Drug delivery to a joint
a technology for delivering drugs and joints, applied in the direction of drugs, antibacterial agents, prostheses, etc., can solve the problems of irreversible damage to nerves or surrounding tissues, no linear, continuous delivery of anesthetics, and significant systemic side effects of anesthetics administered by these methods, etc., to achieve the effect of facilitating elution of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0183] In one example, the drug carrier is provided as a cartridge. The drug may be loaded into the cartridge as a powder, compressed solid, or as granules without a polymer mixture. Alternative the drug may be combined with any suitable biocompatible, biodegradable polymer. Examples of such biocompatible biodegradable polymers useful include: hydroxyaliphatic carboxylic acids, either homo- or copolymers, such as polylactic acid, polyglycolic acid, polylactic glycolic acid; polysaccharides such as cellulose or cellulose derivatives such as ethyl cellulose, cross-linked or uncross-linked sodium carboxymethyl cellulose, sodium carboxymethylcellulose starch, cellulose ethers, cellulose esters such as cellulose acetate, cellulose acetate phthallate, hydroxypropylmethyl cellulose phthallate and calcium alginate, polypropylene, polybutyrates, polycarbonate, acrylate polymers such as polymethacrylates, polyanhydrides, polyvalerates, polycaprolactones such as poly-.epsilon.-caprolactone, po...
example 2
[0184] In one exemplary embodiment, a sustained release device includes a polymeric matrix or liposome from which drug is released by diffusion and / or degradation of the matrix. The release pattern is usually principally determined by the matrix material, as well as by the percent loading, method of manufacture, type of drug being administered and type of device, for example, microsphere. A major advantage of a biodegradable controlled release system over others is that it does not require the surgical removal of the drug depleted device, which is slowly degraded and absorbed by the patient's body, and ultimately cleared along with other soluble metabolic waste products.
[0185] Systemic anesthetics such as methoxyflurane, have been incorporated into liposomes and lecithin microdroplets, for example, as described by Haynes, et al., Anesthesiology 63:490-499 (1985). To date, the liposome and lecithin preparations have not been widely applied in clinical or laboratory practice, because...
example 3
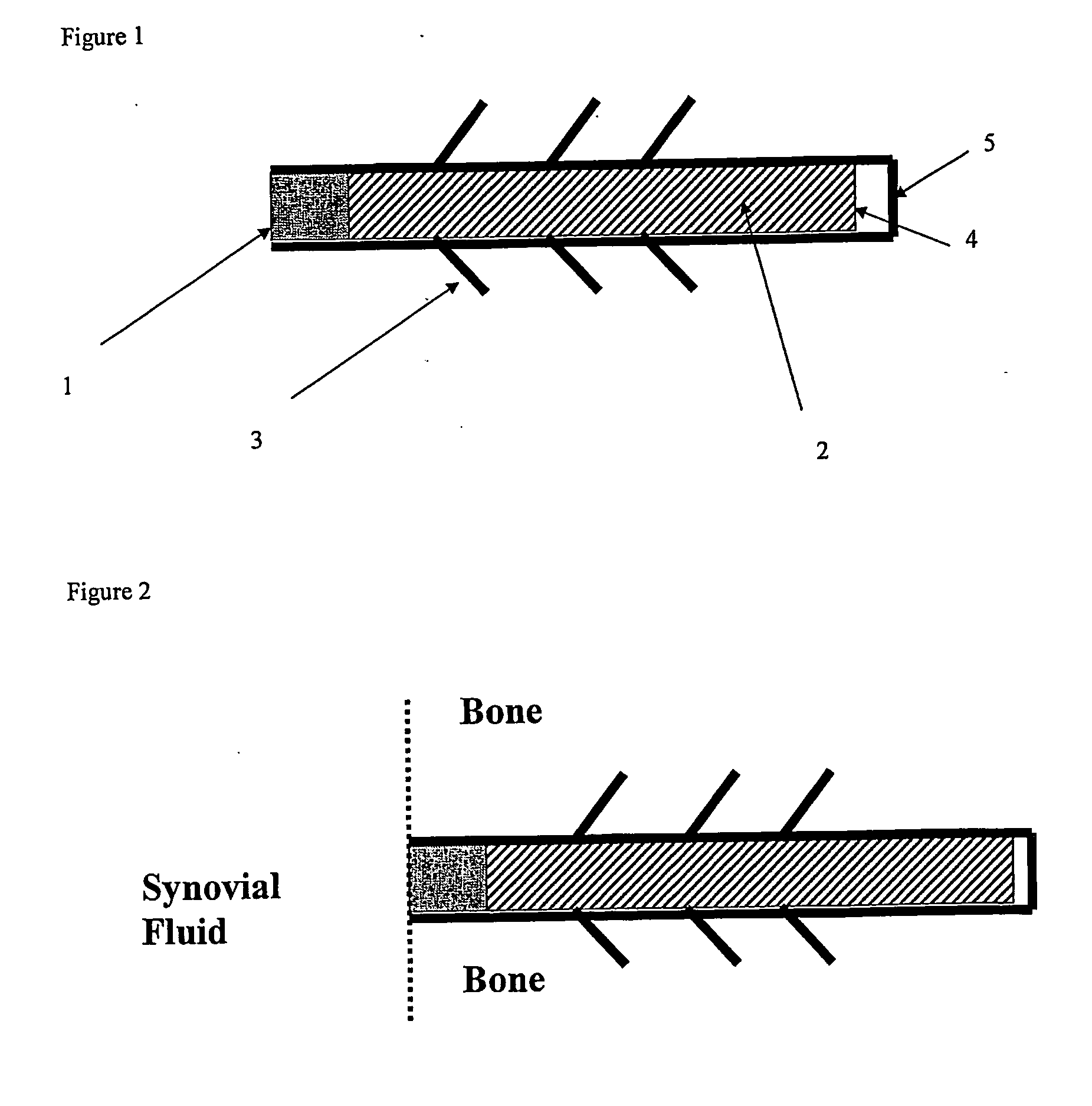
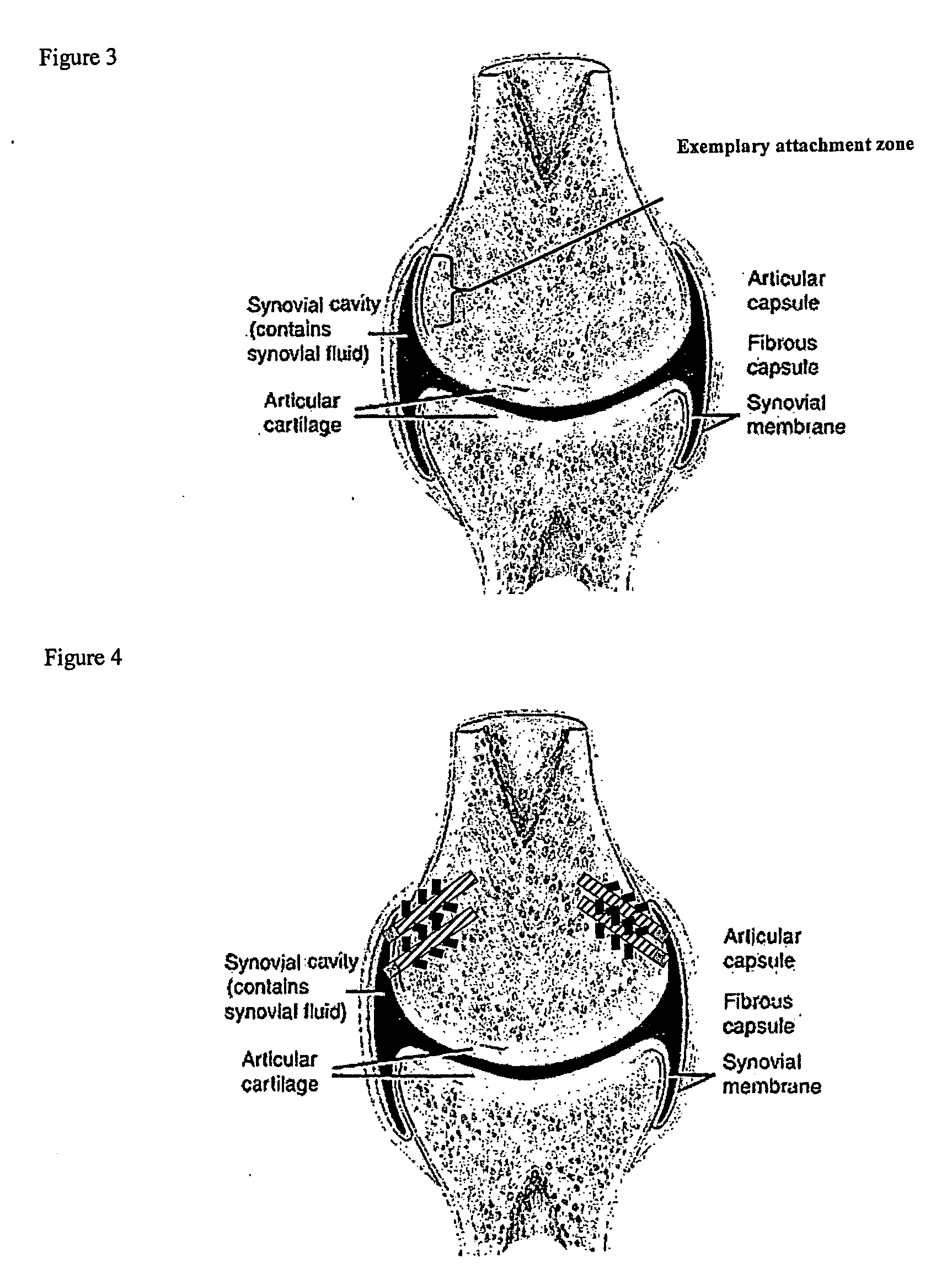
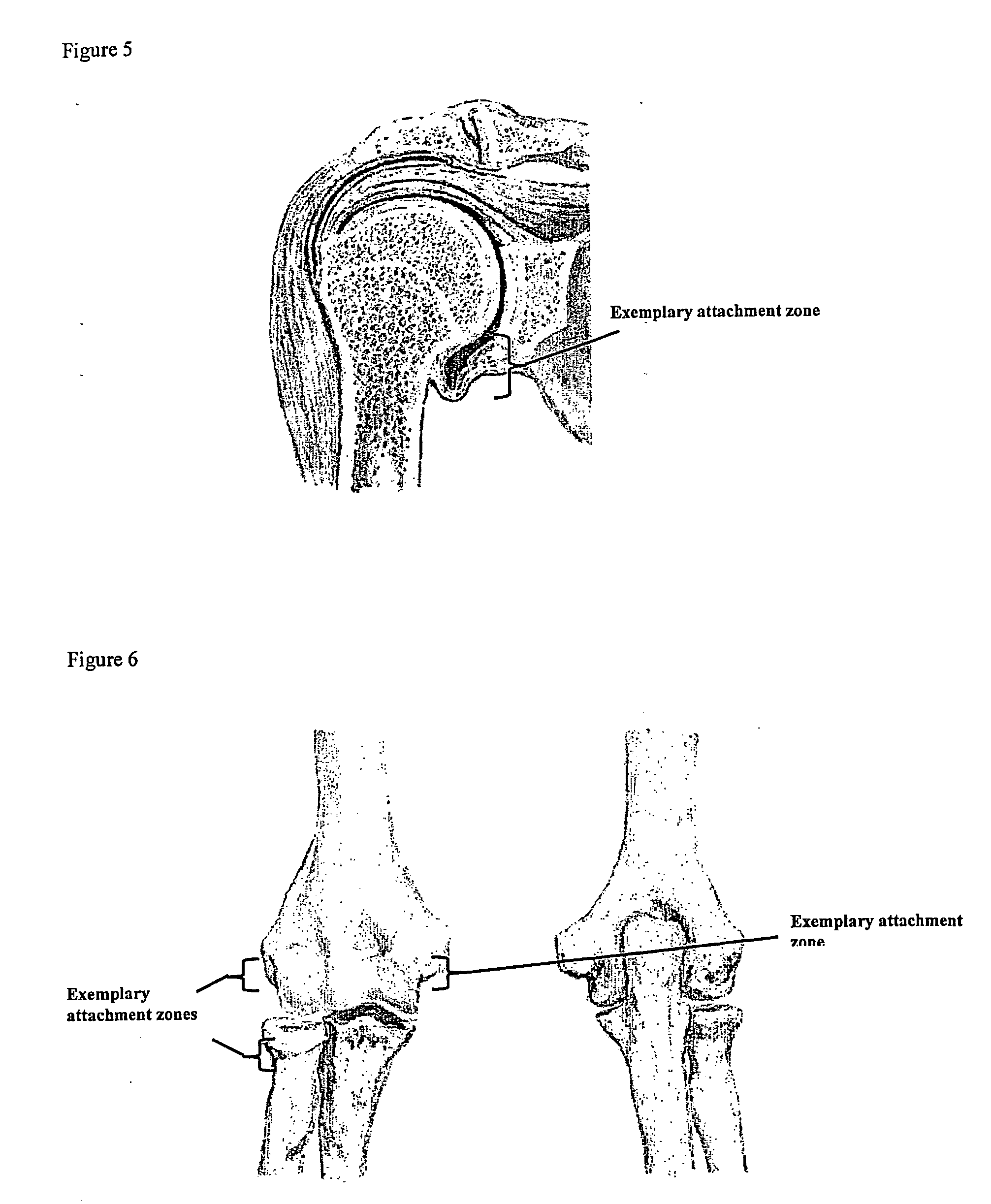
[0186] A biocompatible intra-articular device size for implantation within a joint to continuously deliver a drug within the joint for a period of at least several weeks may include an outer bone anchor construct and an inner core consisting of a drug / polymer reservoir. The drug / polymer reservoir elutes a drug that dissolves in joint fluid through a semipermeable membrane at the synovial fluid interface. The device is implanted in a non-loaded but intra-articular portion of the joint where synovial fluid agitation across the semi-permeable membrane promotes drug elution.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| weight percent | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com