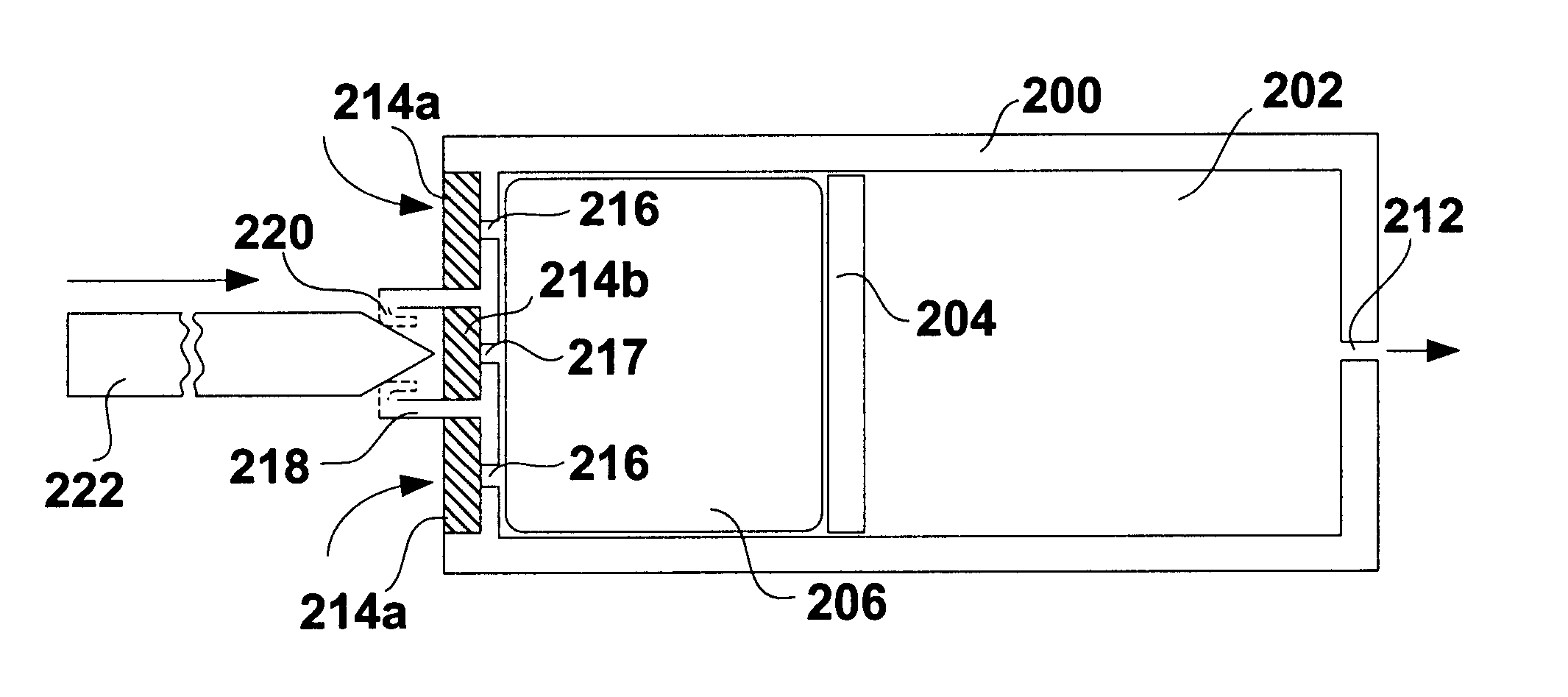
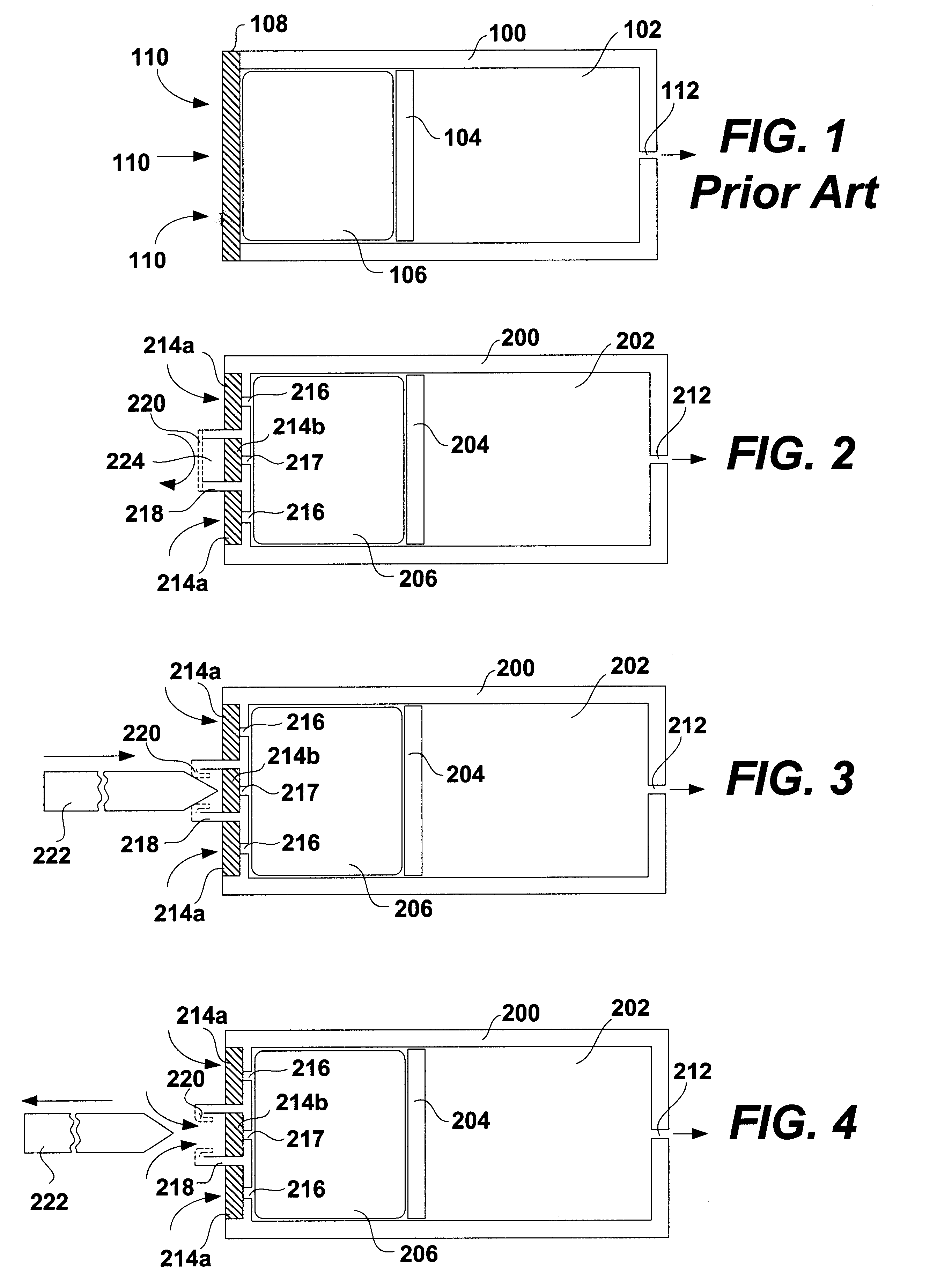
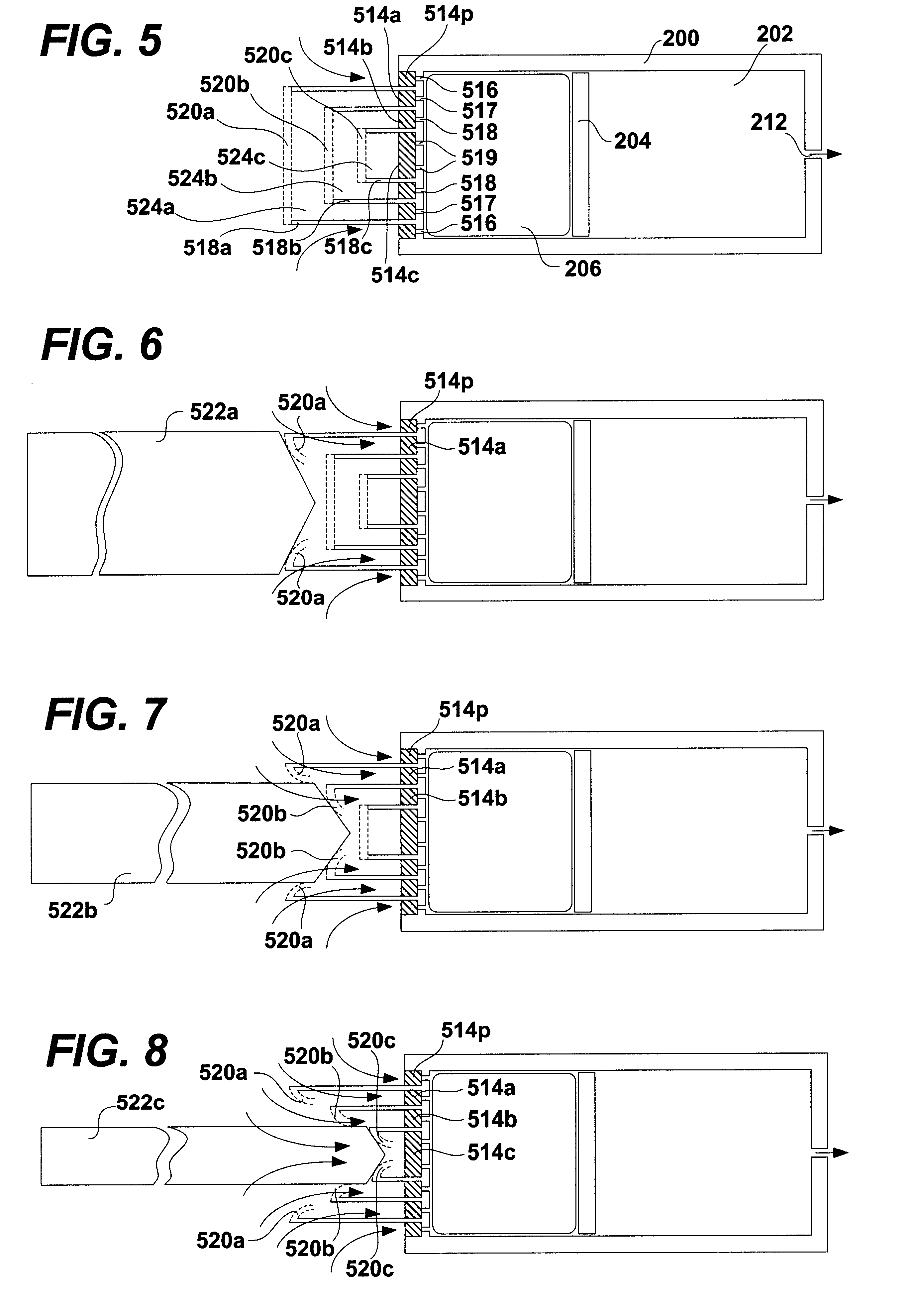
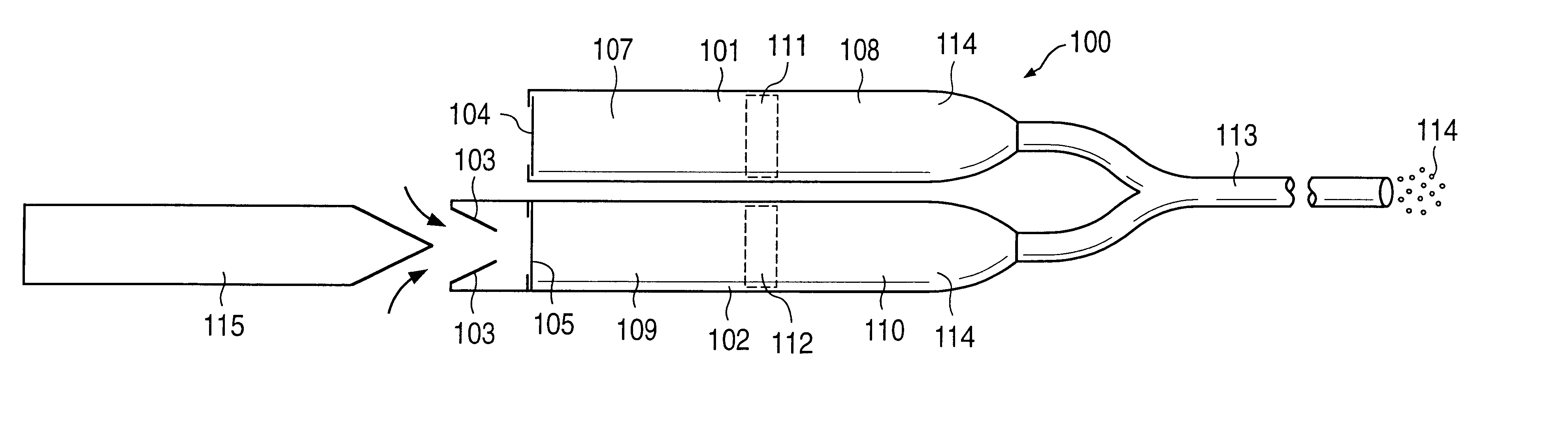
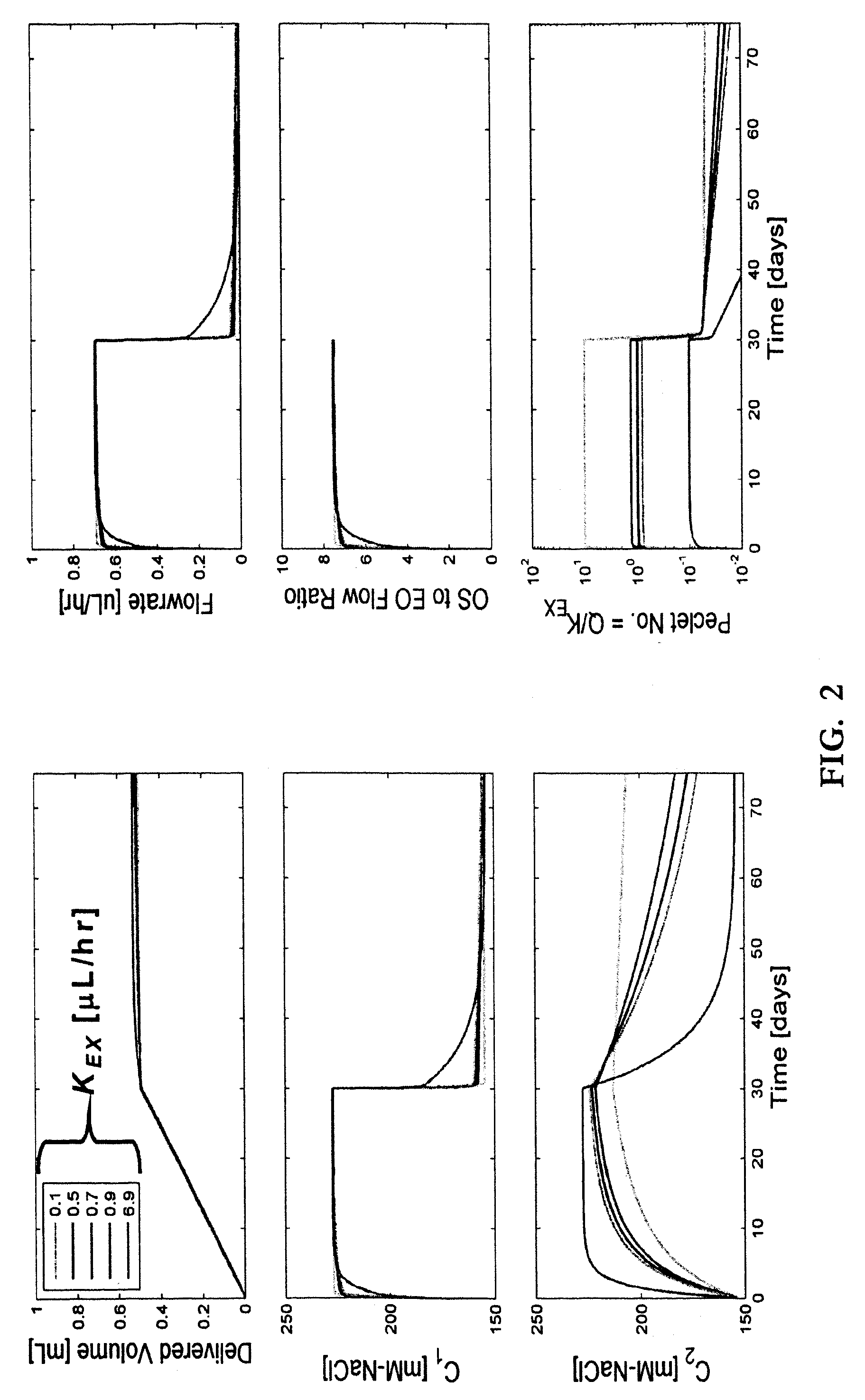
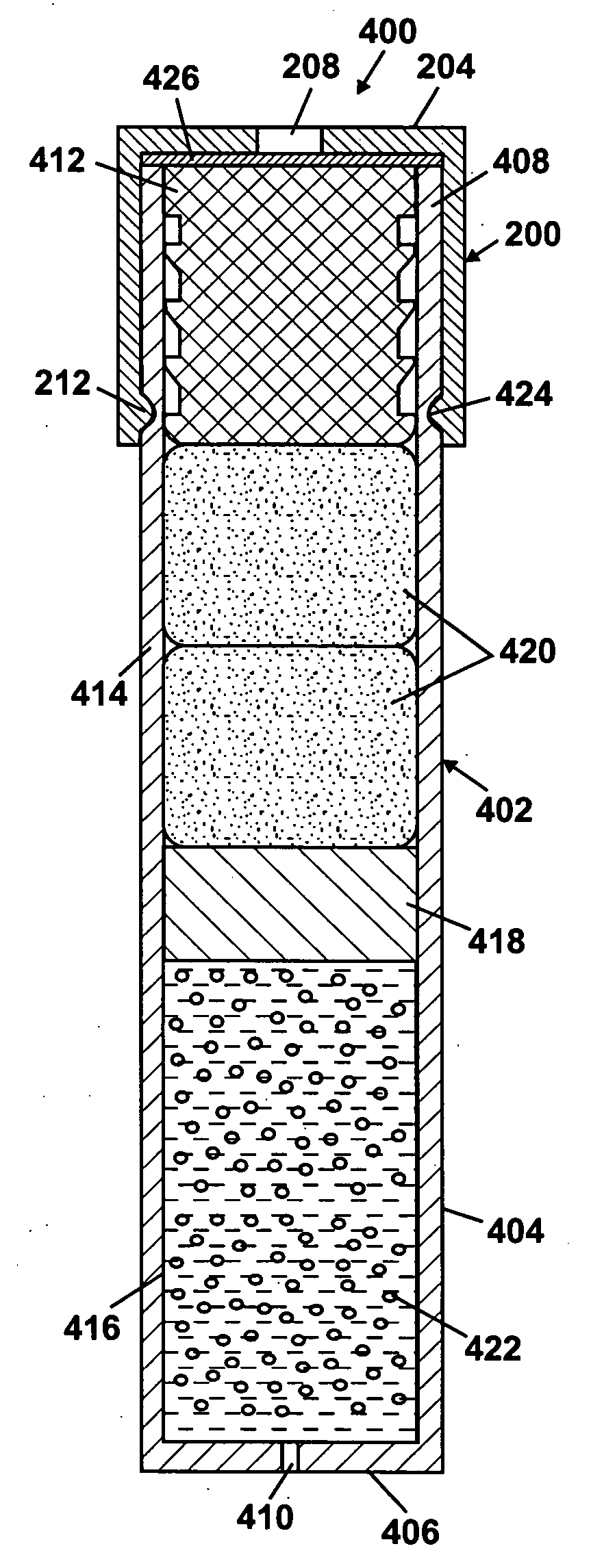
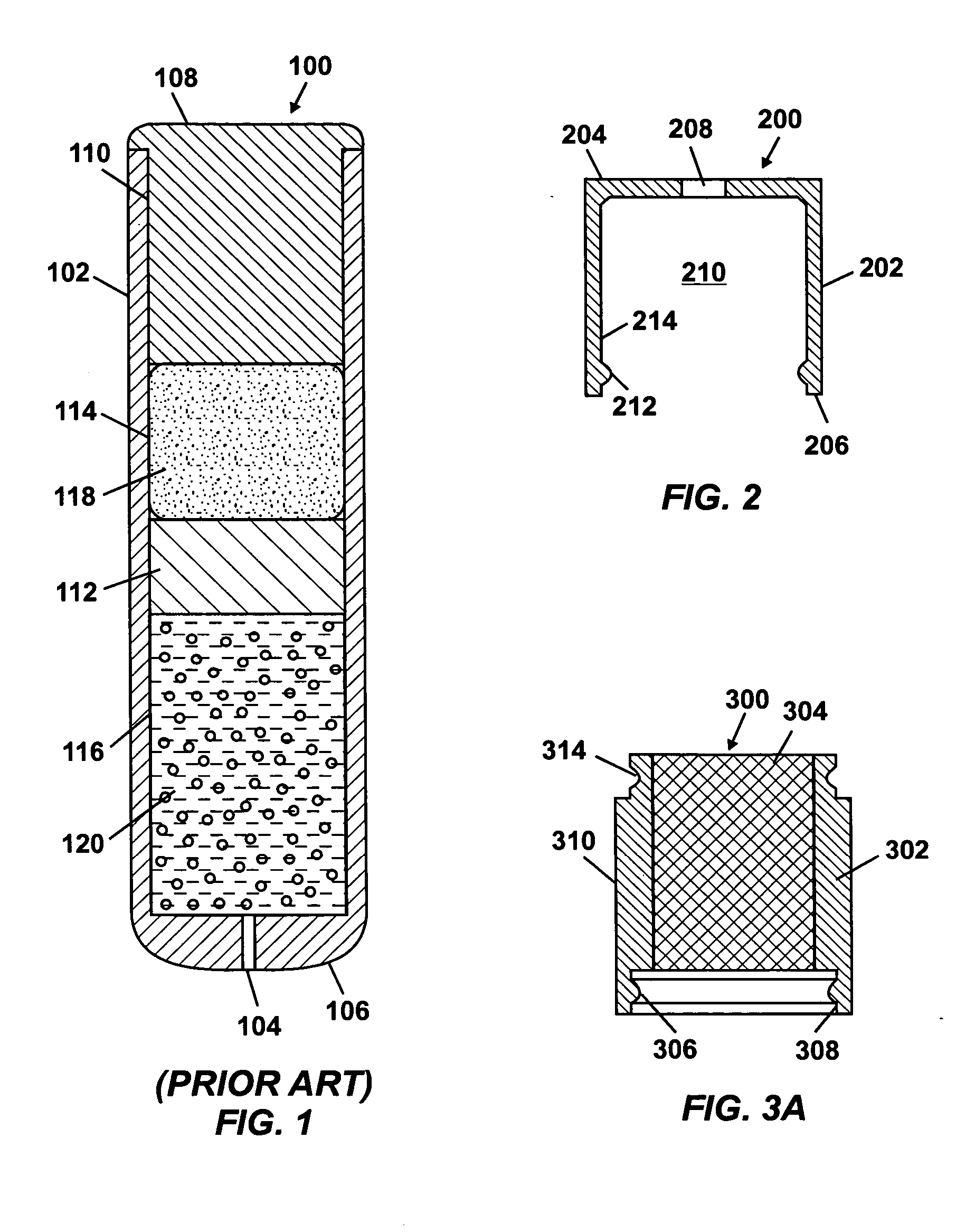
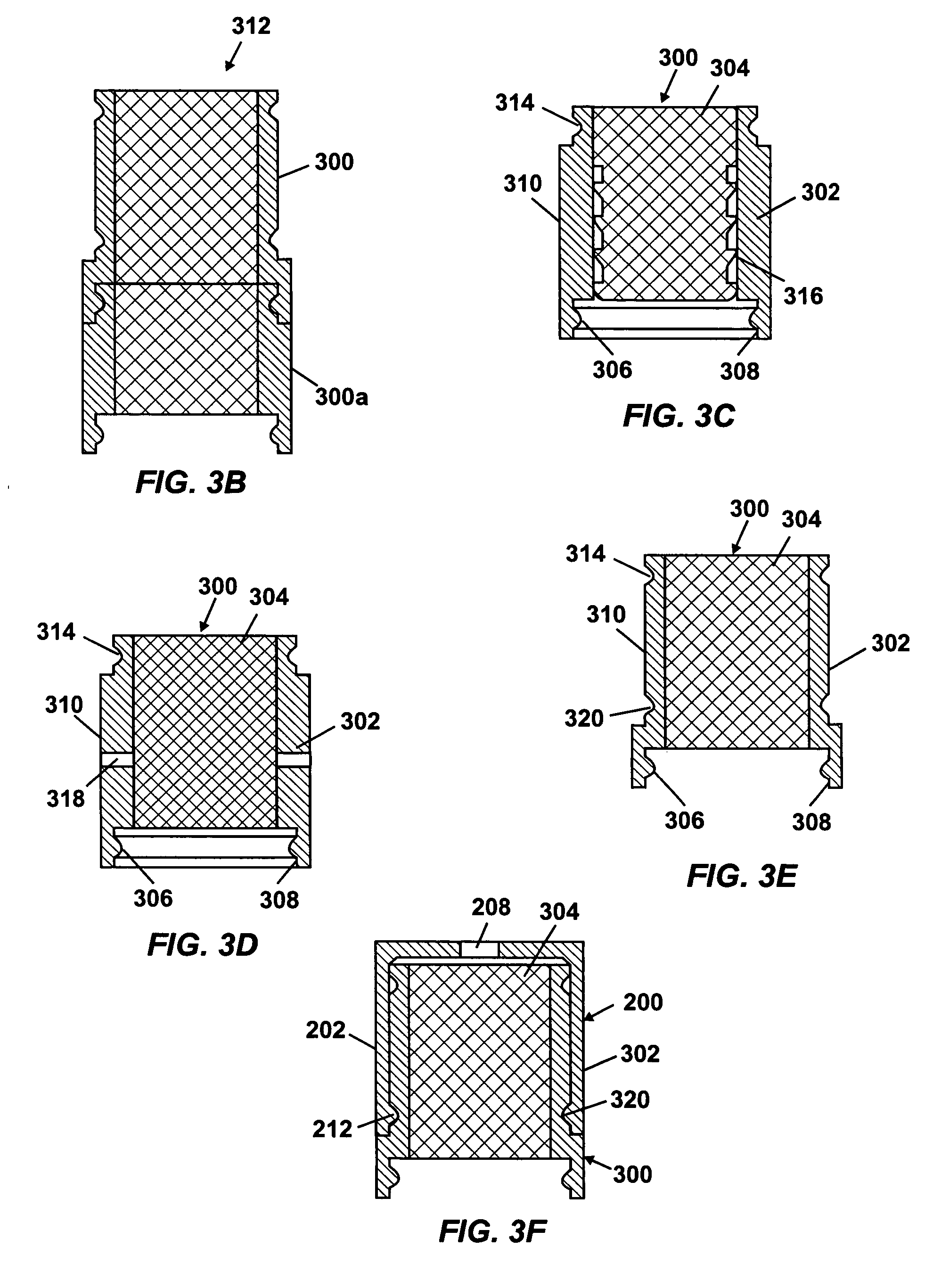
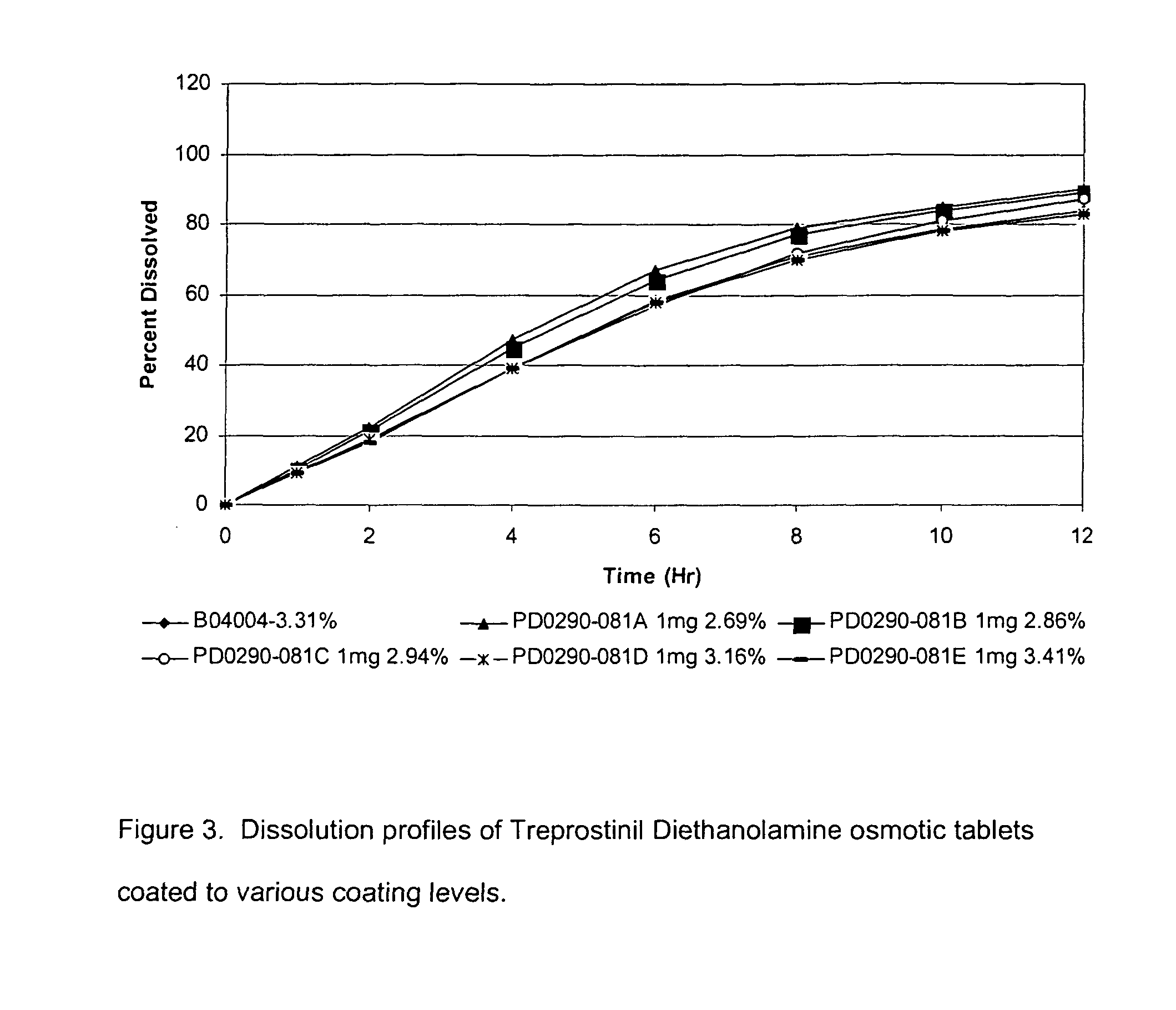
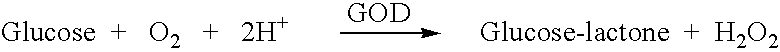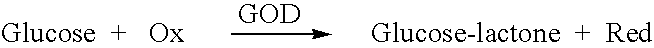Patents
Literature
489 results about "Osmotic pump" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
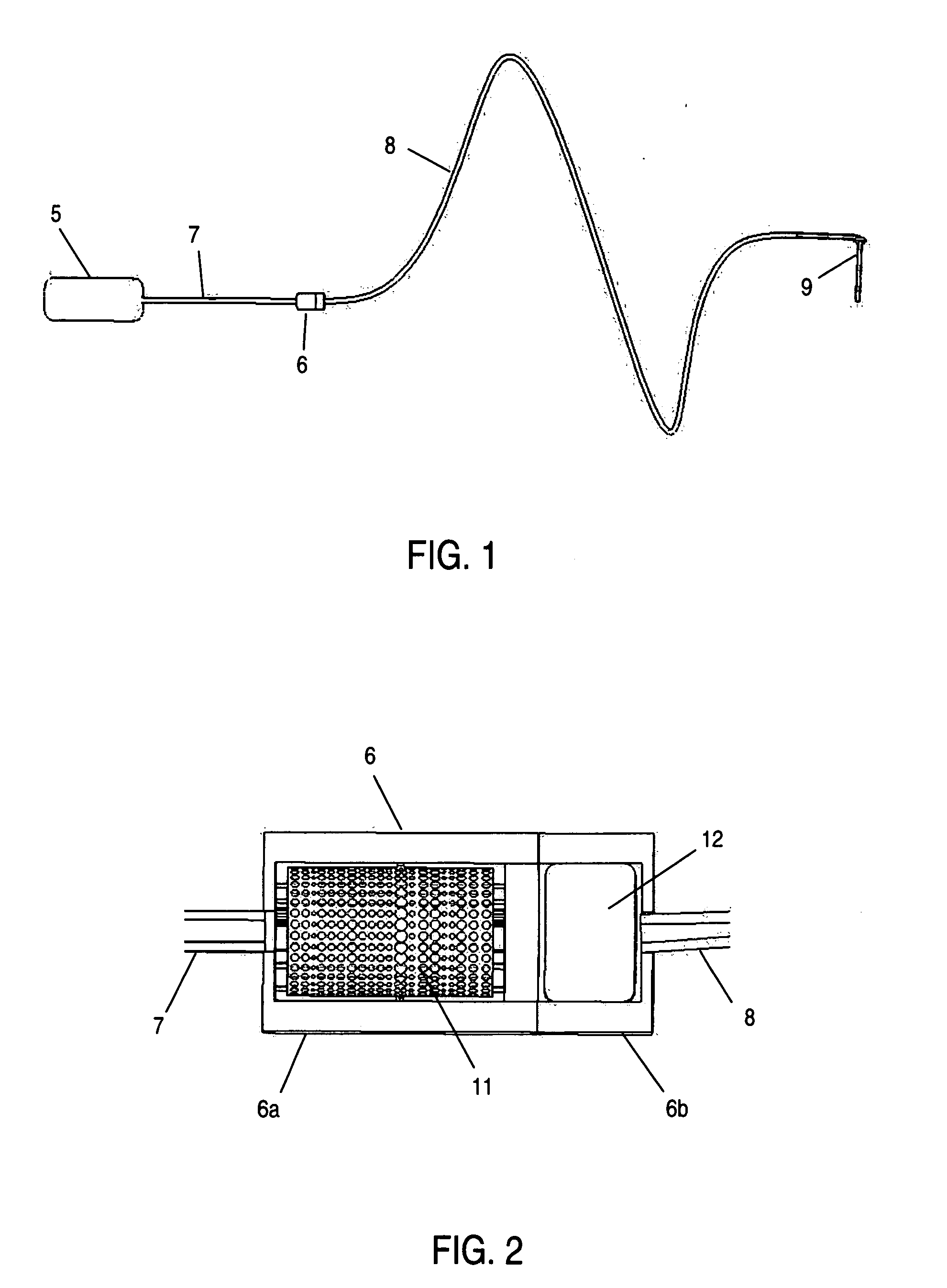
Analyte measurement
InactiveUS20040096959A1Low viscosityMore suitedBioreactor/fermenter combinationsBiological substance pretreatmentsElectrochemical detectorAnalyte
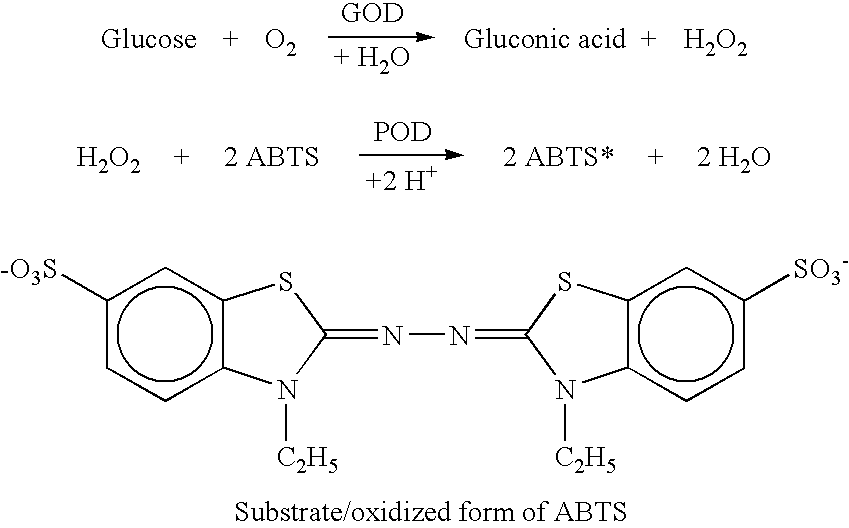
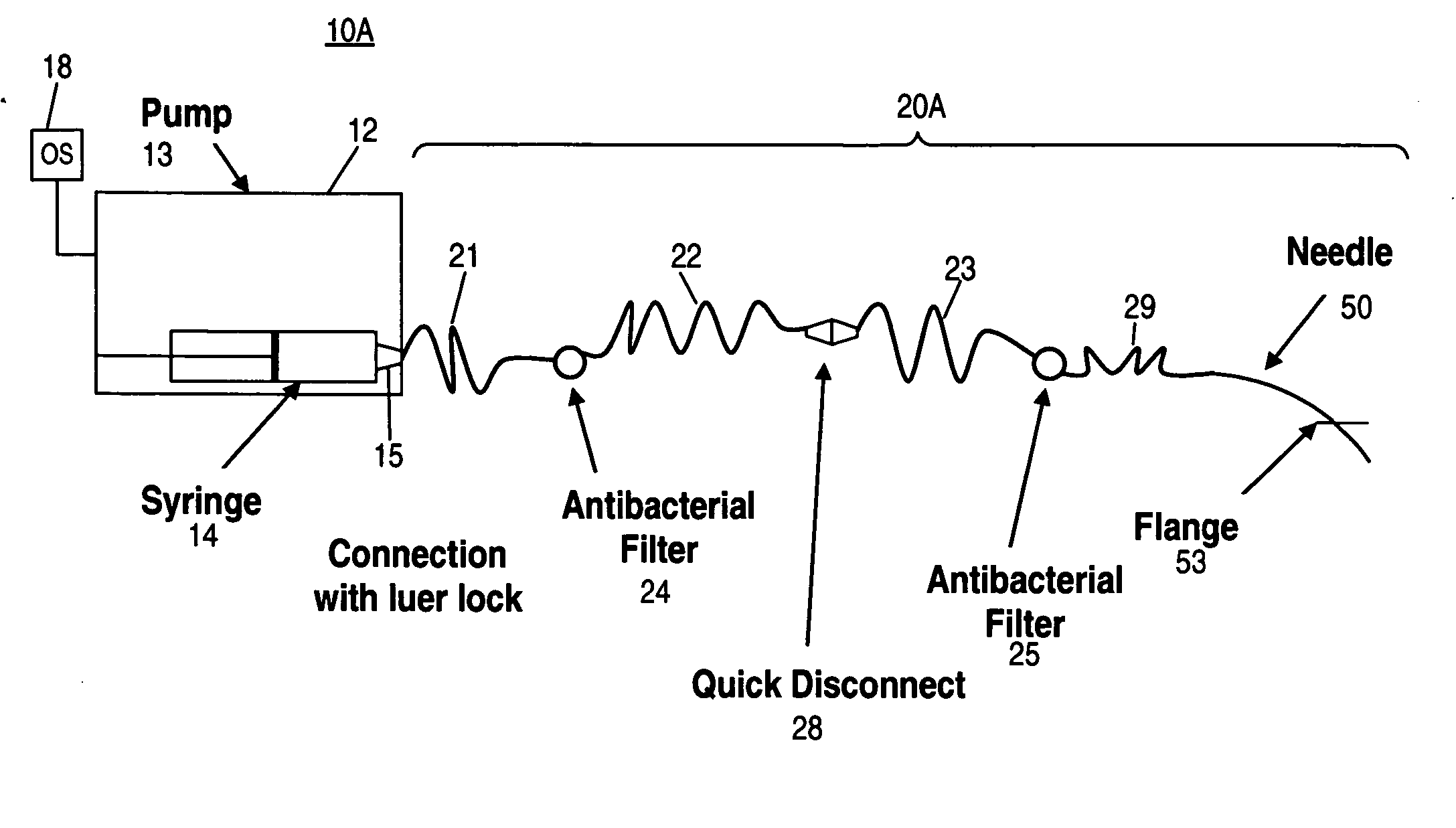
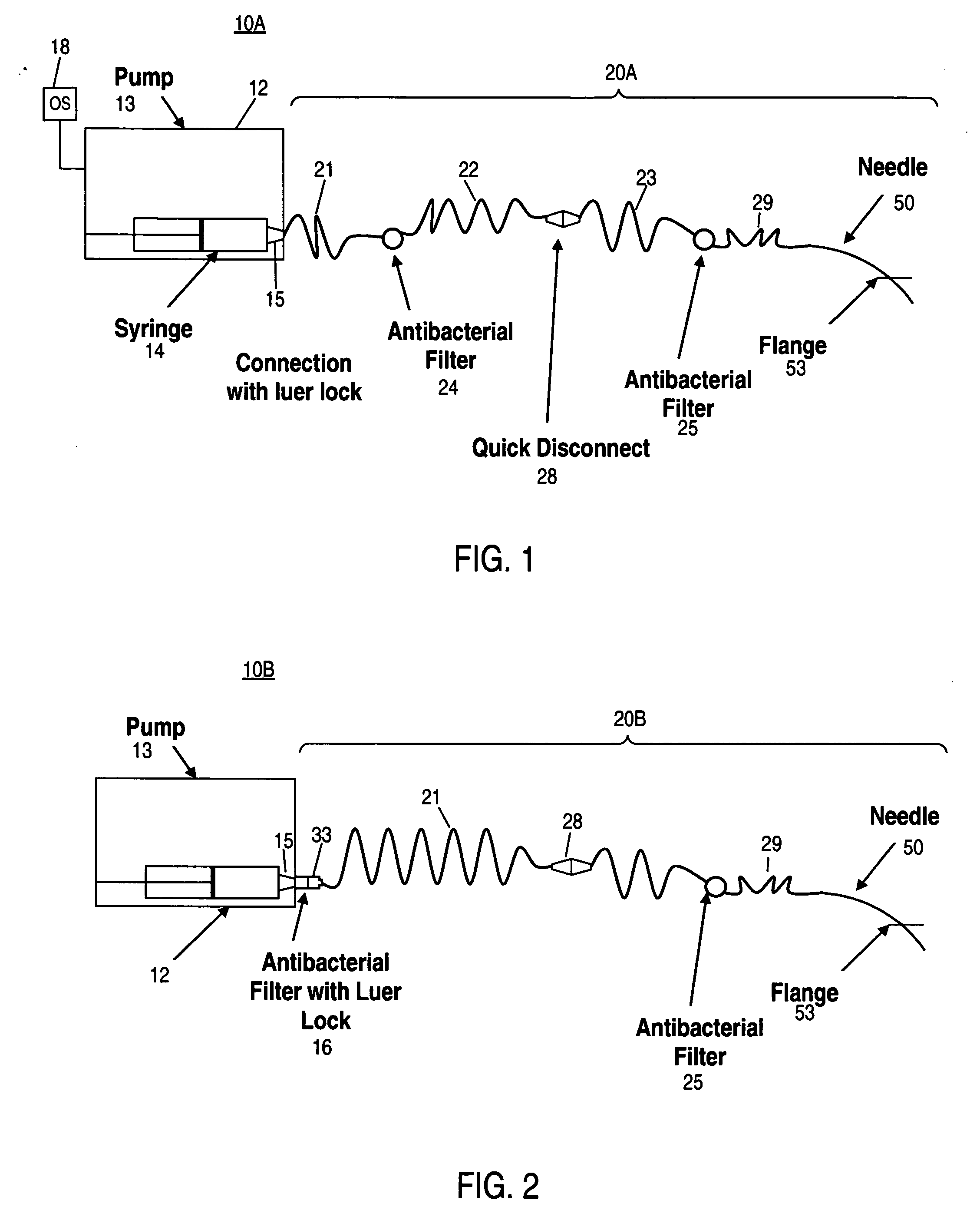
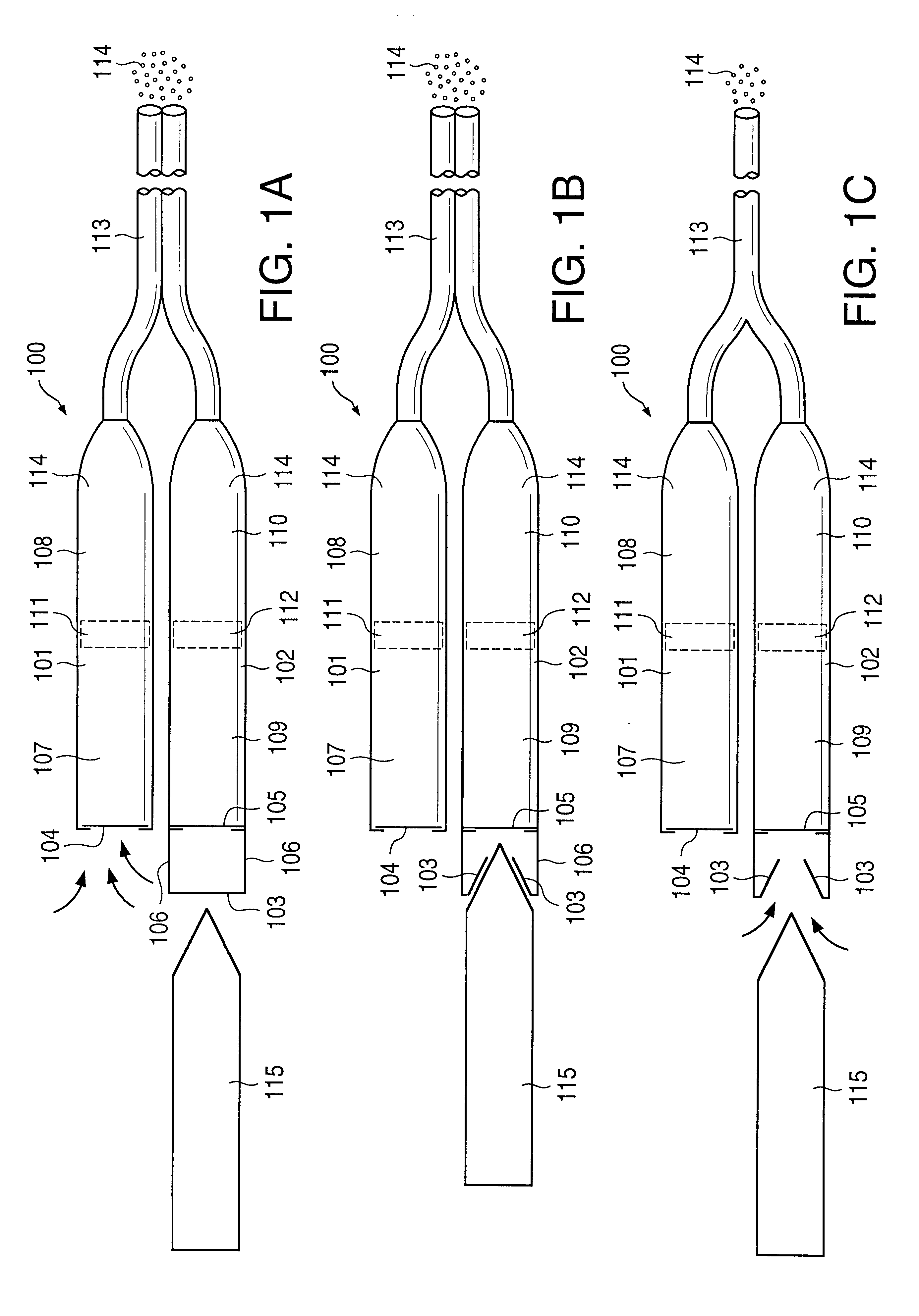
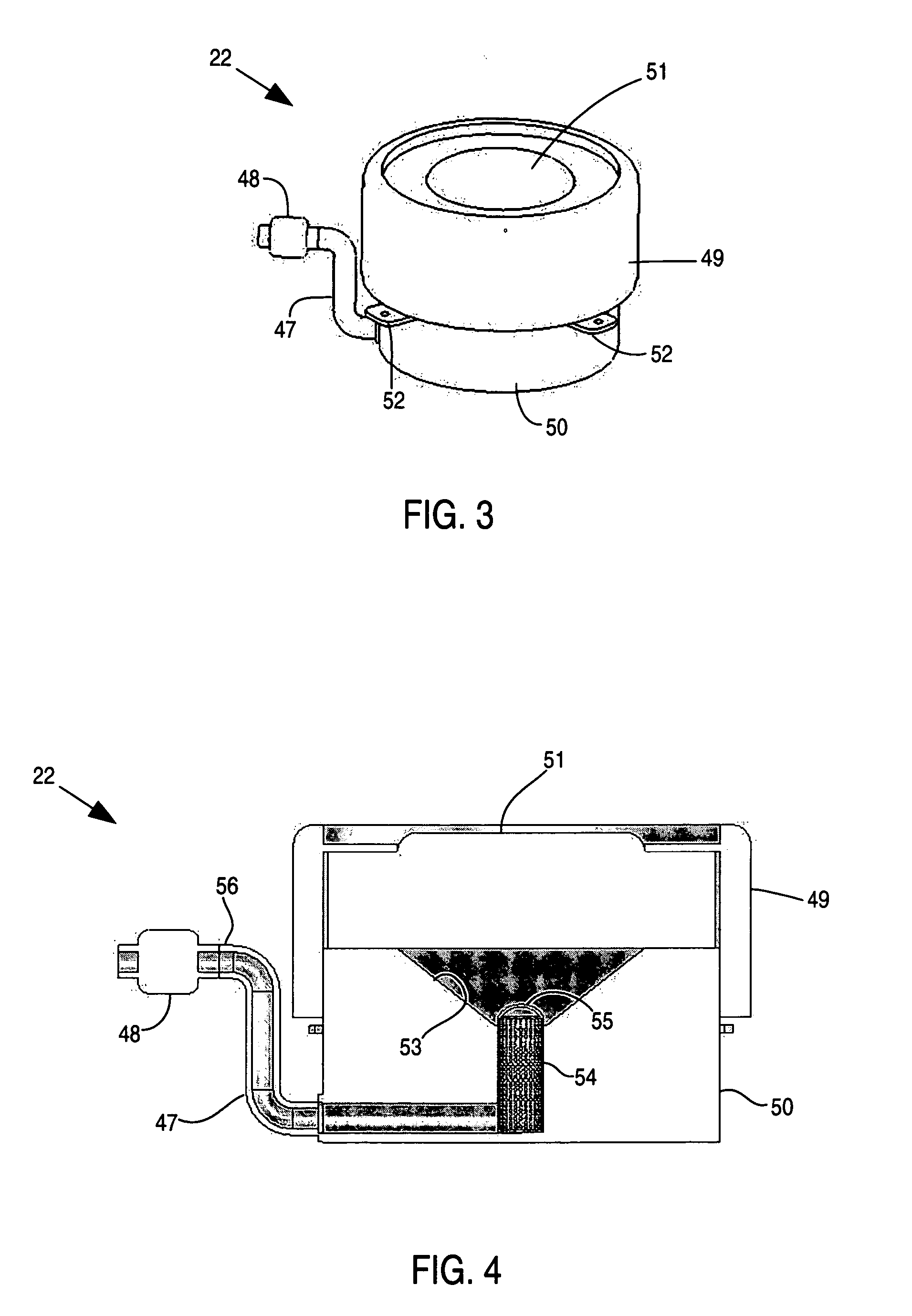
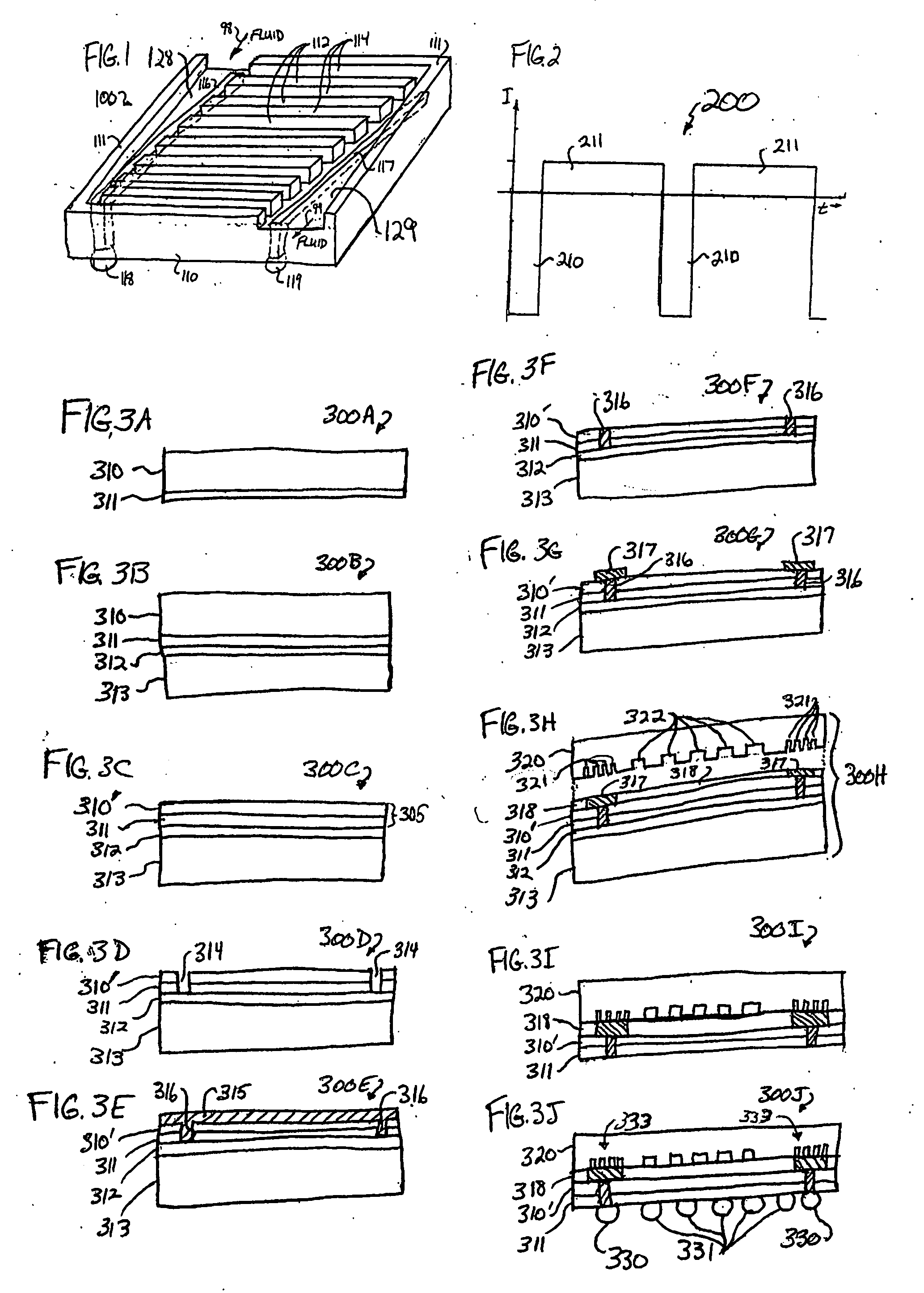
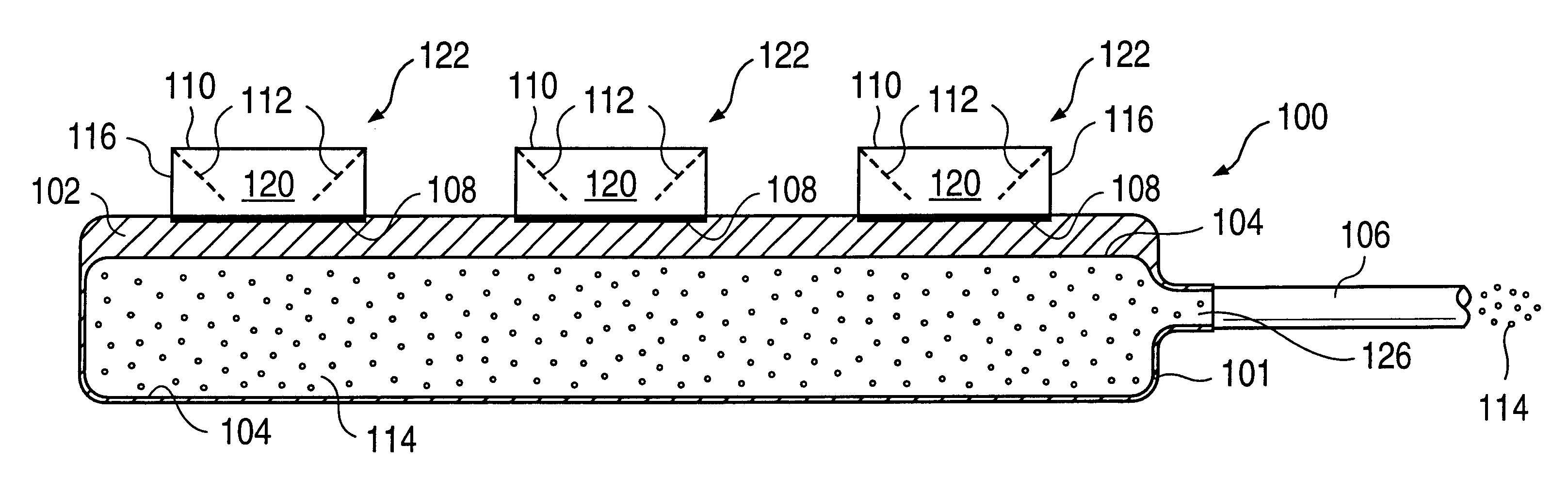
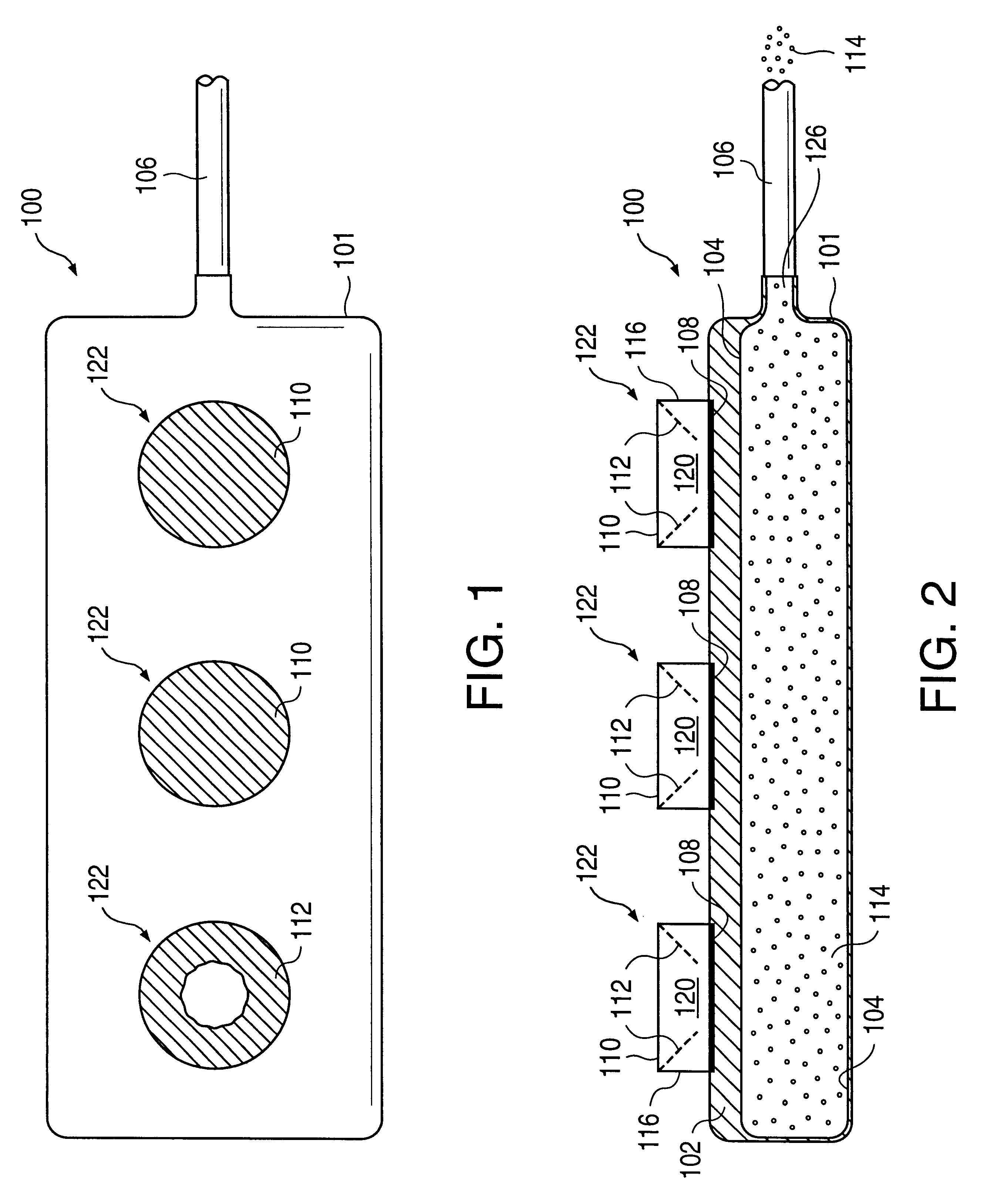
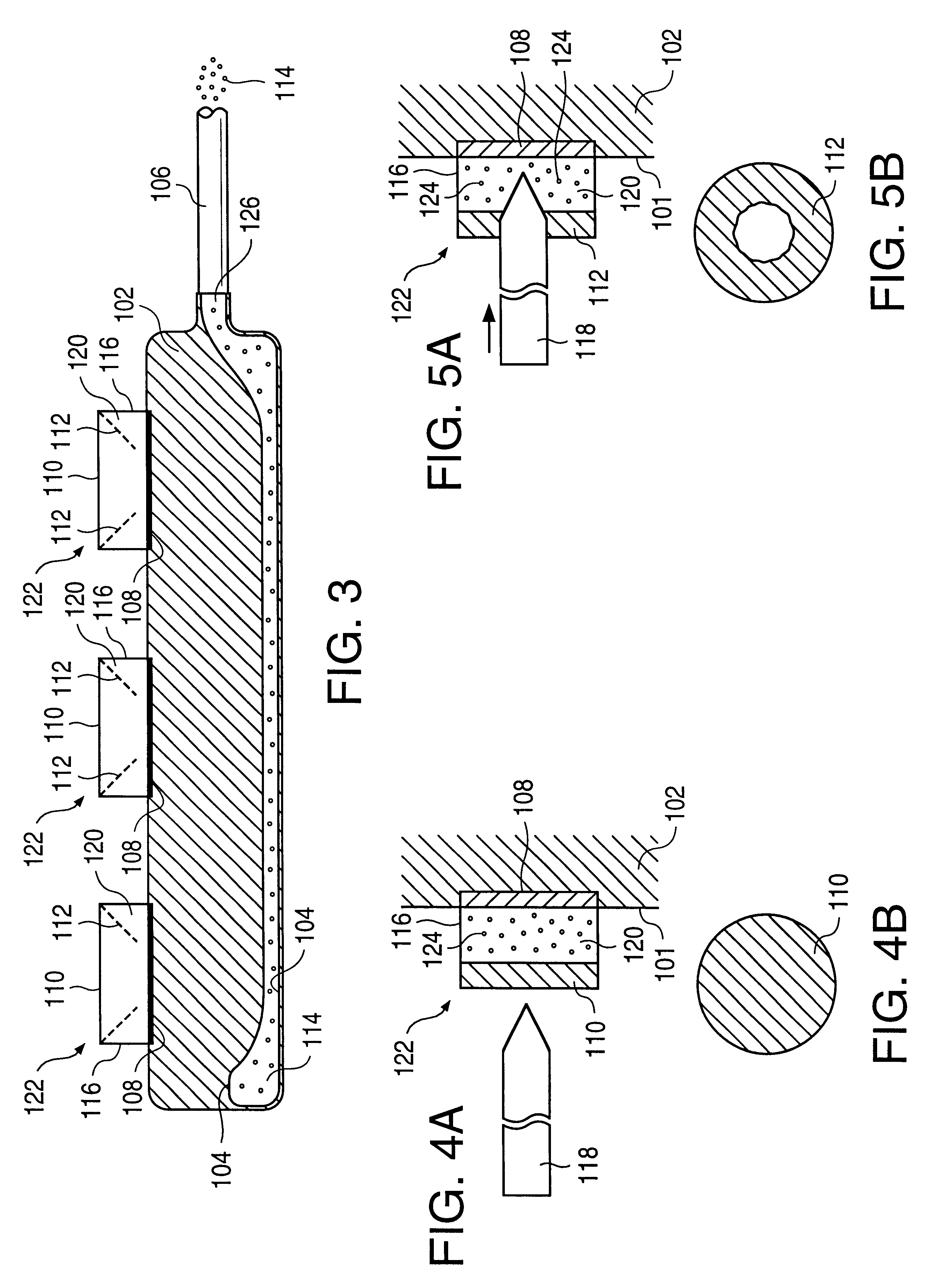
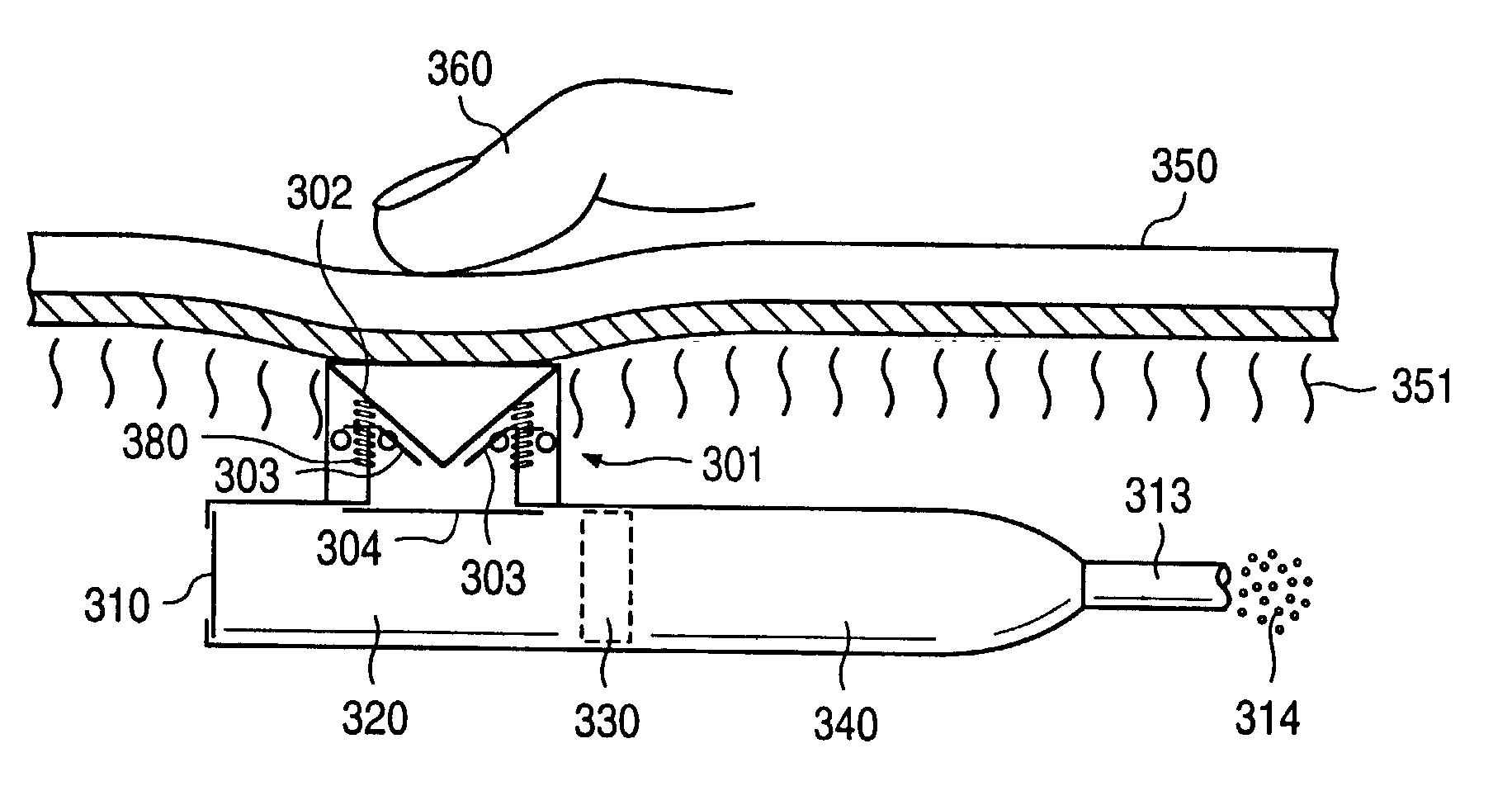
A glucose sensor in the form of a skin patch 2 has a microneedle 4 which painlessly penetrates the skin to draw out interstitial fluid. The interstitial fluid passes to a common entrance port 7. A series of microchannels 8 is provided on the skin patch. The fluid drawn onto the patch is selectively switched between a number of microchannels 8 by means of electro-osmotic pumps 10 and hydrophobic gates 12. Each microchannel 8 has an electrochemical detector 11 for sensing gluocse concentration. Also disclosed is a monlithic device with an integrated lance 83.
Owner:LIFESCAN IP HLDG LLC +1
Apparatus and method for delivering therapeutic and/or other agents to the inner ear and to other tissues
An apparatus may include a needle for sustained delivery of drugs and other agents to the inner ear or other tissues of a human or an animal. The needle can include an insertion stop, and can be placed through the round window membrane or through a surgically-prepared hole in a bone. The needle can be in fluid communication with a port and / or with a micro-infusion or osmotic pump. A cochlear implant electrode can be used instead of a needle.
Owner:NEUROSYSTEC CORP
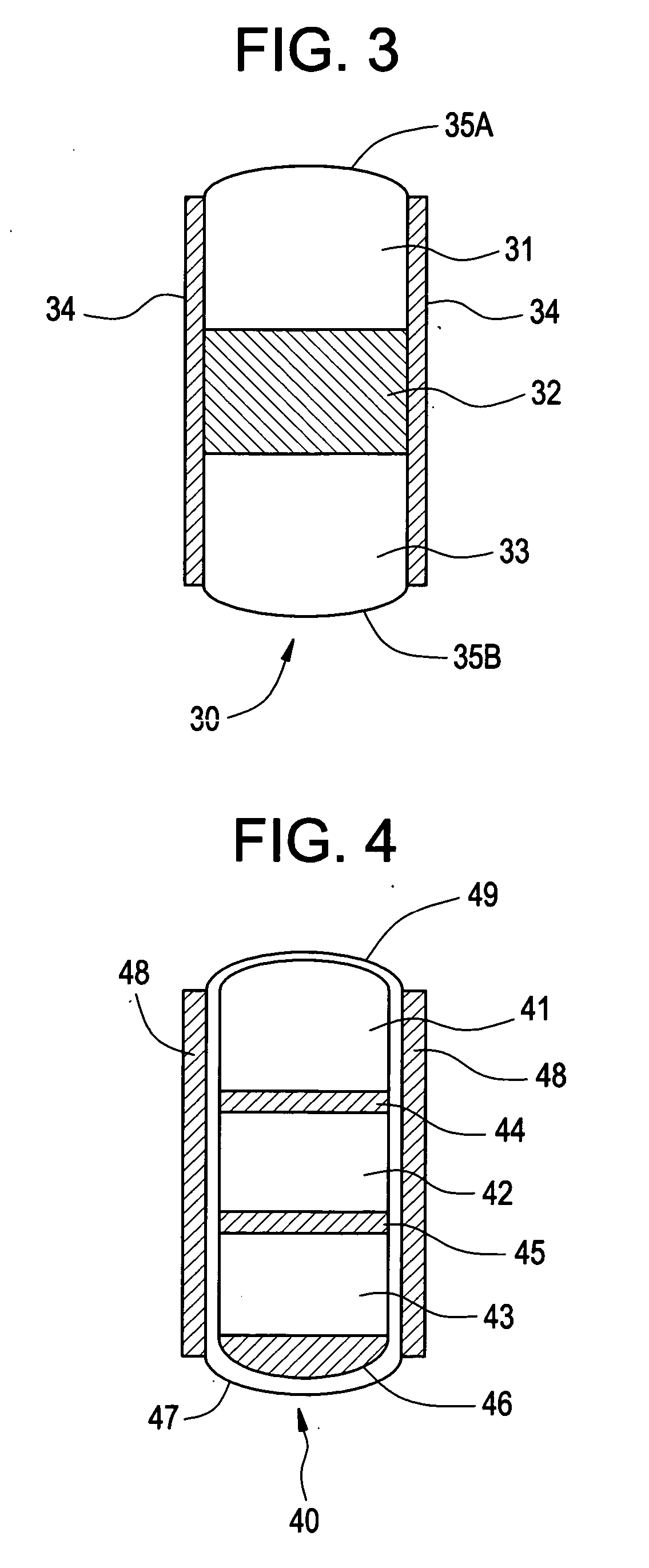
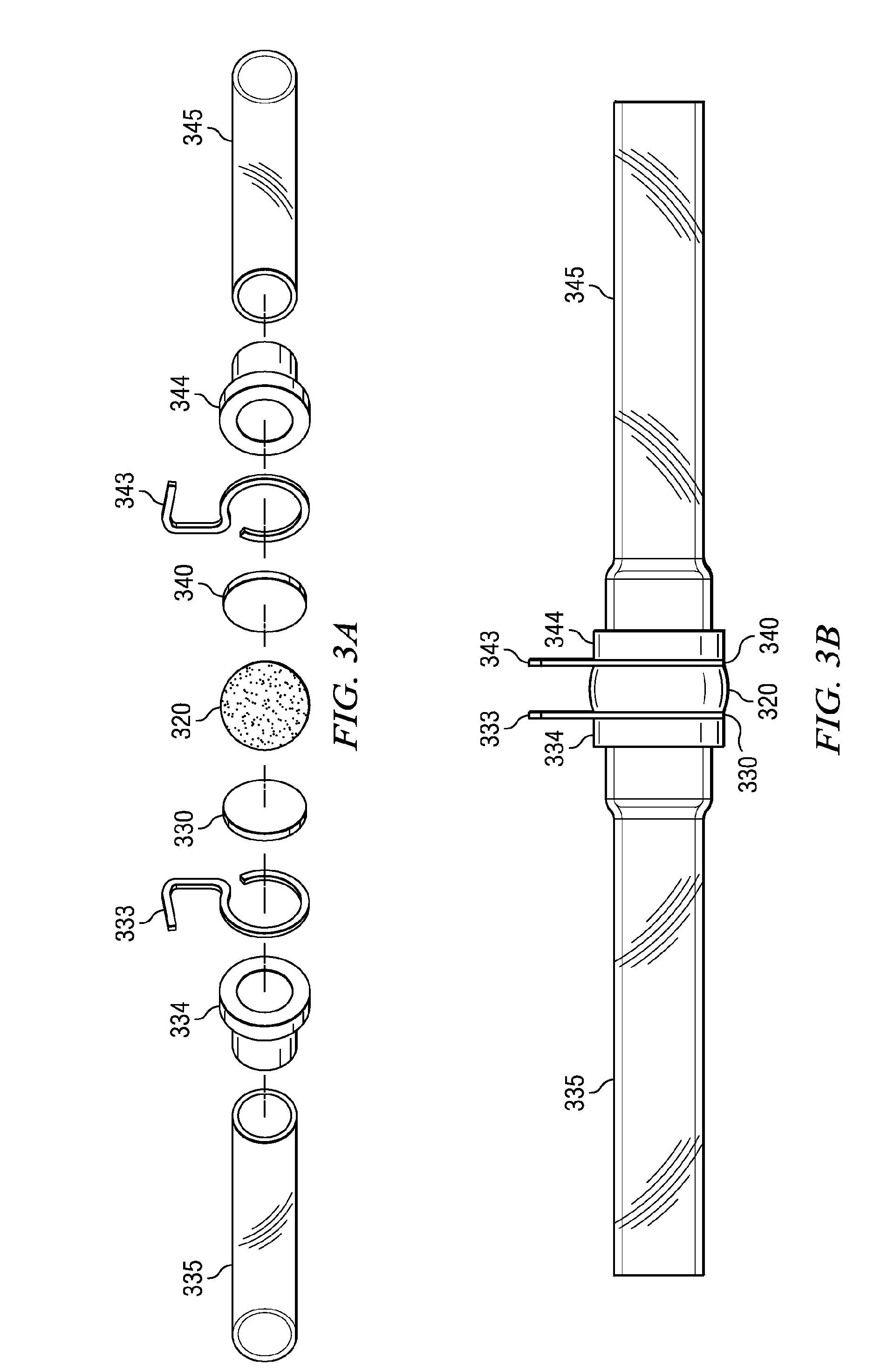
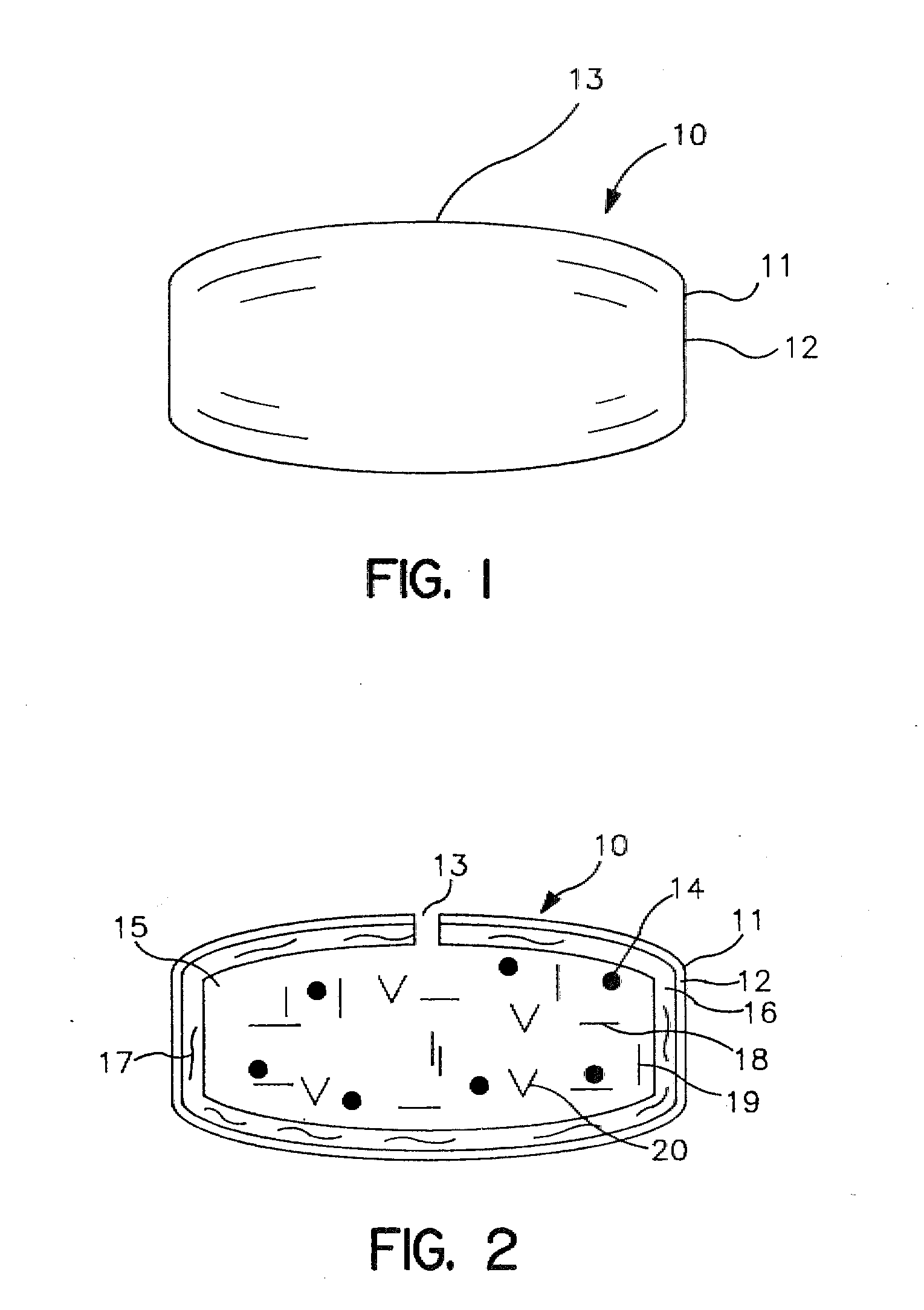
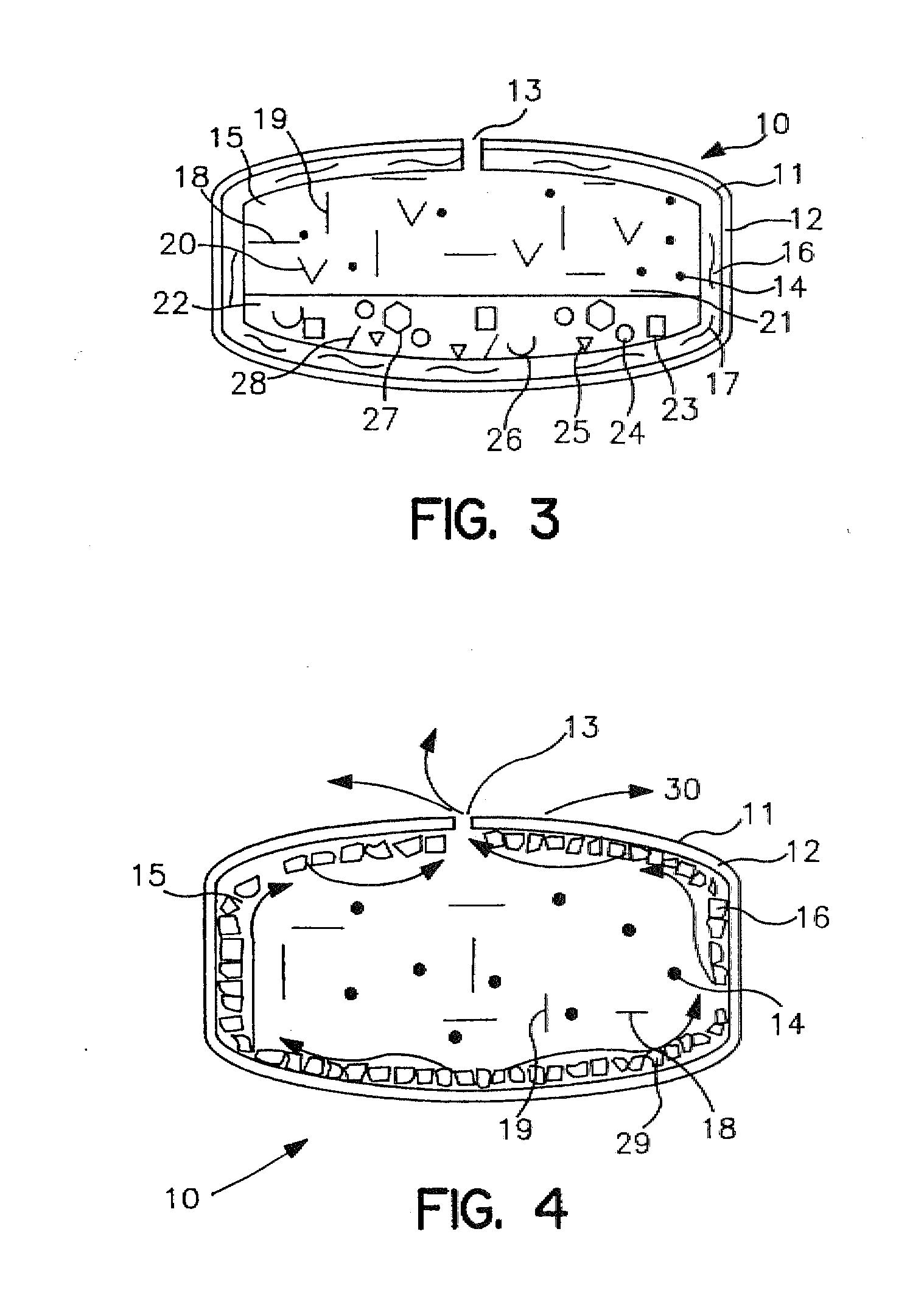
Minimally compliant, volume efficient piston for osmotic drug delivery systems
ActiveUS6939556B2Improve space efficiencyIncrease in sizeMedical devicesPressure infusionDiameter ratioOsmotic pump
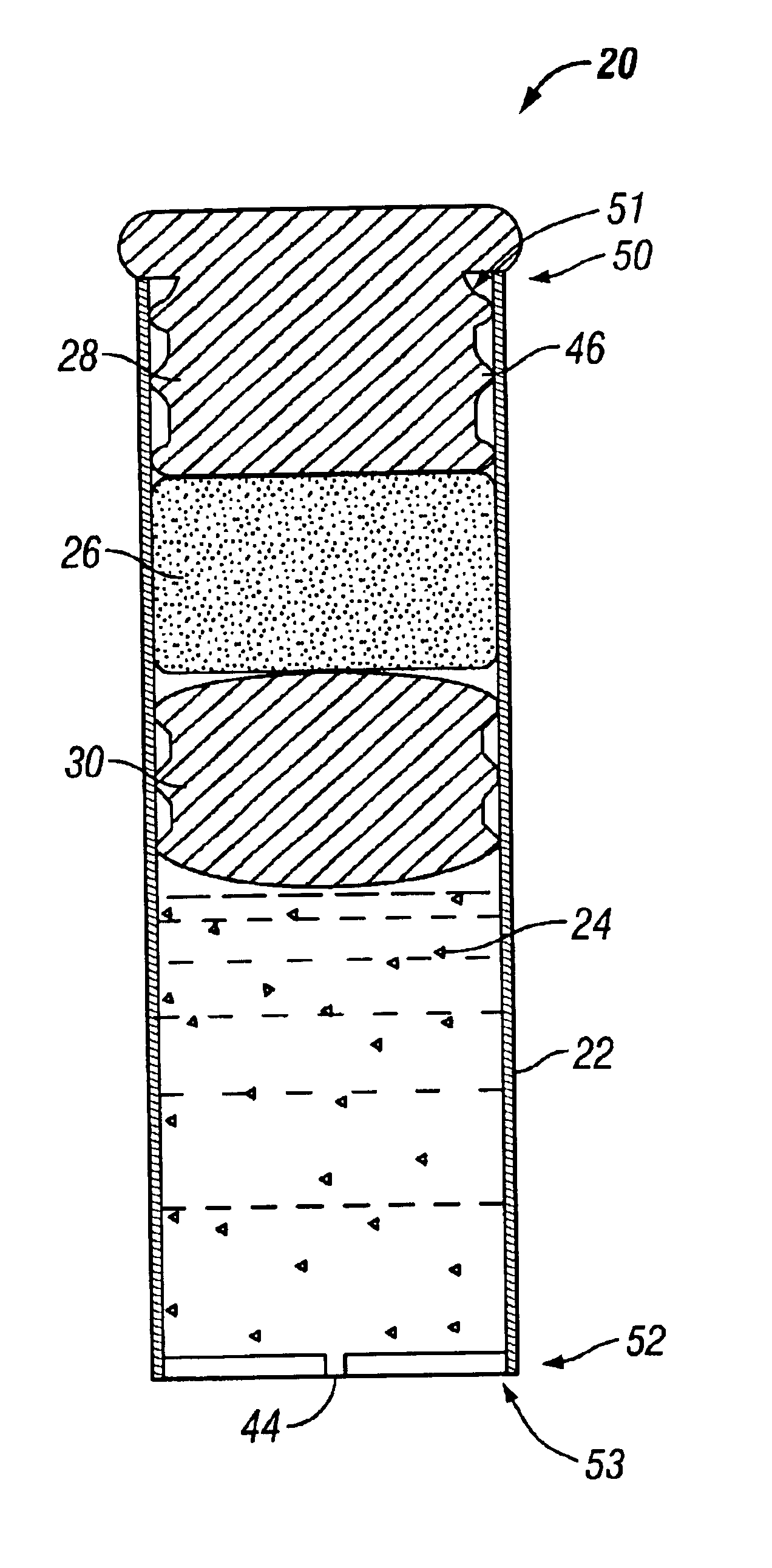
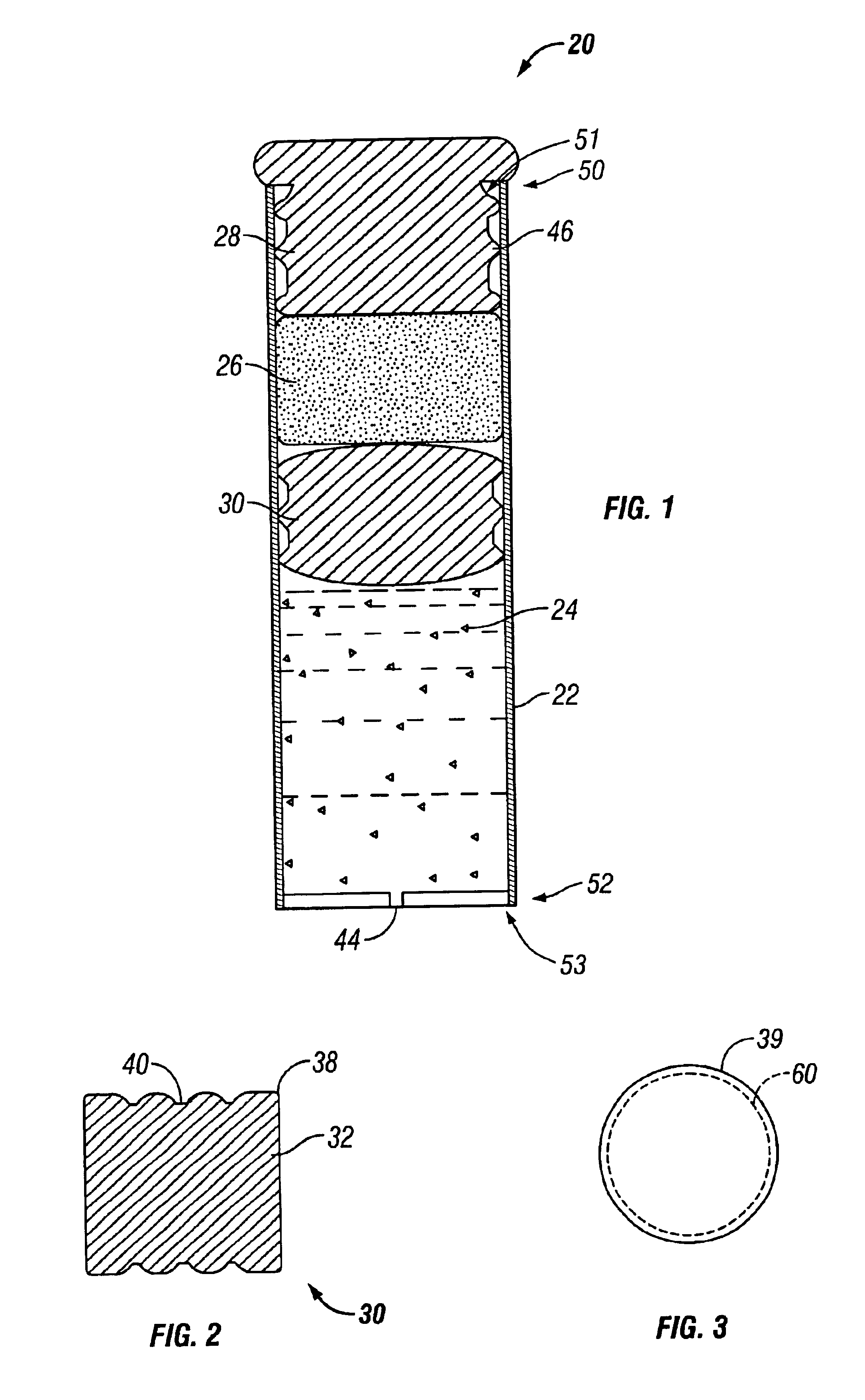
An osmotic pump having a minimally compliant, volume-efficient piston positioned within a capsule is provided. The capsule has an interior surface, a beneficial agent, and an osmotic agent. The piston is movable with respect to an interior surface of the capsule, and defines a movable seal with the interior surface of the capsule. The movable seal separates the osmotic agent from the beneficial agent. The piston has a length-to-total-diameter ratio of about 1.1:1 and a core-diameter-to-total-diameter ratio of about 0.9:1. The piston enables greater beneficial agent and / or osmotic agent payload without increasing the size of the capsule. The osmotic agent imbibes liquid from a surrounding environment through a semipermeable body to cause the piston to move and, in turn, cause delivery of the beneficial agent from the capsule.
Owner:INTARCIA THERAPEUTICS INC
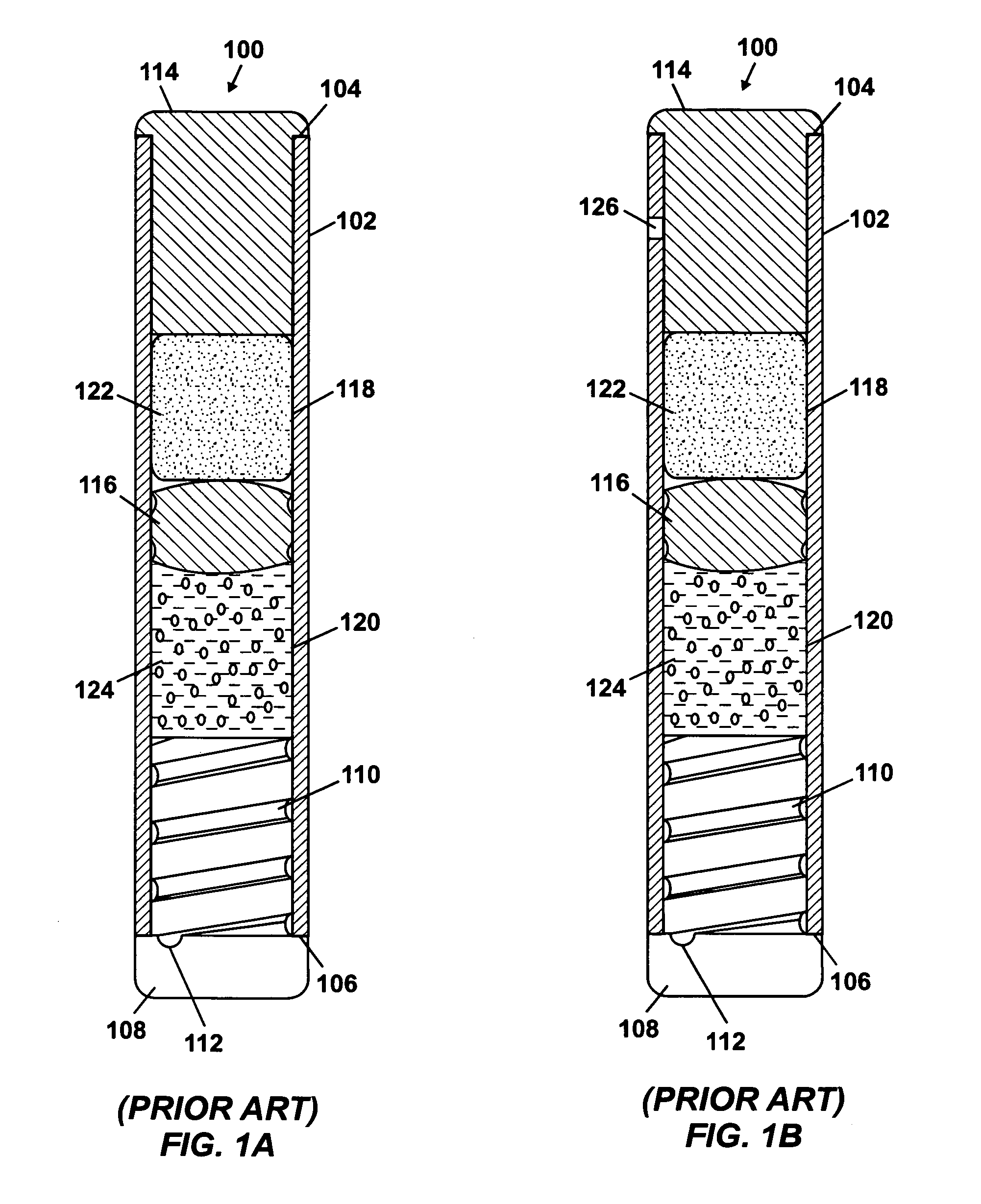
Methods and implantable devices and systems for long term delivery of a pharmaceutical agent
InactiveUS6436091B1Improve delivery rateMedical devicesPharmaceutical delivery mechanismSemipermeable membraneImplanted device
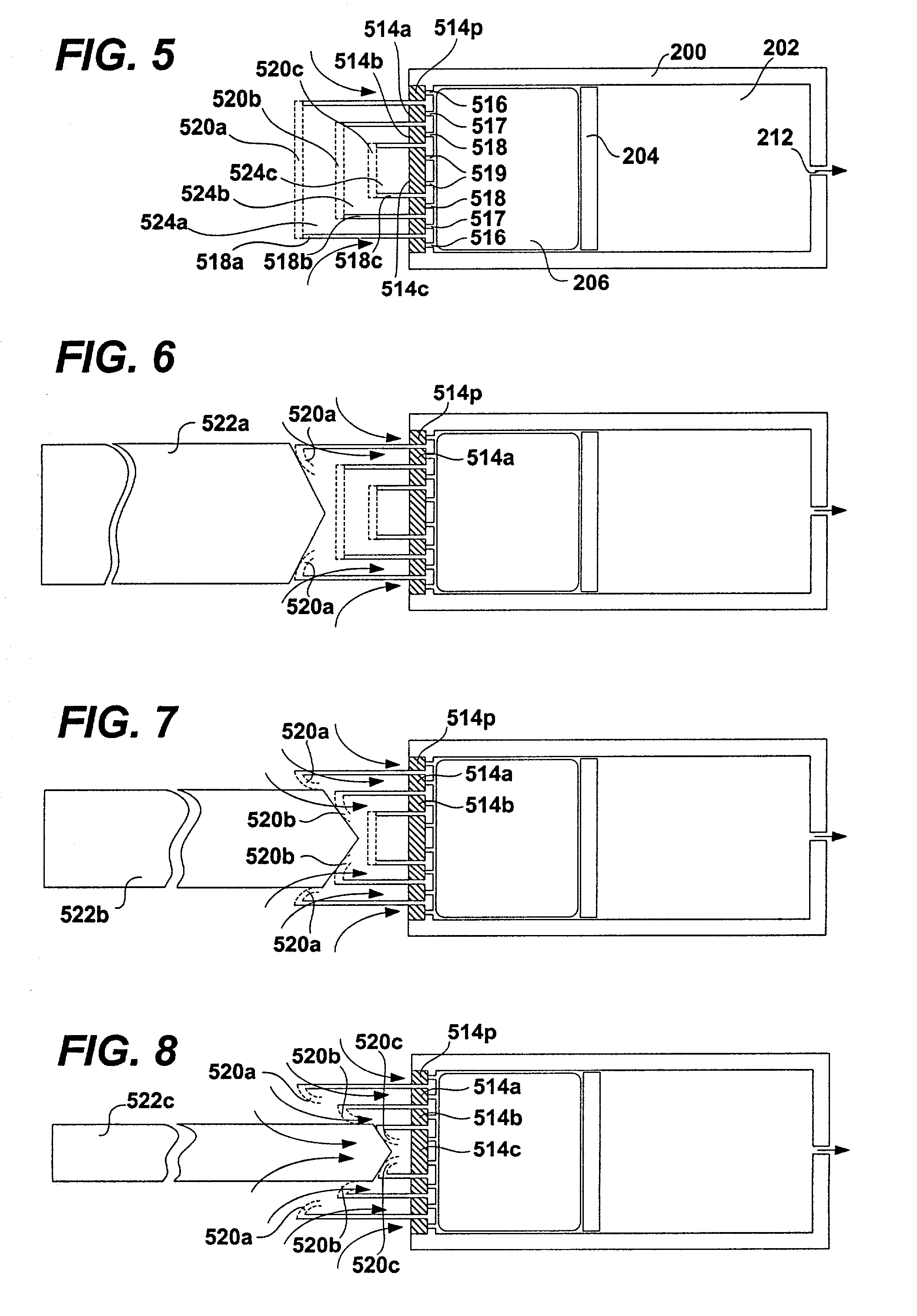
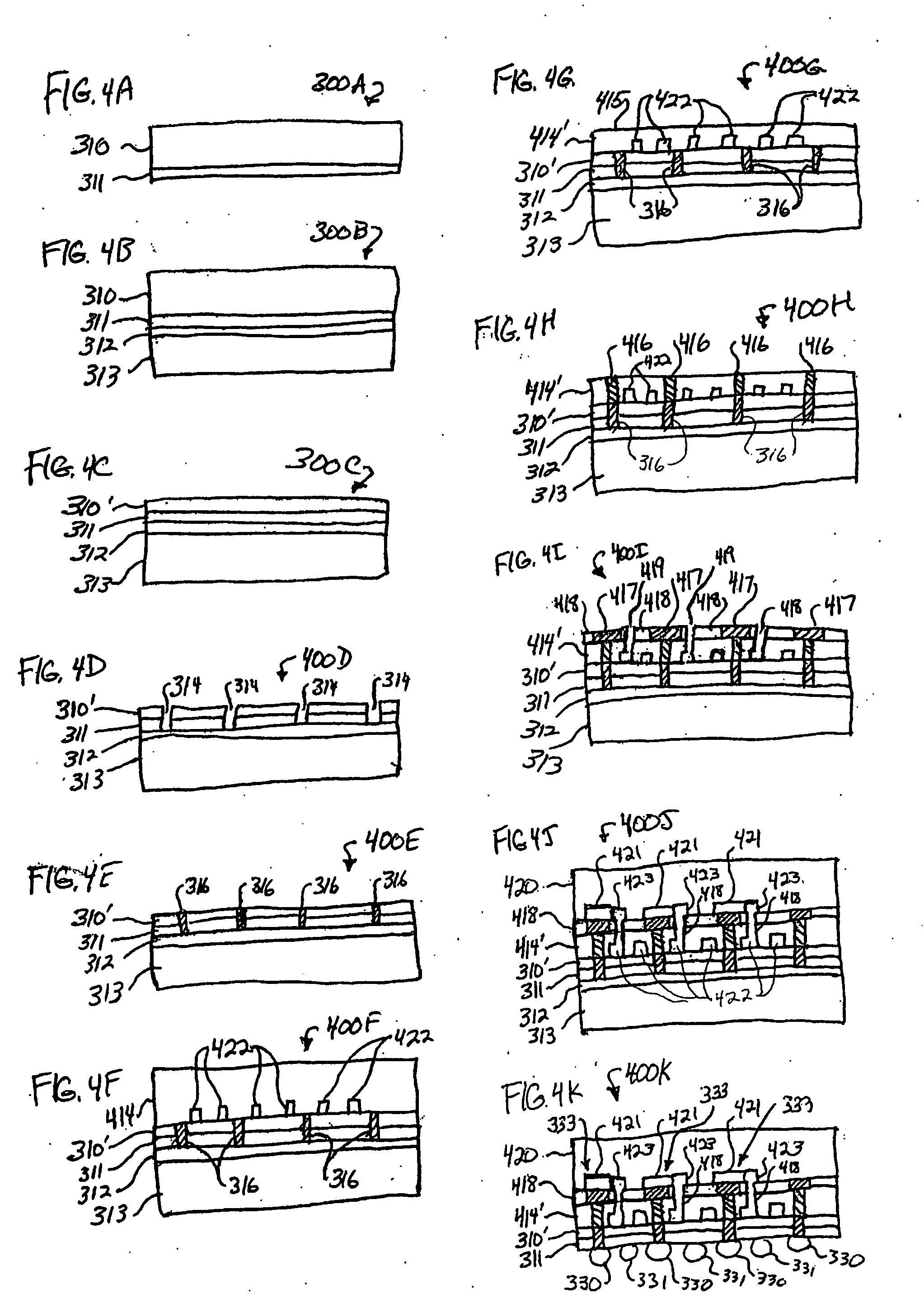
Implantable devices and osmotic pump and catheter systems for delivering a pharmaceutical agent to a patient at selectable rates include an impermeable pump housing and a moveable partition disposed within the housing, the partition dividing the housing into an osmotic driving compartment having an open end and a pharmaceutical agent compartment having a delivery orifice. A plurality of semi permeable membranes may be disposed in the open end of the osmotic driving compartment and a number of impermeable barriers may seal selected ones of the plurality of semi permeable membranes from the patient until breached. Breaching one or more of the impermeable barriers increases the surface area of semi permeable membrane exposed to the patient and controllably increases the delivery rate of the pharmaceutical agent through the delivery orifice and catheter. Each of the plurality of semi permeable membranes may have a selected surface area, composition and / or thickness, to allow a fine-grained control over the infusion rate while the pump is implanted in the patient.
Owner:MICROSOLUTIONS
Osmotic pump drug delivery systems and methods
InactiveUS6471688B1Avoid mixingMedical devicesPharmaceutical delivery mechanismTreatment effectAnalgesics effects
Implantable osmotic pump devices and systems include multiple osmotic pumps and / or semipermeable membranes to extend the useful life cycle and functionality of the drug delivery system. Use of an implantable system including multiple implantable osmotic pumps allows different drugs to be administered from the same implanted system. One or more of the semipermeable membranes of the system may be initially sealed by an overlying impermeable membrane upon implantation of the system into the patient. When the patient develops a tolerance to a first drug or to a first dose of the first drug, the impermeable membrane may be breached, to expose the underlying semipermeable membrane to the osmotic pressure of the patient at the implant site. This causes the infusion rate to increase, thereby providing the patient with the needed relief and / or other desired therapeutic effect. In the case of a multiple pump system, breaching an impermeable membrane may cause the infusion of a second drug. The second drug may potentiate a therapeutic effect (such as an analgesic effect) of the first drug, as is the case with Sufentanil and Clonidine.
Owner:MICROSOLUTIONS
Methods and implantable devices and systems for long term delivery of a pharmaceutical agent
InactiveUS20040111080A1Improve delivery ratePharmaceutical delivery mechanismMedical devicesSemipermeable membraneImplanted device
Implantable devices and osmotic pump and catheter systems for delivering a pharmaceutical agent to a patient at selectable rates include an impermeable pump housing and a moveable partition disposed within the housing, the partition dividing the housing into an osmotic driving compartment having an open end and a pharmaceutical agent compartment having a delivery orifice. A plurality of semi permeable membranes may be disposed in the open end of the osmotic driving compartment and a number of impermeable barriers may seal selected ones of the plurality of semi permeable membranes from the patient until breached. Breaching one or more of the impermeable barriers increases the surface area of semi permeable membrane exposed to the patient and controllably increases the delivery rate of the pharmaceutical agent through the delivery orifice and catheter. Each of the plurality of semi permeable membranes may have a selected surface area, composition and / or thickness, to allow a fine-grained control over the infusion rate while the pump is implanted in the patient.
Owner:MICROSOLUTIONS
Osmotic pump with means for dissipating internal pressure
ActiveUS7207982B2Simple designReducing and minimizing likelihoodMedical devicesPressure infusionInternal pressurePhysical separation
The present invention includes an osmotic pump that includes a means for venting an osmotic composition included in the pump before the internal pressure of the pump has the opportunity to build to such an extent that the pump is structurally compromised, such as when one or more components of the pump are physically separated. The means for venting osmotic material included in an osmotic pump according to the present invention includes a vent that allows the material included in the osmotic composition of the pump to dissipate into an environment of operation at a rate that results in dissipation of the pressure created within the osmotic pump and a reduced potential for subject discomfort or irritation.
Owner:INTARCIA THERAPEUTICS INC
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
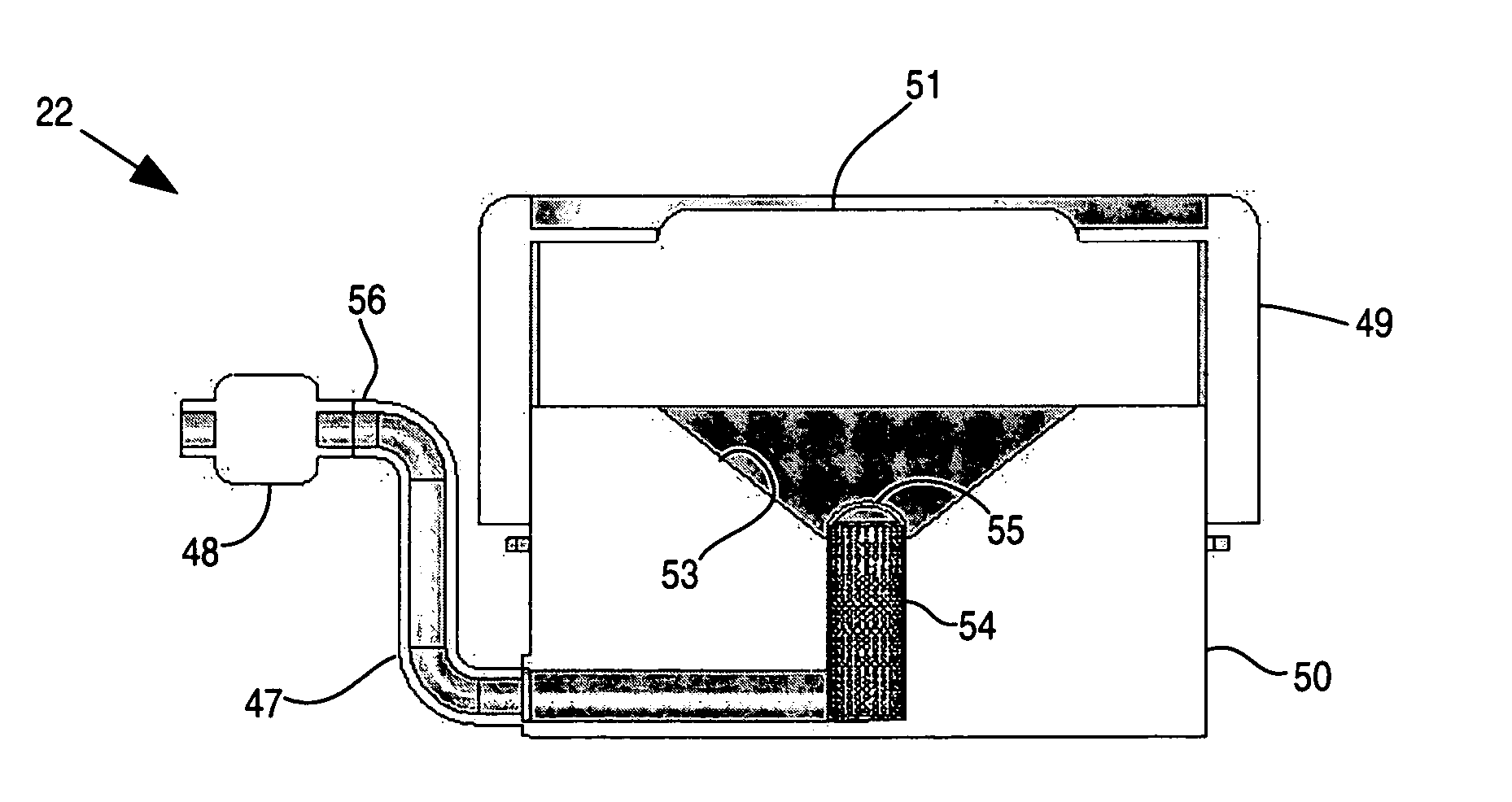
Apparatus and method for delivery of therapeutic and other types of agents
InactiveUS20070255237A1Convenient treatmentHead electrodesFiltering accessoriesOsmotic pumpTarget tissue
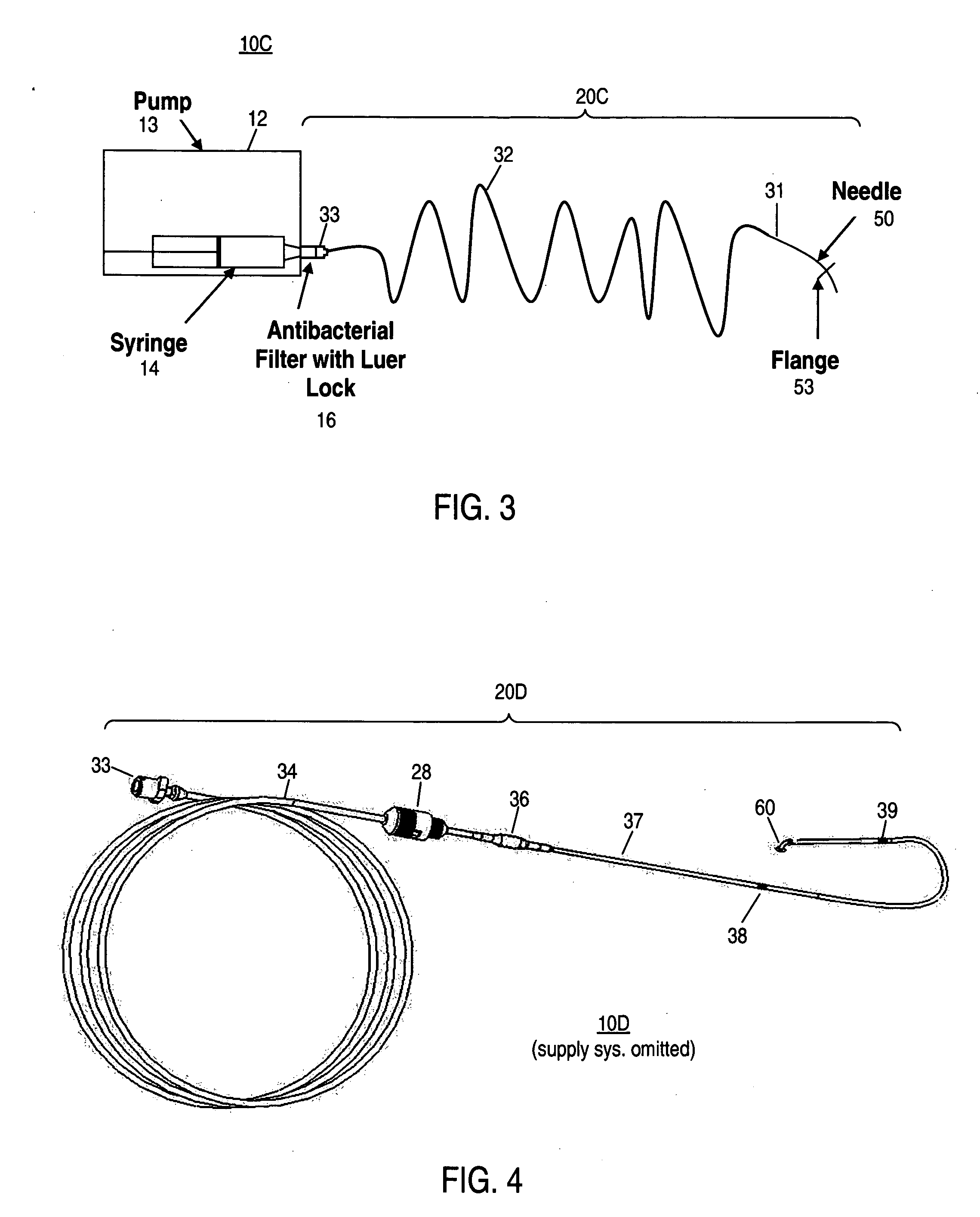
Implantable drug delivery systems target delivery of small volumes of drugs to specific tissues. In some cases, a drug delivery system includes an implantable osmotic pump connected to a drug-containing housing, with that housing connected to a needle, cochlear implant or other type of component for ultimate delivery to the target tissue. In some implementations, a subcutaneous port receives a fluid from an external pump. The port is connected to a needle or other component for delivery of one or more drugs to the target tissue. Both solid and liquid drug formulations can be used. In embodiments using solid drugs, a separate drug vehicle (such as saline) can be used to dissolve a portion of the solid drug, with the drug-loaded vehicle then delivered to the target tissue.
Owner:OTONOMY INC
Osmotically driven active agent delivery device providing an ascending release profile
ActiveUS7241457B2Increase surface areaReduce effective thicknessPressure infusionOsmotic deliveryActive agentWater flow
In one aspect, the present invention is directed to an osmotic pump that automatically provides an ascending release rate of active agent as the osmotic pump functions in an environment of operation and may be designed for implantation within a desired animal or human subject. An osmotic pump according to the present invention includes a reservoir, a rate controlling membrane, an expandable osmotic composition, an active agent formulation and an exit orifice. Once administered to an environment of operation, water passes through the rate controlling membrane and into the osmotic composition, which causes the osmotic composition to expand and expel the active agent formulation through the exit orifice at a rate that is directly proportional to the rate at which water passes through the rate controlling membrane. An osmotic pump according to the present invention permits the flow of water through the rate controlling membrane to increase automatically without the need for manipulation of the osmotic pump after administration. As the flow of water through the rate controlling membrane increases, the rate at which active agent is delivered from the osmotic pump will also increase proportionally.
Owner:INTARCIA THERAPEUTICS INC
Osmotic pump apparatus and associated methods
InactiveUS20080102119A1Small sizeSize reduction requirementsPressure infusionPill deliveryOsmotic pumpBiomedical engineering
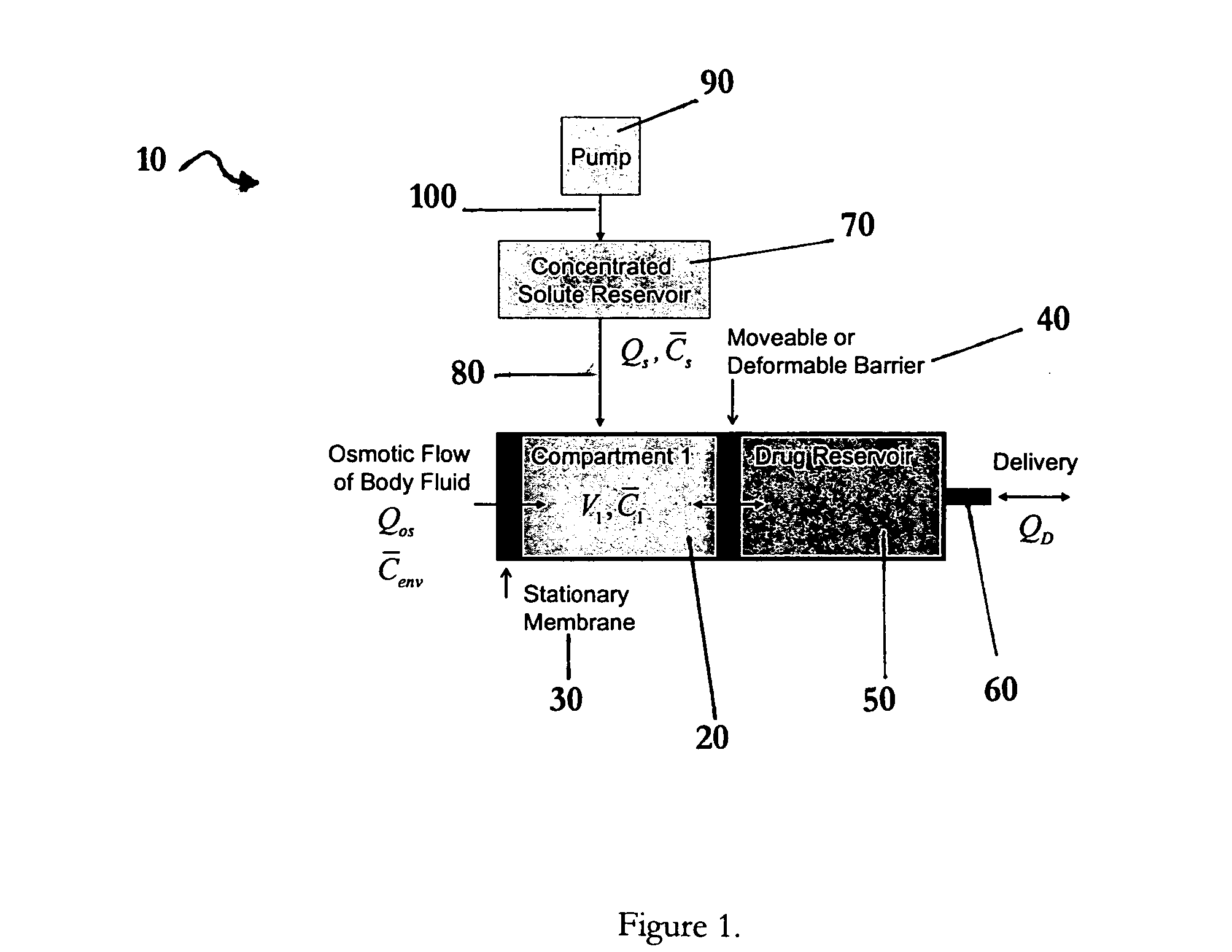
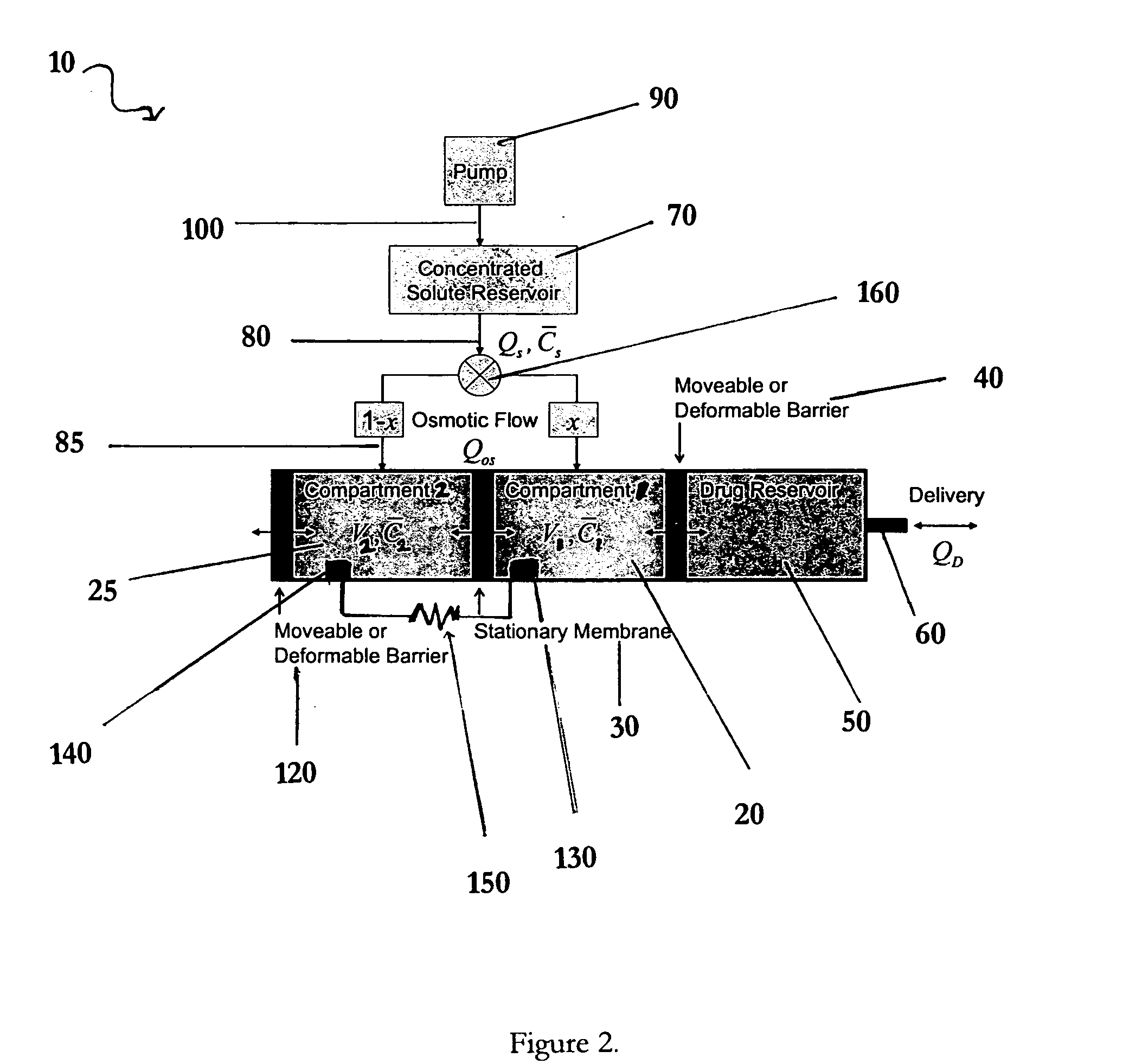
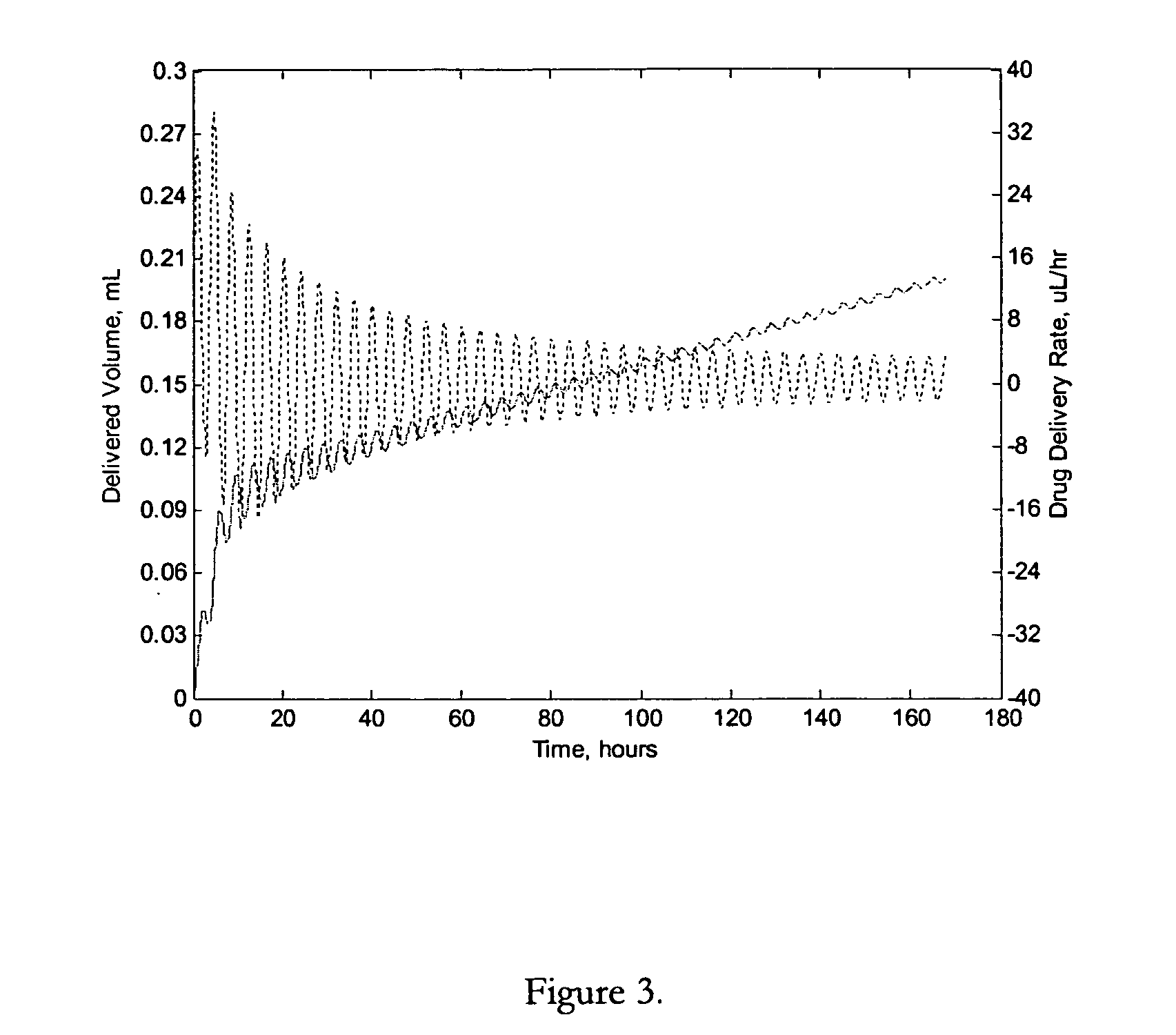
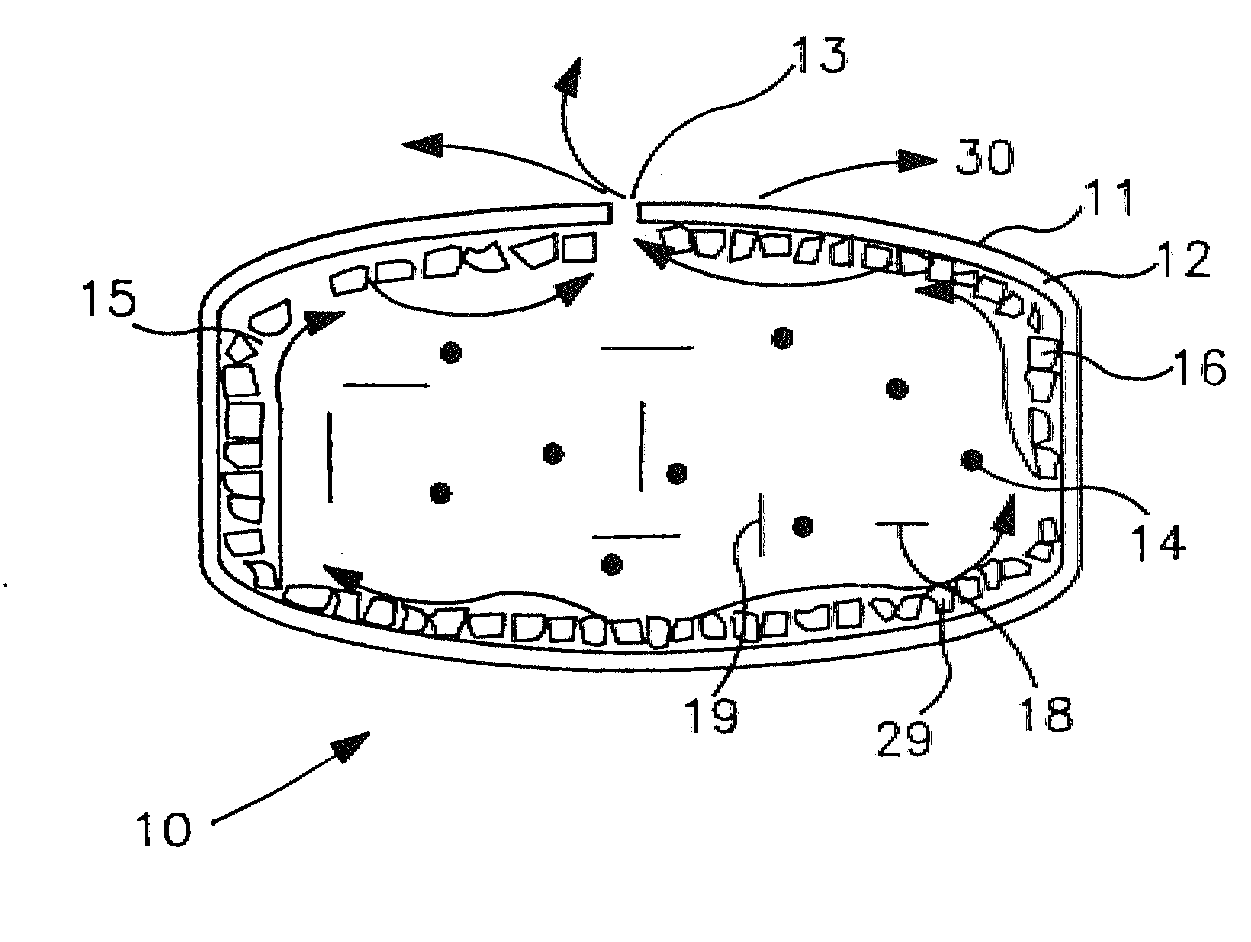
Apparatuses and methods for pumping fluids such as fluid medications are disclosed. Embodiments of the invention provide an osmotic pump fluid delivery apparatus including elements designed to control the fluid delivery rate. Typical embodiments of the invention include an arrangement of elements such as solute reservoirs that can manipulate the solute concentrations within an inner osmotic compartment or compartments of an osmotic pump so as to control fluid delivery from the pump. Other embodiments include sealed electro-osmotic pumps that do not discharge ions into the surroundings or require water from an external source. These embodiments of the invention provide new ways to control fluid delivery in apparatuses that employ osmotic processes to function.
Owner:MEDTRONIC INC
Apparatus and method integrating an electro-osmotic pump and microchannel assembly into a die package
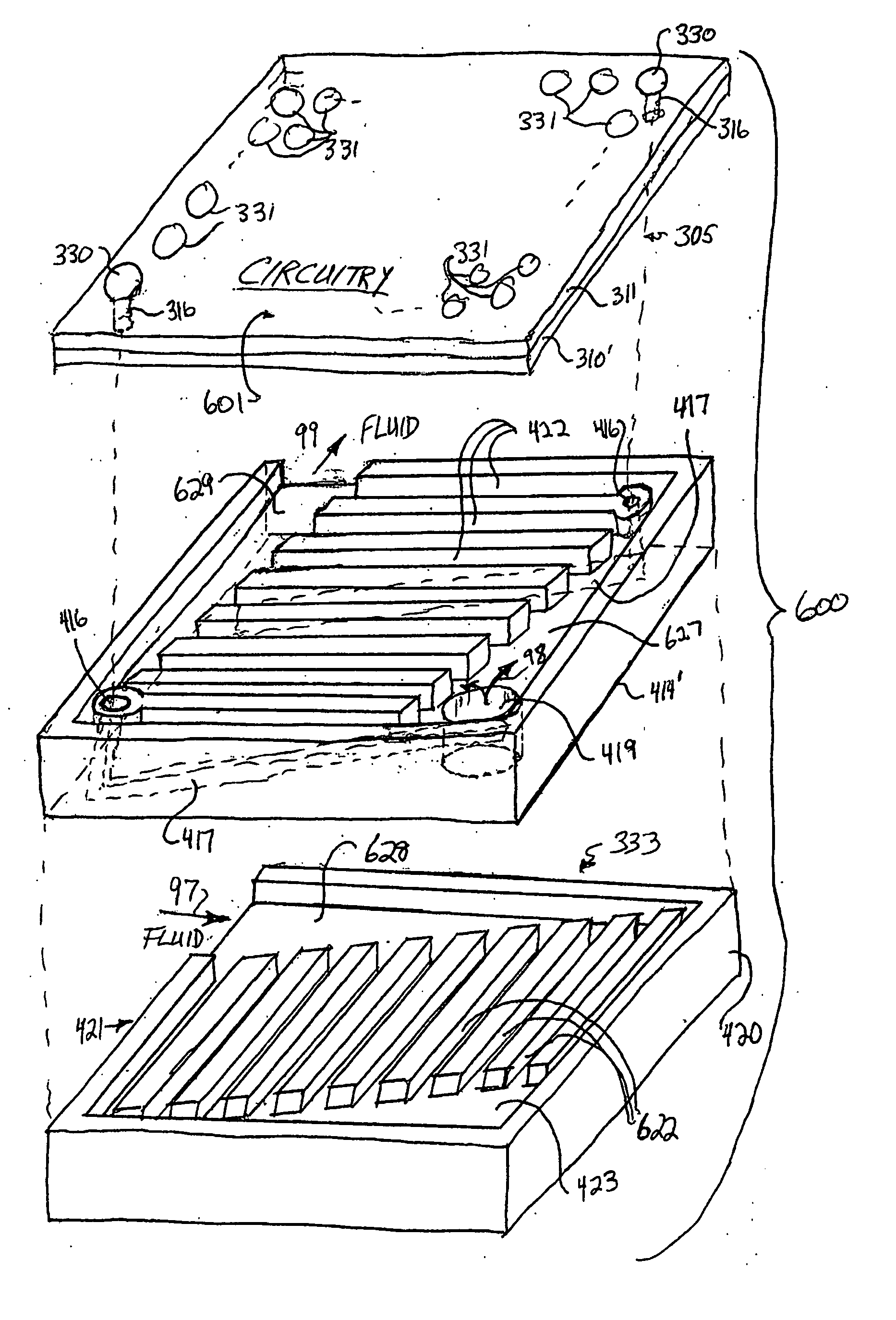
InactiveUS20050139996A1Semiconductor/solid-state device detailsSolid-state devicesEngineeringOsmotic pump
A die package and a method and apparatus for integrating an electro-osmotic pump and a microchannel cooling assembly into a die package.
Owner:INTEL CORP
Osmotic pump apparatus and associated methods
InactiveUS20090281528A1Easy to transportImprove featuresPharmaceutical delivery mechanismPressure infusionOsmotic pumpBiomedical engineering
Owner:MEDTRONIC INC
Modular imbibition rate reducer for use with implantable osmotic pump
InactiveUS20050101943A1Reduce inhalation rateReduce probabilityPill deliveryCapsule deliveryReduction driveReducer
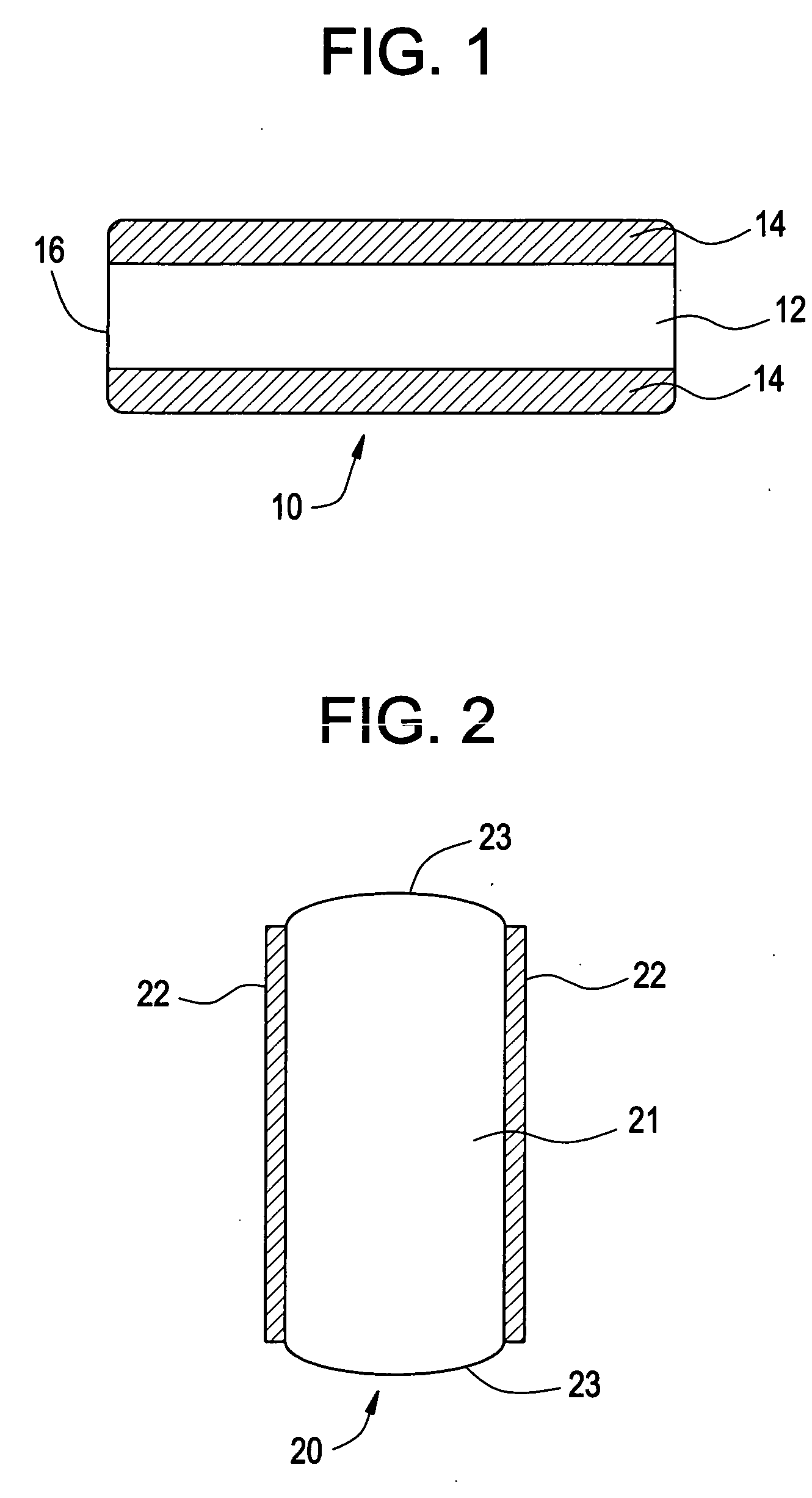
An osmotic pump system includes a capsule having at least one delivery port, a membrane plug retained at an open end of the capsule remote from the delivery port, the membrane plug providing a fluid-permeable barrier between an interior and an exterior of the capsule, and a removable imbibition rate reducer attachable to the capsule. The imbibition rate reducer comprises one or more flow controllers selected from the group consisting of an orifice having a selected size smaller than a surface area of the membrane plug and a membrane having a selected thickness, surface area, radial compression, and permeability. The imbibition rate reducer allows customizable delivery of medicaments.
Owner:INTARCIA THERAPEUTICS INC
Osmotic pump delivery system with flexible drug compartment
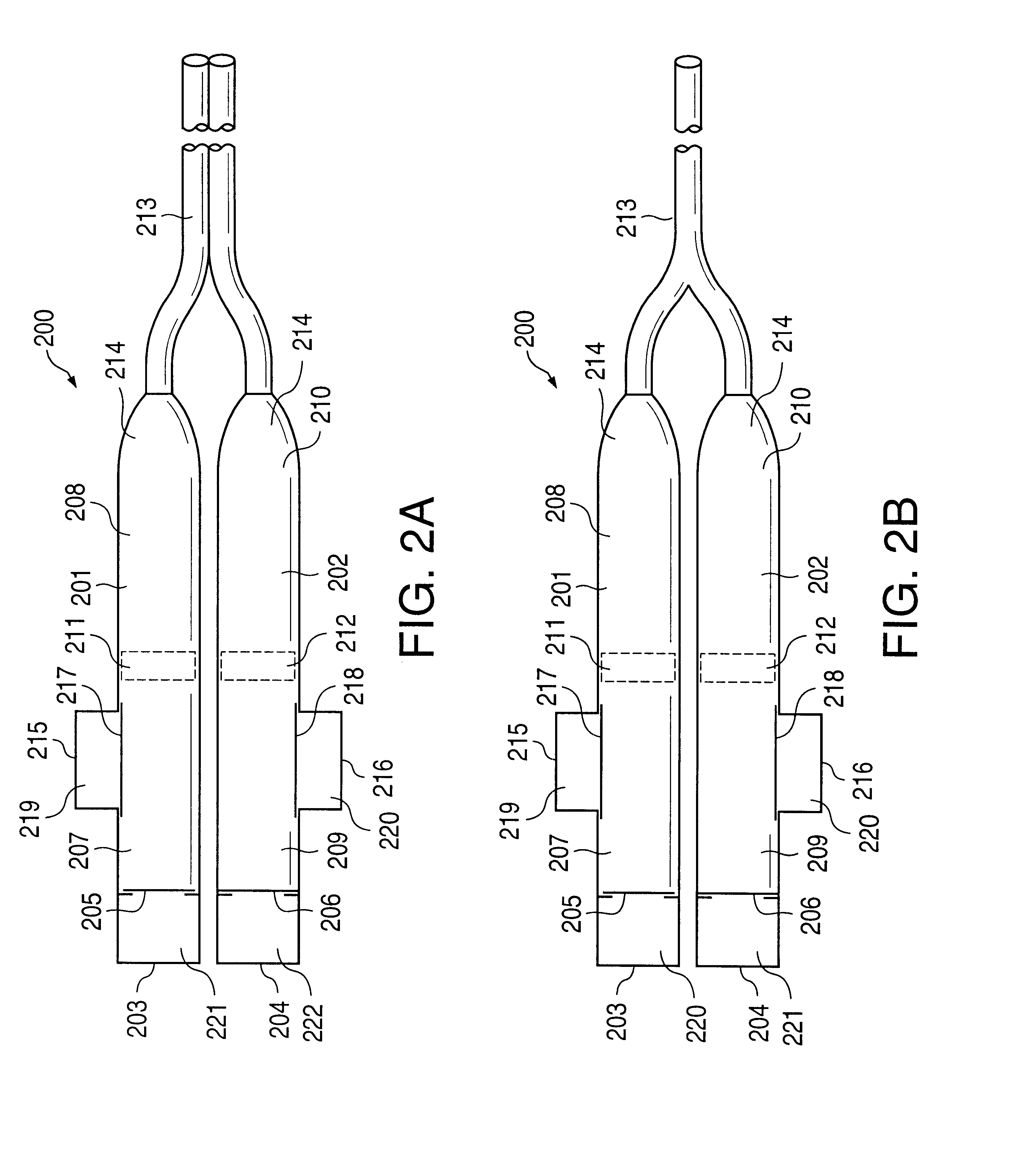
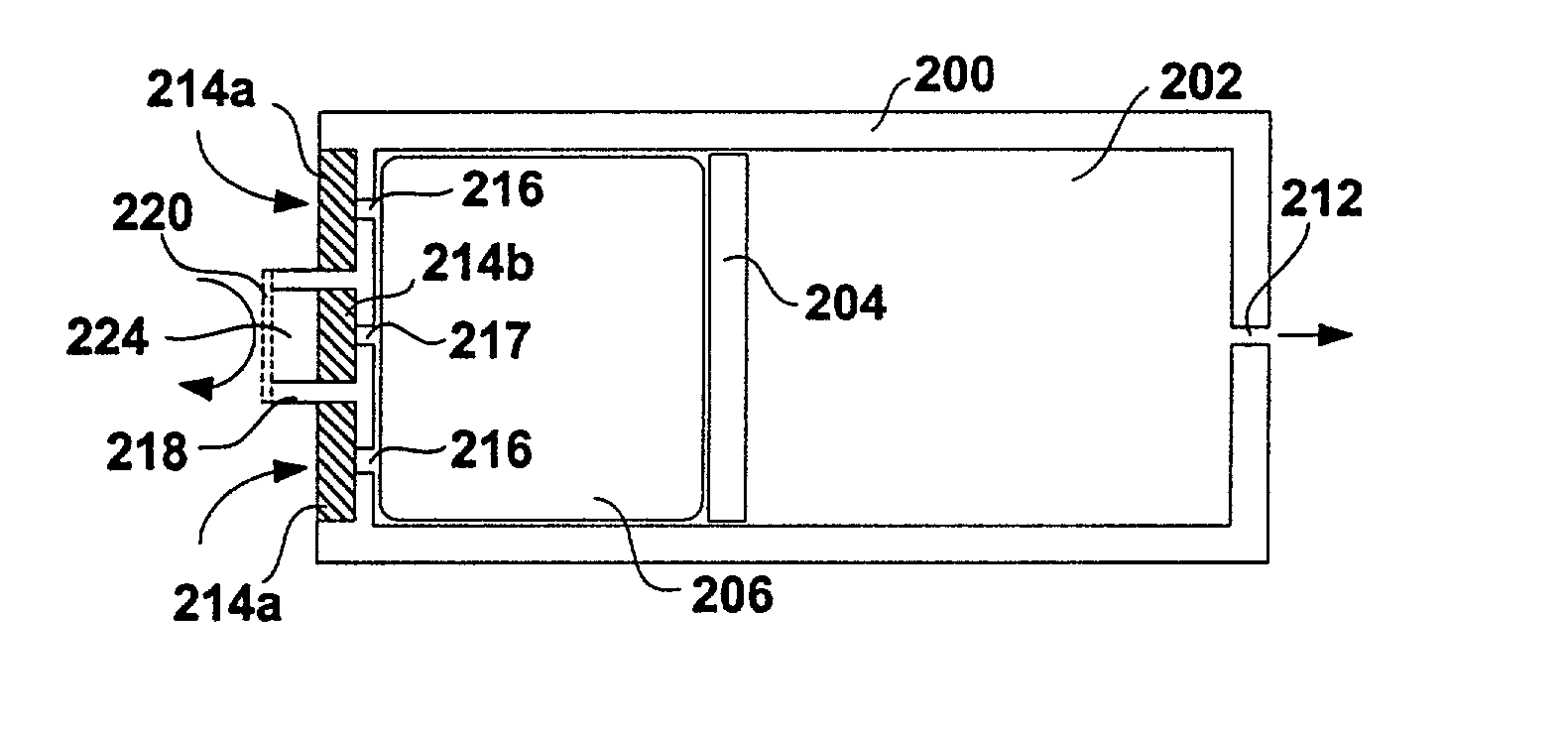
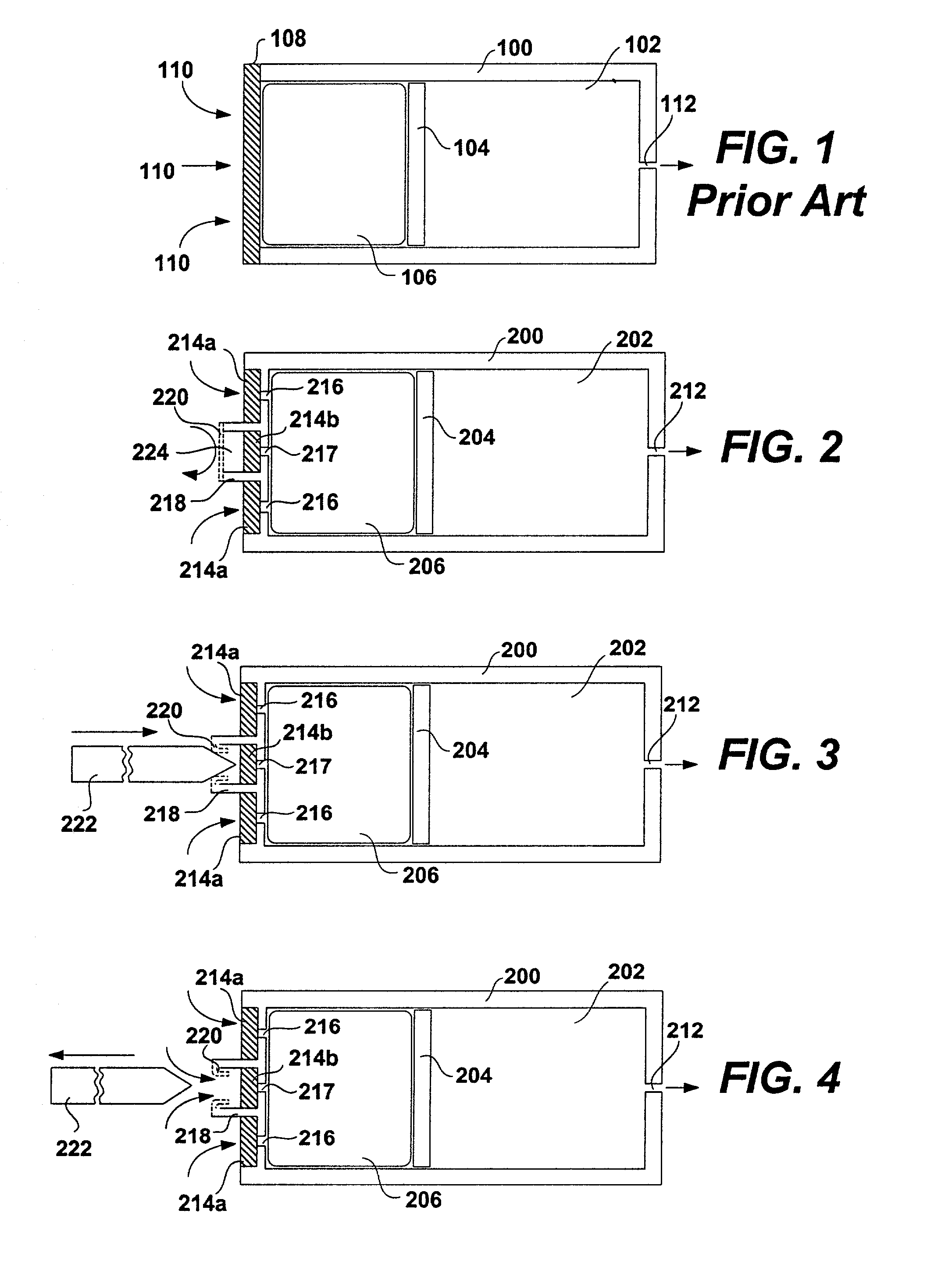
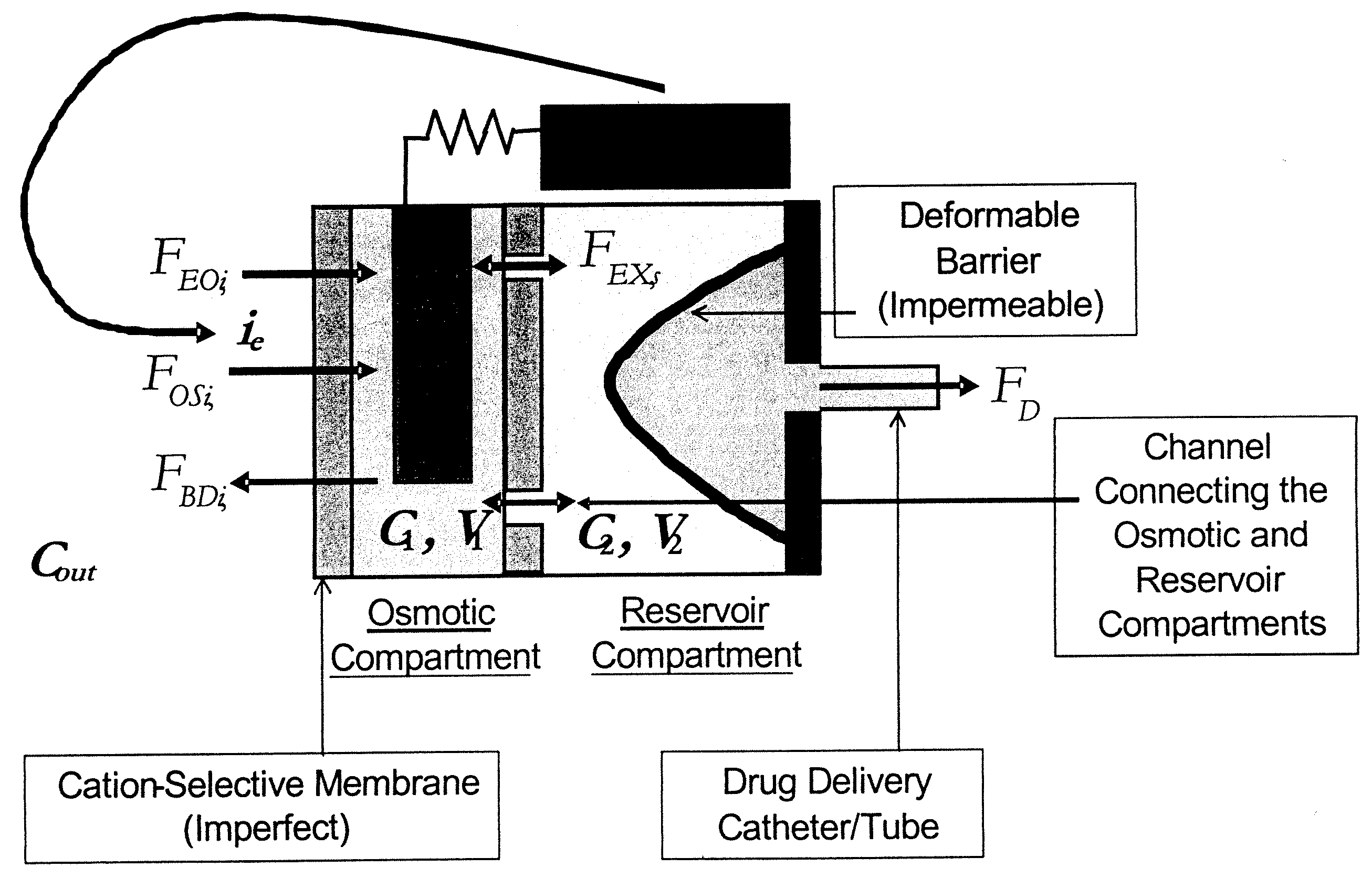
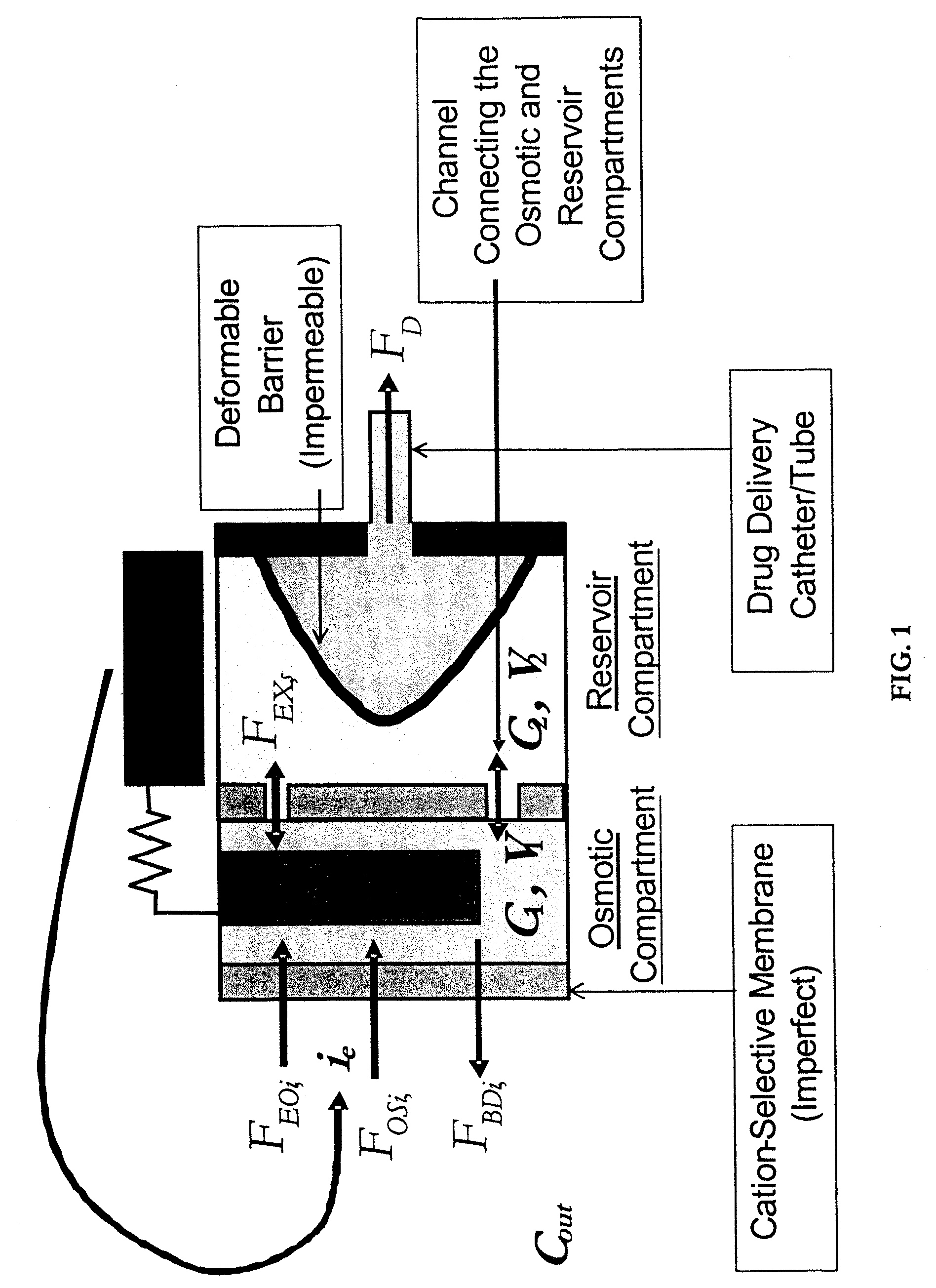
An implantable osmotic pump system includes a rigid pump housing defining an opening adapted to receive a catheter; one or more membrane assemblies fitted to the pump housing; an osmotic engine within the rigid pump housing and a flexible pharmaceutical agent compartment disposed within the pump housing. The flexible pharmaceutical agent compartment is adapted to enclose a volume of a pharmaceutical agent and to cause the pharmaceutical agent to be infused through the opening as water crosses the membrane assembly or assemblies and increases the volume of the osmotic engine. The flexible pharmaceutical agent compartment may include polyethylene teraphthalate (PET), for example, and / or may include a metallic layer such as gold, silver, platinum and / or aluminum, for example, to inhibit the transfer of gas or liquid across the compartment. A catheter may be bonded to the opening of the pump housing and to a corresponding opening in the flexible pharmaceutical agent compartment. The flexible pharmaceutical agent compartment may be free floating inside the pump housing, being only attached to the catheter and / or to the opening of the pump housing. As the present implantable osmotic pump does not include a movable piston, the pump housing may have a cylindrical shape in which the height of the pump housing is less than the diameter thereof, a non-cylindrical shape and / or a shape that conforms to the patient's anatomy at the implant site.
Owner:MICROSOLUTIONS
Extended Release Dosage Form
InactiveUS20070128279A1Satisfies needEnhances fluid fluxBiocideAntipyreticOsmotic pumpBiomedical engineering
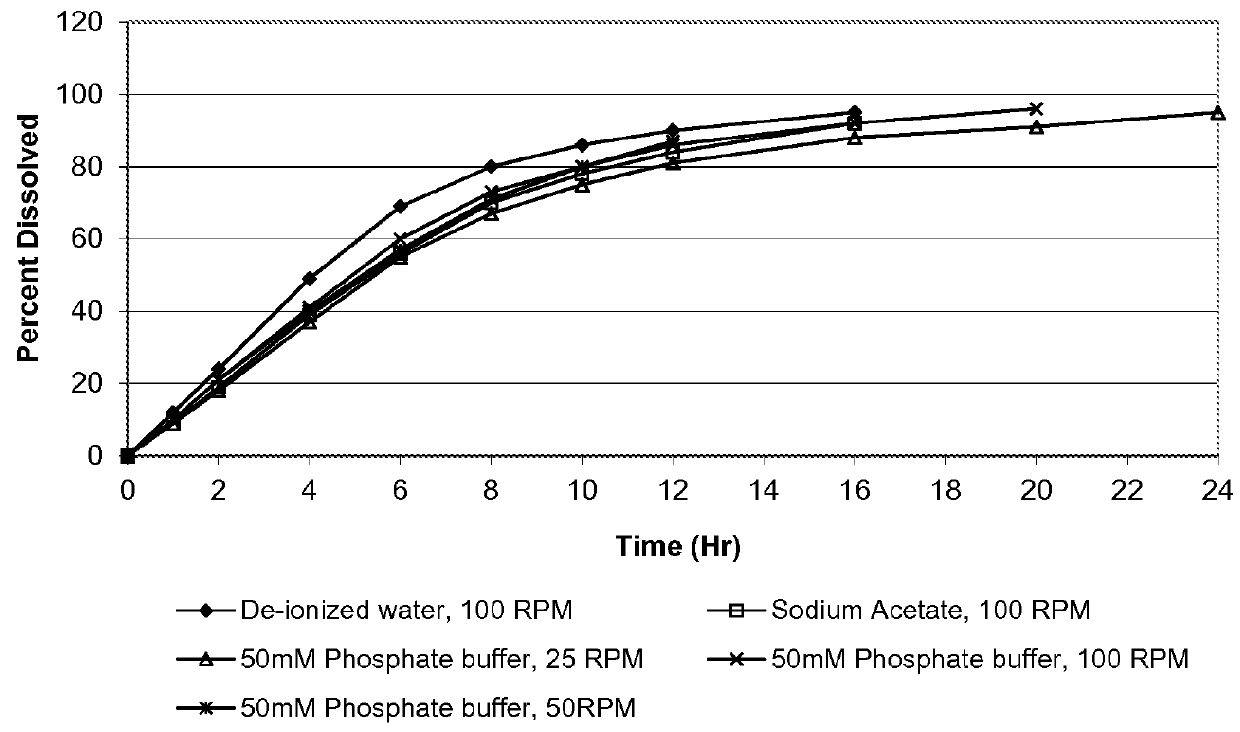
A membrane system comprising an interior wall, a fluid-permeable exterior wall surrounding the interior wall and an internal compartment defined by the membrane system, wherein fluid permeability of the interior wall is responsive to osmolarity of an osmotic core within the internal compartment are disclosed. A controlled release dosage form comprising the membrane system and a process for delivering an osmotically active formulation from an osmotic pump over an extended period of time are also disclosed.
Owner:ALZA CORP
Osmotic drug delivery system
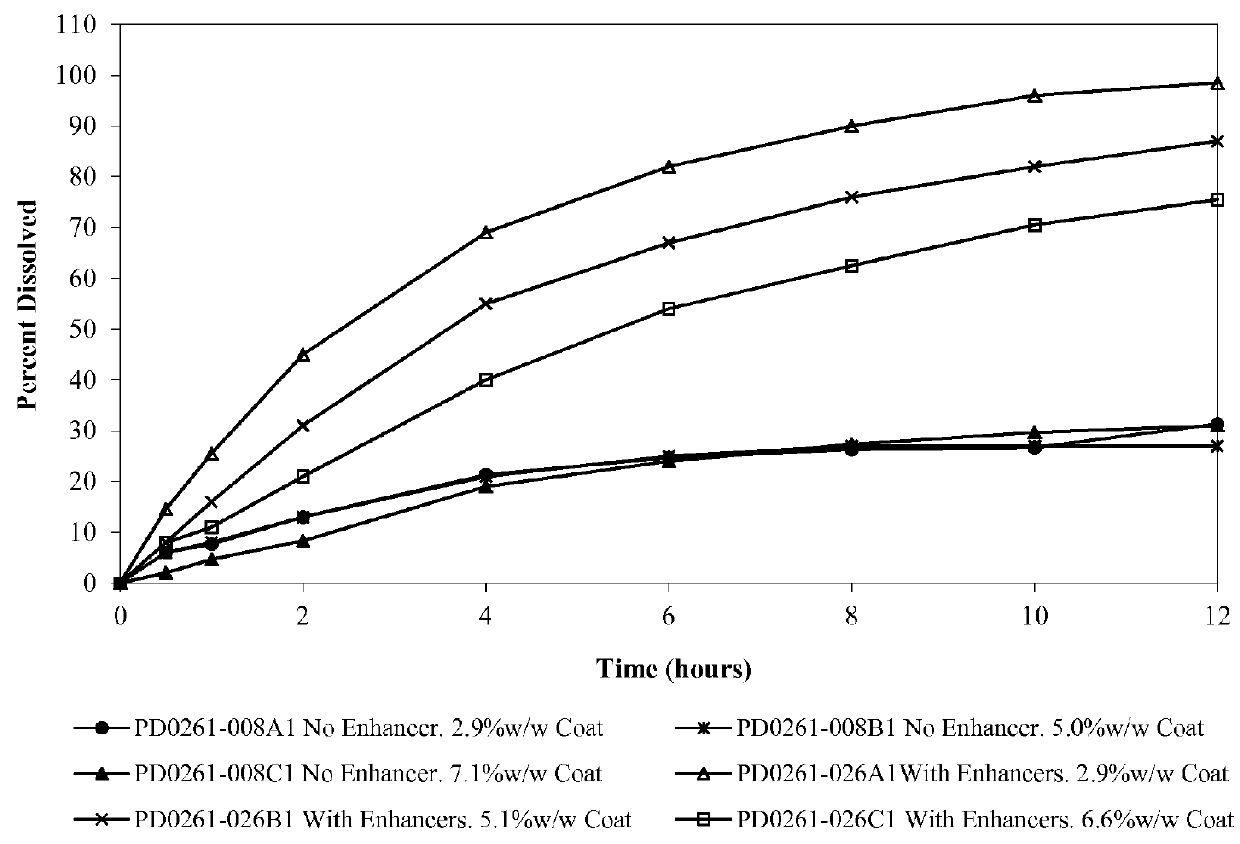
ActiveUS8747897B2Improve solubilityIncrease surface areaBiocideSenses disorderWater soluble drugWater soluble
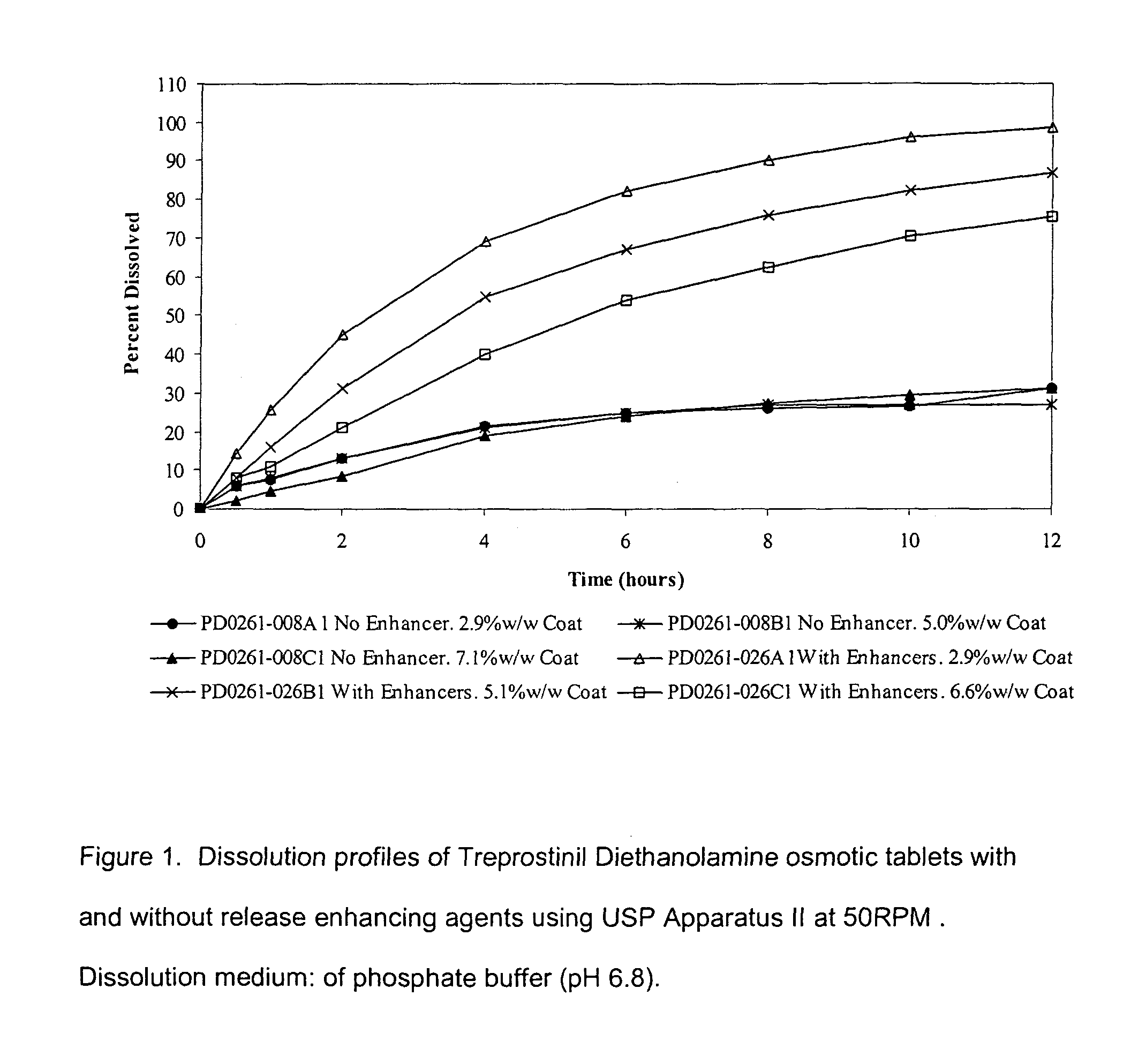
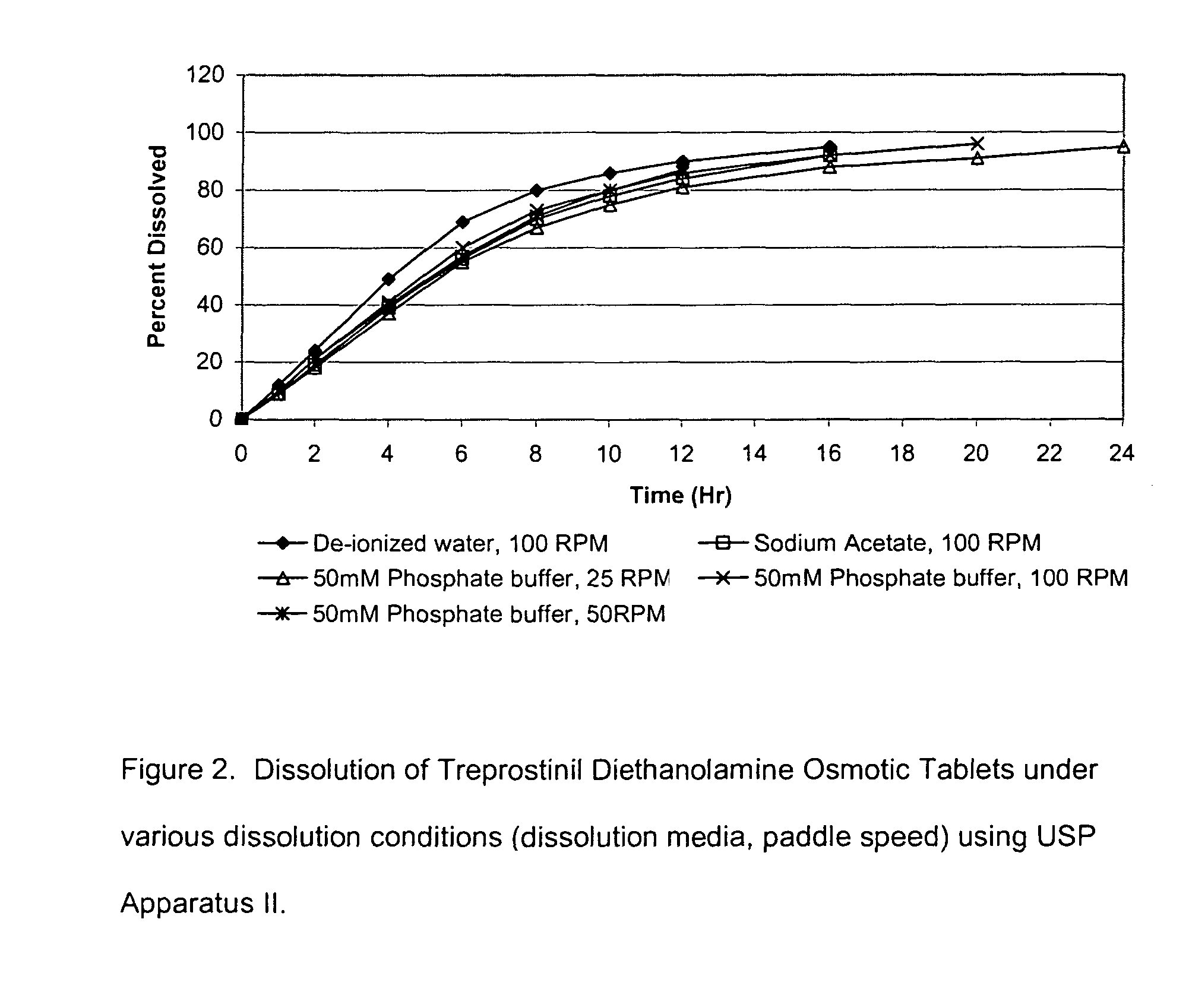
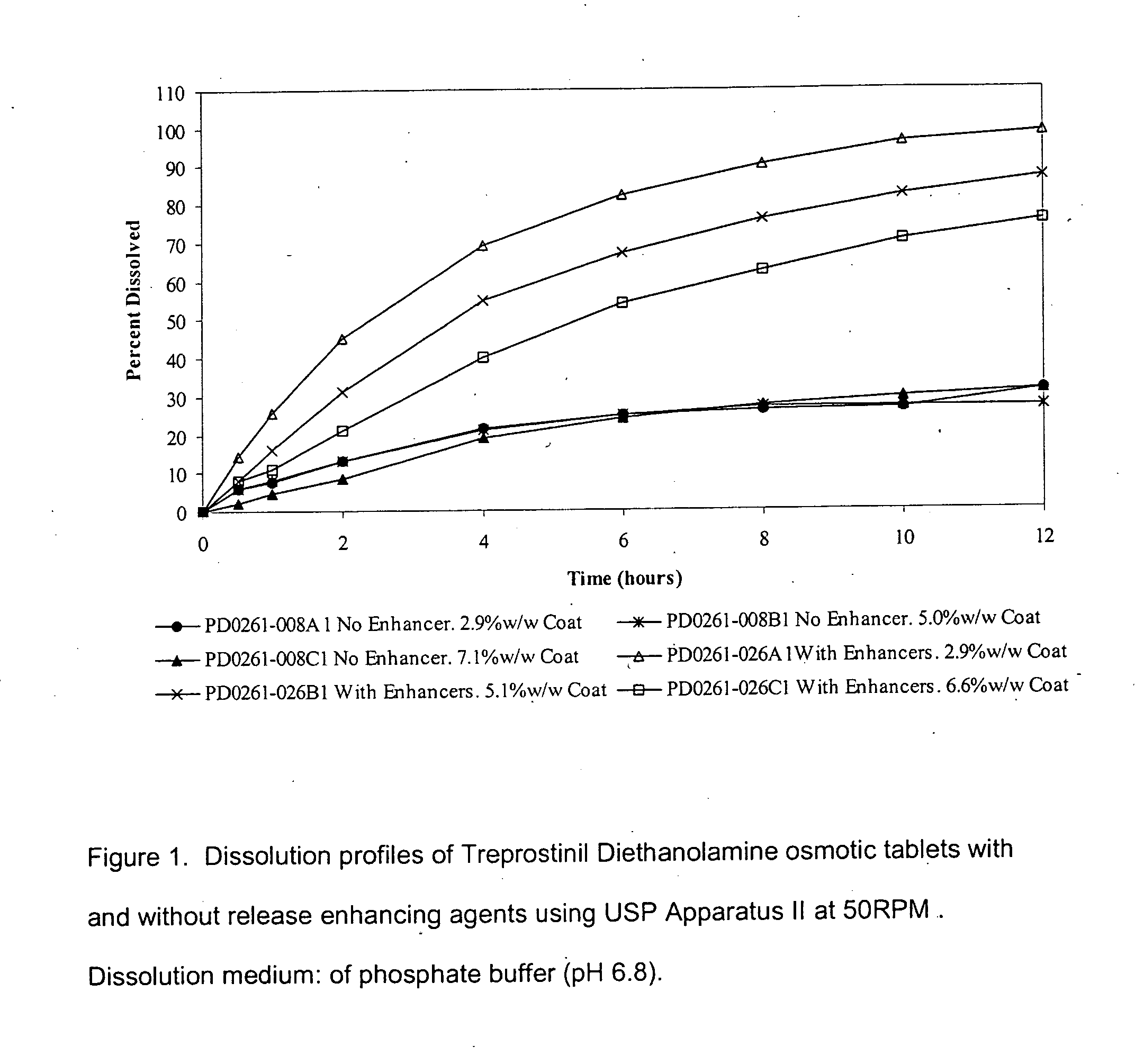
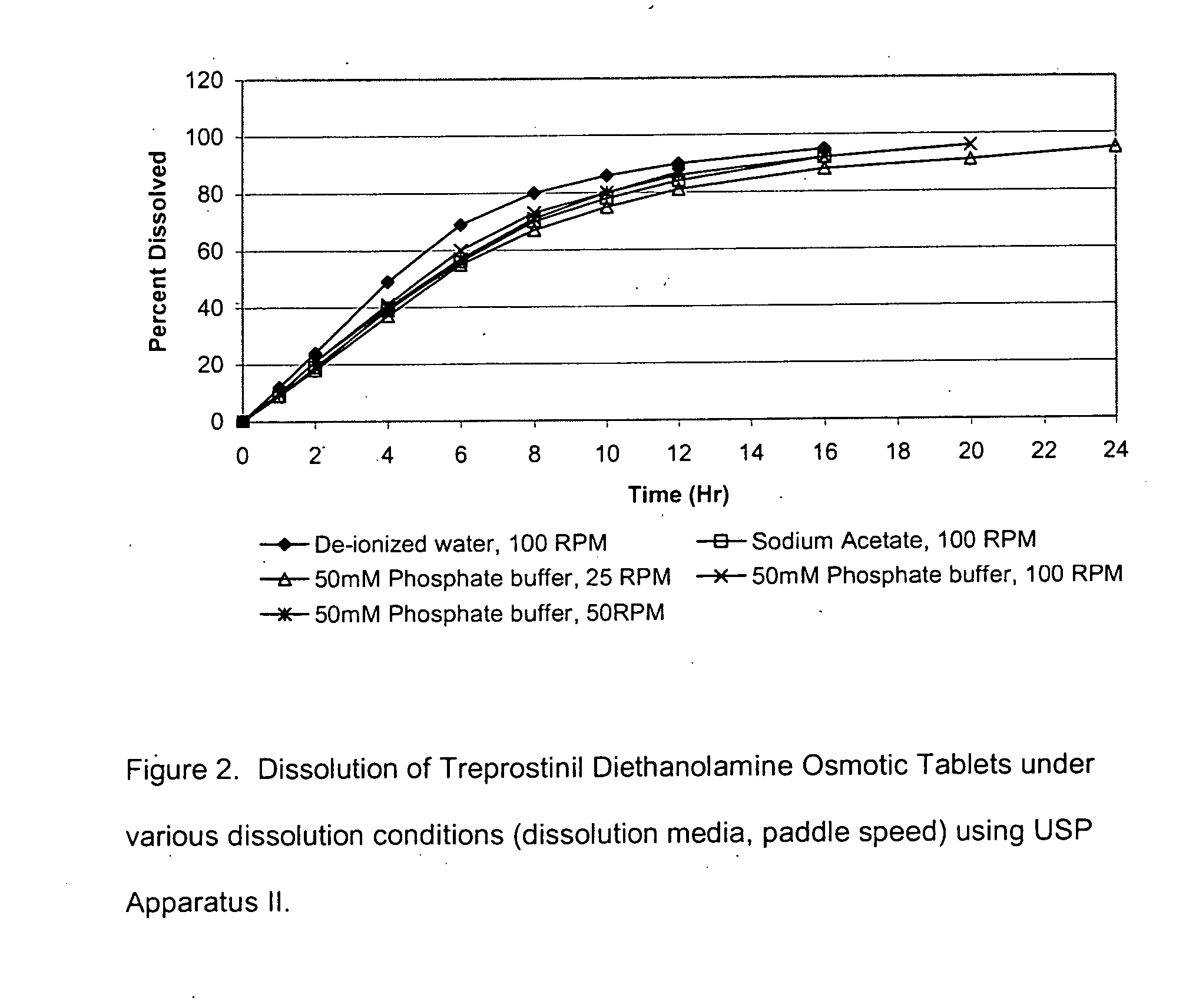
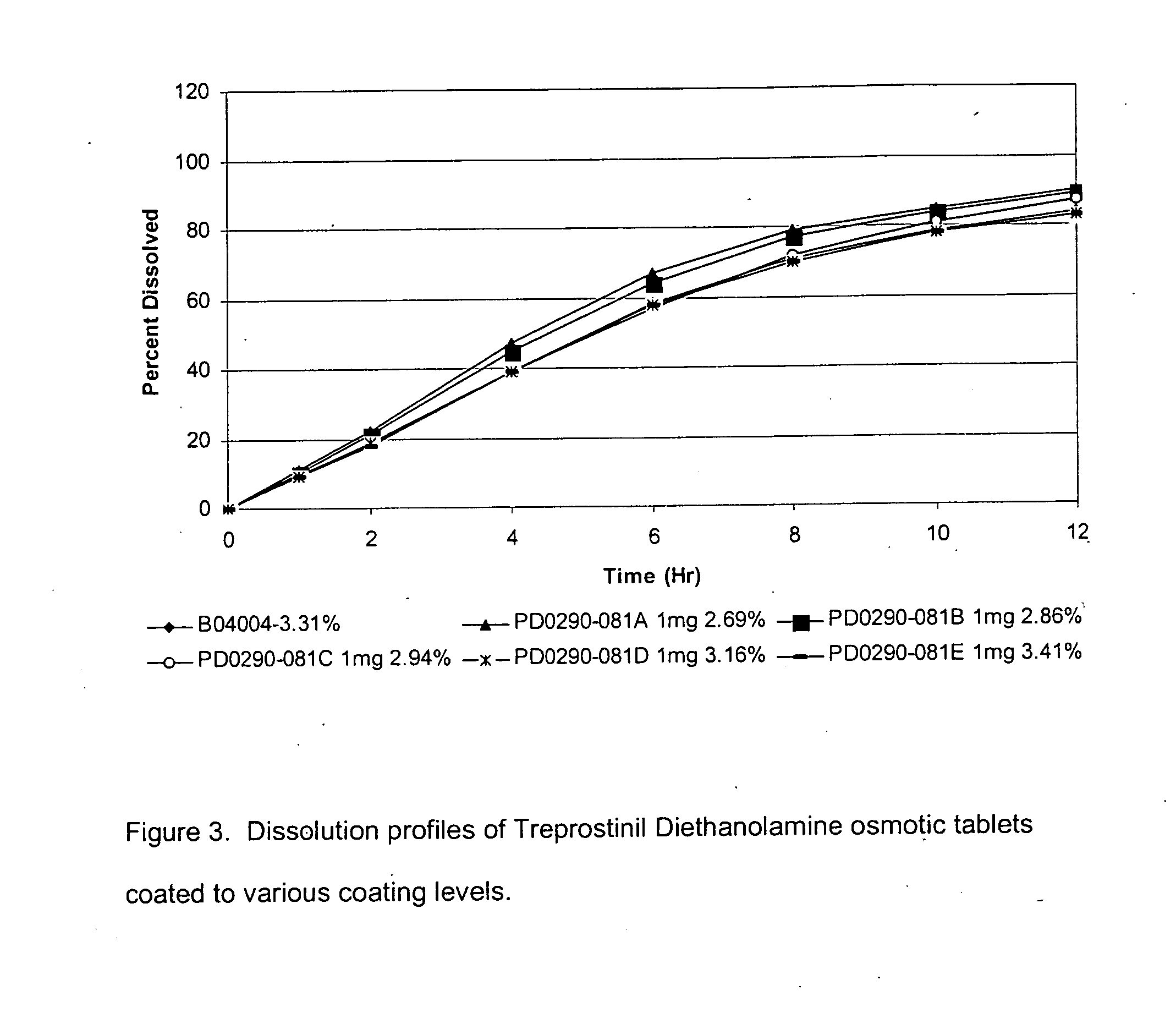
An oral osmotic pharmaceutical delivery system comprises a highly water-soluble drug exhibiting an erratic or an incomplete release profile when formulated in a elementary osmotic pump delivery system and at least one release enhancing agent.
Owner:SUPERNUS PHARM INC
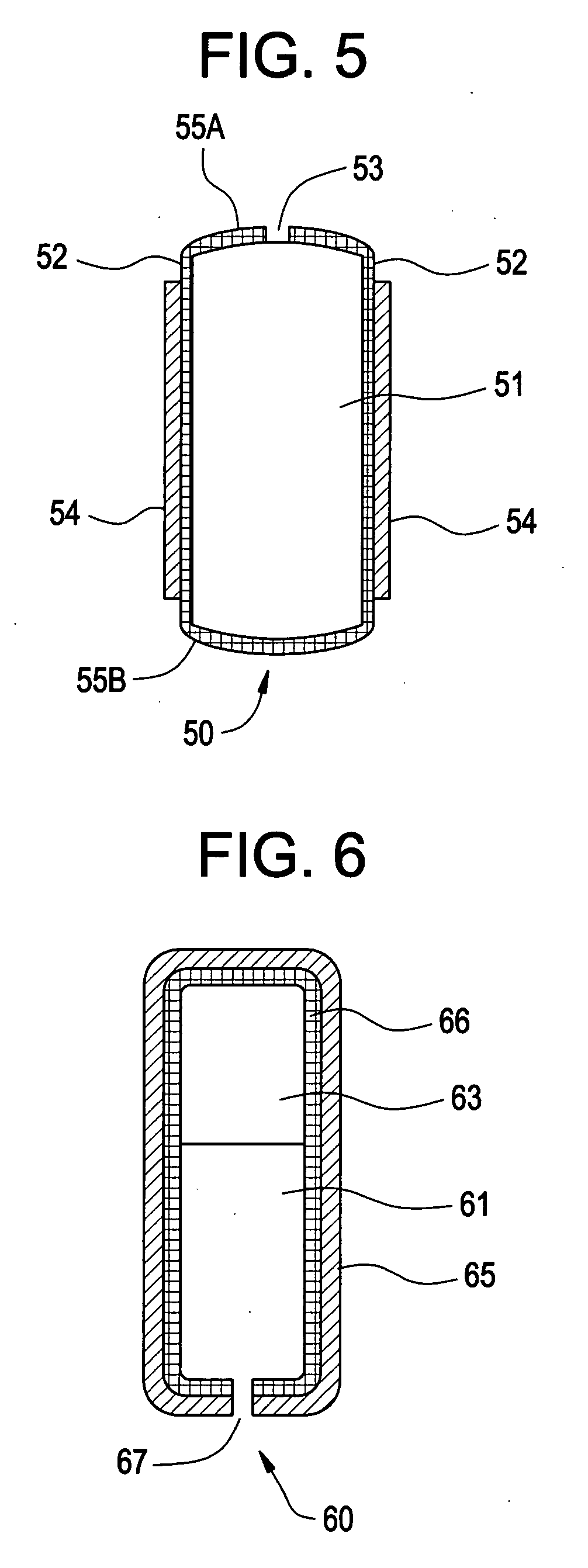
Osmotic pump with self-retaining, fast-start membrane plug
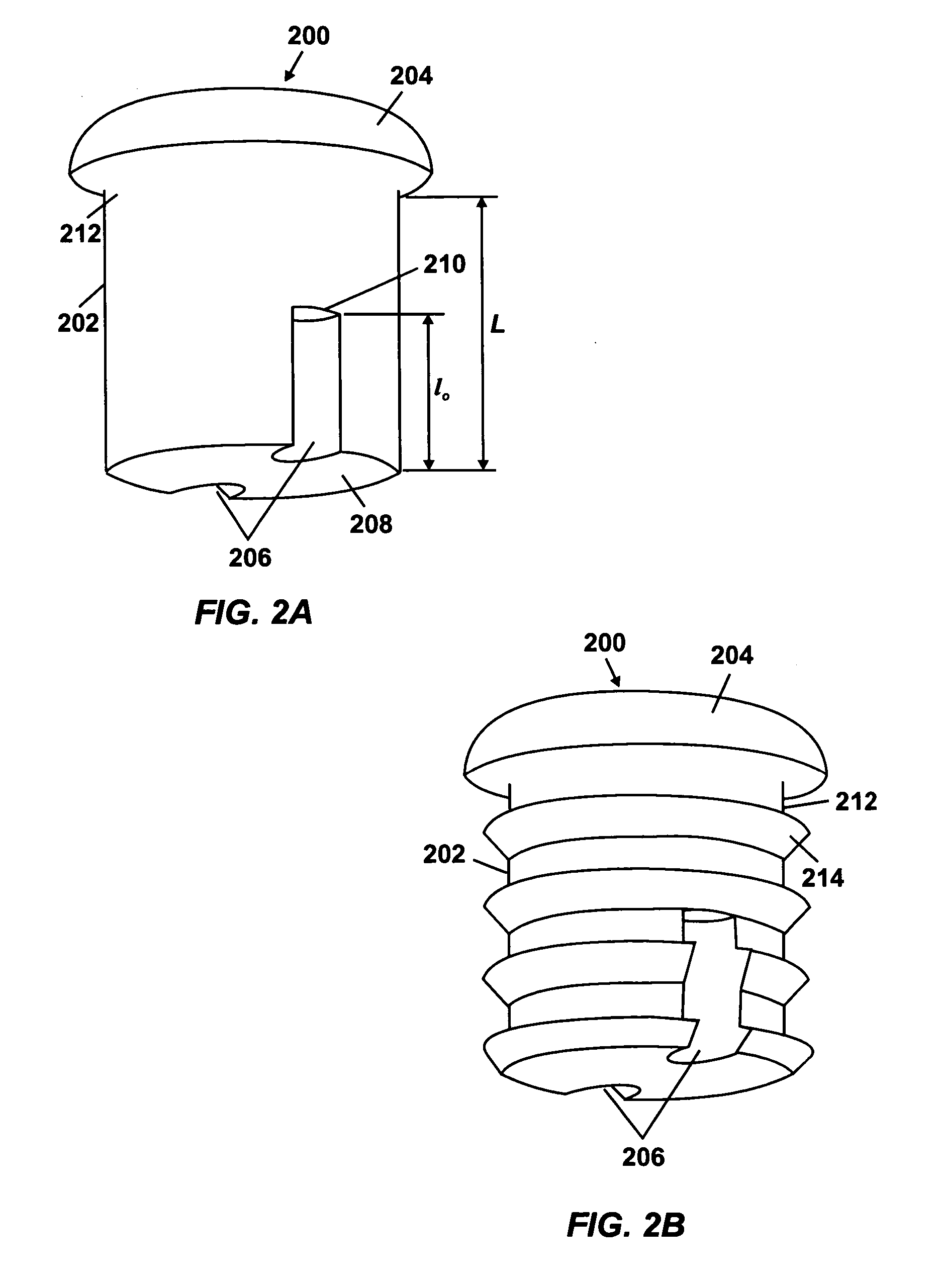
ActiveUS20050095284A1Avoid expulsionMedical devicesCapsule deliveryOsmotic pumpMembrane configuration
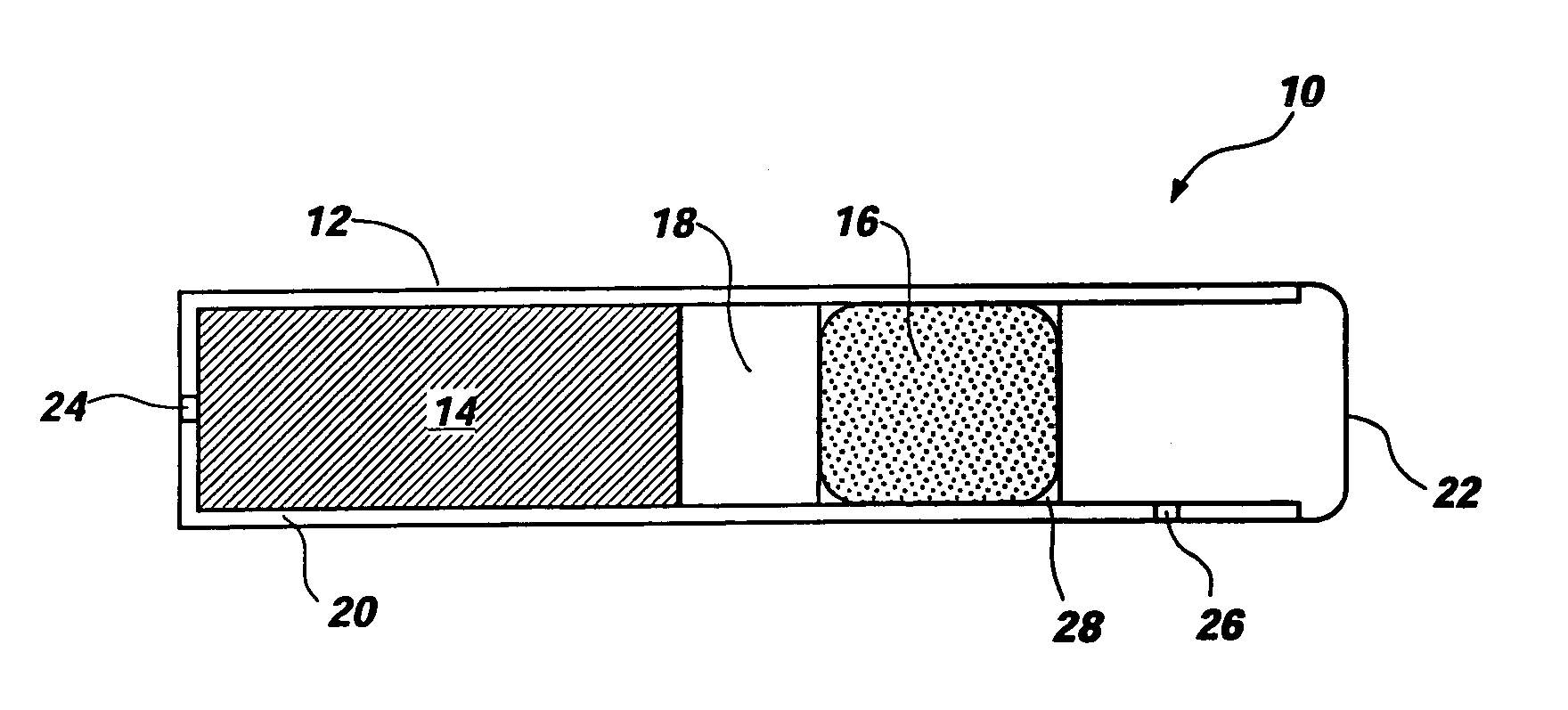
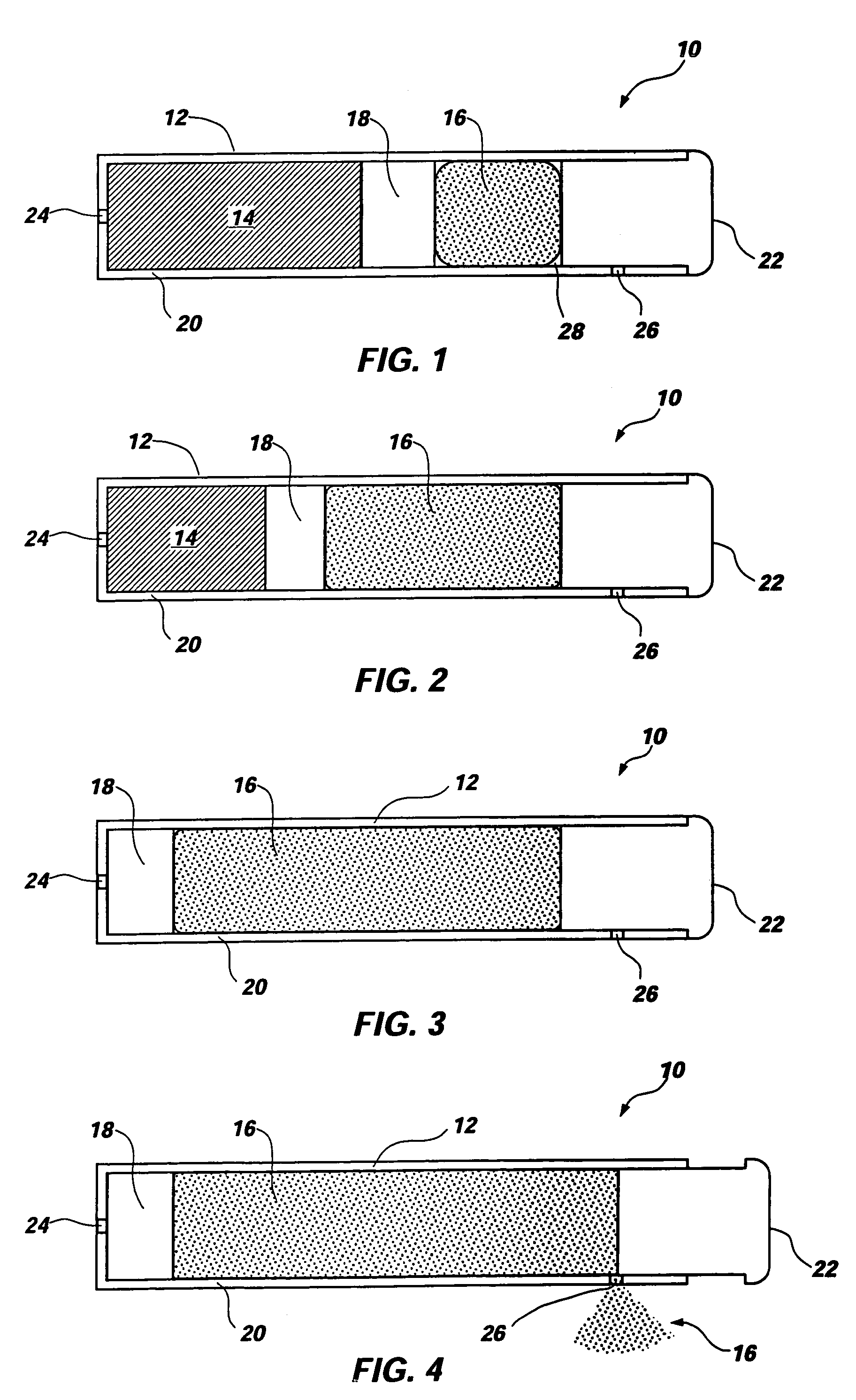
An osmotic pump includes a capsule having at least one delivery port formed at a first end and a membrane plug retained in a second end of the capsule remote from the delivery port to provide a fluid-permeable barrier between an interior and an exterior of the capsule. The membrane plug has a columnar body and at least one slot formed in the columnar body to vent pressure from the interior to the exterior of the capsule when the columnar body extends a predetermined distance relative to the second end of the capsule, thereby preventing expulsion of the membrane plug from the second end.
Owner:INTARCIA THERAPEUTICS INC
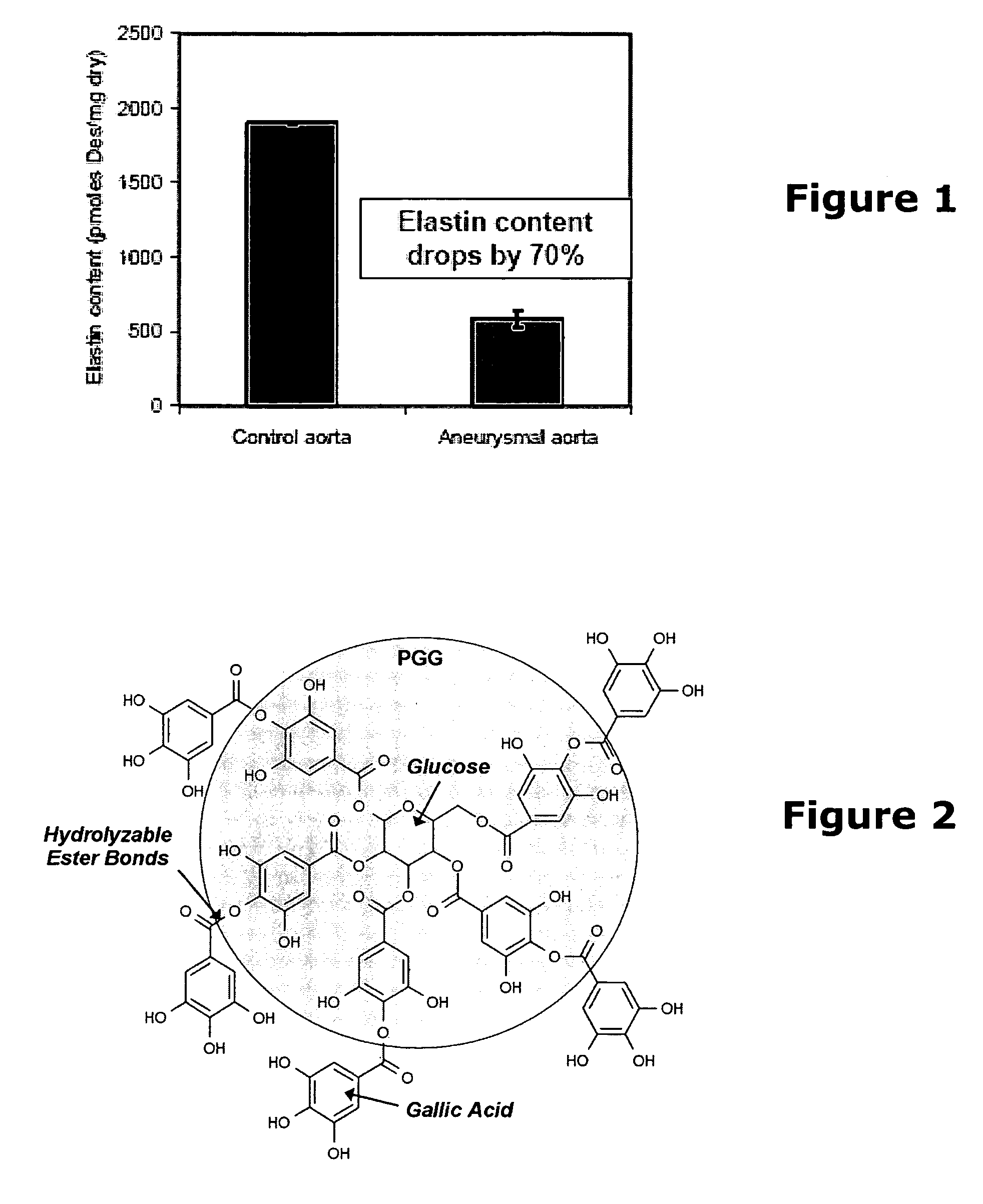
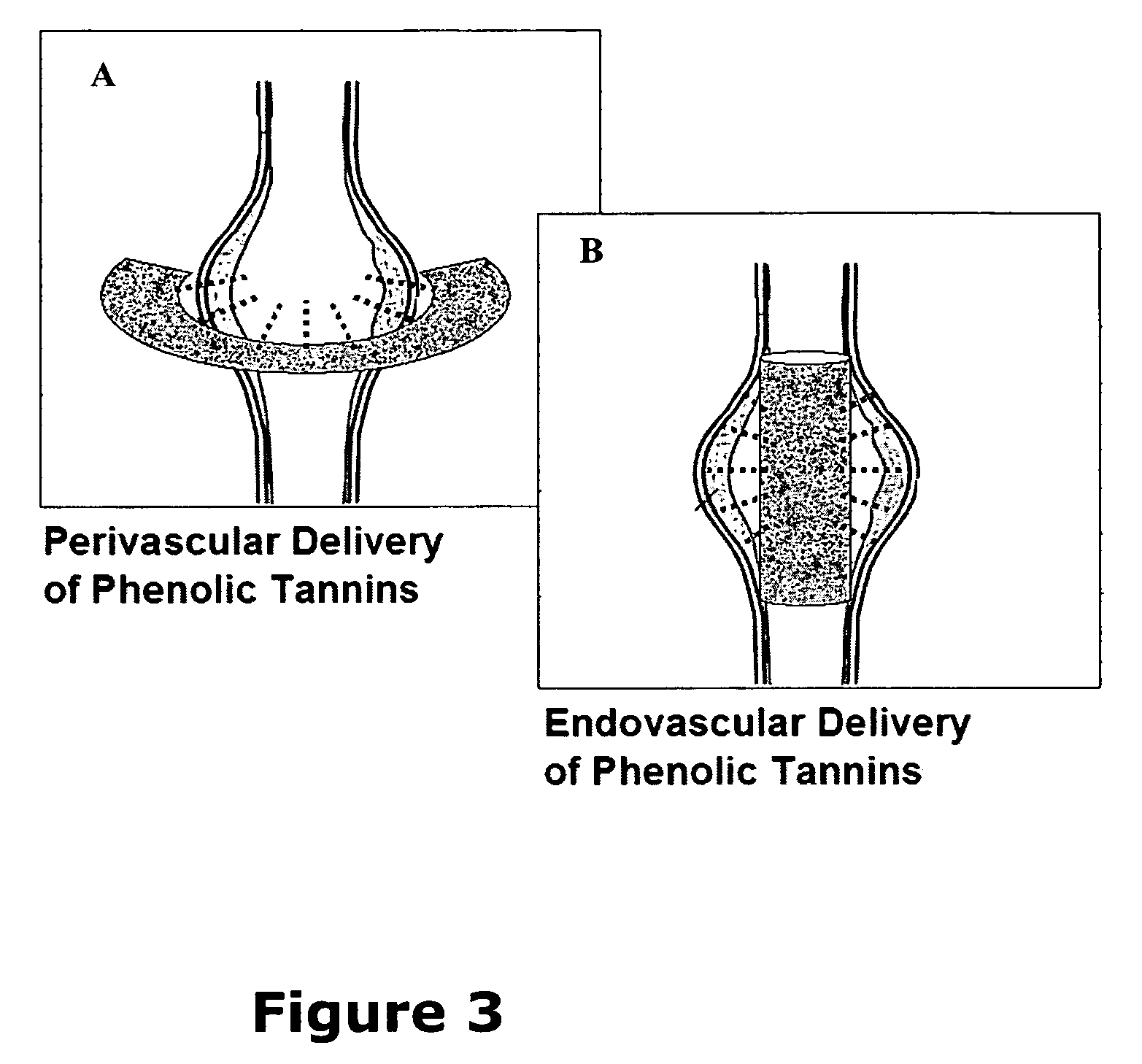
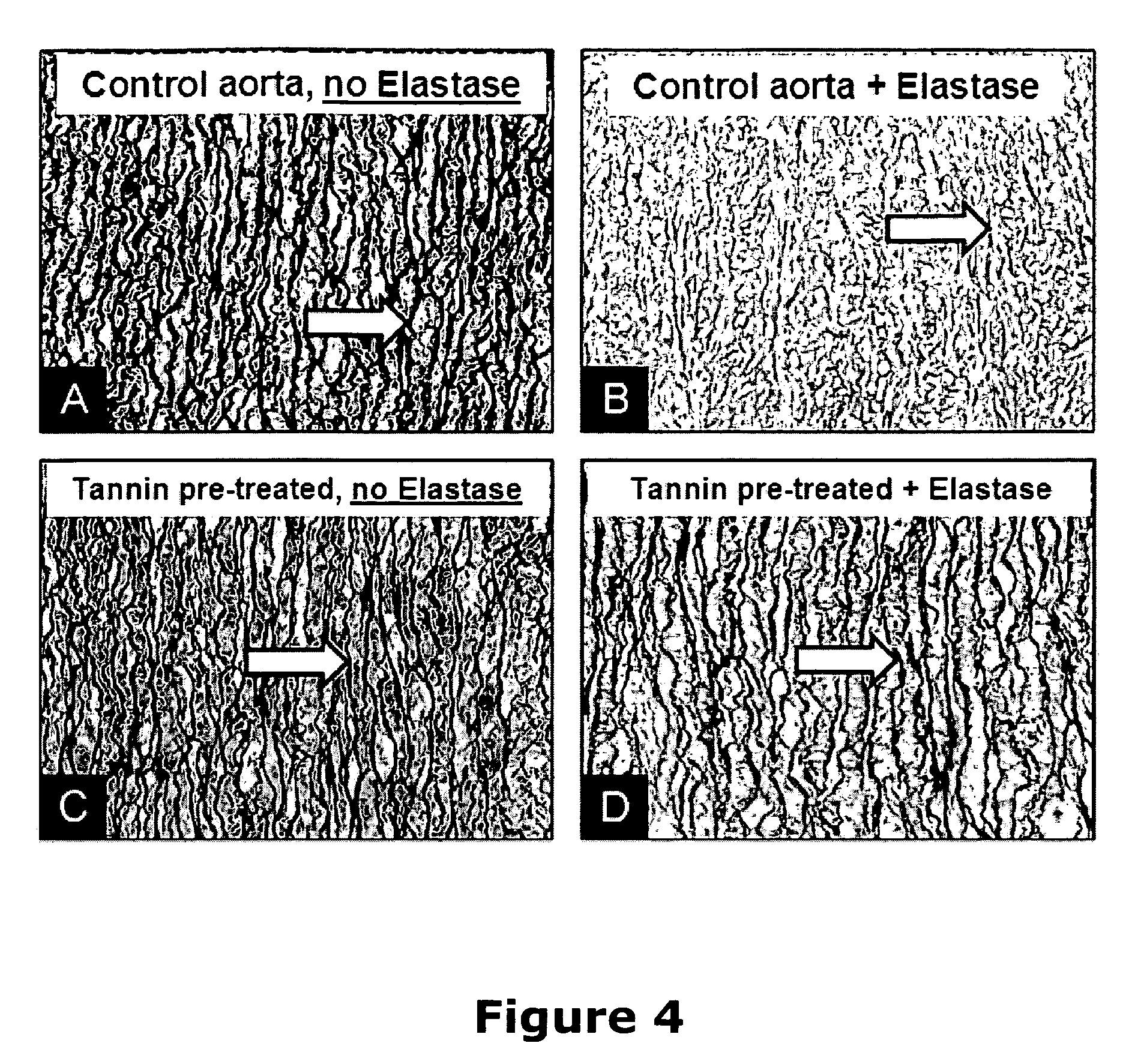
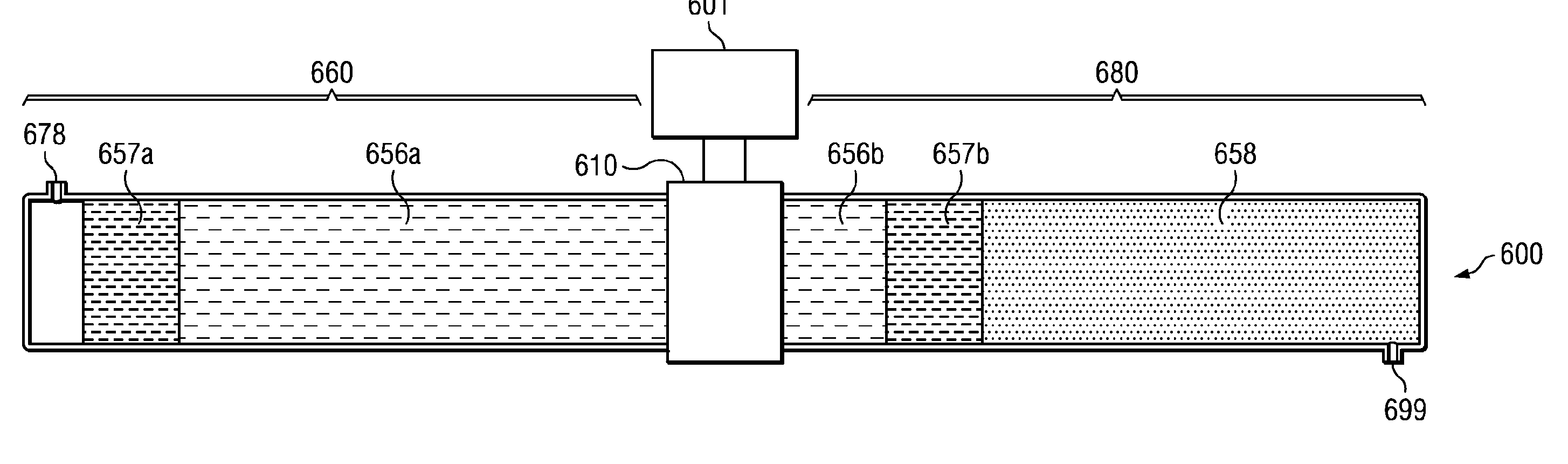
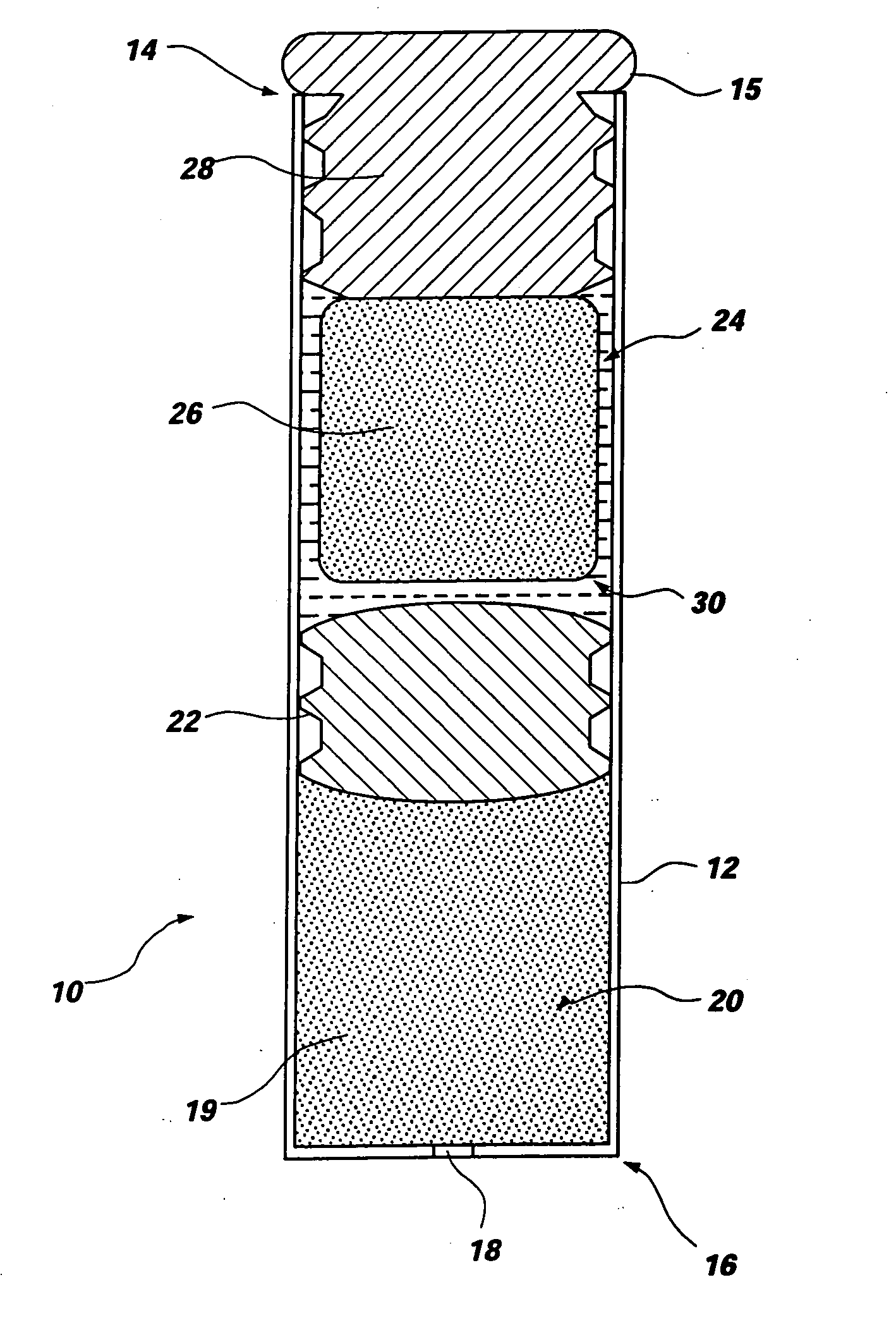
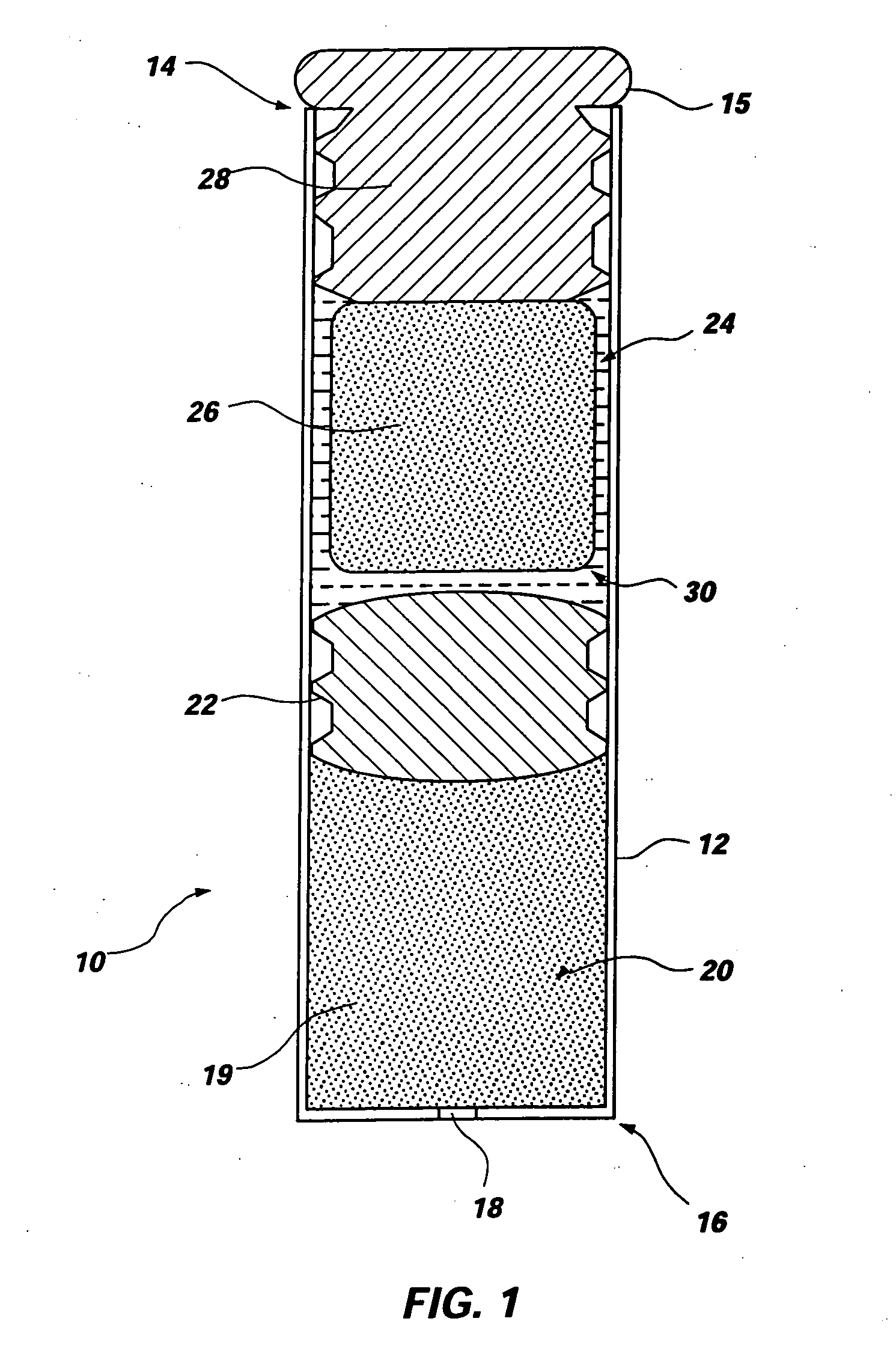
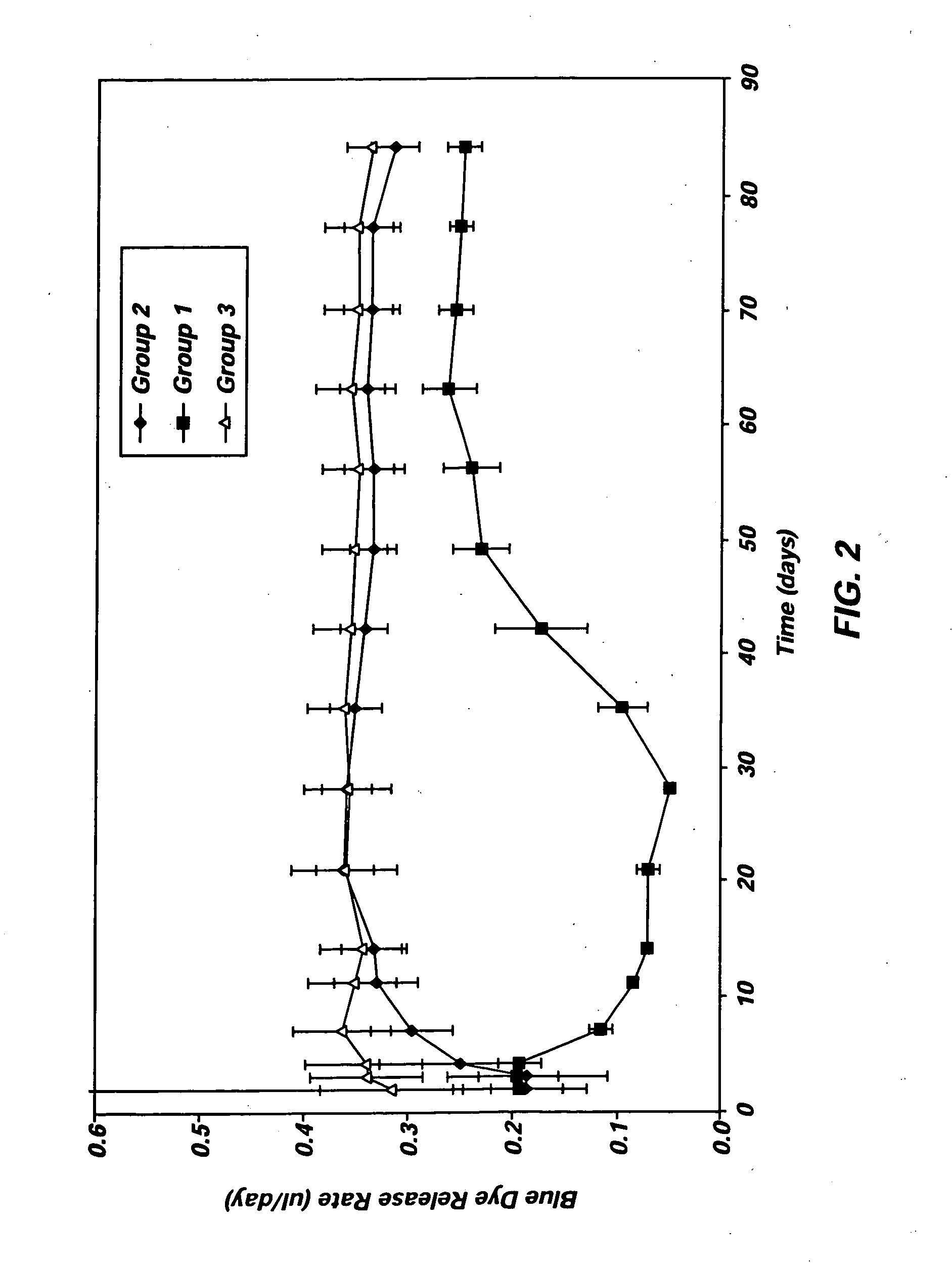
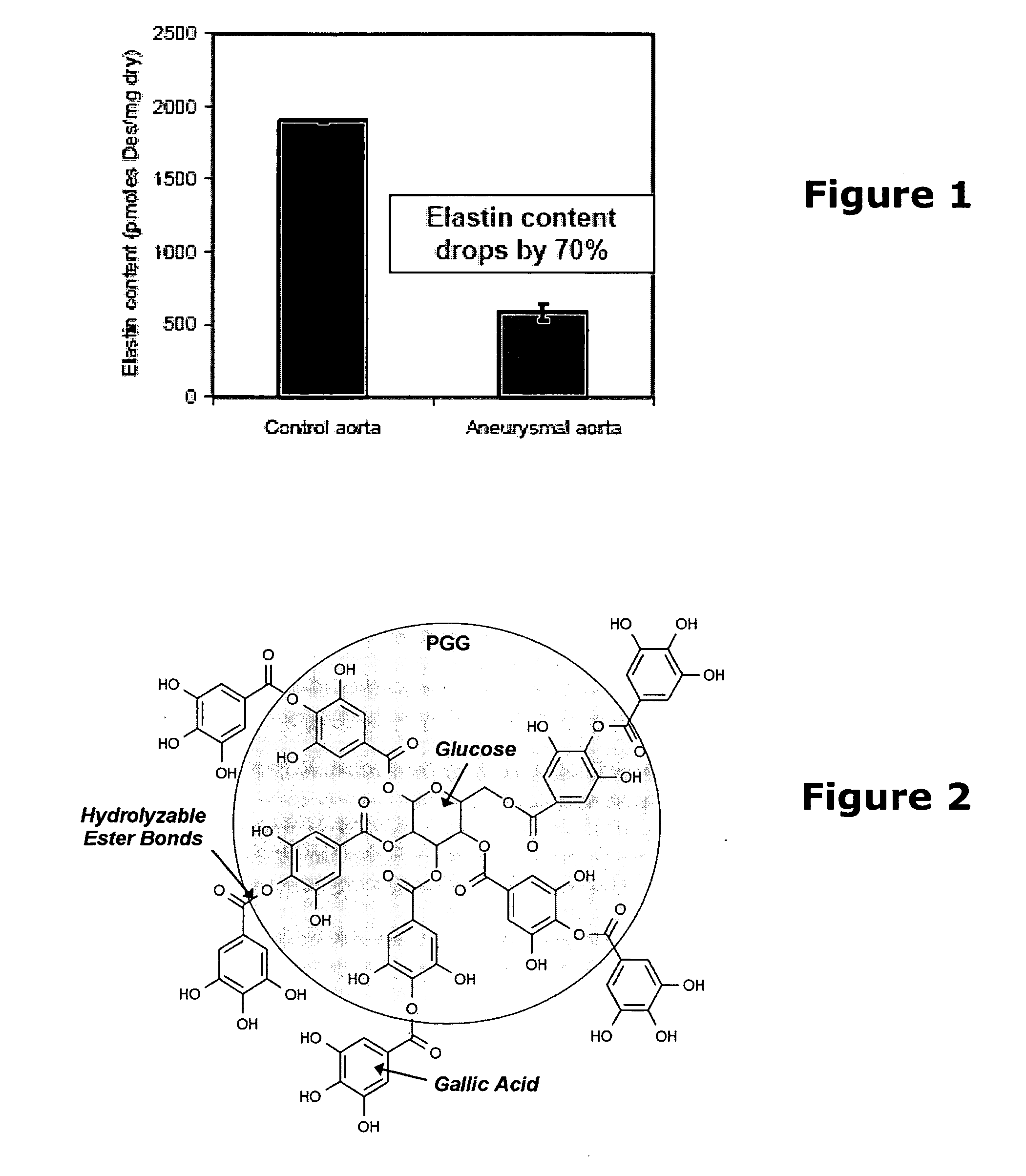
Elastin stabilization of connective tissue
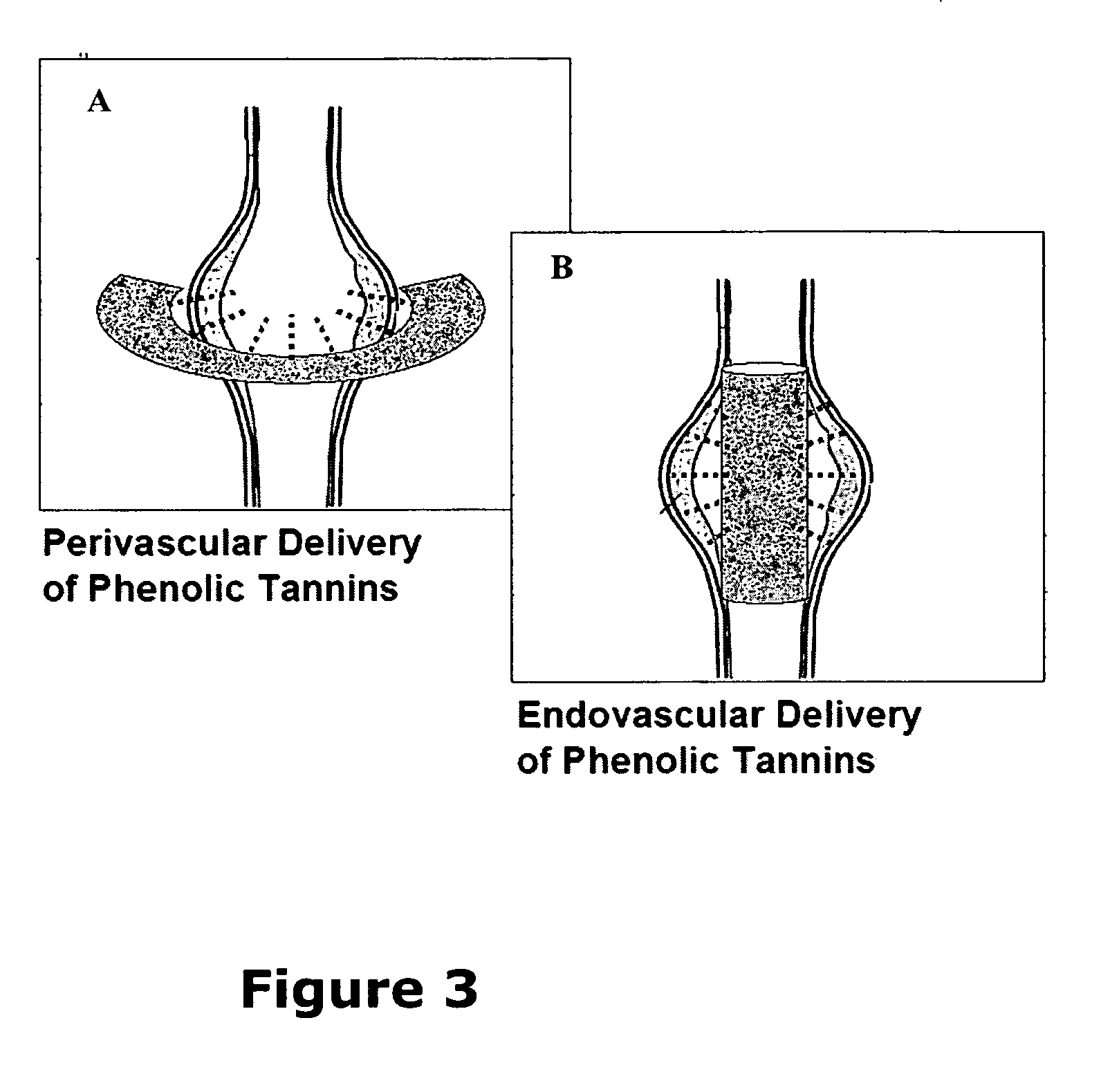
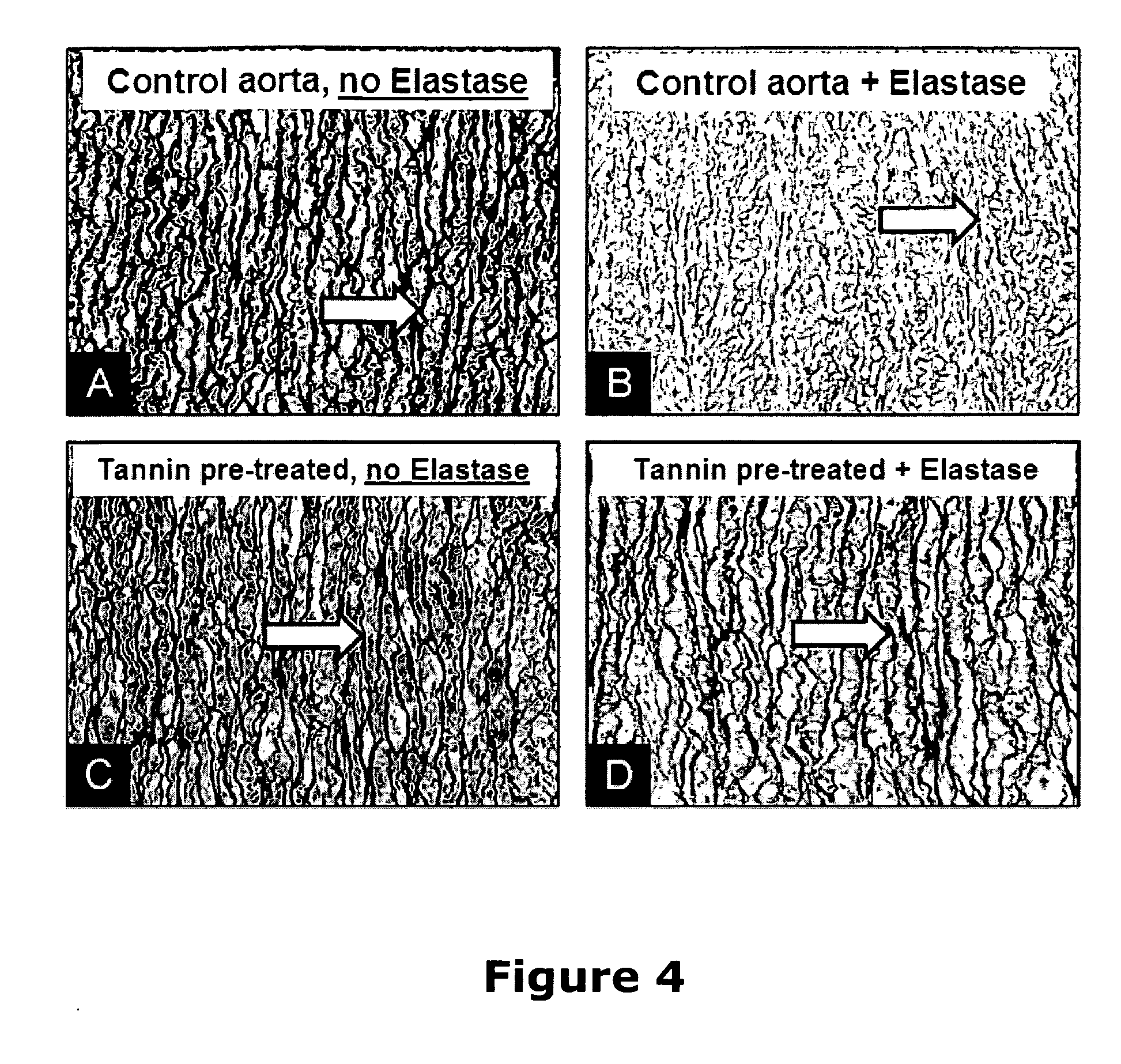
A method and product are provided for the treatment of connective tissue weakened due to destruction of tissue architecture, and in particular due to elastin degradation. The treatment agents employ certain unique properties of phenolic compounds to develop a protocol for reducing elastin degradation, such as that occurring during aneurysm formation in vasculature. According to the invention, elastin can be stabilized in vivo and destruction of connective tissue, such as that leading to life-threatening aneurysms in vasculature, can be tempered or halted all together. The treatment agents can be delivered or administered acutely or chronically according to various delivery methods, including sustained release methods incorporating perivascular or endovascular patches, use of microsphere carriers, hydrogels, or osmotic pumps.
Owner:CLEMSON UNIV RES FOUND
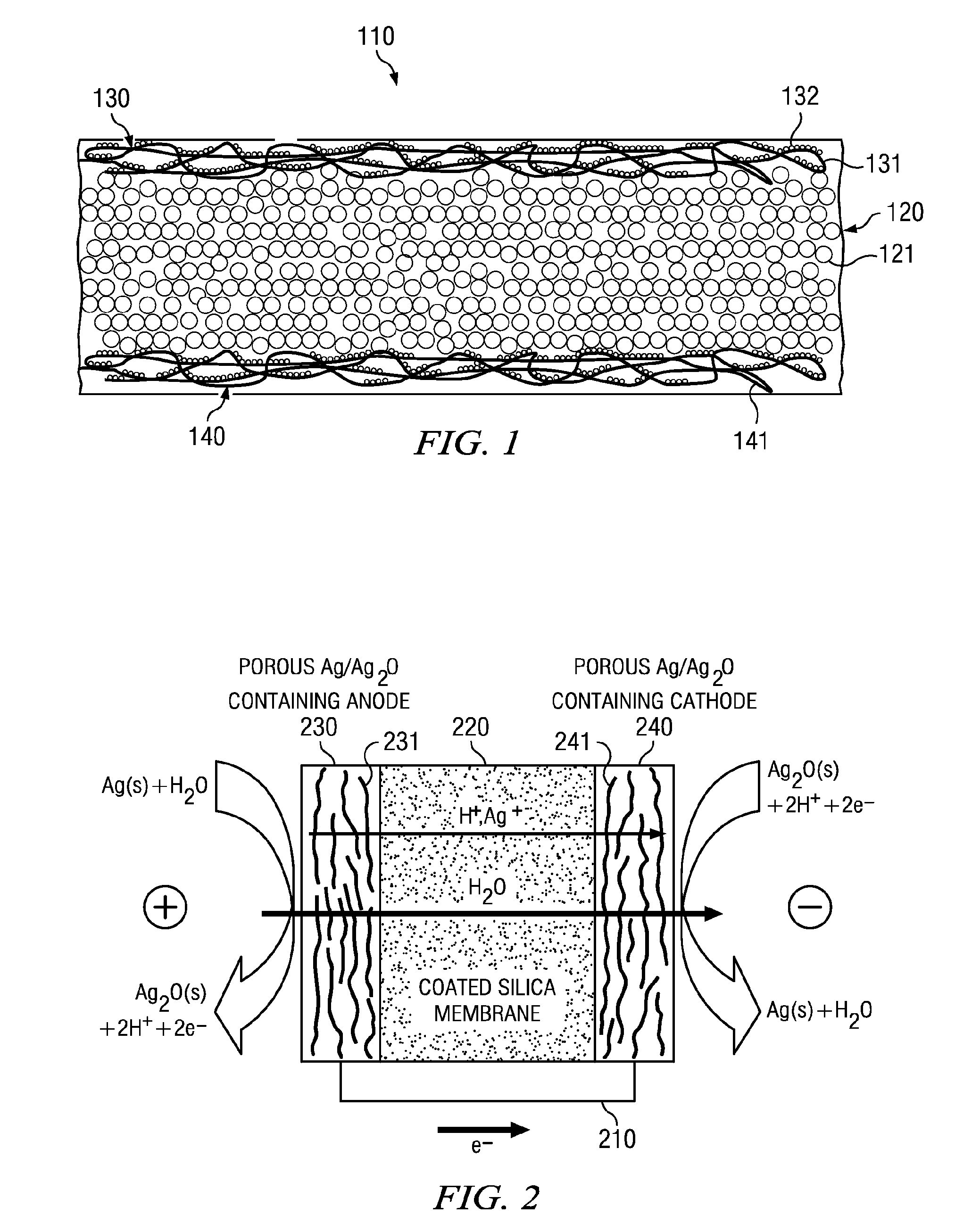
Electro-osmotic pumps, systems, methods, and compositions
The present disclosure relates, according to some embodiments, to compositions, methods, devices, and systems for delivering a composition (e.g., a fluid composition) to a subject. For example, the present disclosure relates to non-gassing, direct current (DC), electro-osmotic pumps in some embodiments. A pump may comprise an anode (e.g., a porous silver / silver oxide anode), a cathode (e.g., a porous silver / silver oxide cathode), and a membrane (e.g., a porous ceramic membrane) positioned at least partially between the anode and the cathode in some embodiments. A pump system may comprise an electro-osmotic pump, a reservoir comprising a pump fluid chamber in fluid communication with the electro-osmotic pump and a delivery fluid chamber in fluid communication with the electro-osmotic pump; a controller assembly in electrical communication with the anode and the cathode; and a cannula and / or a needle in fluid communication with the delivery fluid chamber. A pump fluid may comprise water and / or a delivery fluid may comprise a drug, in some embodiments.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Osmotic pump with self-retaining, fast-start membrane plug
An osmotic pump includes a capsule having at least one delivery port formed at a first end and a membrane plug retained in a second end of the capsule remote from the delivery port to provide a fluid-permeable barrier between an interior and an exterior of the capsule. The membrane plug has a columnar body and at least one slot formed in the columnar body to vent pressure from the interior to the exterior of the capsule when the columnar body extends a predetermined distance relative to the second end of the capsule, thereby preventing expulsion of the membrane plug from the second end.
Owner:INTARCIA THERAPEUTICS INC
Osmotic pump drug delivery systems and methods
InactiveUS20030032947A1Avoid mixingMedical devicesPharmaceutical delivery mechanismTherapeutic effectAnalgesics effects
Owner:MICROSOLUTIONS
Osmotic delivery system and method for decreasing start-up times for osmotic delivery systems
InactiveUS20050010196A1Reduce startup timeEfficient deliveryOrganic active ingredientsPill deliveryMedicineSemipermeable membrane
The present invention includes devices and methods for reducing the start-up time of osmotically driven drug delivery systems capable of delivering a desired drug at a controlled rate over time. In particular, the present invention includes osmotic pumps that have a preloaded membrane, which includes a semipermeable material that has been preloaded with a nonaqueous, incompressible liquid filler that is miscible with water. The present invention further includes methods for making such osmotic pumps. The preloaded membranes included in the osmotic pumps according to the present invention have proven to provide significant decreases in average start-up times relative to osmotic pumps that include semipermeable membranes that are not preloaded.
Owner:INTARCIA THERAPEUTICS INC
Elastin stabilization of connective tissue
A method and product are provided for the treatment of connective tissue weakened due to destruction of tissue architecture, and in particular due to elastin degradation. The treatment agents employ certain unique properties of phenolic compounds to develop a protocol for reducing elastin degradation, such as that occurring during aneurysm formation in vasculature. According to the invention, elastin can be stabilized in vivo and destruction of connective tissue, such as that leading to life-threatening aneurysms in vasculature, can be tempered or halted all together. The treatment agents can be delivered or administered acutely or chronically according to various delivery methods, including sustained release methods incorporating perivascular or endovascular patches, use of microsphere carriers, hydrogels, or osmotic pumps.
Owner:CLEMSON UNIV RES FOUND
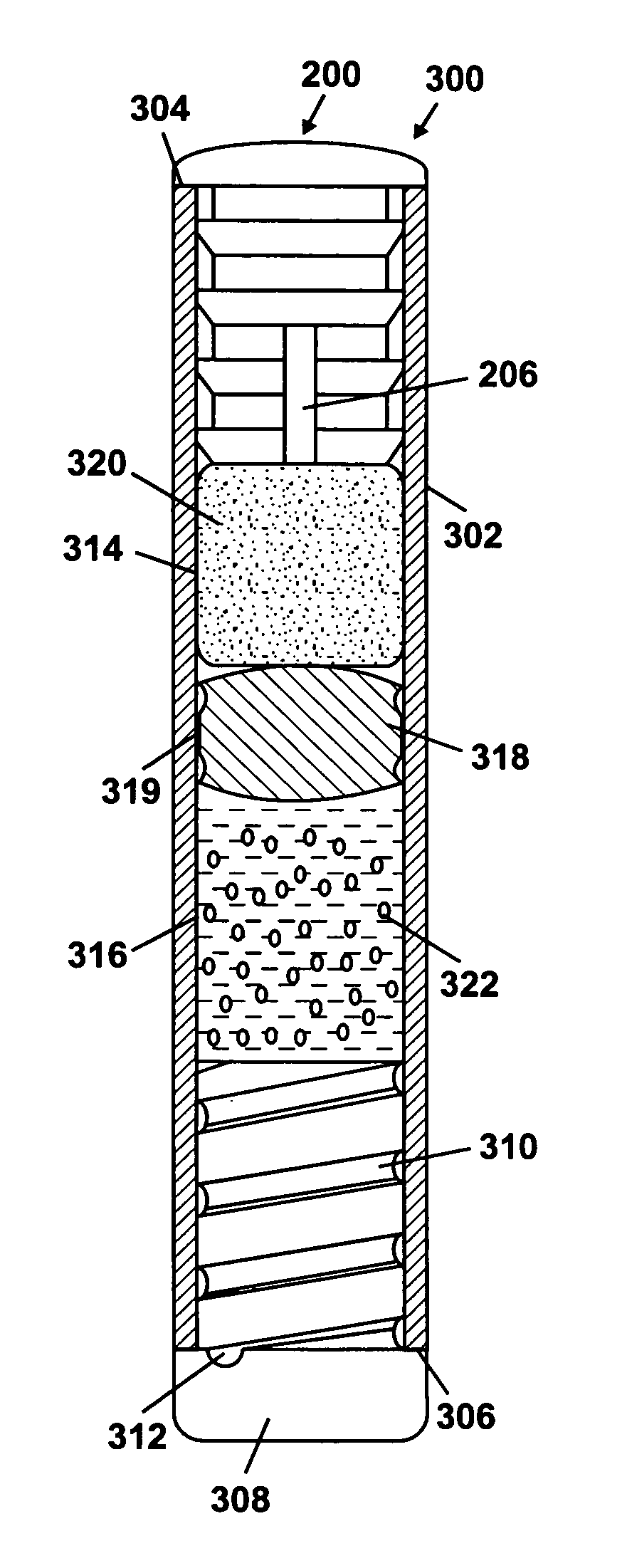
Implantable osmotic pump
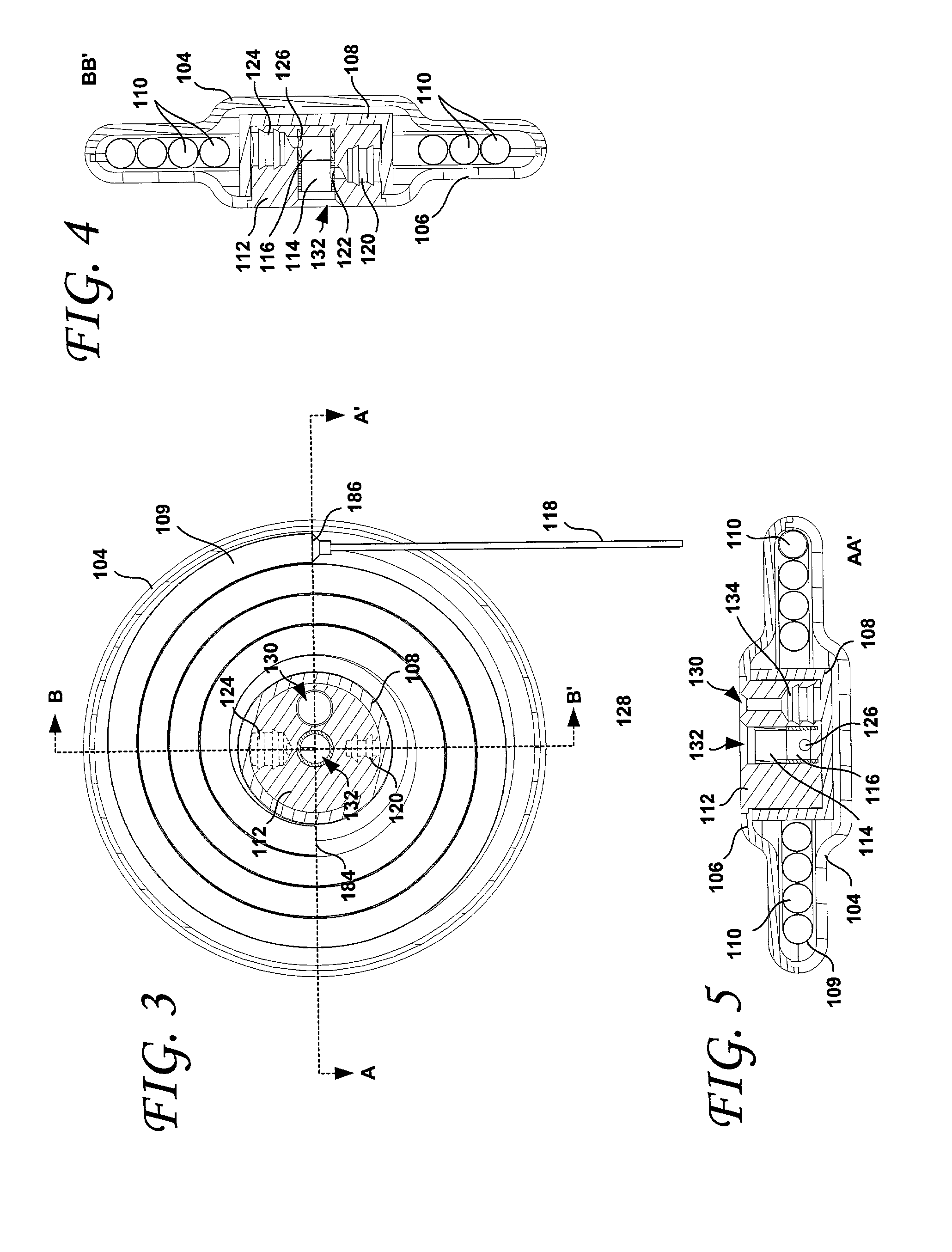
An implantable osmotic pump for delivering a pharmaceutical agent to a patient includes an osmotic engine, a substantially toroidal compartment disposed at least partially around the osmotic engine and a piston disposed within the compartment. The osmotic engine is configured to cause the piston to travel within the compartment and deliver a dose pharmaceutical agent contained within the compartment when the pump is implanted in an aqueous environment. A dose escalation assembly may be fitted to the pump, the dose escalation assembly being adapted to selectively increase the rate at which the pharmaceutical agent is delivered from the pump.
Owner:MICROSOLUTIONS
Osmotic drug delivery system
ActiveUS20070254032A1Good water solubilityPatient compliance is goodBiocideSenses disorderWater solubleWater soluble drug
An oral osmotic pharmaceutical delivery system comprises a highly water-soluble drug exhibiting an erratic or an incomplete release profile when formulated in a elementary osmotic pump delivery system and at least one release enhancing agent.
Owner:SUPERNUS PHARM INC
Sirolimos sustained and controlled release preparation and preparation method thereof
InactiveCN101361703AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsPharmaceutical delivery mechanismSolubilityCyclodextrin
The invention provides a Sirolimus slow controlled release preparation and a preparation method thereof. By adopting the solubilizing methods including a solid dispersion technology or cyclodextrin inclusion technology or adding one or a plurality of surface active agents after micronizing drugs and the like, the solubility is dramatically improved and further the bioavailability is improved, therefore, a matrix type slow controlled release preparation is made by adding one or a plurality of framework materials and other accessories, or a diaphragm-controlled or osmotic pump type slow controlled release preparation is made by adopting slow controlled release materials for coating. The Sirolimus slow controlled release preparation has better solubility and dissolution rate, high bioavailability as well as slow controlled release effect, thus being capable of maintaining steady blood concentration, bringing down the concentration of peak, reducing the occurrence of adverse reaction and improving the safety of clinical medicines, in addition, the raw materials can be acquired easily, the preparation process is simple and feasible with high yield rate and low cost, and the large-scale industrial production can be realized and the economic benefit is marked.
Owner:宋洪涛
Extended release dosage form
InactiveUS20110229533A1Enhances fluid fluxImprove permeabilityBiocideSalicyclic acid active ingredientsOsmotic pumpBiomedical engineering
A membrane system comprising an interior wall, a fluid-permeable exterior wall surrounding the interior wall and an internal compartment defined by the membrane system, wherein fluid permeability of the interior wall is responsive to osmolarity of an osmotic core within the internal compartment are disclosed. A controlled release dosage form comprising the membrane system and a process for delivering an osmotically active formulation from an osmotic pump over an extended period of time are also disclosed.
Owner:ALZA CORP
Osmotic drug delivery system
InactiveUS20140303252A1Improve solubilityIncrease surface areaBiocideSenses disorderWater solubleWater soluble drug
Owner:SUPERNUS PHARM INC
Osmotic pump controlled release preparation composition and preparation thereof
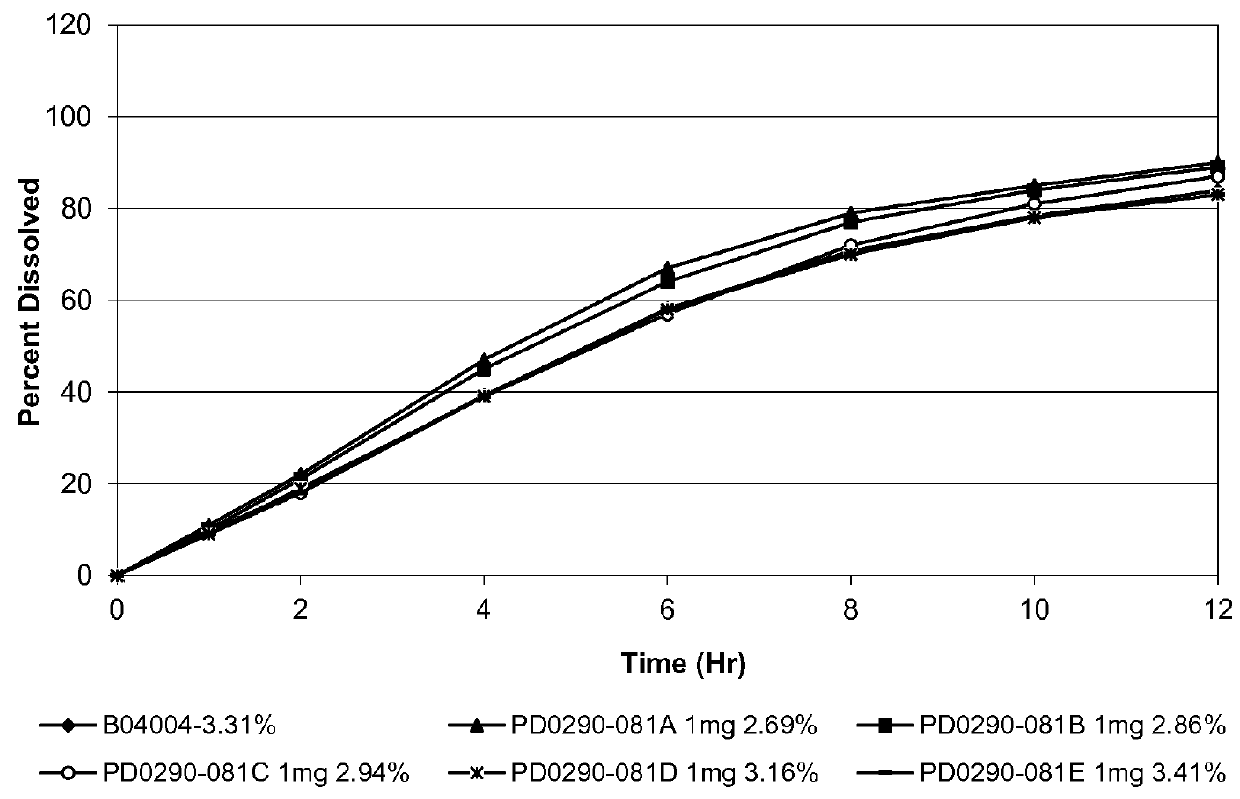
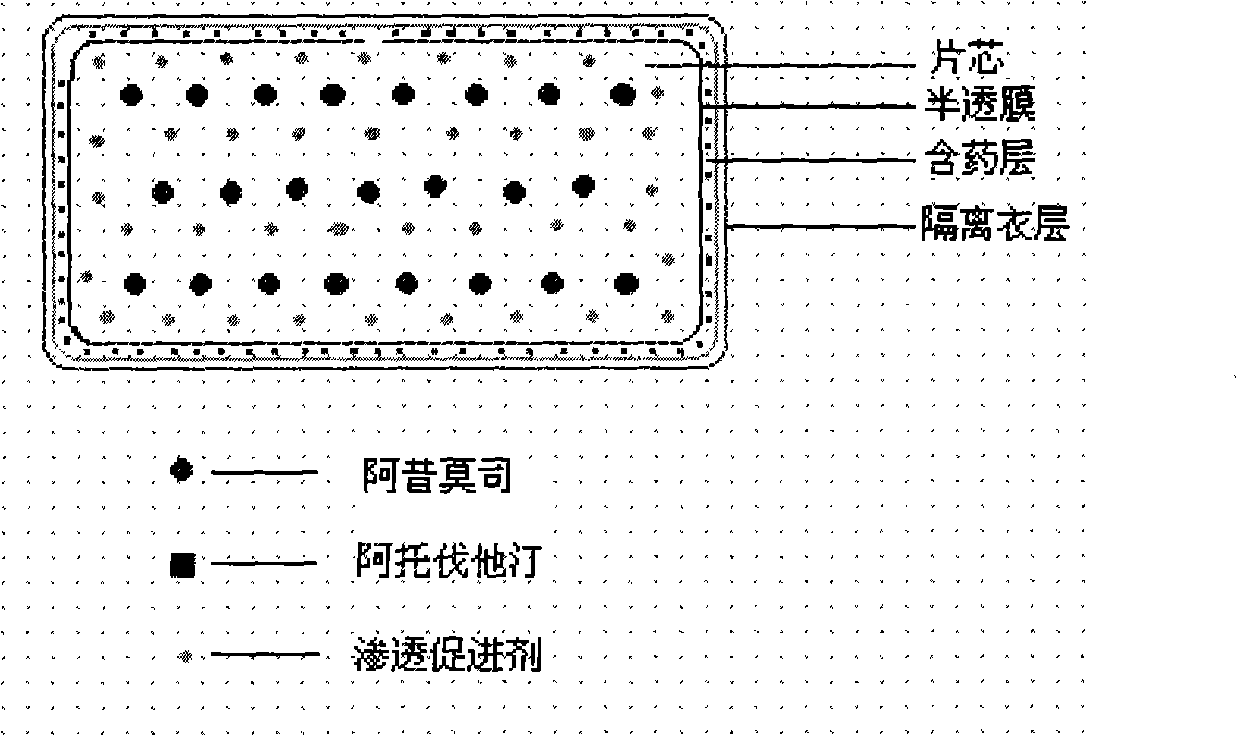
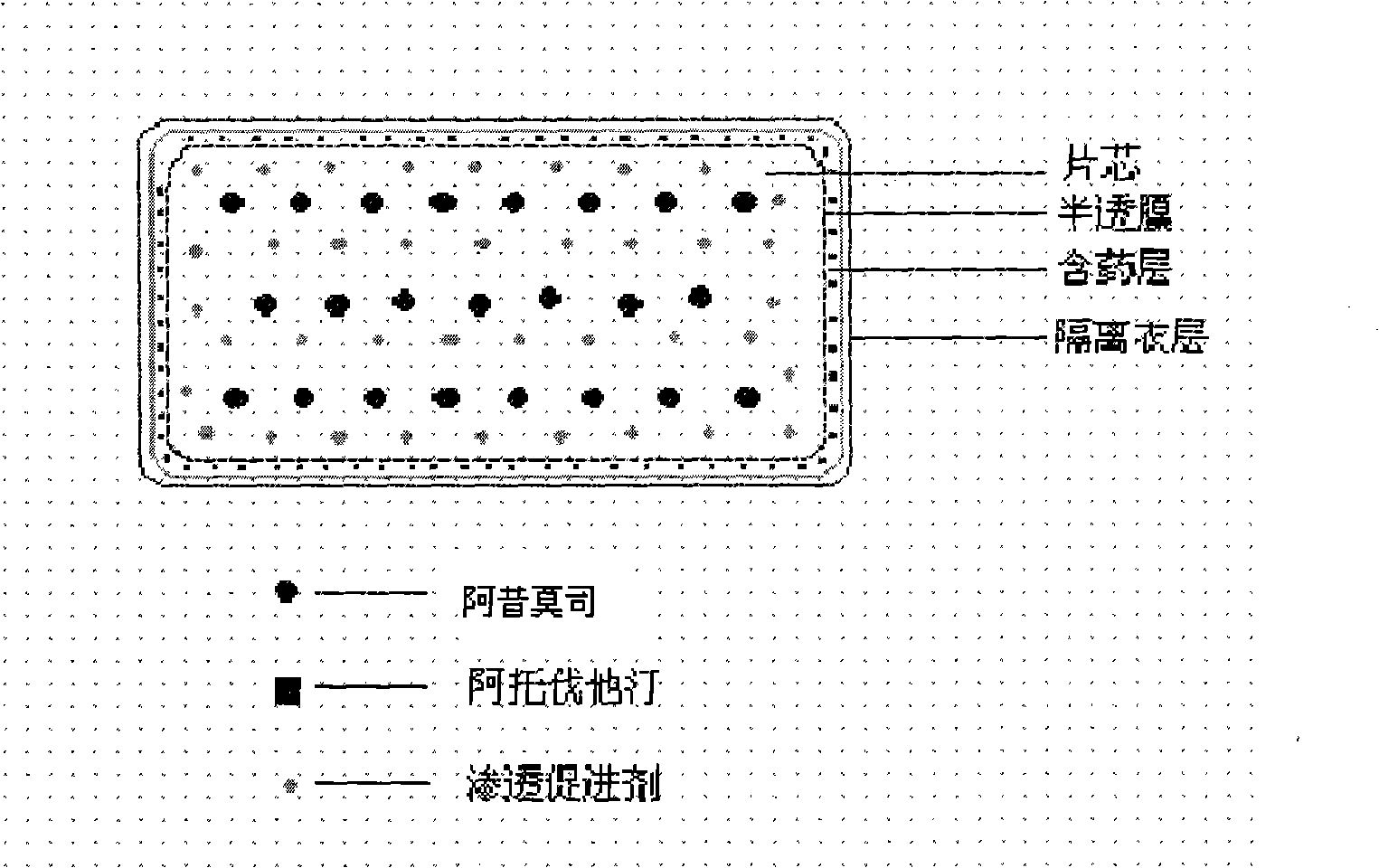
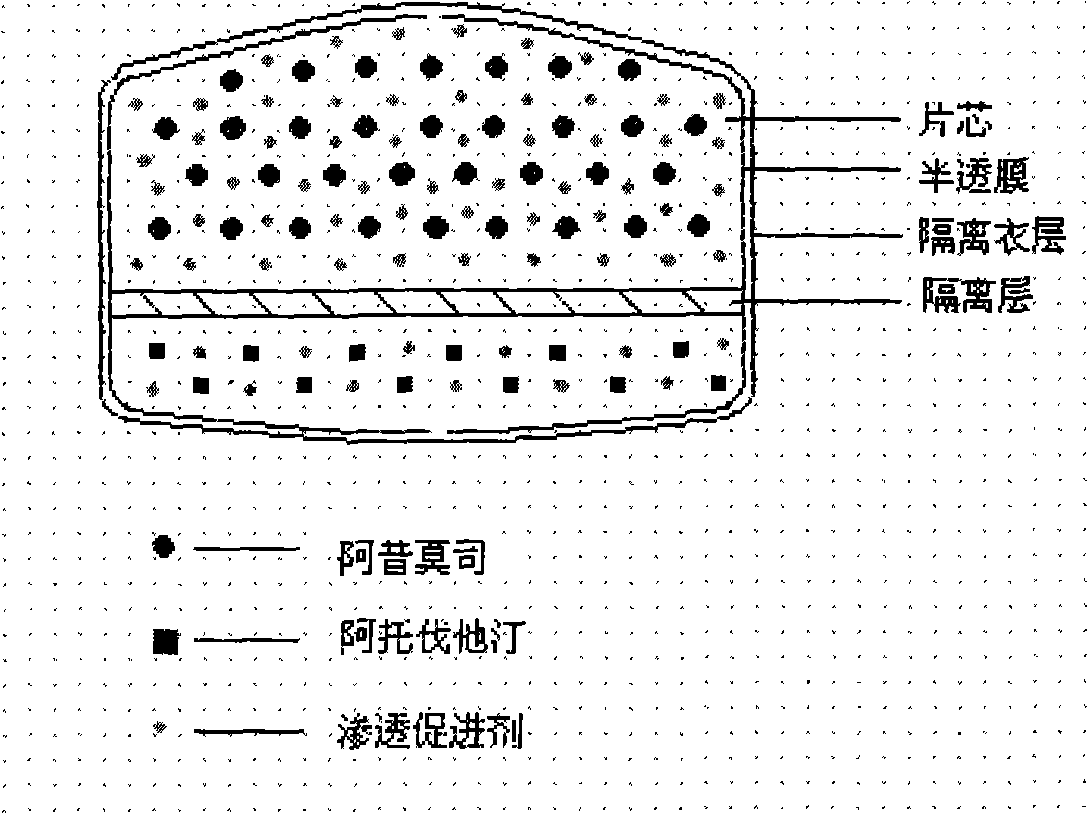
ActiveCN101352426AFully functionalSmall toxicityMetabolism disorderPharmaceutical delivery mechanismSide effectBULK ACTIVE INGREDIENT
The invention relates to an osmotic pump controlled release pharmaceutical composition which contains two medicine active ingredients and is used for treating hyperlipoidemia and a preparation method thereof, the pharmaceutical composition has a nicotinic acids medicine such as acipimox and the other one estatina medicine such as atorvastatin; wherein, the nicotinic acids medicine such as acipimox is a controlled release part, and the estatina medicine such as atorvastatin is a quick release part; or both the acipimox and the atorvastatin are the controlled release part. The compound osmotic pump preparation of the invention has the advantages of comprehensive action, low toxicity and side effects and convenient use.
Owner:LUNAN PHARMA GROUP CORPORATION
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com