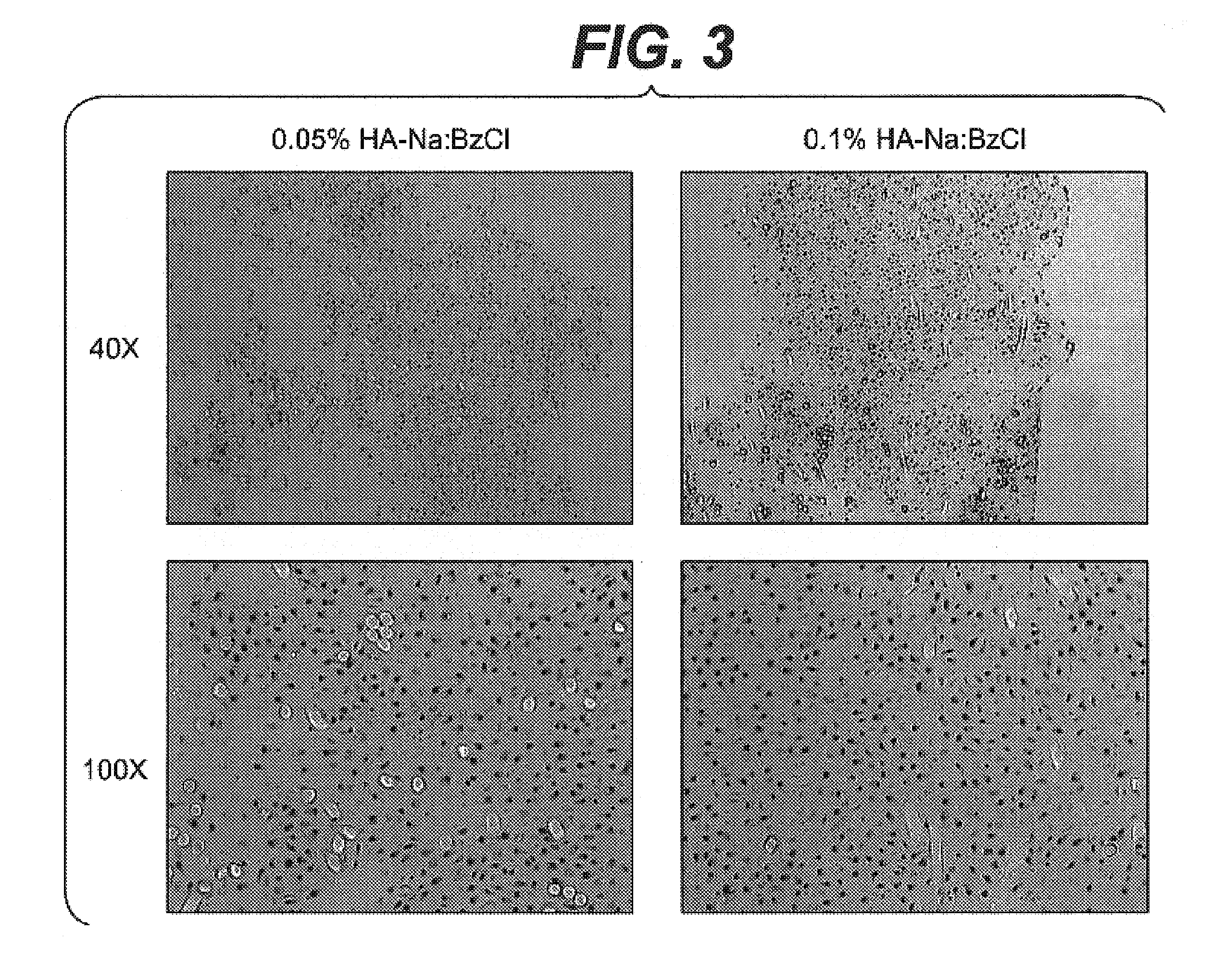
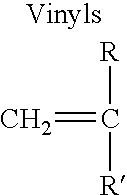Patents
Literature
1428 results about "Hydrophobic polymer" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
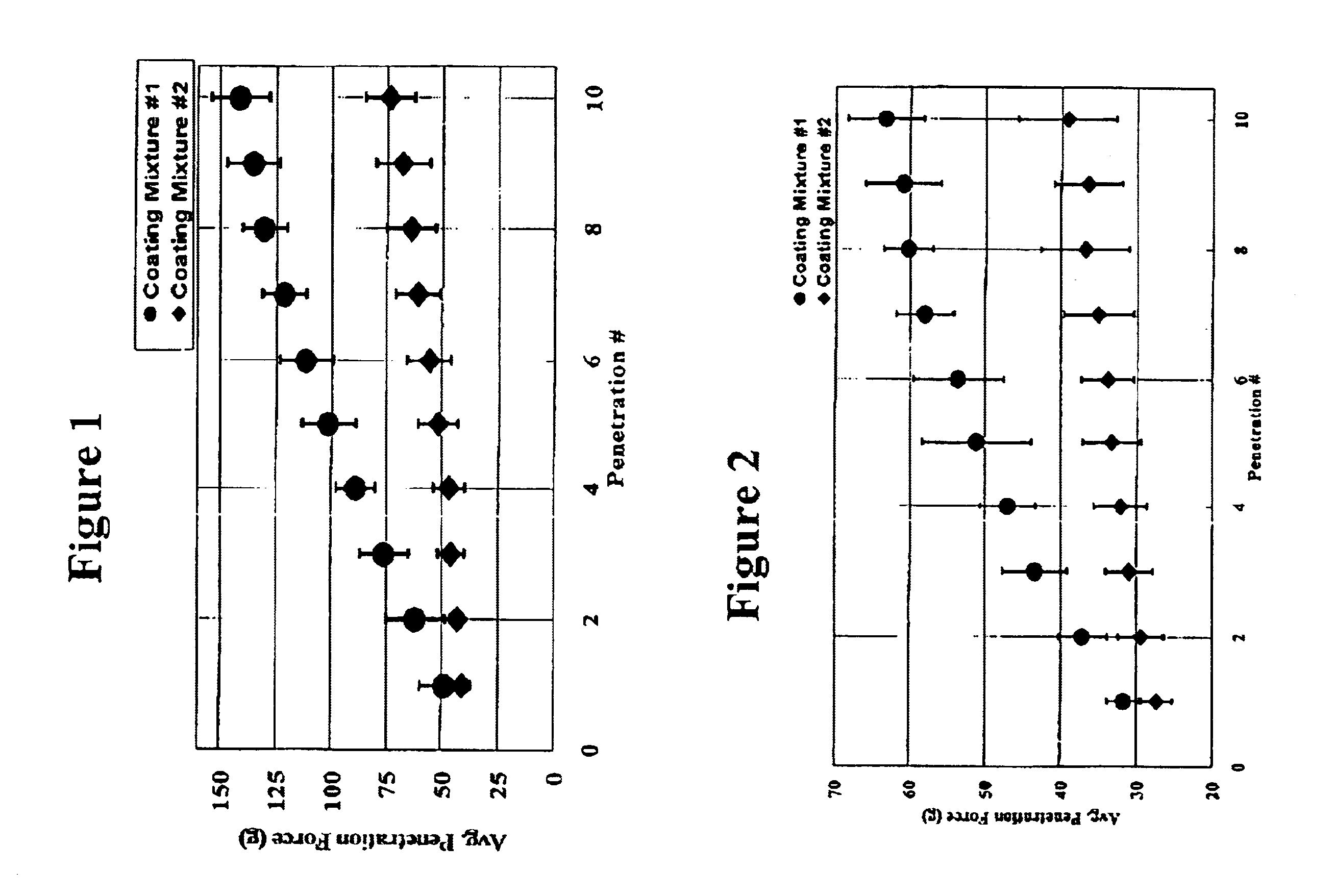
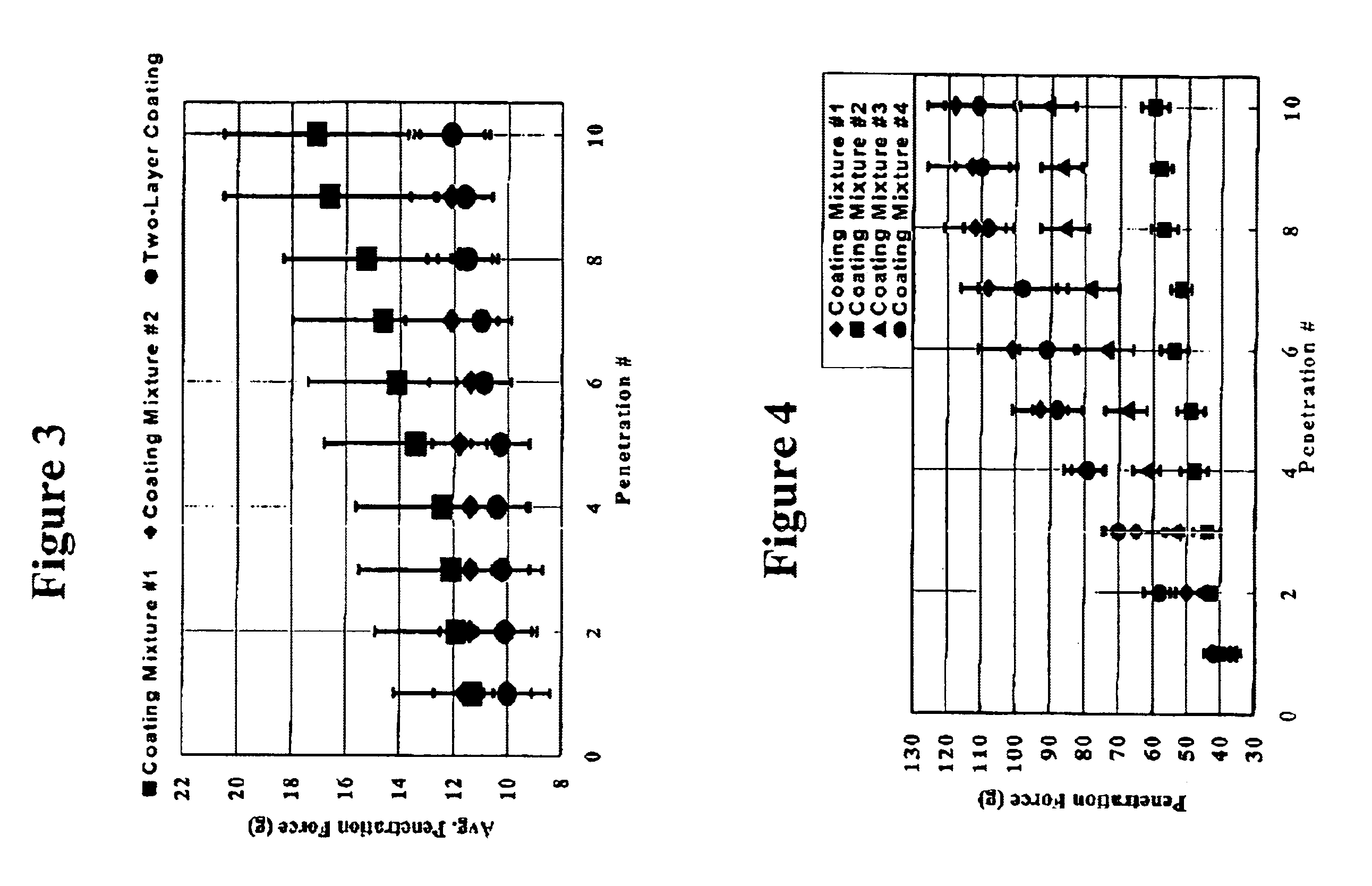
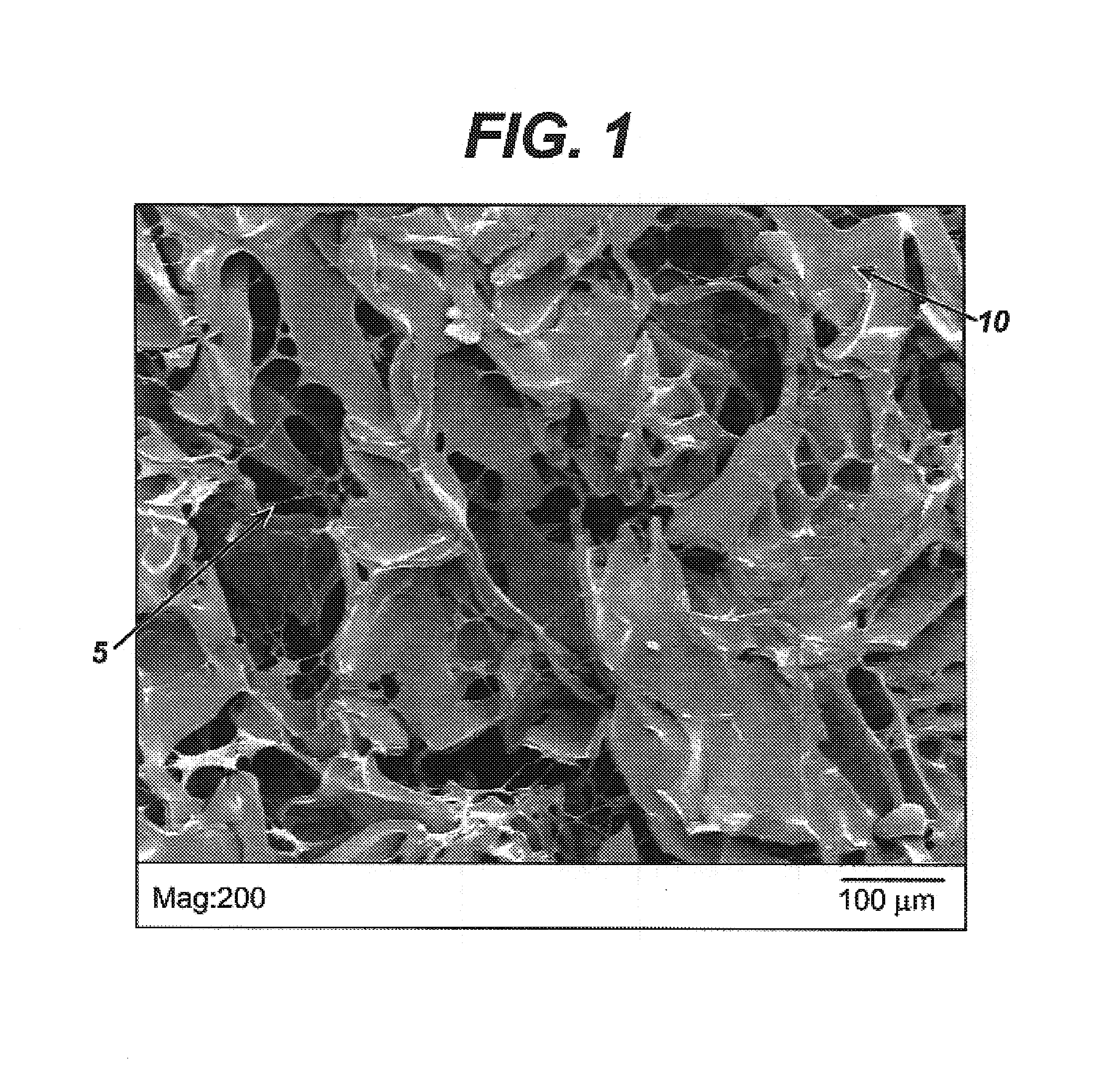
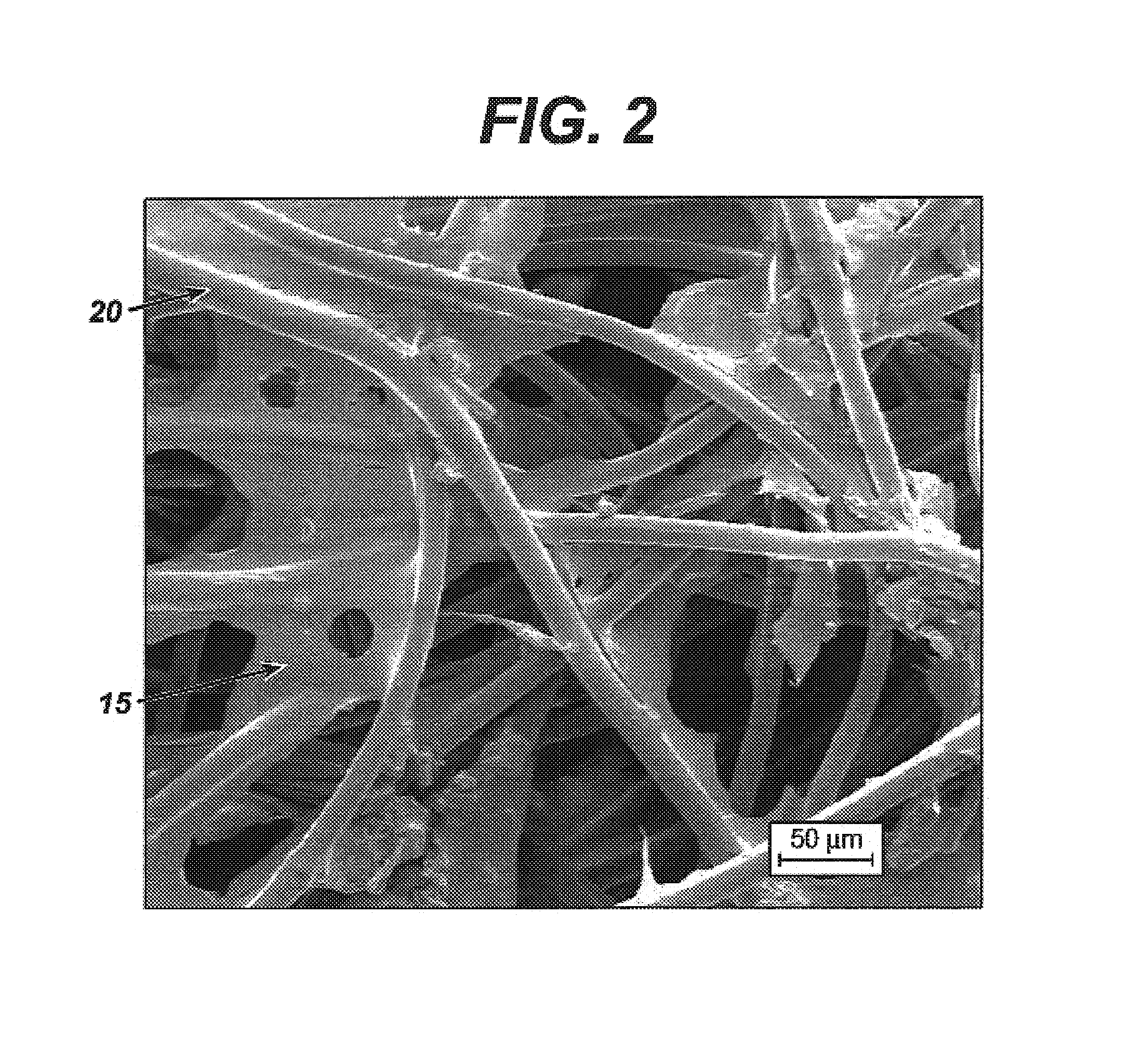
Medical devices having durable and lubricious polymeric coating
A medical device having a contact surface exposed repeatedly to bodily tissue is disclosed. The contact surface is coated with a silicone polymer and one or more non-silicone hydrophobic polymers. The preferred medical device is a surgical needle, and the preferred coating is a polydimethylsiloxane and polypropylene wax hydrocarbon mixture. The incorporation of the non-silicone hydrophobic polymer increases the durability of the coating on the device without sacrificing lubricity.
Owner:ETHICON INC
Modified hyaluronic acid for use in musculoskeletal tissue repair
The present invention includes hyaluronic acid complexes of a monovalent alkali metal salt of hyaluronic acid and a tetra alkyl ammonium halide that are suitable for incorporation with tissue scaffolds that are suitable for use in repair and / or regeneration of muscoloskeletal tissue and that include a biodegradable, porous substrate made from a biodegradable, hydrophobic polymer, where the hyaluronic acid complex is substantially insoluble in water at room temperature, yet soluble in mixtures of organic and aqueous solvents in which the selected hydrophobic polymer is soluble.
Owner:ADVANCED TECH & REGENERATIVE MEDICINE
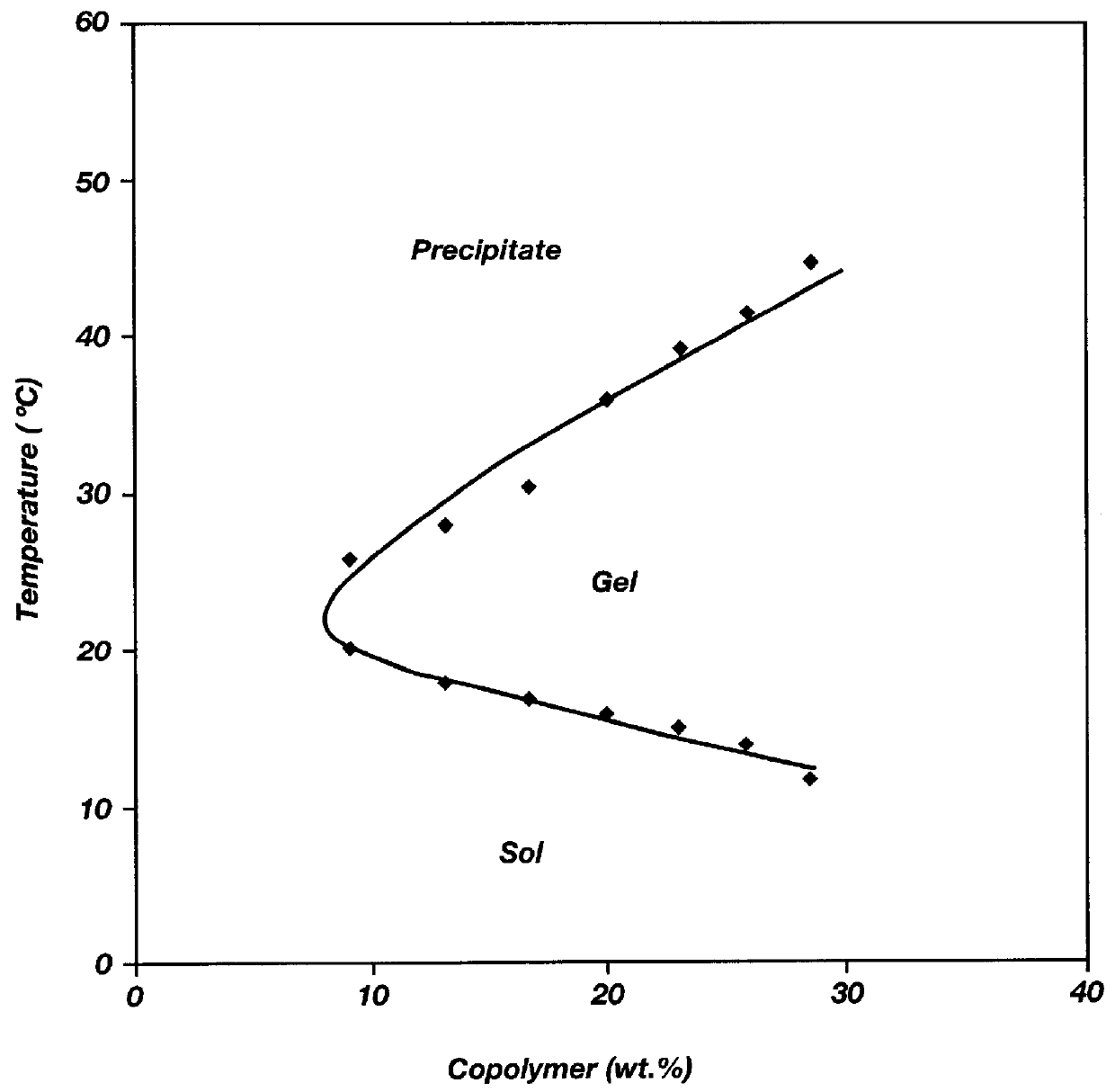
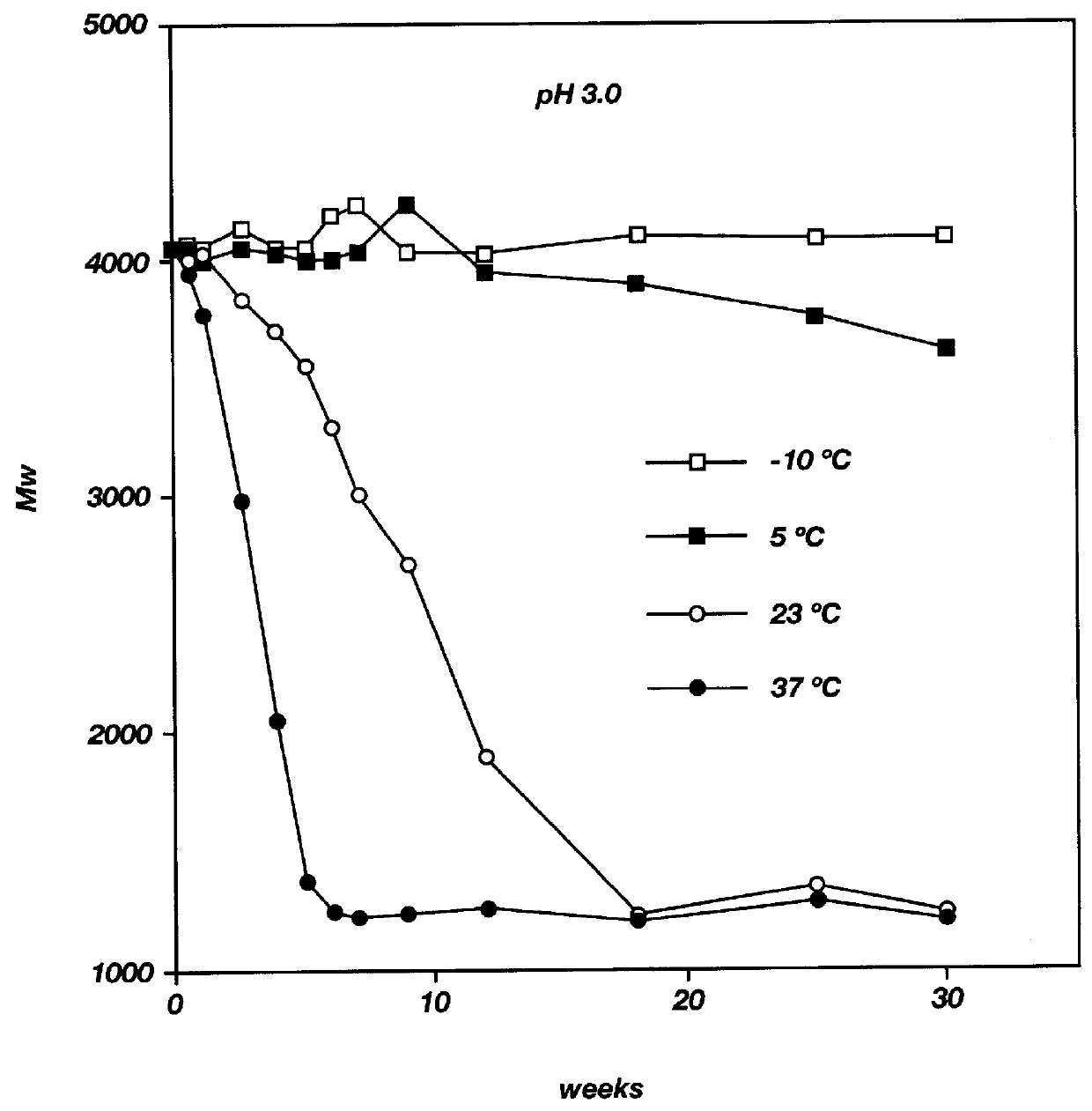
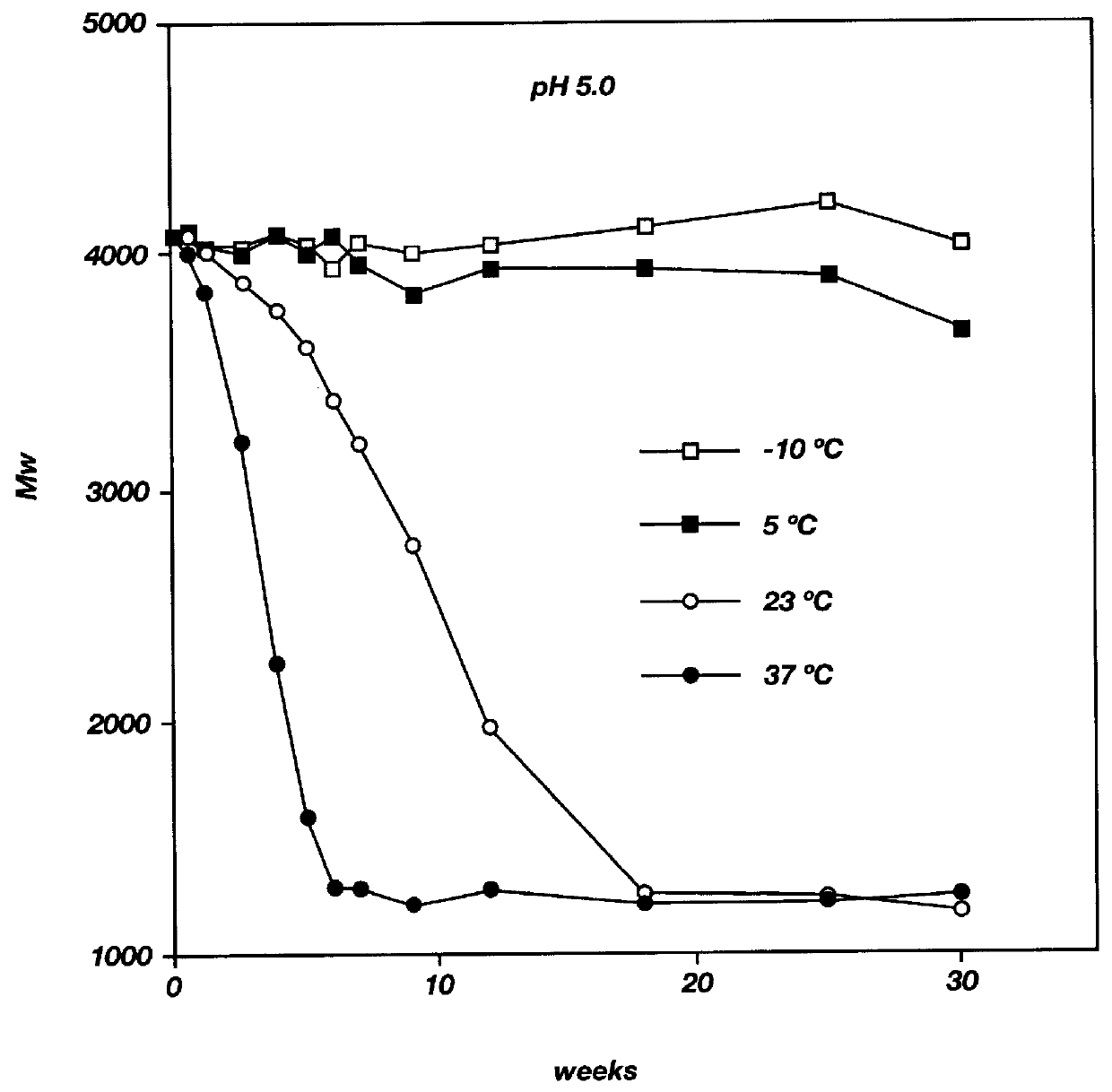
Biodegradable low molecular weight triblock poly (lactide-co-glycolide) polyethylene glycol copolymers having reverse thermal gelation properties
InactiveUS6117949AReduce solubilityReduced stabilityPowder deliveryPeptide/protein ingredientsSolubilityPolymer science
A water soluble biodegradable ABA- or BAB-type triblock polymer is disclosed that is made up of a major amount of a hydrophobic polymer made of a poly(lactide-co-glycolide) copolymer or poly(lactide) polymer as the A-blocks and a minor amount of a hydrophilic polyethylene glycol polymer B-block, having an overall weight average molecular weight of between about 2000 and 4990, and that possesses reverse thermal gelation properties. Effective concentrations of the triblock polymer and a drug may be uniformly contained in an aqueous phase to form a drug delivery composition. At temperatures below the gelation temperature of the triblock polymer the composition is a liquid and at temperatures at or above the gelation temperature the composition is a gel or semi-solid. The composition may be administered to a warm-blooded animal as a liquid by parenteral, ocular, topical, inhalation, transdermal, vaginal, transurethral, rectal, nasal, oral, pulmonary or aural delivery means and is a gel at body temperature. The composition may also be administered as a gel. The drug is released at a controlled rate from the gel which biodegrades into non-toxic products. The release rate of the drug may be adjusted by changing various parameters such as hydrophobic / hydrophilic componenet content, polymer concentration, molecular weight and polydispersity of the triblock polymer. Because the triblock polymer is amphiphilic, it functions to increase the solubility and / or stability of drugs in the composition.
Owner:BTG INT LTD +2
Stent coatings comprising hydrophilic additives
A coating for implantable medical devices and a method for fabricating thereof are disclosed. The coating includes a mixture of a hydrophobic polymer and a polymeric hydrophilic additive, wherein the hydrophobic polymer and the hydrophilic additive form a physically entangled or interpenetrating system.
Owner:ABBOTT CARDIOVASCULAR
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6129933AReduce reunionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
Membranes with controlled permeability to polar and apolar molecules in solution and methods of making same
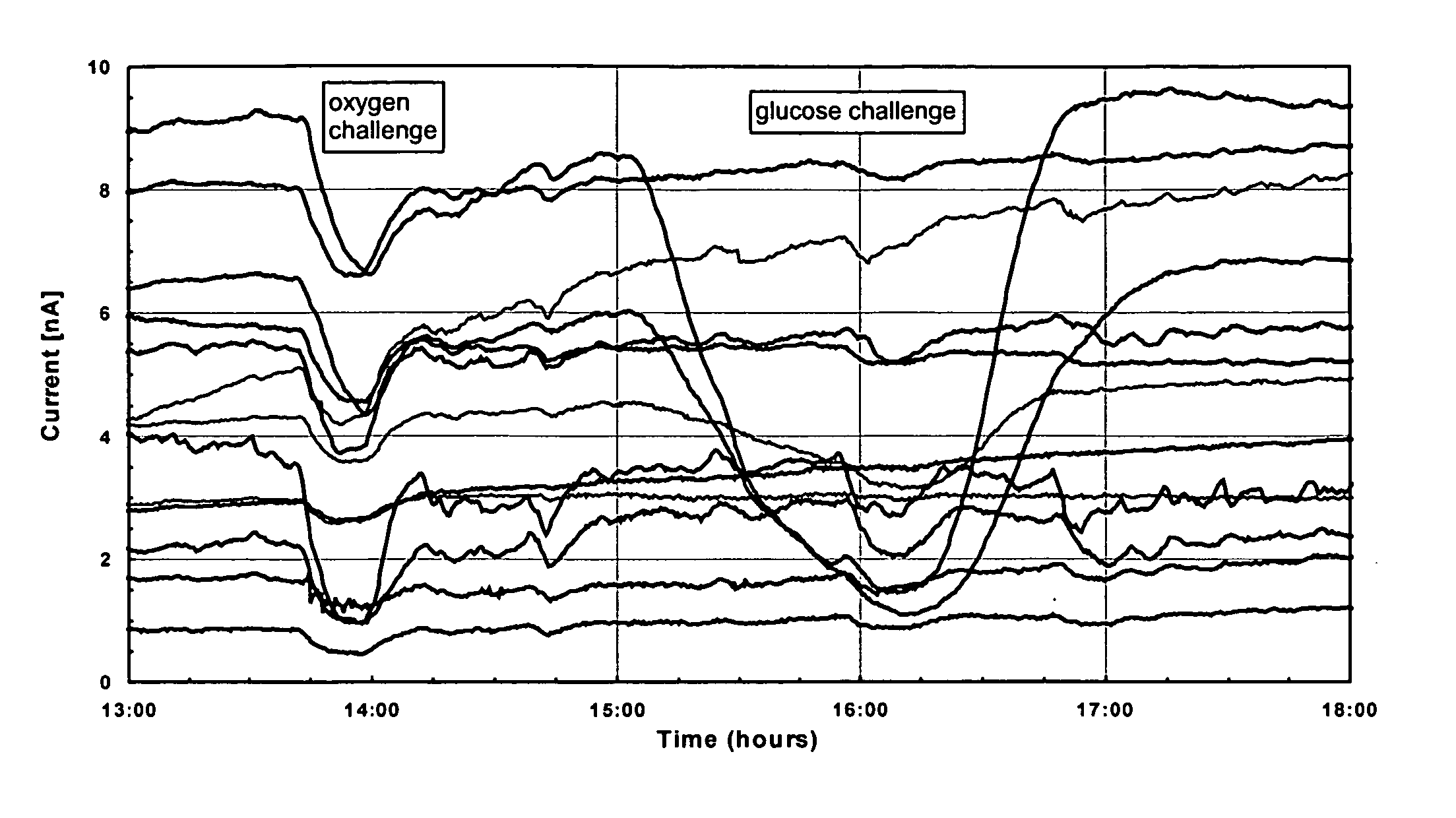
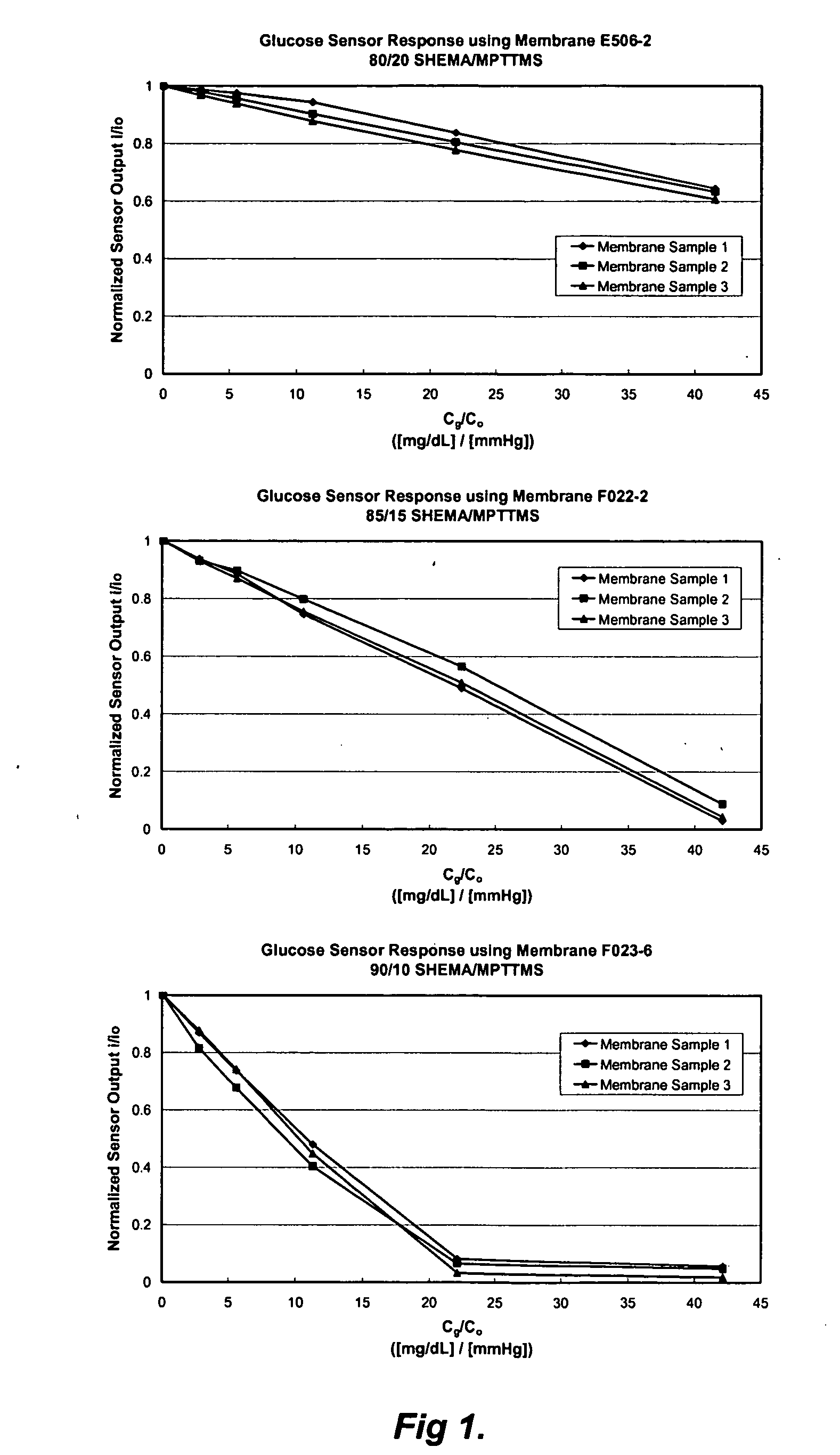
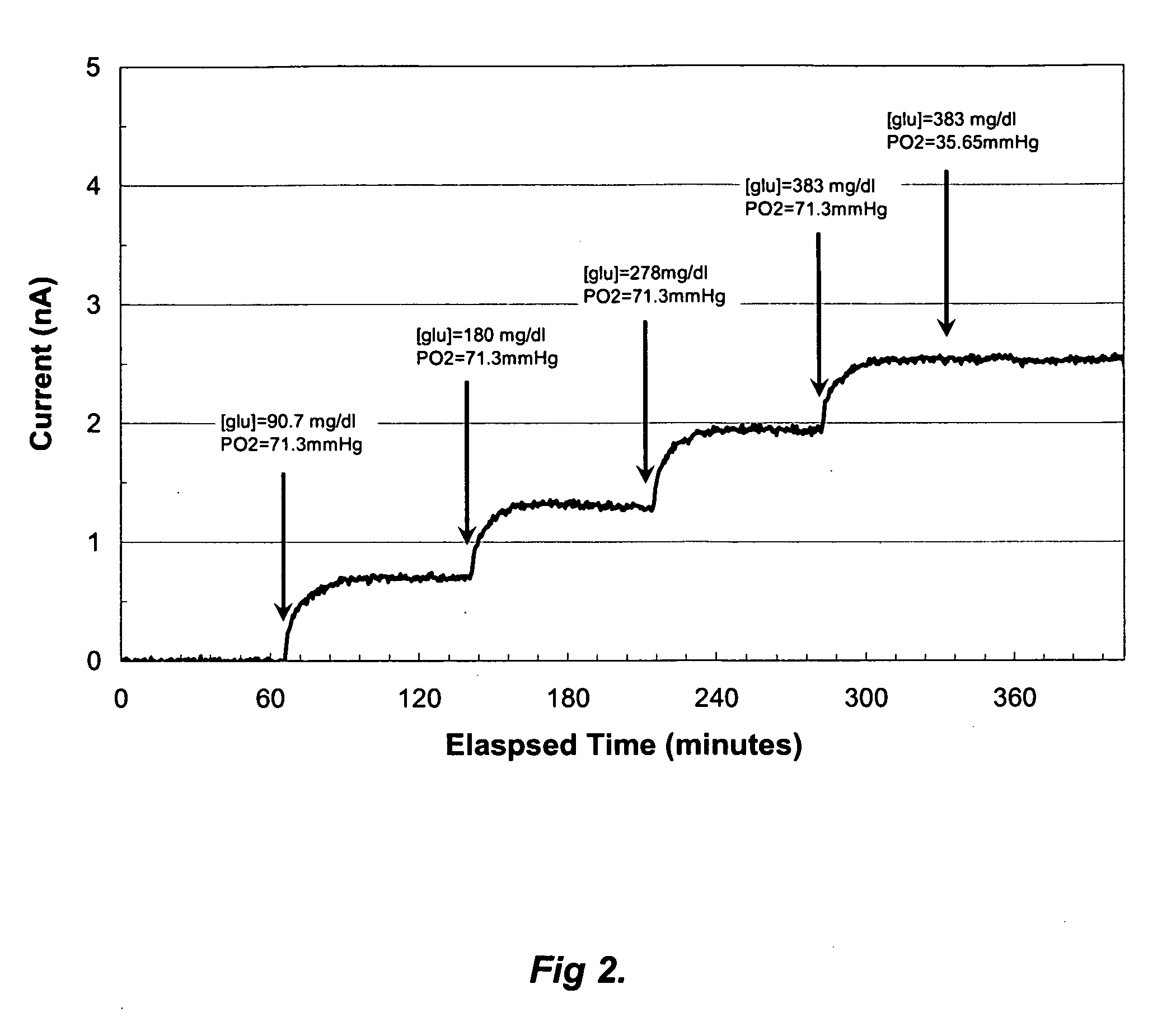
A membrane for use in an implantable glucose sensor including at least one crosslinked substantially hydrophobic polymer and at least one crosslinked substantially hydrophilic polymer; wherein the first and second polymers are different polymers and substantially form an interpenetrating polymer network, semi- interpenetrating polymer network, polymer blend, or copolymer. The membranes are generally characterized by providing a permeability ratio of oxygen to glucose of about 1 to about 1000 in units of (mg / dl glucose) per (mmHg oxygen). Three methods of making membranes from hydrophobic and hydrophilic monomers formed into polymer networks are provided, wherein according to at least two of the methods, the monomers may be substantially immiscible with one another.
Owner:RGT UNIV OF CALIFORNIA
Triclosan-containing medical devices
InactiveUS6106505ALower levelAvoiding undesirably high releaseWrappers shrinkageShrinkage connectionsTriclosanAntiinfective agent
PCT No. PCT / US96 / 20932 Sec. 371 Date Jun. 30, 1998 Sec. 102(e) Date Jun. 30, 1998 PCT Filed Dec. 23, 1996 PCT Pub. No. WO97 / 25085 PCT Pub. Date Jul. 17, 1997The present invention relates to polymeric medical articles comprising the antiinfective agents chlorhexidine and triclosan. It is based, at least in part, on the discovery that the synergistic relationship between these compounds permits the use of relatively low levels of both agents, and on the discovery that effective antimicrobial activity may be achieved when these compounds are comprised in either hydrophilic or hydrophobic polymers. It is also based on the discovery that chlorhexidine free base and triclosan, used together, are incorporated into polymeric medical articles more efficiently. Medical articles prepared according to the invention offer the advantage of preventing or inhibiting infection while avoiding undesirably high release of antiinfective agent, for example into the bloodstream of a subject.
Owner:COLUMBIA UNIV OF THE CITY OF NEW YORK TRUSTEES OF THE
Multi-arm block copolymers as drug delivery vehicles
InactiveUS6838528B2Improve solubilityReduce deliveryPeptide/protein ingredientsNanomedicineSolubilityHydrophilic polymers
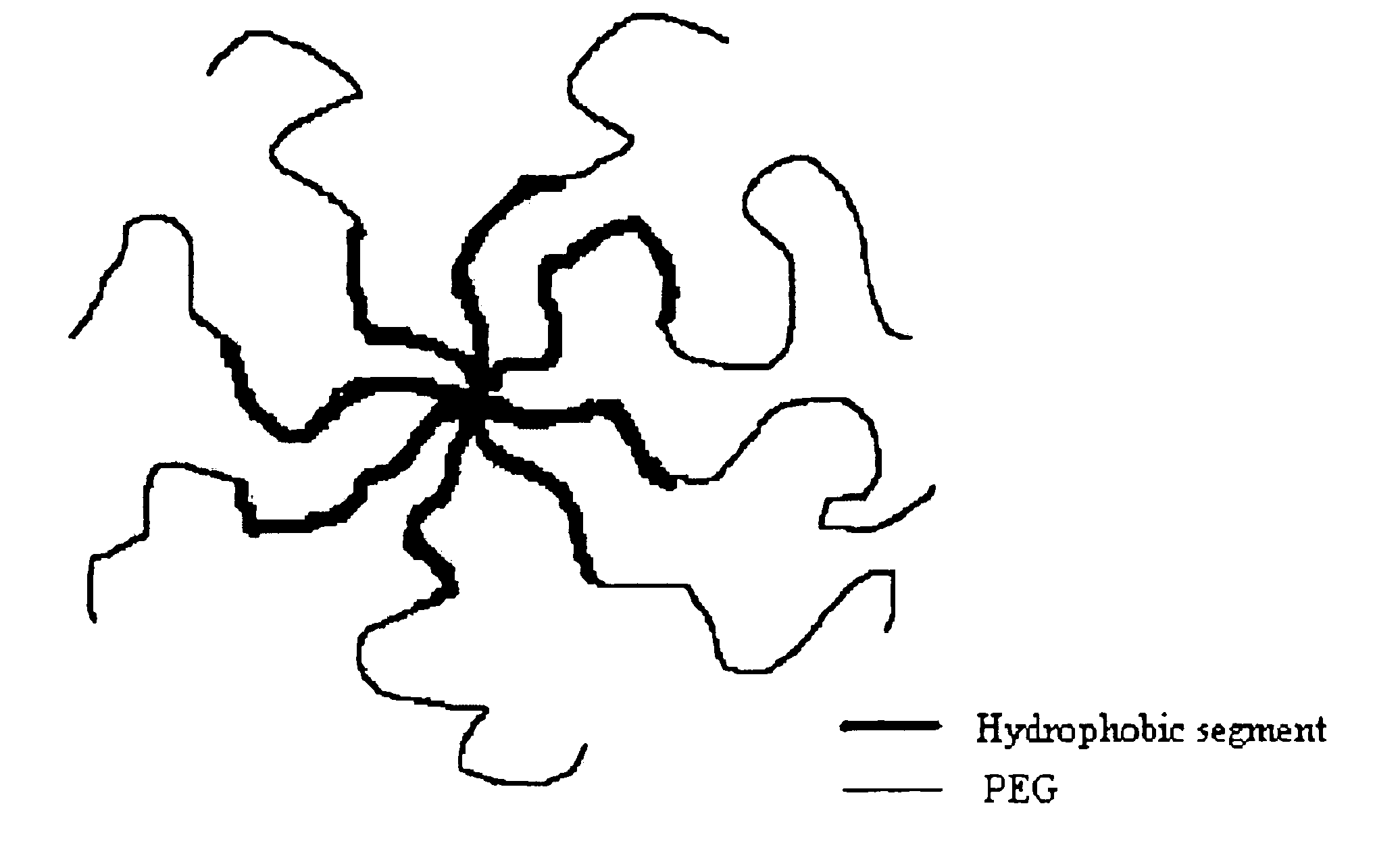
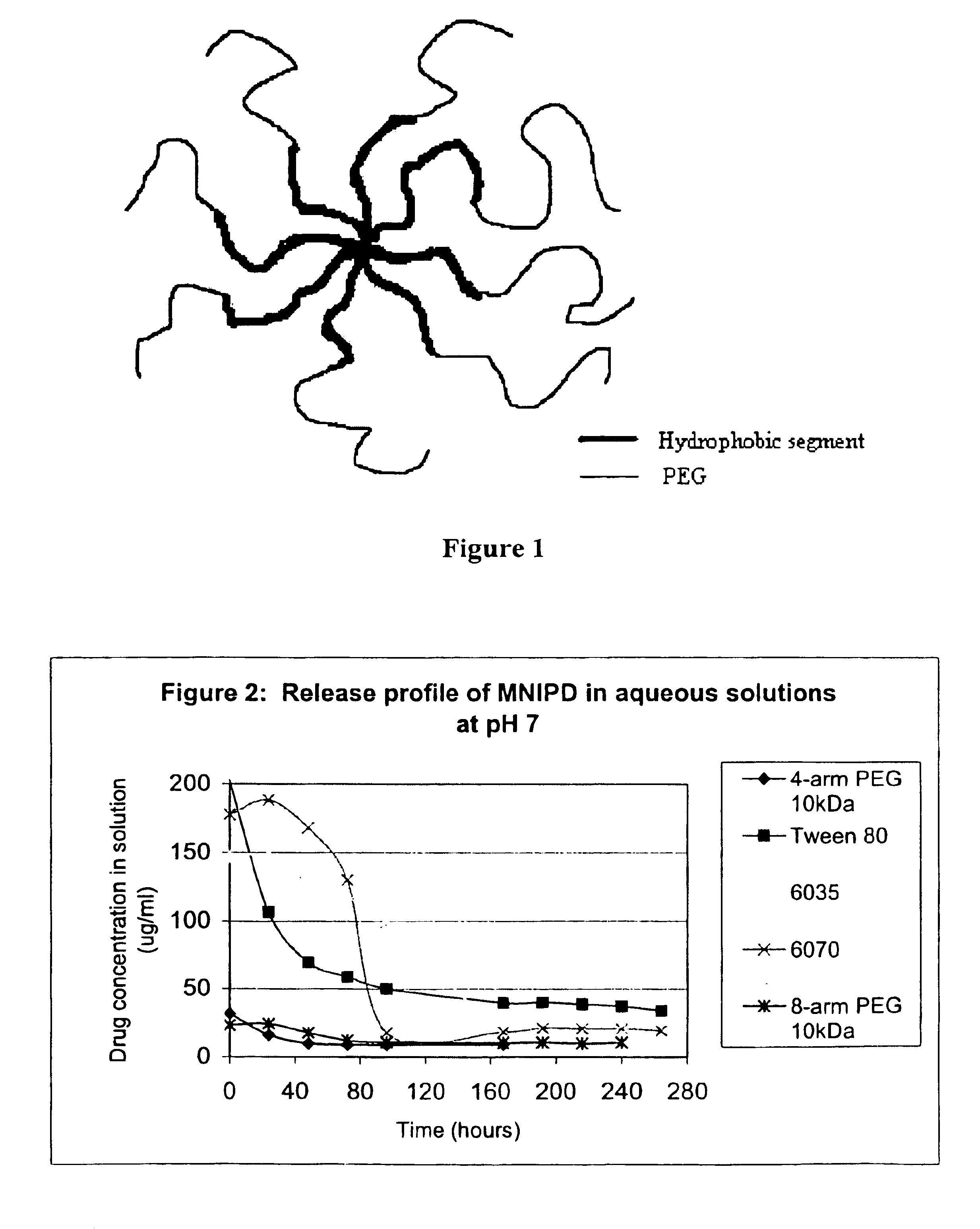
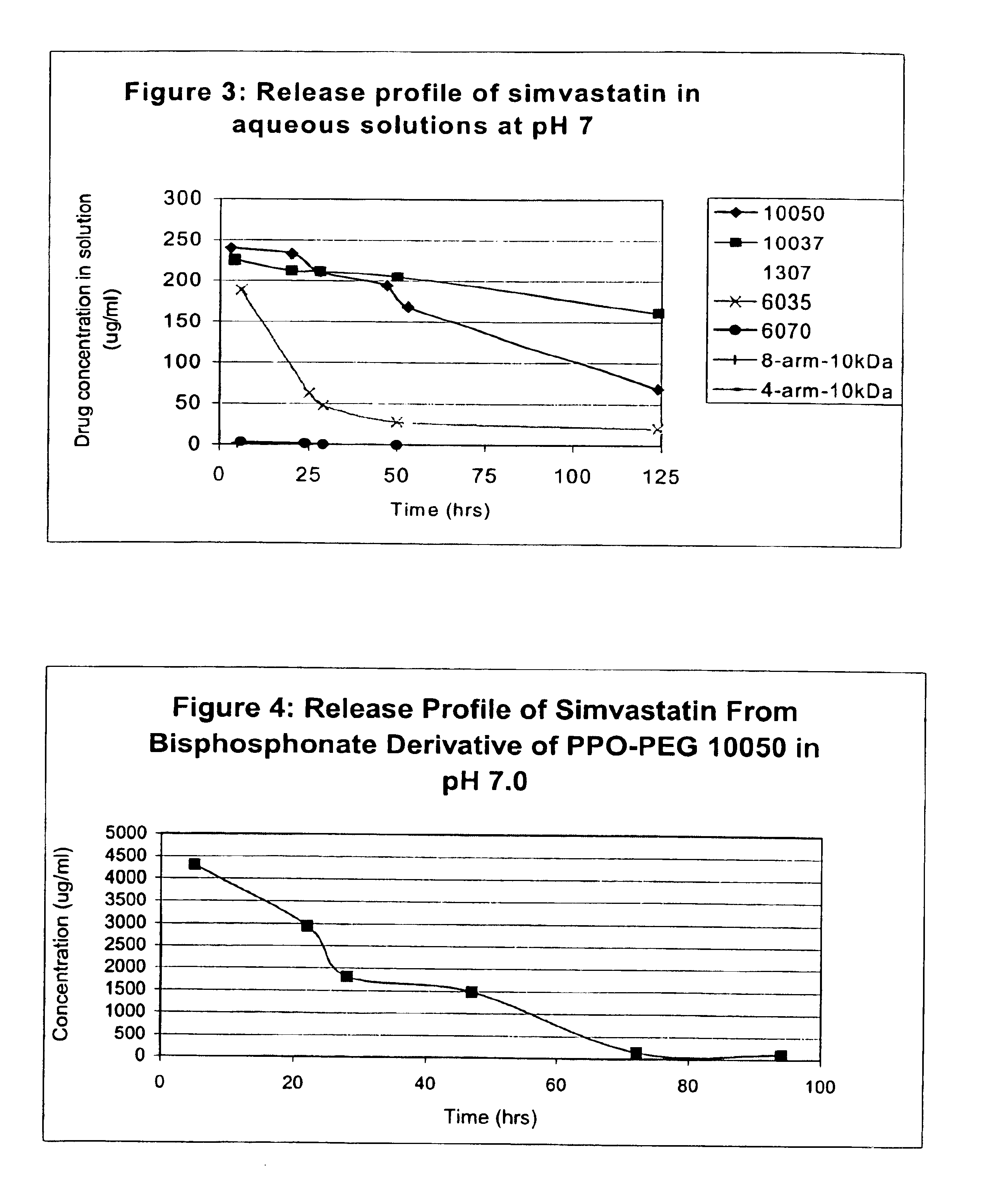
The invention provides multi-arm block copolymers useful as drug delivery vehicles comprising a central core molecule, such as a residue of a polyol, and at least three copolymer arms covalently attached to the central core molecule, each copolymer arm comprising an inner hydrophobic polymer segment covalently attached to the central core molecule and an outer hydrophilic polymer segment covalently attached to the hydrophobic polymer segment, wherein the central core molecule and the hydrophobic polymer segment define a hydrophobic core region. The solubility of hydrophobic biologically active agents can be improved by entrapment within the hydrophobic core region of the block copolymer. The invention further includes pharmaceutical compositions including such block copolymers, methods of making such copolymers and pharmaceutical compositions, and methods of using the block copolymers as drug delivery vehicles.
Owner:NEKTAR THERAPEUTICS INC
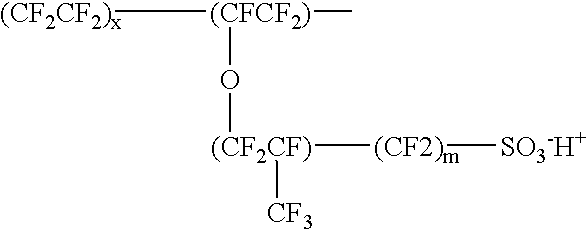
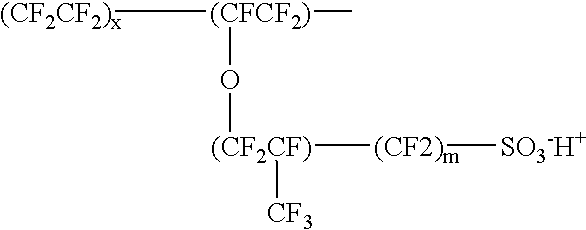
Proton-selective conducting membranes
InactiveUS20020127474A1Easy to operateSelective operationIon-exchanger regenerationSolid electrolyte cellsHydrophobic polymerProton
A membrane comprising: (a) a hydrophobic matrix polymer, and (b) a hydrophilic non-ionic polymer, wherein the hydrophobic polymer and the hydrophilic polymer are disposed so as to form a dense selectively proton-conducting membrane. The microstructure of such a membrane can be tailored to specific functionality requirements, such as proton conductivity vs. proton selectivity, and selectivity to particular species.
Owner:E C R ELECTRO CHEM RES
Biosensor
InactiveUS20050008851A1Baseline at measurement is stabilizedImmobilised enzymesBioreactor/fermenter combinationsHydrophobic polymerBiosensor
It is an object of the present invention to provide a detection surface used for a biosensor wherein nonspecific adsorption is repressed. The present invention provides a biosensor comprising a substrate coated with a hydrophobic polymer.
Owner:FUJIFILM HLDG CORP +1
Layered silicate nanoparticles for controlled delivery of therapeutic agents from medical articles
InactiveUS20050181015A1Improve mechanical propertiesPowder deliverySurgical adhesivesMedicineNanoparticle
Medical articles (for instance, a drug delivery patch or an implantable or insertable medical device) are provided that comprise a release region, which in turn comprises (a) polymeric carrier comprising a polymer (for instance, a hydrophobic polymer) and (b) drug loaded nanoparticles, which are dispersed within the polymeric carrier. The drug loaded nanoparticles comprise a layered silicate material (for instance synthetic or naturally occurring smectite clay nanoparticles) and a therapeutic agent (for instance, a hydrophilic or hydrophobic therapeutic agent). Also described are methods of releasing a therapeutic agent to a patient using such medical articles, and methods of making such medical articles.
Owner:BOSTON SCI SCIMED INC
Bioadhesive compositions and biomedical electrodes containing them
InactiveUS7076282B2Good bioadhesionHigh mechanical strengthElectrocardiographySurgical adhesivesWound dressingHydrophobic polymer
Owner:FIRST WATER
Method for fabricating a coating for a medical device
Methods for fabricating coatings for implantable medical devices are disclosed. The coatings include blends of hydrophilic and hydrophobic polymers. The methods provide for treatment of the coatings to cause enrichment a region close to the outer surface of the coating with the hydrophilic polymers.
Owner:ABBOTT CARDIOVASCULAR
Triclosan-containing medical devices
The present invention relates to polymeric medical articles comprising the antiinfective agents chlorhexidine and triclosan. It is based, at least in part, on the discovery that the synergistic relationship between these compounds permits the use of relatively low levels of both agents, and on the discovery that effective antimicrobial activity may be achieved when these compounds are comprised in either hydrophilic or hydrophobic polymers.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Delayed release pharmaceutical composition containing 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol
InactiveUS20050058706A1Good repeatabilityGood treatment effectOrganic active ingredientsBiocideHydrophobic polymerBULK ACTIVE INGREDIENT
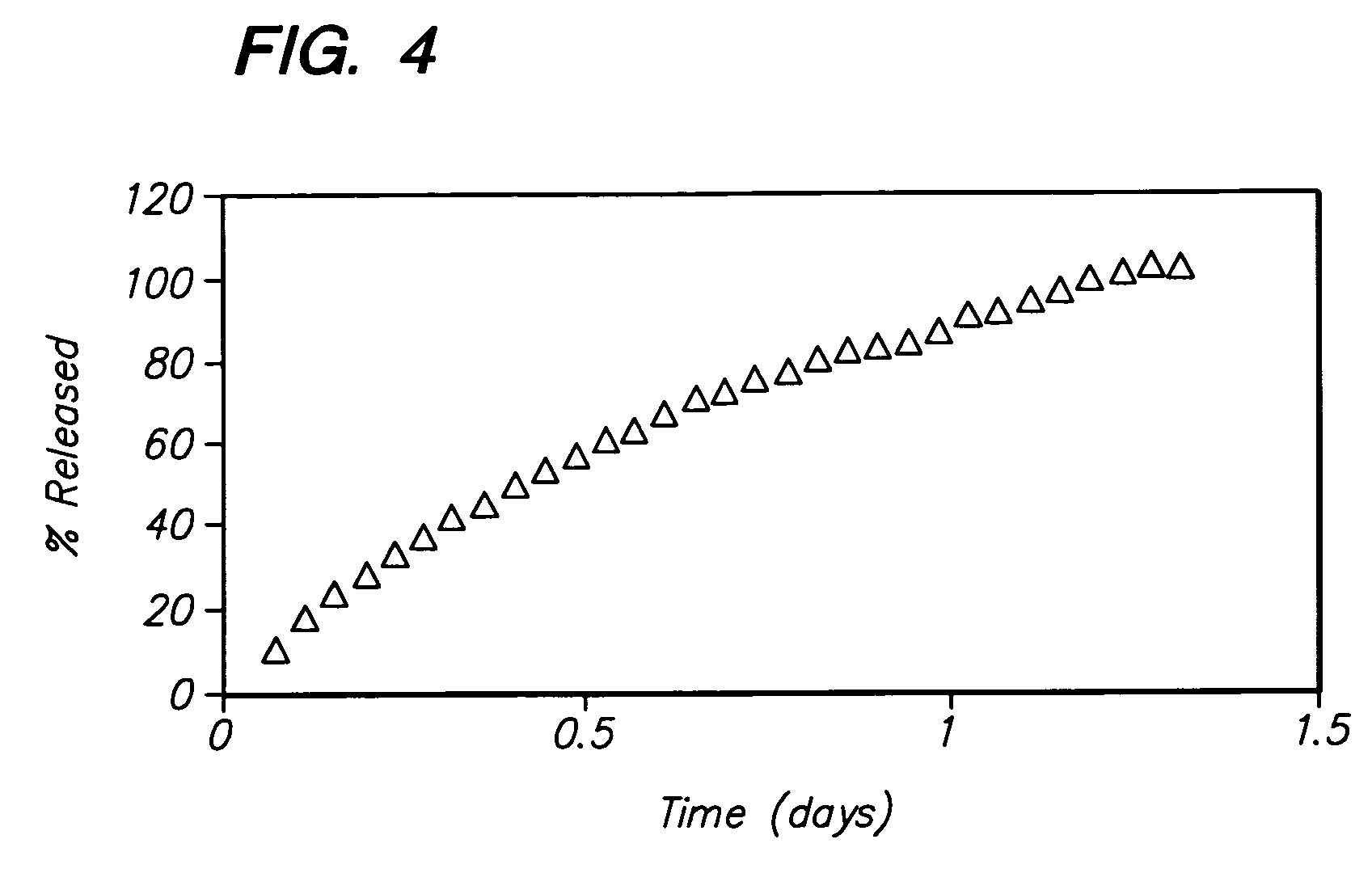
A pharmaceutical formulation for delayed release of the active ingredient 3-(3-dimethylamino-1-ethyl-2-methylpropyl)phenol or a pharmaceutically acceptable salt thereof in a matrix containing between 1 and 80 wt. % of at least one pharmaceutically acceptable hydrophilic or hydrophobic polymer as a matrix forming agent and exhibiting in vivo the following release rate: 3 to 35% by weight (based on 100% by weight active ingredient) 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 0.5 hours; 5 to 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 1 hour; 10 to 75% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 2 hours; 15 to 82% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 3 hours; 30 to 97% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 6 hours; more than 50% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 12 hours; more than 70% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 18 hours, and more than 80% by weight 3-(3-dimethylamino-1-ethyl-2-methyl-propyl)phenol released after 24 hours.
Owner:GRUNENTHAL GMBH
Polymeric drug delivery system for hydrophobic drugs
InactiveUS20050249799A1Low oral bioavailabilityStable against aggregationAntibacterial agentsPowder deliveryHydrophobic polymerImmediate release
An oral delivery system for Class II drugs that have low oral bioavailability due to their insolubility in water and slow dissolution kinetics and method for making such a drug delivery system are disclosed herein. The formulation may be a controlled release or immediate release formulation. The immediate release formulation contains a Class II drug, together with a hydrophobic polymer, preferably a bioadhesive polymer. In one embodiment, the drug and polymer are co-dissolved in a common solvent. The solution is formed into small solid particles by any convenient method, particularly by spray drying. The resulting particles contain drug dispersed as small particles in a polymeric matrix. The particles are stable against aggregation, and can be put into capsules or tableted for administration. The controlled release formulations contain a BCS Class II drug and a bioadhesive polymer. The controlled release formulations may be in the form of a tablet, capsules, mini-tab, microparticulate, or osmotic pump. Enhancement of oral uptake of the drug from use of bioadhesive polymers occurs through (1) increased dissolution kinetics due to stable micronization of the drug, (2) rapid release of the drug from the polymer in the GI tract; and (3) prolonged GI transit due to bioadhesive properties of the polymers. The combination of these effects allows the preparation of a compact, stable dosage form suitable for oral administration of many class II drugs.
Owner:SPHERICS
Bioadhesive polymers with catechol functionality
InactiveUS20050201974A1Good bioadhesionExtended stayNervous disorderPill deliveryArameHydrophobic polymer
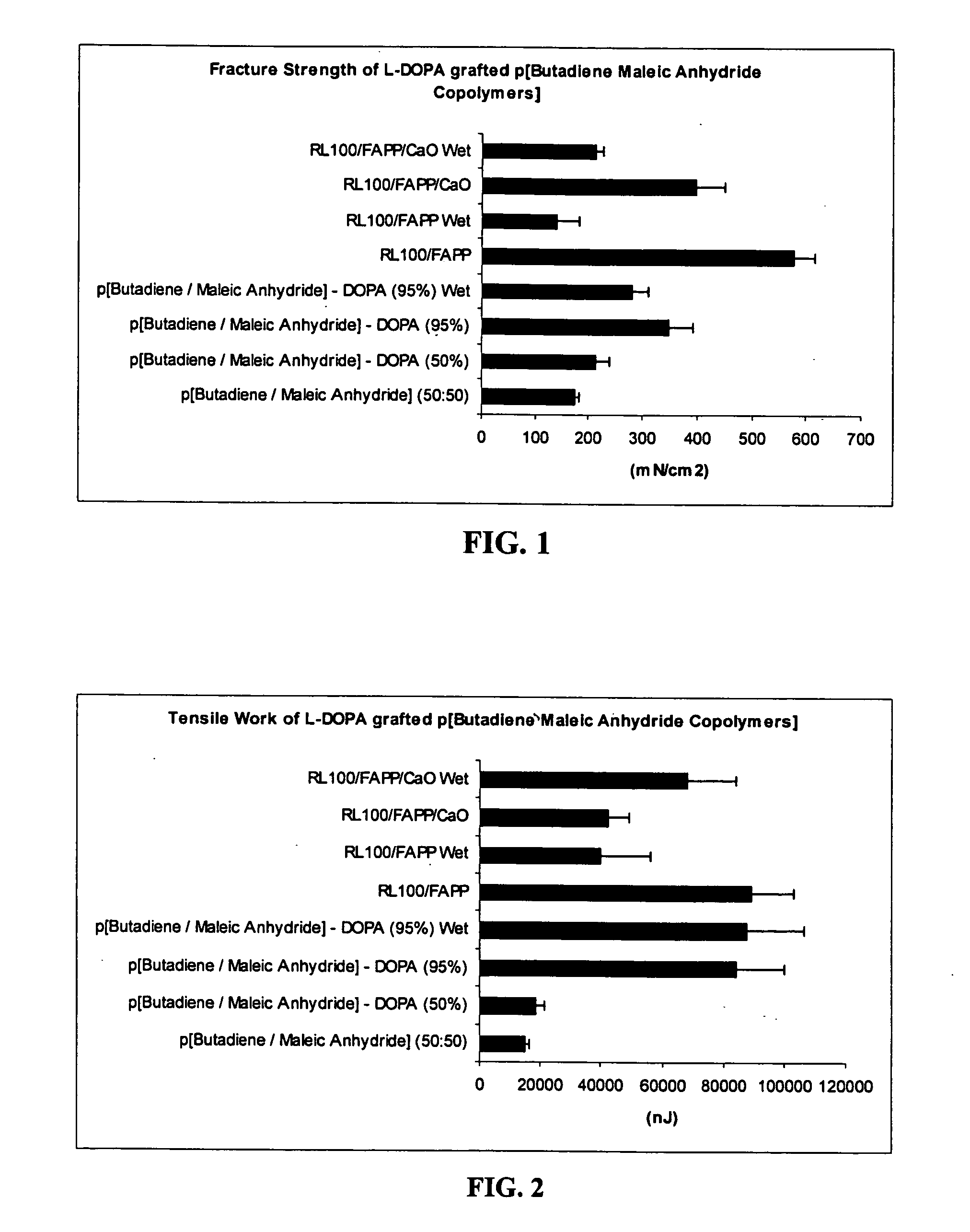
Polymers with improved bioadhesive properties and methods for improving bioadhesion of polymers have been developed. A compound containing an aromatic group which contains one or more hydroxyl groups is grafted onto a polymer or coupled to individual monomers. In one embodiment, the polymer is a biodegradable polymer. In another embodiment, the monomers may be polymerized to form any type of polymer, including biodegradable and non-biodegradable polymers. In some embodiments, the polymer is a hydrophobic polymer. In the preferred embodiment, the aromatic compound is catechol or a derivative thereof and the polymer contains reactive functional groups. In the most preferred embodiment, the polymer is a polyanhydride and the aromatic compound is the catechol derivative, DOPA. These materials display bioadhesive properties superior to conventional bioadhesives used in therapeutic and diagnostic applications. These bioadhesive materials can be used to fabricate new drug delivery or diagnostic systems with increased residence time at tissue surfaces, and consequently increase the bioavailability of a drug or a diagnostic agent. In a preferred embodiment, the bioadhesive material is a coating on a controlled release oral dosage formulation and / or forms a matrix in an oral dosage formulation.
Owner:SPHERICS
Biosensor
ActiveUS20050158850A1Suppresses nonspecific adsorptionSuppressing decreaseBioreactor/fermenter combinationsBiological substance pretreatmentsHydrophobic polymerBiosensor
It is an object of the present invention to provide a biosensor, which is not significantly affected by the baseline fluctuation and suppresses nonspecific adsorption. The present invention provides a biosensor, which comprises a metal surface or metal film coated with a hydrophobic polymer, and has two or more types of different surfaces in a region coated with a hydrophobic polymer.
Owner:FUJIFILM CORP +1
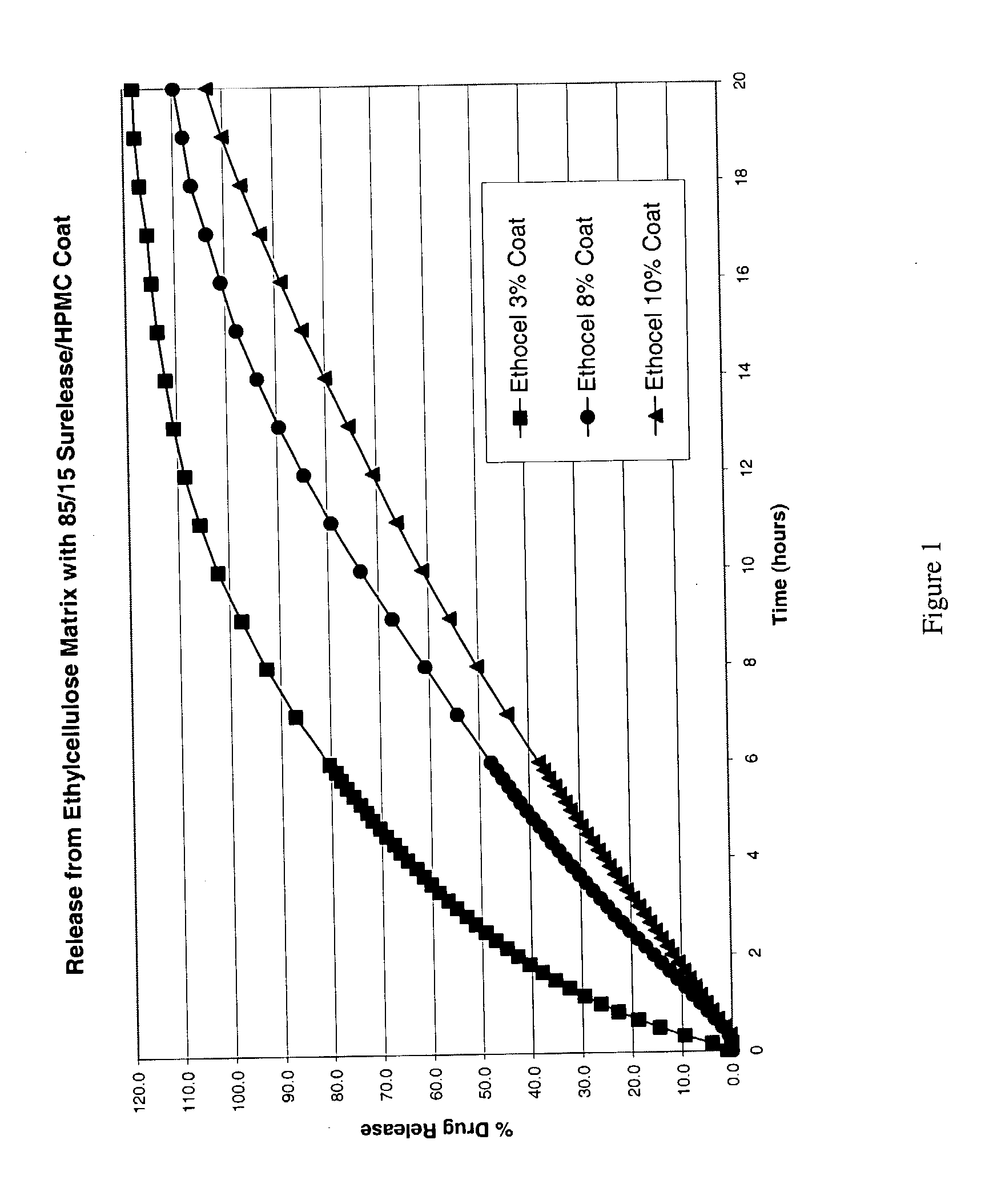
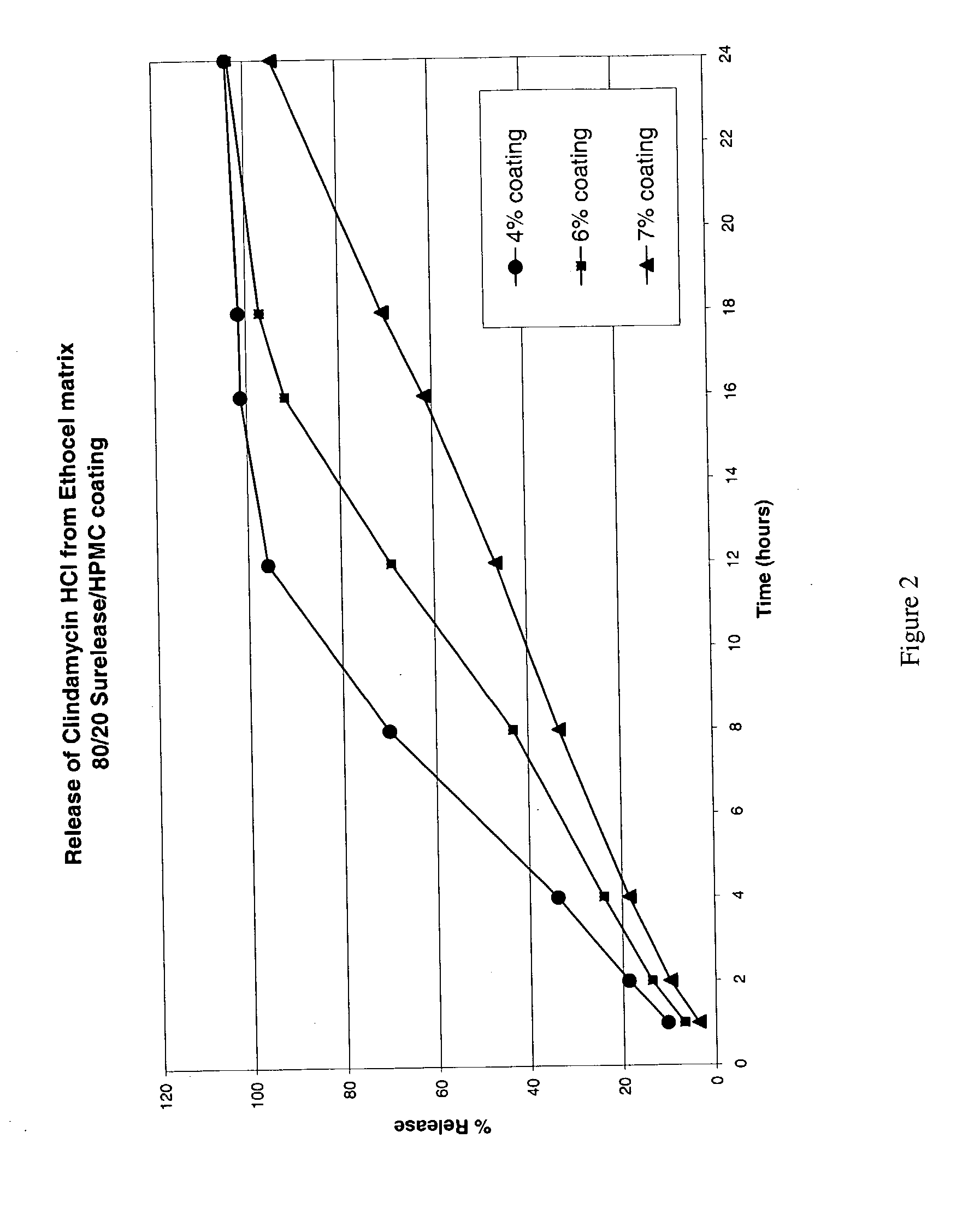
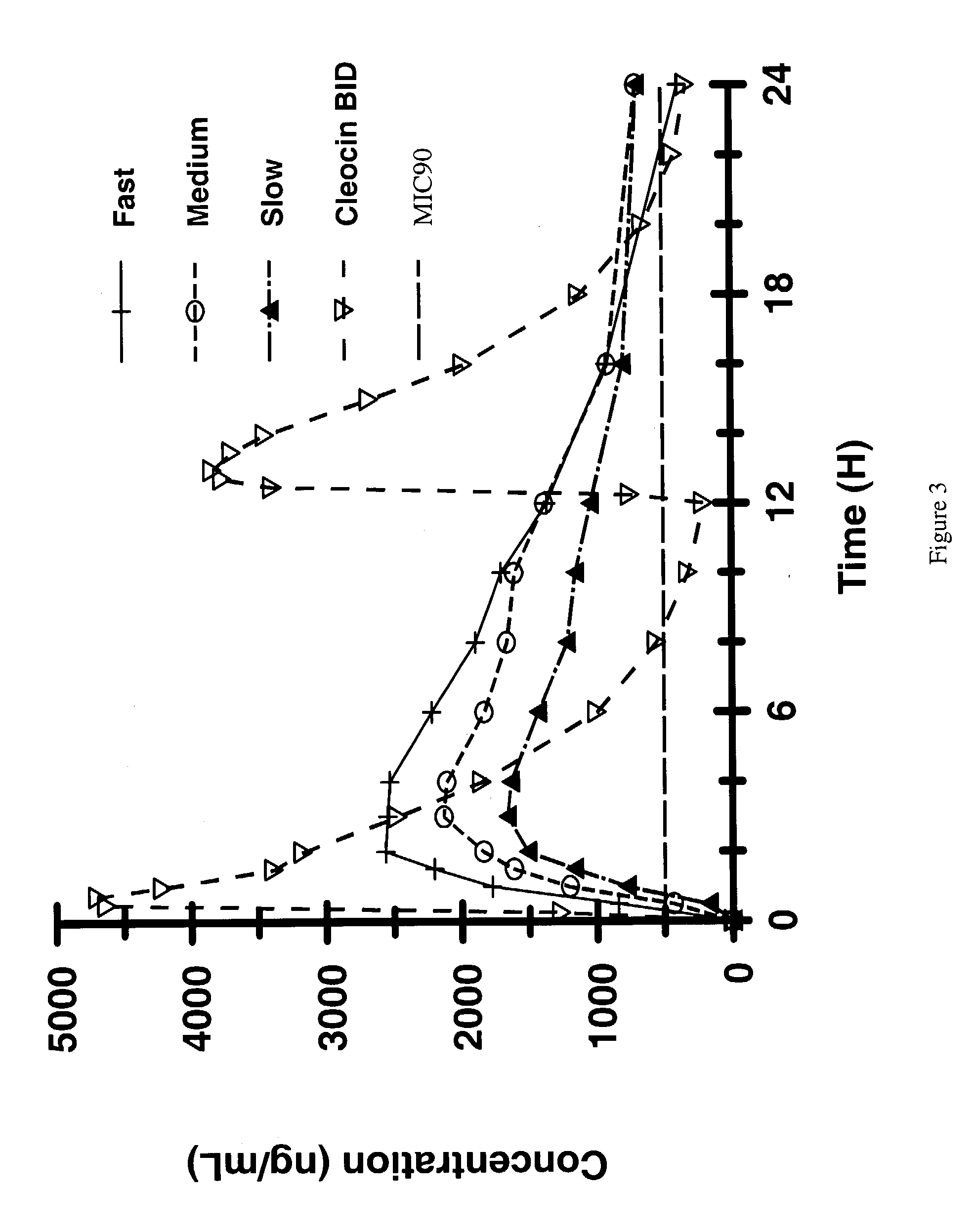
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
Hydrophilic Interpenetrating Polymer Networks Derived From Hydrophobic Polymers
InactiveUS20100010114A1High mechanical strengthReduce coefficient of frictionCosmetic preparationsImpression capsHydrophobic polymerThermoplastic elastomer
A composition of matter comprising a water-swellable IPN or semi-IPN including a hydrophobic thermoset or thermoplastic polymer and an ionic polymer, articles made from such composition and methods of using such articles. The invention also includes a process for producing a water-swellable IPN or semi-IPN from a hydrophobic thermoset or thermoplastic polymer including the steps of placing an ionizable monomer solution in contact with a solid form of the hydrophobic thermoset or thermoplastic polymer; diffusing the ionizable monomer solution into the hydrophobic thermoset or thermoplastic polymer; and polymerizing the ionizable monomers to form a ionic polymer inside the hydrophobic thermoset or thermoplastic polymer, thereby forming the IPN or semi-IPN.
Owner:BIOMIMEDICA
Stabilized controlled release substrate having a coating derived from an aqueous dispersion of hydrophobic polymer
InactiveUS6316031B1Reduce reunionStable productionLiquid surface applicatorsGranular deliveryHydrophobic polymerDissolution
A stabilized solid controlled release dosage form having a coating derived from an aqueous dispersion of ethylcellulose is obtained by overcoating a substrate including a therapeutically active with an aqueous dispersion of ethylcellulose and then curing the coated substrate at a temperature and relative humidity elevated to a suitable level above ambient conditions until the coated dosage form attains a stabilized dissolution profile substantially unaffected by exposure to storage conditions of elevated temperature and / or elevated relative humidity.
Owner:PURDUE PHARMA LP
Silane coating compositions, coating systems, and methods
InactiveUS20070054127A1Synthetic resin layered productsPretreated surfacesHydrophobic polymerSilanes
The invention relates to coating systems having one or more hydrophobic layers bonded to a substrate with a silane composition and methods of making and using the same. In an embodiment, the invention includes an article having a substrate, a base coating layer covalently bonded to the surface of the substrate and a hydrophobic polymer layer disposed on the base coating layer. In an embodiment, the invention includes a method for forming an article including applying a base layer coating solution onto a substrate to form a base layer, applying a hydrophobic polymer layer onto the base layer, and applying actinic energy to the substrate.
Owner:SURMODICS INC
Rate limiting barriers for implantable devices and methods for fabrication thereof
InactiveUS7175873B1Solution value is not highIncrease valueSurgeryPharmaceutical containersRate limitingHydrophobic polymer
A method of coating an implantable medical device, such as a stent, is disclosed. The method includes applying a formulation on a first polymer layer containing a therapeutic substance to form a second layer. The formulation can contain a highly hydrophobic polymer or a solvent which is a poor solvent for the drug or the polymer of the first layer. The formulation can have a low surface tension value or a high Weber number value.
Owner:ABBOTT CARDIOVASCULAR
Disposable urinal system
InactiveUS20020193762A1Easy to storeEasy to useNursing urinalsNon-surgical orthopedic devicesHydrophobic polymerHand held
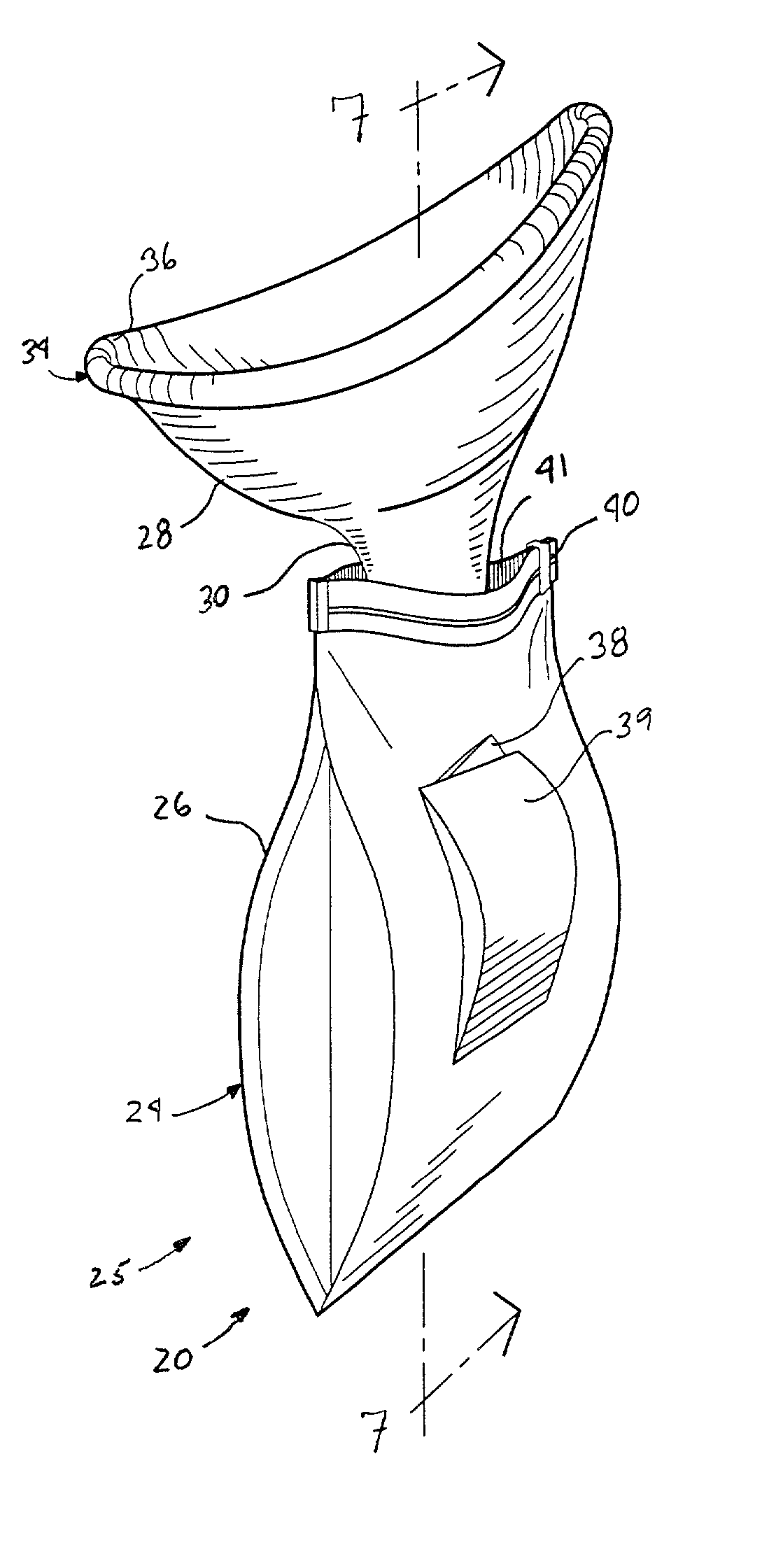
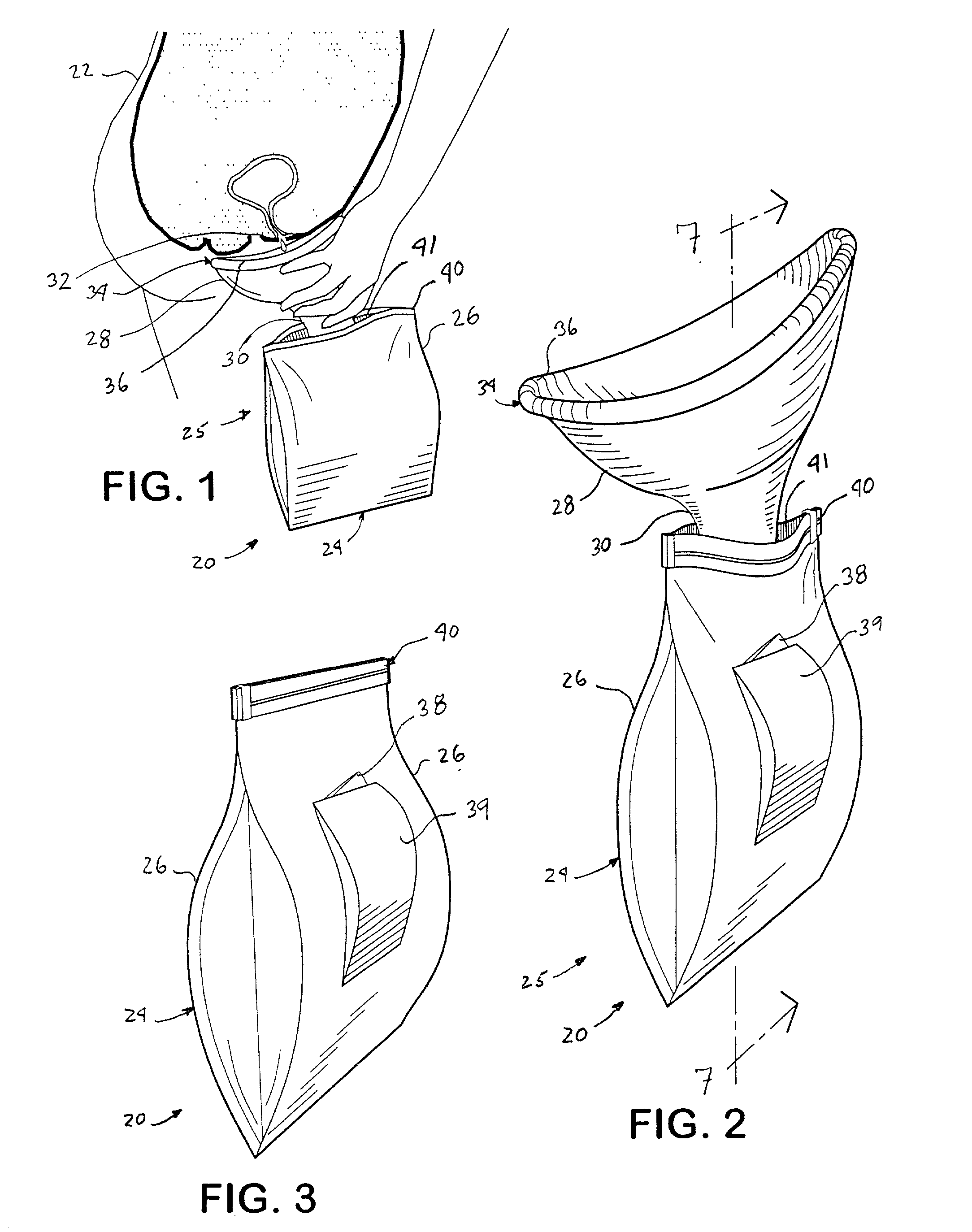
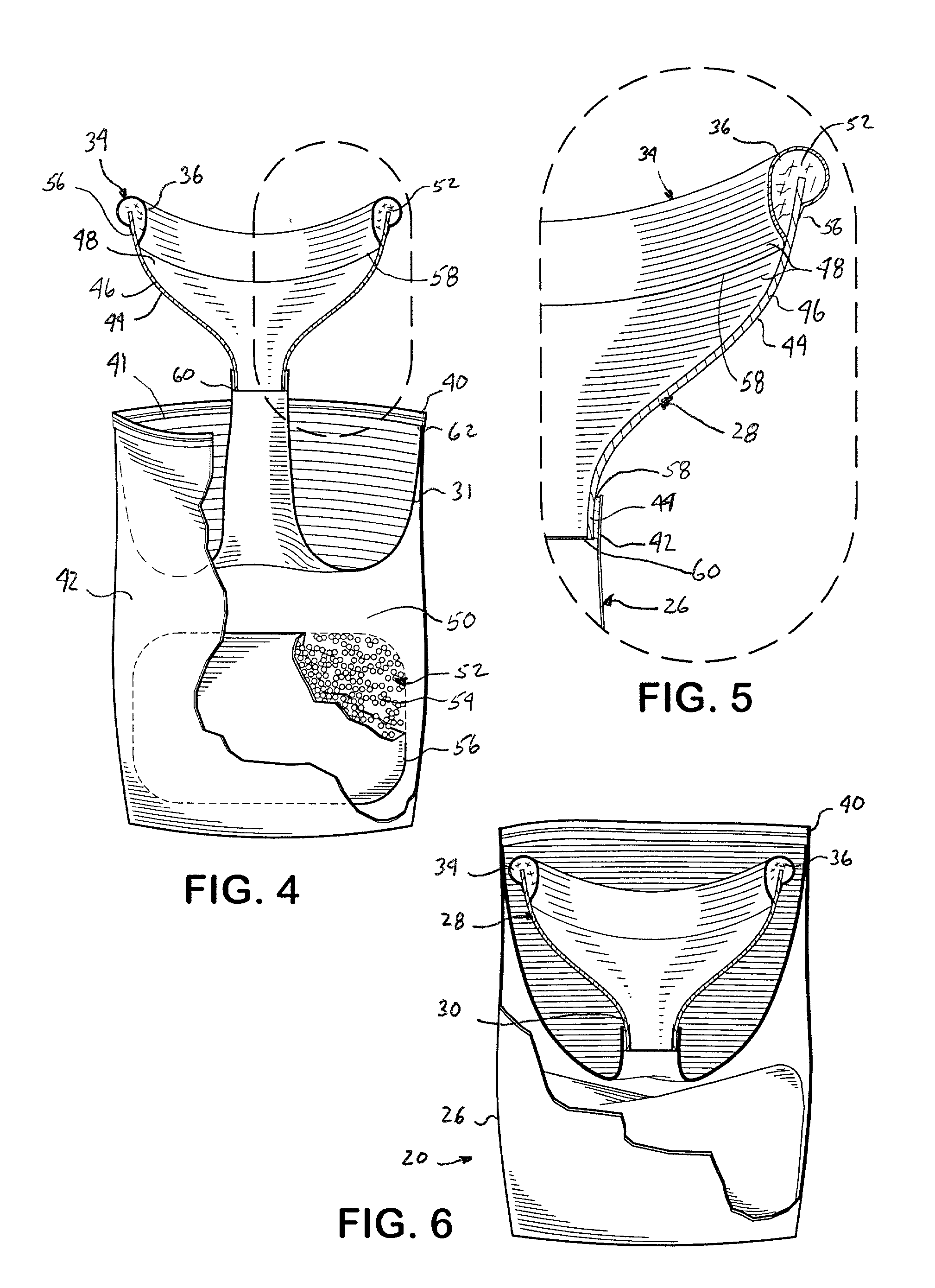
A hand-held disposable portable urinal device particularly well-suited for female use. The easy-to-use device has a leak and splash resistant soft upper seal attached to a retractable funnel, which easily folds back into a sealable bag. The funnel has a neck portion which allows the user to comfortably hold the urinal close to the ureter during use. The disposable urinal bag is odor- and vapor-sealable, and has a hydrophobic polymer containing inner bag for absorbing the urine. In addition, the disposable urinal has a deodorizer activated by the urine upon contact. The disposable urinal is easily transported in a folded state, and preferably comes three in a box. After use, the disposable urinal may be sealed and thrown away.
Owner:SUYDAM KRISTEN V
Solid substrate used for sensors
InactiveUS20050181497A1Good solventPoor solventBioreactor/fermenter combinationsBiological substance pretreatmentsHydrophobic polymerSolid substrate
It is an object of the present invention to provide a solid substrate used for sensors that suppresses nonspecific adsorption and that is able to immobilize a physiologically active substance. The present invention provides A solid substrate used for sensors, wherein two or more different hydrophobic polymer layers are laminated on the solid substrate, and among the above hydrophobic polymer layers, the surface of a layer, which is farthest from the solid substrate, is modified.
Owner:FUJIFILM HLDG CORP +1
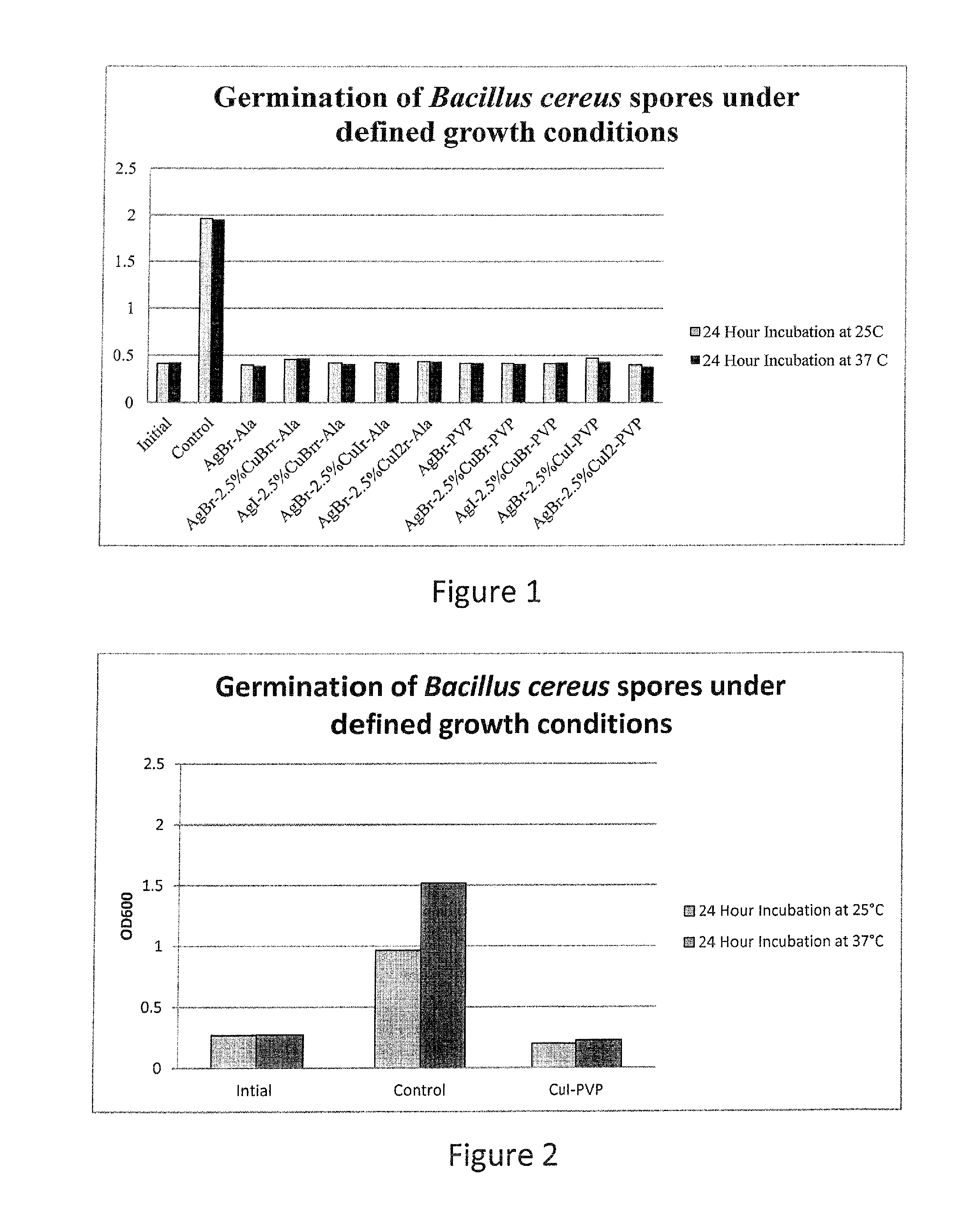
Compositions and methods for antimicrobial metal nanoparticles
InactiveUS20130315972A1Improve efficacyImprove utilizationBiocideInorganic active ingredientsHydrophilic polymersHydrophobic polymer
Compositions having antimicrobial activity contain particles comprising at least one inorganic copper salt; and at least one functionalizing agent in contact with the particles, the functionalizing agent stabilizing the particle in a carrier such that an antimicrobially effective amount of ions are released into the environment of a microbe. The average size of the particles ranges from about 1000 nm to about 3 nm. Preferred copper salts include copper iodide, copper bromide and copper chloride, most preferred being copper iodide. Preferred functionalizing agents comprise materials with a molecular weight of 60 or above, which include amino acids, other acidic materials, thiols, hydrophilic polymers, emulsions of hydrophobic polymers and surfactants. The functionalized particles are preferably made by a grinding process.
Owner:AGIENIC
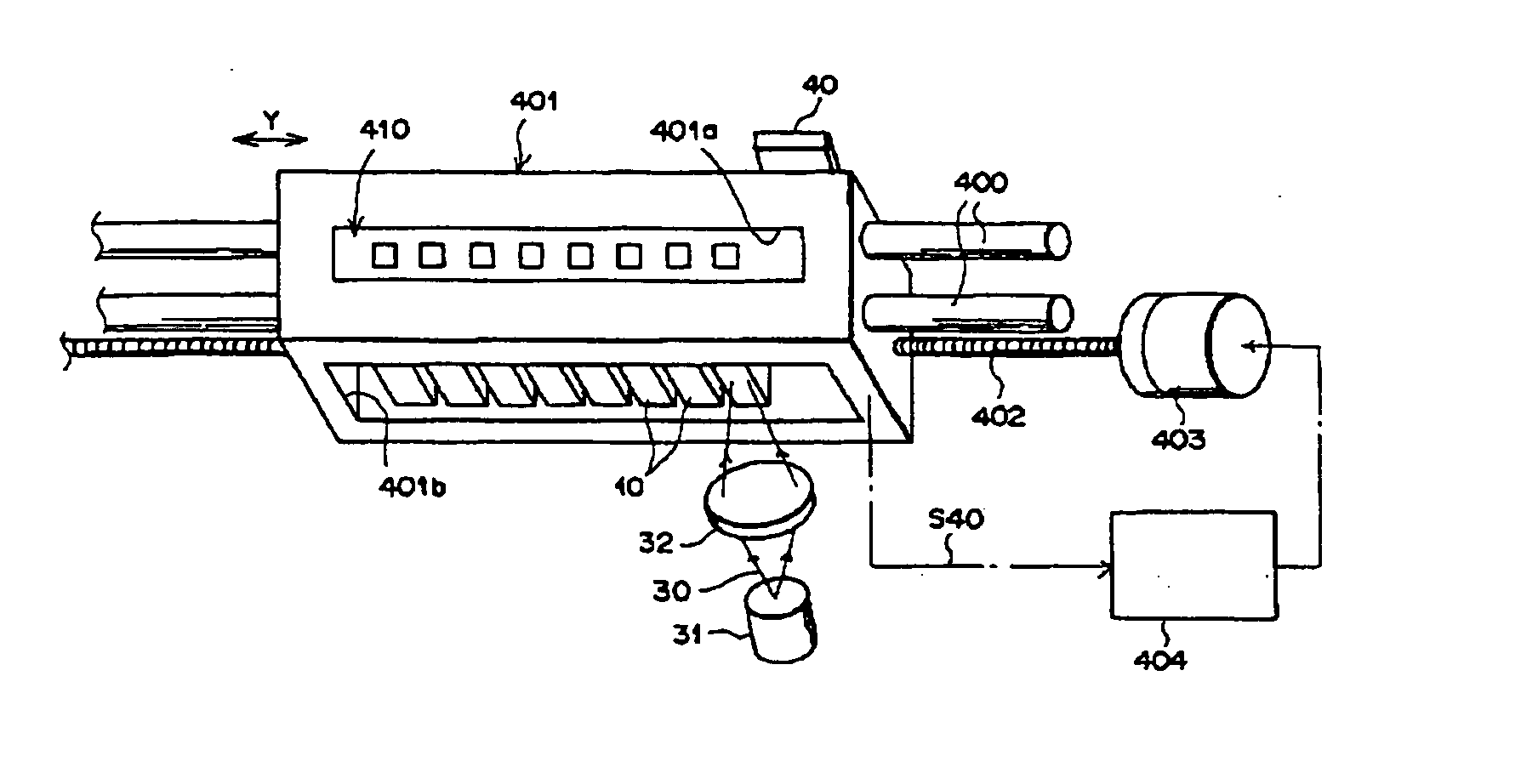
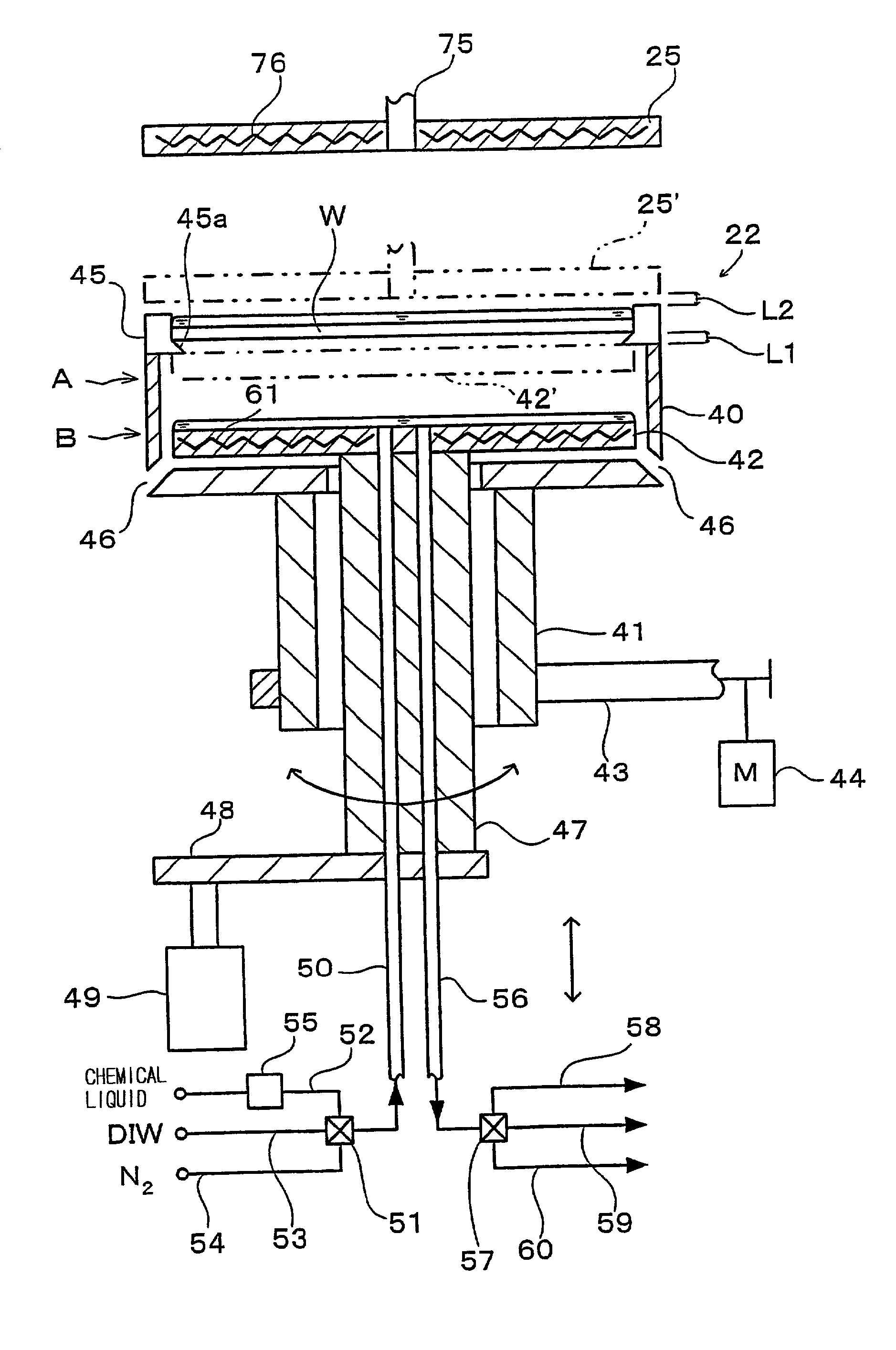
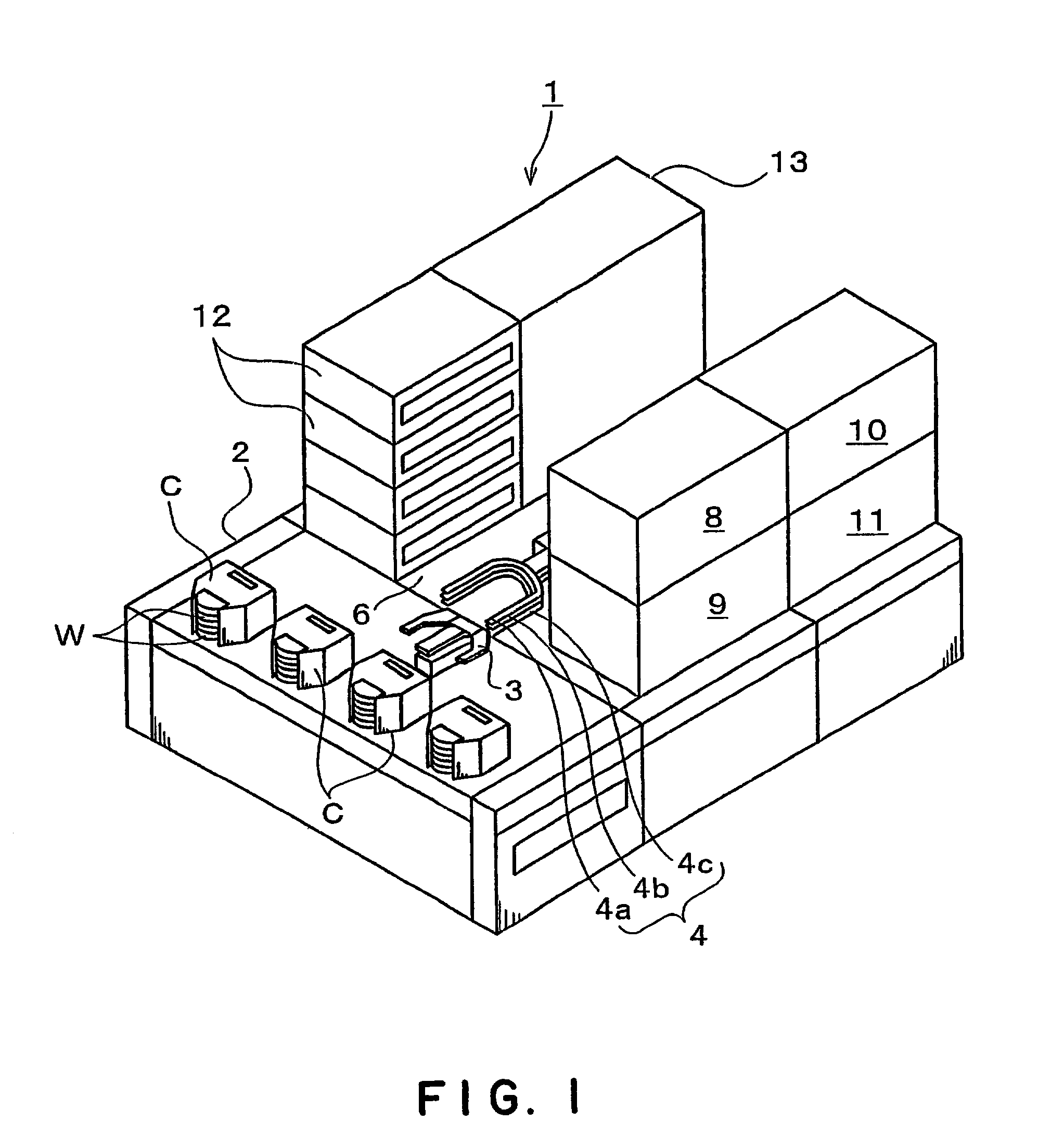
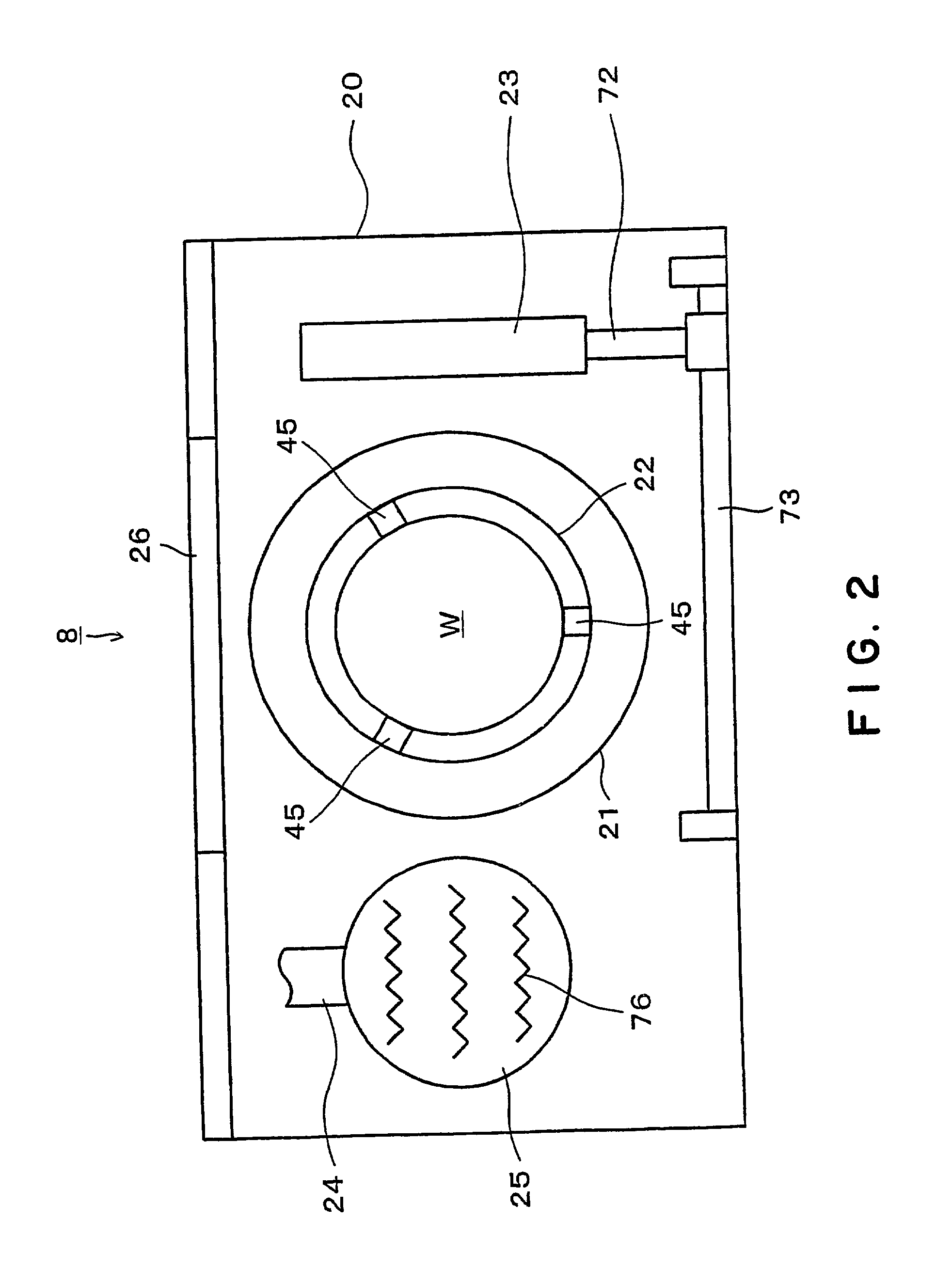
Substrate processing apparatus and substrate processing method
InactiveUS20020096196A1Smooth transferReduce consumptionElectrostatic cleaningSemiconductor/solid-state device manufacturingHydrophobic polymerEngineering
A substrate processing apparatus 8 for feeding a processing liquid and processing a substrate W with the processing liquid comprises holding member 22 for holding the substrate W, and a lower side member 42 which is moved relatively with respect to the back surface of the substrate W held by the holding member 22 between a processing position A near the substrate undersurface and a retreat position B remote from the substrate undersurface. The processing liquid is fed between a gap between the lower side member 42 moved to the processing position A and the back surface of the substrate held by the holding member 22, and the substrate undersurface is processed. A cleaning processing unit 221a as one embodiment of the liquid processing apparatus comprises a spin chuck 22 for holding a wafer W substantially horizontally, a stage 224 substantially horizontally below the wafer W held by the spin chuck 223, and a cleaning liquid discharge openings 241 for feeding a required cleaning liquid into a gap between the wafer W held by the spin chuck 223 and the stage 224. The stage 224 has the surface coated with a hydrophobic polymer so that the surface has wetting which allows a contact angle of the cleaning liquid to be above 50E. A layer of the cleaning liquid is formed in the gap between the wafer W held by the spin chuck 223 and the stage 224 to subject the wafer W to the liquid processing.
Owner:TOKYO ELECTRON LTD
Amphiphilic core-shell latexes
Owner:THE HONG KONG POLYTECHNIC UNIV
Compositions and methods for antimicrobial metal nanoparticles
InactiveUS20120301528A1Good effectBiocideCosmetic preparationsHydrophilic polymersHydrophobic polymer
Embodiments of the invention are directed to a composition having antimicrobial activity comprising particles comprising at least one inorganic copper salt; and at least one functionalizing agent in contact with the particles, the functionalizing agent stabilizing the particle in a carrier such that an antimicrobially effective amount of ions are released into the environment of a microbe. The average size of the particles ranges from about 1000 nm to about 4 nm. Preferred copper salts include copper iodide, copper bromide and copper chloride. Preferred functionalizing agents include amino acids, thiols, hydrophilic polymers, emulsions of hydrophobic polymers and surfactants.
Owner:AGIENIC
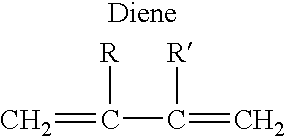
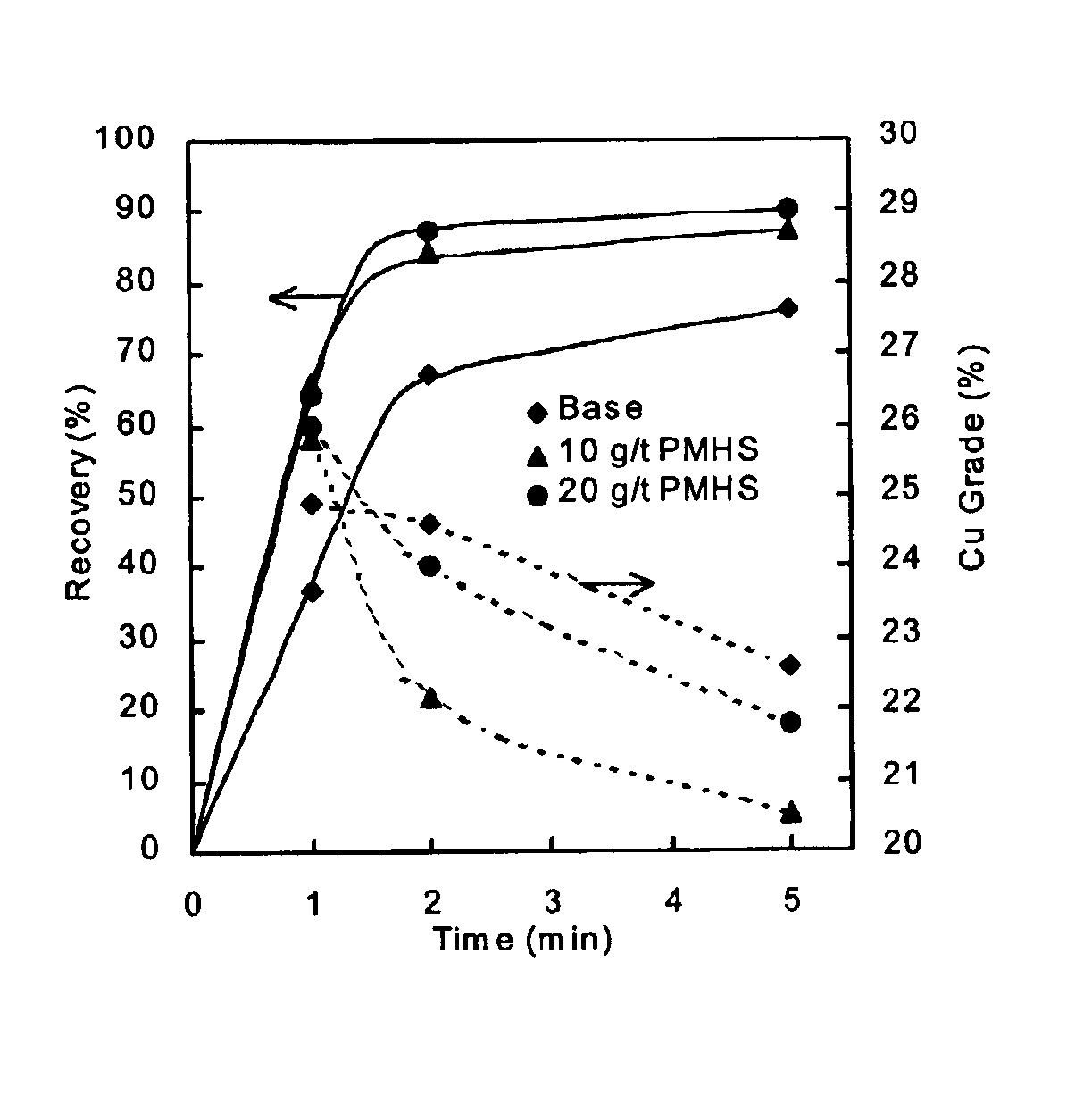
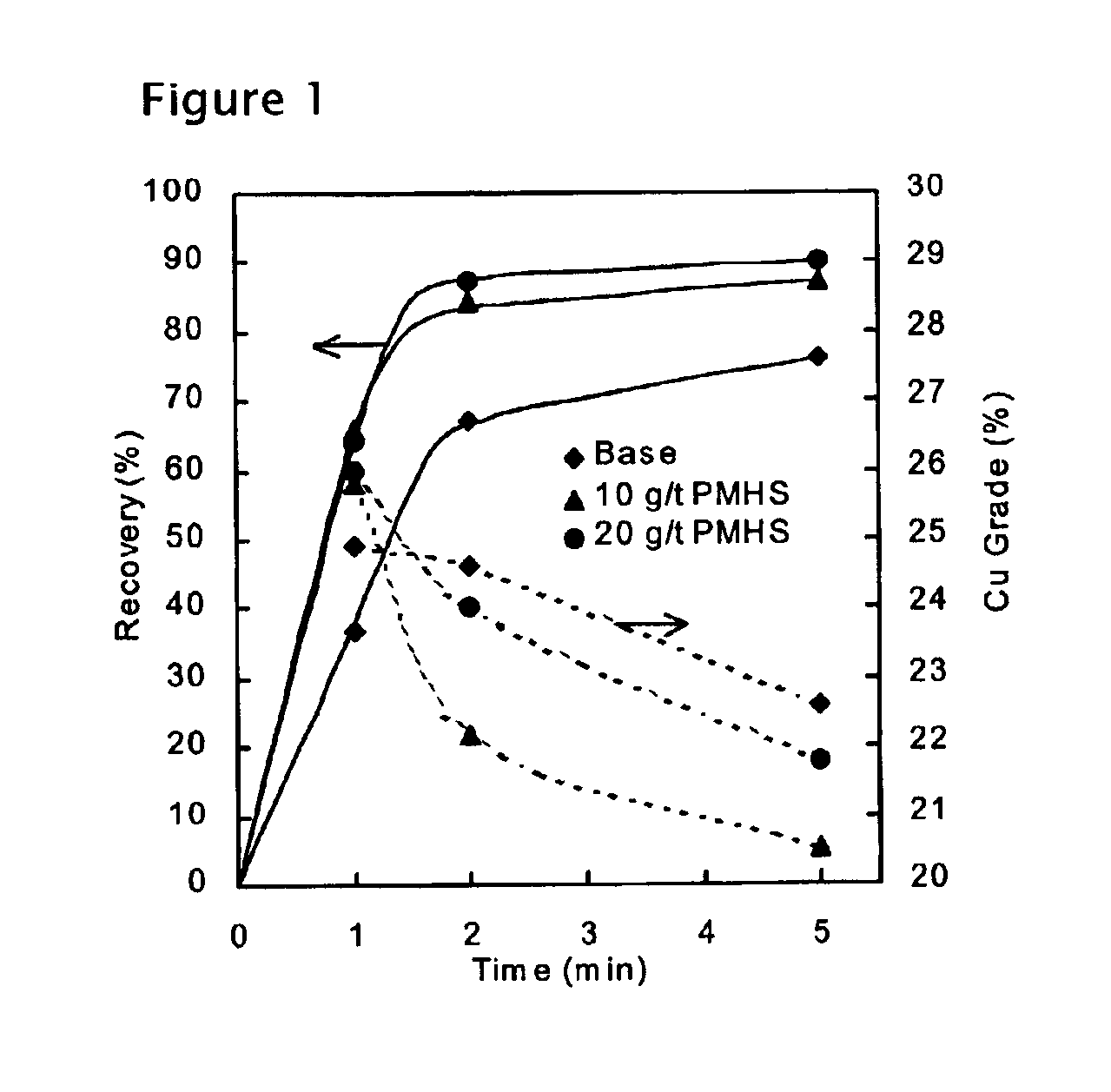
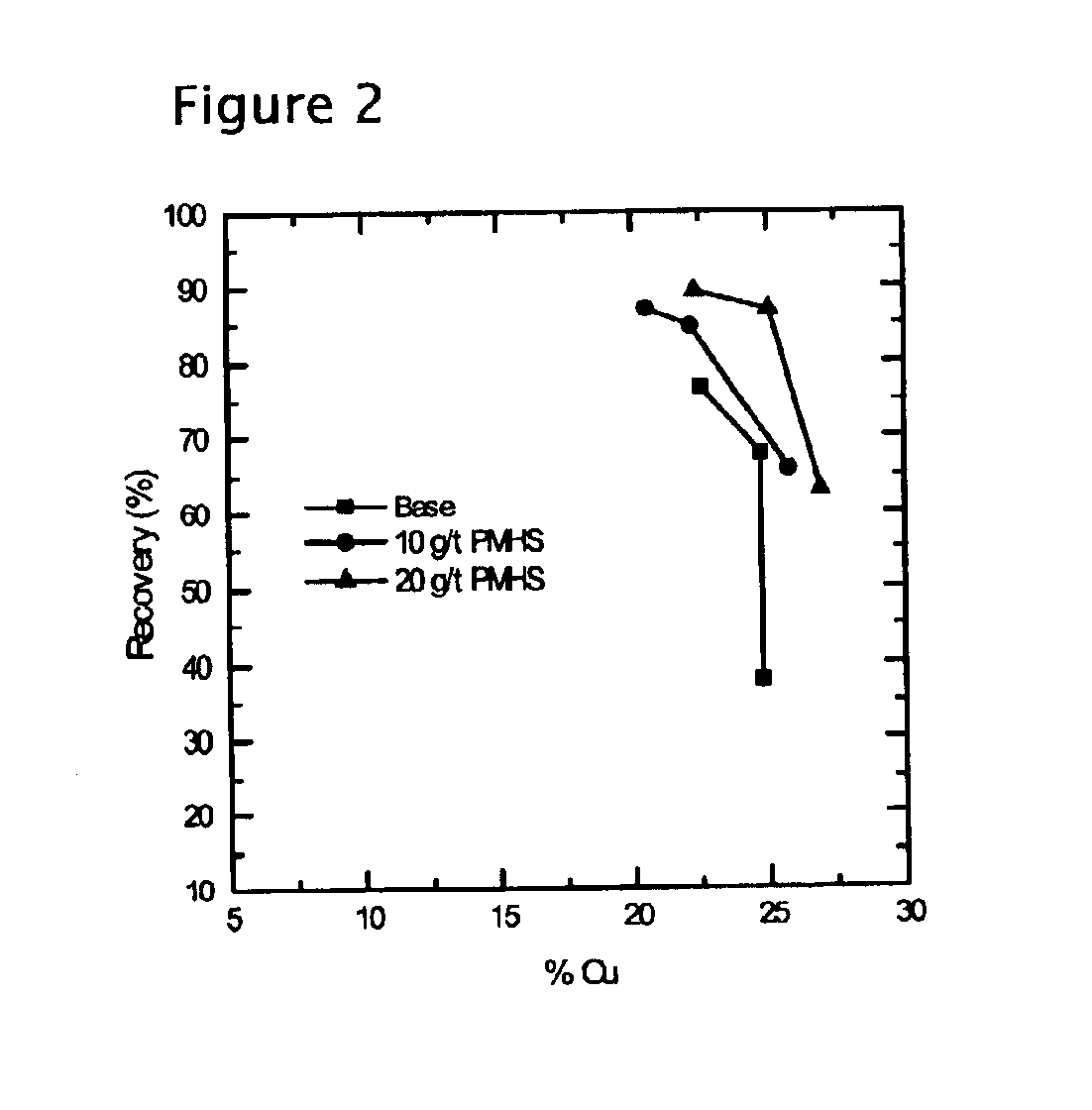
Methods of increasing flotation rate
Methods of increasing the rate of separating hydrophobic and hydrophilic particles by flotation have been developed. They are based on using appropriate reagents to enhance the hydrophobicity of the particles to be floated, so that they can be more readily collected by the air bubbles used in flotation. The hydrophobicity-enhancing reagents include low HLB surfactants, naturally occurring lipids, modified lipids, and hydrophobic polymers. These methods can greatly increase the rate of flotation for the particles that are usually difficult to float, such as ultrafine particles, coarse particles, middlings, and the particles that do not readily float in the water containing large amounts of ions derived from the particles. In addition, new collectors for the flotation of phosphate minerals are disclosed.
Owner:MINERAL & COAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com