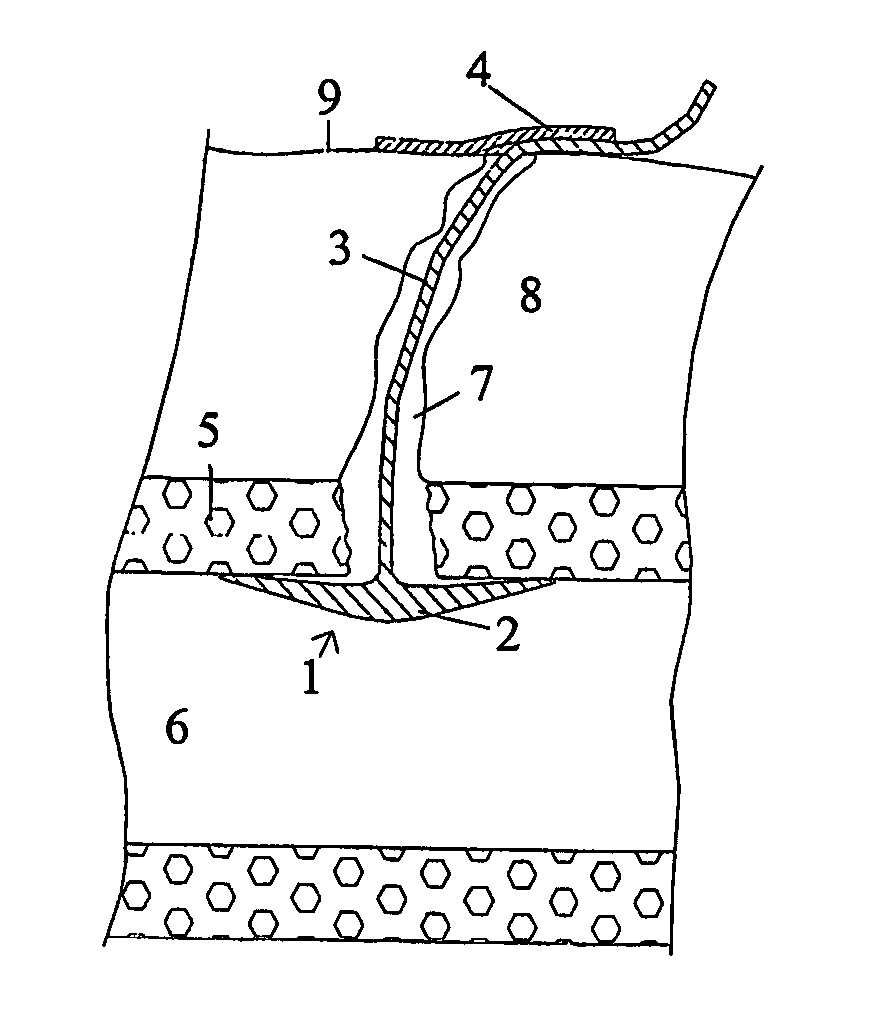
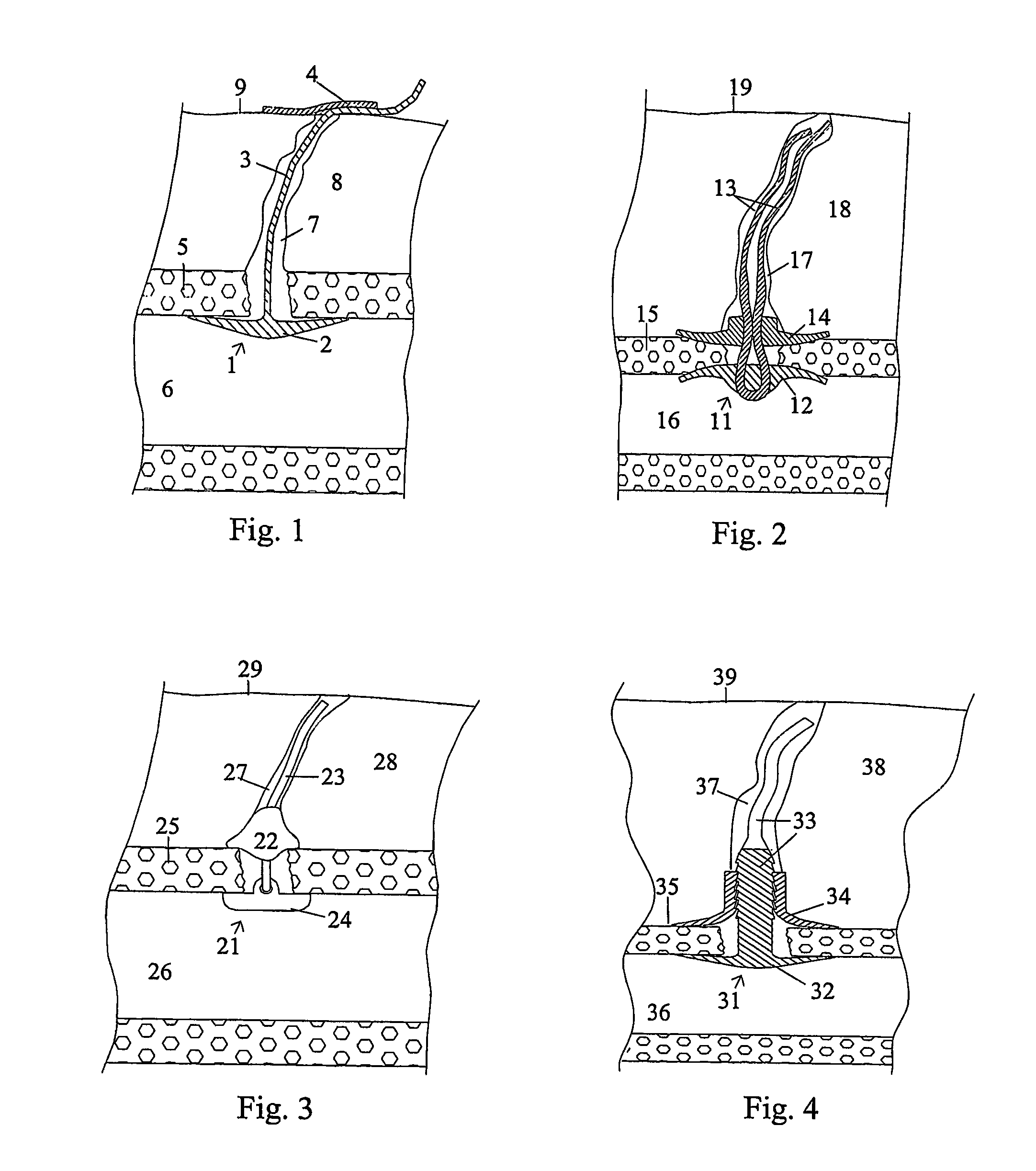
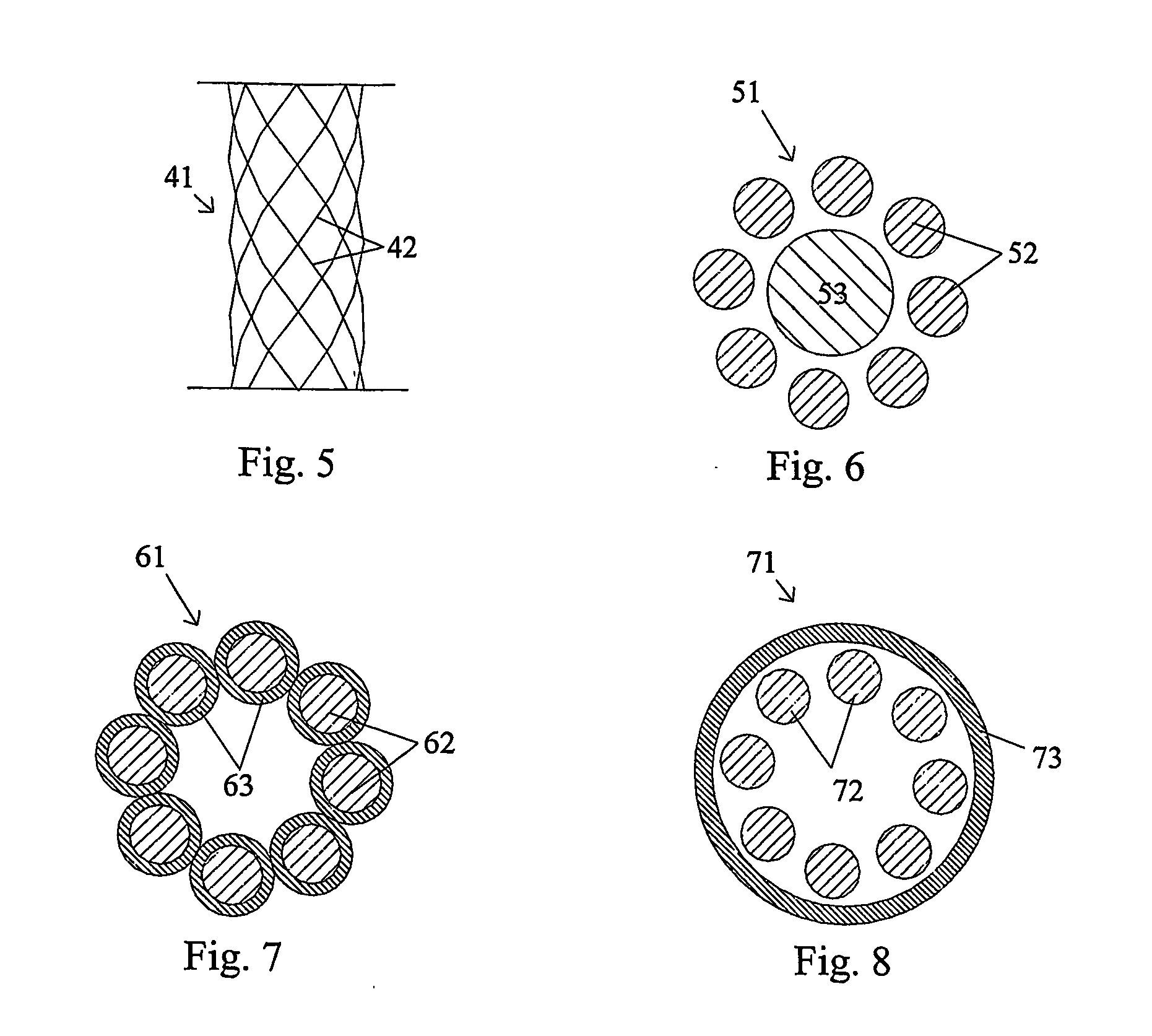
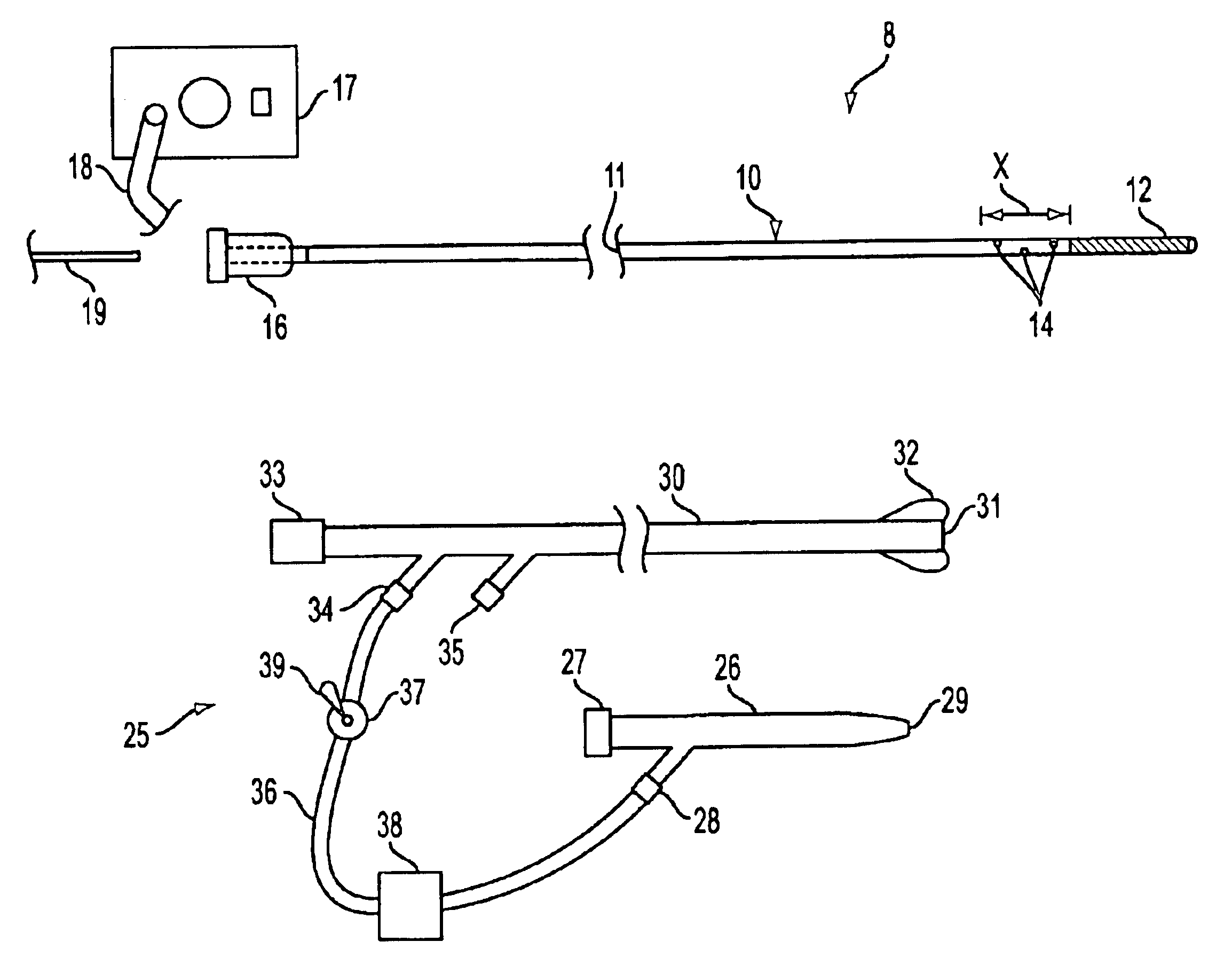
Patents
Literature
1225 results about "Vascular wall" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
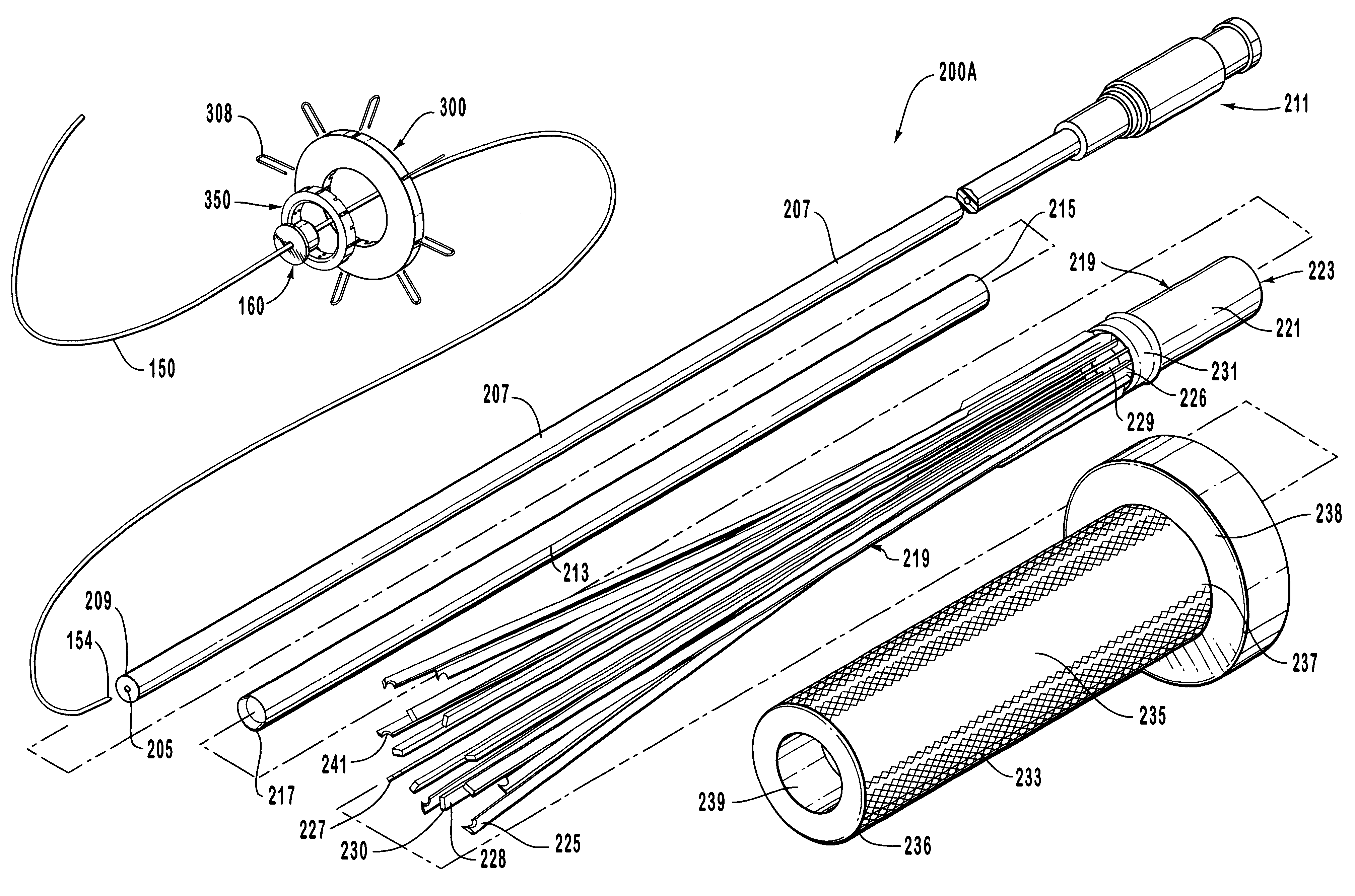
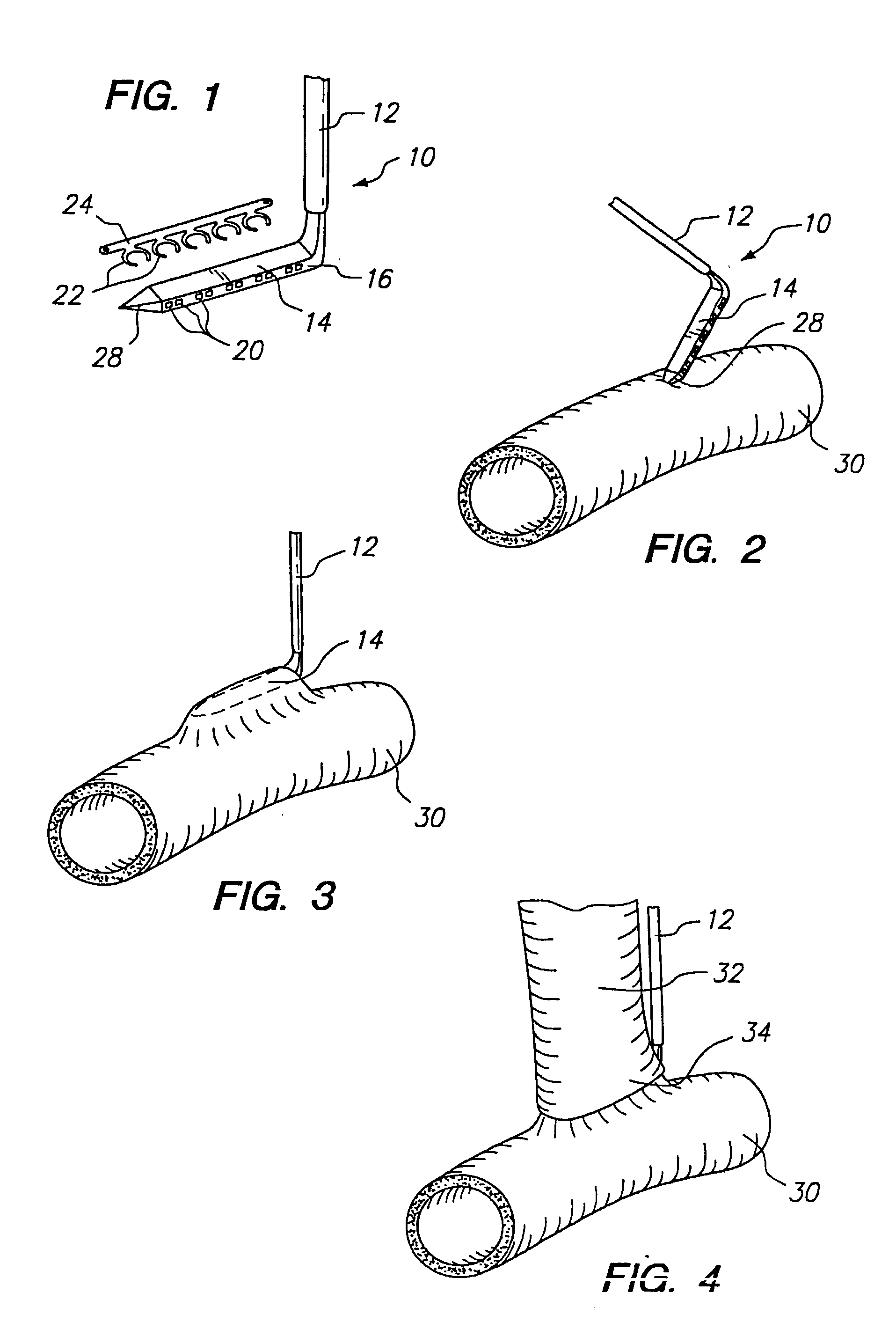
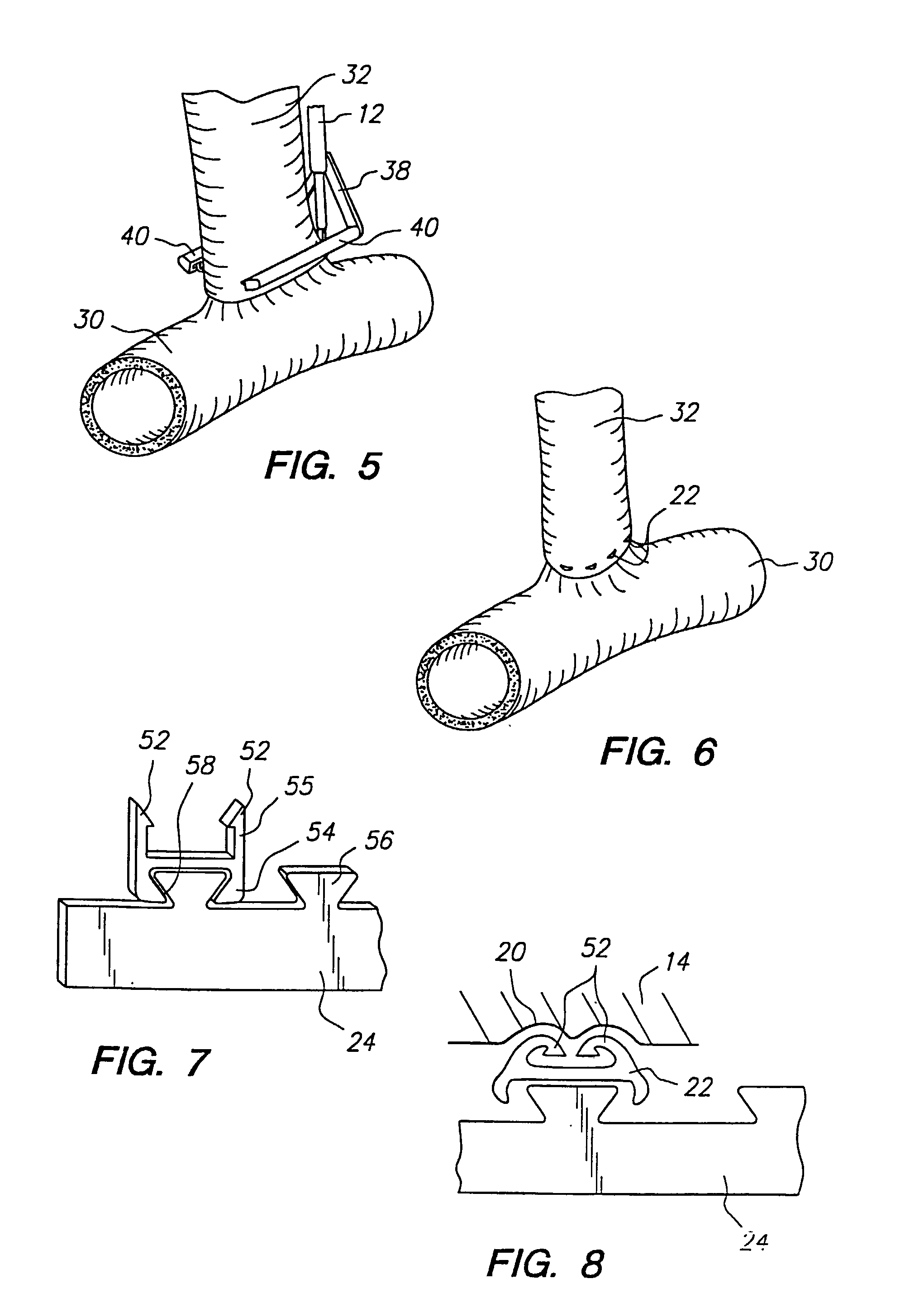
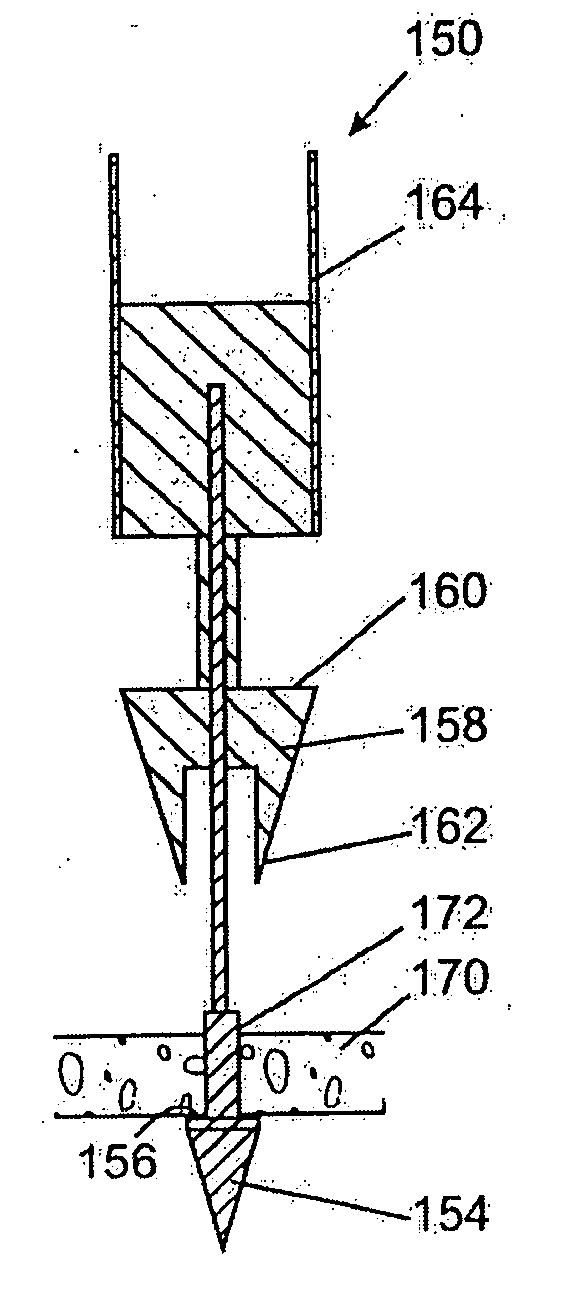
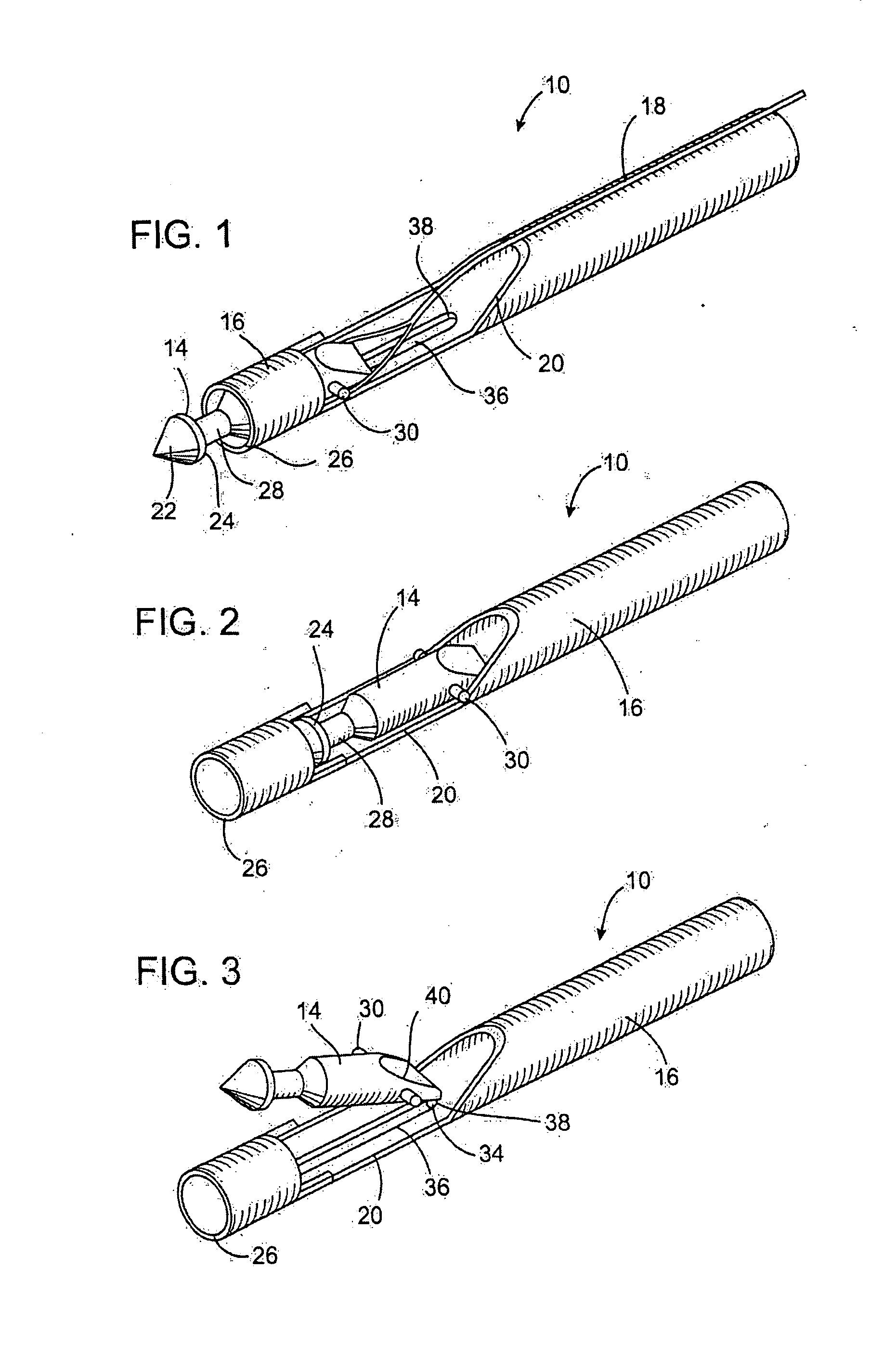
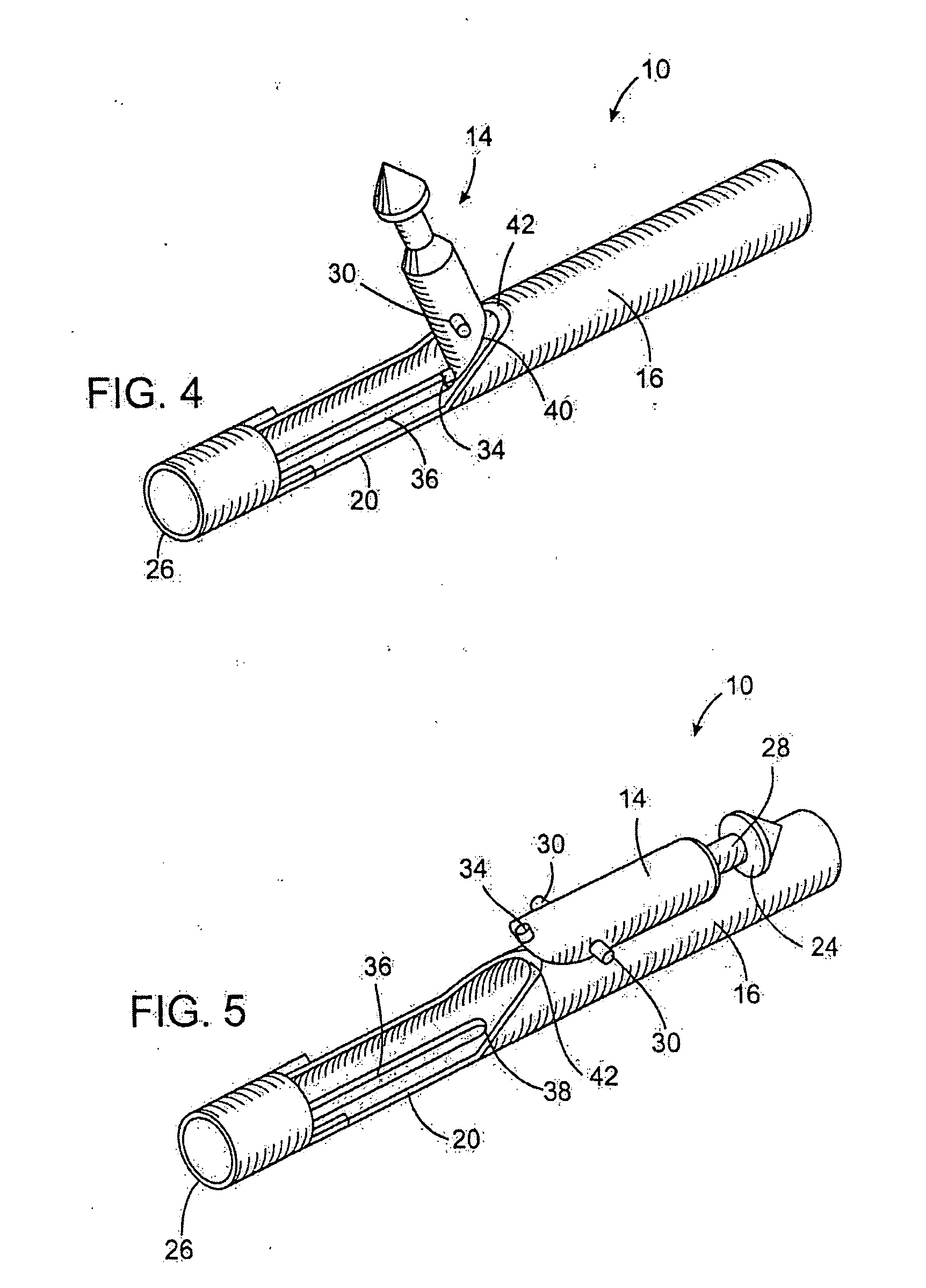
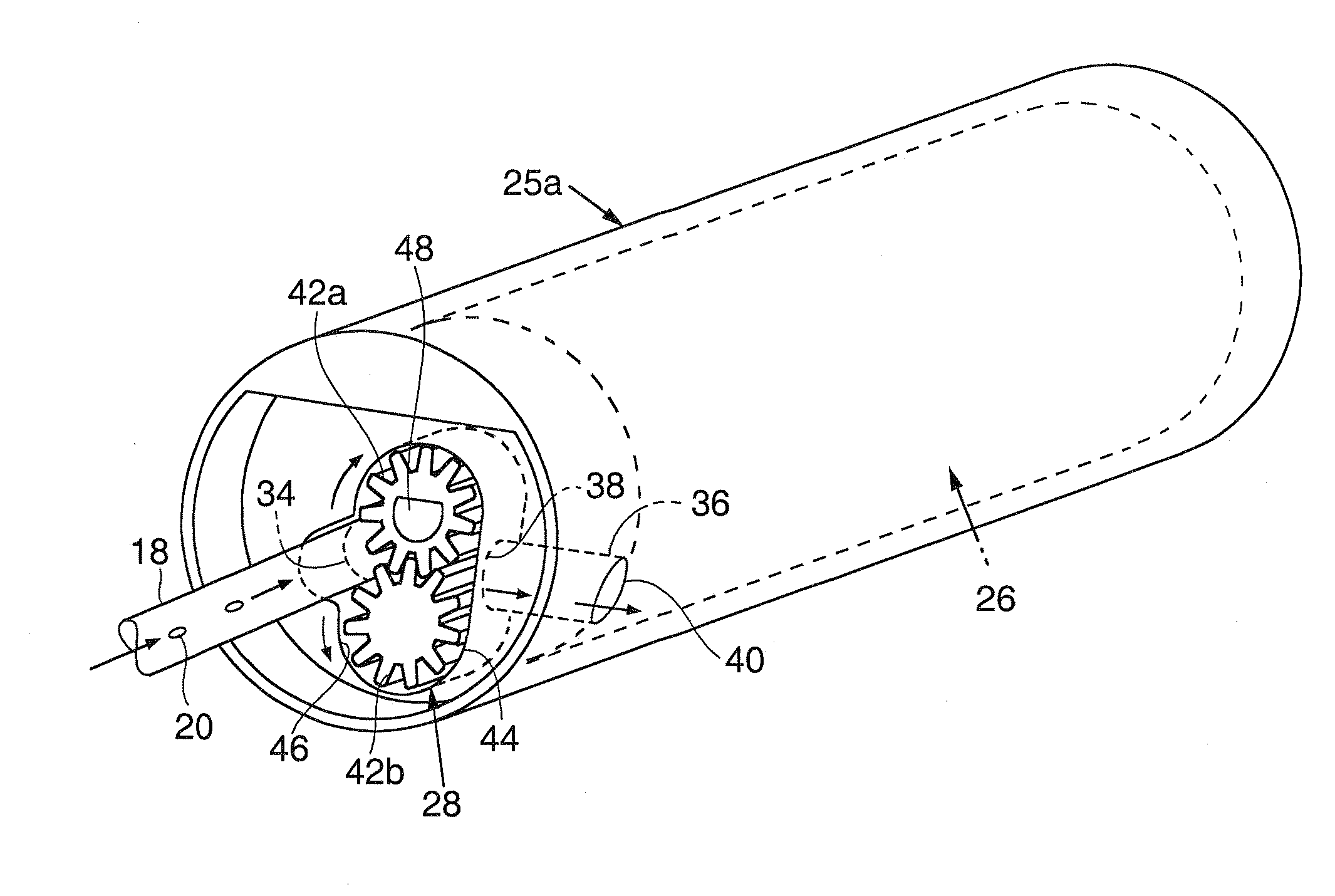
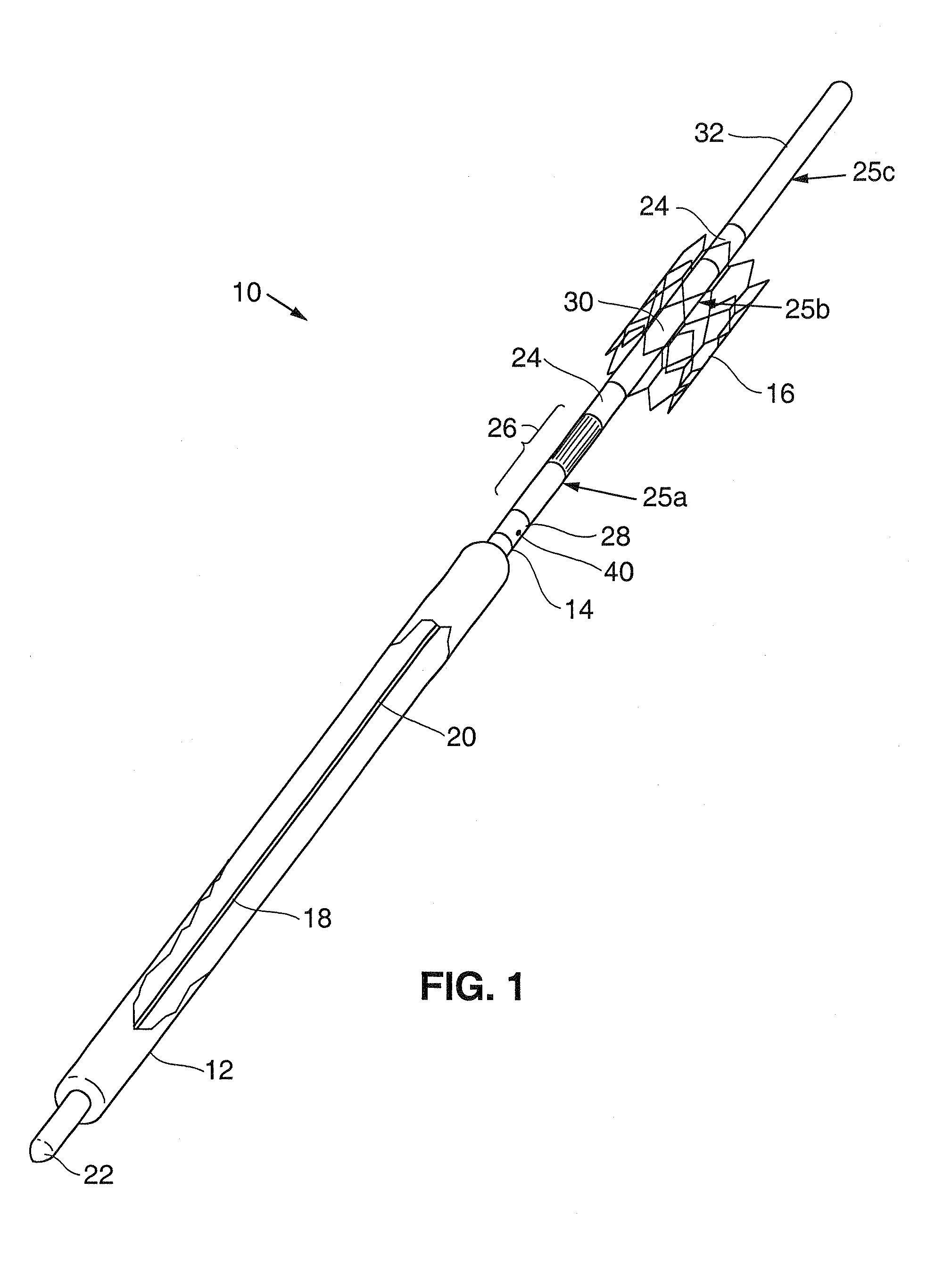
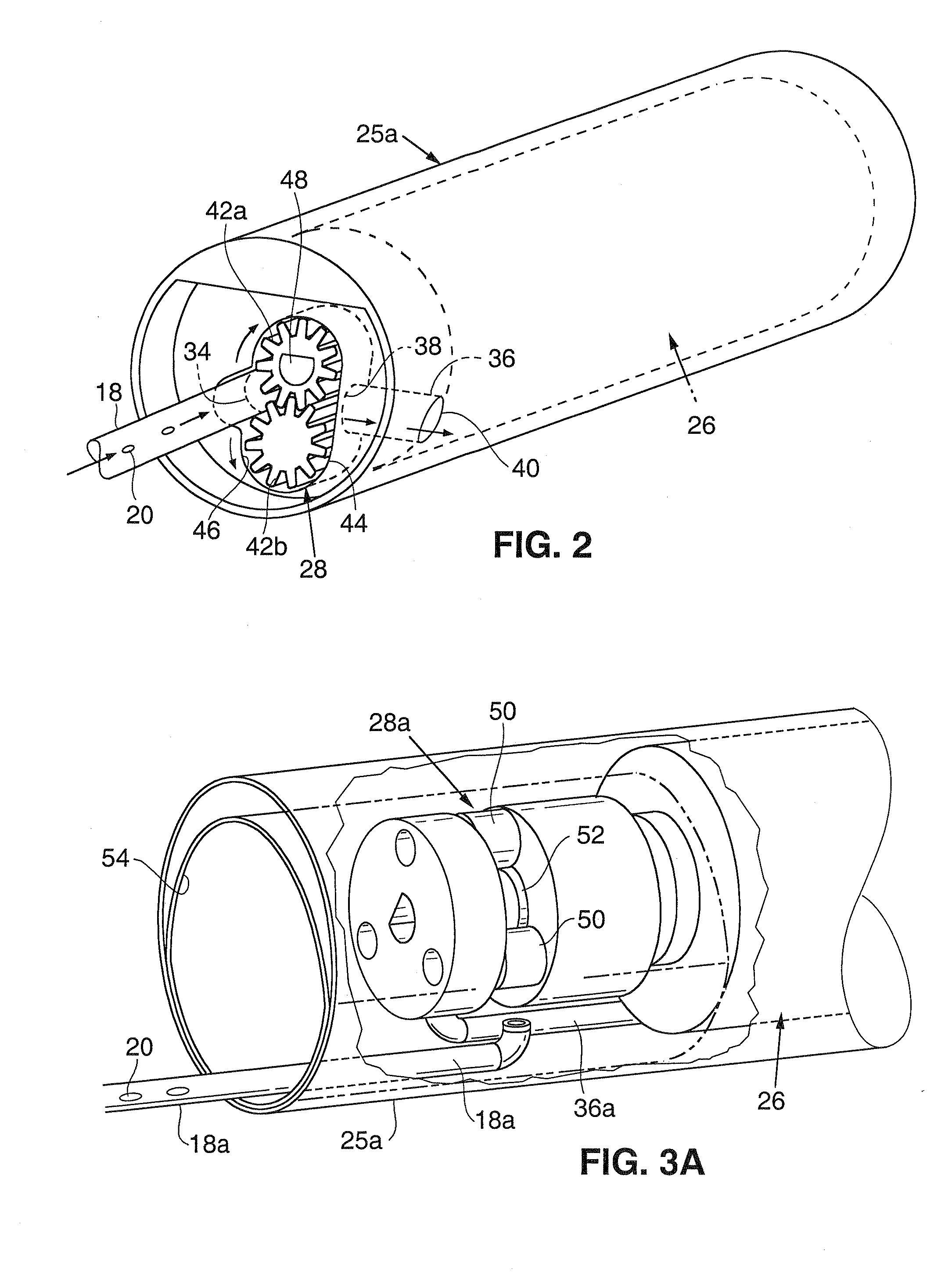
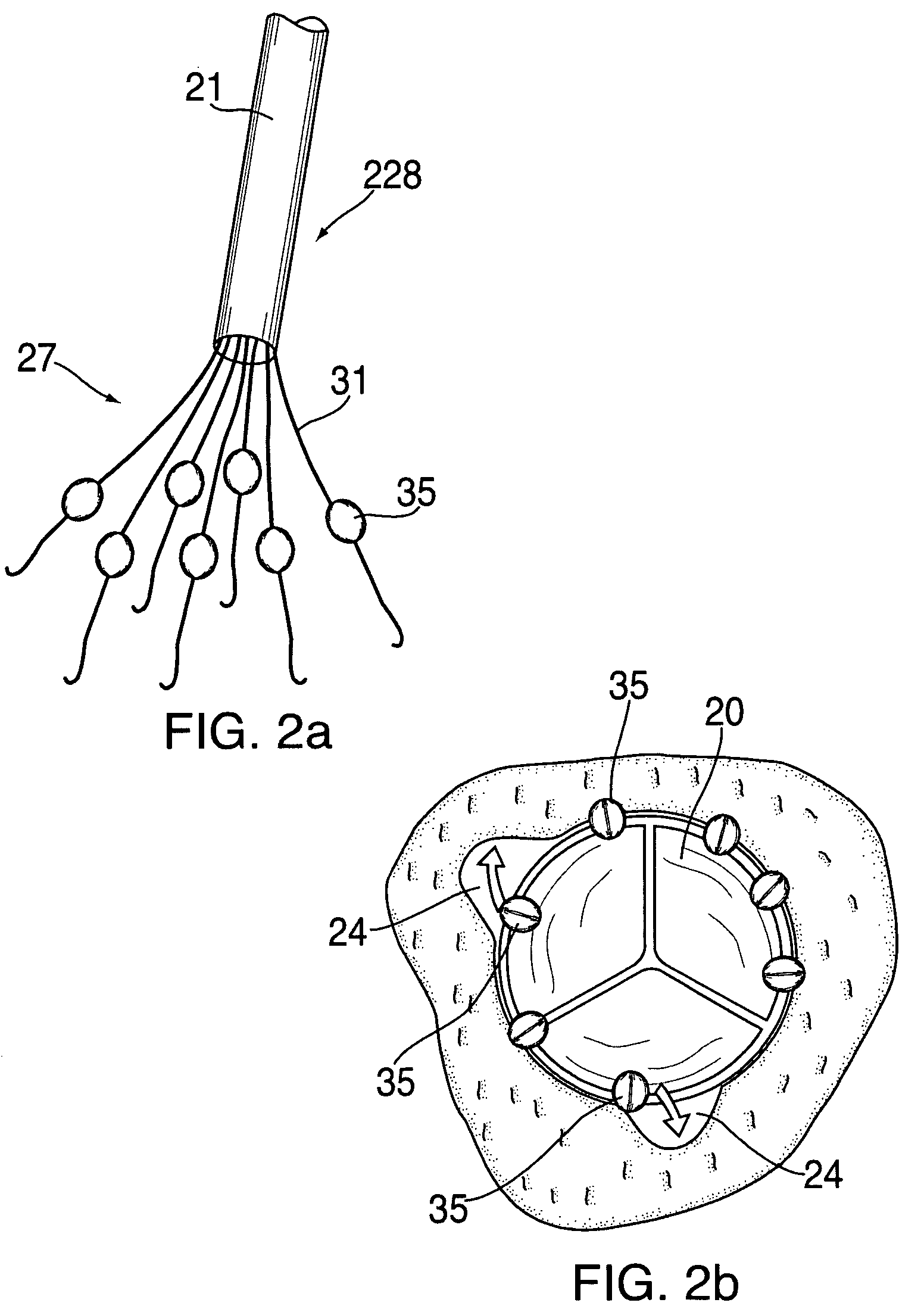
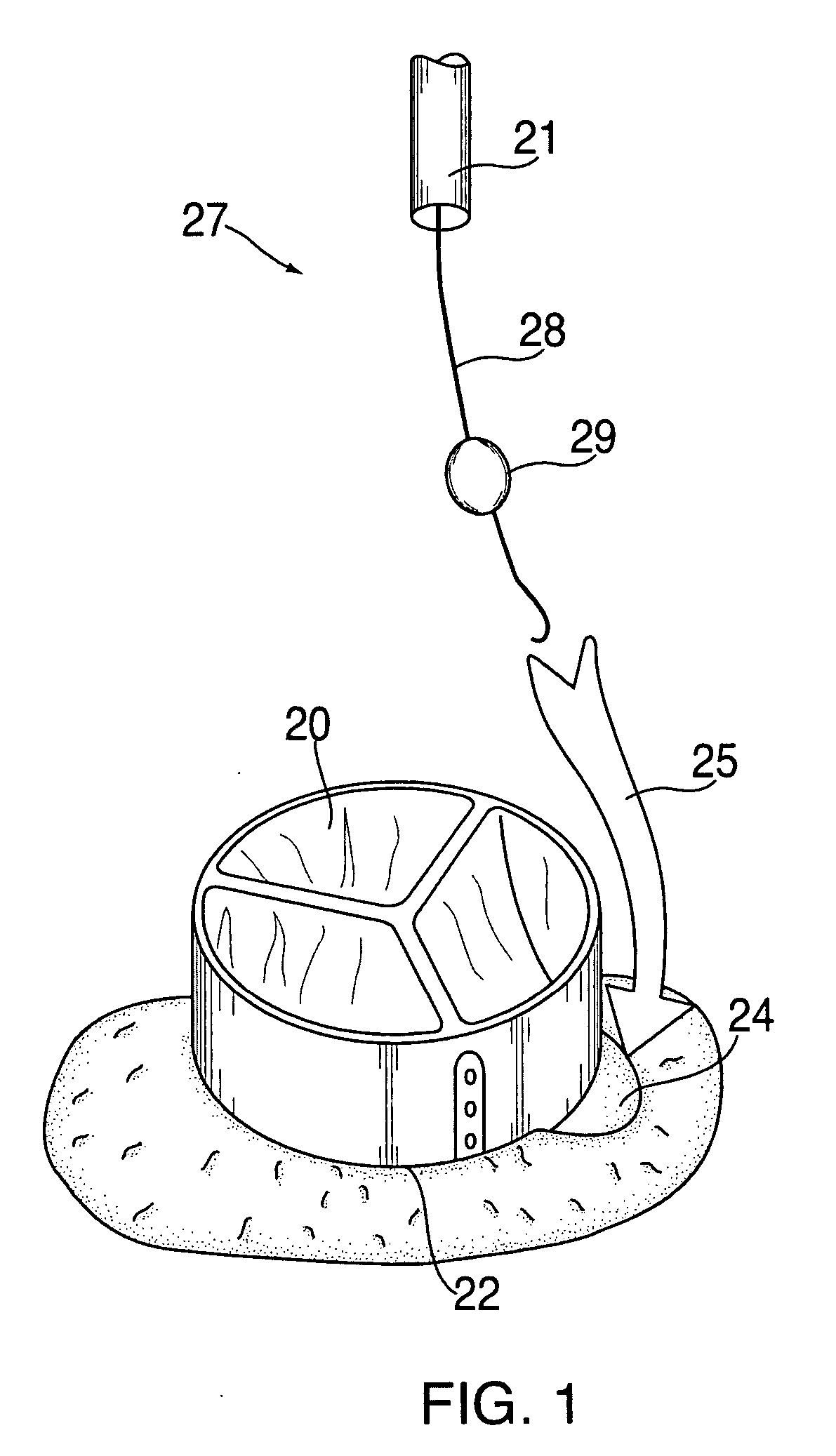
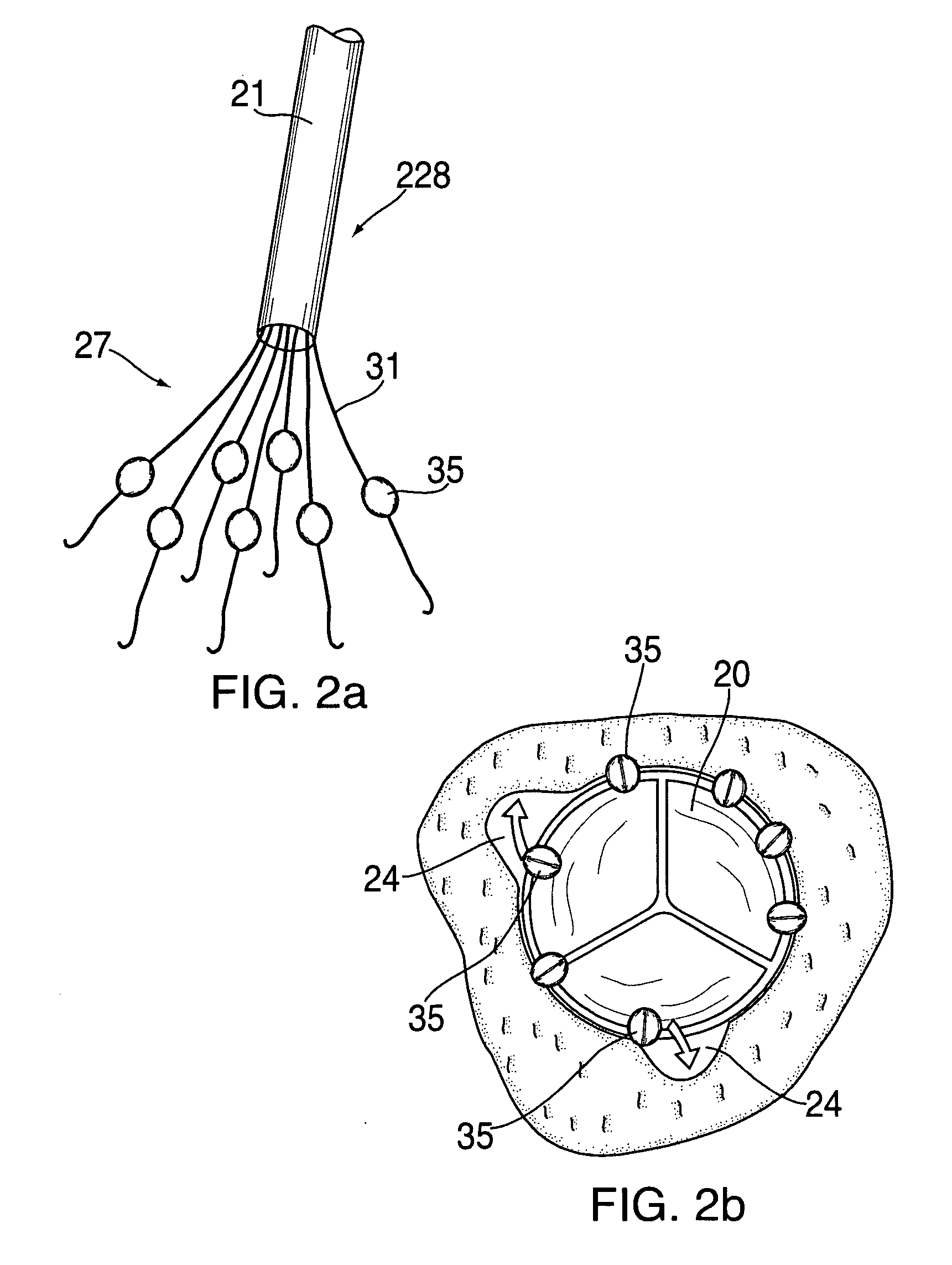
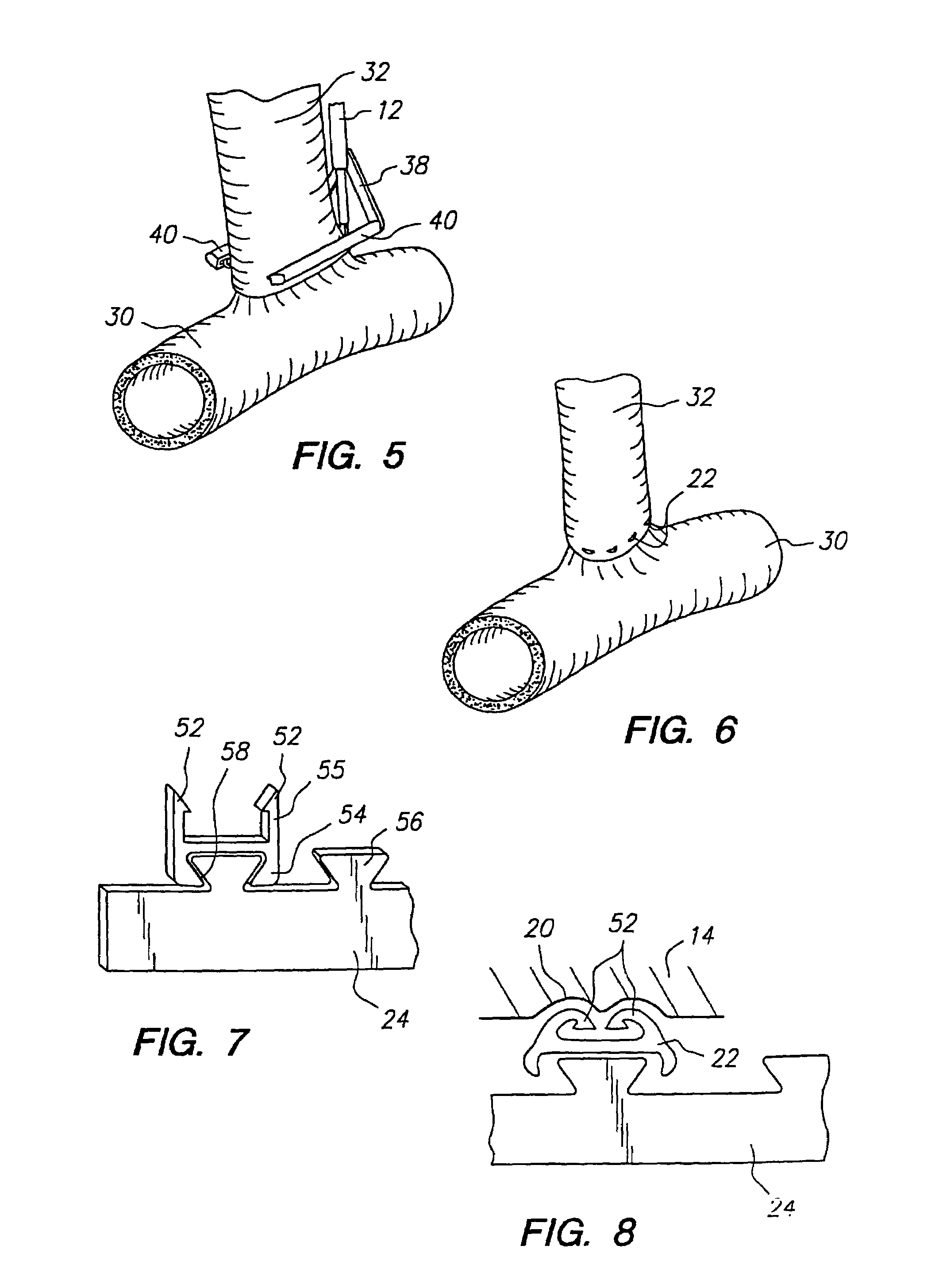
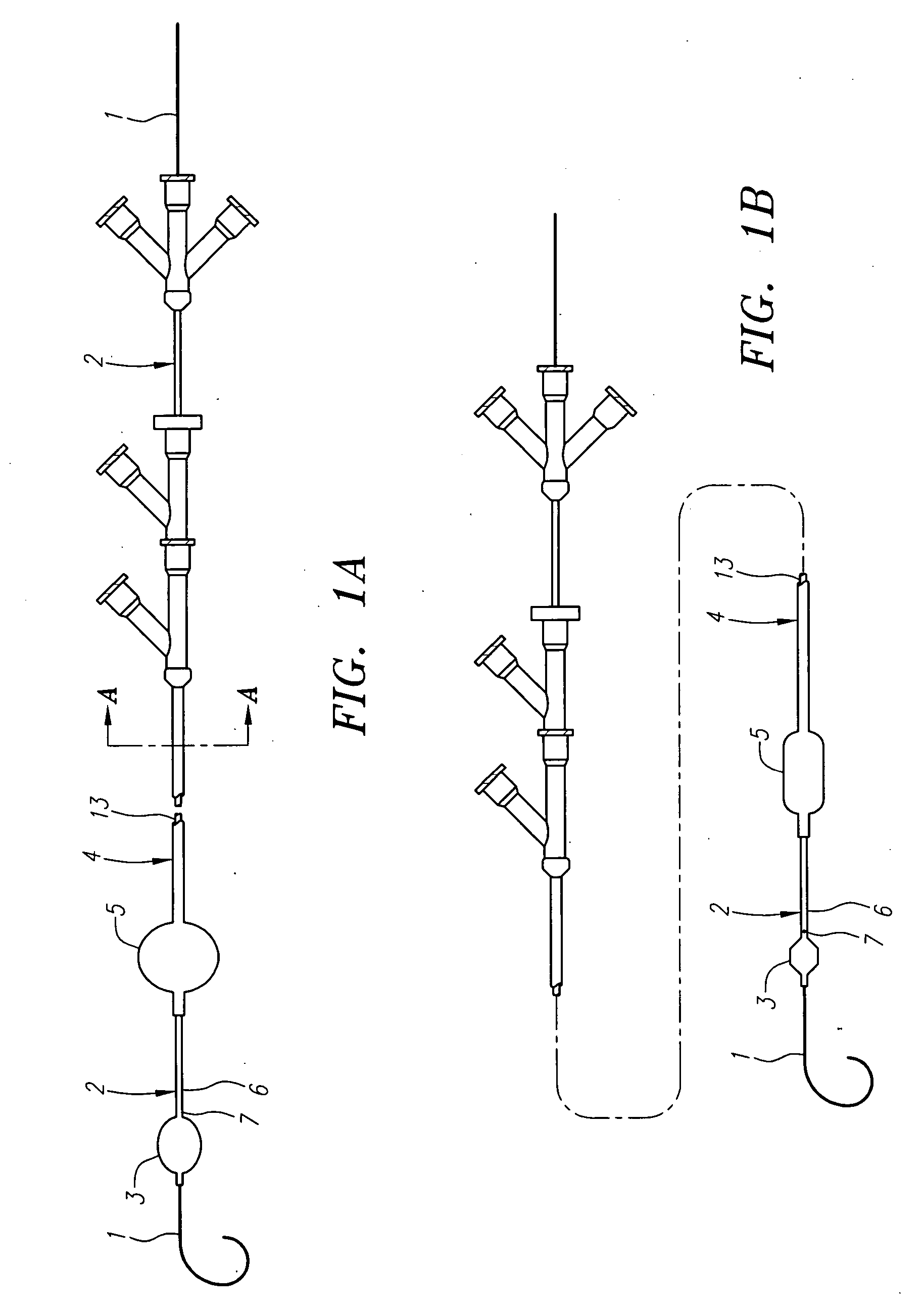
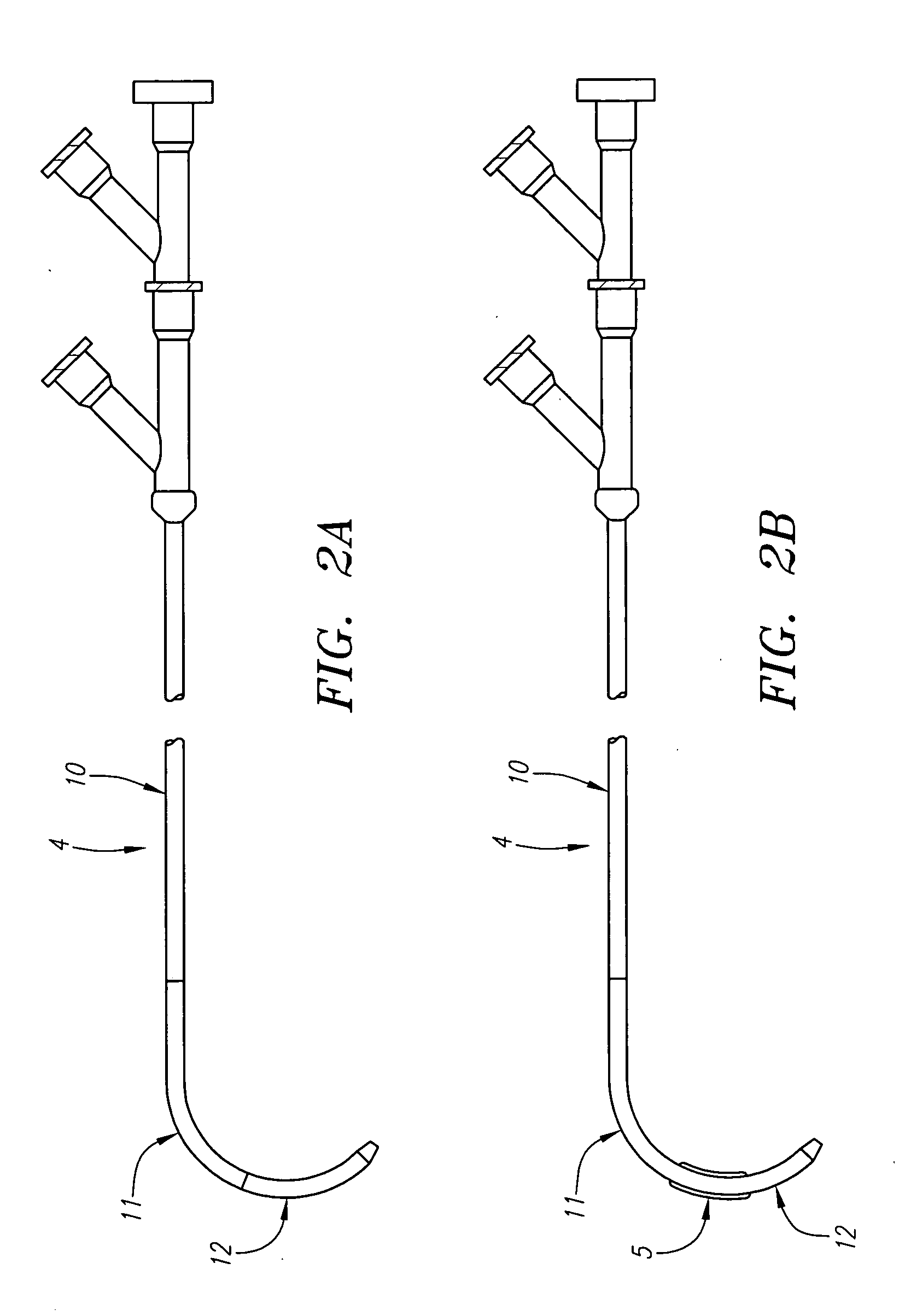
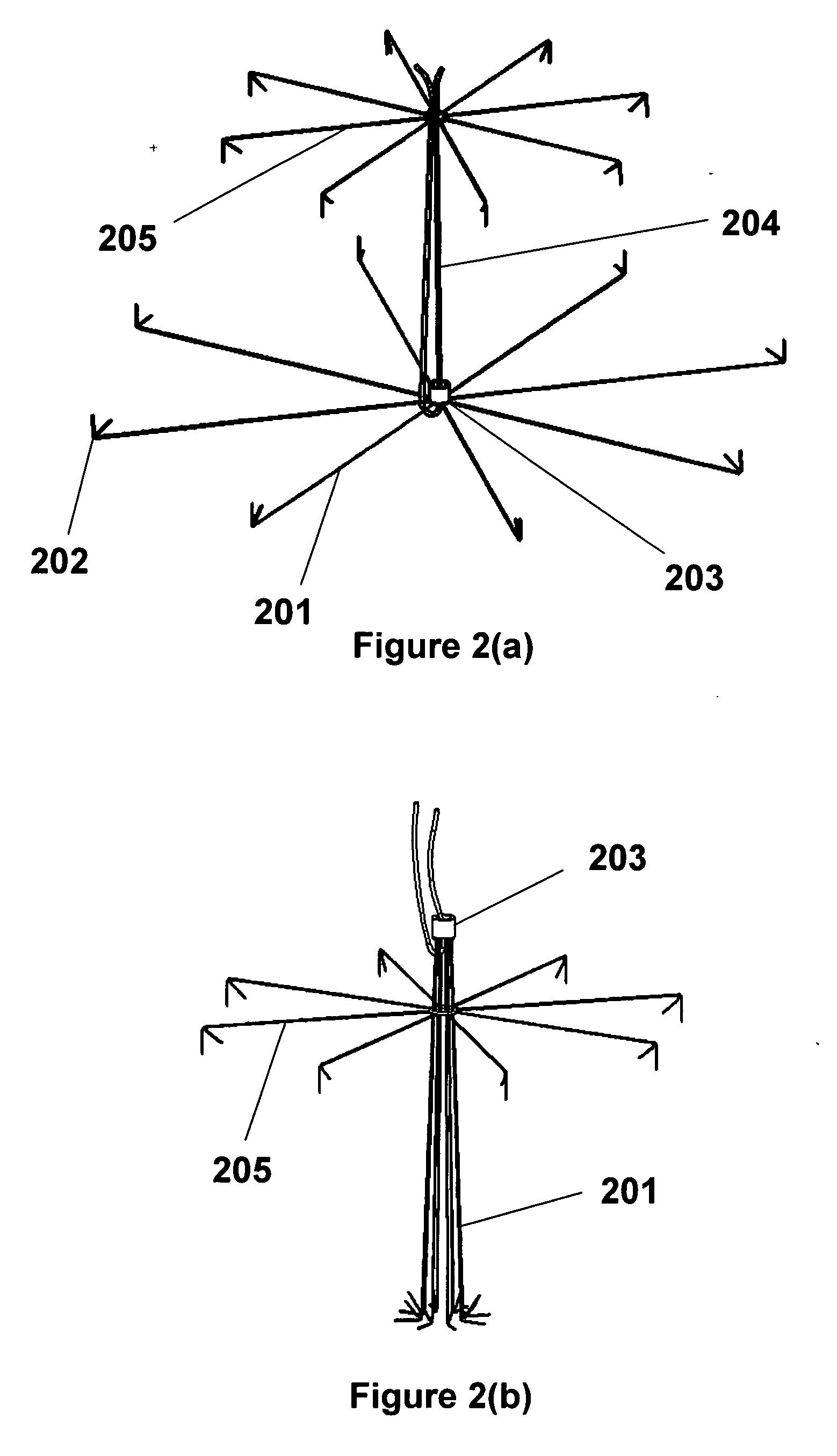
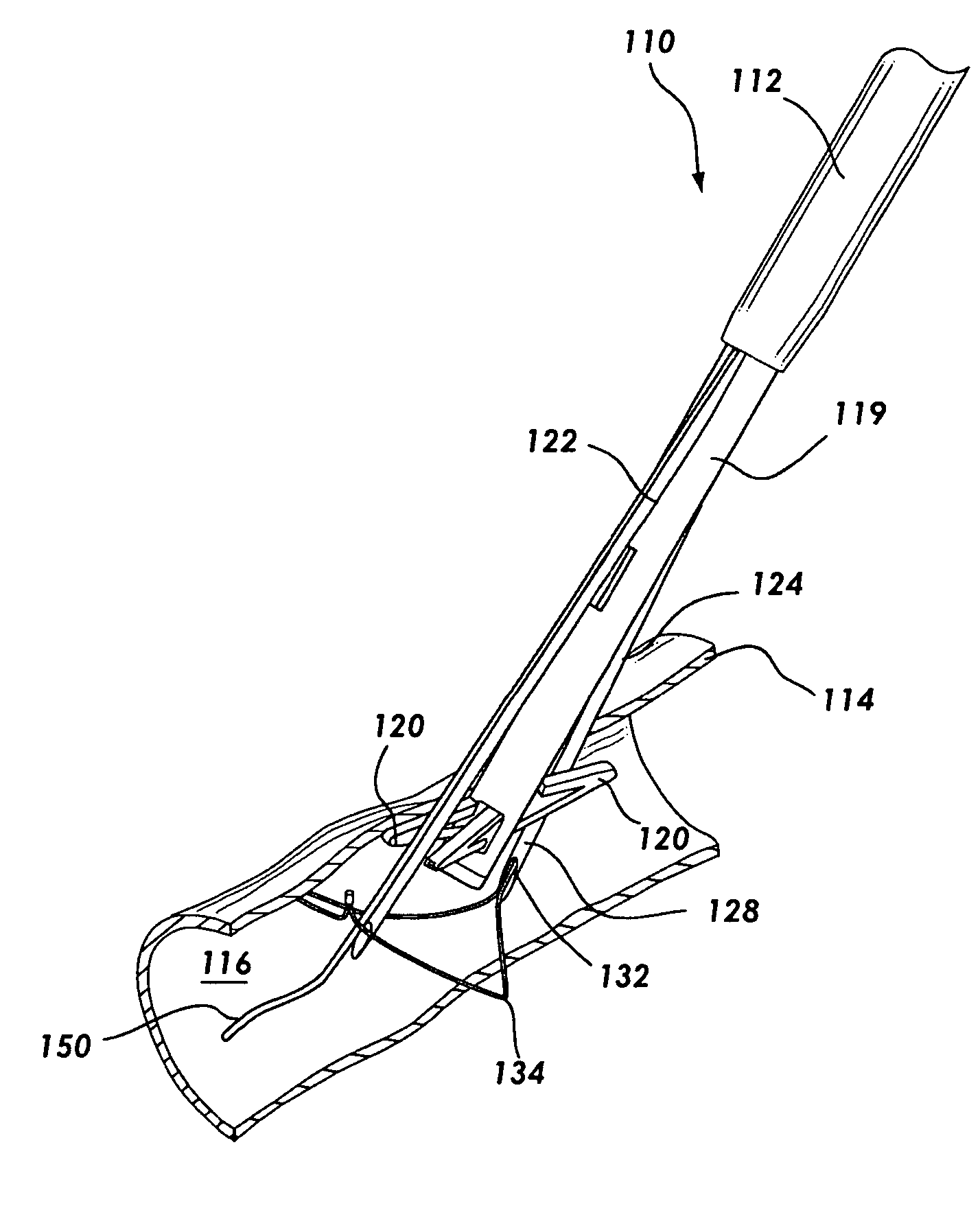
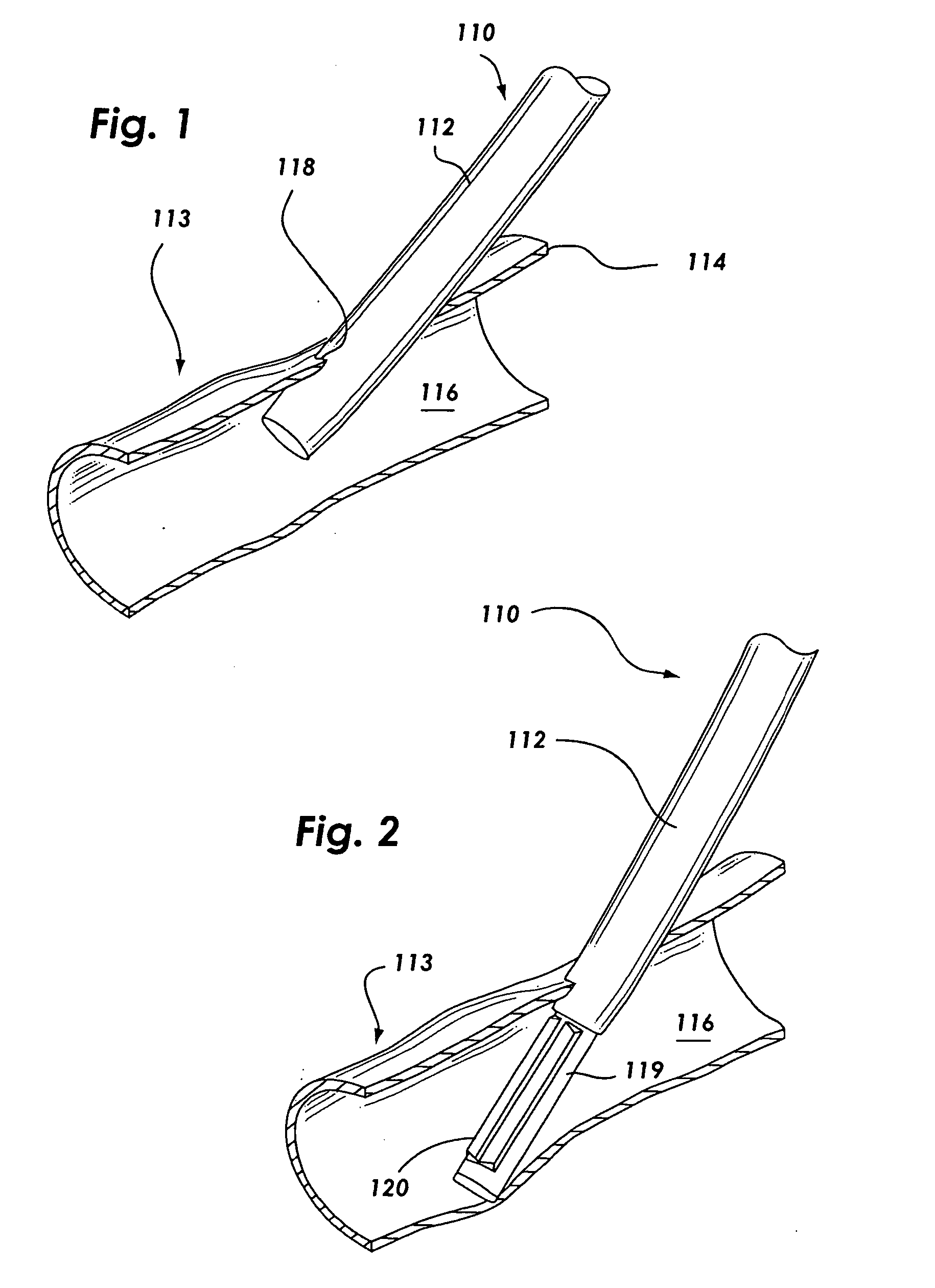
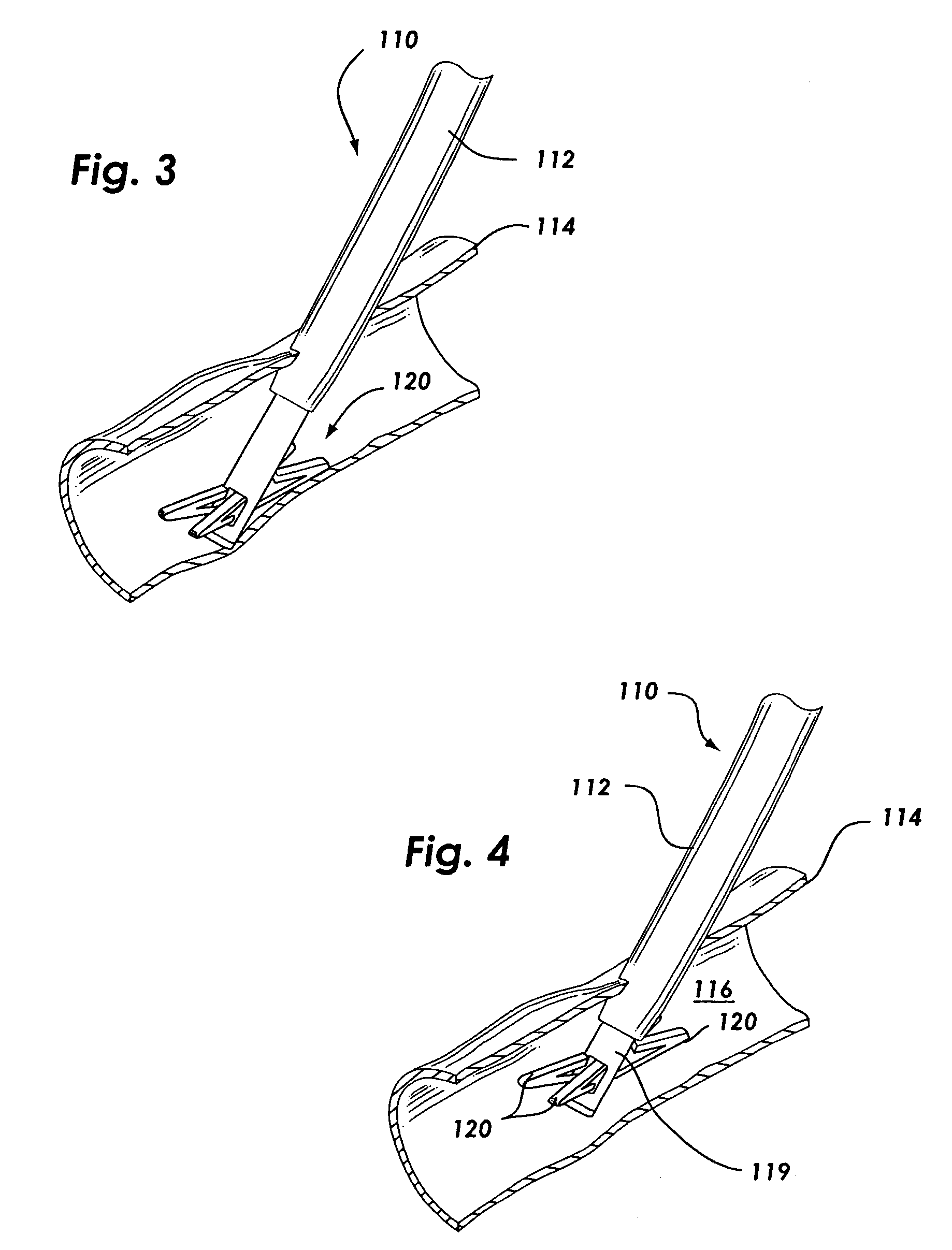
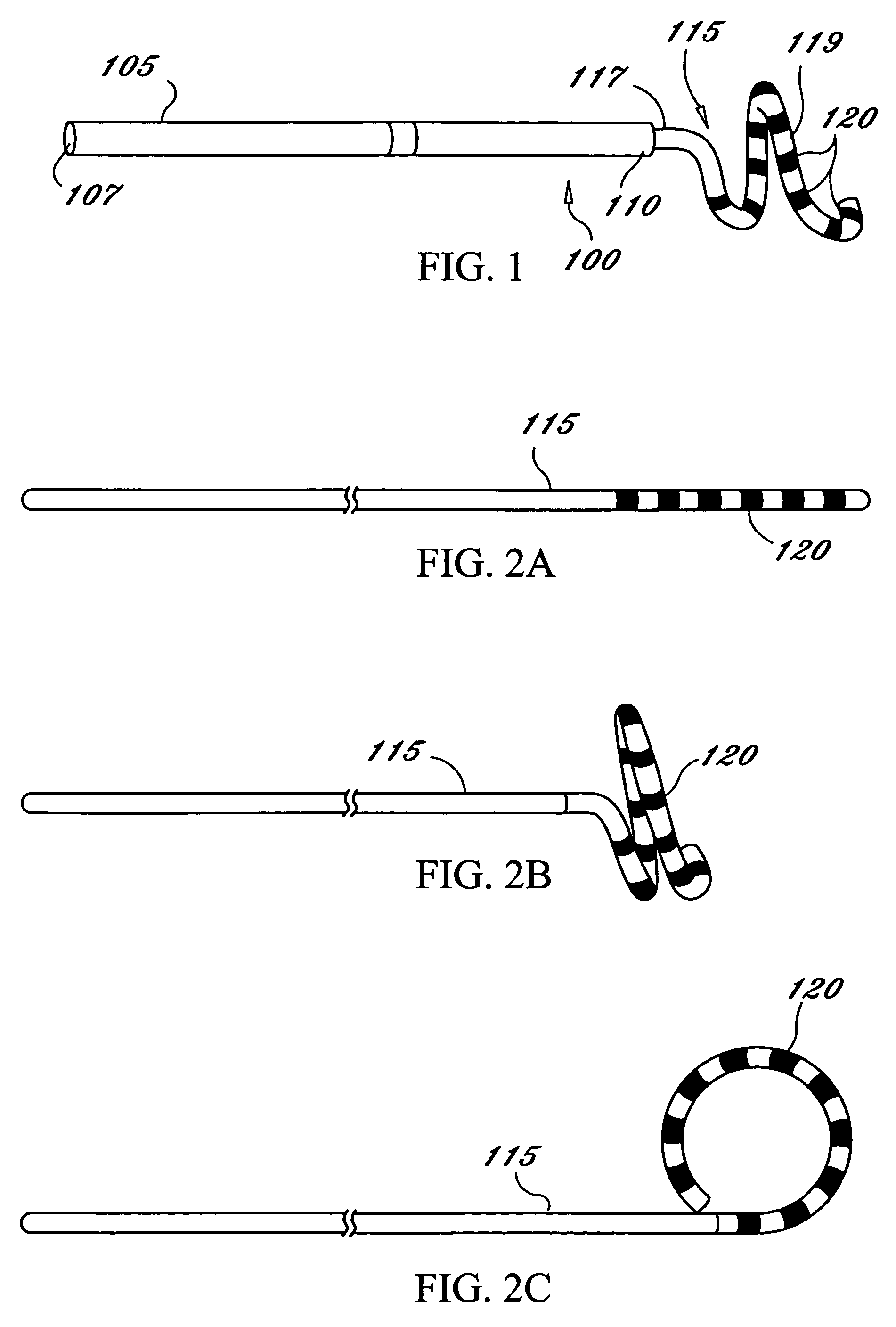
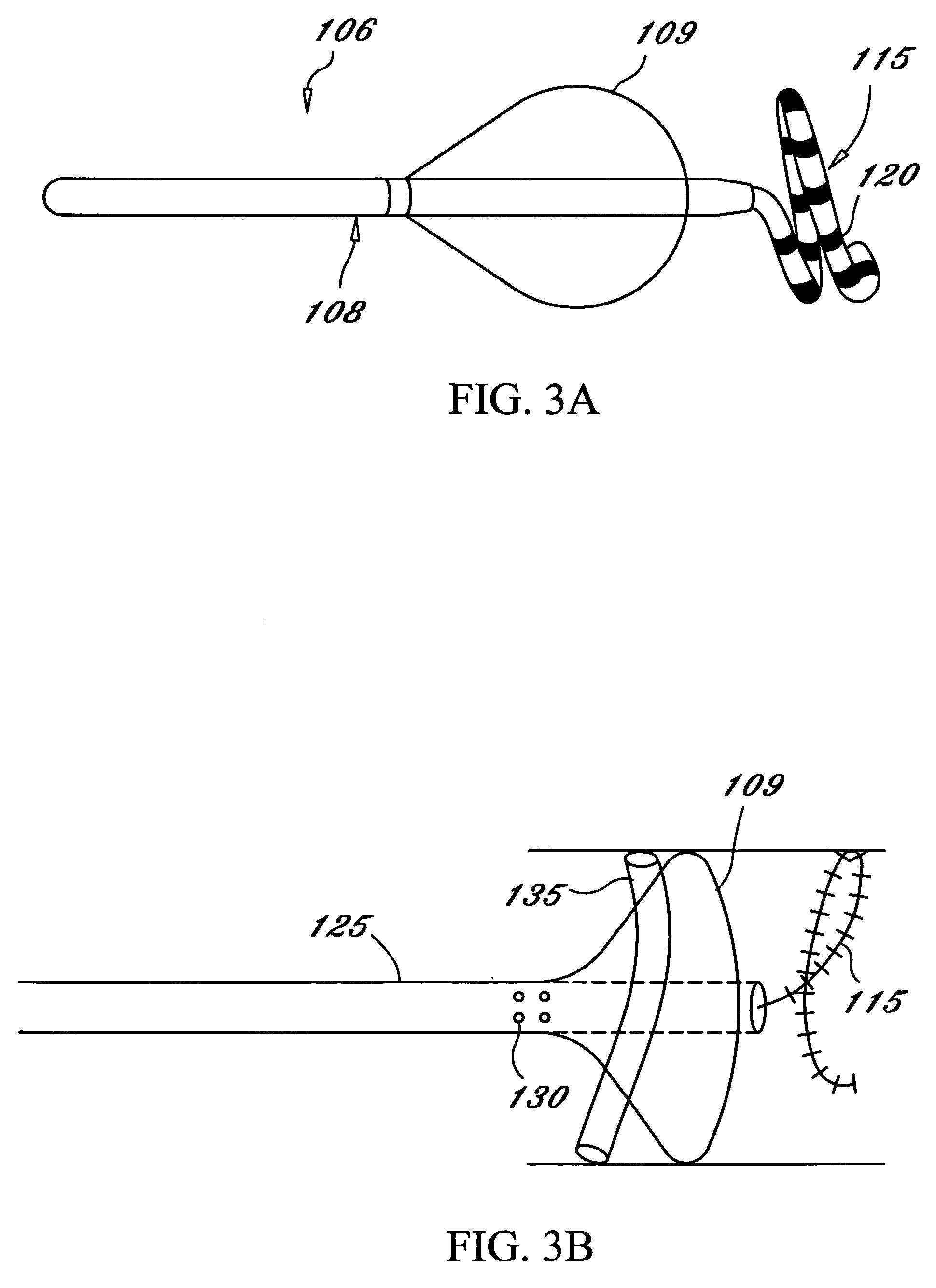
Anastomosis apparatus for use in intraluminally directed vascular anastomosis
InactiveUS6248117B1Surgical needlesSurgical staplesVascular anastomosisMinimally invasive procedures
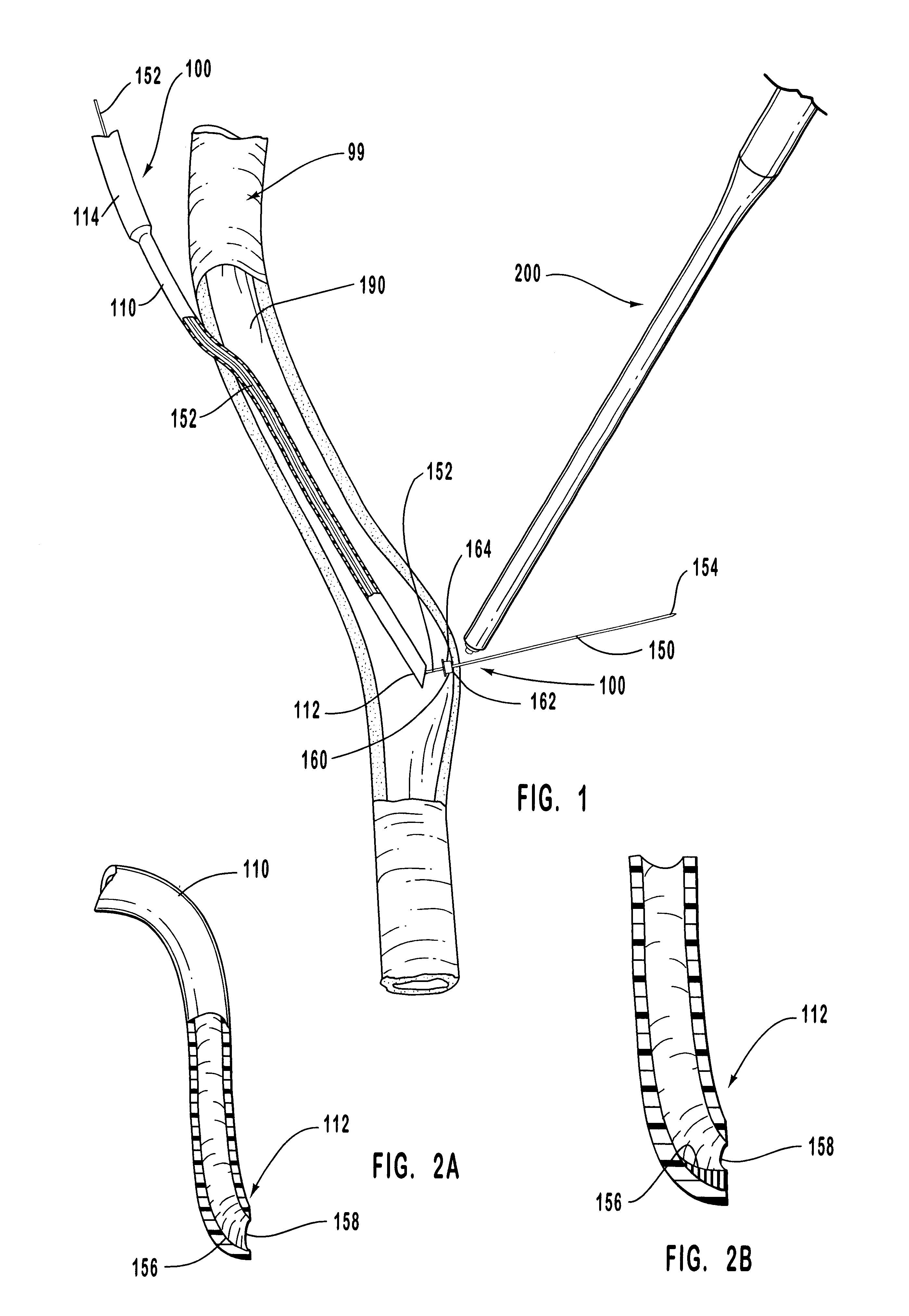
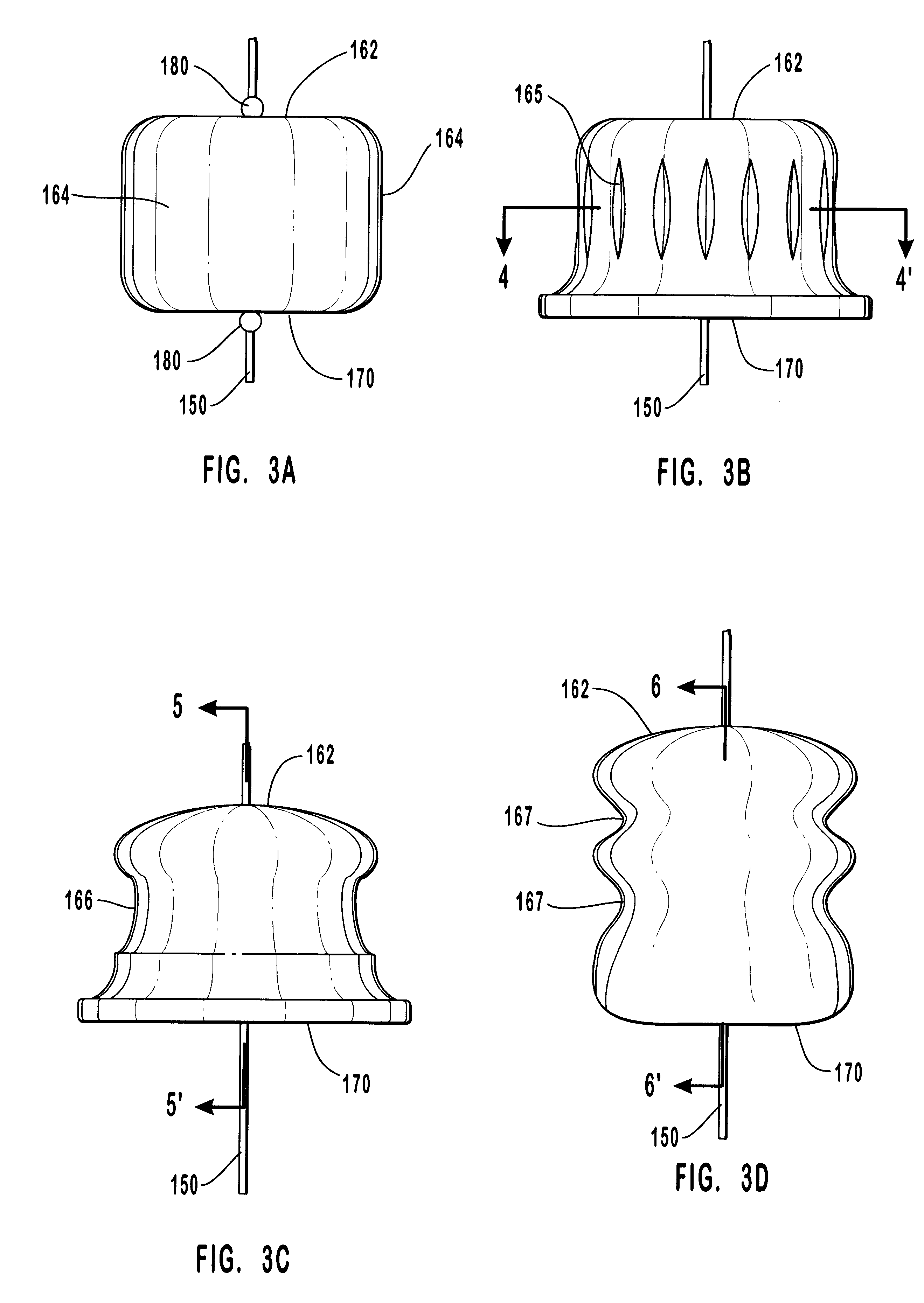
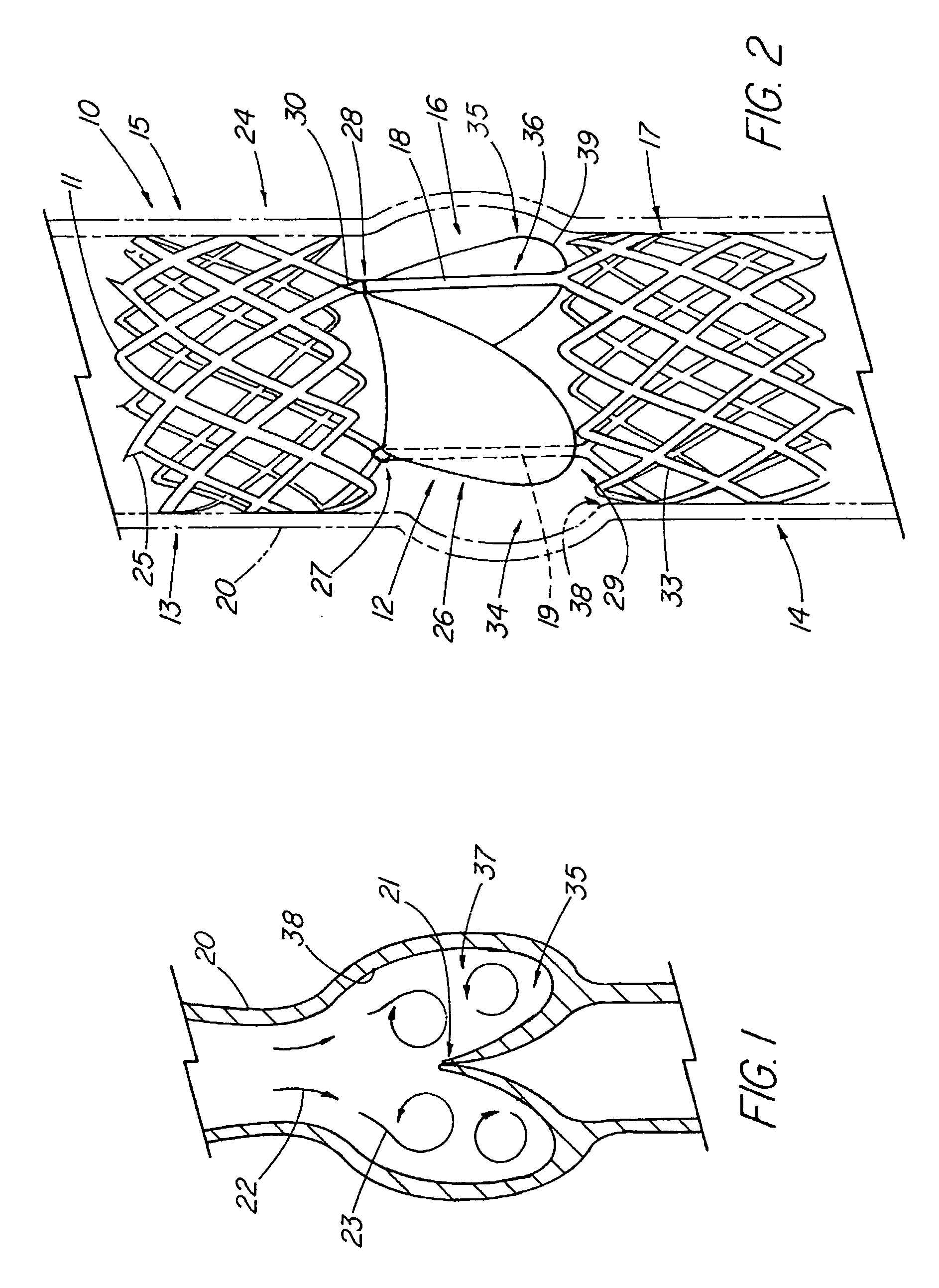
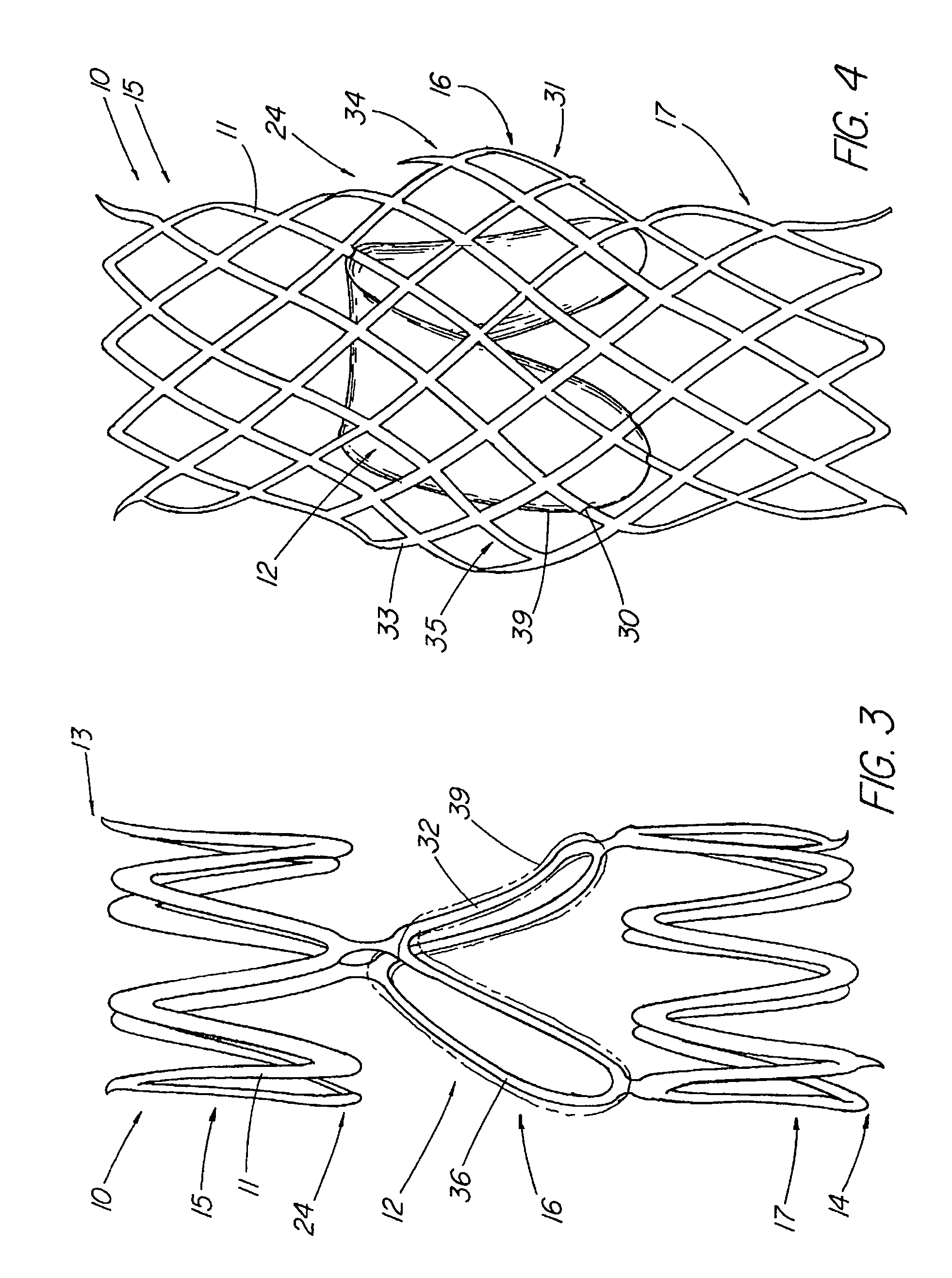
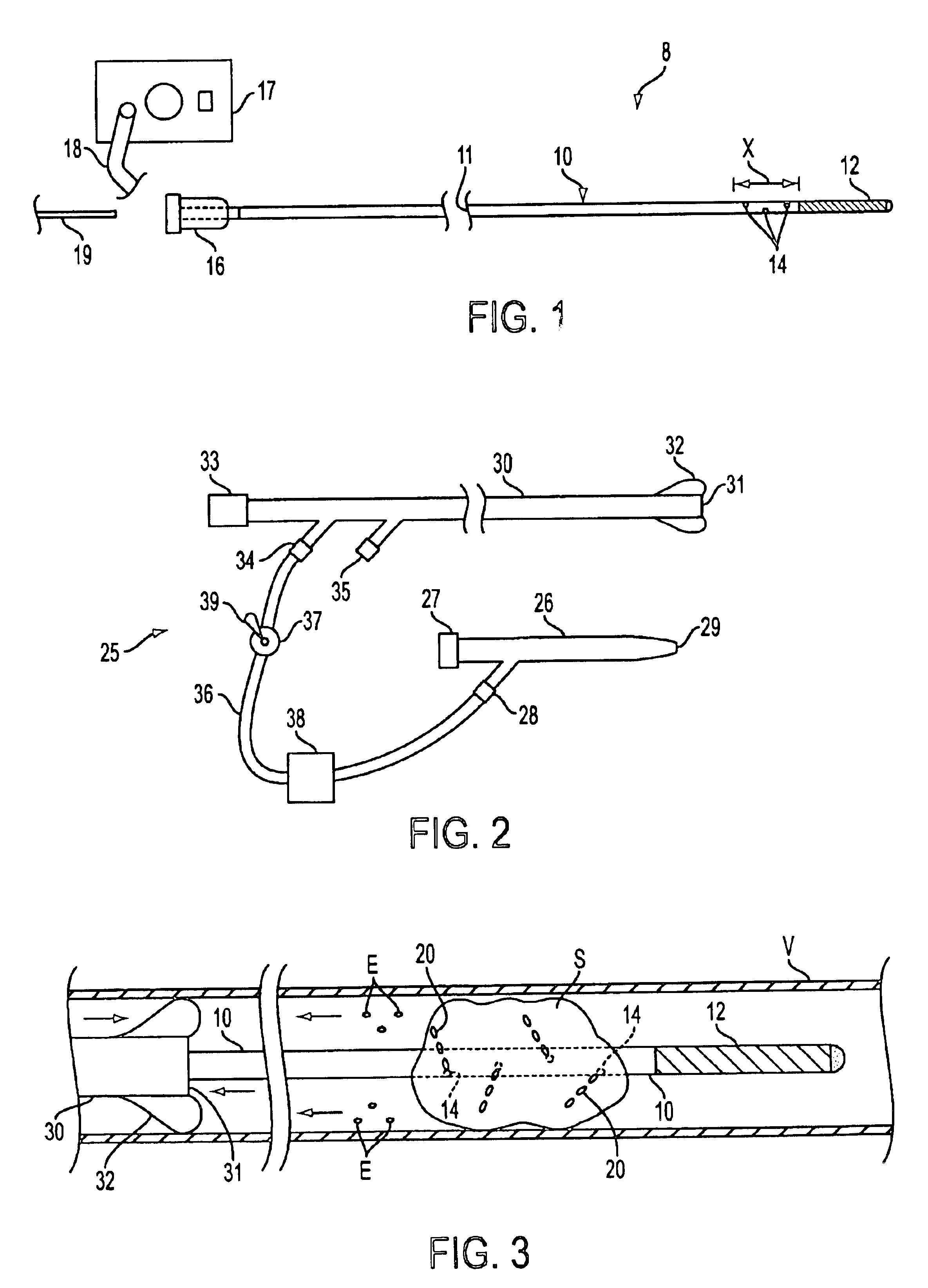
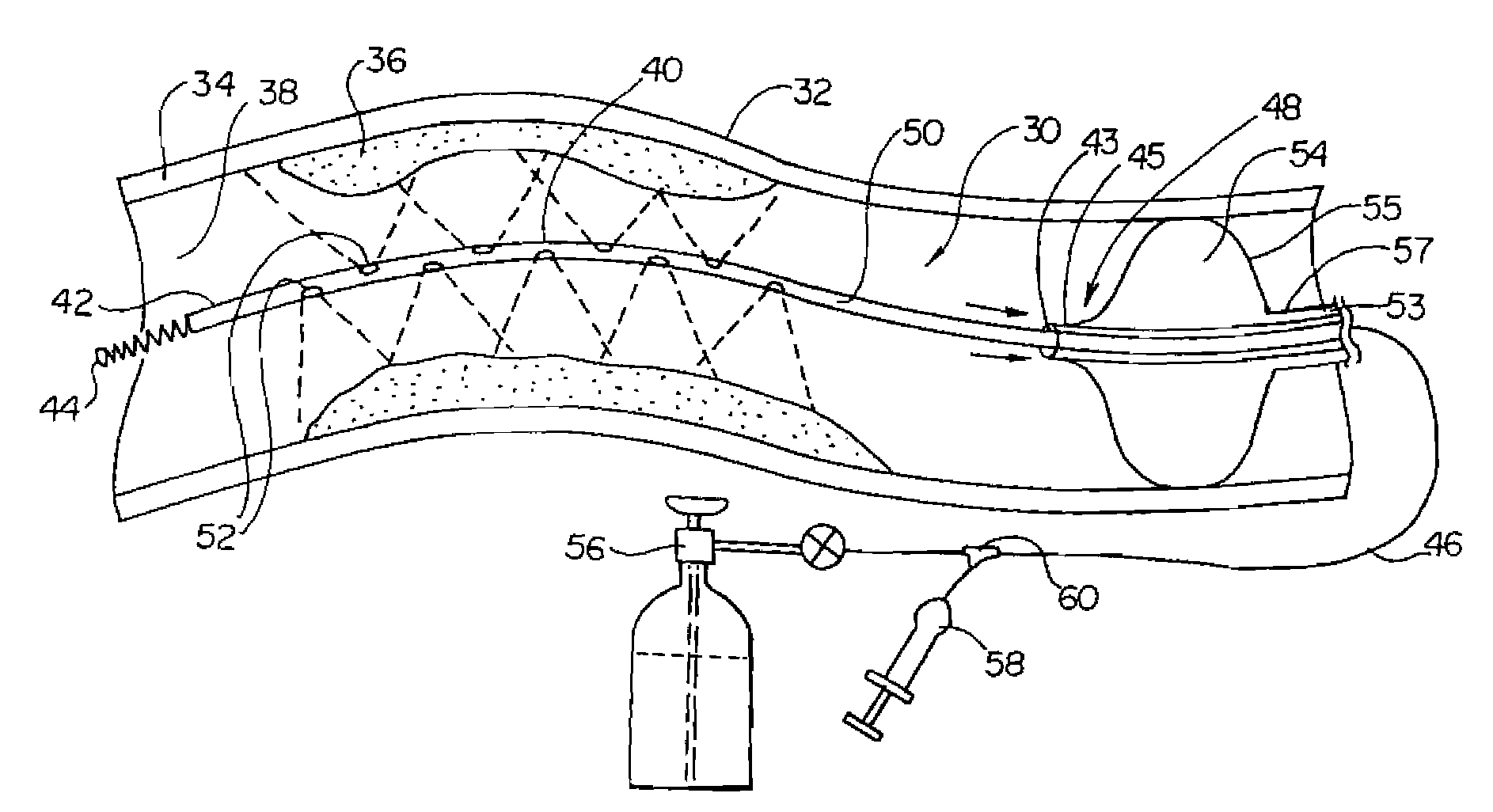
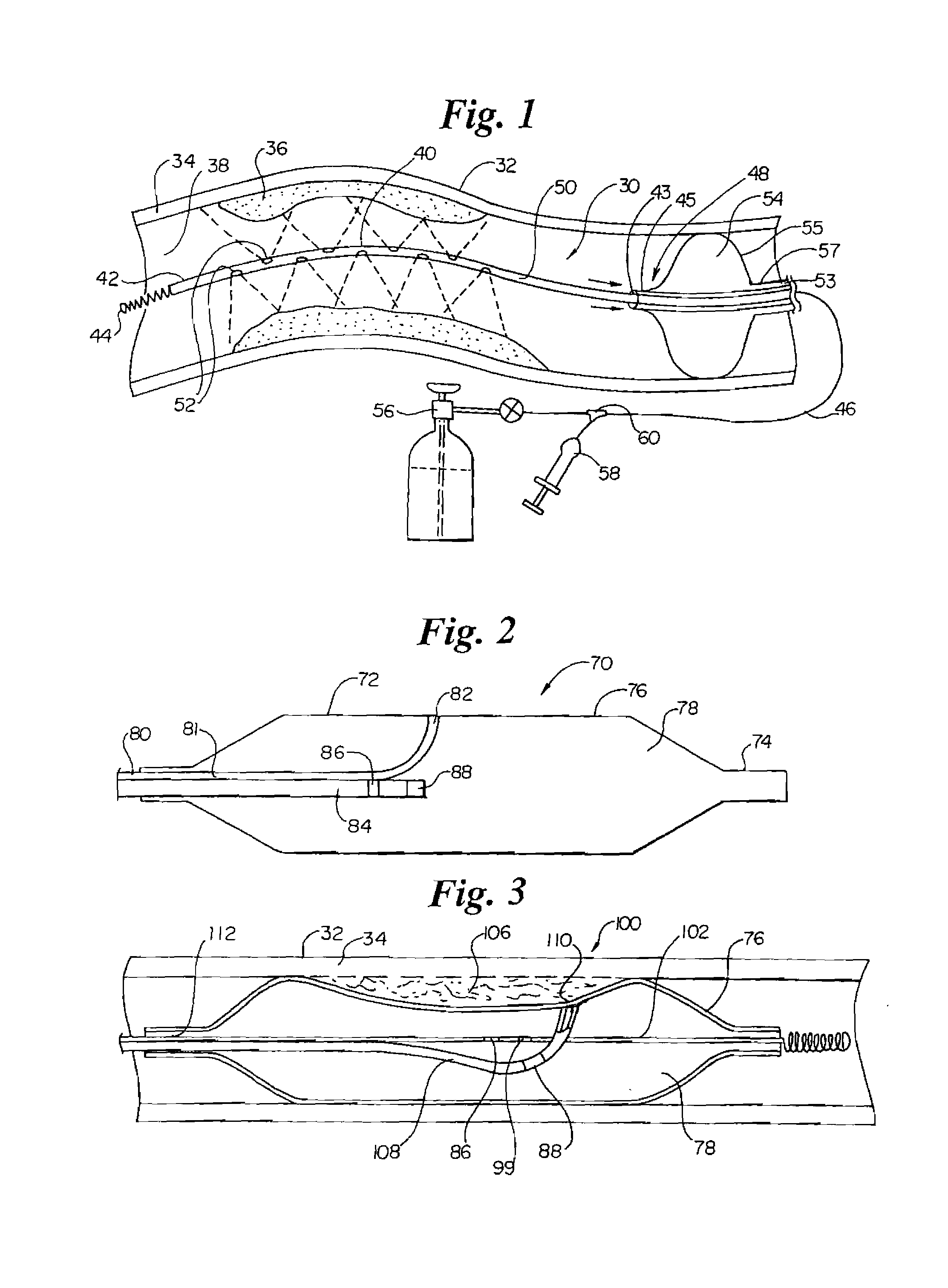
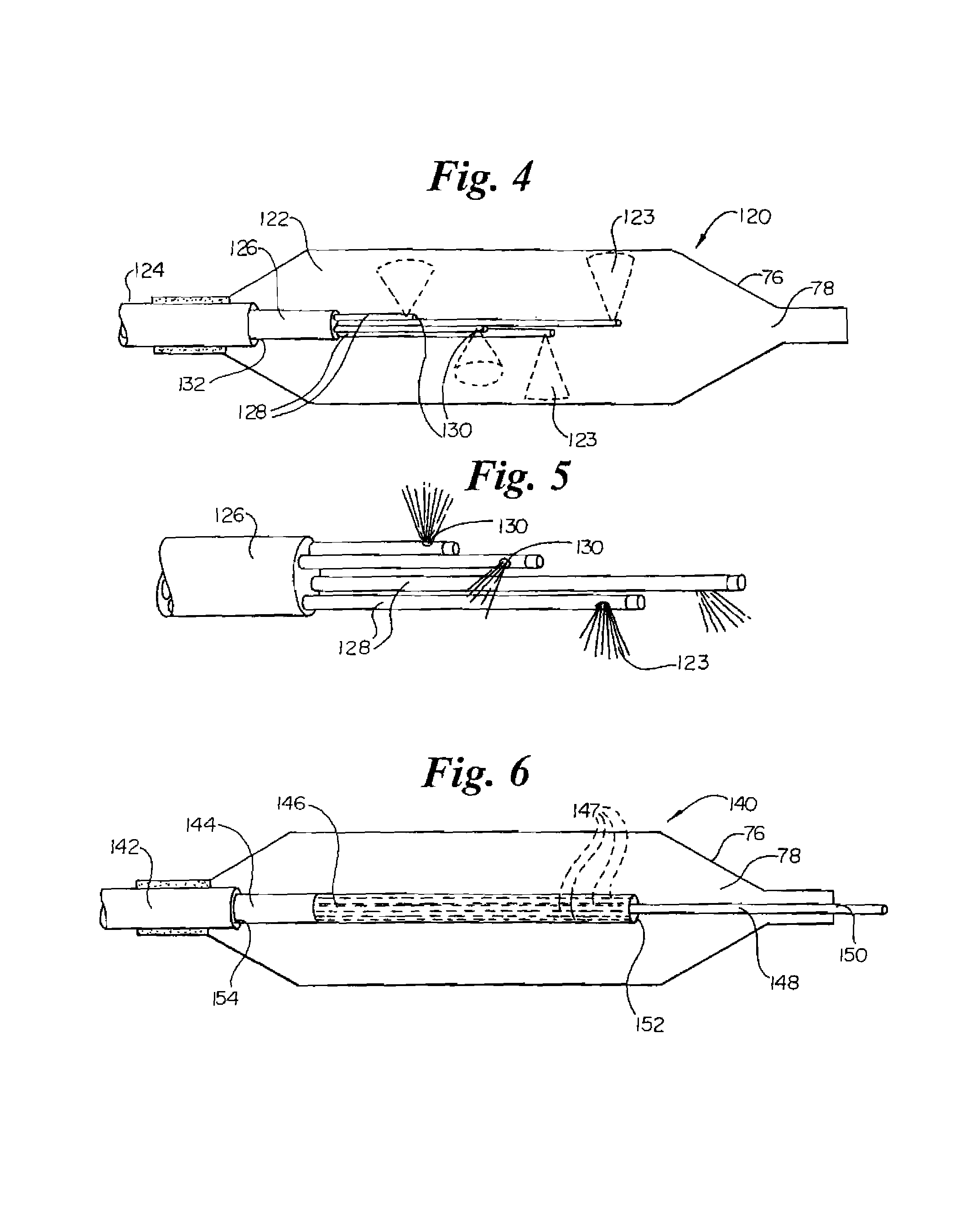
The present invention relates to new and useful apparatus for use with an intraluminally directed anvil apparatus for intraluminally directed vascular anastomosis of an end of a graft vessel to the wall of a receiving blood vessel that is performed according to a minimally invasive procedure. The intraluminally directed vascular anastomosis does not require the interruption of blood flow in the receiving blood vessel and it is versatile enough to suitably combine a variety of cutting, welding, soldering, sealing, and joining techniques such as stapling. The intraluminally directed anvil apparatus comprises an anvil and a wire used for signaling the optimal anastomosis site; this signaling can be performed when the initial exploration is performed. The intraluminally directed anvil apparatus is typically used with a catheter.
Owner:VITAL ACCESS CORP
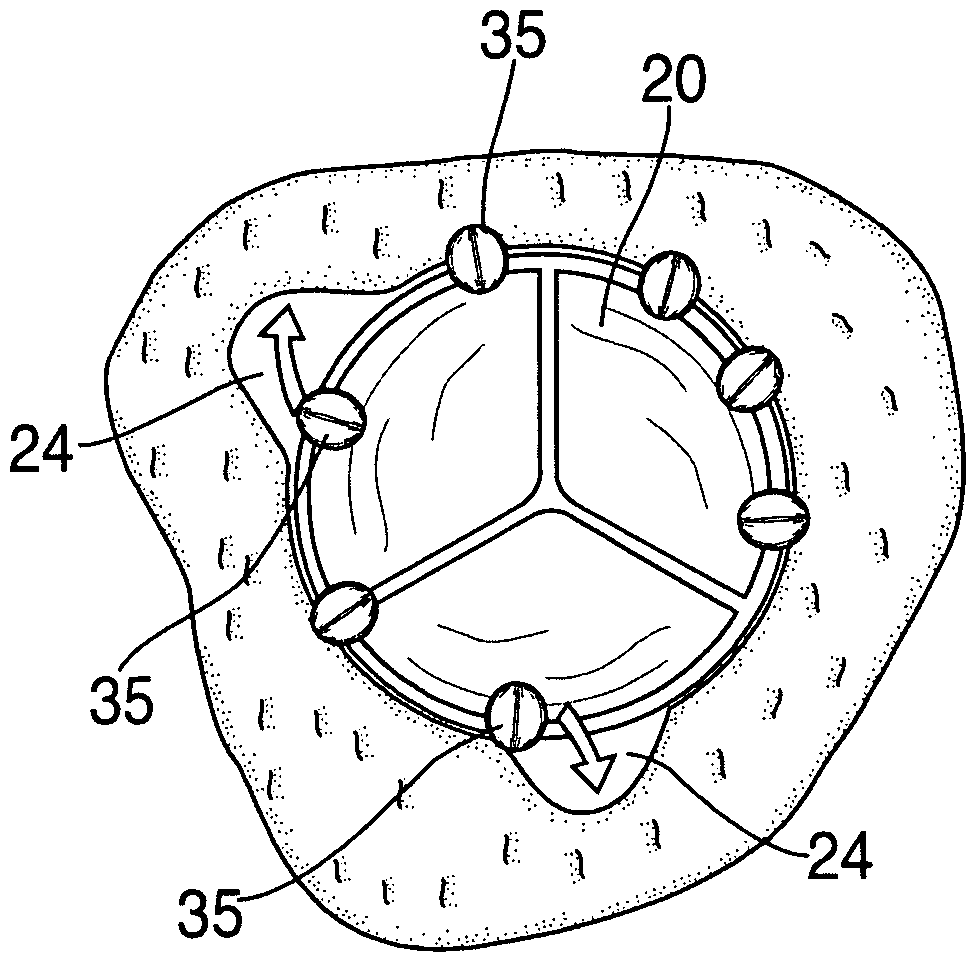
Anastomosis method
An anastomosis system and method uses an anvil to control and support a tissue site during an anastomosis procedure. The anvil is particularly useful for supporting a wall of a coronary artery during attachment of a graft vessel to the coronary artery because the wall of the coronary artery is very thin, difficult to grasp, and susceptible to tearing. In one method, the anvil is inserted into a pressurized or unpressurized target vessel and is pulled against an inner wall of the target vessel causing tenting of the thin tissue of the vessel wall. A graft vessel is then advanced to the anastomosis site and an end of the graft vessel is positioned adjacent and exterior of the target vessel. Staples are inserted through the tissue of the graft vessel and the target vessel by pivoting the arms of a staple holder towards the anvil. When the ends of the staples engage staple bending features on the anvil, the ends of the staples bend over securing the graft vessel and target vessel together. After stapling is complete, an incision is formed in the wall of the target vessel to allow blood flow between the target vessel and the graft vessel.
Owner:AESCULAP AG
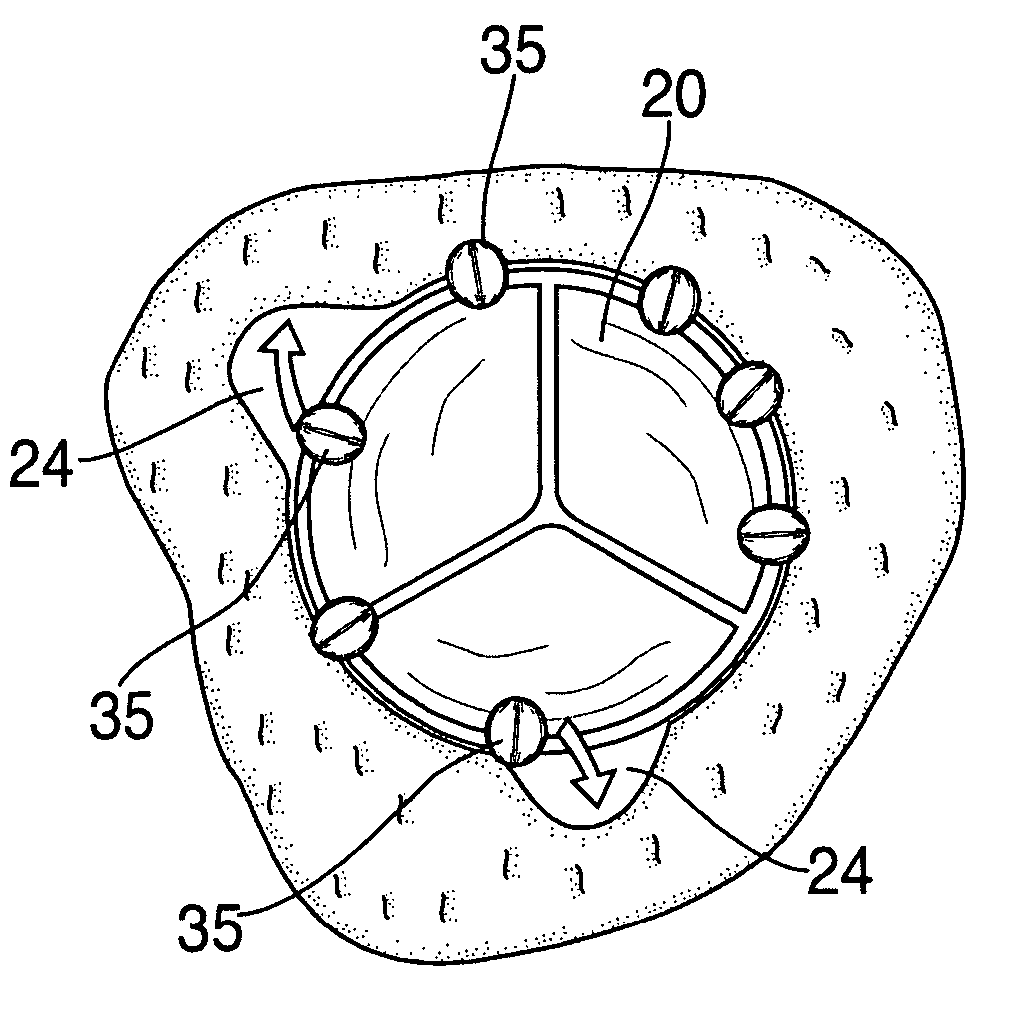
Apparatus and method for fixation of vascular grafts
InactiveUS7351258B2Reduce leakageOvercome disadvantagesStentsBlood vesselsThree vesselsVascular graft
An apparatus for facilitating securement of a vascular graft within a blood vessel, includes a shaft dimensioned for passage within a blood vessel and having an expansion member movable between a contracted condition and an expanded condition and a fastener array comprising at least one fastener disposed about a peripheral portion of the expansion member. The one fastener is deployable into a wall of the blood vessel upon movement of the expansion member to the expanded condition thereof, to thereby engage the vascular graft to secure the vascular graft to a wall of the blood vessel. The fastener array preferably includes a plurality of fasteners. The fasteners may be operatively connected to each other and releasably secured to the peripheral portion of the expansion member.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
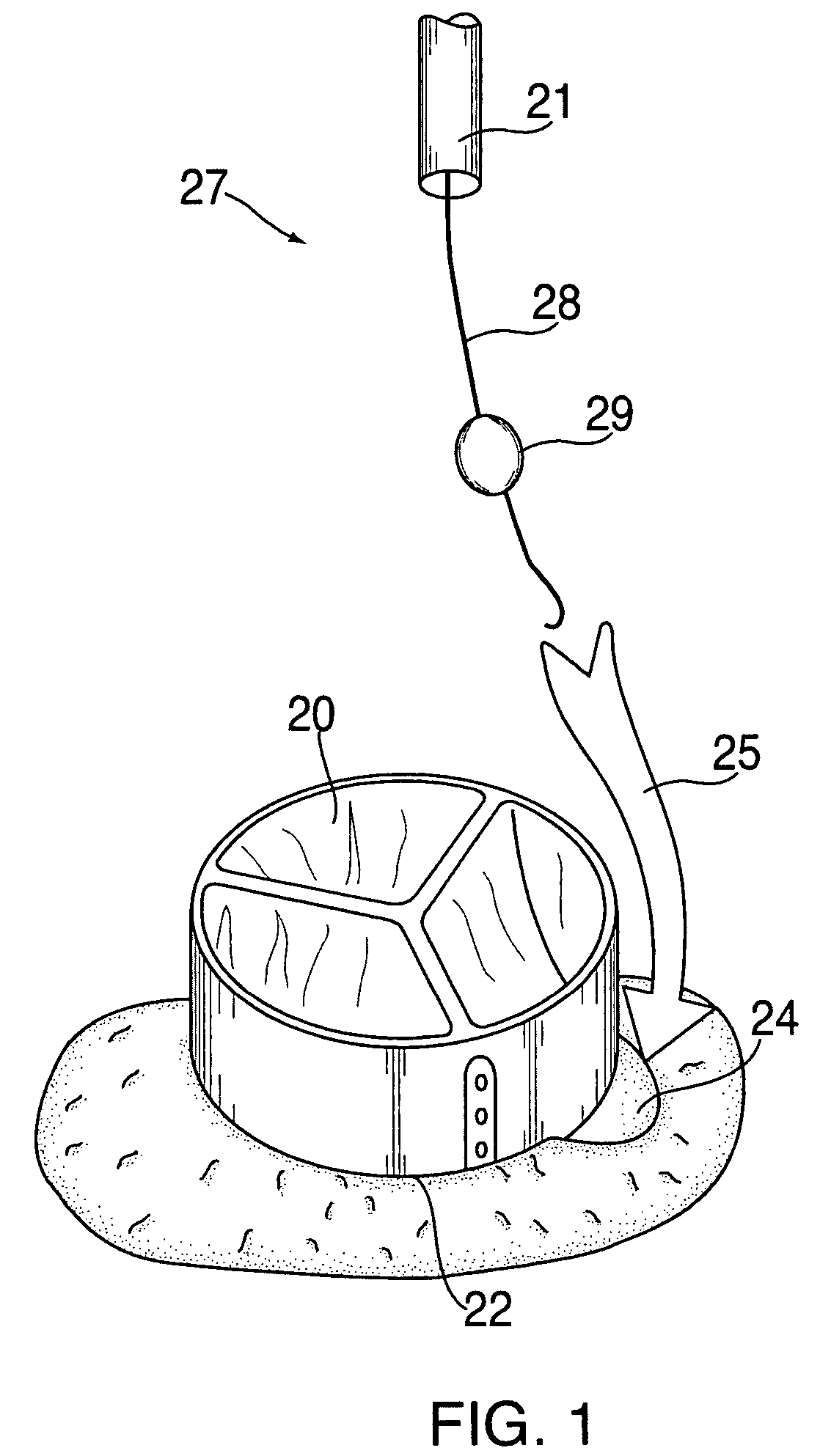
Method for anastomosing vessels
InactiveUS20050154406A1Precise positioningSecurely holdSuture equipmentsDiagnosticsSurgeryBlood vessel
A method for connecting a graft vessel to a target vessel, each vessel having a wall surrounding a lumen, may include providing a connector holder, associating an end of the graft vessel with the connector holder, positioning the connector holder outside of the lumen of the target vessel, outside the lumen of the graft vessel, and in proximity to the outer surface of the wall of the target vessel, and actuating the connector holder to secure the end of the graft vessel to the side of the target vessel.
Owner:AESCULAP AG
Surgical method for treating a vessel wall
Owner:AESCULAP AG
Intravascular delivery system for therapeutic agents
Described herein is a system for intravascular drug delivery system, which includes a reservoir implantable a blood vessel, an intravascular pump fluidly coupled to the reservoir and an anchor expandable into contact with a wall of the blood vessel to retain the system within the vasculature. Delivery conduits may be extend from the reservoir and are positionable at select locations within the vasculature for target drug delivery to select organs or tissues.
Owner:WILLIAMS MICHAEL S +2
Paravalvular leak detection, sealing, and prevention
The present invention provides a series of new percutaneous concepts of paravalvular repairs including identifying the leak location, several repair techniques and finally built-in means for leak prevention, built on percutaneous valves. A catheter-delivered device locates cavities occurring between a prosthetic valve and the wall of the body vessel where the valve is implanted, the cavities producing paravalvular leaks during diastole, the device comprising at least one of a plurality of flexible wires, the wire having attached to it a balloon, wherein the balloon is pulled by the leak through the cavity and wherein the wire then serves to mark the cavity location.
Owner:EDWARDS LIFESCI PVT
Paravalvular leak detection, sealing, and prevention
The present invention provides a series of new percutaneous concepts of paravalvular repairs including identifying the leak location, several repair techniques and finally built-in means for leak prevention, built on percutaneous valves. A catheter-delivered device locates cavities occurring between a prosthetic valve and the wall of the body vessel where the valve is implanted, the cavities producing paravalvular leaks during diastole, the device comprising at least one of a plurality of flexible wires, the wire having attached to it a balloon, wherein the balloon is pulled by the leak through the cavity and wherein the wire then serves to mark the cavity location.
Owner:EDWARDS LIFESCI PVT
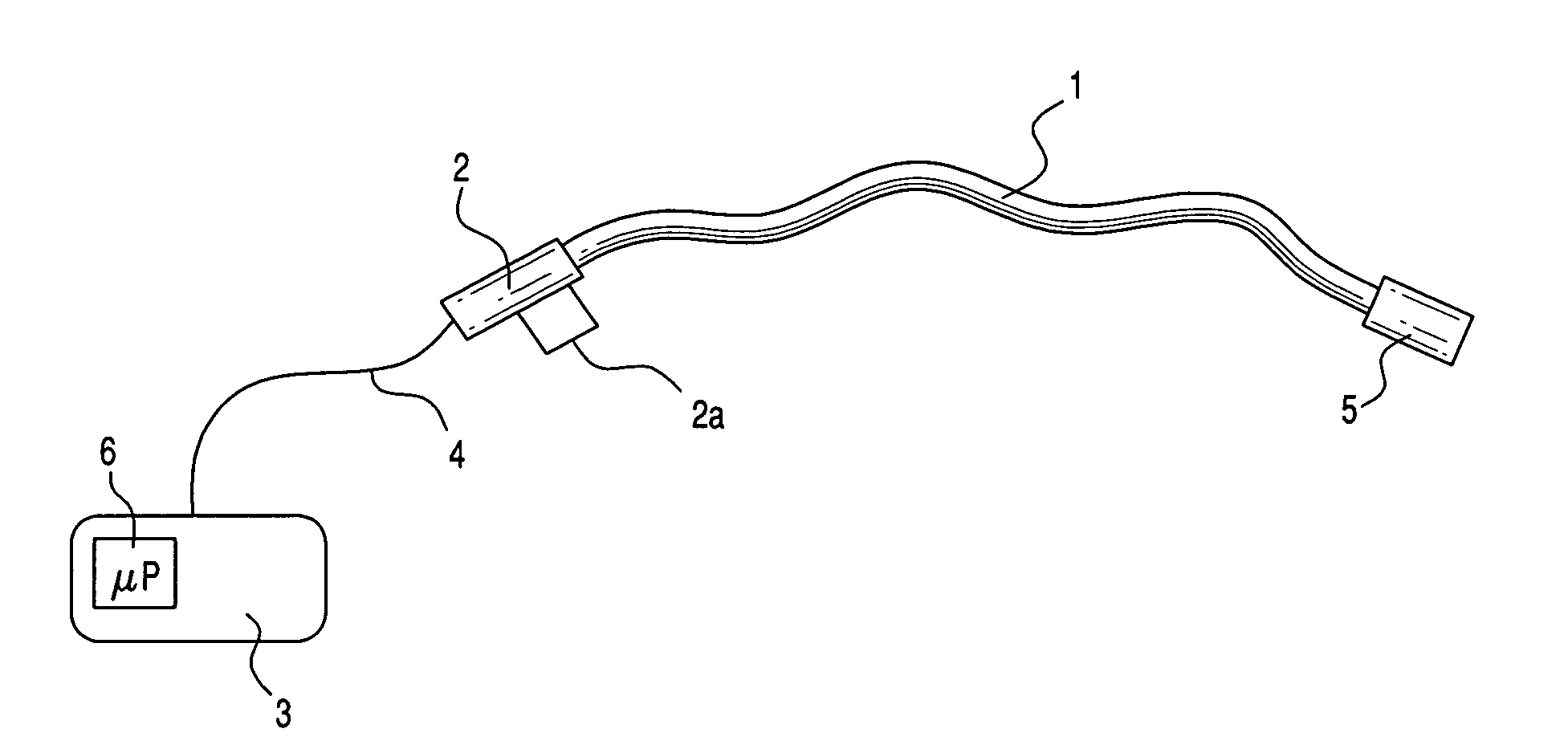
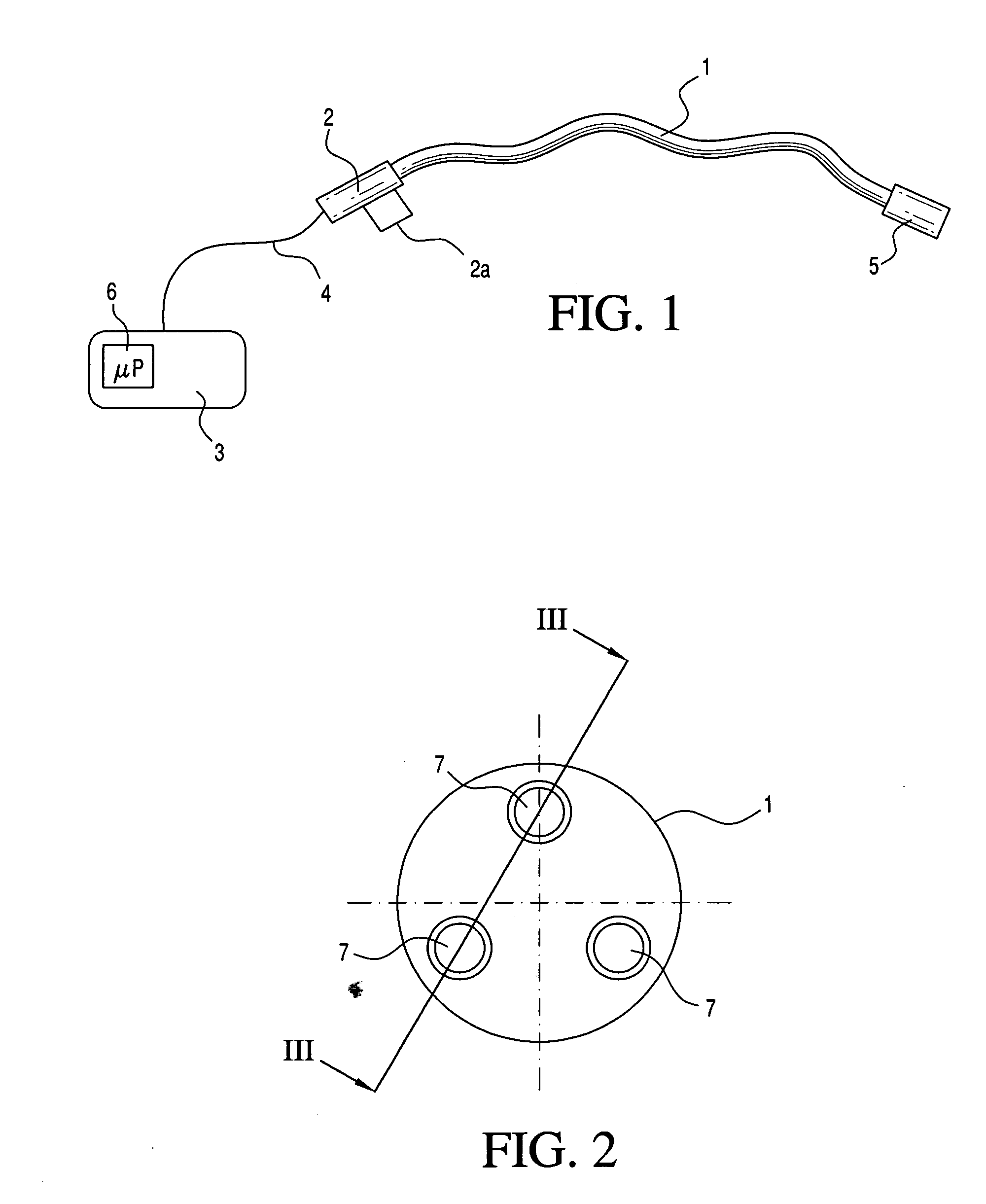
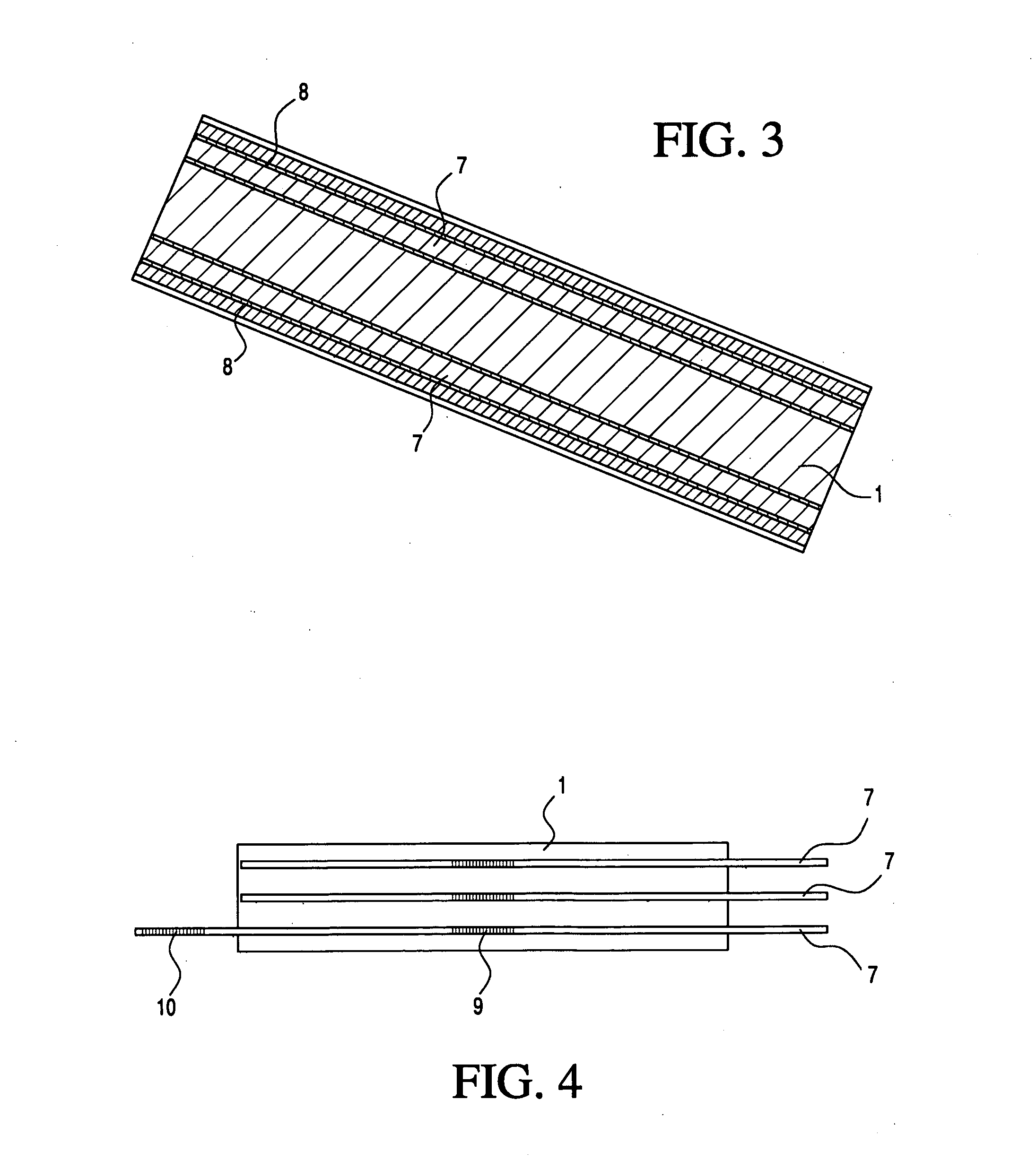
Medical apparatus system having optical fiber load sensing capability
ActiveUS20060200049A1Facilitate speedHelp accuracyStrain gaugePerson identificationRobotic systemsProcess logic
Apparatus is provided for diagnosing or treating an organ or vessel, wherein a deformable body having at least two optical fiber sensors disposed in a distal extremity thereof is coupled to processing logic programmed to compute a multi-dimensional force vector responsive to detected changes in the optical characteristics of the optical fiber sensors arising from deflection of the distal extremity resulting from contact with the tissue of the wall of the organ or vessel. The force vector may be used to facilitate manipulation of the deformable body either directly or automatically using a robotic system.
Owner:ST JUDE MEDICAL INT HLDG SARL
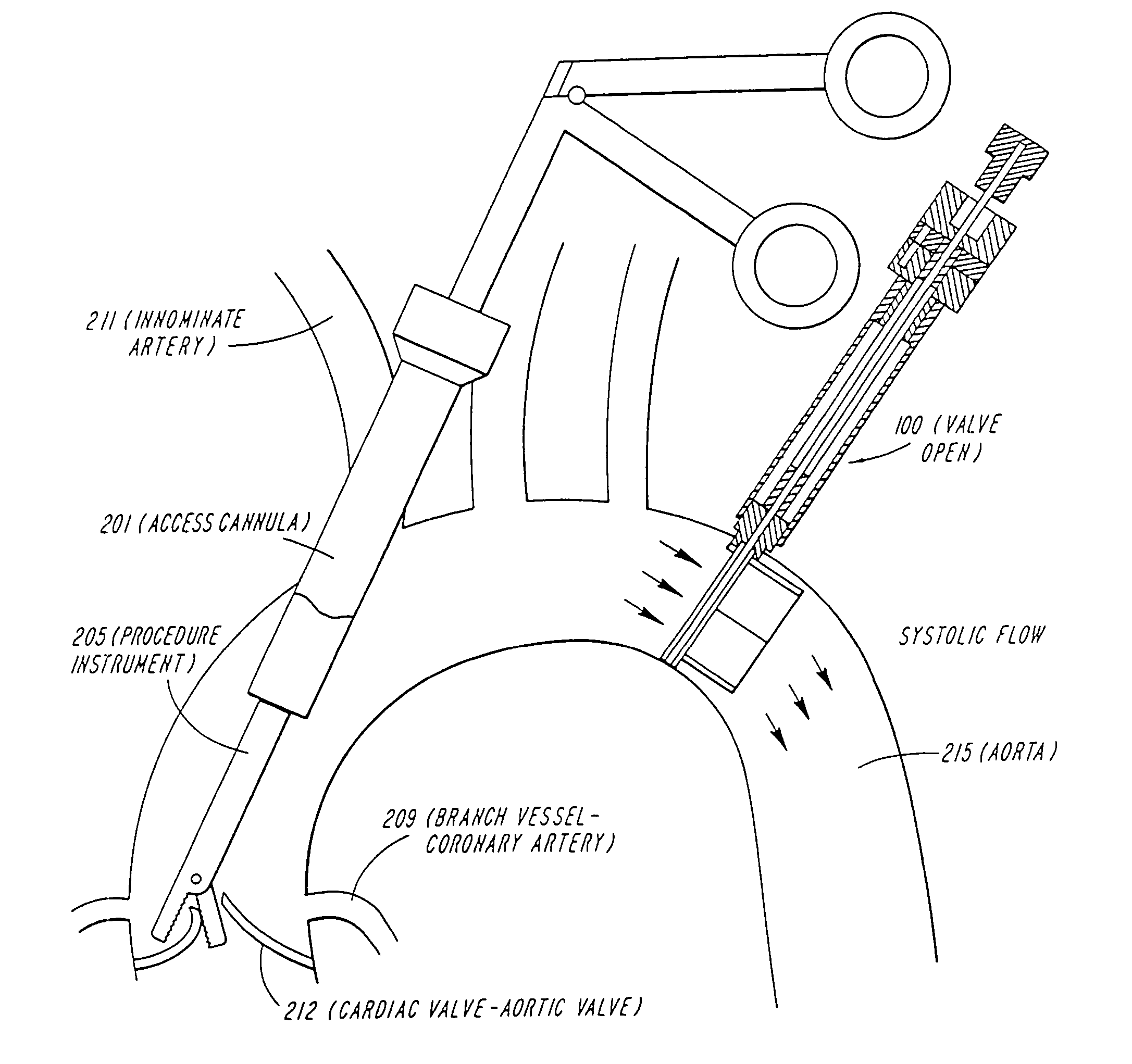
Cardiac valve procedure methods and devices
ActiveUS20050015112A1Improve performanceReduce riskHeart valvesSurgeryCoronary arteriesProsthetic valve
Devices and methods for performing intravascular procedures without cardiac bypass include embodiments of temporary filter devices, temporary valves, and prosthetic valves. The temporary filter devices have a cannula which provides access for surgical tools for effecting repair of cardiac valves. The cannula may have filters which prevent embolitic material from entering the coronary arteries and aorta. The valve devices may also have a cannula for insertion of the valve into the aorta. The valve devices expand in the aorta to occupy the entire flow path of the vessel and operate to prevent blood flow and to permit flow through the valve. The prosthetic valves include valve fixation devices which secure the prosthetic valve to the wall of the vessel. The prosthetic valves are introduced into the vascular system in a compressed state, advanced to the site of implantation, and expanded and secured to the vessel wall.
Owner:MEDTRONIC INC
Vascular treatment method and device
An intravascular catheter with micro spines that penetrate arterial wall to delivery drug or mechanical injury to the vessel wall inducing a “stent” like healing process in the vessel.
Owner:NFOCUS NEUROMEDICAL
Cryogenically enhanced intravascular interventions
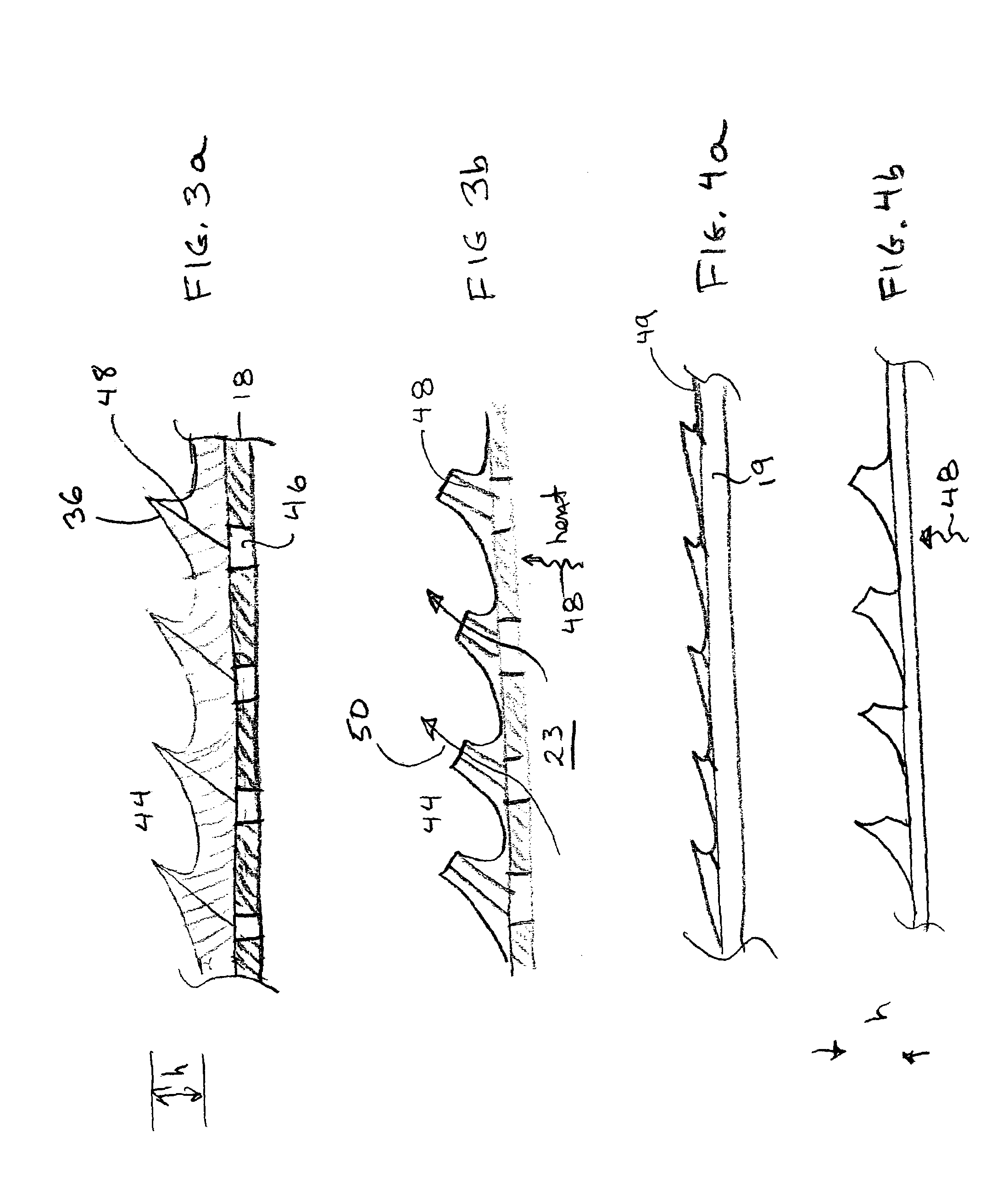
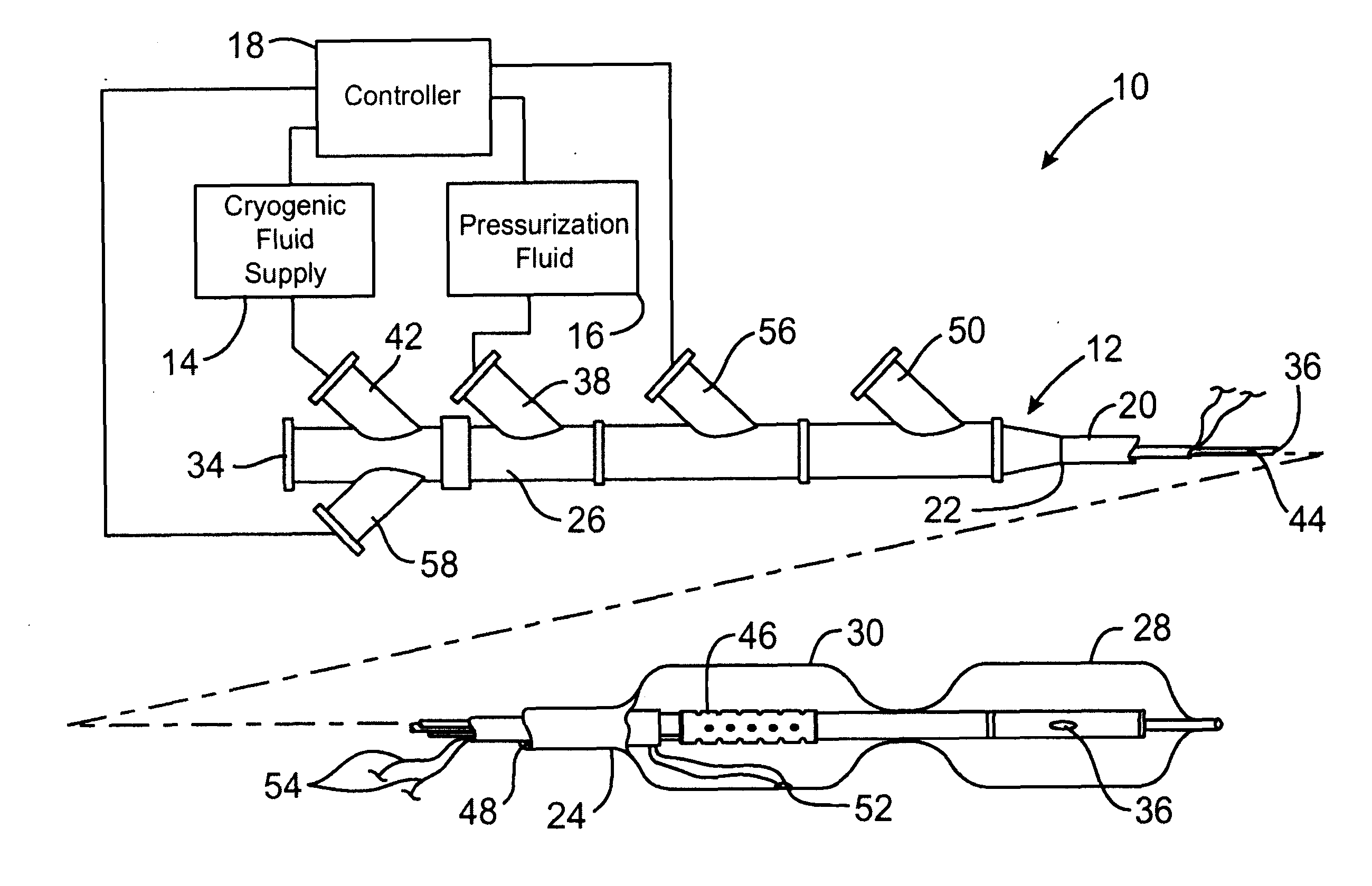
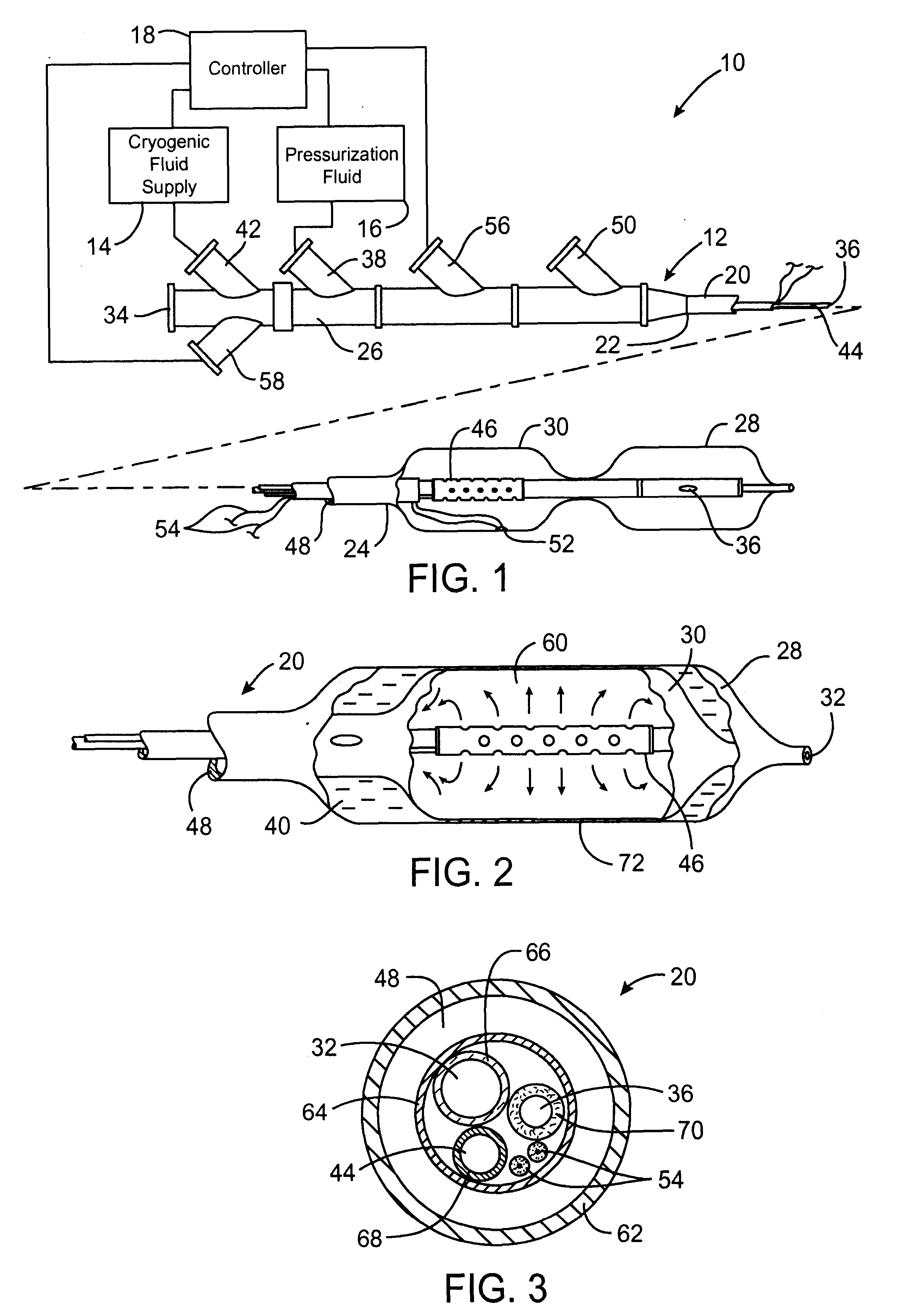
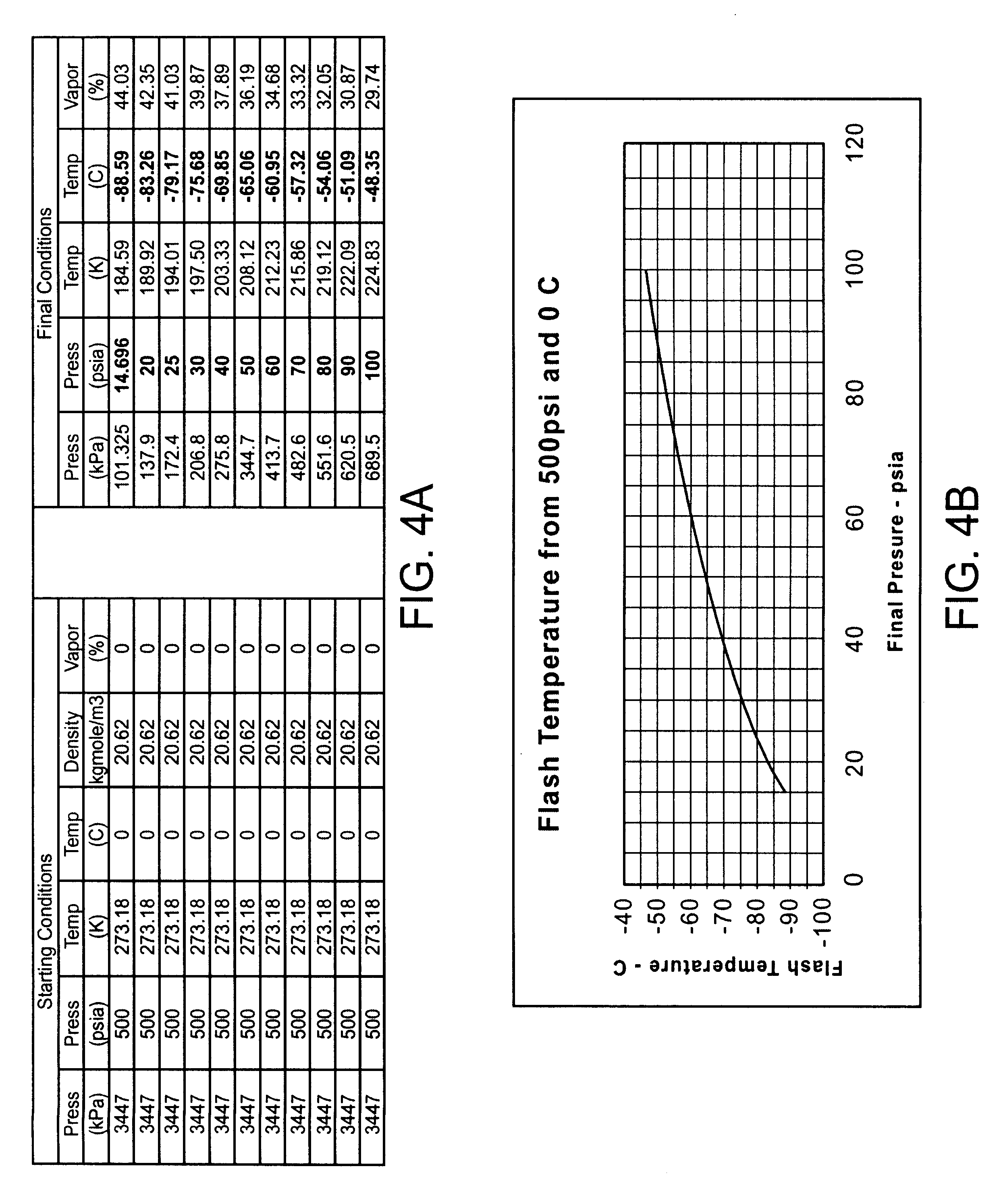
InactiveUS6468297B1Less stenosisLowering indexCatheterSurgical instruments for coolingPercent Diameter StenosisPercutaneous angioplasty
Techniques and devices for treating atherosclerotic disease use controlled cryogenic cooling, often in combination with angioplasty and / or stenting. A combination cryogenic / angioplasty catheter may cool the diseased blood vessel before, during, and / or after dilation. Controlled cooling of the vessel wall reduces actual / observed hyperplasia as compared to conventional uncooled angioplasty. Similar reductions in restenosis may be provided for other primary treatments of the blood vessel, including directional arthrectomy, rotational arthrectomy, laser angioplasty, stenting, and the like. Cooling of vessel wall tissues will often be performed through plaque, and the cooling process will preferably take the thermodynamic effects of the plaque into account.
Owner:BOSTON SCI SCIMED INC
Artificial valve prosthesis with improved flow dynamics
ActiveUS7618447B2Easy to removeMore turbulent flowVenous valvesBlood vesselsVenous ValvesProsthetic valve
An expandable venous valve having a support structure that configured to enlarge the area adjacent to the valve structure such that the flow patterns of retrograde flow are modified in a way that facilitates the flushing of the pockets at the base of the valve area to prevent stagnation of bodily fluid, which in the venous system, can lead to thrombus formation. The enlarged pocket areas can be created by forming an artificial sinus adjacent the valve structure in an unsupported section of vessel wall between two support frame section or the support frame can comprise an expanded-diameter intermediate or proximal section that forms an artificial sinus adjacent the valve structure. In another group of embodiments, the attachment pathway between opposing leaflets and the support frame and / or vessel wall comprises a proximal portion that places the leaflets in extended contact with one another and a distal portion forms a large angle with respect to the adjacent walls such that a large pocket is created at the base of the leaflets. In one embodiment, the attachment pathway extends distally along a pair of substantially parallel longitudinal attachment struts to create an extended leaflet contact area, then angles circumferentially and distally from the former along distal attachment struts to define the bottom edge of the leaflets.
Owner:COOK MEDICAL TECH LLC
Medical apparatus system having optical fiber load sensing capability
ActiveUS20070060847A1Reduces sensor artifactReduction factorStrain gaugePerson identificationLoad sensingEngineering
Apparatus is provided for diagnosing or treating an organ or vessel, wherein a device having at least two optical fiber sensors disposed in a distal extremity thereof is coupled to processing logic programmed to compute a multi-dimensional force vector responsive to detected changes in the optical characteristics of the optical fiber sensors arising from deflection of the distal extremity resulting from contact with the tissue of the wall of the organ or vessel. The force vector may be used to facilitate manipulation of the catheter either directly or automatically using a robotic system.
Owner:ST JUDE MEDICAL INT HLDG SARL
Anastomosis system
An anastomosis system and method uses an anvil to control and support a tissue site during an anastomosis procedure. The anvil is particularly useful for supporting a wall of a coronary artery during attachment of a graft vessel to the coronary artery because the wall of the coronary artery is very thin, difficult to grasp, and susceptible to tearing. In one method, the anvil is inserted into a pressurized or unpressurized target vessel and is pulled against an inner wall of the target vessel causing tenting of the thin tissue of the vessel wall. A graft vessel is then advanced to the anastomosis site and an end of the graft vessel is positioned adjacent and exterior of the target vessel. Staples are inserted through the tissue of the graft vessel and the target vessel by pivoting the arms of a staple holder towards the anvil. When the ends of the staples engage staple bending features on the anvil, the ends of the staples bend over securing the graft vessel and target vessel together. After stapling is complete, an incision is formed in the wall of the target vessel to allow blood flow between the target vessel and the graft vessel.
Owner:AESCULAP AG
Methods and apparatus for localized and semi-localized drug delivery
InactiveUS20050059931A1Turn easilyEasy to navigateStentsBalloon catheterVariable stiffnessThree vessels
A catheter system for localized or semi-localized administration of agents through the wall of a blood vessel is provided. Various catheter system constructions which use at least one expandable occluding device to create an isolated region are provided. Constructions using one catheter and one occlusion device are provided, along with constructions using two catheters and multiple occlusion devices. The catheter system may include a catheter with a variable stiffness along its length. The catheter system may also include a guide wire integrated with an inner catheter. The catheter can infuse the agent into the blood vessel in a pressure regulated manner. Methods for delivery and infusion of the agent within a blood vessel are also provided.
Owner:VENOMATRIX
Double-walled stent system
A double walled stent system particularly suited for treating abnormalities of the right ventricular outflow tract is disclosed having an exterior stent component and an interior stent component. The exterior stent component is secured to the interior stent component in a non-fixed, sliding relationship. The exterior stent component includes a plurality of longitudinally-extending connectors such as straight or sinusoidal bands. The interior stent component has a generally tubular cylindrical body and is centered within the exterior stent component. The stent system has a contracted delivery configuration and a radially expanded configuration for contacting the vessel wall. When deployed, the longitudinally-extending connectors of the exterior stent component come in contact with the vessel wall and fix the stent system to the treatment site. The interior stent component also radially expands but remains centered inside the exterior component and makes little to no contact with the vessel wall.
Owner:MEDTRONIC VASCULAR INC
Devices, systems, and methods for endovascular staple and/or prosthesis delivery and implantation
InactiveUS20090099650A1Reinforce dissectionPromote repairStentsSurgical furnitureProsthesisImplantation Site
Devices, systems, and methods for implanting expandable prostheses in the body lumens rely on stapling or anchoring the prostheses with separately introduced fasteners. The prostheses may be self-expanding or balloon expandable, and may include a single lumen or more than one lumen. After initial placement, a stapling system is introduced within the expanded prosthesis to deploy a plurality of fasteners to at least one prosthesis end. The stapling system may apply a force to the prosthesis to modify the shape of the prosthesis to conform to the shape of the vessel wall. The stapling system can be deflected in one or more distinct steerable segments. A lumen extension or lumens may be coupled to the prosthesis to extend the reach of the prosthesis within the implantation site. Fasteners may also be applied to the lumen extensions.
Owner:MEDTRONIC VASCULAR INC
Clip applier and methods of use
InactiveUS20060020270A1Precise positioningGood hemostasisDiagnosticsStaplesBiomedical engineeringBlood vessel
An apparatus for delivering a closure element into an opening formed in a blood vessel or other body lumen and methods for manufacturing and using same. The apparatus is configured to retain the closure element such that the closure element is disposed substantially within the apparatus. The apparatus also can engage, and position the closure element substantially adjacent to, the blood vessel wall adjacent to the opening. During deployment of the closure element, the apparatus expands the closure element beyond a natural cross-section of the closure element such that the closure element, when deployed, is configured to engage a significant amount of the blood vessel wall and / or tissue. Engaging the blood vessel wall and / or tissue, the closure element is further configured to return to the natural cross-section, thereby drawing the engaged blood vessel wall and / or tissue substantially closed and / or sealed, such that hemostasis within the opening is enhanced.
Owner:INTEGRATED VASCULAR SYST
Embolic filters with controlled pore size
The invention provides a device for filtering emboli from blood flowing through a lumen defined by the walls of a vessel in a patient's body comprising a filter element. The filter is expandable from a collapsed configuration when the filter element is restrained to an expanded configuration when the filter element is unrestrained, and the filter element comprises a self-expanding material having pores. When the filter element is in the expanded configuration, the average pore size is from 30 to 300 microns and the standard deviation of the pore size is less than 20 percent of the average pore size.
Owner:COVIDIEN LP
Wound closure and sealing device
InactiveUS20060173492A1Prevented from appearingStops orSuture equipmentsSurgical needlesEngineeringBlood vessel
Owner:RADI MEDICAL SYST
Infusion catheter having an atraumatic tip
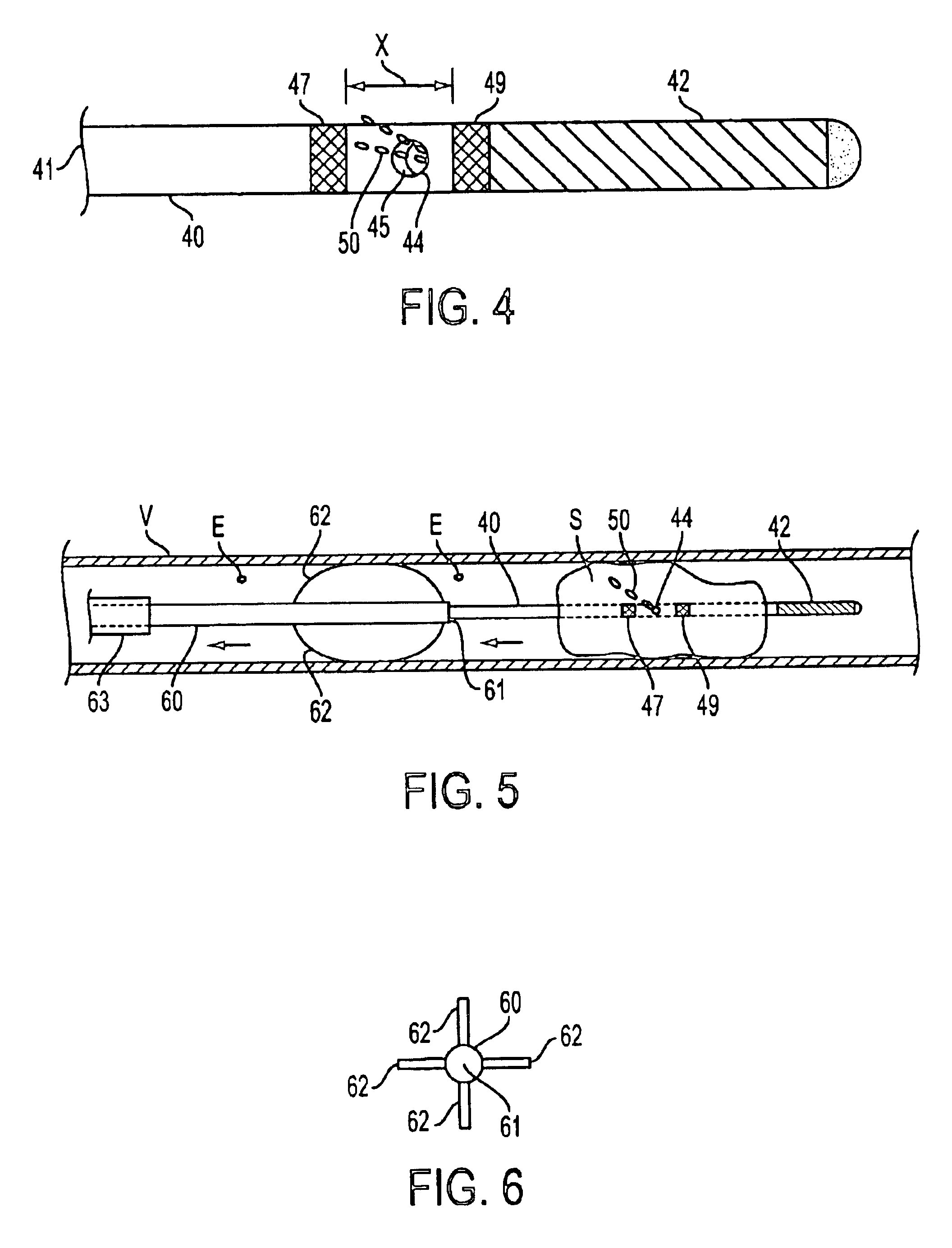
The present invention is directed to apparatus and methods for treating a vascular occlusion by providing an infusion catheter having an atraumatic tip and at least one delivery port configured to infuse fluid into the occlusion. The fluid that is infused dilutes the occlusion and reduces adhesion of the occlusion to an intima of the vessel wall, thereby causing the occlusion to dislodge. Emboli generated in the process are directed into an emboli removal catheter for removal.
Owner:WL GORE & ASSOC INC
Vascular closure methods and apparatuses
InactiveUS20070060895A1Prevent intravascular migrationKeep woundInfusion syringesIntravenous devicesVascular closure deviceVascular device
A vascular closure device comprised of a sheath-delivered expandable, umbrella-like device with structural radial members with terminal and non-terminal hooks that engage the vessel wall. Unlike other vascular closure umbrella-type devices that effect closure by opening of the umbrella to cover an opening, the present invention effects closure of the aperture with closure of the umbrella. The closure can be maintained by a retainer lock that slides down the members, causing contraction, bringing the members into a compressed configuration (e.g., a parallel orientation of linear members) and the wound edges together, permitting immediate vascular closure and healing of the blood vessel. The device can be delivered and recovered by an intravascular sheath.
Owner:ABBOTT CARDIOVASCULAR
Suture based vascular closure apparatus and method incorporating a pre-tied knot
InactiveUS20050121042A1Precise positioningSuture equipmentsSurgical needlesVascular closure deviceVascular device
A pre-tied knot is used in conjunction with a vascular closure device to approximate tissue surrounding an opening in a corporeal vessel. The pre-tied knot is positioned on a proximal end of the suture such that a distal end of the suture can be inserted through the pre-tied knot to complete the knot. A typical medical procedure as contemplated by the present invention is performed through a sheath inserted through an opening in the vessel wall to access the inside of the vessel. The device used to perform the medical procedure is then removed from the sheath and a vascular closure device is inserted through the sheath to position a suture across the vessel opening. The pre-tied knot assists in approximating tissue surrounding the vessel opening.
Owner:ST JUDE MEDICAL PUERTO RICO BV
Cryotreatment device and method
InactiveUS7220257B1Reduce adverse reactionsReduced responseStentsOther blood circulation devicesCoronary artery angioplastyPercent Diameter Stenosis
Devices and methods for cooling vessel walls to inhibit restenosis in conjunction with medical procedures such as coronary artery angioplasty. Stenosed vessel walls can be cooled prior to angioplasty, after angioplasty, or both. The invention is believed to inhibit restenosis through cooling to a temperature near freezing, preferably without causing substantial vessel wall cell death. One catheter device includes a distal tube region having coolant delivery holes radially and longitudinally distributed along the distal region. In some devices, holes spray coolant directly onto the vessel walls, with the coolant absorbed into the blood stream. In other embodiments, a balloon or envelope is interposed between the coolant and the vessel walls and the coolant returned out of the catheter through a coolant return lumen. Some direct spray devices include an occlusion device to restrict blood flow past the region being cooled. Pressure, temperature, and ultrasonic probes are included in some cooling catheters. Pressure control valves are included in some devices to regulate balloon interior pressure within acceptable limits. In applications using liquid carbon dioxide as coolant, the balloon interior pressure can be maintained above the triple point of carbon dioxide to inhibit dry ice formation. Some cooling catheters are coiled perfusion catheters supporting longer cooling periods by allowing perfusing blood flow simultaneously with vessel wall cooling. One coiled catheter is biased to assume a coiled shape when unconstrained and can be introduced into the body in a relatively straight shape, having a stiffening wire inserted through the coil strands.
Owner:BOSTON SCI SCIMED INC
Stent graft with improved proximal end
Disclosed is a stent graft prosthesis comprising a graft portion that includes a main body portion and an cuff portion, the cuff portion generally located at or near the proximal end of the main body portion and extending circumferentially therealong. Stents comprising the graft supporting structure are also attached to graft portion about the proximal end. In one embodiment, the cuff portion comprises material that is folded over the outside surface of the main body portion with an anchoring stent being attached over the cuff and main body portions, extending proximally therefrom. In another series of embodiments, the cuff portion comprises an external sealing zone that extends around the outer main body portion to help prevent leakage of fluids. In one example, the material of the second edge of the cuff portion is frayed to better engage the vessel walls and promote thrombus and / or tissue growth.
Owner:WILLIAM A COOK AUSTRALIA +1
Artificial Valve Prosthesis with Improved Flow Dynamics
ActiveUS20070260327A1Easy to removePrevent stagnantVenous valvesBlood vesselsProsthetic valveVenous Valves
An expandable venous valve having a support structure that configured to enlarge the area adjacent to the valve structure such that the flow patterns of retrograde flow are modified in a way that facilitates the flushing of the pockets at the base of the valve area to prevent stagnation of bodily fluid, which in the venous system, can lead to thrombus formation. The enlarged pocket areas can be created by forming an artificial sinus adjacent the valve structure in an unsupported section of vessel wall between two support frame section or the support frame can comprise an expanded-diameter intermediate or proximal section that forms an artificial sinus adjacent the valve structure. In another group of embodiments, the attachment pathway between opposing leaflets and the support frame and / or vessel wall comprises a proximal portion that places the leaflets in extended contact with one another and a distal portion forms a large angle with respect to the adjacent walls such that a large pocket is created at the base of the leaflets. In one embodiment, the attachment pathway extends distally along a pair of substantially parallel longitudinal attachment struts to create an extended leaflet contact area, then angles circumferentially and distally from the former along distal attachment struts to define the bottom edge of the leaflets.
Owner:COOK MEDICAL TECH LLC
Artificial Heart Valve Stent and Weaving Method Thereof
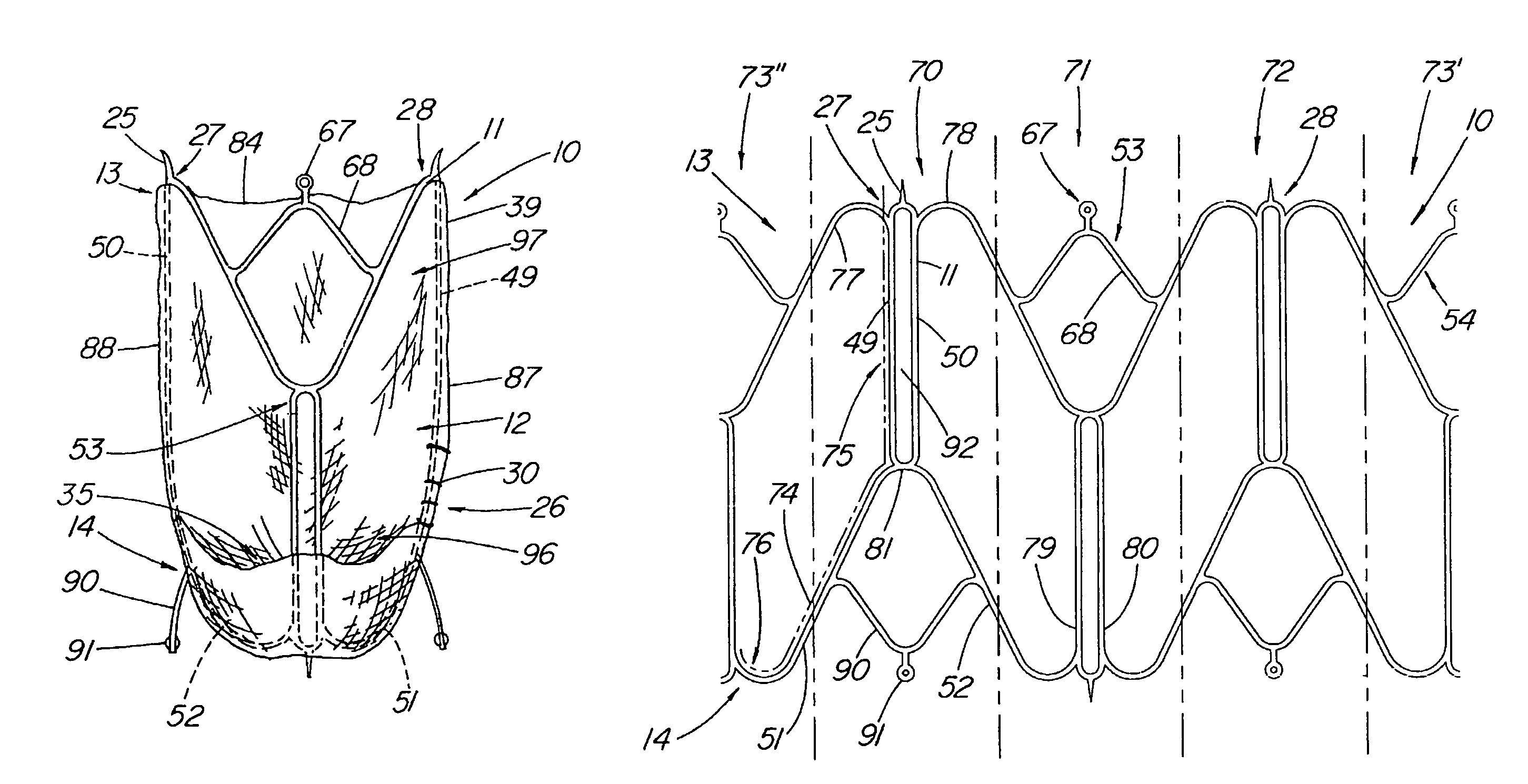
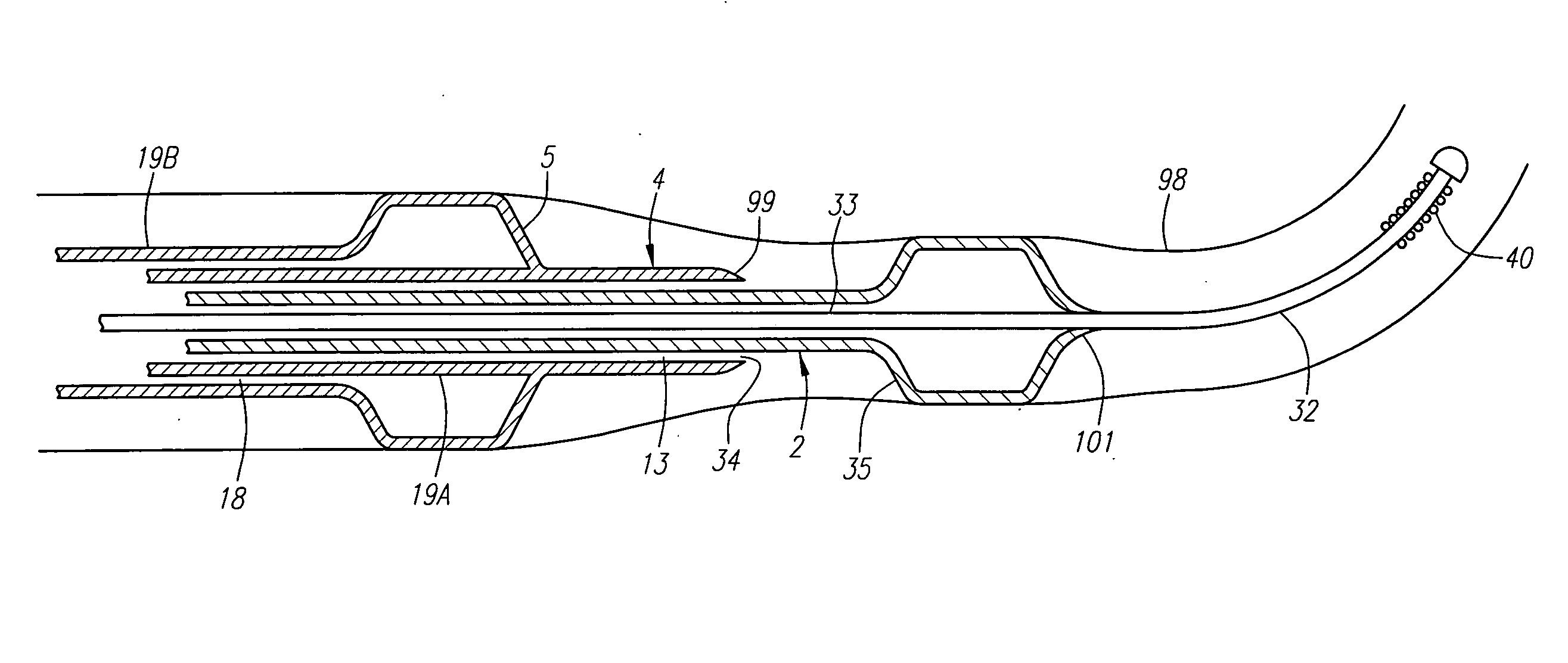
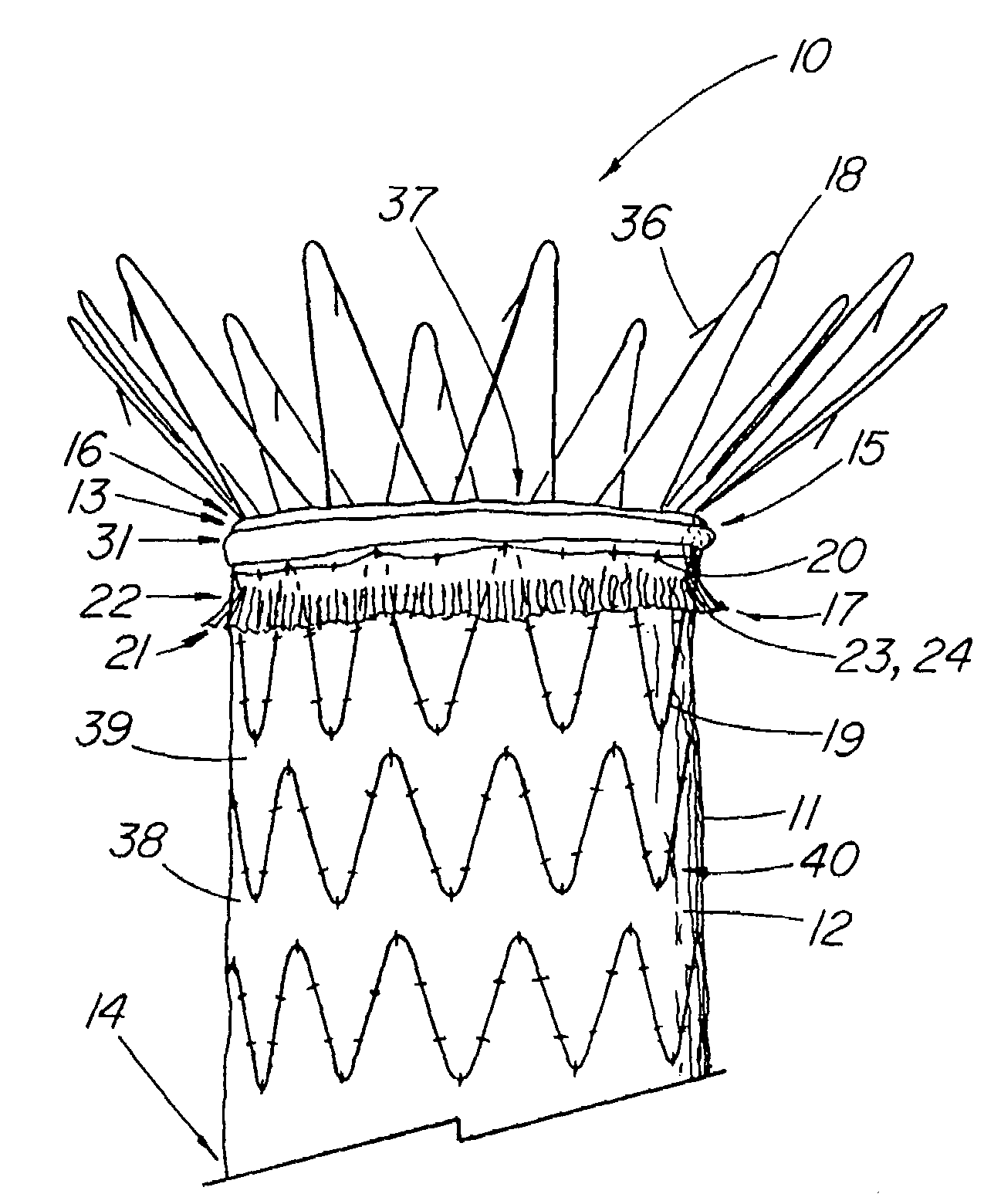
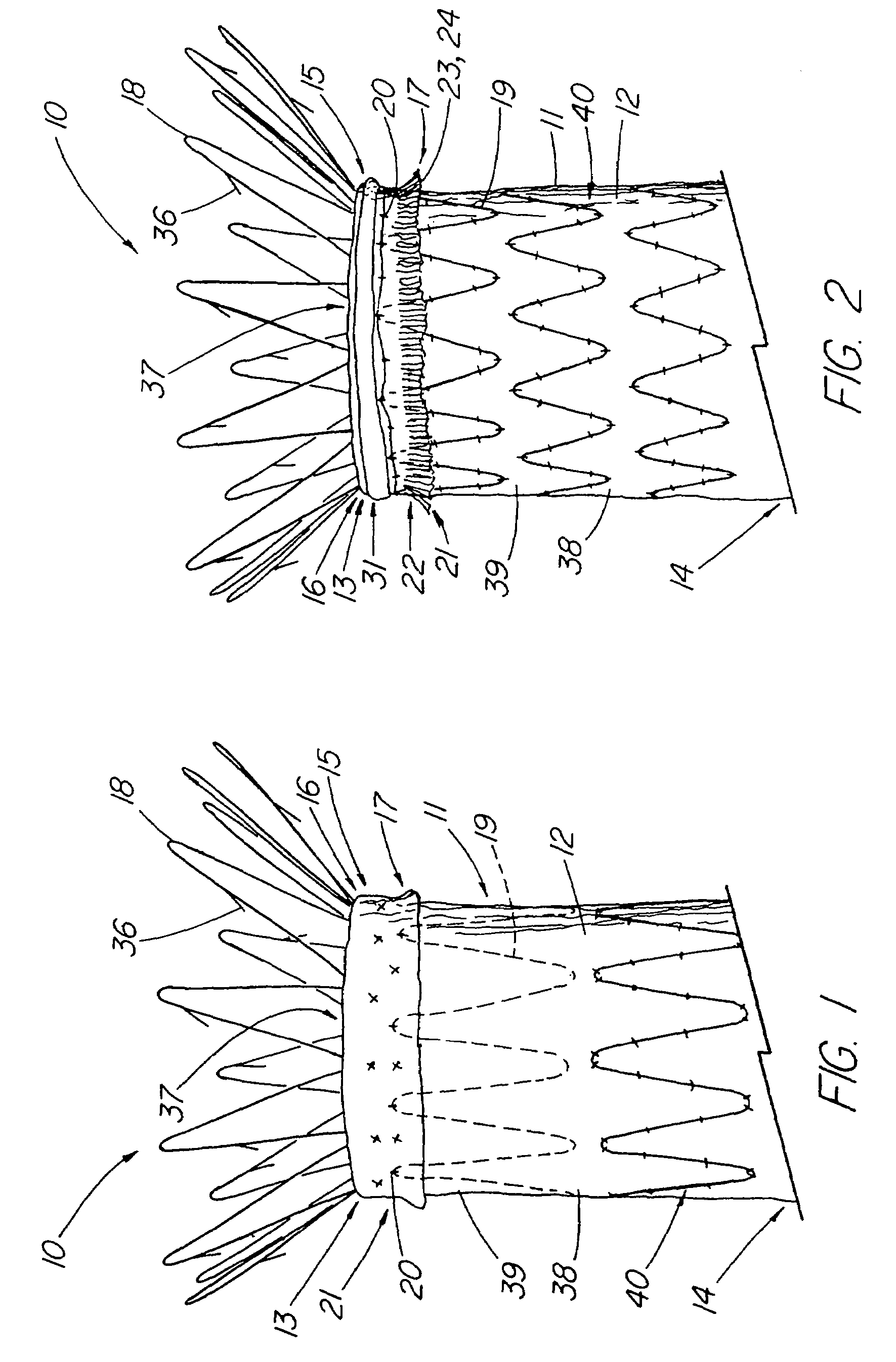
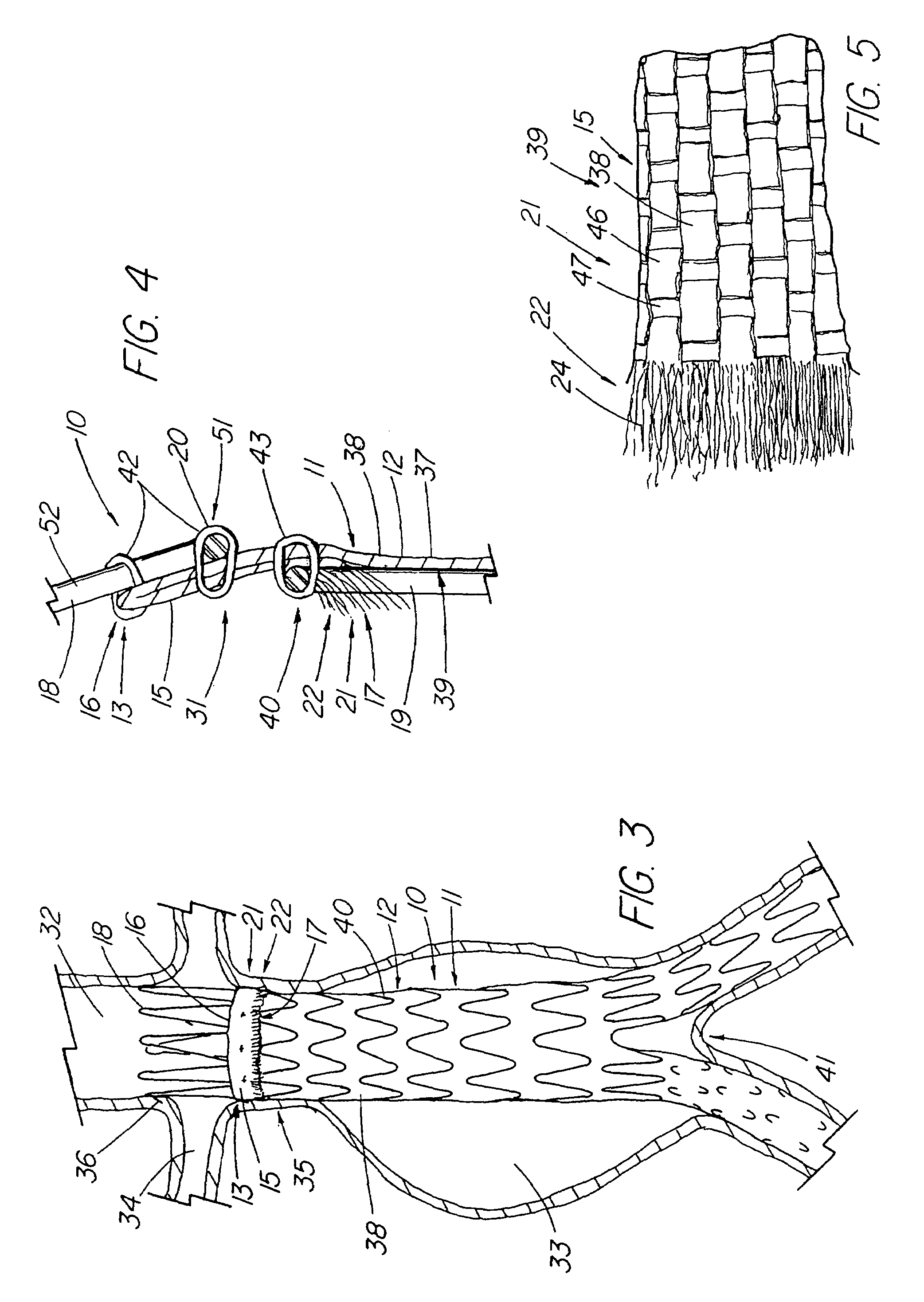
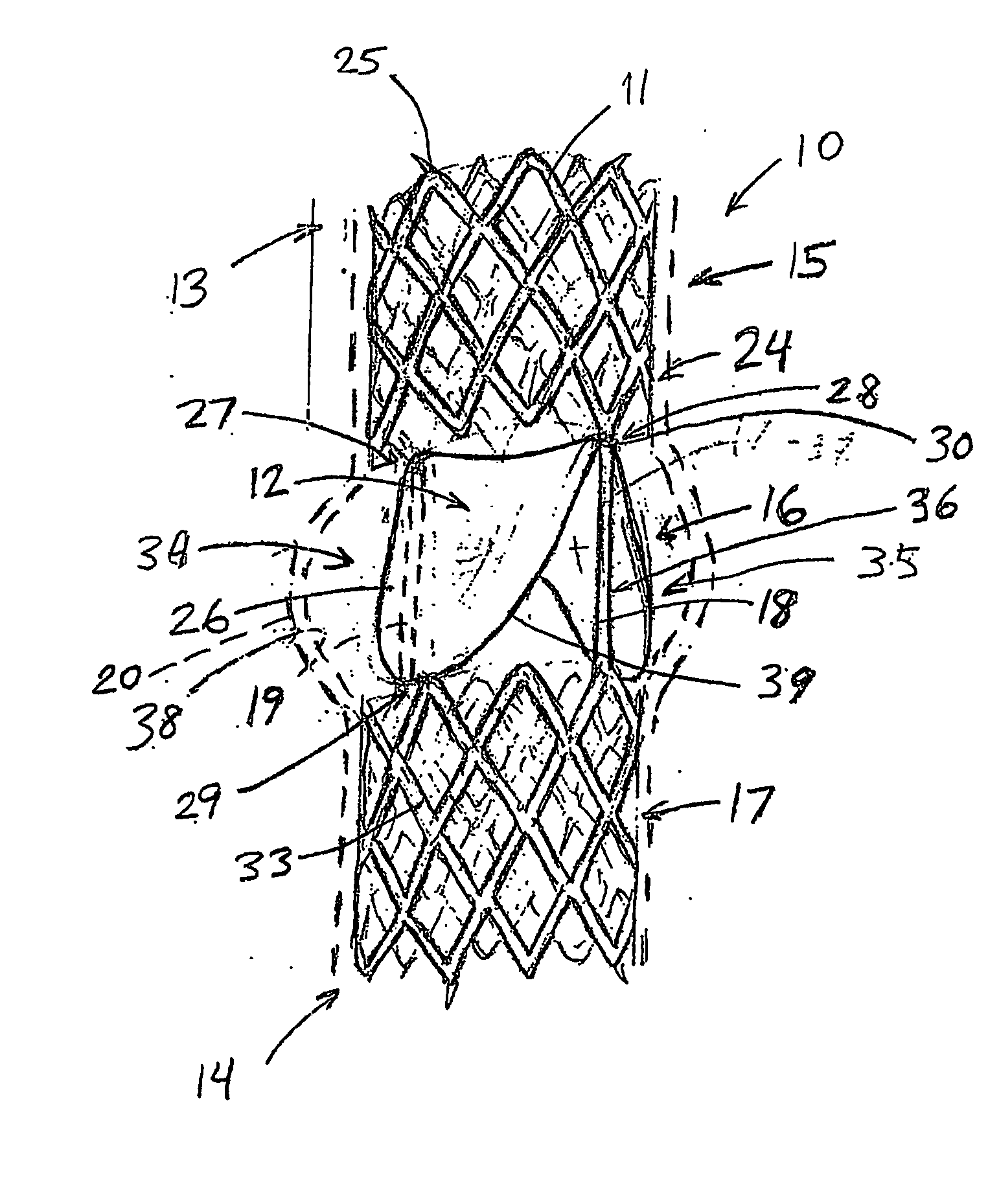
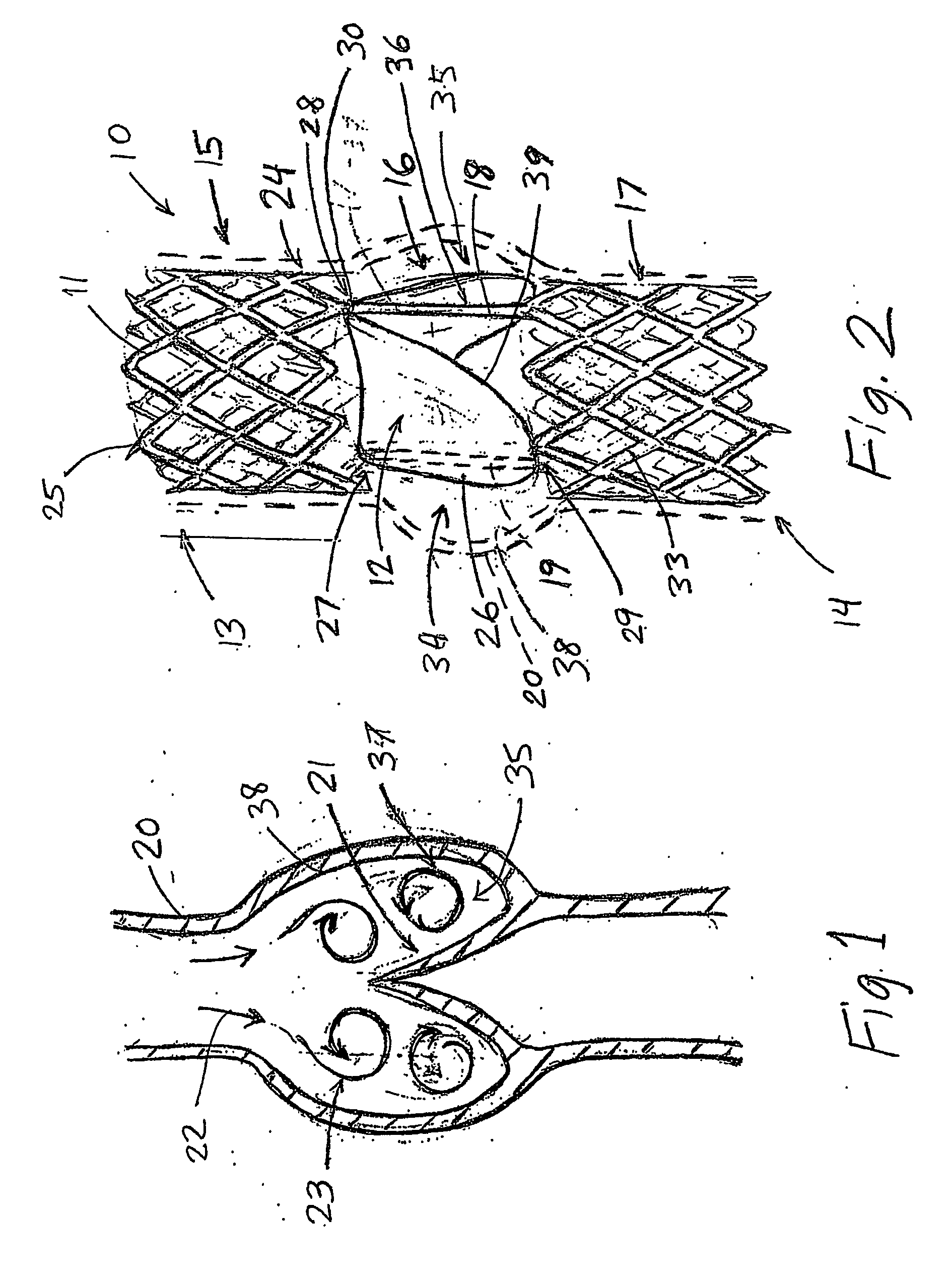
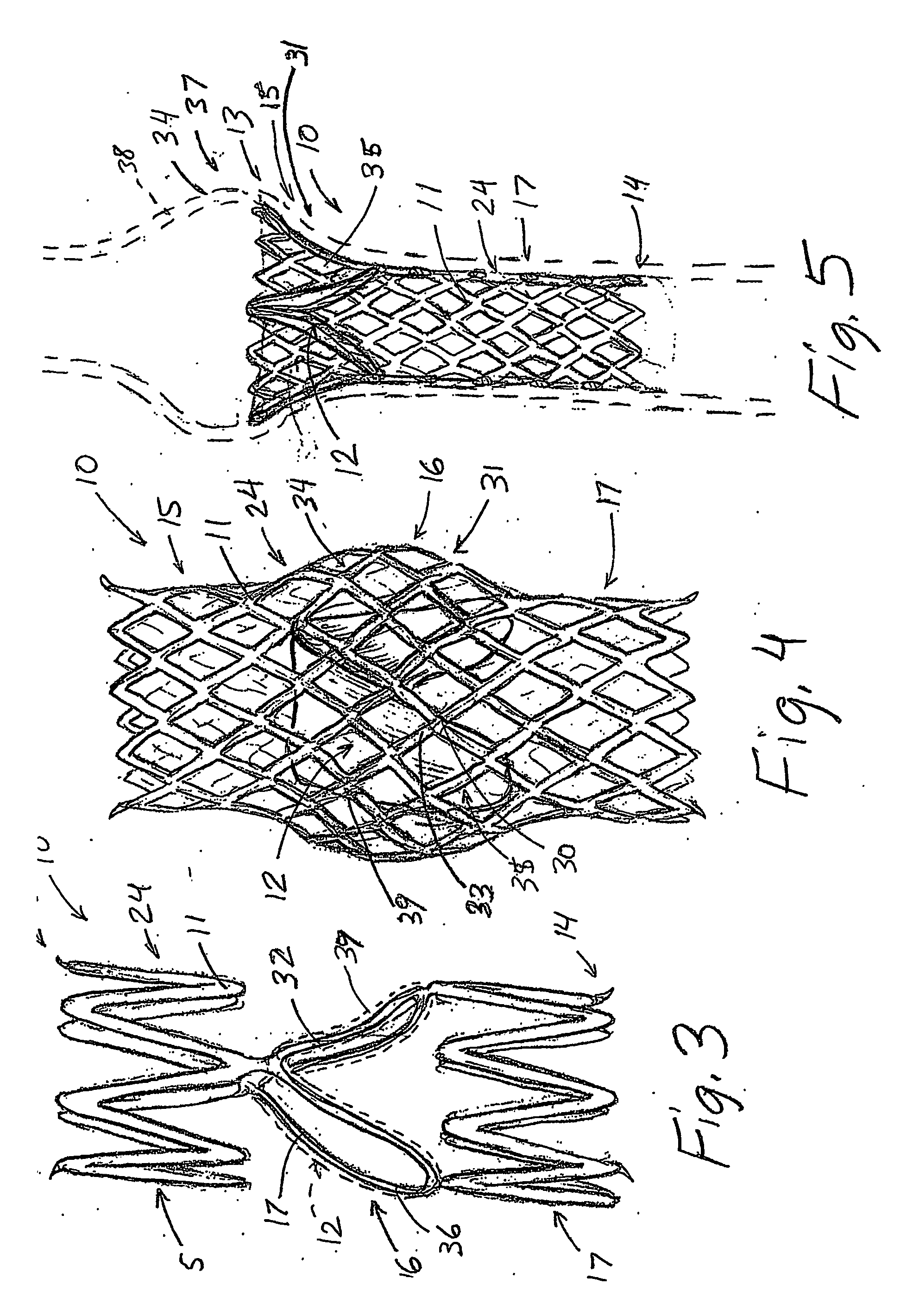
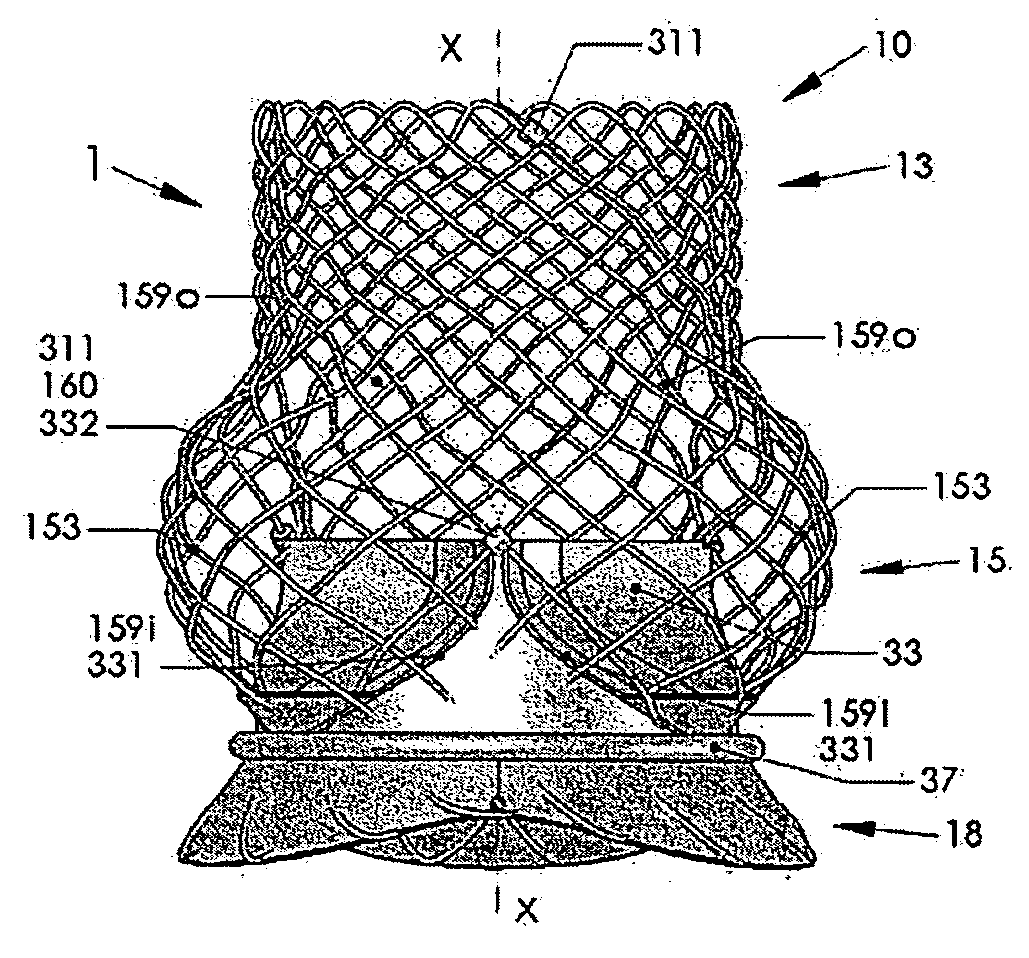
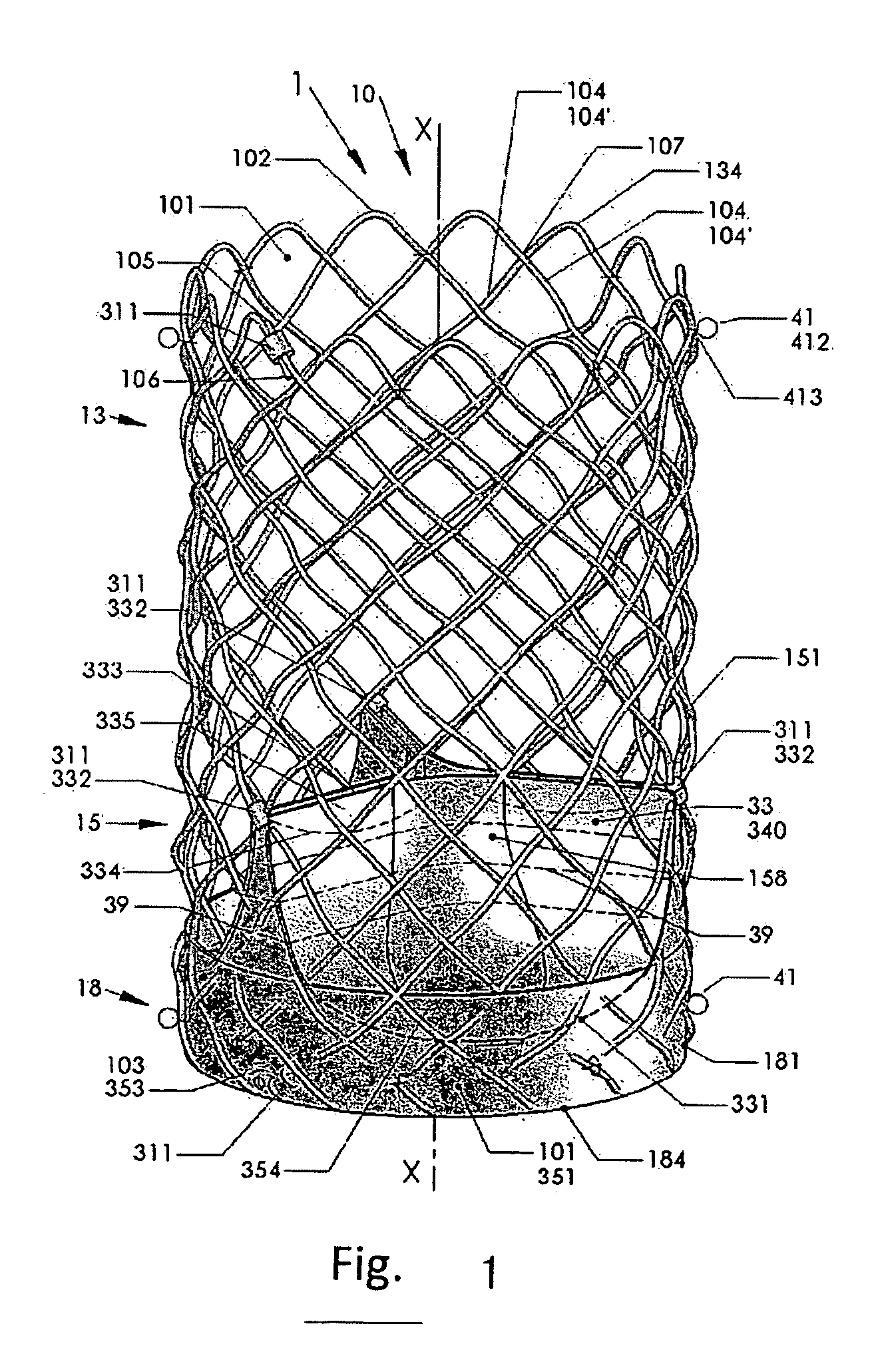
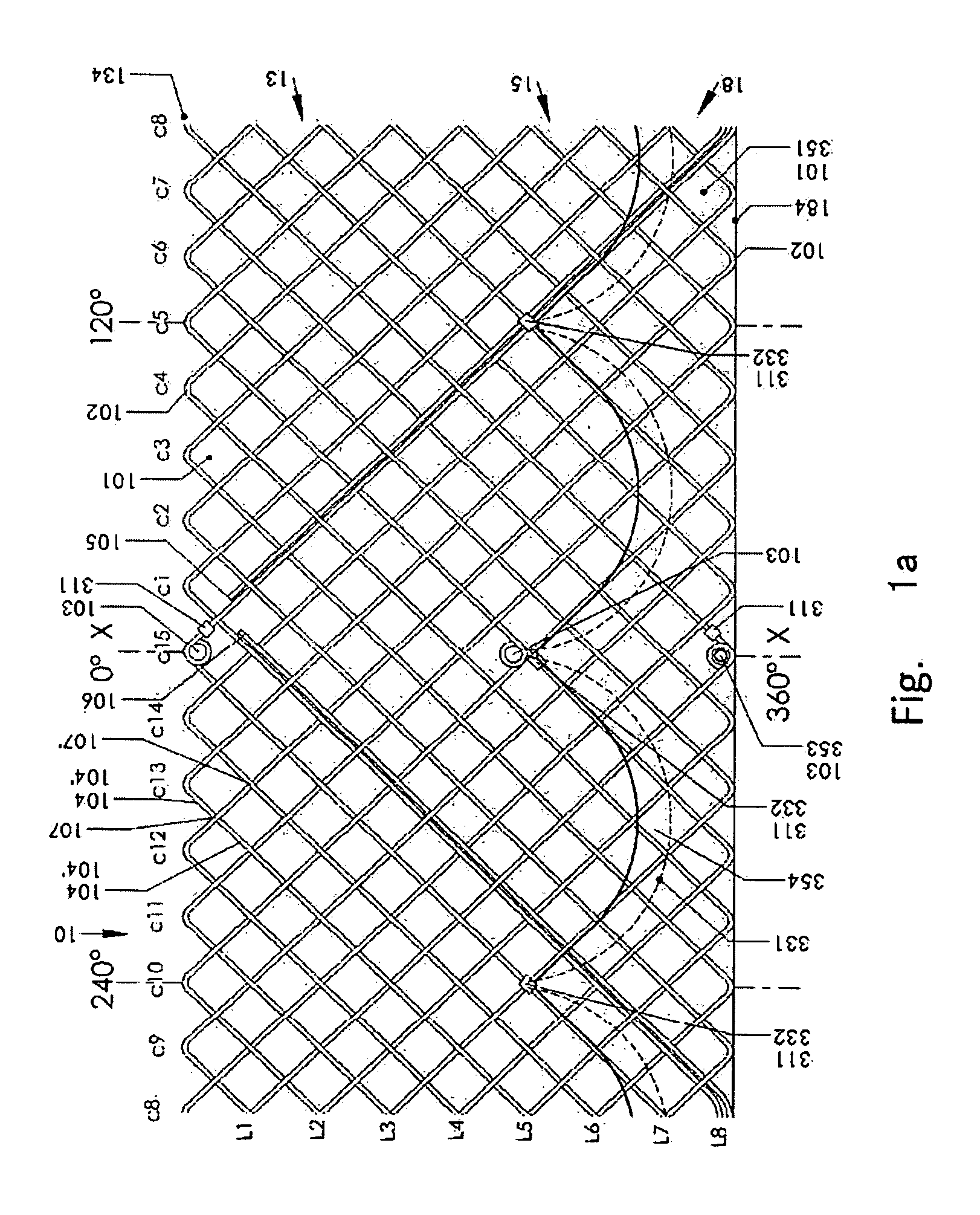
An artificial heart valve and weaving method thereof are disclosed. The valve stent includes a tubular stent (10), valve leaflets (33), sealing membranes (351, 354), x-ray opaque markers (311, 312) and flexible connecting loops (41). The middle segment (15) of the net stent (10) is tubular or drum-shaped, or provided with radial protrusion structures (153), or provided with outer annular structures (155), or provided with outer free tongues (156), or provided with radial protrusion structures (153) and outer free tongues (156). The stent can be made by up and down interweaving the same one elastic metal wire, and also can be made by up and down interweaving different elastic metal wires. Moreover, the structure, shape and function of the valve stent are optimized; in radical compression, the valve can be transported to the right place with the help of interventional device; after expansion, fitted with figure of the vascular wall in the radical and axial direction, the artificial heart valve stent will not produce paravalvular leak; even more after implanting, the valve has a normal effect on prevent the slippage of artificial valve, which is caused by the blood reflux through the closed valve.
Owner:WEN NING +1
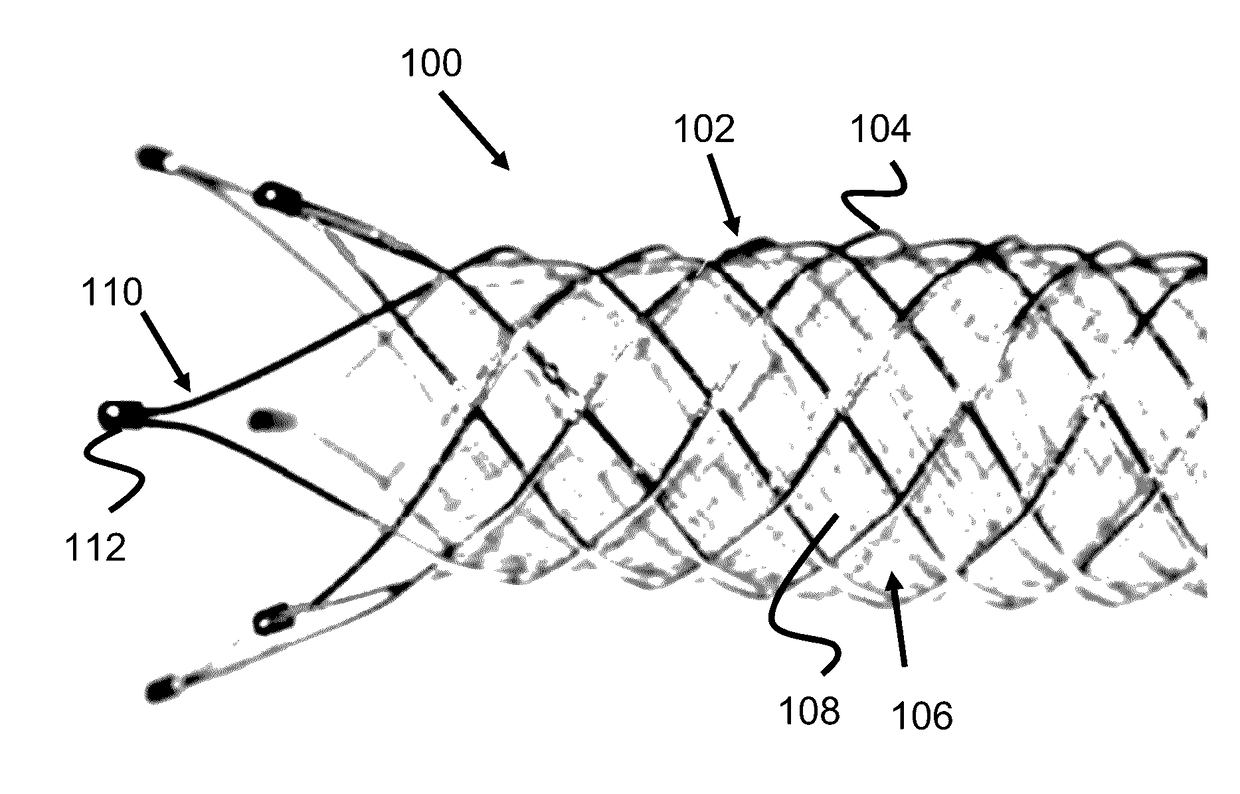
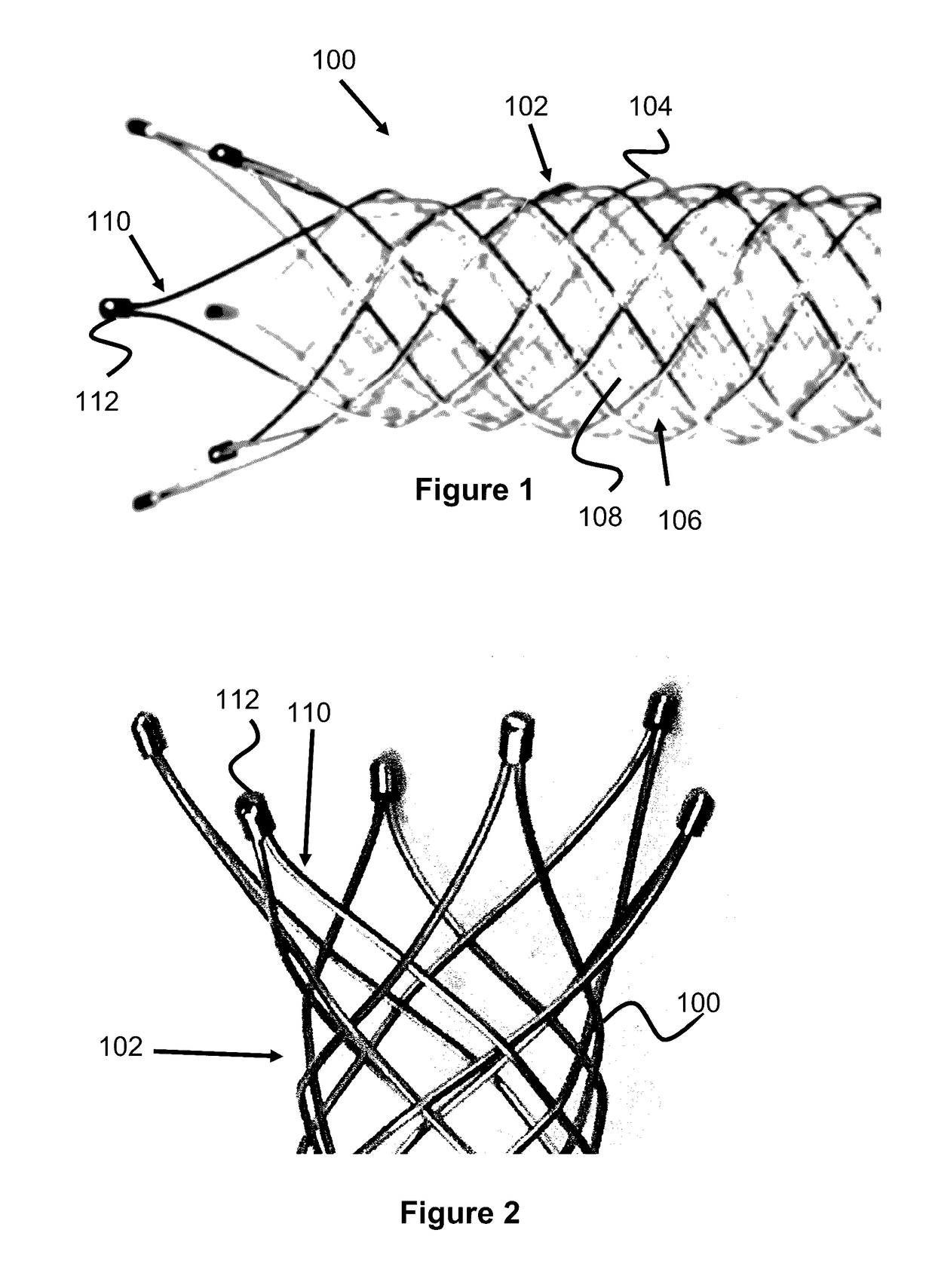
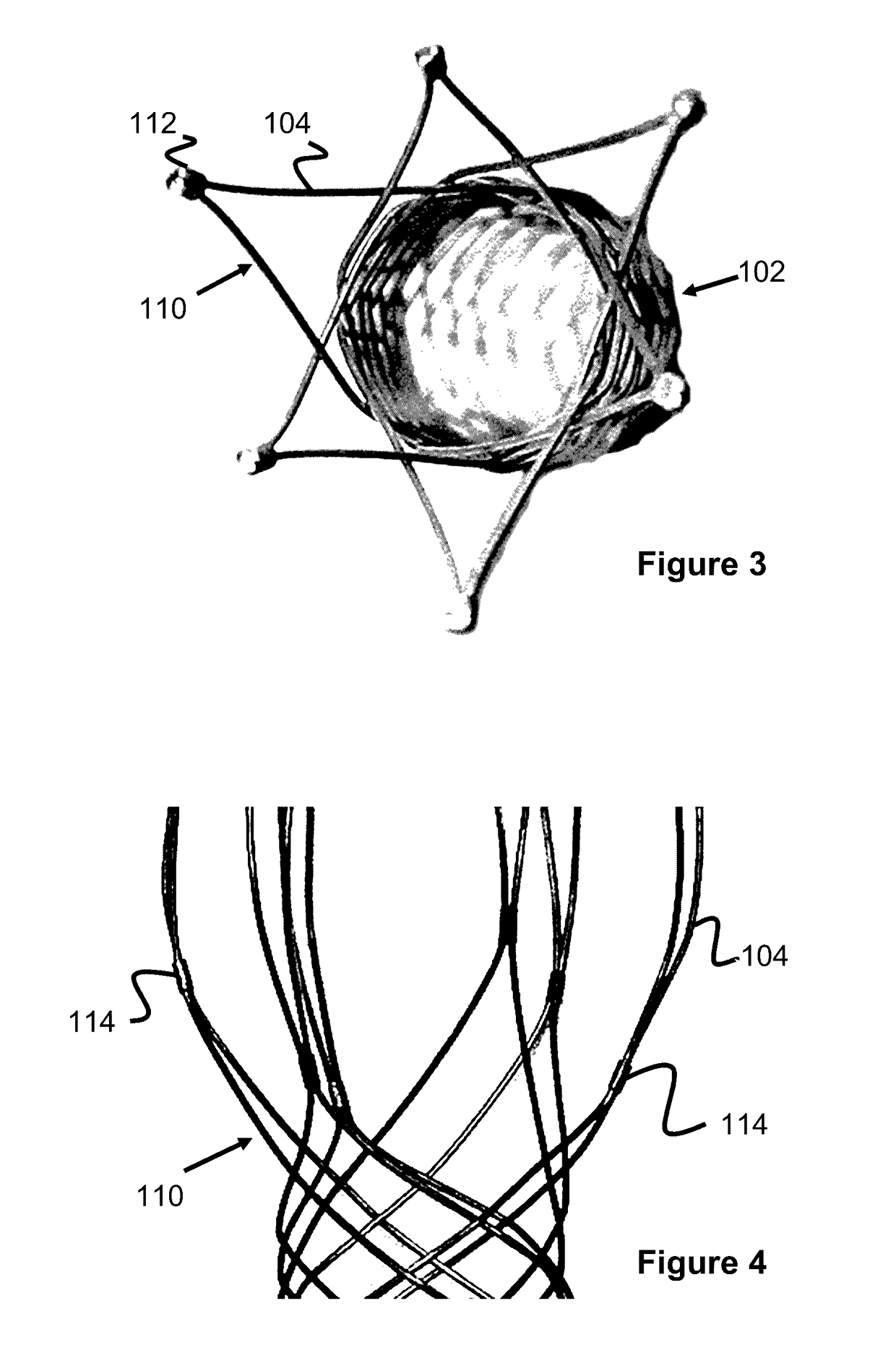
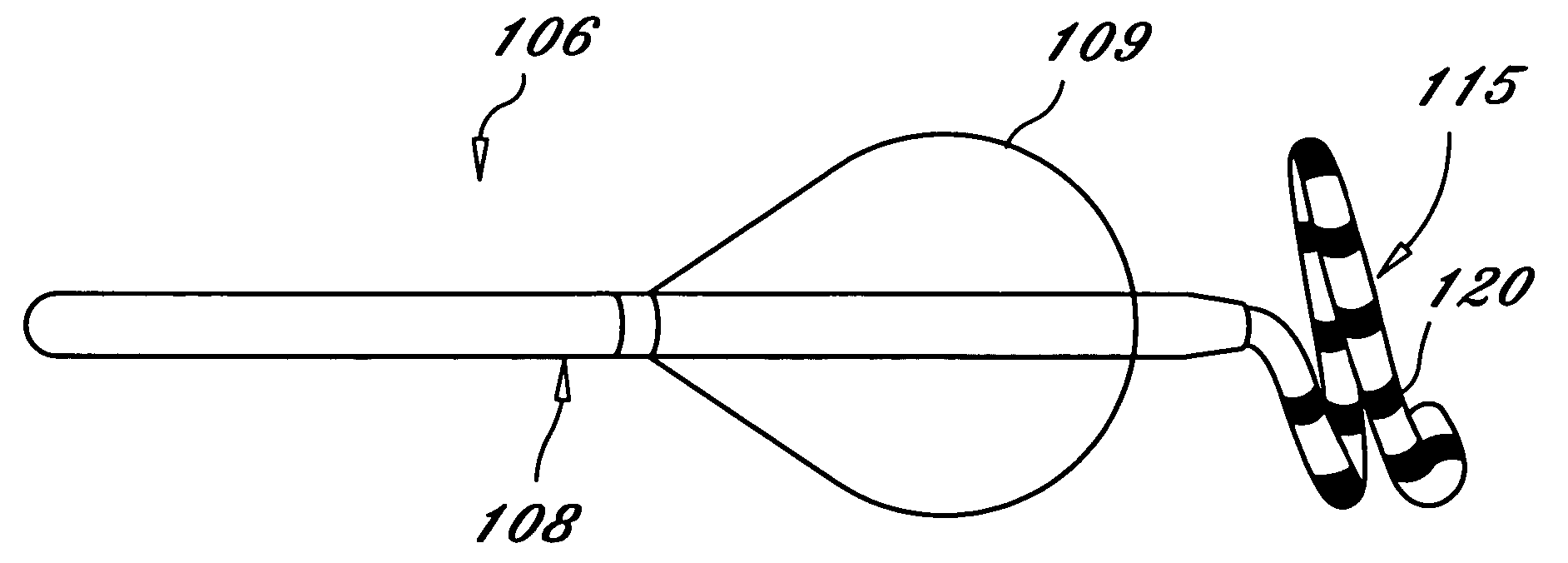
Vessel Prosthesis
ActiveUS20170079812A1Reduce or eliminate any relatively sharp or rough edgesStentsProsthesisVascular prosthesisBlood vessel prosthesis
A vascular prosthesis is described, having blunted wire ends. The blunted wire ends can take on a variety of configurations including end caps, bent ends, curved ends, or eyelets. The novel ends provide a smooth surface to contact the blood vessel walls thereby reducing the risk of trauma during placement of the prosthesis.
Owner:TERUMO KK
Tissue ablation system including guidewire with sensing element
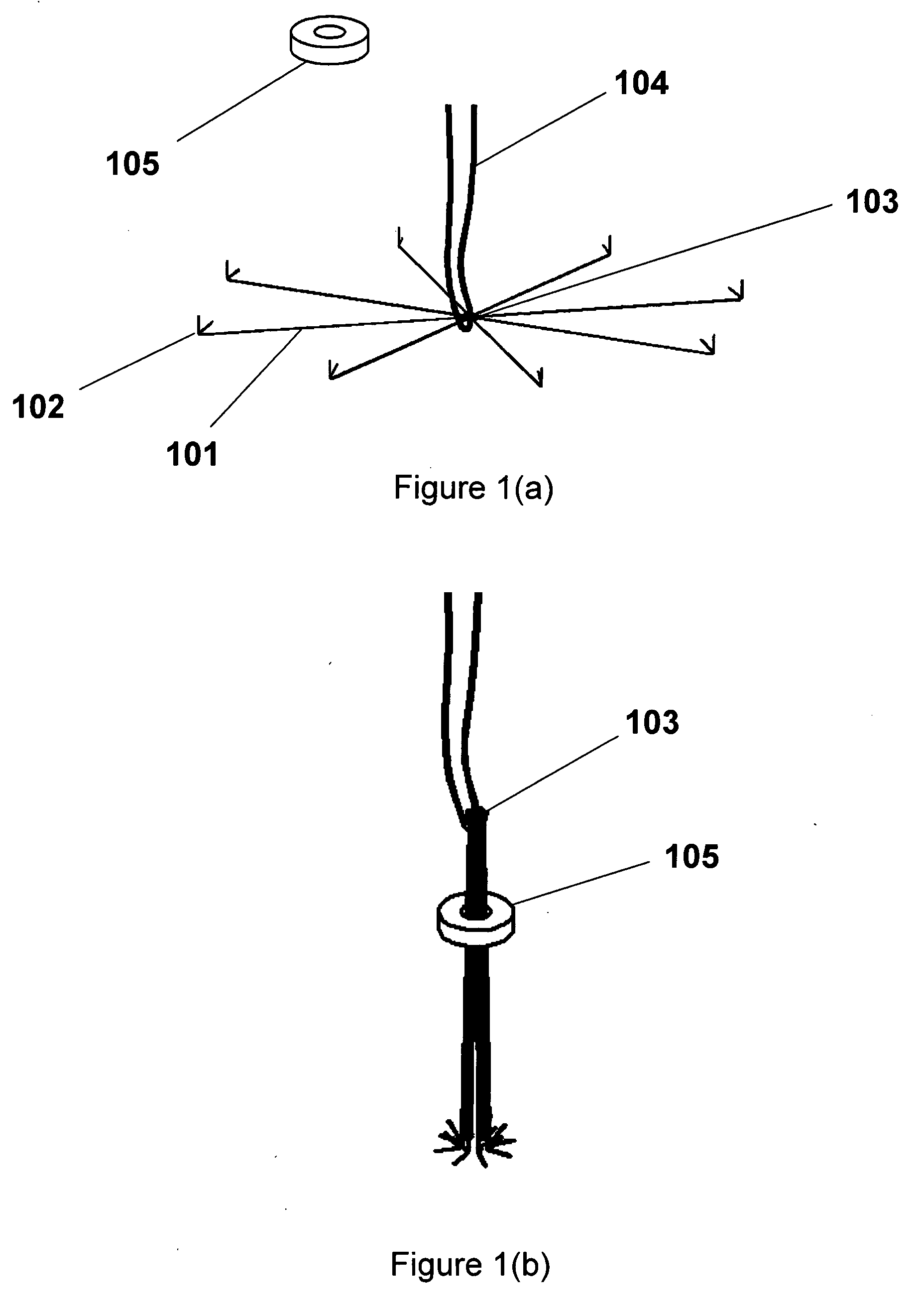
A tissue ablation system for ablating human tissue wherein sensing and ablation procedures are performed and controlled independently. A sensing wire is positioned distally to the ablation region and is adapted to pass thorough the ablation device such that it may move with or independently of the ablation device without obstructing the surface tissue interface of the ablation energy. The ablation device can ablate a substantial portion of a circumferential region of tissue, for example at or near the location where the pulmonary vein extends from the atrium. The tissue ablation system comprises an ablation device comprised of an elongated catheter with a proximal region and a distal region and an ablation element located proximate the distal region of the catheter. A sensing device having an elongated body with a proximal portion and a distal portion is adapted to be positioned within a vessel at or near a vessel ostium, wherein the sensing device is adapted to be slidably received within a lumen of the ablation device. The sensing device, a guide wire for example, may be shaped in various configurations to allow sensing device such as electrodes disposed thereon to contact the vessel wall near the ablation region. In this fashion, the sensing and ablation procedures are de-coupled such that the sensing device does not interfere or obstruct the ablation member's interface with the tissue.
Owner:MEDTRONIC CRYOCATH LP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com