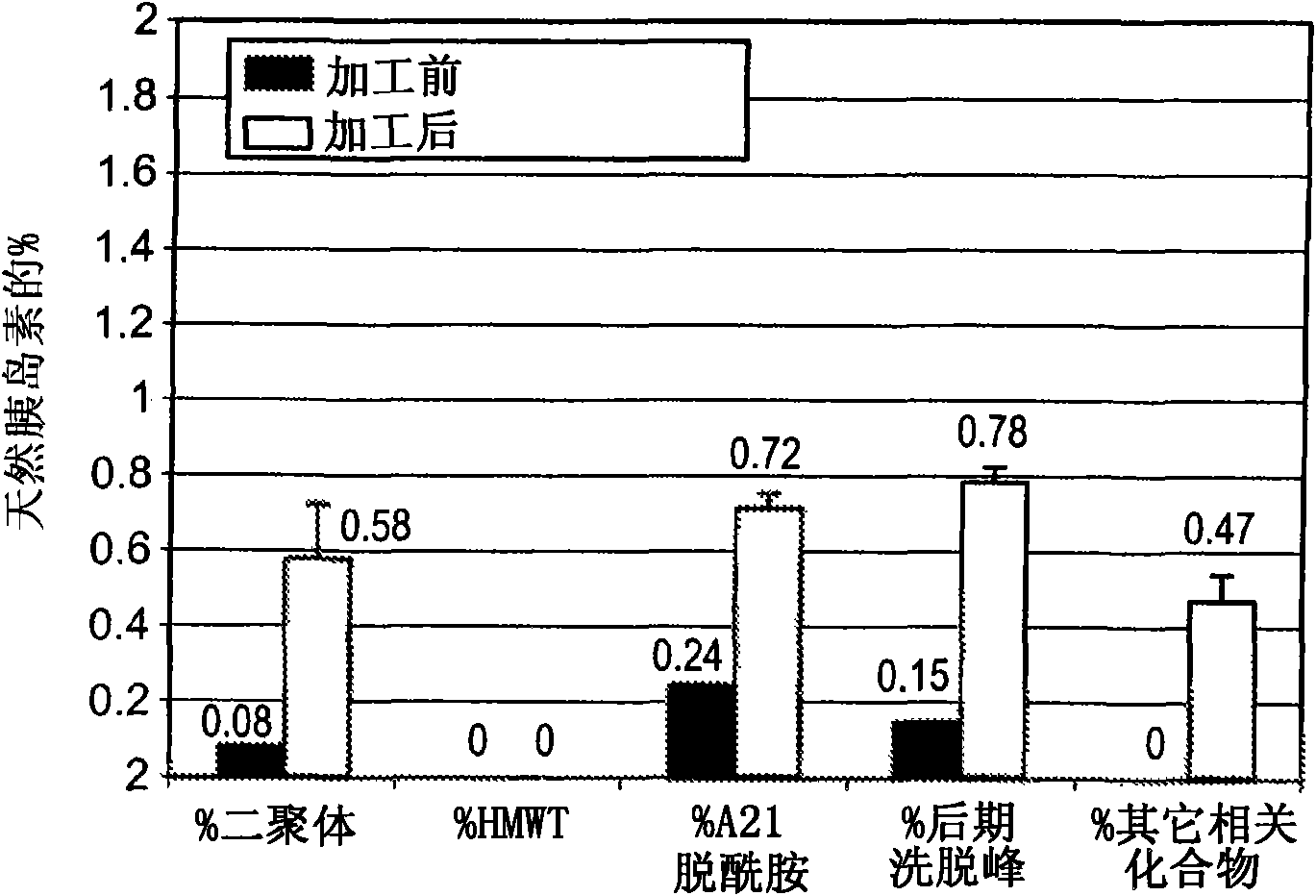
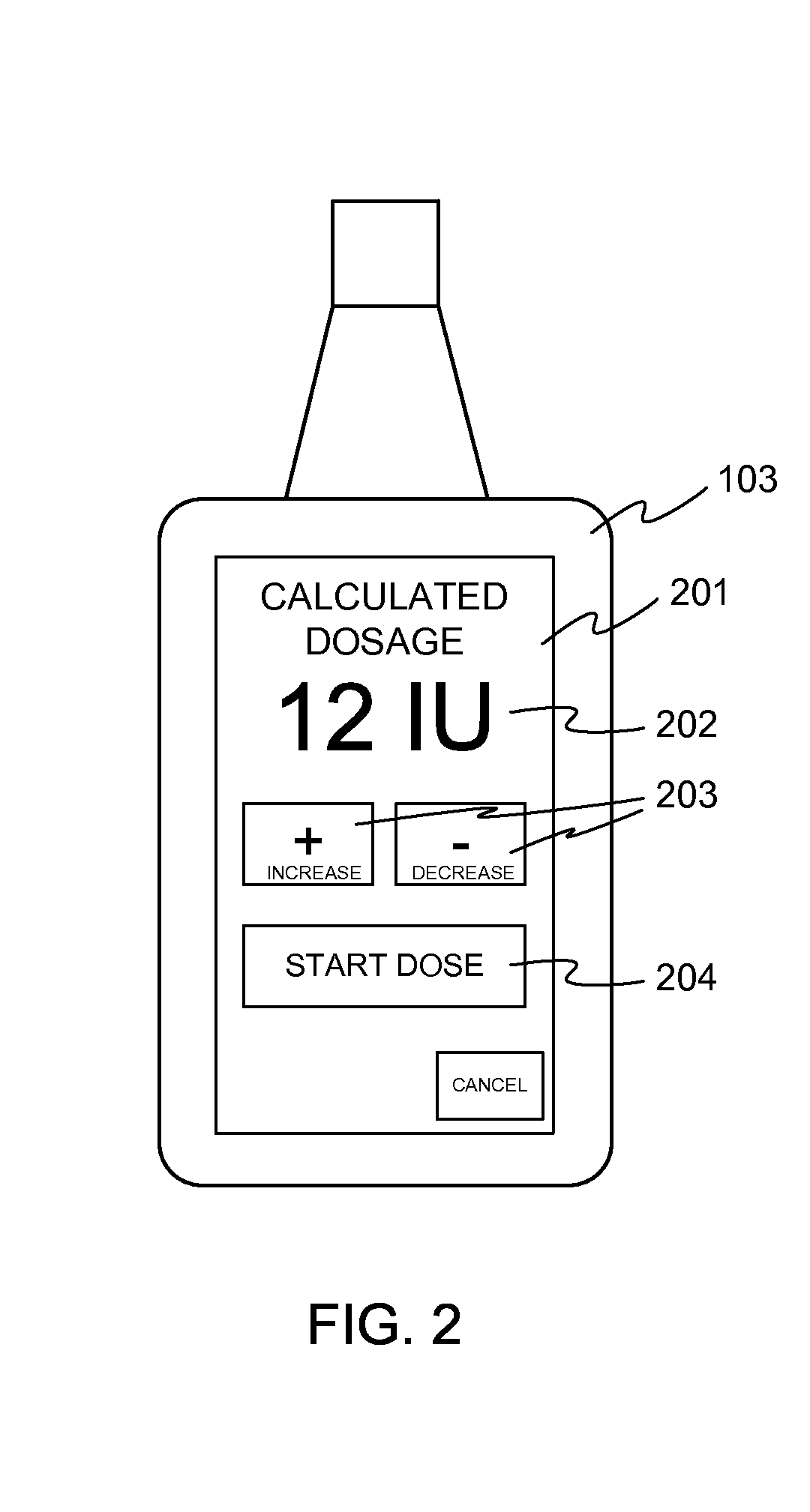
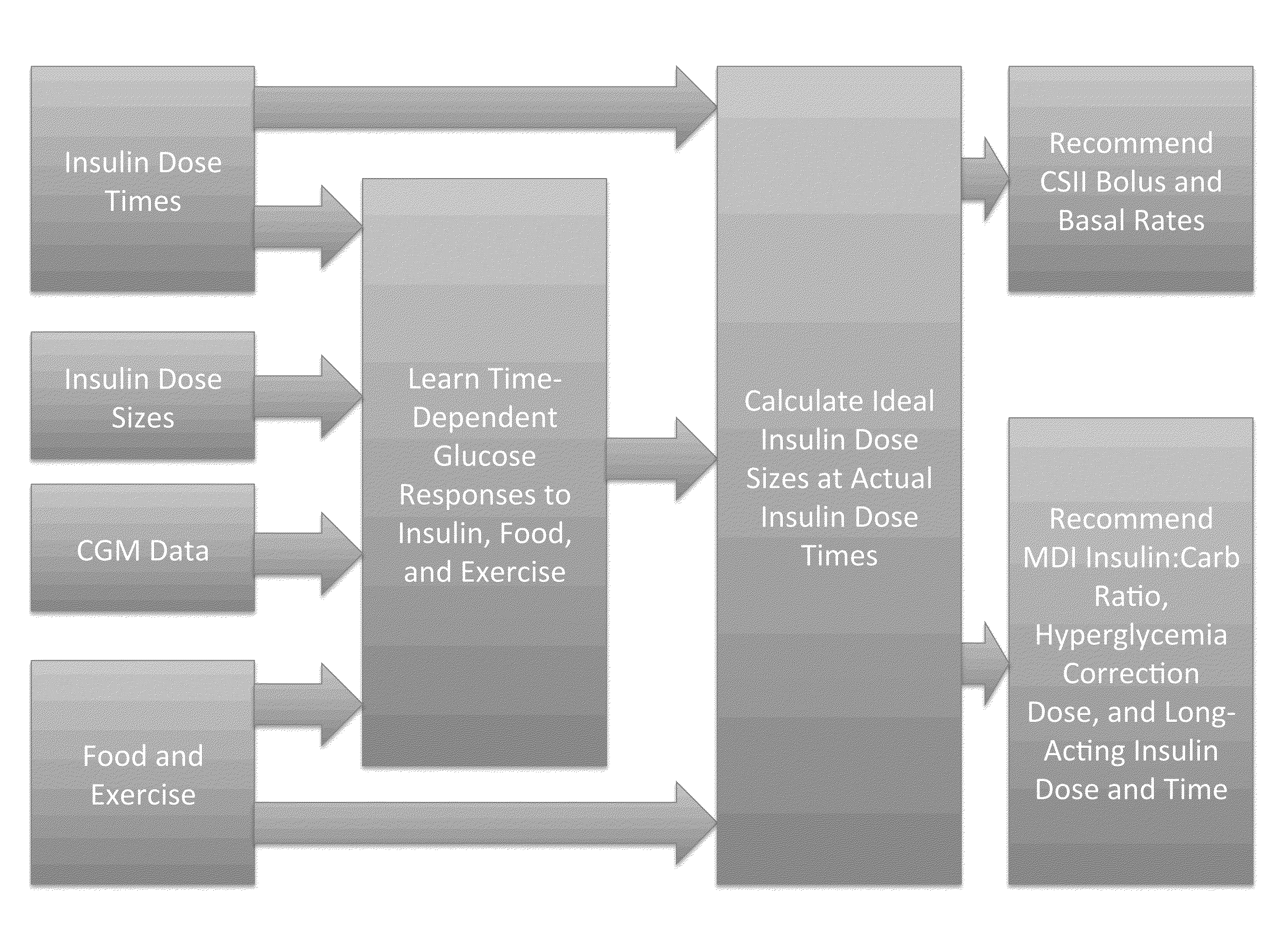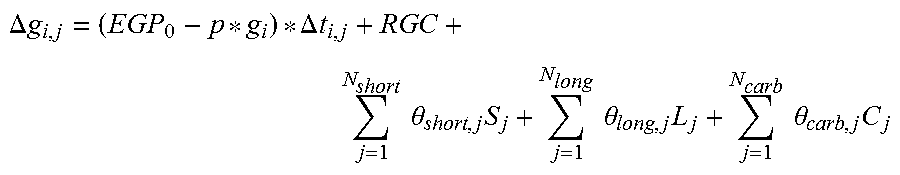Patents
Literature
61 results about "Insulin dose" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
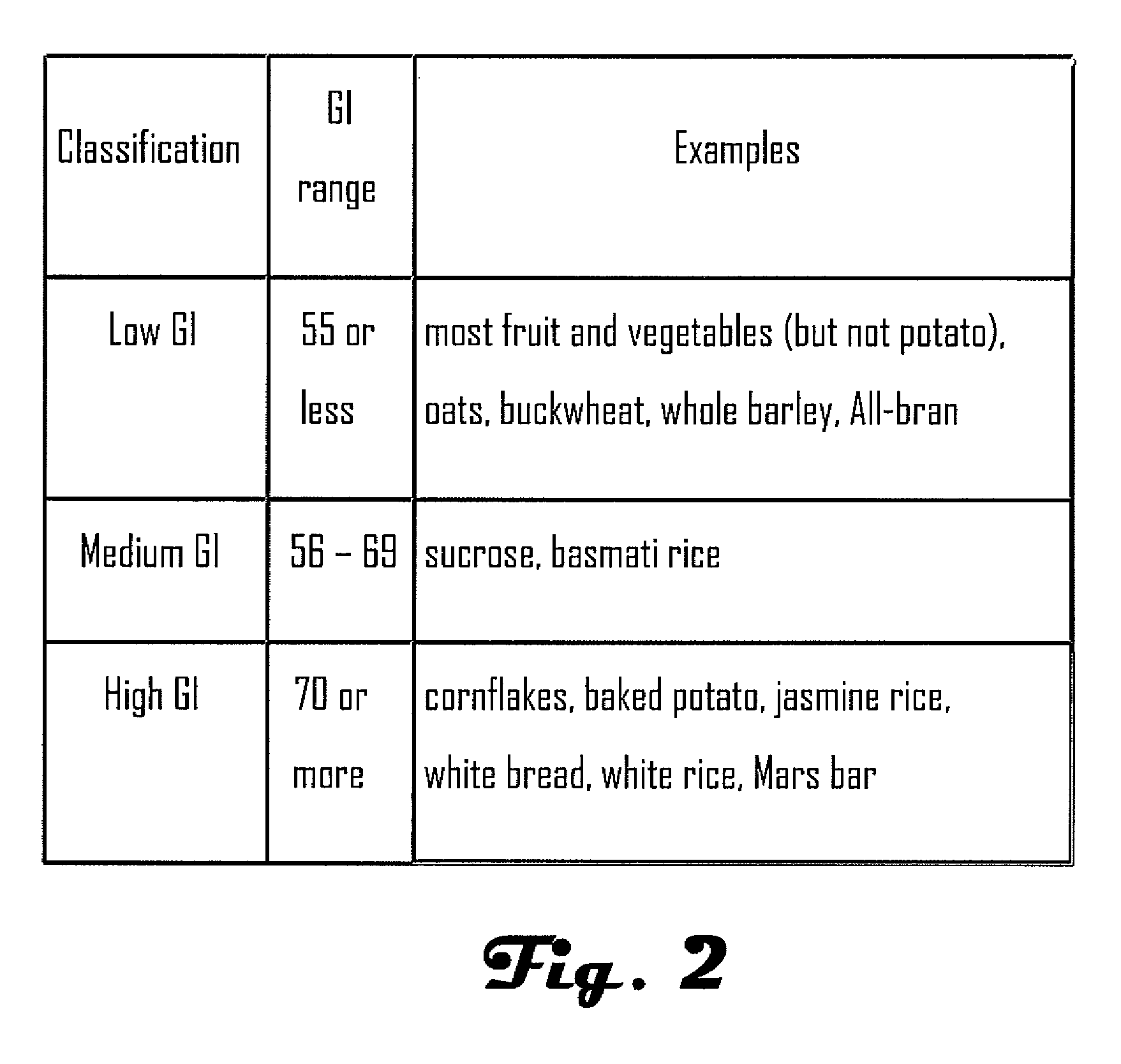
Note: Regular human insulin is available in 2 concentrations: 100 units of insulin per mL (U-100) and 500 units of insulin per mL (U-500) Individualize dose based on metabolic needs and frequent monitoring of blood glucose. -Initial doses are often in the range of 0.2 to 0.4 units/kg/day.
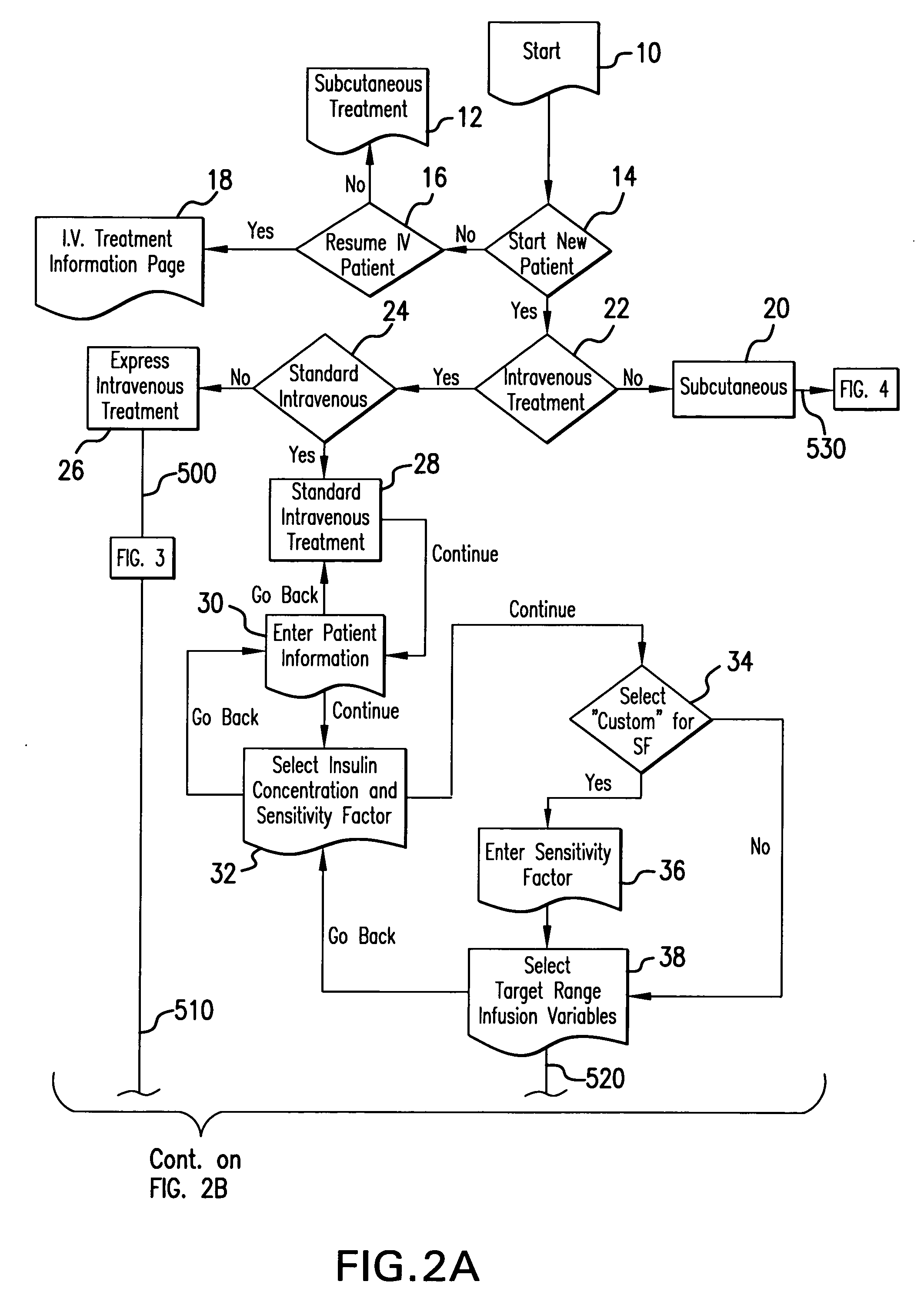
Method for advising patients concerning doses of insulin
A method for guiding a user to select a dose of insulin, including the steps of calculating a firsts pecific dose of insulin by applying information provided by the user to an insulin dose calculation algorithm, wherein such information includes at least the user's current blood glucose level and the user's desired blood glucose level, calculating at least a second specific dose of insulin that is different from the first specific dose, and presenting to the user a range of doses comprising at least two of the specific doses.
Owner:INSULET CORP
Method and system for determining insulin dosing schedules and carbohydrate-to-insulin ratios in diabetic patients
ActiveUS20050049179A1Prevent overshootPeptide/protein ingredientsAutomatic syringesInsulin regimenCompound (substance)
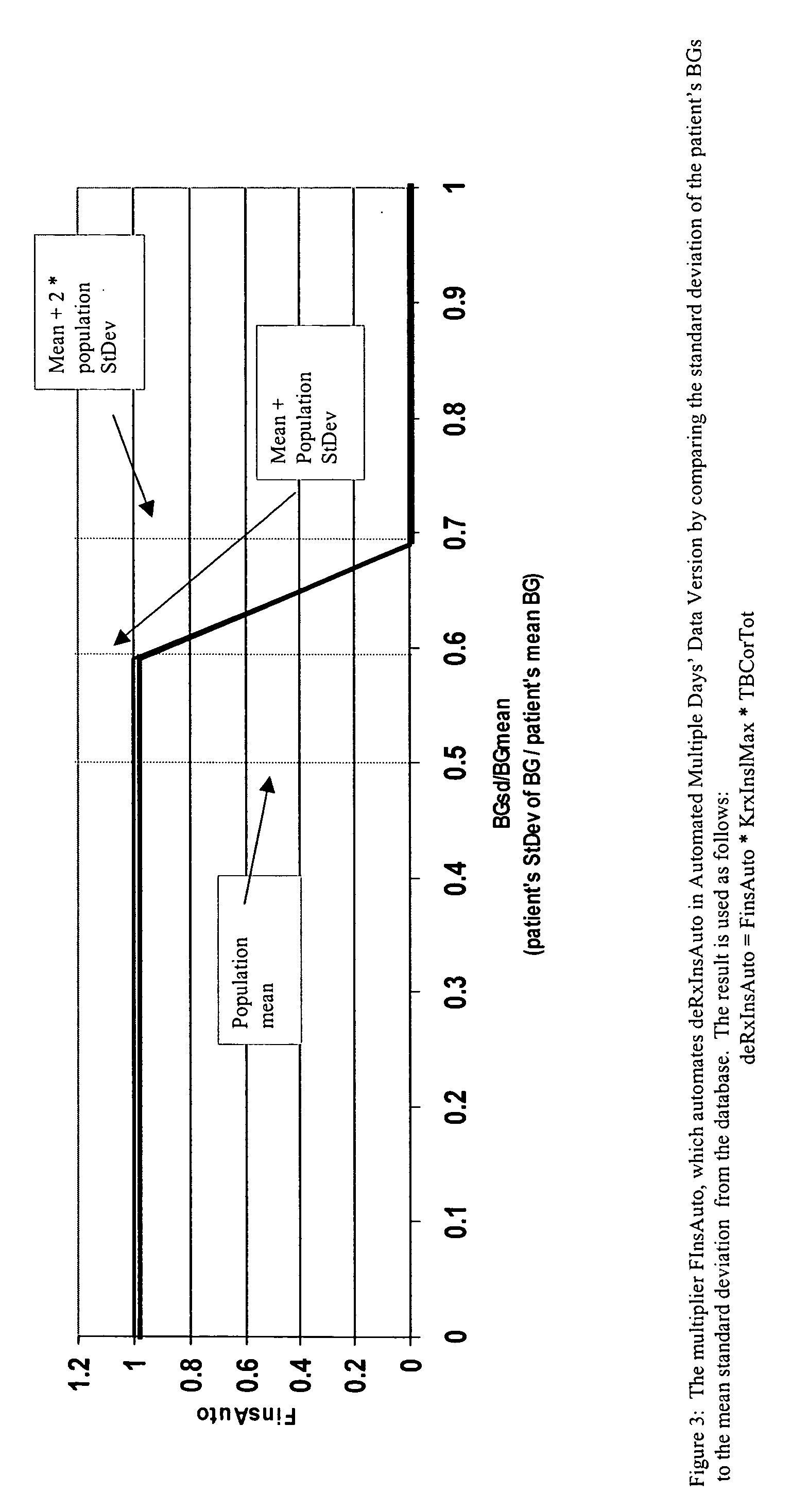
Method for digitally determining the daily insulin regimen for a diabetic patient. The invention divides the patient's day into adjustable time intervals containing basal insulin dosage rates and Carbohydrate-to-Insulin Ratio(s) (for determining meal insulin doses). The invention identifies the Corrective Insulin doses over a time interval as an “error” in the Prescription Insulin (Basal Insulin+Meal Insulin). Methods involve first estimating the change to one of these two components of Prescription Insulin, and then determining the change to the other by subtracting from the error. One method estimates Change in Meal Insulin distributed among intervals proportional to old Meal Insulin. Another method lumps After-Meal Corrective Insulin together with Meal Insulin. Another method splits the interval at the After-Meal Corrective Dose and determines Basal from Time-Boundary Corrective Dose. Data may be obtained from the previous day, and a small fraction of error applied, leading to asymptotic reduction of error. Data may be obtained from recent history, and a larger fraction of error applied by doctor or automatic method.
Owner:ASEKO
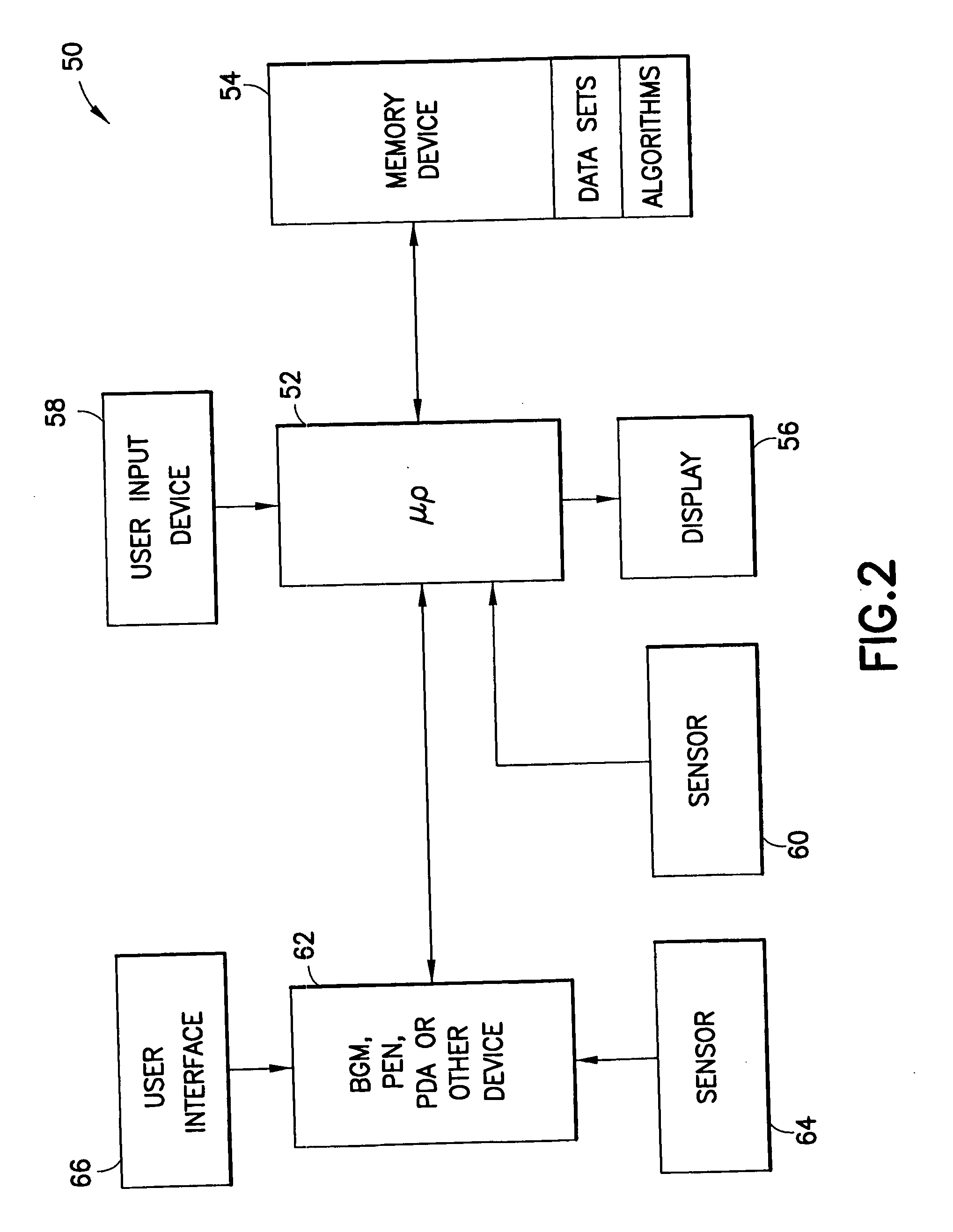
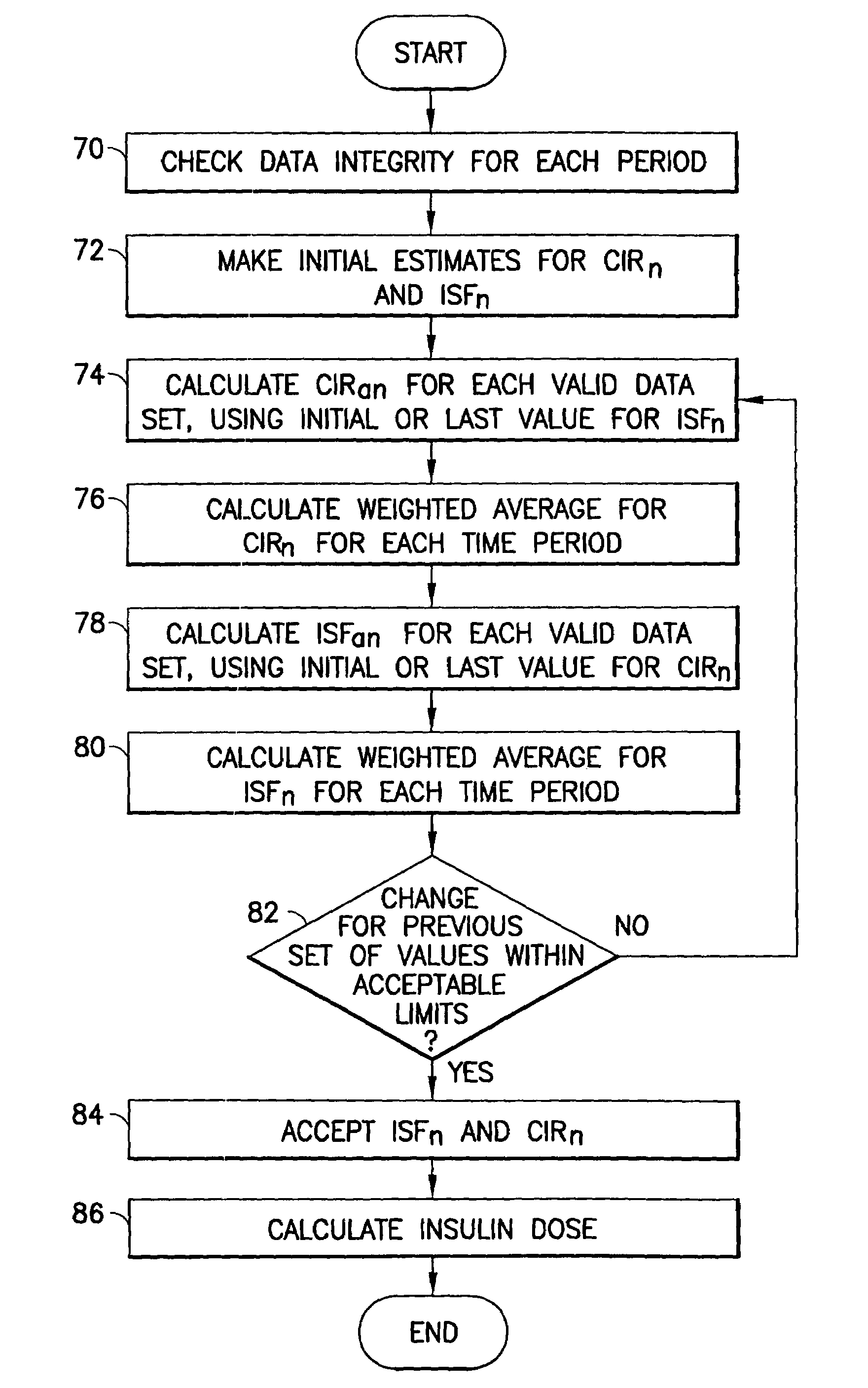
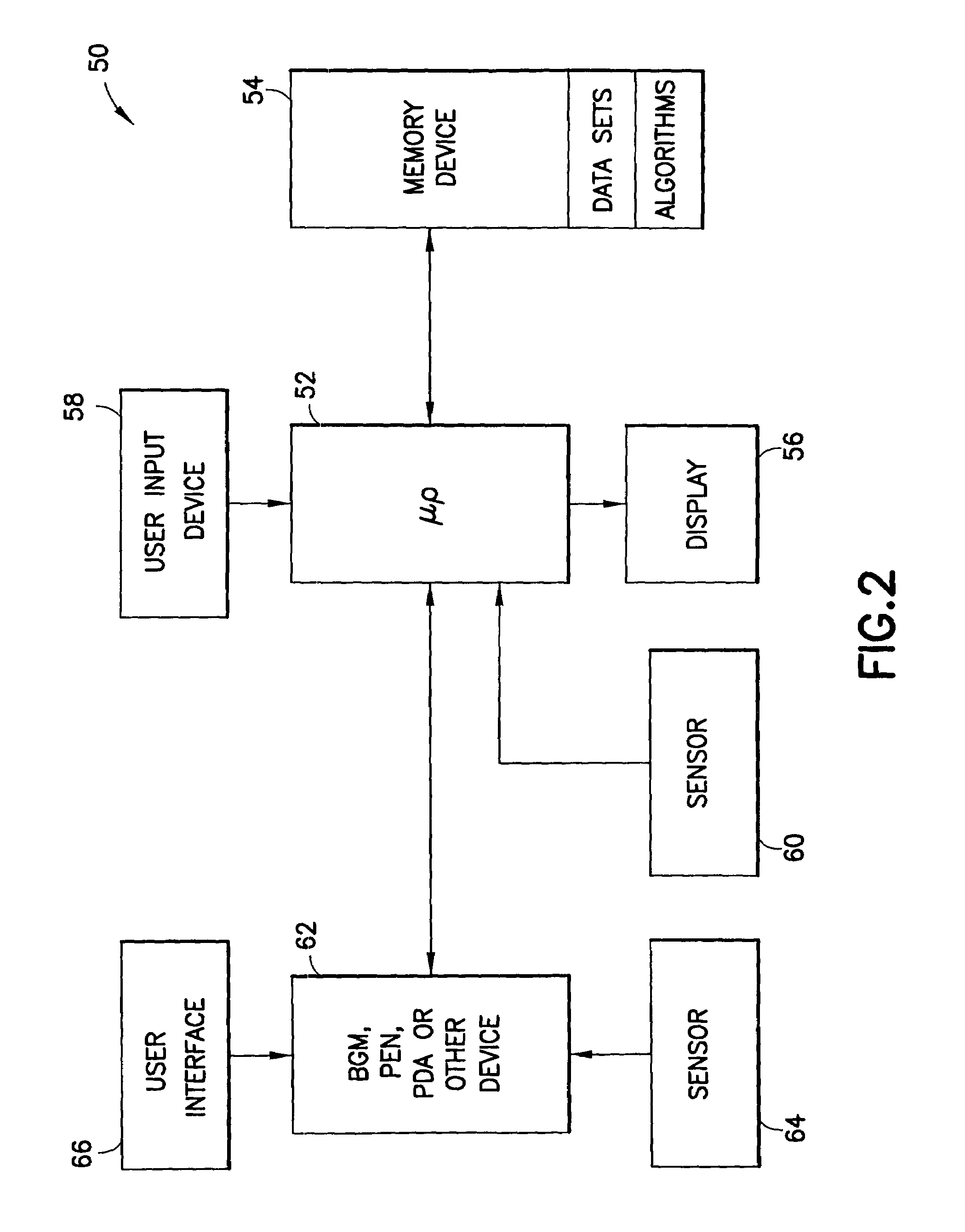
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
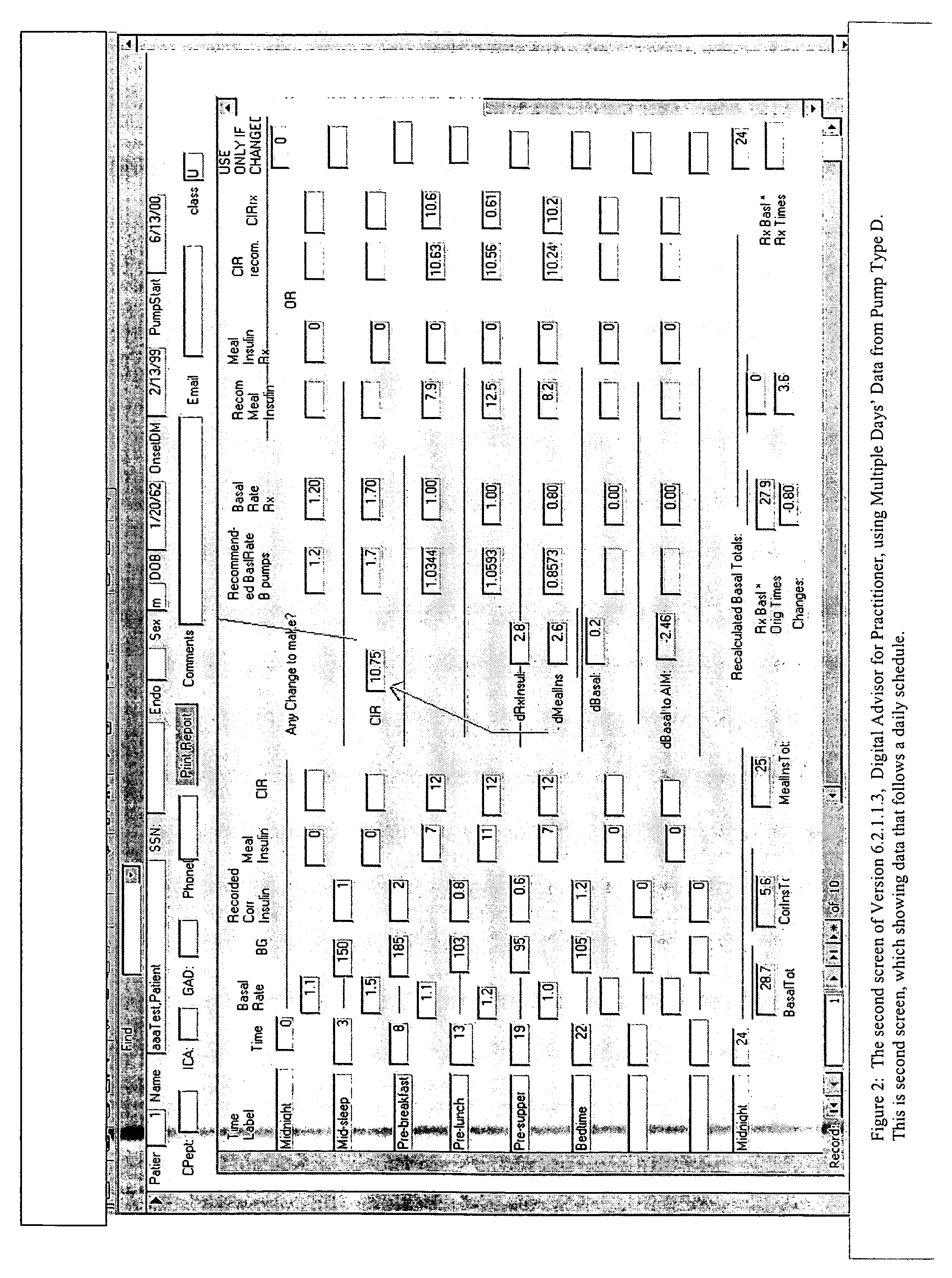
An apparatus and method are provided for determining a patient's carbohydrate to insulin ratio (CIR) and insulin sensitivity factor (ISF), and using these values, along with values for current blood glucose level and deviation from target blood glucose level, for determining insulin dose in view of carbohydrate intake during a particular time period. The apparatus and method employ algorithms that can be implemented in any of a personal computer, personal data assistant, hand held computing device, blood glucose monitor, infusion pump, medication delivery pen, meter, calculator, among other therapeutic, diagnostic or informational devices used for managing a patient's blood glucose levels.
Owner:EMBECTA CORP
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
Owner:EMBECTA CORP
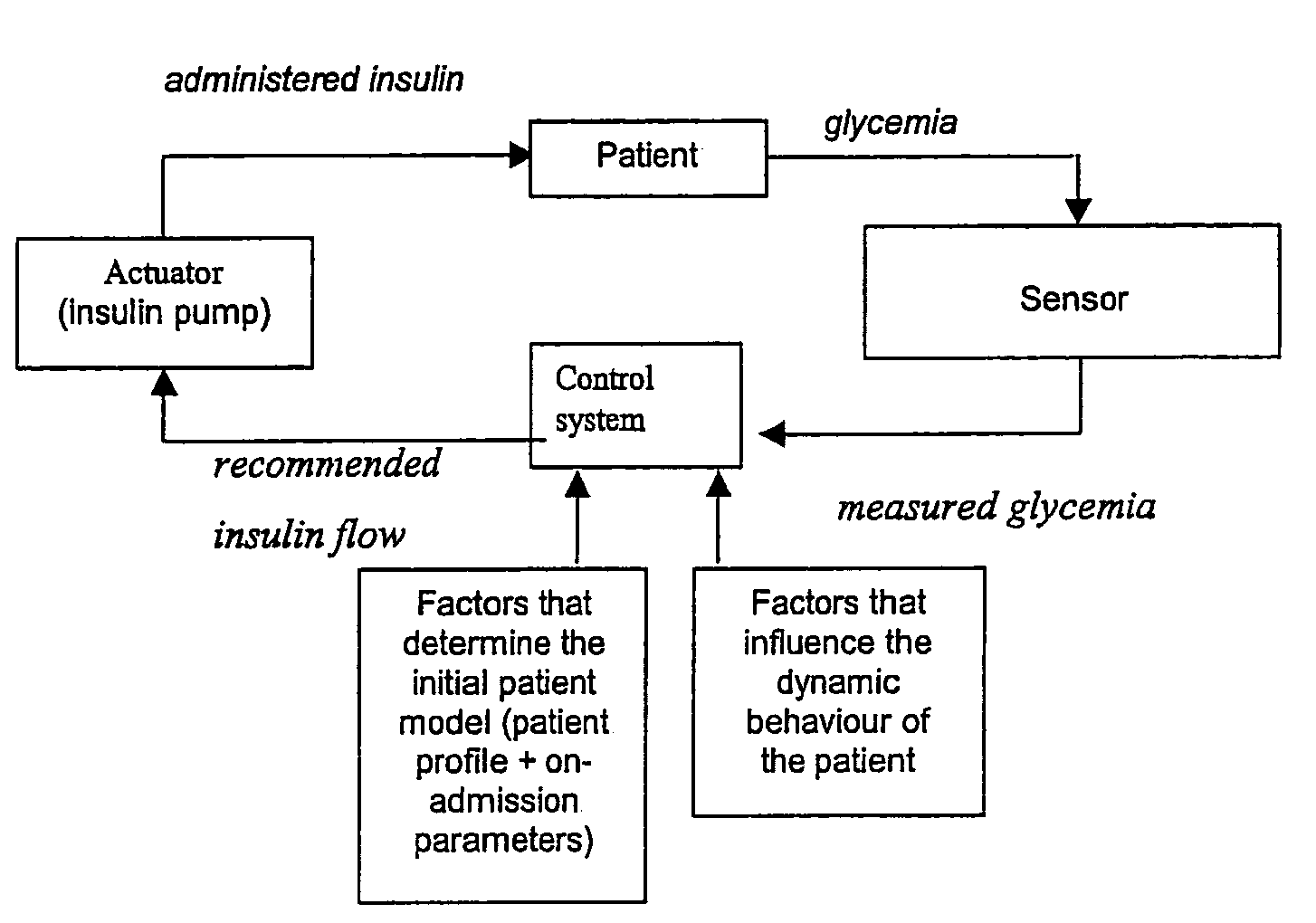
Automatic infusion system based on an adaptive patient model
ActiveUS20050171503A1Ensuring wound repairReduces intensive care and hospital mortality and morbidityMedical devicesPressure infusionPatient modelDisease
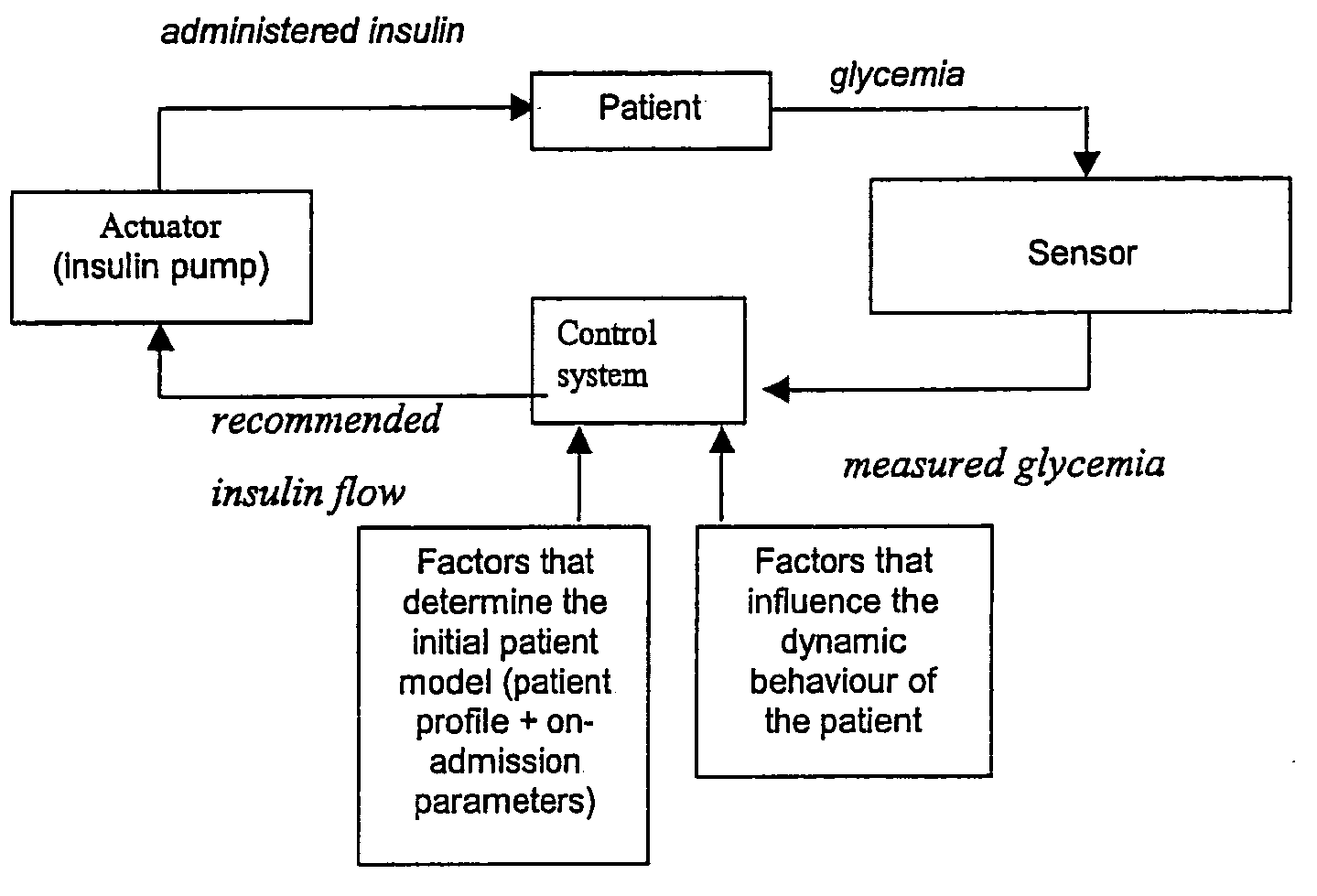
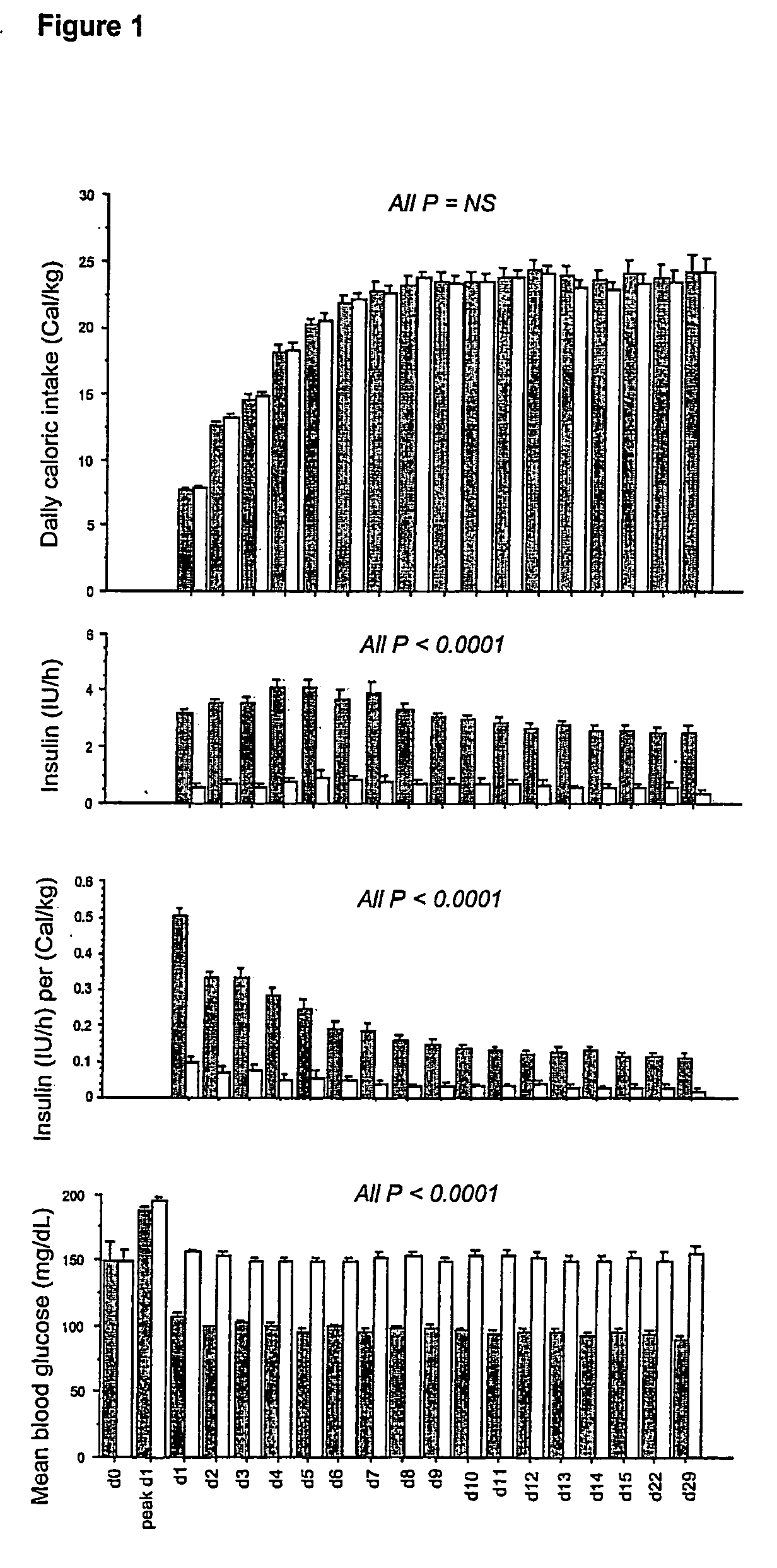
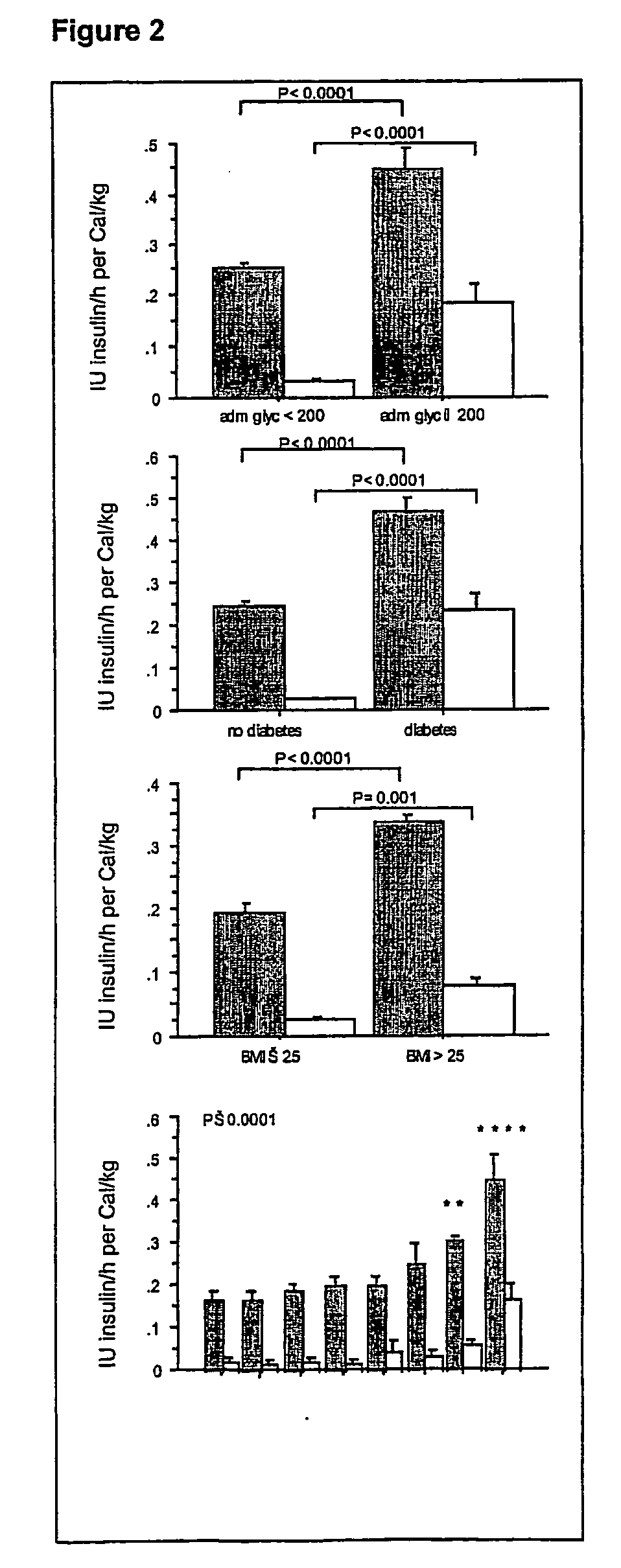
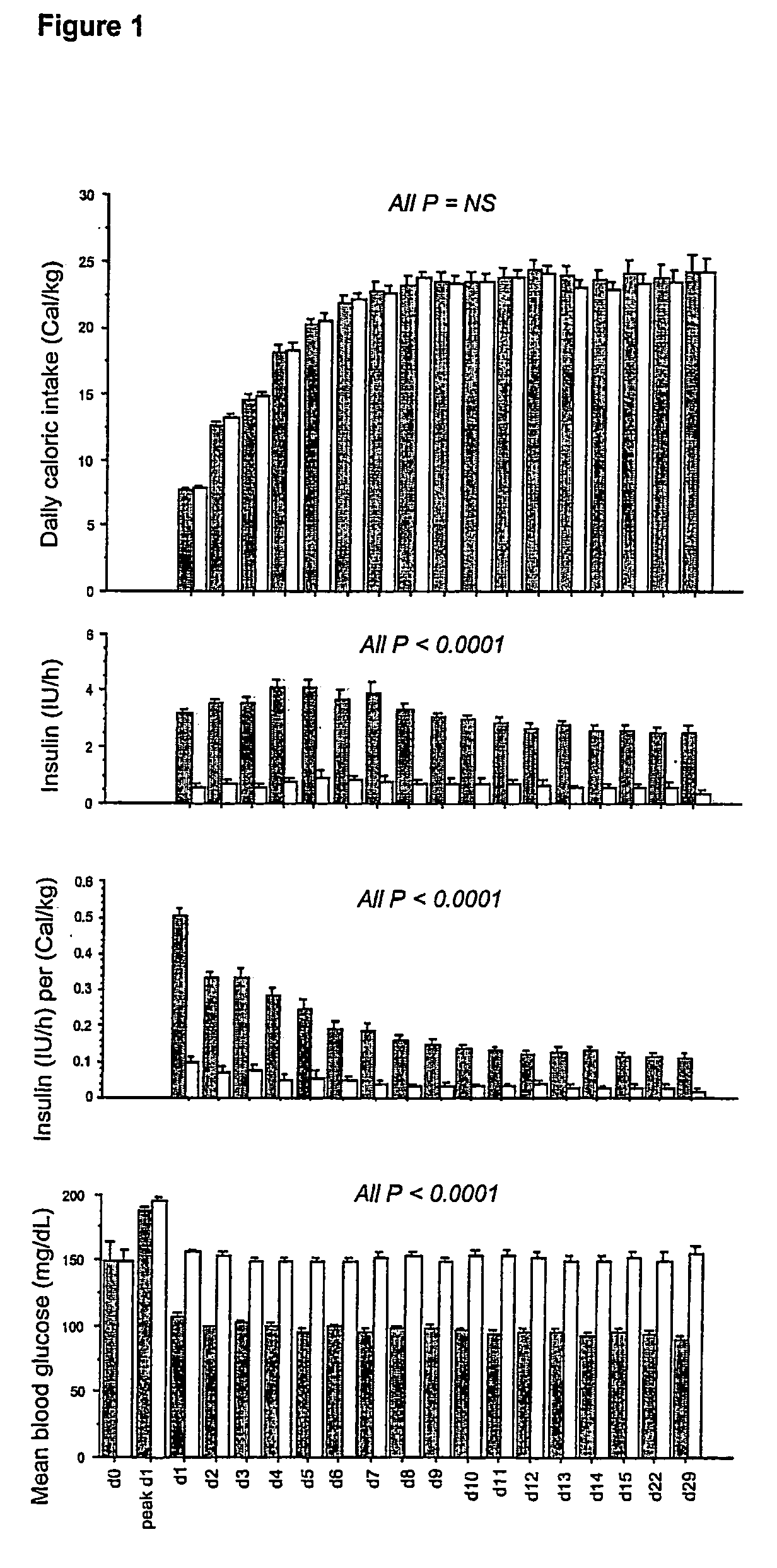
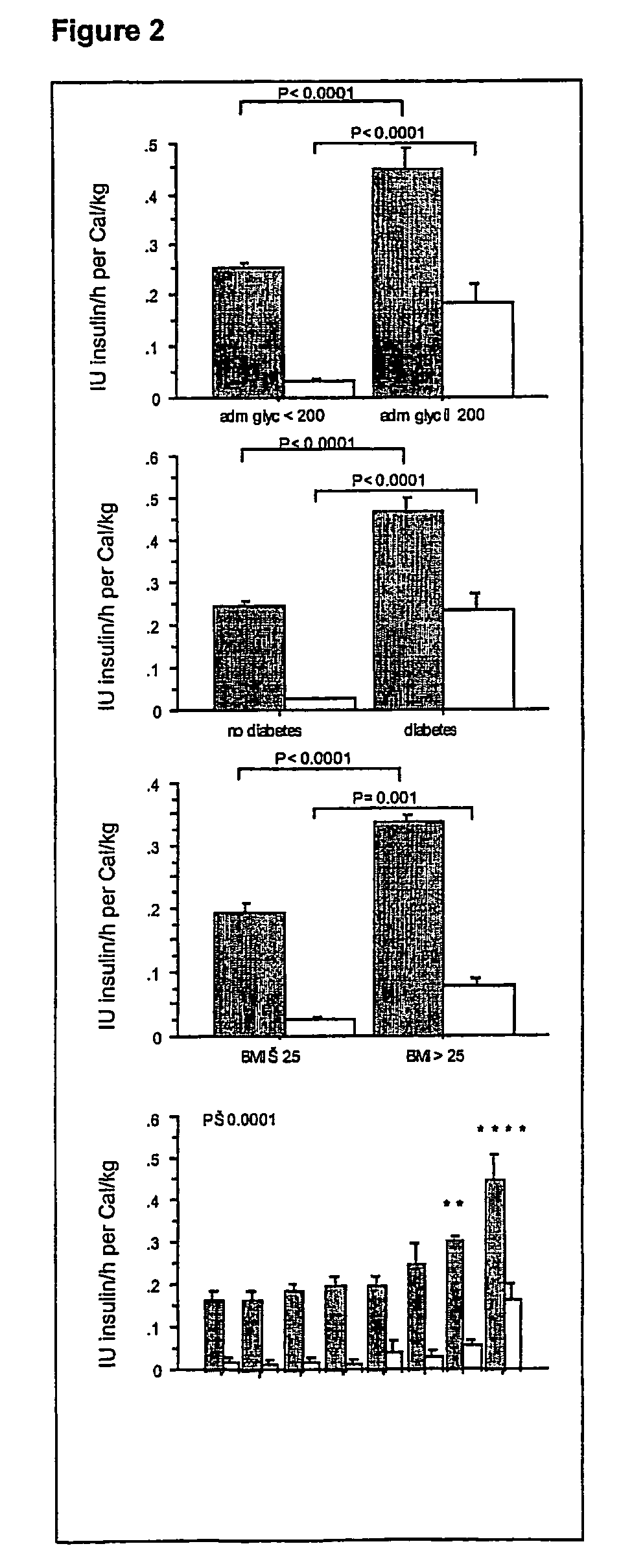
Present invention is a system of blood glucose monitoring and intensive insulin therapy in a ICU for strict maintenance of normoglycemia which reduces intensive care and hospital mortality and morbidity of critically ill adult patients. The findings of present study also reveal factors determining insulin doses needed to maintain normoglycemia as well as the impact of insulin dose versus blood glucose level on the observed outcome benefits have been established. The invention provides a control system that adapts the flow of the insulin infusion based on insulin requirement calculated by blood glucose levels and clinical parameters such as history of diabetes, Body Mass Index, blood glucose level on admission, reason of ICU admission, time in the ICU, type and severity of illness, caloric intake, obesity, drugs affecting insulin sensitivity). This automated insulin monitoring systems significantly reduces the workload and human resource management problems for intensive insulin therapy in patients in the ICU.
Owner:K U LEUVEN RES & DEV
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS7651845B2Accurately predicting insulin bolus dosagesProcess controlPeptide/protein ingredientsDrug and medicationsMonitors blood glucoseGlycemic
Owner:RGT UNIV OF CALIFORNIA
System and method for measuring and predicting insulin dosing rates
InactiveUS20070078314A1Efficient managementDrug and medicationsMedical automated diagnosisPatient dataGlucose polymers
The method and system for managing a patient's blood glucose level predicts an insulin dosing rate to bring a patient's blood glucose level into a preferred target range within a predetermined time interval. The system includes a processor which actuates a blood glucose computer program to measure and predict the patient's blood glucose level. An input mechanism allows for insertion of a preferred target range of the patient's blood glucose level and further permits input of various patient data parameters. The processor calculates the optimum insulin dosing rate for the patient based upon the type of insulin dosing whether it be intravenous dosing and / or subcutaneous dosing. A display mechanism displays the patient dosing parameters and an alarm mechanism alerts a user when the patient's blood glucose level is outside of the preferred patient blood glucose target range.
Owner:GLUCOTEC
Automatic infusion system based on an adaptive patient model
ActiveUS7491187B2Reduces intensive care and hospital mortality and morbiditySafely reachedJet injection syringesMedical devicesPatient modelDisease
Present invention is a system of blood glucose monitoring and intensive insulin therapy in a ICU for strict maintenance of normoglycemia which reduces intensive care and hospital mortality and morbidity of critically ill adult patients. The findings of present study also reveal factors determining insulin doses needed to maintain normoglycemia as well as the impact of insulin dose versus blood glucose level on the observed outcome benefits have been established. The invention provides a control system that adapts the flow of the insulin infusion based on insulin requirement calculated by blood glucose levels and clinical parameters such as history of diabetes, Body Mass Index, blood glucose level on admission, reason of ICU admission, time in the ICU, type and severity of illness, caloric intake, obesity, drugs affecting insulin sensitivity). This automated insulin monitoring systems significantly reduces the workload and human resource management problems for intensive insulin therapy in patients in the ICU.
Owner:K U LEUVEN RES & DEV
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
An apparatus and method are provided for determining a patient's carbohydrate to insulin ratio (CIR) and insulin sensitivity factor (ISF), and using these values, along with values for current blood glucose level and deviation from target blood glucose level, for determining insulin dose in view of carbohydrate intake during a particular time period. The apparatus and method employ algorithms that can be implemented in any of a personal computer, personal data assistant, hand held computing device, blood glucose monitor, infusion pump, medication delivery pen, meter, calculator, among other therapeutic, diagnostic or informational devices used for managing a patient's blood glucose levels.
Owner:EMBECTA CORP
Method of food and insulin dose management for a diabetic subject
InactiveUS7137951B2Lower blood sugar levelsReduce the amount requiredPeptide/protein ingredientsDrug and medicationsINSULIN USEInsulin dose
The invention relates to a method of food and insulin dose management for a diabetic subject, comprising:providing an intended insulin unit value or an intended carbohydrate unit value representing the amount of insulin or carbohydrate intended for intake by the subject; anddetermining the balance value of either insulin units or carbohydrate units needed to balance with the provided unit value and maintain blood sugar in the subject in a target blood sugar range.
Owner:PILARSKI JOSEPH
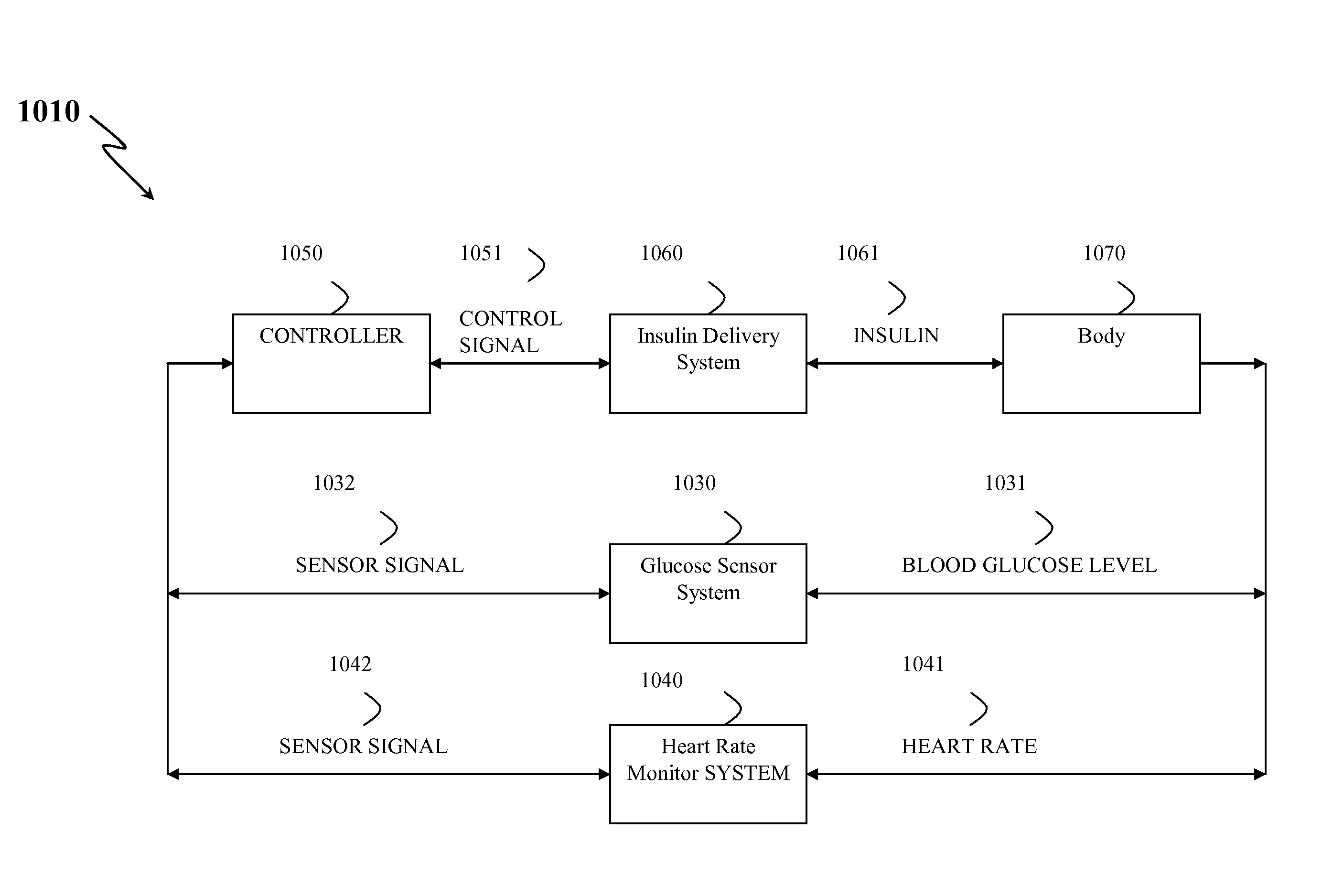
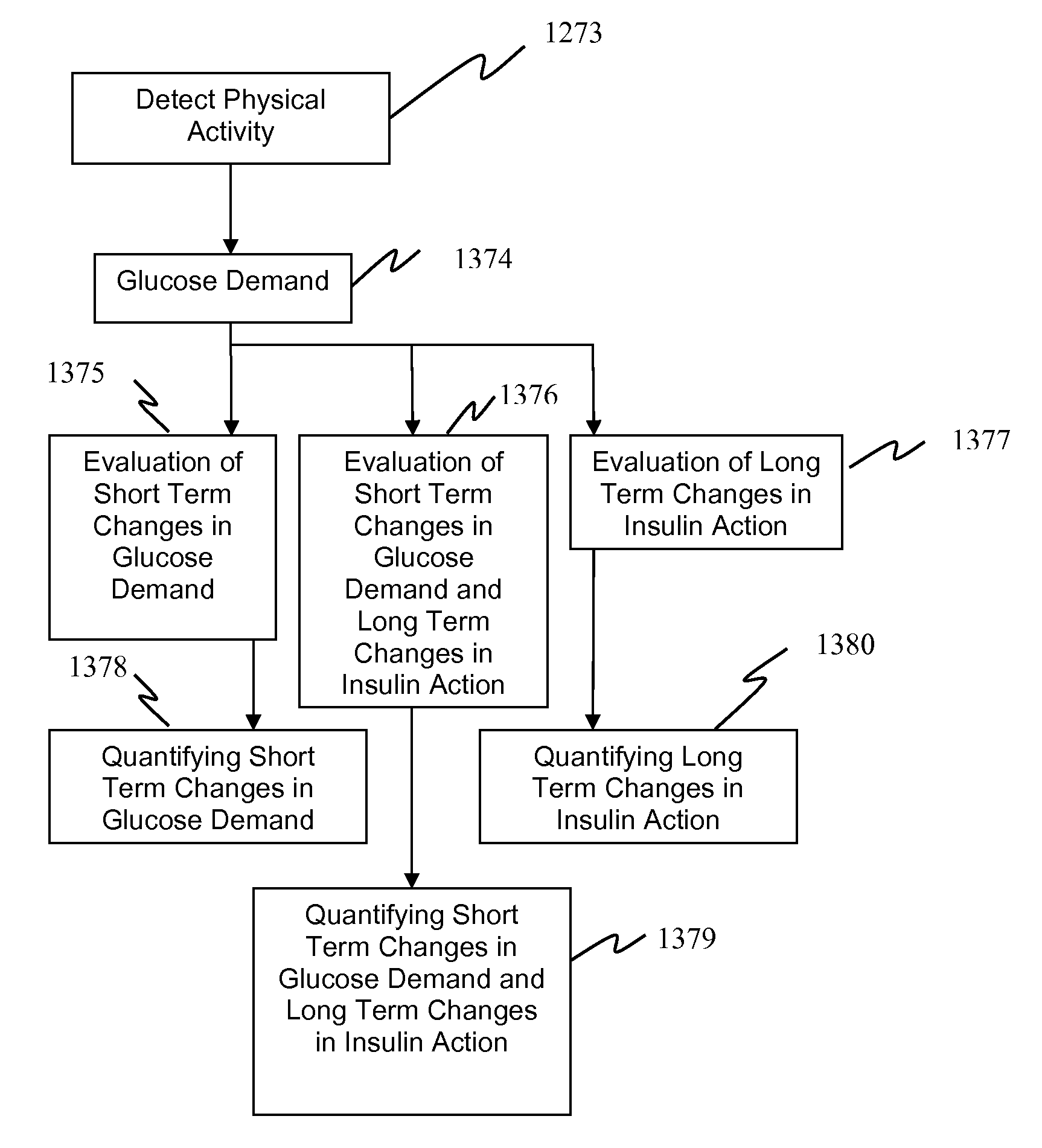
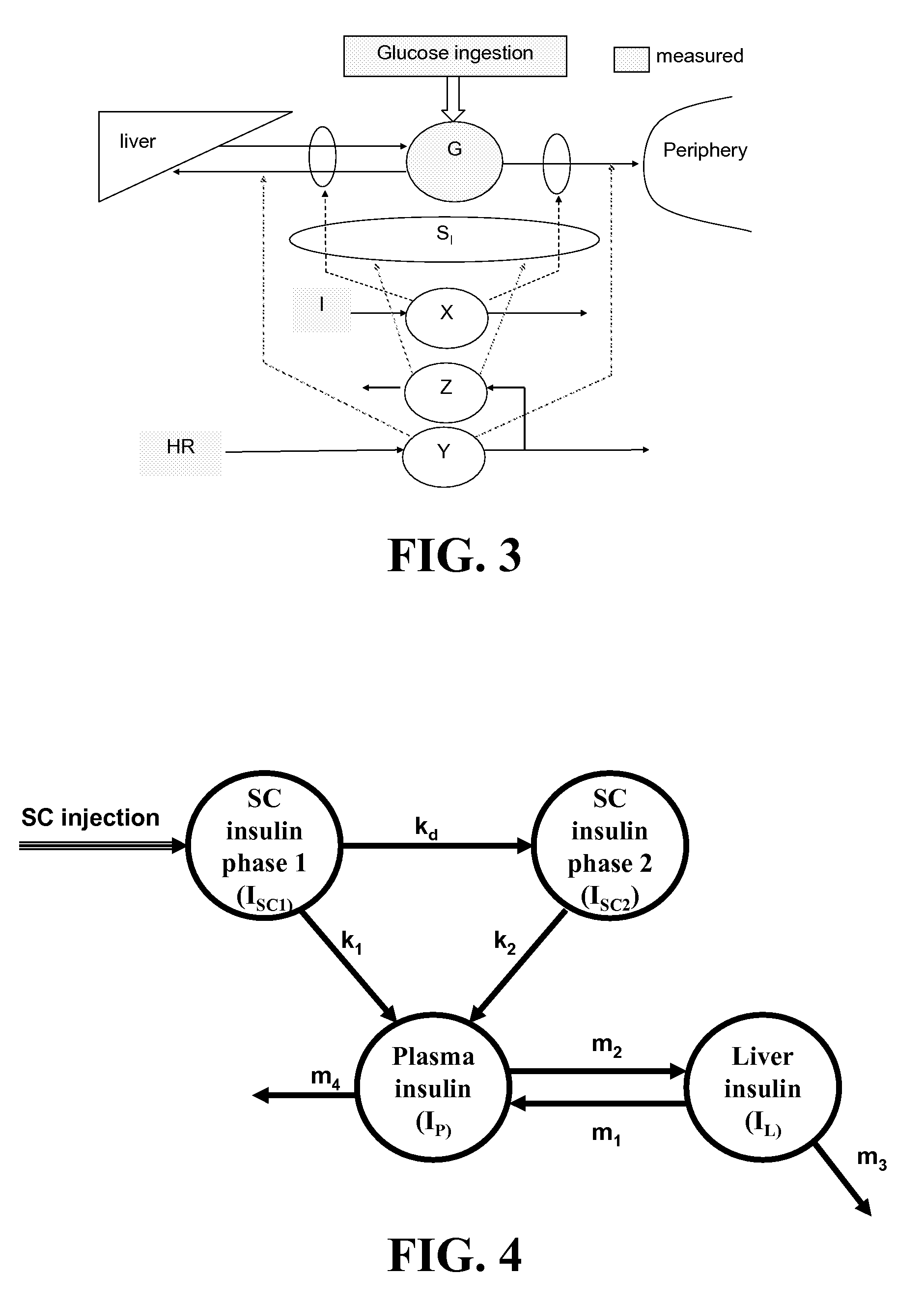
Method, System, and Computer Program Product for the Detection of Physical Activity by Changes in Heart Rate, Assessment of Fast Changing Metabolic States, and Applications of Closed and Open Control Loop in Diabetes
ActiveUS20100057043A1Minimize frequencyAvoid the needMedical simulationDrug and medicationsClosed loopHypoglycemia
A method, system, and computer program product related to the detection of physical activity using changes in heart rate. The method, system, and computer program product evaluates short term glucose demand and long term insulin action due to physical activity. The method, system, and computer program product is further related to the improvement of open and closed loop control of diabetes by accounting for the metabolic changes due to physical activity. The method, system, and computer program product is directed to detecting in real time the short and long term effects of physical activity on insulin action via heart rate analysis, and recommending changes in insulin dosing to compensate for the effects of physical activity. With these recommendations, the open and closed loop control of diabetes can be improved and steps can be taken to prevent hypoglycemia that may result from increased insulin sensitivity due to physical activity.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Clinical variable determination
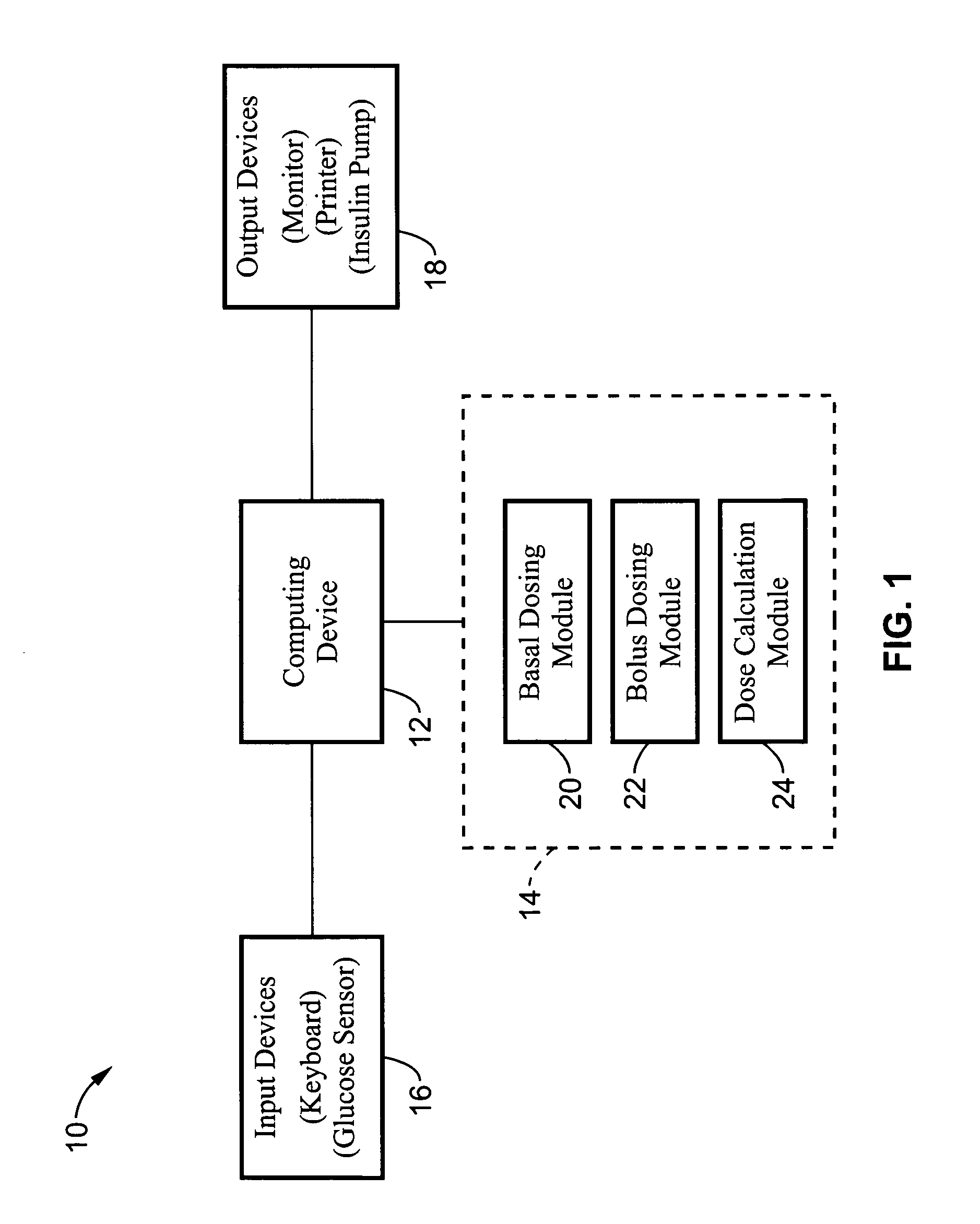
A computer implemented method of determining a clinical variables utilizing an insulin pump that includes initiating blood glucose measurements, initiating ingestion of carbohydrates and receiving input data based on the blood glucose measurements and the ingestion of carbohydrates and utilizing the data to calculate clinical variables. The invention may include presenting instructions to a patient to take various actions and to input various data. The clinical variables determined may be stored in memory and then used to calculate insulin doses and to send a signal to an insulin pump to infuse the insulin dose calculated.
Owner:TANDEM DIABETES CARE INC
Method, System and Computer Program Product for Evaluation of Insulin Sensitivity, Insulin/Carbohydrate Ratio, and Insulin Correction Factors in Diabetes from Self-Monitoring Data
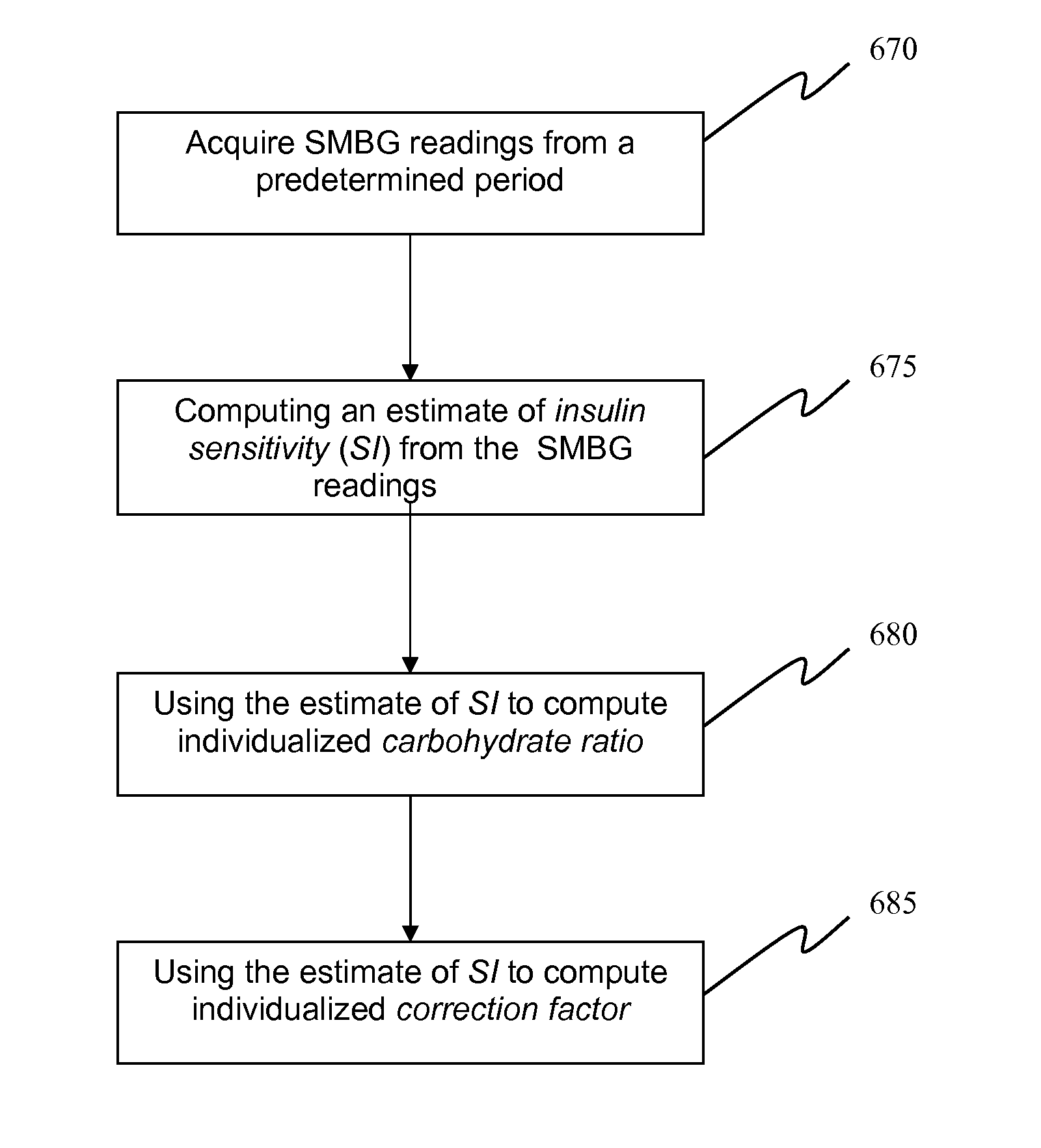
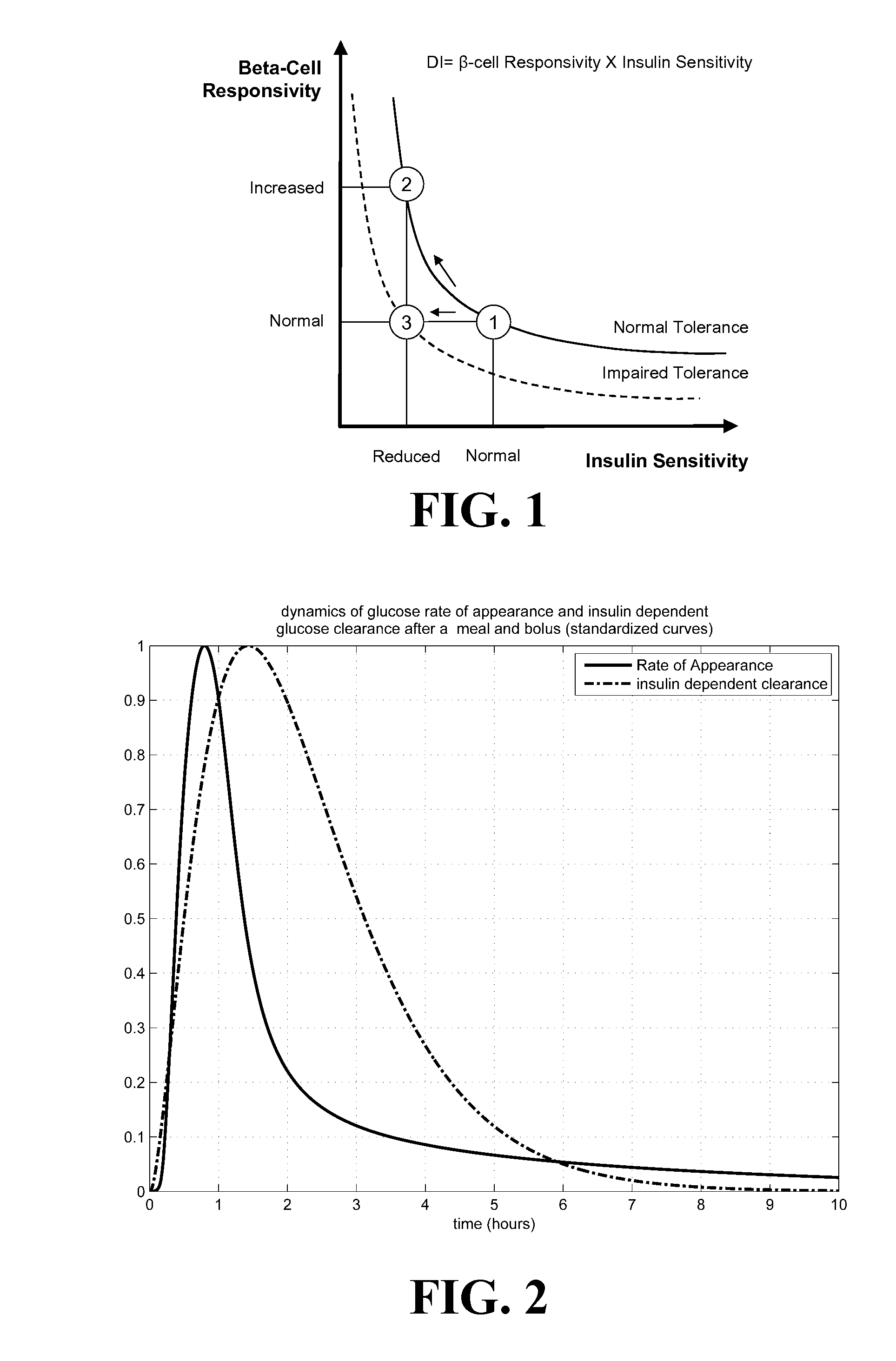
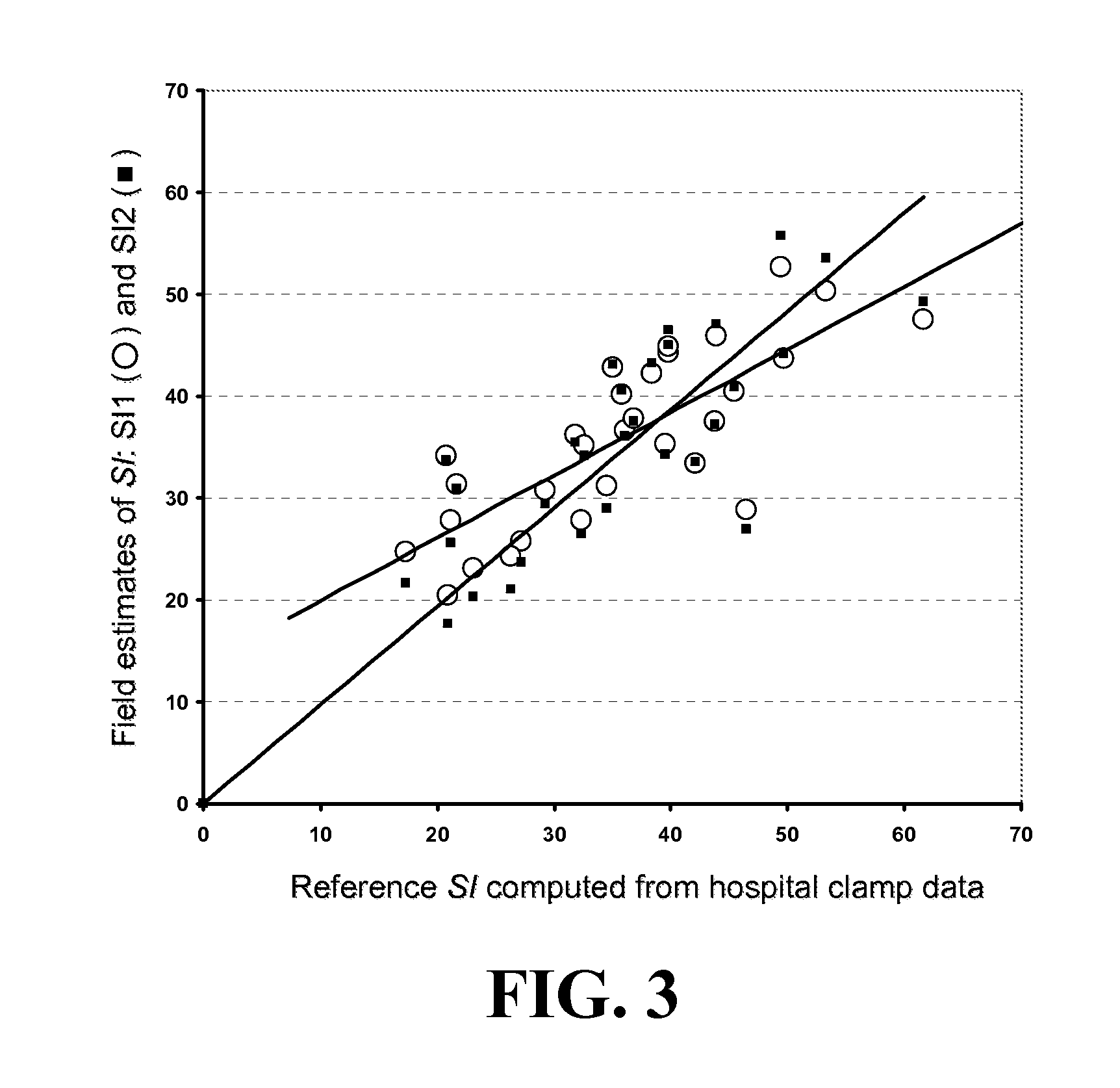
A method, system and computer program product for evaluating or determining a user's insulin sensitivity (SI). An initial step or module may include acquiring SMBG readings from a predetermined period. Another step or module may include computing an estimate of insulin sensitivity (SI) from the SMBG readings. Another step or module may include using the estimate of SI to compute individualized carbohydrate ratio. Additionally, another step or module may include using the estimate of SI to compute individualized correction factor. The computation of the two components of an insulin dose calculator, carbohydrate ratio and correction factor, uses this estimate, which allows the tailoring of carbohydrate ratio and correction factor to the present state of the person.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
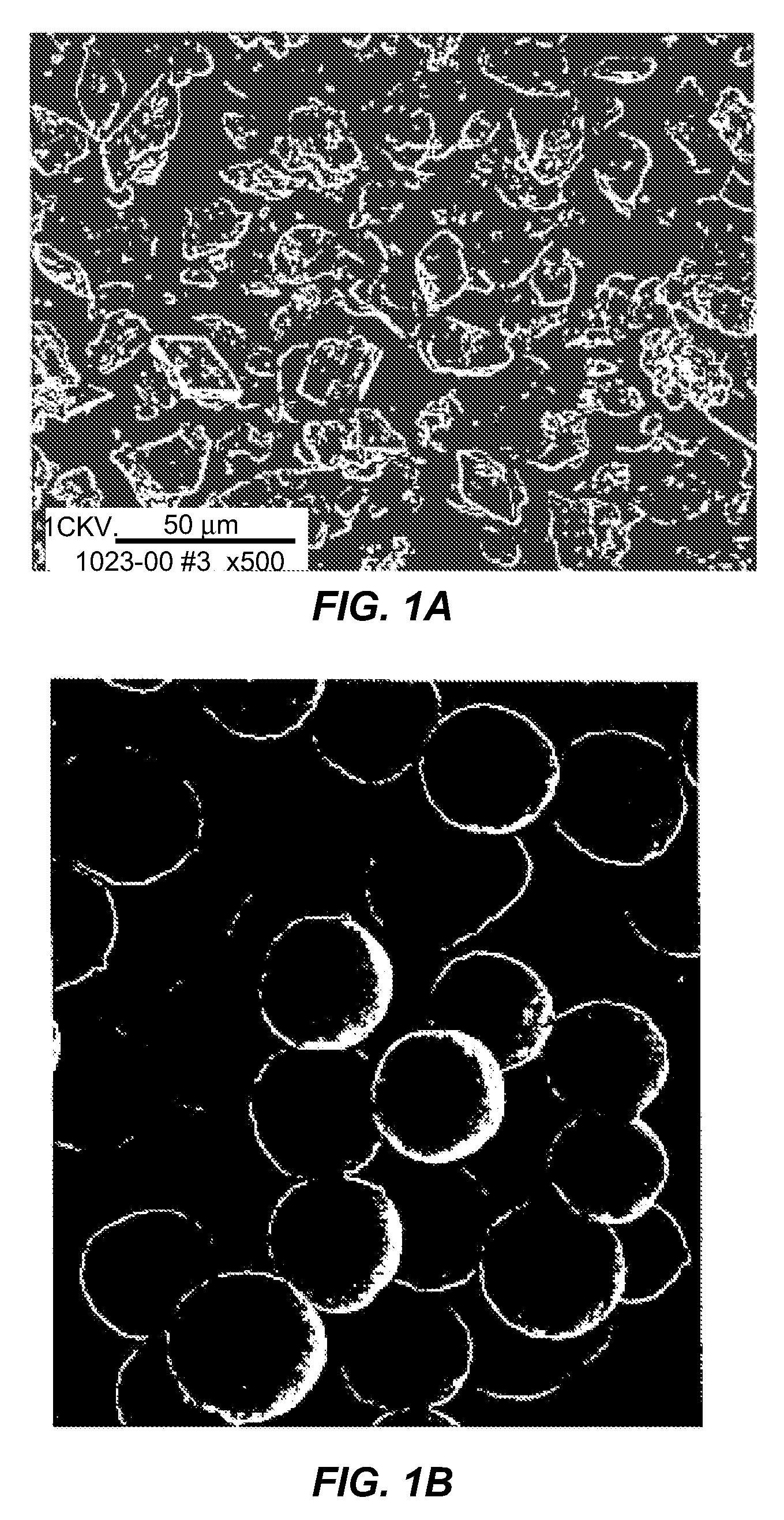
Pulmonary delivery of spherical insulin microparticles
InactiveUS20080026068A1Improved pulmonary application potentialImprove solid stabilityPowder deliveryPeptide/protein ingredientsMedicineClinical trial
Compositions of spherical insulin particles having improved pulmonary application potentials and methods of forming and using these compositions are disclosed in the present application. In one clinical trial with 30 healthy male human subjects, no coughing was observed upon a single pulmonary administration of the spherical insulin particles at an insulin dose of 6.5 mg, nor during the 10-hour post dosing period.
Owner:BAXTER INT INC +1
Method For Defining And Interactively Managing A Treatment For Glycemic Level Control In A Diabetic Patient And Device That Carries Out Said Method
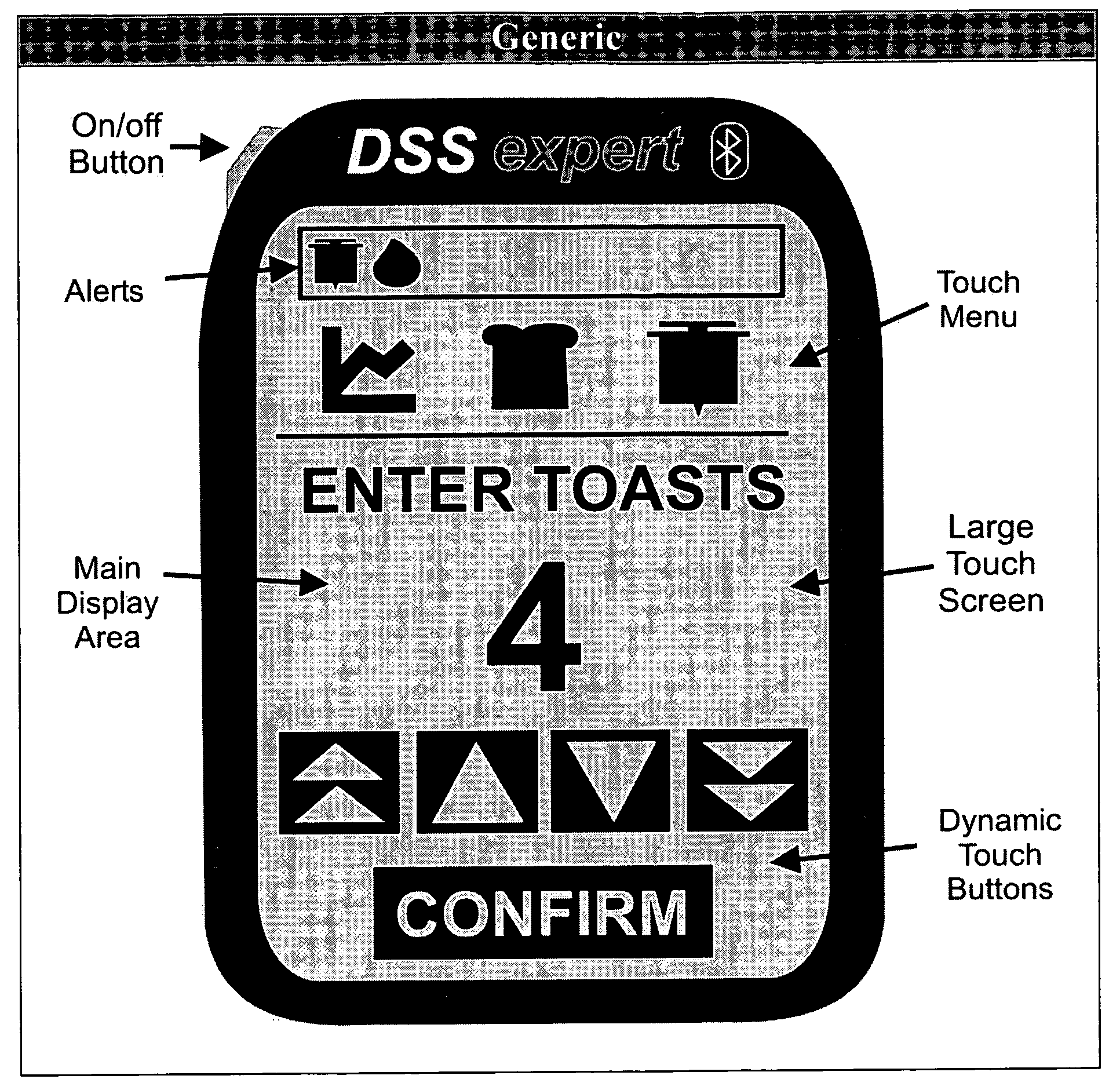
InactiveUS20110119081A1Easy doseIncreasing unmanageably his workloadData processing applicationsDrug and medicationsWork cycleDisplay device
A method for interactive definition and management of a treatment for monitoring blood glucose of a diabetic patient includes performing two distinct, asynchronous and simultaneous, work cycles, having different length: a first local cycle, shorter, includes the steps of acquiring at least one value of said patient's blood glucose by means of a glucometer integrated in a monitoring device, calculating an insulin dosage value to be administered to the patient, by means of a computing algorithm, provided by a specialist doctor, displaying the insulin dosage on a monitoring device display, and defining a date and time for subsequent acquisition of blood glucose level. Furthermore, the system automatically sends glycemic information to the doctor, if they are beyond mean value parameters, time passed from a last contact and number of hypoglycemic value measurements, that the specialist himself can set for each patient and modify over time. A second remote cycle, longer, includes reading the patient's last blood glucose value, periodical running of a procedure for extracting a group of data significant for the patient, related to at least the trend of glycemic level values of the patient and to corresponding insulin doses, recommended on the basis of computation of said algorithm in a predetermined previous period; transmission of the group of significant date to the specialist' doctor's remote computer, by means of a simplified transmitter / receiver module operating via a mobile telephone network, integrated with monitoring device, possibly receiving a set of modified parameters for performing the computing algorithm, or a new computing algorithm, and introduction of such set of modified parameters or of the new algorithm in the managing program.
Owner:BG INFORMATICA
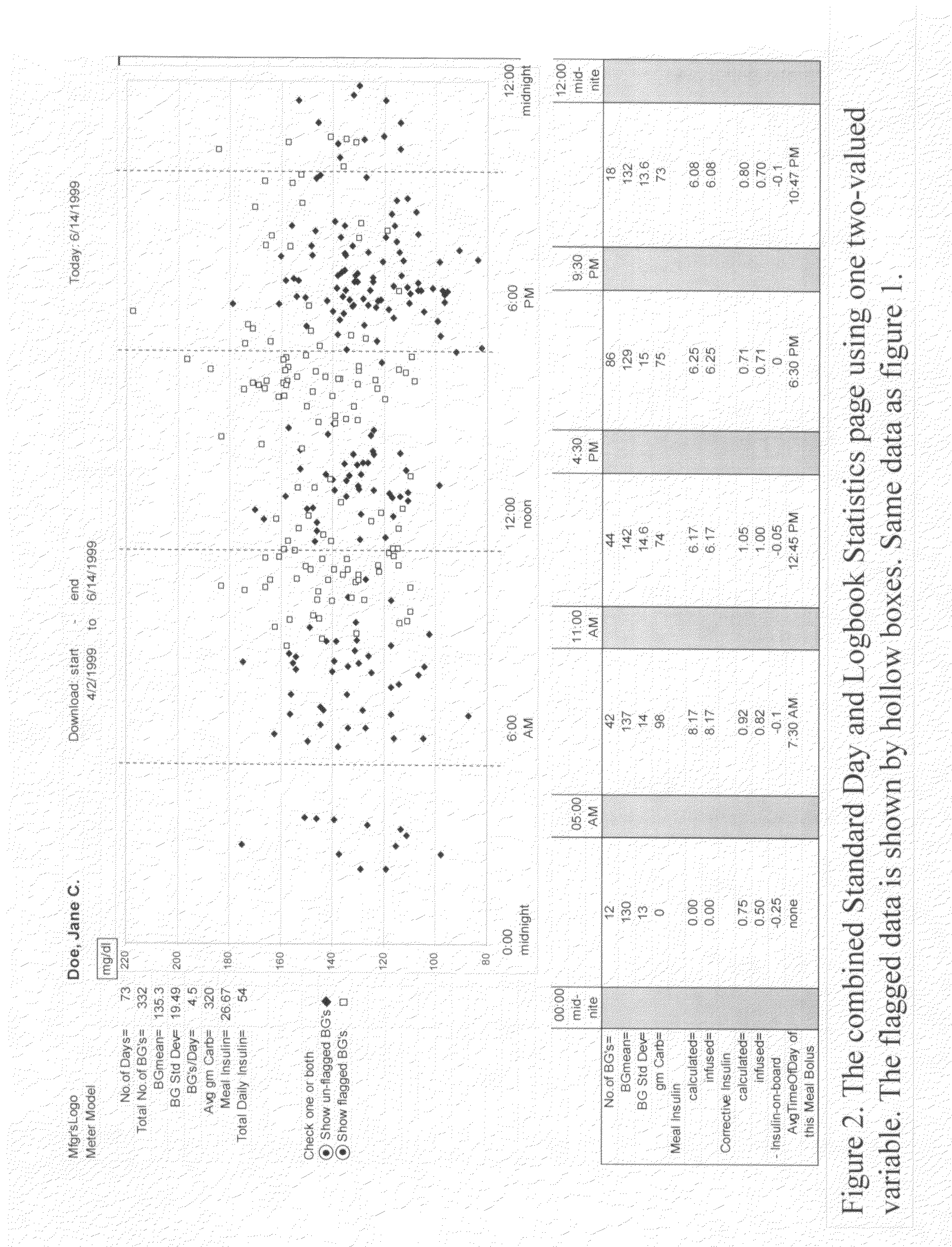
Method and system for categorizing blood glucose tests at test time in a portable device or later in a downloading program and then analyzing the categories separately
InactiveUS20080255707A1Increase currentDrug and medicationsMedical devicesStatistical analysisEmergency medicine
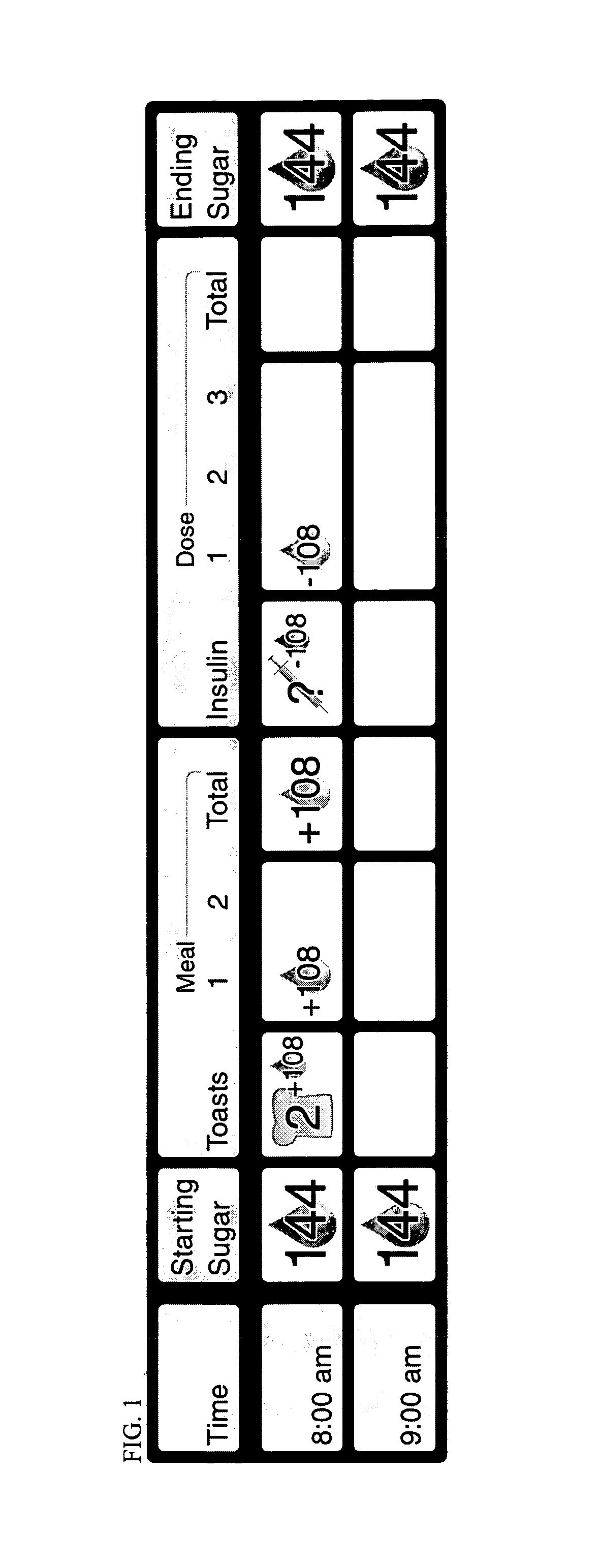
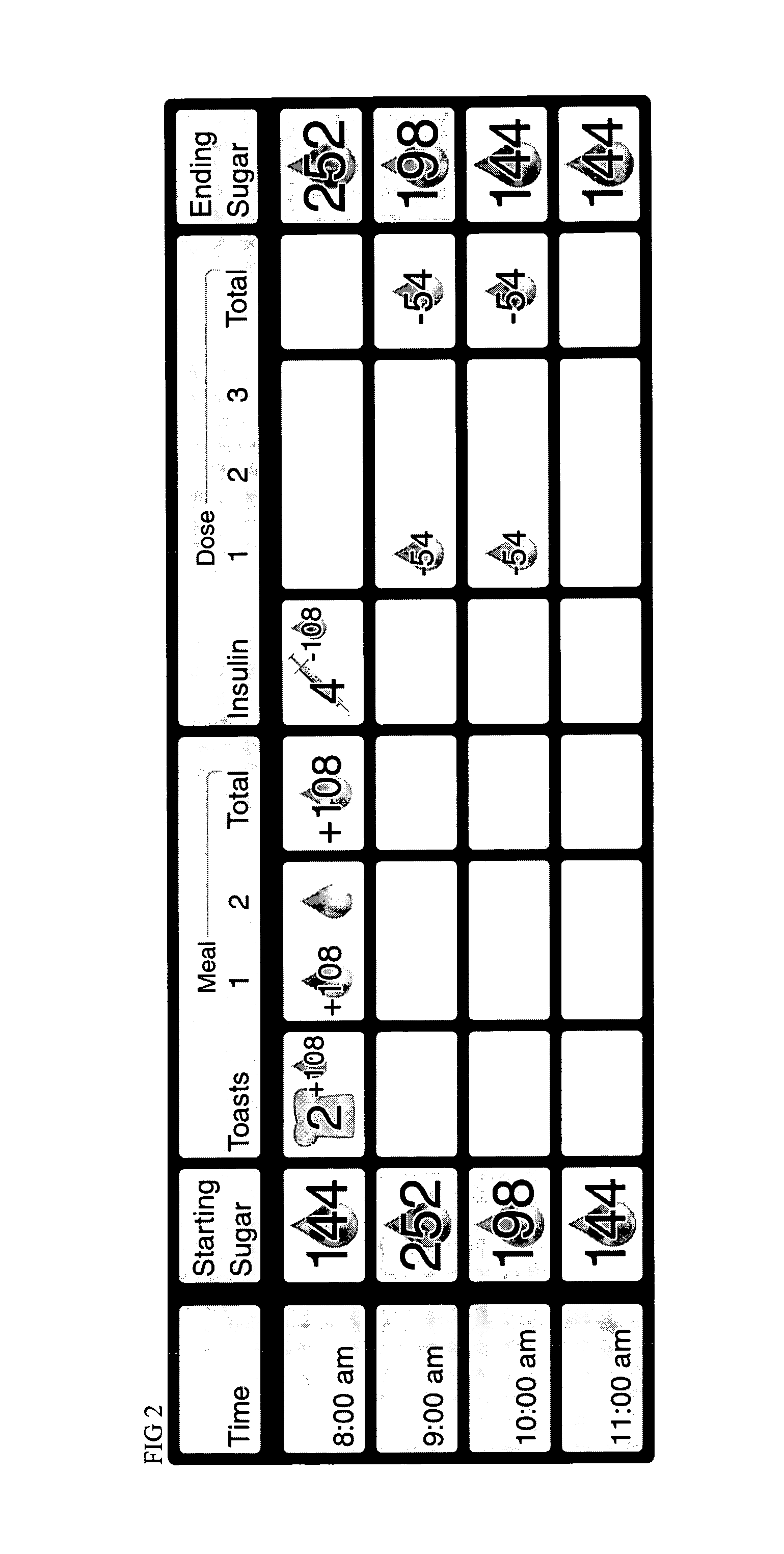
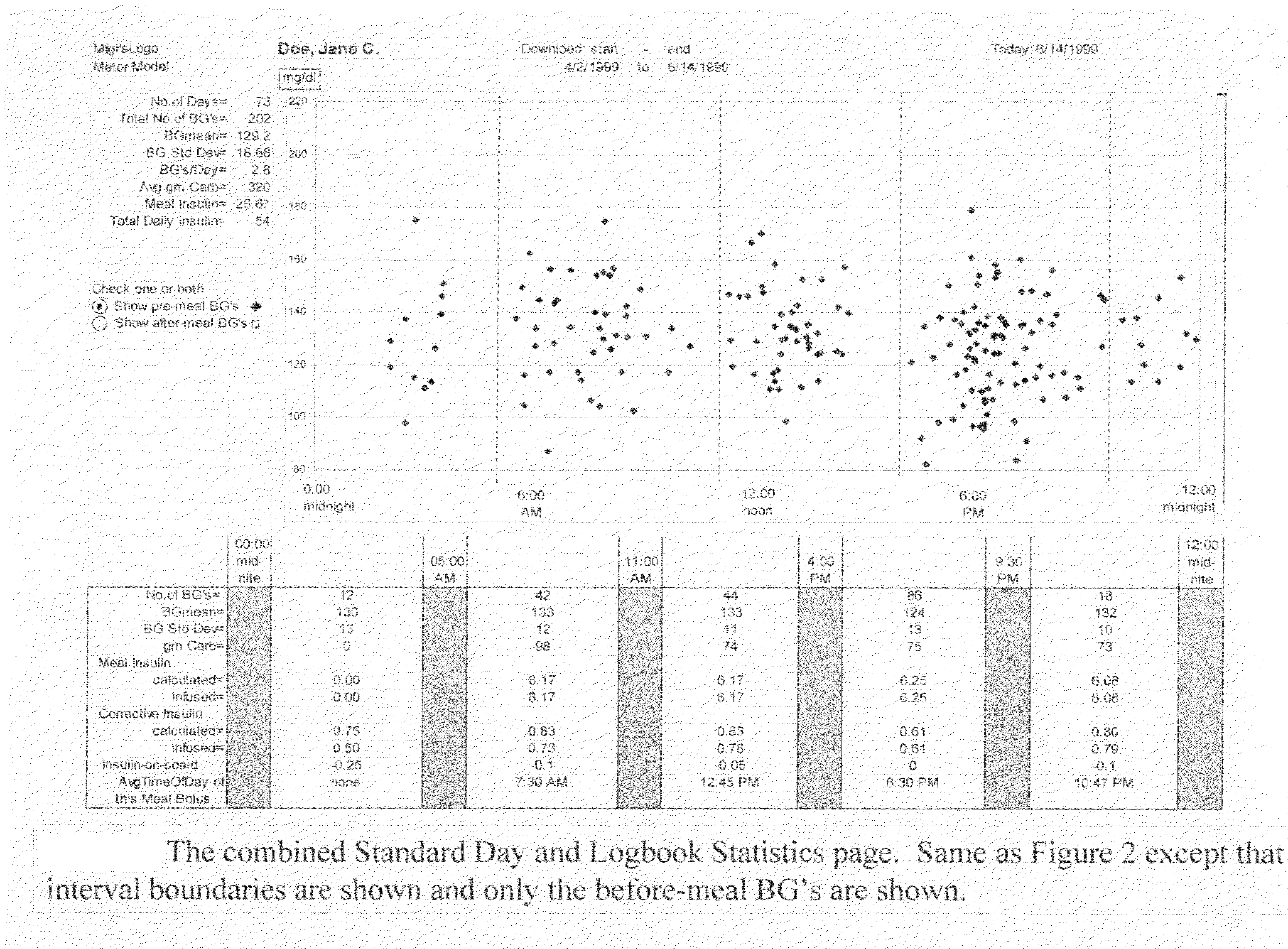
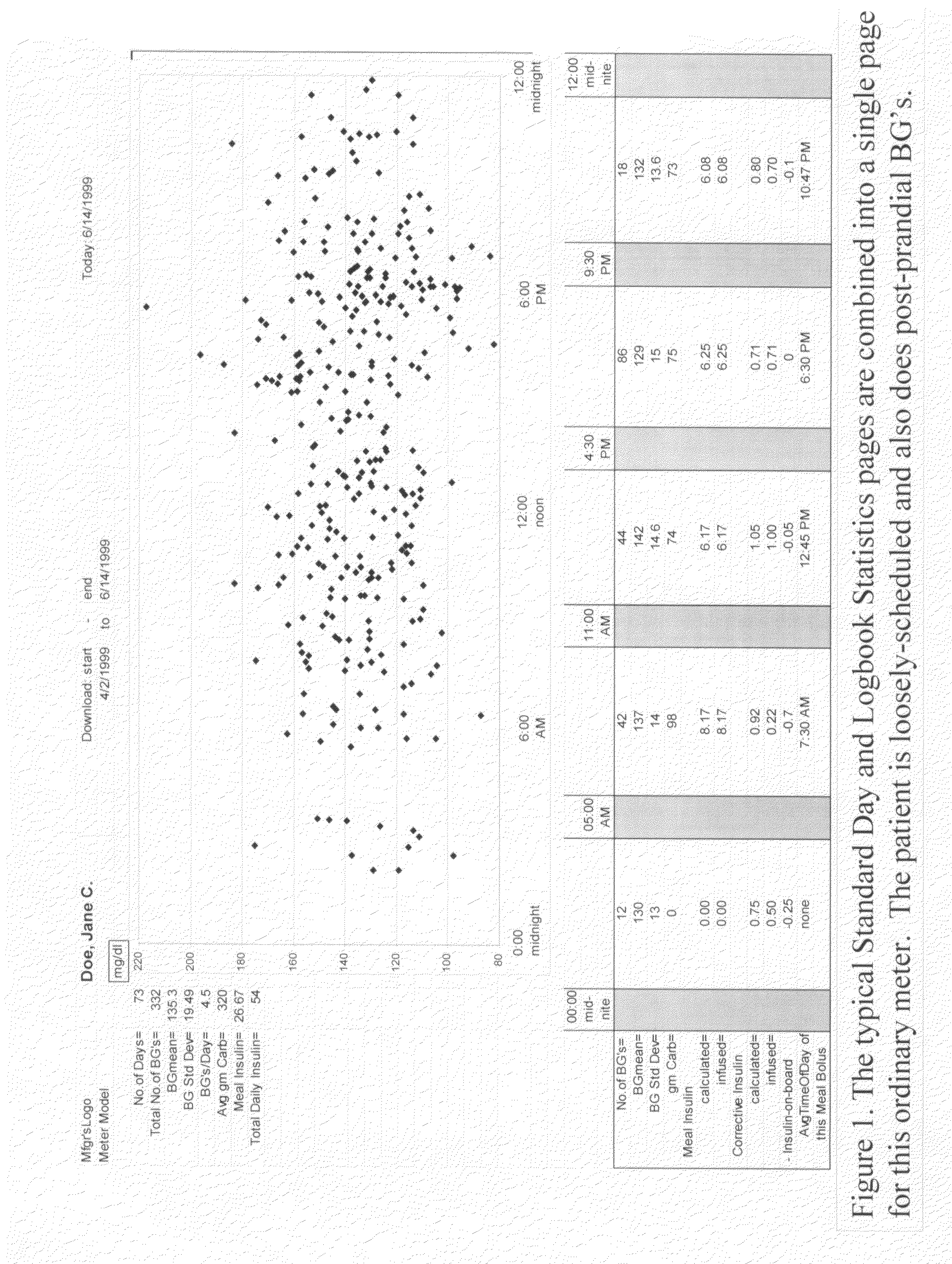
A four-part method for analyzing data of a patient's blood glucose (BG) tests or other parameters e.g. carbohydrate intakes and discrete insulin doses (boluses): 1. An LCD-button on the portable glucose meter or insulin pump, that allows flagging or assigning a category to each blood glucose test at test-time. Examples of categories: pre-meal BG tests, post-meal BG tests. 2. In the computer-based downloading program, features for changing the boundaries of the meal-oriented time-intervals by the faster methods of: a) mouse-dragging the time-boundary lines on a scatter chart, b) placing stretchable rectangles around the data points of interest. 3. Features in the computer-based downloading program, for digitally identifying the time of the meal and digitally assigning the category to the pre-meal BG tests and to the post-meal BG tests. 4. Features in the computer-based downloading program, for statistically analyzing and graphically-displaying the differently-categorized data separately or together in combinations.
Owner:HEBBLEWHITE HARRY R +1
Method, system, and computer program product for the detection of physical activity by changes in heart rate, assessment of fast changing metabolic states, and applications of closed and open control loop in diabetes
ActiveUS8585593B2Minimize frequencyAvoid the needMedical simulationDrug and medicationsClosed loopHypoglycemia
A method, system, and computer program product related to the detection of physical activity using changes in heart rate. The method, system, and computer program product evaluates short term glucose demand and long term insulin action due to physical activity. The method, system, and computer program product is further related to the improvement of open and closed loop control of diabetes by accounting for the metabolic changes due to physical activity. The method, system, and computer program product is directed to detecting in real time the short and long term effects of physical activity on insulin action via heart rate analysis, and recommending changes in insulin dosing to compensate for the effects of physical activity. With these recommendations, the open and closed loop control of diabetes can be improved and steps can be taken to prevent hypoglycemia that may result from increased insulin sensitivity due to physical activity.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Clinical variable determination
A computer implemented method of determining a clinical variables utilizing an insulin pump that includes initiating blood glucose measurements, initiating ingestion of carbohydrates and receiving input data based on the blood glucose measurements and the ingestion of carbohydrates and utilizing the data to calculate clinical variables. The invention may include presenting instructions to a patient to take various actions and to input various data. The clinical variables determined may be stored in memory and then used to calculate insulin doses and to send a signal to an insulin pump to infuse the insulin dose calculated.
Owner:TANDEM DIABETES CARE INC
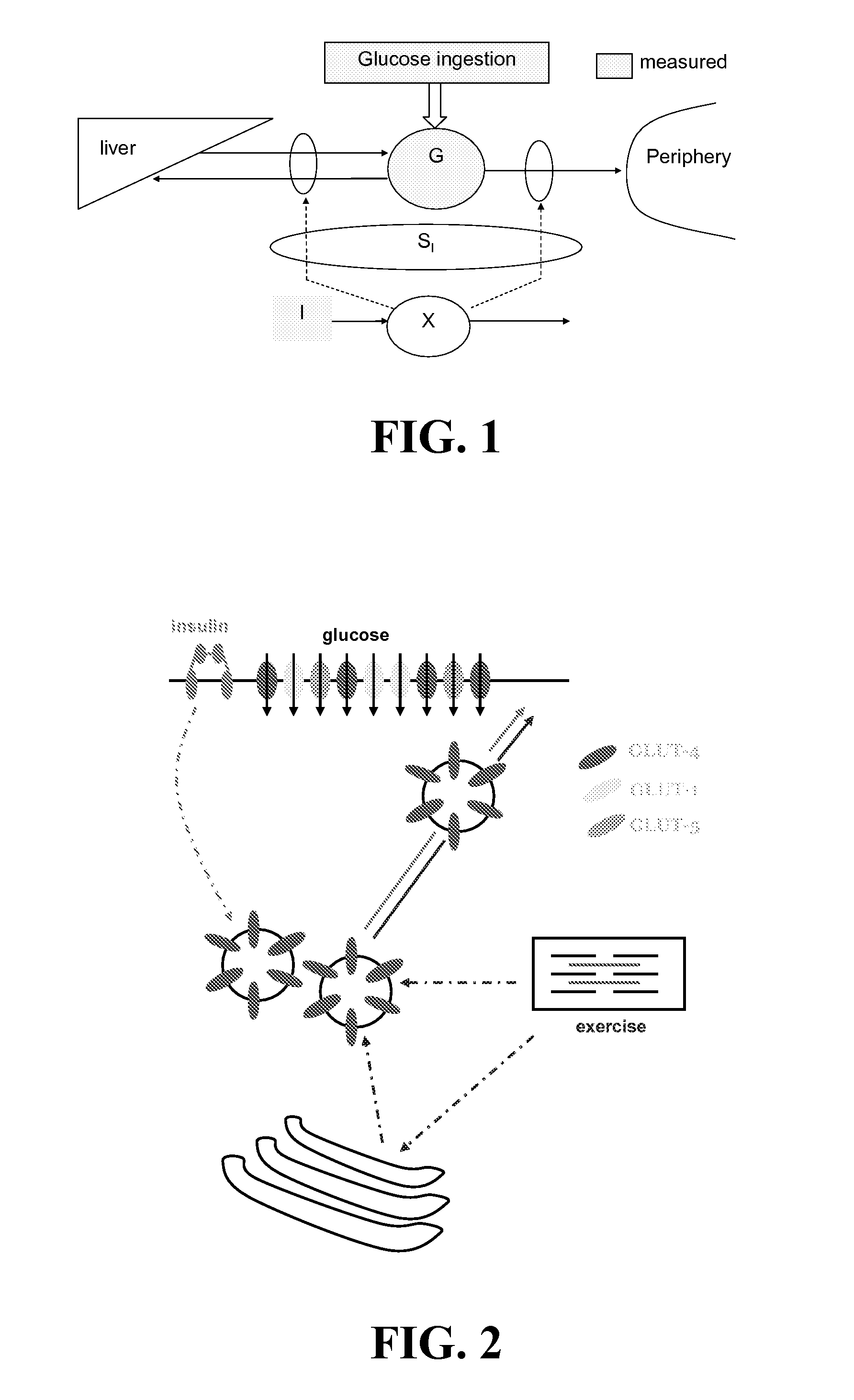
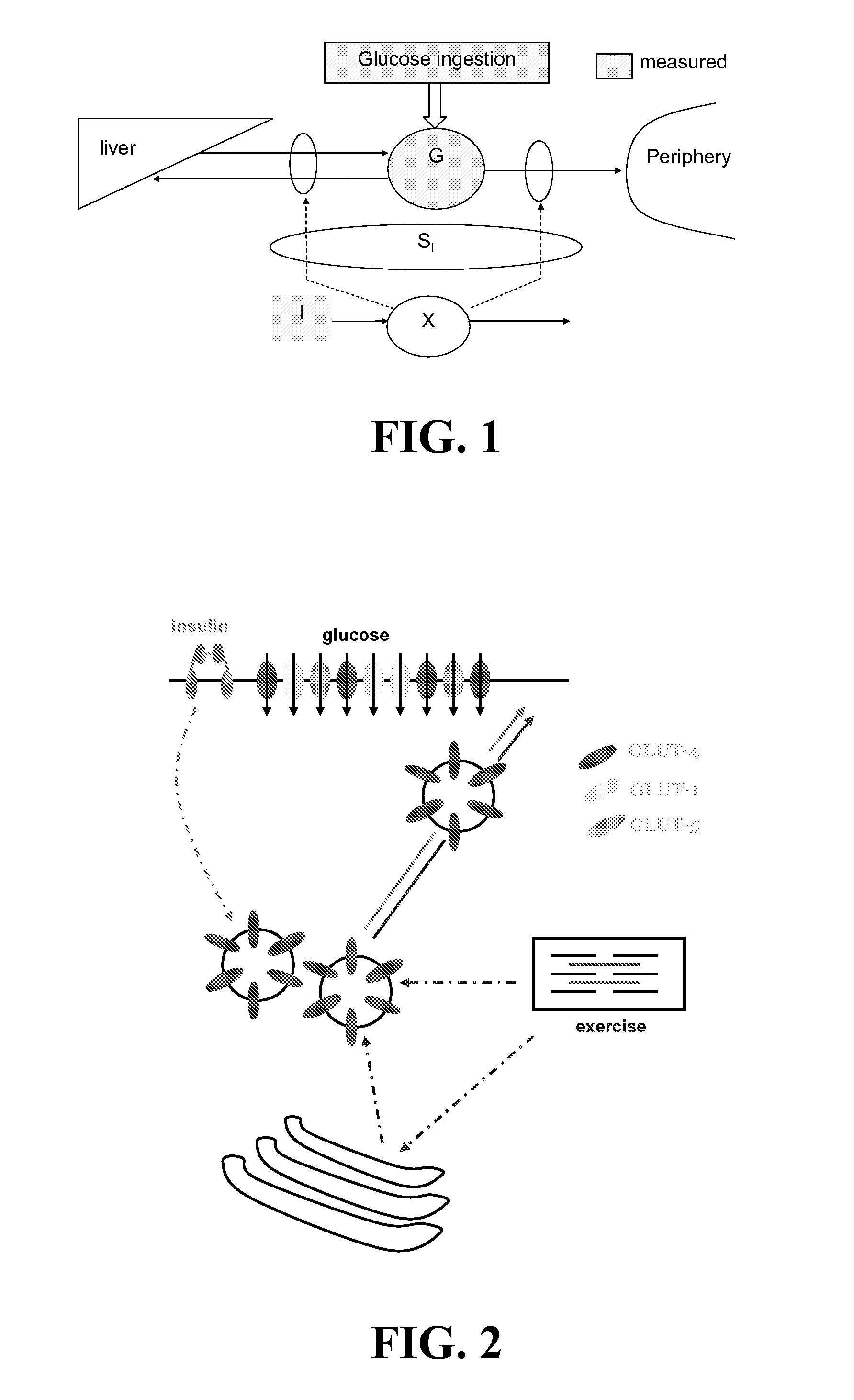
Method System and Device for Assessing Insulin Sensitivity
ActiveUS20080319381A1Low costHighly profitable product for the manufacturerMedical simulationDrug and medicationsPlasma glucose concentrationBlood plasma
A method and a system for determining insulin sensitivity (IS) is described. In one aspect the method and the system can be implemented by receiving a first parameter corresponding to an insulin dose in a subcutaneous tissue; applying a first kinetic model to obtain a plasma insulin concentration based on the first parameter; receiving a second parameter corresponding to a plasma glucose concentration; determining the insulin sensitivity (IS) based on the plasma insulin concentration and the second parameter.
Owner:ROCHE DIABETES CARE INC
Assessing residual insulin time
Methods, systems and devices for assessing residual insulin time of a patient are provided. In one embodiment, the method and the device can be implemented by receiving a confirmation that an insulin dose has been administered to the patient; repetitively receiving a value corresponding to the patient's blood glucose level; identifying at least two consecutively received values based on a predetermined criteria; and, selecting the residual insulin time corresponding to a time period between the confirmation that the insulin dose has been administered and a time corresponding to the identification of the at least two consecutively received values.
Owner:ROCHE DIABETES CARE INC
Novel Administration Regime
ActiveUS20160058840A1Low costEnhancing patient empowermentPeptide/protein ingredientsMetabolism disorderDosing regimenUltra long-acting insulin
The invention relates to a novel administration regime useful in the treatment of diseases or conditions where administration of insulin will be of benefit, as well as to kits for use in the same. In particular, the invention relates to a long-acting or ultra-long acting insulin for use in treating a disease or condition where administration of insulin will be of benefit, wherein the administration of said insulin comprises or consists of the following steps: (a) optionally providing a blood sample to be tested from an individual in need of treatment; (b) taking a single fasting blood (or plasma) glucose measurement from said individual in need of treatment; (c) using the single fasting blood (or plasma) glucose measurement to determine the insulin dose to be administered; and (d) administering the long-acting or ultra-long insulin to the individual at the dose determined in step (c).
Owner:NOVO NORDISK AS
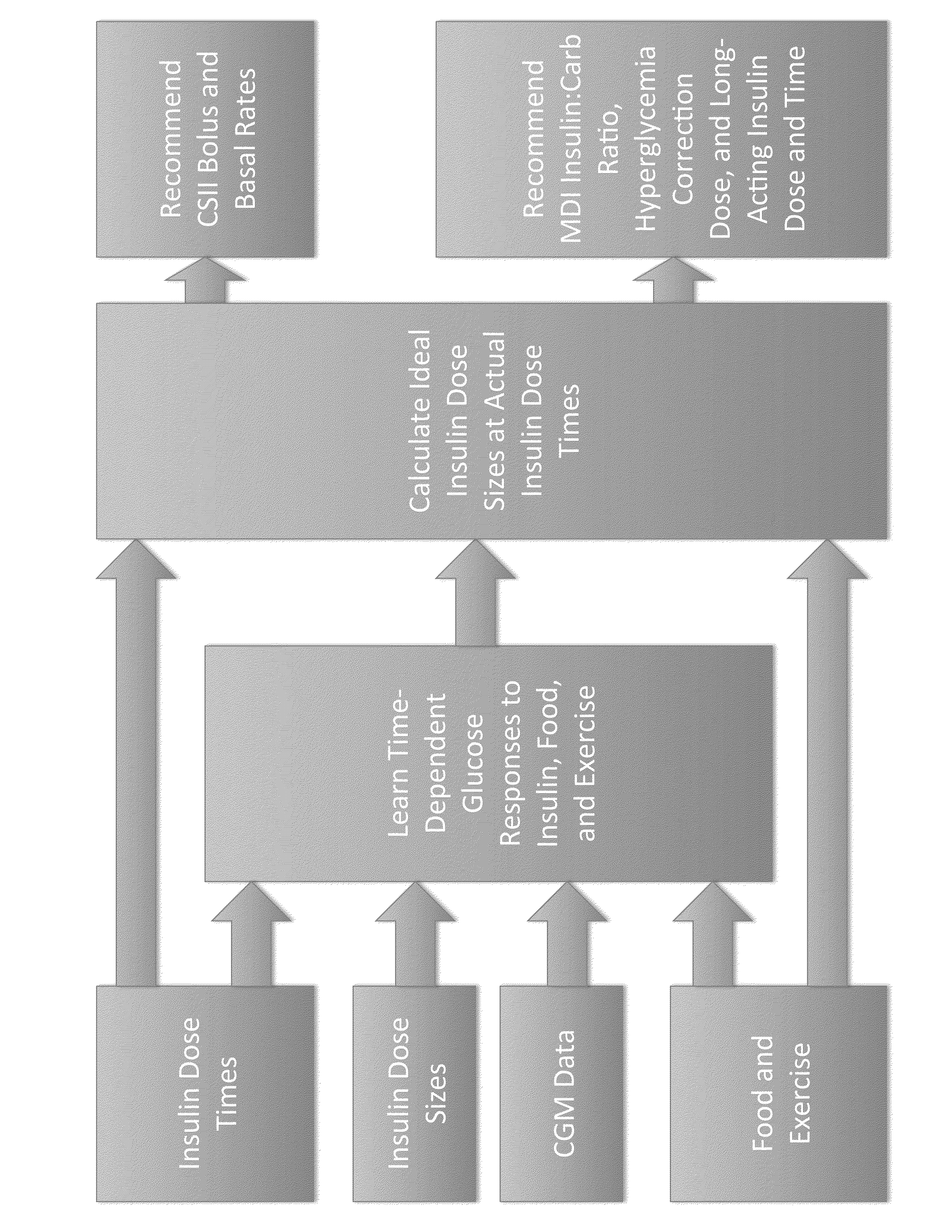
Method to Determine Individualized Insulin Sensitivity and Optimal Insulin Dose by Linear Regression
ActiveUS20160162797A1Accurate doseSensorsProbabilistic networksConcentrations glucoseLinear regression
This invention relates to a method and a device for predicting the glucose concentration of a subject and recommending therapeutic action. The responses of the user's glucose to administered doses of insulin, dietary carbohydrates, and other factors influencing glucose concentration are measured individually for a given user. Once these responses are learned as a function of time, the method and device can receive information about the factors which have been recently or will soon be administered and can recommend which other factors should also be administered.
Owner:INSULET CORP
Pulmonary delivery of spherical insulin microparticles
Compositions of spherical insulin particles having improved pulmonary application potentials and methods of forming and using these compositions are disclosed in the present application. In one clinical trial with 30 healthy male human subjects, no coughing was observed upon a single pulmonary administration of the spherical insulin particles at an insulin dose of 6.5 mg, nor during the 10-hour post dosing period.
Owner:BAXTER INT INC +1
Integration of glucose data to adjust inhaled insulin dose
An insulin dispenser receives blood glucose readings wirelessly from a blood glucose monitor, and calculates an insulin dose based on the historical response of the particular patient to prior insulin doses. The insulin dispenser preferably nebulizes an insulin-containing fluid for inhalation by the patient.
Owner:DANCE BIOPHARM
Apparatus and methods for taking blood glucose measurements and recommending insulin doses
ActiveCN102711899ALow costDrug and medicationsMaterial analysis by electric/magnetic meansBlood Glucose MeasurementGlycemic
Owner:HYGIEIA INC
Analyte testing method and system with safety warnings for insulin dosing
Methods and systems to provide for safeguards in the insulin dosing calculation as part of the diabetes management. The system or method provides a warning if the person with diabetes is calculating a dosing regimen outside of a preselected time period in which certain dosing parameters are customized to the preselected time period.
Owner:LIFESCAN SCOTLAND
Method and system for management of diabetes with a glucose monitor and infusion pump to provide feedback on bolus dosing
Described and illustrated is a system for management of diabetes that includes an infusion pump, glucose sensor and controller with a method programmed into the controller. The infusion pump is configured to deliver insulin to a subject. The system provides the user with the ability to understand the effects of bolus insulin dosing upon their glucose levels. Specifically, the system provides a message that must include: (a) the trend of the user's glucose; (b) the recommended bolus; and (c) the actual bolus.
Owner:LIFESCAN IP HLDG LLC
Medical arrangements and a method for determining parameters related to insulin therapy, predicting glucose values and for providing insulin dosing recommendations
InactiveUS20180296142A1Reduce riskConvenient treatmentPeptide/protein ingredientsDrug and medicationsFinite impulse responseMedicine
The present invention relates to a method for determining a personalized estimate, the magnitude of an intended medical effect, a duration and a shape of this, for a drug, based on sections of data where a target biomarker and dose history have been recorded, wherein the personalized estimate of a finite impulse response model a=[a0, a2 . . . an] and the basal hepatic glucose production Gb is determined according to equations of the present invention. The present invention further relates to a system for controlling insulin delivery to a patient according to the method described in the present invention where the measured biomarker is glucose. The present invention further relates to a medical device performing the calculations according to the present invention.
Owner:DIANOVATOR
Medical device and method for glycemic control
ActiveUS11166650B2Improved carbohydrate calculationDrug and medicationsSensorsGlycemicGlucose control
A medical device and method for determining a dose of insulin to be administered for glycemic control is provided, wherein the dose is stepwise adapted. The method includes determining a blood glucose value, receiving glycemic event information in respect to a predetermined glycemic event, wherein the predetermined glycemic event occurred within a predetermined time interval, receiving a previously adapted dose value stored in a storage unit, and setting an alert based on at least the blood glucose value, the glycemic event information and the previously adapted dose, wherein the alert indicates that the blood glucose value and the predetermined glycemic event are not in a specified relation to the previously adapted dose value.
Owner:SANOFI AVENTIS DEUT GMBH
Preparation and application of glucose-sensitive insulin dosing system
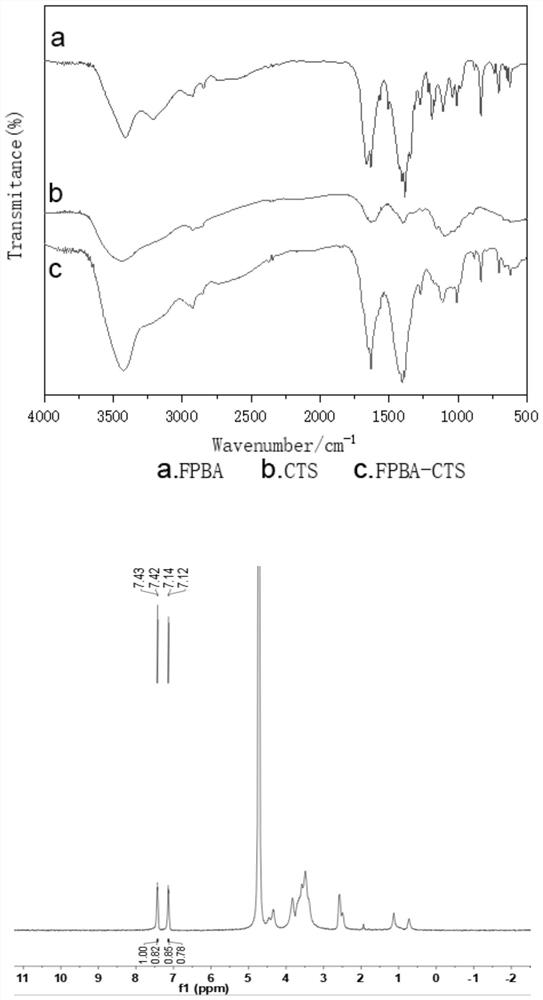
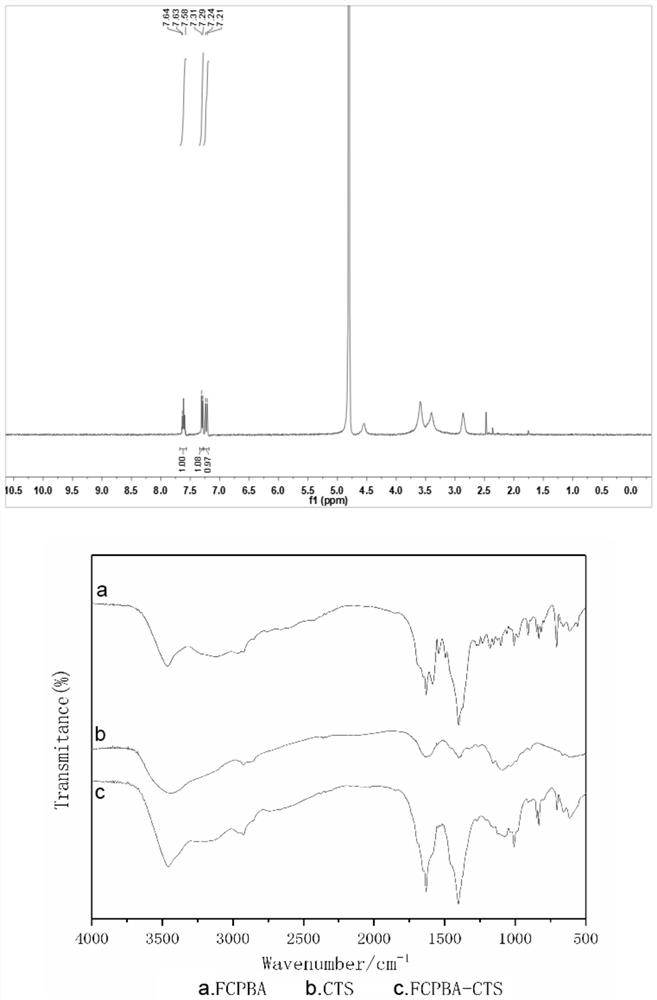
The invention belongs to the field of biomedicines and relates to a preparation method for a glucose-sensitive insulin dosing system and application of the glucose-sensitive insulin dosing system. Theglucose-sensitive insulin dosing system is composed of an inner core and an outer layer, wherein insulin and mesoporous silicon dioxide serve as the inner core, and a glucose-sensitive material capable of responding to different blood glucose concentrations and an anionic polymer for promoting mucus-layer-cross absorption of the system serve as the outer layer. The glucose-sensitive material is selected from 4-formyl phenylboronic acid grafted chitosan or 4-carboxyl-3-fluoro phenylboronic acid grafted chitosan; and a mass ratio of the mesoporous silicon dioxide to the insulin to the 4-formylphenylboronic acid grafted chitosan or 4-carboxyl-3-fluoro phenylboronic acid grafted chitosan is (1-2): (0.5-2): (0.5-2). The anionic polymer for promoting the mucus-layer-cross absorption of the system is selected from phosphoserine. The glucose-sensitive insulin oral-dosing system prepared by the preparation method has a particle size of 90nm to 900nm, and the drug load rate can reach 25% or more and is higher than that of an ordinary organic nano-particle dosing system. The system has an obvious blood glucose reducing effect.
Owner:SHENYANG PHARMA UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com