Patents
Literature
48 results about "Release pattern" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Means of delivering drugs in an ascending zero order release pattern
Disclosed are dosage forms and methods for sustained release of a drug including: a delay layer comprising a polymeric matrix, and microencapsulated drug, wherein the delay layer is substantially free of non-microencapsulated drug; and a second layer including a polymeric matrix, and non-microencapsulated drug matrix; wherein the second layer is located adjacent to the delay layer.
Owner:ALZA CORP
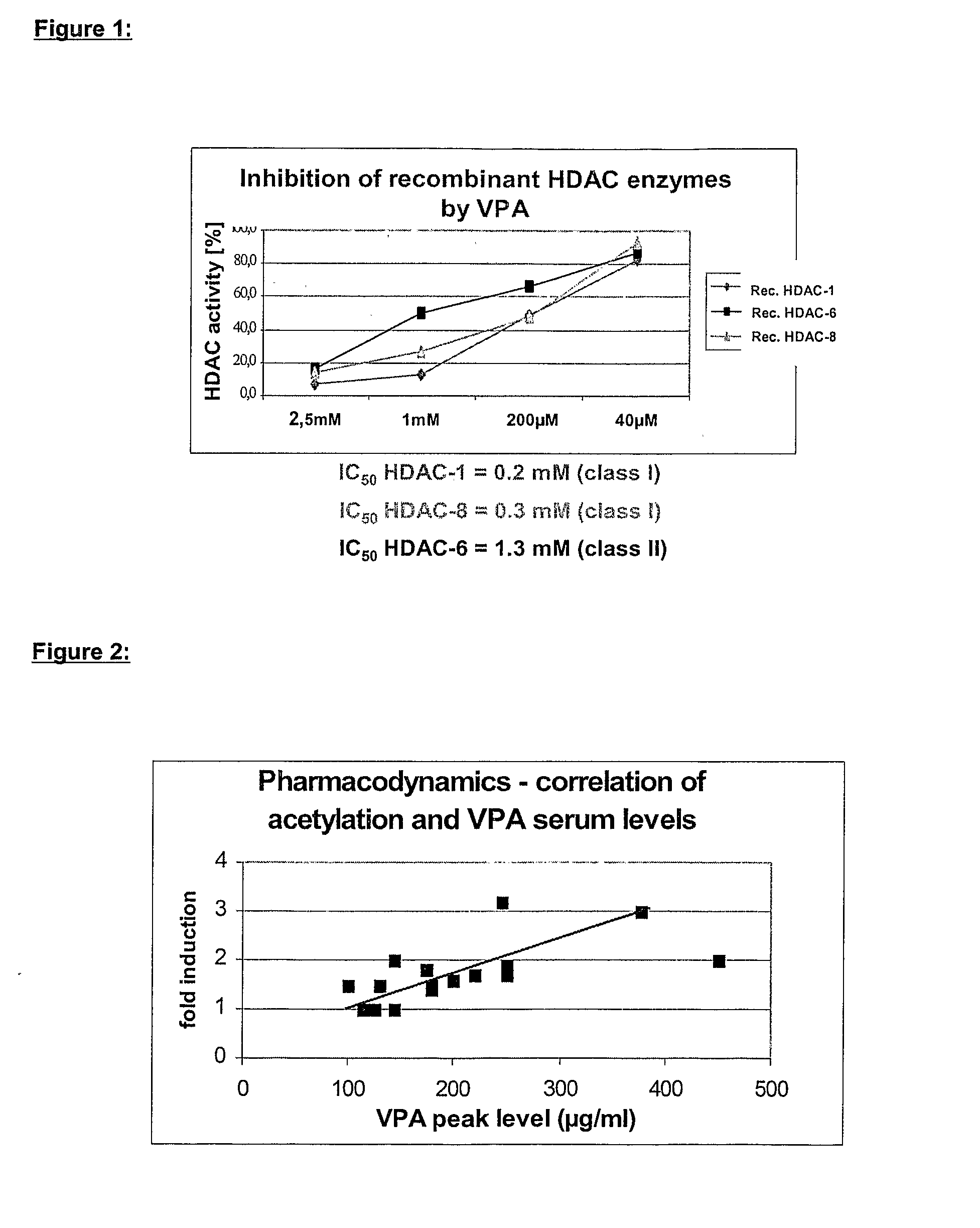
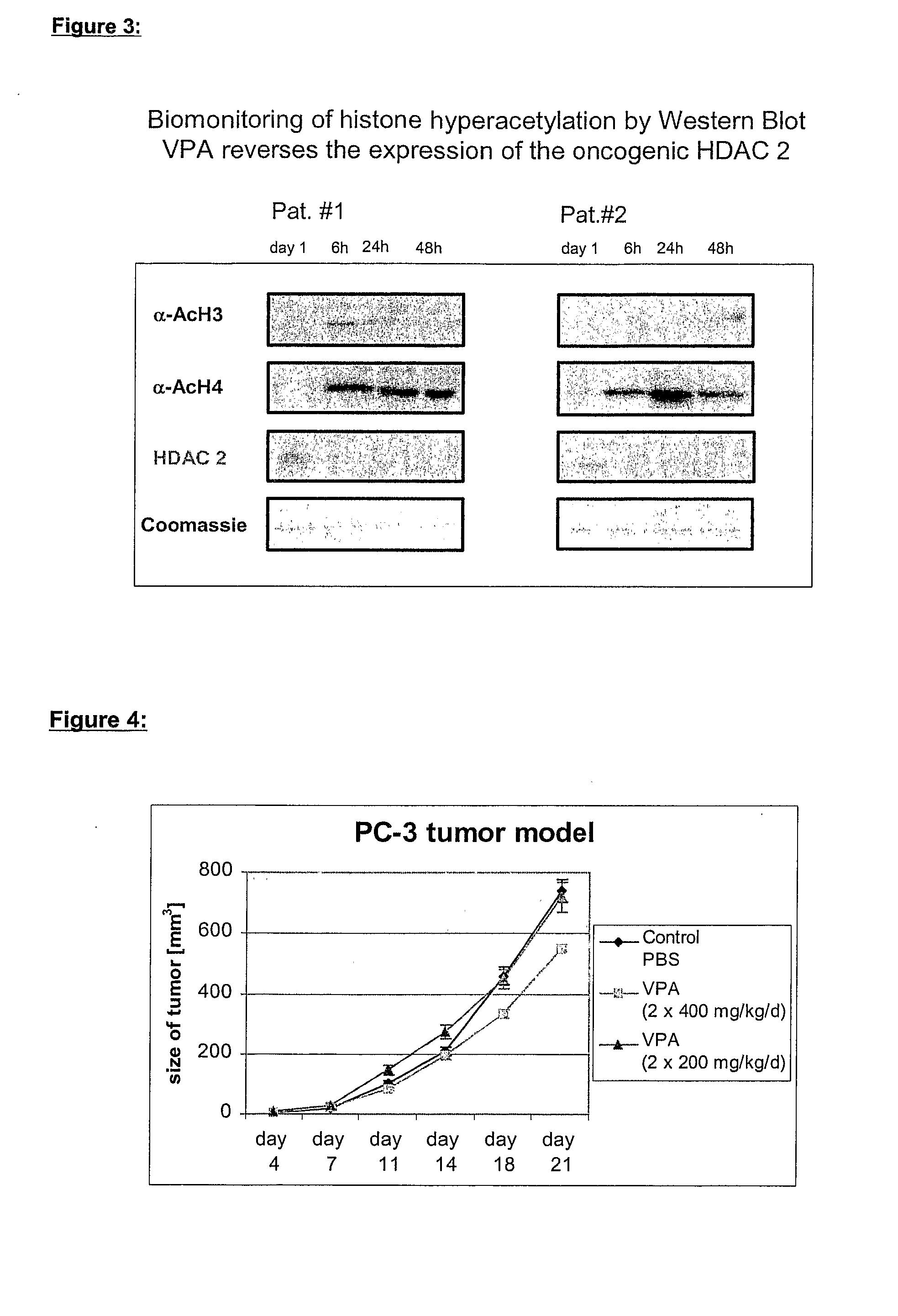
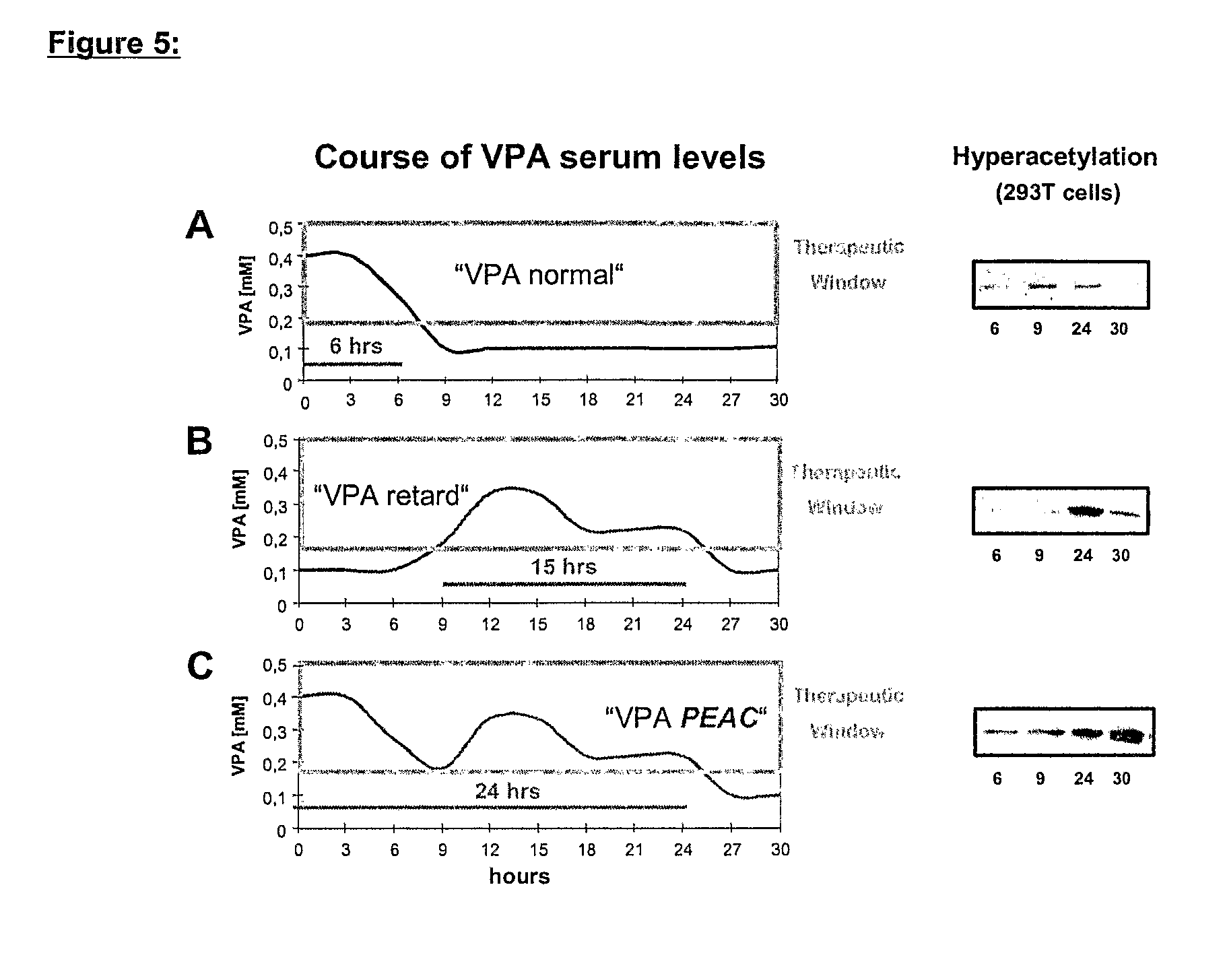
Formulation comprising histone deacetylase inhibitors
InactiveUS20070232528A1Inhibition becomes largerInhibit HDAC target enzymesBiocideSenses disorderValproic AcidApoptosis
The present invention relates to an orally available galenics formulation of Valproic Acid or derivatives thereof exhibiting a specific bi-phasic pharmacokinetic profile optimized for maximum inhibition of histone deacetylases in a therapeutic setting. This specific galenics formulation is designed for the treatment of malignant diseases and diseases associated with hypoacetylation of histones or in which induction of hyperacetylation has a beneficial effect, e.g., by induction of differentiation and / or apoptosis. Due to the bi-phasic release pattern the resulting pharmacokinetic profile is able to inhibit HDAC target enzymes most efficiently and to subsequently induce histone hyperacetylation in a rapid as well as a long-lasting fashion. This profile secures the efficient modulation of a desired target gene expression profile which contributes to the therapeutic benefit.
Owner:TOPOTARGET GERMANY AG +1
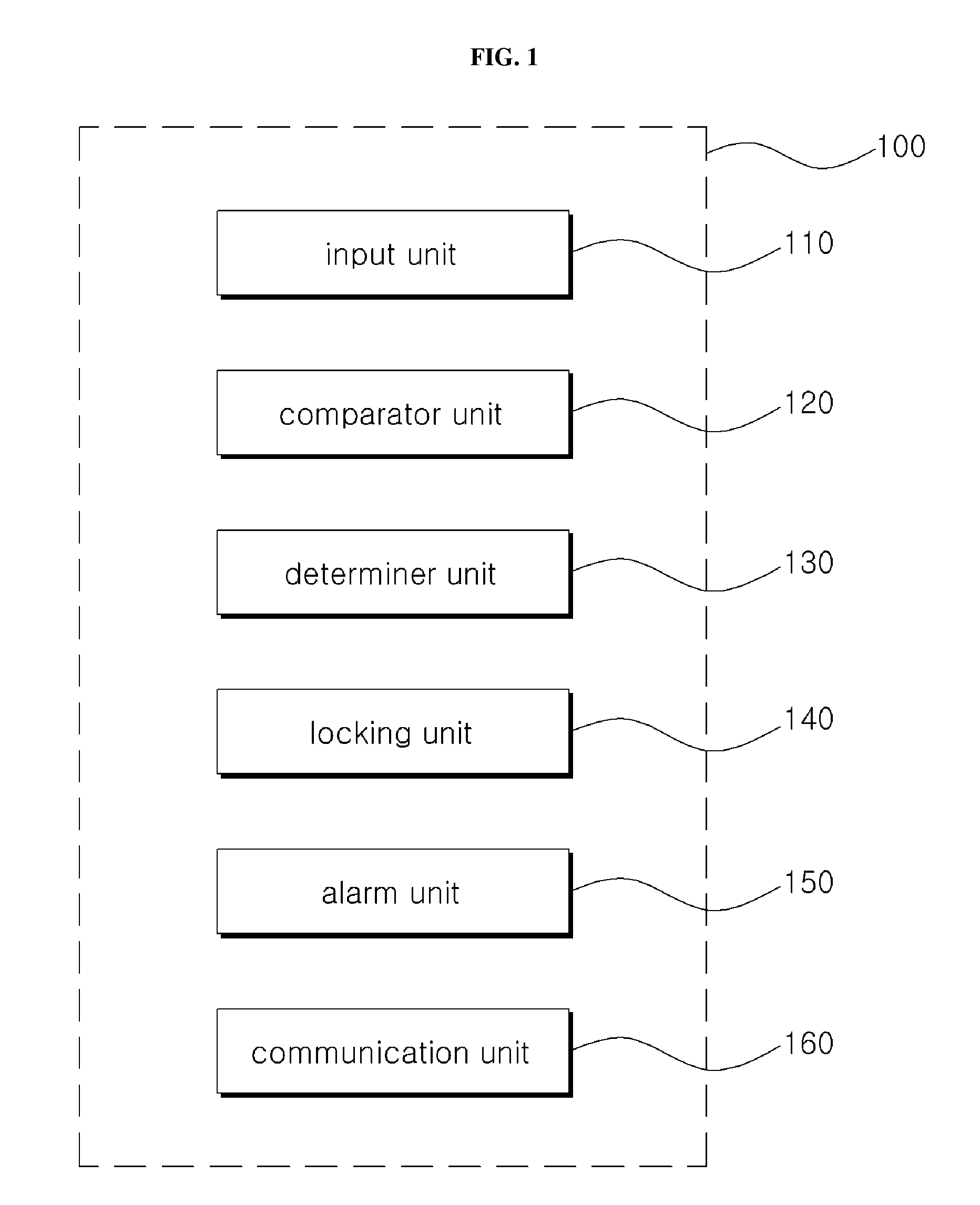
Method for recognizing motion pattern and the apparatus for the same
The present invention relates to a method and apparatus for recognizing a motion pattern formed by a continued contact surface. A method for recognizing a motion patter according to an embodiment of the invention may comprise receiving a motion pattern as input from a user, comparing pattern information of the motion pattern with pattern information of a preset release pattern, and determining a mismatch level of the motion pattern according to the comparison result. According to an embodiment of the invention, if an inputted motion pattern does not match the preset release pattern, the degree of mismatch is determined with different levels, to respond in various ways other than simply maintaining the locked state.
Owner:KOREA UNIV RES & BUSINESS FOUND
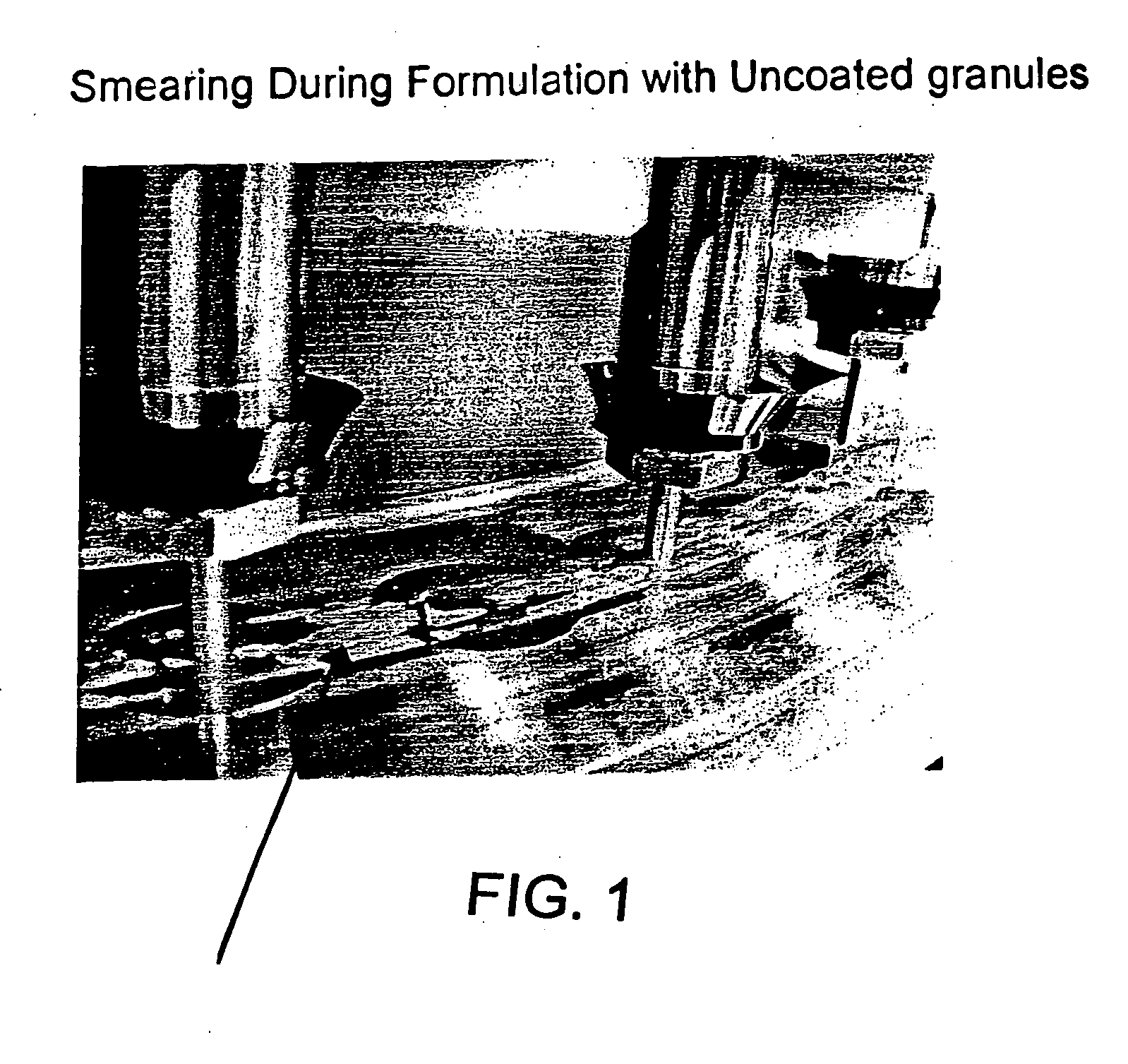
Drug granule coatings that impart smear resistance during mechanical compression
InactiveUS20050175696A1Low dissolution rateExtension of timeBiocideCarbohydrate active ingredientsSolubilityHydrophilic polymers
A drug formulation is disclosed comprising granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a drug formulation consisting of a tablet core made by mechanical compression, wherein said tablet core comprises granules having a substrate and a coating, said granule substrate comprising a solubilizing surfactant or a low solubility therapeutic drug, or both, and said granule coating comprising a hydrophilic polymer. Also disclosed is a dosage form for oral administration of topiramate, comprising a tablet core and an osmotic delivery system. Methods for controlling topiramate release patterns by altering the composition of the topiramate dosage form are also disclosed.
Owner:ALZA CORP
Hologram laminate and hologram label
InactiveUS20060193021A1Reuse can be preventedHinder propertyStampsOther printing matterEngineeringRelease pattern
Owner:DAI NIPPON PRINTING CO LTD
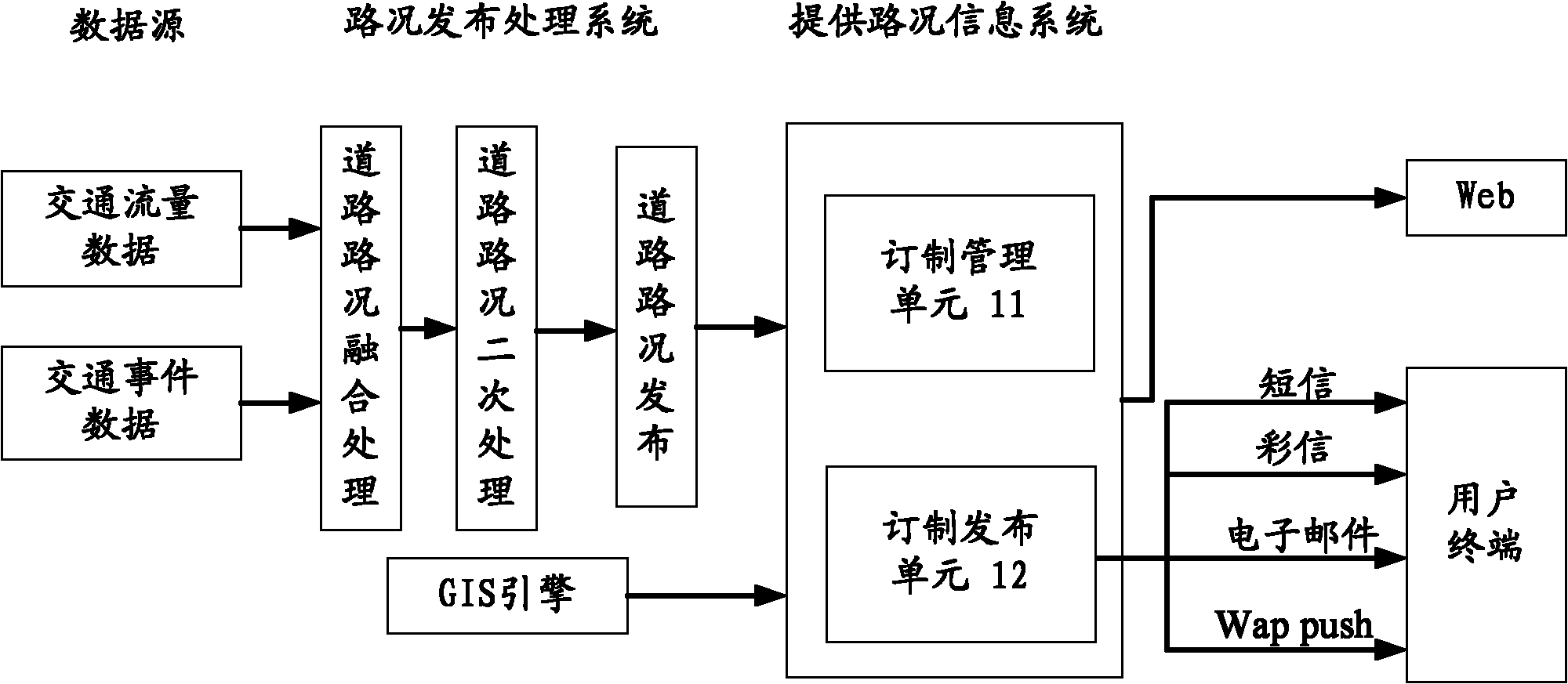
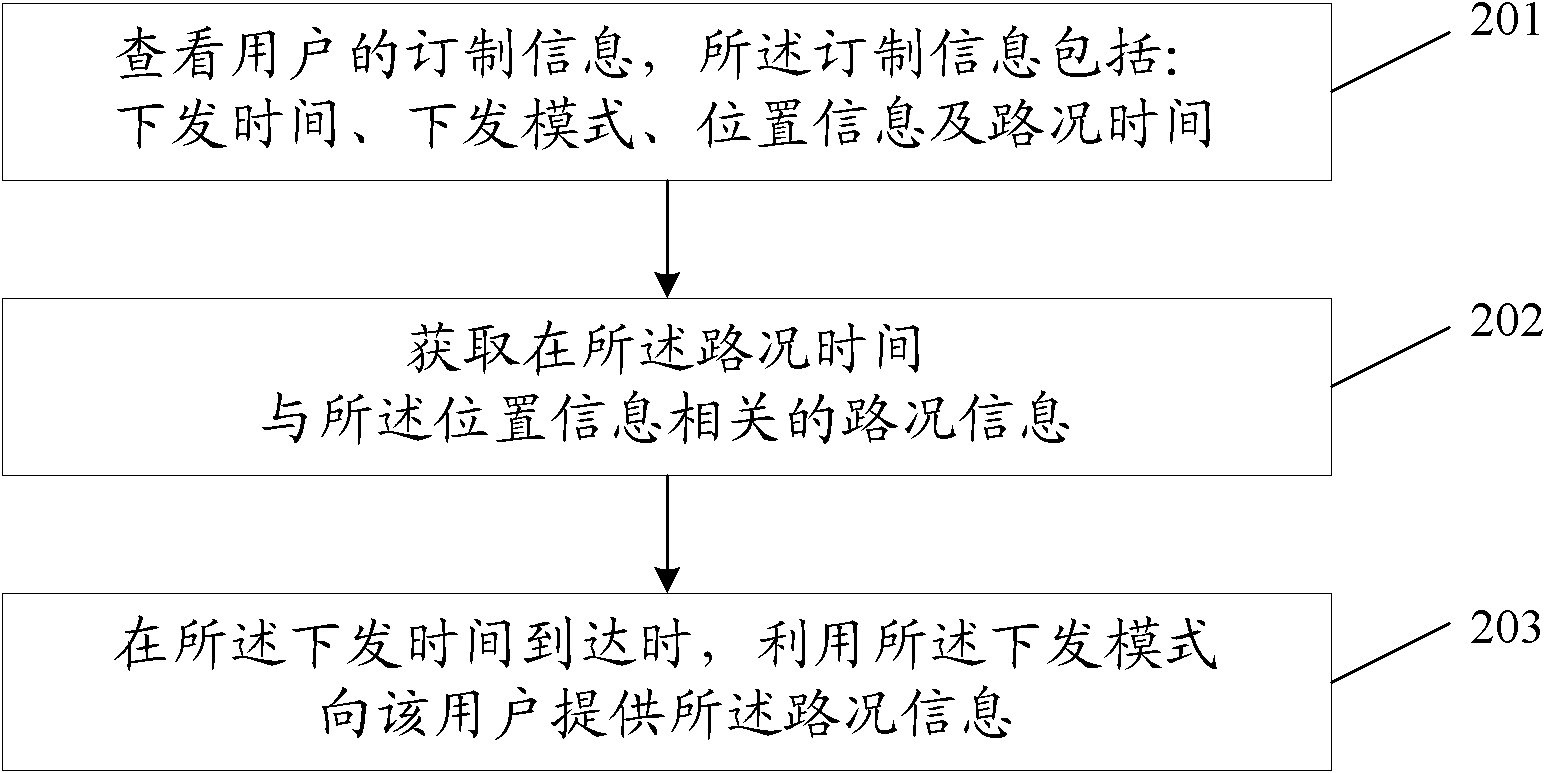
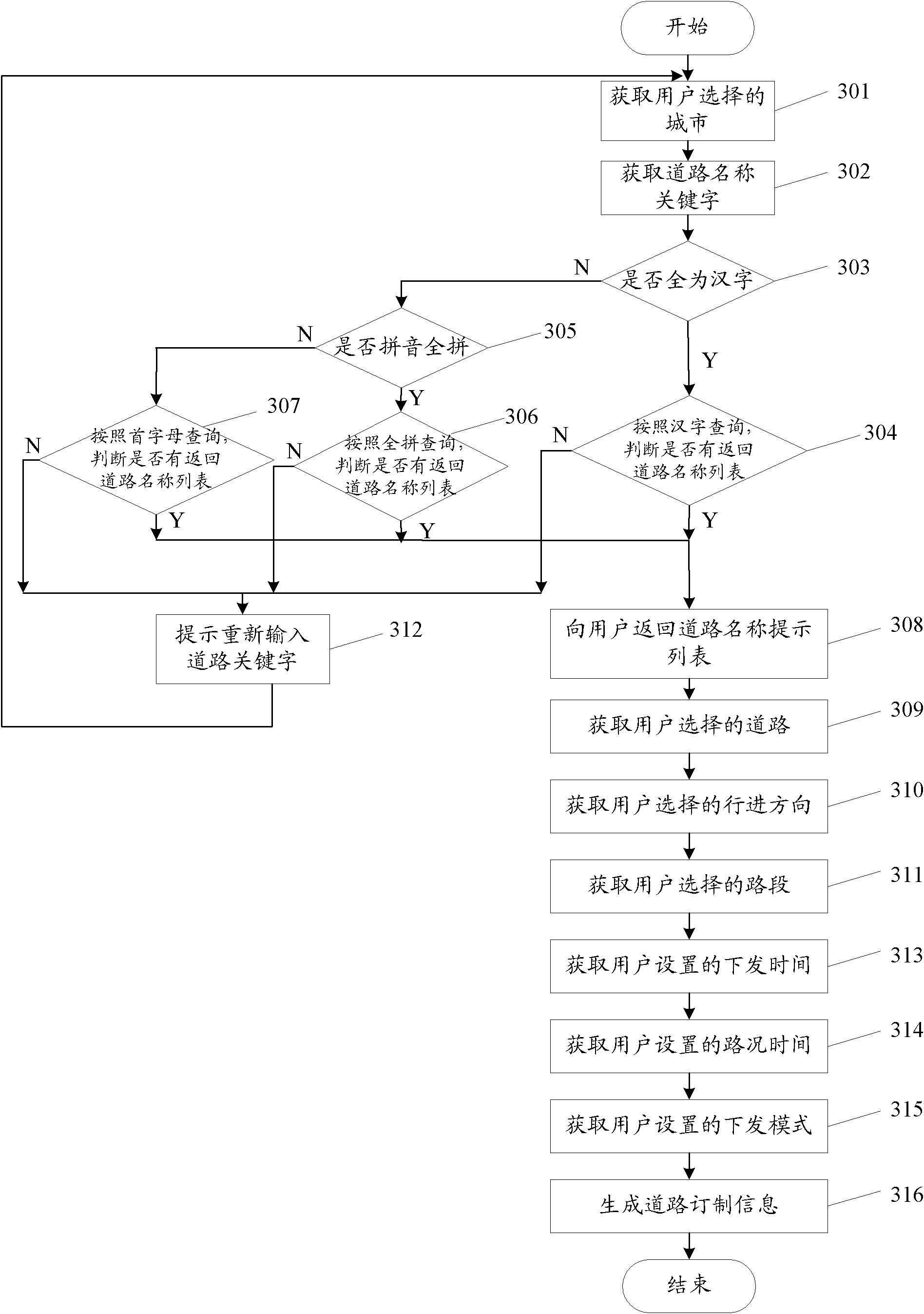
Method, device and system for providing road condition information
ActiveCN102044163AGet goodAvoid Duplicate Query OperationsRoad vehicles traffic controlRelease timeApplication areas
The invention discloses a method, a device and a system for providing road condition information, relates to the field of the processing and application of the road condition information, and is used for avoiding repetitive inquiry operation in the process of acquiring road condition information for a user. The method for providing the road condition information comprises the steps of: viewing ordered information of a user, wherein the ordered information comprises a release time, a release pattern, position information and road condition time; acquiring the road condition information relevant to the position information at the road condition time; and providing the road condition information for the user by utilizing the release pattern when the release time is reached. The method, the device and the system for providing road condition information disclosed by the invention are suitable for the regular acquisition of traffic road condition information for the user.
Owner:CENNAVI TECH
Novel controlled release-niacin formulation
InactiveUS20090069389A1Easy to controlEffective controlBiocideMetabolism disorderControlled releaseBlood concentration
The present invention relates to a controlled-release niacin formulation. In particular, the present invention relates to a controlled-release niacin formulation, comprising niacin; hydroxypropyl methylcellulose; and a carboxyvinyl polymer, in which the carboxyvinyl polymer and hydroxypropyl methylcellulose are contained in a predetermined weight ratio, and to a preparation method thereof.The controlled-release niacin formulation according to the present invention maintains its matrix shape until completion of release, and maintains its release pattern without fluctuation for a desired time period, unlike a commercial formulation. In particular, since niacin formulations are used for long-term treatment of hyperlipidemia, the controlled-release niacin formulation of the present invention, capable of maintaining effective blood concentration and high stability for a long period of time, is very useful.
Owner:SEOUL PHARMA
Method for writing and erasing a non-volatile memory area
InactiveUS6973530B2Guaranteed uptimeReliably determinedMemory adressing/allocation/relocationRead-only memoriesRelease patternOperating system
A method for the writing and erasing of a non-volatile memory area, in which after error-free writing of the memory area, at least one release pattern is written into a predetermined subarea of the memory area. To be able to reliably ascertain aborted erase operations, prior to the erase operation, invalidity patterns are written into two further subareas of the memory area, the subareas provided for this enclosing those subareas reserved for the release patterns. The presence (in part) of invalidity patterns indicates an erase operation not ended correctly. The method can be used advantageously in controllers having non-volatile memories.
Owner:ROBERT BOSCH GMBH
Hologram laminate and hologram label
InactiveUS7085024B2Well formedReuse can be preventedStampsOther printing matterGraphicsRelease pattern
A hologram laminate and a hologram label allow traces of separation of the hologram to be clearly left on both the adherend and the hologram label and hence makes it possible to prevent reuse of the hologram and is excellent in graphical design function and suitable for certification purposes. The hologram laminate has a colored layer, a release pattern, a transparent film, a hologram layer and a surface protective layer laminated on an adherend in the order mentioned. The hologram layer includes a volume hologram layer and a relief hologram layer having a transparent thin-film layer over the surface of a relief interference fringe pattern. When the hologram is separated between the adherend and the transparent film, the colored layer is left on the adherend in a pattern corresponding to the release pattern.
Owner:DAI NIPPON PRINTING CO LTD
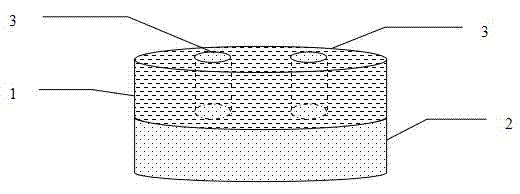
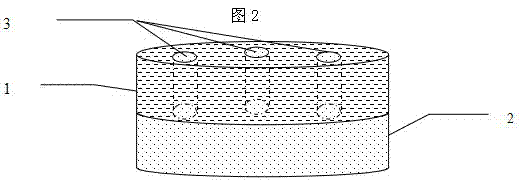
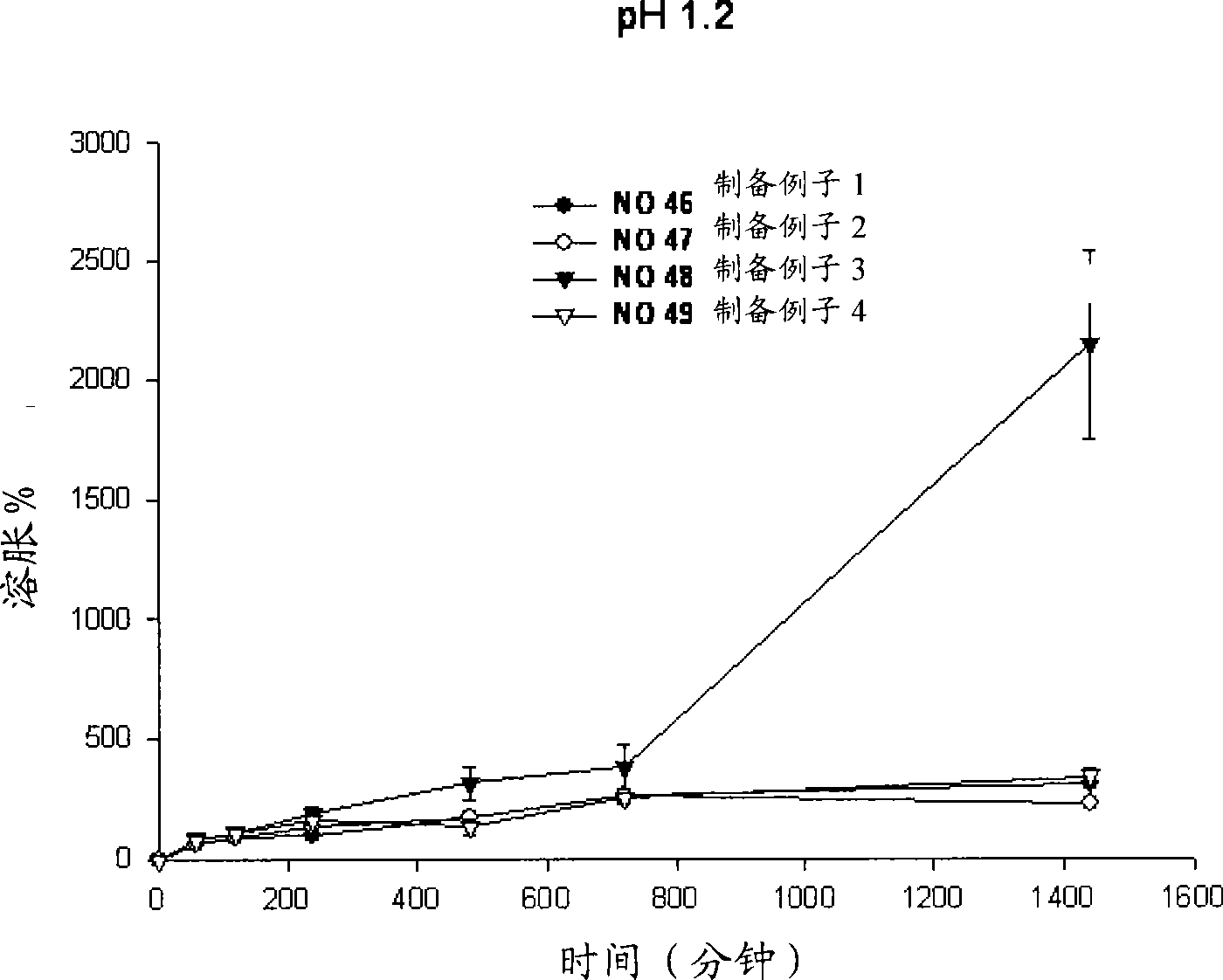
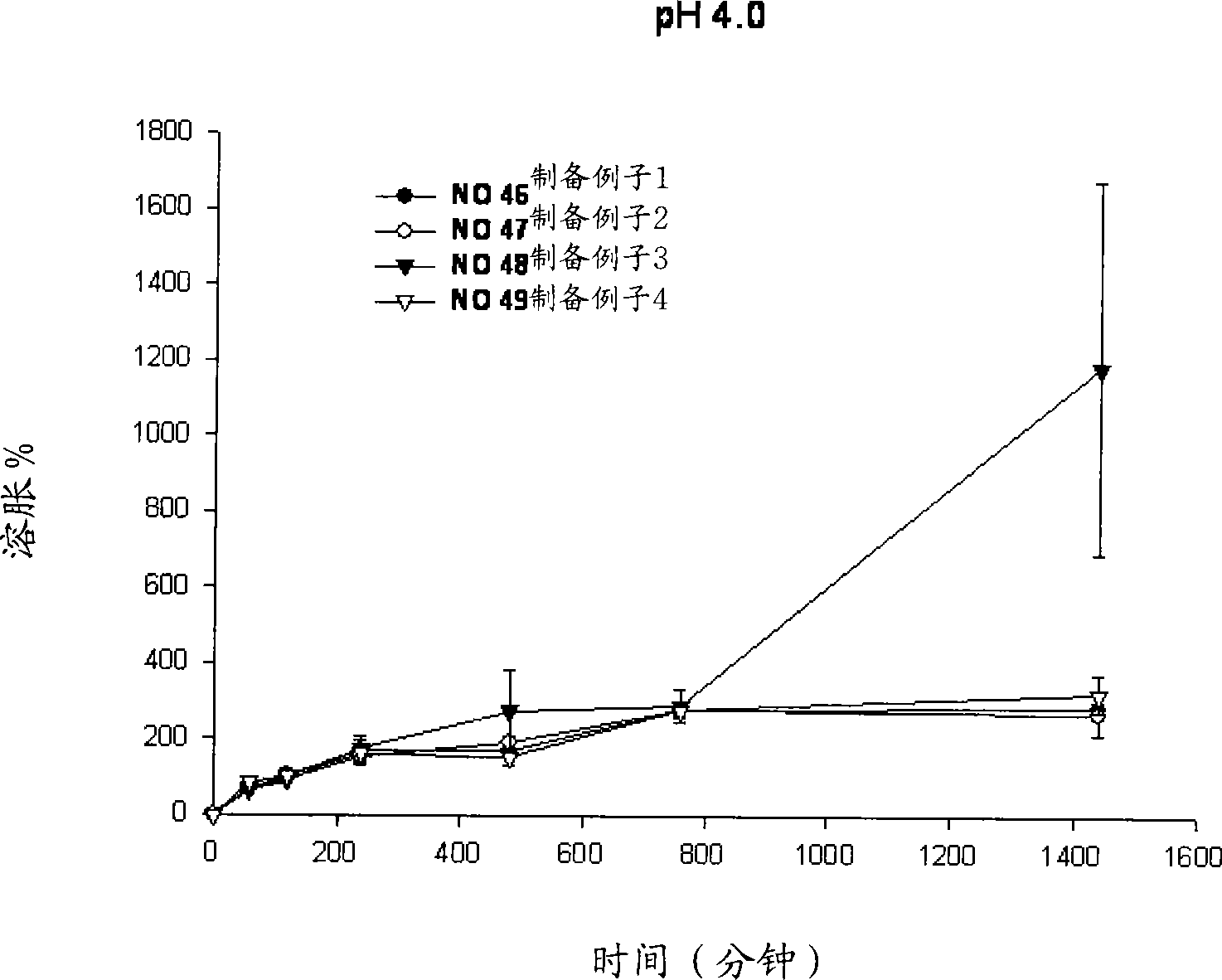
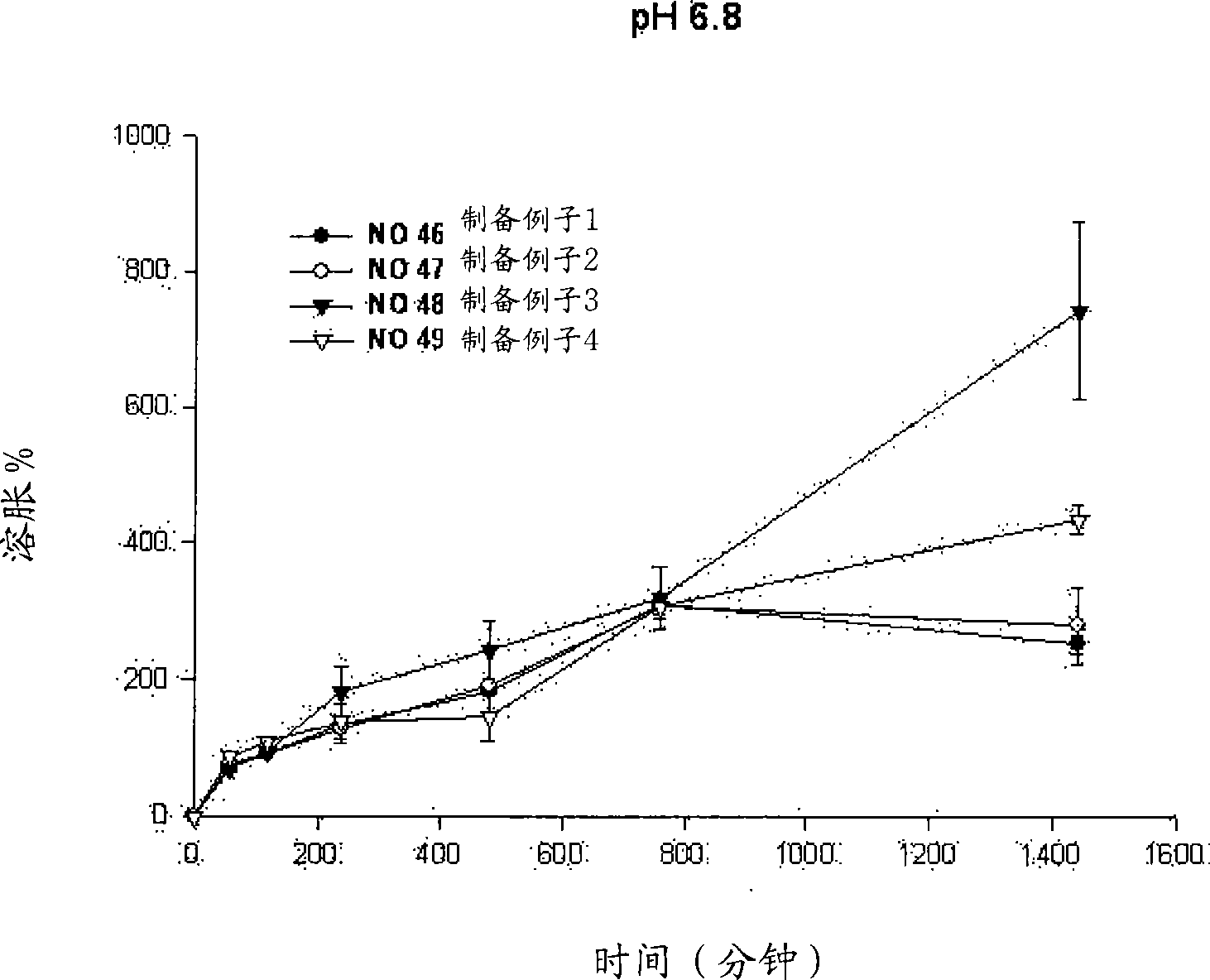
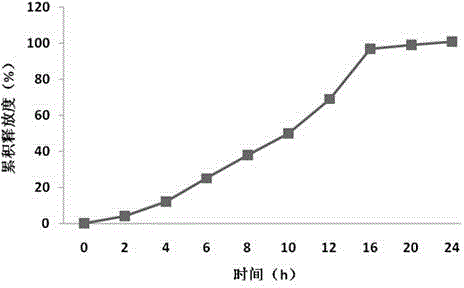
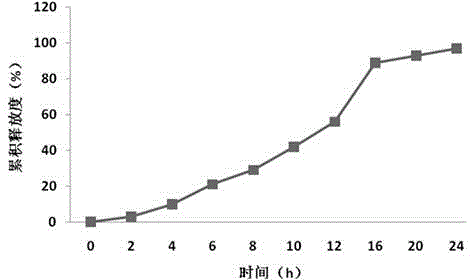
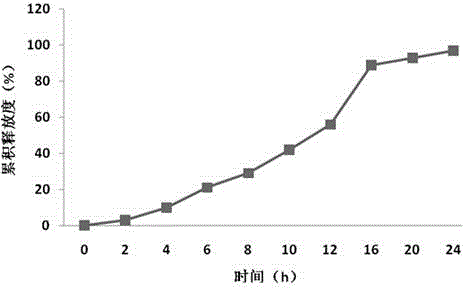
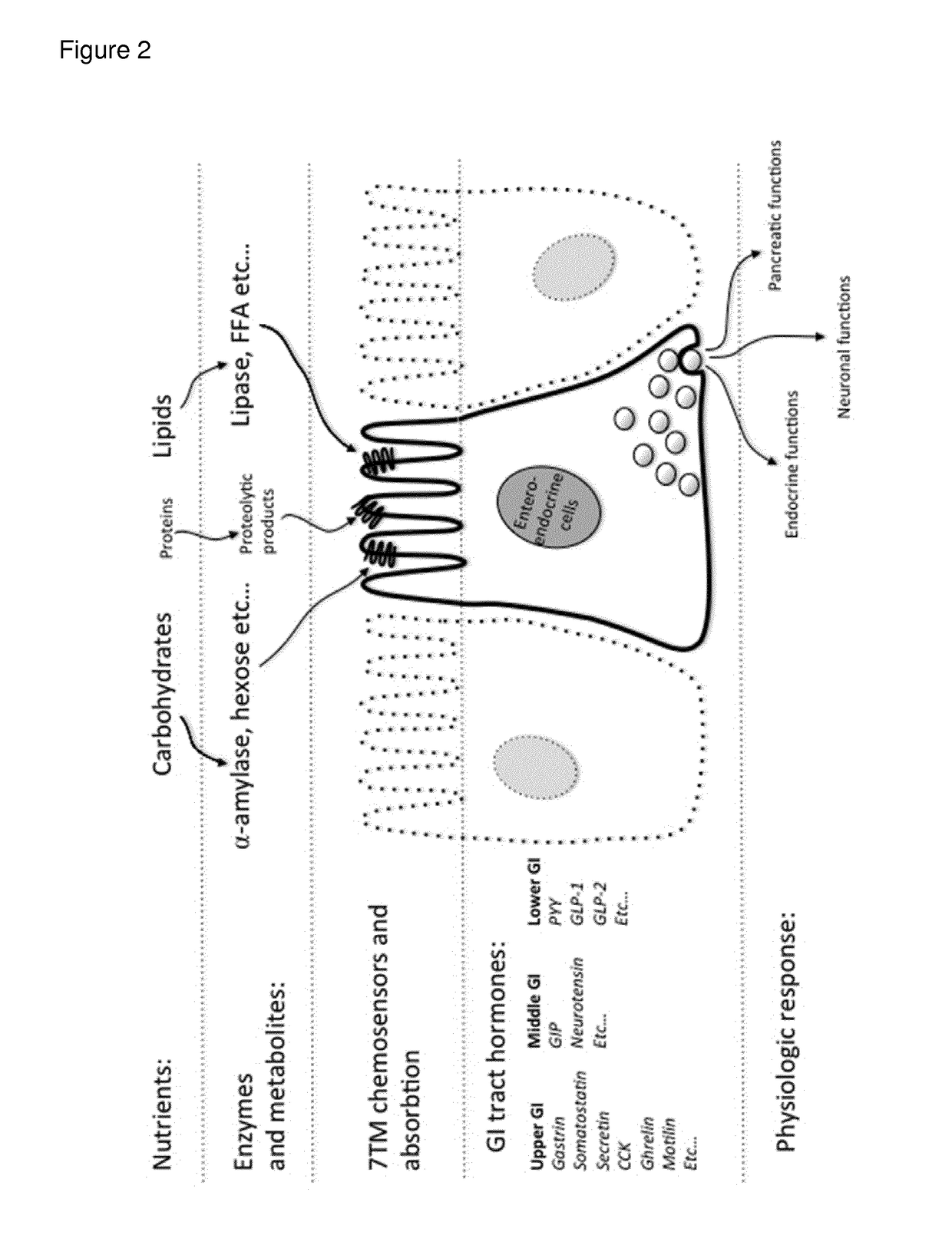
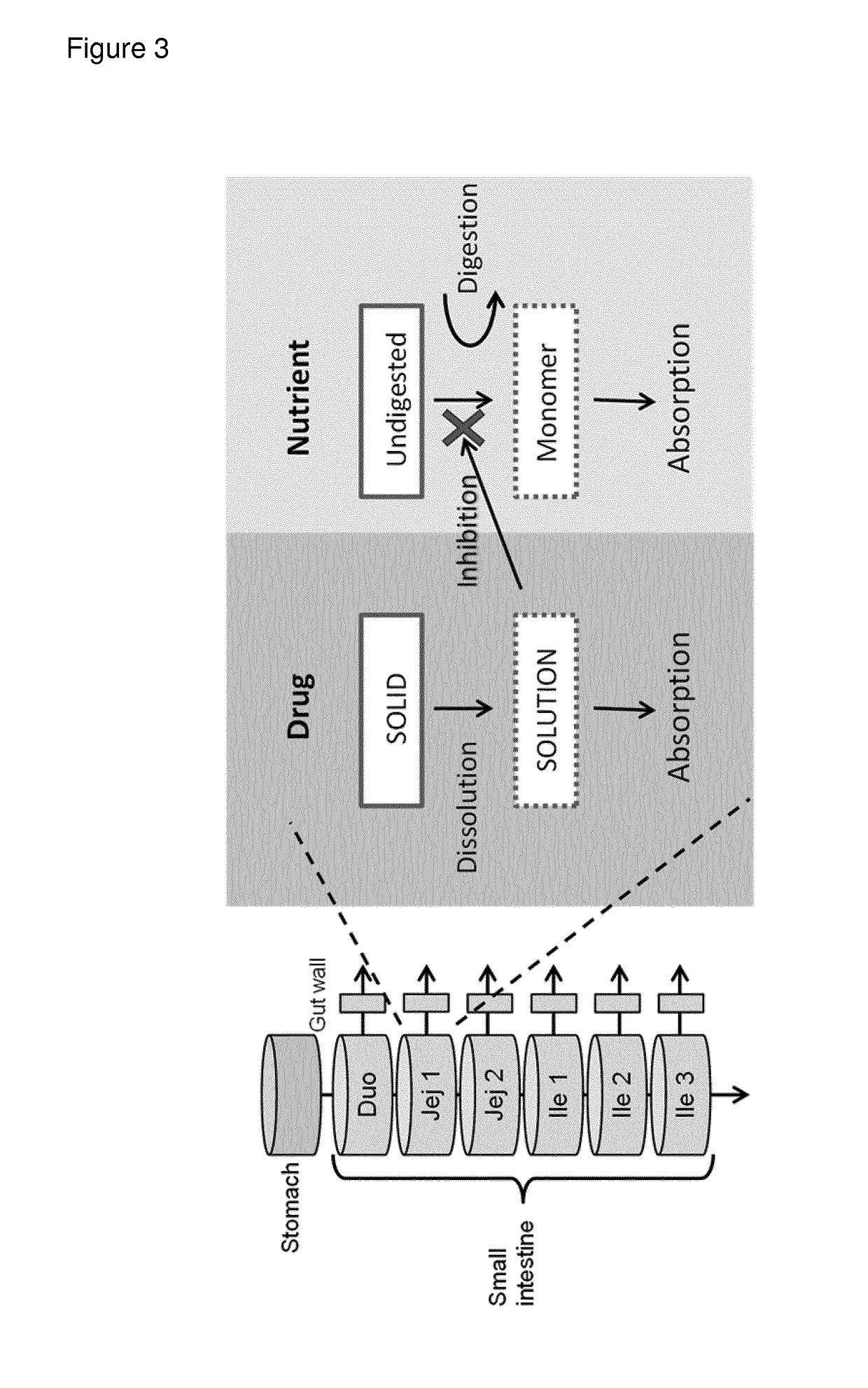
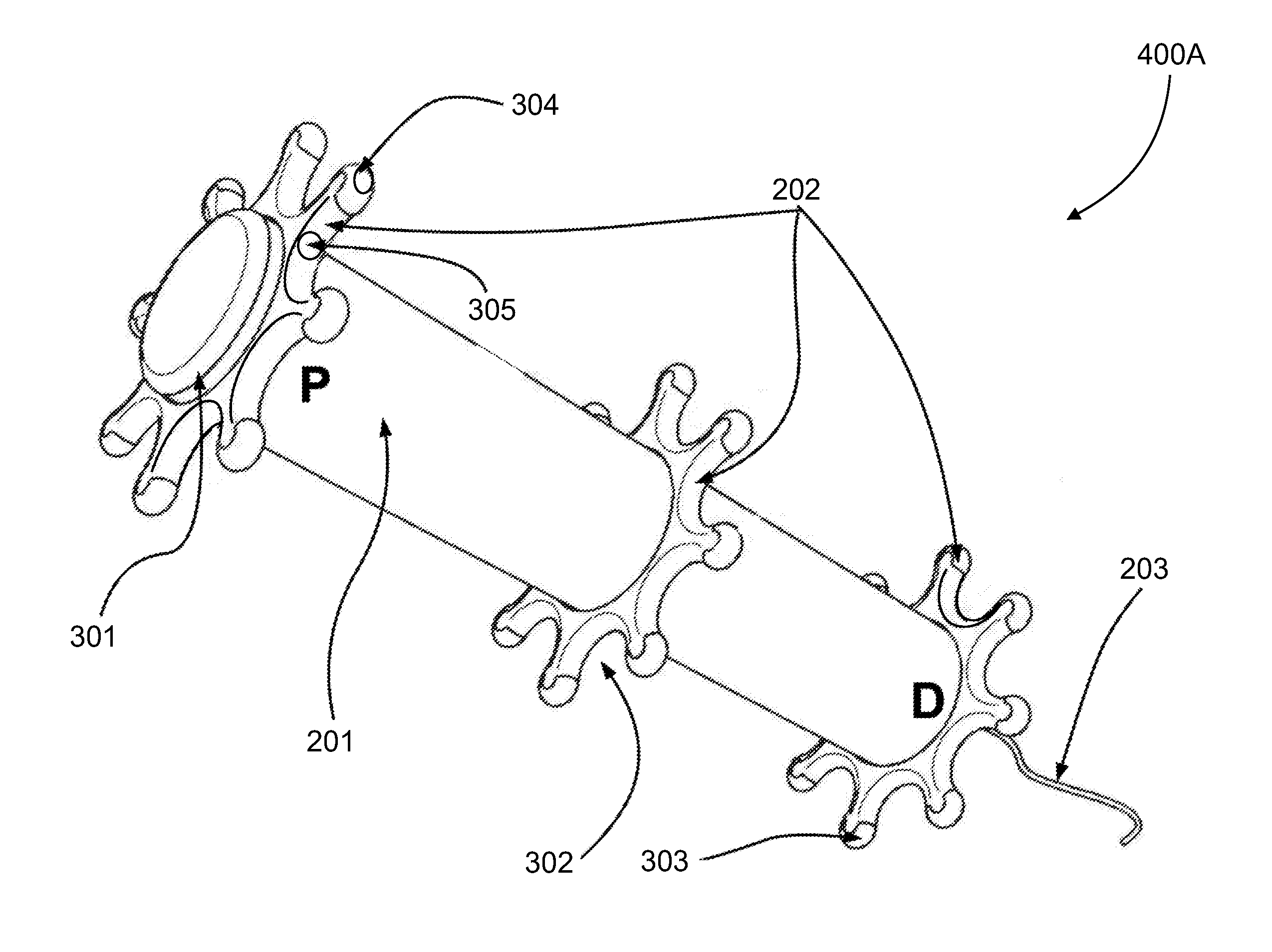
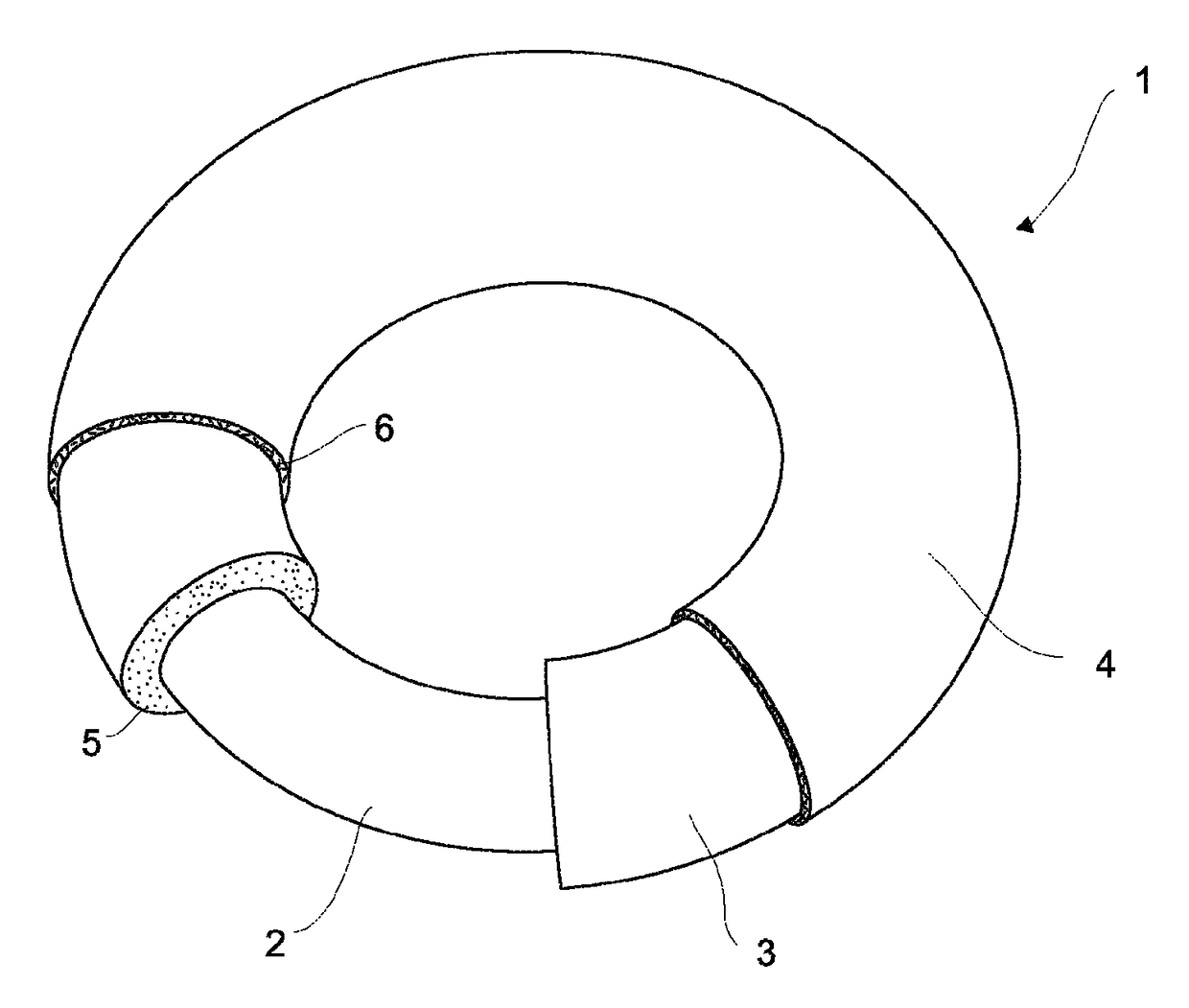
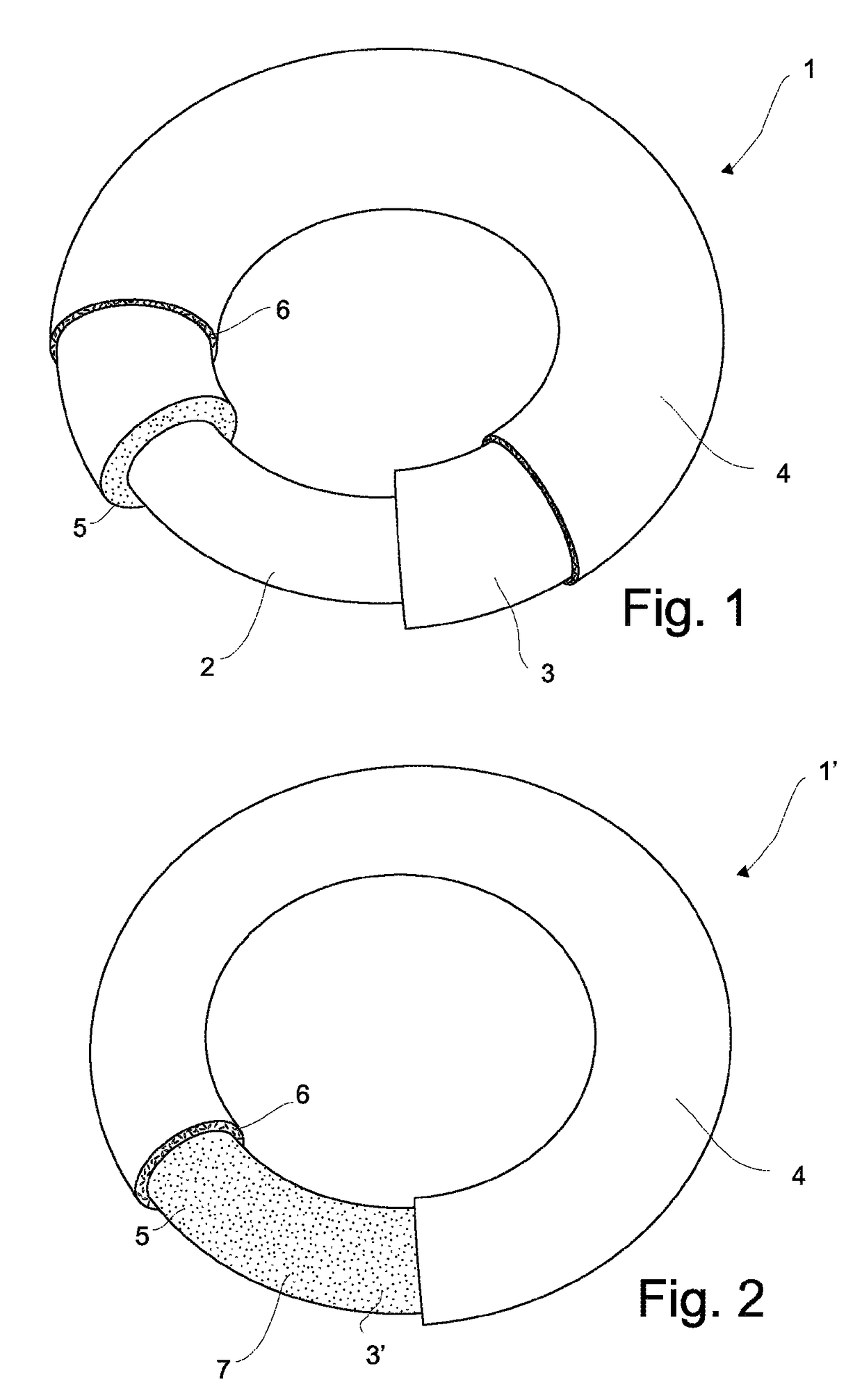
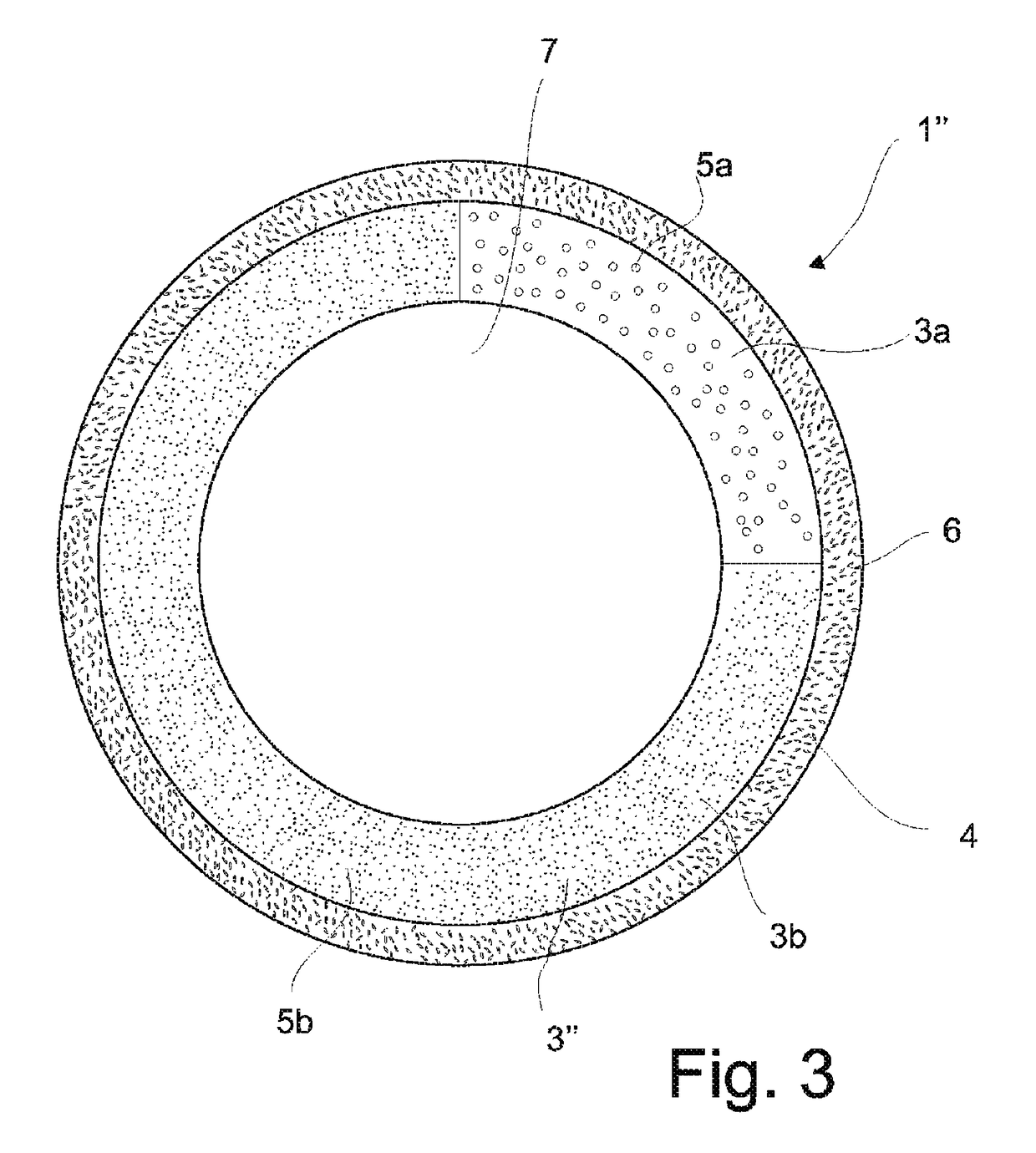
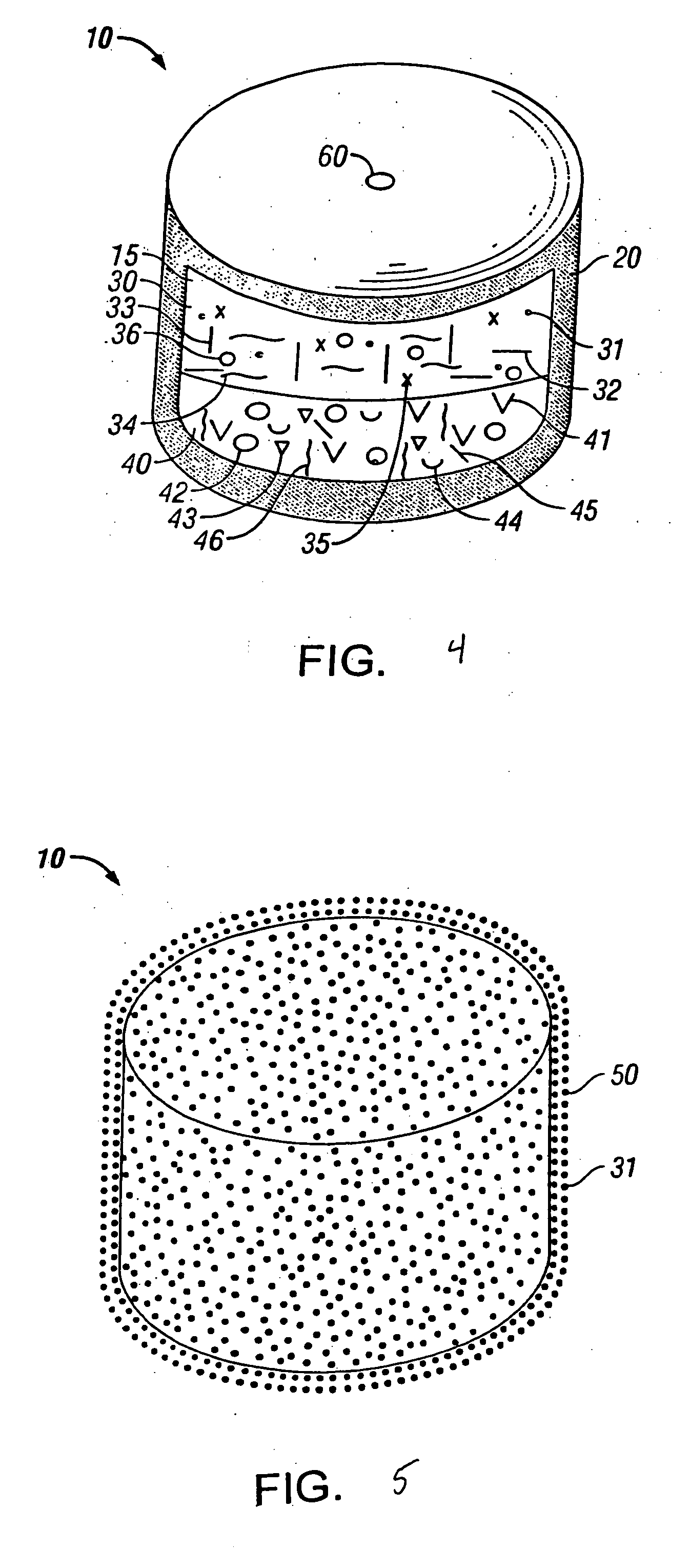
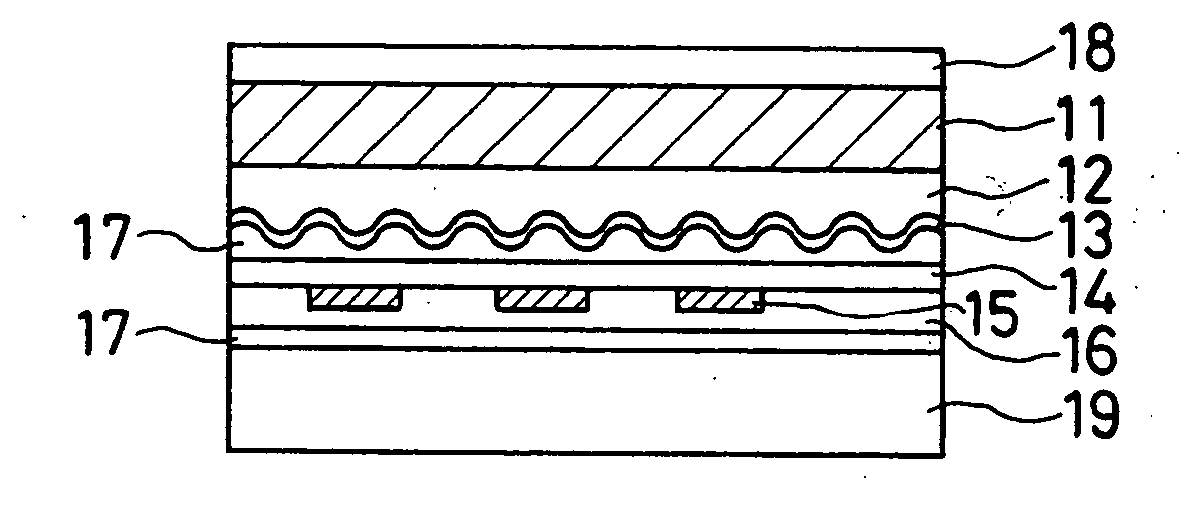
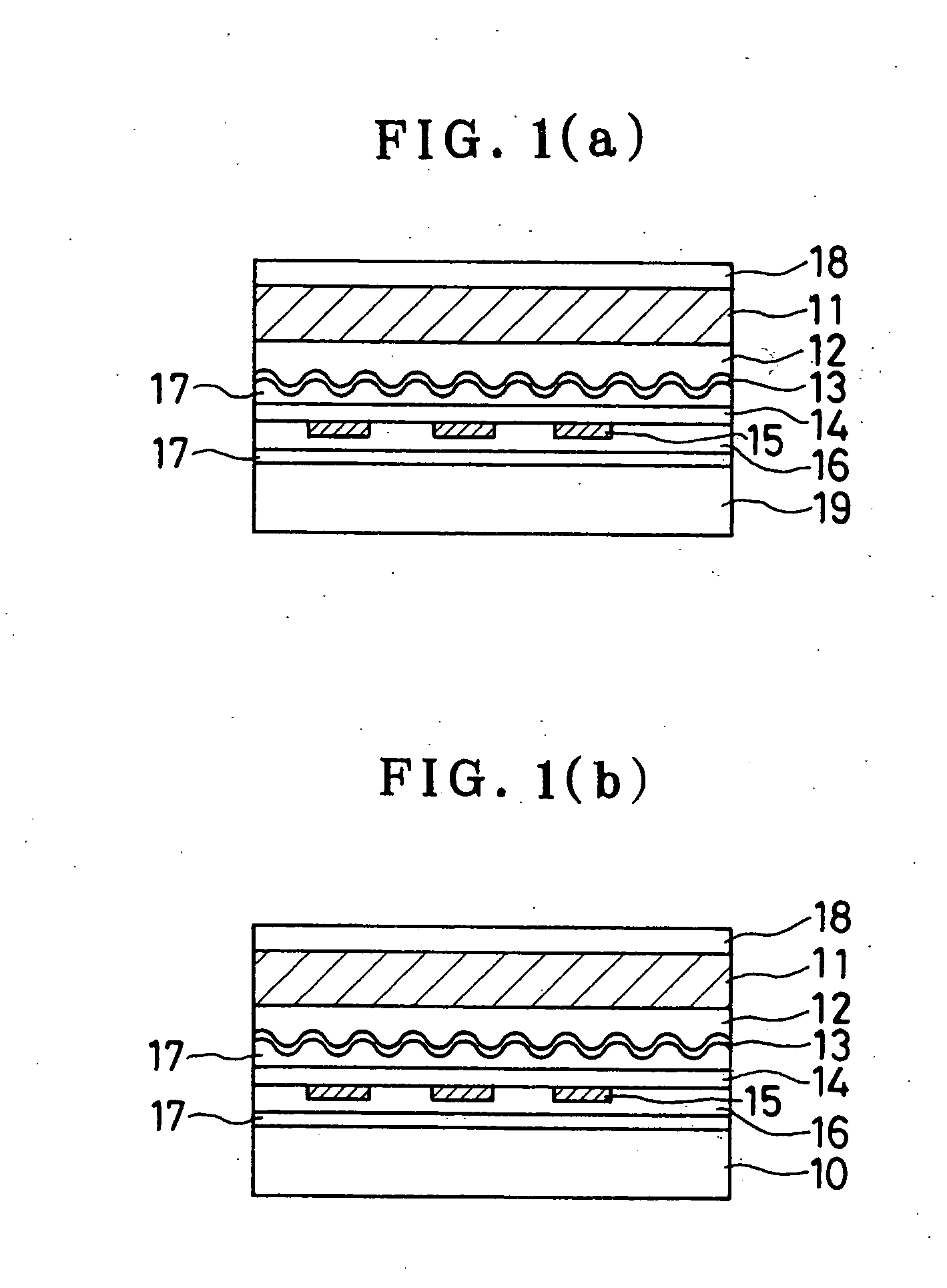
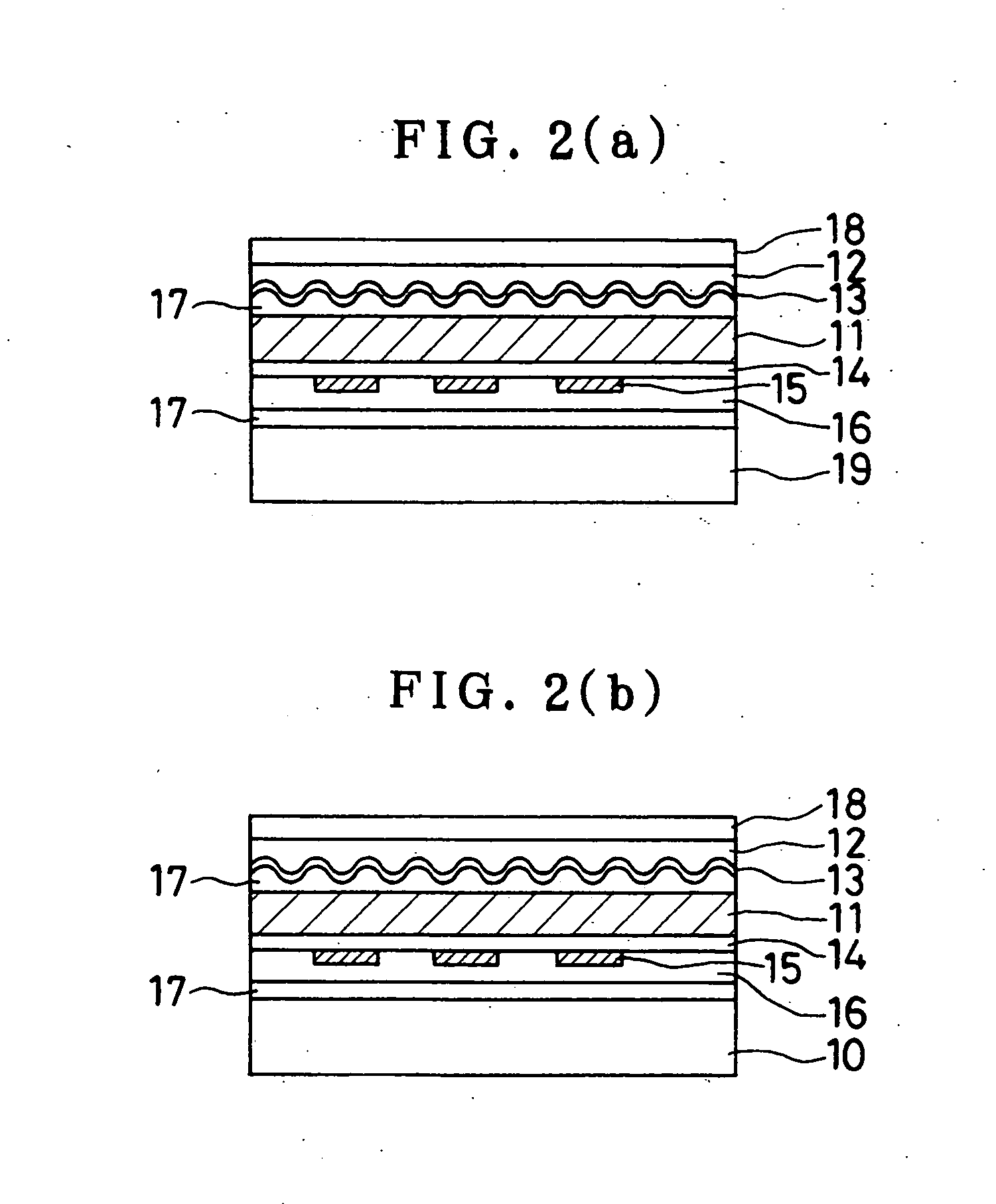
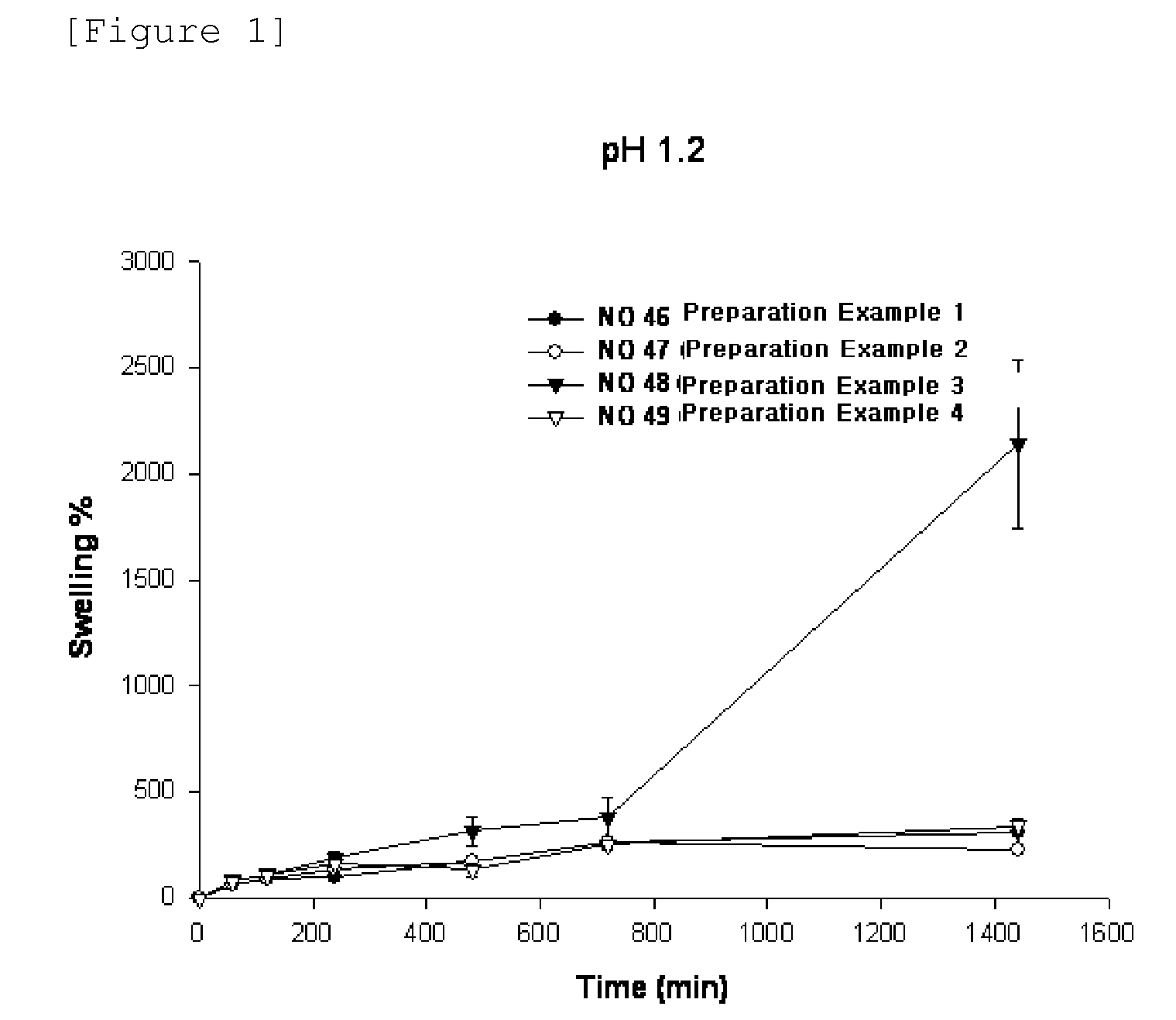
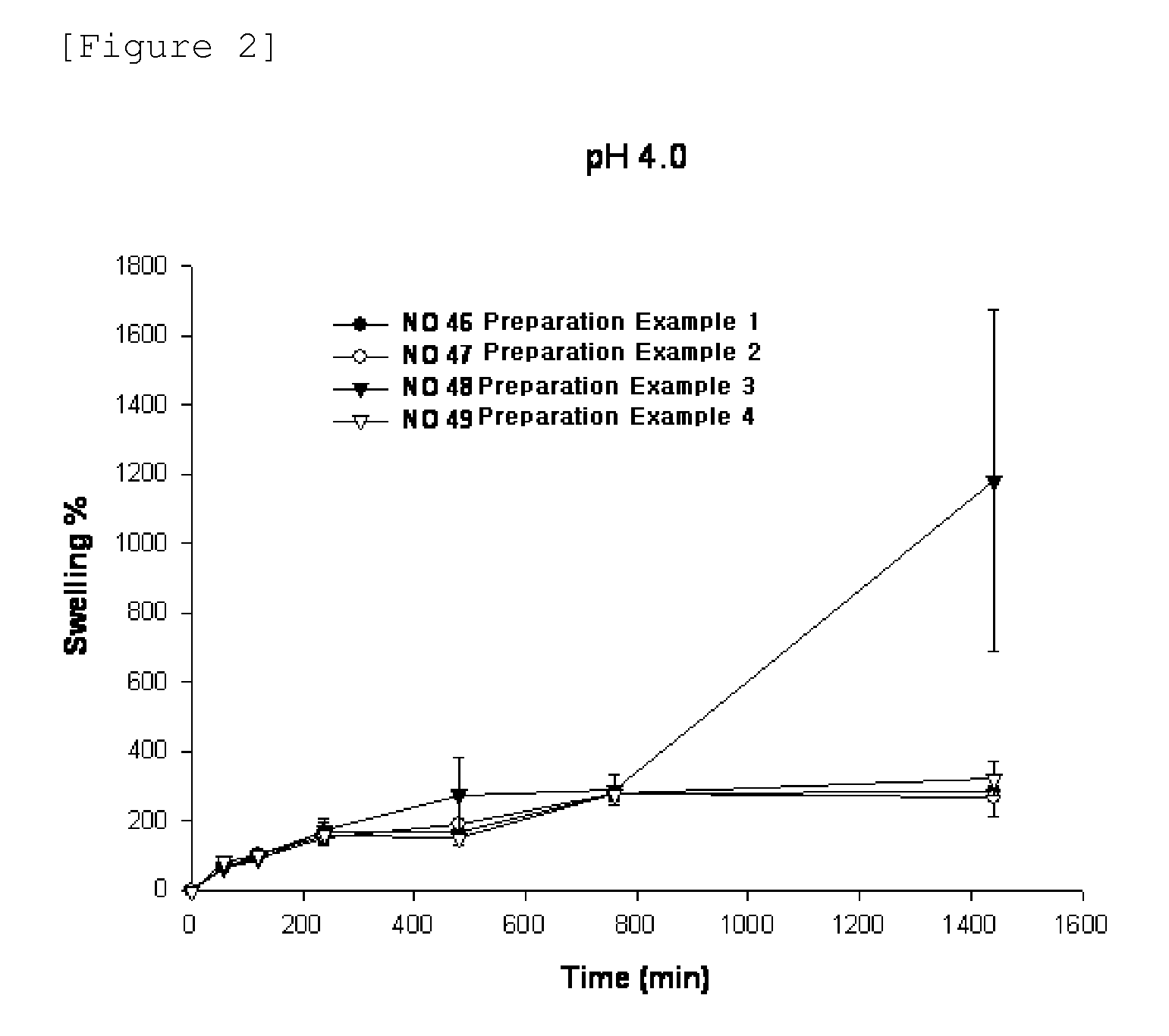
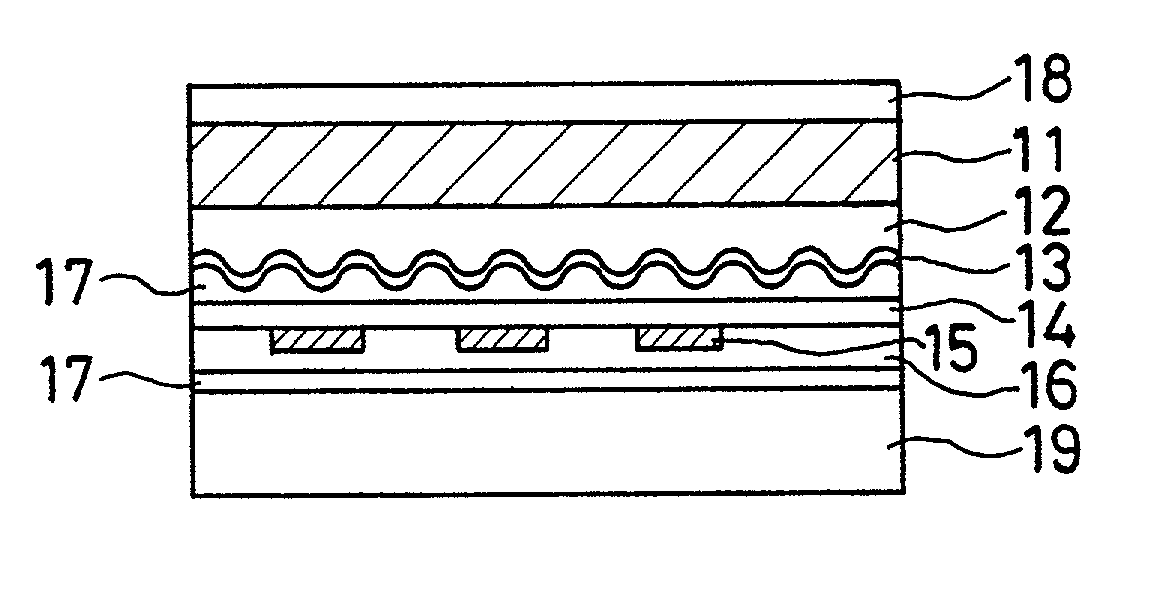
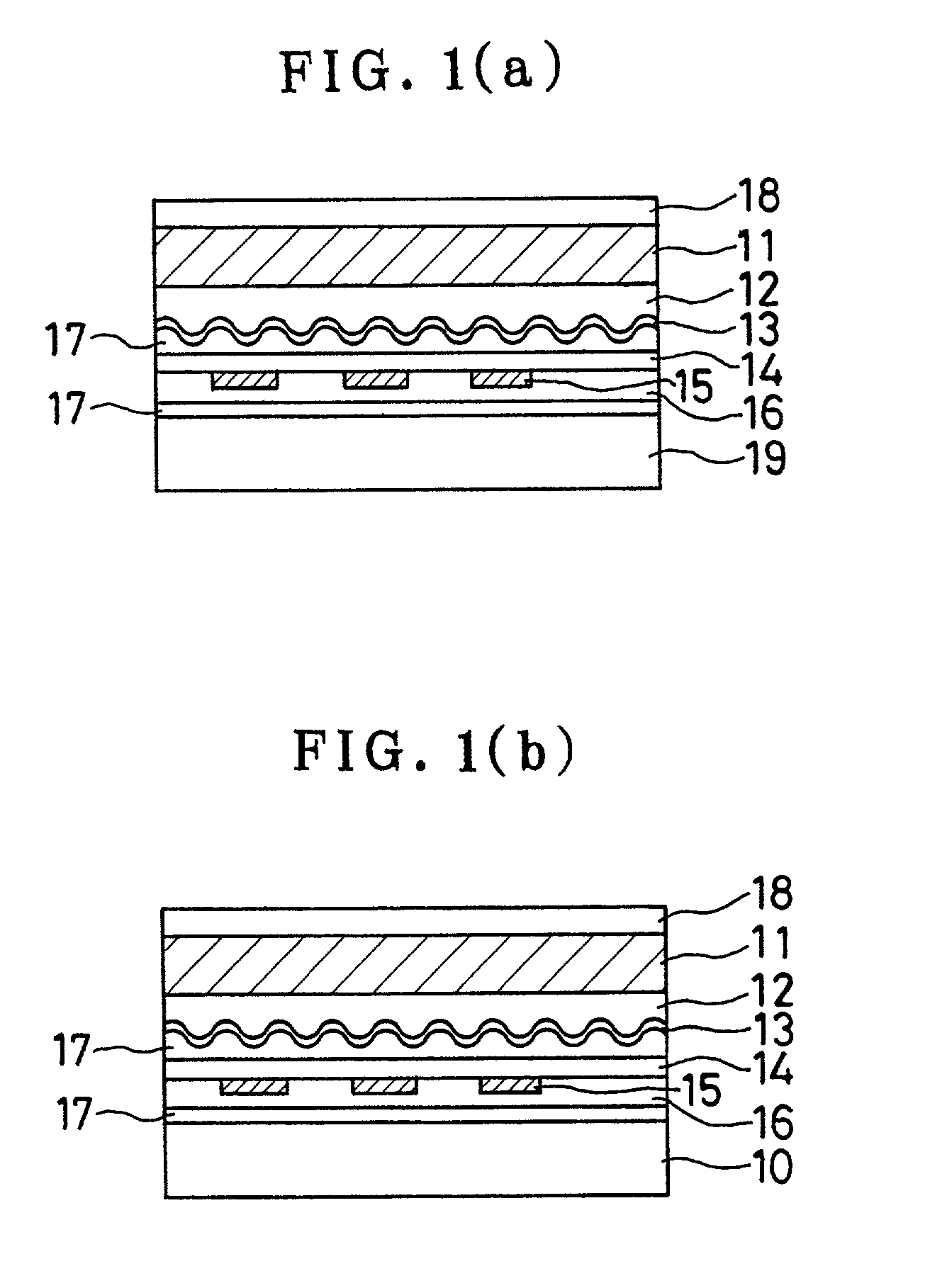
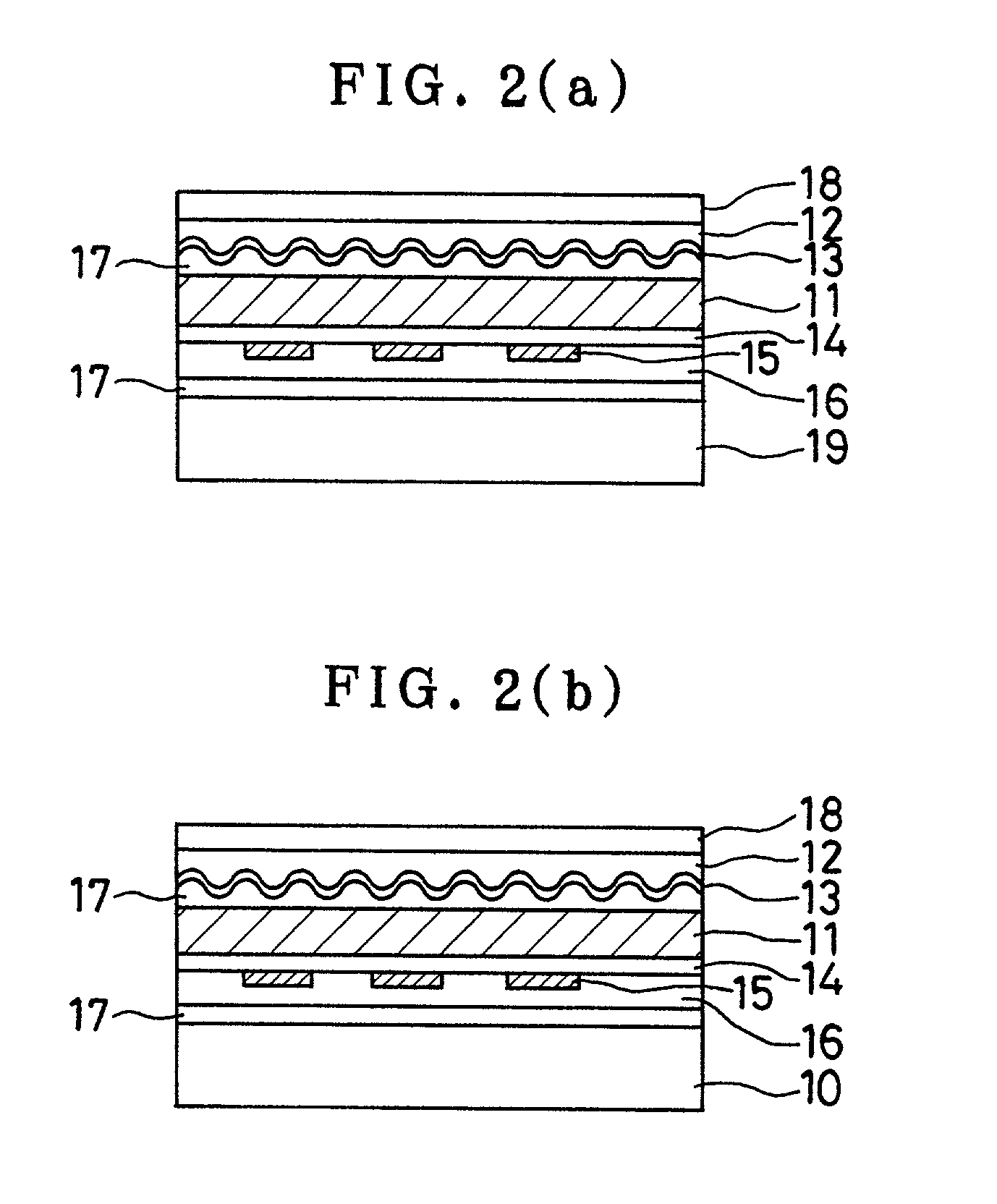
Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient
InactiveUS7041317B2Not cause periodical deterioration and coloringOrganic active ingredientsUrinary disorderWater insolubleBULK ACTIVE INGREDIENT
A sustained-release formulation of 5-acetyl-4,6-dimethyl-2-[2-[4-(2-methoxyphenyl)piperazinyl]ethylamino]pyrimidine trihydrochloride coated with a release-controlling film comprising a water-insoluble polymer film having no hydrophilic group. The formulation of the present invention has such a release pattern that the drug release lasts for 20 hours or more, so that can be appropriately administered for treatment. Furthermore, the formulation itself is so stable that its release pattern does not change with pH and the formulation does not suffer from deterioration, coloration and the like with the lapse of time.
Owner:ONO PHARMA CO LTD
Coated Granular Material and Method for Producing Coated Granular Material
A coated granular material of the present invention includes: a water-soluble granular substance; a coating layer applied to at least a surface of the water-soluble granular substance and containing a thermosetting resin A formed from a resin liquid having a molar ratio A of isocyanate groups to hydroxyl groups of 0.5 to 0.99; and a protection layer applied to the coating layer and containing a thermosetting resin B formed from a resin liquid having a molar ratio B of isocyanate groups to hydroxyl groups of 0.75 to 2.0, wherein the molar ratio B of the protection layer is greater than the molar ratio A of the coating layer. The present coated granular material is capable of achieving both of a short-term release pattern and an initial release limiting function and maintaining good quality.
Owner:CENT GLASS CO LTD
Vaginal carrier for the controlled release of substances
A device that delivers one or more substances into a vaginal lumen according to one of a plurality of release patterns. The device comprises a supporting structure, substance dispensers, and / or coupling elements which are configured to release one or more substances, for example, drugs, in manner designed to optimize treatment of one or more medical conditions.
Owner:ZIV ELAN
Pharmaceutical compositions for release control of methylphenidate
Disclosed is a pharmaceutical composition for release control comprising a plurality of particles for release control. The plurality of particles for release control comprise a core material containing methylphenidate and a polymer coating layer for release control formed on the core material. The plurality of particles for release control are divided into two or more groups based on the average thickness of the polymer coating layer for release control. The particle groups are identical in terms of the composition of the polymer in the polymer coating layer, but are different in terms of the average thickness of the coated layer. The pharmaceutical composition for release control according to the present invention may control the release pattern of methylphenidate contained in the core material as desired, and can be used as an oral formulation in a variety of forms such as orally disintegrating tablets, etc.
Owner:SAMYANG HLDG CORP
Hypnotic double-layer controlled release tablet and preparation method thereof
ActiveCN103690505AImprove physical stabilityTightly boundOrganic active ingredientsNervous disorderSide effectBlood concentration
The invention provides a hypnotic double-layer controlled release tablet, comprising a rapid-release layer and a slow-release layer, wherein the rapid-release layer is provided with pores which are filled with rapid-release particles; the rapid-release layer and the rapid-release particles are composed of hypnotic drug and pharmaceutical excipients; the slow-release layer is composed of a hypnotic drug, a slow-release material and pharmaceutical excipients. The hypnotic double-layer controlled release tablet has the following technical effects that 1) the physical stability of the double-layer tablet provided by the invention is better than that of the common double-layer table, which is advantageous for storage and transportation, and 2) a dissolution test detects that the disintegration time limit of the rapid-release layer of the double-layer tablet provided by the invention ranges from 20 seconds to 30 seconds; and the slow-release layer is in a zero-order release pattern, and therefore the effectiveness and safety of medication for a patient are greatly improved. In the preparation process of the double-layer tablet, the rapid-release particles fill in the pores. The drug release pattern of combined rapid release and slow release guarantees that the rapid-release layer is disintegrated rapidly after the drug is taken; as a result, the blood concentration is capable of quickly reaching the therapeutic window range; the slow-release layer is slowly released so that the therapeutic action is maintained for a long period of time and toxic and side effects are effectively controlled.
Owner:OVERSEAS PHARMA
Accurate information distribution system and method based on Internet
InactiveCN101079870ADoes not affect the normal online experienceTransmissionSpecial data processing applicationsInformation analysisDistribution system
The invention discloses a precise information releasing system and method based on internet, which comprises the following parts: information gathering unit, information analyzing unit, information releasing unit, wherein the information gathering unit is used to gather internet access data of user; the information analyzing unit proceeds statistical analysis for the internet access data gathered by the information gathering unit, which builds the access rule set and user set; the information releasing unit affirms the corresponding user to rule element according to the released information, which proceeds automatic release for directional information without assembling random realizing information at user terminal; when the releasing information doesn' t exist, the normal internet surf is not influenced with different releasing patterns according to different information and user characters.
Owner:沙健松
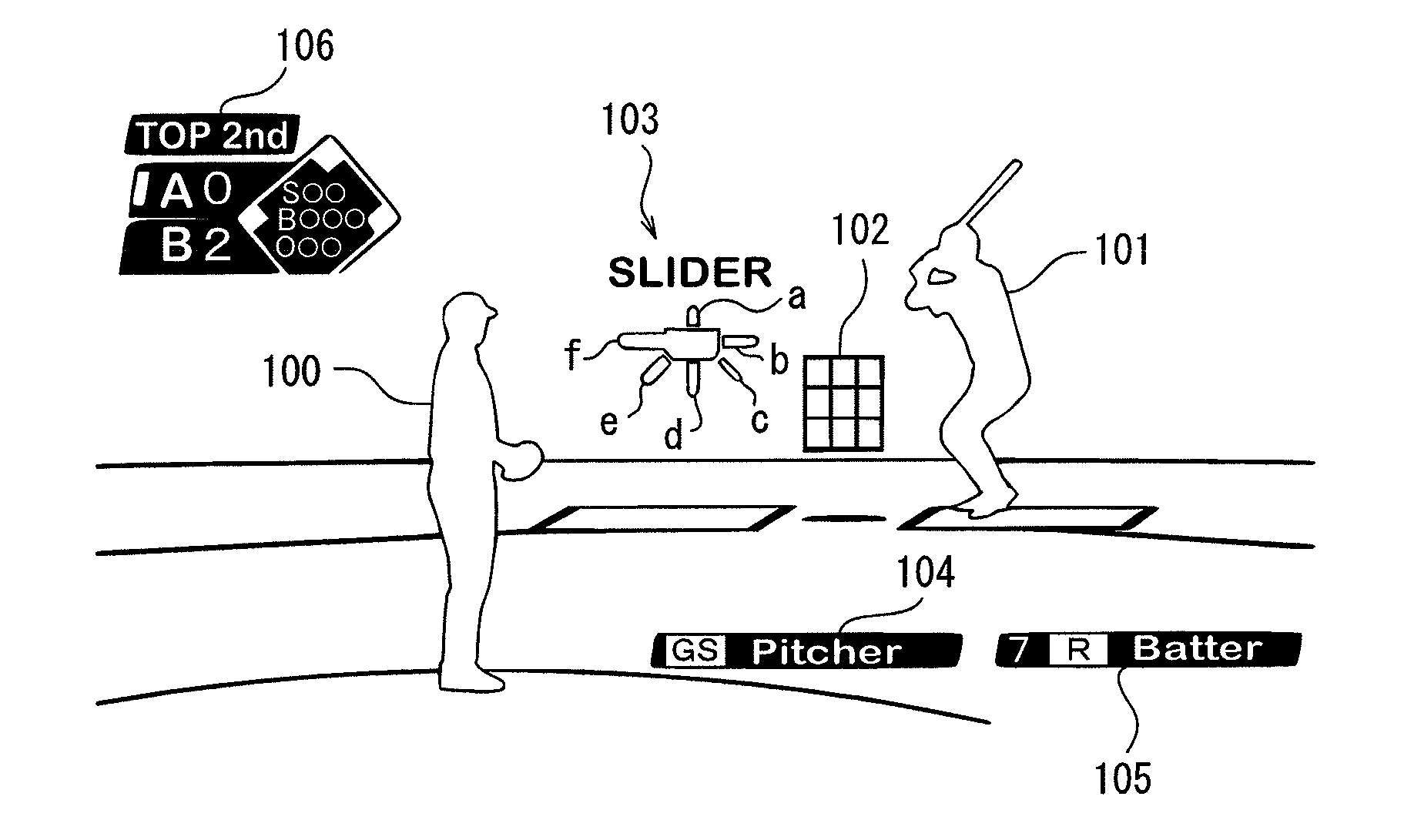
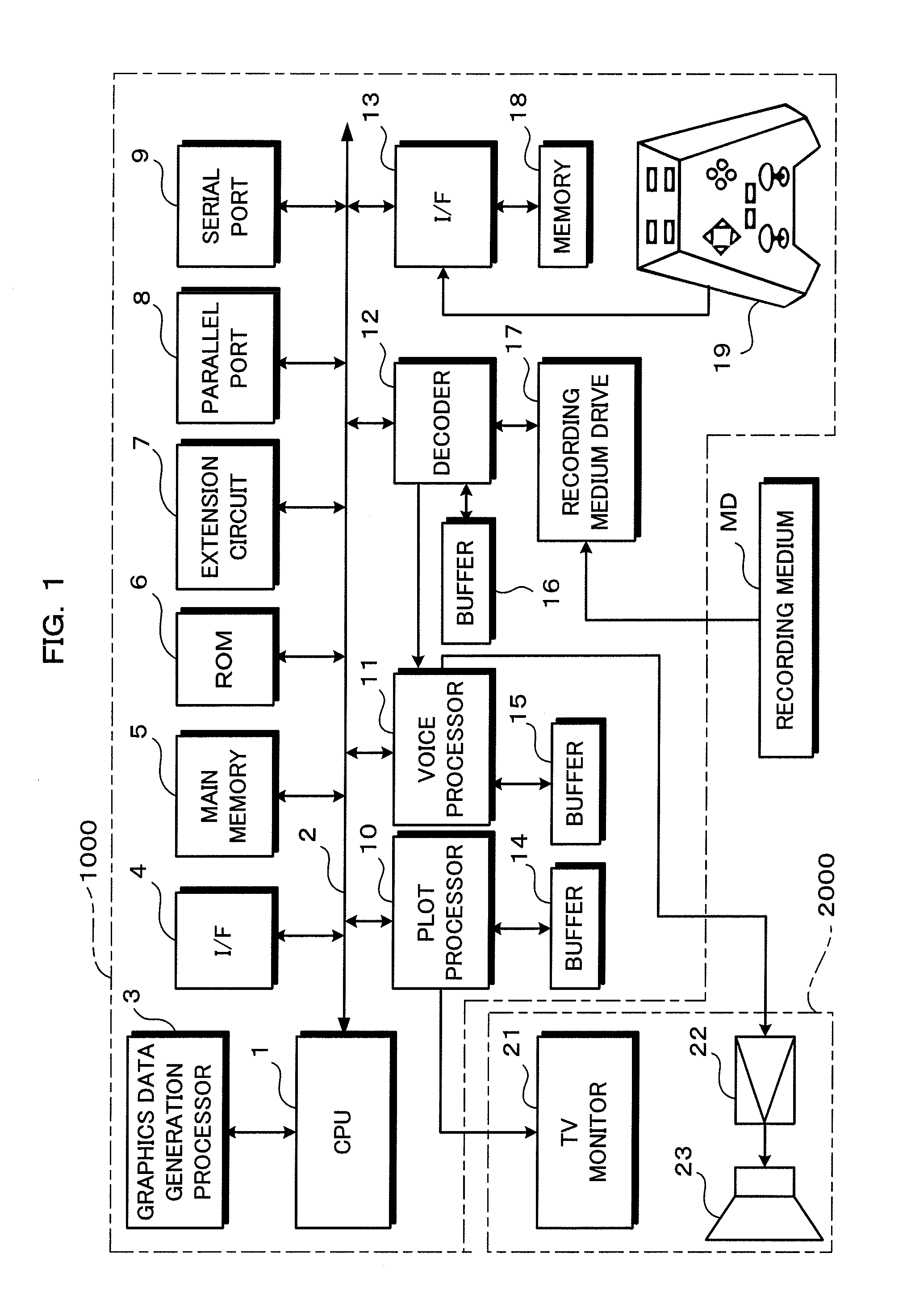
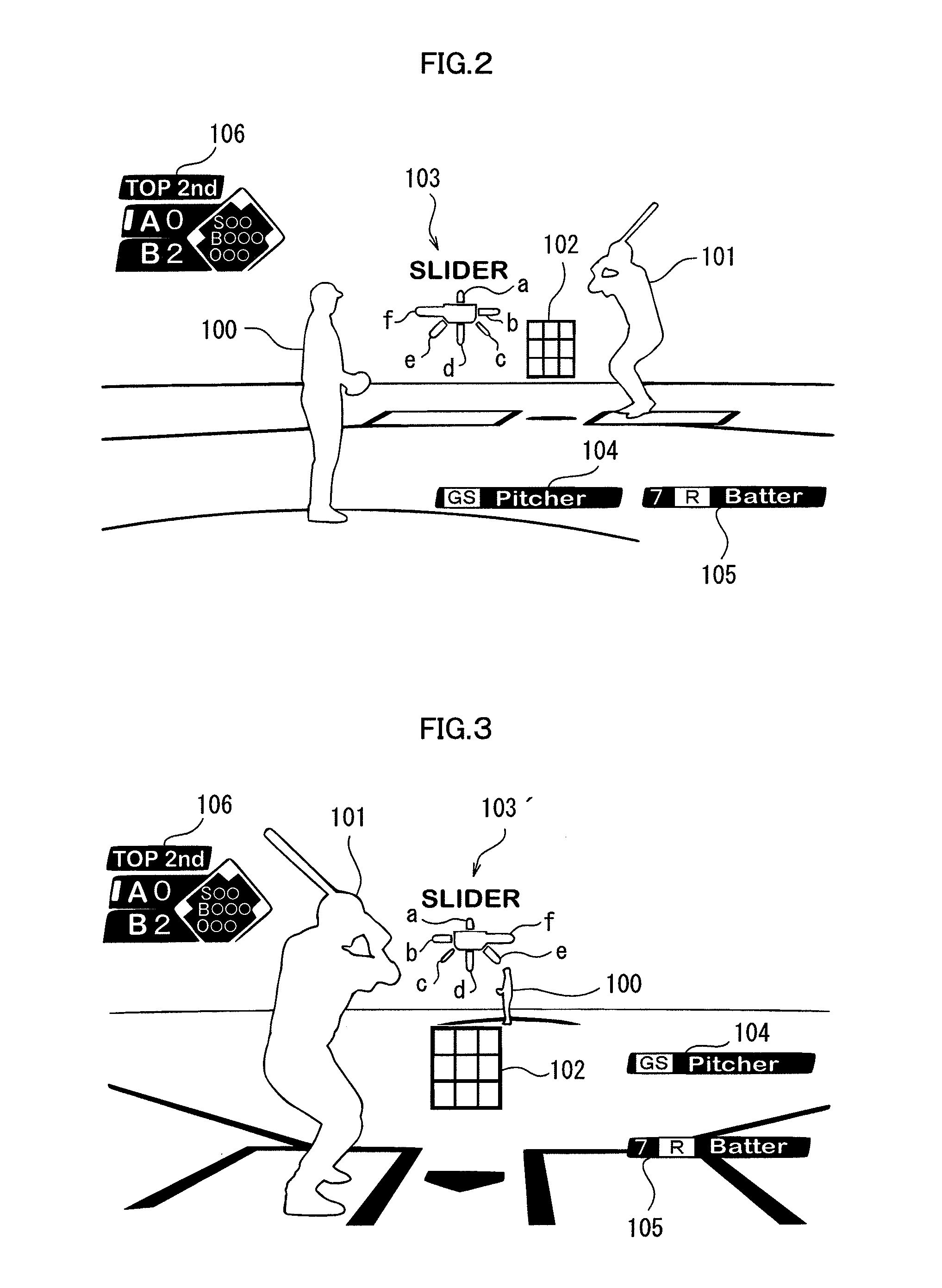
Gaming device, game control method, and recording medium
InactiveUS20120258780A1Reduce difficultyVideo gamesSpecial data processing applicationsPattern matchingSimulation
A gaming device includes a release pattern prediction section which determines a prediction release pattern for the release pattern of the flying object to be released by the first personified character by selecting one of the plurality of release patterns stored in the release pattern storing section based on a selecting operation by a user operating the second personified character; and a difficulty adjustment section, which compares the release pattern determined with the prediction release pattern, and reduces the difficulty for the second character to hit back the flying object released by the first character when the release pattern determined and the prediction release pattern match.
Owner:KONAMI DIGITAL ENTERTAINMENT CO LTD
Novel controlled release-niacin formulation
InactiveCN101380290AHas a water soluble baseOrganic active ingredientsMetabolism disorderBlood concentrationSecondary hyperlipidemia
The present invention relates to a controlled-release niacin formulation. In particular, the present invention relates to a controlled-release niacin formulation, comprising niacin; hydroxypropyl methylcellulose; and a carboxyvinyl polymer, in which the carboxyvinyl polymer and hydroxypropyl methylcellulose are contained in a predetermined weight ratio, and to a preparation method thereof. The controlled-release niacin formulation according to the present invention maintains its matrix shape until completion of release, and maintains its release pattern without fluctuation for a desired time period, unlike a commercial formulation. In particular, since niacin formulations are used for long-term treatment of hyperlipidemia, the controlled-release niacin formulation of the present invention, capable of maintaining effective blood concentration and high stability for a long period of time, is very useful.
Owner:SEOUL PHARMA
A sleeping class double-layer controlled-release tablet and preparation method thereof
ActiveCN103690505BImprove physical stabilityTightly boundOrganic active ingredientsNervous disorderSide effectControl release
Owner:OVERSEAS PHARMA
Posaconazole double-layer osmotic pump controlled release tablet and preparation method thereof
InactiveCN103948558AReduced responseDelay drug resistanceOrganic active ingredientsAntimycoticsIn vivoPharmaceutical formulation
The invention belongs to the field of medicinal preparation and specifically provides a posaconazole double-layer osmotic pump controlled release tablet and a preparation method thereof. The tablet comprises a tablet core and a semipermeable controlled release film. The tablet core comprises a drug containing layer and a boosting layer. The drug containing layer is located inside the controlled release film which is provided with a drug releasing hole. The boosting layer is located inside the controlled release film at a place far away from the drug releasing hole. One end of the water-permeable controlled release film is provided with one or more drug releasing holes. The release curve of the preparation drug shows a zero-order release pattern, and thus the problem of drug resistance caused by long-term use of posaconazole dosage forms on the market is solved. Besides, the problems that the preparation technology is complex, the preparation cost is high, and the drug is unsuitable for industrialized mass production due to the adoption of a hot-melt extrusion technology are solved. Simultaneously, the in vivo-in vitro correlation of the drug is better ensured and the clinical monitoring of plasma concentration is better facilitated.
Owner:JINAN KANGHE MEDICAL TECH
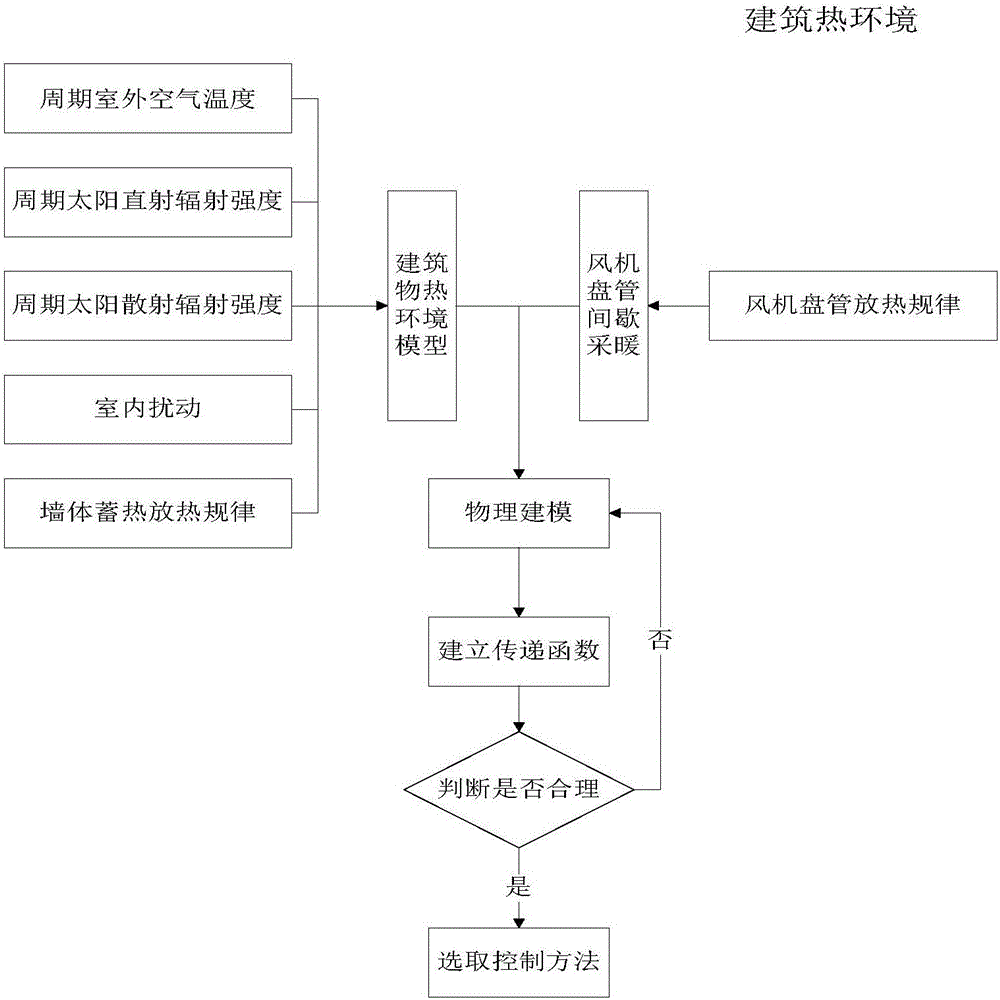
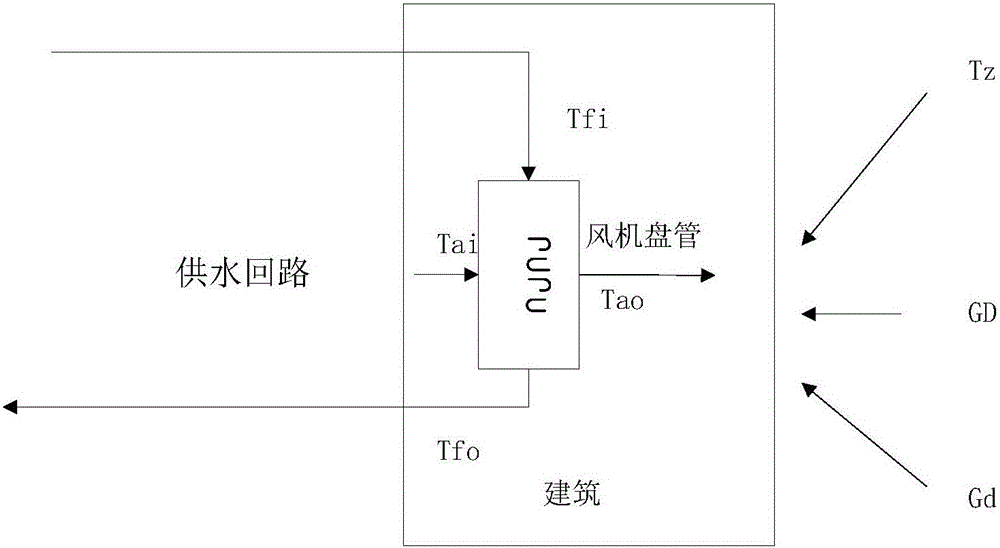
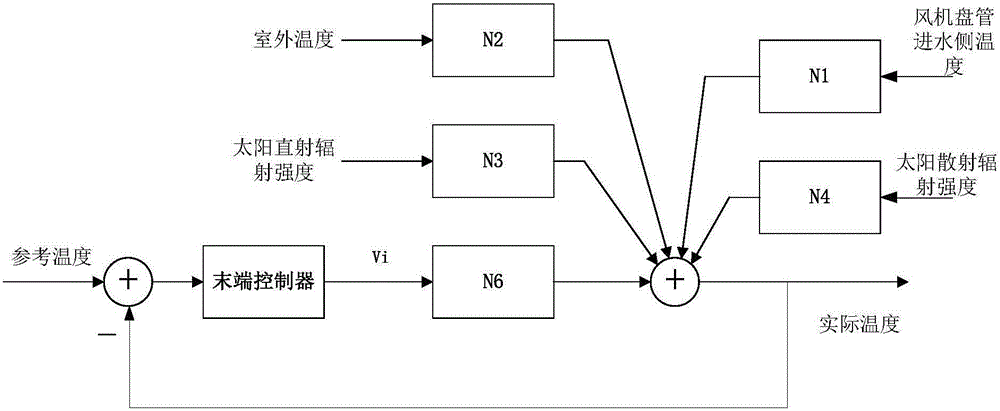
Building thermal environment control modeling method based on physical properties
The invention discloses a building thermal environment control modeling method based on physical properties. The building thermal environment control modeling method determines an hourly thermal environment model of a building through analyzing period outdoor air temperature, solar scattering direct radiation intensity, solar scattering radiation intensity, a wall body heat-storage-release pattern and indoor disturbance, establishes a draught fan coil pipe model through analyzing a heat-releasing pattern of the indoor draught fan coil pipe, establishes the thermal environment control model through analyzing two parts, solves relations between indoor temperature and other influence factors through laplace's transformation, and finally chooses a control variable to control and enables indoor temperature to maintain in a reasonable range. The building thermal environment control modeling method establishes a model on the basis of architectural thermal engineering, combines with thermal engineering characteristics of the draught fan coil pipe, can more accurately describe dynamic characteristics of the architecture thermal environment and provides a model support for subsequent control.
Owner:XI'AN UNIVERSITY OF ARCHITECTURE AND TECHNOLOGY
Pharmaceutical compositions for release control of methylphenidate
Disclosed is a pharmaceutical composition for release control comprising a plurality of particles for release control. The plurality of particles for release control comprise a core material containing methylphenidate and a polymer coating layer for release control formed on the core material. The plurality of particles for release control are divided into two or more groups based on the average thickness of the polymer coating layer for release control. The particle groups are identical in terms of the composition of the polymer in the polymer coating layer, but are different in terms of the average thickness of the coated layer. The pharmaceutical composition for release control according to the present invention may control the release pattern of methylphenidate contained in the core material as desired, and can be used as an oral formulation in a variety of forms such as orally disintegrating tablets, etc.
Owner:SAMYANG HLDG CORP
Vaginal carrier for the controlled release of substances
A device that delivers one or more substances into a vaginal lumen according to one of a plurality of release patterns. The device comprises a supporting structure, substance dispensers, and / or coupling elements which are configured to release one or more substances, for example, drugs, in manner designed to optimize treatment of one or more medical conditions.
Owner:ZIV ELAN
Method of making self-aligned MEMS piezoresistive accelerometer
ActiveCN106564857AAchieve self-alignmentGood symmetryAcceleration measurement using interia forcesDecorative surface effectsElectrical resistance and conductanceLithography process
The invention discloses a method of making a self-aligned MEMS piezoresistive accelerometer, comprising the following steps: making a cavity at the bottom of a silicon substrate through a KOH etching process; growing an oxide layer and a silicon nitride layer on the top of the silicon substrate in sequence from bottom to top; making a piezoresistive pattern and a structure release pattern on the silicon nitride layer at the same time through a lithography process, and etching the silicon nitride layer at the piezoresistive pattern and at the structure release pattern; etching the oxide layer at the piezoresistive pattern, and forming a piezoresistor through an injecting process and an annealing process; etching the oxide layer at the structure release pattern to form a structure release area; deeply etching the silicon substrate in the structure release area to form a movable structure, thus getting an MEMS piezoresistive accelerometer. The piezoresistive pattern and the structure release pattern are formed in the same lithography process. There is no need for equipment to carry out alignment in the machining process. Self-alignment of the piezoresistor and a device structure is realized. Thus, the symmetry of devices is improved, error caused by position deviation of the piezoresistor is reduced, and the performance of devices is improved.
Owner:NORTH ELECTRON RES INST ANHUI CO LTD
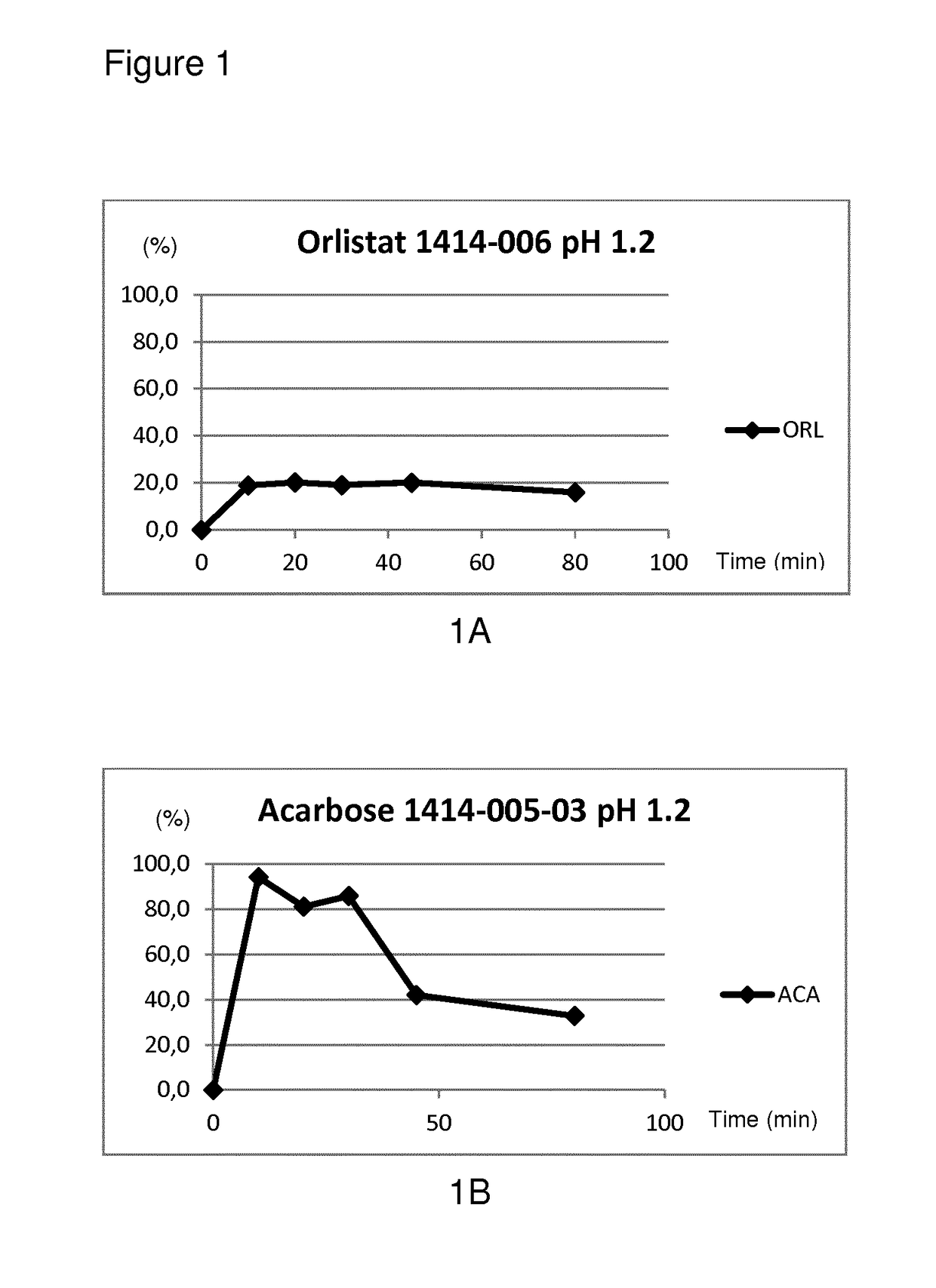
Modified release composition of orlistat and acarbose for the treatment of obesity and related metabolic disorders
ActiveUS20170360715A1Reduce intakeOptimize absorptionOrganic active ingredientsMetabolism disorderOrlistatTotal dose
The present invention relates to a modified-release composition comprising orlistat and acarbose, comprising individually distinct parts with different release patterns: a) a first part, G1, comprising from about 5 to about 70% w / w of the total dose of acarbose, b) a second part, G2A, comprising from about 30 to about 95% w / w of the total dose of acarbose, c) a third part, G2B, comprising from about 10 to about 90% w / w of the total dose of orlistat, and d) a fourth part, G3, comprising from about 10 to about 80% w / w of the total dose of orlistat, and the total concentration of acarbose and orlistat, respectively, in the composition is 100% w / w.
Owner:EMPROS PHARMA AB
Contraceptive medicament preparation containing gestodene
ActiveCN101810626AHigh cumulative releaseOrganic active ingredientsSexual disorderMedicineDistribution characteristic
The invention relates to a contraceptive medicament preparation containing gestodene, which is dosed through natural orifice of human beings or mammals or through transdermal or subcutaneous administration. The gestodene is in a zero order release pattern. The contraceptive medicament preparation contains 0.0005 mg to 0.100 mg of gestodene of daily dose, and one or more kinds of pharmaceutical acceptable excipient or carriers. The contraceptive medicament preparation is characterized in that the gestodene is a crystal or powders with the weight specific surface area more than 100 cm2 / g. The minimal distance between the geometric center of the crystal with more than or equal to 70% of weight proportion and the surface of the preparation is less than or equal to 5 mm. The minimal distance between the geometric center of the powders with more than or equal to 70% of weight proportion and the surface of the preparation is less than or equal to 5 mm. The contraceptive medicinal preparation contains 0.005 mg to 0.050 mg ethinyloestradiol of daily dose. In the invention, the distribution characteristics of the size and the design of the drug is selected, the drug can be in a better zero order release pattern, and the cumulative release rate is higher.
Owner:LIAONING AIMU MEDICAL SCI&TECH
Vaginal carrier for the controlled release of substances
InactiveUS20160287853A1Suppositories deliveryMedical devicesControlled releaseBiomedical engineering
A device that delivers one or more substances into a vaginal lumen according to one of a plurality of release patterns. The device comprises a supporting structure, substance dispensers, and / or coupling elements which are configured to release one or more substances, for example, drugs, in manner designed to optimize treatment of one or more medical conditions.
Owner:ZIV ELAN
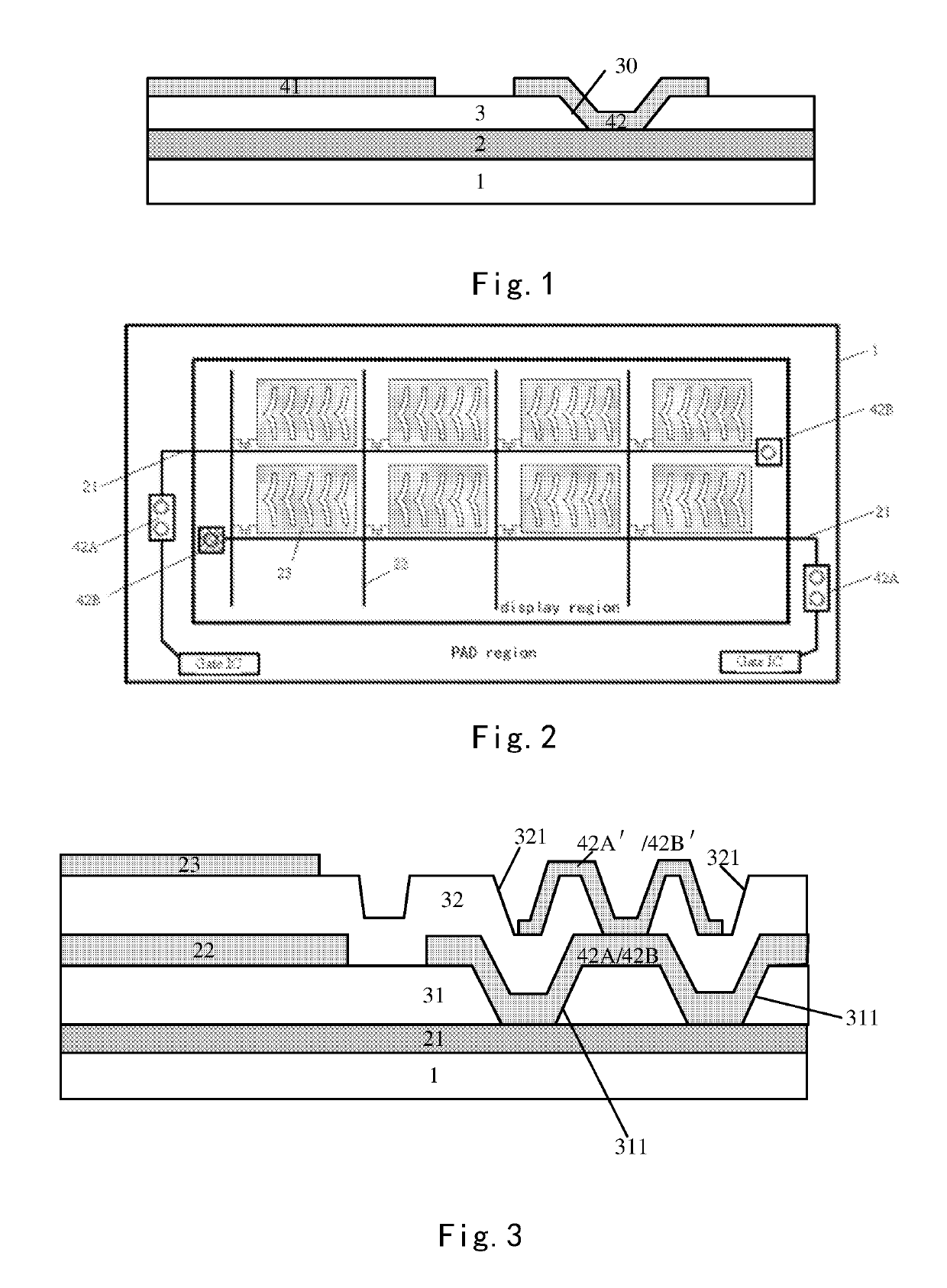
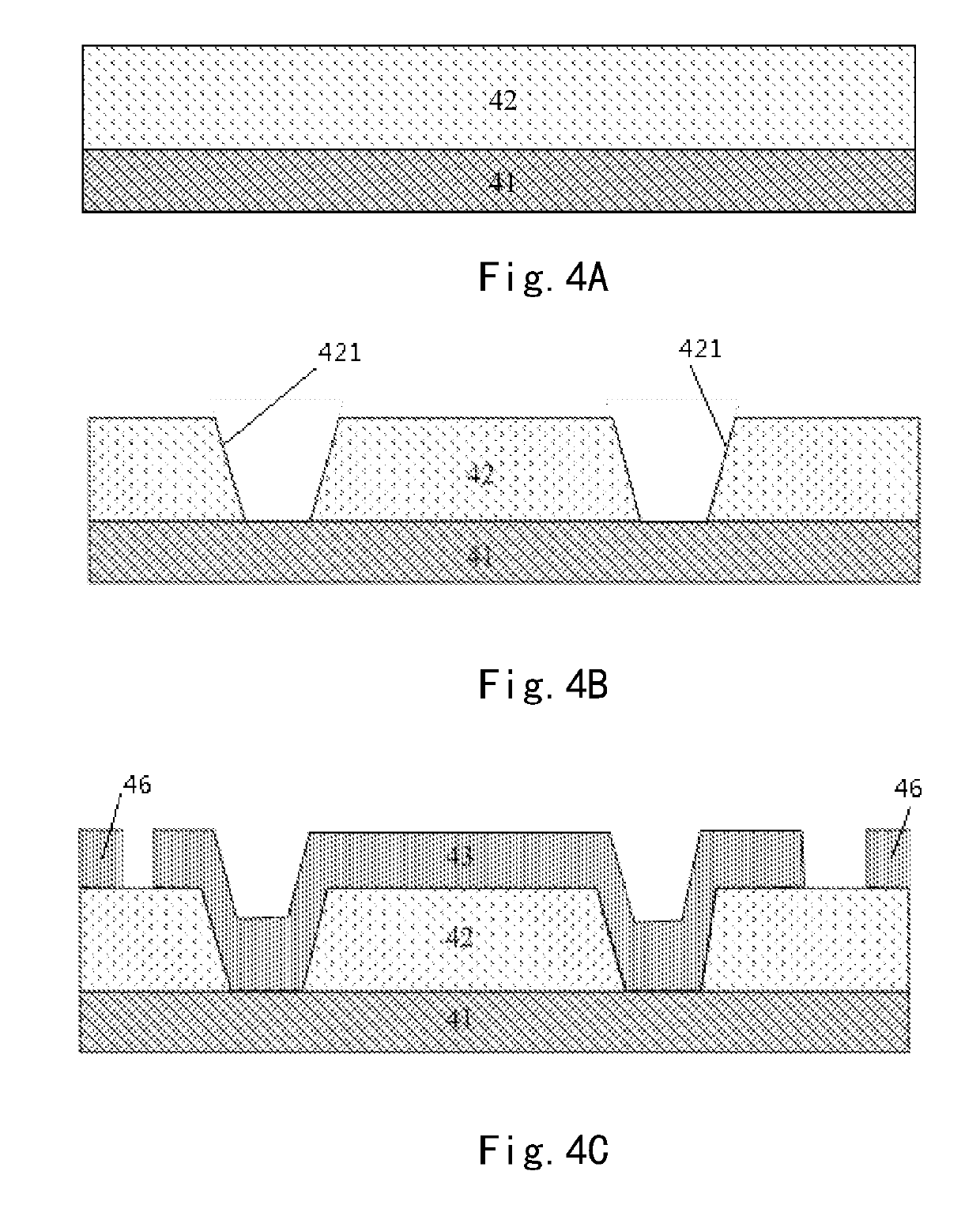
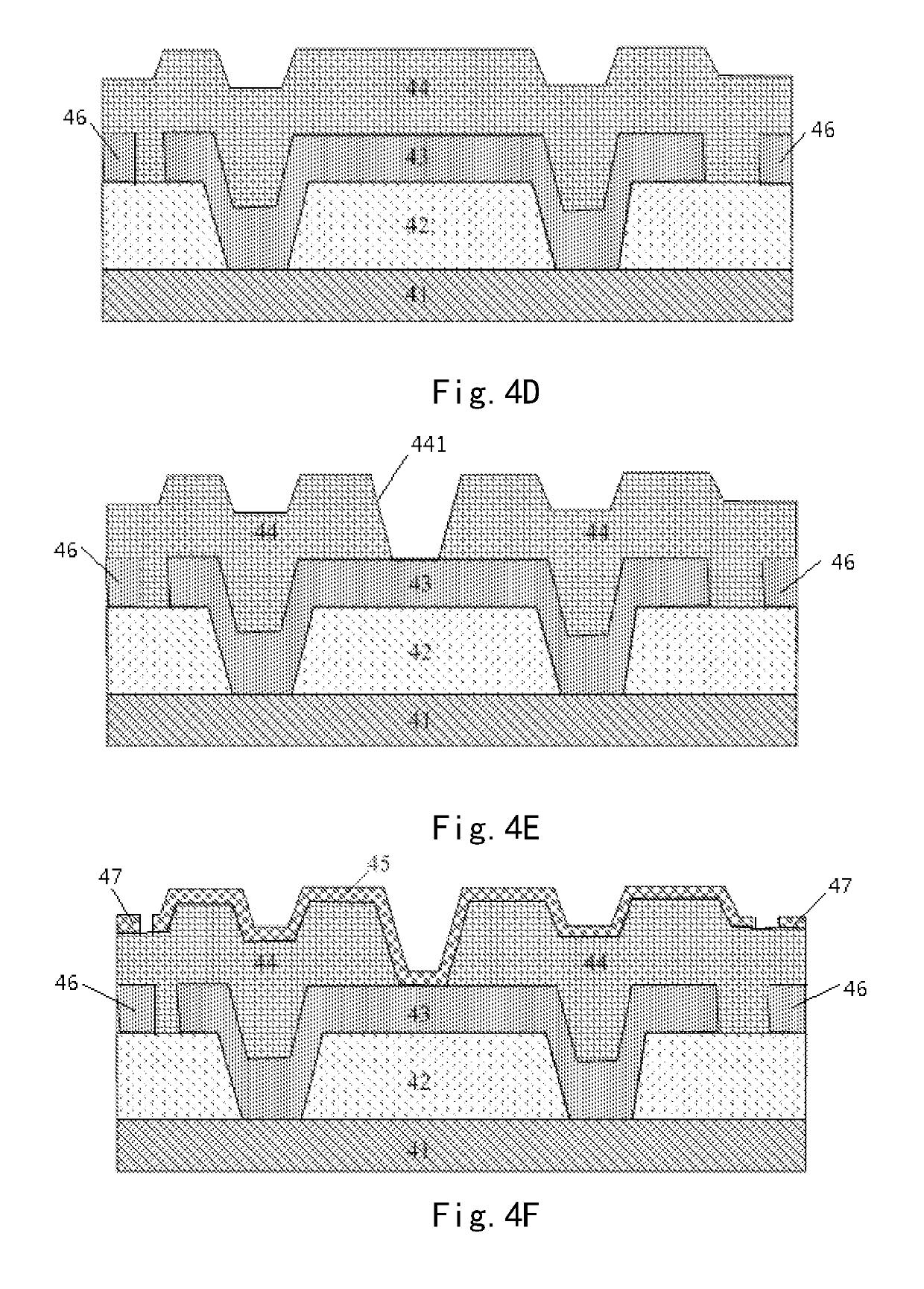
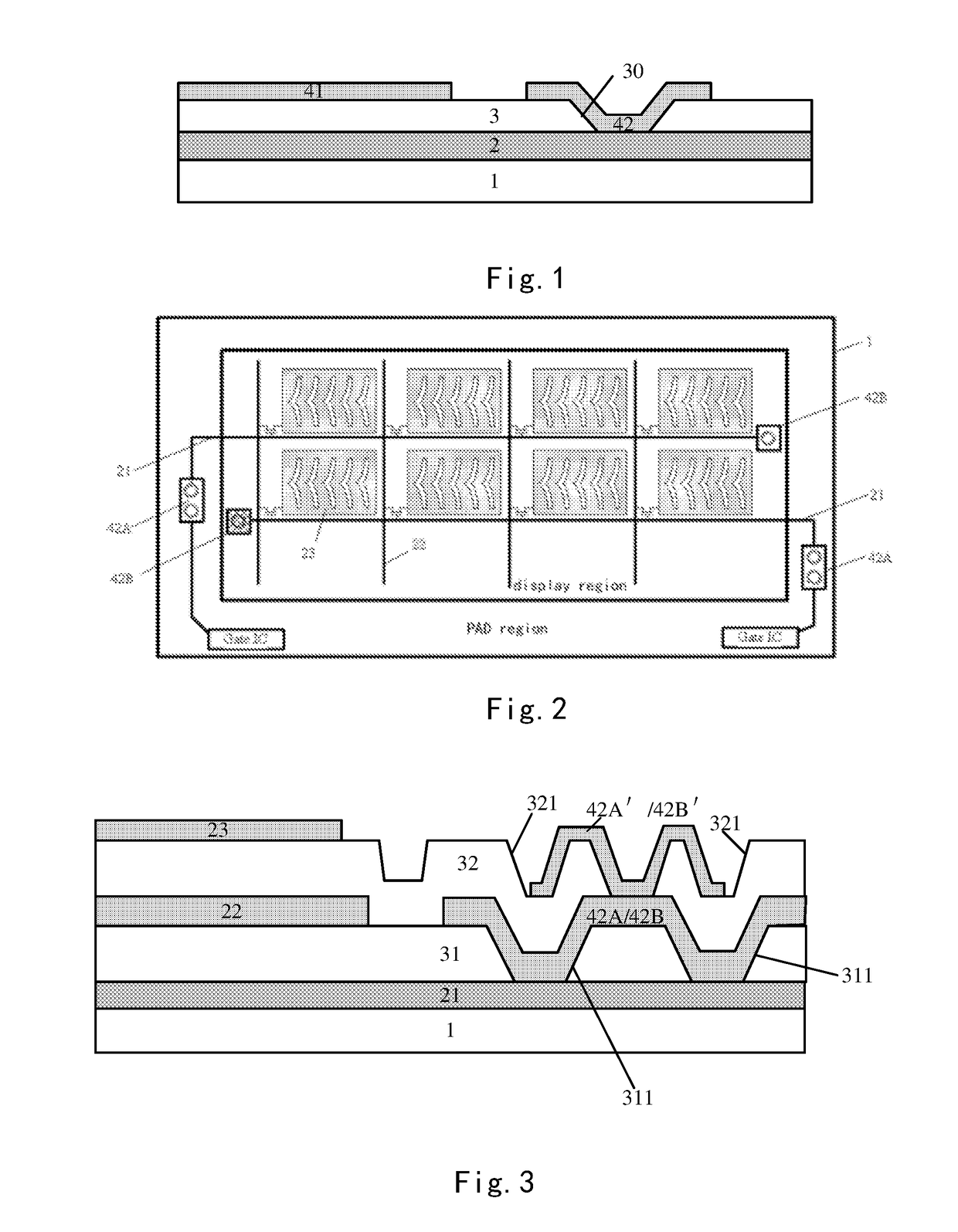
Array substrate with static charge releasing pattern and method for producing the same
ActiveUS10483219B2Eliminate static electricitySemiconductor/solid-state device detailsSolid-state devicesRelease patternStatic Charges
An array substrate includes a metal pattern and an electrically conductive pattern formed sequentially on a base substrate. The electrically conductive pattern is insulated from the metal pattern; and a static charge releasing pattern is formed in a same layer as the electrically conductive pattern and formed by a same material as the electrically conductive pattern, the static charge releasing pattern being insulated from the electrically conductive pattern and electrically connected with the metal pattern.
Owner:BOE TECH GRP CO LTD +1
Intrauterine device and method for reducing the rate of diffusion of active ingredients in said device by adding inert particulates to a polymeric coating
ActiveUS9775905B2Long product lifeEfficient deliveryOrganic active ingredientsInorganic non-active ingredientsMedicineIUD - Intrauterine device
Owner:QPHARMA
Array substrate and method for producing the same
ActiveUS20180047681A1Eliminate static electricitySemiconductor/solid-state device detailsSolid-state devicesRelease patternStatic Charges
Disclosed is an array substrate including: a metal pattern and an electrically conductive pattern formed sequentially on a base substrate, the electrically conductive pattern being insulated from the metal pattern; and a static charge releasing pattern formed in a same layer as the electrically conductive pattern lies and formed by a same material as the electrically conductive pattern, wherein the static charge releasing pattern is insulated from the electrically conductive pattern and electrically connected with the metal pattern.
Owner:BOE TECH GRP CO LTD +1
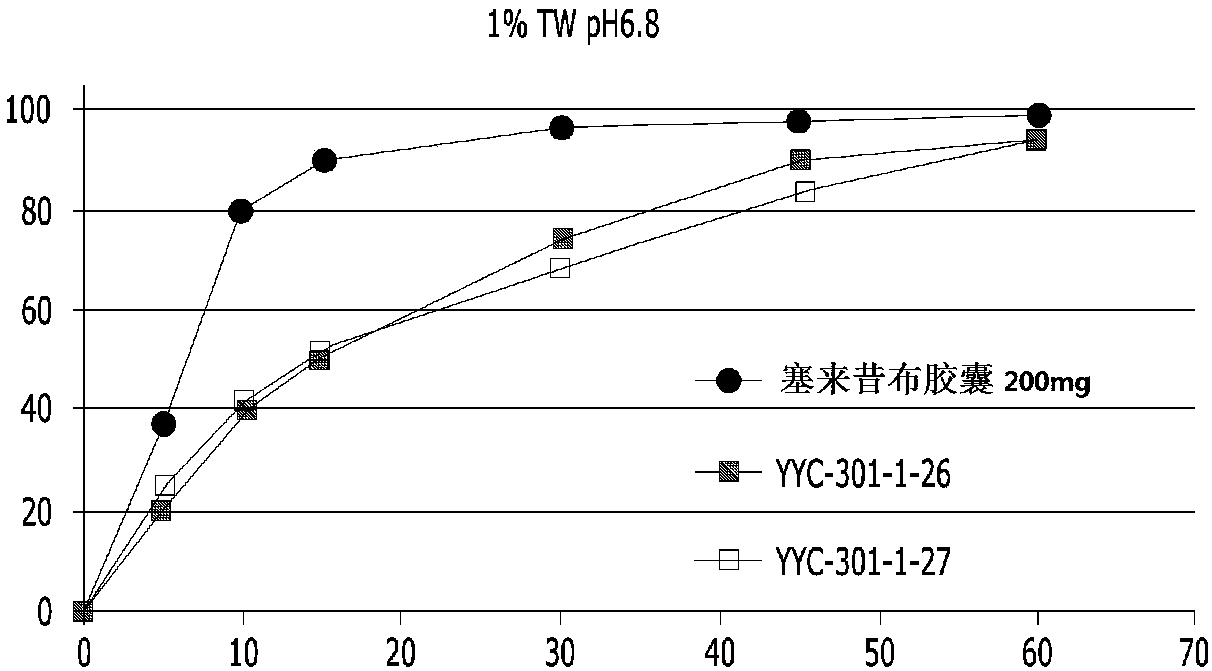
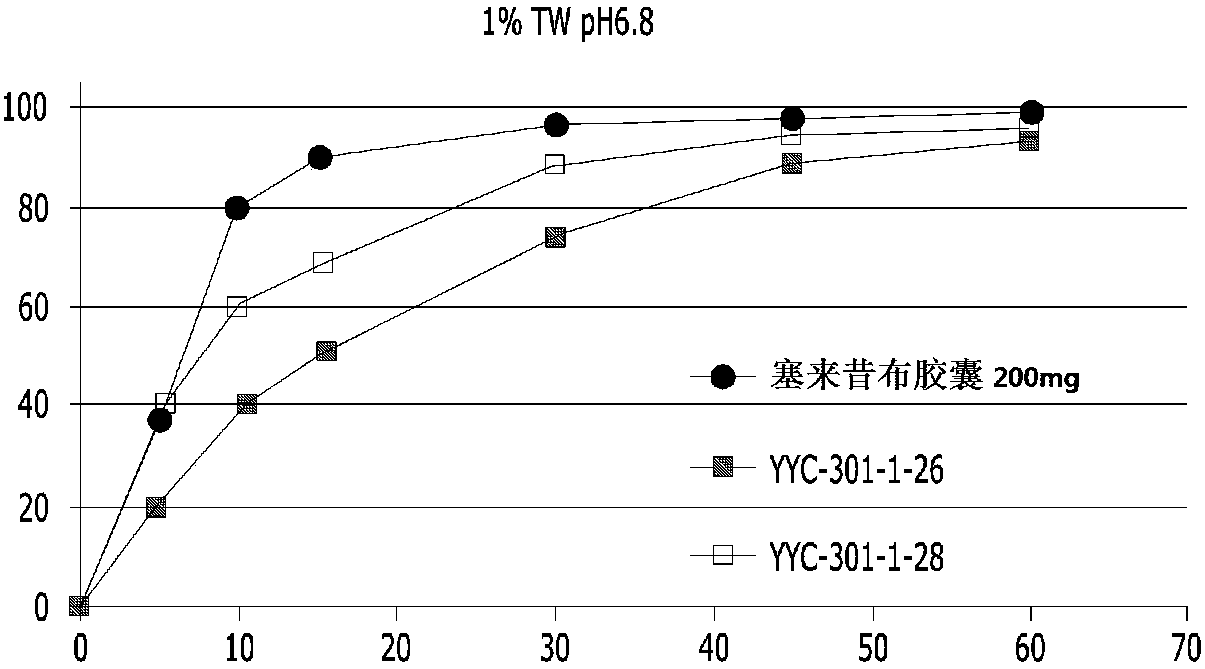
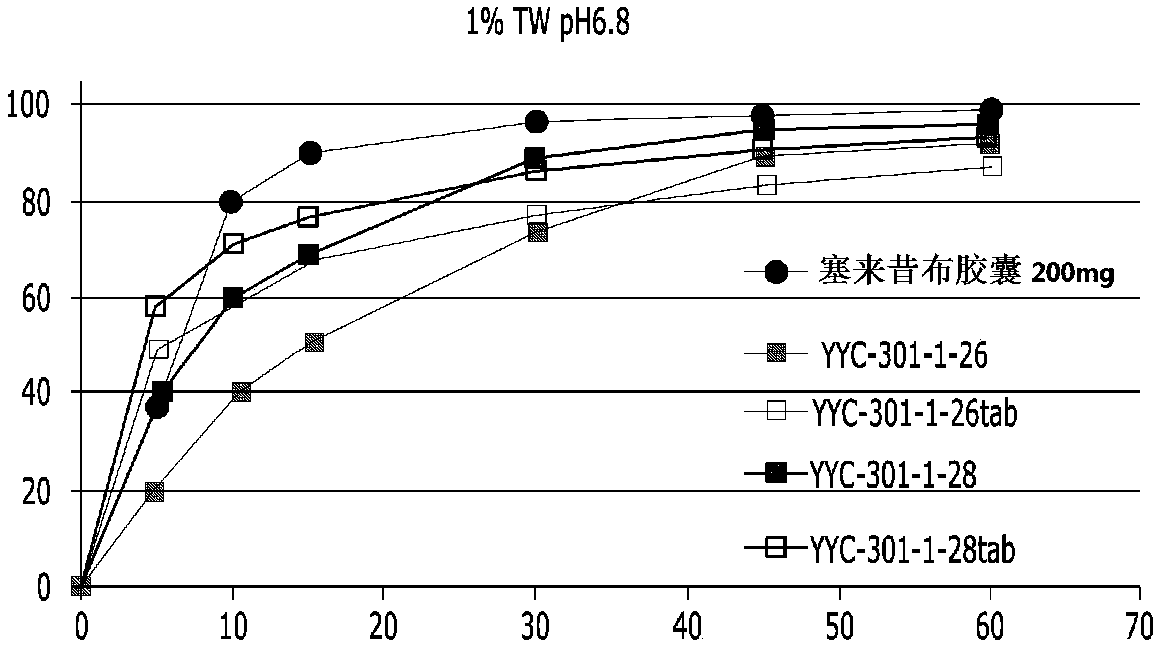
Pharmaceutical composition containing celecoxib and tramadol
InactiveCN108024995AHigh hardnessImprove adhesionNervous disorderAntipyreticPharmaceutical drugBULK ACTIVE INGREDIENT
The present invention relates to a complex containing celecoxib and tramadol. The present invention is designed such that, even though two different active ingredients are prepared into a single dosage form, the release patterns of respective drugs are optimized in the expression of synergy effects, with respect to pain, of the respective drugs, through the new prescription.
Owner:YOO YOUNG PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com

![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00001.png)
![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00002.png)
![Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient Sustained-release preparation containing 5-acetyl-4,6-dimethyl-2[2-[4-(2-methoxyphenyl) piperazinyl]ethylamino] pyrimidine trihydrochloride as active ingredient](https://images-eureka.patsnap.com/patent_img/181e47a5-361c-46e2-bba8-4891943a3203/US07041317-20060509-D00003.png)