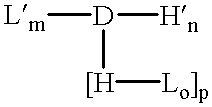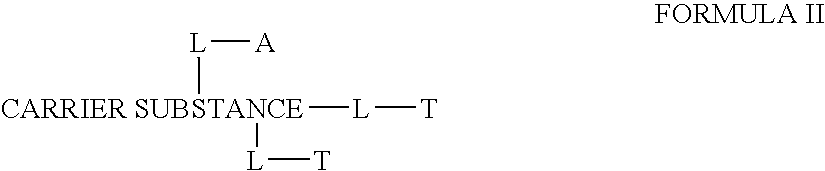Patents
Literature
1284 results about "Insulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Insulin is a protein hormone that is used as a medication to treat high blood glucose. This includes in diabetes mellitus type 1, diabetes mellitus type 2, gestational diabetes, and complications of diabetes such as diabetic ketoacidosis and hyperosmolar hyperglycemic states. It is also used along with glucose to treat high blood potassium levels. Typically it is given by injection under the skin, but some forms may also be used by injection into a vein or muscle.
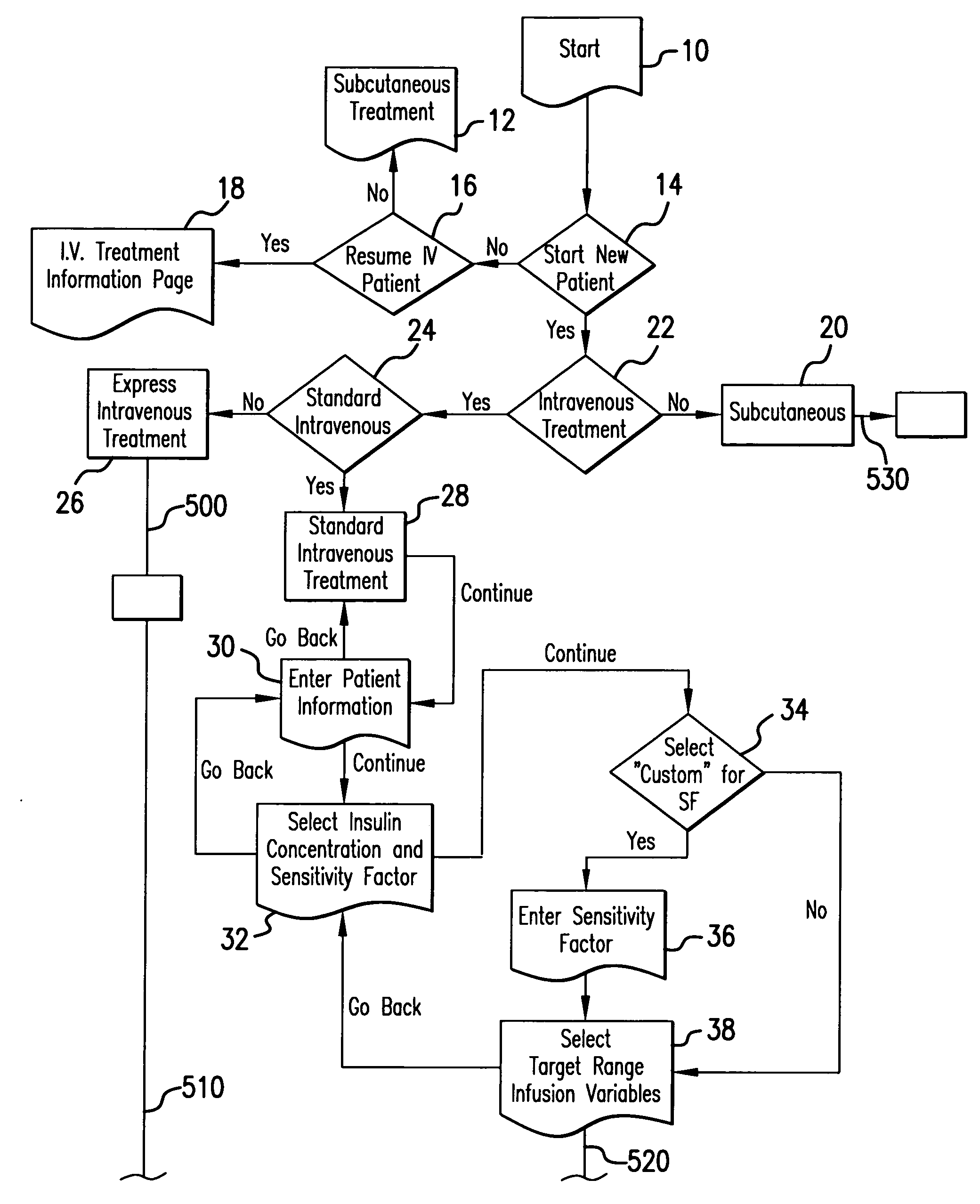
Method for advising patients concerning doses of insulin
A method for guiding a user to select a dose of insulin, including the steps of calculating a firsts pecific dose of insulin by applying information provided by the user to an insulin dose calculation algorithm, wherein such information includes at least the user's current blood glucose level and the user's desired blood glucose level, calculating at least a second specific dose of insulin that is different from the first specific dose, and presenting to the user a range of doses comprising at least two of the specific doses.
Owner:INSULET CORP
Watch controller for a medical device
InactiveUS20070093786A1Minimizes potential for errorEasy to watchMedical devicesPharmaceutical delivery mechanismCommunications systemMonitoring and control
An infusion system that includes a watch controller device and a communication system to transmit the communications from the watch controller device to an infusion device pump that controls delivery of fluids to the user's body. More particularly, these apparatuses and methods are for providing convenient monitoring and control of the infusion pump device in determining the appropriate amount of insulin to deliver.
Owner:MEDTRONIC MIMIMED INC
Amphiphilic drug-oligomer conjugates with hydroyzable lipophile components and methods for making and using the same
InactiveUS6309633B1Reduce deliveryExtended durationAntibacterial agentsOrganic active ingredientsTherapeutic proteinCholesterol
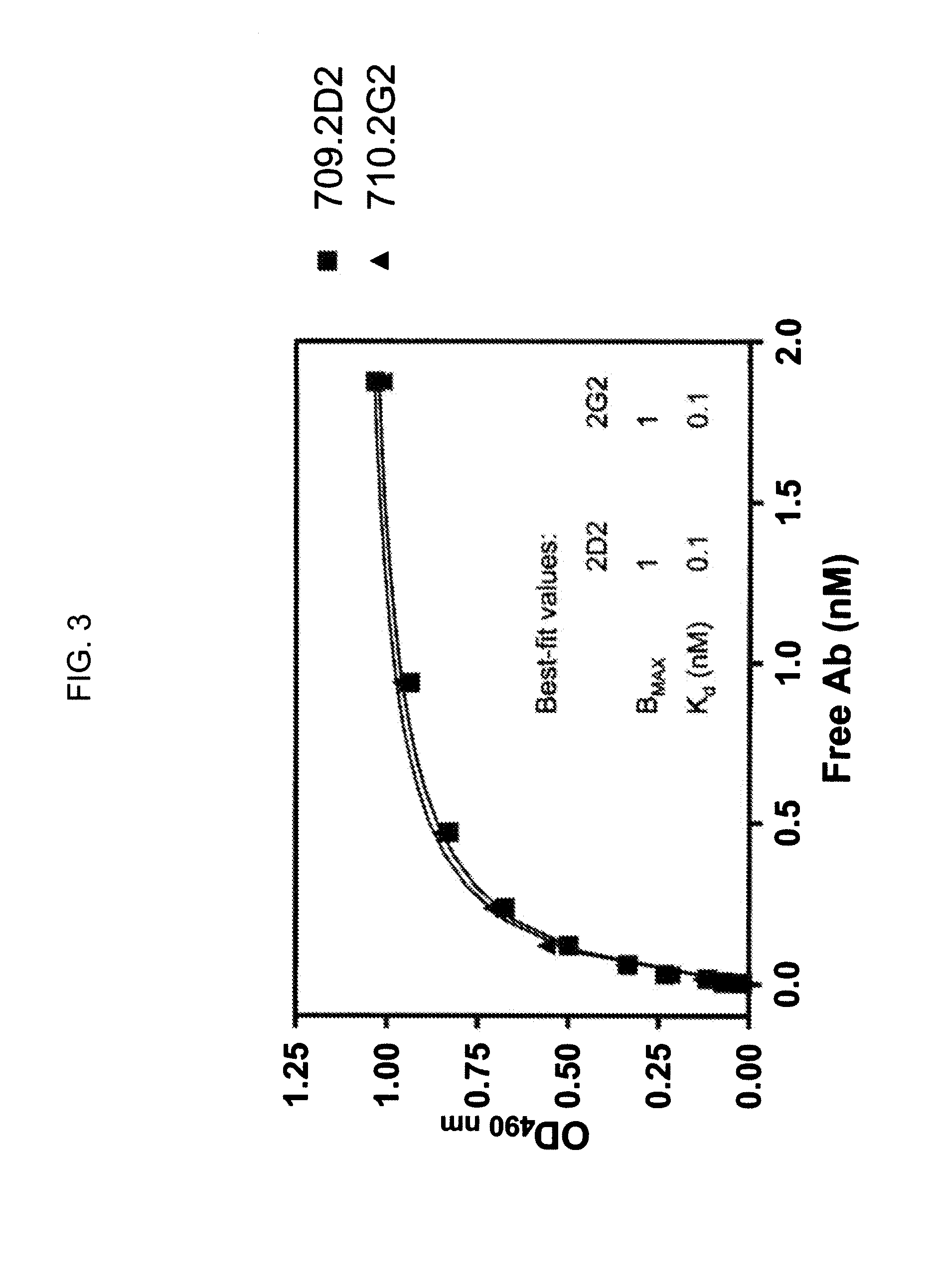
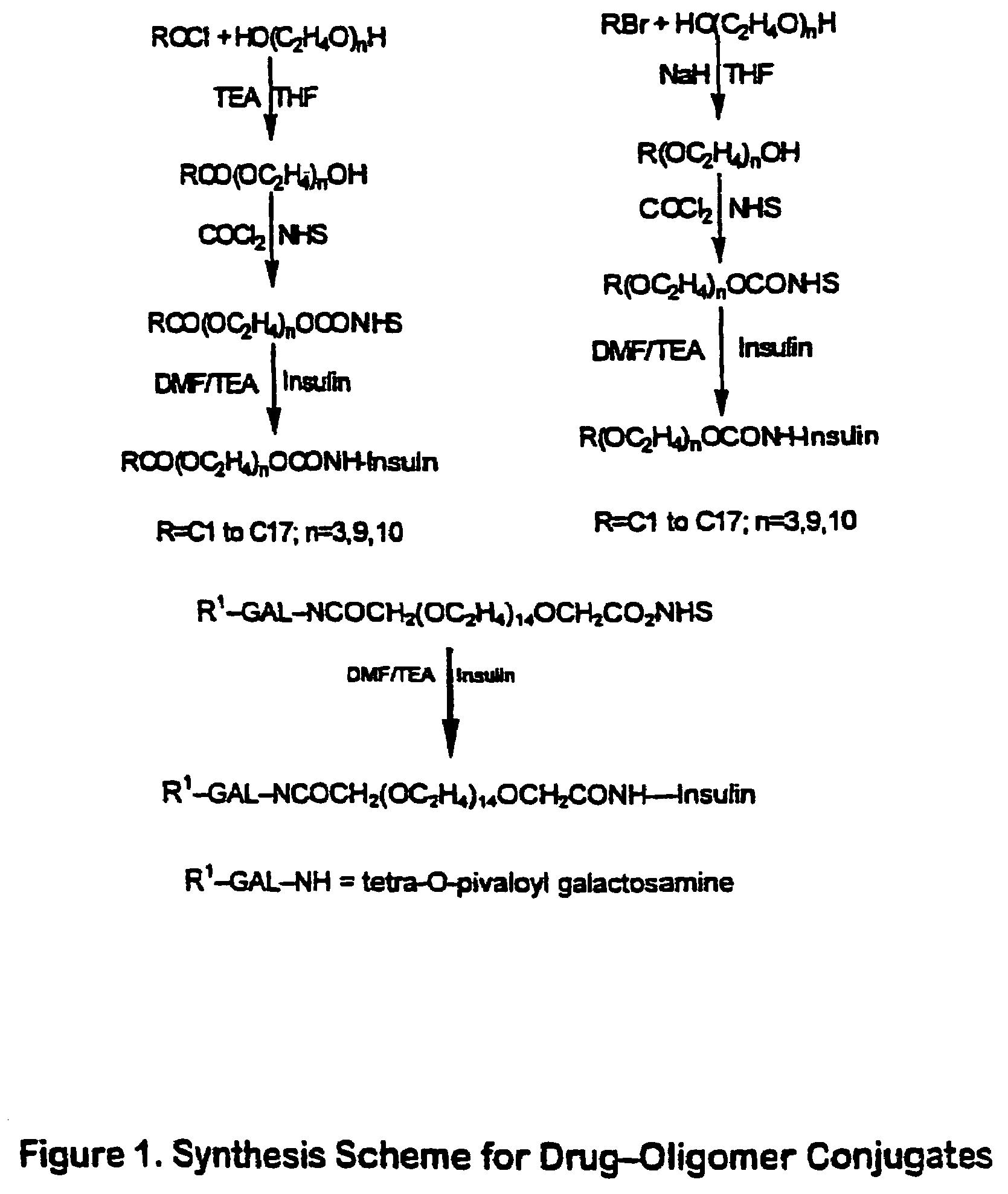
The invention provides a drug-oligomer conjugate having the following general formula:wherein D is a therapeutic drug moiety; H and H' are each a hydrophilic moiety, independently selected from the group consisting of straight or branched PEG polymers having from 2 to 130 PEG subunits, and sugars; L is a lipophilic moiety selected from the group consisting of alkyl groups having 2-26 carbon atoms, cholesterol, adamantane and fatty acids; o is a number from 1 to the maximum number of covalent bonding sites on H; m+n+p together have a value of at least one and not exceeding the total number of covalent bonding sites on D for the -H', -L and -H-L substituents; the H-L bond(s) are hydrolyzable and the D-L' bond(s), when present, are hydrolyzable; the conjugate being further characterized by one of the following: (i) m is 0 and p is at least 1; (ii) n is 0 and p is at least 1; (iii) m and n are each 0 and p is at least 1; (iv) p is 0 and m and n are each at least 1. The therapeutic drug moiety is preferably a therapeutic protein or peptide, preferably insulin or a functional equivalent thereof.
Owner:BIOCON LTD
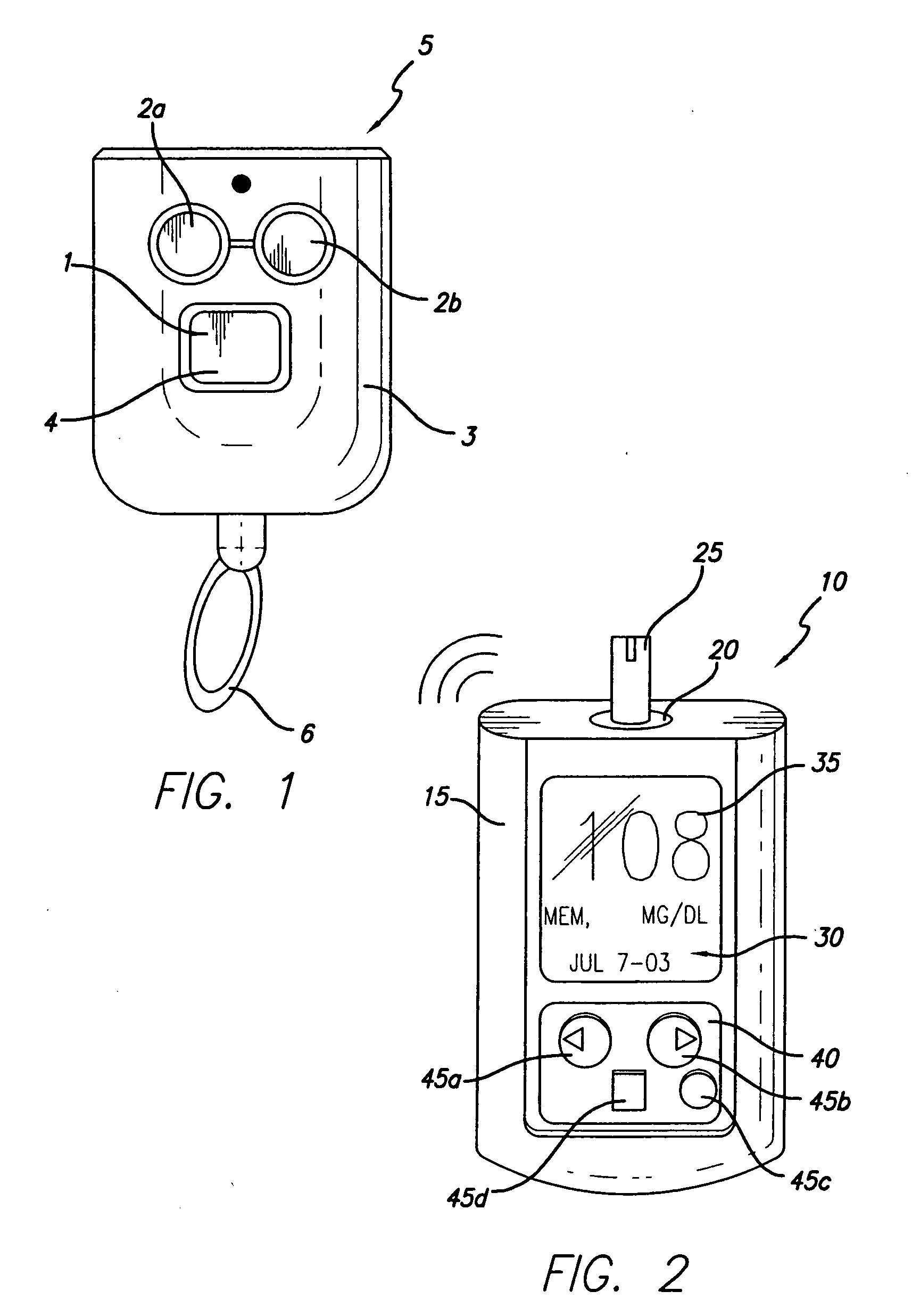
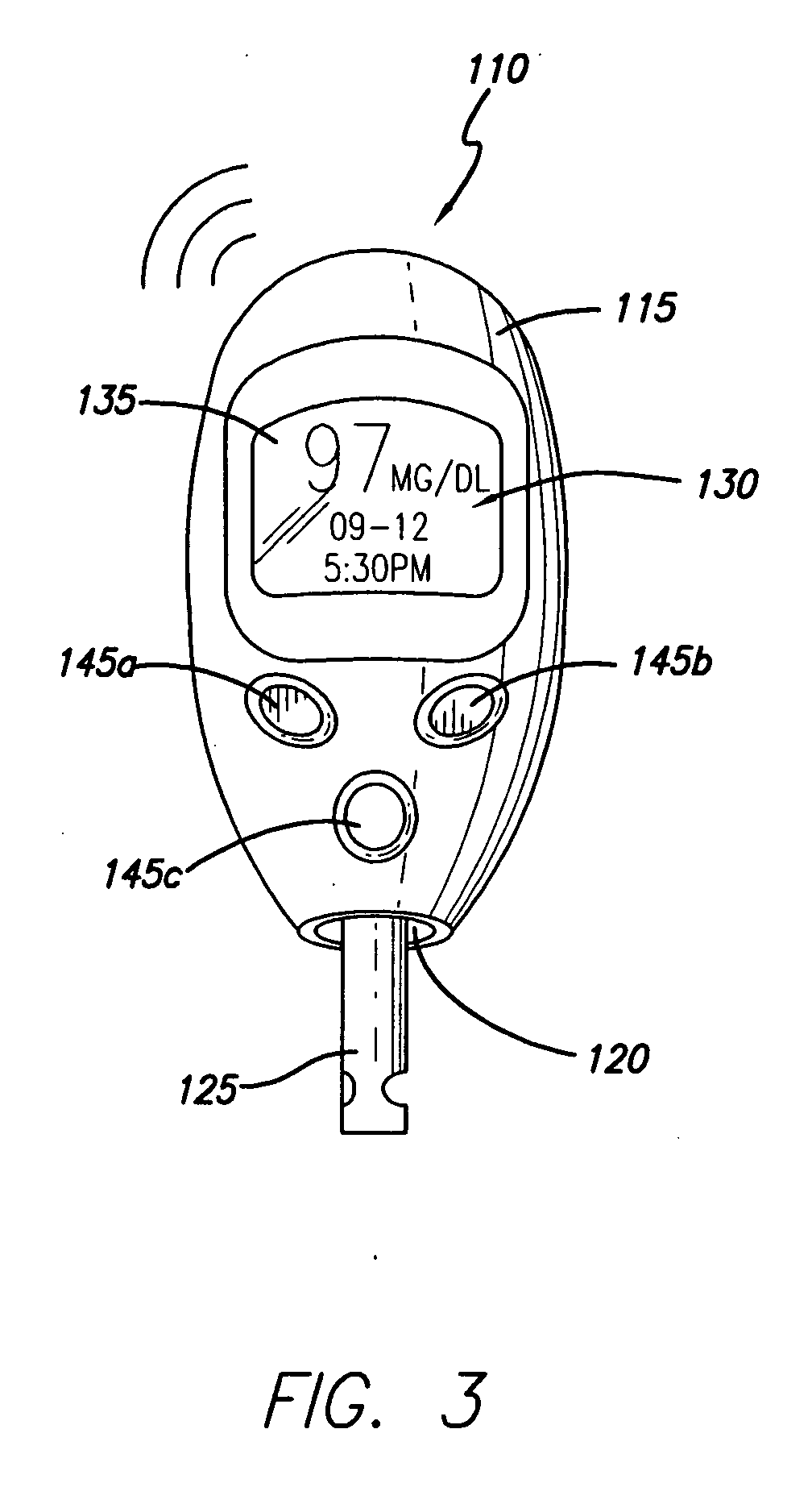
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
Owner:EMBECTA CORP
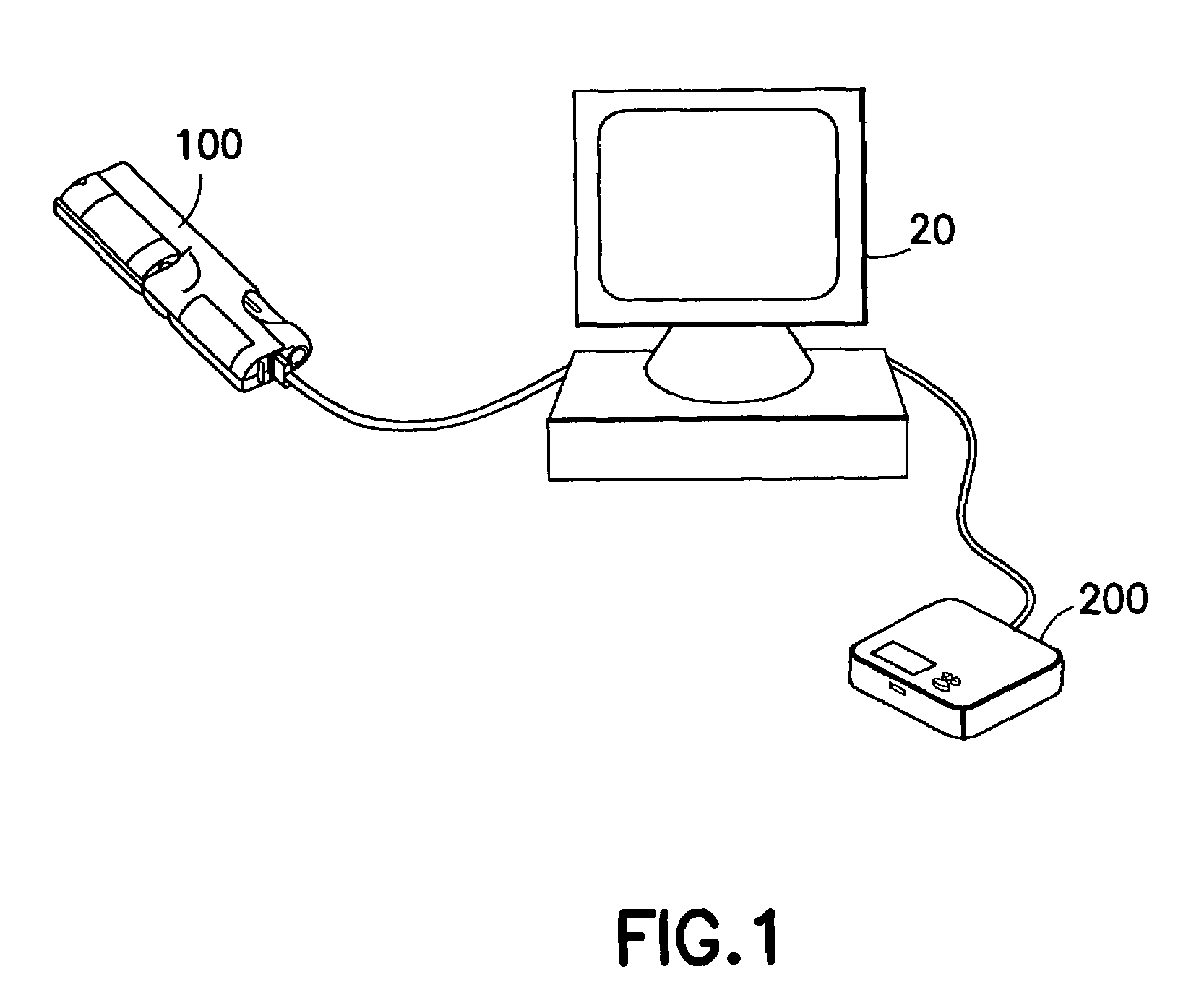
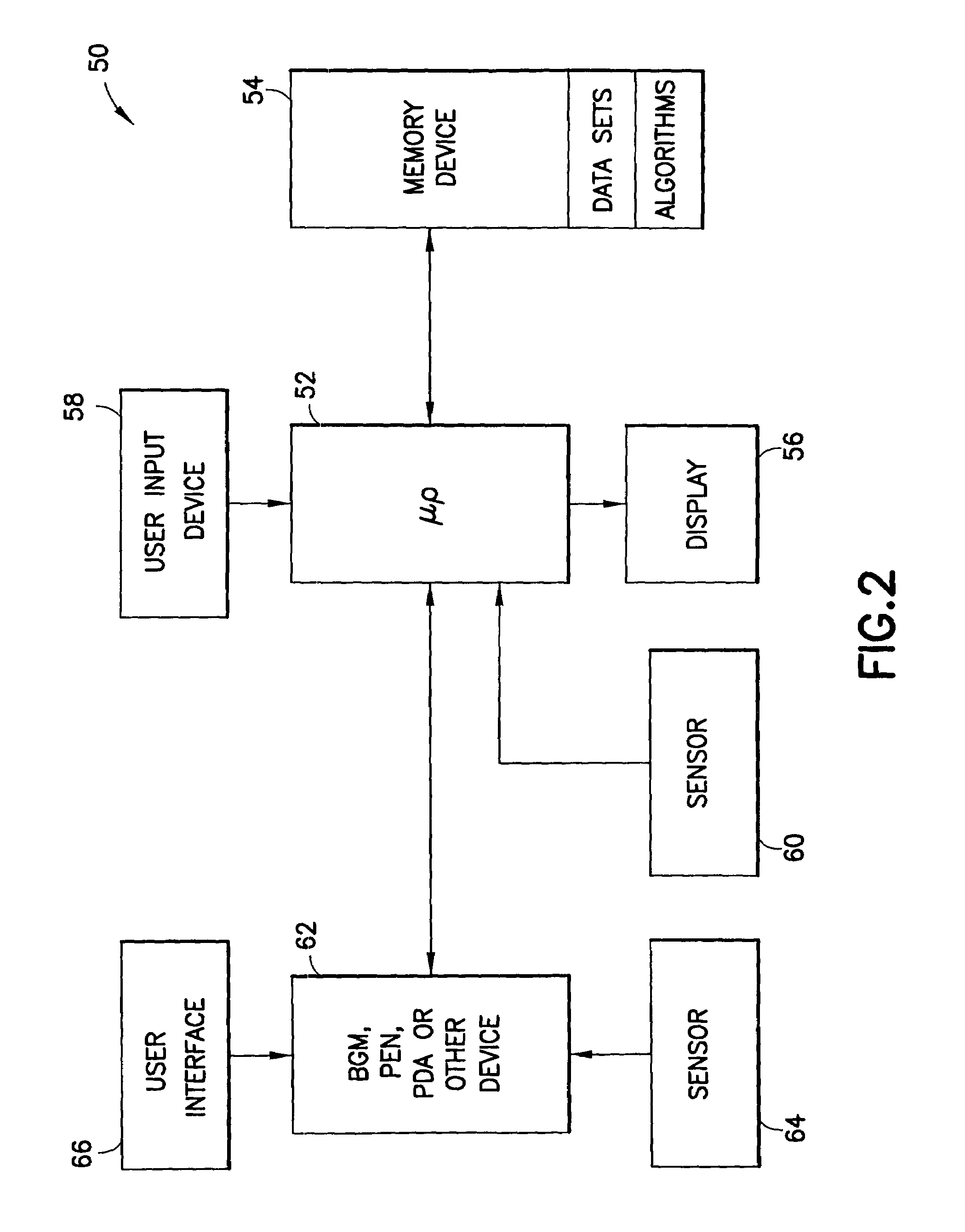
System and method for measuring and predicting insulin dosing rates
InactiveUS20070078314A1Efficient managementDrug and medicationsMedical automated diagnosisPatient dataGlucose polymers
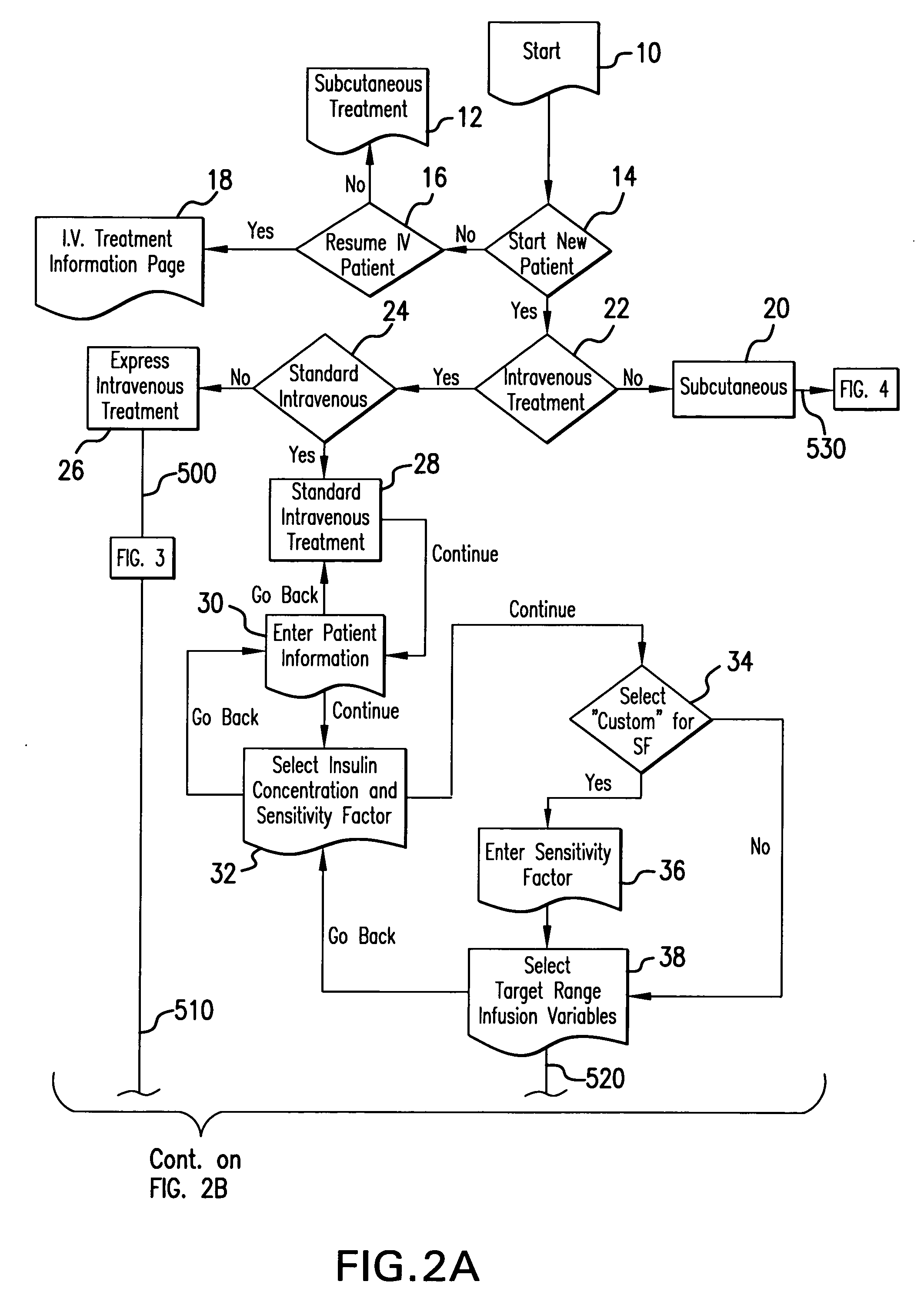
The method and system for managing a patient's blood glucose level predicts an insulin dosing rate to bring a patient's blood glucose level into a preferred target range within a predetermined time interval. The system includes a processor which actuates a blood glucose computer program to measure and predict the patient's blood glucose level. An input mechanism allows for insertion of a preferred target range of the patient's blood glucose level and further permits input of various patient data parameters. The processor calculates the optimum insulin dosing rate for the patient based upon the type of insulin dosing whether it be intravenous dosing and / or subcutaneous dosing. A display mechanism displays the patient dosing parameters and an alarm mechanism alerts a user when the patient's blood glucose level is outside of the preferred patient blood glucose target range.
Owner:GLUCOTEC
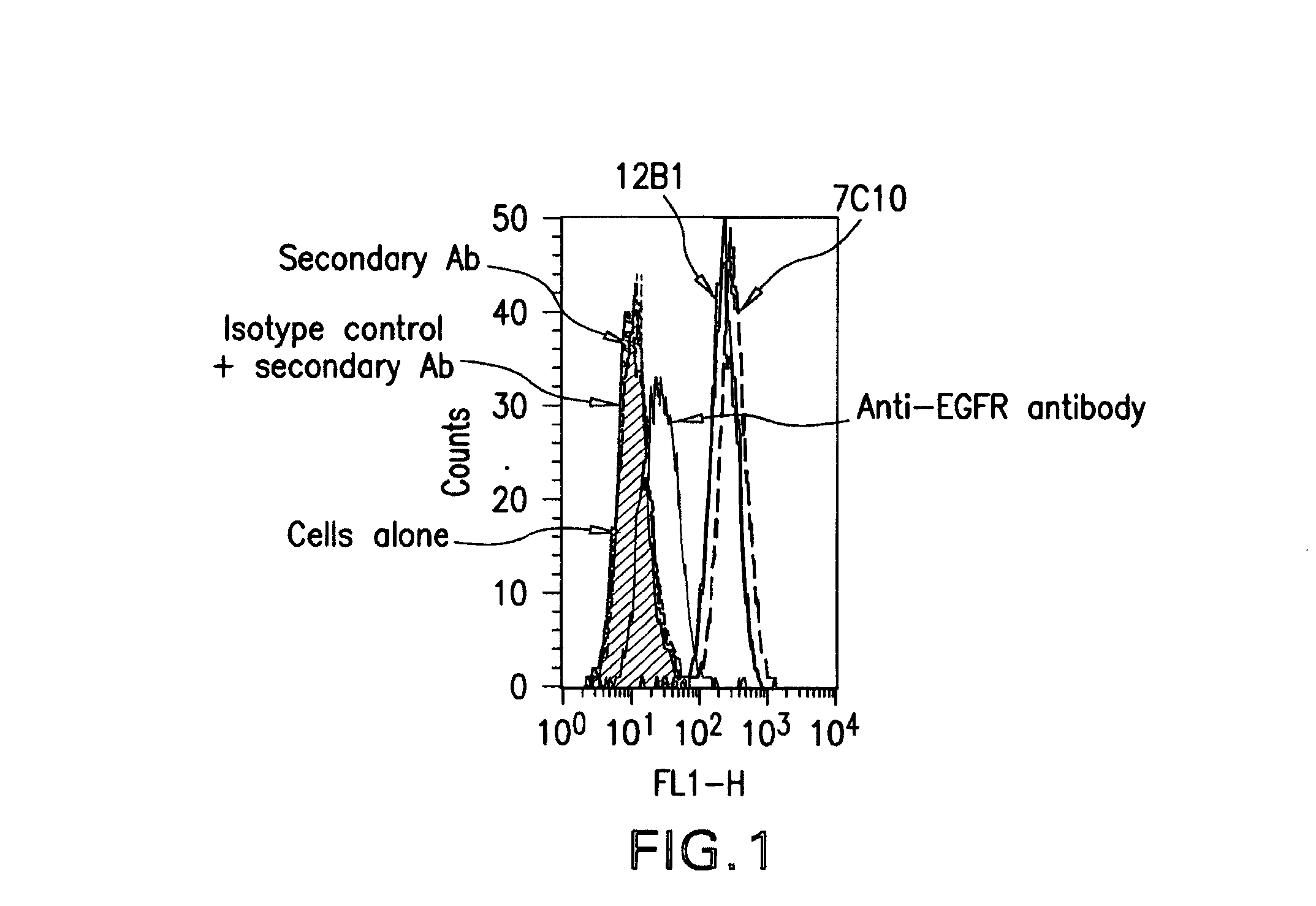
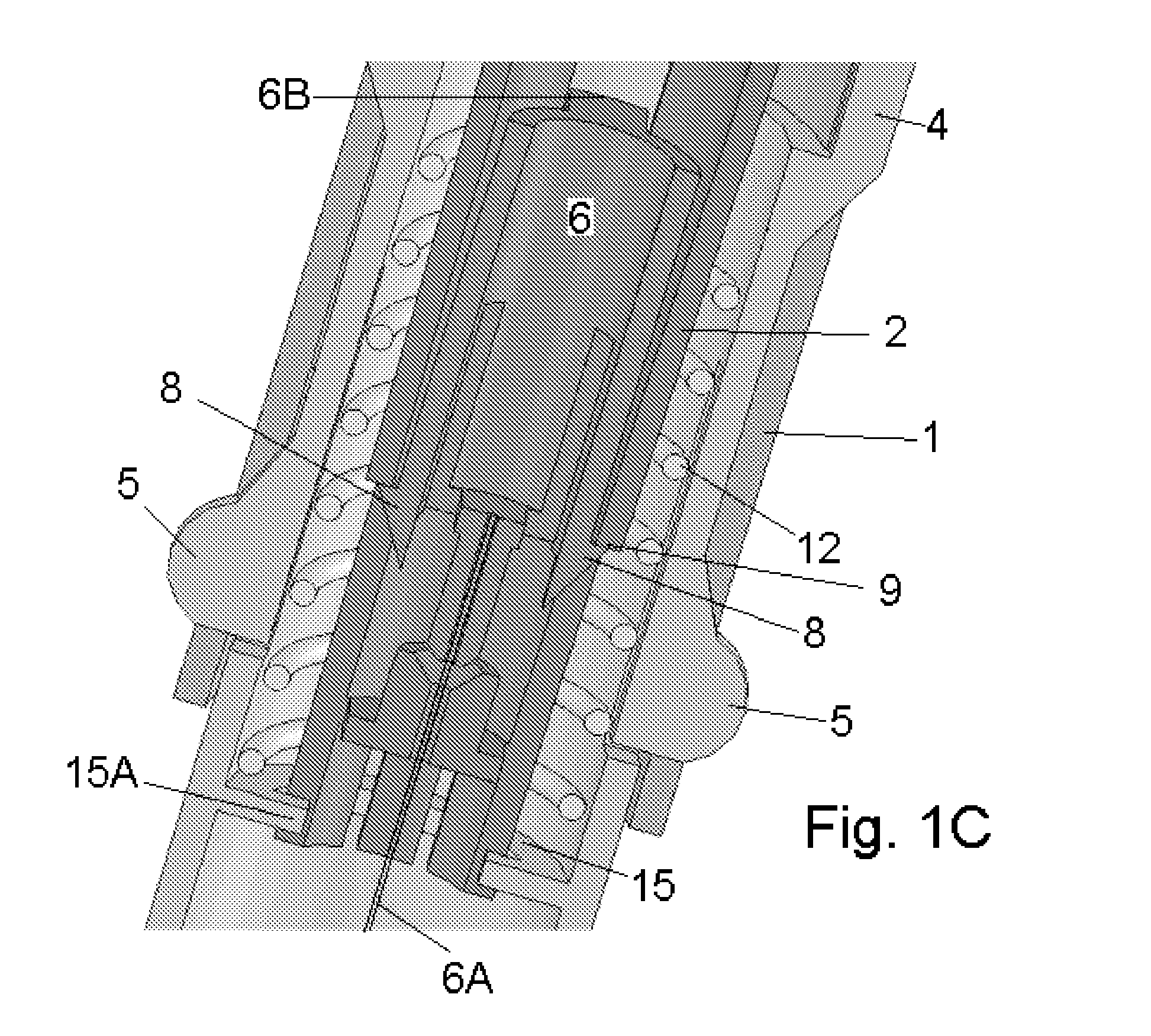
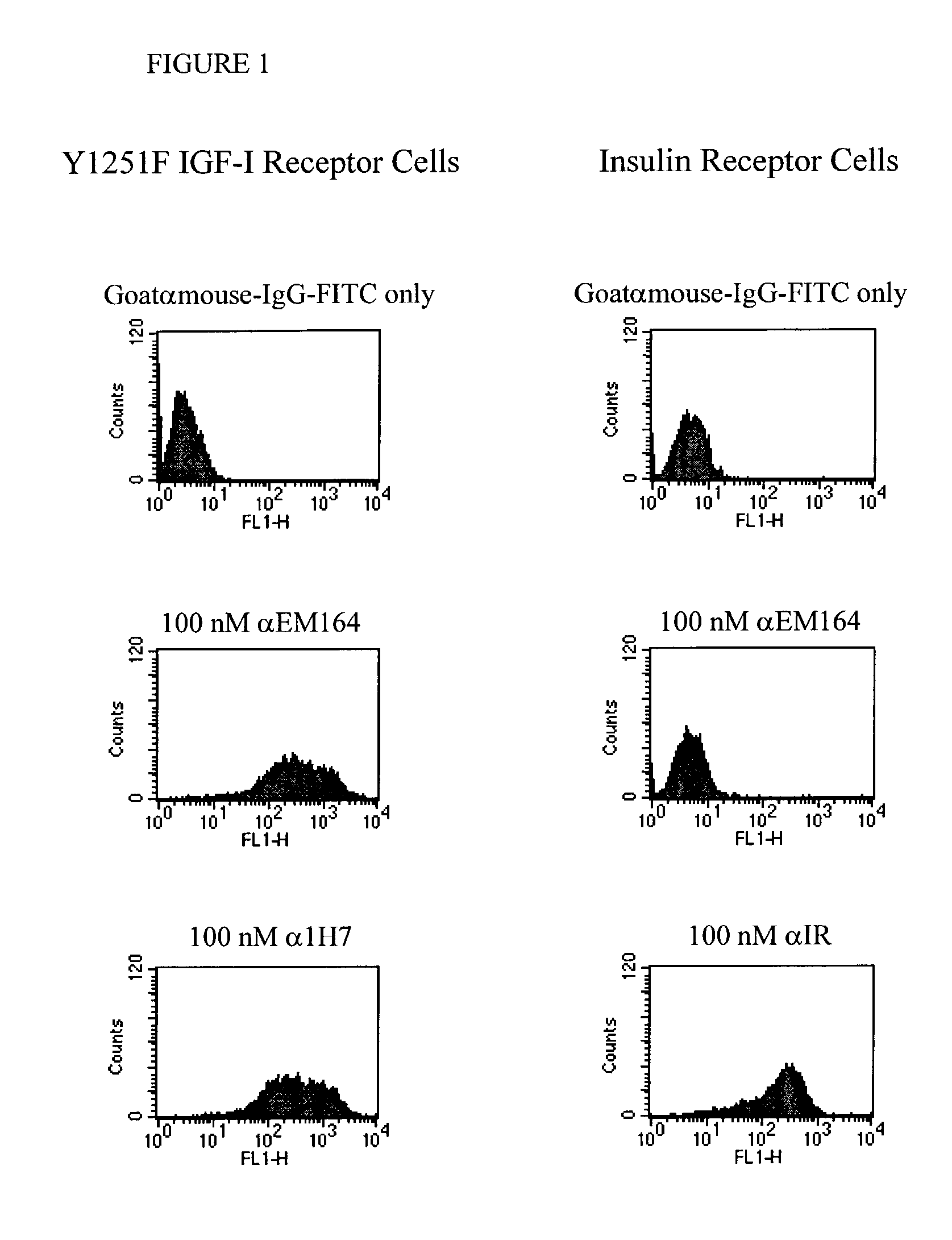
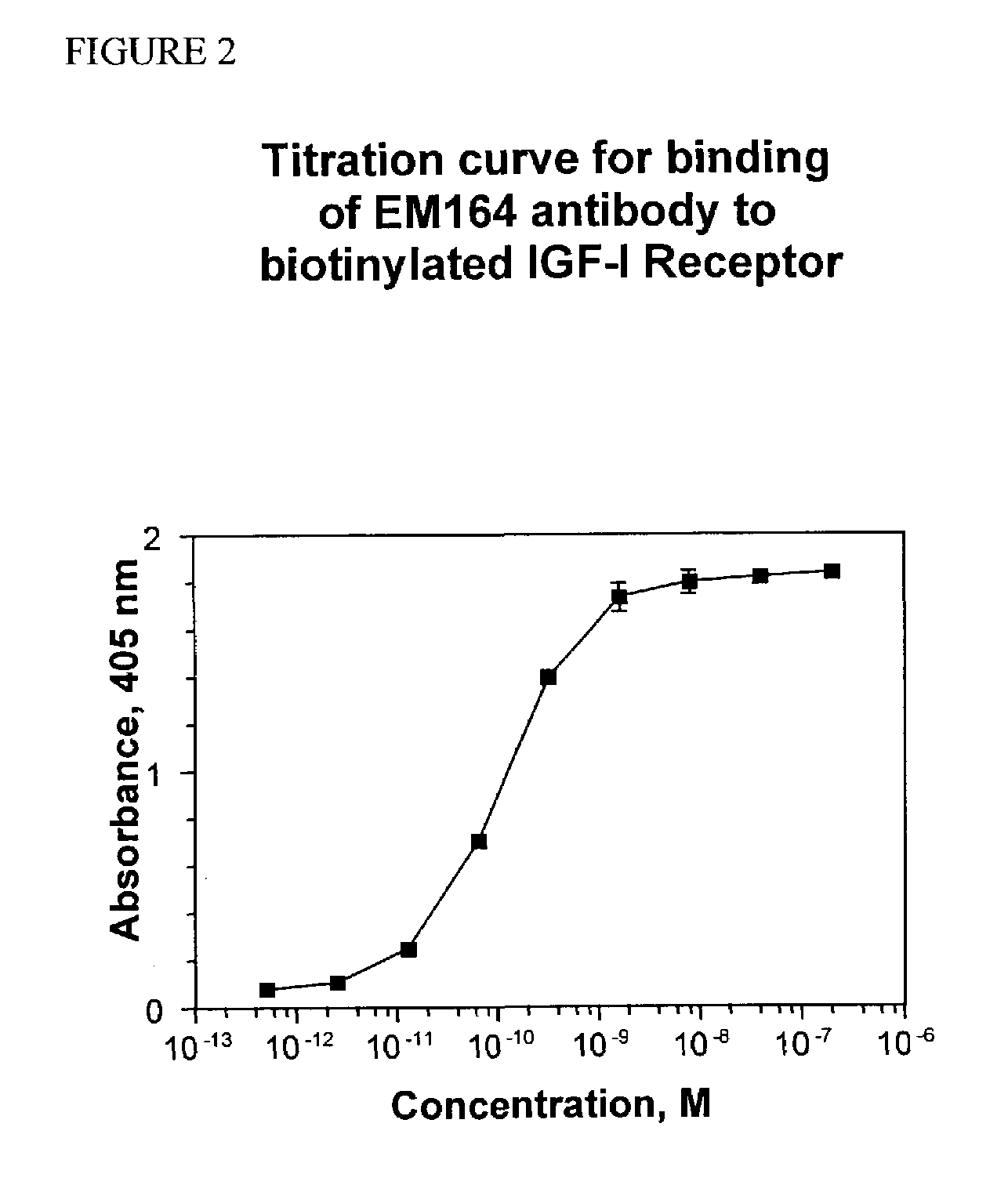
Anti-IGF-I receptor antibody
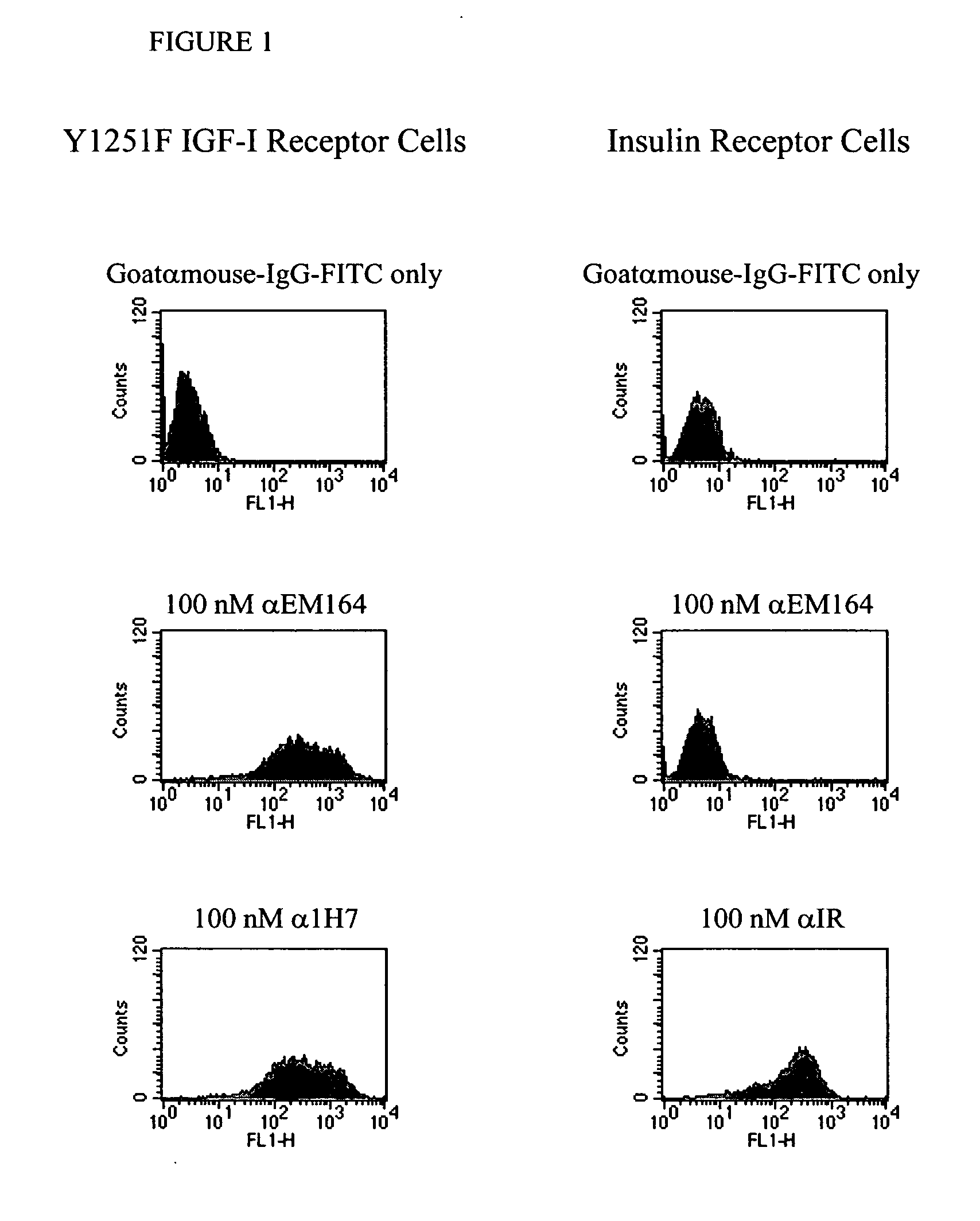
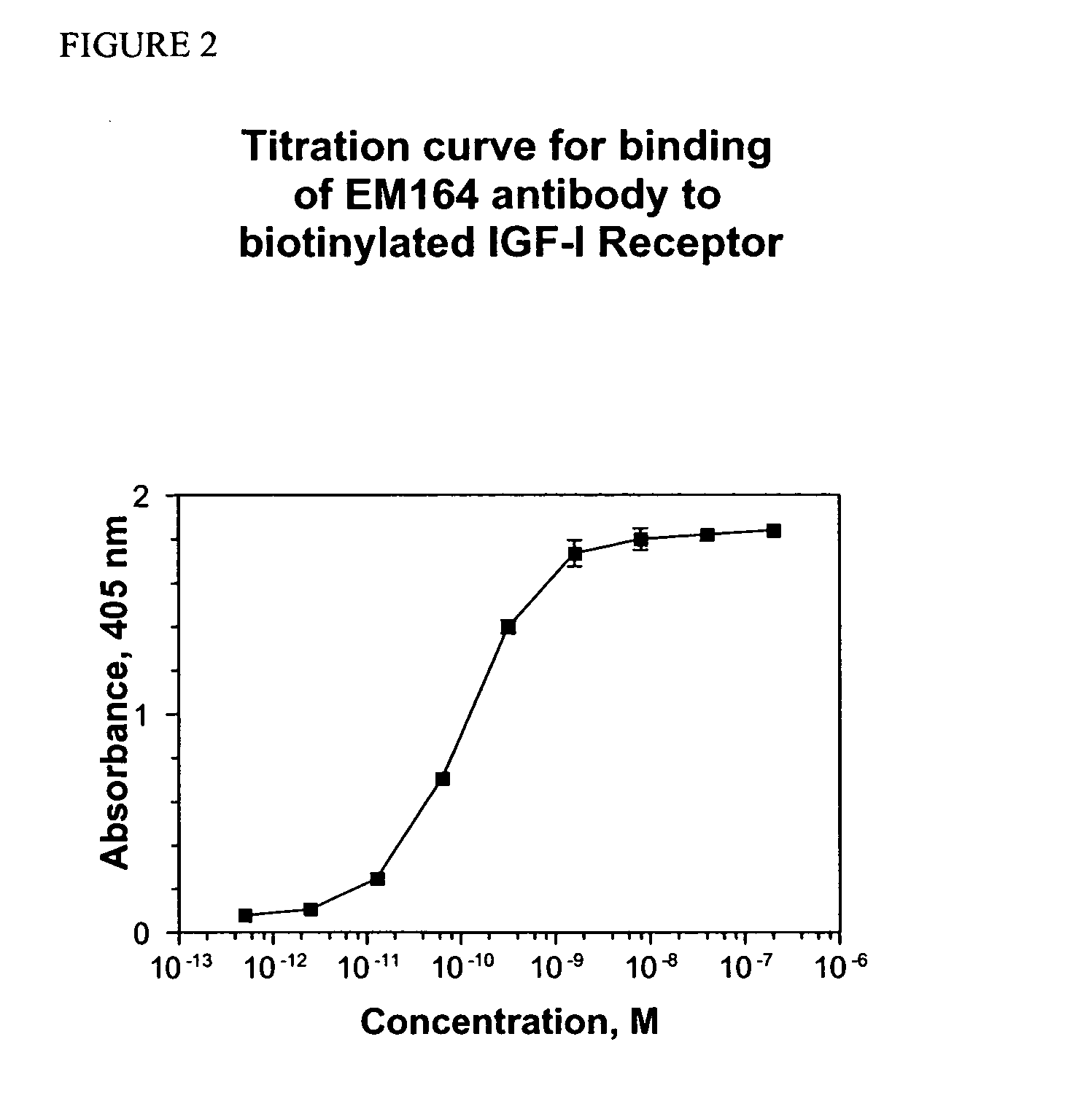
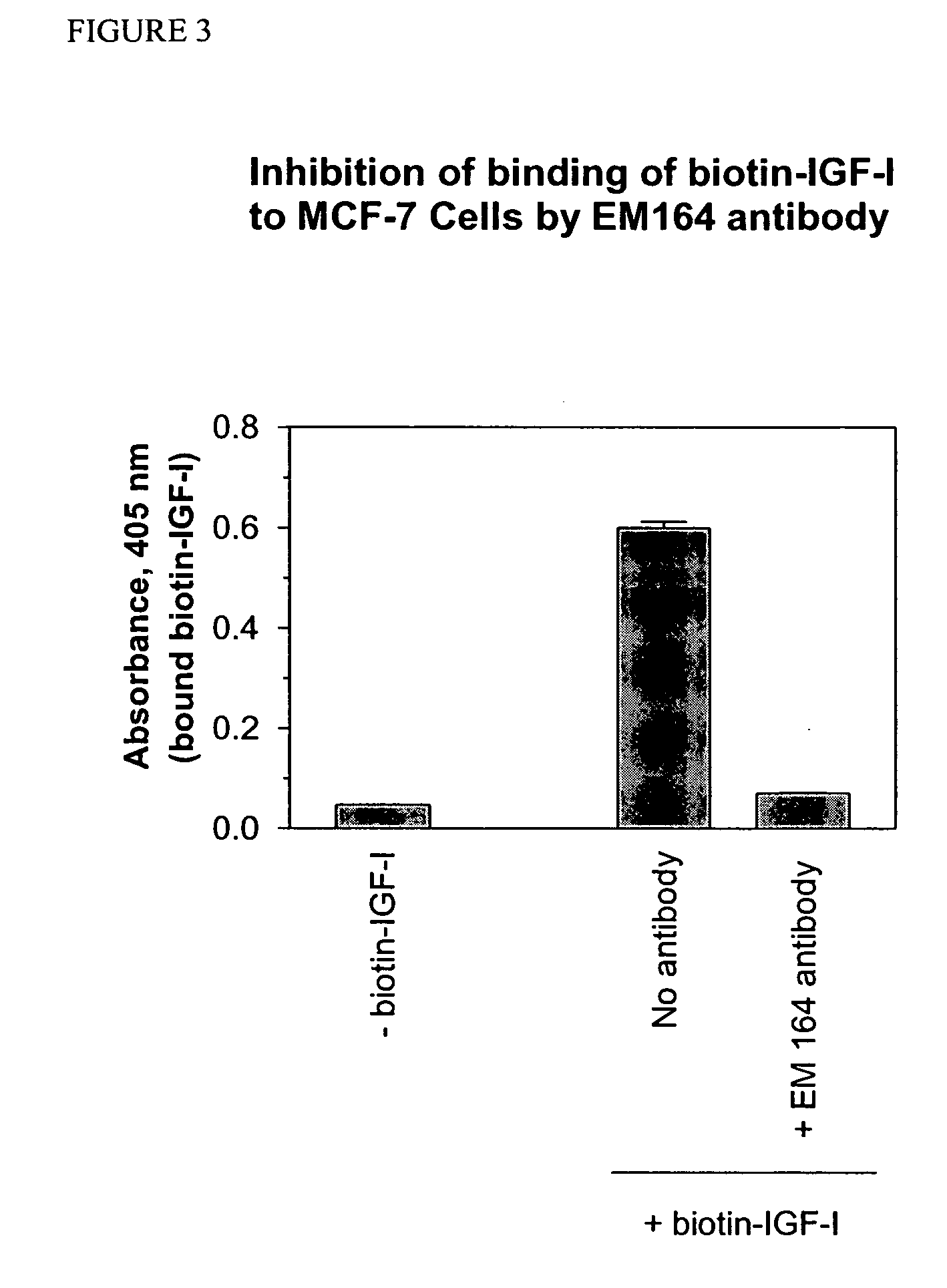
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of same with cytotoxic agents, which specifically bind to, and inhibit, insulin-like growth factor-I receptor, antagonize the effects of IGF-I, IGF-II and serum on the growth and survival of tumor cells, and which are substantially devoid of agonist activity. Said antibodies and fragments thereof may be used, optionally in conjunction with other therapeutic agents, in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma, prostate cancer and pancreatic cancer, and said derivatized antibodies may be used in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
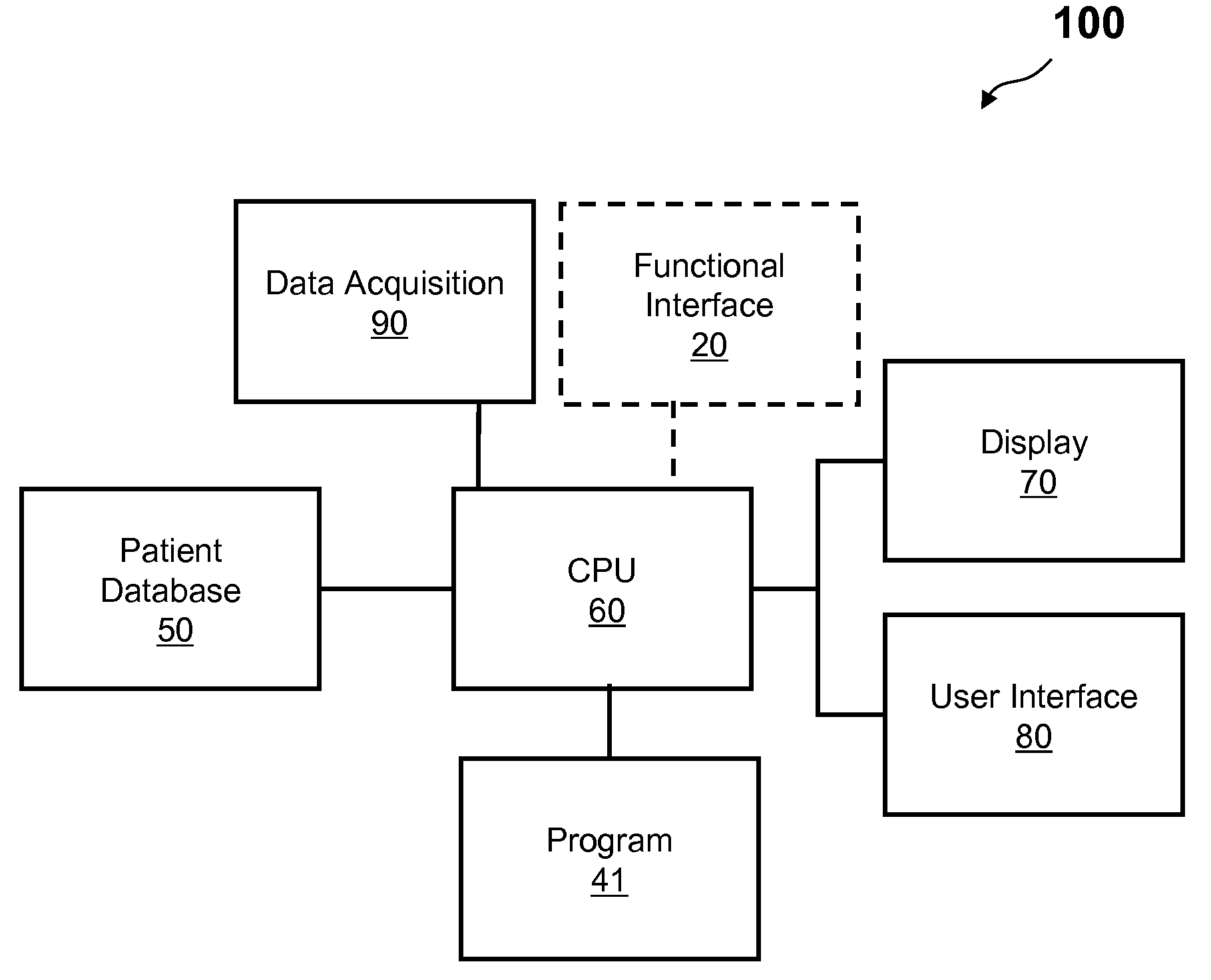
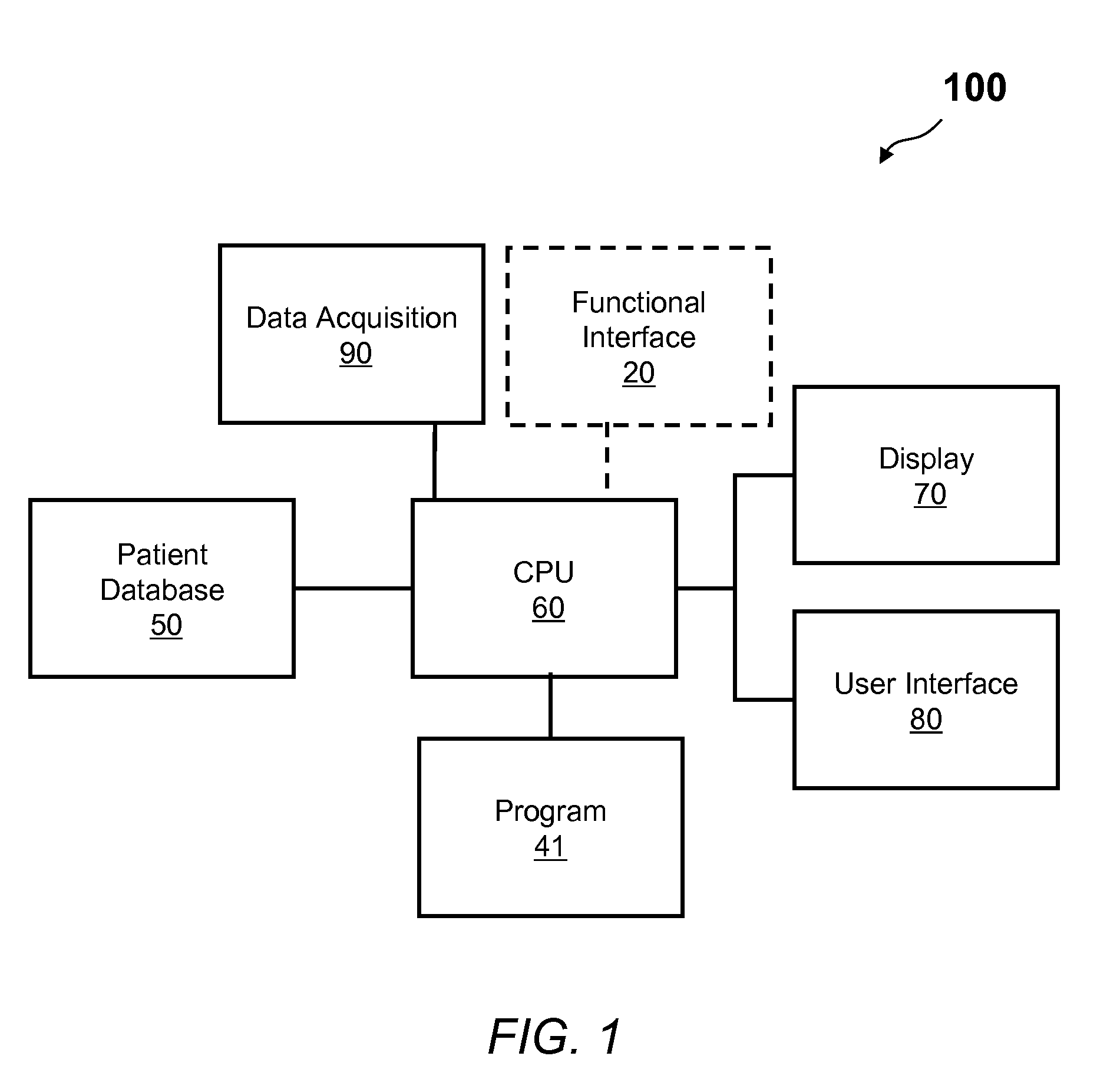
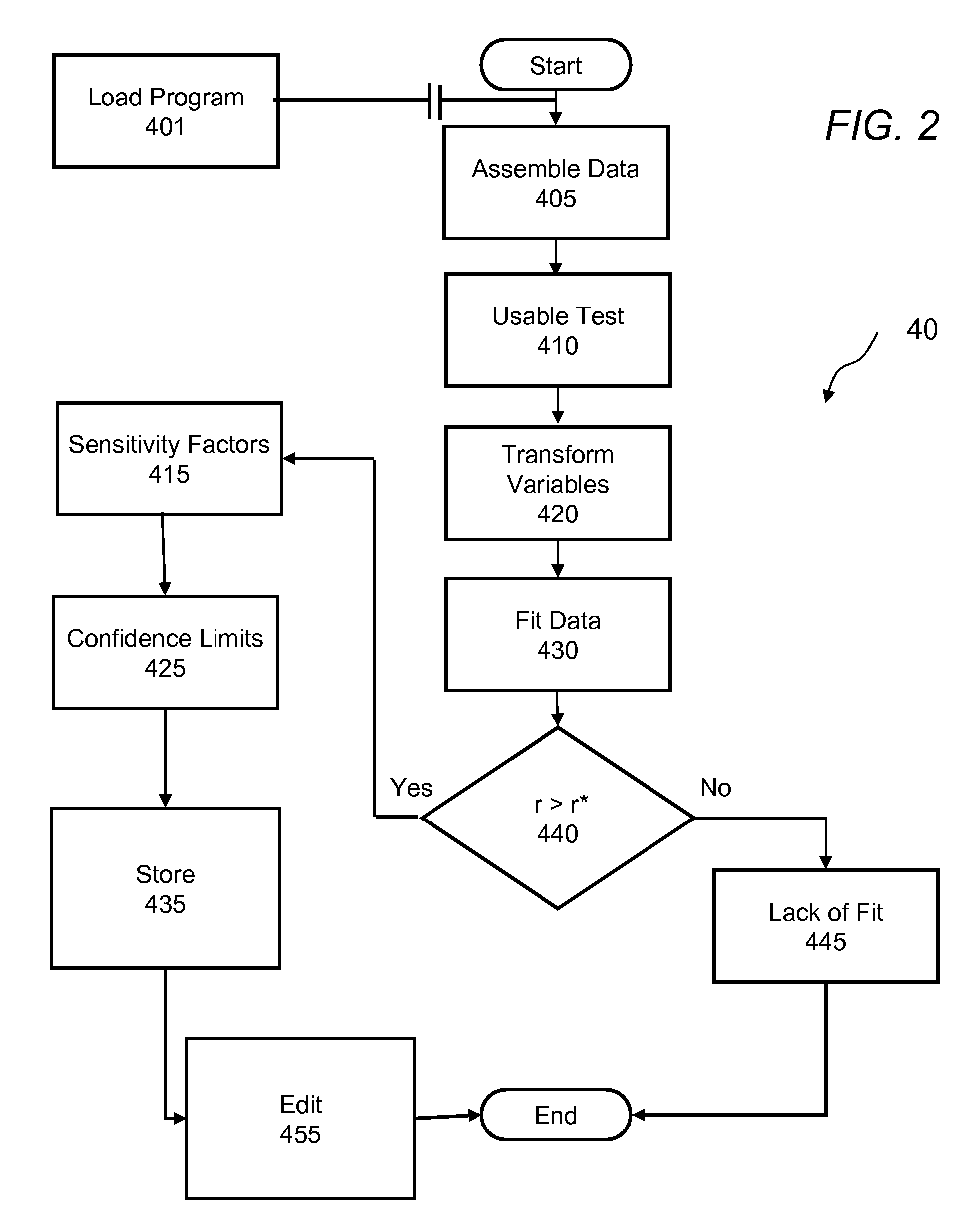
Method and apparatus to calculate diabetic sensitivity factors affecting blood glucose
InactiveUS20100262434A1Reasonable expectationPhysical therapies and activitiesDrug and medicationsGlucose polymersD-Glucose
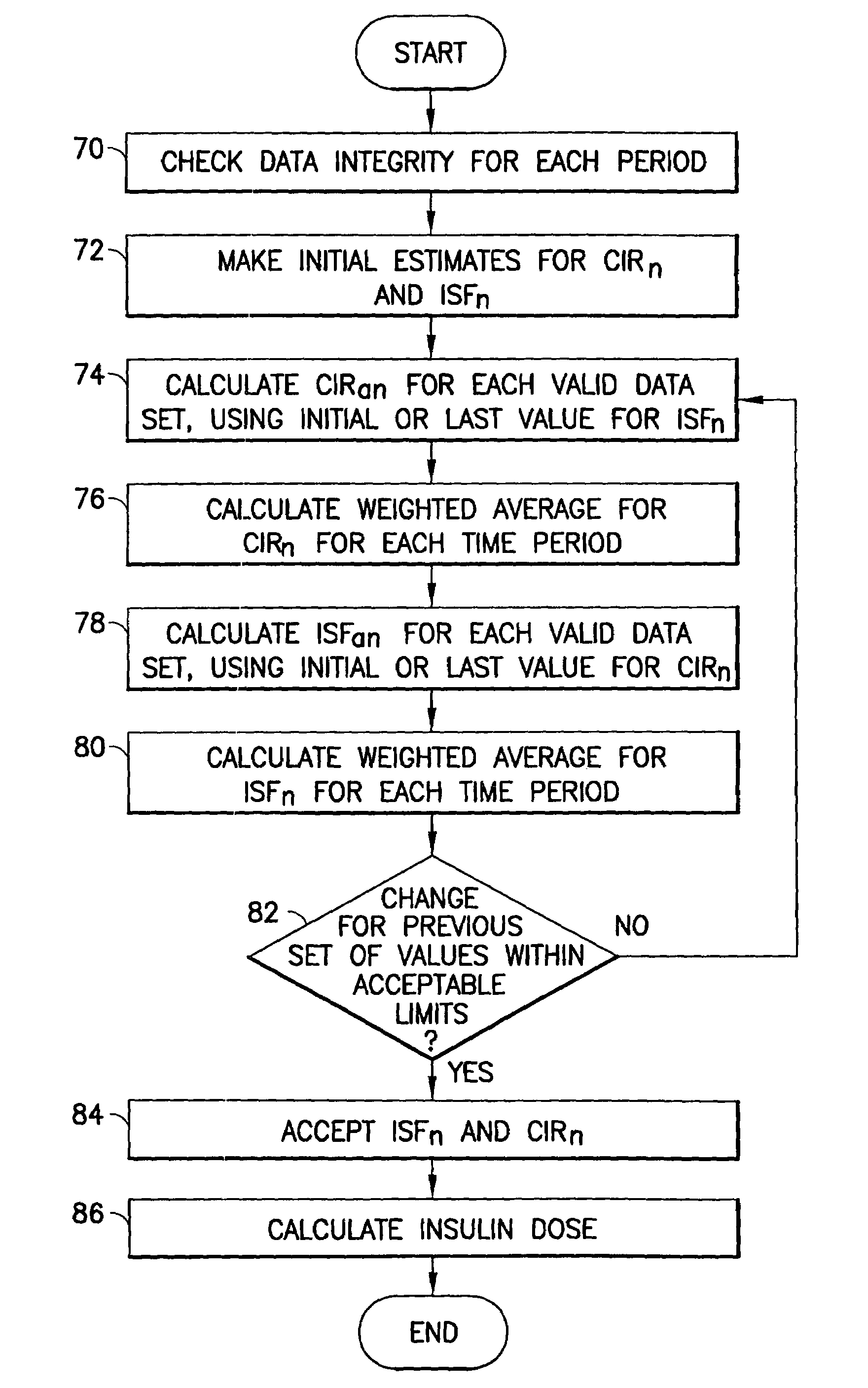
Methods and apparatus are provided for determining a diabetic patient's carbohydrate to insulin ratio (CIR), carbohydrate to blood glucose ratio (CGR), and insulin sensitivity factor (ISF) using the patient's record of blood glucose readings, carbohydrate consumption and insulin doses. The method provides the sensitivity factors that best account for the patient's observed blood glucose changes by linear regression of appropriately transformed variables. An apparatus that can collect and store the blood glucose readings, insulin dosages, and carbohydrate intake data and process these data according to this invention can generate statistically characterized sensitivity factors to advise the diabetic patient on optimal bolus insulin dosages.
Owner:SHAYA STEVEN A
Igf-1r specific antibodies useful in the detection and diagnosis of cellular proliferative disorders
InactiveUS20130084243A1High affinityUseful in detectionAnimal cellsIn-vivo radioactive preparationsDiseaseSingle-Chain Antibodies
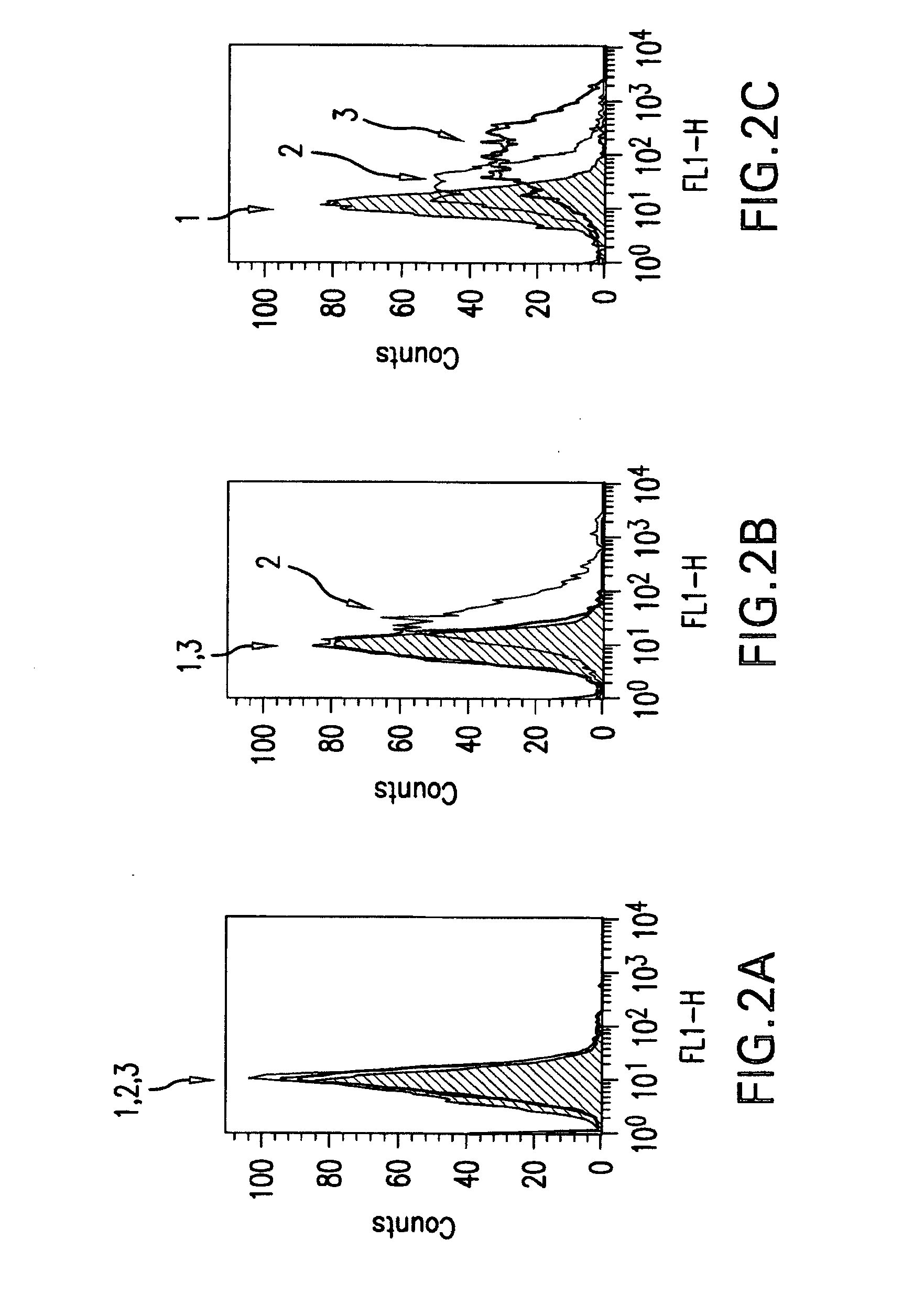
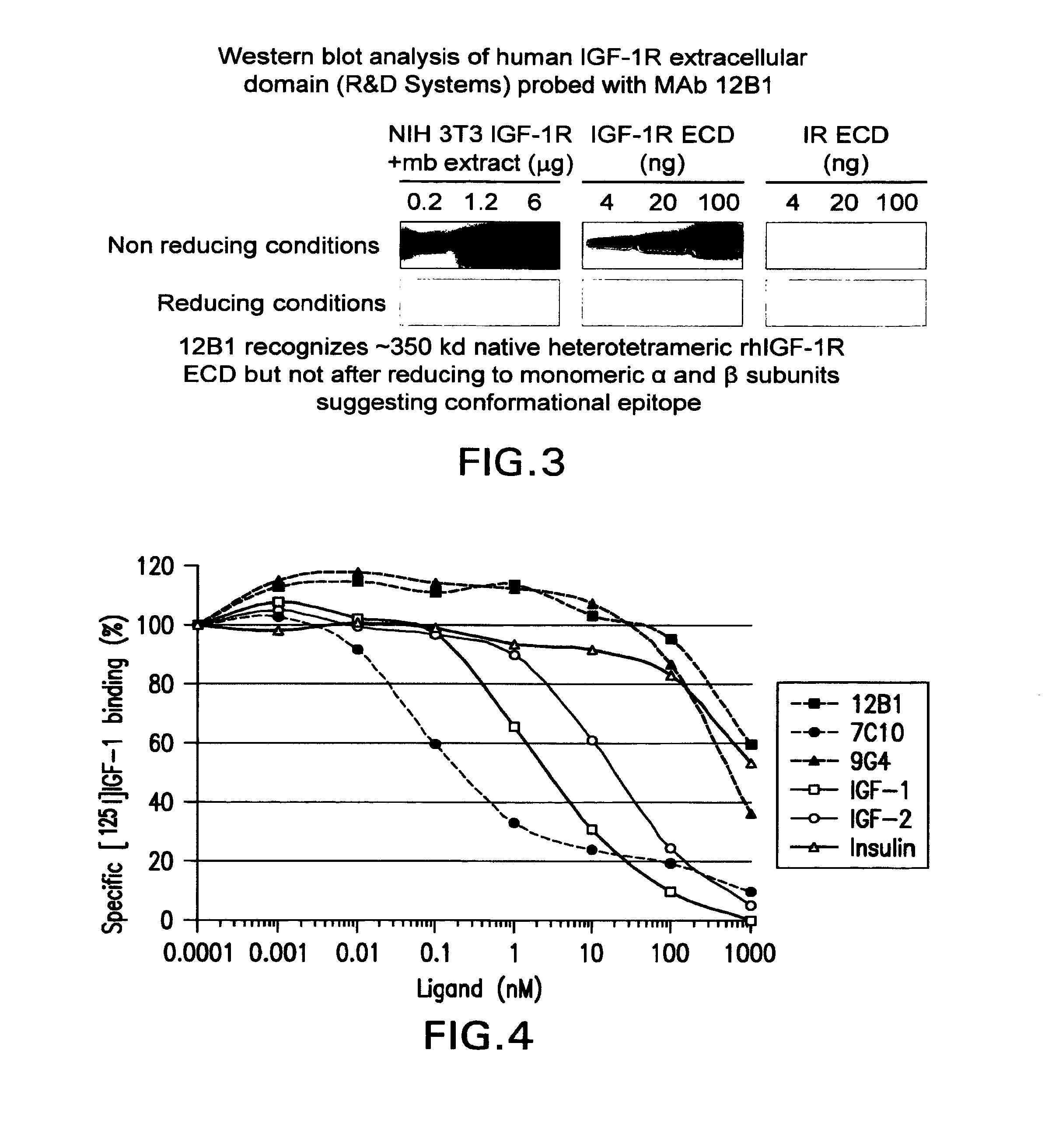
The present invention relates to mammalian antibodies, designated 12B1 and antigen-binding portions thereof that specifically bind to insulin-like growth factor I receptor (IGF-IR), preferably human IGF-IR. Also included are chimeric, bispecific, derivatized, single chain antibodies derived from the antibodies disclosed herein. Nucleic acid molecules encoding the mammalian antibodies as well as methods of use thereof are also disclosed. Also included are pharmaceutical compositions comprising these antibodies and methods of using the antibodies and compositions thereof for treatment and diagnosis of pathological hyperproliferative oncogenic disorders associated with expression of IGf-1R.
Owner:GOETSCH LILIANE +4
Autism treatment
InactiveUS20120128683A1Treat and prevent associated lossMinimally invasiveNervous disorderPeptide/protein ingredientsMedicineNose
A safe and effective treatment to curtail and cure autism spectrum disorders has been described in this invention using insulin, IGF-1, with multiple known adjuvant therapeutic agents, as well as other pharmaceutical, biochemical, nurticeuticals, and biological agents or compounds delivered through the olfactory mucosal region of the nose and external auditory meatus.
Owner:SHANTHA TOTADA R
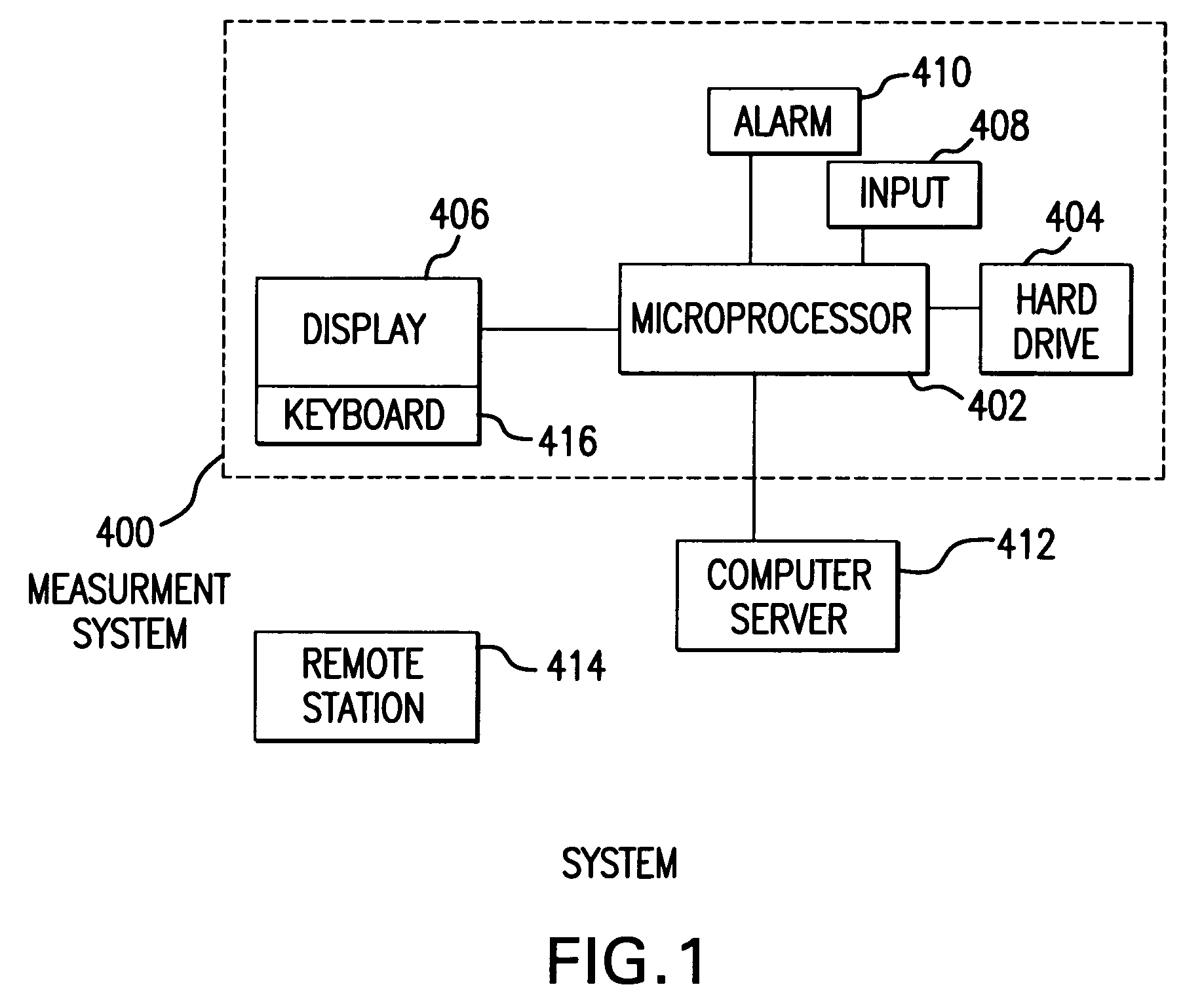
Medical Data Display
InactiveUS20100259543A1Quality is easy to controlMedical simulationDrawing from basic elementsData displayBlood Glucose Measurement
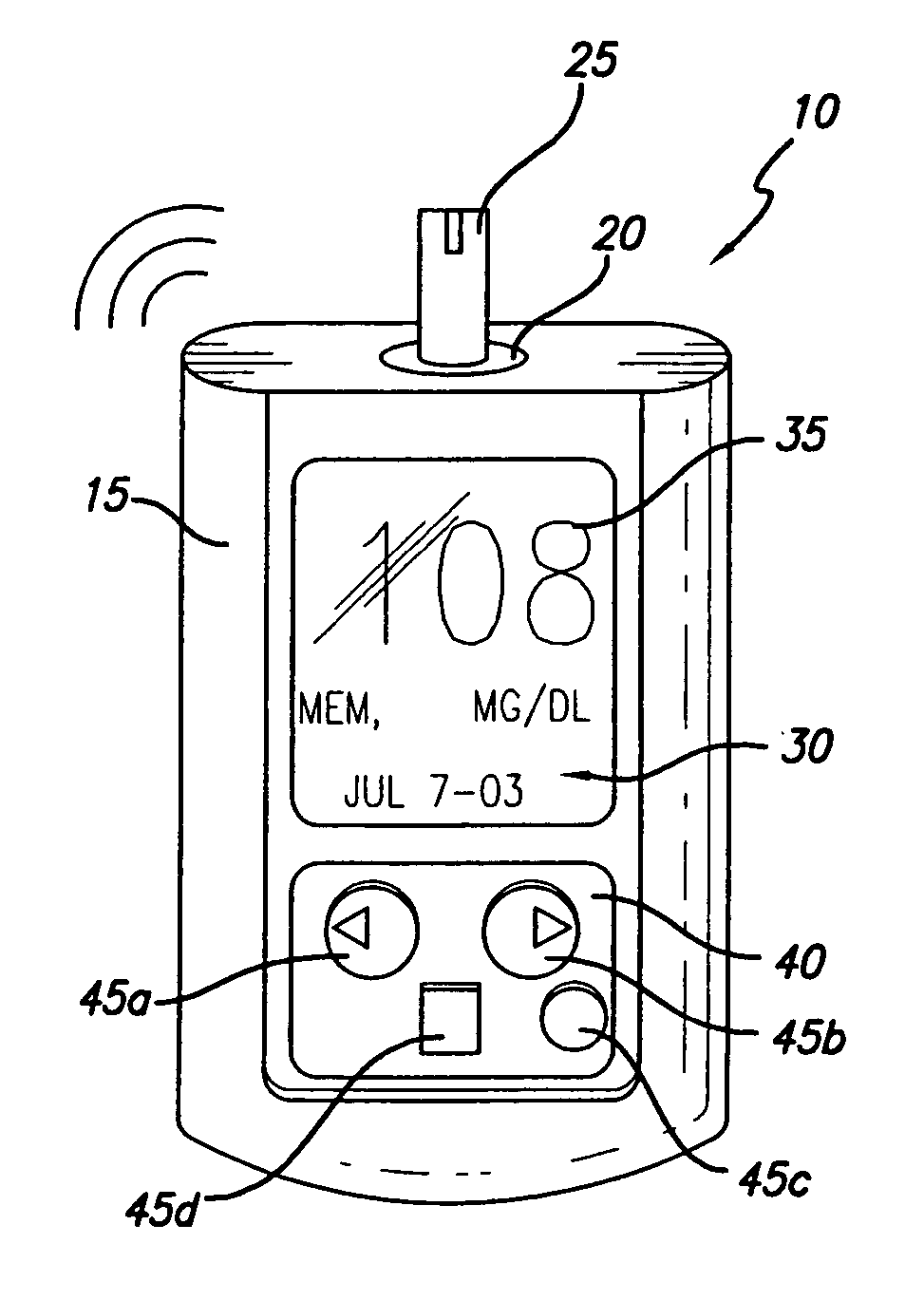
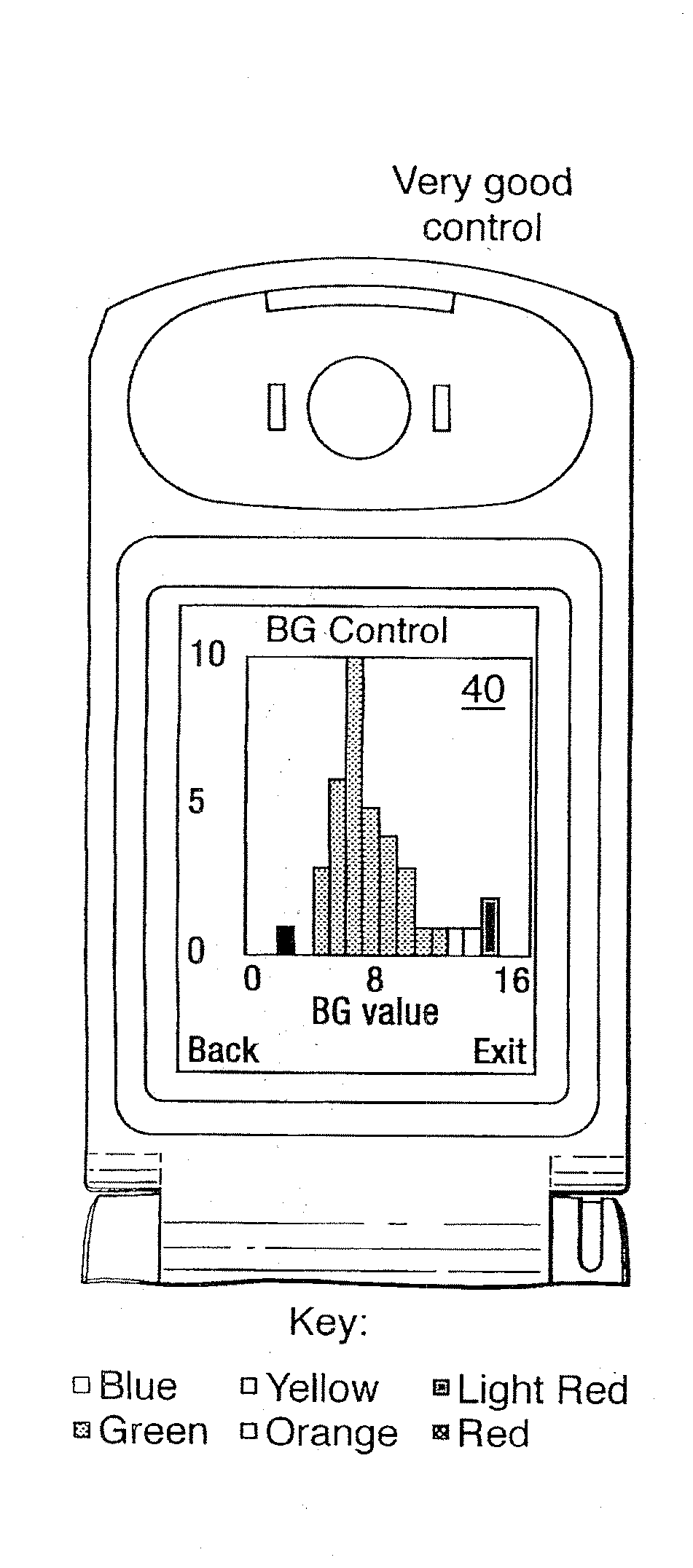
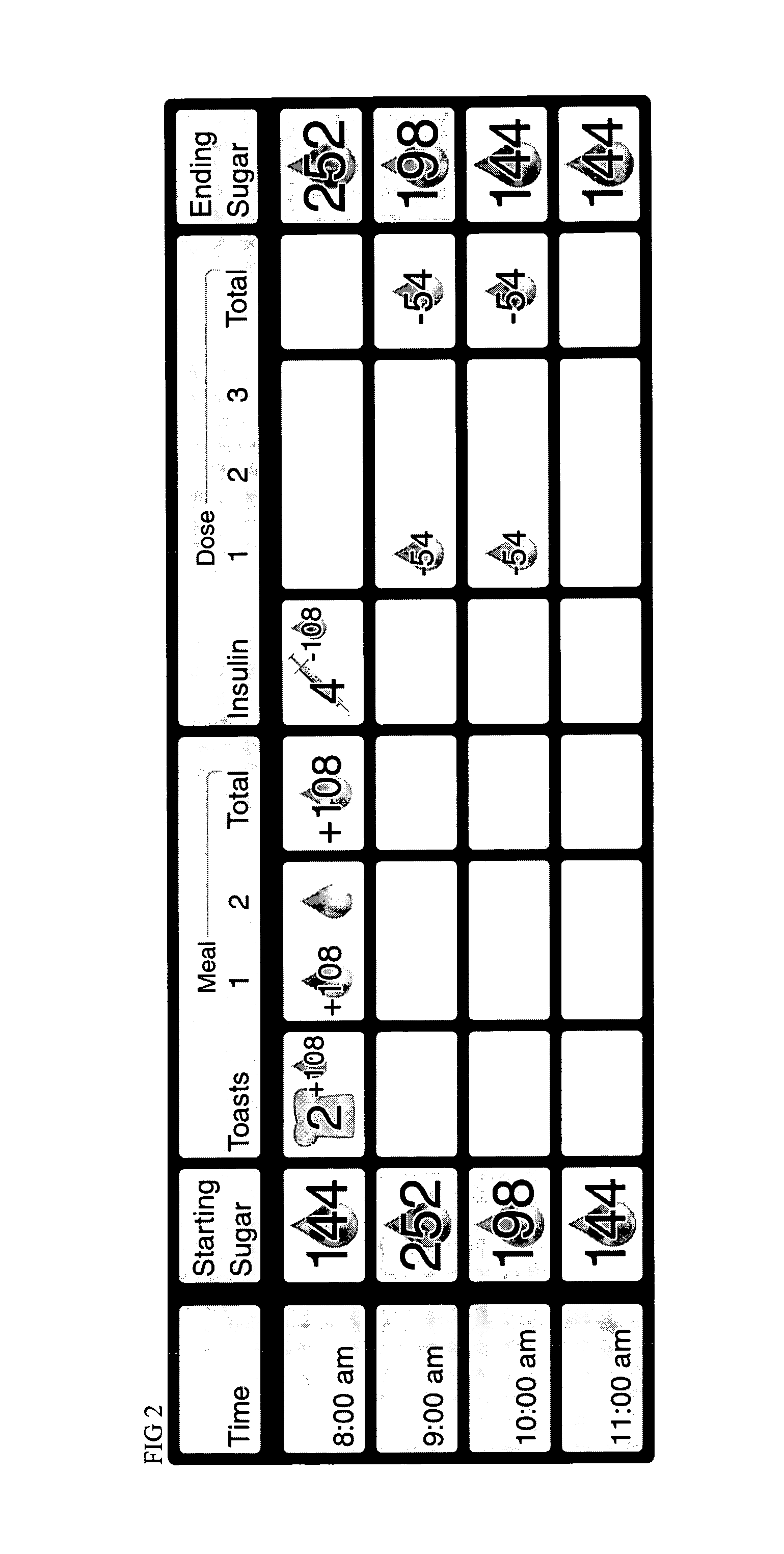
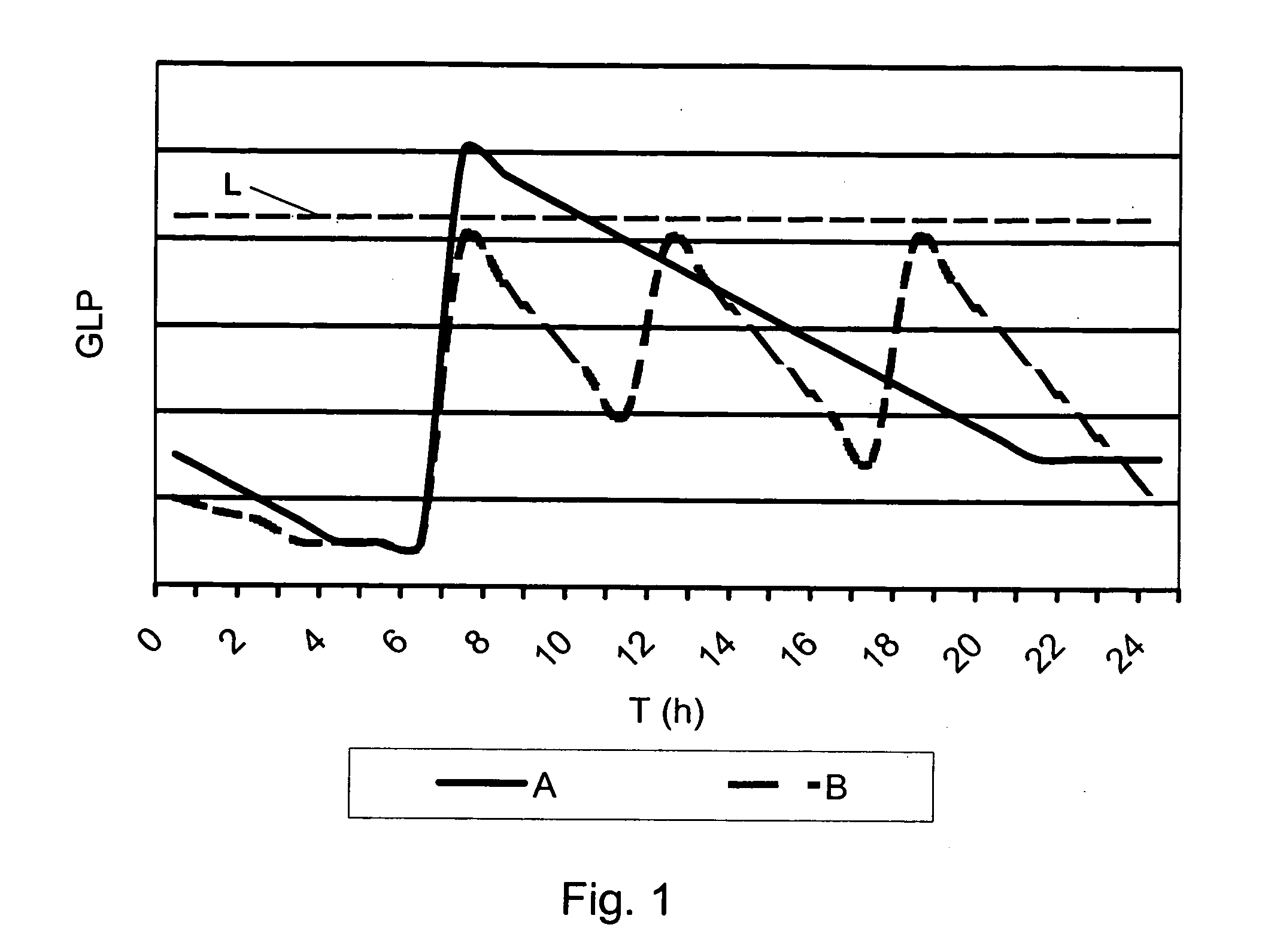
A method of displaying medical data, particularly data representative of the condition of patients suffering from chronic medical conditions such as asthma, diabetes and hypertension. The display consists of two graphical elements, one of which indicates the current value of a parameter indicative of the patient's condition, this being displayed against another graphical element which represents a model of normality for that patient. The graphical element indicating the current condition may be, for example, a needle, against a scale which is constructed according to the patient-specific model of normality. This is particularly advantageous in the case of displays which have a small display area, such as mobile telephones and PDAs. Other forms of display are disclosed, such as histograms with the display being dynamically colour-coded and auto-scaled, or displays including limits which may vary. Another form of display is also disclosed which illustrates administrations of a pharmacological agent and corresponding measurements of the patient's condition, with a visual link such as colour-coding linking the administration to the corresponding condition measurement. For example several days of insulin administration dosages may be displayed alongside several days of blood glucose measurements, with the administrations colour-coded to the corresponding blood glucose measurement, to assist the patient in determining whether the insulin administration is stably controlling their condition.
Owner:E SAN LTD
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonist peptides may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Method of food and insulin dose management for a diabetic subject
InactiveUS7137951B2Lower blood sugar levelsReduce the amount requiredPeptide/protein ingredientsDrug and medicationsINSULIN USEInsulin dose
The invention relates to a method of food and insulin dose management for a diabetic subject, comprising:providing an intended insulin unit value or an intended carbohydrate unit value representing the amount of insulin or carbohydrate intended for intake by the subject; anddetermining the balance value of either insulin units or carbohydrate units needed to balance with the provided unit value and maintain blood sugar in the subject in a target blood sugar range.
Owner:PILARSKI JOSEPH
Multiple canopy
InactiveUS6537249B2Improve reliabilityReduce manufacturing costSurgeryMedical devicesStored energyMorphine
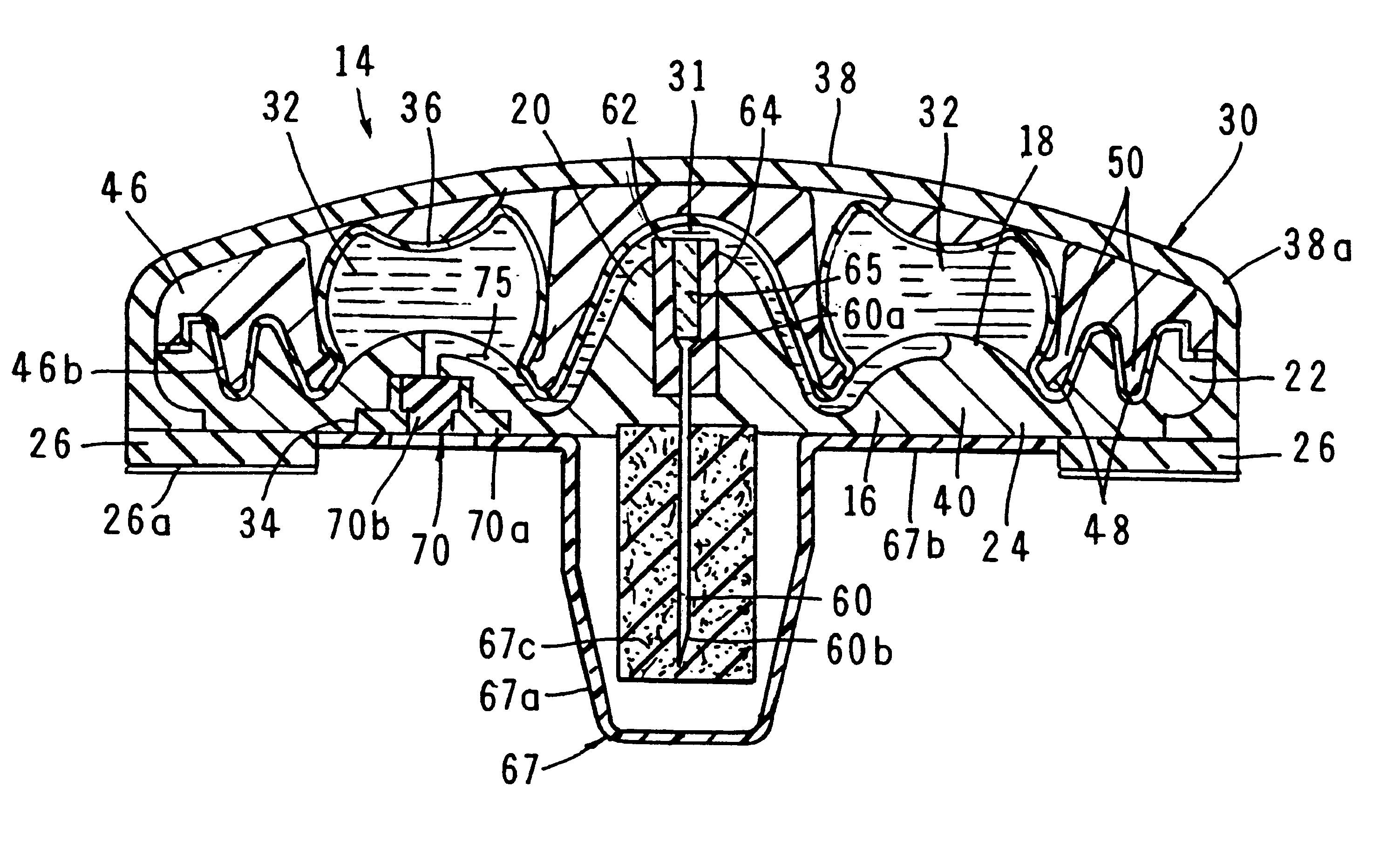
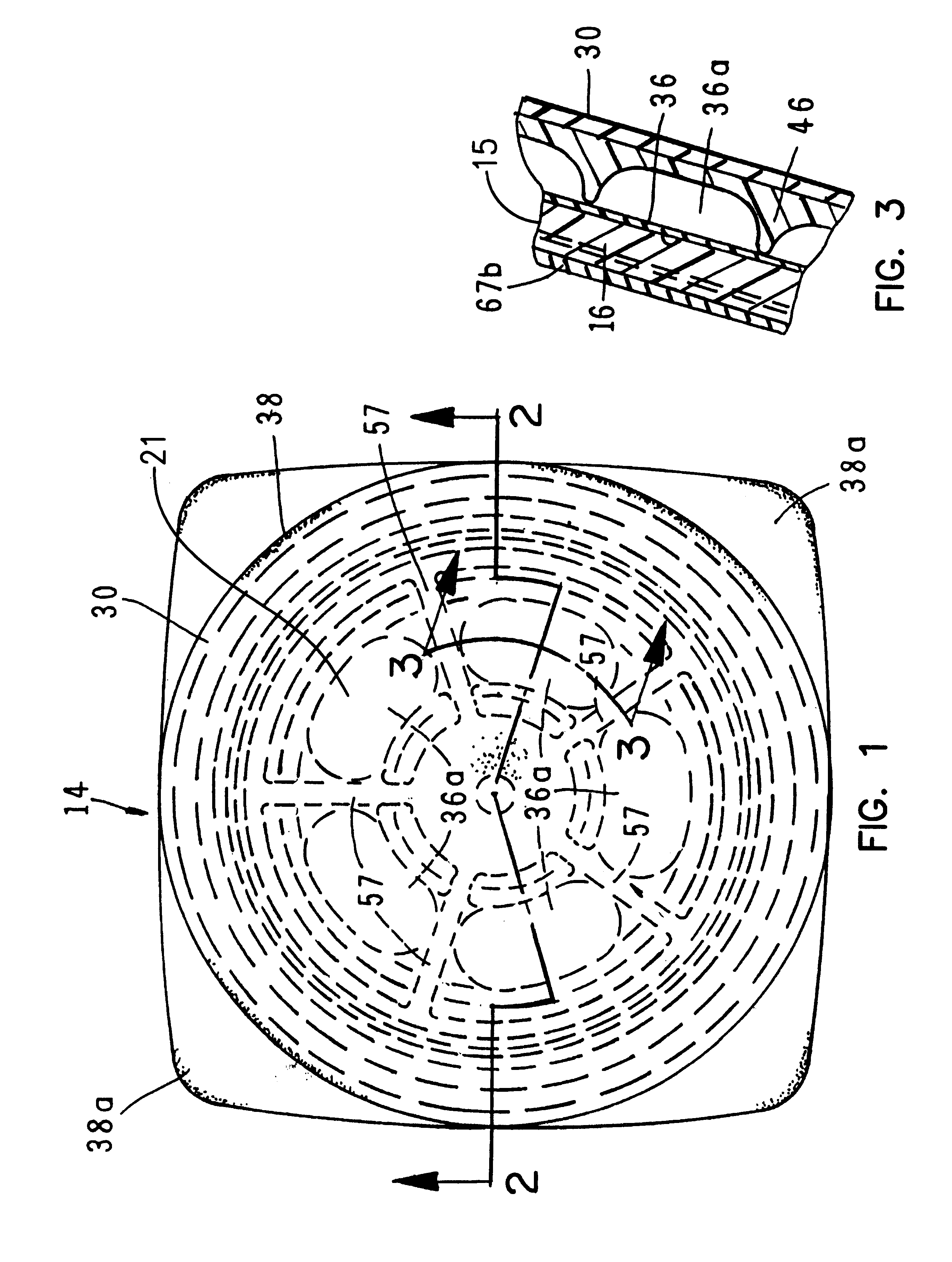
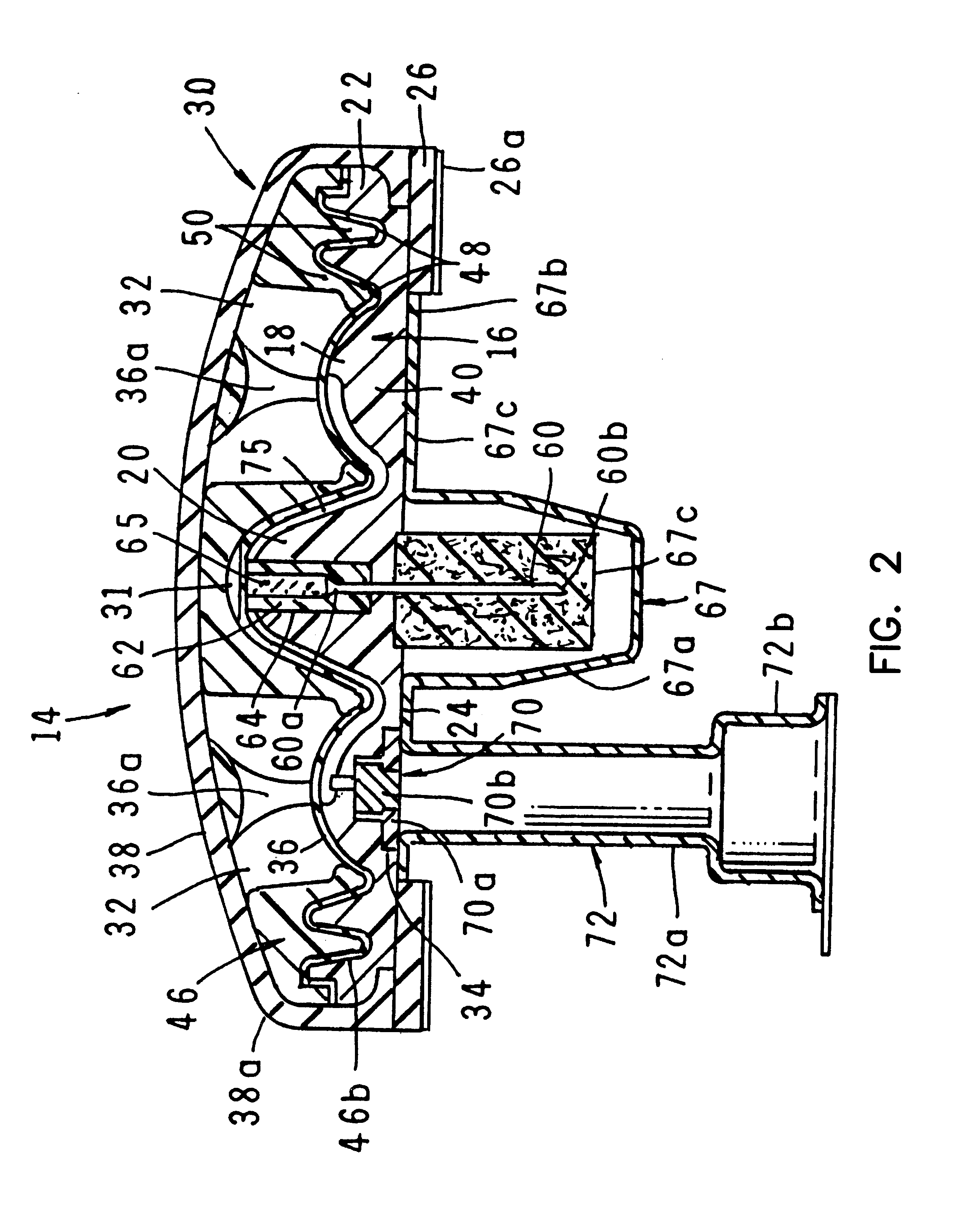
A fluid delivery device having a self-contained stored energy membrane for expelling fluids at a precisely controlled rate, which is of a compact, laminate construction. The device is of very low profile so that it can conveniently be used for the precise delivery of a small volume of pharmaceutical fluids, such as insulin, morphine and the like, into an ambulatory patient at precisely controlled rates over extended periods of time. The device includes strategically configured, multiple fluid chambers to achieve the maximum possible average percent of extension of the membrane and thereby assure adequate fluid delivery pressure.
Owner:PESCADERO BEACH HLDG
Chloroquine coupled antibodies and other proteins with methods for their synthesis
InactiveUS20070166281A1Improve efficacyImprove transportBiocidePeptide/protein ingredientsDrug conjugationTreatment effect
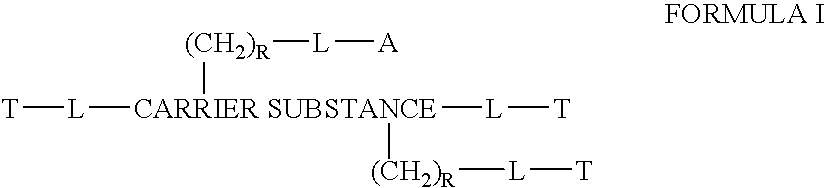
This invention discloses compositions of chloroquine-coupled active agents such as therapeutic antibodies or insulin, including methods for their preparation. The prior art has shown that chloroquines given as free drug in high enough concentration, enhances the release of various agents from cellular endosomes into the cytoplasm. The purpose of these compositions is to provide a controlled amount of chloroquine at the same site where the drug is delivered, thereby reducing the overall dosage needed. The compositions comprise a chloroquine substance coupled to a drug directly or through a variety of pharmaceutical carrier substances. The carrier substances include polysaccharides, synthetic polymers, proteins, micelles and other substances for carrying and releasing the chloroquine compositions in the body for therapeutic effect. The compositions can also include a biocleavable linkage for carrying and releasing the drug for therapeutic or other medical uses. The invention also discloses carrier compositions that are coupled to targeting molecules for targeting the delivery of chloroquine substances and antibody or insulin to their site of action.
Owner:KOSAK KENNETH M
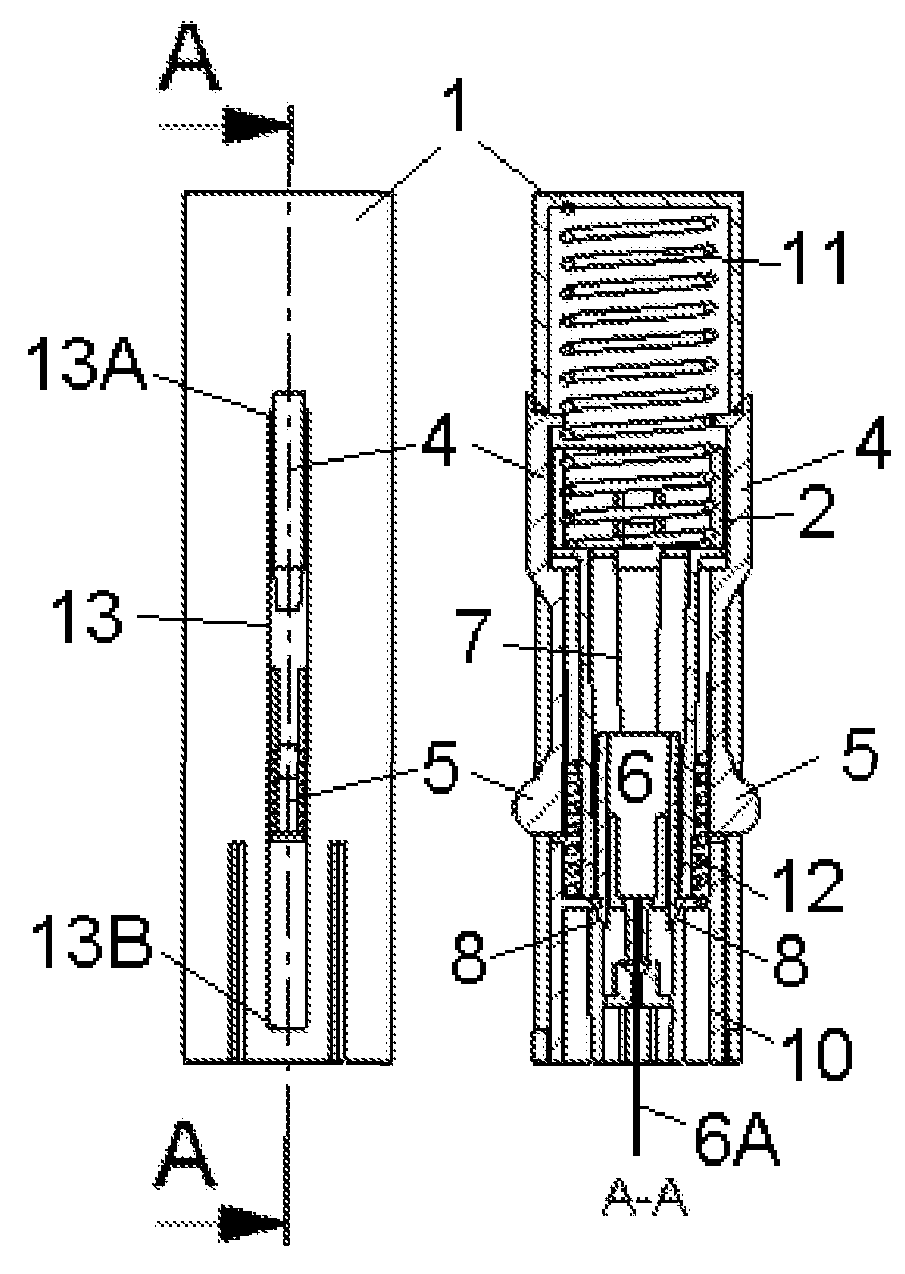
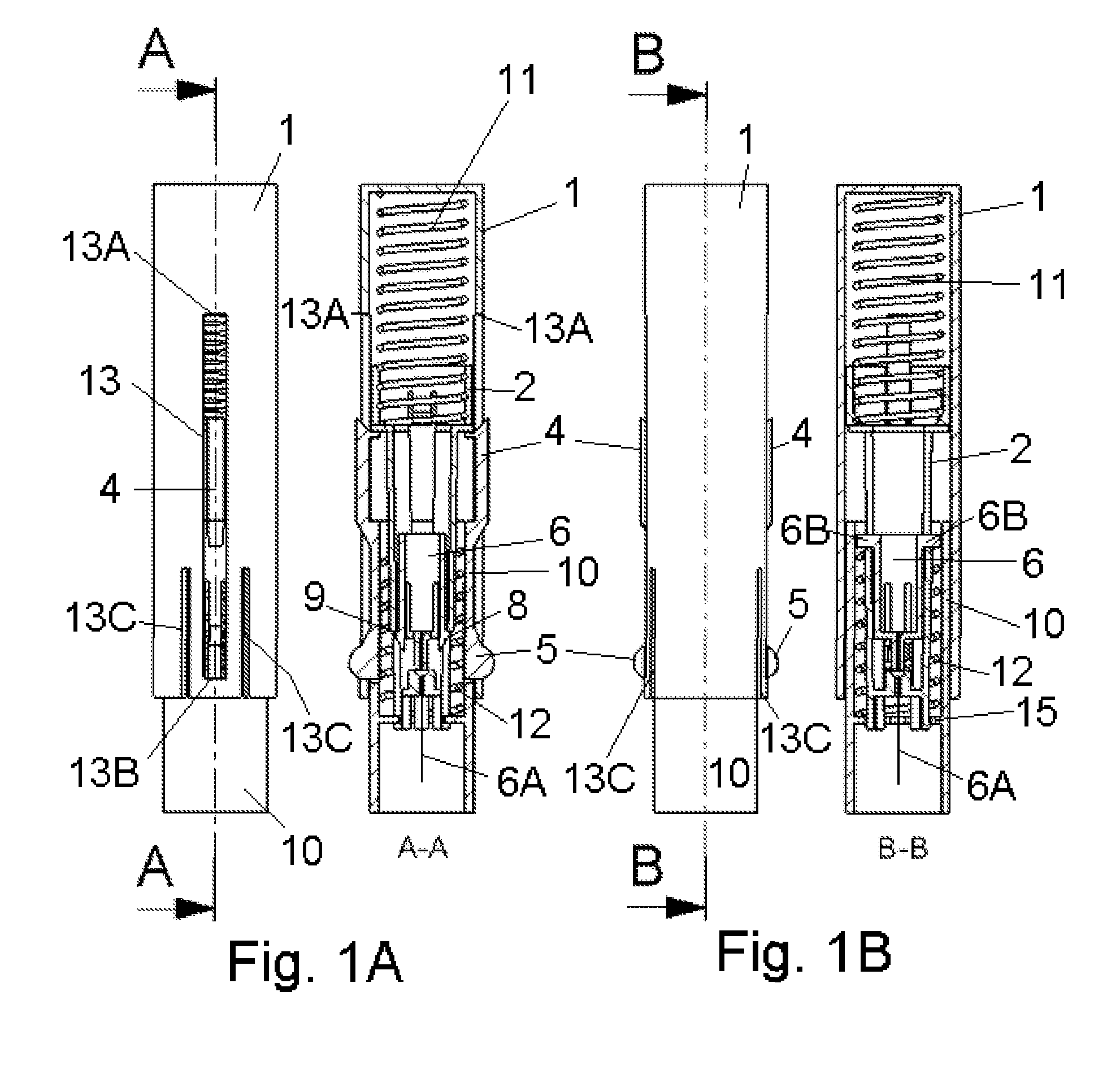
Inserter Having Two Springs
ActiveUS20100228226A1Simple and safe processNon-expensive inserterAutomatic syringesMedical devicesInfusion setBiological activation
The invention relates to an inserter for a medical device e.g. an infusion set or the like for intermittent or continuous administration of a therapeutical substance, such as e.g. insulin. The inserter comprises a needle hub comprising an insertion needle and two elastic elements assuring automatic insertion and automatic retraction of the insertion needle. Activation of the first elastic element (11) cause a penetrating member (6A) to be inserted sub-or transcutaneously into the skin of a patient, and the second elastic element (12) cause the penetrating member (6A) to be retracted from the skin of the patient. The first elastic element (11) is in an unloaded state before activation and upon activation the first elastic element (11) energizes the second elastic element (12).
Owner:UNOMEDICAL AS
Method for producing powder formulation comprising an insulin
The present invention relates to a process for producing a therapeutic powder formulation, comprising (a) providing an acidic aqueous solution comprising an insulin or analoguc or derivative thereof and an enhancer; (b) adjusting the pH to a pH in the range of 4.5 to 7.4; (c) precipitating a product comprising the insulin or analogue or derivative thereof and the enhancer, wherein the precipitation is performed essentially without evaporation of the solution; and (d) removing the water.
Owner:NOVO NORDISK AS
Anti-IGF-I receptor antibody
InactiveUS7538195B2Increase profitOrganic active ingredientsFungiInsulin-like growth factorSynovial sarcoma
Antibodies, humanized antibodies, resurfaced antibodies, antibody fragments, derivatized antibodies, and conjugates of these molecules with cytotoxic agents, which specifically bind to and inhibit insulin-like growth factor-I receptor, antagonize the effects of IGF-I and are substantially devoid of agonist activity toward the insulin-like growth factor-I receptor. These molecules can be conjugated to cytotoxic agents for use in the treatment of tumors that express elevated levels of IGF-I receptor, such as breast cancer, colon cancer, lung cancer, ovarian carcinoma, synovial sarcoma and pancreatic cancer. These molecules can also be labeled for in vitro and in vivo diagnostic uses, such as in the diagnosis and imaging of tumors that express elevated levels of IGF-I receptor.
Owner:IMMUNOGEN INC
Insulin and IGF-1 receptor agonists and antagonists
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Composition and method for treating impaired or deteriorating neurological function
InactiveUS6964969B2Impair actionHigh affinityBiocideHydrocarbon active ingredientsPhosphateAntioxidant
A nutritional supplement composition for normalizing impaired or deteriorating neurological function in humans is composed of: at least one agent which promotes synthesis of ATP and / or creatine phosphate in the body, at least one antioxidant for scavenging free radicals in at least one pathway in the body; at least one agent for normalizing or maintaining membrane function and structure in the body; at least one agent for normalizing or maintaining normal neurotransmitter function in the body; at least one agent for down-regulating cortisol action; and at least one agent for suppressing activation of apoptotic pathways in the body. The composition may further contain one or more of: at least one agent for suppressing inflammation in the body; at least one agent for normalizing or maintaining vascular wall function and structure in the body; at least one agent for normalizing or maintaining function of nerve growth factors and / or neurotropic factors in the body; at least one agent for suppressing toxic metal ionic effects; at least one agent for normalizing or maintaining methyl metabolism in the body; at least one agent for normalizing or maintaining metabolism of insulin and glucose in the body; and at least one agent for up-regulating activity of heat shock proteins in the body. A method for normalizing impaired neurological function in humans modulating nutrient partitioning in a human involves administering the aforementioned composition to the human, preferably on a daily basis, for a therapeutically effective period of time. Preferably, the method further involves having the human follow a stress reduction program, and / or a cognitive retraining program, and / or a dietary program designed to maximize insulin and glucose metabolism.
Owner:MCCLEARY EDWARD LARRY
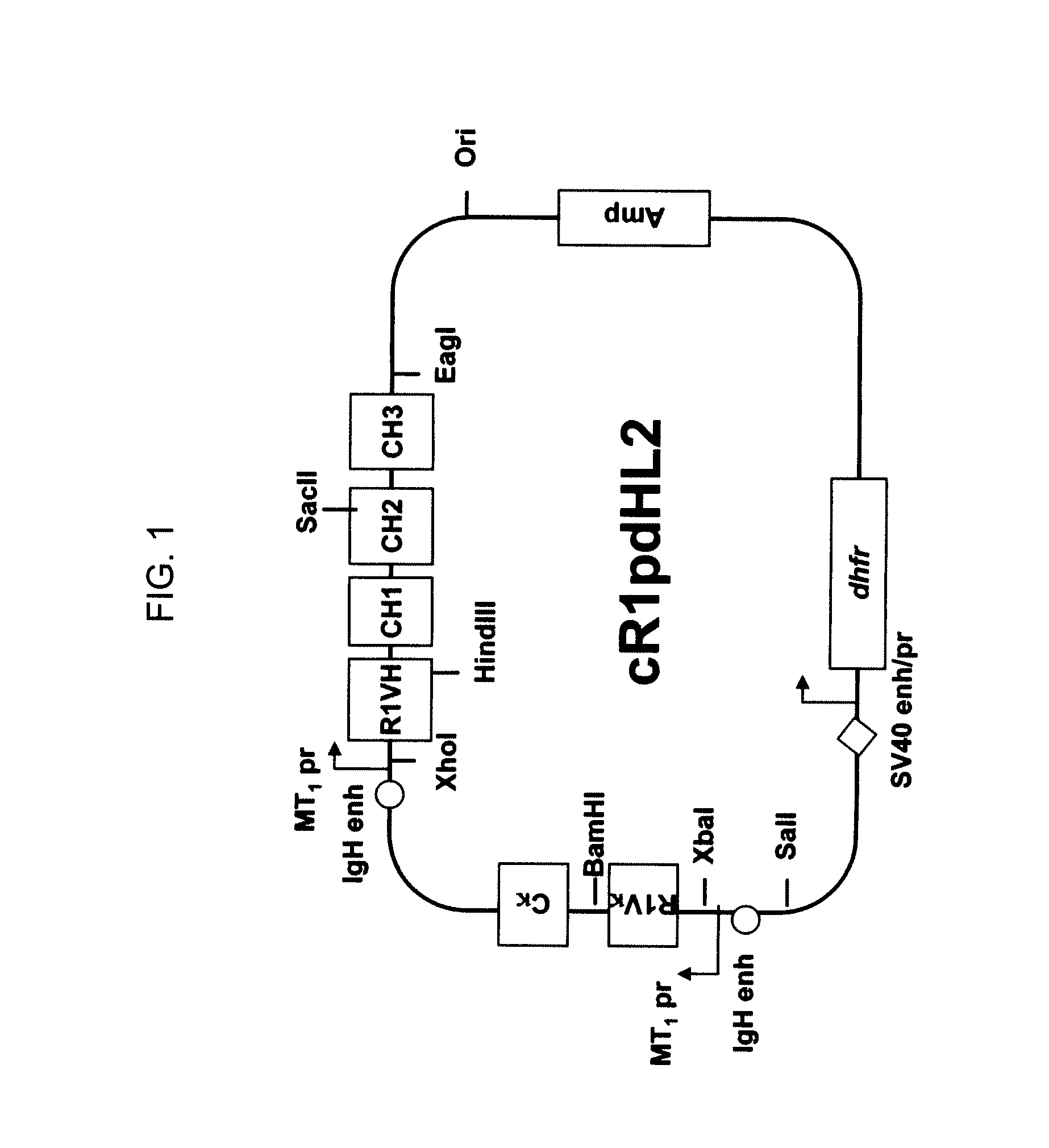
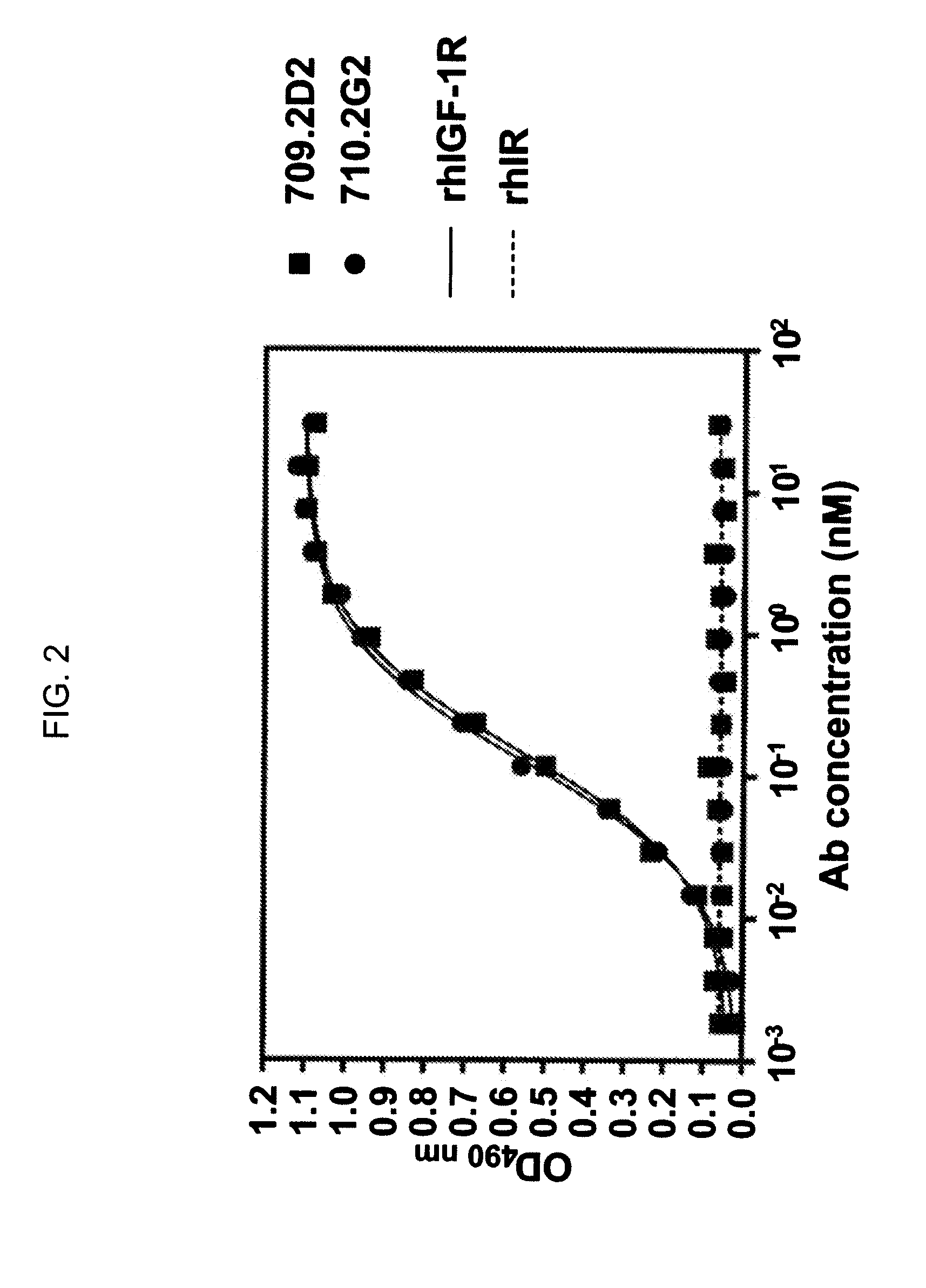
Novel Class of Monospecific and Bispecific Humanized Antibodies that Target the Insulin-like Growth Factor Type I Receptor (IGF-1R)
InactiveUS20100226884A1Organic active ingredientsPeptide/protein ingredientsDiseaseAntiendomysial antibodies
The present invention provides compositions and methods of use of anti-IGF-1R antibodies or antibody fragments. Preferably the antibodies bind to IGF-1R but not IR; are not agonists for IGF-1R; do not block binding of IGF-1 or IGF-2 to isolated IGF-1R, but effectively neutralize activation of IGF-1R by IGF-1 in intact cells; and block binding of an R1 antibody to IGF-1R. The antibodies may be murine, chimeric, humanized or human R1 antibodies comprising the heavy chain CDR sequences DYYMY (SEQ ID NO:1), YITNYGGSTYYPDTVKG (SEQ ID NO:2) and QSNYDYDGWFAY (SEQ ID NO:3) and the light chain CDR sequences KASQEVGTAVA (SEQ ID NO:4), WASTRHT (SEQ ID NO:5) and QQYSNYPLT (SEQ ID NO:6). Preferably the antibodies bind to an epitope of IGF-1R comprising the first half of the cysteine-rich domain of IGF-1R (residues 151-222). The anti-IGF-1R antibodies may be used for diagnosis or therapy of various diseases such as cancer.
Owner:IMMUNOMEDICS INC
Drug-oligomer conjugates with polyethylene glycol components
InactiveUS7030084B2Reduce deliveryExtended durationPeptide/protein ingredientsDepsipeptidesOligomerPolyethylene glycol
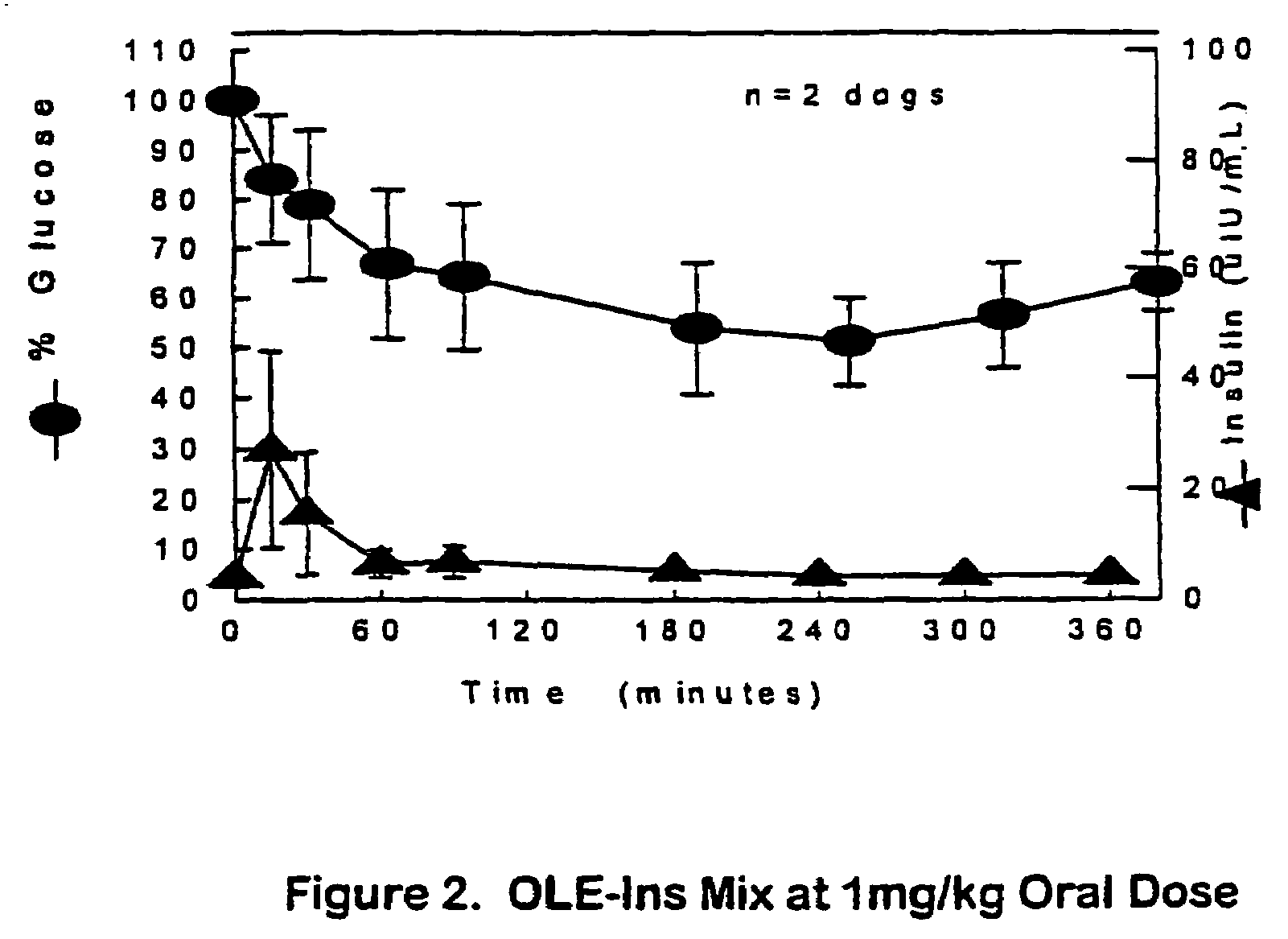
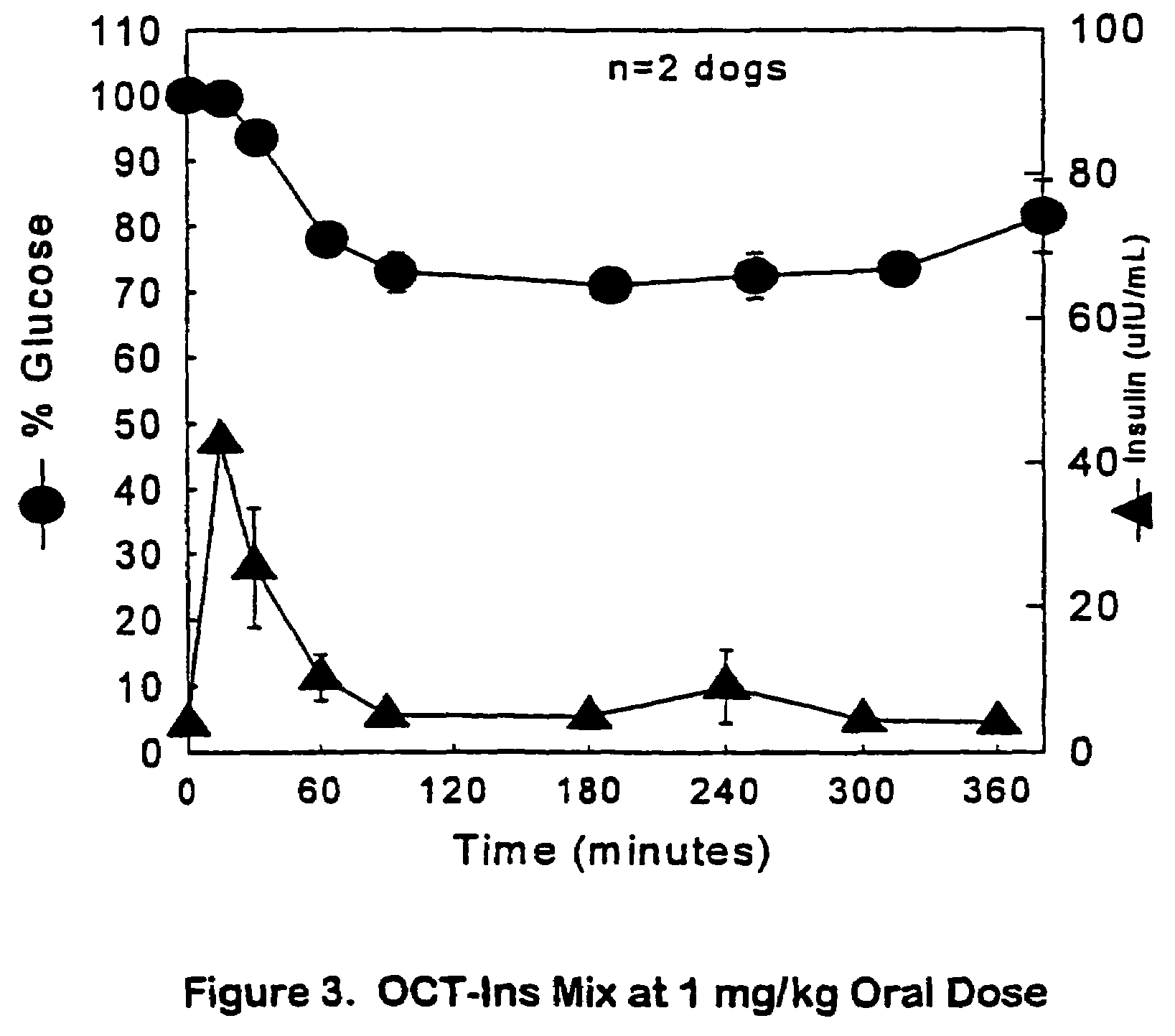
The present invention relates generally to hydrolyzable drug-oligomer conjugates, pharmaceutical compositions comprising such conjugates, and to methods for making and using such conjugates and pharmaceutical compositions. For example, a conjugate of insulin, PEG, and oleic acid can be orally administered.
Owner:BIOCON LTD
Muscle-derived cells (MDCs) for treating muscle- or bone-related injury or dysfunction
The present invention provides muscle-derived cells, preferably myoblasts and muscle-derived stem cells, genetically engineered to contain and express one or more heterologous genes or functional segments of such genes, for delivery of the encoded gene products at or near sites of musculoskeletal, bone, ligament, meniscus, cartilage or genitourinary disease, injury, defect, or dysfunction. Ex vivo myoblast mediated gene delivery of human inducible nitric oxide synthase, and the resulting production of nitric oxide at and around the site of injury, are particularly provided by the invention as a treatment for lower genitourinary tract dysfunctions. Ex vivo gene transfer for the musculoskeletal system includes genes encoding acidic fibroblast growth factor, basic fibroblast growth factor, epidermal growth factor, insulin-like growth factor, platelet derived growth factor, transforming growth factor-β, transforming growth factor-α, nerve growth factor and interleukin-1 receptor antagonist protein (IRAP), bone morphogenetic protein (BMPs), cartilage derived morphogenetic protein (CDMPs), vascular endothelial growth factor (VEGF), and sonic hedgehog proteins.
Owner:UNIVERSITY OF PITTSBURGH
Insulin and IGF-1 receptor agonists and antagonists
InactiveUS6875741B2Peptide/protein ingredientsGenetic material ingredientsBinding sitePancreatic hormone
Peptide sequences capable of binding to insulin and / or insulin-like growth factor receptors with either agonist or antagonist activity and identified from various peptide libraries are disclosed. This invention also identifies at least two different binding sites, which are present on insulin and insulin-like growth factor receptors, and which selectively bind the peptides of this invention. As agonists, certain of the peptides of this invention may be useful for development as therapeutics to supplement or replace endogenous peptide hormones. The antagonists may also be developed as therapeutics.
Owner:NOVO NORDISK AS +1
Biological markers predictive of anti-cancer response to insulin-like growth factor-1 receptor kinase inhibitors
InactiveUS20070065858A1Restore sensitivityHigh sensitivityCompound screeningApoptosis detectionInsulin-like growth factorPancreatic hormone
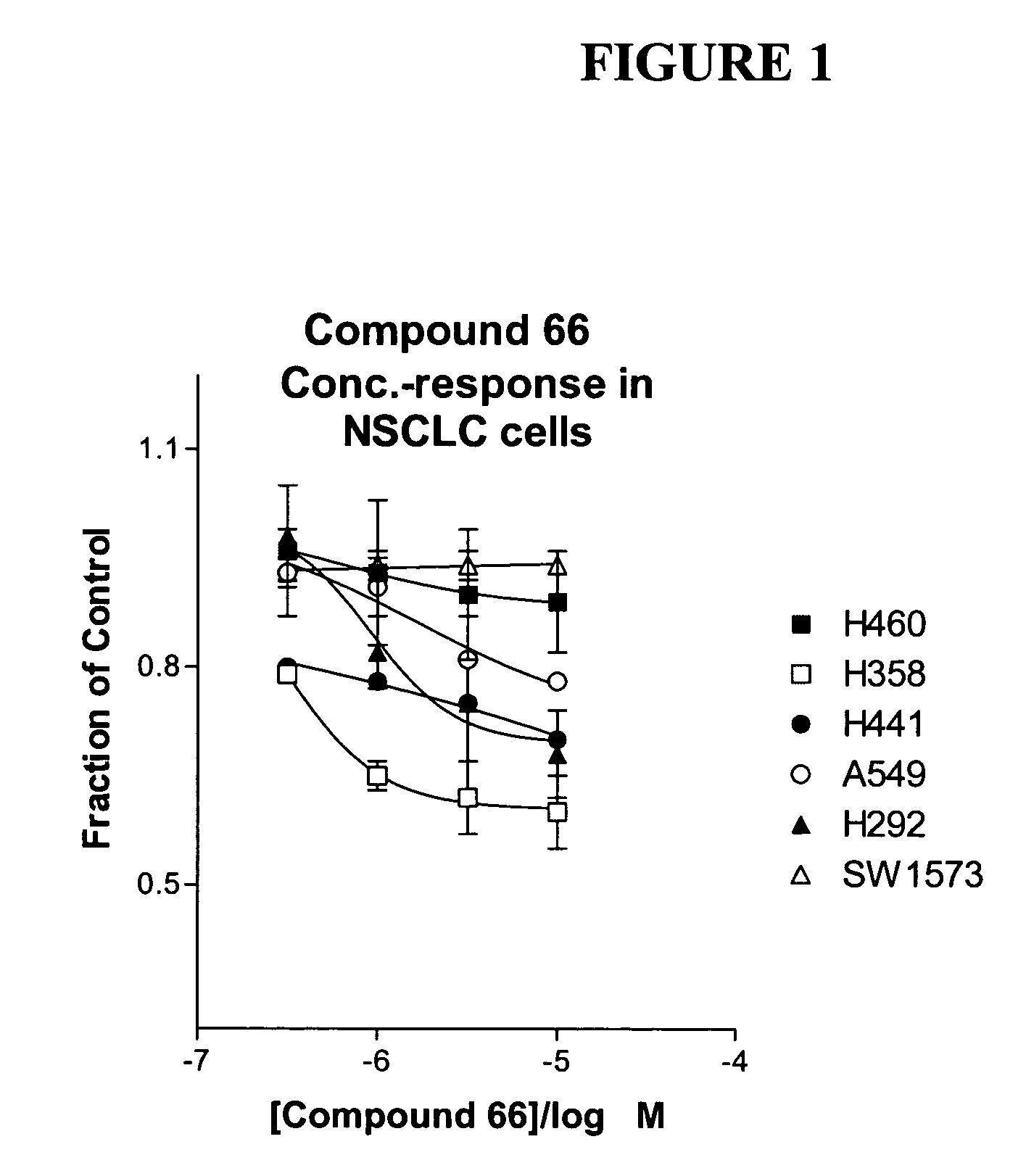
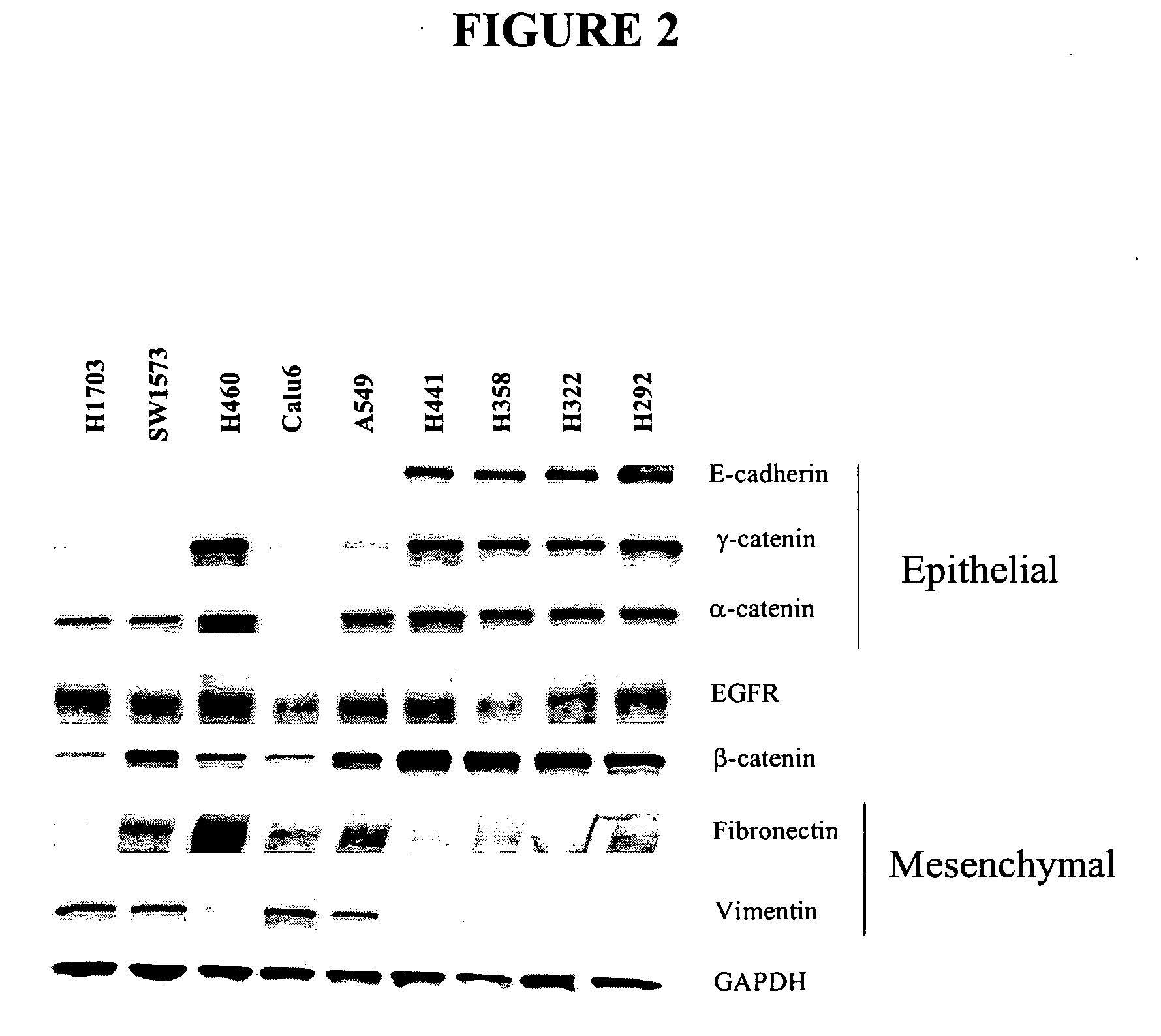
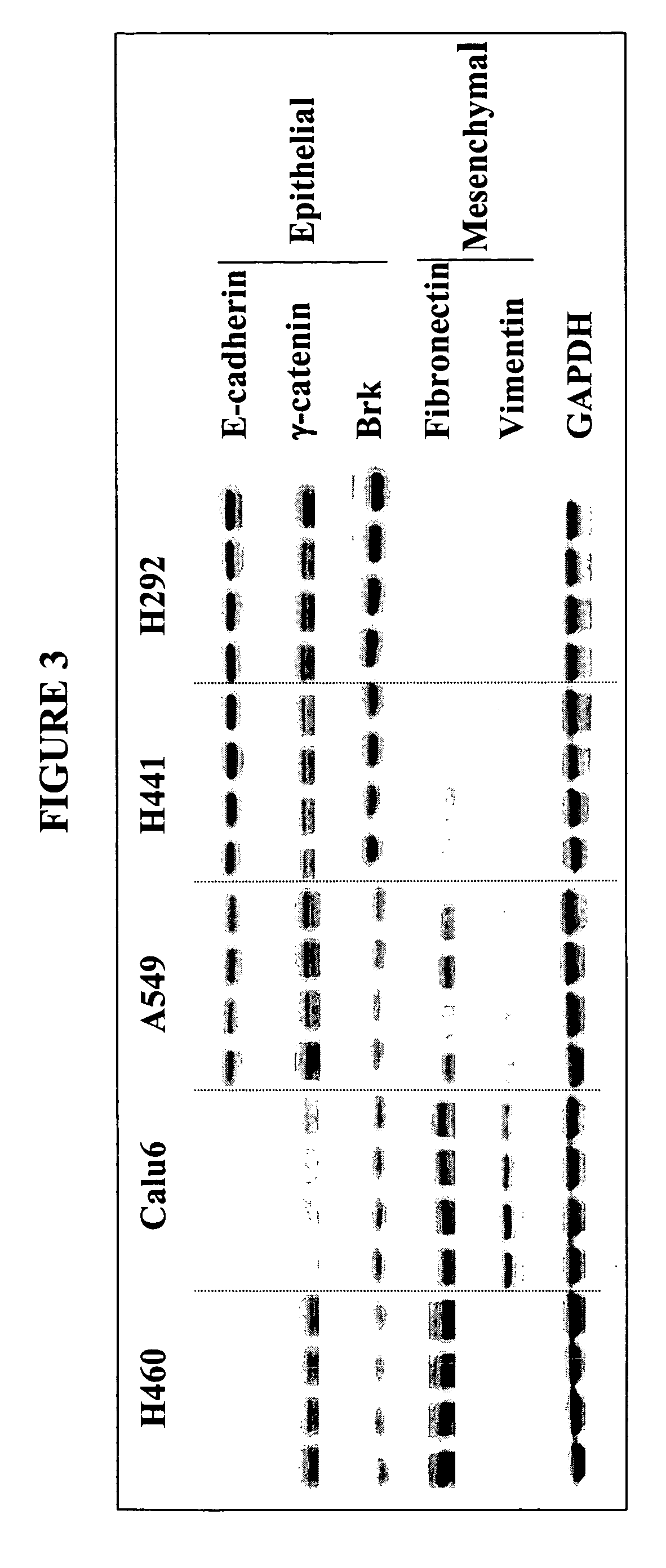
The present invention provides diagnostic and prognostic methods for predicting the effectiveness of treatment of a cancer patient with an IGF-1R kinase inhibitor. Methods are provided for predicting the sensitivity of tumor cell growth to inhibition by an IGF-1R kinase inhibitor, comprising assessing whether the tumor cell has undergone an epithelial to mesenchymal transition (EMT), by determining the expression level of epithelial and / or mesenchymal biomarkers, wherein tumor cells that have undergone an EMT are substantially less sensitive to inhibition by IGF-1R kinase inhibitors. Improved methods for treating cancer patients with IGF-1R kinase inhibitors that incorporate the above methodology are also provided. Additionally, methods are provided for the identification of new biomarkers that are predictive of responsiveness of tumors to IGF-1R kinase inhibitors. Furthermore, methods for the identification of agents that restore the sensitivity of tumor cells that have undergone EMT to inhibition by IGF-1R kinase inhibitors are also provided.
Owner:OSI PHARMA INC
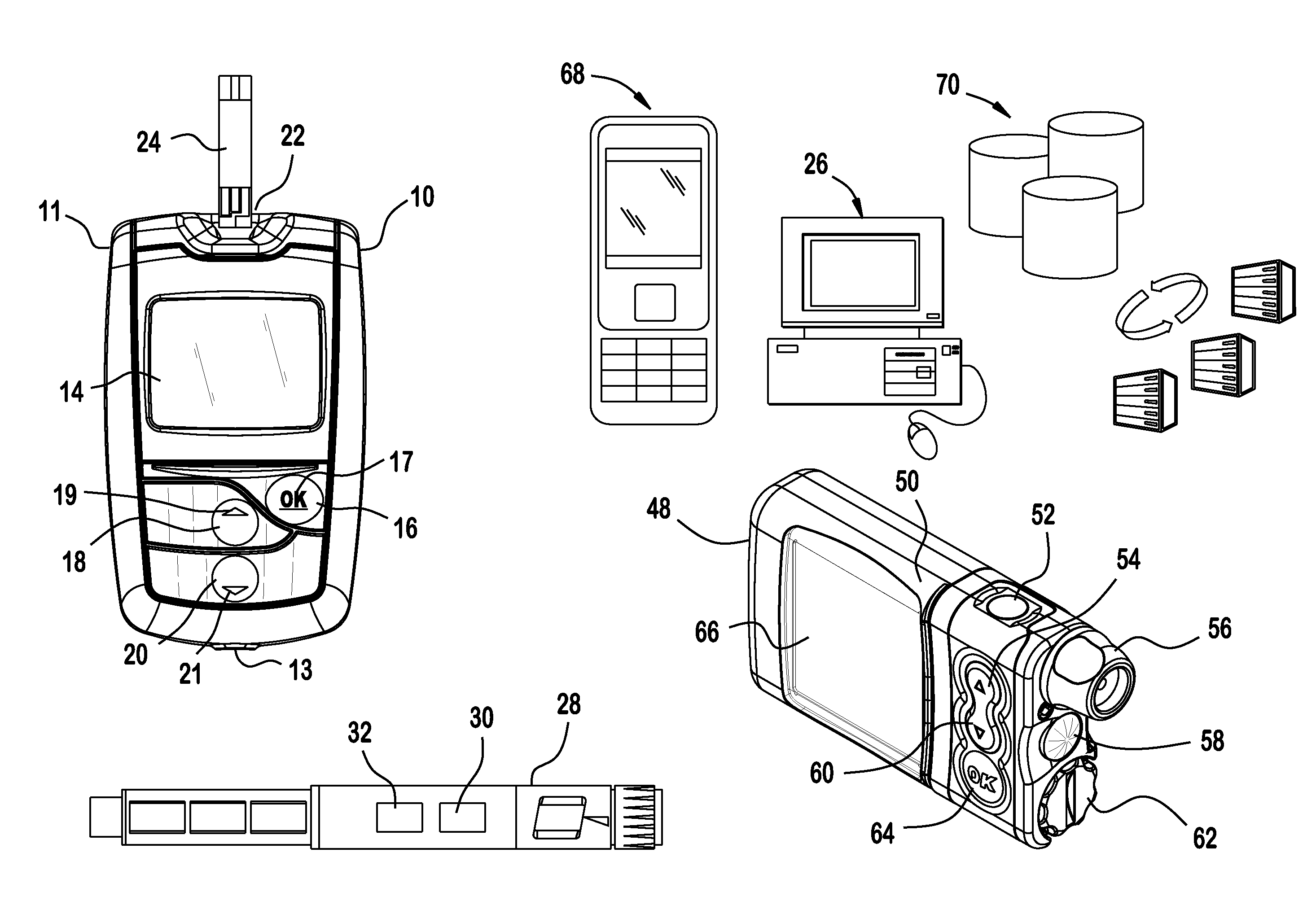
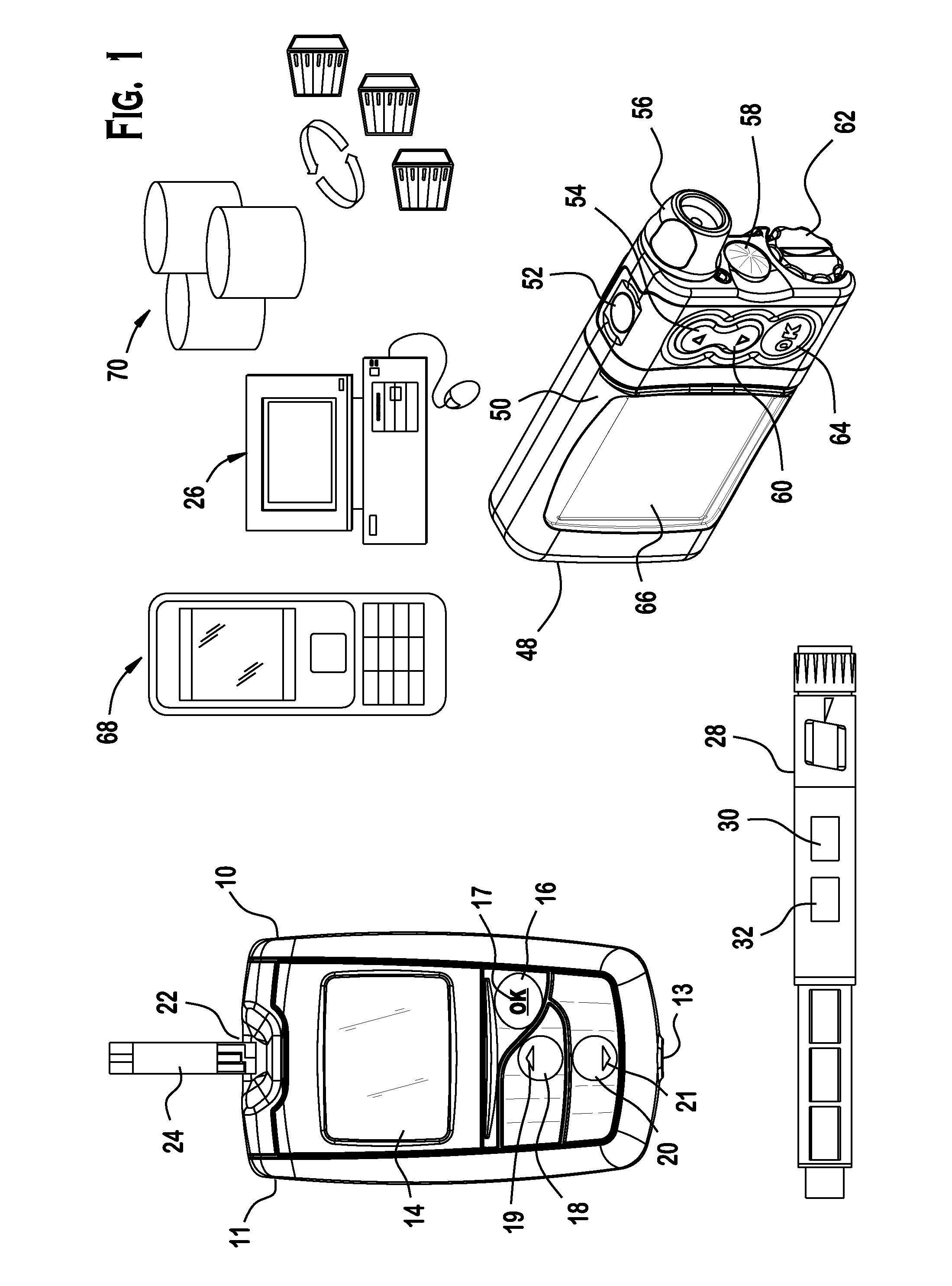
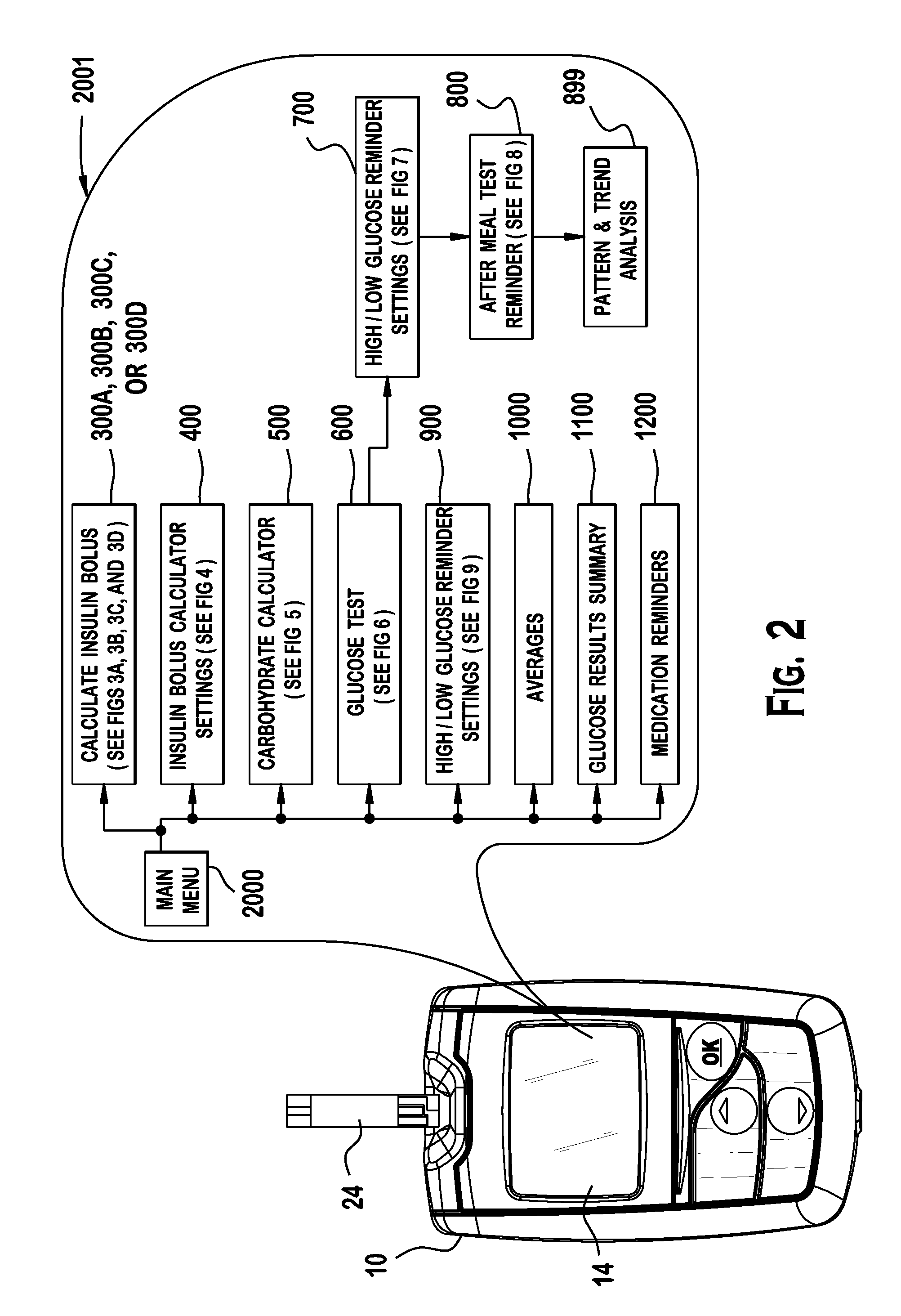
Analyte testing method and device for diabetes mangement
Various embodiments are described and illustrated to calculate an insulin bolus, recommend such bolus, and provide reminder messages for performing an additional glucose test.
Owner:LIFESCAN IP HLDG LLC
Medical product
InactiveUS20060239933A1Powder deliveryPeptide/protein ingredientsPulmonary inhalationMedical product
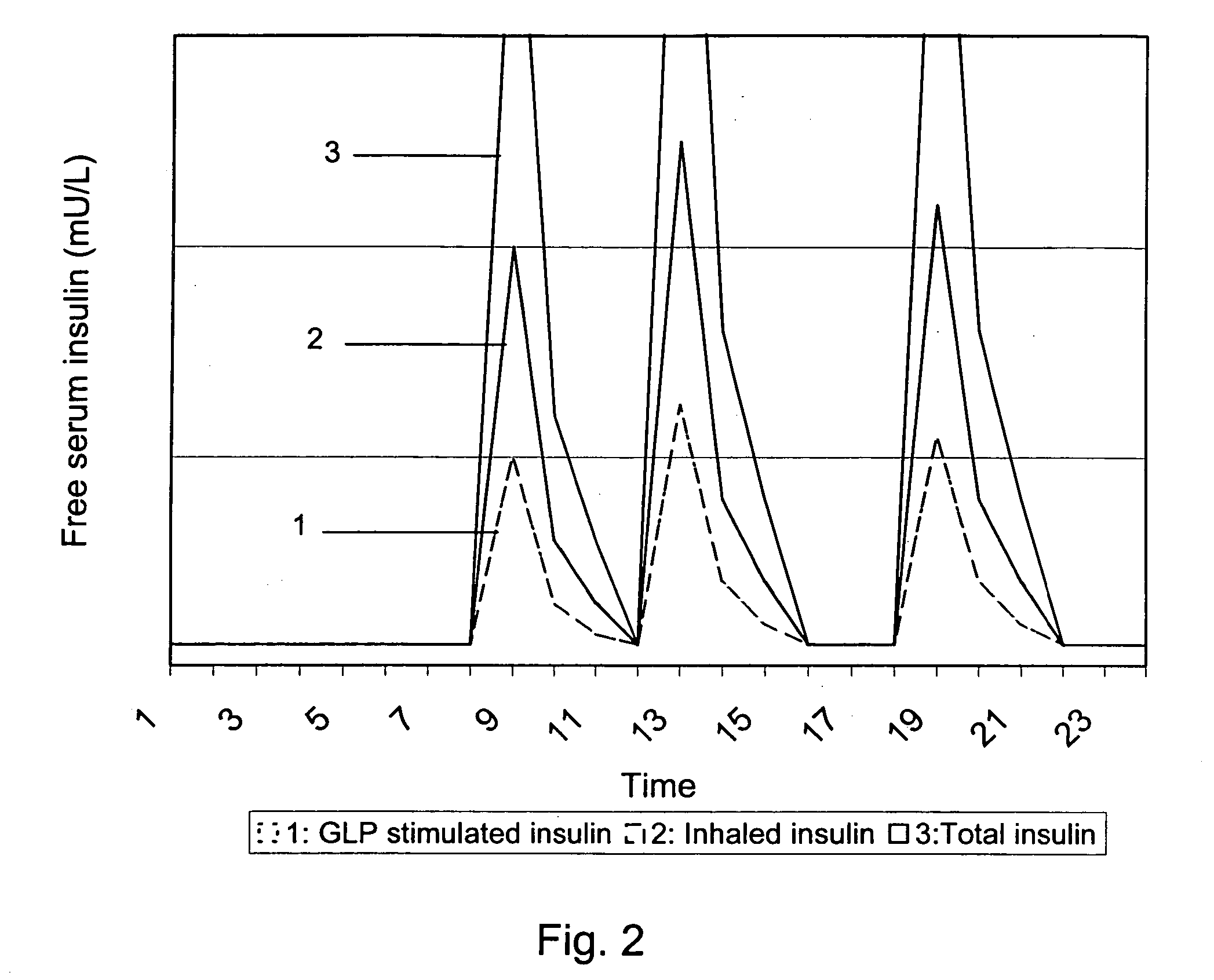
A medical product is disclosed. The medical product contains an accurately metered dose of at least one GLP medicament intended for pulmonary inhalation put into a moisture-tight, high barrier seal container. The medical product optionally also contains a dose of insulin. The container is adapted for application into a dry powder inhaler. The dose loaded in the container is intended for a prolonged delivery by inhalation to the deep lung where the active ingredients are absorbed into the system. Optionally the medical product also may comprise at least one biologically acceptable excipient.
Owner:MEDERIO AG
Compositions and methods for therapuetic agents complexed with calcium phosphate and encased by casein
InactiveUS20020054914A1Improve bioavailabilityBiocideOrganic active ingredientsBiologic AgentsMedicine
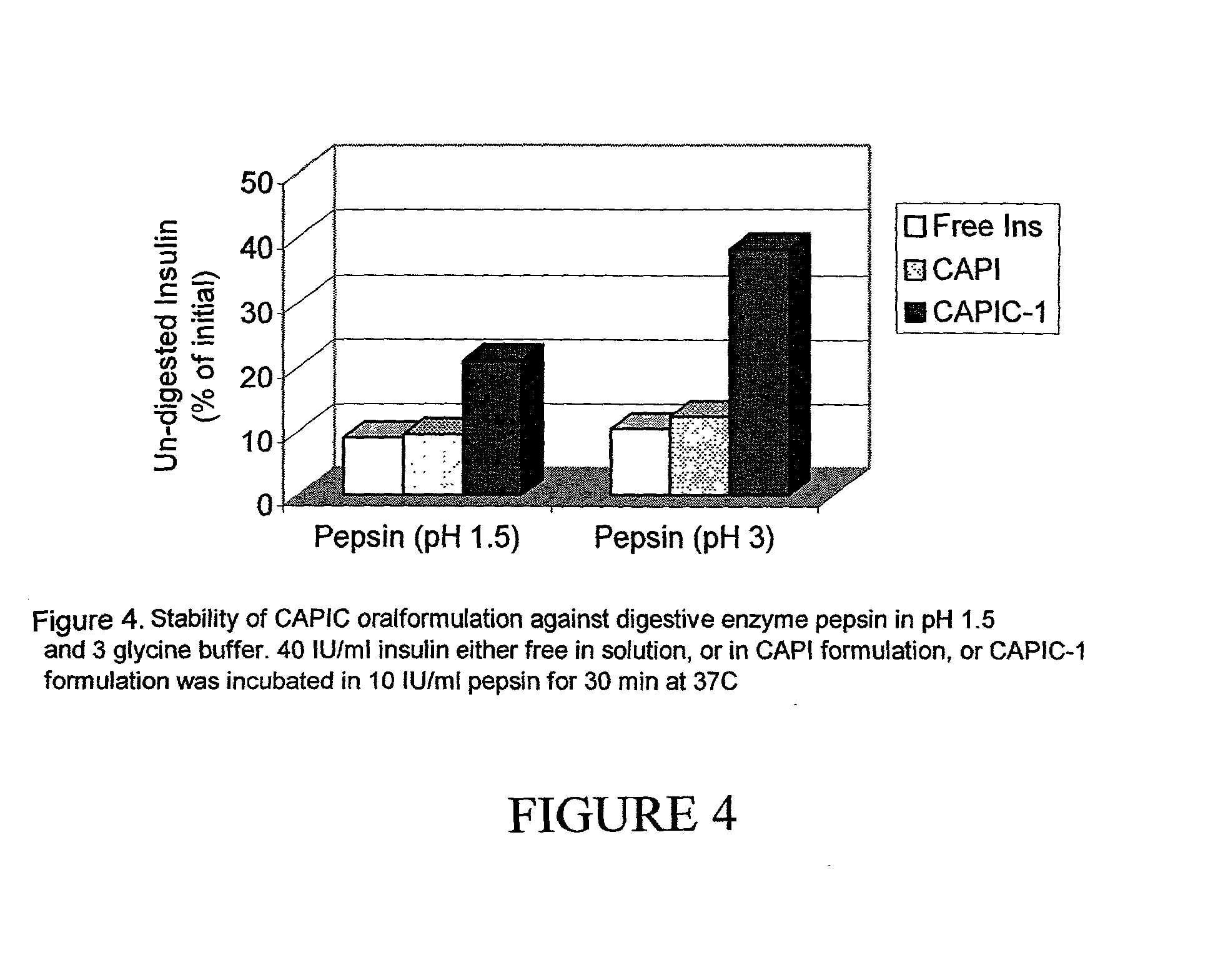
The present invention relates generally to an oral drug delivery system which incorporates a therapeutic bioactive agent with biodegradable calcium phosphate particles, the particles then encapsulated by casein. The resulting particles provide a carrier designed to protect the therapeutic agent in the harsh, acidic environment of the stomach before releasing the agent into the small intestine. The therapeutic agent may be any therapeutically effective agent, such as a natural isolate or synthetic chemical or biological agent, such as a therapeutic agent, and in particular, may be a protein or a peptide such as insulin. Also incorporated with the particles may be additional surface modifying agents to assist binding, controlled release, or to otherwise modify the particles. The particles generally support the therapeutic agent to form controlled- or sustained-release particles for the oral or mucosal delivery of the therapeutic agent over time, wherein the therapeutic agent is incorporated into the structure of the particle core, disposed on the surface of the particle, or both.
Owner:CAPTIVATE PHARMACEUTICALS LLC
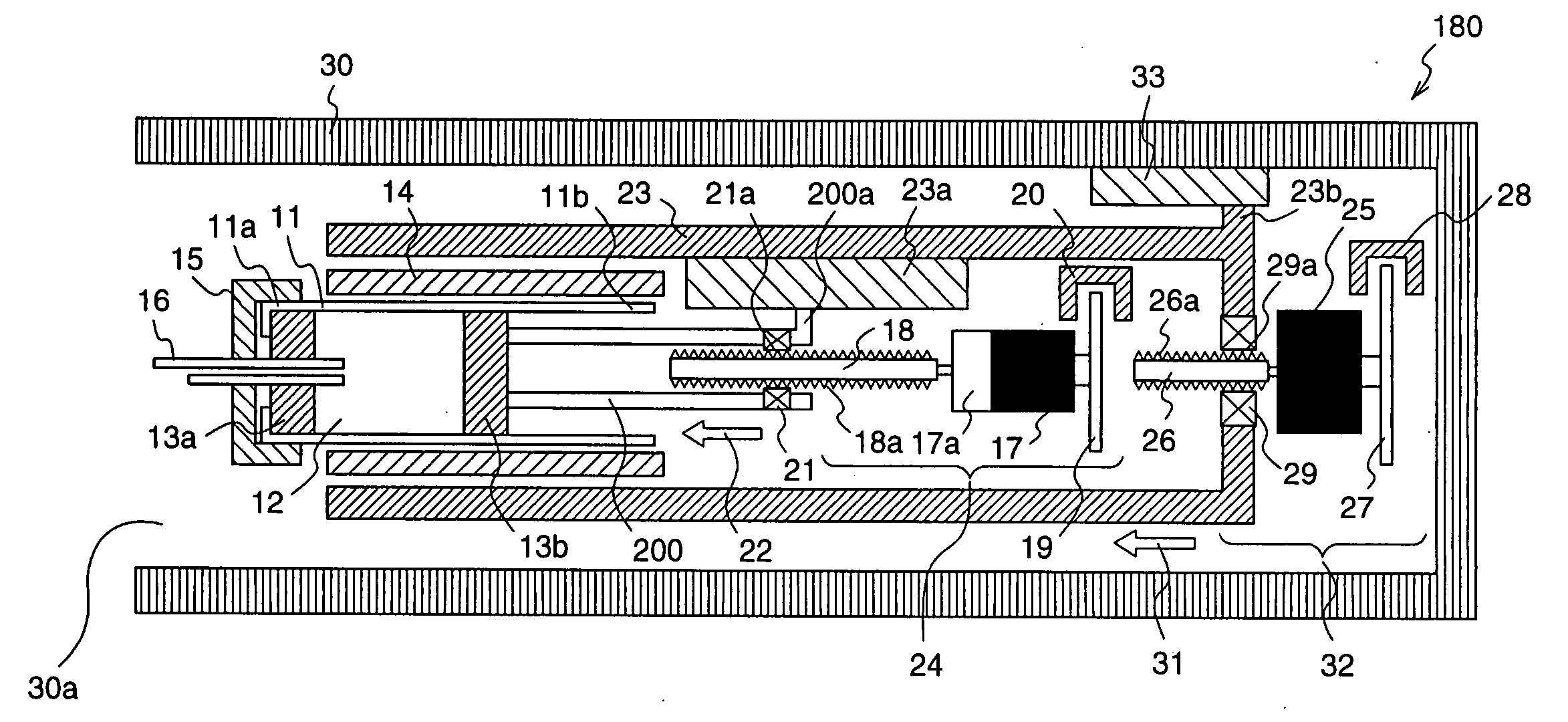
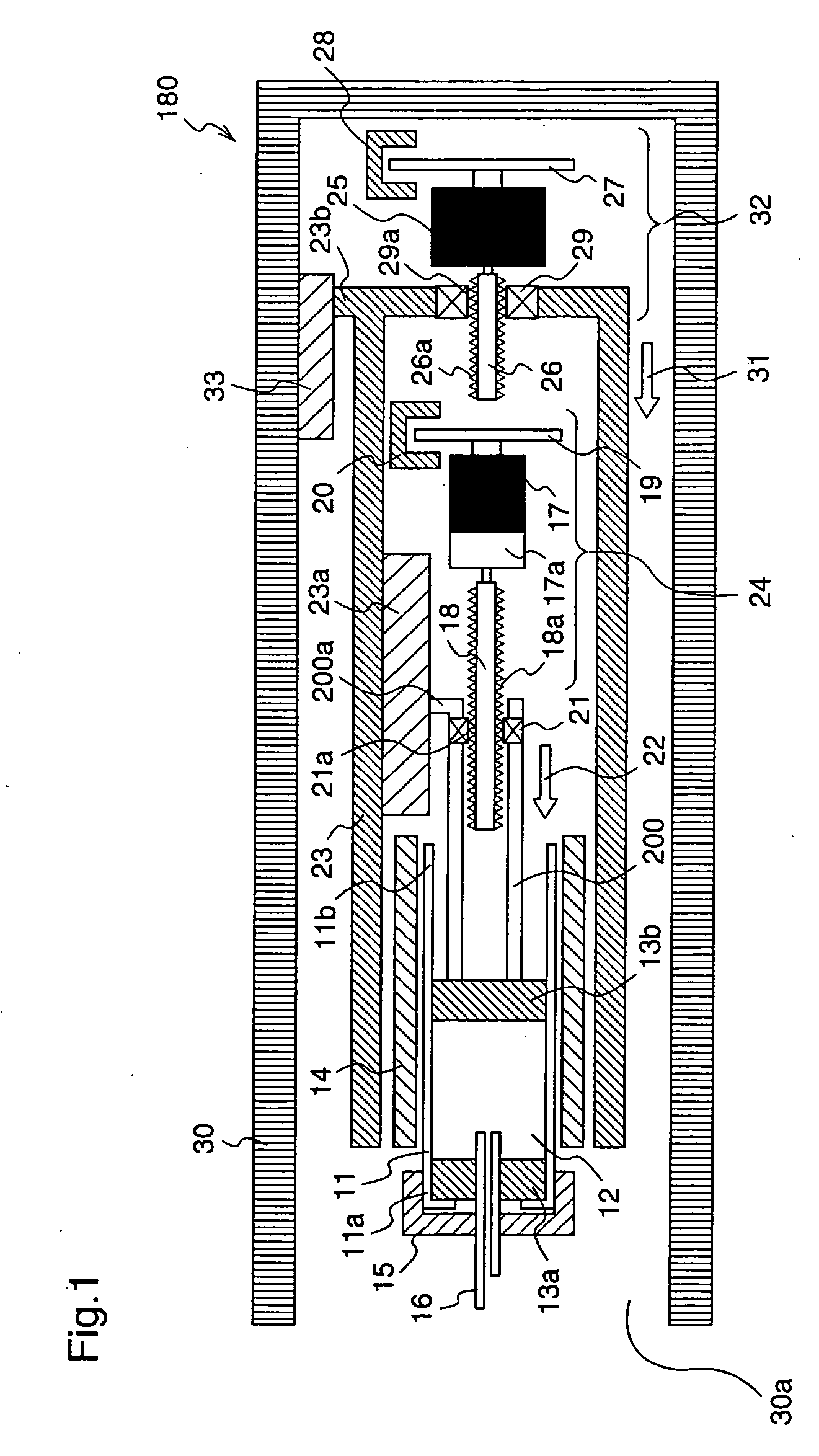
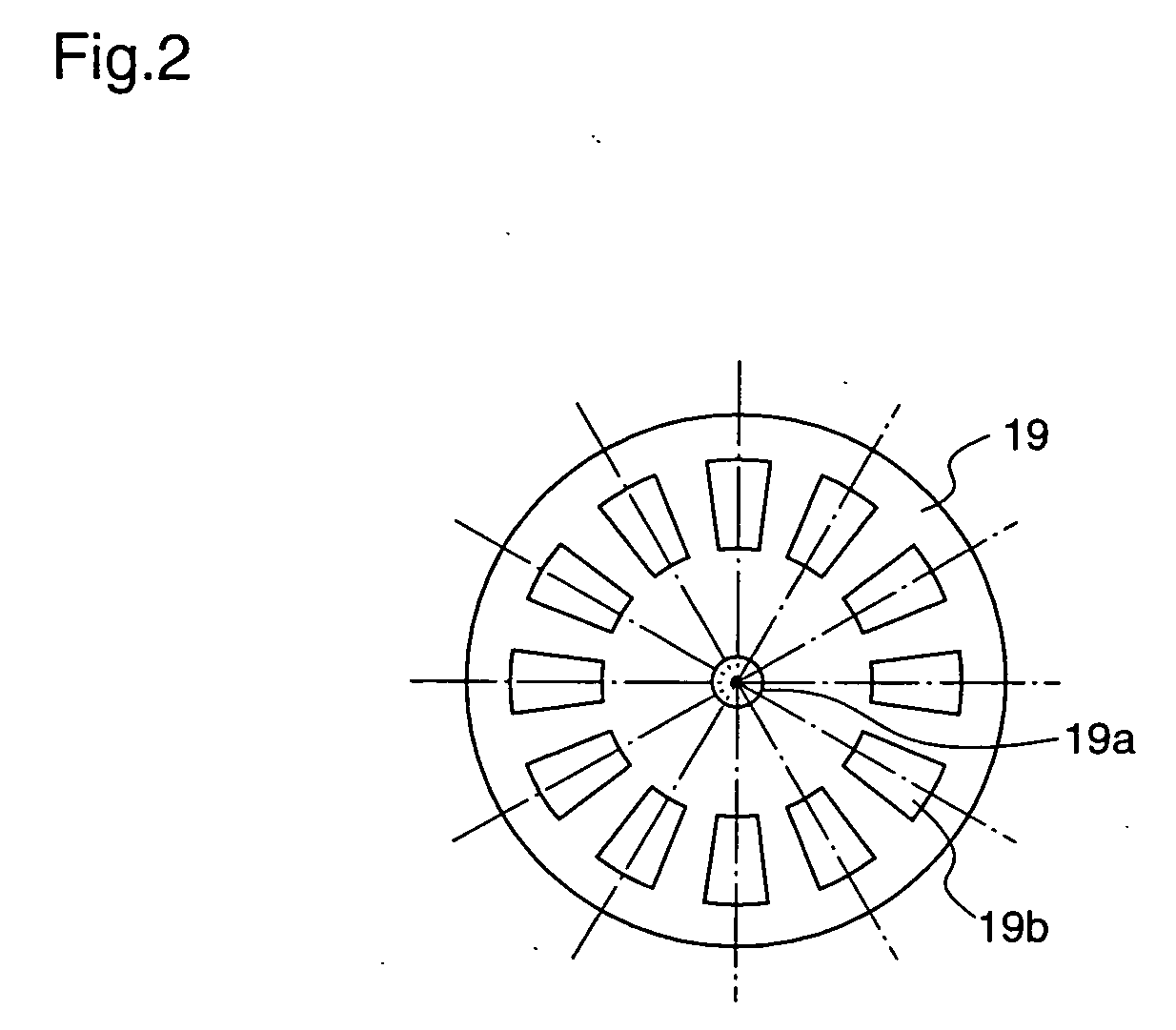
Injection device with puncture function, method for controlling injection device with puncture function, chemical solution administration device, and method for controlling chemical solution administration device
ActiveUS20070066938A1Easy to carryRealize miniaturizationAmpoule syringesElectrotherapyChemical solutionBiomedical engineering
An injection device with puncture function includes, in a single casing 30, a cylindrical cartridge 11 in which insulin 12 is enclosed, a cartridge holder 14 to which the cartridge 11 is inserted, a needle 16 inserted at a front end 11a of the cartridge 11, a reciprocation means 32 for reciprocating the cartridge 11 toward the needle 16, and an extrusion means 23 for extruding the insulin 12 from a rear end11b of the cartridge 11 toward the needle 16, and the motion speed and the motion amount of the reciprocation means 32 are made variable.
Owner:PHC HLDG CORP
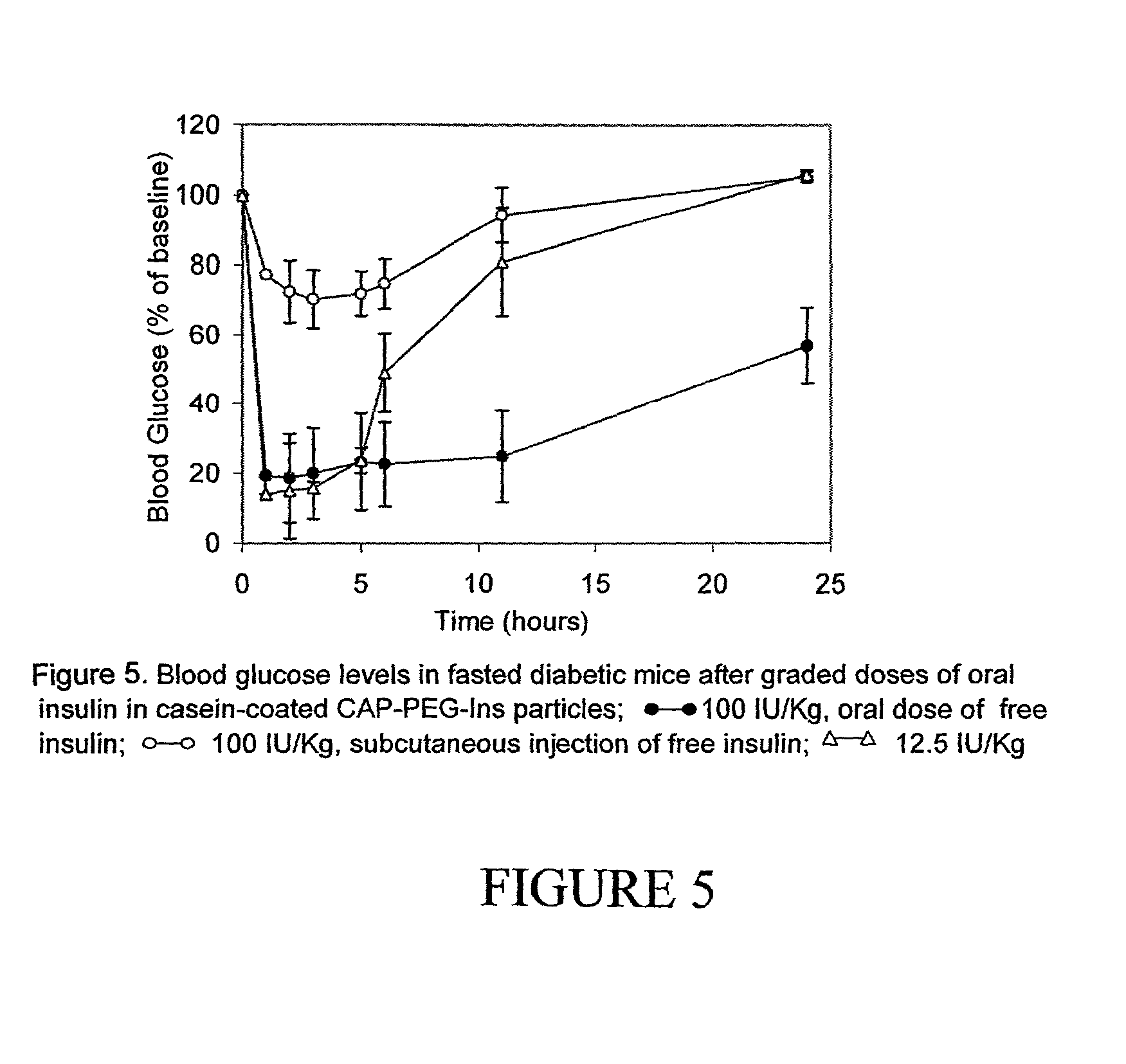
Oral insulin therapy
ActiveUS20060234913A1Reduce morbidityDosing is convenientBiocideOrganic active ingredientsDrugOral medication
Pharmaceutical dosage forms for oral administration to a patient for the treatment of diabetes, comprising insulin and a delivery agent that facilitates insulin transport in a therapeutically effective amount to the bloodstream and that result in a lower incidence of vascular diseases associated with the repeated administration of insulin are disclosed. Also disclosed is a method of attenuating the undesirable incidence of diseases associated with chronic dosing of insulin is provided whereby the oral administration to a patient of insulin along with a suitable delivery agent that facilitates the absorption of insulin from the gastrointestinal tract of the patient in a therapeutically effective amount, for treatment of diabetes.
Owner:NOVO NORDISK NORTH AMERICA OPERATIONS AS
System and method for managing diabetes
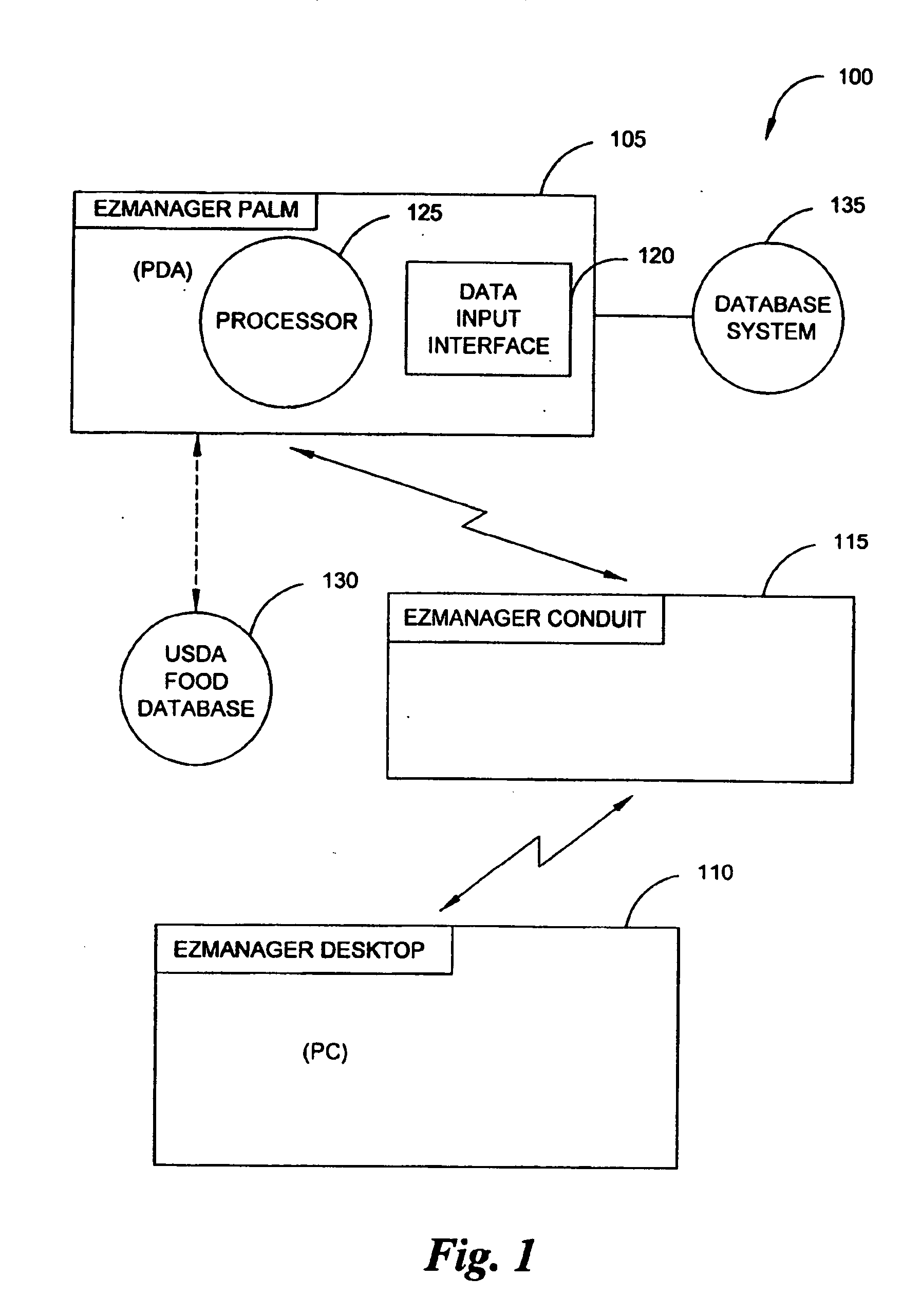
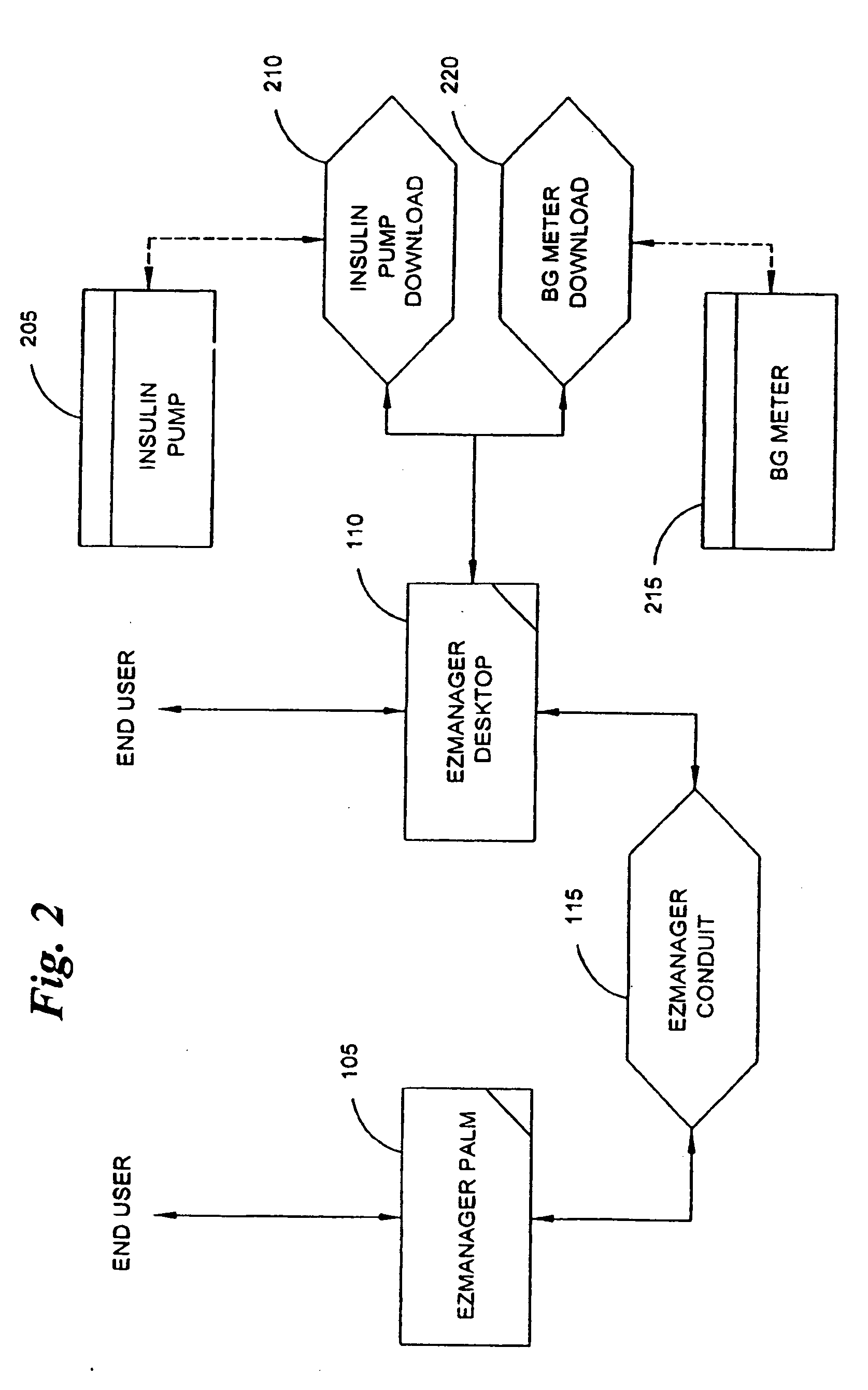
A diabetes management system, an infusion pump, and methods for managing a blood glucose level of a diabetes patient. The infusion pump includes a processor for monitoring an amount of insulin delivered to a patient and an internal database in communication with the processor. The internal database is for storing food information. The diabetes management system includes a blood glucose monitor, a food database, and an infusion pump for delivering insulin to a patient. The food database comprises one or more records of information on various foods. The infusion pump of the diabetes management system includes (i) a processor for monitoring an amount of insulin delivered to the patient and (ii) a second database. The second database is for receiving at least a portion of the information from the food database.
Owner:CROTHALL KATHERINE D +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com