Uracyl spirooxetane nucleosides
a technology of spirooxetane and spirooxetane, which is applied in the field of spirooxetane nucleosides and nucleotides, can solve the problems of high rate of chronic infection, and high rate of chronic infection, and achieves good clinical and economic benefits. , the effect of high side effects of combination therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
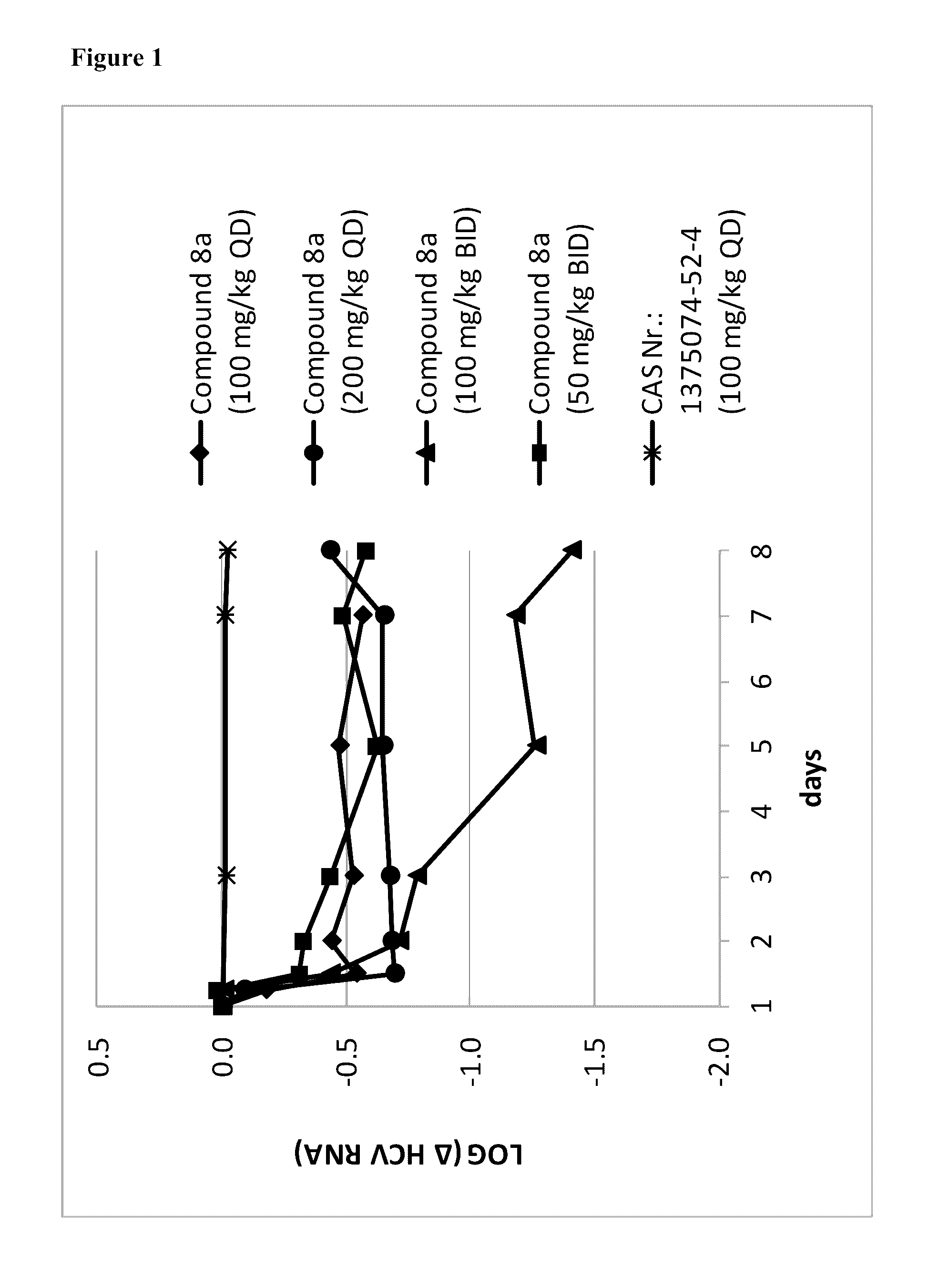
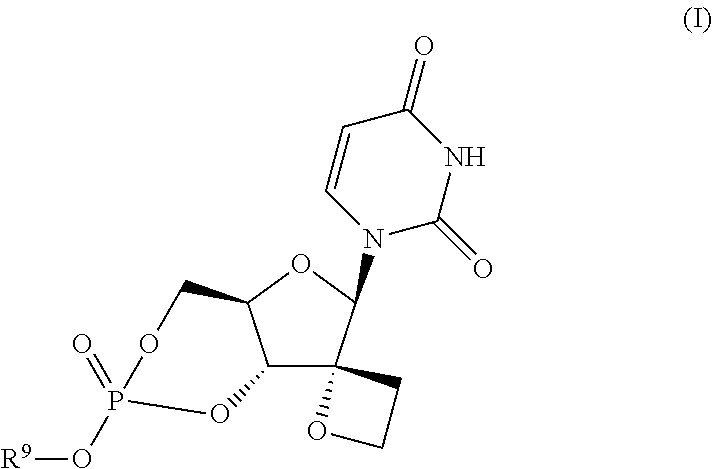
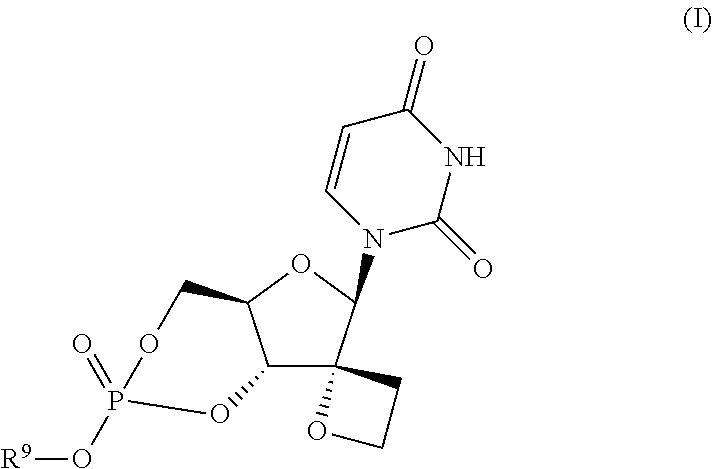
[0057]Synthesis of Compound (8a)
[0058]Synthesis of Compound (2)
[0059]Compound (2) can be prepared by dissolving compound (1) in pyridine and adding 1,3-dichloro-1,1,3,3-tetraisopropyldisiloxane. The reaction is stirred at room temperature until complete. The solvent is removed and the product redissolved in CH2Cl2 and washed with saturated NaHCO3 solution. Drying on MgSO4 and removal of the solvent gives compound (2).
[0060]Synthesis of Compound (3)
[0061]Compound (3) is prepared by reacting compound (2) with p-methoxybenzylchloride in the presence of DBU as the base in CH3CN.
[0062]Synthesis of Compound (4)
[0063]Compound (4) is prepared by cleavage of the bis-silyl protecting group in compound (3) using TBAF as the fluoride source.
[0064]Synthesis of Compound (6a)
[0065]A solution of isopropyl alcohol (3.86 mL,0.05mol) and triethylamine (6.983 mL, 0.05 mol) in dichloromethane (50 mL) was added to a stirred solution of POCl3 (5) (5.0 mL, 0.0551mol) in DCM (50 mL) dropwise over a period o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com