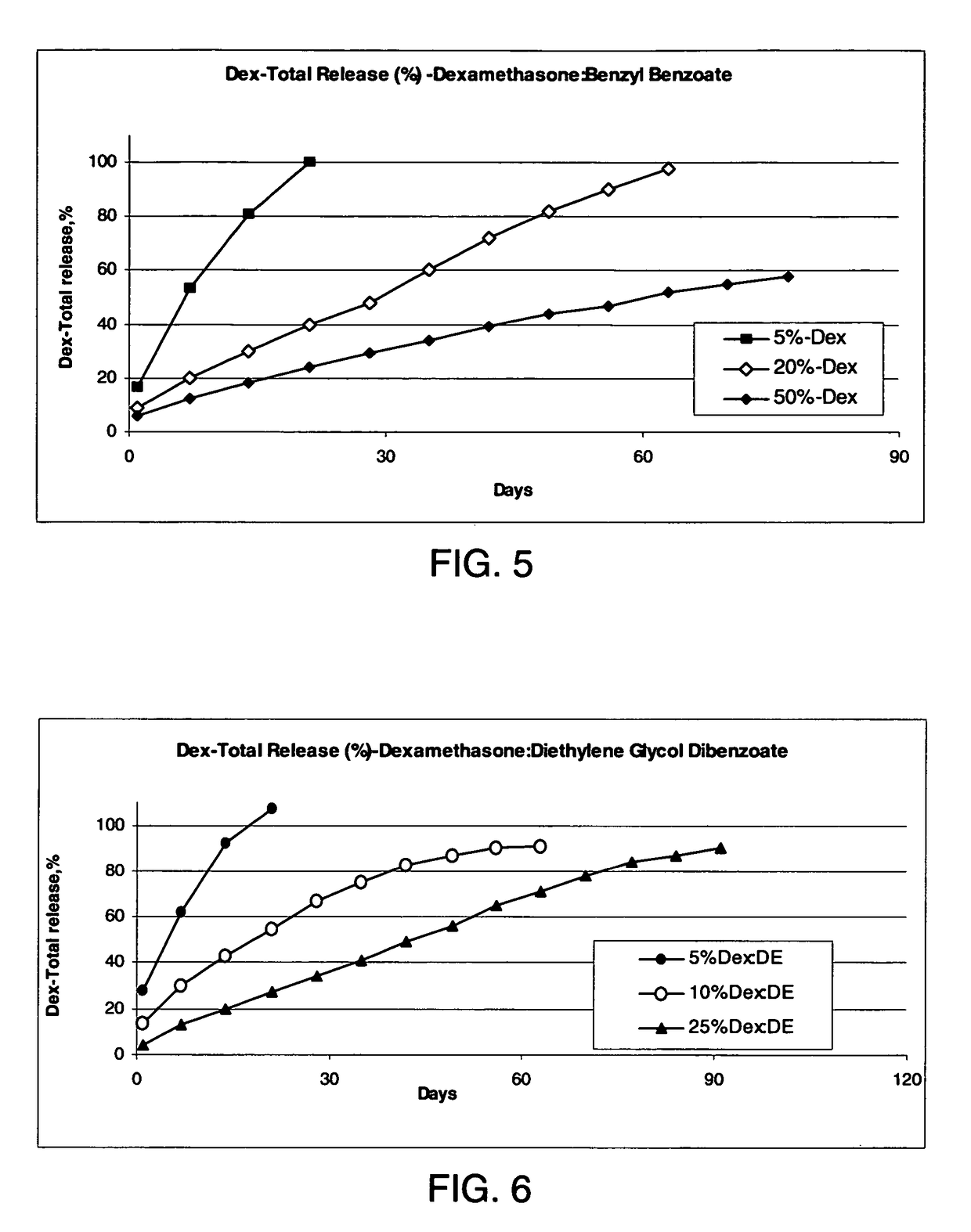
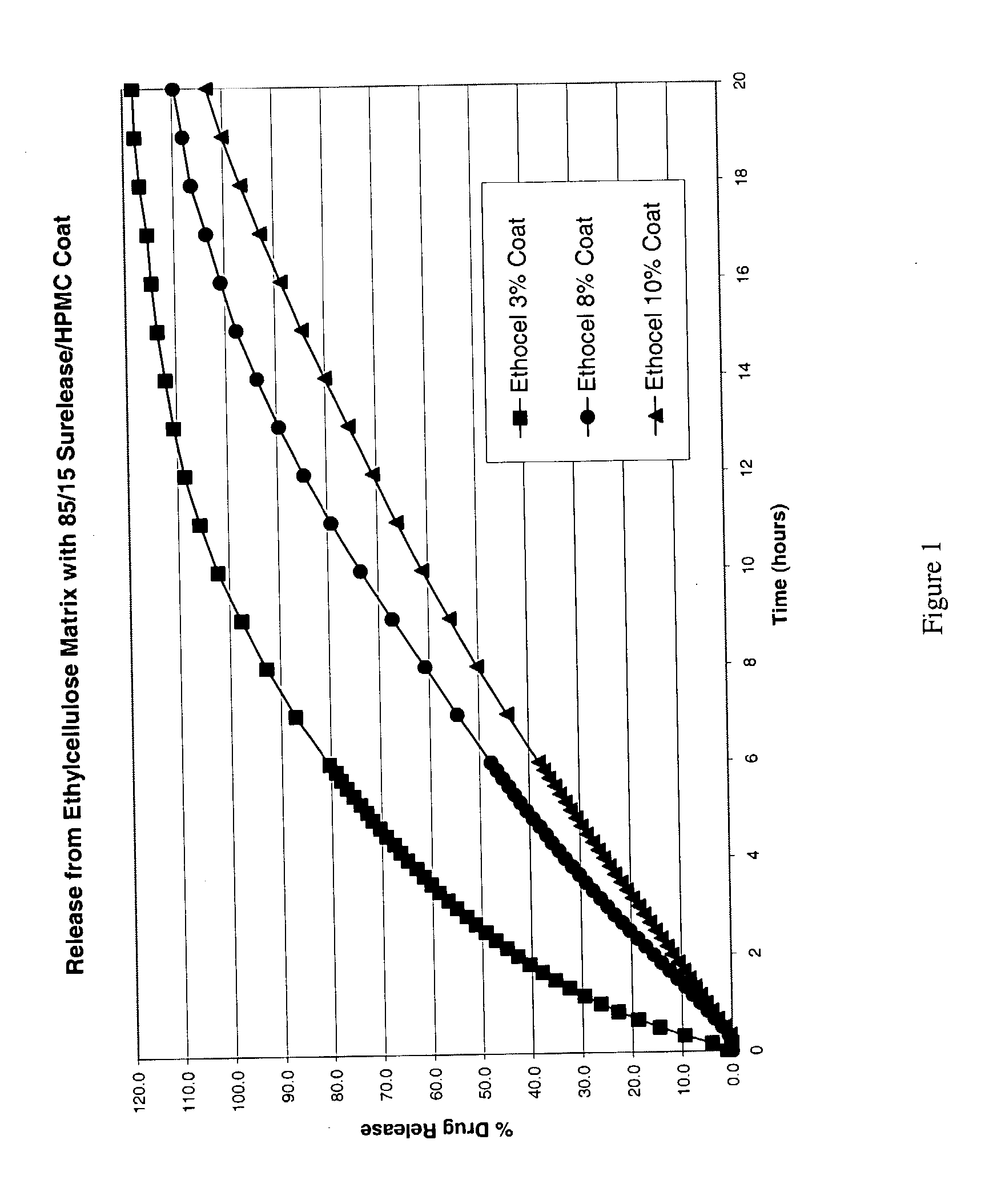
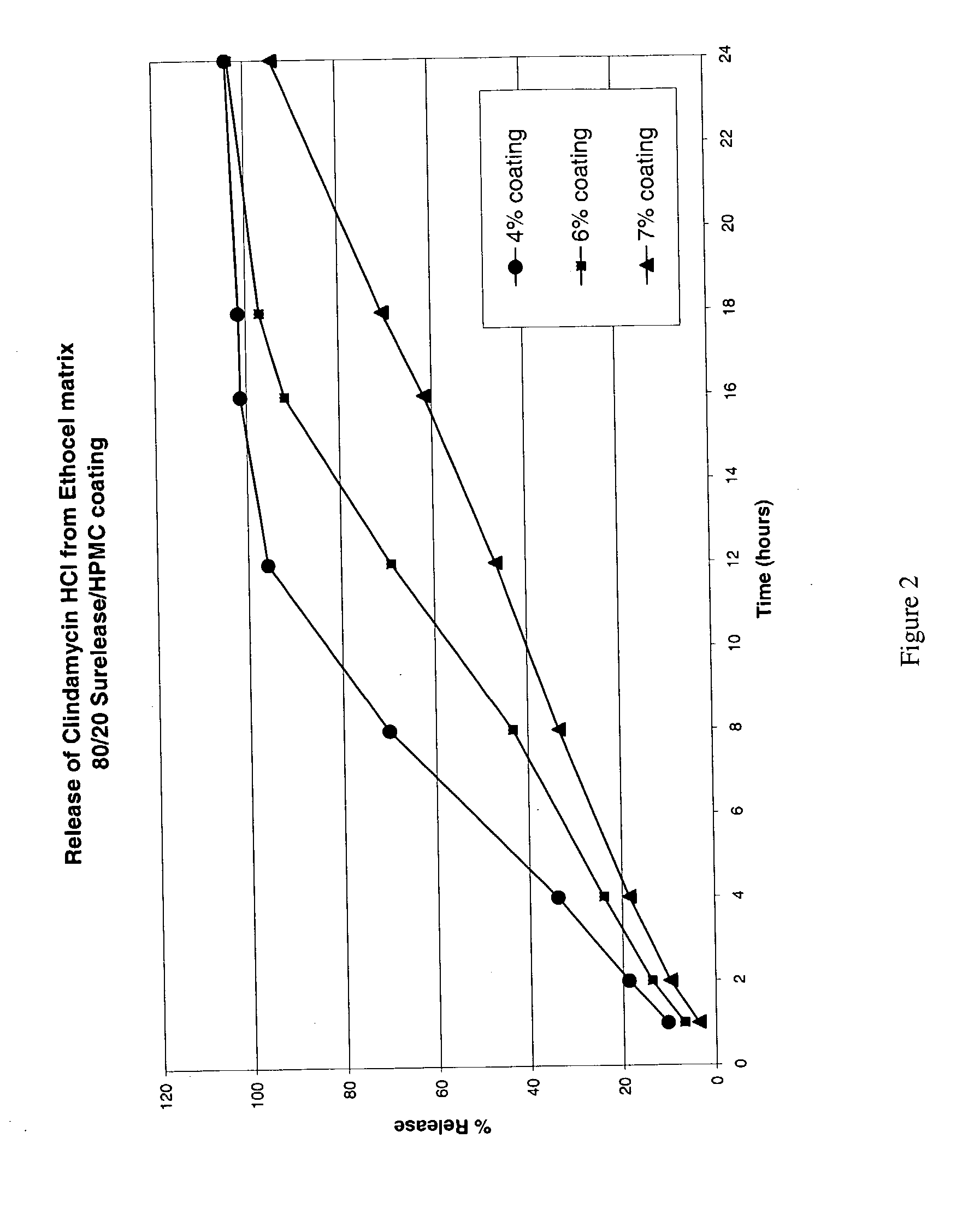
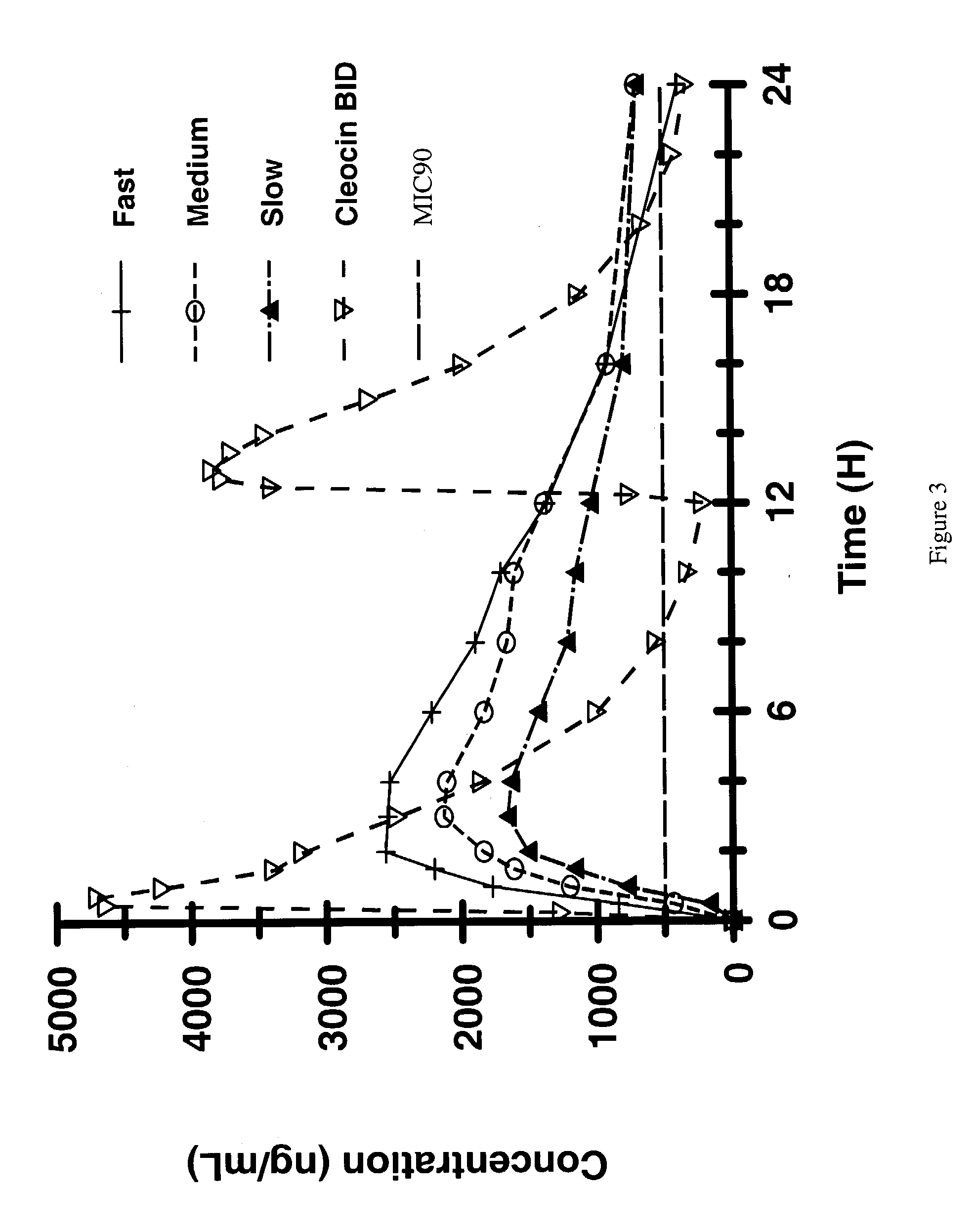
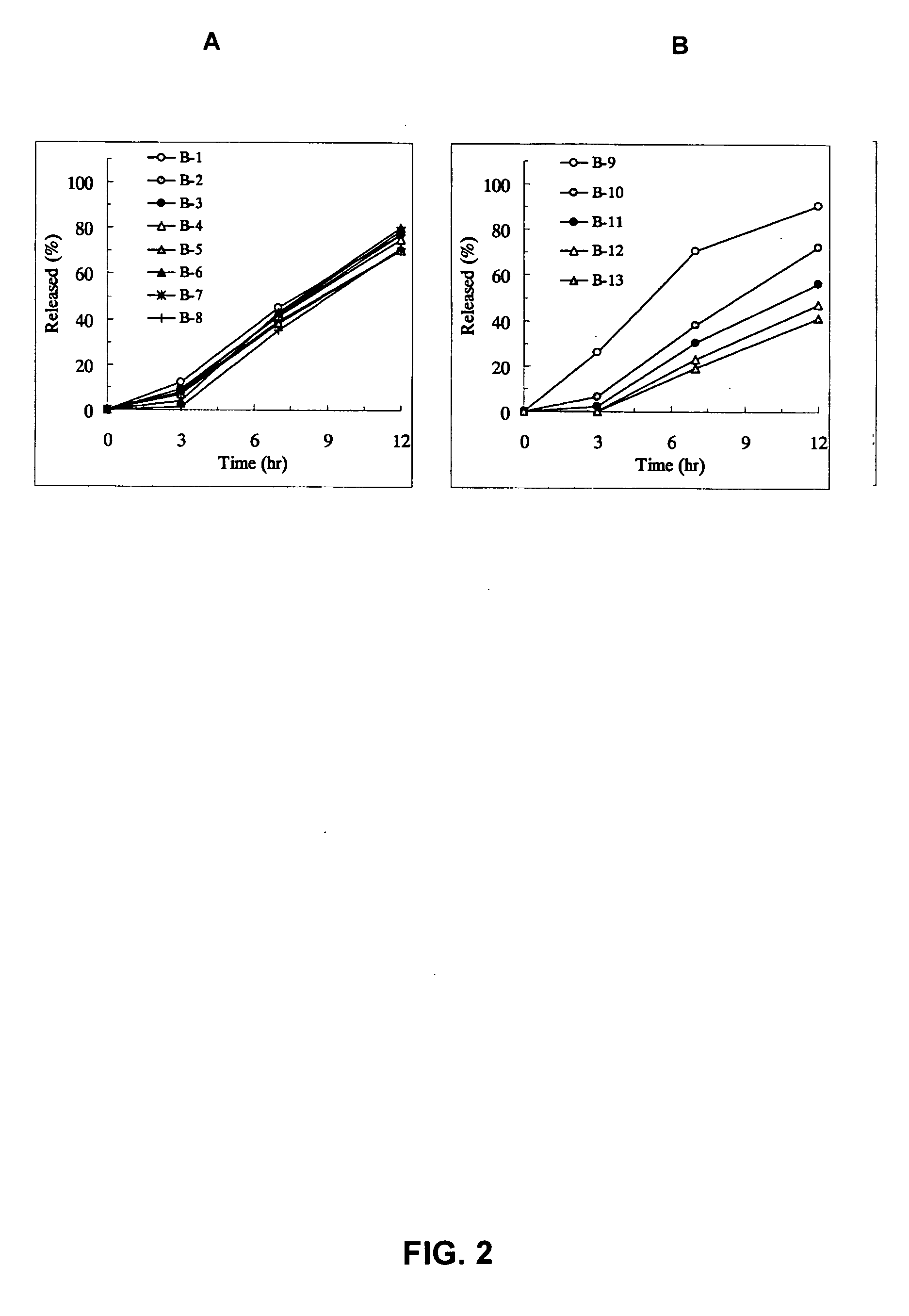
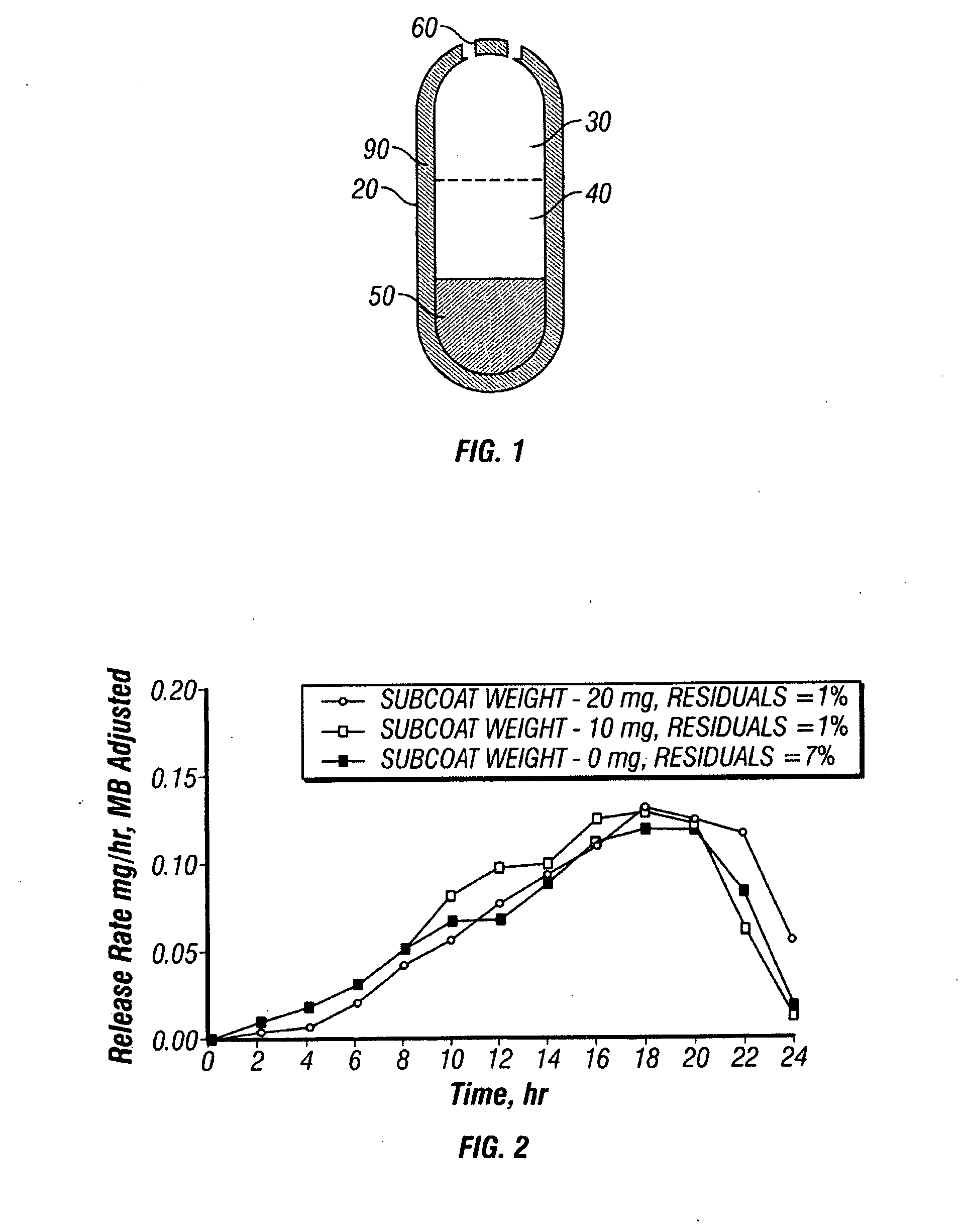
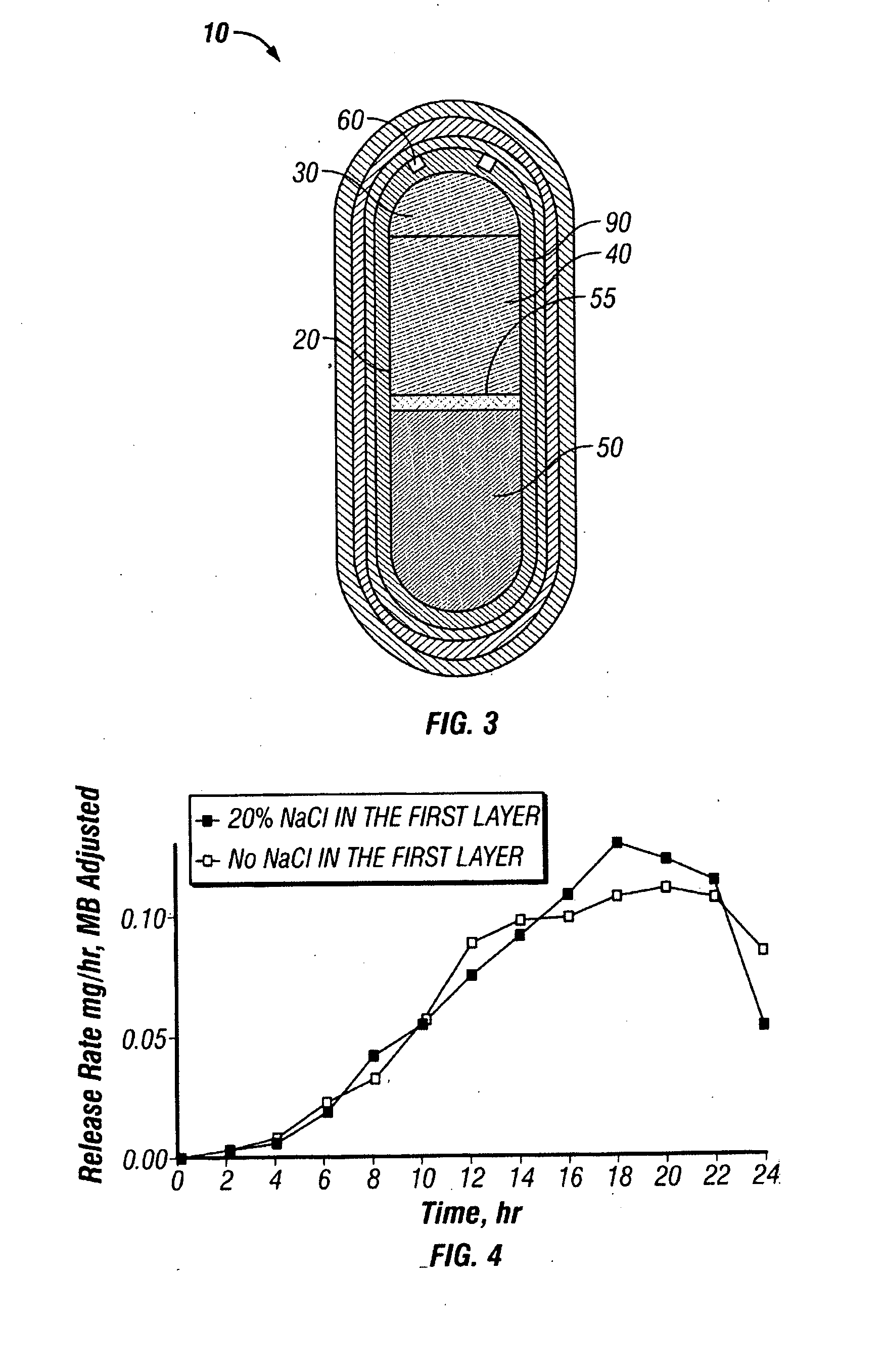
Patents
Literature
314 results about "Sustained release drug" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sustained release's definition is more akin to a "controlled release" rather than "sustained". Extended-release dosage consists of sustained-release (SR) and controlled-release (CR) dosage. SR maintains drug release over a sustained period but not at a constant rate.
Polymer-based, sustained release drug delivery system
InactiveUS20120016467A1Reduce interactionSuture equipmentsAntibacterial agentsRate limitingSolubility
Disclosed is a sustained release system that includes a polymer and a prodrug having a solubility less than about 1 mg / ml dispersed in the polymer. Advantageously, the polymer is permeable to the prodrug and may be non-release rate limiting with respect to the rate of release of the prodrug from the polymer. This permits improved drug delivery within a body in the vicinity of a surgery via sustained release rate kinetics over a prolonged period of time, while not requiring complicated manufacturing processes.
Owner:PSIVIDA INC
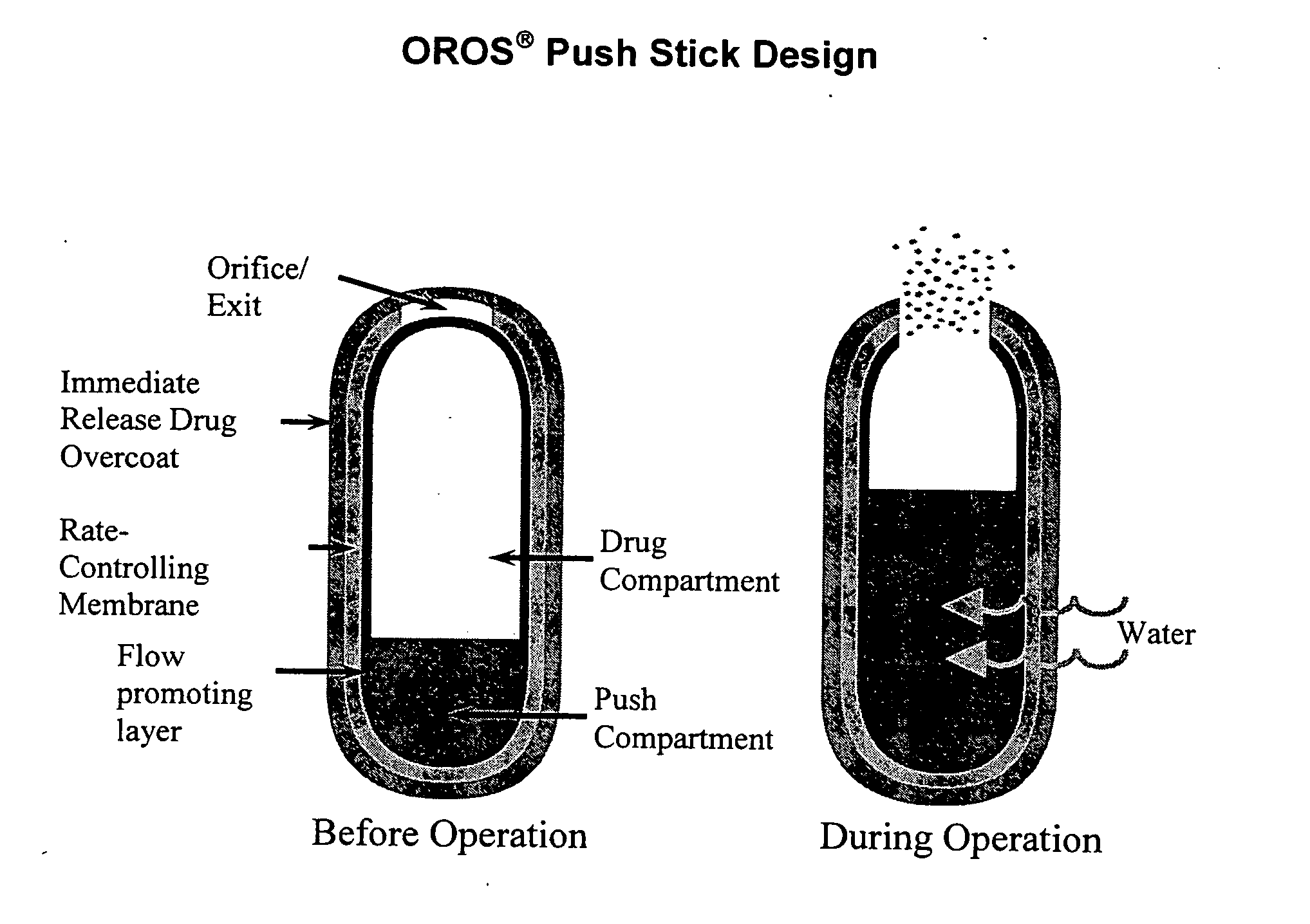
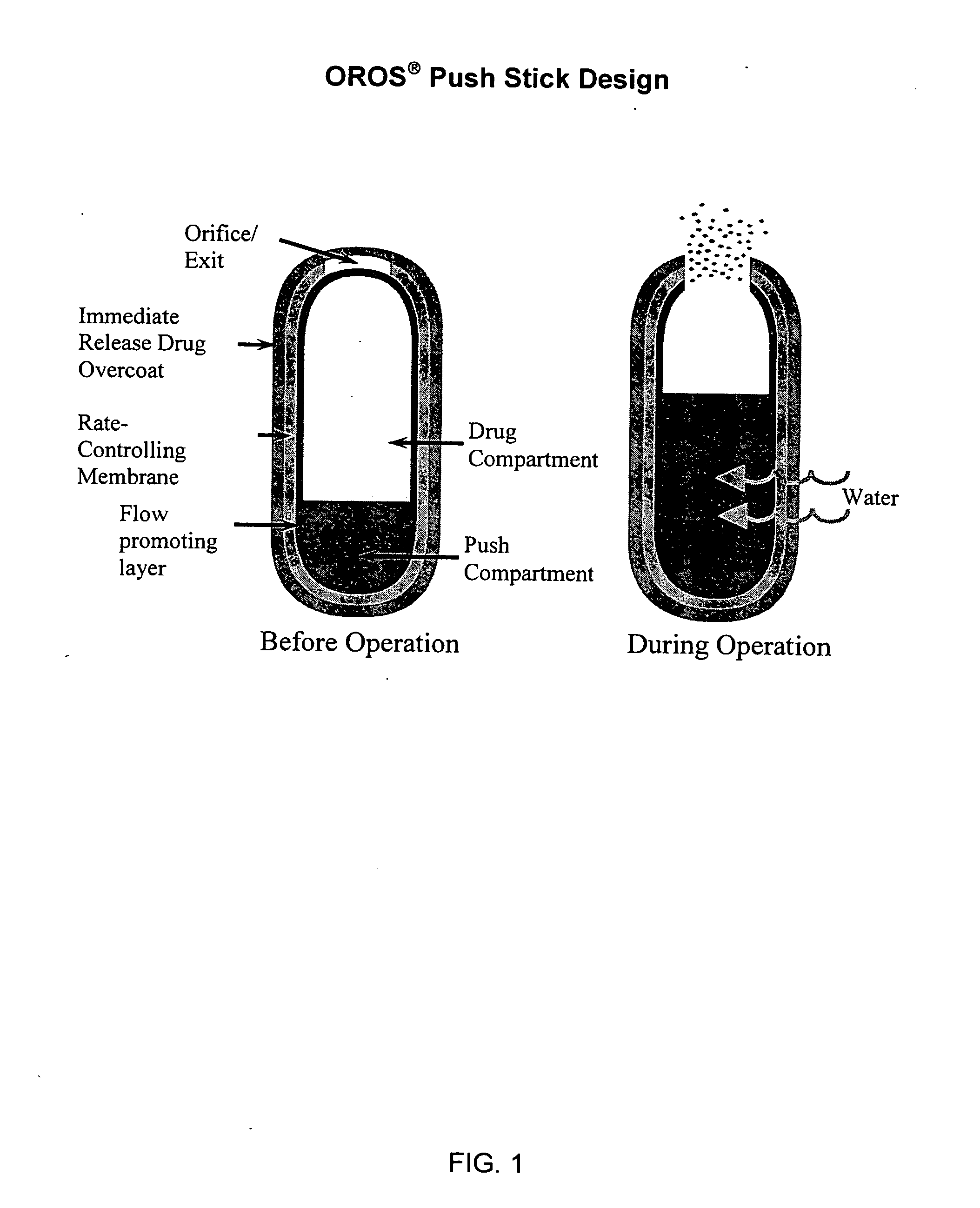
Sustained release drug delivery devices, methods of use, and methods of manufacturing thereof
InactiveUS6375972B1Without of effectWithout riskOrganic active ingredientsSenses disorderBiological bodySustained release drug
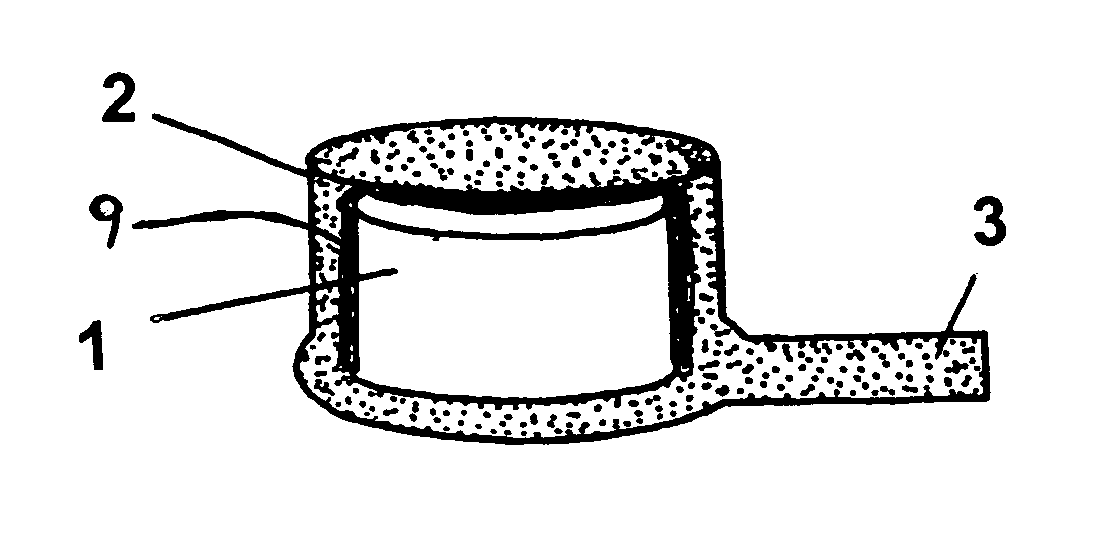
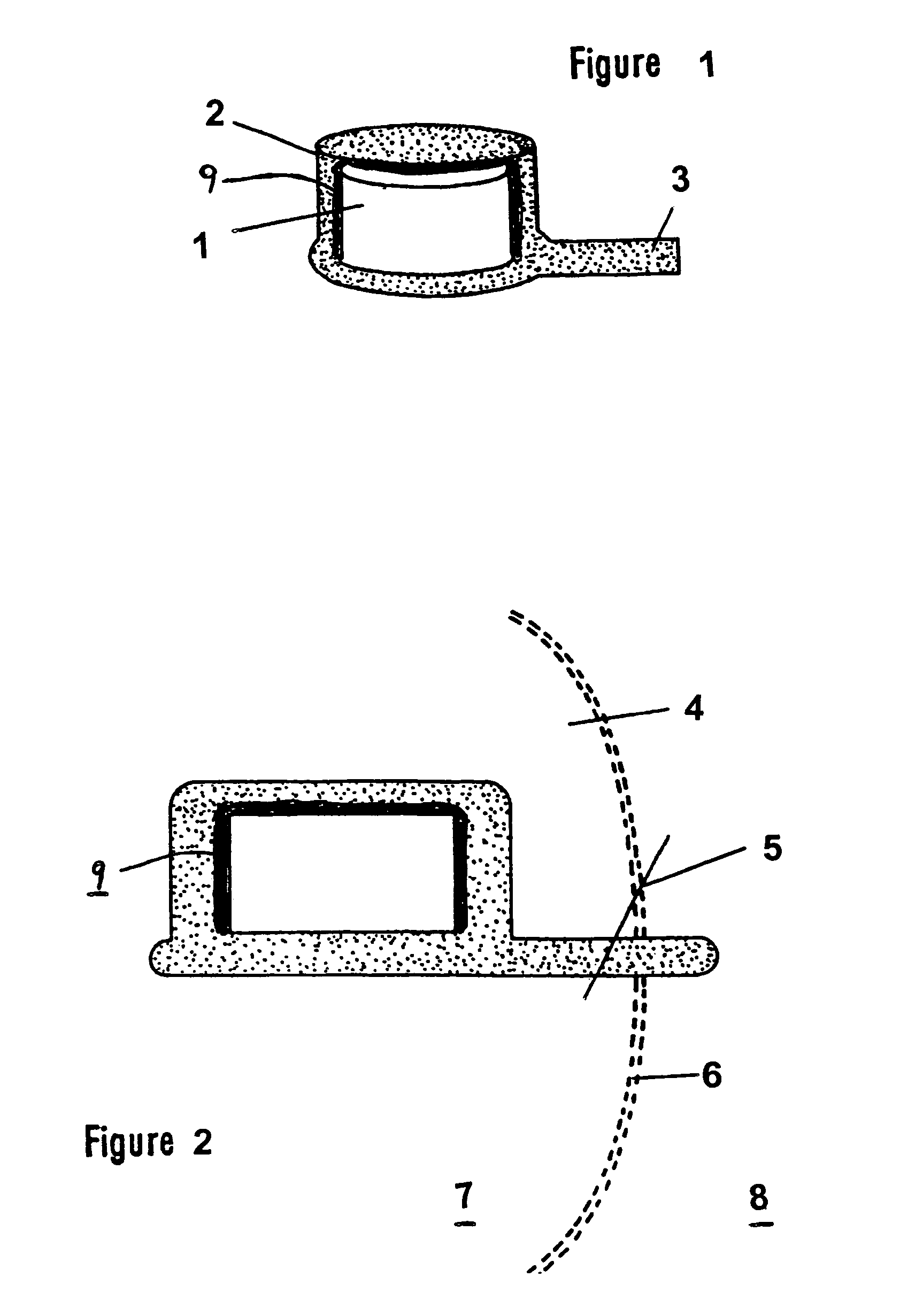
A method and device for treating a mammalian organism to obtain a desired local or systemic physiological or pharmacological effect is provided. The method includes administering a sustained release drug delivery system to a mammalian organism in need of such treatment at an area wherein release of an effective agent is desired and allowing the effective agent to pass through the device in a controlled manner. The device includes an inner core or reservoir including the effective agent, an impermeable tube which encloses portions of the reservoir, and a permeable member at an end of the tube.
Owner:EYEPOINT PHARMA INC
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20050158382A1Reduce the maximumRapid rise in plasma concentrationBiocideNervous disorderImmediate releaseAnalgesic agents
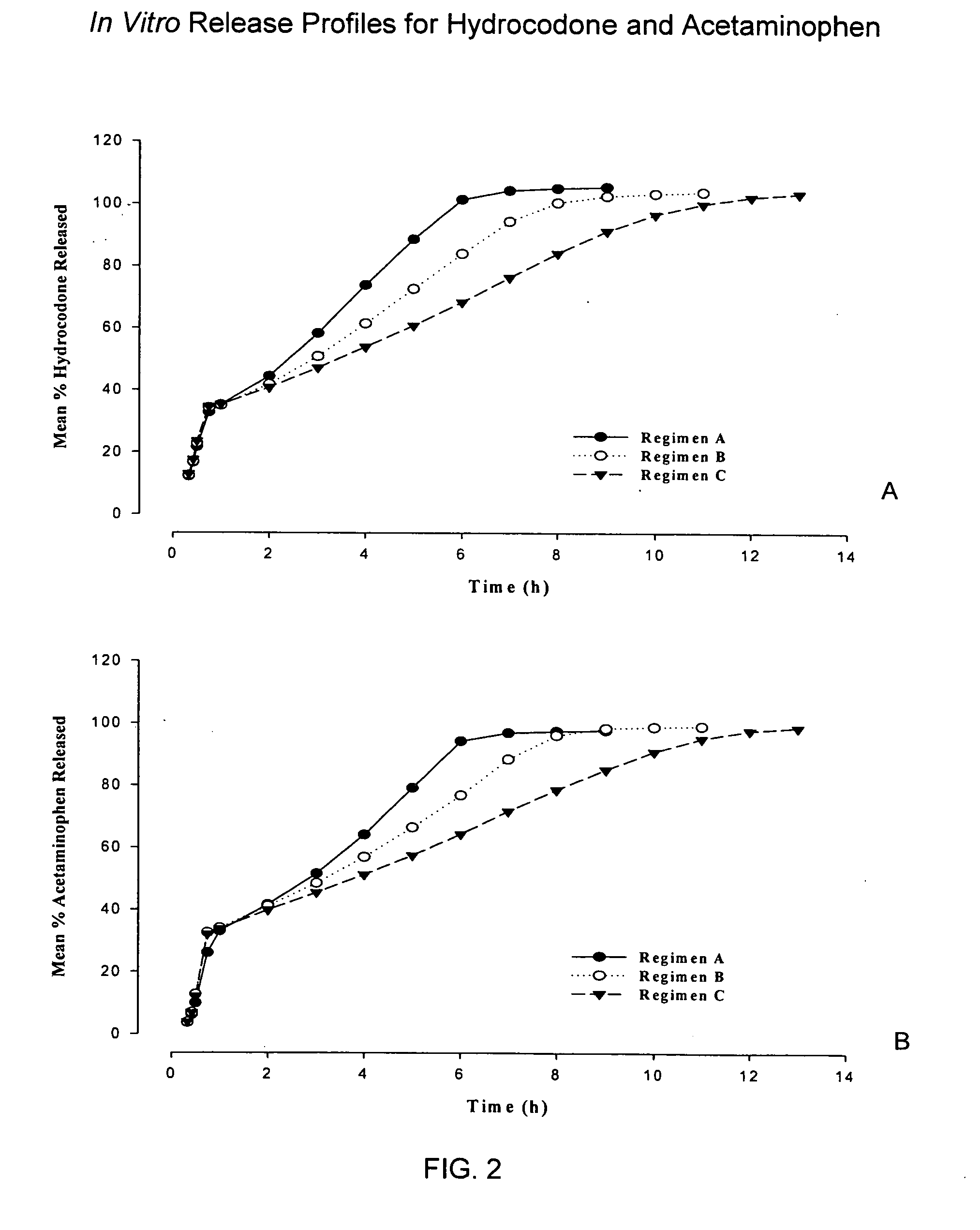
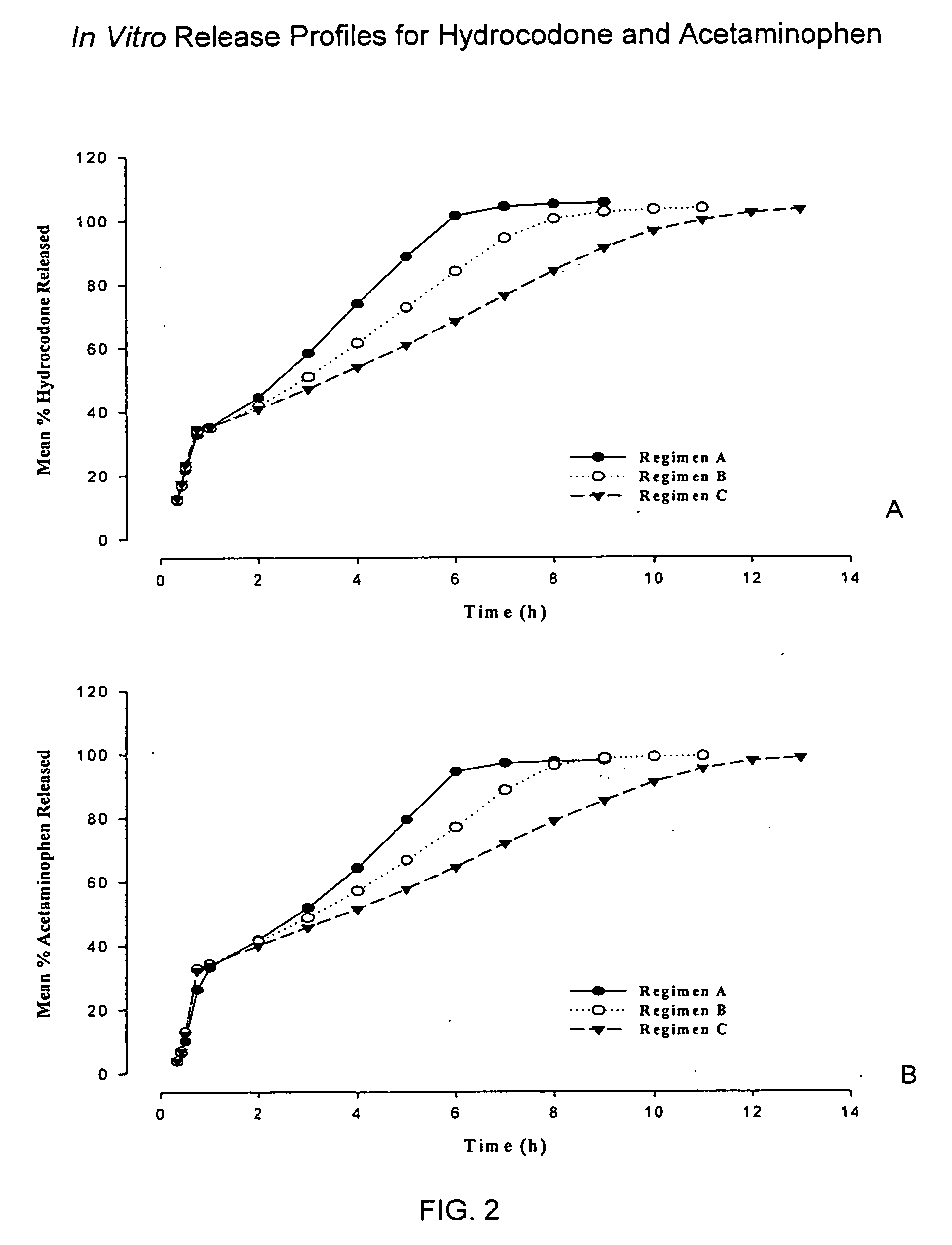
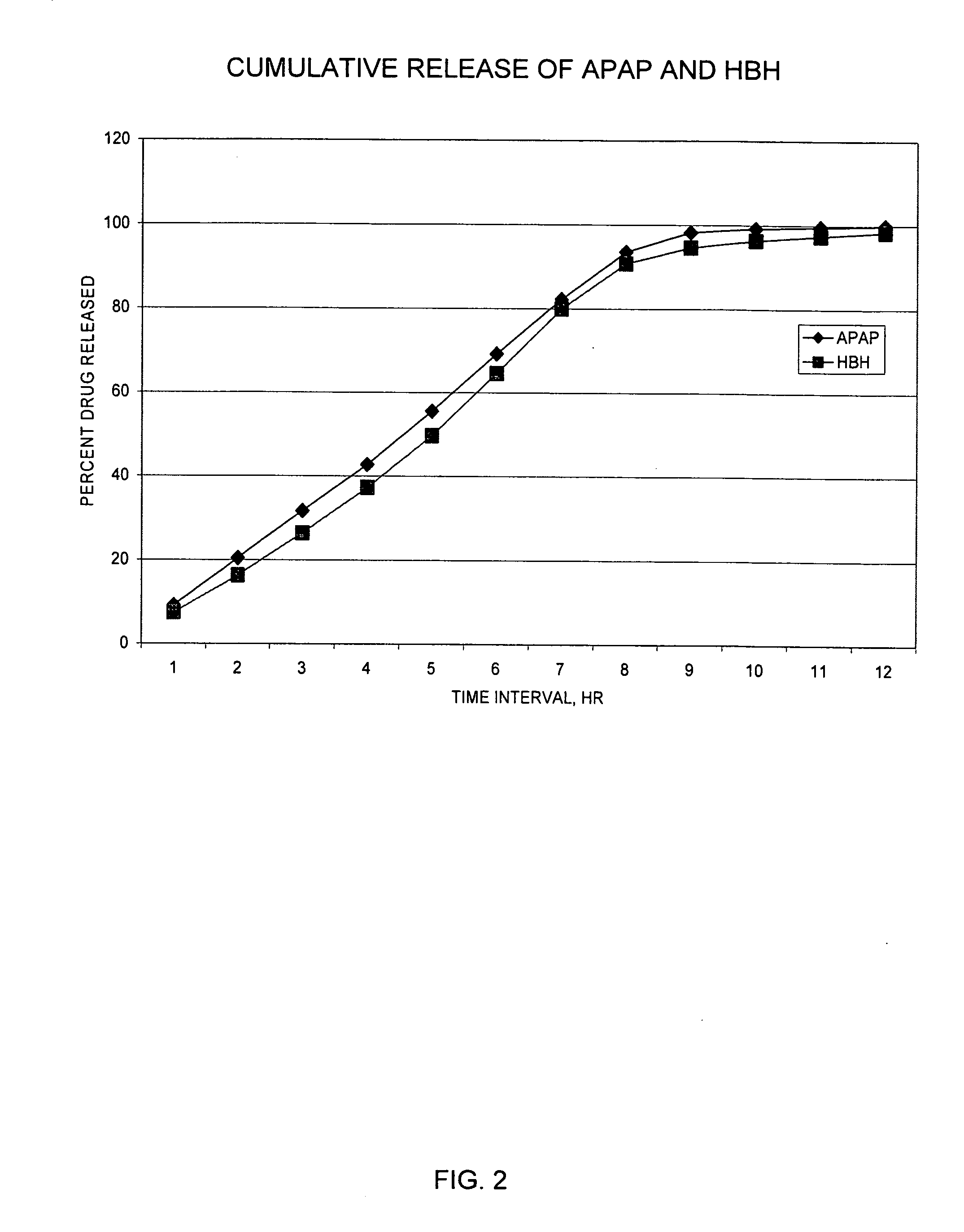
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
Conveniently implantable sustained release drug compositions
ActiveUS20060073182A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Conveniently implantable sustained release drug compositions
InactiveUS20080038316A1Economical and practical and efficientEasy to produceBiocideOrganic active ingredientsDiseaseSustained release drug
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Controlled release formulations of opioid and nonopioid analgesics
InactiveUS20060251721A1Improved ability to treat painLess attentionBiocideNervous disorderImmediate releasePharmaceutical medicine
Sustained release dosage forms for twice daily oral dosing to a human patient for providing relief from pain are provided. The sustained release dosage form comprises an immediate release component and a sustained release component, wherein the immediate release component and the sustained release component collectively contain a therapeutically effective amount of an opioid analgesic and a therapeutically effective amount of nonopioid analgesic. In a preferred embodiment, the nonopioid analgesic is acetaminophen and the opioid analgesic is hydrocodone and pharmaceutically acceptable salts thereof, and in preferred embodiments, the pharmaceutically acceptable salt is bitartrate. The dosage forms produce plasma profiles in a patient characterized by a Cmax for hydrocodone of between about 0.6 ng / mL / mg to about 1.4 ng / mL / mg and an AUC for hydrocodone of between about 9.1 ng*hr / mL / mg to about 19.9 ng*hr / mL / mg (per mg hydrocodone bitartrate administered) and a Cmax for acetaminophen of between about 2.8 ng / mL / mg and 7.9 ng / mL / mg and an AUC for acetaminophen of between about 28.6 ng*hr / mL / mg and about 59.1 ng*hr / mL / mg (per mg acetaminophen administered) after a single dose.
Owner:ALZA CORP
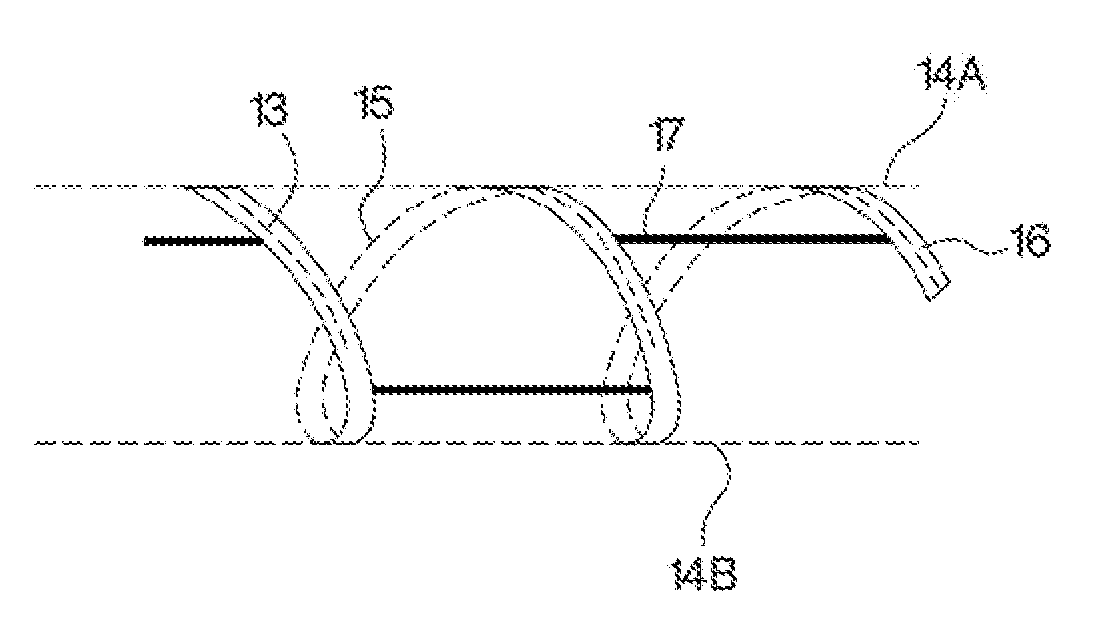
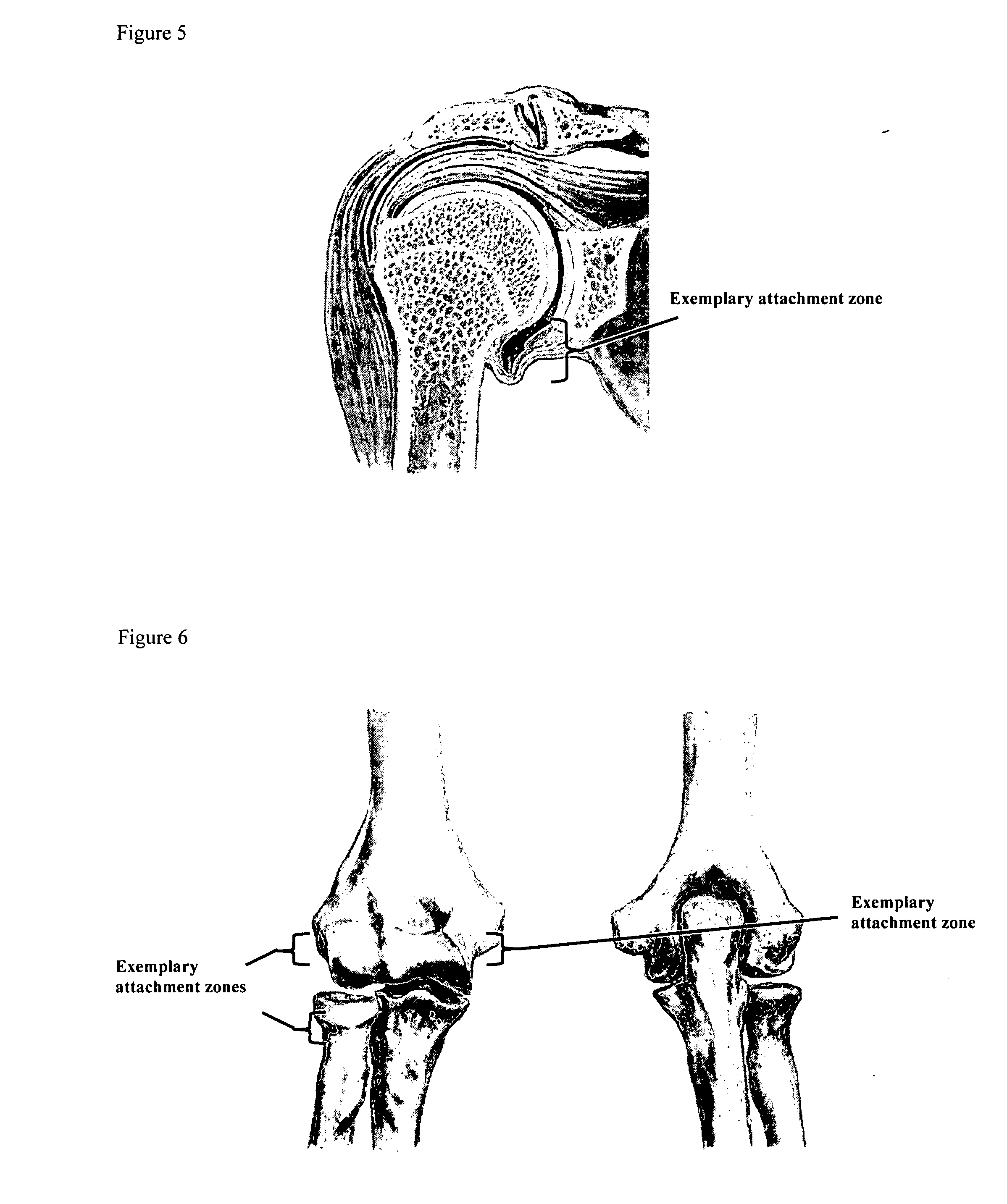
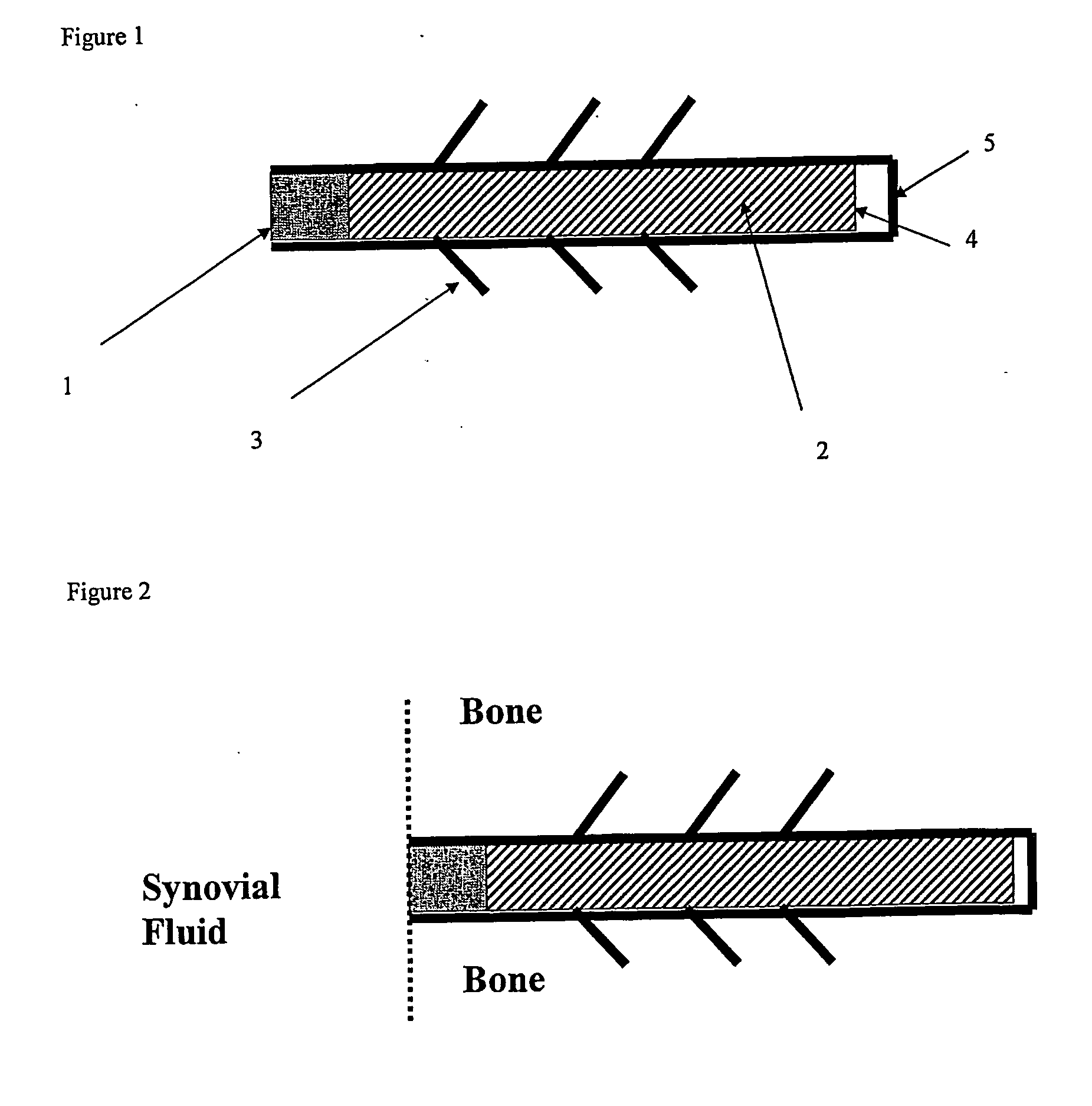
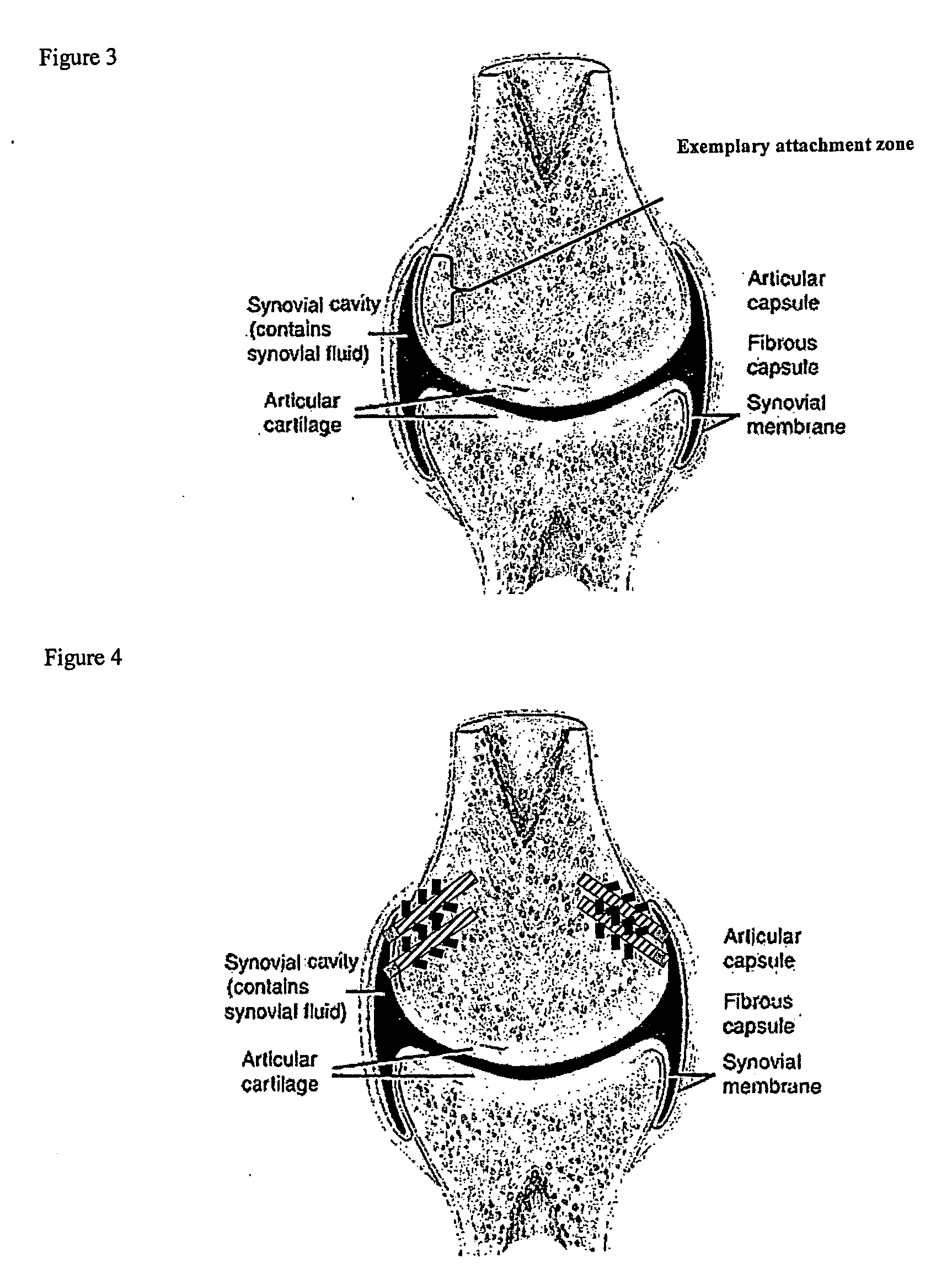
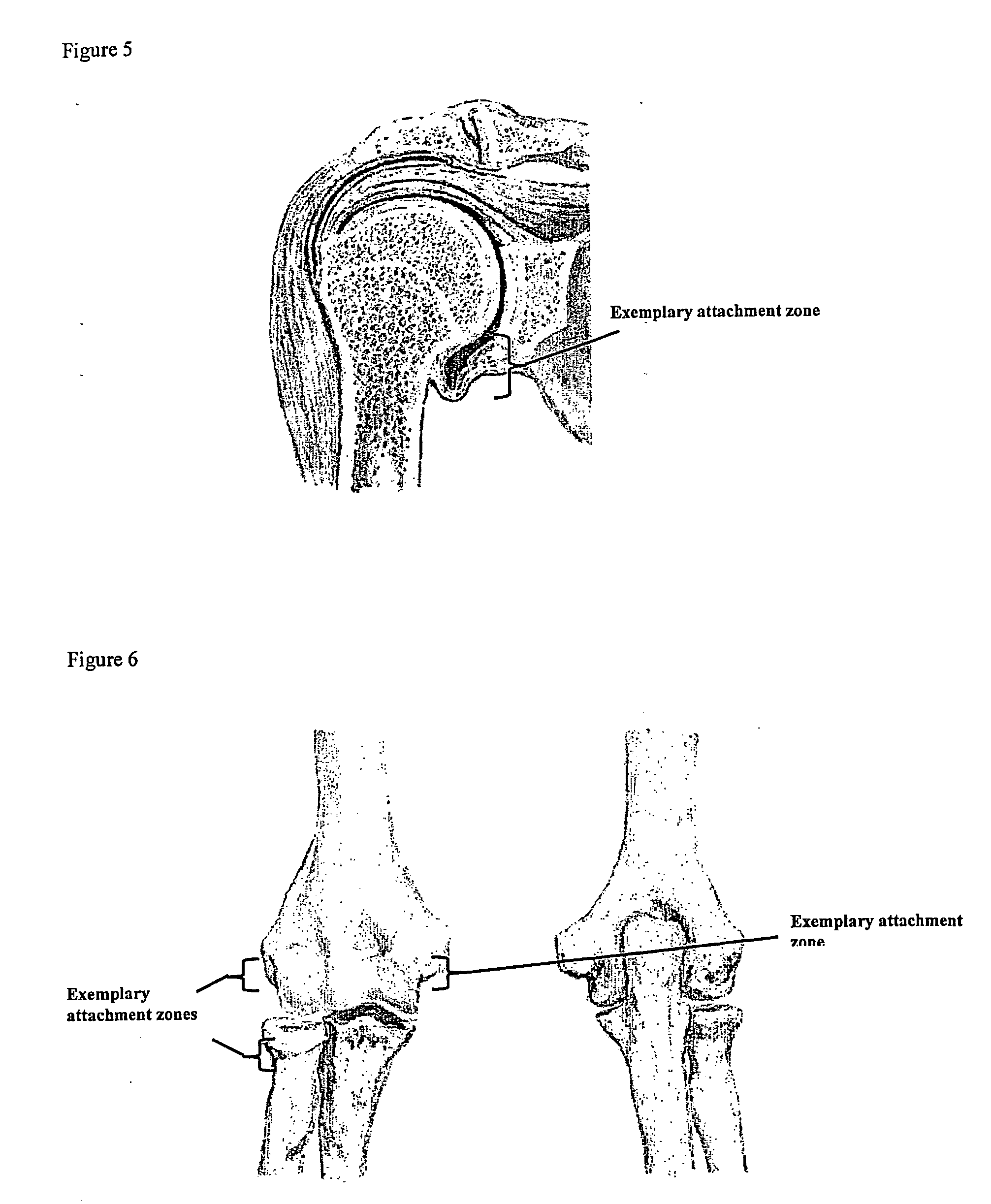
Drug delivery to a joint
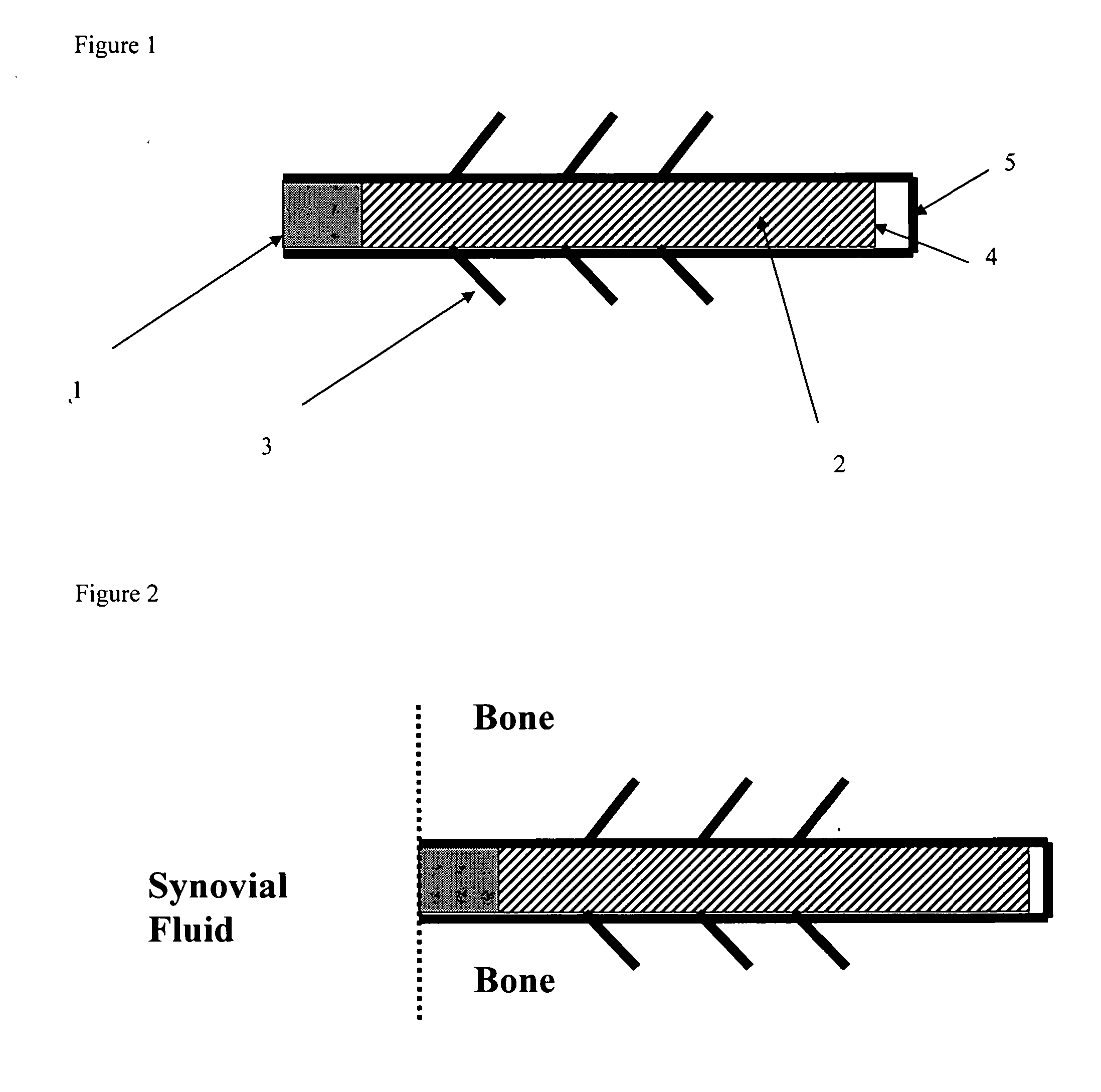
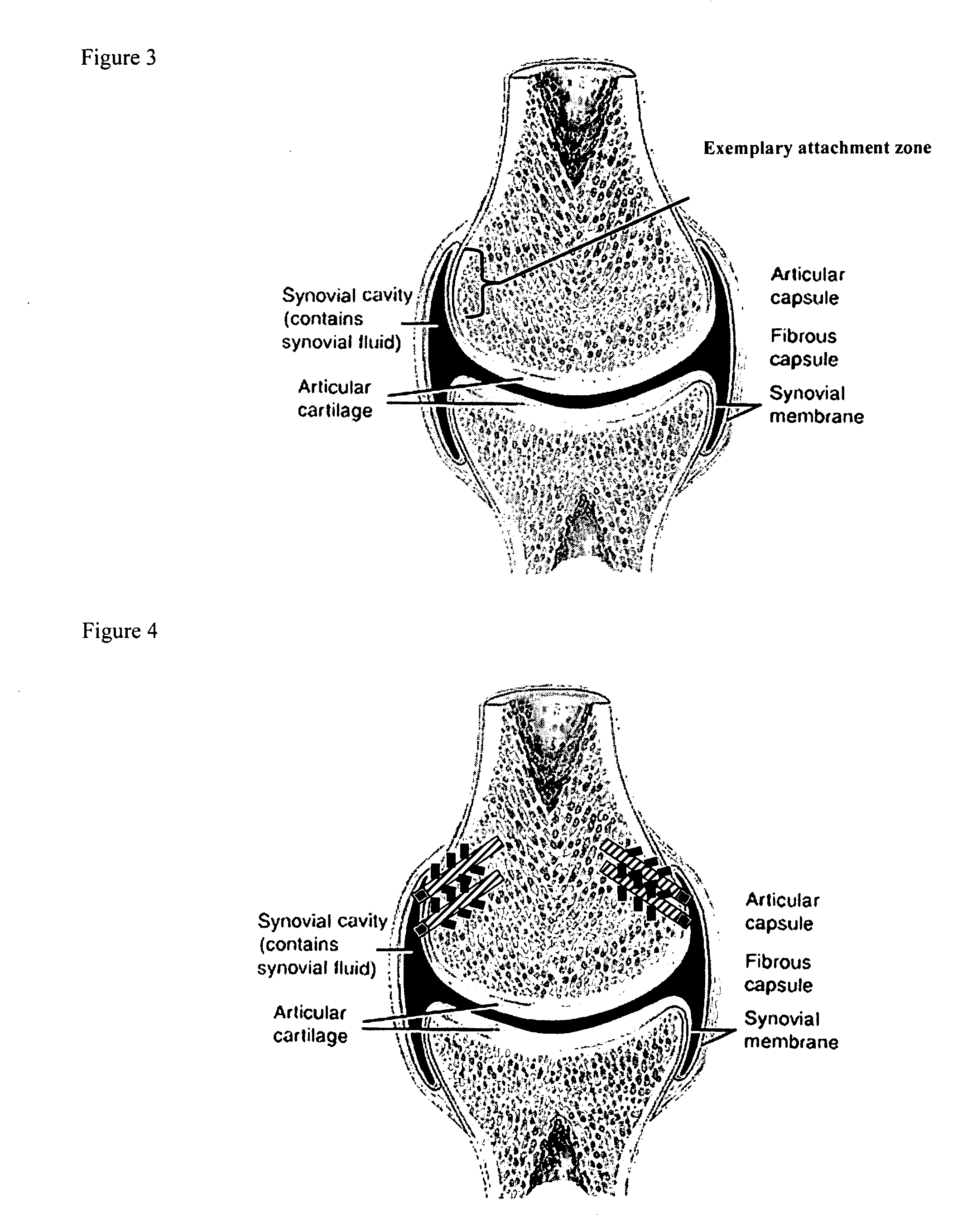
A method of intra-articular drug delivery may include selecting an attachment zone in a synovial joint; affixing a drug release device in the attachment zone, the drug release device comprising a base affixable in the attachment zone, a sustained-release drug carrier, and a drug, the device positioned so that the device releases the drug into the synovial fluid of the synovial joint, and so that agitation of the synovial fluid facilitates elution of the drug from the drug release device.
Owner:NEW YORK SOC FOR THE RUPTURED & CRIPPLED MAINTAINING THE HOSPITAL FOR SPECIAL SURGERY
Sustained release matrix for high-dose insoluble drugs
Sustained release dosage forms of high dose insoluble drugs such as ibuprofen and methods for their manufacture are disclosed.
Owner:PENWEST PHARMA CO
Stent coated with a sustained-release drug delivery and method for use thereof
ActiveUS7279175B2Reduce deliveryReduce solubilitySuture equipmentsAntibacterial agentsSustained release drugDrug compound
An intraluminal medical device comprises a stent having a coating applied to at least part of an interior surface, an exterior surface, or both. The coating comprises a sustained release formulation of a combination of pharmaceutical compounds dispersed within a biologically tolerated polymer composition. The choice of the combination of pharmaceutical compounds are intended to reduce neointimal hyperplasia restenosis.
Owner:PSIVIDA US INC
Conveniently implantable sustained release drug compositions
ActiveUS7906136B2Easily manipulated and injected and implantedEfficient deliveryOrganic active ingredientsBiocideSustained release drugWhole body
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Zero-order sustained release dosage forms and method of making same
InactiveUS20030133982A1High drug loadingReduce releasePowder deliveryBiocideSustained release drugHydrophobic polymer
The present invention relates to zero-order sustained release solid dosage forms suitable for administration of a wide range of therapeutically active medicaments, especially those that are water-soluble, and to a process of making same. The solid dosage form comprises (a) a matrix core comprising ethylcellulose and the active agent and (b) a hydrophobic polymer coating encasing the entire matrix core.
Owner:PHARMACIA CORP
Sustained Release Dosage Forms For Delivery of Agents to an Oral Cavity of a User
Aspects of the invention include a sustained release dosage form that can be administered to an oral cavity, e.g., the mouth. In certain embodiments, the sustained release dosage form is formulated as a lozenge or gum that may be administered to an oral cavity of a user for the purpose of dissolving over a prolonged period of time and thereby delivering an essential oil component therein. In certain embodiments, the sustained release dosage form includes a beneficial agent and, therefore, not only provides for the prolonged delivery of an essential oil component to an oral cavity, but also provides for the sustained release of a beneficial agent thereto. In certain embodiments, the sustained release dosage form includes a biocompatible, water-insoluble polymer, e.g., ethylcellulose and an essential oil component, which are combined in such a manner so as to produce a dosage form that substantially dissolves over a prolonged period of time when positioned within an aqueous environment, such as an oral cavity of a user. In certain embodiments, the sustained release dosage form may include an additional water soluble agent, such as gum arabic, which may be included so as to further provide the dosage form with a desired dissolution characteristic. In certain embodiments, the dosage form may also include a beneficial agent to be delivered to the mouth. Methods of formulating such dosage forms and administering them to an oral cavity for the treatment of an adverse condition are also provided herein.
Owner:BENNES
Novel pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS20080058362A1Reduced plasma concentrationEffective pain managementBiocideAmide active ingredientsDextrorphanSustained release drug
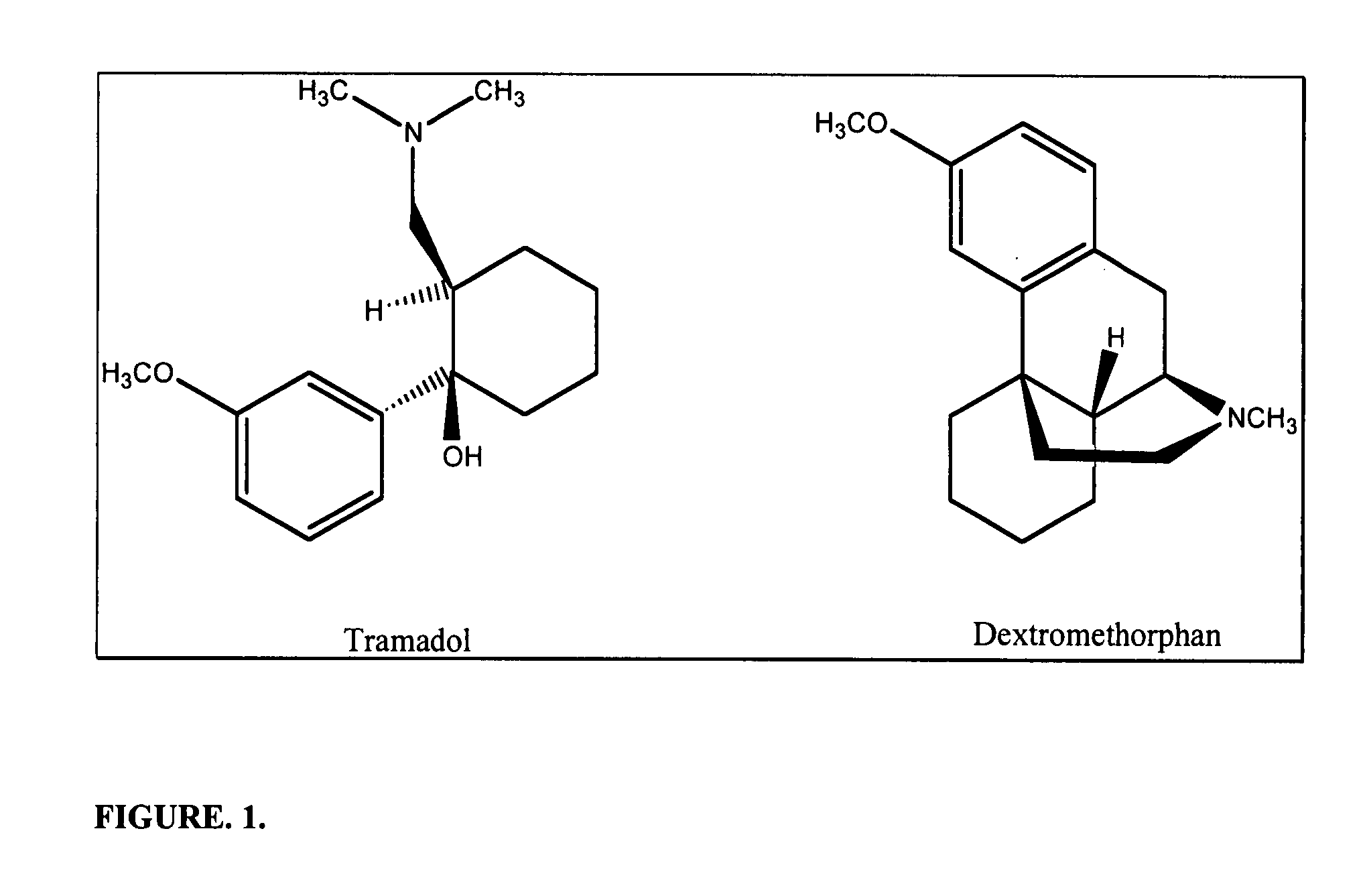
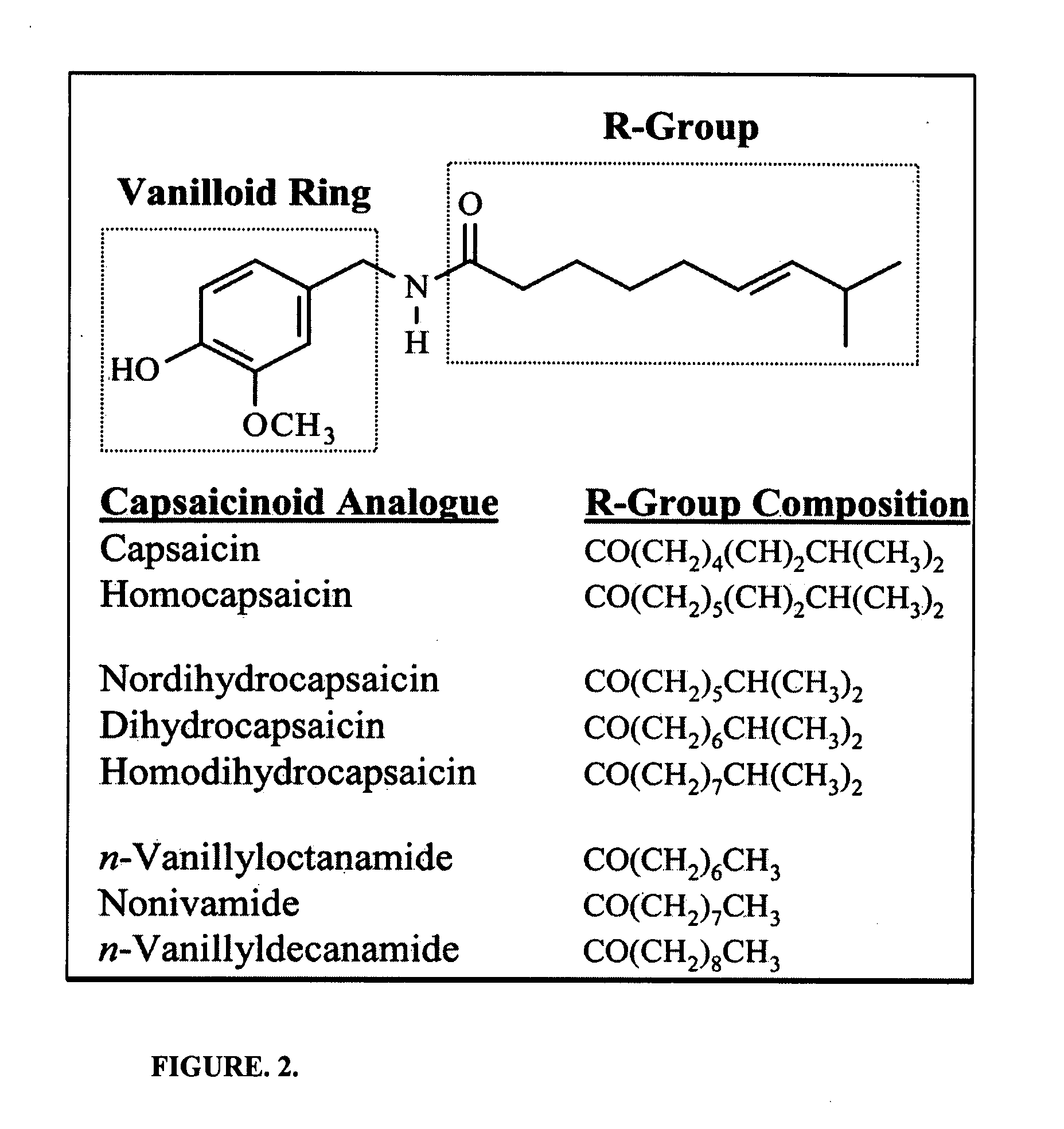
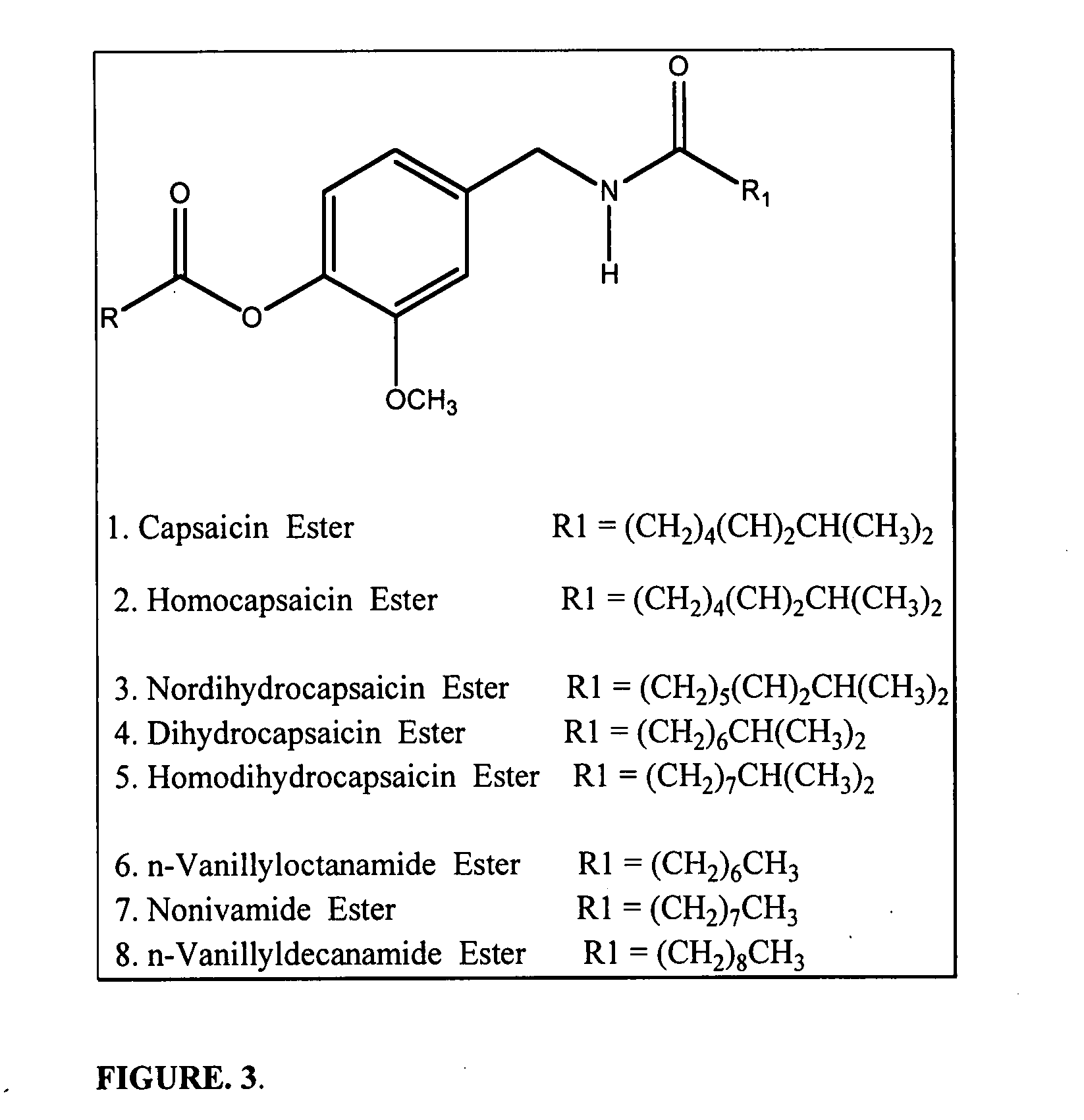
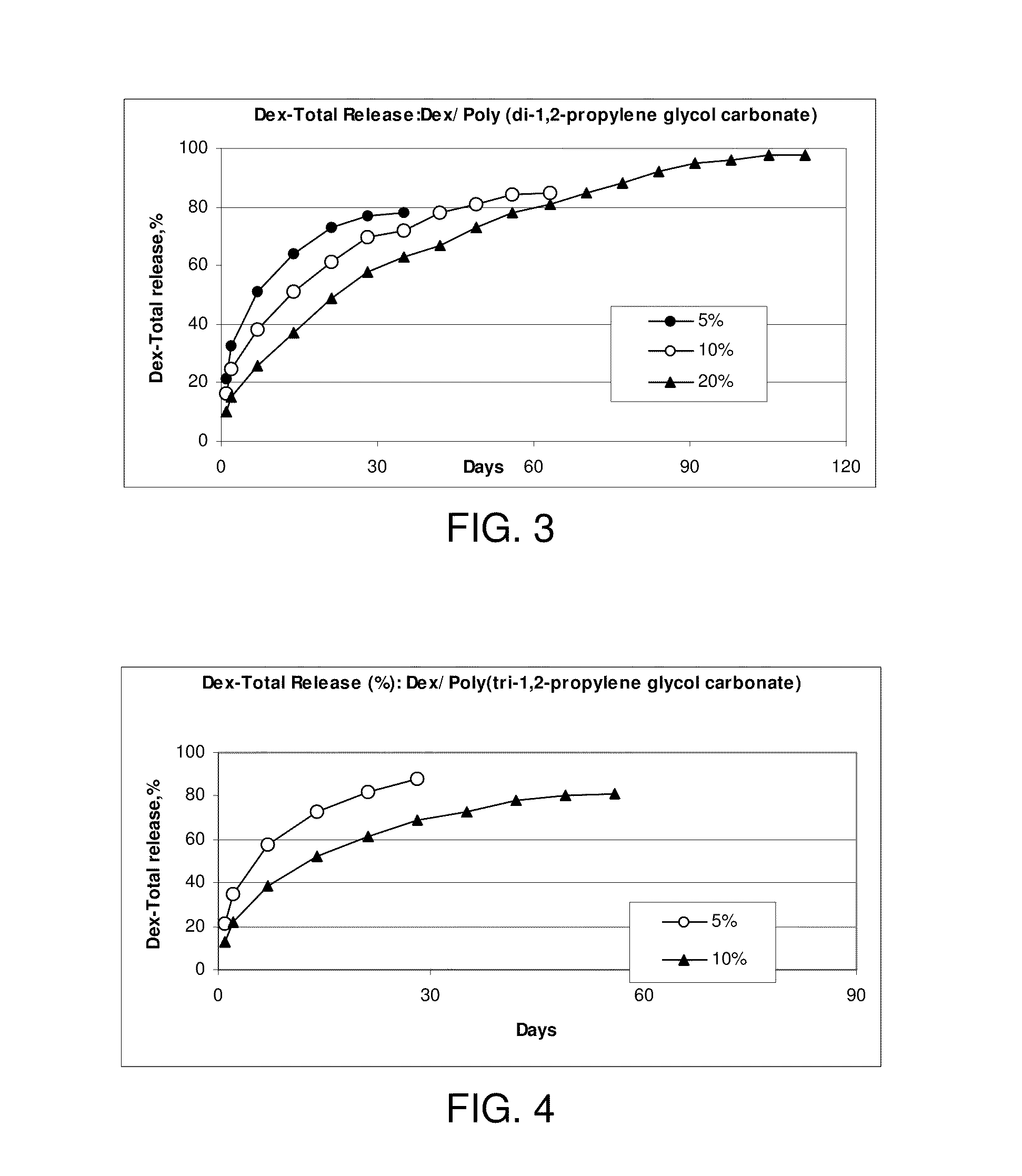
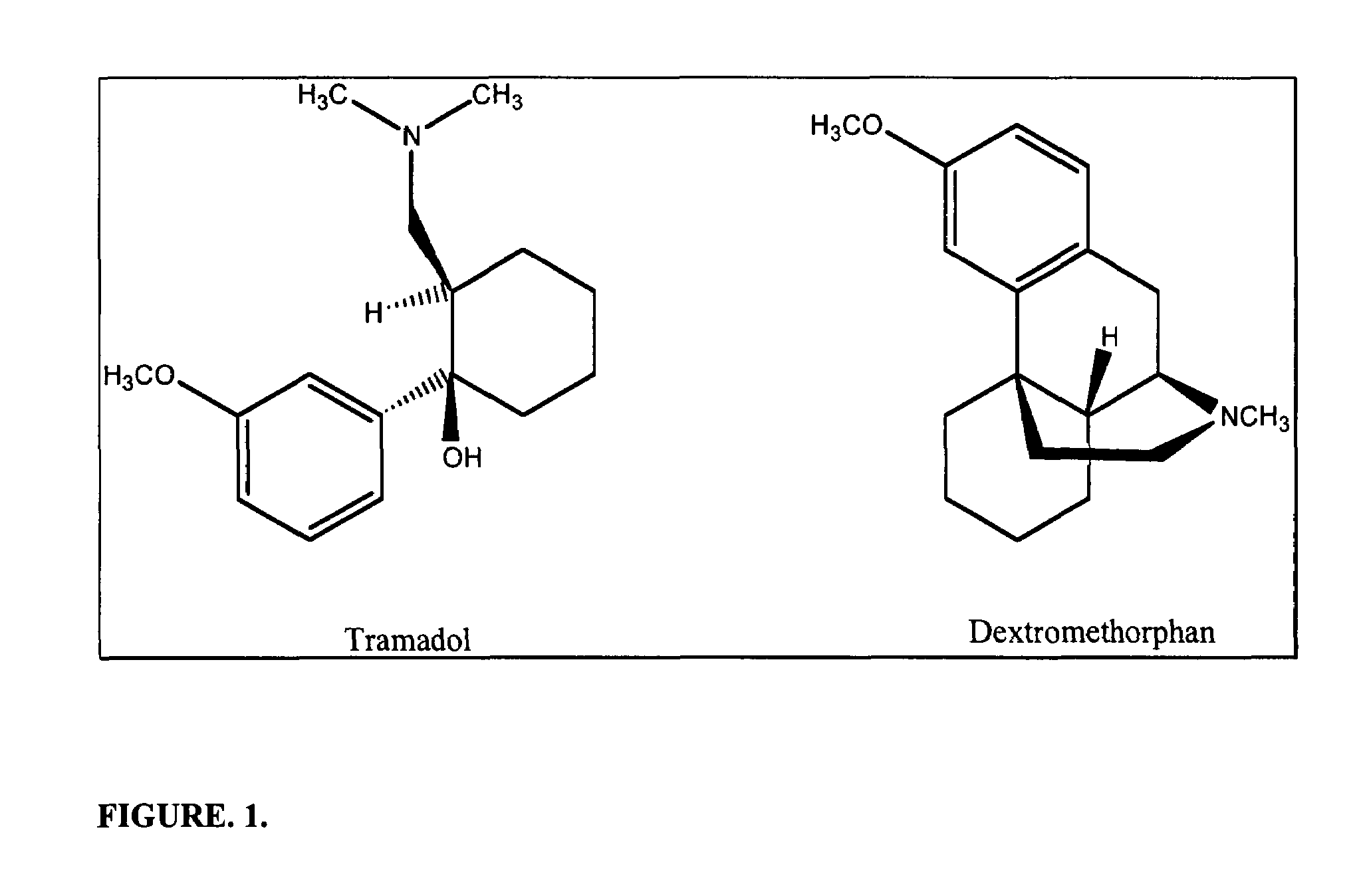
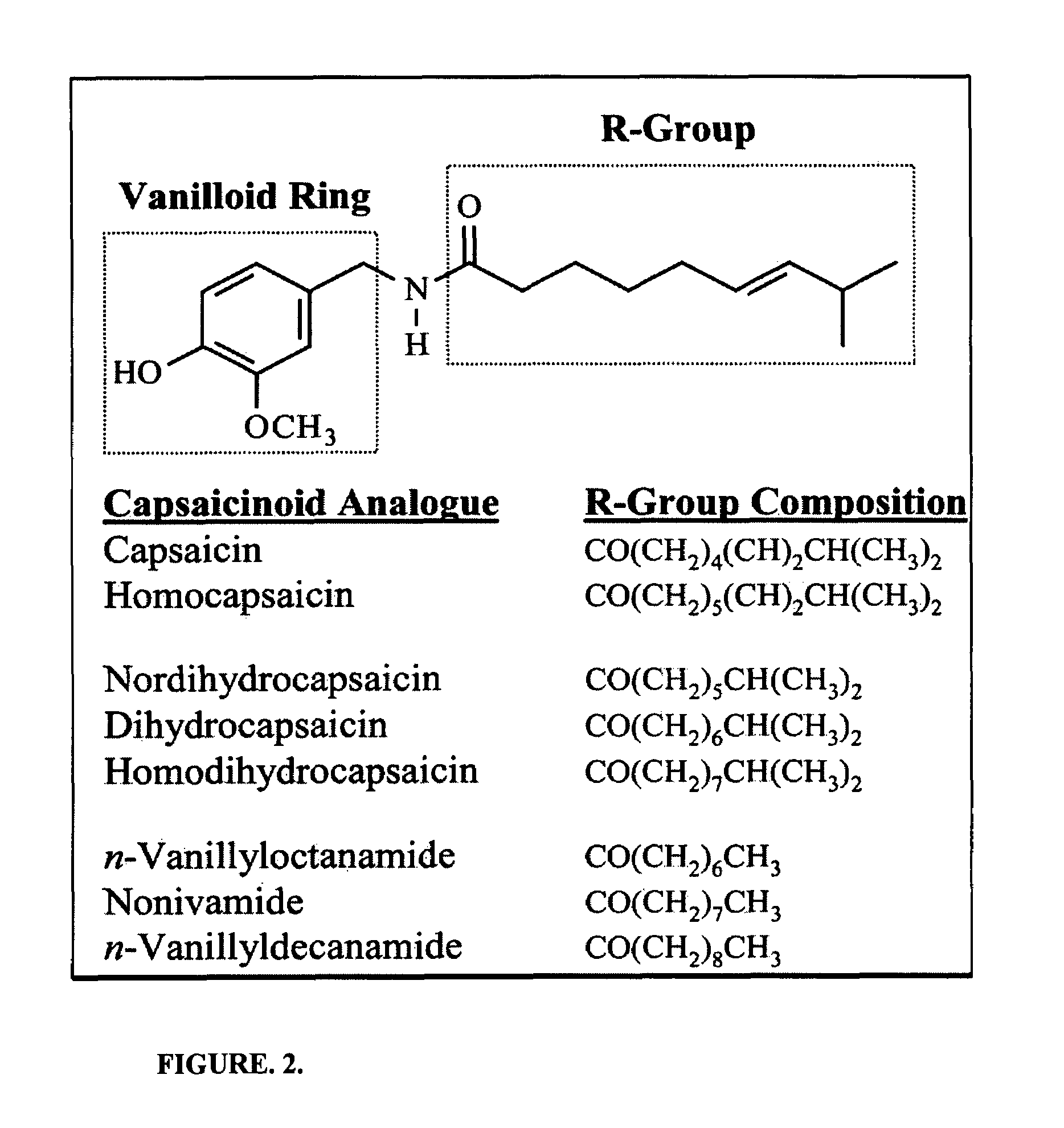
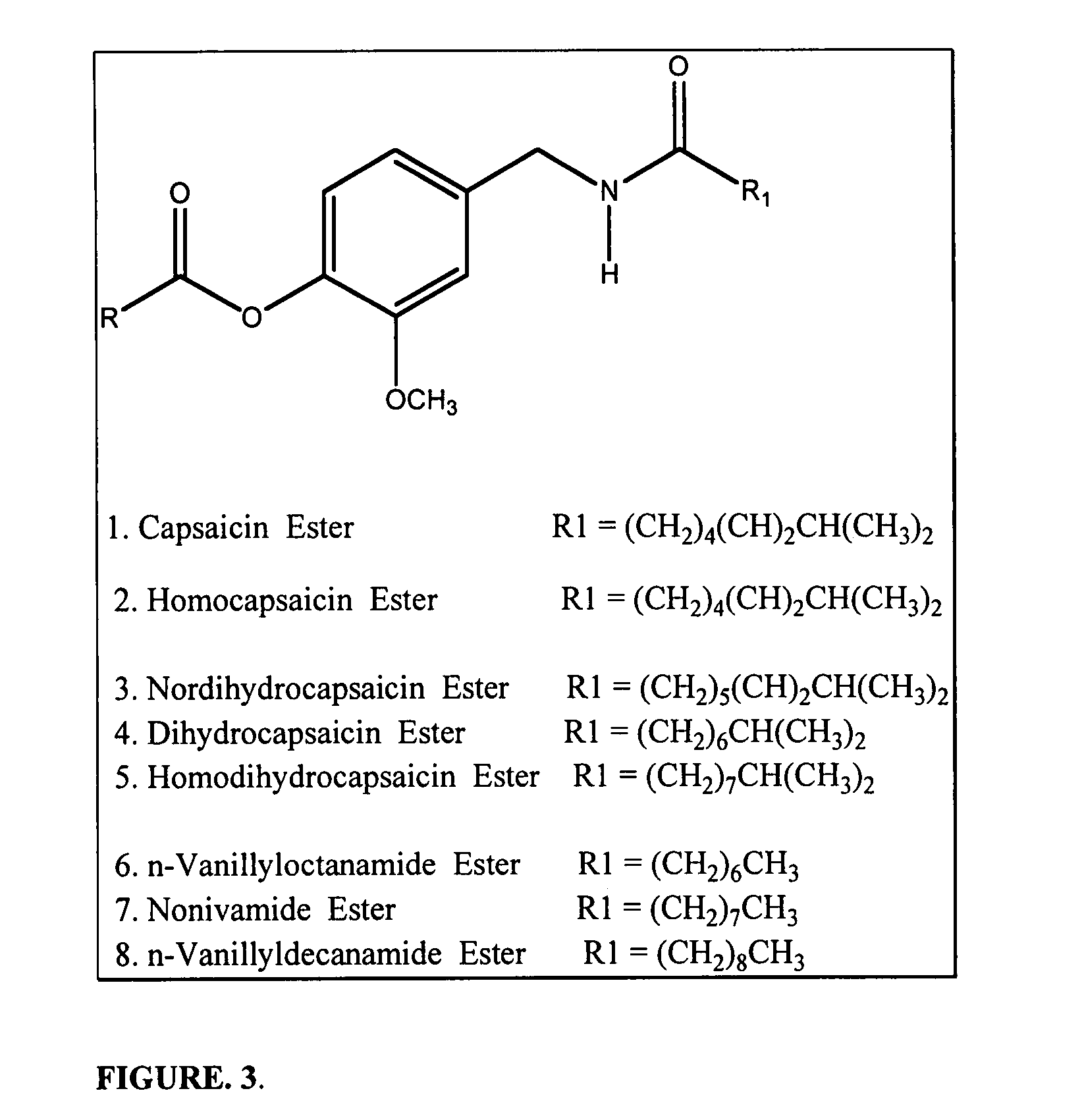
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
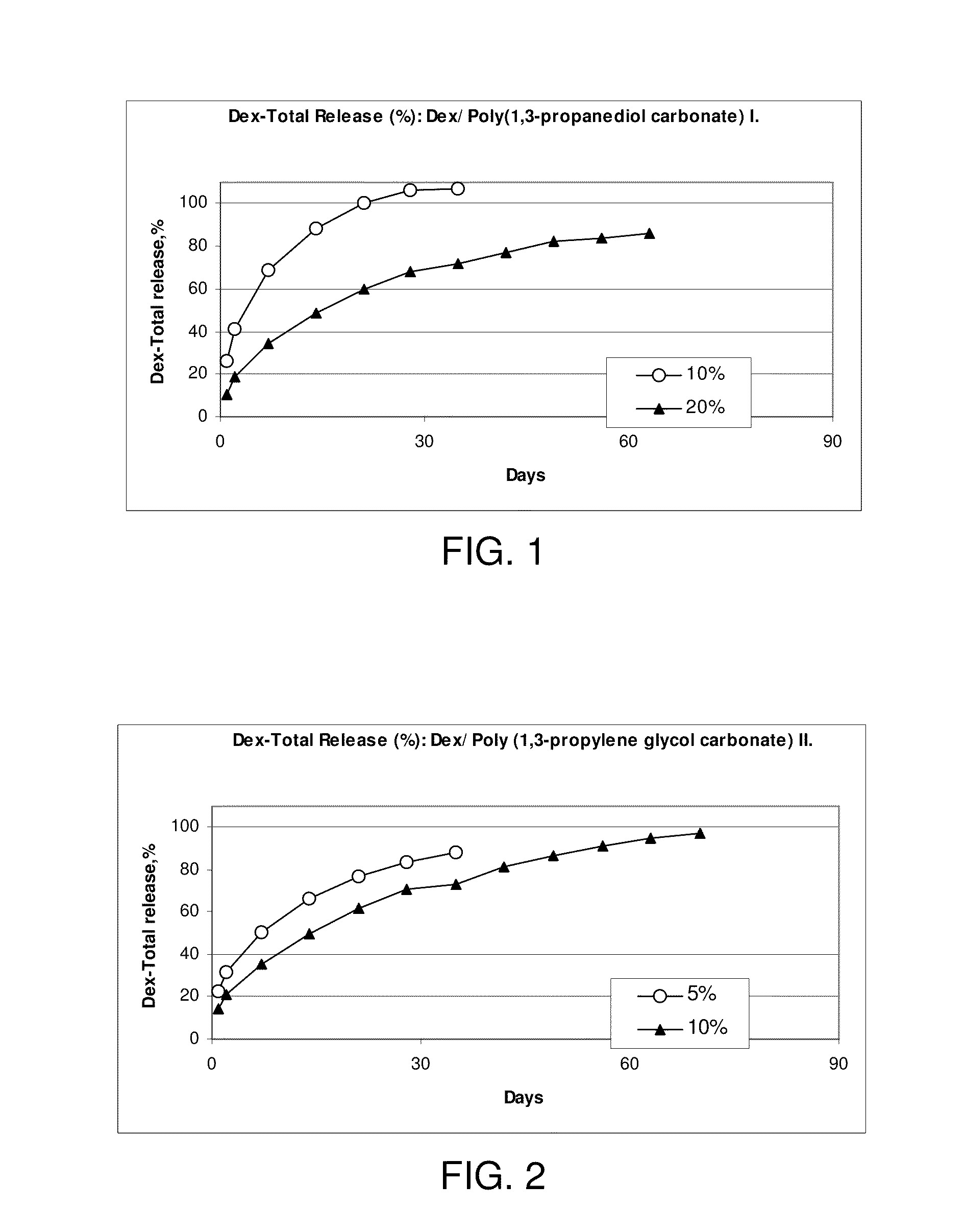
Sustained-release dosage forms of ruxolitinib
The present invention relates to sustained-release formulations and dosage forms of ruxolitinib, or a pharmaceutically acceptable salt thereof, which are useful in the treatment of Janus kinase-associated diseases such as myeloproliferative disorders.
Owner:INCYTE HLDG & INCYTE
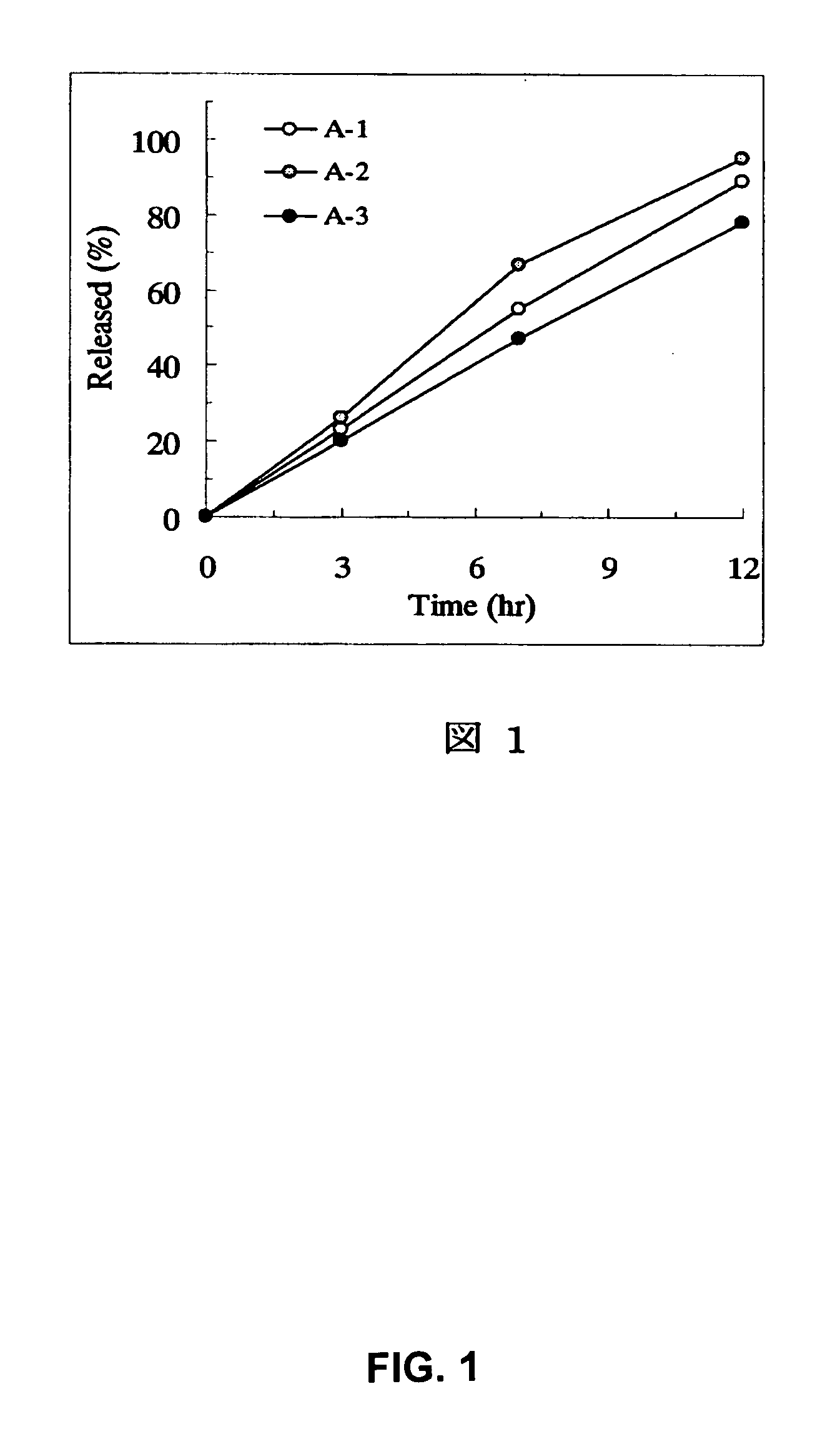
Sustained release preparations
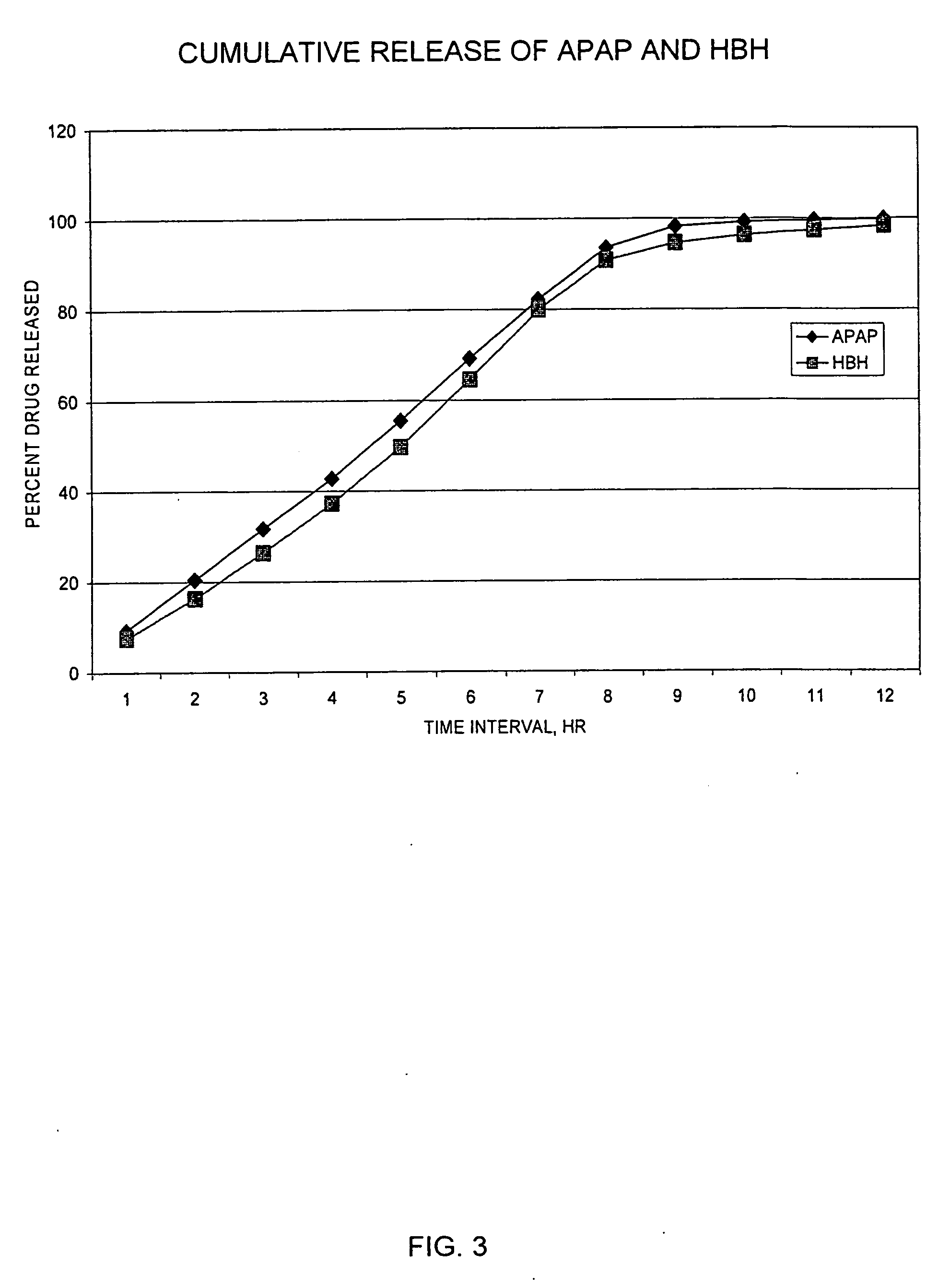
Disclosed are sustained release drug particles suitable for forming sustained release oral pharmaceutical compositions. The sustained release drug particles comprise a drug-ion exchange resin complex and a water-permeable, diffusion barrier surrounding at least a portion of the drug-ion exchange resin complex. The diffusion barrier comprises a film-forming polymer and is free or contains no substantial traces of organic solvent. Also disclosed are oral pharmaceutical compositions, for example, oral suspensions, comprising the sustained release drug particles, a method for the controlled administration of a drug to a patient, and a method for manufacturing the sustained release drug particles. The method of manufacturing involves the use of an aqueous coating composition comprising a water-permeable film-forming polymer such as ethylcellulose.
Owner:MALLINCKRODT INC
Sustained-release drug delivery compositions and methods
InactiveUS20100092562A1Improve stabilityReduce molecular weightPowder deliveryOrganic active ingredientsImmediate releaseDecongestant
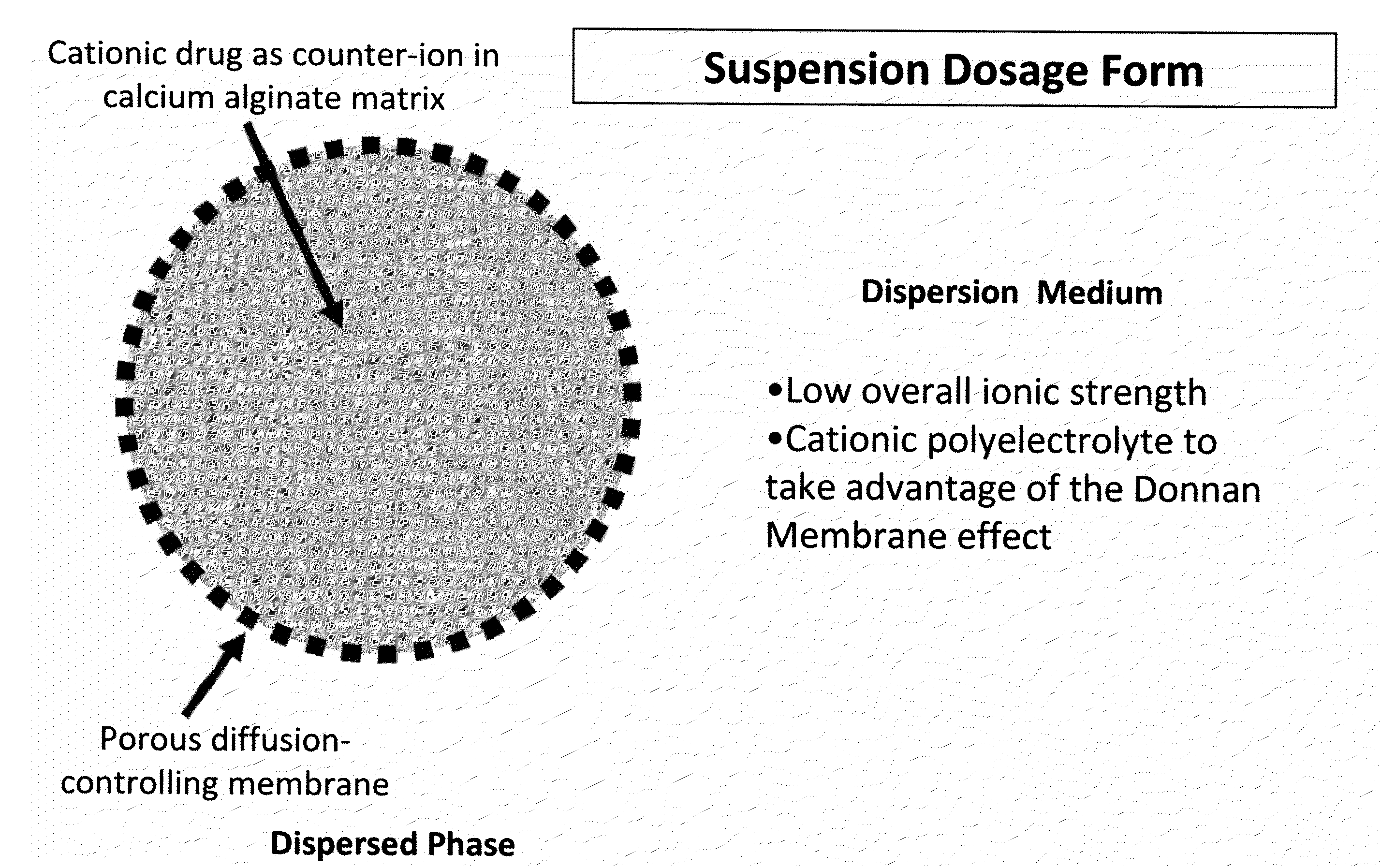
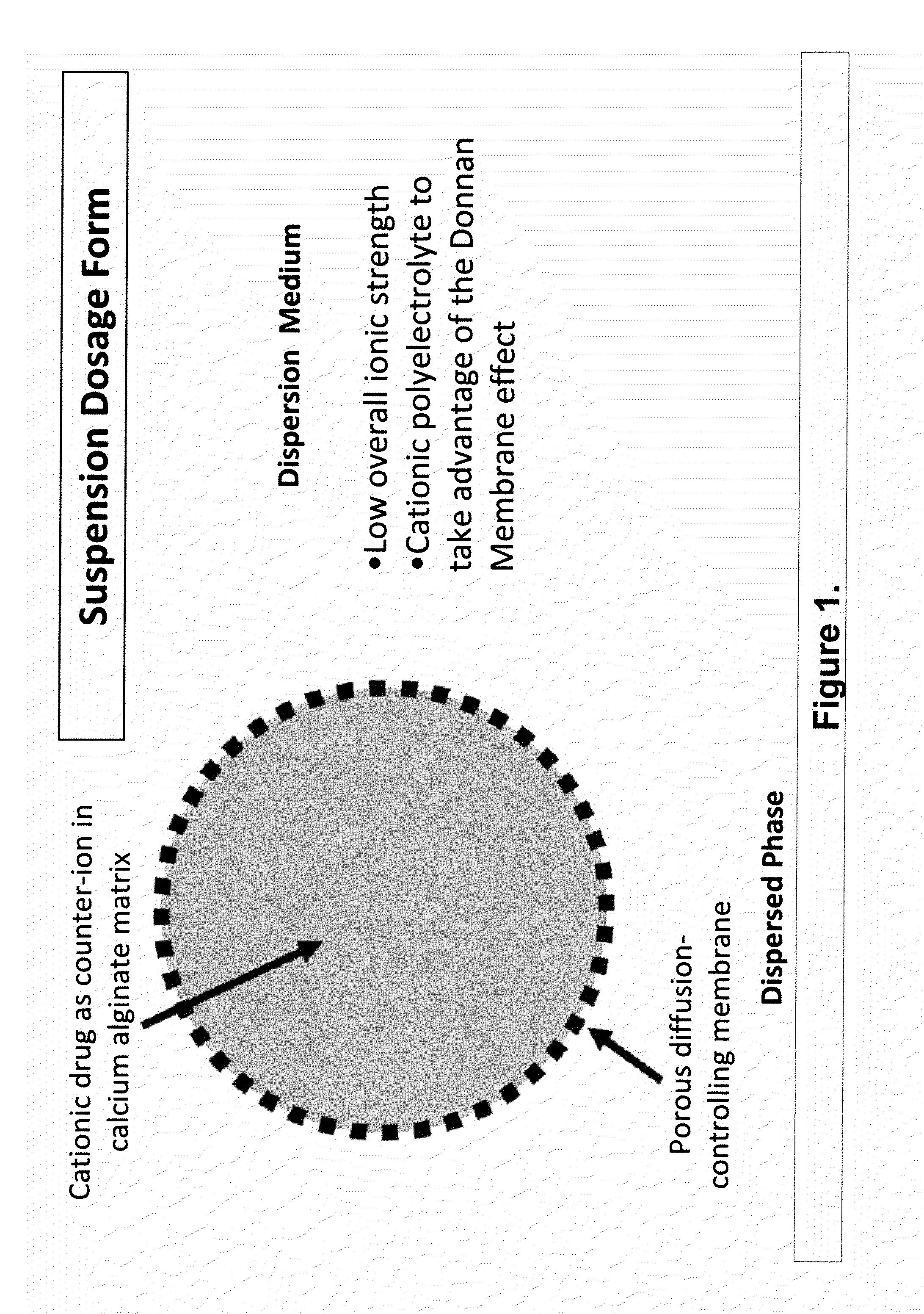
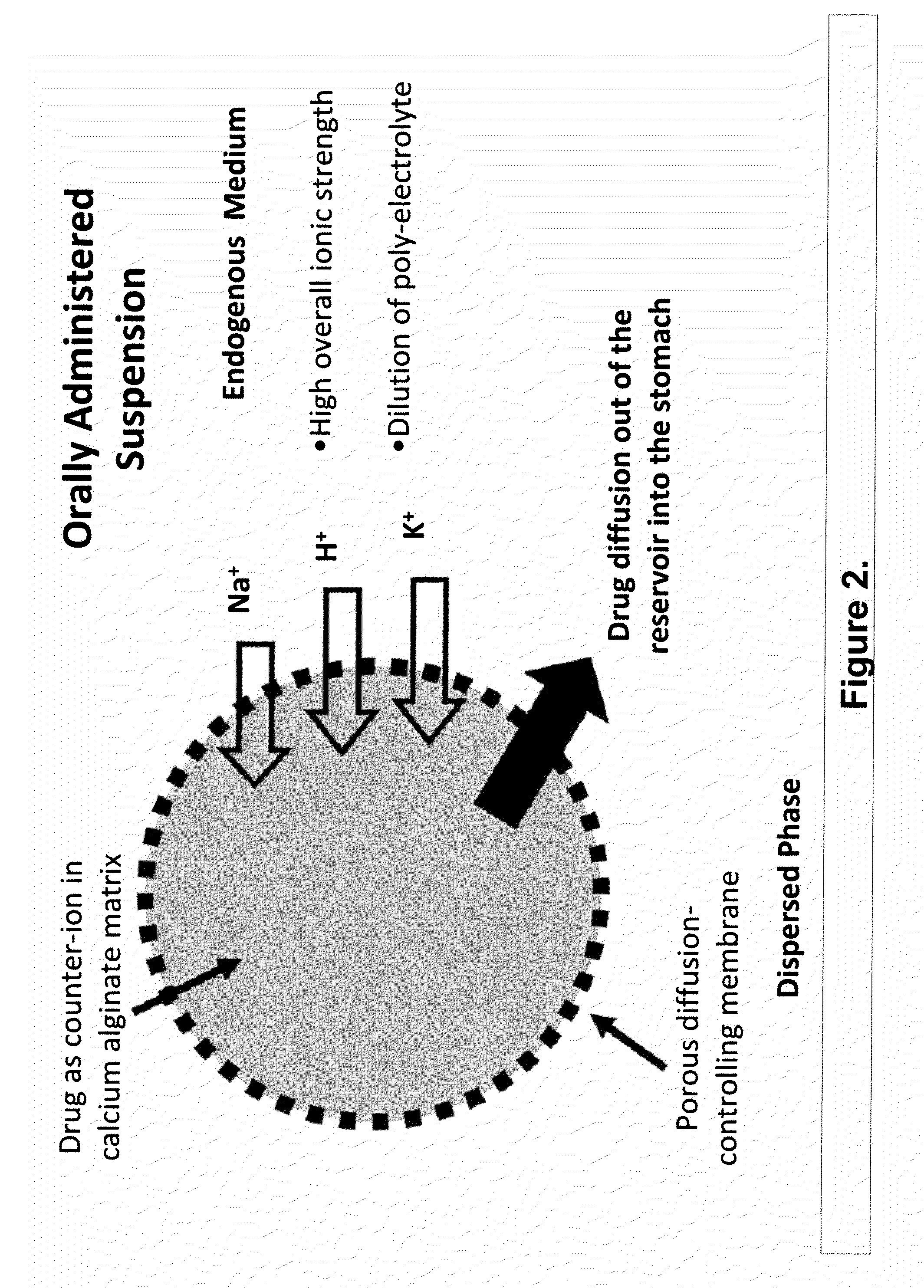
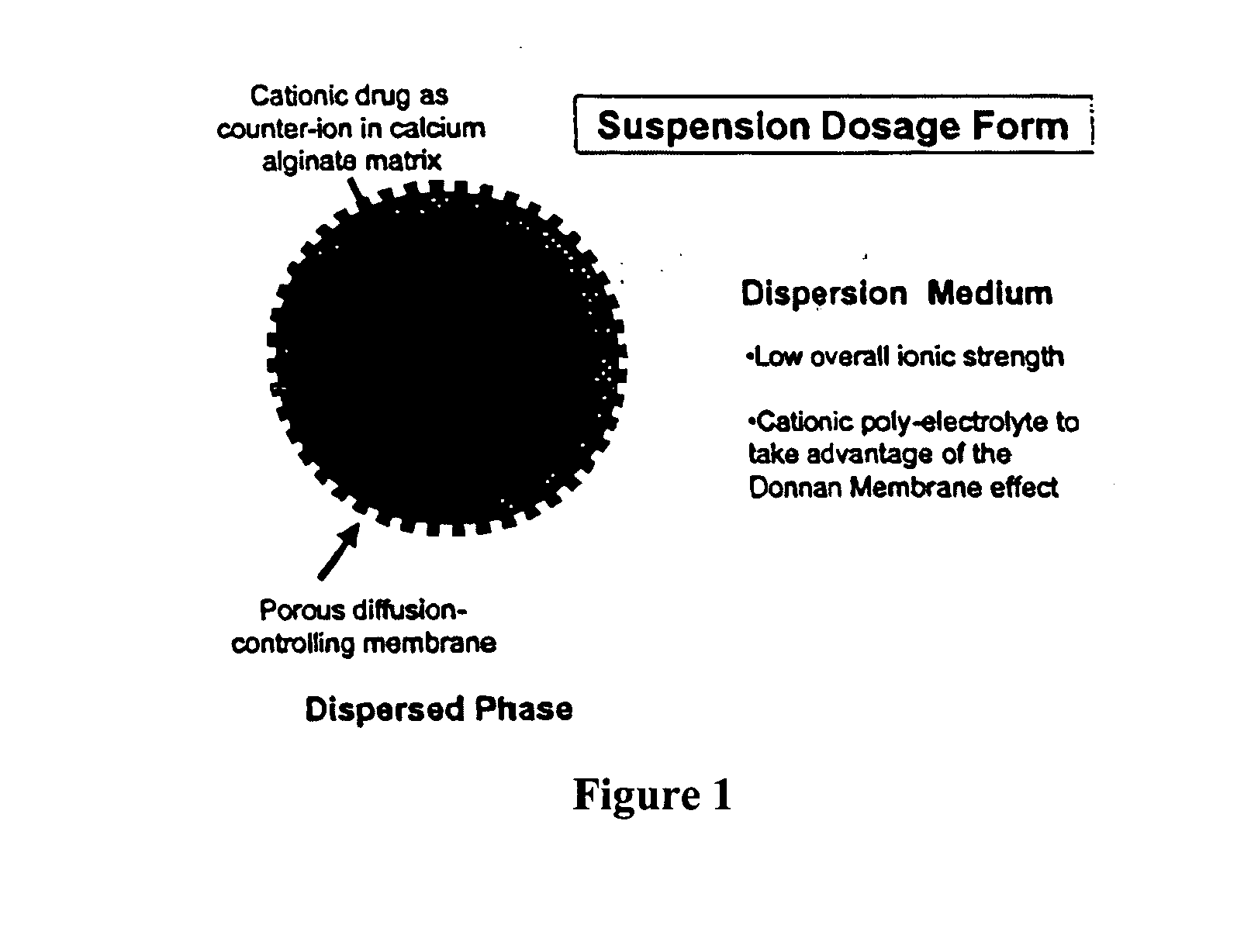
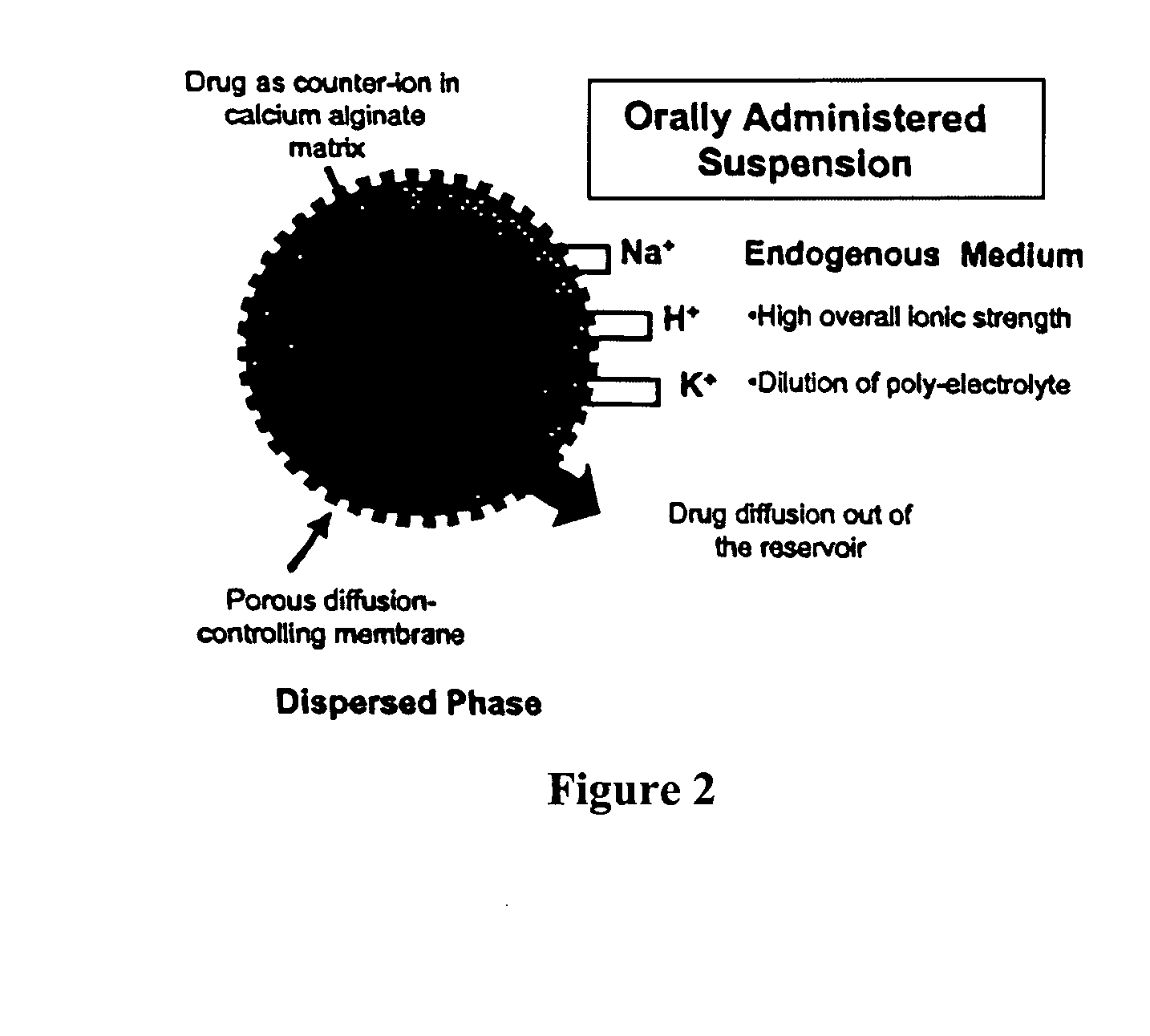
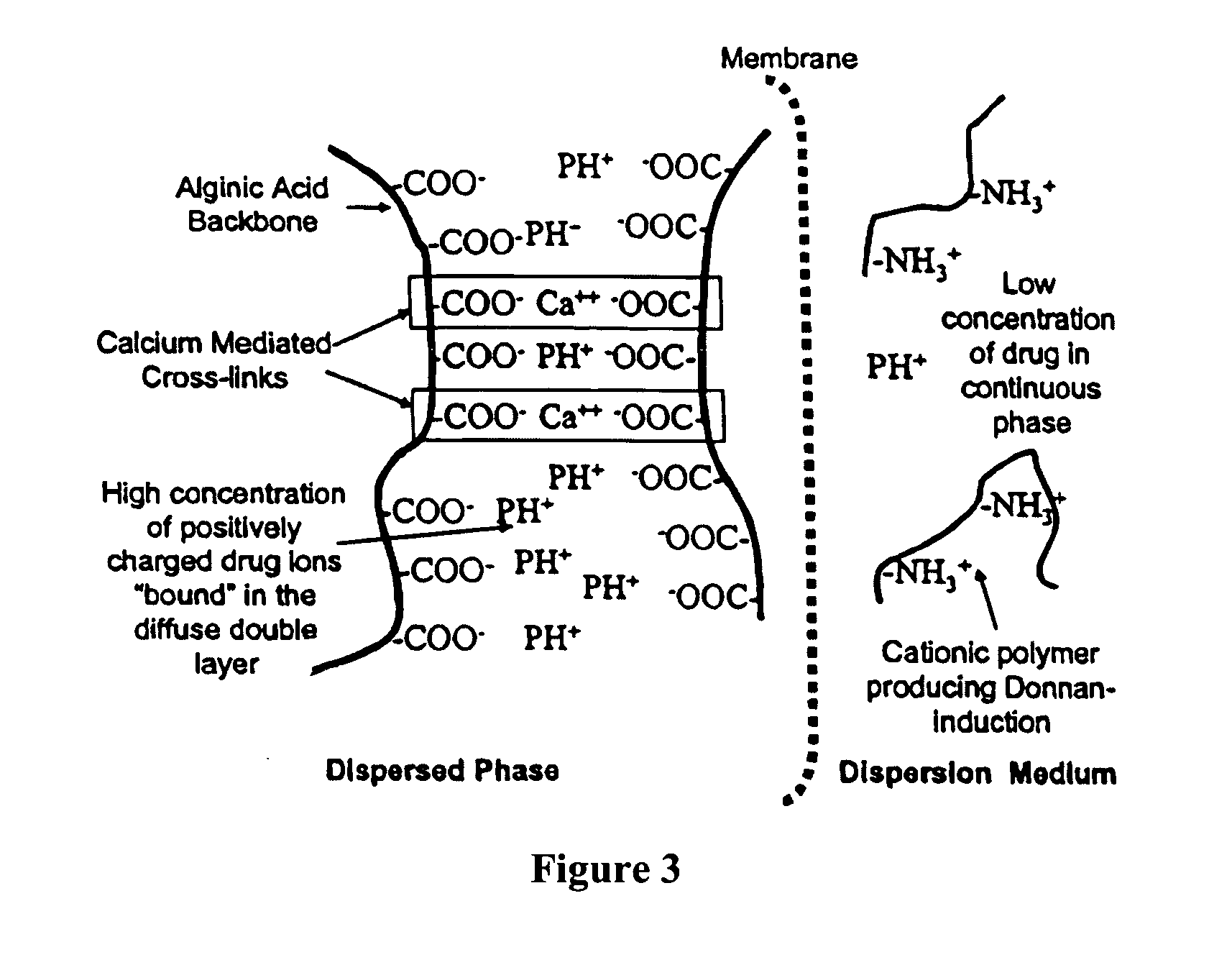
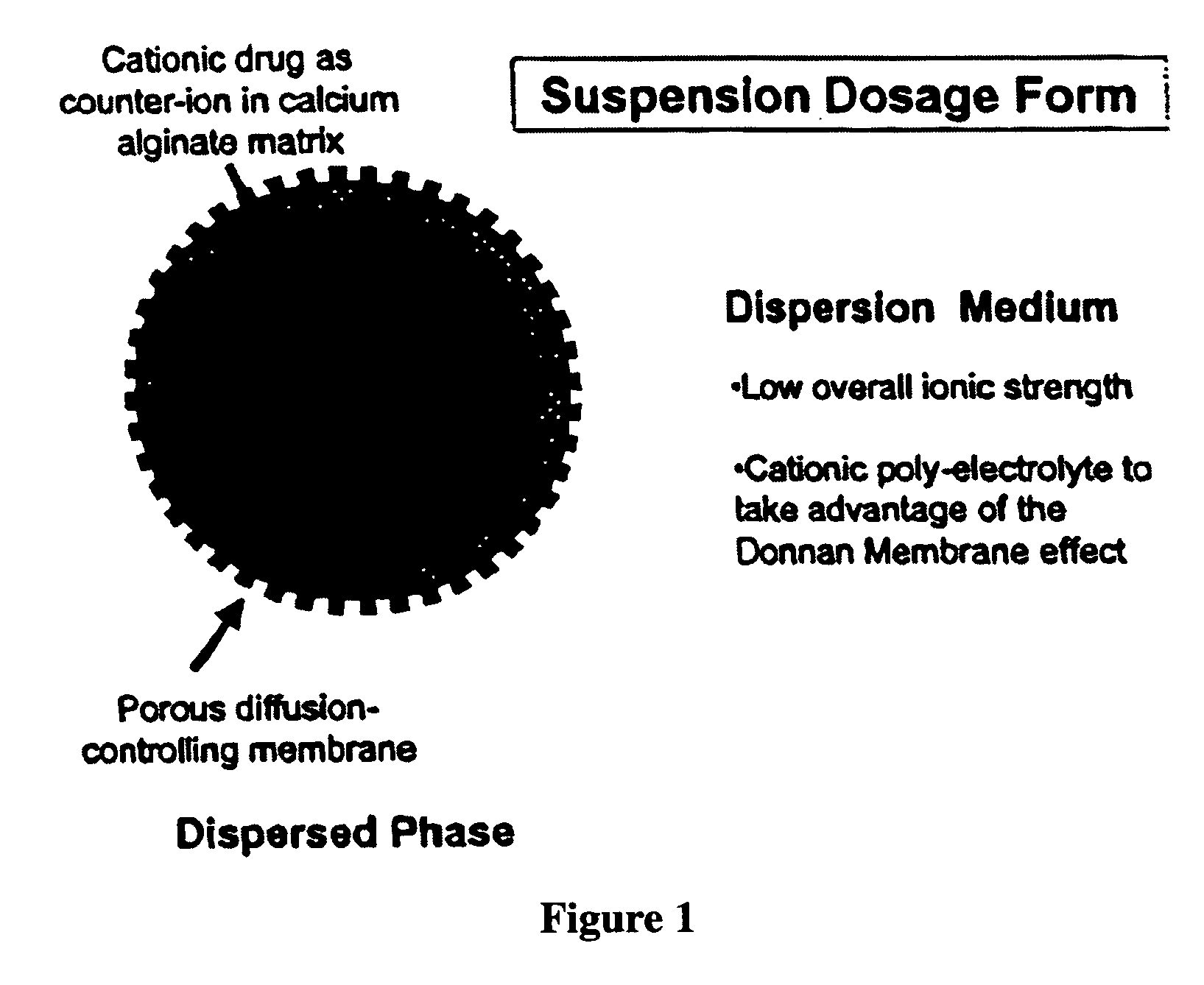
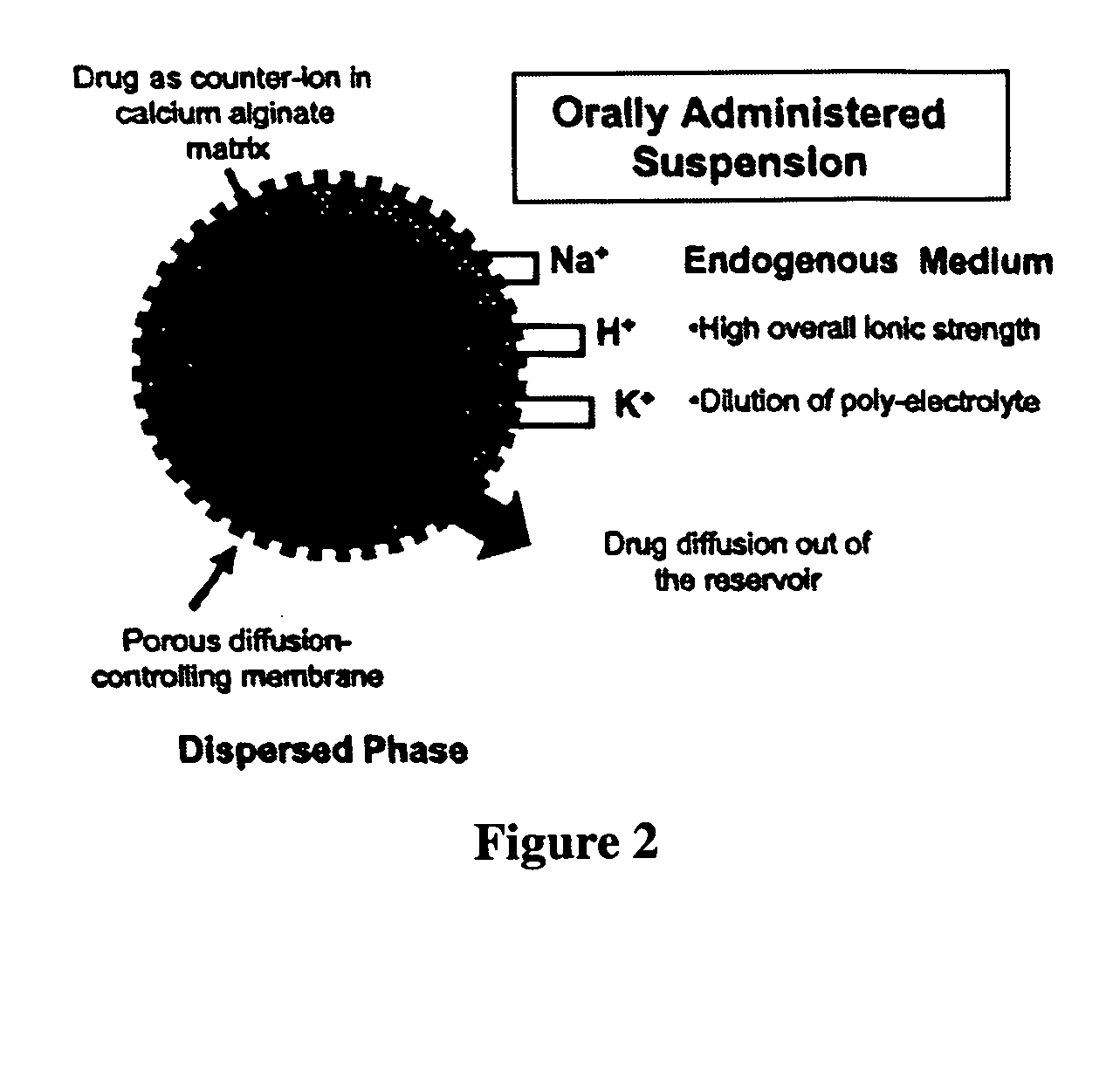
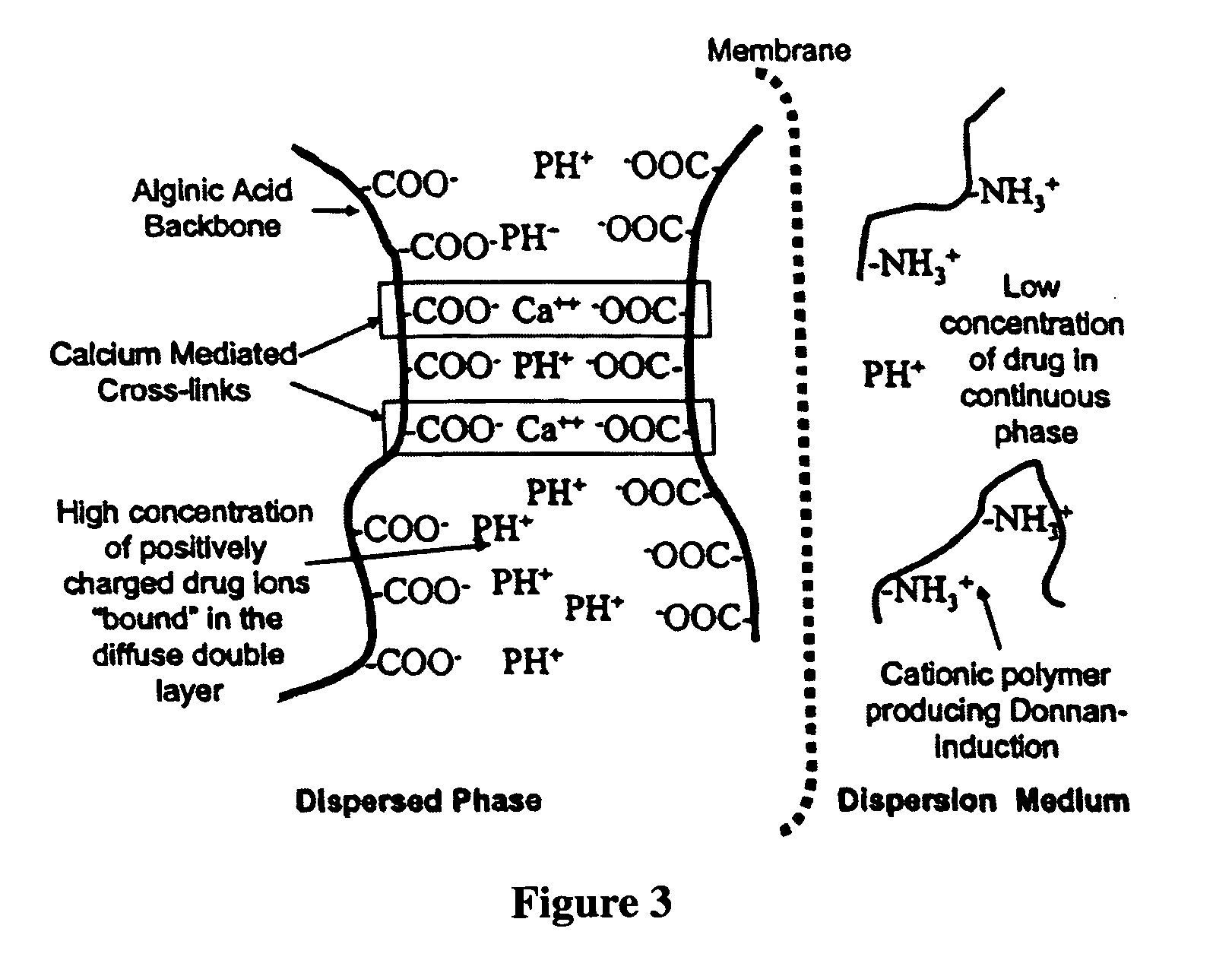
The present invention relates to liquid sustained release suspension dosage forms. In particular, the invention encompasses sustained release compositions comprising a dispersed phase, which contains an ion-exchange matrix drug complex, a diffusion controlling membrane coating and a dispersion medium comprising an excipient capable of impeding water activity such that drug dissolution is inhibited prior to administration. Further, the invention provides for compositions wherein several active ingredients associate in a single bead in the dispersed phase, such that the abuse potential of such active ingredients is reduced. The invention also encompasses sustained release formulations of combination drugs comprising an extended release phase and an immediate release phase. The formulations of the invention may be used to treat a variety of conditions and symptoms, including those that require administration of several drugs, such as cold and allergy symptoms. In one of the embodiments, the sustained release composition combines an antihistamine, an antitussive and a decongestant. The invention further provides for methods of making and using such formulations.
Owner:UPM PHARMA
Conveniently implantable sustained release drug compositions
ActiveUS20110111006A1Easily manipulated and injected and implantedEfficient deliveryBiocideSenses disorderSustained release drugWhole body
This invention provides for biocompatible and biodegradable syringeable liquid, implantable solid, and injectable gel pharmaceutical formulations useful for the treatment of systemic and local disease states.
Owner:RAMSCOR
Pharmaceutical compositions for treating chronic pain and pain associated with neuropathy
InactiveUS7645767B2Reduce concentrationEfficient managementBiocideAmide active ingredientsOpiatePharmaceutical medicine
Chronic pain is alleviated in a mammal suffering there from by administering to the mammal a chronic pain alleviating amount of a nontoxic N-methyl-D-aspartate receptor antagonist such as dextromethorphan, dextrorphan, ketamine or pharmaceutically acceptable salt thereof, in combination with a μ-opiate analgesic such as tramadol or an analogously acting molecular entity, and a capsaicin or an ester of capsaicin, and optionally in sustained release dosage form.
Owner:TRINITY LAB INC
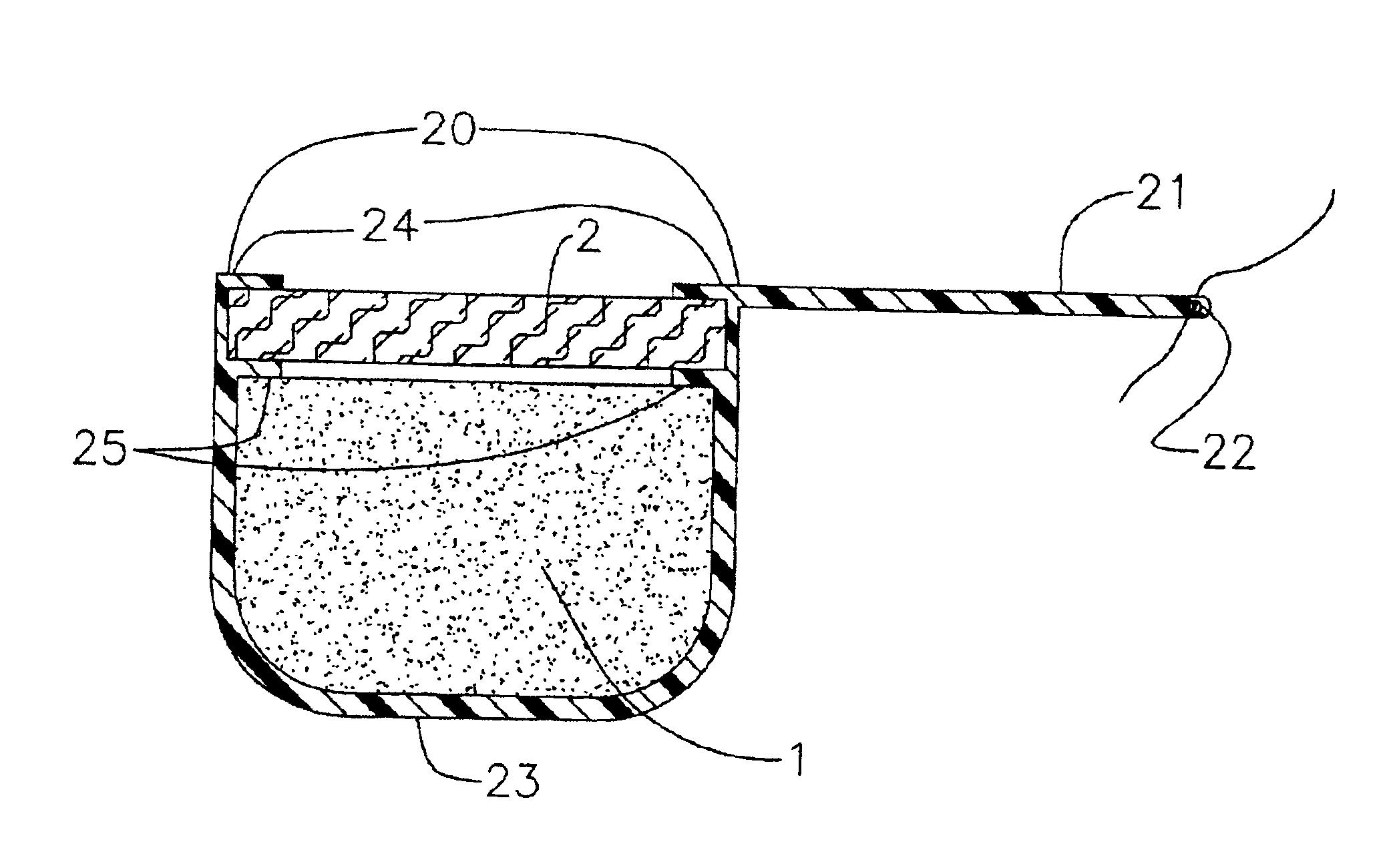
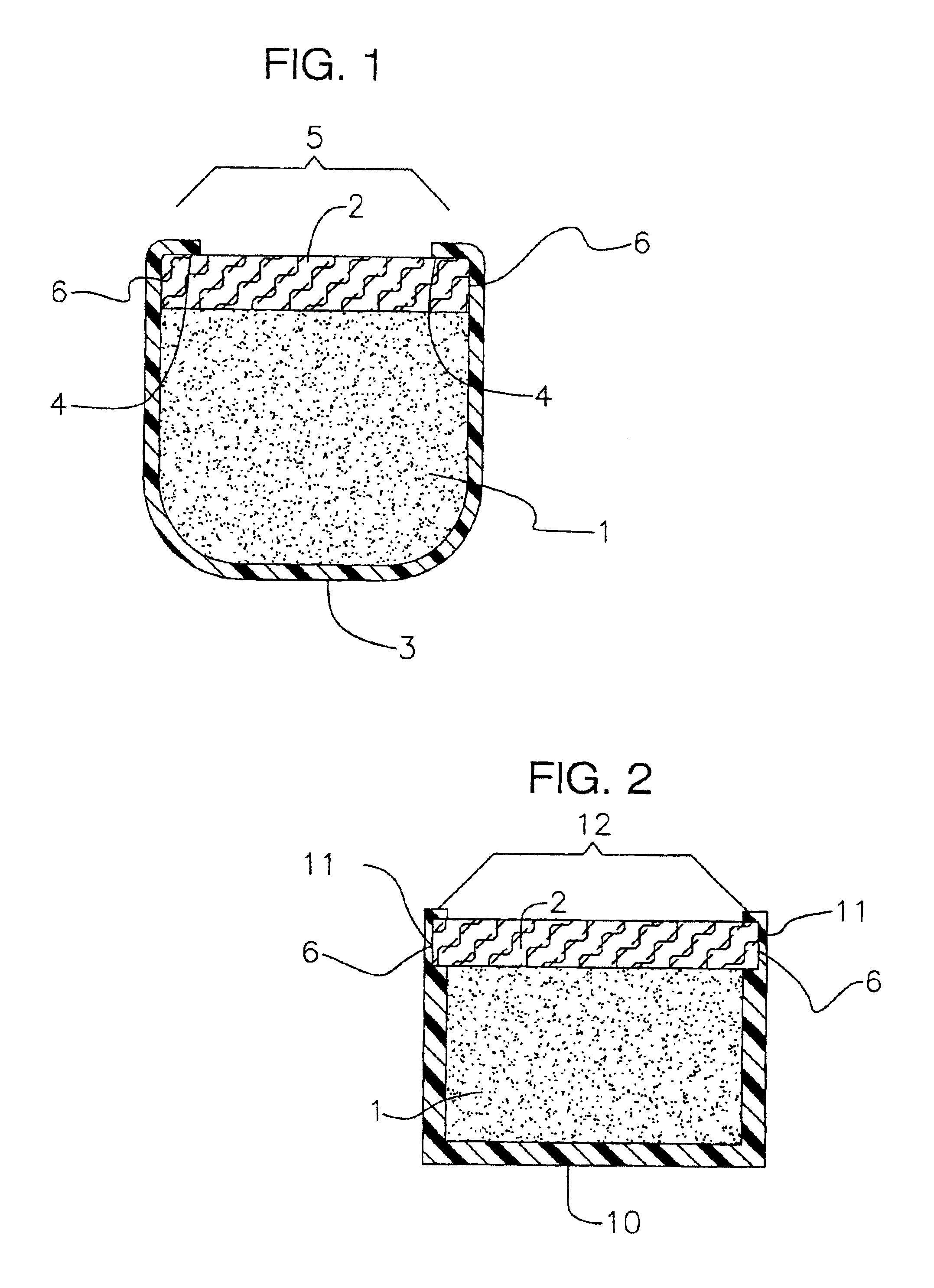
Process for the production of sustained release drug delivery devices
InactiveUS6991808B2Efficiently obtainedEye surgeryPharmaceutical containersSustained release drugMedicine
Disclosed is an improved sustained release drug delivery device and method of producing such device. The device comprises a drug core in an impermeable cup or impermeable coating layer that is adhered to an uncured suture tab and covered with a permeable polymer coating layer that is similar to the makeup of the suture tab. The permeable polymer coating layer that covers the device, covering the impermeable coating layer and at least a portion of the drug core, is cured (after drying) along with the uncured suture tab. The “cocuring” or one step curing process forms a very strong bond between the outer coating layer to the suture tab preventing leaks.
Owner:BAUSCH & LOMB INC
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20050013792A1Change in permeabilityPowder deliveryNervous disorderPolyelectrolyteSustained release drug
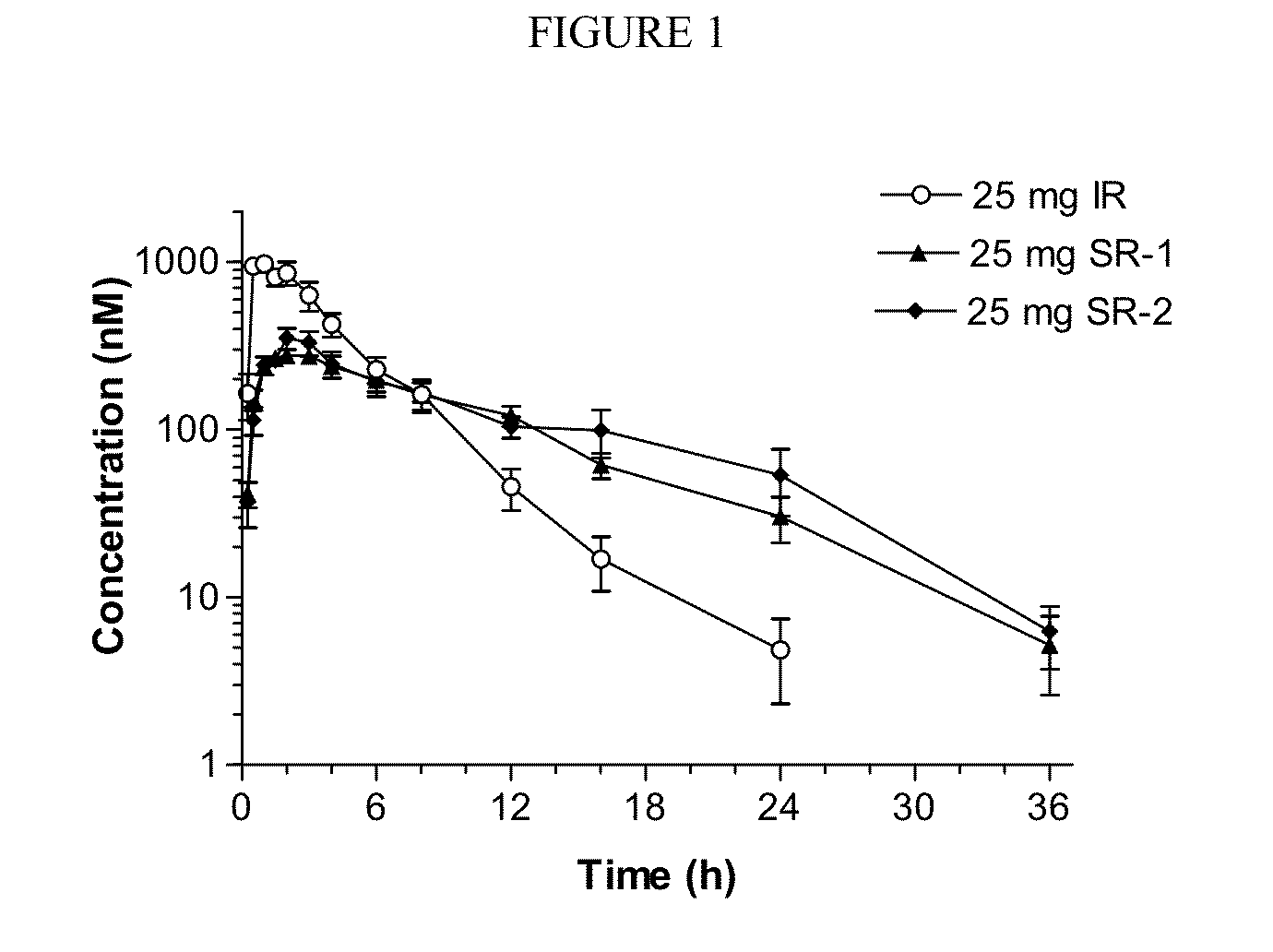
The present invention relates to liquid sustained release suspension dosage forms comprising ionized forms of water-soluble drugs. In particular, the invention encompasses a liquid form controlled release drug composition comprising a dispersed phase comprising an ion-exchange matrix drug complex comprising a pharmaceutically acceptable ion-exchange matrix and a water-soluble electrolytic drug associated with the ion-exchange matrix, wherein the surface charge of the ion-exchange matrix is opposite that of the electrolytic drug and a dispersion medium substantially free of diffusible counterions, further comprising a polyelectrolyte having the same charge as the electrolytic drug. The invention also provides methods for preparing such compositions and methods of treatment.
Owner:MARYLAND UNIV OF BALTIMORE +1
Controlled release formulations exhibiting an ascending rate of release
InactiveUS20070259033A1Low profileReduce solubilityOrganic active ingredientsNervous disorderSustained release drugActive agent
A sustained release dosage form is comprising a pharmaceutically active agent and pharmaceutically acceptable salts thereof and adapted to release as an erodible solid over a prolonged period of time, wherein the dosage form provides an ascending rate of release of the pharmaceutically active agent for at least about 4 hours. The dosage form is able to deliver high doses of poorly soluble or slowly dissolving active agents. When additional pharmaceutically active agents are present, the agents are released from the dosage form at rates that are proportional to the respective weights of each active agent in the dosage form. Methods of using the dosage forms to treat disease or conditions in human patients are also disclosed.
Owner:ALZA CORP
Aqueous sustained-release drug delivery system for highly water-soluble electrolytic drugs
InactiveUS20060134148A1Reduce molecular weightQuick releasePowder deliveryPharmaceutical non-active ingredientsElectrolysisIon exchange
Owner:HOLLENBECK R GARY
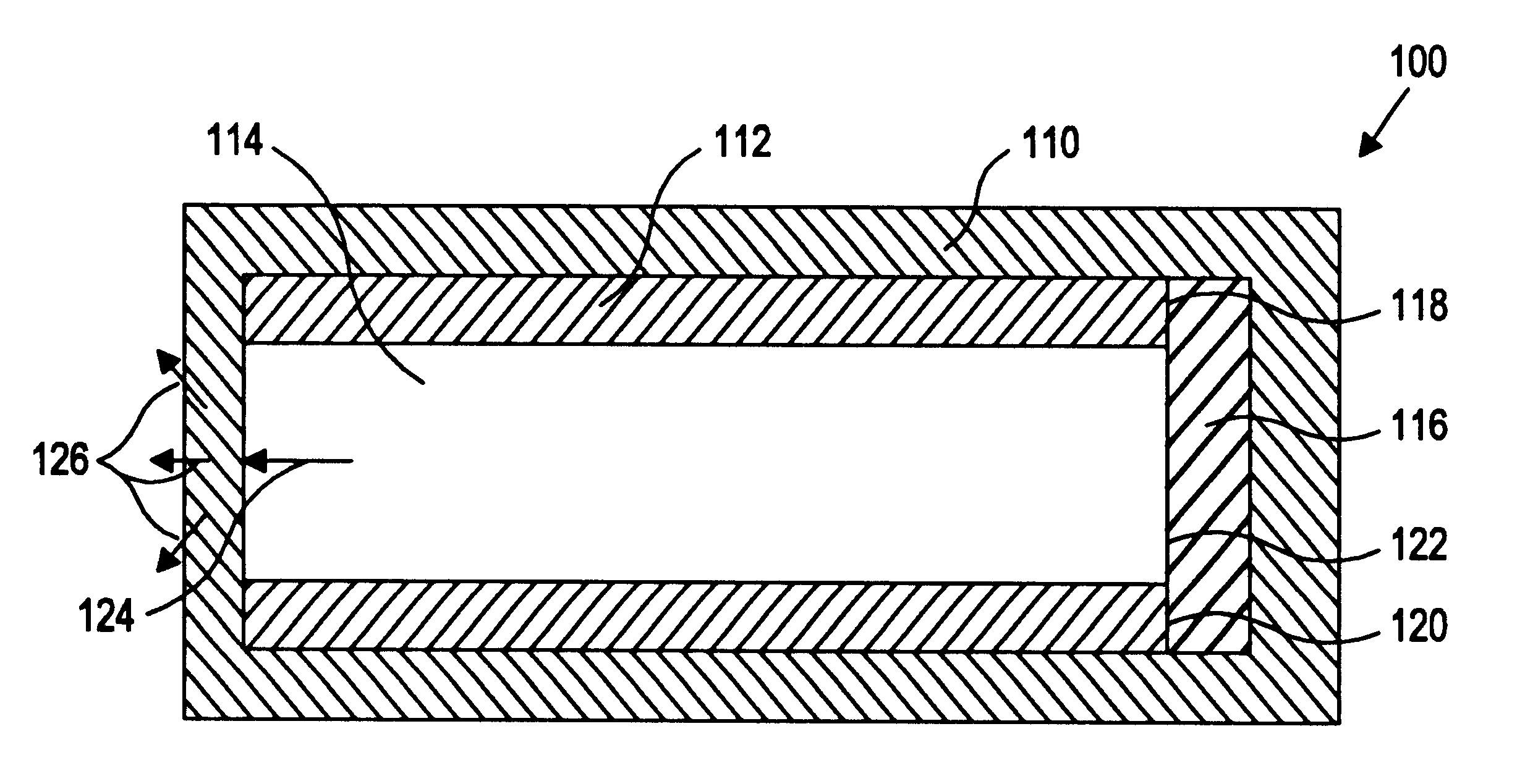
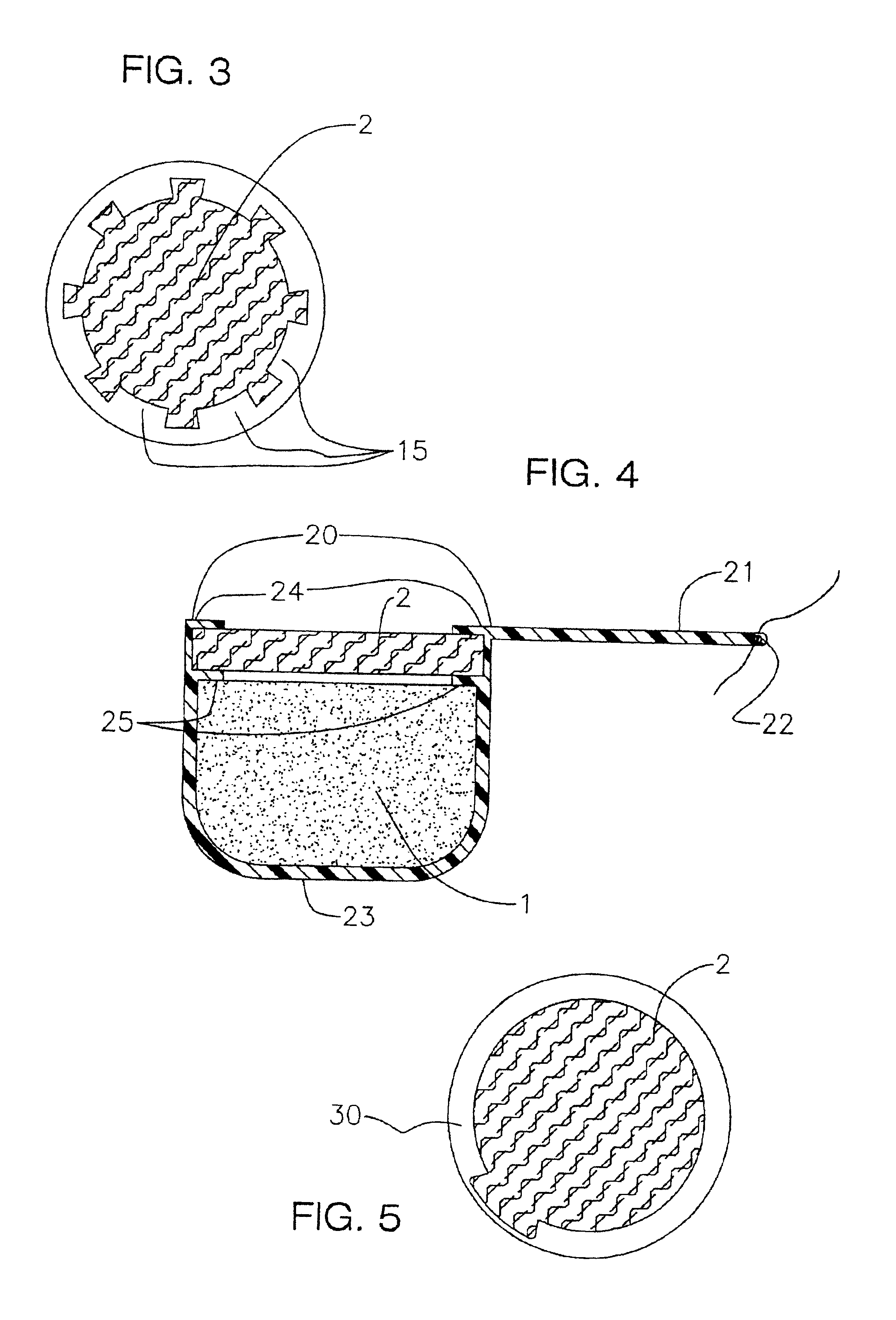
Sustained release drug delivery devices with prefabricated permeable plugs
InactiveUS6964781B2Efficiently obtainedOrganic active ingredientsInorganic non-active ingredientsSustained release drugBiomedical engineering
The present invention is directed to an improved sustained release drug delivery device comprising a drug core, a unitary cup, and a prefabricated permeable plug.
Owner:BAUSCH & LOMB INC
Sustained release pharmaceutical composition
InactiveUS20050100603A1Equivalent and even more efficacyReduce the adverse eventsPowder deliveryBiocideTamsulosin hclSustained release drug
[Problem] As compared with the current oral sustained-release preparation containing tamsulosin hydrochloride which have been supplied to the medical setting at present, it is needed to provide a sustained-release pharmaceutical composition in which the efficacy is equivalent or even better, adverse events such as adverse reactions (e.g., postural hypotension) are reduced, dose can be increased and, if desired, ingestion of food is not limited in the dosage and it is also needed to provide a method for administration of tamsulosin hydrochloride in which the adverse reactions accompanied by therapy or prevention on the basis of an α1 receptor blocking action are reduced. [Means for Resolution] A sustained-release pharmaceutical composition, characterized in that, there are contained tamsulosin or a pharmaceutically acceptable salt thereof and a carrier for a sustained-release pharmaceutical composition and the ratio (Cmin / Cmax ratio) of the plasma tamsulosin concentration at 24 hours after the administration of the preparation per os (Cmin) to the maximum plasma tamsulosin concentration after the administration (Cmax) is about 0.4 or more.
Owner:ASTELLAS PHARMA INC
Methods and dosage forms for controlled delivery of paliperidone and risperidone
Dosage forms and methods for providing a substantially ascending rate of release of paliperidone or risperidone are provided. The sustained release dosage forms provide therapeutically effective average steady-state plasma paliperidone or risperidone concentrations when administered once per day. This once-a-day dosing regimen results in only one peak plasma paliperidone or risperidone concentration occurrence in each 24 hour period. In addition, the peak plasma paliperidone or risperidone concentration occurs at a later time following dose administration and exhibits a lesser magnitude than the peak plasma paliperidone or risperidone concentration that occurs following administration of paliperidone or risperidone in an immediate-release dosage form.
Owner:ALZA CORP
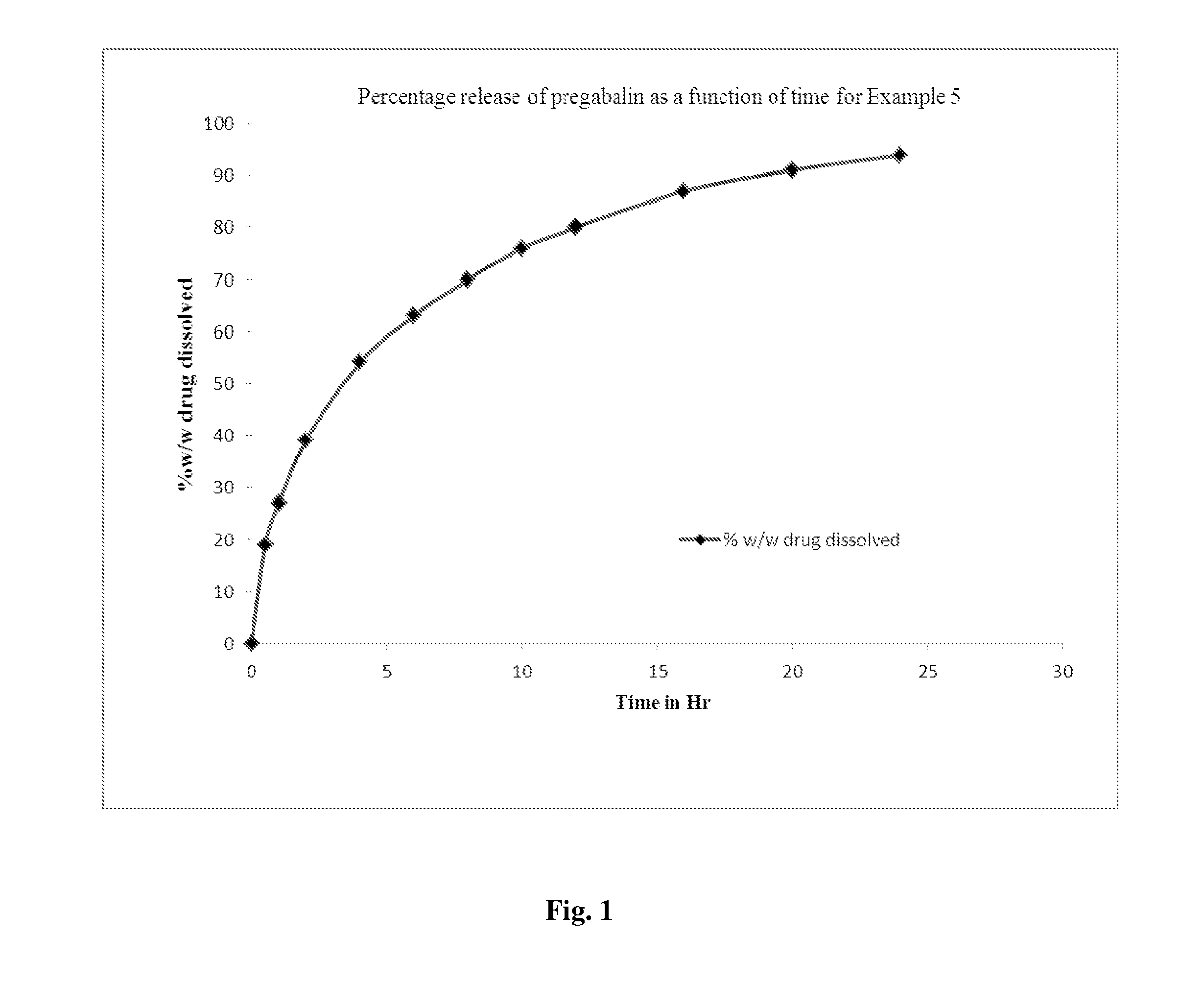
Sustained release pharmaceutical compositions comprising pregabalin
InactiveUS20130280324A1Increase in plasma levelImprove the level ofBiocideOrganic active ingredientsSustained release drugImmediate release
The present invention relates to stable once daily sustained release pharmaceutical compositions comprising pregabalin or pharmaceutically acceptable salts thereof and a pharmaceutically acceptable excipient wherein pharmaceutical composition is bioequivalent to conventional immediate release formulation of pregabalin administered twice daily. The present invention further relates to a composition comprising pregabalin and sugar esters as release retarding agent for maintaining uniform release rate of the drug and process for the preparation of such oral sustained release formulations.
Owner:PANACEA BIOTEC
Drug delivery to a joint
InactiveUS20070053963A1Easy to eluteAntibacterial agentsPowder deliverySustained release drugElution
A method of intra-articular drug delivery may include selecting an attachment zone in a synovial joint; affixing a drug release device in the attachment zone, the drug release device comprising a base affixable in the attachment zone, a sustained-release drug carrier, and a drug, the device positioned so that the device releases the drug into the synovial fluid of the synovial joint, and so that agitation of the synovial fluid facilitates elution of the drug from the drug release device.
Owner:HOSPITAL FOR SPECIAL SURGERY
Ophthalmic lenses capable of sustained drug release and preservative solutions therefor
ActiveUS20060187410A1Prevent elutionStay in shapePowder deliveryEye treatmentHydrophilic monomerMethacrylate
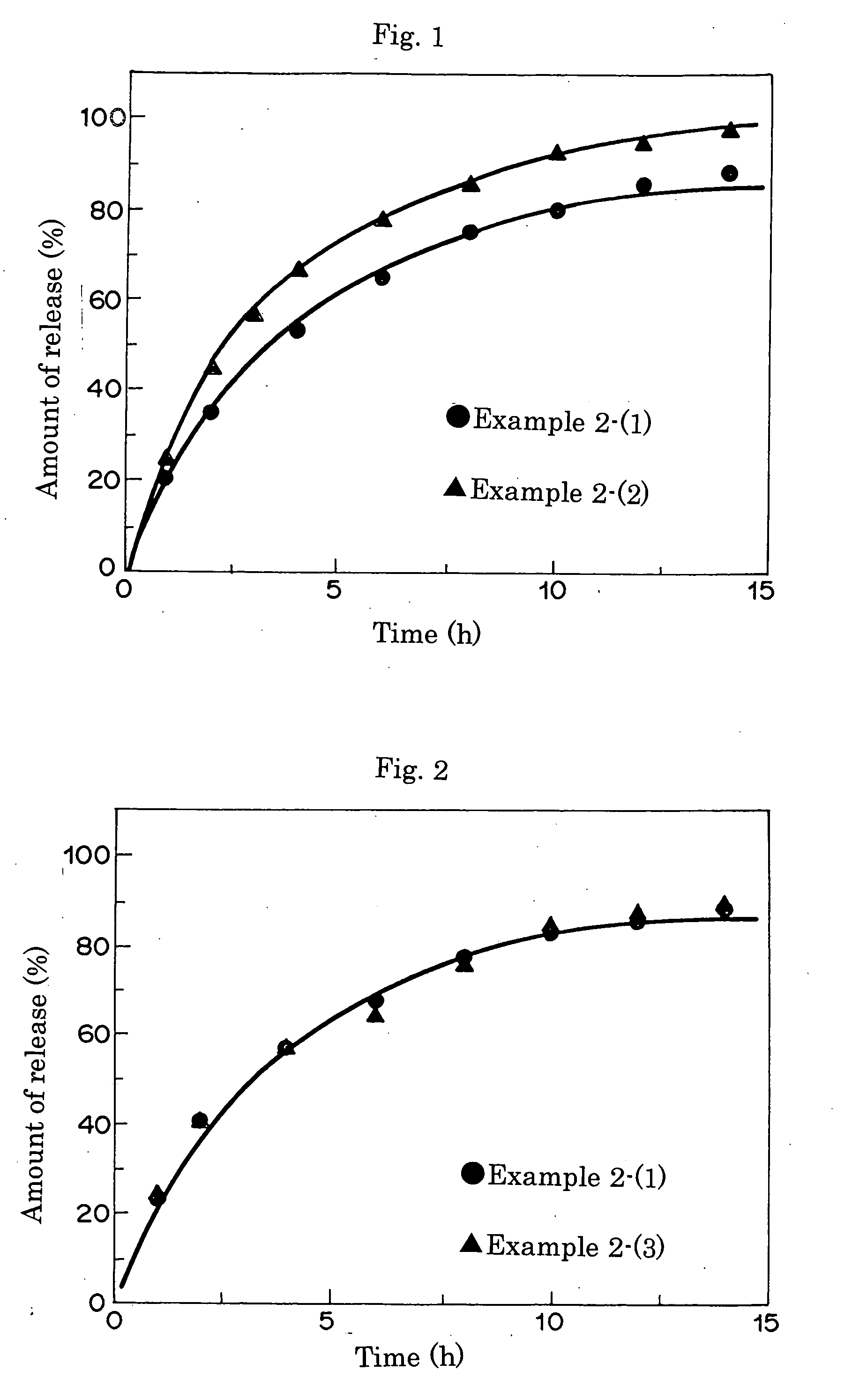
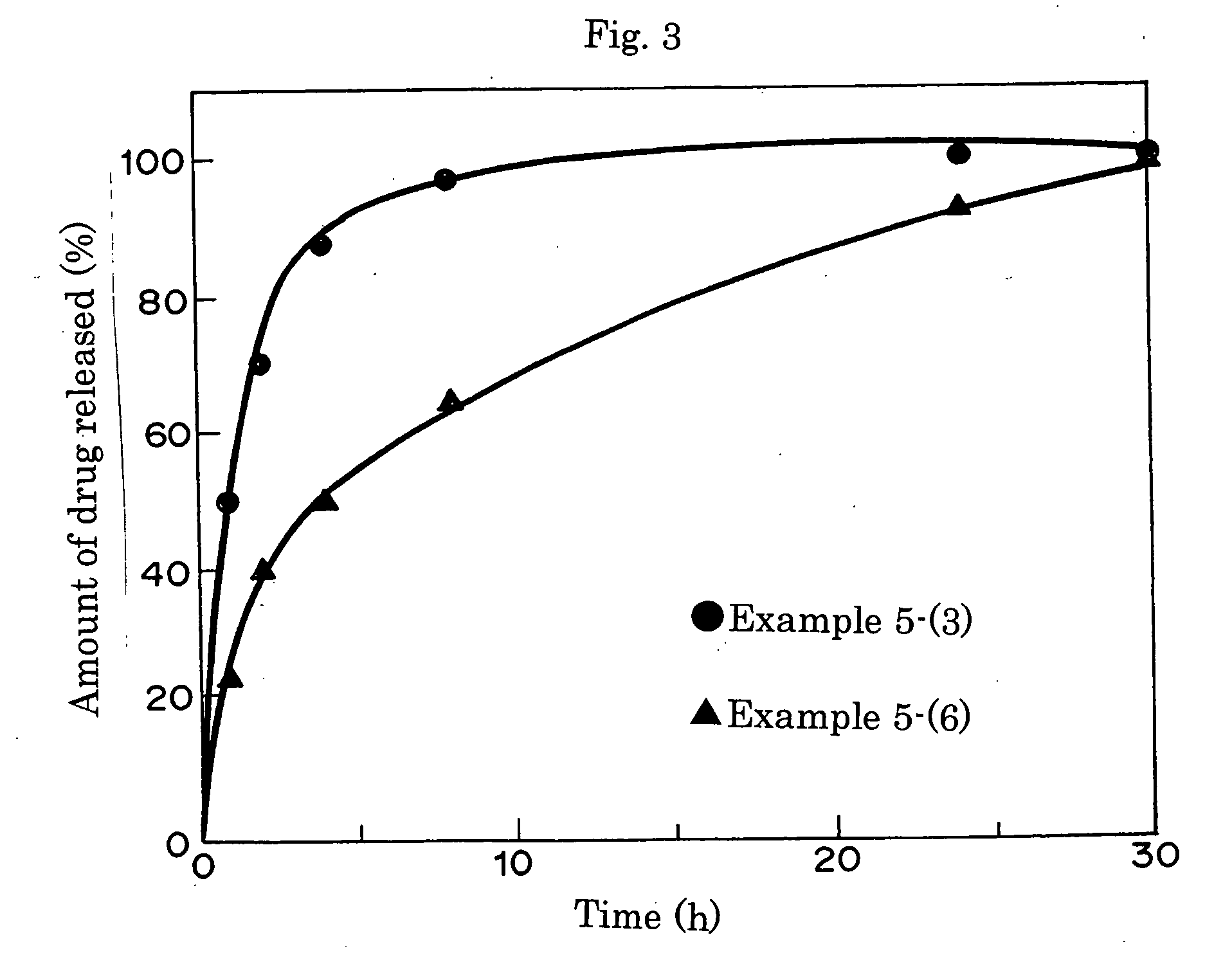
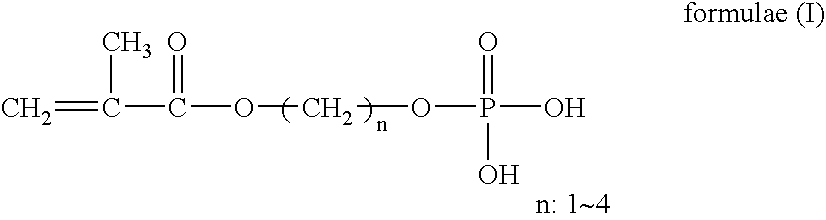
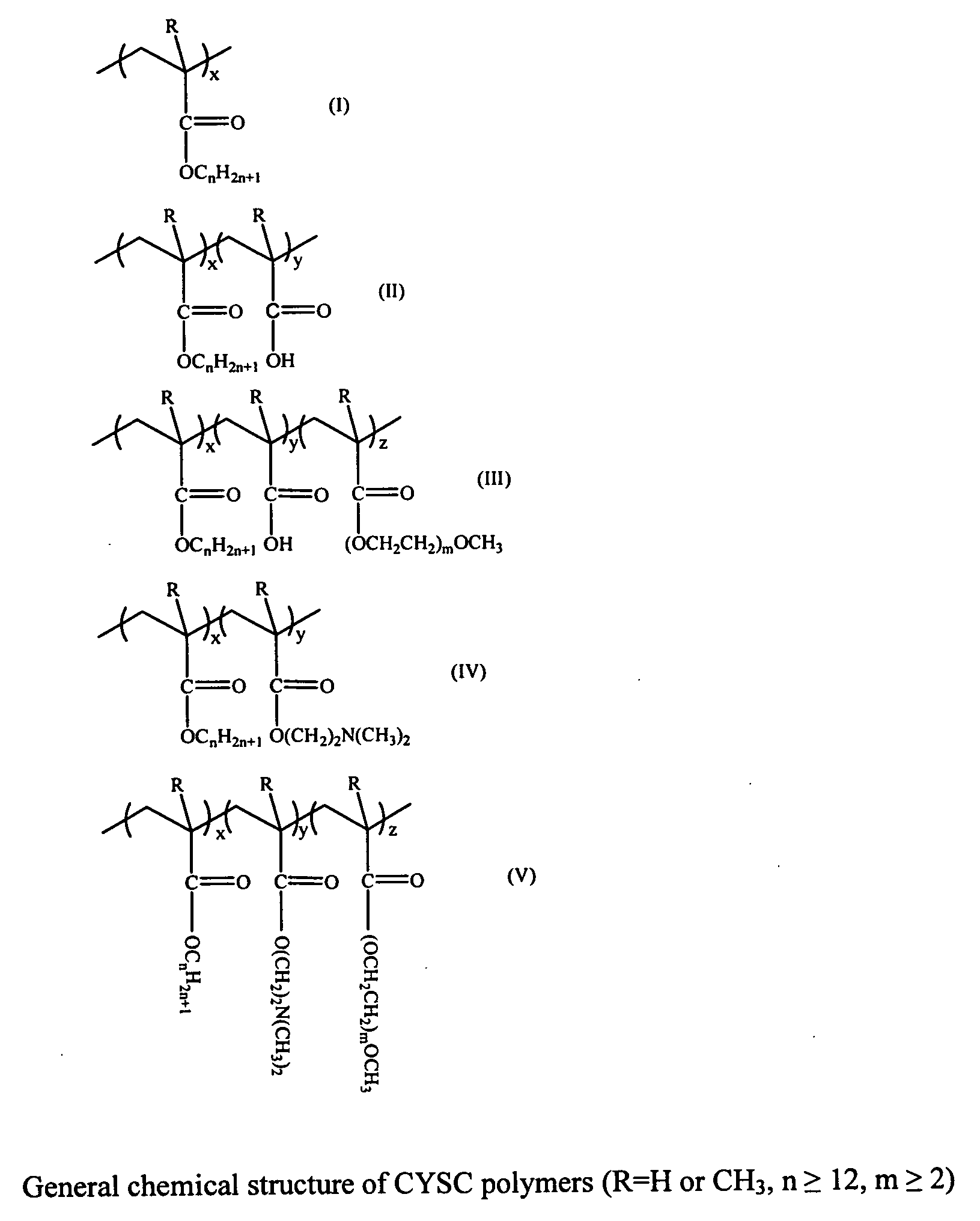
An object of the present invention is to provide a practical ophthalmic lens which has an effect of effectively retaining and sustainedly releasing a drug and has form stability before and after release of the drug, wherein the ionic polymer gel having sustained drug releasability can regulate the amount of the drug included therein, depending on the efficacy of the drug used, and storing solution for a practical ophthalmic lens. The present invention relates to a drug delivery ophthalmic lens comprising a cationic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer having a hydroxyl group in its molecule, at least one member selected from specific phosphate group-containing methacrylates a monomer having a nitrogen atom in its side chain, and a monomer copolymerizable with these components, and also relates to a drug delivery ophthalmic lens comprising an anionic group-containing drug in the inside of a copolymer consisting of a hydrophilic monomer, cationic and anionic monomers, and a monomer copolymerizable with these components, wherein the copolymer contains the anionic monomer in a ratio of 30 to 90 mol % to the cationic monomer, and also relates to storing solution for a practical ophthalmic lens.
Owner:VIEWDLE INC
Delivery of drugs
Formulations of drugs and crystalline side chain polymers to provide controlled and / or sustained release drug formulations.
Owner:LANDEC
Porous silicon drug-eluting particles
ActiveUS20100278931A1Organic active ingredientsPowder deliverySustained release drugDiagnostic agent
The invention provides a biodegradable drug-eluting particle useful for the delivery of diagnostic or therapeutic agents. In certain embodiments, the drug-eluting particle of the invention comprises a biodegradable porous silicon body, a reservoir formed within the porous silicon body having at least one opening to an exterior of the body, wherein the reservoir contains a therapeutic or diagnostic agent, and an agent-permeable seal disposed over the at least one opening. The invention further provides a method for treating a patient to obtain a desired local or systemic physiological or pharmacological effect comprising administering a sustained release drug delivery particle of the invention. The invention also provides methods of fabricating a drug-eluting particle for releasing therapeutic agents
Owner:EYEPOINT PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com