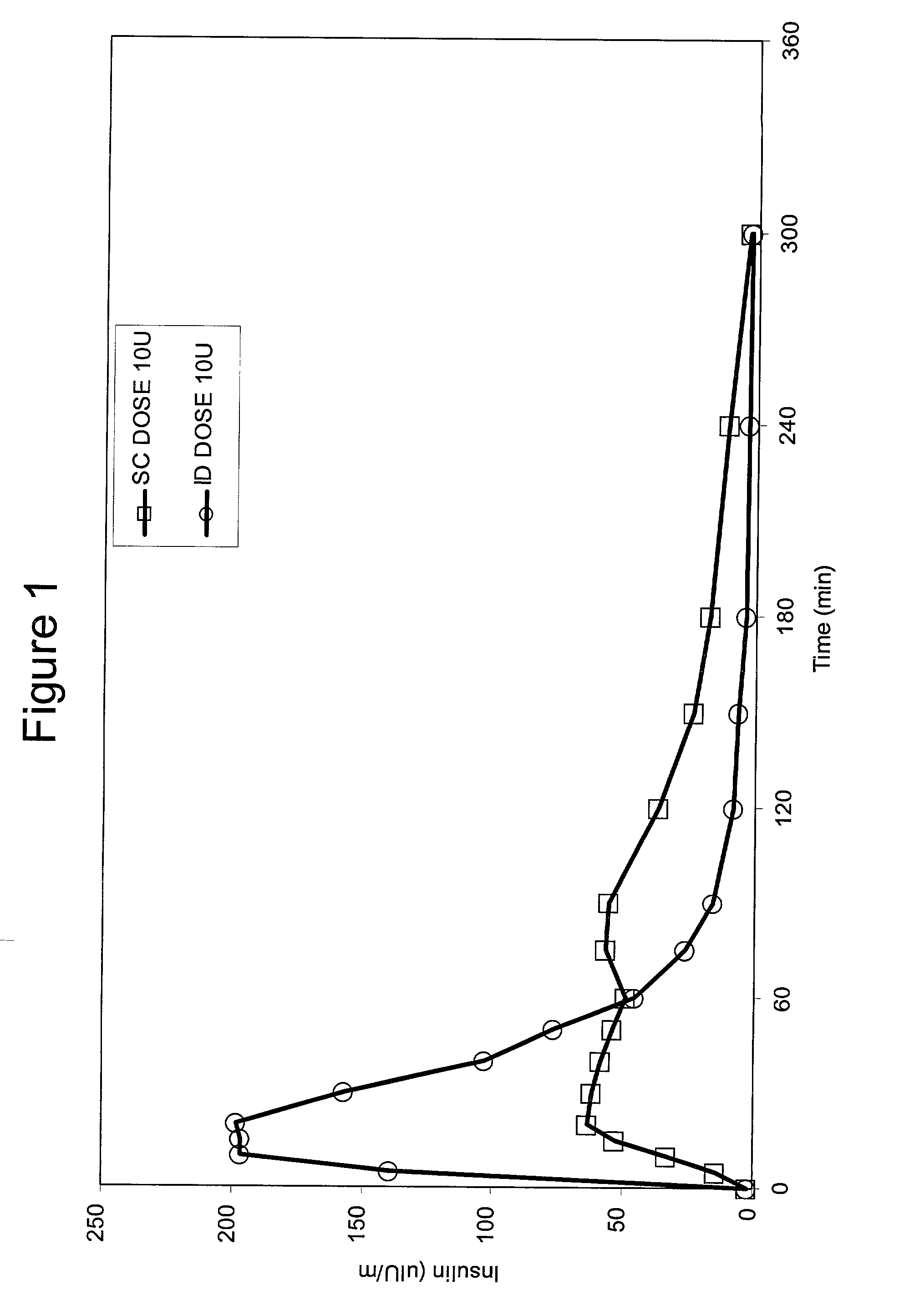
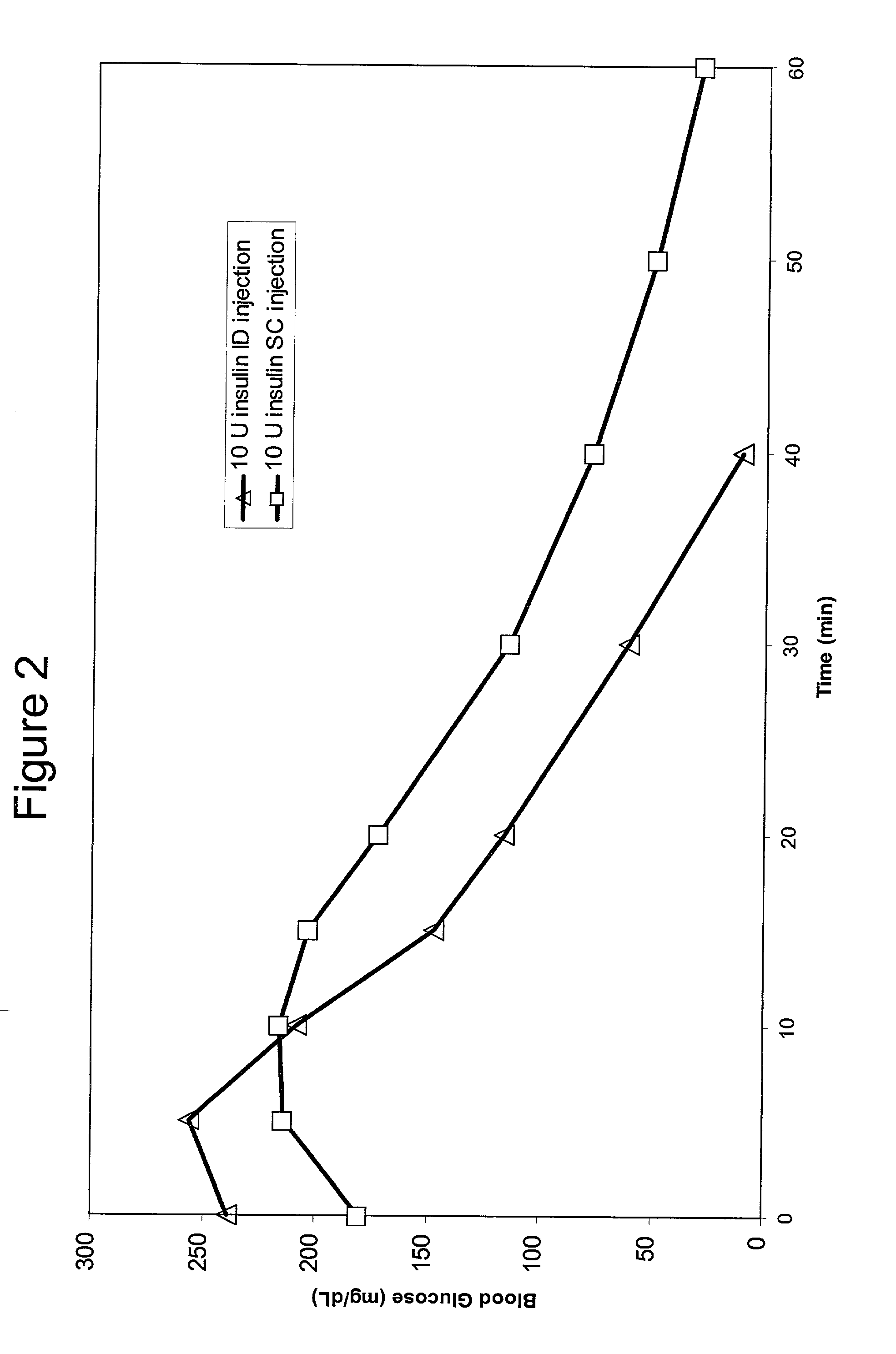
Intradermal delivery of substances
InactiveUS20040073160A1Increase uptakeRapid uptake rateJet injection syringesPeptide/protein ingredientsWhole bodyGrowth hormone
A method for administration of a substance into the dermis of a mammal is disclosed. The method involves administration into the dermis by injection which results in improved systemic absorption relative to that obtained upon subcutaneous administration of the substance. The substance administered may be a growth hormone, a low molecular weight heparin or a dopamine receptor agonist.
Owner:PHARMACIA CORP
Activin-ActRIIa antagonists and uses for promoting bone growth
ActiveUS20090099086A1Reduce expressionPromote bone growthPeptide/protein ingredientsAntibody mimetics/scaffoldsIncreased Bone DensityCancer research
In certain aspects, the present invention provides compositions and methods for promoting bone growth and increasing bone density.
Owner:ACCELERON PHARMA INC
Antagonists of actriib and uses for increasing red blood cell levels
InactiveUS20100028332A1Distribution moreMany formatsPeptide/protein ingredientsAntibody mimetics/scaffoldsRed CellAntagonist
In certain aspects, the present invention provides compositions and methods for increasing red blood cell and / or hemoglobin levels in vertebrates, including rodents and primates, and particularly in humans.
Owner:ACCELERON PHARMA INC
Remodeling and Glycopegylation of Fibroblast Growth Factor (Fgf)
InactiveUS20080176790A1Improve pharmacokineticsCost effectiveOrganic active ingredientsFungiMutantPolynucleotide
The present invention relates to mutants of Fibroblast Growth Factor (FGF), particularly FGF-20 and FGF-21, which contain newly introduced N-linked or O-linked glycosylation site(s). The polynucleotide coding sequences for the mutants, expression cassettes comprising the coding sequences, cells expressing the mutants, and methods for producing the mutants are also disclosed. Further disclosed are pharmaceutical compositions comprising the mutants and method for using the mutants.
Owner:89BIO LTD +1
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption
InactiveUS20030100885A1Increase uptakeRapid onsetOrganic active ingredientsAmpoule syringesWhole bodySystemic absorption
Methods and devices for administration of substances into the intradermal layer of skin for systemic absorption.
Owner:BECTON DICKINSON & CO
Hepatitis c virus inhibitors
ActiveUS20110189129A1Improve pharmacokineticsImproving the pharmacokinetics of drugsBiocidePeptide/protein ingredientsHepatitis c viralPharmaceutical drug
The present invention discloses compounds of Formula (I), and pharmaceutically acceptable salts, esters, or prodrugs thereof:Q-G-A-L-B—W (I),which inhibit RNA-containing virus, particularly the hepatitis C virus (HCV). Consequently, the compounds of the present invention interfere with the life cycle of the hepatitis C virus and are also useful as antiviral agents. The present invention further relates to pharmaceutical compositions comprising the aforementioned compounds for administration to a subject suffering from HCV infection. The invention also relates to methods of treating an HCV infection in a subject by administering a pharmaceutical composition comprising the compounds of the present invention.
Owner:ENANTA PHARM INC
Compositions having enhanced pharmacokinetic characteristics
InactiveUS7491383B2Reduced activityImprove bioavailabilityBiocideSenses disorderAdrenergicFatty acid
Compositions comprising a therapeutic component and an efficacy enhancing component, that enhances the pharmacokinetic disposition of the therapeutic component, are disclosed. The therapeutic component may include an alpha-2-adrenergic agonist and the efficacy enhancing component may include fatty acids. In one embodiment, the therapeutic component and the efficacy enhancing component form a complex.
Owner:ALLERGAN INC
Use of gdf traps to increase red blood cell levels
ActiveUS20120015877A1Distribution moreMany formatsPeptide/protein ingredientsAntibody mimetics/scaffoldsPrimateRed Cell
Owner:ACCELERON PHARMA INC
Medical device applications of nanostructured surfaces
ActiveUS7803574B2Improve adhesionIncrease frictionElectrotherapyNanostructure manufactureFiberNanofiber
This invention provides novel nanofiber enhanced surface area substrates and structures comprising such substrates for use in various medical devices, as well as methods and uses for such substrates and medical devices. In one particular embodiment, a method of administering a composition to a patient is disclosed which comprises providing a composition-eluting device, said composition-eluting device comprising at least a first surface and a plurality of nanostructures attached to the first surface, and introducing the composition-eluting device into the body of the patient.
Owner:RGT UNIV OF CALIFORNIA +1
Methods for dosing an activin-actriia antagonist and monitoring of treated patients
InactiveUS20100015144A1Undesired effectImprove the level ofPeptide/protein ingredientsAntibody mimetics/scaffoldsHematologyAntagonist
In certain aspects, the present invention provides methods for dosing a patient with an activin-ActRIIa antagonist and methods for managing patients treated with an activin-ActRIIa anatagonist. In certain aspects, the methods involve measuring one or more hematologic parameters in a patient.
Owner:ACCELERON PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com