Novel controlled release-niacin formulation
A technology for controlled-release preparations and niacin, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc. Irregular release patterns, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] When preparing the niacin controlled-release preparation of the present invention, the solvent added to the powder mixture includes all solvents that do not affect the activity of the active ingredient niacin, and is usually used in the preparation of granules. This solvent is known in the prior art. Examples thereof may include one or a mixture selected from the group consisting of water, ethanol, isopropanol, glycerol, propylene glycol, and polyethylene glycol, but are not limited thereto. As the liquid solvent, ethanol or a mixed solvent of water and ethanol is preferably used. At this time, when the solvent is water alone or a mixed solvent of water and ethanol, based on the total weight of the drug, the solvent is preferably used in an amount of 5 to 40% by weight, more preferably 10 to 23% by weight. In addition, in a preferred embodiment, the preparation method of the present invention may further include a step of mixing an adhesive in step (a).
[0049] In a specif...
Embodiment 1
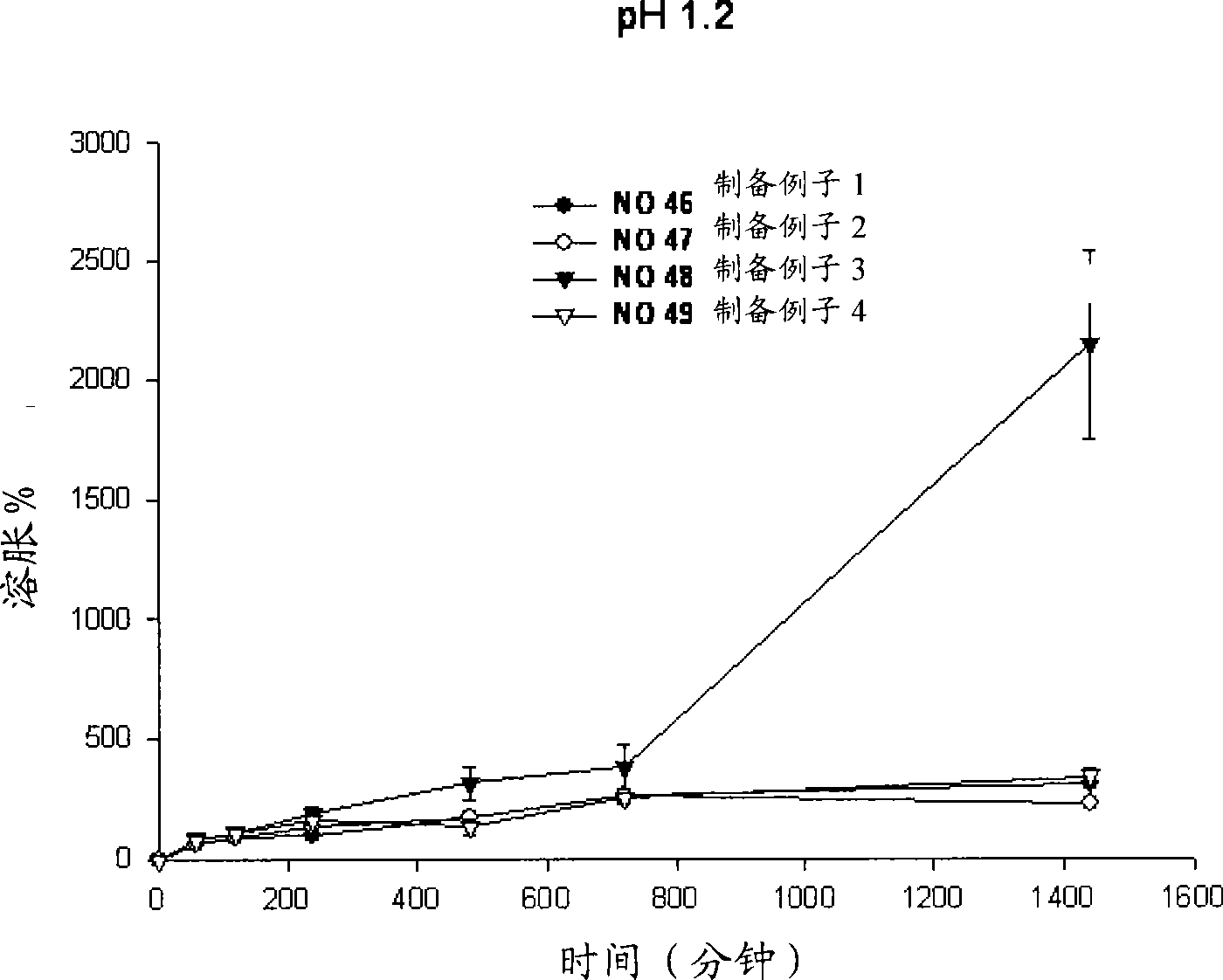
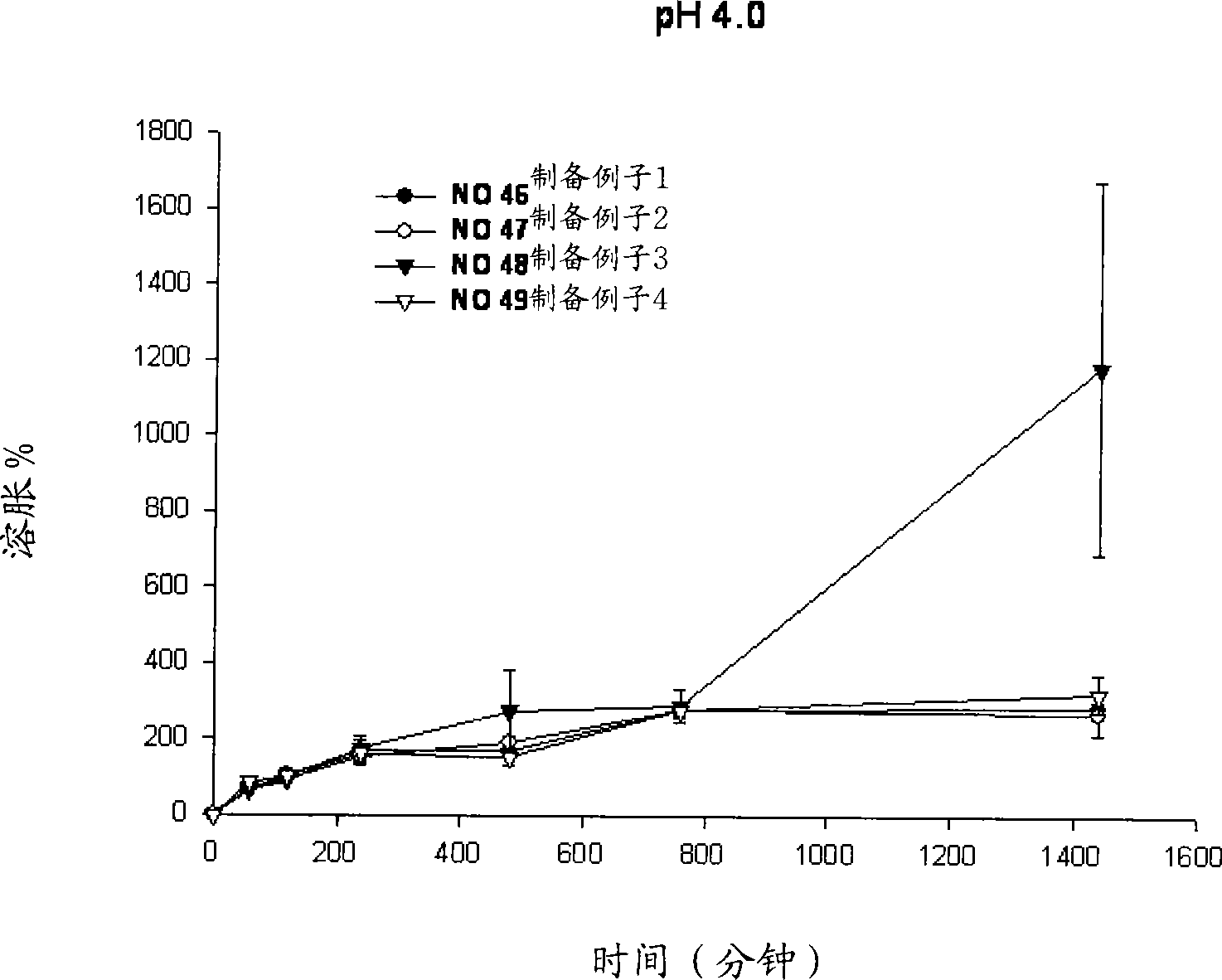
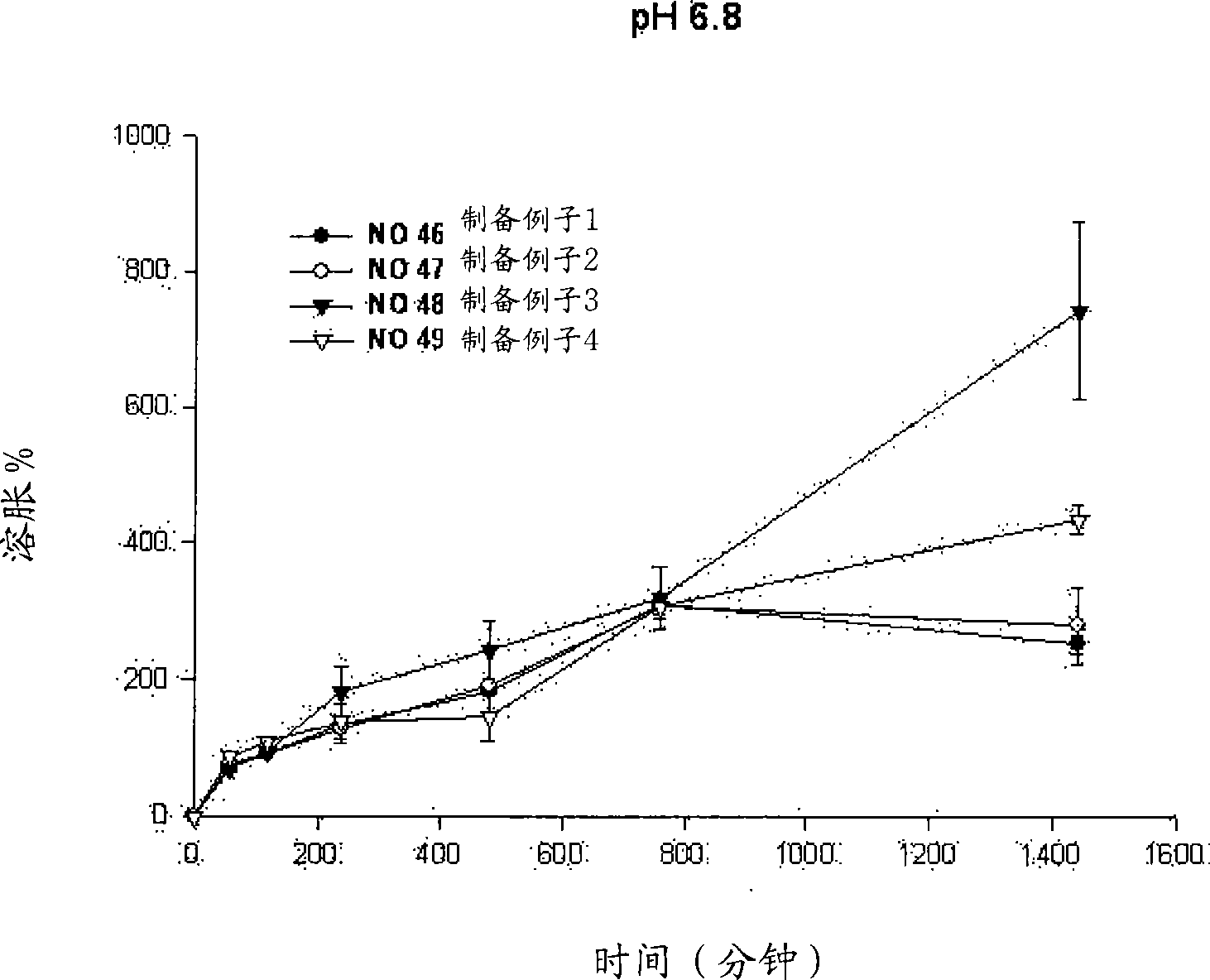
[0053] Example 1: Swelling reagents for different types of polymer substrates
[0054] Perform different types of polymer-based swelling reagents to observe whether the niacin preparation of the present invention releases the drug within the required time period, and maintains its matrix shape during the drug release process.
[0055] Specifically, niacin preparations were prepared according to the following preparation examples 1 to 4.
[0056] Preparation example 1 (No. 46)
[0057] Mix 500.0 mg of niacin as a drug, 90 mg of lactose as an excipient, and 90 mg of microcrystalline cellulose to increase the fluidity of the drug. 170 mg of HPMC2208 (100,000 cps) as a polymer base was added to the powder mixture and mixed uniformly. Subsequently, 0.01 ml of ethanol was sprayed to prepare wet granules.
[0058] The prepared particles were dried in an oven at 60°C and then uniformly ground. Subsequently, 16 mg of magnesium stearate was mixed for the molding of the formulation. Tabl...
Embodiment 2
[0070] Example 2: Comparative dissolution experiment of a commercial formulation and the formulation of the present invention
[0071] A dissolution experiment was conducted to confirm whether the controlled-release niacin formulation and commercial sustained-release formulation of the present invention can maintain their matrix shape for the required time period (24 hours or longer). According to the present invention, a niacin controlled-release preparation containing 500 mg of niacin (components: 500 mg of niacin, 90 mg of lactose, 90 mg of microcrystalline cellulose, 170 mg of HPMC, and 16 mg of magnesium stearate) and commercial cigarettes Acid sustained-release preparation, Niaspanor TM The swelling experiment was carried out at 37°C at 50 rpm and 900 ml of water for 24 hours. The dissolution experiment was performed using the dissolution experiment equipment PT4005956 produced by Pharma Experimental Plant (Germany) and the stirring method of the United States Pharmacopoei...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com