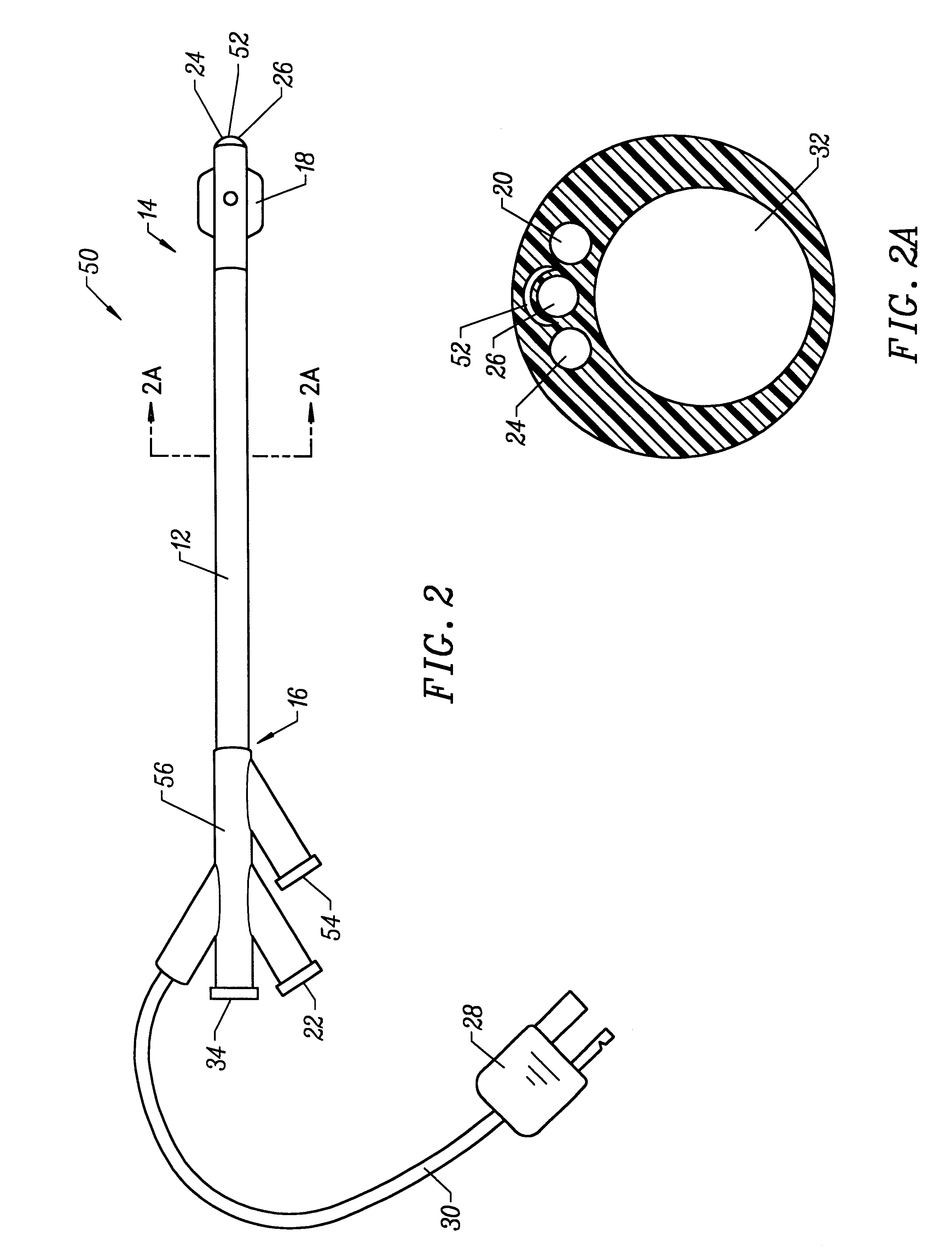
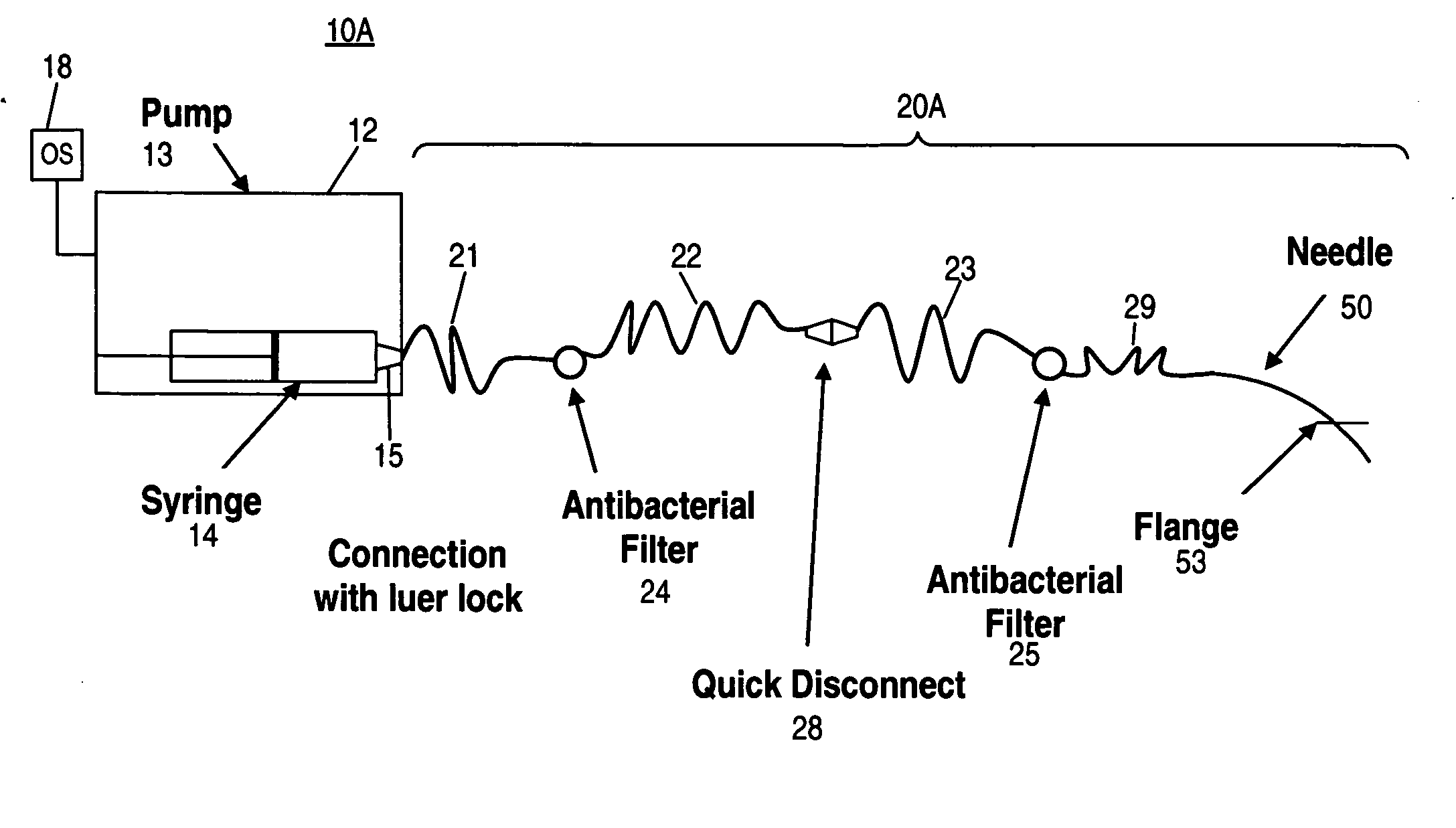
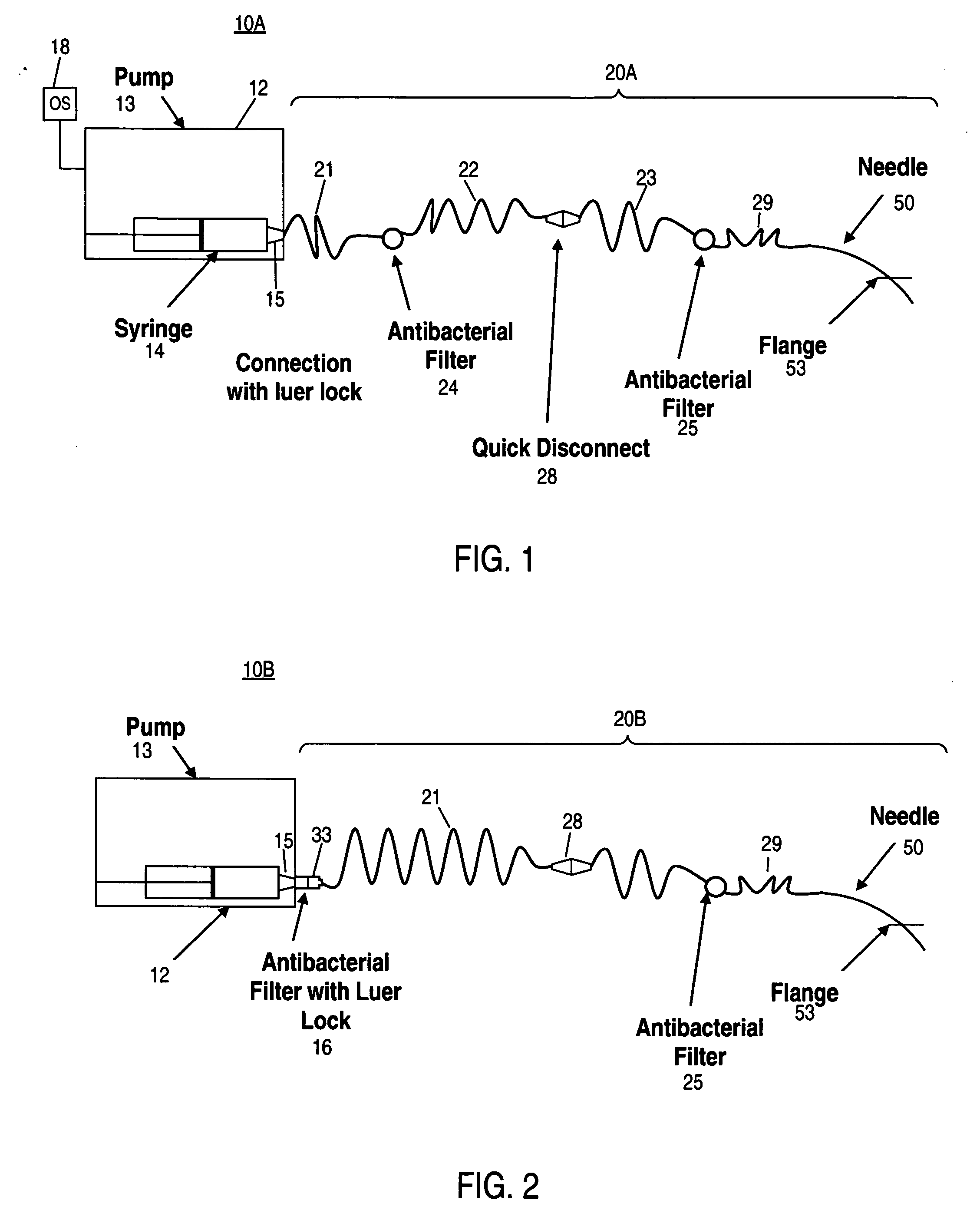
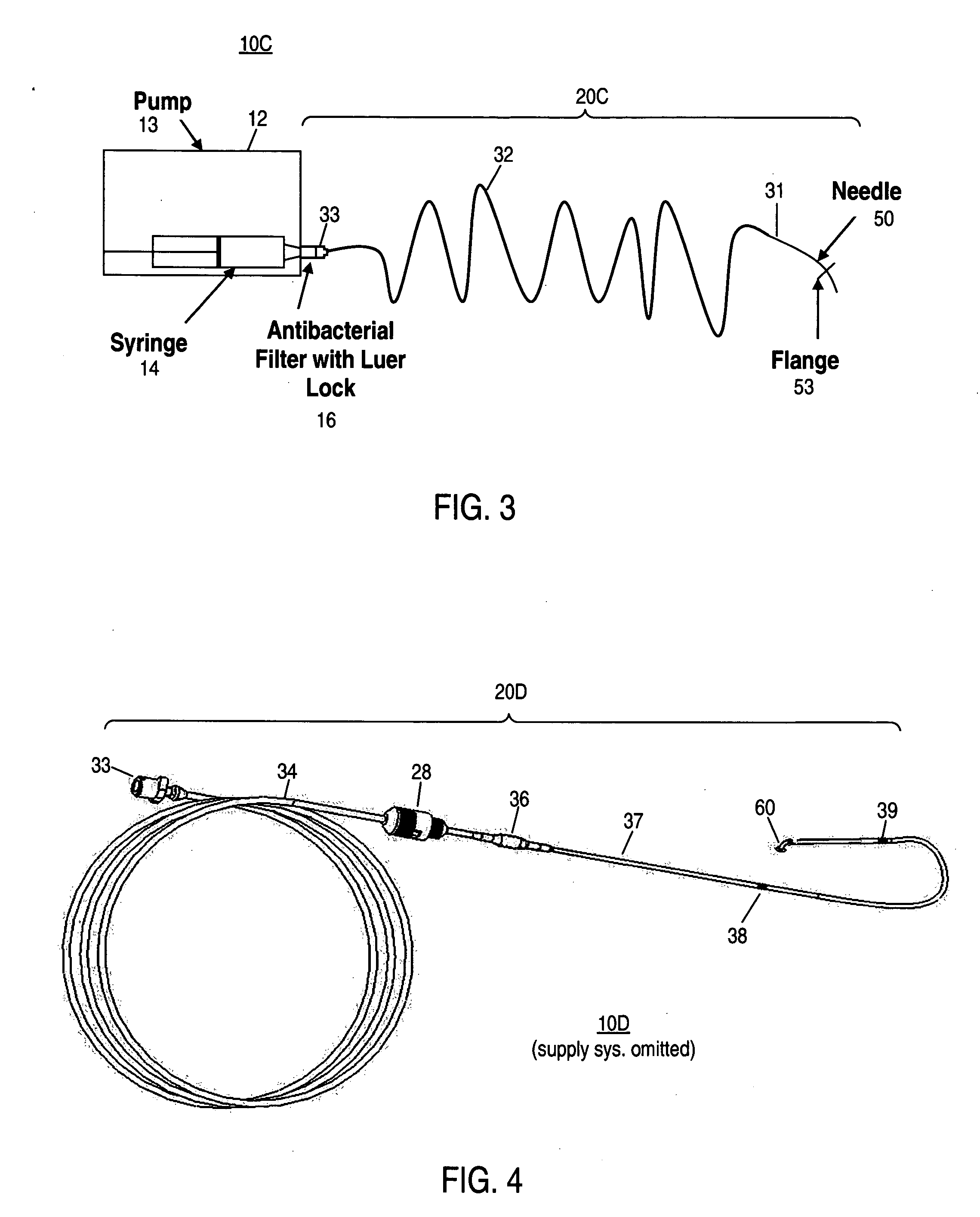
Patents
Literature
7955results about "Infusion syringes" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
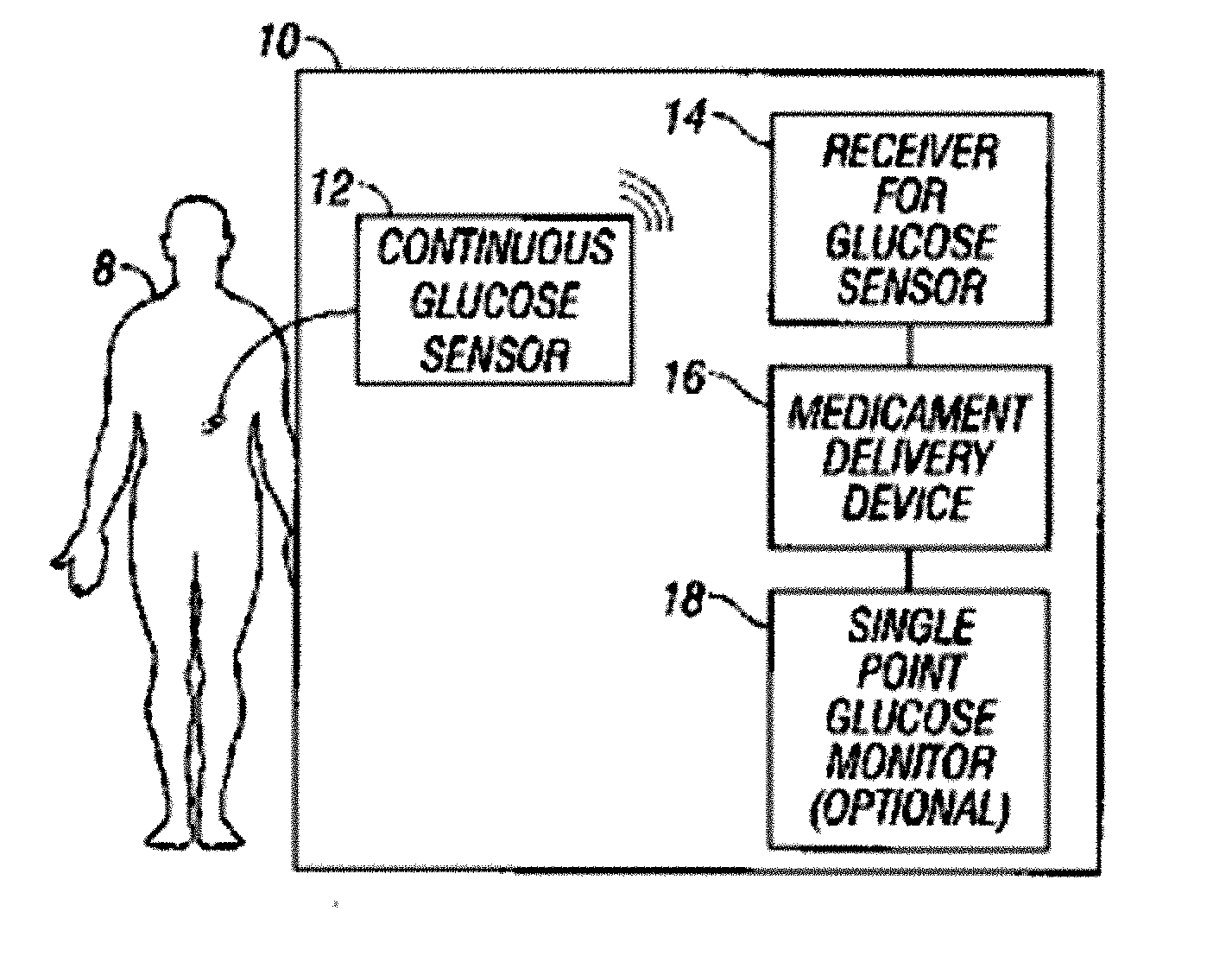
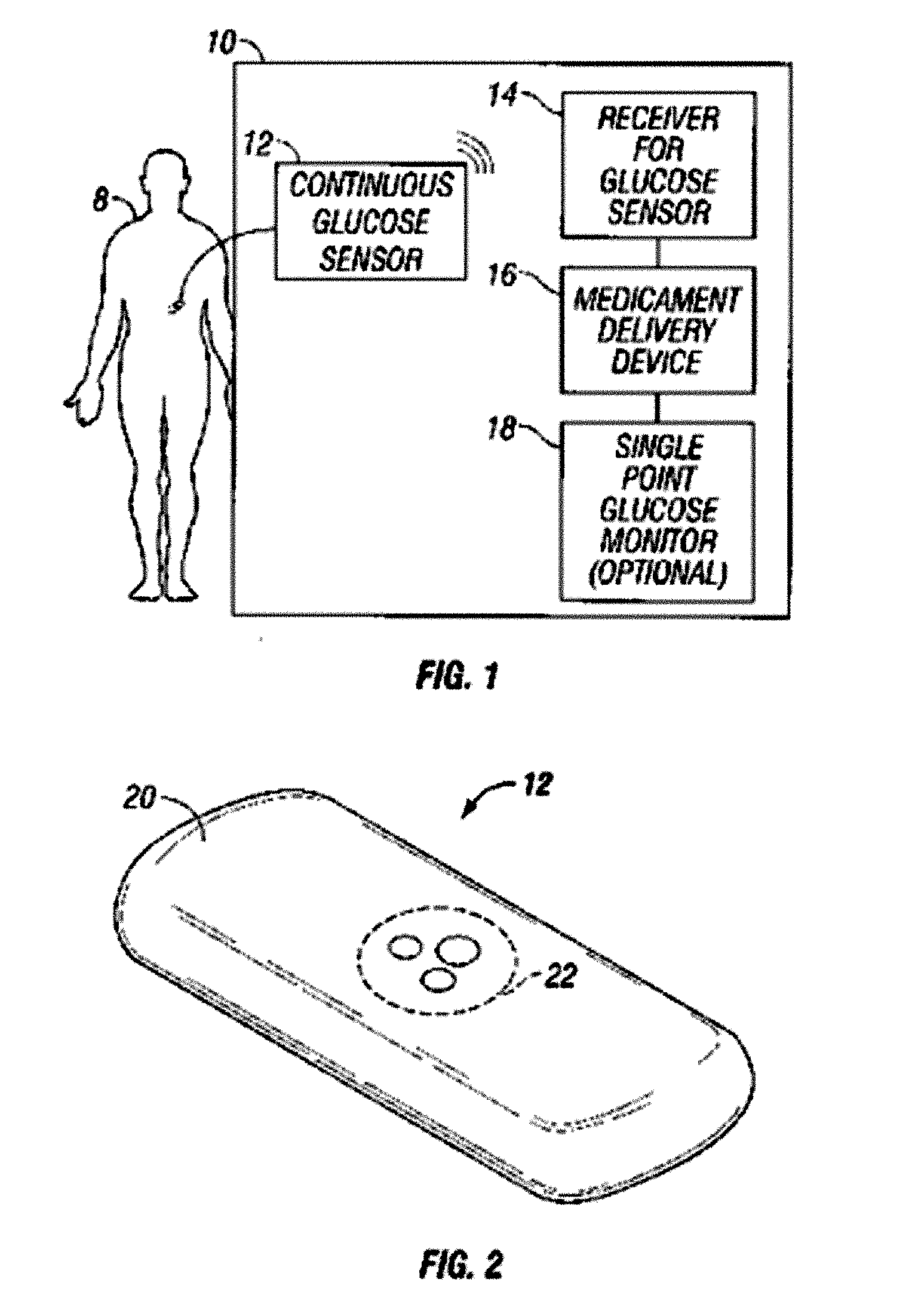
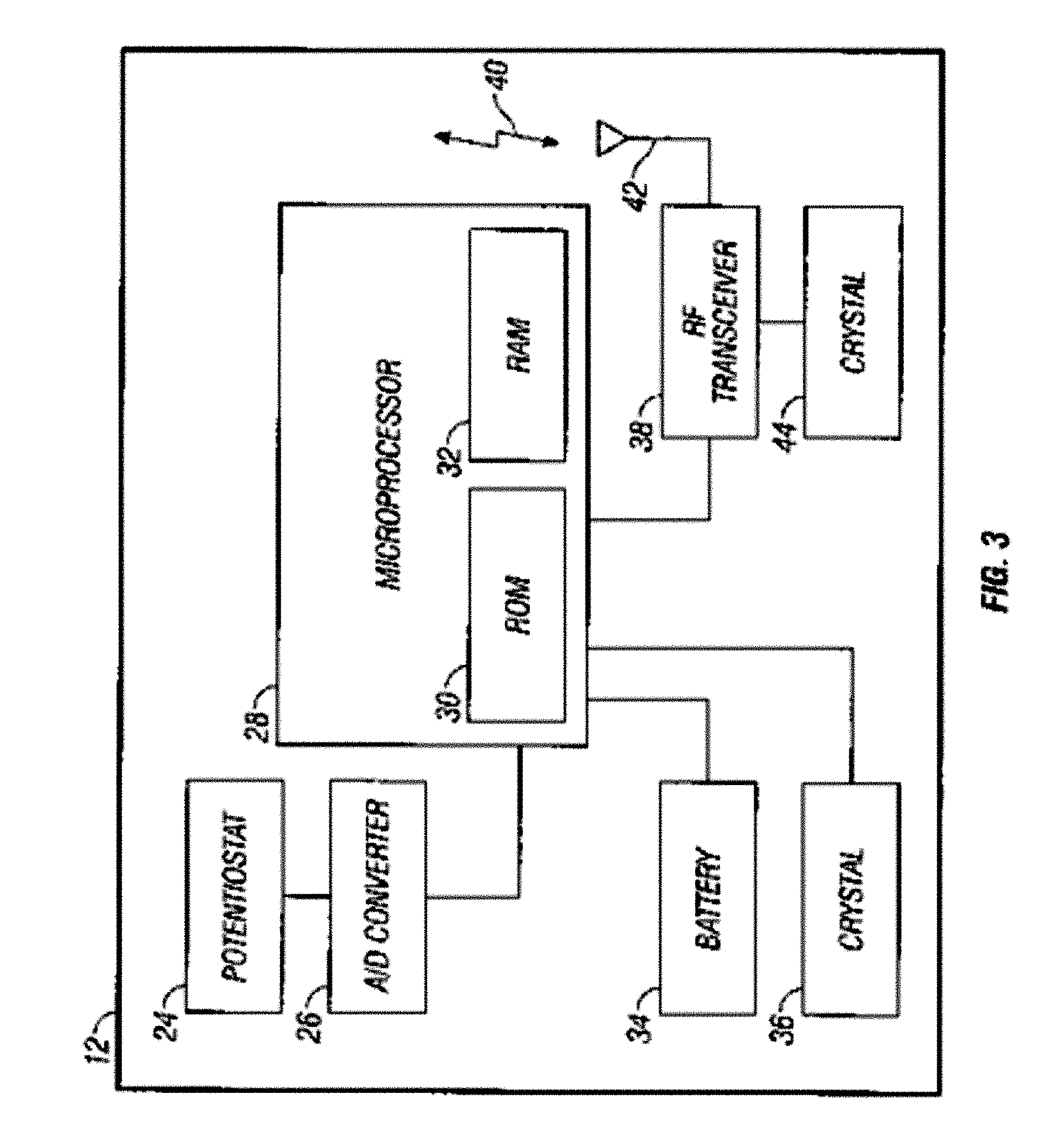
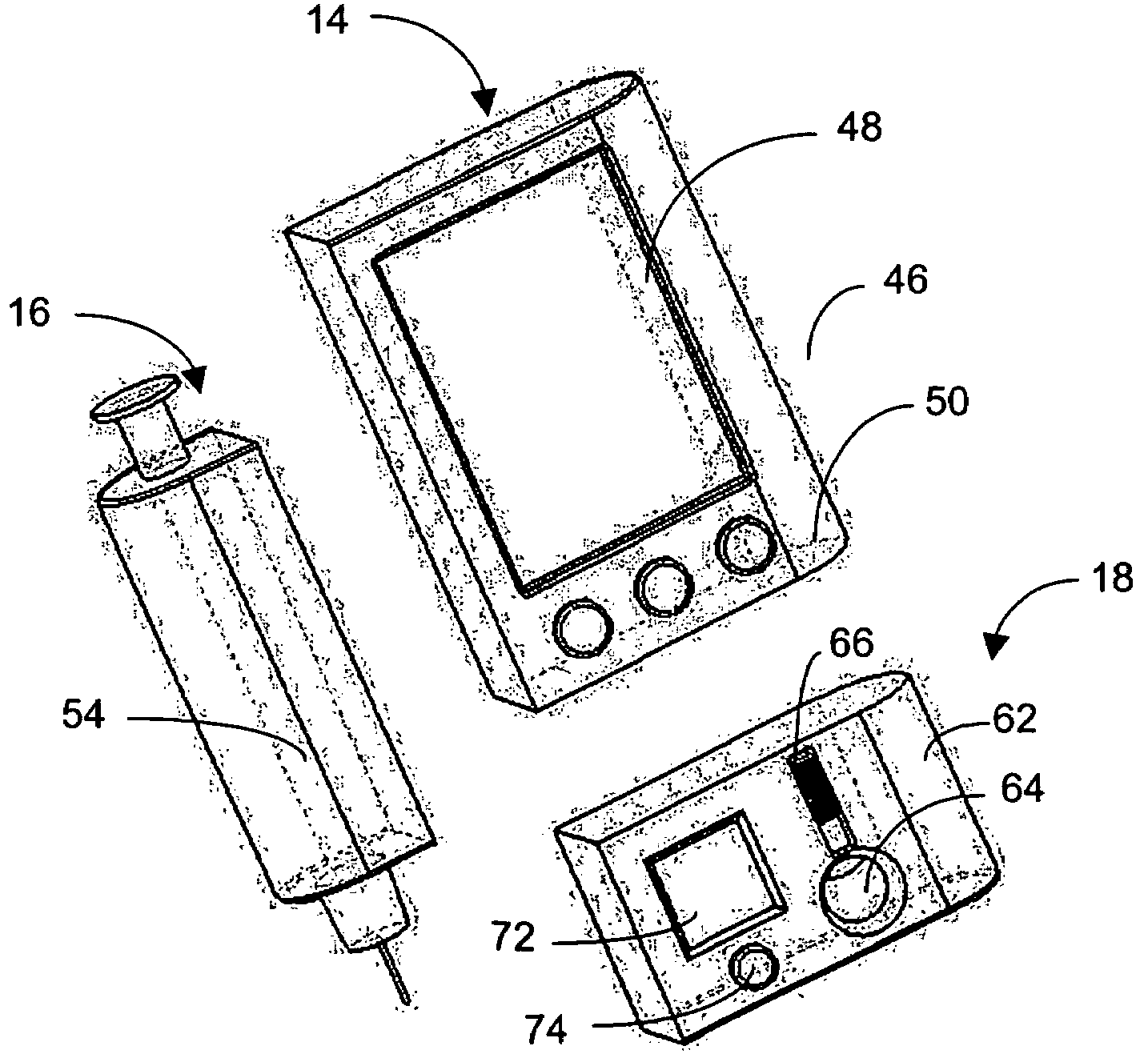
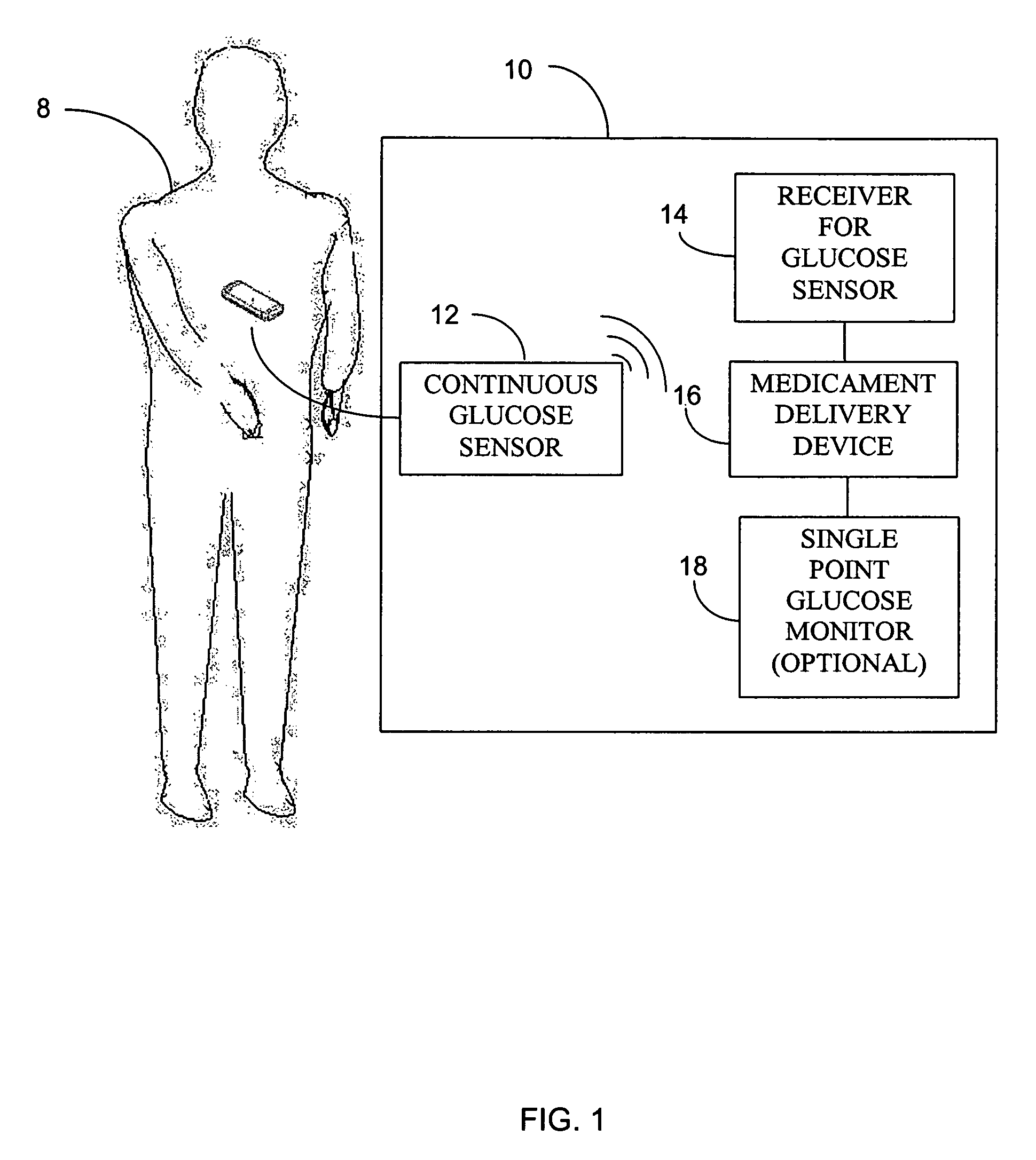
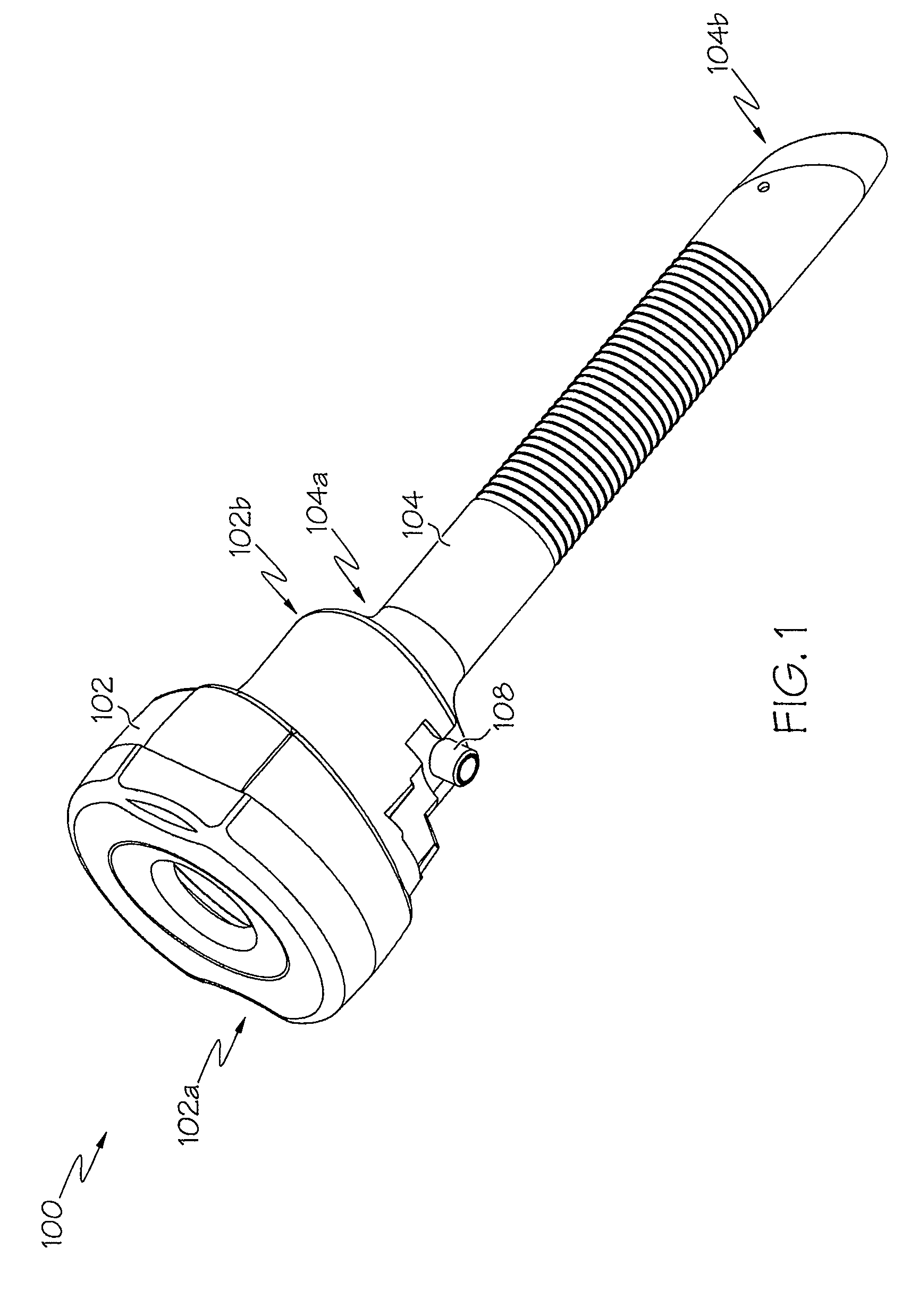
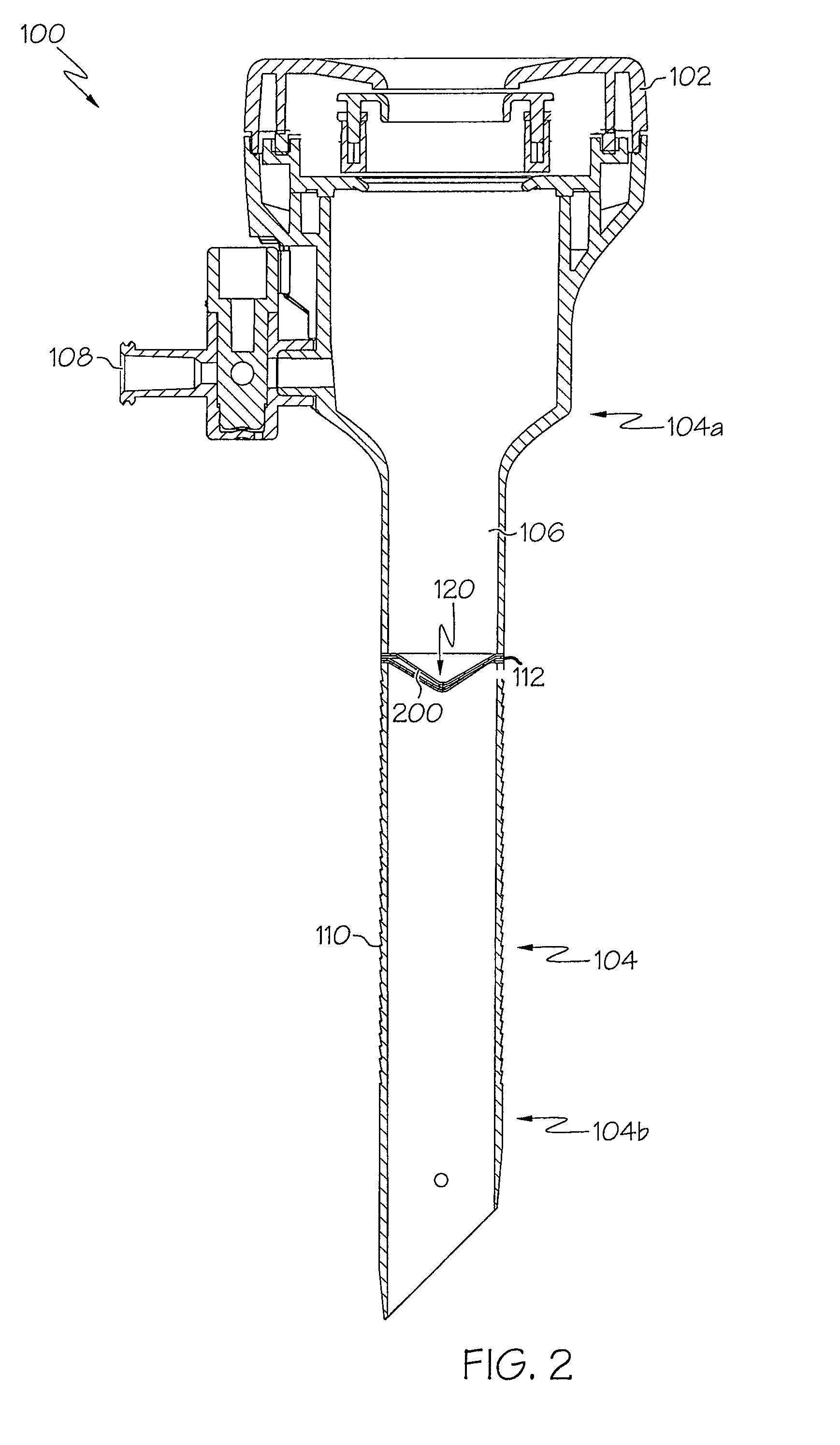
Integrated delivery device for continuous glucose sensor
Abstract of the DisclosureSystems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
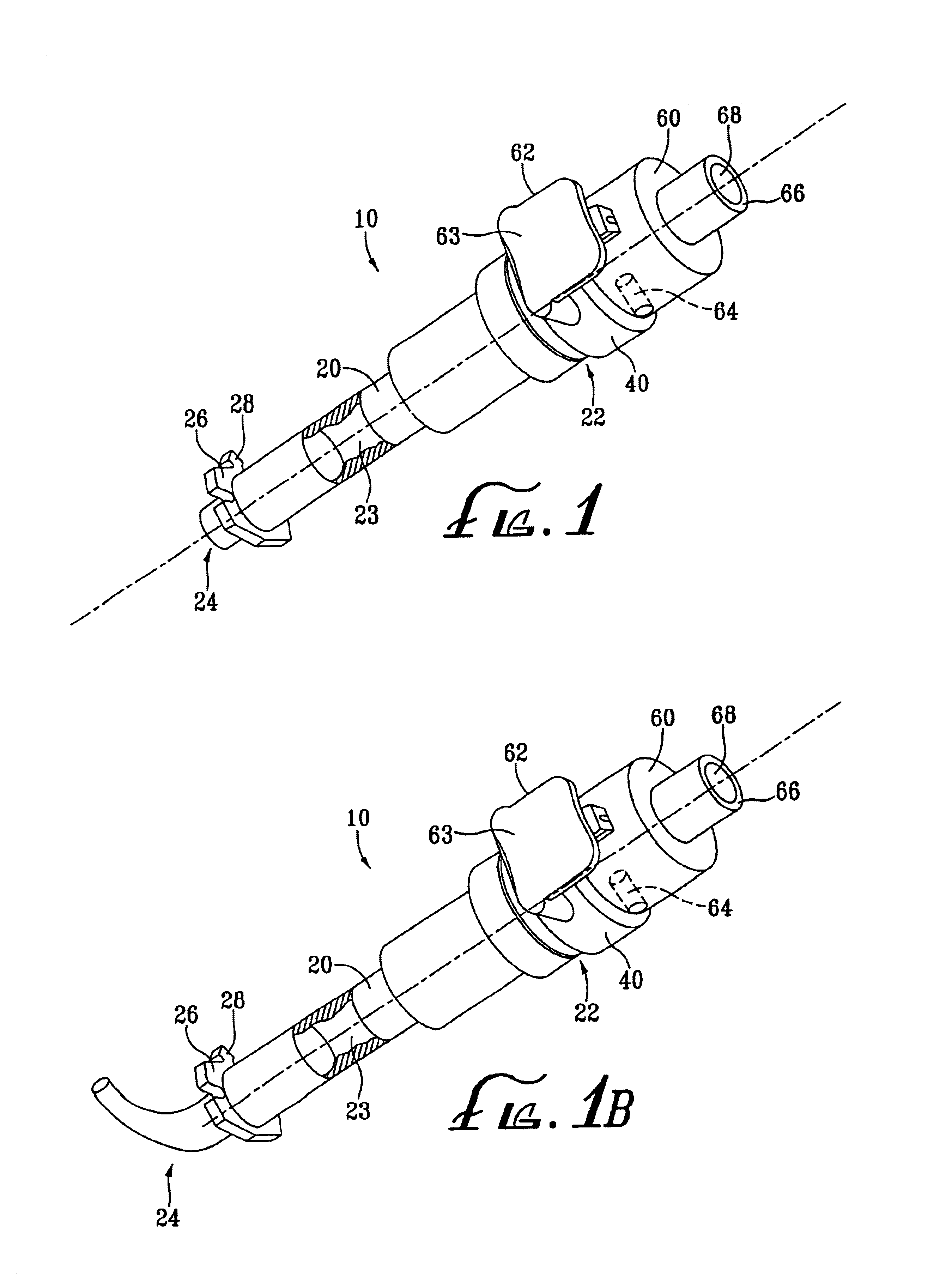
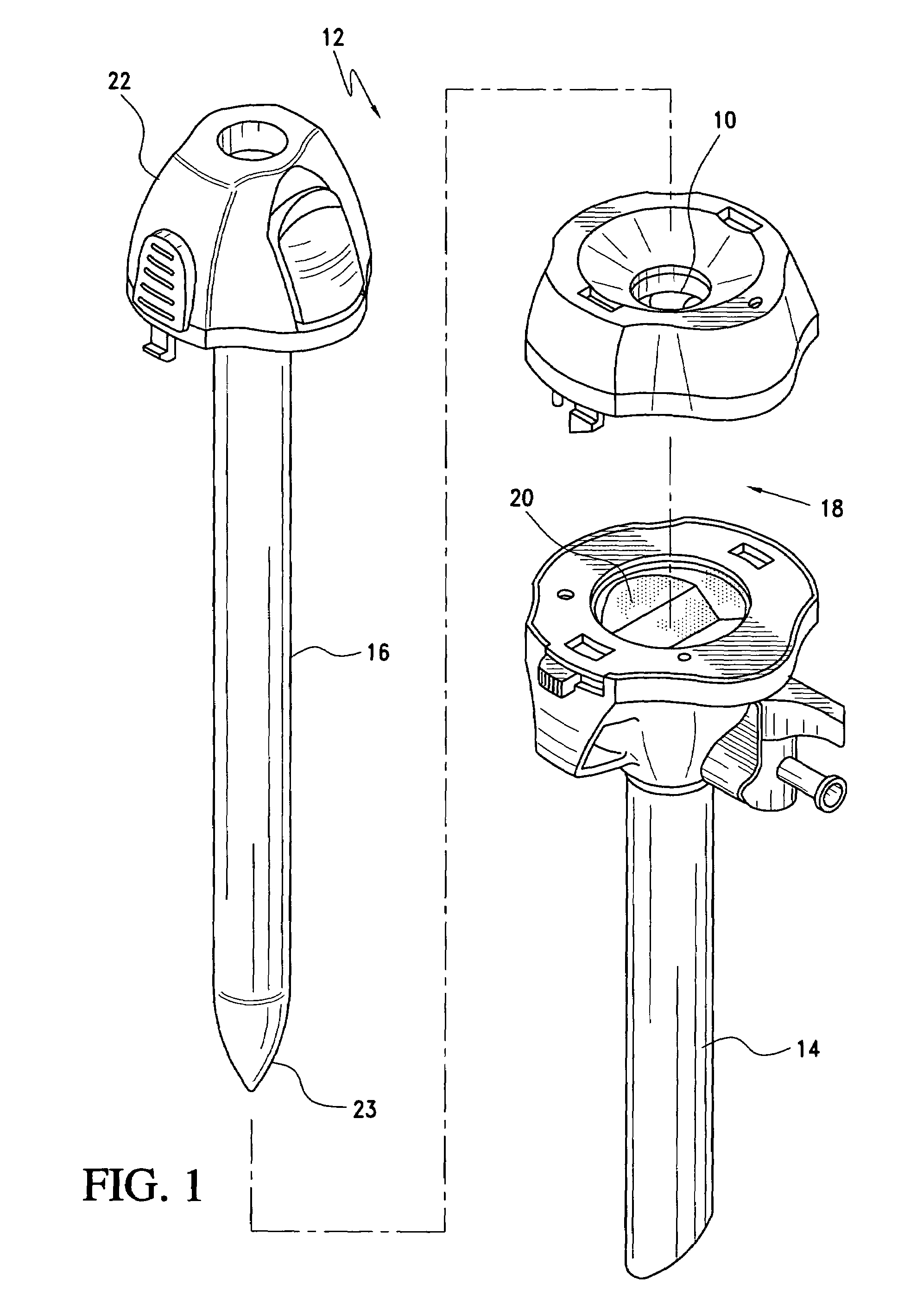
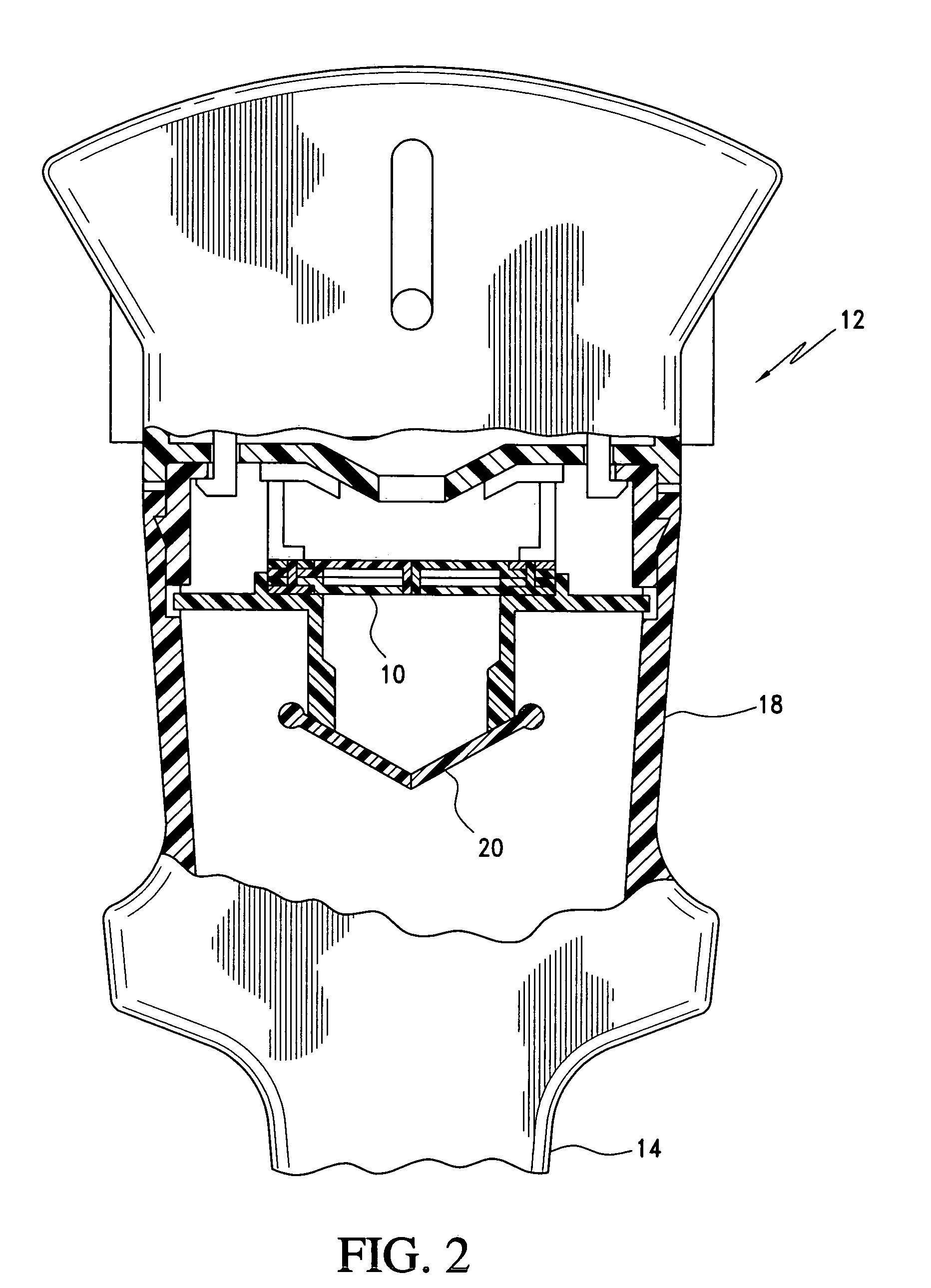
Medical device introducer and obturator
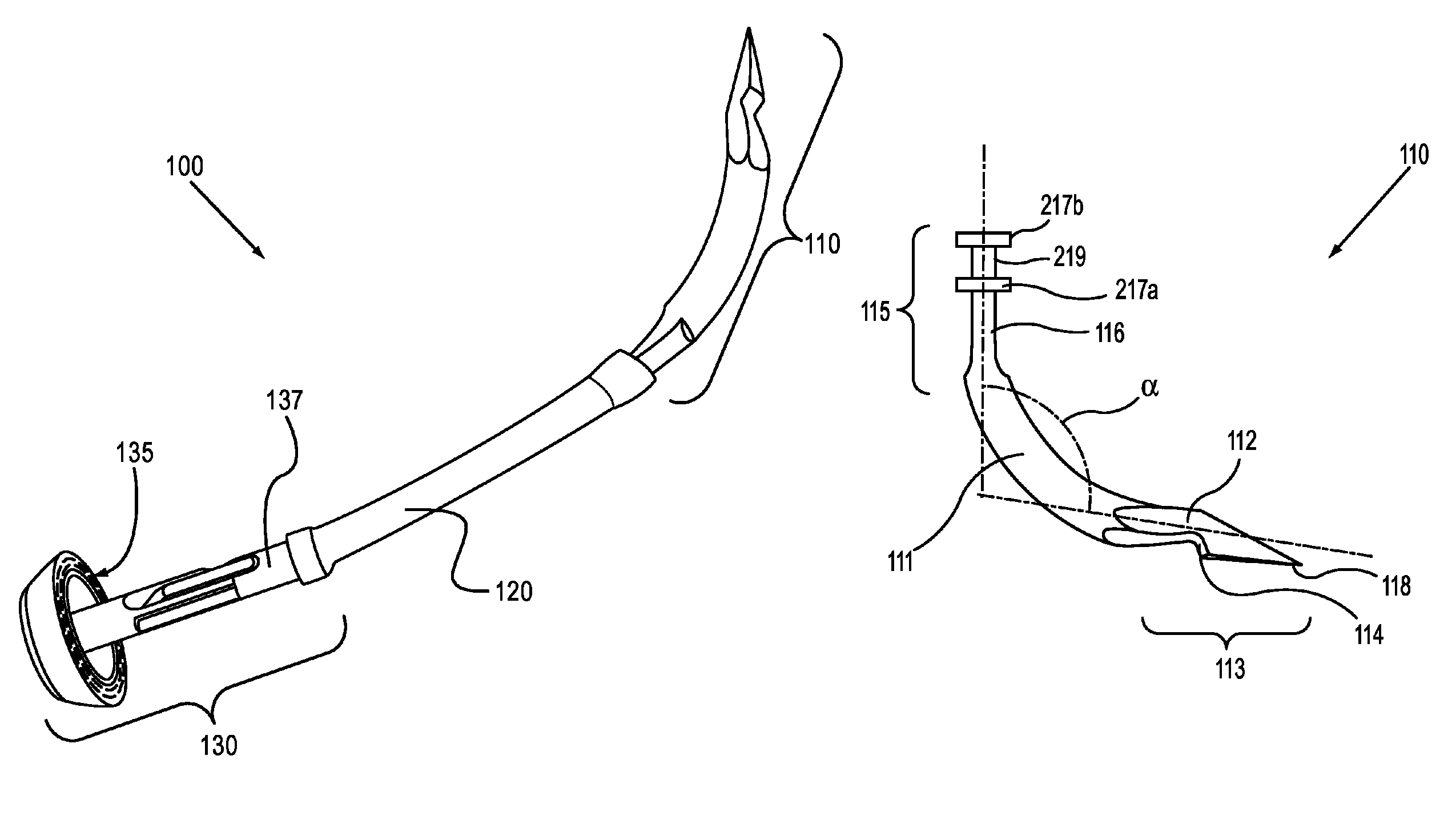
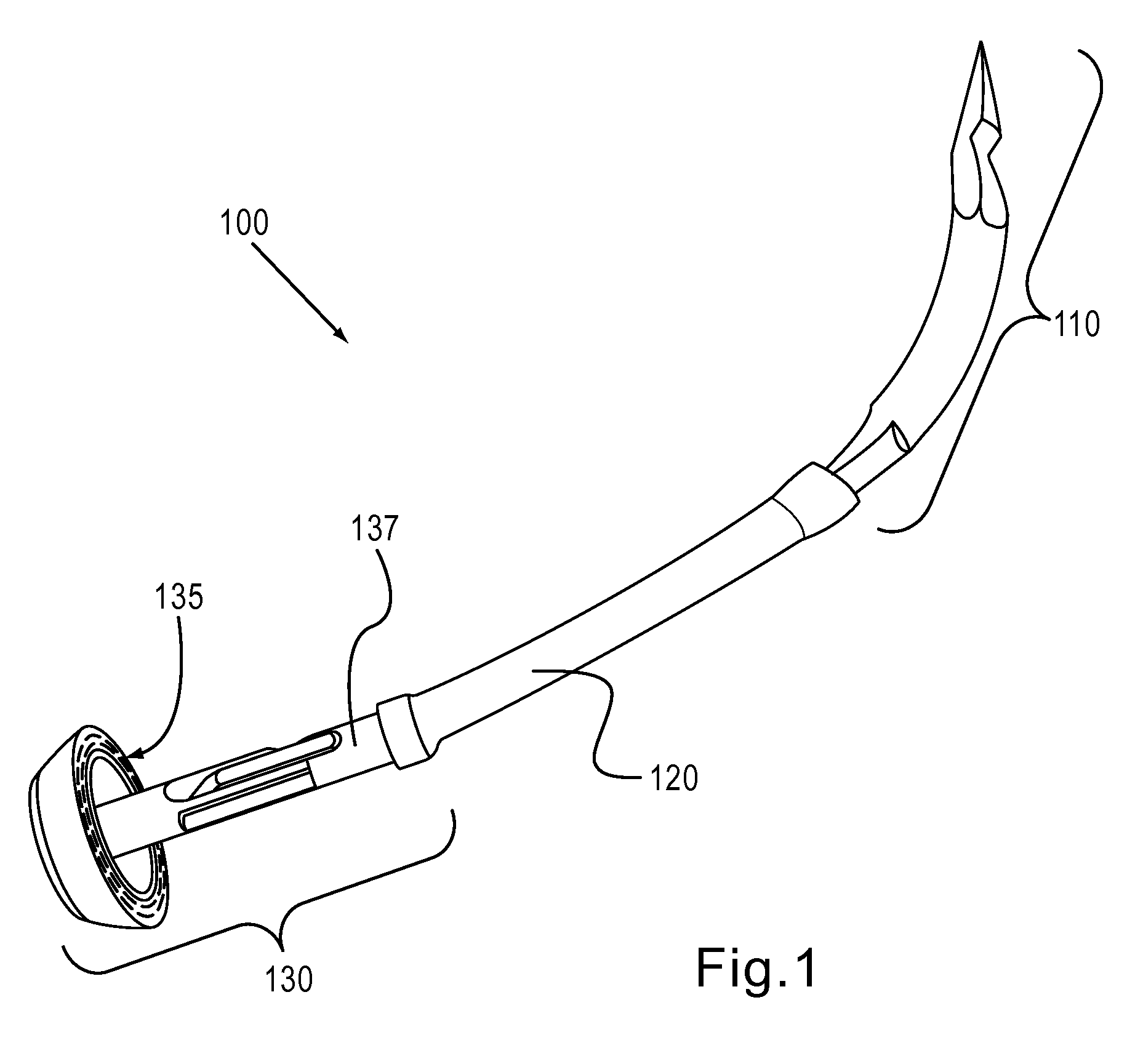
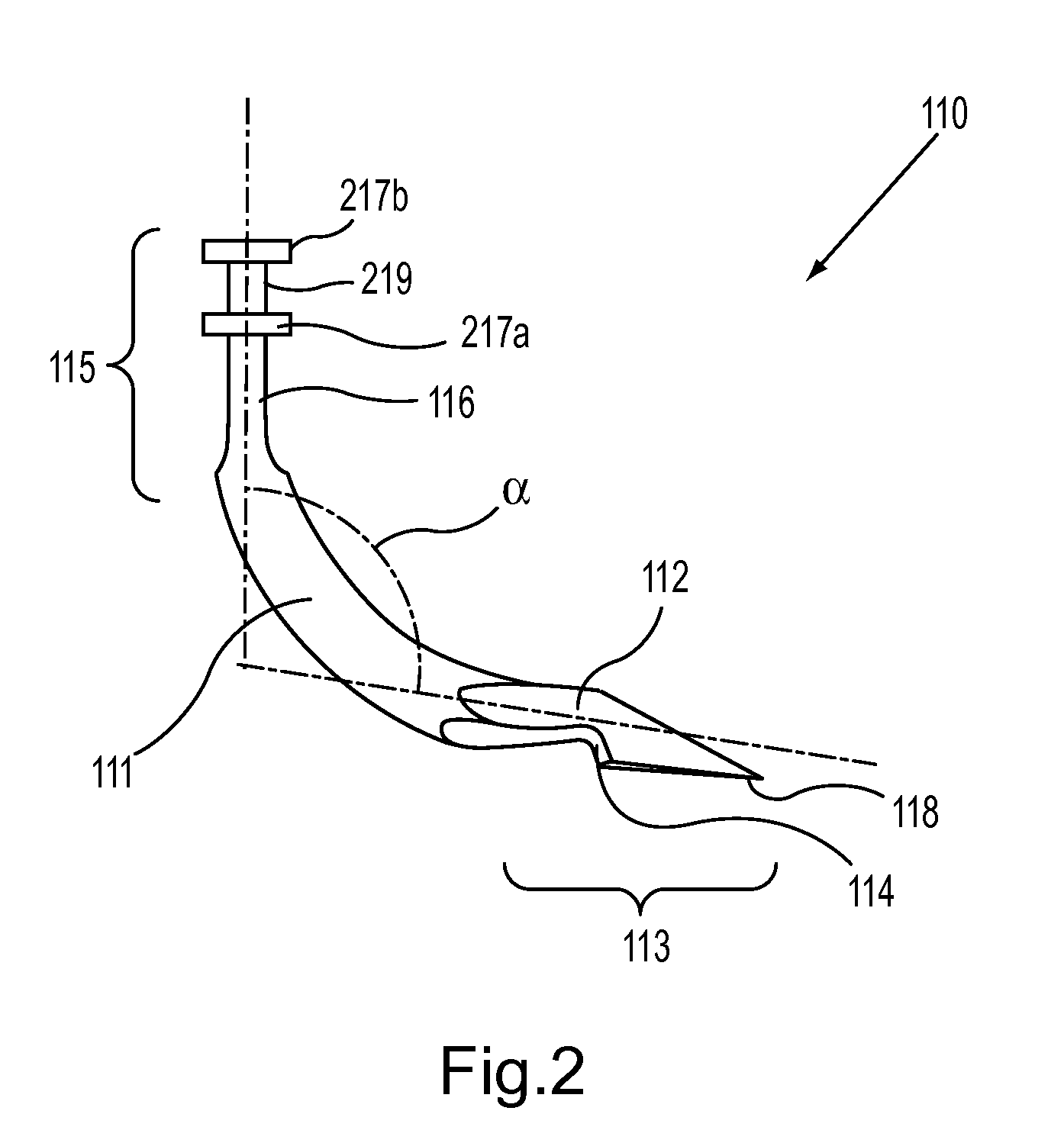
An introducer having an elongate tubular member and a device connector releasably attached to a proximal end of the tubular member. The introducer allows exchange of medical instruments, such as a blood filter and cardioplegia catheter, through a single lumen. An obturator having a retractable blade for making incision on a tissue is insertable through the lumen of the introducer. Methods of using the obturator and the introducer for introducing medical device(s) into body cavity, such as a vessel or cardiac tissue, are also disclosed.
Owner:EDWARDS LIFESCIENCES CORP
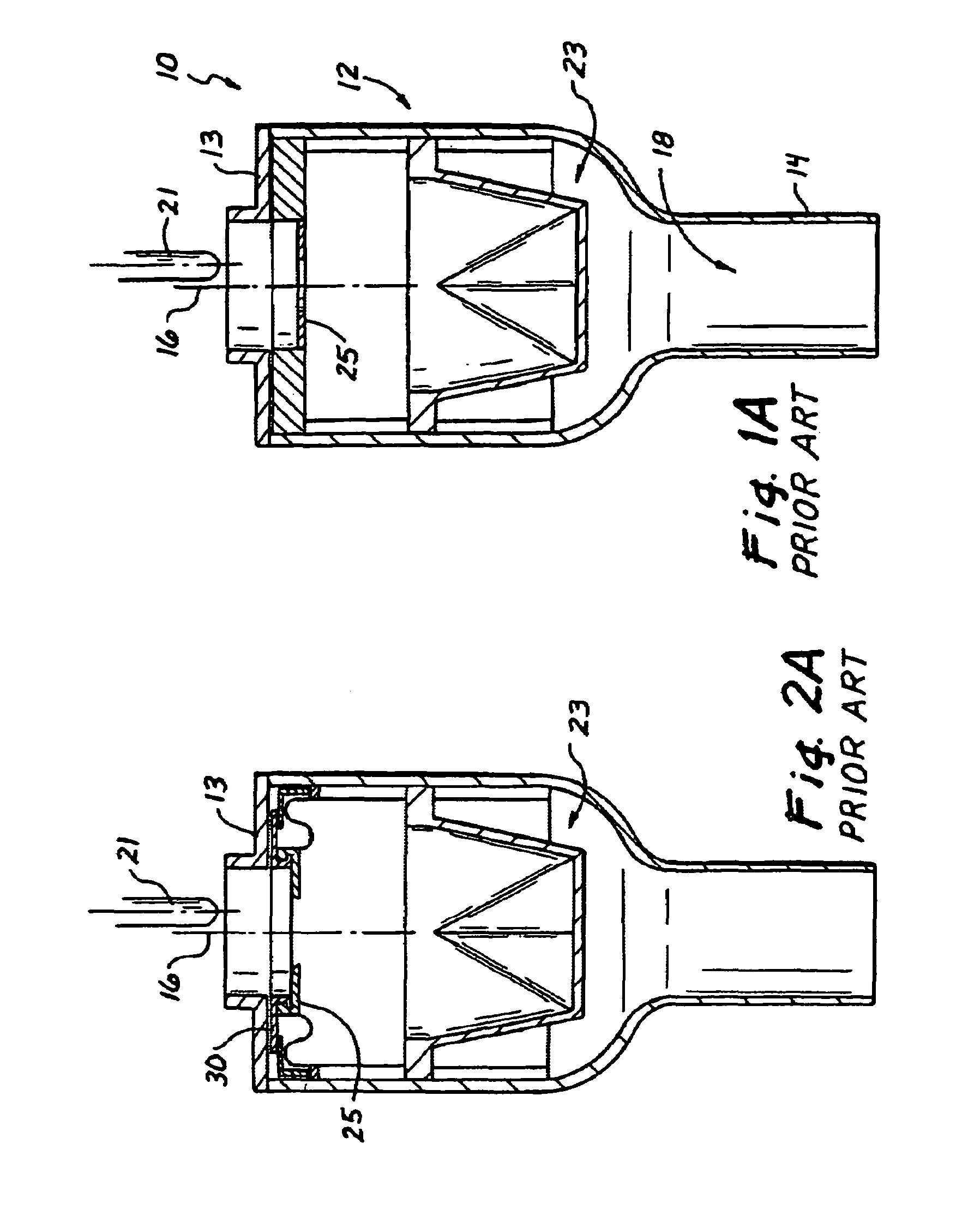
Surgical access device with pendent valve
InactiveUS7083626B2Precise positioningMaximize ease and safetyEar treatmentCannulasSurgical departmentVALVE PORT
A surgical access device, such as a trocar, includes a pendent valve having an elongate structure extending from a proximal end to a septum valve disposed at a distal end. In operation, the elongate structure follows the angle of the instrument to pre-position the septum valve into the path of the instrument where it is not significantly challenged during instrument insertion or manipulation. The pendant valve can be made to float at both the proximal end and the distal end of the elongate structure, to further reduce the vulnerability of the septum valve. Since the valve is less vulnerable to instrument insertion, it can be formed to minimize friction and maximize the functional range of the access device.
Owner:APPL MEDICAL RESOURCES CORP
Integrated delivery device for continuous glucose sensor
Systems and methods for integrating a continuous glucose sensor, including a receiver, a medicament delivery device, and optionally a single point glucose monitor are provided. Manual integrations provide for a physical association between the devices wherein a user (for example, patient or doctor) manually selects the amount, type, and / or time of delivery. Semi-automated integration of the devices includes integrations wherein an operable connection between the integrated components aids the user (for example, patient or doctor) in selecting, inputting, calculating, or validating the amount, type, or time of medicament delivery of glucose values, for example, by transmitting data to another component and thereby reducing the amount of user input required. Automated integration between the devices includes integrations wherein an operable connection between the integrated components provides for full control of the system without required user interaction.
Owner:DEXCOM
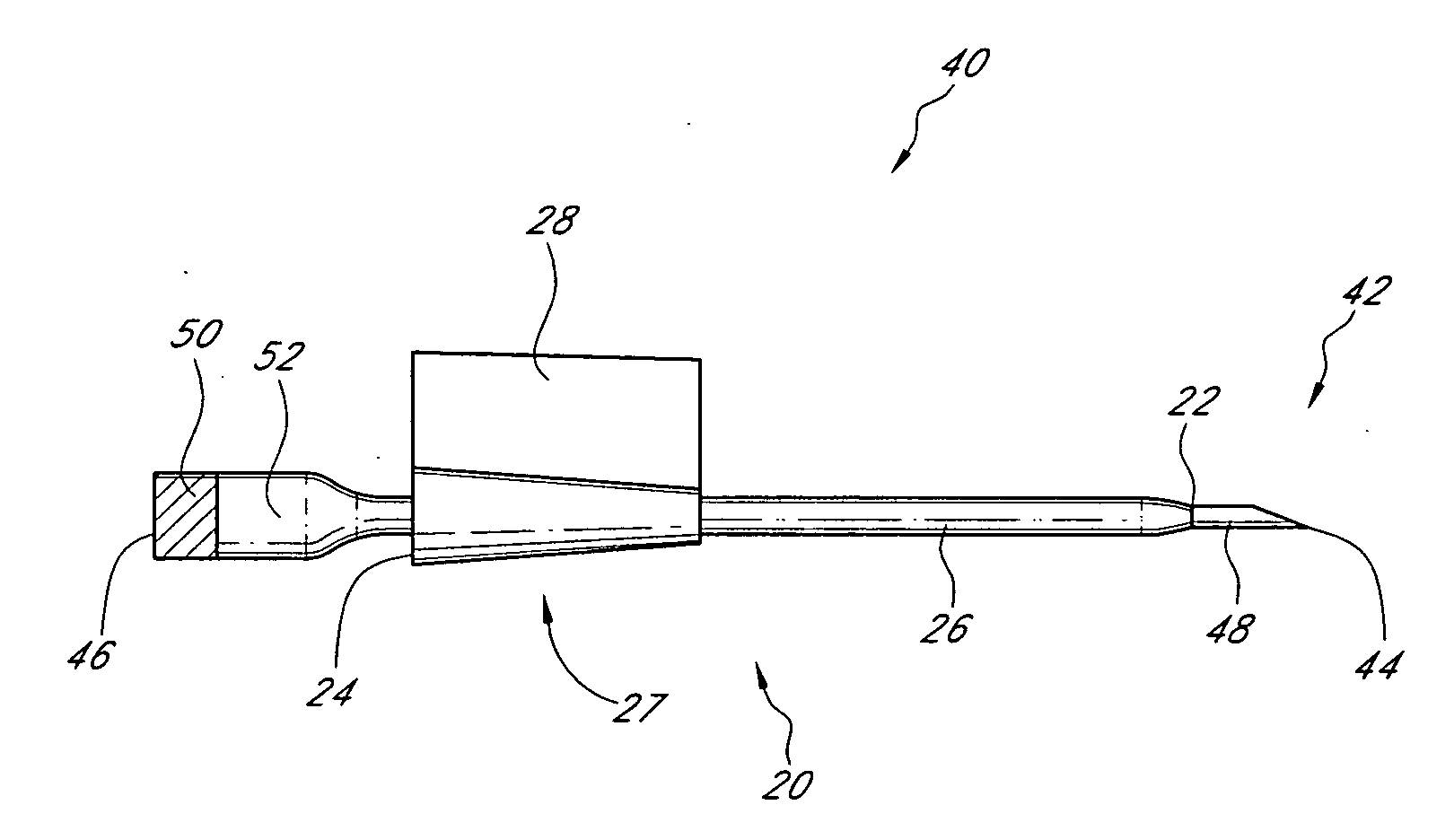
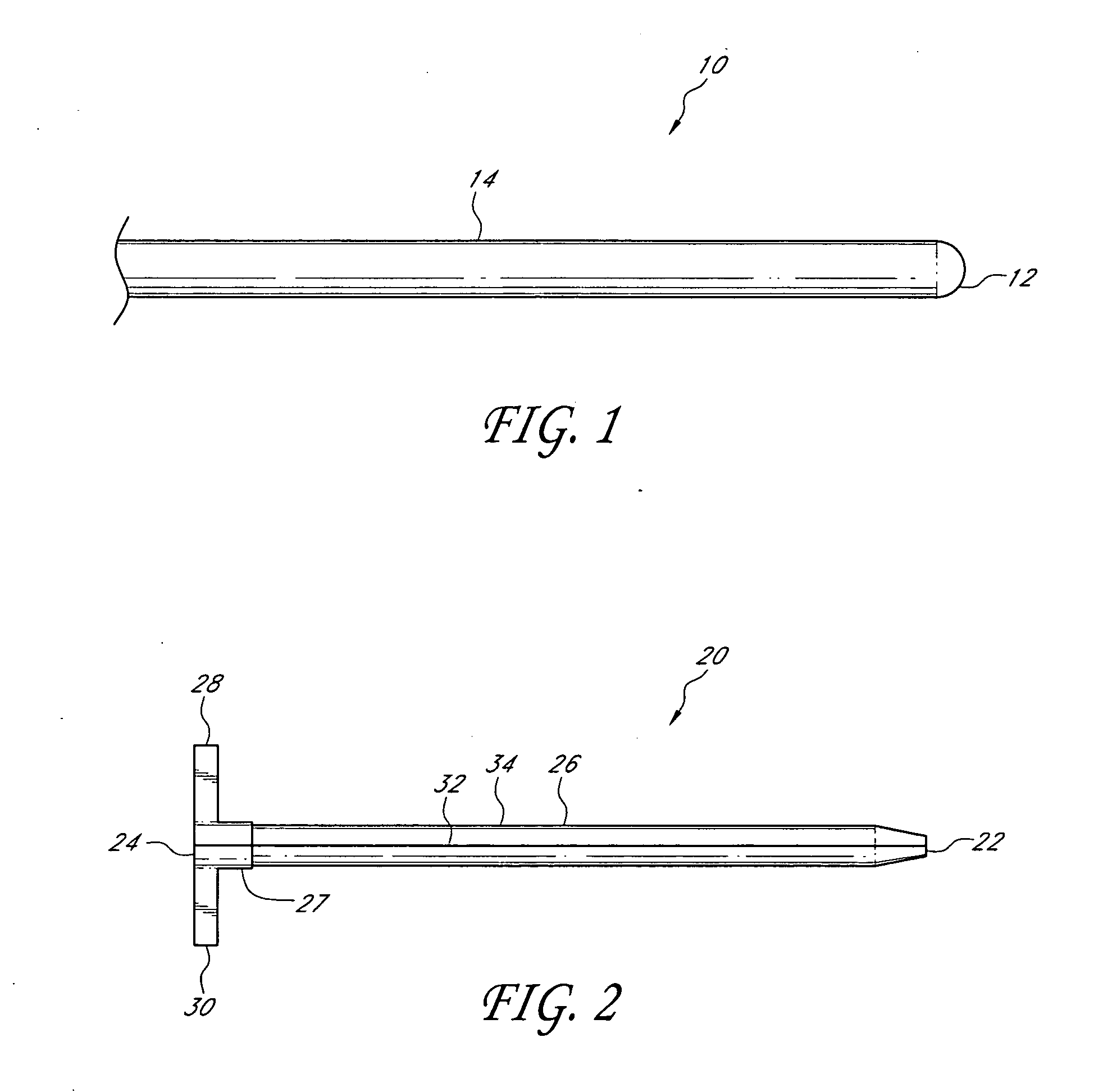
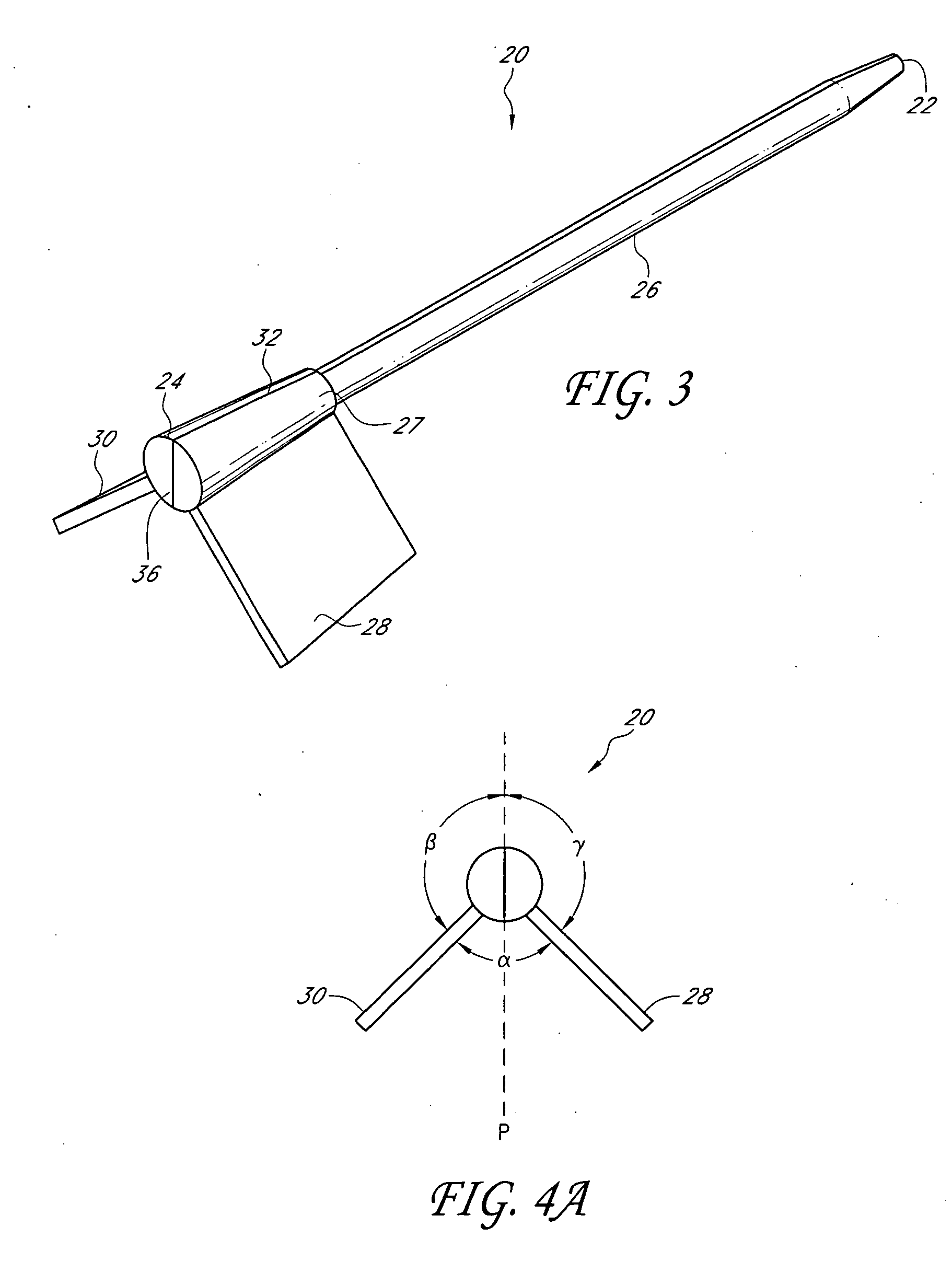
Method and device for tissue removal and for delivery of a therapeutic agent or bulking agent
InactiveUS7806871B2Efficient removalMinimize damageInfusion syringesMedical devicesSurgeryMedical device
According to an aspect of the present invention, a medical device is provided, which comprises the following: (a) a hollow elongate body (e.g., a elongate cylinder, such as a needle) having distal and proximal ends; and (b) a rotatable member comprising a tissue morselizer and an elongate shaft (e.g., an auger-like tissue-drilling bit). The device (i) advances material (e.g., morselated tissue) toward the proximal end of the hollow elongate body when the shaft is rotated in a first direction, and (ii) advances material (e.g., a therapeutic agent and / or a bulking agent) toward the distal end of the hollow elongate body when the shaft is rotated in a second direction that is opposite the first direction. The invention also provides a method of treatment for morselizing and removing tissue from within the patient and creating a void within the patient and introducing a therapeutic agent and / or a bulking agent into the void.
Owner:BOSTON SCI SCIMED INC
Surgical stapler
Methods and devices for closing a puncture wound in a liquid carrying vessel are provided. In one exemplary embodiment, a surgical stapler is provided having a locator tube with an inflatable member formed thereon and adapted to be positioned within a liquid carrying vessel adjacent a puncture wound, and a staple applying apparatus that is slidably disposed on a portion of the locator tube and that is adapted to apply a surgical staple to seal a puncture wound in a liquid carrying vessel.
Owner:ABBOTT CARDIOVASCULAR
Insertion auxiliary implement
An insertion auxiliary implement of the present invention includes: a tubular part into which a flexible endoscope insertion part which is insertable into a body cavity, and one of a treatment tool and a channel into which the treatment tool is insertable, are insertable; and a sealing member which has through holes for supporting the endoscope insertion part and one of the treatment tool and the channel in the tubular part, and which airtightly and movably contacts each of a periphery of the endoscope insertion part, a periphery of the treatment tool or a periphery of the channel, and an inner surface of the tubular part, and thereby maintains airtightness between a distal end and a proximal end inside the tubular part.
Owner:OLYMPUS CORP
Laparoscopic gastric and intestinal trocar
InactiveUS8795308B2Easy to holdReduce the cross-sectional areaInfusion syringesSurgical needlesDistal portionSurgery
A trocar needle includes an elongate body having a distal end portion and a proximal end portion, a penetrating tip formed at the distal end portion of the body, and an attachment portion formed at the proximal end portion for attaching a tether thereto. A grip region can further be provided and can be formed for example, at the proximal end portion of the body to facilitate gripping by a surgical grasping device. Additionally or alternatively, a notch or otherwise reduced cross-sectional area can be provided. Such a feature can be formed, for example, at the distal end portion of the body, arranged proximal from a distal end thereof for enhancing haptic perception by a surgeon when utilizing the needle.
Owner:VALIN ELMER
Surgical access device
Methods and devices for accessing a body cavity are disclosed. In general, a surgical access device is provided that can include a cannula that defines a working channel that is sized and configured to receive a surgical instrument. A seal can be disposed in the cannula of the surgical access device. In one exemplary embodiment, the seal can be positioned at a point in the cannula that is effective to maintain contact between the seal and an instrument inserted therethrough as the instrument is rotated about that point.
Owner:CILAG GMBH INT
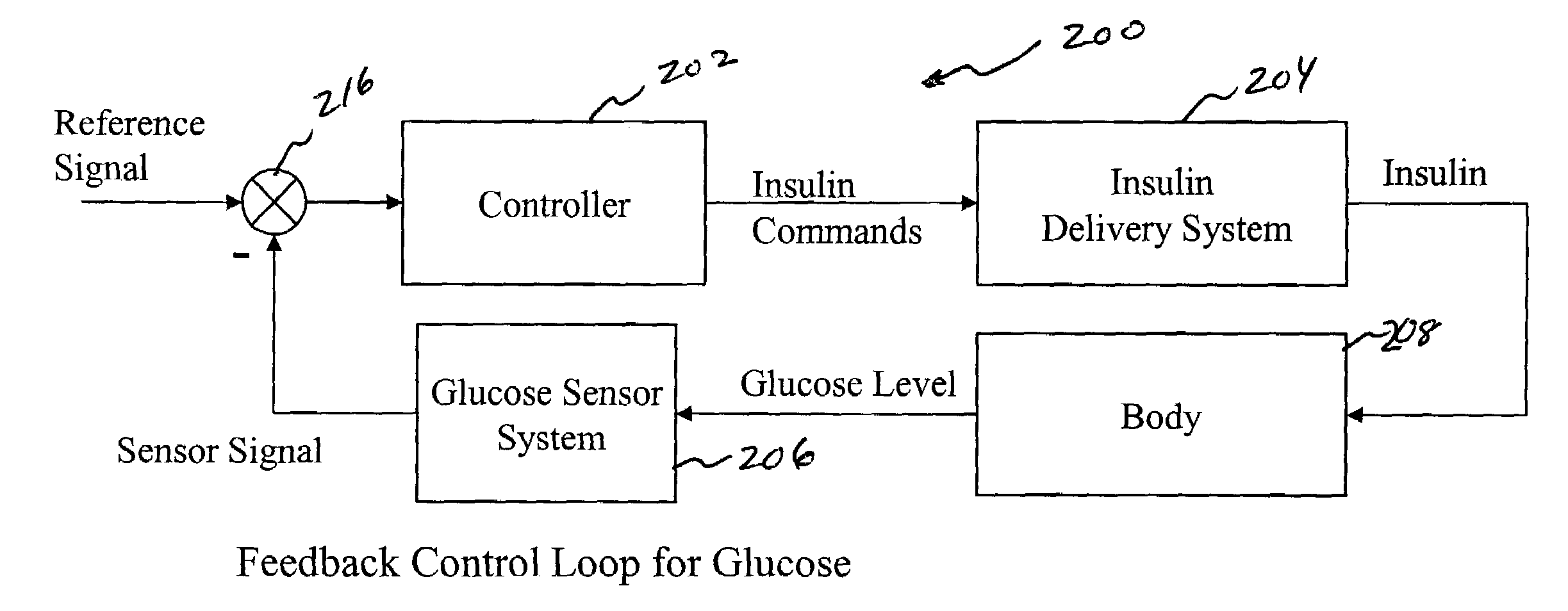
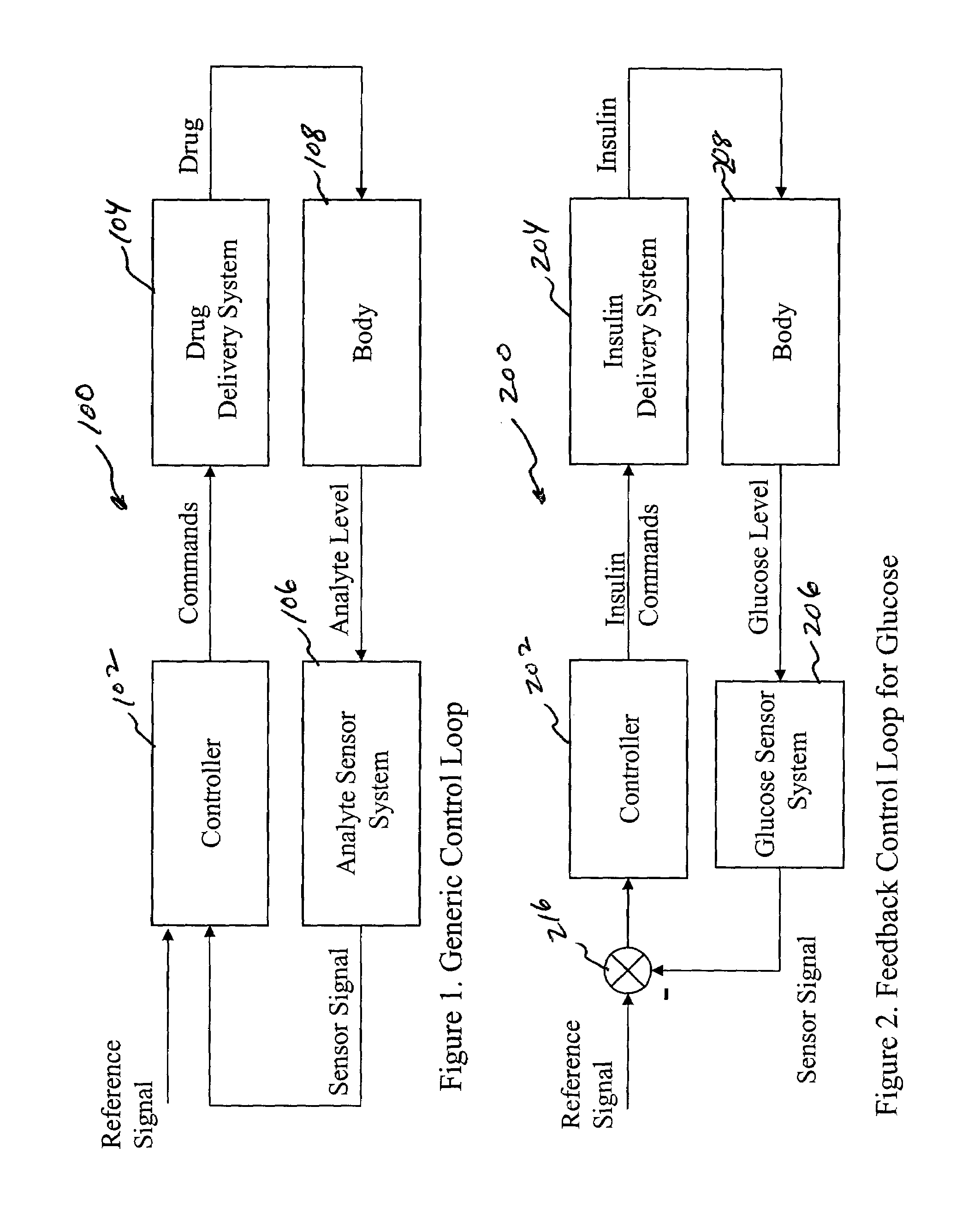
System and method for initiating and maintaining continuous, long-term control of a concentration of a substance in a patient using a feedback or model-based controller coupled to a single-needle or multi-needle intradermal (ID) delivery device
ActiveUS7060059B2Maintaining continuous, long-term control of the blood glucose concentrationsImprove performancePeptide/protein ingredientsDrug and medicationsInsulin infusionClosed loop
A closed loop therapy system for controlling a concentration of a substance, such as blood glucose concentration, in the body of a user. The system and method employ a sensor system that measures a glucose level in the body, a controller that uses the measured glucose levels to generate an output that can be used to automatically or manually control an intradermal insulin infusion system to set a constant or time-varying profile of target blood glucose concentrations in a user, and then infuse an appropriate amount of insulin into the body of the user so as to reach and maintain the target values of the blood glucose concentration.
Owner:BECTON DICKINSON & CO
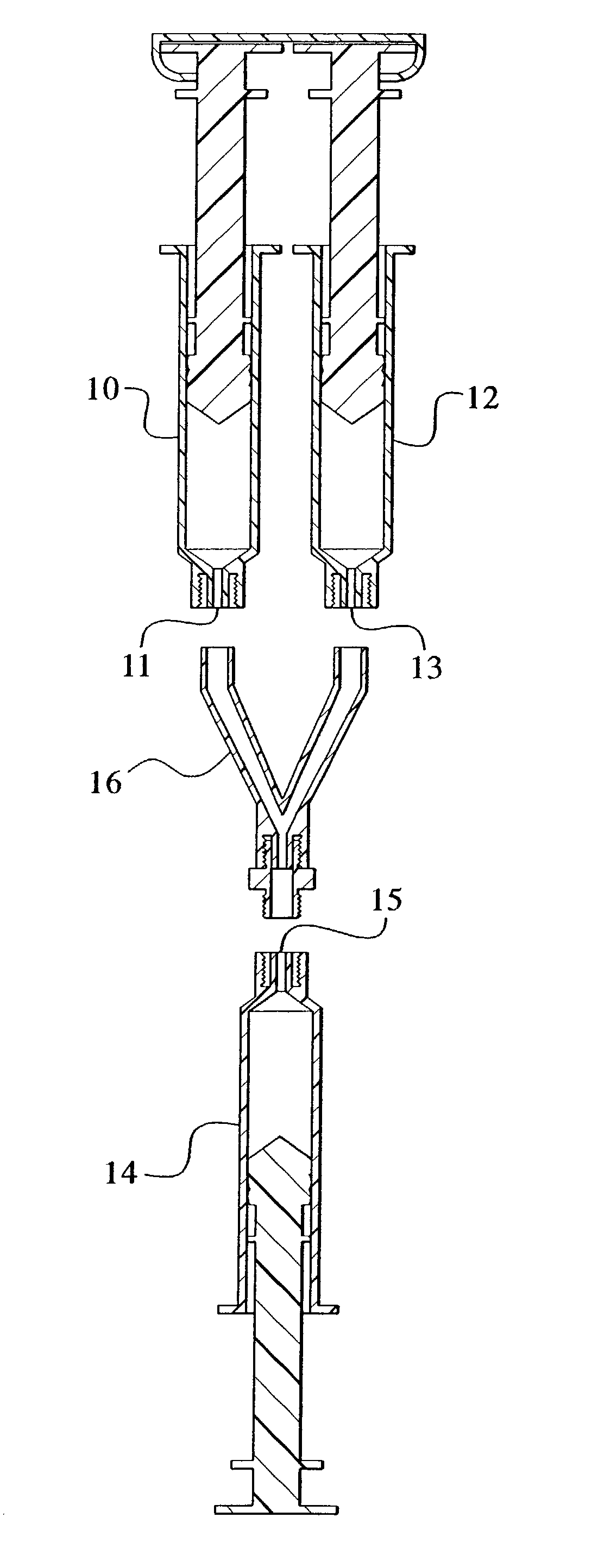
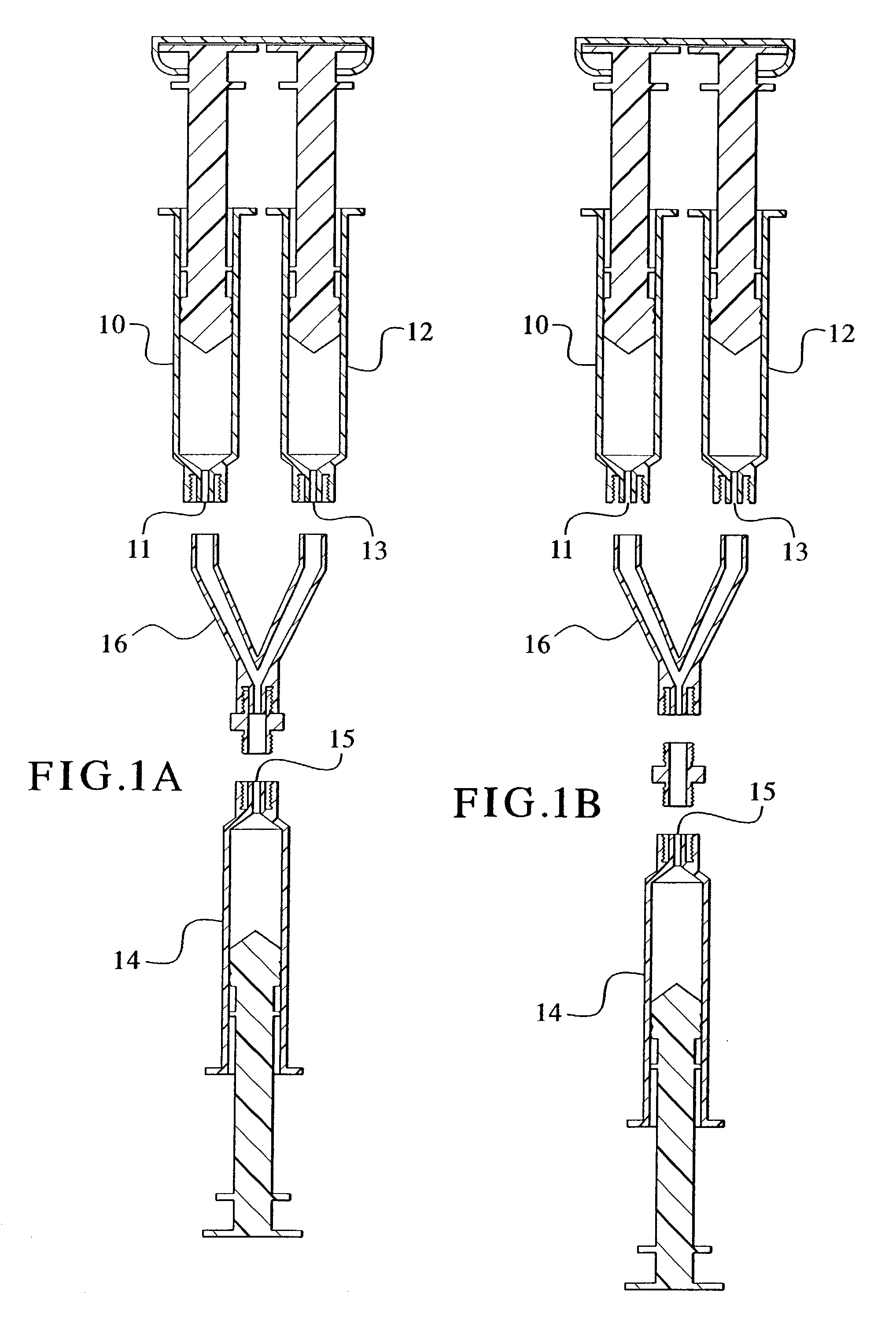
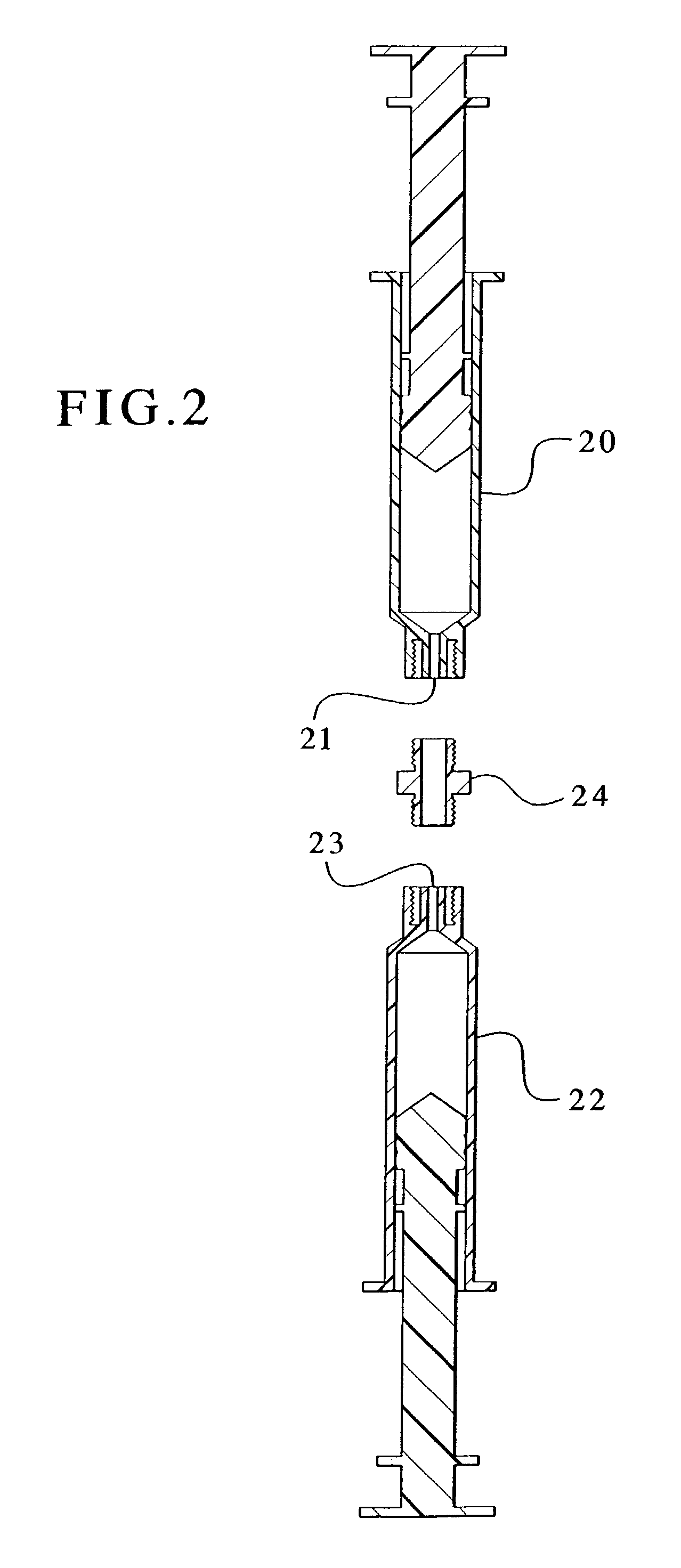
Devices and methods for mixing and extruding medically useful compositions
This invention provides devices and methods for mixing and extruding compositions which are medically and non-medically useful. The devices are particularly useful for mixing substances which are relatively inert when alone but become reactive when mixed. A common feature of all of the devices is that they allow the user to mix and ultimately extrude a composition from a single device which includes a single container or multiple interconnected containers.
Owner:BAXTER INT INC +1
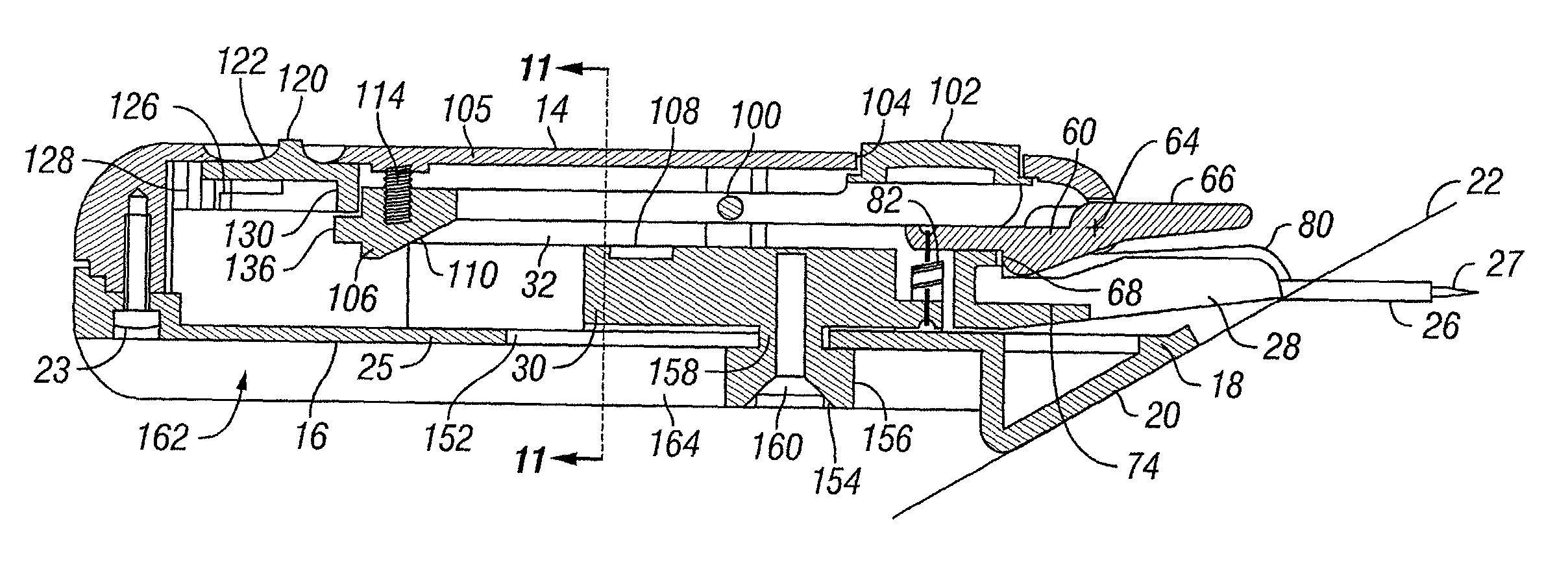
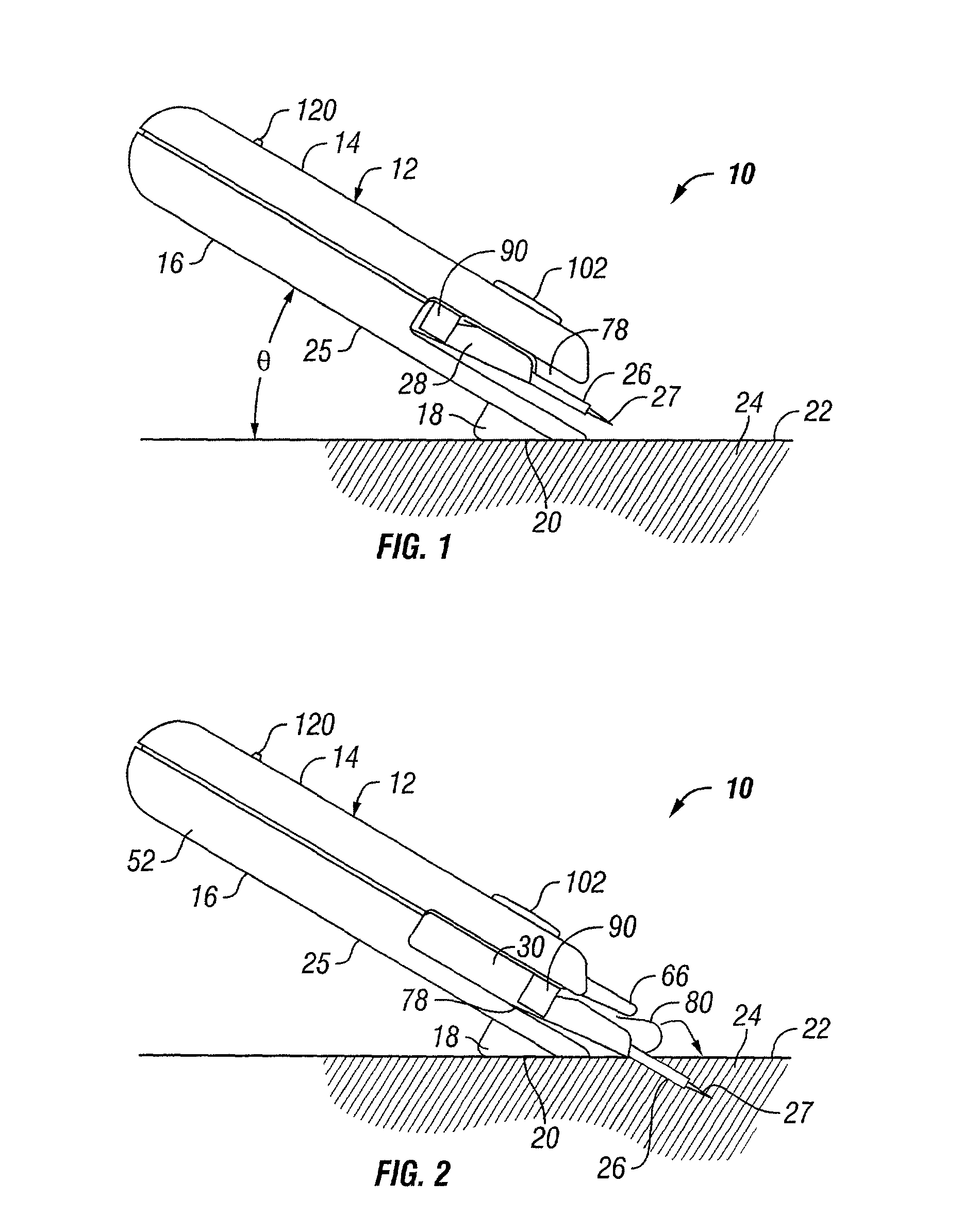
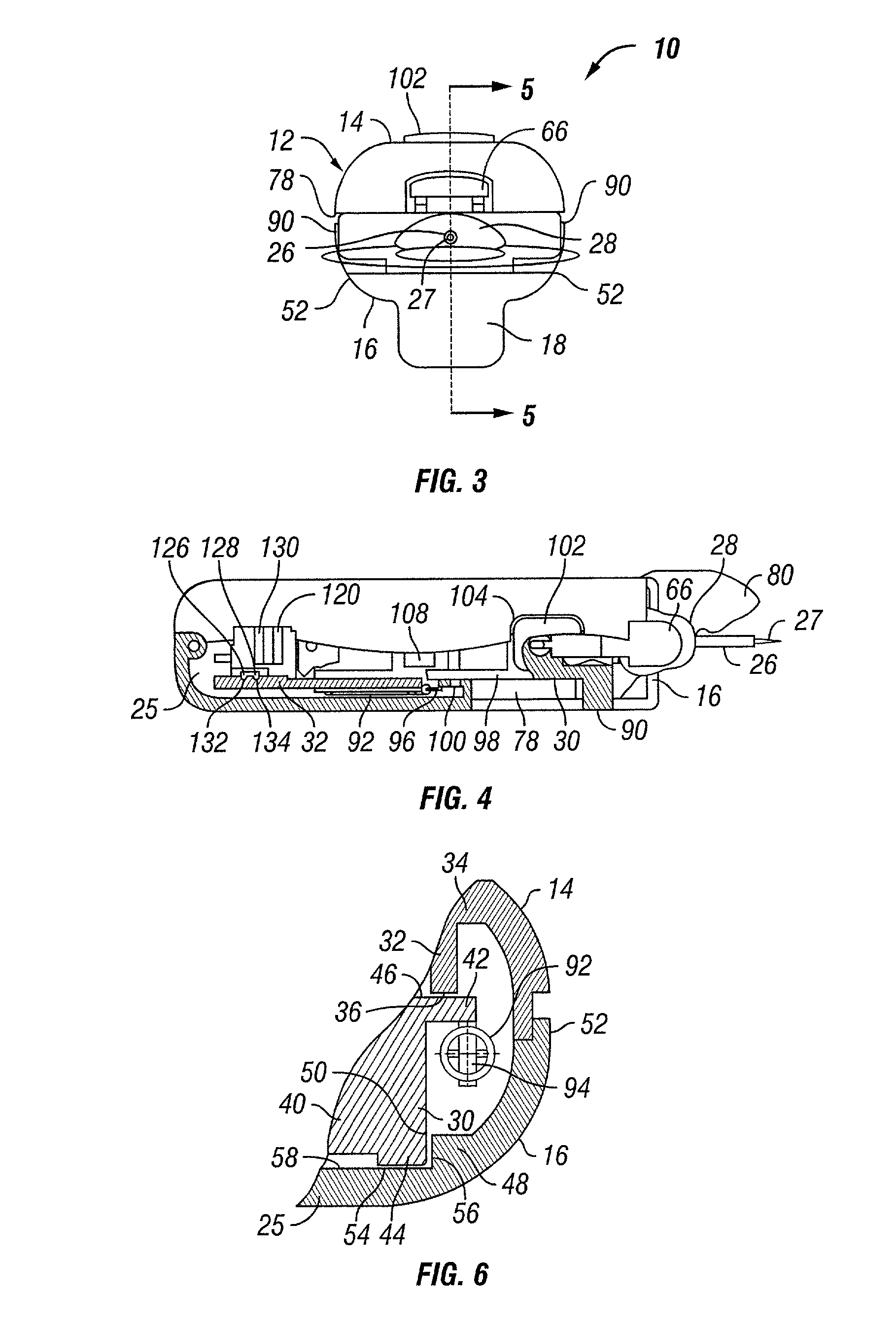
Transcutaneous inserter for low-profile infusion sets
A low-profile inserter for an angled infusion set comprises an inserter housing having a bottom wall, a retainer slidably connected to the inserter housing for movement between retracted and extended positions in a direction substantially parallel with the bottom wall, and a base member connected to the inserter housing. The retainer is adapted to releasably receive a cannula assembly, including a cannula connected to a cannula housing. The base member has a lower surface that is adapted to contact a skin outer surface. The lower surface and bottom wall together form an acute angle. With this arrangement, the cannula can be inserted subcutaneously at the acute angle with respect to the skin outer surface.
Owner:LIFESCAN IP HLDG LLC
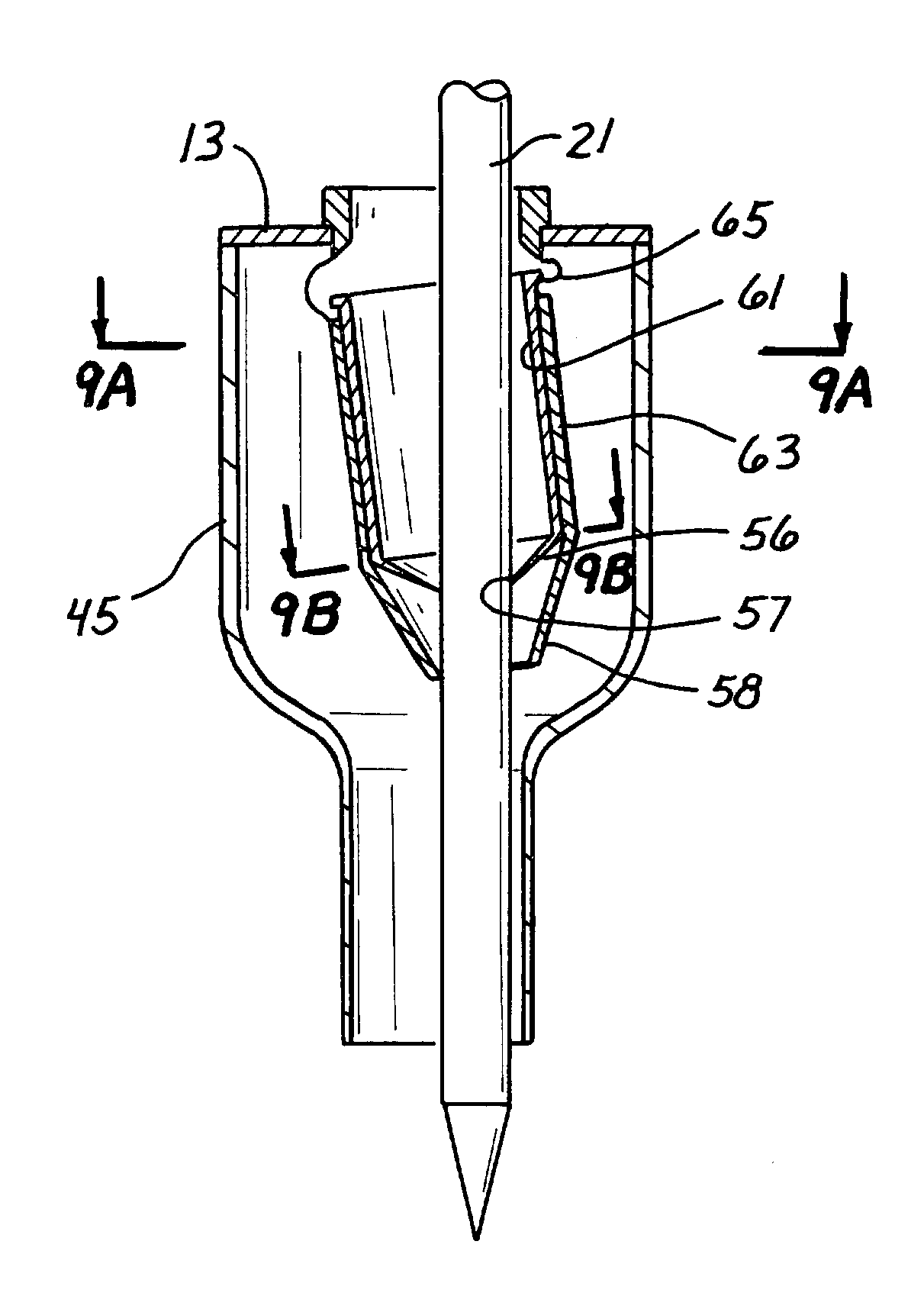
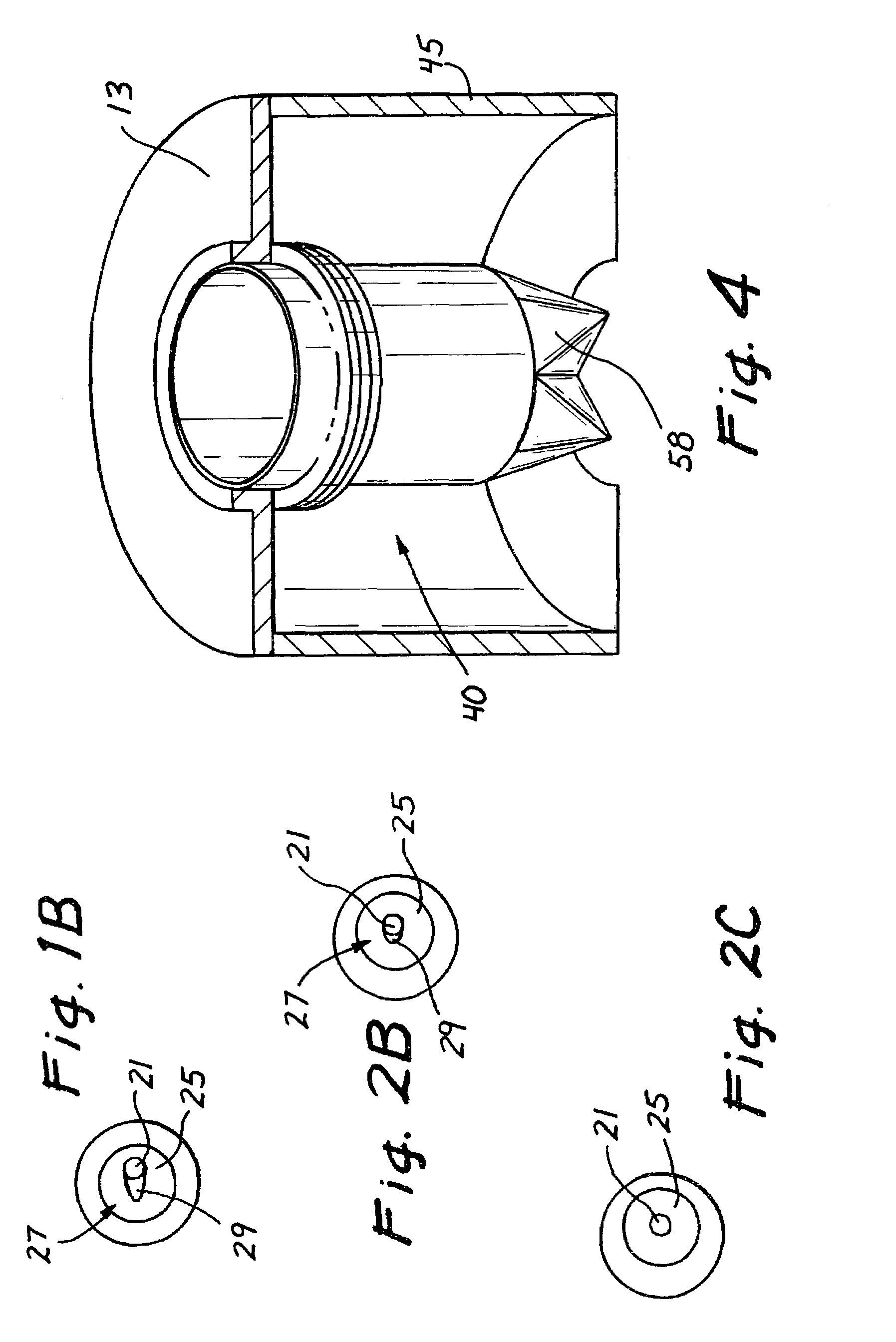
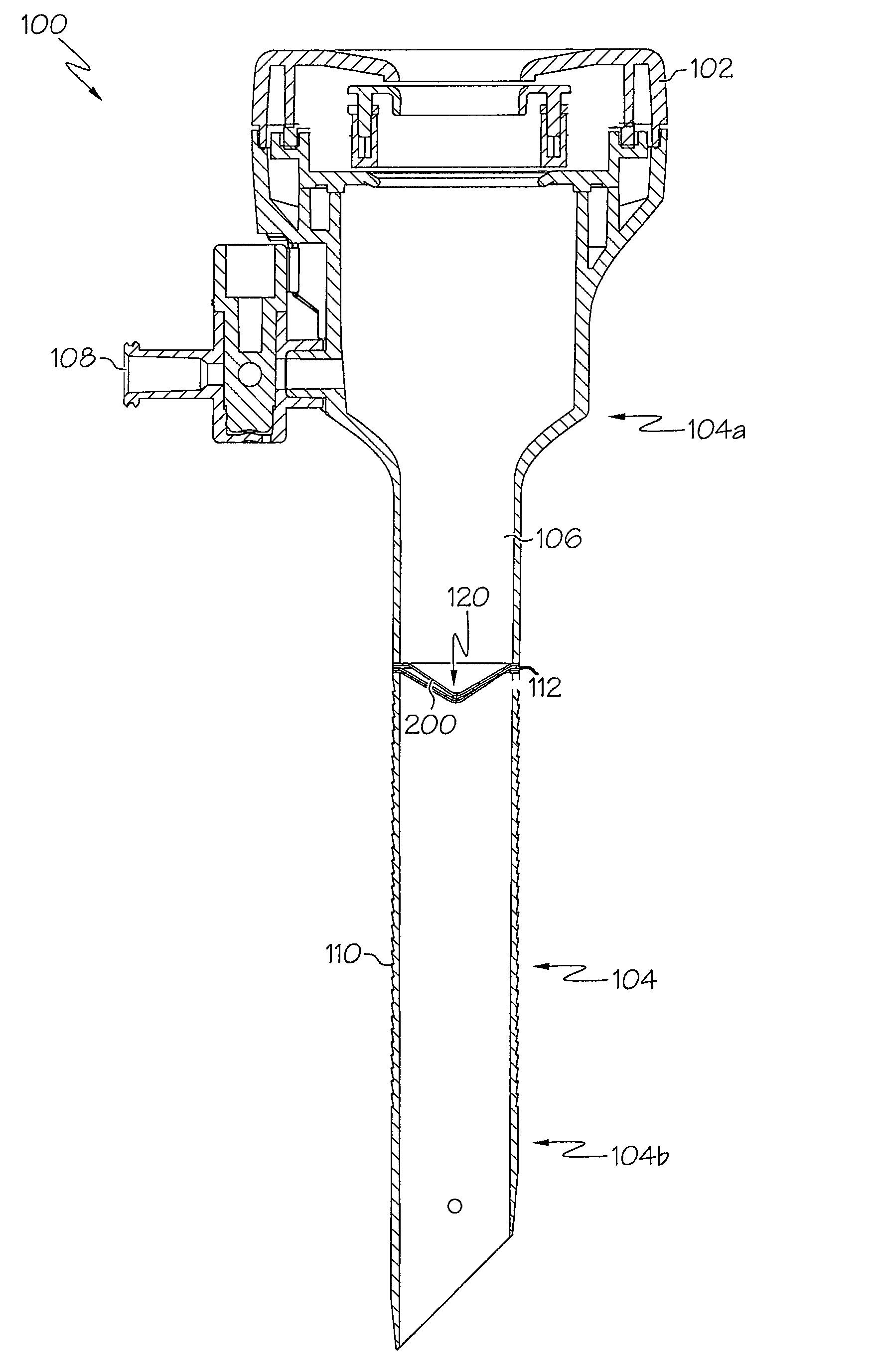
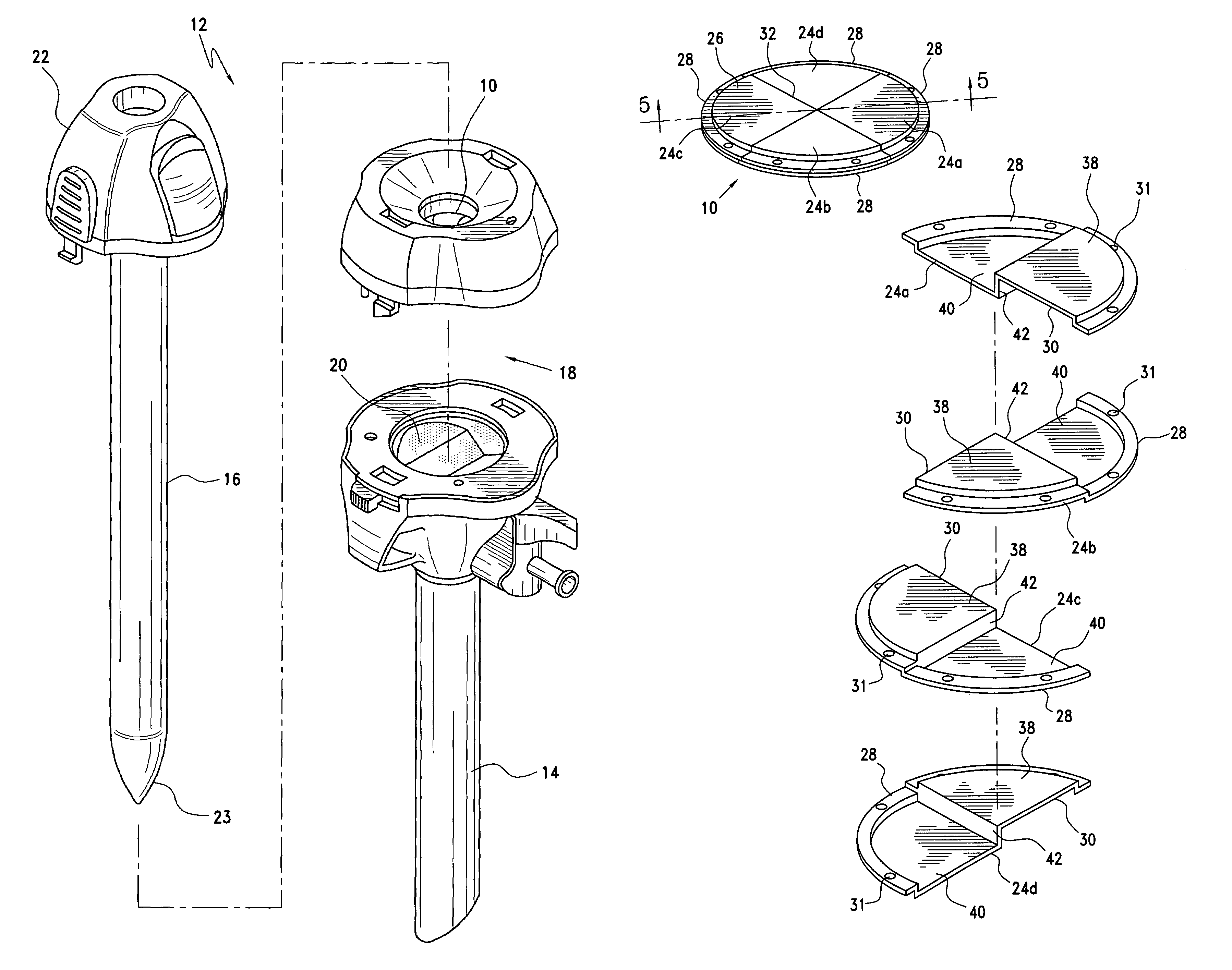
Trocar seal assembly
A seal assembly adapted for use in conjunction with a trocar assembly includes a plurality of seal segments, each seal segment including a peripheral edge and a seam edge. Each seal segment further includes a first section and a second section with a vertical wall connecting the first section and the second section in such a way that the first section is displaced relative to the second section such that the first section of a first seal segment may be placed upon the second section of a second seal segment with the first sections of the first and second seal segments lying in the same plane and the second sections of the first and second seal segments lying in the same plane. The seal segments are assembled in an overlapping woven arrangement to provide a complete seal body without the need for a secondary seal.
Owner:CILAG GMBH INT
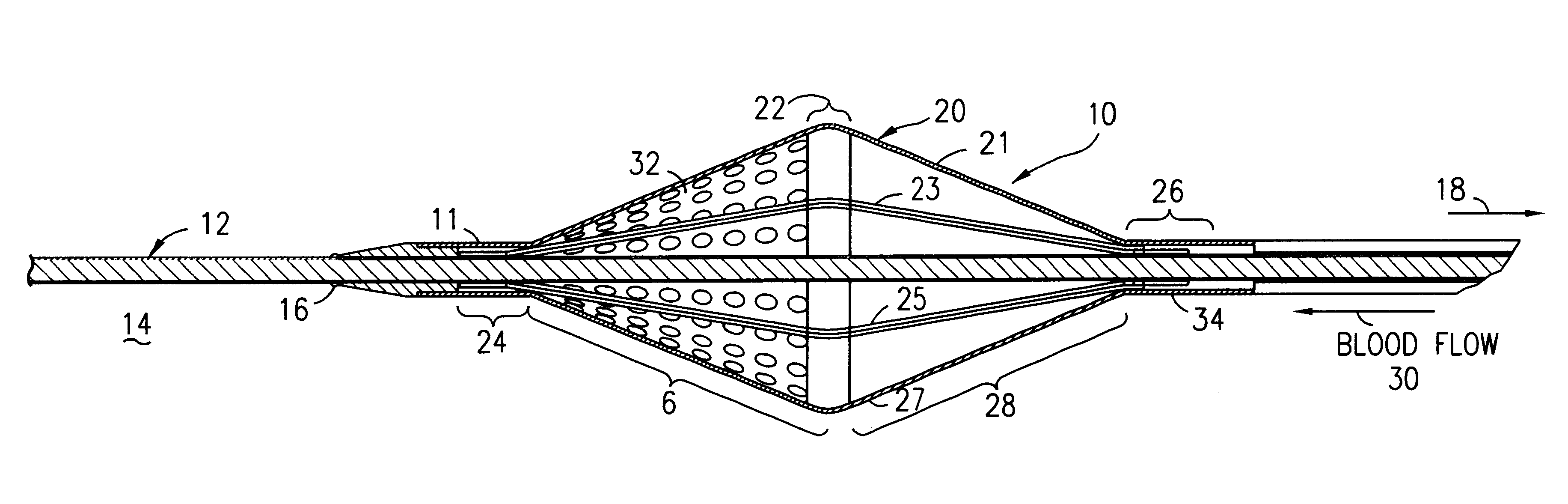
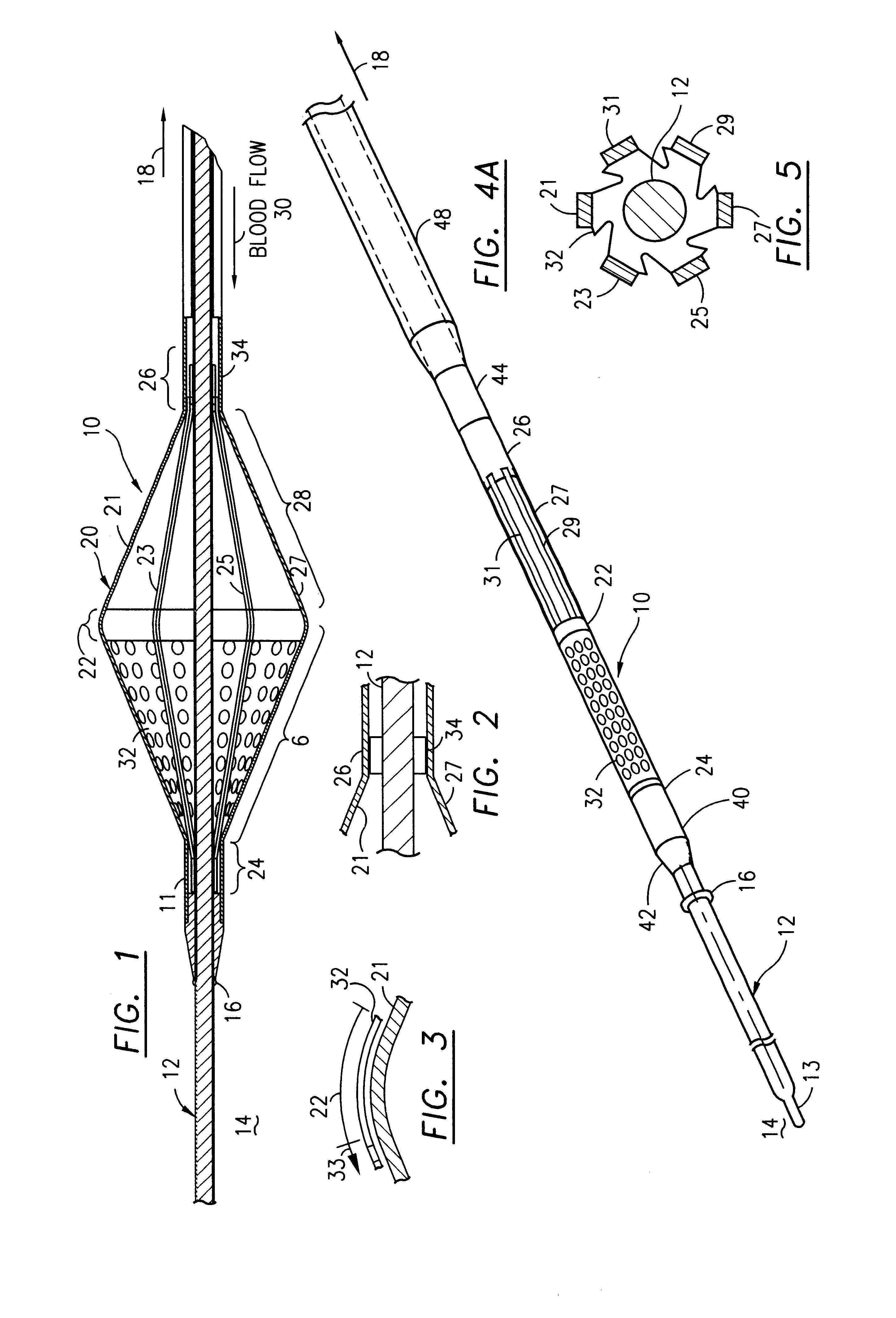
Filter for embolic material mounted on expandable frame
The filter device captures embolic material in a blood vessel and is placed in the blood vessel via a guide wire. The guide wire has a proximal end, a distal end and a stop near its distal end. The filter device includes an expandable frame of frame struts having a closed, radially compact form and an open, radially expanded form. The frame, in the radially expanded form, has frame struts forming a pair of facing frustoconical frame structures. Filter material is attached to one of the pair of frustoconical frame structures. In one embodiment, the filter material is a perforated membrane. The guide wire extends through the expandable frame and the expandable frame is freely movable over the guide wire (likewise, the guide wire is freely movable within the frame), both rotatably and longitudinally, except distally beyond the stop near the distal end of the guide wire. This mobility of the guide wire with respect to the expandable frame enables to guide wire to be guided by the operator through the blood vessel.
Owner:SCION CARDIO VASCULAR
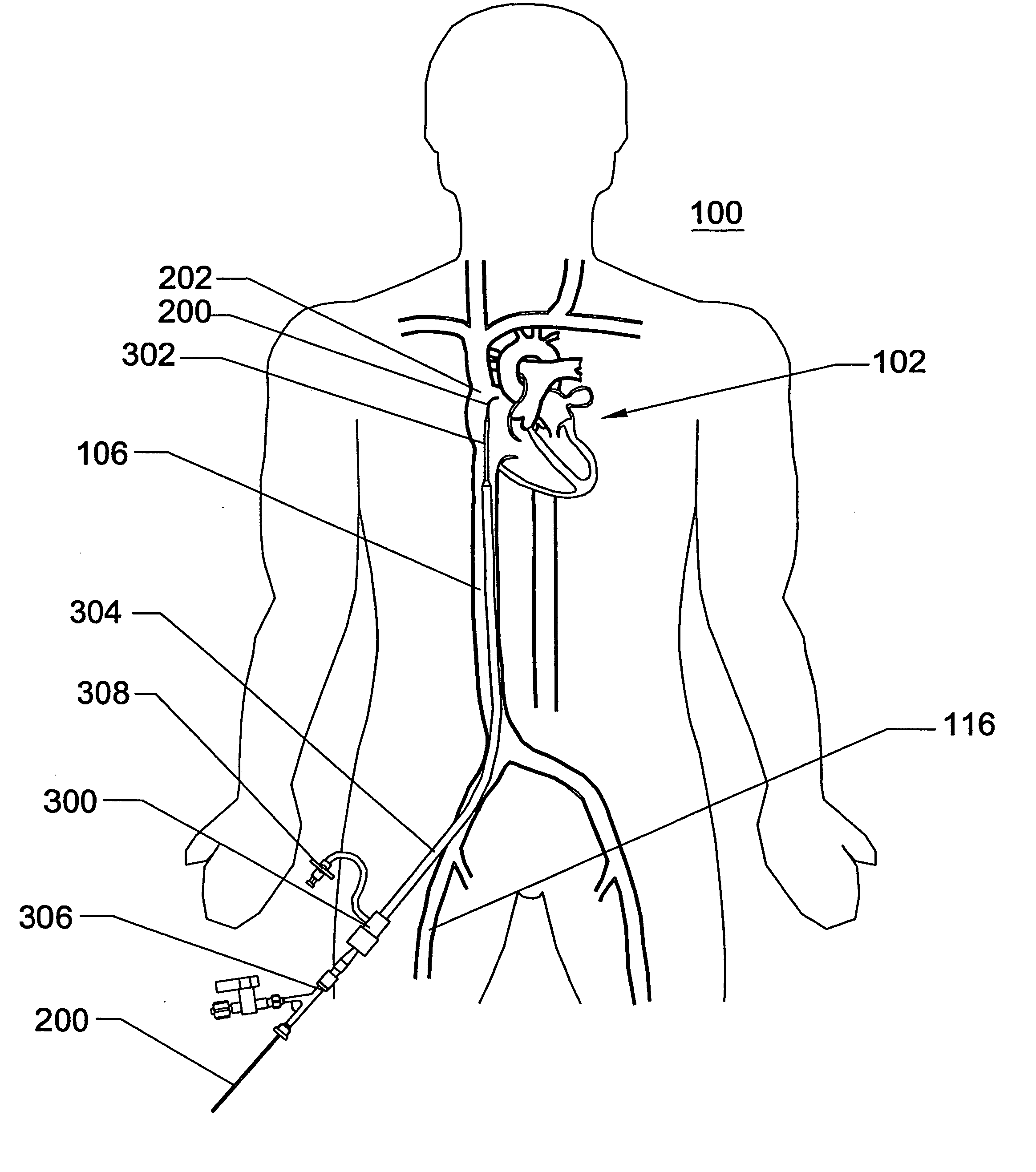
MR-compatible devices
A catheter is used for medical treatments within an organism. The catheter comprises at least one lumen. Within the at least one lumen are at least two microcatheters, with at least one of the at least two microcatheters being connected to a source of liquid material to be delivered to the organism and another of the at least two microcatheters being connected to a system capable of effecting a medical treatment other than delivery of the liquid.
Owner:STANDFORD UNIV +2
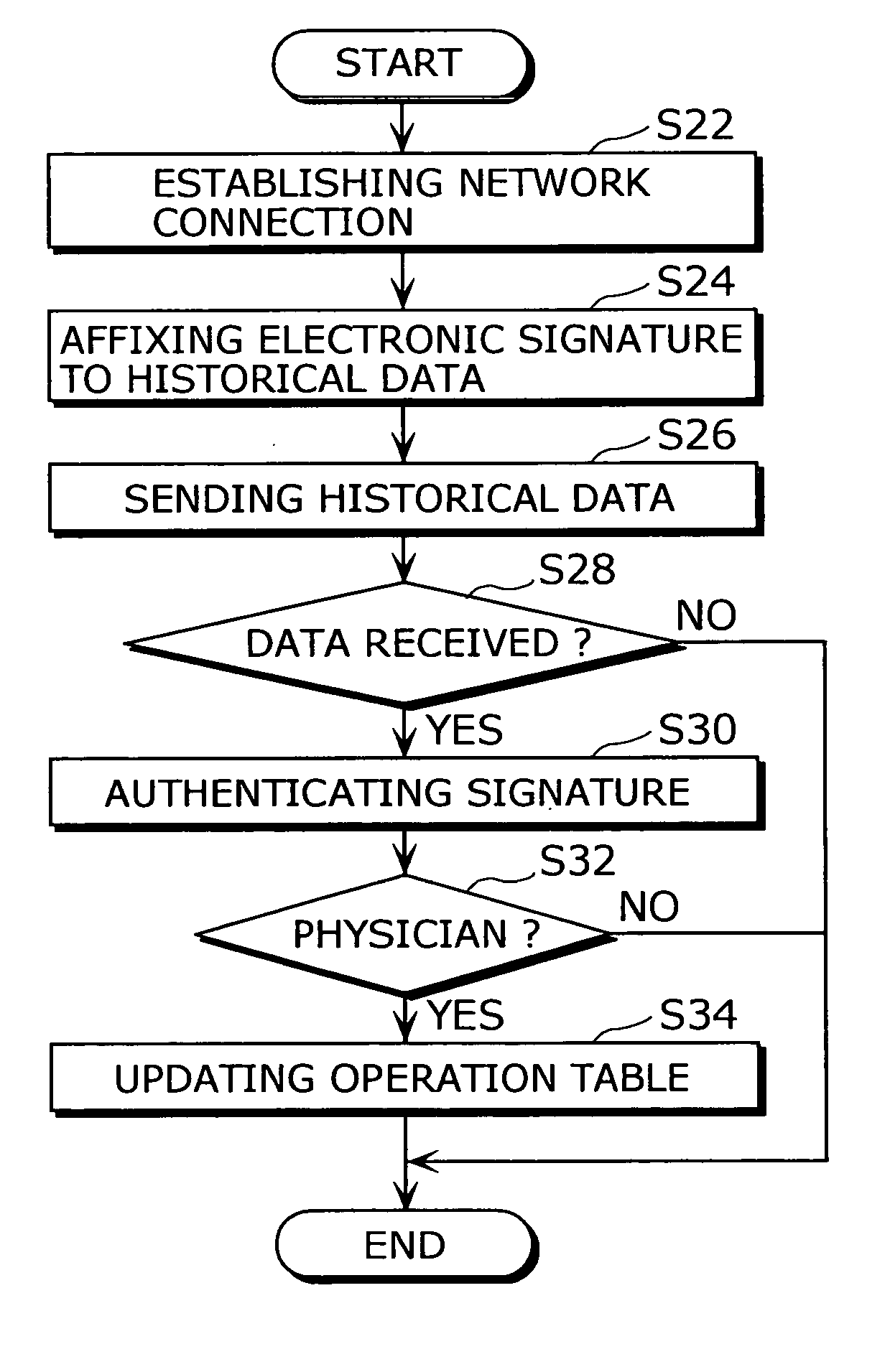
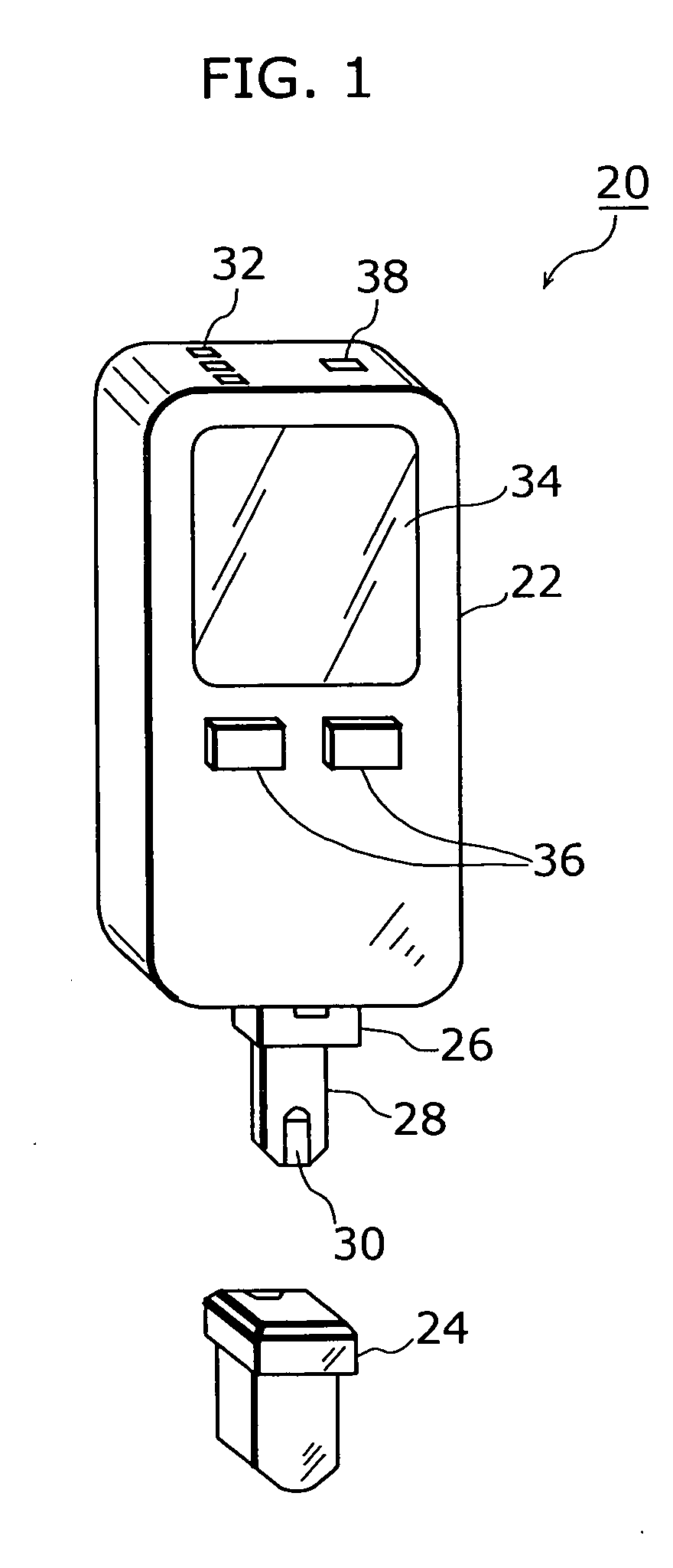
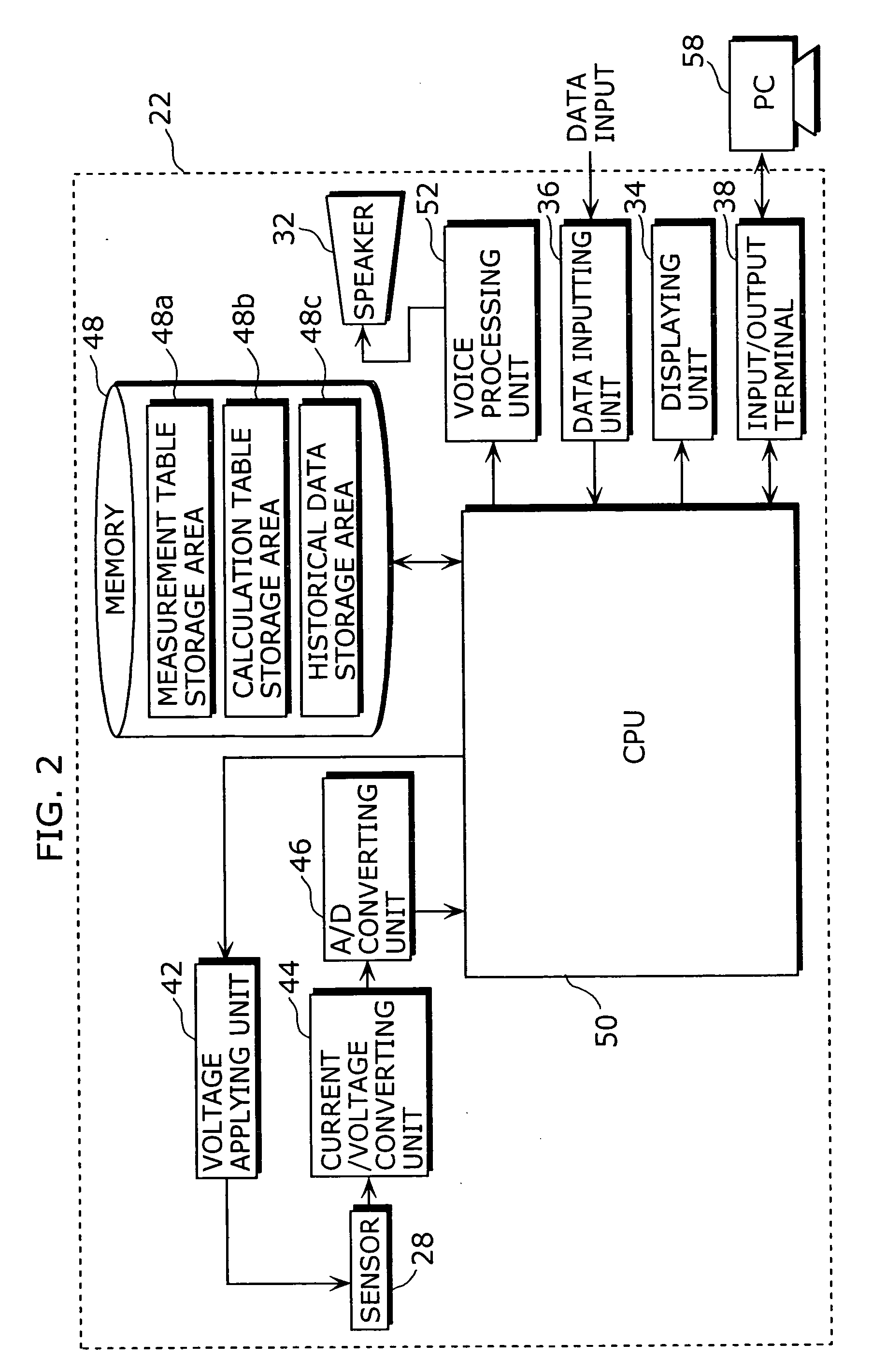
Dosage determination supporting device, injector, and health management supporting system
InactiveUS20050177398A1Improve reliabilityImprove portabilityData processing applicationsInfusion syringesSupporting systemMedicine
A dosage determination supporting apparatus, which is able to precisely determine a dosage in accordance with the health condition of a user, is provided with: a sensor (28) for measuring the blood sugar level obtained from the blood of the user; a memory (48) for storing an operation table showing a correspondence between the blood sugar level and the amount of insulin; a CPU (50) for calculating the amount of insulin corresponding to the blood sugar level, with reference to the operation table stored in the memory (48); a displaying unit (34) for displaying the amount of insulin; and a voice processing unit (52) for performing the voice processing on the amount of insulin and outputting the voice through a speaker (32).
Owner:PANASONIC CORP
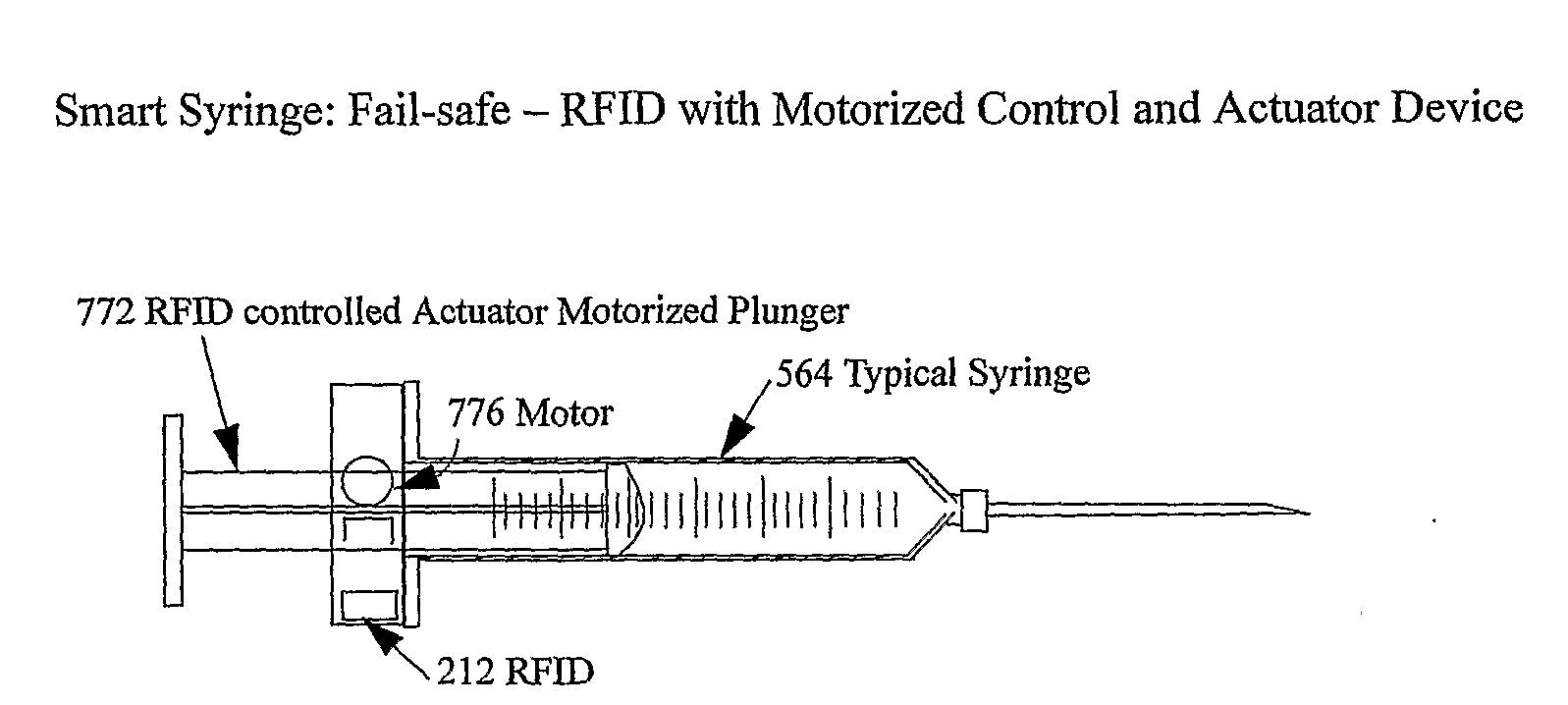
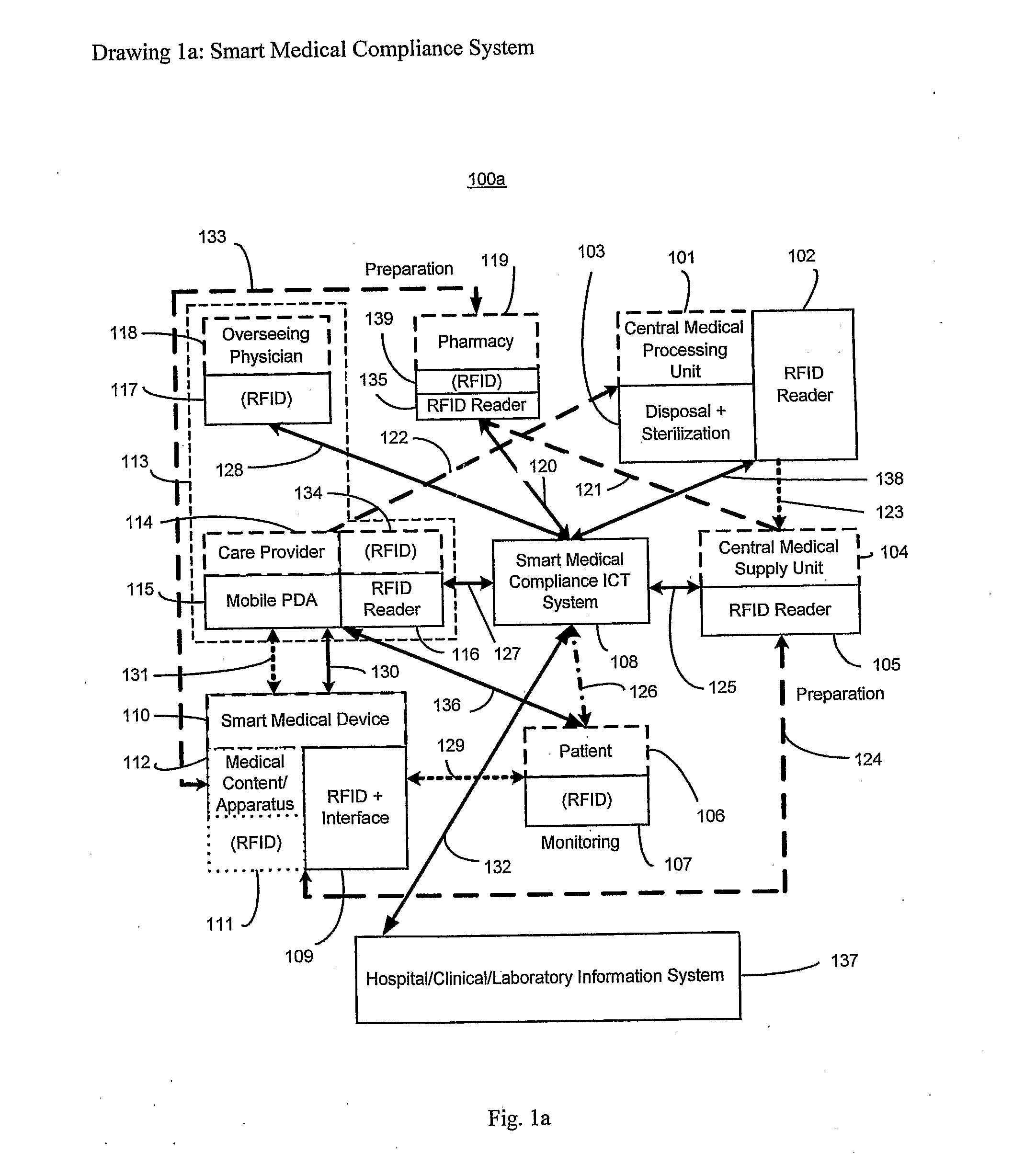
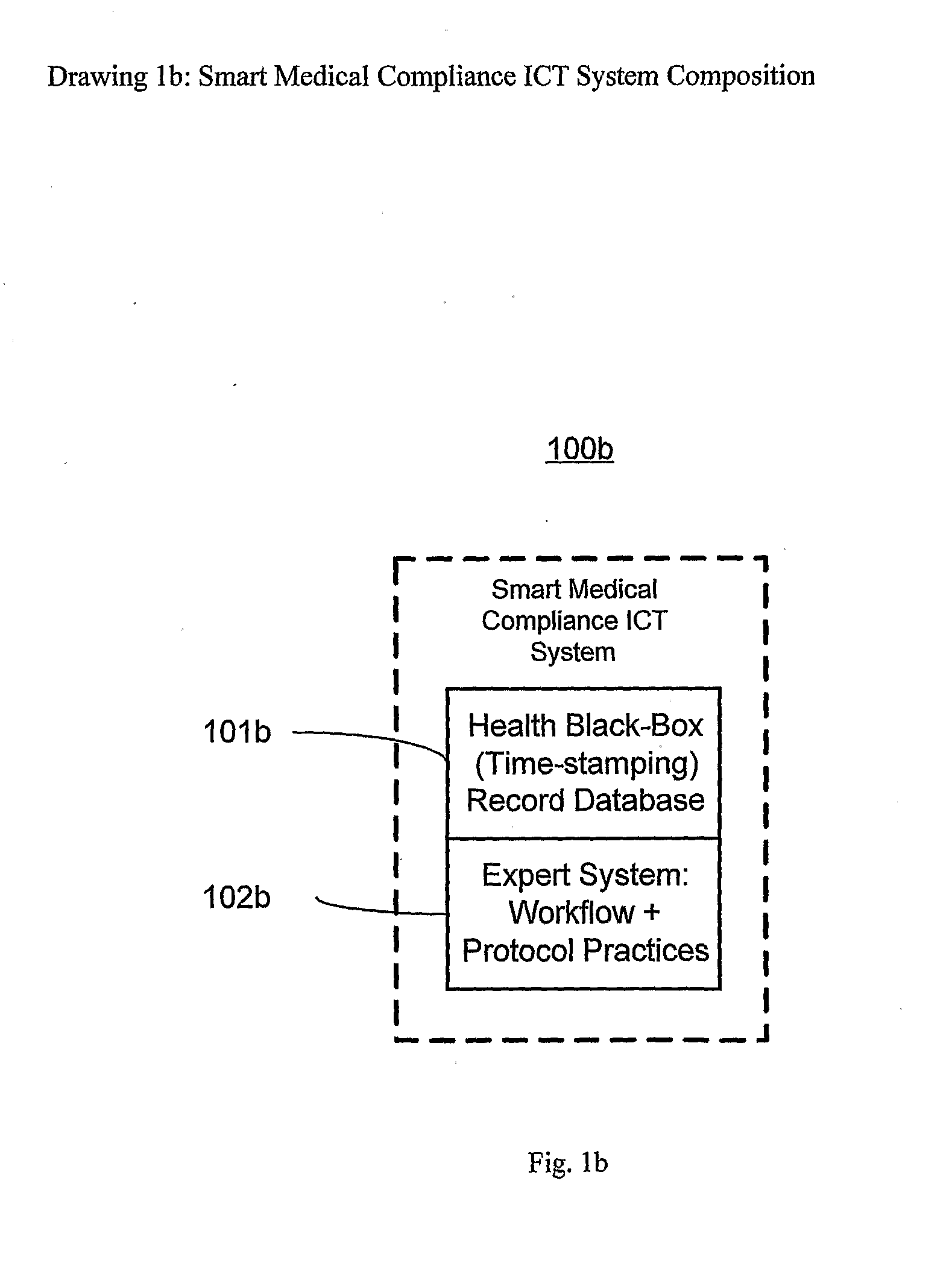
Smart medical compliance method and system
The smart medical compliance method and system invention prevents adverse drug events through the use of protocols that uniquely identifies the patient, care provider, medication and / or medical device that is to be used with radio frequency identification (RFID). The RFID devices incorporate fail-safe locks or indicators that prevent the inadvertent or unauthorized use of medication, medical devices, or medical supplies. The system corroborates, patient, the care provider, the medical device, and the manner in which it is to be used, and authorizes the action to be undertaken through an interface on a personal digital assistant PDA over a wireless communication channel. The system also timestamps events in the equivalent of a medical black box such that records may be kept to further improve patient care and allow an analysis of procedures. In addition, the system includes interfaces to medication preparation and safe disposal. A number of smart devices that interact with the system are also described. These include smart medical containers, smart clamps, smart valves, smart syringes, smart couplers, smart pipettes, and a host of other point of care devices.
Owner:KYAB LULEA
Adhesive Patch Systems and Methods
ActiveUS20080269687A1High bonding strengthReduced adhesion strengthInfusion syringesFiltering accessoriesAdhesion strengthUltimate tensile strength
Various embodiments of the present invention are directed to patches for medical devices. In various embodiments, an adhesive patch of a medical device may have selective areas with adhesive material of varying adhesion strengths. In other embodiments, an adhesive patch of a medical device may include adhesive material that may be activated by a catalyst to increase or decrease the adhesion strength of the adhesive material. In further embodiments, a medical device may include a pierceable membrane containing an agent, the pierceable membrane positioned to be pierced by a needle and to cause some of the agent to be carried to the user-patient.
Owner:MEDTRONIC MIMIMED INC
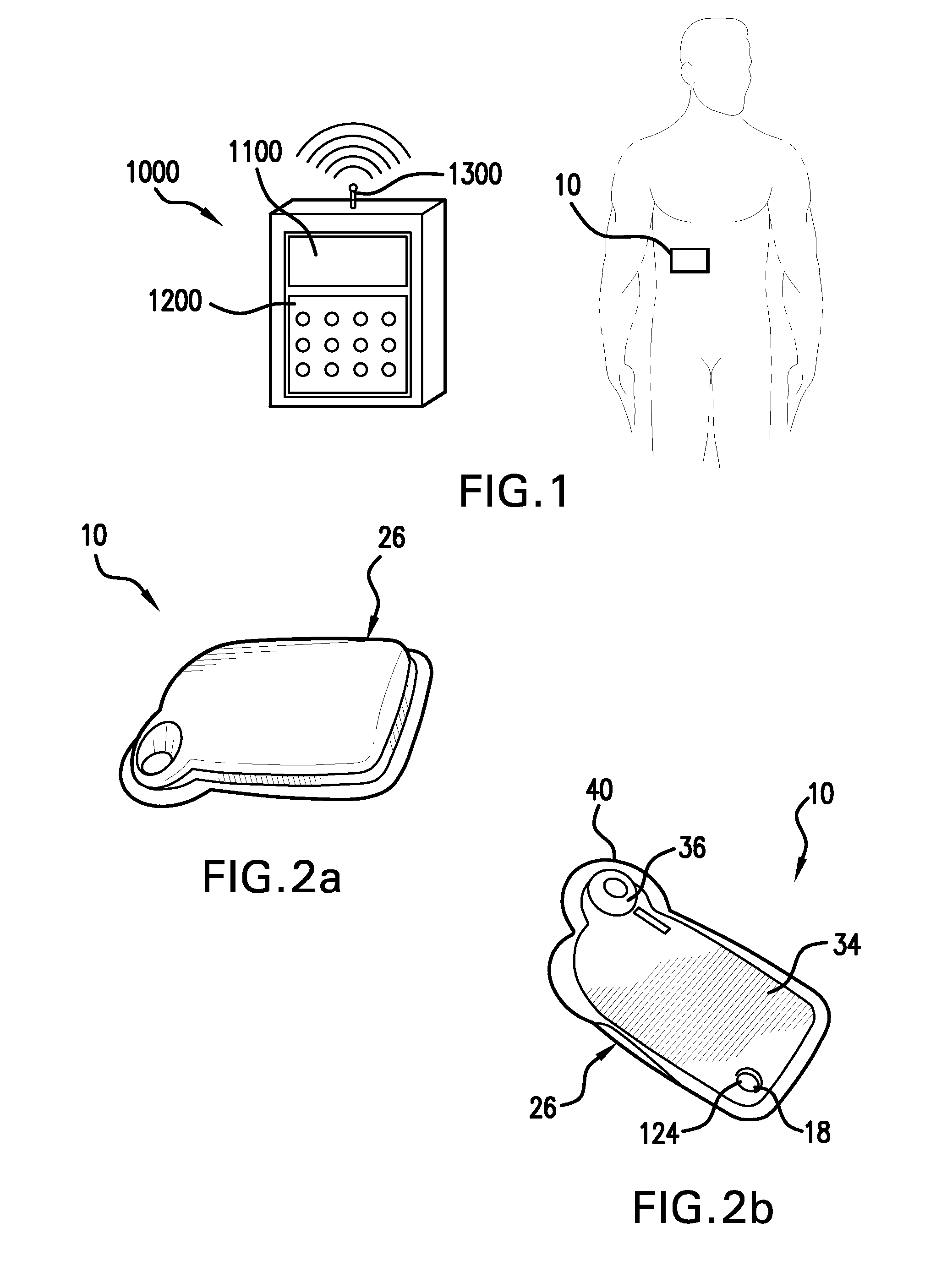
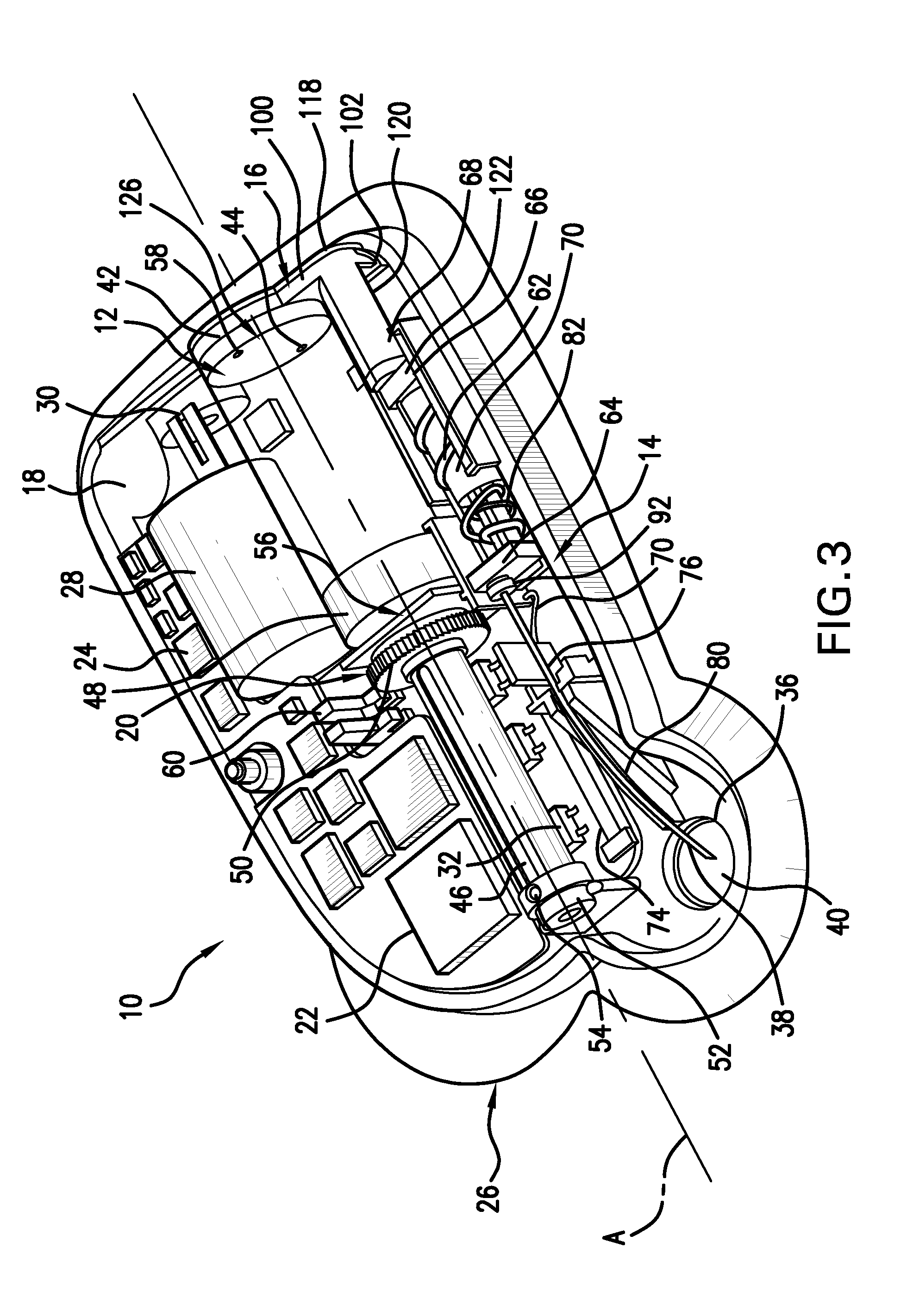
Infusion set
ActiveUS7879010B2Minimise and rule out dangerReduce stepsGuide needlesInfusion syringesMedicineInfusion set
A device for inserting a cannula into tissue, including a cannula, a protective element which can accommodate said cannula, an operating element for moving the cannula out of the protective element, and a holder fixedly connected to the cannula. The invention encompasses a system for connecting a liquid supply to the cannula.
Owner:ROCHE DIABETES CARE INC
Components and methods for patient infusion device
Owner:INSULET CORP
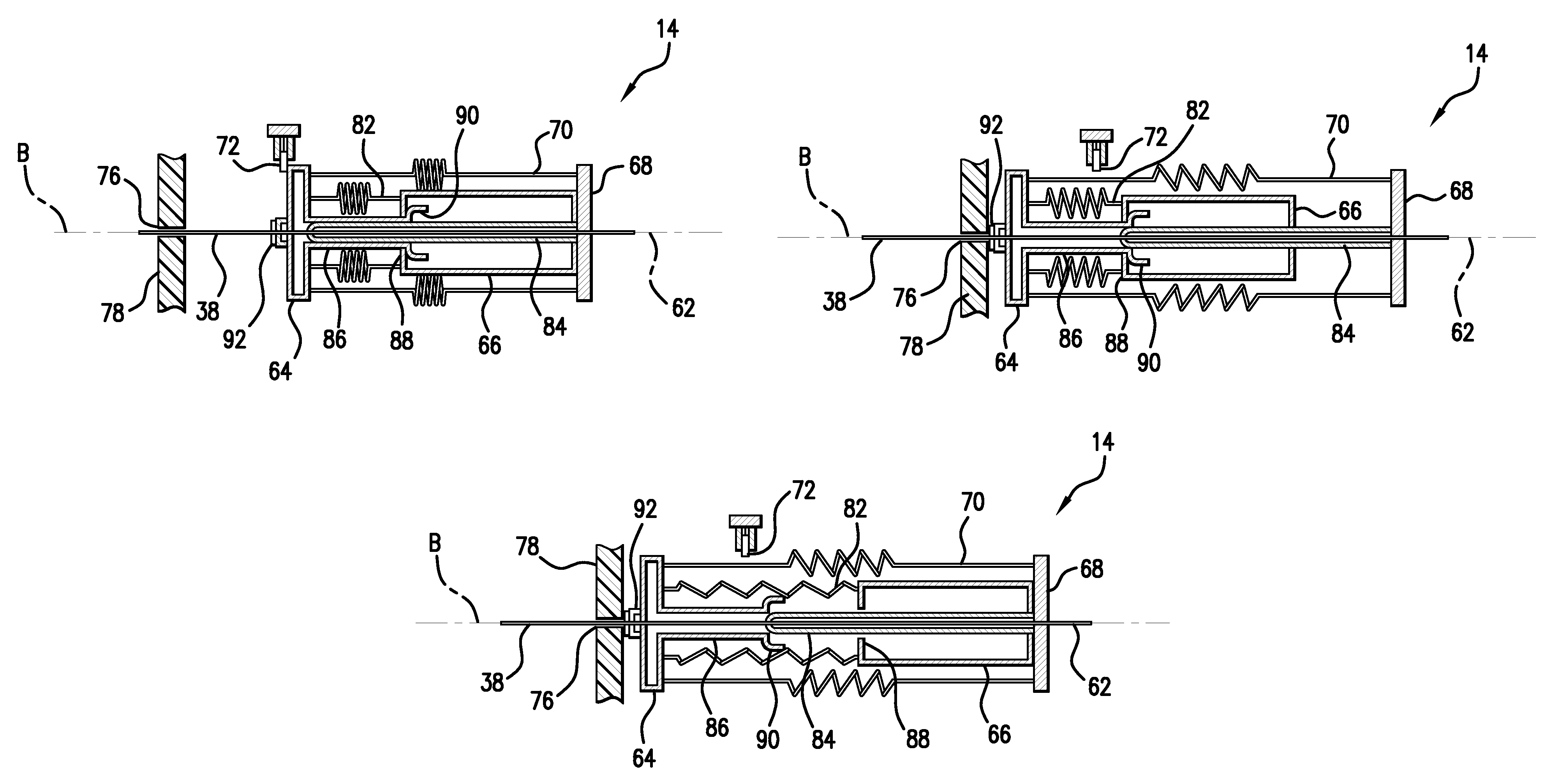
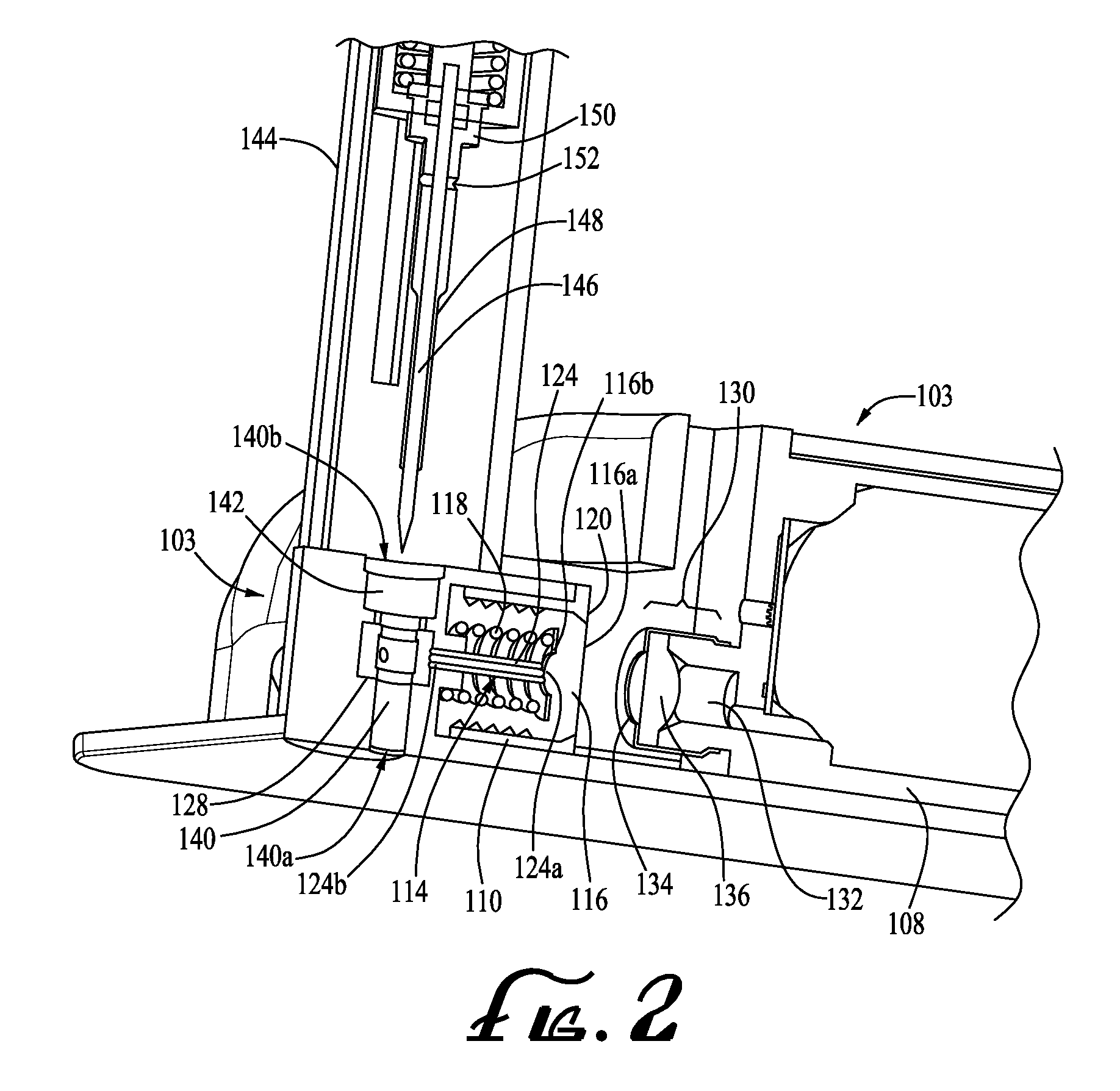
Infusion medium delivery system, device and method with needle inserter and needle inserter device and method
An infusion medium delivery system, device and method for delivering an infusion medium to a patient-user, includes a needle inserter device and method for inserting a needle and / or cannula into a patient-user to convey the infusion medium to the patient-user. The needle inserter device and method operate to insert a needle and cannula into a patient-user's skin and automatically withdraw the needle from the patient-user, leaving the cannula in place and in fluid flow communication with a reservoir. The delivery device may include a base portion and a durable portion connectable to the base portion, and wherein the base portion can be separated from the durable portion and disposed of after one or more specified number of uses. The base portion supports the reservoir and the needle inserter device, while the durable portion supports a drive device for selectively driving the infusion medium out of the reservoir and into the needle and / or cannula.
Owner:MEDTRONIC MIMIMED INC
Apparatus and method for isolated lung access
Apparatus, systems, methods, and kits are provided for isolating a target lung segment and treating that segment, usually by drug delivery or lavage. The systems include at least a lobar or sub-lobar isolation catheter which is introduced beyond a second lung bifurcation (i.e., beyond the first bifurcation in a lobe of the lung) and which can occlude a bronchial passage at that point. An inner catheter is usually introduced through the isolation catheter and used in cooperation with the isolation catheter for delivering and / or removing drugs or washing liquids from the isolated lung region. Optionally, the inner catheter will also have an occluding member near its distal end for further isolation of a target region within the lung.
Owner:PULMONX
Apparatus and method for delivering therapeutic and/or other agents to the inner ear and to other tissues
An apparatus may include a needle for sustained delivery of drugs and other agents to the inner ear or other tissues of a human or an animal. The needle can include an insertion stop, and can be placed through the round window membrane or through a surgically-prepared hole in a bone. The needle can be in fluid communication with a port and / or with a micro-infusion or osmotic pump. A cochlear implant electrode can be used instead of a needle.
Owner:NEUROSYSTEC CORP
Steerable device for accessing a target site and methods
ActiveUS20060167416A1Easy steeringGood drainageInfusion syringesSurgical needlesBending forceBiomedical engineering
A variety of steerable needles, lancets, trocars, stylets, cannulas and systems are provided for examining, diagnosing, treating, or removing tissue from a patient. The steerable needles, trocars, stylets, cannulas and systems also provide a platform for delivery of target materials, such as therapeutics, biologics, polymers, glues, etc., to a target site. An embodiment of the invention includes a steerable device for use in accessing target site in a patient comprising: a steerable member adapted to penetrate tissue; and a steering mechanism adapted to be operated by a user to apply a bending force to bend the steerable member to access the target site.
Owner:EKOS CORP
Methods and devices for diagnostic and therapeutic interventions in the peritoneal cavity
A novel approach to diagnostic and therapeutic interventions in the peritoneal cavity is described. More specifically, a technique for accessing the peritoneal cavity via the wall of the digestive tract is provided so that examination of and / or a surgical procedure in the peritoneal cavity can be conducted via the wall of the digestive tract with the use of a flexible endoscope. As presently proposed, the technique is particularly adapted to transgastric peritoneoscopy. However, access in addition or in the alternative through the intestinal wall is contemplated and described as well. Transgastric and / or transintestinal peritoneoscopy will have an excellent cosmetic result as there are no incisions in the abdominal wall and no potential for visible post-surgical scars or hernias.
Owner:APOLLO ENDOSURGERY INC
Adhesive patch systems and methods
Owner:MEDTRONIC MIMIMED INC
Multiport access device
A trocar having a cannula and a valve housing with a polygonal configuration, provides for a floating septum where the float is encouraged in a first direction and restricted in a second direction. The septum includes multiple septum valves which may function with a comnron zero-closure valve, or individually in a valve assembly with an associated zero-closure valve. Various seals can be configured to prevent blow-back. Cup valves, check valves, and reciprocating valves are contemplated, along with various skirt configurations for maintaining pressurized air within the trocar.
Owner:APPL MEDICAL RESOURCES CORP
Sensor for percutaneous intravascular deployment without an indwelling cannula
The present invention relates to a sensor for percutaneous insertion and intravascular residence without an indwelling cannula. In preferred embodiments, a glucose sensor is inserted into a blood vessel using a removable cannula. After the cannula is removed, the glucose sensor remains within the blood vessel by itself and forms a seal with the patient's tissue.
Owner:MEDTRONIC MIMIMED INC
Expandable trans-septal sheath
InactiveUS20060135962A1Inhibit bindingAvoid interferenceGuide needlesEar treatmentAccess routeDilator
Disclosed is an expandable transluminal sheath, for introduction into the body while in a first, low cross-sectional area configuration, and subsequent expansion of at least a part of the distal end of the sheath to a second, enlarged cross-sectional configuration. The sheath is configured for use in the vascular system. The access route is through the inferior vena cava to the right atrium, where a trans-septal puncture, followed by advancement of the catheter is completed. The distal end of the sheath is maintained in the first, low cross-sectional configuration during advancement through the atrial septum into the left atrium. The distal end of the sheath is expanded using a radial dilator. In one application, the sheath is utilized to provide access for a diagnostic or therapeutic procedure such as electrophysiological mapping of the heart, radio-frequency ablation of left atrial tissue, placement of atrial implants, valve repair, or the like.
Owner:ONSET MEDICAL CORP
Infusion medium delivery device and method with compressible or curved reservoir or conduit
ActiveUS8137314B2Increase fluid pressureReduce internal volumeInfusion syringesFlexible member pumpsCatheterEngineering
A delivery device includes a durable housing portion and a separable disposable portion that selectively engage and disengage from each other. The disposable housing portion secures to the patient-user and may be disposed of after it has been in use for a prescribed period. Components that normally come into contact with a patient-user or with infusion media are supported by the disposable housing portion for disposal after the prescribed use, while the durable housing portion supports other components such as electronics for controlling delivery of infusion media from the reservoir and a drive device and drive linkage.
Owner:MEDTRONIC MIMIMED INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com