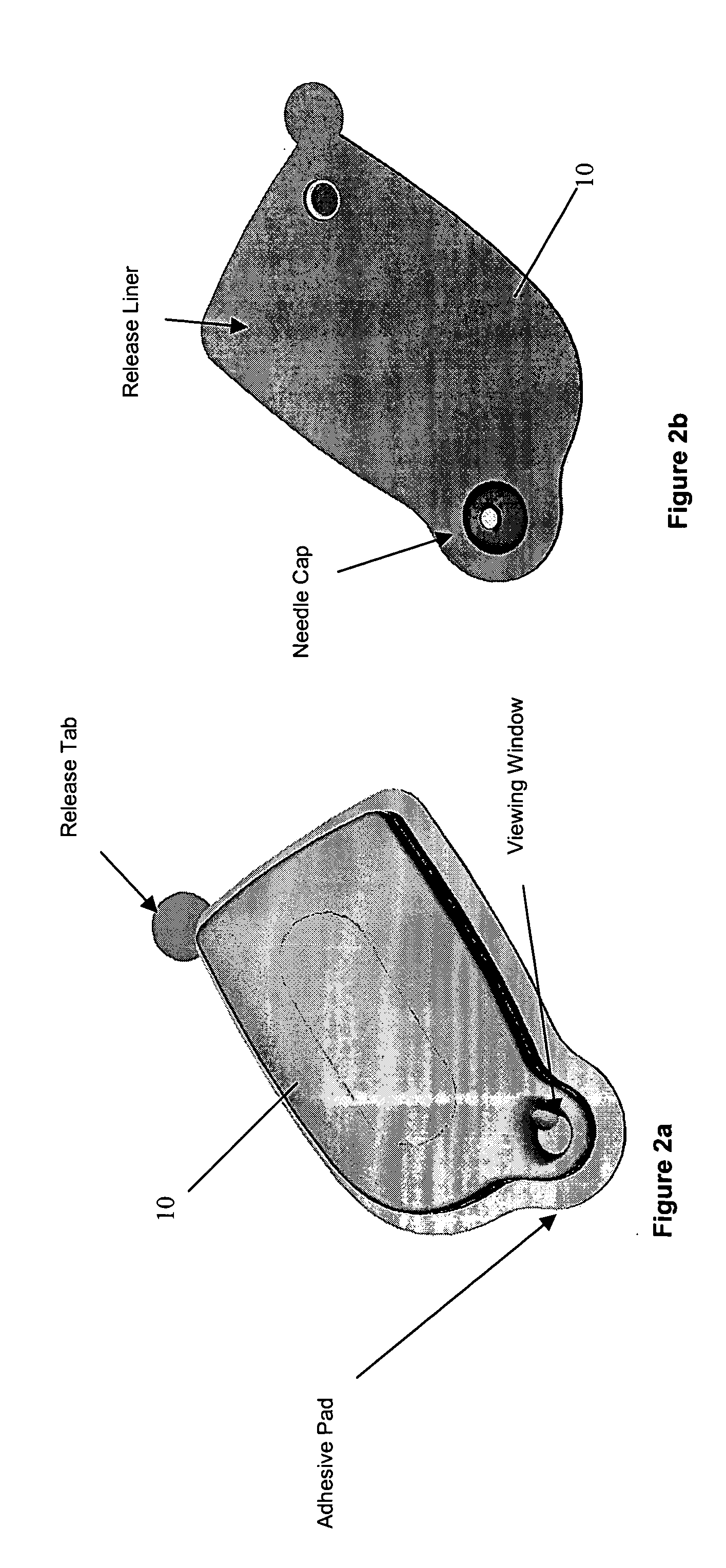
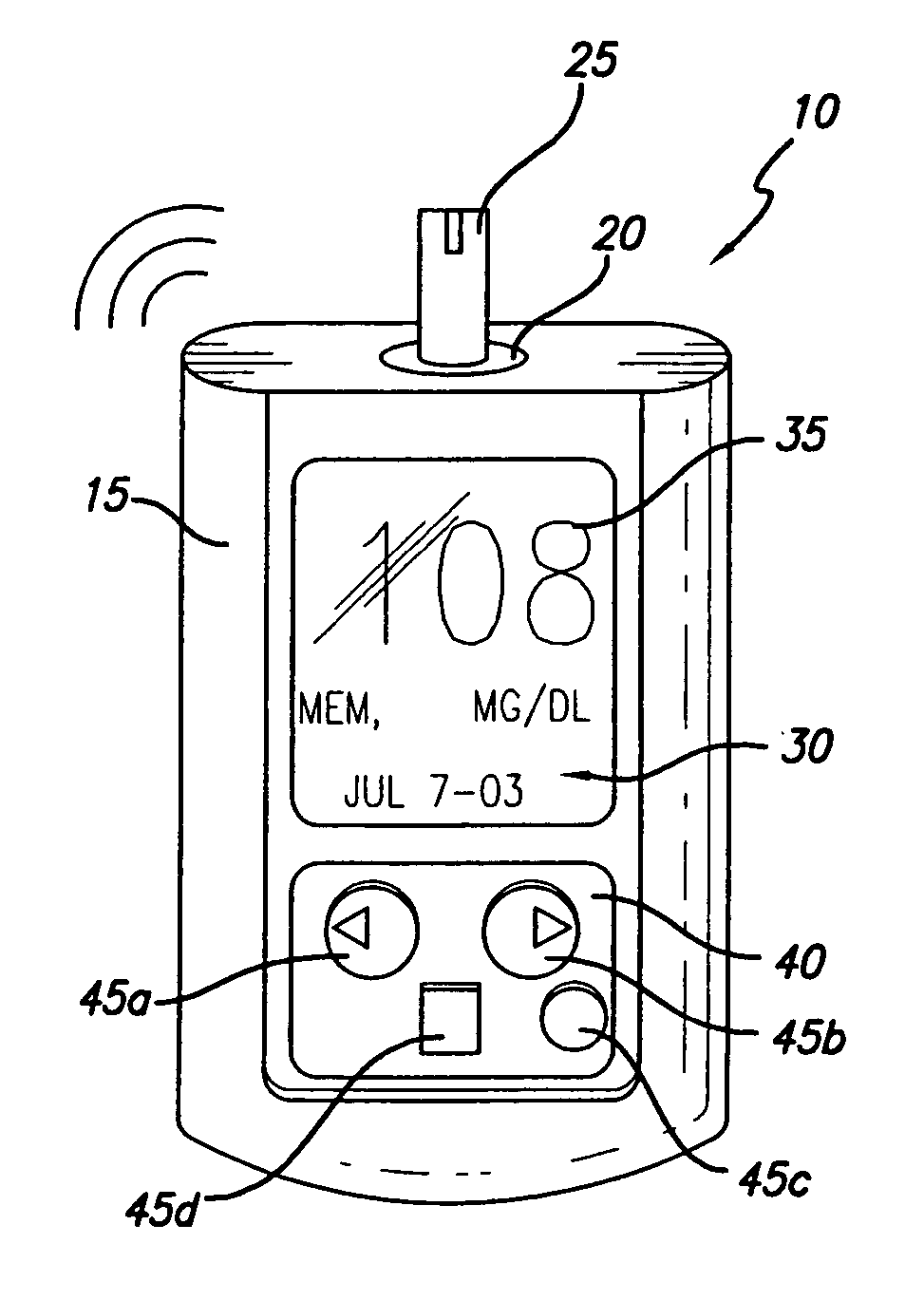
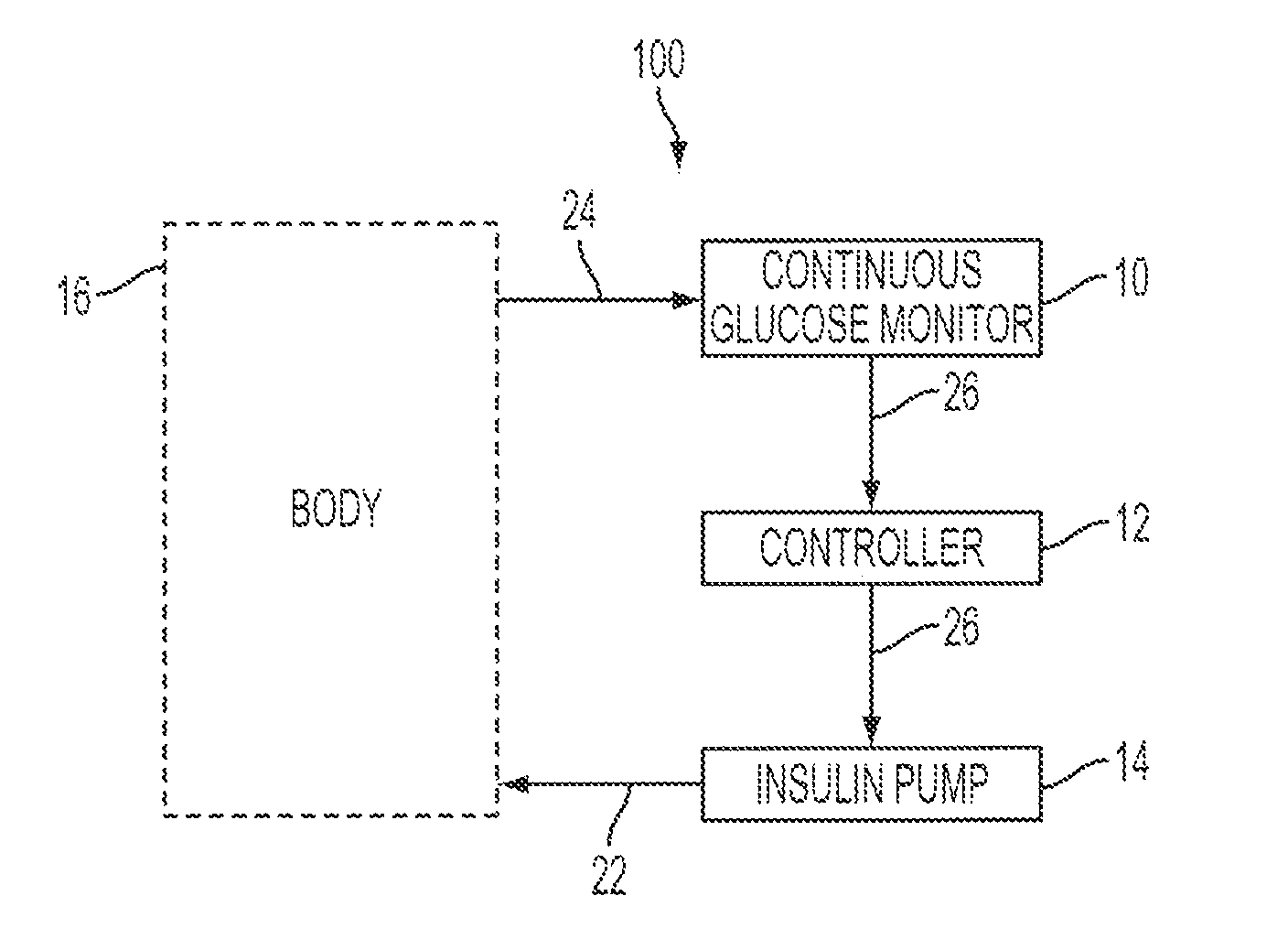
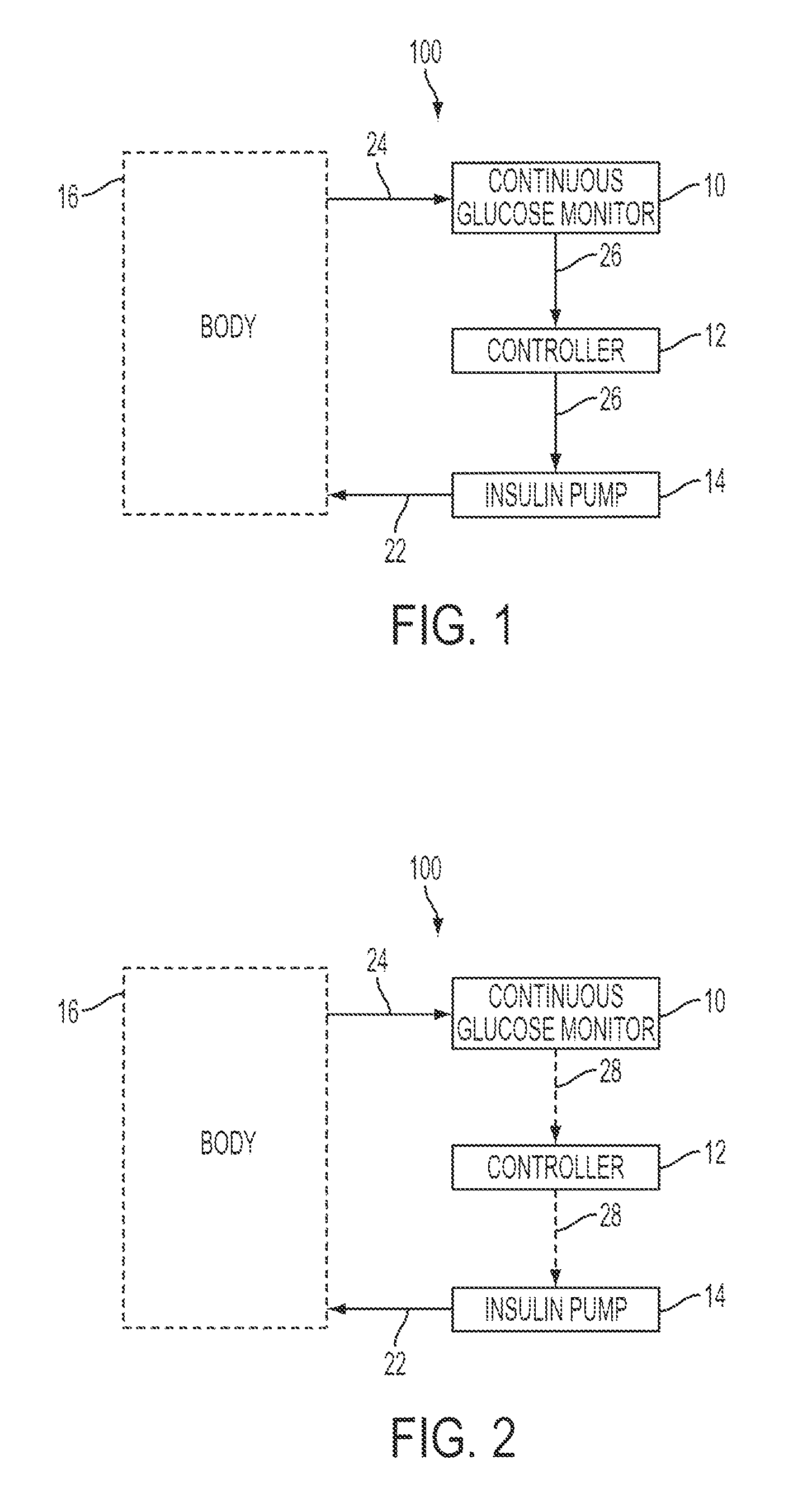
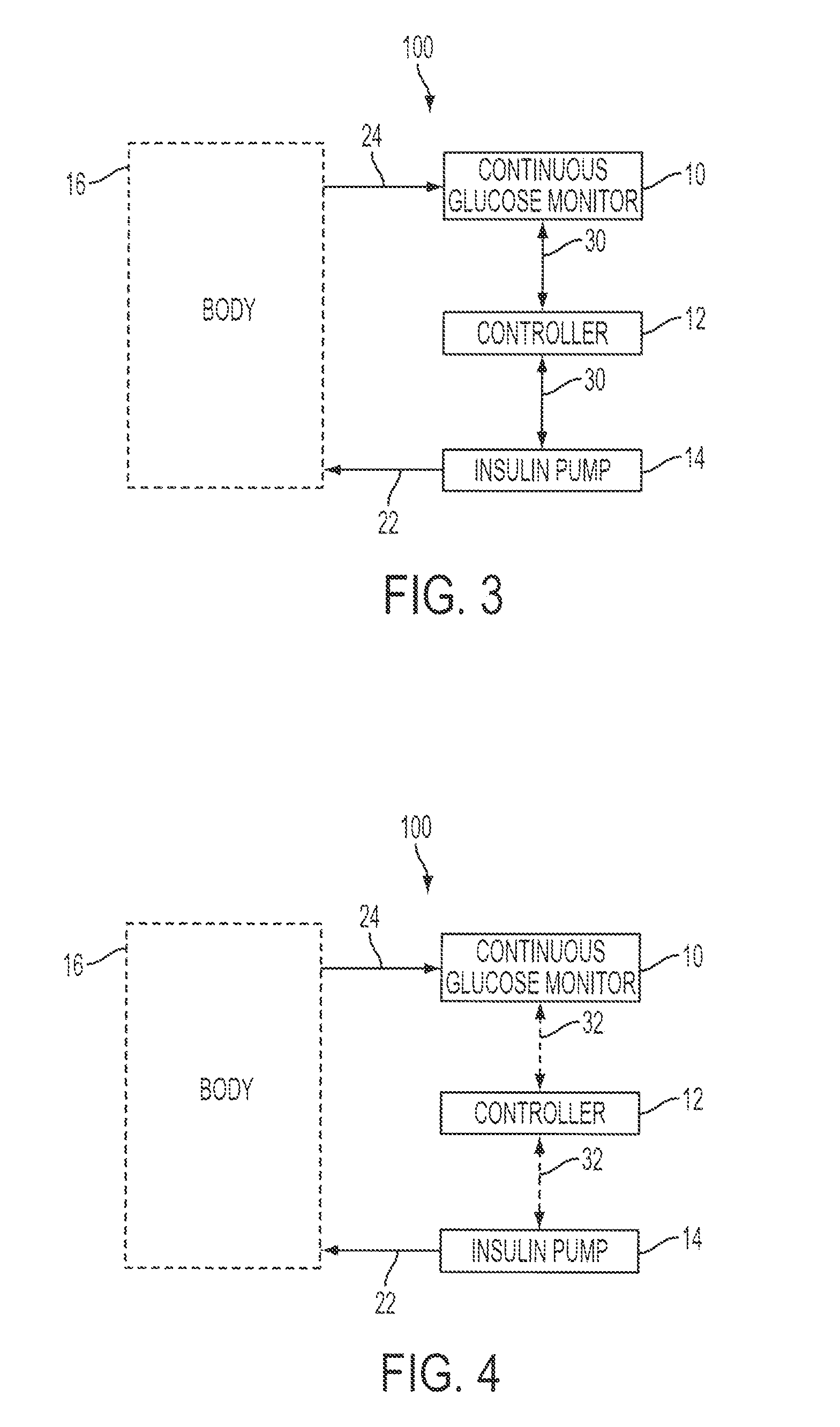
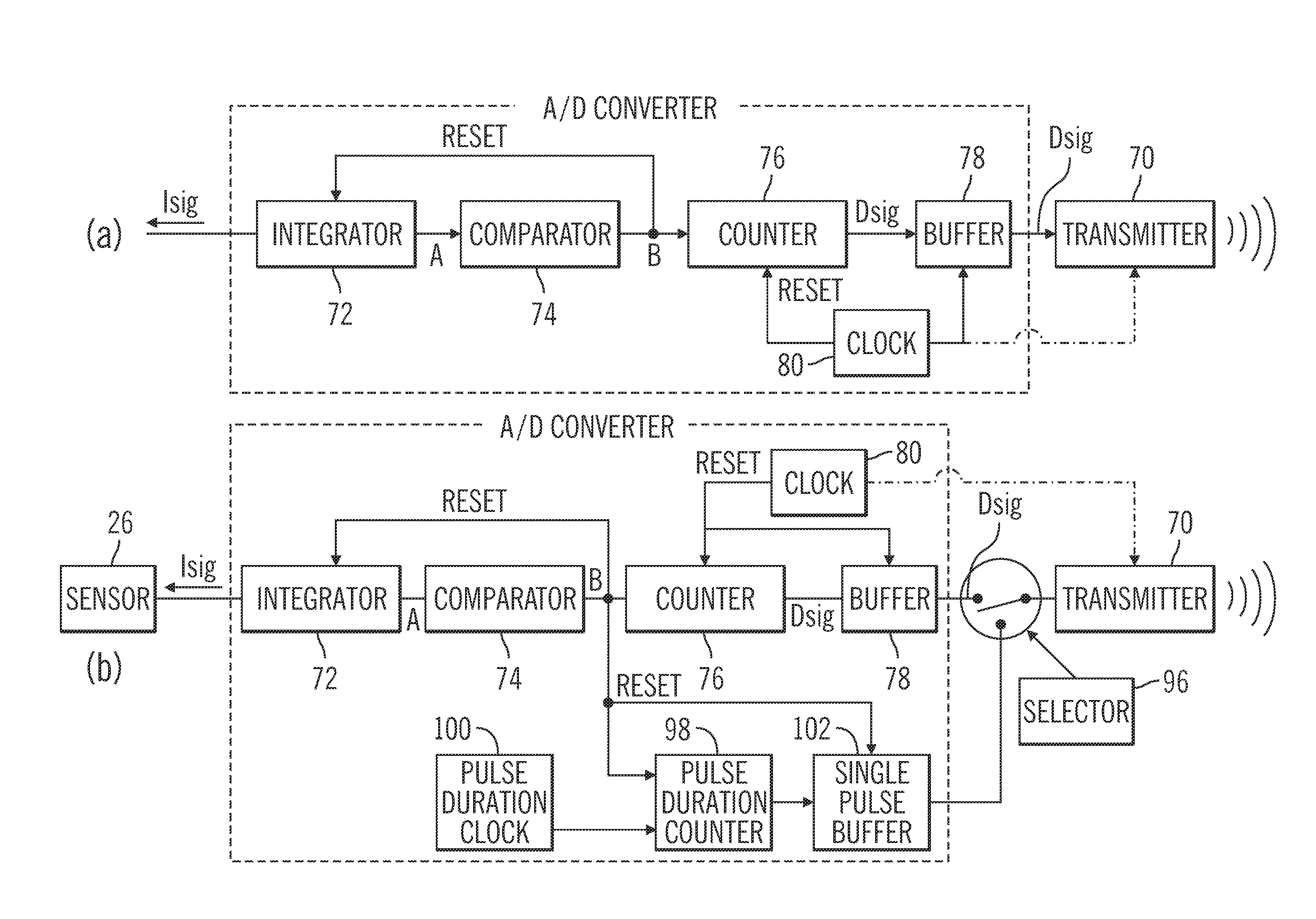
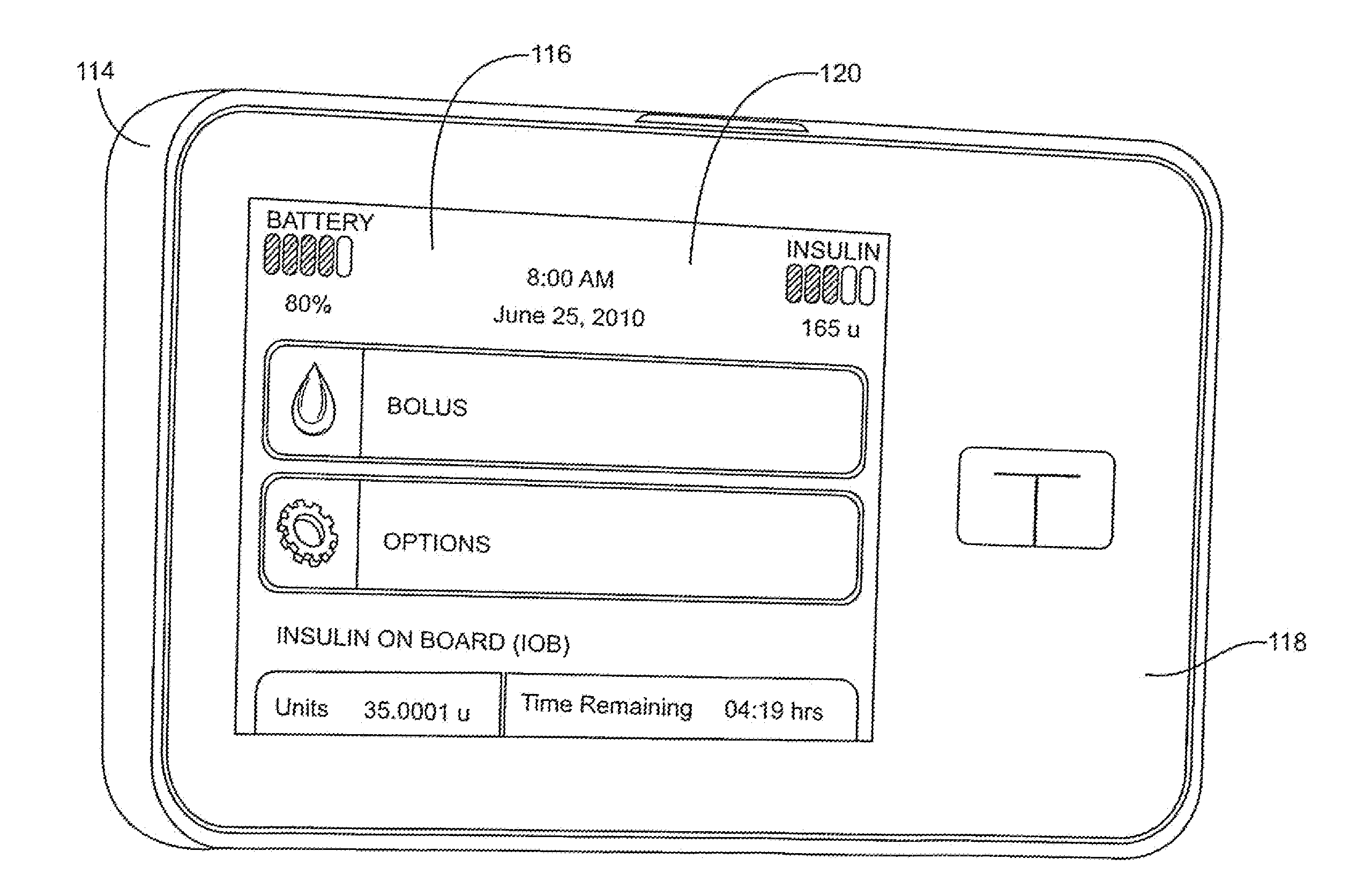
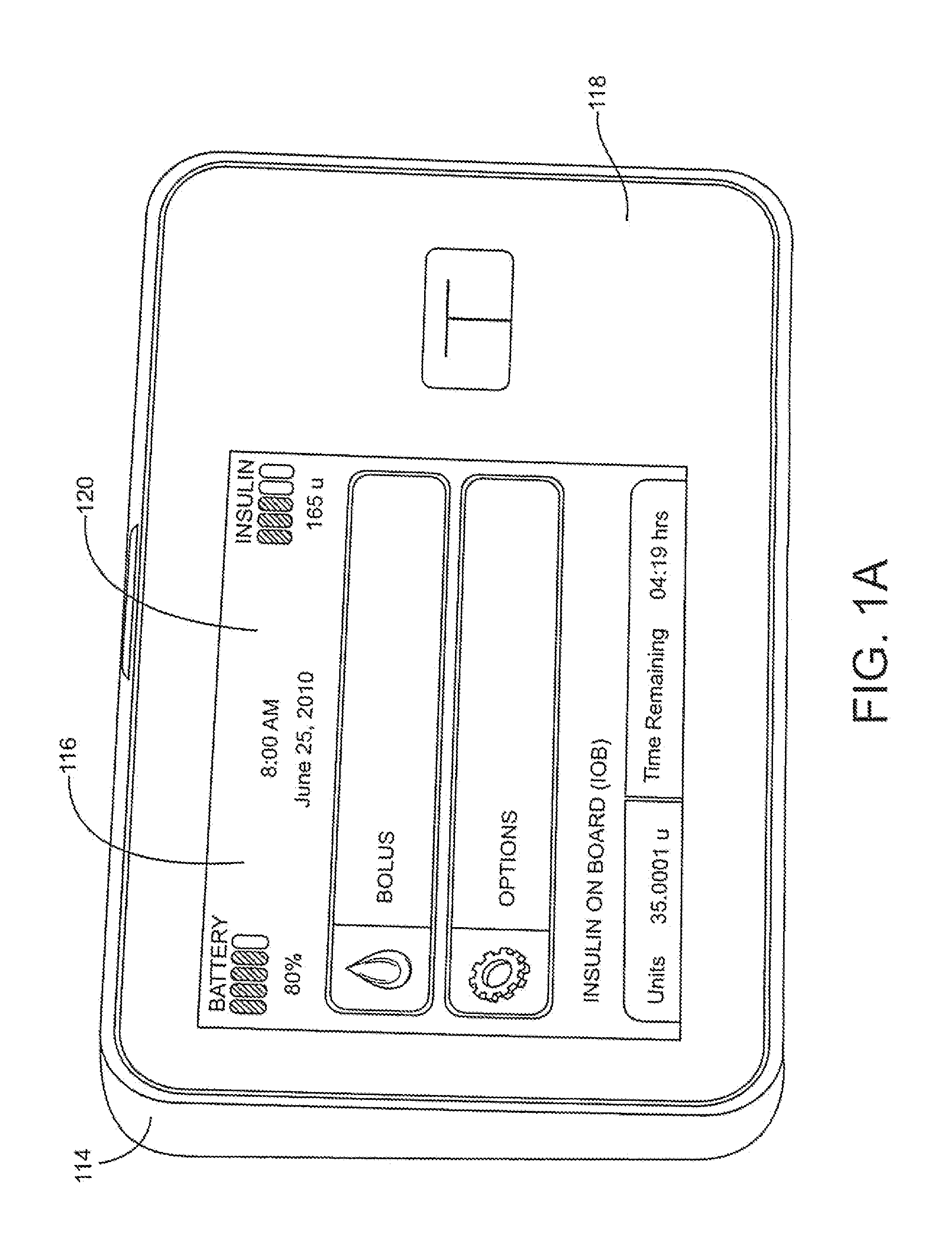
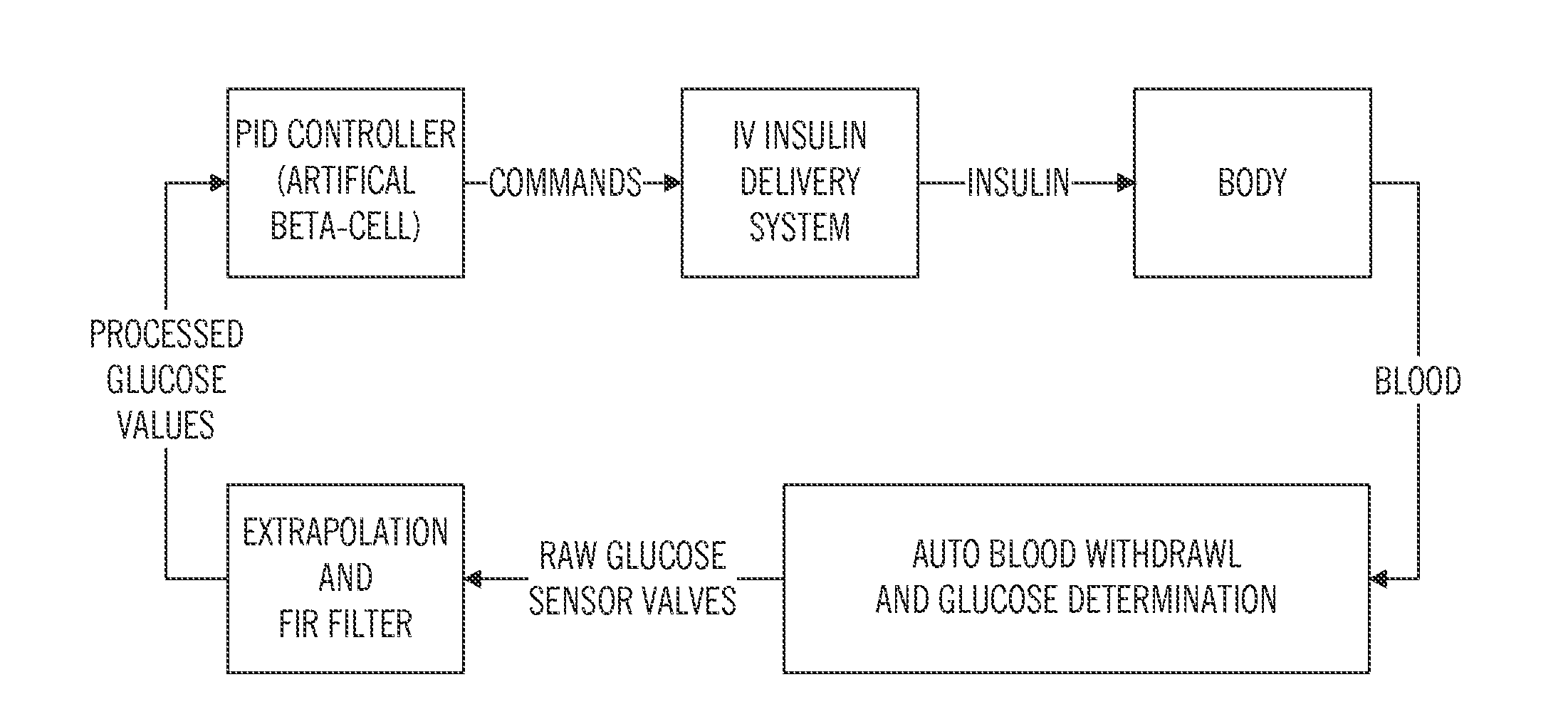
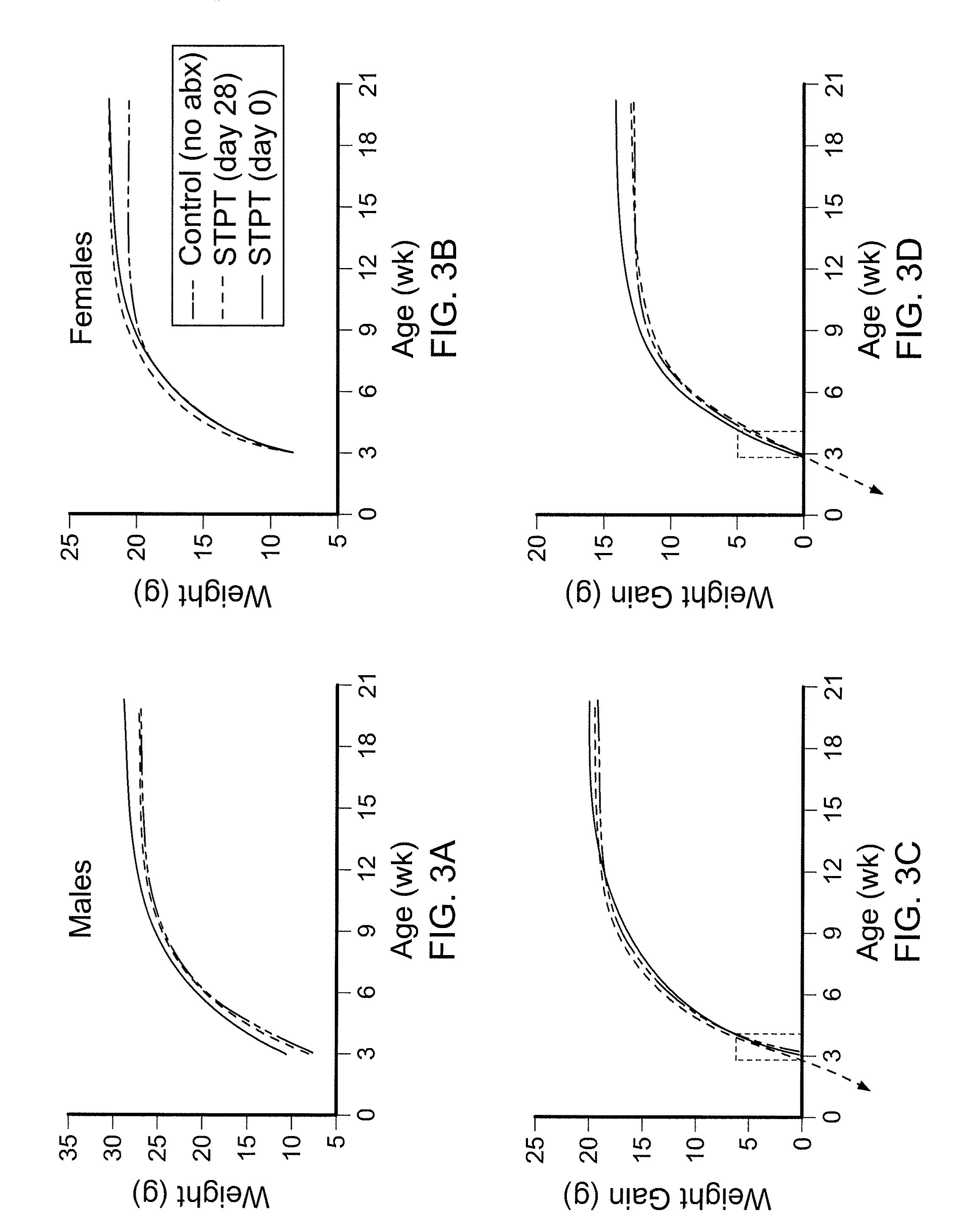Patents
Literature
2239 results about "Insulin humulin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
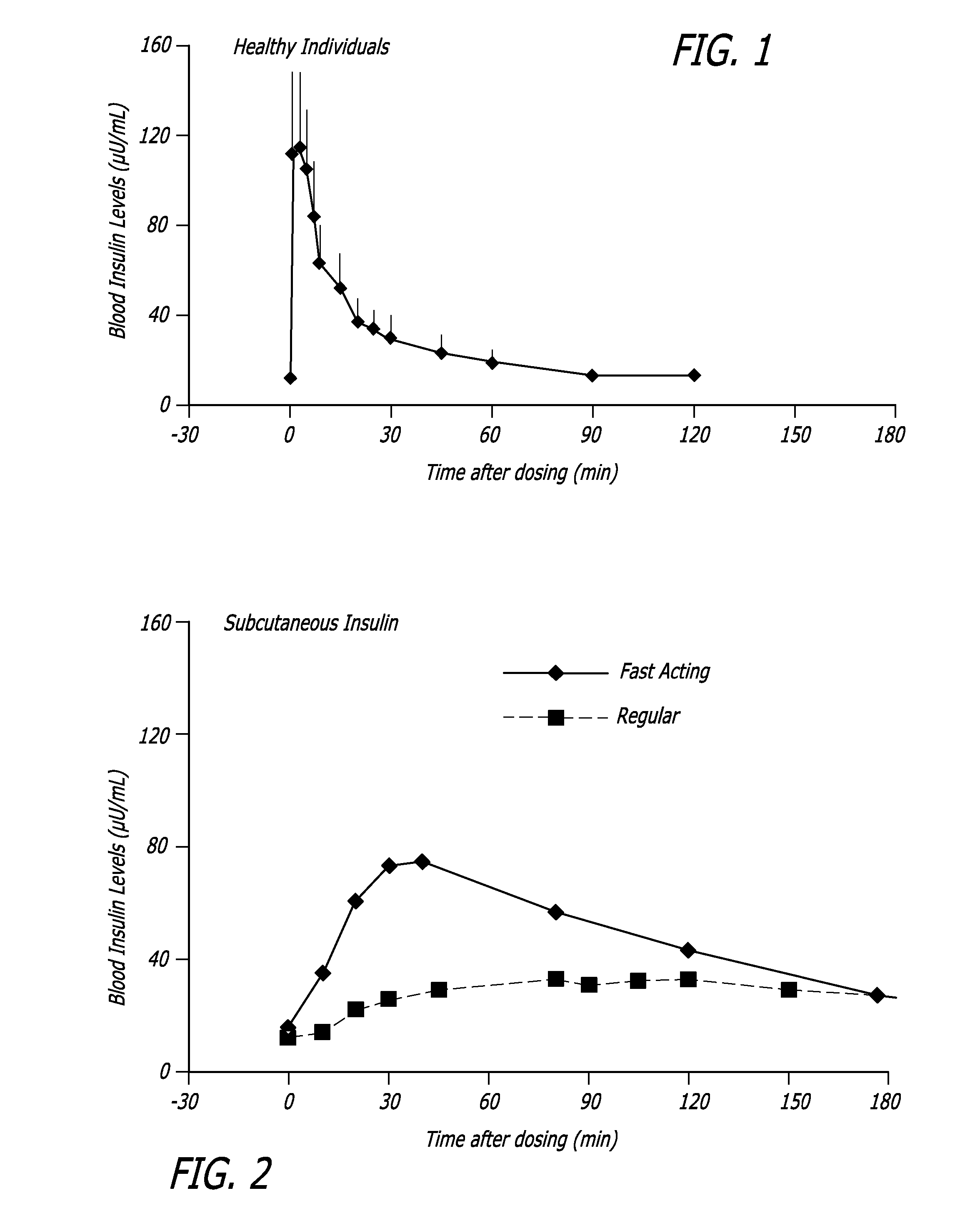
Humulin R is the brand name of a medicine that contains insulin regular (a short-acting form of insulin). Insulin is a hormone that's produced by the body in the pancreas. It works to lower levels of sugar in the blood. This prescription medicine is injected to improve blood sugar control in adults with diabetes.
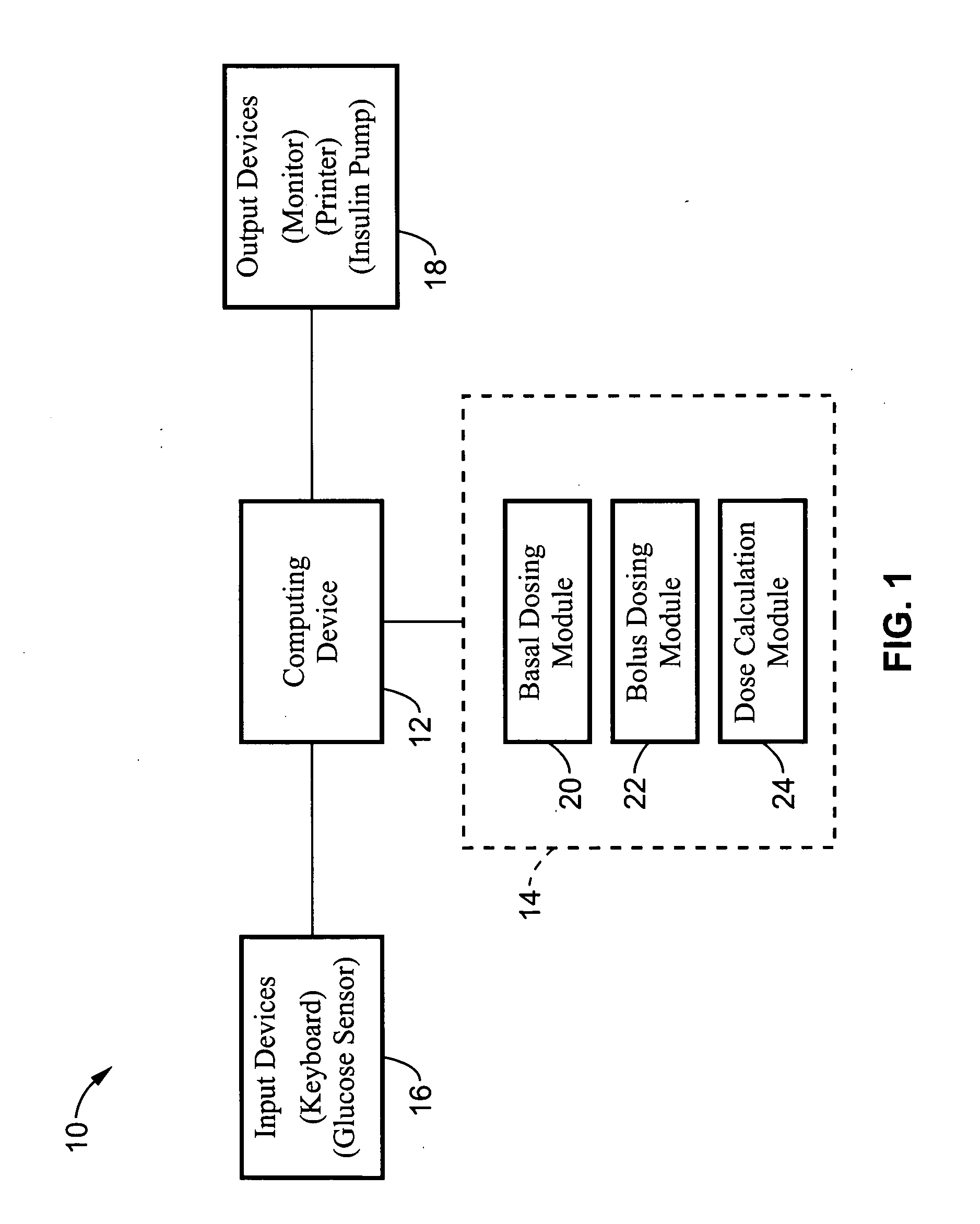
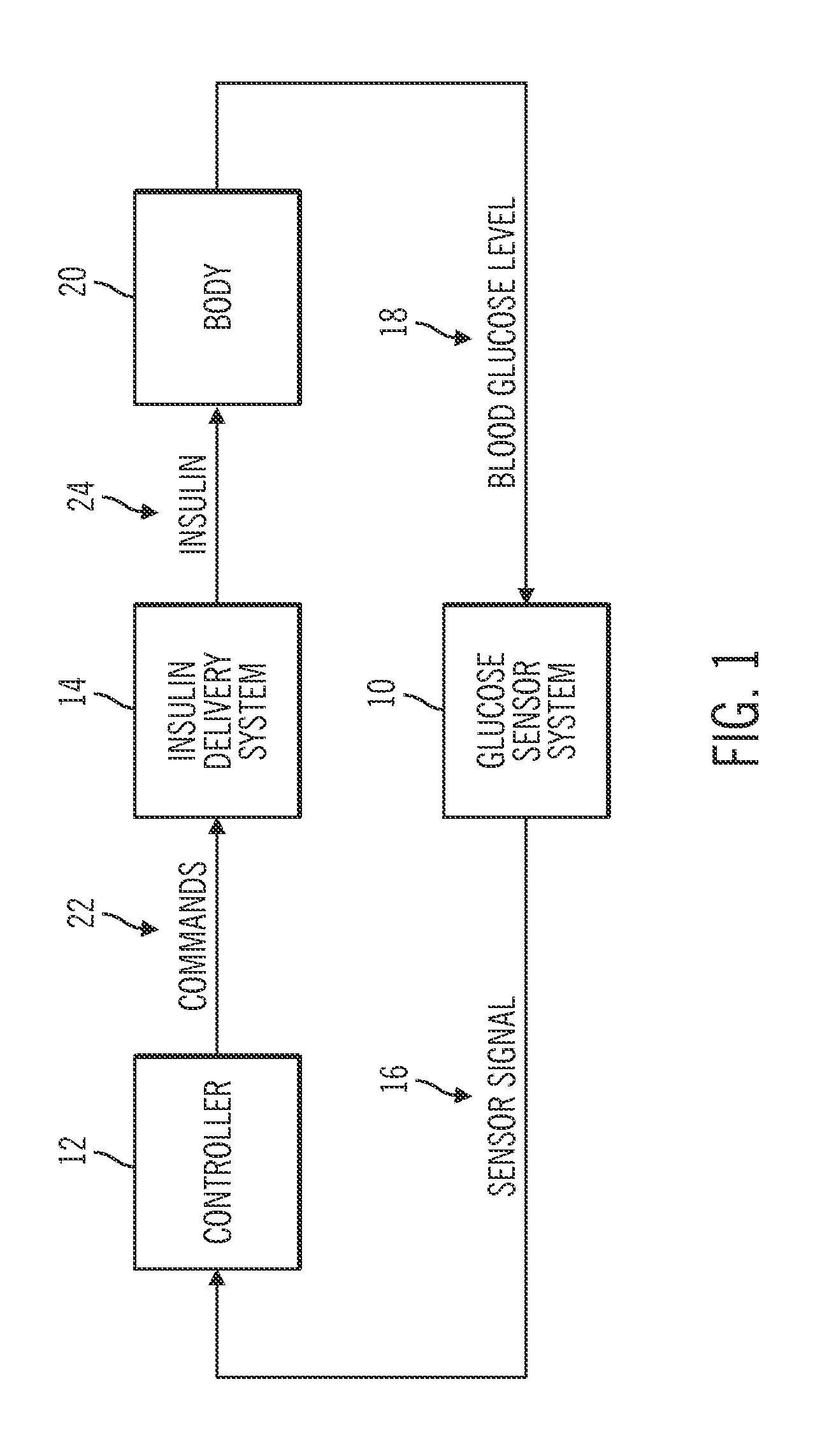
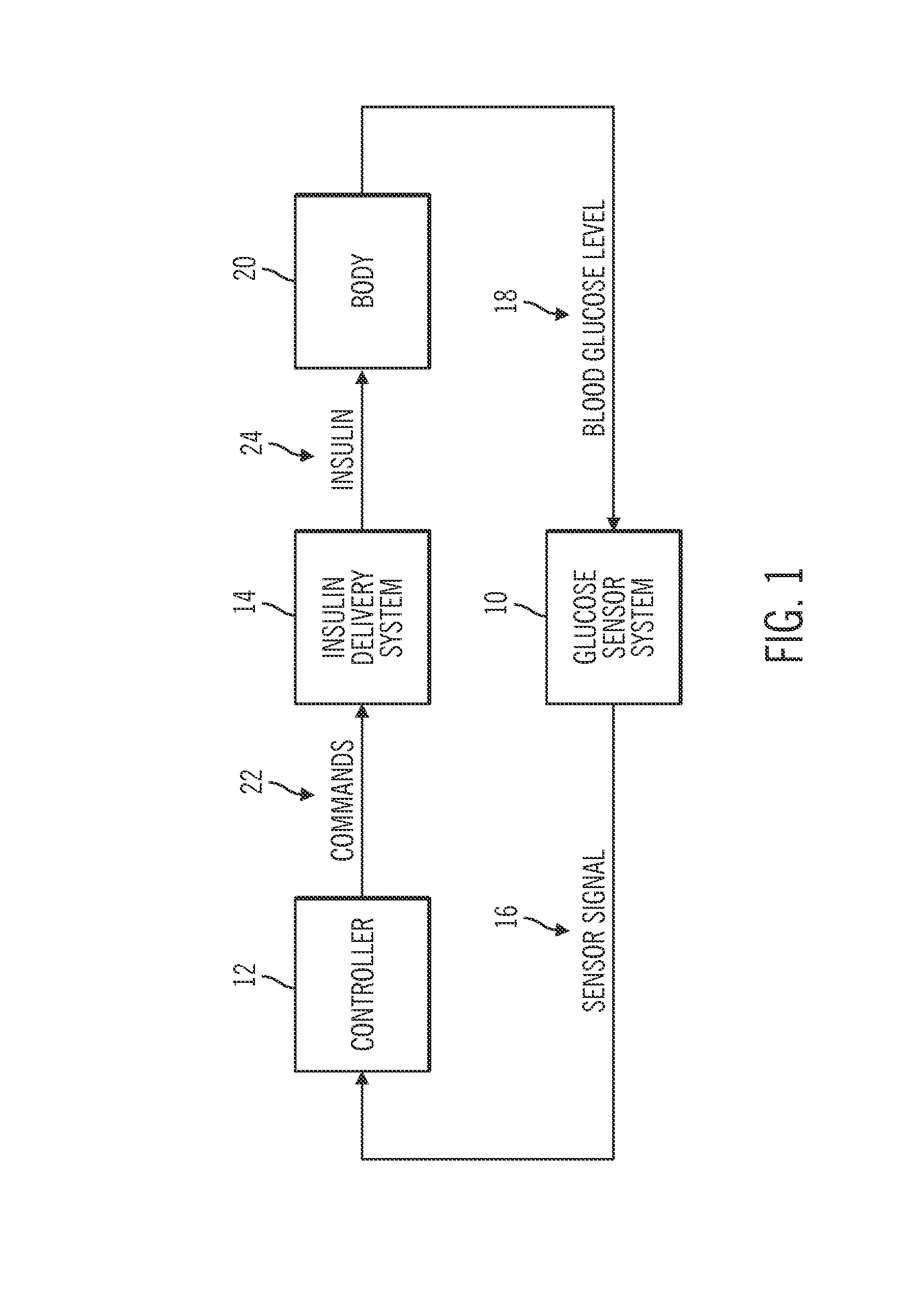
Method for advising patients concerning doses of insulin
A method for guiding a user to select a dose of insulin, including the steps of calculating a firsts pecific dose of insulin by applying information provided by the user to an insulin dose calculation algorithm, wherein such information includes at least the user's current blood glucose level and the user's desired blood glucose level, calculating at least a second specific dose of insulin that is different from the first specific dose, and presenting to the user a range of doses comprising at least two of the specific doses.
Owner:INSULET CORP
Watch controller for a medical device
InactiveUS20070093786A1Minimizes potential for errorEasy to watchMedical devicesPharmaceutical delivery mechanismCommunications systemMonitoring and control
An infusion system that includes a watch controller device and a communication system to transmit the communications from the watch controller device to an infusion device pump that controls delivery of fluids to the user's body. More particularly, these apparatuses and methods are for providing convenient monitoring and control of the infusion pump device in determining the appropriate amount of insulin to deliver.
Owner:MEDTRONIC MIMIMED INC
Microsensors for glucose and insulin monitoring
InactiveUS6893552B1Simultaneous measurementLacking and neededImmobilised enzymesBioreactor/fermenter combinationsOxidative enzymeD-Glucose
A dual sensor for the simultaneous amperometric monitoring of glucose and insulin, wherein the glucose probe is based on the biocatalytic action of glucose oxidase, and the insulin probe is based on the electrocatalytic activity of metal oxide. Further provided is an oxidase enzyme composite electrode with an internal oxygen-rich binder. The present invention also optionally includes metallizing components within the carbon paste to eliminate signals from interfering compounds. The present invention includes embodiments for both in vitro and in vivo uses.
Owner:ARROWHEAD CENT
System for determining insulin dose using carbohydrate to insulin ratio and insulin sensitivity factor
Owner:EMBECTA CORP
Method and apparatus for glucose control and insulin dosing for diabetics
ActiveUS20050272640A1Ensure robustnessAccurately predicting insulin bolus dosagesPeptide/protein ingredientsDrug and medicationsPhysiologyMonitors blood glucose
A computer implemented method and associated apparatus for the combined control of insulin bolus dosing and basal delivery for the goal of achieving normal glycemic response to meals, exercise, stressors, and other perturbations to blood glucose levels. A run-to-run algorithm is used to monitor blood glucose levels and adjust insulin delivery as conditions are varied.
Owner:RGT UNIV OF CALIFORNIA
System and method for measuring and predicting insulin dosing rates
InactiveUS20070078314A1Efficient managementDrug and medicationsMedical automated diagnosisPatient dataGlucose polymers
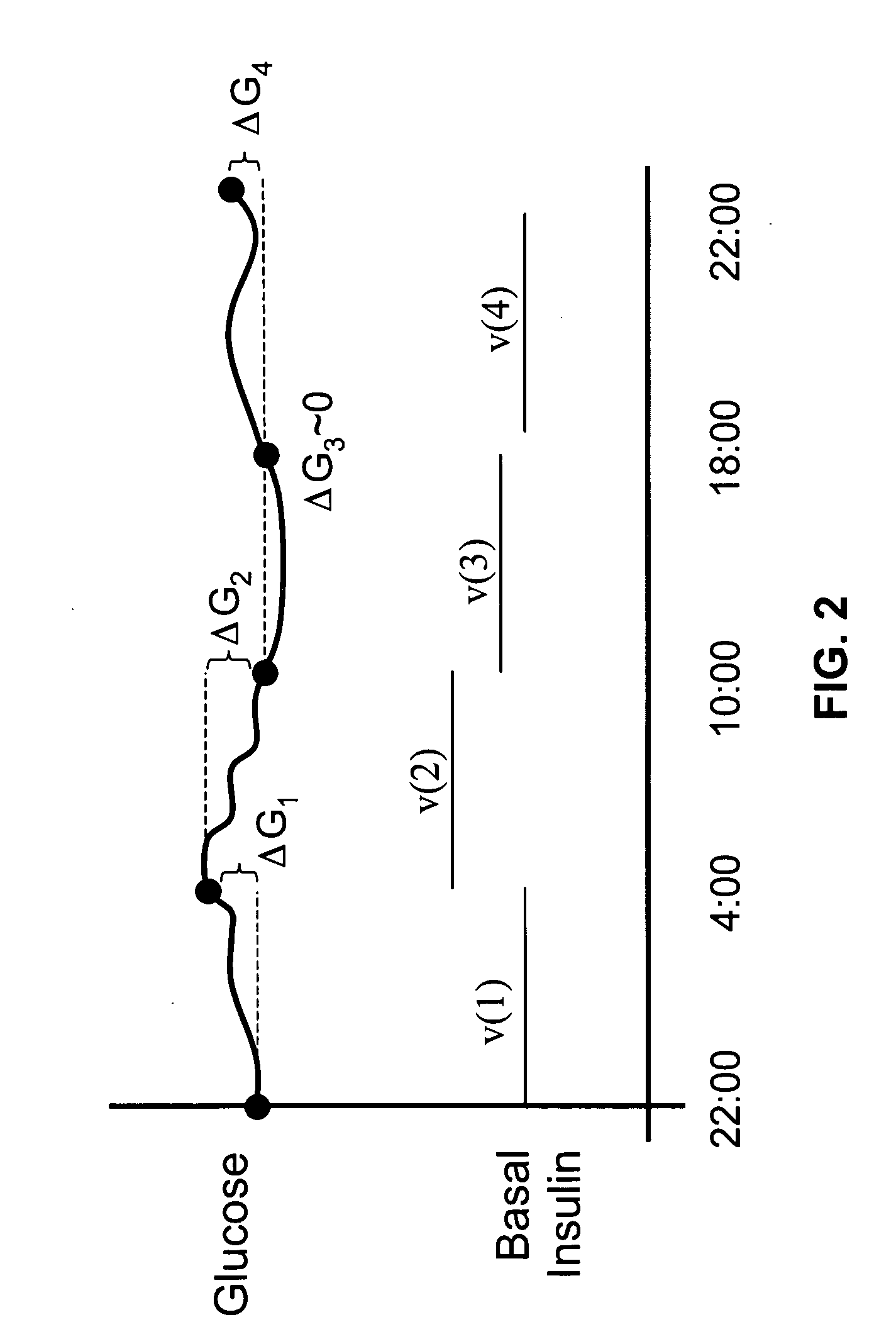
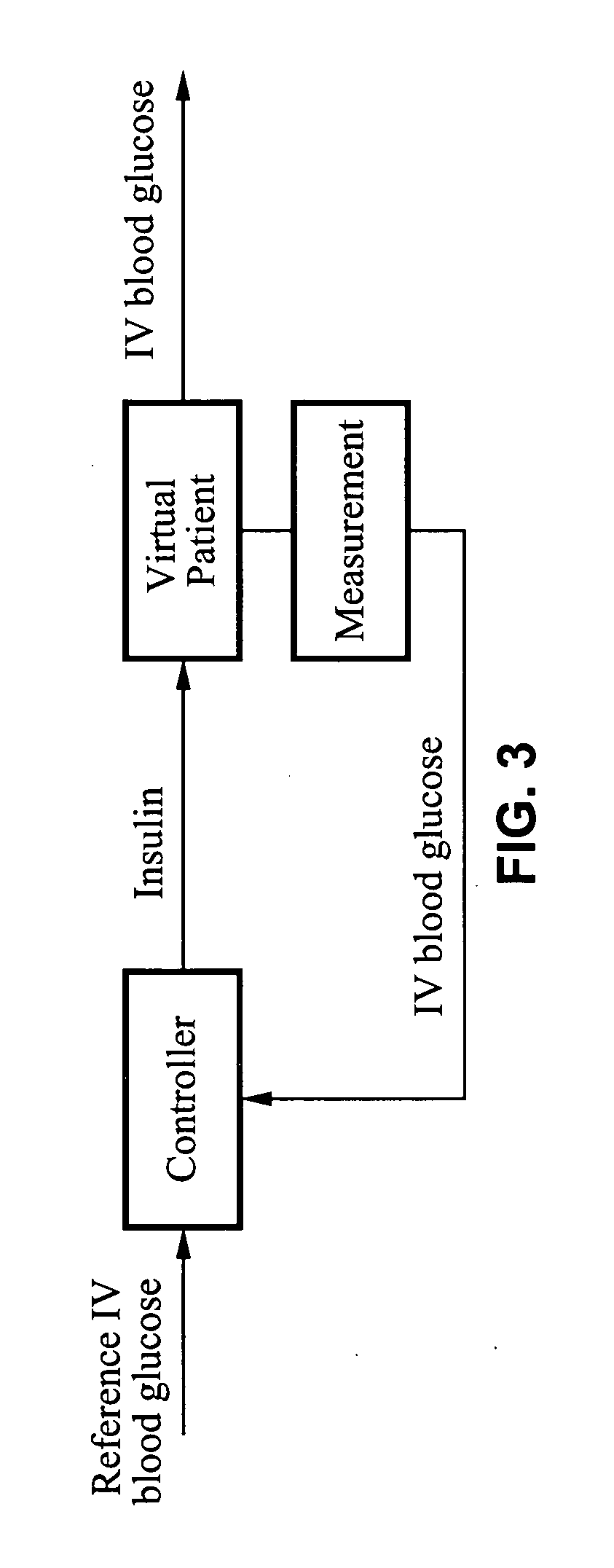
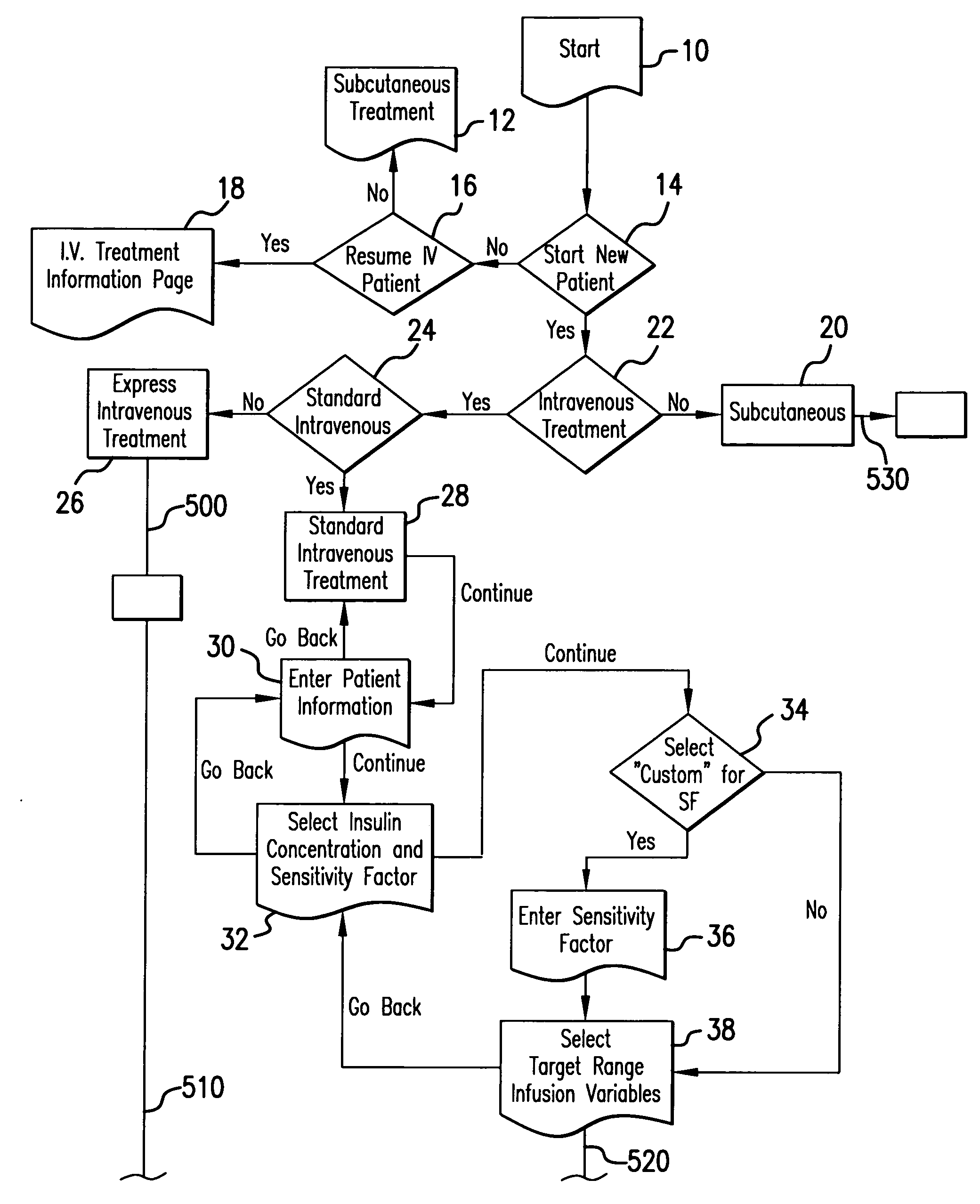
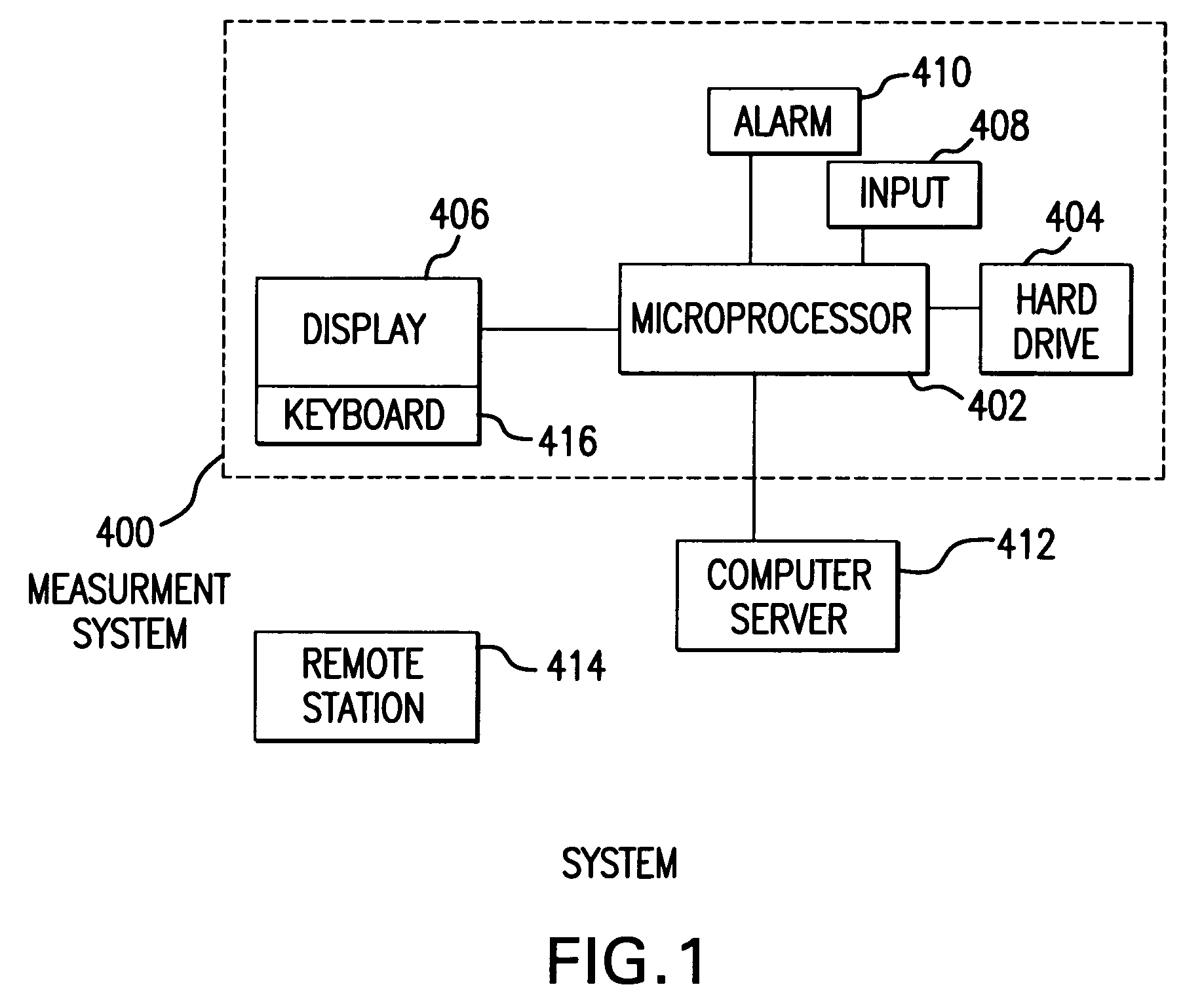
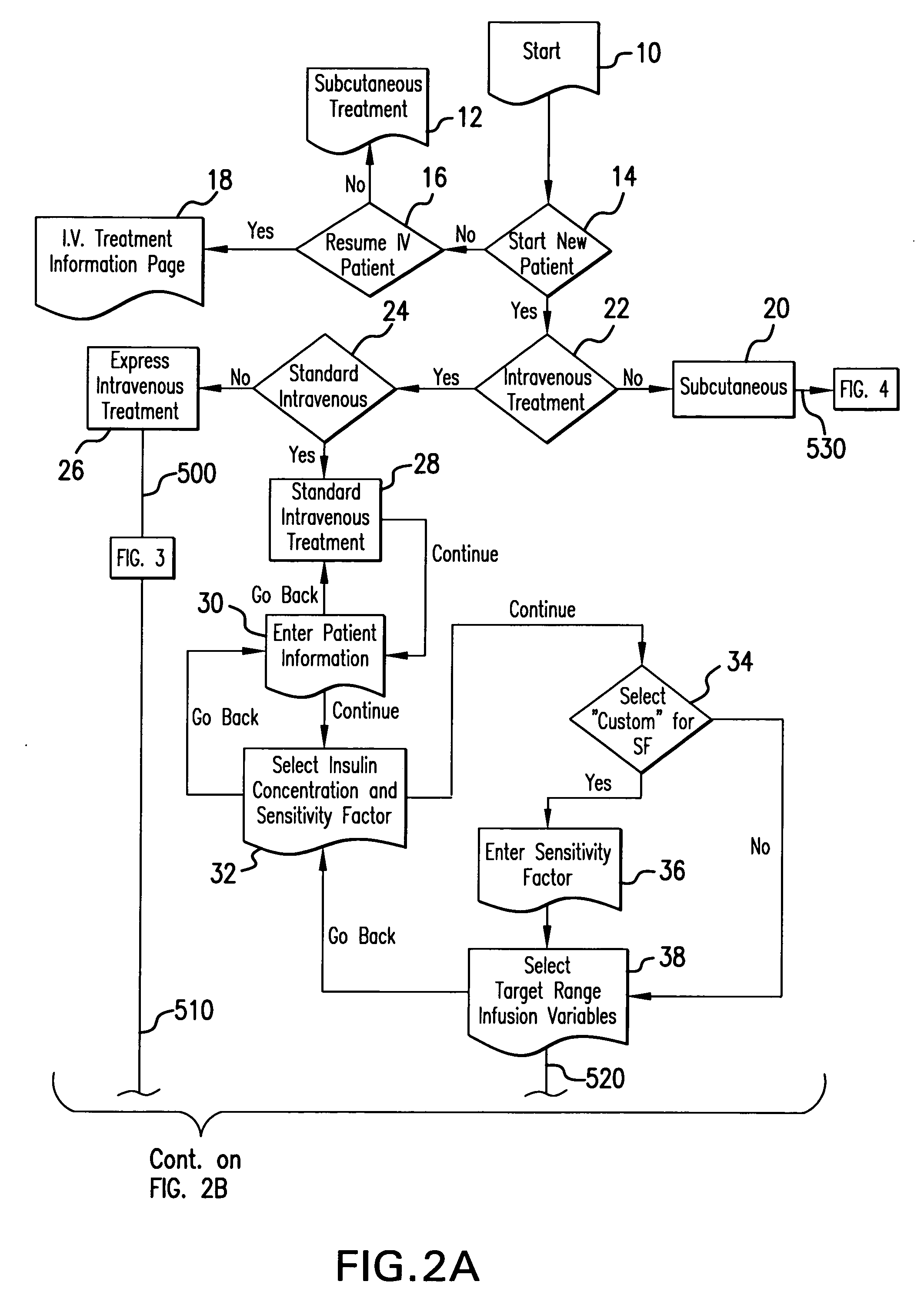
The method and system for managing a patient's blood glucose level predicts an insulin dosing rate to bring a patient's blood glucose level into a preferred target range within a predetermined time interval. The system includes a processor which actuates a blood glucose computer program to measure and predict the patient's blood glucose level. An input mechanism allows for insertion of a preferred target range of the patient's blood glucose level and further permits input of various patient data parameters. The processor calculates the optimum insulin dosing rate for the patient based upon the type of insulin dosing whether it be intravenous dosing and / or subcutaneous dosing. A display mechanism displays the patient dosing parameters and an alarm mechanism alerts a user when the patient's blood glucose level is outside of the preferred patient blood glucose target range.
Owner:GLUCOTEC
Methods of determining pre or post meal time slots or intervals in diabetes management
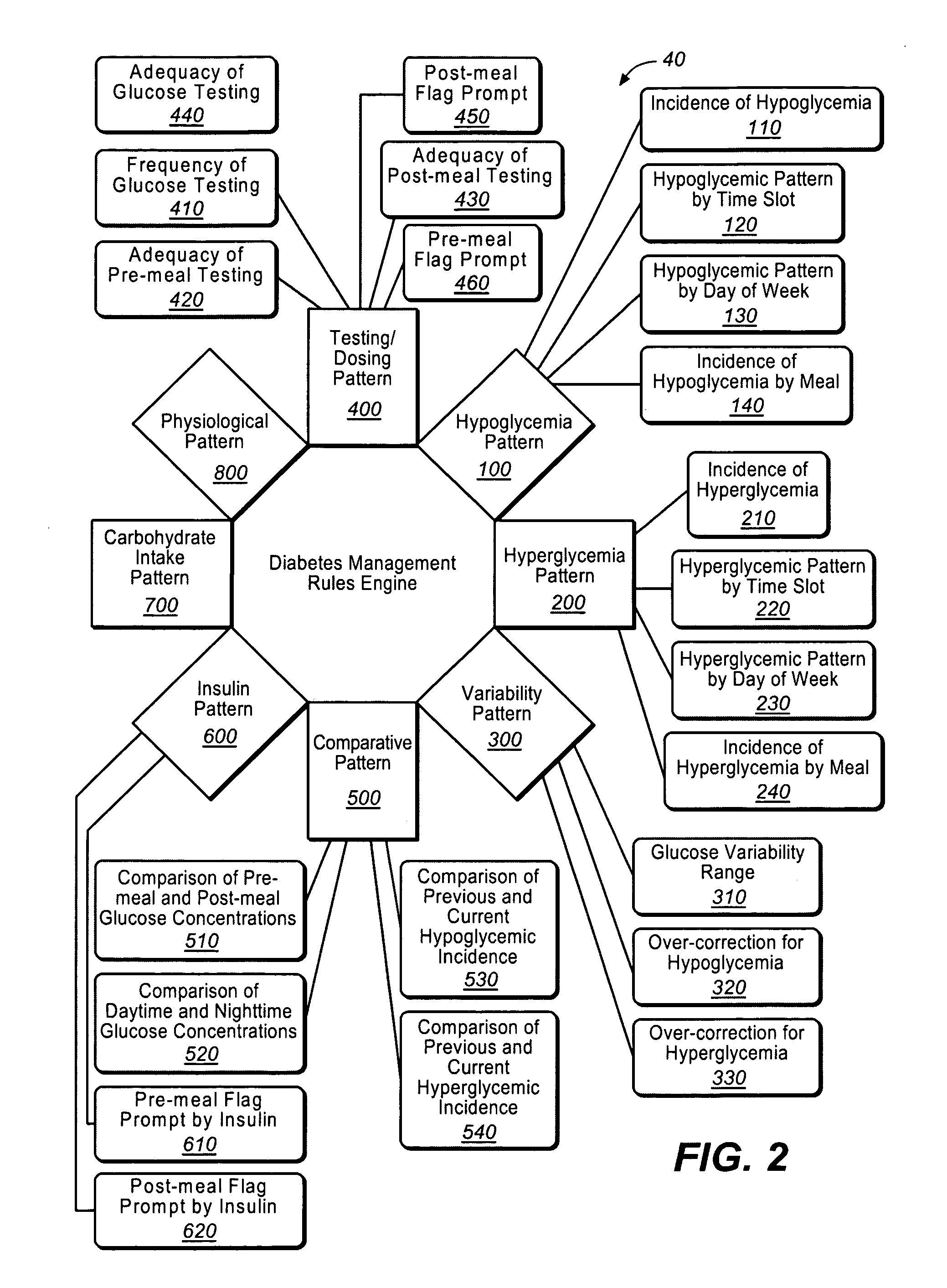
A diabetes management system or process is provided herein that may be used to analyze and recognize patterns for a large number of blood glucose concentration measurements and other physiological parameters related to the glycemia of a patient. In particular, a method of monitoring glycemia in a patient may include storing a patient's data on a suitable device, such as, for example, a blood glucose meter. The patient's data may include blood glucose concentration measurements. The diabetes management system or process may be installed on, but is not limited to, a personal computer, an insulin pen, an insulin pump, or a glucose meter. The diabetes management system or process may identify a plurality of pattern types from the data including a testing / dosing pattern, a hypoglycemic pattern, a hyperglycemic pattern, a blood glucose variability pattern, and a comparative pattern. After identifying a particular pattern with the data management system or process, a warning message may be displayed on a screen of a personal computer or a glucose meter. Other messages can also be provided to ensure compliance of any prescribed diabetes regiments or to guide the patient in managing the patient's diabetes.
Owner:LIFESCAN INC
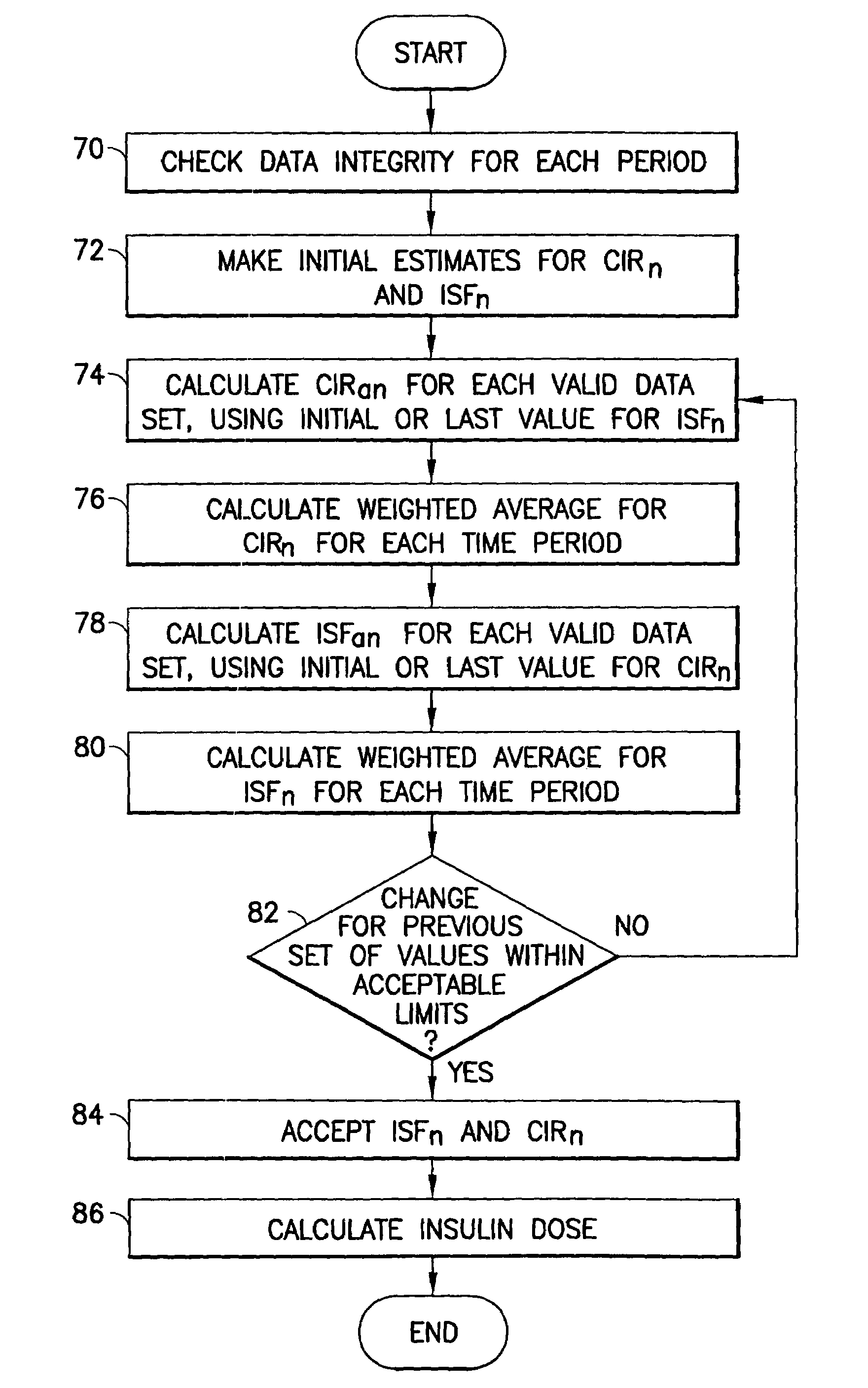
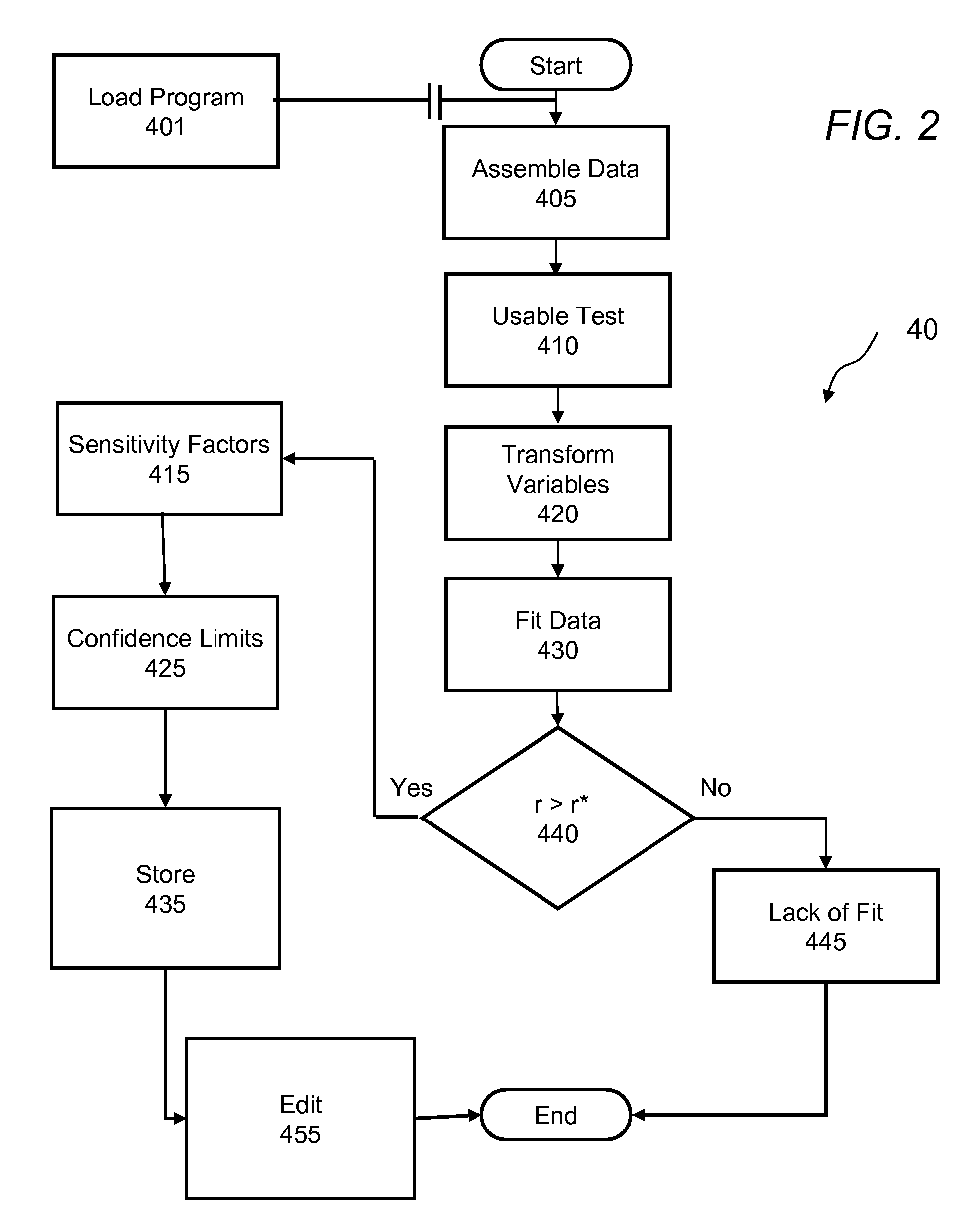
Method and apparatus to calculate diabetic sensitivity factors affecting blood glucose
InactiveUS20100262434A1Reasonable expectationPhysical therapies and activitiesDrug and medicationsGlucose polymersD-Glucose
Methods and apparatus are provided for determining a diabetic patient's carbohydrate to insulin ratio (CIR), carbohydrate to blood glucose ratio (CGR), and insulin sensitivity factor (ISF) using the patient's record of blood glucose readings, carbohydrate consumption and insulin doses. The method provides the sensitivity factors that best account for the patient's observed blood glucose changes by linear regression of appropriately transformed variables. An apparatus that can collect and store the blood glucose readings, insulin dosages, and carbohydrate intake data and process these data according to this invention can generate statistically characterized sensitivity factors to advise the diabetic patient on optimal bolus insulin dosages.
Owner:SHAYA STEVEN A
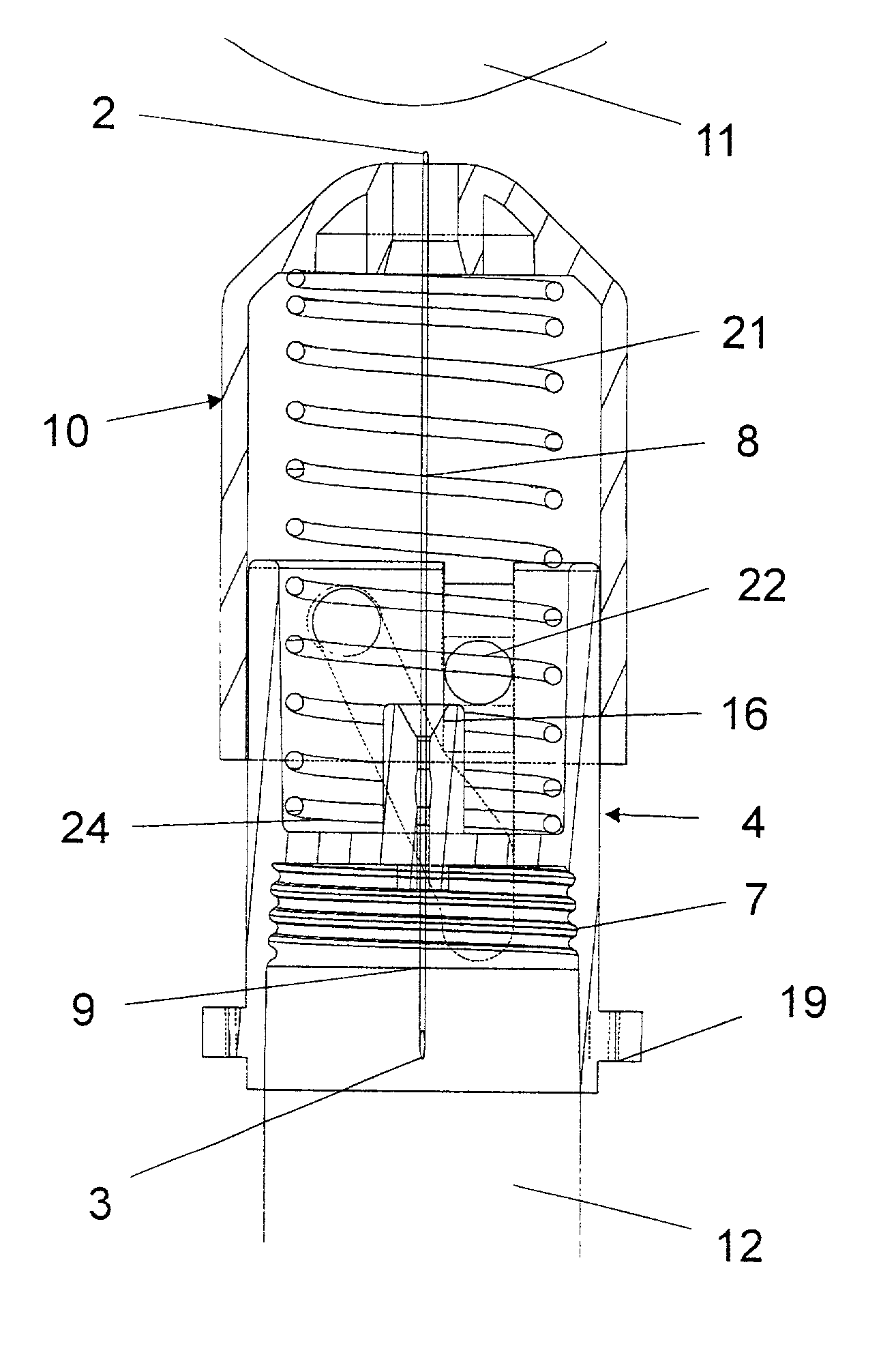
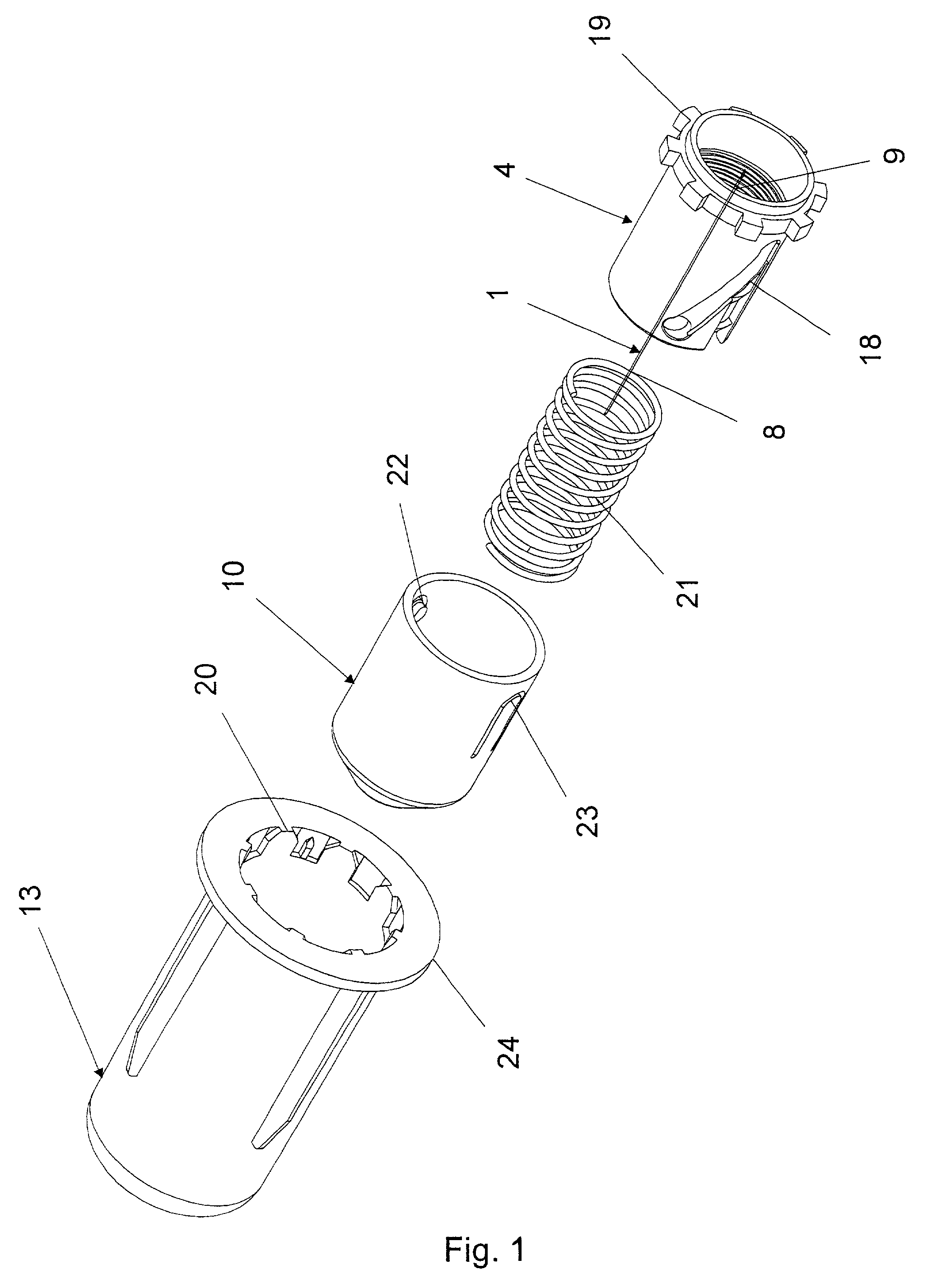
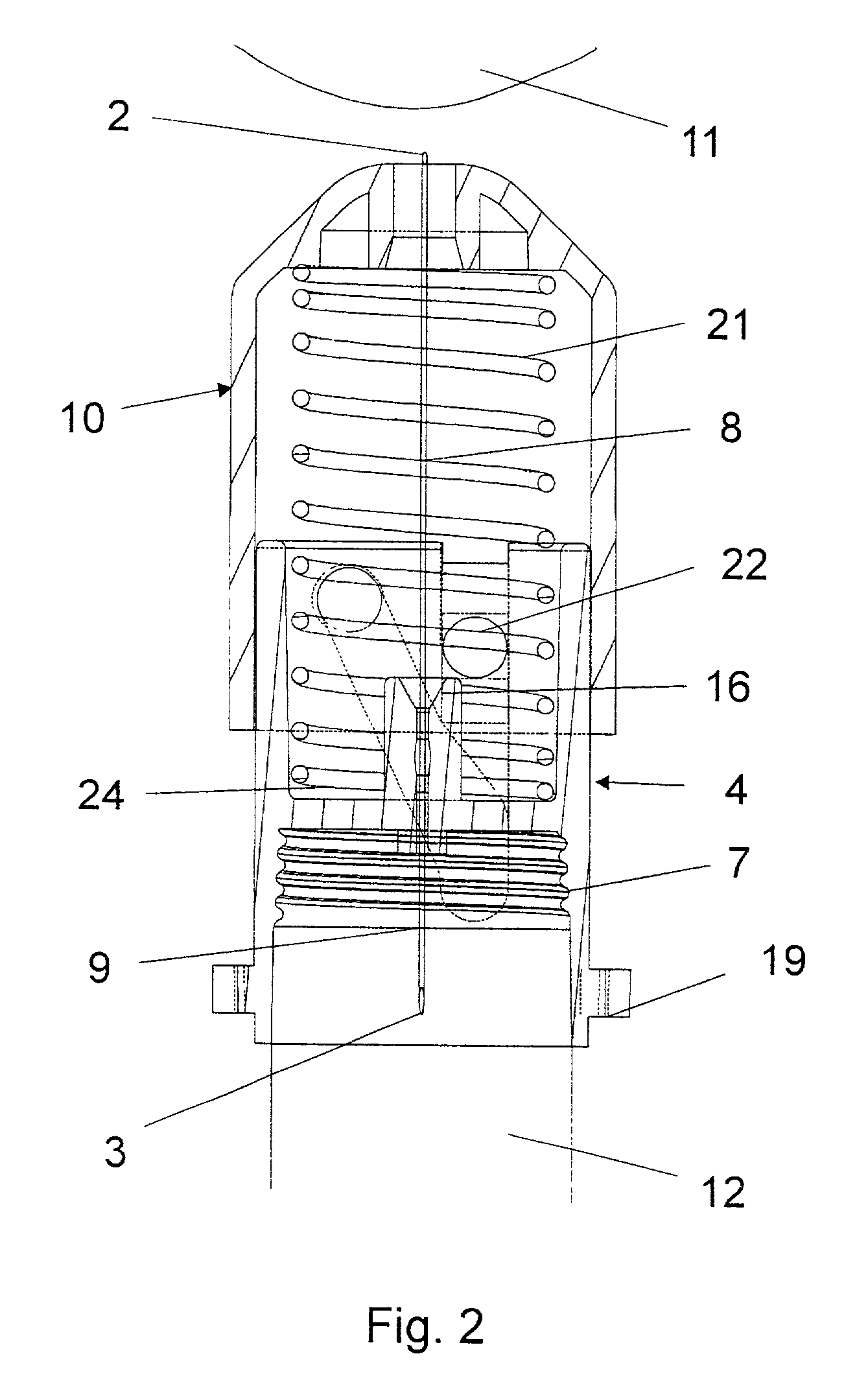
Disposable double pointed injection needle, and an insulin injection system comprising a disposable double pointed injection needle
A disposable double pointed injection needle has a needle hub to which a thin needle cannula is permanently fastened and which needle hub can be mounted on to a syringe comprising a dose setting and injection mechanism and a cartridge containing a liquid medicine to be injected subcutaneously into a human body. The needle hub is provided with a safety shield guided on the outside surface of the needle hub. The safety shield is urged in a direction away from the needle hub by a spring located between the needle hub and the safety shield. The safety shield has a number of protrusions guided in guiding tracks on the outside surface of the needle hub. The guiding tracks are designed such that the safety shield during injection is moved towards the needle hub, and after injection is moved away from the needle hub by the spring and locked in an irreversible position where the safety shield covers the needle cannula and prevents accidental needle stick injuries.
Owner:NOVO NORDISK AS
Methods for modeling insulin therapy requirements
InactiveUS20110098548A1Improve fitShorten the timeMedical simulationDrug and medicationsGlycemicGlucose control
Various methods for improving the use of model based prediction of future blood glucose control in a patient having diabetes are described. A system for processing diabetes related information, including glucose information, for accurately predicting future glucose levels as a function of glucose data, carbohydrate intake, insulin delivery history and exercise history and then providing recommendations related to the predicted future glucose levels, is also described.
Owner:ABBOTT DIABETES CARE INC
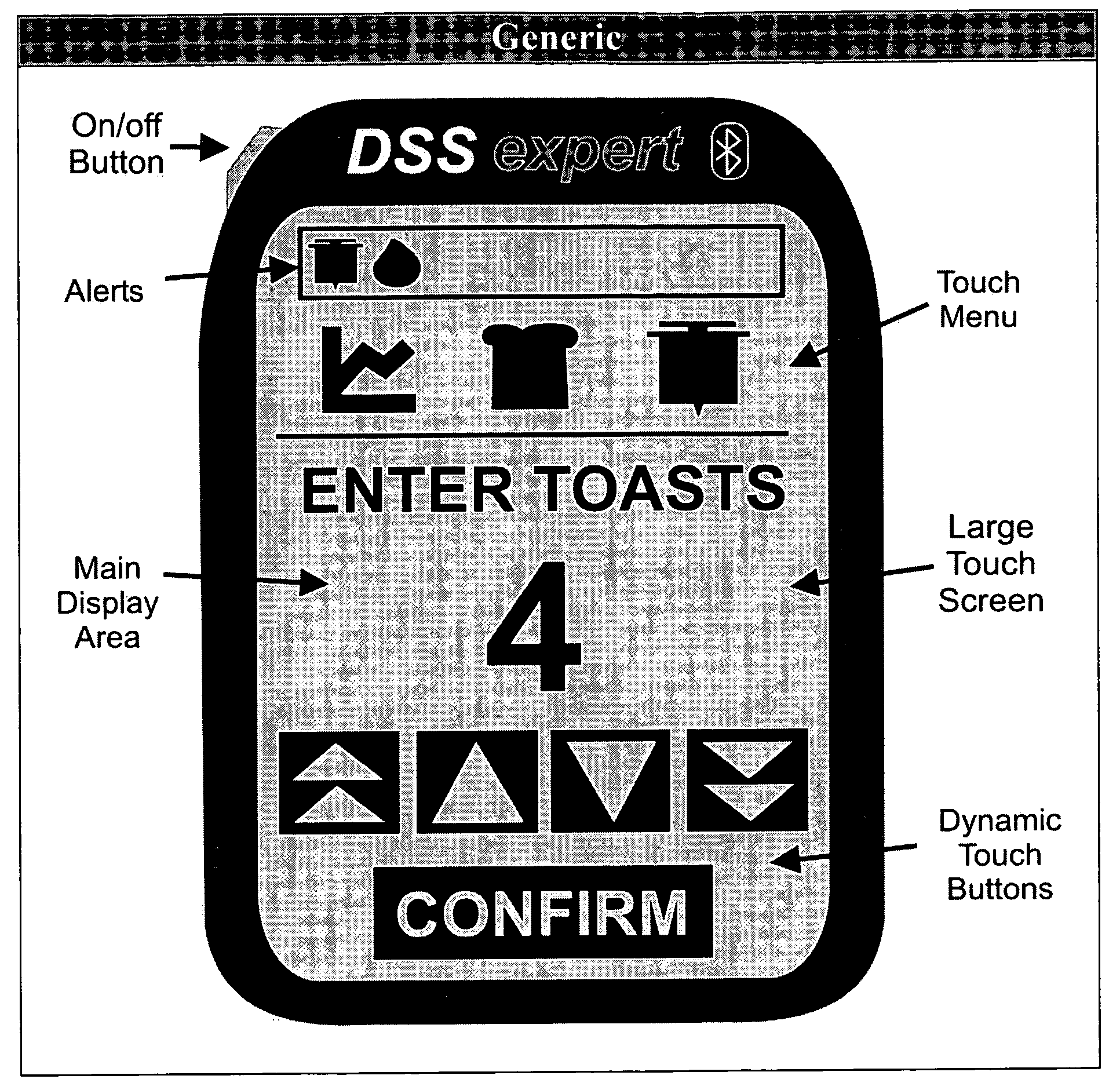
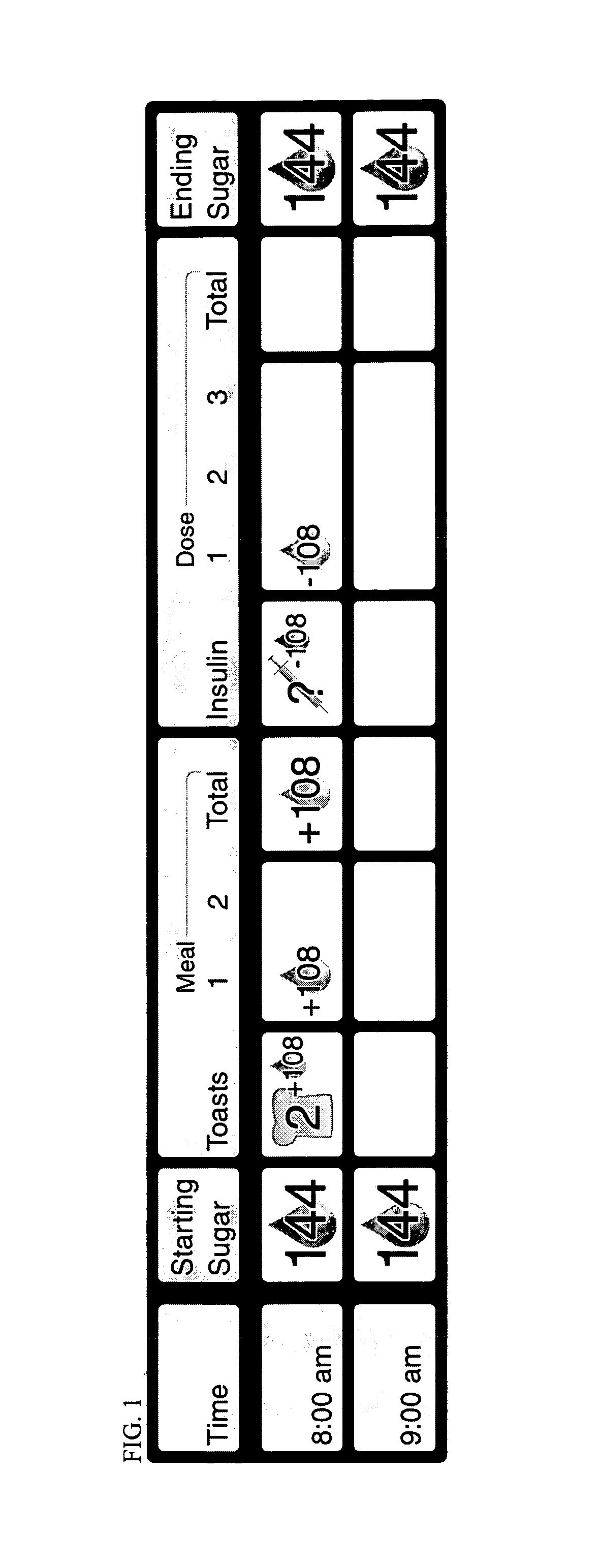
Medical Data Display
InactiveUS20100259543A1Quality is easy to controlMedical simulationDrawing from basic elementsData displayBlood Glucose Measurement
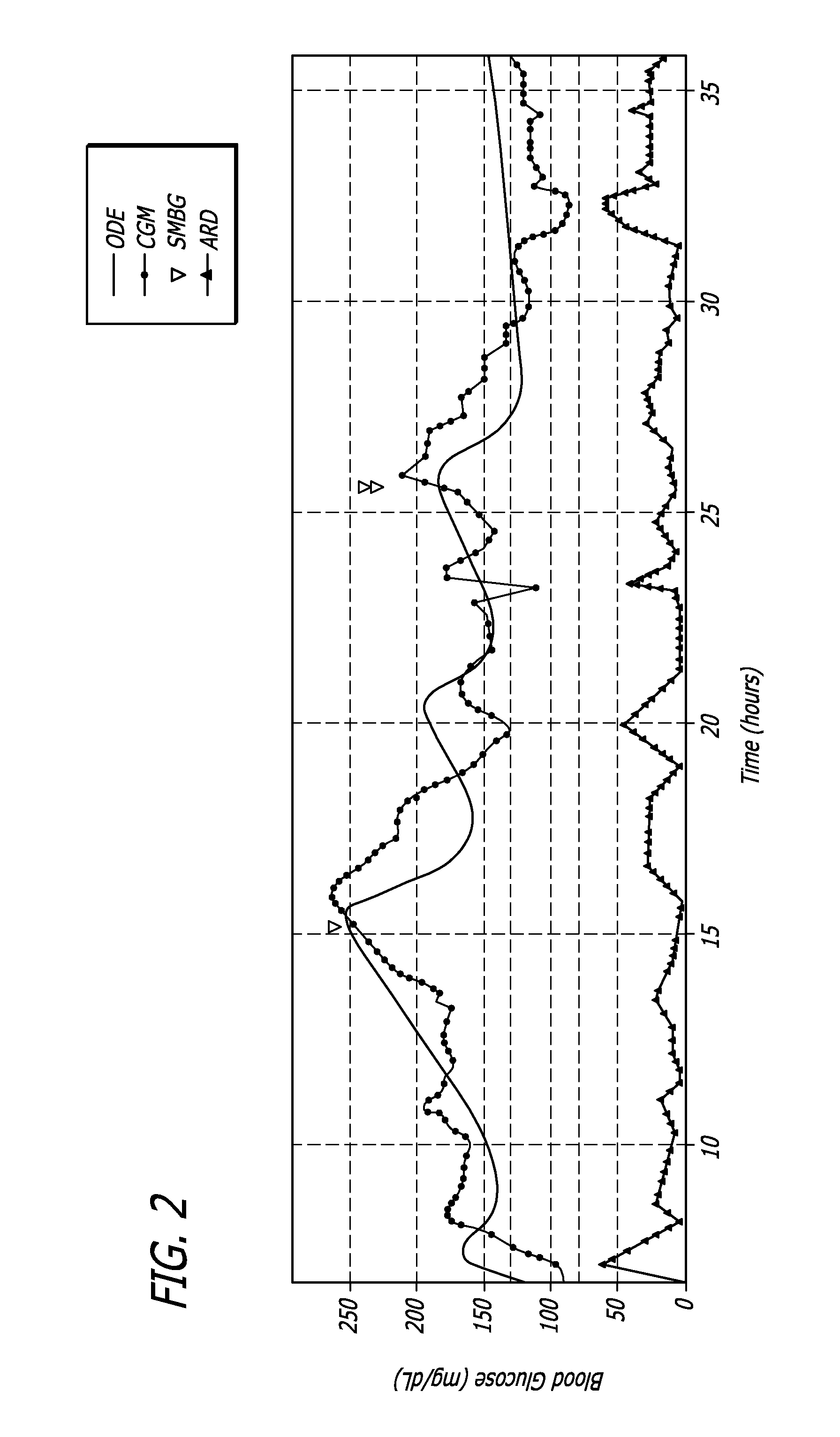
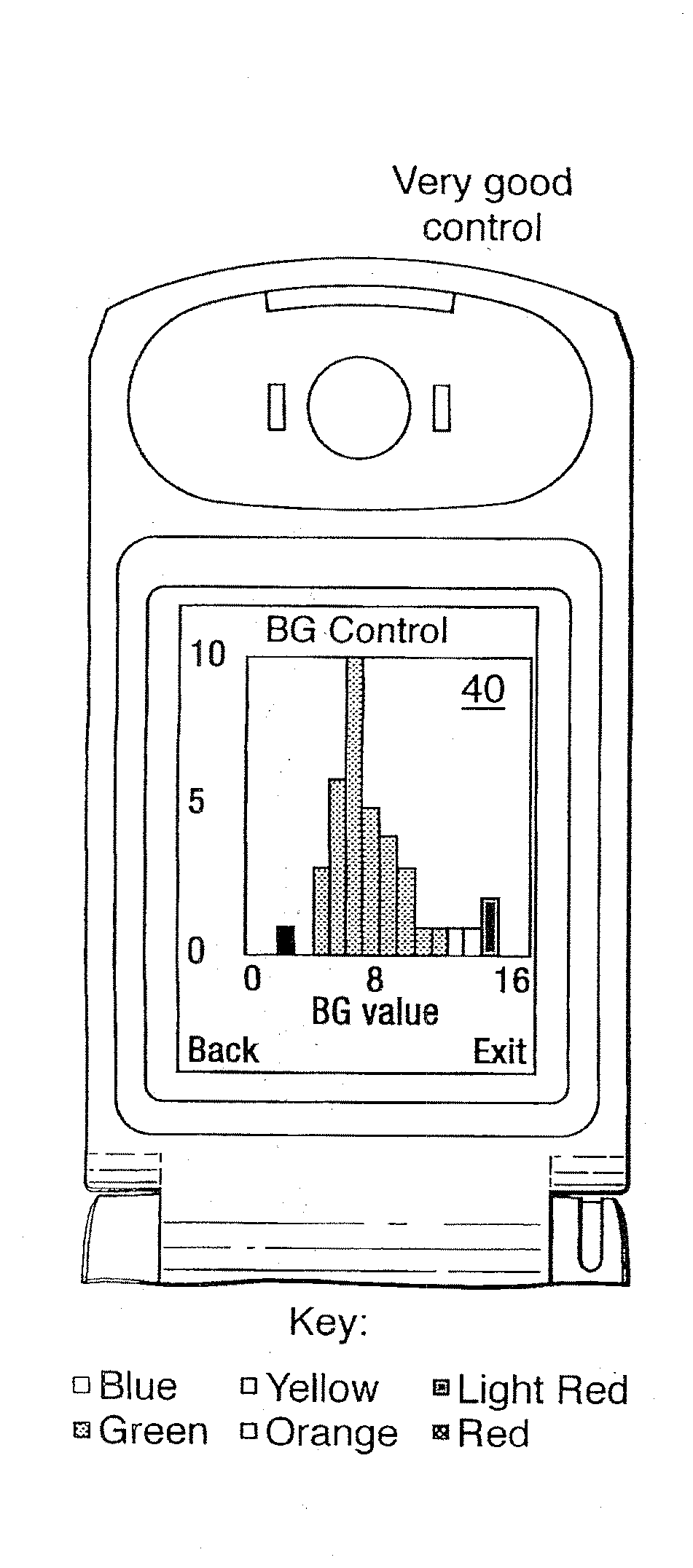
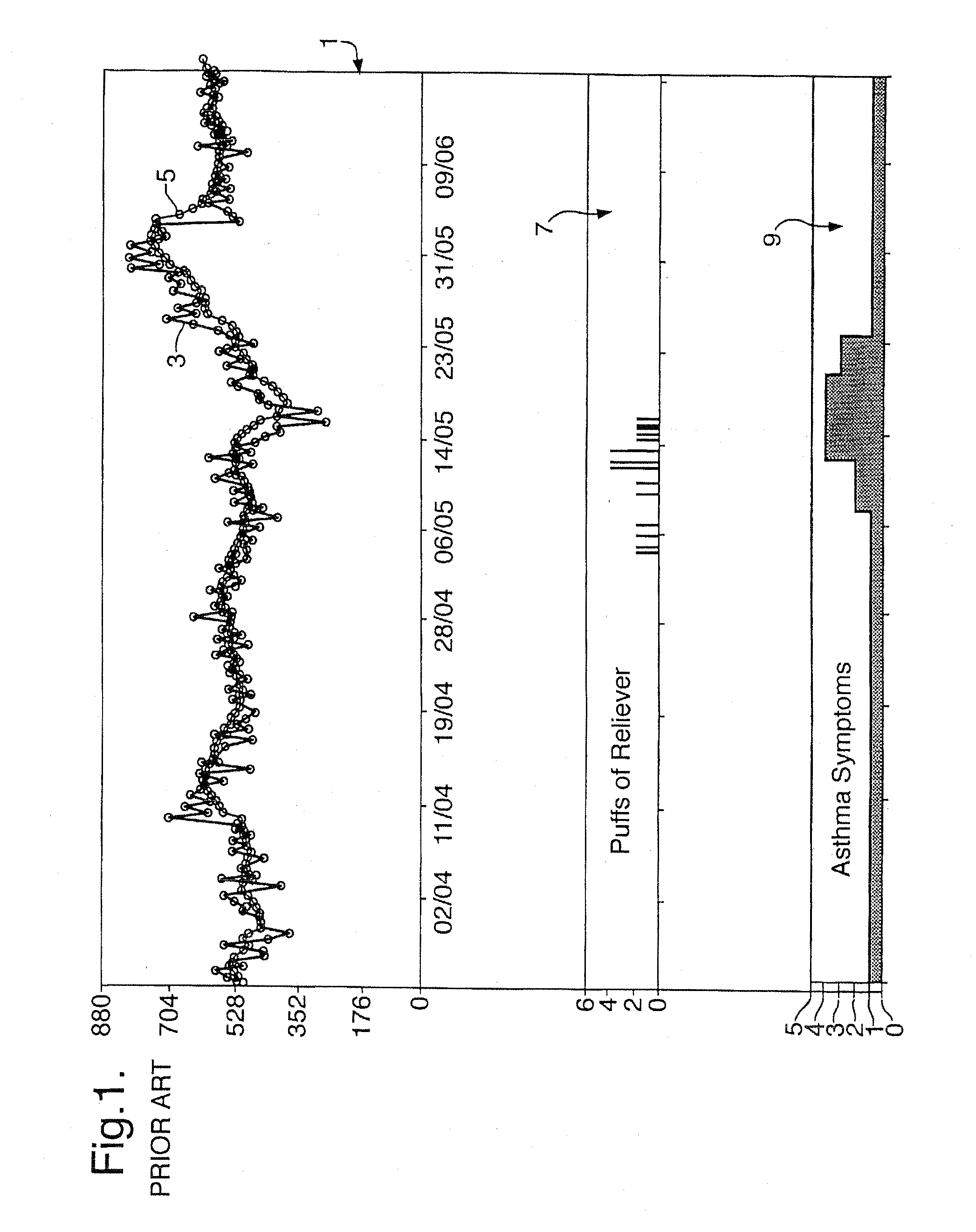
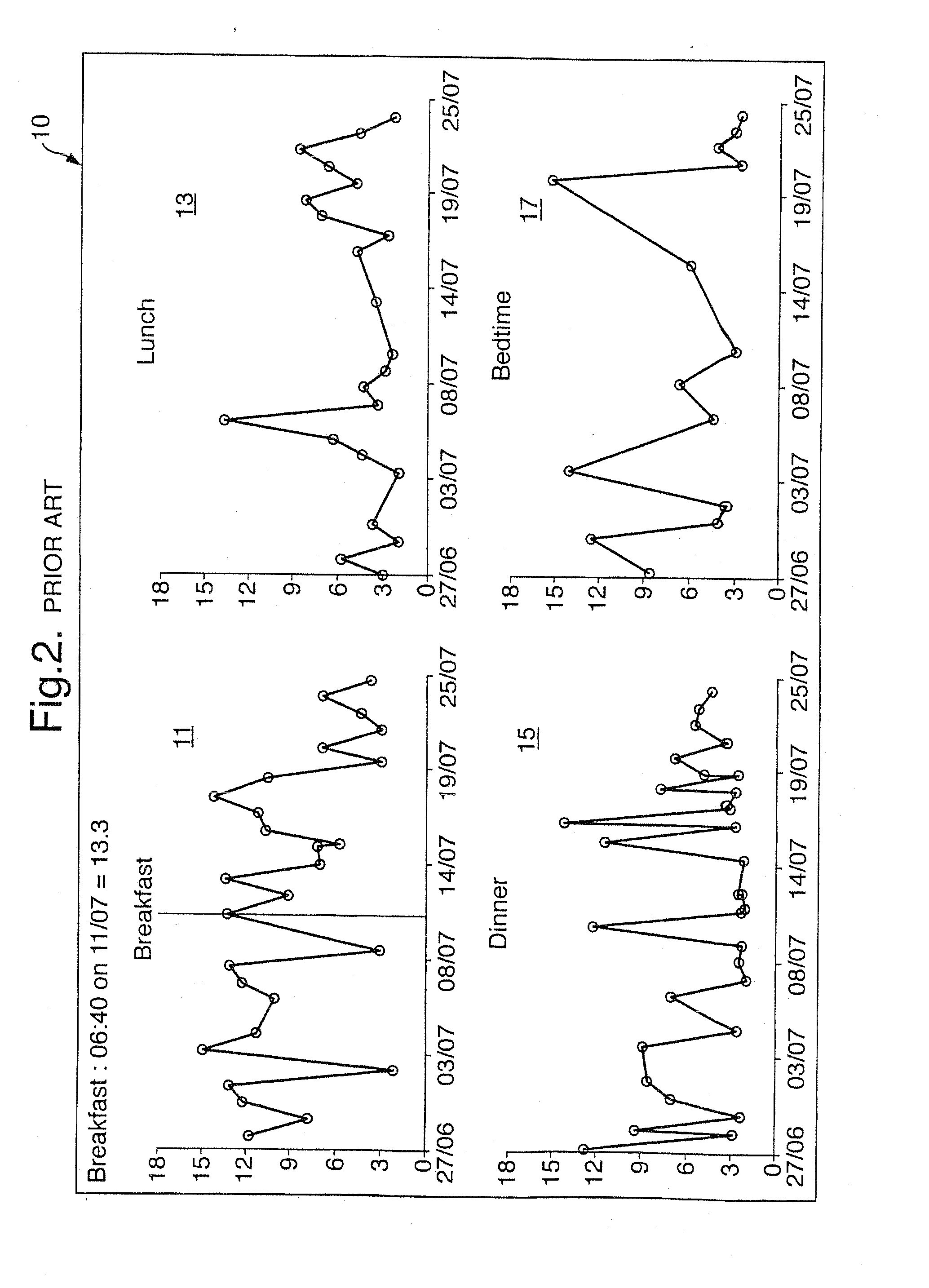
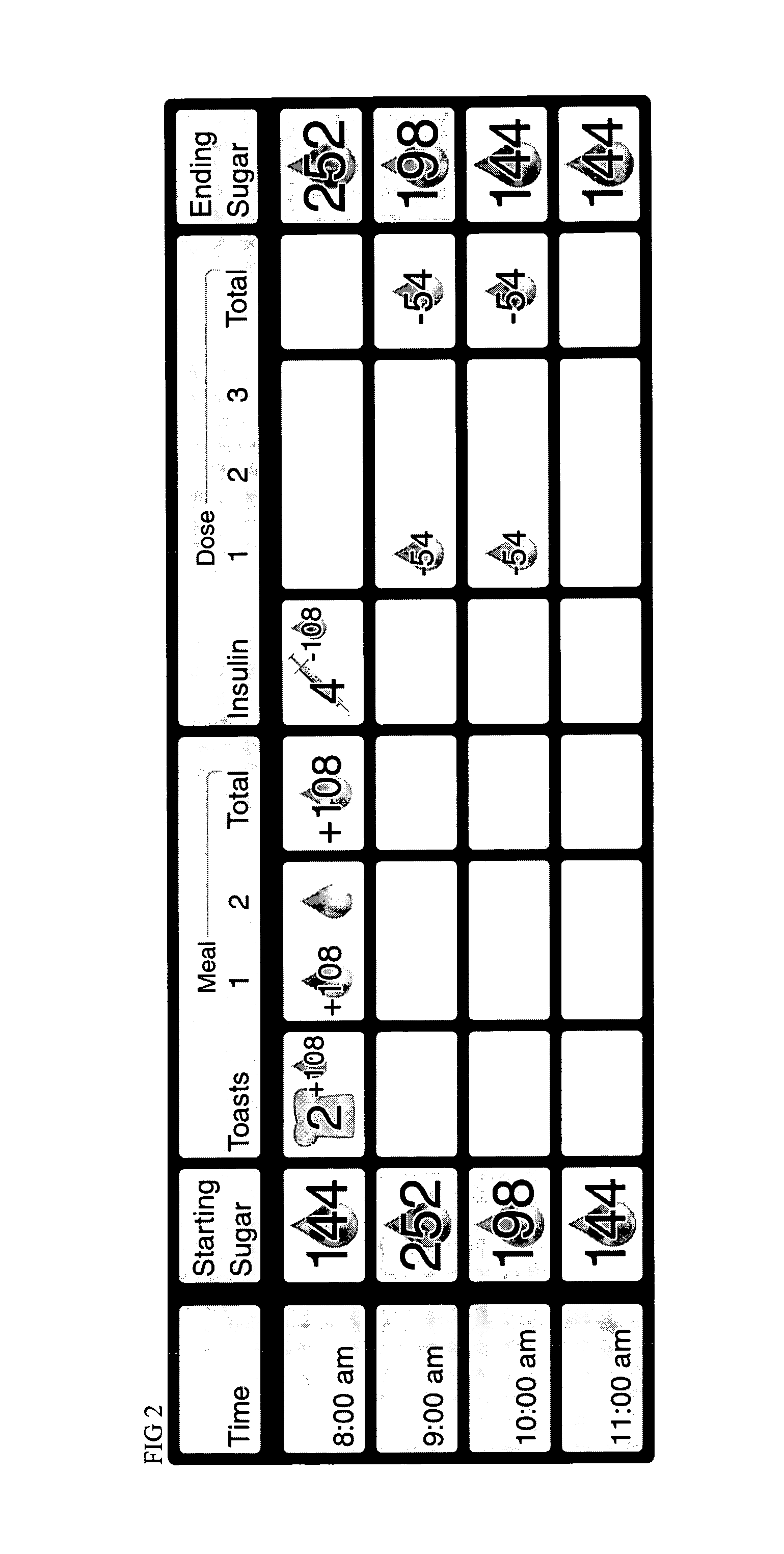
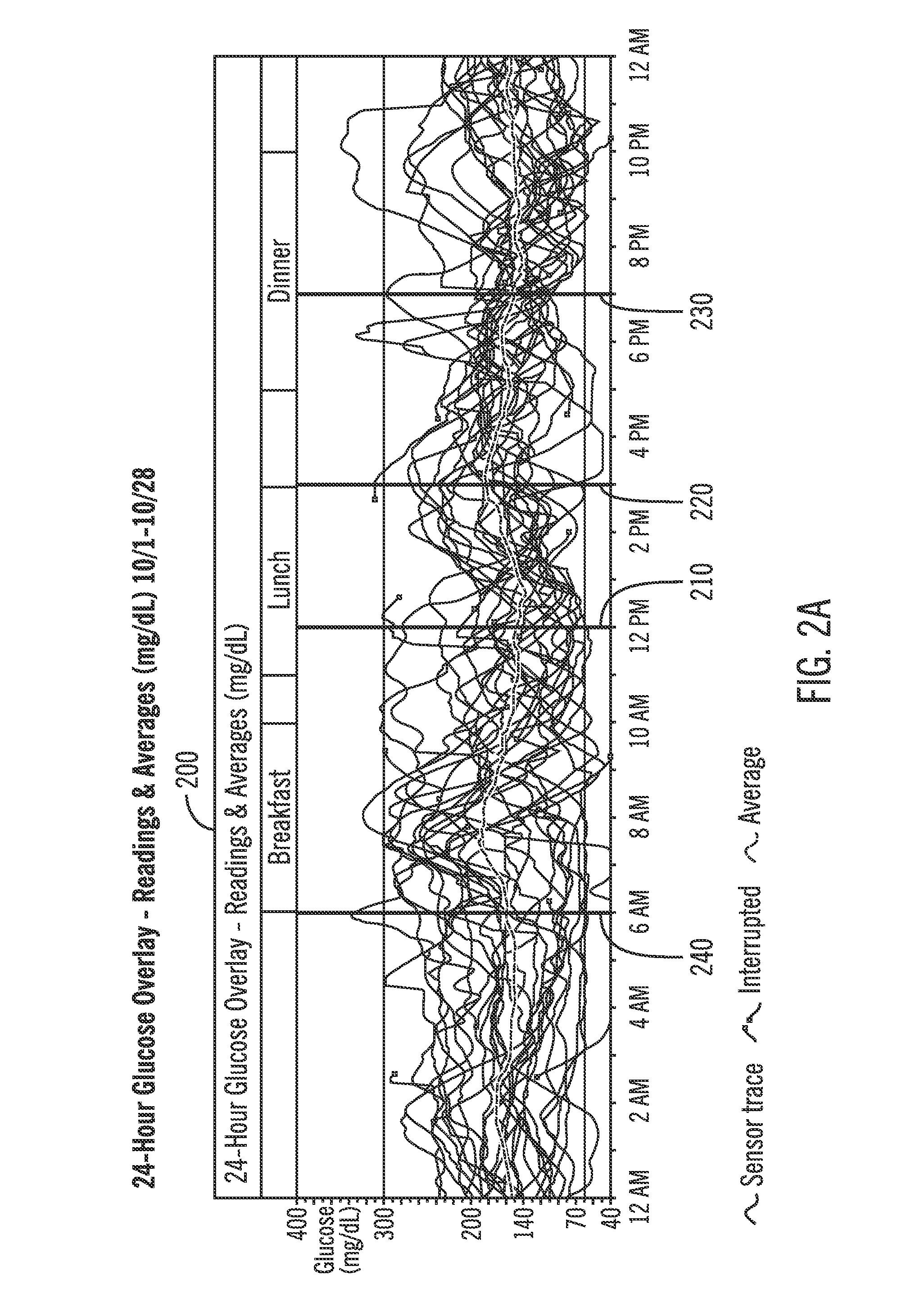
A method of displaying medical data, particularly data representative of the condition of patients suffering from chronic medical conditions such as asthma, diabetes and hypertension. The display consists of two graphical elements, one of which indicates the current value of a parameter indicative of the patient's condition, this being displayed against another graphical element which represents a model of normality for that patient. The graphical element indicating the current condition may be, for example, a needle, against a scale which is constructed according to the patient-specific model of normality. This is particularly advantageous in the case of displays which have a small display area, such as mobile telephones and PDAs. Other forms of display are disclosed, such as histograms with the display being dynamically colour-coded and auto-scaled, or displays including limits which may vary. Another form of display is also disclosed which illustrates administrations of a pharmacological agent and corresponding measurements of the patient's condition, with a visual link such as colour-coding linking the administration to the corresponding condition measurement. For example several days of insulin administration dosages may be displayed alongside several days of blood glucose measurements, with the administrations colour-coded to the corresponding blood glucose measurement, to assist the patient in determining whether the insulin administration is stably controlling their condition.
Owner:E SAN LTD
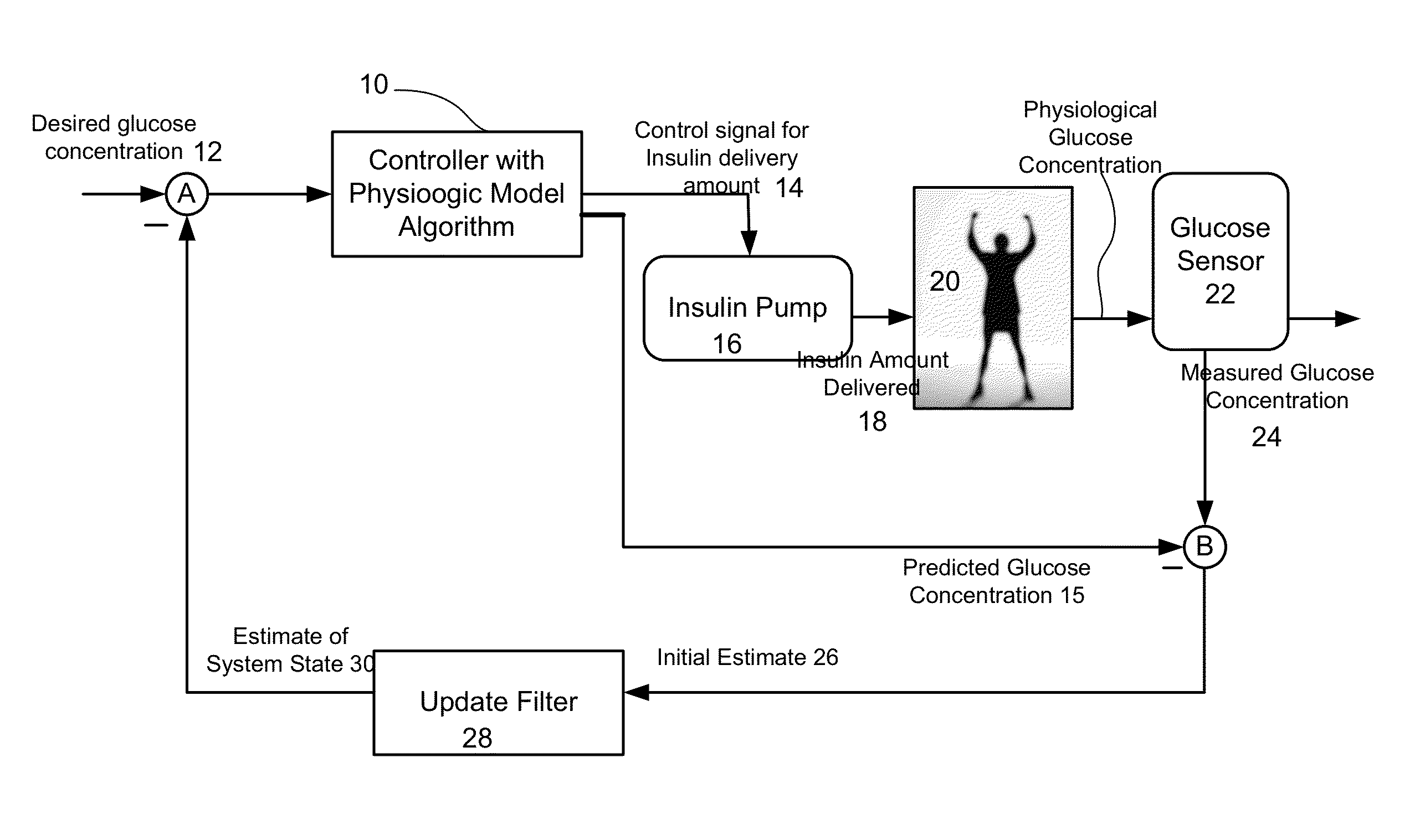
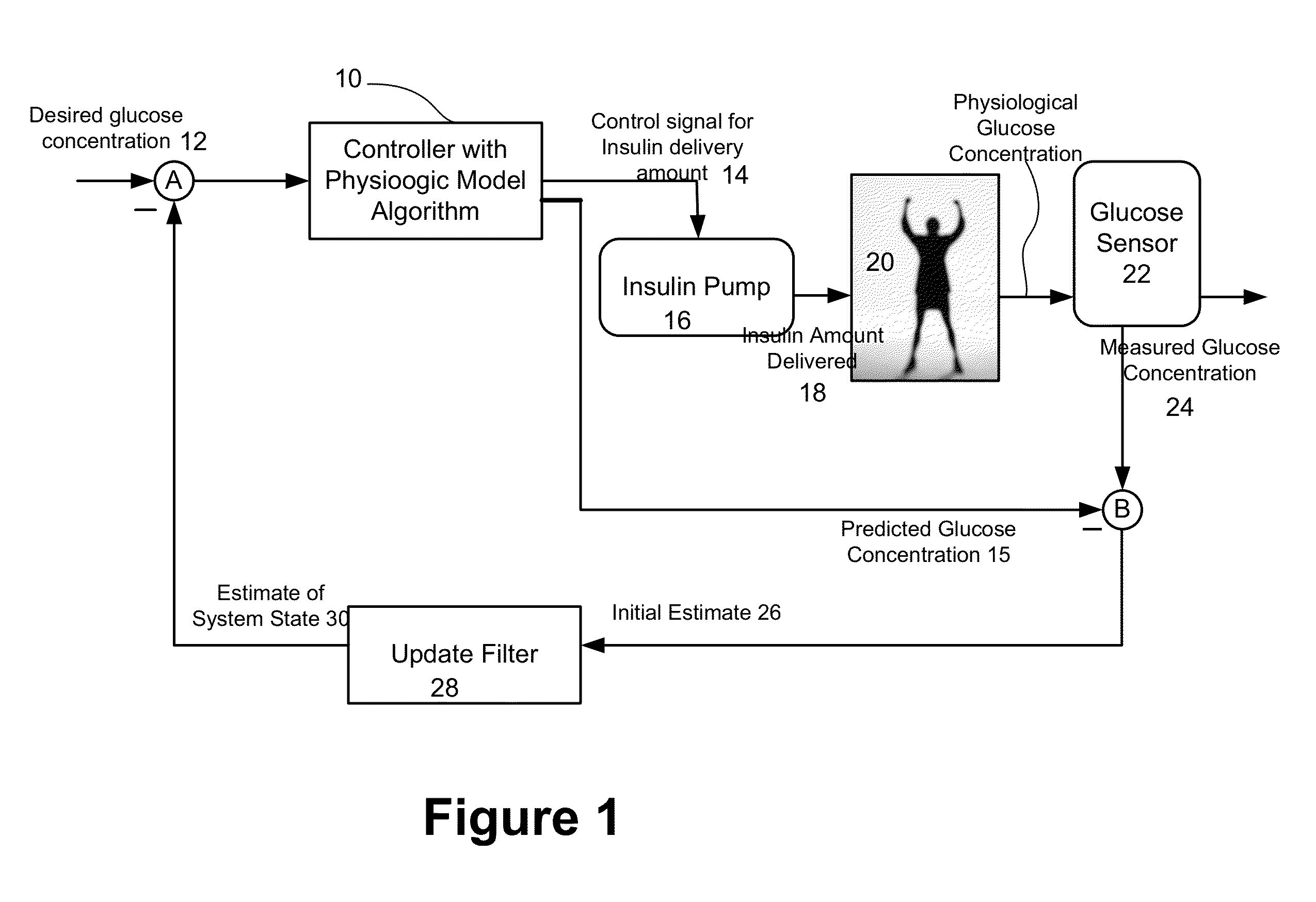
Predictive control based system and method for control of insulin delivery in diabetes using glucose sensing
A system and method for providing optimal insulin injections to a subject, using a controller, a continuous glucose monitor, and an insulin delivery unit is disclosed. The controller possesses a discrete-time, linear model predictive control law, means for sending information to the insulin delivery unit, and means for receiving information from the CGM. The control law implemented is derived from a discrete-time model of glucose insulin dynamics and an aggressiveness parameter. The result is that using only glucose measurements obtained from sensor readings and, prior values of external insulin infusion and meal and exercise announcement the optimal insulin injection necessary to safely regulate blood glucose can be calculated.
Owner:UNIV OF VIRGINIA ALUMNI PATENTS FOUND
Generation and application of an insulin limit for a closed-loop operating mode of an insulin infusion system
A controller for an insulin infusion device includes a processor device and a memory element that cooperate to provide a processor-implemented closed-loop insulin limit module. The insulin limit module is operated to obtain: a fasting blood glucose value of a user; a total daily insulin value of the user; and fasting insulin delivery data that is indicative of insulin delivered to the user during a fasting period. The insulin limit module calculates a maximum insulin infusion rate for the user based on the fasting blood glucose value, the total daily insulin value, and the fasting insulin delivery data. The maximum insulin infusion rate is applicable during a period of closed-loop operation of the insulin infusion device.
Owner:MEDTRONIC MIMIMED INC
Method of food and insulin dose management for a diabetic subject
InactiveUS7137951B2Lower blood sugar levelsReduce the amount requiredPeptide/protein ingredientsDrug and medicationsINSULIN USEInsulin dose
The invention relates to a method of food and insulin dose management for a diabetic subject, comprising:providing an intended insulin unit value or an intended carbohydrate unit value representing the amount of insulin or carbohydrate intended for intake by the subject; anddetermining the balance value of either insulin units or carbohydrate units needed to balance with the provided unit value and maintain blood sugar in the subject in a target blood sugar range.
Owner:PILARSKI JOSEPH
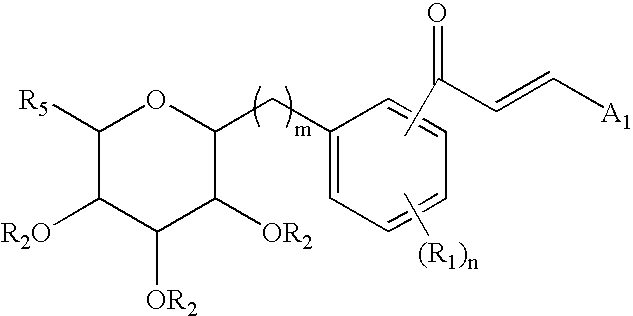
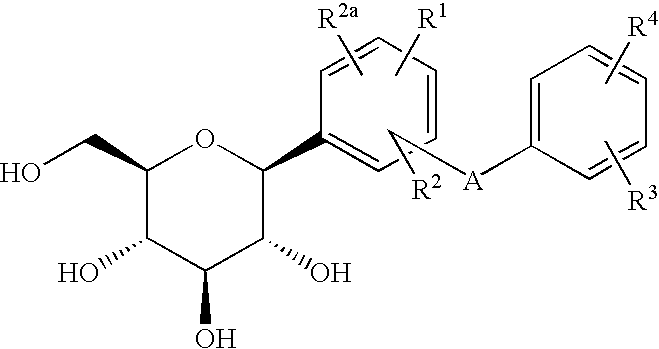
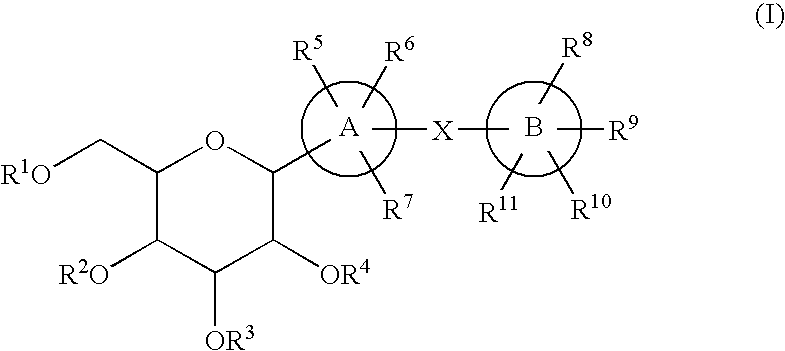
C-glycoside derivatives and salts thereof
The present invention provides C-glycoside derivatives and salts thereof, wherein B ring is bonded to A ring via —X— and A ring is directly bonded to the glucose residue, and it is usable as a Na+-glucose cotransporter inhibitor, especially for a therapeutic and / or preventive agent for diabetes such as insulin-dependent diabetes (type 1 diabetes) and insulin-independent diabetes (type 2 diabetes), as well as diabetes related diseases such as an insulin-resistant diseases and obesity.
Owner:ASTELLAS PHARMA INC +1
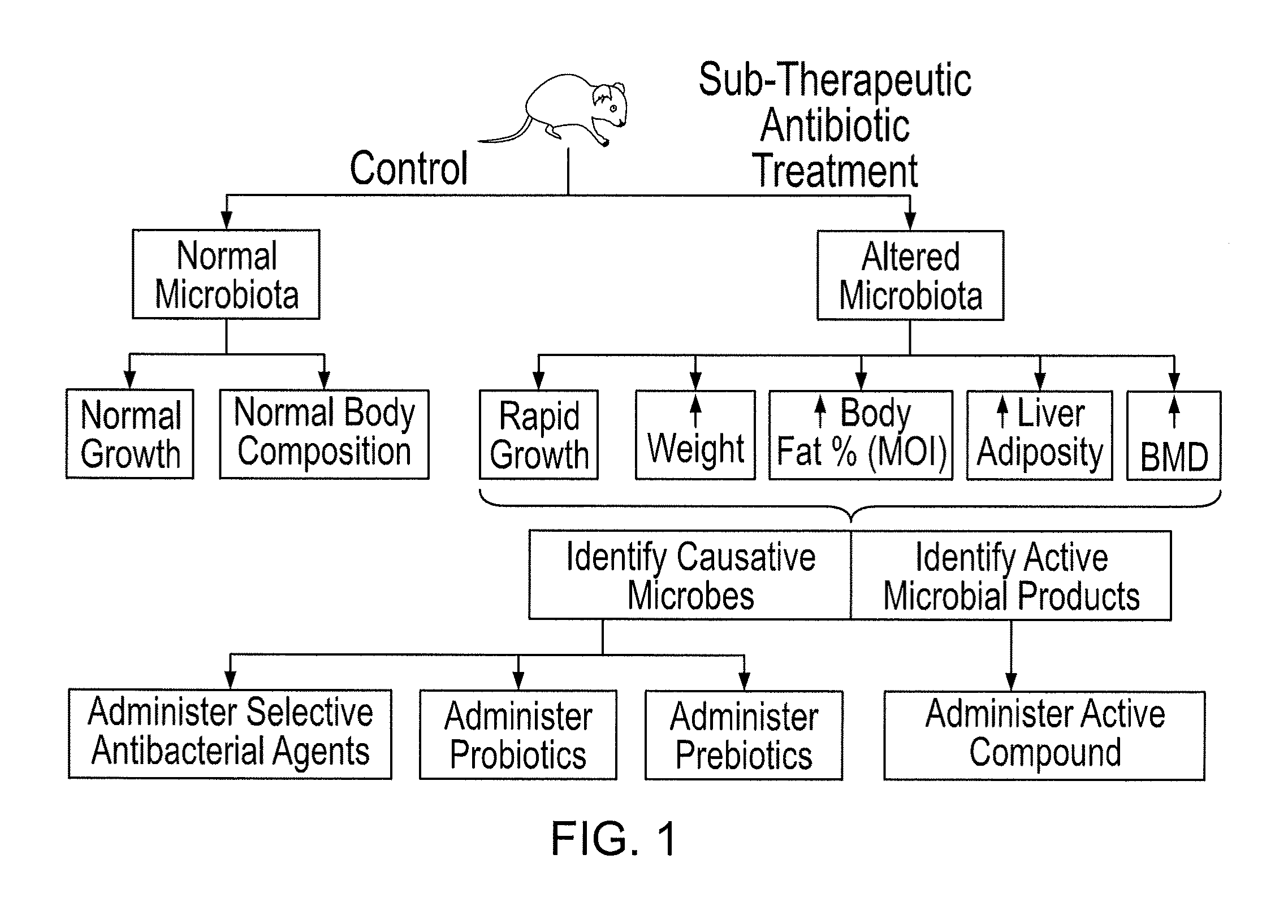
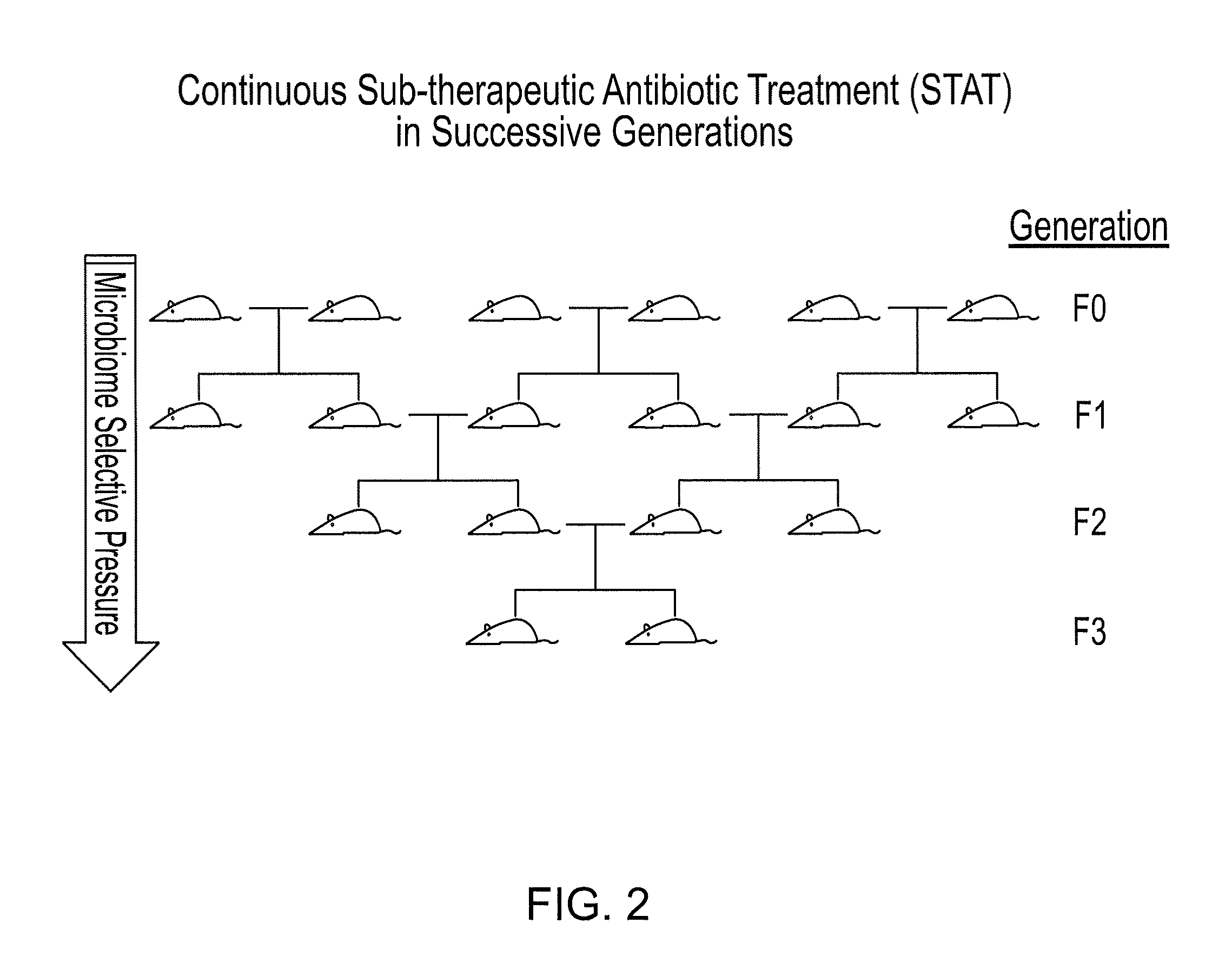
Compositions and methods for treating obesity and related disorders by characterizing and restoring mammalian bacterial microbiota
ActiveUS20110280840A1Increased use of antibioticIncreasing adult height and muscle massBiocideMetabolism disorderIntestinal microorganismsBone formation
The present invention relates to characterizing changes in mammalian gastrointestinal microbiota associated with antibiotic treatment and various disease conditions (such as obesity, metabolic syndrome, insulin-deficiency or insulin-resistance related disorders, glucose intolerance, diabetes, non-alcoholic fatty liver, abnormal lipid metabolism, short stature, osteoporosis, and other disorders of bone formation and mineralization, etc.) and related diagnostic and therapeutic methods. Therapeutic methods of the invention involve the use of probiotics, prebiotics, or narrow spectrum antibiotics / anti-bacterial agents that are capable of restoring healthy mammalian bacterial gastrointestinal microbiota.
Owner:NEW YORK UNIV
Multiple canopy
InactiveUS6537249B2Improve reliabilityReduce manufacturing costSurgeryMedical devicesStored energyMorphine
A fluid delivery device having a self-contained stored energy membrane for expelling fluids at a precisely controlled rate, which is of a compact, laminate construction. The device is of very low profile so that it can conveniently be used for the precise delivery of a small volume of pharmaceutical fluids, such as insulin, morphine and the like, into an ambulatory patient at precisely controlled rates over extended periods of time. The device includes strategically configured, multiple fluid chambers to achieve the maximum possible average percent of extension of the membrane and thereby assure adequate fluid delivery pressure.
Owner:PESCADERO BEACH HLDG
Method for producing powder formulation comprising an insulin
The present invention relates to a process for producing a therapeutic powder formulation, comprising (a) providing an acidic aqueous solution comprising an insulin or analoguc or derivative thereof and an enhancer; (b) adjusting the pH to a pH in the range of 4.5 to 7.4; (c) precipitating a product comprising the insulin or analogue or derivative thereof and the enhancer, wherein the precipitation is performed essentially without evaporation of the solution; and (d) removing the water.
Owner:NOVO NORDISK AS
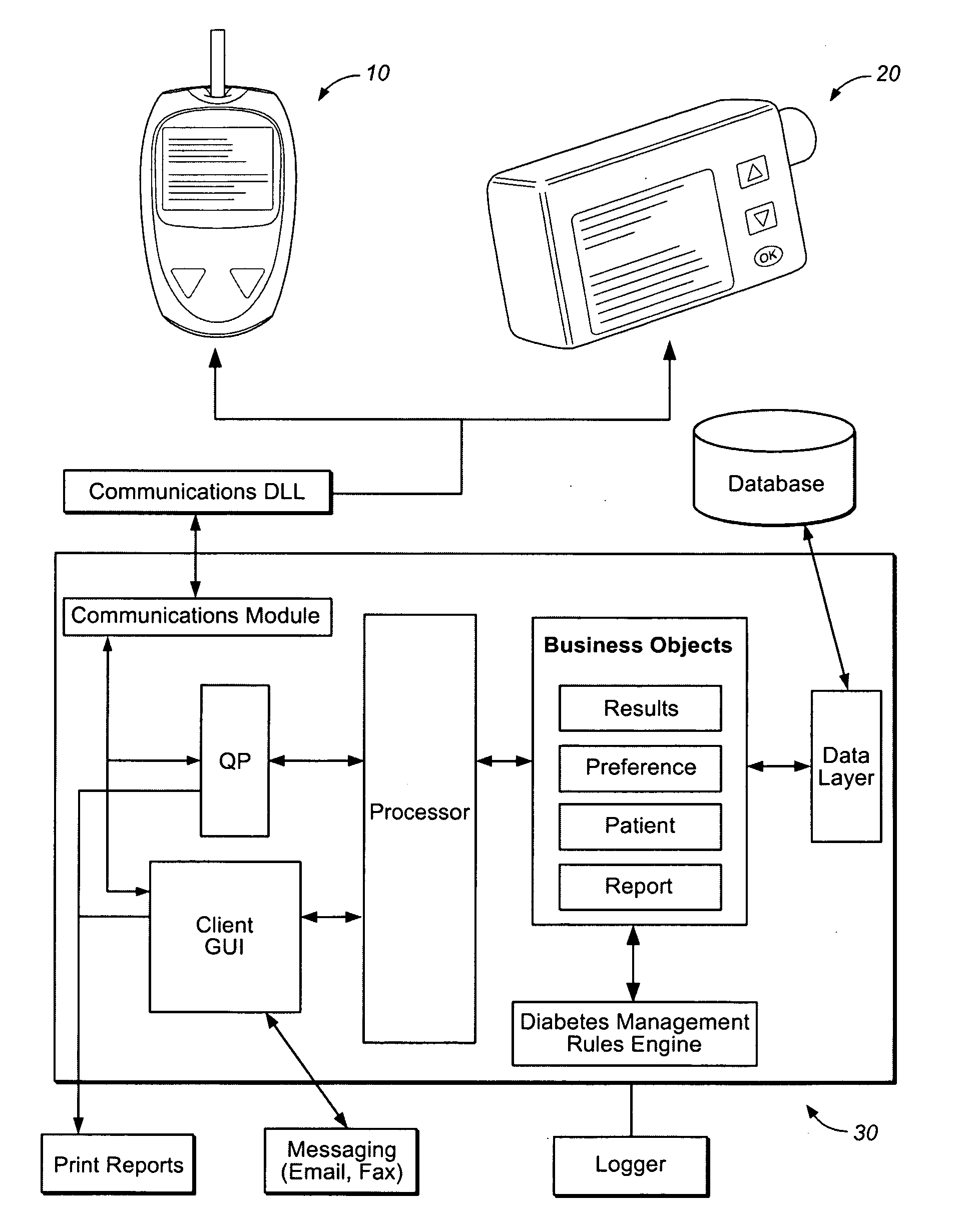
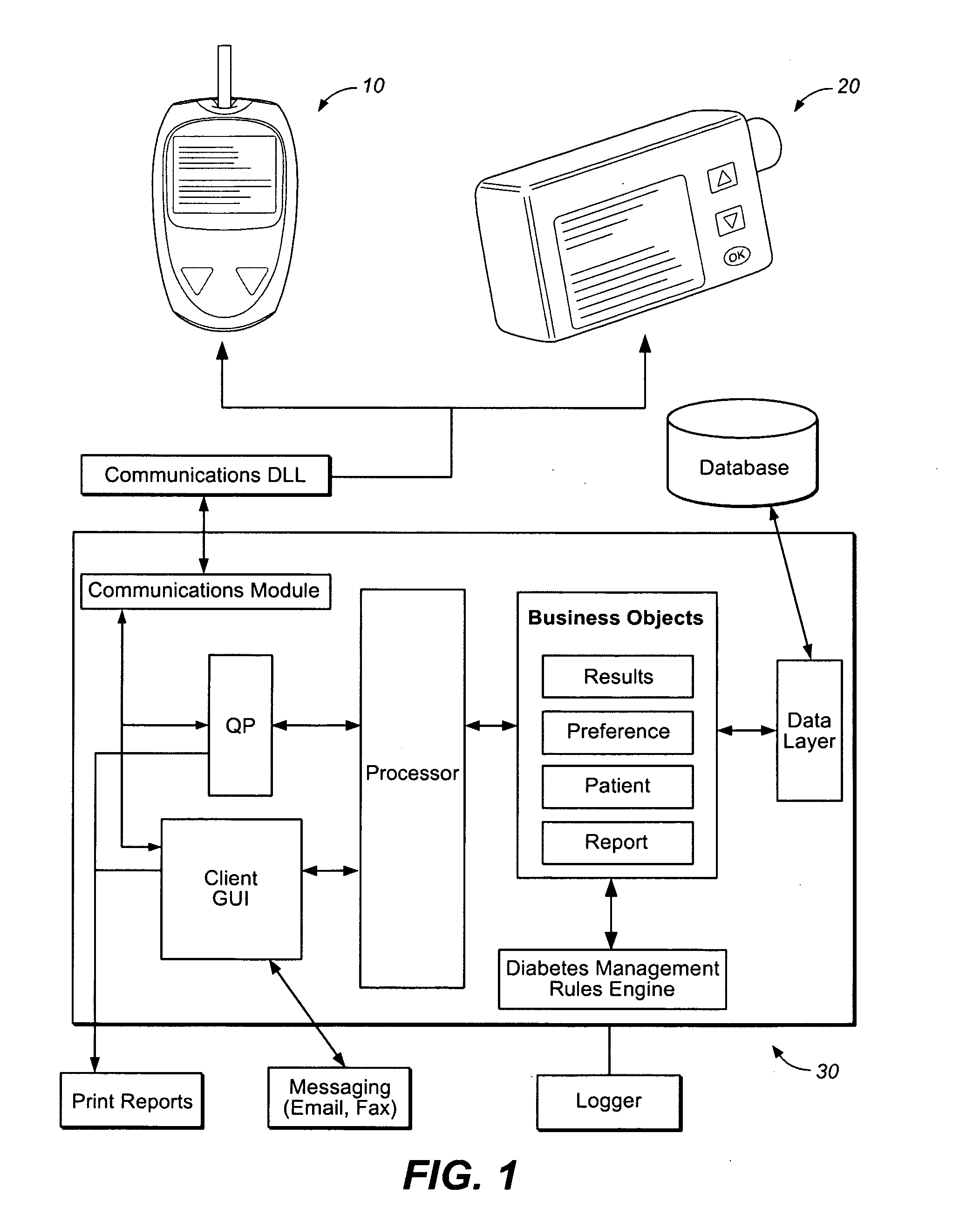
Diabetes therapy management system for recommending adjustments to an insulin infusion device
A method of managing use of an insulin infusion device are provided. The method receives glucose data for a user of the infusion device, wherein the glucose data indicates blood glucose levels of the user for a period of time during which the insulin infusion device is regulating delivery of insulin to the user. The received glucose data is received to detect certain event occurrences, which may be indicative of a correctable basal rate setting of the insulin infusion device and / or indicative of potential maladjustment of a user-specific setting of a bolus calculator setting of the insulin infusion device. The method outputs a recommendation to adjust the basal rate setting and / or the bolus calculator setting as needed to address the detected event occurrences.
Owner:MEDTRONIC MIMIMED INC
Integrated glucose monitor and insulin injection pen with automatic emergency notification
InactiveUS20120046606A1Known techniqueInfusion syringesMedical devicesInsulin injectionDisplay device
A portable insulin injection pen and blood glucose monitoring device is integrated into a single unit for testing and treating diabetes symptoms. The device has a housing of a size suitable for transport in a user's clothing pocket or handbag. Within the housing is a blood glucose monitoring system for receiving a sample of the user's blood and detecting its glucose level, an insulin injection mechanism for administering an insulin injection, and a microprocessor that calculates an insulin dosage appropriate to the detected blood glucose level and sets the insulin injection mechanism to administer the calculated insulin dosage. A communication device automatically informs a remote emergency service provider, such as 911 or an emergency service to which the user has subscribed, if the microprocessor determines that the detected blood glucose level presents a potential danger to the user. The microprocessor also calculates treatment regimens specific to a particular user based on the detected blood glucose level and displays the treatment regimens on an LCD display. In a particularly advantageous embodiment, a GPS receiver within the housing detects the location of the device, and the communication device, which can be a cellular telephone separate from the housing connected wirelessly to the unit via a Bluetooth connection or cellular telephone circuitry within the housing itself, transmits information regarding the location to the remote emergency service.
Owner:THUBAN
Superior control of blood glucose in diabetes treatment
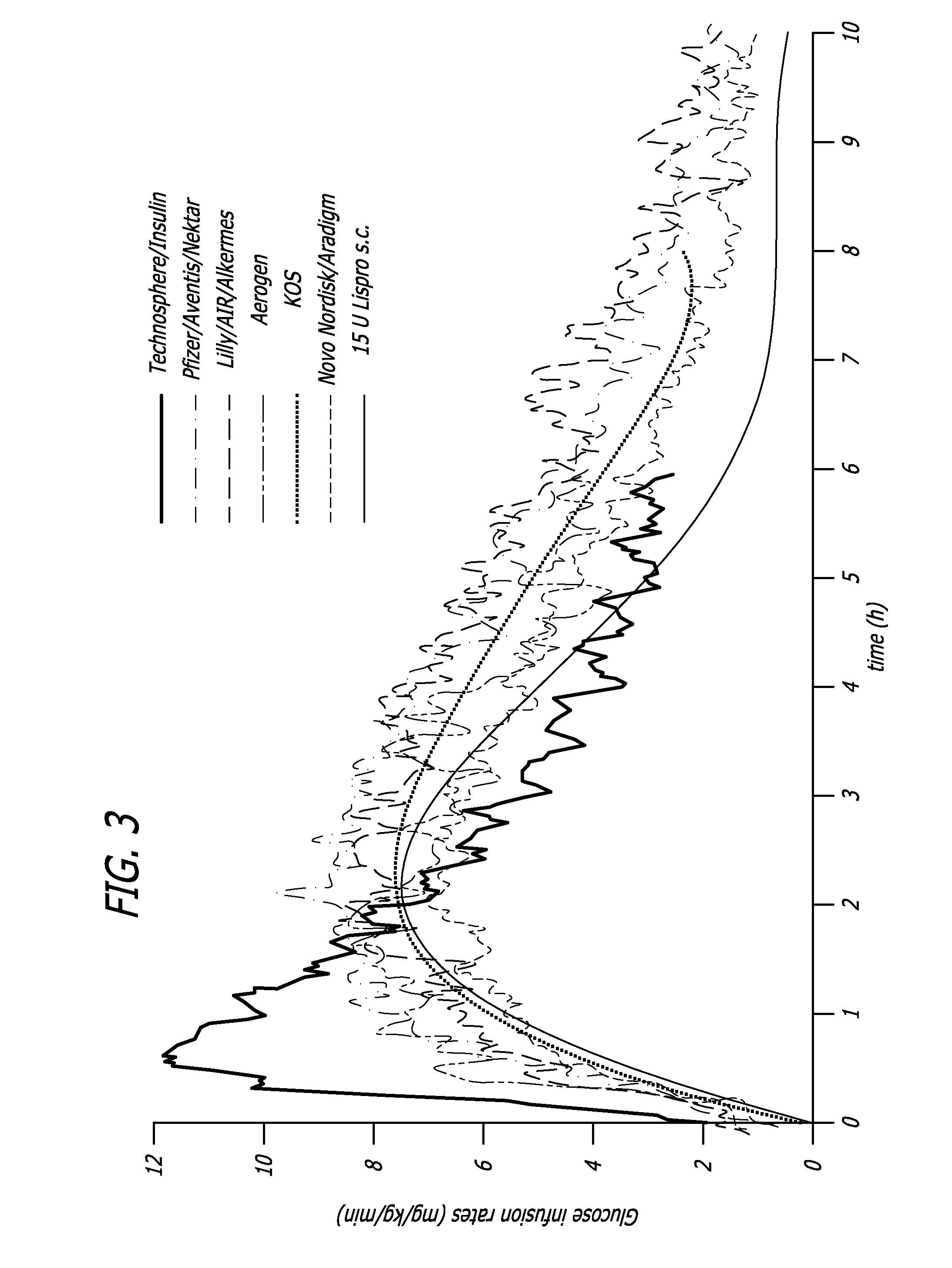
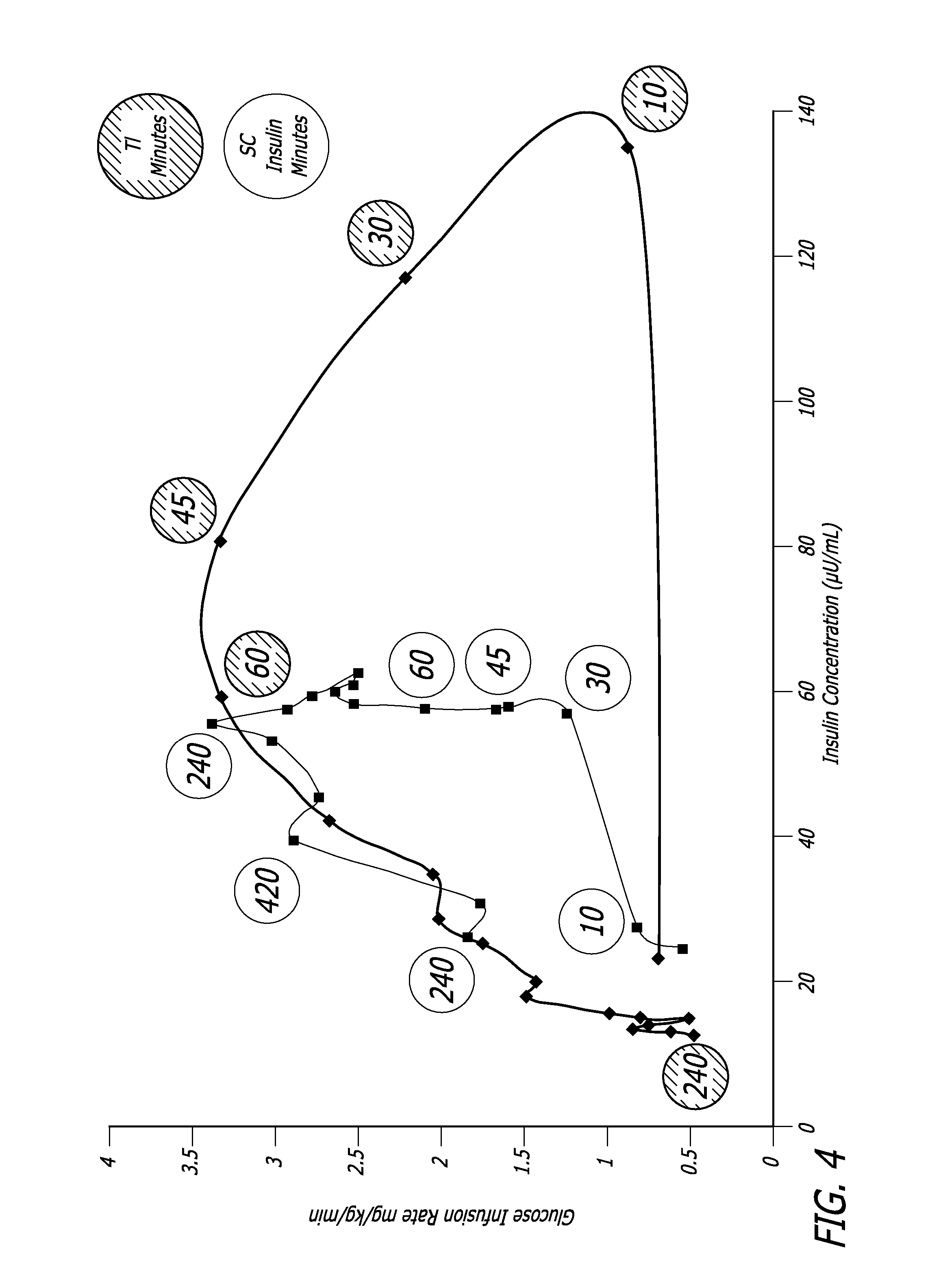
InactiveUS20060239934A1Easy to controlReduce riskOrganic active ingredientsPowder deliveryPostprandial HypoglycemiaGlucose fluctuations
Methods related to the treatment of diabetes and improving the control of blood glucose levels are provided. In particular, methods are provided for effectively reducing postprandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration. Additionally, methods for effectively reducing post-prandial glucose excursions while reducing the incidence of clinically significant late postprandial hypoglycemia by administered an insulin composition in a form suitable for pulmonary administration along with a long-acting basal insulin.
Owner:MANNKIND CORP
Pulmonary insulin crystals
The present invention relates to zinc free insulin crystals having a diameter below 10 mum and to therapeutic powder formulations suitable for pulmonary administration containing such insulin crystals. The crystals of the present invention exhibit a better stability profile than powders of essentially the same composition prepared by spray drying, freeze-drying, vacuum drying and open drying. The therapeutic powder formulations elucidate better flowing properties than corresponding amorphous powder formulations.
Owner:NOVO NORDISK AS
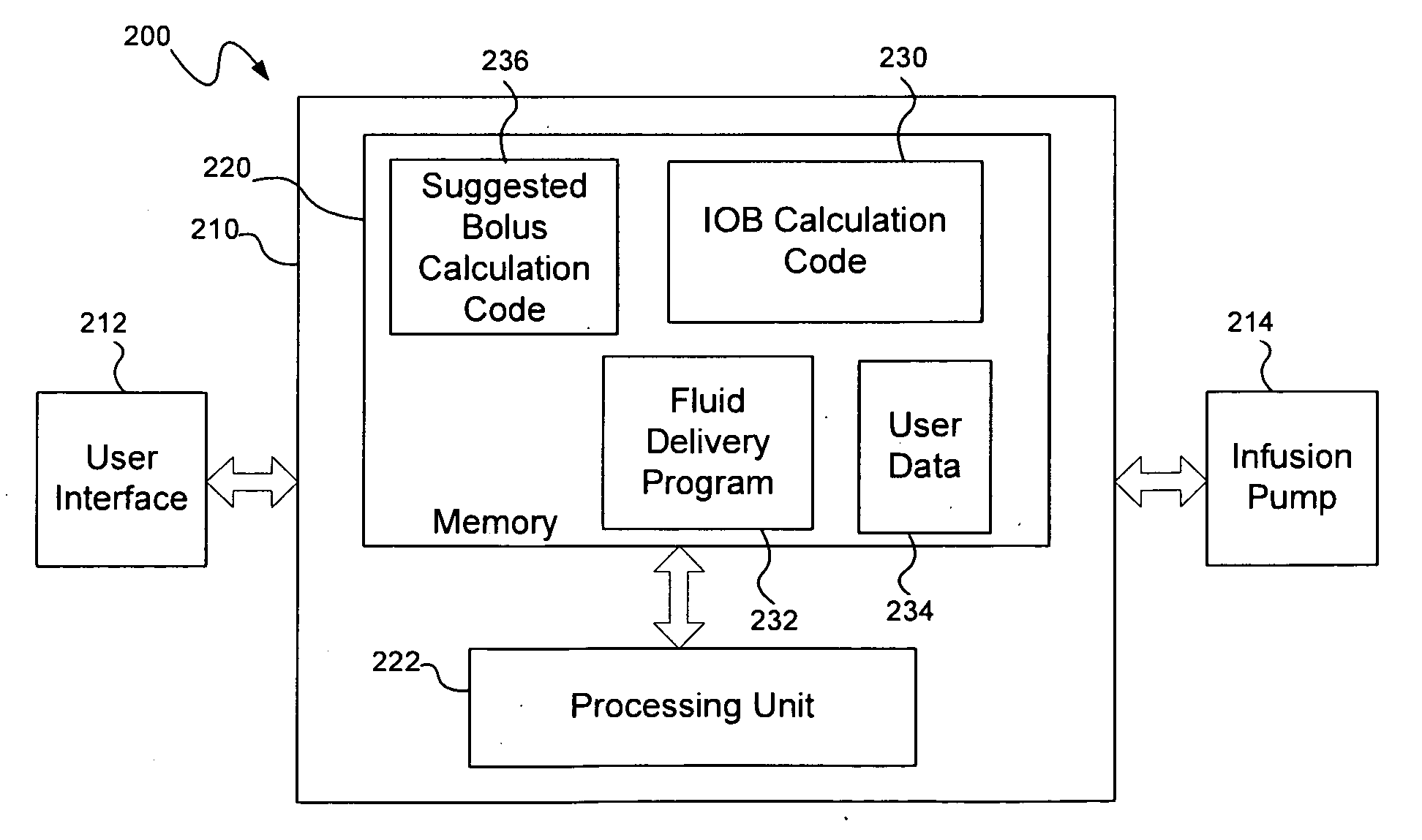
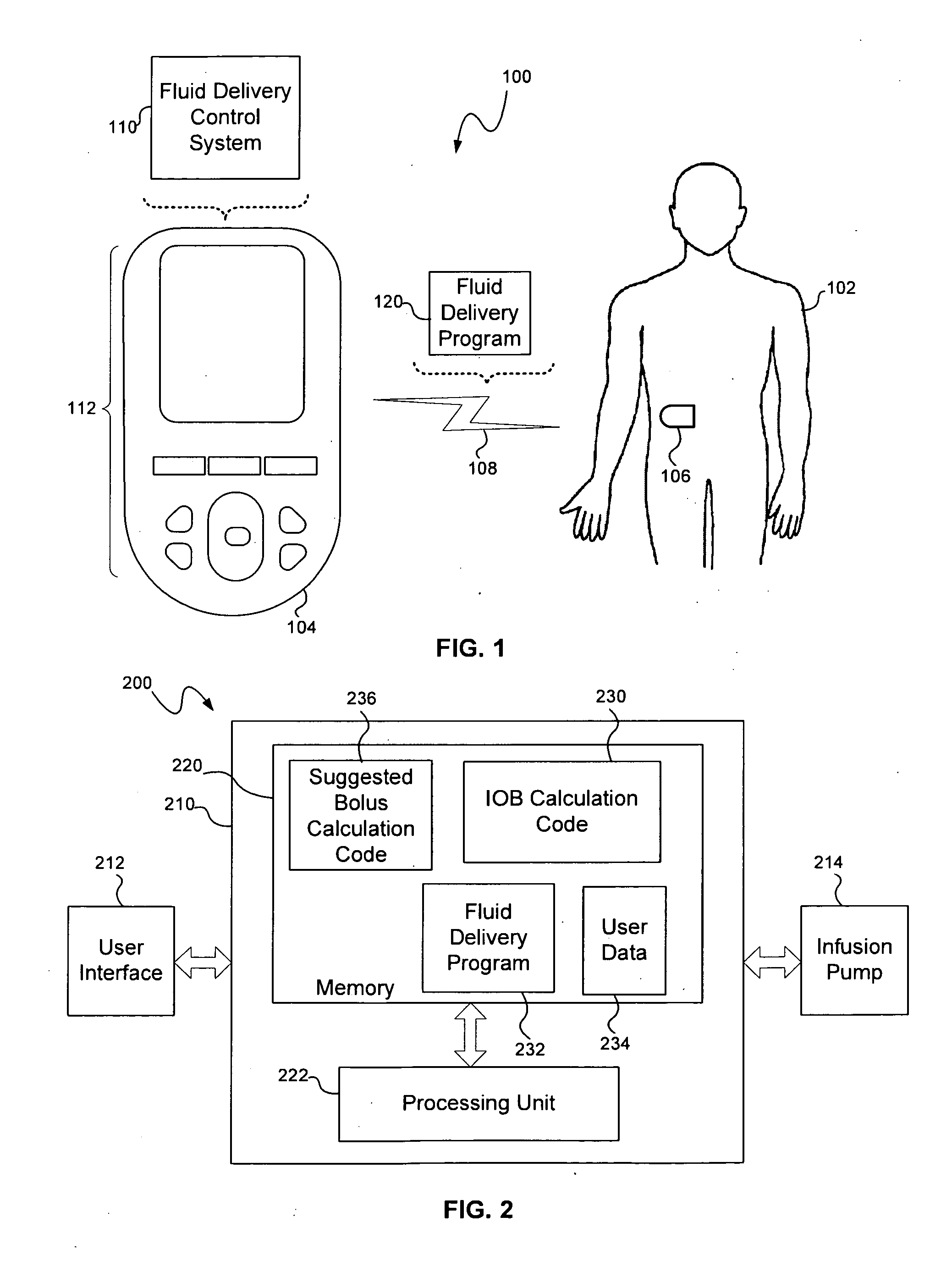
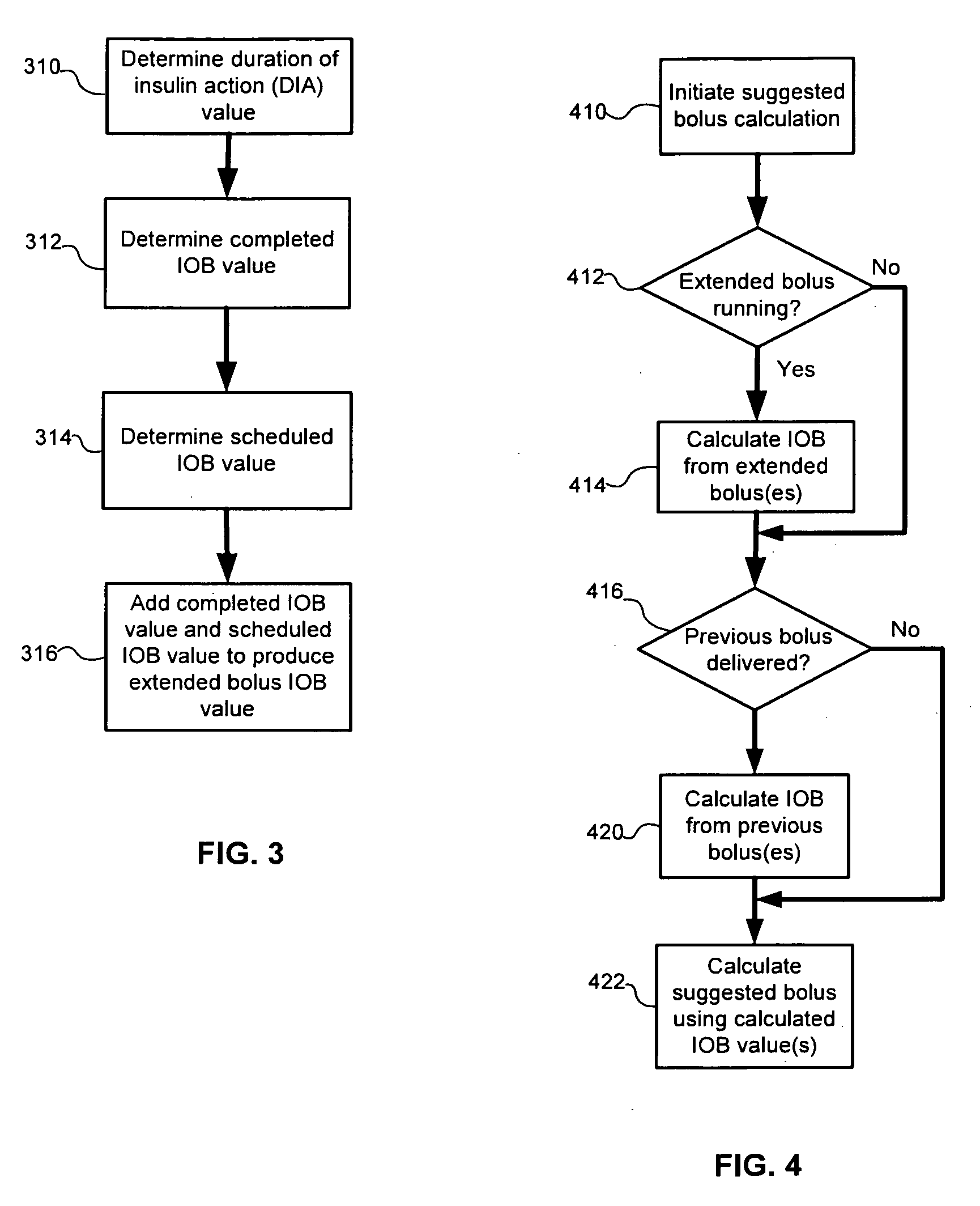
Calculating insulin on board for extended bolus being delivered by an insulin delivery device
A system and method may be used to calculate insulin on board (IOB) for an extended bolus being delivered by an insulin infusion pump. In general, the system and method calculates an extended bolus IOB value for the extended bolus, which takes into account the insulin currently on board from the extended bolus and the insulin scheduled to be delivered by the extended bolus over a subsequent time period equivalent to a duration of insulin action. The extended bolus IOB value may be used to calculate a suggested bolus.
Owner:INSULET CORP
System and method for mitigating risk in automated medicament dosing
ActiveUS20150073337A1Reduce riskPrevent fallingMedical data miningDrug and medicationsContinuous feedbackCvd risk
A portable infusion pump can communicate with glucose monitor, such as a continuous glucose monitor (CGM), to receive continuous feedback relating to a user's blood glucose level during insulin or other medicament therapy and can automatically deliver insulin to a user when the CGM data indicates a need for additional insulin. Due to potential unreliability in the correlation of the CGM data to the user's actual blood glucose level, risk mitigation can be employed to limit the amount of extra insulin that can be delivered by the pump in response to the CGM data.
Owner:TANDEM DIABETES CARE INC
Composition and method for treating impaired or deteriorating neurological function
InactiveUS6964969B2Impair actionHigh affinityBiocideHydrocarbon active ingredientsPhosphateAntioxidant
A nutritional supplement composition for normalizing impaired or deteriorating neurological function in humans is composed of: at least one agent which promotes synthesis of ATP and / or creatine phosphate in the body, at least one antioxidant for scavenging free radicals in at least one pathway in the body; at least one agent for normalizing or maintaining membrane function and structure in the body; at least one agent for normalizing or maintaining normal neurotransmitter function in the body; at least one agent for down-regulating cortisol action; and at least one agent for suppressing activation of apoptotic pathways in the body. The composition may further contain one or more of: at least one agent for suppressing inflammation in the body; at least one agent for normalizing or maintaining vascular wall function and structure in the body; at least one agent for normalizing or maintaining function of nerve growth factors and / or neurotropic factors in the body; at least one agent for suppressing toxic metal ionic effects; at least one agent for normalizing or maintaining methyl metabolism in the body; at least one agent for normalizing or maintaining metabolism of insulin and glucose in the body; and at least one agent for up-regulating activity of heat shock proteins in the body. A method for normalizing impaired neurological function in humans modulating nutrient partitioning in a human involves administering the aforementioned composition to the human, preferably on a daily basis, for a therapeutically effective period of time. Preferably, the method further involves having the human follow a stress reduction program, and / or a cognitive retraining program, and / or a dietary program designed to maximize insulin and glucose metabolism.
Owner:MCCLEARY EDWARD LARRY
Insoluble compositions for controlling blood glucose
InactiveUS6531448B1Easy to controlReduce rateBiocidePeptide/protein ingredientsMedicineDivalent metal
The present invention relates to insoluble compositions comprising a protein selected from the group consisting of insulin, insulin analogs, and proinsulins; a derivatized protein selected from the group consisting of derivatized insulin, derivatized insulin analog, and derivatized proinsulin; a complexing compound; a hexamer-stabilizing compound; and a divalent metal cation. Formulations of the insoluble composition are suitable for both parenteral and non-parenteral delivery for treating hyperglycemia and diabetes. Microcrystal forms of the insoluble precipitate are pharmaceutically analogous to the neutral protamine Hagedorn (NPH) insulin crystal form. Surprisingly, it has been discovered that suspension formulations of such insoluble compositions possess unique and controllable dissolution properties that provide therapeutically advantageous glucodynamics compared with insulin NPH formulations.
Owner:ELI LILLY & CO
Clinical variable determination
A computer implemented method of determining a clinical variables utilizing an insulin pump that includes initiating blood glucose measurements, initiating ingestion of carbohydrates and receiving input data based on the blood glucose measurements and the ingestion of carbohydrates and utilizing the data to calculate clinical variables. The invention may include presenting instructions to a patient to take various actions and to input various data. The clinical variables determined may be stored in memory and then used to calculate insulin doses and to send a signal to an insulin pump to infuse the insulin dose calculated.
Owner:TANDEM DIABETES CARE INC
Safeguarding techniques for a closed-loop insulin infusion system
Processor-implemented methods of controlling an insulin infusion device for a user are provided here. A first method obtains and analyzes calibration factors (and corresponding timestamp data) for a continuous glucose sensor, and regulates entry into a closed-loop operating mode of the infusion device based on the calibration factors and timestamp data. A second method obtains a most recent sensor glucose value and a target glucose setpoint value for the user at the outset of the closed-loop mode. The second method adjusts the closed-loop insulin infusion rate over time, in response to the sensor glucose value and the setpoint value. A third method calculates an upper insulin limit that applies to the insulin infusion rate during the closed-loop mode. The insulin limit is calculated based on a fasting blood glucose value of the user, a total daily insulin value of the user, and fasting insulin delivery data for the user.
Owner:MEDTRONIC MIMIMED INC
Method and system to handle manual boluses or meal events for closed-loop controllers
ActiveUS20140005633A1Negates portionReduce the impactDrug and medicationsMedical devicesGlucose sensorsDiabetes management
Described and illustrated is a diabetes management system that includes an infusion pump, glucose sensor and controller with a method programmed into the controller. The infusion pump is configured to deliver insulin to a subject. The glucose sensor is configured to sense glucose levels in the subject and provide output signals representative of the glucose levels in the subject. The controller is programmed to receive signals from at least one of the glucose sensor and the pump and configured to issue signals to the pump to deliver an amount of insulin determined by a feedback controller that utilizes a model predictive control and also configured to deliver at least the basal amount of insulin whenever the subject has initiated a manual bolus of insulin and a sensed or measured glucose level is at least a first threshold within a first duration of time.
Owner:JDRF INT +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com