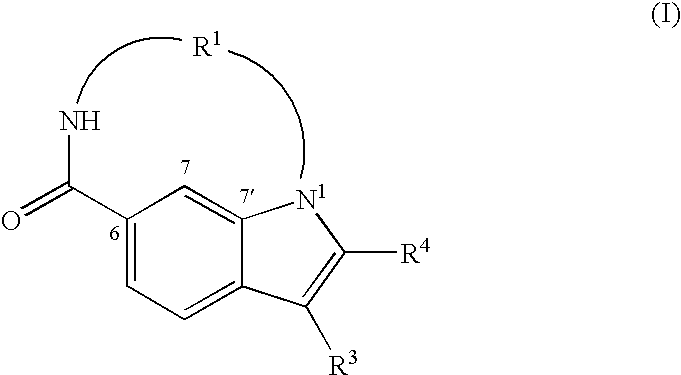
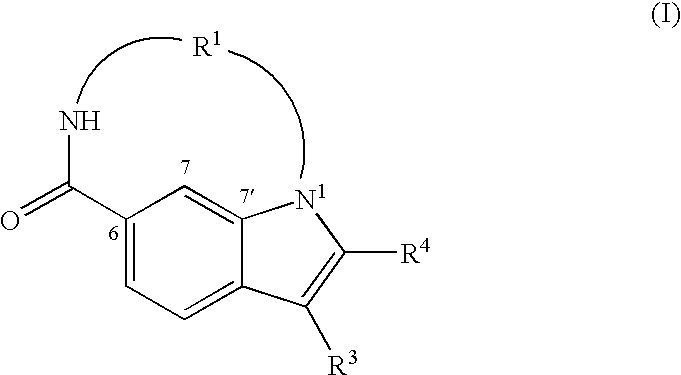
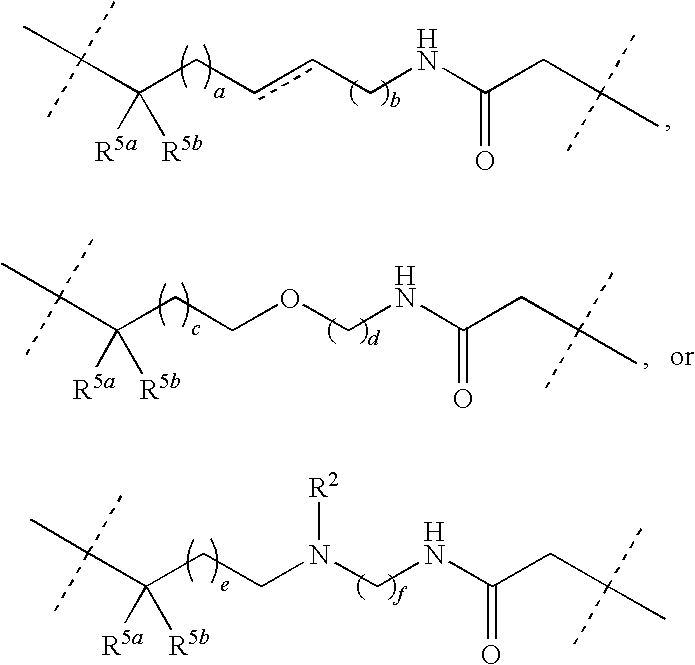
Patents
Literature
75 results about "Hcv treatment" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
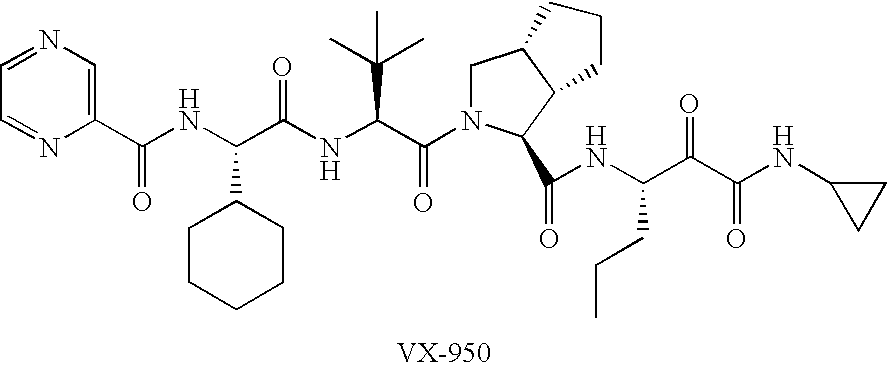
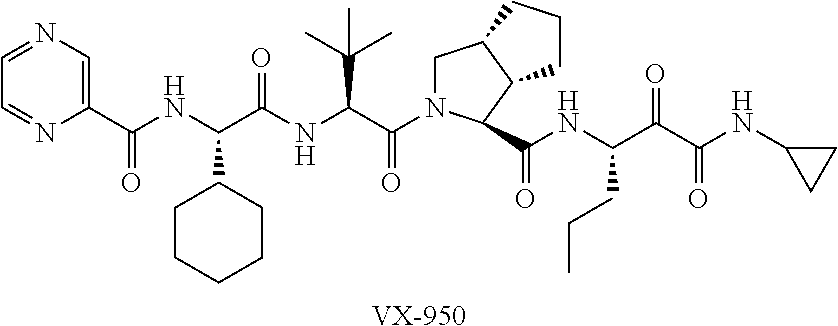
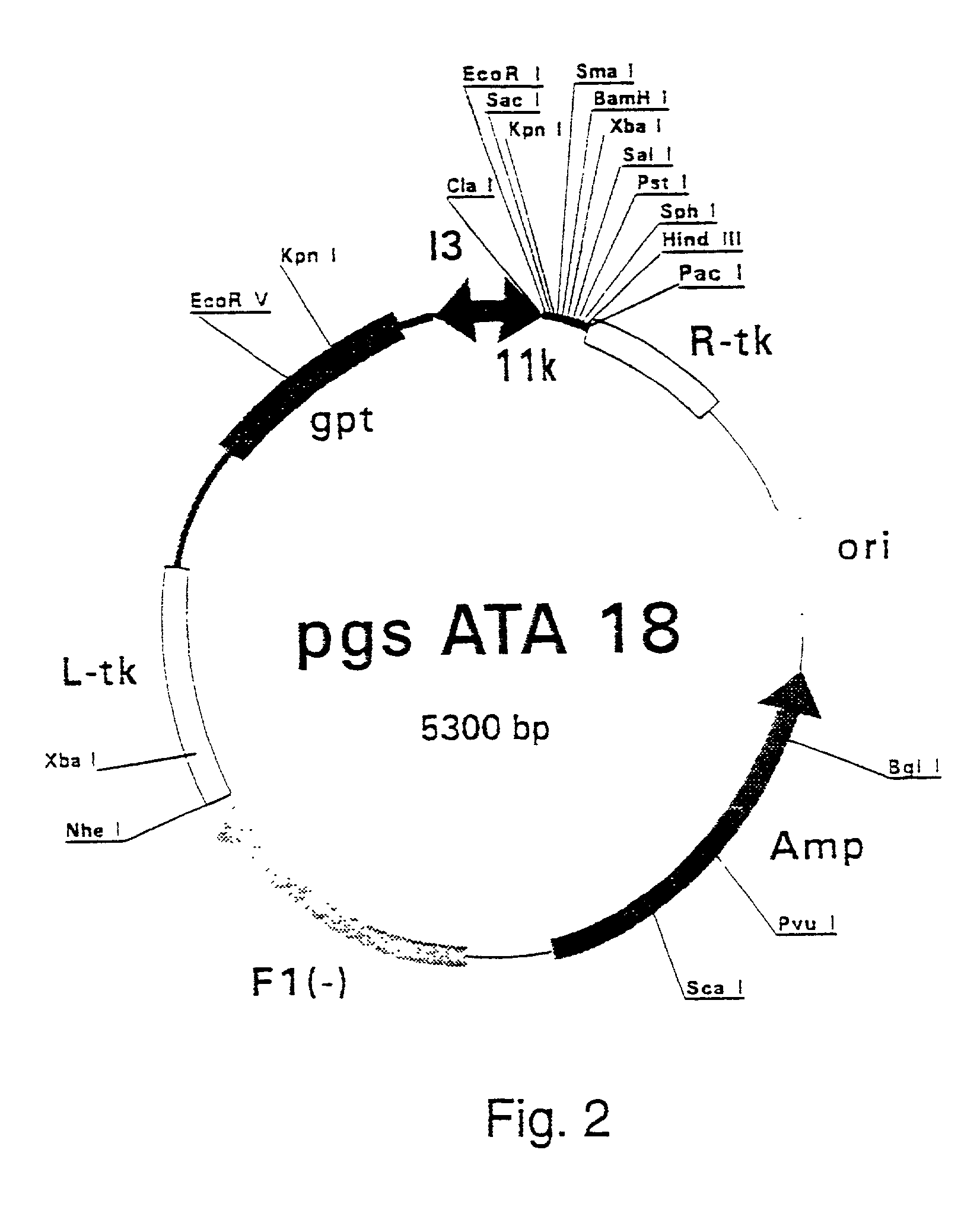
Combinations for HCV treatment
InactiveUS20060003942A1Improve pharmacokineticsImproving the pharmacokinetics of Hepatitis C NS3/4ABiocideOrganic active ingredientsHcv treatmentMonooxygenase
The present invention relates to co-administering a Hepatitis C virus NS3 / 4A protease inhibitor and a cytochrome P450 monooxygenase inhibitor. The combination acts by interfering with the life cycle of the hepatitis C virus and is therefore useful as an antiviral therapy. As such, the combination may be used for treating or preventing Hepatitis C infections in patients. The invention also relates to compositions comprising the combination of inhibitors. The invention also relates to kits and pharmaceutical packs comprising a Hepatitis C virus NS3 / 4A protease inhibitor and a cytochrome P450 monooxygenase inhibitor. The invention also relates to processes for preparing these compositions, combinations, kits, and packs.
Owner:VERTEX PHARMA INC
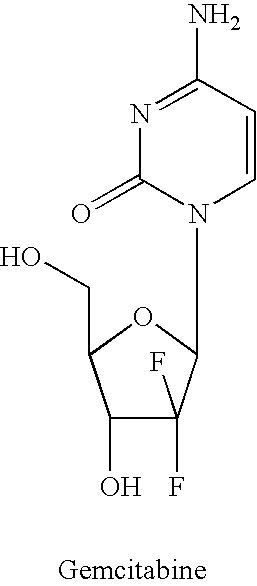
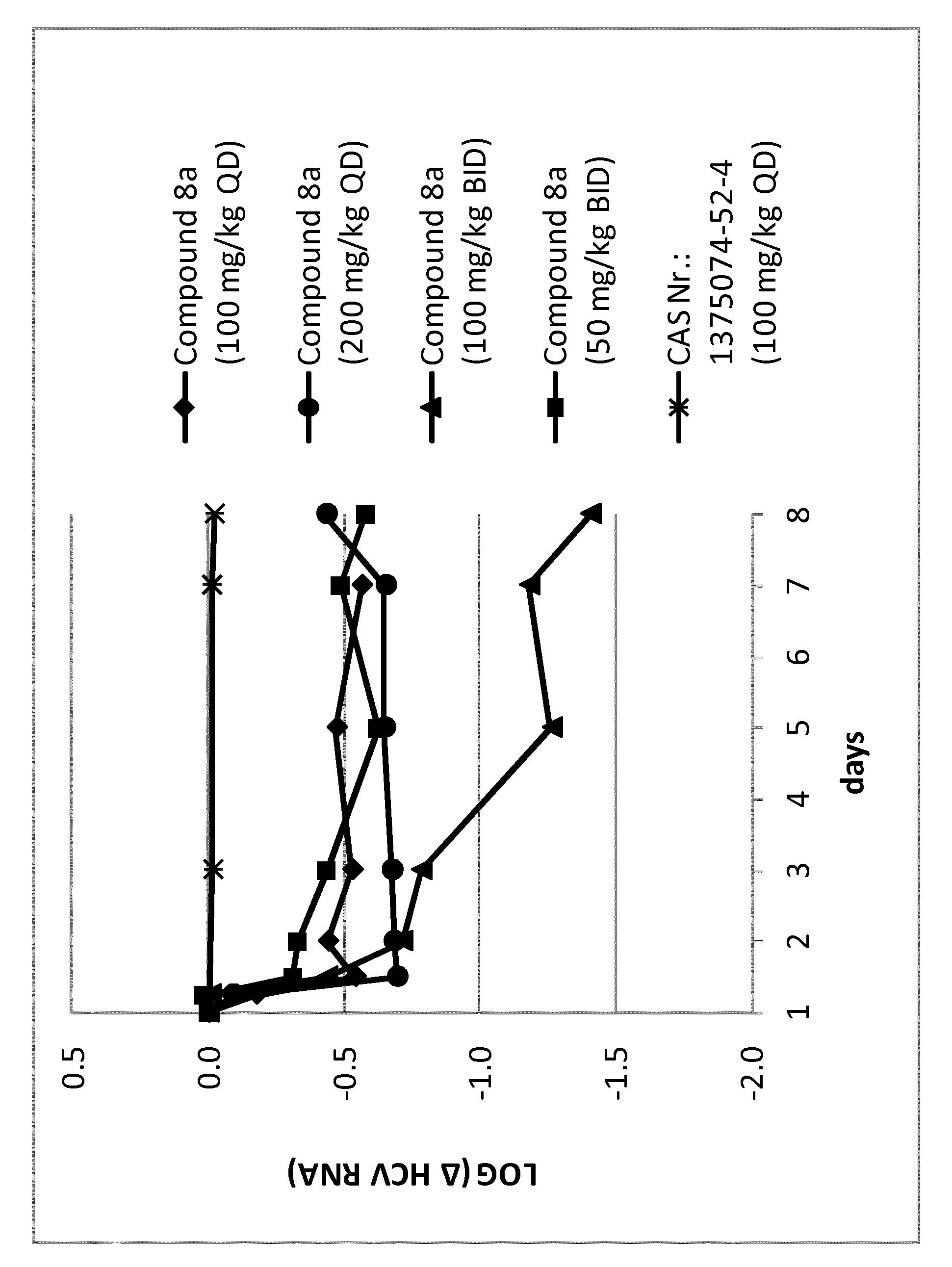
Dosing regimen for gemcitabine HCV therapy
InactiveUS20030225029A1Reduce viral loadRapid and large in viral loadBiocideSugar derivativesDosing regimenHepatitis c viral
A dosage regiment for the treatment of a Flaviviridae infection, including a hepatitis C viral infection, that includes administering gemcitabine (or its salt, prodrug or derivative, as described herein) in a dosage range of approximately 50 mg / m<2 >to about 1300 mg / m<2 >per day for between one and seven days (e.g. 1, 2, 3, 4, 5, 6, or 7 days) followed by cessation of therapy. Viral load is optionally monitored over time, and after cessation, viral rebound is monitored. Therapy is not resumed unless a significant viral load is again observed, and then therapy for 1-7 days and more preferred, 1, 2 or 3 days, is repeated. This therapy can be continued indefinitely to monitor and maintain the health of the patient.
Owner:PHARMASSET
Immunotherapy for chronic hepatitis c virus infection
InactiveUS20110256098A1Increase the number ofReduce in quantitySsRNA viruses positive-sensePeptide/protein ingredientsChronic viral hepatitis CInterferon therapy
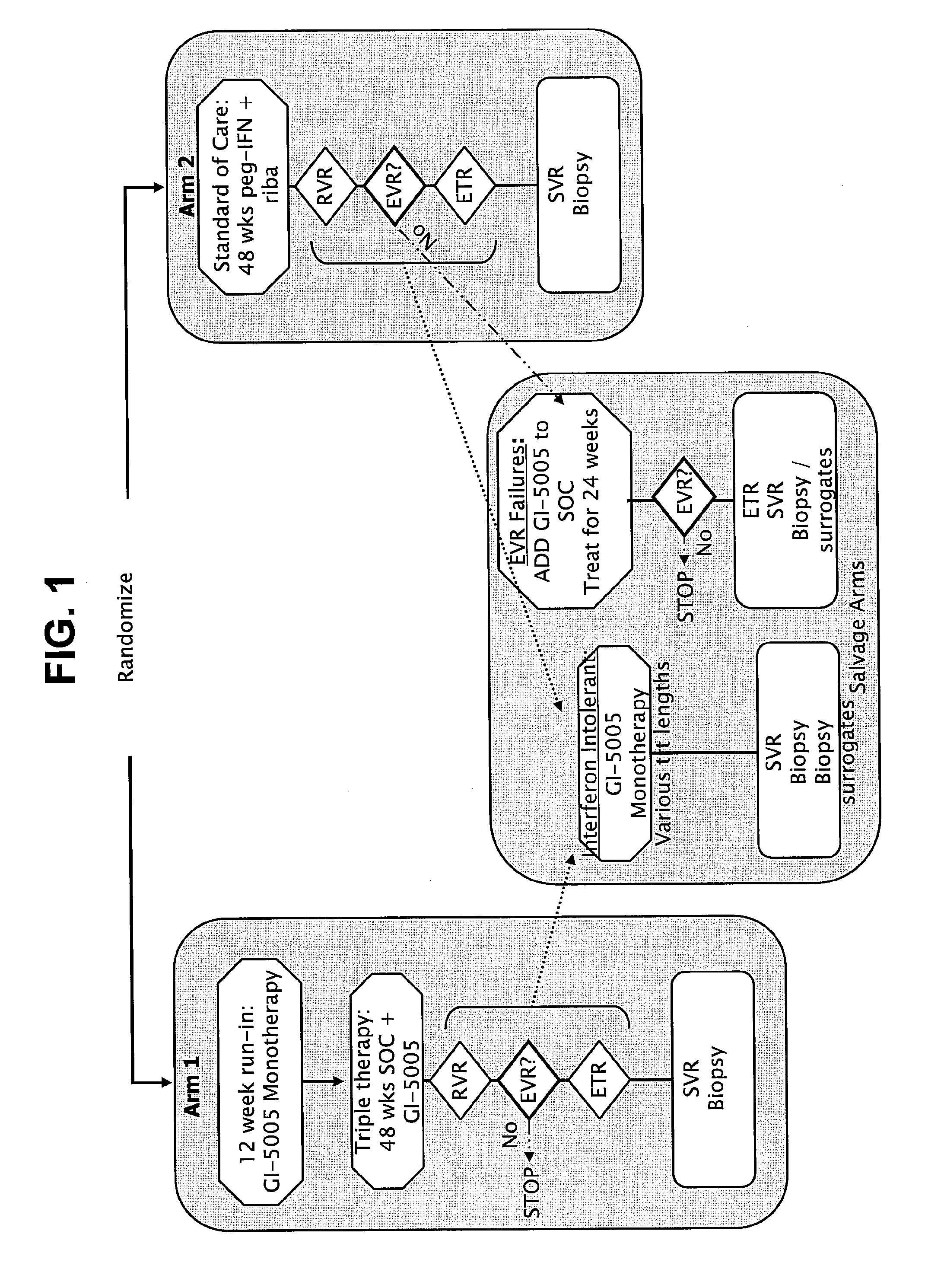
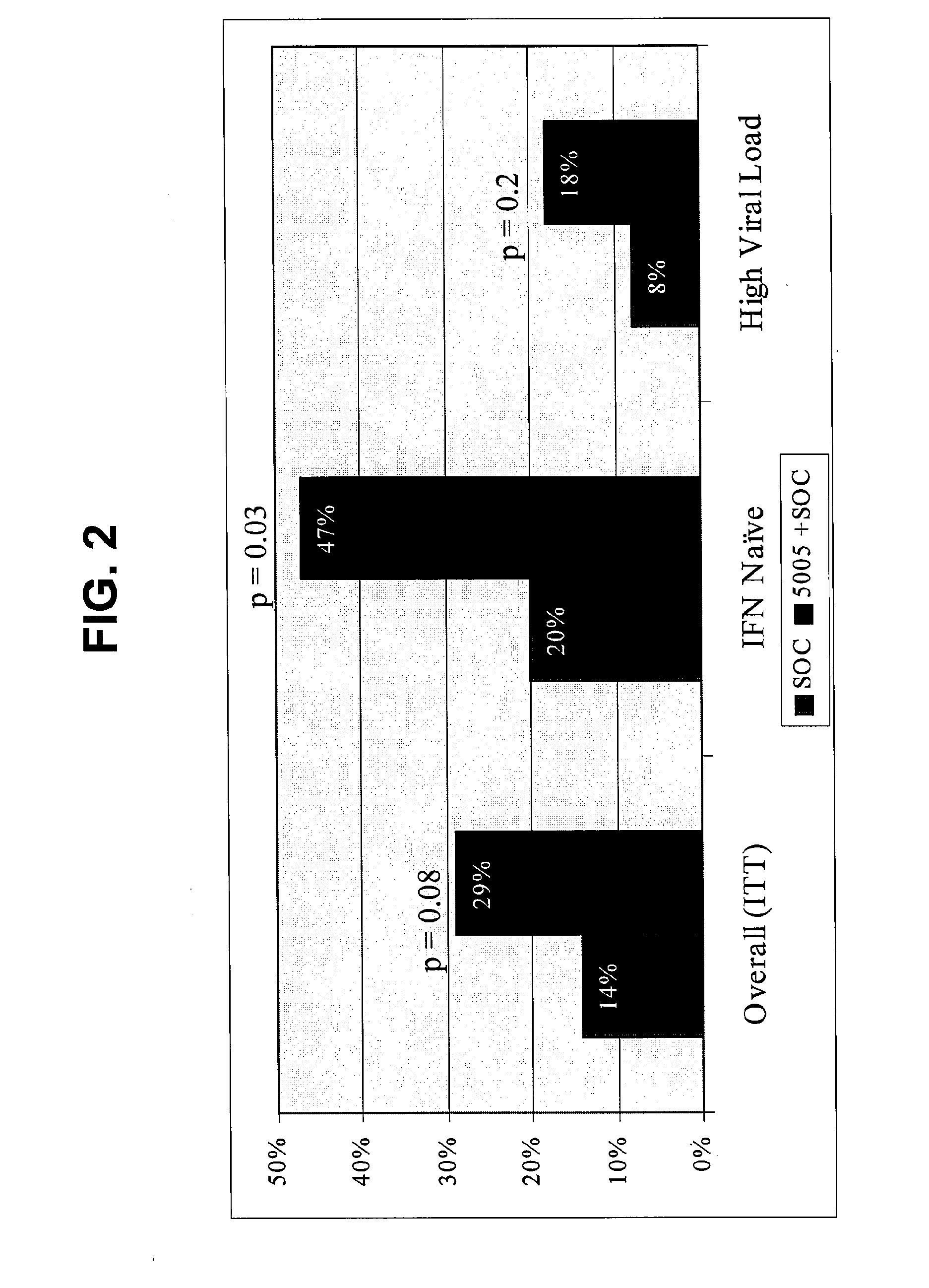
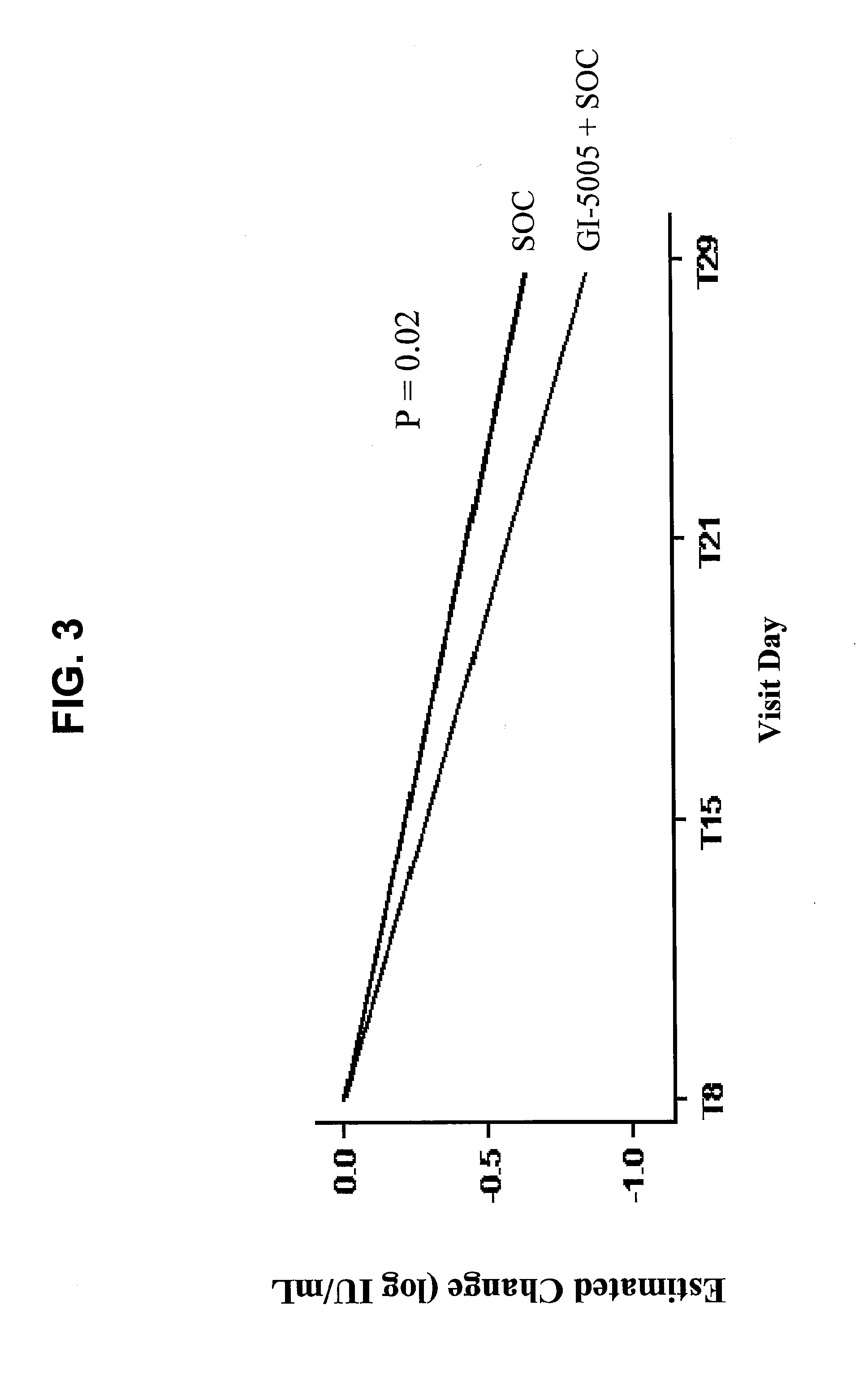
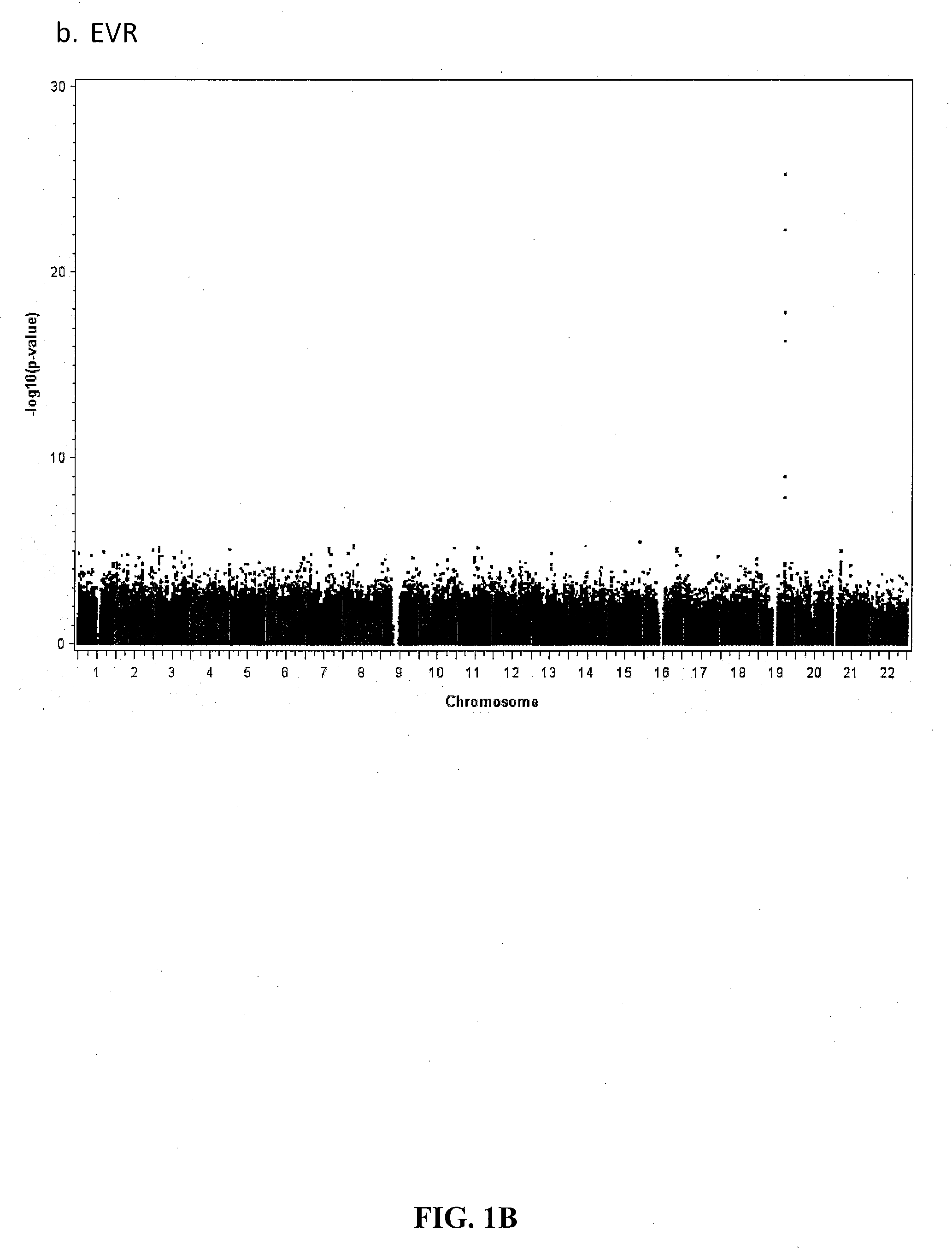
Disclosed are uses of immunotherapeutic compositions in combination with Standard of Care (SOC), or interferon therapy combined with anti-viral therapy, for the improved treatment of chronic hepatitis C virus (HCV) infection and related conditions, including liver function. The compositions, kits and uses of the invention, as compared to the use of SOC therapy alone: improves the rate of early response to therapy as measured by early virologic markers (e.g., RVR and EVR), enlarges the pool of patients who will have sustained responses to therapy over the long term, offers shortened courses of therapy for certain patients, enables “rescue” of patients who are non-responders or intolerant to SOC therapy, improves liver function and / or reduces liver damage in patients, and enables the personalization of HCV therapy for a patient, which can result in dose sparing, improved patient compliance, reduced side effects, and improved long term therapeutic outcomes.
Owner:GLOBE IMMUNE INC
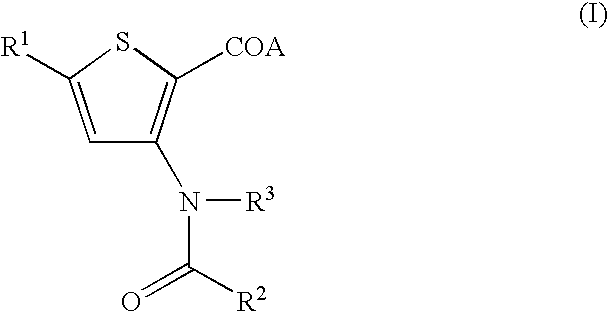
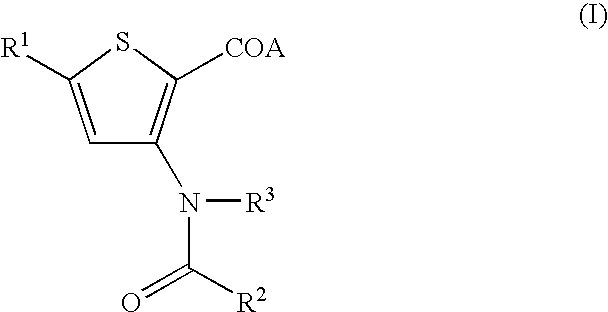
2-carboxy thiophene derivatives as anti viral agents
Anti-viral agents of compounds of Formula (I): wherein A, R1, R2 and R3 are as defined in the specification, processes for their preparation and their use in HCV treatment are provided.
Owner:SMITHKLINE BECKMAN CORP
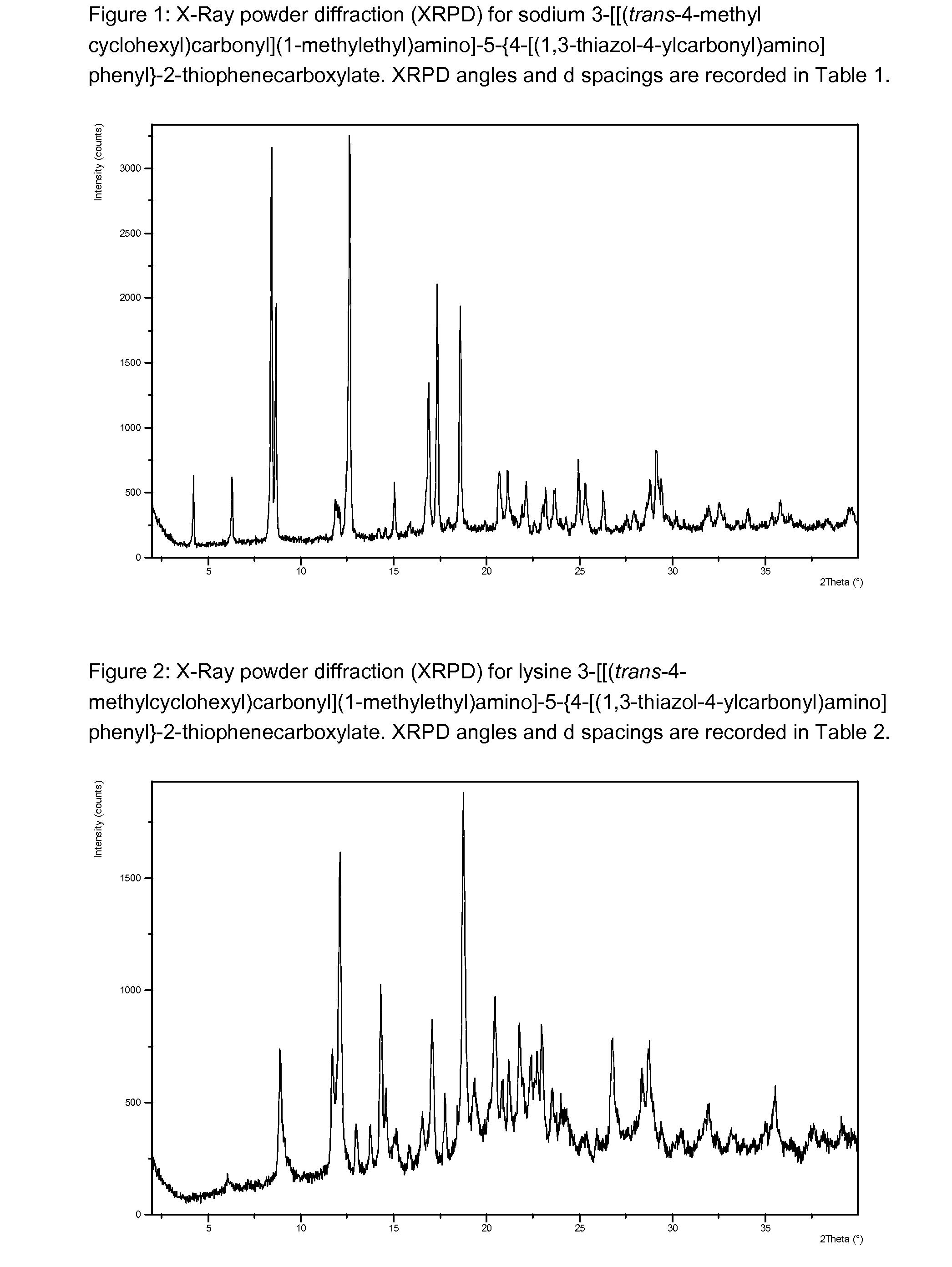
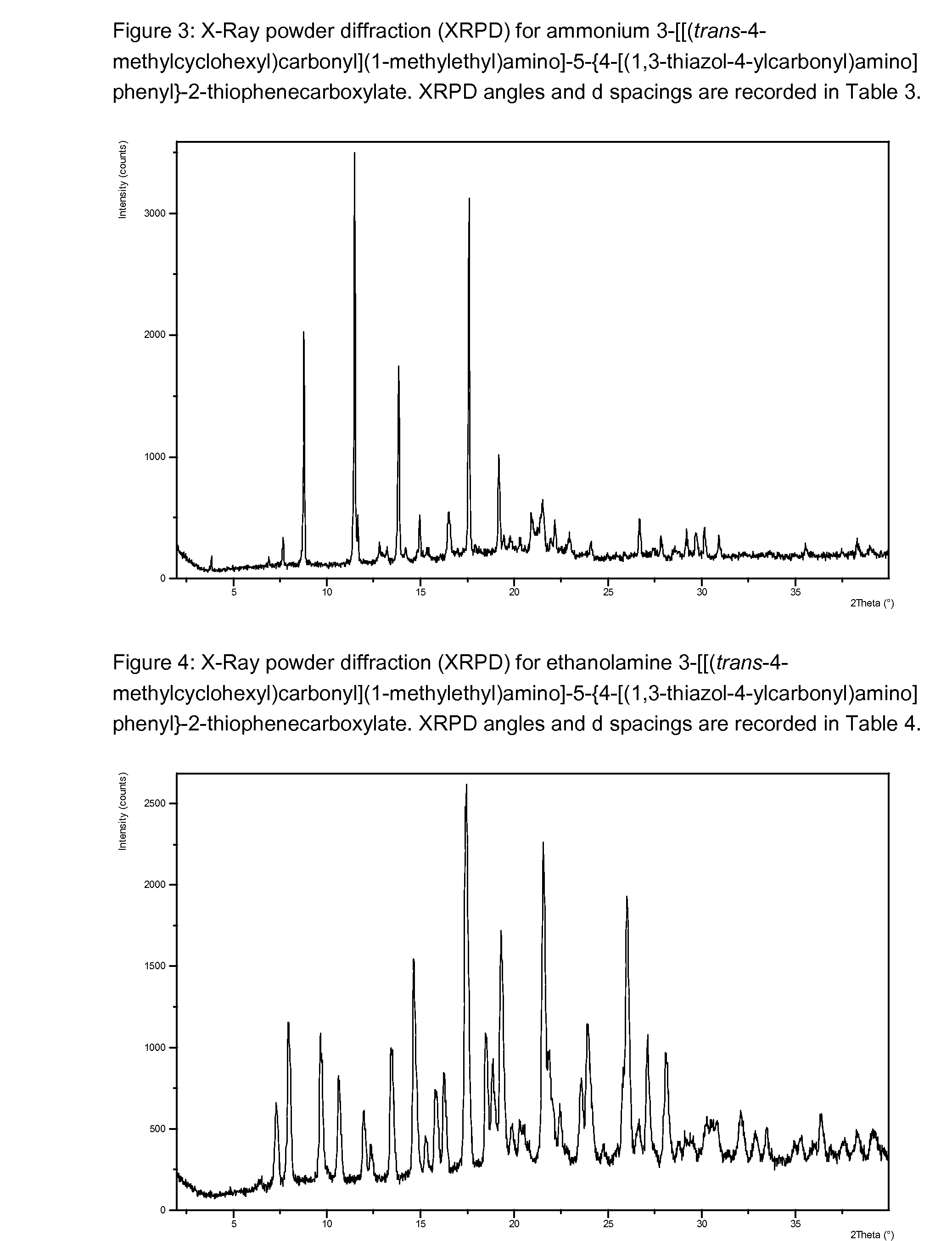
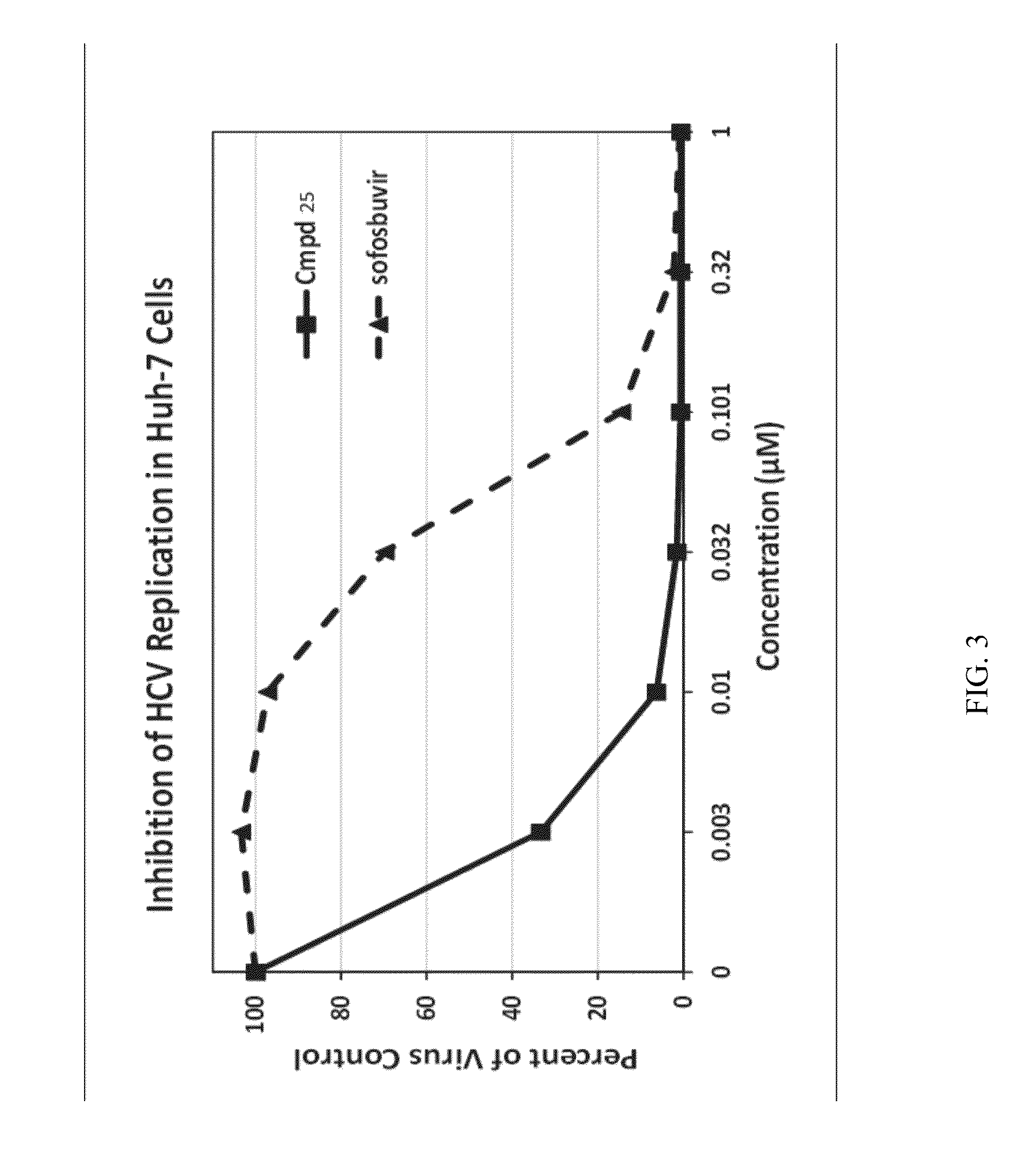
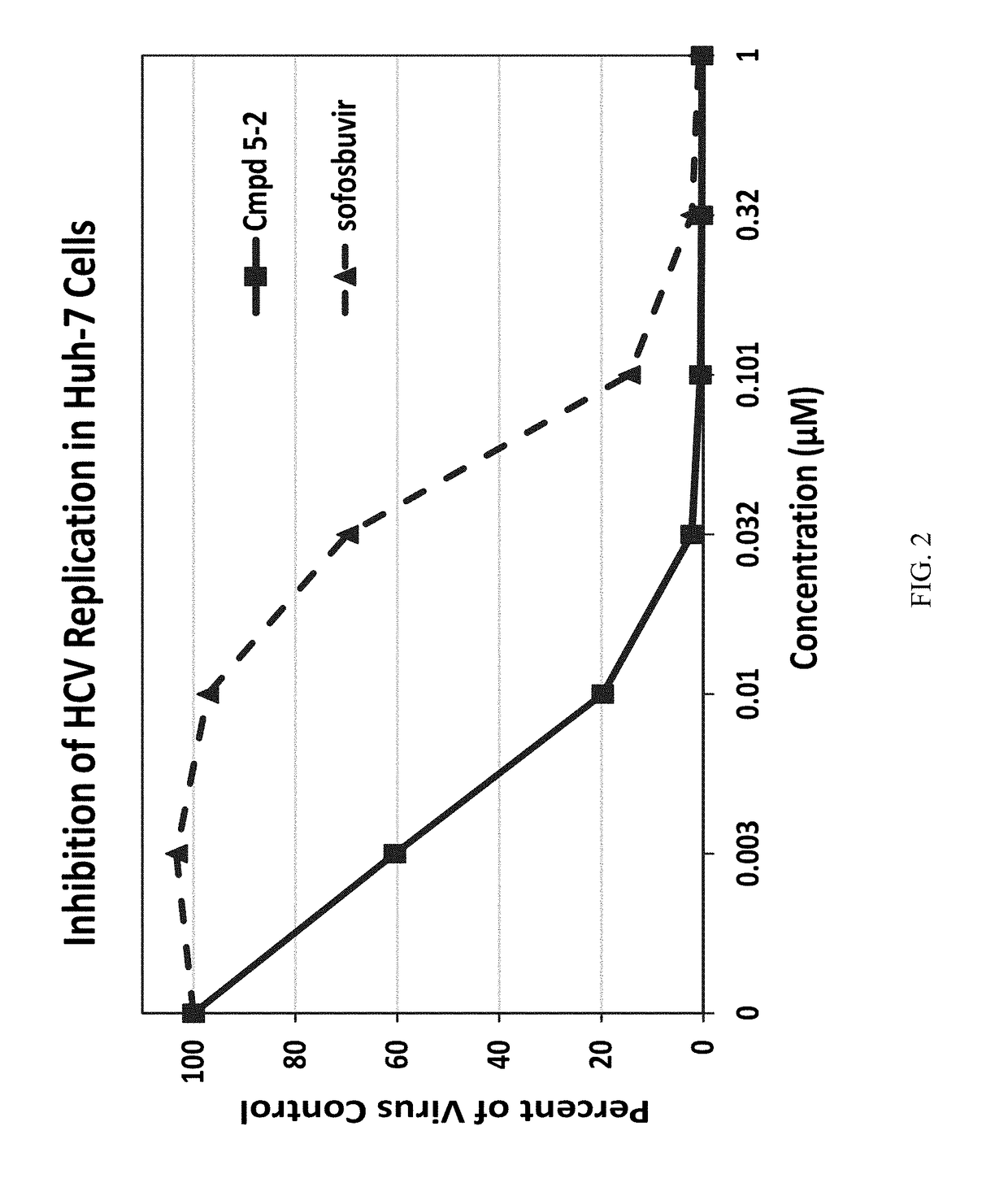
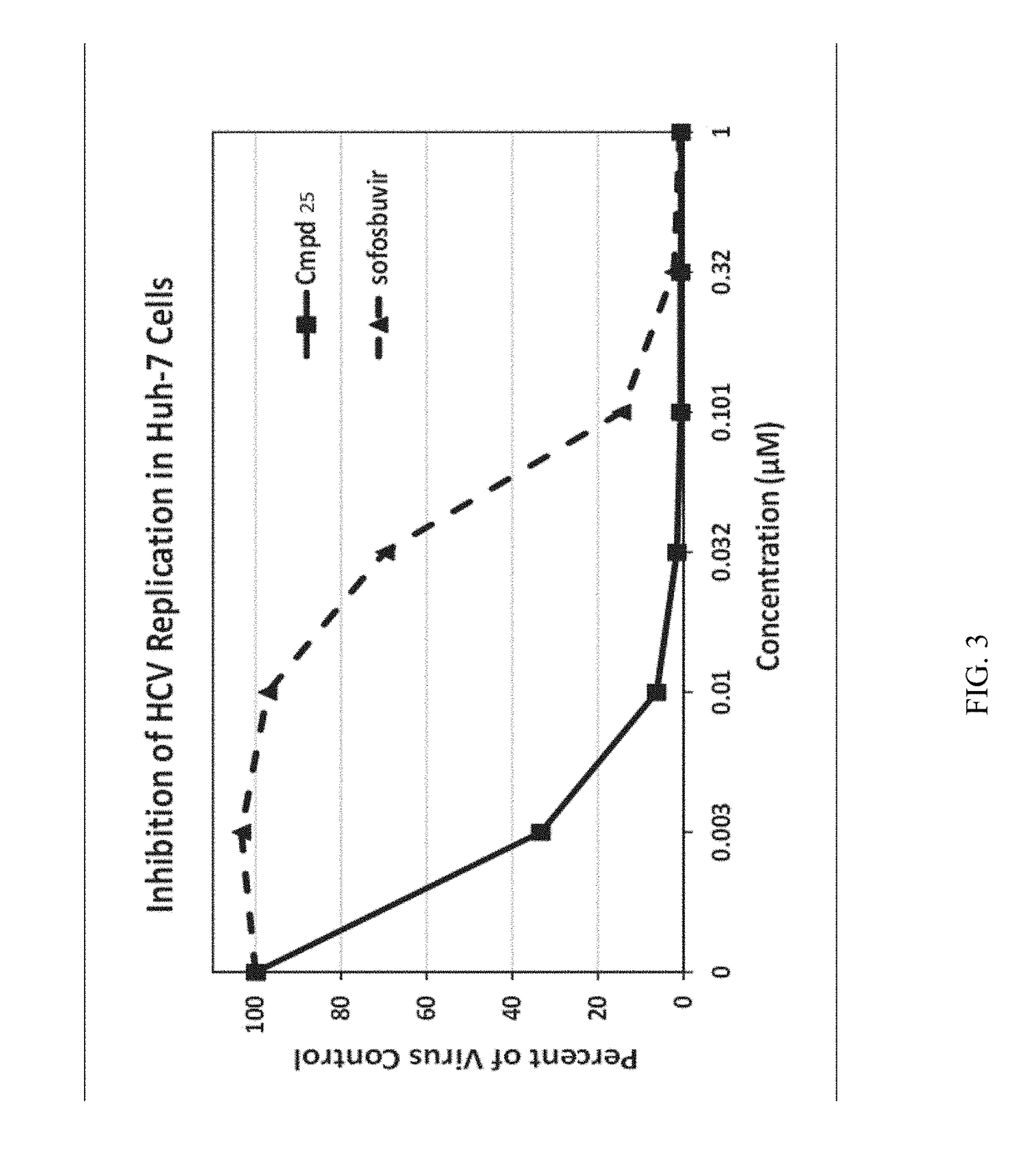
Beta-D-2'-DEOXY-2'-alpha-FLUORO-2'-beta-C-SUBSTITUTED-2-MODIFIED-N6-SUBSTITUTED PURINE NUCLEOTIDES FOR HCV TREATMENT
ActiveUS20160257706A1Strong specificityGood cell permeabilityOrganic active ingredientsSugar derivativesHcv treatmentMedicine
A compound of the structure:or a pharmaceutically acceptable salt or composition thereof for the treatment of a host infected with or exposed to an HCV virus or other disorders more fully described herein.
Owner:ATEA PHARMA INC
β-D-2′-deoxy-2′-α-fluoro-2′-β-C-substituted-2-modified-N6-substituted purine nucleotides for HCV treatment
ActiveUS9828410B2Strong specificityGood cell permeabilityOrganic active ingredientsSugar derivativesHcv treatmentDisease
A compound of the structure:or a pharmaceutically acceptable salt or composition thereof for the treatment of a host infected with or exposed to an HCV virus or other disorders more fully described herein.
Owner:ATEA PHARMA INC
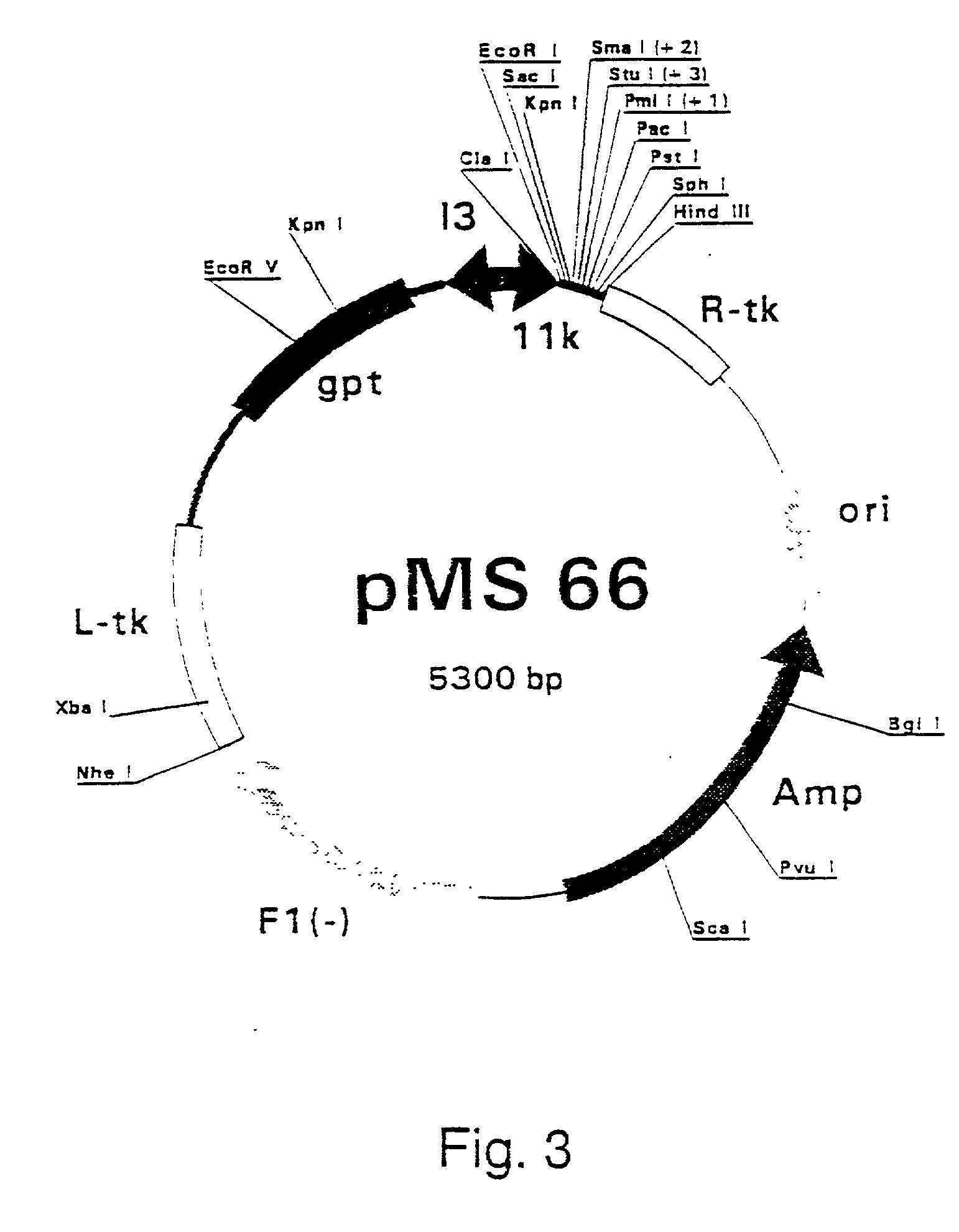
Combinations for HCV Treatment
InactiveUS20100015090A1Improve pharmacokineticsImproving the pharmacokinetics of Hepatitis C NS3/4AOrganic active ingredientsBiocideHcv treatmentMonooxygenase
Owner:VERTEX PHARMA INC
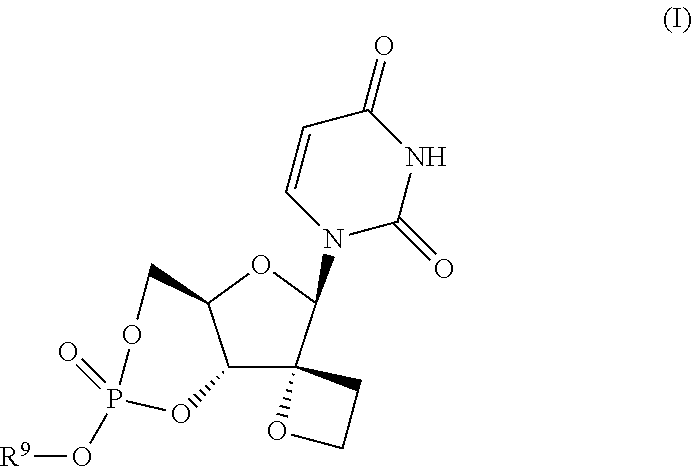
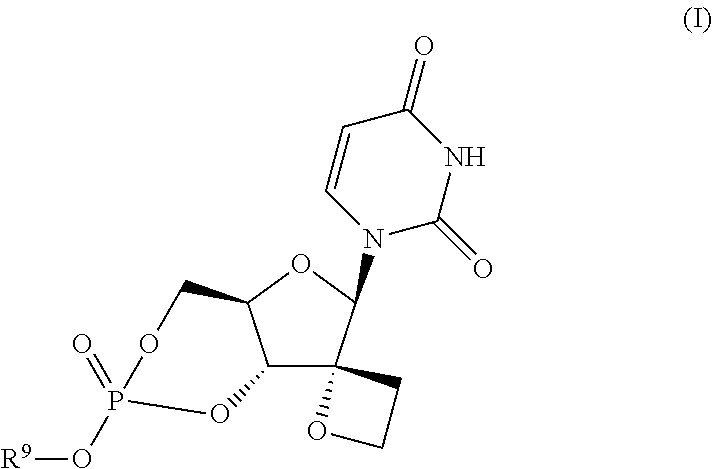
Uracyl spirooxetane nucleosides
The present invention relates to compounds of the formula I: including any possible stereoisomers thereof, wherein R9 has the meaning as defined herein, or a pharmaceutically acceptable salt or solvate thereof. The present invention also relates to processes for preparing said compounds, pharmaceutical compositions containing them and their use, alone or in combination with other HCV inhibitors, in HCV therapy.
Owner:JANSSEN SCI IRELAND UC
Macrocyclic indoles as hepatitis C virus inhibitors
The present invention relates to inhibitors of HCV replication of formula (I), the N-oxide forms, the pharmaceutically acceptable addition salts, the quaternary amines and the stereochemically isomeric forms thereof,wherein R1; R3; and R4 have the meaning defined in the claims.The present invention also relates to processes for preparing said compounds, pharmaceutical compositions containing them and their use in HCV therapy.
Owner:JANSSEN SCI IRELAND UC
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS20040126395A1SsRNA viruses positive-senseViral antigen ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:MAERTENS GEERT +2
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS7101561B2Different reactivityEasy to prepareSsRNA viruses positive-sensePeptide/protein ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:GENIMMUNE NV
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
InactiveUS20030147918A1Reduce the possibilityHigh sensitivitySsRNA viruses positive-sensePeptide/protein ingredientsHcv treatmentDisulphide bonds
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment
Owner:GENIMMUNE NV
Kit for detecting genotyping and IL28-site polymorphism of hepatitis C virus (HCV)
The invention relates to a kit for detecting the genotype and IL28-site polymorphism of a hepatitis C virus (HCV), in particular to a kit for detecting the genotype and IL28-site polymorphism of an HCV, which is prepared by using a nucleic acid reverse dot hybridization technology. The kit comprises an amplification reagent, a low-density chip using a nylon membrane as a carrier and a hybridization reagent, and can simultaneously detect the genotype of the HCV and the IL28B gene polymorphism closely related to the HCV treatment, thereby providing a more reliable basis for the individual hepatitis C treatment.
Owner:DAAN GENE CO LTD
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV
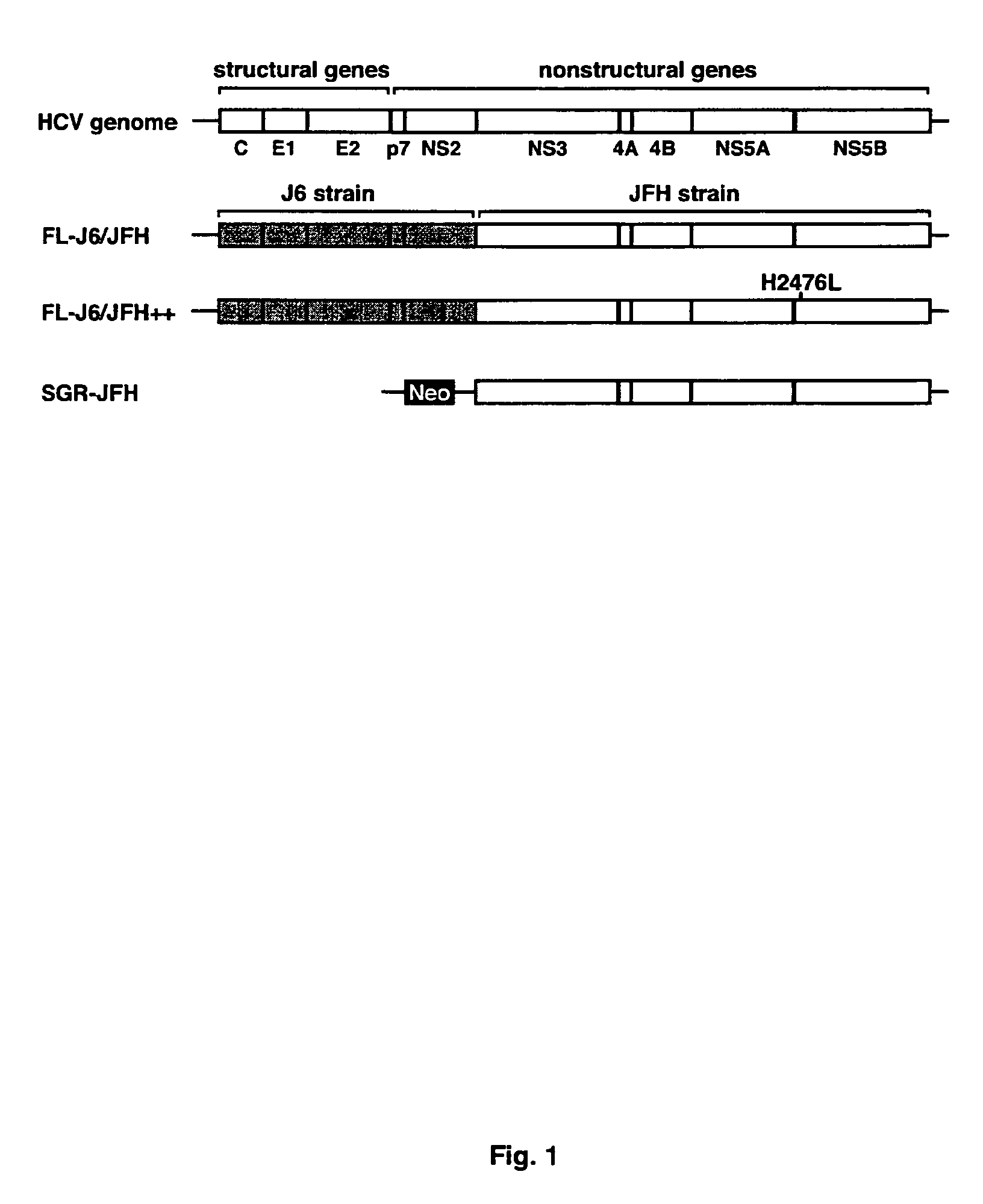
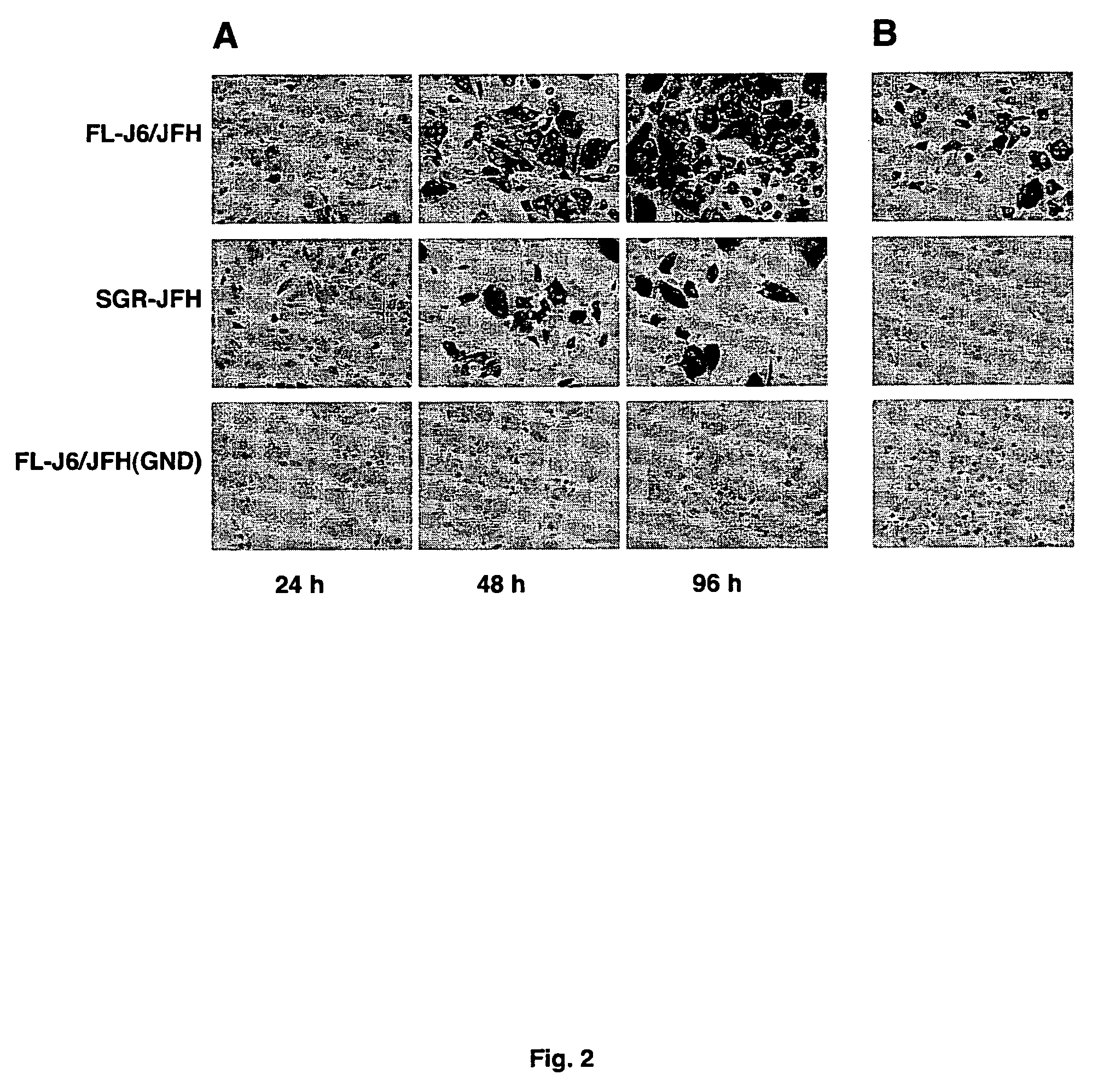
Infectious, chimeric hepatitis C virus, methods of producing the same and methods of use thereof
InactiveUS7674612B2Improve scalabilityCompound screeningSsRNA viruses positive-senseHcv treatmentBiology
The present invention provides infectious recombinant Hepatitis C Viruses (HCV), and vectors, cells and animals comprising the same. The present invention provides methods of producing infectious recombinant HCV, and their use in identifying anti-HCV therapeutic agents, as well as sequences of HCV associated with HCV pathogenesis.
Owner:THE ROCKEFELLER UNIV
Single Nucleotide Polymorphism on Chromosome 15 That Predicts HCV Treatment Responses
InactiveUS20130137084A1Raise the possibilityMicrobiological testing/measurementAntiviralsHcv treatmentDiverse population
The present invention is based on the discovery of associations that exist between single nucleotide polymorphisms (SNPs) on chromosome 15 and virological outcomes in a diverse population of patients with hepatitis C virus (HCV) who received interferon-based treatment.
Owner:ROCHE MOLECULAR SYST INC
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E1 / E2 characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV
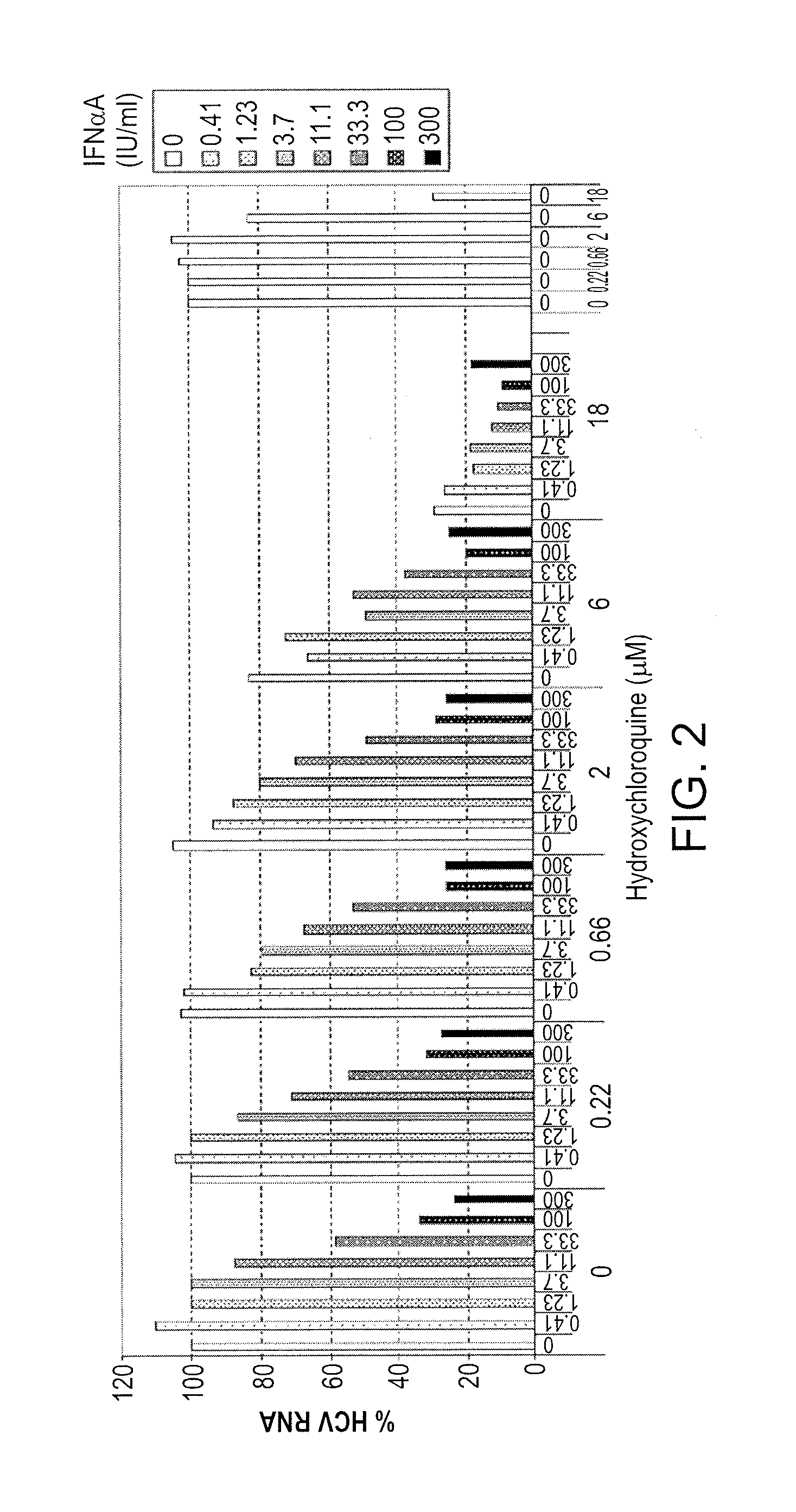
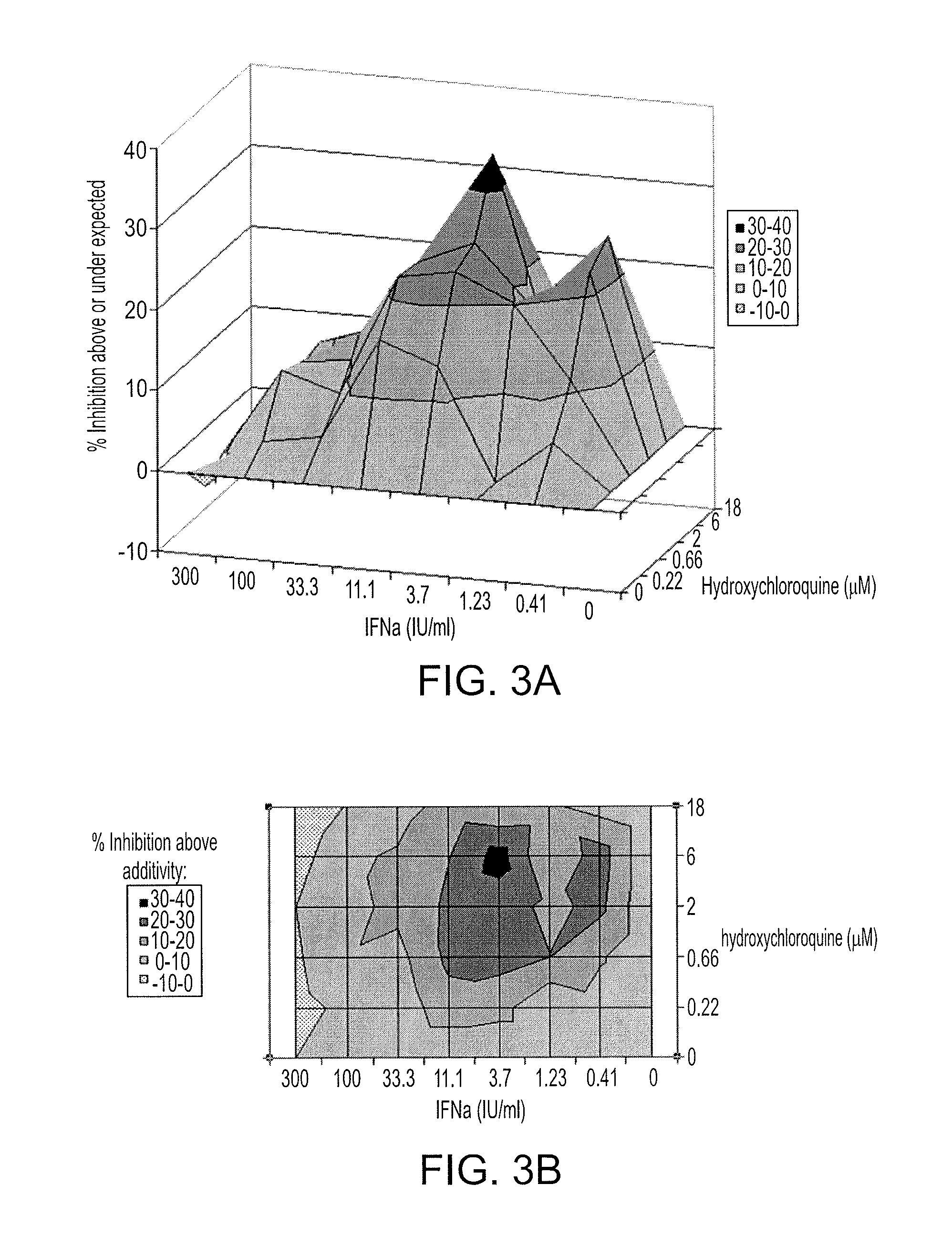
Treatment of hepatitis C virus related diseases using hydroxychloroquine or a combination of hydroxychloroquine and an anti-viral agent
Methods of treating a hepatitis C virus (HCV) related disease, such as HCV infections in subjects non-responsive to anti-HCV therapy, are described herein, comprising administering to the subject a therapeutically effective amount of hydroxychloroquine. An antiviral agent may be co-administered with the hydroxychloroquine. Methods utilizing synergistic combinations of hydroxychloroquine and an antiviral agent are disclosed. Further disclosed are compositions comprising hydroxychloroquine and an antiviral agent, as well as hydroxychloroquine and uses thereof for the treatment of a hepatitis C virus (HCV) related disease.
Owner:PANMED +1
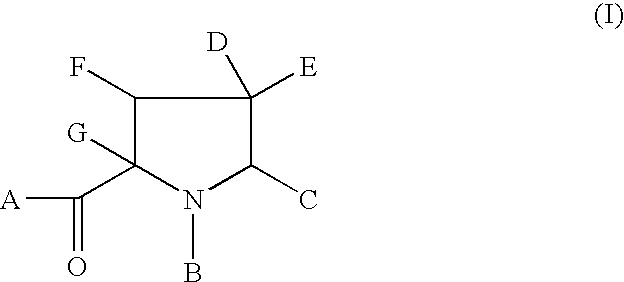
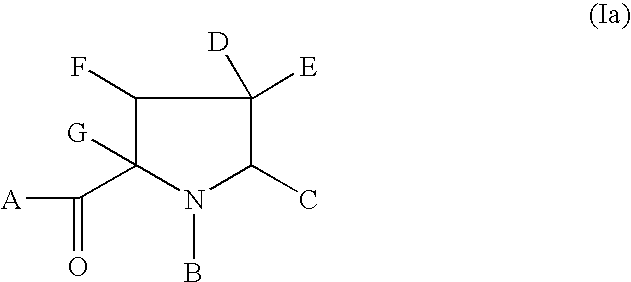
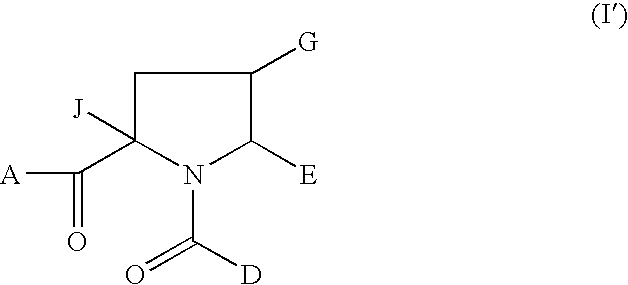
4-(6-membered)-heteroaryl acyl pyrrolidine derivatives as hcv inhibitors
Novel anti-viral agents of Formula wherein: A represents OR1, NR1R2, or R1 wherein R1 and R2 are hydrogen, C1-6alkyl, aryl, heteroaryl, arylalkyl, or heteroarylalkyl; or R1 and R2 together with the nitrogen atom to which they are attached form a 5 or 6 membered saturated cyclic group; B represents C(O)R3 wherein R3 is C1-6alkyl, aryl, heteroaryl, arylalkyl, or heteroarylalkyl; C represents C1-6alkyl, aryl, heteroaryl or heterocyclyl; D represents a saturated or unsaturated optionally substituted 6-membered heterocyclic ring; E represents hydrogen or C1-6-alkyl; F represents hydrogen, C1-6alkyl, aryl or heteroaryl; and G represents hydrogen, C1-6alkyl, heterocyclylalkyl, arylalkyl or heteroarylalkyl; and salts, solvates and esters thereof, processes for their preparation and methods of using them in HCV treatment are provided.
Owner:GLAXO GROUP LTD
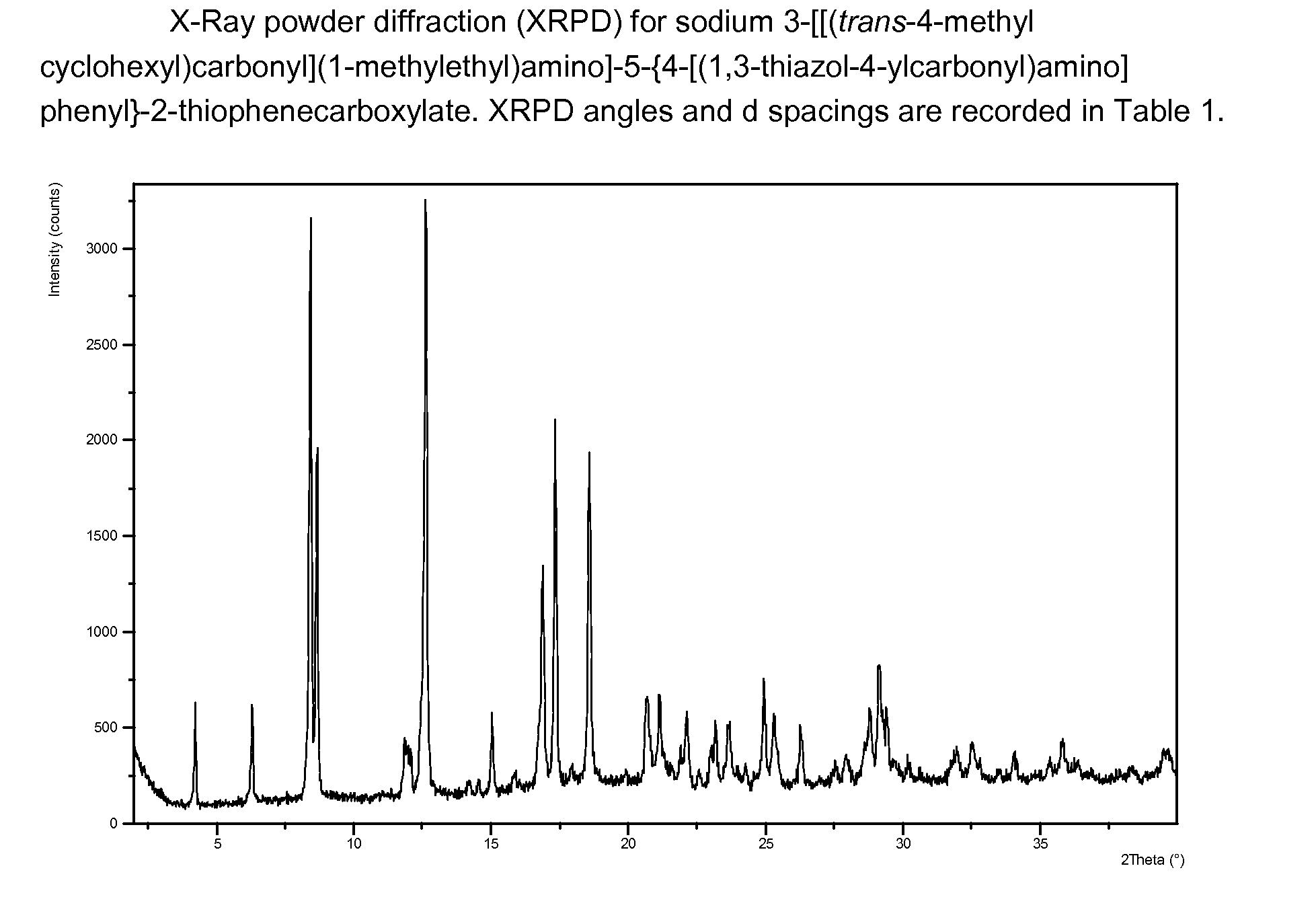
Antiviral 2-Carboxy-Thiophene Compounds
Anti-viral agents of compounds of Formula (I):wherein A, R1, R2 and R3 are as defined in the specification, processes for their preparation and their use in HCV treatment are provided.
Owner:CORFIELD JOHN ANDREW +10
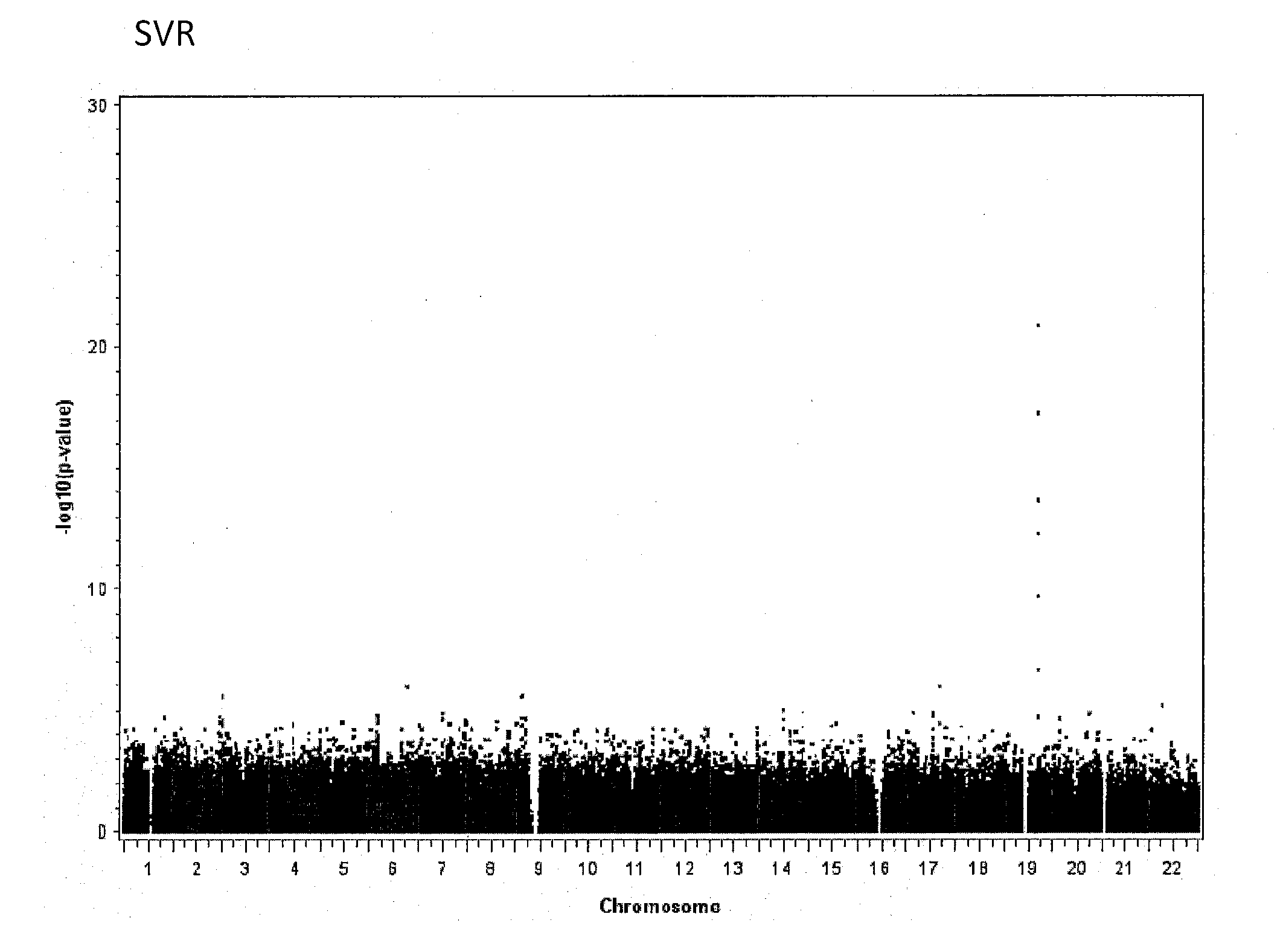
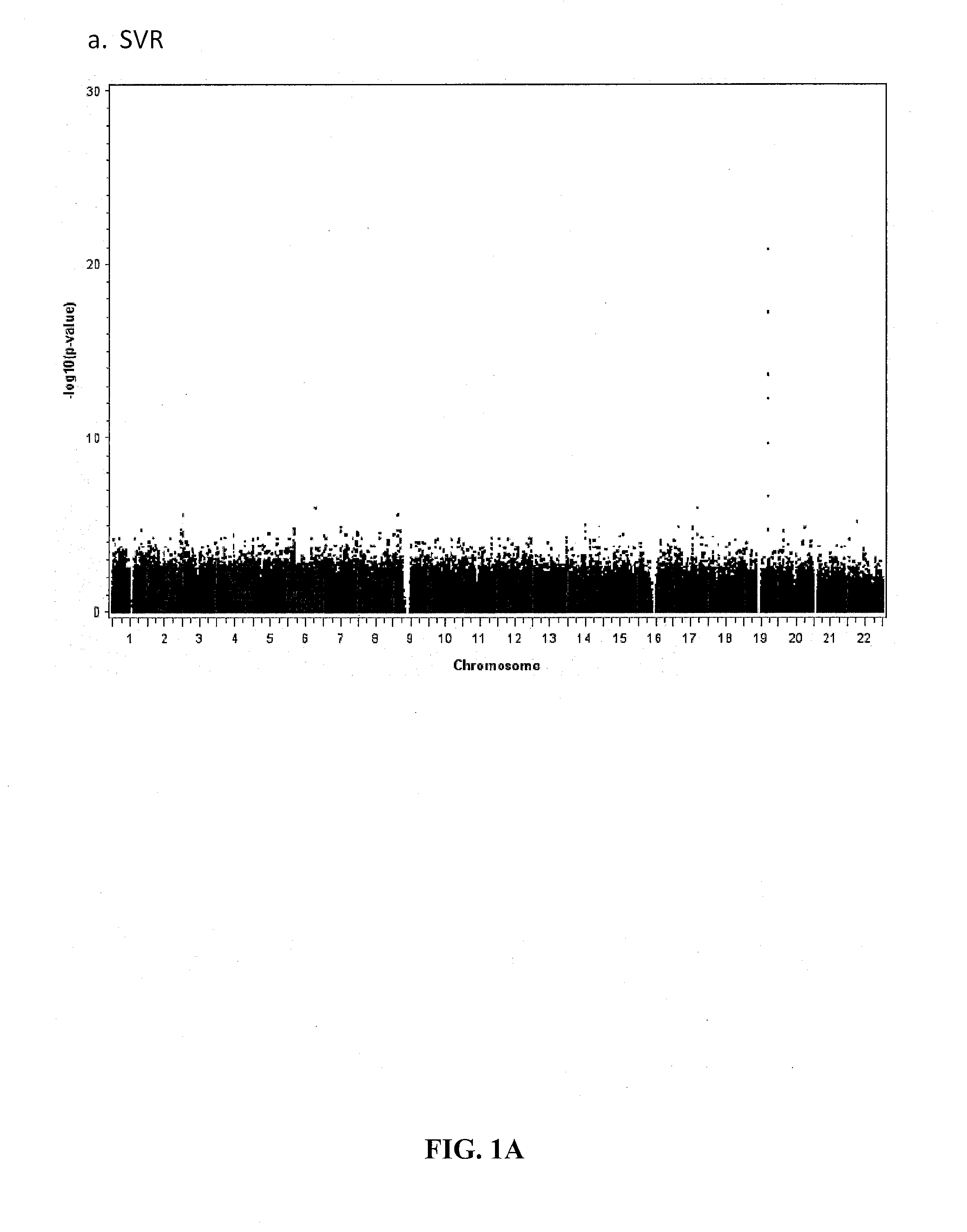
Prediction of Early Virological Response in HCV Treatment
InactiveUS20120094284A1Raise the possibilityShort durationMicrobiological testing/measurementBiological testingHcv treatmentMedicine
The present invention is based on the discovery of associations that exist between single nucleotide polymorphisms (SNPs) on chromosome 19 and virological outcomes in a diverse population of patients with hepatitis C virus (HCV) who received interferon-based treatment.
Owner:ROCHE MOLECULAR SYST INC
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E2 and / or E1 / E2, characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV (NL)
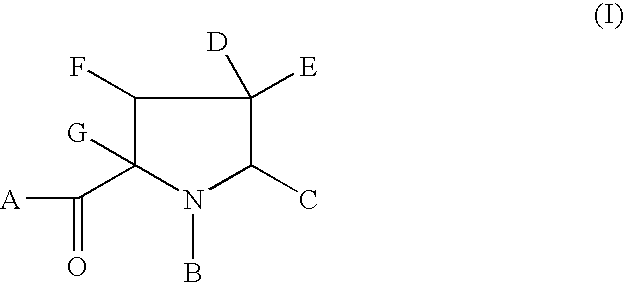
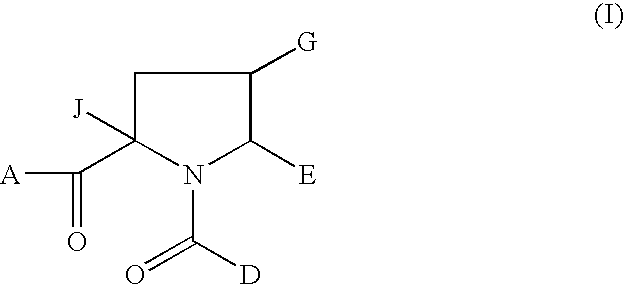
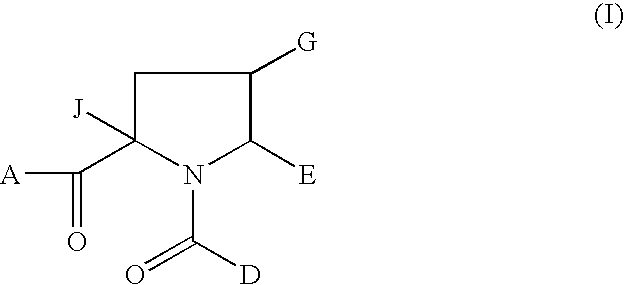
1-Acyl-pyrrolidine derivatives for the treatment of viral infections
Anti-viral agents of Formula (I) wherein: A represents hydroxy; D represents aryl or heteroaryl; E represents hydrogen, C1-6alkyl, aryl, heteroaryl or heterocyclyl; G represents hydrogen or optionally substituted C1-6alkyl; J represents C1-6alkyl, heterocyclylalkyl, arylalkyl or heteroarylalkyl; and salts, solvates and esters thereof; provided that when A is esterified to form —OR where R is selected from straight or branched chain alkyl, aralkyl, aryloxyalkyl, or aryl, then R is other than tert-butyl; processes for their preparation, pharmaceutical compositions comprising them, and methods of using them in HCV treatment are provided.
Owner:GLAXO GROUP LTD
Transgenic mouse models of hepatitis C virus (HCV) and identification of HCV therapeutics
InactiveUS7566812B2Reduce in quantityReduce frequencySsRNA viruses positive-senseVirus peptidesHcv treatmentProteinase activity
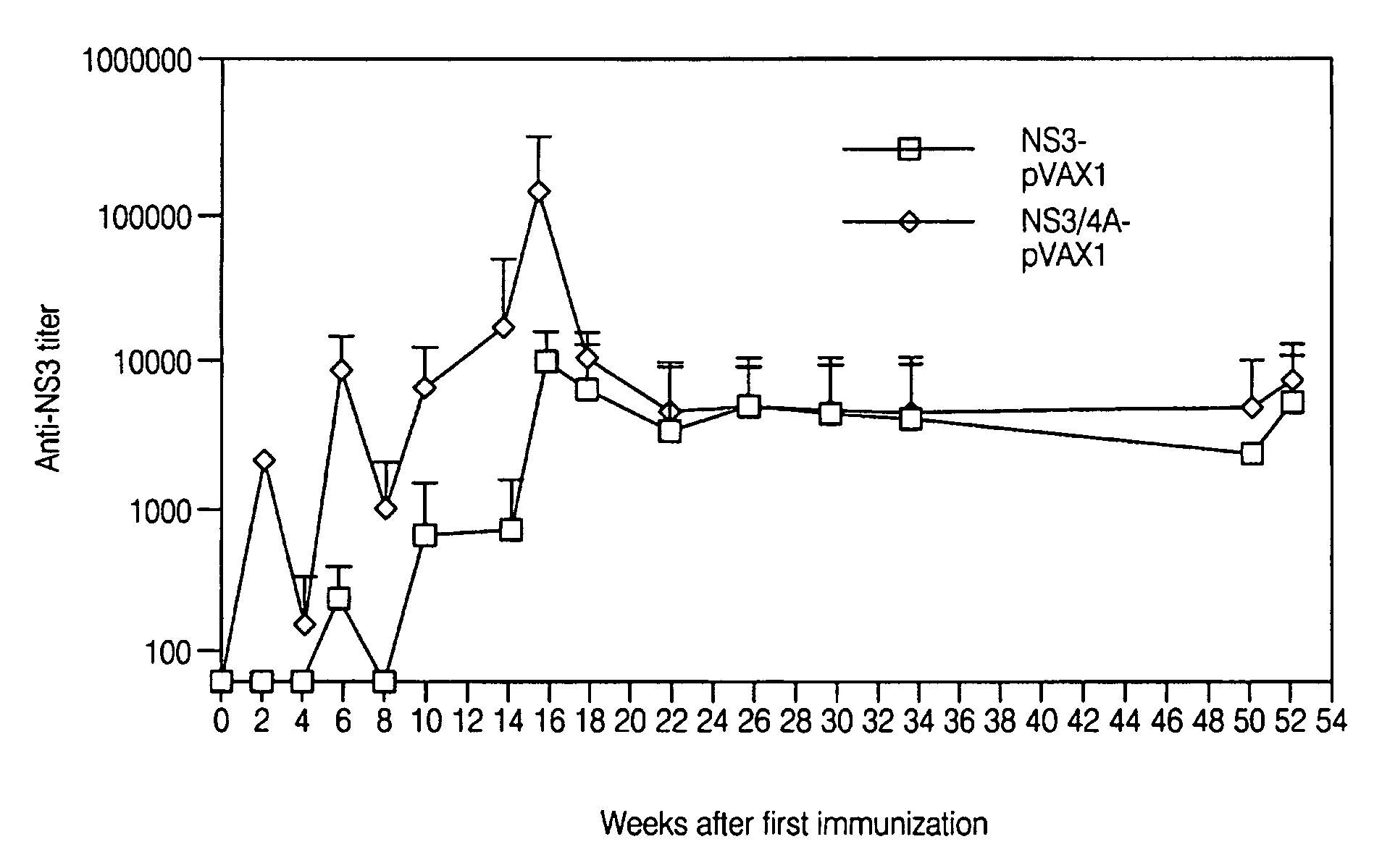
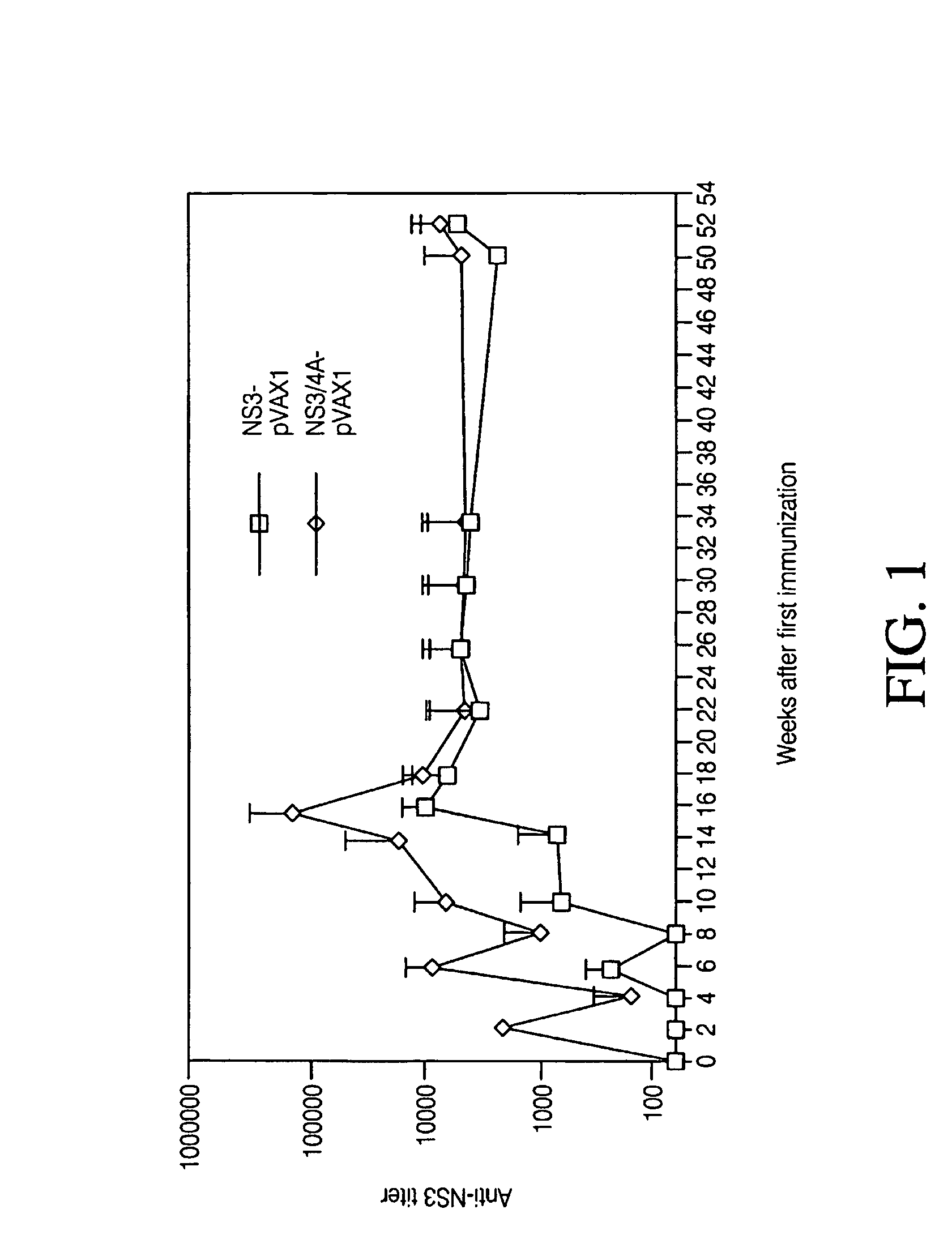
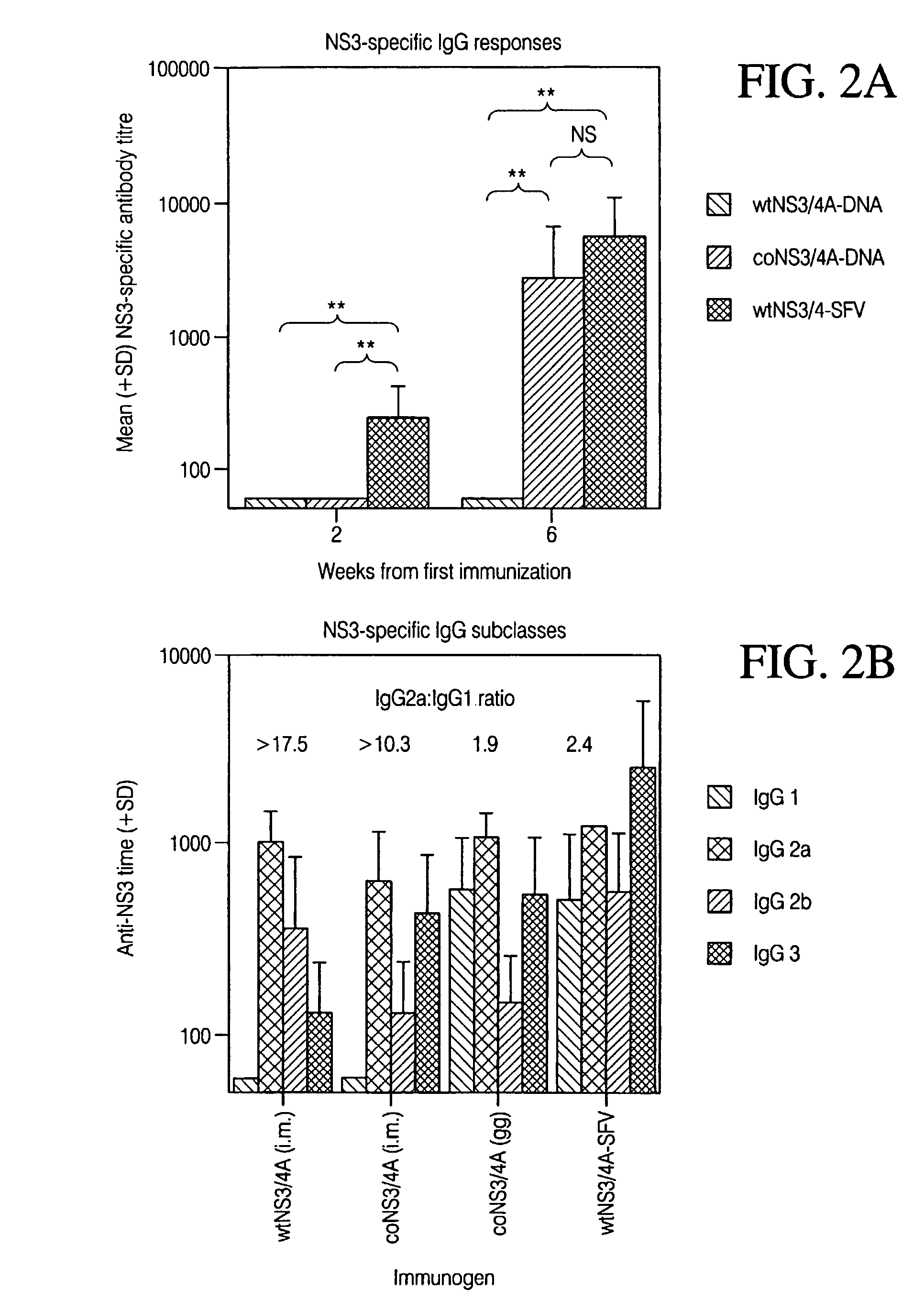
Disclosed herein is the discovery of novel NS3 / 4A compositions with enhanced expression abilities. Embodiments of the invention include codon optimized NS3 / 4A compositions and compositions with the Semliki forest virus replicon. Additional embodiments include transgenic organisms containing these NS3 / 4A compositions, methods or using these transgenic mice to screen and refine drugs, and the drugs refined by these methods. Additional embodiments include protease activity dependent molecules that can indicate the presence or absence of a protease inhibitor.
Owner:TRIPEP
Purified hepatitis C virus envelope proteins for diagnostic and therapeutic use
The present invention relates to a method for purifying recombinant HCV single or specific oligomeric envelope proteins selected from the group consisting of E1 and / or E1 / E2 characterized in that upon lysing the transformed host cells to isolate the recombinantly expressed protein a disulphide bond cleavage or reduction step is carried out with a disulphide bond cleavage agent. The present invention also relates to a composition isolated by such a method. The present invention also relates to the diagnostic and therapeutic application of these compositions. Furthermore, the invention relates to the use of HCV E1 protein and peptides for prognosing and monitoring the clinical effectiveness and / or clinical outcome of HCV treatment.
Owner:INNOGENETICS NV
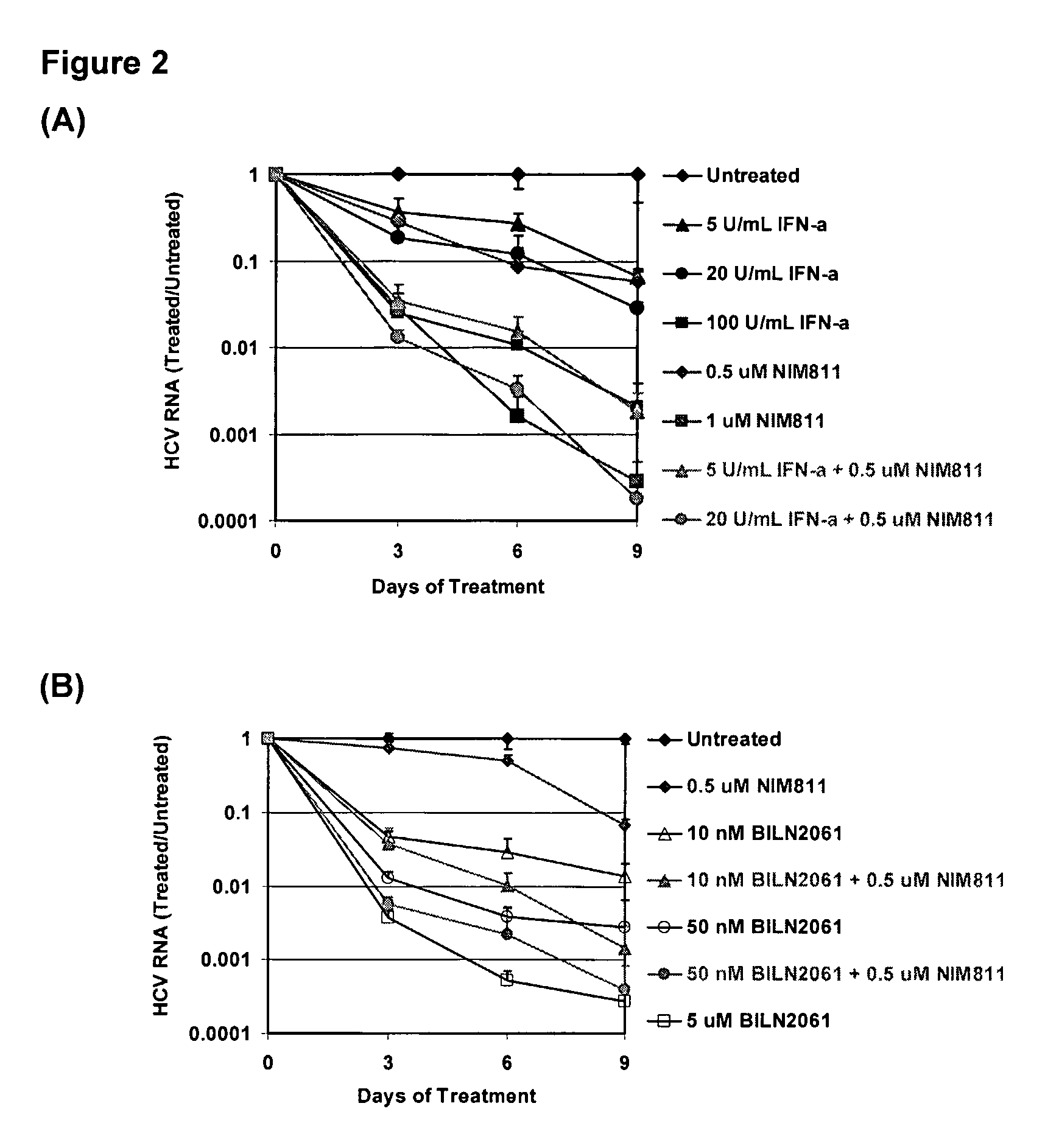
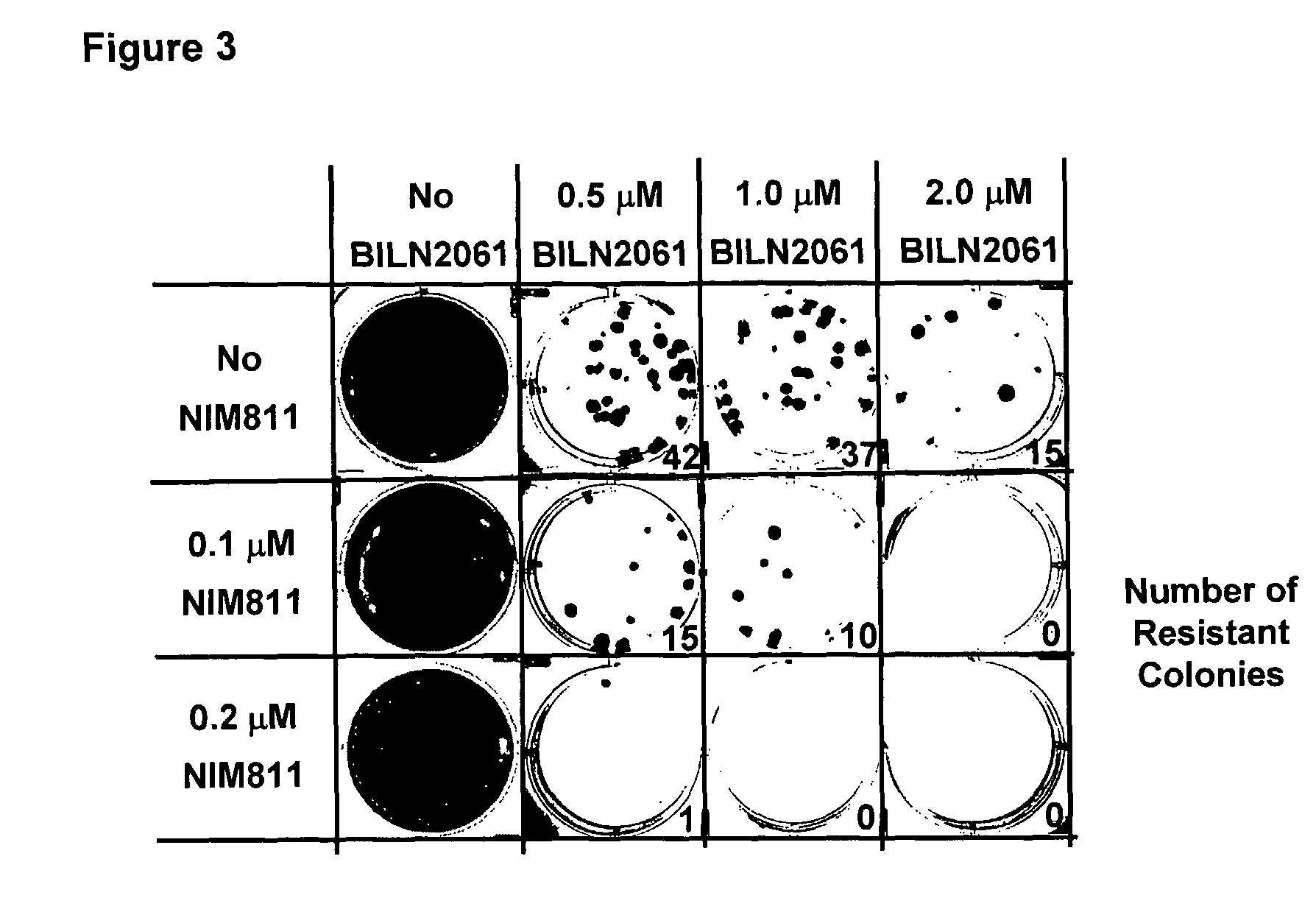
Compositions for HCV treatment
The present invention concerns a pharmaceutical combination comprising a) a first agent which is a non-immunosuppressive cyclophilin-binding cyclosporine, e.g., a compound of formula I and b) a co-agent. Co-agents include, but are not limited to, interferons, a conjugate of interferon, antiviral agents, helicase inhibitors, protease inhibitors, polymerase inhibitors and nucleoside analogs. The instant pharmaceutical combination may be used, e.g., in treating subjects having a flaviviridae infection, e.g., a Hepatitis C infection.
Owner:NOVARTIS AG
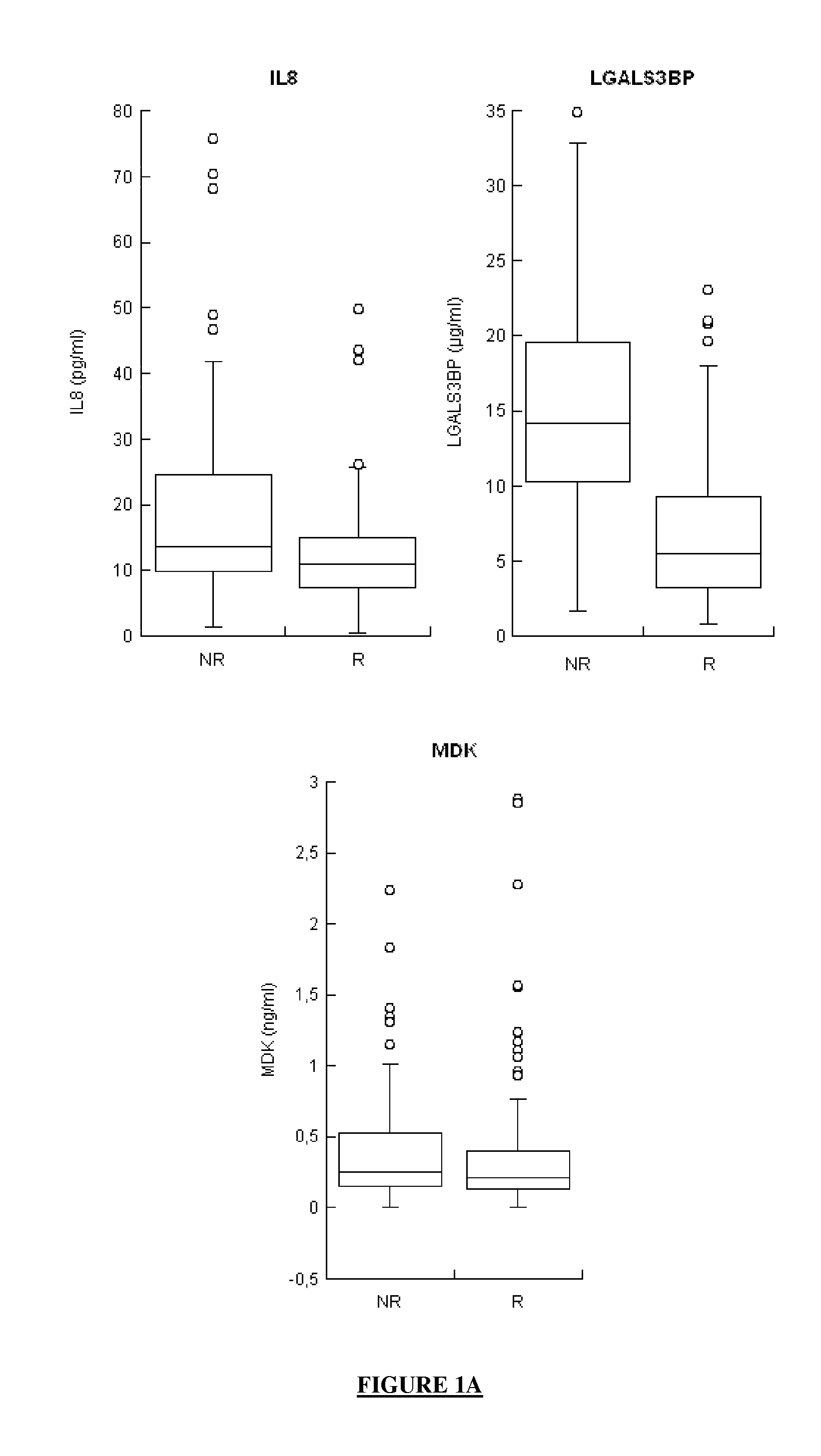
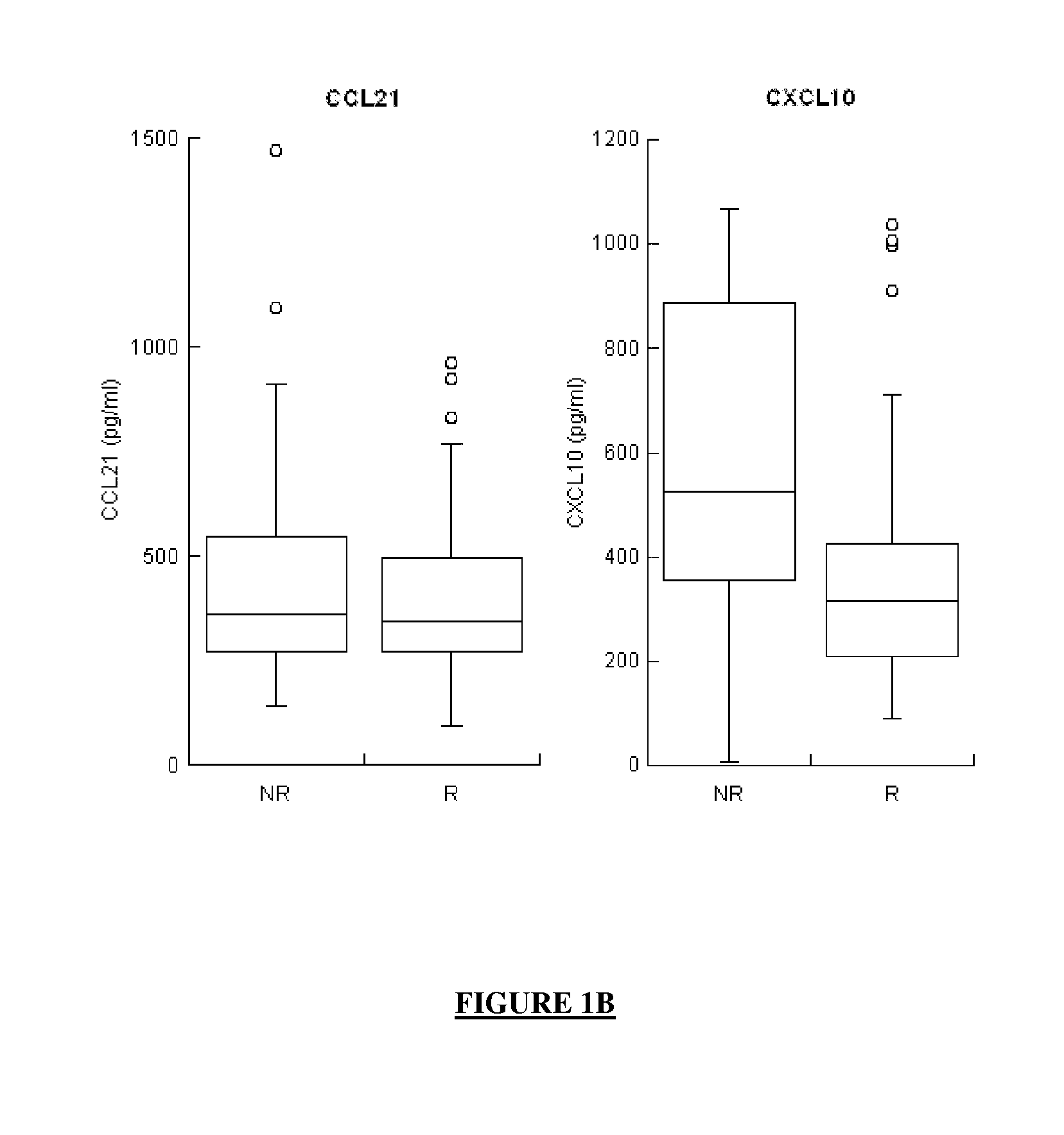
Combination of biomarkers for the prognosis of response or non-response to an Anti-hcv treatment
ActiveUS20130316332A1Improve forecastMicrobiological testing/measurementDisease diagnosisHcv treatmentHigh probability
The application concerns means for predicting whether a subject infected with one or more HCVs has a high probability of responding to an anti-HCV treatment which will comprise the administration of interferon and of ribavirin or whether, in contrast, that subject has a high probability of not responding to that anti-HCV treatment. The means of the invention in particular involve assaying the levels of expression of selected genes, said selected genes being:at least one gene from among MBL2, LGALS3BP and IL8, andat least one gene from among G1P2, CCL21 and CXCL10, andoptionally, at least one gene from among AFP, CRP, CXCL11, CXCL6, CXCL9, FGF7, MDK, MMP2, SFN, TGFB2 and VEGFD.
Owner:BIO RAD EURO GMBH +4
Benzimidazole-imidazole derivatives
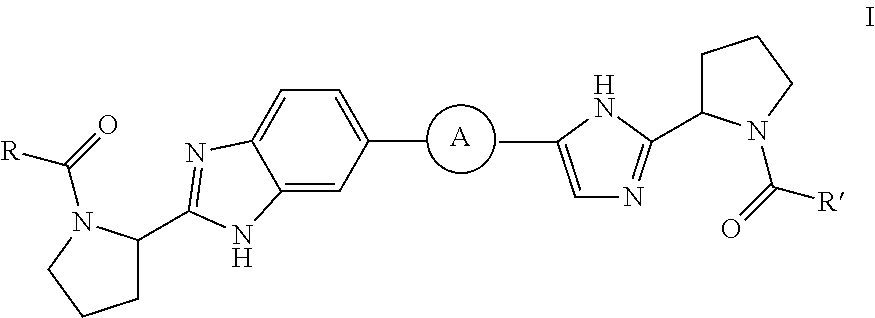
Inhibitors of HCV replication of formula Iincluding stereochemically isomeric forms, and salts, solvates thereof, wherein R and R′ are, each independently, —CR1R2R3, aryl, heteroaryl or heteroC4-6cycloalkyl, whereby aryl and heteroaryl may optionally be substituted with 1 or 2 substituents selected from halo and methyl.The present invention also relates to processes for preparing said compounds, pharmaceutical compositions containing them and their use in HCV therapy.
Owner:JANSSEN SCI IRELAND UC
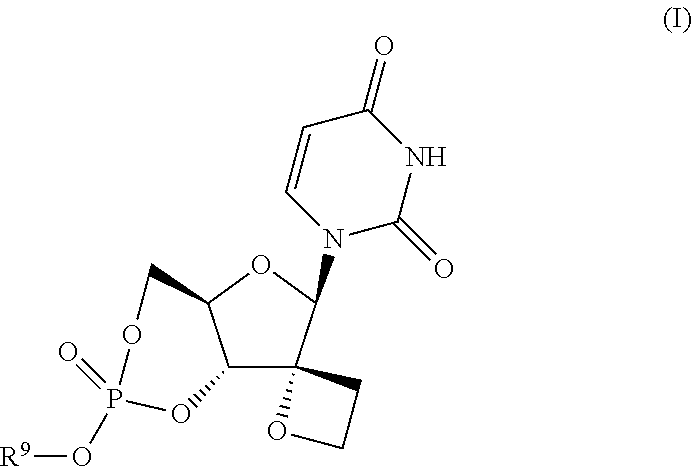
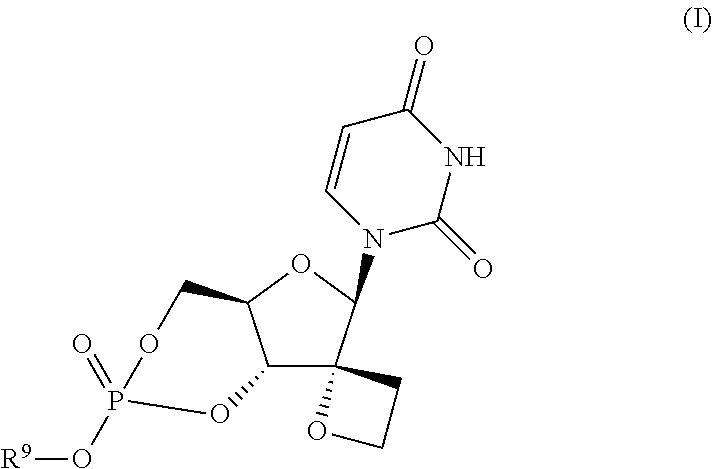
Uracyl spirooxetane nucleosides
The present invention relates to compounds of the formula I:including any possible stereoisomers thereof, wherein R9 has the meaning as defined herein,or a pharmaceutically acceptable salt or solvate thereof.The present invention also relates to processes for preparing said compounds, pharmaceutical compositions containing them and their use, alone or in combination with other HCV inhibitors, in HCV therapy.
Owner:JANSSEN SCI IRELAND UC
Beta-d-2'-deoxy-2'-alpha-fluoro-2'-beta-c-substituted-2-modified-n6-substituted purine nucleotides for hcv treatment
ActiveUS20180030081A1Strong specificityGood cell permeabilityOrganic active ingredientsSugar derivativesHcv treatmentDisease
A compound of the structure:or a pharmaceutically acceptable salt or composition thereof for the treatment of a host infected with or exposed to an HCV virus or other disorders more fully described herein.
Owner:ATEA PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com