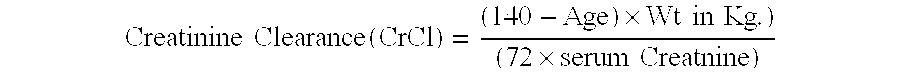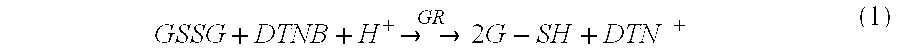Patents
Literature
250 results about "Chronic infection" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Methods and compositions for the treatment of persistent infections
ActiveUS20070122378A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsMicrobiologyPathology
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:DANA FARBER CANCER INST INC +3
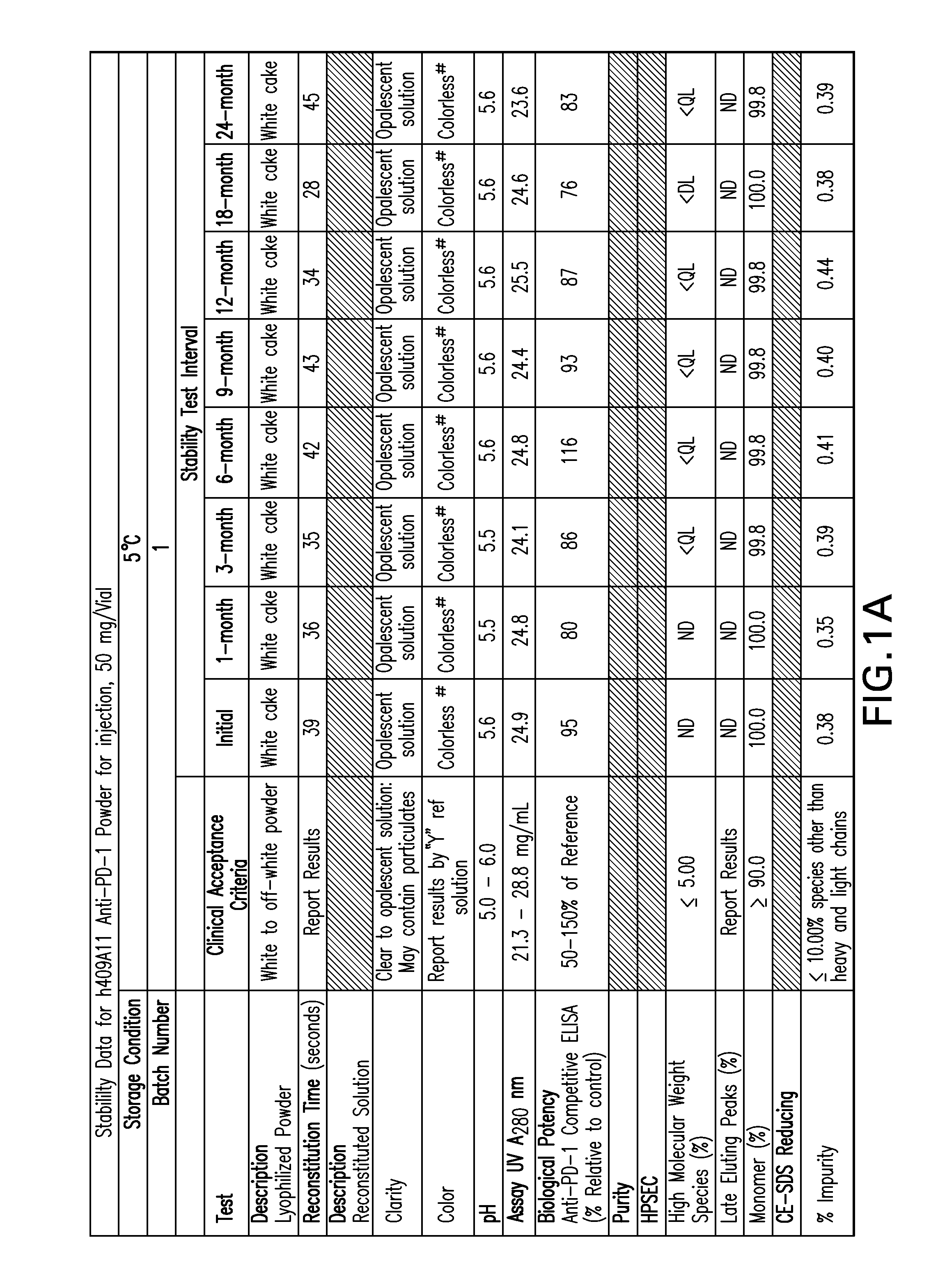
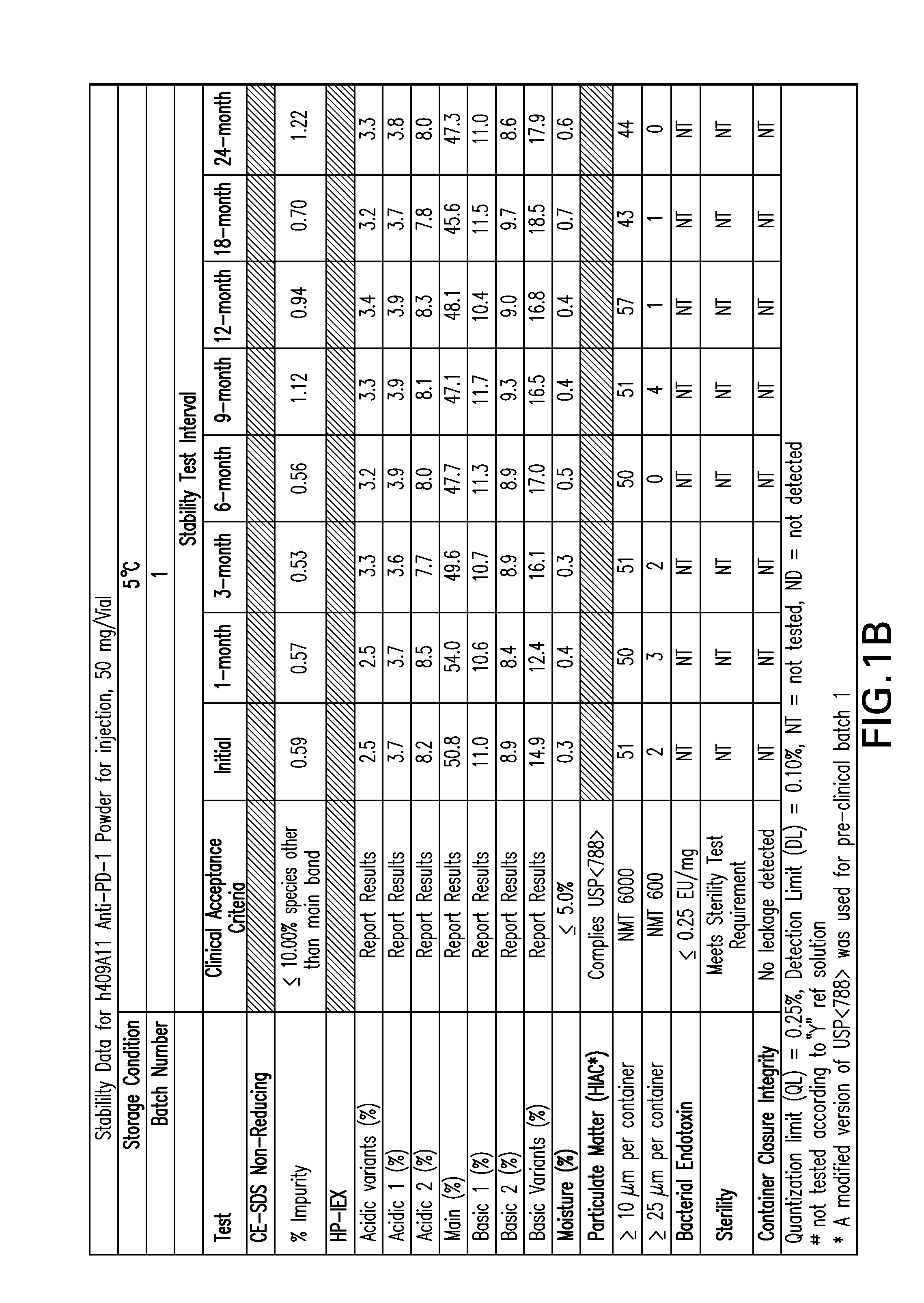
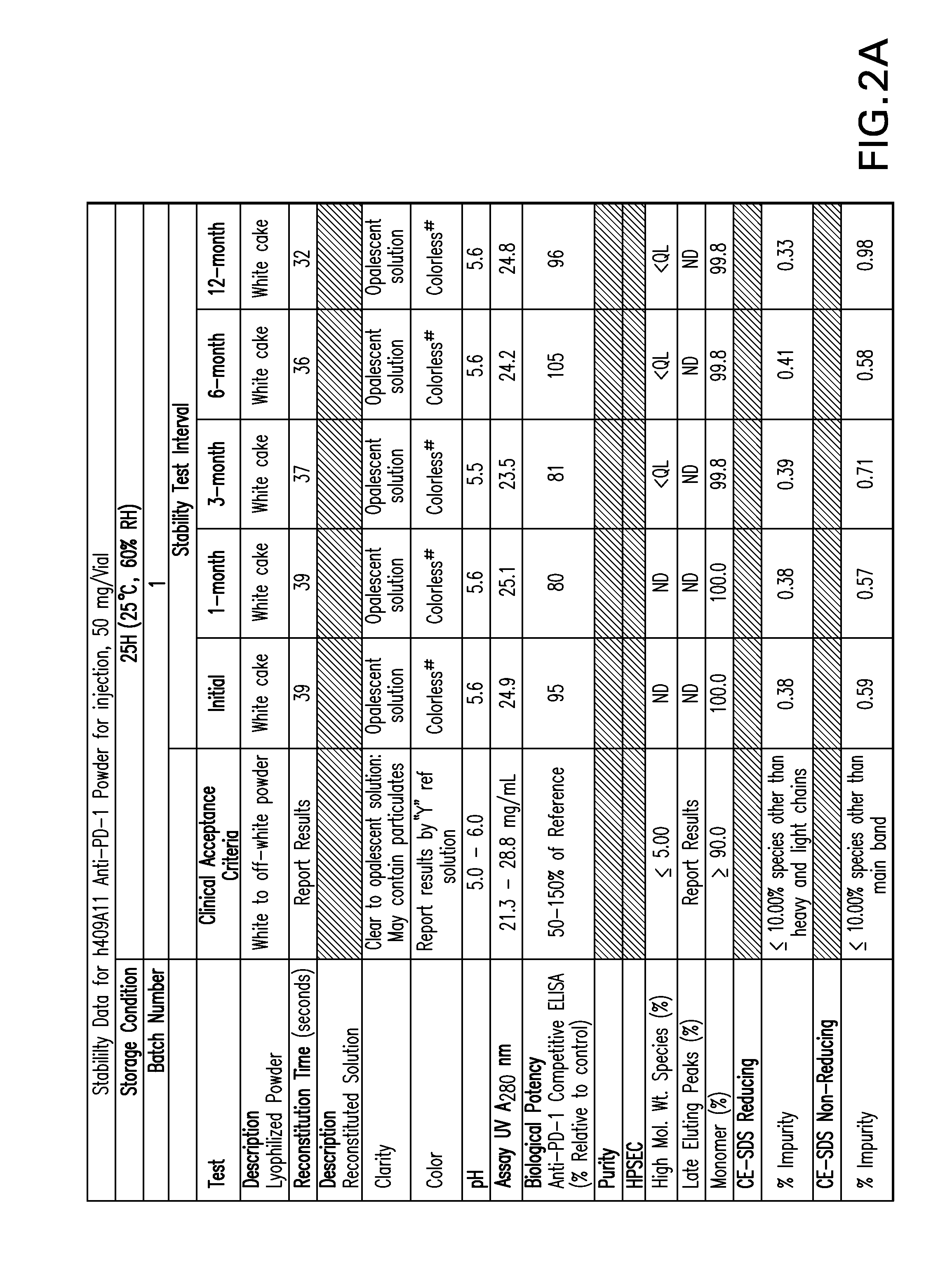
Stable formulations of antibodies to human programmed death receptor pd-1 and related treatments
ActiveUS20140234296A1Immunoglobulins against cell receptors/antigens/surface-determinantsAntiviralsAntigen Binding FragmentProgrammed death
The present invention relates to stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof. The present invention further provides methods for treating various cancers and chronic infections with stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof.
Owner:MERCK SHARP & DOHME LLC
Methods and compositions for the treatment of persistent infections
ActiveUS8652465B2Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsVirologyChronic infection
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:DANA FARBER CANCER INST INC +3
Stable formulations of antibodies to human programmed death receptor PD-1 and related treatments
The present invention relates to stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof. The present invention further provides methods for treating various cancers and chronic infections with stable formulations of antibodies against human programmed death receptor PD-1, or antigen binding fragments thereof.
Owner:MERCK SHARP & DOHME LLC
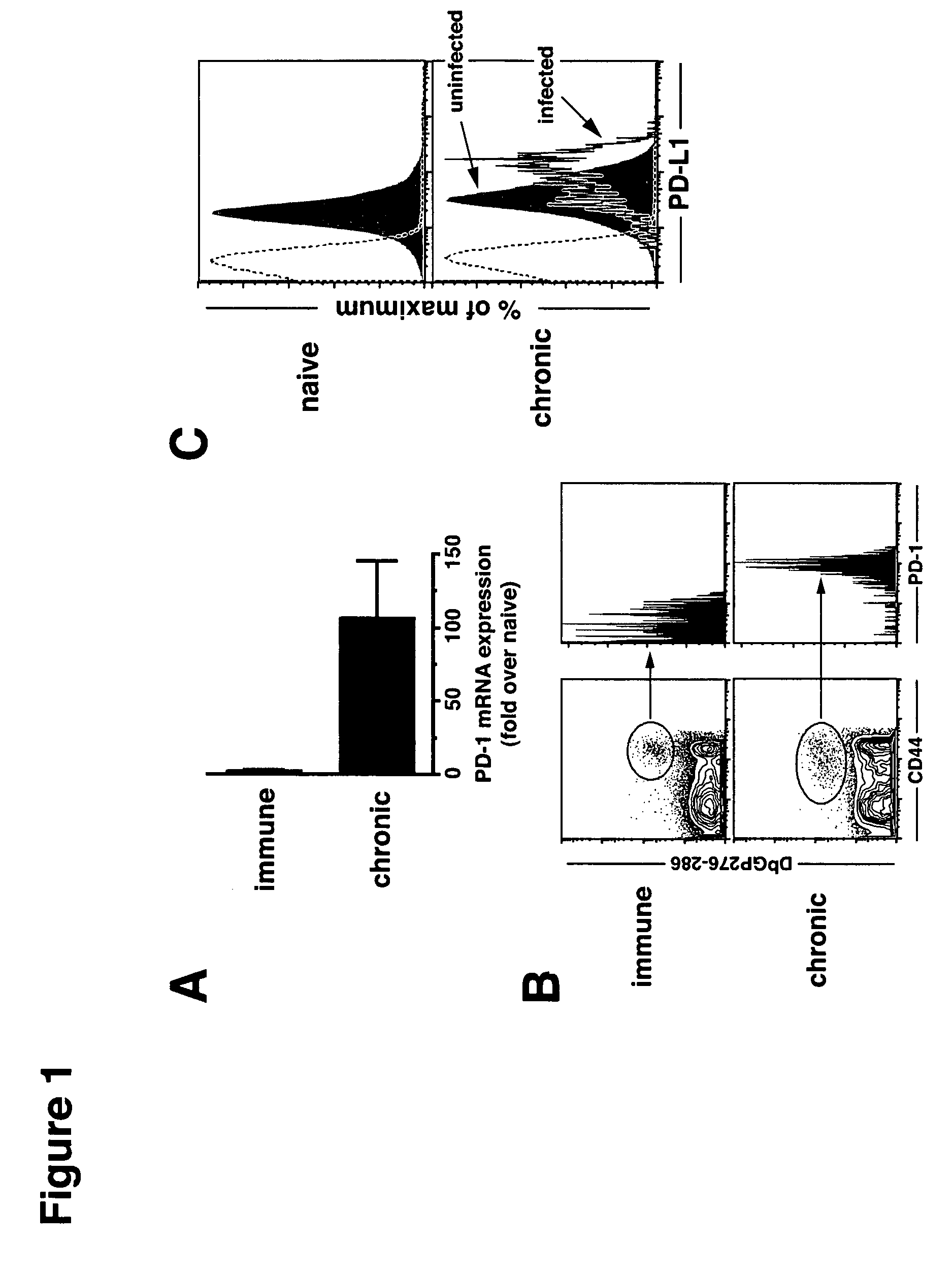
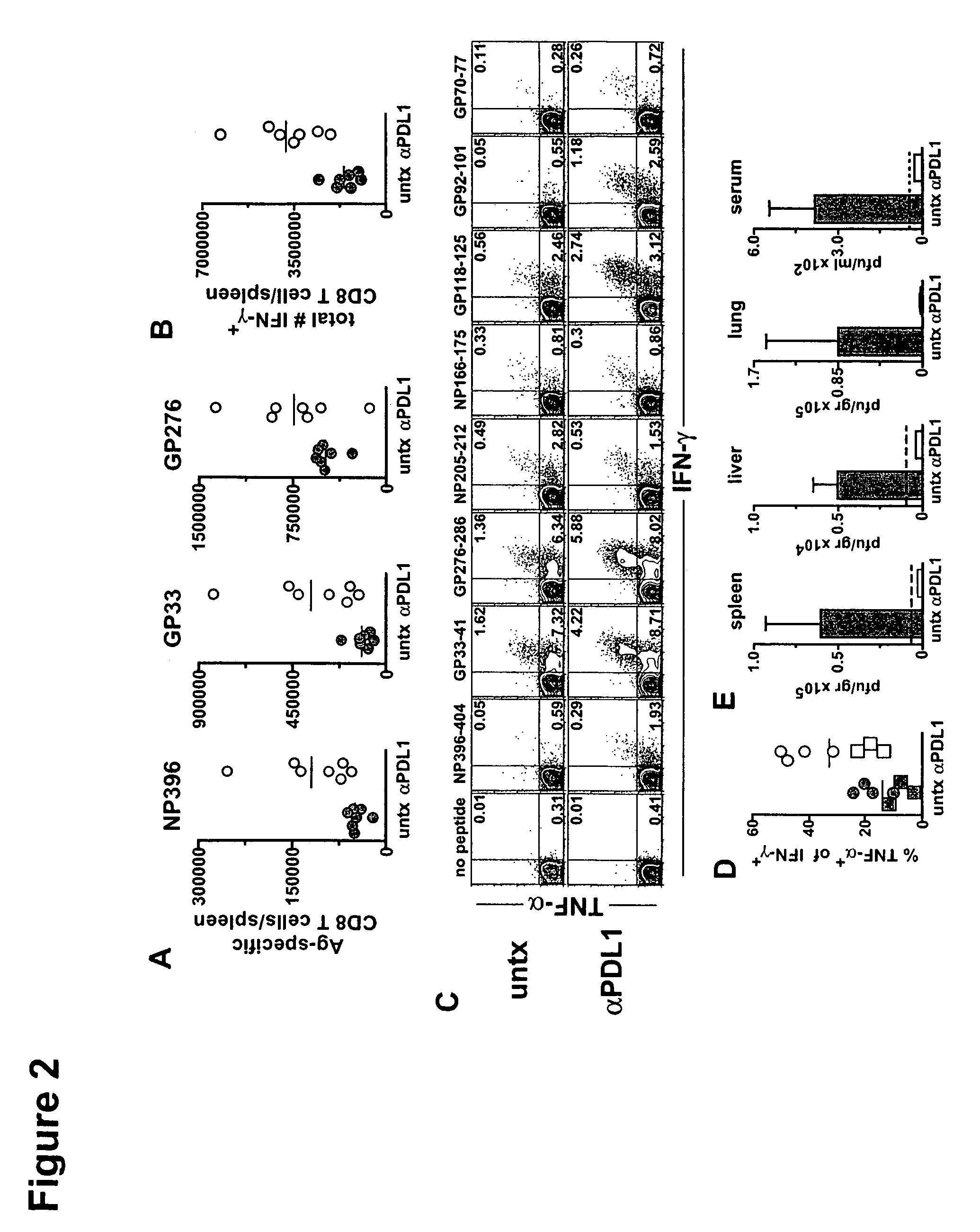
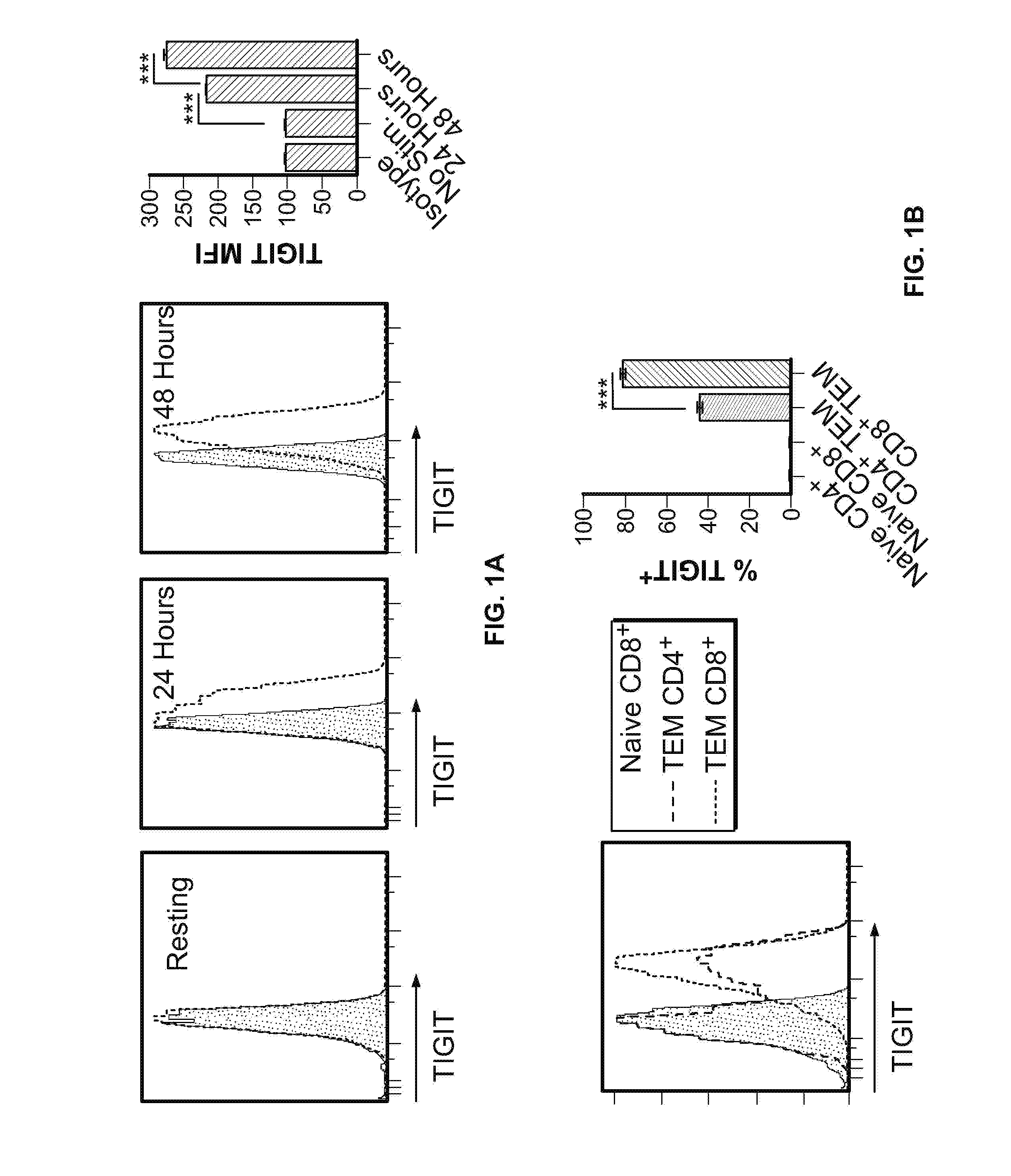
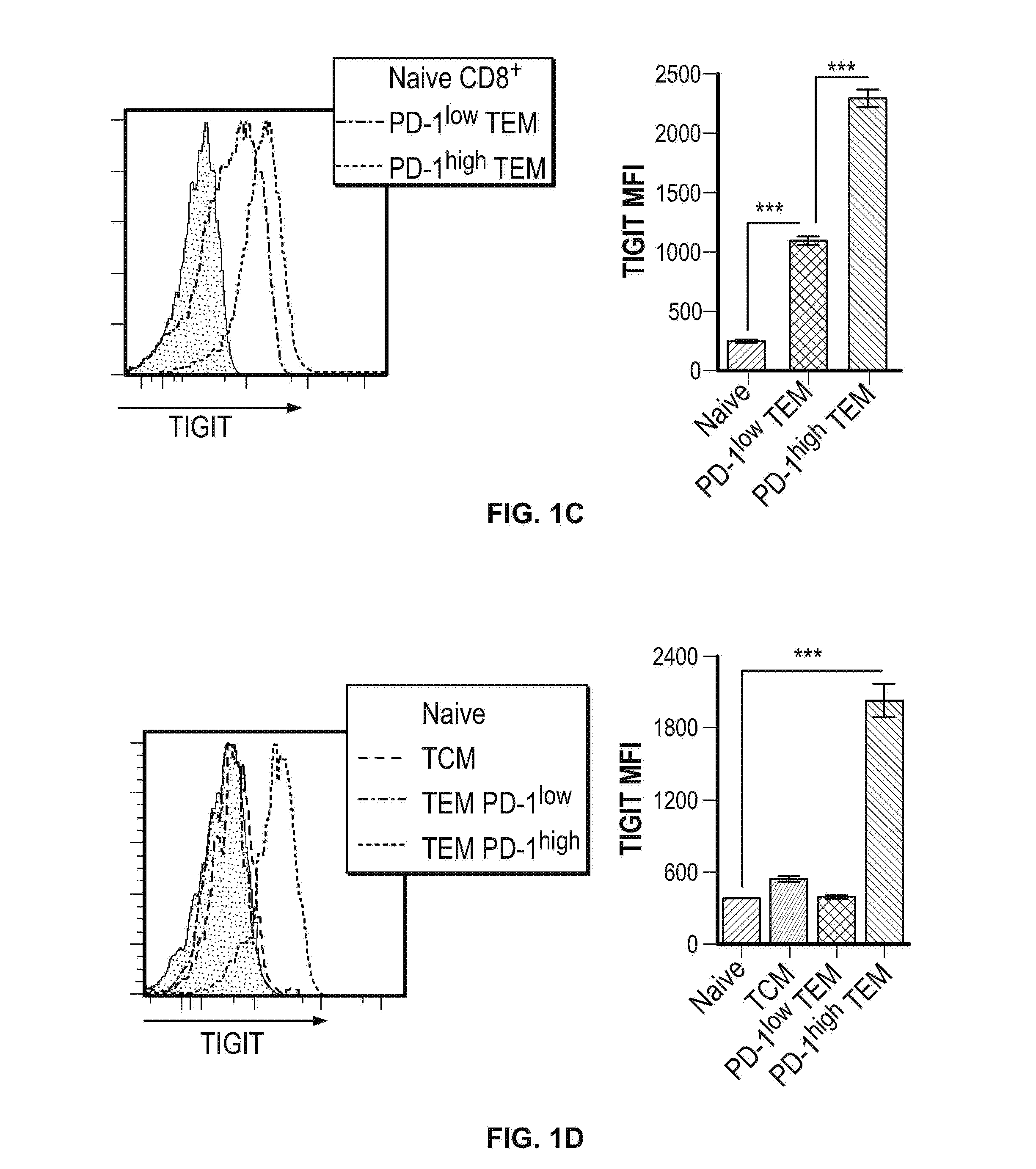
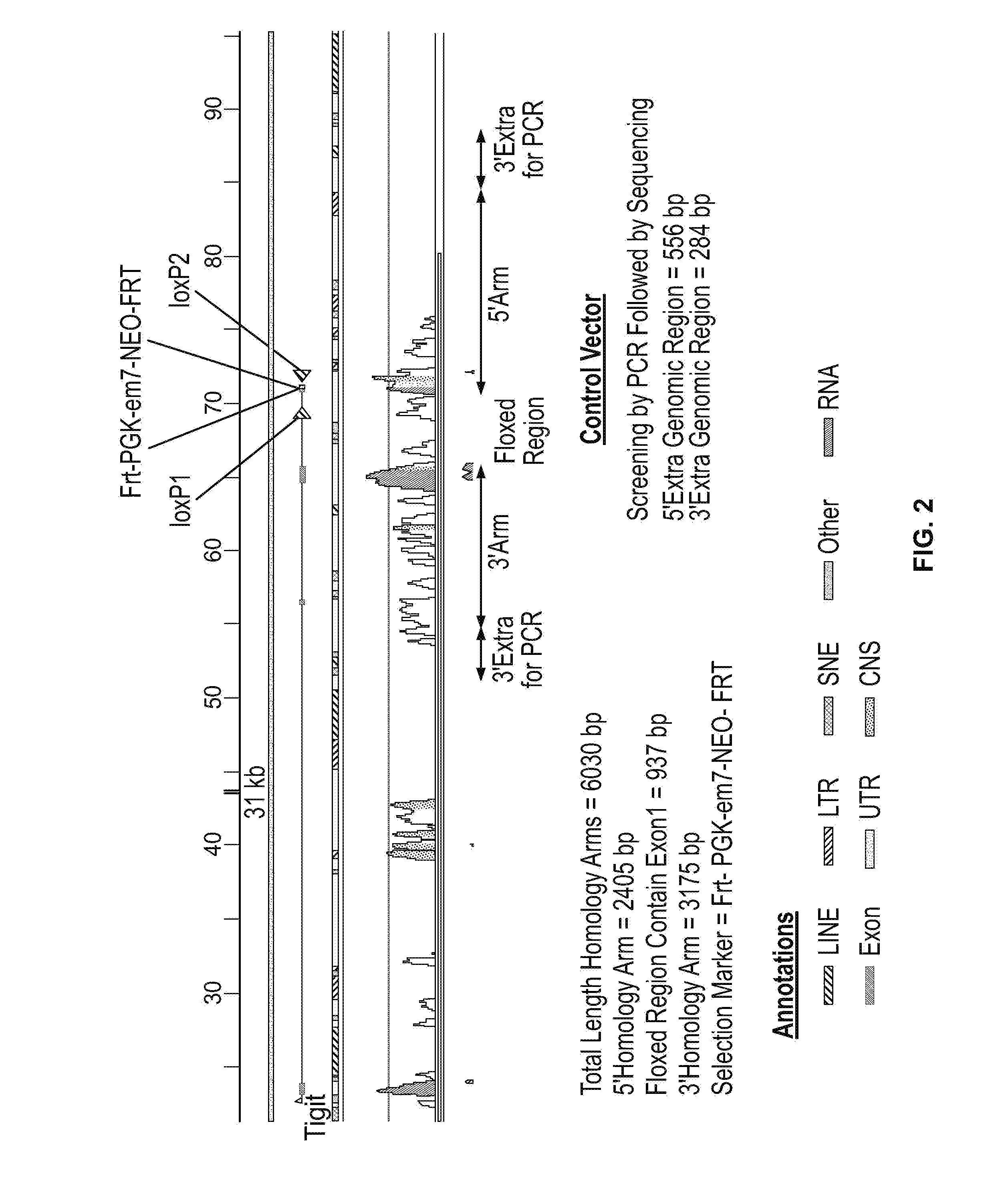
Methods of treating cancer using pd-1 axis binding antagonists and tigit inhibitors
ActiveUS20170044256A1Antibody mimetics/scaffoldsImmunoglobulins against cell receptors/antigens/surface-determinantsOncologyTIGIT
The present invention describes combination treatment comprising a PD-1 axis binding antagonist and an agent that decreases or inhibits TIGIT expression and / or activity and methods for use thereof, including methods of treating conditions where enhanced immunogenicity is desired such as increasing tumor immunogenicity for the treatment of cancer or chronic infection.
Owner:GENENTECH INC
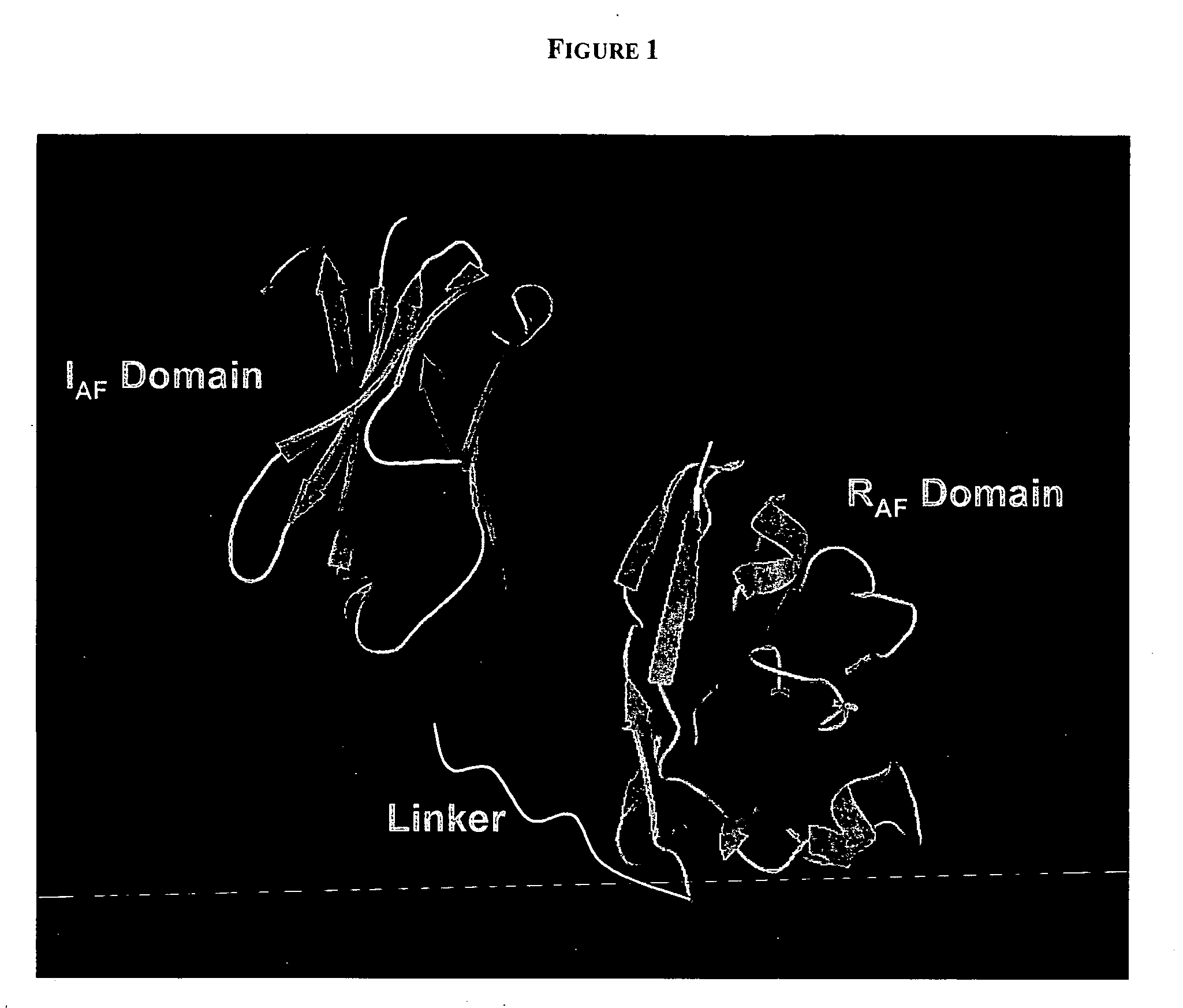
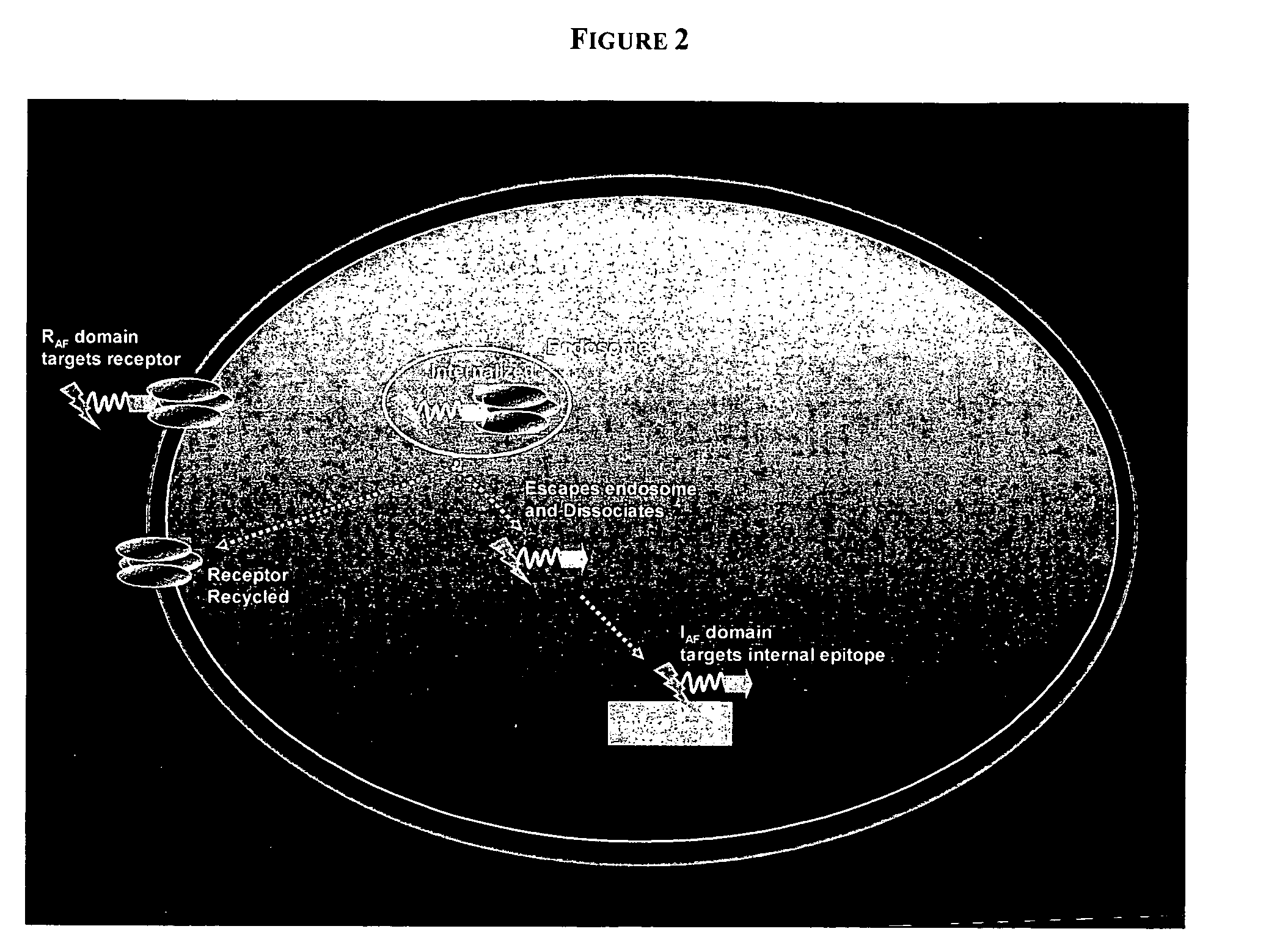
PenetraBodies: receptor-mediated targeted delivery of functionally-active human antibody fragments into cytosol for the treatment of chronic infections and diseases
InactiveUS20060147997A1Small sizeBinding property is not compromisedPeptide librariesBacterial antigen ingredientsCytosolAntibody fragments
The present invention relates to methods of making and using chimeric antibody molecules comprised of at least two domains, namely RAF, and IAF, linked by a flexible peptide linker.
Owner:VIROSYS PHARMA
Compositions and methods useful for modulating immunity, enhancing vaccine efficacy, decreasing morbidity associated with chronic FHV-1 infections, and preventing or treating conjunctivitis
InactiveUS20070280964A1Enhance vaccine efficacyReduce morbidityBiocideSenses disorderTreated animalMicrobiology
Compositions and methods useful for modulating immunity, enhancing vaccine efficacy, decreasing morbidity associated with chronic FHV-1 infections, and / or preventing or treating conjunctivitis in animals. The compositions contain effective amounts of probiotic Enterococcus bacteria and the methods involve administering such compositions to animals alone, in supplements, or in food compositions in amounts suitable for the intended purpose. In certain embodiments, the probiotic is Enterococcus faecium strain NCIMB 10415 (SF68) and the animal is a feline.
Owner:NESTEC SA
Methods of treating cancer using pd-1 axis binding antagonists and tigit inhibitors
Owner:GENENTECH INC
Methods for using JNK inhibitors for treating or preventing disease-related wasting
InactiveUS20040034084A1Inhibit progressPrevent worseningAntibacterial agentsBiocideChronic inflammatory diseaseKidney Failures
The present invention relates to methods useful for the treatment or prevention of disease-related wasting. The methods of the invention comprise the administration of an effective amount of a JNK Inhibitor. In one embodiment, the disease is HIV, AIDS, cancer, end-stage renal disease, kidney failure, chronic heart disease, obstructive pulmonary disease or tuberculosis. The methods can further comprise the administration of a therapeutic or prophylactic agent useful for the treatment or prevention of HIV, AIDS, cancer, end-stage renal disease, kidney failure, chronic heart disease, obstructive pulmonary disease, chronic infectious diseases (e.g., osteoarthritis and bacterial endocarditis), chronic inflammatory diseases (e.g., scleroderma and mixed connective tissue disease) or tuberculosis.
Owner:CELGENE CORP
Antiviral therapy on the basis of RNA interference
InactiveUS20050182010A1Inhibition of replicationGenetic material ingredientsViral/bacteriophage medical ingredientsDouble strandBiology
The invention concerns a gene therapy for treatment of animals and humans which suffer from an infection with a chronic virus such as HIV or HCV. It can also be used prophylactically to prevent chronic infection. The therapy makes use of a nucleotide construct stably integrated in the genome of the target cells of the virus, which is able to produce a single transcript or multiple transcripts capable of forming a double-stranded RNA which inhibits replication of the virus in situ.
Owner:VIRUVATION BV
Combination and method using EDTA, cystine, zinc and selenium for anti-thrombin effect and for anti-platelet aggregation and measurement of efficacy
InactiveUS20020182585A1Increased platelet depositionIncrease blood flowOrganic active ingredientsBiocideEtiologyVitamin C
The invention is for the combination of EDTA, cystine, selenium, Vitamin C, Vitamin E, and zinc for anti-thrombotic effect and for the effect of restoring platelet aggregation, an integral component of thrombus formation, to normal and for the monitoring of the response to therapy with the combination. Methods for use of the components and method for performing the monitoring are included. The combination and method are particularly efficacious for vascular deficiency ailments including atherosclerotic vascular disease, reduction of ischemic cerebal event, complications from surgical procedures including restenosis, neurogenerative disease, and erectile disfunction, and vascular deficiency resulting from etiology of sepsis and chronic infection.
Owner:KINDNESS GEORGE +2
Expanding the T cell repertoire to include subdominant epitopes by vaccination with antigens delivered as protein fragments or peptide cocktails
A convenient way of inducing a broad recognition of dominant and subdominant responses to epitopes of any given antigen of importance for prophylaxis or treatment of a chronic disease is provided. The method involves by immunizing with pools of overlapping fragments (synthetic peptides, e.g., 10-30 mers with 2-20 aa overlap) of the desired antigen in appropriate adjuvants. The T cell repertoire is primed to include not only the immunodominant epitope recognized when the intact molecule is used for immunization and induced by the chronic infection itself, but induce a much broader and balanced response to a number of the subdominant epitopes as well. The vaccination with peptide mix induces a T-cell response that includes response to subdominant epitopes is important for protection against chronic disease that on their own induces a response focused only on immunodominant epitopes. The major advantage of the present invention is that it requires no prior knowledge of the precise localisation and identity of the subdominant epitopes and their recognition in a human population, but expands the T-cell repertoire and thereby the total number of epitopes recognized by specific T cells primed by vaccination from a few immunodominant epitopes to a multiple of epitopes.
Owner:STATENS SERUM INST
Methods and compositions for the treatment of persistent infections and cancer by inhibiting the programmed cell death 1 (pd-1) pathway
ActiveUS20140178370A1Reduced activityReduce expressionAntibacterial agentsOrganic active ingredientsProgrammed cell death 1Chronic infection
The present invention provides methods and compositions for the treatment, prevention, or reduction of persistent infections, such as chronic infections, latent infections, and slow infections and cancer. The methods and compositions of the invention are also useful for the alleviation of one or more symptoms associated with such infections and cancer.
Owner:EMORY UNIVERSITY +3
Silver thiosulfate complex or silver-ammonia complex-containing hygroscopic silver-containing product and preparation method thereof
ActiveCN104083800ABroad spectrum antibacterialHigh antibacterial activityFibre treatmentTwo or more solvent application treatmentAdditive ingredientMoisture absorption
The invention relates to a silver thiosulfate complex or silver-ammonia complex-containing hygroscopic silver-containing product and a preparation method thereof. The invention discloses a hygroscopic silver-containing antibacterial product which comprises a silver thiosulfate complex or a silver-ammonia complex as an antibacterial ingredient, the silver thiosulfate complex or the silver-ammonia complex is evenly distributed and combined on the inner side and / or surface, the hygroscopic silver-containing antibacterial product comprises 0.01-10 wt% of silver, and the moisture absorption ability of the hygroscopic silver-containing antibacterial product is 6g / g and above. The invention also provides a preparation method of the hygroscopic silver-containing antibacterial product as well as a silver-containing antibacterial product dressing prepared from the hygroscopic silver-containing antibacterial product. The hygroscopic silver-containing antibacterial product has a very wide antibacterial spectrum, shows strong antibacterial activity to gram-negative bacteria and gram-positive bacteria, is rapid in effect, has light stability, and can be widely applied to chronic infections exudative wounds.
Owner:FOSHAN UNITED MEDICAL TECH
Agents for the inhibition of virus replication through regulation of protein folding
The invention concerns agents for the treatment of acute and chronic infections with human and animal pathogenic viruses which assemble along the cell membrane and are released through budding on the surface of the cell. Hereunto count especially causative agents of infectious diseases such as AIDS, hepatitis, hemorrhagic fever, SARS, smallpox, measles, polio or the flu. The subjects of the invention are agents that contain inhibitors of the protein folding as active components. Hereunto count inhibitors of cellular folding enzymes (the enzymatic chaperones) as well as substances that disturb the folding of proteins through chemical chaperones. The following substance classes and their derivates belong thereunto: Geldanamycin, Deoxyspergualin, 4-PBA or Herbimycin A. Due to these agents the highly organised processes of the assembly and the proteolytical maturation of virus structure proteins is disturbed. As a result the release and production of infectious decendent viruses is prevented.
Owner:VIROLOGIK GMBH
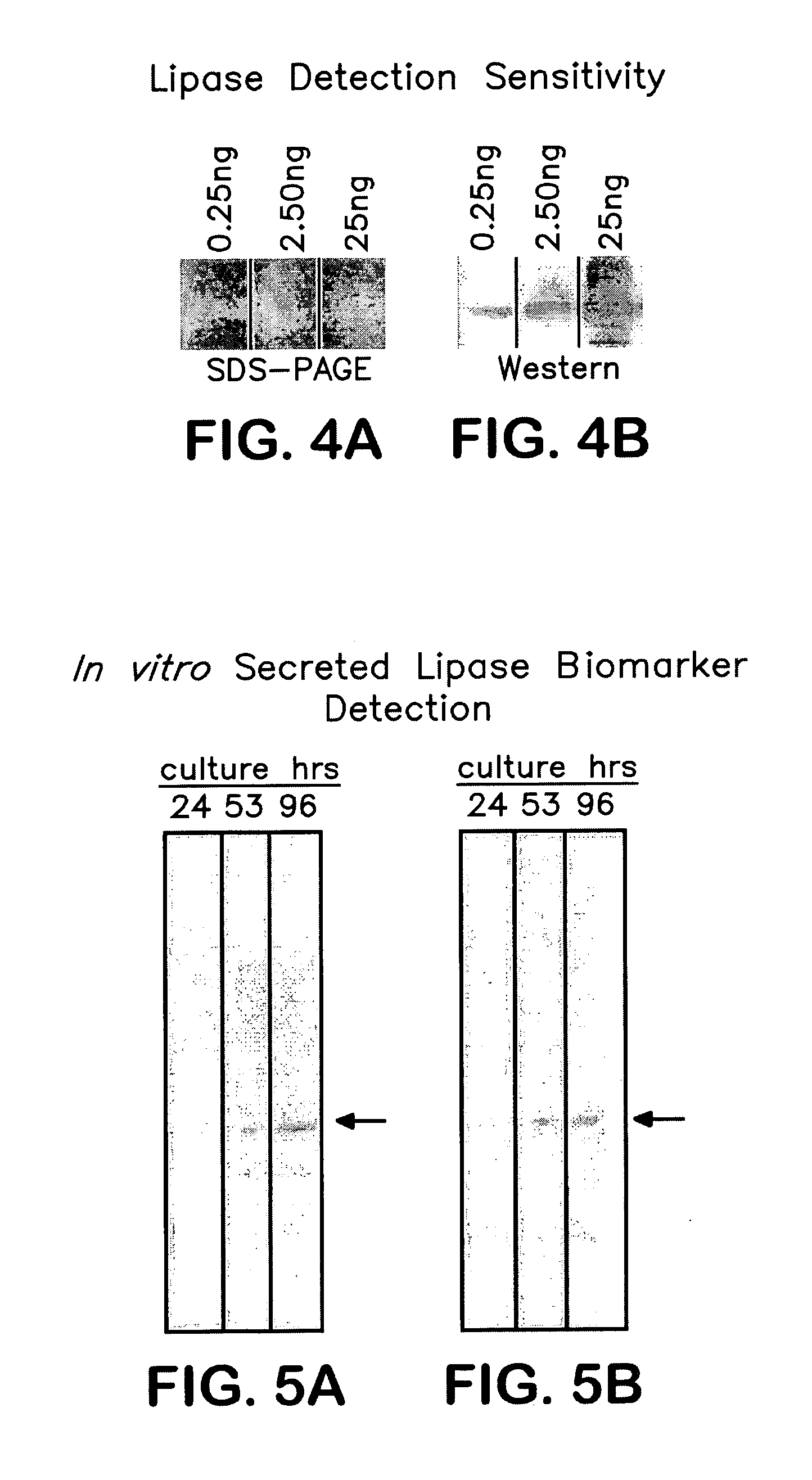
Detection of secreted lipase proteins from Candida species
Methods and devices for the detection of proteins secreted by the pathogenic growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the Candida species, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to lipase proteins whose expression is differentiated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the LIP protein may be detected as well as the LIP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Toxicity T cell position vaccine of the cell for treating Hepatitis B and the preparing method
The invention relates to bio-pharmaceutical engineer technology domain. At present, the therapeutic drugs for HBV infection mainly depend on IFN-Alpha and nucleotide analog, which can not kill virus thoroughly with worse remote therapeutic effect. The invention provides a cytotoxic T cell epitope vaccine for hepatitis B, comprising a fused polypeptide formed by connecting 21 CTL epitopes selected from CTL epitopes data of HBV antigen and two universal epitopes of auxiliary T lymphocyte. Saccharomyces Serevisiae is selected to express the fused antigen and mixes with immunologic adjvant. The said vaccine can activate and enhance the cellular immune response of patient with chronic HBV infection. The vaccine can also promote the immune elimination of HBV, and can be used for treatment of chronic hepatitis B.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
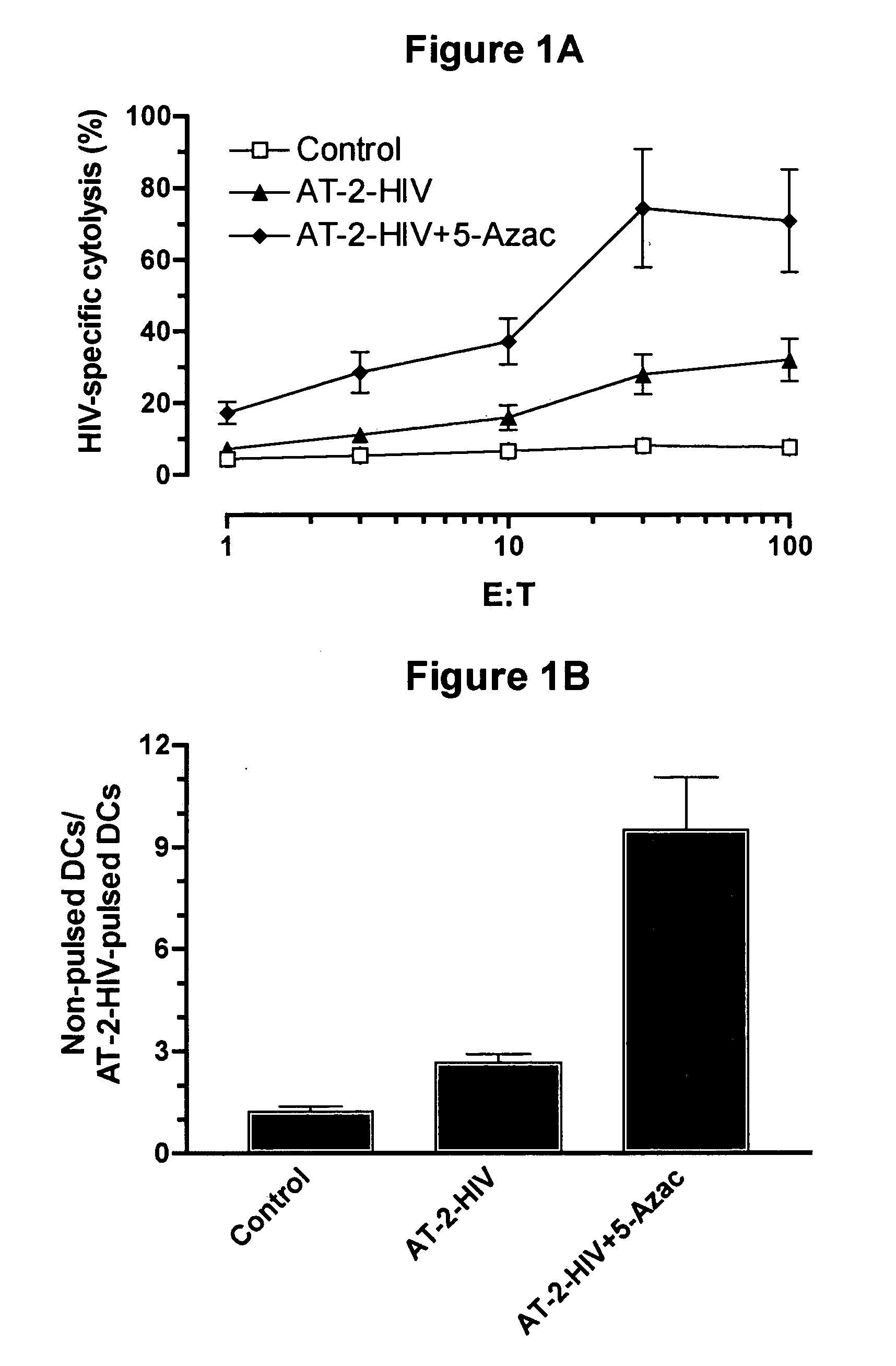
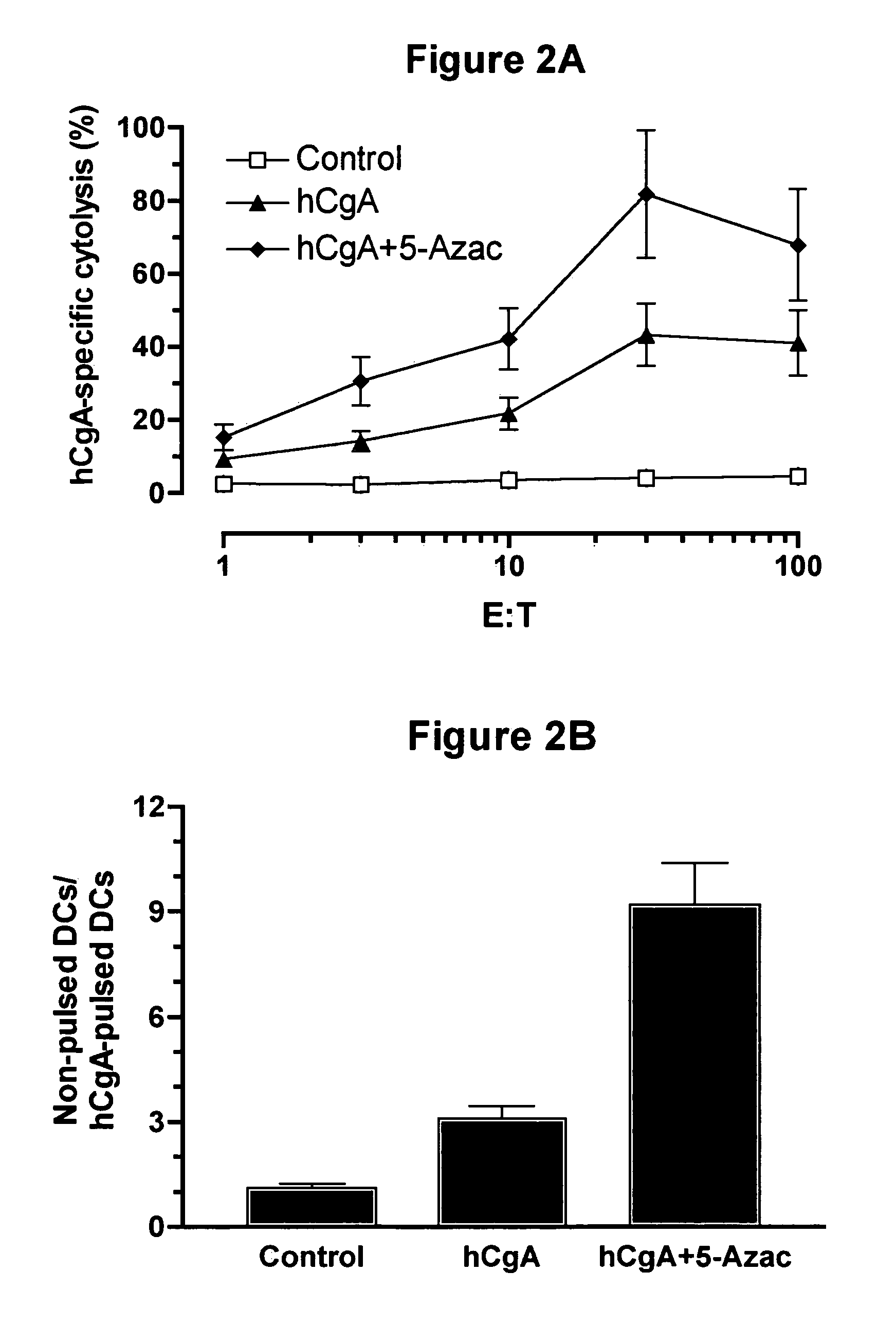
Compositions comprising demethylating agents as enhancers of immunotherapy for the treatment of chronic infections and neoplastic diseases and methods for treating the same
InactiveUS20050266012A1Enhancing antigen-specific cytolysisViral antigen ingredientsGenetic material ingredientsDiseaseImmunotherapy
The invention concerns compositions comprising a demethylationg agent and an antigen for inducing and enhancing antigen-specific immunity and uses thereof. The invention also concerns methods for enhancing antigen-specific immunity by administrating a demethylating agent.
Owner:BIOVAXIM
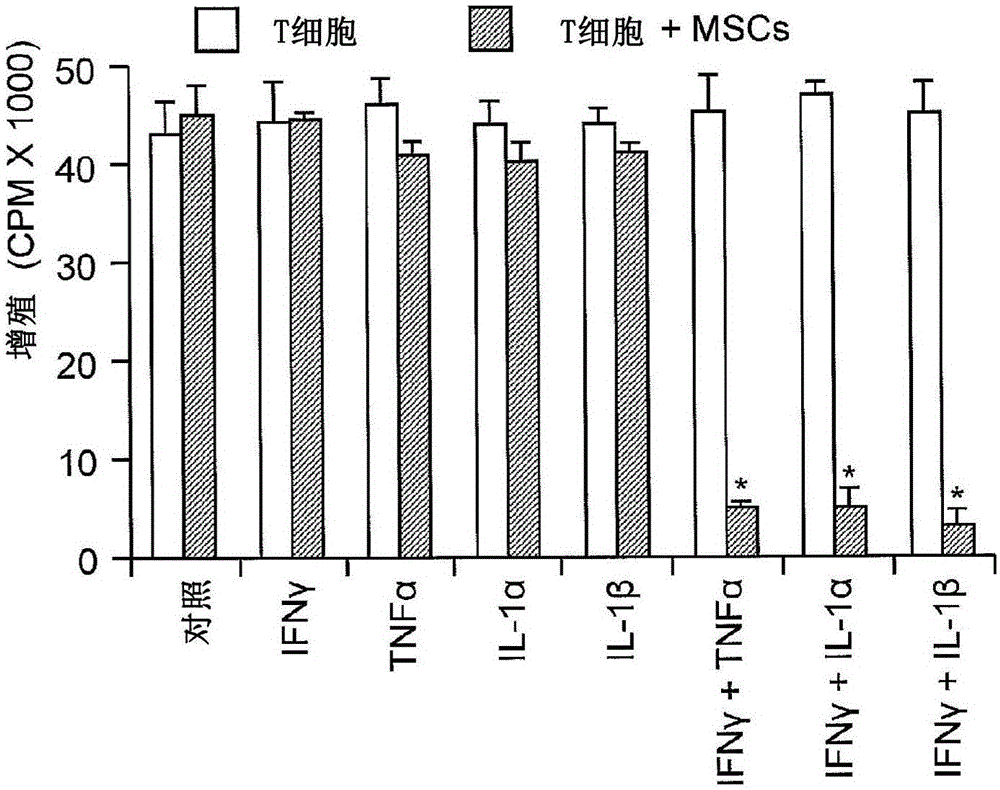
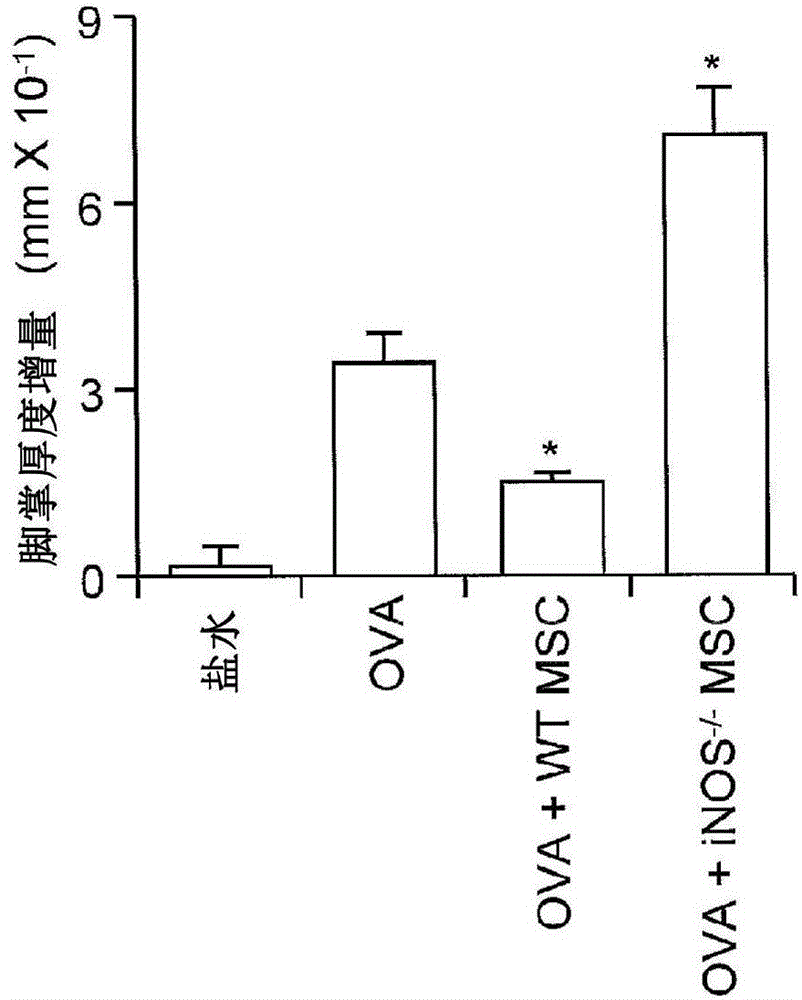
Methods modulating immunoregulatory effect of stem cells
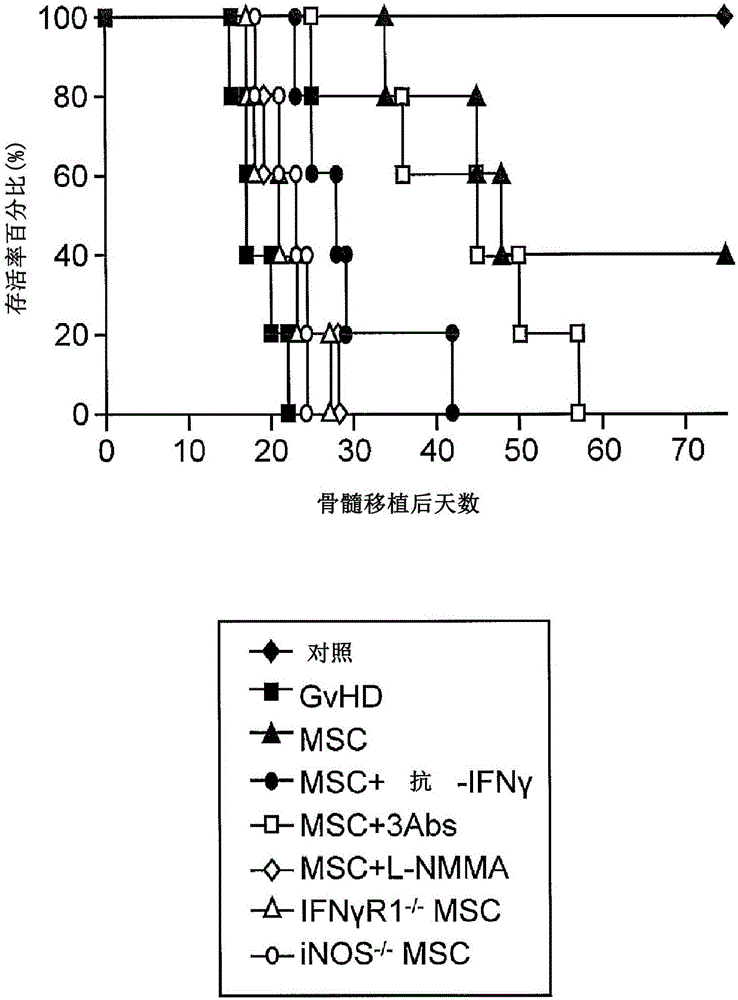
The present invention provides methods or kits with inflammatory cytokines to pretreat 1-ISCs to augment their immune modulatory effect, in prevention and treatment of various diseases such as multiple sclerosis, arthritis, lupus, sepsis, hepatitis, cirrhosis, Parkinson's disease, chronic infections, and GvHD. The present invention relates to novel methods for enhancing the immunosuppressive or the immune stimulatory activities of mesenchymal stem cells (JvfSCs).
Owner:RUTGERS THE STATE UNIV
Liquid dressing capable of sterilizing and repairing wounds and production method of liquid dressing
The invention discloses liquid dressing capable of sterilizing and repairing wounds and a production method of the liquid dressing. The liquid dressing is double-emulsion-system solution using water-soluble amino polysaccharide, sterilizing preservatives and high-molecular polymer as main active constituents and high-molecular emulsifier as the emulsifier. The liquid dressing has the advantages that the liquid dressing can promote wound healing, resist exudation, inhibit bacteria and prevent infection and is applicable to washing or wet dressing treatment of related wounds such as scald wounds, knife wounds and chronic infection wounds. The production method of the liquid dressing includes: preparing water-soluble amino polysaccharide emulsion solution, preparing antibacterial compound emulsion solution, compounding a double-emulsion system and sterilizing.
Owner:GUIZHOU YANGSHENG MEDICAL INSTR
Anti-PD-L1 antibody as well as application, preparation method, kit and drug
ActiveCN107298713AImprove featuresHigh affinityImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsImmunologic disordersAntiendomysial antibodies
The invention discloses an anti-PD-L1 antibody as well as application, a preparation method, a kit and a drug, and relates to the technical fields of biological detection and treatment. The anti-PD-L1 antibody provided by the invention contains a heavy chain variable region and a light chain variable region, wherein the amino acid sequence of the heavy chain variable region is shown in SEQ ID NO. 5, and the amino acid sequence of the light chain variable region is shown in SEQ ID NO. 6. The anti-PD-L1 antibody can be combined with PD-L1 recombinant proteins and inflammation tissue cells or tumor tissue cells expressing PD-L1 molecules via specific recognition, so that the anti-PD-L1 antibody has a relatively high specificity and avidity, can be used for immunoblotting, enzyme-linked immuno sorbent assay, immunohistochemistry and flow cytometry and can also be used for treating diseases of tumors, immunological diseases, chronic infectious diseases and the like.
Owner:联合益康(北京)生物科技有限公司
Liver disease markers
InactiveUS20130225428A1Quantitative precisionPeptide librariesNucleotide librariesDiseaseLiver fibrosis
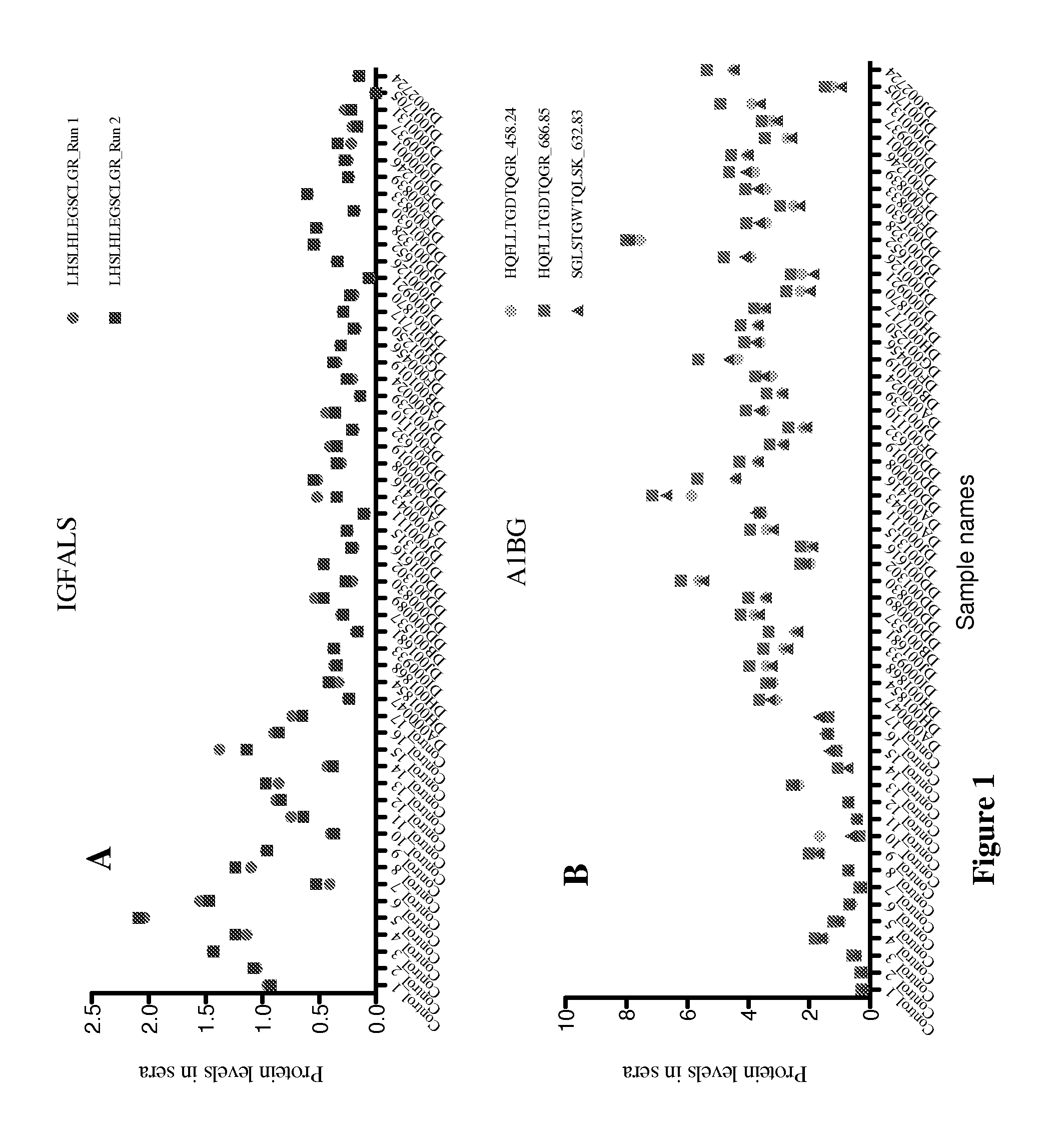
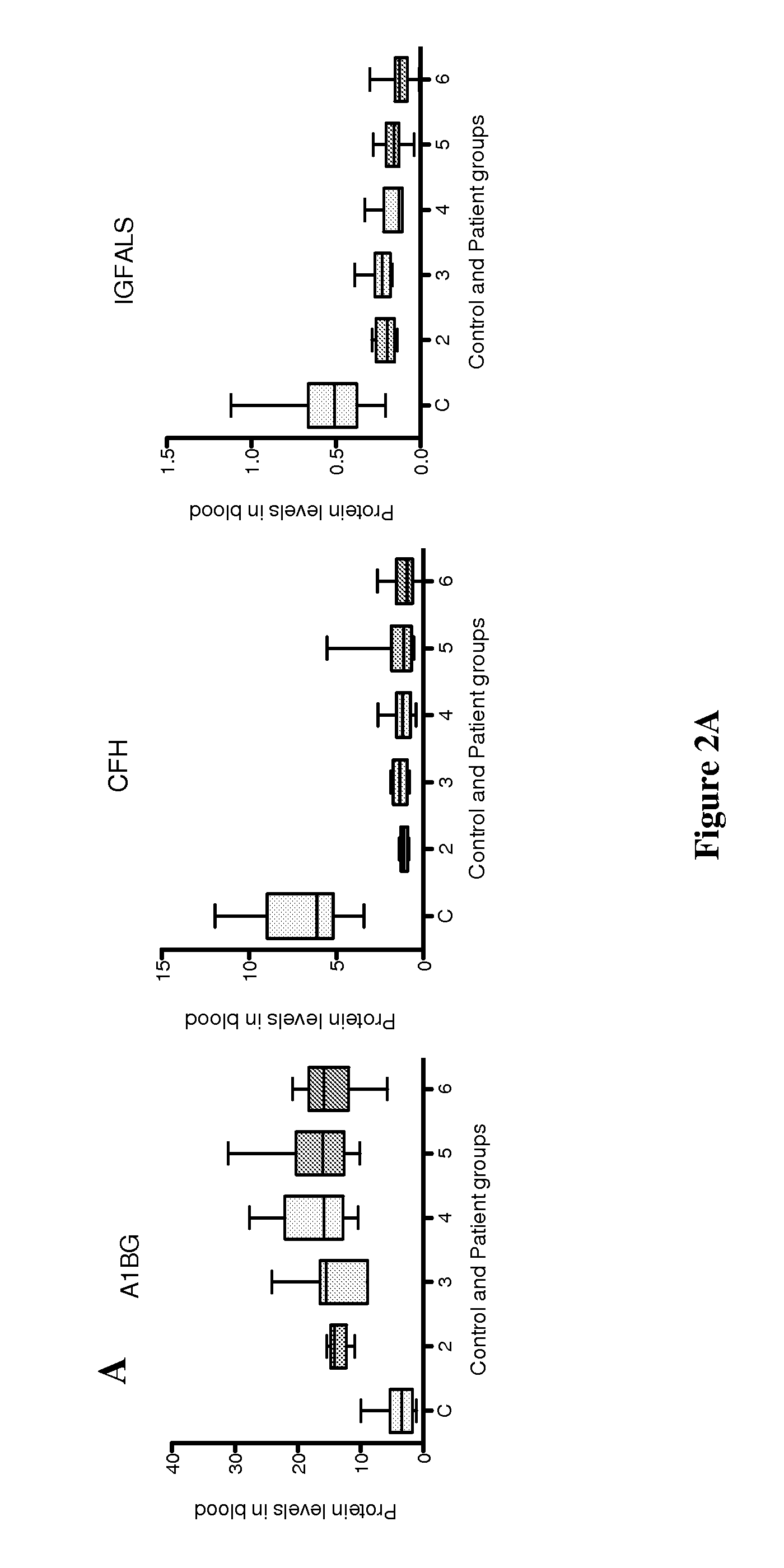
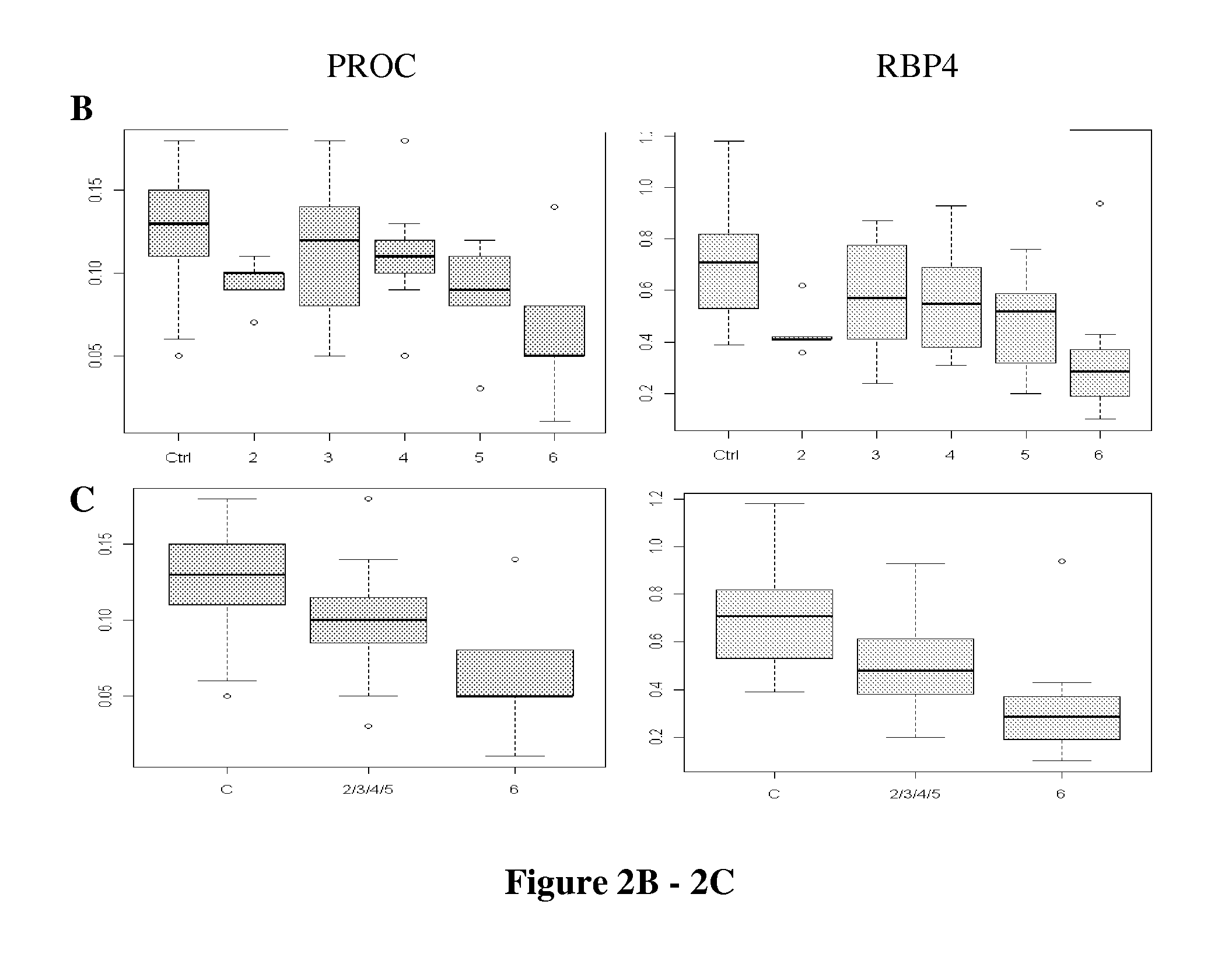
New markers for diseased liver conditions have been identified. A combination of protein C (PROC) and retinol binding protein 4 (RBP4) in blood are biomarkers to distinguish patients at different stages of hepatic fibrosis due, for example, to chronic infection with hepatitis C virus (HCV). Also, alpha-1-B glycoprotein (A1BG), complement factor H (CFH) and insulin-like growth factor binding protein acid labile subunit (IGFALS) distinguish subjects with chronic or acute liver conditions such as hepatitis infection or liver fibrosis from healthy controls.
Owner:INSTITUTE FOR SYSTEMS BIOLOGY
Regulation of toll-like receptors on stem cells
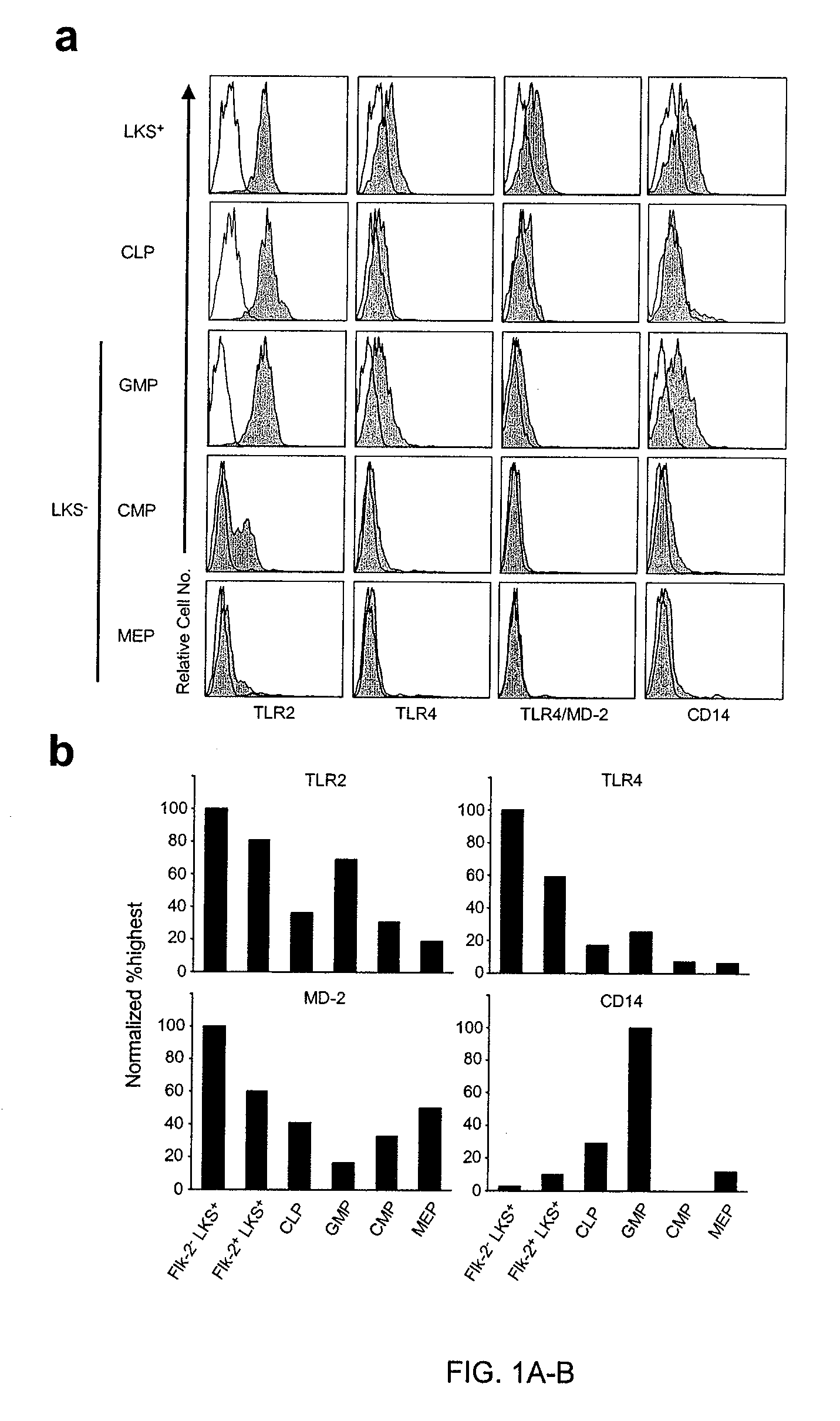
InactiveUS7592003B2Reduce expressionReduce functionBiocideMammal material medical ingredientsProgenitorHematopoietic cell
The discovery of Toll-like receptors (TLRs) on the surface of hematopoietic cells provides new methods for the stimulation and differentiation of various classes of progenitor cells. TLR2 and TLR4 agonists (natural ligands, mimetics, antibodies) are particularly useful in these methods. The cells can be isolated and used for various purpose including tissue regeneration and grafting. In contrast, antagonists of TLRs can be used to protect cells from various insults such as chemo- and radiotherapy, acute and chronic infection, and transplantation by inhibiting activation and differentiation. TLR2, TLR4 and TLR9 pathway antagonists (soluble TLR, mimetics, antibodies) are particularly useful in these methods. Cells can be isolated and used for various purposes including transplantation.
Owner:OKLAHOMA MEDICAL RES FOUND
Detection of secreted aspartyl proteases from Candida species
InactiveUS20070134743A1Bioreactor/fermenter combinationsBiological substance pretreatmentsDisease causeDisease injury
Methods and devices for the detection of proteins secreted by the hyphal growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the species Candida, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to secreted aspartyl protease proteins whose expression is upregulated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the SAP protein may be detected as well as the SAP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
Preparations that potentiate immunogenicity in low immunogenic antigens
ActiveUS20020136735A1Antibacterial agentsBacterial antigen ingredientsImmunogenic peptideVaccine Immunogenicity
This invention discloses means for obtaining immunogenic peptides, polypeptides, proteins, and their corresponding nucleic acid sequences, target cells with vaccine interest, or lysates thereof, without making structural changes in said antigens, through their association with Very Small Size Proteoliposomes. The object of the invention is to provide immunogenic compositions containing peptides, polypeptides, proteins, their corresponding DNA sequences, cells or their lysates and Very Small Size Proteoliposomes (VSSP), which are formed by binding the Outer Membrane Protein Complex (OMPC) of Neisseria meningitidis with gangliosides, by means of hydrophobic links. Additionally, it is stated that these compositions can be formulated alone or in the form of emulsions with the Incomplete Freund's Adjuvant (IFA), and may also be lyophilized. The essence of the invention consists in describing compositions that triggers immunogenicity in low immunogenic antigens, such as growth factor receptors, without imparting structural changes therein. Particularly, this invention refers to preparation of immuno-stimulating compositions capable of generating antigen-specific immune responses, even in immuno-compromised hosts, such as those suffering form cancer or viral or bacterial chronic infections. In said patients, the administration of the vaccine compositions described in this invention has lead to the reestablishment of the functionality of the immune system. Vaccine compositions of this invention can be used to protect or treat infectious, or auto-immune diseases.
Owner:CENT DE INMUNOLOGIA MOLECULAR CENT DE INMUNOLO
Detection of secreted aspartyl proteases from Candida species
Methods and devices for the detection of proteins secreted by the hyphal growth form of Candida species are disclosed. The disclosed devices may constitute a method for the diagnosis of acute or chronic infections, including candidiasis, caused by microorganisms of the species Candida, such as C. albicans, for example. The devices of the present invention incorporate antibodies specific to secreted aspartyl protease proteins whose expression is upregulated upon the conversion of the Candida species from the commensal to the pathogenic form. The antibodies may be used in assays to allow the diagnosis of candidal infections and disease conditions. Either monoclonal antibodies or polyclonal antibodies may be used, and in the case of the monoclonals, the specific epitopes of the SAP protein may be detected as well as the SAP protein itself.
Owner:KIMBERLY-CLARK WORLDWIDE INC
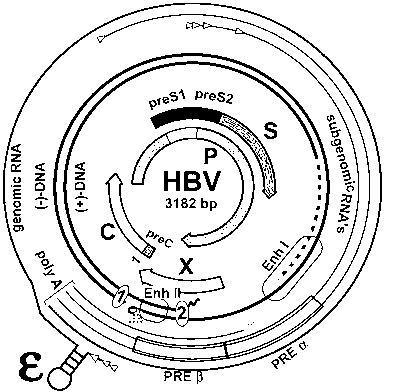
Replicative HBV (Hepatitis B Virus) vector carrying foreign gene and recombinant HBV generated after transfection and corresponding preparation method and application
ActiveCN103173492AGuaranteed high level of replicationPreserve the ability to reinfectMicroorganism based processesViruses/bacteriophagesHepatitis B virusVirus strain
The invention discloses a replicative HBV (Hepatitis B Virus) vector carrying a foreign gene and a recombinant HBV generated after transfection and a corresponding preparation method and an application. The vector separates overlaying genes C and P on an HBV genome by a molecular cloning technique based on originally expressed HBV plasmid to respectively form an integral opened reading frame where a protein translation starting sequence or a protease enzyme cutting site is inserted to respectively guide expression of foreign gene and gene P. The replicative HBV vector carrying the foreign gene transiently transfecting hepatoma carcinoma cell secretes the recombinant HBV. The recombinant HBV prepared from the HBV vector transfection cells provided by the invention can express the foreign gene and maintain the replicative and infecting capacity. The invention is suitable for constructing an HBV chronic infection animal model, an HBV cell model with cccDNA stably and automatically replicated and a traceable HBV strain, researching a molecular mechanism of HBV infection, replication, packaging and the like, and screening anti-HBV novel medicines.
Owner:BEIQIUEN INT PEACE HOSPITAL P L A
Application of RNA and carrier in preparation of product for preventing and/or treating liver cancer
ActiveCN103088061AReduce pathogenicityInhibit liver cancerGenetic material ingredientsDigestive systemChronic hepatitisViral vector
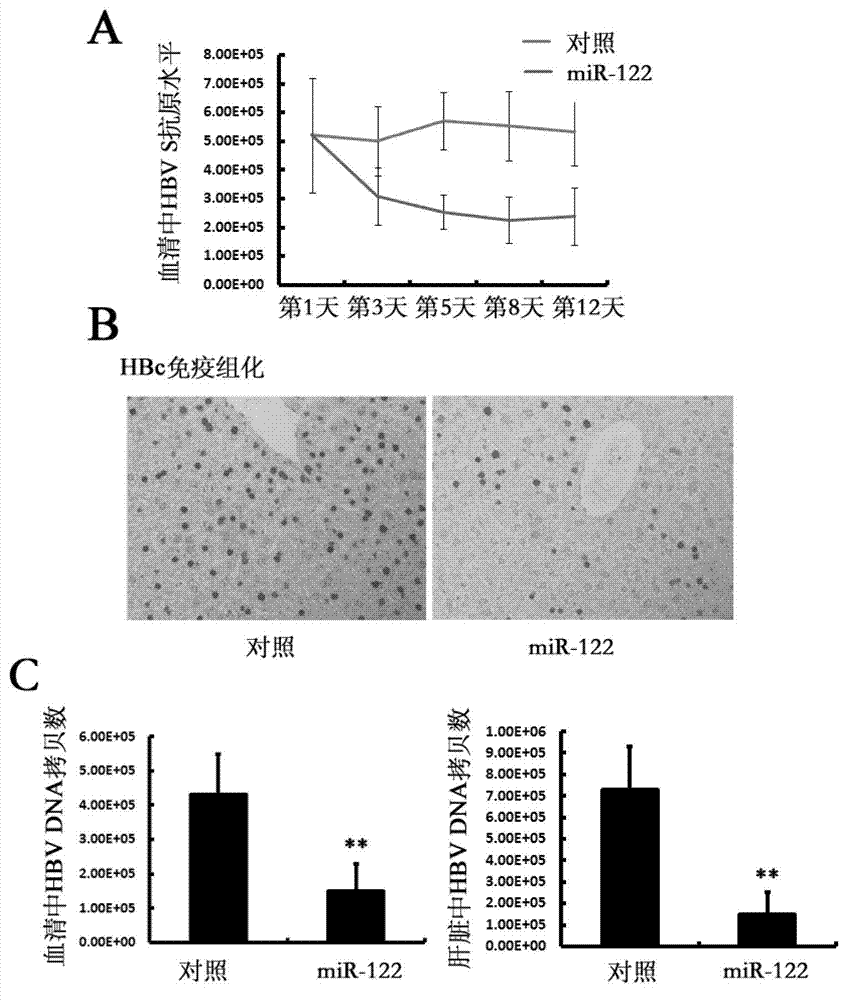
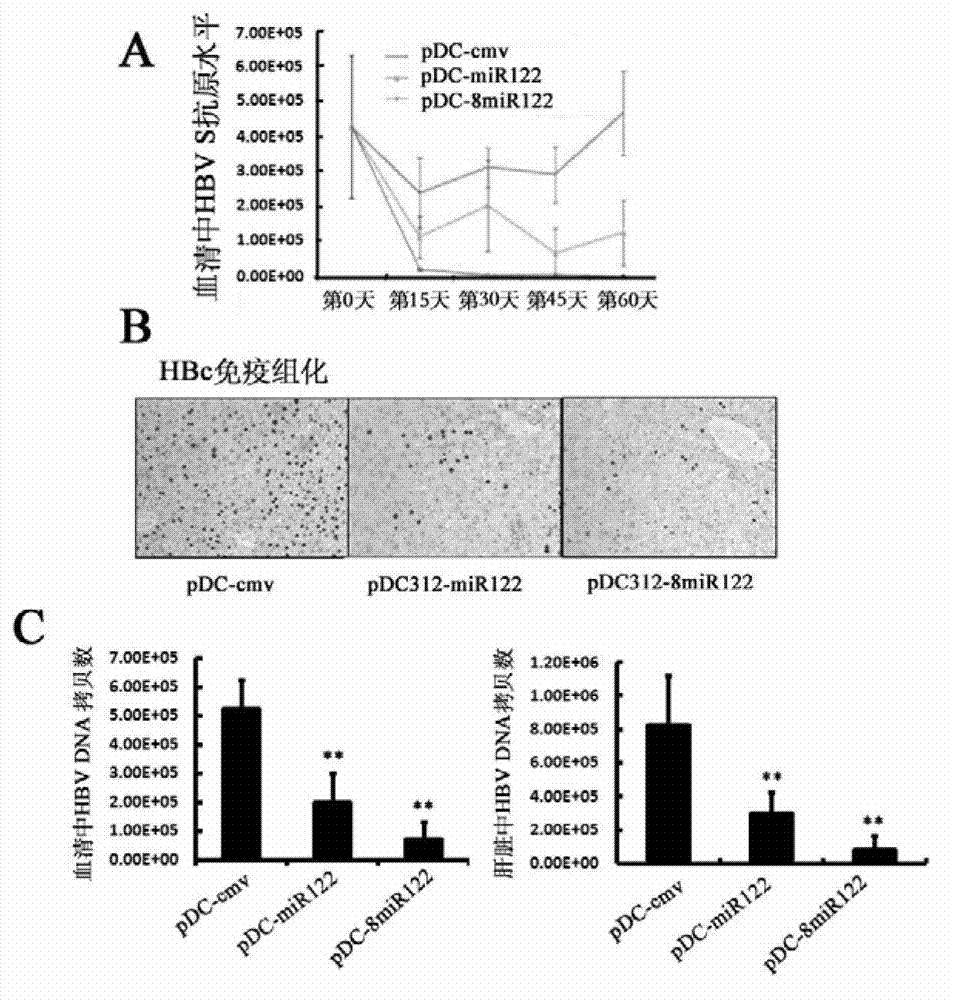
The invention discloses application of RNA and carrier in preparation of a product for preventing and / or treating liver cancer. The invention provides a recombinant adenovirus carrier which is a recombinant adenovirus carrier formed by inserting at least one encoding gene of miR-122 into a pDC312-cmv; and the miR-122 refers to RNA shown in a sequence 1 in a sequence table or RNA encoded by a sequence 3 in the sequence table. The experiment proves that the miR-122 encoding genes are introduced into the adenovirus carrier, the adenovirus is purified and is used for performing an animal experiment, the miR-122 and the recombinant adenovirus carrier or adenovirus can obviously suppress the liver cancer caused by chronic hepatitis B infection, and a novel liver cancer treatment medicine can be developed.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
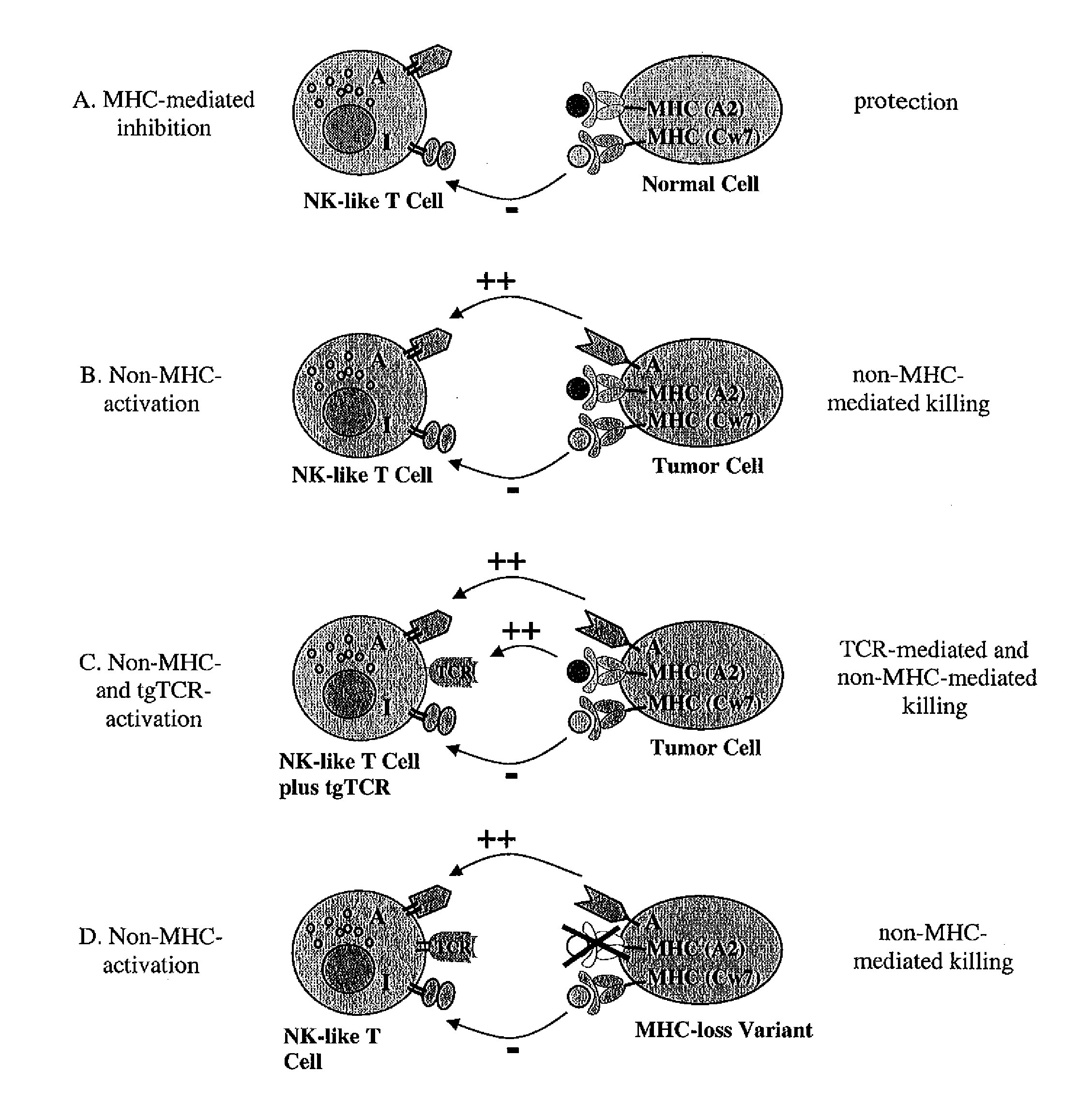
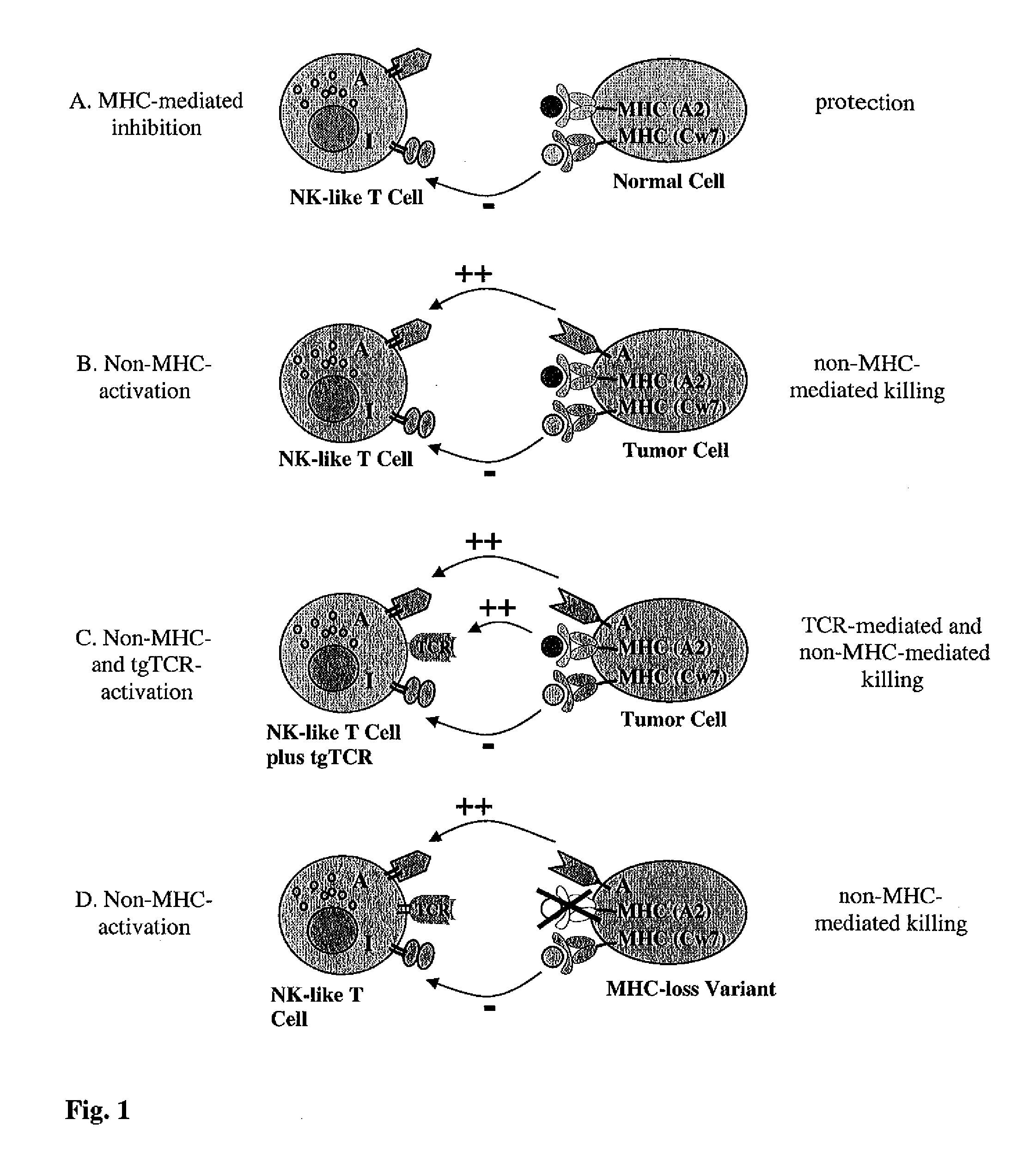
Expression of transgenic t cell receptors in lak-t cells
InactiveUS20110020308A1Short timeQuick buildAntibacterial agentsBiocideAdoptive cellular therapyDisease
The present invention is directed to LAK-T cells, which have been transformed by a transgenic T cell receptor (tg-TCR). The invention is further directed to a method of generating those transgenic T cells, a pharmaceutical composition comprising said cells and the use of the LAK-T cells or of the pharmaceutical composition in the adoptive cell therapy and for treating hematological malignancies or solid tumors or acute or chronic infections or autoimmune diseases.
Owner:HELMHOLTZ ZENT MUNCHEN DEUTES FORSCHUNGSZENT FUR GESUNDHEIT & UMWELT
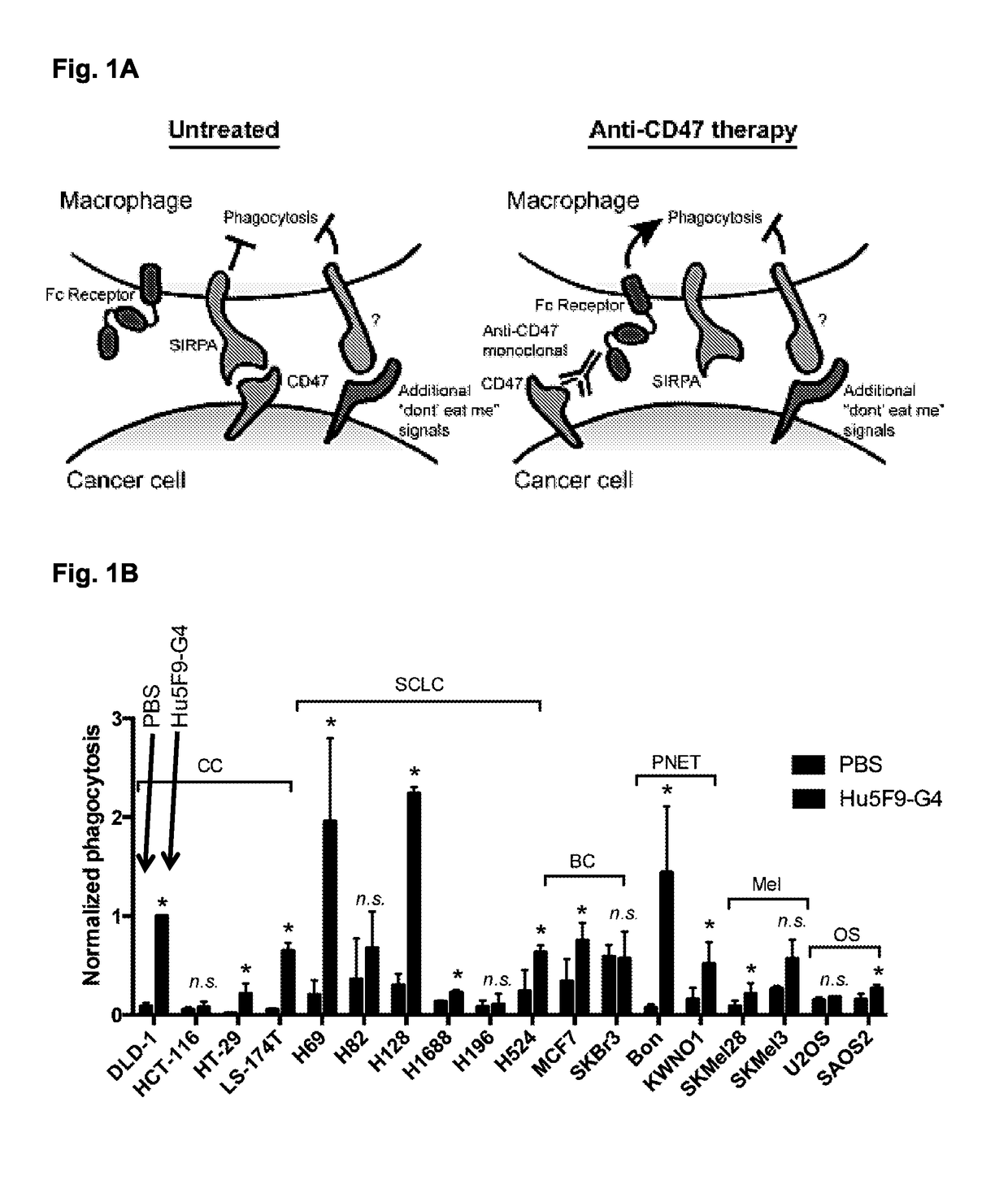
Compositions and methods for inducing phagocytosis of mhc class i positive cells and countering Anti-cd47/sirpa resistance
ActiveUS20180251558A1Reduce in quantityPeptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsMHC class ICo administration
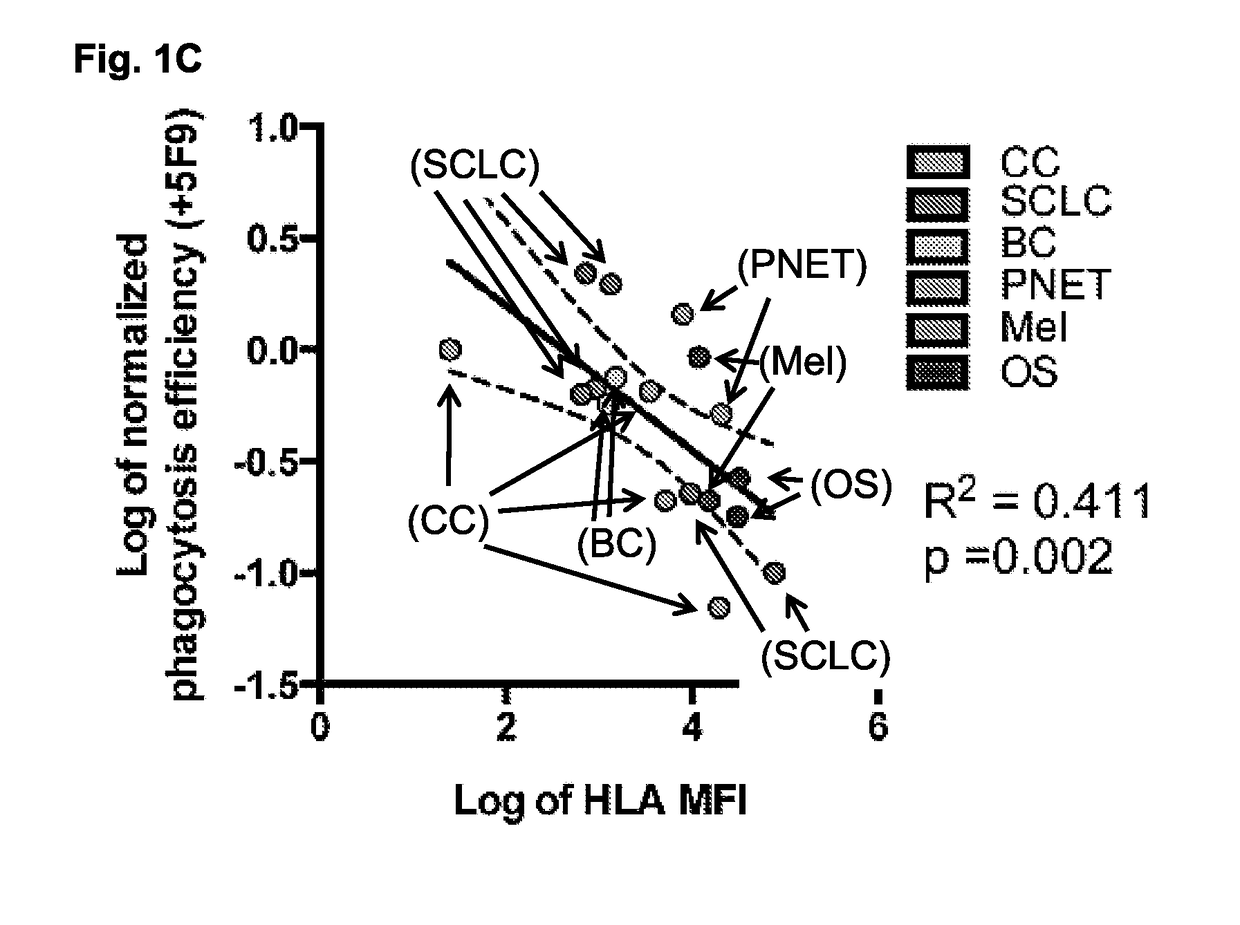
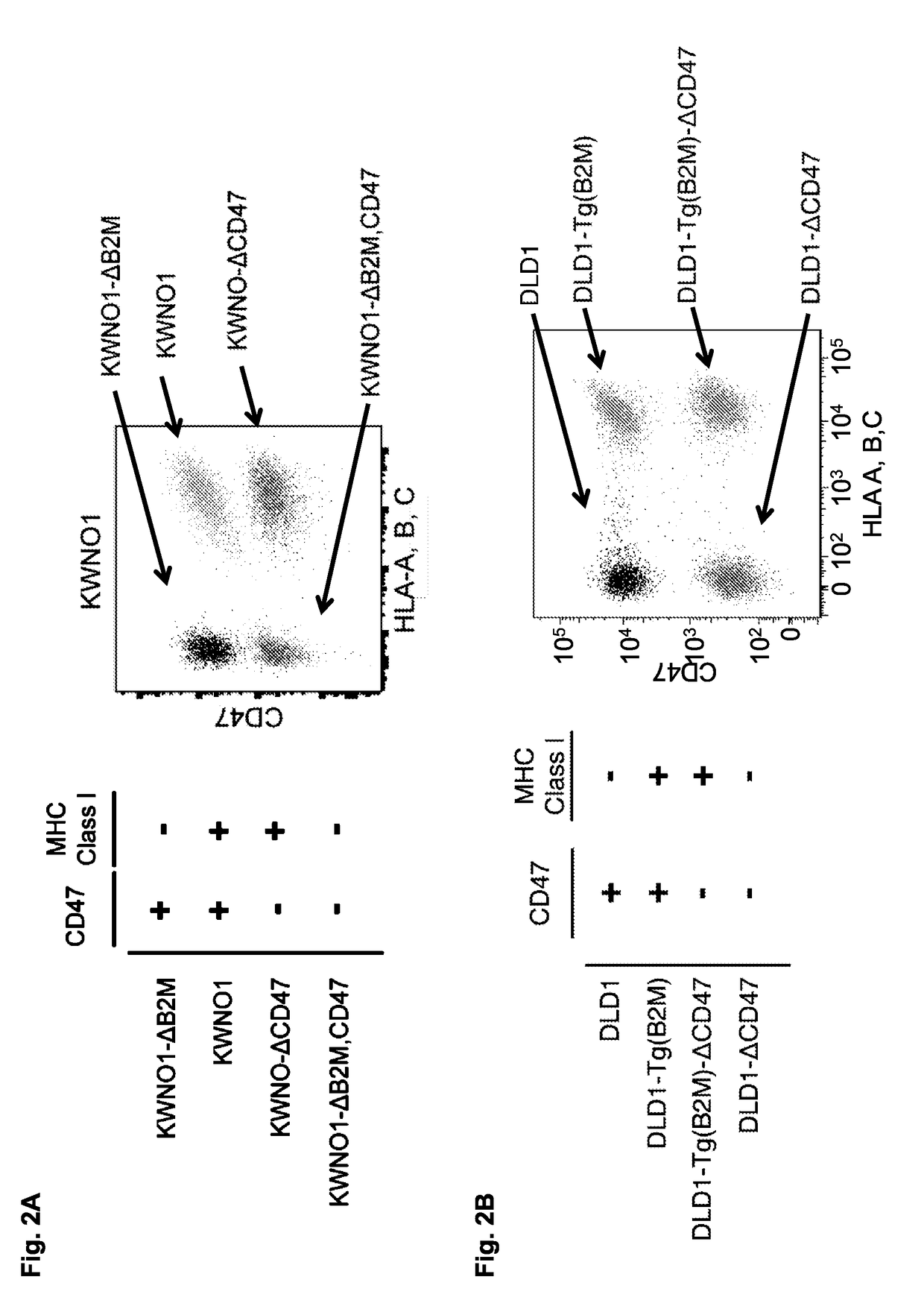
Methods and compositions are provided for inducing phagocytosis of a target cell, treating an individual having cancer, treating an individual having an intracellular pathogen infection (e.g., a chronic infection), and / or reducing the number of inflicted cells (e.g., cancer cells, cells infected with an intracellular pathogen, etc.) in an individual. Methods and compositions are also provided for predicting whether an individual is resistant (or susceptible) to treatment with an anti-CD47 / SIRPA agent. In some cases, the subject methods and compositions include an anti-MHC Class I / LILRB1 agent. In some cases, the subject methods and compositions include an anti-MHC Class I / LILRB1 agent and an anti-CD47 / SIRPA agent (e.g., co-administration of an anti-MHC Class I / LILRB1 agent and an anti-CD47 / SIRPA agent). Kits are also provided for practicing the methods of the disclosure.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com